- Department of Agricultural, Food, Environmental and Animal Science (Di4A), University of Udine, Udine, Italy
Merlot, a grape variety with a rich history and significant genetic diversity, has become one of the most influential cultivars in the global wine industry. Its adaptability to diverse climates has enabled extensive cultivation in major wine-producing regions, including France, Italy, the United States, Australia, and Chile. This adaptability, combined with its ability to produce consistently high-quality wines, underlines the importance of reviewing and understanding its future potential. Merlot originates from a cross between Cabernet Franc and Magdeleine Noire des Charentes, have endowed it with a unique versatility and resilience. These traits have not only facilitated its widespread cultivation but also made it a key player in the face of global viticultural challenges, particularly those posed by climate change. Merlot’s early ripening nature and resistance to certain diseases make it an essential cultivar for regions facing increasingly unpredictable weather patterns. In this context, this review aims to highlight the importance of this grape variety by detailing the factors that contribute to its aromatic complexity and sensory appeal, which make Merlot wines are highly appreciated by both consumers and experts due to its balanced aromatic profile. As the wine industry grapples with the effects of climate change and shifting consumer preferences, understanding Merlot’s strengths and potential becomes even more critical. By integrating genetic, agronomic and oenological perspectives, this work emphasizes Merlot’s current significance and highlights its strategic importance for the future of global viticulture.
Introduction
Merlot, a red wine grape cultivar with a well-documented history rooted in ancient varieties, has evolved over time to become one of the most prominent and widely cultivated grape varieties in the global wine industry. The name “Merlot” first appeared in literature at the end of the 18th century (Viala and Vermorel, 1902–1910). Its name is believed to originate from the French word “Merle,” referring to the species Turdus merula L. (the common blackbird), both due to the dark colour of the grapes, which resembles the bird’s plumage, and because these birds are known to enjoy its early-ripening berries (Rézeau, 1997). Due to its widespread cultivation across ethnographically and linguistically diverse regions, Merlot has historically been known by various names, such as “Medoc Noir.” In France, where the grape reaches its fullest expression, it is also recognized as “Merlau,” “Crabutet Noir” in Switzerland and “Bordeaux” (Bouchereau, 1843; Hardy, 1844; Artozoul et al., 1960; Rézeau, 1997; Galet, 2002; Bettiga, 2003).
By the mid-19th century, Merlot was thoroughly described at the ampelographic level by different authors (Odart, 1845; Rendu, 1857), further solidifying its place in viticultural history. Initial DNA analysis revealed significant genetic similarities between Merlot and other grape varieties such as Cabernet Franc and Carmenere (Clarke and Rand, 2010). Subsequent genetic studies have demonstrated that Merlot is indeed the result of a cross between Cabernet Franc, used as the father, and Magdeleine Noire des Charentes, which served as the mother variety (Boursiquot et al., 2009). More recently, a high-quality phased genome assembly has confirmed this parentage by using both parental genomes as references thereby resolving the previous uncertainties about the maternal lineage (Sichel et al., 2023) (Figure 1A). This diversity is particularly evident in traits such as berry size and the capacity for sugar accumulation during ripening (Lacombe et al., 2012; Sivcev et al., 2018). The international expansion of Merlot began in the mid-19th century and gained significant momentum in the late 20th century. This diffusion was initially driven by its low susceptibility to powdery mildew and its ability to produce high-quality wines. These qualities, combined with its adaptability to diverse climates, led to Merlot being cultivated extensively around the world, with significant plantings in countries such as Italy, the United States, Australia, and Chile (OIV, 2017). Notably, by 2006, Merlot became the most extensively planted black wine grape in France, covering 117,354 hectares (de la Vigne et du Vin, 2007). As an early-ripening variety of dark blue wine grapes, Merlot is highly valued for its softness and fleshy texture, qualities that make it an excellent blending partner, particularly with later-ripening varieties like Cabernet Sauvignon, which is rich in tannins (Robinson et al., 2012).
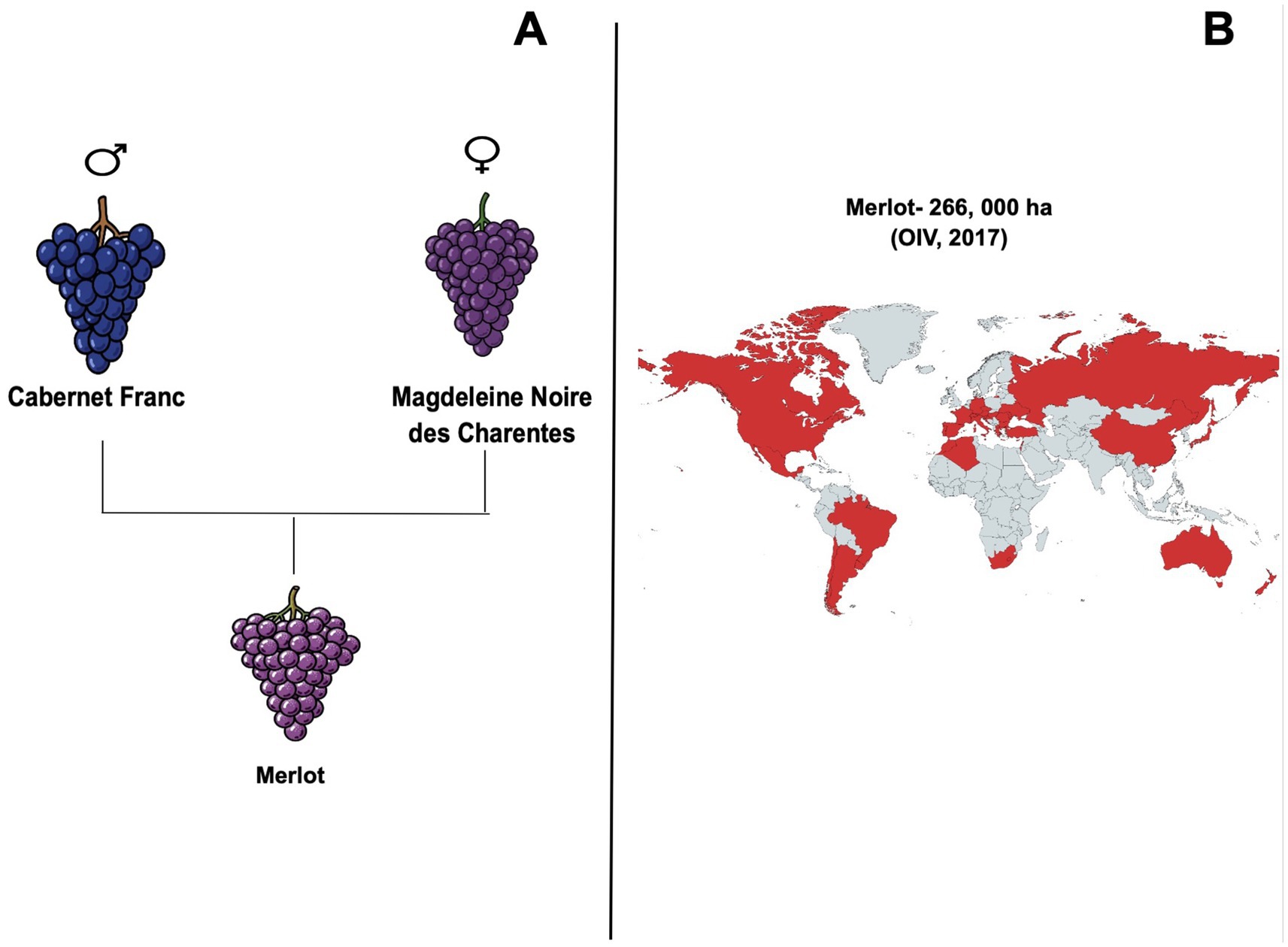
Figure 1. (A) Genetic origin of Merlot from Cabernet Franc (♂) and Magdeleine Noire des Charentes (♀). (B) World distribution of Merlot cultivation in 2017 (266,000 ha; OIV, 2017).
Beyond Europe, Merlot’s significance has been recognized globally, becoming the fourth most widely planted grape variety in the world by 2017, with 266,000 hectares under cultivation, representing approximately 3% of the total global vineyard area (OIV, 2017) (Figure 1B). This international spread has been further facilitated by clonal selection, which has improved both the quantitative and qualitative traits of Merlot, making it a versatile and highly regarded variety in a range of climatic conditions (Boursiquot et al., 2009). Given the importance and multifaceted nature of this grape variety, reviewing the existing literature on Merlot provides valuable insights into its broader impact on global viticulture, making it possible to understand how this grape, with its deep historical roots, has achieved its status as one of the most widely planted varieties in the world. In this sense, this review helps to contextualize Merlot’s significance within the broader landscape of viticulture, providing a foundation for future studies and ensuring that this versatile grape variety continues to play a central role in the wine industry.
Merlot must are typically characterized by high sugar concentration and low acidity, which often results in wines with elevated ethanol levels and reduced freshness (Boursiquot et al., 2009; Hranilovic et al., 2021). This combination of traits presents both opportunities and challenges for winemakers, who must carefully manage these factors to produce balanced, high-quality wines. One of its most famous expressions is Pétrus, the renowned estate in the Pomerol region, which produces wines primarily from Merlot, highlighting the variety’s significance in high-end wine production (Robinson, 1986; Clarke and Rand, 2010). The quality and character of Merlot wines are shaped by a complex interplay of factors, making it essential to understand the elements that contribute to its unique appeal. The aromas of Merlot, ranging from fruity and floral to earthy and herbaceous, are influenced by various. Additionally, the phenolic content of the grapes, responsible for the wine’s colour and structure, is affected by environmental conditions and vineyard management practices. Lastly, the fermentation process, particularly the role of yeast, significantly impacts the wine’s flavour, texture, and overall complexity.
This review aims to synthetize current knowledge on Merlot’s genetic background, viticultural adaptability, and oenological characteristics, with particular attention to its aromatic complexity and potential role in sustainable viticulture under climate change.
Main
The aromatic complexity of Merlot: understanding the role of volatile compounds
In the world of wine, Merlot stands out for its smooth texture and versatile flavor profile—qualities that set it apart from more tannic varieties like Cabernet Sauvignon. Its approachable, fruit-forward profile, with plush tannins and a rounded mouthfeel, contributes to its widespread popularity, appealing to both novice and experienced wine drinkers alike.
Merlot’s aromatic complexity derives from different families of volatile compounds. The main groups include esters, lactones, terpenes, methoxypyrazines, norisoprenoids and higher alcohol acetates which collectively shape the distinctive bouquet of Merlot wines (Arcari et al., 2017; Allamy et al., 2018; Pons et al., 2018; Carrasco-Quiroz, 2020; Cincotta et al., 2021). Sensory descriptors frequently reported include cooked fruits such as prune, peach, and fig, as well as herbaceous notes like ivy and geranium (Pons et al., 2018).
