- 1National Institute of Agricultural Research, Rabat, Morocco
- 2Higher Institute of Nursing Professions and Health Technical (ISPITS), Casablanca, Morocco
- 3Human Nutrition, Bioacives and Oncogenetics Team, Faculty of Sciences, Moulay Ismail University, Meknes, Morocco
- 4Laboratory of Agro-Alimentary and Health, Faculty of Sciences and Techniques, Hassan First University of Settat, Settat, Morocco
- 5Department of Biology, Faculty of Biology, Alexandru Ioan Cuza University of Iasi, Iași, Romania
- 6‘Olga Necrasov’ Center, Department of Biomedical Research, Romanian Academy, Iasi, Romania
- 7Clinical Department, Apollonia University, Iași, Romania
- 8CENEMED Platform for Interdisciplinary Research, “Grigore T. Popa” University of Medicine and Pharmacy of Iasi, Iași, Romania
Introduction: Aphis fabae Scop. (Hemiptera: Aphididae) poses a major threat to Vicia faba L. crops, demanding sustainable control strategies.
Methods: We assessed the single and combined efficacy of two predatory ladybirds—Coccinella septempunctata (CS) and Hippodamia convergens (HC)—with two 10% botanical extracts—Nicotiana glauca (NG) and Ricinus communis (RC) (Solanaceae)—against A. fabae under screenhouse conditions (25 ± 1°C; 60 ± 10% RH). Ten treatments, including imidacloprid (ICP, 20 mL hL−1) as a positive control, were tested in a randomized complete-block design (3 replicates × 7 plants). Ladybirds were released one-week post-treatment.
Results and discussion: Combined treatments were most effective in reducing A. fabae populations. By week 5, egg densities dropped from ~95 to 2.0 (RC + CS treatment) and 3.9 (RC + HC treatment), compared to 15.0 with imidacloprid (ICP). Motile stages declined to 10 (RC + CS treatment) and 15 (RC + HC treatment), versus 45 with ICP treatment. NG-based combinations showed moderate efficacy, while single treatments were less consistent. Ladybird establishment was not affected by extracts. C. septempunctata reached 10.2 motile stages per three leaves per plant in RC + CS treatment by week 5, compared to 8.7 in CS treatment. H. convergens reached 10.0 motile stages per three leaves per plant in RC + HC treatment. Plant visual scores peaked at 9.67 (RC + CS treatment) and 9.35 (RC + HC treatment), outperforming imidacloprid (visual score: 8.25). The synergy between R. communis or N. glauca extracts and ladybird predators offers an effective alternative to imidacloprid for A. fabae control. Field trials and timing optimization are recommended to integrate these tactics into faba-bean IPM programs.
1 Introduction
The black bean aphid, Aphis fabae Scopoli (Hemiptera: Aphididae), is a major pest of over 200 cultivated and ornamental plant species across Europe, Western Asia, South America, and Africa, including faba bean (Vicia faba L.) (Meradsi and Laamari, 2018). It causes direct damage by phloem sap extraction and indirect damage via honeydew, which promotes sooty mold and reduces photosynthesis (Rashedi et al., 2019; Nordey et al., 2021). It is also a key virus vector, with aphids transmitting 275 of approximately 600 known plant viruses (Blackman and Eastop, 2007; Dedryver et al., 2010). In faba bean, yield losses can exceed 50% under severe infestations (Béji et al., 2015). This species prefers young plant tips, causing stunted growth and leaf distortion (Bennour et al., 2021), with damage severity depending on infestation timing and density (Stoddard et al., 2010; Almogdad and Semaškienė, 2021). Rapid population growth results from its short life cycle and telescoping generations (Singh and Singh, 2020). It follows a holocyclic life cycle, overwintering as eggs, with spring migration of winged adults to host plants (Raymond et al., 2001). Populations fluctuate with environmental and biological factors (Hansen et al., 2008). The economic threshold is around 5% infestation (Way et al., 1977), though modern cultivars may be more tolerant (Parker and Biddle, 1998). Aphis fabae undergoes five stages: egg, four nymphal instars, and adult (Saruhan et al., 2015). It alternates between sexual and asexual reproduction. In autumn, winged females lay overwintering eggs on primary hosts after mating; in spring, these hatch into wingless parthenogenetic females (Fericean et al., 2012). Typically, four generations occur per growing season (Cichocka et al., 2002). Population growth is influenced by initial infestation, temperature, light intensity, natural enemies, and intraspecific competition (Way, 1967; Way and Banks, 1967). Climate plays a key role: in cold regions, the species alternates between sexual and asexual phases; in mild regions, it reproduces only by parthenogenesis (Blackman and Eastop, 2007; Béji et al., 2015). The optimal temperature range for population growth on faba bean is 16–24°C (Rochelyn and Satar, 2025). At 15°C, the aphid has the longest development time (11.45 days), greatest longevity (45.68 days), and highest fecundity (89.35 offspring). Development is fastest at 25°C, while at 30°C, lifespan and reproduction drop sharply (Tora et al., 2024). Broad-spectrum insecticides are widely used to control A. fabae. Pyrethroids, such as fenvalerate, are the main insecticides applied as foliar sprays to manage aphid populations (Johnson et al., 2009). Both pyrethroids and neonicotinoids, have proven highly effective against A. fabae under laboratory conditions (Purhematy et al., 2013). According to Ayala et al. (1996), chlorpyrifos methyl combined with cypermethrin, oxydemeton methyl, imidacloprid, and thiometon are among the most efficient insecticides. However, resistance has developed in A. fabae to some chemicals, including partial resistance to pirimicarb and strong resistance to methamidophos (Ioannidis, 2000). Beyond resistance concerns, chemical insecticides pose risks to human health, non-target organisms, and the environment. Aligned with global Integrated Pest Management (IPM) strategies, newer, more selective insecticides—such as mineral oil and spinosad—and biological control agents have been introduced (Stoddard et al., 2010). These selective insecticides reduce harm to beneficial insects and help prevent secondary pest outbreaks (Wilson et al., 2007). Biological control methods are cost-effective and safe for both humans and the environment (Mwanauta et al., 2015). They include natural enemies (predators and parasitoids), botanical insecticides, resistant plant varieties, and cultural practices. Aphid predators comprise 54 species across six insect orders: Coleoptera, Diptera, Hemiptera, Neuroptera, Thysanoptera, and Dermaptera (Bennour et al., 2021). The main insect predators include coccinellids like Hippodamia convergens Guérin-Méneville, Coccinella novemnotata Herbst, and Coccinella septempunctata L., along with chrysopids such as Chrysopa oculata Say and Chrysopa nigricornis (Burmeister) (Bennour et al., 2021). In addition to insect predators, spiders such as Pardosa amentata (Clerck) (Araneae: Lycosidae) and Erigone species (Araneae: Linyphiidae) (Traugott and Symondson, 2008), as well as birds like Apus apus (Linnaeus) (Apodiformes: Apodidae) (Bennour et al., 2021), also prey on A. fabae.
Plant secondary metabolites are important sources of biopesticides and offer an environmentally friendly and safer alternative to chemical insecticides (Isman, 2006). Various plants from different families, including Citrus aurantium L. (Rutaceae), Eucalyptus spp. (Myrtaceae), Euphorbia spp. (Euphorbiaceae), Melia azedarach (Meliaceae), and Sonchus oleraceus (Asteraceae), have been used to control A. fabae (Bennour et al., 2021). Different plant parts—especially leaves, stems, and peels—are utilized, with aqueous and alcoholic extracts being the most common forms (Bennour et al., 2021). Additionally, Ricinus communis (Castor bean) (Euphorbiaceae) and Nicotiana glauca (Tree Tobacco) (Solanaceae) have demonstrated high efficacy against various insect pests worldwide and are readily available in Morocco (El Aalaoui and Sbaghi, 2025). The primary bioactive compounds responsible for their insecticidal effects include ricin (a toxic protein) and ricinine (an alkaloid) in R. communis (Elijah and Somadina, 2020), capsaicin and dihydrocapsaicin in Capsicum annuum, and pyridine alkaloids such as nicotine and anabasine in N. glauca (Alghamdi, 2021a; Alghamdi, 2021b). Crucially, for successful Integrated Pest Management (IPM) programs, the effects of these bio-insecticides on natural enemies must be considered. Recent research highlights that many plant-derived insecticides are selective and less harmful to beneficial predators and parasitoids compared to synthetic pesticides, thus supporting their conservation and enhancing pest control (Regnault-Roger et al., 2020; Desneux et al., 2021). However, compatibility depends on the bioactive compounds, dose, and application methods, requiring localized assessment to optimize IPM outcomes (Sanchez-Bayo and Tennekes, 2020). Combining predators with plant-derived insecticides can improve control of A. fabae by increasing efficiency, reducing the frequency of applications, and minimizing environmental impacts (Abdel-Rahman et al., 2019).
Although research on IPM strategies combining biological and botanical control agents is still limited, existing studies suggest that such combinations can improve insecticidal efficacy and support more sustainable pest control (Abdel-Rahman et al., 2019; El Sayed and Ibrahim, 2020). Despite these findings, IPM in faba bean (Vicia faba) cultivation continues to rely largely on broad-spectrum insecticides. Most previous studies have evaluated control strategies independently, with few exploring their combined effects to fully realize their synergistic potential (Stoddard et al., 2010). In this context, the present study aims to investigate the efficacy of two predatory ladybird species, H. convergens and C. septempunctata, against the black bean aphid Aphis fabae. Additionally, it evaluates the insecticidal activity of plant extracts from R. communis and N. glauca, both individually and in combination with the predators. The goal is to assess their potential as biological control agents and to contribute to the development of more environmentally sound IPM strategies for managing A. fabae in faba bean systems.
2 Materials and methods
2.1 Aphis fabae colony
A colony of A. fabae was established from infested broad bean (Vicia faba L., cv. ‘Aguadulce’) plants collected in fields at Zemamra, Morocco (32°37′48″N, 8°42′0″W; elevation 165 m). The aphids were continuously reared for several generations on healthy V. faba plants (aged 1 month) grown in plastic pots (33 cm diameter × 12 cm height) filled with a substrate mixture of two-thirds fine sand and one-third peat. The colony was maintained in entomological cages (80 cm × 80 cm × 80 cm) constructed with wooden frames and covered with mesh fabric to ensure adequate ventilation. Rearing was conducted under controlled laboratory conditions (26 ± 2°C, 60 ± 10% relative humidity, and a 16:8 h light:dark photoperiod). To sustain the colony, uninfected V. faba plants were regularly introduced when the existing plants showed signs of damage or deterioration. This procedure ensured a continuous and healthy supply of A. fabae individuals for use in experimental trials.
2.2 Predatory ladybirds mass rearing
Individuals of C. septempunctata and H. convergens used in this study were obtained from a colony maintained at the National Institute of Agricultural Research (INRA) insectarium in Zemamra. These predators were kept in entomological cages (80 × 80 × 80 cm) constructed with aluminum frames and covered with mesh fabric to provide proper ventilation. The cages were maintained at 26 ± 2°C, 60 ± 10% relative humidity, and an 8-h light:16-h dark photoperiod. The ladybirds were fed with A. fabae-infested V. faba plants (aged 1 month) (El Aalaoui and Sbaghi, 2023). To ensure sufficient prey for mass rearing, broad bean (V. faba L., cv. ‘Aguadulce’) plants were continuously grown in plastic pots (33 cm diameter × 12 cm height) filled with a mixture of two-thirds fine sand and one-third peat. These plants were cultivated in a glasshouse (11 m × 7 m × 3 m) maintained at 25 ± 4°C and 60 ± 10% relative humidity under natural light at the Regional Center for Agricultural Research of Settat (INRA), Settat, Morocco (32.9538° N, 7.6259° W; elevation 370 m).
