- Institute for Environmental Sciences, University of Koblenz-Landau, Landau, Germany
Although agriculture dominates much of Europe's landscape, there is virtually no information on foraging activity of bats in different crops. Additionally little is known about pesticide exposure of bats and related effects and there are currently no specific regulatory requirements to include bats in European Union pesticide risk assessments for the registration of these chemicals although other mammals are considered. To evaluate the potential pesticide exposure of bats, we studied bat diversity and activity as well as the availability of aerial prey insects in different crops and semi-natural habitats in south-western Germany in a landscape dominated by agriculture. In 300 accumulated sampling nights more than 24,000 bat call sequences were acoustically recorded and, in parallel, almost 110,000 insects of suitable prey sizes were sampled by light traps. A total of 14 bat species were recorded, among them the locally rare and for Germany critically endangered northern bat (Eptesicus nilssonii) and the barbastelle (Barbastella barbastellum), all of them also occurring over agricultural fields. In comparison to agricultural habitats, higher activity levels in forest sites were only found for Myotis species but not for species of the genera Pipistrellus, Eptesicus and Nyctalus. There were no significant differences in the availability of aerial nocturnal insects between forest, meadow and agricultural habitats. Comparing the different agricultural crops, significantly fewer bat call sequences and lower numbers of nocturnal insects were collected above the vineyards compared to orchards, cereal and vegetable fields. Highest activity levels of all bat species were recorded above agricultural fields situated next to forests. Given the high bat activity levels recorded at several agricultural sites, among them orchard and vegetable fields both known for their high pesticide inputs, and the availability of suitable prey insects, we conclude that pesticide exposure via ingestion of contaminated insect prey is possible. This potential risk is currently not considered in the European pesticide risk assessment scheme.
Introduction
Carson's (1962) classic book Silent Spring has immortalized the detrimental effects of organochlorine pesticides on the environment in general and on birds in particular. In the 1960s and 1970s it was also demonstrated that these pesticides were responsible for significant mortality of some bat populations in Europe and the USA (e.g., Jefferies, 1972; Gelusco et al., 1976; Clark et al., 1978). The detrimental highly toxic and persistent pesticides have been replaced by modern pesticides in the European Union and many other countries in the 1970s and 1980s. In recent decades, however, applications of pesticides have even increased even more and, simultaneously, the agricultural landscape heterogeneity has been greatly reduced (Benton et al., 2003). Both aspects of agricultural intensification have been associated with further declines in biodiversity and are sometimes referred to as the Second Silent Spring (e.g., for farmland birds; Krebs et al., 1999). So far, little is known about the relative contribution of habitat loss and use of chemicals to the negative effects on biodiversity. Recently, Geiger et al. (2010) examined the impacts of several factors of agricultural intensification on EU level and identified the use of pesticides as the most consistent to have negative effects on species diversity.
The need for assessing the risk of pesticide exposure on non-target organisms is recognized by regulatory agencies such as the European Food and Safety Authority (EFSA). No authorisation for new pesticides is granted unless a risk assessment demonstrates that no risk for wildlife species occurs when the pesticide is applied under field conditions (European Food Safety Authority, 2009). The current procedure also includes a risk assessment for birds and mammals where, insectivorous mammals are represented by shrews (European Food Safety Authority, 2009). No reference at all is made to bats, although they are reported as being threatened by pesticides (e.g., O'Shea and Johnson, 2009) and comprise one-fifth off all European mammal species with a very specific ecology including hibernation and a low reproduction rate (only a single offspring per year). The reason for this omission is probably related to the scarcity of ecological data and limited knowledge about the occurrence and activity of bats in agricultural crops. However an ecological vulnerability analysis for wildlife using autecological information revealed that bats were among the most vulnerable taxa for studied pesticides (DeLange et al., 2009). Therefore information about the presence of an organism group in or near crops is crucial for the assessment of potential exposure and effects of pesticides.
To estimate the pesticide exposure of bats through ingestion of potentially contaminated insects (oral exposure) we therefore first need to know which species occur in which crop and to what extent. Hence, in this study we recorded bat activity and additionally the availability of nocturnal prey insects in a multitude of agricultural sites and compared them to simultaneous recordings (same sampling night) in nearby habitats know to be used for foraging such as forests and meadows. Furthermore, we examined if recorded bat activity in the agricultural landscape is related to habitat type (i.a. forest, forest edge and open landscape), crop, and nocturnal insect abundance.
Materials and Methods
Study Sites and Sampling
The study was conducted in an agricultural landscape in Rhineland-Palatinate, SW Germany around Landau (Pfalz; 49°11.9064′ N, 8°7.0152′ E). The climate of the region is characterized by an average annual temperature of 10°C and a precipitation of 700 mm. The sampling sites were distributed in 6 sampling areas, being at least 6 km apart from each other. Each sampling area comprised 10 sampling sites, 8 in agricultural fields and one sampling area situated in a forest and another one situated in a meadow (referred to as semi-natural habitats). These were used to compare the recorded activity levels of the examined agricultural fields to activity levels of habitats know to be used for foraging. To allow direct comparison of bat activity in the different habitats, all sites in an area were sampled simultaneously during one night. In order to consider temporal variability each area was surveyed 5 times, resulting in a total of 300 sampling nights (6 areas × 10 sites × 5 nights). All sites were located less than 2.5 km away from the closest village and the closest forest of each area, assuring they were within the home range of all native bat species having their roost sites in settlements or forests. The distance of 2.5 km is based on the foraging range of the common pipistrelle (Pipistrellus pipistrellus), the species with the shortest maximum distance (2.5 km) between foraging sites and roost sites among the native species (Racey and Swift, 1985; Dietz et al., 2007). Agricultural sampling sites (apple orchards, vineyards, cereal-, and vegetable fields) were chosen to reflect the coverage of the different crops in each sampling area. The forest sites were mixed deciduous forest of different age and stand structure with European beech (Fagus sylvatica) being the dominant tree species. The meadow sites were agricultural grasslands with differing management intensities. After analyzing the data we realized that proximity to forest has a high influence on bat activity. Two of the cereal sampling sites were situated 100 m away from a forest. Data of these sites were therefore separately analyzed and termed “forest edge.”
