- 1Laboratory of Evolutionary Entomology, Institute of Biology, University of Neuchâtel, Neuchâtel, Switzerland
- 2Department of Biology, Edmonds Community College, Lynnwood, WA, United States
The domestication of beans has selected for larger seeds in cultivated plants compared to their wild relatives. This has not only resulted in an enhanced resource for humans, but also for the insects that feed on these seeds. Seed beetles that attack wild and cultivated seeds often lay several eggs on a single seed. We hypothesized that the larger seed size of domesticated beans will mitigate the competition among the larvae that hatch from these eggs, with important implications for their growth and survival. To test this we examined how seed size of wild and cultivated Phaseolus lunatus (lima bean) affect the performance of the Mexican bean weevil Zabrotes subfasciatus, an important pest of beans in Mexico. A negative correlation was found between the initial number of eggs on a seed and the weight of female beetles that emerged, but only for the much smaller wild seeds. Similarly, beetle survival was found to be negatively correlated with competition intensity only on wild seeds. Our results imply that by selecting for larger seeds, domestication of P. lunatus has reduced the intensity of intraspecific larval competition of Z. subfasciatus.
Introduction
Increasing evidence shows that plant domestication has altered the strength and nature of their interactions with other organisms (Chen et al., 2015a; Rowen and Kaplan, 2016; Whitehead et al., 2017). Cultivated plants differ from their wild ancestors in a suite of phenotypic traits, collectively known as the domestication syndrome. These include traits related to the ease of cultivation and harvest, as well as morphological and chemical traits that ensure higher yields and enhanced nutritional value. Selection for these traits has commonly resulted in larger tissue mass or organ size, higher nutrient content and decreases in physical defenses and toxic chemical compounds (Meyer et al., 2012). These changes in cultivated plants have been shown to affect the food choices and performance of insects that attack them (Chen et al., 2015a,b, and references therein). This is particularly evident when crops occur in the native range of their wild relatives (Chen et al., this issue), as insects adapted to wild plants are suddenly faced with a more abundant and often more nutritious and less toxic resource.
Phaseolus lunatus (Lima bean), one of the five domesticated species of the genus Phaseolus is of Andean and Mesoamerican origin. Lima beans were domesticated at least twice, one domestication event occurred in the Andean mountains of Ecuador and Northern Peru and a second event in central-western Mexico (Motta-Aldana et al., 2010). Beans went through further domestication events and adapted to a wide variety of climatic regimes and ecological conditions (Martínez-Castillo et al., 2008; Motta-Aldana et al., 2010; Serrano-Serrano et al., 2012; Chacón-Sánchez and Martínez-Castillo, 2017).
Changes resulting from domestication of the genus Phaseolus mainly involve an increase in pod and seed size, decreased shattering, reduction in levels of toxins, such as lectins, lectin-like proteins, and cyanogenic compounds (only in P. lunatus), and an overall increase in proteins and minerals (Delgado-Salinas, 1988; Smartt, 1988; Sotelo et al., 1995). Throughout their distribution range in Mesoamerica, cultivated and wild bean plants coexist in sympatry (Gepts, 1988; Piñero and Eguiarte, 1988; Martínez-Castillo et al., 2014; Silva et al., 2017), allowing for a frequent exchange of insects and pathogens between wild and cultivated forms (Leroi et al., 1990; Lindig-Cisneros et al., 1997; Alvarez et al., 2007; Zaugg et al., 2013). It is well documented that herbivorous insects that achieve pest status usually continue to exist in natural habitats alongside managed ones (Mitchell et al., 2016). Once cultivated beans are harvested and seeds are transported to storage places, they continue to be in close proximity to wild plants and are exposed to the insects that attack them (Alvarez et al., 2005, 2007). Furthermore, human-mediated migration as a result of farmers exchanging or selling seeds in local or regional markets may increase the spread of insects that originate from wild populations (Alvarez et al., 2007). This constant exchange of insects between wild and cultivated populations has important implications for pest pressures in agriculture. This is particularly true for bruchinae beetles that infest cultivated fields in Mexico, for which it has been shown that geographic distance between cultivated and wild populations greatly explains the patterns of infestation rates (Alvarez et al., 2005, 2007). Moreover, if cultivated plants offer a more reliable and nutritious resource than their wild counterparts this can explain why seed beetles thrive in cultivated seeds.