2.3 Plant extracts
Leaf extracts from the local species N. glauca and R. communis were prepared following the method described by Ndereyimana et al. (2020). Leaves were collected from fields in Zemamra, washed, and dried in the shade for 2 weeks. After drying, they were ground into a fine powder using an electric grinder and stored in biodegradable plastic bags. To prepare the extract for field use, 100 grams of powder was mixed directly with 1 L of water and left to steep at room temperature for 24 h. The mixture was then filtered through muslin cloth and diluted with cold water to reach a final volume of 1 L, resulting in a 10% w/v concentration. This concentration corresponds to the recommended field dose for controlling various insect pests in multiple crops (Abdelgader and Elawad, 2022; El Aalaoui and Sbaghi, 2025).
2.4 Insecticide
In this study, Imipower (imidacloprid 35% SC; Nanjing Red Sun Co. Ltd., China) was used as a positive control at a concentration of 20 mL/hL. This concentration was selected based on Saruhan (2018), who demonstrated that 20 mL hL−1 effectively controls black bean aphid populations. Imidacloprid, a neonicotinoid insecticide, is known for its strong systemic activity and high efficacy against A. fabae (Saruhan, 2018; Almogdad and Semaškienė, 2021). Moreover, previous research has shown that imidacloprid not only effectively reduces aphid infestations but also leads to higher crop yields compared to other insecticides such as carbofuran and untreated plants (Al-Naser and Ezz Al-Dden, 2011). Therefore, imidacloprid serves as a reliable benchmark to evaluate alternative control methods in this study.
2.5 Study site and host tissue
The study was conducted in a screenhouse (11 m × 7 m × 3 m), maintained at 25 ± 4°C and 60 ± 10% relative humidity under natural light at the Regional Center for Agricultural Research of Settat (INRA), Settat, Morocco. Prior to the experiments, V. faba plants were grown in plastic pots (33 cm diameter × 12 cm height) filled with a substrate mixture of two-thirds fine sand and one-third peat. These plants were cultivated in the same screenhouse and allowed to develop for one month. The plants were watered as needed throughout the growing period. Each plant was then infested with 100 s-instar A. fabae nymphs (approximately 60 females and 40 males) from the laboratory strain colony. The nymphs were transferred using a fine brush and allowed to establish for 20 days.
2.6 Treatments
The experimental treatments were as follows: T1 – untreated control, T2 – C. septempunctata (CS) alone, T3 –H. convergens alone (HC), T4 –N. glauca at 10% (NG), T5 – R. communis at 10% (RC), T6 – NG + CS, T7 – NG + HC, T8 – RC + CS, T9 – RC + HC, and T10 – Imidacloprid 35% (ICP) at 20 mL/hL % concentration prepared with tap water. The combination of the two predatory ladybirds was not tested. Prior to release, ladybirds were starved for 24 h, and for each plant, ten ovipositing adult females were introduced. Botanical extracts and Imidacloprid were applied at 20 mL per plant using a 1.5 L garden pressure sprayer (Mesto Spritzenfabrik Ernst Stockburger GmbH, Germany). To prevent cross-contamination, each treated plant was enclosed in a ventilated cylindrical cage made of sealed transparent plastic film, with the top covered by coarse mesh organdy fabric to allow airflow. Treatments were arranged in a randomized complete block design with three replicates per treatment, each replicate consisting of 7 Aphis fabae-infested Vicia faba plants, spaced 20 cm apart in a row with 40 cm between rows, totaling 210 plants per experiment. Predatory ladybirds were introduced 1 week after botanical insecticide application to minimize any repellency effects observed in previous study (El Aalaoui and Sbaghi, 2025). The experiment was repeated twice.
2.7 Data collection
Twenty days after infestation and before applying any treatments, three leaves were collected from each treated V. faba plant—one from the top, one from the middle, and one from the bottom. Leaves were cut using scissors, placed individually in paper bags, and transported to the laboratory. These leaves were chosen because they typically have high densities of A. fabae, providing a representative sample of infestation levels. Additionally, these leaf positions are commonly targeted by predatory ladybirds during their nocturnal movements from the plant apex, making them suitable for accurately assessing predation by C. septempunctata and H. convergens (Onzo et al., 2013). Both eggs and motile stages (nymphs and adults) of aphids were counted under a dissecting microscope (Motic).
Monitoring of treatment effectiveness began 1 week after the release of the predatory ladybirds and continued weekly for 5 weeks. Each week, three leaves per treated plant were randomly selected and examined following the same procedure described above. Counts included eggs and motile stages (larvae, nymphs, and adults) of both A. fabae and the predatory ladybirds. All collected ladybird specimens were identified to species level (Zare Khormizi et al., 2013). In addition, weekly assessments of aphid damage were made using a visual rating scale from 0 to 10, where 0 indicates a dead plant and 10 indicates a healthy plant, as described by Gettys et al. (2021). This scale has been widely used to evaluate plant health under various stresses, including herbicide exposure and salinity (Smith et al., 2014; Tootoonchi et al., 2020).
2.8 Statistical analysis
Data were analyzed using R software version 4.3.2. Prior to analysis, normality and homogeneity of variances were tested using the Shapiro–Wilk and Levene’s tests, respectively. To evaluate the effects of treatments and sampling time (week) on A. fabae density, predator abundance, and plant damage scores, a two-way ANOVA was performed, considering treatment and time as fixed factors. When significant differences were detected, means were compared using the Student–Newman–Keuls (SNK) multiple comparison test. Plant damage scores (0–10 scale) were analyzed similarly. A two-sample t-test was used to compare predator densities (eggs and motile stages) between the two ladybird species under the following treatment pairs: T2 –CS vs. T3 – HC; T6 – NG + CS vs. T7 – NG + HC; and T8 – RC + CS vs. T9 – RC + HC. Data were transformed using log10(x + 1) when necessary to satisfy the assumptions of normality and equal variances. Results were visualized to illustrate treatment and time effects on pest densities, predator populations, and plant health.
3 Results
3.1 Efficacy of treatments on Aphis fabae eggs
Before treatment application, there was no significant difference in A. fabae egg density among treatments (F₉,₂₀₀ = 1.1, p = 0.352), confirming homogeneity of initial infestations (Figure 1A). However, from Week 1 onwards, treatment effects were highly significant (Week 1: F₉,₂₀₀ = 728.2, p < 2.2 × 10−16; Week 2: F₉,₂₀₀ = 1694.2, p < 2.2 × 10−16; Week 3: F₉,₂₀₀ = 3077.1, p < 2.2 × 10−16; Week 4: F₉,₂₀₀ = 4931.5, p < 2.2 × 10−16; Week 5: F₉,₂₀₀ = 6992.3, p < 2.2 × 10−16), with all treatments significantly reducing egg densities compared to the control. By week 1, RC + CS (21.62 eggs per plant) and RC + HC (24.00 eggs per 3 leaves per plant) treatments achieved the greatest reductions, followed by NG + CS (28.00 eggs) and NG + HC (30.00 eggs) treatments. This pattern continued through week 5, where RC + CS (2.00 eggs per 3 leaves per plant) and RC + HC (3.90 eggs per 3 leaves per plant) treatments showed the strongest suppression of aphid eggs, while the control treatment remained significantly higher (104.14 eggs per 3 leaves per plant). Intermediate effectiveness was observed for ICP (15.00 eggs per 3 leaves per plant), NG + CS (5.95 eggs per 3 leaves per plant), and NG + HC (8.00 eggs per 3 leaves per plant) treatments. Additionally, prolonged exposure significantly enhanced treatment efficacy in reducing A. fabae egg counts over time. The CS treatment reduced egg density from 95.52 to 38.00 eggs per 3 leaves per plant (F₁,₁₂₀ = 1284.0, p < 2.2 × 10−16), while HC treatment showed a reduction from 93.67 to 39.76 eggs (F₁,₁₂₀ = 1722.6, p < 2.2 × 10−16). Botanical treatments alone also resulted in significant reductions in egg counts: in the NG treatment, egg numbers decreased from 94.52 to 31.57 (F₁,₁₂₀ = 1893.7, p < 2.2 × 10−16), and in the RC treatment, from 94.52 to 25.00 eggs (F₁,₁₂₀ = 2877.4, p < 2.2 × 10−16). Combined treatments were the most effective: NG + CS dropped eggs density from 95.81 to 5.95 eggs (F₁,₁₂₀ = 7361.2, p < 2.2 × 10−16), NG + HC from 94.52 to 8.00 eggs (F₁,₁₂₀ = 6517.2, p < 2.2 × 10−16), RC + CS from 94.57 to 2.00 eggs (F₁,₁₂₀ = 10520.0, p < 2.2 × 10−16), and RC + HC from 94.19 to 3.90 eggs (F₁,₁₂₀ = 8740.9, p < 2.2 × 10−16). The chemical treatment ICP also resulted in a significant reduction from 95.52 to 15.00 eggs (F₁,₁₂₀ = 4159.7, p < 2.2 × 10−16). In summary, all treatments significantly reduced A. fabae egg densities over time, with combined treatments of R. communis extracts and predators showing the greatest and fastest reductions. Treatment effectiveness increased with longer exposure, confirming the cumulative impact of the applied strategies.
