- 1National Institute of Agricultural Research, Rabat, Morocco
- 2Care and Health Biology Team, 2S2D Laboratory, Higher Institute of Nursing Professions and Health Technical (ISPITS), Casablanca, Morocco
- 3Human Nutrition, Bioacives and Oncogenetics Team, Faculty of Sciences, Moulay Ismail University, Meknes, Morocco
- 4Laboratory of Agro-Alimentary and Health, Faculty of Sciences and Techniques, Hassan First University of Settat, Settat, Morocco
- 5Department of Biology, Faculty of Biology, Alexandru Ioan Cuza University, Iasi, Romania
- 6‘Olga Necrasov’ Center, Department of Biomedical Research, Romanian Academy, Iasi, Romania
- 7Clinical Department, Apollonia University, Iasi, Romania
- 8CENEMED Platform for Interdisciplinary Research, “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania
- 9Faculty of Medicine, University of Medicine and Pharmacy “Grigore T. Popa”, Iasi, Romania
Introduction: The potato tuber moth Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae) is a major pest threatening potato crops worldwide.
Methods: This study evaluated the effectiveness of 12 treatments—including biorational insecticides, plant extracts, and their combinations—over 2 years (2024 and 2025) under field conditions. Treatments included Spinosad (200 cc/hL) (SD), mineral oil (1,000 cc/hL) (MO), potassium salts of fatty acids (300 cc/hL) (PFA), Nicotiana glauca (NG) and Ricinus communis (RC) extracts (10% w/v), and their combinations. Applications were made five times per year, starting 3 weeks after planting and repeated every 3 weeks, using a randomized complete block design with two replicates. Parameters measured included leaf damage, larval density, canopy area, tuber number, tuber weight, and total yield.
Results and Discussion: The combination of mineral oil with R. communis consistently resulted in the lowest leaf damage, reducing it to 18.2 and 15.8% at 3 months after the first treatment (MAFT) in 2024 and 2025, respectively. Larval density was also suppressed, with MO + RC reducing larvae to 1.5 (2024) and 1.2 (2025) per plant at 3 MAFT. Canopy area remained stable across treatments in 2024 but decreased significantly in Spinosad combinations in 2025. The highest tuber number (9.4 and 9.16 per plant) and tuber weight (160.85 g and 158.73 g) in 2025 were recorded with MO + NG and MO + RC treatments. Total tuber yield peaked at 43.5 t/ha with MO + NG. These results demonstrate the superior efficacy of mineral oil combined with botanical extracts in reducing pest damage and improving potato productivity.
1 Introduction
Arthropod pests are responsible for approximately 20% of global crop losses, causing financial damage exceeding US$ 470 billion annually (Culliney, 2014). Recent reports indicate that invasive pests and plant diseases can reduce yields by up to 40% each year, leading to economic losses of more than US$ 220 billion (FAO, 2022; C.A.B.I., 2022). Among these, the potato tuber moth Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae) is one of the most destructive pests of potatoes worldwide, particularly in developing countries (Campos and Ortiz, 2020; Naqqash, 2023). It thrives in warm, dry climates and has been reported in Morocco since the mid-20th century (Hanafi, 1999). In North Africa, its populations rise rapidly before harvest, often causing severe damage during storage, with tuber losses reaching up to 100% under poor conditions (Salah et al., 1994; Laarif et al., 2003; Naqqash, 2023). The pest damages both field and stored potatoes, lowering crop quality and increasing the risk of secondary infections (Westedt et al., 1998). It attacks both leaves and tubers, leading to significant yield reductions (Capinera, 2001). Leaf symptoms include blisters, holes, and damage to the stalks, often leading to premature plant death (Hanafi, 1999). Larvae can infest developing tubers either from the foliage or from eggs laid directly on the tubers (Dimsey, 2008). As the larvae bore into the tubers, they create entry points for fungal and bacterial pathogens (Hanafi, 1999). This infestation often continues during harvest and storage (Dimsey, 2008). Under heavy field infestations, foliage destruction can lead to yield losses of up to 70% (Campos and Ortiz, 2020).
The life cycle of P. operculella is highly dependent on temperature and environmental conditions, with the entire cycle completed in about 20 to 30 days under optimal conditions (Raman, 1980). Adult moths live for 10 to 15 days, during which females lay eggs on leaves, stems, tubers, soil, or storage materials (Raman, 1980; Goldson and Emberson, 1985). Individually reared potato tuber moths laid between 0 and 236 eggs over their lifetime, with peak egg-laying occurring 2 to 5 days after emergence and declining significantly by day 7 (Fenemore, 1977). Eggs hatch in 2 to 6 days, and larvae begin feeding internally on plant tissues, passing through four instars over 2–3 weeks (Alvarez et al., 2005). Pupation occurs in soil or within tubers and lasts 6–9 days, although development slows significantly in cooler temperatures (Foot, 1998). In warm climates or non-refrigerated storage, up to 12–18 generations may occur annually, with continuous breeding in stored potatoes (Kabir, 1994; Dimsey, 2008). Peak development occurs at 20–25 °C, especially during hot, dry summers (Raman, 1988; Dimsey, 2008).
Chemical control remains the primary method used by most African farmers to manage potato tuber moth (PTM). Broad-spectrum insecticides, including systemic granules applied at planting and foliar sprays, are commonly employed to reduce pest populations and economic losses (Tsedaley, 2015). In Morocco, commonly used insecticides include azinphos-methyl, malathion, and indoxacarb (Hannour et al., 2017). However, reliance on these chemicals has led to significant issues, such as pest resistance and negative impacts on human health and the environment (Campos and Ortiz, 2020). Chemical control of P. operculella is particularly difficult due to the larvae’s protected tunneling habits and widespread resistance to organophosphates, carbamates, and pyrethroids (Shelton and Wyman, 1979; Shelton et al., 1981; Collantes et al., 1986).
The effective management of P. operculella requires an integrated approach combining cultural, biological, and chemical strategies. Cultural practices such as using healthy seed tubers, crop rotation, eliminating volunteer plants, and maintaining soil coverage can reduce PTM infestations by limiting larval access and preventing egg-laying (Fuglie et al., 1993; Hanafi, 1999; Rondon, 2007). Pheromone-based monitoring and mating disruption provide environmentally friendly tools for tracking pest populations and reducing insecticide use (Von Arx et al., 1987; Larraín et al., 2009). Biological control agents (predators and parasitoids) and entomopathogens (fungi and bacteria) have also shown promise in reducing PTM populations (Douches et al., 2002; Rondon, 2007; Horne and Page, 2009). In addition, transgenic potatoes and host plant resistance contribute to enhancing pest control (Barton et al., 1987; Li et al., 1999). Botanical control methods, such as the use of plant extracts from Lantana camara (Verbenaceae) and Azadirachta indica (neem, Meliaceae), have demonstrated effectiveness in reducing PTM damage by repelling adults or disrupting reproduction (Moawad and Ebadah, 2007; Das et al., 2007). These methods offer a sustainable approach to managing PTM and minimizing the environmental impact of pest control, with host plant resistance and natural enemy conservation being essential components of a robust IPM program.
Over the past 10 years, various biorational insecticides have shown considerable potential in managing P. operculella (Saour, 2008; Clough et al., 2010). Among them, spinosad, derived from Saccharopolyspora spinosa through natural fermentation, contains the active compounds spinosyn A and D—macrocyclic lactones that cause paralysis in insects by disrupting neural transmission via nicotinic acetylcholine and GABA receptors (Salgado, 1998; Thompson et al., 2000). Spinosad is now approved in over 60 countries for use against Lepidopteran pests in a range of crops, including vegetables, fruits, and field crops (Legocki et al., 2010; Wang et al., 2013). Mineral oils act by covering insect spiracles, suffocating them by blocking respiration (Sharaby et al., 2009; Helmy et al., 2012). Insecticidal soaps, composed of potassium salts of fatty acids, disrupt cell membranes after penetrating the insect’s cuticle, leading to dehydration and death (Tsolakis and Ragusa, 2008; Vassiliou, 2008; Lamsal et al., 2023). However, these biorational insecticides face limitations, including reduced persistence under field conditions and potential resistance development (Lamsal et al., 2023). To enhance their effectiveness, combining them with plant extracts—known for their diverse bioactive compounds and environmental safety—offers a promising complementary approach (Isman, 2006).
