- 1State Key Laboratory of Sheep Genetic Improvement and Healthy Breeding, Institute of Animal Husbandry and Veterinary Sciences, Xinjiang Academy of Agricultural and Reclamation Sciences, Shihezi, China
- 2Institute of Animal Sciences (IAS), Chinese Academy of Agricultural Sciences (CAAS), Beijing, China
- 3Shaheed Benazir Bhutto University of Veterinary & Animal Sciences, Sakrand, Sindh, Pakistan
- 4Department of Animal Reproduction & AI Veterinary Research Division, NRC, Cairo, Egypt
Buffalo occupies a leading position as a major livestock commodity and is the primary milk-producing animal in many countries like Italy, China, India, Pakistan, Bangladesh, and Nepal. Buffalo farming emphasizes the significance of effective reproductive strategies. Among effective reproductive strategy, artificial insemination has a significant influence on herd's genetic progress. Nonetheless, buffaloes exhibit unique reproductive behavior, which complicates the insemination process. These animals demonstrate inconsistent periods (ranging from 6–48 h) of mounting acceptance. Therefore, timed artificial insemination (TAI) has surfaced as a useful technique for advancing buffalo breeding initiatives and omits the need for heat detection. TAI enhances reproductive management and genetic progress in buffaloes by synchronizing estrus and optimizing insemination timing. This review focuses on examining buffalo reproductive physiology, particularly emphasizing estrus synchronization protocols, ovulation, and TAI. We also provide a brief description of the factors influencing TAI success, such as hormonal treatments and environmental conditions. This review underscores TAI's importance identifies areas for further research and development and reinforces its central role in sustainable buffalo farming.
1 Introduction
Buffaloes are crucial dairy animals commonly found in warm regions. Over the past 37 years, their global population has notably grown, surpassing 205 million by 2025, 98% of which are found in Asia, 0.7%−0.8% in Africa remarkably in Egypt, 1% and in South America, and Europe 0.2% (1, 2). This short-day seasonal breeder animal shows increased activity as the day length decreases (3). A noticeable factor controlled by melatonin release, in addition to heat stress, is the impact of seasonal breeding patterns on buffalo reproductive activity. However, among female buffaloes, younger animals, such heifers, have a more uniform reproductive function, whereas older animals are more sensitive to such photoperiodic alternance in reproductive efficiency (4). Any disruptions in the reproductive organs that occur at the end of the good reproductive season, between the end of summer and the end of the following winter, and until spring at latitudes above the equator, will actually cause anaetrus in older animals (5). Although melatonin is released all year long, the photoperiod determines how long it lasts, which influences gonadotropin and steroidogenesis, which is a seasonal breeding phenomena in buffaloes (6, 7). By activating its receptors (MTNR1A and MTNR1B) and binding sites in the HPG hypothalamic–pituitarygonadal (HPG) axis, melatonin contributes to sexual development and the restoration of ovarian functions. Melatonin enhances calcium ion influx to GnRH-expressing neurons in GnRH secretion and controls the HPG axis by varying the expression of the gonadotropin gene according to the season (8). This indicates the origin of Buffalo domestication in the Indus Valley (Moen-Jo-Daro) Civilization where calving was synchronized with favorable climatic conditions and abundant food resources marking the early stages of buffalo domestication (9–11). Additionally, while being polyestrous, buffaloes' reproductive effectiveness varies greatly during the year. Buffaloes that give birth during an unfavorable season might not continue ovarian activity until the next advantageous season since buffalo cows show a clear seasonal variation in showing estrus, conception rate, and calving rate (12). When AI is applied outside of the breeding season, the pregnancy rate is impacted by this seasonal reproductive rhythm. The application of AI in buffaloes may increase if hormonal therapies can mitigate some of the challenges associated with seasonality and estrus detection (13). These animals have a unique role in rural livestock farming, particularly in Asia, where their productivity greatly affects the local economy (2, 14), necessitating improvements in reproductive strategies to augment food security, boost farmer income, and foster sustainable rural development (15). Buffaloes add around 13% of the world's milk production (16), with a yearly growth rate exceeding 3.5%, outpacing cow milk production, which has grown by 2.1% (17). India, Pakistan, China, Nepal, and Egypt are the countries with the largest numbers of dairy buffaloes. There are more dairy buffaloes than dairy cows in Nepal and Pakistan (1). Despite buffaloes ability to consume lower-quality food, withstand harsh environments, and resist certain diseases, enhancing the reproductive efficiency of buffalo remains a challenge (18). Silent heat is one of the challenging problems in buffaloes which affects calving intervals due to the failure of the heat detection (19). The efficiency artificial insemination programs in buffalo are highly influenced by heat detection (17).
Artificial insemination is a widely practiced reproductive technique that plays a crucial role in modern livestock breeding programs (20, 21). By enabling the controlled breeding of animals, this technology offers several benefits over natural mating, such as genetic improvement, disease control, and efficient utilization of superior sires (22, 23). In the 1930s, the introduction of AI in cattle raised questions about the optimal timing of insemination (24). Pioneering experiments focused on the ovulation window and insemination timings in dairy cattle demonstrated that the optimal artificial insemination timing is ~12–15 h after the onset of standing heat (25, 26). This groundbreaking discovery established the basis for the AM-PM rule, proposing that a cow detected in standing heat in the morning should undergo artificial insemination in the late afternoon or evening, and conversely, if detected in the evening, insemination should be performed in the morning (27, 28). The AM-PM rule allows adequate time for sperm capacitation and reaching the proper fertilization site in the oviduct (29–31). Given the anatomical and physiological similarities between cattle and water buffalo, AI methods adopted from cattle protocols were applied to water buffalo without proper consideration of ovulation timing (32, 33). The decline in the pregnancy rate due to these practices has decreased the faith of farmers in artificial insemination and modern assisted reproductive technologies (34). Paradoxically, it remains unclear whether the AM-PM rule applies to water buffalo, as this aspect has not yet been systematically investigated (35). Therefore, various protocols to facilitate fixed time artificial insemination (FTAI) have been established to synchronize follicular waves and ovulation within a predetermined timeframe (7, 36).
Extensive research and development concerning estrus and ovulation synchronization in cattle and buffaloes has been conducted, leveraging applications of FTAI (37–39). While extensively applied in cattle breeding its application in buffalo remains limited, particularly in Asia. Factors impacting its efficiency encompass heat stress, heat detection, semen quality, technician expertise, and timing of insemination (34, 40). Brazil's extensive studies on ovulation synchronization regarding FTAI in buffaloes reflect attempts to address estrus detection challenge (41). FTAI presents advantages by simplifying the management and synchronizing estrus cycles, promoting TAI for precise insemination timing, enhancing conception rates, and overall reproductive efficiency (41–43). Recent advancements allowing precise control of ovulation timing mark a substantial leap in improving buffalo reproductive efficiency (12). AI techniques adapted to each species will be necessary to maximize the reproductive physiology of buffalo and ensure their continued agricultural growth in the future (44). Thus, this review study aims to expand our understanding of the reproductive physiology of buffaloes, discuss the various synchronization protocols and explore the factors influencing FTAI success in buffaloes.
2 Reproductive physiology of buffaloes
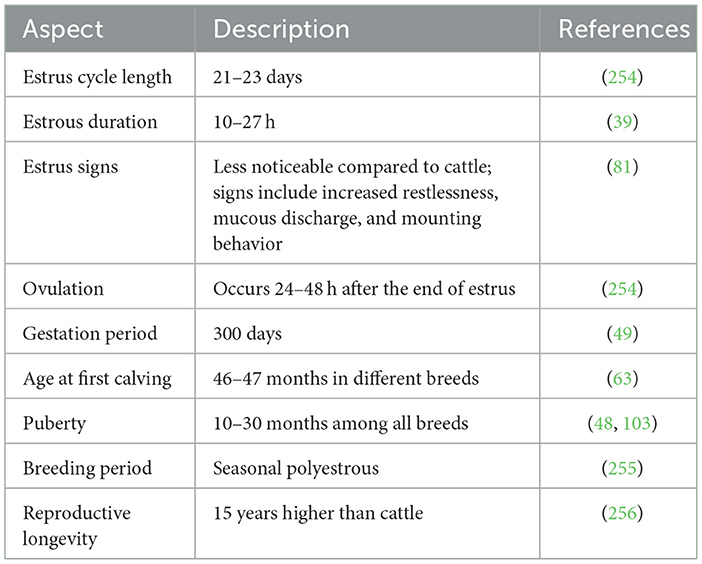
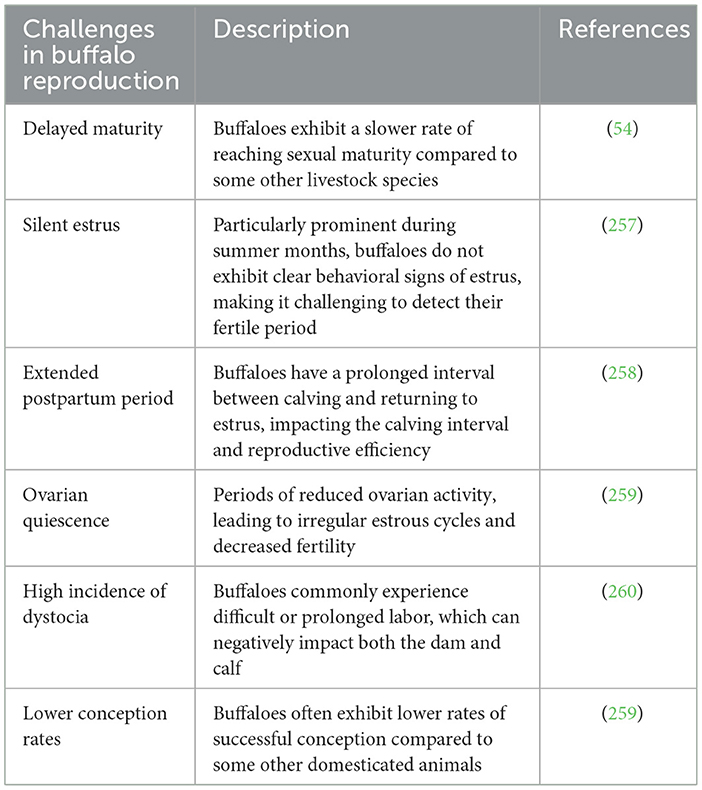
Artificial Insemination (AI) has been made feasible due to advancements in our understanding of the reproductive physiology specific to buffaloes (45). Understanding the reproductive physiology of buffaloes is crucial for implementing effective breeding strategies, including FTAI (46, 47). Water buffaloes, scientifically known as Bubalus bubalis, have distinctive reproductive characteristics that play a significant role in their unique reproductive physiology as outlined in Table 1 (48). The fertility performance and reproductive efficiency of buffaloes in tropical conditions are distinctly influenced by the time of year (49). Buffalo reproduction is affected by several inherent challenges, as illustrated in Table 2 (50). Buffaloes show same advantages of the FTAI and ovulation synchronization already investigated in cattle (51). However, the buffaloes exhibit unique reproductive behavior, the insemination process (39). These animals do not display any homosexual behavior during heat, ensuring the necessity of teaser bull use (45, 52). Likewise, these animals demonstrate inconsistent periods (ranging from 6–48 h) of mounting acceptance (53). Since, AI technology in buffalo is applied at the end of estrus this makes its handling and utilization more challenging (41). The implementation of assisted reproductive technologies has necessitated significant developmental efforts due to the inherent peculiarities in the reproductive physiology of buffaloes (54). These techniques, which have already been established in other species, require adaptation and standardization to suit the unique reproductive characteristics of buffaloes (55, 56).
2.1 Estrous cycle
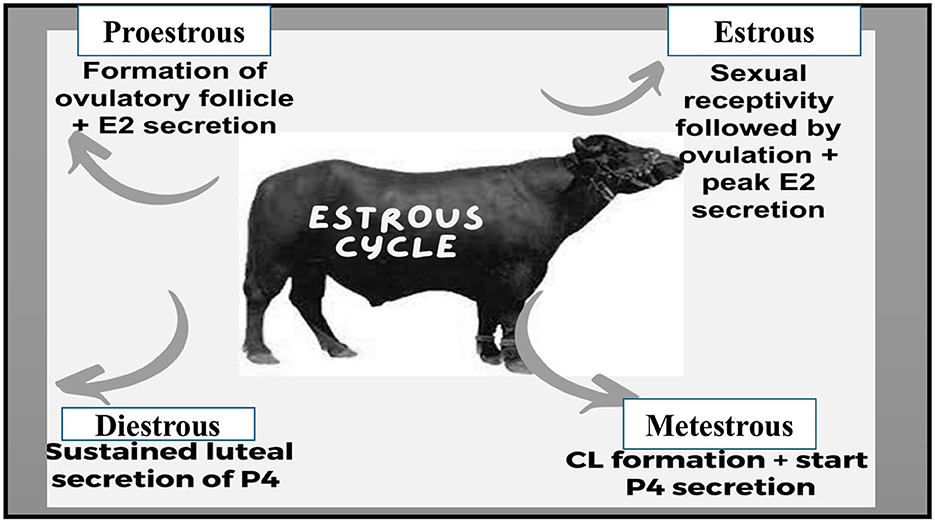
The estrous cycle encompasses the time between the end of one estrus and the onset of the next estrus (57). Water buffaloes are categorized as short-day seasonal polyestrous animals (58–60), although, under some circumstances, they can conceive all year long (61). As in the equator zone, these animals may exhibit estrous cycles throughout the year if the food supply is sufficient to sustain reproductive function (62). No significant difference has been investigated in estrous cycle length among Nilli, Murrah, local, and Crossbreeds (63). The same estrous cycle length of 21.25 ± 2.36 days was observed in Marathwada breed of buffalo (64). Estrous duration of 17–29 h. in local Nili Ravi and Murrah was seen significantly higher than the others (63). Notably, buffaloes become more seasonally polyestrous as they move farther from the equator (65, 66). The estrous cycle of buffaloes consists of two phases: the progesterone phase, also called the luteal phase, and the estrogenic phase, also called the follicular phase. There are two distinct stages in the progesterone dominant (luteal phase) i.e., metestrus and diestrus, and proestrus and estrus stages in the follicular phase as illustrated in Figure 1 (67, 68). The estrous cycle typically spans 16–33 days, with the maximum concentration occurring around days 21–24 (69), with estrus duration lasting around 12–18 h (70). Ovulation typically occurs almost 30 h after the onset of estrus, with variations ranging from 18 to 44 h (51). In contrast to cows, water buffaloes can experience heat for 8–32 h, with fewer heat symptoms (32). Buffalo follicular development follows a wave-like pattern, encompassing stages such as wave emergence (recruitment), growth, selection, dominance, and atresia in each cycle (28). The essential aspects of follicular development align with those observed in cattle (55, 71). Buffalo experience more follicular atresia compared to cattle (72). Preceding an ovulatory wave, it is common to observe 1 or 2 nonovulatory follicular waves (73, 74). Buffalo cows usually experience two to three follicular waves, with buffalo heifers commonly undergoing two-wave cycles (75, 76). Research from India (77), Brazil (78), and Pakistan (79) have indicated that greater populations of buffalo experience two follicular wave activity during the estrous cycle. The two follicular wave cycles are slightly shorter, around 21 days, compared to three-wave cycles which last about 24 days (39). In the 2nd wave dominant follicle's average size is comparable to that in the first wave i.e., 15 mm (80).
2.2 Estrous detection
Silent heat/estrus stands out as the primary reason contributing significantly to the diminished reproductive efficiency observed in buffaloes (81, 82). A rise in gonadotropins shortly before and during the pre-ovulatory surge of estradiol is what causes buffalo's silent heat (83). This happens because progesterone and estradiol concentrations drop during the estrus cycle. Progesterone concentrations therefore lower the peak levels of estradiol around estrus (84). Precise estrus detection is essential for efficient reproductive management, particularly during hand-mating with selected sires (85). Nocturnal behavior is one factor linked to the reduced appearance of estrus signals (86). Uncommon homosexual behavior, varying estrus duration (5–27 h), and unpredictable ovulation timing in buffaloes (24–48 h) following the heat onset are also factors affecting accurate heat detection in buffaloes (61). Traditional estrus indicators in cattle, such as swelling and redness of the vulval mucosa, vaginal mucus secretions, and repeated urination, are unreliable for water buffalo (69, 87). Behavioral manifestations of estrus, such as tail raising, bellowing, and restlessness, are observed in only a limited proportion of buffaloes and are commonly displayed during the night. Identifying precise methods for estrus detection is essential for successful re-insemination of animals that have returned to estrus (88, 89). A few techniques designed for FTAI have been developed to enhance water buffalo reproduction and eliminate the need for heat detection (90). These hormone therapies enable the regulation of luteal and follicular dynamics, estrous and ovulation (synchronization), most critically avoidance of the challenging estrus detection in this species (91–93).