Esters are generally one of the most abundant classes in Merlot wines and (García-Carpintero et al., 2011; Welke et al., 2012; Pereira et al., 2014; Carrasco-Quiroz, 2020; Cincotta et al., 2021). Welke et al. (2012) identified of isoamyl acetate, ethyl lactate, 2-hexen-1-ol, and 3-octenol in Brazilian wines. Later, Arcari et al. (2017) identified 95 volatile compounds in Merlot samples, including those previously reported by Welke et al. (2012). Fare clic o toccare qui per immettere. Ethyl hexanoate, ethyl octanoate, and ethyl decanoate were detected in high concentrations. Their concentrations were also reported to increase under leaf removal in Meditterranean Vineyars, enhancing the varietal expression of Merlot (Cincotta et al., 2021). Within this group, ethyl hexanoate shows the highest odour activity value (OAV) among these esters, associated with green apple and strawberry descriptors (García-Carpintero et al., 2011). Ethyl decanoate, contributes to fruity aroma, has been reported in high concentration in wines from Tangará, whereas ethyl octanoate contributed to the overall aroma profile (Pereira et al., 2014).
Other ester are also involved in the aromatic composition of Merlot. Diethyl succinate and ethyl lactate are associated to creamy and fruity notes (Carrasco-Quiroz, 2020), while isoamyl acetate enhances complexity with banana-like notes (Carrasco-Quiroz, 2020). Additional compounds as ethyl cinnamate, ethyl 2-methylbutanoate, and ethyl isovalerate, impart fruity and spicy notes depending on the harvest year and region (Antalick et al., 2014).
In wine, esters are formed through yeast metabolism via two main pathways, fatty acids acyl-CoA, which leads to the formation of ethyl esters, and acetyl-CoA combined with higher alcohols, which results in acetate esters (Prusova et al., 2022). Several studies have shown that medium chain fatty acids mainly octanoic and decanoic acid act as fermentation inhibitors by reducing intracellular pH and compromising yeast viability (Legras et al., 2010), under stress conditions such low temperatures of fermentation, S. cerevisiae could produce higher content of these compounds (Massera et al., 2021).
Through the acetyl-CoA route, yeast also generates higher alcohol acetates, including ethyl acetate, isoamyl acetate, and phenylethyl acetate. These compounds are particularly relevant to Merlot, as they enrich its fruity and floral dimensions; however, their sensory contribution is strongly concentration-dependent. At moderate levels they enhance aroma complexity, whereas excessive amounts, especially of ethyl acetate, may impart solvent-like notes (García-Carpintero et al., 2011; Peng et al., 2013; Pereira et al., 2014).
Lactone contribute additional to Merlot’s wines sensory attributes δ-decalactone and γ-nonalactone are tipically associated with coconut notes, while c-decalactone impart cooked peach aromas, respectively (Darriet et al., 2001; García-Carpintero et al., 2011).
Although generally found at lower concentrations in red wines compared to white aromatic varieties, terpens still provide important notes to Merlot wines (Ribéreau-Gayon et al., 2006; Arcari et al., 2017). Geraniol provides rose-like aroma, while linalool is associated with floral character and borneol contributing camphor-like notes (Rocha et al., 2007; Ou et al., 2010; Pereira et al., 2014; Arcari et al., 2017; Arcena et al., 2020). The expression of these volatile compounds can also be influenced by factors such as vintage, regional climate, and viticultural practices, results in a multifaceted and dynamic sensory experience that defines Merlot wine (Arcari et al., 2017; Carrasco-Quiroz, 2020; Cincotta et al., 2021).
Methoxypyrazine add a vegetal dimension to Merlot’s aroma. They are nitrogen-containing heterocyclic compounds characterized by extremely low sensory thresholds. Among them, isobutyl methoxypyrazine (IBMP) (Prusova et al., 2022). Although its lower concentrations in Merlot compared to other varieties including Cabernet Sauvignon, Cabernet Franc, and Sauvignon Blanc, IBMP still contributes to its distinctive aromatic profile (Augustyn et al., 1985), in fact is considered one of the most relevant in wines due to its markedly low threshold perception of just 15 ng/L. This compound is responsible for the green or bell pepper aromas commonly found in wines made from various grape varieties (De Boubée et al., 2000). Recent work has also highlighted how viticultural practices and climate conditions modulate IBMP expression in Merlot, underscoring the need for both agronomic and enological strategies to manage these compounds (Pickering et al., 2021).
C13 norisoprenoids represent a further group of aroma-active molecules derived from the oxidative cleavage of carotenoids during grape maturation. β-damascenone, formed add depth to the wine’s aromatic profile with its notes of baked apple, flowers and honey, and α-ionone which imparts notes of raspberry and violet (Kotseridis et al., 1999; Noguerol-Pato et al., 2009; Peng et al., 2013). In summary, Merlot’s aroma arises from the interplay of multiple volatile families, each imparting distinctive sensory trait. This chemical complexity defines the varietals sensory identity and underpins its relevance in both scientific research and winemaking practice.
The impact of climate conditions, vineyard management practices
Climate change linked to global warming presents new challenges for vineyard management, such as reduced precipitation, higher pH levels in grapes, and increased alcohol content (van Leeuwen et al., 2024).
The increase of pH is mainly associated with potassium accumulation in the berries under water stress and high temperatures, which promotes the precipitation of organic acids such as tartaric acid, thereby lowering overall acidity. In parallel, climate change often accelerates sugar accumulation due to faster ripening, leading to higher carbohydrate content in the must. During fermentation, these elevated sugar levels are converted into higher ethanol concentrations, which not only increase the perceived warmth of wines but can also influence chemical equilibria, further affecting acid–base balance and contributing to higher final wine pH (de Mira Orduña, 2010; Van Leeuwen and Destrac-Irvine, 2017).
Merlot has been described as anisohydric variety maintaining stomatal conductance even under limited water availability (Jiang and Zhang, 2012), this trait contributes to its relative adaptability under climate change scenarios, where increased drought frequency and water scarcity are expected (Gutiérrez-Gamboa et al., 2019; Vuerich et al., 2021). Intra-varietal diversity and phenological plasticity further support Merlot’s adaptive potential in different environment (Naidu et al., 2014).
Among adaptive practices, irrigation plays a central role. Moderate irrigation (50% of crop evapotranspiration from the veraison to harvest) was shown to increase berry weight and in wines by improving chromatic properties while also enhancing consumer preference (Ribalta-Pizarro et al., 2024). Similarly, deficit irrigation under semi-arid Mediterranean conditions increased tannins and total polyphenols, particularly in the seeds, demonstrating the importance of water management in optimizing Merlot quality (Chacón-Vozmediano et al., 2021).
In this sense, several studies indicate that these changes impact the phenolic composition of Merlot wines, influencing their colour, stability, and sensory attributes. Merlot wines are known for their rich phenolic profile, including high levels of anthocyanins and other polyphenols crucial for colour stability, and antioxidant properties. This has been highlighted in studies conducted by Ivanova-Petropulos et al. (2015), which demonstrated that Merlot wines are richer than Syrah and Cabernet Sauvignon in total acids and polyphenols, especially anthocyanins, making them deeply coloured, fresh, and suitable for long-term aging. Merlot seeds contain higher quantities of polyphenols and tannins compared to Cabernet Sauvignon (Lorrain et al., 2011), and the grapes contain higher amounts of epicatechin and catechin compared to other cultivars (Sen and Tokatli, 2014).
Furthermore, it has been reported that Merlot wines have higher concentrations of malvidin derivatives, peonidin, 10-hydroxyphenyl-pyranoanthocyanins, and higher acetylated anthocyanin content compared to other varieties, with lower tannin concentrations and reduced astringency and bitterness (Blanco-Vega et al., 2014; González-Neves et al., 2001; Landon et al., 2008; Tudose-Sandu-Ville et al., 2012).
In this context, temperatures play a crucial responsibility in determining the levels of the main phenolic compounds, as the synthesis of anthocyanins is sensitive to temperature. Moderate temperatures favour non-acylated forms, while higher temperatures increase acetylated anthocyanins, known for their superior colour stability (Tarara et al., 2008). In particular, elevated temperatures generally enhance the accumulation of malvidin-3-glucoside, leading to deeper and more stable Merlot colour profiles (Vişan et al., 2020).
Berry temperature, influenced by sunlight and water availability, also significantly impacts phenolic development. Increased sunlight raises berry temperature, while adequate water availability helps regulate berry cooling through transpiration. High temperatures and sunlight can accelerate ripening, potentially leading to higher sugar content but lower phenolic concentrations, which can affect the overall balance and complexity of the Merlot wine (Pavić et al., 2019). Conversely, excessive rainfall can dilute phenolic content, negatively impacting wine quality (Ferrer et al., 2016). Extreme temperatures can stress vines, redirecting resources away from phenolic synthesis and potentially diminishing wine quality. In these conditions, vineyard management practices, such as leaf removal, are crucial in shaping phenolic composition (Figure 2). In Merlot vineyards, this practice has resulted in improved cluster microclimate and enhanced pesticide penetration, thereby reducing Botrytis cinerea severity (Sivilotti et al., 2016). Merlot cultivation in cooler climates like Hawke’s Bay, with excessive crop load and delayed ripening, can be problematic. In these cases, defoliation at veraison has been demonstrated to improve phenolic concentration and increase color intensity, increasing total monomeric anthocyanins, including malvidin-3-glucoside and quercetin-3-glucoside (Mazza et al., 1999; Spayd et al., 2002). This practice has also been reported to significantly impact anthocyanin concentrations and colour intensity in Merlot grapes, with combined treatments like cluster thinning at veraison and basal defoliation showing the most pronounced effects (Di Profio et al., 2011). The timing and method of leaf removal can vary, influencing the effectiveness of the treatment and its impact on wine quality. Defoliation also affects ripeness, impacting total soluble solids and pH, while decreasing titratable acidity, particularly when Merlot crop load is reduced (Karoglan et al., 2014). This type of field treatment has also been reported to have significant effects on the concentration and ratio of other varietal anthocyanins like delphinidin, petunidin, and peonidin (King et al., 2012; Osrečak et al., 2016).