At each site, bat activity and nocturnal insect availability was assessed simultaneously, with the insect traps being at least 50 m away from the batcorders to avoid increased and biased bat activity pattern through attraction of the trap light. Batcorders and light traps were situated at least 40 m from the field edge. The recordings of bat activity and the sampling of nocturnal insects were performed from sunset to sunrise. In a few cases (n = 3) light traps did not work the whole night so that individual samples had to be rejected from the analysis. The study was conducted from the beginning of June until the end of August 2008, coinciding with the lactation period for most European bats (Vaughan et al., 1997). All sampling and recording was conducted in nights with temperatures above 16°C at sunset, no rain and a low wind speed (below 10 km/h).
Bat Activity Measurement
Acoustic measurement of bat activity is a reliable estimate of foraging activity (Russo and Jones, 2003). Bat activity was recorded by using 10 automatic stationary bat detector systems, so-called batcorders (ecoObs GmbH, Nürnberg, Germany) a method suitable to address spatial and temporal variation in bat activity pattern (Stahlschmidt and Brühl, 2012a). Batcorders were installed at a height of 3.5 m above ground and adjusted to the system's standard settings (Runkel, 2008). The sampling points were chosen in a way that assured uncluttered acoustic space within the detection radius of the system, i.e., 10 m (Runkel, 2008). The activity was measured as the number of recorded call-sequences per night. The software bcDiscriminator (ecoObs GmbH, Nürnberg, Germany) was used to automatically determine bat species by their specific calls and to exclude the non-bat recordings. All doubtful determinations were manually identified by using the software bcAnalyze and by comparing sonograms and oscillograms of the calls with images from Skiba (2009). For statistical analyses the individual bat calls were assigned to the following species groups since it was not possible to identify all calls with sufficient probability to species level: Pipistrellus, Eptesicus-Nyctalus and Myotis. Species of the first two groups are predominately aerial hawker while the recorded Myotis species are more adapted to high-clutter environments such as forests. The group Eptesicus-Nyctalus included two genera that have similar food preferences and are also acoustically very similar and cannot be discriminated always with certainty.
Insect Sampling
Simultaneously to the bat recording, we measured the availability of nocturnal aerial insects using unattended light traps. Each light trap consisted of two ultraviolet fluorescent tubes (12V, 15W), two crossed acryl glasses and a plastic bowl hanging below the light and filled with 2 L of water. Three drops of an odorless detergent was added to reduce surface tension and therefore minimize the escaping of caught insects (Hahn et al., 2017). Light traps were positioned at least 50 m within the crop field and installed at a height of 1.8 m. The used light traps have an attraction radius below 15 m as evaluated in previous experiments. To assure that only nocturnal insects were sampled, the traps were automatically activated at dusk and deactivated at dawn. Insects other than Diptera or macro-moths were identified to order, Diptera to sub-order and macro-moths to family level. Furthermore, insect size was measured individually and insects were assigned to defined size classes.
The prey size suitable for Pipistrellus-group is reported to be around 3 mm on average (Barlow, 1997) and mainly <5 mm (Beck, 1995). Thus, the main prey size was considered to be 2–5 mm. The species of the Eptesicus-Nyctalus group differ in their preferred prey, but all of them include small Diptera (the most frequently recorded insect group in our study) in their diet and generally seem to consume different insects in the proportions encountered (Dietz et al., 2007 and references therein). Therefore, insects larger than 2 mm of all orders were considered as potential prey for Eptesicus-Nyctalus. Not all recorded Myotis species are aerial hunters and their prey could not be assessed by the applied insect trapping method. Since it was not possible to identify all Myotis calls with sufficient probability to species level and, consequently to assign them to groups with similar prey preferences, they were excluded from this analysis of food availability and bat activity.
Statistical Analysis
Permutational multivariate analysis of variance (PERMANOVA Anderson, 2001) was used to assess differences in (1) activities of the bat groups (Pipistrellus; Eptesicus-Nyctalus, Myotis) between the different habitat types (forest, forest edge, open landscape), (2) activities of the bat groups between the examined open landscape habitats (meadow, vineyard, cereal fields, vegetable fields, orchards), and (3) the differences in nocturnal insect availability (insects of the size class 2–5 mm, all insects) between the habitats (forest, forest edge, meadow, vineyard, cereal fields, vegetable fields, orchards). PERMANOVA is a non-parametric method that can be used for univariate and multivariate questions. PERMANOVA is a routine for testing the simultaneous response of one or more variables to one or more factors in an analysis of variance (ANOVA) experimental design on the basis of any resemblance measure, using permutation methods. Analysis of variance with permutations (PerANOVA) was used since the data were not normally distributed.
The Euclidean dissimilarity measure was used as the distance metric with 999 permutations for the probability tests for the univariate analysis. The factors (habitat types, open landscape habitats, insect availability) were treated as fixed, the sampling replication were nested within sites. When a factor was identified as significant (at α = 0.05), post-hoc pairwise tests (t-test) were conducted, again using 999 permutations. Analyses were conducted using the software packages PRIMER 6 (version 6.1.13) and PERMANOVA+ (version 1.0.3).
Spearman's coefficient correlation was used to explore relationship between site specific and log transformed mean bat activities of Pipistrellus and Eptesicus-Nyctalus and availabilities of nocturnal insects of the size class 2–5 mm and total number of insects, respectively. These analyses were conducted using SPSS ver. 17 (SPSS, Chicago, USA).
