Abstract
Humans have fundamentally altered the cycling of multiple elements on a global scale. These changes impact the structure and function of terrestrial and aquatic ecosystems, with many implications for human health. Most prior studies linking biogeochemical changes to human health have evaluated the effects of single elements in isolation. However, the relative availability of multiple elements often determines the biological impact of shifts in the concentration of a single element. The balance of multiple elements is the focus of ecological stoichiometry, which highlights the importance of elemental ratios in biological function across all systems and scales of organization. Consequently, ecological stoichiometry is a promising framework to inform research on the links between global changes to elemental cycles and human health. We synthesize evidence that elemental ratios link global change with human health through biological processes occurring at two scales: in the environment (natural ecosystems and food systems) and within the human body. Elemental ratios in the environment impact the key ecosystem processes of productivity and biodiversity, both of which contribute to the production of food, toxins, allergens, and parasites. Elemental ratios in diet impact processes within the human body, including the function and interactions of the immune system, parasites, and the non-pathogenic microbiome. Collectively, these stoichiometric effects contribute to a wide range of non-infectious and infectious diseases. By describing stoichiometric mechanisms linking global change, ecological processes, and human health, we hope to inspire future empirical and theoretical research on this theme.
Introduction
Understanding the effects of global change on human health is a major challenge for the twenty-first century (Leaf, 1989; Whitmee et al., 2015). Anthropogenic activities drive substantial shifts in biogeochemical cycles in ecological systems throughout the world (Peñuelas et al., 2013), many of which can impact human health. For example, application of nitrogen (N) fertilizer directly improves crop yields and reduces malnutrition but indirectly contributes to the prevalence and severity of multiple infectious and non-infectious diseases (Townsend et al., 2003). Similarly, increases in atmospheric carbon dioxide (CO2) concentrations driven by fossil fuel combustion reduce the nutritional quality of food crops and other plants, with myriad human health outcomes (Loladze, 2002).
Biogeochemical effects on human health are typically studied from a single-element perspective. However, the cycling of carbon (C), N, and other elements relevant to health are often linked through biogeochemical mechanisms and shared anthropogenic drivers (Peñuelas et al., 2012), and the ratios of these elements in nature are fundamental to biological function (Sterner and Elser, 2002). Therefore, we need a multi-elemental approach to better understand the mechanisms linking anthropogenic effects on biogeochemical cycles with human health. The balance of multiple elements in nature is the focus of ecological stoichiometry (ES), a research framework that highlights the importance of elemental ratios in biological function across all systems and scales of organization (Sterner and Elser, 2002).
Stoichiometric theory has been applied to many fundamental research topics in ecology and evolution (Hessen et al., 2013; Van de Waal et al., 2018). However, ES has been used relatively infrequently in socio-ecological research (but see Ptacnik et al., 2005; Cease et al., 2015) and has been applied to human health in just a few studies (Table 1). Given the central importance of elemental ratios to biological function, we suggest that ES is a powerful tool to study links between global change and multiple dimensions of human health.
Table 1
| Topic | Finding | References |
|---|---|---|
| Food security and nutrition | C:element ratios in staple crops and other plants increase under elevated CO2. These shifts are expected to contribute to deficiencies in protein, vitamins, and micronutrients. | Loladze, 2002, 2014; Zhu et al., 2018 |
| Cancer | The growth of lung and colon tumor cells may be P limited. Cancer tissues have twice as much P and a lower N:P than non-cancerous reference tissues. | Elser et al., 2006, 2007a |
| Infectious disease vectors | Resource ratios in aquatic ecosystems impact the spatial distribution of larval mosquitoes. Larval diet quality impacts rates of Zika-virus infection and transmission in adult mosquitoes. | Murrell et al., 2011; Paige et al., 2019 |
| Environmental toxins | Se:Hg ratios impact the transfer of Hg through aquatic food webs (including to human food sources) and the effects of Hg within the human body. High environmental As:P ratios disrupt ATP formation. | Drasch et al., 1996; Yang et al., 2012; Walters et al., 2015 |
Examples of prior research on ecological stoichiometry in a human health context.
Here we outline how stoichiometric effects on ecological processes (hereafter, “stoichiometric effects”) link anthropogenic activities, ecological function, and human health. We outline stoichiometric effects at two major scales: in the environment (natural ecosystems and food systems) and within the human body. In the environment, anthropogenic activities including fossil fuel combustion, fertilizer application, land use change, and industrial production drive changes to elemental ratios in air, water, and soil. The alteration of these ratios impacts ecosystem productivity and biodiversity, both of which contribute to the production of food, toxins, allergens, and parasites. Stoichiometric constraints on the quantity and quality of food production have implications for food security, and elemental ratios in human diet impact immune function, metabolism, parasite function, and the microbiome. Collectively, these stoichiometric effects contribute to a wide range of non-infectious and infectious diseases (Figure 1).
Figure 1
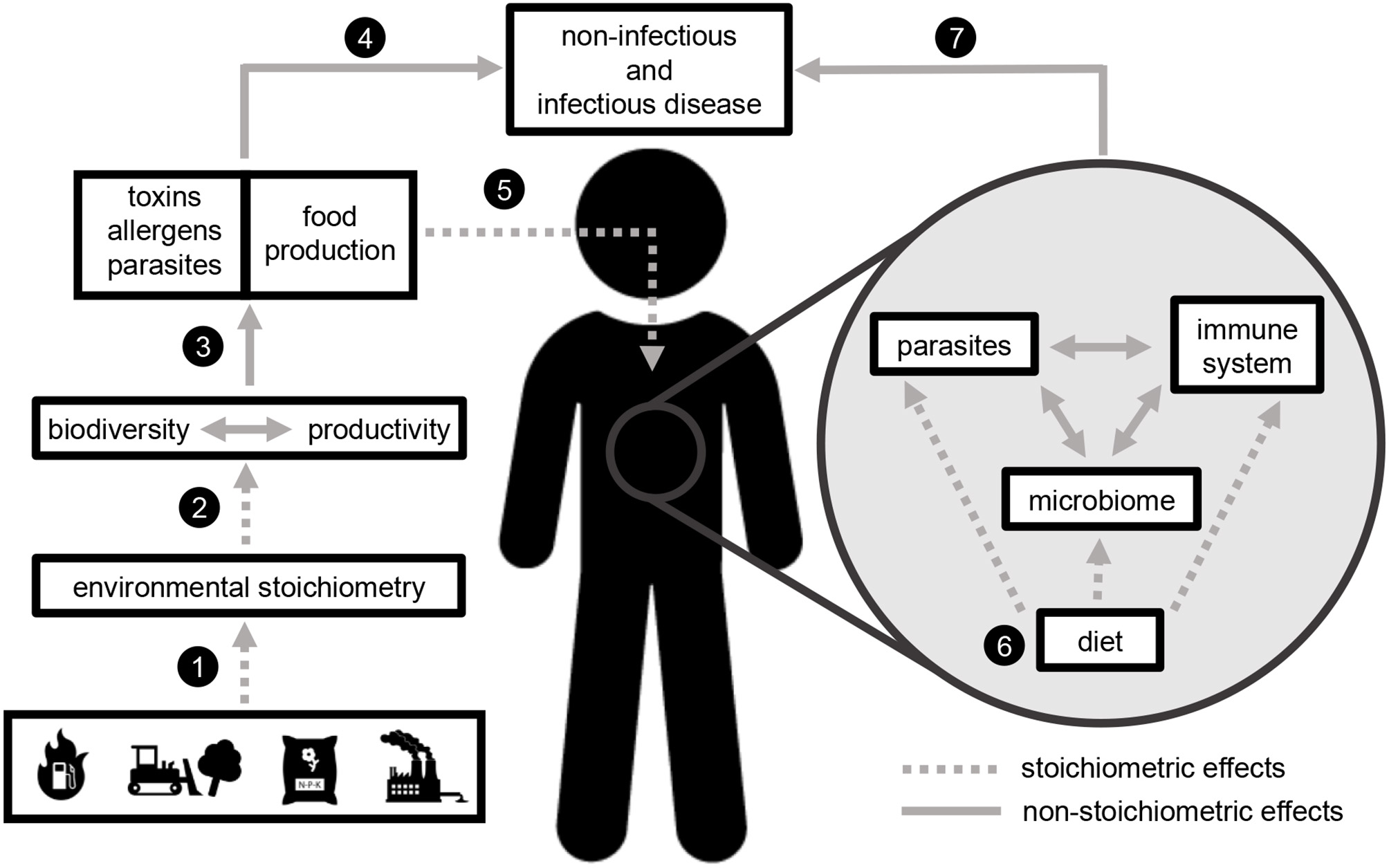
A stoichiometric framework linking global change with human health. Stoichiometric effects (ecological processes mediated by elemental ratios, dashed lines) occur at two scales: in the external environment (to the left of the human figure) and within the human body (gray circle to the right of the human represents internal processes, especially diet-mediated processes within the gut). Global environmental changes (icons represent fossil fuel combustion, land use change, fertilizer application, and industrial pollution) alter the absolute (concentration) and relative (ratios) availability of elements in the environment (1). Environmental stoichiometry impacts the key ecosystem processes of biodiversity and productivity (2). Both of these processes contribute to toxin, allergen, and parasite production (3), all of which can impact human health directly by causing non-infectious and infectious disease (4). Stoichiometric effects on biodiversity and productivity also determine food production in both managed (e.g., agricultural) and unmanaged (e.g., marine and freshwater) systems (3). The quantity and quality of food production can impact human health directly (4), but these impacts may be mediated by stoichiometric effects occurring within the human body (5). Elemental ratios in human diet impact the function of parasites, the non-pathogenic microbiome, and the immune system (6), which collectively influence both non-infectious and infectious disease (7).
By synthesizing evidence that elemental ratios link global change and human health, we hope to inspire future stoichiometric research on this theme. To ensure that this synthesis is accessible to a broad audience, including readers who lack a background in ES, we summarize fundamental aspects of stoichiometric theory (Figure 2) and begin by describing links between human actions, elemental ratios in the environment, and ecological processes that impact human health.
Figure 2
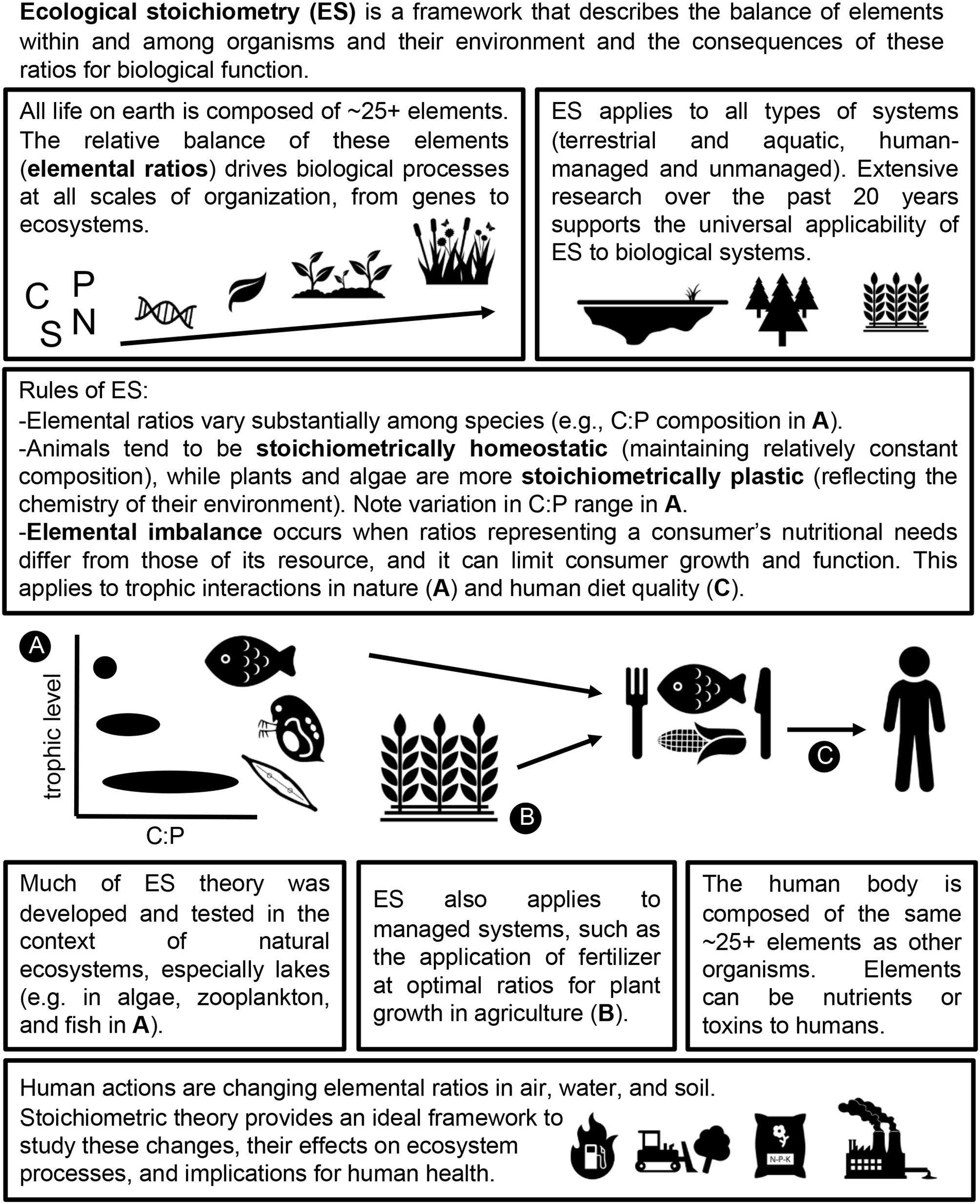
A primer of ecological stoichiometry and an overview of its application to human health.