2.3 Ovulation
The ovulation process in mammals, including buffaloes, is regulated via a complex interplay of hormones (28, 94). Compared to dairy cattle, the pre-ovulatory size of follicles in buffaloes tends to be smaller (78, 95). This is owing to lower estradiol although there may changes in the metabolism of estradiol from circulation between both the buffaloes and cattle (52). The dominant follicle's granulosa cells secrete inhibin, which suppresses the synthesis of follicle-stimulating hormone (FSH). Through inhibin release, the maturation of the dominant follicle and the consequent generation of estrogen are essential for controlling surges in luteinizing hormone (LH). An LH surge required for ovulation is caused by the dominant follicle's growing production of estradiol, which has a positive feedback effect on the pituitary and hypothalamus (96, 97). Investigations into the ovulatory responses of buffaloes after GnRH treatment in FTAI protocol have yielded valuable insights into their reproductive dynamics. It was investigated that the ovulation rate after the first GnRH shot was 60.6%. Animals ovulating after the first GnRH and ovulation treatment displayed larger dominant follicles (0.94 ± 0.17 vs. 0.67 ± 0.24 cm; P < 0.01) at the time of treatment compared to non-ovulating animals. Progesterone (P4) level during the initial GnRH administration did not influence ovulation rate (P > 0.05) (41, 98).
2.4 Puberty
Puberty is the stage during which reproductive organs become functionally developed, and animals gain the ability to release gametes (99, 100). For females, puberty is characterized by the age at which they experience their initial estrus, subsequently leading to ovulation (101). Buffaloes typically at 55%−60% of adult body weight (250–400 kg) attain puberty (102, 103). The age of puberty onset varies significantly, spanning from 18 to 46 months (18, 104). At favorable conditions, the riverine type attains puberty at the age of 15–18, and swamp buffalo at 21–24 months (103, 105). Nutrition, genotype, management, and climate contribute to this variation (106). Animals born in spring attain puberty at 380 kg body weight and 14 months of age (107). In a study of 2020 on 20 animals, it was found that there was a difference in the biochemical profile of delayed puberty animals than normal pubertal heifers (108). During an investigation on the effect of season on age at puberty, it was observed that combined climate factors like temperature and rainfall affected reproductive activity significantly but individual factor effect was not significant (109). Buffalo of India attains puberty at the age of 16 and 40 months but the average time for puberty is 2.5 years according to a report of the Central Institute for Research on Buffalo (110). All breeds exhibit variations in puberty from 10 to 36 months when nutrition and management factors are taken into account (48). Attaining puberty is more closely associated with body weight rather than age (101). Delayed puberty not only defers conception but also diminishes reproductive efficiency, extended calving intervals, and diminished manifestation of estrus consequently extending the unproductive phase (111). Bovine gonadotropin releasing factor significantly affected the buffalo's puberty onset, plasma progesterone concentration, and body weight (112).
2.5 Sexed semen's impact on buffalo reproductive dynamics
The use of sexed semen in buffaloes has significant implications for reproductive dynamics, particularly in enhancing calf sex ratios and improving genetic quality. Compared to cattle bulls, buffalo bulls have only been examined and chosen in the last few decades to furnish semen for artificial insemination. As a matter of fact, the latter have been the first to be the focus of intense selection, with the best bulls being chosen for genetic enhancement and then chosen for their semen quality and freezability. There is still a lot of variation in semen quality, even with the efforts made in recent decades to choose superior buffalo bulls in order to improve the annual genetic merit. If inferior quality semen has been found and shown to be unsuitable for freezing/thawing processing and AI, this variability may significantly impact the use of the best bulls. For instance, buffalo bulls' semen may be more vulnerable to oxidative stress because of increased lipid peroxidation, which is most likely caused by decreased antioxidant enzyme activity (6, 113). In order to ascertain whether pregnancies may be impacted in terms of early embryonic death and whether a season effect was anticipated between the transitional and high breeding seasons, the combined use of sexed semen and AI in pluriparous buffaloes was also investigated (114). Once more, it was determined in that follow-up study that the pregnancy rate is comparable for both unsorted and sorted semen. Furthermore, as compared to the opposite unsexed semen, the use of sexed semen did not change progesterone production or increase embryonic mortality.
The use of sexed semen for conception varied from 35 to 60%, depending on the dam's age and management, according to a review by Thakur and fellows (115). According to certain evaluations, the degree of conception varies between 30 and 80 percent among buffaloes with synchronized estrus and AI from many places and with varying ages (116). According to another study showed that employing sexed semen for buffaloes had an average conception rate of 42.7%, demonstrating its efficacy across breeds and parities (117). Praharani et al. after using sexed semen reported a mean conception rate of 50.7% and a calving rate of 46.2%, with variations based on agroecosystems (118). For genetic modification, sexed semen is essential because it enables the targeted production of female calves, which are frequently more profitable in the dairy industry (119). In addition to increasing buffalo productivity, the use of sexed semen safeguards native breeds against extinction due to shifting climates (120). Despite the benefits of sexed semen for genetic enhancement and reproductive management, issues including reduced blastocyst and cleavage rates in comparison to unsexed semen continue to be a worry (119). This emphasizes how further research is required to maximize its use in buffalo breeding efforts.
2.6 Role of hormones in FTAI
Hormones are biochemical substances produced by the endocrine glands that stimulate other organs of the body to produce chemical secretions (121, 122). These are essential regulators of buffalo reproductive functions (49). Hormonal treatments have been developed to manage luteal and follicular processes, creating opportunities for synchronizing follicle growth and ovulation, a vital aspect for timed artificial insemination during breeding and nonbreeding seasons (78, 123). Here we discuss the key hormones involved.
2.6.1 Gonadotropin-releasing-hormone (GnRH)
GnRH is synthesized in the hypothalamus of the brain and induces the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary gland (124). It acts as a master regulator of reproductive hormones (125, 126). Injecting GnRH during follicular phase of the estrous cycle causes an LH surge, resulting in the ovulation of follicles larger than 9.0 mm (127) or promoting nonviable follicle luteinization, a few days later followed by the emergence of a new wave of follicle growth (128, 129). The simultaneous presence of a mature dominant follicle and corpus luteum at the time of GnRH injection in bovines has been associated with improved ovulation, synchronization, and conception rates (110, 130). Therefore, this hormone improves the conception rate as well as pregnancy rate when used in timed artificial insemination (110, 131).
2.6.2 Follicle stimulating hormone (FSH)
FSH contributes to the growth and development of ovarian follicles (132, 133). This hormone acts as a green line for the follicles to recruit and selection (134, 135). FSH stimulates multiple follicles, typically only one becomes dominant (39). Most of the recruited follicles undergo atresia (136). Follicles that do not undergo atresia get selected and become dominant (137, 138). Peak FSH coincides with LH, averaging around 25 ng/ml (139). Following simultaneous pre-ovulatory surges in the gonadotropins, LH levels experience a sharp decline, while FSH level drops gradually (71). Recent studies have confirmed that in buffaloes, a transient peak of follicle-stimulating hormone in the blood initiates at every follicular wave (52). Weather can also influence peripheral FSH concentrations, with higher FSH/LH ratios during peak breeding seasons (140, 141). During the peak breeding season, the FSH/LH ratio was elevated compared to that in the intermediate and low breeding months (142). Nonetheless, the highest concentration of FSH on the day of estrus remained consistent across both hotter and cooler months (143, 144).
2.6.3 Luteinizing hormone (LH)
LH plays a pivotal role in triggering ovulation in buffaloes (52). After the recruitment of follicles, this hormone is responsible for keeping follicles growing (145). LH surge leads to the release of the oocyte from the dominant follicle. Preovulatory surges in LH have been detected to be similar to those in cattle (146). Peripheral LH levels remain at basal levels throughout the reproductive cycle until the day of estrus, at which point a pre-ovulatory LH surge takes place (71). The period between LH surge and estrus onset is ~8–12 h (45, 147–149). Both of these hormones i.e., FSH and LH are under the control of GnRH which is synthesized and released by the hypothalamus (150).
2.6.4 Progesterone
Following ovulation, the corpus luteum forms on the ovary and acts as an endocrine gland to secrete progesterone (12, 151). Progesterone prepares the uterus for pregnancy, creating an environment conducive to embryonic development (152). Suboptimal nutrition and high environmental temperatures can lead to extended periods of non-breeding (anestrus) in buffaloes (145). Monitoring progesterone metabolites in feces has been shown to effectively reflect corpus luteum functionality, correlating well with blood progesterone levels (153). Additionally, progesterone aids in transporting oocytes in the oviduct and supports early pregnancy, working in harmony with estrogens to stimulate mammary gland tissue growth (154).
2.6.5 Estradiol 17β (E2)
Estrogens, including estradiol 17β (E2), influence the reproductive behavior of females (155). Estrogen is produced by the ovarian follicles and affects the central nervous system, leading to estrus behavior (156). Buffalo plasma E2 profiles resemble those of cattle cows with the highest concentrations before and during preovulatory gonadotropin surges followed by declining levels around the next days of the reproductive cycle (157). In buffalo, the E2 peak precedes the LH peak by a day (158, 159). Weather can influence plasma E2 concentrations, with lower concentrations in hot months compared to cooler months (142, 160). Decreased peak E2 values around estrus, along with lowered P4 concentrations, contribute to a higher occurrence of silent estrus in summer (129, 161). These hormones collaboratively regulate buffalo ovarian follicular growth, ovulation, fertilization, and pregnancy timing.
2.6.6 Prostaglandin F2 alpha (PGF2-alpha)
The large and small luteal cells, two steroidogenic cell types that originate from ruptured follicular granulosa and thecal cell, respectively, make up the mature corpus luteum (162). Prostaglandin F2α (PGF2α) receptors on these large and tiny luteal cells can cause luteal regression when PGF2α binds to them. The luteal tissue undergoes structural alterations after the about 12-h functional period of luteolysis in buffalo, which is reflected in the blood's decreasing progesterone levels (163). In the presence of a responsive CL, which occurs between days 5 and 7 of the estrous cycle in heifers and days 7 to 17 in buffalo cows, prostaglandin F2 alpha and its synthetic analogs efficiently trigger luteolysis (164). Because cows with developed follicles enter estrus earlier than those with immature follicles at the time of treatment, the duration until estrus induction depends on the state of follicle development at the time of PGF2α administration. Procedures for two-dose PGF2α were created to guarantee the presence of responding CL (28). Additionally, PGF2α can shorten the voluntary waiting period, increasing total reproductive efficiency. Fallopian tube function is significantly mediated locally by prostaglandins. They participate in ovulation, fertilization, and the transportation of oocytes and embryos (165). It has been demonstrated earlier that PGF2α at 10 μg/ml markedly increased Caspase 3 expression at 72 h of culture in comparison to other dosages at 24 and 48 h (166).
3 Estrus synchronization
Estrous synchronization is the process of bringing a group of female animals into heat at the same time (167, 168). Estrous synchronization is crucial for coordinating the reproductive processes of multiple buffaloes within a herd (39). This can be achieved by shortening or extending the luteal (progesterone dominant) phase of the reproductive cycle (67, 169). This is accomplished by regulating the luteal phase through the use of progesterone analogs or prostaglandins (170) or managing follicular ovulation and development utilizing diverse combinations of progesterone, prostaglandins, hCG, eCG, GnRH, and estradiol (171). Numerous modern synchronization programs for buffalo have emerged, drawing from research primarily conducted in cattle (35). ES protocols in Buffalo have achieved partial success (172). However, a significant distinction between the buffalo and cattle is that buffaloes experience a pronounced reduction in breeding activity around the hot months of the year, resulting in reduced cyclic ovarian activity (101). This synchronization ensures that a significant number of females are in the desired reproductive stage simultaneously, allowing for efficient insemination procedures and increasing the chances of calving rate and conception rate (173). Instead of relying on visual estrus detection, which can be a significant challenge in buffalo cows, synchronization allows for planned and timely AI, minimizing missed opportunities for conception (174). Hormonal treatments enable the regulation of follicle dynamics and luteal cell functions, estrus, ovulation synchronization, and notably, alleviate the challenging task of estrus detection in this species (33, 35, 45, 170).
4 Fixed time artificial insemination
Fixed time Artificial Insemination is characterized as the process of performing AI at a programmed time following the synchronization of ovulation (175). It's a technology that facilitates AI without detection of heat (33). Understanding the ovarian follicular dynamics, as investigated by ultrasonography, advancements in understanding the hormonal profiles and endocrine control during the reproductive cycle have facilitated the commercial application of FTAI in buffalo population (41, 176). Typically, batches of animals are treated simultaneously with the FTAI according to a prearranged plan or schedule. It essentially means that you can easily determine how many animals to inseminate and when (season, month, and day) is the best time to do it (33). By following ovulation synchronization hormonal protocols, FTAI is made possible (177). This method eliminates the need for detecting estrus and enables breeders to enhance reproductive management through accurate insemination timing (178). FTAI involves several essential components and steps, including estrus synchronization, hormonal treatments, and meticulous insemination timing. Diverse protocols have been devised and applied in buffalo breeding to boost the success (35, 92). These protocols typically employ hormones like progesterone, GnRH, and prostaglandin to manipulate the estrous cycle and prompt ovulation at predetermined times (179–183). The critical interval between standing estrus and ovulation, which is crucial for artificial insemination, was found to be ~30 h in buffaloes (51, 184). The well-known AM-PM rule of insemination under field conditions, formerly developed for cattle has been widely applied to buffaloes (25). According to this, buffaloes have to be inseminated 12–15 h after detecting standing estrus (184, 185). However, there is a common misconception that the onset of heat signs is mistaken for the start of standing estrus, leading to early insemination. This premature insemination might reduce fertility because there's an 8 to 10-h gap between heat signs and actual standing estrus (186). To optimize fertility, buffalo cows should go through insemination 12 h after identifying standing estrus (usually done by observing teaser/bull behavior) otherwise 18–24 h after the heat signs (187). The presence of mucus during artificial insemination serves as an indicator for fertility and the intensity of natural estrus (188, 189). FTAI effectiveness can be enhanced by using ovsynch protocol in cyclic buffaloes during their breeding season and for off breeding season progesterone device with eCG+ GnRH/hCG can be used to enhance FTAI efficacy (41).
4.1 Fixed-TAI protocols
Numerous protocols based on the use of hormones that can act at different points in the hypothalamic-pituitary-ovarian axis have been developed in buffalo to control the estrous cycle and, in some cases, the timing of ovulation (45). The protocols can be classified as heat detection artificial insemination protocols (HDAI) are only PG based and P4 based protocols GnRH based protocols if combined with those protocols are categorized as FTAI protocols.
