- 1Earth Science Department, Paleo[Fab]Lab, University of Florence, Firenze, Italy
- 2Natural History Museum, University of Florence, Firenze, Italy
- 3Georgian National Museum, Tbilisi, Georgia
- 4IPHES, Institut Català de Paleoecologia Humana i Evolució Social, Tarragona, Spain
- 5Area de Prehistoria, Universitat Rovira i Virgili (URV), Tarragona, Spain
- 6ICREA, Barcelona, Spain
In the complex scenario of Plio–Pleistocene mammalian faunal turnovers, recent research on canids has revealed an increasingly higher number of species than previously thought. In this framework, Georgia had a key role in the biogeographic dispersion of fauna from/to Asia, Africa, and Europe. Historically attributed to Canis etruscus, the rich Canis material recovered from Dmanisi possesses certain peculiar cranial and dentognathic features, which cannot be regarded only as intraspecific variability. We revealed closer similarities between the Dmanisi wolf and the younger European Canis mosbachensis, rather than with other Early Pleistocene canids as C. etruscus and Canis arnensis. The discovery of a Canis borjgali sp. nov. in Dmanisi, with characteristics close to those of C. mosbachensis, changes radically the idea of Canis lupus evolution as it is conveyed today, invalidating the paradigm C. etruscus–C. mosbachensis–C. lupus lineage. Furthermore, the geographic position of Dmanisi in the Caucasian area offers interesting insights regarding the Asian canids and their dispersion into Europe and Africa, an aspect still poorly investigated. The exquisite state of preservation of the fossil from Dmanisi combined with novel 3D visualization and a digital imaging technique gives us the opportunity to increase the outreach of the research thanks to user-friendly and free tools. Here, for the first time, we employed augmented reality on a few specimens of C. borjgali sp. nov. through a simple web app. The extraordinary chance offered by these technologies has yet to be implemented in scientific research and dissemination, particularly in paleontology.
Introduction
In the last decades, genetic and paleontological studies have revealed an intricate evolutionary history of the genus Canis (Tedford et al., 2009; Koepfli et al., 2015; Viranta et al., 2017; Zrzavý et al., 2018). The genus Canis Linnaeus, 1758, since its early appearance in North American Late Miocene and its dispersal and radiation throughout Asia, is known to have reached the western part of Europe around 3 Ma (Lacombat et al., 2008), where the first species described is Canis etruscus Forsyth-Major, 1877 from several sites of Europe (Cherin et al., 2014). There is wide consensus considering the modern wolf as the final stage of an evolutionary line starting with the Early Pleistocene C. etruscus through the Early–Middle Pleistocene Canis mosbachensis Soergel, 1925 (see inter alios Brugal and Boudadi-Maligne, 2011). Morphologically modern wolves appear in the early Middle Pleistocene of North America (Tedford et al., 2009) and in several mid Middle Pleistocene Asian and European localities (Bonifay, 1971). In the framework of the Early and Middle Pleistocene radiation of Eurasian mammals, the site of Dmanisi is celebrated for its outstanding record of early Homo Linnaeus, 1758 (Lordkipanidze et al., 2007, 2013; Krijgsman et al., 2019; Rightmire et al., 2019), for the state of preservations of the fossils (allowing molecular analyses on its fossils, Cappellini et al., 2019), and for the pivotal documentation of the biogeographic faunal dispersals between the three continents, Asia, Africa, and Europe. Indeed, the Georgian site offers a unique glimpse on the turnovers that took place around 1.8 Ma (during the late Villafranchian Land Mammal Age) (Ferring et al., 2011; Bartolini Lucenti et al., 2019; Krijgsman et al., 2019). Among the large number of mammalian taxa recovered, carnivores are abundant. At least 13 different species of the five most common families of carnivorans are represented. Of these, Canis (referred to C. etruscus by Vekua, 1995) is by far the most abundant taxon with eight almost complete crania and more than 270 cranial specimens. This rich and remarkably well-preserved sample allowed us to assess its variability, compared to that of other Early and Middle Pleistocene canids. We here suggest an early origin of modern wolves and related species, redescribing the Canis from Dmanisi as a new species.
Materials and Methods
The Comparative Sample
The present study is based on the comparative morphological analysis of the medium-sized Canis from Dmanisi and other wolf-like canids (tribe Canini) of the Early–Middle Pleistocene from Europe. The described fossils are housed at the S. Janashia Museum of Georgia, Georgian National Museum (Tbilisi). As comparative fossil material, the late Villafranchian canids from Eurasia housed at AMNH, AUT, ICP, and IGF (see abbreviation below) were studied. This fossil comparative sample includes specimens of Canis arnensis Del Campana, 1913 and C. etruscus from the Italian sites of Olivola, Upper Valdarno Basin, Coste San Giacomo, and Pantalla (Cherin et al., 2014); Canis apolloniensis Koufos and Kostopoulos (1997) from Apollonia-1 (Koufos, 2018); Canis chihliensis and Canis palmidens from Yushe Basin (Rook, 1993, 1994); remains of C. mosbachensis Soergel, 1925 from Cueva Victoria, Vallparadís (Bartolini Lucenti et al., 2017), Pirro Nord (Petrucci et al., 2013), ‘Ubeidiya (Martínez-Navarro et al., 2009), Untermassfeld (Sotnikova, 2001), and Zhoukoudian (Jiangzuo et al., 2018). The relevant literature on the late Villafranchian canids was inspected (Del Campana, 1913; Crusafont Pairó, 1950; Thenius, 1954; Torre, 1967, 1974, 1979; Bonifay, 1971; Kurtén, 1974; Pons-Moyà and Moyà-Solà, 1978; Pons-Moyà, 1981; Koufos and Kostopoulos, 1997; Wang et al., 1999; Sotnikova, 2001; Garrido and Arribas, 2008; Lacombat et al., 2008; Tedford et al., 2009; Petrucci et al., 2013; Cherin et al., 2014; Koufos, 2014, 2018; Bartolini Lucenti and Rook, 2016; Amri et al., 2017; Mecozzi et al., 2017; Jiangzuo et al., 2018). Extant specimens housed at the AMNH, MZUF, ICP, and MG-GNM (see abbreviation below) were also used for morphological and morphometrical comparisons. We examined specimens of Canis aureus Linnaeus, 1758, Canis latrans Say, 1823, Canis lupaster Hemprich and Ehrenberg, 1828–1834, Canis lupus Linnaeus, 1758, Lupulella adusta (Sundevall, 1847), Lupulella mesomelas (Schreber, 1775), and Lycaon pictus (Temminck, 1820).
The 3D surface scan of the fossil and extant specimens was obtained with the Artec Space Spider high-resolution 3D blue light technology scan at the Museum of Georgia and at the Paleo[Fab]Lab, Earth Science Department of the University of Florence. The 3D surface scans were subsequently elaborated in Artec Studio 12 Professional. The 3D visualization of the cranium D64 and upper and lower teeth comparison models in Augmented Reality was created following the protocols described by several authors (e.g., Etienne, 2017; Carpignoli, 2019) in Visual Studio Code ver. 1.41, using AR.js and A-Frame v. 0.9.2 (www.aframe.io).
Morphometric and Statistical Analyses
Cranial and dental measurements were taken with a digital caliper to the nearest 0.1 mm following von den Driesch (1976) with minor modifications (see abbreviations below).
We performed a principal components analysis (PCA) on a dataset of selected log10-transformed cranial measurements, in order to establish the morphological similarities between various canid species, both extinct (Canis from Dmanisi, and C. etruscus) and extant (C. aureus, C. lupus, C. lupaster, L. adusta, L. mesomelas). We then used a permutational ANOVA on the most explicative principal components to test the statistical significancy of the differences resulting from the analysis.
We compared the material of Canis from Dmanisi with that of other European Late Villafranchian species by means of boxplots, to inspect differences in values distribution, and statistical tests. At this purpose, selected cranial ratios were used to test for statistical difference between the sample of Dmanisi, the other fossil taxa, and the extant species of the genera Canis and Lupulella. We tested these ratios by means of permutational ANOVA computed in R statistical environment (ver. 3.6.). The difference between the samples in dental measures were tested using a permutational multivariate ANOVA (MANOVA), using the pairwise Adonis package (Martinez Arbizu, 2019) computed in R.
Site and Institutional Abbreviations
AMNH, American Museum of Natural History, New York (United States); AUT, Earth Science Department of Aristotle University of Thessaloniki; D, Dmanisi site; ICP, Institut Català de Paleontologia Miquel Crusafont, Universitat Autònoma de Barcelona (Cerdanyola del Vallès, Barcelona, Spain); IGF, Museum of Natural History, Geological and Paleontological section, the University of Florence (Italy); MG-GNM, S. Janashia Museum of Georgia, Georgian National Museum (Tbilisi, Georgia); MZUF, Museum of Natural History, “La Specola” Zoology section, University of Florence (Italy); 0X/D, P, provisional catalog number of unregistered specimens from Dmanisi housed in MG-GNM.
