- 1Institute for Environmental Sciences, RPTU Kaiserslautern—Landau, Kaiserslautern, Germany
- 2Graduate Program in Water Resources and Environmental Engineering, Federal University of Paraná, Curitiba, Brazil
The widespread release of gas bubbles from aquatic sediments (ebullition) has been receiving growing scientific interest because of its globally relevant contribution to methane emissions. Besides being an efficient transport pathway for methane and other gases to the atmosphere, these bubbles have the potential to mobilize resources and pollutants previously buried in the sediment by carrying solutes and particles on their surface. The phenomenon of bubbles transporting substances other than gases is well studied in open water and widely used in technical applications, such as froth flotation or dissolved air floatation. Research on the transport capabilities of natural bubbles forming in, and being released from, aquatic sediments is exceedingly rare. Ebullition resulting from biogenic gas production in sediments is characterized by large spatial and temporal variability and bubble sizes exceed those typically used in technical applications. Here we summarize the current state of research concerning bubble mediated transport (BMT) from aquatic sediments and develop a perspective based on these findings and own experimental results. We present measurements from a shallow reservoir to explore methods to monitor BMT and gather data on ebullition over 1 year. We found consistent bubble size spectra, despite large temporal variations of ebullition fluxes. We highlight some of the inherent difficulties of research in this area and argue that more experiments are needed for improving empirical and mechanistic understanding of BMT.
1 Introduction
The presence of free gas in form of bubbles ranging in size from micrometers to centimeters is ubiquitous in aquatic ecosystems. Bubbles not only affect the physical properties of water, they also facilitate an important transport pathway with relevance for global biogeochemical cycling and climate. Rising bubbles transport gases, as well as particles, solutes and bacteria on their surfaces.
In lack of a consistent definition, we refer to bubble-mediated transport as the transport of gases, solid particles, dissolved substances and living organisms during events of ebullition in aquatic systems. Ebullition, in this context, is the process of gas bubble formation, either in sediment or in free water, followed by their buoyancy-driven rise towards the water surface. Depending on the location of bubble formation and bubble fate, the transport can occur not only within the water column, but also across the sediment-water and air-water interfaces. We will discuss the different sources for bubbles as they form the basis for any transport and in the following chapter we will present what is known about the transport in fresh water bodies.
Near the water surface, bubbles can be generated by entrainment of air due to physical forcing, e.g., by turbulence, wave breaking, or in chute flows. Depending on the difference in partial pressures between atmospheric and dissolved gases, diffusive gas exchange between the bubble and surrounding water enhances the overall air-water gas exchange (Farmer et al., 1993). Rising bubbles are also known to enrich the surface micro layer (SML) at the air-water interface with particulate and dissolved matter (Robinson et al., 2019). This process is known as bubble harvesting or scavenging. The bursting of bubbles upon reaching the surface allows for transport of particulate material into the atmosphere. Aerosols produced in this way can contain a variety of organic and inorganic substances, e.g., Brevi toxins produced during algae blooms (Larrson and Södergren, 1982; Pierce et al., 2003).
Within the water column, gas bubbles can form if the total dissolved gas pressure exceeds the sum of hydrostatic and atmospheric pressure. Near the water surface down to 9 m (∼500% O2 sat. necessary), oxygen produced by photosynthetically active organisms can provide the required supersaturation (Koschorreck et al., 2017). In the ocean or deep lakes like Lake Kivu, the long-term accumulation of geogenic gases, e.g., CO2 and CH4, from the Earth’s crust can provide the necessary gas pressure (Sigurdsson et al., 1987; Pasche et al., 2011).
Under anaerobic conditions within sediments carbon can replace oxygen as an acceptor for electrons and hydrogen in cellular respiration. If the concentration of sequestered methane surpasses the solubility, gas bubbles can form in the sediment (Leifer and Patro, 2002; Bastviken and Likens, 2009). Methane emissions from aquatic ecosystems are of great concern as recent estimates suggest that about half of the global CH4 emissions come from aquatic ecosystems (Rosentreter et al., 2021). About 50% of these emissions are facilitated by ebullition (Tokida et al., 2007; Maeck et al., 2014; McGinnis et al., 2016). Since the bubbles form in the sediment, they have the potential to transport substances and microorganisms from there all the way to the water surface, even in deep and stratified lakes (Delwiche et al., 2020). They can also strip material and gases during their rise. Seepage from natural resource deposits such as oil, gas and volcanic activities presents an abiotic source of bubbles.
While the above-mentioned processes resulting in bubble formation are natural, human activities can be associated with additional sources of bubbles in aquatic ecosystems. At the water surface, vehicle traffic is associated with enhanced turbulence and waves (Nylund et al., 2021). Turbine operation and plunging waters can be an extensive source of supersaturation (Beiningen and Ebel, 1970). Moreover, bubbling with air or oxygen has been a widely applied measure for improving water quality through oxygenation in lakes, fish ponds, and reservoirs (Beutel and Horne, 1999).