A Brief Primer on Global Change and Environmental Stoichiometry
All life on earth is composed of ~25 elements (Kaspari and Powers, 2016), each of which has a unique biogeochemical cycle (Schlesinger and Bernhardt, 2013). Human activity has dramatically changed the absolute and relative availabilities of these elements around the world, which can limit the growth and function of organisms in both terrestrial and aquatic environments.
Fossil fuel combustion has increased the concentration of CO2 in the environment globally, as well as other elements found in fossil fuels such as N and sulfur (S) (Smith et al., 2001). Fertilizer application has increased the availability of N and phosphorus (P) in croplands and adjacent landscapes, including aquatic ecosystems. Commercial fertilizer often includes additional elements, such as potassium (K), calcium (Ca), magnesium (Mg), and S that can also be transported via dust, erosion, or water (Kaspari and Powers, 2016). Biomass burning (to clear land) releases C from plant material back to the atmosphere, but it can also enrich local environments with trace elements, especially K (Sardans and Peñuelas, 2015) and zinc (Zn, Echalar et al., 1995). Industrial production has released trace elements into the environment, such as mercury (Hg) and arsenic (As, Adriano, 1986). These changes in the availability of elements in the environment occur at different spatial scales. For example, the elevation of atmospheric C occurs globally, while N enrichment is a more localized or regional phenomenon (Galloway et al., 2008).
Changes to biogeochemical cycles in aquatic ecosystems are complicated by interactions with soils, plants, and microbes along hydrologic pathways. Fossil fuel combustion has led to increased concentrations of dissolved CO2 in aquatic ecosystems, especially oceans, thereby increasing the availability of C relative to other elements (Caldeira and Wickett, 2003). Terrestrial inputs increase the availability of N and P in freshwaters, but P is preferentially retained by soils relative to N. Nitrogen deposition can increase the P-limitation of aquatic ecosystems due to increasing N:P (Elser et al., 2009) but analysis of rivers across the United States shows that the change in N:P is not unidirectional and is influenced by abiotic factors (Dodds and Smith, 2016). Silica (Si) is also increasing in streams and rivers due to increased erosion, but it can be retained by dams (Humborg et al., 1997, 2006; Carey and Fulweiler, 2012).
These shifts in the availability of multiple elements are not occurring in synchrony across space and time, leading to changes in elemental ratios in the environment. We present a non-exhaustive list of human-driven changes to environmental stoichiometry (Table 2) and briefly discuss their effects on ecosystem productivity and patterns in biodiversity, both of which are linked to multiple dimensions of human health.
Table 2
| Elements and drivers | Elemental ratio altered by global change | Ecological consequences | |
|---|---|---|---|
| C↑ |

|
↑ C:element availability in soil | ↑ C:element of plant tissue(1) |
| ↑ C:N of crops(2) ↑ C stimulates methylation of Hg in water(8) |
↑ herbivory due to compensatory feeding(3) ↓ pollination and seed set(4, 5) ↑ pathogen inoculum due to slow litter decomposition(6, 7) ↑ bioaccumulation of Hg in fish |
||
| ↑ C:element of coastal waters (9) | ↑ HABs | ||
| N↑ |



|
↓ C:N of freshwaters | ↑ microcystin toxin production(10) |
| ↓ C:N coastal flux(9) | ↑ HAB toxin production(11) | ||
| ↓ C:N of crops | ↑ pest populations(12, 13) | ||
| ↑ N:P in water |
↓ growth rates of fish(9) ↑ HABs (11) |
||
| ↑ N:P in soils | ↑ P-limitation of plant production(14) | ||
| ↑ N:element in soil and water | ↓ diversity of plant and marine algal communities(15−19) ↑ K-limitation of crops(8) |
||
| P ↑↓ |


|
↑ N:P in constructed waterways (20) | ↑↓ P-limitation impacts HAB toxin production (21−23) |
| ↑ N:P of inputs to agricultural soils (globally)(24) | ↑ P-limitation of crops (24) | ||
| S↑ |

|
↑ S stimulates methylation of Hg in water(25) | ↑ bioaccumulation of Hg in fish |
| Si ↑↓ |

|
↑ N:Si in aquatic ecosystems(26−28); ↑ Si:As in soil(30) |
↓ fish production(9) ↑ HABs (9) ↓ As in rice (9) |
| Hg↑ |

|
↑ Hg:Se increases retention of methyl-Hg(31) | ↑ Hg bioaccumulation fish (31) |
Human drivers of altered elemental ratios in the environment and ecological consequences relevant to human health.
Global change symbols indicate drivers (fossil fuel combustion, fertilizer application, land and water alteration, industrial production). Numbers refer to publications listed at the bottom of the table. These examples highlight how changes to the cycling of a single element impact elemental ratios in the environment.
Loladze (2002);
Sardans and Peñuelas (2012);
Bezemer and Jones (1998);
Rusterholz and Erhardt (1998);
Lake and Hughes (1999);
Thompson et al. (1993);
Coakley et al. (1999);
Selin (2009);
Ptacnik et al. (2005);
Van de Waal et al. (2009);
Van de Waal et al. (2014);
Bobbink et al. (1998);
Cisneros and Godfrey (2001);
Peñuelas et al. (2013);
Tilman (1996);
Tilman (1997);
Bobbink et al. (2010);
Stevens et al. (2010);
Payne et al. (2013);
Maavara et al. (2015);
Flynn (2002);
Granéli and Johansson (2003);
Pan et al. (1996);
Peñuelas et al. (2012);
Gilmour et al. (1992);
Humborg et al. (2006);
Humborg et al. (1997);
Carey and Fulweiler (2012);
Parsons and Dortch (2002);
Zhang et al. (2017);
Bjerregaard et al. (2011).
Ecosystem Productivity
Primary Productivity
The absolute and relative concentrations of elements in the environment commonly limit the primary productivity of both aquatic and terrestrial ecosystems (Elser et al., 2007b). While traditional perspectives on this topic often focus on a single limiting element (sensu Liebig, 1855), recent studies highlight the importance of co-limitation, the simultaneous limitation of primary productivity by multiple elements, across aquatic and terrestrial ecosystems (Elser et al., 2007b; Harpole et al., 2011; Fay et al., 2015). Primary productivity is often synergistically limited by N and P, such that additions of N and P together increase primary productivity beyond the additive effects expected based on individual inputs of these elements (Elser et al., 2007b; Harpole et al., 2011). Given that life is constructed from over 25 elements, it is also crucial that we expand our view of elemental limitation and co-limitation to include elements beyond N and P (Kaspari and Powers, 2016). The relative availability of elements is also important for productivity in managed systems, such as agriculture, where fertilizer application aims to provide the correct balance of N, P, and other elements to maximize crop yield (Van der Velde et al., 2014).
Consumer Productivity
Elemental ratios in primary producers influence how energy and elements move through food webs to fuel consumer productivity at higher trophic levels (Sterner and Elser, 2002). Mismatches between the ratios of elements required for consumer growth and the elemental ratios of their resources lead to elemental imbalance, which can constrain productivity at any trophic level (Boersma et al., 2008). The effects of elemental imbalance on trophic transfer may be exacerbated when interacting organisms differ in their degree of stoichiometric homeostasis (maintaining consistent organismal stoichiometry despite changes to resource stoichiometry) vs. plasticity (shifting organismal stoichiometry to reflect changes in resource stoichiometry) (Meunier et al., 2014). Algae and terrestrial plants show much higher stoichiometric plasticity than herbivores and other consumers, which can result in substantial elemental imbalance and reduce consumer growth efficiency (Boersma, 2000, Demott et al., 2010). Therefore, changes to elemental ratios in the environment can propagate through food webs to impact productivity at all trophic levels.
Biodiversity
The relative availability of elements in the environment can influence the local composition and richness of ecological communities. Primary producers vary among species in growth response to environmental C:N:P, influencing community composition across ecosystems (Agren et al., 2012). For example, both legumes and N-fixing cyanobacteria have a competitive advantage in low N:P environments (Schindler, 1977; Vitousek and Walker, 1989). Stoichiometric effects on the competitive dominance of primary producers may also impact community composition at higher trophic levels (Singer and Battin, 2007), although stoichiometry is often overlooked as a potential driver of biodiversity patterns.
Stoichiometric effects may also contribute to global declines in species richness. For example, N deposition reduces species diversity in grasslands on a continental scale (Stevens et al., 2010). This is likely driven by both non-stoichiometric (acidification, Stevens et al., 2010) and stoichiometric effects (shifting N:P ratios altering competition; Olde Venterink et al., 2003), which are difficult to separate. The global increase in N availability (primarily driven by N-deposition) without proportionate increases in other elements may contribute to large-scale declines in species richness (sensu Tilman et al., 1982).
Food Security and Water Quality
Two key aims of the United Nations Sustainable Development Goals are to achieve food security and to ensure access to clean water for all people by 2030 (UN General Assembly, 2015). Food security incorporates both food quantity and food quality. Currently, 820 million people suffer from acute food shortage, and ~2 billion experience chronic deficiency of essential nutrients (FAO, 2019). Additionally, 785 million people lack access to potable water, including 144 million who rely on untreated surface water (WHO and UNICEF, 2019). Elemental ratios in air, water, and soil impact both food security and water quality (e.g., Loladze, 2002, Mueller et al., 2012). We summarize stoichiometric effects on crop production, rangeland and pasture-based production of meat and dairy, water quality, and fisheries. Collectively, these stoichiometric effects on food security and water quality propagate to impact both non-infectious and infectious disease.
Food Crop Production
Between 1.2–1.5 billion hectares are used for crop production globally (O'Mara, 2012), and yields have increased consistently from 877 million metric tons in 1961 to 2,351 million tons in 2007 (Tester and Langridge, 2010; Foley et al., 2011). However, ongoing human population growth and changes in consumption patterns necessitate continued increases in crop production, with predicted requirements of more than 4,000 million metric tons in 2050 (Tester and Langridge, 2010; Foley et al., 2011). Given the potential for primary productivity to be limited by the availability of multiple elements, understanding the stoichiometry of food crop production under global change is essential to achieving these production goals.
For example, P-limitation of crops is a global concern that may be exacerbated by stoichiometric effects. In Sub-Saharan Africa and Eastern Europe, soil P availability is a major factor limiting productivity (e.g., 75% of soils in Sub-Saharan Africa are P-deficient; Cordell et al., 2009; Mueller et al., 2012). However, the global availability of mineable P is limited, which will increase prices of P fertilizers (Van Vuuren et al., 2010), exacerbate agricultural P-limitation, and threaten global food production (Cordell et al., 2009). Human-induced increases in environmental C and N availability outpace increases in P availability, intensifying C:N:P imbalances globally (Peñuelas et al., 2013; Carnicer et al., 2015).
