- 1Department of Earth Sciences, Karl-Franzens University Graz, Graz, Austria
- 2Pacific Natural Products Research Centre, The Institute for Applied Sciences, University of the South Pacific, Suva, Fiji
- 3WWF Pacific—Solomon Islands Programme Office, Honiara, Solomon Islands
- 4Marine Programme Oceania, IUCN, Suva, Fiji
Reef rubble from a number of locations in the southwest and central Pacific (Fiji, Kiribati, Solomon Islands) was surveyed for its level of infestation with bioeroding sponges. The assessment was performed by random sampling of reef rubble in shallow water ranging from intertidal to subtidal down to 15 m of water depth. It was found that essentially four styles of bioerosion prevailed: 1) small cavities with roundish cross sections commonly produced by Pione spp. and Cliona cf. macgeachii Holmes; 2) large cavities in the order of cm-size are for example produced by Aka spp., Cliothosa spp., Cliona cf. janitrix Topsent, Cliona cf. ensifera Sollas, and others; 3) multiple etchings which can degrade the interior of coral rubble on a scale of a few cm in diameter are typically produced by both, Cliona orientalis and C. celata species complexes; and 4) large hadromerid sponges are capable of eroding into solid reef rock and produce silt-, sand-, and pebble-sized fragments. Observed was the activity of Spheciospongia solida (Ridley & Dendy) and S. cf. inconstans var. digitata Dendy. These large clionaid sponges are the most efficient eroders on the reef. Species of Pione are abundant at all sites from intertidal to subtidal, and although they are weak bioeroders, they may be the most destructive by number.
Introduction
Bioeroding excavating sponges are known to inhabit a large variety of natural and man-made calcareous substrates. Most commonly these sponges are found in reef limestone (Bak, 1976; Hartman, 1977; Tunnicliffe, 1979) and in skeletons of molluscs (Volz, 1939; Hoeksema, 1983), sometimes causing significant degradation of reefs and death of the organism they infest. The sponges boring into mollusc shells can be a serious pest for cultured oysters (Thomas et al., 1993). The corals or molluscs are not eaten; rather, the sponge is seeking protection for itself by hiding in the hard structures it erodes.
Boring or excavating sponges are of interest for a number of reasons. 1. Their capacity to etch small limestone chips out of hard limestone substrates contributes to the silt-sized sediment fraction of lagoons and other marine carbonate depositional settings (Futterer, 1974; Rützler, 1975; Carballo et al., 2017). 2. The activity of most boring sponges is affecting non-living limestone substrates such as reef rocks, the base of branching coral colonies, or dead shells. The small (mm-sized) holes generated by the sponge render the substrate prone to breakage through wave shock, particularly during high-energy events (e.g., storms) and abrasion. The activity of boring sponges can thus affect the strength of reef frameworks (Neumann, 1966; Acker and Risk, 1985) and the durability of reef clasts. 3. Some species of boring sponges can actively kill coral colonies by overgrowing or undermining and/or dislodging polyps, and have emerged as serious threats to the health of coral reefs. In the Caribbean, for example, the species Cliothosa delitrix (Pang, 1973), C. caribbaea Carter, 1882, and C. varians (Duchassaing de Fonbressin and Michelotti, 1864) are implicated in damage to living coral colonies (Chavez-Fonnegra and Zea, 2007).
Boring sponges can be found all around the world, including the Pacific Ocean from Canada to Mexico and Chile, and in many Pacific Island countries.
Very little is known about boring or excavating sponges in the southwest and central Pacific regions at large although many studies have investigated faunas from elsewhere in the world.
Methods
Random samples of coral rubble and reef clasts were collected by wading, snorkeling, or diving from various shallow water settings in the southwest and central Pacific Ocean (Fiji Islands, Kiribati, Solomon Islands). Samples varied in size from coral branches in no less than 15 cm long to larger chunks of reef rock. The number of samples collected ranged from a few pieces (when the time was limited and logistics complicated) to 50 to 130 samples per site.
More specifically, samples were collected from the following locations:
1) The Nasese tidal flat is a part of Laucala Bay, a shallow triangular estuarine lagoon bordered by Suva, the Fiji Island’s capital city, along its northwest shore. It is situated on the southeastern side of Viti Levu Fiji’s main island.
Nasese tidal flat: 18° 9′41.43″S; 178°25′18.92″E, exposed at low tide, location of Figure 1.

FIGURE 1. (A) Nasese tidal flat at low tide with thousands of large clionaid sponges eroding into the Holocene reef platform. Inset (B) idealized cross section through Spheciospongia solida (Ridley and Dendy, 1886). The base of the sponge can excavate up to 30 cm deep into the sedimentary rock. Inset (C) aggregation of S. solida. Note the closed osculi.
Most of the area to the west of Laucala Bay is composed of soft shores, and their fauna and flora were very well documented by Morton and Raj (1980). Most of the sediment is terrigenous and modified by burrowing infauna, particularly Uca crabs and various worms. The dominant sediment contribution to the tidal flat is from land sources via rivers and drains; a minor component is the resident shell infauna and epifauna and erosion of underlying Holocene carbonates and Suva Marl.
The remains of a Holocene fringing reef can be found in many areas along Suva’s shoreline, either exposed on the tidal flat (Nasese tidal flat, Figure 1A), as rubble along the shore (Nasese and Suva Point), or exposed in dredge spoils (Maritime School). Recent dates of aragonitic shells collected from Nasese and the Maritime School give an age of around 6 300 (+/- 30) years (S. Pohler and L. Zann, unpublished data, 2006). A previous work by Rodda and Nunn (1990) gave dates of 4 400–4 800 (+/− 50) years for a coral head found in front of Government House (Nasese). The Holocene fringing reef is unconformably sitting on Suva Marl on the Nasese tidal platform and is deeply eroded and decaying. The processes of bioerosion in an intertidal setting can be studied here, and a thriving fauna and flora of bioeroding organisms abound at that site. The fossil reef platform dated between 4.800 and 6.300 years old (Rodda and Nunn, 1990) when sea level in the tropical Pacific Ocean was higher by more than one meter (Rodda and Nunn, 1990). The reef is deeply eroded and covered in many places by a thin veneer of muddy epiclastic sand. It is located close to Suva Harbor, and water is turbid and polluted with chemicals and high counts of coliform bacteria. A combination of physical and biological processes facilitates the breakdown of this fossil reef.
2) The Lau Group is located to the east of Fiji (Vanua Levu) and West of Tonga. Four dive sites were visited in 2007 during a sampling campaign by K. Feussner and staff from the Institute of Applied Science (USP) supported by International Cooperative Biodiversity Groups (NIH, Fogarty) in search for marine natural products. They collected reef rubble for the “Boring sponge Project” at four sites.
Site 3: 16°46′20.77″S; 179° 7′4.11″W 10m–15 m depth, Wailagi Lala, passage.
Site 5: 16°59′26.15″S; 179° 4′16.01″W 5 m–16 m, Kikobo Islets, Williamson Reef north of Vanua Balavu.
Site 7: 17° 9′44.20″S; 178°49′11.99″W; 12 m–15 m, Sovu Reef, reef slope east of Vanua Balavu.