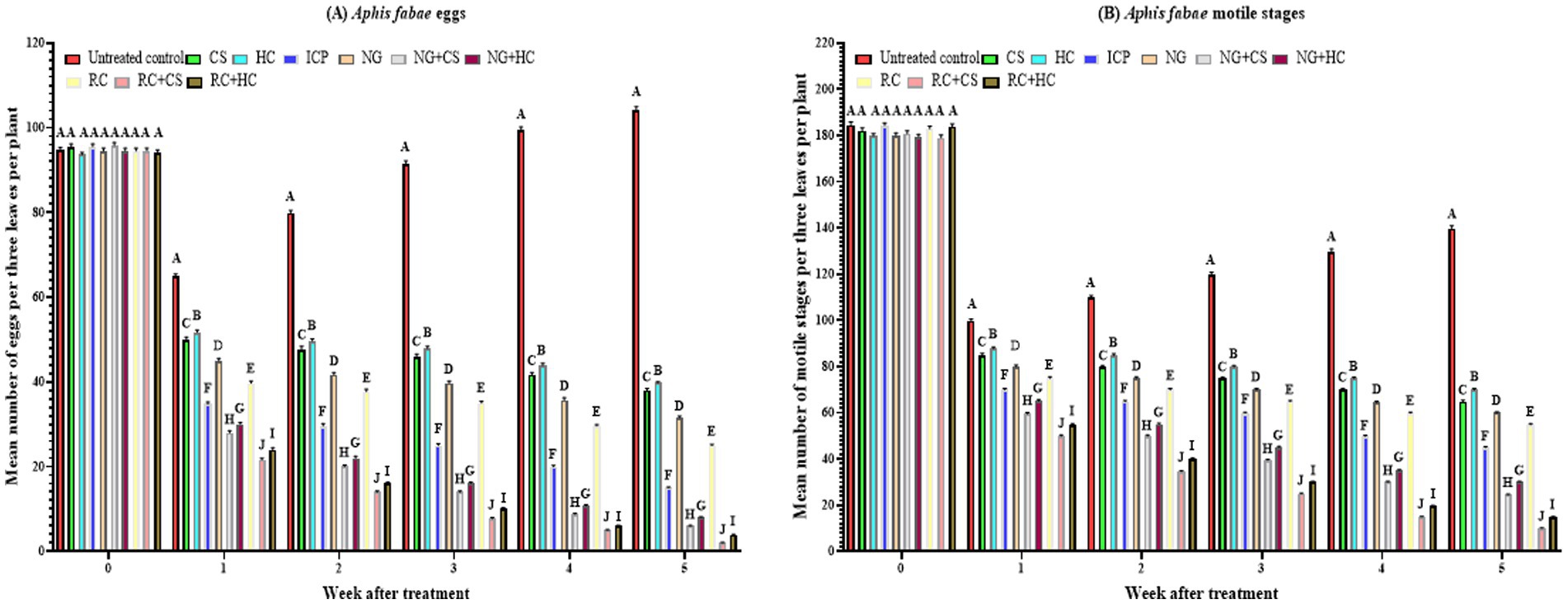
Figure 1. Mean (±SE) densities of eggs (A) and motile stages (B) of Aphis fabae following spray application of Nicotiana glauca and Ricinus communis, and Imidacloprid, and release of predatory ladybirds Coccinella septempunctata and Hippodamia convergens under screenhouse conditions. CS = C. septempunctata alone, HC = H. convergens alone, NG = N. glauca at 10%, RC = R. communis at 10%, and ICP = Imidacloprid 35% at 20 mL/hL % concentration prepared with tap water. Different letters above bars indicate statistical differences (based on the Student–Newman–Keuls test, α = 0.05).
3.2 Efficacy of treatments on Aphis fabae motile stages
Before treatment application, there was no significant difference in A. fabae motile stage density among treatments (Figure 1B). However, from week 1 onwards, treatment effects were highly significant (Week 1: F₉,₂₀₀ = 640.9, p < 2.2 × 10−16; Week 2: F₉,₂₀₀ = 1469.4, p < 2.2 × 10−16; Week 3: F₉,₂₀₀ = 2670.6, p < 2.2 × 10−16; Week 4: F₉,₂₀₀ = 3750.2, p < 2.2 × 10−16; Week 5: F₉,₂₀₀ = 5075.4, p < 2.2 × 10−16), with all treatments significantly reducing densities compared to the control. By week 1, RC + CS (49.90 individuals) and RC + HC (54.90 individuals per 3 leaves per plant) treatments achieved the greatest reductions, followed by NG + CS (59.52 per 3 leaves per plant) and NG + HC (65.09 per 3 leaves per plant) treatments. This trend persisted through week 5, where RC + CS (10.00 individuals per 3 leaves per plant) and RC + HC (15.00 individuals per 3 leaves per plant) treatments maintained the lowest densities, while the control remained significantly higher (139.71 individuals per 3 leaves per plant). Moderate reductions were observed for ICP (45.00 individuals per 3 leaves per plant), NG + CS (24.52 individuals per 3 leaves per plant), and NG + HC (30.00 individuals per 3 leaves per plant) treatments. Additionally, prolonged exposure significantly enhanced treatment efficacy in reducing A. fabae motile stages over time. CS treatment reduced density from 182.05 to 64.81 individuals per 3 leaves per plant (F₁,₁₂₀ = 3646.4, p < 2.2 × 10−16), while HC showed a reduction from 179.81 to 69.86 (F₁,₁₂₀ = 3394.7, p < 2.2 × 10−16). Botanical treatments also led to substantial declines: NG from 179.90 to 60.00 (F₁,₁₂₀ = 4045.1, p < 2.2 × 10−16) and RC from 182.90 to 54.81 (F₁,₁₂₀ = 5124.6, p < 2.2 × 10−16). In NG + CS treatment number of motile stages dropped from 180.81 to 24.52 (F₁,₁₂₀ = 9610.8, p < 2.2 × 10−16), in NG + HC treatment dropped from 179.48 to 30.00 (F₁,₁₂₀ = 9931.0, p < 2.2 × 10−16), RC + CS from 178.95 to 10.00 (F₁,₁₂₀ = 12656.0, p < 2.2 × 10−16), and RC + HC from 183.81 to 15.00 (F₁,₁₂₀ = 12460.0, p < 2.2 × 10−16). The chemical treatment ICP also caused a significant reduction from 184.33 to 45.00 (F₁,₁₂₀ = 6827.7, p < 2.2 × 10−16). Overall, all treatments significantly reduced A. fabae motile stage densities compared to the control, with combined treatments of R. communis extracts and predators (RC + CS, RC + HC) showing the greatest and most sustained reductions. Treatment effectiveness improved over time, demonstrating cumulative suppression of motile stages.
3.3 Effect of treatments on Coccinella septempunctata egg densities
From week 1 onwards, treatment effects were highly significant (week 1: F₂,₆₀ = 21.2, p = 1.10 × 10−7; week 2: F₂,₆₀ = 7.2, p = 0.002; week 3: F₂,₆₀ = 5.6, p = 0.006; week 4: F₂,₆₀ = 4.9, p = 0.011; week 5: F₂,₆₀ = 8.0, p = 8.42 × 10−4), with the RC + CS treatment consistently maintaining the highest egg densities per 3 leaves per plant, followed by NG + CS and CS treatments (Figure 2A). By week 1, RC + CS treatment (7.52 eggs per 3 leaves per plant) had the highest egg density, significantly higher than CS (6.67 eggs per 3 leaves per plant) and NG + CS (6.67 eggs per 3 leaves per plant) treatments. This pattern persisted throughout the study period, with RC + CS treatment showing 6.76 eggs per 3 leaves per plant at week 5, compared to NG + CS (6.33 eggs per 3 leaves per plant) and CS (6.24 eggs per 3 leaves per plant) treatments. At weeks 2 and 4, no significant difference was recorded between RC + CS and NG + CS treatments. For all treatments, egg numbers peaked at week 2, with 7.00 eggs per 3 leaves per plant for CS, 7.30 eggs per 3 leaves per plant for NG + CS, and 7.67 eggs per 3 leaves per plant for RC + CS treatment. Additionally, prolonged exposure revealed a slight but significant decline in egg densities across treatments over time. In the CS treatment, egg counts decreased from 6.67 at week 1 to 6.24 eggs per 3 leaves per plant by week 5 (F₄,₁₀₀ = 8.2, p = 9.8 × 10−6); in the NG + CS treatment, from 6.67 to 6.33 eggs (F₄,₁₀₀ = 12.6, p = 2.4 × 10−8); and in the RC + CS treatment, from 7.52 to 6.76 eggs per 3 leaves per plant (F₄,₁₀₀ = 18.6, p = 1.9 × 10−11). Overall, the RC + CS treatment maintained the highest C. septempunctata egg densities throughout the study, followed by NG + CS and CS treatments. Egg densities peaked at week 2 for all treatments and then gradually declined over time, indicating a slight but significant reduction in egg numbers with prolonged exposure.
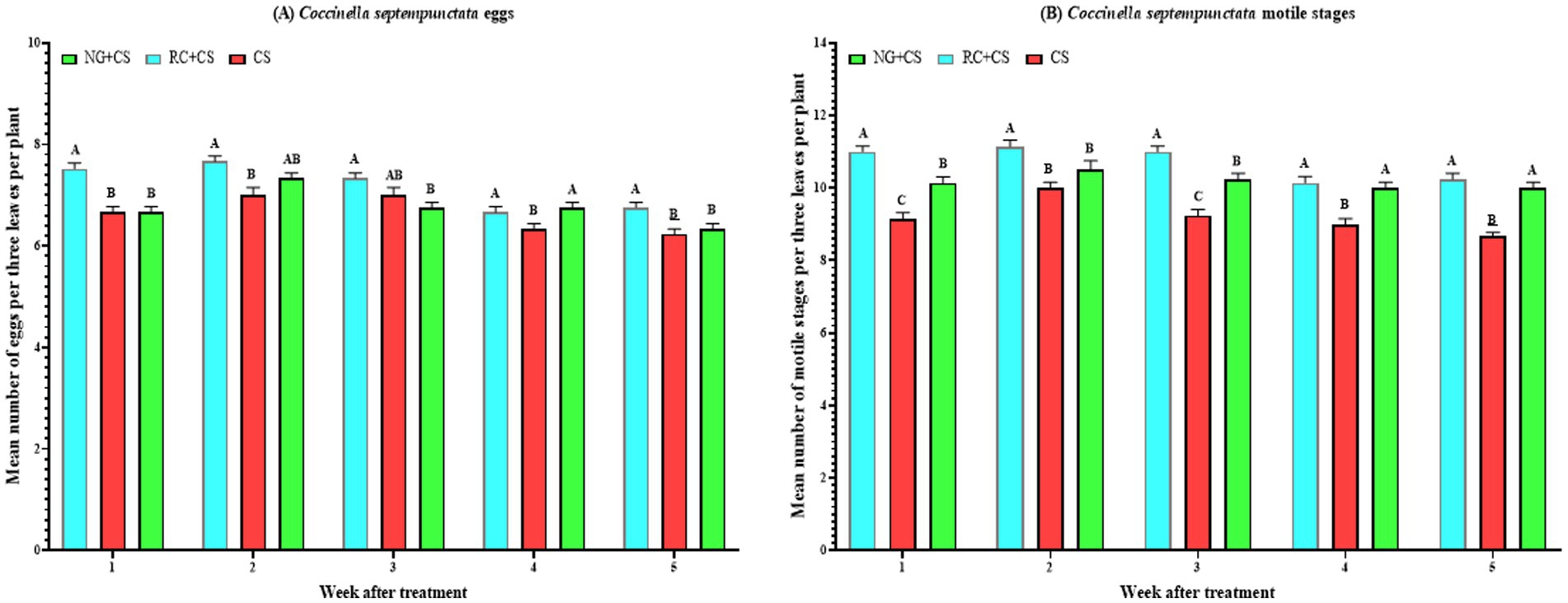
Figure 2. Mean (±SE) densities of eggs (A) and motile stages (B) of Coccinella septempunctata following spray application of Nicotiana glauca and Ricinus communis, and release of predatory ladybird Coccinella septempunctata under screenhouse conditions. CS = C. septempunctata alone, NG = N. glauca at 10%, and RC = R. communis at 10%, prepared with tap water. Different letters above bars indicate statistical differences (based on the Student–Newman–Keuls test, α = 0.05).
