Abstract
Ultramafic soils are typically enriched in nickel (Ni), chromium (Cr), and cobalt (Co) and deficient in essential nutrients, making them unattractive for traditional agriculture. Implementing agromining systems in ultramafic agricultural soils represent an ecological option for the sustainable management and re-valorisation of these low-productivity landscapes. These novel agroecosystems cultivate Ni-hyperaccumulating plants which are able to bioaccumulate this metal in their aerial plant parts; harvested biomass can be incinerated to produce Ni-enriched ash or “bio-ore” from which Ni metal, Ni ecocatalysts or pure Ni salts can be recovered. Nickel hyperaccumulation has been documented in ~450 species, and in temperate latitudes these mainly belong to the family Brassicaceae and particularly to the genus Odontarrhena (syn. Alyssum pro parte). Agromining allows for sustainable metal recovery without causing the environmental impacts associated with conventional mining activities, and at the same time, can improve soil fertility and quality and provide essential ecosystem services. Parallel reductions in Ni phytotoxicity over time would also permit cultivation of conventional agricultural crops. Field studies in Europe have been restricted to Mediterranean areas and these only evaluated the Ni-hyperaccumulator Odontarrhena muralis s.l. Two recent EU projects (Agronickel and LIFE-Agromine) have established a network of agromining field sites in ultramafic regions with different edapho-climatic characteristics across Albania, Austria, Greece and Spain. Soil and crop management practices are being developed so as to optimize the Ni agromining process; field studies are evaluating the potential benefits of fertilization regimes, crop selection and cropping patterns, and bioaugmentation with plant-associated microorganisms. Hydrometallurgical processes are being up-scaled to produce nickel compounds and energy from hyperaccumulator biomass. Exploratory techno-economic assessment of Ni metal recovery by pyrometallurgical conversion of O. muralis s.l. shows promising results under the condition that heat released during incineration can be valorized in the vicinity of the processing facility.
Introduction
Ultramafic soils in Europe
Ultramafic (serpentine) soils are natural metalliferous soils derived from the weathering of ultramafic rocks which comprise at least 70% ferromagnesian (or mafic) minerals (particularly within the olivine and pyroxene groups) and <45% silica (SiO2) (Kruckeberg, 2002). Ultramafic outcrops are found worldwide but with a patchy distribution, covering ~1% of the terrestrial surface (Echevarria, 2018). Major outcrops can be found in temperate (e.g., Alps, Balkans, Turkey, California) and tropical regions (e.g., New Caledonia, Cuba, Brazil, Malaysia, Indonesia).
Ultramafic soils typically present a series of geochemical peculiarities, which include an elevated concentration of magnesium (Mg) and iron (Fe), a low calcium (Ca):Mg ratio and elevated concentrations of trace elements such as nickel (Ni), chromium (Cr), and cobalt (Co). Total Ni concentrations in soils globally range between 2 and 750 mg kg−1 but can reach 3,600 mg kg−1 or more in soils developed over ultramafic rocks (Sparks, 2002). They are also known for their deficiency in macronutrients such as nitrogen (N), potassium (K), or phosphorus (P) and micronutrients such as molybdenum (Mo) or boron (B) (Nkrumah et al., 2016). Since many ultramafic outcrops are steep and rocky their associated soils are often skeletal, with limited organic matter and low water holding capacity (Brooks, 1987). In temperate climates, the most common soil types include high pH Regosols/Leptosols with cambic properties and cation exchange complex dominated by Mg over Ca, to Cambisols with neutral to slightly acidic pH (Echevarria, 2018). This unusual geochemical composition is commonly referred to as the “serpentine syndrome” and creates an inhospitable environment for plant growth. As a result, ultramafic soils often support plant communities with high rates of endemism which have evolved both morphological and physiological adaptations differentiating them from the flora of neighboring areas (Brooks, 1987; Bergmeier et al., 2009). For example, hyperaccumulator plants accumulate extreme amounts of metals within their living tissues. There are ~500 taxa of plants that are known to hyperaccumulate one or more metal(loid)s, and around 90% of these accumulate Ni (van der Ent et al., 2013a; Pollard et al., 2014). Ni-hyperaccumulators accumulate >1,000 mg Ni kg−1 DW matter in their shoots when growing in such metal(loid)-enriched substrates, concentrations that would be toxic to most other plant species (Brooks, 1987). The distribution and taxonomy of European Ni-hyperaccumulators is described in more detail below.
The concept of agromining
The European economy is highly dependent on imports of many raw materials including metals, metalloids, and minerals, with applications in several high-tech industries (ICMM, 2012). Many of these are threatened by supply shortage, due to political instability or rapidly declining ore reserves, and the EU prioritizes research on the extraction, recovery, or recycling of metals from unconventional mineral resources which are not accessible using traditional mining techniques (http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52011DC0021&from=EN). Agromining cultivates plants that accumulate trace metal(loid)s from metal-rich soils or substrates in their shoots, which at the end of the growth period can be harvested and burnt to produce metal(loid)-enriched ash or “bio-ore.” It is considered a commercially viable technique in the case of high-value elements such as Ni, Co, or Au (Chaney et al., 2018). The European Innovation Partnership (EIP) classified Ni as a raw material with high economic importance and Ni agromining was proven to be efficient in the 2000's and became a real market opportunity in 2007 (Chaney et al., 2007; Tang et al., 2012; Bani et al., 2015a). Nickel has been successfully recovered from bio-ores in pure form, as a mineral salt (ANSH, ammonium nickel sulfate hexahydrate), or as eco-catalysts (Simonnot et al., 2018). Nickel-hyperaccumulator plants are ideal candidates for Ni agromining due to their unusual ability to bioconcentrate and purify Ni from ultramafic soils. However, the only large-scale European field trial carried out to date was done in Albania using Odontarrhena muralis sensu lato (syn. Alyssum murale s.l.) (Bani et al., 2007, 2015a,b). In addition to metal extraction, agromining agrosystems can also be managed to provide multiple ecosystem services, such as C sequestration, enhanced soil biodiversity, renewable biomass production, improved agricultural crop productivity (safe edible and non-edible crops) and land restoration (Echevarria et al., 2015). Nickel agromining could also stimulate the rural development of ultramafic regions.
The growing interest in this emerging technology is reflected through the recent funding of two EU projects: Agronickel (Developing Ni agromining on ultramafic land in Europe; funded through the FACCE Surplus–Cofund) and LIFE-Agromine (Cropping hyperaccumulator plants on nickel-rich soils and wastes for the green synthesis of pure nickel compounds; funded by the LIFE Environment and Resources Programme, http://life-agromine.com). These two projects aim to implement agromining at large scale and follow on from the “Agromine” project funded by the ANR (Agence Nationale de la Recherche) in France. Agronickel is dedicated to the selection of adequate nickel crops, optimisation of their biomass productivity and Ni extraction capacity using resource-friendly agriculture (so-called “agroecological” agriculture), and the development of the metal recovery process and synthesis of new Ni-products. The optimal plant cropping pattern and the potential benefits of using plant associated-microbial inoculants or phytohormones will be assessed. On the other hand, through the LIFE-Agromine project the opportunity arose to establish a European network of agromining field-scale demonstrations across distinct geographical areas and under different edapho-climatic conditions. The LIFE-Agromine project aims to demonstrate at a pilot-scale the provision of ecosystem services through agroecological agromining cropping systems, the recovery of valuable Ni-products, as well as the environmental and socio-economic viability of the agromining cycle, including professional training of potential stakeholders. Studies evaluating the environmental, technical, and economic viability of agromining at all stages of the cycle are rare. LIFE-Agromine will carry out a full-cycle assessment of the potential benefits of cropping hyperaccumulator plants on these metalliferous soils for the green synthesis of nickel compounds and provision of ecosystem services.
A synthetic overview of Ni-hyperaccumulators in Europe
Nickel-hyperaccumulators native to Europe are mostly distributed in the southern parts of the continent, where ultramafic outcrops occur at various altitudinal belts and bioclimatic regions. Their diversity follows a west-to-east gradient, with the Iberian Peninsula and Corsica hosting only one or two native species, vs. ca. eight in CS Europe-C Mediterranean and some 30 taxa in SE Europe. Due to the massive distribution of ultramafic soils, the Balkan Peninsula is an outstanding hot-spot of metallophytic flora and serpentine endemism of both paleo- and neo-endemic type (Shallari et al., 1997; Stefanović et al., 2003). Albania and Greece are especially rich in Ni-accumulators, most of them endemics, showing the role of the large ultramafic bodies of the so-called “Dinaric-Hellenid” belt (Bortolotti et al., 2013) as centers of speciation and glacial refugia (Médail and Diadema, 2009). However, the systematics of Ni-accumulators, especially from E Europe is still not completely known, preventing an exact estimation of their diversity to be safely presented.
Undoubtedly, their taxonomic and phylogenetic distribution is highly uneven. All species known so far belong to the Brassicaceae family (crucifers), with a few exceptions reported from the Asteraceae genus Centaurea L. Uneven distribution also occurs within the Crucifers, since all species belong to only two of the ca. 23 tribes of the family, e.g., the Coluteocarpeae and the Alysseae. The former group includes the large genus Noccaea Moench, separated from Thlaspi L. based on phylogenetic evidence (Koch and Al-Shehbaz, 2004; Al-Shehbaz, 2014 and references therein). This includes 11 species that can grow on ultramafic or metal-contaminated soils of Europe and accumulate metals such as Ni and Zn (Table 1). Most of these taxa, however, are of limited interest for agromining applications because of their small biomass production or high ecological specificity (alpine habitats). With four genera and 21 species recognized to date (Table 1), tribe Alysseae is the largest group of Ni-hyperaccumulators in the continent and includes the most promising candidates for Ni-phytoextraction. These are all perennial herbs or small shrubs belonging to three genera, Odontarrhena C.A.Mey., Alyssoides Mill., and Bornmuellera Hausskn. Based on phylogenetic evidence (Cecchi et al., 2010; Rešetnik et al., 2013) the latter is very close to genus Leptoplax O.E. Schulz with the only species L. emarginata, a strict endemic to the serpentines of N Greece and Euboea. In spite of distinctive characters in habit and fruit compared to typical taxa of Bornmuellera (Hartvig, 2002), the occurrence of intergeneric F1 hybrids with partially sympatric B. tymphaea and B. baldaccii led Rešetnik et al. (2013) to include Leptoplax in Bornmuellera, and this was recently accepted in the Alybase treatment (Španiel et al., 2015). Three features convey to B. emarginata a remarkable potential for Ni-phytoextraction, e.g., high accumulation ability (Bani et al., 2010), biomass production (Chardot et al., 2005), and adaptive capacity to different habitats and climates, from low to high altitudes (ca. 300–2100 m a.s.l). Drought tolerance is a fourth feature that makes its use possible also in Mediterranean xeric conditions. The four other taxa of Bornmuellera are also powerful Ni-accumulators restricted to serpentine soils of N Greece, Albania, and Kosovo (Bani et al., 2009), but their smaller stature, biomass production and restriction to alpine habitats (above 1,000 m and up to 2,600 m a.s.l.) represent limiting factors to their use for field applications.
Table 1
| Taxon | Distribution |
|---|---|
| TRIBE ALYSSEAE | |
| Alyssoides utriculata (L.) Medik. | France, Italy, former Yugloslavian countries, Albania, Bulgaria, Greece |
| Bornmuellera baldaccii (Degen) Heywood subsp. baldaccii | Greece |
| B. baldaccii subsp. rechingeri Greuter | Albania |
| B. dieckii Degen | Kosovo |
| B. emarginata (Boiss.) Rešetnik (syn.: Leptoplax emarginata (Boiss.) O.E. Schulz) | Greece |
| B. tymphaea (Hausskn.) Hausskn. | Greece |
| Odontarrhena akamasica (B.L.Burtt) & al. | Cyprus |
| O. argentea (All.) Ledeb. | Italy |
| O. diffusa Jord. & Fourr. | Greece |
| O. fallacina (Hausskn.) Španiel & al. | Greece |
| O. bertolonii (Desv.) Jord. & Fourr. | Italy |
| O. chalcidica (Janka) Španiel & al. | Greece, Albania, FYROM, Kosovo |
| O. cyprica (Nyár.) Španiel & al. | Cyprus |
| O. euboea (Halácsy) Španiel & al. | Greece |
| O. heldreichii (Hausskn.) Španiel & al. | Greece |
| O. lesbiaca P.Candargy | Greece |
| O. muralis (Waldst. & Kit.) Endl. | Bulgaria, Greece, Romania, Serbia |
| O. robertiana (Bernard ex Gren. & Godr.) Španiel & al. | France (Corsica) |
| O. serpyllifolia (Desf.) Jord. & Fourr. | Iberian Peninsula, France |
| O. smolikana (Nyár.) Španiel & al. | Greece, Albania |
| O. troodi (Boiss.) Španiel & al. | Cyprus |
| TRIBE COLUTEOCARPEAE | |
| N. alpestris (Jacq.) Kerguélen | Austria, France, Italy, Switzerland |
| N. boeotica F.K.Mey (Spruner) | Greece |
| N. brachypetala (Jord.) F.K.Mey. | Austria, Czech Rep. Finland, France, Italy, Slovakia, Spain, Sweden, Switzerland |
| N. bulbosa (Spruner) Al-Shehbaz | Greece |
| N. caerulescens (J.Presl & C.Presl) F.K.Mey. | C, W, N Europe |
| N. cepaeifolia (Wulfen) Rchb. | France, Austria, Italy |
| N. epirota (Halácsy) F.K.Mey. | Greece |
| N. goesingensis (Halácsy) F.K.Mey. | Austria, Bosnia and Herzegovina, Bulgaria, Serbia |
| N. graeca (Jord.) F.K.Mey, | Greece |
| N. rotundifolia (L.) Moench | Austria, France, Germany, Italy, Slovenia, Switzerland |
| N. tymphaea (Hausskn.) F.K.Mey. | Albania, Bosnia and Herzegovina, Greece, FYROM |
Nickel-accumulator species in tribes Alysseae and Coluteocarpeae (Brassicaceae) native to Europe (including Cyprus); nomenclature of Noccaea and Alysseae follows respectively Al-Shehbaz (2014) and Spaniel et al. (2015; see also Alybase; http://www.alysseae.sav.sk/checklists/search).
Odontarrhena corsica (Duby) Španiel & al., also a Ni-hyperaccumulator naturalized in Corsica but native to Turkey.
