- 1Research Team: Cell Signaling, Department of Biology, Faculty of Sciences, Moulay Ismail University, Meknes, Morocco
- 2Department of Biology, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, Saudi Arabia
- 3Department of Biology, College of Science, Qassim University, Buraydah, Saudi Arabia
- 4Laboratory of Chemistry-Biology Applied to the Environment, Faculty of Sciences, Moulay Ismail University, Meknes, Morocco
- 5Faculty of Sciences and Techniques of Errachidia, Moulay Ismail University, Errachidia, Morocco
- 6Laboratory of Engineering and Applied Technologies, Higher School of Technology, Sultan Moulay Slimane University, Beni Mellal, Morocco
Medicinal and aromatic plants offer sustainable alternatives to conventional feed additives in heliciculture. In this study, we evaluated dietary inclusion (3% w/w) of Rosmarinus officinalis, Origanum compactum and Thymus zygis subsp. gracilis in Otala tingitana reared under controlled conditions (n = 360). Plant preparations were characterized for proximate composition, total phenolics, flavonoids, antioxidant capacity (DPPH) and antibacterial activity. Over a 142-day trial, supplemented diets maintained comparable final body weight and shell length to controls while enhancing growth rate during the exponential phase and improving feed conversion efficiency, particularly with R. officinalis. Dietary supplementation substantially reduced cumulative mortality (4.4% vs. 22.4% in control) and accelerated sexual maturation (>93% vs. 75.6% in control). Microbiological analyses of snail flesh revealed significant reductions in total aerobic counts, coliforms and Staphylococcus aureus; while Salmonella spp. and Listeria spp. were not detected. These outcomes indicate that 3% inclusion of the tested Moroccan medicinal plants improves survival, growth efficiency, and hygienic quality of O. tingitana without adverse effects on somatic development. Adoption of such phytobiotic supplements could enhance sustainability and food safety in heliciculture; future studies should optimize formulations, elucidate modes of action and assess long-term reproductive and ecological impacts. This work supports translational studies toward commercial feed applications.
1 Introduction
The growing global demand for snails as a novel food source and their diverse applications in industries such as cosmetics and pharmaceuticals have positioned heliciculture as a sustainable and lucrative alternative to wild snail collection (Pissia et al., 2021). However, the industry faces significant challenges. While excessive harvesting and its impact on biodiversity are primary concerns for wild snail populations, infectious diseases represent a major threat to the progress and productivity of snail farming operations. One of the most critical issues in snail farming is the high mortality rate caused by pathogens infections, as snails are susceptible hosts for various parasites throughout their lifecycle (Segade et al., 2013; Pissia et al., 2021). At the same time, the use of antibiotics in animal diets either for growth promotion purposes has been increasingly restricted or banned in the European Union and many other countries due to their role in fostering antibiotic resistance in animals and humans (Schmerold et al., 2023). This has prompted the search for alternative strategies to manage microbial threats without affecting growth performance in farming systems. Evidence suggests that implementing hygienic farming practices and exploring sustainable feed additives could effectively address this issue without relying on the use of antibiotics. Among these alternatives, natural products such as herbs, plant extracts, and essential oils have shown promise in enhancing animal production by mitigating microbial invasions, improving feed conversion, and promoting growth (Steiner and Syed, 2015; Cheng et al., 2024; Fang et al., 2024; Kamble et al., 2024).
Recent farming practices are increasingly adopting natural plant compounds as feed supplements in animal nutrition, influenced by the growing demand for sustainable and health-conscious livestock production. These compounds, often derived from herbs, spices, and plant secondary metabolites, serve as alternatives to synthetic growth promoters and antibiotics (Mahfuz et al., 2021). Bioactive molecules such as essential oils, polyphenols, tannins, and saponins have demonstrated potential in improving animal health, enhancing growth performance, and modulating gut microbiota (Lillehoj et al., 2018; Patra et al., 2019). Essential oils, for example, exhibit antimicrobial properties, reducing pathogenic microorganisms in the gut, while polyphenols act as antioxidants, protecting against oxidative stress and improving immune responses (Gessner et al., 2017; Simitzis, 2017). These natural additives also contribute to better feed utilization, increased nutrient absorption, and reduced environmental impacts, as they minimize nitrogen and methane emissions (Alem, 2024). Additionally, incorporating plant-based feed additives aligns with consumer preferences for organic and antibiotic-free meat products (Franz et al., 2010).
Medicinal and aromatic plants like Rosmarinus officinalis, Origanum compactum, and Thymus zygis subsp. gracilis have shown potent antimicrobial, antioxidant, and antiparasitic activity (Bouyahya et al., 2020; Ed-Dra et al., 2020; Bouymajane et al., 2022a). These plants have been widely studied for their potential to improve animal production as phytobiotics and demonstrated beneficial effects across various animal models. For instance, R. officinalis supplementation in chicken feed has been shown to enhance antioxidant activity and elevate serum protein levels (Zhong and Zhou, 2013). Similarly, O. compactum has been linked to reduced mortality in rabbits (Benlemlih et al., 2020), while the introduction of T. gracilis leaves in ewes’ diet has improved lamb meat quality and reduced bacterial contamination (Nieto et al., 2010). The potential activity of these plants is attributed to their richness in phytochemical compounds such phenolic acids like quinic acid, rosmarinic acid, and caffeic acid, along with flavonoids like hesperidin, luteolin derivatives, and apigenin (Bouymajane et al., 2022b; Chroho et al., 2022; Vladimir-Knežević et al., 2022; Ed-Dra et al., 2024). Additionally, their essential oils are rich in secondary metabolites like thymol, carvacrol, and 1,8-Cineole (Ed-Dra et al., 2020; Al-Maharik et al., 2022; Al-Mijalli et al., 2022; Bouymajane et al., 2022a). These compounds have shown notable efficacy against pathogens such as Listeria monocytogenes, Staphylococcus aureus, and Salmonella Typhimurium, among others.
Morocco has emerged as a leading global producer of land snails, with the sector playing a vital role in both agricultural exports and rural livelihoods. FAOSTAT data indicate that in 2017, Morocco produced 16,520 tons of land snails and accounting for nearly all of North Africa’s total output of 17,505 tons and contributing significantly to the global supply (Caetano et al., 2021). Furthermore, Morocco is one of the countries in the Mediterranean basin with the highest rates of snail consumption, alongside Spain, France, and Portugal. This high demand is driven by snails’ nutritional value, as they are a good source of easily digestible nutrients (Rygało-Galewska et al., 2022; El Khayari et al., 2025). Therefore, Morocco’s snail industry is expected to continue serving as a key pillar of the nation’s agricultural economy while providing an important source of income and employment for many communities across the country.
The breeding of O. tingitana, an endemic edible snail in Morocco, has garnered increasing attention due to its economic and agricultural potential. Recent research has focused on optimizing environmental conditions to improve its growth and reproduction performance. In this regard, experiments in controlled environment indicate that a combination of 20 °C temperature, high relative humidity (around 80%), and a specific photoperiod (16 h of light and 8 h of darkness) enhances mating, egg-laying, and growth performance of O. tingitana snails (El Khayari and Rour, 2021; El Khayari et al., 2023). These optimized conditions allow for up to two complete life cycles annually, significantly boosting productivity and meeting market demands. Additionally, the species has demonstrated resilience under varied environmental factors, showcasing its adaptability to controlled rearing systems. However, microbial and parasitic infections pose a significant threat to O. tingitana, underscoring the need for sustainable solutions to mitigate these challenges.
This study explores the use of R. officinalis, O. compactum, and T. gracilis as feed supplements to improve nutrition, health, and production performance of O. tingitana. The primary objectives are to evaluate the effects of these plants on growth performance, assess their antimicrobial properties, and develop a sustainable heliciculture protocol to address microbial contamination in snail farming and safeguard biodiversity.
2 Materials and methods
2.1 Plant materials and extracts preparation
Three plant species, namely R. officinalis, O. compactum, and T. gracilis, were carefully selected based on previous ethnobotanical investigations and documented bioactivities, with a particular focus on their nutritional and antimicrobial benefits (Ricci et al., 2023). The plant species were cultivated in the Meknes region and purchased from a local market in Meknes, Morocco. Botanically authenticated in our laboratories at Faculty of Sciences, Moulay Ismail University, Meknes, Morocco. Plant materials were air-dried at ambient laboratory conditions to constant weight, milled to a fine powder using a stainless-steel grinder, and stored in airtight, light-protected containers at room temperature until chemical analyses and extract preparation.
