- 1Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, MA, United States
- 2Infectious Disease Institute, Harvard Medical School, Boston, MA, United States
- 3Department of Microbiology and Immunobiology, Harvard Medical School, Boston, MA, United States
Methicillin-resistant Staphylococcus aureus (MRSA) is a common cause of severe and difficult to treat ocular infection. In this study, the population structure of 68 ocular MRSA isolates collected at Massachusetts Eye and Ear between January 2014 and June 2016 was assessed. By using a combination of multilocus sequence typing (MLST) analysis, SCCmec typing and detection of the panton-valentine leukocidin (PVL) gene, we found that the population structure of ocular MRSA is composed of lineages with community and hospital origins. As determined by eBURST analysis of MLST data, the ocular MRSA population consisted of 14 different sequence types (STs) that grouped within two predominant clonal complexes: CC8 (47.0%) and CC5 (41.2%). Most CC8 strains were ST8, harbored type IV SCCmec and were positive for the PVL-toxin (93.7%). The CC5 group was divided between strains carrying SCCmec type II (71.4%) and SCCmec type IV (28.6%). Remaining isolates grouped in 6 different clonal complexes with 3 isolates in CC6 and the other clonal complexes being represented by a single isolate. Interestingly, major MRSA CC5 and CC8 lineages were isolated from discrete ocular niches. Orbital and preseptal abscess/cellulitis were predominantly caused by CC8-SCCmec IV PVL-positive strains. In contrast, infections of the cornea, conjunctiva and lacrimal system were associated with the MDR CC5 lineage, particularly as causes of severe infectious keratitis. This niche specialization of MRSA is consistent with a model where CC8-SCCmec IV PVL-positive strains are better adapted to cause infections of the keratinized and soft adnexal eye tissues, whereas MDR CC5 appear to have greater ability in overcoming innate defense mechanisms of the wet epithelium of the ocular surface.
Introduction
Antimicrobial resistance in human infections has reached alarming levels and has become one of the major public health threats of the twenty first Century (1). Methicillin-resistant Staphylococcus aureus (MRSA) remains a leading cause of antibiotic-resistant infections at many anatomical sites (2, 3). MRSA initially were confined to the hospital environment, but in the mid 1990s began to proliferate in the community (4), and are now leading causes of antibiotic-resistant infections in both settings (5). In US, strains within lineages that constitute clonal complex 5 (CC), notably the USA100 clone, are most commonly hospital-associated (5). USA300, a representative of CC8, has emerged as the most prevalent CA-MRSA clone in the US (5, 6). USA300 has also invaded the hospital setting where it is now a common cause of MRSA infections in American hospitals (7).
Ocular infections caused by MRSA have become increasingly common in the last two decades (8–11). These infections have been associated with serious ocular damage and permanent vision loss (12, 13), including bilateral blindness (14). Despite the growing importance of MRSA in ophthalmology, little is known about the population structure of MRSA causing the most common eye infections, or the microbial and host features that dictate this structure. The eye has extensive defenses for protection of vital structures from constant environmental exposure. These include mechanical barriers (e.g., lids, lashes), a polarized wet epithelium, a secreted tear film containing immunoglobulins and various other antimicrobial factors, mucins (secreted MUC5AC and shed epithelial cell surface-associated transmembrane mucins MUC1, MUC4, and MUC16), and cells of the innate immune system (15–17).
This unique environment of the ocular wet mucosa and its components are expected to act as selective forces that can shape the spatial distribution of microorganisms colonizing and infecting this ocular niche. The study of these ecological and geographical forces, as classically applied in ecology to study the biogeography of life in the natural world can now be combined with refined genetic and genomic epidemiology data to advance our understanding of community structures and distribution of microbes in different body sites (18, 19). We previously reported the genomic characteristics of a divergent cluster of unencapsulated Streptococcus pneumoniae strains that are uniquely tropic and adapted to the conjunctiva (20). These strains carry a set of genes that are absent or substantially different from those encoded within the genomes of encapsulated respiratory strains, which appear to be important for the pathogenesis of epidemic conjunctivitis. We have demonstrated that a unified model of microbial biogeography that incorporates classic ecological principles to explain community assemblage and dynamics can be applied to the understanding of this radical bifurcation in phylogeny and niche subspecialization of the unencapsulated S. pneumoniae conjunctivitis cluster (21). Because MRSA now rank among leading causes of a variety of ocular infections, to gain insight into particular features of importance in the pathogenesis of infection, it was of interest to determine the microscale biogeography of MRSA eye infections, whether dominant genetic lineages were associated with all sites of infection, or if there was evidence of a tissue tropism that would drive a specific population structure. We report that the population structure of ocular MRSA strains isolated at Massachusetts Eye and Ear (MEE) is dominated by the two major clonal complexes that cause infections at other body sites, but exhibit a distinct distribution in the types of infection they cause.
Methods
Bacterial Strains
Protocols for obtaining bacterial isolates collected for infection diagnosis were approved by the MEE Institutional Review Board (IRB). Since this study only included discarded bacterial isolates that were frozen in our pathogen repository, written informed consent was waived by the MEE IRB. In total, 68 consecutive MRSA isolates recovered from January 2014 to June 2016 were analyzed for this study. For patients from whom multiple isolates from the same eye were obtained for infection diagnosis within a period of 6 months, only the first isolate was included. Specimens were obtained by the attending ophthalmologist or resident physician following institutional guidelines and submitted to the clinical laboratory for processing. Suspected S. aureus colonies were routinely identified using a combination of phenotypic methods including detection of coagulase and protein A by latex agglutination, followed by confirmation of species and antimicrobial susceptibility testing using the MicroScan Walkaway 40 Plus System (Beckman Coulter, Brea, CA). Isolates were stored at −80°C in Microbank™ cryopreservative tubes (ProLab Diagnostics). Frozen isolates were cultured twice on blood agar before further testing.
Clinical Data Collection and Statistical Analyses
Demographic data and risk factors for MRSA infection were collected using the IRB-approved Research Electronic Data Capture (REDCap) tool, hosted by MEE and Harvard Medical School (22). General demographic data included age, sex and ethnicity. Ocular comorbidities, including any ophthalmic surgical history, ocular surface disease, eyelid disease, lacrimal system dysfunction, atopy, contact lens use and trauma were collected (see Table 2 legend for full definitions). Patient systemic comorbidities and previous healthcare exposures were also captured. To identify possible healthcare exposures which may potentiate selective pressures for antibiotic-resistant infection, we identified these following groups in our data: patients residing in nursing homes and/or residential facilities; those requiring chronic ambulatory care such as renal replacement therapy (dialysis) and hospital-based infusions; and patients who had either inpatient hospital admission and/or day admission for eye surgery within the preceding 3 prior to developing an MRSA infection. For patients with MRSA keratitis, we recorded the presenting features of the ulcers according to an institution-wide clinical algorithm which mandates the collection of corneal cultures for lesions meeting any of the following criteria: ≥1+ cells in the anterior chamber; ≥2 mm infiltrate and/or the presence of ≥2 satellite lesions; or infiltrate located ≤ 3 mm from the corneal center (23). Simple 2 by 2 tests of proportion (Fischer's exact test) were used to compare CC5 and CC8 groups according to collected categorical variables, while age was compared using the non-parametric Wilcoxon rank-sum test.
