Abstract
As documented by the numerous publications that have appeared in recent years, plastic pollution of the environment and the effects on the respective ecosystems are currently one of the most intensely discussed issues in environmental science and in society at large. Of special concern are the effects of micro- and nano-sized plastics. A key issue in understanding the fate and potential effects of micro- and nano-sized plastics is their dynamic nature, as the size, shape, and charge of the particles change over time. Moreover, due to various biological processes, such as the aggregation of organic material and/or bacteria (“biofouling”), the density of plastic particles that settle in the sediments of aquatic ecosystems may be several orders of magnitudes higher than that in the surrounding waters. Consequently, the risk posed by plastic pollution to benthic fauna is considerably high. Nonetheless, the vast majority of studies examining the effects of microplastics have focused on pelagic organisms so far. We therefore conducted a comprehensive literature review to examine the impact of micro- and nano-sized plastics on benthic invertebrates, including the physical and chemical effects of leaching and the interactions of plastic particles with contaminants. Overall, 330 papers were reviewed for their fulfillment of different criteria (e.g., test species, plastic material, particle shape, particle size, exposure concentration, exposure route, assay type, assay duration), with 49 publications finally included in our survey. A comprehensive gap-analysis on the effects of plastic particles on benthic invertebrates revealed a wide variety of effects triggered by micro- and/or nano-sized plastics but also distinct differences regarding the plastic materials tested, the size fractions applied, the shape of the respective particles, and the exposure routes tested. Our review concludes with a discussion of the important research gaps concerning freshwater ecosystems and recommendations for future areas of research.
Introduction
The pollution of aquatic ecosystems with plastic debris is regarded as one of the most serious environmental issues worldwide. Among this debris, small-sized particles have received increasing attention and are recently of particular concern (e.g., Thompson et al., 2004; Eerkes-Medrano et al., 2015; Rochman et al., 2016). These particles, termed nano- and microplastics, are generally defined by their largest dimensions of 0.001–0.1 μm and 0.1 μm−5 mm, respectively (e.g., Thompson et al., 2004; Moore, 2008) and are both major contributors to plastic pollution in marine as well as freshwater ecosystems (e.g., Thompson et al., 2004; Cole and Galloway, 2015; Chae and An, 2017). Generally, tiny particles could either be manufactured directly for various consumer and industrial applications, serving as primary sources of these particles, or could be derived from the fragmentation of larger plastic particles (e.g., Andrady, 2011; Browne et al., 2011).
An understanding of the environmental fate of small-scale plastic particles is fundamental for the assessment of their potential risks, but this is complicated by the fact that the size, shape, density, and charge of the particles constantly change over time (Galloway et al., 2017). Generally, several plastics, such as Polystyrene (PS), Polyvinylchloride (PVC), or Polyethylene terephthalate (PET), have a specific gravity higher than water, resulting in increased settling rates of these plastic classes in sediments, while plastics with lower densities, such as Low-density polyethylene (LDPE), High-density polyethylene (HDPE), or Polypropylene (PP), are suspected to mainly float in the water column (e.g., Duis and Coors, 2016; Auta et al., 2017). However, due to various biological processes, such as the aggregation of organic material and/or bacteria (“biofouling”), the gravity of plastic particles might become greater, by which their potential to settle in the sediments of aquatic ecosystems is increasing (e.g., Andrady, 2011; Galloway et al., 2017). Subsequently, densities of plastic particles in sediments can become magnitudes higher than in the surrounding waters (Lattin et al., 2004). These processes increase the bioavailability of nano- and microplastics for sediment-inhabiting organisms, especially via ingestion, since the particles are of roughly the same size (or even smaller) as sediment grains (Moore, 2008; Wright et al., 2013a). The ingestion of plastic debris by sediment-dwelling organisms has already been frequently reported and is reviewed elsewhere (e.g., Ivar do Sul and Costa, 2014; Li W. C. et al., 2016; Scherer et al., 2017). Benthic invertebrates are of particular concern, either in marine or freshwater habitats, since they contribute up to 90% of fish prey biomass (e.g., Schindler and Scheuerell, 2002; Weber and Traunspurger, 2015). Hence, for benthic fauna, small-scale plastics may impact trophic energy transfer and/or trophic interactions. However, the vast majority of studies examining the ecotoxicological effects of nano- and microplastics have focused on pelagic rather than benthic organisms so far.
Direct harmful effects of nano- and microplastics may be of physical (mechanical) and/or chemical (toxicological) nature (Barnes et al., 2009; Wright et al., 2013b). The latter include the leaching from plastics of e.g., carcinogenic and endocrine-disrupting contaminants, such as monomers, plastic additives (e.g., Oehlmann et al., 2009; Talsness et al., 2009), and polymer-associated chemicals. In addition, due to their large surface area to volume ratio, small-scale plastic particles can become heavily contaminated, with particle-associated concentrations of the contaminants being several orders of magnitude greater than those in the ambient medium (Mato et al., 2001; Hirai et al., 2011). Among the pollutants with the highest affinity for the hydrophobic surface of plastics are hydrophobic persistent organic pollutants (POPs). After the contaminated particles are ingested by benthic organisms, the possible leaching of associated POPs could result in the bioaccumulation and biomagnification of these chemicals followed by their entry into aquatic food webs (vom Saal et al., 2008).
In this review we assess current knowledge on the effects of nano- and microplastics on benthic invertebrates in aquatic ecosystems. Our assessment is based on a literature analysis of: (i) the impacts on organisms in freshwater and marine environments, (ii) the harmful effects induced by the physical or chemical impacts of plastic particles, (iii) the various particle materials, shapes, and sizes examined, (iv) the exposure matrix and parameters assessed in the respective assays and (v) the interaction of contaminants with nano- and microplastic particles. Subsequently, a gap analysis based on the obtained findings was conducted and areas in need of further research were identified.
Methods
Using the databases Web of Science and Google Scholar, a comprehensive literature review of the physical and chemical effects of leaching processes as well as the interaction of plastic particles with contaminants in terms of their impacts on benthic invertebrates was conducted. The search was based on a query of the key word terms: microplastic* OR nanoplastic* AND benthic* OR benthos* AND invertebrate* AND effect* OR impact* or a combination thereof.
Overall, 330 papers were reviewed, with 49 publications finally included in this survey based upon their relevance to the topic, in agreement with general criteria for peer-reviewed articles and as judged by the authors of this review. Although a comprehensive literature search was carried out, the retrieved studies may not be fully representative of all studies conducted, since the probability that a given study will be published generally increases with the increased statistical significance of its results. This “file drawer problem” was described by Arnqvist and Wooster (1995). However, for consistency, unpublished results were excluded, with only primary literature reports included in the final review process. Additional validity criteria fulfilled by the included publications were distinct characterizations of the respective plastic material as well as the provision of quality criteria in terms of positive and negative controls. Within the selected publications, investigations of two or more different organisms or particles with various characteristics in terms of, e.g., particle material or shape were considered as separate experiments.
The publications were categorized according to the investigated habitat (freshwater or marine) and the impact (physical or chemical) on the benthic invertebrates assessed. For each publication, the following criteria were analyzed: taxon, species, plastic material, particle shape, particle size, exposure concentration, and matrix endpoints investigated. The general review procedure and the effects identified in those studies are summarized in Figure 1.
Figure 1
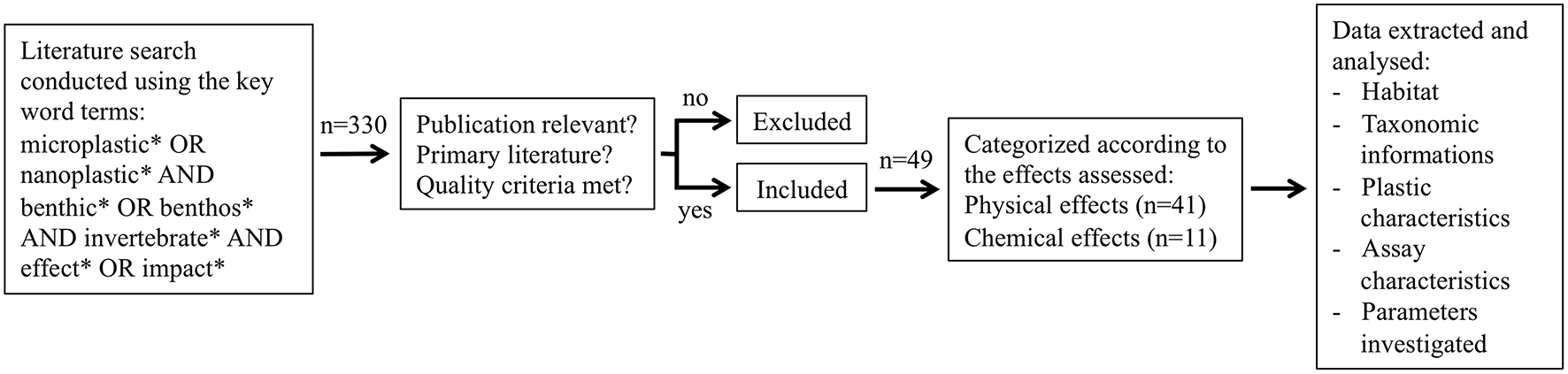
Schematic representation of the review process, publication selection and data analysis.
Results
Physical Impacts
Freshwater Benthic Fauna
The mechanical hazards posed by the ingestion of micro- and nano-sized plastic particles by organisms in freshwater ecosystems were evaluated in 26 experiments reported in 10 publications (Table 1). The most frequently used organisms (38%, n = 10) were arthropods, mainly crustaceans (Figure 2A). The gammarids Gammarus fossarum, Gammarus pulex, and Hyalella azteca were the target in 8 out of the 26 experiments (Table 1), and molluscs and nematodes in 7 (27%) and 6 (23%) of the experiments (Table 1; Figure 2A). By contrast, very little research focused on the physical effects of plastics on annelids (n = 2, 8%) and rotifers (n = 1, 4%; Figure 2A). The former were limited to the effects on Lumbriculus variegatus and Tubifex spp., and the latter to those on the rotifer species Brachionus koreanus (Table 1).
Table 1
| Taxa | Species | Material | Shape | Size | Conc. | Matrix | Parameter | Effects | References |
|---|---|---|---|---|---|---|---|---|---|
| Annelida | Lumbriculus variegatus | PS | Fragments | 20–500 μm | 0.1–40% sed. dw | S | Mortality | No effects on mortality | Redondo-Hasselerharm et al., 2018 |
| Development | No effects on growth | ||||||||
| Behavior | No effects on feeding rate | ||||||||
| Tubifex spp. | PS | Fragments | 20–500 μm | 0.1–40% sed. dw | S | Mortality | No effects on mortality | Redondo-Hasselerharm et al., 2018 | |
| Development | No effects on growth | ||||||||
| Behavior | No effects on feeding rate | ||||||||
| Arthropoda | Asellus aquaticus | PS | Fragments | 20–500 μm | 0.1–40% sed. dw | S | Mortality | No effects on mortality | Redondo-Hasselerharm et al., 2018 |
| Development | No effects on growth | ||||||||
| Behavior | No effects on feeding rate | ||||||||
| Chironomus tepperi | PE | Spheres | 1–4 μm | 500 part. kg−1 | S | Mortality | Significantly increased mortality at 1–4, 10–27, and 43–54 μm | Ziajahromi et al., 2018 | |
| 10–27 μm | Development | Significantly decreased body length at 1–4, 10–27, and 43–54 μm; | |||||||
| 43–54 μm | Significantly decreased length of head capsule at 10–27 μm; | ||||||||
| 100–126 μm | Reduced development of head capsule, mouth parts, and antenna at 10–27 μm; | ||||||||
| Development delay of organisms at 10–27 μm | |||||||||
| Emergence | Significantly lowered emerging rate for all size ranges | ||||||||
| Gammarus fossarum | PS | Spheres | ≤ 5 μm | 12,500 part. ml−1 | AM | Mortality | No effects on mortality | Blarer and Burkhardt-Holm, 2016 | |
| Development | No effects on wet weight | ||||||||
| Behavior | No effects on feeding rate | ||||||||
| Assimilation | No effects on assimilation efficiency | ||||||||
| PA | Fibers | 20 × 500 μm | 2,680 cm−2 | AM | Mortality | No effects on mortality | Blarer and Burkhardt-Holm, 2016 | ||
| Development | No effects on wet weight | ||||||||
| Behavior | No effects on feeding rate | ||||||||
| Assimilation | Significantly decreased assimilation efficiency | ||||||||
| PMMA | Fragments | 32–64 μm | 100,000 part. ind.−1 | AM | Development | Significantly decreased wet weight | Straub et al., 2017 | ||
| 64–125 μm | Behavior | No effects on feeding rate | |||||||
| 125–250 μm | Assimilation | Significantly decreased assimilation efficiency | |||||||
| PHB | Fragments | 32–64 μm | AM | Development | Significantly decreased wet weight | Straub et al., 2017 | |||
| 64–125 μm | Behavior | No effects on feeding rate | |||||||
| 125–250 μm | Assimilation | No effects on assimilation efficiency | |||||||
| Gammarus pulex | PS | Fragments | 20–500 μm | 0.1–40% sed. dw | S | Mortality | No effects on mortality | Redondo-Hasselerharm et al., 2018 | |
| Development | Significantly reduced growth (Ec10: 1.07% sed. dw, | ||||||||
| EC50: 3.57% sed. dw) | |||||||||
| Behavior | No effects on feeding rate | ||||||||
| Hyalella azteca | PS | Fragments | 20–500 μm | 0.1–40% sed. dw | S | Mortality | No effects on mortality | Redondo-Hasselerharm et al., 2018 | |
| Development | No effects on growth | ||||||||
| Behavior | No effects on feeding rate | ||||||||
| PE | Fragments | 10–27 μm | 10–100,000 part. ml−1 | AM | Mortality | Significant, dose-dependent increase in mortality | Au et al., 2015 | ||
| (LOEC: 10,000 part. ml−1,!!! LC50: 4.6 × 104 part. ml−1) | |||||||||
| Development | No effects on dw | ||||||||
| 5,000–20,000 part. ml−1 | Reproduction | Reproduction significantly decreased at 5,000 part. ml−1 (day 28) and | |||||||
| at 10,000 part. ml−1 (day 28 and 42) | |||||||||
| PP | Fibers | 20–75 × 20 μm | 22.5–90 part. ml−1 | AM | Mortality | Significant, dose-dependent increase in mortality | Au et al., 2015 | ||
| (LOEC: 45 part. ml−1,!!! LC50: 71 part. ml−1) | |||||||||
| Development | Significant, dose-dependent decrease in dw (LOEC: 45 part. ml−1) | ||||||||
| Mollusca | Potamopyrus antipodarum | PA | Fragments | 4.64–602 μm | 30 and 70% | Food | Mortality | No effects on mortality | Imhof and Laforsch, 2016 |
| Development | No effects on adult development | ||||||||
| Reproduction | No effects on reproduction | ||||||||
| Mortality | No effects on mortality | ||||||||
| Development | No effects on juvenile development | ||||||||
| PC | Fragments | 4.64–602 μm | 30 and 70% | Food | Mortality | No effects on mortality | Imhof and Laforsch, 2016 | ||
| Development | No effects on adult development | ||||||||