Plants are valuable sources of pest control agents due to their insecticidal, repellent, or growth-inhibitory properties (Zhang et al., 2017; Ropek and Kołodziejczyk, 2019). Storing potatoes with Lantana leaves significantly reduces P. operculella damage (Lal, 1987), while cardamom oil at high concentrations decreases egg hatchability by up to 86.74% (Moawad and Ebadah, 2007). Neem-based products also effectively limit PTM infestation during storage (Siddig, 1987; Tsedaley, 2015). In Morocco, Rosmarinus officinalis (Lamiaceae) essential oils have demonstrated strong protective effects on stored tubers (Hannour et al., 2017). Moreover, native plants like Ricinus communis (Euphorbiaceae) and Nicotiana glauca (Solanaceae), rich in potent compounds such as nicotine, anabasine, and ricin, are known for their insecticidal, antifungal, and antioxidant activities (Zim et al., 2024). These botanicals degrade quickly in the environment, posing less risk to non-target species (Pillmoor et al., 1993). Evaluating the field efficacy of these locally available plants against P. operculella could offer cost-effective and sustainable pest management solutions for farmers. This study assessed the effectiveness of both individual and combined applications of the biorational insecticides—Spinosad, mineral oil, and potassium salts of fatty acids—alongside plant extracts from R. communis and N. glauca for controlling P. operculella in potato fields under field conditions.
2 Materials and methods
2.1 Study site and period
The study was conducted over 2 years (2024 and 2025) on two plots of Solanum tuberosum L. (Desiree variety) in Zemamra, Casablanca-Settat region (33°15′N, 8°30′W). The plots measured 5 hectares in 2024 and 7 hectares in 2025, and were located approximately 10 km apart. Both were managed using standard agricultural practices, with manual weed removal. Planting was carried out in early January (week 1 of each year), and harvesting occurred at the end of April (week 17 of each year). Prior to planting, seed tubers were stored at 4 °C and then exposed to 18–20 °C for a few days to stimulate sprouting. Well-sprouted tubers were selected after 7–10 days. Organic manure was applied at a rate of 30 t/ha and incorporated into the soil through deep plowing (25–30 cm), followed by shallow tillage. Mineral fertilization included 120 kg/ha of ammonium sulfate (21% N), 850 kg/ha of superphosphate (18% P), and 380 kg/ha of potassium sulfate (48% K). A second plowing, at 15 cm depth, was used to incorporate the mineral fertilizer. Tubers were manually planted at a depth of 8–12 cm in rows spaced 60–70 cm apart, with 30–40 cm between plants, using 20 q/ha of seed tubers, corresponding to approximately 50,000 plants per hectare. Crop maintenance included one or two early hoeings, followed by two or three ridgeings. Supplemental nitrogen (ammonium nitrate, 33.5%) was applied at 60 U/ha during the first ridging and again at 60 U/ha during the final ridgeing. Irrigation was provided via drip system every 10 days at a rate of 1.5 L/plant, and was stopped approximately 10 days before harvest. The site is situated in a semi-arid zone, with an average annual rainfall of 330 mm and temperature variations ranging from −1 °C in winter to 40–45 °C in summer. The soil is classified as vertisol, extending to a depth of 1.5 meters, with an alkaline pH (8.6) and the following chemical properties: nitrogen (200 mg/kg), phosphorus (46 mg/kg), potassium (203 mg/kg), molybdenum (1.5 mg/kg), and electrical conductivity of 0.35. Weather data were recorded using an iMetos electronic weather station (iMetos AG/CP/DD 280, Pessl Instruments GmbH, Weiz, Austria). During the first season (2024), the average air temperature was 18.4 °C (ranging from 12.5 °C to 24.7 °C), with a total rainfall of 80.3 mm (ranging from 20.1 mm to 150.3 mm). In the second season (2025), the average air temperature was 19.1 °C (ranging from 13.2 °C to 25.4 °C), and the rainfall was notably higher, totaling approximately 105.7 mm (ranging from 24.5 mm to 162.8 mm), indicating a more pluvial year. The meteorological station was located at a maximum distance of 10 km from the experimental fields.
2.2 Biorational insecticide
The biorational insecticides used in this study were: mineral oil at 1000 cc/hL (Insecticide 101; UPL, India), Spinosad at 200 cc/hL (Tracer®; Dow AgroSciences, USA), and potassium salts of fatty acids at 300 cc/hL (Hamper; Gowan Crop Protection, Italy). These concentrations were chosen based on the average field application rates recommended by the manufacturers. The selection of these doses was also guided by previous research confirming their minimal impact on non-target beneficial organisms (Aramideh and Hosseinzadeh, 2022; El Aalaoui et al., 2025).
2.3 Plant extracts
Leaf extracts from locally available N. glauca and R. communis were prepared based on the procedure outlined by Ndereyimana et al. (2020). Leaves from both plants were gathered from agricultural fields in Zemamra, thoroughly rinsed with water, and left to air-dry in shaded conditions for a period of 2 weeks. Once fully dried, the leaves were finely ground using an electric grinder, and the resulting powder was stored in biodegradable plastic bags until use. For extract preparation, 100 grams of the powdered material were mixed with 1 L of boiling water. The mixture was then removed from the heat source and allowed to infuse for 12 h. Following this, it was filtered through muslin cloth and diluted with cold water to reach a final volume of 1 L, producing a 10% (w/v) solution. This concentration is consistent with field application rates recommended for effective pest control in various crops (Abdelgader and Elawad, 2022; Zim et al., 2024).
2.4 Field experiment
Over the two studied seasons, the following treatments were applied: T1–Untreated control, T2–pinosad at 200 cc/hL (SD), T3–Mineral oil at 1000 cc/hL (MO), T4–potassium salts of fatty acids at 300 cc/hL (PFA), T5–N. glauca at 10% (w/v) (NG), T6–R. communis at 10% (w/v) (RC), T7–SD + NG, T8– SD + RC, T9– MO + NG, T10– MO + RC, T11– PFA + NG, T12–PFA + RC. Each year, the experimental field was divided into 24 microplots, with two replicates per treatment arranged in a randomized complete block design (RCBD). To prevent cross-contamination between treatments, a 6-meter buffer zone separated adjacent microplots. In 2024, microplots measured approximately 1,991.67 m2 and included about 9,958 potato plants, while in 2025, microplots were larger (2,766.67 m2) and contained around 13,834 plants, based on a standard planting density of 50,000 plants per hectare. Infestation by the potato tuber moth occurred naturally, as the pest was widespread in the area. Treatments were applied with a 16 L Matabi Super Green sprayer (Goizper S. Coop., Gipuzkoa, Spain) at a volume rate of 1,000 L/ha. For combination treatments, insecticidal and plant extract solutions were mixed in equal parts (1:1) to reach the desired concentration, based on compatibility and formulation stability tests previously performed in the laboratory, where mixtures were observed for precipitation, phase separation, or color change over 24 h, and no instability was detected. These mixtures were vortexed for 5 min to ensure thorough mixing, and continuously stirred during application to prevent settling. Treatments were administered five times annually, beginning 3 weeks after planting, with subsequent applications spaced 3 weeks apart.
2.5 Data collection and analysis
Throughout both cropping years, the following variables were assessed: percentage of leaves damaged per plant by PTM, larval density (number of PTM larvae per plant), canopy area, number of tubers per plant, average tuber weight (g), and total tuber yield (t/ha).
The canopy area was estimated by approximating the potato canopy as an ellipse using the formula:
× ).
Where width is the horizontal spread of the canopy, height is the vertical height of the plant, and π ≈ 3.1416. This method provides a practical and reliable estimate of canopy coverage, commonly used in field studies to assess plant growth and pest damage (Darabian and Yarahmadi, 2017). Yield per hectare was calculated by averaging the tuber weight per plant and extrapolating based on planting density. To minimize edge effects and ensure representative sampling, data were collected from 10 plants located at the center of each microplot using a systematic zigzag sampling pattern. This sampling method was chosen for its efficiency in capturing field variability while maintaining consistency. The sample size was based on previous studies on root and tuber crops (Darabian and Yarahmadi, 2017; Aramideh and Hosseinzadeh, 2022), which confirmed its adequacy for detecting treatment effects under field conditions. Data collection began 1 week after the first treatment and was carried out monthly over a three-month period for all variables, except for yield-related parameters (total tuber yield, number of tubers per plant, and average tuber weight), which were measured at harvest. The selected three-month period corresponded to the reproductive phase and peak population dynamics of P. operculella. For each year, two one-way analyses of variance (ANOVA) were performed: one to evaluate the main effect of treatment and the other to assess changes over time. Post-hoc comparisons were conducted using the least significant difference (LSD) test. Levene’s test was used to check homogeneity of variances, and data were log-transformed if needed to satisfy normality assumptions. Statistical significance was determined at α = 0.05. All data were analyzed using R software version 4.3.2 and are presented as means ± standard error (SE).
