- 1Independent Investigator, Chicago, IL, United States
- 2Applied Research and Innovation Services, Southern Alberta Institute of Technology, Calgary, AB, Canada
Reduced nitrogen compounds like ammonium or amines are ubiquitous constituents of wastewater. As a source of electrons, they can be oxidized without producing CO2. This makes them ideal for biogas upgrading in microbial power-to-gas processes in wastewater treatment plants as well as for energy storage. Here, we tested the hypothesis whether ammonium can be oxidized to N2 while producing energy-rich chemicals such as H2 or methane. First, we show that ammonium oxidation can be coupled to H2 production in microbial electrolysis cells. We show that with ammonium and water as the only sources of electrons, N2 gas was produced at potentials between +550 and +150 mV vs. a standard hydrogen electrode. Since H2 can neither be stored, nor transported without major upgrades of our infrastructure, we further tested the hypothesis whether wastewater nitrogen can be oxidized and used to produce methane. At a potential of +500 mV, N2 was produced from domestic wastewater while total nitrogen was removed. We compared two different types of anodes, graphite granule drums and carbon brushes, and found that both were comparable in terms of performance. The drums were slightly better in removing chemical oxygen demand, whereas the brushes produced methane faster. Our research shows that nitrogen contained in wastewater can replace water oxidation in electrolytic biogas upgrading.
Introduction
Nitrogen compounds are major contaminants in wastewater, such as domestic, agricultural, and industrial wastewater (Rajasulochana and Preethy, 2016). They comprise predominantly ammonium but also drugs and their degradation products (Petrie et al., 2015). At the same time, 1–2% of our energy is consumed in the Haber-Bosch process capturing this nitrogen from air to produce fertilizer (Chen et al., 2018). An additional 3% of our produced energy is then used to remove these and other compounds from wastewater (McCarty et al., 2011). This energy waste produces 5% of our energy related greenhouse gases (Rothausen and Conway, 2011). In contrast, wastewater can be an energy source (Shizas and Bagley, 2004). Indeed, a small part of its energy is recovered as biogas in anaerobic digesters (AD). During the AD process, organic matter in remnant wastewater sludge is converted into methane by concerted action of microbial communities with acetoclastic methanogenesis as terminal step:
In Equation (1), acetoclastic methanogenesis produces CO2 and methane in a stoichiometric ratio of 1:1. The CO2 reduces the biogas value to almost zero. In consequence, biogas is frequently flared off. One way to remove CO2 is to use CO2 scrubbers (Lindeboom et al., 2013). Alternative substrates can also improve the CO2:CH4 ratio (Siegert et al., 2014a). Nonetheless, carbon compounds will add CO2 to the produced biogas. Injecting H2 into ADs solves this problem (Luo and Angelidaki, 2012). The process is called biogas upgrading. Hydrogen can be generated by water electrolysis which requires expensive catalysts and high energy:
The reason is that water spitting electrolysis in Equation (2) occurs at a voltage of 1.23 V. One way to overcome this is to replace water as an electron donor by ammonium:
The reaction in Equation (3) takes place at only 136 mV with the respective energy savings (calculated from the half cell potentials which were taken from Bratsch, 1989). Besides the energy required for microbial electrolysis, other factors drive costs of BES, such as membranes (Rozendal et al., 2007), electrodes (Siegert et al., 2019), and control hardware (Siegert, 2018). The applied voltage to oxidize ammonium can be much lower than for water splitting with appropriate catalysts in place. In wastewater, such catalysts are microorganisms (Vilajeliu-Pons et al., 2018). Under anaerobic conditions, wastewater methanogens are active and consume the H2 produced:
The reaction in Equation (4) keeps the H2 concentration low, usually below 10 Pa (Thauer et al., 2008). Biocathodic methanogens belong to the genus Methanobacterium (Siegert et al., 2015), which thrives on such low H2 concentrations (Karadagli and Rittmann, 2007). By removing H2, these methanogens can make ammonium oxidation spontaneous:
The low energy gain in Equation (5) is owed to the small voltage of ΔEh = +33 mV of CO2 reduction compared with ammonium oxidation at pH 7 (Figure 1). It is hardly enough to provide the ΔG°′ = +31 kJ mol−1 necessary for ADP phosphorylation. Moreover, the nitrogen bond energy is innately high which requires strong oxidants like O2 (nitrification) or nitrite (anammox). Instead of strong oxidants, a positively poised electrode, e.g., at +500 mV, could provide the activation energy for ammonium oxidation. Such positive redox potentials do not, however, naturally occur in anaerobic environments. Here, we sought to test the hypothesis that ammonium oxidation can be coupled to hydrogenotrophic methanogensis by offering a positive electrode potential without O2. We also demonstrate that this process has an industrial application, such as wastewater treatment.