| Reproduction | No effects on reproduction | ||||||||
| Mortality | No effects on mortality | ||||||||
| Development | No effects on juvenile development | ||||||||
| PET | Fragments | 4.64–602 μm | 30 and 70% | Food | Mortality | No effects on mortality | Imhof and Laforsch, 2016 | ||
| Development | No effects on adult development | ||||||||
| Reproduction | No effects on reproduction | ||||||||
| Mortality | No effects on mortality | ||||||||
| Development | No effects on juvenile development | ||||||||
| PS | Fragments | 4.64–602 μm | 30 and 70% | Food | Mortality | No effects on mortality | Imhof and Laforsch, 2016 | ||
| Development | No effects on adult development | ||||||||
| Reproduction | No effects on reproduction | ||||||||
| Mortality | No effects on mortality | ||||||||
| Development | No effects on juvenile development | ||||||||
| PVC | Fragments | 4.64–602 μm | 30 and 70% | Food | Mortality | No effects on mortality | Imhof and Laforsch, 2016 | ||
| Development | No effects on adult development | ||||||||
| Reproduction | No effects on reproduction | ||||||||
| Mortality | No effects on mortality | ||||||||
| Development | No effects on juvenile development | ||||||||
| Sphaerium corneum | PS | Fragments | 20–500 μm | 0.1–40% sed. dw | S | Mortality | No effects on mortality | Redondo-Hasselerharm et al., 2018 | |
| Development | No effects on growth | ||||||||
| Behavior | No effects on feeding rate | ||||||||
| Dreissena polymorpha | PS | Spheres | 1 and 10 μm | 5 × 105 + 5 × 105 part. l−1, | AM | Cellular response | No effects on GST and SOD activity; | Magni et al., 2018 | |
| 2 × 106 + 2 × 106 part. l−1 | CAT and gpx activity significantly affected at 5 × 105 + 5 × 105 part. l−1; | ||||||||
| No effects on levels of LPO and PCC; | |||||||||
| DOP significantly increased at 5 × 105 + 5 × 105 part. l−1 (day 3) and | |||||||||
| 2 × 106 + 2 × 106 part. L−1 (day 6); | |||||||||
| No effects on SER and GLU; | |||||||||
| No effects on the activity of ache, MAO and micronuclei frequency | |||||||||
| Nematoda | Caenorhabditis elegans | PA | Fragments | ~70 μm | 0.5–10 mg m−2, | AM | Mortality | Significantly increased mortality (LOEC: 0.5 mg m−2) | Lei et al., 2018 |
| 5 mg m−2 | Development | Significantly decreased body length | |||||||
| Reproduction | Number of embryos significantly lowered; No effect on brood size | ||||||||
| Cellular Response | Calcium level in intestines significantly lowered; | ||||||||
| No effect on expression of gst-4 | |||||||||
| PP | Fragments | ~70 μm | 0.5–10 mg m−2, | AM | Mortality | Significantly increased mortality (LOEC: 0.5 mg m−2) | Lei et al., 2018 | ||
| 5 mg m−2 | Development | Significantly decreased body length | |||||||
| Reproduction | Number of embryos significantly lowered; No effect on brood size | ||||||||
| Cellular Response | No effect on calcium level in intestines; | ||||||||
| Expression of gst-4 significantly increased | |||||||||
| PE | Fragments | ~70 μm | 0.5–10 mg m−2, | AM | Mortality | Significantly increased mortality (LOEC: 0.5 mg m−2) | Lei et al., 2018 | ||
| 5 mg m−2 | Development | Significantly decreased body length | |||||||
| Reproduction | Number of embryos significantly lowered; Brood size significantly decreased | ||||||||
| Cellular Response | Calcium level in intestines significantly lowered; | ||||||||
| Expression of gst-4 significantly increased | |||||||||
| PVC | Fragments | ~70 μm | 0.5–10 mg m−2 | AM | Mortality | Significantly increased mortality (LOEC: 1 mg m−2) | Lei et al., 2018 | ||
| Reproduction | Number of embryos significantly lowered; Brood size significantly decreased | ||||||||
| Cellular Response | Calcium level in intestines significantly lowered; | ||||||||
| Expression of gst-4 significantly increased | |||||||||
| Nematoda | Caenorhabditis elegans | PS | Spheres | 0.1 μm | 1–10,000 μg l−1 | AM | Cellular Response | Significant, dose-dependent induction of intestinal ROS production from up 10 μg l−1; | Zhao et al., 2017 |
| Significant, dose-dependent increase in defecation cycle length from up 10 μg l−1 | |||||||||
| Behavior | Significant, dose-dependent decrease of locomotion (head trash, body bend) from up 10 μg l−1 | ||||||||
| Reproduction | Significant, dose-dependent decrease of reproduction (brood size) from up 10 μg l−1 | ||||||||
| Development | Significant, transgenerational effects on F1-generation in terms of intestinal ROS production, | ||||||||
| locomotion behavior and reproduction form up 100 μg l−1 | |||||||||
| Spheres | 0.1 μm | 0.5–10 mg m−2, | AM | Mortality | Significantly increased mortality (LOEC: 0.5 mg m−2), | Lei et al., 2018 | |||
| 1 μm | 5 mg m−2 | Effects significantly size-depending, with slight effects induced by 0.1 μm part., | |||||||
| 5 μm | strongest effects induced by 1 μm part. | ||||||||
| Development | Significantly decreased body length | ||||||||
| Reproduction | Number of embryos significantly lowered for 0.1 and 1 μm part.;Brood size significantly lowered for 0.1 and 1 μm part. | ||||||||
| Cellular Response | Calcium level in intestines significantly lowered for 1 μm;Expression of gst-4 significantly increased | ||||||||
| Rotifera | Brachionus koreanus | PS | Spheres | 0.05 μm | 0.1–20 μg ml−1, | AM | Reproduction | Reproduction time significantly increased for 0.05 μm at 10 μg ml−1; | Jeong et al., 2016 |
| 0.5 μm | 10 μg ml−1 | Fecundity significantly affected for 0.05 and 0.5 μm (LOEC 0.05 μm: 1 μg ml−1, LOEC 0.5 μm: 20 μg ml−1); | |||||||
| 6 μm | Population growth significantly retarded for 0.05 and 0.5 μm | ||||||||
| Life-Span | Life-span significantly affected for 0.05 and 0.5 μm (LOEC 0.05 μm: 0.1 μg ml−1, LOEC 0.5 μm: 20 μg ml−1) | ||||||||
| Cellular Response | ROS level significantly increased under microplastic treatments; | ||||||||
| Significantly affected exposure of antioxidant-related enzymes; | |||||||||
| Phosphorylation status of p-JNK and p-p38 increased for 0.05 μm |
Effects on micro- and nano-sized plastic particles on benthic freshwater invertebrates.
Figure 2
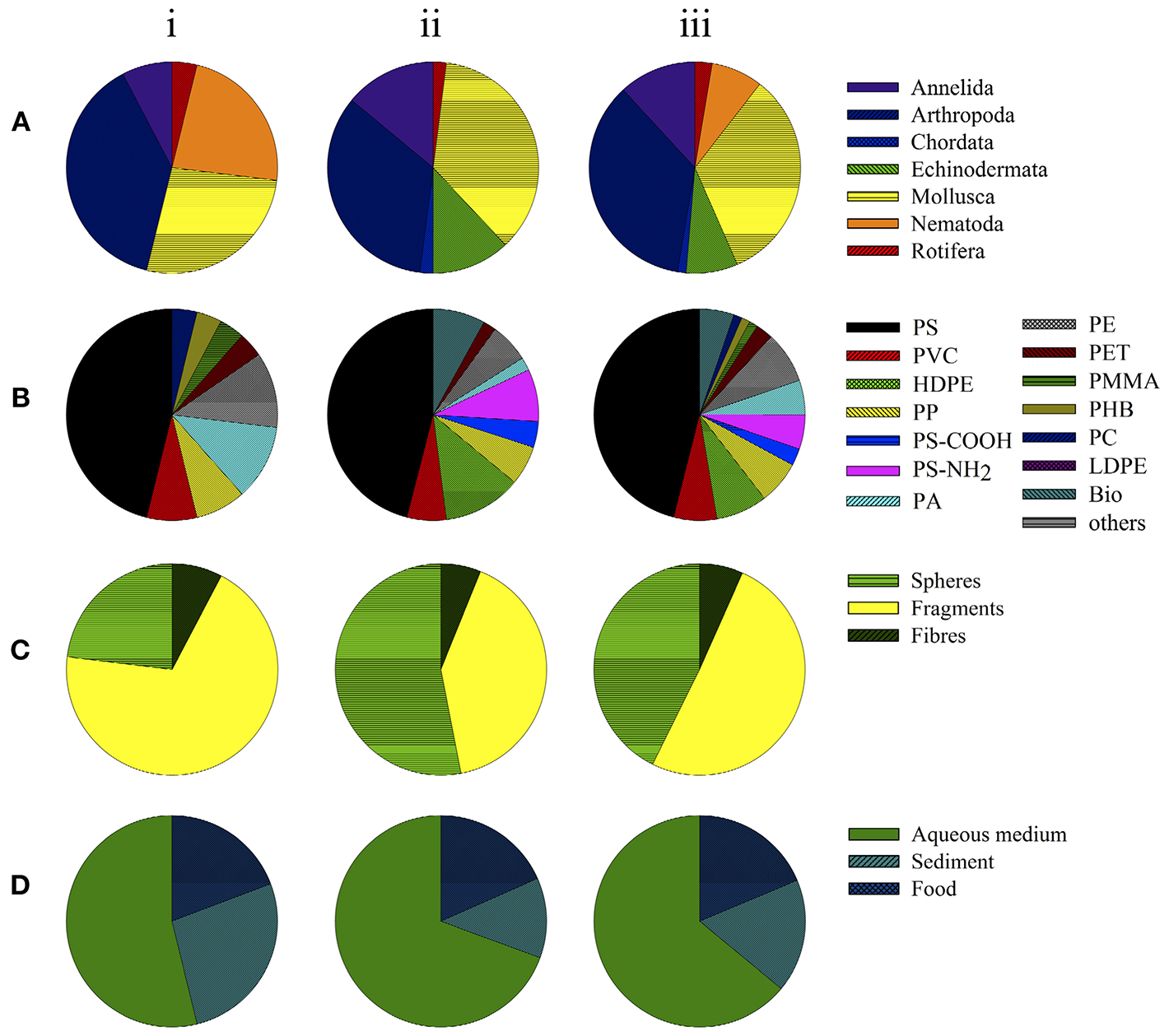
Toxicity assays examining physical effects in organisms in (i) freshwater studies, (ii) marine studies and (iii) total. Data depict the groups of organisms (taxa) used (A), polymer types (B), shape of small-scale plastic particles (C), and matrix or route of exposure (D).
Among the 26 experiments examining the mechanical hazards posed by micro- and nano-sized plastics on benthic organisms in freshwater, 46% (n = 12) focused on polystyrene (PS) particles, 23% (n = 3) on polyamide (PA), 23% (n = 3) on polyethylene (PE), 15% (n = 2) on polyvinyl chloride (PVC), and 15% (n = 2) on polypropylene particles (PP; Figure 2B). The effects of polymethyl methacrylate (PMMA), polyhydroxybutyrate (PHB), polycarbonate (PC), and polyethylene terephthalate (PET) were investigated in single studies (4% each). The vast majority (69%, n = 18) of the freshwater investigations examined the physical effects of microplastic fragments, i.e., non-uniform, irregularly shaped particles, and powders (Figure 2C). In 23% (n = 6; Figure 2C) the effects of spheres, i.e., micro- and/or nano-sized beads, were determined. Two studies (8%) assessed the physical effects of polymer fibers (Figure 2C). Generally, the effects of micro-sized plastic particles (0.1–5,000 μm) were investigated, whereas the toxicity of nano-sized particles (<0.1 μm) was rarely tested (Table 1).
As shown in Figure 2D, in most of the studies on the mechanical hazards of microplastics (54%, n = 14) aqueous medium (AM) was the matrix used to apply the investigated plastic particles, followed by plastic spiked sediments (27%, n = 7) and of food (8%, n = 5).
Mortality
Lethal effects of nano- and micro-sized plastics were investigated in 81% (n = 21) of the studies as displayed in Table 1. Redondo-Hasselerharm et al. (2018) investigated the lethal effects of various PS-fragments in sediments on the annelids L. variegatus and Tubifex spp., the arthropods Asellus aquaticus, G. pulex, and H. azteca and the mollusc Sphaerium corneum, without observing lethal effects on any test organism (Table 1). Similar results were reported by Blarer and Burkhardt-Holm (2016) and Imhof and Laforsch (2016), who neither found significant lethal effects on G. fossarum nor on the mud snail Potamopyrgus antipodarum exposed to a variety of polymer fragments (PA, PC, PET, PS, PVC) offered in different shapes, concentrations, and sizes (Table 1). However, Ziajahromi et al. (2018) and Lei et al. (2018) reported significantly increased mortality rates for Chironumus tepperi and Caenorhabditis elegans when exposed to PE- and PS-spheres varying in size and concentration respectively, with effects being distinctly size-dependent (Table 1). In terms of investigated effects of plastic fragments and fibers, impacts were reported to be rather dose-dependent as observed for PE-fragments and PP-fibers on H. azteca (Au et al., 2015; Table 1) and for PA-, PP-, PE-, and PVC-fragments on C. elegans (Lei et al., 2018; Table 1).
Development
The effects of small-scale plastics on the development of organisms were investigated most frequently, by being of concern in 88% (n = 23) of the respective studies. As reported by Redondo-Hasselerharm et al. (2018), effects of PS-fragments on the development were species-specific, with no significant effects reported for L. variegatus, Tubifex spp., A. aquaticus, H. azteca, and S. corneum, while the growth of G. pulex was significantly affected (Table 1). In the study of Blarer and Burkhardt-Holm (2016), neither PS-spheres nor PA-fibers had significant effects on the development of G. fossarum, while exposure to PMMA- and PHB-fragments in various size-ranges significantly decreased the wet weight of this species (Straub et al., 2017; Table 1). Among the studies focusing on freshwater arthropods, size-dependent effects were reported for PE-spheres again (Ziajahromi et al., 2018), with significantly reduced and delayed development of C. tepperi induced by small particles (Table 1). Effects in a dose-dependent manner were observed by Au et al. (2015), reporting impacts on dry weight of H. azteca when exposed to PE-fragments and PP-fibers in various concentrations (Table 1). However, PA-, PC-, PET-, PS-, and PVC-fragments included in food did not induce any significant effects on the development of P. antipodarum (Imhof and Laforsch, 2016; Table 1), while PA-, PP-, PE-, and PVC-fragments significantly reduced the body length of C. elegans (Lei et al., 2018). The latter study also showed equivalent reductions in the growth of this nematode species by the application of different PS-spheres (Lei et al., 2018; Table 1).
Reproduction
The effects on reproduction were investigated in 50% (n = 13; Table 1) of the analyzed experiments. Significant dose-dependent effects of plastic particles were reported by Au et al. (2015), investigating the impact of PE-particles on the reproduction of H. azteca, and by Zhao et al. (2017), observing dose-depending reductions in brood sizes of C. elegans treated with PS-spheres (Table 1). Additionally, effects were also reported to be size-dependent on C. elegans, as shown by Lei et al. (2018), who reported significant inhibitory effects of smallest PS-spheres applied (Table 1). Comparable size- and dose-dependent effects were also reported for B. koreanus, with a significant prolongation of its reproduction time and a reduced fecundity after exposure to PS nanoparticles (Jeong et al., 2016; Table 1). Additionally, plastic fragments of various origins (PA, PP, PE, and PVC) significantly reduced reproductive success of C. elegans, while only PE- and PVC-fragments affected brood sizes significantly (Lei et al., 2018; Table 1). However, fragments of PA, PC, PET, PS, and PVC had no apparent impact on the reproduction of P. antipodarum (Imhof and Laforsch, 2016; Table 1).
Behavior
Behavioral alterations induced by small-scale plastic particles were investigated in 11 assays, being tantamount with 42% of the experiments described in the included studies (Table 1). No alterations in feeding behavior were observed following the exposure of L. variegatus, Tubifex spp., A. aquaticus, G. pulex, H. azteca, or S. corneum to PS-fragments (Redondo-Hasselerharm et al., 2018), or G. fossarum to PS-spheres (Blarer and Burkhardt-Holm, 2016). Additionally, neither effects of PA-fibers nor of PMMA- or PHB-fragments on G. fossarum could be observed (Blarer and Burkhardt-Holm, 2016; Straub et al., 2017). By contrast, nano-sized PS-spheres significantly reduced the locomotion of C. elegans in a dose-dependent manner (Zhao et al., 2017; Table 1).
Cellular response
Cellular responses, including alterations in gene expression, reactive oxygen species (ROS) production, and enzyme activity, were the assessed end points of 31% (n = 8) of the studies (Table 1). Effects of different PS-spheres at various concentrations on the mussel D. polymorpha were investigated by Magni et al. (2018), analyzing various biomarkers (Table 1). While neither biomarkers of oxidative damage, neuro-genotoxicity, the activities of neuro-enzymes nor the measured frequency of micronuclei were affected, enzyme activity of catalase (CAT), glutathione peroxidase (GPx), as well as measured dopamine levels were significantly altered in treated organisms (Magni et al., 2018; Table 1). The assessed biomarkers of C. elegans showed to be material-dependent with a significant up-regulation of glutathione-S-transferase (GST) after exposure to PE-, PP-, and PVC-fragments, while PA-fragments had no effect, and significantly altered intestinal calcium levels observed in C. elegans exposed to PA-, PE-, and PVC-fragments exclusively (Lei et al., 2018; Table 1). The presence of PS-spheres in various sizes could be shown to induce a general significant up-regulation of GST additionally, whereas measured calcium levels were affected in a size-dependent manner (Lei et al., 2018; Table 1). This size-dependent toxicity of PS-spheres on several cellular biomarkers was also reported by Jeong et al. (2016) for B. koreanus. While the levels of intracellular ROS were generally higher in treated rotifers, size-dependencies were reported for glutathione reductase (GR), superoxide dismutase (SOD), GPx, GST, and the phosphorylation status of mitogen-activated protein kinase (MAPK) signaling proteins with significant effects after the exposure to smallest spheres applied (Jeong et al., 2016; Table 1).