3 Results
3.1 Leaf damage
The different treatments significantly affected the percentage of leaf damage caused by P. operculella on potato during both study years (2024 and 2025) (Table 1). The combined treatment MO + RC consistently resulted in the lowest leaf damage, significantly outperforming all other treatments. By 3 MAFT, this combination reduced leaf damage to 18.2% in 2024 and 15.8% in 2025 (2024: F₁₁,₁₀₈ = 1060.0, p < 0.0001; 2025: F₁₁,₁₀₈ = 1032.0, p < 0.0001). The MO + NG combination also provided strong control, with leaf damage levels of 19.2% (2024) and 16.6% (2025) at 3 MAFT. Combinations of SD with either RC or NG were effective as well, with SD + RC reducing damage to 19.8% (2024) and 17.4% (2025), and SD + NG to 20.7% (2024) and 18.5% (2025). Among individual treatments, MO alone achieved lower leaf damage than SD, with MO recording 24.3% (2024) and 22.8% (2025), compared to SD’s 27.0% (2024) and 25.5% (2025) at 3 MAFT. PFA also showed moderate control. The botanical extracts RC and NG applied alone offered moderate reductions, with RC generally more effective than NG in both years. The untreated control exhibited the highest leaf damage, increasing progressively from 1 to 3 MAFT in both years. In 2024, most treatments showed a slight increase in leaf damage percentage from 1 to 3 MAFT, although this change was generally small and often not significant, especially for treatments involving PFA, SD and their combinations. In 2025, leaf damage mostly remained stable or slightly decreased over time for most treatments, particularly those with MO and its combinations with plant extracts. In contrast, the untreated control showed a significant increase in leaf damage over time in both years.
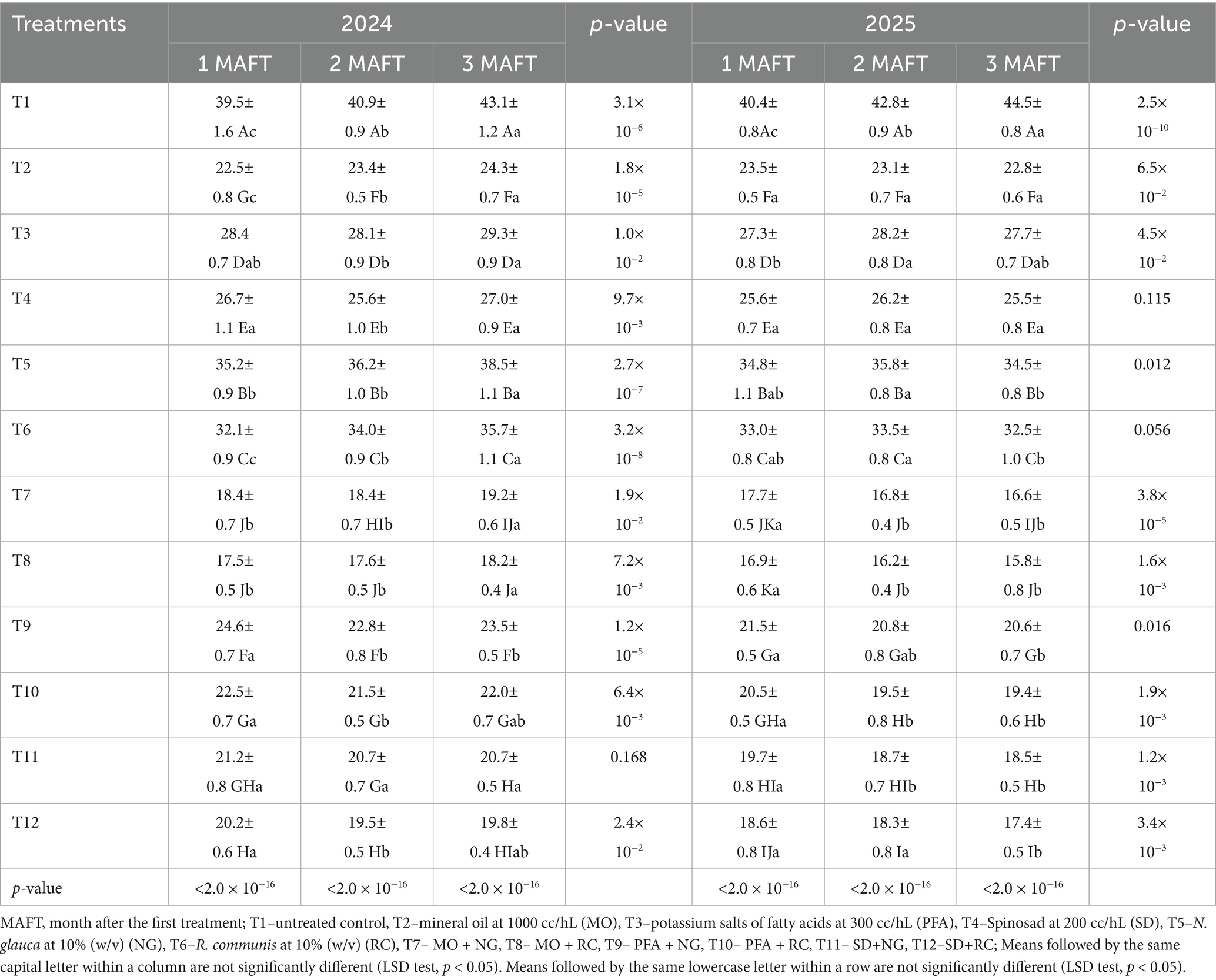
Table 1. Leaf damage (%) (mean ± SE) caused by Phthorimaea operculella on potato (Solanum tuberosum L.) (desiree variety) treated with biorational insecticides, and plant extracts.
3.2 Larval density
The different treatments had a significant effect on the larval density of P. operculella on potato plants during both study years (2024 and 2025) (Table 2). In general, combinations of biorational insecticides with botanical extracts were more effective than individual treatments. The lowest larval densities were recorded in the combined treatments MO + RC and MO + NG, which consistently outperformed all other treatments across all observation times. In 2024, the MO + RC treatment reduced larval numbers to 2.7, 2.6, and 1.5 larvae per plant at 1, 2, and 3 MAFT, respectively. A similar trend was observed in 2025, with larval counts declining to 2.3, 2.2, and 1.2 larvae per plant. The MO + NG treatment also showed strong suppression, reducing larval densities to 3.0, 2.8, and 1.7 in 2024, and 2.6, 2.5, and 1.5 in 2025 (2024: F₁₁,₁₀₈ = 2193.0, p < 0.0001; 2025: F₁₁,₁₀₈ = 164.4, p < 0.0001 at 3 MAFT). Other effective combinations included SD + RC and SD + NG. These combinations significantly reduced larval densities compared to their individual components. SD + RC treatment recorded 3.0, 2.6, and 2.0 larvae per plant in 2024 and 2.8, 2.7, and 1.7 in 2025, respectively. Among individual treatments, MO was more effective than SD and PFA, particularly at 3 MAFT. MO treatment alone reduced larval density to 2.3 in 2024 and 2.0 in 2025 by 3 MAFT. In contrast, SD and PFA achieved moderate reductions, with final larval counts ranging from 2.9 to 3.1 in 2025. RC and NG alone were less effective than when combined with insecticides but still provided better control than the untreated control. The untreated control consistently showed the highest larval densities throughout the observation period, with larval numbers increasing significantly from 1 to 3 MAFT in both years, reaching 6.5 larvae per plant in 2024 and 6.3 in 2025.