Antimicrobial Susceptibility Testing
In vitro susceptibility to ciprofloxacin (Fluka), ofloxacin, levofloxacin (TCI America), moxifloxacin, and besifloxacin (Sigma-Aldrich) was performed by broth microdilution methods according to the Clinical and Laboratory Standards Institute (CLSI) (24). Quality control was performed by testing the S. aureus ATCC 29213 control strain. The interpretative criteria for each antimicrobial agent tested were those published by CLSI (25).
DNA Extraction
DNA extraction was performed using Chelex 100 molecular biology resin (Bio-Rad) as previously described (26). Purified genomic DNA was diluted 1:10, and was assessed for purity and DNA concentration using a Synergy 2 Multi-Mode Plate Reader and Take3 software system (BioTek).
SCCmec Typing
PCR-based genotyping of the chromosomal cassette recombinase (ccr) and mec complexes comprising the SCCmec was determined by a combination of multiplex PCR designed to classify the mec complex and ccr complex using a previously published protocol (27). For each multiplex PCR assay, reference MRSA strains for SCCmec types II (USA100) and IV (USA800), provided by the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) were included. SCCmec was considered nontypeable if mec and/or ccr complex gave no amplification results, if the isolate carried more than one ccr or mec complex, or if there was a mec/ccr complex combination not previously described.
PVL Detection
The presence or absence of the Panton-Valentine Leukocidin (PVL) toxin gene was determined by PCR amplification of the LukS-PV-lukF-PV genes as previously described (28). Reference MRSA strains (provided by NARSA) USA300 and USA100 served as positive and negative controls, respectively.
MLST
Multilocus sequence typing (MLST) was performed for all MRSA isolates using a scheme based on the sequencing of internal fragments of seven S. aureus housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL). The PCR products were purified (QIAquick PCR purification kit; Qiagen), and both strands were sequenced by Genewiz Incorporated (South Plainfield, NJ). The sequences obtained were edited using Geneious R8 and sequence types (STs) were assigned using the S. aureus MLST database (https://pubmlst.org/saureus/). Clonal complexes (CC) were determined using the go eBURST algorithm (http://www.phyloviz.net/goeburst/).
Statistics
Descriptive statistics were calculated using SPSS software (version 25, IBM, Armonk, New York), and proportions were compared by χ2or Fisher exact test, as appropriate. A P value of < 0.05 was considered statistically significant.
Results
A total of 75 MRSA were identified from 281 S. aureus recovered from ocular sites at MEE from January 2014 to June 2016 (overall rate of 26.7%). The proportion of ocular MRSA isolates did not change considerably in 2014 (25.9%) compared to 2015 (22.3%), but was substantially higher during the sampling period of 2016 (37.7%). Of those, 7 MRSA were obtained from second cultures of the same patient eye, and were excluded from further study. The remaining 68 non-duplicate MRSA isolates were then analyzed. Sites of infection from which MRSA were isolated included orbital and preseptal abscess/cellulitis (n = 27), keratitis (n = 14), conjunctivitis (n = 9), lacrimal system infection (n = 8), eyelid margin infections (n = 4), endophthalmitis (n = 2), and miscellaneous (n = 4).
Two Major Clonal Complexes Dominate the Ocular MRSA Population
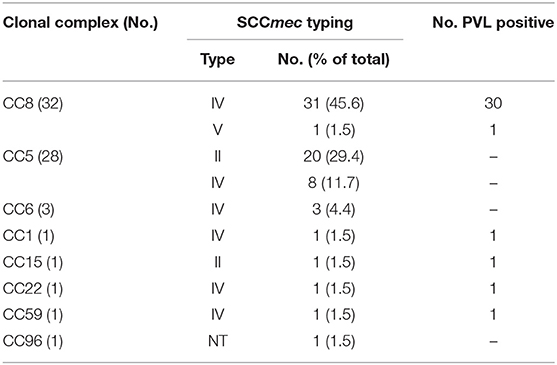
Despite the clinical importance of MRSA, much remains to be learned about the pathogenesis of infection at different anatomical sites on and around the eye. Because the tissues of the eye and adnexa differ widely in host defenses (e.g., wet epithelium vs. keratinized epithelium and soft tissues), it was of interest to know whether some MRSA lineages were enriched in pathogenic features that select for one MRSA lineage over another. By using a combination of multilocus sequence typing analysis, SCCmec typing and detection of the PVL-toxin encoding gene, we found that the population structure of ocular MRSA is diverse, but dominated by the CC5 and CC8 lineages associated with infection at other anatomical sites (4, 5). As determined by eBURST analysis of MLST data (Figure 1), 14 different sequence types (STs) were identified, with most belonging to clonal clusters CC8 (47.0%, n = 32) and CC5 (41.2%, n = 28). The clonal cluster CC6 encompassed 3 strains (4.4%), and 5 strains represented single sequence types. Most CC8 strains (93.7%, n = 30) were ST8, harbored a SCCmec type IV and were positive for the PVL-toxin, common features of the USA300 strain. The CC5 group could be divided into those carrying a SCCmec type II (71.4%, n = 20), which includes isolates with the characteristics of the USA100 clone, and (28.6%, n = 8) SCCmec type IV, typical of the USA800 clone (Table 1).

Figure 1. Go eBurst-based population structure of ocular MRSA strains (n = 68). Each ST is represented by a circle. Lines connect single-locus variants. The black circles represent clonal complex founders. Circles not connected represent singleton STs in this particular population structure.
Age at presentation ranged from 2 to 102 years (median 53.08) with CC5-infected patients being significantly older (median age, 68.05 vs. 35.9, p < 0.001) (Table 2). CC5-infected patients were more frequently subjected to eye surgery (66.7 vs. 16.7%, p < 0.001), especially cataract surgery with implantation of intraocular lenses (44.4 vs. 10%, p = 0.006). Healthcare exposure was more common among patients infected with CC5 strains, including higher rates of topical antibiotic use at presentation (55.6 vs. 10.0%, p < 0.001) and prior to presentation (48.5 vs. 16.7%, p = 0.02). There was also a higher proportion of patients known to require non-acute clinical care and/or residents of aged care facilities in the CC5 group (22.2 vs. 3.3%, p = 0.05).