4.1.1 Prostaglandin-based protocols
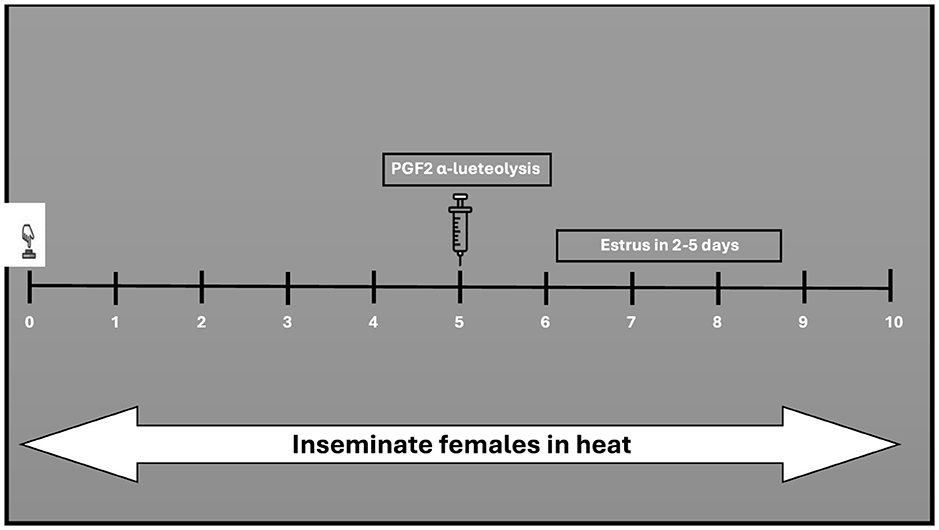
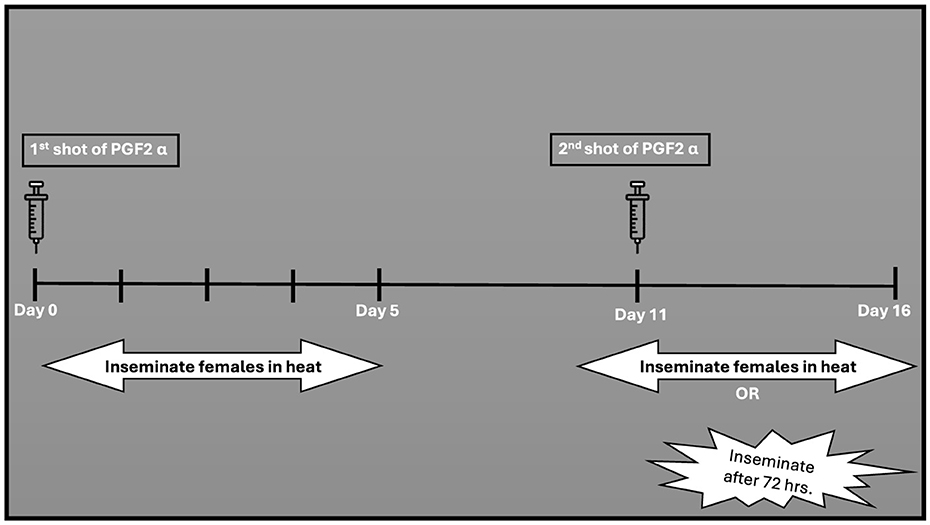
This protocol shortens the luteal phase (190, 191). Prostaglandin (PGF2α) is administered to induce luteolysis (regression of the corpus luteum) in non-pregnant buffaloes (192, 193). In buffalo cows, the PGF2alpha effect is comparable to that studied in cattle (192, 194). Much like the approaches applied in cattle, buffaloes have been subjected to prostaglandin administration, either through a single injection (referred to the one-shot method also known as single shot) or through two injections apart by 11–14 days (195, 196). According to the single-shot method (Figure 2), only animals with a functional CL (5–17 days) of the estrous cycle can receive prostaglandin treatment (67). Research in cattle demonstrated that a single shot PGF2α, administered during an active corpus luteum led to estrus return in 2–3 days (39). In buffaloes, a single injection of PGF2α yielded a response alike to that observed in cattle (164). In the two-shot method, two doses of PGF2α are administered 11 days apart (Figure 3) allowing for estrus synchronization in buffaloes regardless of their specific ovarian status (195). The first injection initiates luteolysis, and the second injection ensures complete regression of the corpus luteum (197). Following the second PGF2α injection, AI is usually performed 48 to 72 h later, as ovulation is expected to occur during this timeframe (82).
4.1.2 Progesterone-based protocols
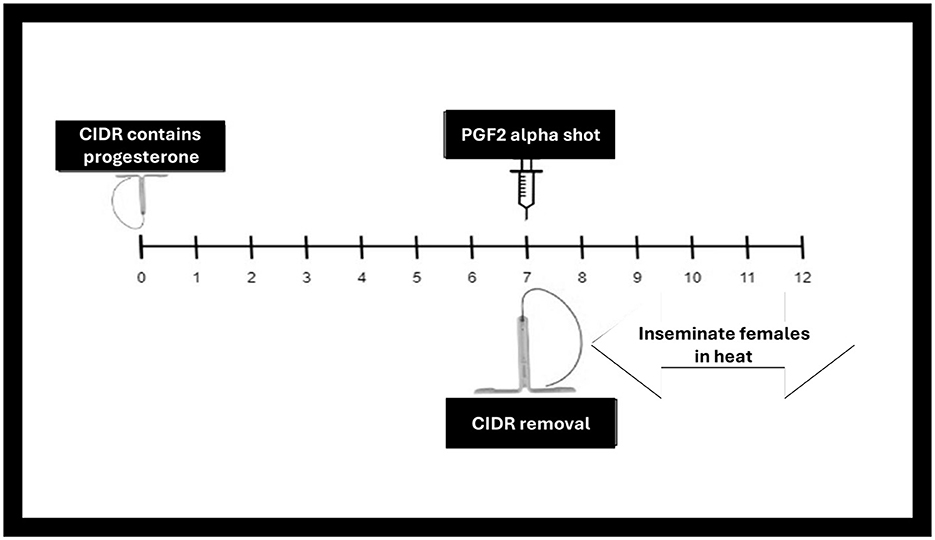
This protocol lengthens the luteal phase (198). Progesterone-based treatment is used to synchronize and control the estrous cycle in buffaloes (Figure 4). One common protocol involves inserting an intravaginal progesterone-releasing device (CIDR) into the reproductive tract for a specific duration (e.g., 10–12 days) (199). It acts as an artificial corpus luteum and mimics the progesterone secretion. When it is removed after 10–12 days, the animal comes into heat (199). If at the time when CIDR is removed, an injection of PGF2α is also administered the animal comes into heat more quickly due to induced luteolysis, followed by AI within a specific timeframe, considering the expected timing of ovulation (143).
4.1.3 Select synch and co synch GnRH-based protocol
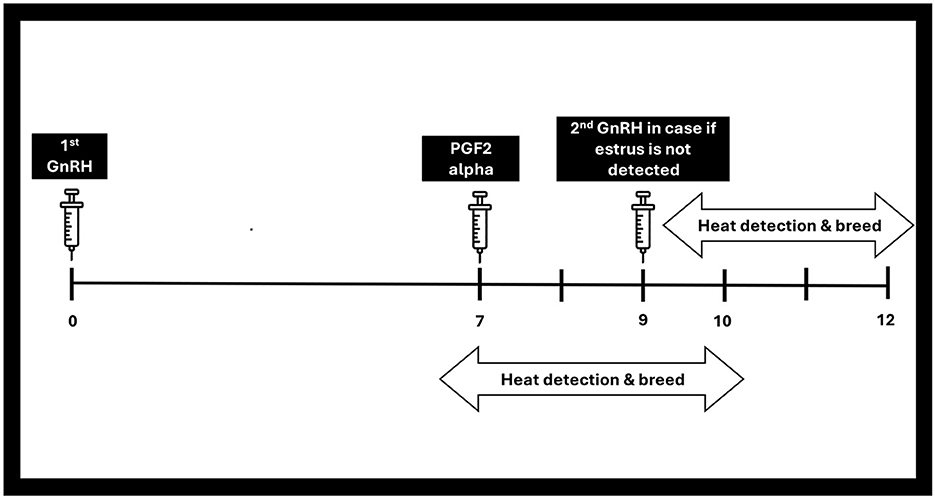
The initial shot of gonadotrophin-releasing hormone (GnRH) prompts ovulation in cyclic females (110), while the following injection of PGF2a facilitates the regression of the corpus luteum, leading to decreased progesterone levels (200). The second shot of GnRH injection facilitates the ovulation process from dominant follicle, which was primed by the initial GnRH treatment (67). This is illustrated in Figure 5 as well. The Co synch protocol is similar to this Ovsynch protocol except that the AI is performed at the 2nd GnRH injection (146, 201).
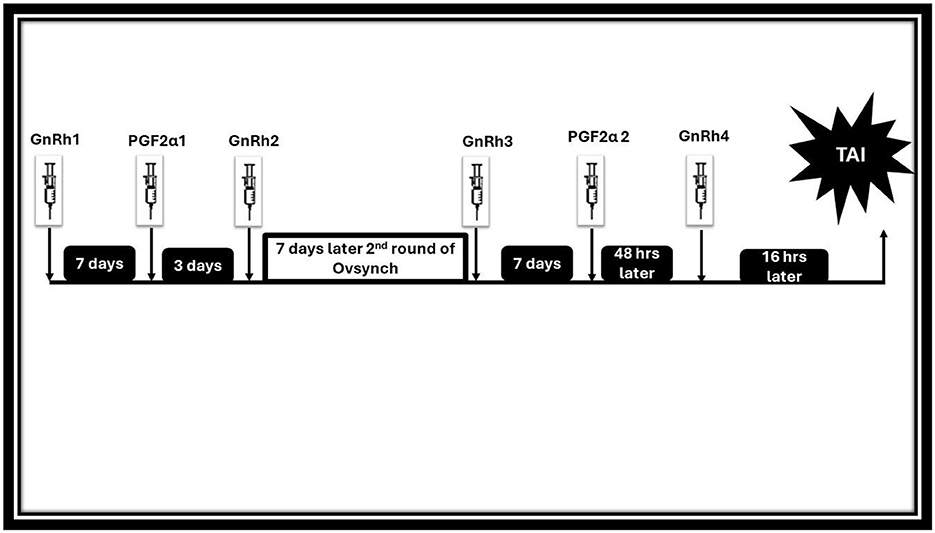
4.1.4 Ovsynch protocols
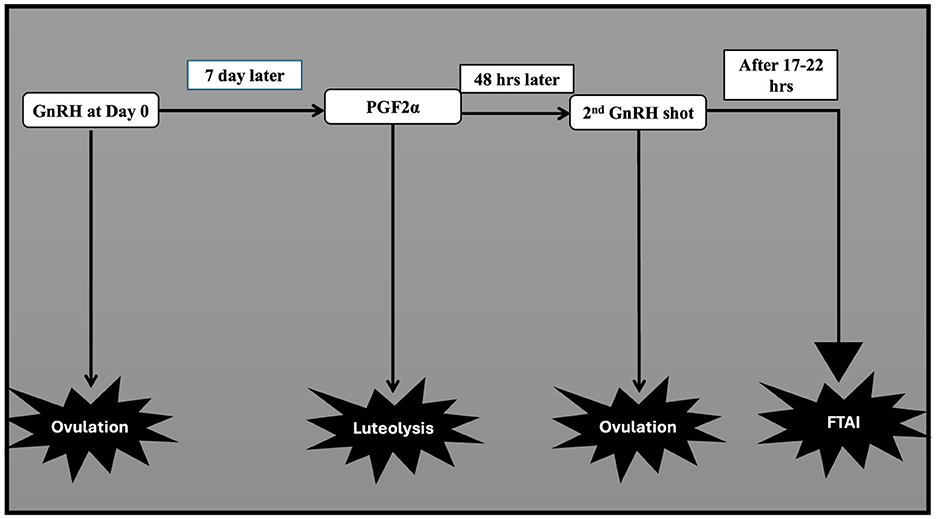
This protocol aims to synchronize ovulation in buffaloes employing GnRH and PGF2α combination (Figure 6). For the FTAI in water buffaloes, this approach has been adopted and is now the most widely used protocol, with multiple published studies supporting its use (33, 182, 202). Firstly, GnRH is administered to induce ovulation of selected dominant follicles. Then PGF2α is injected 7 days later to luteolysis and synchronize follicular wave emergence. A subsequent GnRH injection 48 h after PGF2α administration synchronized ovulation of the newly emerged dominant follicle. AI is performed at a specific time following the second GnRH injection when ovulation is expected (89, 203, 204). Table 3 presents the efficiency of this protocol in the implementation of AI without heat detection in different breeds of buffalo either riverine or swamp in various countries (205–207). The effectiveness of the treatment in buffaloes is predominantly influenced by the breeding period (38). Baruselli (65), employing the Ovsynch protocol, demonstrated a conception rate of 48.8% in buffalo cows around the breeding season and 6.9% in those inseminated during the non-breeding period. More reports utilizing the Ovsynch breeding protocol reported varying conception rates at artificial insemination varying from 56.5% during the breeding season (208) to 36.0%−42.5% during the seasonal anestrus to transition period (209, 210). The variation may be due to a greater proportion of non-cyclic animals stemming from the suboptimal activity of the hypothalamic-pituitary-gonadal axis occurring in buffalo during spring-summer (211). Indeed, studies indicate a better conception rate in cyclic compared to non-cyclic animals (212–214). According to the Ovsynch-FTAI protocol, 78%−90% of buffaloes exhibit synchronized ovulation, and 33%−60% of them get pregnant (65, 205, 209). Because of the substantial embryonic mortality (20%−40%) (215, 216) and anestrus (217) during the non-breeding season, using Ovsynch-FTAI in buffalo during these transitional periods results in a much lower pregnancy rate. When comparing pluriparous buffalo to primiparous buffalo, a greater pregnancy rate is often attained (65). Because of the low ovulatory response to first GnRH and unsynchronized development of new follicular wave Ovsynch-FTAI is not recommended in heifers (45).
4.1.5 Double-Ovsynch protocols
Double-Ovsynch protocols involve two rounds of the Ovsynch protocol to improve synchronization and conception rates (89, 218). The FTAI is performed at a precisely scheduled time following the second GnRH of the second Ovsynch sequence as illustrated in Figure 7 (204, 219). Incorporating GnRH into a pre-synchronization strategy, as implemented in protocols like Ovsynch1, enhances conception rates by targeting the anovular state often observed in cows prior to starting the main Ovsynch protocol (220). The Double Ovsynch protocol resulted in a notably higher conception rate among primiparous cows, achieving 44%, compared to 31% in multiparous cows. This demonstrates the effectiveness of the protocol, particularly in younger, first-time calving cows (221). This protocol has proven effective as a resynchronization protocol, achieving higher conception rates compared to the standard Ovsynch (39% vs. 30%) in cattle. In acyclic heifers and buffaloes a higher conception rate of 83.33% was investigated after using double Ovsynch protocol in Indian Gujrat (222). This effectiveness hinges on having sufficient time to complete the entire protocol without excessively increasing the number of days open (223).
4.2 Factors affecting FTAI in buffaloes
Timed Artificial Insemination in buffaloes relies on a complex interplay of factors to determine FTAI success. This reproductive technique involves synchronization of estrus, precise insemination, and favorable environmental and health conditions for the buffalo. The efficacy of FTAI is influenced by several key factors that encompass hormonal synchronization protocols, the skill of the inseminator, the reproductive health of the buffalo, and environmental elements such as nutrition and stress levels. A thorough understanding and management of these factors are critical in optimizing the success rates of FTAI in buffalo breeding programs. There are several key factors that can influence its success in buffaloes.
4.2.1 Estrous synchronization protocols
Proper timing and administration of hormonal protocols/interventions to synchronize estrus and ovulation increase the likelihood of inseminating buffaloes during their fertile period (224). Estrous synchronization is pivotal in timed artificial insemination, aligning the reproductive cycle of buffaloes for optimal conception (225, 226). Hormonal protocols regulate estrus, ensuring the female buffaloes are at a prime stage for successful insemination. Proper timing and administration of hormones are crucial for synchronization, enhancing the chances of successful breeding (39). How the estrous synchronization protocol is a major influencing factor in FTAI and has yielded varying conception rates is summarized in Table 4.