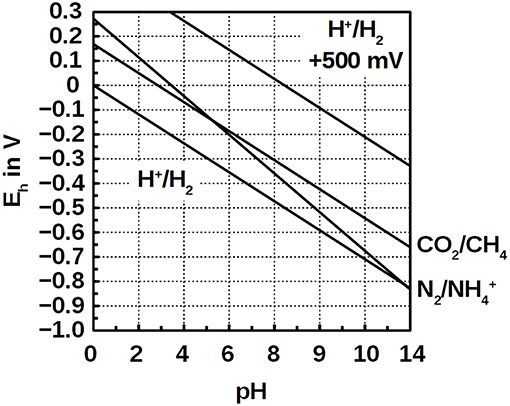
Figure 1. Pourbaix diagram of the redox couples H+/H2, N2/, CO2/CH4, and the hypothetical potential of H+/H2 at +500 mV (order from negative Eh to positive).
Materials and Methods
Microbial Electrolysis Reactor Setup
Two different experiments were conducted. First, ammonium oxidation coupled to hydrogen production was tested. In a second experiment, nitrogen removal from domestic wastewater coupled methane production was tested. For the first experiment, three simple H-cell reactors with Nafion® 117 membranes (effective area: 2.25 cm2) were used as we have described them previously (Siegert et al., 2014b). The cells contained 200 ml of artificial seawater (Siegert et al., 2014a) with 5 mM NH4Cl and water as the only sources of electrons. Each chamber had a head space of about 50 ml. Graphitized carbon brushes (4 × 4 cm) were used as bioanodes (Siegert et al., 2014b). Graphite blocks (2 × 2 × 0.32 cm) connected to titanium wires were used as cathodes. Ag/AgCl reference electrodes (RE-5B, BASi, West Lafayette, IN, USA) were inserted through the butyl rubber septa of the anode chambers. All potentials reported here are expressed vs. a standard hydrogen electrode which has an approximate offset potential to an Ag/AgCl electrode of +210 mV. The cells were operated in fed-batch cycles with the anodes poised at +550 mV for the first 2 cycles, and at +400, +200, or +150 mV, respectively for the last three cycles using a potentiostat. The total duration was ~600 days.
To demonstrate the applicability of bio-electrical ammonium oxidation in wastewater treatment, a second experiment was carried out using domestic wastewater. The experimental setup was similar to that of the first experiment with the exception that there were no membranes and that the cathodes were steel brushes (4 × 4 cm, Gordon Brush, City of Industry, CA, USA; Call et al., 2009). In one setup, the anodes were closed 4 × 4 cm titanium mesh drums filled with graphite granules, corresponding to the 4 × 4 cm carbon brushes. The electrolyte was domestic wastewater. The bio-anodes were poised at +500 mV during all fed-batch cycles. Ammonium was provided by the wastewater which usually contains no nitrate. Each cycle lasted until gas evolution ceased but no longer than 10 days. Prior to the start of all experiments, the head spaces were flushed with argon for at least 5 min. All wastewater experiments were carried out in triplicates except for the single open circuit control.