Others
The parameters assimilation efficiency (n = 4), life-span (n = 1), and emergence (n = 1) were assessed in 23% of the respective experiments together. Studies by Blarer and Burkhardt-Holm (2016) and Straub et al. (2017) on the assimilation efficiency of G. fossarum revealed significant effects induced by PA-fibers and PMMA-fragments, while PS-spheres and PHB-fragments did not show any effects (Table 1). Regarding the parameter life-span, B. koreanus revealed size- and dose-dependent impacts of PS-spheres at various concentrations, with life-span being significantly shortened by the smallest spheres applied (Jeong et al., 2016; Table 1). However, no dependencies in terms of effects could be shown regarding the emergence of C. riparius, being equally lowered by the presence of PE-spheres of various sizes (Ziajahromi et al., 2018).
Marine Benthic Fauna
Mechanically induced effects have also been investigated frequently in marine settings, with 50 studies being included in the review process. Organisms belonging to Mollusca were most commonly used (36%, n = 18; Figure 2A), especially those of the Mytilus complex (mostly M. edulis and M. galloprovincialis), followed by the flat oyster Ostrea edulis and the Pacific oyster Crassostrea gigas, and, in only one study, Atactodea striata (Table 2). Arthropods (34%, n = 17; Figure 2A) were another common target organism, with crustaceans investigated exclusively. These consisted mostly of several decapods, including Carcinus maenas and Palaemonetes pugio, the calanoid species Calanus finmarchicus and C. helgolandicus, as well as Centropages typicus and Parvocalanus crassirostris (13%, n = 3), but also the isopod Idotea emarginata (6%, n = 1) and the cyclopoid species Paracyclopina nana and Tigriopus japonicas (6%, n = 1), as shown in Table 2. Other targets were annelids (14%, n = 7), mostly Arenicola marina, but also Perinereis aibuhitensis and the ragworm Hediste diversicolor. Beside, Echinodermata were investigated frequently (12%, n = 6), mostly Paracentrotus lividus and the sea urchin Tripneustes gratilla (83%, n = 5; Table 2, Figure 2A). Single studies were conducted on the ascidian Ciona robusta and the marine rotifer Brachionus manjavacas (Table 2).
Table 2
| Taxa | Species | Material | Shape | Size | Conc. | Matrix | Parameter | Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Annelida | Arenicola marina | PS | Fragments | 400–1,300 μm | 0.074–7.4% sed. dw | S | Mortality | No effects on mortality | Besseling et al., 2012 |
| Behavior | Significant, dose-dependent decrease of feeding activity | ||||||||
| Development | Significant, dose-dependent decrease of dw, no effects on ww, and AFDW | ||||||||
| PVC | Fragments | 63–500 μm | 0.5–5% sed. dw | S | Egestion | Significantly prolonged gut residence time at 5% sed. | Wright et al., 2013a | ||
| Behavior | Significantly reduced feeding activity at 5% sed. | ||||||||
| Cellular response | Significantly increased phagocytic activity at 0.5 and 5% sed. | ||||||||
| Energy reserves | Significantly reduced energy reserves (J g−1 ww.) at 1 and 5% sed.; | ||||||||
| Significantly lowered lipid reserves for UPVC-exposure | |||||||||
| Fragments | 2.5–316 μm | 0.02–2% sed. ww | S | Mortality | No effects on mortality | Green et al., 2016a | |||
| Development | No effects on dw biomass | ||||||||
| Behavior | Significantly less casts produced at 2% sed. ww | ||||||||
| O2 consumption | Significantly increased O2 consumption at 2% sed. ww | ||||||||
| PLA | Fragments | 1.4–707 μm | 0.02–2% sed. ww | S | Mortality | No effects on mortality | Green et al., 2016a | ||
| Development | No effects on dw biomass | ||||||||
| Behavior | No effects on bioturbation | ||||||||
| O2 consumption | Significantly increased O2 consumption at 2% sed. ww | ||||||||
| HDPE | Fragments | 8.7–478 μm | 0.02–2% sed. ww | S | Mortality | No effects on mortality | Green et al., 2016a | ||
| Development | No effects on dw biomass | ||||||||
| Behavior | No effects on bioturbation | ||||||||
| O2 consumption | Significantly increased O2 consumption at 2% sed. ww | ||||||||
| Perinereis aibuhitensis | PS | Spheres | 8–12 μm | 100 and 1,000 part. ml−1 | AM | Mortality | Smaller spheres increased mortality significantly | Leung and Chan, 2018 | |
| 32–38 μm | Regeneration | Significantly reduced regeneration rate at 1000 part. ml−1, | |||||||
| smaller particles being significantly more detrimental | |||||||||
| Hediste diversicolor | PVC | Fragments | 250 μm | 200 and 2,000 part. kg−1 | S | Mortality | No effects on mortality | Gomiero et al., 2018 | |
| Cellular response | Significantly increased phagocytic activity at 200 part. kg−1 (10 d) | ||||||||
| and 2,000 part. kg−1 (10 and 28 d); | |||||||||
| No effects on extracellular lysosome release, lysosomal | |||||||||
| membrane stability (LMS) and oxyradical production; | |||||||||
| No effects micronuclei frequency and on DNA strand breaks; | |||||||||
| No effects on Lipofuscin content and Catalase activity | |||||||||
| Arthropoda | Calanus finmarchicus | PS | Spheres | 15 μm | 50 and 500 part. ml−1 | AM | Mortality | No effects on mortality | Vroom et al., 2017 |
| Calanus helgolandicus | PS | Spheres | 20 μm | 75 part. ml−1 | AM | Behavior | Feeding rate significantly reduced; Significant shift to small sized prey | Cole et al., 2015 | |
| Mortality | No significant effects on mortality | ||||||||
| Reproduction | No significant effects on egg production rate;Hatching success significantly decreased from up day 6 | ||||||||
| Development | Significantly smaller eggs produced from up day 7 | ||||||||
| O2 consumption | No effects on O2 consumption | ||||||||
| Assimilation | 2-fold greater energetic losses expected | ||||||||
| Carcinus maenas | PP | Fibers | 1,000–5,000 μm | 0.3–1.0% by weight | AM | Behavior | Feeding rate significantly decreased | Watts et al., 2015 | |
| Assimilation | Significantly reduced scope for growth | ||||||||
| PS | Spheres | 8 μm | 106 and 107 part. l−1 | AM | Mortality | No effects on mortality | Watts et al., 2016 | ||
| O2 consumption | O2 consumption significantly reduced (1 h) at 107 PS part. l−1 | ||||||||
| Cellular response | Significant effects on Na+-Ions and Ca2a+-Ions (24 h) at 107 PS part. l−1, | ||||||||
| no effects on K+-Ion concentration; | |||||||||
| Significant, dose-dependent effects on hemocyanin concentration | |||||||||
| No effect on hemolymph protein concentration; | |||||||||
| PS-COOH | Spheres | 8 μm | 106 and 107 part. l−1 | AM | Mortality | No effects on mortality | Watts et al., 2016 | ||
| O2 consumption | No effects on O2 consumption | ||||||||
| Cellular response | No cellular responses induced | ||||||||
| PS-NH2 | Spheres | 8 μm | 106 and 107 part. l−1 | AM | Mortality | No effects on mortality | Watts et al., 2016 | ||
| O2 consumption | No effects on O2 consumption | ||||||||
| Cellular response | No cellular responses induced | ||||||||
| Centropages typicus | PS | Spheres | 7.3 μm | 4,000–25,000 part. ml−1 | AM | Behavior | Significantly, dose-dependently reduced total algae feeding rate | Cole et al., 2013 | |
| Idotea emarginata | PS | Spheres | 10 μm | 12 part. mg food−1 | Food | Mortality | No effects on survival | Hämer et al., 2014 | |
| Behavior | No effects on feeding rate | ||||||||
| Development | No effects on the duration of intermolt periods and growth | ||||||||
| Arthropoda | Idotea emarginata | PS | Fragments | 1–100 μm | 20 part. mg food−1 | Food | Mortality | No effects on mortality | Hämer et al., 2014 |
| Behavior | No effects on feeding rate | ||||||||
| Development | No effects on the duration of intermolt periods and growth | ||||||||
| PA | Fibers | 20–2,500 μm | 0.3 mg part. mg food−1 | Food | Mortality | No effects on mortality | Hämer et al., 2014 | ||
| Behavior | No effects on feeding rate | ||||||||
| Development | No effects on the duration of intermolt periods and growth | ||||||||
| Palaemonetes pugio | PE | Spheres | 35–165 μm | 50,000 part. l−1 | AM | Mortality | Significantly increased mortality by larger particles (>75 μm) | Gray and Weinstein, 2017 | |
| PS | Spheres | 30 and 75 μm | 50,000 part. l−1 | AM | Mortality | Significantly increased mortality by larger particles (75 μm) | Gray and Weinstein, 2017 | ||
| PP | Fragments | 34 and 93 μm | 50,000 part. l−1 | AM | Mortality | Significantly increased mortality by larger particles (93 μm) | Gray and Weinstein, 2017 | ||
| Fibers | 34 and 93 μm | 50,000 part. l−1 | AM | Mortality | Significantly increased mortality | Gray and Weinstein, 2017 | |||
| Paracyclopina nana | PS | Spheres | 0.05 μm | 0.1–20 μg ml−1 | AM | Development | Development significantly delayed for 0.05 μm (LOEC: 10 μg ml−1) | Jeong et al., 2017 | |
| 0.5 μm | Reproduction | Fecundity significantly reduced for 0.05 μm (LOEC: 10 μg ml−1) and | |||||||
| 6 μm | 0.5 μm (LOEC: 20 μg ml−1) | ||||||||
| 20 μg ml−1 | Cellular response | Significant increase in intracellular ROS level by 0.05 μm spheres; | |||||||
| Significantly increased phosphorylation of p-ERK, p-p38 and Nrf2; | |||||||||
| Significantly increased activity of GPx (0.05 μm), GR, GST, and SOD | |||||||||
| Parvocalanus crassirostris | PET | Fragments | 5–10 μm | 10,000–80,000 part. ml−1 | AM | Reproduction | Significantly reduced egg production (LOEC 80,000 part. ml−1) | Heindler et al., 2017 | |
| 20,000 part. ml−1 | Relative population size significantly reduced after 6 and 24 d | ||||||||
| 20,000 part. ml−1 | Cellular response | Significantly down regulated H3 gene expression | |||||||
| Tigriopus japonicus | PS | Spheres | 0.05 μm | 6–313 μg ml−1 | AM | Mortality | No effects on mortality | Lee et al., 2013 | |
| 0.5 μm | 0.125–25 μg ml−1 | Mortality | Survival of F0 generation significantly effected for 0.05 μm | ||||||
| 6 μm | (LOEC: 1.25 μg ml−1, LC50: 2.15 μg ml−1), | ||||||||
| Survival of F1 generation significantly effected for 0.05 μm | |||||||||
| (LOEC: 1.25 μg ml−1, LC50: 0.16 μg ml−1) and 0.5 μm | |||||||||
| (LOEC: 25 μg ml−1, LC50: 23.5 μg ml−1) | |||||||||
| Reproduction | Fecundity of F0 generation significantly decreased for 0.5 μm | ||||||||
| (LOEC: 0.125 μg ml−1, EC50: 0.07 μg ml−1) and 6 μm | |||||||||
| (LOEC: 0.125 μg ml−1, EC50: 0.10 μg ml−1), | |||||||||
| Fecundity of F1 generation significantly decreased for 0.5 μm | |||||||||
| (LOEC: 0.125 μg ml−1, EC50: 0.04 μg ml−1) and 6 μm | |||||||||
| (LOEC: 0.125 μg ml−1, EC50: 0.04 μg ml−1) | |||||||||
| Development | Nauplius phase of F0 generation significantly delayed for 0.05 μm at 1.25 μg ml−1, | ||||||||
| Generation time of F1 generation significantly delayed for 0.05 μm | |||||||||
| at 1.25 μg ml−1 and for 0.5 μm at 25 μg ml−1 (EC50 21.2–21.4 μg ml−1) | |||||||||
| Chordata | Ciona robusta | PS | Spheres | 10 μm | 0.125–25 μg ml−1 | AM | Development | Significantly delayed juvenile development, no effects on larvae | Messinetti et al., 2018 |
| Mortality | No effects on larval and juvenile mortality | ||||||||
| Echinodermata | Paracentrotus lividus | PS | Spheres | 10 μm | 0.125–25 μg ml−1 | AM | Development | Significantly effected body length from up 12.5 μg ml−1, | Messinetti et al., 2018 |
| Significantly reduced body weight at 0.125 μg ml−1, | |||||||||
| Significantly effected arm length at 0.125, 12.5 and 25 μg ml−1 | |||||||||
| Mortality | No effects on larval mortality | ||||||||
| Spheres | 6 μm | 103-105 part. ml−1 | AM | Reproduction | Significantly reduced fertilization rate (LOEC 103 part. ml−1) | Martínez-Gómez et al., 2017 | |||
| Development | Significantly affected larval development of pre-exposed zygotes, | ||||||||
| Significantly reduced larval growth of pre-exposed gametes, | |||||||||
| Significantly affected embryo development at 103 and 104 part. ml−1 | |||||||||
| of virgin PS and at each concentration for aged particles | |||||||||
| PS-COOH | Spheres | 0.04 μm | 25 μg ml−1 | AM | Cellular response | Significantly up regulated Abcb1-genes at 25 μg ml−1 48 hpf | Della Torre et al., 2014 | ||
| 2.5–50 μg ml−1 | Development | No significant effects on development | |||||||
| PS-NH2 | Spheres | 0.05 μm | 3 μg ml−1 | AM | Cellular response | Significantly up regulated cas-8-genes at 3 μg ml−1 24 hpf | |||
| 1–50 μg ml−1 | Development | Significantly increased malformations (EC50 24 hpf 3.82 μg ml−1, 48 hpf 2.61 μg ml−1) | |||||||
| HDPE | Fragments | >0–80 μm | 0.005–5 g l−1 | AM | Development | Significantly affected larval development of pre-exposed zygotes, | Martínez-Gómez et al., 2017 | ||
| Significantly reduced larval growth of pre-exposed gametes, | |||||||||
| Significantly affected embryo development at 103 and 105 part. ml−1 | |||||||||
| of virgin HDPE and at each concentration for aged particles | |||||||||
| Tripneustes gratilla | PE | Spheres | 10–45 μm | 1–300 part. ml−1 | AM | Development | Significantly reduced body width at 300 part. ml−1 | Kaposi et al., 2014 | |
| Mortality | No effects on larval mortality | ||||||||
| Mollusca | Atactodea striata | PS | Fragments | 63–250 μm | 10 and 1,000 part. l−1 | AM | Behavior | Feeding rate significantly reduced at 1,000 part. l−1 | Xu et al., 2017 |
| Assimilation | No effects on absorption efficiency | ||||||||
| O2 consumption | No effects on respiration rate | ||||||||
| Crassostrea gigas | PS | Spheres | 1 and 10 μm | 1–1,000 part. ml−1 | AM | Behavior | No significant effects on feeding rate | Cole and Galloway, 2015 | |
| 100 part. ml−1 | Development | No effects on growth | |||||||
| 2 and 6 μm | 0.023 mg l−1 | Behavior | Significantly increased feeding rate | Sussarellu et al., 2016 | |||||
| Assimilation | Significantly increased absorption efficiency | ||||||||
| Reproduction | Significantly reduced oocyte number | ||||||||
| Development | Significantly reduced oocyte diameter and quality (D-larval yield); | ||||||||
| Significantly reduced sperm velocity; | |||||||||
| Significantly reduced larval growth | |||||||||
| Cellular response | Significantly increased sizes of hyalinocytes and granulocytes; | ||||||||