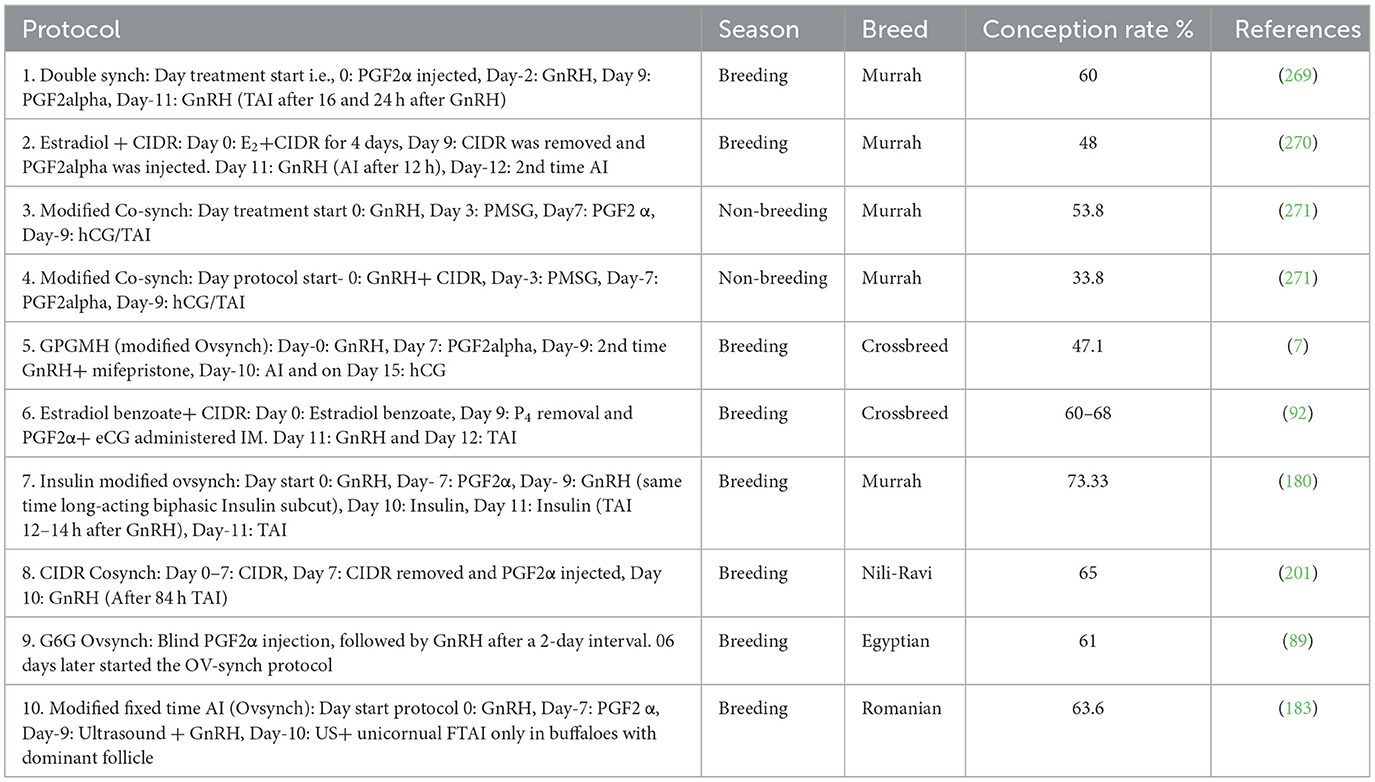
Table 4. Comparison of conception rates across different synchronization protocols in female buffaloes.
4.2.2 Reproductive management
Good reproductive management practices, including proper nutrition, health management, and regular monitoring of estrus behavior, are essential for FTAI success (227, 228). Body condition score (BCS) has an association with productive pregnancy especially during the poor breeding season (229). Farmers need to be aware about such buffaloes exhibiting a low BCS may not exhibit positive responses to various protocols (230). Some authors believe that body condition influence the ovarian cycle of bovine family directly and found an upsurge in the reproductive ovarian cycle of bovine, corresponding to an improvement in the body condition (41, 231). It is advisable to promote efforts aimed at enhancing the nutritional status of the animals ensuring optimal body condition and addressing any reproductive disorders or health issues that can improve the chances of successful AI (173, 232).
4.2.3 Semen quality, AI technique, and genetic factors
The quality of the semen used for FTAI significantly impacts the conception rates (233, 234). Using high-quality semen from genetically superior bulls with good fertility is crucial for maximizing the chances of successful AI in buffaloes (34, 235). An increase in the conception rate up to (63%) using FTAI protocol in Romanian buffaloes was observed by using sexed semen containing 2 million X-chromosome bearing sperm (183). The proficiency and skill of the inseminator in performing AI techniques are crucial for FTAI success (236). Different buffalo breeds may exhibit variations in reproductive characteristics and responses to FTAI protocols. Additionally, genetic factors, including the genetic potential for fertility and reproductive traits, can influence the success of FTAI (229).
4.2.4 Season and stress levels
Buffaloes become sexually activated in response to decreased day length and temperature (237). Seasonality in water buffalo extends postpartum anestrus intervals, adversely impacting the reproductive performance (33). The efficiency of ovulation synchronization protocols for FTAI can be affected by the reproductive seasonality observed in buffaloes (91). During the seasonal anestrus period, buffaloes experience a lack of behavioral estrous, ovulation, and decreased progesterone secretion (238). Consequently, there is an occurrence of ovarian follicular turnover during this time (239). Hormonal treatment can be employed to persuade estrus or ovulation in anestrous cows (41). However, certain buffaloes never respond to the treatment due to low breeding season (111). Various factors may contribute to this phenomenon, but one of the most probable reasons is the follicular status of the animal at the initiation of FTAI protocol (51). The ideal time for treatment can be determined via ovarian activity with ultrasound (240). Reproductive efficacy depends on the breeding season and high conception rate (129, 241). Buffaloes exhibit a seasonal breeding pattern and typically develop sexual activity in response to a decreasing day length, which occurs during later summer to early autumn (9, 242). The breeding season influences the conception rate (50). Buffalo cows treated throughout the breeding season (autumn and winter) shown a higher conception rate compared to those treated throughout the off-breeding season, with rates of 48.8% (472/967) and 6.9% (6/86) respectively (41, 147).
High stress levels, caused by factors such as transportation, handling, or changes in the environment (229), can negatively impact reproductive performance in buffaloes. Heat stress adversely influences the reproductive performance as well as production of Buffalo (243). Buffalo is more vulnerable to heat stress than cattle due to fewer sweat glands and black hair resulting in fertility loss (244). When buffaloes experience heat stress, their consumption and efficiency in utilizing feed are reduced, leading to alterations in the balance of proteins, water, energy, and minerals. Additionally, changes in enzymatic activities and hormone levels negatively impact buffalo reproduction (245). The temperate zone is regarded as the most conducive for enhanced productivity in dairy animals (246). Minimizing stressors and providing a calm and conducive environment can improve FTAI success (247).
4.2.5 Timing in FTAI
Timing is critical in FTAI, as it determines the optimal moment for insemination (26). Insemination is typically performed 48–72 h after the withdrawal of progesterone supplementation or prostaglandin administration when ovulation is expected to occur (52, 248). This allows for the delivery of sperm to the reproductive tract during the fertile window, maximizing the chances of successful fertilization (249). The implementation of FTAI in buffalo breeding programs offers several advantages. It eliminates the need for estrus detection, which can be challenging in buffaloes due to the absence of clear behavioral signs (250). FTAI also allows for the efficient utilization of superior sires by precisely timing insemination (39) and improving the genetic potential of the offspring (251). Additionally, it can enhance reproductive management by enabling breeders to optimize breeding programs, synchronize calving intervals, and increase overall herd productivity (252). These factors can affect the response to hormonal treatments, the synchronization of estrus, and the overall fertility rates. Understanding and managing these factors are crucial for improving timed artificial insemination outcomes in buffalo breeding programs (253).
5 Concluding remarks and suggestions
Fixed-time artificial insemination (FTAI) holds immense potential for optimizing buffalo reproduction, yet its efficacy remains uneven across breeds and seasons. We have discussed various possibilities to initiate and maintain FTAI program under different circumstances according to the specific reproductive physiology of the buffalo and the factors affecting TAI efficiency. As summarized in our review paper's Table 4, slight modifications in ovsynch protocol with FTAI resulted in high conception rate during breeding season but in the protocol no. 5 conception rate was not so high possibly due to cross breed and climate factor or due to genetic differences in follicular dynamics. Insulin modified protocol could be a better choice for crossbreed buffaloes. For cost effective TAI protocol CIDR can be replaced with biodegradable progesterone implants. However, to reduce human error and to refine ovulation window integrated ultrasound-guided FTAI and 5 days later FTAI single hCG shot will be a best possible choice for FTAI. Notably, still there is not any single magical tool or protocol. Therefore, certain adjustments and possible factors affecting FTAI efficiency like season, nutritional, health status, breed of animal and cost of the protocol should be considered. Finally, better understanding of buffalo's reproductive physiology and considering the elements that impact on the effectiveness of FTAI can reinforce the likelihood to achieve better outcomes or provide clearer insight when the results differ from our expectations.
Author contributions
HK: Writing – original draft, Conceptualization. XZhan: Investigation, Writing – review & editing. MR: Formal analysis, Writing – review & editing. MS: Writing – review & editing. AO: Investigation, Writing – review & editing. MP: Writing – review & editing, Validation. MH: Writing – review & editing, Supervision. OT: Writing – review & editing, Formal analysis. PW: Supervision, Writing – review & editing. XZhao: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the earmarked fund for the National Key R&D Program of China (2022YFE0100200), the National Natural Science Foundation of China (32161143032), Specially Appointed Expert Program of Tianchi Talent Program in Xinjiang Province (Xueming Zhao), the earmarked fund for CARS (CARS36-16), and the National Germplasm Center of Domestic Animal Resources and the Agricultural Science and Technology Innovation Program (ASTIP-2016-IAS-06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gateway to Dairy Production and Products. (2025). Available online at: https://www.fao.org/dairy-production-products/dairy/buffaloes/en#:~:text=The%20domestic%20water%20buffalo%20%28Bubalus%20bubalis%29%20contributes%20a,the%20major%20milk%20producing%20animal%20in%20several%20countries (accessed April 29, 2025).
2. Rehman SU, Hassan F-u, Luo X, Li Z, Liu Q. Whole-genome sequencing and characterization of buffalo genetic resources: recent advances and future challenges. Animals. (2021) 11:904. doi: 10.3390/ani11030904
3. Samuel FU, Mada K, Rekwot PI, Mohammed AA. Seasonal perspectives on reproduction of the Buck and Doe. In: Rana T, editor. Elements of Reproduction and Reproductive Diseases of Goats. Hoboken, NJ: John Wiley & Sons, Inc. (2025). p. 71–80. doi: 10.1002/9781394190089.ch7
4. Presicce GA. The Buffalo (Bubalus bubalis)-Production and Research. Sharjah: Bentham Science Publishers (2017). doi: 10.2174/97816810841761170101
5. Presicce GA. Semen sexing in the buffalo (Bubalus bubalis). In:Chauhan MS, Selokar N, editors. Biotechnological Applications in Buffalo Research. Singapore: Springer Singapore (2022). p. 319–32. doi: 10.1007/978-981-16-7531-7_16
6. Cao B, Qin J, Pan B, Qazi IH, Ye J, Fang Y, et al. Oxidative stress and oocyte cryopreservation: recent advances in mitigation strategies involving antioxidants. Cells. (2022) 11:3573. doi: 10.3390/cells11223573
7. Abulaiti A, El-Qaliouby HS, El Bahgy HE, Naseer Z, Ahmed Z, Hua G, et al. a new fixed timed-AI synchronization regimen for swamp and river crossbred buffaloes (Bubalus bubalis). Front Vet Sci. (2021) 8:646247. doi: 10.3389/fvets.2021.646247
8. Yang J, Guo S, Pan B, Qazi IH, Qin J, Zang S, et al. Melatonin promotes in vitro maturation of vitrified-warmed mouse GV oocytes potentially by modulating MAD2 protein expression of SAC component through MTRs. Cryobiology. (2021) 102:82–91. doi: 10.1016/j.cryobiol.2021.07.008
9. Shahzad Q, Waqas M, Pu L, Wadood AA, Xie L, Husna AU, et al. Seasonality and photoperiod influence in vitro production of buffalo embryos. Reprod Domes Anim. (2020) 55:1115–23. doi: 10.1111/rda.13749
10. Hussain T, Babar ME, Nadeem A, Jabeen R, Ali A. Phylogenetic analysis of Kundi buffalo breed of Pakistan through mitochondrial D-loop region. Pak J Zool. (2009) 9:341–246.
11. Wood S, Quinn A, Troupe S, Kingsland C, Lewis-Jones I. Seasonal variation in assisted conception cycles and the influence of photoperiodism on outcome in in vitro fertilization cycles. Hum Fertil. (2006) 9:223–9. doi: 10.1080/14647270600806557
13. Purohit G, de Carvalho N, Soares J, Kahwage P, Garcia A, Sharma R, et al. Bubaline Theriogenology. IVIS (2014).
14. Khalex A-H, Syed-Hussain SS, Ramanoon SZ, Sarbini SR, Hassan MN, Nating WR, et al. Buffalo in Borneo, Sarawak: areview of the current status of the indigenous buffalo industry. J Buffalo Sci. (2021) 10:32–40. doi: 10.6000/1927-520X.2021.10.05
15. Salzano A, Licitra F, D'Onofrio N, Balestrieri ML, Limone A, Campanile G, et al. Space allocation in intensive Mediterranean buffalo production influences the profile of functional biomolecules in milk and dairy products. J Dairy Sci. (2019) 102:7717–22. doi: 10.3168/jds.2019-16656
16. Tsakalidou E, Papadimitriou K. Non-bovine Milk and Milk Products. New York, NY: Academic Press (2016).
17. Singh I, Balhara A. New approaches in buffalo artificial insemination programs with special reference to India. Theriogenology. (2016) 86:194–9. doi: 10.1016/j.theriogenology.2016.04.031
19. Rajesh G, Paul A, Mishra S, Bharati J, Thakur N, Mondal T, et al. Expression and functional role of Bone Morphogenetic Proteins (BMPs) in cyclical corpus luteum in buffalo (Bubalus bubalis). Gen Comp Endocrinol. (2017) 240:198–213. doi: 10.1016/j.ygcen.2016.10.016
20. Shanku E. Cattle breeding practice of the community and evaluation of artificial insemination (AI) after estrus synchronization in wondo genet distirct, sidama zone, snnpr, Ethiopia. Int J Livest Res. (2020) 12:1–15.
21. Fair T, Lonergan P. The oocyte: the key player in the success of assisted reproduction technologies. Reprod Fertil Dev. (2023) 36:133–48. doi: 10.1071/RD23164
22. Degefa T, Jemal J. Manual for Application of Assisted Reproductive Technology Tools for Enhancing Genetic Improvement of Indigenous Cattle Breeds. Bahir Dar: Amhara Regional Agricultural Research Institute (ARARI) (2018).
23. Liu X, Li H, Payan-Carreira R. Breeding Strategies for Healthy and Sustainable Development of Animal Husbandry. Norderstedt: BoD–Books on Demand (2024). doi: 10.5772/intechopen.104143
24. Foote R. The history of artificial insemination: selected notes and notables. J Anim Sci. (2010) 80:1–10. doi: 10.2527/animalsci2002.80E-Suppl_21a
25. Trimberger GW. Breeding Efficiency in Dairy Cattle from Artificial Insemination at Various Intervals Before and After Ovulation. Lincoln, NE: DigitalCommons@University of Nebraska-Lincoln (1948).
26. López-Gatius F. Revisiting the timing of insemination at spontaneous estrus in dairy cattle. Animals. (2022) 12:3565. doi: 10.3390/ani12243565
27. Palomares RA. Estrus detection. In:Hopper RM, editor. Bovine Reproduction. Hoboken, NJ: John Wiley & Sons, Inc (2021). p. 431–46. doi: 10.1002/9781119602484.ch35
28. Das PK, Mukherjee J, Banerjee D. Female reproductive physiology. In:Das PK, Sejian V, Mukherjee J, Banerjee D, editors. Textbook of Veterinary Physiology. Berlin: Springer (2023). p. 513–68. doi: 10.1007/978-981-19-9410-4_22
29. Yanagimachi R. Mechanisms of fertilization in mammals. In:Mastroianni L, Biggers JD, editors. Fertilization and Embryonic Development In Vitro. Cham: Springer (1981). p. 81–182. doi: 10.1007/978-1-4684-4016-4_6
30. Bedford J. Significance of the need for sperm capacitation before fertilization in eutherian mammals. Biol Reprod. (1983) 28:108–20. doi: 10.1095/biolreprod28.1.108
31. Wiebke M, Pieper L, Gürler H, Janowitz U, Jung M, Schulze M. Effect of using liquid semen on fertility in German Holstein Friesian dairy cattle: a randomized controlled clinical trial. Theriogenology. (2023) 199:50–6. doi: 10.1016/j.theriogenology.2023.01.012
32. Drost M. Bubaline versus bovine reproduction. Theriogenology. (2007) 68:447–9. doi: 10.1016/j.theriogenology.2007.04.012
33. Gutiérrez-Añez JC, Camacho de Gutiérrez A, Nava-Trujillo H. Application of fixed-time artificial insemination in water buffaloes. In:Chauhan MS, Selokar N, editors. Biotechnological Applications in Buffalo Research. Cham: Springer (2022). p. 295–318. doi: 10.1007/978-981-16-7531-7_15
34. Anzar M, Farooq U, Mirza M, Shahab M, Ahmad N. Factors affecting the efficiency of artificial insemination in cattle and buffalo in Punjab, Pakistan. Pak Vet J. (2003) 23:106–13.