Site 12: 17°10′18.01″S; 179° 0′8.47″W; 3 m–10 m channel north of Vanua Balavu; Barrier reef, turbid reef between Bavatu Hbr. and Blackswain Point. Dive sites 3 (12 samples with 5 bioeroding sponges), 7 (20 samples with 14 bioeroding sponges), and 12 (20 samples with 8 bioeroding sponges) are located inside of lagoons; dive site 5 (10 samples with 3 bioeroding sponges) is outside of a lagoon on the reef slope. Site 12 is described as a “turbid lagoon” in the vicinity of a harbor. The Lau Islands are small but have a relatively high influx of riverine water laden with soil (possibly enriched with fertilizers) eroded through rainfall from island slopes. Population and industrial wastes are sparse in the region.
3) Nananu-i-Ra is a small island 1.5 km off the coast of Fiji’s main island Viti Levu adjacent to Rakiraki. The island has a land area of 3.5 square kilometers and an elevation of 180 m. The island itself is composed largely of basalt, andesite, and associated epiclastic sediments. A shallow island shelf platform of variable width with coral reefs and calcareous sediments surrounds the land area. Several resorts line the coast. Fieldwork on Nananu-i-Ra was conducted in February 2008 by two of the authors (B.R. and S.P.). Random samples of coral rubble and mollusc shells were collected by wading and snorkeling from shallow subtidal sites on the northeast and southwest sides of Nananu-i-Ra.
Charlie’s Place North (CPN): 17°17′51.28″S; 178°13′14.41″E at 0.5–2 m depth.
Charlie’s Place South (CPS): 17°18′1.02″S; 178°13′7.47″E, at 0.5–2 m depth.
Near shore (On) One Beach: 17°17′26.28″S; 178°13′18.64″E, at 0.5–2 m depth.
Offshore (Oo) One Beach: 17°17′33.81″S; 178°13′27.88″E, in 5 m deep water.
4) Tarawa is located in the Gilbert Group in the central Pacific. At three dive sites, samples of reef rubble were collected with the help of staff from the Ministry of Fisheries & Marine Resource Development (MFMRD) and Naomi Biribo (then MSc candidate at USP) in 2006. Reef rubble for the “Boring sponge Project” was also collected by wading at two additional sites, Fatali’s lagoon and Fatali’s reef flat.
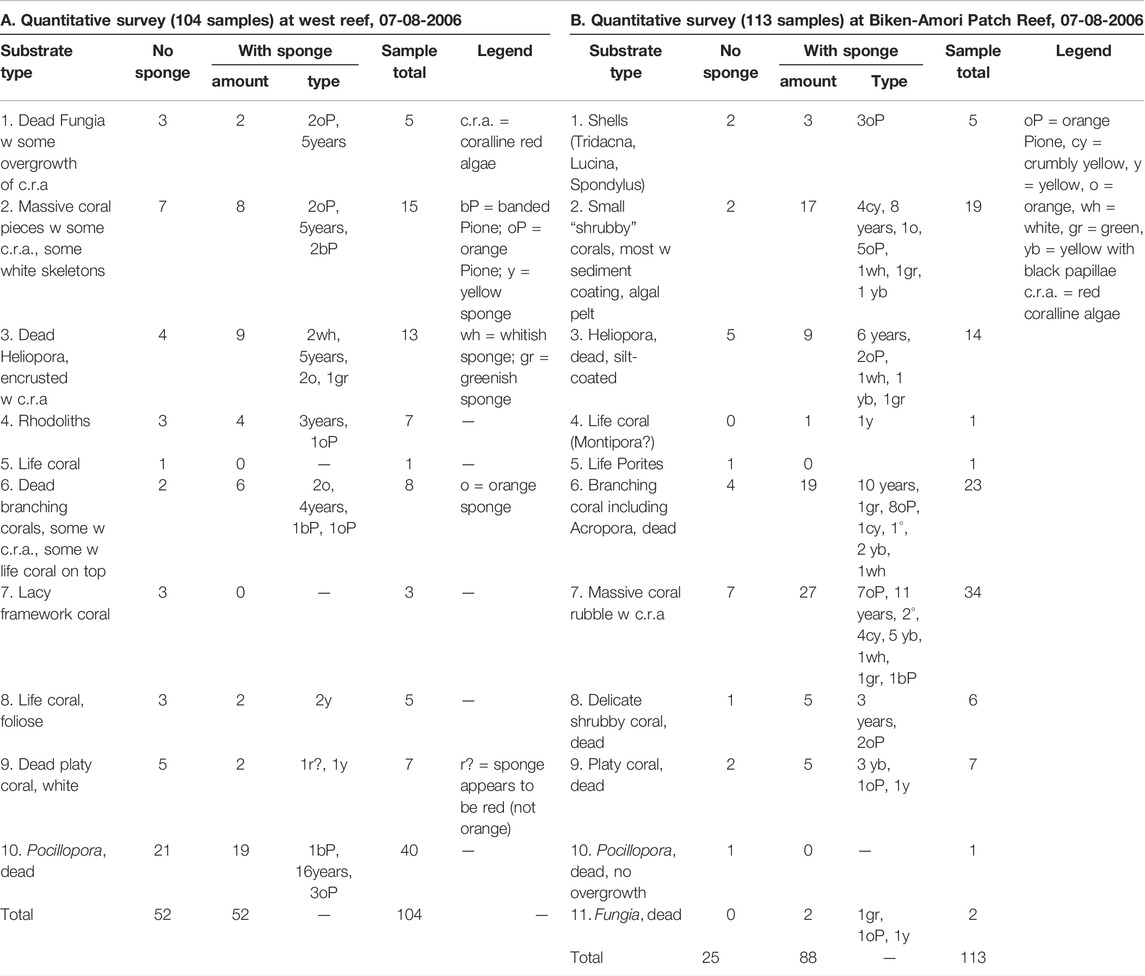
Site 1: 1°34′41.17″N; 172°54′47.52″E; 5–10 m deep, West Reef.
Site 2: 1°29′14.05″N; 173° 0′18.73″E; 5–10 m deep, Biken-Amori.
Site 3: 1°24′47.83″N; 172°55′47.15″E; 5–9 m deep, Betio Passage.
Site 4: 1°20′25.41″N; 172°59′58.60″E; 3–10 m deep; few centimeters at low tide, Fatali’s Lagoon.
Site 5: 1°19′14.47″N; 172°59′23.70″E; few centimeters at low tide, Fatali’s reef flat.
Tarawa has a lagoon that is open to the west, and consequently, exchange rates with water from the open Pacific Ocean are high. Nevertheless, there are pollution issues associated with poor sanitation and high population at South Tarawa. Water quality issues particularly in nearshore lagoonal sites were documented by Graves et al (2021). During our visit, Fatali’s Lagoon showed abundant cyanobacterial growth and Betio Passage had very turbid water with much fine-grained sediment suspended in the water column. These two sites are deemed “polluted” although the turbidity at Betio Passage may be a natural phenomenon.
5) Tabuama Island is an isolated island in the eastern part of Valapata Village (Northwest Vella la Vella Island, Paraso and Java Bays) in the Western Province of the Solomon Islands. The island is not inhabited but was used as a burial ground for the lekasa (Paramount chief) of the tribe that owned this Island since the early 1890s.