| Significant effects on transcript expression in digestive glands, gonads and oocytes; | |||||||||
| Significant effects on the proteome of oocytes | |||||||||
| Mytilus edulis | PLA | Fragments | 0.6–363 μm | 2.5 and 25 μg l−1 | Food | Behavior | Significantly decreased feeding rate at 25 μg l−1 | Green et al., 2016b | |
| HDPE | Fragments | 0.48–316 μm | 2.5 and 25 μg l−1 | Food | Behavior | Significantly decreased feeding rate at 25 μg l−1 | Green et al., 2016b | ||
| Mortality | No effects on mortality | ||||||||
| Fragments | >0–80 μm | 2.5 g l−1 | AM | Cellular response | Significantly increased granulocytoma formation, | von Moos et al., 2012 | |||
| Significantly decreased lysosomal membran stability (LMS) | |||||||||
| Mortality | No effects on mortality | ||||||||
| PS | Spheres | 3 and 9.6 μm | AM | Behavior | No effects on feeding rate | Browne et al., 2008 | |||
| Cellular response | No effects on oxidative status of hemolymph, viability and | ||||||||
| phagocytic activity of hemocytes | |||||||||
| Spheres | 2 and 6 μm | 32 μg l−1 | AM | Cellular response | Significantly increased number of dead hemocytes; | Paul-Pont et al., 2016 | |||
| Significantly increased ROS production; | |||||||||
| Significantly reduced catalase (CAT) activity, | |||||||||
| Significantly reduced lipid peroxidation (LPO), | |||||||||
| Significantly reduced glutathione reductase (GR); | |||||||||
| Significantly effected anti-oxidant gene expression | |||||||||
| Development | Significantly increased histopathological observations | ||||||||
| Spheres | 0.03 μm | 0.1–0.3 g l−1 | AM | Egestion | Significantly increased production of pseudofeces | Wegner et al., 2012 | |||
| Behavior | Significantly decreased feeding rates | ||||||||
| Mytilus galloprovincialis | PS | Spheres | 2 and 6 μm | 32 μg l−1 | AM | Cellular response | Significantly increased number of dead hemocytes; | Paul-Pont et al., 2016 | |
| Significantly increased ROS production; | |||||||||
| Significantly reduced catalase (CAT) activity, | |||||||||
| Significantly reduced lipid peroxidation (LPO), | |||||||||
| Significantly reduced glutathione reductase (GR); | |||||||||
| Significantly effected anti-oxidant gene expression | |||||||||
| Development | Significantly increased histopathological observations | ||||||||
| Fragments | < 100 μm | 1.5 g l−1 | AM | Mortality | No effects on mortality | Avio et al., 2015 | |||
| Cellular response | Significantly decreased LMS | ||||||||
| No effects on hemocytes and phagocytosis activitySignificantly decreased AChE levels in gills | |||||||||
| Significantly inhibited Se-dependent glutathione peroxidase | |||||||||
| PS-NH2 | Spheres | 0.05 μm | 1–50 μg ml−1 | AM | Cellular response | Significantly decreased lysosomal membrane stability (LMS; LOEC 5 μg ml−1) | Canesi et al., 2015 | ||
| Significant increase in lysozyme release (LOEC: 1 μg ml−1) | |||||||||
| Significantly increased ROS production (LOEC: 1 μg ml−1) | |||||||||
| Significantly increased NO production | |||||||||
| Significantly decreased phagocytosis (LOEC: 1 μg ml−1) | |||||||||
| Significantly effected apoptotic parameters (ANX+, ANX+/PI+, TMRE, NAO; LOEC: 50 μg ml−1) | |||||||||
| Spheres | 0.05 μm | 0.001–20 mg l−1 | AM | Development | Significantly decreased larval development (LOEC: 0.01 mg l−1, EC50: 0.142 mg l−1) | Balbi et al., 2017 | |||
| 0.15 mg l−1 | Cellular response | Significantly effected gene expression (CS, CA, EP, ABCB and LYSO) | |||||||
| Mollusca | Mytilus galloprovincialis | PE | Fragments | < 100 μm | 1.5 g l−1 | AM | Mortality | No effects on mortality | Avio et al., 2015 |
| Cellular response | Significantly reduced Granulocytes/Hyalinocytes ratio | ||||||||
| No effects on hemocytes and phagocytosis activity | |||||||||
| Significantly decreased AChE levels in gills | |||||||||
| Ostrea edulis | HDPE | Fragments | 0.48–316 μm | 2.5 and 25 μg l−1 | Food | Behavior | Significantly increased feeding rates at 25 μg l−1 | Green et al., 2016b | |
| Mortality | No effects on mortality | ||||||||
| Fragments | 0.48–316 μm | 80 μg l−1 | Food | Behavior | No effects on feeding rate | Green, 2016 | |||
| Development | No effects on shell growth | ||||||||
| O2 consumption | No effects on respiration rates | ||||||||
| PLA | Fragments | 0.6–363 μm | 2.5 and 25 μg l−1 | Food | Behavior | Significantly increased feeding rates at 2.5 and 25 μg l−1 | Green et al., 2016b | ||
| Mortality | No effects on mortality | ||||||||
| Fragments | 0.6–363 μm | 80 μg l−1 | Food | Behavior | No effects on feeding rate | Green, 2016 | |||
| Development | No effects on shell growth | ||||||||
| O2 consumption | No effects on respiration rates | ||||||||
| Rotifera | Brachionus manjavacas | PS | Spheres | 0.05–3 μm | 0.72–5.74 μg ml−1 | AM | Behavior | Feeding rate dose-dependently reduced by plastic particles, effects size-dependent up to particles of 0.5 μm | Snell and Hicks, 2011 |
| Reproduction | Significantly reduced population growth rate at 1.14 μg ml−1 for 0.05 μm particles (NOEC 0.57 μg ml−1), | ||||||||
| no effects for larger particles; | |||||||||
| No effects on population growth rate of F1 generation |
Effects on micro- and nano-sized plastic particles on benthic marine invertebrates.
Most studies of marine benthic organisms investigated PS exposure, including 10 experiments assessing the mechanical impact of functionalized PS-particles PS-COOH (4%, n = 2) and PS-NH2H (8%, n = 4; Figure 2B; Table 2). PE, specifically, High-density polyethylene (HDPE) particles, was the seconds most frequently tested plastic (12%, n = 6), followed by PVC, PP, and PE (6%, n = 3 each), biodegradable polyactic acid (PLA, 8%), and PA and PET (4% each; Figure 2B; Table 2). In 56% (n = 28) of the marine studies, the studied plastic particles were applied as spheres (Figure 2C and Table 2), with fragments contributing 38% (n = 19; Figure 2C) and polymer fibers 6% (n = 3; Figure 2C).
The main application route was via aqueous medium (70%, n = 35; Figure 2D), while effects of plastic particles applied in sediment and in spiked food were analyzed in only 12% (n = 6) and 18% (n = 9) of the studies, respectively (Figure 2D).
Mortality
Lethal effects were examined in 56% (n = 28; Table 2) of the relevant experiments, by this being the most frequently assessed parameter among the included marine studies. With regard to the model organism A. marina, no effects on mortality could be observed when treated with a variety of microplastics in sediments, including PVC-, PLA-, and HDPE-fragments (Green et al., 2016b; Table 2) as well as PS-spheres (Besseling et al., 2012; Table 2). Even if no effects on mortality could be determined for H. diversicolor exposed to PVC-fragments (Gomiero et al., 2018), Leung and Chan (2018) reported significantly increased mortality of another marine annelid, P. aibuhitensis, by small PS-spheres in a size-depending manner (Table 2). This size-dependency was supported by Lee et al. (2013), reporting significantly increased mortality rates of two generations of T. japonicus when treated with PS-spheres in various sizes (Table 2). However, the mortality rates of other copepod species (C. finmarchicus and C. helgolandicus) were not affected when PS-spheres where applied (Cole et al., 2015; Vroom et al., 2017; Table 2). The same results were obtained by Watts et al. (2016), reporting no effects of PS-spheres on the decapod C. maenas, neither of functionalized nor of non-functionalized particles (Table 2). Additionally, mortality of I. emarginata was also unaffected by PS-spheres, PS-fragments and PA-fibers (Hämer et al., 2014; Table 2). By contrast, Gray and Weinstein (2017) reported increased mortality rates of P. pugio treated with PE- and PS-spheres, PP-fragments, and PP-fibers (Table 2). PP-fibers generally induced significantly increased mortality, while the PE-spheres, PS-spheres, and the PP-fragments affected mortality rates in a size-dependent manner with larger particles being responsible for significant effects exclusively (Table 2). Lethal effects of PS- and PE-spheres were also assessed in Ciona robusta (Chordata; Messinetti et al., 2018), Paracentrotus lividus (Echinodermata; Messinetti et al., 2018) and Tripneustus gratille (Echinodermata; Kaposi et al., 2014; Table 2), with no effects being observed. Among the investigated marine molluscs, no lethal effects induced by a variety of plastic particles could be found (Table 2). Investigations on the lethality of HDPE-fragments in the blue mussel M. edulis and the flat oyster O. edulis failed to show any effects (von Moos et al., 2012; Green et al., 2016a; Table 2), which was also the case regarding O. edulis treated with biodegradable PLA-fragments (Green et al., 2016a). For M. galloprovincialis, neither PS- nor PE-fragments induced mortality as reported by Avio et al. (2015; Table 2).
Development
Effects on the development of benthic organisms in marine environments were examined in 46% of the studies (n = 23), as summarized in Table 2. While PVC-, PLA-, and HDPE-fragments did not induce any negative effects on the development of A. marina (Green et al., 2016b; Table 2), Besseling et al. (2012) reported significant effects on A. marina in terms of dry weight (dw) loss in a dose-dependent manner when exposed to PS-spheres (Table 2). These findings are in line with negative effects induced by PS-spheres on C. helgolandicus (Cole et al., 2015; Table 2). However, neither PS- nor PA-particles of various sized and shapes affected I. emarginata as reported by Hämer et al. (2014; Table 2). Additionally, effects of PS-spheres have further been reported to be size-dependent as indicated for the intergenerational developmental responses of T. japonicas (Lee et al., 2013) and development time of P. nana (Jeong et al., 2017), with both parameters being affected by nano-sized spheres exclusively (Table 2). However, studies examining the impact of small-scale plastics on echinoderms reported significant dose-dependent effects for a variety of applied particles, such as PS-spheres on T. gratilla (Kaposi et al., 2014), and significantly affected development of P. lividus exposed to plain PS-spheres (Martínez-Gómez et al., 2017; Messinetti et al., 2018), nanospheres of functionalized PS particles (PS-NH2; Della Torre et al., 2014) and HDPE-fragments (Martínez-Gómez et al., 2017). In terms of PS-spheres, comparable results were reported for the ascidian C. robusta, with a significant delay in juvenile development but no effects on larvae (Messinetti et al., 2018; Table 2). The impacts of micro- and nanoplastics on molluscan development were examined in C. gigas, M. galloprovincialis, and O. edulis (Table 2). While no effects were reported for C. gigas in a short-term experiment (Cole and Galloway, 2015), enhanced exposure time led to various significantly development parameters (Sussarellu et al., 2016). In terms of M. edulis and M. galloprovincialis, significant effects in a dose-dependent manner were reported for functionalized and non-functionalized PS-spheres (Paul-Pont et al., 2016; Balbi et al., 2017; Table 2), whereas shell growth of O. edulis was neither affected by HDPE- nor PLA-fragments (Green, 2016; Table 2).
Behavior
Behavioral alterations of benthic marine organisms due to nano- and micro-sized plastics were assessed in 46% of the studies (n = 23; Table 2). Studies of potential behavioral alterations induced by plastic particles included an examination of the effects on the bioturbation activity of A. marina exposed to PVC-, PLA- and HDPE-fragments (Green et al., 2016b). However, after 1 month, only the PVC-fragments had induced a reduction in behavior of A. marina, with no effects were observed for any of the other tested polymers (Table 2; Green, 2016). Most of the studies concerning behavioral alterations induced by small-scale plastic particles measured the feeding rate of the test organisms (Table 2). This was the case in studies conducted by Besseling et al. (2012) and Wright et al. (2013a) on the effects of PS-spheres and PVC-fragments in A. marina respectively, with feeding activity being reported to be affected dose-dependently (Table 2). Comparable results were measured for the arthropods C. helgolandicus (Cole et al., 2015) and C. typicus (Cole et al., 2013) respectively, with reduced feeding activities in a dose-dependent manner after exposure to PS-spheres (Table 2). Likewise, dose-dependent effects were also reported regarding the feeding rates of A. striata when treated with PS-spheres (Xu et al., 2017), as well as for PLA- and HDPE-fragments on M. edulis respectively (Green et al., 2016b; Table 2). However, neither PS-fragments or PA-fibers did impact the feeding rates of I. emarginata (Hämer et al., 2014; Table 2), nor did PS-spheres induce behavioral effects on M. edulis (Browne et al., 2008; Table 2). Similarly, Cole and Galloway (2015) did not find any significant effects of PS-spheres on the feeding activity of C. gigas in a short-term experiment, whereas Sussarellu et al. (2016) reported significantly higher algal consumption by oysters exposed for extended periods of time (Table 2). Dose-dependent variations in the impacts of plastic particles on the feeding behavior of O. edulis were reported by Green et al. (2016a,b), with HDPE- and PLA-fragments significantly enhancing feeding rates of mussels at particular concentrations exclusively (Table 2). Exposed to PS-spheres, size dependency was reported for the feeding activity of the rotifer B. manjavacas (Snell and Hicks, 2011), with effects being observed for the smallest particles only (Table 2).
Egestion
Closely linked to changes in feeding behavior are changes in egestion, being assessed in two experiments (2%; Table 2). Dose-dependent effects of PVC-fragments on the gut residence time of food in A. marina were reported by Wright et al. (2013a; Table 2), while the presence of nano-sized PS-spheres generally increased the production of pseudofeces in M. edulis (Wegner et al., 2012; Table 2).