35. Ahmad N, Arshad U. Synchronization and resynchronization strategies to improve fertility in dairy buffaloes. Theriogenology. (2020) 150:173–9. doi: 10.1016/j.theriogenology.2020.01.025
36. Capela JG. Use of FTAI and MOET Reproductive Technologies in an Aberdeen angus Herd. Lisbon: Universidade de Lisboa, Faculdade de Medicina Veterinária (2024).
37. Yousuf MR, Martins J, Husnain A, Riaz U, Riaz H, Sattar A, et al. Effect of oestradiol benzoate on oestrus intensity and pregnancy rate in CIDR treated anoestrus nulliparous and multiparous buffalo. Anim Reprod Sci. (2015) 159:104–8. doi: 10.1016/j.anireprosci.2015.06.003
38. Rossi P, Vecchio D, Neglia G, Di Palo R, Gasparrini B, Michael J, et al. Seasonal fluctuations in the response of Italian Mediterranean buffaloes to synchronization of ovulation and timed artificial insemination. Theriogenology. (2014) 82:132–7. doi: 10.1016/j.theriogenology.2014.03.005
39. Jeyakumar S, Balasubramanian S, Vedamurthy G, Lavanya M, Chethan H, Kumaresan A, et al. Advances in estrous synchronization and timed breeding programs for fertility enhancement in cattle and buffaloes. In:Kumaresan A, Srivastava AK, editors. Current Concepts in Bovine Reproduction. Cham: Springer (2022). p. 119–67. doi: 10.1007/978-981-19-0116-4_9
40. Shenhe L, Jun L, Zipeng L, Tingxian D, ur Rehman Z, Zichao Z, et al. Effect of season and breed on physiological and blood parameters in buffaloes. J Dairy Res. (2018) 85:181–4. doi: 10.1017/S0022029918000286
41. Baruselli PS, Carvalho N, Gimenes L, Crepaldi G. Fixed-time artificial insemination in buffalo. Ital J Anim Sci. (2010) 6(2s):107–18. doi: 10.4081/ijas.2007.s2.107
42. Cofield LG. Improving Reproductive Efficiency and Calving Distribution of Heifers Through Estrus Synchronization, Natural Service, and Fixed-Time Artificial Insemination. Auburn, AL: Auburn University (2020).
43. Leite RF, de Agostini Losano JD, Kawai GKV, Rui BR, Nagai KK, Castiglioni VC, et al. Sperm function and oxidative status: effect on fertility in Bos taurus and Bos indicus bulls when semen is used for fixed-time artificial insemination. Anim Reprod Sci. (2022) 237:106922. doi: 10.1016/j.anireprosci.2022.106922
44. Baruselli PS, de Carvalho NA, Gasparrini B, Campanile G, Michael J. Development, adoption, and impact of assisted reproduction in domestic buffaloes. Animal. (2023) 17:100764. doi: 10.1016/j.animal.2023.100764
45. Neglia G, de Nicola D, Esposito L, Salzano A, D'Occhio MJ, Fatone G. Reproductive management in buffalo by artificial insemination. Theriogenology. (2020) 150:166–72. doi: 10.1016/j.theriogenology.2020.01.016
46. Hufana-Duran D, Duran P, editors. Animal reproduction strategies for sustainable livestock production in the tropics. In: IOP Conference Series: Earth and Environmental Science. London: IOP Publishing (2020). doi: 10.1088/1755-1315/492/1/012065
47. Devkota B, Bohara TP, Yamagishi N. Seasonal variation of anestrus conditions in Buffaloes (Bubalus bubalis) in Southern Nepal. Asian J Anim Vet Adv. (2012) 7:910–4. doi: 10.3923/ajava.2012.910.914
48. Pirondi AN, Teixeira CMC, Lima ES, Valente TNP, Deminicis BB, Bezerra FC, et al. Reproductive characteristics of buffaloes: a review. J Agric Sci. (2019) 11:167. doi: 10.5539/jas.v11n13p167
49. Michael J, Ghuman SS, Neglia G, Della Valle G, Baruselli PS, Zicarelli L, et al. Exogenous and endogenous factors in seasonality of reproduction in buffalo: a review. Theriogenology. (2020) 150:186–92. doi: 10.1016/j.theriogenology.2020.01.044
50. Vale W, Castro S, Chahar S, Neves K, Zicarelli L. Effect of environmental factors on buffalo reproduction. In:Purohit GN, editor. Bubaline Theriogenology. Ithaca, NY: International Veterinary Information Service (2019). p. 1–42.
51. Bhat GR, Dhaliwal GS. Estrus and ovulation synchrony of buffaloes (Bubalus bubalis): a review. Buffalo Bulletin. (2023) 42:239–61. doi: 10.56825/bufbu.2023.4222415
52. Esteves LFMM. Ovulation Synchronization Protocols Study with Italian Mediterranean Buffalo Cows (Bubalus bubalis). Lisbon: Universidade de Lisboa, Faculdade de Medicina Veterinária (2022).
53. Ramesha KP. Controlled breeding and reproductive management in water buffaloes (Bubalus bubalis) using Eazi Breed controlled internal drug release. J S Afr Vet Assoc. (2015) 86:1–5. doi: 10.4102/jsava.v86i1.1064
54. Srirattana K, Hufana-Duran D, Atabay EP, Duran PG, Atabay EC, Lu K, et al. Current status of assisted reproductive technologies in buffaloes. Anim Sci J. (2022) 93:e13767. doi: 10.1111/asj.13767
55. Baruselli PS, de Carvalho JGS, Elliff FM, da Silva JCB, Chello D, de Carvalho NAT. Embryo transfer in buffalo (Bubalus bubalis). Theriogenology. (2020) 150:221–8. doi: 10.1016/j.theriogenology.2020.01.037
56. Mohammed KM. Application of advanced reproductive biotechnologies for buffalo improvement with focusing on Egyptian buffaloes. Asian Pac J Reprod. (2018) 7:193–205. doi: 10.4103/2305-0500.241177
57. Paccola C, Resende C, Stumpp T, Miraglia S, Cipriano I. The rat estrous cycle revisited: a quantitative and qualitative analysis. Anim Reprod. (2018) 10:677–83.
58. Vecchio D, Neglia G, Gasparrini B, Russo M, Pacelli C, Prandi A, et al. Corpus luteum development and function and relationship to pregnancy during the breeding season in the Mediterranean buffalo. Theriogenology. (2012) 77:1811–5. doi: 10.1016/j.theriogenology.2011.12.025
59. Gunwant P, Pandey AK, Kumar A, Singh I, Kumar S, Phogat J, et al. Polymorphism of melatonin receptor (MTNR1A) gene and its association with seasonal reproduction in water buffalo (Bubalus bubalis). Anim Reprod Sci. (2018) 199:51–9. doi: 10.1016/j.anireprosci.2018.10.006
60. Avalos-Rosario I. Effect of Photoperiod and Other Environmental Factors on the Oocyte Populations of Water Buffalo (Bubalus bubalis) in Veracruz state, Mexico. Bangkok: Kasetsart University (2023). doi: 10.56825/bufbu.2023.4225352
61. Perera BM. Reproductive cycles of buffalo. Anim Reprod Sci. (2011) 124:194–9. doi: 10.1016/j.anireprosci.2010.08.022
62. Osman EEI. Evaluation of Ovarian Potential for In vitro Embryo Production in Indian Buffalo at Haryana State, India. Khartoum: Sudan University of Science and Technology (2020).
63. Harun-Or-Rashid M, Sarkar AK, Hasan MMI, Hasan M, Juyena NS. Productive, reproductive, and estrus characteristics of different breeds of buffalo cows in Bangladesh. J Adv Vet Anim Res. (2019) 6:553. doi: 10.5455/javar.2019.f382
64. Mujawar A, Razzaque W, Ramteke S, Patil A, Ali S, Bhikane A, et al. Estrus induction and fertility response in postpartum anoestrus Marathwadi buffaloes using hormonal protocol along with vitamin E and Selenium. Int J Livest Res. (2019) 9:289–96. doi: 10.5455/ijlr.20181113073117
65. Baruselli PS. Control of follicular development applied to reproduction biotechnologies in buffalo. Anais. (2001). p. 128–146.
66. Mandal DK, Kumar M, Tyagi S. Effect of seasons and photoperiods on seminal attributes and sperm morphology in Holstein Friesian × Sahiwal crossbred dairy bulls. Int J Biometeorol. (2022) 66:2223–35. doi: 10.1007/s00484-022-02350-x
67. Pal P, Rayees Dar M. Induction and synchronization of estrus. In:Aral F, Payan-Carreira R, Quaresma M, editors. Animal Reproduction in Veterinary Medicine. London: IntechOpen. (2021). p. 1–14. doi: 10.5772/intechopen.90769
68. Uniyal S, Panda R, Chouhan V, Yadav V, Hyder I, Dangi S, et al. Expression and localization of insulin-like growth factor system in corpus luteum during different stages of estrous cycle in water buffaloes (Bubalus bubalis) and the effect of insulin-like growth factor I on production of vascular endothelial growth factor and progesterone in luteal cells cultured in vitro. Theriogenology. (2015) 83:58–77. doi: 10.1016/j.theriogenology.2014.07.034
69. Purohit G, Rao TK. Estrus Detection in Buffaloes. Ithaca, NY: International Veterinary Information Service (2018). Available online at: https://www.ivis.org/library/bubaline-theriogenology/estrus-detection-buffaloes (accessed May 20, 2025).
70. Sianturi R, Kusumaningrum D, Praharani L, editors. Ovarian dynamics and progesterone profiles during estrus cycle in swamp buffaloes. In: AIP Conference Proceedings. Makassar: AIP Publishing (2023). doi: 10.1063/5.0153945
71. Chaudhari DV, Panchal MT, Dhami AJ, Sarvaiya NP, Pathan MM, Hadiya KK, et al. Follicular dynamics and endocrine profile during normal estrous cycle and early pregnancy in surti buffaloes. Indian J Vet Sci Biotechnol. (2022) 18:1–8. doi: 10.48165/ijvsbt.18.5.01
72. Cheng J, Pan Y, Yang S, Wei Y, Lv Q, Xing Q, et al. Integration of transcriptomics and non-targeted metabolomics reveals the underlying mechanism of follicular atresia in Chinese buffalo. J Steroid Biochem Mol Biol. (2021) 212:105944. doi: 10.1016/j.jsbmb.2021.105944
73. Noseir WM, El-Bawab IE, Hassan WR, Fadel MS. Ovarian follicular dynamics in buffaloes during different estrus synchronization protocols. Vet Sci Dev. (2014) 4:5315. doi: 10.4081/vsd.2014.5315
74. Das G, Kumawat B, Khan F. Ovarian follicular dynamics during estrous cycle and its aberrations during certain reproductive disorders in buffalo. Theriogenology. (2013) 3:37–46.
75. Baldrighi JM, Sá Filho MF, Siqueira A, Visintin JA, Baruselli PS, Assumpção M. Temporal evaluation of follicular dynamics and endocrine patterns of Holstein (Bos taurus), Gir (Bos indicus), and Murrah (Bubalus bubalis) heifers kept under the same nutritional, management and environmental conditions. Theriogenology. (2022) 190:8–14. doi: 10.1016/j.theriogenology.2022.07.006
76. Jan M, Kumar H, Kumar S, Sharma R, Gupta A, Mehrara K. Effect of progesterone administration during growing phase of first dominant follicle on follicular wave pattern in buffalo heifers. Trop Anim Health Prod. (2020) 52:1395–402. doi: 10.1007/s11250-019-02143-2
77. Taneja M, Ali A, Singh G. Ovarian follicular dynamics in water buffalo. Theriogenology. (1996) 46:121–30. doi: 10.1016/0093-691X(96)00147-1
78. Baruselli P, Mucciolo R, Visintin J, Viana W, Arruda R, Madureira E, et al. Ovarian follicular dynamics during the estrous cycle in buffalo (Bubalus bubalis). Theriogenology. (1997) 47:1531–47. doi: 10.1016/S0093-691X(97)00159-3
79. Warriach HM, Ahmad N. Follicular waves during the oestrous cycle in Nili-Ravi buffaloes undergoing spontaneous and PGF2α-induced luteolysis. Anim Reprod Sci. (2007) 101:332–7. doi: 10.1016/j.anireprosci.2007.01.013
80. Abulaiti A, El-Qaliouby HS, Yang L. Evaluation of single and multiple follicular dynamics in chinese crossbred buffalo (riverine × swamp). Intl J Agric Biol. (2018) 21:560–8. doi: 10.17957/IJAB/15.0929
81. Riaz U, Idris M, Ahmed M, Ali F, Yang L. Infrared thermography as a potential non-invasive tool for estrus detection in cattle and buffaloes. Animals. (2023) 13:1425. doi: 10.3390/ani13081425
82. Atabay EC, Maylem ERS, Salazar RL. Enhancing prostaglandin-based estrus synchronization protocol for artificial insemination in water buffaloes. Buffalo Bulletin. (2020) 39:53–60.
83. Bachlaus NK, Arora RC, Prasad A, Pandey RS. Plasma levels of gonadal hormones in cycling buffalo heifers. Indian J Exp Biol. (1979) 17:823–5.
84. Imtiaz B, Nadeem A, Mujahid H, Javed M. Genomic Study of silent heat: a novel mutation in HSD17β1 gene muting the potential of Black Gold of Asia. Pak J Zool. (2024). p. 1–7. doi: 10.17582/journal.pjz/20220817040832
85. Archunan G. Reproductive enhancement in buffalo: looking at urinary pheromones and hormones. Iran J Vet Res. (2020) 21:163.
86. Das GK, Khan FA. Summer anoestrus in buffalo–a review. Reprod Domest Anim. (2010) 45:e483–94. doi: 10.1111/j.1439-0531.2010.01598.x
87. Zambrano-Varon J. Studies in the Superovulatory Response to Gonadotropins and Folliculogenesis of Water Buffalo (Bubalus bubalis). Davis, CA: University of California (2013).
