- Chair of Silviculture, University of Freiburg, Freiburg, Germany
To mitigate negative impacts of drought stress in the face of climate change, mixtures of tree species such as those between European beech (Fagus sylvatica) and silver fir (Abies alba) are assumed to lower risks in forest management. This study investigates the influence of mixing beech and fir on tree growth in general and in particular on tree species responses to the extreme drought event of 2003. For this purpose, we analyzed basal area increment series and carbon isotope composition (δ13C) in wood of ~160 trees from three mixed-species sites in Germany and one site in Croatia. Overall growth performance for both fir and beech increased with proportions of the admixed species when accounting for the interactions with tree size and competition intensity. Mixing improved growth of large trees for both species irrespective of neighborhood density, whereas smaller trees benefitted only in denser neighborhoods. Positive mixing effects on radial growth were more pronounced in fir compared to beech, yet the latter benefitted by admixture of fir with regard to growth recovery following drought. Both the resistance of radial growth against reduction during drought as well as the variation of isotopic composition throughout the drought period were not affected by mixing, indicating that water-use in these two species was not complementary under drought stress. Although trees from both species exhibited growth reductions during the drought, fir maintained higher absolute growth levels than beech during the drought. Both species benefited from growing in mixed neighborhoods but complementary effects depended on tree size and neighborhood density. Mixing fir and beech leads to positive or neutral effects on growth performance of trees, also in response to an extreme drought event. Since increasing tree species richness also spreads the risks associated with extreme events, mixtures of beech and fir can be recommended as a possible alternative for more drought-sensitive stands such as spruce monocultures.
Introduction
Climate models predict rising average temperatures and also more frequent occurrences of extreme climatic events like storms, floods, heat waves, and droughts for the twenty-first century (Pachauri et al., 2014). In forestry, tree species mixtures are viewed as one of the most important approaches to adapt forests to the uncertainties of global change (Bauhus et al., 2017b). A growing number of studies have reported positive relationships between species diversity and forest productivity (Paquette and Messier, 2011; Vila et al., 2013; Jucker et al., 2014; Forrester and Bauhus, 2016) and tree diversity has also been shown to enhance resistance to pest outbreaks (Bauhus et al., 2017a; Jactel et al., 2017). However, whether diverse forests are also better adapted to more frequent and severe drought stress is less clear. A recent review on this topic concluded that there is no systematic relationship between tree diversity and drought tolerance across different forest ecosystems (Grossiord, 2019). However, Grossiord (2019) found that studies which reported that mixed-species forests are more resistant and resilient to drought stress (e.g., Pretzsch et al., 2013; Gazol et al., 2016) were more common than studies reporting negative effects (e.g., Grossiord et al., 2014a; Paquette et al., 2018). In addition, several studies found mixed effects, positive for one and negative for the admixed species (e.g., Condés and Del Río, 2015). Negative effects on drought tolerance may be expected in mixtures that grow faster than the monocultures of participating species since higher growth rates are typically associated with higher transpiration rates (Law et al., 2002; Forrester, 2015). Hence, the relationship between diversity and drought tolerance of trees is less straightforward as for example the more commonly reported positive effect of tree species diversity on resistance to pest outbreaks of specialist herbivore insects (Bauhus et al., 2017a). The inconsistent evidence regarding drought response of mixtures may be related to the fact that it is highly dependent on both tree species identity and site conditions because both jointly determine the absence or presence of mechanisms that lead to complementary water use such as differing species-specific rooting depths or different phenology (Forrester and Bauhus, 2016).
In Central Europe, tree species mixtures are being promoted to replace vulnerable forest stands that have been identified as areas for priority action in the endeavor to adapt forest landscapes to climate change (Bolte et al., 2009). For example, Norway spruce (Picea abies) still dominates large parts of the Central European forest landscape despite its documented susceptibility to droughts and wind throw. Recent observations across Central Europe confirm that the area suitable for Norway spruce cultivation will continue to decline with ongoing changing climate (Hanewinkel et al., 2013) even at higher elevations (Zang et al., 2014). Compared to Norway spruce, European beech (F. sylvatica) and silver fir (A. alba) have been shown to be less susceptible to summer droughts (Zang et al., 2011; Pretzsch et al., 2013; Vitali et al., 2017). Therefore, mixtures of European beech and silver fir, a natural species combination in many European mountain ranges, is currently promoted as an option for montane and upper montane forests in Central Europe (Zang et al., 2011, 2014). The natural distribution range of beech covers most of continental Europe and this species grows in (pure) broadleaved forests or in mixtures with conifer species including silver fir (Ellenberg, 1996). Studies from central and southern Europe have reported both decreasing (e.g., Jump et al., 2006; Gessler et al., 2007; Piovesan et al., 2008) and increasing growth trends for beech over the recent decades (Dittmar et al., 2003; Pretzsch et al., 2014; Tegel et al., 2014). Beech is considered only moderately drought tolerant and toward its southern distribution limit it may be replaced through actively managing for more drought-tolerant Quercus-species (Vitale et al., 2012). Silver fir is native to Europe and has a geographical distribution similar to that of beech but limited to mountainous regions. As fir grew well under the warmer conditions during the mid-Holocene (Tinner et al., 2013; Ruosch et al., 2016), it has been assumed that it would be a good replacement for the more drought-sensitive spruce at higher elevations. For example, in the Black forest, radial growth of spruce was found to more affected by drought than growth of fir and this effect was particularly pronounced at higher altitudes (van der Maaten-Theunissen et al., 2013; Vitali et al., 2017). Likewise, results of a study that included a large number of sites across Southern Germany indicate that fir can maintain growth rates also during severe drought events through its contact to ground water via deep tap roots if sufficient rainfall or snowmelt occurred early in the year (Zang et al., 2011). However, at lower elevations or in regions where high summer temperatures and soil water deficit represent major growth limiting factors, fir has been reported to be also vulnerable to drought (e.g., Battipaglia et al., 2009; Lebourgeois et al., 2010).
Stem growth of beech in mixed stands was reported to be higher than in mono-specific stands for Beech (Bosela et al., 2015; Mina et al., 2018b), and to some extent also for fir (Toigo et al., 2015; Mina et al., 2018b). Yet, with regard to the drought response of these two species, most knowledge stems from mono-specific stands or mixtures with other species. To our knowledge, only two studies have actually assessed the effect of mixing on the drought response of fir-beech mixtures (Lebourgeois et al., 2013; Gazol et al., 2016). In one of these studies, a lower sensitivity of growth to summer drought was found for fir when growing in mixture with beech and the largest benefits occurred at the driest sites (Lebourgeois et al., 2013). Neither of these studies analyzed the growth response of beech to drought when it was mixed with fir, and it remains unclear if benefits for fir came at the expense of beech or if both species benefitted from mixing with regard to their drought response
To study the response of trees to extreme drought events, one can either apply the drought experimentally (Magh et al., 2018), coincidentally measure physiological and growth processes during a drought phase (Isaac-Renton et al., 2018), or use a retrospective approach (Sohn et al., 2013), which was applied in this study. The latter is more suited to study drought influences at multiple sites and thus enable extrapolation of results to a larger population of inference (Gazol et al., 2018). Retrospective approaches analyze the variation of variables contained in the annual growth ring archive of a tree. Radial growth data can be used to understand how tree growth was influenced by forest management and environmental changes, including climate and weather, at multiple spatial and temporal scales. Yet in temperate regions like Central Europe, where tree growth shows less inter-annual variation (Fritts, 1976), stable carbon isotope ratios (δ13C) in wood have been found to be better or useful additional indicators of environmental variations compared to wood growth alone (Farquhar et al., 1989; Saurer et al., 1997; Schleser, 1999). Since drought conditions reduce stomatal aperture and thus leaf-internal CO2 partial pressure, photosynthetic discrimination against heavier 13CO2 of the atmosphere decreases, which leads to higher values of δ13C in wood (Farquhar et al., 1989). Therefore, the difference in δ13C of tree rings formed in wet and dry years is a direct indicator of the level of tree water stress, and this has been used in a number of recent studies to assess the modulating influence of tree species diversity on drought stress in individual species (Grossiord et al., 2014a,b; Forrester et al., 2016). For example, drought stress in trees of a boreal forests, as indicated by stronger increases of δ13C in the dry compared to the wet year, increased with tree species diversity (Grossiord et al., 2014b).
When analyzing the effect of mixtures of species on tree responses to drought events, one has to take into account additional factors that can have confounding effects on growth (Forrester and Bauhus, 2016). In stands with a large variation in tree size, large trees have been reported to be more severely affected by drought in terms of radial growth reductions than smaller trees (McDowell et al., 2011; Bennett et al., 2015; McDowell and Allen, 2015). In addition, transpiration, and therefore susceptibility to drought stress can be higher in overyielding mixtures that have a higher stand density than the monoculture counterparts (Barrufol et al., 2013; Pretzsch and Biber, 2016; Pretzsch and Schutze, 2016). Therefore, it is important to disentangle density from diversity effects when analyzing drought responses of mixed stands (Forrester, 2014).
So far, no study has investigated the effect of mixing on drought sensitivity in terms of both radial growth and isotopic composition for fir and beech. Therefore, the main aim of this study was to examine, if mixing these two species improves the drought tolerance of both species. For this purpose, we analyzed basal area increment (BAI) of 160 trees from three sites in south-western Germany and from one site in Croatia with a particular focus on periods of extreme drought stress. For beech and fir trees growing in neighborhoods of different admixture proportions, we tested whether mixing influenced the resistance, recovery, and resilience of growth to severe soil drought conditions. In addition, we compared the carbon isotope composition (δ13C) among years with different climatic conditions for trees in mixed vs. monospecific neighborhoods. We hypothesized that for both silver fir and European beech:
(1) Benefits of mixing on overall growth are similar for the two species and show similar patterns over time
(2) Mixing leads to a positive effect on the overall growth performance of both species but benefits vary with tree size and neighborhood competition
(3) Drought tolerance of trees is higher in mixed compared to monospecific neighborhoods
Materials and Methods
Study Sites and Stand Selection
Data were collected at four sites, three in the Black Forest in South-western Germany and one that was close to the city of Gospic in Croatia in the Velebit mountains (Table 1). Climate among sites in the Black Forest varies with altitude (ranging from 400 to 860 m.a.s.l.) corresponding to a decrease in mean temperature from 9.8 to 8.6°C and an increase of annual precipitation from 1,130 to 1,370 mm (Table 1). The Croatian site has similar annual precipitation as the highest-elevation site in the Black Forest. Soils at all sites are Cambisols which developed on limestone in Croatia and on paragneiss or sandstone at the German sites. Forest stands were selected for sampling based on the following criteria:
(1) Mixed stands dominated by fir and beech with each species having at least 30% of total stand basal area; percentage of other species below 15% basal area,
(2) Heterogeneous mixing within the stand; including sections with monospecific patches of each species as well as parts with fine-grained tree-wise mixtures,
(3) More or less even-aged and early mature stands, and
(4) Maximum of one thinning intervention in the last 5 years and stumps had to be datable to permit identification of the timing of interventions during the last decade.