3.4 Effect of treatments on Coccinella septempunctata motile stage densities
From week 1 onwards, treatment effects on motile stage densities were significant for the CS and RC + CS treatments, but not for the NG + CS treatment (Figure 2B). In the CS treatment, weekly differences were highly significant (F₄,₁₀₀ = 10.4, p = 4.6 × 10−7), with motile stage density peaking at week 2 (10.00 individuals per 3 leaves per plant) and declining to 8.67 individuals per 3 leaves per plant by week 5. The RC + CS treatment also exhibited significant weekly variation (F₄,₁₀₀ = 8.3, p = 8.08 × 10−6), maintaining the highest overall motile stage densities. Densities remained stable from weeks 1 to 3 (~11.00 individuals) before slightly declining to 10.24 individuals by week 5. In contrast, the NG + CS treatment showed no significant differences across weeks (F₄,₁₀₀ = 1.6, p = 0.190), with densities remaining relatively constant. When comparing treatments within each week, the RC + CS treatment consistently recorded the highest motile stage densities, followed by NG + CS treatment and then CS treatment. At week 1, RC + CS treatment had significantly higher densities (11.00 individuals per 3 leaves per plant) compared to NG + CS (10.14 individuals per 3 leaves per plant) and CS (9.14 individuals per 3 leaves per plant) treatments (F₂,₆₀ = 32.9, p = 2.3 × 10−10). This trend persisted through week 5, where RC + CS (10.24 individuals per 3 leaves per plant) and NG + CS (10.00 individuals per 3 leaves per plant) treatments maintained significantly higher densities than CS treatment (8.67 individuals per 3 leaves per plant) (F₂,₆₀ = 34.1, p = 1.3 × 10−10). In weeks 2 and 3, RC + CS treatment showed significantly higher densities than both CS and NG + CS treatments (week 2: F₂,₆₀ = 9.4, p = 2.8 × 10−4; week 3: F₂,₆₀ = 29.3, p = 1.3 × 10−9). However, at week 4, no significant difference was observed between RC + CS and NG + CS treatments (F₂,₆₀ = 15.5, p = 5.2 × 10−6). Overall, motile stage densities peaked at week 2 for all treatments: CS (10.00 individuals per 3 leaves per plant), NG + CS (10.52 individuals per 3 leaves per plant), and RC + CS (11.14 individuals per 3 leaves per plant). Overall, the RC + CS treatment consistently maintained the highest motile stage densities throughout the study, followed by NG + CS and CS treatments. Motile stage densities peaked at week 2 for all treatments and then generally declined or stabilized over time.
3.5 Effect of treatments on Hippodamia convergens eggs density
From week 1 onwards, treatment effects on H. convergens egg densities varied across treatments and weeks (Figure 3A). In the HC treatment alone, weekly differences in egg density were significant (F₄,₁₀₀ = 6.3, p = 1.6 × 10−4), with egg density peaking at week 2 (6.67 eggs per 3 leaves per plant) and then stabilizing around 6.00–6.33 eggs per 3 leaves per plant through weeks 3 to 5. For the NG + HC treatment, significant but weaker weekly variation was observed (F₄,₁₀₀ = 3.0, p = 0.022), with densities stable at approximately 6.67 eggs per 3 leaves per plant from weeks 1 to 3, then slightly declining from week 4 onward. The RC + HC treatment showed highly significant weekly variation (F₄,₁₀₀ = 5.3, p = 0.001), with the highest egg density at week 2 (7.24 eggs per 3 leaves per plant), followed by a modest decline and stabilization (~6.52–6.67 eggs per 3 leaves per plant) in subsequent weeks. When comparing treatments within each week, egg densities differed significantly on most weeks. At week 1, NG + HC and RC + HC treatments had significantly higher densities (6.67 eggs per 3 leaves per plant each) than HC alone treatment (6.33 eggs) (F₂,₆₀ = 3.3, p = 0.042). In week 2, RC + HC treatment had the highest density (7.24 eggs per 3 leaves per plant), significantly exceeding both HC and NG + HC treatments (each 6.67 eggs per 3 leaves per plant) (F₂,₆₀ = 6.5, p = 0.003). In week 3, densities were similar between NG + HC and RC + HC treatments (each 6.67 eggs per 3 leaves per plant), both generally higher than HC treatment (6.33 eggs per 3 leaves per plant) (F₂,₆₀ = 3.3, p = 0.042). In week 4, RC + HC treatment had the highest density (6.67 eggs per 3 leaves per plant), significantly exceeding both HC and NG + HC treatments (each 6.33 eggs per 3 leaves per plant) (F₂,₆₀ = 3.3, p = 0.042). By week 5, RC + HC and NG + HC treatments maintained higher egg densities (6.52 and 6.33 eggs per 3 leaves per plant, respectively) compared to HC alone treatment (6.00 eggs) (F₂,₆₀ = 8.9, p = 0.000). In summary, the RC + HC treatment generally maintained the highest H. convergens egg densities throughout the study, with egg densities peaking at week 2 across treatments and stabilizing or slightly declining thereafter. NG + HC treatment showed intermediate egg densities, while the HC treatment alone maintained the lowest egg densities over time.
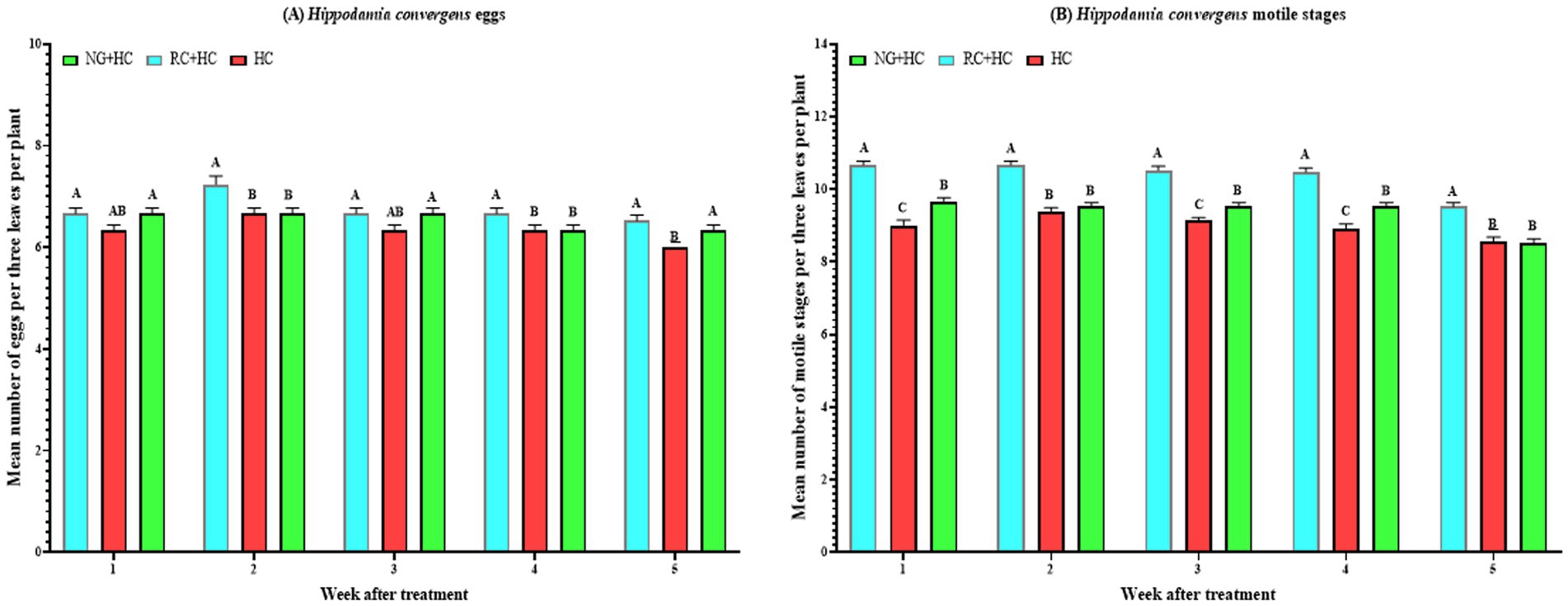
Figure 3. Mean (±SE) densities of eggs (A) and motile stages (B) of Hippodamia convergens following spray application of Nicotiana glauca and Ricinus communis, and release of predatory ladybird Hippodamia convergens under screenhouse conditions. HC = Hippodamia convergens alone, NG = N. glauca at 10%, and RC = R. communis at 10%, prepared with tap water. Different letters above bars indicate statistical differences (based on the Student–Newman–Keuls test, α = 0.05).
3.6 Effect of treatments on Hippodamia convergens motile stages density
From week 1 onwards, treatment effects on H. convergens motile stage densities varied significantly across treatments and weeks (Figure 3B). In the HC treatment alone, weekly differences were significant (F₄,₁₀₀ = 6.2, p = 1.8 × 10−4), with density peaking at week 2 (9.38 individuals per 3 leaves per plant) and gradually declining to 8.57 individuals by week 5. The NG + HC treatment showed highly significant weekly variation (F₄,₁₀₀ = 17.9, p = 4.1 × 10−11), with densities stable at approximately 9.52 individuals from weeks 1 to 4, then decreasing to 8.52 individuals at week 5. The RC + HC treatment also exhibited highly significant weekly differences (F₄,₁₀₀ = 19.4, p = 7.5 × 10−12), with the highest densities recorded during weeks 1 and 2 (10.67 individuals per 3 leaves per plant), followed by a slight decline to 9.52 individuals at week 5. When comparing treatments within each week, motile stage densities differed significantly in all weeks. At week 1, the RC + HC treatment had the highest density (10.67 individuals per 3 leaves per plant), significantly exceeding NG + HC (9.67 individuals per 3 leaves per plant) and HC alone (9.00 individuals per 3 leaves per plant) treatments (F₂,₆₀ = 45.9, p = 8.2 × 10−13). This pattern persisted through week 5, with RC + HC treatment consistently showing the highest densities. Specifically, at week 2, the density of motile stages in the RC + HC treatment (10.67 individuals per 3 leaves per plant) was significantly higher than in the NG + HC (9.52 individuals per 3 leaves per plant) and HC (9.38 individuals per 3 leaves per plant) treatments (F₂,₆₀ = 42.1, p = 3.7 × 10−12). At week 3, densities remained highest in RC + HC (10.52 individuals per 3 leaves per plant), followed by NG + HC (9.52 individuals per 3 leaves per plant) and HC (9.14 individuals per 3 leaves per plant) treatments (F₂,₆₀ = 49.1, p = 2.3 × 10−13). At week 4, RC + HC treatment again had the highest density (10.48 individuals per 3 leaves per plant), significantly greater than NG + HC (9.52 individuals per 3 leaves per plant) and HC (8.90 individuals per 3 leaves per plant) treatments (F₂,₆₀ = 43.2, p = 2.4 × 10−12). By week 5, RC + HC treatment maintained the highest density (9.52 individuals per 3 leaves per plant), significantly higher than HC (8.57 individuals per 3 leaves per plant) and NG + HC (8.52 individuals per 3 leaves per plant) treatments (F₂,₆₀ = 25.7, p = 8.8 × 10−9). In summary, motile stage densities of H. convergens peaked at week 2 for all treatments, with RC + HC treatment consistently supporting the highest densities across all weeks. NG + HC treatment showed intermediate densities, while HC treatment alone maintained the lowest densities over time.