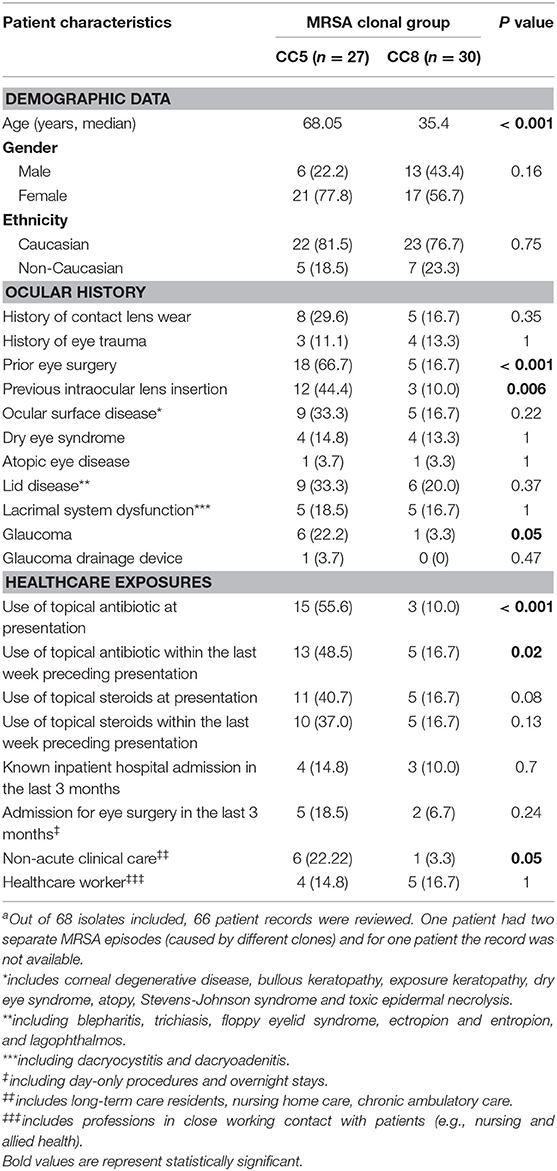
Table 2. Demographic and clinicopathologic data for patients with culture-positive MRSA ocular infections at MEE, 2014–2016 (n = 58).a
Distribution of MRSA Lineages Across Different Ocular Niches
Although S. aureus causes a wide range of human infections, patterns of association of distinct genotypes have been noted with specific types of infection (29–32). The most well documented example is community-acquired skin and soft-tissue infection (SSTI) in the US, where a large majority of cases are caused by the CC8/USA300 lineage (29, 32). In agreement, ocular SSTIs including mainly orbital and preseptal abscess/cellulitis were found in this study to be caused mainly by CC8 SCCmec IV PVL-positive strains, characteristics of the USA300 clone (p < 0.001; Table 3). Interestingly, infections of the wet epithelial tissues of the ocular surface were substantially enriched in the CC5 lineage, which was particularly pronounced for infectious keratitis cases (p < 0.001; Table 3). Keratitis patients frequently presented with potentially sight-threatening corneal ulcers (85.7%) according to the 1,2,3 rule (23) for categorization of the severity of bacterial keratitis (Table 5).
CC5 Strains Preferentially Associated With Infection of the Wet Epithelial Ocular Surface Are Multidrug Resistant
To compare rates of antibiotic resistance among ocular MRSA of various lineages and from the different ocular sites, we tested the sensitivities of all isolates to a panel of clinically relevant antibiotics using an automated microbiology system (MicroScan WalkAway). Overall, moderate to high rates of resistance to erythromycin (88.2%), levofloxacin (54.4%), and clindamycin (42.6%) was found among this MRSA collection. Resistance to gentamycin (2.9%) and tetracycline (1.5%) was rare (Figure 2B). Stratified analysis by clonal complex showed that CC5 strains are significantly more likely to be multidrug resistant (resistance to ≥3 non-beta-lactam antimicrobial classes) than CC8 strains (78.8 vs. 6.1%; p < 0.0001; Figure 2A). Ocular CC5-SCCmec II strains, which include isolates resembling the hospital-adapted clone USA100, were all resistant to erythromycin, clindamycin and levofloxacin (Figure 2B). CC5-SCCmec IV strains, including isolates with the characteristics of the USA800 clone, were frequently resistant to erythromycin (62.5%), levofloxacin (62.5%), and clindamycin (25.0%). While CC5 strains were frequently resistant to ≥3 non-beta-lactam antibiotics, CC8 isolates were usually resistant to only one antibiotic class in addition to beta-lactams, most often to the macrolide erythromycin (Figures 2A,B).
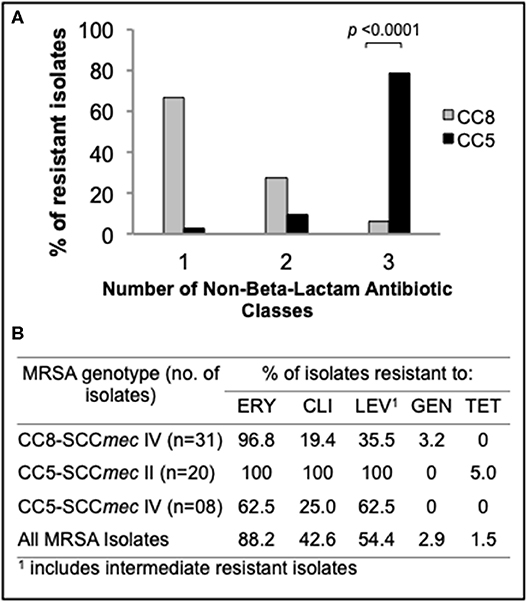
Figure 2. Antimicrobial susceptibility profile of the main ocular MRSA clonal complexes. (A) Frequency (%) of CC5 and CC8 strains resistant to additional non-beta-lactam antibiotic classes. (B) Resistance rates for common antibiotics representing 5 different classes. Statistical significance was determined using Fisher's exact test.
Because topical fluoroquinolone is widely used for empirical treatment of ocular infection, we assessed the in vitro susceptibility of the main ocular MRSA clonal complexes for the most commonly used fluoroquinolones (Table 4). The minimum inhibitory concentrations (MICs) for these agents were determined by reference broth microdilution (24, 25). CC5 isolates were in general highly resistant to the older fluoroquinolones ciprofloxacin, ofloxacin and levofloxacin. All canonical CC5-SCCmec II strains were resistant to oxifloxacin, levofloxacin, ofloxacin and ciprofloxacin with MIC90 values >256 μg/mL. Among CC5-SCCmec IV strains, the MIC90 values ranged from 64 μg/mL for moxifloxacin to >256 μg/mL for levofloxacin, ofloxacin and ciprofloxacin (% of non-susceptible = 62.5% for the 3 drugs). Remarkably, many CC5 isolates were also highly resistant to the newer 8-methoxyfluoroquinolone moxifloxacin (MIC90 64 μg/mL, 62.5% non-susceptible for CC5-SCCmec IV; MIC90 32μg/mL, 100% non-susceptible for CC5-SCCmec II).
