- 1School of Construction and Environment Engineering, Shenzhen Polytechnic, Shenzhen, China
- 2School of Environment and Natural Resource, Renmin University of China, Beijing, China
- 3School of Environmental Science and Engineering, Beijing Forestry University, Beijing, China
The characteristics and performances of catalyst are the key in catalytic ultrasonic treatment of wastewater, and iron based catalysts are known for low cost, high accessibility and safety. This paper reviewed the current research status of iron-based catalysts in water treatment assisted by ultrasound. Zero valent iron, Fe3O4 and iron composited with other metals were analyzed, their behaviors in catalytic sonochemistry were summarized, and the potential catalytic mechanisms were discussed in details. Finally, the future development in this field was proposed.
Introduction
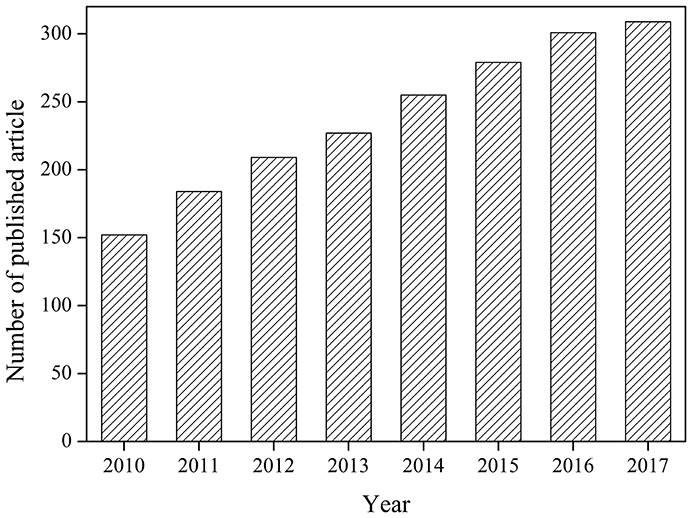
Each year, many kinds of refractory organic pollutants are charged into water (Richardson and Kimura, 2017). These pollutants are hard to be removed by conventional treatment methods, and development of advanced treatment technology is necessary. Ultrasound has attracted great attentions for its safety, easy operation, high degradation efficiency, and free of secondary pollutants (Pokhrel et al., 2016). But removal efficiencies of organic pollutants by ultrasound are sometimes low and the energy consumption is high. Combination with catalyst to form catalytic sono-reactions can improve the efficiency of organic pollutants removal and reduce the energy consumption (Chatel et al., 2016). Many catalysts have been applied in the ultrasonic degradation of pollutants (Descorme, 2017). More and more papers are published in this field from 2010 to 2017 according to search results of the Web of Science (Figure 1).
Among all types of catalysts, iron-based catalysts are of the highest potential due to their low cost, high safety and wide-distribution. Zero valent iron (ZVI), Fe2(SO4)3, FeSO4, FeCl3, and FeOOH have long been used in water and wastewater treatment. Sonication alone achieved 22% diclofenac degradation while combination of FeCeOx improved the efficiency to 81% (Chong et al., 2017). Fe0/TiO2 nano-particle catalytic sonication achieved 100% removal of reactive black 5 in 20 min (Bhaumik et al., 2017).
This paper aims to provide an overview of this developing field of iron-based catalysts in water treatment assisted by ultrasound. The properties, performances, and combination with other metals of iron-based sonocatalysts were discussed. Reaction mechanisms of sonocatalytic degradation of organic pollutants were reviewed. Future development was proposed.
Fe-based Sonocatalysts
Iron element is famous for Fenton's reagent (Fe2++H2O2), a classic advanced oxidation technology. However, hazards associated with the transport, handling and storage of bulk quantities of H2O2 have made the process unsafe and economically challenging. The combination of ultrasound and iron-based catalysts can achieve high degradation efficiency of organic pollutants without H2O2 (Chandran et al., 2014). Iron based sonocatalysts, ZVI, Fe3O4, and iron composited with other metals (lutetium and silver), have been examined. The reaction scheme is based on sonochemistry and Fenton-like reaction. The powerful ultrasound dissociates water to form·OH and H2O2, which then reacts with Fe2+ and Fe3+ ions in sonocatalysts as shown in Equations (1–5) (Jamalluddin and Abdullah, 2014). The catalytic activity of Fe0 is also based on the surface chemistry reactions of Fe0 to initiate the reactions in water, as shown in Equations (6–8). Application of Fe0 as catalyst has a few advantages: enhance mass transfer to the surface of the catalyst, continuous damage of the catalyst surface to create more defects, and cleaning of the catalyst surface (Güyer and Ince, 2011).