Inocula
The inocula for the first experiment were collected from the Atlantic Ocean floor off the coast of Namibia on R/V Meteor cruise M76/1 (Schippers et al., 2012). Living cultures were taken by using 1 ml of gravity core sediment, diluted 1/5 in on-site seawater, and stored over several years. The gravity cores 12803-1 SL (25°45.06S/13°04.20E, water depth 1,942 m, sediment depth 308 cm), 12808-2 SL (26°22.18S/11°53.49E, 3,796 m, 0–431 cm), and 12804-3 SL (27°44.13S/14°14.55E, 1,249 m, 8–88 cm) were used because they contained little organic carbon. They are henceforth referred to as reactors 1, 2, and 3. One ml of each sediment sample was used to inoculate 100 ml of each anode compartment. Every new batch cycle was re-inoculated with 10%v/v of the previous batch cycle resulting in a 1/10 dilution series.
In between batch cycles, the same transfer procedure was done using the wastewater inoculum. The wastewater inoculum was collected at Calgary's Fish Creek Wastewater Treatment Plant, usually during morning hours. The initial seed was enriched with 1% AD sludge to have a source of methanogens from the same plant. After that, AD sludge was not added anymore.
Analytical Procedures
Nitrogen, hydrogen, methane, and oxygen gas concentrations were measured using an SRI 310C gas chromatograph (SRI Instruments, Torrance, CA, USA) with a 6 foot molecular sieve column at 80°C. Chemical oxygen demand (COD) was determined using the HACH COD method 5220 (HACH Company, Loveland, CO, USA). Total nitrogen was measured using the Total Kjeldahl Nitrogen (TKN) method. Other nitrogen species were not determined. Currents and voltages were recorded using potentiostats. All means and standard deviations shown were calculated from the sub populations that remained in 97% confidence intervals of all fed-batch cycles.
Results and Discussion
To test whether ammonium can be oxidized at an anode, we used benthic microbial inocula from the Atlantic Ocean that were depleted in organic carbon (Schippers et al., 2012). In this experiment, N2 evolved in the anodic compartments of all three inoculated reactors with ammonium as the only source of nitrogen, suggesting that ammonium was oxidized to N2 (Figure 2A). At the same time, hydrogen gas evolved in the cathode compartment (Figure 2D). We repeated these experiments with the same set of reactors with similar results. Electrical current across the circuits increased with each batch cycle, even though the anodic potential was reduced (Figure S1). With ammonium and water as the only sources of electrons, hydrogen formation on the cathodes was the result of either ammonium or water oxidation on the anodes. No O2 accumulation was recorded during the experiments, indicating that water was not oxidized. At the anode potentials of +550 mV and with graphite and titanium as electrode material, the oxidation of water and hence the formation of intermediate oxygen is impossible because either the pH must be above 12 (Figure 1) or the partial pressure of O2 below 1 Pa. However, in a parallel O2 control which was a closed serum bottle with butyl rubber stopper that was flushed with argon for >1 h, the residual O2 concentration remained at ~340 Pa. In the electrolysis reactors, the O2 concentration was similar or slightly higher, indicating that the O2 partial pressure was sufficient to inhibit water oxidation. To further test this hypothesis, we reduced the anode potentials of all three reactors to +400, +200, and +150 mV where the O2 partial pressure must be below 0.01 Pa to allow water oxidation. We observed the same effect—implying that ammonium was oxidized anaerobically at the anodes. Moreover, trace O2 from the head spaces would have competed with the anodes as an electron acceptor for ammonium oxidation, bypassing the anodes without current and H2 generation. This was not the case (Figure 2D and Figure S4). While H2 formation during the +550 mV cycles increased, it was lower during the less positive cycles, showing that electrolysis also depended on the redox potential. However, H2 cycling between the cathode and the anode may also be an explanation (Lee and Rittmann, 2010). The coupling of N2 formation with H2 production at ≤ +550 mV suggests that ammonium was oxidized by anaerobic electrogenic microorganisms. The mechanism of this novel type of anaerobic ammonium oxidation is unclear as is the identity of the organisms. From similar experiments, it seems possible that, for example, Planctomycetes (Eyiuche et al., 2017) or Nitrosomonas (Vilajeliu-Pons et al., 2018) were involved.
