- Petroleum Exploration and Production Institute, SINOPEC, Beijing, China
Emulsified behaviors are of importance for chemical flooding. Properties of emulsifiers relate droplets coalescence and transport. Since experiments do not provide access to observables of interest. Numerical simulations with mesoscale scheme pose attractive method to gain insight into emulsifying stability, which is defined as a period of oil droplets movements at fixed ranges. A phase field based lattice Boltzmann model is used which simulate interface behavior. Various parameters including emulsified droplet size, interface thickness, viscosity ratio and density ratio have been discussed. The aim of this study is to provide a critical suggestion that predictive emulsifying behaviors of small oil droplets immersed in water environment.
Introduction
Chemical flooding is an effective technique that plays an important role in oil production at the middle and late stages, in which basic theory is to improve mobility of the hydrocarbon by altering the interfacial conditions. Interfacial tension between oil and water can be realized by adding surfactant. Generally, some additives including high-viscosity polymer are injected with water to improve oil displacement efficiency, and therefore, oil recovery is enhanced. We found that the interfacial phenomenon produced by an emulsifier greatly impacts its emulsifying performance (Sun, Z., et al.). However, the knowledge of the interfacial properties is still lacking (Mohammed and Babadagli) (Sun, Z., et al.) (Cui, Ren, et al.) (Dicharry et al.) (Moran et al.) (Wang et al.) (Temple-Heald et al.) (Cui, Pei, et al.).
Many surfactant studies in oil production have been focused on designs of molecular structures and corresponding evaluations (Dong et al.) (Zhang and Feng) (Ding et al.) (Zhang et al.). In fact, the response time provided by an emulsifier in a target environment is directly and firstly related to its emulsifying performance. However, there are few studies regarding emulsion response time to investigate interfacial behavior. The interfacial interaction can be treated as the attractive/repulsive forces, or as both representing emulsion in numerical models (H.Nour et al.).
Unfortunately, capturing interfacial properties accurately is a challenge, numerically. The interface in numerical treatment has different strategies due to the steep gradients of properties in the normal direction. Here, the lattice Boltzmann equation (LBE) is proposed to study interfacial behavior, which should integrate both microscopic models for the interface and meso-scopic kinetic equations (Lamura et al.) (Song et al.). In the present study, the LBE with interface capturing is used to solve the velocity field, and the basic idea is developed by (Zheng et al.). Instead of recovering the fluid momentum macrodynamic equation (Swift et al.), it has better performance by recovering the fluid velocity based one (Zheng et al.).
Governing Equations of Motion Fluids
Phase Field Theory
A brief outline of this method will be in this section and for more detailed discussion refer to Jacqmin (1999). An order parameter
Where
Where
Where
The surface tension between fluid and fluid is represented as
Cahn and Hillard approximated interfacial diffusion fluxes which is proportional to chemical potential gradients (Zu and He). The interface profile can be described with respect to
Where
Hydrodynamic equations in the phase-field model for incompressible multiphase flows are given by
LBE for a Velocity Based Method
A standard LBE form for hydrodynamic properties is represented as
For particle distribution function in the phase space, it has the microscopic velocity
In the present study, a 2-dimensional 9-velocity (D2Q9) LBM structure is used to solve the field in 2D.
The particle velocity in the D2Q9 structure can be given using Gauss-Hermite quadrature in
Where
To satisfy the function above, the following equilibrium distribution function is.
Introduced as:
For D2Q9 model, weighting factor
The reference speed of sound
Interface Capturing
Interface capturing is proposed to be given as:
Where
The moments of the equilibrium distribution satisfy
Then, density can be calculated according to mass conservation as in Owengrub and Truskinovsky (1998)
The viscosity distribution within interface in the LB is given by
Discretization
The Laplacian of the force terms can be discretized and interfacial force is adopted
Where
Validation
A test with one static droplet is to verify the phase field multiphase lattice Boltzmann model. Initially, a static water droplet is placed in the middle region of a computational domain. Periodic boundary conditions are applied to all boundaries. Physical properties of two phases are given by
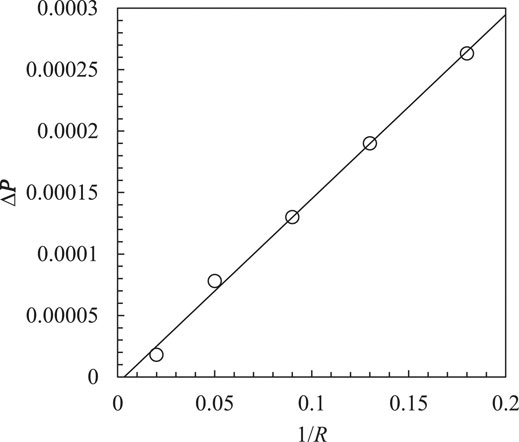
The radius of the droplet
There is a good agreement between simulation results and the theoretical predictions, see Figure 1. The maximum error is less than 2.5%. This indicates that the surface tension simulations in the LB multiphase model have great accuracy.