2.2 Proximate analysis
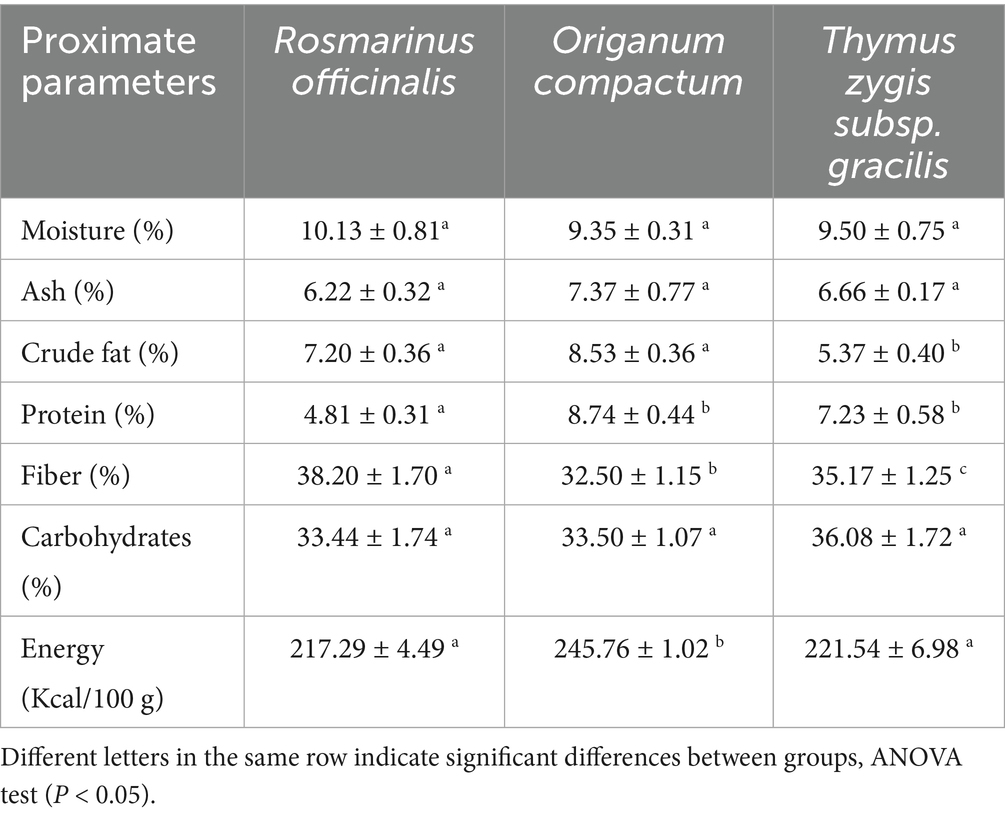
The selected plant species (previously ground to a fine powder) were subjected to proximate analysis following standard methods (AOAC, 2023; Kefale et al., 2023). The contents of moisture, ash, protein, crude fat, fiber, as well as carbohydrates and energy, were determined for each species. Moisture content was measured by drying 5 g of powdered sample at 105 °C. While incineration at 550 °C for 4 was used to determine ash. The Kjeldahl method was used to analyze and quantify protein content, with a nitrogen-to-protein conversion factor of 6.25. The Soxhlet extraction method was used to determine crude fat content. However, fiber content was determined by sequential digestion with sulfuric acid and sodium hydroxide. Carbohydrate content was calculated by subtracting the sum of moisture, crude protein, fat, ash, and fiber contents from 100. The energy content was calculated using the following formula and expressed as kcal per 100 g of dry powder:
2.3 Extracts preparation
Maceration was used for the preparation of extracts. Twenty grams of each plant powder were macerated in 200 mL of a hydroethanolic solution (20:80, v/v) for 24 h at room temperature. Then, the solutions obtained were filtered using Whatman No. 4 filter paper and the solvent was evaporated using a rotary evaporator (Büchi Rotavapor R-200, Flawil, Switzerland). Afterward, the dried extract was collected, yielded, and stored at −20 °C until use.
2.4 Phenolic and flavonoid contents
Folin–Ciocalteu method was used to quatify total phenolic content (TPC) and results were expressed as milligrams of gallic acid equivalent (GAE) per 100 mL of sample (Phong et al., 2022). Additionally, flavonoid content was assessed using aluminum chloride colorimetric method, following the previously published protocol (Phong et al., 2022), and results were expressed in milligrams of quercetin equivalent per 100 mL of juice (mg QE/100 mL). The experiments were conducted in triplicate to ensure the repetability and reproductibility of assays.
2.5 Biological activities
2.5.1 Antioxidant activity
The antioxidant activity provides insight into the ability of the extract to neutralize free radicals, a mechanism of particular interest due to its potential protective role in reducing oxidative stress and thereby preventing disease in animals. The antioxidant activity of the plant extracts was evaluated using the DPPH radical scavenging method, as previously described (Mrabti et al., 2021). Ascorbic acid was used as the standard control, and measurements were performed in triplicate to ensure reliability and reproducibility.
2.5.2 Antibacterial activity
To evaluate the ability of the studied plant extracts to reduce or eliminate bacterial populations during the heliciculture of O. tingitana, in vitro antibacterial assays were carried out using the disc diffusion and microbroth dilution methods, following previously published protocols (Mrabti et al., 2021; Ed-Dra et al., 2025). For this, four bacterial strains, incuding Staphylococcus aureus ATCC 29213, Bacillus subtilis ATCC 6633, Salmonella Typhimurium ATCC 700408, and Escherichia coli ATCC 25922 were selected for this study.
To conduct antibacterial assay, an initial concentration of 500 mg/mL of each plant extract was prepared in sterile distilled water. Then, 20 μL of each extract was applied onto a 6 mm sterile paper disc, which was placed on Petri dishes containing Mueller-Hinton agar (Biokar, Beauvais, France), previously inoculated with bacterial strains and incubated at 37 °C for 24 h (Mrabti et al., 2021). Then, the inhibition zones (including disc diameter) created with each extract were measured in millimeters. Sterile distilled water was used as negative control and chloramphenicol (30 μg) was used as positive control. All assay were conducted in triplicate.
Additionally, the quantification of antibacterial activity was assessed by determining the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) using the microbroth dilution method, as described previously (Mrabti et al., 2021). Subsequently, the antibacterial effect of the extract was evaluated by calculating the MBC/MIC ratio (r). The extract was classified as having a bactericidal effect if r ≤ 4, and a bacteriostatic effect if r > 4 (Jaber et al., 2021).
2.6 Animals and diet formulation
To assess the effects of the three medicinal and aromatic plants as feed supplements in snail nutrition and their potential benefits on the growth and flesh quality of O. tingitana snails, a total of 360 newly hatched snails were reared under well-controlled conditions, maintaining 80% relative humidity, a temperature of 20 °C, and an 8/16-h light/dark cycle. The snails were randomly divided into four groups, each subjected to different dietary conditions: Group A served as the control and received a basal diet consisting solely of flour, Group B was fed with a basal diet supplemented with 3% R. officinalis plant, Group C received a basal diet with 3% O. compactum plant, and Group D was provided with a basal diet containing 3% T. gracilis plant. Each diet was replicated three times in separate rearing enclosures, resulting in a total of 90 snails per dietary condition. Within each rearing box, 30 one-day-old snails were placed, with an average individual body weight of 9.45 ± 0.80 mg and an average size of 2.17 ± 0.33 mm. All procedures involving the handling of snails were conducted in accordance with ethical and scientific standards. The experiments were carried out within controlled laboratory facilities.
2.7 Feeding and growth performance monitoring
The experimental trial was conducted in a randomized design, under homogenous conditions as described previously (El Khayari and Rour, 2021; El Khayari et al., 2023). The initial body weight (BW) and state of the snails were approximately similar across conditions. For monitoring the growth performance, several parameters including: Daily Average Weight Gain (DAWG), Linear Shell Gain (LSL), Daily Average Linear Shell Gain (DASG), Feed Intake (FI), Daily Consumption Rate (DCR), Ecological Efficiency of Growth (EEC), Mortality, and Maturation Time, were continuously recorded daily throughout the 142- day trial. The average of all parameters was calculated and reported every 14 days for each group. The mathematical formulas used to calculate the growth performance parameters were described in previous studies (El Khayari and Rour, 2021; El Khayari et al., 2023).
2.8 Microbial quality of snail flesh
After the 142-day trial, the snails from each feeding condition were euthanized, and their flesh removed for microbiological analysis. The microbiological analysis involved the enumeration of Total Aerobic Mesophilic Flora (TAMF), Total coliforms, Fecal coliforms, Anaerobic Sulfate-Reducing Bacteria (ASRB), Escherichia coli, and Staphylococcus aureus, as well as the detection of Salmonella spp. and Listeria monocytogenes.
For the microbiological analysis, 25 g of snail flesh was mixed with 225 mL of sterile buffered peptone water (Biokar, Beauvais, France) and homogenized for 180 s using a stomacher device (400 Circulator, Seward). Ten-fold serial dilutions were then prepared for microbial enumeration. TAMF were enumerated by inoculating 1 mL of each dilution into Plate Count Agar (PCA, Biokar, Beauvais, France) and incubated at 30 °C for 72 h (ISO 4833-1, 2013). Total and Fecal coliforms were enumerated by inoculating 1 mL of each dilution into Violet Red Bile Lactose (VRBL) agar (Biokar, Beauvais, France), followed by incubation for 24 h at 30 °C and 44 °C, respectively (ISO 4832, 2006). ASRB were enumerated by inoculating 1 mL of each dilution into Tryptone Sulfite (TS) Agar (Biokar, Beauvais, France) and incubated anaerobically at 46 °C for 24 h (ISO 15213-1, 2023). E. coli was enumerated by inoculating 1 mL of each dilution into Tryptone-Bile-Glucoronate (TBX) agar (Biokar, Beauvais, France) and incubated at 44 °C for 24 h (ISO 16649-2, 2001). S. aureus was enumerated by culturing 0.1 mL of each dilution on the surface of Baird Parker Rabbit Plasma Fibrinogen Agar (Biokar, Beauvais, France) followed by incubation at 37 °C for 48 h (ISO 6888-2, 2021). However, the detection of Salmonella and L. monocytogenes in snail flesh was conducted following the previously published protocols (Ed-Dra et al., 2017; Bouymajane et al., 2019). The results obtained were used for a comparative analysis to evaluate the effect of dietary supplementation on the bacterial load in snail flesh. In addition, the results from the four dietary conditions were also compared to those from mature wild snails (n = 30) collected in the El Hajeb region of Morocco (33°41′19.7“N, 5°24′29.7”W). These specimens had an average weight of 5.58 ± 0.7 g and a maximum diameter of 2.7 ± 0.4 cm. The region has annual rainfall of 575.5–576.5 mm, an average annual temperature of 6.930–6.945 °C (NASA POWER), and a photoperiod ranging from 10 L:14D in December to 14 L:10D in June (Van der Klein et al., 2018).
2.9 Statistical analysis
Three replicates were used in the experiments carried out in this research study, and data were expressed as mean values ± SD (standard deviation). Differences were measured using a non-parametric t-test or one-way analysis of variance (ANOVA) followed by Tukey’s test. The p-value < 0.05 was used for significance difference. The statistical analysis was conducted using GraphPad Prism version 9 software (GraphPad, San Diego, CA, USA).