Anatomical Abbreviation
Cranium
AB, height of the cranium without the sagittal crest (inion–basion); BL, basal length of the cranium; CBL, condylobasal length of the cranium; Ect, width across the zygomatic processes of the frontals; ECW, width of the muzzle at level of the upper canine; Eu, greatest neurocranium width; FL, facial length; GPW, greatest palatal width; GWOC, greatest width of occipital condyles; NcL, neurocranial length; PL, palatal length; POCW, smallest width of postorbital constriction; SCL, splanchnocranial length; SH, skull height (with sagittal crest); TL, total length of the cranium (inion-prosthion); Zyg, zygomatic breadth.
Dentition
L, mesiodistal length; LCR, upper cheek toothrow length (P1–M2); LMR, upper molar row length (M1–M2); LPR, upper premolar row length (P1–P4); tdm1, talonid of m1; trm1, trigonid of m1; W, buccolingual width.
Mandible
HR, height of the mandible ramus; LLMR, length of the lower molar row (m1–m3); LLPR, length of the lower premolar row (p1–p4); Mm1B, mandibular corpus breadth below midpoint of m1; Mm1H, mandibular corpus height distal to m1 alveolus; Mp4H, mandibular corpus height distal to p4 alveolus; m1–m2 L, length of the first and second lower molars; p2–p4 L, length of premolar row between p2 and p4.
Systematic Paleontology
Order Carnivora Bowdich, 1821
Family Canidae Fischer, 1817
Subfamily Caninae Fischer, 1817
Tribe Canini Fischer, 1817
Genus Canis Linnaeus, 1758
Canis borjgali sp. nov.
Figures 1, 2, 3; Supplementary Tables S1, S2, S3, S4
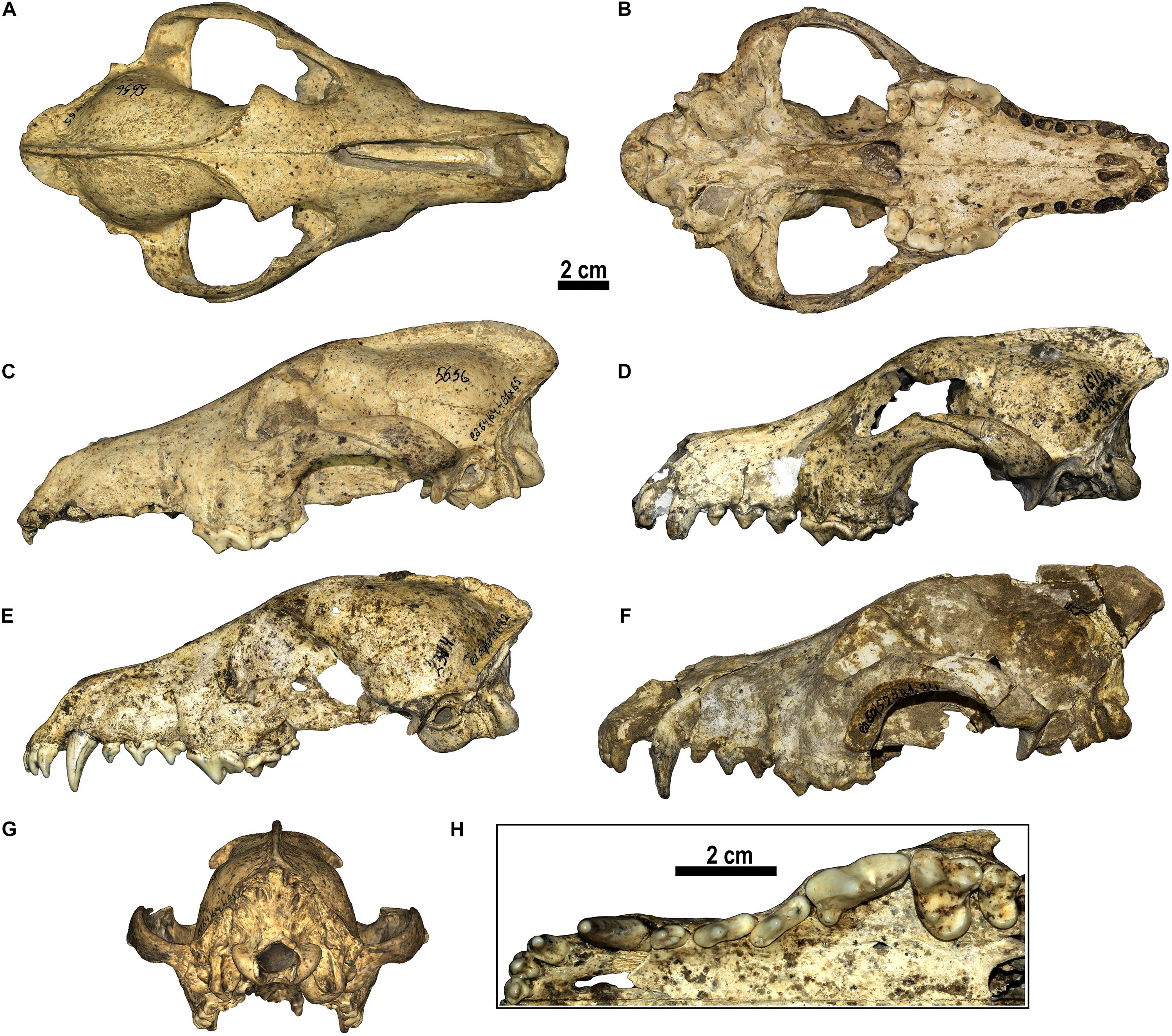
Figure 1. Canis from Dmanisi. (A–C,G) D5656, cranium in (A) dorsal, (B) ventral, (C) left lateral, (G) occipital views. (D) D4510, cranium in left lateral view. (E) D2314, cranium in left lateral view. (F) Dm.50/52.3B1.34, cranium in left lateral view. (H) Detailed view of the occlusal morphology of the upper teeth of Canis from Dmanisi (D2314).
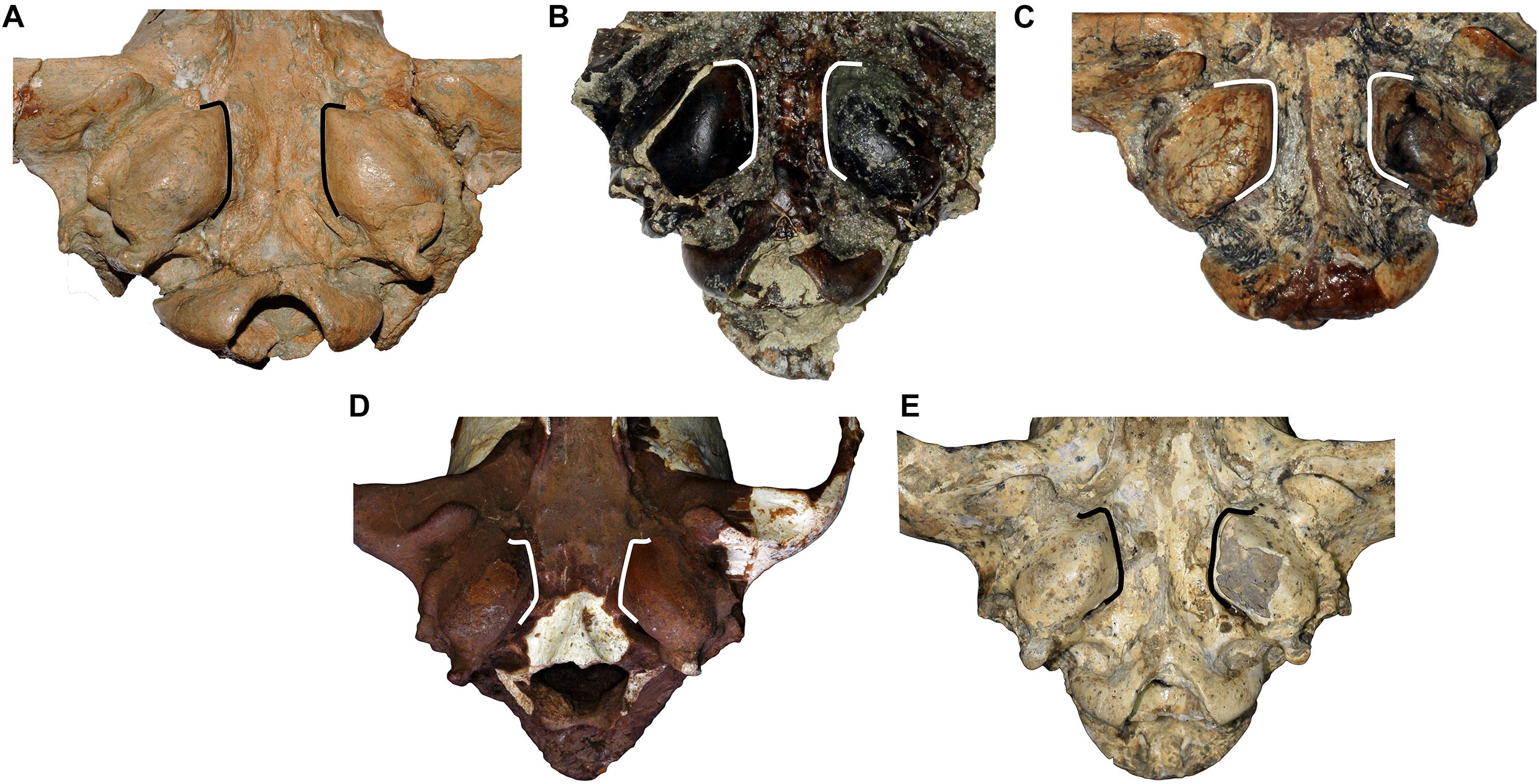
Figure 2. Comparison of the tympanic region of (A) C. etruscus from Upper Valdarno, (B) C. arnensis from Poggio Rosso, (C) C. chihliensis from Yushe Basin, (D) C. mosbachensis from Cueva Victoria, and (E) Canis from Dmanisi. Note the marked divergence of the medial walls of the tympanic bullae of C. mosbachensis and of Canis from Dmanisi. This feature is not documented in the fossil taxa but present in extant wolf-related species (like C. lupus, C. lupaster, C. aureus, etc.).