Numerous studies have shown that seed size greatly influences the oviposition decisions of adult seed beetles, and that size can often be used as a good indicator of seed quality for the developing larvae (Janzen, 1977; Fox and Czesak, 2000; Guedes et al., 2010; Chen et al., 2015b; Oliveira et al., 2015). Indeed, for seeds in the genus Phaseolus, seed size has been found to be the best predictor of oviposition choices (Moreira et al., 2015; Hernandez-Cumplido et al., 2016). Thus, we would predict that, faced with a choice, adult females would preferentially oviposit on cultivated seeds rather than on much smaller wild seeds. We further predict that inside the cultivated seeds the larvae will be exposed to lower levels of conspecific competition, which may be an important reason for the oviposition preference.
We tested this hypothesis with the Mexican bean weevil Zabrotes subfasciatus, and wild and cultivated seeds of Lima bean, P. lunatus. Our specific goal was to test the effects of increased seed size in cultivated varieties on the interaction with the seed beetle. In controlled laboratory experiments using seeds from three cultivated varieties and three wild populations of Lima bean, we investigated the oviposition patterns of adult females and the subsequent performance of their progeny resulting from of seed-size mediated competition among beetle larvae.
Materials and Methods
Seeds
For the experiments we used seeds from three cultivated varieties and three wild populations of P. lunatus (Figure 1). Wild seeds were collected in locations along the Pacific coast of Mexico were Lima bean grows naturally. They are located at: Hidalgo near San Jose Manialtepec (“HGO”; 15.575564, −97.151350), Experimental Campus of the Universidad del Mar (“UMAR”; 15.923366, −97.151892), and near Largartero (“INK”; 15.725127, −96.656343) (as described in Shlichta et al., 2014). We collected seeds from 10 plants per site (only six for HGO).

Figure 1. Seeds of Phaseolus lunatus from domesticated varieties: on the top from left to right with the corresponding mean sizes in mm (± SE); Fordhook 242 (10.87 ± 0.47), Burpee's Best (11.18 ± 0.92) and Jackson Wonder (9.18 ± 0.46). On the bottom line seeds from wild populations, from left to right; HGO (4.59 ± 0.2), Umar (4.43 ± 0.1), and Ink (4.32 ± 0.14).
The following domesticated seed varieties were obtained from W. Atlee Burpee & Co (Warminster, PA, USA): Jackson Wonder, Fordhook 242 Bush Bean and Burpee's Best Pole Bean (we named them “JACK”, “FORD,” and “BURP,” respectively). The choice of these varieties was made based on previous studies with several commercially available cultivated varieties, in which we found that beetles develop well and do not appear to discriminate with respect to their different genetic pool (Shlichta et al. unpublished data). Thus, because we wanted to have extreme variation in seed size in order to test our hypothesis, the choice was made based on this variation and not on their domestication history. These seeds represent a mixture of two and perhaps three genetic pools; “JACK” is of Mesoamerican origin and “FORD” of Andean origin (Nienhuis et al., 1995; Ernest and Kee, 2008), we do not have information regarding the genetic pool “BURP.” Although there is variation in seed size and color among these three cultivated varieties, variation in size is greater between wild and cultivated seeds (Supplementary Figure 1).