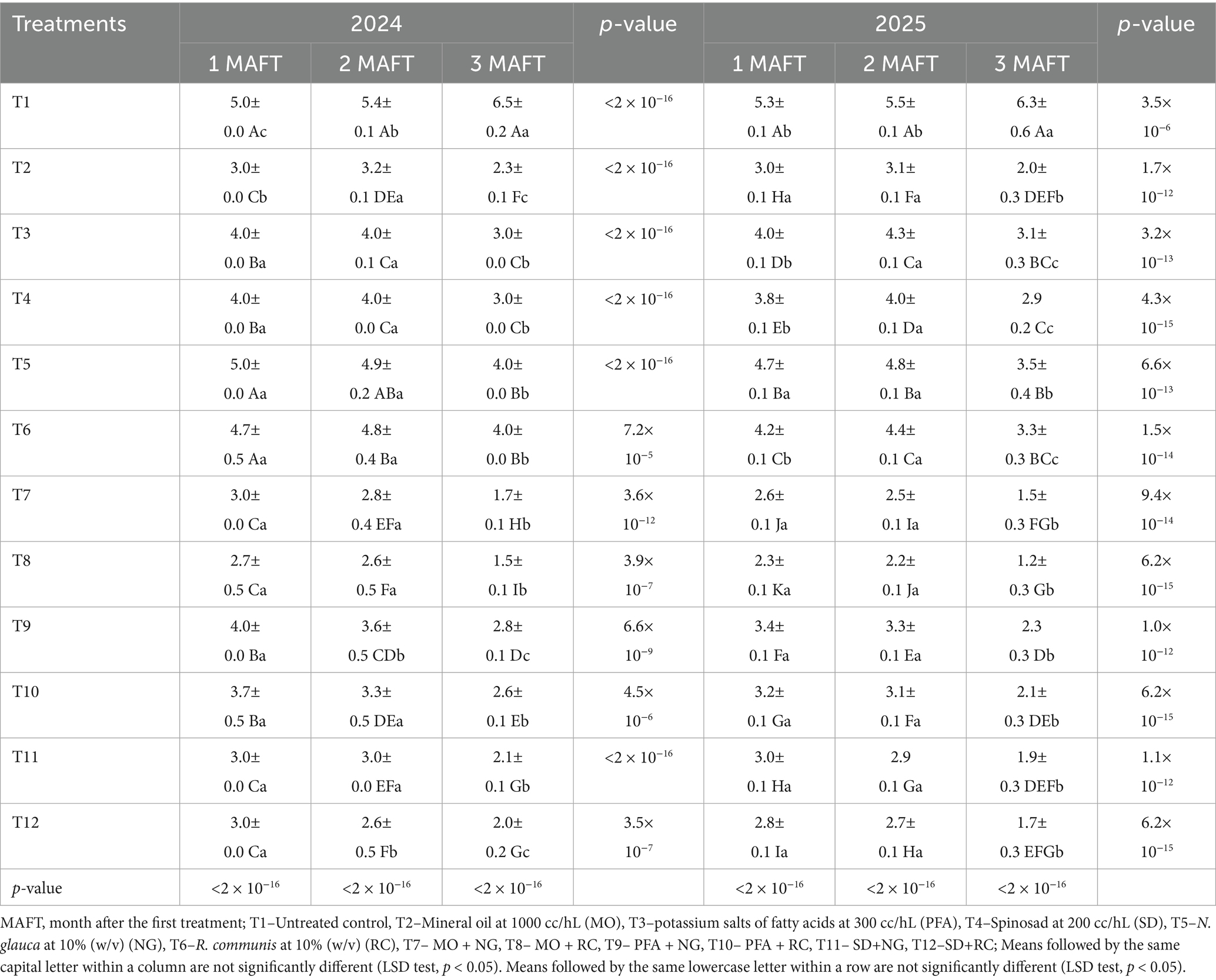
Table 2. Phthorimaea operculella larval density (number of larvae per plant) (mean ± SE) on potato (Solanum tuberosum L.) (desiree variety) treated with treated with biorational insecticides, and plant extracts.
3.3 Plant canopy area
In both 2024 and 2025, the different treatments significantly affected the canopy area (cm2) of potato plants (Table 3). In 2024, there were no significant differences in canopy area among treatments over time (F₁₁,₁₀₈ = 0.122, p = 1.0 at 3 MAFT). Canopy areas across treatments remained relatively stable from 1 to 3 MAFT, with no significant growth trends observed. Plants in the untreated control exhibited a slight, non-significant decrease from 2416.4 cm2 at 1 MAFT to 2135.4 cm2 at 3 MAFT. Similarly, combined treatments such as MO + NG and MO + RC showed only modest increases by 3 MAFT (2423.0 and 2422.0 cm2, respectively), which were not statistically different from other treatments. In contrast, in 2025, treatment effects became more pronounced, with significant differences observed over time (F₁₁,₁₀₈ = 3.72, p = 1.6 × 10–4 at 3 MAFT). Notably, combined treatments SD + NG and SD + RC exhibited a significant reduction in canopy area by 3 MAFT. The SD + NG treatment decreased sharply from 2381.5 cm2 at 1 MAFT to 2170.1 cm2 at 3 MAFT, indicating a possible phytotoxic effect or plant stress. Similarly, SD + RC treatment showed a reduction from 2142.9 cm2 to 2171.4 ± 50.1 cm2 across the season, though the changes were less pronounced. Among the individual treatments, SD, MO, PFA, NG, and RC maintained relatively stable canopy areas across both years, with no significant variations over time. The untreated control also maintained a consistent canopy size in 2025, ranging from 2364.5 to 2351.0 cm2.
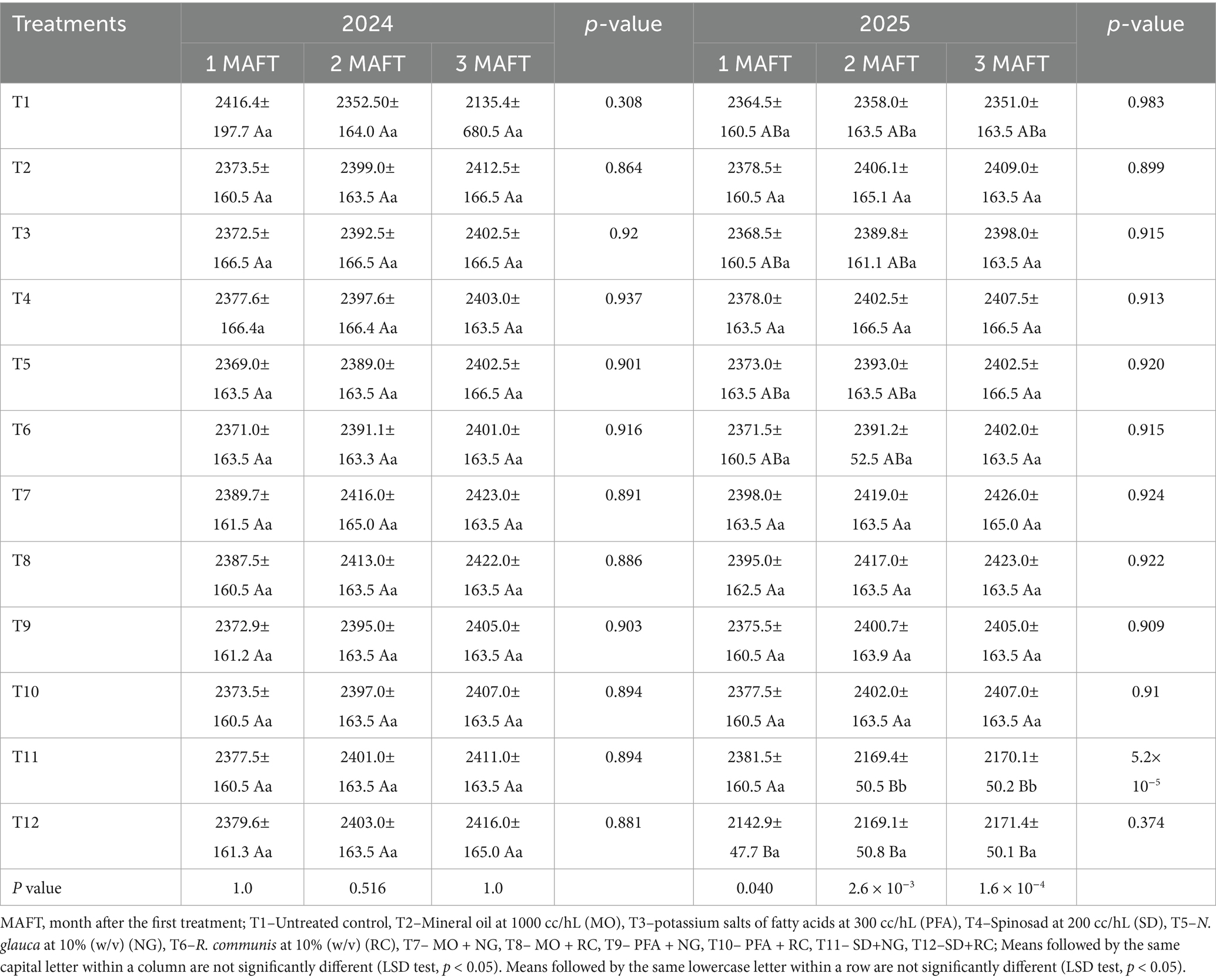
Table 3. Potato (Solanum tuberosum L.) (Desiree variety) plant canopy area (cm2) (calculated as plant height × canopy width, representing the horizontal spread of the foliage) (mean ± SE) under treatments with biorational insecticides, and plant extracts.