Figure 3. Canis from Dmanisi. (A–C) D4871, right hemimandible in (A) buccal, (B) lingual, and (C) occlusal views. (D–F) D5594, right hemimandible in (D) buccal, (E) lingual, and (F) occlusal views. (G–I) D1184, right hemimandible in (G) buccal, (H) lingual, and (I) occlusal views. (J,K) Detailed occlusal morphology of the lower teeth of Canis from Dmanisi: (J) D372, left m3; and (K) D1, left hemimandible.
Etymology. From the Georgian “ბორჯღალი,” IPA: [bɔrdʒʁali], seven-rayed symbol of the sun and eternity typical of Georgia.
Holotype. D64, almost complete skull with I1-M2 and i1-m3.
Hypodigm. See Supplementary Material.
Type Locality and Age: Dmanisi, Georgia; ca 1.80 Ma.
Differential Diagnosis
A medium-sized canid, close or slightly smaller compared to C. etruscus, generally larger than other Villafranchian medium-sized canids. Canis borjgali differs from C. arnensis for the longer nasal bones, the well-developed frontal sinuses, the morphology of the upper molars, and the hypoconid on m1 larger than entoconid in the m1. It differs from C. etruscus for its overall cranial architecture, including the relatively higher cranium, the profile in lateral view, and shorter nasal bone and their morphology. It also differs in dental features, including the presence of accessory cuspulids on the lingual margin of m1 talonid. It is distinguished from C. arnensis and C. etruscus by the caudorostrally divergent medial walls of the tympanic bullae and few other dental features, including the deeper trigon basin compared to the talon basin on M1, the p3 that sets below the alveolar plane of p2 and p4. C. borjgali differs from C. mosbachensis for some less derived features, including the morphology of the second upper molar, the more arched lower toothrow, and larger metaconid on m1. C. borjgali possesses a marked apomorphy, i.e., the medial walls of tympanic bullae that diverge rostral direction.
Description and Comparison
The cranium is moderately elongated (Figure 1). Nasals are long, although not as long as in C. etruscus from Olivola and Pantalla. In lateral view, the profile of the cranium of Canis from Dmanisi is moderately arched, contrasting condition compared to the straight profile of C. etruscus from Olivola and Pantalla, but still less arched than in C. mosbachensis and C. lupus. The postorbital constriction is not marked for the considerable development of the frontal sinuses, which extend caudally toward the frontoparietal suture like in C. chihliensis from Yushe Basin, C. etruscus from Italy, C. mosbachensis from Eurasia, and C. lupus, although reduced compared to the latter (Table 1). The postorbital region is shorter compared to the elongated one of C. lupus, resembling other fossil species (e.g., C. mosbachensis from Cueva Victoria, Untermassfeld, and Zhoukoudian). The supraoccipital shield has the typical Canis triangular shape, although less sharp compared to that of C. mosbachensis from Eurasia (e.g., Cueva Victoria, ntermassfeld, and Zhoukoudian loc. 13) and extant C. lupus (Table 1). In ventral view, the tympanic bullae are oval shaped and moderately inflated. Their medial walls are not parallel, but tend to diverge in caudorostral direction, unlike C. arnensis, C. etruscus, and C. chihliensis from Yushe Basin (Figure 2). There are no diastemata between the upper teeth. The protocone of the upper carnassial is poorly individualized and relatively reduced, condition similar to that found in C. lupus, whereas in C. etruscus from Italy, the protocone is well separated from the rest of the tooth. The P4 also possesses a high and stout paracone, a sharp metastyle, and a strong mesiolingual cingulum. On the M1, the paracone is larger and higher than the metacone as in C. apolloniensis, C. etruscus, C. mosbachensis, and C. lupus (Table 1). As in these species, there is a well-developed metaconule, a high protocone, and a rather individualized protoconule. The protocone basin is deeper and larger than the hypocone basin, like in C. apolloniensis, C. mosbachensis, and C. lupus. The M1 shows a strong and well-developed buccal cingulum, expanded on both the mesial and distal sides, unlike C. lupus in which the cingulum is present but subdued. The morphology of the M2, in occlusal view, tends to be similar to that of C. etruscus, with a large buccal side. Some specimens share this more buccolingually elongated shape resembling the morphology of C. apolloniensis from Apollonia-1, C. lupus, and C. mosbachensis from Eurasia (Table 1).
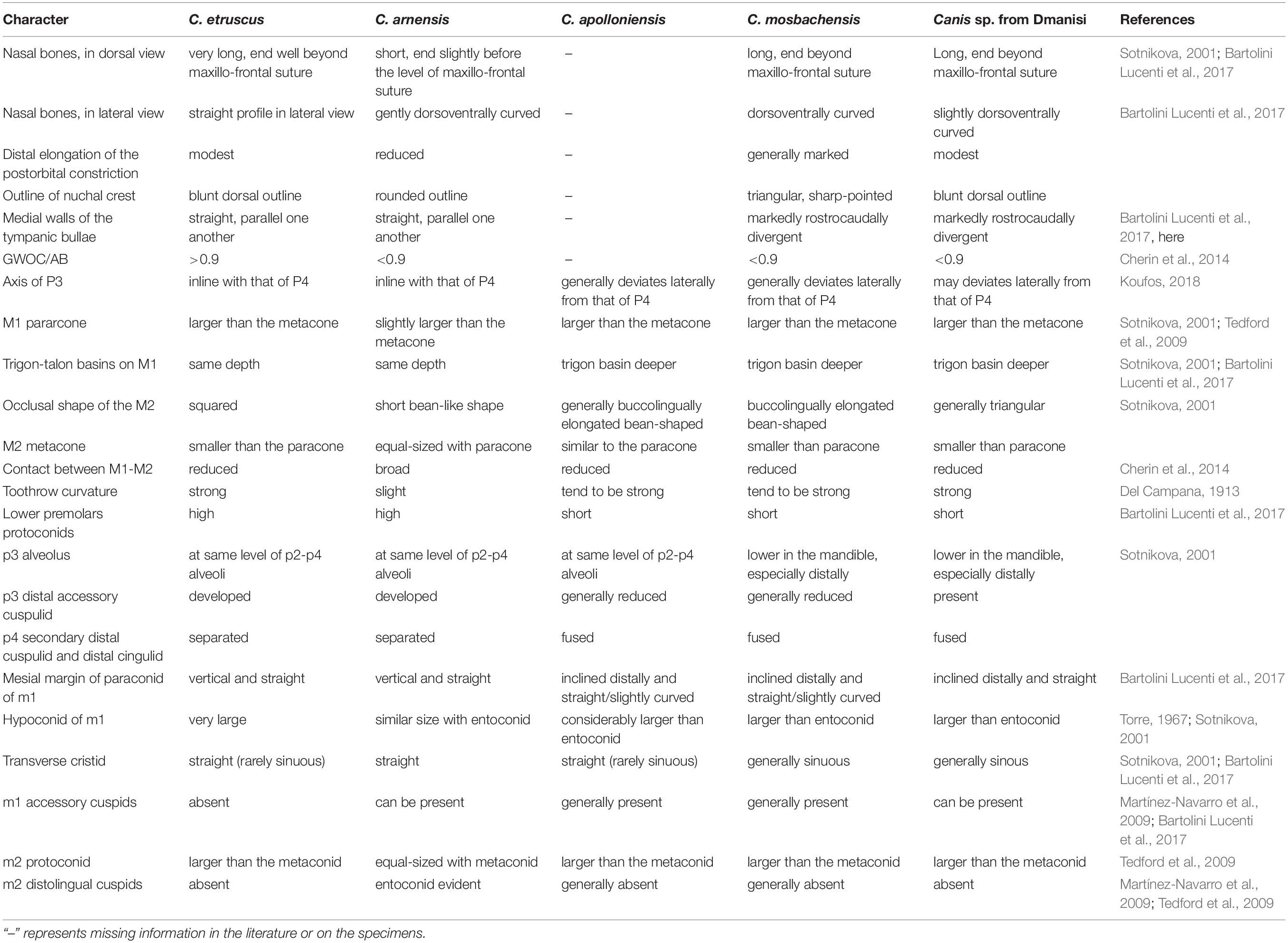
Table 1. Summarizing table of the main craniodental features of C. etruscus, C. arnensis, C. mosbachensis, and C. apolloniensis compared to those of the Canis sp. from Dmanisi.