Odontarrhena has long been considered a section of the genus Alyssum L., but there is morphological and molecular evidence showing that it represents a distinct lineage without direct relationship to Alyssum (Warwick et al., 2008; Cecchi et al., 2010, 2013; Rešetnik et al., 2013). While Ni-accumulation is unknown among the “true” Alyssums, nearly all species and populations of Odontarrhena that grow on ultramafic soil possess this ability. This is the case for a high proportion of the nearly 90 taxa of this genus that range in S Europe, Mediterranean and W Asia (Španiel et al., 2015). Odontarrhena is taxonomically difficult because of the high phenotypic plasticity, the strong incidence of polyploidy and the presence of hybridization processes within at least given groups. Examples of critical species complexes are the two facultative serpentinophytes O. serpyllifolia and O. muralis in the W and E Mediterranean, respectively, in which several names have been adopted to separate the serpentine populations. Both are effective Ni-hyperaccumulators (Bani et al., 2010, 2013; Tumi et al., 2012; Konstantinou and Tsiripidis, 2015; Morais et al., 2015), and the complex of O. muralis s.l. is currently one of the most promising candidates taxa for Ni-agromining because of its biomass production and growth capacity in various field conditions (Bani et al., 2015a,b). Nickel concentrations recorded in field-collected and herbarium material over the last 30 years in the Balkan Peninsula found this species to accumulate the highest Ni concentrations (Bani et al., 2010, 2013). Furthermore, O. muralis s.l. is crucial for the structure of the serpentine vegetation in the Balkans (Bergmeier et al., 2009). While the taxa from Greece are better understood, some of those from Albania, the former Yugoslav Republic of Macedonia (FYROM), and Bulgaria are still poorly known, preventing a correct estimation of the diversity of accumulators in the Balkans to be presented. A recent systematic revision of the genus in Albania (Cecchi et al., 2018) points to the existence of seven taxa, of which six Ni-hyperaccumulators are restricted to ultramafic soils (except for O. chalcidica, facultative serpentinophyte). A polyploid species of likely hybrid origin between O. chalcidica and O. smolikana, originally described from Mt. Smolikas (Greece) as Alyssum decipiens Nyár. and widely distributed on the Albanian ultramafics, could be a promising candidate for phytoextraction because of the high Ni levels, the robust habit and the ability to grow in a wide range of habitats, including anthropogenic ones, from low to high altitudes.
Suitable agroecosystems for Ni-hyperaccumulators
Agromining aims to extract metals from the soil through the implementation of hyperaccumulator cropping systems, to improve soil quality and ecological functions, and to provide complementary income to farmers and local populations. However, climatic conditions, soil properties, and metal bioavailability can limit the production of plant biomass and metal accumulation, and consequently the effectiveness of agromining (van der Ent et al., 2015). Several studies have focused on different agronomic aspects to optimize the metal extraction process.
Adequate fertilization regimes
Due to nutrient deficiencies of ultramafic soils, mineral fertilization has a positive effect on the biomass production of Ni hyperaccumulators such as Odontarrhena (syn. Alyssum) spp. (Bani et al., 2015a; Kidd et al., 2015; Álvarez-López et al., 2016). The application of mineral fertilizers was also found to improve Ni yields (Li et al., 2003a). NPK amendments, coupled with herbicide applications and irrigation management, allowed the production of at least 20 t ha−1 of dry biomass of O. muralis s.l. and 400 kg Ni ha−1 (Chaney et al., 2007). Similarly, after 5 years of field experiments, biomass and Ni yields of a spontaneous cover of O. muralis s.l. in Albania were improved from 0.3 to 9.0 t DW biomass ha−1 and from 1.7 to 105 kg Ni ha−1 after the addition of 120 kg N and 100 kg P, K ha−1 (Bani et al., 2015a). Phosphorus has some effect on biomass yield and Ni uptake by hyperaccumulators (Shallari et al., 2001; Chaney et al., 2008), including the stimulation of flowering of the plants. Additionally, the fractional application of N is advisable to minimize excessive leaching of N to groundwater (Li et al., 2003a; Bani et al., 2015a) and to compensate soil nutrient absorption by the hyperaccumulator plants and maintain biomass production over several years (Kidd et al., 2015; Nkrumah et al., 2016).
The addition of organic amendments can improve soil quality and structure, reduce compaction and erosion, supply essential nutrients, and indirectly stimulate biological activity. Amendment with composted sewage sludges during the cultivation of the Ni-hyperaccumulators O. serpyllifolia and O. bertolonii in ultramafic soil led to a 24- and 62-fold increase, respectively, in biomass production compared to untreated plant covers (Álvarez-López et al., 2016). Similarly, Ghasemi et al. (2018) showed that three Ni-hyperacumulating Odontarrhena spp. produced significantly higher DW yields, improved nutritive status, and increased total Ni phytoextracted when grown in ultramafic soils amended with cow manure compared to NPK-fertilized soils. Alternatively, Saad R. F. et al. (2018b) incorporated green manure from legumes into the soil and found Ni yields were significantly increased following a stimulation of biomass production. The increase in biomass production could be favored by the improvement in soil physical properties, such as structure, porosity, and water retention capacity, but also by the stimulation of microbial activity. Thus, it appears that organic amendments are good candidates to improve agromining, but this strategy needs to be further demonstrated in large-scale field trials (Nkrumah et al., 2016).
Plant cropping patterns
Studies focusing on agricultural crop systems or on grasslands have shown that increased plant diversity enhances soil microbial biomass, activity, and diversity (Benizri and Amiaud, 2005). Plant species coexistence enhances interactions between micro- and macro-organisms in soils. Diversified organic compounds (i.e. rhizodeposits) released from different plants growing together in multi-species vegetation covers, can change the rhizosphere conditions and affect the abundance, functions and diversity of associated microorganisms (Berg and Smalla, 2009). Mixed cropping patterns also increase the diversity of plant litter returned to the soil, increasing diversity of litter-degrading organisms (Brussaard, 2012). Significantly higher values of many biological indicators have been found under multi-species vegetation (Gao et al., 2010).
However, few studies have examined the effect of cultivating metal hyperaccumulators together with other plant species. Some studies focused on the associations of Sedum alfredii + Zea mays (Wei et al., 2011), Sedum alfredii + Alocasia macrorrhiza (Wu et al., 2007), Noccaea caerulescens + Lolium perenne (Jiang et al., 2010), and Noccaea caerulescens + Hordeum vulgare + Lepidium heterophyllum (Gove et al., 2002). Most of these showed that co-cropping with non-metal hyperaccumulator plants can improve the growth of the hyperaccumulator and increase metal extraction. Additionally, co-culture enhanced the size of the microbial community and stimulated certain microbial functional groups (Jiang et al., 2010). The efficiency of nickel phytoextraction in mixed hyperaccumulator covers has also been studied (Lucisine et al., 2014; Rue et al., 2015). Co-cropping Odontarrhena muralis s.l., Noccaea tymphaea, Bornmuellera emarginata and Bornmuellera tymphaea increased biomass (by 21%) and Ni phytoextraction (by 47%) of B. tymphaea in mixed cover compared to its mono-culture (Rue et al., 2015). Moreover, Lucisine et al. (2014) found that the coexistence of different hyperaccumulating plants promoted soil metal bioavailability and modified the genetic and phenotypic structures of the rhizosphere bacterial communities.
Legumes are widely used in conventional agriculture due to their ability to fix atmospheric nitrogen; through the presence of symbiotic N2-fixing Rhizobium bacteria which can convert it into a plant-assimilated form (De Antoni Migliorati et al., 2015). Co-cropping hyperaccumulating plants with legumes could also improve plant growth and consequently metal phytoextraction. Moreover, part of the N fixed is usually transferred to companion plants (Rodrigues et al., 2015). Agromining could benefit from this strategy provided the legume tolerates the metal concentrations present in ultramafic soils (Saad et al., 2016). In a mixed legume-crop cover the rhizosphere microbial communities of this association also differ from those found in mono-cultures and can positively influence nutrient availability and plant uptake (St. Luce et al., 2015; Saad et al., 2017). As a result, improvements in soil quality, structure and biological activity are expected (Stevenson and van Kessel, 1996; Evans et al., 2003). During legume decomposition in co-cropping systems, the N-rich residues constituted a significant quantity of carbon returned to the soil, thus increasing the soil carbon stock by up to 0.6 t ha−1 per year (Kuo et al., 1997). In agromining cropping systems, the presence of legumes also improved soil physical properties, increasing soil porosity and aggregate stability (Saad et al., 2018a). The introduction of legumes into these systems also replaces the need for high inputs of mineral fertilizers, thus promoting agromining as an advanced ecological ‘green’ system.
Plant-microbial associations
Microbial-assisted phytoextraction approaches for the remediation of metal-contaminated or metal-rich soils are relatively recent concepts. Nickel-hyperaccumulators, as all plants, are inhabited by symbiotic microorganisms (Cardinale, 2014). These protect the host from pathogens, stimulate growth, nutrient uptake and transport, attenuate stress (Lugtenberg and Kamilova, 2009; Yang et al., 2009; Rashid et al., 2016) and moderate phenotypic and epigenetic plasticity (Partida-Martínez and Heil, 2011). Extensive research has been dedicated to the identification of microbial inoculants which can be incorporated into phytoextraction systems, for overcoming limitations in these processes due to reduced plant biomass or limited soil metal availability (Khan et al., 2009; Zaidi et al., 2011; Thijs et al., 2016; Kidd et al., 2017a; Benizri and Kidd, 2018).
Plant-bacterial interactions
Plant associated-bacteria are well known to play an important role in plant growth, fitness and nutrient supply. Growth promotion is generally associated with the ability to increase nutrient availability, such as nitrogen (N2-fixing organisms), phosphorus (by solubilization or mineralization through release of organic acids and/or phosphatases), or iron (by releasing Fe(III)-specific chelating agents or siderophores), or via the production of plant hormones or reduction in stress ethylene levels (Khan et al., 2009; Glick, 2010; Benizri and Kidd, 2018). Nickel hyperaccumulating plants have been shown to select for Ni-tolerant bacterial strains in their rhizosphere (Mengoni et al., 2001; Abou-Shanab et al., 2003; Álvarez-López et al., 2016), and these rhizobacteria have recently been linked to the metal hyperaccumulation process itself (Becerra-Castro et al., 2013; Muehe et al., 2015). Bacterial metal solubilization has been attributed to mechanisms such as proton extrusion or the production of complexing agents, e.g., organic acids (Gadd, 2010). Their capacity to enhance soil metal availability and/or plant metal yields offers the possibility to improve agromining efficiency. The results in terms of soil metal removal when using such plant-bacterial associations are promising: numerous examples of enhanced hyperaccumulator plant growth and metal uptake and accumulation can be found after soil, seed or plant inoculation (Kidd et al., 2017a; Benizri and Kidd, 2018). Increases in phytoextracted metal yields have been attributed to either plant growth promotion or the ability of isolates to mobilize metals from less labile soil fractions. Some inoculants have been selected for their ability to enhance Ni yields when applied to more than one plant species and/or soil type (Cabello-Conejo et al., 2014; Durand et al., 2016; Ghasemi et al., 2018).
Overall, inoculation with plant growth-promoting or metal-mobilizing microorganisms is considered a promising approach for increasing metal phytoavailability and the growth and health of “metal crops”. However, the vast majority of these studies have been carried out on a bench-scale and there is still a need for field evaluations of such plant-bacterial associations. Aspects such as the degree of plant species-specificity, the compatibility of the selected host species and inoculant, or the performance of the bacterial strains and their ability to proliferate in the soil, need to be studied under natural field conditions. As part of the Agronickel project, field trials were established in an ultramafic outcrop in NW Spain to test the potential use of three rhizobacterial strains (all originally isolated from rhizosphere of Ni-hyperaccumulating plant species) for improving the establishment and yield of O. muralis s.l. Plant coverage and aerial-biomass (Figure 1), and plant Ni yield (but not shoot Ni concentration), were significantly increased after inoculation with Paenarthrobacter nitroguajacolicus strain LA44 and Pseudoarthrobacter oxydans strain SBA82 (Pardo et al., 2017).
Figure 1

Aerial view of field plots (4 m2) with non-inoculated (A) and inoculated (B)Odontarrhena muralis s.l. Plants were inoculated with Paenarthrobacter nitroguajacolicus strain LA44 (Pardo et al., 2017).
Plant-fungal interactions
Our knowledge of the role of symbiotic fungi in plant adaptation to ultramafic soils is scarce. Available reports indicate that fungal symbionts facilitate plant growth in these environments by affecting Ni uptake and distribution within the plant. The best described group of fungi which has been shown to positively affect plant growth in Ni-enriched soils is the arbuscular mycorrhizal fungi (AMF). Interestingly, there is no unequivocal “mode” of action of fungi (mycorrhiza and others), in terms of their effect on metal uptake and distribution; some species of fungi protect the plant by decreasing Ni uptake, while others increase uptake and translocation from root to shoot (Cao et al., 2008; Rozpądek et al., 2018). Additionally, there is no consensus concerning the mechanism(s) by which fungi alleviate Ni phytotoxicity. It remains open for discussion whether symbiotic fungi improve plant growth under metal toxicity by facilitating water and nutrient acquisition and thus indirectly fine-tuning the host's metal tolerance or by directly upregulating specific metal tolerance mechanisms.
Hyperaccumulators were believed to be unable to form mycorrhiza, until the discovery of AMF in roots of Berkheya coddii, Senecio coronatus and, later, in other Ni-hyperaccumulating species (Turnau and Mesjasz-Przybylowicz, 2003). AMF supported B. coddii by increasing nutrient (K, Fe, Zn, Mn, P, Ca) acquisition and distribution (Orłowska et al., 2013). Co and Ni uptake was lower in mycorrhizal plants. Nevertheless the significantly higher biomass production of mycorrhizal plants resulted in a 20-fold increase in Ni yield per plant. The role of mycorrhizal symbiosis has not yet been studied in European hyperaccumulators, even though there are some candidates from the Asteraceae family that could become a promising tool in phytoextraction. So far, however, the importance of AMF and ectomycorrhiza in ultramafic soils is mainly considered to be the driver of ecosystem diversity, playing an important role in stimulating plant yield in these environments which are rich in Ni and poor in nutrients (such as N, P, or K) but host a unique and rich vegetation.
The non-mycorrhizal Brassicaceae, a family particularly rich in metal-tolerant species, were found to be abundantly inhabited by highly metal-tolerant fungal endophytes (García et al., 2013; Zhang et al., 2014), shown to affect plant metal homeostasis and facilitate vegetation in metalliferous soils (Rozpądek et al., 2018). Their low host specificity and ease in cultivation make them promising candidates for improving the efficiency of phytomining. Ni uptake and most importantly extraction efficiency were positively affected in Brassica juncea inoculated with Trichoderma atroviride strain F6. Additionally, inoculated B. juncea exhibited higher tolerance to metal toxicity (Cao et al., 2008). The mycobiome of Ni-hyperaccumulators from the Odontarrhena and Noccaea genera have been recently studied within the Agronickel project (Turnau et al., 2017). The benefits of available molecular resources for some of these species will allow for studying the role of symbiotic fungi in Ni hyperaccumulation and tolerance more thoroughly. According to preliminary results, plants of both the abovementioned taxa from serpentine soils harbor numerous fungal endophytes. Ninety percent of isolated fungi belong to two classes Dothideomycetes and Sordariomycetes (Class 3 endophytes according to the classification of Rodriguez et al., 2009), the former being most commonly isolated from leaves and the latter from roots (Turnau et al., 2017). Some taxa inhabited both plant roots and shoots. Dark pigmented mycelium of these fungi was visible in cross sections of shoots of hyperaccumulating plants collected in the field; within air spaces of green and healthy leaf tissues and the stem central cylinder. Many of the fungi developed brown/black pigmentation similar to dark septate endophytes (DSE), and considered as Class 4 endophytes (Rodriguez et al., 2009). Members of DSE show extraordinary tolerance to metal toxicity and an ability to accumulate up to 20% DW Pb (Zhang et al., 2008). The DSE Phialocephala fortinii was commonly isolated from roots of Ni-hyperaccumulators, however, it had little phenotypic effect on the host plants (Rozpądek et al., 2017; Ważny et al., 2017).