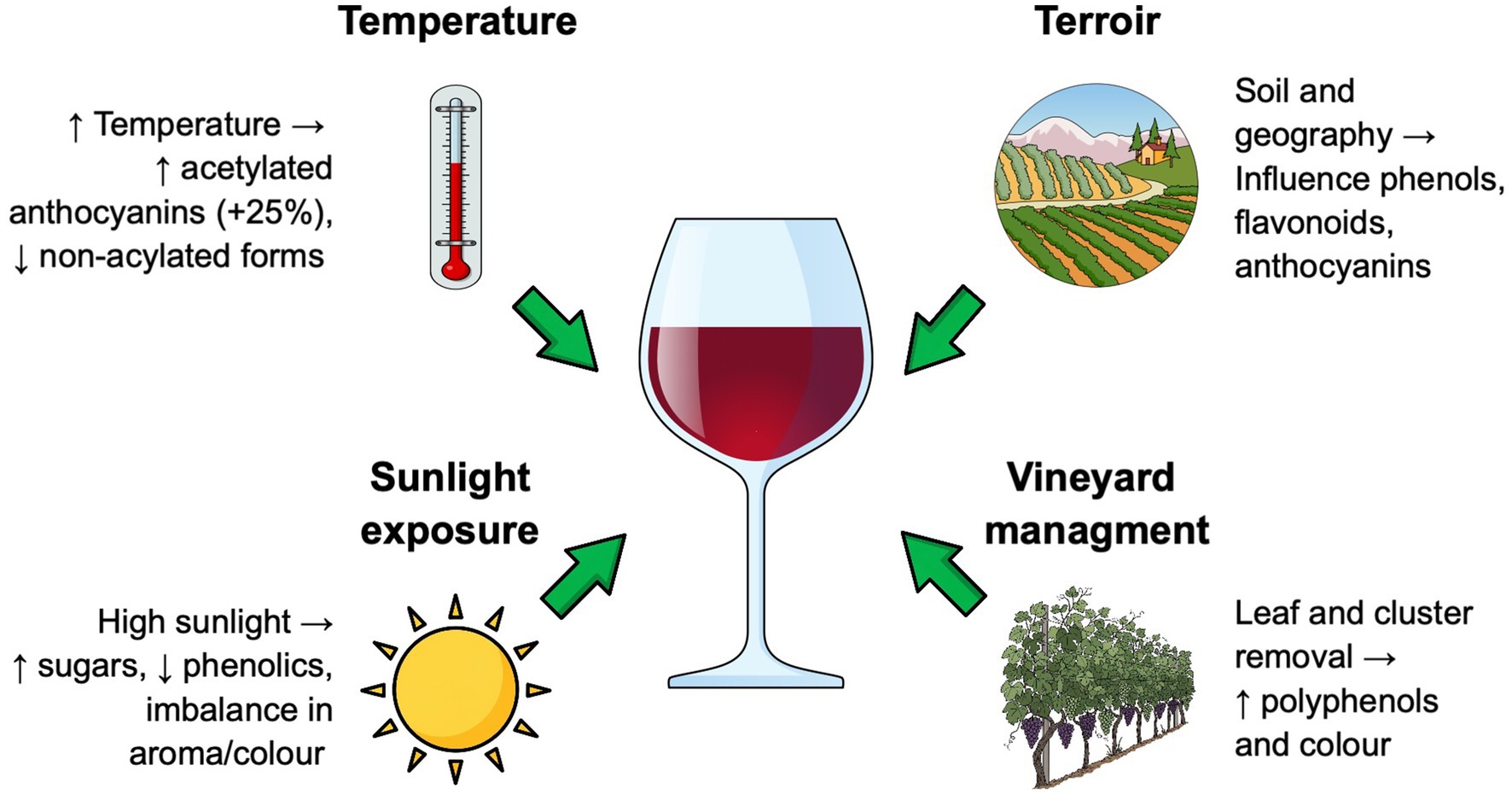
Figure 2. Key environmental, climatic, and agronomic factors and their impact on the organoleptic and sensory characteristics of Merlot wine.
Regional studies reveal variability in phenolic composition, with variations depending on the pedoclimatic conditions of the region. Merlot wines from higher altitudes show increased total phenolic compounds, flavonoids, and anthocyanins (Jin et al., 2017), as in the case of dehydrated Merlot grapes, which shown increased phenolic and mineral contents (Panceri et al., 2015). Moreover, Merlot shows higher variations in trans-resveratrol and trans-piceid concentrations compared to other grape varieties depending on the cultivation area (Kostadinović et al., 2012; Stervbo et al., 2007). Similarly, Merlot wines from southern regions have been reported to contain more catechin, epicatechin, and myricetin compared to those from northern regions (Goldberg et al., 1996).
Although Merlot exhibits several adaptive traits, comparative studies suggest that it may still be less resilient than Cabernet Sauvignon under future climate scenarios. This underlines the need for adaptive measures such as drought-tolerant clones, alternative rootstocks, or relocation to cooler regions (Baltazar et al., 2025).
Effects of pathogens and impact of agronomic treatments on Merlot wine characteristics
The phytosanitary condition of grapes is of fundamental importance to the quality of the obtained grapes. In fact, several authors have identified how different plant pathogens can modify and contribute to the complex aromatic harmony of Merlot (Table 1). Among these, the oomycete Plasmopara viticola has been shown to have a significant impact on the quality of this variety, causing an increase in IBMP (isobutylmethoxypyrazine) in Merlot grapes during infections (Pons et al., 2018).
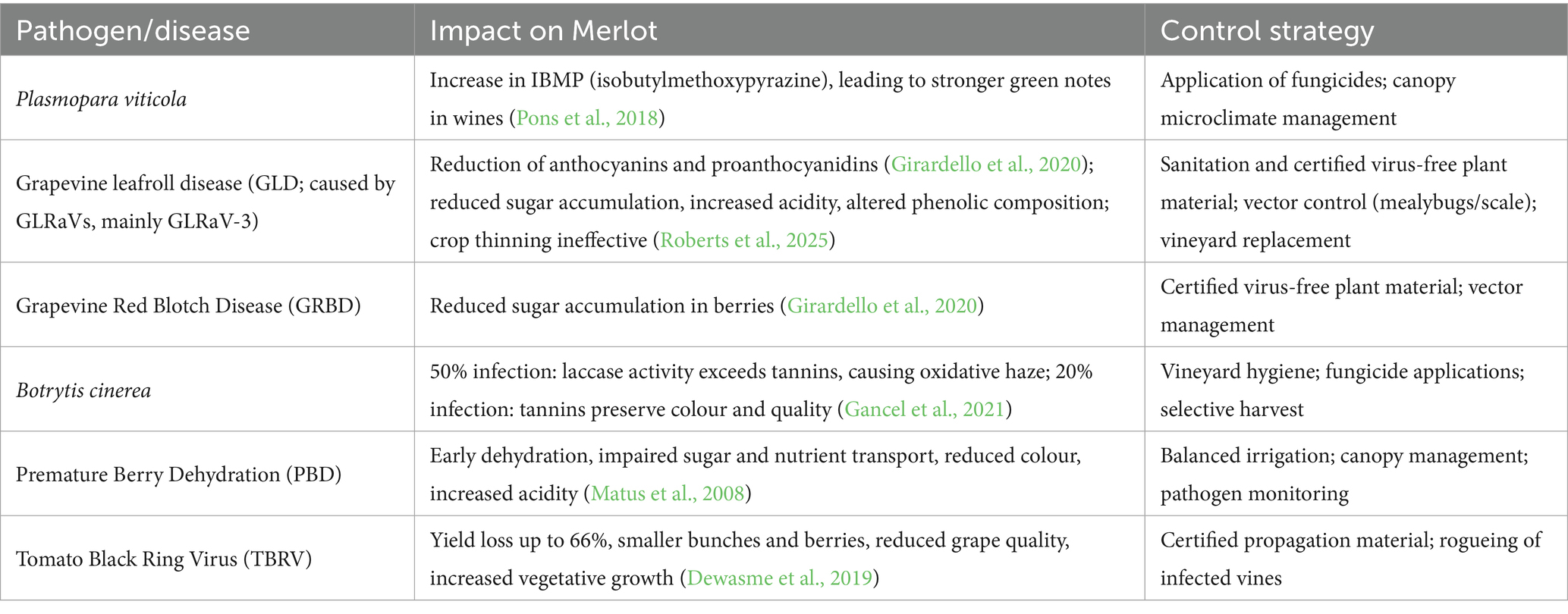
Table 1. Main grapevine pathogens affecting Merlot and their impact on grape and wine quality, with potential control strategies.
Grapevine leafroll disease (GLD) is caused by a complex of Grapevine leafroll-associated viruses (GLRaVs) including GLRaV-1, −2, −3, and −7 (Naidu et al., 2014). Among these, GLRaV is considered the most prelevant and impactful. In Merlot infection reduce anthocyanin and proanthocyanidin concentrations (Girardello et al., 2020) and impair sugars accumulation while increasing acidity and altering phenolic composition (Roberts et al., 2025).
Grapevine red blotch disease (GRBD) also affects Merlot, with reduced sugar accumulation in infected grapes (Girardello et al., 2020). Furthermore, Botrytis cinerea infection affects Merlot wine quality. As reported, at 50% infection by B. cinerea, laccase can overpower tannins, risking oxidative haze, but at 20%, tannins effectively preserve wine colour and quality (Gancel et al., 2021). It has also been reported that Merlot vines exhibit high sensitivity to pre-mature berry dehydration (PBD), a disorder linked to phloem disruption, supposed to arise from pathogenic microorganisms and viruses, which affects fruit development by causing dehydration, disrupting sugar and nutrient transport, and leading to reduced colour and increased acidity in the berries (Matus et al., 2008). Regarding viruses, a study conducted on Merlot vines infected with Tomato Black Ring Virus (TBRV) showed significant yield reductions of up to 66%, with fewer and smaller bunches and berries. Despite slight increases in polyphenols and anthocyanins, which improved wine colour, TBRV resulted in lower grape quality, reduced vegetative growth, and more lateral shoots, leading to complicate vineyard management (Dewasme et al., 2019).
To counteract these pathogens, agronomic treatments are essential. However, several studies have shown that specific chemical compounds used for these practices can significantly affect the chemical composition and sensory attributes of wines (Table 2). For example, chiral tebuconazole residues were reported to significantly impact Merlot wine flavour and colour attributes by altering the levels of volatile compounds. The presence of these residues was also correlated with an increase in acetic acid and changes in concentrations of compounds like 2-heptanol and ethyl butyrate, which negatively impacted the wine’s fruity and floral flavours (Zhao et al., 2022). Additionally, the presence of pyranoanthocyanin derivatives residues resulted in Merlot wines with a more brick-red hue. R-tebuconazole, in particular, had the most detrimental effect on Merlot’s flavour and colour, underscoring the importance of stringent quality control in wine production. Similarly, copper sprayings on vines resulted in a reduction in the varietal aroma of young Merlot wines, particularly affecting volatile thiols (Darriet et al., 2001). Conversely, to improve aromatic components and promote plant development, the use of biostimulants has been gaining ground in recent years (Colautti et al., 2023b). For instance, field application of boron to Merlot grapes was reported to significantly increase the content of anthocyanins, hydroxycinnamic acids, and flavonols, enhancing phenolic metabolism in grape skins and besides modify the phytochemical composition (Arbigaus Bredun et al., 2023) (Figure 3).
