- 1Plant Microbe Interaction Lab, National Institute of Plant Genome Research, New Delhi, India
- 2Plant Molecular Biology, International Center for Genetic Engineering and Biotechnology, New Delhi, India
- 3School of Life Sciences, Jawaharlal Nehru University, New Delhi, India
The imposition of environmental stresses on plants brings about disturbance in their metabolism thereby negatively affecting their growth and development and leading to reduction in the productivity. One of the manifestations of different abiotic and biotic stress conditions in plants is the enhanced production of reactive oxygen species (ROS) which can be hazardous to cells. Therefore, in order to protect themselves against toxic ROS, plant cells employ the anti-oxidant defense system. The ascorbate-glutathione pathway (Halliwell-Asada cycle) is an indispensible component of the ROS homeostasis mechanism of plants. This pathway entails the antioxidant metabolites: ascorbate, glutathione and NADPH along with the enzymes linking them. The ascorbate-glutathione pathway is functional in different subcellular compartments and all the enzymes of this pathway exist as multiple isoforms. The expression of different isoforms of the enzymes of ascorbate-glutathione pathway is developmentally as well as spatially regulated. Moreover, various abiotic and biotic stress conditions modulate the expression of the enzyme- isoforms differently. It is the intricate regulation of expression of different isoforms of the ascorbate-glutathione pathway enzymes that helps in the maintenance of redox balance in plants under various abiotic and biotic stress conditions. The present review provides an insight into the gene families of the ascorbate-glutathione pathway, shedding light on their role in different abiotic and biotic stress conditions as well as in the growth and development of plants.
Introduction
When plants are exposed to various biotic and abiotic stresses, they exhibit characteristic increase in the production of reactive oxygen species (ROS) like singlet oxygen (1O2), superoxide (O•−2), hydrogen peroxide (H2O2) and hydroxyl radical (OH•) (Mittler et al., 2004). These ROS are capable of causing uncontrolled oxidation of various cellular components that can lead to oxidative damage of the cell (Asada, 1999; Dat et al., 2000). Thus, enhanced production of ROS during stress can be hazardous to cells. ROS have also been acknowledged as central players in complex signaling cascades as they act as signals for the activation of various stress-responsive and defense pathways (Knight and Knight, 2001; Mittler et al., 2011). Apart from playing important roles in stress signaling, ROS like H2O2 are also involved in plants' growth and developmental processes like differentiation of cellulose rich cell wall, mediation of aleuronic cell death and stimulation of somatic embryogenesis (Neill et al., 2002; Slesak et al., 2007). Additionally, the transient accumulation of H2O2 following pathogen infection leads to localized programmed cell death or hypersensitive (HR) response and stimulates crosslinking of cell wall proteins, thereby preventing pathogen spread in the plant (Grant and Loake, 2000; Kuzniak and Skłodowska, 2005). Considering the ambivalent role of ROS, a delicate balance between their production and scavenging is of utmost importance for proper growth and development of plants.
Plants have an efficient anti-oxidant defense system which scavenges the excess ROS produced in the cell under different oxidative stress conditions. The anti-oxidant safe guard system in plants comprises of non-enzymatic and enzymatic components (Noctor and Foyer, 1998; Scandalios, 2005). The non-enzymatic components include the major cellular redox buffers: ascorbate (AsA) and glutathione (γ-glutamyl-cysteinyl-glycine, GSH) as well as tocopherol, flavonoids, alkaloids, and carotenoids (Arora et al., 2000; Grace and Logan, 2000; Foyer and Noctor, 2003; Gomathi and Rakkiyapan, 2011). The enzymatic components of the anti-oxidative defense system consist of a number of anti-oxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX) and the enzymes of ascorbate-glutathione (AsA-GSH) cycle namely, ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) (Mittler et al., 2004; Scandalios, 2005). AsA-GSH cycle forms the main H2O2-detoxification system operating in cytosol, chloroplasts and mitochondria of plant cells (Noctor and Foyer, 1998; Shigeoka et al., 2002; Mittler et al., 2004). Since the discovery of the AsA-GSH cycle in the mid-1970s, the enzyme-catalyzed reactions of this pathway have been studied intensively (Noctor and Foyer, 1998; Asada, 1999; Polle, 2001). Studies with mutants and transgenic plants over- or under-expressing enzymes or metabolites of the AsA-GSH pathway have proved very well the co-relation between this pathway and stress tolerance (Gill and Tuteja, 2010). The AsA-GSH cycle not only combats oxidative stress, but also plays an important role in other plant developmental processes (Chen and Gallie, 2006; Eastmond, 2007).
Each enzyme of the AsA-GSH pathway has various subcellular isoforms, which differ from each other with respect to their spatial and temporal expression. Moreover, these isoforms are differentially regulated by different types of stresses. For example, it has been found that under salt stress, various Oryza sativa APX (OsAPX) isoforms are differentially regulated. While some of them are characteristically up-regulated, the others are down-regulated at the same time (Texeira et al., 2006; Yamane et al., 2010). This suggests that the expression of different isoforms of the AsA-GSH pathway enzymes is under intricate regulation. However, the mechanisms underlying the regulation of these isoforms are not completely understood. The present review provides an overview of gene families encoding AsA-GSH pathway in plants and imparts an insight into their role in conferring tolerance to various abiotic and biotic stresses. A brief discussion on the functional importance of this pathway in growth and development of plants is also provided.
The Ascorbate-Glutathione (AsA-GSH) Pathway
The AsA-GSH pathway is composed of four enzymes namely, APX, MDHAR, DHAR and GR (Figure 1) and two anti-oxidants, AsA and GSH. APX, which is the first enzyme of the cycle, detoxifies H2O2 by bringing about the peroxidation of AsA and yielding monodehydroascorbate radical (MDHA). MDHA is either directly reduced back to AsA by MDHAR or undergoes non-enzymatic disproportionation to AsA and dehydroascorbate (DHA). The next step of the cycle involves DHAR mediated reduction of DHA into AsA using GSH as the reductant (Figure 1). DHA can undergo irreversible hydrolysis to 2, 3-diketogulonic acid, if not reduced by DHAR. Thus, DHAR helps in regeneration of AsA and plays an important role in maintaining the cellular AsA pool (Gallie, 2012). Like AsA, the regeneration of GSH is also important. GSH is regenerated from the oxidized glutathione dimers (GSSG) by NADPH-dependent GR (Gill and Tuteja, 2010). The concentration of AsA and GSH varies in different subcellular compartments of the cell (Table 1). Both the redox buffers are known to accumulate in certain cellular compartments under different abiotic stress conditions. The compartment specific role of both the buffers under abiotic stress conditions has been discussed exhaustively in recent reviews (Foyer and Noctor, 2011; Gest et al., 2013; Zechmann, 2014), and we do not focus on this aspect in the present review. The AsA-GSH cycle not only detoxifies toxic H2O2 but also contributes toward the maintenance of cellular AsA and GSH pools in different compartments of the cell. Existing in all the organelles, the AsA-GSH pathway protects the cell from the toxic effects of ROS generated under a variety of abiotic and biotic stresses (Anjum et al., 2010, 2014; Gill and Tuteja, 2010) (Figure 2).
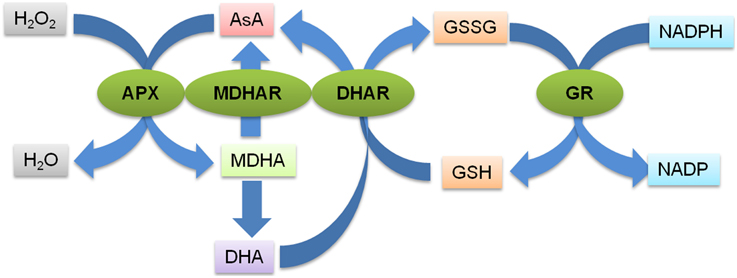
Figure 1. Schematic representation of the AsA-GSH pathway. The first step of the pathway is the detoxification of H2O2 by APX catalyzed peroxidation of AsA which generates MDHA. MDHA is either reduced back to AsA by MDHAR or it undergoes non-enzymatic disproportionation to AsA and dehydroascorbate (DHA). The DHA molecules are reduced to AsA by DHAR using GSH as the reductant. GSH is regenerated from the oxidized glutathione dimers (GSSG) by NADPH-dependent GR. The green ovals indicate the various enzymes catalyzing the different steps of the pathways (indicated by the blue arrows). APX, Ascorbate peroxidase; MDHAR, Monodehydroascorbate reductase; DHAR, Dehydroascorbate reductase; GR, Glutathione reducatse, AsA, Ascorbic acid; GSH, Glutathione; GSSG, oxidized glutathione dimer; MDHA, Monodehydroascorbate; DHA, Dehydroascorbate.
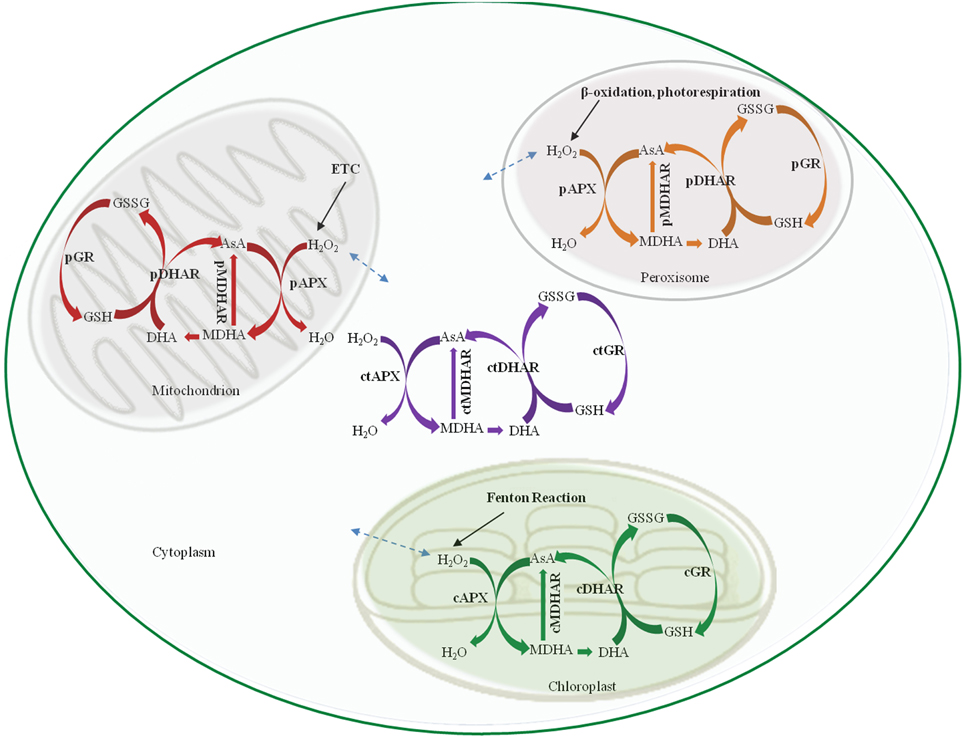
Figure 2. Schematic representation of the AsA-GSH pathway in different sub-cellular compartments. cAPX, cMDHAR, cDHAR, and cGR represent the chloroplastic isoforms; ctAPX, ctMDHAR, ctDHAR, and ctGR stand for the cytoplasmic, mAPX, mDHAR, and mGR indicate the mitochondrial isoforms and pAPX, pMDHAR, pDHAR, and pGR represent the peroxisomal isoforms. H2O2 can freely diffuse between the different organelle as indicated by broken arrows. ETC, Electron transport chain; AsA, Ascorbic acid; GSH, Glutathione; GSSG, oxidized glutathione dimer; MDHA, Monodehydroascorbate; DHA, Dehydroascorbate.
