- 1Institute for Land, Water and Society, Charles Sturt University, Albury, NSW, Australia
- 2ETH-Zurich, Stocker Lab, Institute of Environmental Engineering (IfU), Zurich, Switzerland
- 3Departamento de Zoologia, Instituto de Biociências, UNESP – Universidade Estadual Paulista, São Paulo, Brazil
- 4New South Wales Department of Primary Industries, Port Stephens Fisheries Institute, Taylors Beach, NSW, Australia
- 5Hydrology Group, Pacific Northwest National Laboratory, Richland, WA, United States
- 6JAD Systems LLC, Kingswood, TX, United States
Pumped hydroelectric energy storage (PHES) projects are being considered worldwide to achieve renewable energy targets and to stabilize baseload energy supply from intermittent renewable energy sources. Unlike conventional hydroelectric systems that only pass water downstream, a feature of PHES schemes is that they rely on bi-directional water flow. In some cases, this flow can be across different waterbodies or catchments, posing a risk of inadvertently expanding the range of aquatic biota such as fish. The risk of this happening depends on the likelihood of survival of individuals, which remains poorly understood for turbines that are pumping rather than generating. This study quantified the survival of a globally widespread and invasive poeciliid fish, Eastern gambusia (Gambusia holbrooki), when exposed to three hydraulic stresses characteristic of those experienced through a PHES during the pumping phase. A shear flume and hyperbaric chamber were used to expose fish to different strain rates and rapid and sustained pressurization, respectively. Blade strike models were also used to predict fish survival through a Francis dual turbine/pump. Simulated ranges were based on design and operational conditions provided for a PHES scheme proposed in south-eastern Australia. All gambusia tested survived high levels of shear stress (up to 1,853 s−1), extremely high pressurization (up to 7,600 kPa gauge pressure) and the majority (>93%) were unlikely to be struck by a turbine blade. Given their tolerance to these extreme simulated stresses, we conclude that gambusia will likely survive passage through the simulated PHES scheme if they are entrained at the intake. Therefore, where a new PHES project poses the risk of inadvertently expanding the range of gambusia or similar poeciliid species, measures to minimize their spread or mitigate their ecosystem impacts should be considered.
Introduction
Pumped hydroelectric energy storage (PHES) projects are expanding worldwide, driven by the rising global demand for electricity, political renewable energy targets, security of supply, and upgrades to existing water infrastructure (Yang, 2016). Reversible turbines (usually a Francis dual turbine) pump water to a higher elevation reservoir during periods of low electricity demand. When energy demand is high, water is then released back down to a lower elevation reservoir to turn the turbine (Deane et al., 2010). Often, PHES is deemed an economic and sustainable mechanism to provide a large-scale source of energy and firm capacity for other renewables, in particular wind and solar (Harby et al., 2013).
A key feature of PHES is that it requires large differences in geographical altitude between reservoirs for water movement to occur in both an upstream and downstream direction. Therefore, PHES schemes can facilitate a bi-directional connection, sometimes across different waterbodies and even across different catchments. Discussions relating to the biological implications of such water transfers have occurred for some time (Hauck and Edson, 1976). Concerns have been raised around inter-basin water transfers and the effects on aquatic biota including the loss of biogeographical integrity, loss of biota, alien species introductions, and water quality implications (Davies et al., 1992). Specifically with regards to fish, there is evidence of species transfers (Lampert, 1976), impacts on migration (van Esch, 2012) and injuries and mortality (Hauck and Edson, 1976; van Esch, 2012). If a PHES facilitates the unintentional transfer of alien species to new areas, there can be flow on effects such as the loss of biodiversity, predation, and alterations of food webs (Strayer, 2010; Gallardo et al., 2016). Whether these impacts are realized will depend on whether a species will be entrained and survives passage through a PHES scheme.
Fish that pass through hydropower schemes can be exposed to potentially lethal hydraulic mechanisms. These have been extensively studied for conventional hydropower turbines and include elevated fluid shear and turbulence, turbine blade strike, and rapid and extreme pressure variations (Cada, 2001; Cada et al., 2006; Fu et al., 2016; Boys et al., 2018). Fish survival following exposure to these mortality mechanisms varies among species and life stages and depends on the severity of hydraulic stress (Neitzel et al., 2004; Deng et al., 2005; Boys et al., 2016a), fish morphology and swimming ability (Coutant and Whitney, 2000; Deng et al., 2007), and their position in the water column (Silva et al., 2018). In general, while blade strike likelihood is lower for small fish (Deng et al., 2007), the opposite occurs for shear stress, with eggs and larvae being more vulnerable to injury than larger fish (Navarro et al., 2019). Additionally, injuries and mortality tend to proportionally increase with higher shear stress levels (Deng et al., 2010; Navarro et al., 2019).
Due to the complex flow patterns near turbine blades, not all fish passing through conventional hydroelectric turbines are exposed to lethal shear stress or blade strike (Deng et al., 2007). Shear and blade strike exposure depends on the route taken by the fish through the turbine system (Fu et al., 2016). In comparison, all fish passing conventional hydroelectric turbines are exposed to rapid decompression, and the magnitude depends largely on turbine design and operation (Brown et al., 2012a,b). This rapid decompression can result in predictable levels of injury and mortality of fish of all life stages; eggs, larvae, juveniles, and adults (Boys et al., 2016a,b; Pflugrath et al., 2018). It is likely that the insights gained from other studies concerning conventional turbine passage can be directly applied to PHES when turbines are in the generation phase (passing water downstream). It is, however, unlikely that this information is transferable to PHES schemes that are operating in the pumping phase (passing water uphill). The hydraulic conditions created by a turbine are very different during the generating phase, compared to when it is pumping. For instance, whilst a rapid and transient exposure to negative pressures is likely at a generating turbine, fish passing a pumping turbine will experience a rapid compression that will be sustained and slowly released as the water body travels toward the upper reservoir. Unlike the effects of rapid decompression on fish, there have been few studies investigating fish survival following exposure to sustained pressurization (see Belaud and Barthelemy, 1973; Lampert, 1976; Sebert and MacDonald, 1993). While mortality has been observed in fish species exposed to high pressures (Sebert and MacDonald, 1993), in some cases fish do survive pressure in excess of 5,000 kPa (Lampert, 1976). The available evidence suggests the effects of compression vary among species and life stage, the rate at which the compression occurs, the time held at pressure and water temperature. Currently, the effects of compression have not been examined across a broad suite of fish families, and compression rates within PHES schemes are considerably greater than what has been examined on any fish species in the available literature.
As more PHES projects are approved for installation globally, it is imperative to resolve whether fish are likely to survive their hydraulic conditions. This is even more critical when the pumping involves inter-basin transfers of water that could result in range expansions of both invasive and non-invasive fish. In this study, the survival of the poeciliid fish, Eastern gambusia (Gambusia holbrooki; Girard, 1859, referred to as gambusia herein), was evaluated using simulations of shear stress, extreme pressurization, and blade strike likely to be experienced when passing through a 2,000 MW PHES scheme being proposed for south-eastern Australia.
Gambusia are a globally distributed species and are considered invasive in many areas, including Australia. They are capable of establishing populations when transferred to new locations (García-Berthou et al., 2005), where they have the potential to cause environmental damage and threaten small-bodied native fish (Rowe et al., 2008; MacDonald et al., 2012; Carmona-Catot et al., 2013). To evaluate the survival likelihood of adult gambusia when passing a PHES scheme during the pumping phase, the following tests were simulated: (1) fish were exposed to a range of shear strains representative of those estimated to occur when passing through draft tubes and close to turbine blades to quantify how survival changed; (2) fish were exposed to extreme pressurization simulating a PHES pumping phase; and (3) the likelihood that a gambusia would be struck by a turbine blade when passing through a Francis turbine was predicted using blade strike models. Ranges tested for each stressor were based on design parameters and operational conditions provided by the entity that commissioned the PHES at the time of publishing, and assumed that fish entrainment would occur at the site.
Materials and Methods
Study Species and Husbandry
Adult gambusia (see Table 1 for size ranges) were collected from water storage dams at Charles Sturt University, Albury (New South Wales, Australia), between September and October 2018 with a 4 mm mesh seine net. Fish were transported a few 100 m in 100 L bins filled with dam water to Charles Sturt University Fish Laboratory (CSUFL) where experiments were conducted. On arrival, gambusia were placed in a 1,000 L quarantine tank and monitored for a week prior to release into additional 1,000 L holding tanks. To minimize stress-related diseases following transport, fish were given a prophylactic salt treatment (5 ppt for 1 week) and maintained at 2.5 ppt throughout the study. Chlorinated town water supply, treated with Safe™ (1 g/1,000 L) to remove chlorine, was used in the holding tanks and recirculated and filtered with a Polygeyser Bead Filter (AST Endurance Model 4000, Aquaculture Systems Technologies, New Orleans). Gambusia were maintained on a diet of Artemia spp. nauplii and held for 5–7 days prior to experimentation. Water quality was monitored daily (Table 2).