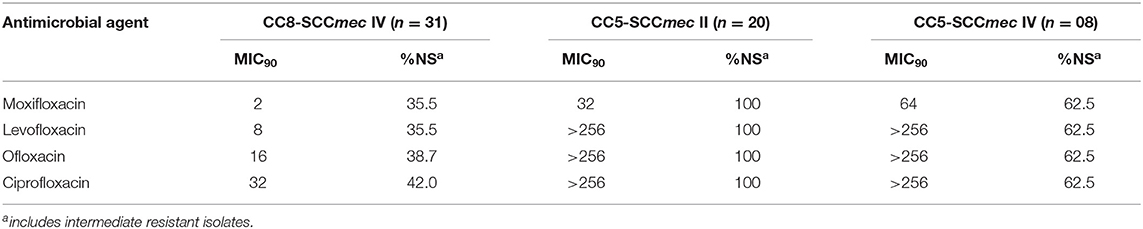
Table 4. In vitro susceptibility profile for topically used fluorquinolones among ocular MRSA isolates according to the clonal group.
Discussion
The population structure of S. aureus isolates from human infections is highly diverse (33). However, only a small proportion of these lineages have become successful MRSA clones that are now widely disseminated in both community and hospital settings (5). These epidemic lineages cause a variety of human infections, with some showing strong tropisms for specific body niches (29–32).
The eye has developed unique mechanisms to protect its delicate and exceptionally important structures against constant external disturbance (15–17). Because of this, we postulated that the uniqueness of this environment could be a driving force shaping the population structure of ocular MRSA infections, in a similar manner as reported for Streptococcus pneumoniae (20). To begin testing this hypothesis, we selected a collection of consecutive and non-duplicate MRSA strains prospectively isolated from a variety of eye infections, representing two major and distinct localizations: (i) the ocular surface and (ii) the ocular adnexal soft tissues. We found that the ocular MRSA population was dominated by two major clonal complexes that are also common causes of MRSA infections in other body sites: CC8 (47.0%) and CC5 (41.2%). The distribution of lineages within these clonal complexes followed a pattern of enrichment that was split into the main ocular niches tested, with preseptal and orbital abscess/cellulitis being predominantly associated to CC8, while ocular surface infections were frequently caused by CC5 strains, with a significant enrichment in keratitis (Table 3).
The basis for the discernable tropism of CC5 strains for the ocular surface is unknown, but viewed in light of the findings of others, we can develop a testable model. Strains grouped within CC5, notably the USA100 and USA800, are well established as major causes of healthcare-related infections in the US (5). CC5 strains are often replete with acquired antibiotic resistances (34, 35), and were implicated in the first 12 cases of frank vancomycin resistant S. aureus stemming from independent acquisitions of the vanA gene from Enterococcus spp. (36). Despite their hospital association, CC5-MRSA strains also occur in the community, potentially bridged by long-term care facilities. The nasal reservoir of MRSA of long-term care facility residents mirrors the molecular epidemiology of US hospitals, with CC5-related strains being predominant (37). Data from national surveillance programs show that CC5 lineages USA100 and USA800 are now widely disseminated in the community, even among noninstitutionalized individuals with no known risk factors for nasal colonization with these hospital-adapted clones (38). Although CC5 strains are frequently found among carriers in the community, they are only occasionally associated with community-acquired infection (32). In our population, despite the community origins of the patients in which ocular infections occurred, CC5 strains predominated as causes of infection of the wet epithelial surface of the eye. Keratitis in our series was predominantly caused by strains resembling the USA100 lineage, and mostly presented as severe and potentially sigh-threatening infections as determined the “1, 2, 3-Rule” (23). The severity of CC5-caused keratitis points toward the possible existence of virulence factors that could be particularly associated with corneal damage. In addition to carrying a variety of antimicrobial resistance genes, CC5 strains also posses a constellation of virulence genes (35). Of particular interest is the enterotoxin gene cluster (egc), which represents a unique group of enterotoxins with superantigen activities, and seems to be particularly enriched among CC5 strains (35), while being completely absent in CC8 strains (36, 39). Epidemiological observations have found an association of egc-encoded enterotoxins in the development of corneal ulcer in patients with atopic keratoconjunctivitis (40). Whether this locus is associated with exacerbation of the ocular surface inflammatory response and aggravation of the corneal damage is yet to be fully elucidated.
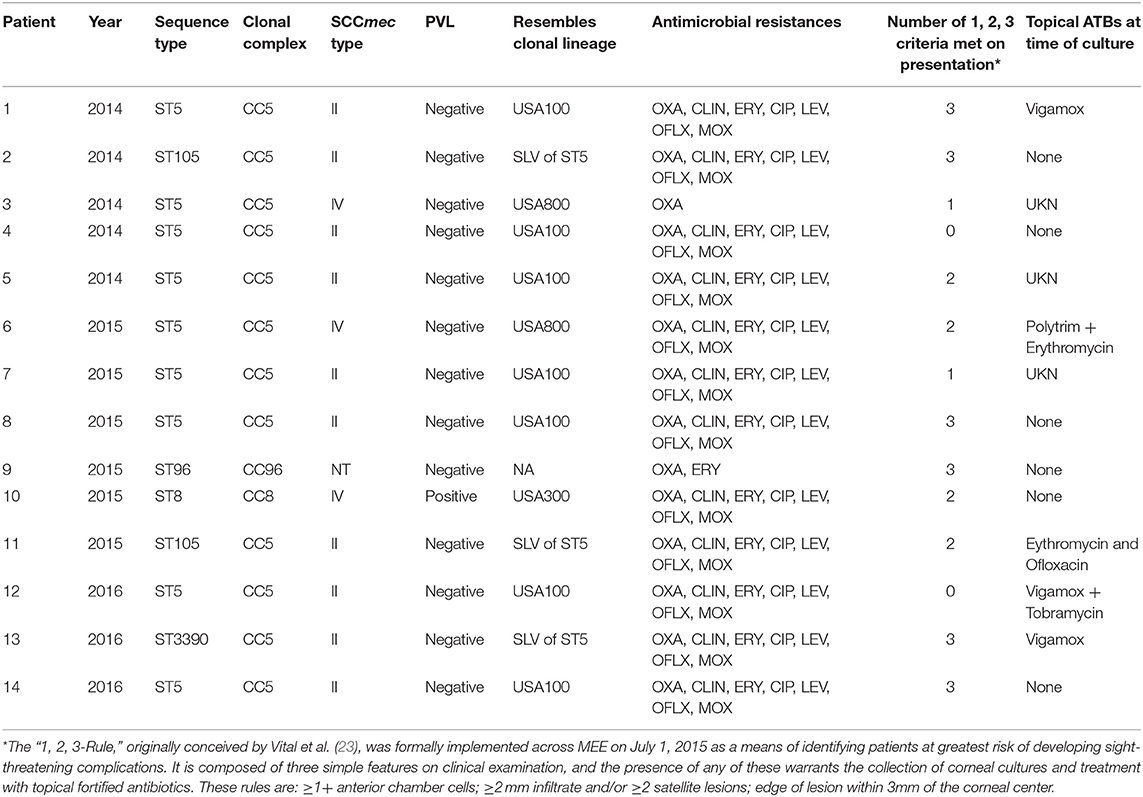
Empirical use of topical broad-spectrum antibiotics remains the first-line treatment for bacterial keratitis (41) and is commonly initiated with a topical newer fluoroquinolone ophthalmic solution (e.g., moxifloxacin 0.5%) (42). CC5 strains display generally higher fluoroquinolone MICs (non-susceptibility rate for moxifloxacin of 89.3%, MIC90 64 μg/mL) (Table 4). However, CC8-SCCmec IV strains associated with ocular infections at other sites were also often resistant to the commonly used fourth-generation fluoroquinolone, moxifloxacin (non-susceptibility rate using systemic breakpoints of 35.5%, MIC90 2 μg/mL). The influence of degrees of resistance on population structure is unclear. In light of its pharmacokinetics on the ocular surface, neither CC5 nor CC8 would be predicted to respond to topical moxifloxacin therapy. Based on pharmacokinetic data for topical moxifloxacin in the cornea of pigmented rabbits (43), we calculated PK/PD (AUC0−24h/MIC90 ratio) indices for the main MRSA lineages examined in our study (Figure S1). Although the index was higher for CC8 strains compared to CC5, all the indices were far below the PK/PD target that predicts clinical efficacy as determined for systemic infections (44) and also for keratitis treated with fluoroquinolone (45). In addition, a review of the medical records of 14 patients with MRSA keratitis included in this study showed that only 4/14 had prior exposure to a fluoroquinolone (Vigamox, n = 2; erythromycin plus ofloxacin, n = 1; and Vigamox plus tobramycin, n = 1), with no information available for 3 patients (Table 5). Together, the data on prior topical antibiotic use and the calculated PK/PD indices for the ocular MRSA lineages discounts the role of direct fluoroquinolone selection as the driver of the CC5 predominant population structure associated with keratitis.