Global shifts in the cycling of C and N impact food security through additional stoichiometric effects on food quantity and quality. Increases in atmospheric CO2 concentrations tend to increase crop yields (Ainsworth and Long, 2005; Kimball, 2016), with positive impacts on food quantity. However, crops produced under elevated CO2 exhibit increased C:element ratios (e.g., C:N, P, Se, Fe, or Zn), lowering nutritional quality (Loladze, 2002). Nitrogen deposition or over-fertilization can leach K from soils and increase the likelihood of plant K-limitation (Sardans and Peñuelas, 2015), which can alter plant biomass allocation and increase drought vulnerability (Cakmak et al., 1994). Additionally, the availability of many elements in soil (including N, P, K, Zn, and others) can impact the prevalence and severity of infectious disease in crops through several mechanisms, with substantial consequences for yields (Dordas, 2008; Veresoglou et al., 2013). These effects have not previously been studied from a stoichiometric perspective, although elemental ratios mediate many infectious disease processes (Sanders and Taylor, 2018). Overall, human-induced shifts in the ratios of multiple elements in the environment lead to changes in both the quantity and quality of food crop production.
Rangeland and Pasture-Based Production of Meat and Dairy
Grasslands account for ~60% of agricultural land world-wide and support global meat and dairy production (O'Mara, 2012). Like crop production, the productivity of grasslands depends on both the relative and absolute availability of elements in the environment. Elevated atmospheric CO2 and N deposition generally increase grassland productivity (Thornton et al., 2009; Hatfield and Prueger, 2011; Chapman et al., 2012), but the effects of CO2 also contribute to increases in plant C:N that represent decreased forage quality for livestock (Craine et al., 2017; Augustine et al., 2018).
Water Quality
The relative balance of N, P, and other elements in aquatic ecosystems are major drivers of algal bloom dynamics, and anthropogenic inputs of these elements threaten the quality of surface water as it pertains to human health. N:P ratios have long been viewed as drivers of algal blooms (Schindler, 1977), and more recent work has shown that the relative availability of dissolved elements can influence water quality in a variety of ways. For example, low N:P ratios provide a competitive advantage for N-fixing cyanobacteria (Mazur-Marzec et al., 2006), which can form harmful algal blooms (HABs). Elemental ratios in water impact both the abundance of harmful algae or flagellates and the amount of toxins they produce (increasing toxin production in low C:N, low C:P, and high N:P waters; Townsend et al., 2003; Ptacnik et al., 2005, Van de Waal et al., 2009; Anderson et al., 2012). Toxins from HABs have threatened drinking water supplies of entire cities (e.g., Toledo, USA and Wuxi, China; Liu et al., 2011; Steffen et al., 2017), and the specific effects of HABs on human health are discussed in the non-infectious disease section below.
Fisheries
Fish and shellfish (hereafter, “fish”) represent ~5% of global protein consumption (FAO, 2000). However, fish provide a major source of essential fatty acids, vitamins, and other nutrients, thereby playing an important role in global food quality (Golden et al., 2016). The threshold of sustainable harvests in natural systems is strongly linked to primary productivity and trophic transfer efficiencies through food webs (Brander, 2007; Boyce et al., 2010), both of which are subject to stoichiometric constraints (Winder et al., 2009). Eutrophication resulting from anthropogenic changes to biogeochemical cycles can alter fish production in both marine and freshwater systems, and HABs can lead to fish kills (Camargo and Alonso, 2006; Anderson et al., 2012). Eutrophication also impacts the trophic transfer of fatty acids through food webs, reducing the nutritional value of fish for human consumption (Taipale et al., 2016).
Non-infectious Disease
Elemental ratios link global change with non-infectious disease (encompassing a broad suite of physical and mental health outcomes) at two scales: through the effects of nutrition on processes occurring within the human body and through environmental processes occurring outside the body (Figure 3).
Figure 3
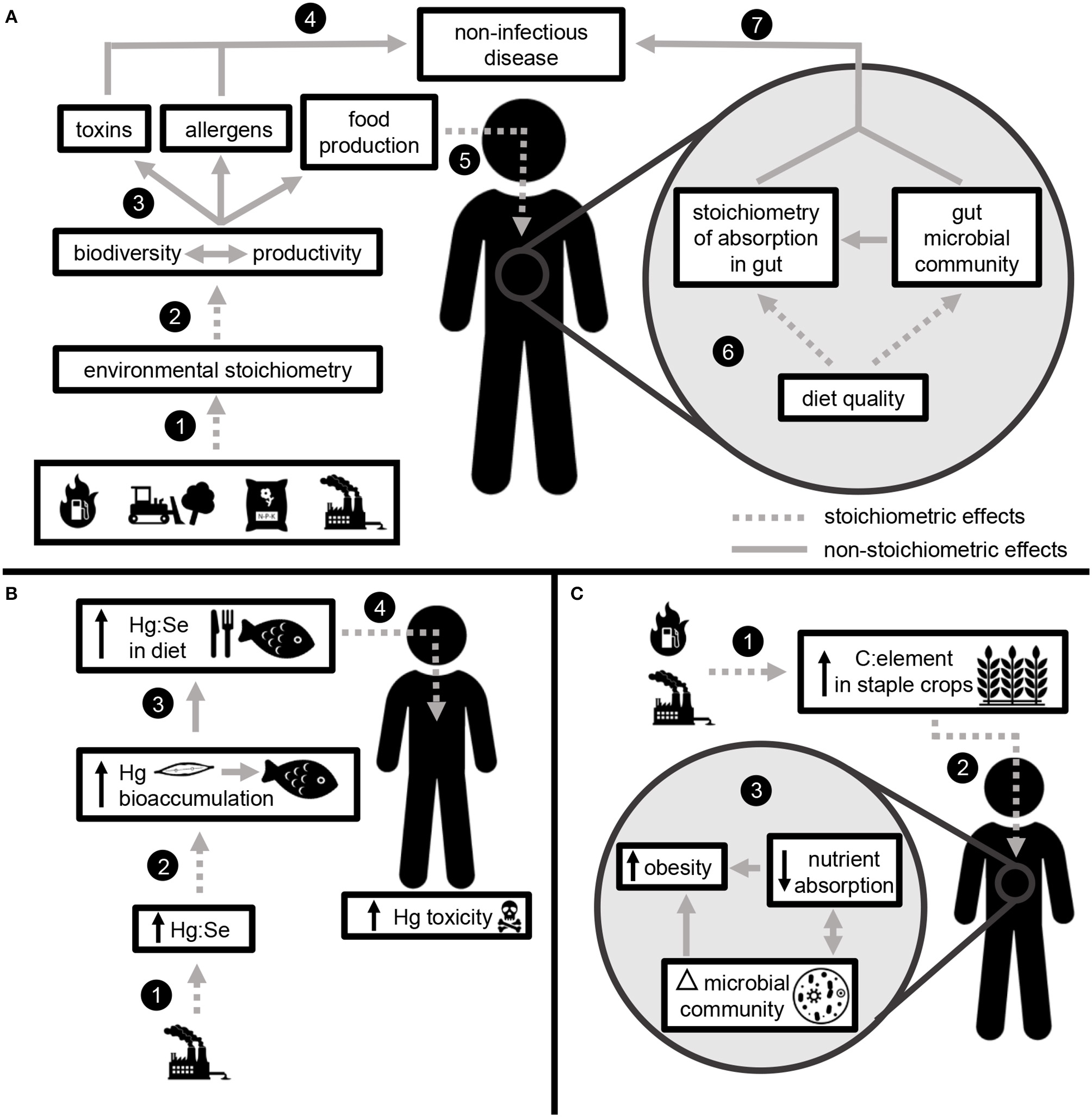
Elemental ratios link global change with non-infectious disease through stoichiometric effects occurring in the environment and within the human body. (A) Fossil fuel combustion, land use change, fertilizer application, and industrial pollution change the relative availability of elements in the environment (1). Environmental stoichiometry impacts the key ecosystem processes of biodiversity and productivity (2), both of which contribute to the production of toxins, allergens, and food (3). Allergens and toxins directly contribute to non-infectious disease (4), while the effects of food quality are mediated by stoichiometric effects within the body (5). Elemental ratios in human diet impact the efficiency of nutrient absorption and the structure and function of the gut microbiome (6), both of which mediate the effects of nutrition on non-infectious disease (7). (B) Example: Industrial production increases the concentrations of Hg in the environment, which leads to higher Hg:Se (1). The degree to which Hg bioaccumulates in aquatic organisms is mediated by Hg:Se (2), leading to greater Hg consumption through human diet (3). Following consumption, Hg:Se within the body impacts Hg toxicity (4). (C) Example: Anthropogenic increases in CO2 lead to increased C:element in food crops (1). Elemental ratios in diet (2) may lead to shifts the gut microbiome and impact nutrient absorption, both of which contribute to obesity (3).
Nutritional Stoichiometry and Non-infectious Disease
Traditional views of human nutrition focus on how imbalances between dietary macromolecules and nutritional requirements impact health, including a broad range of non-infectious diseases. Ecological stoichiometry provides a parallel perspective on this imbalance, with the focus on elemental ratios rather than macromolecules. While ES is a reductionist approach that does not capture the full complexity of nutritional biochemistry, elemental ratios provide a common currency to link food production in the environment with diet quality and its consequences for health.
Dietary Stoichiometry and Physical Health
Perhaps the clearest stoichiometric link between global change and human dietary health is evidence that elevated atmospheric CO2 leads to higher C:element ratios in plants, including staple crops (Loladze, 2002, 2014). This reduction in crop quality means that consumption of staple crops in the future will correspond to higher caloric intake relative to nutritional value (as protein, micronutrient, and vitamin content). This change may contribute to caloric overconsumption, a problem that already drives many major public health crises, including global diabetes and obesity epidemics (James, 2008; Zimmet et al., 2010). Increasing C:element ratios in crops may also contribute to a broad range of health effects caused by mineral and vitamin deficiencies. Along with decreases in Ca, K, Mg, Fe, Zn, and Cu concentrations (Loladze, 2014), elevated CO2 leads to declines in many essential vitamins in rice (Zhu et al., 2018). Among the B vitamins which decline under elevated CO2, the more N-rich forms (such as folate and thiamine) show the greatest reductions in concentration (Zhu et al., 2018).
Changes to elemental ratios in diet can also impact human health through overconsumption of micronutrients and interactions among elements that impact their absorption within the gut. For example, most people in the United States consume far more than the recommended daily allowance of P, and overconsumption of P is associated with increased mortality rates (Chang et al., 2014). However, P content is not required on the labels of packaged foods, making consumption difficult to monitor (Calvo et al., 2014). The overconsumption of P may be particularly important given the high concentrations of readily absorbed inorganic P present in many processed foods (Ritz et al., 2012), which are absorbed much more efficiently (80–100%) in the gut than organically bound P forms (40–60%; Calvo et al., 2014). Stoichiometric effects may underlie some of the observed links between P overconsumption and negative health outcomes. For example, the ratio of dietary P:Ca is important to maintaining normal physiological function and bone health, independently of the consumption of P and Ca individually (Brot et al., 1999; Ito et al., 2011; Lee et al., 2014). Similar stoichiometric effects on other dietary micronutrients are also possible. For example, interactions among Fe, Zn, and Cu impact their absorption and bioavailability within the gut (Sandström, 2001). Overall, these examples suggest that a stoichiometric perspective can help to link global changes in food production, human diet quality, and the effects of nutrition on non-infectious disease.
Diet stoichiometry may also have consequences for cancer due to the elemental requirements of tumor growth. One particularly interesting prior application of ES to human health tested the growth rate hypothesis in tumor dynamics. The growth rate hypothesis (GRH) states that fast growing cells have lower N:P requirements than slower growing cells due to the high P content of ribosomal RNA (Elser et al., 1996, 2003). Rapidly growing lung and colon tumors had 2-fold higher P content and lower N content than normal tissue (although this was not the case for kidney or liver tumors), suggesting some degree of P limitation in cancer cells (Elser et al., 2006, 2007a). While these studies did not specifically test the effects of dietary P content on tumor dynamics, the ultimate source of P used by cancer cells is human diet. High concentrations of inorganic P in food lead to increased tumor proliferation and growth in mice (Jin et al., 2009), which may be due to the P demand of rapidly-dividing cancer cells. Additional research on the GRH in the context of cancer should test the effects of elemental ratios in diet on tumor dynamics, which is especially relevant given the many effects of global change on food quality.