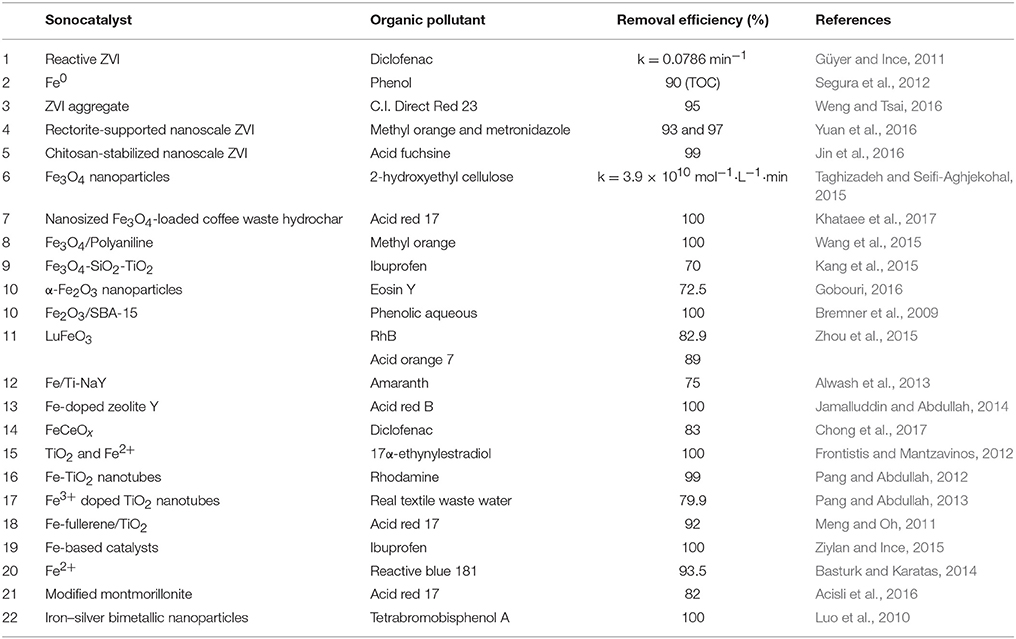
Table 1 summarizes the typical iron-based sonocatalysts. The dechlorination of Fe0 for complicated chlorinated compounds is often incomplete and with low efficiency. Luo et al. (2010) found that the Ag/Fe catalyst was quite effective for the degradation of chlorinated organics, and silver also provide some disinfection effect.
When nonmagnetic catalysts (e.g., TiO2) are employed, their recovery is a troublesome issue. Super-paramagnetic Fe3O4 facilitates fast recovery/re-dispersion of the catalyst by simply switching on/off an external magnet (Richardson and Kimura, 2017), which is the most effective and simplest method to enhance the recovery and reuse of catalysts (Kang et al., 2015). Taghizadeh and Seifi-Aghjekohal (2015) found that the sonocatalytic activity of Fe3O4 was the best among Fe3O4, Rutile-TiO2, ZnO, and Anatase-TiO2 nanoparticles. The proposed reason was that Fe3O4 enhanced the ·OH radical generation by the electron transfer between iron ions and H2O molecules.
The activity of ZVI and Fe3O4 is promoted in acidic solution. However, the reuse property is poor due to severe iron leaching in acidic condition. Besides, acidic pH limits the practical application of sonocatalysts. Therefore, iron composites catalysts have been proposed. Zhou et al. (2015) found that the pH value had a small effect on the sonocatalytic degradation of RhB by LuFeO3, and the LuFeO3 particles exhibited a good structural stability with no structural change before and after sonication. Alwash et al. (2013) found that Fe/Ti-NaY kept its high activity in the decolorization of amaranth after three times of reuse; X-ray diffraction proved that the catalyst was stable after reuse and maintained its crystallinity. Another method to improve the stability of catalyst is doping the active component on supports like graphene and zeolite (Rakmae et al., 2016). Even at neutral and alkaline medium, a good decolorization and degradation efficiency could still be achieved in the present of Fe-doped zeolite Y under ultrasound. Fe (III)/Y demonstrated good catalytic efficiency, low Fe leaching, and good reusability (Jamalluddin and Abdullah, 2014).