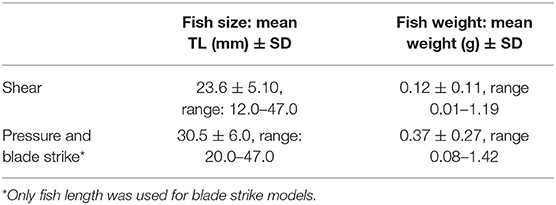
Table 1. Summary of adult gambusia measurements used for shear, pressure, and blade strike assessments.
PHES Simulation
The tests conducted in the present study were based on specifications related to a PHES scheme under development in south-eastern Australia. The simulations were based on the assumption that fish were entrained into the PHES from the lower elevation storage reservoir and pumped to the upper, higher elevation storage reservoir while being exposed to a head differential of 7,600 kPa. During a passage event, a fish passes through a Francis reversible turbine (simulated in the blade strike models) and through a series of structures before ascending to the upper reservoir (Figure 1). The profile and total passage time simulated for the pressure tests reflected all six turbines operating to represent the most extreme scenario for a passing fish (Table 3; Figure 2). The shear strain rates tested (up to 1,853 s−1) were designed to incorporate the range of levels known to be generated by PHES schemes, where rapidly flowing water passes near internal structures of the turbines, such as trash racks, draft tubes, wicket gates, and stay vanes.
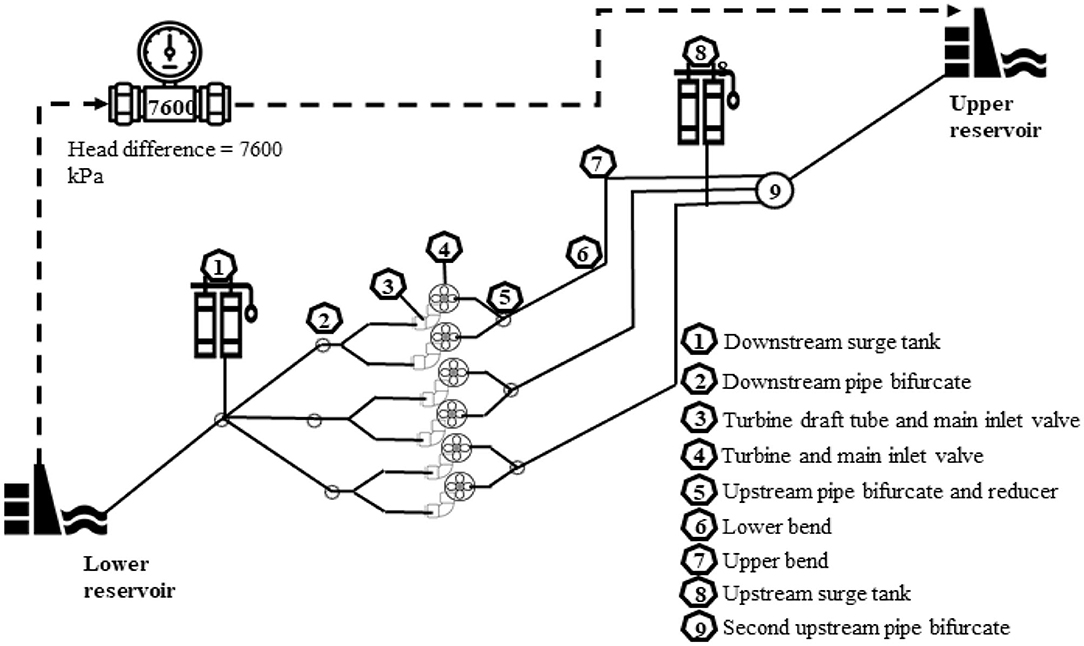
Figure 1. Schematic of the pumped hydroelectric storage informing the head difference between lower reservoir and upper reservoir during the pumping phase and the main structures (numbers 1–9) where pressure changes will occur through the scheme. Note that the head difference was estimated for the scenario where the lower reservoir was operating at full supply level and upper reservoir was at the minimum operating level.
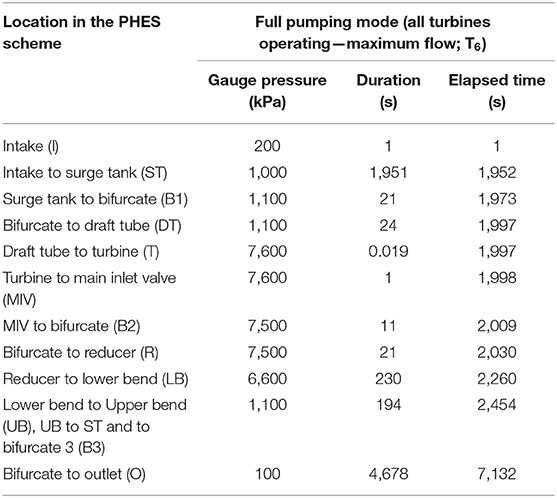
Table 3. Expected pressure changes when pumping from the lower reservoir to the upper reservoir with all six turbines operating (T6), ranging from 100 kPa to a maximum of 7,600 kPa.
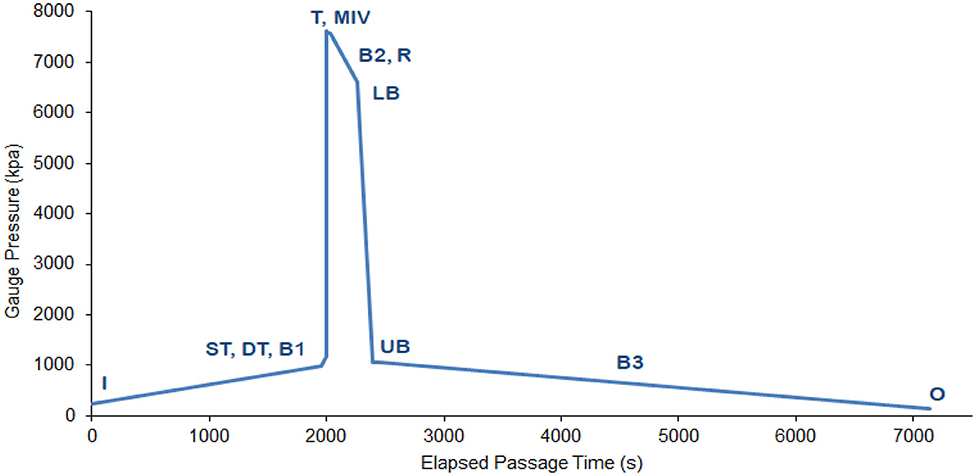
Figure 2. Pressure profile simulated in the hyperbaric chamber at full pumping capacity (all six turbines operating). See Table 3 for location labels and a full description of pressures and transient times.
Shear Experiments
Gambusia were exposed to one of five shear strain rates using a flume consisting of a cylindrical Plexiglas chamber connected to a submerged jet at one end and a fiberglass reservoir tank at the other (Figures 3, 4; for a detailed description see Boys et al., 2014; Navarro et al., 2019). On entry to the chamber, a conical nozzle reduced the flow diameter from 15 to 5 cm over a distance of 26 cm (Figure 4). This effectively accelerated the flow to allow a shear environment to be generated and quantified at the position where water within the flume was entrained in the jet stream (based on Neitzel et al., 2000). Nozzle flow rates were manually adjusted to create different jet velocities which produced various strain rates. The maximum strain rate was achieved at the maximum velocity that could be generated in the shear flume. Velocity testing established in Navarro et al. (2019) found that the highest area of strain across all flow rates was between 20 and 30 mm from the jet centerline. For this reason, fish were introduced into the jet through a polycarbonate tube positioned 30 mm above the jet centerline and at an angle of ~30° to prevent contact of fish with the tube on exit. A small flow of water was used in the delivery tube to transition the fish into the jet.