Ascorbate Peroxidase
APX (EC 1.11.1.11) is the first enzyme of the AsA-GSH pathway. It catalyzes the conversion of H2O2 to H2O and O2 using AsA as specific electron donor (Asada, 1999). APX, thus, prevents the accumulation of toxic levels of H2O2 in the cell. APX belongs to class I peroxidase family of proteins which are characterized by the presence of heme prosthetic groups. APXs are extremely sensitive to the concentration of AsA, which is reflected by the rapid decline in their activity at very low concentration (less than 20 μM) of AsA (Shigeoka et al., 2002). The enzyme has been identified in a number of higher plants, algae and cyanobacteria (reviewed in Caverzan et al., 2012). APX gene family comprises of different isoenzymes with different characteristics. Till date, five APX isoforms namely, cytosolic, mitochondrial, peroxisomal/glyoxysomal and chloroplastic have been identified in plants (Dąbrowska et al., 2007). In Arabidopsis thaliana, the reported eight isoenzymes of APX can be categorized into three groups: soluble cytosolic (APX1, APX2, and APX6), (Dąbrowska et al., 2007); Panchuk et al., 2002) (Table 2). Similarly, the identification of APX gene family in Lycopersicum esculentum revealed the presence of seven APX genes: three cytosolic, two peroxisomal, and two chloroplastic (Najami et al., 2008). In O. sativa, eight members of the APX gene family have been reported; encoding two cytosolic, two peroxisomal, three chloroplastic, and one mitochondrial isoforms (Texeira et al., 2004, 2006). Mitochondrial APX isoforms have also been reported in Solanum tuberosum and Pisum sativum (Jimenez et al., 1997; Leonardis et al., 2000).
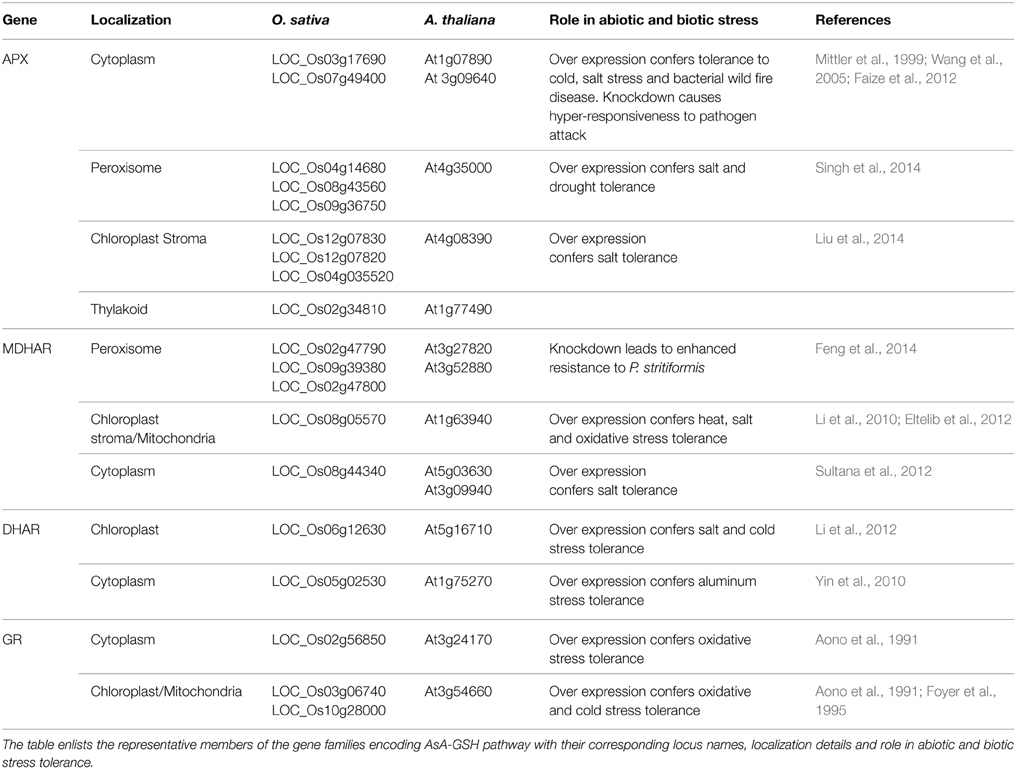
Table 2. AsA-GSH pathway gene families in A. thaliana and O.sativa and their role in abiotic and biotic stress tolerance.
The APX isoforms are stress sensitive and are regulated by nearly all kinds of abiotic and biotic stress conditions (Shigeoka et al., 2002). The expression of APX isoforms can be activated by specific factors such as pathogen attack, mechanical pressure, injury, ultraviolet (UVB) radiation, water deficiency, salt stress, low or high temperature, atmospheric pollution, and excess metal ions (reviewed in Shigeoka et al., 2002; Dąbrowska et al., 2007). The stress conditions also modulate the kinetic properties of the enzyme. For example, the exposure of A. thaliana wild type and flavonoid mutant (tt5) plants to UVB radiation led to a significant decrease in KAsAm as well as synthesis of new isoforms of cytosolic APX (Rao et al., 1996). The over-expression of APX has been shown to confer tolerance to various abiotic stresses (Xu et al., 2008; Sun et al., 2010; Sato et al., 2011). For example, Jatropha curcas plants over-expressing a chloroplastic APX were found to be salt tolerant (Liu et al., 2014). Similarly, over-expression of the peroxisomal APX from the halophyte Salicornia brachiata conferred salt and drought stress tolerance to transgenic Arachis hypogea plants (Singh et al., 2014). Transgenic L. esculentum plants over-expressing cytosolic APX exhibited improved tolerance to chilling, salinity, heat and UVB stress (Wang et al., 2005, 2006). A. thaliana vtc mutants deficient in AsA are reported to be hypersensitive to drought stress (Pastori et al., 2003; Faize et al., 2011).
Monodehydroascorbate Reductase
MDHAR (EC 1.6.5.4) recycles MDHA molecules into AsA. The exposure of plants to environmental stress conditions like high light leads to very quick oxidation of AsA to MDHA in chloroplast (Polle, 2001). It is, therefore, necessary for the survival of plants that MDHA is reduced back to regenerate AsA. In chloroplast, MDHA is reduced to AsA by photoreduced ferredoxin at a high rate and this is likely to constitute the main pathway of AsA regeneration in the vicinity of the thylakoid membrane. Away from the thylakoid membrane, reduction of MDHA can occur via two enzymes in the AsA-GSH pathway; DHAR and MDHAR (Asada, 1999). MDHAR reduces MDHA directly by using NAD(P)H as an electron donor. Alternatively, two molecules of MDHA can react non-enzymatically to generate AsA and DHA. The majority of MDHA is, however, found to be reduced by MDHAR (Polle, 2001). MDHAR enzyme activity is found across the entire plant and animal kingdom. Plant MDHARs exhibit high level of sequence similarity with prokaryotic flavoenzymes. MDHAR activities are reported to be present in algae (Haghjou et al., 2009), bryophytes (Lunde et al., 2006) and in all higher plants (Yoon et al., 2004; Leterrier et al., 2005). Higher plants' MDHARs belong to a multigene family constituting several sub-cellular isoforms. MDHAR activity has been detected in several cell compartments, such as chloroplasts, mitochondria, peroxisomes and cytosol (Jimenez et al., 1997; López-Huertas et al., 1999; Mittova et al., 2003; Kavitha et al., 2010). In A. thaliana, six isoforms of MDHAR are present among which two are peroxisomal, two are cytosolic and one is dually targeted chloroplastic/mitochondrial isoform (Lisenbee et al., 2005) (Table 2). The L. esculentum MDHAR family consists of three isoforms (Stevens et al., 2007). A total of three cytosolic isoforms of MDHARs have been reported in the moss Physcomitrella patens (Lunde et al., 2006). Physcomitrella apparently lacks any chloroplastic isoform indicating that AsA reduction in the plant exclusively occurs in cytosol (Drew et al., 2007).
In order to protect against the deleterious effects of ROS, the AsA pools are required to be maintained in a reduced state. Thus, ascorbate reductases like MDHARs, which are responsible for the reduction of AsA have considerable roles in stress tolerance. The activity of MDHAR proteins as well as MDHAR gene expression has been found to be differentially affected by various stress conditions. The increase in MDHAR activity has been reported in stress conditions like salinity, high light, UV radiation, boron toxicity and low temperature (Mittova et al., 2003; Cervilla et al., 2007). Transgenic studies have also confirmed the vital role of this enzyme in conferring tolerance to various abiotic stresses. For example, over-expression of A. thaliana MDHAR in Nicotiana tabacum enhanced tolerance of transgenic plants to ozone, salt and dehydration stress (Eltayeb et al., 2007). The over-expression of Acanthus ebracteatus cytoplasmic and Malpighia glabra chloroplastic MDHAR genes improved salt stress tolerance in O. sativa and N. tabacum, respectively (Eltelib et al., 2012; Sultana et al., 2012). Similarly, over-expression of chloroplastic MDHAR from L. esculentum and Avicennia marina, respectively, was shown to confer resistance to high temperature and methyl viologen-mediated oxidative stress in transgenic L. esculentum (Li et al., 2010) and to salt stress in transgenic N. tabacum plants (Kavitha et al., 2010).