Insects
The Mexican bean weevil Z. subfasciatus, native to Mesoamerica, attacks seeds of several wild and cultivated species in the genus Phaseolus throughout Mexico, Central and South America (Credland and Dendy, 1992; Benrey et al., 1998; Romero and Johnson, 2000), It is considered one of the most important pests in bean cultivation and storage (Birch et al., 1985; Leroi et al., 1990), not only in the Americas but also in tropical regions of Asia and Africa (Davies, 1972). Females glue their eggs on the seed coat and upon emergence, first instar larvae bore into the seed, where they feed, develop, pupate and then emerge as adults (Benrey et al., 1998).
This beetle is particularly suited to test our hypothesis because females do not avoid seeds with previously laid eggs and may lay many eggs on a single seed, even when seed availability is not limited. Indeed, a single seed has been observed to present up to 63 eggs lay by multiple females (Teixeira and Zucoloto, 2012), even though larval survival under these conditions is highly unlikely (Cuny, personal observation). Once larvae enter the seed, they are confined to it for their entire development until adulthood. If several larvae are inside the seed, they can experience high levels of competition for both space and food resource.
Zabrotes subfasciatus has been reared in our lab for several years on cultivated seeds of Phaseolus vulgaris (Vivien Paille red Kidney, obtained from MultiFood, 3238 Gals, Switzerland; see Campan and Benrey, 2006 for details on the rearing). To control for inbreeding effects, every year new field-collected individuals from Mexico are added to the colony and allow to mix for several generations before being used in experiments. All the insects described in this experiment were <4 days old.
Experimental Protocol
Five seeds of one of the varieties or populations were placed in a plastic Petri dish (28 × 23 × 5 mm, Semadeni AG, A4686). Ten Petri dishes were set up for each variety or population (60 in total). One male and one female beetle were introduced into each dish for 5 days, after which the number of eggs laid on each seed was counted and the seeds were individually stored in falcon tubes at 28°C. Beetles complete their development on average in 25 days. Dishes were checked daily and we recorded: larval survival (number of adults that emerged divided by the initial number of eggs laid on the seed), adult sex (determined from elytra patterns and size; Oliveira et al., 2015) and weight (to the nearest 0.01 mg with an analytical balance Mettler AE163, Switzerland). In parallel, in order to confirm the size difference between wild and cultivated lima bean seeds, 20 uninfested seeds (20 seeds per cultivated variety and per wild population) were weighed and measured using a binocular magnifier with an ocular scale.
Finally, we conducted an experiment to evaluate the effect of seed size on female oviposition independent of other factors linked to bean domestication. Seeds from each cultivated variety and wild population were selected and divided in two groups; small and large (chosen from the available natural variation within each seed type). Two seeds of different size from the same variety or population were placed in a Petri dish (as described in the previous experiment), and one male and one female beetle were introduced. Three days later, we counted the number of eggs laid on each seed. Based on previous studies, we know that a 3-day period is sufficient for beetles to make an oviposition choice and at the same time assures that not to many eggs are laid on a single seed (Campan and Benrey, 2006).
Statistical Analysis
Data were analyzed using SAS (SAS Institute, 2002)1. SAS Institute Inc., statistical package. Assumptions of normality and homoscedasticity were tested before each test. Linear mixed models (PROC MIXED) or generalized linear mixed models (PROC GLIMMIX), followed by a post-hoc analysis (Tukey) were used to compare data on seed size, weight, the number of eggs laid on the seeds, adult sex ratio and survival. Correlations have been tested using Pearson or Spearman correlations tests (PROC CORR). Seeds and Petri dishes were included as random factors in the models and seed domestication status, as well as seed varieties and population nested in domestication status were included as fixed factors (to account for natural variation among the three cultivated varieties and the three wild populations). Seeds with only one egg were not included in the analysis of beetle survival. Females being generally heavier than males, their weight was analyzed separately. For the experiment performed to test the relationship between seed size and number of eggs within each cultivated variety or wild population, seeds with no eggs were excluded from the analysis.
