- 1Pediatric Biochemistry Unit, Department of Pediatrics, Post Graduate Institute of Medical Education & Research (PGIMER), Chandigarh, India
- 2Newborn Unit, Department of Pediatrics, Post Graduate Institute of Medical Education & Research (PGIMER), Chandigarh, India
Introduction: Necrotizing enterocolitis (NEC) is an inflammatory bowel disease that primarily affects preterm infants. Predisposing risk factors for NEC include prematurity, formula feeding, anemia, and sepsis. To date, no studies have investigated the gut microbiota of preterm infants with NEC in India.
Method: In the current study, shotgun metagenomic sequencing was performed on fecal samples from premature infants with NEC and healthy preterm infants (n = 24). Sequencing was conducted using the NovaSeq X Plus platform, generating 2 × 150 bp paired-end reads. The infants were matched based on gestational age and postnatal age.
Result: The median time to NEC diagnosis was 9 days (range: 1–30 days). Taxonomic analysis revealed a high prevalence of Enterobacteriaceae at the family level, with the genera Klebsiella and Escherichia particularly prominent in neonates with NEC. No statistically significant differences in alpha or beta diversity were observed between stool samples from infants with and without NEC. Linear regression analysis demonstrated that Enterobacteriaceae were significantly more abundant in stool samples from infants with NEC than without NEC (q < 0.05). Differential abundance analysis using Linear Discriminant Analysis Effect Size (LEfSe) identified Klebsiella pneumoniae and Escherichia coli as enriched in the gut microbiota of preterm infants with NEC. Functional analysis revealed an increase in genes associated with lipopolysaccharide (LPS) O-antigen, the type IV secretion system (T4SS), the L-rhamnose pathway, quorum sensing, and iron transporters, including ABC transporters, in stool samples from infants with NEC.
Conclusion: The high prevalence of Enterobacteriaceae and enrichment of LPS O-antigen and T4SS genes may be associated with NEC in Indian preterm infants.
Introduction
Necrotizing enterocolitis (NEC) is a devastating inflammatory disease that significantly contributes to morbidity and mortality in preterm infants. The incidence of NEC is approximately 7% (95% CI: 6–8%) among very low birth weight (VLBW) infants (Alsaied et al., 2020). Risk factors for NEC, such as formula feeding, maternal and neonatal antibiotic exposure, and premature birth, have been shown to induce alterations in gut microbiota (Thänert et al., 2020). The immature intestines of preterm infants, when exposed to inappropriate bacterial colonization alongside a hyperactivated immune system, may drive the development of NEC (Mara et al., 2018). Dysbiosis of the gut microbiota has been observed in individuals with NEC even beyond five years of age (Magnusson et al., 2024). The severity of dysbiosis correlates with the stage of NEC and is more pronounced in patients who undergo surgical treatment (Magnusson et al., 2024). These findings demonstrate how the sequelae of NEC lead to persistent perturbations in the gut microbiota of affected children.
Despite two decades of extensive research using next-generation sequencing techniques, the specific microbial communities responsible for the development of NEC have not yet been identified. Several factors contribute to this challenge, including heterogeneity among studies, such as variations in the timing of sample collection, duration of clinical presentations, and differences in sequencing methods—ranging from targeting variable regions of the 16S rRNA gene to whole-genome bacterial sequencing. Additionally, the gut microbiota of preterm infants is influenced by environmental factors related to hospital admission, antibiotic use, feeding methods, medications, and other supportive interventions (Thänert et al., 2024). During hospitalization, feed intolerance, sepsis, and other comorbidities commonly co-occur with NEC, further contributing to alterations in the gut microbiota. Probiotics have been shown to restore dysbiosis in infants, thereby reducing the incidence of NEC (Samara et al., 2022). Disruptions in the gut microbiota during the early weeks of life in preterm infants are associated with an increased risk of NEC.
Several studies conducted in high-income countries have established an association between the microbiome and NEC (Thänert et al., 2020; Tarracchini et al., 2021); however, data from low- and middle-income countries remain scarce. Investigating the gut microbiota is valuable not only for identifying microbial biomarkers but also for developing bacterial-mediated therapies, such as fecal microbiota transplantation and bacteriophage treatment. In this study, we employed a shotgun metagenomic approach to characterize the gut microbiome and identify functional metabolic pathways as the primary and secondary outcomes in fecal samples from preterm infants with NEC.
Methods
Participant recruitment
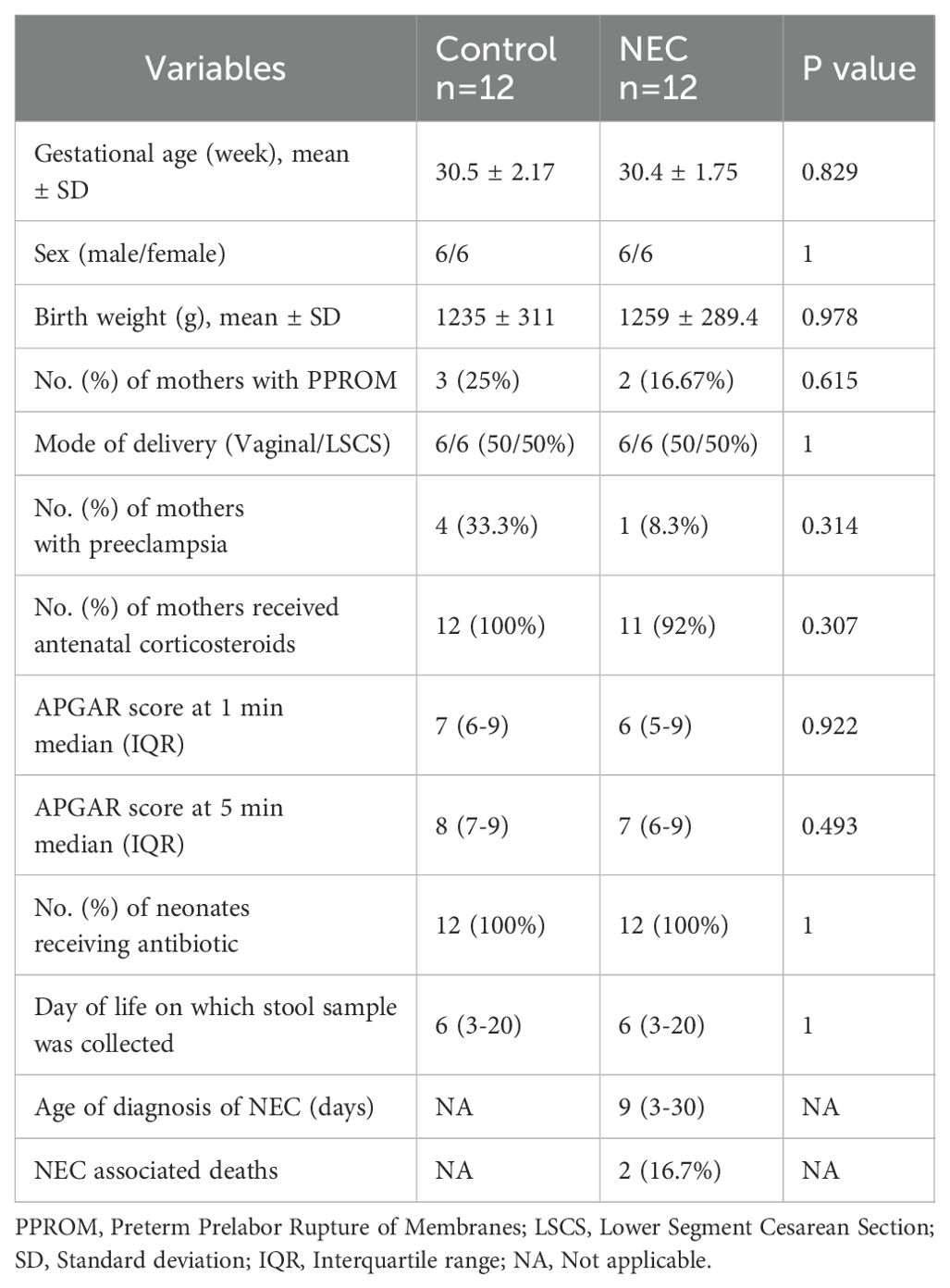
This prospective observational cohort study was conducted from September 2022 to January 2025 at a tertiary care neonatal unit in Northern India. Preterm infants born between 26 and 32 weeks of gestation, with a birth weight of less than 1500 grams, and admitted to the NICU were recruited. Participants’ demographic information and clinical details were collected prospectively until discharge or death using a standardized proforma. NEC was diagnosed according to the Bell staging criteria and classified as cases. Healthy preterm infants matched for gestational and postnatal age were recruited as controls. The study was approved by the Institutional Ethics Committee (IEC) of the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, and was conducted in accordance with the Declaration of Helsinki.