3.7 Effect of treatments on treated plant visual quality
By week 1, the treatment effect became highly significant (F₉,₂₀₀ = 498.2, p < 2.2 × 10−16), with RC + CS (visual quality score: 8.80) and RC + HC (8.68) treatments being the most effective treatments, followed by NG + CS (8.20) and NG + HC (7.87) treatments. In contrast, the control (5.50), HC (6.10), and CS (6.20) treatments showed the lowest scores. This pattern was further reinforced in week 2 (F₉,₂₀₀ = 628.7, p < 2.2 × 10−16), where RC + CS (9.10), RC + HC (8.89), and NG + CS (8.49) treatments significantly outperformed all other treatments, while the control remained the least effective (5.68). In week 3, the same top-performing treatments maintained their superiority (F₉,₂₀₀ = 650.5, p < 2.2 × 10−16), with RC + CS, RC + HC, and NG + CS treatments achieving visual quality scores of 9.29, 9.02, and 8.73, respectively. The control (6.10), HC (6.30), and CS (6.58) treatments continued to score significantly lower. Week 4 analysis (F₉,₂₀₀ = 694.5, p < 2.2 × 10−16) confirmed that RC + CS (9.50) and RC + HC (9.27) treatments remained the most effective, followed by NG + CS (8.85) and NG + HC (8.52) treatments, while the control (6.20) and HC (6.51) were among the least effective. By week 5, the treatment effect peaked (F₉,₂₀₀ = 708.5, p < 2.2 × 10−16), with RC + CS (9.67), RC + HC (9.35), NG + CS (8.87), and NG + HC (8.73) treatments continuing to lead. The control remained the lowest (6.51), significantly different from all other treatments. Across weeks, all treatments exhibited significant improvement in plant visual quality scores over time. In the CS treatment, the score rose from 5.15 before treatment application to 6.69 in week 5 (F₅,₁₂₀ = 120.8, p < 2.2 × 10−16), and in the HC treatment from 5.25 to 6.58 (F₅,₁₂₀ = 74.3, p < 2.2 × 10−16). The NG treatment showed a steady increase from 5.27 to 7.31 (F₅,₁₂₀ = 193.7, p < 2.2 × 10−16), while NG + CS rose from 5.59 to 8.87 (F₅,₁₂₀ = 383.8, p < 2.2 × 10−16), and NG + HC from 5.46 to 8.73 (F₅,₁₂₀ = 370.4, p < 2.2 × 10−16). In the ICP treatment, the score improved from 5.59 to 8.25 (F₅,₁₂₀ = 255.8, p < 2.2 × 10−16); in the RC treatment from 5.38 to 7.74 (F₅,₁₂₀ = 250.3, p < 2.2 × 10−16); in the RC + CS treatment from 5.27 to 9.67 (F₅,₁₂₀ = 332.4, p < 2.2 × 10−16); and in the RC + HC treatment from 5.34 to 9.35 (F₅,₁₂₀ = 563.5, p < 2.2 × 10−16). These results clearly demonstrate that combined treatments involving RC and NG, particularly when paired with CS or HC, provided the most consistent and effective improvement in plant visual quality over time, significantly outperforming the control and single-component treatments. In summary, plant visual quality improved significantly across all treatments over time, with RC + CS and RC + HC treatments consistently achieving the highest scores. Treatments combining RC or NG with CS or HC were notably more effective than single-component and control treatments in maintaining superior plant appearance.
3.8 Comparison of Coccinella septempunctata and Hippodamia convergens densities across treatments
The densities of C. septempunctata (CS) and H. convergens (HC) were compared under three different treatments: CS alone versus HC alone, NG + CS versus NG + HC (where NG = N. glauca at 10%), and RC + CS versus RC + HC (where RC = R. communis at 10%). When comparing CS alone to HC alone, CS had higher egg densities at weeks 1 (6.67 vs. 6.30), 3 (7.00 vs. 6.30), and 5 (6.24 vs. 6.00), while motile stage densities were mostly similar except at week 2 where CS was higher (10.00 vs. 9.38) (Table 1). In the NG treatments, NG + CS showed greater egg densities than NG + HC at weeks 2 (7.33 vs. 6.70) and 4 (6.76 vs. 6.30). Motile stages were also consistently more abundant in NG + CS across weeks 1 to 5, with a notable difference at week 5 (10.00 vs. 8.50) (Table 2). For the RC treatments, RC + CS had higher egg densities than RC + HC during the first 3 weeks, such as at week 1 (7.52 vs. 6.67). Motile stage densities were higher in RC + CS compared to RC + HC at weeks 2, 3, and 5 (for example, week 5: 10.24 vs. 9.50) (Table 3). These results indicate that C. septempunctata generally exhibits higher egg and motile stage densities than H. convergens, when combined with N. glauca or R. communis (Figure 4).
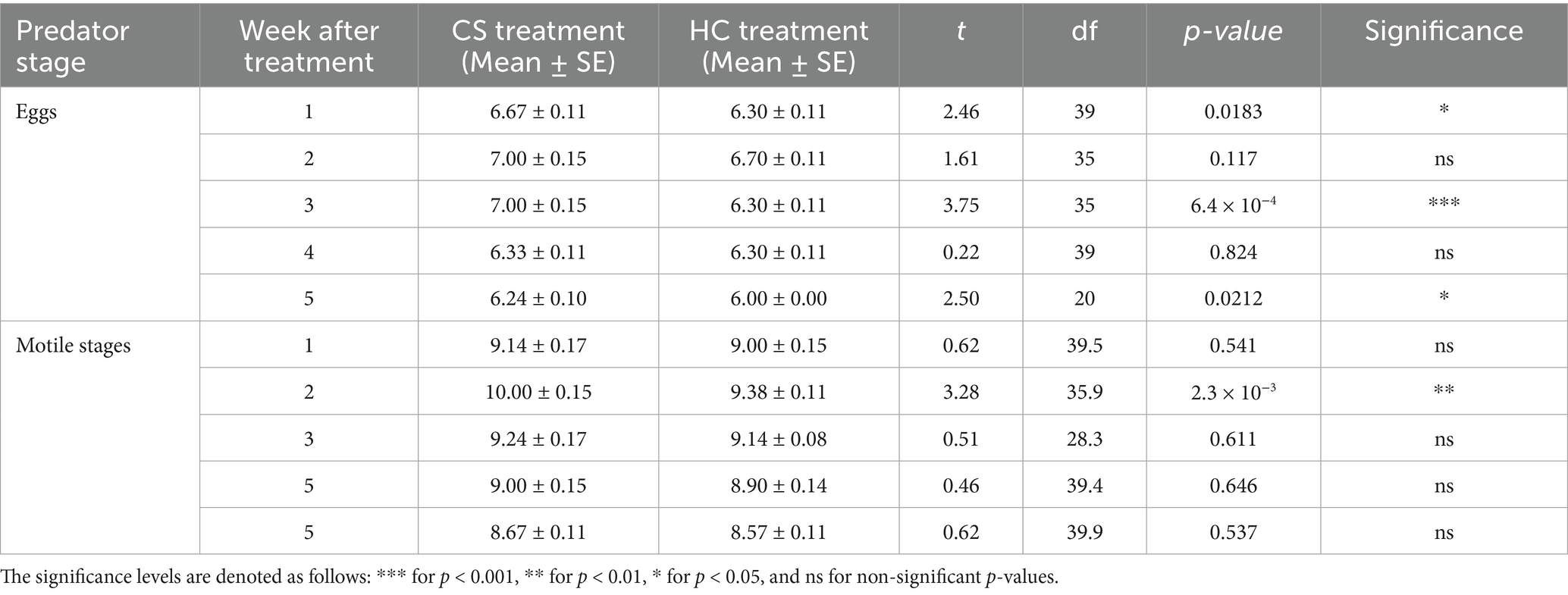
Table 1. Comparison of Coccinella septempunctata (CS) and Hippodamia convergens (HC) densities (eggs and motile stages per 3 leaves per plant) between treatment T2 (C. septempunctata alone, CS) and treatment T3 (H. convergens alone, HC).
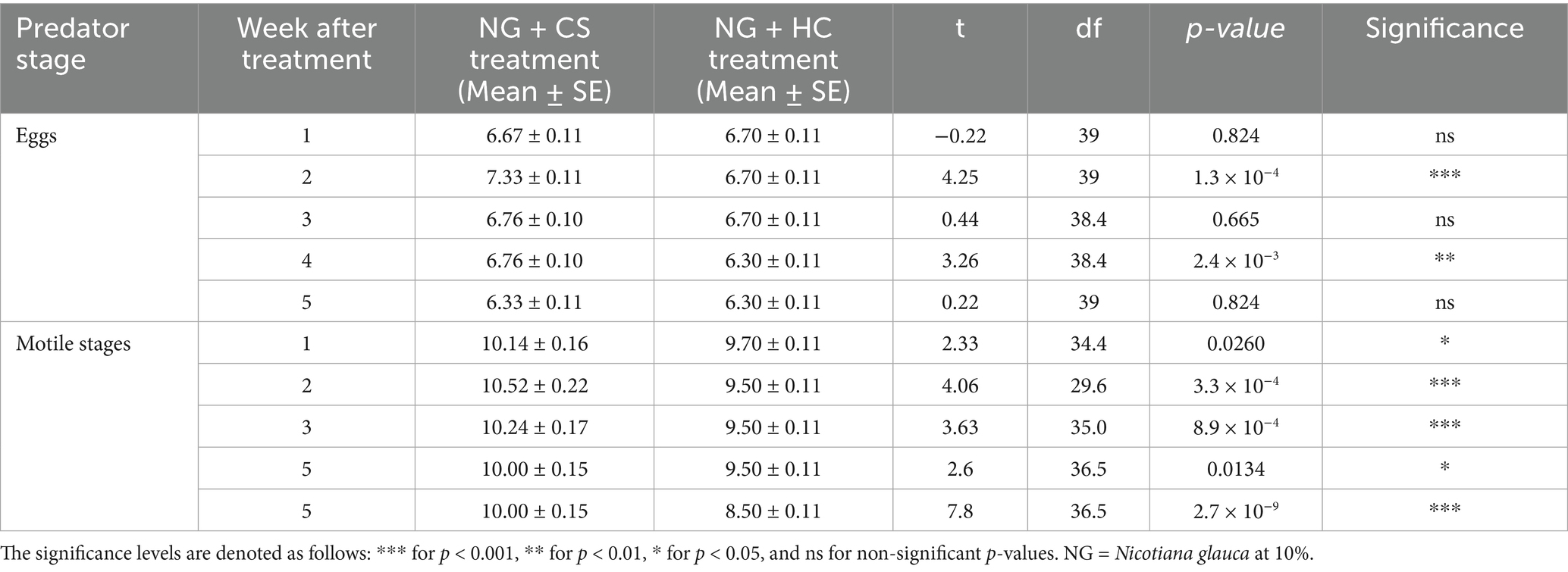
Table 2. Comparison of Coccinella septempunctata (CS) and Hippodamia convergens (HC) densities (eggs and motile stages per 3 leaves per plant) between treatment T6 – NG + CS and treatment T7 – NG + HC.