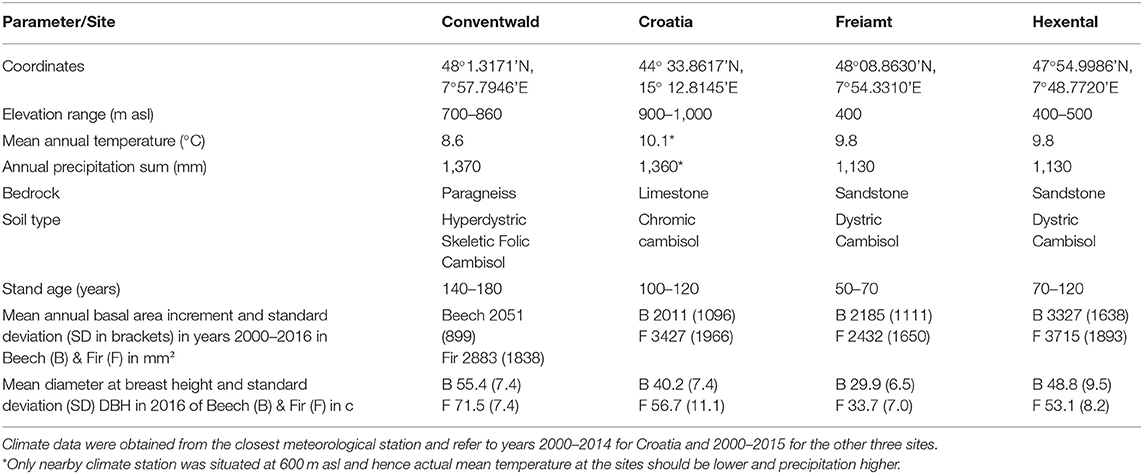
Table 1. Site and stand description for the three study sites in Germany and the fourth site in Croatia.
Data Collection, Tree-Ring Analyses, and Identification of the Drought Event
At each site, approximately twenty trees per species were selected evenly across three groups of neighborhood composition reflecting differing mixture proportions of fir and beech comprising trees with mostly conspecific neighbors, trees being surrounded by an even mix of the two species, and trees surrounded by heterospecific neighbors. Additional selection criteria for sample trees were that they had to (1) be of at least co-dominant status; (2) show no visible signs of injuries, damage, or loss in vitality; (3) have not more than one tree of a third species in their neighborhood; (4) have neighborhoods of comparable density (+/– closed conditions), and (5) not be part of the neighborhood of the next sample tree to avoid spatial correlation. The minimum distance between the selected trees within each stand varied among stands depending on their age and size. For each sample (= focal) tree, we recorded tree-ID, species, diameter at breast height (DBH) and extracted at that height two cores with an increment borer from perpendicular directions starting upslope and going in clockwise direction. Assuming that spatial extent of above ground competition also reflects below ground competition, trees were considered to be actual neighbors, and hence competitors of the central tree, if their crown interfered with the crown of the central trees. For each neighbor, we determined species identity and DBH and measured the distance to the central tree to determine neighborhood density and composition.
All increment cores were air-dried and then sanded with increasingly finer sandpaper. The polished cores were scanned and visually cross-dated with the WinDENDRO software (Regent Instruments Inc.). Next, the cross-validation software COFECHA (Grissino-Mayer, 2001) was used to check for possible cross-dating mistakes. We averaged the two cores per tree, assuming cross-sectional incremental areas of concentric shape. Mean annual basal area increments (BAI, mm2 year−1) of trees were calculated from annual radial increments (mm). Values before 2000 were excluded from further analyses to avoid the confounding impact of natural and thinning-related changes in the neighborhood, which could not be reconstructed prior to 2000 for the majority of stands. Since basal area increment is closely related to crown dimensions, which are rather stable in mature trees, detrending these data was not considered necessary for the short time period of investigation (2000–2016) (Sohn et al., 2016).
The well-documented Pan-European drought event of 2003, also obvious in precipitation and temperature data at the study sites (Figure 1), was selected for analyzing the effect of mixing on the drought response of growth.
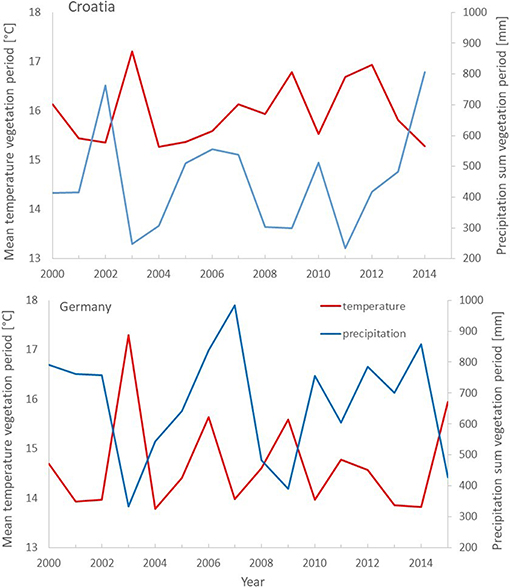
Figure 1. Climate diagrams for two weather stations in Gospic in Croatia (top) and Conventwald in Germany (bottom) depicting mean temperature (red) and sum of precipitation (blue) of the vegetation period (May to September) from 2000 to 2014 (15).
Analyses of Stable Carbon Isotopes
The carbon isotopic composition of dried wood samples was analyzed for all sample trees for a period of three consecutive years: 2002, 2003 and 2004, which included the drought event of 2003 as well as 1 year before and after the drought event. Measurements of δ13C were done using a DeltaPlus isotope ratio mass spectrometer (Thermo Fisher, Bremen, Germany) coupled via a ConFlowIII open-split to an elemental analyzer (Carlo Erba 1100 CE analyzer; Thermo Fisher Scientific, Rodano, Italy). For more details regarding the measurement procedure, see Werner et al. (1999), Werner and Brand (2001) and Brooks et al. (2003). δ13C values were calculated using the following equation,
where 13Rsample is the 13C/12C ratio of the sample, and 13Rstandard denotes the 13C/12C ratio of the standard. Values are expressed in per mil (%0) by multiplying the δ value with the factor 1000 (Coplen, 2011; Brand et al., 2012). The δ13C values are given on the δ13CIAEA-603–LSVEC scale by analyzing the samples against a calibrated in-house-standard (Acetanilide: −30.06 ± 0.05%0). A quality control standard (Caffeine: −40.46 %0) was interspersed between samples. The daily precision of the sequences was equal to or better than 0.1%0.
Data Analyses
To test our hypotheses (H1–H3), three types of analyses were conducted:
(1) Temporal analyses to test for the overall effect of mixing on growth complementarity of the two species over time (H1).
(2) Neighborhood analyses to determine the effect of mixing and other growth-relevant parameters on tree growth for a period for which exact data on neighborhood competition and composition were available (H2).
(3) Drought response analyses to test the influence of mixing on drought tolerance of trees (H3).
General Modeling Framework
To account for the replicated structure (species, trees and sites and years) and hence inherently correlated errors in our dataset, we used linear mixed-effects models (LMMs) to determine the effect of mixing on our response variables for all three analyses. The random structure of the mixed models (random effects, temporal autocorrelation, variance structure) was optimized in the presence of all fixed effects by comparing the AIC of models with different random structures using restricted maximum likelihood (REML) estimation following the procedure described by Zuur et al. (2009).
Fixed effects were selected only for the neighborhood analyses while a fixed set of predictors was used in both temporal and drought analyses (Table 2). All continuous, fixed effects were centered and scaled. The validity of model assumptions was evaluated using graphical tools (i.e., residual, autocorrelation and quantile-quantile plots). Models were fit with R (R Core Team, 2014), using the package nlme (Pinheiro et al., 2019) to allow for the specification of variance functions, to address heteroscedasticity and to model temporal autocorrelation. P-values were calculated with the package lmerTest based on Satterthwaite approximations of the degrees of freedom to test for the significance of the fixed effects (Kuznetsova et al., 2017). The modeling framework for each of the three analyses is summarized in the following sections and more detailed descriptions are presented in Supplementary Materials 1, 2.
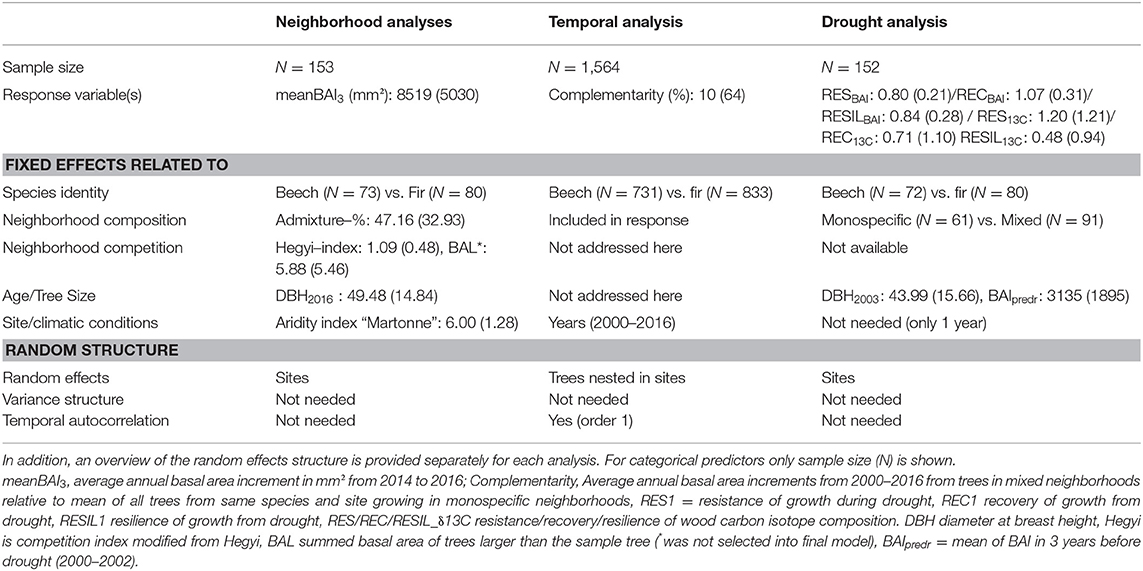
Table 2. Summary statistics (means and standard deviation SD in brackets) for response variables and (continuous) predictors used in the three models related neighborhood analysis (testing H2), temporal analysis (testing H1), and drought response analysis (testing H3).
Temporal Analysis: Testing H1
To examine if growth of the two tree species was positively affected by mixing and if mixing effects persisted over time, we calculated the response variable complementarity of annual growth (BAI) of the period 2000–2016 as a modification of the mixing response suggested by Vitali et al. (2018) and Forrester et al. (2013) as:
where BAIMix is the annual BAI of trees growing in mixed neighborhoods, and BAIMono is the average value of annual BAI of all trees in monospecific neighborhoods from the same species and site (as the mixed tree). Hence, the average growth over time of trees experiencing interspecific interactions is compared to that of trees which are subjected to intraspecific interactions. Complementarity values were transformed using the BoxCox-distribution to approach more normal distributions (see Supplementary Material 2). The mixed model included only Species and Year plus their interaction as fixed effects (Table 2). The optimal random structure was found to be trees nested in sites and an autocorrelation structure of order 1 (AC function) was included to take account of temporal autocorrelation of repeated measures (Table 2, Supplementary Material 2). In addition, temporal trends of BAI series were tested separately for each site and species using linear regression.