3 Results
3.1 Proximate analysis
To evaluate the nutritional properties of the studied plant species, we conducted a physicochemical characterization, and the results are summarized in Supplementary Table S1. Our findings indicate that the plant species were rich in nutritional compounds, with no significant differences in moisture, ash, and carbohydrates content (p > 0.05). T. gracilis exhibited lower level of crude fat (5.37 ± 0.40%) compared to other plant species (p < 0.05). However, R. officinalis exhibited a lower level of protein (4.81 ± 0.31%) and a higher level of fiber (38.20 ± 1.70%) (p < 0.05). O. compactum exhibited signifcantly higher energy (245.76 ± 1.02 Kcal/100 g), followed by T. gracilis (221.54 ± 6.98/100 g) and R. officinalis (217.29 ± 4.49 Kcal/100 g) (Table 1).
3.2 Bioactive compounds
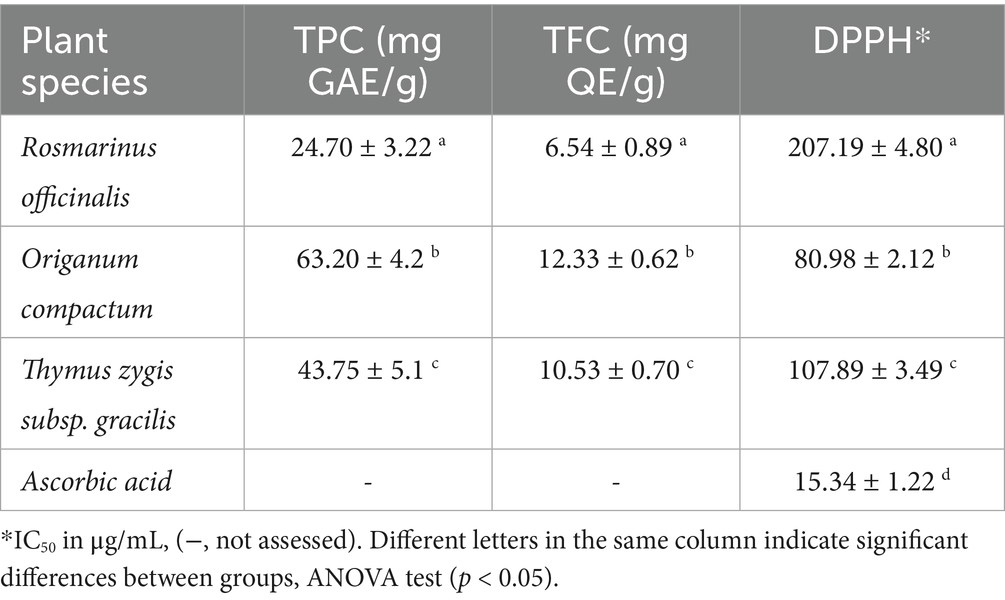
The bioactive compounds in the studied plant species were determined using hydroethanolic maceration extracts and results were presented in Supplementary Table S2. Our results revealed that O. compactum extract had the highest TPC and TFC, with values of 63.20 ± 4.2 mg GAE/g and 12.33 ± 0.62 mg QE/g, respectively (p < 0.05). This was followed by T. gracilis extract with TPC of 43.75 ± 5.1 mg GAE/g and TFC of 10.53 ± 0.70 mg QE/g, and R. officinalis extract with TPC of 24.70 ± 3.22 mg GAE/g and TFC of 6.54 ± 0.89 mg QE/g. These differences in bioactive compound content likely influenced antioxidant activity. O. compactum exhibited the strongest antioxidant capacity, as indicated by the lowest DPPH IC50 value (90.28 ± 1.14 μg/mL). T. gracilis showed moderate activity with IC50 of 127.89 ± 3.49 μg/mL, while R. officinalis had the lowest antioxidant activity with IC50 of 207.19 ± 4.80 μg/mL. However, ascorbic acid showed an IC50 of 15.34 ± 1.22 μg/mL (Table 2).
3.3 Anibacterial activity
Antibacterial activity was assessed using the disc diffusion and microbroth dilution methods, and the results are presented in Figure 1 and Table 3.
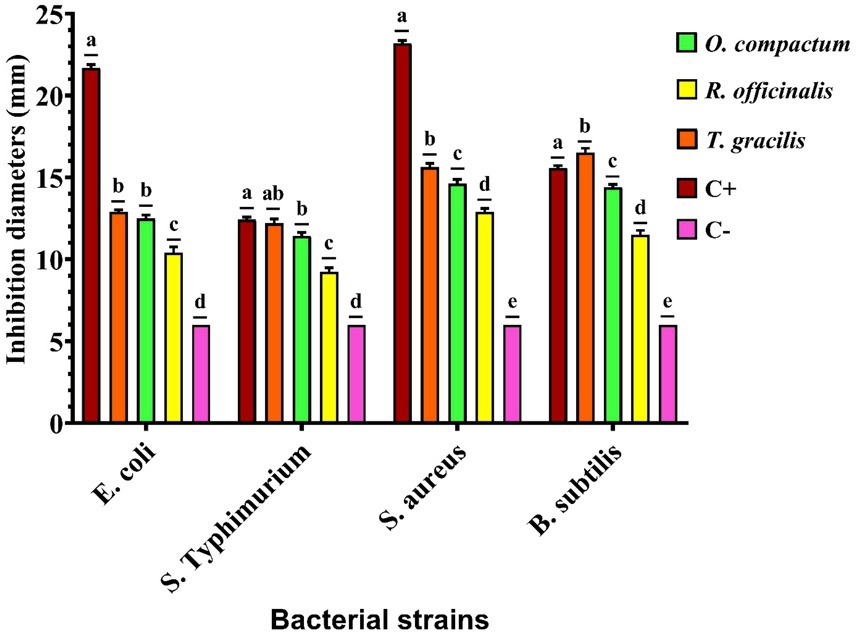
Figure 1. Inhibitory diameters of Rosmarinus officinalis, Origanum compactum, and Thymus zygis subsp. gracilis extracts. C+, positive control (chloramphenicol); C−, negative control (sterile distilled water). Different letters indicate significant differences between groups, ANOVA test (p < 0.05).
The disc diffusion method revealed that T. gracilis extract exhibited slightly higher antibacterial activity, with inhibition zones ranging from 15.63 ± 0.23 mm to 16.53 ± 0.25 mm against Gram-positive bacteria and 12.20 ± 0.26 mm to 12.90 ± 0.12 mm against Gram-negative bacteria. This was followed by O. compactum extract, which showed inhibition zones of 14.40 ± 0.17 mm to 14.63 ± 0.25 mm against Gram-positive bacteria and 11.43 ± 0.21 mm to 12.50 ± 0.20 mm against Gram-negative bacteria. In contrast, R. officinalis extract displayed the lowest activity, with inhibition diameters of 11.50 ± 0.26 mm to 12.90 ± 0.20 mm against Gram-positive bacteria and 9.23 ± 0.25 mm to 10.40 ± 0.36 mm against Gram-negative bacteria (Supplementary Table S3).
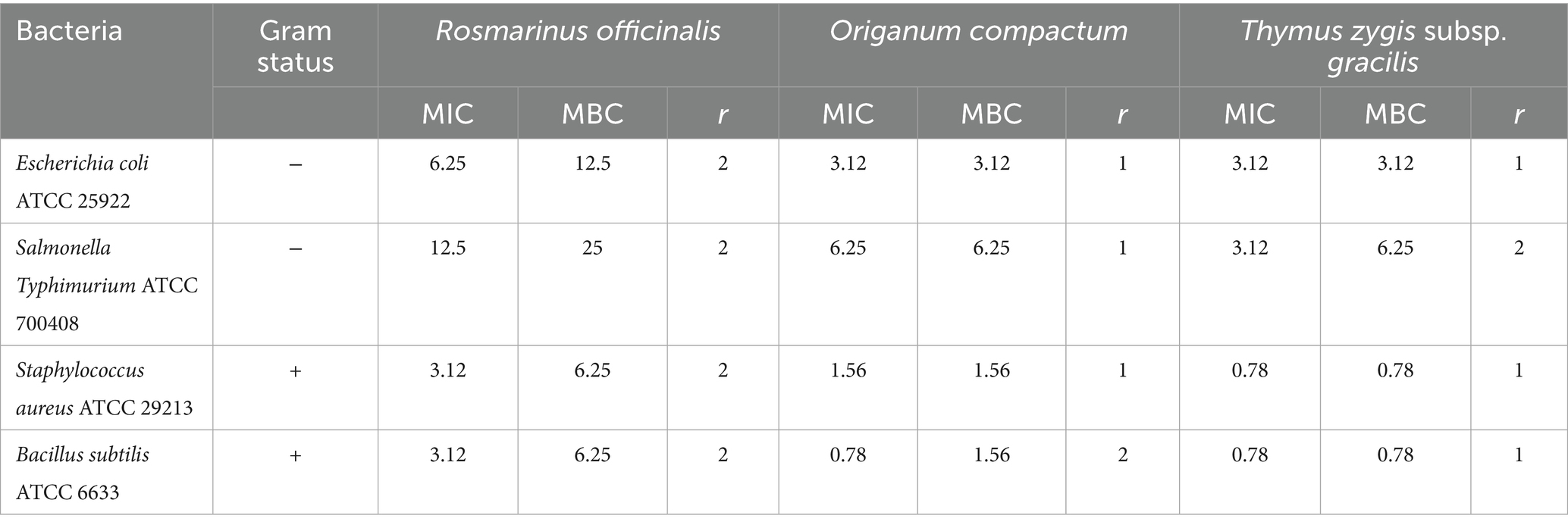
Similarly, the microbroth dilution assay confirmed the antibacterial activity of the tested extracts. T. gracilis exhibited a MIC of 0.78 mg/mL against Gram-positive bacteria and 3.12 mg/mL against Gram-negative bacteria. O. compactum showed MIC values between 0.78 and 1.56 mg/mL (Gram-positive) and 3.12 to 6.25 mg/mL (Gram-negative). Meanwhile, R. officinalis extract had a MIC of 3.12 mg/mL against Gram-positive bacteria and 6.25 to 12.5 mg/mL against Gram-negative bacteria.
Additionally, the determination of the minimum bactericidal concentration (MBC) indicated that all extracts possessed a bactericidal effect, with an MBC/MIC ratio of less than 4 (Table 3).