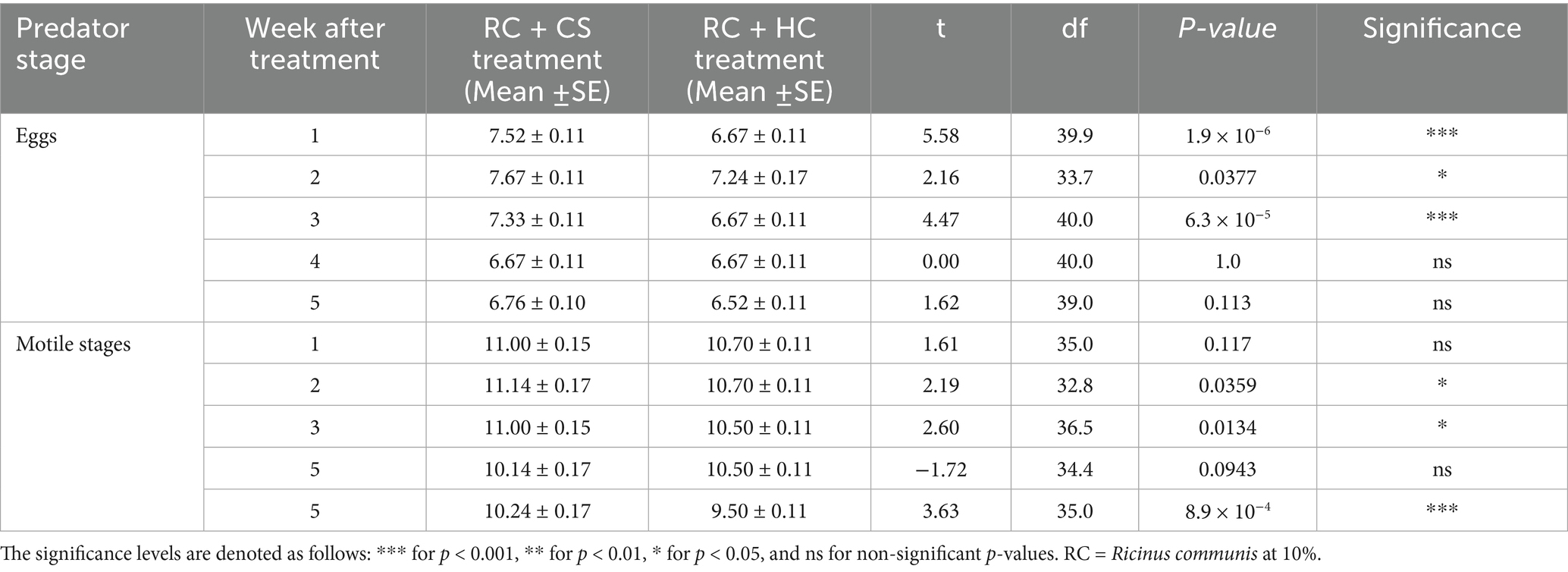
Table 3. Comparison of Coccinella septempunctata (CS) and Hippodamia convergens (HC) densities (eggs and motile stages per 3 leaves per plant) between treatment T8 – RC + CS and T9 – RC + HC.

Figure 4. Visual quality of Vicia faba L. plants after spray application of Nicotiana glauca and Ricinus communis, and Imidacloprid, and release of predatory ladybirds Coccinella septempunctata and Hippodamia convergens under screenhouse conditions. CS = C. septempunctata alone, HC = H. convergens alone, NG = N. glauca at 10%, RC = R. communis at 10%, and ICP = Imidacloprid 35% at 20 mL/hL % concentration prepared with tap water. Visual quality is rated on a scale from 0 to 10, where 0 represents dead; 5 denotes fair quality with acceptable form and color, minimal chlorosis or necrosis; and 10 indicates excellent quality, healthy and robust with optimal color and form. Different letters above bars indicate statistical differences (based on the Student–Newman–Keuls test, α = 0.05).
4 Discussion
This study evaluated the individual and combined efficacy of two predatory ladybird species, C. septempunctata (CS) and H. convergens (HC), botanical insecticides from N. glauca (NG) and R. communis (RC), and the chemical insecticide imidacloprid (ICP) in controlling A. fabae populations on V. faba plants. Our findings demonstrate that all treatments significantly reduced aphid densities and improved plant health compared to untreated controls, with combined treatments—particularly those pairing predatory ladybirds with botanical extracts—producing the most substantial and consistent suppression of aphid populations and enhancement of plant visual quality over time. The results confirm the effectiveness of C. septempunctata and H. convergens against A. fabae, aligning with previous studies (Shannag and Obeidat, 2008).
Across all 5 weeks of observation, C. septempunctata consistently outperformed H. convergens in reducing both aphid egg and motile stage densities, as well as in maintaining higher predator reproduction and motile stage populations. This was evident not only in the CS versus HC treatments, but also in the combined treatments (NG + CS vs. NG + HC; RC + CS vs. RC + HC). These findings suggest that CS possesses superior predation efficiency, reproductive output, and possibly better adaptation to local environmental conditions. The superior performance of CS aligns with earlier studies that have documented its higher fecundity, voracity, and broader prey spectrum compared to other coccinellid species (Hodek and Honek, 1996). Furthermore, the relatively lower performance of H. convergens may be attributed to its smaller body size, which can limit its predation efficiency. As noted by Blum (1981), in the absence of prey defenses, predator effectiveness is largely shaped by prey specificity and the relative size of the predator to its prey.
Furthermore, R. communis consistently showed higher efficacy than N. glauca in reducing populations of A. fabae and improving the visual quality of V. faba plants. This trend was evident both when applied alone and in combination with predatory ladybirds. The superior performance of R. communis is likely related to its rich content of toxic secondary metabolites, including ricin, ricinine, alkaloids, and flavonoids, which exhibit strong insecticidal and repellent properties (Chouhan et al., 2021). Previous studies have demonstrated that R. communis effectively controls pests such as Dactylopius opuntiae Cockerell (Hemiptera: Dactylopiidae) (da Silva Santos et al., 2016), Aedes aegypti Linnaeus (Diptera: Culicidae) (Neves et al., 2014), and Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae) (Ramos-López et al., 2010). The toxicity of R. communis seeds is mainly due to ricin, a potent protein toxin (Chouhan et al., 2021). Additionally, flavonoids and other secondary metabolites contribute by damaging insect cells and disrupting DNA, RNA, and protein synthesis (Randhir et al., 2004). Nicotiana glauca also showed insecticidal effects but was less effective across all parameters measured. It has been reported to affect pests such as Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) (Alghamdi, 2021a; Alghamdi, 2021b), Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) (Abdelgader and Elawad, 2022), and D. opuntiae (Zim et al., 2025). The relatively lower efficacy of N. glauca in this study may be due to lower concentrations of active compounds, differences in the mode of action, environmental factors during application, or pest susceptibility. Moreover, toxicity of botanical extracts varies according to the plant parts used, since concentrations of bioactive chemicals differ among plant tissues (Santiago et al., 2008).
Taken together, the ranking of treatment efficacy based on A. fabae suppression and plant health improvement followed the order: C. septempunctata > H. convergens > R. communis > N. glauca. This consistent hierarchy throughout the experimental period highlights the superior and sustained predation by ladybird species, which actively locate and consume aphids across all developmental stages. In contrast, the botanical extracts demonstrated lower efficacy, likely due to their limited persistence and rapid degradation under field conditions, as reflected in the less pronounced aphid suppression observed. These findings suggest that while botanical insecticides offer environmentally friendly options, their practical effectiveness may be constrained by factors such as reduced residual activity and slower action, which were evident in our study. Future work should focus on optimizing formulations to improve stability and efficacy, thereby enhancing their role in integrated pest management strategies.
The results of this study revealed a clear additive or synergistic effect when predatory ladybirds were combined with botanical extracts, particularly R. communis combined with C. septempunctata and H. convergens. These combined treatments consistently achieved the greatest reductions in both A. fabae eggs and motile stages, surpassing the efficacy of single treatments and the chemical insecticide imidacloprid throughout the monitoring period. The improved efficacy likely results from complementary modes of action: direct predation by ladybirds reduces aphid populations, while botanical extracts exert repellent, toxic, or anti-feeding effects on the pest (Acheuk et al., 2017; Kayange et al., 2019). These findings align with many studies demonstrating that botanical insecticides enhance biological control by reducing pest pressure with minimal impact on beneficial natural enemies. Azadirachta indica A. Juss. (Meliaceae) exhibits systemic action within plants, rapid environmental degradation, and low toxicity to predators (Kunbhar et al., 2018). In this study, predator abundance was higher in botanical treatments than in the chemical pesticide treatment, indicating acute safety for natural enemies. Similarly, Melia azedarach L. (Meliaceae) extracts did not show significant toxicity to insect pests or their natural enemies. These observations are consistent with Kraiss and Cullen (2008), who reported that azadirachtin is safer for adults of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Patel et al. (2009) documented increased activity of coccinellid beetles in mustard crops treated with neem oil formulations. Smitha and Giraddi (2006) also concluded that botanical pesticides are generally safer for predatory coccinellids. Ma et al. (2000) found that biorational pesticides, including azadirachtin and bifenthrin, were effective against Helicoverpa armigera (Hübner) and H. punctigera (Wallengren) (Lepidoptera: Noctuidae) while being safer for coccinellids and Chrysoperla carnea Stephens (Neuroptera: Chrysopidae), unlike synthetic chemicals which were highly toxic. Additionally, Qi et al. (2001) reported that azadirachtin at concentrations of 50–200 ppm did not affect predation rates of coccinellid beetles. Several studies have confirmed the minimal adverse effects of botanical insecticides on non-target natural enemies under laboratory and field conditions (Chaudhary et al., 2017; Kunbhar et al., 2018). These combined findings support the integration of predatory ladybirds with botanical extracts as an effective, environmentally safe approach for aphid management.
Both C. septempunctata and H. convergens demonstrated significant predation activity, leading to marked reductions in aphid populations. However, C. septempunctata generally exhibited higher egg and motile stage densities than HC across treatments, particularly when combined with botanical extracts, suggesting a greater reproductive potential or better adaptation under the experimental conditions. This observation is consistent with prior research indicating that C. septempunctata may have higher fecundity and more robust establishment on aphid-infested plants (Hodek and Honek, 1996; Obrycki et al., 2000). Interestingly, while both predator species effectively reduced aphid populations, the differences in their population dynamics and abundance suggest potential niche differentiation or variable responses to botanical extracts. For instance, NG + CS treatments maintained higher predator densities than NG + HC, which may reflect differential sensitivity or preference to chemical cues from N. glauca extracts. Similarly, in RC + CS treatments, C. septempunctata densities were significantly higher than in RC + HC at several time points, including a highly significant difference in egg density during week 1. These consistent patterns across treatments support the superior reproductive performance and adaptability of C. septempunctata in the presence of botanical insecticides. Such findings have important implications for biocontrol strategies, as they suggest that C. septempunctata may be more effective than H. convergens in maintaining stable populations when combined with plant-based products. Therefore, integrating this predator with compatible botanicals can enhance the sustainability and efficacy of IPM programs. This highlights the importance of carefully selecting predator-plant extract combinations in integrated pest management (IPM) programs to maximize predator efficacy and survival (Poderoso et al., 2016; Vikas, 2024; Hyder et al., 2025).
Although imidacloprid reduced aphid densities, its effectiveness was generally lower than that of combined botanical and biological treatments, especially those based on R. communis. This could potentially be due to sublethal effects on predatory ladybirds or aphid resistance to neonicotinoids (Matsuura and Nakamura, 2014; Bass et al., 2015). Additionally, imidacloprid did not improve plant visual quality as much as combined treatments, highlighting the benefits of integrating biocontrol agents with botanical insecticides. These results support IPM approaches that effectively control pests while reducing environmental and non-target risks linked to synthetic chemicals (Isman, 2020).