Neighborhood Analyses: Testing H2
To identify the most important growth-controlling variables for the two species at the tree neighborhood level, we analyzed the effect of admixing on tree growth while accounting for additional confounding factors. As response variable, we calculated mean annual growth by averaging annual values of BAI of the years 2014–2016 (meanBAI3) for all focal trees as this is more indicative of growth performance than BAI of a single year (2016). (In addition, mean BAI of the last 3 years was highly correlated to BAI in 2016 and to mean BAI of the last 5 years, r >0.9, see Supplementary Material 3). The log transformation was applied on the response variable meanBAI3 to obtain normal residuals. After determining the optimal random structure, we formulated the full hypothesis as:
In Equation (3), “*” denotes that main effect and interactions of the respective variables are considered in the model, and the “1|x” notation denotes a random intercept with grouping variable x. The variables in Equation (3) refer to:
(a) Admixed_prop: Admixture proportions (%) based on Hegyi-index
(b) Hegyi: a modified version of the competition index according to Hegyi (Lee and Gadow, 1997)
(c) BAL: Basal area of trees larger than the focal tree in m2
(d) Martonne: an aridity-index (Martonne, 1926)
(e) DBH: tree size at breast height in cm
(f) Species: binary, fir, and beech
(g) Site: location of the measurement
The variables (b–e) are confounding predictors, which were included in the full model because the annual variation of radial growth is known to depend on fluctuations of environmental and stand-related factors (Monserud and Sterba, 1996; Danescu et al., 2016). These confounding predictors and the model selection procedure leading to the final model shown in Table 2 are described in detail in Supplementary Materials 1, 3.
Analyses of the Tree-Level Drought Response: Testing H3
The effect of mixing on drought tolerance of beech and fir trees was analyzed using the following response variables regarding the growth and isotopic variation throughout the drought period. For tree growth, we calculated three drought response variables by dividing the observed growth into resistance of radial growth to drought (RES), its recovery from drought (REC), and the resilience to drought (RESIL) as suggested by Lloret et al. (2011) as
where value DY is the annual basal area increment (BAI) of each tree during the drought year (DY) 2003, value preDY is the BAI during the year(s) before the drought, and value postDY is the BAI during the year(s) following the drought of individual trees. In order increase the robustness of our results, we calculated the Lloret-indices based on both one and 2 year-long pre- and post-drought periods. We restricted this period to 2 years before and after the drought for calculating RES1/2BAI, REC1/2BAI, and RESIL1/2BAI to avoid any influence of the heavy and partial masting that took place in fir and beech in 2006. The six response variables were relatively independent from each other (r <0.67 see Supplementary Material 4) and hence should contain unique information. To obtain normal residuals, a log transformation was applied on all indices (except for RESILBAI calculated with 2 year periods, which could be directly used without any transformation).
For analyses of carbon isotopic composition (δ13C) in tree-rings, we selected the same years (2002–2004) that were used to calculate growth responses to drought. Analogous to calculations of growth resistance and following the analysis done by Schaefer et al. (2017), the drought resistance of δ13C (RES13C) was quantified as the ratio between the value of the dry year and the wet pre-DY (Equation 4a) so that, a higher value of RES13C reflects a smaller increase in δ13C in the dry year indicating a lower stomatal response and thus a lower level of drought stress. In addition, the recovery and resilience of δ13C following drought was calculated as the ratio between the post-DY 2004 and the DY 2003 (REC13C, Equation 4b) and between the post-DY 2004 and the pre-DY 2002 (RESIL13C, Equation 4c). The ratios RES13C and RESIL13C could be directly used, without transformation, as response variables in our models while REC13C had to be log-transformed to obtain a normal distribution.
As we did not extract increment cores from the neighbors of our central trees, and therefore could not calculate DBH of these trees in the past, actual data on neighborhood composition and competition was available only for the time of sampling in 2016. Therefore, we could not calculate neighborhood characteristics for the drought year 2003 and had to use a different set of predictors as used in the neighborhood analyses (Table 2 and more details on predictors in Supplementary Material 1).
To visualize the overall mixing effect on growth and isotopic response to drought, the complementarity of drought responses was calculated using the same framework as used for BAI (Equation 1) according to Vitali et al. (2018) as:
where RespMix is the average value of each of the drought response indices (resistance, recovery, and resilience of BAI and 13C) of each tree in mixed neighborhoods, and RespMono is the average value of either response of all trees in mono-specific neighborhoods from the same species and site.
Results
General Results
Annual mean BAI was higher in fir than beech in most years at the two higher altitude sites (Conventwald and Croatia, Figure 2) while the two species showed similar absolute growth rates at the two lower altitude sites (Hexental and Freiamt) throughout the period 2000–2016. A growth decline during the 2003 drought was visible for both species at all sites (Figure 2). Fir displayed a second growth depression in 2006, most likely due to a strong masting event and this decline was even more pronounced than the one in 2003 at the two lower sites (Freiamt and Hexental) (Figure 2). At the time of sampling, mean DBH of fir was significantly larger than that of beech at the two higher altitude sites (Conventwald, Croatia) while at the 2 lower sites trees of the two species had comparable average DBH (Supplementary Material 5). Yet, the competition index was similar for both species at all sites (Supplementary Material 5).
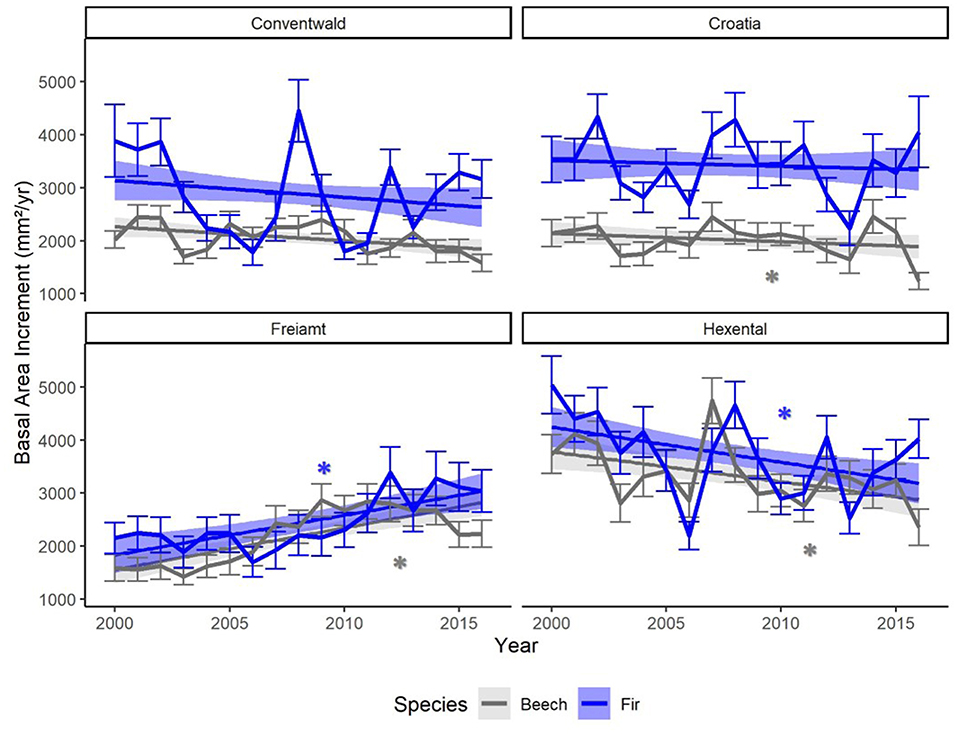
Figure 2. Means of annual basal area increments (BAI) of trees from two species (fir: blue and beech: gray) at four sites (Conventwald, Croatia, Freiamt, Hexental), from 2000 to 2016. Thin bars represent standard deviation. Trend-lines and bands represent smoothed conditional means using linear model (geom_smooth function, ggplot2 in R). Stars indicate a significant trend (p < 0.05) over time, which was detected based on linear regression of BAI vs. Year, separately for each site and species.
For beech, decreasing BAI trends over time are visible at two sites (Hexental and Croatia), while fir shows decreasing growth only at the Hexental site (Figure 2). Both species exhibited positive BAI trends at the Freiamt site but this is most likely an age-related effect as trees at this site were considerably younger than at the other sites (Table 1).
Mixing Effect on Growth and Over Time (H1)
Across all sites, fir had positive values for complementarity of BAI (mean annual growth of all trees in mixed vs. monospecific neighborhoods) with 20% higher growth in mixed as in monospecific neighborhoods when averaging values of years 2000–2016 (range 3–37%) (Figure 3). In contrast, for the same period overall lower values of BAI complementarity—including negative ones—were observed in beech leading to a net zero mean (from −9 to +9%) when averaging complementarity values of all years (Figure 3 and Supplementary Material 5).
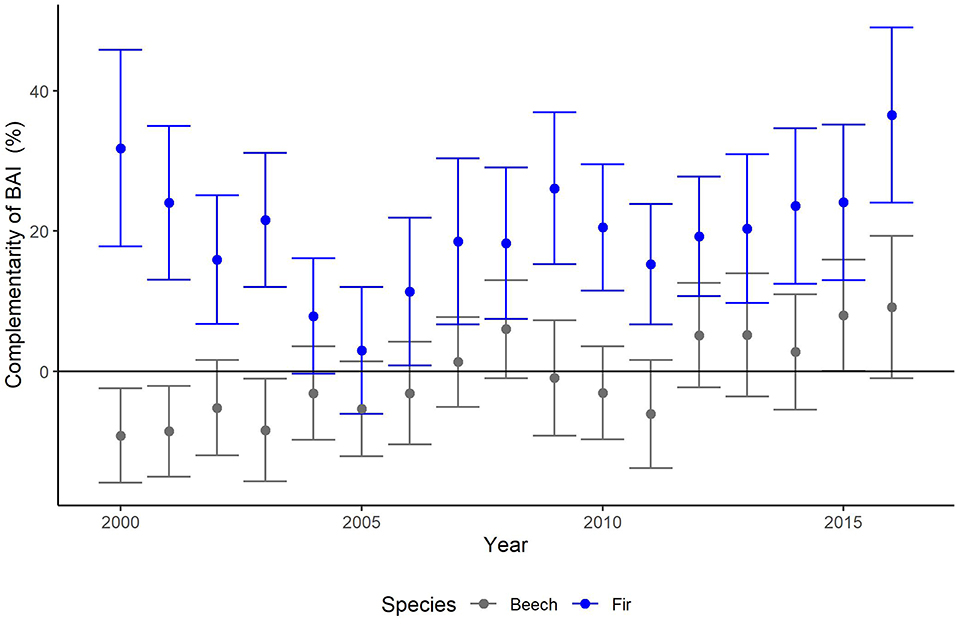
Figure 3. Annual means and SE (thin bars) of complementarity (%) of basal area increments (BAI) of beech (gray) and fir (blue) for years 2000–2016. Complementarity of BAI reflects the average growth (BAI) of trees experiencing interspecific interactions compared to that of trees, which are subjected to intraspecific interactions (see Equation 2 for calculation).
After applying the BoxCox-transformation (to obtain normal residuals), complementarity of BAI shows an increasing trend regardless of species (p < 0.01) (Table 3). The selection of random effect structure (using REML) led to trees nested in sites as the most important random effect with a moderate signal among sites and trees (Supplementary Material 2): Since complementarity values have, after transformation, approximately a range of 21, the SD of 2 for trees nested in sites represents 10% of the range of the response and the SD of 1.4 for residual error represent 7% of the response range. As the variance explained by the fixed effects in the mixed model was very low (Table 3, . only 0.03) and in view of the considerable proportion of the variability of the response absorbed by random effects, we looked at the site-specific patterns of growth complementarity over time. At three sites, fir showed positive values of growth complementarity in the majority of years (Figure 4) except for three negative values at the Conventwald site from 2005 to 2007 (Figure 4). However, at the fourth site (Hexental) negative values of growth complementarity in fir were observed (Figure 4). Interestingly this is the only site, where values of growth complementarity were consistently positive and even increased over time for beech (Figure 4). At the remaining three sites, growth complementarity of beech was either consistently negative (Croatia) or fluctuated around zero (Conventwald and Freiamt) (Figure 4).