3.4 Body weight, linear shell length, and average daily weight gain
The animals that received various feed supplements demonstrated comparable BW and LSL to the control group (Figures 2A,D). Throughout the experiment, growth patterns were consistent and favorable, characterized by an initial phase of exponential growth lasting until the 14th week, followed by a slower growth phase until the end of the trial. During the exponential growth phase, the supplemented groups exhibited higher ADWG and DALSG compared to the control group (Figures 2C,F). However, no significant differences were observed in the final BW and LSL measurements across the dietary treatments (Figures 2B,E). The control group, which was fed the basal diet, achieved the highest growth performance, with a final BW of 3411.75 mg/snail and a maximum shell length of 24.92 mm.
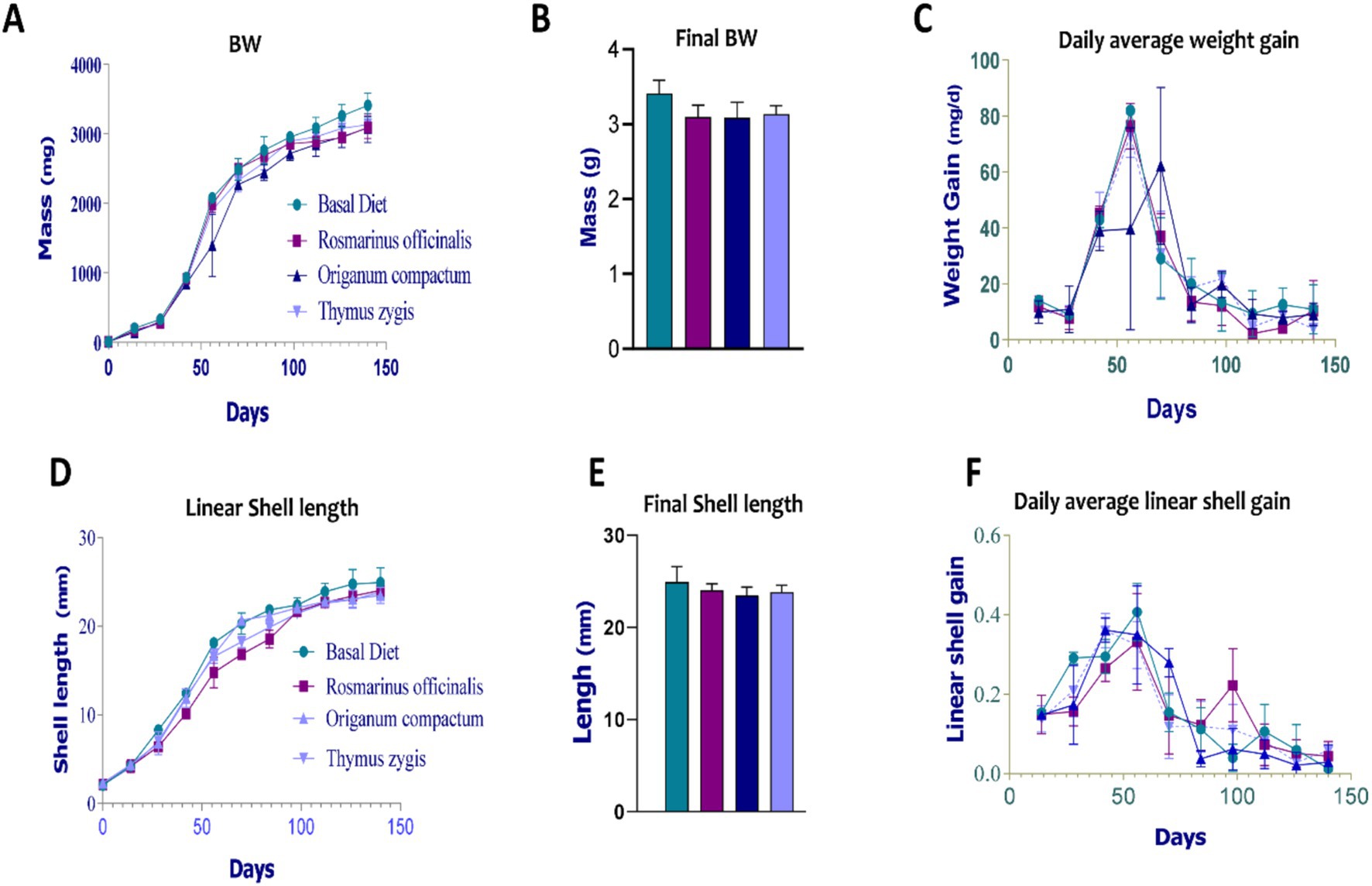
Figure 2. The effect of feed supplementation on the growth parameters of Otala tingitana snails. (A) The mass evolution of body weight (BW); (B) The maximal body wight (BW); (C) Daily Average weight gain; (D) The linear shell length (LSL) evolution; (E) The maximal shell length; (F) Daily average linear shell gain (DALSG).
3.5 Feed intake, daily consumption rate, and ecological efficiency of growth
Given the critical role of FI and feed conversion as key parameters influencing animal production and farming performance, this study also assessed the impact of diet on these parameters to better understand their effects on appetite and the efficiency of livestock production in O. tingitana snails. FI steadily increased across all groups over time (Figure 3A), with no significant differences observed among the four dietary treatments (p < 0.05). The highest FI was recorded in the control group at the end of the experiment (57.35 mg/day/snail).
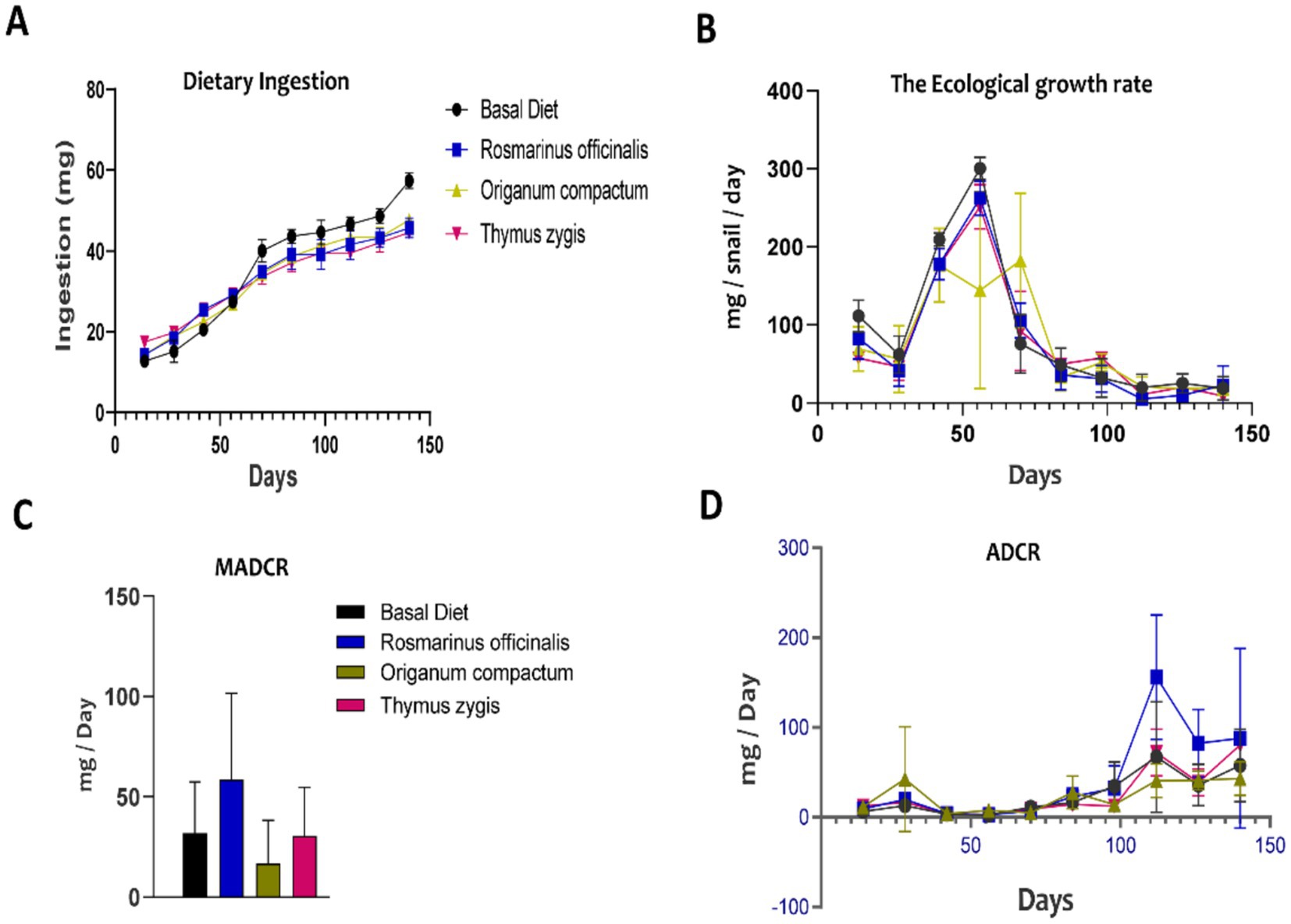
Figure 3. The effect of feed supplementation on dietary ingestion and the ecological growth rate of the Otala tingitana snail. (A) Feed intake (or dietary ingestion); (B) The ecological growth rates; (C) The maximal average daily consumption rate (MADCR); (D) The average daily consumption rate (ADCR) evolution.
The DCR, representing the feed consumed per day relative to the animal’s weight, remained relatively stable across all four diet groups throughout the experiment (Figure 3D). This stability supports the observed linear correlation between feed consumption and BW development. Among the groups, snails fed a diet supplemented with R. officinalis exhibited the highest average daily consumption rate (ADCR) at 58.59%, while the control group had an ADCR of 31.94%. The groups supplemented with T. gracilis and O. compactum had ADCRs of 30.57 and 16.68%, respectively (Figure 3C). Furthermore, the ecological efficiency of growth was significantly higher during the first 8 weeks of the trial, demonstrating a consistent increase. However, after this period, the growth-to-consumption ratio declined sharply, reaching very low efficiency during the final 6 weeks (Figure 3B).