Dehydroascorbate Reductase
AsA, which is a major anti-oxidant in plants, is oxidized to DHA via successive reversible electron transfers with MDHA as a free radical intermediate. DHA, so produced, is reduced to AsA by DHAR with GSH as an electron donor (EC 1.8.5.1). DHAR is the key enzyme to regenerate AsA. DHARs have been isolated and characterized from higher plants like A. thaliana, N. tabacum and agricultural crops such as oleracea, O. sativa and Pennisetum glaucum (Urano et al., 2000; Shimaoka et al., 2000; Ushimaru et al., 2006; Pandey et al., 2014). In A. thaliana five different DHARs (At1g19550, At1g19570, At1g75270, At5g36270, At5g16710) have been identified, with their presence either in an organelle (chloroplast or mitochondrion) or in the cytosol (Chew et al., 2003) (Table 2). Recently the At1g19570 isoform has been found to be associated with peroxisomes (Kataya and Reumann, 2010). Two different DHAR isoforms have been discovered in Spinacia oleracea leaves with one isoform located in chloroplasts whereas the other being present in the sub-cellular compartment other than chloroplasts (Shimaoka et al., 2000). DHAR activity has also been found in mitochondria, chloroplasts and peroxisomes of both leaf and root cells of the cultivated L. esculentum (M82) and its wild salt-tolerant relative, L. pennellii (Lpa) (Mittova et al., 2000). Two DHAR genes encoding for cytosolic and chloroplastic DHARs have also been identified in Eucalyptus spp. (Teixeira et al., 2005).
DHAR also plays an important role in abiotic stress tolerance and its expression is activated by a number of abiotic stress factors (Ali et al., 2005; Lu et al., 2008; Fan et al., 2014). Moreover, enhanced tolerance to various abiotic stresses was observed in plants over-expressing DHAR (Kwon et al., 2003; Ushimaru et al., 2006; Wang et al., 2010). For example, the over-expression of A. thaliana cytosolic DHAR has been shown to impart tolerance to aluminum stress in transgenic N. tabacum plants (Yin et al., 2010). In yet another report, it was shown that the over expression of DHAR which led to enhanced AsA accumulation conferred oxidative and salt stress tolerance to L. esculentum plants (Li et al., 2012). The simultaneous expression of chloroplastic O. sativa DHAR and E. coli GR in N. tabacum plants resulted in enhanced tolerance to salt and cold stress (Le Martret et al., 2011).
Additionally, DHAR plays an important role in plant growth and development (Chen and Gallie, 2006). The lack of DHAR resulted in the quick loss of AsA from O. sativa plants and led to slower rate of leaf expansion consequently affecting plant growth and development (Ye et al., 2000).
Glutathione Reductase
GR (NADPH: oxidized glutathione oxidoreductase; EC 1.6.4.2) maintains the cellular redox state by regenerating the reduced form of GSH, thereby, maintaining the balance between reduced GSH and AsA pools (Noctor and Foyer, 1998; Reddy and Raghavendra, 2006). GR is a flavo-protein oxidoreductase ubiquitously present in both prokaryotes and eukaryotes (Romeo-Puertas et al., 2006). The protein has been purified and characterized from a variety of organisms (Rao and Reddy, 2008; Achary et al., 2014). Although localized mainly in the chloroplasts, the enzyme is also found in cytosol (Edwards et al., 1990), mitochondria and peroxisomes (Jimenez et al., 1997; Romeo-Puertas et al., 2006).
Multiple isoforms of GR have been reported in a number of plants (Edwards et al., 1990; Lascano et al., 2001; Contour-Ansel et al., 2006; Rao and Reddy, 2008; Trivedi et al., 2013). Modulation in the expression profile of various GR isoforms have been known to occur under various stress conditions (reviewed in Yousuf et al., 2012; Gill et al., 2013). Transgenic N. tabacum plants over-expressing E. coli GR in the cytoplasm and chloroplast exhibited enhanced GR activity and tolerance to methyl viologen-mediated oxidative stress (Aono et al., 1991, 1993). Similarly, the over-expression of GR in chloroplasts of N. tabacum plants led to enhanced accumulation of GSH and AsA and the transgenic plants were found to be more tolerant to high light and chilling stress (Foyer et al., 1995). Overproduction of chloroplastic GR led to reduced photoinhibition under chilling stress in transgenic Gossypium hirsutum plants (Kornyeyev et al., 2003). Transgenic N. tabacum plants with reduced expression of GR were shown to display enhanced sensitivity to oxidative stress (Ding et al., 2009).
AsA-GSH Pathway in Chloroplasts
The AsA-GSH cycle plays a critical role in maintaining ROS homeostasis in chloroplasts. These organelles are devoid of catalases and the AsA-GSH cycle acts as the major H2O2 metabolizing pathway in these photosynthetic organelles. The photoreduction of O2 in chloroplast via photosystem–I (PSI) leads to the formation of superoxide ions, which rapidly dismutate to H2O2 spontaneously or by the action of superoxide dismutases (Asada, 1999). Chloroplasts contain relatively higher levels of AsA and GSH as compared to the other sub cellular organelles (Noctor and Foyer, 1998; Gest et al., 2013; Zechmann, 2014). Thus, the AsA-GSH pathway in chloroplast is imperative in protecting it from the deleterious effects of excess ROS production. Among the four enzymes of the AsA-GSH pathway in chloroplasts, the chloroplastic APX (chAPX) which consists of thylakoid (tAPX) and stromal (sAPX) isoforms scavenges the H2O2 generated during photosynthesis. The stromal and thylakoid-bound APXs have been identified and purified from several plant species (Ishikawa et al., 1996, 1998). tAPX is characterized by the presence of an extended C-terminal sequence that makes it 5 KDa larger than the sAPX (Asada, 1999). This sequence is responsible for binding of the protein to the membrane. sAPX has been shown to be predominantly important for photo-protection in young leaves. tAPX and sAPX isoforms are apparently functionally redundant and contribute to oxidative stress tolerance in chloroplasts. A sudden exposure to high light stress in tapx and sapx double mutant of A. thaliana led to a characteristic decline in the photochemical efficiency of PSII (Kangasjärvi et al., 2008). Likewise, the over-expression of tAPX in N. tabacum plants helped in maintaining photosynthetic efficiency of plants under high light and low temperature stress, thereby, substantiating the role of chloroplastic APX in stress resistance (Yabuta et al., 2002). The MDHA formed in the lumen by the oxidation of AsA disproportionates to DHA and moves into the stroma through the thylakoid membrane. MDHA produced by both stromal and thylakoid bound APX isoforms is reduced by stromal MDHAR. MDHAR has not been reported in the lumen of chloroplast (Obara et al., 2002). Along with the regeneration of AsA from MDHA, chloroplastic MDHAR also brings about the photo-reduction of dioxygen to O•−2 in absence of MDHA (Miyake et al., 1998; López-Huertas et al., 1999). DHAR and GR activities convert the DHA translocated from the lumen and the DHA generated in the stroma to AsA (Asada, 1999).
AsA-GSH Pathway in Mitochondria
The presence and activity of AsA-GSH cycle enzymes in mitochondria of plant cells have been established, and this cycle plays an important role in protecting mitochondrion against the toxic ROS regularly produced in respiratory chain reactions (Leonardis et al., 2000; Chew et al., 2003; Mittova et al., 2004; Lázaro et al., 2013). The mitochondrial AsA-GSH cycle deals with both photosynthetic as well as stress-induced oxidative stress (Jimenez et al., 1997). The mitochondrial AsA-GSH cycle also plays an important role in eliminating the mitochondrial-derived radicals, thereby protecting the heme of leghemoglobin in N2-fixing legume root nodules (Iturbe-Ormaetxe et al., 2001; Loscos et al., 2008). The mitochondrial APX is known to be membrane-localized in plants (Leonardis et al., 2000; Iturbe-Ormaetxe et al., 2001). The best collective evidence for the presence of MDHAR, DHAR, and GR in mitochondria is from P. sativum leaves (Jimenez et al., 1997) and Phaseolus valgaris nodules (Iturbe-Ormaetxe et al., 2001).
AsA-GSH Pathway in Cytoplasm
In A. thaliana, the cytosolic AsA-GSH pathway is characterized by the presence of one cytosolic APX (APX1), with an additional stress inducible APX (APX2) (Panchuk et al., 2002), along with the other enzymes (Mittler et al., 2004). It has been shown that the cytosolic APX imparts cross compartment protection of the other sub-cellular organellar APXs like mitochondrial APX, thylakoidal and stromal APXs hinting toward the fact that cytosolic AsA-GSH pathway plays an important role in protecting the other organelles during periods of stress (Davletova et al., 2005). Notably, cytosolic APX accounts for up to 0.9% of the total soluble protein of nodules and is particularly abundant in infected cells and nodule parenchyma of Medicago sativa (Dalton et al., 1998).
AsA-GSH Pathway in Peroxisome
Peroxisomes are single membrane-bound subcellular organelles being involved in production as well as the degradation of H2O2 and are sites for photorespiration, fatty acid β-oxidation, glyoxylate cycle and ureide metabolism (Corpas et al., 2001; Mano and Nishimura, 2005). The four enzymes of the AsA-GSH cycle, APX, MDHAR, DHAR and GR have been reported to be expressed in peroxisomes of roots and leaves of P. sativum and L. esculentum (Jimenez et al., 1997; Mittova et al., 2000; Leterrier et al., 2005). The presence of reduced AsA and GSH, and their oxidized forms, DHA and GSSG, respectively, was demonstrated by high performance liquid chromatography (HPLC) analysis in intact peroxisomes of P. sativum leaves (Jimenez et al., 1997). cDNAs encoding peroxisomal APX have been isolated from Gossypium spp. (Bunkelmann and Trelease, 1996), A. thaliana (Zhang et al., 1997) and S. oleracea (Ishikawa et al., 1998). The deduced amino acid sequence of peroxisomal APX has a high degree of identity with cytosolic APX, but it has a C-terminal amino acid extension containing a single, putative membrane−spanning region (Mullen et al., 1999). DHAR and GR were also found in the soluble fraction of peroxisomes, whereas membrane bound APX proteins have been shown to be present in P. sativum, Cucurbita maxima, and L. esculentum (Yamaguchi et al., 1995; Bunkelmann and Trelease, 1996; López-Huertas et al., 1999).