Stoichiometric effects on nutrition and related health outcomes may be most severe among low-income populations (within or among nations) because people in more affluent populations have a greater ability to make dietary choices. For populations obtaining the majority of their nutrients from staple crops, increasing C:element ratios are more likely to lead to diseases stemming from malnutrition. Similarly, as low-income populations continue to increase their consumption of processed foods (Monteiro et al., 2013), the stoichiometric mismatch between calories and micronutrients is likely to increase (Chopra et al., 2002).
Dietary Stoichiometry and Mental Health
The inefficiency of current medical treatments for common mental illnesses such as major depression, along with emerging evidence from longitudinal datasets, has led to a renewed interest in the field of nutritional psychiatry (Sarris et al., 2015). Recent meta-analyses have shown clear links between poor diet and cognitive impairment, dementia, Alzheimer's disease, and especially depression (Psaltopoulou et al., 2013; Lai et al., 2014; Jacka et al., 2017). Adults with mood disorders have been shown to increase functioning when consuming diets high in micronutrients (Davison and Kaplan, 2012; Jacka et al., 2017), especially Mg (Black et al., 2015; Tarleton and Littenberg, 2015). It is fair to assume that decreased nutritional quality, such as increased C:Mg in staple crops, is likely to have pronounced effects on mental health. Therefore, any stoichiometric shifts in diet quality that are relevant to physical health outcomes should also be evaluated in the context of mental health.
Microbiome Mediation of Diet-Health Links
The functional composition of the human gut microbiome is inextricably linked with the physiological processes governing many aspects of health. Microbial community composition within the gut has been linked to a broad array of non-infectious diseases, including cancer (Schwabe and Jobin, 2013), obesity (Mathur and Barlow, 2015), diabetes (Barlow et al., 2015), allergies (McKenzie et al., 2017), depression and anxiety disorders (Foster and McVey Neufeld, 2013), autism (Mulle et al., 2013), and a suite of neurological disorders (Tremlett et al., 2017).
Resource stoichiometry impacts the composition and function of microbial communities across a broad suite of environments (Cherif and Loreau, 2007; Hibbing et al., 2010; Larsen et al., 2019), but this has not yet been explored in the human gut. Both dietary fiber intake (Hamaker and Tuncil, 2014) and dietary N (Holmes et al., 2017) have clear effects on gut microbial community composition and function, suggesting that dietary C:element ratios play a role in shaping the microbiome and its effects on health. Additionally, the GRH predicts that faster-growing microbes should dominate in environments with greater relative P availability (Elser et al., 2003), but this has not been tested in the context of human dietary P intake and the microbiome. Future studies are necessary to determine the extent to which elemental ratios in human diet impact the functional composition of the microbiome and how these effects propagate to human health.
Environmental Stoichiometry and Non-infectious Disease
Elemental ratios also link non-infectious disease with global change through ecological processes that occur in terrestrial and aquatic ecosystems (Figure 3). Stoichiometric effects on the productivity and biodiversity of these systems impact human health through HABs, pollen production, and toxin exposure.
Harmful Algal Blooms
As previously discussed, elemental ratios in water (especially N:P) can drive both the abundance of phytoplankton involved in HABs and the amount of toxins they produce (Van de Waal et al., 2009; Anderson et al., 2012). Toxins from HABs can have both direct and indirect impacts on human health, including endocrine, neurological, and digestive effects (Paerl and Otten, 2013). Depending on the causative organism, exposure to HABs toxins can cause nausea, abdominal pain, diarrhea, respiratory distress, chills, fever, memory loss, seizures, paralysis, and coma (Backer, 1995).
Pollen Production
Elemental ratios in the environment are important drivers of plant productivity (as discussed previously), and stoichiometric effects on pollen production may impact human health through both allergies and effects on pollinator populations that are essential to the production of some food crops. Soil N and P and atmospheric CO2 contribute to pollen production rate and pollen grain size (Lau and Stephenson, 1993, 1994; Lau et al., 1995; Perez-Moreno and Read, 2001), which may exacerbate pollen allergies. Although pollen production has not been specifically linked to elemental ratios in the environment, the frequency of co-limitation in terrestrial ecosystems suggests that stoichiometric effects on plant productivity may underlie pollen production. Stoichiometric effects on biodiversity loss may also contribute to pollen allergies; biodiversity loss has been linked to increased prevalence of allergies and inflammatory diseases in urban settings (Hanski et al., 2012).
Biogeochemical shifts can also impact pollen quality, with implications for pollinator health and food crop production. For example, elevated atmospheric CO2 led to increased pollen C:N in both historical and experimental analyses, which corresponds to a decline in pollen protein content (Ziska et al., 2016). Pollen protein is essential to bee diet, so this stoichiometric shift in pollen quality may have negative effects on bee health and the pollination of food crops that are integral to human diet.
Toxin Exposure
While a large portion of ES literature focuses on ratios of C, N, and P, the ratios of many other elements may impact human health through exposure to toxins. For example, selenium (Se) concentrations in water impact the amount of Hg transferred through food webs (e.g., Sørmo et al., 2011; Walters et al., 2015), thus mediating human exposure to Hg via fish consumption. Following Hg consumption, the amount of Se and Hg circulating through and released from the body also depends on their relative concentrations (Drasch et al., 1996), suggesting that Hg:Se ratios mediate the toxic effects of Hg.
The health effects of arsenic (As), another toxic element that is globally prevalent and linked to a wide range of non-infectious diseases (Amini et al., 2008; Naujokas et al., 2013), are also mediated by its relative balance with other elements. For example, As (in the form of arsenate) has the same shape as phosphate, a molecule essential for all life (Finnegan and Chen, 2012). Consequently, As can be taken into cells in place of P and decouple processes that require high amounts of P, like the formation of adenosine triphosphate (ATP, the main currency of energy in humans). The toxic effects of As are greater when P is low since increased demand for P leads to greater As acquisition (Rodriguez Castro et al., 2015). Arsenic uptake by organisms (and potential exposure to humans) is further complicated by the stoichiometry of additional elements. For example, As uptake by freshwater microbial communities is controlled by N:P ratios (rather than P concentration alone, MacNeill, 2019), and Si:As ratios in soil determine the degree of As accumulation in rice (Zhang et al., 2017). Overall, these examples demonstrate that complex stoichiometric effects can mediate the human health outcomes associated with exposure to toxic elements.
Infectious Disease
Anthropogenic changes to N and P cycles are associated with increased prevalence of many infectious diseases in human hosts (McKenzie and Townsend, 2007; Johnson et al., 2010). Although previous research has used stoichiometric theory to link biogeochemical cycles with parasite and pathogen infection in a range of non-human hosts (Aalto et al., 2015; Sanders and Taylor, 2018), this topic has not been explored in the context of human health. We outline stoichiometric effects linking infectious disease in humans with global change at two scales: through the effects of nutrition on parasite-host interactions within the human host and through environmental processes outside the human host (Figure 4). Hereafter, we use “parasite” to refer to a broad suite of pathogenic organisms (e.g., viruses, protozoa, bacteria, and helminths) that infect human and non-human hosts.
Figure 4
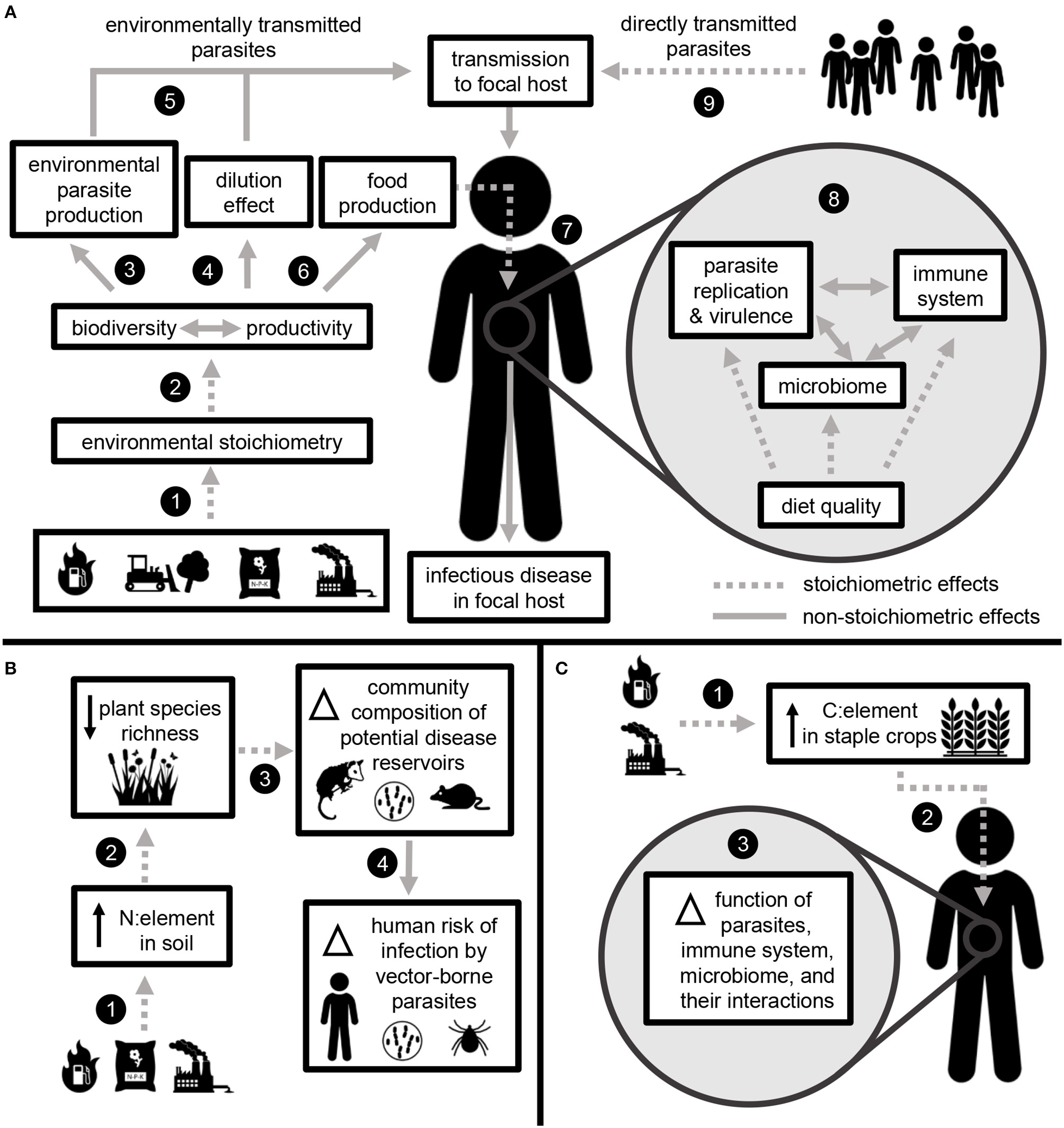
Elemental ratios link global change with infectious disease through stoichiometric effects occurring in the environment and within the human body. (A) Fossil fuel combustion, land use change, fertilizer application, and industrial pollution change the relative availability of elements in the environment (1). Environmental stoichiometry impacts the key ecosystem processes of biodiversity and productivity (2). These processes impact the production of environmentally transmitted parasite species (3). Biodiversity may mediate parasite transmission to human hosts via the dilution effect (4). Together, these processes impact the rate at which human hosts are exposed to environmentally transmitted parasites (5). Biodiversity and ecosystem productivity also impact food production (6), and elemental ratios in human diet (7) lead to stoichiometric effects on infectious disease development within the body. Diet stoichiometry impacts the function of parasites, the non-pathogenic microbiome, and the immune system (8), which collectively determine the development of infectious disease within an individual host. The same within-host processes also apply to the development of disease caused by directly transmitted parasites (species that do not require environmental replication) (9). (B) Example: Human-driven changes to N availability increase N:element ratios in soil (1), which can contribute to declines in plant community diversity (2). Changes in plant diversity may alter the richness and composition of consumer communities, including potential reservoir hosts for infectious diseases of humans (3). The diversity of host communities may impact disease vector abundance and human risk of infection through a dilution effect (4). (C) Example: Anthropogenic increases in CO2 lead to increased C:element in food crops (1), which corresponds to reduced nutritional quality (as dietary C:element) (2). Within a human host, changes to diet stoichiometry may impact the function of parasites, the microbiome, the immune system, and their interactions. Collectively, these functions determine the development of infectious disease (3).