3.4 Number of tubers per plant
The number of tubers per plant was significantly affected by the different treatments during both study years (2024 and 2025) (Figure 1A). Combined applications consistently outperformed individual treatments across both years. Among these, the combinations treatments MO + NG, MO + RC, and SD + RC recorded the highest tuber numbers, averaging 8.91, 8.89, and 8.62 in 2024, and 9.40, 9.16 and 9.15 in 2025, respectively (2024: F₁₁,₁₀₈ = 37.25, p < 0.0001; 2025: F₁₁,₁₀₈ = 35.13, p < 0.0001). Among individual applications, MO treatment consistently showed the best performance, with 8.20 tubers in 2024 and 8.78 in 2025, followed by SD, RC, PFA, and NG treatments. The untreated control exhibited the lowest tuber numbers, with 6.50 and 6.95 in 2024 and 2025, respectively. Pairwise t-tests revealed statistically significant year-to-year differences in several treatments, including the untreated control, where tuber production increased from 2024 to 2025.
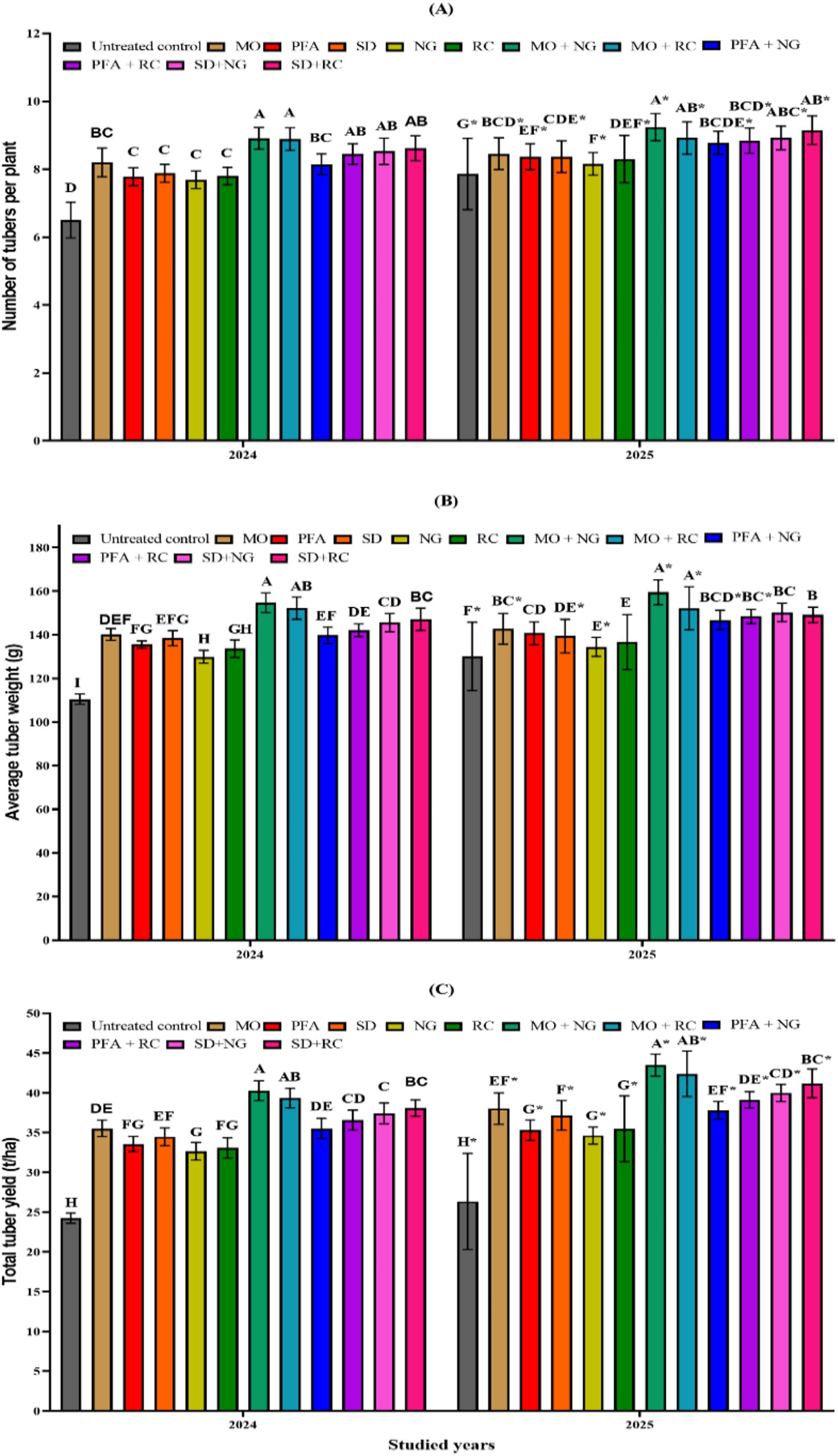
Figure 1. Number of tubers per plant (A), average tuber weight (g) (B), and total tuber yield (t/ha) (C) of potato (Solanum tuberosum L.) (mean ± SE) under treatments with biorational insecticides and plant extracts. For each year, different letters above the bars indicate statistically significant differences (LSD test, α = 0.05). For each treatment, an asterisk (*) indicates a significant difference between the two study years (2024 and 2025) according to t-tests at α = 0.05. Mineral oil at 1000 cc/hL (MO), potassium salts of fatty acids at 300 cc/hL (PFA), Spinosad at 200 cc/hL (SD), Nicotiana glauca at 10% (w/v) (NG), Ricinus communis at 10% (w/v) (RC).
3.5 Average tuber weight
The average tuber weight per plant was significantly affected by the treatments in both years 2024 and 2025 (Figure 1B). Combined applications consistently resulted in higher tuber weights compared to individual treatments. The combinations of MO + NG and MO + RC produced the highest tuber weights, averaging 154.75 g and 152.24 g in 2024, and 160.85 g and 158.73 g in 2025, respectively (2024: F₁₁,₁₀₈ = 99.9, p < 0.0001; 2025: F₁₁,₁₀₈ = 65.04, p < 0.0001). Among the individual treatments, MO alone yielded the highest tuber weights, averaging 140.20 g in 2024 and 145.99 g in 2025, followed by SD, PFA, RC, and NG. The untreated control showed the lowest tuber weights, averaging 110.50 g in 2024 and 115.57 g in 2025. Pairwise t-tests revealed significant differences between years for most treatments, with the exception of PFA, RC, and SD + RC treatments, indicating improved tuber weight performance in 2025 compared to 2024.
3.6 Total tuber yield
The total tuber yield (t/ha) was significantly affected by the different treatments in both years 2024 and 2025 (Figure 1C). Combined applications consistently resulted in higher yields compared to individual treatments. The combined treatments MO + NG and MO + RC recorded the highest yields, averaging 40.29 t/ha and 39.35 t/ha in 2024, and 43.50 t/ha and 42.40 t/ha in 2025, respectively (2024: F₁₁,₁₀₈ = 135.1, p < 0.0001; 2025: F₁₁,₁₀₈ = 167.7, p < 0.0001). Among the individual treatments, MO alone resulted in the highest yields, with averages of 35.54 t/ha in 2024 and 38.03 t/ha in 2025, followed by SD, RC, PFA, and NG. The untreated control consistently produced the lowest yields, with 24.25 t/ha in 2024 and 26.35 t/ha in 2025. Pairwise t-tests revealed significant year-to-year differences in nearly all treatments, with notably higher yields in 2025 compared to 2024. The most significant improvements were observed in MO + RC treatment (t = −5.5908, df = 18.0, p = 2.6 × 10−5), MO + NG (t = −5.8618, df = 18.0, p = 1.5 × 105), and SD + RC treatments (t = −5.8618, df = 18.0, p = 1.5 × 10−5), indicating enhanced productivity under field conditions in the second year.
