- 1Pediatric Pulmonology & Respiratory Intermediate Care Unit, Sleep and Long-Term Ventilation Unit, Academic Department of Pediatrics, Bambino Gesù Children's Hospital, Rome, Italy
- 2Pediatric Pulmonology & Regional Reference Centre for Pediatric Respiratory Failure and Cystic Fibrosis, Regina Margherita's Hospital, AOU Città della Salute e della Scienza, Turin, Italy
Central hypoventilation (CH) is a quite rare disorder caused by some congenital or acquired conditions. It is featured by increased arterial concentration of serum carbon dioxide related to an impairment of respiratory drive. Patients affected by CH need to be treated by mechanical ventilation in order to achieve appropriate ventilation and oxygenation both in sleep and wakefulness. In fact, in severe form of Congenital Central Hypoventilation Syndrome (CCHS) hypercarbia can be present even during the day. Positive pressure ventilation via tracheostomy is the first therapeutic option in this clinical condition, especially in congenital forms. Non-Invasive ventilation is a an option that must be reserved for more stable clinical situations and that requires careful monitoring over time.
Introduction
Central hypoventilation (CH) is a quite rare disorder caused by some congenital or acquired conditions. It is featured by increased arterial concentration of serum carbon dioxide (PaCO2) related to an impairment in respiratory drive (1). Diagnosis is not easy to do, as hypoventilation occurs mainly during sleep, but it must be made as early as possible, as hypoventilation can give serious long-term complications.
Pathophysiology
Hypoventilation refers to an increased PaCO2 due to inadequate gas exchange. This concept is summarized in the equation PaCO2 = K x VCO2/VA where K is a constant. It reads like this: PaCO2 is directly proportional to the body's CO2 production (VCO2) and inversely proportional to alveolar ventilation (VA). In other words, PaCO2 increases when CO2 production increases or alveolar ventilation decreases.
The breathing system is regulated by a set of receptors sensitive to changes in partial pressure of oxygen (PaO2), PaCO2 and hydrogen potential (pH), as well as other factors, such as the stretching of bronchial smooth muscle cells. Central chemoreceptors, located bilaterally below the ventro-lateral surface of the bulb, respond to small changes in PaCO2. Peripheral chemoreceptors are located in structures called glomas, which are located at the bifurcation of the common carotid artery (carotid glomas), and at the aortic arch (aortic glomas) and are sensitive to changes in PaO2, PaCO2 and pH (2, 3).
In healthy subjects, PaCO2 is the main ventilation stimulating factor. If PaCO2 increases, ventilation initially increases with a corresponding greater tidal volume, followed by an increase in respiratory rate. In case of hypoxia, ventilation initially increases but then decreases over time (4).
During sleep, the resistance of the upper airways increases, muscle tone decreases (in non-REM sleep) until it is completely abolished in sleep with rapid eye movement (REM), when breathing is maintained by the diaphragm only. During non-REM sleep, ventilation decreases and causes a slight increase in PaCO2 and a decrease in PaO2 compared to wakefulness. In the REM phase, breathing is superficial and irregular and the respiratory response of the respiratory centers to O2 and CO2 is reduced (5, 6).
Clinical Aspects
CH can be due to congenital or acquired conditions and the onset of symptoms can occur at different times depending on the underlying pathology. It's hard to find specific signs or symptoms diagnostic of central sleep apnea (CSA) and even more of hypoventilation, so clinicians must be aware that this pathway can occur in the following diseases.
Congenital central hypoventilation syndrome (CCHS), also known as Ondine's curse, is a rare condition that causes primary alveolar hypoventilation, firstly described in 1970 (7). In 2003 Amiel and collaborators (8) discovered the gene responsible of the disease, which is the paired-like homeobox 2B (PHOX2B), located on chromosome 4p12. This gene is a transcription factor which plays a central role in the differentiation of the neural lineage of autonomic nervous system. Most mutations occur de novo in CCHS patients, but inheritance can derive by parents with an autosomic dominant pattern with incomplete penetrance or with a mosaicism. Majority (90%) of patients with this congenital condition have a polyalanine expansion mutations (PARMs) in exon 3 (9). Other patients present nonsense, missense, frameshift or stop codon mutations in exon 1, 2, and 3, defined as non-polyalanine expansion mutations (NPARMs) (10). Over time, knowledge of the gene let us understand that PHOX2B mutation determines the phenotype of the patients (11, 12) and must guide care choices.