The Island has a total approximate size of 1.2 km2 and an elevation of ∼1.5 m above sea level. The island is perched on a reef platform with a fringing reef (mostly rocky substrate) to the west that drops off sharply into 70 m deep water a short distance from the shoreline. Tidal currents are strong, and high waves can reach close to the island shore. The wider reef platform is bordered by a passage into the bay area to the north, to the east a blue hole is evident, and to the south, another island (Bolo Udu) connects to Kokolope on the main island. Samples were collected at low tide from North, East, and South Tabuama Island. The western part of the Island was not considered because not much rubble was evident there.
This fieldwork was carried out by (then) local student Henry Kaniki for a marine studies research project. Funding was by USP (University of the South Pacific). The sampling campaign took place from the 6th of July to the 18th of July 2009.
Fieldwork was carried out with the help of nine villagers from Pusiju, south Vella la Vella Island. The samples collected were mostly of scleractinian branching corals which litter the reef platform (Acropora sp.) Since it was the lowest tide season (June–July) in this area, it was convenient to snorkel only at a certain depth (∼2 m) for the collection of samples. The approximate measurements of the rubbles collected were 15—20 cm in length.
Site 1: 7°41′54.67″S; 156°45′28.79″E, North Tabuama Island, ∼2 m depth at low tide.
Site 2: 7°41′59.37″S; 156°45′30.49″E East Tabuama Island, ∼2 m depth at low tide.
Site 3: 7°42′1.68″S; 156°45′30.67″E South Tabuama Island, ∼2 m depth at low tide.
Following collection samples were inspected for the presence of sponge papillae and then split several times to find sponges. Rubble with sponges was photographed with scale, and sample number. Most samples were kept in a deep freeze initially but later dried for permanent storage. Sponge collections are housed in the Marine Studies Collection of the University of the South Pacific, Suva, Fiji.
Spicule preparations were made using nitric acid or bleach following methods outlined by Hooper (2003).
Taxonomically, most of our sponges are retained in open nomenclature with “confer” (cf.), indicating that the suggested species is, in our opinion, the closest possible assignment that would need to be formally evaluated. Some species are thought to be part of a species complex, indicated by the expression “species complex.”
Results
Bioerosion by Large Excavating Sponges: Case Study From Nasese Tidal Flat, Laucala Bay, Fiji Islands
The Nasese intertidal carbonate platform is characterized by aggregations of large clionaid sponges (Figure 1A): Spheciospongia cf. inconstans var. digitata (Dendy 1887) and Spheciospongia solida (Ridley and Dendy 1886) (Figure 1B). The latter sponge was determined by Tendal (1969) based on materials collected from the vicinity of Suva, possibly near Lami Suburb (Ridley and Dendy, 1887). The large excavating sponges S. cf inconstans var. digitata and S. solida inhabit with up to 15 colonies per square meter the western side of the Nasese tidal platform where mud content and riverine influx are low. The sponges are exposed to air, heat, strong UV light, and rain at low tide. The sponge bodies are deeply rooted in the sediment (Figure 1C) and incorporate the products of their etching, sand, and small rubble in their tissues. This fortification probably protects them from being eaten by Hawksbill Turtles whose diet includes sponges (Brandis et al., 2014). Upon the death of the sponge, the carbonate particles will be released, and contribute to the sediment budget of the lagoon and to the erosion of the old Holocene reef platform. The bioerosion products are silt-sized chips, sand, and limestone pebbles; hence, these sponges are very efficient eroders.
There were 182 pieces of coral rubble collected on the platform of which 85% yielded small excavating sponges; 28% of the infested samples contained Pione cf. margaritifera Dendy, 1905, whereas 72% of the cavities were made by Pione cf. carpenteri (Hancock, 1867). Infestation levels are high at this site.
Infestation of Reef Rubble at a Pristine Site: Case Study From the Lau Group, Fiji Islands
The calculation of infestation rates of the 62 pieces of rubble collected near Vanua Balavu in percent indicates that at Site 3, 41.7% of the rubble contained boring sponges; Site 5 contained 33.3%; Site 7 contained 70%; and Site 12 yielded 48.4% boring sponges. These results suggest that the highest infestation rate with excavating sponges is at Site 7 which would be expected to be a quite pristine site with regard to nutrient influx because it is far from the large island Vanua Balavu. Although the samples (20 pieces of rubble) are too sparse for a definite statement, it is likely that prolific sponge bioerosion here is caused by the abundance of a suitable substrate such as reef rubble from branching corals. Most of the samples collected were fragments of branching, massive corals, and “boxwork”-type coralline algae. Samples are more or less encrusted by coralline red algae, algal turf, and various non-skeletal epizoans and epiphytes. Encrusting coralline red algal plaques are noted because they have a detrimental effect on boring sponges by overgrowing the papillae and killing the sponge.
The Lauan boring sponge fauna (Table 1) is dominated by species of the genus Cliona with 17 specimens belonging to four different species, followed by Pione spp. with eight specimens (belonging probably to two different species). The genus Cliothosa is represented by only one species (C. cf. hancocki) with one specimen. The four specimens of Cornulella? were found in samples labeled with “p” for purple. This refers to the distinctive color of the sponge tissue. Cornulella-type spicules were sparse in the samples and mixed with other spicules. Consequently, the assignment to this genus is tentative.

TABLE 1. Samples collected and types of bioerosion from the Lau Group, with preliminary genus/species determinations of excavating sponges.
Large cavities are produced only by Cliothosa and Cliona cf. caesia with five specimens. Weak excavators dominate with 21 specimens. Multiple etchings are produced by the purple poecilosclerid sponge Cornulella? sp. indet. (4 specimens).
One characteristic Cliona species which generates large cavities in Lauan waters (Figure 2) has a spicule composition comprising oxeas with pointy tips, small and large tylostyles with thin necks, and pointy tips; malformed crooked shapes of tylostyles occur frequently (Figure 2 Inset). This sponge resembles Cliona (?) caesia described by Schönberg (2000). It was initially placed in Pione, and later in Cliona, but an assignment to Volzia Rosell and Uriz, 1997 was also considered. Our specimens do not show the characteristic blue center of the papillae observed by Schönberg (2000).
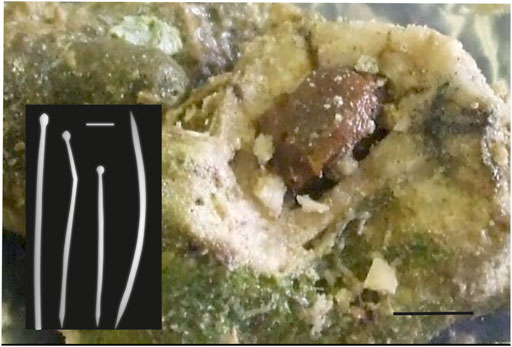
FIGURE 2. Cliona cf. caesia (Schönberg, 2000) from Lau Basin, scale bar is 1 mm long. Sample LB′07-s12-4fy. The sponge shown above sits in a dead coral sample, in which the sponge color is brownish in color but was more yellow before deep freezing. The species creates large cavities. Inset shows drawings of the characteristic spicule; from left to right: larger tylostyles are present and between 185–200 μm long; malformed tylostyle with kink; oxea with pointy tips; small spicule is 150 μm long, scale bar equals 30 μm. Same magnification for all spicules.