Nutritional Stoichiometry Shapes Parasite-Host Interactions and Disease Development
The effects of human nutrition on infectious disease have received extensive consideration (Field et al., 2002; Cunningham-Rundles et al., 2005), though not yet from a stoichiometric perspective. Within a human host, we can view parasites, the non-pathogenic microbiome, and human immune cells as consumers that require specific resource quality to function optimally (Smith and Holt, 1996). The collective function of the consumers in this scenario determines the symptomatic response observed as infectious disease. Ecological stoichiometry provides a common currency to link elemental ratios in human diet with nutritional impacts on infectious disease development through several mechanisms (Figure 4).
Effects of Host Nutrition on Parasite Function
Parasites are ecological consumers whose survival, growth, and reproduction are limited by the quality of resources provided by their hosts (Smith, 2007). From a stoichiometric perspective, the rates of these physiological processes may depend on elemental ratios originating from host diet. Several core concepts from stoichiometric theory may contribute to our understanding of links between parasite function and human diet: the GRH, threshold elemental ratios, and stoichiometrically-mediated interactions among species.
The GRH provides a promising avenue to link human diet quality with parasite function. The rapid growth rates of parasites relative to their hosts suggests that parasitic taxa should be limited by C:P or N:P within hosts. Experimental evidence from other disease systems supports this prediction: viral production increased in algal hosts grown at low C:P (Clasen and Elser, 2007), and bacterial infection rates increased when zooplankton hosts were fed a low C:P diet (Frost et al., 2008). While the GRH has not been tested for parasites infecting human hosts, understanding links between dietary C:P or N:P and parasite growth are especially relevant given the overconsumption of P in many human diets (Razzaque, 2011).
However, parasites vary widely in elemental ratios across species (Paseka and Grunberg, 2019), so the limitation of parasite growth rate by P is unlikely to be universal. For example, fungal parasites infecting cyanobacteria had the highest replication rate when hosts were grown at high N:P, suggesting that N is more limiting to these parasites than P (Frenken et al., 2017). The concept of threshold elemental ratios (TERs) provides a framework for predicting how nutritionally-limited consumers respond to variation in resource quality (Boersma and Elser, 2006). In contrast to the GRH, TERs are not specific to P but could instead be applied to any elemental ratio. A consumer's TER represents an “ideal” resource ratio where consumer growth is equally limited by both elements under consideration. The cost of processing excess elements is predicted to create a hump-shaped response of growth rate to diet quality around the TER (Boersma and Elser, 2006). TERs have not been explored as a means to study stoichiometric limitation of parasite growth, but this concept could aid in making specific predictions about how parasites will respond to shifts in human diet.
Finally, ES may also provide a framework for predicting how human diet influences the outcome of interspecific interactions among parasites and the non-pathogenic microbiome. These organisms interact through complex mechanisms, and the composition and function of the microbiome can limit or promote infectious disease (Baümler and Sperandio, 2016). As described earlier, we lack critical data on potential effects of elemental ratios in host diet on the structure and function of the human microbiome and its effects on disease.
Nutritional Controls on the Immune System
There is ample evidence that human diet regulates infectious disease through immune function (Field et al., 2002; Cunningham-Rundles et al., 2005), though this has not been studied in the context of ES. From a stoichiometric perspective, human immune cells can be viewed as consumers that function optimally at specific elemental ratios. For this reason, applying the concept of TERs to immune cells (and comparing host immune TERs to parasite TERs) could help to draw links between human diet quality, optimal immune function, and defense against infectious disease.
Environmental Stoichiometry Mediates Human Infection Risk
Elemental ratios also link infectious disease with global change through processes occurring in terrestrial and aquatic ecosystems that mediate human exposure to environmentally transmitted parasites (Figure 4). Stoichiometric effects on ecosystem productivity shape the population dynamics of consumers, including parasites that require development outside the human host. We use “environmental parasites” to refer to species that replicate during free-living stages in the environment and those that replicate within vector or intermediate host species.
Population Dynamics of Parasites, Intermediate Hosts, and Vectors Impact Human Risk of Exposure
Stoichiometric effects on ecosystem productivity will impact parasites that have free-living stages in the environment (i.e., those that live independently of a host or vector). For example, Vibrio cholerae, the causative agent of cholera, lives as a heterotrophic bacterium in aquatic environments when outside the human host and is therefore impacted by environmental resource availability (Cottingham et al., 2007). Associations between algal blooms and cholera outbreaks have been observed for decades (Epstein, 1993; Colwell, 1996), yet it remains unclear what mechanisms link elemental availability, primary production, and V. cholerae population dynamics. Elemental ratios in aquatic ecosystems may regulate V. cholerae directly by providing resources required for growth or indirectly by promoting the growth of attachment surfaces (phytoplankton, zooplankton, and macrophytes) on which the bacteria aggregate during blooms (Cottingham et al., 2007). Elemental ratios constrain the growth of non-pathogenic bacteria in aquatic ecosystems and mediate algal bloom dynamics (Sterner and Elser, 2002), suggesting that stoichiometric effects may impact the population dynamics of V. cholerae and other human parasites with free-living environmental stages.
The effects of elemental ratios on ecosystem productivity can also constrain the distribution and population dynamics of vectors or intermediate hosts, thereby limiting the populations of their associated parasite species. For example, elemental ratios in aquatic ecosystems impact the distribution and population dynamics of larval mosquitoes (Murrell et al., 2011; Yee et al., 2015), which in turn determine the populations of adult mosquitoes that transmit a wide array of viruses and protozoan parasites.
In addition to the number of vectors or hosts in the environment, the quality of these organisms as resources for parasites may also be mediated by stoichiometric effects. Like parasites within human hosts, environmental parasites may be nutrient limited within intermediate hosts or vectors. Evidence from several disease systems indicates that shifts in the stoichiometry of the environment can cascade to alter the elemental composition or other aspects of host and vector physiology, thus altering the resources available to parasites and mediating transmission risk (Sanders and Taylor, 2018). For example, mosquitoes that consumed high quality diets as larvae matured into adults that were higher in %N, which corresponded to lower rates of Zika virus infection and transmission potential (Paige et al., 2019). While the mechanism behind this shift is unknown, it may reflect elemental requirements of mosquito immune function. These studies on mosquito vectors are a rare demonstration of the importance of elemental ratios for vector population dynamics, traits, and transmission of a human virus, and this topic represents an important direction for future research in additional disease systems. Like within-host disease dynamics, we suggest that TERs and the GRH are core stoichiometric concepts that should be used to study the effects of elemental ratios on environmental parasites, intermediate hosts, and vectors.
Ecological Communities Mediate Parasite Transmission to Human Hosts
In addition to the effects of environmental stoichiometry on the direct interactions between environmental parasites and their intermediate hosts and vectors, broader properties of ecological communities also have important implications for infectious disease. Stoichiometric effects on species richness and composition may mediate human infection risk through the dilution effect, a well-supported, inverse relationship between infectious disease and biodiversity (Keesing et al., 2006; Ostfeld and Keesing, 2012). There is robust support for the dilution effect across a broad range of infectious disease systems, including human diseases (Civitello et al., 2015). The effects of environmental stoichiometry on human infectious disease as mediated by community richness and composition have not been studied, though results of a grassland experiment suggest that N-fertilization can reduce the strength of the dilution effect and lead to greater levels of fungal disease in plant communities (Liu et al., 2017).
Overall, stoichiometric constraints on ecosystem productivity and biodiversity may mediate the rate at which humans encounter infective parasite stages produced in the environment. Future research on how elemental ratios cascade through food webs to impact parasite production and transmission will provide new opportunities to link global change with infectious disease risk.
Discussion
Limitations of a Stoichiometric Approach to Human Health
Ecological stoichiometry is a powerful framework for studying the effects of global change on human health because it enhances our understanding of how elements and energy are transferred across scales of biological organization. However, one limitation to the application of ES is that organisms (including humans) cannot assimilate all molecular forms of elemental nutrients. For example, humans require N in complex molecules because we are only able to synthesize half of the amino acids required for physiological function. Such nuance is lost when considering only the C:N ratios (or N content alone) of human diet. Nevertheless, a stoichiometric approach can often complement more traditional considerations of dietary macromolecules. For example, the elevation of C:N content in rice corresponds to decreased concentrations of N-rich B vitamins (Zhu et al., 2018). In this case, stoichiometric and macromolecular perspectives provide complementary insight into links between global change and human nutrition.
However, some biogeochemical effects of global change on human health can be effectively characterized using a single-element approach. For example, nitrate leaching due to over-fertilization has numerous human health implications (Townsend et al., 2003). The human health effects of nitrate in groundwater are largely a function of N alone, not N:P or another stoichiometric interaction. Similarly, the health effects of toxic elements such as Hg are better expressed as Hg concentration in the body than as an C:Hg ratio. However, the ratios of Hg with other elements (such as Se:Hg) influences human health risk through stoichiometric processes both in the environment and within the human body. While a reductionist, stoichiometric approach has limitations, the interdisciplinary, cross-scale power of ES also holds great potential to inform human health research.
Stoichiometric Insight for Human Health Under Global Change
We have outlined many potential mechanisms through which elemental ratios link changing biogeochemical cycles with food security and water quality, as well as a wide array of infectious and non-infectious diseases in humans. However, we note that stoichiometrically-explicit data on this topic are generally scarce. We advocate for future research to harness the power of ES by testing some of its core tenets in the context of human health. This review is not an exhaustive exploration of these ideas, the current literature, or potential future directions. Instead, we hope that this work will inspire future research on the application of stoichiometric theory to human health under global change.
Statements
Author contributions
RP conceived the original idea for this review and coordinated manuscript completion. All authors (RP, AB, KM, AB, and CS) contributed to development and writing. Author order is random after first.
Funding
Woodstoich 4 was supported by the National Science Foundation DEB-1840408. RP was was supported by the National Socio-Environmental Synthesis Center (SESYNC) under funding received from the National Science Foundation DBI-1639145.
Acknowledgments
This manuscript is a product of Woodstoich 4, and we thank Jim Elser and Michelle Evans-White for organizing a groovy and productive event for early career researchers. Thank you to our three reviewers, whose comments and suggestions led to substantial improvements on this manuscript. We thank Zoe Cardon for her sage advice and enthusiasm throughout Woodstoich. Thank you to artists at the Noun Project (https://thenounproject.com; Supplementary Information 1) for icons used in tables and figures and to Steven Ross Davidson for help assembling figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00378/full#supplementary-material
References
1
Aalto S. L. Decaestecker E. Pulkkinen K. (2015). A three-way perspective of stoichiometric changes on host-parasite interactions. Trends Parasitol. 31, 333–340. 10.1016/j.pt.2015.04.005
2
Adriano D. C. (1986). Trace elements in the terrestrial environment. New York, NY: Spring-Verlag. 10.1007/978-1-4757-1907-9
3
Agren G. I. Wetterstedt J. Å. M. Billberger M. F. (2012). Nutrient limitation on terrestrial plant growth–modeling the interaction between nitrogen and phosphorus. New Phytol. 194, 953–960. 10.1111/j.1469-8137.2012.04116.x
4
Ainsworth E. A. Long S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol.165, 351–371. 10.1111/j.1469-8137.2004.01224.x
5
Amini M. Abbaspour K. C. Berg M. Winkel L. Hug S. J. Hoehn E. et al . (2008). Statistical modeling of global geogenic arsenic contamination in groundwater. Environ. Sci. Technol.42, 3669–3675. 10.1021/es702859e
6
Anderson D. M. Cembella A. D. Hallegraeff G. M. (2012). Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Ann. Rev. Mar. Sci. 4, 143–176. 10.1146/annurev-marine-120308-081121
7
Augustine D. J. Blumenthal D. M. Springer T. L. Lecain D. R. Gunter S. A. Derner J. D. (2018). Elevated CO2 induces substantial and persistent declines in forage quality irrespective of warming in mixedgrass prairie. Ecol. Appl.28, 721–735. 10.1002/eap.1680
8
Backer L. C. (1995). Manual on Harmful Marine Microalgae, Monographs on Oceanographic Methodology,2nd Edn. Paris: UNESCO Publishing.