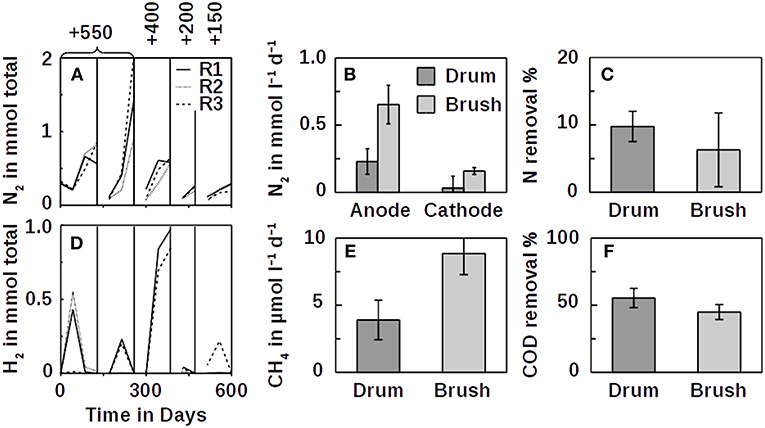
Figure 2. Anodic N2 accumulation (A, mineral medium; B, wastewater), nitrogen removal from wastewater (C), cathodic H2 production (D, mineral medium) and CH4 production (E, wastewater), and COD removal from wastewater (F). Nitrogen production of the drum reactors in (B) is shown with the open circuit control subtracted. No such control was performed for the brushes. Voltages are against a standard hydrogen electrode (SHE). Individual fed-batch cycles in (A,D) are separated by vertical lines. Poised potentials in (A,D) are shown on top vs. an SHE in mV. Lines connecting individual data points are not interpolations and merely indicate trends. The anode drum was a titanium mesh cylinder filled with graphite granules, and the anode brush was a conventional carbon brush. Errors are standard deviations of their means within 97% confidence intervals.
As shown in the above experiments using mineral medium, the oxidation of ammonium can be coupled to abiotic H2 production at cathodes (Figures 2A,D). To link H2 formation to methane production coupled to ammonium oxidation, we tested the possibility of using real domestic wastewater with an average COD of 0.3 g l−1. Because methane gas is a cheap commodity, we compared inexpensive graphite granules in titanium mesh drums to more expensive carbon brush anodes (Call and Logan, 2008). The N2 evolution rate in the electrical treatments of wastewater using these drums was about 1.3 times faster (1.3±0.1 mmol l−1 d−1, n = 6) compared with the open circuit controls (1.0±0.3 mmol l−1 d−1, n = 4). Dinitrogen gas also evolved more rapidly at the anodes than at the cathodes (Figure 2B). The ratio of NN2:NTKN was 8.4 ± 8.4 (n = 8) for the poised potential drum anodes (open circuit: 1.2 ± 0.2, n = 3) and 1.0 ± 0.9 (n = 6) for the brush anodes. Ideally, this ratio should be 1. The higher ratio for the drum anodes can be explained by out-gassing of entrapped nitrogen during the experiments or nitrogen impurities of the graphite. Initially, the COD in the drum reactors increased by about 20% (brushes 7%), indicating that impurities did initially contribute to the mass balance. With nitrate absent from wastewater under anaerobic conditions, the results suggest that at least some TKN was removed from the wastewater at the anode in an oxidative process forming N2 as shown in the first experimental setups. However, it seems also possible that some ammonium was oxidized independent from the anode, as the open circuit control indicated. These oxidation products, such as nitrite (Kostera et al., 2010), could have been denitrified to N2 at the cathode. Indeed, some N2 formed at the cathodes (Figure 2B), but might as well have diffused over from any of the anodes. Overall, the two anode types, drum and brush, were comparable in terms of their performance. While with the drums, nitrogen was removed slightly faster than with the brushes (Figure 2C), methane production was better using the brush anodes (Figure 2E). With 56 ± 8% COD removal (n = 5), the drums, however, performed better than the brushes which removed 45 ± 6% (n = 4; Figure 2F). Despite the removal of half the COD and 10% the TKN, only 1.3 ± 0.3% (n = 6) of the electrons were recovered as hydrogen and methane using the drums (brushes: 2.6 ± 0.3%, n = 7). While this explains the discrepancy in the nitrogen mass balance compared with methane (Figures 2B,E), the reason for this loss of electrons may be explained by competing processes involving iron cycling at the steel cathodes (Figure S5). The potential charge contribution of ammonium oxidation relative to COD removal was 28 ± 4% (n = 4) at the drum anodes (open circuit 22 ± 2%, n = 3), compared with 12 ± 3% (n = 6) at the brushes. The electron recovery rate indicates active redox processes that bypassed the circuit. As time progressed, however, more electrons were transferred across the electrical circuits (Figures S2, S3, S6).