88. Galvão K, Federico P, De Vries A, Schuenemann GM. Economic comparison of reproductive programs for dairy herds using estrus detection, timed artificial insemination, or a combination. J Dairy Sci. (2013) 96:2681–93. doi: 10.3168/jds.2012-5982
89. Othman MA, Elshalofy AS, Abou-Ahmed MM, Ghallab ARM. The fertility outcomes of egyptian buffalo cows after ovsynch and presynch-ovsynch protocols. J Appl Vet Sci. (2023) 8:28–36. doi: 10.21608/javs.2023.218656.1246
90. Roy K, Prakash B. Changes in endocrine profiles during ovsynch and ovsynch plus norprolac treatment in Murrah buffalo heifers at hot summer season. Trop Anim Health Prod. (2009) 41:677–87. doi: 10.1007/s11250-008-9241-3
91. de Carvalho NA, Soares JG, Baruselli PS. Strategies to overcome seasonal anestrus in water buffalo. Theriogenology. (2016) 86:200–6. doi: 10.1016/j.theriogenology.2016.04.032
92. Monteiro BM, de Souza DC, Vasconcellos GS, Corrêa TB, Vecchio D, de Sá Filho MF, et al. Ovarian responses of dairy buffalo cows to timed artificial insemination protocol, using new or used progesterone devices, during the breeding season (autumn-winter). Anim Sci J. (2016) 87:13–20. doi: 10.1111/asj.12400
93. Gutiérrez-Añez JC, Palomares RA, Jiménez-Pineda JR, Camacho AR, Portillo-Martínez GE. Pregnancy rate in water buffalo following fixed-time artificial insemination using new or used intravaginal devices with two progesterone concentrations. Trop Anim Health Prod. (2018) 50:629–34. doi: 10.1007/s11250-017-1479-1
94. Répási A, Szelényi Z, Melo De Sousa N, Beckers JF, Nagy K, Szenci O. Effect of ovulation rate and timing of ovulation after different hormone treatments on pregnancy rate in dairy cows. Pol J Vet Sci. (2019) 22:355–62. doi: 10.24425/pjvs.2019.129228
95. Singh SK, Mehrotra S, Chandra P, Patra M, Kumar B, Kumar H, et al. Effect of pre-ovulatory follicle size on pregnancy rate in murrah buffaloes. Int J Curr Microbiol Appl Sci. (2020) 9:2968–72. doi: 10.20546/ijcmas.2020.908.333
96. Devi MG. Triggers in controlled ovarian hyperstimulation. Fertil Sci Res. (2023) 10:183–7. doi: 10.4103/fsr.fsr_40_23
97. Kauffman AS. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front Neurosci. (2022) 16:953252. doi: 10.3389/fnins.2022.953252
98. Kumar S, Kumar G, Gahalot SC. Anestrous in buffaloes and different treatment regimens: a mini review. Int J Curr Microbiol Appl Sci. (2019) 8:1162–79. doi: 10.20546/ijcmas.2019.803.138
99. Kenny D, Heslin J, Byrne C. Early onset of puberty in cattle: implications for gamete quality and embryo survival. Reprod Fertil Dev. (2018) 30:101–17. doi: 10.1071/RD17376
100. Duittoz A, Kenny D. Early and late determinants of puberty in ruminants and the role of nutrition. Animal. (2023) 17:100812. doi: 10.1016/j.animal.2023.100812
101. Sharma R, Jerome A, Purohit G. Reproductive physiology of the male and female buffalo. In:Purohit GN, editor. Bubaline Theriogenology. Ithaca, NY: International Veterinary Information Service (2014) p. 614.
102. Mondal G, Talukdar P, Das TK, Bhakat M, Mohini M. Influence of metabolizable energy and protein levels on age and weight at puberty in male buffalo. Indian J Anim Sci. (2021) 91:487–91. doi: 10.56093/ijans.v91i6.115452
103. Balamurugan B, Mehrotra S, Kumar V, Ramamoorthy M. Studies on age at puberty, service period, gestation period and calving interval in Vrindavani, Tharparkar cattle and Murrah buffalo. J Pharm Innov. (2020) 9:186–90.
104. Bertoni A, Napolitano F, Mota-Rojas D, Sabia E, Álvarez-Macías A, Mora-Medina P, et al. Similarities and differences between river buffaloes and cattle: health, physiological, behavioral and productivity aspects. J Buffalo Sci. (2020) 9:92–109. doi: 10.6000/1927-520X.2020.09.12
105. Samad M. A systematic review of research findings on buffalo health and production published during the last six decades in Bangladesh. J Vet Med One Health Res. (2020) 2:01–62. doi: 10.36111/jvmohr.2020.2(1).0016
106. Deka N, Nath K, Deka B, Bhuyan D, Saikia B, Das G, et al. Comparative study on genital status, energy balance and mineral content of swamp buffalo (Luit) heifers and cows under organized system of rearing in Assam, India. Pharm Innov J. (2020) SP-9:36–40.
107. Plansky V, Dimitrov D. Puberty Age and Body Weight of the Water Buffalo Heifers. Sofia: Faculty of Veterinary Medicine, University of Forestry (2020).
108. Pothireddy S, Honparkhe M, Singh G, Ahuja A, Dhindsa S, Singh P. Comparison of certain blood plasma biochemical profiles between normal and delayed pubertal buffalo heifers. J Entomol Zool Stud. (2020) 8:323–5.
109. Anwar M. Effect of calving season and climatic factors on age at puberty, service period and successful mating in Nili-Ravi buffalo. Buffalo Bull. (2021) 40:475–84.
110. Uddin AM, Petrovski KR, Song Y, Garg S, Kirkwood RN. Application of exogenous GnRH in food animal production. Animals. (2023) 13:1891. doi: 10.3390/ani13121891
111. Warriach H, McGill D, Bush R, Wynn P, Chohan K. A review of recent developments in buffalo reproduction—a review. Asian-Australas J Anim Sci. (2015) 28:451. doi: 10.5713/ajas.14.0259
112. Gupta SK, Singh P, Shinde KP, Lone SA, Kumar N, Kumar A. Strategies for attaining early puberty in cattle and buffalo: a review. Agric Rev. (2016) 37:160–7. doi: 10.18805/ar.v37i2.10741
113. Nair SJ, Brar A, Ahuja C, Sangha S, Chaudhary K. A comparative study on lipid peroxidation, activities of antioxidant enzymes and viability of cattle and buffalo bull spermatozoa during storage at refrigeration temperature. Anim Reprod Sci. (2006) 96:21–9. doi: 10.1016/j.anireprosci.2005.11.002
114. Campanile G, Vecchio D, Neglia G, Bella A, Prandi A, Senatore EM, et al. Effect of season, late embryonic mortality and progesterone production on pregnancy rates in pluriparous buffaloes (Bubalus bubalis) after artificial insemination with sexed semen. Theriogenology. (2013) 79:653–9. doi: 10.1016/j.theriogenology.2012.11.020
115. Thakur A, Birthal PS. Sexed semen technology for cattle breeding: an interpretative review on its performance, and implications for India's dairy economy. Agric Econ Res Rev. (2023) 36:53–64. doi: 10.5958/0974-0279.2023.00004.6
116. Praharani L, Sianturi RG. Inbreeding depression and alternative solution in buffaloes. WARTAZOA Indones Bull Anim Vet Sci. (2018) 28:1–12. doi: 10.14334/wartazoa.v28i1.1744
117. Sharma T, Gupta R, Gautam A, Giram P, Madan J. Effectiveness and performance of “Sexcel”-ABS sexed semen, in dairy heifers, cows and buffaloes in field conditions in different agro-climatic zones of India. J Anim Res. (2019) 9:499–504. doi: 10.30954/2277-940X.04.2019.1
118. Praharani L, Kusumaningrum D, Muttaqin Z, Sianturi R, Rusdiana S, Talib C, et al, editors. Pregnancy and calving rate of artificial insemination with sexed semen in swamp buffaloes under field conditions. In: IOP Conference Series: Earth and Environmental Science. London: IOP Publishing (2024). doi: 10.1088/1755-1315/1377/1/012077
119. Huan LK, Qing LY, Zhang M, Gan YX. SPERM sexing in buffalo using flow cytometry. In: 10th World Buffalo Congress and 7th Asian Buffalo Congress, Hilton Phuket Arcadia Resort and Spa, Phuket, Thailand, 6–8 May (2013).
120. Yata VK, Singh SK, Kumar S, Mohanty TK, Mohanty AK. Use of Sexed Semen for Genetic Improvement of Indigenous Dairy Cattle and Buffaloes Productivity. Cheyenne, WY: Remedy Publications LLC (2022). doi: 10.56093/ijans.v92i7.105407
121. Butnariu M, Sarac I. Biochemistry of hormones that influences feelings. Ann Pharm Drug. (2019) 1:1001.
122. Stárka L, Dušková M. What is a hormone? Physiol Res. (2020) 69:S183. doi: 10.33549/physiolres.934509
123. Baruselli PS, Soares JG, Gimenes LU, Monteiro BM, Olazarri M, Carvalho NAT. Control of buffalo follicular dynamics for artificial insemination, superovulation and in vitro embryo production. Buffalo Bull. (2013) 32:160–7.
124. Delli V, Silva MS, Prévot V, Chachlaki K. The KiNG of reproduction: kisspeptin/nNOS interactions shaping hypothalamic GnRH release. Mol Cell Endocrinol. (2021) 532:111302. doi: 10.1016/j.mce.2021.111302
125. Kah O, Lethimonier C, Somoza G, Guilgur L, Vaillant C, Lareyre J-J. GnRH and GnRH receptors in metazoa: a historical, comparative, and evolutive perspective. Gen Comp Endocrinol. (2007) 153:346–64. doi: 10.1016/j.ygcen.2007.01.030
126. Saleh AA, Hassanine NN, Taha TK, Rashad AM, Sharaby MA. Molecular regulation and genetic basis of gonadotropin-releasing hormone genes: a review. Appl Vet Res. (2023) 2:2023017. doi: 10.31893/avr.2023017
127. Saleh M, Holtz W.. LH pattern and ovarian response in ovsynch-synchronized superovulated goats induced to ovulate with GnRH or hCG. Theriogenology. (2022) 185:61–9. doi: 10.1016/j.theriogenology.2022.03.020
128. Shah SH, Memon AA, Kaka A, Tunio AN, Channo A, Kalwar Q, et al. Evaluation of ovsynch estrus synchronization in Holstein Friesian and Holstein Friesian-Jersey crossbred cows at District Thatta, Sindh, Pakistan. Pak J Zool. (2023) 1–18. doi: 10.17582/journal.pjz/20220523070520
129. Pursley J, Mee M, Wiltbank M. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. (1995) 44:915–23. doi: 10.1016/0093-691X(95)00279-H
130. Barański W, Nowicki A, Zduńczyk S, Tobolski D. Effect of repeated low doses of GnRH on follicular development and ovulation in anovulatory dairy cows with follicle growth to emergence size. Polish J Vet Sci. (2022) 25:391–6. doi: 10.24425/pjvs.2022.142022
131. Pacelli C, Barile VL, Sabia E, Casano AB, Braghieri A, Martina V, et al. Use of GnRH treatment based on pregnancy-associated glyco-proteins (PAGs) levels as a strategy for the maintenance of pregnancy in buffalo cows: a field study. Animals. (2022) 12:2822. doi: 10.3390/ani12202822
132. Li L, Shi X, Shi Y, Wang Z. The signaling pathways involved in ovarian follicle development. Front Physiol. (2021) 12:730196. doi: 10.3389/fphys.2021.730196
133. Zhang X-Q, Zhang L-J, Zhu X-L, Xu H, Luo Y-Q, Yao L, et al. The clinical efficacy of three different follicle-stimulating hormones for follicle growth and development in long-protocol controlled ovarian hyperstimulation treatment. Drug Des Dev Ther. (2021) 15:3573–80. doi: 10.2147/DDDT.S316189
134. Richard S, Zhou Y, Jasoni CL, Pankhurst MW. Ovarian follicle size or growth rate can both be determinants of ovulatory follicle selection in mice. Biol Reprod. (2024) 110:130–9. doi: 10.1093/biolre/ioad134
135. Sen U. Real-time assessment of the superovulatory effect of FSH and eCG with laparoscopy at different seasons in Akkaraman ewes. Pol J Vet Sci. (2020) 23:291–9. doi: 10.24425/pjvs.2020.133644
136. Manasa Varra GKV, Ramesh H, Suchitra B, Sudha G, Pooja C. Cellular and molecular pathways associated with ovarian physiology and estrus cycle in buffaloes. Hormones. (2022) 53:66.
137. Yao Y, Song X, Zhai Y, Liu S, Lu J, Xu X, et al. Transcriptome analysis of sheep follicular development during prerecruitment, dominant, and mature stages after FSH superstimulation. Domest Anim Endocrinol. (2021) 74:106563. doi: 10.1016/j.domaniend.2020.106563
138. Currin L, Baldassarre H, Bordignon V. In vitro production of embryos from prepubertal holstein cattle and mediterranean water buffalo: problems, progress and potential. Animals. (2021) 11:2275. doi: 10.3390/ani11082275
139. Arrebola FA, Torres-Martell R, González-Casquet O, Meza-Herrera CA, Pérez-Marín CC. Periovulatory hormonal profiles after estrus induction and conception rate by fixed-time AI in Payoya goats during the anestrous season. Animals. (2022) 12:2853. doi: 10.3390/ani12202853
140. Arya J, Madan M. Post-partum gonadotropins in suckled and weaned buffaloes. Indian Vet J. (2001) 78:406–9.
141. An G, Chen X, Li C, Zhang L, Wei M, Chen J, et al. Pathophysiological changes in female rats with estrous cycle disorder induced by long-term heat stress. BioMed Res Int. (2020) 2020:4701563. doi: 10.1155/2020/4701563
142. Vijayalakshmy K, Verma R, Rahman H, Prasad Yadav H, Virmani M, Kumar D, et al. Factors influencing seasonal anestrus in buffaloes and strategies to overcome the summer anestrus in buffaloes. Biol Rhythm Res. (2020) 51:907–14. doi: 10.1080/09291016.2018.1558740
143. Murugavel K, Antoine D, Raju M, López-Gatius F. The effect of addition of equine chorionic gonadotropin to a progesterone-based estrous synchronization protocol in buffaloes (Bubalus bubalis) under tropical conditions. Theriogenology. (2009) 71:1120–6. doi: 10.1016/j.theriogenology.2008.12.012
144. Succu S, Sale S, Ghirello G, Ireland J, Evans A, Atzori A, et al. Exposure of dairy cows to high environmental temperatures and their lactation status impairs establishment of the ovarian reserve in their offspring. J Dairy Sci. (2020) 103:11957–69. doi: 10.3168/jds.2020-18678
145. Sethi M, Shah N, Mohanty Tk, Bhakat M, Dewry Rk, Yadav Dk, et al. The induction of cyclicity in postpartum anestrus buffaloes: a review. J Exp Zool India. (2021) 24:989–97. Available online at: https://connectjournals.com/03895.2021.24.989
146. Kouamo J. Current concepts for estrus synchronization in bovine. Rev Mar Sci Agron Vét. (2017) 5:456–62.
147. Zicarelli L, editor News on buffalo cow reproduction. In: Proceedings 5th World Buffalo Congress, Royal Palace, Caserta, Italy. (1997).
148. Ramesh V, Devi LS, Joshi V, Mech M, Khate K, Khan MH. Ovarian follicular dynamics, hormonal profiles and ovulation time in Mithun cows (Bos frontalis). Reprod Domest Anim. (2022) 57:1218–29. doi: 10.1111/rda.14196
149. Seren E, Parmeggiani A, Campanile G, editors. The Control of Ovulation in Italian buffalo. Proc of the Symposium Reproduction and Animal Breeding. Milan: Advances and Strategy (1995).
150. Casteel CO, Singh G. Physiology, Gonadotropin-releasing Hormone. Treasure Island, FL: StatPearls Publishing (2025).
151. Duncan WC. The inadequate corpus luteum. Reprod Fertil. (2021) 2:C1–7. doi: 10.1530/RAF-20-0044
152. Beal JR, Ma Q, Bagchi IC, Bagchi MK. Role of endometrial extracellular vesicles in mediating cell-to-cell communication in the uterus: a review. Cells. (2023) 12:2584. doi: 10.3390/cells12222584
153. Hankele A-K, Rehm K, Berard J, Schuler G, Bigler L, Ulbrich SE. Progestogen profiling in plasma during the estrous cycle in cattle using an LC-MS based approach. Theriogenology. (2020) 142:376–83. doi: 10.1016/j.theriogenology.2019.10.005
154. Kitilit jk. Reproductive Hormone Concentrations and Histology of the Hypothalamus, Pituitary, Ovary, Uterus and Liver of Rabbits Fed on Aflatoxin Laced Diets. Eldoret: University of Eldoret (2022).