9
Barlow G. M. Yu A. Mathur R. (2015). Role of the gut microbiome in obesity and diabetes mellitus. Nutr. Clin. Pract.30, 787–797. 10.1177/0884533615609896
10
Baümler A. J. Sperandio V. (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature535, 85–93. 10.1038/nature18849
11
Bezemer T. M. Jones T. H. (1998). Plant-insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos82, 212–222. 10.2307/3546961
12
Bjerregaard P. Fjordside S. Hansen M. G. Petrova M. B. (2011). Dietary selenium reduces retention of methyl mercury in freshwater fish. Environ. Sci. Technol. 45, 9793–9798. 10.1021/es202565g
13
Black L. J. Allen K. L. Jacoby P. Trapp G. S. Gallagher C. M. Byrne S. M. et al . (2015). Low dietary intake of magnesium is associated with increased externalising behaviours in adolescents. Public Health Nutr. 18, 1824–1830. 10.1017/S1368980014002432
14
Bobbink R. Hicks K. Galloway J. Spranger T. Alkemade R. Ashmore M. et al . (2010). Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol. Appl. 20, 30–59. 10.1890/08-1140.1
15
Bobbink R. Hornung M. Roelofs J. G. M. (1998). The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J. Ecol. 86, 717–738. 10.1046/j.1365-2745.1998.8650717.x
16
Boersma M. (2000). The nutritional quality of P-limited algae for Daphnia. Limnol. Oceanogr. 45, 1157–1161. 10.4319/lo.2000.45.5.1157
17
Boersma M. Aberle N. Hantzsche F. M. Schoo K. L. Wiltshire K. H. Malzahn A. M. (2008). Nutritional limitation travels up the food chain. Int. Rev. Hydrobiol. 93, 479–488. 10.1002/iroh.200811066
18
Boersma M. Elser J. J. (2006). Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology87, 1325–1330. 10.1890/0012-9658(2006)87[1325:TMOAGT]2.0.CO;2
19
Boyce D. G. Lewis M. R. Worm B. (2010). Global phytoplankton decline over the past century. Nature466, 591–596. 10.1038/nature09268
20
Brander K. M. (2007). Global fish production and climate change. Proc. Natl. Acad. Sci. U.S.A. 104, 19709–19714. 10.1073/pnas.0702059104
21
Brot C. Jørgensen N. Madsen O. R. Jensen L. B. Sørensen O. H. (1999). Relationships between bone mineral density, serum vitamin D metabolites and calcium:phosphorus intake in healthy perimenopausal women. J. Intern. Med. 245, 509–516. 10.1046/j.1365-2796.1999.00474.x
22
Cakmak I. Hengeler C. Marschner H. (1994). Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 45, 1245–1250. 10.1093/jxb/45.9.1245
23
Caldeira K. Wickett M. E. (2003). Anthropogenic carbon and ocean pH. Nature425:365. 10.1038/425365a
24
Calvo M. S. Moshfegh A. J. Tucker K. L. (2014). Assessing the health impact of phosphorus in the food supply: issues and considerations. Adv. Nutr. 5, 104–113. 10.3945/an.113.004861
25
Camargo J. A. Alonso A. (2006). Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ. Int. 32, 831–849. 10.1016/j.envint.2006.05.002
26
Carey J. C. Fulweiler R. W. (2012). Human activities directly alter watershed dissolved silica fluxes. Biogeochemistry111, 125–138. 10.1007/s10533-011-9671-2
27
Carnicer J. Sardans J. Stefanescu C. Ubach A. Bartrons M. Asensio D. et al . (2015). Global biodiversity, stoichiometry and ecosystem function responses to human-induced C-N-P imbalances. J. Plant Physiol. 172, 82–91. 10.1016/j.jplph.2014.07.022
28
Cease A. J. Capps K. A. Gates K. K. McCrackin M. L. Nidzgorski D. A. (2015). Consumer-driven nutrient dynamics in urban environments: the stoichiometry of human diets and waste management. Oikos124, 931–948. 10.1111/oik.02391
29
Chang A. R. Lazo M. Appel L. J. Gutierrez O. M. Grams M. E. (2014). High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. [Erratum appears in Am J Clin Nutr. 2017 Apr;105(4):1021; PMID: 28373320]. Am. J. Clin. Nutr.99, 320–327. 10.3945/ajcn.113.073148
30
Chapman S. C. Chakraborty S. Dreccer M. F. Howden S. M. (2012). Plant adaptation to climate changeopportunities and priorities in breeding. Crop Pasture Sci. 63, 251–268. 10.1071/CP11303
31
Cherif M. Loreau M. (2007). Stoichiometric constraints on resource use, competitive interactions, and elemental cycling in microbial decomposers. Am. Nat. 169, 709–724. 10.1086/516844
32
Chopra M. Galbraith S. Darnton-Hill I. (2002). A global response to a global problem: the epidemic of overnutrition. Bull. World Health Organ. 80, 952–958. 10.1590/S0042-96862002001200009
33
Cisneros J. J. Godfrey L. D. (2001). Midseason pest status of the cotton aphid (homoptera: aphididae) in california cotton - is nitrogen a key factor?Environ. Entomol. 30, 501–510. 10.1603/0046-225X-30.3.501
34
Civitello D. J. Cohen J. Fatima H. Halstead N. T. Liriano J. McMahon T. et al . (2015). Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl. Acad. Sci. U.S.A.112, 8667–8671. 10.1073/pnas.1506279112
35
Clasen J. L. Elser J. J. (2007). The effect of host Chlorella NC64A carbon:phosphorus ratio on the production of Paramecium bursaria Chlorella Virus-1. Freshw. Biol. 52, 112–122. 10.1111/j.1365-2427.2006.01677.x
36
Coakley S. M. Scherm H. Chakraborty S. (1999). Climate change and plant disease management. Annu. Rev. Phytopathol. 37, 399–426. 10.1146/annurev.phyto.37.1.399
37
Colwell R. R. (1996). Global climate and infectious disease: the cholera paradigm. Science274, 2025–2031. 10.1126/science.274.5295.2025
38
Cordell D. Drangert J.-O. White S. (2009). The story of phosphorus: global food security and food for thought. Glob. Environ. Chang. 19, 292–305. 10.1016/j.gloenvcha.2008.10.009
39
Cottingham K. L. Chiavelli D. A. Taylor R. K. (2007). Environmental microbe and human pathogen: the ecology and microbiology of Vibrio cholerae. Front. Ecol. Environ. 1:80. 10.1890/1540-9295(2003)001[0080:EMAHPT]2.0.CO;2
40
Craine J. M. Elmore A. Angerer J. P. (2017). Long-term declines in dietary nutritional quality for North American cattle. Environ. Res. Lett.12:044019. 10.1088/1748-9326/aa67a4
41
Cunningham-Rundles S. McNeeley D. F. Moon A. (2005). Mechanisms of nutrient modulation of the immune response. J. Allergy Clin. Immunol. 115, 1119–1128. 10.1016/j.jaci.2005.04.036
42
Davison K. M. Kaplan B. J. (2012). Nutrient intakes are correlated with overall psychiatric functioning in adults with mood disorders. Can. J. Psychiatry57, 85–92. 10.1177/070674371205700205
43
Demott W. R. Mckinney E. N. Tessier A. J. (2010). Ontogeny of digestion in Daphnia: implications for the effectiveness of algal defenses. Ecology91, 540–548. 10.1890/08-2103.1
44
Dodds W. K. Smith V. H. (2016). Nitrogen, phosphorus, and eutrophication in streams. Inl. Waters6, 155–164. 10.5268/IW-6.2.909
45
Dordas (2008). Role of nutrients in controlling plant diseases in sustainable agriculture: a review. Agron. Sust. Devel. 28, 33–46. 10.1051/agro:2007051
46
Drasch G. Wanghofer E. Roider G. Strobach S. (1996). Correlation of mercury and selenium in the human kidney. J. Trace Elem. Med. Biol.10, 251–254. 10.1016/S0946-672X(96)80043-5
47
Echalar F. Gaudichet A. Cachier H. Artaxo P. (1995). Aerosol emissions by tropical forest and savanna biomass burning: characteristic trace elements and fluxes. Geophys. Res. Lett. 22, 3039–3042. 10.1029/95GL03170
48
Elser J. Bracken M. Cleland E. Gruner D. S. Harpole W. S. Hillebrand H. et al . (2007b). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. 10.1111/j.1461-0248.2007.01113.x
49
Elser J. J. Acharya K. Kyle M. Cotner J. Makino W. Markow T. et al . (2003). Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943. 10.1046/j.1461-0248.2003.00518.x
50
Elser J. J. Andersen T. Baron J. S. Bergström A. K. Jansson M. Kyle M. et al . (2009). Shifts in lake N: P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science326, 835–837. 10.1126/science.1176199
51
Elser J. J. Dobberfuhl D. R. MacKay N. A. Schampel J. H. (1996). Organism size, life history, and N:P stoichiometry. BioScience46, 674–684. 10.2307/1312897
52
Elser J. J. Kyle M. M. Smith M. S. Nagy J. D. (2007a). Biological stoichiometry in human cancer. PLoS ONE2:e1028. 10.1371/journal.pone.0001028
53
Elser J. J. Nagy J. D. Kuang Y. (2006). Biological stoichiometry: an ecological perspective on tumor dynamics. Bioscience53, 1112–1120. 10.1641/0006-3568(2003)053[1112:BSAEPO]2.0.CO;2
54
Epstein P. R. (1993). Algal blooms in the spread and persistence of cholera. BioSystems31, 209–221. 10.1016/0303-2647(93)90050-M
55
FAO (2000). The Sustainable Contribution of Fisheries to Food Security. Rome: Food and Agriculture Organization of the United Nations.
56
FAO (2012). The State of the World Fisheries and Aquaculture. Rome: Food and Agriculture Organization of the United Nations.
57
FAO (2019). The State of Food Security and Nutrition in the World. Rome: Food and Agriculture Organization of the United Nations.