In conclusion, our research shows that ammonium oxidation can be coupled to H2 and subsequent methane production. Different inocula and electrode designs can be used to carry out these processes. They are useful for bio-electric CO2 conversion as well as for wastewater treatment.
Author Contributions
MS invented the process, designed the experiments, collected the ocean samples and the data, prepared all figures, and wrote the manuscript. AT collected the wastewater samples and the data.
Funding
Parts of this research were funded by the Natural Sciences and Engineering Research Council of Canada Engage Grant for Colleges 508739-17, and the Deutsche Forschungsgemeinschaft (DFG) priority program IODP/ODP.
Conflict of Interest Statement
MS applied for patents on parts of this research under PCT/CA2018/051366 and PCT/IB2018/052671.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ken Omotani of ARIS at SAIT. We are also indebted to Calgary's Fish Creek Wastewater Treatment Plant staff and the R/V Meteor crew for being always supportive. The ocean samples were a gift from Prof. Axel Schippers of the Bundesanstalt für Geowissenschaften und Rohstoffe in Hannover, for which we owe him our debt of gratitude.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2019.00017/full#supplementary-material
References
Bratsch, S. G. (1989). Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 18, 1–21. doi: 10.1063/1.555839
Call, D., and Logan, B. E. (2008). Hydrogen production in a single chamber microbial electrolysis cell (MEC) lacking a membrane. Environ. Sci. Technol. 42, 3401–3406. doi: 10.1021/es8001822
Call, D., Merrill, M. D., and Logan, B. E. (2009). High surface area stainless steel brushes as cathodes in microbial electrolysis cells (MECs). Environ. Sci. Technol. 43, 2179–2183. doi: 10.1021/es803074x
Chen, J. G., Crooks, R. M., Seefeldt, L. C., Bren, K. L., Bullock, R. M., Darensbourg, M. Y., et al. (2018). Beyond fossil fuel–driven nitrogen transformations. Science 360:eaar6611. doi: 10.1126/science.aar6611
Eyiuche, N. J., Asakawa, S., Yamashita, T., Ikeguchi, A., Kitamura, Y., and Yokoyama, H. (2017). Community analysis of biofilms on flame-oxidized stainless steel anodes in microbial fuel cells fed with different substrates. BMC Microbiol. 17:145. doi: 10.1186/s12866-017-1053-z
Karadagli, F., and Rittmann, B. E. (2007). Thermodynamic and kinetic analysis of the H2 threshold for Methanobacterium bryantii M.o.H. Biodegradation 18, 439–452. doi: 10.1007/s10532-006-9073-7
Kostera, J., McGarry, J., and Pacheco, A. A. (2010). Enzymatic interconversion of ammonia and nitrite: the right tool for the job. Biochemistry 49, 8546–8553. doi: 10.1021/bi1006783
Lee, H.-S., and Rittmann, B. E. (2010). Significance of biological hydrogen oxidation in a continuous single-chamber microbial electrolysis cell. Environ. Sci. Technol. 44, 948–954. doi: 10.1021/es9025358
Lindeboom, R. E. F., Ferrer, I., Weijma, J., and van Lier, J. B. (2013). Silicate minerals for CO2 scavenging from biogas in autogenerative high pressure digestion. Water Res. 47, 3742–3751. doi: 10.1016/j.watres.2013.04.028
Luo, G., and Angelidaki, I. (2012). Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 109, 2729–2736. doi: 10.1002/bit.24557
McCarty, P. L., Bae, J., and Kim, J. (2011). Domestic wastewater treatment as a net energy producer – can this be achieved? Environ. Sci. Technol. 45, 7100–7106. doi: 10.1021/es2014264