3.6 Sexual maturation and short generation time
Mature snails began to emerge in the 16th week across all groups, with the percentage of mature individuals progressively increasing over time. By the end of the experiment, Group B (feed supplemented with R. officinalis) exhibited the highest maturity rate at 95.55%, followed closely by Group D (feed supplemented with T. gracilis) at 94.44% and Group C (feed supplemented with O. compactum) at 93.33%. In contrast, Group A (control) recorded the lowest percentage of mature snails, at 75.55% (Figure 4).
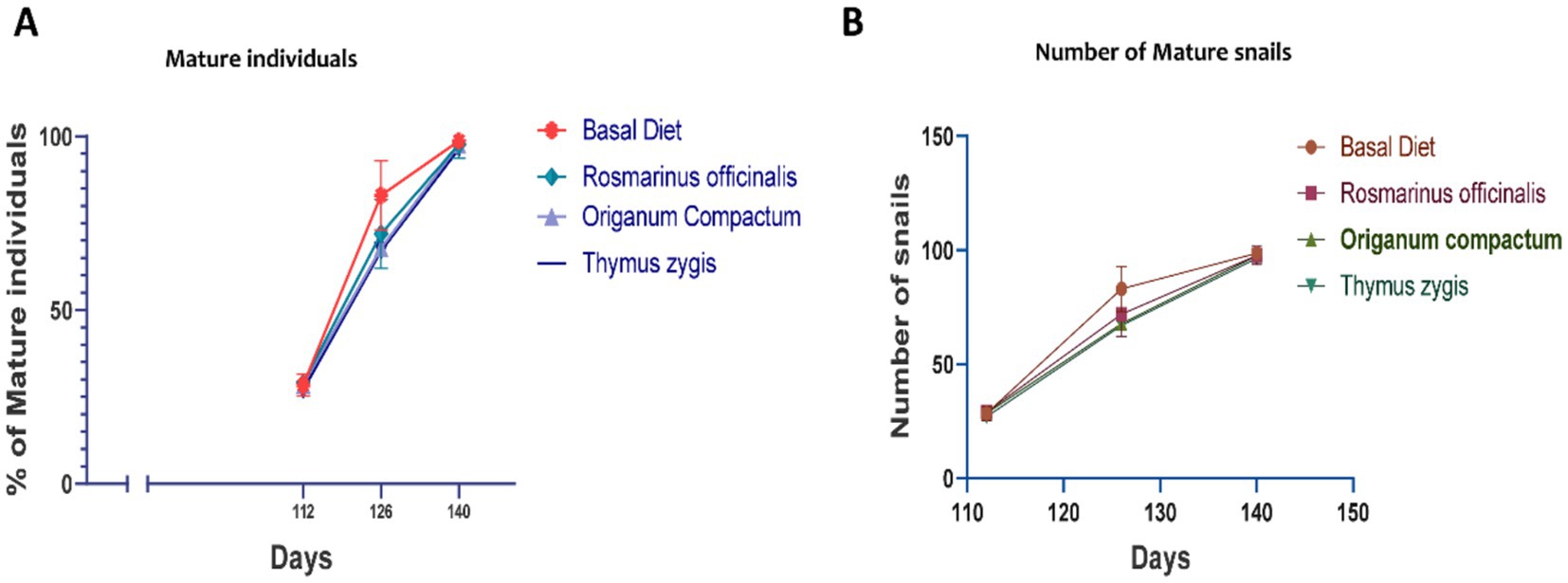
Figure 4. Maturation of Otala tingitana snails (A). The number and the percentage of mature individuals was monitored and recorded each 14 days (B). Generally mature snails appear from the 16 weeks and exponentially doubled in the following four weeks.
3.7 Survival improvement and mortality rate
Throughout the experimental trial, snail mortality across different age groups was closely monitored, as it is a critical factor affecting farming efficiency and productivity. The results revealed a significant improvement in survival rates among snails fed diets supplemented with herbs. Notably, all three herbs (R. officinalis, T. gracilis, and O. compactum) exhibited strong effects in enhancing the survival of O. tingitana snails (Figure 5A). In contrast to the control group, which was fed a basal diet and experienced a cumulative mortality rate of 22.44%, the herb-supplemented groups showed markedly lower mortality rates. Snails fed R. officinalis exhibited the lowest mortality at 4.44%, while those in the T. gracilis and O. compactum groups had mortality rates of 5.55 and 6.66%, respectively (Figure 5B).
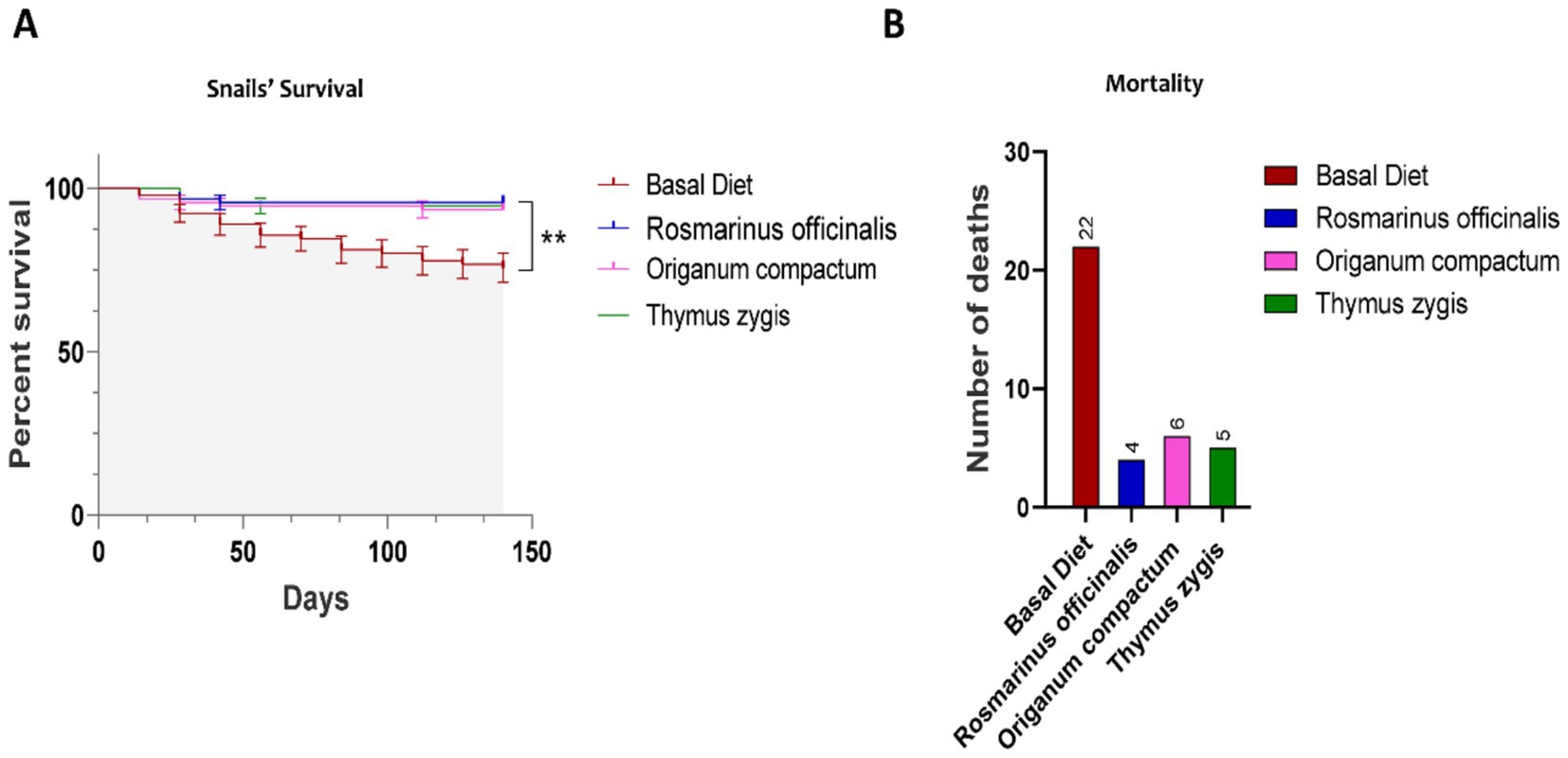
Figure 5. The effect of feed supplementation on the mortality rate of Otala tingitana snails. Feed supplementation with herbs decreased mortality rate of Otala tingitana snails (A). The overall number of deaths varied significantly between basal diet and other diet conditions (B) (n = 90).
3.8 Effect of feed supplements on microbiological quality of snail flesh
The effect of herbal supplementation on bacterial contamination in snails was assessed by analyzing the bacterial load in snail flesh at the end of the experimental trial. Flesh samples from the different dietary groups were meticulously examined and results were summarized in Table 4.