Our results are consistent with a report of 30 cases of S. aureus keratitis in Japan, that also found CC5 strains to be the predominant cause of MRSA-infected ulcers (46). CC8 isolates were more frequently isolated from the healthy conjunctival sac (46). Together, these findings suggest an enhanced ability of CC5 to endure selective pressures and colonize the cornea and adjacent tissues. Lactoferrin, one the most abundant tear proteins with antimicrobial activity is inhibitory for S. aureus, including MRSA, but this activity varies among clinical isolates from different sites of isolation (47, 48). In one study, clinical S. aureus isolates causing bloodstream infections (50%) were frequently resistant to lactoferrin concentrations ≥ 20 μM, while isolates from conjunctivitis (33%) and SSTI (13%) were less often resistant (47). Since these infections are caused by distinct MRSA lineages, with CC5 strains being commonly associated to bloodstream infections (49), variations across different genotypes may contribute to increased resistance to this tear antimicrobial compound, especially among CC5. Similarly, the antimicrobial activity of phospholipase A2 (PLA2), an enzyme that has been found to be the major tear molecule with bactericidal activity against staphylococci, varies against methicillin-susceptible and -resistant isolates, with MRSA being more resistant to its action following short incubations (50), pointing toward a variance of this enzyme in its ability to kill S. aureus according to the genetic background. Further, previous reports have demonstrated that S. aureus strains may be differently equipped to bind to and invade ocular tissues in vitro and in a rabbit model of conjunctivitis and keratitis (51, 52), suggesting the existence of specific sets of adhesive surface proteins that enhance S. aureus ability to bind and invade the ocular tissues in a strain-specific manner.
A recent study of S. aureus keratitis in South Florida (53) found both CC5 (40%) and CC8 (37.3%) MRSA among isolates, clearly showing that although CC5 strains are most common, CC8 strains in other circumstances are also capable of causing keratitis. Among our patients, 12 out of 14 (85.7%), were classified as potentially sight-threatening MRSA keratitis cases according to an institution-wide grading system based on the “1, 2, 3” rule (23). For these patients, corneal cultures were unequivocally positive at diagnostic levels for the pathogen. We do not yet know whether the differences between studies stem from levels of disease severity, or other factors such as association with contact lens wear. Larger studies with well-defined criteria for enrollment will be important for determining conditions that favor infection by various pathogenic, antibiotic resistant lineages of S. aureus.
In contrast to the wet epithelial surface of the eye, CC8 MRSA strains, typified by the USA300 lineage, predominate as causes of infections of the keratinized epithelium and eye soft tissues. Lineage enrichment has been also reported in patients with bloodstream infection with haematogenous complications (30) infective endocarditis (31) and respiratory tract infections (54). Among our patients, CC8 strains were the predominant causes of preseptal and orbital abscess/cellulitis (22 out of 27 cases), consistent with their known tropism for infecting keratinized epithelium and soft tissues (29, 32). Among the remaining abscess/cellulitis cases, only one was caused by a CC5 isolate and 3 by other lineages (Table 3). In the early 2000s, the USA300 clone appeared in outbreaks of community-acquired SSTIs in otherwise healthy people (55, 56). Because of its ability to rapidly spread through person-to-person contact and readily compete with commensal skin flora (56), the USA300 lineage quickly predominated as the main cause of SSTIs (29, 32), and a significant cause of severe invasive infections (6). USA300 is generally more virulent, causing infections of greater severity and associated with worse outcome compared to other MRSA clones (57, 58). The aggressiveness of USA300 type strains is thought to be associated to the carriage of a variety of virulence factors, including the pore-forming toxin PVL, which is predominantly found in CC8 strains (59), and has been linked to the ability of this strain to cause necrotizing infections (60).
Orbital and preseptal abscesses are SSTIs commonly caused by Staphylococcus aureus (61, 62), and MRSA rates appear to be rising (63). Severe cases have been reported, including orbital and periorbital necrotizing fasciitis (64–66), necrotizing conjunctivitis with orbital invasion (12) and orbital cellulitis with bilateral involvement that progressed to blindness (14). A prospective study (2012–2015) of children and adolescents presenting with staphylococcal periorbital and orbital cellulitis in Houston found that most of the S. aureus isolates in their population were methicillin-resistant (67%) and were genetically related to the USA300 clone (78%) (67). Similarly, in a series of 11 patients presenting with culture-positive MRSA infections of the eye and orbit in San Francisco, most (82%) were caused by USA300 (13). Preseptal and orbital abscess/cellulitis were among the most common manifestations in these cases, some presenting with extensive necrosis of the eyelid and orbital tissues.