One of the most commonly encountered fungal endophytes in Noccaea species was Embelisia thlaspsis (Dothideomycetes; Rozpądek et al., 2017). A Ni-adapted strain of this fungus alleviated metal toxicity: the plants accumulated less stress protective anthocyanins when exposed to elevated Ni concentrations in the soil (Figure 2A), even though Ni acquisition by symbiotic plants was significantly higher (Figure 2B). Additionally, the root system of inoculated plants was more developed; showing that co-cultivation with E. thlaspis resulted in significant lateral root elongation (Figure 2C). The possible utilization of fungal endophytes in improving phytoextraction efficiency of Ni-hyperaccumulating Brassicaceae is currently under investigation. Preliminary results indicate that these microorganisms may indeed be used to support plant vegetation and Ni accumulation.
Figure 2
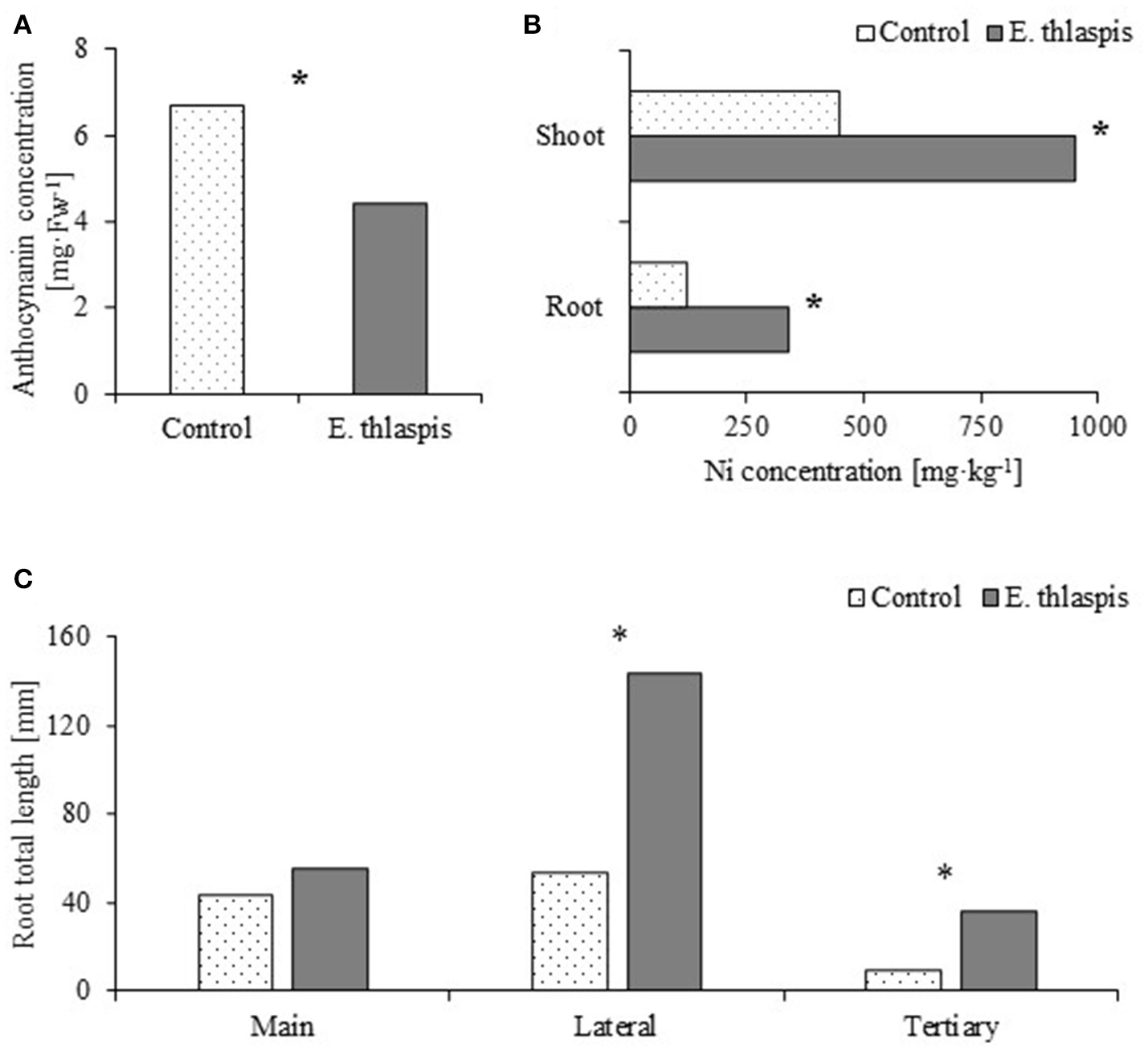
The beneficial effect of endophytic fungus Embellisia thlaspis on Noccaea caerulescens. (A) Anthocyanin concentration [analyzed according to Fukumoto and Mazza (2000)]. (B) Ni accumulation in plant root and shoot (analyzed by Atomic Absorption Spectrometry). (C) Changes in root architecture. Statistical significances were evaluated with the t-test, P ≤ 0.0.05, N = 5 (in A,C) and N = 50 (in B). Plants were grown in sand mixed with perlite (1:1; v:v) for 50 days in a vegetation chamber with a 16 h photoperiod and 21/17°C day/night temperature. Ten day-old seedlings were inoculated with 3 ml of mycelium suspended in water (OD600 = 0.15). Fourteen days after inoculation the substrate was supplemented with 150 μM of NiSO4 in Hoagland solution. Plants were irrigated with Hoagland solution twice per week. *Indicates statistical significance between inoculated and control. Data based on Rozpądek et al. (2017).
European network of agromining field sites
The Agronickel and Life-Agromine projects established a network of pilot-scale field sites in ultramafic regions across western, central and southern Europe in order to cover a range in climatic and edaphic conditions (Figure 3 and Table 2; Echevarria et al., 2017). Experimental field plots have been set-up to demonstrate the benefits of incorporating organic amendments, legumes or bioinoculants into agromining agrosystems with the aim to maximize plant yields and Ni removal at the same time as improving soil quality and functioning.
Figure 3
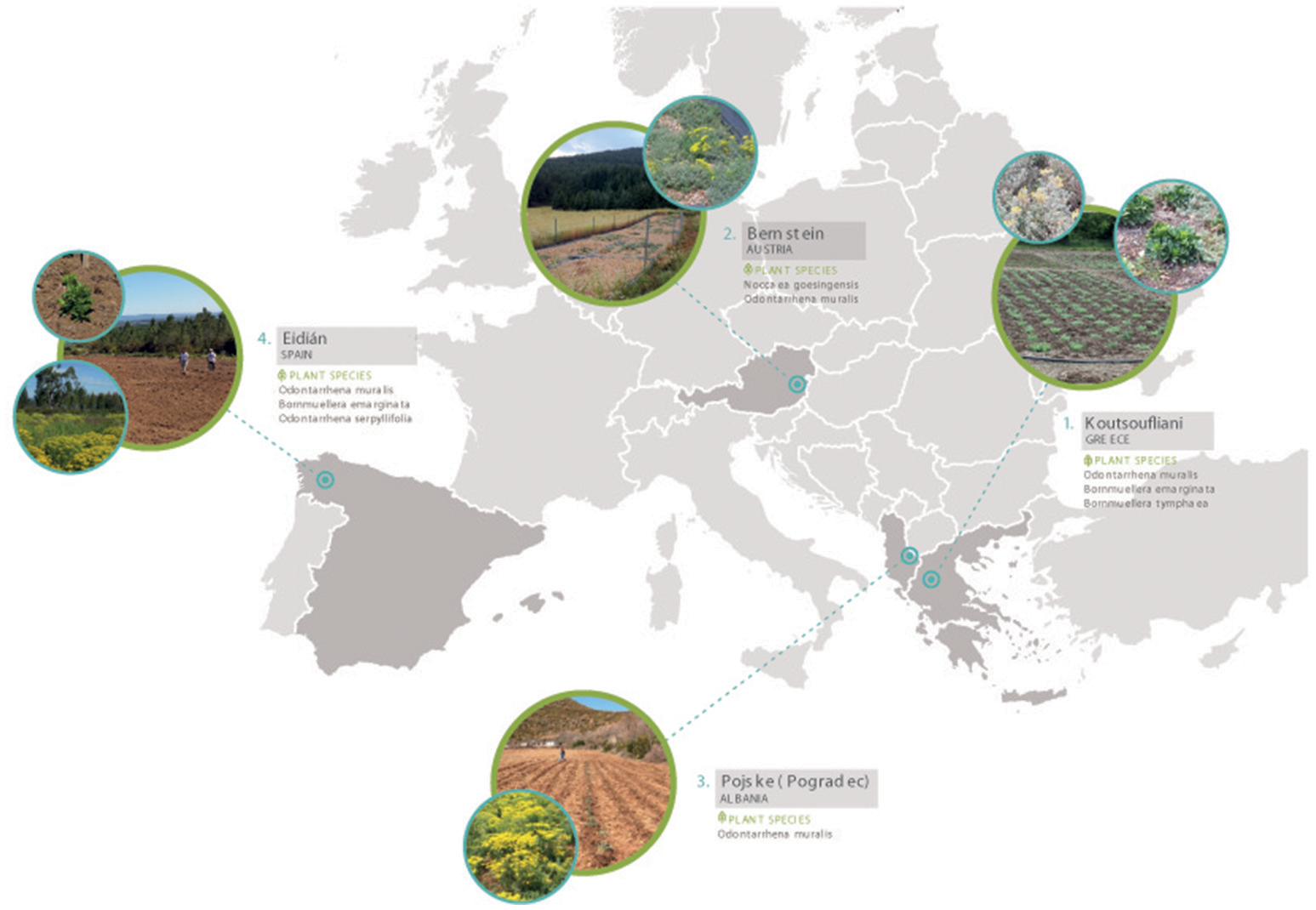
European network of nickel agromining field sites.
Table 2
| Country | Pojskë | Bernstein | Koutsoufliani | Eidián |
|---|---|---|---|---|
| Site | Albania | Austria | Greece | Spain |
| Coordinates | 40°59′N 20°38′E | 47°24′45.2″N 16°16′04.7″E | 39°51′37.8″N 21°18′50.2″E | 42°49′54.2″N 8°00′13.4″W |
| Altitude (m) | 700 | 620 | 930 | 430 |
| SOIL GENERAL PROPERTIES | ||||
| Texture | Clay | Sandy loam | Silty clay | Sandy loam |
| pHH2O | 7.5 ± 0.2 | 6.1 ± 0.1 | 7.2 ± 0.0 | 5.8 ± 0.1 |
| CEC (cmolc kg−1) | 38.9 ± 1.1 | 15.9 ± 2.0 | − | 4.9 ± 1.0 |
| TOC (g kg−1) | 28.4 ± 4.0 | 22.4 ± 0.5 | − | 52.6 ± 8.0 |
| TN (g kg−1) | 2.3 ± 0.3 | 2.2 ± 0.5 | − | 3.0 ± 0.4 |
| P Olsen (mg kg−1) | <0.05 | 9.0 ± 1.5 | 4.6 ± 0.4 | 4.5 ± 0.3 |
| Ca-Total (g kg−1) | 3.9 ± 0.4 | 6.7 ± 0.2 | 7.0 ± 0.3 | 7.6 ± 0.6 |
| Mg-Total (g kg−1) | 60.0 ± 2.5 | 103.0 ± 8.6 | 138.0 ± 3.0 | 45.1 ± 2.3 |
| K-Total (mg kg−1) | 4500 ± 250 | 905 ± 70 | 1863 ± 34 | 438 ± 25 |
| Fe-Total (g kg−1) | 98.0 ± 0.2 | 64.0 ± 1.2 | − | 71.0 ± 10.7 |
| Ni-Total (mg kg−1) | 3140 ± 60 | 1450 ± 180 | 2347 ± 37 | 967 ± 13 |
| Cr-Total (mg kg−1) | 1600 ± 160 | 1840 ± 140 | − | 1263 ± 65 |
| Co-Total (mg kg−1) | 207.0 ± 12.0 | 113.0 ± 4.2 | − | 77.5 ± 3.3 |
| Soil Ni availability | ||||
| Ni-DTPA (mg kg−1) | 124.0 ± 6.0 | 38.4 ± 4.6 | 71.1 ± 2.3 | 36.8 ± 13.8 |
| Ni-Sr(NO3)2 (mg kg−1) | − | 0.53 ± 0.07 | 0.80 ± 0.05 | 1.20 ± 0.27 |
| CLIMATE CHARACTERISTICS | ||||
| Climate type | Sub-mediterranean continental | Warm-summer humid continental | Sub-mediterranean continental | Humid temperate |
| Mean annual precipitation (mm) | 700 | 718 | 666 | 1200 |
| Mean annual temperature (°C) | 10.6 | 8.3 | 12.4 | 12.1 |
Soil physicochemical and climate characteristics of the field sites included in the Life-Agromine network (mean values ± SE).
CEC, cation exchange capacity; TOC, total organic C; TN, total N.
Ultramafic areas in Albania occupy ~11% of the total surface area; geologically they vary from partly serpentinized peridotite (harzburgite) to serpentinite (Bani et al., 2014; Estrade et al., 2015). Long-term agromining studies have been carried out intermittently over the period 2007–2017 in the Pogradec district (Pojskë), E Albania (Figure 3 and Table 2; Bani et al., 2007, 2015a,b). The current land use in the area is low-productivity agriculture (pasture or arable land) into which agromining could be successfully incorporated. These studies optimized the agronomic aspects of the agromining chain for these Balkan agricultural landscapes. In-situ agromining experiments using the native O. muralis s.l. were conducted from 2005 to 2009 and from 2012 to 2014, and continued as part of the Life-Agromine project in 2016–2017. The effects of fertilization, herbicide application, soil tillage, crop establishment and plant density (natural cover vs. sown crop), and competition between plant species, have been assessed. Over the period 2005 to 2009, the optimal cropping system, with split-N fertilization, irrigation and post-emergent herbicide (FocusTM ultra) treatment, progressively achieved a biomass production of 9.0 t ha−1 and Ni phytoextraction yield of 105 kg Ni ha−1 (Bani et al., 2007, 2015a,b; Figure 4). Maximum biomass was obtained with a density of 4 plants m−2 (10.2 t ha−1 in 2013 and 8.8 t ha−1 in 2014); the drop in biomass observed in 2014 was probably due to the competition between planted O. muralis s.l. and its own spontaneous recruits. The highest Ni yields were achieved in 2013 with 112 kg ha−1 (Figure 4). New plots evaluate the application of organic amendments (sheep and pig manure): biomass production in 2017 was 9.96 t ha−1 in fertilized plots and Ni yield reached its maximum of 139.3 kg ha−1. Phenological studies have shown that, in this region, Ni bioaccumulation is maximal during the mid-flowering stage which was then set as the recommended harvesting time (mid-June) (Bani et al., 2015a; Estrade et al., 2015).