58
Fay P. A. Prober S. M. Harpole W. S. Knops J. M. H. Bakker J. D. Borer E. T. et al . (2015). Grassland productivity limited by multiple nutrients. Nat. Plants1:15080. 10.1038/nplants.2015.80
59
Field C. J. Johnson I. R. Schley P. D. (2002). Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 71, 16–32. 10.1189/jlb.71.1.16
60
Finnegan P. M. Chen W. (2012). Arsenic toxicity: the effects on plant metabolism. Front. Physiol.3, 1–18. 10.3389/fphys.2012.00182
61
Flynn K. J. (2002). Toxin production in migrating dinoflagellates: a modelling study of PSP producing Alexandrium. Harmful Algae1, 147–155. 10.1016/S1568-9883(02)00028-8
62
Foley J. A. Ramankutty N. Brauman K. A. Cassidy E. S. Gerber J. S. Johnston M. et al . (2011). Solutions for a cultivated planet. Nature478, 337–342. 10.1038/nature10452
63
Foster J. A. McVey Neufeld K. A. (2013). Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci.36, 305–312. 10.1016/j.tins.2013.01.005
64
Frenken T. Wierenga J. Gsell A. S. van Donk E. Rohrlack T. Van de Waal D. B. (2017). Changes in N:P supply ratios affect the ecological stoichiometry of a toxic cyanobacterium and its fungal parasite. Front. Microbiol. 8, 1–11. 10.3389/fmicb.2017.01015
65
Frost P. C. Ebert D. Smith V. H. (2008). Responses of a bacterial pathogen to phosphorus limitation of its aquatic invertebrate host. Ecology89, 313–318. 10.1890/07-0389.1
66
Galloway J. Erisman J. W. Trends R. Galloway J. N. Townsend A. R. Erisman J. W. et al . (2008). Transformation of the nitrogen cycle : recent trends, questions, and potential solutions. Science.320, 889–892. 10.1126/science.1136674
67
Gilmour C. C. Henry E. A. Ralph M. (1992). Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol. 26, 2281–2287. 10.1021/es00035a029
68
Golden C. D. Allison E. H. Cheung W. W. L. Dey M. M. Halpern B. S. McCauley D. J. et al . (2016). Nutrition: fall in fish catch threatens human health. Nature534, 317–320. 10.1038/534317a
69
Granéli E. Johansson N. (2003). Increase in the production of allelopathic substances by Prymnesium parvum cells grown under N- or P-deficient conditions. Harmful Algae2, 135–145. 10.1016/S1568-9883(03)00006-4
70
Hamaker B. R. Tuncil Y. E. (2014). A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 426, 3838–3850. 10.1016/j.jmb.2014.07.028
71
Hanski I. Von Hertzen L. Fyhrquist N. Koskinen K. Torppa K. Laatikainen T. et al . (2012). Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. U.S.A.109, 8334–8339. 10.1073/pnas.1205624109
72
Harpole W. S. Ngai J. T. Cleland E. E. Seabloom E. W. Borer E. T. Bracken M. E. S. et al . (2011). Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862. 10.1111/j.1461-0248.2011.01651.x
73
Hatfield J. L. Prueger J. H. (2011). Agroecology: implications for plant response to climate change, in Crop Adaptations to Climate Change, eds YadavS. S.ReddenR. J.HatfieldJ. L.Lotze-CampenH.HallA. E. (West Sussex: John Wiley & Sons), 27–43. 10.1002/9780470960929.ch3
74
Hessen D. O. Elser J. J. Sterner R. W. Urabe J. (2013). ecological stoichiometry: an elementary approach using basic principles. Limnol. Oceanogr. 58, 2219–2236. 10.4319/lo.2013.58.6.2219
75
Hibbing M. E. Fuqua C. Parsek M. R. Peterson S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25. 10.1038/nrmicro2259
76
Holmes A. J. Chew Y. V. Colakoglu F. Cliff J. B. Klaassens E. Read M. N. et al . (2017). Diet-microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 25, 140–151. 10.1016/j.cmet.2016.10.021
77
Humborg C. Ittekkot V. Cociasu A. Bodungen B. V. (1997). Effect of danube river dam on black sea biogeochemistry and ecosystem structure. Nature386, 385–388. 10.1038/386385a0
78
Humborg C. Pastuszak M. Aigars J. Siegmund H. Mörth C. M. Ittekkot V. (2006). Decreased silica land-sea fluxes through damming in the Baltic Sea catchment - Significance of particle trapping and hydrological alterations. Biogeochemistry77, 265–281. 10.1007/s10533-005-1533-3
79
Ito S. Ishida H. Uenishi K. Murakami K. Sasaki S. (2011). The relationship between habitual dietary phosphorus and calcium intake, and bone mineral density in young Japanese women: a cross-sectional study. Asia Pac. J. Clin. Nutr. 20, 411–417.
80
Jacka F. N. O'Neil A. Opie R. Itsiopoulos C. Cotton S. Mohebbi M. et al . (2017). A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 15:23. 10.1186/s12916-017-0791-y
81
James W. P. T. (2008). The epidemiology of obesity: the size of the problem. J. Intern. Med. 263, 336–352. 10.1111/j.1365-2796.2008.01922.x
82
Jin H. Xu C.-X. Lim H.-T. Park S.-J. Shin J.-Y. Chung Y.-S. et al . (2009). High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 179, 59–68. 10.1164/rccm.200802-306OC
83
Johnson P. T. J. Townsend A. R. Cleveland C. C. Gilbert P. M. Howarth R. W. McKenzie V. J. et al . (2010). Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecol. Appl.20, 16–29. 10.1890/08-0633.1
84
Kaspari M. Powers J. S. (2016). Biogeochemistry and geographical ecology: embracing all twenty-five elements required to build organisms. Am. Nat. 188, S62–S73. 10.1086/687576
85
Keesing F. Holt R. D. Ostfeld R. S. (2006). Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. 10.1111/j.1461-0248.2006.00885.x
86
Kimball B. A. (2016). Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol.31, 36–43. 10.1016/j.pbi.2016.03.006
87
Lai J. S. Hiles S. Bisquera A. Hure A. J. McEvoy M. Attia J. (2014). A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 99, 181–197. 10.3945/ajcn.113.069880
88
Lake J. C. Hughes L. (1999). Nectar production and floral characteristics of Tropaeolum majus L. grown in ambient and elevated carbon dioxide. Ann. Bot. 84, 535–541. 10.1006/anbo.1999.0949
89
Larsen M. L. Wilhelm S. W. Lennon J. T. (2019). Nutrient stoichiometry shapes microbial coevolution. Ecol. Lett. 22, 1009–1018. 10.1111/ele.13252
90
Lau T.-C. Lu X. Koide R. T. Stephenson A. G. (1995). Effects of soil fertility and mycorrhizal infection on pollen production and pollen grain size of Cucurbita pepo (Cucurbitaceae). Plant Cell Environ. 18, 169–177. 10.1111/j.1365-3040.1995.tb00350.x
91
Lau T.-C. Stephenson A. G. (1994). Effects of soil phosphorus on pollen production, pollen size, pollen phosphorus content, and the ability to sire seeds in Cucurbita pepo (Cucurbitaceae). Springer-Verlag7, 215–220. 10.1007/BF00232740
92
Lau T. C. Stephenson A. G. (1993). Effects of soil nitrogen on pollen production, pollen grain size, and pollen performance in Cucurbita pepo (Cucurbitaceae). Am. J. Bot. 80, 763–768. 10.1002/j.1537-2197.1993.tb15292.x
93
Leaf A. (1989). Potential health effects of global climatic and environmental changes. N. Engl. J. Med. 321, 1577–1583. 10.1056/NEJM198912073212305
94
Lee K.-J. Kim K.-S. Kim H.-N. Seo J.-A. Song S.-W. (2014). Association between dietary calcium and phosphorus intakes, dietary calcium/phosphorus ratio and bone mass in the Korean population. Nutr. J.13:114. 10.1186/1475-2891-13-114
95
Liebig J. (1855). Principles of Agricultural Chemistry. London: Walton & Maberly.
96
Liu X. Lyu S. Sun D. Bradshaw C. Zhou S. (2017). Species decline under nitrogen fertilization reduces community-level resistance to fungal diseases. Proc. R. Soc. B Biol. Sci. 284:20162621. 10.1098/rspb.2016.2621
97
Liu Y. Chen W. Li D. Huang Z. Shen Y. Liu Y. (2011). Cyanobacteria-/cyanotoxin-contaminations and eutrophication status before Wuxi Drinking Water Crisis in Lake Taihu, China. J. Environ. Sci. 23, 575–581. 10.1016/S1001-0742(10)60450-0
98
Loladze I. (2002). Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry?Trends Ecol. Evol. 17, 457–461. 10.1016/S0169-5347(02)02587-9
99
Loladze I. (2014). Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife3:e03233. 10.7554/eLife.02245
100
Maavara T. Parsons C. T. Ridenour C. Stojanovic S. Dürr H. H. Powley H. R. et al . (2015). Global phosphorus retention by river damming. Proc. Natl. Acad. Sci. U.S.A.112, 15603–15608. 10.1073/pnas.1511797112
101
MacNeill K. L. (2019). Drivers of Nutrient and Toxic Element Cycles in Tropical and Temperate Montane Streams. Ithaca, NY: Cornell University.
102
Mathur R. Barlow G. M. (2015). Obesity and the microbiome. Exp. Rev. Gastroenterol. Hepatol.9, 1087–1099. 10.1586/17474124.2015.1051029
103
Mazur-Marzec H. Krezel A. Kobos J. Plinski M. (2006). Toxic Nodularia spumigena blooms in the coastal waters of the Gulf of Gdansk: a ten-year survey. Oceanologia48, 255–273.
104
McKenzie C. Tan J. Macia L. Mackay C. R. (2017). The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 278, 277–295. 10.1111/imr.12556
105
McKenzie V. J. Townsend A. R. (2007). Parasitic and infectious disease responses to changing global nutrient cycles. Ecohealth4, 384–396. 10.1007/s10393-007-0131-3
106
Meunier C. L. Malzahn A. M. Boersma M. (2014). A new approach to homeostatic regulation: towards a unified view of physiological and ecological concepts. PLoS ONE9, 3–9. 10.1371/journal.pone.0107737
107
Monteiro C. A. Moubarac J.-C. Cannon G. Ng S. W. Popkin B. (2013). Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 14, 21–28. 10.1111/obr.12107
108
Mueller N. D. Gerber J. S. Johnston M. Ray D. K. Ramankutty N. Foley J. A. (2012). Closing yield gaps through nutrient and water management. Nature490, 254–257. 10.1038/nature11420
109
Mulle J. G. Sharp W. G. Cubells J. F. (2013). The gut microbiome: a new frontier in autism research. Curr. Psychiatry Rep. 15:337. 10.1007/s11920-012-0337-0
110
Murrell E. G. Damal K. Lounibos L. P. Juliano S. A. (2011). Distributions of competing container mosquitoes depend on detritus types, nutrient ratios, and food availability. Ann. Entomol. Soc. Am. 104, 688–698. 10.1603/AN10158
111
Naujokas M. F. Anderson B. Ahsan H. Vasken Aposhian H. Graziano J. H. Thompson C. et al . (2013). The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect. 121, 295–302. 10.1289/ehp.1205875
112
Olde Venterink H. Wassen M. Verkroost A. De Ruiter P. (2003). Species richness–productivity patterns differ between N-, P-, and K-limited wetlands. Ecology84, 2191–2199. 10.1890/01-0639
113
O'Mara F. P. (2012). The role of grasslands in food security and climate change. Ann. Bot. 110, 1263–1270. 10.1093/aob/mcs209
114
Ostfeld R. S. Keesing F. (2012). Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst. 43, 157–182. 10.1146/annurev-ecolsys-102710-145022
115
Paerl H. W. Otten T. G. (2013). Harmful cyanobacterial blooms: causes, consequences, and controls. Microb. Ecol. 65, 995–1010. 10.1007/s00248-012-0159-y
116
Paige A. S. Bellamy S. K. Alto B. W. Dean C. L. Yee D. A. (2019). Linking nutrient stoichiometry to Zika virus transmission in a mosquito. Oecologia19, 1–10. 10.1007/s00442-019-04429-6
117
Pan Y. Subba Rao D. V. Mann K. H. (1996). Changes in domoic acid production and cellular chemical composition of the toxigenic diatom Pseudo-nitschia multiseries under phosphate limitation. J. Phycol. 32, 371–381. 10.1111/j.0022-3646.1996.00371.x
118
Parsons M. L. Dortch Q. (2002). Sedimentological evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnol. Oceanogr. 47, 551–558. 10.4319/lo.2002.47.2.0551
119
Paseka R. E. Grunberg R. L. (2019). Allometric and trait-based patterns in parasite stoichiometry. Oikos128, 102–112. 10.1111/oik.05339
120
Payne R. J. Dise N. B. Stevens C. J. Gowing D. J. Duprè C. Dorland E. et al . (2013). Impact of nitrogen deposition at the species level. Proc. Natl. Acad. Sci. U.S.A. 110, 984–987. 10.1073/pnas.1214299109
121
Peñuelas J. Poulter B. Sardans J. Ciais P. van der Velde M. Bopp L. et al . (2013). Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 4, 1–10. 10.1038/ncomms3934
122
Peñuelas J. Sardans J. Rivas-ubach A. Janssens I. A. (2012). The human-induced imbalance between C, N and P in Earth's life system. Glob. Chang. Biol. 18, 3–6. 10.1111/j.1365-2486.2011.02568.x
123
Perez-Moreno J. Read D. J. (2001). Exploitation of pollen by mycorrhizal mycelial systems with special reference to nutrient recycling in boreal forests. Proc. R. Soc. B Biol. Sci. 268, 1329–1335. 10.1098/rspb.2001.1681
124
Psaltopoulou T. Sergentanis T. N. Panagiotakos D. B. Sergentanis I. N. Kosti R. Scarmeas N. (2013). Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann. Neurol. 74, 580–591. 10.1002/ana.23944
125
Ptacnik R. Jenerette G. D. Verschoor A. M. Huberty A. F. Solimini A. G. Brookes J. D. (2005). Applications of ecological stoichiometry for sustainable acquisition of ecosystem services. Oikos109, 52–62. 10.1111/j.0030-1299.2005.14051.x
126
Razzaque M. S. (2011). Phosphate toxicity: new insights into an old problem. Clin. Sci. 120, 91–97. 10.1042/CS20100377
127
Ritz E. Hahn K. Ketteler M. Kuhlmann M. K. Mann J. (2012). Phosphate additives in food—a health risk. Dtsch. Arztebl. Int. 109, 49–55. 10.3238/arztebl.2012.0049
128
Rodriguez Castro M. C. Urrea G. Guasch H. (2015). Influence of the interaction between phosphate and arsenate on periphyton's growth and its nutrient uptake capacity. Sci. Total Environ. 503–504, 122–132. 10.1016/j.scitotenv.2014.06.094
129
Rusterholz H. P. Erhardt A. (1998). Effects of elevated CO2 on flowering phenology and nectar production of nectar plants important for butterflies of calcareous grasslands. Oecologia113, 341–349. 10.1007/s004420050385
130
Sanders A. J. Taylor B. W. (2018). Using ecological stoichiometry to understand and predict infectious diseases. Oikos127, 1399–1409. 10.1111/oik.05418
131
Sandström B. (2001). Micronutrient interactions: effects on absorption and bioavailability. Br. J. Nutr. 85:S181. 10.1079/BJN2000312
132
Sardans J. Peñuelas J. (2012). The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Phys.160, 1741–1761. 10.1104/pp.112.208785
133
Sardans J. Peñuelas J. (2015). Potassium: a neglected nutrient in global change. Glob. Ecol. Biogeogr. 24, 261–275. 10.1111/geb.12259
134
Sarris J. Logan A. C. Akbaraly T. N. Amminger G. P. Balanzá-Martínez V. Freeman M. P. et al . (2015). Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry2, 271–274. 10.1016/S2215-0366(14)00051-0
135
Schindler D. W. (1977). Evolution of phosphorus limitation in lakes. Science195, 260–262. 10.1126/science.195.4275.260
136
Schlesinger W. H. Bernhardt E. S. (2013). Biogeochemistry: An Analysis of Global Change,3rd Edn. San Diego, CA: Academic Press.