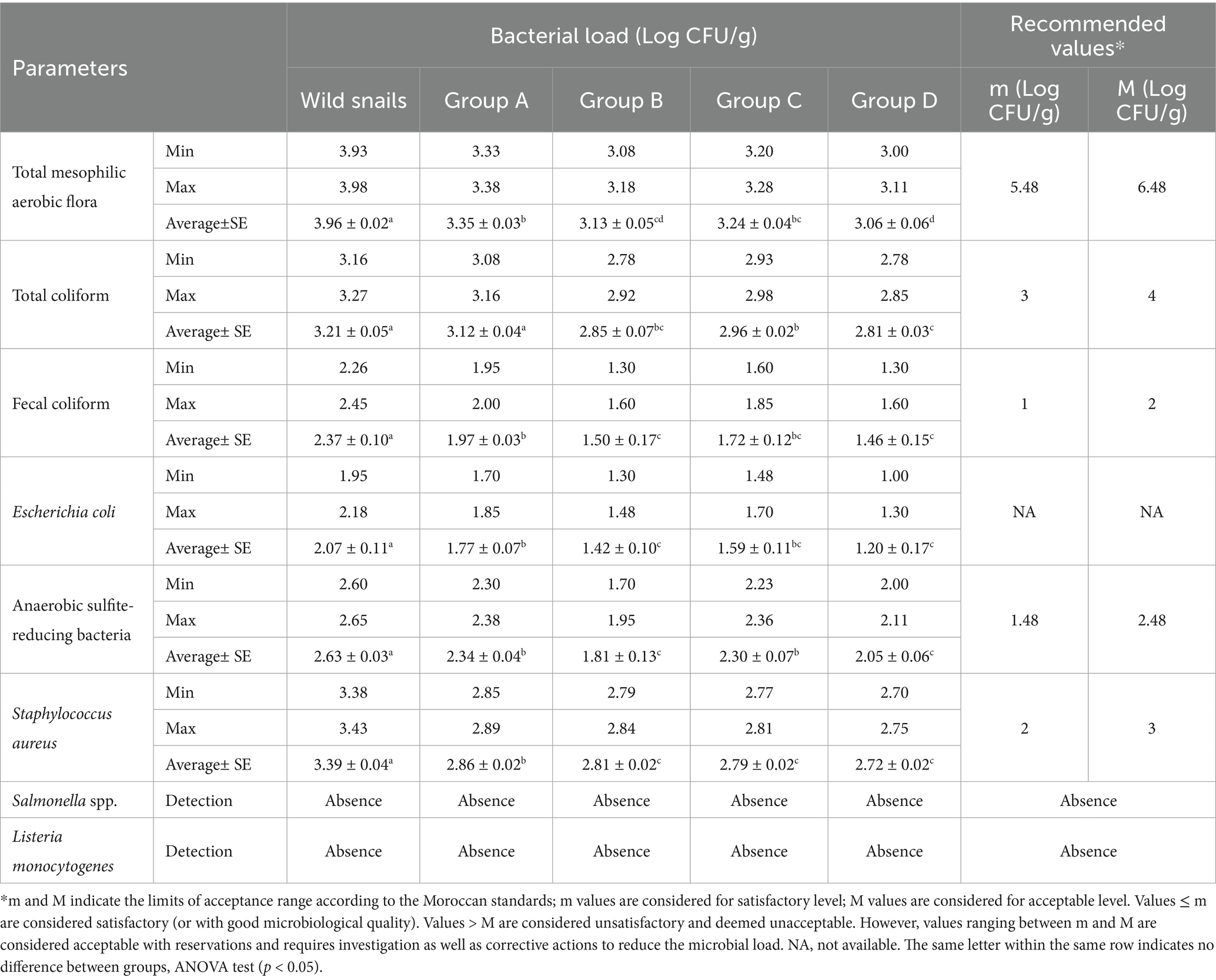
Microbiological analysis revealed that all snails reared under controlled conditions (group A, B, C, and D) exhibited acceptable microbiological loads for all the parameters studied. In contrast, wild snails collected from free environments demonstrated unacceptable microbiological quality, particularly for fecal coliforms, ASRB, and S. aureus (Table 4). These findings underscore the benefits of controlled rearing environments in improving the microbiological quality of snail flesh.
Moreover, snails fed with R. officinalis (group B), O. compactum (group C), and T. gracilis (group D) showed a significant reduction in microbial load for all the parameters studied compared to wild snails (p < 0.05). Notably, snails in Groups B and D, which were fed with R. officinalis and T. gracilis, respectively, demonstrated significant reductions in all measured microbial parameters compared to the reference group receiving a basal diet (Group A) (p < 0.05). Meanwhile, Group C, fed with O. compactum, showed a significant reduction in total coliforms and S. aureus levels compared to the reference group (p < 0.05). Furthermore, all the analyzed samples revealed no detection of Salmonella and L. monocytogenes.
4 Discussion
Heliciculture, as an emerging sector of sustainable livestock farming, has gained popularity in recent years thanks to the high nutritional and functional value of snail meat. It is an abundant source of high-quality protein, essential amino acids, minerals, and bioactive compounds, while being low in carbohydrates. This makes it particularly suitable for certain diets, especially for people with diabetes (El Khayari et al., 2025; Etukudo et al., 2025). However, the development of this sector is hampered by various constraints, including the vulnerability of snails to microbial contamination. This not only affects the hygiene and safety of the meat, but also leads to high mortality rates in farms, thereby reducing productivity and overall profitability (Garkov et al., 2025). Therefore, developing optimal feeding practices and adding natural dietary supplements that can boost growth performance, improve meat quality, and fortify resistance against microbial pathogens are necessary to overcome these constraints.
Furthermore, Aromatic and medicinal plants are valued for their abundance of nutrients and secondary metabolites, which justify their positive impacts in animal feed. This research revealed substantial concentrations of carbohydrates, fiber, proteins, and lipids in the three plant species examined, with differences observed between species. These results are consistent with the observations of El Finou et al. (2023), Elsherif et al. (2023), and Anwar et al. (2024). The studies also demonstrated a high concentration of phenolic compounds, especially in O. compactum (63.20 ± 4.2 mg GAE/g), linked to a powerful antioxidant action (IC₅₀ = 90.28 ± 1.14 μg/mL) and significant antibacterial effects, particularly for T. gracilis against Gram-positive bacteria, consistent with the observations of Silva et al. (2020), Chroho et al. (2022), and Francolino et al. (2023).
In this study, adding 3% R. officinalis, O. compactum, and T. gracilis to the basic diet did not cause any significant changes in the growth criteria of O. tingitana (such as body weight, shell diameter, and shell length). This lack of effect can be attributed, on the one hand, to the already perfect nutritional quality of the basic diet, which probably satisfied the species’ basic needs, thus reducing the extent of quantifiable supplements on growth. This confirms the conclusions of Rygało-Galewska et al. (2022), highlighting the need for an appropriate nutritional composition of the flour used in heliciculture, both in terms of quantity and quality. In addition, the secondary metabolites contained in the plants examined (polyphenols, flavonoids, essential oils) may have limited bioavailability or act primarily on physiological mechanisms not specifically associated with somatic growth, such as metabolic adjustment, immunity enhancement, or oxidative stress management. In line with this assumption, our results indicate that the addition of plants helped to reduce mortality, improve reproductive performance, and optimize meat quality. This suggests that the major impact of these plants is manifested more through physiological and metabolic regulation than through a direct increase in body weight.
These results are consistent with previous research that demonstrated that the same plant supplements promoted reproductive indices in Cryptomphalus aspersus (El Khayari et al., 2024). Similarly, Merlin et al. (2024) reported that T. vulgaris supplementation promoted growth and reproduction in Archachatina marginata snails. In addition, various studies have demonstrated the benefits of medicinal and aromatic plants in animal nutrition, proving their ability to optimize zootechnical performance in several species (Pliego et al., 2022; Tadese et al., 2022). It is particularly noteworthy in this research that the use of these plant-based supplements has jointly reduced mortality and bacterial contamination in O. tingitana meat. These findings are consistent with the results of Lemjallad et al. (2019), who demonstrated that R. officinalis reduced mortality and microbial load while improving protein concentration and controlling specific catalase activity in snails. Overall, our research shows that these three types of plants contribute significantly to the survival and health of snails. This confirms previous studies on other species that emphasize the paramount importance of diet and hygiene in reducing mortality rates (Cabaret et al., 1988; Milinsk et al., 2003).
This research highlights the multifactorial importance of medicinal and aromatic plants (R. officinalis, T. gracilis, and O. compactum) when incorporated into the diet of O. tingitana. In addition to their beneficial nutritional profile, these plants contain a wide variety of bioactive compounds such as polyphenols, flavonoids, terpenes, and phenolic acids that can jointly influence the physiology, immune system health, and intestinal microbiology of snails. The significant reduction in microbial load detected in the flesh, particularly for TAMF, total and fecal coliforms, E. coli, ASRB, and S. aureus, indicates that these plants have a direct antimicrobial action, potentially regulated by modifying bacterial membranes, blocking enzymes essential for microbial replication, and adjusting the expression of pathogenic genes (Ed-Dra et al., 2021, 2024). In addition, the possible prebiotic action of these compounds encourages the establishment of a healthy gut microbiota, including bacteria such as Lactobacillus and Bifidobacterium. The latter optimizes nutrient assimilation, strengthens the intestinal barrier, and positively influences the innate and adaptive immune response (Kamble et al., 2024; Zheng et al., 2024).
Metabolites such as flavonoids, polysaccharides, carotenoids, and tannins have antioxidant and anti-inflammatory activity that helps reduce oxidative stress, protects cell proteins, and preserves tissue integrity, while improving metabolic performance and reproduction (Ponnampalam et al., 2022; Elkomy et al., 2023). These effects are accentuated by enzyme regulation, particularly that of catalase and other internal antioxidant systems, which help reduce the energy cost associated with stress and inflammatory reactions (Patra et al., 2019; Mahfuz et al., 2021). Together, these processes explain the reduction in mortality and improvement in meat quality. This shows that plant supplements not only act as sources of nutrition, but also influence physiology, metabolism, and resistance to agents.
These studies show that incorporating medicinal and aromatic plants into the snails’ diet is a strategic method for sustainable snail farming, providing both zootechnical and health benefits. By minimizing the use of antibiotics and chemical additives, this method complies with responsible farming standards and contributes to improving food safety, productivity, and animal welfare, while enhancing the nutritional and microbiological profile of meat intended for human consumption. These results highlight the need for further research into the molecular and metabolic processes of plant extracts, with the aim of better understanding the interactions between diet, intestinal health, and productivity in O. tingitana and other snail species.
5 Conclusion
This study underscores the significant benefits of R. officinalis, O. compactum, and T. gracilis as feed supplements for O. tingitana snails reared under controlled conditions. These medicinal plants demonstrated remarkable potential to enhance microbiological quality and improve survival rates without adverse effects. Supplementing snail feed with a 3% inclusion of these herbs effectively reduces microbial loads, offering protection against pathogenic bacteria. However, while the herbal supplements positively enhanced microbiological quality and survival, they did not significantly influence growth parameters, maturity, and development rates in the snails.