Figure 4
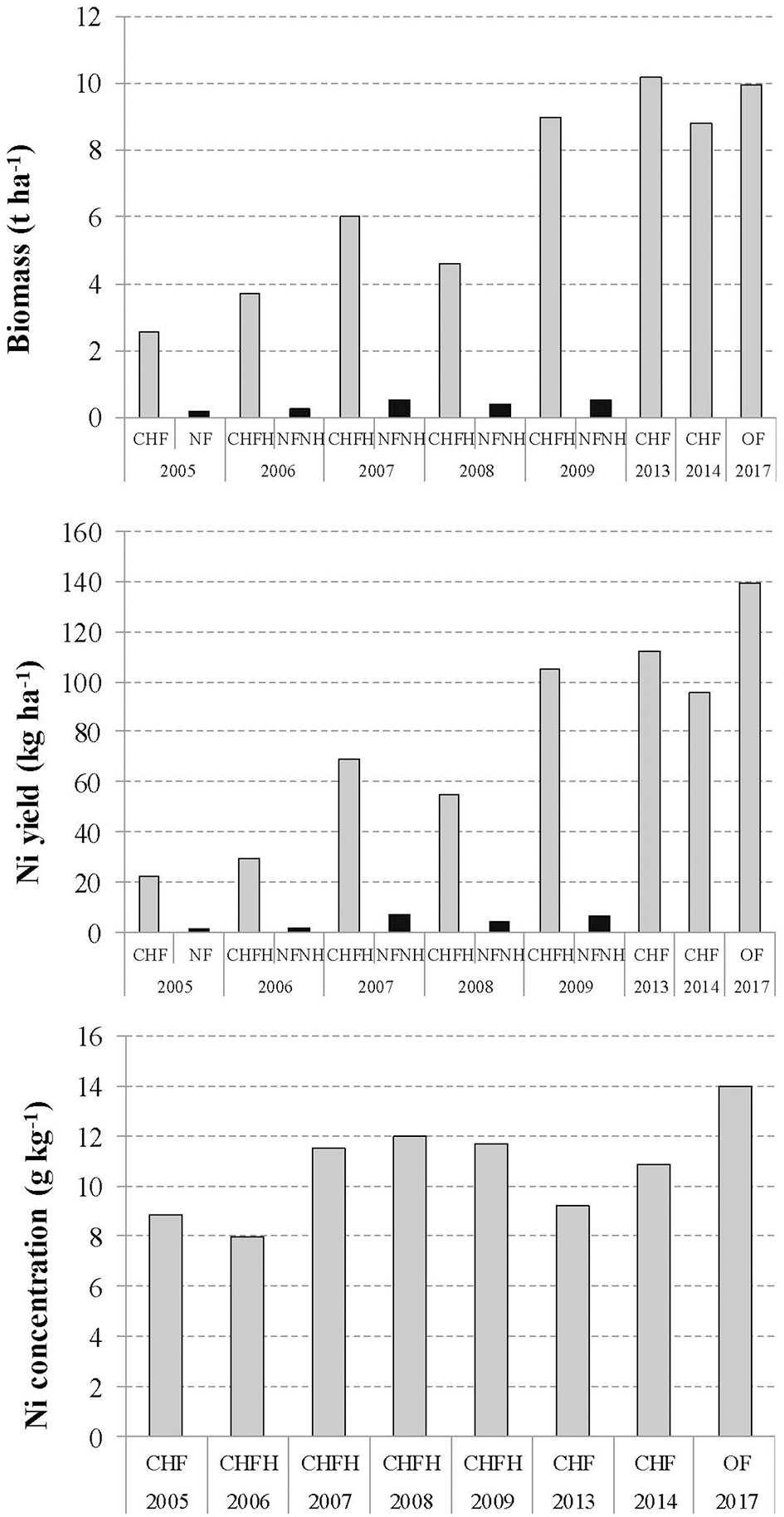
Evolution of biomass production, Ni phytoextracted yield and shoot Ni concentration of Odontarrhena muralis s.l. during 8 years of a field study in Pojska, Pogradec (Albania). CHF-chemical fertilizer, NF-no fertilizer, H-herbicide, OF-organic fertilizer. Modified with permission from Echevarria (2018); Bani and Echevarria (in press) and Bani et al. (2007, 2015a).
New agromining field trials have been established in May 2017 in the Pindus Mountain Range in Greece, which runs along the borders of Thessaly, Epirus, and W Macedonia, and hosts a large number of serpentine outcrops and many serpentinophytes (see section A Synthetic Overview of Ni-Hyperaccumulators in Europe). Experimental plots are located in Koutsoufliani, near the village Panagia at the borders of the above-mentioned regions (NW Greece) on an abandoned field with a mean total Ni concentration of 2,347 mg kg−1 (Table 2 and Figure 3; Echevarria et al., 2017). Odontarrhena muralis s.l. is a constant member of the herbaceous vegetation. The ongoing field assessments are evaluating the Ni agromining capacity of three Ni-hyperaccumulators (O. muralis s.l., B. tymphaea, and B. emarginata) in monoculture plots (three replicate 50 m2 plots/species). Additional plots assess the effects of inorganic fertilization or goat manure addition on the growth and Ni bioaccumulation of O. muralis s.l. and B. emarginata, as well as the benefits of co-cultivating or rotating these hyperaccumulators with the legume Medicago sativa. Harvesting is programmed for June 2018. Results so far indicate maximum plant survival in the organic-amended plots (95–97%) compared to either the inorganic NPK-fertilized plots (15–20%) or co-culture/rotation treatments (3–38%) (Echevarria et al., 2017). The high plant mortality was attributed to elevated temperatures during transplantation and prolonged drought (exacerbated by the slope) during the summer period of 2017 (despite periodic irrigation). The addition of organic amendment appears to have a positive effect on plant resistance to drought and general adverse conditions.
The Austrian field site is located in the province of Burgenland, coinciding with one of the main ultramafic outcrops in this country (Wenzel and Jockwer, 1999). Experimental plots were set up in autumn 2016 on an arable field with a mean total Ni concentration of 1,450 mg kg−1 (Table 2 and Figure 3; Ridard et al., 2017). The first experimental period lasted from October 2016 to September 2017. Odontarrhena muralis s.l., the main agromining crop in the Life-Agromine project, was compared with the indigenous Noccaea goesingensis. The effects of different plant cropping patterns (co-culture with Lotus corniculatus), planting densities or soil amendments (addition of elemental sulfur) on plant yields and Ni bioaccumulation are currently being assessed. The pre-harvest average shoot Ni concentration of O. muralis s.l. was 12,400 mg kg−1 with average shoot DW yields of 3.77 t ha−1. In contrast, N. goesingensis clearly showed a lower accumulation and estimated biomass production (7,900 mg Ni kg−1 and 2.90 t ha−1, respectively; Ridard et al., 2017). The second vegetation period in 2018 will only cultivate O. muralis s.l. due to the temperate climate and shorter growing season in the Bernstein area the vegetation period for O. muralis s.l. was adapted; i.e. planting in spring and harvesting in autumn. Interestingly, flowering was already observed a few weeks before harvesting, i.e., ~5 months after planting. Further treatments (testing different plant densities and fertilization regimes) for optimizing the yield and thus the total content of phytoextracted Ni are planned.
In Spain, the experimental site is located in the Melide ultramafic complex close to the village of Eidián in Galicia (NW Spain; Table 2 and Figure 3), one of the principal periodite outcrops in the Iberian Peninsula together with Andalusia (S Spain) and Trás-os-Montes (NE Portugal). Agricultural crops growing in the ultramafic soils of this region have been shown to accumulate considerable amounts of Ni and Cr (Fernandez et al., 1999). The Ni-hyperaccumulator O. serpyllifolia is a feature of fallow fields at this site. Field trials are evaluating the viability of O. muralis s.l., O. serpyllifolia and B. emarginata for agromining purposes. Monoculture plots (50 m2) were established in 2015 to initially test the two Mediterranean hyperaccumulators, O. muralis s.l. and B. emarginata (Pardo et al., 2018). Soil was fertilized with gypsum (1,000 kg ha−1) and inorganic NPK fertilizers (120:120:150 kg ha−1) and both species planted at a density of 4 plants m−2 following Bani et al. (2015b). The elevated precipitation which is characteristic of this region (European humid-temperate climate) led to waterlogging which, together with competition from weeds (mainly Poaceae), significantly affected plant survival: after one growth season (June 2015–May 2016) plant mortality of up to 50–60% was recorded in some plots. Nonetheless, all surviving plants showed good growth and a healthy aspect. The final plant DW yields (1.0 ± 0.3 and 0.7 ± 0.2 t DW ha−1 for O. muralis s.l. and B. emarginata, respectively) were significantly lower than those obtained by Bani et al. (2015b) for O. muralis s.l. after 8-years of cultivation in Albania but of a similar magnitude to the yields obtained during the first years after implementing agromining at this site (Bani et al., 2007). Odontarrhena muralis s.l. (4.2 kg Ni ha−1) produced a slightly higher Ni yield than B. emarginata (3.0 kg Ni ha−1). As observed by Bani and colleagues, Ni bioaccumulation was maximal at the mid-flowering stage and this was the case for both species, confirming that this is the optimal stage for harvesting, not only for O. muralis s.l., but also for B. emarginata. Moreover, Ni accumulation was strongly compartmentalized with the main contribution to Ni yields coming from the plant leaves, especially the leaves of flowering stems, but the contribution from flowers and fruits was also significant in the case of B. emarginata. Implementing the agromining system increased soil nutrient availability, and modified microbial community structure and metabolic activity (Pardo et al., 2018). Bacterial community structure and diversity are being monitored over time and compared with surrounding soils where agromining was not implemented. The soil bacterial communities are dominated by Proteobacteria, Actinobacteria, Acidobacteria, and Chloroflexi, and after one growth season the agromining crops modified the relative abundance of some phyla (increasing Proteobacteria, Bacteroidetes, and Nitrospirae and reducing Acidobacteria and Planctomycetes) (Pardo et al., 2018).
Implementing a drainage system and weed control during the second year of cultivation (2016–2017) had a dramatic effect on plant survival and biomass production: plant yields were up to 10-fold higher in the case of O. muralis s.l. (reaching close to 10 t ha−1 in some plots) and 7-fold higher for B. emarginata (close to 5 t ha−1) (Kidd et al., 2017b). In 2017 the endemic O. serpyllifolia was also cultivated but showed a significantly lower biomass yield than O. muralis s.l., but a similar magnitude to that of B. emarginata (1.0–5.9 t ha−1) (Kidd et al., 2017b). These preliminary results suggest that O. muralis s.l. has more potential for agromining on this site. However, crop management practices (e.g., plant density) could be optimized so as to improve biomass yields of B. emarginata or O. serpyllifolia, since both species presented similar shoot Ni concentrations to O. muralis s.l. At the same site, ongoing field experiments are evaluating the benefits of co-cropping systems and the application of cow manure instead of NPK fertilizers. After 1 year of cultivation, co-cropping of O. muralis s.l. with the legume Vicia sativa had positive effects on plant growth and Ni removal: hyperaccumulator biomass and Ni yields were increased by 417% (0.82 t ha−1) and 493% (7.93 kg ha−1), respectively, compared to non-fertilized mono-cropping (0.16 t ha−1 and 1.32 kg ha−1) (Figure 5; Saad et al., 2017). However, no significant differences were found between co-cropping and fertilized (NH4NO3) mono-cropping treatments. After a second year of cultivation, yields were doubled in all treatments but plant biomass production and phytoextracted Ni was highest in the co-cropping treatment (2.01 t ha−1 and 13.6 kg ha−1, Saad et al., 2017). On the other hand, parallel experiments showed that after one growth season the addition of cow manure did not significantly modify plant yields of either O. muralis s.l. or B. emarginata compared to NPK fertilization (Kidd et al., 2017b). The Ni yield obtained using either fertilization regime is currently being determined, as well as any differences in soil fertility or microbial community diversity and activity.
Figure 5
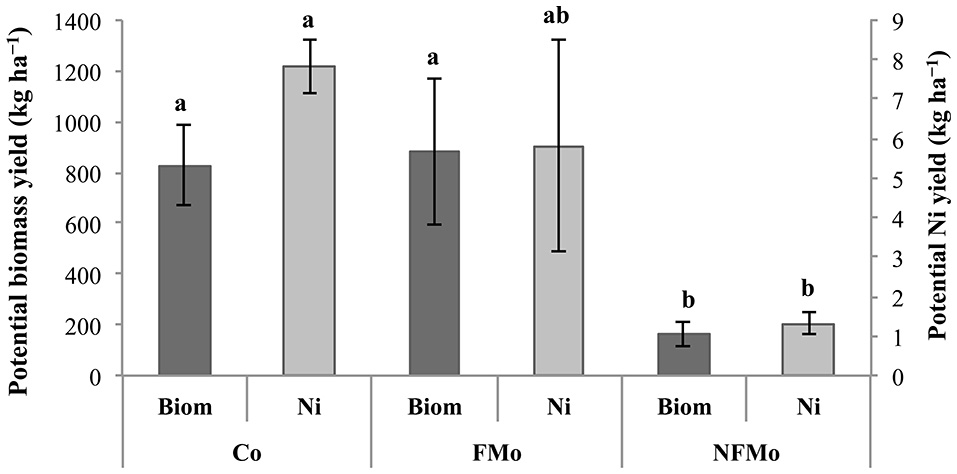
Potential biomass (Biom) and Ni yields (Ni) of Odontarrhena muralis s.l. shoots (kg ha−1). “Co”, “FMo” and “NFMo” correspond to co-cropping with the legume, fertilized mono-cropping, and non-fertilized mono-cropping, respectively. Means ± standard error followed by different letters are significantly different at p < 0.05 (Duncan's multiple range test).
Processing biomass from Ni-hyperaccumulator plants
The whole nickel (Ni) agromining chain consists in two stages: (1) the cultivation of hyperaccumulator plants to obtain sufficient aerial biomass with a high Ni concentration, and (2) the transformation of the biomass to obtain valuable end products. In step 1, the dry plants are burnt to remove organic matter and concentrate Ni by a factor of around 12. The resulting ash is a real bio-ore, containing up to 20 wt % of Ni. In step 2, it has been proven possible to obtain a bunch of Ni compounds (e.g., Ni metal, Ni-based catalysts, Ni salts or oxides) by hydrometallurgical processes (Simonnot et al., 2018). Energy is potentially another end product of agromining. The Agronickel aims to investigate new routes for optimizing the valorization of the hyperaccumulator biomass in terms of energy and metal recovery. The Life-Agromine project aims to up-scale the process, to produce nickel compounds and energy from hyperaccumulator biomass, especially O. muralis s.l. at demonstration levels.
Nickel recovery and reactor design
The up-scaled process is the patented synthesis of ANSH (ammonium nickel sulfate hexahydrate) from the biomass of O. muralis s.l. (Barbaroux et al., 2012). The process consists of (1) washing the ashes with water to remove soluble K (around 80% of the total K content), (2) transferring Ni into the aqueous phase by sulfuric acid leaching and (3) precipitating and purifying ANSH (Figure 6). Each step has been thoroughly investigated to assess the influence of the process parameters (e.g., stirring speed or reaction time) on process efficacy and salt purity (Zhang et al., 2016; Houzelot et al., 2017a,b). However, up-scaling also requires technical adjustments and process intensification. The process has been designed through batch reactor experiments, but its implementation at a larger scale requires a different strategy. The main options are (1) moving from discontinuous batchwise to continuous process or (2) increasing the treatment capacity by using reactors in parallel. This second option is considered here and applied to ash washing and ash leaching.