155. Wojnarowski K, Podobiński P, Cholewińska P, Smoliński J, Dorobisz K. Impact of estrogens present in environment on health and welfare of animals. Animals. (2021) 11:2152. doi: 10.3390/ani11072152
156. Nwankudu O. Endocrine, reproductive, neurophysiologic and extraneous activities of estrogen in vertebrates. Nig Vet J. (2020) 41:85–107. doi: 10.4314/nvj.v41i2.2
157. Shahbazi Y, Malekinejad H, Tajik H. Determination of naturally occurring estrogenic hormones in cow's and river buffalo's meat by HPLC-FLD method. J Food Drug Anal. (2016) 24:457–63. doi: 10.1016/j.jfda.2016.02.014
158. RJ M. Recruitment of follicles for superovulation. Comp Contin Educ Pract Veter. (1994) 16:127–30. doi: 10.1080/0158037940160201
159. Neglia G, Natale A, Esposito G, Salzillo F, Adinolfi L, Zicarelli L, et al. Follicular dynamics in synchronized Italian Mediterranean buffalo cows. Ital J Anim Sci. (2007) 6:611–4. doi: 10.4081/ijas.2007.s2.611
160. Voss A, Fortune J. Levels of messenger ribonucleic acid for cholesterol side-chain cleavage cytochrome P-450 and 3 beta-hydroxysteroid dehydrogenase in bovine preovulatory follicles decrease after the luteinizing hormone surge. Endocrinology. (1993) 132:888–94. doi: 10.1210/en.132.2.888
161. Hussein F, Metwally K, El-Nemr HS. reproductive performance of buffalo-heifers under stressful condition. Alex J Vet Sci. (2024) 80:178804. doi: 10.5455/ajvs.178804
162. Tsai S-J, Wiltbank MC. Prostaglandin F2α regulates distinct physiological changes in early and mid-cycle bovine corpora lutea. Biol Reprod. (1998) 58:346–52. doi: 10.1095/biolreprod58.2.346
163. Punetha m, kumar s, paul a, jose b, bharati j, sonwane a, et al. Deciphering the functional role of egr1 in prostaglandin f2 alpha induced luteal regression applying crispr in corpus luteum of buffalo. Biol Res. (2021) 54:1–10. doi: 10.1186/s40659-021-00333-7
164. Gabriel RRG, Atabay EP, Apolinario JPR, Ortiz JGM, Atabay EC, Orden EA. Effects of different prostaglandin (pgf2α) analogues during synchronization of ovulation in dairy buffaloes (Bubalus bubalis). Phil J Vet Anim Sci. (2019) 45:178–86.
165. De Carvalho JGS, de Carvalho NAT, de Souza DC, Júnior BM, Macedo GG, Vieira LM, et al. Administration of PGF2α during the periovulatory period increased fertilization rate in superovulated buffaloes. Theriogenology. (2020) 145:138–43. doi: 10.1016/j.theriogenology.2019.11.010
166. Paul A, Bharati J, Punetha M, Kumar S, Vidyalakshmi G, Chouhan VS, et al. Transcriptional regulation of thrombospondins and its functional validation through CRISPR/Cas9 mediated gene editing in corpus luteum of water buffalo (Bubalus bubalis). Cell Physiol Biochem. (2019) 52:532–52. doi: 10.33594/000000038
167. Yizengaw L. Review on estrus synchronization and its application in cattle. Int J Adv Res Biol Sci. (2017) 4:67–76. doi: 10.22192/ijarbs.2017.04.04.010
168. Ozis Altincekic S, Koyuncu M. Reproductive performance with short-time controlled internal drug release (CIDR)-based synchronization protocol for fixed-time artificial insemination in nulliparous and primiparous Saanen goats. Pol J Vet Sci. (2022) 25:13–8. doi: 10.24425/pjvs.2022.140835
169. Arshad U, Qayyum A, Hassan M, Husnain A, Sattar A, Ahmad N. Effect of resynchronization with GnRH or progesterone (P4) intravaginal device (CIDR) on Day 23 after timed artificial insemination on cumulative pregnancy and embryonic losses in CIDR-GnRH synchronized Nili-Ravi buffaloes. Theriogenology. (2017) 103:104–9. doi: 10.1016/j.theriogenology.2017.07.054
170. Neglia G, Capuano M, Balestrieri A, Cimmino R, Iannaccone F, Palumbo F, et al. Effect of consecutive re-synchronization protocols on pregnancy rate in buffalo (Bubalus bubalis) heifers out of the breeding season. Theriogenology. (2018) 113:120–6. doi: 10.1016/j.theriogenology.2018.01.020
171. Arya D, Goswami R, Sharma M. Estrous synchronization in cattle, sheep and goat. Multidiscip Rev. (2023) 6:2023001. doi: 10.31893/multirev.2023001
172. Ghuman S, Honparkhe M, Singh J, Dhami D, Kumar A, Nazir G, et al. Fertility response using three estrus synchronization regimens in lactating anestrous buffaloes. Indian J Anim Sci. (2012) 82:162. doi: 10.56093/ijans.v82i12.25676
173. Purohit G, Thanvi P, Pushp M, Gaur M, Saraswat C, Arora A, et al. Estrus synchronization in buffaloes: prospects, approaches and limitations. Pharm Innov J. (2019) 8:54–62.
174. Mekonnin AB. Monitoring and Improving Reproductive Performance of Crossbred Dairy Cattle in Tigray Region, Ethiopia [Ph.D. Thesis]. Edinburgh: University of Edinburgh (2017).
175. Monteiro PL, Consentini CEC, Andrade JPN, Beard AD, Garcia-Guerra A, Sartori R, et al. Research on timed AI in beef cattle: past, present and future, a 27-year perspective. Theriogenology. (2023) 211:161–71. doi: 10.1016/j.theriogenology.2023.07.037
176. Maria G, Giuseppinamaria T, Barile V, Antonio B. Overview on reproductive endocrine aspects in buffalo. J Buffalo Sci. (2012) 1:126–38. doi: 10.6000/1927-520X.2012.01.02.01
177. Sales J, Carvalho J, Crepaldi G, Soares J, Girotto R, Maio J, et al. Effect of circulating progesterone concentration during synchronization for fixed-time artificial insemination on ovulation and fertility in Bos indicus (Nelore) beef cows. Theriogenology. (2015) 83:1093–100. doi: 10.1016/j.theriogenology.2014.12.009
178. Baruselli PS, Ferreira RM, Colli MHA, Elliff FM, Sá Filho MF, Vieira L, et al. Timed artificial insemination: current challenges and recent advances in reproductive efficiency in beef and dairy herds in Brazil. Anim Reprod. (2018) 14:558–71. doi: 10.21451/1984-3143-AR999
179. Carvalho NATd, Carvalho JGSd, Sales JNdS, Macari RC, Baruselli PS. Different times to perform timed artificial insemination when using a P4/E2/eCG-based protocol in buffalo. Ciênc Rural. (2020) 50:e20190784. doi: 10.1590/0103-8478cr20190784
180. Bisen A, Shukla SN, Shukla MK, Mishra A. Fertility response using timed insemination protocols in sub-oestrus buffaloes. J Anim Res. (2018) 8:417–21. doi: 10.30954/2277-940X.06.2018.13
181. Akhtar MS, Ullah S, Farooq AA, Mazhar M, Murtaza S. Pregnancy rate in lactating buffaloes treated with or without estradiol after estrus synchronization protocols at timed AI. Buffalo Bull. (2013) 32:366–9.
182. Rathore R, Sharma R, Phulia S, Mudgal V, Jerome A, Ghuman S, et al. Comparative efficacy of oestrus synchronization protocols in buffalo (Bubalus bubalis). Trop Anim Health Prod. (2017) 49:1377–82. doi: 10.1007/s11250-017-1337-1
183. Ciornei SG, Roşca P. Upgrading the fixed-time artificial insemination (FTAI) protocol in Romanian buffaloes. Front Vet Sci. (2023) 10:1265060. doi: 10.3389/fvets.2023.1265060
184. Dorji D, Rai GR, Tamang NB. Responses of local buffaloes to estrus synchronization and fixed timed artificial insemination in subtropical Bhutan. Bhutan J Anim Sci. (2021) 5:18–26.
185. Ravikumar K, Selvaraju M, Napolean E, Prakash S. Effect of Ovsynch protocol on conception in postpartum buffaloes during peak and low breeding seasons. Pharm Innov J. (2021) SP-10:590–3.
186. Perry G, Ketchum J, Epperson K, Quail L. Improving reproductive management in cow herds. Clin Theriogenol. (2022) 14:174–92.
187. Riaz U, Hassan M, Husnain A, Naveed MI, Singh J, Ahmad N. Effect of timing of artificial insemination in relation to onset of standing estrus on pregnancy per AI in Nili-Ravi buffalo. Anim Reprod. (2018) 15:1231. doi: 10.21451/1984-3143-AR2017-0015
188. Loeffler S, De Vries M, Schukken Y, De Zeeuw A, Dijkhuizen A, De Graaf F, et al. Use of AI technician scores for body condition, uterine tone and uterine discharge in a model with disease and milk production parameters to predict pregnancy risk at first AI in Holstein dairy cows. Theriogenology. (1999) 51:1267–84. doi: 10.1016/S0093-691X(99)00071-0
189. Kumbhar UB, Deshpande VP, Gulavane SU, Gaikwad SM. Enhancement of conception rate through luteotropic hormones with special reference to metabolic, biochemical profile and cervical mucus properties in non-infectious repeat breeding Murrah buffaloes. Buffalo Bull. (2020) 39:61–74.
190. Coffman EA, Pinto CR. A review on the use of prostaglandin F2α for controlling the estrous cycle in mares. J Equine Vet Sci. (2016) 40:34–40. doi: 10.1016/j.jevs.2016.01.008
191. Barański W, Nowicki A, Zduńczyk S. Comparison of efficacy of Ovsynch protocol to single PGF2α administration in treatment of individual dairy cows with post-service subestrus. Pol J Vet Sci. (2021) 24:351–4. doi: 10.24425/pjvs.2021.137672
192. Shah KB, Tripathy S, Suganthi H, Rudraiah M. Profiling of luteal transcriptome during prostaglandin F2-alpha treatment in buffalo cows: analysis of signaling pathways associated with luteolysis. PLoS ONE. (2014) 9:e104127. doi: 10.1371/journal.pone.0104127
193. Gugssa T. Effects of prostaglandin administration frequency, artificial insemination timing and breed on fertility of cows and heifers in eastern zone of Tigray region, Ethiopia. Mekelle: Mekelle Univeristy (2015).
194. Roy B, Moniruzzaman M, Pasha M, Modak A, Alam M, Islam M, et al. PGF 2α induced estrus synchronization of native buffaloes in Bangladesh. Bangladesh J Anim Sci. (2021) 50:80–5. doi: 10.3329/bjas.v50i2.58134
195. Barile VL. Technologies related with the artificial insemination in buffalo. J Buffalo Sci. (2012) 1:139–46. doi: 10.6000/1927-520X.2012.01.02.02
196. Colazo MG, Mapletoft RJ, A. review of current timed-AI (TAI) programs for beef and dairy cattle. Can Vet J. (2014) 55:772.
197. Drugociu D, Roşca P, Ciornei S. Induction of Estrus in Buffalo by Luteolysis Management (Single PGF and Dual Administration). Lucrari Stiintifice-seria Medicina Veterinara (2019) p. 62.
198. Kumar PR, Singh SK, Kharche SD, Govindaraju CS, Behera BK, Shukla SN, et al. Anestrus in cattle and buffalo: Indian perspective. Adv Anim Vet Sci. (2014) 2:124–38. doi: 10.14737/journal.aavs/2014/2.3.124.138
199. Ganesh K, Selvaraju M, Madheswaran R, Balasubramaniam GA. Resumption of postpartum reproductive cyclicity and pregnancy rates after treatment with CIDR plus PGF. Vet Arh. (2022) 92:559–76. doi: 10.24099/vet.arhiv.1200
200. Pandit A, Poudel S, Gautam M, Shah S. Status of estrus synchronization in Nepal. Vet Sci: Res Rev. (2022) 8:81–9. doi: 10.17582/journal.vsrr/2022/8.2.81.89
201. Haider S, Chishti GA, Mehmood MU, Jamal MA, Mehmood K, Shahzad M, et al. The effect of GnRH administration/insemination time on follicular growth rate, ovulation intervals, and conception rate of Nili Ravi buffalo heifers in 7–day-CIDR Co-synch. Trop Anim Health Prod. (2021) 53:558. doi: 10.1007/s11250-021-03003-8
202. Sharma R, Phulia S, Jerome A, Singh I. Ovsynch Plus protocol improves ovarian response in anovular Murrah buffaloes in low-breeding season. Reprod Domest Anim. (2017) 52:1030–5. doi: 10.1111/rda.13020
203. Gupta K, Shukla S, Inwati P, Shrivastava O. Fertility response in postpartum anoestrus buffaloes (Bubalus bubalis) using modified Ovsynch based timed insemination protocols. Vet World. (2015) 8:316. doi: 10.14202/vetworld.2015.316-319
204. Hoque MN, Talukder AK, Akter M, Shamsuddin M. Evaluation of ovsynch protocols for timed artificial insemination in water buffaloes in Bangladesh. Turkish J Vet Anim Sci. (2014) 38:418–24. doi: 10.3906/vet-1302-35
205. Paul V, Prakash B. Efficacy of the Ovsynch protocol for synchronization of ovulation and fixed-time artificial insemination in Murrah buffaloes (Bubalus bubalis). Theriogenology. (2005) 64:1049–60. doi: 10.1016/j.theriogenology.2005.02.004
206. Warriach HM, Channa AA, Ahmad N. Effect of oestrus synchronization methods on oestrus behaviour, timing of ovulation and pregnancy rate during the breeding and low breeding seasons in Nili-Ravi buffaloes. Anim Reprod Sci. (2008) 107:62–7. doi: 10.1016/j.anireprosci.2007.06.007
207. Chaikhun T, Tharasanit T, Rattanatep J, De Rensis F, Techakumphu M. Fertility of swamp buffalo following the synchronization of ovulation by the sequential administration of GnRH and PGF2alpha combined with fixed-timed artificial insemination. Theriogenology. (2010) 74:1371–6. doi: 10.1016/j.theriogenology.2010.06.007
208. de Araujo Berber RC, Madureira EH, Baruselli PS. Comparison of two Ovsynch protocols (GnRH versus LH) for fixed timed insemination in buffalo (Bubalus bubalis). Theriogenology. (2002) 57:1421–30. doi: 10.1016/S0093-691X(02)00639-8
209. Neglia G, Gasparrini B, Di Palo R, De Rosa C, Zicarelli L, Campanile G. Comparison of pregnancy rates with two estrus synchronization protocols in Italian Mediterranean Buffalo cows. Theriogenology. (2003) 60:125–33. doi: 10.1016/S0093-691X(02)01328-6
210. Barile V, Pacelli C, De Santis G, Penna L, Veloccia C, Allegrini S, et al, editors. Fixed time artificial insemination in buffalo using two different hormonal schedule for oestrus synchronization. In: Preliminary results. Proc 7th World Buffalo Congress, Manila, Philippine, October (2004).
211. Barile V, Terzano G, Pacelli C, Todini L, Malfatti A, Barbato O, et al. peak and ovulation after two different estrus synchronization treatments in buffalo cows in the daylight-lengthening period. Theriogenology. (2015) 84:286–93. doi: 10.1016/j.theriogenology.2015.03.019
212. De Rensis F, Ronci G, Guarneri P, Nguyen BX, Presicce G, Huszenicza G, et al. Conception rate after fixed time insemination following ovsynch protocol with and without progesterone supplementation in cyclic and non-cyclic Mediterranean Italian buffaloes (Bubalus bubalis). Theriogenology. (2005) 63:1824–31. doi: 10.1016/j.theriogenology.2004.07.024
213. Ali A, Fahmy S. Ovarian dynamics and milk progesterone concentrations in cycling and non-cycling buffalo-cows (Bubalus bubalis) during Ovsynch program. Theriogenology. (2007) 68:23–8. doi: 10.1016/j.theriogenology.2007.03.011
214. Karen AM, Darwish SA. Efficacy of Ovsynch protocol in cyclic and acyclic Egyptian buffaloes in summer. Anim Reprod Sci. (2010) 119:17–23. doi: 10.1016/j.anireprosci.2009.12.005
215. Campanile G, Neglia G. Embryonic mortality in buffalo cows. Ital J Anim Sci. (2007) 6:119–29. doi: 10.4081/ijas.2007.s2.119
216. Campanile G, Neglia G, Michael J. Embryonic and fetal mortality in river buffalo (Bubalus bubalis). Theriogenology. (2016) 86:207–13. doi: 10.1016/j.theriogenology.2016.04.033
217. Campanile G, Baruselli PS, Neglia G, Vecchio D, Gasparrini B, Gimenes LU, et al. Ovarian function in the buffalo and implications for embryo development and assisted reproduction. Anim Reprod Sci. (2010) 121:1–11. doi: 10.1016/j.anireprosci.2010.03.012
218. Consentini CE, Abadia T, Galindez JP, Lopes AL, Emerich Y, Ferro PP, et al. (To be Submitted for Journal of daIry scieNce): Fertility Programs for lactaTing Dairy Cows: A Novel Presynch+ Timed AI Program (Double e-synch) Produces Similar Ovarian Dynamics, Synchronization, and Fertility as Double-Ovsynch. Piracicaba: “Luiz de Queiroz” College of Agriculture (2022). p. 65.