Infestation of Reef Rubble at a Populated Site: Case Study From Nananu-i-Ra, Northern Viti Levu, Fiji Islands
At Charlie’s Place North (CPN), 58 pieces of the rubble of dead branching coral, massive coral, shells, and boxwork-type coralline red algae were collected. Forty were without a sponge (= 69%) and eighteen were with boring sponges (= 31%) (3). Sixteen different sponges were found to belong to five different genera.
At Charlie’s Place South (CPS), 100 pieces of rubble were collected (Table 2). Of those, 22 were without bioeroding sponges and 33 with bioeroding sponges (Table 3). In total, 34 excavating sponges were found to belong to five different genera. Only two different types of rubble were found on random collection: branching coral fragments and boxwork-type coralline algae. The latter did not contain any boring sponges; all sponges are limited to the coral fragments.
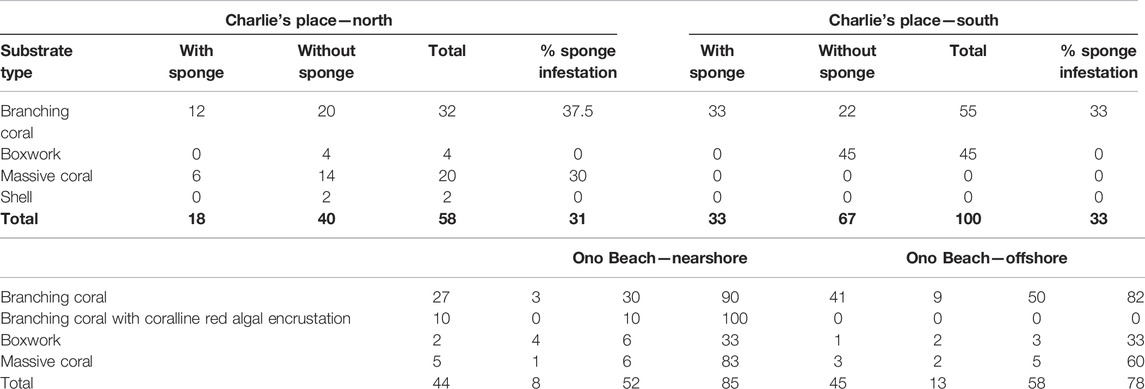
TABLE 2. Substrate types and sponge infestation on Nananu-i-Ra at Charlie’s Place North, Charlie’s Place South, One Beach nearshore, and One Beach offshore.
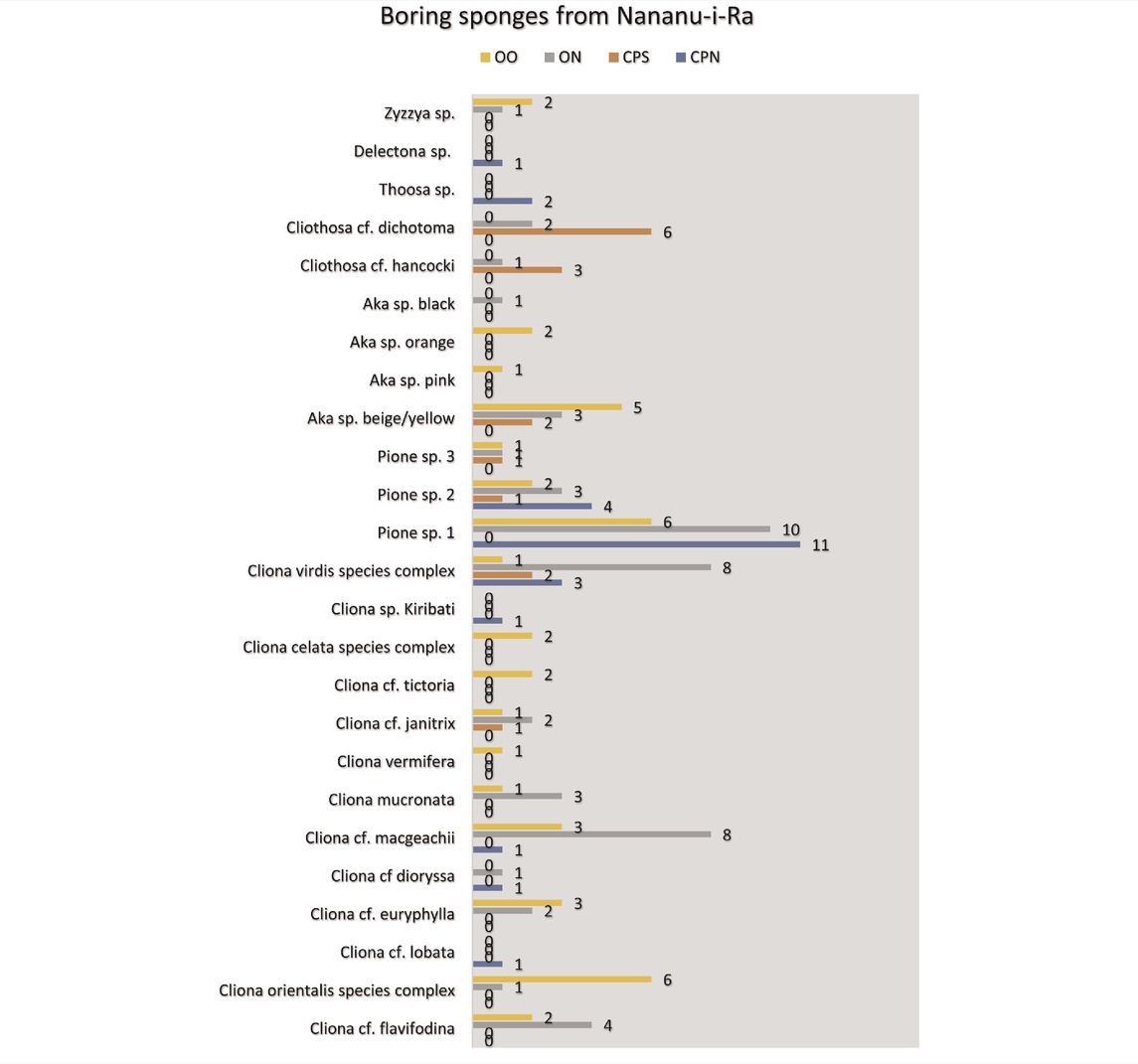
TABLE 3. Preliminary species list of excavating sponges from Nananu-i-Ra, Fiji. CPN = Charlie’s Place North, CPS = Charlie’s Place South, ON = One Beach nearshore and OO = One Beach offshore.
At One Beach nearshore, 52 pieces of rubble were collected (Table 2). Four different substrates types were distinguished: branching coral fragments, branching coral with coralline red algal encrustation (Br.c. w c.r.a.), boxwork-type coralline red algae, and massive coral fragments. Branching corals without (or minor) coralline red algal overgrowth are the most common type, 90% with sponges infested. The least suitable sites for boring sponges are boxwork coralline algal fragments with only 33% infestation.
Eight were without sponge (= 15%) and 44 pieces were with sponge (= 85%). There were 53 sponges that were found to belong to six different genera (Table 3). The infestation level at this site is generally high.