Results
Measurements of seed size and weight confirmed that cultivated seeds are significantly (~60%) larger and heavier than wild seeds [Figure 2, N = 40, F(1.74) = 172.8, p < 0.001, and N = 40, F(1.74) = 281, p < 0.001 for size and weight, respectively]. The fixed factor of population and variety nested within seed domestication status was significant for seed size and weight [F(1.74) = 6.63, p = 0.002, and F(1.74) = 59.9, p < 0.001, respectively].
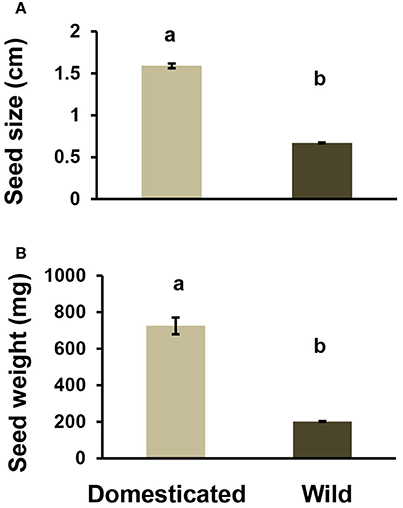
Figure 2. (A) Mean size of domesticated and wild bean seeds. [N = 40, F(1.74) = 172.8, p < 0.001]. (B) Mean weight of domesticated and wild bean seeds. [N = 40, F(1.74) = 281, p < 0.001]. Different letters indicate a significant difference. Bars are means ± SE.
Female beetles laid significantly more eggs (2-fold) on seeds from cultivated varieties than on wild seeds [Figure 3, Nwild = 41, Ncultivated = 51, F(1.233) = 13.32; p < 0.001]. We also found a significant effect of population and variety nested within seed domestication status on the number of eggs laid per seed [F(4.236) = 28.86, p < 0.001]. Finally, within each variety and population, the relationship between seed size and number of eggs laid was not significant (Supplementary Figure 2). This suggests that the variation in seed size within wild or cultivated seeds is not large enough to influence ovipositing females.
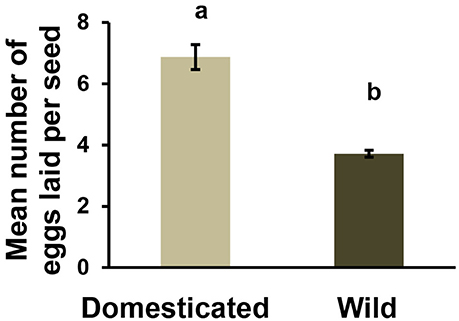
Figure 3. Mean number of eggs laid per domesticated and wild seed in a no-choice experiment. [N = 121, F(1.233) = 13.32; p < 0.001]. Different letters indicate a significant difference. Bars are means ± SE.
Larval survival (expressed as the percentage of adults that emerged per seed) was negatively correlated with the number of eggs laid on wild seeds (Figure 4, N = 51, r = −0.32, p = 0.023), but no significant correlation was found for survival on cultivated seeds (N = 43, r = −0.21, p = 0.17). Similarly, female weight was negatively correlated with the competition intensity (expressed as the number of eggs per the seed) when they developed in wild seeds (Figure 5B, N = 39, r = −0.48, p = 0.002), but not in cultivated seeds (Figure 5A, N = 27, r = −0.19, p = 0.34). However, male weight was only marginally significant correlated with number of eggs on in wild seeds (Figure 5B, N = 37, r = −0.32, p = 0.056) and this correlation was also not significant in cultivated seeds (Figure 5A, N = 34, r = −0.018, p = 0.9). Finally, we did not find a difference in the sex ratio of beetles that emerged from domesticated or wild seeds [F(1.79) = 0.03, p = 0.868; Supplementary Figure 3], nor a significant effect among cultivated varieties or wild populations [F(4.79) = 0.74, p = 0.57].