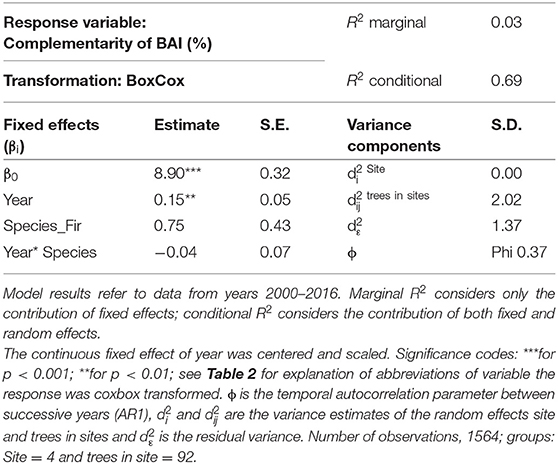
Table 3. Parameter estimates with standard errors (S.E.) of linear mixed model fit for temporal analyses by REML t-tests using Satterthwaite approximations to degrees of freedom for complementarity of BAI.
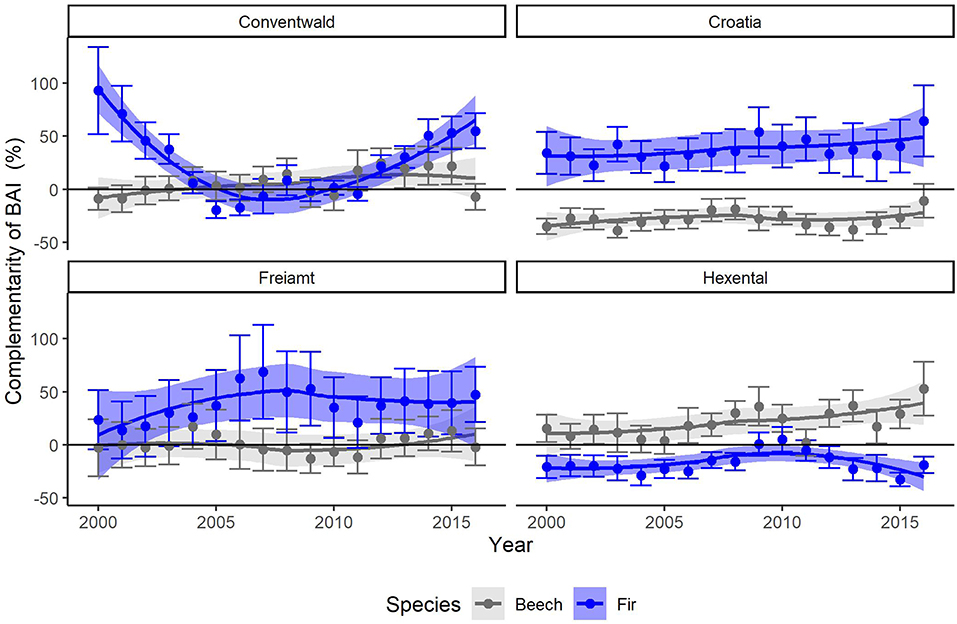
Figure 4. Annual means and SE (thin bars) of complementarity (%) of basal area increments (BAI) of beech (gray) and fir (blue) for years 2000–2016 for the 4 sites (Conventwald, Croatia, Freiamt, and Hexental). Bands represent loess-smoothed conditional means (geom_smooth function with ‘loess' and formula “y ~ x” with span = 1, ggplot2 in R). Complementarity of BAI reflects the average growth (BAI) of trees experiencing interspecific interactions compared to that of trees which are subjected to intraspecific interactions (see Equation 2 for calculation).
Drivers of Tree Growth at the Neighborhood Level (H2)
Average tree growth from 2014 to 2016 (meanBAI3) increased significantly with admixture proportions for fir at two sites while no relationship between meanBAI3 and admixture proportions was found for beech at any site (Figure 5). However, results of the most parsimonious mixed model indicate that in the presence of several confounding factors, meanBAI3 was positively related to admixture proportions in both species (p < 0.05) (Table 4). This highlights the need to take into account additional growth-relevant factors. Results of the mixed model indicated a direct positive effect of DBH on meanBAI3 (p < 0.001), and a direct negative effect of the competition index on meanBAI3 (p < 0.01). In addition, model outcomes showed two significant positive interactions of the relationship between admixture proportion and meanBAI3 with DBH and the Hegyi-index (both p < 0.001). The selection of the random effect structure (using REML) led to site as the most important random effect. The random effect variances showed a moderate variability of the signal among sites. Since meanBAI3 values assumed after transformation approximately a range of 3.6, the standard deviation of 0.40 represents 11% of the range of the response (likewise the standard deviation of 0.40 for the residual error represents 11% of the response range).
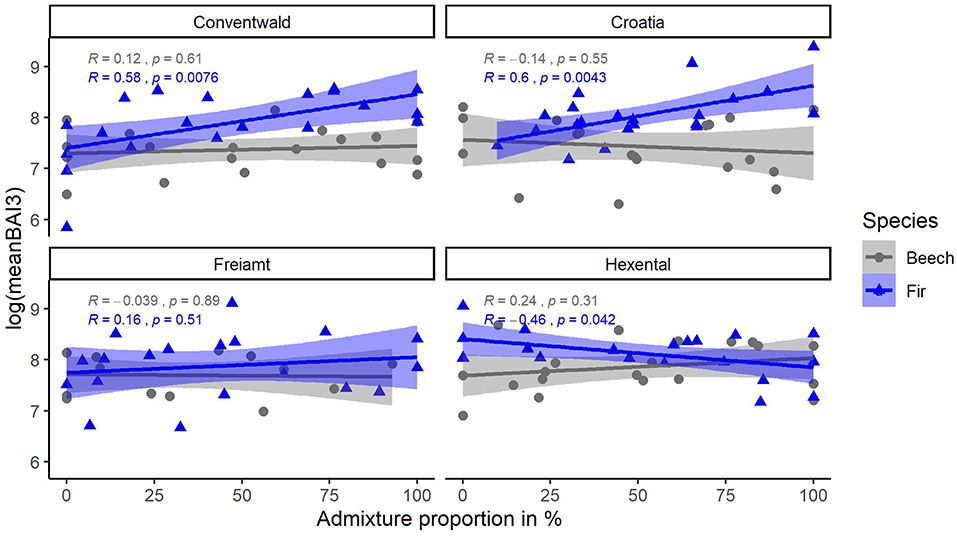
Figure 5. Relationship of (transformed) response variable meanBAI3 (average BAI of last 3 years) with admixture proportions for beech (gray) and fir (blue) trees at 4 sites (Conventwald, Croatia, Freiamt, and Hexental). Pearson-r and p-values refer to results of linear regression for each species and site separately, N = 14–20.
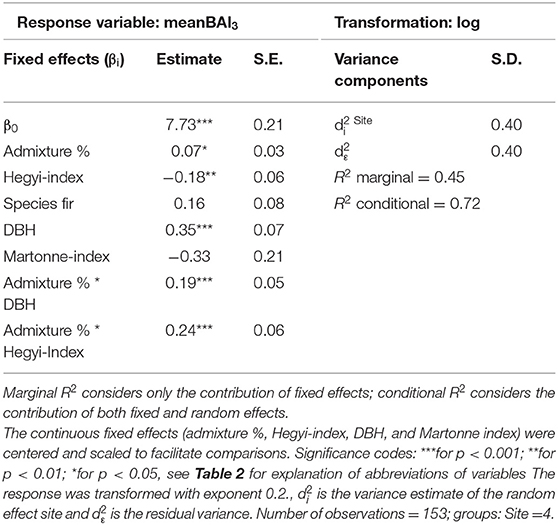
Table 4. Parameter estimates with standard errors (S.E.) of the best linear mixed model fit for neighborhood analyses by REML t-tests using Satterthwaite approximations to degrees of freedom.
To obtain a visual comparison of the interactive effects of tree size and competition with mixing, we plotted model predictions of the relationship between the response meanBAI3 with admixture proportions for (a) trees of different dimension in terms of DBH (at average levels of competition) and (b) trees of different levels of neighborhood competition (at average levels of DBH). For the Martonne index, we used the median value. As model predictions were similar for fir and beech (as indicated by the lack of interaction between admixing and species, Table 4), both species were combined for these predictions.
The relationship between admixture proportions and basal area growth became more positive with increasing tree size; switching from negative to positive for a DBH-value of ~50 cm (Figure 6A). Likewise, the relationship between admixture proportions and basal area growth became more positive with increasing competition levels; switching from negative to positive for a Hegyi-value around 1 (Figure 6B). In heterospecific neighborhoods (>70% admixed), tree growth became even higher in denser compared to more open conditions (Figure 6B). Note that due to the negative relationship between the competition index and DBH (Supplementary Material 5) high levels of competition (Hegyi-values of >1.5) were observed only in neighborhoods of trees with a DBH below 45 cm so that our finding of a positive effect of admixing at high competition levels is limited to smaller trees. Based on model predictions, we can summarize that basal area growth increases with admixture proportions in larger trees irrespective of competition levels (due to smaller absolute ranges of the Hegyi-index) and for smaller trees in denser neighborhoods (Figures 6A,B).
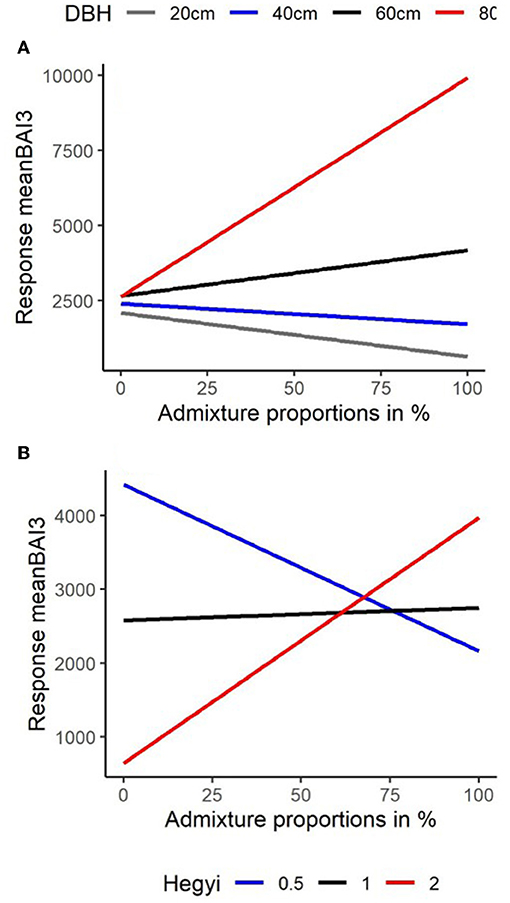
Figure 6. Comparison of model predictions (see Table 4) for the relationship between the (back-transformed) response meanBAI3 (y-axis) (average BAI from 2014 to 2016) with increasing admixture proportions (x-axis) in % for (A) trees of different dimensions in terms of DBH and (B) for 3 levels of neighborhood competition Hegyi = 0.5 (blue), Hegyi = 1.0 (Black), and Hegyi = 2.0 (Red).