Offshore from One Beach in about 5 m water depth, 58 random pieces of coral rubble were collected by divers (Table 2). Most of the rubble was from branching corals, and sample sizes ranged from 20 cm long fragments to large (1–2 kg) fragments. A few samples were small dead or partially dead massive Porites colonies and anastomosing (“boxwork-type”) coralline red algal fragments which are typically found at the reef front. Most of the fragments were from the surrounding patch reef, but according to local divers, a few fragments may have been transported from further offshore by a recent storm because the parent corals and coralline red algae were not seen in situ at the site. Branching coral fragments yield the highest number of sponges with 82 percent, followed by massive coral fragments. Only 33% infestation level occurs in boxwork coralline algal samples, but these fragments were generally sparse at this site.
Infestation levels here were overall high at 78%, and many samples had more than one sponge in them so that the 45 pieces of rubble actually yielded 50 boring sponges belonging to 4 different genera (Table 3).
In the samples from Nananu-i-Ra, seven different genera of boring sponges were found (Table 3). Eleven species of Cliona were identified of which those belonging to the C. viridis species complex were the most abundant (21 specimens). The sponges from this group form eventually large cavities, but often, initially excavations with crumbly fabric are found (Figure 3). Crumbly textures are also produced by C. celata Grant, 1826 species complex, C. cf. tinctoria Schönberg, 2000, C. cf. euryphylle Topsent, 1888, C. orientalis Thiele, 1900 species complex.

FIGURE 3. A sponge species which probably belongs to the Cliona viridis species complex forms multiple small joined excavations, rendering the interior of the limestone piece with a crumbling texture which easily breaks. This is the most common sponge on Nananu-i-Ra and quite an efficient bioeroder, although initially excavations are small.
Also, common are Cliona cf. macgeachi Holmes, 2000 (12 specimens) (Figure 4). Pione spp. features prominently. Both are weak bioeroders making small cavities. Also, small cavities are only produced by Delectona sp., Thoosa sp., Cliona mucronata, Sollas, 1878, and C. vermifera Hancock, 1867. Large cavities are produced by the Cliothosa spp., the Aka spp., Cliona cf. janitrix Topsent, 1932 (Figures 5A–C), and C. cf. dioryssa (Laubenfels, 1950). The large clionaid sponges such as Spheciospongia were not seen but may be present at other locations in the vicinity.
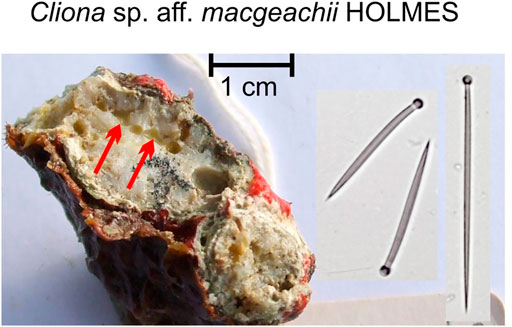
FIGURE 4. Cliona cf. macgeachii Holmes, 2000, a species that is a weak bioeroder, here in a branching coral fragment. This sponge is very common in shallow intertidal settings and is close to a species described from the Caribbean. The red arrows point to the pale yellow sponge cavities. The species is characterized by tylostyles in two size classes (right: short tylostyles, 115 and 116 μm long, far right: long tylostyle, 194 μm long). Microscleres are absent.
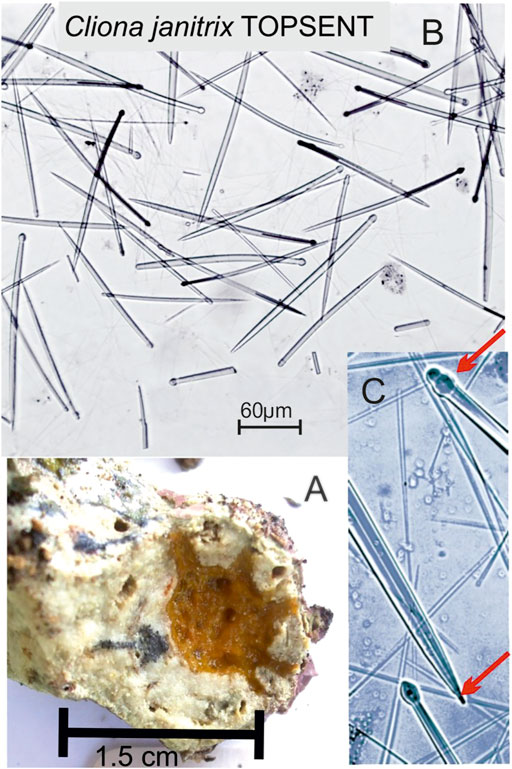
FIGURE 5. (A) Cliona cf. janitrix Topsent, 1932 from Nananu-i-Ra is a vivid orange sponge which can make large cavities. (B) Tylostyles as megascleres and not microscleres. The spicules are 250–300 μm long. (C) Characteristic mammilated or stepped tips (red arrow, right bottom) and “mucronate” tyle measuring 10 μm in diameter (red arrow, right top).
Bioerosion by Sponges From Tarawa, Kiribati
The reef rubble collected from various sites at Tarawa is diverse (Tables 4, 5) and consists of rubble from various growth forms of corals including the conspicuous blue coral Heliopora sp. Rhodoliths, and shells are rare.
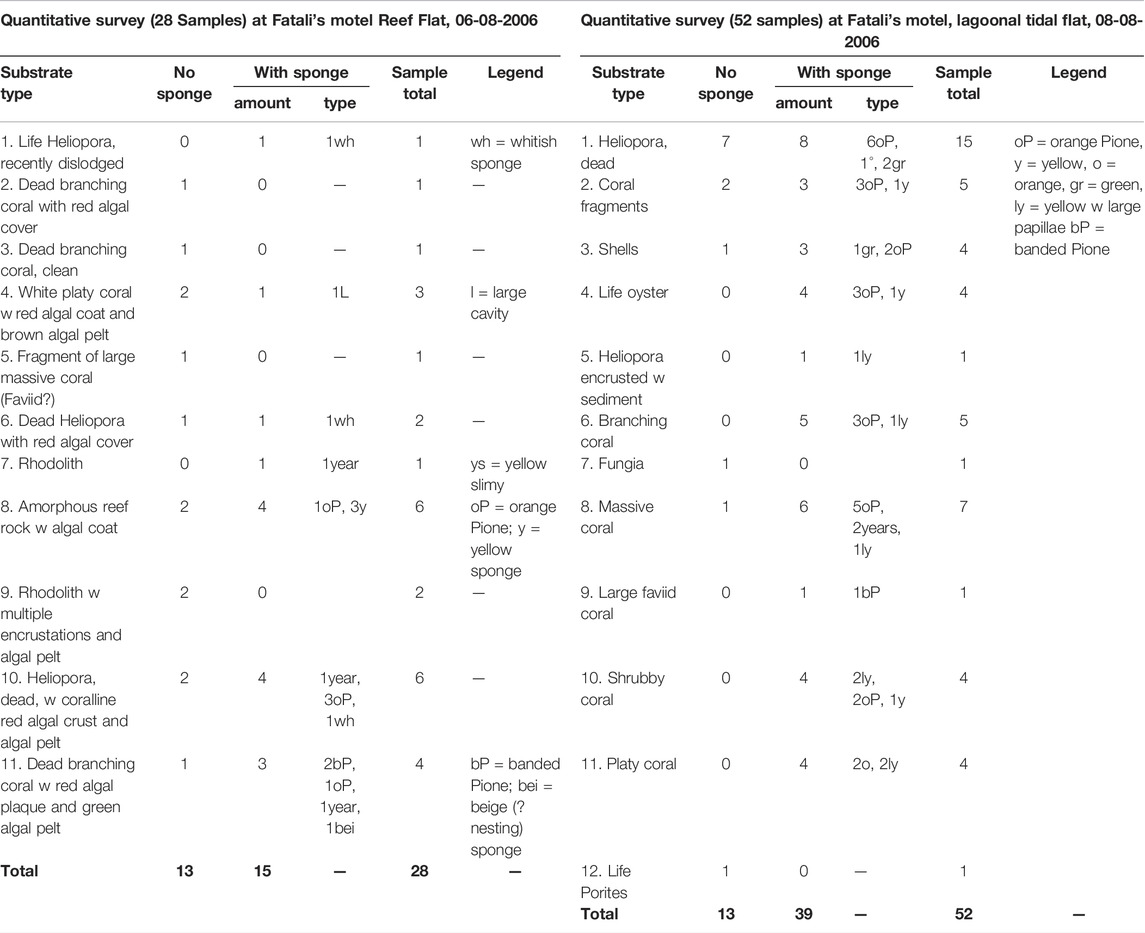
TABLE 5. Substrate types and sponge infestation at Fatali’s Motel reef flat and lagoon, Tarawa, Kiribati.