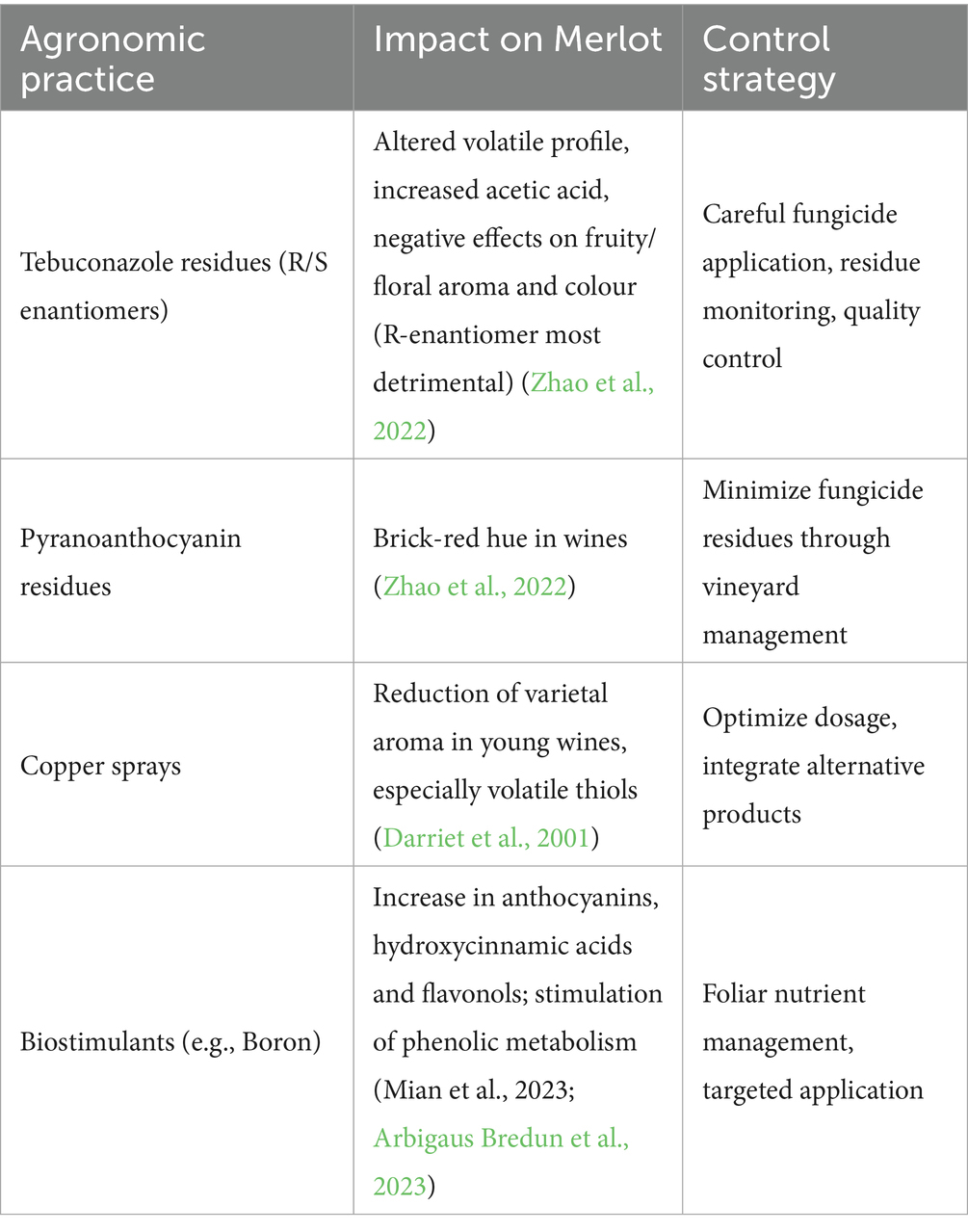
Table 2. Agronomic practices influencing Merlot grape and wine quality, their impact on composition and potential management strategies.
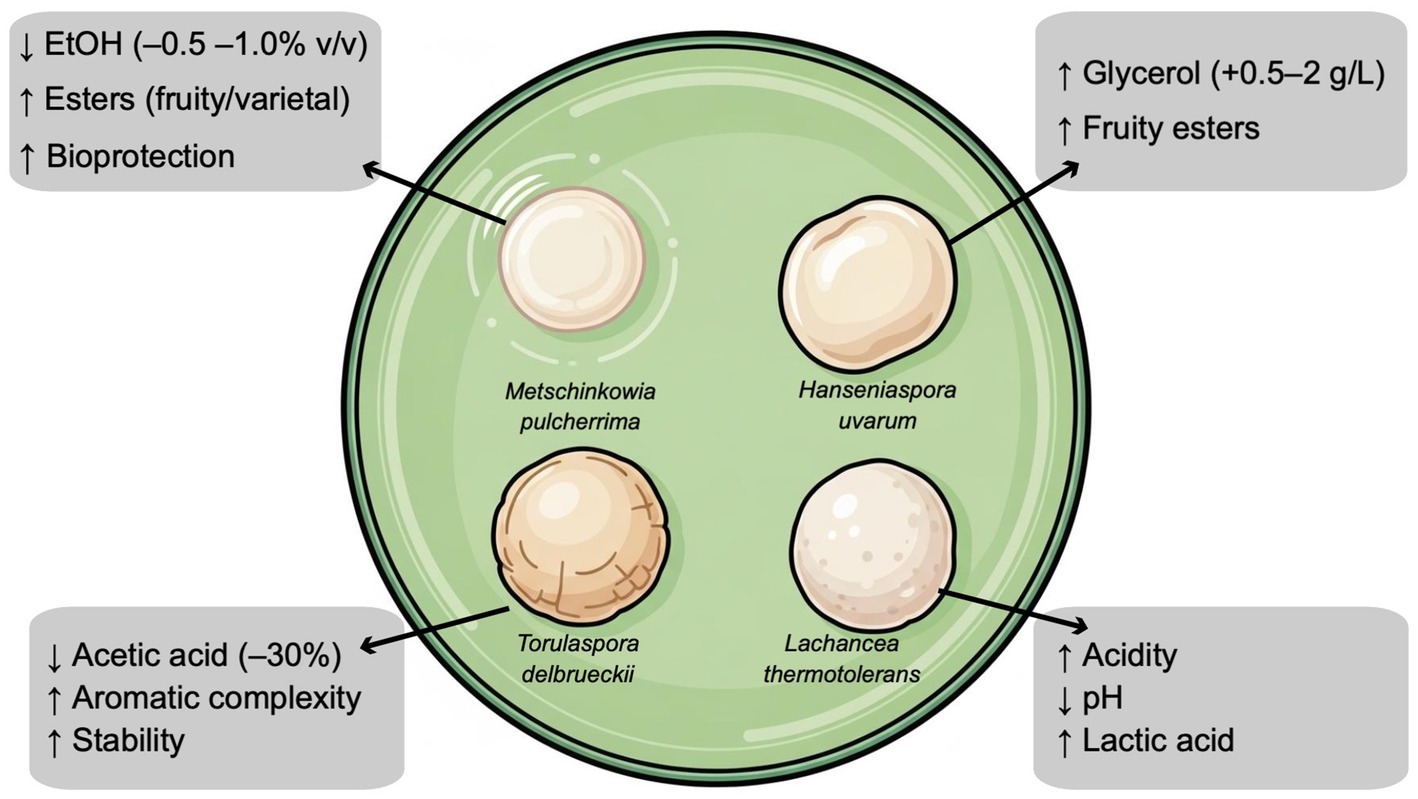
Figure 3. Key non-Saccharomyces yeast strains whose fermentative effects have been specifically studied in the vinification of Merlot grapes.
The impact of winemaking techniques on Merlot quality
Winery technologies are crucial in Merlot vinification as they significantly impact its organoleptic characteristics. From fermentation management to the selection of aging materials, each technical aspect helps shaping the wine’s aromatic profile and taste.
For example, it has been observed that bentonite, a widely used fining agent in wineries for clarification, can influence the concentration of rare earth elements in Merlot wine based on its origin, thereby impacting the wine’s chemical profile (Tatár et al., 2007). Additionally, combining bentonite with polyvinylpolypyrrolidone (PVPP) and plant proteins (PPI) as fining agents significantly affected monomeric flavanols, turbidity, and anthocyanin content in Merlot wines. Optimal results were achieved with a combination of 25% PPI, 43% PVPP, and 32% bentonite (Ficagna et al., 2020). Activated charcoal has also been shown to be particularly effective in reducing smoke taint in Merlot wines, enhancing fruit characteristics without significantly altering colour or acidity (Fudge et al., 2012).
Another technique, ultrasound application, has yielded contrasting results for Merlot. Xie et al. (2023) highlighted the benefits of combining ultrasound with low-temperature pre-treatment, noting improvements in anthocyanin and phenolic acid levels, aroma, and reduction of undesirable odours, suggesting strong application potential. Similarly, Maier et al. (2024) reported that high-power ultrasound treatment improved the extraction of polyphenolic compounds during Merlot grape maceration. Conversely, Ignat et al. (2016) found that traditional fermentation and rotating tank maceration techniques produced the most balanced anthocyanin levels in Merlot, while ultrasound maceration was less effective. The study also observed varying percentages of free anthocyanins, with malvidin being the most prevalent.
In addition to these cellar technologies, extraction dynamics during maceration are also important in shaping Merlot’s aromatic profile. A recent study investigated the impact of varying seed-to-skin ratio in Merlot and found that a higher proportion of seeds led to Merlot wines with significantly increased tannin content and anthocyanin concentration, resulting in stronger colour density and a higher phenolic index (Makalisa et al., 2025).
The evolution of phenolic compounds and colour in Merlot and Marselan dry red wines throughout winemaking and aging was also studied (Zhang et al., 2024). These authors found minimal phenolic leaching during cold maceration, with rapid release during alcoholic fermentation, leading to increased pyran anthocyanins and polymeric pigments, which remain high through malolactic fermentation and storage. The wines’ colour during aging is primarily influenced by anthocyanins and F-A polymeric pigments, with the red color of Merlot being closely linked to the presence of pinotins. Increased concentrations of flavan-3-ols have been correlated with enhanced color stability, indicating their potential role in mitigating color loss and instability.
Fermentation dynamics and yeast influence in Merlot wines
Grape-associated microbiota differs considerably across viticultural regions under the influence of environmental factors such as soil and climate. In Merlot these microbial signatures were distinctive enough to classify grapes by geographical origin providing strong evidence for their role in microbial terroir (Bokulich et al., 2014). Further studies confirmed this regional specificity. In Southern Brazil Hanseniaspora uvarum, Issatchenkia terricola, Saturnispora diversa and Starmerella bacillaris were identified as the dominant species on Merlot berries, showing biodiversity comparable to Cabernet Sauvignon (Mattos Rocha et al., 2022). The relevance of microbial communities extends beyond regional diversity to the fermentation process itself. In Merlot musts, distinct dynamics have been reported during spontaneous fermentations, notably the high presence of Pichia anomala during fermentations, leading to wines with lower alcohol percentages and increased glycerol concentrations (Clavijo et al., 2010; Li et al., 2010; Varela et al., 2017).
During spontaneous fermentation, the microbial succession has been extensively investigated. Culture-independent analyses reveled that in the early stages of alcoholic fermentation non-Saccharomyces yeast are predominant before being progressively replaced by S. cerevisiae (Zott et al., 2008).
Another distinctive feature of Merlot fermentations is the high prevalence of killer strains during spontaneous fermentation possibly due to greater initial yeast populations on the berries compared to varieties like Cabernet Sauvignon (Renouf et al., 2008). The dynamics of these killer yeasts play a crucial role, with Merlot fermentations showing a predominance of killer strains from early stages, unlike Malbec, where a mixed population of sensitive and killer strains was observed (Sangorrín et al., 2001).