Overall, these findings highlight the potential of these three Moroccan medicinal and aromatic plants as phytobiotics in snail farming, offering a natural alternative to chemical treatments and antibiotics. Nevertheless, further advanced studies are needed to refine formulations, investigate synergistic effects among the plants, and elucidate their mechanisms of action.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by ethics committee from the Moulay Ismail University, Meknes. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AEK: Conceptualization, Data curation, Investigation, Methodology, Software, Visualization, Writing – original draft. AA: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. ER: Writing – review & editing, Conceptualization, Investigation, Project administration, Resources, Supervision, Validation. EA: Data curation, Formal analysis, Writing – review & editing. FR: Conceptualization, Project administration, Validation, Writing – review & editing. TT: Data curation, Software, Visualization, Writing – review & editing. AB: Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. AE-D: Data curation, Formal analysis, Methodology, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2501).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1670337/full#supplementary-material
References
Alem, W. T. (2024). Effect of herbal extracts in animal nutrition as feed additives. Heliyon 10:e24973. doi: 10.1016/j.heliyon.2024.e24973
Al-Maharik, N., Jaradat, N., Hawash, M., Al-Lahham, S., Qadi, M., Shoman, I., et al. (2022). Chemical composition, antioxidant, antimicrobial and anti-proliferative activities of essential oils of Rosmarinus officinalis from five different sites in Palestine. Separations 9:339. doi: 10.3390/separations9110339
Al-Mijalli, S. H., Mrabti, N. N., Ouassou, H., Sheikh, R. A., Assaggaf, H., Bakrim, S., et al. (2022). Chemical composition and antioxidant, antimicrobial, and anti-inflammatory properties of Origanum compactum Benth essential oils from two regions: in vitro and in vivo evidence and in silico molecular investigations. Molecules 27:7329. doi: 10.3390/molecules27217329
Anwar, F., Mahrye,, Khan, R., Qadir, R., Saadi, S., Gruczynska-Sekowska, E., et al. (2024). Exploring the biochemical and nutra-pharmaceutical prospects of some Thymus species – a review. Chem. Biodivers. 21:e202400500. doi: 10.1002/CBDV.202400500
AOAC (2023). Official Methods of Analysis, 22nd Edition. AOAC International. Available online at: https://www.aoac.org/official-methods-of-analysis/ (Accessed March 21, 2025).
Benlemlih, M., Barchan, A., Aarab, A., Bakkali, M., Arakrak, A., and Laglaoui, A. (2020). Effect of dietary dried fennel and oregano and thyme supplementation on zootechnical parameters of growing rabbit. J. World's Poult. Res. 10, 332–337. doi: 10.36380/SCIL.2020.WVJ43
Bouyahya, A., Zengin, G., Belmehdi, O., Bourais, I., Chamkhi, I., Taha, D., et al. (2020). Origanum compactum Benth., from traditional use to biotechnological applications. J. Food Biochem. 44:e13251. doi: 10.1111/JFBC.13251
Bouymajane, A., Filali, F. R., Benhallam, F., Ed-dra, A., El Allaoui, A., Chaiba, A., et al. (2019). Quantitative and qualitative microbial diversity of the raw cow’s milk sold by street trading in Meknes, Morocco. Malays. J. Microbiol. 15, 425–431. doi: 10.21161/mjm.180079
Bouymajane, A., Filali, F. R., Ed-Dra, A., Aazza, M., Nalbone, L., Giarratana, F., et al. (2022a). Chemical profile, antibacterial, antioxidant, and anisakicidal activities of Thymus zygis subsp. gracilis essential oil and its effect against Listeria monocytogenes. Int. J. Food Microbiol. 383:109960. doi: 10.1016/j.ijfoodmicro.2022.109960
Bouymajane, A., Filali, F. R., El Majdoub, Y. O., Ouadik, M., Abdelilah, R., Cavò, E., et al. (2022b). Phenolic compounds, antioxidant and antibacterial activities of extracts from aerial parts of Thymus zygis subsp. gracilis, Mentha suaveolens and Sideritis incana from Morocco. Chem. Biodivers. 19:e202101018. doi: 10.1002/CBDV.202101018
Cabaret, J., Morand, S., Aubert, C., and Yvore, P. (1988). Snail farming: a survey of breeding management, hygiene and parasitism of the garden snail, Helix aspersa müller. J. Molluscan Stud. 54, 209–214. doi: 10.1093/mollus/54.2.209
Caetano, D. B., Miranda, A., Lopes, S. A., Paiva, J. B., Rodrigues, A. J., Videira, A., et al. (2021). Nutritional and toxicity profiles of two species of land snail, Theba pisana and Otala lactea, from Morocco. J. Food Compos. Anal. 100:103893. doi: 10.1016/j.jfca.2021.103893
Cheng, Y., Liu, S., Wang, F., Wang, T., Yin, L., Chen, J., et al. (2024). Effects of dietary Terminalia chebula extract on growth performance, immune function, antioxidant capacity, and intestinal health of broilers. Animals 14:746. doi: 10.3390/ANI14050746/S1
Chroho, M., Bouymajane, A., Aazza, M., Oulad El Majdoub, Y., Cacciola, F., Mondello, L., et al. (2022). Determination of the phenolic profile, and evaluation of biological activities of Hydroethanolic extract from aerial parts of Origanum compactum from Morocco. Molecules 27:5189. doi: 10.3390/molecules27165189
Ed-Dra, A., Abdallah, E. M., Sulieman, A. M. E., and Anarghou, H. (2024). Harnessing medicinal plant compounds for the control of Campylobacter in foods: a comprehensive review. Vet. Res. Commun. 48, 2877–2900. doi: 10.1007/S11259-024-10455-4
Ed-Dra, A., Filali, F. R., Lo Presti, V., Zekkori, B., Nalbone, L., Bouymajane, A., et al. (2020). Chemical composition, antioxidant capacity and antibacterial action of five Moroccan essential oils against Listeria monocytogenes and different serotypes of Salmonella enterica. Microb. Pathog. 149:104510. doi: 10.1016/j.micpath.2020.104510
Ed-Dra, A., Filali, F. R., Presti, V. L., Zekkori, B., Nalbone, L., Elsharkawy, E. R., et al. (2021). Effectiveness of essential oil from the artemisia herba-alba aerial parts against multidrug-resistant bacteria isolated from food and hospitalized patients. Biodiversitas 22, 2995–3005. doi: 10.13057/biodiv/d220753
Ed-Dra, A., Nalbone, L., Shahat, A. A., Laaraj, S., Farihi, A., Moujane, S., et al. (2025). Antilisterial activity of Thymus vulgaris essential oil: in vitro, in situ, and in silico investigations. Microb. Pathog. 204:107557. doi: 10.1016/j.micpath.2025.107557
Ed-Dra, A., Filali, F. R., Karraouan, B., El Allaoui, A., Aboulkacem, A., and Bouchrif, B. (2017). Prevalence, molecular and antimicrobial resistance of Salmonella isolated from sausages in Meknes, Morocco. Microb. Pathog. 105, 340–345. doi: 10.1016/j.micpath.2017.02.042
El Finou, H., Bammou, M., Zaid, A., and El rhaffari, l. (2023). Nutritional composition, sensory quality and bioactivity of a dietary supplement based on the Origanum compactum Benth and Foeniculum vulgare mill aerial part. J. Food Nutr. Res. 62, 236–244.
El Khayari, A., and Rour, E. (2021). Combined effects of air temperature, photoperiod and humidity on Otala tingitana snails’ breeding (Mollusca, Gastropoda, Helicidae). Molluscan Res. 41, 316–323. doi: 10.1080/13235818.2021.2004574
El Khayari, A., Rour, E., Bouymajane, A., Mzoudi, N., and Chenni, Y. (2024). Use of medicinal plants to enhance the breeding and microbiological quality of the flesh in the snail Cryptomphalus aspersus. Invertebr. Reprod. Dev. 68, 27–35. doi: 10.1080/07924259.2024.2330950
El Khayari, A., Rour, E., Chbihi Kaddouri, A., and Mzoudi, N. (2025). Edible snail production for food security: an appraisal using Morocco as a model country. Invertebr. Reprod. Dev. 69, 91–101. doi: 10.1080/07924259.2025.2474400
El Khayari, A., Rour, E., and Mzoudi, N. (2023). Tracking the growth of the edible Moroccan snail, Otala tingitana (Paladilhe, 1875), raised under controlled conditions. Molluscan Res. 43, 144–151. doi: 10.1080/13235818.2023.2211528
Elkomy, A. E., El-Saadany, A. S., Shreif, E. Y., Bayoumi, A. A., Abd El-Maged, M. H., Alagawany, M., et al. (2023). Anise and grape seed oils as a feed additive to improve the performance, immune response, and antioxidant activity and reduce caecal pathogenic microbes of quail. Arch Anim Breed 66, 379–390. doi: 10.5194/AAB-66-379-2023
Elsherif, K. M., Alhlbad, E. A. A., and Ewlad-Ahmed, A. M. (2023). Phytochemical screening, antioxidant activity, and mineral profiling of Rosmarinus officinalis L. from Msallata region (Libya). Scientific J. Faculty Sci. Sirte Univ. 3, 9–17. doi: 10.37375/sjfssu.v3i2.1543
Etukudo, O. M., Inyang, A. B., Nkantion, U. N., Patani, I., and Okon, B. (2025). Snail farming in Nigeria: tapping into the economic potential of heliculture. A review. J. Agric. Ext. Rural Dev. 2, 42–54. doi: 10.31248/jaere2025.018
Fang, C., Tang, X., Zhang, Q., Yu, Q., Deng, S., Wu, S., et al. (2024). Effects of dietary Lonicera flos and Sucutellaria baicalensis mixed extracts supplementation on reproductive performance, umbilical cord blood parameters, colostrum ingredients and immunoglobulin contents of late-pregnant sows. Animals 14:2054. doi: 10.3390/ani14142054
Francolino, R., Martino, M., Caputo, L., Amato, G., Chianese, G., Gargiulo, E., et al. (2023). Phytochemical constituents and biological activity of wild and cultivated Rosmarinus officinalis Hydroalcoholic extracts. Antioxidants 12:1633. doi: 10.3390/antiox12081633
Franz, C., Baser, K. H. C., and Windisch, W. (2010). Essential oils and aromatic plants in animal feeding – a European perspective. A review. Flavour Fragr. J. 25, 327–340. doi: 10.1002/FFJ.1967
Garkov, M., Fidan, H., and Nikovska, K. (2025). Land snail consumption: microbiological safety considerations, best practices and regulations. BIO Web Conf. 170:04002. doi: 10.1051/bioconf/202517004002
Gessner, D. K., Ringseis, R., and Eder, K. (2017). Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr (Berl) 101, 605–628. doi: 10.1111/JPN.12579
ISO 15213-1 (2023). Microbiology of the food chain horizontal method for the detection and enumeration of Clostridium spp. part 1: enumeration of sulfite-reducing Clostridium spp. by colony-count technique. Available online at: https://www.iso.org/standard/67050.html (Accessed December 15, 2024).