Petrie, B., Barden, R., and Kasprzyk-Hordern, B. (2015). A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 72, 3–27. doi: 10.1016/j.watres.2014.08.053
Rajasulochana, P., and Preethy, V. (2016). Comparison on efficiency of various techniques in treatment of waste and sewage water – a comprehensive review. Resour. Technol. 2, 175–184. doi: 10.1016/j.reffit.2016.09.004
Rothausen, S. G. S. A., and Conway, D. (2011). Greenhouse-gas emissions from energy use in the water sector. Nat. Clim. Chang. 1, 210–219. doi: 10.1038/nclimate1147
Rozendal, R., Hamelers, H. V. M., Molenkamp, R. J., and Buisman, C. J. N. (2007). Performance of single chamber biocatalyzed electrolysis with different types of ion exchange membranes. Water Res. 41, 1984–1994. doi: 10.1016/j.watres.2007.01.019
Schippers, A., Kock, D., Höft, C., Köweker, G., and Siegert, M. (2012). Quantification of microbial communities in subsurface marine sediments of the Black Sea and off Namibia. Front. Microbiol. 3:16. doi: 10.3389/fmicb.2012.00016
Shizas, I., and Bagley, D. M. (2004). Experimental determination of energy content of unknown organics in municipal wastewater streams. J. Energy Eng. 130, 45–53. doi: 10.1061/(ASCE)0733-9402(2004)130:2(45)
Siegert, M. (2018). A scalable multi-channel software potentiostat. Front. Energy Res. 6:131. doi: 10.3389/fenrg.2018.00131
Siegert, M., Sitte, J., Galushko, A., and Krüger, M. (2014a). “Starting up microbial enhanced oil recovery,” in Geobiotechnology II, eds. A. Schippers, F. Glombitza, and W. Sand (Berlin: Springer), 1–94. doi: 10.1007/10_2013_256
Siegert, M., Sonawane, J. M., Ezugwu, C. I., and Prasad, R. (2019). “Economic assessment of nano-materials in bio-electrical water treatment,” in Advanced Research in Nanosciences for Water Technology, eds. R. Prasad, and T. Karchiyappan (Cham: Springer), 1–23. doi: 10.1007/978-3-030-02381-2_1
Siegert, M., Yates, M. D., Call, D. F., Zhu, X., Spormann, A., and Logan, B. E. (2014b). Comparison of nonprecious metal cathode materials for methane production by electromethanogenesis. ACS Sustain. Chem. Eng. 2, 910–917. doi: 10.1021/sc400520x
Siegert, M., Yates, M. D., Spormann, A. M., and Logan, B. E. (2015). Methanobacterium dominates biocathodic archaeal communities in methanogenic microbial electrolysis cells. ACS Sustain. Chem. Eng. 3, 1668–1676. doi: 10.1021/acssuschemeng.5b00367
Thauer, R. K., Kaster, A.-K., Seedorf, H., Buckel, W., and Hedderich, R. (2008). Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6, 579–591. doi: 10.1038/nrmicro1931
Keywords: bio-electrical system, anaerobic ammonium oxidation, microbial electrolysis cell, power-to-gas, biogas upgrading, wastewater treatment, methanogenesis, hydrogen evolution reaction
Citation: Siegert M and Tan A (2019) Electric Stimulation of Ammonotrophic Methanogenesis. Front. Energy Res. 7:17. doi: 10.3389/fenrg.2019.00017
Received: 14 November 2018; Accepted: 08 February 2019;
Published: 28 February 2019.
Edited by:
Lars Ditlev Mørck Ottosen, Aarhus University, DenmarkReviewed by:
Xu Wang, Wuhan University, ChinaPascal E. Saikaly, King Abdullah University of Science and Technology, Saudi Arabia
Copyright © 2019 Siegert and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Siegert, bWljaGFlbEBzaWVnZXJ0Lm9yZw==
 Michael Siegert
Michael Siegert Allen Tan
Allen Tan