The mandible of Canis from Dmanisi generally has a moderately deep horizontal ramus (Figure 3), similar in dorsoventral height and morphology to that of C. etruscus from Italy, unlike C. arnensis from Upper Valdarno, in which it is more slender. The postcanine toothrow is slightly arched (buccally convex at the p4–m1 commissure) as generally in C. apolloniensis, C. etruscus, C. mosbachensis, and C. lupus, whereas it is moderately arched in C. arnensis from Upper Valdarno (Table 1). Diastemata are absent between the lower premolars. In Canis from Dmanisi, the p3 generally sets in the mandible below the alveolar level of the p2 and p4, as in C. mosbachensis and C. lupus (Figure 3 and Table 1). The p3 possesses a distal accessory cuspulid, generally absent in C. apolloniensis and C. mosbachensis. The p4 occlusal shape is wide and oval similar to C. mosbachensis from Eurasia compared the more slender shape of C. apolloniensis from Apollonia-1, C. arnensis from Upper Valdarno. The m1 paraconid is higher than the p4 protoconid, like in C. apolloniensis from Apollonia-1, C. mosbachensis from Eurasia, and C. lupus, in contrast to C. arnensis and C. etruscus. In the lower carnassial, the metaconid is large and individualized from the protoconid. It is larger compared to C. apolloniensis from Apollonia-1, C. mosbachensis from Eurasia, and C. lupus, which possess a reduced metaconid, closely attached to the large protoconid. Nevertheless, the lower carnassial is less developed compared to C. arnensis and C. etruscus. On the talonid, the hypoconid is larger than the entoconid, although the difference between them is reduced compared to that visible in C. lupus and C. etruscus (Figure 3 and Table 1). The transverse cristid between the m1 hypoconid and entoconid is evident and generally sinuous, as in C. mosbachensis and C. lupus, unlike C. arnensis or C. etruscus from Italy. The m1 entoconid is more developed compared to C. apolloniensis and C. mosbachensis, similarly to C. etruscus. Accessory cuspulids may be present on the lingual side of the talonid, like in C. apolloniensis and C. mosbachensis from Eurasia. The m2 protoconid is larger compared to the metacone, unlike C. arnensis (Table 1). Distally on the m2, there is a hypoconid and a lingual cristid, generally with no accessory cuspulids, as in C. apolloniensis and C. mosbachensis (although in the latter, the m2 occlusal shape is generally more rectangular). The m3 has two cuspulids, with the buccal larger than the lingual one as in C. etruscus from Italy and C. mosbachensis from Europe, as opposed to C. arnensis (which has two equal-sized cuspids) and C. lupus (with a single and large cuspid).
Results
Principal Component Analysis
The results of the PCA on cranial variables are reported in Figure 4. The PC1, which accounts for most of the variance (95.8%), has positive and similar loading for all the analyzed original variables (Supplementary Table S5), thus being positively influenced by cranial shape. Along the PC1, the modern wolves separate from other extant canids (jackals and golden wolves), the former on the positive end whereas the latter on the negative end. Extinct species (Canis from Dmanisi and C. etruscus) occupy an intermediate position, slightly overlapping with the modern wolf variation. C. etruscus variability on this axis is relatively limited, and its variance is included in that of Canis from Dmanisi. The PC2 only accounts for 1.5% of total variance, being dominated positively by greatest nasal length, splanchnocranial length, and facial length, whereas negatively by the skull height with the sagittal crest, the neurocranial length, and the width of the muzzle across the upper canines. On this axis, C. etruscus is considerably separated from the range of the other canids; therefore, it is visibly distinguished from Canis from Dmanisi. The results of the ANOVA on the PC1 is reported in Supplementary Table S6. The analysis confirms the significant difference between C. lupus and the other extant canids visible in Figure 4. Both C. etruscus and Canis from Dmanisi are different from the extant canids, yet there is no statistical difference between them on the PC1 (F = 0.2576, p = 0.99923), as visible from Figure 4.
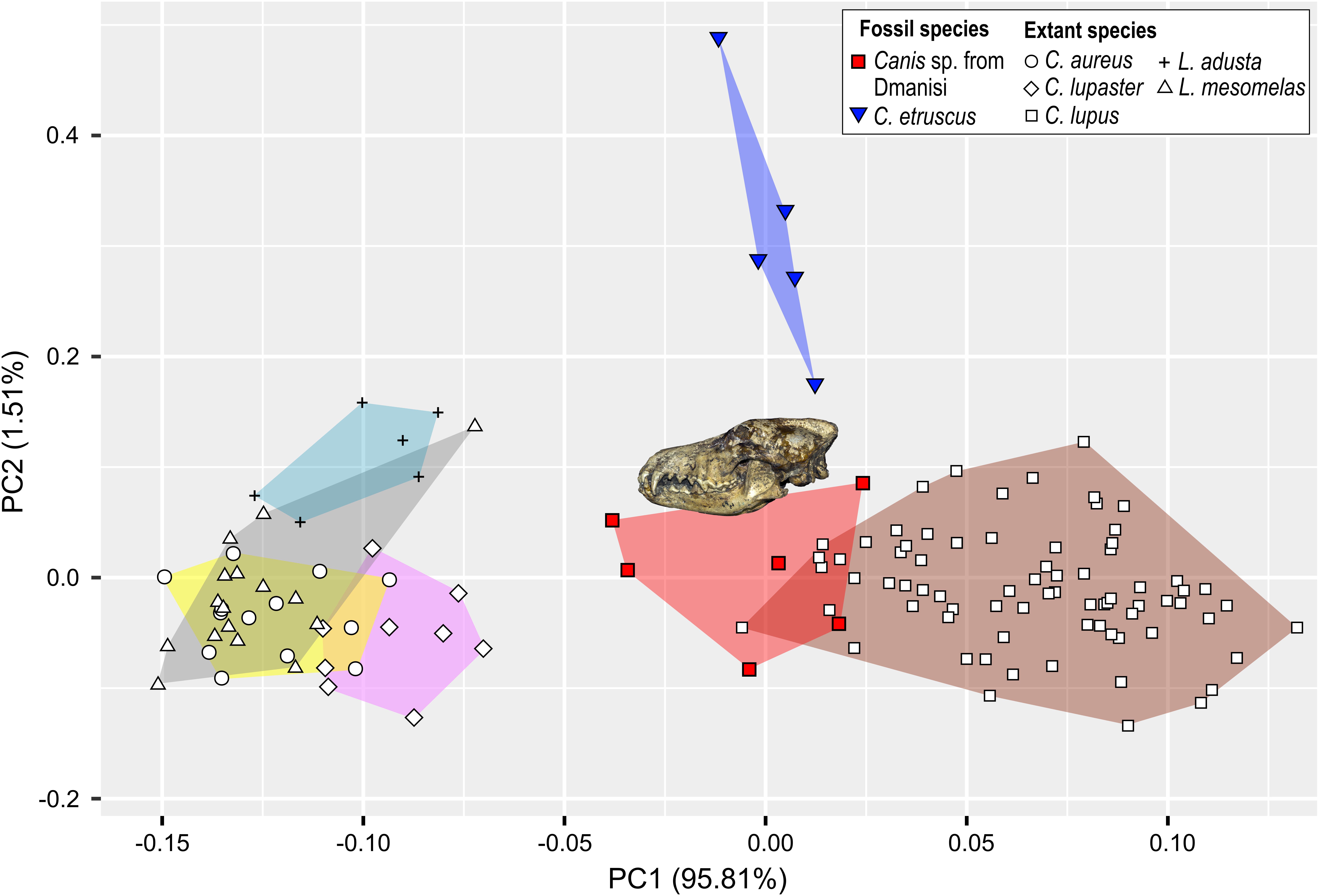
Figure 4. Principal components analysis (PCA) based on selected log-transformed cranial measurements on extant and fossil species of the general Canis and Lupulella. Symbols are explained in the legend.