Reef rubbles from Betio Passage had the lowest percentage of boring sponges with 35%, followed by West reef (50%) and Fatali’s reef flat (Table 5) with 54%. The highest number of excavations was found at Fatali’s Lagoon (75%) and Biken Amori (78%) (Table 4).
The determination of sponges from Kiribati proved difficult because many samples had excavations filled with dead sponges, chips, and mixed spicules. In addition, most Cliona species could only be determined to the genus level (Table 6). Others seemed familiar, such as Cliothosa cf. hancocki (Topsent, 1888) and the very abundant Cliona celata species complex. Several species of Pione are present. Noteworthy is the occurrence of mixtures of spicules from Cliona mucronata and C. cf. ecaudis Topsent, 1932 (Figure 6). The latter species is here nowhere seen as a clean sample with only the blunt pointed ecaudis spicules. One sample only from the Tuamotus yielded such a clean spicule composition (pers. obs. 2008).
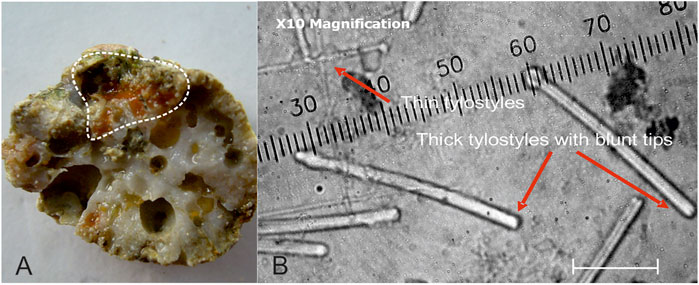
FIGURE 6. (A) Weakly bioeroding red-orange sponge in a piece of branching coral (delineated by dashed white line). (B) Spicules of the sample are characteristic of Cliona cf. ecaudis Topsent, 1932 with blunt rounded tips. In all samples from Kiribati, they are mixed with C. mucronata Sollas, 1878 spicules with mamillated tips. Scale is in micrometer. Sample K-BP′06-128 from Betio Passage, Tarawa, Kiribati.
Bioerosion by Sponges: Case Study of Sponges Forming Large Cavities, Tabuama Island, Vella Lavella, Solomon Islands
From Tabuama Island North, 140 samples were collected, from Tabuama Island East, 130, and from Tabuama Island South, 90. In total, 360 samples were collected. The highest sponge infestation was found at Tabuama South with 52%, followed by Tabuama East with 47%, and the least number of sponges was found at Tabuama North with 35%.
To date, only two sponge species with the largest cavities were investigated. These were Cliona cf. ensifera Sollas, 1878 (10 specimens) and Cliothosa cf. dichotoma Calcinai et al., 2000 (10 specimens, Figure 7). C. cf. ensifera makes cavities 4–7 mm high (mean 4 mm) and 7–12 mm wide (mean 10 mm). C. cf. dichotoma excavates cavities 1.2–4 mm high (mean 4 mm) and 7–16 mm wide (mean 10 mm). These two species are conspicuous elements of the excavating sponge fauna of Tabuama Island.
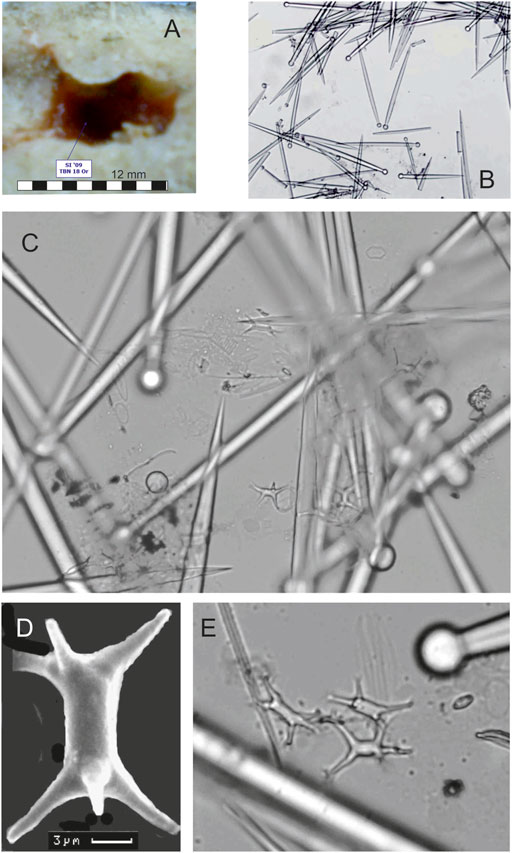
FIGURE 7. (A) Cavity of Cliothosa cf. dichotoma Calcinai et al., 2000. (B) Ensiform tylostyles vary in length from 140 to 170 µm (x10). (C) Small amphiasters with an axis of about 7 µm long. (D) Scanning electron microscope image of ramose amphiaster with a characteristic absence of split ends. (E) Ramose amphiasters (x70).
Discussion and Conclusion
The tropical reefs and associated settings contain numerous genera and species of boring sponges ranging from 10 to 90% infestation levels. On average, every second coral fragment contains one or more boring sponges. Living and attached colonies have fewer endolithic sponges with higher numbers associated with partial mortality of the coral. So far, the destructive species Cliothosa delitrix (Pang, 1973) which is of great concern in the Caribbean has not been found in Fiji or other surveyed regions (Kiribati, Solomon Islands). There seems to be only a weak link between eutrophy and sponge infestation because presumably pristine sites such as Dive site 7 at Lau and One Beach offshore at Nananu-i-Ra can also have high infestation levels. Low levels of infestation are correlated with coralline red algal overgrowth of substrates or boxwork coralline red algal fragments. Only a few “specialist” species are commonly found in reef rubble encrusted by coralline red algae and mature rhodoliths have no boring sponges at all. “High-diversity” rubble, that is, rubble with many different types of encrusters and overgrowths, tends to be good hosts for boring sponges. Rubble surrounded on all sides with algal plaques appears after some residence time of the rubble on the sea bed and requires some wave energy that overturns the particles regularly. Such rubble is not a good substrate for boring sponges. Plaques of coralline red algae seem to suffocate endolithic sponges by overgrowing them. Only one species, Cliona cf. macgeachii consistently survives in substrates overgrown by coralline red algae. This observation was also made by Holmes (2000) in the Caribbean. Fresh accumulations of reef rubble after recent storms open a “window of opportunity” for excavating sponges which closes when slow-growing coralline red algae begin to compete for space and eventually render the substrate unsuitable for boring sponges.