Results and Discussions
The parameters in the model are specified as dimensionless ratios between oil and water including viscosity and density ratios that represent external conditions. Interface thickness reflects the chemical properties of various surfactants. Radius depicts the size of the potential emulsified droplet.
Simulations of the binary droplet collision were carried out. Slip and no penetration boundary conditions are imposed at all boundaries of the computational domain. Two droplets are initially located at a far-center distance of 4
Head-On Collisions and Pre-coalescence of Binary Droplets
In order to evaluate the effects of various dominant parameters on emulsifier performance, a measurement is proposed. Two moving oil droplets immersed in water (light fluid) have interfacial behavior with the following procedures, see Figure 2. Initially, two bodies move from far afield. When the two reach the region where the distance between the two body centers is smaller than 2R, the first time is reckoned in as
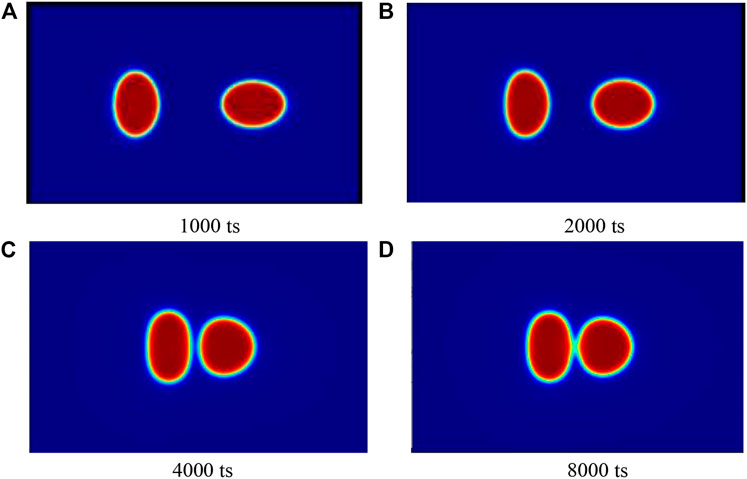
FIGURE 2. Temporal snapshots of two moving droplets within the time window from start time point to contact time point:
To quantify the balance between surface tension and interactive strength numerically, the selection of time window is defined as effective moving time. Here, effective moving time starts from the distance between droplets smaller than two radiuses to the nearest boundary distance equal to twice interface thickness,
Quantitative Measurements of Time Windows
To advise engineers to select one surfactant with good expectation, the effects of various parameters on the time window have been studied quantitatively.
Sizes of Existing Droplets,
Surface tension is used to reflect the interactive strength of the two emulsified droplets and has significant effect on two phase interfacial behavior, and thereby the time window within effective contact distance between two droplets is measured as defined in Head on Collisions and Pre Coalescence of Binary Droplets. In order to keep surface tension acting on two phase systems at a reasonable level, various parameters are evaluated. In Figures 3, 4, effective time windows are decreased with the increase of magnitudes of surface tensions in systems with different radiuses and surface thicknesses. Weak interfacial strengths from two phases induce large time windows in which emulsifiers perform small surface tensions that are convenient for managing and controlling the cost of emulsifying stability.