Patients with CCSH often present apnea and cyanosis in neonatal period, edema and signs of right heart failure associated with pulmonary hypertension, tachycardia and sweating during sleep, early breath holding spells, episodes of unexplained convulsions, severe Apparent Life-Threatening Events (13).
CCHS is also related to some tumors of neural crest origin (ganglioneuroma, neuroblastoma, ganglioneuroblastoma), or symptoms attributed to abnormal development of neural crest cells such as the following manifestations: ophtalmologic (anisocoria, strabismus), cardiovascular (alterations of cardiac rhythm or blood pressure disregulation), endocrinologic (hyperinsulinism, hypoglycemia, hyperglycemia), gastrointestinal (constipation, Hirschprung's disease) (14).
Pathophysiology of hypoventilation in CCHS is not clear yet. Breathing and autonomic dysregulation can be related to alterations of the brain, showed by functional or structural MRI, but it is not well understood if they can be determined in neurogenesis consequent to genetic mutations or secondary to hypoxic, hypercarbic, or perfusion damage (15). Moreover there are multiple impaired processes which can be causative factors in the onset of respiratory symptoms.
Rapid-onset obesity with hypothalamic dysfunction, hypoventilation and autonomic dysregulation (ROHHAD) is a rare disorder presenting in childhood with rapid weight gain, hypothalamic endocrine dysfunction, and severe hypoventilation. Clinical features include ophthalmologic abnormalities, altered thermoregulation, gastrointestinal dysmotility, behavior disorders, altered pain perception and tumor of neural crest origin (16). Respiratory phenotype of ROHHAD may initially present with OSA and only develop central hypoventilation later (17). Despite many clinical aspects in common with CCHS, mutations of PHOX2B are lacking in ROHHAD patients (18). A recent paper support the hypothesis of a possible aberrant immune process in pathogenesis of the disease (19).
Prader-Willi syndrome (PWS) is a genetic disorder due to loss of function of specific genes in chromosome 15. It is characterized by poor muscle tone often associated to feeding problems during infancy; in childhood an insatiable appetite often leads to obesity; patients are typically affected by mild to moderate intellectual impairment, behavioral problems, short stature, hypogonadism with genital hypoplasia, incomplete pubertal development (20).
PWS patients present impairments in ventilatory control: absent or altered hypoxic ventilatory response, reduced hypercapnic ventilatory response in obese subjects, altered pulmonary mechanics due to hypotonia, respiratory muscle weakness, kyphoscoliosis, and obesity. The phenotype of sleep disordered breathing evolves over time from a central pattern in infants to an obstructive one in older children and often hesitates in excessive daytime sleepiness (21). Long-term treatment with growth hormone is indicated for children with PWS but it may determine worsening of sleep-disordered breathing soon after the initiation; therefore an evaluation by polysomnography within the first 3–6 months of starting therapy should be repeated (22).
Other congenital diseases, as Familial dysautonomia (23), Arnold Chiari malformations (24), achondroplasia (25), disorders affecting mitochondrial metabolism (26) may result in central hypoventilation, within a wider spectrum of sleep disordered breathing. These patients must be investigated as well as the increasing population with acquired central hypoventilation which is constituted by those previously healthy children presenting damage of respiratory centers in the brain. Gangliogliomas and consequences from neurosurgical procedures were found mostly represented (27), but central nervous system infections, encephalitis, trauma, and other central nervous system tumors can be other onset reasons.
Diagnosis
Hypoventilation is caused by insufficient alveolar ventilation which causes altered blood gas values. American Academy of Sleep Medicine (AASM) (28, 29) scored hypoventilation during sleep when >25% of the total sleep time as measured by either the arterial PCO2 or surrogate is spent with a PCO2 > 50 mmHg. The process of arterial blood is the gold standard method to diagnose hypoventilation but its use during sleep is difficult and normally not available in a sleep laboratory. Therefore, AASM stated that an elevated PaCO2 obtained immediately after waking allows diagnosis of hypoventilation during sleep. So, surrogate measures such as transcutaneous PCO2 (PtcCO2) and end-tidal PCO2 (PETCO2) are commonly used and considered acceptable methods for assessing pediatric alveolar hypoventilation.