Statistical Analyses
In both cranial and dental measures, Canis from Dmanisi is close to C. etruscus and other Early Pleistocene canids of Eurasia like C. mosbachensis and C. apolloniensis, and comparable to smaller subspecies of C. lupus (e.g., Canis lupus arabs or Canis lupus pallipes; Supplementary Material, Supplementary Tables S1–S4). Nevertheless, the cranial ratios (SCL/TL, GNL/TL, GWOC/AB, SH/TL) show significant difference between C. etruscus and Canis from Dmanisi (Figure 5). C. etruscus is considerably different in cranial proportions as opposed to the others species, fossil, and extant ones. The ratios of Canis from Dmanisi are more similar to C. mosbachensis and C. lupus. C. arnensis differs significantly from C. etruscus in three ratios (SCL/TL, GNL/TL, GWOC/AB) and cannot be distinguished from Canis from Dmanisi in these ratios. Nevertheless, the relative height of the skull is significantly different between C. arnensis and Canis from Dmanisi. The analysis of variance on the relative length of the nasals (Supplementary Table S7), the most numerous of the considered ratios, revealed to be statistically significant between Canis from Dmanisi and C. etruscus (F = −0.102498; p ≪ 0.01; Supplementary Table S7). The MANOVA on dental measures (Supplementary Tables S8, S9) confirms the statistical difference between Canis from Dmanisi and C. etruscus (upper teeth: F = 33.403; p = 0.04496; lower teeth: F = 19.4091, p = 0.04496), on one side, and no difference between the former and C. mosbachensis, on the other. As for cranial ratios, the dental measures of C. arnensis are partially similar to Canis from Dmanisi, as the lower teeth values of the two species are significantly different (F = 33.403; p = 0.04496; Supplementary Table S9).
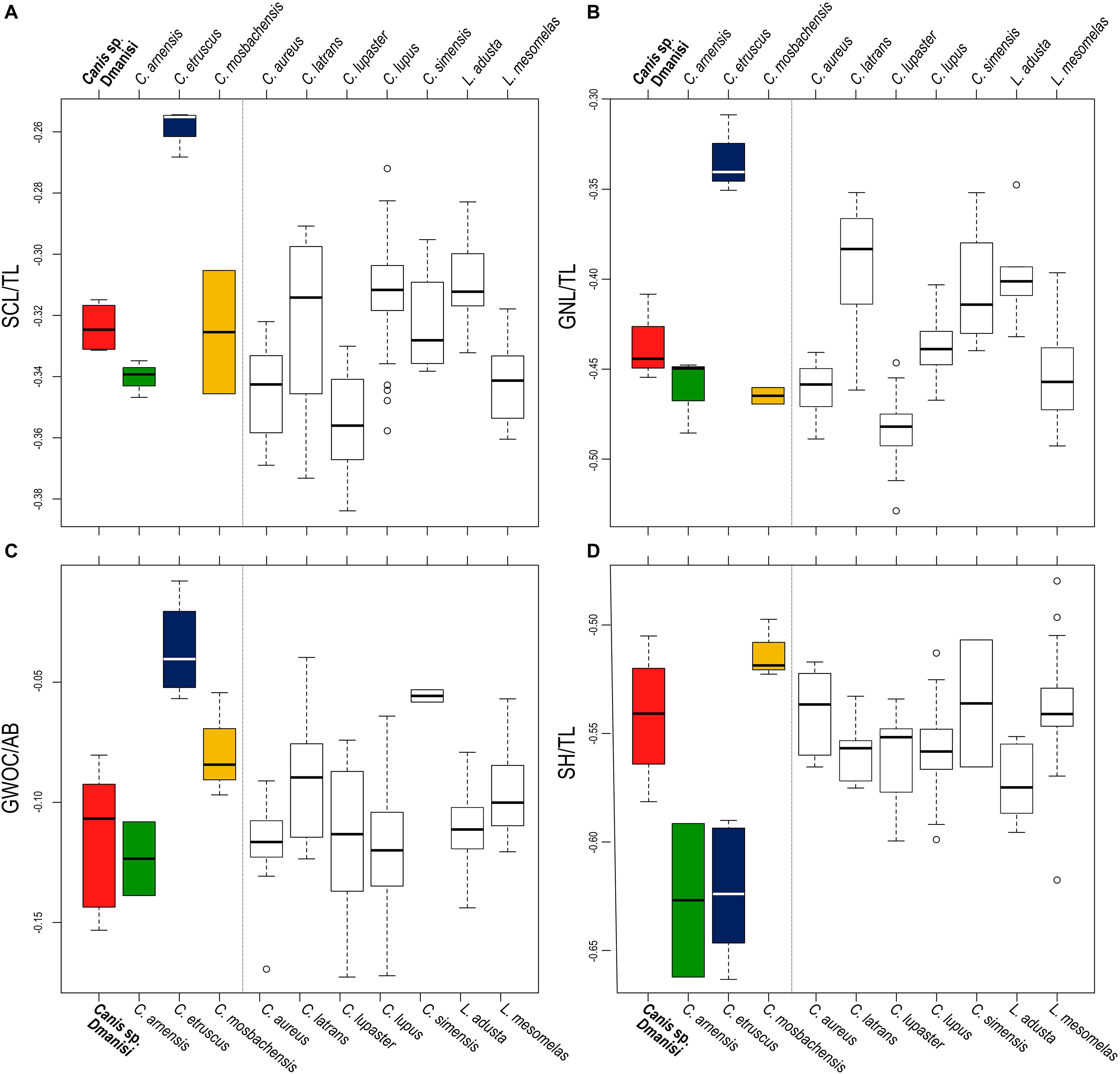
Figure 5. Boxplots of selected cranial ratios: (A) relative length of the splanchnocranium, ScL/TL; (B) relative length of the nasals, GNL/TL; (C) width of the occipital condyles relative to the height of the cranium, GWOC/AB; (D) relative height of the cranium (including the sagittal crest), SH/TL. Gray dashed line separates the fossil species from the extant ones.
Discussion
Taxonomic Attribution of Canis From Dmanisi and Implications for the Evolution of Canini
The results of the ANOVA on the PC1, the axis explaining the majority of the variability of the PCA (Figure 4), show no statistical distinction between C. etruscus and Canis sp. from Dmanisi (Supplementary Table S6). Nevertheless, the separation between these species in the plot of the principal component analysis is along the PC2 (as evident from Figure 4). The cranial ratios (SCL/TL, GNL/TL, GWOC/AB, and SH/TL) shown in Figure 5 take into consideration those values that influence the dispersion of the specimens on this axis. A permutational ANOVA test on the GNL/TL, the most represented of the ratios and the most important parameter of PC2, clearly supports the distinction of C. etruscus. The result acknowledges the peculiarity of C. etruscus compared to other known fossil and extant species as noted by Cherin et al. (2014), including the sample from Dmanisi (Supplementary Table S7). The distinction between Canis sp. from Dmanisi and C. etruscus is confirmed also by the MANOVA on upper and lower teeth (Supplementary Tables S8, S9). Therefore, despite being considered as synonyms (Vekua, 1995), C. etruscus remarkably differs from the Georgian sample in both morphometrics and ratios measure (Figure 5 and Supplementary Tables S7–S9). In this light, the material of Canis from Dmanisi cannot be ascribed to C. etruscus. Several of the morphometric and morphological results point out to a closer affinity of Canis from Dmanisi to the late Early Pleistocene Canis ex gr. mosbachensis (C. mosbachensis, e.g., from Untermassfeld, Sotnikova, 2001; Iberian Peninsula, Bartolini Lucenti et al., 2017; Pirro Nord, Petrucci et al., 2013; C. mosbachensis variabilis from Zhoukoudian localities, Jiangzuo et al., 2018; and C. apolloniensis from Apollonia-1, Koufos, 2018), rather than to C. etruscus. In addition to the affinity with these fossil taxa, Canis from Dmanisi shares also affinities with the group of wolf-related canids (see Lindblad-Toh et al., 2005), e.g., the caudorostrally divergent tympanic bullae; p3 alveolus at a lower level than p2–p4 (Figures 1–3). Indeed, the divergent walls of the bullae is shared by C. lupus, other wolf-related canids, and C. mosbachensis (see Bartolini Lucenti et al., 2017), as opposed to any other extinct species (Figures 1–3). Nevertheless, some other features of the sample from Dmanisi are more primitive, e.g., blunt or subrounded dorsal outline of the nuchal crest, reduced caudal elongation of the postorbital region; relatively developed crushing surface of the molars; and presence of an accessory cuspulid on p3. This combined pattern of features (similar to both primitive- and derived-like canids), unlike any other early Early Pleistocene canids, supports the ascription of the sample to a new species, C. borjgali sp. nov.