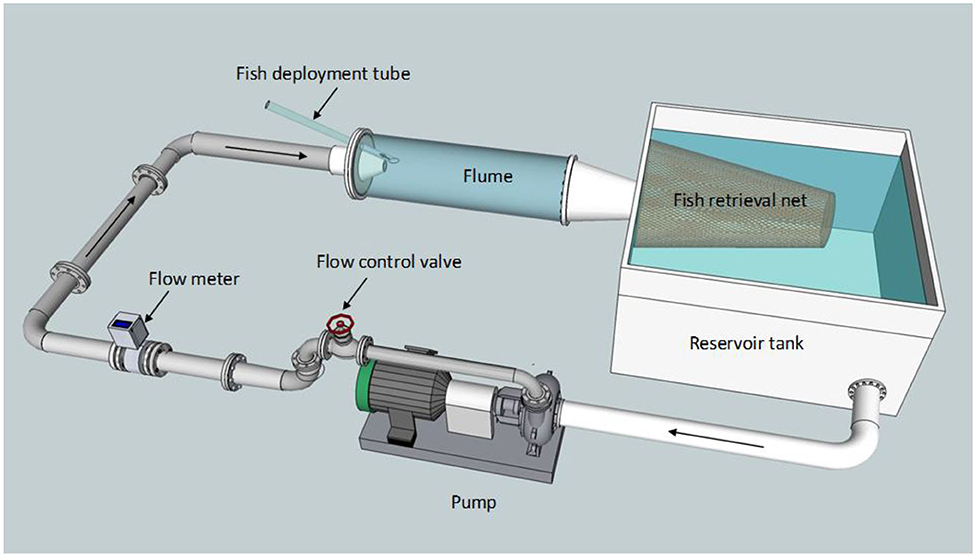
Figure 3. Schematic of the shear flume that was used to expose gambusia to simulated strain rates. Fish are released in the fish deployment tube, exposed to a jet in the flume then collected in the retrieval net before being transferred to holding facilities. Adapted from Boys et al. (2014), used with author permission.
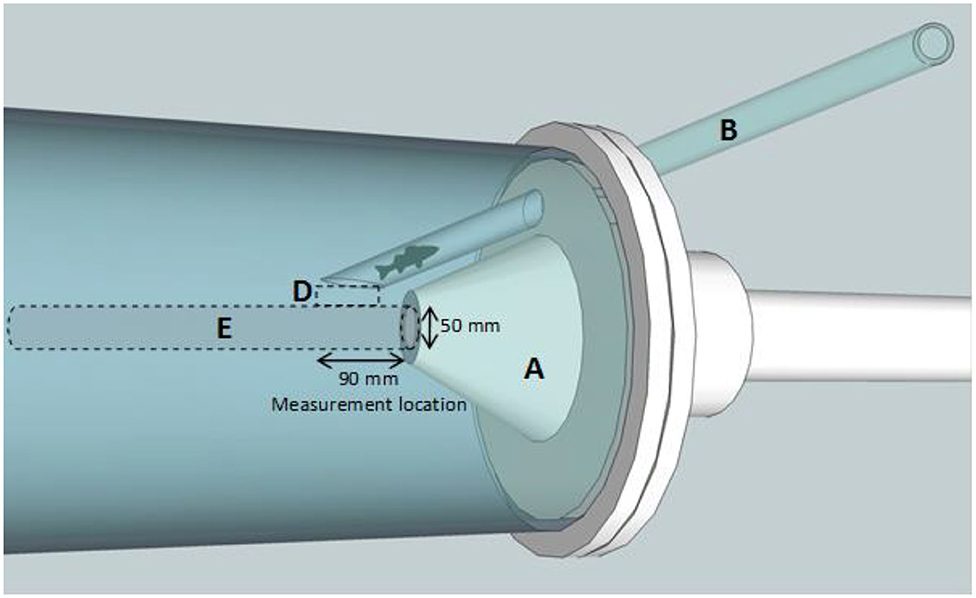
Figure 4. Schematic of the conical nozzle of the shear flume used to reduce the diameter of the flow, effectively accelerating the flow to generate a shear environment. Adapted from Boys et al. (2014), used with author permission. (A) Conical nozzle. (B) Fish delivery tube. (D) Fish exposure to edge of jet and shear forces and (E) Flow-establishment zone.
Strain rates were defined as the maximum strain rate that we can assume a fish was exposed to within the zone of flow establishment (as per Neitzel et al., 2004). Mean jet velocities for the flow rates applied (Table 4) were obtained using a total tube connected to a pressure gauge ranging from 0 to 45 psi (with 10 psi increments) positioned 90 mm from the nozzle in the center of the jet. Pressure gauge readings (psi) were converted to meters of head and jet velocities calculated using Bernoulli's equation
where H is the total head (m), v is the velocity (m s−1) and g is the gravitational constant (m2 s−1). Once mean jet velocities were obtained, shear strain rates were calculated using the equation suggested by Neitzel et al. (2004)
where v is mean water velocity and y is the distance perpendicular to the force. To provide a fine scale measurement of the shear strain rate at the width of the fish (Neitzel et al., 2004), distance (y) was defined as 10 mm for adult gambusia.
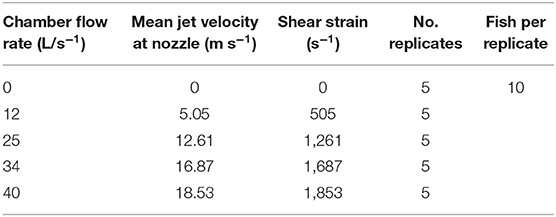
Table 4. Shear chamber flow rates, mean jet velocities and calculated shear strain rates with a summary of experimental treatments and fish size.
Shear strain rates tested ranged from 505 to 1,853 s−1, with five replicates for each strain rate treatment (Table 4). Each replicate consisted of a group of 10 individual gambusia (see Table 1 for fish sizes) that were dip-netted from holding tanks and inserted into the delivery tube for exposure to the shear environment. A “0” shear strain was applied as a control to consider any potential handling effects, and involved delivering fish via a duplicated deployment tube that was directed into a fish retrieval net. Following shear exposure, gambusia were collected from the fish retrieval net for each test group and immediately assessed for survival. Each fish was then placed with others from its replicate test group in a 16 cm by 16 cm mesh basket floating within the holding tank and survival was reassessed 24 h later. Fish that swam and fed freely during the post-experimental monitoring period were deemed to have survived. After the experiment, all fish still alive were euthanized in 100 mg L−1 benzocaine and then measured [total length (TL) in mm] and weighed (to the nearest 0.01 of a gram).
Pressure Experiments
A purposely-built hyperbaric chamber (Figure 5) capable of generating extremely fast positive pressure transients (>200 kPa per ms) and able to achieve up to 9,000 kPa (with 0.1% accuracy in pressure regulation) was used to simulate the pressure profile experienced by a fish as it would travel through the PHES, from the intake to outlet (Table 3 and Figure 1). The chamber was manufactured to meet the compression spike of the simulation while maintaining a stable environment (oxygen and temperature levels).
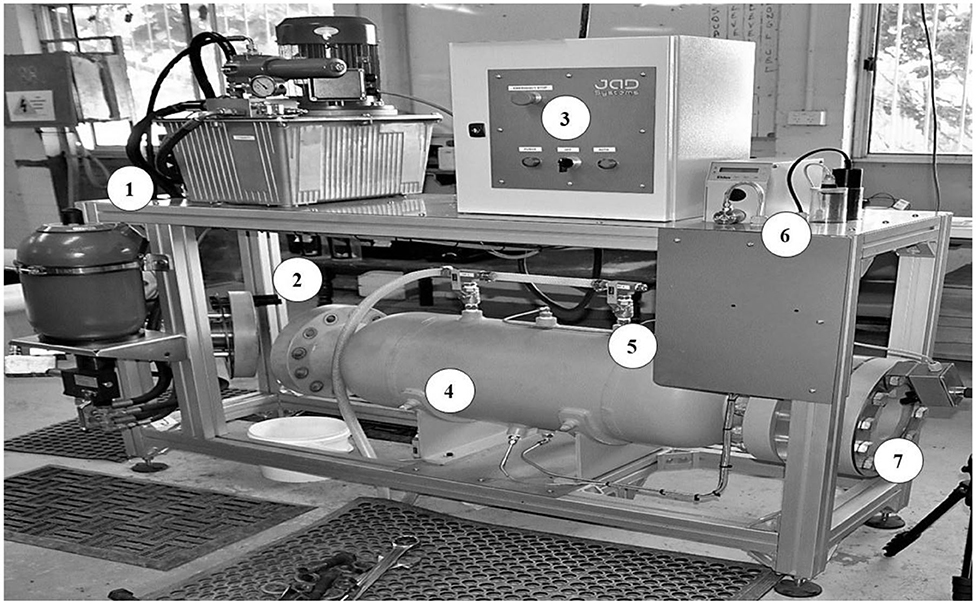
Figure 5. Diagram of the pressure chamber and labeled components: (1) hydraulic pump and cylinder, (2) removable flange where fish capsules were inserted, (3) control panel, (4) pressure vessel, (5) pressure sensor, (6) oxygen and temperature sensor and (7) fixed viewing window.