Results
Bat Activity
In 300 sampling nights a total of 24,012 call sequences were recorded, corresponding to 14 species (Table 1). About 66.6% of them were assigned to Pipistrellus, 26.3% to Eptesicus-Nyctalus, 6.1% to Myotis, and 0.3% to Plecotus. Barbabastella barbastellus was only recorded 3 times. The remaining 0.6% sequences were unidentifiable and thus excluded from the analysis. By far the most detected species was Pipistrellus pipistrellus with 65.0% of all recorded call sequences.
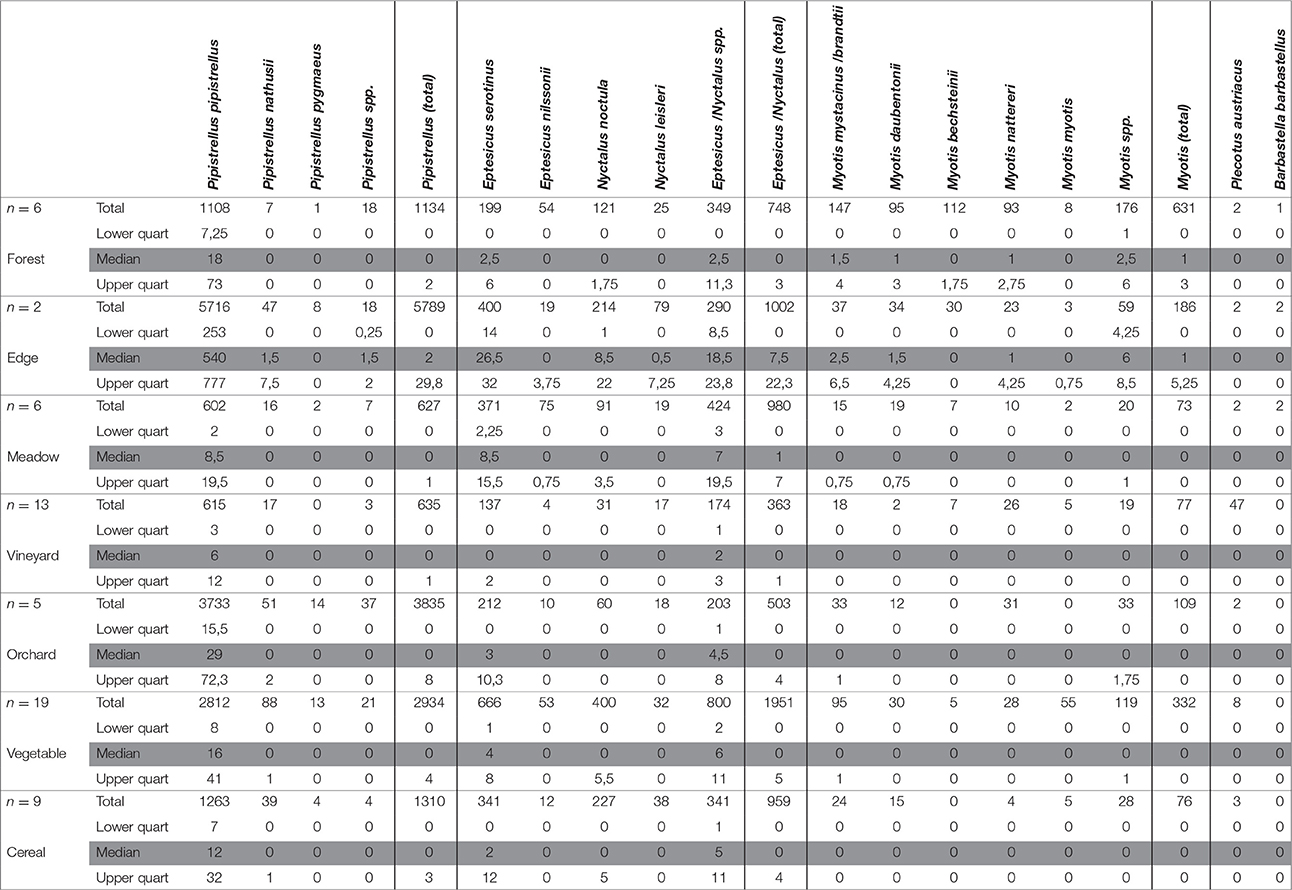
Table 1. Total number, median, and lower and upper quartile of bat call sequences per habitat of 5 sampling nights per site for all sites (forest, n = 6; forest edge, n = 2; meadow, n = 6; vineyard, n = 13; orchard, n = 5; vegetable, n = 19; cereal, n = 9).
Apart from the common pipistrelle, Nathusius's bat (Pipistrellus nathusii) and the midge bat (Pipistrellus pygmaeus) of the genus Pipistrellus were detected (Table 1). On average, the highest numbers of total Pipistrellus call sequences were recorded at forest edges, the lowest numbers above vineyards (Table 1). Relatively high numbers were detected in the orchards while forests, meadows, cereal and vegetable fields were used to similarly extents (Table 1).
In the species group Eptesicus-Nyctalus we recorded the serotine (Eptesicus serotinus), the northern bat (Eptesicus nilssonii), the noctule (Nyctalus noctula), and Leisler's bat (Nyctalus leisleri). The highest median numbers of call sequences of all Eptesicus-Nyctalus were recorded at the forest edges. For all species of that group similar activities were detected in forests and open landscape habitats (Table 1).
The differences in activity levels between habitat types (forest, forest edge, open landscape) were significant for the groups Pipistrellus and Eptesicus-Nyctalus (PERMANOVA: P > 0.005 in both cases). Pair-wise comparisons (PERMANOVA) showed no differences between open landscape and forest (P = 0.883 and P = 0.401, respectively), between forest edge and forest (P = 0.036 and P = 0.062, respectively) but between forest edge and open landscape (P = 0.005 and P = 0.003, respectively), caused by the high number of recorded call sequences for both groups at the forest edge habitats (Table 1).