Sediments in freshwater and costal marine systems often carry the legacy of industrialization in the form of pollutants (Rheinheimer, 1998; Atgin et al., 2000; Li et al., 2007). Heavy metals, polycyclic aromatic hydrocarbons (PAH), and microplastic, are some of the prominent examples (Rabodonirina et al., 2015). The potential of bubbles to mobilize such materials is known from industrial processes, waste water treatment or mineral and oil processing (Smith, 1989; Saththasivam et al., 2016; Temesgen et al., 2017; Han et al., 2022). Therefore, knowledge about the potential of ebullition to reintroduce these materials into the water is vital for understanding sediment-water interactions in rivers, lakes and reservoirs. Sun et al. (2021) estimated that ebullition facilitate flux of PAH from lake sediments on a global scale. According to their work this flux could potentially overcompensate (118%) the global deposition of PAHs into lake sediments (Sun et al., 2021). Detailed theories and experimental work though are mostly available for describing the interactions of rising gas bubbles with suspended particles in technical applications (i.e., particle scavenging, or stripping), with scarce studies considering natural processes. The potential of bubbles to transport particles is related to size and surface charge of the particles, as well as on the ionic composition and pH of the water (Edzwald, 2010). For optimizing operation, gas bubbles in technical applications are typically small (around 100 µm or less), at least one order of magnitude smaller than bubbles sizes reported for sediment ebullition (Delwiche and Hemond, 2017). Existing knowledge therefore applies mostly to particle stripping, but not to the transport of particles from the sediment by CH4 bubbles forming in the sediment. This process is more complex, because the bubbles are in contact with a large variety of particles sizes and particle properties, compared to stripping from a homogeneous suspension.
The specific focus of this perspective is on bubble mediated transport of sediment particles by methane ebullition. In the following section, we review existing experimental studies, before we present our own field experiments, aiming to understand the potential of ebullition in a shallow reservoir to transport particles and heavy metals through the water column.
2 Current advances
Early work demonstrated that sediment can rerelease previously adsorbed polychlorinated biphenyls (PCBs) and in the presence of ebullition. These contaminants can then reach the water surface and become aerosolized (Larrson and Södergren, 1982). Yuan et al. (2007, 2009) performed laboratory experiments and modelled fluxes of phenanthrene (a polycyclic aromatic hydrocarbon PAH) from the sediment to the air-water interface, (Yuan et al., 2007, 2009). Their laboratory setup consisted of a column with sediment at the bottom, an optional cap of sand, a water column, and a final layer of hexane on top of the water. The hexane layer was an extraction phase for the phenanthrene. Methane gas was injected at constant rates into the sediment; information on bubble size were not provided. Their experiments showed that sediment resuspension by rising bubbles was an important factor for contaminant release. The authors further concluded that at natural rates of biogenic gas generation in sediments (<1 L m−2 d−1), resuspension rates and facilitated release rates of solid-associated contaminants are small in comparison to fluxes expected from bioturbation.
Viana et al. (2012) examined BMT in 14 different urban water bodies, (Viana et al., 2012). Natural bubbles were collected using an inverted funnel functioning as a bubble trap. To collect particles and PAHs, glass wool was fixed at the outlet of the funnel and at the outside as a reference. Ebullition was measured as the total accumulated gas volume during the sampling period and showed high variability between 2 to 450 mmol m−2 d−1 (approx. 0.05–10.82 L m−2 d−1). Significant fluxes of relevant PAHs and certain heavy metals were recorded. The analysis indicates that metals are more so associated with resuspended particles. The transport of PAHs appears to be more complex with a combination of sorption to particles as well as transport directly by bubbles. In an additional study, benthic fluxes of PAHs and metals were compared to ebullition enhanced fluxes from sediments in a contaminated waterway (Viana et al., 2018). Ebullitive fluxes were significant and exceeded benthic fluxes, for some metals and PAHs by one order of magnitude.
Delwiche et al. (2020) studied heavy metal and particle transport by gas bubbles in an urban lake, (Delwiche et al., 2020). Their analysis focused on the potential transport of cyanobacteria. Bubbles released upon disturbance of the sediment were collected, funneled through an optical bubble sensor, which detects the number and size of individual gas bubbles (Delwiche and Hemond, 2017) and then into a particles collection cup. The field measurements were complemented by laboratory experiments in a 15 m tall column. Metals were analyzed with inductively coupled plasma mass spectrometer (ICP-MS) and cyanobacteria were quantified with quantitative PCR (qPCR). They found that the concentrations of heavy metals and cyanobacterial cells in bubble-transported particulate material were similar to those in the bulk sediment. Bubble-mediated heavy metal transport appeared to be a small component in lake-wide cycling, whereas BMT of cyanobacteria could contribute substantially to their recruitment into the surface layer.