Sample collection
The fecal samples from infants were collected in sterile containers during the first month of life as described in previous studies (Devarajalu et al., 2024; Devarajalu et al., 2025). Fecal samples from control subjects were collected on days of life matched to those of the NEC group. Participants were recruited after obtaining written informed consent from one of the parents. Stool samples were stored in an ultra-low temperature freezer (-80 °C) until further processing.
Whole genome sequencing
Genomic DNA was isolated from approximately 100 milligrams of fecal specimens using the QIAamp PowerFecal Pro DNA Kit (Qiagen Inc., Germany). The purity of the extracted DNA was assessed by 1% agarose gel electrophoresis. DNA concentration was determined using the Qubit DNA High Sensitivity (HS) assay (Invitrogen). A paired-end DNA library was prepared using the Twist EF Library Prep Kit (Illumina, Inc., USA). Briefly, the DNA was first fragmented to the desired size, then end-repaired and mono-adenylated at the 3’ end in a single enzymatic reaction. Next, adapters were ligated to the DNA fragments using a T4 DNA ligase-based reaction. Following ligation, the fragments were prepared as substrates for PCR-based indexing in the subsequent step. During PCR, barcodes were incorporated using unique primers for each sample, enabling multiplexing. All prepared libraries were assessed for fragment distribution using a 5300 Fragment Analyzer system (Agilent Technologies, Inc., CA, United States). The resulting libraries were pooled and diluted to achieve optimal loading concentrations. Finally, the pooled libraries were loaded onto a NovaSeq X Plus system (Illumina, Inc., CA, United States) to generate 150 bp paired-end reads. The average read count of the samples is 22.8 million. Sequencing statistics are shown in Supplementary Table 1.
Sequencing and mapping
The adapter sequences were trimmed using the fastq-mcf tool (version 1.04.803). The trimmed reads were then aligned to the human genome (hg19) using BWA (version 0.7.12) to remove human contamination. The remaining unaligned reads were de novo assembled with MEGAHIT (version 1.2.9). The resulting assembled genome was used for open reading frame (ORF) prediction and annotation with Prodigal (version 2.6.3). Taxonomic classification was performed using datasets from the National Center for Biotechnology Information (NCBI), and functional pathways were identified from the predicted ORFs using SEED protein classification in MEGAN 6 (Huson et al., 2016).
Microbiome composition and statistical analysis
Alpha diversity metrics, including observed counts and the Shannon index, were calculated using the Phyloseq package (McMurdie and Holmes, 2013). Differences in alpha diversity between groups were assessed using the Wilcoxon rank-sum test. Beta diversity metrics were computed with the Vegan package. A permutational analysis of variance (PERMANOVA) was performed using the adonis2 function from the pairwise package to identify variation between groups by fitting Bray-Curtis and Jaccard distance matrices separately, incorporating covariates, with 999 permutations (Oksanen et al., 2001).
Differential bacterial abundance between variables was analyzed using Linear Discriminant Analysis (LDA) Effect Size (LEfSe) with an LDA threshold of 4, implemented through the MicrobiomeMarker package (Cao et al., 2022). Additionally, a linear regression model employing the LinDA and MaAsLin2 packages was used to identify taxa and functional pathways associated with necrotizing enterocolitis (NEC) (Mallick et al., 2021; Zhou et al., 2022). Multiple testing corrections, Benjamini-Hochberg (BH) was applied, with a significance threshold of q < 0.05. Welch’s t-test was conducted for differential functional analysis using STAMP software (Parks et al., 2014). Correlation and network analyses were performed using the Network Construction and Comparison for Microbiome Data (NetCoMi) package (Peschel et al., 2021). All statistical analyses were conducted using R software (version 4.4.2), and figures were generated using the ggplot2, pheatmap, and MicrobiomeStat packages.
Results
Baseline demographic characteristics
The baseline demographic information is presented in Table 1. The NEC and control groups were matched for gestational and postnatal ages. One sample (NEC) was excluded from downstream analysis due to low sequencing read quality.
Microbial richness and diversity
Microbial richness, assessed using alpha diversity, showed no significant differences in observed operational taxonomic units (OTUs) and Shannon index between infants with NEC and the control group (Figure 1). Alpha diversity indices did not differ significantly when analyzed against other covariates, including mode of delivery (vaginal vs. LSCS), sex (male vs. female), birth weight (<1000 g vs. >1000 g), and gestational age (<28 weeks vs. 28–32 weeks). Furthermore, no statistically significant differences or effect size variations were observed between NEC and control groups for beta diversity, as measured by both Bray-Curtis and Jaccard distance metrics (Figure 2). PERMANOVA analysis revealed that mode of delivery accounted for a greater variance in beta diversity than other covariates within our cohort (Table 2).