Significant differences in activity patterns between the different habitats of the open landscape (crops and meadows) were also found for the groups Pipistrellus and Eptesicus-Nyctalus (PERMANOVA: P = 0.011 and P = 0.005, respectively). Pair-wise comparisons revealed that the vineyards differ in number of Pipistrellus call sequences from all other open landscape habitats (Table 2), revealing lowest activity levels. The same pattern was found for Eptesicus-Nyctalus with the exception that there was no difference in activity between the vineyards and orchards (Table 2).
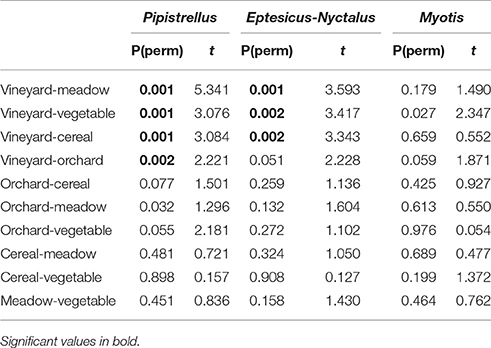
Table 2. Results of pairwise comparisons (PERMANOVA) of activity levels of the 3 bat groups (Pipistrellus, Eptesicus-Nyctalus, Myotis, see above) between the different open landscape habitats (meadow, vineyard, orchard. vegetable, cereal).
The genus Myotis was represented by the whiskered bat (Myotis mystacinus and brandtii), Daubenton's bat (Myotis daubentonii), Bechstein's bat (Myotis bechsteinii), Natterer's bat (Myotis nattereri) and the greater mouse-eared bat (Myotis myotis). All Myotis species showed high activity for forest and forest edge habitats with the exception of the greater mouse-eared bat with slightly higher activity over vegetable fields (Table 1). Bechstein's bat was almost exclusively recorded in forests and at forest edges (Table 1). Mean number of call sequences of the gray long-eared bat (Plecotus austriacus) was highest over vineyards (Table 1). The barbastelle (B. barbastellus) was only recorded twice at forest edges and once in a forest (Table 1).
Activity levels between habitat types were different for Myotis (PERMANOVA: P = 0.001). Pair-wise comparison (PERMANOVA) demonstrated no differences between forest edge and forest (P = 0.918) but between open landscape and forest (P = 0.001) and between open landscape and forest edge (P = 0.003) which could be attributed to the low activity levels recorded at the open landscape. No differences were found between Myotis call sequences at the different open landscape habitats (PERMANOVA: P = 0.162), which were lower compared to those in the forests and at the forest edges (Table 1).
When comparing the summed bat activity pattern for the five nights of all examined habitats which were simultaneously recorded in each sampling area, the highest activity levels were recorded at forest edges (sampling areas 1 and 2), over vegetable fields (sampling areas 3 and 4), an orchard (sampling area 5) and within a forest (sampling area 6).
Food Availability
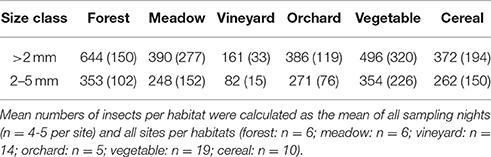
In total 109,264 insects with body size larger than 2 mm were trapped in 281 sampling nights (70,735 of them were assigned to the size class 2–5 mm). More than 70% of the sampled insects were assigned to the order Diptera. On average, the highest numbers of insects larger than 2 mm were recorded in forest habitats (Table 3). Numbers of insects of the size class 2–5 mm were highest in vegetable fields and forests (Table 3). For both size groups the lowest numbers of insects were collected in vineyards (Table 3). Availability of total nocturnal insects larger than 2 mm and insects of the size classes 2–5 mm, representing suitable prey for Eptesicus-Nyctalus and Pipistrellus, respectively, differed significantly between habitats (PERMANOVA: P = 0.002 and P = 0.001, respectively). Pair-wise comparisons revealed that this could be attributed to vineyards which differed from forest, meadow and other crops by lower insect abundances while no differences between the other three habitats were found (Table 4).
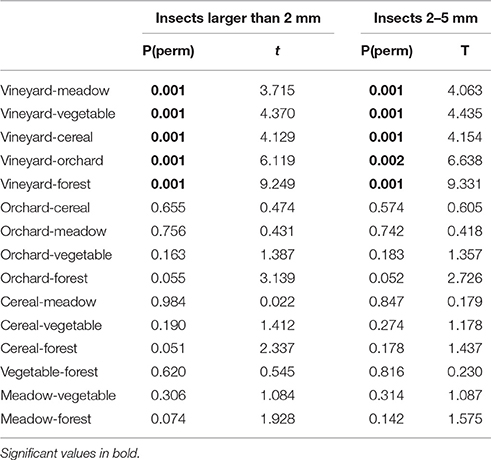
Table 4. Results of pairwise comparisons (PERMANOVA) of numbers of nocturnal insects (insects larger than 2 mm; insects sized between 2 and 5 mm) between the different habitats (forest, meadow, vineyard, orchard, vegetable, cereal).
A significant positive correlation was found between site specific Pipistrellus activity and insect availability of the size class 2–5 mm (rs = 0.340, p = 0.007, n = 60; Figure 1A) and site specific Eptesicus-Nyctalus activity and all insects larger than 2 mm (rs = 0.484, p = 0.001, n = 60, Figure 1B).