Treatment efficacy improved over time, with the strongest aphid control and plant health observed in later weeks, especially in RC + CS and RC + HC treatments. Delaying predator release by one week likely prevented negative effects from botanical residues, supporting effective establishment. The stable predator populations indicate that the botanical extracts used were safe at applied doses, supporting their use in IPM programs (Snyder, 2019). In the present study, treated plant visual quality scores closely followed aphid suppression patterns, with combined treatments achieving the highest ratings. This demonstrates the practical effectiveness of RC + CS and RC + HC in maintaining plant health and reducing pest damage, supporting their potential for sustainable agricultural use.
While the study provides robust evidence for the efficacy of combining botanical extracts with predatory ladybirds, it did not test combinations involving both ladybird species simultaneously. Future work should explore whether synergistic interactions between C. septempunctata and H. convergens further enhance aphid suppression, as mixed predator assemblages may exploit complementary hunting strategies and reduce prey escape (Lundgren, 2009; Sethuraman and Obrycki, 2024). Additionally, field trials are necessary to validate these findings under variable environmental conditions and pest pressure. Long-term assessments of predator survival, botanical extract persistence, and potential non-target effects will be essential to optimize application protocols and ensure sustainability.
5 Conclusion
This study demonstrates that combining predatory ladybirds (C. septempunctata and H. convergens) with botanical extracts from R. communis and N. glauca significantly reduces A. fabae populations and improves plant health better than using either method alone or imidacloprid. Among treatments, R. communis combined with predators was the most effective, and C. septempunctata generally showed higher predator numbers than H. convergens. These results support using integrated biological and botanical control as a safer, more sustainable alternative to chemical pesticides. Future work should validate these findings in field conditions, test combined use of both ladybird species, and refine application methods to maximize pest control and crop protection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ME: Writing – original draft. FK: Writing – review & editing. SR: Writing – review & editing. AC: Writing – review & editing. CA: Writing – review & editing. VB: Writing – review & editing. MS: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research of this study was supported by the National Institute of Agricultural Research (INRA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelgader, M. A. A., and Elawad, L. M. E. (2022). Insecticidal activity of chewing tobacco (Nicotiana glauca) against cotton mealy bug (Phenacoccus solenopsis) (Homoptera: Pseudococcidae). Int. J. Innov. Sci. Eng. Technol. 9, 49–54.
Abdel-Rahman, R. S., Ismail, I. A. E., Mohamed, T. A., Hegazy, M. E. F., and Abdelshafeek, K. A. (2019). Laboratory and field evaluation of certain wild plant extracts against Aphis fabae scop. (Homoptera: Aphididae) and its predators. Bull. Natl. Res. Cent. 43, 1–5. doi: 10.1186/s42269-019-0084-z
Acheuk, F., Lakhdari, W., Abdellaoui, K., Belaid, M., Allouane, R., and Halouane, F. (2017). Phytochemical study and bioinsecticidal effect of the crude ethanolic extract of the Algerian plant Artemisia judaica L. (Asteraceae) against the black bean aphid, Aphis fabae scop. Poljoprivreda i Sumarstvo 63:95. doi: 10.17707/AgricultForest.63.1.11
Alghamdi, A. A. (2021a). Impact of the invasive plant species Nicotiana glauca toxins on the larvae of the invasive insect species Rhynchophorus ferrugineus: a damaging pest of date palm trees in Saudi Arabia. Saudi J. Biol. Sci. 28, 1154–1157. doi: 10.1016/j.sjbs.2020.11.051
Alghamdi, A. A. (2021b). Phytoconstituents screening and antimicrobial activity of the invasive species Nicotiana glauca collected from Al-Baha region of Saudi Arabia. Saudi J. Biol. Sci. 28, 1544–1547. doi: 10.1016/j.sjbs.2020.12.034
Almogdad, M., and Semaškienė, R. (2021). The occurrence and control of black bean aphid (Aphis fabae scop.) in broad bean. Zemdirb.-Agric. 108, 165–172. doi: 10.13080/z-a.2021.108.022
Al-Naser, Z., and Ezz Al-dden, D. (2011). The chemical control of black bean aphid (Aphis fabae Scopoli) and their effects on morphological characters and yield of broad bean (Vicia faba L.). Arab J. Arid Environ 6, 89–96. (In Arabic)
Ayala, J., Perez-de-San-Roman, C., Ortiz, A., and Juanche, J. (1996). Chemical control of Myzus persicae (Sulz.) and Aphis fabae (scop.) (Homoptera: Aphididae) on sugar beet with aphicides applied at sowing and as foliar sprays. Bol. Sanid. Veg. Plagas. 22, 731–740.
Bass, C., Denholm, I., Williamson, M. S., and Nauen, R. (2015). The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 121, 78–87. doi: 10.1016/j.pestbp.2015.04.004
Béji, B., Bouhachem-Boukhris, S., Bouktila, D., Mezghani-Khemakhem, M., Rezgui, S., Kharrat, M., et al. (2015). Identification of sources of resistance to the black bean aphid, Aphis fabae Scopoli, in faba bean, Vicia faba L., accessions. J. Crop Prot. 4, 217–224.
Bennour, C., Belgacem, A. B., and Nasr, H. B. A. (2021). Review of the management of Aphis fabae Scopoli (Hemiptera: Aphididae). J. Oasis Agric. Sustain. Dev. 3, 32–44.
Blackman, R.L., and Eastop, V.F. (2007) Taxonomic Issues H.F. Emdenvan and R. Harrington Aphids as crop pests CABI Wallingford, UK 1–29
Chaudhary, S., Kanwar, R. K., Sehgal, A., Cahill, D. M., Barrow, C. J., Sehgal, R., et al. (2017). Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Front. Plant Sci. 8:610. doi: 10.3389/fpls.2017.00610
Chouhan, H. S., Swarnakar, G., and Jogpal, B. (2021). Medicinal properties of Ricinus communis: a review. Int. J. Pharm. Sci. Res. 12, 3632–3642.
Cichocka, E., Leszczyński, B., Ciepiela, A. A. P., and Goszczyński, W. (2002). Response of Aphis fabae scop. To different broad bean cultivars. Electron. J. Pol. Agric. Univ. 5, 1–7.
da Silva Santos, A. C., Oliveira, R. L. S., da Costa, A. F., Tiago, P. V., and de Oliveira, N. T. (2016). Controlling Dactylopius opuntiae with fusarium incarnatum–equiseti species complex and extracts of Ricinus communis and Poincianella pyramidalis. J. Pest. Sci. 89, 539–547. doi: 10.1007/s10340-015-0689-4
Dedryver, C. A., Le Ralec, A., and Fabre, F. (2010). The conflicting relationships between aphids and men: a review of aphid damage and control strategies. C. R. Biol. 333, 539–553. doi: 10.1016/j.crvi.2010.03.009
Desneux, N., Han, P., Mansour, R., Arnó, J., Brévault, T., Campos, M. R., et al. (2021). Integrated pest management of Tuta absoluta: practical implementations across different world regions. J. Pest. Sci. 95, 17–39. doi: 10.1007/S10340-021-01442-8
El Aalaoui, M., and Sbaghi, M. (2023). Comparative study of development, survival and reproductive performances of Coccinella septempunctata and Hippodamia convergens feeding on artificial diets. Int. J. Trop. Insect Sci. 43, 1957–1966. doi: 10.1007/s42690-023-01095-3
El Aalaoui, M., and Sbaghi, M. (2025). Integrated pest management of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) on sugar beet. Sugar Tech., 1–12. doi: 10.1007/s12355-025-01614-1
El Sayed, Y. A., and Ibrahim, S. A. (2020). Toxicological studies and histopathological changes on black bean aphid, Aphis craccivora induced by entomopathogenic fungi, Metarhizium anisopliae and Purpureocillium lilacinum. Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control. 12, 185–196.
Elijah, J. P., and Somadina, C. R. (2020). Phytochemical and toxicity analysis of Ricinus communis. Asian J. Biol. 3, 34–41.
Fericean, L. M., Rada, O., and Ostan, M. (2012). The behaviour, life cycle and biometrical measurements of Aphis fabae. Res. J. Agric. Sci. 44, 31–37.
Gettys, L. A., Thayer, K. L., and Sigmon, J. W. (2021). Evaluating the effects of acetic acid and d-limonene on four aquatic plants. HortTechnology 31, 225–233. doi: 10.21273/HORTTECH04769-20
Hansen, L. M., Lorentsen, L., and Boelt, B. (2008). How to reduce the incidence of black bean aphids (Aphis fabae scop.) attacking organically grown field beans (Vicia faba L.) by growing partially resistant bean varieties and by intercropping field beans with cereals. Acta Agric. Scand. Sect. B Soil Plant Sci. 58, 359–364.
Hodek, I., and Honek, A. (1996). Ecology of Coccinellidae. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Hyder, M., Ali, F., Ghafar, M. A., Bukero, A., ul Haq, I., Lodhi, A. M., et al. (2025). Toxicity of ethanolic plant extracts to Aphis gossypii, Bemisia tabaci, and Frankliniella occidentalis and selectivity to Coccinella septempunctata and Menochilus sexmaculatus. Neotrop. Entomol. 54:87. doi: 10.1007/s13744-025-01298-y
Ioannidis, P. (2000). Resistance of Aphis fabae and Myzus persicae to insecticides in sugar beets. In Proceedings of the 63rd Congress of the International Institute for Beet Research, Belgium: International Institute for Beet Research. 497–504
Isman, M. B. (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. doi: 10.1146/annurev.ento.51.110104.151146
Isman, M. B. (2020). Botanical insecticides in the twenty-first century—fulfilling their promise? Annu. Rev. Entomol. 65, 233–249. doi: 10.1146/annurev-ento-011019-025010
Johnson, K. D., O’Neil, M. E., Ragsdale, D. W., Difonzo, C. D., Swinton, S. M., Dixon, P. M., et al. (2009). Probability of cost-effective management of soybean aphid (Hemiptera: Aphididae) in North America. J. Econ. Entomol. 102, 2101–2108.
Kayange, C. D., Njera, D., Nyirenda, S. P., and Mwamlima, L. (2019). Effectiveness of Tephrosia vogelii and Tephrosia candida extracts against common bean aphid (Aphis fabae) in Malawi. Adv. Agric. 2019, 1–6. doi: 10.1155/2019/6704834
Kraiss, H., and Cullen, E. M. (2008). Insect growth regulator effects of azadirachtin and neem oil on survivorship, development and fecundity of Aphis glycines (Homoptera: Aphididae) and its predator, Harmonia axyridis (Coleoptera: Coccinellidae). Pest Manag. Sci. 64, 660–668. doi: 10.1002/ps.1541
Kunbhar, S., Rajput, L. B., Gilal, A. A., Channa, G. A., and Sahito, J. G. M. (2018). Impact of botanical pesticides against sucking insect pests and their insect predators in brinjal crop. J. Entomol. Zool. Stud. 6, 83–87.
Lundgren, J. G. (2009). “Plant-incorporated pest resistance and natural enemies” in Relationships of natural enemies and non-prey foods. ed. J. G. Lundgren (New York, NY, USA: Springer Science + Business Media B.V), 309–331.