Drought Response of Tree Growth (H3)
Beech showed significantly lower annual growth (BAI) in dry compared to normal years (P < 0.05) while BAI of fir was similar in dry and normal years (Supplementary Material 5). Fir exhibited significantly higher basal area growth than beech irrespective of climatic conditions (Supplementary Material 5). Growth (BAI) decreased on average by 18–20% for both beech and fir during the drought year compared to the previous year(s) across all sites but resistance varied considerably across sites with growth reductions ranging from only ~5% for beech at the Freiamt site up to ~30% for beech at the Hexental site (Supplementary Material 5). Results of the mixed models indicate that growth resistance during the 2003 drought was similar for the two species and did not differ between mixed and monospecific neighborhoods (Table 5, Figures 7A,B). Similarly, recovery of growth following drought and growth resilience to drought was similar in mixed and monospecific neighborhoods and neither recovery nor resilience of growth was different between the two species (Table 5). However, there was a significant interaction between species and mixing category for growth recovery 1 indicating faster recovery in beech than in fir in mixed neighborhoods (Table 5 and effect plots in Supplementary Material 2). This is also in line with the higher complementarity of recovery in beech compared to fir when comparing all trees across all sites (Figure 7). In addition, results of the mixed models indicate no direct effect of tree size on either RES BAI, REC BAI, or RESIL BAI but a significant interaction indicating that the relationship of RECBAI and RESILBAI with tree size is more negative in mixed compared to monospecific neighborhoods (Table 5 and effect plots in Supplementary Material 2). However, the proportion of variation explained by our selected predictors (all fixed effects) to test the third hypothesis was overall low and ranged from 2 to 29% (see R2 marginal values in Table 5). The selection of random effect structure (using REML), pointed to site as the most important random effect and the random effect variances showed a low variability of the signal among sites (The standard deviation for site represents merely <7% of the range of the responses, and the standard deviation for residual error represents <18% of the response range).
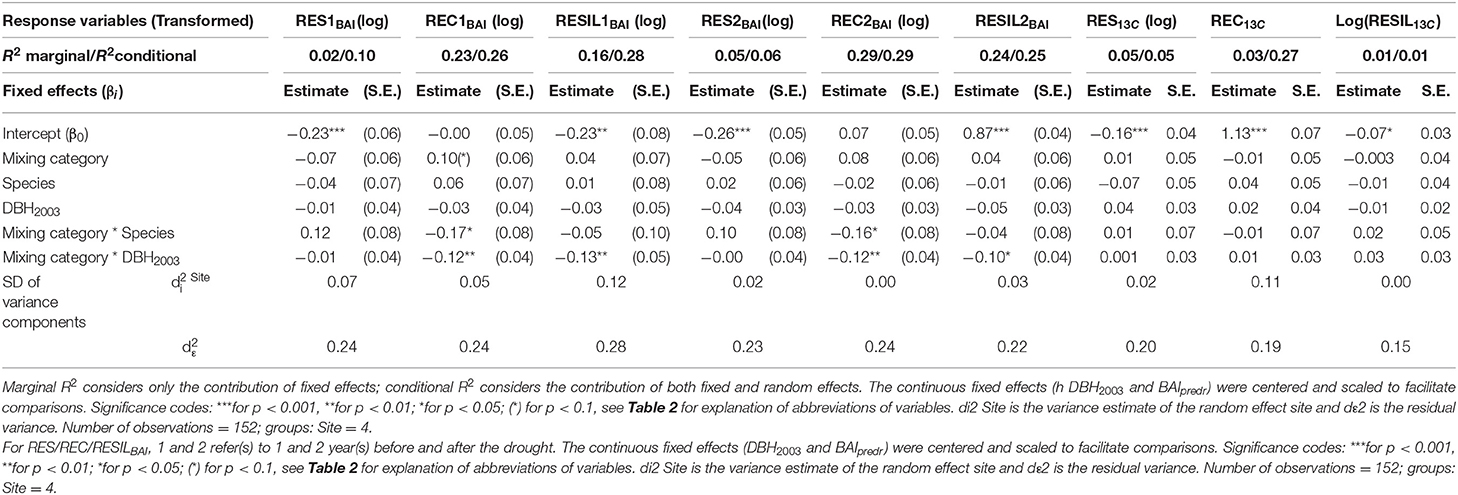
Table 5. Parameter estimates with standard errors (S.E.) of linear mixed model fit for drought analyses by REML t-tests using Satterthwaite approximations to degrees of freedom for the response variables reflecting growth and isotopic response to 2003 drought: resistance (RES), recovery (REC), and resilience (RESIL) of BAI and δ13C.
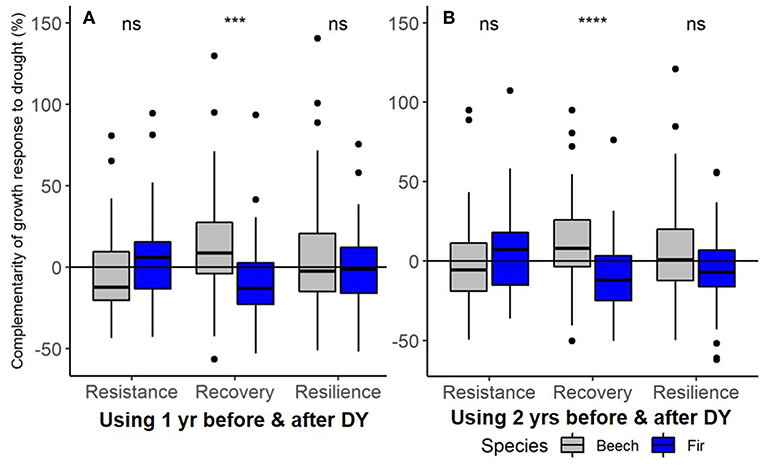
Figure 7. Complementarity effects of mixing on growth responses to the 2003-drought of European beech (gray) and silver fir (blue) in terms of resistance during drought (RES), recovery following drought (REC), and resilience to drought (RESIL) using (A) one and (B) two year(s) in the pre- and post-drought period. Stars (***P < 0.001 and ****P < 0.0001) indicate significant differences between the 2 species for each index and “ns” indicates no significant difference between the 2 species (P > 0.05) based on t-tests. Note that T-test was done for with transformed (∧0.25) data while figure depicts raw data.
Drought Response of Isotopic Composition (H3)
The inter-annual variation of δ13C follows a similar pattern in both species with a significant increase from 2002 to the drought year (2003) and a subsequent significant decrease from the drought to the post-drought year (2004) (Supplementary Material 5). This pattern was consistent across sites (Supplementary Material 5). Mean annual values of δ13C were significantly higher for fir as for beech in all years (p < 0.001, Supplementary Material 5) and at all sites (except for the site Hexental in 2003). Values of δ13C in each year were similar for trees in monospecific and mixed neighborhoods for both species (Figure 8). This is line with results of the mixed models, which indicated that resistance, recovery and resilience of δ13C was similar for the two species and for trees growing in mixed or monospecific neighborhoods (Table 5, Supplementary Material 5). Across all sites and trees, complementarity of isotopic response to drought (resistance, recovery and resilience of δ13C) was similar in beech and fir (Figure 9).
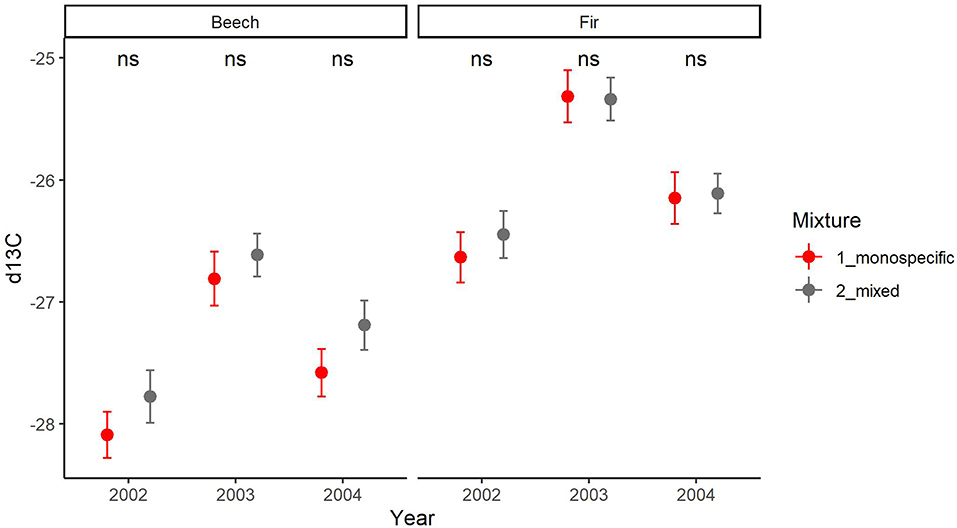
Figure 8. Comparison of means (SE depicted as thin bars) of carbon isotopic composition δ13C in wood of the years 2002, 2003, and 2004 between trees growing in monospecific (gray) and mixed (red) neighborhoods for beech (left) and fir (right). ns indicates that there is no significant difference between mixed and monospecific neighborhoods within species based on t-test.
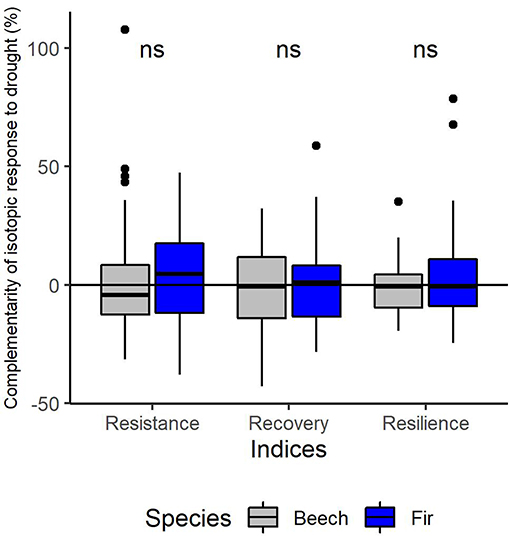
Figure 9. Complementarity effects of mixing on isotopic response to the 2003-drought of European beech (gray) and silver fir (blue) in terms of resistance during drought (RES), recovery following drought (REC) and resilience to drought (RESIL). “ns” Indicates no significant difference (P > 0.05) between the 2 species for each index based on t-tests.
Results of the mixed models indicate that there is neither a direct effect of DBH on the isotopic drought indices nor an interactive one of DBH with mixing category (Table 5). The selection of random effect structure (using REML), again pointed to site as the most important random effect and the random effect variances showed a low to moderate variability of the signal among sites. The standard deviation for site represents merely 0–9% of the range of the responses. The standard deviation for residual error was higher representing 15–17% of the response range. The variance explained by the selected fixed effects in the isotope models was very low with 1–5% (R2-marginal values in Table 5).
Discussion
Results of this study highlight the importance of incorporating data on actual neighborhood composition and competition when examining the effects of mixing on growth performance of individual species. Positive effects of mixing on overall growth performance were more pronounced in fir than in beech, yet the latter benefitted more from admixture of fir with regard to the growth recovery following drought. In contrast to our assumptions, both the growth resistance during drought as well as the variation in isotopic composition throughout the drought period were not affected by mixing. In the following we will first discuss our results in the same order as our hypotheses; regarding (1) effects of mixing on the overall and temporal growth performance of trees, (2) the interactive effect of mixing with other growth-relevant factors on overall growth performance and (3) how mixing affected the drought response of trees.