The transport of microbes via methane bubbles was also studied at a natural seep site off the coast of California (Schmale et al., 2015; Jordan et al., 2020). Methane oxidizing bacteria (MOB) were collected in a bubble catcher, a steel frame with a glass cylinder suspended inside, which is outfitted with valve and stop-cocks. A variant has an additional gas outlet below the glass cylinders opening for artificial ebullition. Ebullition rates were characterized from video files, and the quantification of MOB was accomplished with the CARD FISH method [see (Kubota, 2013)] and the identification via DNA analysis. The field measurements showed the transport of bacteria form the sediment into the water column above vent sites. Interestingly, the rate of transport was not proportional to the rate of gas release. Instead, the lower volumetric bubble rates showed higher transport rates. It is speculated that higher volumetric flows don’t allow a sufficient resupply of the bubble path with microorganisms (Jordan et al., 2020).
McLinn and Stolzenburg (2009) investigated the transport of tar droplets from contaminated sediments to the water surface in the Penobscot River (USA), (McLinn and Stolzenburg, 2009). Floating tar droplets were collected directly at the water surface and, in the case of a visible hydrocarbon sheen, teflon nets were used for sampling. Bubble rates were determined by collecting rising gas in transparent polyethylene tubes at locations of high ebullition. The occurrence of tar at the surface was found to correlate with water temperature and water level, both are known to be major driving factors for biogenic methane production and bubble release (Maeck et al., 2014). A concise overview of all the experimental parameters and studied systems can be found in Table 1.
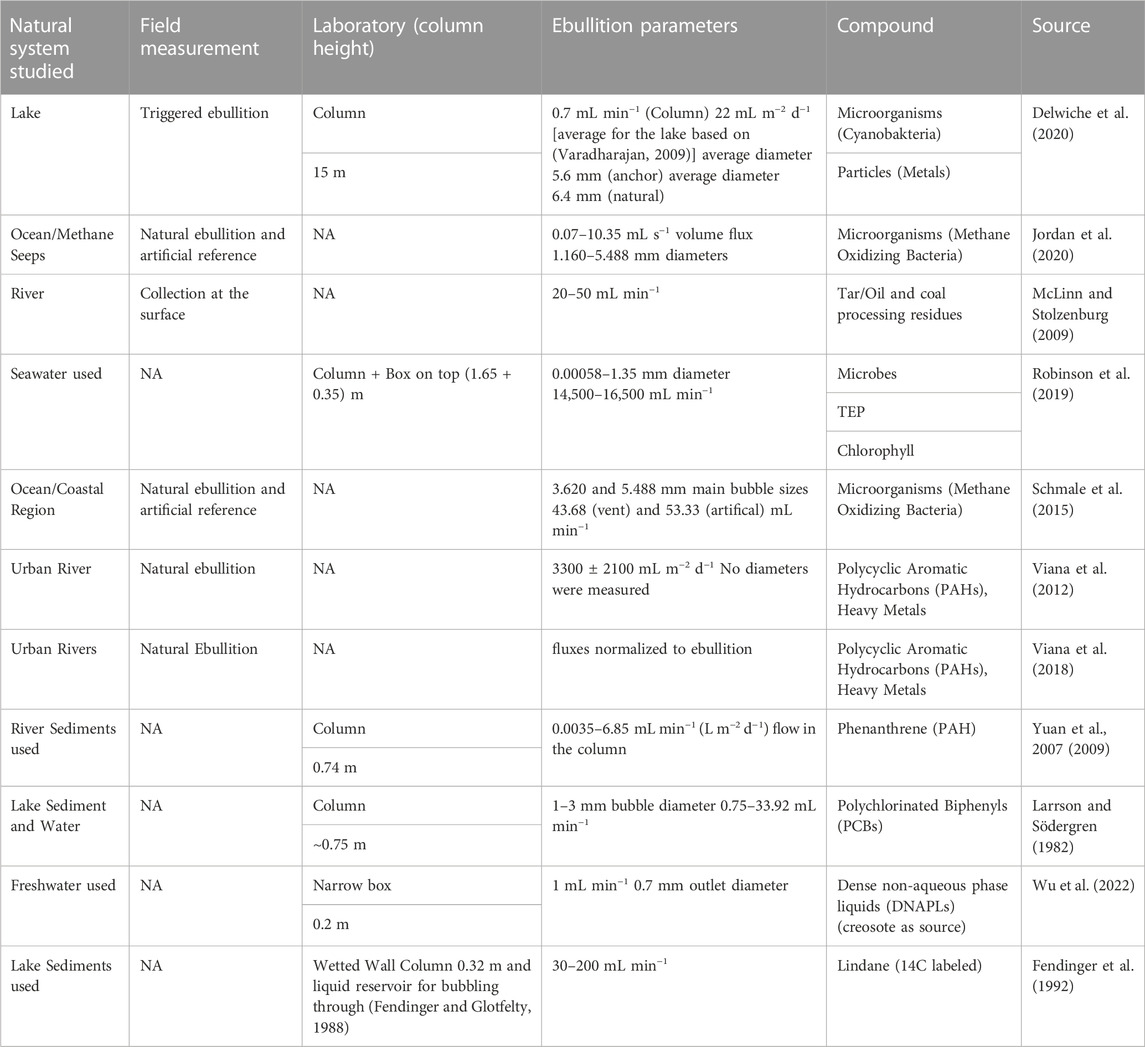
TABLE 1. Previous works examining bubble-mediated transport of solutes and particles by bubbles released from aquatic sediments in field and in laboratory settings.