Iron has been used to modify TiO2 and formed new sonocatalysts. TiO2 nano-tubes possess a relatively wide energy band gap (3.2 or 3.0 eV in Anatase or Rutile phase) and fast recombination rate of charge carriers (Richardson and Kimura, 2017). One possible solution to this problem is introducing suitable transition metals such as Fe, Cr, or Co into TiO2 to form a new sonocatalyst with narrower band gap and longer lifetime of charge carriers (Pang and Abdullah, 2013).
Potential Mechanisms of Sonocatalytic Degradation of Organic Pollutants
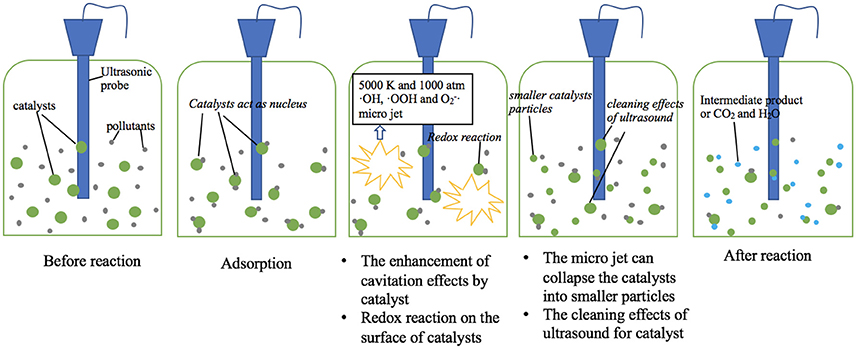
The main mechanism of ultrasound is cavitation effects, which can be enhanced by addition of sonocatalysts. Some organic pollutants are adsorbed on the surface of catalysts, thus increases the removal rate due to shorter reaction path. Meanwhile, ultrasound enhances the redox reaction between catalyst and organic pollutants. Potential mechanisms of sonocatalytic degradation of organic pollutants are shown in Figure 2.
Cavitation Effects
The most import sonocatalytic mechanism lays in the cavitation of water during ultrasonic irradiation (Wang et al., 2015). The cavitation involves the processes of formation (nucleation), rapid growth (expansion), and violent collapse (implosion) of cavitation bubbles. Such a violate collapse of cavitation bubbles cause local high temperature and pressure (up to 5,000 K and 1,000 atm) and the emission of light (sono-luminescence), and generate free radicals including ·OH, ·OOH, and (Pokhrel et al., 2016). The free radicals can react with target contaminants.
Mechanical effect is a main advantage of ultrasound irradiation (Alwash et al., 2013). Ultrasound can promote the mass transfer, which leads the contact of pollutants with free radical or the catalyst more sufficiently. In addition, the micro jet can collapse the catalyst into smaller particles, which offer a higher surface area, thus enhances the surface reaction.
The Enhancement of Cavitation Effects by Catalyst
Catalyst particles in water can act as nucleus for cavitation bubbles (Zhao et al., 2014). Once the catalyst particles sizes are in the same order of magnitude with the size of the cavitation bubbles, catalyst particles can form extra nucleus of cavitation bubbles. Extra nucleus generates more cavitation bubbles, causes stronger cavitation effects, leading to higher degradation efficiency.
Adsorption of Catalysts
Organic pollutants can be adsorbed on the surface of catalysts due to electrostatic attraction and sonication (Thangavel et al., 2015). The adsorbed pollutants then react with the sono-generated free radicals from/around the catalyst. Moreover, the concentration of free radicals is high on the surface of catalysts and the adherence of organic pollutants upon the catalyst can shorten the path for radicals/cavities to decompose the pollutants. Then the degradation rate of organic pollutants is improved.
Redox Reaction between Sonocatalyst and Organic Pollutants
Organic pollutants can react with the surface of sonocatalyst. The free radicals produced by ultrasound improve the redox reaction between sonocatalyst and organic pollutants, which accelerates the removal of organic pollutant. Free radicals react with polyvalent metal sonocatalyst such as iron-based composites, which may produce some new valence state sonocatalyst to accelerate the reaction (Chong et al., 2017).