Cellular response
The cellular responses of marine organisms to small-scale plastics were assessed in 36% (n = 18) of the studies (Table 2). PVC-fragments induced significantly increased phagocytic activity of immune cells in A. marina after chronic exposure, which was also the case for H. diversicolor as the proportions of phagocytic cells among coelomic fluid cells increased significantly in worms exposed to PVC particles (Gomiero et al., 2018). Heindler et al. (2017) examined the cellular responses of the arthropod P. crassirostris to PET-fragments with Histone 3 (H3) gene expression was significantly down regulated after 6 days but significant alterations were no longer detected after 18 days of recovery (Table 2). Additionally, Hsp7p0-like gene expression was not affected neither after 6 days of exposure nor at the end of the recovery phase (Heindler et al., 2017). Jeong et al. (2017) examined the molecular responses of the marine arthropod P. nana in terms of the activation of mitogen-activated protein kinase (MAPK), p38 and nuclear factor erythroid 2-related factor 2 (Nrf2), ROS levels, and the activities of the antioxidant enzymes GPx, glutathione reductase (GR), GST, and SOD. For ROS levels, phosphorylation of the MAPK protein extracellular signal-regulated kinase (p-ERK) as well as p38 and Nrf2, significant size-depending alterations were reported with smallest spheres inducing significant increases (Table 2). Additionally, size-dependencies were analyzed in antioxidant enzymes, with GPx activity was significantly enhanced in P. nana exposed to smallest PS-spheres exclusively, while the activities of GPx, GR, GST, and SOD were generally up regulated after plastic exposure of the copepod (Table 2). By contrast, there was no difference in the phosphorylation status of phosphorylated c-Jun N-terminal kinase (Jeong et al., 2017). Watts et al. (2016) also examined the impact of PS, exposing the crab C. maenas to non-functionalized and functionalized (PS, PS-COOH, and PS-NH2) spheres. Exposure to neutrally charged spheres resulted in significant effects on several hemolymph constituents and significantly reduced hemocyanin concentrations in a dose-dependent manner, while protein concentrations in the hemolymph of the crabs remained unchanged (Table 2). Overall, the exposure of C. maenas to carboxyl- or amino-coated plastics had no significant effect on any assessed parameters (Table 2; Watts et al., 2016). The impact of functionalized spheres was also assessed by Della Torre et al. (2014), who treated P. lividus with nanoparticles of PS-COOH and PS-NH2 (Table 2). PS-COOH spheres significantly up-regulated Abcb1 gene expression, whereas exposure to the amino-coated spheres induced an up-regulation of cas-8. No other effects on further analyzed stress genes (14-3-3ε, Abcc5, cas8, and p-38 MAPK) were observed (Table 2). Sussarellu et al. (2016) investigated the effects of PS-spheres on the mollusc C. gigas by analyzing its hyalinocyte and granulocyte sizes. Both hemocyte types were significantly larger in exposed organisms and the oxidative activity of these cells was altered (Table 2). Additionally, transcriptomic and proteomic analyses revealed further plastic-induced effects on C. gigas: within digestive glands, two clusters of transcripts exhibited similar expression patterns (up and down regulated), with glucocorticoid stimulus, fatty acid catabolic processes, respiratory burst, and cellular response to mechanical stimulus as the main significantly enriched Gene Ontology (GO) biological processes (Sussarellu et al., 2016). In gonads, the expression of transcripts related to glutamine biosynthetic processes, the positive regulation of insulin secretion and of epithelial cell proliferation, and ovarian follicle cell–cell adhesion were among the significantly enriched GO biological processes (Sussarellu et al., 2016). In the transcript of oocytes, the significantly enriched GO biological processes were proteolysis, embryo development, and ion binding. In addition, two abundant protein spots, identified as arginine kinase, were detected in the proteome. The expression of this enzyme was significantly lower in exposed oysters, while the expression of the protein severin was higher in their oocytes (Sussarellu et al., 2016). Regarding effects of irregularly shaped HDPE-fragments on the blue mussel M. edulis, von Moos et al. (2012) reported a significant increase in granulocytoma formation and a significantly decreased destabilization time of lysosomes, while no effects on biomarkers of oxyradical damage (lipofuscin accumulation) or on neutral lipid content could be found (Table 2). No effects on the oxidative status of the hemolymph or in hemocyte viability and phagocytic activity were measured by Browne et al. (2008), analyzing M. edulis treated with PS-spheres. However, Paul-Pont et al. (2016) exposed organisms from the Mytilus-complex (M. edulis and M. galloprovincialis) to PS-spheres and reported the percentage of dead hemocytes, ROS production and the activity of anti-oxidant enzyme, CAT, and lipid peroxidation (LPO), being significantly affected, while phagocytosis as well as hemocyte and granulocyte concentrations have been unaffected (Table 2). In terms of gene expression, only gill mRNA levels changed significantly in response to the PS-spheres, specifically, lys levels were enhanced and cat levels significantly reduced (Table 2). After depuration, both the percentage of dead hemocytes and phagocytosis capacity were significantly higher; granulocyte concentrations and hemocyte counts were significantly lower, GST, and SOD activities were significantly increased, and LPO activity significantly decreased in exposed vs. control mussels. By contrast, after depuration there were no effects on phagocytosis activity, ROS production, and hyalinocyte concentration (Table 2). Expression of the genes sod and pk in the gills of the mussels was increased, whereas that of the genes cat and pgp was significantly decreased in the gills and digestive glands, respectively (Table 2). Using M. galloprovincialis as the target organism, impacts of amino-coated (PS-NH2) nano-sized particles were assessed by Canesi et al. (2015) and Balbi et al. (2017). Hemocyte functional parameters (lysosomal membrane stability, lysosomal enzyme release, phagocytosis, ROS, and nitric oxide (NO) production) and apoptotic parameters (mitochondrial membrane potential and cardiolipin peroxidation) were evaluated by Canesi et al. (2015), with significant dose-dependent decreases measured in lysosomal membrane stability (LMS) at highest concentrations tested (Table 2). However, lysosomal enzyme release, phagocytosis, and ROS production reacted more sensitive with significant alterations measured even at lowest particle concentrations tested (Canesi et al., 2015; Table 2). Additionally, NO production was also increased, with the amount varying depending on the incubation time (Canesi et al., 2015). In terms of apoptotic parameters measured, significant effects could only be measured at highest concentrations applied (Canesi et al., 2015; Table 2). Balbi et al. (2017) evaluated the effects induced on the transcription of genes related to neuroendocrine signaling (serotonin receptor, 5-HTR), antioxidant defense (CAT, SOD), biotransformation (GST, ABC transporter p-glycoprotein [ABCB]), biomineralization (extrapallial protein [EP], carbonic anhydrase [CA]), autophagy, growth and metabolism (mammalian target of rapamycin [mTor]), apoptosis (p53), the immune response (Toll-like receptor I isoform [TLR-i]), shell formation (chitin synthetase [CS]), and the immune response/intracellular digestion lysozyme (LYSO). A time-dependent response of genes were reported, with significant up-regulation of CS and a significant decrease in LYSO after 24 h and a general down-regulation of all genes after 48 h, with significant alterations in the transcription of CS, CA, EP, ABCB, and LYSO (Balbi et al., 2017; Table 2). Cellular responses of M. galloprovincialis induced by PS- and PE-fragments were investigated by Avio et al. (2015) by assessing the levels of tissue biomarkers of immunological alterations (granolocytes/hyalinocytes ratio, phagocytosis activity, and LMS in hemocytes), neurotoxic responses (acetylcholinesterase [AChE] in hemocytes and gills), cellular, and oxidative stress biomarkers in digestive tissues (acyl-CoA oxidase [AOX], anti-oxidant defenses [CAT, GST, glutathione reductase, total glutathione], total oxyradical scavenging capacity [TOSC], lysosomal latency period [LP], malondialdehyde [MDA], lipofuscin, and neutral lipids), as well as genotoxic effects in hemolymph (DNA strand breaks, micronuclei frequency (MN), and nuclear alterations (NA). Additionally, transcriptional analyses were conducted for organisms treated with the PS-particles exclusively (Avio et al., 2015). Among the immunological responses of hemocytes, there was no significant change in phagocytosis whereas the granulocytes/hyalinocytes ratio decreased significantly in mussels exposed to PE-fragments and LMS decreased significantly in those treated with PS-fragments (Table 2). Although there were no obvious genotoxic effects in hemolymph, both the PE- and the PS-fragments induced neurotoxic effects in gills, including a significant decrease in AChE (Table 2). Alterations in biomarkers of cellular and oxidative stress included the significant inhibition of Se-dependent glutathione peroxidases in mussels exposed to the PS-fragments, with a similar trend determined for CAT (Table 2). Overall, the analysis of transcriptional responses identified a total of 2,143 genes differentially expressed in response to PS exposure, with 1,062 of those genes being down-regulated and 1,081 being up-regulated (Avio et al., 2015).
O2 consumption
The effects induced by the mechanical hazards of micro- and nano-sized plastics on oxygen (O2) consumption by marine organisms were examined in 20% (n = 10) of the included experiments (Table 2). Alterations in the O2 consumption of the annelid A. marina treated with PVC-, PLA-, and HDPE-fragments were analyzed by Green et al. (2016a), reporting dose-dependent effects with significantly increased respiration rates at highest concentrations tested (Table 2). As reported by Watts et al. (2016), O2 consumption rates of C. maenas were significantly reduced by the highest applied concentration of plain PS-spheres, whereas there were no significant changes following exposure to functionalized PS-COOH and PS-NH2 spheres (Watts et al., 2016; Table 2). However, no effects of plain PS-spheres on O2 consumption were reported for C. helgolandicus (Cole et al., 2015; Table 2). Additionally, no significant changes in O2 consumption induced by fragments of PS, HDPE, and PLA were measured for A. striata and O. edulis (Green, 2016; Xu et al., 2017; Table 2).
Reproduction
The mechanical hazards affecting reproduction (egg production, fecundity, fertilization rates, oocyte number, and population size and growth rate) were assessed in 7 (14%) of the marine studies (Table 2). Heindler et al. (2017) reported significant dose-dependent effects of PET-fragments on the reproduction of P. crassirostris, with population size being more sensitive than egg production (Table 2). Population size was also investigated by Snell and Hicks (2011) assessing two generations of the rotifer B. manjavacas treated with PS-spheres. For these particles, distinct size-depending effects were observed with significantly reduced population growth rates for the smallest particles tested (Table 2). Size-dependent effects of PS-spheres were also found in T. japonicus, with significant effects on the fecundity of organisms exposed to larger spheres at lowest concentrations tested (Lee et al., 2013; Table 2). However, significant effects of PS-spheres have additionally been reported in terms of fertilization rates of P. lividus (Martínez-Gómez et al., 2017) and the decline in numbers of oocytes of C. gigas (Sussarellu et al., 2016; Table 2).
Assimilation
Sussarellu et al. (2016) examined the effects of PS-spheres on energy budgets of C. gigas, showing absorption efficiency being significantly higher in exposed than in control oysters (Table 2). However, PS-fragments did not alter absorption efficiency of A. striata (Xu et al., 2017). By contrast, the energy budget of the marine arthropod C. maenas showed to be rather sensitive toward exposure with PP-fibers, with a reduction in growth capacity noted at each of the applied concentrations (Watts et al., 2015). Wright et al. (2013a) showed a dose-dependent decrease in total available energy reserves of A. marina following exposure to PVC-fragments, with significant reductions at highest concentrations tested, as well as significantly lowered lipid reserves and comparable trends for proteins and sugar reserves (Table 2). Based on a conceptual carbon budget model, significant energy losses were also reported in the copepod C. helgolandicus treated with PS-spheres (Cole et al., 2015; Table 2).
Regeneration
Finally, the regeneration potential of P. aibuhitensis after PS-spheres exposure was investigated by Leung and Chan (2018), reporting significant decreases in regeneration rates, especially in worms exposed to the smallest particles (Table 2).
Chemical Impacts
Leachates
Freshwater benthic fauna
Among the 24 experiments focusing on leachates of micro- and nanoplastics, a single one (4%) targeted a freshwater organism (Table 3). Zhao et al. (2017) exposed C. elegans to PS-spheres in various concentrations, examining the effects on cellular responses, behavior, and reproduction. However, dose-dependent effects on each assessed parameter were reported with significant alterations at the highest concentration applied (Table 3).
Table 3
| Habitat | Taxa | Species | Material | Shape | Conc. | Matrix | Leaching | Parameter | Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Marine | Arthropoda | Amphibalanus amphitrite | HDPE | Fragments | 0.004–0.5 m2m l−1 | AM | 24 h | Mortality | Significantly increased mortality (LOEC: 0.1 m2m l−1) | Li H. X. et al., 2016 |
| Development | Significantly inhibited settlement (LOEC: 0.004 m2m l−1) | |||||||||
| LDPE | Fragments | 0.004–0.5 m2m l−1 | AM | 24 h | Mortality | Significantly increased mortality (LOEC: 0.1 m2m l−1) | Li H. X. et al., 2016 | |||
| Development | Significantly inhibited settlement (LOEC: 0.004 m2m l−1) | |||||||||
| PC | Fragments | 0.004–0.5 m2m l−1 | AM | 24 h | Mortality | Significantly increased mortality (LOEC: 0.1 m2m l−1) | Li H. X. et al., 2016 | |||
| Development | Significantly inhibited settlement (LOEC: 0.004 m2m l−1) | |||||||||
| PET | Fragments | 0.004–0.5 m2m l−1 | AM | 24 h | Mortality | Significantly increased mortality (LOEC: 0.1 m2m l−1) | Li H. X. et al., 2016 | |||
| Development | Significantly inhibited settlement (LOEC: 0.004 m2m l−1) | |||||||||
| PP | Fragments | 0.004–0.5 m2m l−1 | AM | 24 h | Mortality | Significantly increased mortality (LOEC: 0.1 m2m l−1) | Li H. X. et al., 2016 | |||
| Development | Significantly inhibited settlement (LOEC: 0.004 m2m l−1) | |||||||||
| PS | Fragments | 0.004–0.5 m2m l−1 | AM | 24 h | Mortality | Significantly increased mortality (LOEC: 0.5 m2m l−1) | Li H. X. et al., 2016 | |||
| Development | Significantly inhibited settlement (LOEC: 0.004 m2m l−1) | |||||||||
| PVC | Fragments | 0.004–0.5 m2m l−1 | AM | 24 h | Mortality | Significantly increased mortality (LOEC: 0.1 m2m l−1) | Li H. X. et al., 2016 | |||
| Development | Significantly inhibited settlement (LOEC: 0.004 m2m l−1) | |||||||||
| Nitocra spinipes | Bio-bag | Fragments | 100 g l−1 | AM | 72 h | Mortality | Significantly induced mortality (LC10 Bio: 24 g l−1 (0 h), 12 g l−1 (96 h), 29 g l−1 (192 h), | Bejgarn et al., 2015 | ||
| 18 g l−1 (288 h); LC50 Bio: >100 g l−1 (0 h), 24 g l−1 (96 h), 36 g l−1 (192 h), 26 g l−1 (288 h)) | ||||||||||
| Bio-PET | Fragments | 100 g l−1 | AM | 72 h | Mortality | No effects on mortality | Bejgarn et al., 2015 | |||
| HDPE | Fragments | 100 g l−1 | AM | 72 h | Mortality | No effects on mortality | Bejgarn et al., 2015 | |||
| LDPE | Fragments | 100 g l−1 | AM | 72 h | Mortality | No effects on mortality | Bejgarn et al., 2015 | |||
| PET | Fragments | 100 g l−1 | AM | 72 h | Mortality | No effects on mortality | Bejgarn et al., 2015 | |||
| PLA | Fragments | 100 g l−1 | AM | 72 h | Mortality | No effects on mortality | Bejgarn et al., 2015 | |||
| PP | Fragments | 100 g l−1 | AM | 72 h | Mortality | LC10 PP: 37 g l−1 (192 h), 16 g l−1 (288 h), LC50 PP: 71 g l−1 (192 h), 21 g l−1 (288 h) | Bejgarn et al., 2015 | |||
| PS | Fragments | 100 g l−1 | AM | 72 h | Mortality | No effects on mortality | Bejgarn et al., 2015 | |||
| PUR | Fragments | 100 g l−1 | AM | 72 h | Mortality | LC10 PUR: 26 g l−1 (0 h), 40 g l−1 (96 h), >100 g l−1 (192 h), | Bejgarn et al., 2015 | |||
| LC5C0 PUR: 85 g l−1 (0 h), >100 g l−1 (96 h), >100 g l−1 (192 h) | ||||||||||
| PVC | Fragments | 100 g l−1 | AM | 72 h | Mortality | LC10 PVC: 16–43 g l−1 (0 h), 23–35 g l−1 (96 h), 12–87 g l−1 (192 h), 11 g l−1 (288 h), | Bejgarn et al., 2015 | |||
| LC50 PVC: 35–>100 g l−1 (0 h), 35–60 g l−1 (96 h), 24–52 g l−1 (192 h), 21 g l−1 (288 h) | ||||||||||
| Rubber | Fragments | 100 g l−1 | AM | 72 h | Mortality | LC1C0 Rubber: 6 g l−1 (0 h), 6 g l−1 (96 h), LC50 PUR: 8 g l−1 (0 h), 7 g l−1 (96 h) | Bejgarn et al., 2015 | |||
| Echinodermata | Lytechinus variegatus | PE | Fragments | 250 ml l−1 | AM | 24 h | Development | Significantly affected embryo development in the pellet-water interface assay, | Nobre et al., 2015 | |
| Significantly affected embryo development in elutriate assay for virgin pellets exclusively | ||||||||||
| Paracentrotus lividus | PS | Spheres | 103-105 part. ml−1 | AM | 30 d | Development | Significantly affected embryo development | Martínez-Gómez et al., 2017 | ||
| HDPE | Fragments | 0.005–5 g l−1 | AM | 30 d | Development | Significantly affected embryo development in leachate assays of 0.005 and 5 g l−1 | Martínez-Gómez et al., 2017 | |||
| Mollusca | Perna perna | PP | Fragments | 50–200 ml l−1 | AM | 24 h | Development | Significantly affected embryo development in leachate assays with PP (LOEC 50 ml l−1), | Gandara E Silva et al., 2016 | |
| Effects significantly higher in tube assays compared to beaker assays, | ||||||||||
| PP | Fragments | 200 ml l−1 | AM | 24 h | Development | Significantly affected embryo development in leachate assays, | Gandara E Silva et al., 2016 | |||
| unknown | Effects of beach-collected fragments significantly higher compared to PP fragments | |||||||||
| Freshwater | Arthropoda | Ceriodaphnia dubia | PS | Fragments | 4 EPS cups l−1 | AM | 30 min | Mortality | Significantly increased mortality | Thaysen et al., 2018 |
| Reproduction | Significantly decreased total number of offspring; | |||||||||
| Delay in time to first brood | ||||||||||
| Nematoda | Caenorhabditis elegans | PS | Spheres | 10–10,000 μg l−1 | AM | 1 w | Cellular response | Significantly reduced intestinal ROS production at 10,000 μg l−1 | Zhao et al., 2017 | |
| Behavior | Significantly decreased locomotion (head trash, body bend) at 10,000 μg l−1 | |||||||||
| Reproduction | Significantly decreased reproduction (brood size) at 10,000 μg l−1 |
Effects of plastic leachates on benthic invertebrates.