3 Particulate matter transport in a shallow reservoir
In-situ measurements were conducted at the upstream region of Wupper reservoir (Latitude 51.16°N and longitude 7.33°E), a shallow impoundment of 2 m average depth. Quasi continuous ebullition measurements were conducted using optical bubble detectors as in (Delwiche and Hemond, 2017), see Figure 1A for schematic and yielded an average flux of 0.04 L m−2 d−1. Despite the strong temporal dynamics of ebullition fluxes, the bubble size distributions remained similar throughout 500 days of measurements, with an overall median diameter around 6.4 mm and daily median values varying between 2 to 10 mm (Figure 1B). The measurements during the continuous instrument deployment were often interrupted by clogging of the bubble sensor, but showed variations between months and sampling locations. Because of the apparent transport happening, the decision was made to equip bubble detectors with a sedimentation trap at the outlet of the detector for 5 months, where solid material carried by bubbles would deposit. To ensure that the collected material was transported by bubbles, additional traps were installed, in which the bottom of the funnel was blocked, but with additional holes at the side, allowing for exchange with flowing water. These traps consistently contained less material than traps with open funnels, indicating that biological growth within the trap was not a dominant source of particulate matter (Figure 1C).
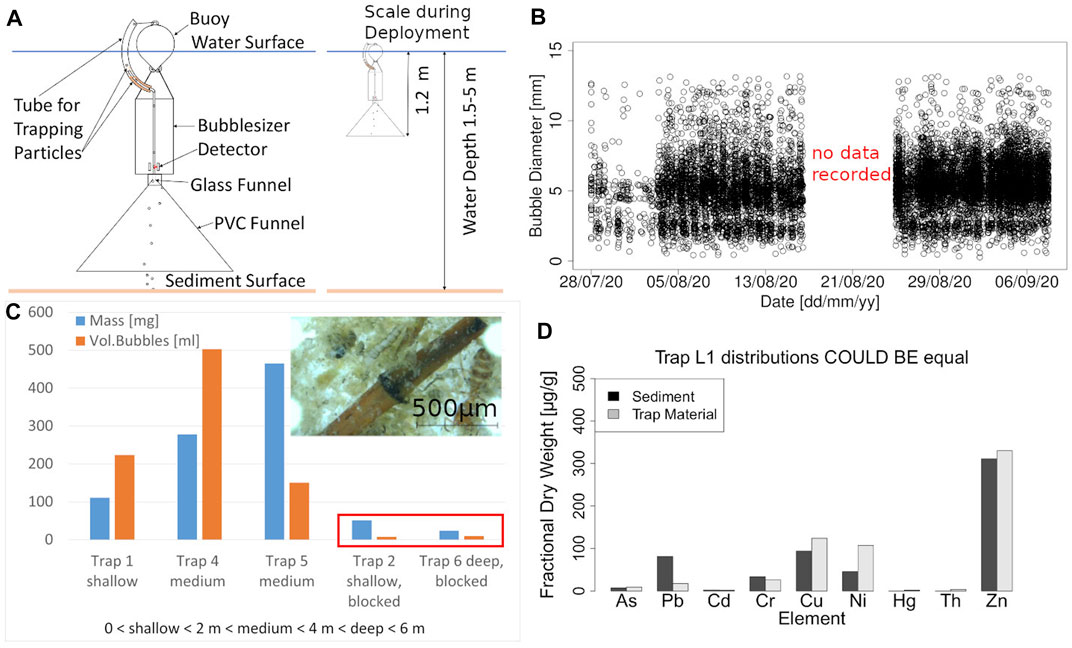
FIGURE 1. (A) Deployment schematics of an optical bubble sensor. (B) Time series of bubble size distributions (each symbol represents a single bubble) measured in a freshwater reservoir. Note that the periodicity in time (approx. 6 h) results from intermittent measurement intervals. The bi-modal size distribution is most likely caused by the funnel geometry, which leads to merging of bubbles into larger ones within the glass funnel. (C) Bar chart showing the mass of collected material in May 2021 in blue as well as the volume of bubbles in orange during the same time. In the red rectangle are two traps that served as a reference for the accumulation of material without bubbles as well as the background of the bubble detection. All values below 50 are most likely not caused by ebullition. The smaller picture shows material found in in the bubble trap. We monitored ebullition over the course of 1 year in a pre-dam at the Wupper River in Germany with optical bubble traps based on Delwiche and Hemond (2017), (Delwiche and Hemond, 2017). After half of a year collection tubes for particulate matter were added. The collected material was dried, weighed and analyzed by ICP-MS to compare the composition of metal ions in the samples with previous sediment analysis. (D) Comparison of metal concentrations in dried material found in the bubble trap and in the sediment of the reservoir.