PtcCO2 has a good correlation with PaCO2, but its response time to acute changes in ventilation is longer than the PETCO2. The American Association for Respiratory Care Clinical Practice Guidelines (30) recommend that arterial blood gas values be compared to transcutaneous readings, in order to verify them in acute situations. PtcCO2 is a very useful device in order to monitor the adequacy of ventilation (31).
Monitoring of exhaled CO2 (capnography) is very used in children, but it is not accurate with low tidal volume and fast respiratory rates, as in infants or in acute distress, because a defined plateau in expiratory curve is not available. Usually PaCO2 is higher of PETCO2 between 2 and 7 mmHg (28). End-tidal PCO2 monitoring cannot be used during application of supplemental oxygen or during mask ventilation because exhaled gas sample is diluted and in case of mouth breathing.
Polysomnography is the gold standard to score central, obstructive or mixed events. Usually apneas recur during REM sleep, but in CCHS the events occur during NREM one. Central apnea is defined by cessation of airflow without respiratory effort and duration of 20 s or longer, or duration of two breaths during baseline breathing and association with an arousal or ≥3% oxygen desaturation, or, for infants younger than 1 year of age, duration of two breaths during baseline breathing and association with a decrease in heart rate to <50 beats per minute for at least 5 s or <60 beats per minute for 15 s (29).
Management
Clinicians must be aware of the different therapeutic options and be able to choose the correct one, even because the amount of ventilator assistance required in these syndromes is extremely variable.
Ventilation
In patients requiring continuous ventilation, positive-pressure ventilation via tracheostomy is the most common and effective method of treatment (1). American Thoracic Society statement still advice this ventilation option in the first years of life for CCHS patients (13) since ventilator system becomes more stable with age.
If possible, tracheostomy tube should be cuffless and smaller than airways dimension, in order to reduce the risk of tracheomalacia and to consent the utilization of speaking-valve when patient is not ventilated.
Non-invasive positive-pressure ventilation (NPPV) provides adequate ventilation through a mask; this modality can be chosen in patients requiring ventilation only during sleep. Its major benefit is the avoidance of tracheostomy and its complications. Nasal masks are the most used, as in all populations that perform long-term ventilation. Bilevel positive airway pressure (BiPAP) ventilation mode is the most used as it provides variable continuous flow, with fixed inspiratory and expiratory positive airway pressure whose difference is proportional to tidal volume. As children with central hypoventilation syndromes don't trigger the ventilator adequately during sleep, the timed mode with a set respiratory rate is to be preferred.
Recently intelligent volume-assured pressure support (iVAPS), which can modulate pressure support to ensure constant alveolar ventilation (32) has been proponed for CCHS patients. The rationale for the proposal is that, since these patients have variable ventilation between REM and NREM sleep, pressure is automatically modulated to better control carbon dioxide levels throughout the night (33). Some manufacturers indicate that iVAPS is FDA cleared for patients weighing more than 66 lb (>30 kg). A 10 months old CCHS infant report shows that, in case of availability of an adequate nasal mask, of a correct education of the parents and of a mid-face hypoplasia prevention program, it is possible to use NIV in AVAPS mode (average volume assured pressure support), which it is similar to iVAPS, even in younger children. This ensures less oscillation of the PaCO2 values during sleep (34).
Mid-face hypoplasia is a frequent complication of mask ventilation, especially if introduced in infancy. Provide two different masks with different points of pressure on the face is a good strategy, but also a closely follow up by pediatric maxillofacial surgeon and orthodontist should be performed.
An increasing number of children with CCHS have been successfully transitioned from invasive ventilation to BiPAP ventilation and recently a proposal of an algorithm for decannulation was published (35). By the moment there are not specific indications about optimal time to switch from tracheostomy to NPPV because it is a choice closely linked to the patient and his family, but ending the decannulation program before adolescence can be a good option.
Negative-pressure ventilation (NPV) causes inspiration as it creates a negative inspiratory pressure around the chest of the patient, using a bell of his appropriate size.
A small group of CCHS children passed from invasive ventilation to negative pressure ventilation removing tracheostomy (36) but its use is really limited because of non-portability, obliged supine position during sleep, skin irritation, uncomfort, risk of obstructive sleep apneas relied to asynchrony between vocal cords opening and thoracic inspiratory efforts (1).