The chamber consisted of a 100 L pressure vessel. Fish were inserted in one end through a removable ASME B16.1 Class 600 15 cm flange that could then be sealed by bolting the flange to the chamber. Within this removable flange was a hydraulic ram for water displacement. At the other end of the chamber was a thick acrylic viewing port (Figure 5). The chamber was filled with water using a submersible pump (Ozito Model: PSDW-750 Submersible Pump). Once a pressure profile was initiated, no replacement water entered the chamber. Therefore, the chamber included a bleed off valve where water could be periodically removed to test dissolved oxygen levels and a 10,000 kPa dosing pump to move the sampled water back into the chamber or to oxygenate water before being returned to the chamber. A 23,000 kPa 20 L hydrogen charged accumulator was used to store the large amount of energy required to move the hydraulic ram to rapidly create a desired pre-programmed pressure change in the system. Rapid pressure changes were implemented by a high-end embedded Moog Motion Controller (Moog Inc., USA, http://www.moog.com) moving a high bandwidth Moog hydraulic ram/valve combination to accurately and rapidly displace water in the test chamber. Measurement of all signals (e.g., dissolved oxygen, temperature, and pressure) was achieved using Beckhoff Digitization Hardware (Beckhoff Automation GmbH & Co. KG, Germany, https://www.beckhoff.com/). Thermo-couple temperature channels were calibrated using a Fluke 725 Multifunction Process Calibrator (Fluke Corporation, Washington, https://us.flukecal.com/) to simulate the sensor characteristics. The dissolved oxygen sensor (RDP-Pro X, In-Situ, Fort Collins, Colorado USA, https://in-situ.com/us/) was calibrated with the calibration tool and simulation solution provided by the supplier.
The pressure profile simulated in this study was that expected to be experienced by a fish moving from the intake of the lower reservoir, through the PHES scheme and to the upper reservoir (Figure 1). The scenario was based on full pumping capacity with all six turbines operating simultaneously at full speed resulting in a total passage time of 2 h (~7,133 seconds) (Figure 2). Under these conditions, the highest pressure achieved would be 7,600 kPa at the turbines and the lowest 100 kPa (Table 3). Pressure changes and travel times tested were characteristic of the lower reservoir being at full supply level (FSL) and the upper reservoir at minimum operating level (MOL) (Table 3). By testing this scenario, a conservative approach was taken to assessing the risk of gambusia survival, because the pressure profiles would be the most extreme. That is, if gambusia survived this scenario, they would also likely survive all other operating scenarios.
Each pressure trial involved adding 10 gambusia (see Table 1 for fish sizes) to each of the two cylindrical Perspex capsules (length 350 mm, diameter 100 mm) that contained water from the holding tanks. The 20 fish were sealed within the capsules using Velcro-attached mesh (1.5 mm2 mesh) “lids” so that they could not escape but oxygenated water could exchange between the capsules and chamber. Both capsules were inserted into the partially filled chamber, ensuring that no air bubbles were trapped in or around each cylinder. The chamber was then sealed and filled with the same water the gambusia were housed in using a submersible pump (Ozito Model: PSDW-750 Submersible Pump). Once full, the chamber was purged to remove any air bubbles and a computer program with graphical user interface (GUI; LabView) was used to control the chamber hardware to run the pre-programmed pressure profile.
Five replicate test groups of 10 gambusia per capsule (20 per replicate) were exposed to the pressure scenario. Five control replicates were also performed that consisted of the identical handling protocol but without exposing fish to any pressure change. At the end of the experiment, the chamber was drained into an intermediary holding tank using an in-line pump (Ozito Model: TRP-650 Transfer Water Pump), the end flange unbolted and the Perspex cylinders removed. The computer program saved pressure, dissolved oxygen and temperature data for each trial, and these data were examined to confirm the pressure profile that fish were exposed to and to verify oxygen and temperature stability in the chamber throughout the experiment. Gambusia were collected from the capsules and immediately assessed for survival, then placed into a holding tank that contained baskets for each experimental replicate and held for 24 h to reassess survival as a percentage for each test group replicate. All fish still alive after 24 h were euthanized in 100 mg L−1 benzocaine and measured following the same procedure as for the shear experiments.
Statistical Analysis
Immediate and 24 h mean survival was pooled and presented as the probability of survival of adult gambusia after 24 h for the shear and pressure experiments. For the shear experiments, a binary logistic regression analysis (logit link) was used to test whether survival probability (after 24 h) was influenced by the shear strain rate with specimens classed as dead (0) or alive (1). Predicted survival rates and Wald confidence intervals were generated and the survival rates presented graphically. For the pressure experiments, the mean survival of gambusia was calculated as a percentage immediately after exposure and after 24 h for each experimental group (control and pressure). All analyses were performed using the Statistical Analysis Software (SAS) package (SAS Institute, Cary, NC, USA).
Blade Strike Model
Based on the assumption that fish must pass through a turbine runner leading edge plane after the sweep of one blade and before the sweep of the next one to avoid the strike, Von Raben (1957) proposed the first deterministic blade strike model. Turnpenney et al. (2000) later defined the “water length” as the distance between two successive blades along the flow line and derived the blade strike model for Kaplan turbines. Fish aligned with the flow lines and longer than the water length would be struck by the runner blades. Since fish can enter the turbine in random orientations rather than aligning with flow lines, which can shorten the apparent fish length, the deterministic model would maximize the blade strike risk.
Strike probability was given by:
Deng et al. (2007) defined “critical passage time” as the time between sweeps of two successive blades. Considering the time a fish needs to pass safely through the plane of the leading edges of the runner blades, Deng et al. (2007) introduced a stochastic blade strike model evaluating the validity of using blade strike modeling as an estimate of the biological performance of several large Kaplan turbines. The blade strike model used here for Francis turbines is derived following Deng et al. (2007), to approximate strike probabilities for the PHES. First, the surface of the imaginary cylinder of a Francis turbine wicket gate exit was calculated (Figure 6):
where Rwgc is the radius of the imaginary cylinder of the wicket gate exit and hwgis the wicket gate height. For the proposed PHES turbine, the turbine runner leading edge cylinder is adjacent to the imaginary cylinder of the wicket gate exit due to its high head (>600 m) and low specific speed, so
where R1 is the turbine runner leading edge radius. Radial velocity at the wicket exit is
Assuming that the angle of a fish at the wicket exit is the same as the wicket gate opening angle θ, then the time a fish needs to safely pass through the imaginary cylinder of the turbine runner leading edges is
where l is the fish length.
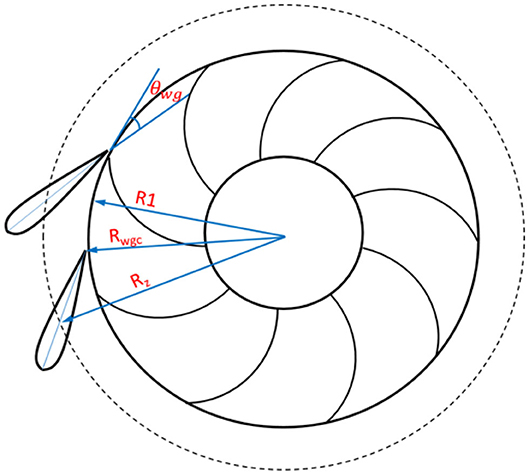
Figure 6. Schematic showing the wicket gate angle and radius of the imaginary cylinder of the wicket gate exit.
The “critical passage time” tcr, is the time between sweeps of two successive blades expressed as:
where n is the number of runner blades and N is the runner speed in revolutions per minute (RPM).
A fish will be struck by a turbine blade if it does not pass through the imaginary cylinder of the turbine runner leading edges within the “critical passage time” tcr, so the probability of blade strike can be expressed as:
For the current simulation, because of the high rotation speed of the Francis turbine and lack of experimental data on blade strike injury for gambusia, the approach taken was to consider the worst case scenario and it was assumed that all fish struck by a blade will be mortally injured.