Role of Gene Families of AsA-GSH Pathway in Abiotic Stresses
Drought Stress
Drought stress leads to the production of ROS (mainly H2O2) in chloroplasts and mitochondria of plant cells (Dat et al., 2000). Drought stress causes varied effects on the enzymes of the AsA-GSH cycle, the response being dependent on the plant species, the developmental and metabolic state of plant, and the duration and intensity of the stress (Sofo et al., 2010). In majority of cases, drought stress led to an increase in the activity of enzymes of AsA-GSH cycle (Reddy et al., 2004; Sofo et al., 2005; Pukacka and Ratajczak, 2006; Bian and Jiang, 2009). For example, desiccation of recalcitrant seeds of Acer saccharinum was characterized by increased O−2 and H2O2 production, elevation in AsA and GSH contents as well as increased activity of the AsA-GSH enzymes (Pukacka and Ratajczak, 2006). Similarly, subjecting five Morus alba cultivars to drought stress led to an increase in the activity of AsA-GSH cycle enzymes (Reddy et al., 2004). During prolonged drought treatment in Prunus spp, the activities of the AsA-GSH enzymes were up-regulated, AsA/DHA ratio was decreased and the ratio of GSH/GSSG was increased suggesting an important role of the AsA-GSH pathway in combating drought stress (Sofo et al., 2005). Polyethylene glycol (PEG) induced drought stress to Cucumis sativus seedling roots led to increased activity of APX. However, the activities of DHAR and MDHAR first decreased (24 h) and then increased. The activity of GR was found to decrease at all time points (Fan et al., 2014). Drought stress differentially affected the antioxidant levels in the genotypes of plants which were contrasting with respect to drought tolerance. For example, the drought tolerant cultivars exhibited enhanced antioxidant enzyme activity under drought stress in comparison with sensitive cultivars of Dendranthema grandiflorum (Sun et al., 2013). The effect of drought stress on different isoforms of AsA-GSH cycle genes is extremely variable among different plant species. For example, drought stress was shown to decrease the activity of cytosolic isoform of APX whereas it led to increased activity of the chloroplastic isoform in Helianthus annuus. In the same study, it was shown that drought stress did not affect the activity of both the cytosolic and chloroplastic isoforms of APX in Sorghum bicolor (Zhang and Kirkham, 1996).
Salt Stress
In plants, salinity stress leads to cellular dehydration, which enhances the production of ROS causing oxidative stress and thereby leading to enhanced expression of ROS scavenging enzymes. The expression levels of all enzymes of AsA-GSH pathway have been shown to be affected by salt stress (Mittova et al., 2004; Jebara et al., 2005). However, activities of AsA-GSH pathway enzymes were found to be differentially altered by salinity stress in the salt tolerant and sensitive varieties. For example, O. sativa L. cv. Pokkali which is a salt-tolerant genotype, showed enhanced activity of AsA-GSH cycle enzymes, whereas, the salt-sensitive, O. sativa L. cv. BRRI dhan 29 exhibited decreased APX activity, increased DHAR activity and unchanged MDHAR and GR activity (Hossain et al., 2013). However, salinity stress in Triticum aestivum and O. sativa resulted in increased activities of MDHAR (Sairam et al., 2002; Vaidyanathan et al., 2003). All the isoforms of MDHAR, viz. mitochondrial, peroxisomal, chloroplastic, and cytosolic have been found to be sensitive to salt stress. For instance, salinity stress leads to increased activities of mitochondrial and peroxisomal MDHARs in Lycopersicon pennellii, which is a salt tolerant wild variety (Mittova et al., 2003). An increased GR activity has been reported in the roots and leaf of Cicer arientinum under salt stress (Eyidogan and Oz, 2005).
Temperature Stress
High temperature in plants enhances the generation of ROS, consequently inducing oxidative stress (Yin et al., 2008). Under high temperature, RuBisCO can lead to the enhanced production of H2O2 as a result of its oxygenase reaction (Kim and Portis, 2004). Tolerance to heat stress has been ascribed to elevated antioxidant enzymes' activity in many crop plants (Rainwater et al., 1996; Sairam et al., 2000; Sato et al., 2001; Rizhsky et al., 2002; Vacca et al., 2004; Almeselmani et al., 2006). The AsA-GSH pathway was found to be upregulated in response to heat stress in Malus domestica as reflected by increased gene expression and activities of APX, DHAR and GR enzymes (Ma et al., 2008). Under heat stress, the response of antioxidant enzymes activity varied amongst different genotypes of plants. For example, the analysis of gene expression of APX in a thermo-tolerant and thermo-susceptible variety of Brassica spp, T. aestivum, and Vigna radiata revealed increased activity of the enzyme under heat stress in all the genotypes. However, the elevation in transcript level was found to be higher in case of thermo-tolerant genotypes (Almeselmani et al., 2006; Rani et al., 2013). Heat stress induced elevation in transcript level of APX has also been reported in Poa pratensis by He and Huang (2007). Similar to APX, GR activity was also found to be enhanced by 50% in thermo-tolerant and 33% in thermo-susceptible genotypes of Brassica spp under heat stress (Rani et al., 2013). Exposure of N. tabacum cell suspension to elevated temperature (55°C) also resulted in increased GR activity (Locatto et al., 2009). However, Ma et al. (2008) reported the initial increase and then decrease in GR activity in M. domestica leaves during prolonged exposure to heat stress. The activities of DHAR and GR were also found to be increased under heat stress in temperature sensitive orchid Phalaenopsis (Ali et al., 2005). The activity of MDHAR was found to be repressed under heat stress in the same study. This study also indicated a differential effect of heat on the activity of the antioxidant enzymes in roots and shoots. For example, the activity of GR was doubled at 40°C in leaf but was drastically reduced in roots at the same temperature. The authors attributed the decrease in GR activity in roots to reduced availability of NADPH (Ali et al., 2005).
Similar to heat stress, low temperature stress also induces H2O2 production in cells (Suzuki and Mittler, 2006) and is known to up-regulate transcripts, protein level and activities of different ROS-scavenging enzymes (Prasad et al., 1994; Saruyama and Tanida, 1995; Sato et al., 2001). In Pinus spp, enhanced freezing tolerance during cold acclimation was characterized by elevated levels of APX, GR, MDHAR, and DHAR (Tao et al., 1998). In leaves of Eupatorium adenophorum, the activity of APX and GR increased with decreasing temperatures. However, upon cold stress treatment to leaves of thermo-tolerant E. odoratum, the activity of APX reached a peak value at 15°C and then declined, whereas GR activity was not affected. MDHAR activity in leaves of the cold-treated E. adenophorum was not significantly different from the controls, whereas the activity was found to be decreased in leaves of E. odoratum. DHAR activity in leaves of the two species was found to increase with both heat and cold stresses (Lu et al., 2008).
Role of Gene Families of AsA-GSH Pathway in Biotic Stress
The production of ROS constitutes one of the first responses of plant cells to infection (Torres et al., 2006). The apoplastic generation of ROS occurs mainly by enzymes like membrane NADPH-dependent oxidase, cell wall peroxidase or polyamine oxidases (Bolwell et al., 2002). ROS generated upon pathogen attack can either enhance the harmful effect of infection or may contribute to plant defense by causing hypersensitive response (Levine et al., 1994). ROS can also serve as signal molecules for the activation of local and systemic resistance (Grant and Loake, 2000; Kuzniak and Skłodowska, 2005). The ROS-mediated plant defense response is further more complex and is dependent on factors like the life style of pathogen (biotrophy/necrotrophy), the type of plant–pathogen interaction (compatible/incompatible interactions) and the stage of plant development (Govrin and Levine, 2000; Huckelhoven and Kogel, 2003). For maintaining ROS homeostasis, it becomes important to have an intricate and tightly regulated balance between ROS production and removal. Pathogen induced changes in antioxidant enzyme levels have been shown in a number of plants (Table 3). For example, in Hordeum vulgare leaves challenged with the powdery mildew fungus, Blumeria graminis, the fungal infection led to a significant decrease in APX and GR activity in whole-leaf extracts of resistant variety but caused no significant change in the susceptible one. However, there was no change in the activities of MDHAR and DHAR (Vanacker et al., 1998). Kuzniak and Skłodowska (2005) showed that Botrytis cinerea infection differentially affected the AsA-GSH gene families in L. esculentum. Upon infection, APX activity was found to increase in chloroplasts and decrease in mitochondria and peroxisomes 2 days after infection (dpi). The activity of peroxisomal MDHAR increased considerably at 1 dpi followed by subsequent decrease in activities of all MDHAR isoforms. A significant reduction in the activity of DHAR was observed in whole leaf extract at all time points. The chloroplastic DHAR activity was not affected, whereas the mitochondrial and peroxisomal DHAR activities were distinctly decreased starting from the third day after pathogen challenge. The GR activity on the other hand was found to increase in the chloroplasts. The peroxisomal and mitochondrial GR activities were repressed in response to infection by the pathogen. The decline in the activity of mitochondrial and peroxisomal isoforms points toward the “fungus-promoted precocious senescence” that led to the disease development (Kuzniak and Skłodowska, 2005). Similarly, Sesamum orientale plants, upon infection with the fungus Alternaria sesami displayed an initial increase in the activity of APX, MDHAR, and GR followed by a gradual decrease in the corresponding activities (Shereefa and Kumaraswamy, 2014). The expression of cytosolic MDHAR and DHAR was shown to be upregulated in A. thaliana seedlings co-cultivated with the root-colonizing endophytic fungus Piriformospora indica suggesting an important role of the enzyme in the maintenance of mutualistic plant- fungal interaction (Vadassery et al., 2009). However, knockdown of T. aestivum MDHAR resulted in improved resistance to Puccinia striiformis in wheat (Feng et al., 2014) suggesting that plants with compromised activity of the antioxidant enzymes have improved resistance against pathogens.
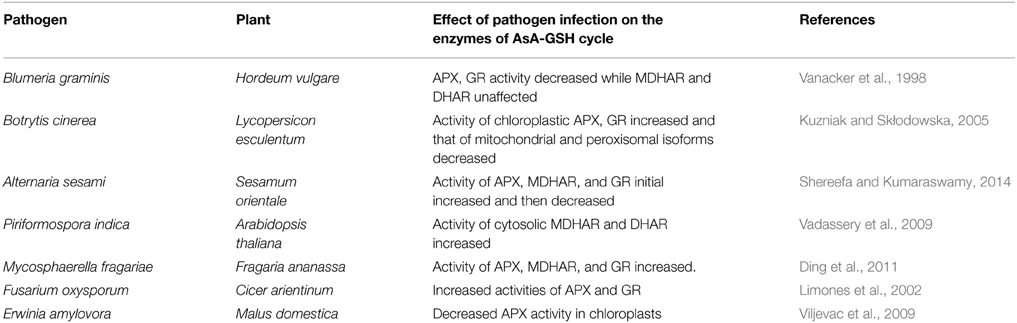
Table 3. Representative examples of modulation of plant antioxidant activities by different pathogens.