At the beginning of the seasonal field sampling, sediment samples were collected and analyzed for heavy metals. We compared the fractional content of heavy metals in the sediment with that in the particulate material collected by the bubble traps using ICP-MS. Figure 1D) shows an example of this analysis and the following discussion only applies to results of this first sampling in the first month. In this case no statistically significant difference (t-test, p = 0.42) could be detected, suggesting that both samples could have the same source. Although not being statistically significant, lead and nickel concentrations differed by up to 100%, with Ni being highly enriched and Pb being underrepresented in the material from the bubble trap. It could be reasoned that granules of Pb containing sediment suspended in the bulk water would lose more Pb to the water before analysis than sediment at the river bed. The surplus of Ni and to a lesser degree Cu and Zn most likely stem from additional organic matter that also accumulated in the traps. For later samplings (not shown) in the following month statistical test still gave similar results although the bar charts did not show as obvious of a pattern.
The total dry weight of the particulate matter collected in the bubble traps over deployment periods of around 30 days was generally below a maximum of 1 g (0.042 g m−2 d−1), while the total volume of gas detected was below max. Volume of 1 L (0.04 L m−2 d−1) in 90% of cases. The corresponding rate of BMT of 1 mg mL−1 is about one to two orders of magnitude higher than the values reported in (Delwiche et al., 2020), which included field sampling and laboratory experiments.
Preliminary tests of the authors showed that the water exchange caused by bubbles passing through a trap alone can lead to substantial sedimentation from turbid water but a comparable turbidity to lab tests was not observed in the reservoir. Consequently, the most likely source of additional material in the bubble traps is organic matter. The studied reservoir contains large amounts of waterweed in the shallower parts. Fragments of these plants as well as floating algae could drift up the funnels and such plant material was indeed found in the traps (Figure 1C). The second main source could be swimming or migrating animals. We found living segmented, insectoid and worm-like animals. It is assumed that they migrated up along the funnel surface through the bubble-sizer. Another source of animal material were shells of insects, for example, corixidae, additionally legs, torsos, heads and other fragments of unidentified insect bodies could also be found. Organisms in freshwater are known to perform diurnal vertical migration (Stich and Lampert, 1981). It is likely that migrating organisms would be caught in the funnel when they are moving upwards and subsequently trapped. This effect should vary with the seasons, being more pronounced in the warmer months with increasing biological activity. For the first sampling from April to May 2021 the analytical results showed a generally similar pattern between sediment and trap material. In the following months, results showed greater differences and the bar plots did no longer appear visually similar. This could indicate increasing biological activity, since metals accumulate in different ratios in living matter compared to sediment. The data was too limited though for a definitive analysis.
Another important factor to consider is the natural bubble size spectrum that was found. During the 1 year of measurements, the mean diameter was 6.9 and the median 6.3 mm, compared to a mean of bubble size of 4.6 mm (Delwiche and Hemond, 2017), 5.7 mm (Ostrovsky et al., 2008) and 5.9 mm (DelSontro et al., 2015). The difference in mean diameters could in part be caused by bubble coalescence in the trap, especially for high ebullition rates and bubble plumes. Experimental data and mathematical models in the context of dissolved/dispersed air floatation show higher transport efficiency for smaller bubbles, typically at the micrometer scale (Edzwald, 2010; Coward et al., 2015). Additionally, the transport of material through the funnel of the bubble trap leads to two issues. The first is material getting stuck, which is evidenced by data gaps (Figure 1A). The second problem is the potential false positive detection of rising bubbles. The principle of the optical bubble sensor is to detect a loss of signal at its internal light barriers. This means, particles can be counted as bubbles passing through the detector which would reduce the transport flux by adding false volume. Considering all factors, it is never the less assumed that the measured transport rate is very likely still overestimated due to biological factors.
4 Discussion and perspective
Monitoring sediment ebullition and BMT in the field has been proven difficult. The higher the transport rates the easier their analytical quantification, but there is also an increased risk of material blocking or distorting bubble detection. Separating BMT and other modes of transport, like migrating zooplankton is still quite complex during long term monitoring considering the current available methods. Additionally, due to spatial heterogeneity of ebullition, extrapolating between sampling locations or across different water bodies can lead to large over- or underestimates (de Mello et al., 2018; Wik et al., 2018).