The new identity of Canis from Dmanisi casts some doubts on the current interpretation of the origin of modern wolf. The wide consensus in scientific literature supports that C. lupus and its lineage originates during the Early Pleistocene with the primitive C. etruscus (Torre, 1967; Kurtén, 1968; Musil, 1972; Sotnikova, 2001; Sotnikova and Rook, 2010), from which derived C. mosbachensis in the second half of the Early Pleistocene to first half of the Middle Pleistocene. Despite the taxonomical debate (Mecozzi et al., 2017), C. mosbachensis is widely considered the ancestor of C. lupus (in addition to the former: Brugal and Boudadi-Maligne, 2011; Sardella et al., 2014). Sotnikova and Rook (2010) were the first to suggest a possible alternative classification for the Canis of Dmanisi and the plausible ancestry of C. lupus. Here, such an interpretation is supported, as C. etruscus appears more primitive than C. borjgali and should probably be regarded as one of the earliest taxa to disperse into Europe (around the end of the Pliocene–early Pleistocene). On the contrary, C. borjgali might represent the ancestor or, in any case, a close-allied taxon of the clade of more derived species of Eurasian Canini (e.g., C. ex gr. mosbachensis, C. lupus). The morphological and morphometrical similarity between the C. borjgali and C. mosbachensis supports the idea of close relationship between the two species. Some authors (Bartolini Lucenti et al., 2017; Jiangzuo et al., 2018) reinterpreted the idea of direct ancestry between C. mosbachensis and C. lupus, putting forward the idea that C. mosbachensis, as a large-ranged and time-spanned species, might actually be related to the crown species of the wolf clade (i.e., C. latrans, C. lupaster) rather than only to C. lupus. This might find support in recent molecular (e.g., Koepfli et al., 2015; Viranta et al., 2017) and paleontological evidence (Tedford et al., 2009). Koepfli et al. (2015) estimated the time of divergence between the C. lupaster and the C. latrans–C. lupus clade around 1.3 Ma and between C. latrans and C. lupus around 1.1 Ma. This suggests that the Early Pleistocene was a time of high diversification in modern canids, as is also confirmed by fossil record. Tedford et al. (2009) reported the early presence of C. lupus in the early Middle Pleistocene deposits of Alaska. In the broader and more intricate framework of the evolutionary history of crown-Canini, the privileged geographical position of the Caucasian region reveals its importance in canids’ evolutionary history, as a noted by Sotnikova and Rook (2010). Indeed, the Caucasus sets as a crossroads between three continents, and its peculiar biotic evolution testifies to its role of key region in evolution of Old World faunas since Miocene times (Krijgsman et al., 2019). The discovery of a previously undescribed taxon in Dmanisi, with some modern features as opposed to C. etruscus, close but more primitive compared to C. mosbachensis, changes radically the idea of C. lupus evolution as it is conveyed today, invalidating the paradigm C. etruscus–C. mosbachensis–C. lupus lineage. As probable ancestor of C. ex gr. mosbachensis, C. borjgali sp. nov. might also represent one of the latest common ancestors of the crown clade of modern Canis species (C. latrans, C. lupaster, C. lupus).
Augmented Reality: Opportunities and Perspectives
The exquisite conservation of the fossil specimens of C. borjgali offers the perfect subject for the application of 3D and digital imaging techniques, like Augmented Reality (AR), thanks to the use of Artec Space Spider. AR allows interacting with computer-generated objects (sensu lato, from 2D pictures, videos to 3D objects) in the real world contexts in real time, allowing the users to perceive the real environment surrounding the digital rendering (Azuma, 1997; Zhou et al., 2008; Lee, 2012). Thus, differing from Virtual Reality (VR), in which users are immersed in a digital/computer-generated environment, AR can be considered a mixed-reality experience (Bower et al., 2014). It is not a brand-new technology, as its first applications date back to the 1990s. Currently, AR applications cover a diverse range of fields, from medicine to manufacturing, from aeronautics to entertainment and tourism (Bower et al., 2014). Particularly in educational sciences, AR has revealed its pedagogical potential, as much research documented (see Lee, 2012; Akçayır and Akçayır, 2017 and references therein). Nevertheless, no or little use has been made in scientific research, and its potentiality to paleontology and museology seems widely underestimated (at the very least, in Italian institutions). After the virtuous case of “Virtual Showcase” (Bimber et al., 2001, 2002), a multiuser AR display for museal exhibitions, no other use is reported in the literature. We propose here the first application of AR as support to paleontological research. Of the wide range of possible types of AR applications (see Bower et al., 2014’s review), a marker-based one was chosen (for further discussion see Kan et al., 2011) to allow remote and independent use by any interested user. The application of easy-to-use, open-source, and marker-based tools like AR.js and A-Frame allows to expand the accessibility to superb fossil specimens like D64 (Figure 6). A smartphone, a tablet, or any device with a camera and internet connection would suffice to grant access to the actual 3D morphology of the specimens and their tiniest features, such as dental cuspules/cuspulids. The same tool can be used to perform digital comparisons between different species or specimens, like in Figures 7, 8, where the upper and lower morphology of the teeth of C. etruscus, C. borjgali, and C. lupus can be compared as easily as would be with the three real specimens. Moreover, the use of a marker consent to even compare the 3D digital objects implemented in the app to real ones placed near the marker (for instance to a cranium of an extant canid, e.g., C. lupus, Figure 9), to directly see similarities and/or differences. In short, following the motto of the A-Frame team, “show, don’t tell.”
Figure 6. QR code linking to the web app for Augmented Reality (AR) visualization of the 3D model of the skull D64. Instructions: Scan the QR code on the left; open the link; allow the browser to access the camera of your device; point the camera toward the marker (on the right); and wait for the model to load (up to 10 seconds). It is possible to turn the device around the marker (or to move the marker) to see different parts of the model. Best visualization performances can be achieved by printing the markers, rather pointing at them on screens. Refer to Supplementary Material for common issues.
Figure 7. QR code and Augmented Reality (AR) marker showing 3D comparison between the upper teeth morphologies of C. etruscus from Upper Valdarno (blue), Canis from Dmanisi (red), and extant C. lupus (grayish). Instructions: Scan the QR code on the left; open the link; allow the browser to access the camera of your device; point the camera toward the marker (on the right); and wait for the model to load (up to 10 seconds). It is possible to turn the device around the marker (or to move the marker) to see different parts of the model. Best visualization performances can be achieved by printing the markers, rather pointing at them on screens. Refer to Supplementary Material for common issues.
Figure 8. QR code and Augmented Reality (AR) marker showing 3D comparison between the lower teeth morphologies of C. etruscus from Upper Valdarno (blue), Canis from Dmanisi (red), and extant C. lupus (grayish). Instructions: Scan the QR code on the left; open the link, allow the browser to access the camera of your device; point the camera toward the marker (on the right); and wait for the model to load (up to 10 seconds). It is possible to turn the device around the marker (or to move the marker) to see different parts of the model. Best visualization performances can be achieved by printing the markers, rather pointing at them on screens. Refer to Supplementary Material for common issues.

Figure 9. Example of the visualization of the Augmented Reality web app in a comparison between a real cranium (that of an extant C. lupus) and the digital model D64 of C. borjgali (as visible from Figure 6). The digital specimen is not at the same scale of the real specimen.