Role of Gene Families of AsA-GSH Pathway in Physiological and Developmental Processes of Plants
Apart from the important role in protecting the plants from the stress induced ROS, the enzymes of AsA-GSH pathway also play a part in growth and development of plants. AsA and GSH have been known to play important roles in organ developmental processes of plants (Arrigoni and De Tullio, 2002). The peroxisomal MDHAR in A. thaliana has been shown to be important in mobilization of lipid reserves during early growth following germination by removing H2O2 generated by β-oxidation (Eastmond, 2007). The transcript profiles of certain enzymes of the pathway are known to be spatially and developmentally regulated. Expression of A. thaliana cytosolic APX (APX1) in leaves and roots is relatively high as compared to the cytosolic APX2 isoforms (Panchuk et al., 2005; Hruz et al., 2008). A. thaliana apx1 mutant plants exhibit delayed development, late flowering and altered stomatal responses (Pnueli et al., 2003). The study of Correa-Aragunde et al. (2013) suggests the participation of APX1 in the redistribution of H2O2 accumulation during root growth and lateral root development in A. thaliana. The transcripts of APX1 in Ipomoea batata were detected clearly in leaves, weakly in stems, and not in non-storage and storage roots. The expression level appeared to be higher in mature leaves than in immature leaves, suggesting its growth-stage specific expression (Park et al., 2004). Expression of APX2, another cytosolic isoform was found to be limited to bundle sheath cells in leaves exposed to excess light (Fryer et al., 2003). Like APX, DHAR also plays an important role in developmental processes. It has been reported that suppression of DHAR expression results in a preferential loss of chlorophyll a and less CO2 assimilation, resulting in decreased rate of leaf expansion, reduced foliar dry weight and premature leaf aging. Furthermore, the over-expression of DHAR which led to reduced lipid peroxidation in the transgenic plants led to delayed leaf aging in O. sativa (Chen and Gallie, 2006).
Summary and Perspectives
Despite their deleterious effects, ROS at low concentrations play crucial roles in stress perception, regulation of photosynthesis, pathogen recognition, programmed cell death, and plant development. The antioxidant enzymes of AsA-GSH pathway help in maintaining ROS homeostasis in cells by avoiding the potential cytotoxicity of ROS and allowing them to function as signal molecules. Considering the different levels and intensities of AsA and GSH production in the different organelles of cell under normal and stress conditions, the regulation of antioxidant enzymes also differs. There are different subcellular isoforms of each of the antioxidant enzymes and each isoform differentially responds to different stress and developmental cues. The mechanism of regulation of each isoforms by different stresses and developmental stages is yet to be completely understood. Further studies are required to decipher the complex regulation of expression of different isoforms of the AsA-GSH pathway enzymes in order to bolster our understanding of ROS homeostasis in plants. Understanding the intricate regulation of the various isoforms under various stress conditions can facilitate deeper insights into the stress tolerance mechanism of plants. This will also help in designing better strategies for the development of plants with improved abiotic and biotic stress tolerance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge financial support from Department of Science and Technology to PP and Department of Biotechnology to AVM. The authors also deeply acknowledge the support from National Institute of Plant Genome Research and International Centre for Genetic Engineering and Biotechnology.
References
Achary, V. M., Reddy, C. S., Pandey, P., Islam, T., Kaul, T., and Reddy, M. K. (2014). Glutathione reductase a unique enzyme: molecular cloning, expression and biochemical characterization from the stress adapted C4 plant, Pennisetum glaucum (L.) R. Br. Mol. Biol. Rep. doi: 10.1007/s11033-014-3832-z. [Epub ahead of print].
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ali, M. B., Hahn, E. J., and Paek, K. Y. (2005). Effects of temperature on oxidative stress defense systems, lipid peroxidation and lipoxygenase activity in Phalaenopsis. Plant Physiol. Biochem. 43, 213–222. doi: 10.1016/j.plaphy.2005.01.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Almeselmani, M., Deshmukh, R. K., Sairam, R. K., Kushwaha, S. R., and Singh, T. P. (2006). Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 171, 382–388. doi: 10.1016/j.plantsci.2006.04.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anjum, N. A., Gill, S. S., Gill, R., Hasanuzzaman, M., Duarte, A. C., Pereira, E., et al. (2014). Metal/metalloid stress tolerance in plants: role of ascorbate, its redox couple, and associated enzymes. Protoplasma 251, 1265–1283. doi: 10.1007/s00709-014-0636-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anjum, N. A., Umar, S., and Chan, M. T. (2010). Ascorbate-Glutathione Pathway and Stress Tolerance in Plants. Dordrecht: Springer.
Aono, M., Kubo, A., Saji, H., Natori, T., Tanaka, K., and Kondo, N. (1991). Resistance to active oxygen toxicity of transgenic Nicotiana tabacum that expresses the gene for glutathione reductase from Escherichia coli. Plant Cell Physiol. 32, 691–698.
Aono, M., Kubo, A., Saji, H., Tanaka, K., and Kondo, N. (1993). Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol. 34, 129–135.
Arora, T., Byrem, M., Nair, M. G., and Strasburg, G. M. (2000). Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 373, 102–109. doi: 10.1006/abbi.1999.1525
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arrigoni, O., and De Tullio, M. C. (2002). Ascorbic acid, much more than just an antioxidant. Biochim. Biophys. Acta. 1569, 1–9. doi: 10.1016/S0304-4165(01)00235-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Asada, K. (1999). The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. doi: 10.1146/annurev.arplant.50.1.601
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bian, S., and Jiang, Y. (2009). Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of kentucky bluegrass in response to drought stress and recovery. Sci. Hort. 120, 264–270. doi: 10.1016/j.scienta.2008.10.014
Bolwell, G. P., Bindschedler, L. V., Blee, K. A., Butt, V. S., Davies, D. R., Gardner, S. L., et al. (2002). The apoplastic oxidative burst in response to biotic stress in plants: a tree component system. J. Exp. Bot. 53, 1367–1376. doi: 10.1093/jexbot/53.372.1367
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bunkelmann, J. R., and Trelease, R. N. (1996). Ascorbate peroxidase—a prominent membrane protein in oilseed glyoxysomes. Plant Physiol. 110, 589–598. doi: 10.1104/pp.110.2.589
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Caverzan, A., Passaia, G., Rosa, S. B., Ribeiro, C. W., Lazzarotto, F., and Margis-Pinheiro, M. (2012). Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 35, 1011–1019. doi: 10.1590/S1415-47572012000600016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cervilla, L. M., Blasco, B., Rìos, J. J., Romero, L., and Ruiz, J. M. (2007). Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to boron toxicity. Ann. Bot. 100, 747–756. doi: 10.1093/aob/mcm156
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, Z., and Gallie, D. R. (2006). Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 142, 775–787. doi: 10.1104/pp.106.085506
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chew, O., Whelan, J., and Millar, A. H. (2003). Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 278, 46869–46877. doi: 10.1074/jbc.M307525200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Contour-Ansel, D., Torres-Franklin, M. L., Cruz, D. E. C. M. H., D'Arcy-Lameta, A., and Zuily-Fodil, Y. (2006). Glutathione reductase in leaves of cowpea: cloning of two cDNAs, expression and enzymatic activity under progressive drought stress, desiccation and abscisic acid treatment. Ann. Bot. 98, 1279–1287. doi: 10.1093/aob/mcl217
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corpas, F. J., Barroso, J. B., and del Río, L. A. (2001). Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 6, 145–150. doi: 10.1016/S1360-1385(01)01898-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Correa-Aragunde, N., Foresi, N., Delledonne, M., and Lamattina, L. (2013). Auxin induces redox regulation of ascorbate peroxidase 1 activity by S- nitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J. Exp. Bot. 64, 3339–3349. doi: 10.1093/jxb/ert172
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dąbrowska, G., Kata, A., Goc, A., Szechyńska-Hebda, M., and Skrzypek, E. (2007). Characteristics of the plant ascorbate peroxidase family. Acta Biol. Cracov. Ser. Bot. 49, 7–17.