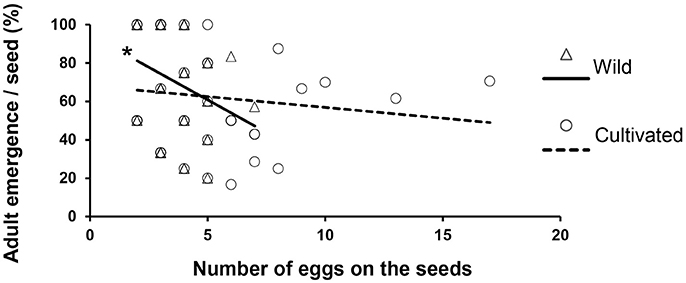
Figure 4. Percentage of adults that emerged from each seed calculated as, number of adults that emerged from the seed/number of eggs laid on the seed * 100. Dashed line indicates linear regression for beetles that developed in cultivated seeds (N = 43, Spearman r = −0.21, P = 0.17) and solid line shows linear regression for beetles that developed in wild seeds (N = 51, Spearman r = −0.32, P = 0.023).
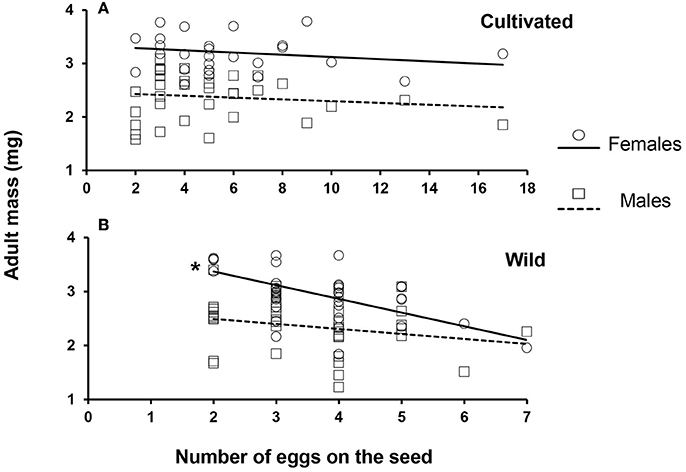
Figure 5. Correlation between the weight of adult females (circles) and males (squares) that emerged from (A) domesticated and (B) wild seeds carrying different egg densities (as a proxy of the intensity of larval competition inside the seed). Solid lines indicate linear regression for female beetles and dashed lines show linear regression for males. A significant correlation was found for females emerging from wild seeds (N = 39, Spearman r = −0.48, P = 0.002; non on cultivated seeds P = 0.34) whereas, for the males no significant correlation was found (P = 0.056 wild seeds; P = 0.9 cultivated seeds). *Indicates significant difference p < 0.05.
Discussion
For pulse crops, larger seed size is one of the major agronomic traits that were selected for during domestication (Evans, 1993; Fuller, 2007). Larger seeds not only result in larger yields (Kluyver et al., 2017), but have also been associated with increases in germination success and seedling competitive ability and survival (Westoby et al., 2002). However, increases in seed size also have been repeatedly shown to be correlated with an increase in the likelihood of herbivore attack (reviewed in Chen et al., 2015b). Here, we found again support for this hypothesis; female beetles laid more eggs on the larger cultivated seeds of Lima bean than on the smaller wild seeds. Further, our results support the hypothesis that larger seeds offer a better resource for the Mexican bean weevil and as a consequence mitigate the intensity and negative effects of larval competition. In addition to and despite the higher number of eggs laid on cultivated seeds, more and larger adults emerged from these seeds.