In patients with central hypoventilation syndromes, a second ventilator in the home is necessary, in the event of technical failure. Moreover, if ventilation is continuous, a power generator or a continuous energy supply according with local energy company must be available.
Diaphragm Pacing
Diaphragm pacing can generate breathing using the child's own diaphragmatic contraction by the electrical stimulation of the phrenic nerve (37). This device is very useful in patients with central hypoventilation syndrome requiring ventilator support 24 h a day. Contrary to adults, in children ventilation is still necessary as diaphragm needs a rest some hours a day but, moreover, because complete airway obstruction after pacing implantation is described as upper airway muscle contraction is not synchronous with paced inspiration (38).
These patients require careful and structured management over time. In Figure 1 we suggested the tests to be performed over time for central hypoventilation.
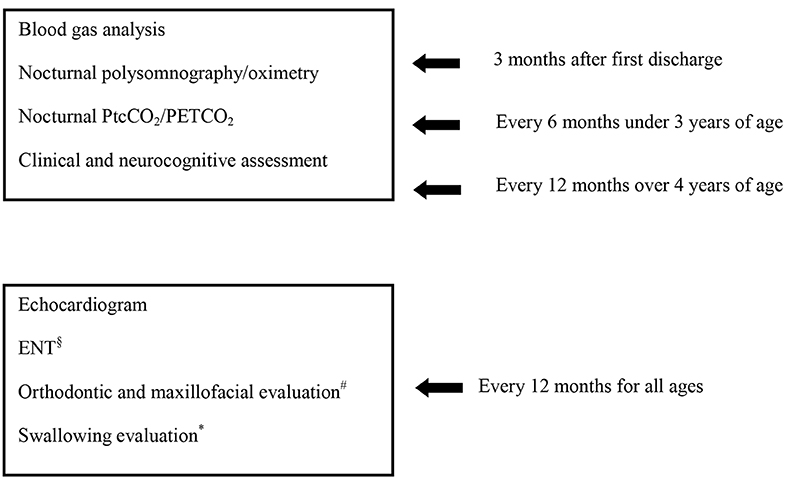
Figure 1. Follow up program for clinically stable patients with central hypoventilation. §In case of tracheostomy. #In case of NIV, after 3 years of age. *Evaluation carried out as needed.
Obviously, the pathologies underlying hypoventilation will require specific follow up for the disease and its complications.
Last but not least, the follow up must be individualized on the basis of the specific characteristics of the patient, with a prompt response in the event of exacerbations or clinical worsening.
Conclusion
Central hypoventilation syndrome is the clinical manifestation of a heterogeneous group of pathologies. Therefore, clinician must adequately treat respiratory impairment, but also other aspects related to primary disease with a multidisciplinary approach.
Ventilation must be suited individually, basing on clinical pathway, on the amount of daily time spent in ventilation, on patient and family decision, if possible. Non-invasive ventilation has multiple advantages, but it requires a considerable effort and needs a careful monitoring over time.
Author Contributions
MP designed the review in collaboration with IE. MG and ER contributed to the content. EB and RC revised the content of review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cielo CM, Marcus CL. Central hypoventilation syndromes. Sleep Med Clin. (2014) 9:105–18. doi: 10.1016/j.jsmc.2013.10.005
2. Kc P, Martin RJ. Role of central neurotransmission and chemoreception on airway control. Respir Physiol Neurobiol. (2010) 173:213–22. doi: 10.1016/j.resp.2010.03.020
3. Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol. (2010) 95:657–67. doi: 10.1113/expphysiol.2009.049312
4. Gozal D, Kheirandish-Gozal L. Disorders of breathing during sleep. In: Wilmott RW, editor. Kendig and Chernick's Disorders of the Respiratory Tract in Children. Philadelphia, PA: Elsevier (2012). p. 1067–86. doi: 10.1016/B978-1-4377-1984-0.00077-2
5. Colrain IM, Trinder J, Fraser G, Wilson GV. Ventilation During Sleep Onset. J Appl Physiol. (1985) 63:2067–74. doi: 10.1152/jappl.1987.63.5.2067
6. Housley GD. Recent insights into the regulation of breathing. Auton Neurosci. (2011) 164:3–5. doi: 10.1016/j.autneu.2011.08.002
7. Mellins RB, Balfour HH, Turino GM, Winters RW. Failure of automatic control of ventilation (Ondine's curse). Medicine. (1970) 49:487–504. doi: 10.1097/00005792-197011000-00003
8. Amiel J, Laudier B, Attié-Bitach T, Trang H, de Pontual L, Gener B, et al. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. (2003) 33:459–61. doi: 10.1038/ng1130
9. Weese-Mayer DE, Rand CM, Berry-Kravis EM, Jennings LJ, Loghmanee DA, Patwari PP, et al. Congenital central hypoventilation syndrome from past to future: model for translational and transitional autonomic medicine. Pediatr Pulmonol. (2009) 44:521–35. doi: 10.1002/ppul.21045