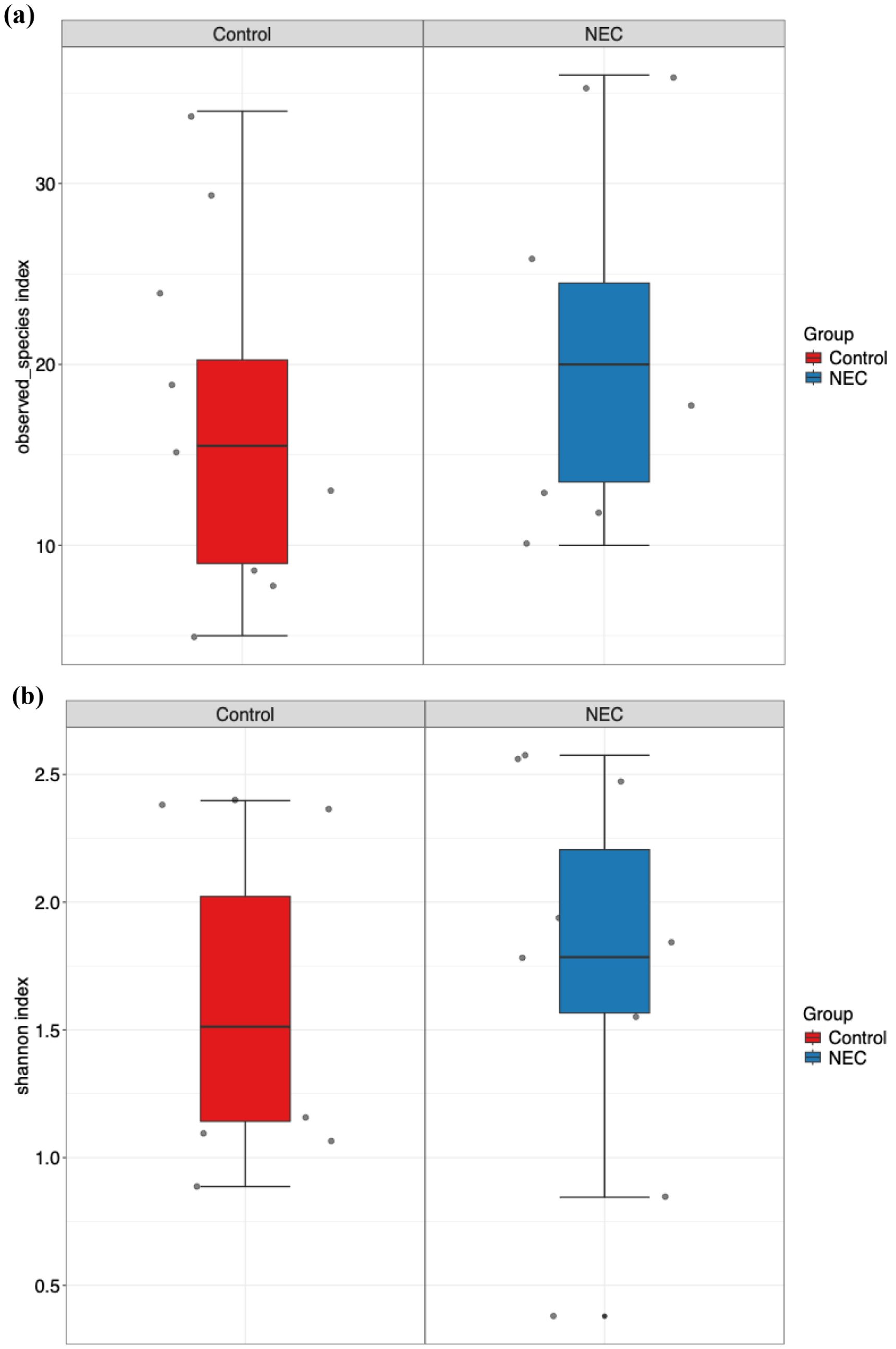
Figure 1. Box and whisker plot represents alpha diversity index (a) observed ASVs and (b) Shannon index for NEC and control groups.

Figure 2. Principal coordinate analysis (PCoA) plots showing the beta diversity with (a) Bray-Curtis and (b) Jaccard measures for NEC and control groups.
Taxonomic composition and differential abundance
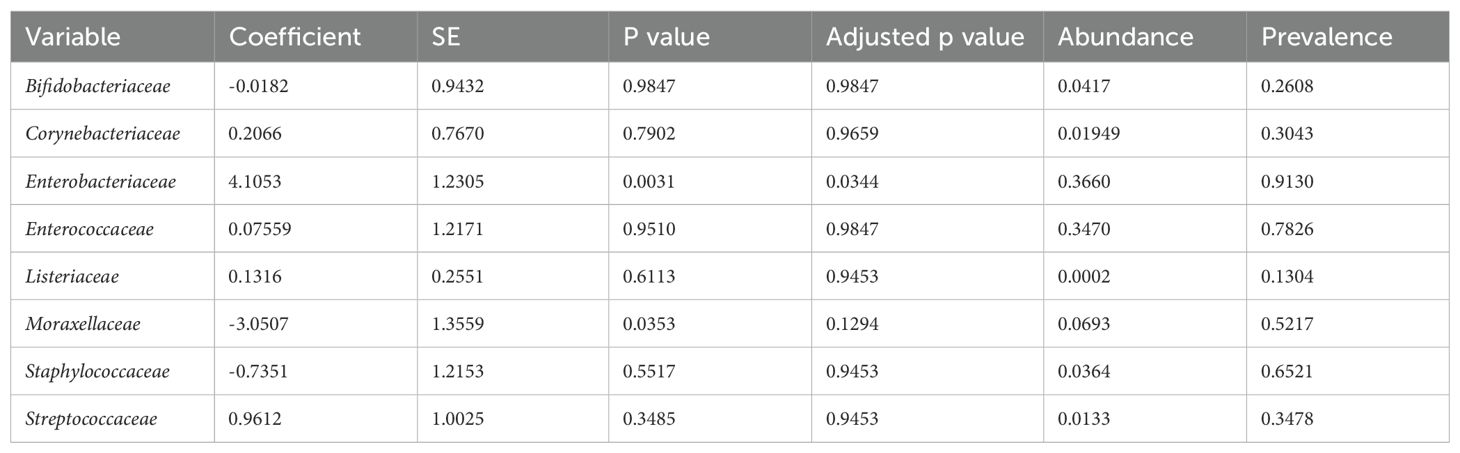
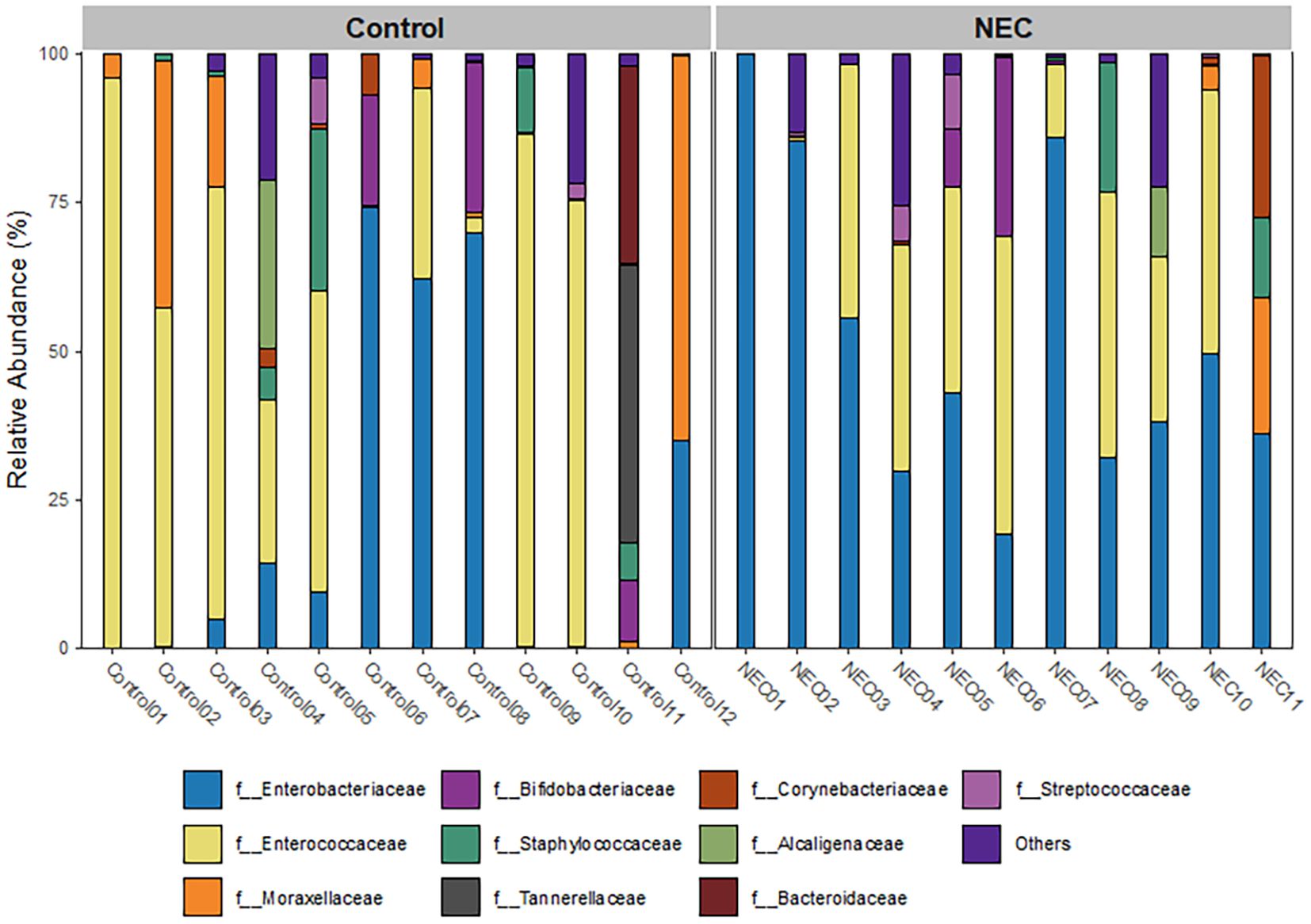
The taxonomic composition at the phylum level indicated that Pseudomonadota, Bacillota, and Actinomycetota were the predominant phyla during the first week of life. An increased abundance of Pseudomonadota was observed in preterm infants with NEC, accounting for 47% of the total phyla (Figure 3a). The two major families identified in the gut of preterm infants during the first week were Enterobacteriaceae and Enterococcaceae, which together comprised 60% of the total taxonomic families (Figure 