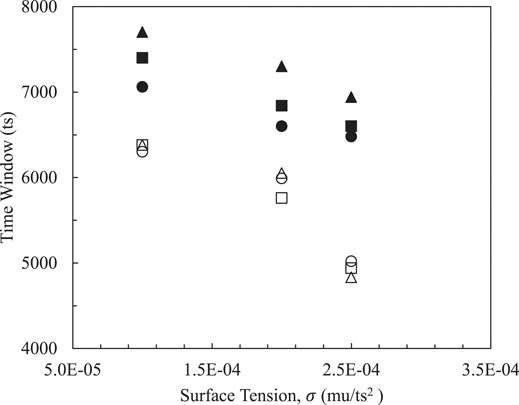
FIGURE 3. Effects of droplets sizes on time windows: circles (
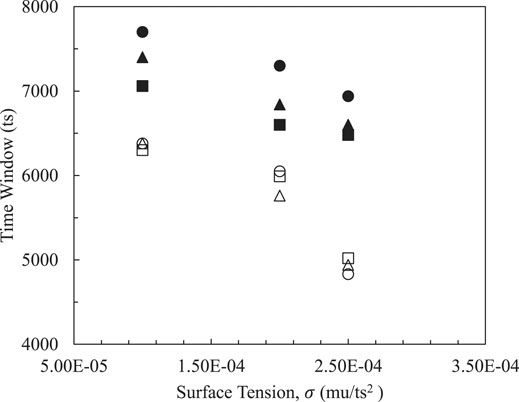
FIGURE 4. Effects of surface thicknesses on time windows with
Figures 3, 4 both show the same trends on sizes of dispersed phases in terms of the effective time window. Smaller droplets have a wider time window due to increased total surface energy. Therefore, smaller ones are able to perform better stability and improve flow capability. On the other hand, larger droplets remain with a short duration that is used to govern contact behavior.
Shells of oil droplets and dimensions of emulsified droplets relate to emulsifying performance from chemical components of a specified emulsifier. To assess the effects of surface thicknesses and droplet radius on the time window, two thicknesses were specified on droplets surfaces as 5 and 10 lattice units and three droplets R = 15, 25 and 35 lattice unit (lu) are simulated, respectively. As the interface thickness is increased (empty symbols), the overlapped symbols indicate that sizes of internal phases have little effect on effective contact time. Meanwhile, if the thin shells with small surface thicknesses are attached onto oil droplets, the impact of the existing droplet dimension becomes significant, especially when surface tension is small. This means that the emulsifier with a capability of forming small oil droplets and a thick interface would be recommended. Furthermore, slopes of systems with thicker interfaces in both figures point out that it is sensitive to interface thickness as it changes faster at relatively high surface tension coefficients from 2.0
Effect of Density Ratio, μ∗
In Figure 5, differences in viscosities between oil and water are dimensionlized as
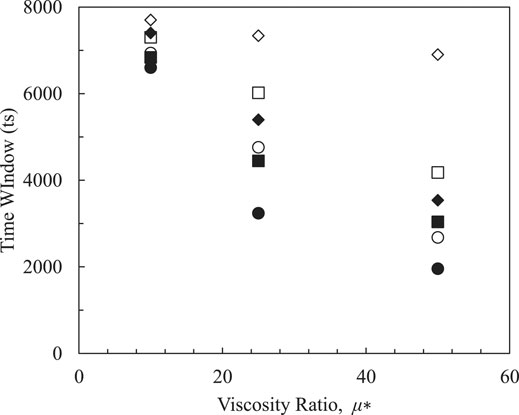
FIGURE5. Effects of viscosity ratio
Effect of Density Ratio
Density is one of the most remarkable parameters in oil production. Density ratios in our LB simulations are varied from 0.8 to 1.4 recovering ranges of light to heavy oil extraction in reality. Simulation results from Figure 6 demonstrate that emulsifiers having increasing probability of emulsifying smaller droplets are recommended which not only show good emulsifying performance, but also are beneficial to manage the emulsifying process due to the large time windows left for engineers. Generally, physical properties of oil and water are fixed, small slopes (empty symbols) of systems with small droplets impact on time windows denote that increased density ratios between oil and water that can ensure relatively long durations of being dispersed. Therefore, the emulsifier carried by water should be designed to increase the density of the external phase comparing the density of the internal phase. This is to say that the less difference in density between the internal and external phases, the better emulsifying performance should be expected.