Deterministic Model
A deterministic model was applied to gambusia at three given turbine discharges: minimum, mid-point and maximum flow. The corresponding wicket gate opening angles were 13°, 20°, and 21°, respectively. A deterministic model lacks probability, that is, it predicts a single unique estimate for each combination of input values. Since fish orientation relative to the flow direction ranges from 0° to 90°, the arithmetic mean of 45° was used as the relative orientation of a fish to the flow direction, and the apparent length of a fish (see Table 1 for fish sizes) was calculated as fish length*cos (45°). In this deterministic model, blade strike predictions were a function of fish length and radial injection location, runner geometry, runner rotation rate, and axial flow.
Stochastic Model
Stochastic models use randomized data based on the specific distribution for each independent variable used as parameter inputs (Deng et al., 2007). The stochastic version of the model was implemented using @RISK software (the Palisade Corporation, Ithaca, New York). The software allows users to define distributions for any or all independent variables so that the variation in variable values is propagated through calculations and is reflected in model predictions. The Monte Carlo simulation method and 10,000 realizations were used for all analyses. In this analysis, three variables were assigned distributions of possible values in simulations: wicket gate angle, fish size, and fish orientation relative to the flow direction. Wicket gate angle was assigned a uniform distribution ranging from the minimum flow angle to the maximum flow angle. The discharge was linear interpolated using the given flow information. Fish size was assigned a normal distribution. Fish orientation relative to flow direction was assigned a uniform distribution from 0° to 90°. Sensitivity and scenario analysis reports were performed using the @RISK software to identify the input distributions most critical to the predicted results. The higher the regression coefficient between the input and the output, the more significant the input is in determining the value of the output.
Results
Shear Experiments
There was a significant relationship between strain rate and survival of gambusia (χ2 = 21.7, df = 1, p < 0.0001). At strain rates of about 1,000 s−1, the survival of gambusia decreased by 16% (Odds Ratio = 0.998, Figure 7). At the maximum strain rate tested (1,853 s−1), there was still an estimated 80% survival.
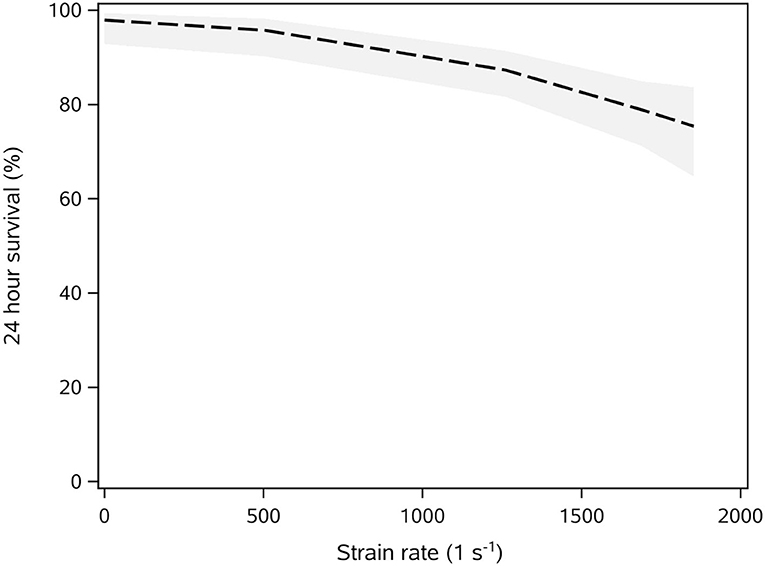
Figure 7. Relationship between shear strain rate and the probability of survival of adult gambusia (after 24 h) determined from a binary logistic regression analysis (logit link). See Table 4 for a summary of the experimental replicates for each shear strain tested. The line is predicted survival ±95% confidence interval (gray shading). Note the maximum strain included on the model was 1,853 (s−1).
Pressure Experiments
Results for adult gambusia exposed to the pressure change profile with travel time equivalent to all six turbines operating at full capacity showed a 100% survival rate after 24 h in both the control and pressure exposure test groups.
Blade Strike Modeling
Using a deterministic model that included turbine discharge and fish length, the probability of blade strike for gambusia ranged between 2.4 and 6.1%, with overall survival estimates ranging from 93.9 to 97.6% (Table 5). Smaller gambusia (20 mm) had similar blade strike probability under all modeled flow scenarios (2.4–2.6%). Larger gambusia (47 mm) had the highest probability of being struck by a blade with 5.7% at both minimum and maximum flow, and 6.1% at mid-flow. The blade strike probability from the stochastic model ranged from 0.2 to 8.3%, with the mean value at 2.9%. The sensitivity analysis for the stochastic model showed that the standardized regression coefficient (r) for fish size was 1.00, indicating that fish size contributed more significantly to the probability of blade strike than wicket gate angle (r = 0.02).
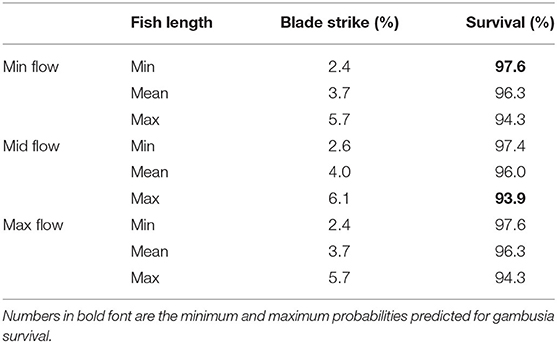
Table 5. Predictions of the likelihood of a blade strike for gambusia and associated survival (%) through a Francis turbine using a deterministic model for a range of discharges [minimum, mid-point and maximum flow] and fish lengths (TL; minimum 20.0 mm, mean 30.5 mm, maximum 47.0 mm).
Discussion
Most adult gambusia survived elevated fluid shear, extremely high pressurization, and most were predicted unlikely to be struck by a turbine blade. Therefore, there is a high possibility that the majority of adult gambusia will survive passage through the 2,000 MW PHES scheme during the pumping phase if entrained in the turbines.
Shear Stress
The majority of gambusia survived exposure to all shear strain treatments tested, even at the maximum rates tested. Elevated levels of shear are known to be harmful to other fish species (Neitzel et al., 2000) and mortality thresholds have been established under similar laboratory conditions for several species in the USA, such as salmonids [e.g., juvenile rainbow trout and steelhead (Oncorhynchus mykiss), juvenile Spring and Fall Chinook salmon (O. tshawytscha)], and American shad (Alosa sapidissima) (Johnson, 1970; Neitzel et al., 2004); and for Mekong species [e.g., blue gourami (Trichopodus trichopterus) and iridescent shark (Pangasianodon hypophthalmus) Colotelo et al., 2018]. Mortality thresholds for these species generally ranged between 800 and 1,200 s−1, with blue gourami being the least tolerant (852 s−1) (Colotelo et al., 2018) and steelhead being the most tolerant [i.e., having no reported mortality at the highest strain tested (≥1,008 s−1)] (Neitzel et al., 2004). In comparison to these species, our results indicate that gambusia are a relatively tolerant species to shear strain, owing perhaps to their small size and body morphology (Neitzel et al., 2000). Our results from the shear experiments and other similar laboratory studies (e.g., Neitzel et al., 2004; Deng et al., 2005, 2010; Colotelo et al., 2018) have broadened our understanding of the effects of shear forces on a variety of fishes and indicate that there are species-specific responses, which generally relate to fish body morphology sensitivities. What is consistent from these other studies and the present study is, however, the probability of survival decreases with the severity of shear forces.
Naturally occurring fluid shear in freshwater ecosystems is generally below 200 s−1, and aquatic organisms have developed adaptations to these levels (Vogel, 1994; Neitzel et al., 2000). PHES schemes create situations of extremely elevated and potentially lethal levels of shear that can occur in areas such as in a boundary layer near turbine structures including the stay vane, wicket gate, and runner blade leading edge (Neitzel et al., 2004). However, a large proportion of the total number of fish passing through the PHES may not be exposed to lethal or injurious strain rates (Neitzel et al., 2004; Cada et al., 2006). Computational fluid dynamics (CFD) models can be applied to fully understand the shear environment that a fish will encounter during passage and to quantify the proportion of fish survival through the PHES scheme. While our laboratory study looks at a simplistic single exposure, in reality, fish may be exposed to multiple events that could be more likely to kill them. Thus, our simulation may overestimate gambusia survival following shear exposure in the PHES scheme. However, computational modeling for conventional hydroturbines indicates that levels exceeding 1,000 s−1 occur in <10% of the overall draft tube area (McEwan and Scobie, 1992). Therefore, the maximum shear values tested in the present study were “extreme” and many fish may not be exposed to levels this high, depending on the path they take through the turbine.