Mixing Effects on Overall Growth Performance and Over Time
Results of the neighborhood analyses indicate increasing growth with admixture proportions for both species, which is in accordance with our first hypothesis and other recent studies. Radial increment of beech and fir in mixtures responded positively to mixing but this was not directly compared with trees experiencing strictly monospecific conditions (Bosela et al., 2015). Several studies reported that growth of European beech increases, compared to mono-specific situations, in neighborhoods with other species (Pretzsch et al., 2010, 2013; Bosela et al., 2015; Toigo et al., 2015; Metz et al., 2016). Likewise, higher growth rates were observed for firs in functionally diverse neighborhoods when admixed with several species including beech (Gazol et al., 2016) and spruce (Vallet and Pérot, 2011). However, results of two other studies based on periodic stand-level inventories in mixtures of beech and fir indicated either no effects of mixing on growth (BAI) of either species (Del Rio et al., 2014) or positive mixing effects for beech at all sites, whereas positive effects for fir were restricted to sites of lower productivity (Toigo et al., 2015).
Our finding of positive mixing effects on growth of fir and beech indicates that for both species intra-specific competition was greater than inter-specific competition. We can only speculate about potential mechanisms behind the observed growth complementarity. Owing to their strongly different traits such as in crown and root architecture, leaf morphology, phenology, etc. they may use the resources in a complementary way or even facilitate each other (Forrester and Bauhus, 2016; Magh et al., 2018). In case of beech, mixing benefits have been commonly linked to a reduction in strong intra-specific competition and the development of larger crowns (Pretzsch and Biber, 2005; Dieler and Pretzsch, 2013; Mina et al., 2018a). Positive mixing effects on growth performance of fir may be the result of increased crown light capture in spring and autumn when beech is leafless (Ishii and Asano, 2010; Lebourgeois et al., 2010). Whether or not mixing may improve water or nutrient availability of the two species cannot be ascertained on the basis of this study.
In accordance with the second part of our hypothesis, beech displayed more negative growth (BAI) trends compared to fir across our sites but the temporal trajectories of BAI complementarity were positive and did not differ between the two species. Similarly, another European-wide study has reported declining growth trends in European beech but not in silver fir growing in mixed stands for a similar period, concluding that fir is less susceptible to warmer and drier conditions than beech (Bosela et al., 2018). Our findings with regard temporal trends of complementarity and growth should be interpreted with caution, however, as we analyzed only the most recent 16 years. In addition, our study shows that such broad trends may not occur at every site and that variability among sites in magnitude and direction is very high.
Mixing Benefits for Both Species Increase With Tree Size and Neighborhood Density
Results of our neighborhood analyses clearly demonstrate that overall growth performance for both fir and beech increases with admixture proportions when considering the interactions of admixing with tree size and with competition intensity; this is in full agreement with our second hypothesis. Hence, both trees size and the level of neighborhood competition are key factors in shaping species interactions. According to the estimates of our mixed model, the strongest effect, which explained most of the growth variation, was actually derived from the variation in DBH among trees and sites (note that size differences in our study are mainly due to age differences and not due to differences in canopy status). Our finding that mixing benefits increased with tree size in beech is in agreement with results of two recent studies, which reported increasing mixing benefits for beech with tree size/stand age in combination with spruce (Houpert et al., 2018) and pine (Forrester et al., 2017).
Mixing benefits for growth performance increased not only with tree size in both species but this relationship was additionally modulated by competition intensity in tree neighborhoods. This is an indication that for beech-fir mixtures, positive effects of competitive reduction outweigh any negative effects caused by interspecific competition. Two other studies reported also increasing complementarity with stand density in fir and spruce mixtures for both species (Forrester et al., 2013; Mina et al., 2018a). Likewise, the complementarity effect was greater at higher stand densities for beech in mixtures with pine (Condés et al., 2013; Bello et al., 2019).
Increasing complementarity with stand density is more likely to occur in mixtures where interactions lead to improved light capture (Forrester et al., 2013). Under these conditions, positive as well as negative species interactions have been found to be weaker at lower densities and hence complementarity may initially increase with stand density to a certain point (Forrester et al., 2013), which is in agreement with our results. Only at very high densities competition may outweigh any complementarity effects (Forrester et al., 2013). As our stands were at least 60 years old and because we did not sample trees of lower canopy status but only (co-)dominant individuals, it is likely that we never crossed the threshold after which negative density effects on complementarity appear. Since neighborhood density was comparable for trees growing in mixed and monospecific neighborhoods, we can rule out that the mixing effect was mediated by differences in stand density, which is often the case (Forrester, 2014).
Growth and Isotopic Response to Drought
According to our expectations, both species responded with substantial growth reductions of 20% on average to the drought in 2003. Although trees from both species exhibited similar growth resistance, fir maintained higher absolute growth levels as beech during the drought. The similar increase of 1.5%0 δ13C on average in both species indicates that during the drought year stomatal aperture and hence conductance was strongly and similarly controlled (Farquhar et al., 1989). In accordance with findings of Schaefer et al. (2017), we found a lower δ13C in the broadleaved compared to the evergreen species indicating a higher ci due to higher stomatal conductance and/or a lower rate of photosynthesis and accordingly different intrinsic water use efficiencies in the two species. Still, the comparable growth and isotopic response during the 2003 drought points toward a similar physiology and/or a similar length/timing of the wood formation period i.e., similar cambial activity, in these two shade-tolerant species (Zang et al., 2014). In contrast to our findings, Zang et al. (2014) found higher values of resistance, recovery and resilience in fir compared to beech. However, they analyzed several drought events and sites across a larger climatic gradient.
Mixing Did Not Affect the Resistance of Growth and δ13C During Drought
In disagreement with our third hypothesis, there was no indication for complementary water-use between these two species during a period of severe water limitations. However, absolute growth (BAI) during the drought year was significantly higher in fir than in beech. The lack of mixing benefits on growth resistance and isotopic response to drought for either of the two species is in accordance with several other studies for beech and fir (Grossiord et al., 2014b); fir (Lebourgeois et al., 2013; Gazol et al., 2016); and beech (Metz et al., 2016; Schaefer et al., 2017). In contrast, results of two other studies indicate mixing benefits for growth resistance in fir in mixture with spruce (Dănescu et al., 2018; Vitali et al., 2018).
Similar response to drought in mixed and monospecific neighborhoods indicate that the entire rooted soil profile dried out during the extreme drought year 2003 and that there was no advantage of deeper-rooted species. Under these conditions, any potential presence of mechanisms related to complementary water-use, such as spatial or temporal stratification, would have been insufficient to buffer soil moisture reductions (Forrester and Bauhus, 2016; Schaefer et al., 2017).
Viewed from another perspective, the finding of comparable growth and resistance of isotopic composition indicates that the overall faster growth of trees in mixed compared to monospecific neighborhoods was not a disadvantage for either of the species during drought (Forrester, 2014) as has been reported for other species combinations (Metz et al., 2016).
Mixing Improves Growth Recovery Following Drought in Beech
The complementarity of growth recovery was significantly higher in beech than in fir. This finding of greater mixing benefits for beech than for companion species is in agreement with other studies (Mölder and Leuschner, 2014; Metz et al., 2016). In contrast to our finding, fir trees growing in more functionally diverse stands recovered more quickly (Gazol et al., 2016).
The positive effects of fir trees on growth recovery of beech imply lower competitive stress once water became less limiting in the post-drought year. We can only speculate about the actual mechanisms behind the competitive reduction in beech in the year following the drought event. One possible explanation could be that beech fine-root systems with lower construction costs can recover more quickly from the drought (Meier and Leuschner, 2008)
The faster growth recovery of beech in mixed compared to monospecific neighborhoods was not accompanied by a higher recovery of carbon isotopic composition. This inconsistency suggests that growth processes are to some extent decoupled from physiological processes, perhaps through mixing-related changes in allocation patterns to above- or below-ground tissues at the tree level (Forrester and Bauhus, 2016). However, we cannot ascertain if and to what extent mixing beech and fir did indeed lead to structural adaptations at the tree level. The absence of a mixing effect on the recovery of δ13C may be the result of simultaneous changes in assimilation and stomatal conductance, which both affect δ13C but in opposing directions (Barbour et al., 2002).
Neither the resilience of growth and of δ13C was affected by species or mixing which is most likely due to persisting water shortages in 2004. This year was relatively dry at all sites, which is also indicated by δ13C values and growth not returning to pre-drought levels at the majority of sites.
Tree Size Affects the Growth Response Following Drought
Mixing benefits for growth recovery and resilience varied with tree size whereas no such interaction was found for growth resistance and the isotopic drought response. The relationship of recovery and resilience of growth with tree size was more negative in mixed compared to monospecific neighborhoods. This is in contrast with our findings on general growth performance where mixing benefits increased with tree size. However, results of a recent meta-analysis also indicate increasing drought impacts on stem growth with tree size (Bennett et al., 2015). We are not aware of a study that actually tested the combined effects of mixing and tree size on the drought response of trees and we will refrain from any further interpretations due to the low amount of variance explained by fixed plus random effects in all our drought response models (low R2s). The incorporation of climactic and/or neighborhood data referring to the pre-drought, drought and post-drought period may have improved the variance explained by our drought models considerably [see for example (Dănescu et al., 2018) but finding the best (most parsimonious model)] was not the aim of our study as we were interested in the overall mixing effect on growth and isotopic response of our tree to the extreme Pan-European drought in 2003.
Conclusion
Both species benefited from growing in mixed neighborhoods but complementarity effects were dependent on tree size and neighborhood density. Results of this study demonstrate that mixing silver fir and European beech leads to positive or neutral effects on growth performance of trees also in relation to an extreme drought event. Our results demonstrate that mixing fir and beech offers no advantages for mitigating growth responses during periods of extreme water shortage. However, mixing fir and beech can help to improve the growth recovery following drought in beech but not in fir. In addition, faster growth rates of trees of both species in mixed compared to monospecific neighborhoods have no disadvantages for their response to drought. Therefore, mixtures of beech and fir may be considered at appropriate sites as an alternative for more drought-sensitive Norway spruce forests.
Data Availability Statement
The datasets generated for this study are available at: https://freidok.uni-freiburg.de.
Author Contributions
JB conceived the study and acquired funding for the project. JB and JS designed the study and discussed and interpreted the results and contributed to the writing of the final manuscript. JS carried out data collection, performed the analysis, and drafted the manuscript.