The Cleaning Effects of Ultrasound for Catalyst
The cleaning effect of ultrasound for catalysts refers to the phenomenon that ultrasound waves in the ultrasonic system continuously remove the intermediates or by-products from the surface of catalysts to reactive the surface (Alwash et al., 2013). The cleaning effects of ultrasound are based on the cavitation effects. Ultrasound generates many bubbles in water and they collapse fast, then the catalysts are cleaned by the shock wave. Such an ultrasonic regeneration of the sonocatalyst is of great advantage via removing the contaminants and decomposition of toxic organic pollutants (Wang et al., 2015).
Conclusions and Perspectives
In this mini-review, the typical iron-based sonocatalysts are summarized. Their sonocatalytic activity is different for different organic pollutants. The special property for iron-based sonocatalysts is magnetism, which is beneficial to separate catalysts from water. The mechanisms of sonocatalytic degradation of organic pollutants involve both ultrasound irradiation and sonocatalyst. In the future, more works will be done on this developing field and following issues might be of great values:
(1) Produce better sonocatalysts
Obviously the sonocatalytic efficiency depends on the type of catalyst. Development of novel catalyst with higher efficiency has always been the hot topic, and better understanding of the sonocatalytic mechanisms is also necessary to promote the application of the sonocatalytic technology.
(2) Pay more attention to the safety and cost efficiency
Most researchers only investigate the activity of sonocatalyst, but pay little attention on the economy and safety of sonocatalyst. If the materials for preparing sonocatalyst are rare or expensive and the preparation method is complex, these sonocatalysts are difficult to be applied. The stability and reusability of sonocatalyst also need be considered, which are relative to the service life of sonocatalyst. the selectivity of sonocatalysts
(3) Enhance the selectivity of sonocatalysts
In some situations, trace toxic substances and high concentration of non-poisonous pollutants co-exist in water, the sonocatalyst must have good selectivity, but little investigation is available on this subject, which needs be addressed.
Author Contributions
NZ collected and read papers and contributed partially to the Introduction and mainly to Table 1; GX collected and read papers and contributed to paper writing; XL contributed greatly to the writing of Potential Mechanisms of Sonocatalytic Degradation of Organic Pollutants section; JZ contributed to the paper design and refine; PZ contributed to paper design and data analysis; GZ was in charge of the whole writing.
Funding
Authors thanks financial support from National Key R&D Program of China (2017YFC0504405), Shenzhen Science and Technology Committee (JSKF20150901115155532), and Beijing Natural Science Foundation (8172028).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acisli, O., Khataee, A., Karaca, S., and Sheydaei, M. (2016). Modification of nanosized natural montmorillonite for ultrasound-enhanced adsorption of Acid Red 17. Ultrason. Sonochem. 31, 116–121. doi: 10.1016/j.ultsonch.2015.12.012
Alwash, A. H., Abdullah, A. Z., and Ismail, N. (2013). Investigation on the catalytic behavior of Fe loaded on encapsulated titanium for sonocatalytic degradation of amaranth : characterization and reusability study. Sci. Res. 2, 100–109. doi: 10.4236/mrc.2013.23015
Basturk, E., and Karatas, M. (2014). Advanced oxidation of Reactive Blue 181 solution: a comparison between Fenton and Sono-Fenton Process. Ultrason. Sonochem. 21, 1881–1885. doi: 10.1016/j.ultsonch.2014.03.026