Conclusion and Perspectives
The extensive record of Canis from Dmanisi is outstanding both for its state of preservation and abundance. Several morphological and morphometric patterns challenge the previous attribution to C. etruscus and favor the ascription of the taxon to a new species, C. borjgali sp. nov. The record of Dmanisi has already changed our understanding of evolutionary history of different species, like in the case of the rhino “Stephanorhinus” (Cappellini et al., 2019) or of the genus Homo (Rightmire et al., 2019 and reference therein) as the first discovery of the earliest Out-of-Africa hominids. The description of C. borjgali sp. nov. offers a new picture of the evolutionary history of modern wolves and wolf-like canids. Instead of considering the rather primitive species C. etruscus as the ancestor of C. lupus, the similarity between C. borjgali and C. mosbachensis suggests to acknowledge in the species from Dmanisi the ancestor of modern wolf-related canids. Furthermore, the present research reports the first application of Augmented Reality to a scientific paper in paleontology. Although especially used with pedagogical and educational purposes, scientific articles might benefit from the employment of AR contents thanks to its high interactivity and attractivity. Digital visualizations could be used to convey and communicate more easily the discoveries of a research, especially in life and physical sciences. In paleontology, the use of AR of digitalized fossil specimens in scientific articles might allow to increase the accessibility to superb fossils like D64 (Figure 6) without compromising the right and ownerships of institutions (museums or research ones) on these specimens. Undoubtedly, any 3D object, resulting from any different source (laser scan, CT scans, etc.), can be used. The implementation of web-based AR is valuable to other researchers, as it allows them to operate their observations of the 3D model better and more precisely than using 2D photos or even stereophotography. Relative height of cusps and cuspids, development of the crests, and depth of depressions are all features that become directly observable and other workers might make their own opinion and verify the finding of the paper. Digital comparisons between different species (Figures 7, 8) and comparisons with real specimens placed near the marker (Figure 9). All of this can be achieved simply using a mobile device (smartphones, tablets, etc.). Digital technologies dealing with AR are constantly advancing while its potentials are already high; we just need to keep the pace.
Data Availability Statement
The datasets generated for this study can be found in the ZooBank (urn:lsid:zoobank.org:pub:93965532-1069-44E1-9430-DCC5706EA312).
Author Contributions
SB has studied the material together with the support of BM-N, MB, and DL. SB and MB digitalized the fossil specimens. SB designed the paper and wrote the manuscript with contributions from BM-N. DL and MB critically reviewed several parts and improved the first draft.
Funding
The Georgian-Italian collaborative project is supported by the Italian Ministry of Foreign Affairs (MAECI, DGSP-VI) and the Italian Embassy in Georgia. BM-N is supported by grant CGL2016-80975-P of the Spanish Ministry of Science, grant GENCAT 2017SGR 859 of the Generalitat de Catalunya, and MB by the grant FR-18-27262 of the Shota Rustaveli Georgian Science Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are indebted to the kindness and availability of the curators who granted access to the collections of their institutions and museum: P. Agnelli of MZUF; E. Cioppi of IGF; J. Galkin, and J. Meng of the AMNH, G. Koufos of AUT. The authors thank Prof. Rook for his constant help and support and for his advice and suggestions on the first draft of the manuscript; M. Nitti and B. Rossi of Bluefactor for their support on web developing; Paleo[Fab]Lab thanks TBNET SOLUZIONI 3D (Arezzo) for their support and kind availability; Dr. F. Carotenuto and Dr. G. Sansalone for their reviews, which greatly improved the first version of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2020.00131/full#supplementary-material
References
Akçayır, M., and Akçayır, G. (2017). Advantages and challenges associated with augmented reality for education: a systematic review of the literature. Edu. Res. Rev. 20, 1–11.
Amri, L., Bartolini Lucenti, S., Mtimet, M. S., Karoui-Yaakoub, N., Ros-Montoya, S., Espigares, M. P., et al. (2017). Canis othmanii sp. nov. (Carnivora, Canidae) from the early Middle Pleistocene site of Wadi Sarrat (Tunisia). CR Palevol. 16, 774–782.
Bartolini Lucenti, S., Alba, D. M., Rook, L., Moyà-Solà, S., and Madurell-Malapeira, J. (2017). Latest Early Pleistocene wolf-like canids from the Iberian Peninsula. Quat. Sci. Rev. 162, 12–25.
Bartolini Lucenti, S., Bukhsianidze, M., Lordkipanidze, D., Martínez-Navarro, B., and Rook, L. (2019). “The carnivorans guild from the Early Pleistocene site of Dmanisi (Georgia),” in Proceedings of the XXXV Jornadas de Paleontología SEP, Baza.
Bartolini Lucenti, S., and Rook, L. (2016). A review on the late Villafranchian medium-sized canid Canis arnensis based on the evidence from Poggio Rosso (Tuscany. Italy). Quat. Sci. Rev. 151, 58–71.
Bimber, O., Gatesy, S. M., Witmer, L. M., Raskar, R., and Encarnação, L. M. (2002). Merging fossil specimens with computer-generated information. Computer 35, 25–30.
Bimber, O., Fröhlich, B., Schmalstieg, B. D., and Encarnação, L. M. (2001). The virtual showcase. IEEE Comput. Graph. Appl. 21, 48–55.
Bonifay, M. F. (1971). Carnivores quaternaires du Sud-Est de la France. New York, NY: Éditions du Muséum.
Bowdich, T. E. (1821). An Analysis of the Natural Classifications of Mammalia: For the Use of Students and Travellers. Boston, MA: J. Smith.
Bower, M., Howe, C., McCredie, N., Robinson, A., and Grover, D. (2014). Augmented Reality in education–cases, places and potentials. Edu. Media Int. 51, 1–15.
Brugal, J. P., and Boudadi-Maligne, M. (2011). Quaternary small to large canids in Europe: taxonomic status and biochronological contribution. Quat. Int. 243, 171–182.
Cappellini, E., Welker, F., Pandolfi, L., Ramos-Madrigal, J., Samodova, D., Rüther, P. L., et al. (2019). Early Pleistocene enamel proteome from Dmanisi resolves Stephanorhinus phylogeny. Nature 574, 103–107. doi: 10.1038/s41586-019-1555-y
Carpignoli, N. (2019). AR.js – The Simplest Way to get Cross-Browser Augmented Reality on the Web. Available online at: https://medium.com/swlh/ar-js-the-simplest-way-to-get-cross-browser-augmented-reality-on-the-web-10cbc721debc (accessed May 11, 2019).
Cherin, M., Bertè, D. F., Rook, L., and Sardella, R. (2014). Re-defining Canis etruscus (Canidae, Mammalia): a new look into the evolutionary history of Early Pleistocene dogs resulting from the outstanding fossil record from Pantalla (Italy). J. Mamm. Evol. 21, 95–110.
Crusafont Pairó, M. (1950). El primer representante del género Canis en el Pontiense eurasiatico (Canis cipio nova sp.). Bol. R. Soc. Esp. Hist. Nat. 48, 43–51.
Del Campana, D. (1913). I cani pliocenici di Toscana. Tipografia successori fratelli Nistri. Palaeont. It. 19, 189–254.
Etienne, J. (2017). AR-Code: a Fast Path to Augmented Reality. Available at: https://medium.com/arjs/ar-code-a-fast-path-to-augmented-reality-60e51be3cbdf (accessed April 4, 2017).
Ferring, R., Oms, O., Agustí, J., Berna, F., Nioradze, M., Shelia, T., et al. (2011). Earliest human occupations at Dmanisi (Georgian Caucasus) dated to 1.85–1.78 Ma. Proc. Natl. Acad. Sci. U.S.A. 108, 10432–10436. doi: 10.1073/pnas.1106638108
Forsyth-Major, C. I. (1877). Considerazioni sulla fauna dei mammiferi pliocenici e post-pliocenici della Toscana. Atti Soc. Tosc. Sci. Nat. Mem. 3, 207–227.
Garrido, G., and Arribas, A. (2008). Canis accitanus nov. sp., a new small dog (Canidae, Carnivora, Mammalia) from the Fonelas P-1 Plio–Pleistocene site (Guadix basin, Granada, Spain). Géobios 41, 751–761. doi: 10.1093/mmy/myx007
Hemprich, F. G., and Ehrenberg, C. G. (1828–1834). Symbolae Physicae, seu Icones et Descriptiones Corporum Naturalium Novorum aut Minus Cognitorum Quae ex Itineribus per Libyam, Aegyptium, Nubiam, Dongalam, Syriam, Arabiam et Habessiniam, Pars Zoologica II, Anima. Berlin: Officina Academica.
Jiangzuo, Q., Liu, J., Wagner, J., Dong, W., and Chen, J. (2018). Taxonomical revision of fossil Canis in Middle Pleistocene sites of Zhoukoudian. Beijing, China and a review of fossil records of Canis mosbachensis variabilis in China. Quat. Int. 482, 93–108.
Kan, T. W., Teng, C. H., and Chen, M. Y. (2011). “QR code based augmented reality applications,” in Handbook of Augmented Reality, ed. F. Borko (New York, NY: Springer), 339–354.
Koepfli, K. P., Pollinger, J., Godinho, R., Robinson, J., Lea, A., Hendricks, S., et al. (2015). Genome-wide evidence reveals that African and Eurasian golden jackals are distinct species. Current Biol. 25, 2158–2165. doi: 10.1016/j.cub.2015.06.060
Koufos, G. D. (2018). New Material and Revision of the Carnivora, Mammalia from the Lower Pleistocene Locality Apollonia 1, Greece. Quaternary 1:6.