3b). The abundance of Enterobacteriaceae was significantly higher in the gut of infants with NEC than without NEC, as demonstrated by linear regression analysis (Table 3). There was an increased prevalence of the genera Escherichia and Klebsiella and a decreased prevalence of Enterococcus in preterm infants with NEC. Linear regression analysis indicated a significant increase in the abundance of the genus Klebsiella in NEC cases. Heatmap analysis revealed that either Escherichia or Klebsiella was present in 82% of NEC cases compared to 33.3% of controls (Figure 4). Krona plots showed that the abundance of Klebsiella pneumoniae in the NEC and control groups was 24% and 8% of the total microbial OTUs, respectively, while Escherichia coli accounted for 21% in the NEC cases and 13% in controls (Figure 5).
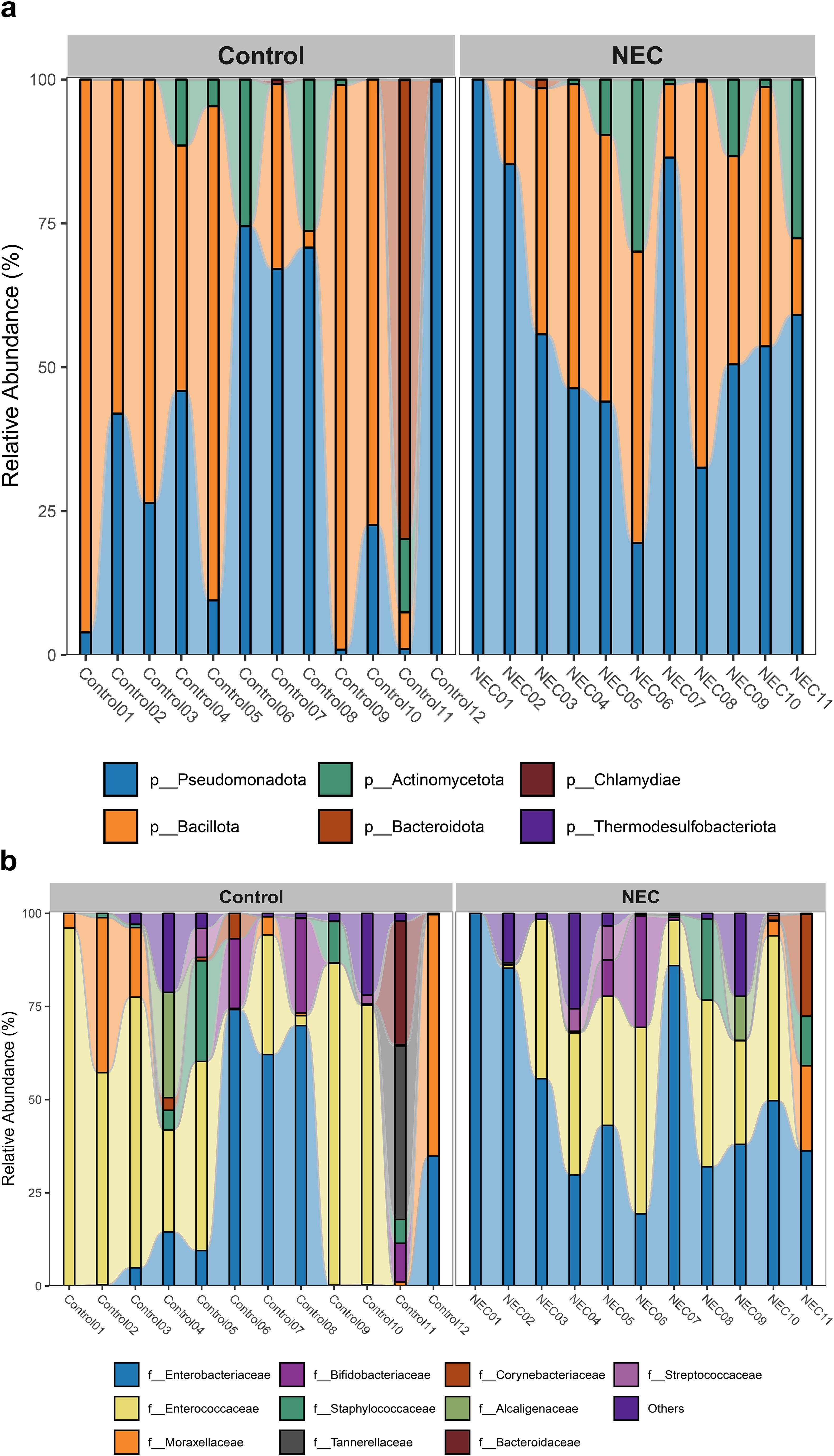
Figure 3. Bar plot represents taxonomic composition of both NEC and controls at (a) phylum and (b) family level.