Earlier studies with Phaseolus beans and various species of Bruchinae beetles, support our findings that seed size largely explains the observed patterns of oviposition and larval performance (Paukku and Kotiaho, 2008; Moreira et al., 2015; Oliveira et al., 2015; Hernandez-Cumplido et al., 2016). In a study aimed at examining the role of cyanogenic glycosides of Lima bean seeds on beetle performance, Shlichta et al. (unpublished data) conducted an experiment similar to the one described here but allowing only one larva of Z. subfasciatus to develop in each seed. They found that in the absence of larval competition within the seed, whether seeds were wild or cultivated did not affect the survival and average weight of the emerging adults. In another study with wild Lima bean seeds, Hernandez-Cumplido et al. (2016) found that under field and laboratory conditions, beetles laid more eggs on larger seeds. Also, using seeds from different wild bean populations, Moreira et al. (2015) found that two Bruchinae species, Acanthoscelides obtectus and Z. subfasciatus, laid more eggs and had higher survival on the larger seeds of P. coccineus than on the smaller seeds of P. vulgaris.
For seed beetles, seed size can be a reliable indicator of seed quality (Fox and Czesak, 2000; Cope and Fox, 2003). For example, Cope and Fox (2003) found that when females of the seed beetle, Callosobruchus maculatus were presented with seeds of varying sizes, they distributed their eggs in a manner that maximized resource availability for all offspring. C. maculatus rejects seeds that already carry eggs (Messina and Renwick, 1985). For these insects, the presence of previously laid eggs can therefore also serve as a good indicator of the quality of the seed, as it reflects the level of competition that their offspring will face inside the seed. For Z. subfasciatus this appears not to be always the case (Campan and Benrey, 2006). Although females prefer to oviposit on uninfested seeds, if they do not have a choice, they will oviposit on seeds that already have eggs (Teixeira and Zucoloto, 2012, M. Cuny, personal observation). Even if the probability of larvae surviving under high egg densities is very low. For females of this species, it seems advantageous to rely on cues such as seed size that will help minimize larval competition and maximize lifetime fitness. Limited amounts of resource inside the seed for the developing larvae will not only affect the intensity of competition and subsequent survival, but also the size of the emerging adults, with important consequences for their fitness. Female fitness is dependent on their fecundity, which is directly dependent on body size (Dendy and Credland, 1991; Colegrave, 1993; Callejas, 1996), while male size although not so directly linked to reproductive success, can affect mating success (Savalli and Fox, 1998). Earlier studies with C. maculatus found that seed size and the initial number of eggs on the seed influenced the weight of emerging adults (Credland et al., 1986; Giga and Smith, 1991; Colegrave, 1995). For Z. subfasciatus, we found that seed size mostly affects female but not male size and only on the smaller wild seeds. This result can be explained by the overall smaller size of males (on average 30% smaller and lighter than females), implying that they may be less limited by the availability of resources for development and thus not as affected by larval competition inside the seeds.
It is important to note that the cultivated and wild seeds used in this study do not only differ in their size, but also in other traits that are part of the domestication syndrome of Phaseolus beans resulting from adaptations to cultivation, harvesting practices and human preferences. These other changes in bean traits can all have an influence on beetle oviposition decisions and larval performance. Wild seeds are harder, have a thicker testa and an inconspicuous dark brown color, whereas cultivated seeds have been selected for faster germination, hence are softer and have a thinner seed coat permeable to water and there is a vast color variation among varieties. Physical features of the seeds are known to affect beetle oviposition behavior and the ability of larvae to burrow into the seed (Chavan et al., 1997; Plaza, 2001; Boeke et al., 2004). Similarly, nutritional and defense chemical compounds present in the testa and inside the seed are known to interfere with the development and affect the survival of seed beetles (Goossens et al., 2000; Moraes et al., 2000; Silva et al., 2004), and their concentrations can differ between wild and cultivated accessions (Sotelo et al., 1995; Zaugg et al., 2013). Particularly, for Z. subfasciatus, earlier studies have documented differences in its performance when reared on cultivated or wild beans (Schoonhoven et al., 1983; Benrey et al., 1998; Campan and Benrey, 2006), as well as differential performance of beetles on wild seed populations that vary in their protein or phenolic content (Moreira et al., 2015; Hernandez-Cumplido et al., 2016). These differences in physical and chemical traits between wild and cultivated seeds will undoubtedly influence the oviposition decisions and performance of the Mexican bean weevil. Yet, our results unequivocally demonstrate that the difference in seed size between cultivated and wild seeds plays a major role in the oviposition and performance differences. Although we cannot completely disentangle seed size from other factors associated with the domestication status of the seeds, one key finding of this study is that the larger seed size of cultivated beans, independent of their genetic pool of origin, mitigates the potential negative effects of larval intraspecific competition, a process that in nature controls the size of populations (Begon et al., 2009). This additional consequence of bean domestication implies that the presence of bean fields in areas where wild beans occur naturally provides new ecological opportunities for associated insects. The expansion to a new and more profitable resource favors individuals that exploit these novel resources that provide conditions of relaxed competition (Van Valen, 1965). Yet caution should be taken to extrapolate our results to natural situations. The transferability of these results to the field would require additional measurements on variation in seed size and insect oviposition in natural conditions.