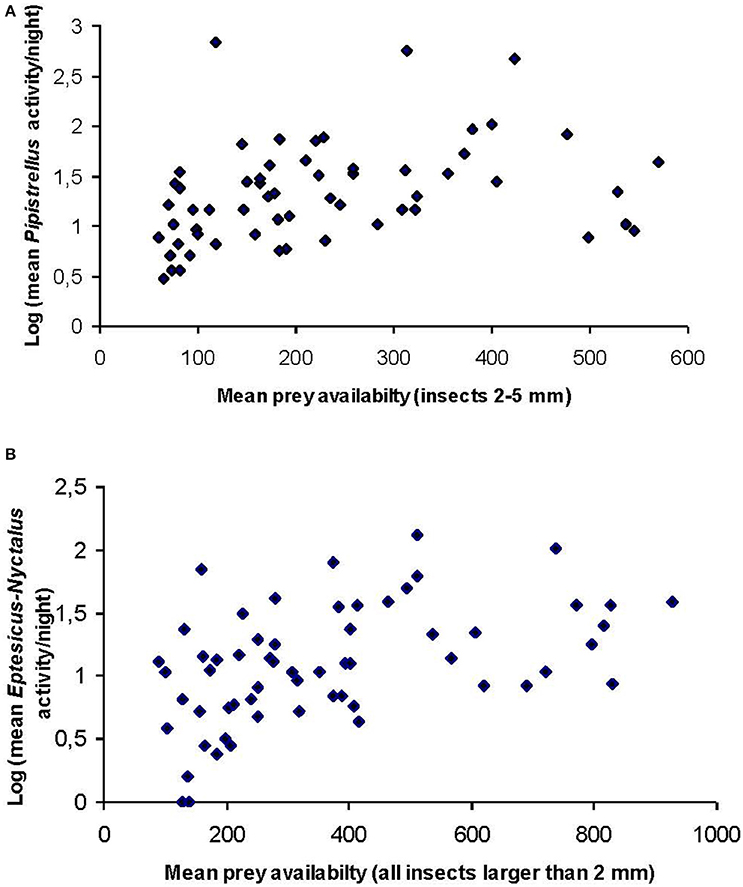
Figure 1. Scatter plots of site-specific mean (n = 5 nights per site) bat activity of (A) Pipistrellus and (B) Eptesicus-Nyctalus against site-specific availability of the corresponding prey groups. Data were log transformed to account for the high variability at sites and sampling nights.
Discussion
Farmland is the most widespread terrestrial wildlife habitat in Europe, covering 43% of the EU member states' surface area (Geiger et al., 2010). For bats, however, little is known about the role of agricultural crop fields as foraging habitats (Park, 2015). In contrary, the use of freshwater habitats or deciduous forests, both generally representing only small portions of most European landscapes, are well studied (e.g., Stahlschmidt et al., 2012; Zehetmair et al., 2015). Some studies have reported an avoidance of intensively managed agricultural fields by bats (Walsh and Harris, 1996; Vaughan et al., 1997). However, results of Vaughan et al. (1997) showed that bat activity levels over arable land in Great Britain were statistically lower for most bat species compared to their activities over water surfaces (i.e., rivers and lakes) but were comparable to the examined non-arable terrestrial habitats (different kinds of grassland and woodland). Water habitats are rare within most European agricultural landscapes while in contrast arable land constituting more than 40% of the available habitat (Walsh and Harris, 1996). Therefore, the predominant arable land, even if disproportionately more scarcely used by bats, may play an important and currently underestimated role as a foraging habitat. Wickramasinghe et al. (2003) compared bat activity across conventional and organic agricultural land and recorded higher activity on organic farms. However, subsequently, it was demonstrated that these differences were only due to higher activity over water habitats of the farms but not over land habitats (Davy et al., 2007). In one study in United Kingdom even higher bat activity levels were demonstrated on conventional farms when compared to farms using less intensive agricultural practices (Fuentes-Montemayor et al., 2011). Relatively large numbers of foraging attempts were recorded in some arable fields (Russo and Jones, 2003). Kalda et al. (2014) demonstrated the importance of woody habitats such as linear tree lines or solitary trees for bats in the agricultural landscape of Estonia. A recent study in Germany showed that open-space specialists foraged more intensively above agricultural fields during the migration period, while edge-space specialists foraged also during the energy demanding period of lactation (Heim et al., 2016). Here open-space and edge-space foraging migratory bats were recorded more often on farmland and arable fields than narrow-space foraging and regionally moving species which are able to forage within dense vegetation.
However, none of the aforementioned studies provides details about the crops in order to allow any conclusion about potential exposure of bats to pesticides. The present study is the first detailed investigation of the diversity and activity of European bats in different agricultural crops using a standardized approach that allows comparison to non-cropped semi-natural habitats.
Bat Activity
All 14 bat species recorded in the different habitats of the six sampling areas were also detected over agricultural fields, among them the northern bat, a species reported locally as facing extinction and the rare barbastelle which was not yet recorded in this region of Rhineland Palatinate (König and Wissing, 2007). Activity at a sampling site does not necessarily reflect its quality as a foraging habitat since quality is also reflected by the number of bat individuals present which depends on roost site availability and the distance to them. Therefore comparisons of site-specific activity levels of different habitat types on a large spatial scale are problematic (Hayes, 2000). However automated bat recording and our study design with several sampling sites in different habitats grouped in a sampling area within the home-range to potential roost sites (both housing and forests) for all occurring species, allows the direct comparison of activity levels between the different habitats.
The activity levels of the recorded species of the genera Pipistrellus, Eptesicus and Nyctalus, all of them being predominately aerial hawker, did not significantly differ between agricultural sites, forests and meadow habitats. Higher activity levels over agricultural fields than those in the simultaneously examined meadows and forests could even be demonstrated in several cases (fruit orchards, vegetable fields).
The activity levels of both aerial hawker groups (Pipistrellus and Eptesicus-Nyctalus) were correlated with suitable prey insect availability indicating that they use the agricultural sites for foraging. In accordance to the significant lower insect abundances found at the vineyards activity levels of the aerial hawkers were also significantly lower over vineyards compared to all other crop types.
In contrast, higher activity levels in the forests and significantly reduced activity in the open landscape were found for the Myotis species. Most of the recorded Myotis species are known to take their prey mainly (Natterer's and Bechstein's bat) or at least partly (Whiskered and Brandt's bat) by gleaning from vegetation (Dietz et al., 2007 and references therein). Bats using this foraging strategy are more adapted to high-clutter environments such as forests (e.g., in regards to their echolocation), but not to open landscape habitats (Aldridge and Rautenbach, 1987). Exceptions are the greater mouse-eared bat which almost exclusively feeds on carabid beetles and Daubenton's bat, a species adapted to take prey from water surfaces (Dietz et al., 2007 and references therein).