Figure 6

Process description for the preparation of ANSH from Odontarrhena muralis s.l. at the pilot scale.
The investigation of the batchwise process has shown that:
The efficacy of ash washing only depends on the solid/liquid (S/L) weight ratio and ash wettability. S/L ratio must not overcome 20% to allow stirring, above 20% suspension viscosity is too high.
During the leaching step (2 M sulfuric acid), a part of the protons is involved in ash neutralization (initial pH around 13) and another part in Ni leaching. Around 30% of initial H+ have not reacted (final pH around 0.6), but they are essential to increase nickel extraction yield. The leachate has to be neutralized before ANSH precipitation, which leads to the consumption of alkali.
To increase the treatment capacity while saving chemical reagents (and limiting proton loss during leaching), it is proposed to develop a discontinuous process using reactors in parallel.
Theoretical aspects for the design of parallel batch reactors
The proposed configuration is composed of J units in parallel (Figure 7). Unit #i (1 ≤ i ≤ J) is made up of two batch reactors in series, the first one (Wi) for ash washing and the second one (Li) for ash leaching. A mass m of ash and a volume Vw of water are introduced into reactor W1; after a washing time of ca 15 min, ash (without K) is transferred into reactor L1, a part of the solution (Vw') is introduced into W2 and the remaining part (Vw”) is removed. To simplify, we assume that the amount of water transferred from W1 to L1 with the ash is negligible (ash is dry). Leaching is run in reactor L1 by adding a volume VL of sulphuric acid at the concentration C1. At the end of the leaching (2 h at 90 °C), we obtain the ash (Ash1, without K and Ni) and a volume VL” of leachate rich in Ni. Reactor W2 is fed by the same mass m of ash, the volume Vw' of solution exiting from reactor W1, and the volume Vw” of water and so on. The same method is applied to the leaching reactors, the concentration of inlet acid being adjusted. The proposed configuration enables us to increase the concentration of K from reactor W1 to W2 and so on, and the concentration of Ni from L1 to L2 and so on, until reaching a limit value. In this way, the concentrations of K and Ni increase without changing the S/L ratio. The main advantage of this configuration is to lead to identical solutions exiting the reactors when the limit value is reached. At the exit of the complete system, we obtain a volume (Vw'+J Vw”) of solution rich in K and a volume (VL'+J VL”) of leachate rich in Ni. The solution rich in K is an effluent, which could be used for other purposes, and the leachate rich in Ni is treated to obtain the ANSH salt. The problem to be solved is to determine how many units are needed to reach the limit value of the concentration.
Figure 7
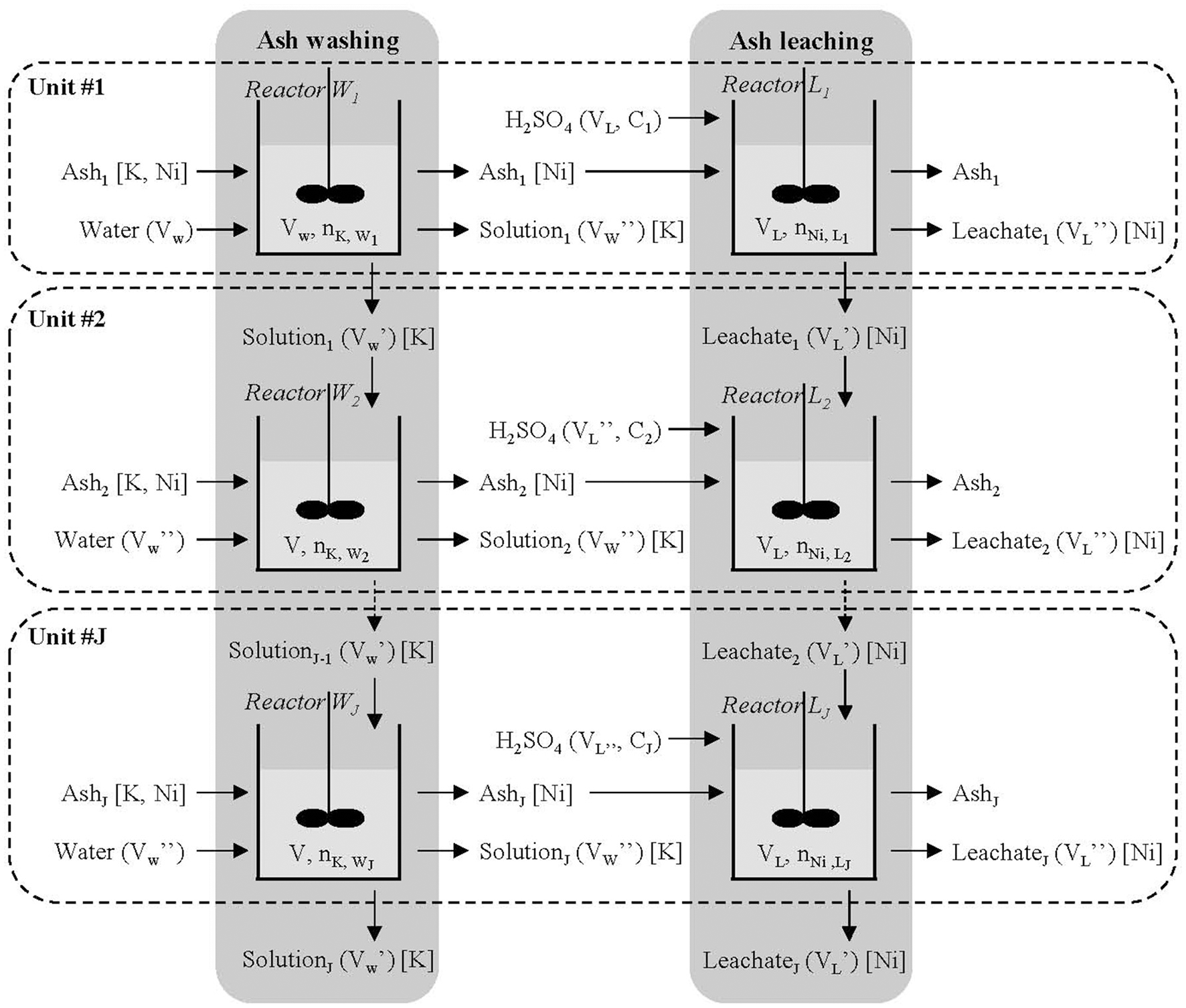
Parallel batch configuration for Odontarrhena muralis s.l. ash washing and leaching. Liquid properties are indicated in brackets and targeted elements in square brackets.
Application to ANSH preparation
Previous experiments have shown that K and Ni cannot be concentrated more than twice compared to the concentrations obtained after ash washing and leaching. Otherwise they would precipitate in the ashes, mainly in the sulfate form.
In the proposed configuration, choosing Vw' = Vw” = 0.5 Vw and VL' = VL” = 0.5 VL, mass balances show that at least 6 units in parallel are needed to reach the limit value. If we compare this with the case where Vw' = VL' = 0, half of the water and 30% of the sulphuric acid are saved. As a consequence, the amount of alkaline solution required for leachate neutralization decreases as well.
To conclude, this section has shown how ash washing and leaching can be up-scaled to intensify the process, reach a high efficacy and save chemicals.
Energy recovery
The calorific values of hyperaccumulators from the Brassicaceae family have been monitored to assess the interest in energy recovery during agromining. HHVs (higher heating values) obtained using a calorimetric bomb with dried ground biomass of O. muralis s.l. and B. emarginata were 16.7 and 17.4 MJ kg−1, respectively (Hazotte et al., 2017). These values are comparable with HHV of wood pellets (20 MJ kg−1; Rollinson and Williams, 2016).
Pellet manufacturing may improve combustion efficiency and facilitate plant handling and storage. However, this will be a challenge when using biomass from this hyperaccumulator family. First tests have shown that, unlike wood, the low content of lignin leads to a low pellet stability. The addition of a binder may limit this effect, but inevitably, it produces Ni dilution in ashes, as well as an increase in operating costs.
Biomass combustion upscaling requires mandatory gas control, especially for SOx and NOx emissions. However, it is also necessary to take care of Ni emissions, especially in fly ash. In France, this element is grouped with Sb, Cr, Co, Cu, Sn, Mn, V, and Zn, and the total flow rate must not exceed 25 g h−1, with <5 mg m−3. A 20 kWh boiler has been set up, with a combustion capacity of 7 kg biomass per hour (Figure 6). Its functioning is being validated and emissions analyses will be performed under standard guidelines NF EN 14385. Other parameters which should be taken into account during agromining development include the biomass chloride content, which might be a criterium for choosing hyperaccumulators. Hydrochloric acid formation during combustion may significantly cause corrosion of boilers. For example, O. muralis s.l. dried biomass contains around 0.25% Cl, which is more than wood (0.01–0.03%) but less than straw and grass (0.4–0.8%). This value is close to that of Miscanthus (Obernberger et al., 2006).
Economic and environmental aspects of agromining
Economics of agromining
Until today, research on the economics of the agromining chain focuses on costs and benefits from a business perspective. Most of the literature considers Ni as the extraction target, although other elements such as Ge, Re and Au have also attracted attention (Harris et al., 2009; Novo et al., 2015; Rentsch et al., 2016). Aspects being discussed can be grouped as follows: (i) characteristics of the metal market; (ii) process flow diagrams; (iii) inventory of costs, and revenues; and (iv) sensitivity analyses. These topics correspond to the elements of a techno-economic analysis as proposed by Van Dael et al. (2015), and will be discussed below.
Since 1989 Ni prices have fluctuated between 2 and 13 USD lb−1 (4 and 23 EUR kg−1) with a sharp peak of 23 USD lb−1 (40 EUR kg−1) right before the start of the financial crisis in the summer of 2007 (InfoMine, 2018). According to Li et al. (2003b), Ni cannot be agromined economically at the lower Ni prices. Therefore, the production of Ni compounds with much higher value, such as ANSH could be a better alternative. Current prices of ANSH depend on the purity and the amount: 97.50 EUR for 500 g with 98% purity, and 134 EUR for 25 g with 99.999% purity (Sigma-Aldrich, 2018).
Ideally the released energy during biomass incineration should be valorized; however, this depends on the vicinity of energy demand to the location of the processing facility. Hence, small-scale mobile units should be compared to large-scale central processing. The former requires measures for quality control of the produced bio-ores when it is mixed with another feedstock by local smallholders, whereas the latter needs carefully optimized storage facilities. As an alternative to the pyrometallurgical thermo-chemical treatment for the production of Ni, the hyperaccumulators can be treated hydrometallurgically (as discussed above) for the production of the higher value Ni compounds.
So far, costs have not been reported in detail. Harris et al. (2009) mention the cost of producing the Ni-hyperaccumulator Berkheya codii, but not for O. muralis s.l. Bani et al. (2015a) estimated land rental and production costs for agromining with O. muralis s.l. to be 150 USD ha−1 yr−1 and 390 USD ha−1 yr−1, respectively. Nkrumah et al. (2016) compare intensive agromining systems with extensive systems and assume its production costs in 2016 to be 1074 USD ha−1 yr−1 and 600 USD ha−1 yr−1, respectively. The latter is comparable to the cost of 500 USD ha−1 yr−1 mentioned by van der Ent et al. (2013b).
Moreover, both Bani et al. (2015a) and Nkrumah et al. (2016) expect 20 % of the Ni value to cover the cost of the metal recovery process, which would result in a profit of ca. 990 USD ha−1 yr−1. Although the production costs in van der Ent et al. (2013b) are comparable, they expect higher net economic gains of 1900 USD ha−1 yr−1. This difference can be explained by higher expected Ni uptake: 150 kg ha−1 yr−1 in Indonesia compared to 105 kg ha−1 yr−1 in Albania. Li et al. (2003b) mention that 25% of the Ni value would cover the cost of metal recovery, and license and royalty fees, resulting in a net profit of ca. 1800 USD ha−1 yr−1. In fact, the estimate of Li et al. (2003b) corresponds to a Ni recovery cost of 1.75 USD kg−1, whereas the one of Bani et al. (2015a) corresponds to 3.4 USD kg−1. This indicates that the cost of the processing part of the process flow still needs thorough investigation as one would actually expect the cost to decrease over time due to the learning effect.
To conclude, a techno-economic model for the agromining chain should consist of two stages: (i) cultivation and (ii) conversion. To determine the cultivation costs, one should include land rental, land preparation, seeds, fertilizers, chemicals, irrigation, etc. The Ni metal or Ni compound extraction cost is determined by the cost of the subsequent pyro- or hydro-metallurgical process. To date, no literature exists containing detailed costing of the conversion stage; nevertheless, supporting information can be found in Kuppens (2012) for the incineration plant, in Crundwell et al. (2011) for Ni smelting, and in Rodrigues et al. (2016) and Zhang et al. (2016) for the Ni compounds. Finally, the techno-economic model should be customized to country-specific data reflecting differences in Ni concentrations in the soils, hyperaccumulator yields and prices. Preliminary calculations for the pyrometallurgical process for Ni agromining show promising results under the condition that heat released during incineration can be valorized close to the processing facility.
Environmental assessment
Although agromining is focused on the use of plants to recover valuable metals out of natural or man-polluted soils, it remains an anthropogenic process, at the intersection between agriculture and soil remediation: it needs to be assessed in terms of its sustainability and global environmental impact. Life Cycle Assessment (LCA) is the most recognized method for this type of assessment. LCA is a systematic tool which obeys the rules specified by International Standards (ISO, 2006a,b) and has seen many applications in the field of agriculture (for food crops Keyes et al., 2015; Ingrao et al., 2018; etc. and energy crops Arodudu et al., 2017; Hoekman and Broch, 2018; etc.) or remediation of contaminated soil (Suer and Andersson-Sköld, 2011; Lemming et al., 2012). This type of analyses was used to assess the combination of phytoremediation of contaminated soils with energy production by Witters et al. (2012) and Kuppens et al. (2015) for willow, rapeseed and maize, and by Nsanganwimana et al. (2014) for Miscanthus x giganteus. Vigil et al. (2015) confirmed that biomass valorization was necessary to make this soil remediation technique sustainable. In the case of Ni-agromining, studies applying LCA to assess the environmental impact of this process are rare. To the best of our knowledge, only one study (Rodrigues et al., 2016) can be found in the literature. Rodrigues et al. (2016) emphasized that energy from O. muralis s.l. ashing should be recovered to make the process really environmentally beneficial.
The basic impacts which are generally considered in LCA are related to natural resources (depletion of water, abiotic and renewable resources for irrigation, fertilizers production, energy, etc.), natural environment (land use, climate change, eutrophication, acidification, ecotoxicity, etc.) and human health. In the case of soil remediation it has been proposed to combine LCA with a specific local health assessment for decontamination operators to guide the selection of sustainable remediation processes (Hou et al., 2017). According to Rodrigues et al. (2016), cultivation techniques in agromining should be selected to limit soil erosion, either due to rainfall or wind, as erosion favors the transport and dispersion of metal-laden particles in the environment.