![Two circular charts display the proportional distribution of bacteria. Chart a) highlights Escherichia coli (21%) and Klebsiella pneumoniae (24%) as prominent, with additional bacteria shown in lesser proportions. Chart b) emphasizes Escherichia coli (13%) and [Candida] glabrata (9%) with other microorganisms present in smaller percentages. The charts use gradient coloring to differentiate categories and both have a central label “all.](https://www.frontiersin.org/files/Articles/1649384/fcimb-15-1649384-HTML/image_m/fcimb-15-1649384-g005.jpg)
Figure 5. A Krona plot showing composition at species levels for NEC and controls. (a) NEC. (b) Control.
Differential abundance analysis using LEfSe revealed that Klebsiella pneumoniae and Klebsiella quasipneumoniae were more prevalent in infants with NEC, whereas unclassified Acinetobacter species were more abundant in the non-NEC group (Figure 6).
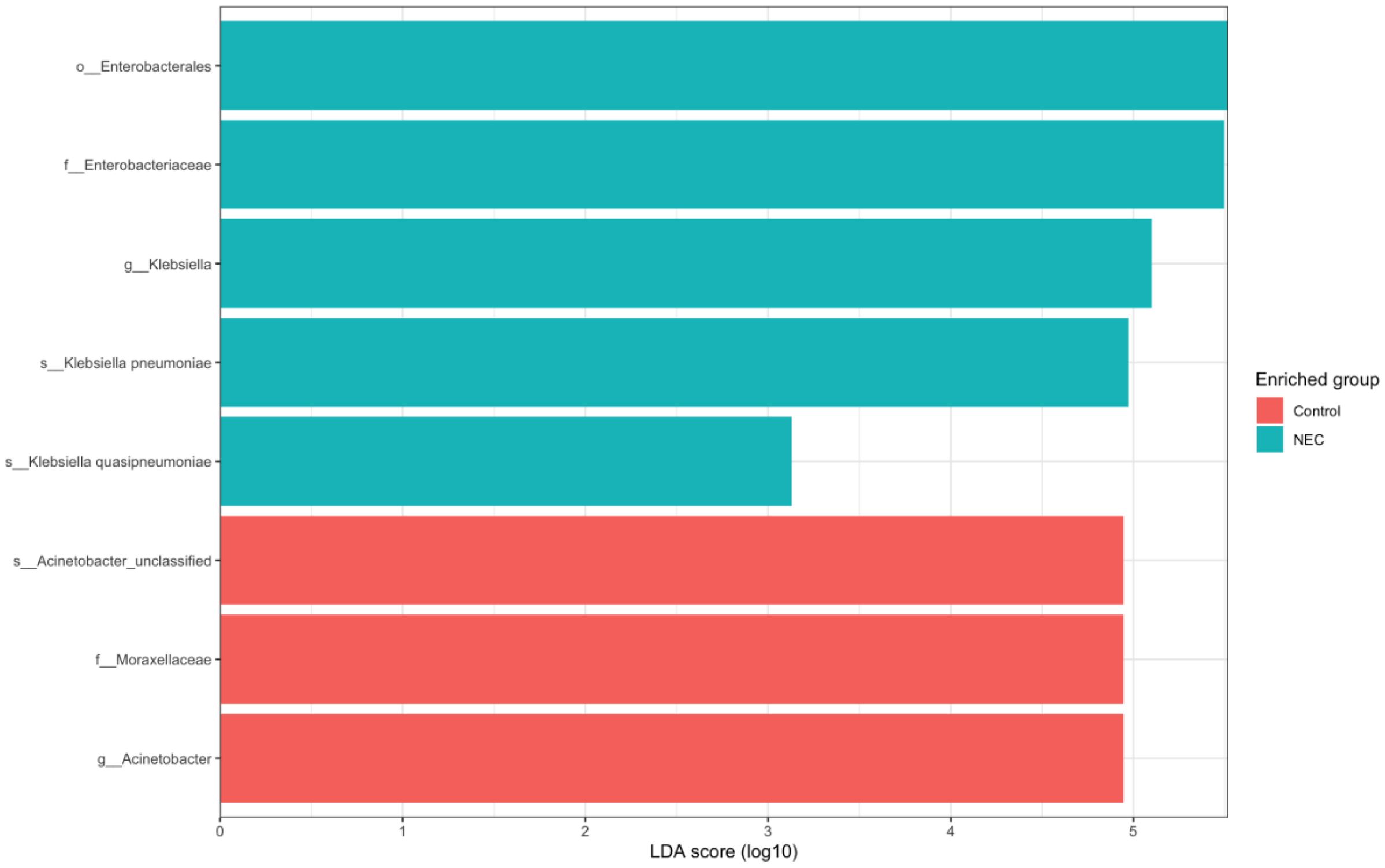
Figure 6. Bar stack plot showing LEfSe results of differential abundance at species levels between NEC and Controls (p < 0.05, LDA > 4).
Correlation of microbial community
The relationships between microbial communities at the genus level were evaluated using Pearson’s correlation analysis. A strong positive correlation was observed between Finegoldia and Cutibacterium as well as between Paraburkholderia and Clostridioides. Weak positive correlations were found between Shigella and Klebsiella, and between Escherichia and Klebsiella. Additionally, weak negative correlations were noted between Bifidobacterium and Enterococcus and between Enterobacter and Acinetobacter (Supplementary Figure 1).