These naturally occurring antagonistic interactions within Merlot fermentation have inspired modern bioprotection strategies, where selected non-Saccharomyces yeast are employed to limit spoilage microorganism while reducing sulphur dioxide (SO2). In this sense, bioprotection was successfully tested on Merlot wines, which limited the oxidation of must during fermentation and provided protection against undesirable microorganisms, such as acetic acid bacteria, helping to preserve the wine’s sensory qualities (Windholtz et al., 2021a). A specific study examined the sensory profiles of sulphite-free wines made with and without bioprotection over two years. The results showed that wines treated with bioprotection, as well as those without sulphites, displayed intense notes of “Fresh blackcurrant,” “Cooked black cherries,” “Mint,” and “Coolness” differing from wines made with the addition of SO2. The findings showed that these yeasts effectively dominated the pre-fermentation environment, significantly influencing the final wine aroma. Specifically, bioprotection encouraged the formation of linear esters, while sequential inoculation promoted the production of acetate esters from higher alcohols, contributing to a more pronounced fruity aroma. Sensory analyses confirmed that the use of non-Saccharomyces yeasts enhanced the fruity qualities of the wines, further highlighting their aromatic benefits (Windholtz et al., 2021a). Another study investigated the use of Metschnikowia pulcherrima and Meyerozyma guilliermondii in combination with S. cerevisiae for fermenting Merlot must. The research revealed a reduction in ethanol levels in wines in which M. pulcherrima was involved, compared to those fermented solely with S. cerevisiae. However, fermentations inoculated with M. guilliermondii resulted in higher ethyl acetate levels, though sensory analysis showed no detrimental effect on wine quality (Aplin et al., 2021). The bioprotective effect of non-Saccharomyces yeasts also relates to their ability to consume dissolved oxygen, with M. pulcherrima and Torulaspora delbreuckii proving particularly effective in limiting the growth of spoilage bacteria such as Glucanobacter oxydans. Notably, M. pulcherrima consumed oxygen more rapidly than S. cerevisiae, underscoring its potential for use in bioprotection during winemaking (Windholtz et al., 2023a). The addition of bioprotective yeasts such as M. pulcherrima and T. delbrueckii early in the winemaking process helps prevent must deterioration by reducing the presence of filamentous fungi. Temperature control during pre-fermentation being essential to maximize their protective effects (Windholtz et al., 2021b). Altogether, these result confirm that bioprotection enhances aroma, stabilizes must, and allows a reduction in Merlot winemaking (Alexandre et al., 2023; Windholtz et al., 2023b).
Furthermore, it is essential to characterize microbial populations for their impact on the organoleptic and safety aspects of the product. One major concern in Merlot production is the potential presence of biogenic amines (BAs). Different levels of biogenic amines, including spermidine, serotonin, putrescine, and cadaverine, have been reported in Merlot wines depending on the inoculation strategy of alcoholic and malolactic fermentation starters (Manfroi et al., 2009). Other studies have highlighted that the co-inoculation of yeast and lactic acid bacteria during malolactic fermentation in Merlot wines can reduce the content of cadaverine and tyramine (Cañas et al., 2012). The composition of microbial populations in Merlot can also be influenced by vineyard and cellar practices, highlighting the importance of management choices in modulating yeast communities and their functional impact (Colautti et al., 2023a). In this context the use of indigenous yeast strains for wine fermentation is crucial as it enhances the unique character of the terroir, promotes a more natural and sustainable fermentation process, and preserves vineyard biodiversity (Nisiotou et al., 2018). Concrete examples in Merlot support this approach. The isolation and characterization of autochthonous S. cerevisiae strains confirmed that indigenous populations can provide distinctive contributions, with one strain (7F) producing wines with superior colour, aroma intensity, and fruity character.(Ut et al., 2022). Similarly, in Northwestern Argentina, ten non-Saccharomyces species were isolated from Merlot grapes, with H. uvarum as the most abundant. Selected strain, including H. uvarum, H. vinae and Metschnikowia pulcherrima exhibited favorable oenological traits such as moderate SO2 tolerance, low volatile acidity production and enzymatic activity related to aroma release (Mendoza et al., 2019). Beyond these examples interest in non-Saccharomyces yeasts has significantly increased due to their technological potential (Borren and Tian, 2020; Maicas and Mateo, 2023). Yeasts like Hanseniaspora spp. and Starmerella spp., dominant in spontaneous fermentations, are known to significantly contribute to the production of aromatic compounds such as higher alcohols, esters, and terpenes, thus enhancing the aromatic complexity of Merlot wines (Renault et al., 2015). In addition, several non-Saccharomyces yeasts secrete enzymes such as β-glucosidases and proteases, which can significantly affect the final aroma of Merlot wines by interacting with grape-derived precursors, a notable example is H. vineae which has been associated with the production of phenyl ethyl acetate, contributing fruity, floral, and honey-like notes, although its impact depends on the competitive dynamics within Merlot must (Lleixà et al., 2016). Taken together, these findings emphasise that yeast selection in Merlot winemaking is not only critical for fermentation performance but also decisive in defining the aromatic identity and overall sensory quality of the wine (Figure 3). Despite these promising perspectives, the application of non-Saccharomyces yeasts in Merlot winemaking at industrial scale still faces important limitations. Most studies have been conducted under laboratory or pilot-scale conditions, and large-volume fermentations often show lower reproducibility and stability (Jolly et al., 2014; Padilla et al., 2016). In Merlot specifically, M. pulcherrima has been associated with reduced ethanol levels, but fermentations involving M. guilliermondii resulted in increased ethyl acetate, which could negatively impact wine quality (Aplin et al., 2021). Moreover, non-Saccharomyces strains are frequently outcompeted by S. cerevisiae, requiring high inoculation rates and strict management to ensure persistence. While recent studies on bioprotection have confirmed their potential for reducing SO₂ and controlling spoilage bacteria (Windholtz et al., 2023b; Alexandre et al., 2023), these approaches still demand further validation under commercial winemaking conditions.
Potential of Merlot wine and grape pomace extracts in oxidative stress and neuroprotection
Several studies have highlighted the benefits of drinking moderate quantities of wine responsibly (Buljeta et al., 2023). Beneficial effects have been particularly observed in red wines, including Merlot, due to their high levels of antioxidant compounds (Landrault et al., 2001). Majkić et al. (2019) explored using a cell culture in vitro model the anti-inflammatory properties of Merlot wines, observing a reduction in Prostaglandin E2 (PGE2, involved in inflammation, fever, and pain) production by up to 65.5% and Thromboxane A2 (TXA2, crucial for blood clotting and vascular tone) by up to 47.9%. These reductions suggest potential cardiovascular benefits similar to a low dose of aspirin. However, no direct correlation was found between the wines’ phenolic content and their anti-inflammatory effects, indicating that other compounds or synergistic effects might be at play. Martín et al. (2011) further demonstrated that Merlot red wine can protect human astrocytoma cells from oxidative damage caused by the Fenton reaction, which generates harmful radicals. This finding is significant as it implies that Merlot’s antioxidants might help protect against cell damage related to various diseases, including cancer, potentially leading to new dietary strategies for improving health and preventing disease.
Additionally, the potential to extract valuable active compounds from this cultivar’s grapes has been demonstrated, offering an opportunity to add value to the industry’s by-products due to their high phenol and antioxidant content (Díaz et al., 2022). For example, it was reported that pressed Merlot red wine extract has higher neuroprotective activity than free run wine due to the greater extraction of polyphenols, including quercetin, catechin, and procyanidins from the grape pomace. In particular, quercetin was found to be effective in preventing PC12 cell death and reducing the overproduction of reactive oxygen species (ROS) (Martín et al., 2012). Moreover, Merlot grape pomace hydroalcoholic extract, rich in anthocyanins, could improve oxidative and inflammatory states in arthritis patients (Gonçalves et al., 2017). These extracts also exhibited antimicrobial activity against various pathogens, including E. coli and methicillin-resistant S. aureus (Corrêa et al., 2017). Similarly, other extracts such as flavonoids, flavones, hydroxybenzoic acid derivatives, hydroxycinnamic acid derivatives, and ferulic acid methyl ester from Merlot grape pomace, showed effective antimicrobial activity mainly against Gram-positive bacteria (Ghendov-Mosanu et al., 2022).
Conclusion
Thanks to its distinctive organoleptic qualities, which include a fruity aromatic profile and a smooth, approachable taste, Merlot captivates a wide spectrum of consumers. This broad appeal has fuelled its expansion beyond traditional regions like Italy, Spain, and France to major international markets, including the United States, Australia, and Chile. In recent years, its popularity has also surged in Asia, particularly in China. The Asian market is becoming increasingly important for the wine industry, with growing demand for both imported and locally produced wines. Merlot aligns perfectly with the preferences of these emerging consumers, who often favour rounder, fruitier wines with lower alcohol content and softer tannins compared to more robust red varieties. This makes Merlot particularly suited to the expanding middle class in Asia, where an evolving wine culture is fostering new opportunities.
Merlot’s adaptability to various geographical regions further strengthens its global reach. Its ability to thrive in diverse terroirs, combined with early ripening and disease resistance, makes it a strategic choice for vineyards grappling with the unpredictable weather patterns exacerbated by climate change.
As Merlot’s presence continues to grow, ongoing research into this variety will be essential. With consumers increasingly focused on sustainability and ecological practices, future studies must prioritize environmentally friendly viticulture. Exploring the use of biostimulants and examining the role of environmental microbiomes, both areas where Merlot has seen limited research, will be crucial to developing sustainable farming practices that minimize chemical inputs.
Equally important are the advancements in winemaking technology. Emphasizing the role of native microbial flora can help winemakers create wines that authentically reflect the unique characteristics of their region, offering a point of distinction in a highly competitive global market. Further research into these indigenous microorganisms could lead to the development of new starter cultures that improve fermentation control and efficiency. In particular, isolating yeast strains that can withstand higher fermentation temperatures without sacrificing quality would support the industry’s push towards more energy-efficient production methods. Additionally, reducing the use of traditional additives like SO2 by identifying effective bioprotective strains could lead to wines with a smaller environmental footprint, responding to the demand for more natural and low-intervention products.
Author contributions
EG: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft. AC: Visualization, Writing – review & editing. MP: Investigation, Validation, Writing – review & editing. GC: Methodology, Validation, Writing – review & editing. LI: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by EU Next-GenerationEU – PNRR M4C2, Investimento 1.5 – D.D. 1058 23/06/2022, ECS00000043 (iNEST). This manuscript reflects only the authors' views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. AI was used to improve the English language of the manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alexandre, H., Puyo, M., and Tourdot-Maréchal, R. (2023). “Bioprotection in winemaking” in New advances in Saccharomyces (London, UK: IntechOpen).