Marine benthic fauna
All of the remaining studies (96%, n = 23) on chemical hazards posed by leachates of plastics examined marine organisms (Table 3), including arthropods (75%, n = 18), echinoderms (13%, n = 3), and molluscs (8%, n = 1; Figure 3A). In 13% (n = 3) of those studies, effects were examined on leachates of PS, HDPE, PP, and biodegradable plastics (each 12%, n = 3; Figure 3B). Leachates of LDPE, PVC, and PET were assessed in two studies each (8% of the investigations; Figure 3B). PE, PC, PUR, rubber, and an unknown plastic polymer were investigated in single studies (4%, n = 1; Figure 3B). The leachates derived from plastic fragments were assessed in 22 of the 23 studies, while a single study used spheres (4%; Figure 3C). All of the studies were conducted in aqueous medium and mainly assessed mortality (60%, n = 18) and/or development (40%, n = 12; Table 3).
Figure 3
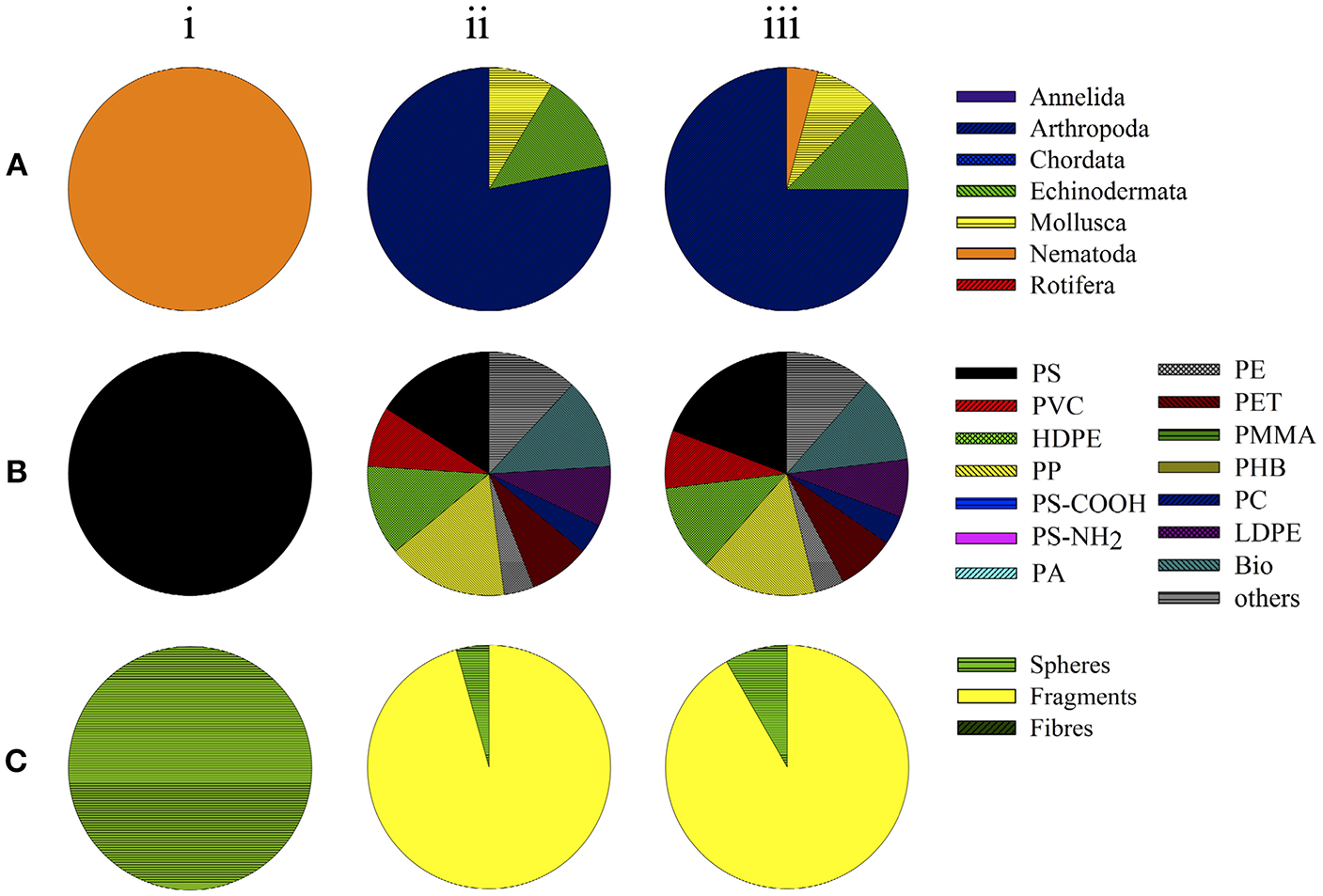
Toxicity assays examining chemical effects by leachates of plastic particles in organisms in (i) freshwater studies, (ii) marine studies, and (iii) total. Data depict the groups of organisms (taxa) used (A), polymer types (B), and shape of small-scale plastic particles applied (C).
Mortality
Li H. X. et al. (2016) investigated the impact of various plastic polymers (HDPE, LDPE, PC, PET, PP, PS, and PVC), applied as fragments, on the mortality of juvenile A. amphitrite (Table 3). However, mortality increased in a polymer- and dose-dependent manner, with distinct effects in barnacles at low concentrations of HDPE, LDPE, PC, PET, PP, and PVC, while PS induced significant effects at the highest concentration tested exclusively (Table 3). Bejgarn et al. (2015) also found polymer-dependent differences in their examination on the mortality of N. spinipes induced by leachates of HDPE, LDPE, PET, PP, PS, PUR, PVC, rubber, biodegradable, and unknown polymers (Table 3). Additionally, the same authors compared the effects of selected plastics that had been artificially aged via irradiance with a xenon lamp (wavelength 300–800 nm, 765 W m2) to simulate natural sunlight (Bejgarn et al., 2015; Table 3). However, acute toxicity toward N. spinipes was not induced by leachates of 62% of the plastic materials used (n = 13), while effects were measured in the remaining 38% (n = 8), before and/or after their irradiation (Bejgarn et al., 2015; Table 3). Generally, no common trend in toxicity, as a function of irradiation time, could be observed for the various polymers (Table 3).
Development
The development of juvenile A. amphitrite, measured as settlement success, was investigated by Li H. X. et al. (2016) exposing the organisms to various fragments of HDPE, LDPE, PC, PET, PP, PS, and PVC. Settlement was dose-dependently affected for all polymers investigated, being significantly reduced at the lowest concentrations applied (Table 3). Nobre et al. (2015) exposed embryos of L. variegatus to leachates of PE-fragments in a pellet-water interface assay and an elutriate assay. Anomalous larval development was observed in sea urchins assayed by either method, together with significant reductions in the proportions showing normal development patterns (Table 3). In comparable assays conducted by Gandara E Silva et al. (2016), P. perna was exposed to beach-collected pellets and to commercially available virgin PP-fragments. Exposure to the PP-fragments resulted in malformations or dead embryos in a dose-dependent manner, with significant effects at the lowest concentration tested (Table 3). Moreover, the beach-collected pellets were significantly more toxic than the PP-fragments in a comparative assay (Table 3). Embryonic development was also assessed by Martínez-Gómez et al. (2017), by treating zygotes of P. lividus with leachates of PS-spheres and HDPE-fragments, with abnormalities in the embryonic development being recorded for both polymers applied (Table 3).
Interaction With Chemicals
Studies on effects induced by the interaction of micro- and nanoplastics with pollutants on benthic organisms are scarce. The eight experiments included in the present review were conducted with marine organisms exclusively, three (38%) using annelids and arthropods and two (25%) using molluscs (Figure 4A). Plastic particles mainly consisted of PS (50%, n = 4; Figure 4B) but PVC and PE were examined as well (25% each, n = 2 each). These particles were applied as fragments and spheres in four studies each (Figure 4C). Among the chemicals investigated, polycyclic aromatic hydrocarbons (PAHs) were most frequently tested, followed by polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), pharmaceuticals and personal care products (PCPPs) as well as nonylphenol ethoxylates (NPEOs; Figure 4D). The reviewed assays were conducted in aqueous medium or sediment in 70% of the studies, while exposure via the diet applied was assessed in two studies only (Figure 4E).
Figure 4
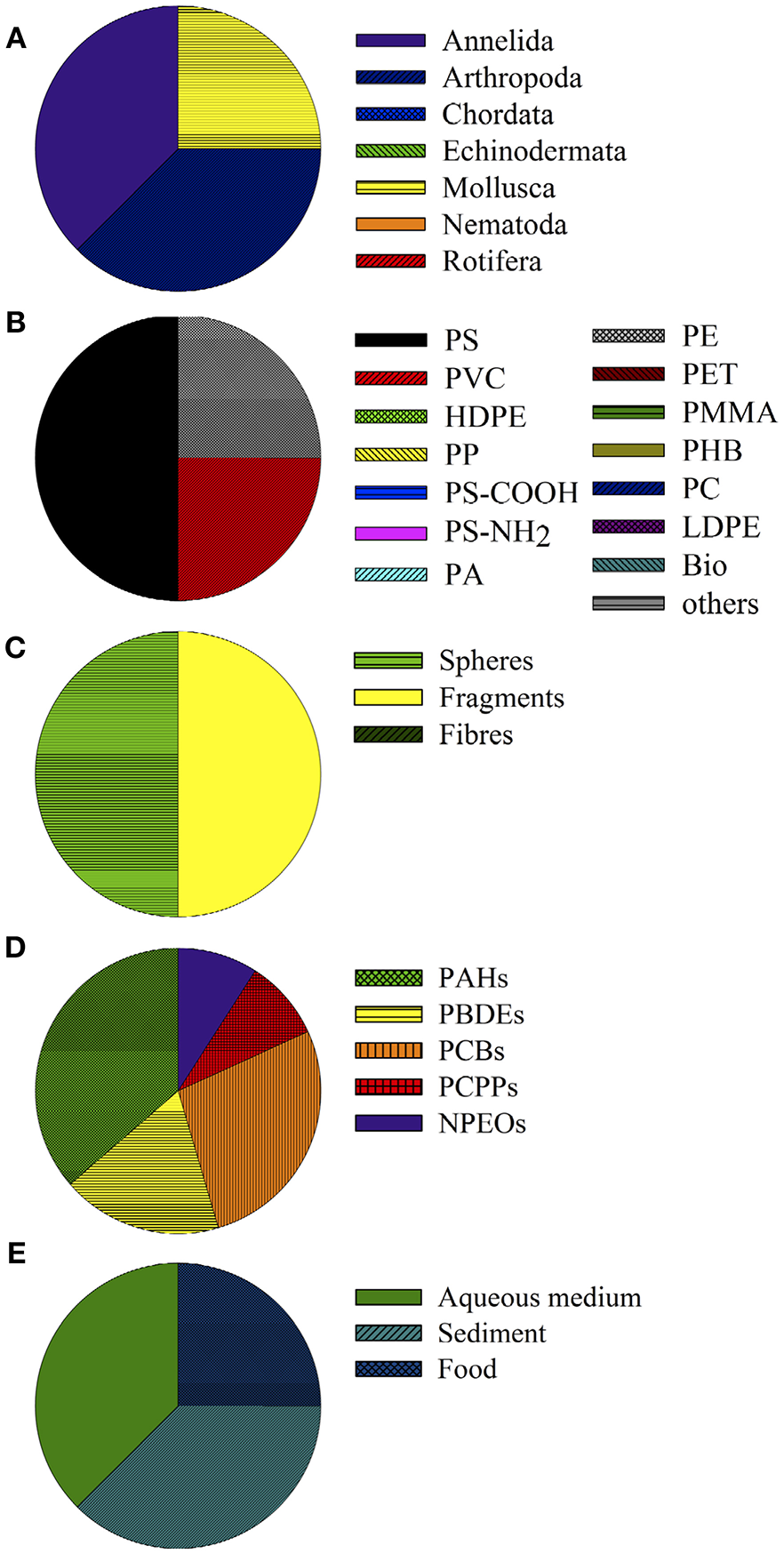
Toxicity assays examining chemical effects posed by the interaction of chemicals and plastic particles in marine benthic invertebrates. Data depict the groups of organisms (taxa) used (A), polymer types (B), shape of particles applied (C), POPs used as co-contaminant (D), and matrix or route of exposure (E).
Development
Devriese et al. (2017) examined the developmental alterations in N. norvegicus after exposure to PE- and PS-spheres cross-contaminated with PCBs (applied as congeners 28, 52, 101, 118, 138, 153, 180, CB29, CB112, and CB140). After 3 weeks of exposure, no significant effects on the wet weight (ww), carapace length, and condition of exposed lobsters could be found (Table 4). Species of the Mytilus complex were treated with PS and the PAH fluoranthene (FLU) over 7 days, followed by 7 days of depuration (Paul-Pont et al., 2016). At day 7, a significant increase in developmental effects, seen as histopathological lesions/abnormalities, was demonstrated in mussels exposed to microplastic particles and FLU, alone or in combination (Paul-Pont et al., 2016; Table 4). After 7 days of depuration, the observed effects were still significant for the combined exposure treatments (Paul-Pont et al., 2016; Table 4).
Table 4
| Habitat | Phylum | Species | Material | shape | PM conc. | Chem. | Chem. conc. | Matrix | Parameter | Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marine | Annelida | Arenicola marina | PS | Fragments | 0.074–7.4% sed. dw | PCBs | 1.84 μg kg−1 | S | Accumulation | Significantly increased accumulation of PCBs | Besseling et al., 2012 |
| Behavior | No effects on feeding activity | ||||||||||
| Mortality | No effects on mortality | ||||||||||
| PVC | Fragments | 50 mg l−1 | Nonylphenol | 0.69 μg g−1 | S | Accumulation | Significantly less chemicals accumulated under PVC exposure | Browne et al., 2013 | |||
| Phenanthrene | 0.11 μg g−1 | Mortality | Significantly increased mortality by Triclosan, | ||||||||
| Triclosan | 57.3 μg g−1 | no effects by Nonylphenol, Phenanthrene, PBDE-47 | |||||||||
| PBDE-47 | 9.49 μg g−1 | Cellular response | Significantly reduced phagocytic activity by Nonylphenol, no effects by Phenanthrene, PBDE-47 and Triclosan; | ||||||||
| Significantly reduced oxidative status by Phenanthrene, no effects by Nonylphenol, Phenanthrene PBDE-47 | |||||||||||
| Behavior | Significantly reduced feeding activity by Triclosan, no effects by Nonylphenol, Phenanthrene PBDE-47 | ||||||||||
| Hediste diversicolor | PVC | Fragments | 200 and 2,000 part. kg−1 | B(a)P | 4.58 μg kg−1 | S | Accumulation | Significantly increased accumulation of B(a)P | Gomiero et al., 2018 | ||
| Mortality | No effects on mortality | ||||||||||
| Cellular response | Significantly decreased phagocytic activity at 200 part. kg−1 (28 d), and at 2,000 part. kg−1 (10 and 28 d); | ||||||||||
| No effects on extracellular lysosome release; | |||||||||||
| Significantly decreased mitochondrial activity at 200 part. kg−1 (28 d) and at 2,000 part. kg−1 (10 and 28 d); | |||||||||||
| Significantly decreased lysosomal membrane stability (LMS); | |||||||||||
| Significantly increased oxyradical production | |||||||||||
| Significantly increased micronuclei frequency and DNA strand breaks; | |||||||||||
| Significantly increased Lipofuscin content (28 d) and | |||||||||||
| Catalase activity (10 d) | |||||||||||
| Arthropoda | Allorchestes compressa | PE | Fragments | 0.1 g l−1 | PBDEs | 50 and 500 ng g−1 MP | AM | Accumulation | Significantly lowered accumulation of PBDEs, | Chua et al., 2014 | |
| Higher-brominated PBDEs accumulated in greater rates than lower-brominated congeners | |||||||||||
| Nephrops norvegicus | PE | Spheres | 3.1 mg l−1 | PCBs | 8.7 μg g−1 MP | Food | Accumulation | Limited effects of PE on accumulation of PCBs | Devriese et al., 2017 | ||
| Development | No effects on condition factor and nutritional state | ||||||||||
| PS | Spheres | 3.1 mg l−1 | PCBs | 8.7 μg g−1 MP | Food | Accumulation | No effects of PS on accumulation of PCBs | Devriese et al., 2017 | |||
| Development | No effects on condition factor and nutritional state | ||||||||||
| Mollusca | Mytilus edulis | PS | Spheres | 32 μg l−1 | Fluoranthene | 30 μg l−1 | AM | Accumulation | No effects on accumulation after 7 d, significantly higher concentrations after depuration (7 d) | Paul-Pont et al., 2016 | |
| Development | Significantly higher histopathological lesions after depuration | ||||||||||
| Cellular response | Lowered no. of dead hemocytes; | ||||||||||
| Significantly decreased phagocytosis activity and ROS production; | |||||||||||
| No effects on hemocyte and granulocyte concentration; | |||||||||||
| Significantly reduced CAT activity and LPO, | |||||||||||
| Significantly increased SOD, GST and GR activity after depuration; | |||||||||||
| Significantly increased cat levels and increased in pk and sod, | |||||||||||
| Significantly increased cat expression after depuration and lower levels of pk, gpx, idp, sod, and amylase | |||||||||||
| Mytilus galloprovincialis | PS | Spheres | 32 μg l−1 | Fluoranthene | 30 μg l−1 | AM | Accumulation | No effects on accumulation after 7 d, significantly higher concentrations after depuration (7 d) | Paul-Pont et al., 2016 | ||
| Development | Significantly higher histopathological lesions after depuration | ||||||||||
| Cellular response | Lowered no. of dead hemocytes; | ||||||||||
| Significantly decreased phagocytosis activity and ROS production; | |||||||||||
| No effects on hemocyte and granulocyte concentration; | |||||||||||
| Significantly reduced CAT activity and LPO, | |||||||||||
| Significantly increased SOD, GST and GR activity after depuration; | |||||||||||
| Significantly increased cat levels and increased in pk and sod, | |||||||||||
| Significantly increased cat expression after depuration and lower levels of pk, gpx, idp, sod, and amylase |
Effects of contaminated plastic particles on benthic (marine) invertebrates.