ISO 16649-2 (2001). Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli. Part 2: Colony-count technique at 44 degrees C using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. Available online at: https://www.iso.org/standard/29824.html (Accessed December 15, 2024).
ISO 4832 (2006). Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of coliforms: Colony-count technique. Available online at: https://www.iso.org/standard/38282.html (Accessed December 15, 2024).
ISO 4833-1 (2013). Microbiology of the food chain. Horizontal method for the enumeration of microorganisms. Part 1: Colony count at 30 °C by the pour plate technique. Available online at: https://www.iso.org/standard/53728.html (Accessed December 15, 2024).
ISO 6888-2 (2021). Microbiology of the food chain. Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species). Part 2: method using rabbit plasma fibrinogen agar medium. Available online at: https://www.iso.org/standard/76673.html (Accessed December 15, 2024).
Jaber, H., Oubihi, A., Ouryemchi, I., Boulamtat, R., Oubayoucef, A., Bourkhiss, B., et al. (2021). Chemical composition and antibacterial activities of eight plant essential oils from Morocco against Escherichia coli strains isolated from different Turkey organs. Biochem. Res. Int. 2021:685800. doi: 10.1155/2021/6685800
Kamble, M. T., Gallardo, W., Salin, K. R., Pumpuang, S., Chavan, B. R., Bhujel, R. C., et al. (2024). Effect of Moringa oleifera leaf extract on the growth performance, hematology, innate immunity, and disease resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae biotype 2. Animals 14:953. doi: 10.3390/ANI14060953
Kefale, B., Delele, M. A., Fanta, S. W., and Mekonnen Abate, S. (2023). Nutritional, physicochemical, functional, and textural properties of red pepper (Capsicum annuum L.), red onion (Allium cepa), ginger (Zingiber officinale), and garlic (Allium sativum): main ingredients for the preparation of spicy foods in Ethiopia. J. Food Qual. 2023:3916692. doi: 10.1155/2023/3916692
Lemjallad, L., Chabir, R., Kandri Rodi, Y., El Ghadraoui, L., Ouazzani Chahdi, F., and Errachidi, F. (2019). Improvement of Heliciculture by three medicinal plants belonging to the Lamiaceae Family. Sci. World J. 2019, 1–7. doi: 10.1155/2019/2630537
Lillehoj, H., Liu, Y., Calsamiglia, S., Fernandez-Miyakawa, M. E., Chi, F., Cravens, R. L., et al. (2018). Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 49, 1–18. doi: 10.1186/S13567-018-0562-6
Mahfuz, S., Shang, Q., and Piao, X. (2021). Phenolic compounds as natural feed additives in poultry and swine diets: a review. J. Anim. Sci. Biotechnol. 12:1, 1–18. doi: 10.1186/S40104-021-00565-3
Merlin, T. G., Jua, J. J., Ferdinand, N., and Joseph, T. (2024). Enhancing giant African land snail (Archachatina marginata) production with Thymus vulgaris (Lamiaceae) in the south-west region of Cameroon. Pubvet 18:e1589–e1589. doi: 10.31533/pubvet.v18n05e1589
Milinsk, M. C., Das Graças Padre, R., Hayashi, C., De Souza, N. E., and Matsushita, M. (2003). Influence of diets enriched with different vegetable oils on the fatty acid profiles of snail Helix aspersa maxima. Food Chem. 82, 553–558. doi: 10.1016/S0308-8146(03)00010-4
Mrabti, H. N., Bouyahya, A., Ed-Dra, A., Kachmar, M. R., Mrabti, N. N., Benali, T., et al. (2021). Polyphenolic profile and biological properties of Arbutus unedo root extracts. Eur. J. Integr. Med. 42:101266. doi: 10.1016/j.eujim.2020.101266
Nieto, G., Díaz, P., Bañón, S., and Garrido, M. D. (2010). Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Sci. 85, 82–88. doi: 10.1016/J.meatsci.2009.12.009
Patra, A. K., Amasheh, S., and Aschenbach, J. R. (2019). Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds – a comprehensive review. Crit. Rev. Food Sci. Nutr. 59, 3237–3266. doi: 10.1080/10408398.2018.1486284
Phong, H. X., Viet, N. T., Quyen, N. T. N., Van Thinh, P., Trung, N. M., and Ngan, T. T. K. (2022). Phytochemical screening, total phenolic, flavonoid contents, and antioxidant activities of four spices commonly used in Vietnamese traditional medicine. Mater Today Proc 56, A1–A5. doi: 10.1016/J.MATPR.2021.12.142
Pissia, M., Matsakidou, A., and Kiosseoglou, V. (2021). Raw materials from snails for food preparation. Future Foods 3:100034. doi: 10.1016/J.FUFO.2021.100034
Pliego, A. B., Tavakoli, M., Khusro, A., Seidavi, A., Elghandour, M. M. M. Y., Salem, A. Z. M., et al. (2022). Beneficial and adverse effects of medicinal plants as feed supplements in poultry nutrition: a review. Anim. Biotechnol. 33, 369–391. doi: 10.1080/10495398.2020.1798973
Ponnampalam, E. N., Kiani, A., Santhiravel, S., Holman, B. W. B., Lauridsen, C., and Dunshea, F. R. (2022). The importance of dietary antioxidants on oxidative stress, meat and Milk production, and their preservative aspects in farm animals: antioxidant action, animal health, and product quality—invited review. Animals 12:3279. doi: 10.3390/ANI12233279
Ricci, A., Lazzi, C., and Bernini, V. (2023). Natural antimicrobials: a reservoir to contrast Listeria monocytogenes. Microorganisms 11:2568. doi: 10.3390/microorganisms11102568
Rygało-Galewska, A., Zglińska, K., and Niemiec, T. (2022). Edible snail production in Europe. Animals 12:2732. doi: 10.3390/ani12202732
Schmerold, I., van Geijlswijk, I., and Gehring, R. (2023). European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharm. Sci. 189:106473. doi: 10.1016/J.EJPS.2023.106473
Segade, P., García-Estévez, J., Arias, C., and Iglesias, R. (2013). Parasitic infections in mixed system-based heliciculture farms: dynamics and key epidemiological factors. Parasitology 140, 482–497. doi: 10.1017/S0031182012001795
Silva, A. M., Martins-Gomes, C., Souto, E. B., Schäfer, J., Santos, J. A., Bunzel, M., et al. (2020). Thymus zygis subsp. zygis an endemic portuguese plant: phytochemical profiling, antioxidant, anti-proliferative and anti-inflammatory activities. Antioxidants 9:482. doi: 10.3390/antiox9060482
Simitzis, P. E. (2017). Enrichment of animal diets with essential oils—a great perspective on improving animal performance and quality characteristics of the derived products. Medicine 4:35. doi: 10.3390/medicines4020035
Steiner, T., and Syed, B. (2015). Phytogenic feed additives in animal nutrition. Dordrecht: Springer, 403–423.
Tadese, D. A., Song, C., Sun, C., Liu, B., Liu, B., Zhou, Q., et al. (2022). The role of currently used medicinal plants in aquaculture and their action mechanisms: a review. Rev. Aquac. 14, 816–847. doi: 10.1111/RAQ.12626
Van der Klein, S. A. S., Bédécarrats, G. Y., and Zuidhof, M. J. (2018). The effect of rearing photoperiod on broiler breeder reproductive performance depended on body weight. Poult. Sci. 97, 3286–3294. doi: 10.3382/ps/pey199
Vladimir-Knežević, S., Perković, M., Zagajski Kučan, K., Mervić, M., and Rogošić, M. (2022). Green extraction of flavonoids and phenolic acids from elderberry (Sambucus nigra L.) and rosemary (Rosmarinus officinalis L.) using deep eutectic solvents. Chem. Pap. 76, 341–349. doi: 10.1007/S11696-021-01862-X/METRICS
Zheng, Y., Qin, C., Wen, M., Zhang, L., and Wang, W. (2024). The effects of food nutrients and bioactive compounds on the gut microbiota: a comprehensive review. Foods 13:1345. doi: 10.3390/FOODS13091345
Keywords: food safety, medicinal and aromatic plants, microbiological quality, mortality rate, Otala tingitana , phytobiotics
Citation: El Khayari A, Alhudhaibi AM, Rour E, Abdallah EM, Rhazi Filali F, Taha TH, Bouymajane A and Ed-Dra A (2025) Sustainable heliciculture of Otala tingitana in controlled environments using plant-based feed supplements. Front. Sustain. Food Syst. 9:1670337. doi: 10.3389/fsufs.2025.1670337
Edited by:
Marian Burducea, Alexandru Ioan Cuza University, RomaniaReviewed by:
Ercüment Genç, Ankara University, TürkiyeElijah Ige Ohimain, Niger Delta University, Nigeria
Copyright © 2025 El Khayari, Alhudhaibi, Rour, Abdallah, Rhazi Filali, Taha, Bouymajane and Ed-Dra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdelmajid El Khayari, YWIuZWxraGF5YXJpQGVkdS51bWkuYWMubWE=; Abdulrahman Mohammed Alhudhaibi, YW1hbGh1ZGhhaWJpQGltYW11LmVkdS5zYQ==
 Abdelmajid El Khayari
Abdelmajid El Khayari Abdulrahman Mohammed Alhudhaibi
Abdulrahman Mohammed Alhudhaibi Elhabib Rour1
Elhabib Rour1 Emad M. Abdallah
Emad M. Abdallah Tarek H. Taha
Tarek H. Taha Abdelaziz Ed-Dra
Abdelaziz Ed-Dra