In spite of LCA normalization, there are still questions arising regarding the way to deal with land use (LU) and its change (LUC). This is a critical issue in the study of any system in which an agricultural process is developed. Land surface and functions should be taken into account, but it is difficult to reach a consensus on the descriptors that should be used: there is a growing awareness of the need to improve the cause-effect chain models related to ecosystem services provision in life cycle inventories (Othoniel et al., 2016). Morais and Delerue-Matos (2010) pointed out the difficulty of spatial and temporal differentiation of local impacts assessment, especially when taking into account the prediction of long-term toxicity effects.
Generally speaking any land use is seen as negative (Koellner et al., 2013). However in the case of soil remediation the problem is more complex as the ultimate goal is to improve the soil properties and functions. In the case of agromining of ultramafic soils the initial land state is natural. The contamination stage is outwith the scope of the agromining process if this is carried out on soil polluted due to human activity.
Soil carbon sequestration is one of the descriptors used to assess land use change: the development of a clear soil carbon accounting method is still under discussion for agricultural processes (Goglio et al., 2015). In particular the timing of soil CO2 emissions during long-term agromining should be taken into account, together with agricultural management practices (fertilization, tillage, crop rotation, etc.; Levasseur et al., 2010). Experiments are undertaken to monitor C status in agromining experimental fields at the four locations as well as greenhouse gas emissions at two of the four sites (AT and ES). The data obtained from these experiments will help a better estimation of these issues in the LCA. Another descriptor largely used to assess land use is biodiversity. Gabel et al. (2016) reviewed twenty-two different biodiversity impact assessment methods susceptible to be used in LCA of agricultural processes. Most of them were not initially developed for these processes and questions still arise as to which biodiversity aspects should be considered, which indicators should be used and how references should be selected. A conceptual model of effect of land intervention (occupation, transformation) has been proposed by Curran et al. (2016): the effect on ecosystem quality is assessed through indicators of biodiversity damage potential, at the local and regional scales. In agromining the introduction of non-endemic species will be detrimental to local biodiversity: local plants have developed at least tolerance to metals, if not metal accumulation properties.
Since most metal hyperaccumulators discussed so far are angiosperms, another ecosystem function is also relevant: pollination. Although necessary for maintaining endemic species, pollen transport may favor dissemination of non-endemic species. Furthermore high-metal contents in flowers could affect the behavior of honey bees (Di et al., 2016; Nikolić et al., 2016), with potential incorporation of metals in honey and propolis (Matin et al., 2016). Meindl and Ashman (2017) discussed the effect of soil chemistry on pollen germination in a Ni-hyperaccumulator, Streptanthus polygaloides. However, O. muralis s.l. has always been a widespread weed (“Pulë verdhë” in Albanian) in agricultural ultramafic areas of the Pogradec district: wild and domesticated honey bees have dealt with it for at least several millennia since Antiquity. Opening the landscape by clearing the native woodland vegetation has probably provoked a boost in the populations of O. muralis s.l., which is not very common in the native maquis (Bani et al., 2014). More knowledge about the potential incorporation of agromined metals in the human food chain is desirable (Herrero-Latorre et al., 2017). However, there might be a positive effect on the pollination service since O. muralis s.l. is a strong nectar producer and stimulates pollinating insects), and also because there is absolutely no need to use insecticides with this crop (insecticides may have a negative impact on pollinating insects populations).
The application of LCA to agromining processes is not yet fully developed. The methodology already applied to agroprocesses as well as to soil remediation serve as starting points, but certain aspects related to land use and land use changes in a context of using local/non-local species in their native/non-native habitat will require specific adjustments and research efforts.
Final remarks
Agromining offers new possibilities for the recovery of strategic metals from natural resources. This review focused on natural metalliferous soils, but agromining can also be applied to secondary resources derived from industrial activities. The exceptional capacity of hyperaccumulator plants to bioaccumulate metals in their harvestable tissues at commercial ore grade levels makes these techniques viable. The EU initiatives summarized here demonstrate at field scale the potential for Ni agromining on ultramafic soils across a range in climatic and edaphic conditions. Nickel agromining in these areas represent a new form of agriculture which can generate income from low-productivity agricultural land derived from ultramafic bedrock. Agromining efficiency can be optimized using appropriate agronomic practices, with positive effects on soil health and quality, as well as the generation of wider services (e.g., biodiversity conservation, C sequestration, etc.).
Statements
Author contributions
PK, ÁP-F, BR-G, RS, and EB carried out the experimental work in the agromining field site in Spain. AB, J-LM, and GE have been involved in the development of agromining in the Albanian field site. CR, TR, and MP carried out experimental work in the Austrian field site, and MK and DK established the agromining experimental plots in Greece. PK coordinated the overall preparation of the manuscript, and all authors contributed to the writing of the manuscript. GE coordinates the LIFE-Agromine and Agronickel projects.
Acknowledgments
The authors thank the financial support received by the LIFE Environment and Resource Efficiency Programme (Life Agromine; LIFE15 ENV/FR/000512), ERA-NET FACCE Surplus (Agronickel; ID71), Agence Nationale pour la Recherche (ANR15-SUSF-0003-RA), Spanish Ministerio de Economía, Industria y Competitividad (PCIN-2017-028) and Polish National Centre for Research and Development (FACCE SURPLUS/I/AGRONICKEL/02/2016).
Conflict of interest
Authors AT and JK were employed by the company alchemia-nova GmbH. This company is also doing research in the area of phytomining. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Abou-ShanabR. I.DelormeT. A.AngleJ. S.ChaneyR. L.GhanemK.MoawadH.et al. (2003). Phenotypic characterization of microbes in the rhizosphere of Alyssum murale. Int. J. Phytoremediat. 5, 367–379. 10.1080/15226510309359043
2
Al-ShehbazI. A. (2014). A synopsis of the genus Noccaea (Coluteocarpeae, Brassicaceae). Harvard P. Bot. 19, 25–51. 10.3100/hpib.v19iss1.2014.n3
3
Álvarez-LópezV.Prieto-FernándezÁ.Becerra-CastroC.MonterrosoC.KiddP. S. (2016). Rhizobacterial communities associated with the flora of three serpentine outcrops of the Iberian Peninsula. Plant Soil403, 233–252. 10.1007/s11104-015-2632-0
4
Álvarez-LópezV.Prieto-FernándezÁ.Cabello-ConejoM. I.KiddP. (2016). Organic amendments for improving biomass production and metal yield of Ni-hyperaccumulating plants. Sci. Total Environ. 548–549, 370–379. 10.1016/j.scitotenv.2015.12.147
5
AroduduO.HelmingK.WiggeringH.VoinovA. (2017). Towards a more holistic sustainability assessment framework for agro-bioenergy systems -A review. EIA Review62, 61–75. 10.1016/j.eiar.2016.07.008
6
BaniA.EchevarriaG. (in press). How to improve agromining cropping systems by fertilizing with organic amendments in Albanian ultramafic landscapes?Int. J. Phytoremediation. [Epub ahead of print].
7
BaniA.EchevarriaG.MullajA.ReevesR. D.MorelJ. L.SulçeS. (2009). Ni hyperaccumulation by Brassicaceae in serpentine soils of Albania and NW Greece. Northeast Nat. 16, 385–404. 10.1656/045.016.0528
8
BaniA.EchevarriaG.Montargès-PelletierE.GjokaF.SulçeS.MorelJ. L. (2014). Pedogenesis and nickel biogeochemistry in a typical Albanian ultramafic toposequence. Environ. Monitor. Assess. 186, 4431–4444. 10.1007/s10661-014-3709-6
9
BaniA.EchevarriaG.SulçeS.MorelJ. L. (2015a). Improving the agronomy of Alyssum murale for extensive phytomining: a five-year field study. Int. J. Phytoremediat. 17, 117–127. 10.1080/15226514.2013.862204
10
BaniA.EchevarriaG.SulceS.MorelJ. L.MullaiA. (2007). In-situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site (Albania). Plant Soil293, 79–89. 10.1007/s11104-007-9245-1
11
BaniA.EchevarriaG.ZhangX.BenizriE.LaubieB.MorelJ. L.et al. (2015b). The effect of plant density in nickel phytomining field experiments with Alyssum murale in Albania. Aust. J. Bot. 63, 72–77. 10.1071/BT14285
12
BaniA.ImeriA.EchevarriaG.PavlovaD.ReevesR. R.MorelJ. L.et al. (2013). Nickel hyperaccumulation in the serpentine flora of Albania. Fresenius Environ. Bull. 22, 1792–1801.
13
BaniA.PavlovaD.EchevarriaG.MullajA.ReevesR. D.MorelJ. L.et al. (2010). Nickel hyperaccumulation by species of Alyssum and Thlaspi (Brassicaceae) from the ultramafics of Balkans. Bot. Serb.34, 3–14.
14
BarbarouxR.PlasariE.MercierG.SimonnotM.-O.MorelJ. L.BlaisJ.-F. (2012). A new process for nickel ammonium disulfate production from ash of the hyperaccumulating plant Alyssum murale. Sci. Total Environ. 423, 111–119. 10.1016/j.scitotenv.2012.01.063
15
Becerra-CastroC.KiddP.KuffnerM.Prieto-FernándezÁ.HannS.MonterrosoC.et al. (2013). Bacterially induced weathering of ultramafic rock and its implications for phytoextraction. Appl. Environ. Microbiol. 79, 5094–5103. 10.1128/AEM.00402-13
16
BenizriE.AmiaudB. (2005). Relationship between plants and soil microbial communities in fertilized grasslands. Soil Biol. Biochem. 37, 2055–2064. 10.1016/j.soilbio.2005.03.008
17
BenizriE.KiddP. S. (2018). “The role of the rhizosphere and microbes associated with hyperaccumulator plants in metal accumulation,” in Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants, eds Van der EntA.EchevarriaG.BakerA. J. M.MorelJ. L. (Cham: Springer International Publishing), 157–188.
18
BergG.SmallaK. (2009). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68, 1–13. 10.1111/j.1574-6941.2009.00654.x
19
BergmeierE.KonstantinouM.TsiripidisI.SykoraK. (2009). Plant communities on metalliferous soils in northern Greece. Phytocoenologia39, 411–438. 10.1127/0340-269X/2009/0039-0411
20
BortolottiV.ChiariM.MarroniM.PandolfiL.PrincipiG.SaccaniL. (2013). Geodynamic evolution of ophiolites from Albania and Greece (Dinaric-Hellenic belt): one, two, or more oceanic basins?Int. J. Earth Sci. 102, 783–811. 10.1007/s00531-012-0835-7
21
BrooksR. R. (1987). Serpentine and its Vegetation: a Multidisciplinary Approach. London; Sydney, NSW: Dioscorides Press; Croom Helm.
22
BrussaardL. (2012). “Ecosystem services provided by the soil biota,” in Soil Ecology and Ecosystem Services, eds WallD. H.BardgettR. D.Behan-PelletierV.HerrickJ. E.JonesT. H.RitzK.SixJ.StrongD. R.van der PuttenW.H. (Oxford, UK: Oxford University Press), 45–58.
23
Cabello-ConejoM. I.Becerra-CastroC.Prieto-FernándezÁ.MonterrosoC.Saavedra-FerroA.MenchM.et al. (2014). Rhizobacterial inoculants can improve nickel phytoextraction by the hyperaccumulator Alyssum pintodasilvae. Plant Soil379, 35–50. 10.1007/s11104-014-2043-7
24
CaoL.JiangM.ZengZ.DuA.TanH.LiuY. (2008). Trichoderma atroviride F6 improves phytoextraction efficiency of mustard (Brassica juncea (L.) Coss. var. foliosa Bailey) in Cd, Ni contaminated soils. Chemosphere71, 1769–1773. 10.1016/j.chemosphere.2008.01.066
25
CardinaleM. (2014). Scanning a microhabitat: plant-microbe interactions revealed by confocal laser microscopy. Front. Microbiol.5:94. 10.3389/fmicb.2014.00094
26
CecchiL.BettariniI.ColziI.CoppiA.EchevarriaG.PazzagliL.et al. (2018). The genus Odontarrhena (Brassicaceae) in Albania: Taxonomy and Nickel accumulation in a critical group of metallophytes from a major serpentine hot-spot. Phytotaxa. 351, 1–28. 10.11646/hytotaxa.351.1.1.
27
CecchiL.ColziI.CoppiA.GonnelliC.SelviF. (2013). Diversity and biogeography of Ni-hyperaccumulators of Alyssum section Odontarrhena (Brassicaceae) in the central western Mediterranean: evidence from karyology, morphology and DNA sequence data. Bot. J. Linn. Soc.173, 269–289. 10.1111/boj.12084
28
CecchiL.GabbrielliR.ArnetoliM.GonnelliC.HaskoA.SelviF. (2010). Evolutionary lineages of nickel hyperaccumulation and systematics in European Alysseae (Brassicaceae): evidence from nrDNA sequence data. Ann. Bot.106, 751–767. 10.1093/aob/mcq162
29
ChaneyR. L.AngleJ. S.BroadhurstC. L.PetersC. A.TapperoR. V.SparksD. L. (2007). Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J. Environ. Qual. 36, 1429–1443. 10.2134/jeq2006.0514
30
ChaneyR. L.BakerA. J. M.MorelJ. L. (2018). “The long road to developing agromining/phytomining,” in Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants, eds Van der EntA.EchevarriaG.BakerA. J. M.BakerJ. L. (Cham: Springer International Publishing), 1–17.
31
ChaneyR. L.ChenK. Y.LiY. M.AngleJ. S.BakerA. J. M. (2008). Effects of calcium on nickel tolerance and accumulation in Alyssum species and cabbage grown in nutrient solution. Plant Soil311, 131–140. 10.1007/s11104-008-9664-7
32
ChardotV.MassouraS. T.EchevarriaG.ReevesR. D.MorelJ. L. (2005). Phytoextraction potential of the nickel hyperaccumulators Leptoplax emarginata and Bornmuellera tymphaea. Int. J. Phytoremediat. 7, 323–335. 10.1080/16226510500327186
33
CrundwellF. K.MoatsM. S.RamachandranV.RobinsonT. G.DavenportW. G. (2011). Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals. Oxford; Amsterdam: Elsevier.
34
CurranM.de SouzaD. M.AntónA.TeixeiraR. F.MichelsenT.Vidal-LegazB.et al. (2016). How well does LCA model land use impacts on biodiversity? A comparison with approaches from ecology and conservation. Environ. Sci. Technol. 50, 2782–2795. 10.1021/acs.est.5b04681
35
De Antoni MiglioratiM.BellM.GraceP. R.ScheerC.RowlingsD. W.LiuS. (2015). Legume pastures can reduce N2O emissions intensity in subtropical cereal cropping systems?Agric. Ecosyst. Environ. 204, 27–39. 10.1016/j.agee.2015.02.007
36
DiN.HladunK. R.ZhangK.LiuT. X.TrumbleJ. T. (2016). Laboratory bioassays on the impact of cadmium, copper and lead on the development and survival of honeybee (Apis mellifera L.) larvae and foragers. Chemosphere152, 530–538. 10.1016/j.chemosphere.2016.03.033
37
DurandA.PiuttiS.RueM.MorelJ. L.EchevarriaG.BenizriE. (2016). Improving nickel phytoextraction by co-cropping hyperaccumulator plants inoculated by plant growth promoting rhizobacteria. Plant Soil399, 179–192. 10.1007/s11104-015-2691-2
38
EchevarriaG. (2018). “Genesis and behaviour of ultramafic soils and consequences for nickel biogeochemistry,” in Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants, eds Van der EntA.EchevarriaG.BakerA. J. M.MorelJ. L. (Cham: Springer International Publishing), 135–156.