4 Discussion
Previous studies showed that the tested biorational insecticides and plant extracts were effective against P. operculella in laboratory and semi-field trials (Sharaby et al., 2009; Saour et al., 2014). However, confirming their effectiveness in real field conditions was essential, since laboratory results do not always reflect field performance (Ndereyimana et al., 2020). This two-year field study conducted in the semi-arid Casablanca-Settat region, Morocco demonstrated that combining mineral oil with botanical extracts—especially R. communis and N. glauca—significantly reduced leaf damage and P. operculella larval density, showing greater effectiveness than single treatments or other combinations. The strong effectiveness of the mineral oil combined with R. communis or N. glauca likely comes from a dual action: mineral oil suffocates insects by blocking respiration and reducing egg viability (Helmy et al., 2012), while the bioactive compounds in R. communis and N. glauca have toxic or repellent effects on larvae by disrupting cellular functions and inhibiting vital processes (Randhir et al., 2004; Abdelgader and Elawad, 2022; Zim et al., 2024). This combination consistently lowered leaf damage to 15–18% and reduced larval numbers to fewer than 2 per plant 3 months after treatment, effectively controlling P. operculella during its critical reproductive stage. Nicotiana glauca has demonstrated insecticidal activity against pests like Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae), and Dactylopius opuntiae (Cockerell) (Hemiptera: Dactylopiidae) (Abdelgader and Elawad, 2022; Zim et al., 2024). Similarly, R. communis affects species such as Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), and D. opuntiae, with its seeds notably toxic due to ricin content (Neves et al., 2014; Zim et al., 2024). Comparable effects have been observed using orange peel oil against both sexes of P. operculella (Sharaby et al., 2009).
Spinosad combined with botanical extracts also provided strong control but was slightly less effective than mineral oil combinations. Spinosad, a bacterial-derived insecticide targeting insect nervous systems, is recognized for its efficacy and safety on beneficial organisms. Several authors have reported excellent ovicidal activity of spinosad against eggs of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae), Cactoblastis cactorum (Berg), Acrobasis vaccinii Riley (Lepidoptera: Pyralidae), and Plutella xylostella (L.) (Lepidoptera: Plutellidae) (Bloem et al., 2005; Temerak, 2005; Wise et al., 2010; Mahmoud et al., 2011). El-Barkey et al. (2009) found a 52% reduction in egg hatching of Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) when treated with Radiant SC 12% (second-generation spinosad) at the LC50 level. However, Pérez et al., 2007 reported no ovicidal effect of spinosad on eggs of Aedes aegypti (L.) (Diptera: Culicidae). Spinosad causes lower mortality compared to emamectin on larvae and adults of beneficial species like Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Coccinella undecimpunctata L. (Coleoptera: Coccinellidae) (Aramideh and Hosseinzadeh, 2022). Medina et al. (2003) also reported no direct or indirect negative effects on C. carnea. On the other hand, De Castro et al. (2018) observed toxicity of spinosad to both Spodoptera exigua Hübner (Lepidoptera: Noctuidae) and its predator Podisus maculiventris (Say) (Heteroptera: Pentatomidae). To ensure effective pest management while protecting natural enemies, dosage, application timing, and infestation levels must be carefully managed (Ibarra-Cortés et al., 2018). Moreover, spinosad has a steep dose–response curve, and some natural enemies, particularly parasitoids, may be highly susceptible to its effects (Williams et al., 2003). The addition of botanical extracts may enhance efficacy by providing complementary insecticidal compounds or prolonging residual activity. However, the slightly higher leaf damage and larval densities compared to mineral oil mixtures suggest that mineral oil’s physical mode of action provides more sustained suppression under the semi-arid conditions of this study.
Individual applications of mineral oil, spinosad, and potassium salts of fatty acids provided moderate control of P. operculella, reducing damage and larval counts compared to the untreated control but falling short of the effectiveness achieved by combined treatments. This supports previous findings that insecticides with a single mode of action can be more effective when combined with botanical or biopesticidal agents to improve pest control (Aramideh and Hosseinzadeh, 2022; El Aalaoui et al., 2025). Plant extracts (R. communis and N. glauca) applied alone showed moderate efficacy, with R. communis consistently outperforming N. glauca. This may be related to differences in the phytochemical profiles of the two species, where R. communis is known for potent bioactive compounds such as ricinoleic acid and other secondary metabolites with insecticidal properties (Abdelgader and Elawad, 2022). The 10% (w/v) extract concentration, while effective, likely requires optimization to maximize control as a standalone treatment. The temporal dynamics of leaf damage and larval density over the three-month monitoring period revealed treatment stability, especially in the mineral oil plus botanical extract combinations. This suggests that these treatments provide sustained control over the potato tuber moth population during its peak reproductive phase. In contrast, the untreated control consistently exhibited increased leaf damage and larval density over time, reflecting the pest’s natural population growth in the absence of intervention.
Agronomically, the improved pest control translated into better canopy development, higher tuber numbers per plant, increased average tuber weight, and greater total yield in treated plots, underscoring the practical benefits of these integrated treatments. These results confirm that managing P. operculella effectively reduces yield losses and improves overall crop performance under semi-arid conditions typical of the Casablanca-Settat region.
The use of biorational insecticides and botanical extracts aligns with sustainable pest management goals by reducing reliance on synthetic chemicals and mitigating risks to non-target organisms and the environment. Moreover, the compatibility and mixing procedures developed for combining treatments offer practical application protocols for growers seeking integrated, eco-friendly management strategies. While the selected biorational insecticides and botanical extracts are generally considered to have low toxicity toward beneficial and non-target organisms (Aramideh and Hosseinzadeh, 2022; El Aalaoui et al., 2025), further studies are required to evaluate potential impacts on non-target species under field conditions, including natural predators, pollinators, and soil microfauna. Environmental factors such as temperature, humidity, and sunlight may influence formulation stability and exposure levels, making dedicated field assessments essential for confirming ecological safety. Year-to-year comparisons indicated that pest control efficacy and crop yield were generally improved during the second season (2025). This improvement may be attributed to more favorable environmental conditions—specifically, slightly higher average temperatures (19.1 °C vs. 18.4 °C) and increased rainfall (105.7 mm vs. 80.3 mm)—which likely supported better plant development and may have enhanced the effectiveness or persistence of certain treatments. Furthermore, refinements in treatment application techniques or the cumulative suppressive effect of repeated treatments across seasons may have contributed to these positive outcomes.
The significant increases observed in yield parameters during 2025 underscore the importance of consistent and integrated pest management strategies. In line with this, Biondi et al. (2018) reported that ineffective control of Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in tomato crops can lead to severe yield losses, potentially reaching 100%, thus emphasizing the critical need for effective and sustained pest control measures. The present findings support this perspective, suggesting that integrated and seasonally adaptive approaches are essential for minimizing pest impact and optimizing crop productivity over time.
5 Conclusion
In conclusion, this study provides robust evidence that integrating mineral oil with botanical extracts, particularly R. communis, offers a promising, sustainable approach for managing P. operculella in potato production under semi-arid conditions. Such integrated strategies are critical for enhancing food security while minimizing environmental impact in the context of increasing pest pressure and climate variability. Future research should focus on further refining extract concentrations, application timing, and frequency to optimize control efficacy and cost-effectiveness. Additionally, long-term field trials examining the impact on beneficial insect populations and potential resistance development would provide valuable insights for broader implementation. Investigating the mode of action and potential synergistic mechanisms between mineral oil and botanical extracts may also help improve formulation and application strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ME: Writing – original draft. FK: Writing – review & editing. SR: Writing – review & editing. AC: Writing – review & editing. CA: Writing – review & editing. BN: Writing – review & editing. VB: Writing – review & editing. MS: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research of this study was supported by the National Institute of Agricultural Research (INRA), Morocco.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelgader, M. A. A., and Elawad, A. (2022). Insecticidal activity of chewing tobacco (Nicotiana glauca) against cotton mealy bug (Phenacoccus solenopsis) (Homoptera: Pseudococcidae). Int. J. Innov. Sci. Eng. Technol. 9, 49–54.
Alvarez, J.M., Dotseth, E., and Nolte, P. (2005). Potato tuberworm: a threat for Idaho potatoes. University of Idaho Extension Bulletin CIS1125. Available online at: http://www.info.ag.uidaho.edu/pdf/CIS/CIS1125.pdf (Accessed August 26, 2025)
Aramideh, S., and Hosseinzadeh, A. (2022). Effects of some chemical and biological insecticides on beet armyworm (Spodoptera exigua Hübner) (Lepidoptera: Noctuidae) and natural enemies in sugar beet fields. Trop. Agric. 99, 27–37.