Dalton, D. A., Joyner, S. L., Becana, M., Iturbe-Ormaetxe, I., and Chatfield, J. M. (1998). Enhanced antioxidant defenses in the peripheral cell layers of legume root nodules. Plant Physiol. 116, 37–43. doi: 10.1104/pp.116.1.37
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dat, J., Vandenabeele, S., Vranová, E., Van Montagu, M., Inzé, D., and Van reusegem, F. (2000). Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 57, 779–795. doi: 10.1007/s000180050041
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Davletova, S., Rizhsky, L., Liang, H., Shengqiang, Z., Oliver, D. J., Coutu, J., et al. (2005). Cytosolic Ascorbate Peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17, 268–281. doi: 10.1105/tpc.104.026971
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ding, L., Charles, M. T., Carissea, O., Tsao, R., Dube, C., and Khanizadeh, S. (2011). Changes in ascorbate–glutathione pathway enzymes in response to Mycosphaerella fragariae infection in selected strawberry genotypes. Arch. Phytopathology Plant Protect. 44, 712–725. doi: 10.1080/03235400903266297
Ding, S., Lu, Q., Zhang, Y., Yang, Z., Wen, X., Zhang, L., et al. (2009). Enhanced sensitivity to oxidative stress in transgenic tobacco plants with decreased glutathione reductase activity leads to a decrease in ascorbate pool and ascorbate redox state. Plant Mol. Biol. 69, 577–592. doi: 10.1007/s11103-008-9440-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drew, D. P., Lunde, C., Lahnstein, J., and Fincher, G. B. (2007). Heterologous expression of cDNAs encoding monodehydroascorbate reductases from the moss, Physcomitrella patens and characterization of the expressed enzymes. Planta 225, 945–954. doi: 10.1007/s00425-006-0394-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eastmond, P. J. (2007). MONODEHYDROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and post germinative growth in Arabidopsis. Plant Cell 19, 1376–1387. doi: 10.1105/tpc.106.043992
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Edwards, E. A., Rawsthorne, S., and Mullineaux, P. M. (1990). Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 180, 278–284. doi: 10.1007/BF00194008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eltayeb, A. E., Kawano, N., Badawi, G. H., Kaminaka, H., Sanekata, T., Shibahara, T., et al. (2007). Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225, 1255–1264. doi: 10.1007/s00425-006-0417-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eltelib, H. A., Fujikawa, Y., and Esaka, M. (2012). Overexpression of the acerola (Malpighia glabra) monodehydroascorbate reductase gene in transgenic tobacco plants results in increased ascorbate levels and enhanced tolerance to salt stress. S. Afr. J. Bot. 78, 295–301. doi: 10.1016/j.sajb.2011.08.005
Eyidogan, F., and Oz, M. T. (2005). Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol. Plant 29, 485–493. doi: 10.1007/s11738-007-0059-9
Faize, M., Burgos, L., Faize, L., Petri, C., Barba-Espin, G., Díaz-Vivancos, P., et al. (2012). Modulation of tobacco bacterial disease resistance using cytosolic ascorbate peroxidase and Cu,Zn-superoxide dismutase. Plant Pathol. 61, 858–866. doi: 10.1111/j.1365-3059.2011.02570.x
Faize, M., Burgos, L., Faize, L., Piqueras, A., Nicolas, E., Barba-Espin, G., et al. (2011). Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J. Exp. Bot. 62, 2599–2613. doi: 10.1093/jxb/erq432
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fan, H. F., Ding, L., Du, C. X., and Wu, X. (2014). Effect of short-term water deficit stress on antioxidative systems in cucumber seedling roots. Bot. Stud. 55:46. doi: 10.1186/s40529-014-0046-6
Feng, H., Wang, X., Zhang, Q., Fu, Y., Feng, C., Wang, B., et al. (2014). Monodehydroascorbate reductase gene, regulated by the wheat PN-2013 miRNA, contributes to adult wheat plant resistance to stripe rust through ROS metabolism. Biochim Biophys Acta 1839, 1–12. doi: 10.1016/j.bbagrm.2013.11.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Foyer, C. H., and Noctor, G. (2003). Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 119, 355–364. doi: 10.1034/j.1399-3054.2003.00223.x
Foyer, C. H., and Noctor, G. (2011). Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155, 2–18. doi: 10.1104/pp.110.167569
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Foyer, C. H., Souriau, N., Perret, S., Lelandais, M., Kunert, K. J., Pruvost, C., et al. (1995). Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 109, 1047–1057. doi: 10.1104/pp.109.3.1047
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fryer, M. J., Ball, L., Oxborough, K., Karpinski, S., Mullineaux, P. M., and Baker, N. R. (2003). Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 33, 691–705. doi: 10.1046/j.1365-313X.2003.01656.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gallie, D. R. (2012). The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 64, 433–443. doi: 10.1093/jxb/ers330
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gest, N., Gautier, H., and Stevens, R. (2013). Ascorbate as seen through plant evolution: the rise of a successful molecule? J. Exp. Bot. 64, 33–53. doi: 10.1093/jxb/ers297
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gill, S. S., Anjum, N. A., Hasanuzzaman, M., Gill, R., Trivedi, D. K., Ahmad, I., et al. (2013). Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 70, 204–212. doi: 10.1016/j.plaphy.2013.05.032
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gomathi, R., and Rakkiyapan, P. (2011). Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int. J. Plant Physiol. Biochem. 3, 67–74. Available online at: http://www.academicjournals.org/journal/IJPPB/article-abstract/BF9CCD910257
Govrin, E. M., and Levine, A. (2000). The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 10, 751–757. doi: 10.1016/S0960-9822(00)00560-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grace, S. G., and Logan, B. A. (2000). Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. B Biol. Sci. 355, 1499–1510. doi: 10.1098/rstb.2000.0710
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grant, J. J., and Loake, G. J. (2000). Role of reactive oxygen intermediates and cognate redox signalling in disease resistance. Plant Physiol. 124, 21–29. doi: 10.1104/pp.124.1.21
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Haghjou, M. M., Shariati, M., and Smirnoff, N. (2009). The effect of acute high light and low temperature stresses on the ascorbate–glutathione cycle and superoxide dismutase activity in two Dunaliella salina strains. Physiol. Plant. 135, 272–280. doi: 10.1111/j.1399-3054.2008.01193.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
He, Y., and Huang, B. (2007). Protein changes during heat stress in three kentucky bluegrass cultivars differing in heat tolerance. Crop Sci. 47, 2513–2520. doi: 10.2135/cropsci2006.12.0821
Hossain, M. A., Ismail, M. R., Uddin, M. K., Islam, M. Z., and Ashrafuzzaman, M. (2013). Efficacy of ascorbate-glutathione cycle for scavenging H2O2 in two contrasting rice genotypes during salinity stress. Aust. J. Crop Sci. 7, 1801–1808.
Hruz, T., Laule, O., Szabo, G., Wessendorp, F., Bleuler, S., Oertle, L., et al. (2008). Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. 2008:420747. doi: 10.1155/2008/420747
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huckelhoven, R., and Kogel, K. H. (2003). Reactive oxygen intermediates in plant–microbe interactions: who is who in powdery mildew resistance? Planta 216, 891–902. doi: 10.1007/s00425-003-0973-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ishikawa, T., Yoshimura, K., Sakai, K., Tamoi, M., Takeda, T., and Shigeoka, S. (1998). Molecular characterization and physiological role of a glyoxysome-bound ascorbate peroxidase from spinach. Plant Cell Physiol. 39, 23–34. doi: 10.1093/oxfordjournals.pcp.a029285
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ishikawa, T., Sakai, K., Yoshimura, K., Takeda, T., and Shigeoka, S. (1996). cDNAs encoding spinach stromal and thylakoid bound ascorbate peroxidase, differing in the presence or absence of their 3'-coding regions. FEBS Lett. 384, 289–293. doi: 10.1016/0014-5793(96)00332-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Iturbe-Ormaetxe, I., Matamoros, M. A., Rubio, M., Dalton, D. A., and Becana, M. (2001). The antioxidants of legume nodule mitochondria. Mol. Plant-Microbe Interact. 14, 1189–1196. doi: 10.1094/MPMI.2001.14.10.1189
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jebara, S., Jebara, M., Limam, F., and Aouani, M. E. (2005). Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 162, 929–936. doi: 10.1016/j.jplph.2004.10.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jimenez, A., Hernandez, J. A., del Rio, L. A., and Sevilla, F. (1997). Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 114, 275–284.
Kangasjärvi, S., Lepisto, A., Hännikäinen, K., Piippo, M., Luomala, E. M., Aro, E. M., et al. (2008). Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem. J. 412, 275–285. doi: 10.1042/BJ20080030
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kataya, A. R., and Reumann, S. (2010). Arabidopsis glutathione reductase 1 is dually targeted to peroxisomes and the cytosol. Plant Signal. Behav. 5, 171. doi: 10.4161/psb.5.2.10527
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kavitha, K., George, S., Venkataraman, G., and Parida, A. (2010). A salt-inducible chloroplastic monodehydroascorbate reductase from halophyte Avicennia marina confers salt stress tolerance on transgenic plants. Biochimie 92, 1321–1329. doi: 10.1016/j.biochi.2010.06.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, K., and Portis, J. (2004). Oxygen-dependent H2O2 production by Rubisco. FEBS Lett. 571, 124–128. doi: 10.1016/j.febslet.2004.06.064
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Knight, H., and Knight, M. R. (2001). Abiotic stress signaling pathways: specificity and cross-talk. Trends Plant Sci. 6, 262–267. doi: 10.1016/S1360-1385(01)01946-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kornyeyev, D., Logan, B. A., Payton, P., Allen, R. D., and Holaday, A. S. (2003). Elevated chloroplastic glutathione reductase activities decrease chilling-induced photoinhibition by increasing rates of photochemistry, but not thermal energy dissipation, in transgenic cotton. Funct. Plant Biol. 30, 101–110. doi: 10.1071/FP02144
Kuzniak, E., and Skłodowska, M. (2005). Compartment-specific role of the ascorbate–glutathione cycle in the response of tomato leaf cells to Botrytis cinerea infection. J. Exp. Bot. 56, 921–933. doi: 10.1093/jxb/eri086
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kwon, S. Y., Choi, S. M., Ahn, Y. O., Lee, H. S., Lee, H. B., Park, Y. M., et al. (2003). Enhanced stress-tolerance of transgenic tobacco plants expressing a human DHAR gene. J. Plant Physiol. 160, 347–353. doi: 10.1078/0176-1617-00926
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lascano, H. R., Casano, L. M., Melchiorre, M. N., and Trippi, V. S. (2001). Biochemical and molecular characterization of wheat chloroplastic glutathione reductase. Biol. Plant 44, 509–516. doi: 10.1023/A:1013726200294
Lázaro, J. J., Jiménez, A., Camejo, D., Iglesias-Baena, I., Martí Mdel, C., Lázaro-Payo, A., et al. (2013). Dissecting the integrative antioxidant and redox systems in plant mitochondria. Effect of stress and S-nitrosylation. Front. Plant Sci. 4:460. doi: 10.3389/fpls.2013.00460
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Le Martret, B., Poage, M., Shiel, K., Nugent, G. D., and Dix, P. J. (2011). Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotech. J. 9, 661–673. doi: 10.1111/j.1467-7652.2011.00611.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leonardis, S., Dipierro, N., and Dipierro, S. (2000). Purification and characterization of an ascorbate peroxidase from potato tuber mitochondria. Plant Physiol. Biochem. 38, 773–779. doi: 10.1016/S0981-9428(00)01188-8
Leterrier, M., Corpas, F. J., Barroso, J. B., Sandalio, L. M., and del Rio, L. A. (2005). Peroxisomal Monodehydroascorbate Reductase. Genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiol. 138, 2111–2123. doi: 10.1104/pp.105.066225
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. J. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593. doi: 10.1016/0092-8674(94)90544-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, F., Wu, Q. Y., Sun, Y. L., Wang, L. Y., Yang, X. H., and Meng, Q. W. (2010). Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol. Plant. 139, 421–434. doi: 10.1111/j.1399-3054.2010.01369.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, Q., Li, Y., Li, C., and Yu, X. (2012). Enhanced ascorbic acid accumulation through overexpression of dehydroascorbate reductase confers tolerance to methyl viologen and salt stresses in tomato. Czech J. Genet. Plant Breed. 48, 74–86. Available online at: http://www.agriculturejournals.cz/web/cjgpb.htm?volume=48&firstPage=74&type=publishedArticle