All examined bat groups showed remarkably high activity levels over agricultural fields located next to forests. Forest edges in general are known to be used for foraging by bat species that avoid navigating through structurally complex habitats as well as those that avoid the open landscape (Walsh and Harris, 1996; Morris et al., 2010).
Food Availability
Abundances of insects of the examined size classes did not differ between forest, meadow and most agricultural habitats. This appears to be in contrast to other studies reporting insect abundances and diversity being negatively associated with agricultural intensification (e.g., Benton et al., 2002; Wickramasinghe et al., 2004). However, we compared abundance of nocturnal insects with more than 70% being Diptera. Diptera are collected in our light trap design (water filled bowl as collector) whereas this group might be underrepresented in other designs. In a study by Nielsen et al. (1994) the occurrence of Diptera was not significantly impacted by pesticide use and, while tillage has been reported as a disturbance factor for terrestrial Diptera, some species are even specialized on the initial stages of succession after tillage (Frouz, 1999). Thus some Diptera species may be less affected by agricultural intensification and occur in high abundances in crop fields. The main factors affecting the occurrence of Diptera with terrestrial larval stages are the organic matter content and the moisture of the soil (Frouz, 1999). The soils of vegetable fields are especially rich in organic matter due to the remnants of the former crops (up to 3 different vegetable cultures per year). In combination with the presence of permanently wet soils due to irrigation, vegetable fields appear to provide the most suitable conditions of the examined crops for Diptera leading in several cases to insect abundances even exceeding those measured simultaneously at nearby forest sites. The soils of the cereals fields are also relatively rich in organic matter due to the remnants of former crops while the orchards are poorer in this regard. Vineyards, however, do not provide suitable conditions for most Diptera since their soils are rather dry.
Potential Exposure to Pesticides
Given the high bat activity levels recorded at several agricultural sites and the availability of suitable prey insects, an uptake of pesticides through consumption of potentially contaminated food items after pesticide application is possible. Especially high bat activity levels were recorded in several apple orchards, a crop known for high pesticide input. Commercial apple plantations in Germany received for example applications of 30 pesticides (22 fungicides and 8 insecticides) in 2007 (Roßberg and Harzer, 2015). Because of the vegetation structure suitable for gleaning, orchards were the only crop where Natterer's and Brandt's bat were recorded on a regular basis. Since the estimation of the exposure requires information on pesticide residues on bat-specific food items, a follow-up study (Stahlschmidt and Brühl, 2012b) was performed in one of the apple orchards where high bat activity levels were demonstrated. According to the preferences of the recorded bat guilds the residue pattern of different nocturnal arthropod groups were examined following applications of insecticides. The highest residue values were measured on foliage-dwelling arthropods which may result in a risk for all bat species that, even to a small extent, include foliage-dwelling arthropods in their diet (Stahlschmidt and Brühl, 2012b). Chlorpyrifos, the insecticide used in this exposure study, was also evaluated in the first toxicity study performed with a bat species (Big brown bat, Eptesicus fuscus) and flight impairment was one of the endpoints (Eidels et al., 2016). The authors conclude that the field relevant applications of this insecticide “could present bats with dietary concentrations consistent with adverse effects.”
Considering the high bat activity levels recorded over several vegetable fields indicating a good foraging habitat and the pesticide input in these crops (Roßberg, 2007), a study of pesticide residue patterns on nocturnal insects is strongly suggested to get a realistic estimate for the risk of pesticide exposure. The mean number of call sequences per night of the greater mouse-eared bat, a species almost exclusively feeding on carabid beetles (Beck, 1995), was highest above vegetable fields. Ground-dwelling arthropods such as carabid beetles may exhibit high pesticide residues especially after ground-directed applications in the afternoon. A massive die-off of juvenile greater mouse-eared bats which was attributed to the application of an organophosphate to potato fields and apple orchards in Germany (Hofmann, 1991) already demonstrated that this species is threatened by pesticide exposure. While in the orchards most of the airborne small insects were non-Diptera such as small moths (Hahn et al., 2017), Diptera were the predominant group in the vegetable fields. Since it has been shown that Diptera larvae can accumulate significant amounts of chemicals (Eitminavichiute et al., 1982; Park et al., 2009), food residue patterns in vegetable fields may differ from those measured in the orchard. Research is required to examine if such an accumulation of modern and less persistent pesticides takes place in Diptera developing in agricultural soils, especially in vegetable fields where wet soils may increase the contact of the larvae with pesticides.
Bat activity was rather low over the vineyards with the exception of the gray long-eared bat. While availability of nocturnal insects in general was lower in vineyards compared to the other agricultural habitats, higher abundances of nocturnal moths of the family Noctuidae (Hahn et al., 2017), on which the gray long-eared bat is almost exclusively preying (Bauerová, 1982), were recorded. In the residue study performed in the apple orchard (Stahlschmidt and Brühl, 2012b) large moths exhibited the lowest pesticide residues of all examined arthropods groups, revealing the lowest risk for bat species mainly feeding on them. Therefore, similar low residue pattern on the moths and a low risk for the gray long-eared bat feeding on them are expected in vineyards.
Remarkably high activity levels of all examined bat groups were detected over agricultural fields located next to forests. Given that in agricultural landscapes most forest edges are situated next to crop fields, a thorough examination of the potential pesticide exposure is necessary and special risk mitigation methods for those habitats may be required. Forest edges function as windbreaks which potentially could concentrate large densities of contaminated insects after pesticide application. The northern bat and the barbastelle were in this study predominantly recorded at the forest edges. Both are rare species and a potential risk due to pesticide exposure could have severe impacts on their populations. Research is also required if Bechstein's bat and the brown long-eared bat (Plecotus auritus), both forest inhabiting bats exclusively taking their prey by gleaning, are using orchards situated next to forests for foraging since a high risk is expected due to the elevated residue values of foliage-dwelling arthropods in orchards (Stahlschmidt and Brühl, 2012b).