Barton, K. A., Whiteley, H. R., and Yang, N. (1987). Bacillus thuringiensis endotoxin expressed in transgenic Nicotiana tabacum provides resistance to lepidopteran insects. Plant Physiol. 85, 1103–1109. doi: 10.1104/pp.85.4.1103
Biondi, A., Guedes, R. N. C., Wan, F. H., and Desneux, N. (2018). Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: past, present, and future. Annu. Rev. Entomol. 63, 239–258. doi: 10.1146/annurev-ento-031616-034933
Bloem, S., Mizell, R. F. III., Bloem, K. A., Hight, S. D., and Carpenter, J. E. (2005). Laboratory evaluation of insecticides for control of the invasive cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 88, 395–400. doi: 10.1653/0015-4040(2005)88[395:LEOIFC]2.0.CO;2
C.A.B.I. (2022). Global burden of crop loss. Centre for Agriculture and Bioscience International. Available online at: https://www.cabi.org/projects/global-burden-of-crop-loss/ (Accessed 5 August 2025).
Campos, H., and Ortiz, O. (2020). The potato crop: Its agricultural, nutritional and social contribution to humankind. Cham, Switzerland: Springer Nature, 518.
Clough, G. H., Rondon, S. I., DeBano, S. J., David, N., and Hamm, P. B. (2010). Reducing tuber damage by potato tuberworm (Lepidoptera: Gelechiidae) with cultural practices and insecticides. J. Econ. Entomol. 103, 1306–1311. doi: 10.1603/EC09065
Collantes, L. G., Raman, K. V., and Cisneros, F. H. (1986). Effect of six synthetic pyrethroids on two populations of potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae), in Peru. Crop Prot. 5, 355–357. doi: 10.1016/0261-2194(86)90116-X
Culliney, T. W. (2014). “Crop losses to arthropods” In: Integrated pest management: pesticide problems. eds. D. Pimentel and R. Peshin. Springer Dordrecht. vol. 3, 201–225.
Darabian, K., and Yarahmadi, F. (2017). Field efficacy of azadirachtin, chlorfenapyr, and Bacillus thuringiensis against Spodoptera exigua (Lepidoptera: Noctuidae) on sugar beet crop. J. Entomol. Res. Soc. 19, 45–52.
Das, P. D., Raina, R., Prasad, A. R., and Sen, A. (2007). Electroantennogram responses of the potato tuber moth, Phthorimaea operculella (Lepidoptera; Gelechiidae) to plant volatiles. J. Biosci. 32, 339–349. doi: 10.1007/s12038-007-0033-0
De Castro, A. A., Legaspi, J. C., Tavares, W. S., Meagher, R. L. J. R., Miller, N. W., Kanga, L., et al. (2018). Lethal and behavioral effects of synthetic and organic insecticides on Spodoptera exigua and its predator Podisus maculiventris. PLoS One 13:e0206789.
Douches, D. S., Li, W., Zarka, K., Coombs, J., Pett, W., Grafius, E., et al. (2002). Development of Bt-cry5 insect-resistant potato lines 'Spunta-G2' and 'Spunta-G3'. HortScience 37, 1103–1107. doi: 10.21273/HORTSCI.37.7.1103
El Aalaoui, M., Rammali, S., Bencharki, B., and Sbaghi, M. (2025). Efficacy of biorational insecticides and entomopathogenic fungi for controlling Cassida vittata Vill. (Coleoptera: Chrysomelidae) in sugar beet crops. Neotrop. Entomol. 54, 1–13.
El-Barkey, N. M., Amer, A. E., and Kandeel, M. A. (2009). Ovicidal activity and biological effects of radiant and hexaflumuron against eggs of pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae). Egypt Acad. J. Biol. Sci. A Entomol. 2, 23–36.
FAO. (2022). Food and agriculture data. Food and agriculture Organization of the United Nations. Available online at: https://www.fao.org/faostat/en/#home (Accessed 10 February 2022).
Fenemore, P. G. (1977). Oviposition of potato tuber moth, Phthorimaea operculella (lepidoptera: gelechiidae) – fecundity in relation to mated state, age, and pupal weight. NZ J. Zool. 4, 187–191.
Foot, M. (1998). Potato tuber moth life cycle. Horticulture and Food Research Institute of New Zealand Ltd. Available online at: http://www.hortnet.co.nz/publications/hortfacts/hf401015.htm (Accessed July 23, 2025).
Fuglie, K., Ben Salah, H., Essamet, M., Ben Temime, A., and Rahmouni, A. (1993). The development and adoption of integrated pest management of the potato tuber moth, Phthorimaea operculella (Zeller), in Tunisia. Int. J. Trop. Insect Sci. 14, 501–509. doi: 10.1017/S174275840001420X
Goldson, S.L., and Emberson, R.M. (1985). The potato moth Phthorimaea operculella (Zeller): its habits, damage potential and management. Spec. Publ., Agron. Soc. N. Z., Christchurch, New Zealand, 61–66.
Hanafi, A. (1999). Integrated pest management of potato tuber moth in field and storage. Potato Res. 42, 373–380.
Hannour, K., Boughdad, A., Maataoui, A., and Bouchelta, A. (2017). Chemical composition and toxicity of Moroccan Rosmarinus officinalis (Lamiaceae) essential oils against the potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). J. Mater. Environ. Sci. 8, 758–769.
Helmy, E. I., Kwaiz, F. A., and El-Sahn, O. M. N. (2012). The usage of mineral oils to control insects. Egypt Acad. J. Biol. Sci. 5, 167–174.
Horne, P., and Page, J. (2009). “IPM in Australian potato crops and the threat from potato psyllid” in Solanaceous crops, Psyllids & Liberibacter: Proceedings of the workshop held at the 7th world potato congress. ed. W. Nelson (Christchurch, New Zealand).
Ibarra-Cortés, K. H., González-Hernández, H., Guzmán-Franco, A. W., Ortega-Arenas, L. D., Villanueva-Jiménez, J. A., and Robles-Bermúdez, A. (2018). Interactions between entomopathogenic fungi and Tamarixia radiata (Hymenoptera: Eulophidae) in Diaphorina citri (Hemiptera: Liviidae) populations under laboratory conditions. J. Pest. Sci. 91, 373–384. doi: 10.1007/s10340-017-0870-z
Isman, M. B. (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. doi: 10.1146/annurev.ento.51.110104.151146
Kabir, A. (1994). Laboratory studies on the oviposition and generation production of the potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Bangladesh J. Zool. 22, 25–28.
Laarif, A., Fattouch, S., Essid, W., Marzouki, N., Salah, H. B., and Hammouda, M. H. B. (2003). Epidemiological survey of Phthorimaea operculella granulosis virus in Tunisia. Bull. OEPP 33, 335–338. doi: 10.1046/j.1365-2338.2003.00656.x
Lal, L. (1987). Studies on natural repellents against potato tuber moth (Phthorimaea operculella Zeller) in country stores. Potato Res. 30, 329–334. doi: 10.1007/BF02357672
Lamsal, B., Magar, S. K., Bhusal, K. K., and Bharati, S. (2023). A study on the efficacy of different botanicals against potato tuber moth (Phthorimaea operculella) in stored potatoes. Int. J. Quant. Res. Model. 4, 185–190. doi: 10.46336/ijqrm.v4i4.531
Larraín, P. S., Guillon, M., Kalazich, J., Graña, F., and Vásquez, C. (2009). Effect of pheromone trap density on mass trapping of male potato tuber moth Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae), and level of damage on potato tubers. Chil. J. Agric. Res. 69, 281–285.
Legocki, J., Polec, I., and Zelechowski, K. (2010). Contemporary trends in development of active substances possessing pesticidal properties: spinosyn insecticides. Pestycydy/Pesticides 1–4, 59–71.
Li, W., Zarka, K., Douches, D. S., Coombs, J., Pett, W., and Grafius, E. J. (1999). Co-expression of potato PVY coat protein and cryV-Bt genes in potato. J. Am. Soc. Hortic. Sci. 123, 218–223.
Mahmoud, M., Garjan, A. S., and Abbasipour, H. (2011). Ovicidal effect of some insecticides on the diamondback moth, Plutella xylostella (lepidoptera: plutellidae). Chil. J. Agric. Res. 71, 226–230.
Medina, P., Budia, F., Estal, P. D., and Viñuela, E. (2003). Effects of three modern insecticides, pyriproxyfen, spinosad, and tebufenozide, on survival and reproduction of Chrysoperla carnea adults. Ann. Appl. Biol. 142, 55–61.
Moawad, S. S., and Ebadah, I. M. A. (2007). Impact of some natural plant oils on some biological aspects of the potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Egypt. Agric. Biol. Sci. 3, 119–123.