39
EchevarriaG.BakerA. J. M.BenizriE.MorelJ. L.van der EntA.HouzelotV.et al. (2015). “Agromining for nickel: a complete chain that optimizes ecosystem services rendered by ultramafic landscapes,” in Mineral Resources in a Sustainable World, Vol 1–5, eds Andre-MayerA. S.CathelineauM.MuchezP. (Nancy), 1465–1467.
40
EchevarriaG.BaniA.BenizriE.KiddP. S.KisserJ.KonstantinouM.et al. (2017). “LIFE AGROMINE: a European demonstration project for Ni agromining,” in Proceedings of the 14th International Phytotechnologies Conference, Session 4E - Part 1 - Bioremediation and bioeconomy (Montreal, QC), 25–29.
41
EstradeN.CloquetC.EchevarriaG.SterckemanT.DengT. H. B.TangY. T.et al. (2015). Weathering and vegetation controls on nickel isotope fractionation in surface ultramafic environments (Albania). Earth Planet Sci. Lett. 423, 24–25. 10.1016/j.epsl.2015.04.018
42
EvansJ.ScottG.LemerleD.KaiserA.OrchardB.MurrayG. M.et al. (2003). Impact of legume ≪ break ≫ crops on the yield and grain quality of wheat and relationship with soil mineral N and crop N content. Aust. J. Agric. Res. 54, 777–788. 10.1071/AR02224
43
FernandezS.SeoaneS.MerinoA. (1999). Plant heavy metal concentrations and soil biological properties in agricultural serpentine soils. Commun. Soil Sci. Plant Anal.30, 1867–1884.
44
FukumotoL. R.MazzaG. (2000). Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 48, 3597–3604. 10.1021/jf000220w
45
GabelV. M.MeierM. S.KöpkeU.StolzeM. (2016). The challenges of including impacts on biodiversity in agricultural life cycle assessments. J. Environ. Manage. 181, 249–260. 10.1016/j.jenvman.2016.06.030
46
GaddG. M. (2010). Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology156, 609–643. 10.1099/mic.0.037143-0
47
GaoY.ZhouP.MaoL.ZhiY.ZhangC.ShiW. (2010). Effects of plant species coexistence on soil enzyme activities and soil microbial community structure under Cd and Pb combined pollution. J. Environ. Sci. 22, 1040–1048. 10.1016/S1001-0742(09)60215-1
48
GarcíaE.AlonsoÁ.PlatasG.SacristánS. (2013). The endophytic mycobiota of Arabidopsis thaliana. Fungal Divers. 60, 71–89. 10.1007/s13225-012-0219-0
49
GhasemiZ.GhaderianS. M.Rodríguez-GarridoB.KiddP. S. (2018). Plant species-specificity and effects of bioinoculants and fertilization on plant performance for nickel phytomining. Plant Soil.10.1007/s11104-017-3553-x
50
GlickB. R. (2010). Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 28, 367–374. 10.1016/j.biotechadv.2010.02.001
51
GoglioP.SmithW. N.GrantB. B.DesjardinsR. L.McConkeyB. G.CampbellC. A.et al. (2015). Accounting for soil carbon changes in agricultural life cycle assessment (LCA): a review. J. Clean. Prod. 104, 23–39. 10.1016/j.jclepro.2015.05.040
52
GoveB.HutchinsonJ. J.YoungS. D.CraigonJ.McGrathS. P. (2002). Uptake of metals by plants sharing a rhizosphere with the hyperaccumulator Thlaspi caerulescens. Int. J. Phytoremediat4, 267–281. 10.1080/15226510208500087
53
HarrisA. T.NaidooK.NokesJ.WalkerT. O. (2009). Indicative assessment of the feasibility of Ni and Au phytomining in Australia. J. Clean. Prod. 17, 194–200. 10.1016/j.jclepro.2008.04.011
54
HartvigP. (2002). “Ptilotrichum; Leptoplax; Bornmuellera,” in Flora Hellenica 2, eds StridA.TanK. (Ruggell: Gantner Verlag), 230–234.
55
HazotteC.LaubieB.RamezS.RueM.BenizriE.EchevarriaG.et al. (2017). “Caractérisation de plantes hyperaccumulatrices pour la production d'énergie et de sels de nickel,” in SFGP 2017 – 16ème Congrès de la Société Française de Génie des Procédés (Nancy), 11–13.
56
Herrero-LatorreC.Barciela-GarciaJ.Garcia-MartinS.Pena-CrecenteR. M. (2017). The use of honeybees and honey as environmental bioindicators for metals and radionuclides: a review. Environ. Rev. 25, 463–480. 10.1139/er-2017-0029
57
HoekmanS. K.BrochA. (2018). Environmental implications of higher ethanol production and use in the U.S.: a literature review. Part II – Biodiversity, land use change, GHG emissions, and sustainability. Renew. Sust. Energ. Rev. 81, 3159–3177. 10.1016/j.rser.2017.05.052
58
HouD.QuS.RigbyM.O'ConnorD. (2017). Incorporating life cycle assessment with health risk assessment to select the 'greenest' cleanup level for Pb contaminated soil. J. Clean. Prod. 162, 1157–1168. 10.1016/j.jclepro.2017.06.135
59
HouzelotV.LaubieB.PontvianneS.SimonnotM.-O. (2017a). Effect of up-scaling on the quality of ashes obtained from hyperaccumulator biomass to recover Ni by agromining. Chem. Eng. Res. Des. 120, 26–33. 10.1016/j.cherd.2017.02.002
60
HouzelotV.RancB.LaubieB.SimonnotM.-O. (2017b). Agromining of hyperaccumulator biomass: study of leaching kinetics of extraction of nickel, magnesium, potassium, phosphorus, iron, and manganese from Alyssum murale ashes by sulfuric acid. Chem. Eng. Res. Des. 129, 1–11. 10.1016/j.cherd.2017.10.030
61
ICMM (2012). The Role of Mining in National Economies. Available online at http://www.icmm.com
62
InfoMine (2018). Historical Nickel Prices and Price Chart. Available online at: http://www.infomine.com/investment/metal-prices/nickel/all/(January 26, 2018).
63
IngraoC.LicciardelloF.PecorinoB.MuratoreG.ZerboA.MessineoA. (2018). Energy and environmental assessment of a traditional durum-wheat bread. J. Clean. Prod. 171, 1494–1509. 10.1016/j.jclepro.2017.09.283
64
ISO (International Organization for Standardization) (2006a). 14040 - Environmental Management - Life Cycle Assessment - Principles and Framework.
65
ISO (International Organization for Standardization) (2006b). 14044 - Environmental Management - Life Cycle Assessment - Requirements and Guidelines.
66
JiangC.WuQ. T.SterckemanT.SchwartzC.SirgueyC.OuvrardS.PerrigueyJ.et al. (2010). Co-planting can phytoextract similar amounts of cadmium and zinc to mono-cropping from contaminated soils. Ecol. Eng. 36, 391–395. 10.1016/j.ecoleng.2009.11.005
67
KeyesS.TyedmersP.BeazleyK. (2015). Evaluating the environmental impacts of conventional and organic apple production in Nova Scotia, Canada, through life cycle assessment. J. Clean. Prod. 104, 40–51. 10.1016/j.jclepro.2015.05.037
68
KhanM. S.ZaidiA.WaniP. A.OvesM. (2009). Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 7, 1–19. 10.1007/s10311-008-0155-0
69
KiddP. S.Álvarez-LópezV.Becerra-CastroC.Cabello-ConejoM.Prieto-FernándezÁ. (2017a). “Potential role of plant-associated bacteria in plant metal uptake and implications in phytotechnologies,” in Advances in Botanical Research, Vol 83, (London, UK: Academic Press), 87–126.
70
KiddP. S.PardoT.Soto-VázquezJ. L.Prieto-FernándezÁ.Rodriguez-GarridoB.SaadR.et al. (2017b). “Developing agromining systems in ultramafic soils of NW Spain with Mediterranean Ni-hyperaccumulating plant species,” in Proceedings of the 9th International Conference on Serpentine Ecology (Tirana).
71
KiddP.MenchM.Álvarez-LópezV.BertV.DimitriouI.Friesl-HanlW.et al. (2015). Agronomic practices for improving gentle remediation of trace element-contaminated soils. Int. J. Phytoremediat. 17, 1005–1037. 10.1080/15226514.2014.1003788
72
KochM.Al-ShehbazI. A. (2004). Taxonomic and phylogenetic evaluation of the American ”Thlaspi” species: identity and relationship to the Eurasian genus Noccaea. Syst. Bot. 29, 375–384. 10.1600/036364404774195566
73
KoellnerT.de BaanL.BeckT.BrandãoM.CivitB.MargniM.et al. (2013). UNEP-SETAC guideline on global land use impact assessment on biodiversity and ecosystem services in LCA. Int. J. Life Cycle Assess. 18, 1188–1202. 10.1007/s11367-013-0579-z
74
KonstantinouM.TsiripidisI. (2015). “Heavy metal uptake by species from metalliferous sites in Northern Greece,” in Proceedings of 13th SGA Biennial Meeting 2015, Vol. 4 (Nancy), 1477–1480.
75
KruckebergA. R. (2002). “The influences of lithology on plant life,” in Geology and Plant Life: The Effects of Landforms and Rock Type on Plants (Seattle,WA; London: Univ Wash Press), 160–181.
76
KuoS.SainjuU. M.JellumE. J. (1997). Winter cover crop effects on soil organic carbon and carbohydrate in soil. Soil. Sci. Soc. Am. J. 61, 145–152. 10.2136/sssaj1997.03615995006100010022x
77
KuppensT. (2012). Techno-Economic Assessment of Fast Pyrolysis for the Valorisation of Short Rotation Coppice Cultivated for Phytoextraction, Ph.D. dissertation, Hasselt University.
78
KuppensT.Van DaelM.VanreppelenK.ThewysT.YpermanJ.CarleerR.et al. (2015). Techno-economic assessment of fast pyrolysis for the valorization of short rotation coppice cultivated for phytoextraction. J. Clean. Prod. 88, 336–344. 10.1016/j.jclepro.2014.07.023
79
LemmingG.ChambonJ. C.BinningP. J.BjergP. L. (2012). Is there an environmental benefit from remediation of a contaminated site? Combined assessments of the risk reduction and life cycle impact of remediation. J. Environ. Manage. 112, 392–403. 10.1016/j.jenvman.2012.08.002
80
LevasseurA.LeasgeP.MargniM.DeschênesL.SamsonR. (2010). Considering time in LCA: dynamic LCA and its application to global warming impact assessments. Environ. Sci. Technol. 44, 3169–3174. 10.1021/es9030003
81
LiY. M.ChaneyR. L.BrewerE. P.AngleJ. S.NelkinJ. (2003a). Phytoextraction of nickel and cobalt by hyperaccumulator Alyssum species grown on nickel-contaminated soils. Environ. Sci. Technol. 37, 1463–1468. 10.1021/es0208963
82
LiY.-M.ChaneyR.BrewerE.RosebergR.AngleJ. S.BakerA.et al. (2003b). Development of a technology for commercial phytoextraction of nickel: economic and technical considerations. Plant Soil249, 107–115. 10.1023/A:1022527330401
83
LucisineP.EchevarriaG.SterckemanT.VallanceJ.ReyP.BenizriE. (2014). Effect of hyperaccumulating plant cover composition and rhizosphere-associated bacteria on the efficiency of nickel extraction from soil. Appl. Soil Ecol. 81, 30–36. 10.1016/j.apsoil.2014.04.011
84
LugtenbergB.KamilovaF. (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63, 541–556. 10.1146/annurev.micro.62.081307.162918
85
MatinG.KargarN.BuyukisikaH. B. (2016). Bio-monitoring of cadmium, lead, arsenic and mercury in industrial districts of Izmir, Turkey by using honey bees, propolis and pine tree leaves. Ecol. Eng. 90, 331–335. 10.1016/j.ecoleng.2016.01.035
86
MédailF.DiademaK. (2009). Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 36, 1333–134510.1111/j.1365-2699.2008.02051.x
87
MeindlG. A.AshmanT. L. (2017). Effects of soil metals on pollen germination, fruit production, and seeds per fruit differ between a Ni hyperaccumulator and a congeneric nonaccumulator. Plant Soil420, 493–50310.1007/s11104-017-3425-4
88
MengoniA.BarzantiR.GonnelliC.GabbrielliR.BazzicalupoM. (2001). Characterization of nickel-resistant bacteria isolated from serpentine soil. Environ. Microbiol. 3, 691–698. 10.1046/j.1462-2920.2001.00243.x
89
MoraisI.CamposJ. S.FavasP. J. C.PratasJ.PitaF.PrasadM. N. V. (2015). Nickel accumulation by Alyssum serpyllifolium subsp. lusitanicum (Brassicaceae) from serpentine soils of Bragança and Morais (Portugal) ultramafic massifs: plant-soil relationships and prospects for phytomining. Aust. J. Bot. 63, 17–30. 10.1071/BT14245
90
MoraisS. A.Delerue-MatosC. (2010). A perspective on LCA application in site remediation services: critical review of challenges. J. Hazard. Mater. 175, 12–22. 10.1016/j.jhazmat.2009.10.041
91
MueheE. M.WeigoldP.AdaktylouI. J.Planer-FriedrichB.KraemerU.KapplerA.et al. (2015). Rhizosphere microbial community composition affects cadmium and zinc uptake of the metal-hyperaccumulating plant Arabidopsis halleri. Appl. Environ. Microbiol. 81, 2173–2181. 10.1128/AEM.03359-14
92
NikolićT. V.KojićD.OrcićS.BatinićD.VukašinovićEBlagojevićD. P.et al. (2016). The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere164, 98–105. 10.1016/j.chemosphere.2016.08.077
93
NkrumahP. N.BakerA. J. M.ChaneyR. L.ErskineP. D.EchevarriaG.MorelJ. L.et al. (2016). Current status and challenges in developing nickel phytomining: an agronomic perspective. Plant Soil406, 55–69. 10.1007/s11104-016-2859-4
94
NovoL. B.MahlerC. F.GonzálezL. (2015). Plants to harvest rhenium: scientific and economic viability. Environ. Chem. Lett. 13, 439–445. 10.1007/s10311-015-0517-3
95
NsanganwimanaF.PourrutB.MenchM.DouayF. (2014). Suitability of Miscanthus species for managing inorganic and organic contaminated land and restoring ecosystem services. A review. J. Environ. Manage. 143, 123–134. 10.1016/j.jenvman.2014.04.027
96
ObernbergerI.BrunnerT.BärnthalerG. (2006). Chemical properties of solid biofuels - significance and impact. Biomass Bioenergy30, 973–982. 10.1016/j.biombioe.2006.06.011
97
OrłowskaE.PrzybyłowiczW.OrlowskiD.MongwaketsiN. P.TurnauK.Mesjasz-PrzybyłowiczJ. (2013). Mycorrhizal colonization affects the elemental distribution in roots of Ni-hyperaccumulator Berkheya coddii roessler. Environ. Pollut. 175, 100–109. 10.1016/j.envpol.2012.12.028
98
OthonielB.RuganiB.HeijungsR.BenettoE.WithagenC. (2016). Assessment of life cycle impacts on ecosystem services: promise, problems, and prospects. Environ. Sci. Technol. 50, 1077–1092. 10.1021/acs.est.5b03706
99
PardoT.BenizriE.SaadR.Rodríguez-GarridoB.EchevarriaG.Prieto-FernándezÁ.et al. (2017). “Assessment of the use of bacterial inoculants for improving the agromining potential of Ni-hyperaccumulating plant species at field-scale,” in Proceedings of the Annual Congress on Soil Sciences (Madrid) (Accessed December 4–5, 2017).