Funding
Financial support by the Federal Minister of Agriculture (BML) and the Federal Minister of Environment (BMU) via the Federal Institute of Agriculture and Nutrition (BLE) in the frame of the project Buchen-Tannen-Mischwälder zur Anpassung von Wirtschaftswäldern an Extremereignisse des Klimawandels (BuTaKli) within the program Waldklimafondsis gratefully acknowledged (Grant No. FKZ/28W-C-1-069-01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the help of district forester Johannes Wiesler from the community Hexental in Breisgau-Hochschwarzwald for his personal support and the provision of an additional sampling site. The authors thank the forestry districts Breisgau-Hochschwarzwald and Emmendingen in Germany and Mladen Ivankovic from the Croatian Forest Research Institute Gopsic for supporting this study by providing data on tree composition of the forest stands. We also thank Nanja Unger, Nanja Unger, Fekxi Lindicke, Carlos Kuhlmann, Florentin Jäger, and Bárbara Magdalena San Martín for their assistance in fieldwork. We express our gratitude to Sven Hofmann and Nanja Unger for the many hours spend in the lab during sample preparation and processing of growth data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2019.00079/full#supplementary-material
References
Barbour, M. M., Walcroft, A. S., and Farquhar, G. D. (2002). Seasonal variation in delta13C and delta18O of cellulose from growth rings of Pinus radiata. Plant Cell Environ. 25, 1483–1499. doi: 10.1046/j.0016-8025.2002.00931.x
Barrufol, M., Schmid, B., Bruelheide, H., Chi, X., Hector, A., Ma, K., et al. (2013). Biodiversity promotes tree growth during succession in subtropical forest. PLoS ONE 8:e81246. doi: 10.1371/journal.pone.0081246
Battipaglia, G., Saurer, M., Cherubini, P., Siegwolf, R. T. W., and Cotrufo, M. F. (2009). Tree rings indicate different drought resistance of a native (Abies alba Mill.) and a nonnative (Picea abies (L.) Karst.) species co-occurring at a dry site in Southern Italy. For. Ecol. Manage. 257, 820–828. doi: 10.1016/j.foreco.2008.10.015
Bauhus, J., Forrester, D. I., Gardiner, B., Jactel, H., Vallejo, R., and Pretzsch, H. (2017a). “Ecological stability of mixed-species forests,” in Mixed-Species Forests. Ecology and Management, eds H. Pretzsch, D. I. Forrester, and J. Bauhus (Berlin, Heidelberg: Springer), 337–382. doi: 10.1007/978-3-662-54553-9_7
Bauhus, J., Forrester, D. I., and Pretzsch, H., (eds.). (2017b). “Mixed-Species Forests: the development of a forest management paradigm,” in Mixed-Species Forests. Ecology and Management, (Berlin; Heidelberg: Springer), 1–25. doi: 10.1007/978-3-662-54553-9_1
Bello, J., Vallet, P., Perot, T., Balandier, P., Seigner, V., Perret, S., et al. (2019). How do mixing tree species and stand density affect seasonal radial growth during drought events? For. Ecol. Manage. 432, 436–445. doi: 10.1016/j.foreco.2018.09.044
Bennett, A. C., McDowell, N. G., Allen, C. D., and Anderson-Teixeira, K. J. (2015). Larger trees suffer most during drought in forests worldwide. Nat. Plants 1:15139. doi: 10.1038/nplants.2015.139
Bolte, A., Ammer, C., Löf, M., Madsen, P., Nabuurs, G. -J., Schall, P., et al. (2009). Adaptive forest management in central Europe: climate change impacts, strategies and integrative concept. Scand. J. For. Res. 24, 473–482. doi: 10.1080/02827580903418224
Bosela, M., Lukac, M., Castagneri, D., Sedmák, R., Biber, P., Carrer, M., et al. (2018). Contrasting effects of environmental change on the radial growth of co-occurring beech and fir trees across Europe. Sci. Total Environ. 615, 1460–1469. doi: 10.1016/j.scitotenv.2017.09.092
Bosela, M., Tobin, B., Šeben, V., Petráš, R., and Larocque, G. R. (2015). Different mixtures of Norway spruce, silver fir, and European beech modify competitive interactions in central European mature mixed forests. Can. J. For. Res. 45, 1577–1586. doi: 10.1139/cjfr-2015-0219
Brand, W. A., and Coplen, T. B. (2012). Stable isotope deltas: tiny, yet robust signatures in nature. Isotopes Environ. Health Stud. 48, 393–409. doi: 10.1080/10256016.2012.666977
Brooks, P. D., Geilmann, H., Werner, R. A., and Brand, W. A. (2003). Improved precision of coupled delta13C and delta15N measurements from single samples using an elemental analyzer/isotope ratio mass spectrometer combination with a post-column six-port valve and selective CO2 trapping; improved halide robustness of the combustion reactor using CeO2. Rapid Commun. Mass Spectrom. 17, 1924–1926. doi: 10.1002/rcm.1134
Condés, S., and Del Río, M. (2015). Climate modifies tree interactions in terms of basal area growth and mortality in monospecific and mixed Fagus sylvatica and Pinus sylvestris forests. Eur. J. For. Res. 134, 1095–1108. doi: 10.1007/s10342-015-0912-0
Condés, S., Del Rio, M., and Sterba, H. (2013). Mixing effect on volume growth of Fagus sylvatica and Pinus sylvestris is modulated by stand density. For. Ecol. Manage. 292, 86–95. doi: 10.1016/j.foreco.2012.12.013
Coplen, T. B. (2011). Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 25, 2538–2560. doi: 10.1002/rcm.5129
Danescu, A., Albrecht, A. T., and Bauhus, J. (2016). Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 182, 319–333. doi: 10.1007/s00442-016-3623-4
Dănescu, A., Kohnle, U., Bauhus, J., Sohn, J., and Albrecht, A. T. (2018). Stability of tree increment in relation to episodic drought in uneven-structured, mixed stands in southwestern Germany. For. Ecol. Manage. 415-416, 148–159. doi: 10.1016/j.foreco.2018.02.030
Del Rio, M., Schutze, G., and Pretzsch, H. (2014). Temporal variation of competition and facilitation in mixed species forests in Central Europe. Plant Biol. 16, 166–176. doi: 10.1111/plb.12029
Dieler, J., and Pretzsch, H. (2013). Morphological plasticity of European beech (Fagus sylvatica L.) in pure and mixed-species stands. For. Ecol. Manage. 295, 97–108. doi: 10.1016/j.foreco.2012.12.049
Dittmar, C., Zech, W., and Elling, W. (2003). Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—a dendroecological study. For. Ecol. Manage. 173, 63–78. doi: 10.1016/S0378-1127(01)00816-7
Ellenberg, H. (1996). Vegetation Mitteleuropas mit den Alpen. Stuttgart: kologischer, dynamischer und historischer Sicht.
Farquhar, G. D., Ehleringer, J. R., and Hubick, K. T. (1989). Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537. doi: 10.1146/annurev.pp.40.060189.002443
Forrester, D. I. (2014). The spatial and temporal dynamics of species interactions in mixed-species forests. From pattern to process. For. Ecol. Manage. 312, 282–292. doi: 10.1016/j.foreco.2013.10.003
Forrester, D. I. (2015). Transpiration and water-use efficiency in mixed-species forests versus monocultures: effects of tree size, stand density and season. Tree Physiol. 35, 289–304. doi: 10.1093/treephys/tpv011
Forrester, D. I., Ammer, C., Annighöfer, P. J., Avdagic, A., Barbeito, I., Bielak, K., et al. (2017). Predicting the spatial and temporal dynamics of species interactions in Fagus sylvatica and Pinus sylvestris forests across Europe. For. Ecol. Manage. 405, 112–133. doi: 10.1016/j.foreco.2017.09.029
Forrester, D. I., and Bauhus, J. (2016). A review of processes behind diversity—productivity relationships in forests. Curr. For. Rep. 2, 45–61. doi: 10.1007/s40725-016-0031-2
Forrester, D. I., Bonal, D., Dawud, S., Gessler, A., Granier, A., Pollastrini, M., et al. (2016). Drought responses by individual tree species are not often correlated with tree species diversity in European forests. J. Appl. Ecol. 53, 1725–1734. doi: 10.1111/1365-2664.12745
Forrester, D. I., Kohnle, U., Albrecht, A. T., and Bauhus, J. (2013). Complementarity in mixed-species stands of Abies alba and Picea abies varies with climate, site quality and stand density. For. Ecol. Manage. 304, 233–242. doi: 10.1016/j.foreco.2013.04.038
Gazol, A., Camarero, J., and Gomez-Aparicio, J. L. (2016). Functional diversity enhances silver fir growth resilience to an extreme drought. J. Ecol. 104, 1063–1075. doi: 10.1111/1365-2745.12575
Gazol, A., Camarero, J. J., Sangüesa-Barreda, G., and Vicente-Serrano, S. M. (2018). Post-drought resilience after forest die-off: shifts in regeneration, composition, growth and productivity. Front. Plant Sci. 9:1546. doi: 10.3389/fpls.2018.01546
Gessler, A., Keitel, C., Kreuzwieser, J., Matyssek, R., Seiler, W., and Rennenberg, H. (2007). Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 21, 1–11. doi: 10.1007/s00468-006-0107-x
Grissino-Mayer, H. D. (2001). Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Res. 57, 2162–4585.
Grossiord, C. (2019). Having the right neighbors: how tree species diversity modulates drought impacts on forests. N. Phytol. doi: 10.1111/nph.15667. [Epub ahead of print].
Grossiord, C., Granier, A., Gessler, A., Jucker, T., and Bonal, D. (2014a). Does drought influence the relationship between biodiversity and ecosystem functioning in boreal forests? Ecosystems 17, 394–404. doi: 10.1007/s10021-013-9729-1
Grossiord, C., Granier, A., Ratcliffe, S., Bouriaud, O., Bruelheide, H., Checko, E., et al. (2014b). Tree diversity does not always improve resistance of forest ecosystems to drought. Proc. Natl. Acad. Sci. USA. 111, 14812–14815. doi: 10.1073/pnas.1411970111
Hanewinkel, M., Cullmann, D. A., Schelhaas, M. -J., Nabuurs, G. -J., and Zimmermann, N. E. (2013). Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 3, 203–207. doi: 10.1038/nclimate1687
Houpert, L., Brigitte, R., David Forrester, I., Marco, M., and Markus, H. O. (2018). Mixing effects in Norway Spruce—European beech stands are modulated by site quality, stand age and moisture availability. Forests 9:83. doi: 10.3390/f9020083
Isaac-Renton, M., Montwé, D., Hamann, A., Spiecker, H., Cherubini, P., and Treydte, K. (2018). Northern forest tree populations are physiologically maladapted to drought. Nat. Commun. 9:5254. doi: 10.1038/s41467-018-07701-0
Ishii, H., and Asano, S. (2010). The role of crown architecture, leaf phenology and photosynthetic activity in promoting complementary use of light among coexisting species in temperate forests. Ecol. Res. 25, 715–722. doi: 10.1007/s11284-009-0668-4
Jactel, H., Bauhus, J., Boberg, J., Bonal, D., Castagneyrol, B., Gardiner, B., et al. (2017). Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 3, 223–243. doi: 10.1007/s40725-017-0064-1
Jucker, T., Bouriaud, O., Avacaritei, D., Dănilă, I., Duduman, G., Valladares, F., et al. (2014). Competition for light and water play contrasting roles in driving diversity-productivity relationships in Iberian forests. J. Ecol. 102, 1202–1213. doi: 10.1111/1365-2745.12276
Jump, A. S., Hunt, J. M., and Peñuelas, J. (2006). Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 12, 2163–2174. doi: 10.1111/j.1365-2486.2006.01250.x
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Law, B. E., Falge, E., Gu, L., Baldocchi, D. D., Bakwin, P., Berbigier, P., et al. (2002). Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agri. For. Meteor. 113, 97–120. doi: 10.1016/S0168-1923(02)00104-1
Lebourgeois, F., Gomez, N., Pinto, P., and Mérian, P. (2013). Mixed stands reduce Abies alba tree-ring sensitivity to summer drought in the Vosges mountains, western Europe. For. Ecol. Manage. 303, 61–71. doi: 10.1016/j.foreco.2013.04.003
Lebourgeois, F., Rathgeber, C. B. K., and Ulrich, E. (2010). Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). J. Veg. Sci. 21, 364–376. doi: 10.1111/j.1654-1103.2009.01148.x
Lee, W. K., and Gadow, K. V. (1997). Iterative bestimmung der konkurrenzbäume in Pinus densiflora beständen. Allg. Forst Jagdztg. 168, 41–45.