Priority in future research should be to establish empirical relationships and mechanistic models between bubble characteristics and observed particle fluxes. Early works like (Larrson and Södergren, 1982) treated ebullition rather as a black box mechanism to observe transport and therefor the results are mostly just the comparison between ebullition and its absence. Accordingly, future investigations should aim at the mechanistic side of BMT. These studies need to differentiate between direct transport of particles being attached to bubbles versus particle resuspension and turbulence-driven transport through the water column. Klein (2006) pointed out from laboratory experiments the sediment suspended by ebullition enhanced solute flux, (Klein, 2006).
Water depth is certainly an important parameter when considering BMT. In laboratory experiments, particles that are attached to rising bubbles can be observed using optical techniques, such as shadowgraphy (Trudel et al., 2018), or one could transform bacteria to express fluorescent proteins to visualize their transport by methane bubbles. To assess the flow driven transport, techniques from research into dissolved air flotation and fluid mechanics could be utilized, e.g., shadowgraphy, particle image velocimetry, laser-induced fluorescence, turbidity sensors, etc., In the field, information about the size, frequency and origin of the bubbles can be collected in order to compare to lab experiments. Optical bubble sensors are readily applicable, but are more limited in their results than imaging techniques. While the latter are more precise, they require extensive technical equipment and computational effort. Nevertheless, mechanistic studies will be of great value as they provide the knowledge for precise modelling and extrapolation to field conditions.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
MS original draft, investigation. LM review and editing, investigation. AL review and editing, supervision.
Funding
The study was financially supported by the German Research Foundation (Grant No. LO 1150/16).
Acknowledgments
We thank Christoph Bors for his support during the field work and construction of the bubblesizers and the department of Environmental and Soil Chemistry for providing the ICP-MS analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Atgin, R. S., El-Agha, O., Zararsız, A., Kocataş, A., Parlak, H., and Tuncel, G. (2000). Investigation of the sediment pollution in izmir bay: Trace elements. Spectrochim. Acta Part B At. Spectrosc. 55, 1151–1164. doi:10.1016/S0584-8547(00)00231-7
Bastviken, D., and Likens, G. E. (2009). Encyclopedia of Inland waters. Oxford, United Kingdom: Oxford Academic Press.
Beiningen, K. T., and Ebel, W. J. (1970). Effect of john day dam on dissolved nitrogen concentrations and salmon in the columbia river, 1968. Trans. Am. Fish. Soc. 99, 664–671. doi:10.1577/1548-8659(1970)99<664:EOJDDO>2.0.CO;2
Beutel, M. W., and Horne, A. J. (1999). A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv. Manag. 15, 285–297. doi:10.1080/07438149909354124
Coward, T., Lee, J. G., and Caldwell, G. S. (2015). The effect of bubble size on the efficiency and economics of harvesting microalgae by foam flotation. J. Appl. Phycol. 27, 733–742. doi:10.1007/s10811-014-0384-5
de Mello, N. A. S. T., Brighenti, L. S., Barbosa, F. A. R., Staehr, P. A., and Bezerra Neto, J. F. (2018). Spatial variability of methane (CH4) ebullition in a tropical hypereutrophic reservoir: Silted areas as a bubble hot spot. Lake Reserv. Manag. 34, 105–114. doi:10.1080/10402381.2017.1390018
DelSontro, T., McGinnis, D. F., Wehrli, B., and Ostrovsky, I. (2015). Size does matter: Importance of large bubbles and small-scale hot spots for methane transport. Environ. Sci. Technol. 49, 1268–1276. doi:10.1021/es5054286
Delwiche, K., Gu, J., Hemond, H., and Preheim, S. P. (2020). Vertical transport of sediment-associated metals and cyanobacteria by ebullition in a stratified lake. Biogeosciences 17, 3135–3147. doi:10.5194/bg-17-3135-2020
Delwiche, K., and Hemond, H. F. (2017). An enhanced bubble size sensor for long-term ebullition studies. Limnol. Oceanogr. Methods 15, 821–835. doi:10.1002/lom3.10201
Edzwald, J. K. (2010). Dissolved air flotation and me. Water Res. 44, 2077–2106. doi:10.1016/j.watres.2009.12.040