100
PardoT.Rodríguez-GarridoB.SaadR. F.Soto-VázquezJ. L.Loureiro-Viñ-asM.Prieto-FernándezÁ.et al. (2018). Assessing the agromining potential of Mediterranean nickel-hyperaccumulating plant species at field-scale in ultramafic soils under humid-temperate climate. Sci. Tot. Environ. 630, 275–286. 10.1016/j.scitotenv.2018.02.229
101
Partida-MartínezL. P.HeilM. (2011). The microbe-free plant: fact or artifact?Front. Plant Sci. 2:100. 10.3389/fpls.2011.00100
102
PollardA. J.ReevesR. D.BakerA. J. (2014). Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci. 217–218, 8–17. 10.1016/j.plantsci.2013.11.011
103
RashidM. I.MujawarL. H.ShaahzadT.AlmeelbiT.IsmailI. M.OvesM. (2016). Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 183, 26–41. 10.1016/j.micres.2015.11.007
104
RentschL.AubelI. A.SchreiterN.HöckM.BertauM. (2016). PhytoGerm: extraction of germanium from biomass – an economic pre-feasibility study. J. Business Chem. 13, 47–58.
105
RešetnikI.SatovicZ.SchneeweissG. M.LiberZ. (2013). Phylogenetic relationships in Brassicaceae tribe Alysseae inferred from nuclear ribosomal and chloroplast DNA sequence data. Mol. Phylogenet. Evol. 69, 772–786. 10.1016/j.ympev.2013.06.026
106
RidardC.RosenkranzT.KiddP.EchevarriaG.PuschenreiterM. (2017). “Evaluating Ni phytomining efficiency on Austrian serpentine soils in a long-term field experiment,” in International Workshop: Geochemical Cycle of Ni, Co and Sc: From Mining Exploration to Ecotoxicity, Vol. 22, eds CathelineauC.BoironM-C.EchevarriaG.FilippovL.CloquetC.GiamberiniL.SamperA.AbildtrupI. (Nancy).
107
RodriguesJ.HouzelotV.FerrariF.EchevarriaG.LaubieB.MorelJ.-L.et al. (2016). Life cycle assessment of agromining chain highlights role of erosion control and bioenergy. J. Clean. Prod. 139, 770–778. 10.1016/j.jclepro.2016.08.110
108
RodriguesM.DimandeP.PereiraE. L.FerreiraQ. I.FreitasS.CorreiaC. M.et al. (2015). Early-maturing annual legumes: an option for cover cropping in rainfed olive orchards. Nutr. Cycl. Agroecosys. 103, 153–166. 10.1007/s10705-015-9730-5
109
RodriguezR. J.WhiteJ. F.Jr.ArnoldA. E.RedmanR. S. (2009). Fungal endophytes: diversity and functional roles. New Phytol. 182, 314–330. 10.1111/j.1469-8137.2009.02773.x
110
RollinsonA. N.WilliamsO. (2016). Experiments on torrefied wood pellet: study by gasification and characterization for waste biomass to energy applications. R. Soc. Open Sci. 3:150578. 10.1098/rsos.150578
111
RozpądekP.DomkaA.WażnyR.NosekM.JedrzejczykR.TokarzK.et al. (2018). How does the endophytic fungus Mucor sp. improve Arabidopsis arenosa vegetation in the degraded environment of a mine dump?Environ. Exp. Bot. 147, 31–42. 10.1016/j.envexpbot.2017.11.009
112
RozpądekP.JanickaM.JedrzejczykR.WażnyR.DomkaA.TurnauK. (2017). “Can fungal endophytes be used to improve Thlaspi growth in ultramafic soils?,” in Proceedings of the 9th International Conference on Serpentine Ecology, (Tirana), 177.
113
RueM.VallanceJ.EchevarriaG.ReyP.BenizriE. (2015). Phytoextraction of nickel and rhizosphere microbial communities under mono- or multispecies hyperaccumulator plant cover in a serpentine soil?Aust. J. Bot. 63, 92–102. 10.1071/BT14249
114
SaadR. F.KobaissiA.AmiaudB.RuelleJ.BenizriE. (2018a). Changes in physicochemical characteristics of a serpentine soil and in root architecture of a hyperaccumulating plant cropped with a legume. J. Soil. Sediment. 18, 1994–2007. 10.1007/s11368-017-1903-1
115
SaadR. F.KobaissiA.MachinetG.VilleminG.EchevarriaG.BenizriE. (2018b). Crop rotation associating a legume and the nickel hyperaccumulator Alyssum murale improves the structure and biofunctioning of an ultramafic soil. Ecol. Res. 10.1007/s11284-017-1526-4. [Epub ahead of print].
116
SaadR.KobaissiA.GouxX.EchevarriaG.KiddP.BenizriE. (2017). “Developing Ni-agromining by associating Alyssum murale with a leguminous plant: an in-situ experiment on an ultramafic site in Spain,” in The 9th International Conference On Serpentine Ecology (Albania), 172.
117
SaadR.KobaissiA.RobinC.EchevarriaG.BenizriE. (2016). Nitrogen fixation and growth of Lens culinaris as affected by nickel availability: a pre-requisite for optimization of agromining. Environ. Exp. Bot. 131, 1–9. 10.1016/j.envexpbot.2016.06.010
118
ShallariS.EchevarriaG.SchwartzC.MorelJ. L. (2001). Availability of nickel in soils for the hyperaccumulator Alyssum murale (Waldst. & Kit.). S. Afr. J. Sci. 97, 568–570.
119
ShallariS.SchwartzC.HaskoA.MorelJ. L. (1997). Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Tot. Environ. 209, 133–142. 10.1016/S0048-9697(98)80104-6
120
Sigma-AldrichS. (2018). Ammonium Nickel(II) Sulfate Hexahydrate. Available online at: https://www.sigmaaldrich.com (January 26, 2018).
121
SimonnotM.-O.VaughanJ.LaubieB. (2018). “Processing of bio-ore to products,” in Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants, eds Van der EntA.EchevarriaG.BakerA. J. M.MorelJ. L. (Cham: Springer International Publishing), 39–52.
122
ŠpanielS.KempaM.Salmerón-SánchezE.Fuertes-AguilarJ.Francisco MotaJ.Al-ShehbazI. A.et al. (2015). AlyBase – database of names, chromosome numbers, and ploidy levels of Alysseae (Brassicaceae), with a new generic concept of the tribe. Plant System. Evol. 301, 2463–2491. 10.1007/s00606-015-1257-3
123
SparksD. L. (2002). Environmental Soil Chemistry. San Diego, CA: Academic Press.
124
St. LuceM.GrantC. A.ZebarthB. J.ZiadiN.O'DonovanJ. T.BlackshawR. E.et al. (2015). Legumes can reduce economic optimum nitrogen rates and increase yields in a wheat-canola cropping sequence in western Canada. Field Crops Res. 179, 12–25. 10.1016/j.fcr.2015.04.003
125
StefanovićV.TanK.IatrouG. (2003). Distribution of the endemic Balkan flora on serpentine I. – Obligate serpentine endemics. Plant Syst. Evol. 242, 149–170. 10.1007/s00606-003-0044-8
126
StevensonF. C.van KesselC. (1996). The nitrogen and non-nitrogen rotation benefits of pea to succeeding crops. Can. J. Plant. Sci. 76, 735–745. 10.4141/cjps96-126
127
SuerP.Andersson-SköldY. (2011). Biofuel or excavation? -Life cycle assessment (LCA) of soil remediation options. Biomass Bioenergy35, 969–981. 10.1016/j.biombioe.2010.11.022
128
TangY.-T.DengT-H-B.WuQ-H.WangS-Z.QiuR-L.WeZ-B.et al. (2012). Designing cropping systems for metal-contaminated sites: a review. Pedosphere22, 470–488. 10.1016/S1002-0160(12)60032-0
129
ThijsS.SillenW.RineauF.WeyensN.VangronsveldJ. (2016), Towards an enhanced understanding of plant-microbiome interactions to improve phytoremediation: engineering the metaorganism. Front. Microbiol. 7:341. 10.3389/fmicb.2016.00341
130
TumiA. F.MihailovićN.GajićB. A.NiketićM.TomovićG. (2012). Comparative study of hyperaccumulation of nickel by Alyssum murale. Populations from the ultramafics of Serbia. Pol. J. Environ. Stud. 21, 1855–1866.
131
TurnauK.Mesjasz-PrzybylowiczJ. (2003). Arbuscular mycorrhiza of Berkheya coddii and other Ni-hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza13, 185–190. 10.1007/s00572-002-0213-6
132
TurnauK.RozpądekP.JedrzejczykR.WażnyR.DomkaA. (2017). “Hyperaccumulators as a model to study plant-fungal interactions,” in Proceedings of the 9th International Conference on Serpentine Ecology (Tirana), 86.
133
Van DaelM.KuppensT.LizinS.Van PasselS. (2015). “Techno-economic assessment methodology for ultrasonic production of biofuels,” in Production of Biofuels and Chemicals with Ultrasound, eds ZhenF.SmithL. R.XinhuaQ. (Dordrecht), 317–345.
134
van der EntA.BakerA. J.ReevesR. D.ChaneyR. L.AndersonC. W.MeechJ. A.et al. (2015). Agromining: farming for metals in the future?Environ. Sci. Technol. 49, 4773–4780. 10.1021/es506031u
135
van der EntA.BakerA. J. M.van BalgooyM. M. J.TjoaA. (2013b). Ultramafic nickel lateries in Indonesia (Sulawesi, Halmahera): Mining, nickel hyperaccumulators and opportunities for phytomining. J. Geochem. Explor. 128, 72–79. 10.1016/j.gexplo.2013.01.009
136
van der EntA.BakerA. M.ReevesR.PollardA. J.SchatH. (2013a). Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil362, 319–334. 10.1007/s11104-012-1287-3
137
VigilM.Marey-PérezM. F.Martinez HuertaG.Álvarez CabalV. (2015). Is phytoremediation without biomass valorization sustainable? —Comparative LCA of landfilling vs. anaerobic co-digestion. Sci. Tot. Environ. 505, 844–850. 10.1016/j.scitotenv.2014.10.047
138
WarwickS. I.SauderC. A.Al-ShehbazI. A. (2008). Phylogenetic relationships in the tribe Alysseae (Brassicaceae) based on nuclear ribosomal ITS DNA sequences. Botany86, 315–336. 10.1139/B08-013
139
WażnyR.RozpądekP.JedrzejczykR.JanickaM.LichtsheidlI.TurnauK. (2017). “Endophytic fungal mycobiota in hyperaccumulating Thlaspi,” in Proceedings of the 9th International Conference on Serpentine Ecology (Tirana), 179.
140
WeiZ. B.GuoX. F.WuQ. T.LongX. X.PennC. J. (2011). Phytoextraction of heavy metals from contaminated soil by co-cropping with chelator application and assessment of associated leaching risk. Int. J. Phytoremdiat. 13, 717–729. 10.1080/15226514.2010.525554
141
WenzelW. W.JockwerF. (1999) Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environ. Pollut.104, 145–155.
142
WittersN.MendelsohnR. O.Van SlyckenS.WeyensN.SchreursE.MeersE.et al. (2012). Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: energy production and carbon dioxide abatement. Biomass Bioenergy38, 454–469. 10.1016/j.biombioe.2011.08.016
143
WuQ. T.HeiL.WongJ. W.SchwartzC.MorelJ. L. (2007). Co-cropping for phyto-separation of zinc and potassium from sewage sludge. Chemosphere68, 1954–1960. 10.1016/j.chemosphere.2007.02.047
144
YangJ.KloepperJ. W.RyuC. M. (2009). Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14, 1–4. 10.1016/j.tplants.2008.10.004
145
ZaidiA.OvesM.AhmadE.KhanM. S. (2011). “Importance of free-living fungi in heavy metal remediation,” in Biomanagement of Metal-Contaminated Soils, eds KhanM. S.ZaidiA.GoelR.MusarratJ. (Springer Science Publishers), 479–494.
146
ZhangQ.ZhangJ.YangL.ZhangL.JiangD.ChenW.et al. (2014). Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol. Control72, 98–108.
147
ZhangX.LaubieB.HouzelotV.PlasariE.EchevarriaG.SimonnotM.-O. (2016). Increasing purity of Ammonium nickel sulfate hexahydrate and production sustainability in nickel phytomining process. Chem. Eng. Res. Des. 106, 26–32. 10.1016/j.cherd.2015.12.009
148
ZhangY.ZhangY.LiuM.ShiX.ZhaoZ. (2008). Dark septate endophyte (DSE) fungi isolated from metal polluted soils: their taxonomic position, tolerance, and accumulation of heavy metals in vitro. J. Microbiol. 46, 624–632. 10.1007/s12275-008-0163-6
Summary
Keywords
Alyssum s.l., hydrometallurgy, Leptoplax emarginata, Odontarrhena, phytomining, serpentine soils
Citation
Kidd PS, Bani A, Benizri E, Gonnelli C, Hazotte C, Kisser J, Konstantinou M, Kuppens T, Kyrkas D, Laubie B, Malina R, Morel J-L, Olcay H, Pardo T, Pons M-N, Prieto-Fernández Á, Puschenreiter M, Quintela-Sabarís C, Ridard C, Rodríguez-Garrido B, Rosenkranz T, Rozpądek P, Saad R, Selvi F, Simonnot M-O, Tognacchini A, Turnau K, Ważny R, Witters N and Echevarria G (2018) Developing Sustainable Agromining Systems in Agricultural Ultramafic Soils for Nickel Recovery. Front. Environ. Sci. 6:44. doi: 10.3389/fenvs.2018.00044
Received
28 February 2018
Accepted
17 May 2018
Published
08 June 2018
Volume
6 - 2018
Edited by
Rocio Millán, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas, Spain
Reviewed by
Anna De Marco, Università degli Studi di Napoli Federico II, Italy; M Oves, King Abdulaziz University, Saudi Arabia
Updates
Copyright
© 2018 Kidd, Bani, Benizri, Gonnelli, Hazotte, Kisser, Konstantinou, Kuppens, Kyrkas, Laubie, Malina, Morel, Olcay, Pardo, Pons, Prieto-Fernández, Puschenreiter, Quintela-Sabarís, Ridard, Rodríguez-Garrido, Rosenkranz, Rozpądek, Saad, Selvi, Simonnot, Tognacchini, Turnau, Ważny, Witters and Echevarria.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra Susan Kidd pkidd@iiag.csic.es
This article was submitted to Agroecology and Land Use Systems, a section of the journal Frontiers in Environmental Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.