Koufos, G. D. (2014). The Villafranchian carnivoran guild of Greece: implications for the fauna, biochronology and paleoecology. Integr. Zool. 9, 444–460. doi: 10.1111/1749-4877.12061
Koufos, G. D., and Kostopoulos, D. (1997). New carnivore material from the Plio-Pleistocene of Macedonia (Greece) with a description of a new canid. Münchn. Geowiss. Abh. 34, 33–63.
Krijgsman, W., Tesakov, A., Yanina, T., Lazarev, S., Danukalova, G., Van Baak, C. G. C., et al. (2019). Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution. Earth Sci. Rev. 188, 1–40.
Lacombat, F., Abbazzi, L., Ferretti, M. P., Martínez-Navarro, B., Moullé, P. E., Palombo, M. R., et al. (2008). New data on the Early Villafranchian fauna from Vialette (Haute-Loire, France) based on the collection of the Crozatier Museum (Le Puy-en-Velay, Haute-Loire, France). Quat. Int. 179, 64–71.
Lindblad-Toh, K., Wade, C. M., Mikkelsen, T. S., Karlsson, E. K., Jaffe, D. B., Kamal, M., et al. (2005). Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438:803. doi: 10.1038/nature04338
Linnaeus, C. (1758). Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis. Synonymis, Locis. Tomus I. Editio Decima, Reformata. Stockholm: Laurentius Salvius.
Lordkipanidze, D., de León, M. S. P., Margvelashvili, A., Rak, Y., Rightmire, G. P., Vekua, A., et al. (2013). A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo. Science 342, 326–331. doi: 10.1126/science.1238484
Lordkipanidze, D., Jashashvili, T., Vekua, A., de León, M. S. P., Zollikofer, C. P., Rightmire, G. P., et al. (2007). Postcranial evidence from early Homo from Dmanisi. Georgia. Nature 449:305. doi: 10.1038/nature06134
Martinez Arbizu, P. (2019). pairwiseAdonis: Pairwise Multilevel comparison using Adonis. R package version 0.3.
Martínez-Navarro, B., Belmaker, M., and Bar-Yosef, O. (2009). The large carnivores from ‘Ubeidiya (early Pleistocene, Israel): biochronological and biogeographical implications. J. Hum. Evol. 56, 514–524. doi: 10.1016/j.jhevol.2009.02.004
Mecozzi, B., Iurino, D. A., Berte, D. F., and Sardella, R. (2017). Canis mosbachensis (Canidae, Mammalia) from the Middle Pleistocene of Contrada Monticelli (Putignano, Apulia, southern Italy). B. Soc. Paleontol. Ital. 56:72.
Petrucci, M., Cipullo, A., Martínez-Navarro, B., Rook, L., and Sardella, R. (2013). The late Villafranchian (Early Pleistocene) carnivores (Carnivora. Mammalia) from Pirro Nord (Italy). Palaeontogr. A 298, 113–145. doi: 10.1590/0001-3765201420140314
Pons-Moyà, J. (1981). El Canis etruscus Major (Carnivora, Mammalia) del Villafranquiense terminal de la Cueva Victoria. Endins 8, 43–46.
Pons-Moyà, J., and Moyà-Solà, S. (1978). La fauna de carnivoros del Pleistoceno medio (Mindel) de la Cueva Victoria (Cartagena, Murcia). Acta Geol. Hisp. 13, 54–58.
Rightmire, G. P., Margvelashvili, A., and Lordkipanidze, D. (2019). Variation among the Dmanisi hominins: multiple taxa or one species? Am. J. Phys. Anthr. 168, 481–495. doi: 10.1002/ajpa.23759
Rook, L. (1993). I cani dell’Eurasia dal Miocene Superiore al Pleistocene Medio. Ph.D. dissertation, Modena-Bologna-Firenze and Roma “La Sapienza” Universities, Italy.
Rook, L. (1994). The Plio-Pleistocene Old World Canis (Xenocyon) ex gr. falconeri. Boll. Soc. Paleontol. It. 33, 71–82.
Sardella, R., Bertè, D., Iurino, D. A., Cherin, M., and Tagliacozzo, A. (2014). The wolf from Grotta Romanelli (Apulia, Italy) and its implications in the evolutionary history of Canis lupus in the Late Pleistocene of Southern Italy. Quat. Int. 328, 179–195.
Say, T. (1823). Account of an Expedition from Pittsburgh to the Rocky Mountains, Performed in the Years 1819 and ‘20, by Order of the Hon. J. C. Calhoun, Sec’y of War: under the Command of Major Stephen H. Long. From the Notes of Major Long, Mr. T. Say, and Other Gentlemen of the Exploring Party, ed. E. James (Philadelphia, PA: H.C. Carey and I. Lea), 503.
Schreber, J. C. D. (1775). Die Säugethiere in Abbildungen nach der Natur mit Beschreibungen. Erlangen: Wolfgang Walther.
Soergel, W. (1925). Die Säugetierfauna des altdiluvialen Tonlages von Jockgrim in des Pfalz. Zeits. Deut. Geol. Ges. 77, 405–438.
Sotnikova, M., and Rook, L. (2010). Dispersal of the Canini (Mammalia, Canidae: Caninae) across Eurasia during the late Miocene to early Pleistocene. Quat. Int. 212, 86–97.
Sotnikova, M. V. (2001). “Remains of Canidae from the lower Pleistocene site of Untermassfeld,” in Das Pleistozän von Untermassfeld bei Meiningen (Thüringen) Teil. 2, ed. R. D. Kahlke (Habelt Verlag: Bonn), 607–632.
Sundevall, C. J. (1847). Nya Mammalia från Sydafrika. Ofv. K. Svenska Vet.-Akad. Forhandl. Stockholm 3, 118–121.
Tedford, R. H., Wang, X., and Taylor, B. E. (2009). Phylogenetic systematics of the North American fossil caninae (Carnivora: Canidae). Bull. Am. Mus. Nat. Hist. 2009, 1–218.
Temminck, C. J. (1820). Sur le genre Hyène, et description d’une espèce nouvelle, découverte en Afrique. Ann. Gen. Sci. Phys. 3, 46–57.
Thenius, E. (1954). Die Caniden (Mammalia) aus dem Altquartär von Hundsheim (Niederösterreich) nebst Bemerkungen zur stammesgeschichte der Gattung Cuon. N. Jb. Geol. Paläontol. 99, 230–286.
Torre, D. (1974). Affinità dentali del cane della grotta di l’Escale. Riv. Ital. Paleontol. 80, 147–156.
Torre, D. (1979). The Ruscinian and Villafranchian dogs of Europe. Boll. Soc. Paleontol. Ital. 18, 162–165.
Vekua, A. (1995). Die Wirbeltierfauna des Villafranchium von Dmanisi und Ihre biostratigraphishe bedeutung. Jb. Röm.-Germ. Zentralmus. Mainz 42, 77–180.
Viranta, S., Atickem, A., Werdelin, L., and Stenseth, N. C. (2017). Rediscovering a forgotten canid species. BMC Zool. 2:6. doi: 10.1186/s40850-017-0015-0
von den Driesch, A. (1976). A guide to the measurement of animal bones from archaeological sites. Peabody Mus. Bull. 1, 1–137.
Wang, X., Tedford, R. H., and Taylor, B. E. (1999). Phylogenetic systematics of the Borophaginae (Carnivora. Canidae). Bull. Am. Mus. Nat. Hist. 243, 1–391.
Zhou, F., Duh, H. B. L., and Billinghurst, M. (2008). “Trends in augmented reality tracking, interaction and display: a review of ten years of ISMAR,” in Proceedings of the 7th IEEE/ACM International Symposium on Mixed and Augmented Reality, Cambridge, UK.
Keywords: Canidae, Carnivora, Georgia, augmented reality, early Pleistocene, morphology
Citation: Bartolini Lucenti S, Bukhsianidze M, Martínez-Navarro B and Lordkipanidze D (2020) The Wolf From Dmanisi and Augmented Reality: Review, Implications, and Opportunities. Front. Earth Sci. 8:131. doi: 10.3389/feart.2020.00131
Received: 13 January 2020; Accepted: 07 April 2020;
Published: 15 May 2020.
Edited by:
Pasquale Raia, University of Naples Federico II, ItalyReviewed by:
Francesco Carotenuto, University of Naples Federico II, ItalyGabriele Sansalone, University of New England, Australia
Copyright © 2020 Bartolini Lucenti, Bukhsianidze, Martínez-Navarro and Lordkipanidze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saverio Bartolini Lucenti, c2F2ZXJpby5iYXJ0b2xpbmlsdWNlbnRpQHVuaWZpLml0
 Saverio Bartolini Lucenti
Saverio Bartolini Lucenti Maia Bukhsianidze
Maia Bukhsianidze Bienvenido Martínez-Navarro4,5,6
Bienvenido Martínez-Navarro4,5,6