Limones, C. G., Hervás, A., Navas-Cortés, J. A., Jiménez-Dıìaz, R. M., and Tenaa, M. (2002). Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp.ciceris. Physiol. Mol. Plant Pathol. 61, 325–333. doi: 10.1006/pmpp.2003.0445
Lisenbee, C. S., Lingard, M. J., and Trelease, R. N. (2005). Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant J. 43, 900–914. doi: 10.1111/j.1365-313X.2005.02503.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, Z., Bao, H., Cai, J., Han, J., and Zhou, L. (2014). A novel thylakoid ascorbate peroxidase from Jatropha curcas enhances salt tolerance in transgenic tobacco. Int. J. Mol. Sci. 15, 171–185. doi: 10.3390/ijms15010171
Locatto, V., DePinto, M. C., and De Gera, L. (2009). Different involvement of the mitochondrial, plastidal and cytosolic ascorbate glutathione redox enzymes in heat shock responses. Physiol. Plant. 135, 296–306. doi: 10.1111/j.1399-3054.2008.01195.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
López-Huertas, E., Corpas, F. J., Sandalio, L. M., and Del Río, L. A. (1999). Characterization of membrane polypeptides from pea leaf peroxisomes involved in superoxide radical generation. Biochem. J. 337, 531–536. doi: 10.1042/0264-6021:3370531
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Loscos, J., Matamoros, M. A., and Becana, M. (2008). Ascorbate and Homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol. 146, 1282–1292. doi: 10.1104/pp.107.114066
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lu, P., Sang, W. G., and Ma, K. P. (2008). Differential responses of the activities of antioxidant enzymes to thermal stresses between two invasive Eupatorium species in China. J. Integr. Plant Biol. 50, 393–401. doi: 10.1111/j.1744-7909.2007.00583.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lunde, C., Baumann, U., Shirley, N. J., Drew, D. P., and Fincher, G. B. (2006). Gene structure and expression pattern analysis of three monodehydroascorbate reductase (Mdhar) genes in Physcomitrella patens: implications for the evolution of the MDHAR family in plants. Plant Mol. Biol. 60, 259–275. doi: 10.1007/s11103-005-3881-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ma, Y. H., Ma, F. W., Zhang, J. K., Li, M. J., Wang, Y. H., and Liang, D. (2008). Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci. 175, 761–766. doi: 10.1016/j.plantsci.2008.07.010
Mano, S., and Nishimura, M. (2005). Plant peroxisomes. Vitam. Horm. 72, 111–154. doi: 10.1016/S0083-6729(05)72004-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittler, R., Herr, E. H., Orvar, B. L., van Camp, W., Willekens, H., Inzé, D., et al. (1999). Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc. Natl. Acad. Sci. U.S.A. 96, 14165–141670. doi: 10.1073/pnas.96.24.14165
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittler, R., Vanderauwera, S., Gollery, M., and Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittler, R., Vanderauwera, S., Suzuki, N., Tognetti, G. M. V. B., Vandepoele, K., Gollery, M., et al. (2011). ROS signaling: the new wave? Trends Plant Sci. 16, 300–309. doi: 10.1016/j.tplants.2011.03.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittova, V., Guy, M., Tal, M., and Volokita, M. (2004). Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 55, 1105–1113. doi: 10.1093/jxb/erh113
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittova, V., Tal, M., Volokita, M., and Guy, M. (2003). Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 6, 845–856. doi: 10.1046/j.1365-3040.2003.01016.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittova, V., Volokita, M., Guy, M., and Tal, M. (2000). Activities of SOD and the ascorbate-glutathione cycle enzymes in subcellular compartments in leaves and roots of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant. 110, 42–51. doi: 10.1034/j.1399-3054.2000.110106.x
Miyake, C., Schreiber, U., Hormann, H., Sano, S., and Asada, K. (1998). The FAD-enzyme monodehydroascorbate radical reductase mediates photoproduction of superoxide radicals in spinach thylakoid membranes. Plant Cell Physiol. 39, 821–829. doi: 10.1093/oxfordjournals.pcp.a029440
Mullen, R. T., Lisenbee, C. S., Miernyk, J. A., and Trelease, R. N. (1999). Peroxisomal membrane ascorbate peroxidase is sorted to a membranous network that resembles a subdomain of the endoplasmic reticulum. Plant Cell 11, 2167–2185. doi: 10.1105/tpc.11.11.2167
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Najami, N., Janda, T., Barriah, W., Kayam, G., Tal, M., Guy, M., et al. (2008). Ascorbate Gene family in tomato: its identification and characterization. Mol. Genet. Genom. 279, 171–182. doi: 10.1007/s00438-007-0305-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neill, S. J., Desikan, R., Clarke, A., Hurst, R. D., and Hancock, J. T. (2002). Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53, 1237–1247. doi: 10.1093/jexbot/53.372.1237
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Noctor, G., and Foyer, C. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 249–279. doi: 10.1146/annurev.arplant.49.1.249
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Obara, K., Sumi, K., and Fukuda, H. (2002). The use of multiple transcription starts causes the dual targeting of Arabidopsis putative monodehydroascorbate reductase to both mitochondria and chloroplasts. Plant Cell Physiol. 43, 697–705. doi: 10.1093/pcp/pcf103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Panchuk, I. I., Volkov, R. A., and Schoffl, F. (2002). Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 129, 838–853. doi: 10.1104/pp.001362
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Panchuk, I. I., Zentgraf, U., and Volkov, R. A. (2005). Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana. Planta 222, 926–932. doi: 10.1007/s00425-005-0028-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pandey, P., Achary, V. M., Kalasamudramu, V., Mahanty, S., Reddy, G. M., and Reddy, M. K. (2014). Molecular and biochemical characterization of dehydroascorbate reductase from a stress adapted C4 plant, pearl millet [Pennisetum glaucum (L.) R. Br]. Plant Cell Rep. 33, 435–445. doi: 10.1007/s00299-013-1544-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Park, S. Y., Ryu, S. H., Jang, I. C., Kwon, S. Y., Kim, J. G., and Kwak, S. S. (2004). Molecular cloning of a cytosolic ascorbate peroxidase cDNA from cell cultures of sweet potato and its expression in response to stress. Mol. Genet. Genomics. 271, 339–346. doi: 10.1007/s00438-004-0986-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pastori, G. M., Kiddle, G., Antoniw, J., Bernard, S., Veljovic-Jovanovic, S., Verrier, P. J., et al. (2003). Leaf vitamin C contents modulate plant defense transcripts and regulate genes controlling development through hormone signaling. Plant Cell 15, 939–951. doi: 10.1105/tpc.010538
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pnueli, L., Liang, H., Rozenberg, M., and Mittler, R. (2003). Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 34, 187–203. doi: 10.1046/j.1365-313X.2003.01715.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Polle, A. (2001). Dissecting the superoxide dismutase–ascorbate peroxidase–glutathione pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol. 126, 445–462. doi: 10.1104/pp.126.1.445
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prasad, T. K., Anderson, M. D., Martin, B. A., and Stewart, C. R. (1994). Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6, 65–74. doi: 10.1105/tpc.6.1.65
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pukacka, S., and Ratajczak, E. (2006). Antioxidative response of ascorbate glutathione pathway and metabolites to desiccation of recalcitrant Acer saccharinum seeds. J. Plant Physiol. 163, 1259–1266. doi: 10.1016/j.jplph.2005.10.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rainwater, D. T., Gossett, D. R., Millhollon, E. P., Hanna, H. Y., Banks, S. W., and Lucas, M. C. (1996). The relationship between yield and the antioxidant defense system in tomatoes grown under heat stress. Free Radic. Res. 25, 421–435. doi: 10.3109/10715769609149065
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rani, B., Dhawan, K., Jain, V., Chhabra, M. L., and Singh, D. (2013). High Temperature Induced Changes in Antioxidative Enzymes in Brassica juncea(L) Czern & Coss. Available online at: http://www.australianoilseeds.com/__data/assets/pdf_file/0003/6861/46_High_temperature_induced_changes_in_antioxidative_enzymes_in_Brassica_juncea.pdf (Accessed Decenber 28, 2013).
Rao, A. S. V. C., and Reddy, A. R. (2008). “Glutathione reductase: a putative redox regulatory system in plant cells,” in Sulfur Assimilation and Abiotic Stress in Plants, eds N. A. Khan, S. Singh, and S. Umar (Berlin: Verlag; Springer), 111–147.
Rao, M. V., Paliyath, G., and Ormrod, D. P. (1996). Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 110, 125–136. doi: 10.1104/pp.110.1.125
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reddy, A. R., Chaitanya, K. V., Jutur, P. P., and Sumithra, K. (2004). Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ. Exp. Bot. 52, 33–42. doi: 10.1016/j.envexpbot.2004.01.002
Reddy, A. R., and Raghavendra, A. S. (2006). “Photooxidative stress,” in Physiology and Molecular Biology of Stress Tolerance in Plants, eds K. V. M. Rao, A. S. Raghavendra, and K. J. Reddy (Netherlands: Springer), 157–186.
Rizhsky, L., Liang, H., and Mittler, R. (2002). The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 130, 1143–1151. doi: 10.1104/pp.006858
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Romeo-Puertas, M. C., Corpas, F. J., Sandalio, L. M., Leterrier, M., Rodriguez-Serrano, M., del Rio, L. A., et al. (2006). Glutathione reductase from pea leaves:response to abiotic stress and characterization of the peroxisomal isoenzyme. New Phytol. 170, 43–52. doi: 10.1111/j.1469-8137.2006.01643.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sairam, K., Rao, K. V., and Srivastava, G. C. (2002). Differential response of wheat genotypes to long-term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 163, 1037–1046. doi: 10.1016/S0168-9452(02)00278-9
Sairam, R. K., Srivastava, G. C., and Saxena, D. C. (2000). Increased antioxidant activity under elevated temperatures, a mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 43, 245–251. doi: 10.1023/A:1002756311146
Saruyama, H., and Tanida, M. (1995). Effect of chilling on activated oxygen-scavenging enzymes in low temperature-sensitive and – tolerant cultivars of rice (Oryza sativa L.). Plant Sci. 109, 105–113. doi: 10.1016/0168-9452(95)04156-O
Sato, Y., Masuta, Y., Saito, K., Murayama, S., and Ozawa, K. (2011). Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep. 30, 299–406. doi: 10.1007/s00299-010-0985-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sato, Y., Murakami, T., Funatsuki, H., Matsuba, S., Saruyama, H., and Tanida, M. (2001). Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J. Exp. Bot. 52, 145–151. doi: 10.1093/jexbot/52.354.145
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scandalios, J. G. (2005). Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 38, 995–1014. doi: 10.1590/S0100-879X2005000700003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shereefa, L. A. H., and Kumaraswamy, M. (2014). Reactive oxygen species and ascorbate–glutathione interplay in signaling and stress responses in Sesamum orientale L. against Alternaria sesami (Kawamura) Mohanty and Behera. J. Saudi Soc. Agri. Sci. doi: 10.1016/j.jssas.2014.04.007
Shigeoka, S., Ishikawa, T., Tamoi, M., Miyagawa, Y., Takeda, T., Yabuta, Y., et al. (2002). Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 53, 1305–1319. doi: 10.1093/jexbot/53.372.1305
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shimaoka, T., Yokota, A., and Miyake, C. (2000). Purification and characterization of chloroplast dehydroascorbate reductase from spinach leaves. Plant Cell Physiol. 41, 1110–1118. doi: 10.1093/pcp/pcd035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Singh, N., Mishra, A., and Jha, B. (2014). Ectopic over-expression of peroxisomal ascorbate peroxidase (SbpAPX) gene confers salt stress tolerance in transgenic peanut (Arachis hypogaea). Gene 547, 119–125. doi: 10.1016/j.gene.2014.06.037
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Slesak, I., Libik, M., Karpinska, B., Karpinski, S., and Miszalski, Z. (2007). The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim Pol. 54, 39–50. Available online at: http://psjd.icm.edu.pl/psjd/element/bwmeta1.element.bwnjournal-article-abpv54p39kz?q=aaf60356-3128-4d0a-b093-d57a82b6d4ee$1&qt=IN_PAGE
Sofo, A., Cicco, N., Paraggio, M., and Scopa, A. (2010). “Regulation of the ascorbate–glutathione cycle in plants under drought stress,” in Ascorbate-Glutathione Pathway and Stress Tolerance in Plants, eds N. A. Anjum, M. T. Chan, and S. Umar (Netherlands: Springer), 137–189.