Functional pathway analysis
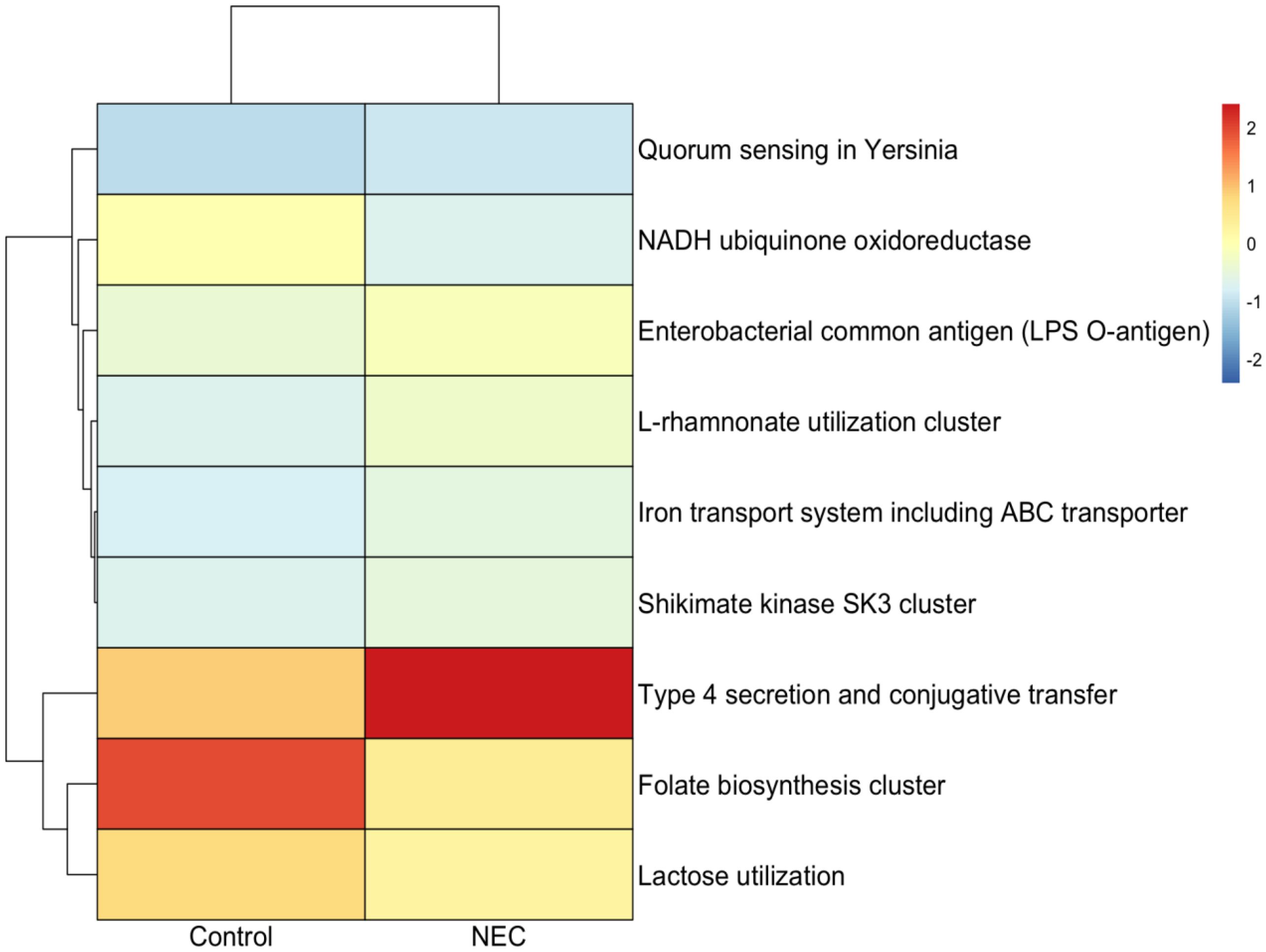
Functional analysis using SEED classification revealed that pathways associated with type IV secretion systems, conjugative transfer, Enterobacterial common antigen (LPS O-antigen), quorum sensing in Yersinia, the L-rhamnose pathway, and iron transport systems—including ABC transporters and the Shikimate kinase SK3 cluster—were significantly enriched in infants with NEC compared to controls (Figures 7, 8). These pathways were significant in both linear regression analysis and Welch’s t-test using the STAMP tool (Supplementary Figure 2). Additionally, genes involved in folate biosynthesis and lactose utilization were reduced in the NEC group compared to the control group.

Figure 7. Significant functional pathways between NEC and control groups. Student t test was performed.
Discussion
The present study identified that Enterobacteriaceae were significantly more abundant in the stool samples of infants with NEC. Functional analysis revealed an increased abundance of genes involved in the biosynthesis of lipopolysaccharide (LPS) O-antigen, the type IV secretion system (T4SS), the L-rhamnose pathway and quorum sensing in the stool samples of infants with NEC. To the best of our knowledge, this is the first study to examine gut microbial communities using whole-genome shotgun sequencing in Indian preterm infants with NEC.
PERMANOVA analysis demonstrated that the mode of delivery influenced the beta diversity of the gut microbiome. However, the differential bacterial abundance identified through linear regression analysis between NEC and control groups was not affected by the mode of delivery. This is likely because the number of infants delivered vaginally and by C-section was equal in both the NEC and control groups.
The median gestational age of infants with NEC in our cohort was 30.5 ± 2.17 weeks, comparable to that reported in Western studies evaluating the gut microbiome in these infants, which showed a median gestational age of 30.1 ± 2.4 weeks (Pammi et al., 2017). No significant differences were observed between the NEC and control groups in alpha diversity indices or beta diversity measures. This finding is consistent with previous meta-analyses on the subject (Pammi et al., 2017).
Differential abundance analysis at the family level revealed elevated levels of Enterobacteriaceae and Pseudomonadaceae in the fecal samples of infants with NEC. However, the increase in Pseudomonadaceae did not reach statistical significance after p-value adjustment. The overrepresentation of Enterobacteriaceae in fecal samples from infants with NEC has been documented in studies using both culture-based and molecular techniques (Millar et al., 1992; Marolda and Valvano, 1995; Brower-Sinning et al., 2014). Pseudomonadaceae has also been associated with NEC and sepsis in preterm infants (Morrow et al., 2013; Wang et al., 2024). A previous study demonstrated that colonization with uropathogenic E. coli (UPEC) is associated with NEC-related mortality (76%) (Ward et al., 2016). In contrast, the current study found that only 21% of the infants were colonized with E. coli.