Antalick, G., Perello, M.-C., and De Revel, G. (2014). Esters in wines: new insight through the establishment of a database of French wines. Am. J. Enol. Vitic. 65, 293–304. doi: 10.5344/ajev.2014.13133
Allamy, L., Darriet, P., and Thibon, C. (2018). Impact of oxygenation on wine aroma: Chemical and sensory aspects. OENO One 52, 291–305. doi: 10.20870/oeno-one.2018.52.4.2041
Aplin, J. J., Paup, V. D., Ross, C. F., and Edwards, C. G. (2021). Chemical and sensory profiles of merlot wines produced by sequential inoculation of Metschnikowia pulcherrima or Meyerzyma guilliermondii. Fermentation 7:126. doi: 10.3390/fermentation7030126
Arbigaus Bredun, M., Sartor, S., Pretto Panceri, C., Chaves, E. S., and Maria Burin, V. (2023). Changes in phytochemical composition of merlot grape and wine induced by the direct application of boron. Food Res. Int. 163:112258. doi: 10.1016/j.foodres.2022.112258
Arcari, S. G., Caliari, V., Sganzerla, M., and Godoy, H. T. (2017). Volatile composition of merlot red wine and its contribution to the aroma: optimization and validation of analytical method. Talanta 174, 752–766. doi: 10.1016/j.talanta.2017.06.074
Arcena, M. R., Kebede, B., Leong, S. Y., Silcock, P., and Oey, I. (2020). Feasibility of using integrated fingerprinting, profiling and chemometrics approach to understand (bio) chemical changes throughout commercial red winemaking: A case study on merlot. Food Res. Int. 127:108767. doi: 10.1016/j.foodres.2019.108767
Artozoul, L., Poutaraud, A., and Ollivier, D. (1960). Les cépages du Bordelais: étude ampélographique et œnologique. Paris: Institut Technique de la Vigne et du Vin.
Augustyn, O. P. H., Rapp, A., and Van Wyk, C. J. (1985). Some volatile aroma components of Vitis vinifera L. cv. Sauvignon blanc. S. Afr. J. Enol. Vitic. 3. doi: 10.21548/3-2-2382
Baltazar, M., Castro, I., and Gonçalves, B. (2025). Adaptation to climate change in viticulture: the role of varietal selection—A review. Plants 14:104. doi: 10.3390/plants14010104
Bettiga, L. J. (2003). Wine grape varieties in California. Agriculture and Natural Resources, Publication: University of California, 3419.
Blanco-Vega, D., Gómez-Alonso, S., and Hermosín-Gutiérrez, I. (2014). Identification, content and distribution of anthocyanins and low molecular weight anthocyanin-derived pigments in Spanish commercial red wines. Food Chem. 158, 449–458. doi: 10.1016/j.foodchem.2014.02.154
Bokulich, N. A., Thorngate, J. H., Richardson, P. M., and Mills, D. A. (2014). Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 111, E139–E148. doi: 10.1073/pnas.1317377110
Borren, E., and Tian, B. (2020). The important contribution of non-Saccharomyces yeasts to the aroma complexity of wine: a review. Foods 10:13. doi: 10.3390/foods10010013
Bouchereau, J. (1843). Notice sur les principaux cépages cultivés dans le département de la Gironde. Bordeaux: Imprimerie de Gounouilhou.
Boursiquot, J.-M., Lacombe, T., Laucou, V. V., Julliard, S., Perrin, F. X., Lanier, N., et al. (2009). Parentage of merlot and related winegrape cultivars of southwestern France: discovery of the missing link. Aust. J. Grape Wine Res. 15, 144–155. doi: 10.1111/j.1755-0238.2008.00041.x
Buljeta, I., Pichler, A., Šimunović, J., and Kopjar, M. (2023). Beneficial effects of red wine polyphenols on human health: comprehensive review. Curr. Issues Mol. Biol. 45, 782–798. doi: 10.3390/cimb45020052
Cañas, P. M. I., Pérez-Martín, F., Romero, E. G., Prieto, S. S., and De Los Llanos Palop Herreros, M. (2012). Influence of inoculation time of an autochthonous selected malolactic bacterium on volatile and sensory profile of Tempranillo and merlot wines. Int. J. Food Microbiol. 156, 245–254. doi: 10.1016/j.ijfoodmicro.2012.03.033
Carrasco-Quiroz, M., et al. (2020). Influence of grape ripeness and leaf removal on the volatile composition and sensory attributes of Merlot wines. Food Res. Int. 137:109459. doi: 10.1016/j.foodres.2020.109459
Chacón-Vozmediano, J. L., Martínez-Gascueña, J., García-Romero, E., Gómez-Alonso, S., García-Navarro, F. J., and Jiménez-Ballesta, R. (2021). Effects of water stress on the phenolic compounds of ‘merlot’ grapes in a semi-arid mediterranean climate. Horticulturae 7:161. doi: 10.3390/horticulturae7070161
Clavijo, A., Calderón, I. L., and Paneque, P. (2010). Diversity of Saccharomyces and non-Saccharomyces yeasts in three red grape varieties cultured in the Serranía de Ronda (Spain) vine-growing region. Int. J. Food Microbiol. 143, 241–245. doi: 10.1016/j.ijfoodmicro.2010.08.010
Cincotta, F., Verzera, A., Prestia, O., Tripodi, G., Lechhab, W., Sparacio, A., et al. (2021). Influence of leaf removal on grape, wine and aroma compounds of Vitis vinifera L. cv. Merlot under Mediterranean climate. Eur. Food Res. Technol. 248, 403–413. doi: 10.1007/s00217-021-03885-w
Colautti, A., Civilini, M., Contin, M., Celotti, E., and Iacumin, L. (2023a). Organic vs. conventional: impact of cultivation treatments on the soil microbiota in the vineyard. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1242267
Colautti, A., Golinelli, F., Iacumin, L., Tomasi, D., Cantone, P., and Mian, G. (2023b). Triacontanol (long-chain alcohol) positively enhances the microbial ecology of berry peel in Glera yet promotes the must total soluble sugars content. OENO One 57, 133–146. doi: 10.20870/oeno-one.2023.57.2.7507
Corrêa, R. C. G., Haminiuk, C. W. I., Barros, L., Dias, M. I., Calhelha, R. C., Kato, C. G., et al. (2017). Stability and biological activity of merlot (Vitis vinifera) grape pomace phytochemicals after simulated in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 36, 410–417. doi: 10.1016/j.jff.2017.07.030
Darriet, P., Bouchilloux, P., Poupot, C., Bugaret, Y., Clerjeau, M., Sauris, P., et al. (2001). Effects of copper fungicide spraying on volatile thiols of the varietal aroma of sauvignon blanc, Cabernet Sauvignon and Merlot wines. EurekaMag. Available online at: https://eurekamag.com/research/003/740/003740089.php
De Boubée, D. R., Van Leeuwen, C., and Dubourdieu, D. (2000). Organoleptic impact of 2-methoxy-3-isobutylpyrazine on red Bordeaux and Loire wines. Effect of environmental conditions on concentrations in grapes during ripening. J. Agric. Food Chem. 48, 4830–4834. doi: 10.1021/jf000181o
de Mira Orduña, R. (2010). Climate change associated effects on grape and wine quality and production. Food Res. Int. 43, 1844–1855. doi: 10.1016/j.foodres.2010.05.001
Dewasme, C., Mary, S., Darrieutort, G., Audeguin, L., Van Helden, M., and Van Leeuwen, C. (2019). Quantitative and qualitative impact of tomato black ring virus (TBRV) on merlot and cabernet franc. OENO One 53. doi: 10.20870/oeno-one.2019.53.2.2429
Di Profio, F., Reynolds, A. G., and Kasimos, A. (2011). Canopy management and enzyme impacts on merlot, cabernet franc, and cabernet sauvignon. Part II. Wine composition and quality. Am. J. Enol. Vitic. 62, 152–168. doi: 10.5344/ajev.2010.10035
Díaz, N., Aqueveque, P. M., Vallejos-Almirall, A., Radrigán, R., Zúñiga-López, M. C., and Folch-Cano, C. (2022). Antioxidant compound adsorption in Polyvinylpolypyrrolidone from Chilean Carménère, cabernet sauvignon, and merlot grape pomaces as potential by-products. Antioxidants 11:2017. doi: 10.3390/antiox11102017
Ferrer, M., Echeverría, G., and Carbonneau, A. (2016). Effect of berry weight and its components on the contents of sugars and anthocyanins of three varieties of Vitis vinifera L. under different water supply conditions. S. Afr. J. Enol. Vitic. 35:1. doi: 10.21548/35-1-989
Ficagna, E., Gava, A., Rossato, S. B., Rombaldi, C. V., and Borsato, D. (2020). Effect on merlot red wine of fining agents mixture: application of the simplex centroid design. Food Sci. Technol. 40, 729–735. doi: 10.1590/fst.18719
Fudge, A. L., Schiettecatte, M., Ristic, R., Hayasaka, Y., and Wilkinson, K. L. (2012). Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 18, 302–307. doi: 10.1111/j.1755-0238.2012.00200.x
Gancel, A.-L., Vignault, A., Pilard, E., Miramont, C., Jourdes, M., Fermaud, M., et al. (2021). Impacts of added oenological tannins on red wine quality to counteract Botrytis infection in merlot grapes. OENO One 55, 381–402. doi: 10.20870/oeno-one.2021.55.2.4623
García-Carpintero, E. G., Sánchez-Palomo, E., Gallego, M. A. G., and González-Viñas, M. A. (2011). Volatile and sensory characterization of red wines from cv. Moravia Agria minority grape variety cultivated in La Mancha region over five consecutive vintages. Food Res. Int. 44, 1549–1560. doi: 10.1016/j.foodres.2011.04.022
Ghendov-Mosanu, A., Cojocari, D., Balan, G., Patras, A., Lung, I., Soran, M.-L., et al. (2022). Chemometric optimization of biologically active compounds extraction from grape Marc: composition and antimicrobial activity. Molecules 27:1610. doi: 10.3390/molecules27051610
Girardello, R. C., Cooper, M. L., Lerno, L. A., Brenneman, C., Eridon, S., Sokolowsky, M., et al. (2020). Impact of grapevine red blotch disease on cabernet sauvignon and merlot wine composition and sensory attributes. Molecules 25:3299. doi: 10.3390/molecules25143299
Goldberg, D. M., Ng, E., Yan, J., Karumanchiri, A., Soleas, G. J., and Diamandis, E. P. (1996). Regional differences in resveratrol isomer concentrations of wines from various cultivars. J. Wine Res. 7, 13–24. doi: 10.1080/09571269608718057
Gonçalves, G. A., Soares, A. A., Correa, R. C. G., Barros, L., Haminiuk, C. W. I., Peralta, R. M., et al. (2017). Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 33, 408–418. doi: 10.1016/j.jff.2017.04.009
González-Neves, G., Gómez-Cordovés, C., and Barreiro, L. (2001). Anthocyanic composition of Tannat, cabernet sauvignon and merlot young red wines from Uruguay. J. Wine Res. 12, 125–133. doi: 10.1080/09571260120095030
Gutiérrez-Gamboa, G., Pérez-Donoso, A. G., Pou-Mir, A., Acevedo-Opazo, C., and Valdés-Gómez, H. (2019). Hydric behaviour and gas exchange in different grapevine varieties (Vitis vinifera L.) from the Maule Valley (Chile). South African Journal of Enology and Viticulture 40, 1–11. doi: 10.21548/42-2-3224
Hardy, A. (1844). Monographie du cépage Merlot et des principaux cépages du Médoc. Bordeaux: Imprimerie de Crugy.
Hranilovic, A., Albertin, W., Capone, D. L., Gallo, A., Grbin, P. R., Danner, L., et al. (2021). Impact of Lachancea thermotolerans on chemical composition and sensory profiles of merlot wines. Food Chem. 349:129015. doi: 10.1016/j.foodchem.2021.129015
Ignat, G., Balan, G. G., Sandu, I., and Ville, S. T. S. (2016). Study of phenolic compounds in merlot red wines obtained by different technologies. ResearchGate. Available online at: https://www.researchgate.net/publication/309576661_Study_of_phenolic_compounds_in_Merlot_red_wines_obtained_by_different_technologies
Ivanova-Petropulos, V., Ricci, A., Nedelkovski, D., Dimovska, V., Parpinello, G. P., and Versari, A. (2015). Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chem. 171, 412–420. doi: 10.1016/j.foodchem.2014.09.014
Jiang, B., and Zhang, Z. W. (2012). Comparison on phenolic compounds and antioxidant properties of cabernet sauvignon and merlot wines from four wine grape-growing regions in China. Molecules 17, 8804–8821. doi: 10.3390/molecules17088804
Jin, X.-D., Wu, X., and Liu, X. (2017). Phenolic characteristics and antioxidant activity of merlot and cabernet sauvignon wines increase with vineyard altitude in a high-altitude region. S. Afr. J. Enol. Vitic. 38:2. doi: 10.21548/38-2-1068
Jolly, N. P., Varela, C., and Pretorius, I. S. (2014). Not your ordinary yeast: non- Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 14, 215–237. doi: 10.1111/1567-1364.12111
Karoglan, M., Osrečak, M., Maslov, L., and Kozina, B. (2014). Effect of cluster and berry thinning on merlot and cabernet sauvignon wines composition. Czech J. Food Sci. 32, 470–476. doi: 10.17221/598/2013-cjfs
King, P. D., McClellan, D. J., and Smart, R. E. (2012). Effect of severity of leaf and crop removal on grape and wine composition of merlot vines in Hawke’s bay vineyards. Am. J. Enol. Vitic. 63, 500–507. doi: 10.5344/ajev.2012.12020
Kostadinović, S., Wilkens, A., Stefova, M., Ivanova, V., Vojnoski, B., Mirhosseini, H., et al. (2012). Stilbene levels and antioxidant activity of Vranec and merlot wines from Macedonia: effect of variety and enological practices. Food Chem. 135, 3003–3009. doi: 10.1016/j.foodchem.2012.06.118
Kotseridis, Y., Baumes, R. L., and Skouroumounis, G. K. (1999). Quantitative determination of free and hydrolytically liberated β-damascenone in red grapes and wines using a stable isotope dilution assay. J. Chromatogr. A 849, 245–254. doi: 10.1016/s0021-9673(99)00540-3
Lacombe, T., Boursiquot, J.-M., Laucou, V., Di Vecchi-Staraz, M., Peros, J.-P., and This, P. (2012). Large-scale parentage analysis in an extended set of grapevine cultivars (Vitis vinifera L.). Theor. Appl. Genet. 126, 401–414. doi: 10.1007/s00122-012-1988-2
Landon, J. L., Weller, K., Harbertson, J. F., and Ross, C. F. (2008). Chemical and sensory evaluation of astringency in Washington state red wines. Am. J. Enol. Vitic. 59, 153–158. doi: 10.5344/ajev.2008.59.2.153
Landrault, N., Poucheret, P., Ravel, P., Gasc, F., Cros, G., and Teissedre, P.-L. (2001). Antioxidant capacities and Phenolics levels of French wines from different varieties and vintages. J. Agric. Food Chem. 49, 3341–3348. doi: 10.1021/jf010128f
Legras, J.-L., Merdinoglu, D., Cornuet, J. M., and Karst, F. (2010). Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 16, 2091–2102. doi: 10.1111/j.1365-294X.2007.03266.x
Li, S.-S., Cheng, C., Li, Z., Chen, J.-Y., Yan, B., Han, B.-Z., et al. (2010). Yeast species associated with wine grapes in China. Int. J. Food Microbiol. 138, 85–90. doi: 10.1016/j.ijfoodmicro.2010.01.009
Lleixà, J., Martín, V., Portillo, M. d. C., Carrau, F., Beltran, G., and Mas, A. (2016). Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 7:338. doi: 10.3389/fmicb.2016.00338
Lorrain, B., Chira, K., and Teissedre, P. L. (2011). Phenolic composition of merlot and cabernet-sauvignon grapes from Bordeaux vineyard for the 2009-vintage: comparison to 2006, 2007 and 2008 vintages. Food Chem. 126, 1991–1999. doi: 10.1016/j.foodchem.2010.12.062
Maicas, S., and Mateo, J. J. (2023). The life of Saccharomyces and non-Saccharomyces yeasts in drinking wine. Microorganisms 11:1178. doi: 10.3390/microorganisms11051178
Maier, A., Padureanu, V., Lupu, M., Canja, C., Branescu, G. R., Padureanu, C., et al. (2024). Effect of high-power ultrasound treatment on bioactive compound content and chromatic characteristics of red wines : Doaj.Org.
Majkić, T. M., Torović, L. D., Lesjak, M. M., Četojević-Simin, D. D., and Beara, I. N. (2019). Activity profiling of Serbian and some other European merlot wines in inflammation and oxidation processes. Food Res. Int. 121, 151–160. doi: 10.1016/j.foodres.2019.03.033
Makalisa, A., Aleixandre-Tudo, J.-L., and du Toit, W. J. (2025). Investigating the phenolic composition of merlot and shiraz grape extracts and wines produced from grapes with different seed-to-skin ratios. S. Afr. J. Enol. Vitic. doi: 10.21548/46-6672
Manfroi, L., Silva, P. H. A., Rizzon, L. A., Sabaini, P. S., and Glória, M. B. A. (2009). Influence of alcoholic and malolactic starter cultures on bioactive amines in merlot wines. Food Chem. 116, 208–213. doi: 10.1016/j.foodchem.2009.02.034
Martín, S., González-Burgos, E., Carretero, M. E., and Gómez-Serranillos, M. P. (2011). Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chem. 128, 40–48. doi: 10.1016/j.foodchem.2011.02.074
Martín, S., González-Burgos, E., Carretero, M. E., and Gómez-Serranillos, M. P. (2012). Protective effects of merlot red wine extract and its major polyphenols in Pc12 cells under oxidative stress conditions. J. Food Sci. 78. doi: 10.1111/1750-3841.12000
Massera, A., Assof, M., Sari, S., Ciklic, I., Mercado, L., Jofré, V., et al. (2021). Effect of low temperature fermentation on the yeast-derived volatile aroma composition and sensory profile in merlot wines. LWT 142:111069. doi: 10.1016/j.lwt.2021.111069
Mattos Rocha, R. K., Andrioli, J., Scariot, F. J., Schwarz, L. V., Longaray Delamare, A. P., and Echeverrigaray, S. (2022). Yeast diversity in cabernet-sauvignon and merlot grapes grown in the highlands of southern Brazil. OENO One 56, 101–110. doi: 10.20870/oeno-one.2022.56.2.4660
Matus, J. T., Vega, A., Loyola, R., Serrano, C., Cabrera, S., and Arce-Johnson, P. (2008). Phytoplasma and virus detection in commercial plantings of Vitis vinifera cv. Merlot exhibiting premature berry dehydration. Electron. J. Biotechnol. 11. doi: 10.2225/vol11-issue5-fulltext-8
Mazza, G., Fukumoto, L., Delaquis, P., Girard, B., and Ewert, B. (1999). Anthocyanins, Phenolics, and color of cabernet franc, merlot, and pinot noir wines from British Columbia. J. Agric. Food Chem. 47, 4009–4017. doi: 10.1021/jf990449f
Mendoza, L. M., Vega-Lopez, G. A., Fernández de Ullivarri, M., and Raya, R. R. (2019). Population and oenological characteristics of non-Saccharomyces yeasts associated with grapes of northwestern Argentina. Arch. Microbiol. 201, 235–244. doi: 10.1007/s00203-018-1601-4
Naidu, R., Rowhani, A., Fuchs, M., Golino, D., and Martelli, G. P. (2014). Grapevine Leafroll: A complex viral disease affecting a high-value fruit crop. Plant Dis. 98, 1172–1185. doi: 10.1094/PDIS-08-13-0880-FE
Nisiotou, A., Sgouros, G., Mallouchos, A., Nisiotis, C.-S., Michaelidis, C., Tassou, C., et al. (2018). The use of indigenous Saccharomyces cerevisiae and Starmerella bacillaris strains as a tool to create chemical complexity in local wines. Food Res. Int. 111, 498–508. doi: 10.1016/j.foodres.2018.05.035
Noguerol-Pato, R., González-Barreiro, C., Cancho-Grande, B., and Simal-Gándara, J. (2009). Quantitative determination and characterisation of the main odourants of Mencía monovarietal red wines. Food Chem. 117, 473–484. doi: 10.1016/j.foodchem.2009.04.014
Odart, C. A. P. (1845). Ampélographie; ou.OIV. (2017). Distribution of the world’s grapevine varieties.
OIV (2017). Distribution of the world’s grapevine varieties : Organisation Internationale de la Vigne et du Vin Available online at: https://www.oiv.int/public/medias/5888/en-distribution-of-the-worlds-grapevine-varieties.pdf.
Osrečak, M., Karoglan, M., and Kozina, B. (2016). Influence of leaf removal and reflective mulch on phenolic composition and antioxidant activity of merlot, Teran and Plavac Mali wines (Vitis vinifera L.). Sci. Hortic. 209, 261–269. doi: 10.1016/j.scienta.2016.07.005
Ou, C., Du, X., Shellie, K., Ross, C., and Qian, M. C. (2010). Volatile compounds and sensory attributes of wine from cv. Merlot (Vitis vinifera L.) grown under differential levels of water deficit with or without a kaolin-based, foliar Reflectant particle film. J. Agric. Food Chem. 58, 12890–12898. doi: 10.1021/jf102587x
Padilla, B., Gil, J. V., and Manzanares, P. (2016). Past and future of non-Saccharomyces yeasts: from spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 7:411. doi: 10.3389/fmicb.2016.00411
Panceri, C. P., De Gois, J. S., Borges, D. L. G., and Bordignon-Luiz, M. T. (2015). Effect of grape dehydration under controlled conditions on chemical composition and sensory characteristics of cabernet sauvignon and merlot wines. LWT 63, 228–235. doi: 10.1016/j.lwt.2015.02.014
Pavić, V., Kujundžić, T., Kopić, M., Jukić, V., Braun, U., Schwander, F., et al. (2019). Effects of defoliation on phenolic concentrations, antioxidant and antibacterial activity of grape skin extracts of the varieties Blaufränkisch and merlot (Vitis vinifera L.). Molecules 24:2444. doi: 10.3390/molecules24132444
Peng, C.-T., Wen, Y., Tao, Y.-S., and Lan, Y.-Y. (2013). Modulating the formation of meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 61, 1542–1553. doi: 10.1021/jf3043874
Pereira, V., Cacho, J., and Marques, J. C. (2014). Volatile profile of Madeira wines submitted to traditional accelerated ageing. Food Chem. 162, 122–134. doi: 10.1016/j.foodchem.2014.04.039
Pickering, G. J., Willwerth, J., Botezatu, A., and Thibodeau, M. (2021). Prevalence and Management of Alkyl-Methoxypyrazines in a changing climate: Viticultural and oenological considerations. Biomolecules 11:1521. doi: 10.3390/biom11101521
Pons, A., Mouakka, N., Deliere, L., Crachereau, J. C., Davidou, L., Sauris, P., et al. (2018). Impact of Plasmopara viticola infection of merlot and cabernet sauvignon grapes on wine composition and flavor. Food Chem. 239, 102–110. doi: 10.1016/j.foodchem.2017.06.087
Prusova, B., Humaj, J., Sochor, J., and Baron, M. (2022). Formation, losses, preservation and recovery of aroma compounds in the winemaking process. Fermentation 8:93. doi: 10.3390/fermentation8030093
Renault, P., Coulon, J., De Revel, G., Barbe, J.-C., and Bely, M. (2015). Increase of fruity aroma during mixed T. Delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 207, 40–48. doi: 10.1016/j.ijfoodmicro.2015.04.037
Rendu, V. (1857). Ampélographie française: comprenant la statistique, la description des meilleurs cépages, l’analyse chimique du sol, et les procédés de culture et de vinification des principaux vignobles de la France. Paris: Imprimerie Impériale.
Renouf, V., Claisse, O., and Lonvaud-Funel, A. (2008). Understanding the microbial ecosystem on the grape berry surface through numeration and identification of yeast and bacteria. Aust. J. Grape Wine Res. 11, 316–327. doi: 10.1111/j.1755-0238.2005.tb00031.x
Rézeau, C. (1997). Dictionnaire des noms de cépages de France: Étymologie et histoire. Paris: CNRS Éditions.
Ribalta-Pizarro, C., Muñoz, P., and Munné-Bosch, S. (2024). The wine quality of merlot relies in irrigation supplementation and spotlights sustainable production constraints in Mediterranean-type ecosystems. Aust. J. Grape Wine Res. 2024. doi: 10.1155/2024/5001343
Ribéreau-Gayon, P., Dubourdieu, D., Donèche, B., and Lonvaud, A. (2006). Handbook of enology. Volume 1: the microbiology of wine and Vinifications (2nd edition, Vol. 1) Chichester, UK: Wiley.
Roberts, A., Hart, M., Usher, K., and Úrbez-Torres, J. R. (2025). Role of cluster thinning and viral load on the effects of grapevine leafroll disease in merlot and cabernet sauvignon in British Columbia, Canada. Am. J. Enol. Vitic. 76:0760004. doi: 10.5344/ajev.2024.24052
Robinson, J., Harding, J., and Vouillamoz, J. (2012). Wine Grapes: A complete guide to 1,368 vine varieties, including their origins and flavours. London: HarperCollins Publishers.
Rocha, S. M., Coelho, E., Zrostlíková, J., Delgadillo, I., and Coimbra, M. A. (2007). Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry of monoterpenoids as a powerful tool for grape origin traceability. J. Chromatogr. A 1161, 292–299. doi: 10.1016/j.chroma.2007.05.093
Sangorrín, M. P., Zajonskovsky, I. E., Lopes, C. A., Rodríguez, M. E., De Van Broock, M. R. G., and Caballero, A. C. (2001). Killer behaviour in wild wine yeasts associated with Merlot and Malbec type musts spontaneously fermented from Northwestern Patagonia (Argentina). J. Basic Microbiol. 41, 105–113. doi: 10.1002/1521-4028(200105)41:2
Sen, I., and Tokatli, F. (2014). Authenticity of wines made with economically important grape varieties grown in Anatolia by their phenolic profiles. Food Control 46, 446–454. doi: 10.1016/j.foodcont.2014.06.015
Sichel, V., Sarah, G., Girollet, N., Laucou, V., Roux, C., Roques, M., et al. (2023). Chimeras in merlot grapevine revealed by phased assembly. BMC Genomics 24:396. doi: 10.1186/s12864-023-09453-8
Sivcev, B., Rankovic-Vasic, Z., Petrovic, A., Jancic, R., and Milisic, K. (2018). Fruit characteristics of the merlot clones in Belgrade wine growing region, Serbia. J. Adv. Plant Sci., 1,:1. Available online at: http://fulltext.scholarena.co/Fruit-Characteristics-of-the-Merlot-Clones-in-Belgrade-Wine-Growing-Region-Serbia.php
Sivilotti, P., Herrera, J. C., Lisjak, K., Česnik, H. B., Sabbatini, P., Peterlunger, E., et al. (2016). Impact of leaf removal, applied before and after flowering, on anthocyanin, tannin, and Methoxypyrazine concentrations in ‘merlot’ (Vitis vinifera L.) grapes and wines. J. Agric. Food Chem. 64, 4487–4496. doi: 10.1021/acs.jafc.6b01013
Spayd, S. E., Tarara, J. M., Mee, D. L., and Ferguson, J. C. (2002). Separation of Sunlight and Temperature Effects on the Composition of Vitis vinifera cv. Merlot Berries. Am. J. Enol. Vitic. 53, 171–182. doi: 10.5344/ajev.2002.53.3.171
Stervbo, U., Vang, O., and Bonnesen, C. (2007). A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 101, 449–457. doi: 10.1016/j.foodchem.2006.01.047
Tarara, J. M., Lee, J., Spayd, S. E., and Scagel, C. F. (2008). Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 59, 235–247. doi: 10.5344/ajev.2008.59.3.235
Tatár, E., Mihucz, V. G., Virág, I., Rácz, L., and Záray, G. (2007). Effect of four bentonite samples on the rare earth element concentrations of selected Hungarian wine samples. Microchem. J. 85, 132–135. doi: 10.1016/j.microc.2006.05.009
Tudose-Sandu-Ville, Ş., Cotea, V. V., Colibaba, C., Nechita, B., Niculaua, M., and Codreanu, M. (2012). Phenolic compounds in merlot wines obtained through different technologies in Iaşi vineyard, Romania. Cercet. Agron. Moldova 45, 89–98. doi: 10.2478/v10298-012-0068-8
Ut, C., Berbegal, C., Lizama, V., Polo, L., García, M. J., Andrés, L., et al. (2022). Isolation and characterisation of autochthonous Saccharomyces cerevisiae from ‘Pago’ merlot wines of Utiel-Requena (Spain) origin. Aust. J. Grape Wine Res. 28, 330–346. doi: 10.1111/ajgw.12536
Van Leeuwen, C., and Destrac-Irvine, A. (2017). Modified grape composition under climate change conditions requires adaptations in the vineyard. OENO One 51, 147–154. doi: 10.20870/oeno-one.2017.51.2.1647
van Leeuwen, C., Sgubin, G., Bois, B., Ollat, N., Swingedouw, D., and Zito, S. (2024). & Gambetta, G. A. (2024). Climate change impacts and adaptations of wine production. Nat. Rev. Earth Environ. 5, 258–275. doi: 10.1038/s43017-024-00521-5
Varela, C., Barker, A., Tran, T., Borneman, A., and Curtin, C. (2017). Sensory profile and volatile aroma composition of reduced alcohol merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 252, 1–9. doi: 10.1016/j.ijfoodmicro.2017.04.002
Viala, P., and Vermorel, V. (1902–1910). Ampélographie: Description des cépages cultivés en France et dans les pays étrangers. Paris: Masson et Cie.
Vişan, L., Radiana, M., Tamba-Berehoiu, R.-M., and Constantinescu, D.G. (2020). Studies on the influence of climate conditions on the quality of merlot wines. ResearchGate. Available online at: https://www.researchgate.net/publication/340720325_STUDIES_ON_THE_INFLUENCE_OF_CLIMATE_CONDITIONS_ON_THE_QUALITY_OF_MERLOT_WINES
Vuerich, M., Braidotti, R., Sivilotti, P., Alberti, G., Casolo, V., Braidot, E., et al. (2021). Response of merlot grapevine to drought is associated to adjustments of growth and nonstructural carbohydrates allocation in above and underground organs. Water 13:2336. doi: 10.3390/w13172336
Welke, J. E., Manfroi, V., Zanus, M., Lazarotto, M., and Zini, C. A. (2012). Characterization of the volatile profile of Brazilian merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 1226, 124–139. doi: 10.1016/j.chroma.2012.01.002
Windholtz, S., Nioi, C., Coulon, J., and Masneuf-Pomarede, I. (2023a). Bioprotection by non-Saccharomyces yeasts in oenology: evaluation of O2 consumption and impact on acetic acid bacteria. Int. J. Food Microbiol. 405:110338. doi: 10.1016/j.ijfoodmicro.2023.110338
Windholtz, S., Nioi, C., Thibon, C., Bécquet, S., Vinsonneau, E., Coulon, J., et al. (2023b). Bioprotection as an alternative to SO2 in the pre-fermentation phase. Bordeaux, France: IVES Technical Reviews, Vine and Wine.
Windholtz, S., Redon, P., Lacampagne, S., Farris, L., Lytra, G., Cameleyre, M., et al. (2021a). Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT 149:111781. doi: 10.1016/j.lwt.2021.111781
Windholtz, S., Vinsonneau, E., Farris, L., Thibon, C., and Masneuf-Pomarède, I. (2021b). Yeast and filamentous Fungi microbial communities in organic red grape juice: effect of vintage, maturity stage, SO2, and bioprotection. Front. Microbiol. 12:748416. doi: 10.3389/fmicb.2021.748416
Xie, Q., Tang, Y., Wu, X., Luo, Q., Zhang, W., Liu, H., et al. (2023). Combined ultrasound and low temperature pretreatment improve the content of anthocyanins, phenols and volatile substance of merlot red wine. Ultrason. Sonochem. 100:106636. doi: 10.1016/j.ultsonch.2023.106636
Zhang, H.-L., Xia, N.-Y., Yao, X.-C., Duan, C.-Q., and Pan, Q.-H. (2024). Effects of Phenolic Evolution on Color Characteristics of Single-Cultivar Vitis vinifera L. Marselan and Merlot Wines during Vinification and Aging. Foods 13:494. doi: 10.3390/foods13030494
Zhao, S., Li, M., Simal-Gandara, J., Tian, J., Chen, J., Dai, X., et al. (2022). Impact of chiral tebuconazole on the flavor components and color attributes of Merlot and Cabernet Sauvignon wines at the enantiomeric level. Food Chem. 373:131577. doi: 10.1016/j.foodchem.2021.131577
Keywords: Merlot, wine, winemaking, fermentation, climate change, polyphenols, volatile compounds, bioprotection
Citation: Gridello E, Colautti A, Pellegrini M, Comi G and Iacumin L (2025) The resilient Merlot: from global growth to sustainable viticulture and winemaking in the age of climate change. Front. Sustain. Food Syst. 9:1675782. doi: 10.3389/fsufs.2025.1675782
Edited by:
Nicolás Oscar Soto-Cruz, TecNM-Instituto Tecnológico de Durango, MexicoReviewed by:
Jesús Bernardo Páez Lerma, TECNM/I. T. DURANGO, MexicoVinicius Caliari, Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina, Brazil
Copyright © 2025 Gridello, Colautti, Pellegrini, Comi and Iacumin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michela Pellegrini, bWljaGVsYS5wZWxsZWdyaW5pQHVuaXVkLml0
 Emma Gridello
Emma Gridello Andrea Colautti
Andrea Colautti Michela Pellegrini
Michela Pellegrini Giuseppe Comi
Giuseppe Comi Lucilla Iacumin
Lucilla Iacumin