Bhaumik, M., Maity, A., and Gupta, V. K. (2017). Synthesis and characterization of Fe0/TiO2 nano-composites for ultrasound assisted enhanced catalytic degradation of reactive black 5 in aqueous solutions. J. Colloid Interface Sci. 506, 403–414. doi: 10.1016/j.jcis.2017.07.016
Bremner, D. H., Molina, R., Martínez, F., Melero, J. A., and Segura, Y. (2009). Degradation of phenolic aqueous solutions by high frequency sono-Fenton systems (US-Fe2O3/SBA-15-H2O2). Appl. Catal. B 90, 380–388. doi: 10.1016/j.apcatb.2009.03.028
Chandran, H. T., Thangavel, S., Jipsa, C. V., and Venugopal, G. (2014). Study on inorganic oxidants assisted sonocatalytic degradation of Resazurin dye in presence of β-SnWO4nanoparticles. Mater. Sci. Semicond. Process. 27, 212–219. doi: 10.1016/j.mssp.2014.06.021
Chatel, G., Novikova, L., and Petit, S. (2016). How efficiently combine sonochemistry and clay science? Appl. Clay Sci. 119, 193–201. doi: 10.1016/j.clay.2015.10.019
Chong, S., Zhang, G., Wei, Z., Zhang, N., Huang, T., and Liu, Y. (2017). Sonocatalytic degradation of diclofenac with FeCeOx particles in water. Ultrason. Sonochem. 34, 418–425. doi: 10.1016/j.ultsonch.2016.06.023
Descorme, C. (2017). Catalytic wastewater treatment: oxidation and reduction processes. Recent studies on chlorophenols. Catal. Today 297, 324–334. doi: 10.1016/j.cattod.2017.03.039
Frontistis, Z., and Mantzavinos, D. (2012). Sonodegradation of 17-ethynylestradiol in environmentally relevant matrices: laboratory-scale kinetic studies. Ultrason. Sonochem. 19, 77–84. doi: 10.1016/j.ultsonch.2011.06.016
Gobouri, A. A. (2016). Ultrasound enhanced photocatalytic properties of α-Fe2O3 nanoparticles for degradation of dyes used by textile industry. Res. Chem. Intermed. 42, 5099–5113. doi: 10.1007/s11164-015-2347-0
Güyer, G. T., and Ince, N. H. (2011). Degradation of diclofenac in water by homogeneous and heterogeneous sonolysis. Ultrason. Sonochem. 18, 114–119. doi: 10.1016/j.ultsonch.2010.03.008
Jamalluddin, N. A., and Abdullah, A. Z. (2014). Low frequency sonocatalytic degradation of Azo dye in water using Fe-doped zeolite y catalyst. Ultrason. Sonochem. 21, 743–753. doi: 10.1016/j.ultsonch.2013.10.008
Jin, X., Zhuang, Z., Yu, B., Chen, Z., and Chen, Z. (2016). Functional chitosan-stabilized nanoscale zero-valent iron used to remove acid fuchsine with the assistance of ultrasound. Carbohydr. Polym. 136, 1085–1090. doi: 10.1016/j.carbpol.2015.10.002
Kang, K., Jang, M., Cui, M., Qiu, P., Na, S., Son, Y., et al. (2015). Enhanced sonocatalytic treatment of ibuprofen by mechanical mixing and reusable magnetic core titanium dioxide. Chem. Eng. J. 264, 522–530. doi: 10.1016/j.cej.2014.10.106
Khataee, A., Kayan, B., Kalderis, D., Karimi, A., Akay, S., and Konsolakis, M. (2017). Ultrasound-assisted removal of Acid Red 17 using nanosized Fe3O4-loaded coffee waste hydrochar. Ultrason. Sonochem. 35, 72–80. doi: 10.1016/j.ultsonch.2016.09.004
Luo, S., Yang, S., Wang, X., and Sun, C. (2010). Reductive degradation of tetrabromobisphenol A over iron-silver bimetallic nanoparticles under ultrasound radiation. Chemosphere 79, 672–678. doi: 10.1016/j.chemosphere.2010.02.011
Meng, Z. D., and Oh, W. C. (2011). Sonocatalytic degradation and catalytic activities for MB solution of Fe treated fullerene/TiO2 composite with different ultrasonic intensity. Ultrason. Sonochem. 18, 757–764. doi: 10.1016/j.ultsonch.2010.10.008
Pang, Y. L., and Abdullah, A. Z. (2012). Effect of low Fe3+ doping on characteristics, sonocatalytic activity and reusability of TiO2 nanotubes catalysts for removal of Rhodamine B from water. J. Hazard. Mater. 235–236, 326–335. doi: 10.1016/j.jhazmat.2012.08.008