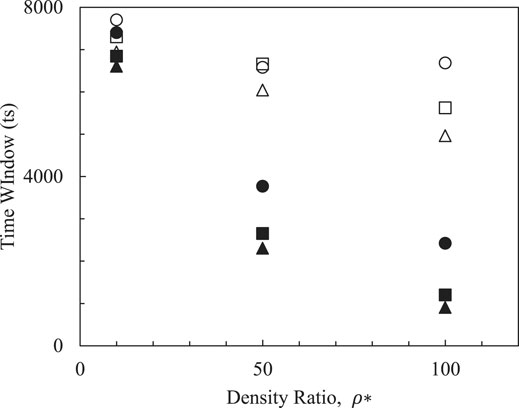
FIGURE 6. Effects of density ratio
Remark Conclusions
In this study, an effective time window is defined to measure emulsifying properties via staying times of dispersed phases. Interfacial tension, interfacial thickness, and differences of density and viscosity between dispersed and continuous phases have been simulated for the management and design of the emulsifiers.
1)Emulsifiers with a capability of forming small droplets and a thin interface outside are suggested, since small time windows indicate the good emulsifying performance can keep the droplets dispersed longer.
2)Small interfacial tensions provided by emulsifiers are still desired.
3)Decreasing differences of viscosities and densities between oil and water are also advised to design a binary emulsifying system.
4)A suitable emulsifier is not controlled by only one parameter. The difficulty in designing better performing emulsifiers relies on balancing these various parameters.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
XL has contributed to simulation and datat analysis, JF has worked on double check manuscript, and BJ has worked for supervisor.
Funding
This work is financially supported by Chinese National Key Research and Development Program (No.SQ2018YFA070028) and SINOPEC Technical Project (No. P21085-18).
Conflicts of Interest
Authors XL, JF, BJ are employed by SINOPEC.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cui, Guodong., Pei, Shufeng., Rui, Zhenhua., Dou, Bin., Ning, Fulong., and Wang, Jiaqiang. (2020). Whole Process Analysis of Geothermal Exploitation and Power Generation from a Depleted High-Temperature Gas Reservoir by Recycling CO2. Energy 217, 119340 doi:10.1016/j.energy.2020.119340
Cui, Guodong., Ren, Shaoran., Bin, Dou., and Fulong, Ning. (2020). Geothermal Energy Exploitation from Depleted High-Temperature Gas Reservoirs by Recycling CO2: The Superiority and Existing Problems. Geosci. Front. in press, doi:10.1016/j.gsf.2020.08.014
Dicharry, C., Arla, D., Sinquin, A., Graciaa, A., and Bouriat, P. (2006). Stability of Water/Crude Oil Emulsions Based on Interfacial Dilatational Rheology. J. Colloid Interf. Sci. 297 (2), 785–791. doi:10.1016/j.jcis.2005.10.069
Ding, D., Sun, Z., and Xu, M. (1998). Properties of Surface Film of Oil Components. J. Univ. Petroleum 22 (3), 82–83
Dong, Z., Lin, M., Wang, H., and Li, M. (2010). Influence of Surfactants Used in Surfactant-Polymer Flooding on the Stability of Gudong Crude Oil Emulsion. Pet. Sci. 7 (2), 263–267. doi:10.1007/s12182-010-0031-y
Jacqmin, D. (1999). Calculation of Two-phase Navier-Stokes Flows Using Phase-Field Modeling. J. Comput. Phys. 155 (1), 96–127. doi:10.1006/jcph.1999.6332
Lamura, A., Gonnella, G., and Yeomans, J. M. (1999). A Lattice Boltzmann Model of Ternary Fluid Mixtures. Europhys. Lett. 45 (3), 314–320. doi:10.1209/epl/i1999-00165-4
Mohammed, M., and Babadagli, T. (2021). New Insights into the Interfacial Phenomena Occurring between Hydrocarbon Solvent and Heavy Oil. J. Pet. Sci. Eng. 196, 108022. doi:10.1016/j.petrol.2020.108022
Moran, K., Yeung, A., and Masliyah, J. (2006). The Viscoplastic Properties of Crude Oil-Water Interfaces. Chem. Eng. Sci. 61 (18), 6016–6028. doi:10.1016/j.ces.2006.05.026
Nour, H. A., Yunus, R. M., and Anwaruddin, H. (2007). Water-in-Crude Oil Emulsions: Its Stabilitzation and Demulsification. J. Appl. Sci. 15, 12–130