Nonetheless, these findings have important evolutionary and applied implications. Divergent selective factors that act on the plants and insects associated with wild and cultivated bean populations can lead to specialization and in extreme cases genetic differentiation and host race formation (Alvarez et al., 2007; Laurin-Lemay et al., 2013; Kenyon et al., 2015). There is further evidence for our bruchid-bean system that shows that bean domestication has selected for different behaviors in host use, not only in seed beetles, but also in the natural enemies of these beetles (Benrey et al., 1998; Campan and Benrey, 2004; Aebi et al., 2008). Yet, strong human-mediated dispersion of cultivated beans and these associated organisms will most likely result in continuous genetic mixing and will prevent selection for divergent behaviors that could lead to genetic differentiation of insects specializing on wild or cultivated seeds (Alvarez et al., 2007; Laurin-Lemay et al., 2013).
Finally, it is important to emphasize that studies in regions where cultivated plants coexist with their wild relatives allow us to understand the interplay between natural and human-mediated selection and how they interact to shape the present-day associations between plants and insects in agricultural and natural systems (Chen et al. this issue). For our study system this is also important from an applied perspective, as beans are a major staple food in many countries of Mesoamerica as well as in other regions of the world (FAO, 2013). The development of strategies that will allow us control pests in this important crop might be facilitated by unraveling the changes in interactions among insects and plants that resulted from plant domestication.
Author Contributions
The three authors conceived and designed the experiment and participated to the writing of the paper; MC performed the experiments and analyzed the data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Johanna Gendry for her assistance with data collection. Ted Turlings and two reviewers made useful suggestions that helped to improve the manuscript. This research was financially supported by the Swiss National Science Foundation (Project No. 31003A_127364) awarded to BB.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2017.00145/full#supplementary-material
Footnotes
1. ^Statistical Analysis System (2002).
References
Aebi, A., Shani, T., Hansson, C., Contreras-Garduño, J., Mansion, G., and Benrey, B. (2008). The potential of native parasitoids for the control of Mexican bean beetles: a genetic and ecological approach. Biol. Control 47, 289–297. doi: 10.1016/j.biocontrol.2008.07.019
Alvarez, N., Hossaert-McKey, M., Restoux, G., Delgado-Salinas, A., and Benrey, B. (2007). Anthropogenic effects on population genetics of phytophagous insects associated with domesticated plants. Evolution 61, 2986–2996. doi: 10.1111/j.1558-5646.2007.00235.x
Alvarez, N., McKey, D., Hossaert–McKey, M., Born, C., Mercier, L., and Benrey, B. (2005). Ancient and recent evolutionary history of the bruchid beetle, Acanthoscelides obtectus Say, a cosmopolitan pest of beans. Mol. Ecol. 14, 1015–1024. doi: 10.1111/j.1365-294X.2005.02470.x
Begon, M., Mortimer, M., and Thompson, D. J. (2009). Population Ecology: A Unified Study of Animals and Plants. Hoboken, NJ: John Wiley & Sons.
Benrey, B., Callejas, A., Rios, L., