- 1Department of Sustainable Food Systems and Development, Faculty of Natural and Agricultural Sciences, University of the Free State, Bloemfontein, South Africa
- 2Centre of Competence in Environmental Biotechnology, Department of Environmental Sciences, College of Agriculture and Environmental Sciences, University of South Africa, Johannesburg, South Africa
- 3The Bioenergy Consortium, College of Agriculture and Environmental Sciences, University of South Africa, Johannesburg, South Africa
- 4Department of Life and Consumer Sciences, College of Agriculture and Environmental Sciences, University of South Africa, Johannesburg, South Africa
Anaerobic co-digestion (AcoD) has emerged as an effective technology for treating poultry manure (PM) and food waste (FW), converting these organic materials into valuable biogas and bio-fertilizers. The management of PM and FW has become increasingly critical due to environmental challenges associated with their disposal, which often leads to pollution and greenhouse gas (GHG) emissions. By employing AcoD, both PM and FW can be processed simultaneously, optimizing resource utilization and enhancing waste management (WM) strategies. This strategy relies on the use of sustainable waste-to-energy technology (WET) that harnesses agricultural biomass as a clean, renewable, and carbon-neutral energy source. The advantages of AcoD over traditional anaerobic digestion (AD) include improved biogas yields, better nutrient balance, and enhanced microbial stability due to the diverse substrate mix. Co-digesting animal-based wastes, such as PM, with plant-based wastes like FW enables more efficient degradation of organic matter, leading to increased methane production. Various studies have demonstrated the successful co-digestion of PW with various types of FW, resulting in significant increases in methane yields, as a potent renewable energy source. This review critically highlights the characteristics and benefits of AcoD, focusing on the synergistic effects that various FW substrates have when paired with PM. Essential process parameters influencing microbial activity, such as pH, organic loading rate (OLR), temperature, carbon-to-nitrogen (C/N) ratio, and hydraulic retention time, are discussed for optimizing biogas production (BGP). The findings underscore the greenness of AcoD as an eco-friendly approach, reducing dependency on fossil fuels, and mitigating environmental pollution, thereby contributing to sustainable WM practices and BGP.
1 Introduction
Co-digestion of animal waste, such as poultry manure (PM), and food waste (FW), particularly through anaerobiosis [anaerobic co-digestion (AcoD)], presents a significant opportunity for sustainable waste management (WM) and Biogas production (BGP), contributing to nutrient recycling and reducing greenhouse gas (GHG) emissions (Kadam et al., 2024; Adnane et al., 2024). This approach relies on sustainable waste-to-energy technology (WET) that harnesses agricultural biomass as a clean, renewable, and carbon-neutral energy source.
Food wastes (FWs) are fractions of edible and non-edible foods removed from the food supply chain (FSC). Approximately 1.3 billion tons of global food production are lost or wasted yearly along the FSC, which encompasses every stage from initial production to final consumption (Food and Nations, 2012). These wastes comprise crop products like fruits, vegetables, and cereals, as well as animal products, primarily meat and dairy, along with other agricultural food items. The global volume of FW is projected to rise steadily, particularly in sub-Saharan Africa, as the world’s population is expected to hit 9.6 billion by 2050 (Bajželj, 2015). If proper WM strategies, such as nutrient recycling, are not implemented, this surge in FW will not only exacerbate food scarcity issues but also lead to serious environmental consequences, including increased GHG emissions and greater pressure on WM systems. For instance, (Ganesh et al., 2022) reported that fruits and vegetables are among the most widely consumed food products, and their wastes constitute 42% of the global FW. In another report from the World Wide Fund for Nature, the South Africa report (WWF-SA, 2017) reveals that South Africa produces 31 million tonnes of food annually, of which 10 million tonnes are wasted. The information went further to state that the wastage includes fruits and vegetables (44%), grains (26%), and meat (15%). The remaining 15% are combinations of oilseeds, roots, and tubers. In the same vein, a study conducted by Cronjé et al. (2018) amongst 100 households in Kimberly, South Africa, showed that bananas, apples, and avocados are the most wasted fruits, while tomatoes, potatoes, and cabbage are the most wasted vegetables. In another study, Esparza et al. (2020) noted that fruit and vegetable wastes (FVWs) occupy an ever-increasing space in waste treatment facilities and landfills.
Based on the reported findings, they affirmed that FVW valorisation resulted in extracting bioactive compounds, producing enzymes and exopolysaccharides, synthesizing bioplastics and biopolymers, and producing biofuels. However, through traditional activities, these FVWs are poorly disposed of in landfills or rivers. Global warming, depletion of the ozone layer, acid rain, and public health concerns are some of the adverse environmental effects of greenhouse gas (GHG) emissions from these wastes (Sahoo et al., 2021). According to Cantera et al. (2018), landfills generate GHGs such as methane (CH4), sulfides, ammonia (NH3), carbon dioxide (CO2), among others, that contribute to global warming. The methane gas produced has an impact 25 times greater than that of CO2. Parvin and Tareq (2021) also note that toxic substances called leachates generated from these landfills pose a great risk to public health as they filter gradually through the soil to pollute the groundwater and subsequently migrate into the surface water. These raise an environmental concern about the need to find a sustainable solution to FVWs.
Poultry manure (PM), characterized by its nitrogen-rich content stemming from poultry litter, feather waste, and spilled feeds, is one of the driest and bulkiest manures generated in intensive agricultural practices (Lakshmi et al., 2020; Lynch et al., 2013). However, while PM, as a product of poultry farming, serves as a vital source of nutrients and plays a major role in crop production as an organic fertilizer, it also poses considerable environmental challenges, including soil and water pollution resulting from waste management issues (Muduli et al., 2019). This waste can lead to detrimental effects on both human and animal health when not properly managed. To mitigate these environmental risks, co-digesting PM anaerobically with FVWs presents a promising approach (Adnane et al., 2024). This proposition not only reduces pollution risks but also transforms agricultural wastes into useful resources.
The social and environmental implications of FVWs reflect a pressing sustainability issue, as outlined in the United Nations’ 2030 Agenda (De Moraes et al., 2020). By integrating the digestion of PM and FVWs, it becomes possible to produce beneficial materials while simultaneously contributing to several sustainable development goals (SDGs), including poverty alleviation (SDG 1), zero hunger (SDG 2), promoting health and well-being (SDG 3), ensuring clean water and sanitation (SDG 6), and generating affordable and clean energy (SDG 7) (Biermann et al., 2017).
Anaerobic digestion (AD) is an economically feasible, eco-friendly, and socially acceptable biological process that transforms complex substrates into biogas and digestates in the absence of oxygen (Vats et al., 2020). The biogas generated is a combination of CO2, CH4, and minute quantities of other gases. Researchers (Náthia-Neves et al., 2018) found that AD, whether applied singularly or in a co-digestion framework, can serve as a source of clean energy since the biogas produced typically contains 50–75% CH4 gas, depending on the substrate used. The CH4 gas produced can be used as a substitute for natural gas, while the digested biomass can be used as fertilizers rich in nitrogen, phosphorus, and potassium. This capacity to produce renewable energy and nutrient-rich fertilizers further highlights the ecological advantages of AD, especially AcoD, over traditional WM. For instance, Arif et al. (2018) affirmed that AD could be used to reduce GHG emissions in organic waste treatment. It will still provide sustainable alternative by-products such as biogas for clean energy production and beneficial materials for composting and fertilizing.
The utilization of AcoD, which involves the combined processing of animal manure, such as PM, and FW, for WM and biogas generation, has seen substantial growth over the past decade. Initial studies established the groundwork for the development of AcoD, highlighting its environmental benefits, cost efficiency, and sustainability. Innovative advancements in AD, including anaerobic mono-digestion (AMD) and AcoD methods, have spurred its widespread adoption throughout this decade. This trend is evidenced by a notable rise in published research on the subject, as illustrated in Figure 1.
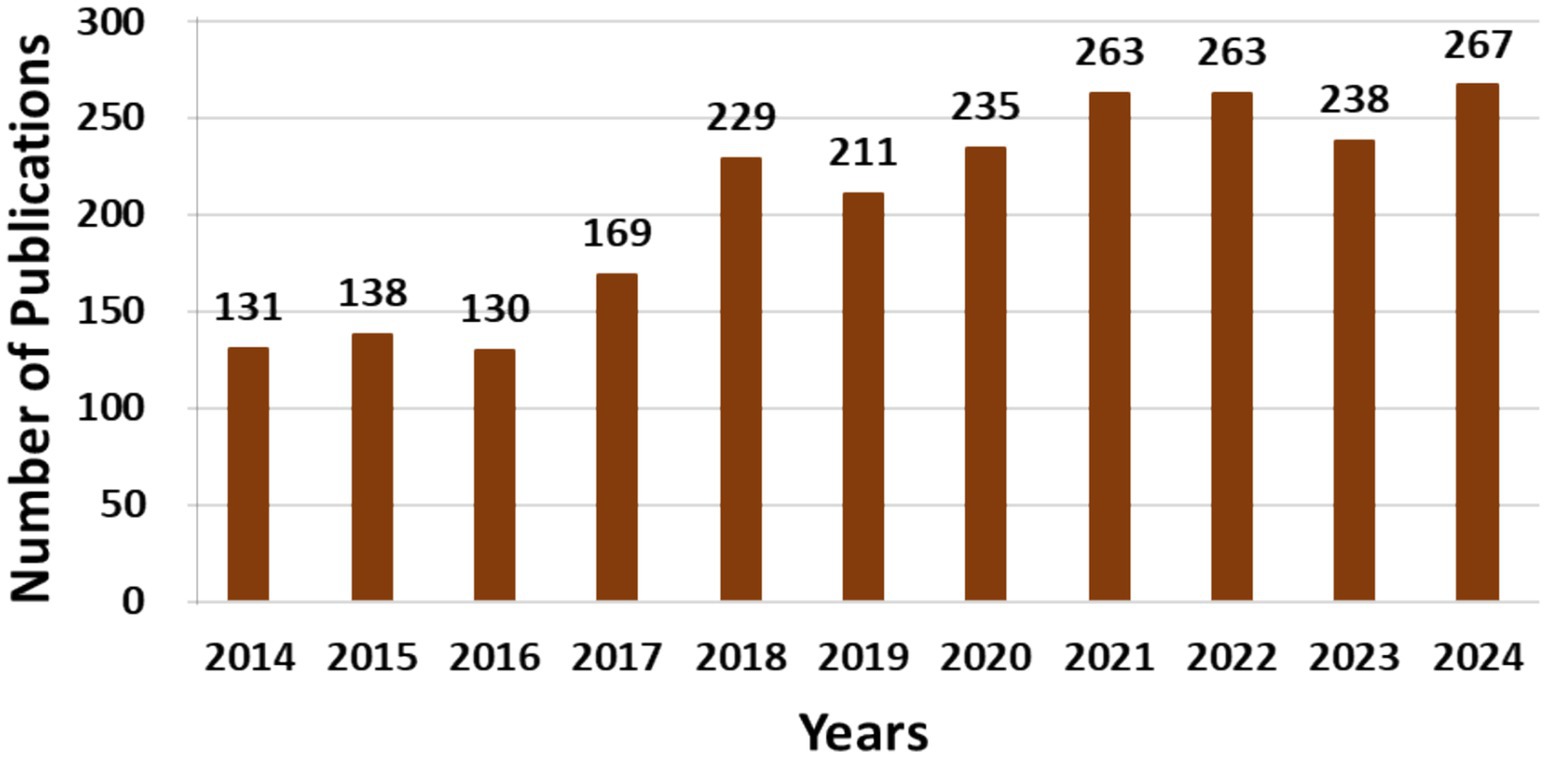
Figure 1. During a decade (2014–2024), several publications were generated on the use of anaerobic digestion (including AcoD) for BGP. Search keywords: “anaerobic co-digestion for biogas production.” Scopus (February 2025).
This critical review aims to investigate studies on BGP from the AcoD of poultry and food wastes (P&FWs), while also exploring the benefits and drawbacks of AMD or single-substrate digestion (SSD) in BGP, thereby providing insights into the role of AcoD as a sustainable solution for WM and biogas generation.
2 Poultry manure
The use of animal waste, such as poultry manure (PM), as a substrate for BGP over decades has gained more interest as it lowers GHG emissions, enhances nutrient re-circulation, and reduces dependence on fossil energy (Ahlberg-Eliasson et al., 2021; Holm-Nielsen et al., 2009; Hou et al., 2017). Choosing animal manure for biogas depends on the animal type and management practices. Furthermore, the suitability of such manure is dependent on the water content, herd size, and biogas potential (Liebetrau et al., 2021). PM ranks the highest among the common substrates for BGP. About 0.5 m3 of biogas (58% CH4) has been reported to be produced by 1 kg of organic matter in PM (Tawfik et al., 2023). This has been attributed to their high potential for BGP arising from their high content of total solids, volatile matter, and high biodegradability rate, as shown in Figure 2 (Maj, 2022; Tawfik et al., 2023).
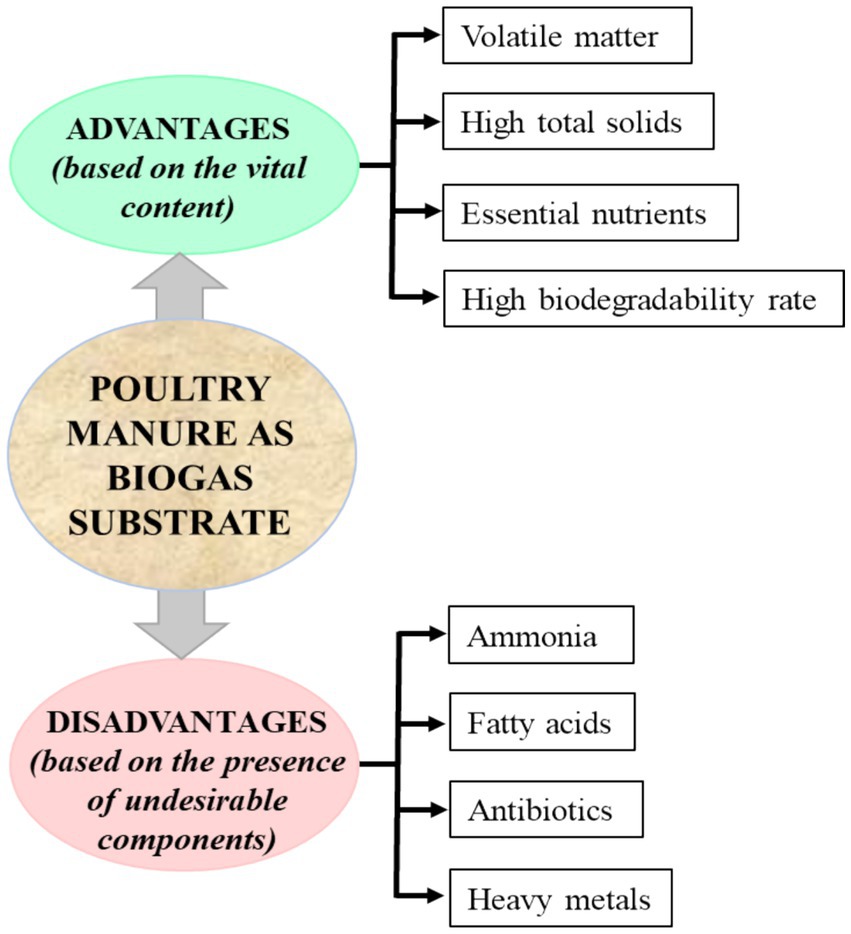
Figure 2. Advantages and disadvantages of poultry manure (PM) as a biogas substrate (Tawfik et al., 2023).
Studies on the proximate composition of PM revealed moisture content ranging from 8.84 to 9.62% moisture content, 21.60–39.01% ashes, 54.33–57.51% volatile matter, 90.3% dry matter, 2.61% fat content, and 16.09% crude fiber (Simbolon et al., 2019; Inna et al., 2022; Usman et al., 2019), as shown in Table 1.
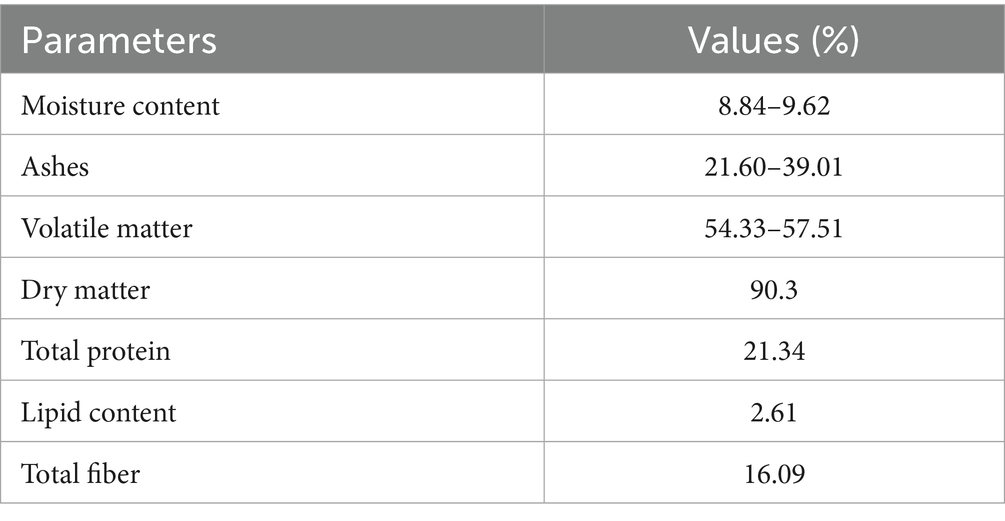
Table 1. Proximate composition of poultry manure (Simbolon et al., 2019; Inna et al., 2022; Usman et al., 2019).
Moisture: The high moisture content of poultry manure is of great importance to BGP as it enhances the breakdown of nutrients and substrate decomposition, contributing to an efficient rate of CH4 production and final yield (Zobeashia et al., 2021; Czekała et al., 2023; Liotta, 2013).
Crude protein: The high crude protein content of PM also enhances its BGP, as protein-rich substrates have been reported to have a higher yield of methane (Kovács et al., 2015; Nwokolo et al., 2020).
Volatile solid: This is the organic fraction of the total solid of a substrate, and it is a major determinant of the amount of biogas that can be produced (Mrosso et al., 2023; Orhorhoro et al., 2017). It also contributes to the efficiency of the fermentation process (Mrosso et al., 2023, Orhorhoro et al., 2017).
Crude fiber: The fiber contents of substrates have been linked to enhanced BGP, as their fermentation has been linked to the production of soluble sugars, which increases the energy content required for the production of biogas (Chomini et al., 2019).
Dry matter: The high dry matter content of poultry substrate makes it easy to undergo isolation, which increases methane yield (Kreuger et al., 2011).
Fat content: The low-fat content of PM is advantageous to its use as a substrate for BGP. Fatty acids have been implicated in decreased BGP owing to their toxicity to the fermenting microbes, which causes a drop in pH (Nwokolo et al., 2020). This leads to the failure of the fermentation system (Pramanik et al., 2019; Nwokolo et al., 2020).
The high ash content of PM measures its total elemental composition. The composition elements include zinc, selenium, nitrogen, potassium, calcium, molybdenum, phosphorus, manganese, cobalt, iron, and copper (Dróżdż et al., 2020), and of these, calcium and potassium have been reported to be the major metals (Quiroga et al., 2010). These elements are important in the degradation of organics into bioenergy by metabolizing microorganisms (Tawfik et al., 2023). However, PM has been reported for its low concentrations of trace elements, such as copper and zinc, which may affect its microbial degradation in producing methane (Tawfik et al., 2023; Quiroga et al., 2010).
3 Food waste
Food waste (FW) has been acknowledged as an important contributor to environmental pollution and public health issues, as it is highly biodegradable and contributes to the high organic content of solid waste (Plazzotta et al., 2017; Shi et al., 2018). Their biodegradability can be attributed to the high organic contents consisting of carbohydrates (starch and cellulose), proteins, and lipids, as well as high moisture content (Liu R. et al., 2022; Perin et al., 2020). FW includes leftovers from restaurants, canteens, and homes, as well as waste from food processing.
According to the Food and Agriculture Organization (FAO), FWs are produced annually from about 33% of the edible parts of human food (FAO, 2011). The European Union (EU) wastes nearly 89 million tons of food each year, a critical issue that requires urgent action (Stenmarck et al., 2016), while about 195, 22, and 90 million tons of FW are produced annually in China, Japan, and the United States (US), respectively (Thi et al., 2015). Agricultural production wastes are the major sources of FW in industrialized nations. They arise mostly from overproduction and post-harvest assessment of crops based on quality criteria demanded by retailers (FAO, 2011; Segrè and Falasconi, 2011). In developing countries, poor production and distribution of agricultural produce, leading to post-harvest loss and accumulation of perishable commodities, account for most of the FW (Plazzotta et al., 2017).
The use of FW as a substrate for BGP has been adopted in some countries to manage the environmental and public health threats it poses. Thus, providing a significant economic opportunity for sustainable development in addition to addressing the problem of sustainable waste management and lessening environmental pollution (Sarkar et al., 2021). In China, food waste has the ability to contribute up to 30.3% of the annual natural gas demand, which is equivalent to 70 billion m3 of methane production (Rajendran et al., 2014; Shi et al., 2018). The BGP potential of food wastes can be attributed to their organic composition, which includes volatile solids (70–90%, dry mass) (Perin et al., 2020). Figure 3 presents the proximate composition of different FWs.
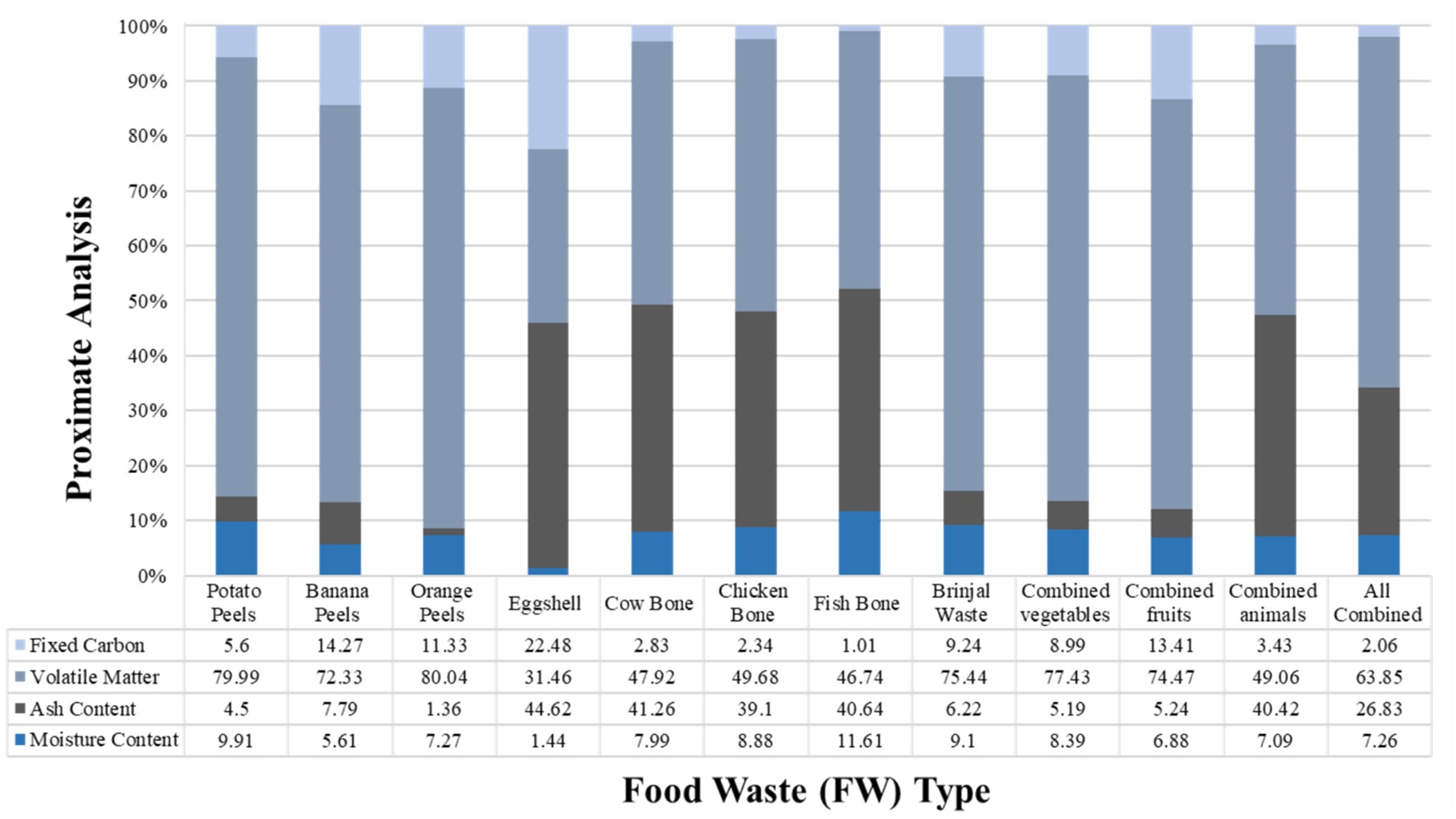
Figure 3. Proximate composition of various food wastes (Tawfik et al., 2023).
There are certain concerns regarding the use of FW as a single substrate for BGP. These have been attributed to the low pH values, low C/N ratio, and high nitrogen content (Perin et al., 2020). During digestion, the fraction of food waste that has low total solids and high levels of volatile fatty acids is hydrolyzed rapidly, leading to acidification and inhibition of the digestion process (Shen et al., 2013; Plazzotta et al., 2017). The low C/N ratio and high nitrogen cause rapid accumulation of ammonia. Ammonia has been implicated in the inhibition of the methanogenic activities of digestive microbes (Feng et al., 2021; Shi et al., 2018). The sodium, volatile, and long-chain fatty acids contents of food wastes also contribute to the inhibition of methanogenic activities (Feng et al., 2021; Pavi et al., 2017). The high simple sugar content also instigates rapid acidification and inhibition of methanogenic activity (Pavi et al., 2017). All these effects lead to a suppressed organic loading rate (OLR) and methane production.
The advantages and disadvantages of FW as a mono-substrate for BGP are presented in Figure 4.
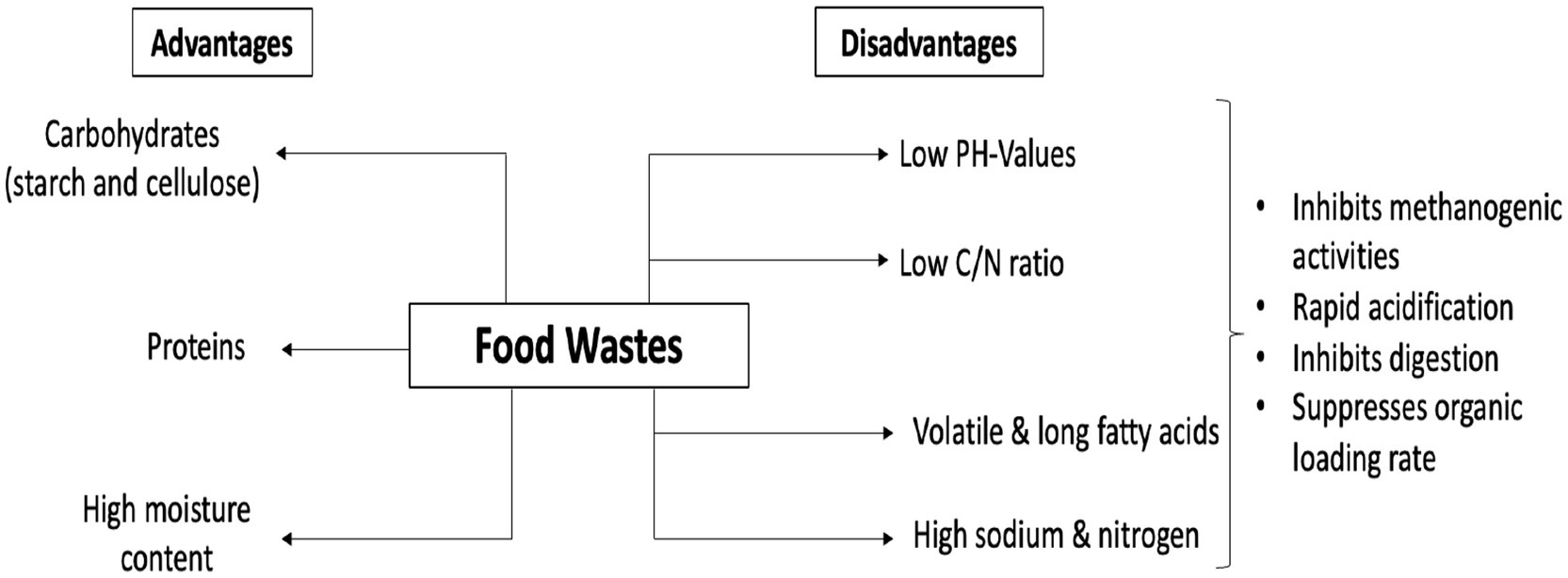
Figure 4. Advantages and disadvantages of food waste (FW) as a mono-substrate for BGP (Islam et al., 2023).
4 Biogas, compositions, and production
As a renewable energy source, biogas is produced from biomass used for domestic cooking, machinery, electricity, and other energy applications (Persson and Jönsson, 2007). It is composed of CO2 and CH4, which are generated by the microbial decomposition of organic materials such as human and animal excretory products (manures) and plant materials under anaerobic conditions (Raven and Gregersen, 2007; Olugasa et al., 2014). Biogas also contains other gases such as NH3, nitrogen (N2), gaseous hydrocarbons, hydrogen sulfide (H2S), oxygen (O2), and Si–O–Si bond-containing organic compounds like siloxanes (Awe et al., 2017; Alhassan et al., 2019).
As described in Figures 5, 6, the production of biogas involves several processes, including the methanation of biomass and organic waste materials derived from anaerobically digested sewage sludge, landfills, commercial composting, biomass gasification, and the AcoD of animal farm manure combined with agro-food waste (AFW) (Awe et al., 2017, Alhassan et al., 2019). The resulting residual digestates can also serve as fertilizers for agriculture.
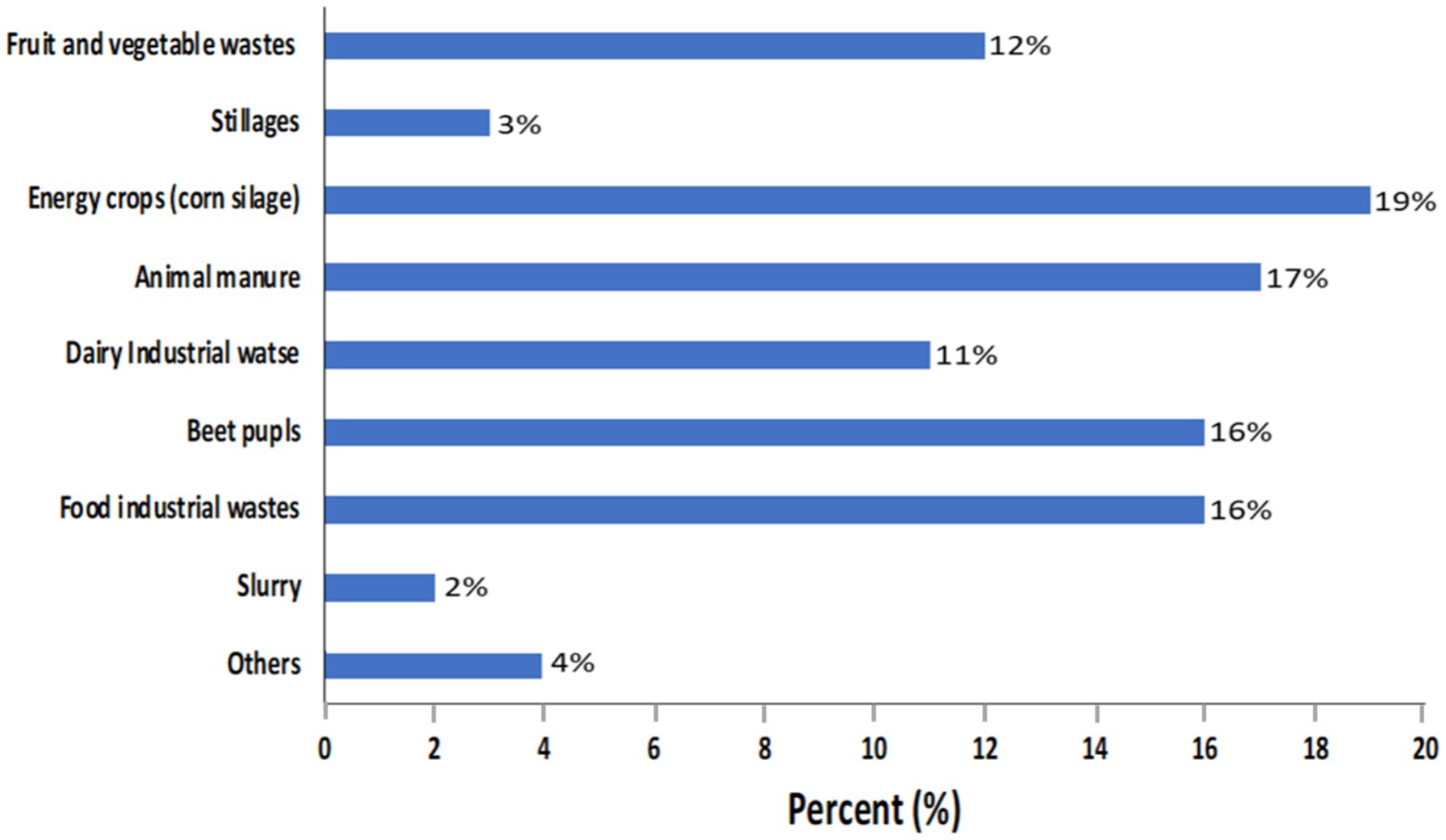
Figure 5. Substrate utilization in BGP in Poland 2021 (Ignatowicz et al., 2023).
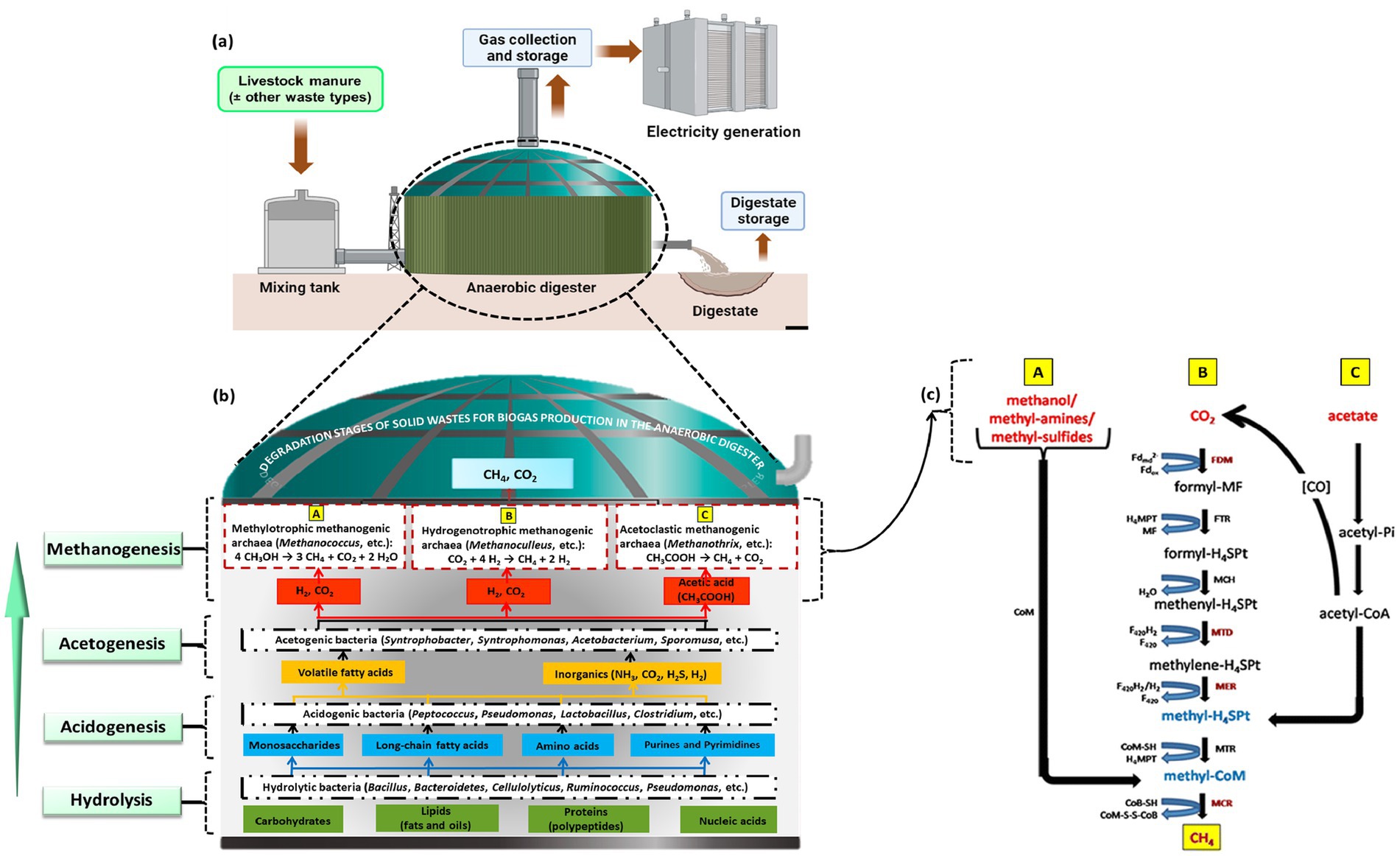
Figure 6. (a) Graphical illustration of a conventional AD reactor system (Kadam et al., 2024). (b) Stages of AD in BGP with some modifications (Adnane et al., 2024). (c) Biochemical pathways to produce CH4 from different starting materials during AD: A—methylotrophic methanogenesis, B—hydrogenotrophic methanogenesis, and C—acetoclastic or acetotrophic methanogenesis (Goswami et al., 2016). MF: methanofuran; CHO-MF: formylmethanofuran; Fd2−red: reduced ferrodoxin; Fdox: oxidized ferrodoxin; FDM (W/Mo-FMD)-(tungsten/molybdenum-dependent) formylmethanofuran dehydrogenase; H4MPT: tetrahydromethanopterin; FTR: formylmethanofuran: tetrahydromethanopterin formyltransferase; CHO–H4SPT: formylmethanofuran; MCH: N5,N10-methenyl tetrahydromethanopterin cyclohydrolases; methenyl-H4SPT+: methenyl tetrahydromethanopterin; F420H2: reduced cofactor F420; MTD: coenzyme F420-dependent N5,N10-methylene tetrahydromethanopterin dehydrogenase; CH2 = H4SPT: methylene tetrahydromethanopterin; MER: N5,N10-methylene tetrahydromethanopterin reductase; CH3–H4SPT: methyl tetrahydromethanopterin; CoM–SH: coenzyme M; MTR: N5-methyl tetrahydromethanopterin: coenzyme M methyltransferase; CH3–S–CoM: methyl coenzyme M; CoB–SH: coenzyme B; MCR: methyl coenzyme M reductase; CoM–S–S–CoB: coenzyme M-HTP heterodisulfide.
4.1 Compositions of biogas
As shown in Table 2, biogas produced from household waste is composed of CH4 (50–60%), CO2 (34–38%), N2 (0–5%), H2O (6%), and O2 (0–1%), with traces of H2 and H2S (NE, 2009; Liu et al., 2006). Biogas from agro waste has been reported to consist of CH4 (50–80%), CO2 (30–50%), N2 (0–1%), H2 (0–2%), H2S (0.70%), carbon monoxide (CO) (0–1%), O2 (0–1%) and H2O (6%), with a trace of NH3 (NE, 2009; Chen et al., 2015).
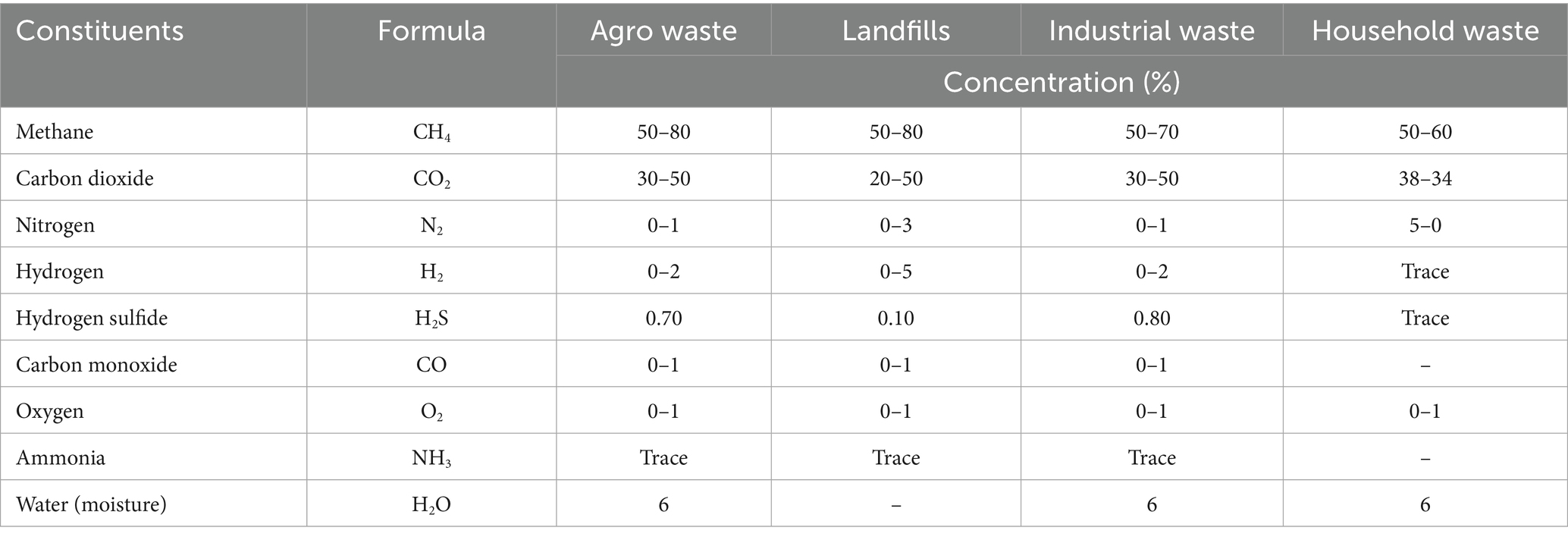
Table 2. Composition of biogas (Markoš, 2011; Chen et al., 2015; Liu et al., 2006; NE, 2009).
In addition, biogas from landfills is composed of CH4 (50–80%), CO2 (20–50%), N2 (0–3%), H2 (0–5%), H2S (0.10%), CO (0–1%), and O2 (0–1%), with a trace of NH3 (Chen et al., 2015). Meanwhile, the biogas generated from industrial waste is composed of CH4 (50–70%), CO2 (30–50%), N2 (0–1%), H2 (0–2%), H2S (0.80%), CO (0–1%), and O2 (0–1%), with a trace of NH3 (Chen et al., 2015).
Several factors, such as substrate composition and concentration, pH, and temperature, have been reported to affect the composition of biogas (Dobre et al., 2014).
4.2 Substrate for biogas production
Studies have shown that biogas production (BGP) is greatly dependent on the type of substrate. This can be attributed to the carbohydrate, fat, protein, and other organic contents of the substrates (Alhassan et al., 2019). Plant and animal products constitute the bulk of these substrates, which can readily undergo fermentation. Substrates for BGP are grouped into mono-substrates and co-substrates. Mono-substrates comprise various organic materials, including animal manure, slurry, and some other animal digestive contents, particularly those of ruminants. These substrates possess the fermentation capacity attributed to the presence of methane-producing bacteria (Ignatowicz et al., 2023). Co-substrates are substrates added to digesters to suppress inhibition, improve process efficiency, and attain proper hydration (Ignatowicz et al., 2023).
The common substrates for BGP are FVWs, slurry, food industrial wastes, beet pulps, dairy industrial wastes, animal manure, energy crops, and stillages (Alhassan et al., 2019; Ignatowicz et al., 2023). As shown in Figure 5, maize or corn silage (19%), as an energy crop, was the largest utilized substrate for BGP in Poland in 2021 (Ignatowicz et al., 2023). This was followed by animal manure (17%), food industrial waste (16%), and beet pulp (16%).
5 Anaerobic digestion
Anaerobic digestion (AD) is a biochemical process involving the disintegration of organic matter by methanogenic organisms in the absence of oxygen (O2) to generate a gaseous mixture referred to as biogas (Felton et al., 2014; Uddin and Wright, 2023). The produced biogas consists of methane (50–80%), carbon dioxide (20–50%), carbon monoxide, hydrogen, nitrogen, hydrogen sulfide, and water vapor (Felton et al., 2014). The microorganisms that play a role in AD form a large and diverse consortium that works together to catalyze the digestion process (Kunatsa and Xia, 2022).
5.1 Stages of anaerobic digestion
The anaerobic digestion (AD) process consists of four biochemical stages, vis-à-vis, hydrolysis, acidogenesis, acetogenesis, and methanogenesis, as illustrated in Figure 6.
Hydrolysis: This step, in the presence of water, involves the breakdown of inputted feedstocks consisting of complex polymers such as carbohydrates (starch and cellulose), proteins, and lipids into sugars, amino acids, and fatty acids (Uddin and Wright, 2023; Menzel et al., 2020). This step is catalyzed by hydrolytic enzymes such as cellulase, lipase, amylase, and pectinase (Uddin and Wright, 2023). Equation 1 summarizes the hydrolytic reaction.
Acidogenesis: After hydrolysis, acidogenic bacteria break the hydrolytic products into ketones, alcohols (mainly methanol and ethanol), short-chain volatile acids, CO2, and H2 (Rezapoor and Rahimpour, 2023; Uddin and Wright, 2023). This step is catalyzed by hydrogenases (Hendriks et al., 2018). The acidogenic reactions are summarized in Equations 2–4.
Acetogenesis: In this stage, acetogenic bacteria catalyze the transformation of acidogenic products and long-chain fatty acids from the hydrolysis stage into acetate (CH3COO−), CO2, and H2 (Laçın et al., 2023). Acetogens catalyze this reaction (Gould, 2015). The acetogenic reactions are summarized in Equations 5–7.
Methanogenesis: This last stage of anaerobic digestion is carried out by acetotrophic or acetoclastic, hydrogenotrophic or hydrogenophilic, and methylotrophic methanogenic bacteria and is catalyzed by methanogens (Uddin and Wright, 2023; Enzmann et al., 2018). Acetotrophic bacteria, such as Methanosaeta, Methanothrix, and Methanosarcina species, convert acetate into methane (CH4) and CO2 (Equation 8), while hydrogenotrophic bacteria, such as Methanospirillum, Methanobacterium, Methanobrevibacter, Methanoculleus, and Methanothermobacter, utilize formate or H2 to reduce CO2 into CH4 (Equation 9) (Uddin and Wright, 2023). Meanwhile, methylotrophic bacteria, such as Methanobacterium, Methanococcus, Methanomassiliicoccus, Methanosarcina, and Methanosalsus, produce CH4 through the utilization of methylated compounds like methanol, methylamine, and methyl sulfides (Equation 10).
5.2 Optimum process conditions for microorganisms in anaerobic digestion
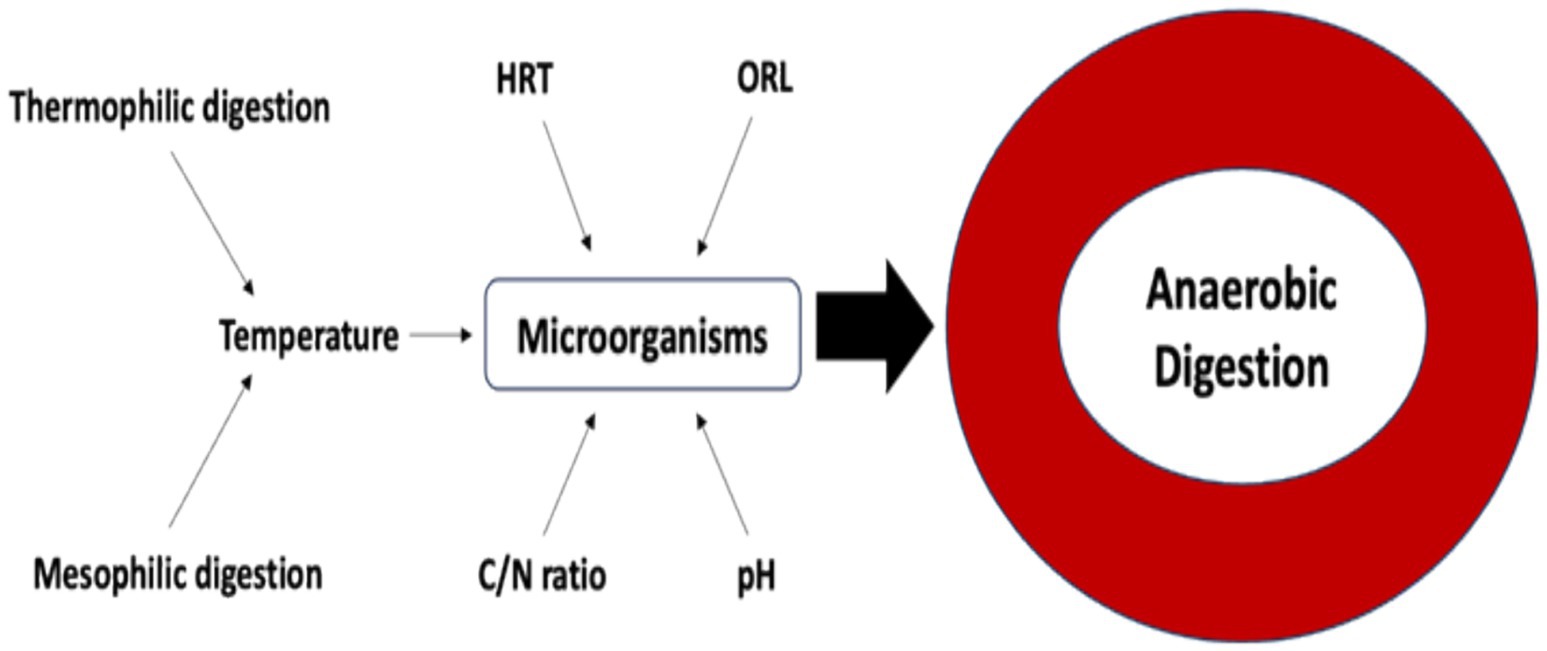
Based on reported investigations, AD is mediated by microorganisms (Caruso et al., 2019). These microorganisms need to be maintained in optimum conditions to ensure effective AD. These conditions include pH, organic loading rate (OLR), temperature, carbon/nitrogen (C/N) ratio, and hydraulic retention time (HRT), as shown in Figure 7.
5.2.1 Influence of pH
pH is a measure of the acidity or alkalinity of a solution, which significantly influences anaerobic digestion (AD). Optimal activity of hydrolytic and acidogenic bacteria occurs within a pH range of 5.5–6.5, while methanogens perform best around a neutral pH of 7. Conversely, acidogenic bacteria favor higher pH levels (Latif et al., 2017; Uddin and Wright, 2023).
During AD, fluctuations in pH can affect process stability and microbial activity, particularly that of methanogens (Vargas-Estrada et al., 2022). The efficiency of CH4 production is closely linked to pH, as CH4 formation naturally neutralizes the pH (Carotenuto et al., 2020). Since the AD process involves four stages (Section 5.1), each stage requires specific pH conditions for the optimal activities of the associated microbes. Maintaining an optimal pH, generally between 5.5 and 7.5, is critical for maximizing CH4 yield (Vargas-Estrada et al., 2022). Deviations from this range can alter microbial populations, impacting overall biogas production. For instance, low pH can lead to volatile fatty acid (VFA) accumulation and process inhibition, while high pH may favor NH3 formation, which can be toxic to microbes (Vargas-Estrada et al., 2022). Regarding the effect of pH, controlling initial pH through substrate selection or co-digestion is a practical approach to optimize AD, with adjustments often aiming for a pH around 7 to ensure stable and efficient operation.
5.2.2 Influence of OLR
The organic loading rate (OLR) indicates the amount of organic material introduced per unit volume of the digester (Ahmad et al., 2021). OLR (measured in kg VS m−3 d−1) is a critical parameter in designing the digestion system, influencing factors such as digester size and type, as it reflects the daily input of volatile solids (VS) or organic dry matter (ODM) per cubic meter of reactor volume. It can be calculated using the formula stated in Equation 11 (Adnane et al., 2024).
where Q is the daily feedstock flow rate, VS is the influent volatile solids concentration, and V is the reactor volume. Higher OLR values can enhance biogas production, reduce heating energy needs, and lower the required reactor size, but overloading may cause VFA accumulation and acidification, risking reactor failure. Excessive OLR can also impair heat transfer, cause uneven mixing, and damage circulating pumps beyond their capacity (Uddin and Wright, 2023).
Notably, co-digesting pig manure with sugar beet byproduct at an OLR of 11.2 gVS/L/day under mesophilic conditions achieved the highest methane productivity within 6 days (Adnane et al., 2024).
5.2.3 Influence of temperature
Temperature is a fundamental factor influencing microbial activity in anaerobic digestion. Two primary operating temperature ranges, namely mesophilic (35–40 °C) and thermophilic (55–60 °C), are recognized for optimal microbial performance (Gaby et al., 2017; Uddin and Wright, 2023). Thermophilic digestion accelerates feed degradation, reduces hydraulic retention time (HRT), and enhances biogas production, but it tends to be less stable and demands additional energy input. In contrast, mesophilic systems operate at moderate temperatures, offering greater stability and lower operational costs, making them the most commonly used in commercial applications (Uddin and Wright, 2023).
AD can also occur under psychrophilic conditions (<20 °C), which are suitable for cold environments and are energy-efficient due to the absence of heating requirements (Adnane et al., 2024). Higher temperatures generally lead to faster reaction kinetics, pathogen deactivation, and increased solubility of organic matter, resulting in higher biogas yields, up to 41% more than cryophilic conditions. However, thermophilic processes are more susceptible to environmental disturbances and NH3 or volatile fatty acid (VFA) accumulation, potentially inhibiting microbial activity (Adnane et al., 2024). Integrating optimal temperatures with an appropriate C/N ratio and pH mitigates NH3 toxicity, especially in co-digestion systems.
Overall, mesophilic digestion is favored for its balance of stability, efficiency, and lower sensitivity to inhibitors, while psychrophilic systems are advantageous in cold climates with minimal heating costs (Adnane et al., 2024).
5.2.4 Influence of C/N ratio
The carbon-to-nitrogen (C/N) ratio reflects the balance between organic (energy source) and nitrogen (growth/metabolism) in the substrate, serving as an important indicator of nutrient availability for AD. A low C/N ratio indicates higher nitrogen content, which can lead to increased NH3 production, raising the pH and inhibiting microbial activity (Induchoodan et al., 2022). Conversely, a high C/N ratio suggests nitrogen deficiency, resulting in suppressed microbial metabolism and reduced biogas production (BGP) (Induchoodan et al., 2022; Uddin and Wright, 2023).
Optimal C/N ratios for efficient AD are generally reported between 20 and 30 (Rajlakshmi et al., 2023), with 25 often cited as ideal (Klassen et al., 2016). Higher ratios above 35 can cause low CH4 yields and NH3 buildup, impairing methanogenic bacteria due to nitrogen deficiency. Conversely, very low ratios can be lethal to microbes, disrupting the digestion process. Combining carbon-rich and nitrogen-rich substrates in AD is a common strategy to achieve a balanced C/N ratio, enhance biogas yield, and improve overall process efficiency (Ajeej et al., 2015).
5.2.5 Influence of HRT
Hydraulic retention time (HRT) refers to the average duration that feedstock (both solids and liquids) remains within a digester, ensuring sufficient contact with microbial communities for effective digestion (Uddin and Wright, 2023). To achieve optimal biogas production (BGP), substrates must stay in the reactor long enough to allow complete breakdown processes (Shi et al., 2017; Uddin and Wright, 2023).
In continuous flow systems like continuously stirred tank reactors (CSTRs), where there is no recycling, the HRT equals the sludge retention time (SRT). When influent streams contain high solids content, longer HRTs are necessary to maximize biogas yields and process efficiency (Khanal, 2008). Essentially, HRT indicates the treatment duration, with longer times enabling better contaminant removal due to increased microbial contact. This leads to higher degradation efficiency and improved energy recovery from the waste (Sawyerr et al., 2019).
5.2.6 Influence of substrates/nutrients
The biochemical and nutrient composition of substrates is a critical factor influencing the performance, biogas output, and stability of AcoD and any other anaerobic digestion processes. Selecting suitable (co-)substrates depends largely on their nutrient profiles, with protein-rich wastes from animal manure, meat processing, and slaughterhouses offering high CH4 potential due to their elevated nitrogen content (Adnane et al., 2024). However, microbial breakdown of these substrates can lead to NH3 accumulation, which, at levels above 4 g/L, can inhibit methanogenic activity and destabilize the process (Adnane et al., 2024).
Incorporating carbohydrate-rich substrates like lignocellulosic residues and food waste is an effective strategy to mitigate NH3-related issues. Lipid-rich materials, such as slaughterhouse waste and effluents from oil industries, have higher theoretical methane yields but pose challenges due to the production of long-chain fatty acids (LCFAs) (Abomohra et al., 2022). Excessive LCFAs can hinder methanogenesis, cause operational problems like clogging, and reduce overall process efficiency, making co-digestion a valuable approach to balance nutrient content and minimize inhibitory effects (Adnane et al., 2024).
5.2.7 Influence of stirring or mixing
Stirring or mixing plays a key physical parameter in anaerobic digestion, significantly influencing biogas production efficiency. It promotes consistent and intimate contact between microorganisms, nutrients, and the organic substrate, enhancing microbial activity (Adnane et al., 2024). Additionally, stirring helps release trapped gas bubbles, improving gas flow and collection. It also prevents the formation of scum, sediments, crusts, and foam, maintaining optimal reactor conditions. Furthermore, stirring dilutes toxic compounds within the digester, reducing inhibitory effects (Adnane et al., 2024). Overall, it ensures a uniform environment, supporting the optimal growth and function of anaerobic bacteria.
6 Anaerobic co-digestion
Anaerobic co-digestion (AcoD) of animal manure and other substrates has been shown to increase BGP. This has been attributed to increased volatile solids concentration, substrate degradability, optimal pH, provision of microbial nutrients and anaerobic microorganisms, and inhibition of chemical toxicity, such as NH3 production (Tawfik et al., 2023; Kunatsa and Xia, 2022; Kunatsa et al., 2022). Co-digestion with poultry manure has been shown to enhance the C/N ratio and provision of methanogenic microorganisms, thereby leading to increased methane production (Tawfik et al., 2023; Minale and Worku, 2014). This is advantageous to FWs as they are low in nitrogen (Odejobi et al., 2023). When co-digested with FWs, poultry manure increases the amount of hydrogenotrophic methanogens, such as Methanoculleus and Methanothermobacter, which enhance AD and contribute to increased BGP (Tawfik et al., 2023).
Table 3 provides a summary of research findings regarding the composition, volume, and quantity of biogas produced through PM and FW co-digestion.
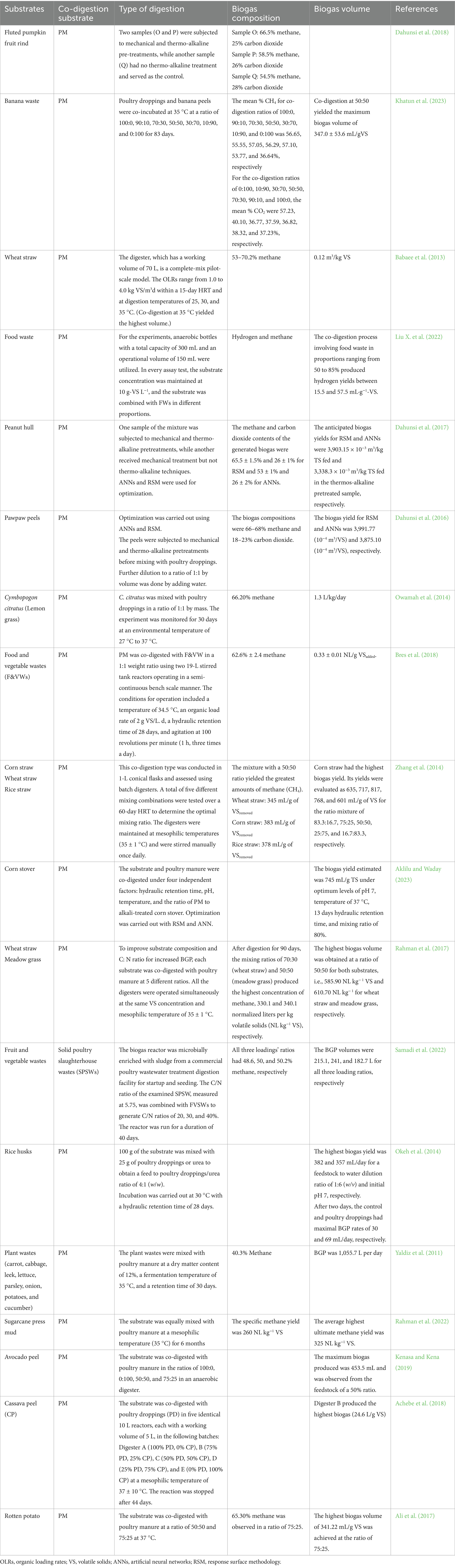
Table 3. Summary of studies on biogas composition and volumes generated from PM and FW co-digestion.
6.1 Pros and cons of anaerobic digestion
The widespread use of AD methods offers a wide range of advantages, which can be summarized as follows (Reith et al., 2003):
• Energy production: AD processes generate an energy source through CH4 production.
• Low energy consumption: AD technology typically requires minimal energy. At room temperature, the energy needs range from 0.05 to 0.1 kWh per cubic meter (equivalent to 0.18–0.36 MJ/m3), largely dependent on pumping and effluent recycling requirements.
• Reduced solid waste: The process significantly decreases the volume of solids that must be managed. The excess sludge produced during AD is considerably lower than that from aerobic methods when based on biodegradable chemical oxygen demand (COD).
• Simplified dewatering: Sludge dewatering is facilitated through anaerobic processes.
• Stabilization of raw waste: This treatment stabilizes raw waste effectively.
• Minimal odor: The end products of AD are relatively free of odors.
• Nutrient retention: There is nearly complete retention of crucial nutrients, such as phosphorus, nitrogen, and potassium.
• High loading capacity: Advanced AD systems are capable of handling substantial organic loads, with capacities 30 g COD/L/day at 30 °C and 50 g COD/L/day at 40 °C, particularly for medium-strength, primarily soluble wastewater.
• Long-term sludge preservation: Anaerobic sludge can be stored for extended periods without the need for feeding.
• Lower construction costs: The costs associated with building anaerobic treatment facilities are comparatively low.
• Compact footprint: Anaerobic systems require less space than traditional treatment systems.
However, there are also certain drawbacks associated with AD processes, as stated below.
• Sensitivity to chemicals: Methanogenic bacteria, which are crucial to the process, are highly sensitive to various chemical substances. While some anaerobic organisms can adapt to these chemicals, this sensitivity poses challenges.
• Time-consuming start-up: Initiating an anaerobic treatment system without adequate seed sludge can be slow due to the slow growth rates of anaerobic bacteria.
• Odor issues with sulfur compounds: When treating wastewater containing sulfurous compounds, the process can produce odors due to sulfide formation. A widely adopted approach to address this problem is the incorporation of a microaerophilic post-treatment phase, which facilitates the conversion of sulfide into elemental sulfur.
6.2 Pros and cons of anaerobic co-digestion
The major advantages of AcoD over the single-substrate AD are stated below (Kunatsa and Xia, 2022).
• Enhanced biogas production: AcoD often results in increased CH4 yields due to synergistic effects among different substrates (co-substrates). This thus enhances energy recovery efficiency.
• Improved process stability: AcoD promotes consistent operation by balancing the C/N ratio (nutrient profile) and supplementing essential trace elements, ensuring a stable microbial environment.
• Reduction of inhibitory substances: AcoD dilutes toxic compounds, such as NH3, lignin derivatives, long-chain fatty acids, and heavy metals, to levels below inhibitory thresholds, thereby safeguarding microbial activity.
• Broader substrate flexibility: AcoD enables the utilization of various organic wastes, such as food waste and manure, promoting waste valorization and reducing disposal challenges.
• Nutrient balance optimization: AcoD addresses nutrient deficiencies or imbalances typical in mono-digestion, leading to more efficient and stable digestion processes.
• Increased organic loading: Combining multiple substrates allows for higher concentrations of biodegradable organic matter, which boosts overall biogas production potential.
• Production of nutrient-rich digestate: The resulting co-digestate from the AcoD process is rich in nutrients, making it suitable for agricultural applications and contributing to sustainable nutrient recycling or waste management.
• Improved process resilience: As mentioned earlier, the diverse nutrient profile mitigates inhibitory effects and supports robust microbial activity, ensuring consistent digestion performance.
Although AcoD offers numerous advantages, it also presents certain limitations, which are summarized below (Kunatsa and Xia, 2022; Sembera et al., 2019).
• Complexity of process management: Managing multiple substrates can complicate process control and monitoring of AcoD.
• Potential for inhibitory interactions: Certain substrate combinations may produce inhibitory compounds or imbalances if not properly managed.
• Accumulation of solids within the digester: Solids accumulation from indigestible materials like glass and metals that may be present in food waste (FW) reduces digester volume and shortens hydraulic retention time (HRT), leading to decreased CH4 production, system clogging, equipment damage, and lower digestate quality.
• Decline in HRT: Reduced HRT due to solids buildup hampers treatment efficiency, affects digestate dewaterability, and compromises CH4 recovery and sludge stabilization.
• Nitrogen accumulation: High protein co-substrates, such as FW and dairy byproducts, can cause nitrogen backload, increase ammonium levels, and risk free-ammonia inhibition.
• Digestate sludge dewatering and drying: Shortened HRT also results in higher volumes of solids that require drying and disposal, increasing operational costs.
• Economic and logistical challenges: Variability in substrate availability and the need for pre-treatment can increase operational complexity and costs.
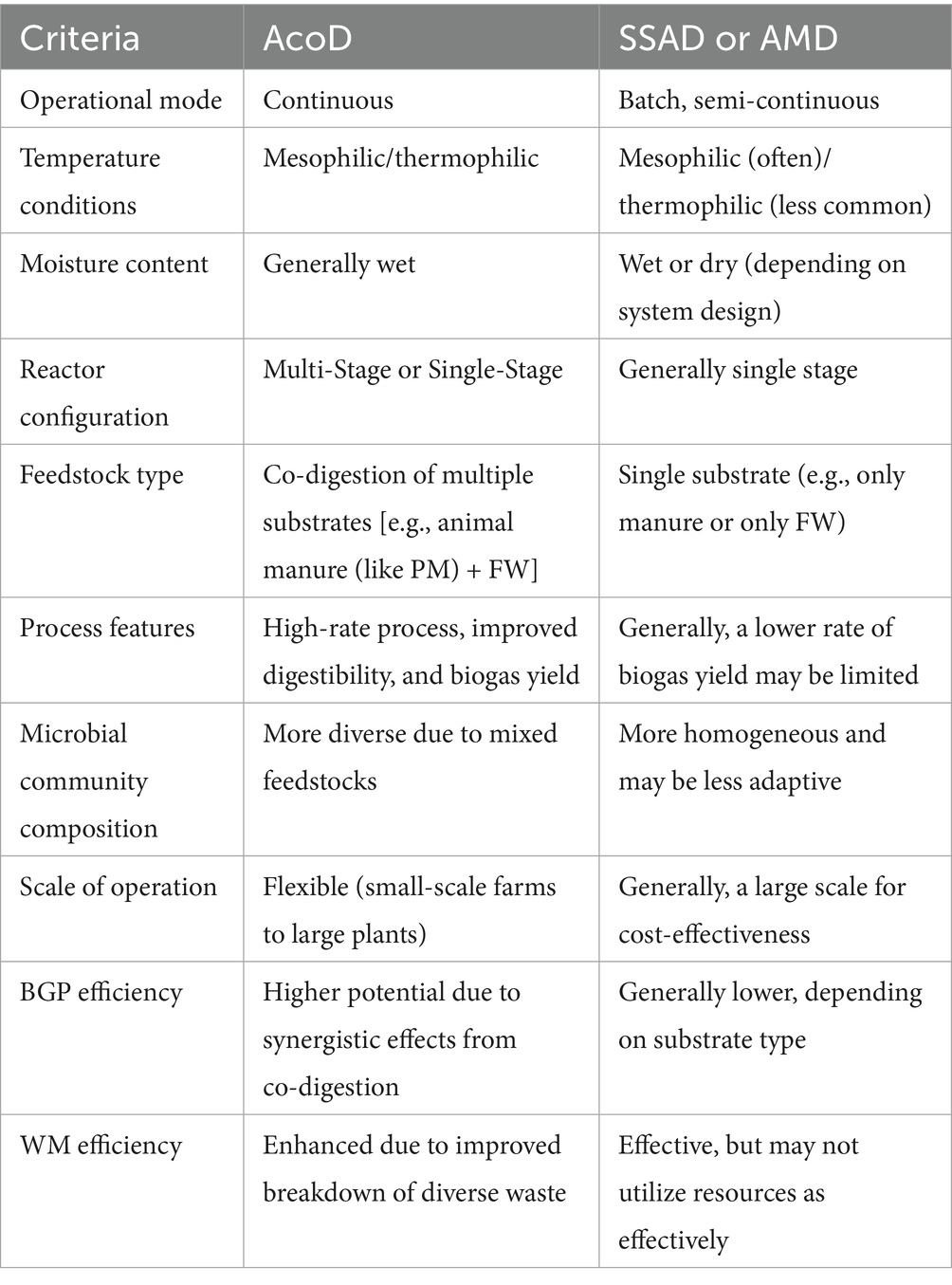
6.3 Comparison of AcoD with other forms of AD
Anaerobic co-digestion (AcoD) stands out from other forms of AD, such as single-substrate AD (SSAD) or AMD, in several key aspects toward WM strategies and BGP. These aspects include continuous operational mode, which maximizes the efficiency of processing multiple substrates simultaneously, unlike traditional methods (like AMD), which operate in batch or semi-continuous modes that restrict throughput. Additionally, AcoD can adapt to both mesophilic and thermophilic temperature conditions, while thriving on wet feedstocks, and its flexible reactor configuration allows for either single-stage or multi-stage designs to enhance biogas yields. In contrast, some conventional systems, such as AMD, focus narrowly on SSD. Furthermore, AcoD’s distinctive microbial community composition benefits from the diverse organic sources it processes, often resulting in a higher BGP rate compared to low-rate conventional AD (or AMD), and it can be implemented effectively at both small and large scales, making it a versatile choice for optimizing BGP across different applications.
Table 4 highlights the comparable features between AcoD and other forms of AD, especially SSAD or AMD, using animal manure, such as PM, and FWs for WM and BGP.
According to data from various studies (Kunatsa and Xia, 2022), as summarized in Table 5, co-digestion substrates tend to yield greater biogas production than mono-digestion processes. Combining lignocellulosic wastes with animal manure can mitigate the challenges associated with their recalcitrance by utilizing the native microbial populations in manure, thereby boosting biogas output. The selection of different substrate combinations and mixing ratios influences both the volume of biogas produced and methane concentrations. Therefore, further research is essential to identify optimal feedstock combinations and blend ratios to maximize biogas yields.
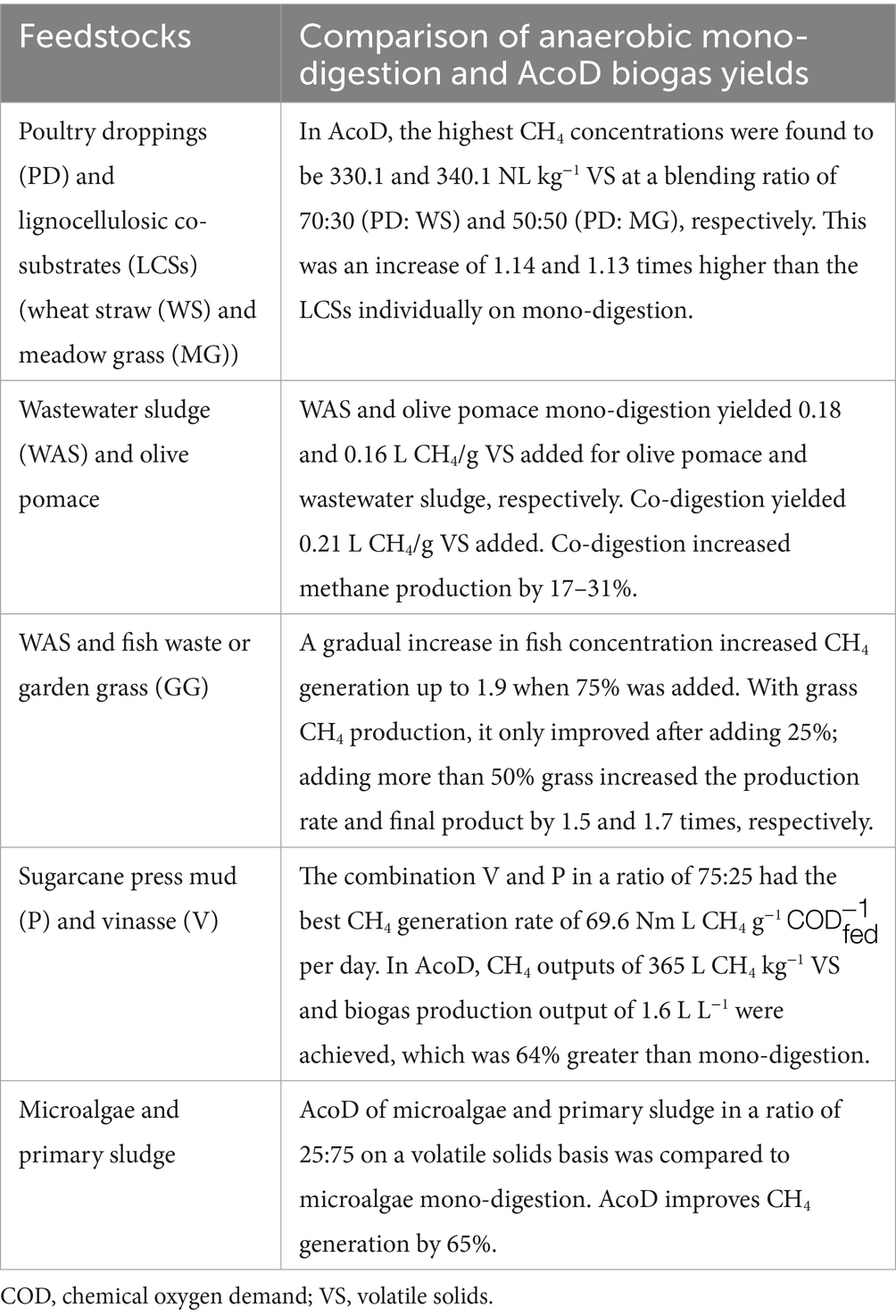
Table 5. Data from various studies on the AcoD effects on biogas yield (Kunatsa and Xia, 2022).
7 Challenges and future directions
7.1 Challenges
Anaerobic digestion processes, especially AcoD of poultry manure and food wastes (PM & FWs), face challenges that can hinder BGP. Variability in the composition and quality of these wastes affects production efficiency. Moreover, optimizing conditions like pH, temperature, and carbon-to-nitrogen (C/N) ratio for diverse waste mixtures is complicated. The elevated nitrogen and ammonia levels in PM can also inhibit microbial activity, reducing biogas yields.
Additionally, the high initial setup and operational costs of AcoD systems pose economic challenges, limiting their broader adoption and commercial viability in the renewable energy sector.
7.2 Future directions
Future advancements in AcoD of PM&FWs for BGP require several strategic directions. Establishing standardized protocols for different waste mixtures will help tackle existing challenges in waste-to-energy initiatives. Further, research should prioritize biotechnology innovations, particularly the use of genetically engineered microbes to boost digestion efficiency. Investments in affordable reactor designs could enhance the feasibility of small-scale operations, fostering broader participation. Life Cycle Assessments (LCAs) are necessary to assess the ecological benefits of the process.
Furthermore, an extensive review of the techno-economic analysis is crucial to evaluate the process’s viability from both technical and economic standpoints. It is crucial to encourage policymakers to advocate for or support waste-to-energy initiatives. By implementing these strategies, the overall effectiveness and adoption of BGP from organic waste can be improved, contributing to greater environmental sustainability and optimal resource utilization.
8 Conclusion
BGP from agro-food biomass (AFB) residues or wastes via AcoD serves as a promising and innovative alternative bioenergy source compared to AMD, with this review emphasizing the significant advantages and efficiency of AcoD of PM and FWs. AcoD not only aids in generating clean energy, thereby diminishing dependence on fossil fuels and associated costs, but it also plays a crucial role in mitigating environmental pollution, leading to economic benefits and improved public health. This method responds effectively to the urgent need for healthy diets by converting waste into energy while simultaneously tackling critical challenges such as food insecurity and environmental degradation. Ultimately, this narrative review underscores the significant potential of integrating PM&FWs into a sustainable approach that can foster energy-resilient communities and promote overall sustainability.
Author contributions
AA: Conceptualization, Methodology, Writing – original draft. TM: Funding acquisition, Supervision, Writing – review & editing. CR: Writing – review & editing. IM: Project administration, Writing – review & editing. JN: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This review is based on a research project that received funding from the National Research Foundation (NRF) under Grant Number 138093, awarded to TM, as well as from the DSI and the TIA under Grant Number 2022/FUN252/AA, also awarded to TM.
Acknowledgments
We extend our gratitude to the Department of Sustainable Food Systems and Development at the University of the Free State, as well as the Centre for Competence in Environmental Biotechnology at the University of South Africa (UNISA), for their ongoing support. Additionally, we acknowledge the National Research Foundation (NRF) of South Africa (NRF Grant Number: 138093, awarded to TM), the Department of Science and Innovation (DSI) of South Africa, and the Technology Innovation Agency (TIA) for funding the research project related to this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abomohra, A., Faisal, S., Ebaid, R., Huang, J., Wang, Q., and Elsayed, M. (2022). Recent advances in anaerobic digestion of lipid-rich waste: challenges and potential of seaweeds to mitigate the inhibitory effect. Chem. Eng. J. 449:137829. doi: 10.1016/j.cej.2022.137829
Achebe, C. H., Onokpite, E., and Onokwai, A. O. (2018). Anaerobic digestion and co-digestion of poultry droppings (Pd) and cassava peels (Cp): comparative study of optimal biogas production. J. Eng. Appl. Sci. 12, 87–93. doi: 10.13140/RG.2.2.18134.10561/1
Adnane, I., Taoumi, H., Elouahabi, K., Lahrech, K., and Oulmekki, A. (2024). Valorization of crop residues and animal wastes: anaerobic co-digestion technology. Heliyon 10:e26440. doi: 10.1016/j.heliyon.2024.e26440
Ahlberg-Eliasson, K., Westerholm, M., Isaksson, S., and Schnürer, A. (2021). Anaerobic digestion of animal manure and influence of organic loading rate and temperature on process performance, microbiology, and methane emission from digestates. Front. Energy Res. 9:740314. doi: 10.3389/fenrg.2021.740314
Ahmad, I., Abdullah, N., Chelliapan, S., Krishnan, S., Koji, I., and Yuzir, A. (2021). Effect of organic loading rate on the performance of modified anaerobic baffled reactor treating landfill leachate containing heavy metals. Mater Today Proc 46, 1913–1921. doi: 10.1016/j.matpr.2021.02.027
Ajeej, A., Thanikal, J. V., Narayanan, C., and Kumar, R. S. (2015). An overview of bio augmentation of methane by anaerobic co-digestion of municipal sludge along with microalgae and waste paper. Renew. Sust. Energ. Rev. 50, 270–276. doi: 10.1016/j.rser.2015.04.121
Aklilu, E. G., and Waday, Y. A. (2023). Optimizing the process parameters to maximize biogas yield from anaerobic co-digestion of alkali-treated corn Stover and poultry manure using artificial neural network and response surface methodology. Biomass Convers. Biorefinery 13, 12527–12540. doi: 10.1007/s13399-021-01966-0
Alhassan, K. A., Abdullahi, B. T., and Shah, M. M. (2019). A review on biogas production as the alternative source of fuel. J. Appl. Adv. Res. 4, 61–65. doi: 10.21839/jaar.2019.v4i2.266
Ali, S., Shah, T. A., Afzal, A., and Tabbassum, R. (2017). Evaluating the co-digestion effects on chicken manure and rotten potatoes in batch experiments. Int. J. Biosci. 10, 150–159. doi: 10.12692/ijb/10.6.150-159
Arif, S., Liaquat, R., and Adil, M. (2018). Applications of materials as additives in anaerobic digestion technology. Renew. Sust. Energ. Rev. 97, 354–366. doi: 10.1016/j.rser.2018.08.039
Awe, O. W., Zhao, Y., Nzihou, A., Minh, D. P., and Lyczko, N. (2017). A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valoriz. 8, 267–283. doi: 10.1007/s12649-016-9826-4
Babaee, A., Shayegan, J., and Roshani, A. (2013). Anaerobic slurry co-digestion of poultry manure and straw: effect of organic loading and temperature. J. Environ. Health Sci. Eng. 11, 1–6. doi: 10.1186/2052-336X-11-15
Bajželj, B. (2015). Why we need to address food demand. Cambridge, England; University of Cambridge.
Biermann, F., Kanie, N., and Kim, R. E. (2017). Global governance by goal-setting: the novel approach of the UN sustainable development goals. Curr. Opin. Environ. Sustain. 26, 26–31. doi: 10.1016/j.cosust.2017.01.010
Bres, P., Beily, M. E., Young, B. J., Gasulla, J., Butti, M., Crespo, D., et al. (2018). Performance of semi-continuous anaerobic co-digestion of poultry manure with fruit and vegetable waste and analysis of digestate quality: a bench scale study. Waste Manag. 82, 276–284. doi: 10.1016/j.wasman.2018.10.041
Cantera, S., Muñoz, R., Lebrero, R., López, J. C., Rodríguez, Y., and García-Encina, P. A. (2018). Technologies for the bioconversion of methane into more valuable products. Curr. Opin. Biotechnol. 50, 128–135. doi: 10.1016/j.copbio.2017.12.021
Carotenuto, C., Guarino, G., D’amelia, L. I., Morrone, B., and Minale, M. (2020). The peculiar role of C/N and initial pH in anaerobic digestion of lactating and non-lactating water buffalo manure. Waste Manag. 103, 12–21. doi: 10.1016/j.wasman.2019.12.008
Caruso, M. C., Braghieri, A., Capece, A., Napolitano, F., Romano, P., Galgano, F., et al. (2019). Recent updates on the use of agro-food waste for biogas production. Appl. Sci. 9:1217. doi: 10.3390/app9061217
Chen, X. Y., Vinh-Thang, H., Ramirez, A. A., Rodrigue, D., and Kaliaguine, S. (2015). Membrane gas separation technologies for biogas upgrading. RSC Adv. 5, 24399–24448. doi: 10.1039/C5RA00666J
Chomini, M. S., Peter, M. K., Ayodele, A. O., and Mazeli, P. (2019). Effects of anaerobic co-digestion of organic wastes on biogas yield and some proximate characteristics of their by- products. Eur. J. Eng. Technol. 7, 57–67. doi: 10.17660/th2019/070713916
Cronjé, N., Van Der Merwe, I., and Müller, I.-M. (2018). Household food waste: a case study in Kimberley, South Africa. J. Consum. Sci. 46:1–9. doi: 10.10520/EJC-15f4ff1682
Czekała, W., Nowak, M., and Bojarski, W. (2023). Characteristics of substrates used for biogas production in terms of water content. Fermentation 9:449. doi: 10.3390/fermentation9050449
Dahunsi, S., Oranusi, S., and Efeovbokhan, V. (2017). Pretreatment optimization, process control, mass and energy balances and economics of anaerobic co-digestion of Arachis hypogaea (Peanut) hull and poultry manure. Bioresour. Technol. 241, 454–464. doi: 10.1016/j.biortech.2017.05.152
Dahunsi, S. O., Oranusi, S., Efeovbokhan, V. E., Zahedi, S., Ojediran, J. O., Olayanju, A., et al. (2018). Biochemical conversion of fruit rind of Telfairia occidentalis (fluted pumpkin) and poultry manure. Energy Sources Part A Recover Util. Environ. Eff. 40, 2799–2811. doi: 10.1080/15567036.2018.1511651
Dahunsi, S., Oranusi, S., Owolabi, J., and Efeovbokhan, V. (2016). Mesophilic anaerobic co-digestion of poultry dropping and Carica papaya peels: modelling and process parameter optimization study. Bioresour. Technol. 216, 587–600. doi: 10.1016/j.biortech.2016.05.118
De Moraes, C. C., De Oliveira Costa, F. H., Pereira, C. R., Da Silva, A. L., and Delai, I. (2020). Retail food waste: mapping causes and reduction practices. J. Clean. Prod. 256:120124. doi: 10.1016/j.jclepro.2020.120124
Dobre, P., Nicolae, F., and Matei, F. (2014). Main factors affecting biogas production-an overview. Rom. Biotechnol. Lett. 19, 9283–9296. doi: 10.1016/j.rser.2014.10.055
Dróżdż, D., Wystalska, K., Malińska, K., Grosser, A., Grobelak, A., and Kacprzak, M. (2020). Management of poultry manure in Poland–current state and future perspectives. J. Environ. Manag. 264:110327. doi: 10.1016/j.jenvman.2020.110327
Enzmann, F., Mayer, F., Rother, M., and Holtmann, D. (2018). Methanogens: biochemical background and biotechnological applications. AMB Express 8:1. doi: 10.1186/s13568-017-0531-x
Esparza, I., Jiménez-Moreno, N., Bimbela, F., Ancín-Azpilicueta, C., and Gandía, L. M. (2020). Fruit and vegetable waste management: conventional and emerging approaches. J. Environ. Manag. 265:110510. doi: 10.1016/j.jenvman.2020.110510
Felton, G., Lansing, S., Moss, A., and Klavon, K. (2014). Anaerobic digestion: basic processes for biogas production. Fact sheet. Maryland, USA; University of Maryland.
Feng, K., Wang, Q., Li, H., Du, X., and Zhang, Y. (2021). Microbial mechanism of enhancing methane production from anaerobic digestion of food waste via phase separation and pH control. J. Environ. Manag. 288:112460. doi: 10.1016/j.jenvman.2021.112460
Food and Nations, A. O. O. T. U. (2012). “Towards the future we want: End hunger and make the transition to sustainable agricultural and food systems” in Policy paper for the Rio+ 20 Conference 13 to 22 June 2012 (Rome: FAO).
Gaby, J. C., Zamanzadeh, M., and Horn, S. J. (2017). The effect of temperature and retention time on methane production and microbial community composition in staged anaerobic digesters fed with food waste. Biotechnol. Biofuels 10, 1–13. doi: 10.1186/s13068-017-0989-4
Ganesh, K. S., Sridhar, A., and Vishali, S. (2022). Utilization of fruit and vegetable waste to produce value-added products: conventional utilization and emerging opportunities-a review. Chemosphere 287:132221. doi: 10.1016/j.chemosphere.2021.132221
Goswami, R., Chattopadhyay, P., Shome, A., Banerjee, S. N., Chakraborty, A. K., Mathew, A. K., et al. (2016). An overview of physico-chemical mechanisms of biogas production by microbial communities: a step towards sustainable waste management. 3 Biotech 6:72. doi: 10.1007/s13205-016-0395-9
Gould, M. C. (2015). “Bioenergy and anaerobic digestion” in Bioenergy, Amsterdam, Netherlands: Elsevier 297–317.
Hendriks, A., Van Lier, J., and De Kreuk, M. (2018). Growth media in anaerobic fermentative processes: the underestimated potential of thermophilic fermentation and anaerobic digestion. Biotechnol. Adv. 36, 1–13. doi: 10.1016/j.biotechadv.2017.08.004
Holm-Nielsen, J. B., Al Seadi, T., and Oleskowicz-Popiel, P. (2009). The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 100, 5478–5484. doi: 10.1016/j.biortech.2008.12.046
Hou, Y., Velthof, G. L., Lesschen, J. P., Staritsky, I. G., and Oenema, O. (2017). Nutrient recovery and emissions of ammonia, nitrous oxide, and methane from animal manure in Europe: effects of manure treatment technologies. Environ. Sci. Technol. 51, 375–383. doi: 10.1021/acs.est.6b04524
Ignatowicz, K., Filipczak, G., Dybek, B., and Wałowski, G. (2023). Biogas production depending on the substrate used: a review and evaluation study—European examples. Energies 16:798. doi: 10.3390/en16020798
Induchoodan, T., Haq, I., and Kalamdhad, A. S. (2022). Factors affecting anaerobic digestion for biogas production: a review. Adv. Organic Waste Manag., 223–233. doi: 10.1016/B978-0-323-85792-5.00020-4
Inna, S., Henriette, A. Z., Boukar, H., Cornelius, T., Cârâc, G., Mihaela, R. D., et al. (2022). Compositional characteristics and theoretical energy potential of animal droppings from Adamawa region of Cameroon. Biomass Convers. Biorefinery, 1–13. doi: 10.1007/s13399-022-03320-4
Islam, M. R., Wang, Q., Guo, Y., Wang, W., Sharmin, S., and Ebere Enyoh, C. (2023). Physico-chemical characterization of food wastes for potential soil application. PRO 11:250. doi: 10.3390/pr11010250
Kadam, R., Jo, S., Lee, J., Khanthong, K., Jang, H., and Park, J. (2024). A review on the anaerobic co-digestion of livestock manures in the context of sustainable waste management. Energies 17:546. doi: 10.3390/en17030546
Kenasa, G., and Kena, E. (2019). Optimization of biogas production from avocado fruit peel wastes co-digestion with animal manure collected from juice vending house in Gimbi town, Ethiopia. Ferment. Technol. 8, 1–6. doi: 10.4172/2167-7972.1000153
Khanal, S. K. (2008). Anaerobic biotechnology for bioenergy production. Principles and application. Ames, Iowa: Willey and Blackwell, 161–186.
Khatun, M. L., Nime, J., Nandi, R., Alam, M. M., and Saha, C. K. (2023). Co-digestion of poultry droppings and banana waste for maximizing biogas production in Bangladesh. Fuel 346:128346. doi: 10.1016/j.fuel.2023.128346
Klassen, V., Blifernez-Klassen, O., Wobbe, L., Schlüter, A., Kruse, O., and Mussgnug, J. H. (2016). Efficiency and biotechnological aspects of biogas production from microalgal substrates. J. Biotechnol. 234, 7–26. doi: 10.1016/j.jbiotec.2016.07.015
Kovács, E., Wirth, R., Maróti, G., Bagi, Z., Nagy, K., Minárovits, J., et al. (2015). Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour. Technol. 178, 254–261. doi: 10.1016/j.biortech.2014.08.111
Kreuger, E., Nges, I. A., and Björnsson, L. (2011). Ensiling of crops for biogas production: effects on methane yield and total solids determination. Biotechnol. Biofuels 4, 1–8. doi: 10.1186/1754-6834-4-44
Kunatsa, T., and Xia, X. (2022). A review on anaerobic digestion with focus on the role of biomass co-digestion, modelling and optimisation on biogas production and enhancement. Bioresour. Technol. 344:126311. doi: 10.1016/j.biortech.2021.126311
Kunatsa, T., Zhang, L., and Xia, X. (2022). Biogas potential determination and production optimisation through optimal substrate ratio feeding in co-digestion of water hyacinth, municipal solid waste and cow dung. Biofuels 13, 631–641. doi: 10.1080/17597269.2020.1835452
Laçın, K., Çaloğlu, B., and Binay, B. (2023). “Anaerobic digestion methods for the production of fuels” in Bioenergy engineering (Amsterdam, Netherlands. Elsevier).
Lakshmi, V., Aruna Devi, D., and Jhansi Rani, K. (2020). “Wealth from poultry waste” in Waste management as economic industry towards circular economy, London, United Kingdom: Springer Nature. 67–76.
Latif, M. A., Mehta, C. M., and Batstone, D. J. (2017). Influence of low pH on continuous anaerobic digestion of waste activated sludge. Water Res. 113, 42–49. doi: 10.1016/j.watres.2017.02.002
Liebetrau, J., O’shea, R., Wellisch, M., Lyng, K.-A., Bochmann, G., Mccabe, B. K., et al. (2021). Potential and utilization of manure to generate biogas in seven countries. Iea Bioenergy Task 6, 1–53. doi: 10.17028/rd.searche.7354445.v1
Liotta, F. (2013). Bio-methanation tests and mathematical modelling to assess the role of moisture content on anaerobic digestion of organic waste. Cassino, Italy: Université Paris-Est.
Liu, D., Liu, D., Zeng, R. J., and Angelidaki, I. (2006). Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res. 40, 2230–2236. doi: 10.1016/j.watres.2006.03.029
Liu, X., Yang, Y., Wu, N., Wei, Y., Shan, H., and Zhao, H. (2022). Co-production of biohydrogen and biomethane from chicken manure and food waste in a two-stage anaerobic fermentation process. Appl. Biochem. Biotechnol. 194, 3706–3720. doi: 10.1007/s12010-022-03945-1
Liu, R., Zhang, K., Chen, X., and Xiao, B. (2022). Effects of substrate organic composition on mesophilic and thermophilic anaerobic co-digestion of food waste and paper waste. Chemosphere 291:132933. doi: 10.1016/j.chemosphere.2021.132933
Lynch, D., Henihan, A. M., Bowen, B., Lynch, D., Mcdonnell, K., Kwapinski, W., et al. (2013). Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 49, 197–204. doi: 10.1016/j.biombioe.2012.12.009
Maj, I. (2022). Significance and challenges of poultry litter and cattle manure as sustainable fuels: a review. Energies 15:8981. doi: 10.3390/en15238981
Markoš, J. (2011). Mass transfer in chemical engineering processes. Rijeka, Croatia: BoD–Books on Demand.
Menzel, T., Neubauer, P., and Junne, S. (2020). Role of microbial hydrolysis in anaerobic digestion. Energies 13:5555. doi: 10.3390/en13215555
Minale, M., and Worku, T. (2014). Anaerobic co-digestion of sanitary wastewater and kitchen solid waste for biogas and fertilizer production under ambient temperature: waste generated from condominium house. Int. J. Environ. Sci. Technol. 11, 509–516. doi: 10.1007/s13762-013-0255-7
Mrosso, R., Mecha, A. C., and Kiplagat, J. (2023). Characterization of kitchen and municipal organic waste for biogas production: effect of parameters. Heliyon 9:e16360. doi: 10.1016/j.heliyon.2023.e16360
Muduli, S., Champati, A., Popalghat, H. K., Patel, P., and Sneha, K. (2019). Poultry waste management: an approach for sustainable development. Int. J. Adv. Sci. Res. 4, 8–14.
Náthia-Neves, G., Berni, M., Dragone, G., Mussatto, S., and Forster-Carneiro, T. (2018). Anaerobic digestion process: technological aspects and recent developments. Int. J. Environ. Sci. Technol. 15, 2033–2046. doi: 10.1007/s13762-018-1682-2
NE. (2009). Biogas renewable energy [Online]. Naskeo Environnement. Available online at: https://www.biogas-renewable-energy.info/index.html (Accessed July 8 2024).
Nwokolo, N., Mukumba, P., Obileke, K., and Enebe, M. (2020). Waste to energy: a focus on the impact of substrate type in biogas production. PRO 8:1224. doi: 10.3390/pr8101224
Odejobi, O. J., Ajala, O. O., and Osuolale, F. N. (2023). Anaerobic co-digestion of kitchen waste and animal manure: a review of operating parameters, inhibiting factors, and pretreatment with their impact on process performance. Biomass Convers. Biorefinery 13, 5515–5531. doi: 10.1007/s13399-021-01626-3
Okeh, O. C., Onwosi, C. O., and Odibo, F. J. C. (2014). Biogas production from rice husks generated from various rice mills in Ebonyi state, Nigeria. Renew. Energy 62, 204–208. doi: 10.1016/j.renene.2013.07.006
Olugasa, T. T., Odesola, I., and Oyewola, M. (2014). Energy production from biogas: a conceptual review for use in Nigeria. Renew. Sust. Energ. Rev. 32, 770–776. doi: 10.1016/j.rser.2013.12.013
Orhorhoro, E. K., Ebunilo, P. O., and Sadjere, G. E. (2017). Experimental determination of effect of total solid (Ts) and volatile solid (Vs) on biogas yield. Am. J. Mod. Energy 3, 131–135. doi: 10.11648/j.ajme.20170306.13
Owamah, H., Alfa, M., and Dahunsi, S. (2014). Optimization of biogas from chicken droppings with Cymbopogon citratus. Renew. Energy 68, 366–371. doi: 10.1016/j.renene.2014.02.006
Parvin, F., and Tareq, S. M. (2021). Impact of landfill leachate contamination on surface and groundwater of Bangladesh: a systematic review and possible public health risks assessment. Appl Water Sci 11:100. doi: 10.1007/s13201-021-01431-3
Pavi, S., Kramer, L. E., Gomes, L. P., and Miranda, L. A. S. (2017). Biogas production from co-digestion of organic fraction of municipal solid waste and fruit and vegetable waste. Bioresour. Technol. 228, 362–367. doi: 10.1016/j.biortech.2017.01.003
Perin, J. K. H., Borth, P. L. B., Torrecilhas, A. R., Da Cunha, L. S., Kuroda, E. K., and Fernandes, F. (2020). Optimization of methane production parameters during anaerobic co-digestion of food waste and garden waste. J. Clean. Prod. 272:123130. doi: 10.1016/j.jclepro.2020.123130
Persson, M., and Jönsson, O. (2007). Biogas-a renewable fuel for the transport sector for the present and the future. J. Swedish Gas Center. 1–34.
Plazzotta, S., Manzocco, L., and Nicoli, M. C. (2017). Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 63, 51–59. doi: 10.1016/j.tifs.2017.02.013
Pramanik, S. K., Suja, F. B., Zain, S. M., and Pramanik, B. K. (2019). The anaerobic digestion process of biogas production from food waste: prospects and constraints. Bioresour. Technol. Rep. 8:100310. doi: 10.1016/j.biteb.2019.100310
Quiroga, G., Castrillón, L., Fernández-Nava, Y., and Marañón, E. (2010). Physico-chemical analysis and calorific values of poultry manure. Waste Manag. 30, 880–884. doi: 10.1016/j.wasman.2009.12.016
Rahman, M. A., Møller, H. B., Saha, C. K., and Alam, M. M. (2022). The effect of temperature on the anaerobic co-digestion of poultry droppings and sugar mill press mud. Biofuels 13, 139–147.
Rahman, M. A., Møller, H. B., Saha, C. K., Alam, M. M., Wahid, R., and Feng, L. (2017). Optimal ratio for anaerobic co-digestion of poultry droppings and lignocellulosic-rich substrates for enhanced biogas production. Energy Sustain. Dev. 39, 59–66. doi: 10.1080/17597269.2019.1649902
Rajendran, K., Kankanala, H. R., Martinsson, R., and Taherzadeh, M. J. (2014). Uncertainty over techno-economic potentials of biogas from municipal solid waste (Msw): a case study on an industrial process. Appl. Energy 125, 84–92. doi: 10.1016/j.apenergy.2014.03.041
Rajlakshmi, J., Jadhav, D., Dutta, S., Sherpa, K., Jayaswal, K., Saravanabhupathy, S., et al. (2023). “Codi gestion processes of waste: status and perspective” in Bio-based materials and waste for energy generation and resource management. Amsterdam, Neitherlands: Elsevier.
Raven, R. P., and Gregersen, K. H. (2007). Biogas plants in Denmark: successes and setbacks. Renew. Sust. Energ. Rev. 11, 116–132. doi: 10.1016/j.rser.2004.12.002
Reith, J., Wijffels, R. H., and Barten, H. (2003). Bio-methane and bio-hydrogen: status and perspectives of biological methane and hydrogen production.
Rezapoor, P., and Rahimpour, M. R. (2023). Animal waste to energy, technologies, economics, and challenges. Amsterdam, Neitherlands: Elsevier.
Sahoo, A., Sarkar, S., Lal, B., Kumawat, P., Sharma, S., and De, K. (2021). Utilization of fruit and vegetable waste as an alternative feed resource for sustainable and eco-friendly sheep farming. Waste Manag. 128, 232–242. doi: 10.1016/j.wasman.2021.04.050
Samadi, M. T., Leili, M., Rahmani, A., Moradi, S., and Godini, K. (2022). Anaerobic co-digestion using poultry slaughterhouse and vegetable wastes to enhance biogas yield: effect of different C/N ratios. Biomass Convers. Biorefinery, 14:1–9. doi: 10.1007/s13399-022-03501-1
Sarkar, O., Santhosh, J., Dhar, A., and Mohan, S. V. (2021). Green hythane production from food waste: integration of dark-fermentation and methanogenic process towards biogas up-gradation. Int. J. Hydrog. Energy 46, 18832–18843. doi: 10.1016/j.ijhydene.2021.03.053
Sawyerr, N., Trois, C., Workneh, T. S., and Okudoh, V. (2019). An overview of biogas production: fundamentals, applications and future research. Int. J. Energy Econ. Policy 9, 105–116. doi: 10.32479/ijeep.7375
Segrè, A., and Falasconi, L. (2011). Il libro nero dello spreco in Italia: il cibo. Oristano, Italy: Edizioni Ambiente.
Sembera, C., Macintosh, C., Astals, S., and Koch, K. (2019). Benefits and drawbacks of food and dairy waste co-digestion at a high organic loading rate: a Moosburg Wwtp case study. Waste Manag. 95, 217–226. doi: 10.1016/j.wasman.2019.06.008
Shen, F., Yuan, H., Pang, Y., Chen, S., Zhu, B., Zou, D., et al. (2013). Performances of anaerobic co-digestion of fruit & vegetable waste (Fvw) and food waste (Fw): single-phase vs. two-phase. Bioresour. Technol. 144, 80–85. doi: 10.1016/j.biortech.2013.06.099
Shi, X.-S., Dong, J., Yu, J., Yin, H., Hu, S., Huang, S., et al. (2017). Effect of hydraulic retention time on anaerobic digestion of wheat straw in the semicontinuous continuous stirred-tank reactors. Biomed. Res. Int. 2017:1–6. doi: 10.1155/2017/2457805
Shi, X., Guo, X., Zuo, J., Wang, Y., and Zhang, M. (2018). A comparative study of thermophilic and mesophilic anaerobic co-digestion of food waste and wheat straw: process stability and microbial community structure shifts. Waste Manag. 75, 261–269. doi: 10.1016/j.wasman.2018.02.004
Simbolon, L., Pandey, D., Horvat, A., Kwapinska, M., Leahy, J., and Tassou, S. (2019). Investigation of chicken litter conversion into useful energy resources by using low temperature pyrolysis. Energy Procedia 161, 47–56. doi: 10.1016/j.egypro.2019.02.057
Stenmarck, Â., Jensen, C., Quested, T., Moates, G., Buksti, M., Cseh, B., et al. (2016). Estimates of European food waste levels. Wageningen, Netherlands: Ivl Swedish Environmental Research Institute.
Tawfik, A., Eraky, M., Osman, A. I., Ai, P., Zhou, Z., Meng, F., et al. (2023). Bioenergy production from chicken manure: a review. Environ. Chem. Lett. 21, 2707–2727. doi: 10.1007/s10311-023-01618-x
Thi, N. B. D., Kumar, G., and Lin, C.-Y. (2015). An overview of food waste management in developing countries: current status and future perspective. J. Environ. Manag. 157, 220–229. doi: 10.1016/j.jenvman.2015.04.022
Uddin, M. M., and Wright, M. M. (2023). Anaerobic digestion fundamentals, challenges, and technological advances. Phys. Sci. Rev. 8, 2819–2837. doi: 10.1515/psr-2021-0068
Usman, S., Ogbe, K., Oguche, J., Momoh, T., and Omale, S. (2019). Utilization of poultry waste as feed and supplementary feed for fish growth. J. Appl. Sci. Environ. Manag. 23, 627–631. doi: 10.4314/jasem.v23i4.8
Vargas-Estrada, L., Longoria, A., Arenas, E., Moreira, J., Okoye, P. U., Bustos-Terrones, Y., et al. (2022). A review on current trends in biogas production from microalgae biomass and microalgae waste by anaerobic digestion and co-digestion. Bioenergy Res. 15, 77–92. doi: 10.1007/s12155-021-10276-2
Vats, N., Khan, A. A., and Ahmad, K. (2020). Options for enhanced anaerobic digestion of waste and biomass—a review. J. Biosyst. Eng. 45, 1–15. doi: 10.1007/s42853-019-00040-y
WWF-SA. (2017). Food Loss and Waste: Facts and Futures [Online]. WWF-SA. Available online at: https://wwfafrica.awsassets.panda.org/downloads/Wwf_Food_Loss_and_Waste_Web.pdf (Accessed June 2 2024).
Yaldiz, O., Sozer, S., Caglayan, N., Ertekin, C., and Kaya, D. (2011). Methane production from plant wastes and chicken manure at different working conditions of a one-stage anaerobic digester. Energy Sources Part A Recovery Util. Environ. Eff. 33, 1802–1813. doi: 10.1080/15567030903419463
Zhang, T., Yang, Y., Liu, L., Han, Y., Ren, G., and Yang, G. (2014). Improved biogas production from chicken manure anaerobic digestion using cereal residues as co-substrates. Energy Fuel 28, 2490–2495. doi: 10.1021/ef500262m
Keywords: poultry manure, food waste, anaerobic co-digestion, biogas, waste management
Citation: Ayantokun AS, Matambo TS, Rashama C, Van der Merwe I and Van Niekerk JA (2025) A critical review of food waste and poultry manure anaerobic co-digestion: an eco-friendly valorization for sustainable waste management and biogas production. Front. Sustain. Food Syst. 9:1695945. doi: 10.3389/fsufs.2025.1695945
Edited by:
Airton Kunz, EMBRAPA Swine and Poultry, BrazilReviewed by:
Akash Phillip, IIMT University, IndiaIyyadurai Mariappan, Institute of Microbial Technology (CSIR), India
Copyright © 2025 Ayantokun, Matambo, Rashama, Van der Merwe and Van Niekerk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tonderayi S. Matambo, bWF0YW10c0B1bmlzYS5hYy56YQ==
†ORCID: Ayandeji Sunday Ayantokun, http://orcid.org/0000-0003-4163-7094
Tonderayi S. Matambo, http://orcid.org/0000-0001-9432-3772
 Ayandeji Sunday Ayantokun
Ayandeji Sunday Ayantokun Tonderayi S. Matambo
Tonderayi S. Matambo Charles Rashama
Charles Rashama Ismari Van der Merwe1
Ismari Van der Merwe1