Collectively, these results are consistent with various MRSA lineages being enriched for properties that enhance their ability to resist defenses and competitive pressures, and colonize and infect either the wet epithelial surface or the ocular adnexal tissues. We propose that many of the features that have allowed CC5-type MRSA to adapt and be transmitted in the hospital environment, and readily acquire antibiotic resistances, endows them with properties that enhance their ability to resist robust ocular surface defenses and infect the cornea. In a comparative analysis of the genomes of the CC5 strains that had acquired vancomycin resistance from enterococci, we identified a constellation of traits with the S. aureus pathogenicity island as likely involved with their ability to persist in a mixed infection and acquire resistances by horizontal gene transfer (36). These same traits may well enhance their survival despite the defenses of the wet epithelial surface. In contrast, CC8-SCCmec IV PVL-positive strains are already well known to have features that enhance their ability to colonize the skin and compete effectively for that niche with coagulase negative strains, including the ACME locus (68). We believe these features account for their association with infection of the keratinized surfaces of the ocular adnexa. Direct testing of isogenic mutants will be required to identify the major contributors to pathogenesis of various anatomical sites of the eye by MRSA, and to develop new approaches for mitigating that common threat. It is important to note that these findings represent the population structure of a relatively small ocular MRSA population isolated at the Massachusetts Eye and Ear, and validation of our results using a larger bacterial collection and isolates from other locations would be warranted.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
PB, LU, JC, and MG contributed conception and design of the study and wrote sections of the manuscript. PB and LU organized the database and performed the statistical analysis. PB wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
This work was supported in part by NEI grant EY024285, and the Harvard-wide Program on Antibiotic Resistance NIAID grant AI083214. Funding agencies had no role in study design, data analysis, decision to publish or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Rick Body, James Cadorette and other medical technologists from the Clinical Microbiology Laboratory at MEE for their support in creating a microbial repository of strains isolated from ocular and otolaryngology infections.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00204/full#supplementary-material
Figure S1. PK/PD (AUC0−24h/MIC90 ratio) indices for the main MRSA clones isolated in our study. AUC0−24h data was derived from pharmacokinetic studies of topical moxifloxacin in the cornea of pigmented rabbits (43). Dashed line indicates the PK/PD target that predicts clinical efficacy in keratitis patients treated with topical fluoroquinolone (45).
References
1. World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance. (2014).
2. Klein EY, Mojica N, Jiang W, Cosgrove SE, Septimus E, Morgan DJ, et al. Trends in methicillin-Resistant staphylococcus aureus hospitalizations in the united states, 2010–2014. Clin Infect Dis. (2017) 65:1921–3. doi: 10.1093/cid/cix640
3. Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, et al. Vital signs: epidemiology and recent trends in methicillin-Resistant and in methicillin-Susceptible staphylococcus aureus bloodstream infections - united states. MMWR Morb Mortal Wkly Rep. (2019) 68:214–9. doi: 10.15585/mmwr.mm6809e1
4. van Duin D, Paterson DL. Multidrug-Resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am. (2016) 30:377–90. doi: 10.1016/j.idc.2016.02.004
5. DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant staphylococcus aureus in the genomics era. J Clin Invest. (2009) 119:2464–74. doi: 10.1172/JCI38226
6. David MZ, Daum RS. Community-associated methicillin-resistant staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. (2010) 23:616–87. doi: 10.1128/CMR.00081-09
7. Diekema DJ, Richter SS, Heilmann KP, Dohrn CL, Riahi F, Tendolkar S, et al. Continued emergence of uSA300 methicillin-resistant staphylococcus aureus in the united states: results from a nationwide surveillance study. Infect Control Hosp Epidemiol. (2014) 35:285–92. doi: 10.1086/675283
8. Asbell PA, Colby KA, Deng S, McDonnell P, Meisler DM, Raizman MB, et al. Ocular tRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. (2008) 145:951–8. doi: 10.1016/j.ajo.2008.01.025
9. Asbell PA, Sahm DF, Shaw M, Draghi DC, Brown NP. Increasing prevalence of methicillin resistance in serious ocular infections caused by staphylococcus aureus in the united states: 2000 to 2005. J Cataract Refract Surg. (2008) 34:814–8. doi: 10.1016/j.jcrs.2008.01.016
10. Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the united states: five-Year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. (2015) 133:1445–54. doi: 10.1001/jamaophthalmol.2015.3888
11. Cavuoto K, Zutshi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivitis in south florida. Ophthalmology. (2008) 115:51–6. doi: 10.1016/j.ophtha.2007.03.076
12. Brown SM, Raflo GT, Fanning WL. Transconjunctival orbital invasion by methicillin-resistant staphylococcus aureus. Arch Ophthalmol. (2009) 127:941–2. doi: 10.1001/archophthalmol.2009.144
13. Rutar T, Chambers HF, Crawford JB, Perdreau-Remington F, Zwick OM, Karr M, et al. Ophthalmic manifestations of infections caused by the uSA300 clone of community-associated methicillin-resistant staphylococcus aureus. Ophthalmology. (2006) 113:1455–62. doi: 10.1016/j.ophtha.2006.03.031
14. Rutar T, Zwick OM, Cockerham KP, Horton JC. Bilateral blindness from orbital cellulitis caused by community-acquired methicillin-resistant staphylococcus aureus. Am J Ophthalmol. (2005) 140:740–2. doi: 10.1016/j.ajo.2005.03.076
15. Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. (2008) 8:477–83. doi: 10.1097/ACI.0b013e32830e6b04
16. McClellan KA. Mucosal defense of the outer eye. Surv Ophthalmol. (1997) 42:233–46. doi: 10.1016/S0039-6257(97)00090-8
17. Sack RA, Nunes I, Beaton A, Morris C. Host-defense mechanism of the ocular surfaces. Biosci Rep. (2001) 21:463–80. doi: 10.1023/A:1017943826684
18. Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. (2012) 336:1255–62. doi: 10.1126/science.1224203
19. Martiny JB, Bohannan BJ, Brown JH, Colwell RK, Fuhrman JA, Green JL, et al. Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol. (2006) 4:102–12. doi: 10.1038/nrmicro1341
20. Valentino MD, McGuire AM, Rosch JW, Bispo PJ, Burnham C, Sanfilippo CM, et al. Unencapsulated streptococcus pneumoniae from conjunctivitis encode variant traits and belong to a distinct phylogenetic cluster. Nat Commun. (2014) 5:5411. doi: 10.1038/ncomms6411
21. Ung L, Bispo PJM, Bryan NC, Andre C, Chodosh J, Gilmore MS. The best of all worlds: streptococcus pneumoniae conjunctivitis through the lens of community ecology and microbial biogeography. Microorganisms. (2019) 8:46. doi: 10.3390/microorganisms8010046
22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
23. Vital MC, Belloso M, Prager TC, Lanier JD. Classifying the severity of corneal ulcers by using the “1, 2, 3” rule. Cornea. (2007) 26:16–20. doi: 10.1097/ICO.0b013e31802b2e47
24. Clinical and Laboratory Standard Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition. CLSI document M07-A9. Wayne, PA: Clinical and Laboratory Standard Institutes. (2012).
25. Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute (2018).
26. Bispo P, Höfling-Lima A, Pignatari A. Characerization of ocular methicillin- resistant staphylococcs epidermidis isolates belonging predominantly to clonal complex 2 subcluster iI. J Clin Microbiol. (2014) 52:1412–7. doi: 10.1128/JCM.03098-13
27. Kondo Y, Ito T, Ma X, Watanabe S, Kreiswirth B, Etienne J, et al. Combination of multiplex pCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicro Agents Chem. (2007) 51:264–74. doi: 10.1128/AAC.00165-06
28. David MZ, Boyle-Vavra S, Zychowski DL, Daum RS. Methicillin-susceptible staphylococcus aureus as a predominantly healthcare-associated pathogen: a possible reversal of roles? PLoS ONE. (2011) 6:e18217. doi: 10.1371/journal.pone.0018217
29. Albrecht VS, Limbago BM, Moran GJ, Krishnadasan A, Gorwitz RJ, McDougal LK, et al. Staphylococcus aureus colonization and strain type at various body sites among patients with a closed abscess and uninfected controls at US Emergency Departments. J Clin Microbiol. (2015) 53:3478–84. doi: 10.1128/JCM.01371-15
30. Fowler VG Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, et al. Potential associations between hematogenous complications and bacterial genotype in staphylococcus aureus infection. J Infect Dis. (2007) 196:738–47. doi: 10.1086/520088
31. Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, et al. Methicillin-susceptible staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis. (2011) 204:704–13. doi: 10.1093/infdis/jir389
32. Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, et al. Comparison of staphylococcus aureus from skin and soft-tissue infections in uS emergency department patients, 2004 and 2008. Clin Infect Dis. (2011) 53:144–9. doi: 10.1093/cid/cir308
33. Wurster JI, Bispo PJM, Van Tyne D, Cadorette JJ, Boody R, Gilmore MS. Staphylococcus aureus from ocular and otolaryngology infections are frequently resistant to clinically important antibiotics and are associated with lineages of community and hospital origins. PLoS ONE. (2018) 13:e0208518. doi: 10.1371/journal.pone.0208518
34. Aanensen DM, Feil EJ, Holden MT, Dordel J, Yeats CA, Fedosejev A, et al. Whole-Genome sequencing for routine pathogen surveillance in public health: a population snapshot of invasive staphylococcus aureus in europe. MBio. (2016) 7:16. doi: 10.1128/mBio.00444-16
35. Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant staphylococcus aureus. PLoS ONE. (2011) 6:e17936. doi: 10.1371/journal.pone.0017936
36. Kos VN, Desjardins CA, Griggs A, Cerqueira G, Van Tonder A, Holden MT, et al. Comparative genomics of vancomycin-resistant staphylococcus aureus strains and their positions within the clade most commonly associated with methicillin-resistant S. aureus hospital-acquired infection in the United States. MBio. (2012) 3:12. doi: 10.1128/mBio.00112-12
37. Hudson LO, Reynolds C, Spratt BG, Enright MC, Quan V, Kim D, et al. Diversity of methicillin-resistant staphylococcus aureus strains isolated from residents of 26 nursing homes in orange county, california. J Clin Microbiol. (2013) 51:3788–95. doi: 10.1128/JCM.01708-13
38. Tenover FC, McAllister S, Fosheim G, McDougal LK, Carey RB, Limbago B, et al. Characterization of staphylococcus aureus isolates from nasal cultures collected from individuals in the united states in 2001 to 2004. J Clin Microbiol. (2008) 46:2837–41. doi: 10.1128/JCM.00480-08
39. van Belkum A, Melles DC, Snijders SV, van Leeuwen WB, Wertheim HF, Nouwen JL, et al. Clonal distribution and differential occurrence of the enterotoxin gene cluster, egc, in carriage- versus bacteremia-associated isolates of Staphylococcus aureus. J Clin Microbiol. (2006) 44:1555–7. doi: 10.1128/JCM.44.4.1555-1557.2006
40. Fujishima H, Okada N, Dogru M, Baba F, Tomita M, Abe J, et al. The role of staphylococcal enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy. (2012) 67:799–803. doi: 10.1111/j.1398-9995.2012.02818.x
41. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. (2017) 124:1678–89. doi: 10.1016/j.ophtha.2017.05.012
42. Hsu HY, Nacke R, Song JC, Yoo SH, Alfonso EC, Israel HA. Community opinions in the management of corneal ulcers and ophthalmic antibiotics: a survey of 4 states. Eye Contact Lens. (2010) 36:195–200. doi: 10.1097/ICL.0b013e3181e3ef45
43. Proksch JW, Ward KW. Ocular pharmacokinetics/pharmacodynamics of besifloxacin, moxifloxacin, and gatifloxacin following topical administration to pigmented rabbits. J Ocul Pharmacol Ther. (2010) 26:449–58. doi: 10.1089/jop.2010.0054
44. Segreti J, Jones RN, Bertino JS Jr. Challenges in assessing microbial susceptibility and predicting clinical response to newer-generation fluoroquinolones. J Ocul Pharmacol Ther. (2012) 28:3–11. doi: 10.1089/jop.2011.0072
45. Wilhelmus KR. Evaluation and prediction of fluoroquinolone pharmacodynamics in bacterial keratitis. J Ocul Pharmacol Ther. (2003) 19:493–9. doi: 10.1089/108076803322473042
46. Hayashi S, Suzuki T, Yamaguchi S, Inoue T, Ohashi Y. Genotypic characterization of Staphylococcus aureus isolates from cases of keratitis and healthy conjunctival sacs. Cornea. (2014) 33:72–6. doi: 10.1097/ICO.0b013e3182a4810f
47. Aguila A, Herrera AG, Morrison D, Cosgrove B, Perojo A, Montesinos I, et al. Bacteriostatic activity of human lactoferrin against staphylococcus aureus is a function of its iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol Med Microbiol. (2001) 31:145–52. doi: 10.1111/j.1574-695X.2001.tb00511.x
48. Chen PW, Jheng TT, Shyu CL, Mao FC. Synergistic antibacterial efficacies of the combination of bovine lactoferrin or its hydrolysate with probiotic secretion in curbing the growth of meticillin-resistant staphylococcus aureus. J Med Microbiol. (2013) 62(Pt 12):1845–51. doi: 10.1099/jmm.0.052639-0