Farmer, D., McNeil, C., and Johnson, B. (1993). Evidence for the importance of bubbles in increasing air–sea gas flux. Nature 361, 620–623. doi:10.1038/361620a0
Fendinger, N. J., Adams, D. D., and Glotfelty, D. E. (1992). The role of gas ebullition in the transport of organic contaminants from sediments. Sci. total Environ. 112, 189–201. doi:10.1016/0048-9697(92)90187-W
Fendinger, N. J., and Glotfelty, D. E. (1988). A laboratory method for the experimental determination of air-water Henry’s law constants for several pesticides. Environ. Sci. Technol. 22, 1289–1293. doi:10.1021/es00176a007
Han, G., Chen, S., Su, S., Huang, Y., Liu, B., and Sun, H. (2022). A review and perspective on micro and nanobubbles: What they are and why they matter. Miner. Eng. 189, 107906. doi:10.1016/j.mineng.2022.107906
Jordan, S. F. A., Treude, T., Leifer, I., Janßen, R., Werner, J., Schulz-Vogt, H., et al. (2020). Bubble-mediated transport of benthic microorganisms into the water column: Identification of methanotrophs and implication of seepage intensity on transport efficiency. Sci. Rep. 10, 4682–4715. doi:10.1038/s41598-020-61446-9
Klein, S. (2006). Sediment porewater exchange and solute release during ebullition. Mar. Chem. 102, 60–71. doi:10.1016/j.marchem.2005.09.014
Koschorreck, M., Hentschel, I., and Boehrer, B. (2017). Oxygen ebullition from lakes. Geophys. Res. Lett. 44, 9372–9378. doi:10.1002/2017GL074591
Kubota, K. (2013). CARD-FISH for environmental microorganisms: Technical advancement and future applications. Microbes Environ. 28, 3–12. doi:10.1264/jsme2.ME12107
Larrson, P., and Södergren, A. (1982). Transport of PCBs in aquatic laboratory model ecosystems from sediment to the atmosphere via the surface microlayer. Ambio 111, 41–45. doi:10.2307/4312751
Leifer, I., and Patro, R. K. (2002). The bubble mechanism for methane transport from the shallow sea bed to the surface: A review and sensitivity study. Cont. Shelf Res. 22, 2409–2428. doi:10.1016/S0278-4343(02)00065-1
Li, Q., Wu, Z., Chu, B., Zhang, N., Cai, S., and Fang, J. (2007). Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environ. Pollut. 149, 158–164. doi:10.1016/j.envpol.2007.01.006
Maeck, A., Hofmann, H., and Lorke, A. (2014). Pumping methane out of aquatic sediments – ebullition forcing mechanisms in an impounded river. Biogeosciences 11, 2925–2938. doi:10.5194/bg-11-2925-2014
McGinnis, D. F., Bilsley, N., Schmidt, M., Fietzek, P., Bodmer, P., Premke, K., et al. (2016). Deconstructing methane emissions from a small northern European river: Hydrodynamics and temperature as key drivers. Environ. Sci. Technol. 50, 11680–11687. doi:10.1021/acs.est.6b03268
McLinn, E. L., and Stolzenburg, T. R. (2009). Ebullition-facilitated transport of manufactured gas plant tar from contaminated sediment. Environ. Toxicol. Chem. Int. J. 28, 2298–2306. doi:10.1897/08-603.1
Nylund, A. T., Arneborg, L., Tengberg, A., Mallast, U., and Hassellöv, I.-M. (2021). In situ observations of turbulent ship wakes and their spatiotemporal extent. Ocean Sci. 17, 1285–1302. doi:10.5194/os-17-1285-2021
Ostrovsky, I., McGinnis, D. F., Lapidus, L., and Eckert, W. (2008). Quantifying gas ebullition with echosounder: The role of methane transport by bubbles in a medium-sized lake. Limnol. Oceanogr. Methods 6, 105–118. doi:10.4319/lom.2008.6.105
Pasche, N., Schmid, M., Vazquez, F., Schubert, C. J., Wüest, A., Kessler, J. D., et al. (2011). Methane sources and sinks in lake Kivu. J. Geophys. Res. Biogeosciences 116, G03006. doi:10.1029/2011JG001690
Pierce, R. H., Henry, M. S., Blum, P. C., Lyons, J., Cheng, Y. S., Yazzie, D., et al. (2003). Brevetoxin concentrations in marine aerosol: Human exposure levels during a karenia brevis harmful algal bloom. Bull. Environ. Contam. Toxicol. 70, 161–165. doi:10.1007/s00128-002-0170-y
Rabodonirina, S., Net, S., Ouddane, B., Merhaby, D., Dumoulin, D., Popescu, T., et al. (2015). Distribution of persistent organic pollutants (PAHs, Me-PAHs, PCBs) in dissolved, particulate and sedimentary phases in freshwater systems. Environ. Pollut. 206, 38–48. doi:10.1016/j.envpol.2015.06.023
Rheinheimer, G. (1998). Pollution in the baltic sea. Naturwissenschaften 85, 318–329. doi:10.1007/s001140050508
Robinson, T.-B., Giebel, H.-A., and Wurl, O. (2019). Riding the plumes: Characterizing bubble scavenging conditions for the enrichment of the sea-surface microlayer by transparent exopolymer particles. Atmosphere 10, 454. doi:10.3390/atmos10080454