Owengrub, J., and Truskinovsky, L. (1998). Quasi-Incompressible Cahn-Hilliard Fluids and Topological Transitions. Proc. R. Soc. A: Math. Phys. Eng. Sci. 454, 2617–2654. doi:10.1098/rspa.1998.0273
Song, W., Yin, Y., Landry, C. J., Prodanovic, M., Qu, Z., and Yao, J. (2021). A Local-Effective-Viscosity Multirelaxation-Time Lattice Boltzmann Pore-Network Coupling Model for Gas Transport in Complex Nanoporous Media. SPE J. 26 (01), 461–481. doi:10.2118/203841-pa
Sun, Z., Li, X., Liu, W., Zhang, T., He, M., and Nasrabadi, H. (2020). Molecular Dynamics of Methane Flow Behavior through Realistic Organic Nanopores under Geologic Shale Condition: Pore Size and Kerogen Types. Chem. Eng. J. 398 (124341), 1–10. doi:10.1016/j.cej.2020.124341
Sun, Z., Shi, J., Wu, K., Zhang, T., Feng, D., and Li, X. (2019). Effect of Pressure-Propagation Behavior on Production Performance: Implication for Advancing Low-Permeability Coalbed-Methane Recovery. SPE J. 24 (02), 681–697. doi:10.2118/194021-pa
Swift, M. R., Orlandini, E., Osborn, W. R., and Yeomans, J. M. (1996). Lattice Boltzmann Simulations of Liquid-Gas and Binary Fluid Systems, Phys. Rev. E, 54, 5, 5041–5052. doi:10.1103/physreve.54.5041
Temple-Heald, C., Davies, C., Wilson, N., and Readman, N. (2014). Developing New Surfactant Chemistry for Breaking Emulsions in Heavy Oil. J. Pet. Technology 66 (01), 30, doi:10.2118/0114-0030-jpt
Tóth, G. I., and Kvamme., B. (2015). Phase Field Modelling of Spinodal Decomposition in the Oil/Water/Asphaltene System, Phys. Chem. Chem. Phys. 17. (31), 20259–20273. doi:10.1039/c5cp02357b
Van Der Sman, R. G. M., and Meinders, M. B. J. (2016). Analysis of Improved Lattice Boltzmann Phase Field Method for Soluble Surfactants, Computer Phys. Commun. 199, 12–21. doi:10.1016/j.cpc.2015.10.002
Wang, H. Y., Liu, Ai-qin., and Wen, Xin-min. (2008). Factors Influencing Interfacial Electric Properties of Produced Water from Oilfield Jining: Journal of the University of Petroleum, 143–146+151
Zhang, Bingzhu., and Feng, Shubo. (2010). Advances in the Modelling and Simulation of Emulsion Polymerisation. Int. J. Model. 11 (Nov), 262–273. doi:10.1504/ijmic.2010.037038
Zhang, J. Y., Wang, X. P., Liu, H. Y., Tang, J. A., and Jiang, L. (1998). Interfacial Rheology Investigation of Polyacrylamide-Surfactant Interactions. Colloids Surf. A: Physicochemical Eng. Aspects 132 (1), 9–16. doi:10.1016/s0927-7757(97)00151-9
Zheng, H. W., Shu, C., Chew, Y. T., and Sun, J. H. (2008). Three-dimensional Lattice Boltzmann Interface Capturing Method for Incompressible Flows. Int. J. Numer. Meth. Fluids 56, 1653–1671. doi:10.1002/fld.1563
Keywords: emulsion, phase field model, LBM, time window, surfactant
Citation: Li X, Fang J and Ji B (2021) Interface Properties in Binary Fluid Using Lattice Boltzmann Method. Front. Earth Sci. 9:753529. doi: 10.3389/feart.2021.753529
Received: 05 August 2021; Accepted: 21 September 2021;
Published: 19 October 2021.
Edited by:
Wenhui Song, China University of Petroleum (Huadong), ChinaReviewed by:
Guodong Cui, China University of Geosciences Wuhan, ChinaQing You, China University of Geosciences, China
Copyright © 2021 Li, Fang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingyu Ji, amlieS5zeWt5QHNpbm9wZWMuY29t
 Xiaoqi Li
Xiaoqi Li Jichao Fang
Jichao Fang