The shallow fringing reefs of Viti Levu are subjected to high wave stress, and here coralline red algae are particularly successful residents on “rolling stones” (i.e., coral rubble overgrown by multiple generations of coralline algae), suppressing the bioeroding activity of endolithic sponges.
High levels of sponge infestation can be linked to the abundance of “free” substrate available for the settlement of boring sponge larvae. Areas with large amounts of coarse and very coarse coral rubble with soft algal cover are the most favored sites; hence, a link can be established to recent bleaching events and storms or tropical depressions which dislodged and killed many corals, providing abundant settling sites for boring sponges.
Within the surveyed regions, most common boring sponges belong to the Cliona viridis species complex and the Cliona celata species complex. Cliona cf. macgeachii Holmes, 2000 and Pione spp. are frequently found. The determination of Pione species is hampered by taxonomic problems; that is, the genus and its species are in urgent need of revisions supported by molecular analyses as exemplified by Barucca et al. (2007). Generally, Pione species are commonly found at intertidal sites where other genera are rare or absent. They seem to be very hardy and adapted to the harsh conditions. This can also be said about the species of Spheciospongia at Nasese.
It is noteworthy that there seem to be many sponge species present which also occur in the Caribbean (such as Cliona flavifodina Rützler, 1974, Cliona janitrix Topsent, 1932), whereas others are very similar to faunas from the Indian Ocean (Annandale, 1915; Burton, 1937; Thomas, 1973, 1979). The land bridge between North- and South America formed about 3 million years ago and ship traffic through the Panama Canal began in 1914. Calcareous biofouling organisms attached to ships’ hulls may have traveled from the Caribbean into the Pacific carrying with them early stages of excavating sponges. Studies of sponge spicules preserved in Holocene reef rubble and/or molecular studies could resolve the question of the cosmopolitan versus regional nature of sponge populations.
Very little overlap is seen with faunas described from other tropical Pacific regions such as Indonesia and Vietnam (Calcinai et al., 2005; Calcinai et al., 2006); however, more detailed taxonomic and molecular may show that some of the new species described by these authors are close to or con-specific with northern Pacific species. An example for this could be Cliothosa dichotoma (Calcinai et al., 2000). Cliona utricularis Calcinai et al., 2005 from Indonesia seems close to our C. cf. janitrix.
In a more regional context, a comparison between faunas from Nananu-i-Ra (Lau), and Kiribati (Tarawa) suggests that the islands further to the east (Vanuabalavu, Lau) and northeast (Kiribati) have lower diversity of sponge faunas. Additional collections (not here presented) by one of the authors (S.P.) from Tonga, Tuvalu, and Suva Barrier reef support this suggestion. The relatively more isolated location of the Lauan Islands and Kiribati may explain the impoverished fauna. The problem with determining sponges from Kiribati may be a result of high endemicity.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Numerous people have helped with collecting and processing boring sponges from the region. The Nasese project was competently helped by Rusila Savou and Seini Tawakelevu. Lauan sample preparations were made by Vavo’ou Faktaufon. For the project on Nananu-i-Ra, we wish to thank the owners of Charlie’s Place for their support with boats and diving, Sharon Raj and Analaisa Ranadi Pohler are thanked for help with processing samples. In Kiribati, we are indebted to staff of the Ministry of Fisheries & Marine Resource Development (MFMRD) in Tarawa and Naomi Biribo-Atauea for facilitating the study in numerous ways. On Vella la Vella, Western Province in the Solomon Islands, we thank the villagers from Pusiju and Valapata for their tireless efforts in collecting raw materials from the field. All authors and particularly SP appreciate financial support from the University of the South Pacific. KF’s work was in addition supported by the International Cooperative Biodiversity Groups (NIH, Fogarty). We also thank the reviewer for the helpful suggestions and corrections. The authors acknowledge the financial support by the University of Graz.
References
Acker, K. L., and Risk, M. J. (1985). Substrate Destruction and Sediment Production by the Boring Sponge Cliona Caribbaea on Grand Cayman Island. J. Sediment. Petrology 55, 705–711. doi:10.1306/212f87c4-2b24-11d7-8648000102c1865d
Bak, R. P. M. (1976). The Growth of Coral Colonies and the Importance of Crustose Coralline Algae and Burrowing Sponges in Relation with Carbonate Accumulation. Neth. J. Sea Res. 10, 285–337. doi:10.1016/0077-7579(76)90009-0
Barucca, M., Azzini, F., Bavestrello, G., Biscotti, M. A., Calcinai, B., Canapa, A., et al. (2007). The Systematic Position of Some Boring Sponges (Demospongiae, Hadromerida) Studied by Molecular Analysis. Mar. Biol. 151, 529–535. doi:10.1007/s00227-006-0486-y
Brandis, R. G. V., Mortimer, J. A., Reilly, B. K., van Soest, R. W. M., and Branch, G. M. (2014). Diet Composition of Hawksbill Turtles (Eretmochelys Imbricata) in the Republic of the Seychelles. West. Indian Ocean. J. Mar. Sci. 13, 81–91.
Burton, M. (1937). The Littoral Fauna of Krusadai Island in the Gulf of Manaar. Bull. Madras Govt. Mus. (n.s.) Nat. Hist. Sect. 1, 1–58.
Calcinai, B., Azzini, F., Bavestrello, G., Cerrano, C., Pansini, M., and Thung, D. (2006). Boring Sponges from Ha Long Bay, Tonkin Gulf, Vietnam. Zool. Stud. 45, 201–212.
Calcinai, B., Bavestrello, G., and Cerrano, C. (2005). Excavating Sponge Species from the Indo-Pacific Ocean. Zool. Stud. 44, 5–18.
Calcinai, B., Cerrano, C., Sarà, M., and Bavestrello, G. (2000). Boring Sponges (Porifera, Demospongiae) from the Indian Ocean. Italian J. Zoology 67 (2), 203–219. doi:10.1080/11250000009356314
Carballo, J. L., Ovalle-Beltrán, H., Yáñez, B., Bautista-Guerrero, E., and Nava-Bravo, H. (2017). Assessment of the Distribution of Sponge Chips in the Sediment of East Pacific Ocean Reefs. Mar. Ecol. 38 (1), e12390. doi:10.1111/maec.12390
Carter, H. J. (1882). Some Sponges from the West Indies and Acapulco in the Liverpool Free Museum Described, with General and Classificatory Remarks. Ann. Mag. Nat. Hist. 9, 266–301. doi:10.1080/00222938209459039
Chavez-Fonnegra, A., and Zea, S. (2007). “Observations on Coral Undermining by the Caribbean Excavating Sponge Cliona Delitrix (Demospongiae, Hadromerida),” in Porifera Research: Biodiversity, Innovation and Sustainability. 7th International Sponge Symposium. Editors M. R. Custódio, G. Lôbo-Hajdu, E. Hajdu, and G. Muricy (Museu Nacional, Rio de Janeiro, Serie Livros), 7–13.
Dendy, A. (1905). “Report on the Sponges Collected by Professor Herdman at Ceylon, in 1902,” in Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar. 3 (Supplement 18). Editor W.A. Herdman (London: Royal Society), 57–246.
Dendy, A. (1887). XVI.-The Sponge-Fauna of Madras. A Report on a Collection of Sponges Obtained in the Neighbourhood of Madras by Edgar Thurston, Esq. Ann. Mag. Nat. Hist. 20, 153–165. doi:10.1080/00222938709460032
Duchassaing de Fonbressin, P., and Michelotti, G. (1864). Spongiaires de la mer Caraibe. Natuurkundige Verh. Holl. Maatsch. Wet. Haarlem 21 (2), 1–124.
Futterer, D. K. (1974). Significance of the Boring Sponge Cliona for the Origin of Fine Grained Material of Carbonate Sediments. J. Sediment. Res. 44, 79–84. doi:10.1306/74d72987-2b21-11d7-8648000102c1865d
Grant, R. E. (1826). Notice of a New Zoophyte (Cliona Celata Gr.) from the Firth of Forth. Edinb. New Philosophical J. 1, 78–81.
Graves, C. A., Powell, A., Stone, M., Redfern, F., Biko, T., and Devlin, M. (2021). Marine Water Quality of a Densely Populated Pacific Atoll (Tarawa, Kiribati): Cumulative Pressures and Resulting Impacts on Ecosystem and Human Health. Mar. Pollut. Bull. 163, 111951. doi:10.1016/j.marpolbul.2020.111951
Hancock, A. (1867). XXXVI.-Note on the Excavating Sponges; with Descriptions of Four New Species. Ann. Mag. Nat. Hist. 19 (3112), 229–242. doi:10.1080/00222936708679766
Hoeksema, B. W. (1983). Excavating Patterns and Spiculae Dimensions of the Boring Sponge Cliona Celata from SWNetherlands. Senck Marit. 15, 55–85.
Holmes, E. K. (2000). Effects of Eutrophication on Bioerosion Sponge Communities with the Description of New West Indian Sponges, Cliona Spp. (Porifera: Hadromerida: Clionidae). Invertebr. Biol. 119, 125–138.
Hooper, J. N. A. (2003). Sponge Guide. - 'sponguide'. Guide to Sponge Collection and Identification. South Brisbane: Queensland Museum. Available at: https://www.qm.qld.gov.au.
Laubenfels, M. W. d., and Hindle, D. E. (1950). The Porifera of the Bermuda Archipelago. Trans. Zoological Soc. Lond. 27 (1), 1–154. doi:10.1111/j.1096-3642.1950.tb00227.x
Morton, J., and Raj, U. (1980). The Shore Ecology of Suva and South Viti Levu. Fiji: Institute of Marine Resources, University of the South Pacific.
Neumann, A. C. (1966). Observations on Coastal Erosion in Bermuda and Measurements of the Boring Rate of the Sponge, Cliona Lampa 1,2. Limnol. Oceanogr. 11, 92–108. doi:10.4319/lo.1966.11.1.0092
Pang, R. K. (1973). The Systematics of Some Jamaican Excavating Sponges (Porifera). Postilla 161, 1–75. doi:10.5962/bhl.part.24559
Ridley, S. O., and Dendy, A. (1887). Report on the Monaxonida Collected by H.M.S. 'Challenger' during the Years 1873-76. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the Years 1873–76. Zoology 20, 1.
Ridley, S. O., and Dendy, A. (1886). XXXIV.-Preliminary Report on the Monaxonida Collected by H.M.S. 'Challenger'. Ann. Mag. Nat. Hist. 18, 325–351. doi:10.1080/00222938609459982
Rodda, P., and Nunn, P. D. (1990). Emerged Microatolls off Government House. Suva: Fiji Mineral Resources Department.
Rosell, D., and Uriz, M. J. (1997). Phylogenetic Relationships within the Excavating Hadromerida (Porifera), with a Systematic Revision. Cladistics 13 (4), 349–366. doi:10.1111/j.1096-0031.1997.tb00324.x
Schönberg, C. H. L. (2000). Bioeroding Sponges Common to the Central Great Barrier Reef: Descriptions of Three New Species, Two New Records, and Additions to Two Previously Described Species. Senckenberg. Maritima. 30 (3-6), 161–221.
Sollas, W. J. (1878). On Two New and Remarkable Species of Cliona. Ann. Mag. Nat. Hist. 1, 54–66. doi:10.1080/00222937808682289
Tendal, O. S. (1969). Demospongiae from the Fiji Islands. Vidensk. Meddelelser fra Dan. naturhistorisk Foren. 132, 3l–34.
Thomas, P. A. (1973). “Boring Sponges of the Reefs of Gulf of Mannar and Palk Bay,” in Symposium on Corals and Coral Reefs, Mandapam Camp. Editors C. Mukundan, and C.S.G. Pillai (Tervuren, Belgium: Marine Biological Association of India), 333–362.
Thomas, P. A. (1979). Studies on Sponges of the Mozambique Channel. I. Sponges of Inhaca Island. II. Sponges of Mambone and Paradise Islands. Annls. Mus. R. Afr. Cent. (Zool.) 227, 1–73.
Thomas, R., Ramadoss, K., and Vincent, S. G. (1993). Response to Claims: Counter-claims. J. Mar. Biol. Ass. India 35, 146–159. doi:10.1007/978-1-349-22644-3_7
Topsent, E. (1888). Contribution à l'étude des Clionides. Archives de Zoologie expérimentale générale 2, 1–165.
Topsent, E. (1932). Notes sur des clionides. Archives de Zoologie expérimentale générale 74 (28), 549–579.
Tunnicliffe, V. (1979). The Role of Boring Sponges in Coral Fracture. Biol. Des. Spongiaires 291, 309–315.
Keywords: bioerosion, Fiji Islands, Solomon Islands, Kiribati, Porifera
Citation: Pohler SML, Feussner K, Kaniki HV, Rashni B and Wendt H (2022) Patterns of Substrate Bioerosion by Excavating Sponges From the Southwest and Central Pacific Ocean. Front. Earth Sci. 10:914319. doi: 10.3389/feart.2022.914319
Received: 06 April 2022; Accepted: 25 April 2022;
Published: 04 July 2022.
Edited by:
Eduardo Jesús Mayoral Alfaro, University of Huelva, SpainReviewed by:
Susana Enríquez, National Autonomous University of Mexico, MexicoBarbara Calcinai, Polytechnic University of Marche, Italy
Copyright © 2022 Pohler, Feussner, Kaniki, Rashni and Wendt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanne M. L. Pohler, c3VzYW5uZS5wb2hsZXJAdW5pLWdyYXouYXQ=
 Susanne M. L. Pohler1*
Susanne M. L. Pohler1* Klaus Feussner
Klaus Feussner Bindiya Rashni
Bindiya Rashni