Klebsiella pneumoniae and Klebsiella quasipneumoniae were the dominant species identified in infants with NEC in our study. A previous study found that K. pneumoniae and Klebsiella oxytoca are associated with NEC (Paveglio et al., 2000; Thänert et al., 2024). K. quasipneumoniae is a recently classified subspecies of K. pneumoniae. Numerous studies have reported that K. quasipneumoniae acquires antimicrobial resistance genes (ARGs) and is associated with hospital-acquired infections (Mathers et al., 2019). K. quasipneumoniae has been detected in the rectal and fecal samples of preterm infants admitted to the neonatal intensive care unit (NICU) (Crellen et al., 2019; Chen et al., 2020). Previous studies showed that Clostridium neonatale and Clostridium perfringens were associated with NEC onset (Tarracchini et al., 2021). However, we did not found any association between Clostridium and NEC in our cohort.
Functional pathway analysis revealed that pathways associated with type IV secretion systems (T4SS), LPS O-antigen, quorum sensing in Yersinia, the L-rhamnose pathway, and iron transport systems including ABC transporters and the shikimate kinase SK3 cluster, were significantly increased in NEC. T4SS facilitates horizontal gene transfer among bacteria, promoting the dissemination of antibiotic resistance genes and the delivery of virulence factors. The LPS O-antigen is a conserved surface antigen found in the Enterobacteriaceae family. The increased abundance of Enterobacteriaceae in NEC samples may result in elevated levels of LPS antigens. LPS antigen-induced activation of Toll-like receptor 4 (TLR4) pathway is a well-characterized mechanism contributing to intestinal inflammation and necrosis in animal models of NEC as well as patients (Shaw et al., 2021). The L-rhamnose pathway is involved in the synthesis of LPS O-antigen in Gram-negative bacteria, including Enterobacteriaceae (Marolda and Valvano, 1995). The shikimate kinase SK3 pathway is crucial for the synthesis of aromatic amino acids, such as tyrosine and tryptophan, which are essential for bacterial growth and survival, including in E. coli (Ely and Pittard, 1979). Moreover, shikimate kinase is a target for the therapeutic inhibition of multi-resistant strains, including K. pneumoniae (Li et al., 2025). Overall, the functional pathways identified in this study highlight that Enterobacteriaceae and their metabolic functions contribute to the pathogenesis of NEC.
The limitations of this study include its single-center design and small sample size. However, to our knowledge, this is the first study in India to use a shotgun metagenomic approach to investigate the association of gut microbiota in fecal samples of infants with NEC.
Conclusion
Enterobacteriaceae were more abundant in stool samples from infants with NEC than infants without NEC. Differential abundance analysis using Linear Discriminant Analysis Effect Size (LEfSe) identified Klebsiella pneumoniae and Escherichia coli as enriched in the gut microbiota of preterm infants with NEC. Functional analysis revealed increased expression of genes associated with the LPS O-antigen, the Type IV secretion system, the L-rhamnose pathway, quorum sensing, and iron transporters, including ABC transporters, in the NEC samples.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI–PRJNA1304237.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee, PGIMER. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PD: Formal analysis, Methodology, Investigation, Conceptualization, Funding acquisition, Writing – original draft. SA: Conceptualization, Writing – review & editing, Supervision, Project administration. JKu: Methodology, Resources, Writing – review & editing. SD: Writing – review & editing, Conceptualization. JKa: Writing – review & editing, Project administration, Methodology, Formal analysis, Supervision, Visualization, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by a grant from the Department of Health Research, Ministry of Health and Family Welfare, Government of India, awarded to Prabavathi under Women Scientist Scheme, Human Resource Development (R.12013/13/2022-HR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1649384/full#supplementary-material
References
Alsaied, A., Islam, N., and Thalib, L. (2020). Global incidence of Necrotizing Enterocolitis: a systematic review and Meta-analysis. BMC Pediatr. 20, 344. doi: 10.1186/s12887-020-02231-5
Brower-Sinning, R., Zhong, D., Good, M., Firek, B., Baker, R., Sodhi, C. P., et al. (2014). Mucosa-associated bacterial diversity in necrotizing enterocolitis. PloS One 9, e105046. doi: 10.1371/journal.pone.0105046
Cao, Y., Dong, Q., Wang, D., Zhang, P., Liu, Y., and Niu, C. (2022). microbiomeMarker: an R/Bioconductor package for microbiome marker identification and visualization. Bioinformatics 38, 4027–4029. doi: 10.1093/bioinformatics/btac438
Chen, Y., Brook, T. C., Soe, C. Z., O’Neill, I., Alcon-Giner, C., Leelastwattanagul, O., et al. (2020). Preterm infants harbour diverse Klebsiella populations, including atypical species that encode and produce an array of antimicrobial resistance- and virulence-associated factors. Microbial Genomics 6, e000377. doi: 10.1099/mgen.0.000377
Crellen, T., Turner, P., Pol, S., Baker, S., Nguyen, T. N.T., Stoesser, N., et al. (2019). “Transmission dynamics and control of multidrug-resistant Klebsiella pneumoniae in neonates in a developing country,” in eLife. (Cambridge CB2 1AW, UK: eLife Sciences Publications, Ltd). Available online at: https://elifesciences.org/articles/50468.