Pressurization
Most adult gambusia survived the extreme and rapid pressurization to be expected during passage through the pumping phase of the PHES scheme, and at levels not tested on fish before. This was necessary to simulate the conditions that are likely to be experienced in PHES schemes. The results were similar to those observed by others previously for lower pressure exposures. Redfin perch (Perca fluviatilis), grayling (Thymallus thymallus), carp (Cyprinus carpio), roach (Leuciscus rutilus), whitefish (Coregoma sp.), and rainbow trout all had high survival to a rapid increase in pressure up to ~5,070 kPa (Lampert, 1976). The direct effects of high pressures on fish have been observed to cause immobilization during pressurization, with recovery immediately after pressure is released (Rowley, 1955). Such immobilization was observed in the gambusia tested, along with erratic swimming. Similar to previous studies, normal swimming behavior resumed shortly after exposure. Thus, rapid compression is unlikely to contribute to high mortality in gambusia.
Blade Strike
Considering the operating scenario tested in the present study, and based on deterministic modeling of blade strike effects, the probability of a fish being struck by a blade was low and the associated survival rate of fish was expected to be high (>93%). It is also believed that this may be an underestimate of the probability of survival since the model predictions were based on the conservative assumption that all blade strikes would lead to mortality (i.e., the empirical factor “mutilation ratio” was set at 100%) (Von Raben, 1957).
Other studies have similarly estimated blade strikes from models, but generally under different turbine geometries. Nonetheless, with careful comparisons, they do identify a similar trend of low mortality (high survival). For example, the findings from the present study are consistent with strike mortality estimated from a Francis turbine at the Stornorrfors Dam on the Ume River (Sweden), ranging from 5.3 to 9.7% for juvenile salmon and trout but less than that for adult salmon and trout (ranging between 25.2 and 45.3%) (Ferguson et al., 2008). Similarly the mortality from blade strike at a large Kaplan turbine at Bonneville Dam on the Columbia River (USA) ranged from 3.4 to 4.3% for juvenile salmon (Deng et al., 2007).
The present study is unique in that it has modeled the survival of one of the smallest fish to date. Our results are consistent with the general notion that blade strike is related to overall fish size; the smaller the fish, the less likely it will be struck by a rotating blade (van Esch, 2012; Romero-Gomez and Richmond, 2014; van Esch and Spierts, 2014). The quantitative survival predictions for small fish are likely to be further underestimated owing to drag-vs-inertia effects where their large surface area to mass ratio may help them to be pulled around the blade rather than colliding with it. While it is acknowledged that turbine blade strike impacts should be empirically assessed to validate the modeling predictions presented in this study, this requires a functioning pumped hydroelectric simulator, at a scale close to a PHES turbine. This work could be extended to assess the percentage of struck fish that end up dying (i.e., the mutilation rate).
Although the shear, blade strike, and/or pressure stressors related to turbine passage may not be immediately lethal, fish may die from secondary effects such as physical injuries (e.g., an abrasion or hemorrhage) or later succumb to the effects of the injury or disease (Cada, 2001; Neitzel et al., 2004). Swimming impairments such as disorientation, loss of equilibrium and erratic swimming can leave fish susceptible to predation immediately following hydroelectric passage (Walker et al., 2016). For example, (Groves, 1972) reported that fish exposed to a shear environment were disoriented but regained “normal capacities” within 5–30 min. Similarly, Neitzel et al. (2000) subjected rainbow trout to predators after they were exposed to strain rates of 688 s−1 and found they were more susceptible to predation. Collectively these data suggest that exposure to strain rates causing disorientation could result in indirect mortality. During compression experiments, we observed that fish quickly became negatively buoyant (i.e., sank) once under pressure and therefore these fish would generally be unable to maintain their position in the water column whilst being transported to the turbine. They would likely contact continuously through the tunnel wall but the stress and impacts of such passage could not be tested within the current study.
Our independent assessments were designed to simulate some of the physical conditions encountered by gambusia as they pass through a PHES, allowing us to isolate specific factors that may influence fish survival. The specifications and combination of experiments provided here are one step toward assessing the risk of species transfer through the PHES scheme prior to its construction. We acknowledge our study is limited by the assumption that a fish subjected to one of these stressors (e.g., shear) does not become more susceptible to being negatively impacted by other hydroelectric-related stressors (e.g., blade strike and/or pressure changes or even multiple sources of shear during passage). For example, a fish that has become disorientated following exposure to shear stress could become more vulnerable to blade strike. It may well be that a fish exposed to shear, blade strike or pressure could be injured or stressed (Coutant and Whitney, 2000) and the cumulative and combined exposure to multiple stressors could result in reduced survival. One study investigated the component sources of entrainment mortality for juvenile Gambusia affinis using a nuclear power plant simulator, by exposing the fish to different combinations of pump speed and water temperatures (Cada et al., 1980). While the stressors were different to what we tested, their study showed that although a single factor (e.g., thermal shock or shear forces) may not have a major effect on entrained fishes, its influence may still be exerted through interaction with other stress factors that are a part of the entrainment experience. In any case, G. affinis suffered relatively low mortalities, even when combined with thermal shocks of 10°C above ambient temperatures (Cada et al., 1980). Gambusia survival may be lower due to interacting hydraulic variables in a PHES scheme, and these warrant empirical testing. Nonetheless, gambusia did have high survival for the individual stressors tested in the present study (shear strain, extreme pressurization and blade strike) and can potentially survive multiple stressors (Cada et al., 1980), thus a precautionary approach with regard to management measures should be applied.
The present study focuses on passage survival and was conducted based on the assumption that adult gambusia will become entrained in the PHES scheme. For a more complete assessment of the likelihood that a fish species will establish a new population with the facilitation of a PHES scheme, there are three key components: (1) entrainment risk, including the location of the intake relative to the target species' habitat (Huang et al., 2015; Langford et al., 2016), (2) passage survival through the PHES scheme, and (3) suitable habitat in the new environment (in the present case, the upper reservoir). The susceptibility of a particular species to entrainment can be examined using a range of approaches, including using existing knowledge of species ecology and habitat requirements, obtaining empirical data on the spatio-temporal distribution and swimming ability/rheotactic response of the target species, using computational fluid dynamics (CFD) modeling around the intake (Coutant and Whitney, 2000; Przybilla et al., 2010), laboratory and field experiments (Mussen et al., 2013, 2014), and acoustic telemetry (Stuart et al., 2010; Martins et al., 2014) or underwater cameras (Silva et al., 2018). To predict whether the fish species will establish a population in the new environment following survival through a PHES, screening tools that incorporate species life history and environmental tolerances can be applied (Kolar, 2004). Furthermore, the present study could be repeated to include a survival assessment of the early life stages of gambusia, as they are also a potential mechanism of dispersal.
Conclusion
Gambusia had high survival when exposed to individual stressors tested in the present study (shear strain, extreme pressurization and blade strike). Thus, there is more than a marginal risk that gambusia will survive passage to potentially colonize the upper reservoir of a PHES scheme, should they be entrained at the intake. Where a new PHES scheme poses the risk of inadvertently expanding the range of gambusia or similar poeciliid species, measures to minimize their spread or mitigate their ecosystem impacts should be considered. Prior to the consideration of any mitigation or management measures, however, an assessment of their applicability to the site and species of interest is required, followed by experimentation using both laboratory and field conditions, if warranted. Ideally, these measures should be a key consideration during the design and environmental planning phase of a PHES development.
Data Availability Statement
The datasets presented in this article are not readily available. Certain datasets generated for this study may be available on request to the corresponding author. Data associated with confidential information relating to specific design of the turbines has not been made public and may not be released. Requests to access the datasets should be directed to Katherine Doyle, a2Fkb3lsZUBjc3UuZWR1LmF1.
Ethics Statement
The animal study was reviewed and approved by Charles Sturt University Animal Care and Ethics.
Author Contributions
LS, LB, and CB conceived the concept of the study. LB and CB were responsible for the design of the shear testing facilities. JdP designed and built the pressure chamber. LS, LB, CB, NN, and KD designed the experimental procedures. KD, NN, LS, EB, and LB conducted the experiments. KD, NN, LS, EB, LB, and CB wrote and reviewed the manuscript. WR conducted the statistical analysis. ZD and TF developed and generated the blade strike models. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Snowy Hydro Limited with additional support from New South Wales Department of Primary Industries and Charles Sturt University. Open access publication fees were provided through a competitive grant process from the Institute for Land, Water and Society, Charles Sturt University with support from Snowy Hydro Limited. The work gives an independent and scientific account of potential upgrade works.