Lloret, F., Keeling, E. G., and Sala, A. (2011). Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120, 1909–1920. doi: 10.1111/j.1600-0706.2011.19372.x
Magh, R. -K., Grün, M., Knothe, V. E., Stubenazy, T., Tejedor, J., Dannenmann, M., et al. (2018). Silver-fir (Abies alba MILL.) neighbors improve water relations of European beech (Fagus sylvatica L.), but do not affect N nutrition. Trees 32, 337–348. doi: 10.1007/s00468-017-1557-z
Martonne, E. D. (1926). L'indice d'aridité. Bull. Assoc. Geogr. Fr. 3, 3–5. doi: 10.3406/bagf.1926.6321
McDowell, N. G., and Allen, C. D. (2015). Darcy's law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 5, 669–672. doi: 10.1038/nclimate2641
McDowell, N. G., Bond, B. J., Dickman, L. T., Ryan, M. G., and Whitehead, D. (2011). “Relationships between tree height and carbon isotope discrimination,” in. Size- and Age-Related Changes in Tree Structure and Function, eds F. C. Meinzer, B. Lachenbruch, and T. E. Dawson (Dordrech: Springer), 255–286. doi: 10.1007/978-94-007-1242-3_10
Meier, I. C., and Leuschner, C. (2008). Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Change. Biol. 14, 2081–2095. doi: 10.1111/j.1365-2486.2008.01634.x
Metz, J., Annighofer, P., Schall, P., Zimmermann, J., Kahl, T., Schulze, E. -D., et al. (2016). Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Change. Biol. 22, 903–920. doi: 10.1111/gcb.13113
Mina, M., del Río, M., Huber, M. O., Thürig, E., and Rohner, B. (2018a). The symmetry of competitive interactions in mixed Norway spruce, silver fir and European beech forests. J. Veg. Sci. 29, 775–787. doi: 10.1111/jvs.12664
Mina, M., Huber, M. O., Forrester, D. I., Thürig, E., and Rohner, B. (2018b). Multiple factors modulate tree growth complementarity in Central European mixed forests. J. Ecol. 106, 1106–1119. doi: 10.1111/1365-2745.12846
Mölder, I., and Leuschner, C. (2014). European beech grows better and is less drought sensitive in mixed than in pure stands. Tree neighbourhood effects on radial increment. Trees 28, 777–792. doi: 10.1007/s00468-014-0991-4
Monserud, R. A., and Sterba, H. (1996). A basal area increment model for individual trees growing in even- and uneven-aged forest stands in Austria. For. Ecol. Manage. 80, 57–80. doi: 10.1016/0378-1127(95)03638-5
Pachauri, R. K., Allen, M. R., Barros, V. R., Broome, J., Cramer, W., Christ, R., et al. (2014). Climate change 2014: Synthesis Report. Geneva: Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change IPCC.
Paquette, A., Hector, A., Castagneyrol, B., Vanhellemont, M., Koricheva, J., Scherer-Lorenzen, M., et al. (2018). A million and more trees for science. Nat. Ecol. Evol. 2, 763–766. doi: 10.1038/s41559-018-0544-0
Paquette, A., and Messier, C. (2011). The effect of biodiversity on tree productivity: from temperate to boreal forests. Glob. Ecol. Biogeogr. 20, 170–180. doi: 10.1111/j.1466-8238.2010.00592.x
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D, and R Core Team. (2019). nlme: Linear and Nonlinear Mixed Effects Models. Available online at: https://CRAN.R-project.org/package=nlme (accessed September 27, 2019).
Piovesan, G., Biondi, F., Di Filippo, A., Alessandrini, A., and Maugeri, M. (2008). Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Change. Biol. 14, 1265–1281. doi: 10.1111/j.1365-2486.2008.01570.x
Pretzsch, H., and Biber, P. (2005). A re-evaluation of Reineke's rule and stand density index. For. Sci. 51, 304–320. doi: 10.1093/forestscience/51.4.304
Pretzsch, H., and Biber, P. (2016). Tree species mixing can increase maximum stand density. Can. J. For. Res. 46, 1179–1193. doi: 10.1139/cjfr-2015-0413
Pretzsch, H., Biber, P., Schütze, G., Uhl, E., and Rötzer, T. (2014). Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 5:4967. doi: 10.1038/ncomms5967
Pretzsch, H., Block, J., Dieler, J., Dong, P. H., Kohnle, U., Nagel, J., et al. (2010). Comparison between the productivity of pure and mixed stands of Norway spruce and European beech along an ecological gradient. Ann. For. Sci. 67:712. doi: 10.1051/forest/2010037
Pretzsch, H., and Schutze, G. (2016). Effect of tree species mixing on the size structure, density, and yield of forest stands. Eur. J. For. Res. 135, 1–22. doi: 10.1007/s10342-015-0913-z
Pretzsch, H., Schutze, G., and Uhl, E. (2013). Resistance of European tree species to drought stress in mixed versus pure forests: evidence of stress release by inter-specific facilitation. Plant Biol. 15, 483–495. doi: 10.1111/j.1438-8677.2012.00670.x
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna, Austria. Available online at: http://www.R-project.org/ (accessed September 27, 2019).
Ruosch, M., Spahni, R., Joos, F., Henne, P. D., ven der Knaap, W. O., and Tinner, W. (2016). Past and future evolution of Abies alba forests in Europe - comparison of a dynamic vegetation model with palaeo data and observations. Glob. Chang. Biol. 22, 727–740. doi: 10.1111/gcb.13075
Saurer, M., Borella, S., Schweingruber, F., and Siegwolf, R. (1997). Stable carbon isotopes in tree rings of beech: climatic versus site-related influences. Trees 11, 291–297. doi: 10.1007/s004680050087
Schaefer, C., Grams, T., Roetzer, T., Feldermann, A., and Pretzsch, H. (2017). Drought stress reaction of growth and Δ13C in tree rings of European beech and Norway spruce in monospecific versus mixed stands along a precipitation gradient. Forests 8:177. doi: 10.3390/f8060177
Schleser, G.-H. (1999). Isotope signals as climate proxies: the role of transfer functions in the study of terrestrial archives. Quat. Sci. Rev. 18, 927–943. doi: 10.1016/S0277-3791(99)00006-2
Sohn, J. A., Gebhardt, T., Ammer, C., Bauhus, J., Häberle, K.-H., Matyssek, R., et al. (2013). Mitigation of drought by thinning: short-term and long-term effects on growth and physiological performance of Norway spruce (Picea abies). For. Ecol. Manage. 308, 188–197. doi: 10.1016/j.foreco.2013.07.048
Sohn, J. A., Hartig, F., Kohler, M., Huss, J., and Bauhus, J. (2016). Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecol. Appl. 26, 2190–2205. doi: 10.1002/eap.1373
Tegel, W., Seim, A., Hakelberg, D., Hoffmann, S., Panev, M., Westphal, T., et al. (2014). A recent growth increase of European beech (Fagus sylvatica L.) at its Mediterranean distribution limit contradicts drought stress. Eur. J. For. Res. 133, 61–71. doi: 10.1007/s10342-013-0737-7
Tinner, W., Colombaroli, D., Heiri, O., Henne, P. D., Steinacher, M., Untenecker, J., et al. (2013). The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecol. Monogr. 83, 419–439. doi: 10.1890/12-2231.1
Toigo, M., Vallet, P., Perot, T., Bontemps, J. D, Piedallu, C., and Courbaud, B. (2015). Overyielding in mixed forests decreases with site productivity. J. Ecol. 103, 502–512. doi: 10.1111/1365-2745.12353
Vallet, P., and Pérot, T. (2011). Silver fir stand productivity is enhanced when mixed with Norway spruce: evidence based on large-scale inventory data and a generic modelling approach. J. Veg. Sci. 22, 932–942. doi: 10.1111/j.1654-1103.2011.01288.x
van der Maaten-Theunissen, M., Kahle, H. -P., and van der Maaten, E. (2013). Drought sensitivity of Norway spruce is higher than that of silver fir along an altitudinal gradient in southwestern Germany. Ann. For. Sci. 70, 185–193. doi: 10.1007/s13595-012-0241-0
Vila, M., Carrillo-Gavilan, A., Vayreda, J., Bugmann, H., Fridman, J., Grodzki, W., et al. (2013). Disentangling biodiversity and climatic determinants of wood production. PLoS ONE 8:e53530. doi: 10.1371/journal.pone.0053530
Vitale, M., Mancini, M., Matteucci, G., Francesconi, F., Valenti, R., and Attorre, F. (2012). Model-based assessment of ecological adaptations of three forest tree species growing in Italy and impact on carbon and water balance at national scale under current and future climate scenarios. iFor. Biogeosci. For. 5, 235–246. doi: 10.3832/ifor0634-005
Vitali, V., Büntgen, U., and Bauhus, J. (2017). Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob. Chang. Biol. 23, 5108–5119. doi: 10.1111/gcb.13774
Vitali, V., Forrester, D. I., and Bauhus, J. (2018). Know your neighbours: drought response of norway spruce, silver fir and douglas fir in mixed forests depends on species identity and diversity of tree neighbourhoods. Ecosystems 5:145. doi: 10.1007/s10021-017-0214-0
Werner, R. A., and Brand, W. A. (2001). Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 15, 501–519. doi: 10.1002/rcm.258
Werner, R. A., Bruch, B. A., and Brand, W. A. (1999). ConFlo III - an interface for high precision δ13C and δ15N analysis with an extended dynamic range. Rapid Commun. Mass Spectrom. 13, 1237–1241. doi: 10.1002/(SICI)1097-0231(19990715)13:13<1237::AID-RCM633>3.0.CO;2-C
Zang, C., Hartl-Meier, C., Dittmar, C., Rothe, A., and Menzel, A. (2014). Patterns of drought tolerance in major European temperate forest trees: climatic drivers and levels of variability. Glob. Chang. Biol. 20, 3767–3779. doi: 10.1111/gcb.12637
Zang, C., Rothe, A., Weis, W., and Pretzsch, H. (2011). Zur Baumarteneignung bei Klimawandel: ableitung der Trockenstress-Anfälligkeit wichtiger Waldbaumarten aus Jahrringbreiten. Allg. Forst. Jagdzt. 182, 98–112.
Keywords: climate change, water-use efficiency, neighborhood analysis, radial growth, dendro-ecology, resistance, recovery, resilience
Citation: Schwarz JA and Bauhus J (2019) Benefits of Mixtures on Growth Performance of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Increase With Tree Size Without Reducing Drought Tolerance. Front. For. Glob. Change 2:79. doi: 10.3389/ffgc.2019.00079
Received: 16 September 2019; Accepted: 11 November 2019;
Published: 26 November 2019.
Edited by:
David Findlay Scott, University of British Columbia Okanagan, CanadaReviewed by:
John L. Campbell, Forest Service (USDA), United StatesMark David Tomer, Agricultural Research Service (USDA), United States
Copyright © 2019 Schwarz and Bauhus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia A. Schwarz, anVsaWEuc2Nod2FyekB3YWxkYmF1LnVuaS1mcmVpYnVyZy5kZQ==
 Julia A. Schwarz
Julia A. Schwarz Jürgen Bauhus
Jürgen Bauhus