Conclusion
The present study demonstrated that abundances of suitable prey insects for aerial hunting bats in orchards, vegetable and cereal fields are comparable to nearby forests and meadows, the latter known to be used as foraging habitats by bats. Since high bat activity was recorded in orchards and arable fields, crops that are known for elevated pesticide inputs, an exposure through ingestion of pesticide contaminated insects is especially likely. The following scenarios indicate a risk of pesticide exposure for bats: gleaners foraging in orchards, bats preying on soil arthropods in vegetable fields, aerial hawkers feeding on Diptera over vegetable fields, and bat species foraging along forest edges situated next to agricultural fields. In addition to studies on the pesticide contamination of bat food items as a basis for the development of a realistic risk assessment approach for this group, telemetry studies are needed to gain insights in individual foraging patterns in agricultural habitats.
Author Contributions
CB and PS designed the study. PS and MH collected the data. PS anaylsed the data. PS, MH, and CB wrote the manuscript.
Funding
We thank the Ministry of Science Rhineland-Palatinate for financial support in the project “Ecotoxicology in the agricultural landscape: from molecule to measures.”
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the farmers in the region for access to their land and local authorities for granting permission for road access. We are also thankful for the comments provided by five reviewers that helped to improve the manuscript.
References
Aldridge, H. D. J. N., and Rautenbach, I. L. (1987). Morphology, echolocation and resource partitioning in insectivorous bats. J. Anim. Ecol. 56, 763–778. doi: 10.2307/4947
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Barlow, K. E. (1997). The diets of two phonic types of the bat Pipistrellus pipistrellus in Britain. J. Zool. 243, 597–609. doi: 10.1111/j.1469-7998.1997.tb02804.x
Bauerová, Z. (1982). Contribution to the trophic ecology of the grey long-eared bat, Plecotus austriacus. Folia Zool. Brno. 31, 113–122.
Benton, T. G., Bryant, D. M., Cole, L., and Crick, H. Q. P. (2002). Linking agricultural practice to insect and bird populations: a historical study over three decades. J. Appl. Ecol. 39, 673–687. doi: 10.1046/j.1365-2664.2002.00745.x
Benton, T. G., Vickery, J. A., and Wilson, J. D. (2003). Farmland biodiversity: is habitat heterogeneity the key? TREE 18, 182–188. doi: 10.1016/S0169-5347(03)00011-9
Clark, D. R. Jr., LaVal, R. K., and Swineford, D. M. (1978). Dieldrin-induced mortality in an endangered species, the gray bat (Myotis griscescens). Science 199, 1357–1359. doi: 10.1126/science.564550
Davy, C. M., Russo, D., and Fenton, M. B. (2007). Use of native woodlands and traditional olive groves by foraging bats on a Mediterranean island: consequences for conservation. J. Zool. 273, 397–405. doi: 10.1111/j.1469-7998.2007.00343.x
DeLange, H. J., Lahr, J., Van der Pol, J. C., Wessels, Y., and Faber, J. H. (2009). Ecological vulnerability in wildlife: an expert judgement and multicriteria analysis tool using ecological traits to assess relative impact of pollutants. Environ. Toxicol. Chem. 28, 2233–2240. doi: 10.1897/08-626.1
Dietz, C., von Helversen, O., and Nill, D. (2007). Handbuch der Fledermäuse Europas und Nordwestafrikas. Stuttgart: Kosmos Naturführer.
Eidels, R. R., Sparks, D. W., Whitaker, J. O., and Sprague, C. A. (2016). Sub-lethal effects of chlorpyrifos on big brown bats (Eptesicus fuscus). Arch. Environ. Contam. Toxicol. 71, 322–335. doi: 10.1007/s00244-016-0307-3
Eitminavichiute, I. S., Strazdiene, V., Kadyte, B. A., and Vanagas, J. J. (1982). The accumulation of benzophosphate in soil animals. Pedobiologia (Jena). 24, 23–28.
European Food Safety Authority (2009). Guidance document on risk assessment for birds and mammals on request from EFSA. EFSA J. 7, 1438. doi: 10.2903/j.efsa.2009.1438
Frouz, J. (1999). Use of soil dwelling Diptera (Insecta, Diptera) as bioindicators: a review of ecological requirements and response to disturbance. Agric. Ecosys. Environ. 74, 167–186. doi: 10.1016/S0167-8809(99)00036-5
Fuentes-Montemayor, E., Goulson, D., and Park, K. J. (2011). Pipistrelle bats and their prey do not benefit from widely applied agri-environment management prescriptions. Biol. Conserv. 144, 2233–2246. doi: 10.1016/j.biocon.2011.05.015
Geiger, F., Bengtsson, J., Berendse, F., Weisser, W. W., Emmerson, M., Morales, M. B., et al. (2010). Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 11, 97–105. doi: 10.1016/j.baae.2009.12.001
Gelusco, K. N., Altenbach, J. S., and Wilson, D. E. (1976). Bat mortality: pesticide poisoning and migratory stress. Science 194, 184–186. doi: 10.1126/science.959845
Hahn, M., Brühl, C. A., and Stahlschmidt, P. (2017). Nocturnal insects in different crops and the potential food provision for bat species. Mainzer Naturwissenschaftliches Archiv. 53, 5–20.
Hayes, J. P. (2000). Assumptions and practical considerations in the design and interpretation of echolocation-monitoring studies. Acta Chiropterologica 2, 225–236.
Heim, O., Schröder, A., Eccard, J., Jung, K., and Voigt, C. C. (2016). Seasonal activity patterns of European bats above intensively used farmland. Agric. Ecosyst. Environ. 233, 130–139. doi: 10.1016/j.agee.2016.09.002
Hofmann, K. (1991). Vergiftung junger mausohren (Myotis myotis) durch Pflanzenschutzmittel. Nyctalus 4, 85–87.