Conflict of Interest
JdP was employed by the company JAD Systems LLC. LB is a guest editor on the research topic Balancing Hydropower and Freshwater Environments in the Global South.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge Geoff Gibson and Mark Evans (both from CSU Department of Facilities Management) who assisted with the setup, maintenance, and transportation of the experimental apparatus including raising additional funds to complete the establishment of the fish laboratory. Dr. Brett Pflugrath (Pacific Northwest National Laboratory) assisted with an initial literature review of pressure effects on fish and later Dr. Miao Li (CSU Faculty of Business, Justice and Behavioral Sciences) provided advice regarding calibration of the shear flume. We also thank Cameron McGregor and Jarrod McPherson for setting up the hatchery and experimental apparatus, and Anthony Fowler, Isabelle Thiebaud, and C. McGregor for fish collection. All work was conducted in accordance with Charles Sturt University Animal Care and Ethics Permits A18055 and A18015; the NSW Group Biosecurity Permit OUT18/8784 and NSW Individual Biosecurity Permit OUT18/14635; and the NSW Scientific Collection Permit P01/0059(A)-3.0.
References
Belaud, A., and Barthelemy, L. (1973). Effects of hydrostatic pressure (31 to 101 ATA) on eels (Anguilla anguilla L.). IRCS Cardiovas. Syst. 73:11.
Boys, C. A., Navarro, A., Robinson, W., Fowler, A., Chilcott, B., Miller, B., et al. (2014). Downstream Fish Passage Criteria for Hydropower and Irrigation Infrastructure in the Murray-Darling Basin. Fisheries Final Report Series. NSW DPI Fisheries, Nelson Bay, NSW, 117.
Boys, C. A., Pflugrath, B., Mueller, M., Pander, J., Deng, Z. D., and Geist, J. (2018). Physical and hydraulic forces experienced by fish passing through three different low-head hydropower turbines. Mar. Freshwater Res. 69, 1934–1944. doi: 10.1071/MF18100
Boys, C. A., Robinson, W., Miller, B., Pflugrath, B., Baumgartner, L. J., Navarro, A., et al. (2016a). A piecewise regression approach for determining biologically relevant hydraulic thresholds for the protection of fishes at river infrastructure. J. Fish Biol. 88, 1677–1692. doi: 10.1111/jfb.12910
Boys, C. A., Robinson, W., Miller, B., Pflugrath, B., Baumgartner, L. J., Navarro, A., et al. (2016b). How low can they go when going with the flow? Tolerance of egg and larval fishes to rapid decompression. Biol. Open 5, 786–793. doi: 10.1242/bio.017491
Brown, R. S., Carlson, T. J., Gingerich, A. J., Stephenson, J. R., Pflugrath, B. D., Welch, A. E., et al. (2012a). Quantifying mortal injury of juvenile chinook salmon exposed to simulated hydro-turbine passage. T. Am. Fish. Soc. 141, 147–157. doi: 10.1080/00028487.2011.650274
Brown, R. S., Pflugrath, B. D., Colotelo, A. H., Brauner, C. J., Carlson, T. J., Deng, Z. D., et al. (2012b). Pathways of barotrauma in juvenile salmonids exposed to simulated hydroturbine passage: Boyle's law vs. Henry's law. Fisher. Res. 121, 43–50. doi: 10.1016/j.fishres.2012.01.006
Cada, G. F. (2001). The development of advanced hydroelectric turbines to improve fish passage survival. Fisheries 26, 14–23. doi: 10.1577/1548-8446(2001)026<0014:TDOAHT>2.0.CO;2
Cada, G. F., Loar, J., Garrison, L., Fisher, R., and Neitzel, D. (2006). Efforts to reduce mortality to hydroelectric turbine-passed fish: locating and quantifying damaging shear stresses. Environ. Manage. 37, 898–906. doi: 10.1007/s00267-005-0061-1
Cada, G. F., Suffern, J. S., Kumar, K. D., and Solomon, J. A. (1980). “Investigations of entrainment mortality among larval and juvenile fishes using a power plant simulator,” in National Workshop on Entrainment and Impingement (San Francisco, CA), 30.
Carmona-Catot, G., Magellan, K., and Garcia-Berthou, E. (2013). Temperature-specific competition between invasive mosquitofish and an endangered cyprinodontid fish. PLoS ONE 8:e54734. doi: 10.1371/journal.pone.0054734
Colotelo, A. H., Mueller, R. P., Harnish, R. A., Martinez, J. J., Phommavong, T., Phommachanh, K., et al. (2018). Injury and mortality of two mekong river species exposed to turbulent shear forces. Mar. Freshwater Res. 69, 1945–1953. doi: 10.1071/MF18126
Coutant, C. C., and Whitney, R. R. (2000). Fish behavior in relation to passage through hydropower turbines: a review. T. Am. Fish. Soc. 129, 351–380. doi: 10.1577/1548-8659(2000)129<0351:FBIRTP>2.0.CO;2
Davies, B. R., Thoms, M., and Meador, M. (1992). An assessment of the ecological impacts of inter-basin water transfers, and their threats to river basin integrity and conservation. Aquat. Conserv. 2, 325–349. doi: 10.1002/aqc.3270020404
Deane, J. P., Gallachóir, B. Ó., and McKeogh, E. (2010). Techno-economic review of existing and new pumped hydro energy storage plant. Renew. Sust. Energ. Rev. 14, 1293–1302. doi: 10.1016/j.rser.2009.11.015
Deng, Z. D., Carlson, T. J., Ploskey, G. R., Richmond, M. C., and Dauble, D. D. (2007). Evaluation of blade-strike models for estimating the biological performance of kaplan turbines. Ecol. Model. 208, 165–176. doi: 10.1016/j.ecolmodel.2007.05.019
Deng, Z. D., Guensch, G. R., McKinstry, C. A., Mueller, R. P., Dauble, D. D., and Richmond, M. C. (2005). Evaluation of fish-injury mechanisms during exposure to turbulent shear flow. Can. J. Fish Aquat. Sci. 62, 1513–1522. doi: 10.1139/f05-091
Deng, Z. D., Mueller, R. P., Richmond, M. C., and Johnson, G. E. (2010). Injury and mortality of juvenile salmon entrained in a submerged jet entering still water. N. Am. J. Fish. Manage. 20, 623–628. doi: 10.1577/M09-153.1
Ferguson, J. W., Ploskey, G. R., Leonardsson, K., Zabel, R. W., and Lundqvist, H. (2008). Combining turbine blade-strike and life cycle models to assess mitigation strategies for fish passing dams. Can. J. Fish. Aquat. Sci. 65, 1568–1585. doi: 10.1139/F08-078
Fu, T., Deng, Z. D., Duncan, J. P., Zhou, D., Carlson, T. J., Johnson, G. E., et al. (2016). Assessing hydraulic conditions through Francis turbines using an autonomous sensor device. Renew. Energ. 99, 1244–1253. doi: 10.1016/j.renene.2016.08.029
Gallardo, B., Clavero, M., Sánchez, M. I., and Vilá, M. (2016). Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biol. 22, 151–163. doi: 10.1111/gcb.13004
García-Berthou, E., Alcaraz, C., Pou-Rovira, Q., Zamora, L., Coenders, G., and Feo, C. (2005). Introduction pathways and establishment rates of invasive aquatic species in Europe. Can. J. Fish. Aquat. Sci. 62, 453–463. doi: 10.1139/f05-017
Groves, A. B. (1972). Effects of Hydraulic Shearing Actions on Juvenile Salmon (Summary Report). Seattle, WA: National Marine Fisheries Service, Northwest Fisheries Center.
Harby, A., Sauterleute, J., Korpås, M., Killingtveit, Å., Solvang, E., and Nielsen, T. (2013). “Pumped storage hydropower,” in Transition to Renewable Energy Systems, eds D. Stolten, and V. Scherer (John Wiley & Sons), 1008.
Hauck, F. R., and Edson, Q. A. (1976). Pumped storage: it's significance as an energy source and some biological ramifications. T. Am. Fish. Soc. 105, 158–164. doi: 10.1577/1548-8659(1976)105<158:PS>2.0.CO;2
Huang, B., Zhu, D. Z., Shao, W., Fu, J., and Rui, J. (2015). Forebay hydraulics and fish entrainment risk assessment upstream of a high dam in China. J. Hydro Environ. Res. 9, 91–103. doi: 10.1016/j.jher.2014.03.002
Johnson, R. L. (1970). Fingerling Fish Mortalities at 57.5 fps. Report 22. Portland, OR: U.S. Army Corps of Engineers, North Pacific Division.