Sofo, A., Dichio, B., Xiloyannis, C., and Masia, A. (2005). Antioxidant defences in olive trees during drought stress: changes in activity of some antioxidant enzymes. Funct. Plant Biol. 32, 45–53. doi: 10.1071/FP04003
Stevens, R., Buret, M., Duffe, P., Garchery, C., Baldet, P., Rothan, C., et al. (2007). Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations Plant Physiol. 143, 1943–1953. doi: 10.1104/pp.106.091413
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sultana, S., Khew, C. Y., Morshed, M. M., Namasivayam, P., Napis, S., and Ho, C. L. (2012). Overexpression of monodehydroascorbate reductase from a mangrove plant (AeMDHAR) confers salt tolerance on rice. J. Plant Physiol. 169, 311–318. doi: 10.1016/j.jplph.2011.09.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, J., Gu, J., Zeng, J., Han, S., Song, A. P., Chen, F. D., et al. (2013). Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 161, 249–258. doi: 10.1016/j.scienta.2013.07.015
Sun, W. H., Duan, M., Shu, D. F., Yang, S., and Meng, Q. W. (2010). Over-expression of StAPX in tobacco improves seed germination and increases early seedling tolerance to salinity and osmotic stresses. Plant Cell Rep. 29, 917–926. doi: 10.1007/s00299-010-0878-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Suzuki, N., and Mittler, R. (2006). Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol. Plant. 126, 45–51. doi: 10.1111/j.0031-9317.2005.00582.x
Tao, D. L., Oquist, G., and Wingsle, G. (1998). Active oxygen scavengers during cold acclimation of Scots pine seedlings in relation to freezing tolerance. Cryobiology 37, 38–45. doi: 10.1006/cryo.1998.2096
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Teixeira, F. K., Menezes-Benavente, L., Galvão, V. C., and Margis-Pinheiro, M. (2005). Multigene families encode the major enzymes of antioxidant metabolism in Eucalyptus grandis L. Genet. Mol. Biol. 28, 529–538. doi: 10.1590/S1415-47572005000400007
Texeira, F. K., Menezes-Benavente, L., Galvao, V. C., Margis, R., and Margis-Pinheiro, M. (2006). Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different sub cellular compartments. Planta 224, 300–314. doi: 10.1007/s00425-005-0214-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Texeira, F. K., Menezes-Benavente, L., Margis, R., and Margis-Pinheiro, M. (2004). Analysis of the molecular evolutionary history of the Ascorbate peroxidase gene family: inferences from the rice genome. J. Mol. Evol. 59, 761–770. doi: 10.1007/s00239-004-2666-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Torres, M. A., Jones, J. D. G., and Dang, J. L. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378. doi: 10.1104/pp.106.079467
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Trivedi, D. K., Gill, S. S., Yadav, S., and Tuteja, N. (2013). Genome-wide analysis of glutathione reductase (GR) genes from rice and Arabidopsis. Plant Signal. Behav. 8:e23021 doi: 10.4161/psb.23021
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Urano, J., Nakagawa, T., Maki, Y., Masumura, T., Tanaka, K., Murata, N., et al. (2000). Molecular cloning and characterization of a rice dehydroascorbate reductase. FEBS Lett. 466, 107–111. doi: 10.1016/S0014-5793(99)01768-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ushimaru, T., Nakagawa, T., Fujioka, Y., Daicho, K., Naito, M., Yamauchi, Y., et al. (2006). Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J. Plant Physiol. 163, 1179–1184. doi: 10.1016/j.jplph.2005.10.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vacca, R. A., de Pinto, M. C., Valenti, D., Passarella, S., Marra, E., and De Gara, L. (2004). Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco BrightYellow 2 cells. Plant Physiol. 134, 1100–1112. doi: 10.1104/pp.103.035956
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vadassery, J., Tripathi, S., Prasad, R., Varma, A., and Oelmüller, R. (2009). Monodehydroascorbate reductase 2 and dehydroascorbate reductase 5 are crucial for a mutualistic interaction between Piriformospora indica and Arabidopsis. J. Plant Physiol. 166, 1263–1274. doi: 10.1016/j.jplph.2008.12.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vaidyanathan, H., Sivakumar, P., Chakrabarty, R., and Thomas, G. (2003). Scavenging of reactive oxygen species in NaCl stressed rice (Oryza sativa L.) differential response in salt tolerant and sensitive varieties. Plant Sci. 165, 1411–1418. doi: 10.1016/j.plantsci.2003.08.005
Vanacker, H., Carver, T. L. W., and Foyer, C. H. (1998). Pathogen induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 117, 1103–1114. doi: 10.1104/pp.117.3.1103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Viljevac, M., Dugalic, K., Stolfa, I., Dermic, E., Cvjetkovic, B., Sudar, R., et al. (2009). Biochemical basis of apple leaf resistance to Erwinia amylovora infection. Food Technol. Biotechnol. 47, 281–287. Available online at: http://www.ftb.com.hr/index.php/archives/64-volume-47-issue-no-3/204
Wang, Y., Wisniewski, M., Meilan, R., Cui, M., and Fuchigami, L. (2006). Transgenic tomato (Lycopersicon esculentum) overexpressing cAPX exhibits enhanced tolerance to UV-B and heat stress. J. Appl. Hortic. 8, 87–90. Available online at: http://horticultureresearch.net/journal_pdf/200687-90.pdf
Wang, Y., Wisniewski, M., Meilan, R., Cui, M., Webb, R., and Fuchigami, L. (2005). Over-expression of cytosolic ascorbate peroxidase in tomato confers tolerance to chilling and salt stress. J. Am. Soc. Hortic. Sci. 130, 167–173. Available online at: http://journal.ashspublications.org/content/130/2/167.abstract?ijkey=637b016bf001ec4160f78db977ad2dc9e55f935d&keytype2=tf_ipsecsha
Wang, Z., Xiao, Y., Chen, W., Tang, K., and Zhang, L. (2010). Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J. Int. Plant Biol. 52, 400–409. doi: 10.1111/j.1744-7909.2010.00921.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xu, W. F., Shi, W. M., Ueda, A., and Takabe, T. (2008). Mechanisms of salt tolerance in transgenic Arabidopsis thaliana carrying a peroxisomal ascorbate peroxidase gene from barley. Pedosphere 18, 486–495. doi: 10.1016/S1002-0160(08)60039-9
Yabuta, Y., Motoki, T., Yoshimura, K., Takeda, T., Ishikawa, T., and Shigeoka, S. (2002). Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 32, 915–925 doi: 10.1046/j.1365-313X.2002.01476.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yamaguchi, K., Mori, H., and Nishimura, M. (1995). A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol. 36, 1157–1162.
Yamane, K., Mitsuya, S., Taniguchi, M., and Miyake, H. (2010). Transcription profiles of genes encoding catalase an ascorbate peroxidase in rice leaf tissues under salinity. Plant Prod. Sci. 13, 164–168. doi: 10.1626/pps.13.164
Ye, X. D., Al-Babili, S., Klöti, A., Zhang, J., Lucca, P., Beyer, P., et al. (2000). Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287, 303–305. doi: 10.1126/science.287.5451.303
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yin, H., Chen, Q. M., and Yi, M. F. (2008). Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul. 54, 45–54. doi: 10.1007/s10725-007-9227-6
Yin, L., Wang, S., Eltayeb, A. E., Uddin, M. I., Yamamoto, Y., Tsuji, W., et al. (2010). Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231, 609–621. doi: 10.1007/s00425-009-1075-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoon, H. S., Lee, H., Lee, I. A., Kim, K. Y., and Jo, J. (2004). Molecular cloning of the monodehydroascorbate reductase gene from Brassica campestris and analysis of its mRNA level in response to oxidative stress. Biochim. Biophys. Acta 1658, 181–186. doi: 10.1016/j.bbabio.2004.05.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yousuf, P. Y., Hakeem, K. U. R., Chandna, R., and Ahmad, P. (2012). “Role of glutathione reductase in plant abiotic stress,” in Abiotic Stress Responses in Plants, eds P. Ahmad and M. N. V. Prasad (New York, NY: Springer), 149–158.
Zechmann, B. (2014). Compartment-specific importance of glutathione during abiotic and biotic stress. Front. Plant Sci. 5:566. doi: 10.3389/fpls.2014.00566
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, J., and Kirkham, M. B. (1996). Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 132, 361–373. doi: 10.1111/j.1469-8137.1996.tb01856.x
Zhang, H., Wang, J., Allen, R. D., Nickel, U., and Goodman, H. M. (1997). Cloning and expression of an Arabidopsis gene encoding a putative peroxisomal ascorbate peroxidase. Plant Mol. Biol. 34, 967–971. doi: 10.1023/A:1005814109732
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: reactive oxygen species, abiotic stress, redox homeostasis, ascorbate-glutathione pathway, isoforms, gene families
Citation: Pandey P, Singh J, Achary VMM and Reddy MK (2015) Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 3:25. doi: 10.3389/fenvs.2015.00025
Received: 18 January 2015; Accepted: 13 March 2015;
Published: 31 March 2015.
Edited by:
Naser A. Anjum, University of Aveiro, PortugalReviewed by:
Brahma B. Panda, Berhampur University, IndiaAshwani Pareek, Jawaharlal Nehru University, India
Dibyendu Talukdar, Raja Peary Mohan College (Affiliated to University of Calcutta), India
Copyright © 2015 Pandey, Singh, Achary and Reddy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prachi Pandey, International Center for Genetic Engineering and Biotechnology, Aruna Asaf Ali Marg, New Delhi, Delhi 110067, IndiacHJhY2hpcG5keUBnbWFpbC5jb20=
 Prachi Pandey
Prachi Pandey Jitender Singh
Jitender Singh V. Mohan Murali Achary
V. Mohan Murali Achary Mallireddy K. Reddy
Mallireddy K. Reddy