Cellular response
The cellular responses of the lugworm A. marina to sediment co-contamination with nonylphenol (NPEO), Triclosan (PCPP), phenanthrene (PAH), and PBDE-47 (PBDE) combined with PVC-fragments were studied by Browne et al. (2013; Table 4). The phagocytic activity of A. marina decreased significantly in treatments consisting of sediment, PVC and NPEO, but also in those in which the sediments were spiked with NPEO alone (Browne et al., 2013). None of the additionally examined POPs induced significant effects on phagocytic activity (Table 4). An assay of the coelomic fluid of the lugworm showed that the ingestion of PVC-contaminated sediment resulted in a >30% lower oxidative stress response, while exposure to pollutants and additives through desorption from PVC had no effect (Table 4). Gomiero et al. (2018) investigated cellular responses of H. diversicolor exposed to PVC-fragments in combination with the PAH benzo(a)pyrene (B(a)P) in sediments over 10 and 28 days. Phagocytic activity was significantly reduced in organisms exposed for 28 days to B(a)P and both PVC concentrations, and for 10 days to B(a)P and highest concentrations of fragments (Table 4). Although B(a)P-exposure alone reduced phagocytic activity, the effects were significant only in combination with the plastics (Gomiero et al., 2018). In addition, mitochondrial activity and LMS were significantly decreased and oxyradical production significantly increased in H. diversicolor after combined exposure (Table 4). Again, the effect patterns were similar in organisms exposed to B(a)P alone, but combined contamination greatly intensified the observed alterations (Gomiero et al., 2018). In that same study, the ability of plastic particles to increase dispersion and the effects of genotoxic pollutants, such as B(a)P, were investigated by scoring the increments of micronuclei formation and DNA strand breaks in the coelomocytes of H. diversicolor. The results supported those obtained for mitochondrial activity, LMS, and oxyradical production, with co-contamination enhancing the effects of POP alone (Gomiero et al., 2018). An investigation of the effects on the lipofuscin content and CAT activity of H. diversicolor likewise showed enhancement by co-contamination, with significantly higher values in organisms treated with B(a)P and PVC than in controls and in worms exposed to sediments spiked only with B(a)P (Table 4).
Paul-Pont et al. (2016) focused on hemocyte parameters, enzyme activities and gene expression in M. edulis and M. galloprovincialis exposed to PS-spheres and FLU. Compared to organisms treated with FLU alone, significant decreases in phagocytic activity and ROS production after combined exposure were observed, together with decreased numbers of dead hemocytes. By contrast, hemocyte and granulocyte concentrations did not differ between treatments (Table 4). CAT activity and LPO were also significantly reduced under combined exposure vs. FLU alone (Table 4). Gene expression was also altered after combined vs. single exposure, evidenced by significantly higher cat levels in gills and the very high levels of pk and sod mRNA (Table 4). After depuration, single exposure to FLU induced a reduction in cat mRNA, and combined exposure a significant increase (Table 4). The expression of sod, gpx, idp, pk, and amylase increased significantly under FLU exposure, but not in organisms treated with both PS and FLU (Table 4).
Mortality
An effect of combined exposure on mortality was determined in three studies (Table 4). Browne et al. (2013) reported the increased mortality of A. marina exposed to a combination of PVC-fragments and PCPP, but not to co-contamination with NPEO, PAH, or PBDE-47 (Table 4). Besseling et al. (2012) and Gomiero et al. (2018) investigated the effects on the mortality of A. marina or H. diversicolor in treatments consisting of PS-spheres combined with PCBs and PVC-fragments with B(a)P, respectively, with no alterations being observed under any of the conditions tested (Table 4).
Behavior
Behavioral alterations, measured as effects on feeding activity, in the lugworm A. marina were assessed by Besseling et al. (2012) and by Browne et al. (2013) with contrary results obtained: while there were no alterations in feeding activity following combined exposure to PS-fragments and PCBs or to PVC-fragments and NPEO, PAH, and PBDE-47, treatments consisting of PVC-particles combined with PCPP reduced feeding activity significantly (Table 4).
Accumulation
POP accumulation and the consequences of POP co-contamination with plastic particles were examined in every study included in this part of the review (Table 4). Browne et al. (2013) reported less NPEO, PAH, PCPP, and PBDE-47 accumulation in A. marina exposed to the chemicals together with PVC-fragments. These results were supported by those of Chua et al. (2014) in their study of A. compressa. The authors found less accumulation of PBDEs when in combination with PE-fragments. However, higher-brominated PBDEs accumulated at higher rates than did lower-brominated congeners (Table 4). A polymer-dependent effect on POP accumulation was shown by Devriese et al. (2017), who reported significantly increased body concentrations of PCBs in the tail tissues of N. norvegicus exposed to PE-spheres. Co-exposure with PS-spheres, however, did not have any effects on PCB accumulation, independent of the particle size tested (Table 4). By contrast, Besseling et al. (2012) reported a significant, but non-dose-dependent increase in PCB accumulation in A. marina exposed to sediments additionally spiked with PS-fragments (Table 4). Effects of co-contamination on accumulation were also reported for PAH and PVC-fragments, as Gomiero et al. (2018) showed a significant increase in accumulation of B(a)P in the body of H. diversicolor under combined exposure (Table 4). In addition, Paul-Pont et al. (2016) reported time-dependent effects on the accumulation of FLU induced by PS-spheres for M. edulis and M. galloprovincialis (Table 4). While no effects on accumulation were found after 7 days of exposure, body concentrations in both mussels were significantly higher after depuration (Table 4).
Conclusion and Gap-Analysis
The present review is a comprehensive analysis of studies investigating the (eco) toxicological effects of micro- and nanoplastics on benthic invertebrates in marine and in freshwater ecosystems. However, 80% (n = 39) of the respective publications analyzed referred to marine organisms and only 20% (n = 10) to freshwater organisms. Moreover, the toxic effects induced by leachates of plastic debris on freshwater organisms were analyzed in a single study (Zhao et al., 2017; Table 3), whereas the interactive hazards posed by micro- and nanoplastics and POPs in freshwaters have yet to be addressed at all (Table 4). Most of the benthic organisms assessed belonged to the macrofauna, investigated in 71% of freshwater studies and 99% of marine studies, with arthropods and molluscs as the most widely used organisms (72% of all studies). By contrast, despite their indisputable ecological relevance, organisms belonging to the micro- or meiofauna have been widely neglected. However, these groups of organisms should be of particular concern regarding nano- and micro-sized plastics, especially due to their key functions in aquatic ecosystems, and necessarily be included in further research.
Among the polymers used in the various assays, PS was the most commonly used: 42 vs. <10% each for other polymers, including PP, HDPE, and PVC (Figures 2B, 3B, 4B). Overall, more polymer types were used in studies investigating the hazards posed by plastics on marine than on freshwater organisms, and only PS was used in the leachate study in freshwater (Figure 3B). Even though PS is one of the most commonly found polymers in nature (e.g., Barnes et al., 2009; Browne et al., 2010; Klein et al., 2015), other types of plastics present in the environment should also be comprehensively assessed, especially regarding the specific gravity of different plastic classes (e.g., Duis and Coors, 2016; Auta et al., 2017) and the associated individual risk to pose hazards on benthic organisms. Additionally, different polymers vary in their POP-sorption capacity, with consistently higher concentrations of PCBs and PAHs absorbing to HDPE, LDPE, and PP than to PET and PVC, while PAH sorbs more to PE than to PP or PVC (Rochman et al., 2013; Bakir et al., 2014). Hence, the effects of both the polymer and the associated contaminants have to be considered when assessing the potential risks of small-scale plastics in aquatic ecosystems. Effects of weathering or aging have been shown to affect the superficial characteristics of plastic particles and should also be taken into account for assessing potential risks of plastics under environmental relevant conditions. In studies assessing effects posed by non-functionalized vs. functionalized particles comparatively, with coated particles serving as surrogates for naturally altered plastics (Della Torre et al., 2014; Watts et al., 2016), differences in effects were reported. However, these studies investigated physical hazards posed by small-sized plastic particles exclusively, while chemical effects induced might be even more pronounced due to altered surface characteristics.
Regarding the various shapes of plastic particles used to investigate the environmental burden posed, fragments were the most frequent shape (60% of overall studies), whereas spheres still accounted for around 36% of the analyzed studies, and fibers for 5% only. However, particles of primary sources, mainly manufactured as spheres, are of minor relevance in nature and rather irregularly-shaped particles, resulting from the fragmentation of larger plastic items and of materials containing synthetic polymers, are much more prevalent (e.g., Duis and Coors, 2016). By this review, most obvious imbalances could be revealed regarding chemical effects posed by small-scale plastic particles on benthic freshwater organisms again, with spheres being investigated exclusively in terms of potential impacts induced by leachates. Regarding the interaction of chemicals with micro- and/or nanoplastics, the number of studies examining effects of co-contaminations is not representing environmental relevant circumstances, by using fragments and spheres equally (Figure 4C).
Furthermore, the exposure of benthic organisms to plastics in nature is doubtless dominated by particles in the sediments, both in marine and in freshwater ecosystems (Moore, 2008; Wright et al., 2013b; Galloway et al., 2017); however, in the vast majority of the reviewed studies exposure was via the aqueous phase (Figures 2D, 4E; Table 3). Studies investigating the effects of leachates on benthic organisms were conducted in aqueous medium exclusively (Table 3), while particles were applied in more relevant matrices, sediment or food, in terms of studies on physical hazards and the interaction with POPs (Figures 2D, 4E). However, the scope of further scientific research should be broadened to include investigations under realistic exposure scenarios, mainly by using the ecologically most relevant exposure matrix for the particular organism group tested.
Even though the reviewed studies generally investigated effects of particles in a huge size range, the vast majority of studies applied microplastics, defined as particles ranging between 0.1 μm and 5 mm respectively (e.g., Thompson et al., 2004; Moore, 2008; Tables 1–4). However, in those studies assessing effects of nano- and micro-sized particles comparatively, distinct size-dependent effects were reported with increased impacts of nanoplastics, both in marine studies (Snell and Hicks, 2011; Lee et al., 2013; Jeong et al., 2017) as well as in experiments concerning freshwater organisms (Jeong et al., 2016; Lei et al., 2018). Irrespectively of the recent technical obstacles concerning the detection of nano-sized particles and the resulting uncertainties about natural concentrations, a subsequent degradation of plastic debris into nano-sized particles is widely accepted (e.g., Andrady, 2011; Lambert et al., 2013; Lambert and Wagner, 2016). By this, distinct research gaps concerning effects of nanoplastics on benthic organisms could be revealed.
The current lack of methodological standardization and harmonization greatly hampers inter-study comparisons, as already noted by Van Cauwenberghe et al. (2015). Generally, small-scale plastics have been tested in concentrations several orders of magnitude higher than current known environmental concentrations. While such approaches may be useful in identifying the general hazards posed by plastic particles, little information is provided on the actual impacts on benthic organisms. However, due to current detection limits for small-scale plastic particles, concrete measures of realistic concentrations are rarely available for particles >10 μm and not available for smaller particles (<10 μm).
Even though a general comparison between the various assessed parameters in terms of their susceptibility toward nano- and microplastic exposure is difficult due to varying experimental conditions, sub-lethal parameters indicated to be more sensitive than mortality (Tables 1–4). This should be taken into account for upcoming research on this highly relevant topic. Furthermore, single-species tests were the preferred investigation design and the exposures were mostly short or of intermediate duration. However, long-term exposure and interspecific interactions characterize natural conditions; accordingly, the possible effects on the food chain and on the reproductive system of exposed organisms should also be investigated. Such studies are crucial due to the potential impact of small-scale plastics on community structure and population dynamics but also, indirectly, on higher trophic levels. More realistic model ecosystems and controlled experimental conditions would enable explorations of the effects of plastics on whole benthic communities. Extended plastic exposures, even at the low concentrations currently found in nature, may also lead to multi-generational effects on populations and communities.
In conclusion, the present review identified several shortcomings that have limited a comprehensive risk assessment of the impact of micro- and nanoplastics, as well as future areas of research:
- Few studies have focused on the organisms in freshwater ecosystems, especially chemical effects are widely neglected so far.
- In both marine and freshwater systems, micro- and meiobenthic organisms must be more extensively assessed.
- Greater attention should be devoted to micro- and nano-sized plastics whose polymer composition, shape, surface properties, and exposure routes are those characterizing plastic particles contaminating the natural environment.
- Nano-sized particles should be of concern when assessing potential effects of plastics.
- Long-term assays of multiple species (e.g., model ecosystems) should be conducted to examine effects with higher ecological relevance.
- Standardization of concentrations and exposure conditions are needed together with quality assessments to obtain more reliable and comparable data.
Statements
Author contributions
AH contributed conception and design of the review. AH, M-TM, HF, and WT organized and contributed to the database. AH wrote the first draft of the manuscript. M-TM, HF, and WT contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research (BMBF) as part of the Project MikroPlaTaS—Microplastics in Dams and Reservoirs: Sedimentation, Spread, Effects (BMBF grant 02W22WPL1L448D88D). We further acknowledge support for the Article Processing Charge by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of Bielefeld University. Additionally, we would like to thank Catherine Mouneyrac and the reviewers for their comments and editorial work on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
AndradyA. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull.62, 1596–1605. 10.1016/j.marpolbul.2011.05.030
2
ArnqvistG.WoosterD. (1995). Meta-analysis: synthesizing research findings in ecology and evolution. Find. Ecol. Evol. 10, 236–240. 10.1016/S0169-5347(00)89073-4
3
AuS. Y.BruceT. F.BridgesW. C.KlaineS. J. (2015). Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem.34, 2564–2572. 10.1002/etc.3093
4
AutaH. S.EmenikeC. U.FauziahS. H. (2017). Distribution and importance of microplastics in the marine environment : a review of the sources, fate, effects, and potential solutions. Environ. Int.102, 165–176. 10.1016/j.envint.2017.02.013
5
AvioC. G.GorbiS.MilanM.BenedettiM.FattoriniD.D'ErricoG.et al. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut.198, 211–222. 10.1016/j.envpol.2014.12.021
6
BakirA.RowlandS. J.ThompsonR. C. (2014). Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar. Coast. Shelf Sci.140, 14–21. 10.1016/j.ecss.2014.01.004
7
BalbiT.CamisassiG.MontagnaM.FabbriR.FranzellittiS.CarboneC.et al. (2017). Impact of cationic polystyrene nanoparticles (PS-NH2H) on early embryo development of Mytilus galloprovincialis: effects on shell formation. Chemosphere186, 1–9. 10.1016/j.chemosphere.2017.07.120
8
BarnesD. K.GalganiF.ThompsonR. C.BarlazM. (2009). Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R Soc. B Biol. Sci.364, 1985–1998. 10.1098/rstb.2008.0205
9
BejgarnS.MacLeodM.BogdalC.BreitholtzM. (2015). Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes. Chemosphere132, 114–119. 10.1016/j.chemosphere.2015.03.010
10
BesselingE.WegnerA.FoekemaE. M.van Den Heuvel-GreveM. J.KoelmansA. A. (2012). Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol.47, 593–600. 10.1021/es3s02763x33x
11
BlarerP.Burkhardt-HolmP. (2016). Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ. Sci. Pollut. Res.23, 23522–23532. 10.1007/s1s1356-016-75844-2
12
BrowneM. A.CrumpP.NivenS. J.TeutenE. L.TonkinA.GallowayT. S.et al. (2011). Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ. Sci. Technol.45, 9175–9179. 10.1021/es201811s
13
BrowneM. A.DissanayakeA.GallowayT. S.LoweD. M.ThompsonR. C.. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol.42, 5026–5031.