Rosentreter, J. A., Borges, A. V., Deemer, B. R., Holgerson, M. A., Liu, S., Song, C., et al. (2021). Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat. Geosci. 14, 225–230. doi:10.1038/s41561-021-00715-2
Saththasivam, J., Loganathan, K., and Sarp, S. (2016). An overview of oil–water separation using gas flotation systems. Chemosphere 144, 671–680. doi:10.1016/j.chemosphere.2015.08.087
Schmale, O., Leifer, I., Deimling, J. S. V., Stolle, C., Krause, S., Kießlich, K., et al. (2015). Bubble transport mechanism: Indications for a gas bubble-mediated inoculation of benthic methanotrophs into the water column. Cont. Shelf Res. 103, 70–78. doi:10.1016/j.csr.2015.04.022
Sigurdsson, H., Devine, J. D., Tchua, F. M., Presser, F. M., Pringle, M. K. W., and Evans, W. C. (1987). Origin of the lethal gas burst from lake monoun, cameroun. J. Volcanol. Geotherm. Res. 31, 1–16. doi:10.1016/0377-0273(87)90002-3
Smith, R. W. (1989). Flotation of algae, bacteria and other microorganisms. Mineral Process. Extr. Metallurgy Rev. 4, 277–299. doi:10.1080/08827508908952640
Stich, H.-B., and Lampert, W. (1981). Predator evasion as an explanation of diurnal vertical migration by zooplankton. Nature 293, 396–398. doi:10.1038/293396a0
Sun, T., Li, W., and Yin, K. (2021). Estimation of total flux of polycyclic aromatic hydrocarbons facilitated by methane ebullition into water column from global lake sediments. Water Res. 204, 117611. doi:10.1016/j.watres.2021.117611
Temesgen, T., Bui, T. T., Han, M., Kim, T., and Park, H. (2017). Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 246, 40–51. doi:10.1016/j.cis.2017.06.011
Tokida, T., Miyazaki, T., Mizoguchi, M., Nagata, O., Takakai, F., Kagemoto, A., et al. (2007). Falling atmospheric pressure as a trigger for methane ebullition from peatland. Glob. Biogeochem. Cycles 21. doi:10.1029/2006GB002790
Trudel, N., Mörs, F., Hlawitschka, M. W., Wirz, D., Lichti, M., Bajohr, S., et al. (2018). Ressourceneffiziente Methanolsynthese im Blasensäulenreaktor. Chem. Ing. Tech. 90, 1143–1144. doi:10.1002/cite.201855025
Varadharajan, C. (2009). Magnitude and spatio-temporal variability of methane emissions from a eutrophic freshwater lake. Cambridge, MA, USA: Massachusetts Institute of Technology.
Viana, P., Yin, K., and Rockne, K. (2018). Comparison of direct benthic flux to ebullition-facilitated flux of polycyclic aromatic hydrocarbons and heavy metals measured in the field. J. Soils Sediments 18, 1729–1742. doi:10.1007/s11368-017-1893-z
Viana, P., Yin, K., and Rockne, K. J. (2012). Field measurements and modeling of ebullition-facilitated flux of heavy metals and polycyclic aromatic hydrocarbons from sediments to the water column. Environ. Sci. Technol. 46, 12046–12054. doi:10.1021/es302579e
Wik, M., Johnson, J. E., Crill, P. M., DeStasio, J. P., Erickson, L., Halloran, M. J., et al. (2018). Sediment characteristics and methane ebullition in three subarctic lakes. J. Geophys. Res. Biogeosciences 123, 2399–2411. doi:10.1029/2017JG004298
Wu, J., Garcia, A. N., and Mumford, K. G. (2022). Ebullition-facilitated mobilization of trapped dense non-aqueous phase liquid at residual saturation from sandy sediments. J. Environ. Manag. 317, 115448. doi:10.1016/j.jenvman.2022.115448
Yuan, Q., Valsaraj, K. T., and Reible, D. D. (2009). A model for contaminant and sediment transport via gas ebullition through a sediment cap. Environ. Eng. Sci. 26, 1381–1391. doi:10.1089/ees.2008.0269
Keywords: bubble size spectra, reservoir monitoring, heavy metal transport, sediment remobilization, freshwater bubbles
Citation: Schwarz M, Marcon L and Lorke A (2023) Quantifying bubble-mediated transport by ebullition from aquatic sediments. Front. Earth Sci. 11:1113349. doi: 10.3389/feart.2023.1113349
Received: 01 December 2022; Accepted: 13 April 2023;
Published: 25 April 2023.
Edited by:
Tobias Goldhammer, Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), GermanyReviewed by:
Timothy Nie Hunter, University of Leeds, United KingdomKyle Delwiche, University of California, Berkeley, United States
Copyright © 2023 Schwarz, Marcon and Lorke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Schwarz, c2Nod2Fyem1pY2hhZWxAdW5pLWxhbmRhdS5kZQ==
 Michael Schwarz
Michael Schwarz Lediane Marcon
Lediane Marcon Andreas Lorke
Andreas Lorke