Naqqash, M. N. (2023). Insect-pests of potato: history, production, current trends, and future prospects. Elsevier, 1–144.
Ndereyimana, A., Nyalala, S., Murerwa, P., and Gaidashova, S. (2020). Field efficacy of entomopathogens and plant extracts on Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) infesting tomato in Rwanda. Crop Prot. 134, 105–183.
Neves, R. T., Rondon, J. N., Silva, L. I. M., Peruca, R. D., Ítavo, L. C. V., Carvalho, C. M. E., et al. (2014). Efeito larvicida de Ricinus communis L. Rev. Eletrônica Gestão. Educ. Tecnol. Ambient. 18, 127–131.
Pérez, C. M., Marina, C. F., Bond, J. G., Rojas, J. C., Valle, J., and Williams, T. (2007). Spinosad, a naturally derived insecticide, for control of Aedes aegypti (Diptera: Culicidae): efficacy, persistence, and elicited oviposition response. J. Med. Entomol. 44, 631–638. doi: 10.1603/0022-2585(2007)44[631:SANDIF]2.0.CO;2
Pillmoor, J. B., Wright, K., and Terry, A. S. (1993). Natural products as a source of agrochemicals and leads for chemical synthesis. Pestic. Sci. 39, 131–140. doi: 10.1002/ps.2780390206
Raman, K. V. (1980). Potato tuber moth. Technical information bulletin 3. Lima, Peru: International Potato Center.
Raman, K. V. (1988). Control of potato tuber moth Phthorimaea operculella with sex pheromones in Perú. Agric. Ecosyst. Environ. 21, 85–89.
Randhir, R., Lin, Y. T., and Shetty, K. (2004). Stimulation of phenolics, antioxidant, and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 39, 637–646. doi: 10.1016/S0032-9592(03)00197-3
Rondon, S.I. (2007). New emerging pests in the Pacific northwest. Oregon State University Integrated Pest Management. Available online at: http://oregonstate.edu/potatoes/ipm/insects/emerging.htm (Accessed 15 March 2007).
Ropek, D., and Kołodziejczyk, M. (2019). Efficacy of selected insecticides and natural preparations against Leptinotarsa decemlineata. Potato Res. 62, 85–95. doi: 10.1007/s11540-018-9398-8
Salah, H. B., Fuglie, K., Temime, A. B., Rahmouni, A., and Cheikh, M. (1994). Utilisation du virus de la granulose de la teigne de la pomme de terre et du Bacillus thuringiensis dans la lutte intégrée contre Phthorimaea operculella Zell. (Lepidoptera: Gelechiidae) en Tunisie. Ann. Natl. Inst. Agron. Tunis. 67, 1–20.
Salgado, V. L. (1998). Studies on the mode of action of spinosad: insect symptoms and physiological correlates. Pestic. Biochem. Physiol. 60, 91–102. doi: 10.1006/pest.1998.2332
Saour, G. (2008). Effect of thiacloprid against the potato tuber moth Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). J. Pest. Sci. 81, 3–8.
Saour, G., Ismail, H., Jassem, I., and Tamer, S. (2014). Biorational insecticides against the potato tuber moth (Lepidoptera: Gelechiidae) on stored potatoes. Adv. Hortic. Sci. 28, 146–152. doi: 10.36253/ahsc-18401
Sharaby, A., Abdel-Rahman, H., and Moawad, S. (2009). Biological effects of some natural and chemical compounds on the potato tuber moth, Phthorimaea operculella Zell. (Lepidoptera: Gelechiidae). Saudi J. Biol. Sci. 16, 1–9. doi: 10.1016/j.sjbs.2009.07.001
Shelton, A. M., and Wyman, J. A. (1979). Time of tuber infestation and relationships between pheromone catches of adult moths, foliar larval populations, and tuber damage by the potato tuberworm. J. Econ. Entomol. 72, 599–601. doi: 10.1093/jee/72.4.599
Shelton, A. M., Wyman, J. A., and Mayor, A. J. (1981). Effects of commonly used insecticides on the potato tuberworm and its associated parasites and predators in potatoes. J. Econ. Entomol. 74, 303–308. doi: 10.1093/jee/74.3.303
Siddig, S. A. (1987). “A proposed pest management program including neem treatments for combating potato pests in Sudan” in Natural pesticides from the neem tree (Azadirachta indica A. Juss) and other tropical plants. Proceedings of the 3rd international neem conference. eds. H. Schmutterer and K. R. S. Ascher (Kenya: Nairobi), 449–459.
Temerak, S. A. (2005). Ovicidal activity of the natural bio-product spinosad through field observation of tagged egg masses of Spodoptera littoralis on cotton in five governorates of Egypt. Assiut J. Agric. Sci. 36, 85–95.
Thompson, G. D., Dutton, R., and Sparks, T. C. (2000). Spinosad—a case study: an example from a natural product discovery programme. Pest Manag. Sci. 56, 696–702.
Tsedaley, B. (2015). Integrated management of potato tuber moth (Phthorimaea operculella) (Zeller) in field and storage. J. Biol. Agric. Healthc. 5, 134–144.
Tsolakis, H., and Ragusa, S. (2008). Effects of a mixture of vegetable and essential oils and fatty acid potassium salts on Tetranychus urticae and Phytoseiulus persimilis. Ecotoxicol. Environ. Saf. 70, 276–282. doi: 10.1016/j.ecoenv.2007.10.001
Vassiliou, V. A. (2008). A study of applied research methods and techniques for landscape arthropods: the crape myrtle aphid Tinocallis kahawaluokalani (Kirkaldy) (Hemiptera: Aphididae), in Texas. J. Agric. Urban Entomol. 25, 205–221. doi: 10.3954/1523-5475-25.3.205
Von Arx, R., Goueder, J., Cheikh, M., and Ben Temime, A. (1987). Integrated control of potato tubermoth Phthorimaea operculella (Zeller) in Tunisia. Int. J. Trop. Insect Sci. 8, 989–994. doi: 10.1017/S1742758400023298
Wang, D., Wang, Y. M., Liu, H. Y., Xin, Z., and Xue, M. (2013). Lethal and sublethal effects of spinosad on Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 106, 1825–1831. doi: 10.1603/EC12220
Westedt, A. L., Douches, D. S., Pett, W., and Grafius, E. J. (1998). Evaluation of natural and engineered resistance mechanisms in Solanum tuberosum for resistance to Phthorimaea operculella (lepidoptera: gelechiidae). J. Econ. Entomol. 91, 552–556. doi: 10.1093/jee/91.2.552
Williams, T., Valle, J., and Vinuela, E. (2003). Is the naturally derived insecticide spinosad compatible with insect natural enemies. Biocontrol Sci. Tech. 13, 459–475. doi: 10.1080/0958315031000140956
Wise, J. C., Jenkins, P. E., Vander, Poppen, R., and Isaacs, R. (2010). Activity of broad-spectrum and reduced-risk insecticides on various life stages of cranberry fruitworm (Lepidoptera: Pyralidae) in highbush blueberry. J. Econ. Entomol. 103, 1720–1728. doi: 10.1603/EC10079
Zhang, Y. N., He, P., Xue, J. P., Guo, Q., Zhu, X. Y., Fang, L. P., et al. (2017). Insecticidal activities and biochemical properties of Pinellia ternata extracts against the beet armyworm Spodoptera exigua. J. Asia Pac. Entomol. 20, 469–476. doi: 10.1016/j.aspen.2017.03.003
Keywords: pest control, botanical insecticides, field trials, potato yield, damage reduction, sustainable agriculture
Citation: El Aalaoui M, Kamal FZ, Rammali S, Ciobica A, Albert C, Novac B, Burlui V and Sbaghi M (2025) Field evaluation of botanical and biorational insecticides for sustainable management of Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae) in potato cultivation. Front. Sustain. Food Syst. 9:1672799. doi: 10.3389/fsufs.2025.1672799
Edited by:
Matteo Balderacchi, Independent Researcher, Piacenza, ItalyReviewed by:
Rayappa Balikai, University of Agricultural Sciences, IndiaAlessandra Marieli Vacari, University of Franca, Brazil
Copyright © 2025 El Aalaoui, Kamal, Rammali, Ciobica, Albert, Novac, Burlui and Sbaghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Albert, YWxiaWRlbnQ3MkB5YWhvby5jb20=; Bodgan Novac, Ym9kZ2FubnZjQGdtYWlsLmNvbQ==
 Mohamed El Aalaoui1
Mohamed El Aalaoui1 Fatima Zahra Kamal
Fatima Zahra Kamal Alin Ciobica
Alin Ciobica