Ma, D. L., Gordh, G., and Zalucki, M. P. (2000). Toxicity of biorational insecticides to Helicoverpa spp. (Lepidoptera: Noctuidae) and predators in cotton field. Int. J. Pest Manag. 46, 237–240. doi: 10.1080/096708700415580
Matsuura, A., and Nakamura, M. (2014). Development of neonicotinoid resistance in the cotton aphid Aphis gossypii (Hemiptera: Aphididae) in Japan. Appl. Entomol. Zool. 49, 535–540. doi: 10.1007/s13355-014-0289-4
Meradsi, F., and Laamari, M. (2018). Behavioral and biological responses of black bean aphid (Aphis fabae, Scopoli, 1763) on seven Algerian local broad bean cultivars. Acta Agric. Slov. 111, 535–543. doi: 10.14720/aas.2018.111.3.02
Mwanauta, R. W., Mtei, K. M., and Ndakidemi, P. A. (2015). Potential of controlling common bean insect pests (bean stem maggot (Ophiomyia phaseoli), ootheca (ootheca bennigseni) and aphids (Aphis fabae)) using agronomic, biological and botanical practices in field. Agric. Sci. 6, 489–497.
Ndereyimana, A., Nyalala, S., Murerwa, P., and Gaidashova, S. (2020). Field efficacy of entomopathogens and plant extracts on Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) infesting tomato in Rwanda. Crop Prot. 134:105183. doi: 10.1016/j.cropro.2020.105183
Neves, R. T., Rondon, J. N., Silva, L. I. M., Peruca, R. D., Ítavo, L. C. V., Carvalho, C. M. E., et al. (2014). Efeito larvicida de Ricinus communis L. Rev. Eletron. Gest. Educ. Tecnol. Ambient. 8, 127–131.
Nordey, T., Boni, S. B., Agbodzavu, M. K., Mwashimaha, R., Mlowe, N., Ramasamy, S., et al. (2021). Comparison of biological methods to control Aphis fabae Scopoli (Hemiptera: Aphididae) on kalanchoe crops in East Africa. Crop Prot. 142:105520. doi: 10.1016/j.cropro.2020.105520
Obrycki, J.J., Elliott, N.C., and Giles, K.L. (2000). Coccinellid introductions: potential for and evaluation of nontarget effects. In Nontarget effects of biological control. New York, NY: Springer Science+Business Media, pp. 127–145.
Onzo, A., Bello, I. A., and Hanna, R. (2013). Effects of the entomopathogenic fungus Neozygites tanajoae and the predatory mite Typhlodromalus aripo on cassava green mite densities: screenhouse experiments. BioControl 58, 397–405. doi: 10.1007/s10526-013-9508-0
Parker, W.E., and Biddle, A.J. (1998) Assessing the damage caused by black bean aphid (Aphis fabae) on spring beans. In Brighton Crop Protection Conference. Brighton, UK: Pests & Diseases 1998, pp. 1077–1082
Patel, B. S., Patel, I. S., and Patel, G. M. (2009). Evaluation of different eco-friendly modules for the management of mustard aphid, Lipaphis erysimi (Kalt.) in North Gujarat. J. Oilseed Res. 26, 679–680.
Poderoso, J. C. M., Correia-Oliveira, M. E., Chagas, T. X., Zanuncio, J. C., and Ribeiro, G. T. (2016). Effects of plant extracts on developmental stages of the predator Podisus nigrispinus (Hemiptera: Pentatomidae). Fla. Entomol. 99, 113–116. doi: 10.1653/024.099.0121
Purhematy, A., Ahmadia, K., and Moshrefi, M. (2013). Toxicity of thiacloprid and fenvalerate on the black bean aphid, Aphis fabae, and biosafety against its parasitoid, Lysiphlebus fabarum. J. Biopest. 6:207.
Qi, B., Gordon, G., and Gimme, W. (2001). Effects of neem-fed prey on the predacious insects Harmonia conformis (Boisduval) (Coleoptera: Coccinellidae) and Mallada signatus (schneider) (Neuroptera: Chrysopidae). Biol. Control 22, 185–190. doi: 10.1006/bcon.2001.0965
Ramos-López, M. A., Pérez, S., Rodríguez-Hernández, G. C., Guevara-Fefer, P., and Zavala-Sanchez, M. A. (2010). Activity of Ricinus communis (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). Afr. J. Biotechnol. 9, 1359–1365.
Randhir, R., Lin, Y. T., and Shetty, K. (2004). Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 39, 637–646. doi: 10.1016/S0032-9592(03)00197-3
Rashedi, A., Rajabpour, A., Rasekh, A., and Zandi-Sohani, N. (2019). Interactions between host plant, Aphis fabae, and its natural enemies, Orius albidipennis and Lysiphlebus fabarum in a tritrophic system. J. Asia Pac. Entomol. 22, 847–852. doi: 10.1016/j.aspen.2019.07.001
Raymond, B., Searle, J. B., and Douglas, A. E. (2001). On the processes shaping reproductive isolation in aphids of the Aphis fabae (scop.) complex (Aphididae: Homoptera). Biol. J. Linn. Soc. 74, 205–215. doi: 10.1111/j.1095-8312.2001.tb01387.x
Regnault-Roger, C., Hemetsberger, S., and Buchbauer, G. (2020). “Use of essential oils in agriculture” in Handbook of essential oils (United States: CRC Press), 873–917.
Rochelyn, D.O.N.A., and Satar, S. (2025). Thermal effects on the growth and developmental time of bean Aphis fabae (Hemiptera: Aphididae), Int. J. Trop. Insect Sci. doi: 10.21203/rs.3.rs-5220155/v1
Sanchez-Bayo, F., and Tennekes, H. A. (2020). Time-cumulative toxicity of neonicotinoids: experimental evidence and implications for environmental risk assessments. Int. J. Environ. Res. Public Health 17:1629. doi: 10.3390/ijerph17051629
Santiago, G. P., Pádua, L. E. M., Silva, P. R. R., Carvalho, E. M. S., and Maia, C. B. (2008). Efeitos de extratos de plantas na biologia de Spodoptera frugiperda (J. E. Smith, 1797) mantida em dieta artificial. Cienc. Agrotec. 32, 792–796.
Saruhan, I. (2018). Efficacy of some entomopathogenic fungi against Aphis fabae Scopoli (Hemiptera: Aphididae). Egypt J. Biol. Pest Control, 28, 89. doi: 10.1186/s41938-018-0096-2
Saruhan, I., Senyer, N., Ayvaz, T., Kayhan, G., Ergun, E., Odabas, M. S., et al. (2015). The estimation of adult and nymph stages of Aphis fabae (Hemiptera: Aphididae) using artificial neural network. Entomol. News 125, 12–19. doi: 10.3157/021.125.0104
Sethuraman, A., and Obrycki, J. J. (2024). Population genomics and demographic modeling enhance our understanding of trophic level interactions in biological control. Biol. Control 196:105585. doi: 10.1016/j.biocontrol.2024.105585
Shannag, H. K., and Obeidat, W. M. (2008). Interaction between plant resistance and predation of Aphis fabae (Homoptera: Aphididae) by Coccinella septempunctata (Coleoptera: Coccinellidae). Ann. Appl. Biol. 152, 331–337. doi: 10.1111/j.1744-7348.2008.00220.x
Singh, R., and Singh, G. (2020). “Aphids” in In ecofriendly Pest Management for Food Security. ed. Omkar (London, UK: Academic Press), 105–182.
Smith, H. C., Ferrell, J. A., and Koschnick, T. J. (2014). Flurprimidol performance on ornamental species in relation to trimming time and method of application. HortScience 49, 1305–1308. doi: 10.21273/HORTSCI.49.10.1305
Smitha, M. S., and Giraddi, R. S. (2006). Safety of pesticidal sprays to natural enemies in chilli (Capsicum annuum L.). J. Biol. Control. 20, 7–12.
Snyder, W. E. (2019). Give predators a complement: conserving natural enemy biodiversity to improve biocontrol. Biol. Control 135, 73–82. doi: 10.1016/j.biocontrol.2019.04.017
Stoddard, F. L., Nicholas, A. H., Rubiales, D., Thomas, J., and Villegas-Fernández, A. M. (2010). Integrated pest management in faba bean. Field Crop Res. 115, 308–318. doi: 10.1016/j.fcr.2009.07.002
Tootoonchi, M., Gettys, L. A., Thayer, K. L., Markovich, I. J., Sigmon, J. W., and Sadeghibaniani, S. (2020). Ecotypes of aquatic plant Vallisneria americana tolerate different salinity concentrations. Diversity 12:65. doi: 10.3390/d12020065
Tora, T. I., Biswas, A. P., Amin, M. R., Hassan, J., and Rahman, M. M. (2024). Exploring developmental plasticity in Aphis fabae (Hemiptera: Aphididae) across varied temperature conditions. J. Entomol. Zool. Stud. 12, 116–122.
Traugott, M., and Symondson, W. O. C. (2008). Molecular analysis of predation on parasitized hosts. Bull. Entomol. Res. 98, 223–231. doi: 10.1017/S0007485308005968
Vikas, R. R. (2024). Agroecological approaches to sustainable development. Front. Sustain. Food Syst. 8:1405409.
Way, M. J. (1967). The nature and causes of annual fluctuations in numbers of Aphis fabae scop. On field beans (Vicia faba). Ann. Appl. Biol. 59, 175–188. doi: 10.1111/j.1744-7348.1967.tb04427.x
Way, M. J., and Banks, C. J. (1967). Intra-specific mechanisms in relation to the natural regulation of numbers of Aphis fabae scop. Ann. Appl. Biol. 59, 189–205. doi: 10.1111/j.1744-7348.1967.tb04428.x
Way, M. J., Cammell, M. E., Alford, D. V., Gould, H. J., Graham, C. W., Lane, A., et al. (1977). Use of forecasting in chemical control of black bean aphid, Aphis fabae scop., on spring-sown field beans, Vicia faba L. Plant Pathol. 26, 1–7.
Wilson, M., Moshitzky, P., Laor, E., Ghanim, M., Horowitz, A. R., and Morin, S. (2007). Reversal of resistance to pyriproxyfen in the Q biotype of Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 63, 761–768. doi: 10.1002/ps.1403
Zare Khormizi, M., Biranvand, A., and Shakarami, J. (2013). The faunistic survey of lady beetles (Coleoptera, Coccinellidae) in the Mehriz region (Yazd Province), Iran. Bull. Iraq Nat. Hist. Mus. 12, 43–51.
Keywords: biocompatibility, secondary metabolites, polyphagous predators, trophic interactions, functional response, sustainability assessment
Citation: El Aalaoui M, Kamal FZ, Rammali S, Ciobica A, Albert C, Burlui V and Sbaghi M (2025) Synergistic control of Aphis fabae Scop. (Hemiptera: Aphididae) on faba bean using botanical extracts and predatory ladybirds. Front. Sustain. Food Syst. 9:1672706. doi: 10.3389/fsufs.2025.1672706
Edited by:
Miguel Angel García-Parra, National Open and Distance University, ColombiaReviewed by:
Wang Jie, Beijing Academy of Agriculture and Forestry Sciences, ChinaArley Villamizar, Industrial University of Santander, Colombia
Copyright © 2025 El Aalaoui, Kamal, Rammali, Ciobica, Albert, Burlui and Sbaghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Albert, YWxiaWRlbnQ3MkB5YWhvby5jb20=
 Mohamed El Aalaoui
Mohamed El Aalaoui Fatima Zahra Kamal
Fatima Zahra Kamal Said Rammali3,4
Said Rammali3,4 Alin Ciobica
Alin Ciobica