Kolar, C. (2004). Risk assessment and screening for potentially invasive fishes. New Zeal. J. Mar. Fresh. 38, 391–397. doi: 10.1080/00288330.2004.9517247
Lampert, W. (1976). Experiments on the resistance of fish to rapid increase in hydrostatic pressure. J. Fish Biol. 8, 381–383. doi: 10.1111/j.1095-8649.1976.tb03966.x
Langford, M. T., Zhu, D. Z., and Leake, A. (2016). Upstream hydraulics of a run-of-the-river hydropower facility for fish entrainment risk assessment. J. Hydraul. Eng. 142:05015006. doi: 10.1061/(ASCE)HY.1943-7900.0001101
MacDonald, J. I., Tonkin, Z. D., Ramsey, D. S., Kaus, A. K., King, A. K., and Crook, D. A. (2012). Do invasive eastern gambusia (Gambusia holbrooki) shape wetland fish assemblage structure in south-eastern Australia? Mar. Freshwater Res. 63, 659–671. doi: 10.1071/MF12019
Martins, E. G., Gutowsky, L. F. G., Harrison, P. M., Flemming, J. E. M., Jonsen, I. D., Zhu, D. Z., et al. (2014). Behavioural attributes of turbine entrainment risk for adult resident fish revealed by acoustic telemetry and state-space modelling. Anim. Biotelem. 2, 1–13. doi: 10.1186/2050-3385-2-13
McEwan, D., and Scobie, G. (1992). Estimation of the Hydraulic Conditions Relating to Fish Passage Through Turbines. Glasgow: Report to National Engineering Laboratory.
Mussen, T. D., Cocherell, D., Hockett, Z., Ercan, A., Bandeh, H., Kavvas, M. L., et al. (2013). Assessing juvenile chinook salmon behaviour and entrainment risk near unscreened water diversions: large flume simulations. T. Am. Fish. Soc. 142, 130–142. doi: 10.1080/00028487.2012.720633
Mussen, T. D., Cocherell, D., Poletto, J. B., Reardon, J. S., Hocket, Z., Ercan, A., et al. (2014). Unscreened water-diversion pipes pose an entrainment risk to the threatened green sturgeon, Acipenser medirostris. PLoS ONE 9:e86321. doi: 10.1371/journal.pone.0086321
Navarro, A., Boys, C. A., Robinson, W., Baumgartner, L. J., Miller, B., Deng, Z. D., et al. (2019). Tolerable ranges of fluid shear for early life-stage fishes: implications for safe fish passage at hydropower and irrigation infrastructure. Mar. Freshwater Res. 70, 1503–1512. doi: 10.1071/MF18131
Neitzel, D. A., Dauble, D. D., Cada, G. F., Richmond, M. C., Guensch, G. R., Mueller, R.P., et al. (2004). Survival estimates for juvenile fish subjected to a laboratory-generated shear environment. T. Am. Fish. Soc. 133, 447–454. doi: 10.1577/02-021
Neitzel, D. A., Richmond, M. C., Dauble, D. D., Mueller, R. P., Moursund, R. A., Abernethy, C. S., et al. (2000). Laboratory Studies on the Effects of Shear on Fish. Richland: Pacific Northwest National Laboratory.
Pflugrath, B. D., Boys, C. A., and Cathers, B. (2018). Predicting hydraulic structure-induced barotrauma in Australian fish species. Mar. Freshwater Res. 69, 1954–1961. doi: 10.1071/MF18137
Przybilla, A., Kunze, S., Rudert, A., Bleckmann, H., and Brücker, C. (2010). Entraining in trout: a behavioural and hydrodynamic analysis. J. Exp. Biol. 213, 2976–2986. doi: 10.1242/jeb.041632
Romero-Gomez, P., and Richmond, M. C. (2014). Simulating blade-strike on fish passing through marine hydrokinetic turbines. Renew. Energ. 71, 401–413. doi: 10.1016/j.renene.2014.05.051
Rowe, D. K., Moore, A., Giorgetti, A., Maclean, C., Grace, P., Wadhwa, S., et al. (2008). Review of the Impacts of Gambusia, Redfin Perch, Tench, Roach, Yellowfin Goby and Streaked Goby in Australia. Prepared for the Australian Government Department of the Environment, Water, Heritage and the Arts. Available online at: www.environment.gov.au/biodiversity/publications/index.html
Sebert, P., and MacDonald, A. G. (1993). “Fish,” in Effects of High Pressure on Biological Systems, eds R. E. Marquis, A. G. MacDonald, A. C. Hall, D. M. Pickles, J. J. Kendig, Y. Grossman, et al. (Berlin: Springer Verlag), 147–196.
Silva, L. G. M., Beirão, B. V., Falcão, R. C., de Castro, A. L., and Dias, E. W. (2018). It's a catfish! Novel approaches are needed to study the effects of rapid decompression on benthic species. Mar. Freshwater Res. 69, 1922–1933. doi: 10.1071/MF18267
Strayer, D. L. (2010). Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biol. 55, 152–174. doi: 10.1111/j.1365-2427.2009.02380.x
Stuart, I. G., Koehn, J. D., O'Brien, T. A., McKenzie, J. A., and Quinn, G. (2010). Too close for comfort: a fishway exit and a hydro-power station inlet. Mar. Freshwater Res. 61, 23–33. doi: 10.1071/MF08340
Turnpenney, A., Clough, S., Hanson, K., Ramsey, R., and McEwan, D. (2000). Risk Assessment for Fish Passage Through Small, Low-Head Turbines. Fawley Aquatic; Department of Trade and Industry: London.
van Esch, B. P. M. (2012). Fish injury and mortality during passage through pumping stations. J. Fluid Eng. 134:071302. doi: 10.1115/1.4006808
van Esch, B. P. M., and Spierts, I. L. Y. (2014). Validation of a model to predict fish passage mortality in pumping stations. Can. J. Fish. Aquat. Sci. 71, 1910–1923. doi: 10.1139/cjfas-2014-0035
Vogel, S. (1994). Life in Moving Fluids: The Physical Biology of Flow, 2nd Edn. Princeton, NJ: Princeton University Press.
Von Raben, K. (1957). Regarding the problem of mutilations of fishes by hydraulic turbines. Die Wasserwirtschaft 4, 97–100.
Walker, R. W., Ashton, N. K., Brown, R. S., Liss, S. A., Colotelo, A. H., Beirão, B. V., et al. (2016). Effects of a novel acoustic transmitter on swimming performance and predator avoidance of juvenile chinook salmon: determination of a size threshold. Fish. Res. 176, 48–54. doi: 10.1016/j.fishres.2015.12.007
Keywords: pumped hydropower, renewable energy, invasive species, Francis turbine, pressure, shear strain, mosquito fish (Gambusia spp.)
Citation: Doyle KE, Ning N, Silva LGM, Brambilla EM, Boys CA, Deng ZD, Fu T, du Preez JA, Robinson W and Baumgartner LJ (2020) Gambusia holbrooki Survive Shear Stress, Pressurization and Avoid Blade Strike in a Simulated Pumped Hydroelectric Scheme. Front. Environ. Sci. 8:563654. doi: 10.3389/fenvs.2020.563654
Received: 19 May 2020; Accepted: 30 September 2020;
Published: 23 October 2020.
Edited by:
José Francisco Gonçalves Júnior, University of Brasilia, BrazilReviewed by:
Alban Kuriqi, Instituto Superior Técnico, PortugalCarlos Bernardo Mascarenhas Alves, Bio-Ambiental Consultoria, Brazil
Copyright © 2020 Doyle, Ning, Silva, Brambilla, Boys, Deng, Fu, du Preez, Robinson and Baumgartner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine E. Doyle, a2Fkb3lsZUBjc3UuZWR1LmF1
 Katherine E. Doyle
Katherine E. Doyle Nathan Ning
Nathan Ning Luiz G. M. Silva
Luiz G. M. Silva Eduardo M. Brambilla
Eduardo M. Brambilla Craig A. Boys1,4
Craig A. Boys1,4 Z. Daniel Deng
Z. Daniel Deng Tao Fu
Tao Fu Jan A. du Preez
Jan A. du Preez Wayne Robinson
Wayne Robinson Lee J. Baumgartner
Lee J. Baumgartner