Jefferies, D. J. (1972). Organochlorine insecticide residue in British bats and their significance. J. Zool. Lond. 166, 245–263. doi: 10.1111/j.1469-7998.1972.tb04088.x
Kalda, O., Kalda, R., and Liira, J. (2014). Multi-scale ecology of insectivorous bats in agricltural landscapes. Agric. Ecosyst. Environ. 199, 105–113. doi: 10.1016/j.agee.2014.08.028
Krebs, J. R., Wilson, J. D., Bradbury, R. B., and Siriwardena, G. M. (1999). The second silent spring? Nature 400, 611–612. doi: 10.1038/23127
Morris, A. D., Miller, D. A., and Kalcounis-Rueppell, M. C. (2010). Use of forest edges by bats in a managed pine forest landscape. J. Wildlife Manag. 74, 26–34. doi: 10.2193/2008-471
Nielsen, B. E., Nielsen, B. L., Axelsen, J., and Elmegaard, N. (1994). Winter abundance of soil Diptera larvae in arable soil. Pedobiologica 38, 208–221.
O'Shea, T. J., and Johnson, J. J. (2009). “Environmental contaminants and bats: investigating exposure and effects,” in Ecological and Behavioral Methods for the Study of Bats, 2nd Edn., eds T. H. Kunz and S. Parsons (Baltimore: Johns Hopkins University Press), 500–528.
Park, K. J. (2015). Mitigating the impacts of agriculture on biodiversity: bats and their potential role a bioindicators. Mamm. Biol. 80, 191–204. doi: 10.1016/j.mambio.2014.10.004
Park, K. J., Müller, C. T., Markman, S., Swinscow-Hall, O., Pascoe, D., and Buchanan, K. L. (2009). Detection of endocrine disrupting chemicals in aerial invertebrates at sewage treatment works. Chemosphere 77, 1459–1464. doi: 10.1016/j.chemosphere.2009.08.063
Racey, P. A., and Swift, S. M. (1985). Feeding ecology of Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) during pregnancy and lactation. I. Foraging behaviour. J. Appl. Ecol. 54, 205–215. doi: 10.2307/4631
Roßberg, D. (2007). NEPTUN oder “Wie oft wird gespritzt.” Gesunde Pflanze 59, 55–65. doi: 10.1007/s10343-007-0146-2
Roßberg, D., and Harzer, U. (2015). Survey on application of chemical pesticides in apple farming. J. Kulturpflanzen 67, 85–91. doi: 10.5073/JfK.2015.03.01
Runkel, V. (2008). Mikrohabitatnutzung Syntoper Waldfledermäuse. Ph.D. thesis. Universität Erlangen-Nürnberg.
Russo, D., and Jones, G. (2003). Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: conservation implications. Ecography 26, 197–209. doi: 10.1034/j.1600-0587.2003.03422.x
Skiba, R. (2009). Europäische Fledermäuse-Kennzeichen, Echoortung und Detektoran-Wedung. Die neue Brehm-Bücherei. Westarp Wissenschaften-Verlagsgesellschaft Hohenwarsleben. Magdeburg: VerlagsKG Wolf.
Stahlschmidt, P., and Brühl, C. A. (2012a). Bats as bioindicators–the need of a standardized method for acoustic bat activity surveys. Methods Ecol. Evol. 3, 503–508. doi: 10.1111/j.2041-210X.2012.00188.x
Stahlschmidt, P., and Brühl, C. A. (2012b). Bats at risk? Bat activity and insecticide residue analysis of food items in an apple orchard. Environ. Toxicol. Chem. 31, 1556–1563. doi: 10.1002/etc.1834
Stahlschmidt, P., Pätzold, A., Ressl, L., Schulz, R., and Brühl, C. A. (2012). Constructed wetlands support bats in agricultural landscapes. Basic Appl. Ecol. 13, 196–203. doi: 10.1016/j.baae.2012.02.001
Vaughan, N., Jones, G., and Harris, S. (1997). Habitat use by bats (Chiroptera) assessed by the means of a broad-band acoustic method. J. Appl. Ecol. 34, 716–730. doi: 10.2307/2404918
Walsh, A. L., and Harris, S. (1996). Foraging habitat preferences of vespertilionid bats in Britain. J. Appl. Ecol. 33, 508–518. doi: 10.2307/2404980
Wickramasinghe, L. P., Harris, S., Jones, G., and Jennings, N. V. (2004). Abundance and species richness of nocturnal insects on organic and conventional farms: effects of agricultural intensification on bat foraging. Conserv. Biol. 18, 1283–1292. doi: 10.1111/j.1523-1739.2004.00152.x
Wickramasinghe, L. P., Harris, S., Jones, G., and Vaughan, N. (2003). Bat activity and species richness on organic and conventional farms: impact of agricultural intensification. J. Appl. Ecol. 40, 984–993. doi: 10.1111/j.1365-2664.2003.00856.x
Keywords: chiroptera, crops, pesticide, risk assessment
Citation: Stahlschmidt P, Hahn M and Brühl CA (2017) Nocturnal Risks-High Bat Activity in the Agricultural Landscape Indicates Potential Pesticide Exposure. Front. Environ. Sci. 5:62. doi: 10.3389/fenvs.2017.00062
Received: 12 August 2016; Accepted: 22 September 2017;
Published: 18 October 2017.
Edited by:
Veerle L.B. Jaspers, Norwegian University of Science and Technology, NorwayReviewed by:
Markus Wagner, NERC Centre for Ecology & Hydrology, United KingdomRichard Shore, CEH, United Kingdom
Davi Castro Tavares, State University of Norte Fluminense, Brazil
Copyright © 2017 Stahlschmidt, Hahn and Brühl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carsten A. Brühl, YnJ1ZWhsQHVuaS1sYW5kYXUuZGU=
 Peter Stahlschmidt
Peter Stahlschmidt Melanie Hahn
Melanie Hahn Carsten A. Brühl
Carsten A. Brühl