Devarajalu, P., Kumar, J., Dutta, S., Attri, S. V., and Kabeerdoss, J. (2024). Gut microbiota of preterm infants in the neonatal intensive care unit: a study from a tertiary care center in northern India. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1329926
Devarajalu, P., Kumar, J., Dutta, S., Attri, S. V., and Kabeerdoss, J. (2025). Gut microbiota alteration in healthy preterm infants: an observational study from tertiary care center in India. Microorganisms 13, 577. doi: 10.3390/microorganisms13030577
Ely, B. and Pittard, J. (1979). Aromatic amino acid biosynthesis: regulation of shikimate kinase in Escherichia coli K-12. J. Bacteriol 138, 933–943. doi: 10.1128/jb.138.3.933-943.1979
Huson, D. H., Beier, S., Flade, I., Górska, A., El-Hadidi, M., Mitra, S., et al. (2016). MEGAN community edition - interactive exploration and analysis of large-scale microbiome sequencing data. PloS Comput. Biol. 12, e1004957. doi: 10.1371/journal.pcbi.1004957
Li, Y., Kumar, S., and Zhang, L. (2025). Shikimate kinase 1 from klebsiella pneumoniae as a new drug target enzyme: insights from comparative modeling and molecular dynamics. Indian J. Pharm. Educ. Res. 58, 1110–1120. doi: 10.5530/ijper.58.4.122
Magnusson, A., Jabbari Shiadeh, S. M., Ardalan, M., Swolin-Eide, D., and Elfvin, A. (2024). Gut microbiota differences in five-year-old children that were born preterm with a history of necrotizing enterocolitis: A pilot trial. iScience 27, 110325. doi: 10.1016/j.isci.2024.110325
Mallick, H., Rahnavard, A., McIver, L. J., Ma, S., Zhang, Y., Nguyen, L. H., et al. (2021). Multivariable association discovery in population-scale meta-omics studies. PloS Comput. Biol. 17, e1009442. doi: 10.1371/journal.pcbi.1009442
Mara, M. A., Good, M., and Weitkamp, J.-H. (2018). Innate and adaptive immunity in necrotizing enterocolitis. Semin. Fetal Neonatal Med. 23, 394–399. doi: 10.1016/j.siny.2018.08.002
Marolda, C. L. and Valvano, M. A. (1995). Genetic analysis of the dTDP-rhamnose biosynthesis region of the Escherichia coli VW187 (O7:K1) rfb gene cluster: identification of functional homologs of rfbB and rfbA in the rff cluster and correct location of the rffE gene. J. Bacteriol 177, 5539–5546. doi: 10.1128/jb.177.19.5539-5546.1995
Mathers, A. J., Crook, D., Vaughan, A., Barry, K. E., Vegesana, K., Stoesser, N., et al. (2019). Klebsiella quasipneumoniae Provides a Window into Carbapenemase Gene Transfer, Plasmid Rearrangements, and Patient Interactions with the Hospital Environment. Antimicrobial Agents Chemotherapy 63, e02513-18. doi: 10.1128/aac.02513-18
McMurdie, P. J. and Holmes, S. (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One 8, e61217. doi: 10.1371/journal.pone.0061217
Millar, M. R., MacKay, P., Levene, M., Langdale, V., and Martin, C. (1992). Enterobacteriaceae and neonatal necrotising enterocolitis. Arch. Dis. Child 67, 53–56. doi: 10.1136/adc.67.1_spec_no.53
Morrow, A. L., Lagomarcino, A. J., Schibler, K. R., Taft, D. H., Yu, Z., Wang, B., et al. (2013). Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 1, 13. doi: 10.1186/2049-2618-1-13
Oksanen, J., Simpson, G. L., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., et al. (2001). vegan: Community Ecology Package. 2.6-8. doi: 10.32614/CRAN.package.vegan
Pammi, M., Cope, J., Tarr, P. I., Warner, B. B., Morrow, A. L., Mai, V., et al. (2017). Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31. doi: 10.1186/s40168-017-0248-8
Parks, D. H., Tyson, G. W., Hugenholtz, P., and Beiko, R. G. (2014). STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. doi: 10.1093/bioinformatics/btu494
Paveglio, S., Ledala, N., Rezaul, K., Lin, Q., Zhou, Y., Provatas, A. A., et al. (2020). Cytotoxin-producing Klebsiella oxytoca in the preterm gut and its association with necrotizing enterocolitis. Emerg. Microbes Infect. 9, 1321–1329. doi: 10.1080/22221751.2020.1773743
Peschel, S., Müller, C. L., von Mutius, E., Boulesteix, A.-L., Depner, M., Boulesteix, A.-L., and Depner, M. (2021). NetCoMi: network construction and comparison for microbiome data in R. Briefings Bioinf. 22, bbaa290. doi: 10.1093/bib/bbaa290
Samara, J., Moossavi, S., Alshaikh, B., Ortega, V. A., Pettersen, V. K., Ferdous, T., et al. (2022). Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe 30, 696–711.e5. doi: 10.1016/j.chom.2022.04.005
Shaw, A. G., Sim, K., Rose, G., Wooldridge, D. J., Li, M.-S., Misra, R. V., et al. (2021). Premature neonatal gut microbial community patterns supporting an epithelial TLR-mediated pathway for necrotizing enterocolitis. BMC Microbiol. 21, 225. doi: 10.1186/s12866-021-02285-0
Tarracchini, C., Milani, C., Longhi, G., Fontana, F., Mancabelli, L., Pintus, R., et al. (2021). Unraveling the microbiome of necrotizing enterocolitis: insights in novel microbial and metabolomic biomarkers. Microbiol. Spectr. 9, e01176–e01121. doi: 10.1128/Spectrum.01176-21
Thänert, R., Keen, E. C., Dantas, G., Warner, B. B., and Tarr, P. I. (2020). Necrotizing enterocolitis and the microbiome: current status and future directions. J. Infect. Dis. 223, S257–S263. doi: 10.1093/infdis/jiaa604
Thänert, R., Schwartz, D. J., Keen, E. C., Hall-Moore, C., Wang, B., Shaikh, N., et al. (2024). Clinical sequelae of gut microbiome development and disruption in hospitalized preterm infants. Cell Host Microbe 32, 1822–1837.e5. doi: 10.1016/j.chom.2024.07.027
Wang, Y., Jiang, K., Xia, Q., Kang, X., Wang, S., Yu, J.-H., et al. (2024). Exploration of pathogenic microorganism within the small intestine of necrotizing enterocolitis. World J. Pediatr. 20, 165–172. doi: 10.1007/s12519-023-00756-0
Ward, D. V., Scholz, M., Zolfo, M., Taft, D. H., Schibler, K. R., Tett, A., et al. (2016). Metagenomic sequencing with strain-level resolution implicates uropathogenic E. coli in necrotizing enterocolitis and mortality in preterm infants. Cell Rep. 14, 2912–2924. doi: 10.1016/j.celrep.2016.03.015
Keywords: Necrotizing enterocolitis, gut microbiota, preterm infants, shotgun metagenomics, LPS O-antigen, TLR4, type IV secretion system (T4SS), India
Citation: Devarajalu P, Attri SV, Kumar J, Dutta S and Kabeerdoss J (2025) Characterization of gut microbiota signatures in Indian preterm infants with necrotizing enterocolitis: a shotgun metagenomic approach. Front. Cell. Infect. Microbiol. 15:1649384. doi: 10.3389/fcimb.2025.1649384
Received: 18 June 2025; Accepted: 13 August 2025;
Published: 11 September 2025.
Edited by:
Valeriy Poroyko, Laboratory Corporation of America Holdings (LabCorp), United StatesReviewed by:
Dhirendra Kumar Singh, University of North Carolina at Chapel Hill, United StatesGiuseppe De Bernardo, Ospedale Buon Consiglio Fatebenefratelli, Italy
Copyright © 2025 Devarajalu, Attri, Kumar, Dutta and Kabeerdoss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jayakanthan Kabeerdoss, amF5YWthbnRoYW5ra0BnbWFpbC5jb20=; ay5qYXlha2FudGhhbkBwZ2ltZXIuZWR1Lmlu
†These authors have contributed equally to this work and share first authorship
 Prabavathi Devarajalu
Prabavathi Devarajalu Savita Verma Attri
Savita Verma Attri Jogender Kumar
Jogender Kumar Sourabh Dutta
Sourabh Dutta Jayakanthan Kabeerdoss
Jayakanthan Kabeerdoss