137
Schwabe R. F. Jobin C. (2013). The microbiome and cancer. Nat. Rev. Cancer13, 800–812. 10.1038/nrc3610
138
Selin N. E. (2009). Global biogeochemical cycling of mercury: a review. Annu. Rev. Environ. Resour. 34, 43–63. 10.1146/annurev.environ.051308.084314
139
Singer G. A. Battin T. J. (2007). Anthropogenic subsidies alter stream consumer-resource stoichiometry, biodiversity, and food chains. Ecol. Appl. 17, 376–389. 10.1890/06-0229
140
Smith S. J. Pitchera H. Wigley T. M. L. (2001). Global and regional anthropogenic sulfur dioxide emissions. Glob. Planet. Change29, 99–119. 10.1016/S0921-8181(00)00057-6
141
Smith V. (2007). Host resource supplies influence the dynamics and outcome of infectious disease. Integr. Comp. Biol. 47, 310–316. 10.1093/icb/icm006
142
Smith V. H. Holt R. D. (1996). Resource competition and within-host disease dynamics. Trends Ecol. Evol. 11, 386–389. 10.1016/0169-5347(96)20067-9
143
Sørmo E. G. Ciesielski T. M. Øverjordet I. B. Lierhagen S. Eggen G. S. Berg T. et al . (2011). Selenium moderates mercury toxicity in free-ranging freshwater fish. Environ. Sci. Technol.45, 6561–6566. 10.1021/es200478b
144
Steffen M. M. Davis T. W. Mckay R. M. L. Bullerjahn G. S. Krausfeldt L. E. Stough J. M. A. et al . (2017). Ecophysiological examination of the lake erie microcystis bloom in 2014: linkages between biology and the water supply shutdown of Toledo, OH. Environ. Sci. Technol.51, 6745–6755. 10.1021/acs.est.7b00856
145
Sterner R. W. Elser J. J. (2002). Ecological Stoichiometry: The Biology of Elements From Molecules to the Biosphere. Princeton, NJ: Princeton University Press. 10.1515/9781400885695
146
Stevens C. J. Dupr C. Dorland E. Gaudnik C. Gowing D. J. G. Bleeker A. et al . (2010). Nitrogen deposition threatens species richness of grasslands across Europe. Environ. Pollut. 158, 2940–2945. 10.1016/j.envpol.2010.06.006
147
Taipale S. Vuorio K. Strandberg U. Kahilainen K. Järvinen M. Hiltunen M. et al . (2016). Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int.96, 156–166. 10.1016/j.envint.2016.08.018
148
Tarleton E. K. Littenberg B. (2015). Response: re: magnesium intake and depression in adults. J. Am. Board Fam. Med. 28, 683–684. 10.3122/jabfm.2015.05.150194
149
Tester M. Langridge P. (2010). Breeding technologies to increase crop production in a changing world. Science818, 818–822. 10.1126/science.1183700
150
Thompson G. B. Brown J. K. M. Woodwars F. I. (1993). The effects of host carbon dioxide, nitrogen and water supply on the infection of wheat by powdery mildew and aphids. Plant. Cell Environ. 16, 687–694. 10.1111/j.1365-3040.1993.tb00487.x
151
Thornton P. K. van de Steeg J. Notenbaert A. Herrero M. (2009). The impacts of climate change on livestock and livestock systems in developing countries: a review of what we know and what we need to know. Agric. Syst. 101, 113–127. 10.1016/j.agsy.2009.05.002
152
Tilman D. (1997). The influence of functional diversity and composition on ecosystem processes. Science277, 1300–1302. 10.1126/science.277.5330.1300
153
Tilman D. Kilham S. Kilham P. (1982). Phytoplankton community ecology: the role of limiting nutrients. Annu. Rev. Ecol. Syst. 13, 349–372. 10.1146/annurev.es.13.110182.002025
154
Tilman G. D. (1996). Biodiversity: population versus ecosystem stability. Ecology77, 350–363. 10.2307/2265614
155
Townsend A. R. Howarth R. W. Bazzaz F. A. Booth M. S. Cleveland C. C. Collinge S. K. et al . (2003). Human health effects of a changing global nitrogen cycle. Front. Ecol. Environ. 1, 240–246. 10.1890/1540-9295(2003)001[0240:HHEOAC]2.0.CO;2
156
Tremlett H. Bauer K. C. Appel-Cresswell S. Finlay B. B. Waubant E. (2017). The gut microbiome in human neurological disease: a review. Ann. Neurol.81, 369–382. 10.1002/ana.24901
157
UN General Assembly (2015). Transforming Our World: The 2030 Agenda for Sustainable Development. New York, NY: United Nations.
158
Van de Waal D. Smith V. H. Declerck S. A. J. Stam E. C. M. Elser J. J. (2014). Stoichiometric regulation of phytoplankton toxins. Ecol. Lett. 17, 736–742. 10.1111/ele.12280
159
Van de Waal D. B. Elser J. J. Martiny A. C. Sterner R. W. Cotner J. B. (2018). Progress in ecological stoichiometry. Front. Microbiol. 9, 1–5. 10.3389/978-2-88945-621-5
160
Van de Waal D. B. Verspagen J. M. H. Lürling M. Van Donk E. Visser P. M. Huisman J. (2009). The ecological stoichiometry of toxins produced by harmful cyanobacteria: an experimental test of the carbon-nutrient balance hypothesis. Ecol. Lett. 12, 1326–1335. 10.1111/j.1461-0248.2009.01383.x
161
Van der Velde M. Folberth C. Balkovic J. Ciais P. Fritz S. Janssens I. A. et al . (2014). African crop yield reductions due to increasingly unbalanced Nitrogen and Phosphorus consumption. Glob. Chang. Biol. 20, 1278–1288. 10.1111/gcb.12481
162
Van Vuuren D. P. Bouwman A. F. Beusen A. H. W. (2010). Phosphorus demand for the 1970–2100 period: a scenario analysis of resource depletion. Global Environ. Change Hum. Policy Dimen.20, 428–439. 10.1016/j.gloenvcha.2010.04.004
163
Veresoglou S. D. Barto E. K. Menexes G. Rillig M. C. (2013). Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathol. 62, 961–969. 10.1111/ppa.12014
164
Vitousek P. Walker L. (1989). Biological invasion by Myrica faya in Hawai'i: plant demography, nitrogen fixation, ecosystem effects. Ecol. Monogr.59, 247–265. 10.2307/1942601
165
Walters D. M. Rosi-Marshall E. Kennedy T. A. Cross W. F. Baxter C. V. (2015). Mercury and selenium accumulation in the Colorado River food web, Grand Canyon, USA. Environ. Toxicol. Chem. 34, 2385–2394. 10.1002/etc.3077
166
Whitmee S. Haines A. Beyrer C. Boltz F. Capon A. G. De Souza Dias B. F. et al . (2015). Safeguarding human health in the Anthropocene epoch: report of the rockefeller foundation-lancet commission on planetary health. Lancet386, 1973–2028. 10.1016/S0140-6736(15)60901-1
167
WHO and UNICEF (2019). Progress on Household Drinking Water, Sanitation, and Hygiene 2000–2017: Special Focus on Inequalities. New York, NY: United Nations Children's Fund and World Health Organization.
168
Winder M. Reuter J. E. Schladow S. G. (2009). Lake warming favours small-sized planktonic diatom species. Proc. R. Soc. B Biol. Sci. 276, 427–435. 10.1098/rspb.2008.1200
169
Yang H.-C. Fu H.-L. Lin Y.-F. Rosen B. P. (2012). Pathways of arsenic uptake and efflux. Curr. Top. Membr.69, 325–358. 10.1016/B978-0-12-394390-3.00012-4
170
Yee D. A. Kaufman M. G. Ezeakacha N. F. (2015). How diverse detrital environments influence nutrient stoichiometry between males and females of the co-occurring container mosquitoes Aedes albopictus, Ae. aegypti, and Culex quinquefasciatus. PLoS ONE10, 1–18. 10.1371/journal.pone.0133734
171
Zhang M. Zhao Q. Xue P. Zhang S. Li B. Liu W. (2017). Do Si/As ratios in growth medium affect arsenic uptake, arsenite efflux and translocation of arsenite in rice (Oryza sativa)?Environ. Pollut. 229, 647–654. 10.1016/j.envpol.2017.06.078
172
Zhu C. Zhu J. Jiang Q. Xu X. Liu G. Kobayashi K. et al . (2018). Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 4, 1–9. 10.1126/sciadv.aaq1012
173
Zimmet P. Alberti K. G. Shaw J. (2010). Diabetes Epidemic. Macmillian Mag. Ltd.414, 782–787. 10.1038/414782a
174
Ziska L. H. Pettis J. S. Edwards J. Hancock J. E. Tomecek M. B. Clark A. et al . (2016). Rising atmospheric CO2 is reducing the protein concentration of a floral pollen source essential for north American bees. Proc. R. Soc. B Biol. Sci.283:20160414. 10.1098/rspb.2016.0414
Summary
Keywords
ecological stoichiometry, biological stoichiometry, public health, global change, food security, water quality, nutrition, disease
Citation
Paseka RE, Bratt AR, MacNeill KL, Burian A and See CR (2019) Elemental Ratios Link Environmental Change and Human Health. Front. Ecol. Evol. 7:378. doi: 10.3389/fevo.2019.00378
Received
21 July 2019
Accepted
20 September 2019
Published
10 October 2019
Volume
7 - 2019
Edited by
James Joseph Elser, University of Montana, United States
Reviewed by
Robert Ptacnik, Wasser Cluster Lunz, Austria; Irakli Loladze, Bryan College of Health Sciences, United States; Krista Capps, University of Georgia, United States
Updates
Copyright
© 2019 Paseka, Bratt, MacNeill, Burian and See.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel E. Paseka rachelpaseka@gmail.com
This article was submitted to Behavioral and Evolutionary Ecology, a section of the journal Frontiers in Ecology and Evolution
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.