Pang, Y. L., and Abdullah, A. Z. (2013). Fe3+ doped TiO2 nanotubes for combined adsorption-sonocatalytic degradation of real textile wastewater. Appl. Catal. B 129, 473–481. doi: 10.1016/j.apcatb.2012.09.051
Pokhrel, N., Vabbina, P. K., and Pala, N. (2016). Sonochemistry: science and engineering. Ultrason. Sonochem. 29, 104–128. doi: 10.1016/j.ultsonch.2015.07.023
Rakmae, S., Keawkumay, C., Osakoo, N., Montalbo, K. D., de Leon, R. L., Kidkhunthod, P., et al. (2016). Realization of active species in potassium catalysts on zeolite NaY prepared by ultrasound-assisted impregnation with acetate buffer and improved performance in transesterification of palm oil. Fuel 184, 512–517. doi: 10.1016/j.fuel.2016.07.035
Richardson, S. D., and Kimura, S. Y. (2017). Emerging environmental contaminants: challenges facing our next generation and potential engineering solutions. Environ. Technol. Innov. 8, 40–56. doi: 10.1016/j.eti.2017.04.002
Segura, Y., Martínez, F., Melero, J. A., Molina, R., Chand, R., and Bremner, D. H. (2012). Enhancement of the advanced Fenton process (Fe0/H2O2) by ultrasound for the mineralization of phenol. Appl. Catal. B 113–114, 100–106. doi: 10.1016/j.apcatb.2011.11.024
Taghizadeh, M. T., and Seifi-Aghjekohal, P. (2015). Sonocatalytic degradation of 2-hydroxyethyl cellulose in the presence of some nanoparticles. Ultrason. Sonochem. 26, 265–272. doi: 10.1016/j.ultsonch.2014.12.014
Thangavel, S., Raghavan, N., Kadarkarai, G., Kim, S. J., and Venugopal, G. (2015). Graphene-oxide (GO)-Fe3+ hybrid nanosheets with effective sonocatalytic degradation of Reactive Red 120 and study of their kinetics mechanism. Ultrason. Sonochem. 24, 123–131. doi: 10.1016/j.ultsonch.2014.11.019
Wang, Y., Gai, L., Ma, W., Jiang, H., Peng, X., and Zhao, L. (2015). Ultrasound-assisted catalytic degradation of methyl orange with Fe3O4 /polyaniline in near neutral solution. Ind. Eng. Chem. Res. 54, 2279–2289. doi: 10.1021/ie504242k
Weng, C. H., and Tsai, K. L. (2016). Ultrasound and heat enhanced persulfate oxidation activated with Fe0 aggregate for the decolorization of C.I. Direct Red 23. Ultrason. Sonochem. 29, 11–18. doi: 10.1016/j.ultsonch.2015.08.012
Yuan, N., Zhang, G., Guo, S., and Wan, Z. (2016). Enhanced ultrasound-assisted degradation of methyl orange and metronidazole by rectorite-supported nanoscale zero-valent iron. Ultrason. Sonochem. 28, 62–68. doi: 10.1016/j.ultsonch.2015.06.029
Zhao, H., Zhang, G., and Zhang, Q. (2014). MnO2/CeO2 for catalytic ultrasonic degradation of methyl orange. Ultrason. Sonochem. 21, 991–996. doi: 10.1016/j.ultsonch.2013.12.002
Zhou, M., Yang, H., Xian, T., Yang, Y., and Zhang, Y. (2015). Sonocatalytic activity of LuFeO3 crystallites synthesized via a hydrothermal route. Chin. J. Catal. 36, 1987–1994. doi: 10.1016/S1872-2067(15)60941-X
Keywords: ultrasound, iron, sonocatalyst, organic pollutants, mechanisms
Citation: Zhang N, Xian G, Li X, Zhang P, Zhang G and Zhu J (2018) Iron Based Catalysts Used in Water Treatment Assisted by Ultrasound: A Mini Review. Front. Chem. 6:12. doi: 10.3389/fchem.2018.00012
Received: 06 November 2017; Accepted: 15 January 2018;
Published: 02 February 2018.
Edited by:
Md Ahmaruzzaman, National Institute of Technology, Silchar, IndiaReviewed by:
Steve Suib, University of Connecticut, United StatesVasco D. B. Bonifácio, Instituto Superior Técnico, Universidade de Lisboa, Portugal
Copyright © 2018 Zhang, Xian, Li, Zhang, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangming Zhang, emdtQHJ1Yy5lZHUuY24=
Jia Zhu, NzU2Njg3NEBxcS5jb20=
 Nan Zhang1,2
Nan Zhang1,2 Guang Xian
Guang Xian Xuemei Li
Xuemei Li Panyue Zhang
Panyue Zhang Guangming Zhang
Guangming Zhang