14
BrowneM. A.GallowayT. S.ThompsonR. C. (2010). Spatial patterns of plastic debris along Estuarine shorelines. Environ. Sci. Technol.44, 3404–3409. 10.1021/es903784e
15
BrowneM. A.NivenS. J.GallowayT. S.RowlandS. J.ThompsonR. C. (2013). Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol.23, 2388–2392. 10.1016/j.cub.2013.10.012
16
CanesiL.CiacciC.BergamiE.MonopoliM. P.DawsonK. A.PapaS.et al. (2015). Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res.111, 34–40. 10.1016/j.apsusc.2016.12.004
17
ChaeY.AnY. J. (2017). Effects of micro- and nanoplastics on aquatic ecosystems: Current research trends and perspectives. Mar. Pollut. Bull.124, 624–632. 10.1016/j.marpolbul.2017.01.070
18
ChuaE. M.ShimetaJ.NugegodaD.MorrisonP. D.ClarkeB. O. (2014). Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, allorchestes compressa. Environ. Sci. Technol.48, 8127–8134. 10.1021/es405717z
19
ColeM.GallowayT. S. (2015). Ingestion of nanoplastics and microplastics by pacific oyster larvae. Environ. Sci. Technol.49, 14625–14632. 10.1021/acs.est.5b55b0b4099
20
ColeM.LindequeP.FilemanE.HalsbandC.GallowayT. S. (2015). The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol.49, 1130–1137. 10.1021/es5s04525u55u
21
ColeM.LindequeP.FilemanE.HalsbandC.GoodheadR.MogerJ.et al. (2013). Microplastic ingestion by zooplankton. Environ. Sci. Technol.47, 6646–6655. 10.1021/es4s00663f33f
22
Della TorreC.BergamiE.SalvatiA.FaleriC.CirinoP.DawsonK. A.et al. (2014). Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos paracentrotus lividus. Environ. Sci. Technol.48, 12302–12311. 10.1021/es5s02569w99w
23
DevrieseL. I.De WitteB.VethaakA. D.HostensK.LeslieH. A. (2017). Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): an experimental study. Chemosphere186, 10–16. 10.1016/j.chemosphere.2017.07.121
24
DuisK.CoorsA. (2016). Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur.28, 1–25. 10.1186/s1s2302-015-0069-y
25
Eerkes-MedranoD.ThompsonR. C.AldridgeD. C. (2015). Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res.75, 63–82. 10.1016/j.watres.2015.02.012
26
GallowayT. S.ColeM.LewisC. (2017). Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol.1:116. 10.1038/s4s1559-017-0116
27
Gandara E SilvaP. P.NobreC. R.ResaffeP.PereiraC. D. S.GusmãoF. (2016). Leachate from microplastics impairs larval development in brown mussels. Water Res.106, 364–370. 10.1016/j.watres.2016.10.016
28
GomieroA.StrafellaP.PelliniG.SalvalaggioV.FabiG. (2018). Comparative effects of ingested PVC micro particles with and without adsorbed Benzo(a)pyrene vs. spiked sediments on the cellular and sub cellular processes of the benthic organism hediste diversicolor. Front. Mar. Sci.5, 1–12. 10.3389/fmars.2018.00099
29
GrayA. D.WeinsteinJ. E. (2017). Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem.36, 3074–3080. 10.1002/etc.3881
30
GreenD. S. (2016). Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ. Pollut.216, 95–103. 10.1016/j.envpol.2016.05.043
31
GreenD. S.BootsB.O'ConnorN. E.ThompsonR. (2016a). Microplastics affect the ecological functioning of an important biogenic habitat. Environ. Sci. Technol.51, 68–77. 10.1021/acs.est.6b66b0b4496
32
GreenD. S.BootsB.SigwartJ.JiangS.RochaC. (2016b). Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ. Pollut.208, 426–434. 10.1016/j.envpol.2015.10.010
33
HämerJ.GutowL.KöhlerA.SaborowskiR.HämerJ.GutowL.et al. (2014). Fate of Microplastics in the Marine Isopod Idotea emarginata. Environ. Sci. Technol.48, 13451–13458. 10.1021/es5s01385y55y
34
HeindlerF. M.AlajmiF.HuerlimannR.ZengC.NewmanS. J.VamvounisG.et al. (2017). Toxic effects of polyethylene terephthalate microparticles and Di(2-ethylhexyl)phthalate on the calanoid copepod, Parvocalanus crassirostris. Ecotoxicol. Environ. Saf.141, 298–305. 10.1016/j.ecoenv.2017.03.029
35
HiraiH.TakadaH.OgataY.YamashitaR.MizukawaK.SahaM.et al. (2011). Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull.62, 1683–1692. 10.1016/j.marpolbul.2011.06.004
36
ImhofH. K.LaforschC. (2016). Hazardous or not – Are adult and juvenile individuals of Potamopyrgus antipodarum affected by non-buoyant microplastic particles?Environ. Pollut.218, 383–391. 10.1016/j.envpol.2016.07.017
37
Ivar do SulJ. A.CostaM. F. (2014). The present and future of microplastic pollution in the marine environment. Environ. Pollut.185, 352–364. 10.1016/j.envpol.2013.10.036
38
JeongC. B.KangH. M.LeeM. C.KimD. H.HanJ.HwangD. S.et al. (2017). Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2f pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep.7, 1–11. 10.1038/srep4p1323
39
JeongC. B.WonE. J.KangH. M.LeeM. C.HwangD. S.HwangU. K.et al. (2016). Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol.50, 8849–8857. 10.1021/acs.est.6b66b0b1441
40
KaposiK. L.MosB.KelaherB. P.DworjanynS. A. (2014). Ingestion of microplastics has limited impact on a marine larva. Environ. Sci. Technol.48:1638. 10.1021/es4s04295e55e
41
KleinS.WorchE.KnepperT. P. (2015). Occurrence and spatial distribution of microplastics in river shore sediments of the rhine-main area in Germany. Environ. Sci. Technol.49, 6070–6076. 10.1021/acs.est.5b55b0b0492
42
LambertS.SinclairC. J.BradleyE. L.BoxallA. B. A. (2013). Effects of environmental conditions on latex dergadation in aquatic systems. Sci. Total Environ.447, 225–234. 10.1016/j.scitotenv.2012.12.067
43
LambertS.WagnerM. (2016). Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere145, 265–268. 10.1016/j.chemosphere.2015.11.078
44
LattinG. L.MooreC. J.ZellersA. F.MooreS. L.WeisbergS. A. (2004). A comparison of neustonic plastic and zooplankton at different depths near the southern California shore. Mar. Pollut. Bull.49, 291–294. 10.1016/j.marpolbul.2004.01.020
45
LeeK.ShimW. J.KwonO. Y.KangJ. (2013). Size-dependent effects of micro polystyrene particles in the marine copepod tigriopus japonicus. Environ. Sci. Technol.47, 11278–11283. 10.1021/es4s01932b22b
46
LeiL.WuS.LuS.LiuM.SongY.FuZ.et al. (2018). Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ.619–620, 1–8. 10.1016/j.scitotenv.2017.11.103
47
LeungJ.ChanK. Y. K. (2018). Microplastics reduced posterior segment regeneration rate of the polychaete Perinereis aibuhitensis. Mar. Pollut. Bull.129, 782–786. 10.1016/j.marpolbul.2017.10.072
48
LiH. X.GetzingerG. J.FergusonP. L.OrihuelaB.ZhuM.RittschofD. (2016). Effects of toxic leachate from commercial plastics on larval survival and settlement of the barnacle amphibalanus amphitrite. Environ. Sci. Technol.50, 924–931. 10.1021/acs.est.5b55b0b2781
49
LiW. C.TseH. F.FokL. (2016). Plastic waste in the marine environment: a review of sources, occurence and effects. Sci. Total Environ.566–567, 333–349. 10.1016/j.scitotenv.2016.05.084
50
MagniS.GagnéF.AndréC.Della TorreC.AuclairJ.HananaH.et al. (2018). Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ.631–632, 778–788. 10.1016/j.scitotenv.2018.03.075
51
Martínez-GómezC.LeonV. M.CallesS.Gomáriz-OlcinaM.VethaakA. D. (2017). The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar. Environ. Res.130, 69–76. 10.1016/j.marenvres.2017.06.016
52
MatoY.IsobeT.TakadaH.KanehiroH.OhtakeC.KaminumaT. (2001). Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol.35, 318–324. 10.1021/es0s010498
53
MessinettiS.MercurioS.ParoliniM.SugniM.PennatiR. (2018). Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ. Pollut.237, 1080–1087. 10.1016/j.envpol.2017.11.030
54
MooreC. J. (2008). Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res.108, 131–139. 10.1016/j.envres.2008.07.025
55
NobreC. R.SantanaM. F. M.MalufA.CortezF. S.CesarA.PereiraC. D. S.et al. (2015). Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull.92, 99–104. 10.1016/j.marpolbul.2014.12.050
56
OehlmannJ.Schulte-OehlmannU.KloasW.JagnytschO.LutzI.KuskK. O.et al. (2009). A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R Soc. B Biol. Sci.364, 2047–2062. 10.1098/rstb.2008.0242
57
Paul-PontI.LacroixC.González FernándezC.HégaretH.LambertC.Le GoïcN.et al. (2016). Exposure of marine mussels Mytilus spp. to polystyrene microplastics: toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut.216, 724–737. 10.1016/j.envpol.2016.06.039
58
Redondo-HasselerharmP. E.FalahudinD.PeetersE. T. H. M.KoelmansA. A. (2018). Microplastic effect thresholds for freshwater benthic macroinvertebrates. Environ. Sci. Technol.52, 2278–2286. 10.1021/acs.est.7b77b0b5367
59
RochmanC. M.BrowneM. A.UnderwoodA. J.Van FranekerJ. A.ThompsonR. C.Amaral-ZettlerL. A. (2016). The ecological impact of marine debris: unraveling the demonstrated evidence from what i sperceived. Ecology97, 302–312. 10.1890/14-2070.1
60
RochmanC. M.HohE.HentschelB. T.KayeS. (2013). Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ. Sci. Technol.47, 1646–1654. 10.1021/es3s03700s00s
61
SchererC.BrennholtN.ReifferscheidG.WagnerM. (2017). Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci. Rep.7, 1–9. 10.1038/s4s1598-017-171911-7
62
SchindlerD. E.ScheuerellM. D. (2002). Habitat coupling in lake ecosystems. Oikos98, 177–189. 10.1034/j.16000-0706.2002.980201.x
63
SnellT. W.HicksD. G. (2011). Assessing toxicity of nanoparticles using Brachionus manjavacas (Rotifera). Environ. Toxicol.26, 146–152. 10.1002/tox.20538
64
StraubS.HirschP. E.Burkhardt-HolmP. (2017). Biodegradable and petroleum-based microplastics do not differ in their ingestion and excretion but in their biological effects in a freshwater invertebrate Gammarus fossarum. Int. J. Environ. Res. Public Health14:4070774. 10.3390/ijerph1h4070774
65
SussarelluR.SuquetM.ThomasY.LambertC.FabiouxC.PernetM. E. J.et al. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci.113, 2430–2435. 10.1073/pnas.1519019113
66
TalsnessC. E.AndradeA. J. M.KuriyamaS. N.TaylorJ. A.vom SaalF. S. (2009). Components of plastic: experimental studies in animals and relevance for human health. Philos. Trans. R Soc. B Biol. Sci.364, 2079–2096. 10.1098/rstb.2008.0281
67
ThaysenC.StevackK.RuffoloR.PoirierD.De FrondH.DeVeraJ.et al. (2018). Leachate from expanded polystyrene cups is toxic to aquatic invertebrates (Ceriodaphnia dubia). Front. Mar. Sci.5:71. 10.3389/fmars.2018.00071
68
ThompsonR. C.OlsonY.MitchellR. P.DavisA.RowlandS. J.JohnA. W.et al. (2004). Lost at sea: where is all the plastic?Science304:838. 10.1126/science.1094559
69
Van CauwenbergheL.DevrieseL.GalganiF.RobbensJ.JanssenC. R. (2015). Microplastics in sediments: a review of techniques, occurence and effects. Mar. Environ. Res.111, 5–17. 10.1016/j.marenvres.2015.06.007
70
vom SaalF. S.ParmigianiS.PalanzaP. L.EverettL. G.RagainiR. (2008). The plastic world: Sources, amounts, ecological impacts and effects on development, reproduction, brain and behavior in aquatic and terrestrial animals and humans. Environ. Res.108, 127–130. 10.1016/j.envres.2008.03.008
71
von MoosN.Burkhardt-HolmP.KoehlerA. (2012). Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol.46, 327–335. 10.1021/es3s02332w22w
72
VroomR. J. E.KoelmansA. A.BesselingE.HalsbandC. (2017). Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut.231, 987–996. 10.1016/j.envpol.2017.08.088
73
WattsA. J.UrbinaM. A.GoodheadR.MogerJ.LewisC.GallowayT. S. (2016). Effect of microplastic on the gills of the shore crab carcinus maenas. Environ. Sci. Technol.50, 5364–5369. 10.1021/acs.est.6b66b0b1187
74
WattsA. J. R.UrbinaM. A.CorrS.LewisC.GallowayT. S. (2015). Ingestion of plastic microfibers by the crab carcinus maenas and its effect on food consumption and energy balance. Environ. Sci. Technol.49, 14597–14604. 10.1021/acs.est.5b55b0b4026
75
WeberS.TraunspurgerW. (2015). The effects of predation by juvenile fish on the meiobenthic community structure in a natural pond. Freshw. Biol.60, 2392–2409. 10.1111/fwb.12665
76
WegnerA.BesselingE.FoekemaE. M.KamermansP.KoelmansA. A. (2012). Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ. Toxicol. Chem.31, 2490–2497. 10.1002/etc.1984
77
WrightS. L.RoweD.ThompsonR. C.GallowayT. S. (2013a). Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol.23, 1031–1033. 10.1016/j.cub.2013.10.068
78
WrightS. L.ThompsonR. C.GallowayT. S. (2013b). The physical impacts of microplastics on marine organisms: a review. Environ. Pollut.178, 483–492. 10.1016/j.envpol.2013.02.031
79
XuX. Y.LeeW. T.ChanA. K. Y.LoH. S.ShinP. K. S.CheungS. G. (2017). Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar. Pollut. Bull.124, 798–802. 10.1016/j.marpolbul.2016.12.027
80
ZhaoL.QuM.WongG.WangD. (2017). Transgenerational toxicity of nanopolystyrene particles in the range of μg L-1i11in the nematode: Caenorhabditis elegans. Environ. Sci. Nano4, 2356–2366. 10.1039/c7ce77en0n0707h77h
81
ZiajahromiS.KumarA.NealeP. A.LeuschF. D. L. (2018). Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut.236, 425–431. 10.1016/j.envpol.2018.01.094
Summary
Keywords
microplastic, nanoplastic, sediment, marine, freshwater, invertebrates, toxicity
Citation
Haegerbaeumer A, Mueller M-T, Fueser H and Traunspurger W (2019) Impacts of Micro- and Nano-Sized Plastic Particles on Benthic Invertebrates: A Literature Review and Gap Analysis. Front. Environ. Sci. 7:17. doi: 10.3389/fenvs.2019.00017
Received
17 September 2018
Accepted
23 January 2019
Published
15 February 2019
Volume
7 - 2019
Edited by
Catherine Mouneyrac, UCO Angers, France
Reviewed by
Bruno B. Castro, University of Minho, Portugal; Alessio Gomiero, NORCE Norwegian Research Centre, Norway
Updates
Copyright
© 2019 Haegerbaeumer, Mueller, Fueser and Traunspurger.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arne Haegerbaeumer a.haegerbaeumer@uni-bielefeld.de
This article was submitted to Environmental Toxicology, a section of the journal Frontiers in Environmental Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.