219. Li Z, Luan S, Yan L, Xie C, Lian Z, Yang M, et al. Effect of Double-Ovsynch and Presynch-Ovsynch on postpartum ovarian cysts and inactive ovary in high-yielding dairy cows. Front Vet Sci. (2024) 11:1348734. doi: 10.3389/fvets.2024.1348734
220. Santos V, Carvalho P, Maia C, Carneiro B, Valenza A, Fricke P. Fertility of lactating Holstein cows submitted to a Double-Ovsynch protocol and timed artificial insemination versus artificial insemination after synchronization of estrus at a similar day in milk range. J Dairy Sci. (2017) 100:8507–17. doi: 10.3168/jds.2017-13210
221. Astiz S, Fargas O. Pregnancy per AI differences between primiparous and multiparous high-yield dairy cows after using Double Ovsynch or G6G synchronization protocols. Theriogenology. (2013) 79:1065–70. doi: 10.1016/j.theriogenology.2013.01.026
222. Raval R, Vala K, Padodara R, Dhami A, Kavani F. Evaluation of double ovsynch protocol in acyclic Jaffarabadi heifers and buffaloes with respect to ovarian dynamics, hormonal profile and fertility. Indian J Anim Res. (2021) 55:879–88. doi: 10.18805/ijar.B-4154
223. Giordano J, Wiltbank M, Guenther J, Pawlisch R, Bas S, Cunha A, et al. Increased fertility in lactating dairy cows resynchronized with Double-Ovsynch compared with Ovsynch initiated 32 d after timed artificial insemination. J Dairy Sci. (2012) 95:639–53. doi: 10.3168/jds.2011-4418
224. Peralta-Torres JA, Torres-Chablé OM, Segura-Correa JC, Ojeda-Robertos NF, Chay-Canul AJ, Luna-Palomera C, et al. Ovarian dynamics of buffalo (Bubalus bubalis) synchronized with different hormonal protocols. Trop Anim Health Prod. (2020) 52:3475–80. doi: 10.1007/s11250-020-02381-9
225. Atabay EP. Synchronization of Ovulation and Fixed Time AI in Water Buffaloes: Recent Development and Insights on Philippine Initiatives. Nueva Ecija: International Training Course on Dairy Herd Improvement by the Use of Reproductive Biotechnologies, Philippine Carabao Center, Science City of Munoz (2015). p. 13.
226. Bota A, Bujdei H, Chiorean R. Oestrus synchronization and artificial insemination at fixed times protocols in dairy buffaloes. Sci Pap. (2021) 54:75.
227. Cardoso Consentini CE, Wiltbank MC, Sartori R. Factors that optimize reproductive efficiency in dairy herds with an emphasis on timed artificial insemination programs. Animals. (2021) 11:301. doi: 10.3390/ani11020301
228. Dominguez M. Effects of body condition, reproductive status and breed on follicular population and oocyte quality in cows. Theriogenology. (1995) 43:1405–18. doi: 10.1016/0093-691X(95)00126-S
229. Devkota B, Shah S, Gautam G. Reproduction and fertility of buffaloes in Nepal. Animals. (2022) 13:70. doi: 10.3390/ani13010070
230. Devkota B. Association of nutritional status to reproductive performance in buffaloes. J Agric For Univ. (2018) 2:1–7.
231. Cavalieri J, Fitzpatrick L. Artificial insemination of Bos indicus heifers: the effects of body weight, condition score, ovarian cyclic status and insemination regimen on pregnancy rate. Aust Vet J. (1995) 72:441–7. doi: 10.1111/j.1751-0813.1995.tb03485.x
232. Akhtar HMB, Mehmood S, Aziz U, Andrabi SMH, Riaz MN. Factors affecting longevity and reasons for culling in dairy cattle and buffalo. J Agric Vet Sci. (2023) 2:251–70. doi: 10.55627/agrivet.002.03.0439
233. Almeida J, Brito M, Neves B, Becerra V, Auler P, Hadad J, et al. Use of cooled buffalo semen as a strategy to increase conception rates in fixed-time artificial insemination programs during unfavorable reproductive periods. Arq Bras Med Vet Zootec. (2021) 73:560–70. doi: 10.1590/1678-4162-12142
234. Ivanova MG, Gradinarska DG, Tsvetkov TS, Kirilova IV, Georgiev BA. Chromatography analysis of seminal plasma proteins in buffalo semen samples with high and low cryotolerance. Pol J Vet Sci. (2019) 22:11–6. doi: 10.24425/pjvs.2018.125617
235. Patil S, Kumar P, Singh G, Bala R, Jerome A, Patil C, et al. ‘Semen dilution effect'on sperm variables and conception rate in buffalo. Anim Reprod Sci. (2020) 214:106304. doi: 10.1016/j.anireprosci.2020.106304
236. Yilma T. Estrus Response of Boran and Boran-Holstein Crossed Cattle to pgf2α and Pregnancy Rates to Sexed and Conventional Semen, Holeta, Ethiopia. Addis Ababa: Addis Ababa University (2020).
237. Zicarelli L. Enhancing reproductive performance in domestic dairy water buffalo (Bubalus bubalis). In:Lucy M, Pate J, Smith M, Spencer T, editors. Reproduction in Domestic Ruminants VII. UkRBQHdpbGV5LmNvbQ== (2011). p. 443–55. doi: 10.7313/UPO9781907284991.034
238. Sarath T, Singh S, Arunmozhi N, Rajkumar R, Saxena A, Agarwal S. Characterization of ovarian follicular development and steroid profile during estrous cycle and seasonal anestrous in buffalo. Indian J Anim Sci. (2016) 86:28–31. doi: 10.56093/ijans.v86i1.54995
239. Kabir ME, Islam MS, Dadok F, Islam MR, Rahman A-NMI, Naim Z. Influence of season on the ovarian biometry, oocyte yield and quality in buffaloes. Asian J Res Anim Vet Sci. (2021) 8:152–8. doi: 10.9734/ajravs/2021/v4i4179
240. De Rensis F, Lopez-Gatius F. Protocols for synchronizing estrus and ovulation in buffalo (Bubalus bubalis): a review. Theriogenology. (2007) 67:209–16. doi: 10.1016/j.theriogenology.2006.09.039
241. Nava-Trujillo H, Valeris-Chacin R, Morgado-Osorio A, Zambrano-Salas S, Tovar-Breto L, Quintero-Moreno A. Reproductive performance of water buffalo cows: a review of affecting factors. J Buffalo Sci. (2020) 9:133–51. doi: 10.6000/1927-520X.2019.08.03.15
243. Omran FI. Impact of frequent heat stress on physiological and productive performance of buffalo dams during the dry period of the Nile Valley climate. Egypt J Agric Res. (2023) 101:151–65.
244. Bhakat M, Maiti S, Mohanty T, Mondal G, Baithalu R. Impact of climate change on dairy production. Clim Resil Anim Husb. (2021) 104:104–23.
245. Reswati R, Purwanto B, Priyanto R, Manalu W, Arifiantini R, editors. Reproductive performance of female swamp buffalo in West Sumatra. In: IOP Conference Series: Earth and Environmental Science. London: IOP Publishing (2021). doi: 10.1088/1755-1315/748/1/012025
246. Dash S, Chakravarty A, Singh A, Upadhyay A, Singh M, Yousuf S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: a review. Vet World. (2016) 9:235. doi: 10.14202/vetworld.2016.235-244
247. Fernandez-Novo A, Pérez-Garnelo SS, Villagrá A, Pérez-Villalobos N, Astiz S. The effect of stress on reproduction and reproductive technologies in beef cattle-a review. Animals. (2020) 10:2096. doi: 10.3390/ani10112096
248. Patel A, Patel J, Dhami A, Prajapati A, Sarvaiya N. Efficacy of doublesynch, estradoublesynch and progesterone based protocols for improving fertility in anestrus Surti buffaloes. Indian J Dairy Sci. (2018) 71:84–8. Available online at: http://epubs.icar.org.in/ejournal/index.php/IJDS/article/view/72776/31963
249. Haider M, Hassan M, Khan A, Husnain A, Bilal M, Pursley J, et al. Effect of timing of insemination after CIDR removal with or without GnRH on pregnancy rates in Nili-Ravi buffalo. Anim Reprod Sci. (2015) 163:24–9. doi: 10.1016/j.anireprosci.2015.09.010
250. Carvalho N, Soares J, Baruselli PS. Evolution and perspectives of timed artificial insemination (TAI) programs in Brazil-A review. Indian J Anim Reprod. (2018) 39:1–8.
251. Baruselli PS, de Abreu LÂ, de Paula VR, Albertini S, dos Santos GFF, Rebeis LM, et al. Breeding for sustainability: how reproductive biotechnologies can help buffalo farmers combat climate change. Rev Cient Fac Vet. (2023) 33. doi: 10.52973/rcfcv-wbc011
252. Ciornei SG, Ucar O, Lopes G, Cenariu M. Perspectives in the biotechnology of artificial insemination in ruminants. Front Vet Sci. (2024) 11:1376794. doi: 10.3389/fvets.2024.1376794
253. Mastromonaco GF, Gonzalez-Grajales AL. Reproduction in female wild cattle: influence of seasonality on ARTs. Theriogenology. (2020) 150:396–404. doi: 10.1016/j.theriogenology.2020.02.016
254. Mancheno-Herrera A, Almeida-López F, Tello-Flores L. Estrous cycle of buffaloes bubalus bubalis determined with a digital estrus and ovulation detector. Ann For Res. (2023) 66:2969–79.
255. Hirabhai PK, Hirjibhai TP, Hirjibhai SH, Bhagvanbhai VK. Seasonal variation in semen quality and conception rate of Jaffarabadi buffalo bulls (Bubalus bubalis) in India. Buffalo Bull. (2022) 41:431–9. doi: 10.56825/bufbu.2022.4133642
256. Mehla R. Effect of non-genetic factors on various Reproduction traits in Murrah Buffaloes. Indian J Dairy Sci. (2018) 71:193–97.
257. Javed M, Nadeem A. Hassan F-u, Mujahid H, Rehman SU. Genomic analysis of arginine vasopressin gene in riverine buffalo reveals its potential association with silent estrus behavior. Mol Biol Rep. (2022) 49:9315–24. doi: 10.1007/s11033-022-07776-5
258. Mota-Rojas D, Bragaglio A, Braghieri A, Napolitano F, Domínguez-Oliva A, Mora-Medina P, et al. Dairy buffalo behavior: calving, imprinting and allosuckling. Animals. (2022) 12:2899. doi: 10.3390/ani12212899
259. Jan M, Kumar H, Kumar S, Malla W, Sharma R. Comparative biochemical profiles, utero-ovarian function, and fertility of the postpartum buffalo with and without subclinical endometritis. Trop Anim Health Prod. (2021) 53:1–11. doi: 10.1007/s11250-020-02502-4
260. Juneja R, Jhamb D, Gaur M, Sharma SK, Sain A. A retrospective study on causes of dystocia in cattle and buffaloes at referral Centre in South Rajasthan. Indian J Vet Sci Biotechnol. (2023) 19:104–6. doi: 10.48165/ijvsbt.19.2.21
261. Yotov S, Atanasov A, Ilieva Y. Therapy of ovarian inactivity in postpartum Bulgarian Murrah buffaloes by PRID and Ovsynch estrus synchronization protocols. Asian Pac J Reprod. (2012) 1:293–9. doi: 10.1016/S2305-0500(13)60095-0
262. Kharel C, Devkota B, Sah S, Karki R. Evaluation of ovsynch protocol on reproductive performance of anestrous buffaloes during breeding season in Chitwan, Nepal. Nepalese Vet J. (2017) 34:41–50. doi: 10.3126/nvj.v34i0.22902
263. Oropeza AJ, Rojas ÁF, Velazquez MA, Muro JD, Márquez YC, Vilanova LT. Efficiency of two timed artificial insemination protocols in Murrah buffaloes managed under a semi-intensive system in the tropics. Trop Anim Health Prod. (2010) 42:1149–54. doi: 10.1007/s11250-010-9539-9
264. Shah S, Gautam G, Kharel C, Lamsal D, Pandeya Y, Devkota B, editors. Response of novel hormonal protocol in anestrus buffaloes during different breeding seasons. In: Proceedings of International Buffalo Symposium 2017, Chitwan, Nepal, November 15–18 (2017).
265. Atanasov A, Yotov S, Antonov A, Kolev P. Induction of oestrus and conception rates in Bulgarian Murrah buffaloes after fixed-time artificial insemination (a preliminary study). Bulg J Vet Med. (2011) 14:165–70.
266. Mirmahmoudi R, Prakash B. The endocrine changes, the timing of ovulation and the efficacy of the Doublesynch protocol in the Murrah buffalo (Bubalus bubalis). Gen Comp Endocrinol. (2012) 177:153–9. doi: 10.1016/j.ygcen.2012.03.004
267. Wagan SA, Memon AA, Kalwer Q, Korejo RA, Khokhar TA, Khoso ZA, et al. Efficacy of estrous synchronization protocols ovsynch and cosynch in kundhi buffalo heifers. IOSR J Agric Vet Sci. (2019) 12:9–11. doi: 10.9790/2380-1202020911
268. Derar R, Hussein H, Fahmy S, El-Sherry T, Megahed G. The effect of parity on the efficacy of an ovulation synchronization (Ovsynch) protocol in buffalo (Bubalus bubalis). Anim Reprod. (2018) 9:52–60.
269. Pushp MK, Tiwari RK, Meena DS. Comparative analysis of two protocols (Ovsynch and double synch) in postpartum cyclical and anoestrus buffaloes. Int. J. Vet. Sci. Anim. Husbandry. (2024) 9:481–4.
270. Bhat G, Dhaliwal G, Ghuman S, Honparkhe M. Comparative efficacy of E-17β and GnRH administration on day 0 of a controlled internal drug release (CIDR) based protocol on synchrony of wave emergence, ovulation and conception rates in Murrah buffalos (Bubalus bubalis). Iran J Vet Res. (2015) 16:53.
Keywords: buffalo, estrous synchronization, reproductive cycle, TAI, FTAI protocols, fixed-time artificial insemination (FTAI)
Citation: Kolachi HA, Zhang X, Rahimoon MM, Shahzad M, Oluwaseun AJ, Panhwar MI, Hassan MF, Tawfik Kandil OM, Wan P and Zhao X (2025) Fixed-time artificial insemination technology in buffaloes: a review. Front. Vet. Sci. 12:1586609. doi: 10.3389/fvets.2025.1586609
Received: 03 March 2025; Accepted: 09 May 2025;
Published: 02 June 2025.
Edited by:
Kangfeng Jiang, Yunnan Agricultural University, ChinaReviewed by:
Stefan Gregore Ciornei, Iasi, University of Life Science (IULS), RomaniaChao Xu, Jilin Agricultural University, China
Copyright © 2025 Kolachi, Zhang, Rahimoon, Shahzad, Oluwaseun, Panhwar, Hassan, Tawfik Kandil, Wan and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueming Zhao, emhhb3h1ZW1pbmdAY2Fhcy5jbg==; Pengcheng Wan, d2FucGNAaG90bWFpbC5jb20=
 Hubdar Ali Kolachi
Hubdar Ali Kolachi Xiaomeng Zhang2
Xiaomeng Zhang2 Muhammad Shahzad
Muhammad Shahzad Ayantoye Jesse Oluwaseun
Ayantoye Jesse Oluwaseun Xueming Zhao
Xueming Zhao