10. Parodi S, Bachetti T, Lantieri F, Di Duca M, Santamaria G, Ottonello G, et al. Parental origin and somatic mosaicism of PHOX2B mutations in congenital central hypoventilation syndrome. Hum Mutat. (2008) 29:206–16. doi: 10.1002/humu.9516
11. Matera I, Bachetti T, Puppo F, Di Duca M, Morandi F, Casiraghi GM, et al. PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset central hypoventilation syndrome. J Med Genet. (2004) 41:373–80. doi: 10.1136/jmg.2003.015412
12. Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE. Congenital central hypoventilation syndrome, PHOX2B mutations and phenotype. Am J Respir Crit Care Med. (2006) 174:1139–44. doi: 10.1164/rccm.200602-305OC
13. Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med. (2010) 181:626–44. doi: 10.1164/rccm.200807-1069ST
14. Bishara J, Keens TG, Perez IA. The genetics of congenital central hypoventilation syndrome: clinical implications. Appl Clin Genet. (2018) 11:135–44. doi: 10.2147/TACG.S140629
15. Patwari PP, Carroll MS, Rand CM, Kumar R, Harper RM, Weese-Mayer DE. Congenital central hypoventilation syndrome and the PHOX2B gene: a model of respiratory and autonomic dysregulation. Respir Physiol Neurobiol. (2010) 173:322–35. doi: 10.1016/j.resp.2010.06.013
16. Patwari PP, Wolfe LF. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: review and update. Curr Opin Pediatr. (2014) 26:487–92. doi: 10.1097/MOP.0000000000000118
17. Reppucci D, Hamilton J, Yeh EA, Katz S, Al-Saleh S, Narang I. ROHHAD syndrome and evolution of sleep disordered breathing. Orphanet J Rare Dis. (2016) 11:106–14. doi: 10.1186/s13023-016-0484-1
18. Rand CM, Patwari PP, Rodikova EA, Zhou L, Berry-Kravis EM, Wilson RJA, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: analysis of hypothalamic and autonomic candidate genes. Pediatr Res. (2011) 70:375–78. doi: 10.1203/PDR.0b013e318229474d
19. Giacomozzi C, Guaraldi F, Cambiaso P, Niceta M, Verrillo E, Tartaglia M, et al. Anti-hypothalamus and anti-pituitary auto-antibodies in ROHHAD syndrome: additional evidence supporting an autoimmune etiopathogenesis. Horm Res Paediatr. (2019) 92:124–32. doi: 10.1159/000499163
20. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-willi syndrome. Genet Med. (2012) 14:10–26. doi: 10.1038/gim.0b013e31822bead0
21. Gillett ES, Perez IA. Disorders of sleep and ventilatory control in prader-willi syndrome. Diseases. (2016) 4:23–34. doi: 10.3390/diseases4030023
22. Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Sandahl Christiansen J 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop Participants. Growth hormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in prader-willi syndrome. J Clin Endocrinol Metab. (2013) 98:E1072–87. doi: 10.1210/jc.2012-3888
23. Bernardi L, Hilz M, Stemper B, Passino C, Welsch G, Axelrod FB. Respiratory and cerebrovascular responses to hypoxia and hypercapnia in familial dysautonomia. Am J Respir Crit Care Med. (2003) 167:141–49. doi: 10.1164/rccm.200207-677OC
24. Yates JF, Troester MM, Ingram DG. Sleep in children with congenital malformations of the central nervous system. Curr Neurol Neurosci Rep. (2018) 18:38–48. doi: 10.1007/s11910-018-0850-6
25. Afsharpaiman S, Saburi A, Waters KA. Respiratory difficulties and breathing disorders in achondroplasia. Paediatr Respir Rev. (2013) 14:250–55. doi: 10.1016/j.prrv.2013.02.009
26. Koo P, Sethi JM. Metabolic myopathies and the respiratory system. Clin Chest Med. (2018) 39:401–10. doi: 10.1016/j.ccm.2018.02.001
27. Felix O, Amaddeo A, Olmo Arroyo J, Zerah M, Puget S, Cormier-Daire V, et al. Central sleep apnea in children: experience at a single center. Sleep Med. (2016) 25:24–28. doi: 10.1016/j.sleep.2016.07.016
28. Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine (2007).
29. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. (2012) 8:597–619. doi: 10.5664/jcsm.2172
30. Restrepo RD, Hirst KR, Wittnebel L, Wettstein R. AARC clinical practice guideline: transcutaneous monitoring of carbon dioxide and oxygen: 2012. Respir Care. (2012) 57:1955–62. doi: 10.4187/respcare.02011
31. Lambert LL, Baldwin MB, Velasco Gonzalez C, Lowe GR, Willis JR. Accuracy of transcutaneous CO2 values compared with arterial and capillary blood gases. Respir Care. (2018) 63:907–12. doi: 10.4187/respcare.05936
32. Khayat A, Medin D, Syed F, Moraes TJ, Bin-Hasan S, Narang I, et al. Intelligent volume-assured pressured support (iVAPS) for the treatment of congenital central hypoventilation syndrome. Sleep Breath. (2017) 21:513–19. doi: 10.1007/s11325-017-1478-5
33. Zaidi S, Gandhi J, Vatsia S, Smith NL, Ali Khan S. Congenital central hypoventilation syndrome: an overview of etiopathogenesis, associated pathologies, clinical presentation, and management. Auton Neurosci. (2018) 210:1–9. doi: 10.1016/j.autneu.2017.11.003
34. Saddi V, Teng A, Thambipillay G, Allen H, Pithers S, Sullivan C. Nasal mask average volume-assured pressure support in an infant with congenital central hypoventilation syndrome. Respirol Case Rep. (2019) 7:e00448–50. doi: 10.1002/rcr2.448
35. Paglietti MG, Porcaro F, Sovtic A, Cherchi C, Verrillo E, Pavone M, et al. Decannulation in children affected by congenital central hypoventilation syndrome: a proposal of an algorithm from two European centers. Pediatr Pulmonol. (2019) 54:1663–69. doi: 10.1002/ppul.24448
36. Hartmann H, Jawad MH, Noyes J, Samuels MP, Southall DP. Negative extrathoracic pressure ventilation in central hypoventilation syndrome. Arch Dis Child. (1994) 70:418–23. doi: 10.1136/adc.70.5.418
37. Nicholson KJ, Nosanov LB, Bowen KA, Kun SS, Perez IA, Keens TJ, et al. Thoracoscopic placement of phrenic nerve pacers for diaphragm pacing in congenital central hypoventilation syndrome. J Pediatr Surg. (2015) 50:78–81. doi: 10.1016/j.jpedsurg.2014.10.002
Keywords: central hypoventilation, arterial concentration of serum carbon dioxide, central apnea, non-invasive ventilation, children
Citation: Paglietti MG, Esposito I, Goia M, Rizza E, Cutrera R and Bignamini E (2020) Long Term Non-invasive Ventilation in Children With Central Hypoventilation. Front. Pediatr. 8:288. doi: 10.3389/fped.2020.00288
Received: 03 April 2020; Accepted: 07 May 2020;
Published: 19 June 2020.
Edited by:
Bülent Taner Karadag, Marmara University, TurkeyReviewed by:
Zuzana Rennerova, Slovak Medical University, SlovakiaGiuseppe Pingitore, ASL Roma, Italy
Copyright © 2020 Paglietti, Esposito, Goia, Rizza, Cutrera and Bignamini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabetta Bignamini, ZWxpc2FiZXR0YS5iaWduYW1pbmlAZ21haWwuY29t
 Maria Giovanna Paglietti
Maria Giovanna Paglietti Irene Esposito2
Irene Esposito2 Renato Cutrera
Renato Cutrera Elisabetta Bignamini
Elisabetta Bignamini