49. Chua T, Moore CL, Perri MB, Donabedian SM, Masch W, Vager D, et al. Molecular epidemiology of methicillin-resistant staphylococcus aureus bloodstream isolates in urban detroit. J Clin Microbiol. (2008) 46:2345–52. doi: 10.1128/JCM.00154-08
50. Qu XD, Lehrer RI. Secretory phospholipase a2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. (1998) 66:2791–7. doi: 10.1128/IAI.66.6.2791-2797.1998
51. McCormick CC, Caballero AR, Balzli CL, Tang A, Weeks A, O'Callaghan RJ. Diverse virulence of staphylococcus aureus strains for the conjunctiva. Curr Eye Res. (2011) 36:14–20. doi: 10.3109/02713683.2010.523194
52. Tang A, Balzli CL, Caballero AR, McCormick CC, Taylor SD, O'Callaghan RJ. Staphylococcus aureus infection of the rabbit cornea following topical administration. Curr Eye Res. (2012) 37:1075–83. doi: 10.3109/02713683.2012.716485
53. Peterson JC, Durkee H, Miller D, Maestre-Mesa J, Arboleda A, Aguilar MC, et al. Molecular epidemiology and resistance profiles among healthcare- and community-associated staphylococcus aureus keratitis isolates. Infect Drug Resist. (2019) 12:831–43. doi: 10.2147/IDR.S190245
54. Booth MC, Pence LM, Mahasreshti P, Callegan MC, Gilmore MS. Clonal associations among staphylococcus aureus isolates from various sites of infection. Infect Immun. (2001) 69:345–52. doi: 10.1128/IAI.69.1.345-352.2001
55. Begier EM, Frenette K, Barrett NL, Mshar P, Petit S, Boxrud DJ, et al. A high-morbidity outbreak of methicillin-resistant staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. (2004) 39:1446–53. doi: 10.1086/425313
56. Kazakova SV, Hageman JC, Matava M, Srinivasan A, Phelan L, Garfinkel B, et al. A clone of methicillin-resistant staphylococcus aureus among professional football players. N Engl J Med. (2005) 352:468–75. doi: 10.1056/NEJMoa042859
57. Kempker RR, Farley MM, Ladson JL, Satola S, Ray SM. Association of methicillin-resistant staphylococcus aureus (MRSA) uSA300 genotype with mortality in mRSA bacteremia. J Infect. (2010) 61:372–81. doi: 10.1016/j.jinf.2010.09.021
58. Kreisel KM, Stine OC, Johnson JK, Perencevich EN, Shardell MD, Lesse AJ, et al. USA300 methicillin-resistant staphylococcus aureus bacteremia and the risk of severe sepsis: is uSA300 methicillin-resistant staphylococcus aureus associated with more severe infections? Diagn Microbiol Infect Dis. (2011) 70:285–90. doi: 10.1016/j.diagmicrobio.2011.03.010
59. Brown ML, O'Hara FP, Close NM, Mera RM, Miller LA, Suaya JA, et al. Prevalence and sequence variation of panton-valentine leukocidin in methicillin-resistant and methicillin-susceptible staphylococcus aureus strains in the united states. J Clin Microbiol. (2012) 50:86–90. doi: 10.1128/JCM.05564-11
60. Chi CY, Lin CC, Liao IC, Yao YC, Shen FC, Liu CC, et al. Panton-Valentine leukocidin facilitates the escape of staphylococcus aureus from human keratinocyte endosomes and induces apoptosis. J Infect Dis. (2014) 209:224–35. doi: 10.1093/infdis/jit445
61. Carniciu AL, Chou J, Leskov I, Freitag SK. Clinical presentation and bacteriology of eyebrow infections: the massachusetts eye and ear infirmary experience (2008-2015). Ophthalmic Plast Reconstr Surg. (2017) 33:372–5. doi: 10.1097/IOP.0000000000000797
62. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. (2007) 144:497–501. doi: 10.1016/j.ajo.2007.04.049
63. Amato M, Pershing S, Walvick M, Tanaka S. Trends in ophthalmic manifestations of methicillin-resistant staphylococcus aureus (MRSA) in a northern california pediatric population. J AAPOS. (2013) 17:243–7. doi: 10.1016/j.jaapos.2012.12.151
64. Gurdal C, Bilkan H, Sarac O, Seven E, Yenidunya MO, Kutluhan A, et al. Periorbital necrotizing fasciitis caused by community-associated methicillin-resistant staphylococcus aureus periorbital necrotizing fasciitis. Orbit. (2010) 29:348–50. doi: 10.3109/01676831003697509
65. Shield DR, Servat J, Paul S, Turbin RE, Moreau A, de la Garza A, et al. Periocular necrotizing fasciitis causing blindness. JAMA Ophthalmol. (2013) 131:1225–7. doi: 10.1001/jamaophthalmol.2013.4816
66. Singam NV, Rusia D, Prakash R. An eye popping case of orbital necrotizing fasciitis treated with antibiotics, surgery, and hyperbaric oxygen therapy. Am J Case Rep. (2017) 18:329–33. doi: 10.12659/AJCR.902535
67. Foster CE, Yarotsky E, Mason EO, Kaplan SL, Hulten KG. Molecular characterization of staphylococcus aureus isolates from children with periorbital or orbital cellulitis. J Pediatric Infect Dis Soc. (2018) 7:205–9. doi: 10.1093/jpids/pix036
68. Planet PJ, LaRussa SJ, Dana A, Smith H, Xu A, Ryan C, et al. Emergence of the epidemic methicillin-resistant staphylococcus aureus strain uSA300 coincides with horizontal transfer of the arginine catabolic mobile element and speG-mediated adaptations for survival on skin. MBio. (2013) 4:e00889–13. doi: 10.1128/mBio.00889-13
Keywords: MRSA, Ocular infection, Molecular Epidemiology, Tissue tropism, biogeography of infections
Citation: Bispo PJM, Ung L, Chodosh J and Gilmore MS (2020) Hospital-Associated Multidrug-Resistant MRSA Lineages Are Trophic to the Ocular Surface and Cause Severe Microbial Keratitis. Front. Public Health 8:204. doi: 10.3389/fpubh.2020.00204
Received: 01 November 2019; Accepted: 05 May 2020;
Published: 03 June 2020.
Edited by:
Filipa Grosso, University of Porto, PortugalCopyright © 2020 Bispo, Ung, Chodosh and Gilmore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael S. Gilmore, bWljaGFlbF9naWxtb3JlQG1lZWkuaGFydmFyZC5lZHU=
 Paulo J. M. Bispo
Paulo J. M. Bispo Lawson Ung
Lawson Ung James Chodosh
James Chodosh Michael S. Gilmore1,2,3*
Michael S. Gilmore1,2,3*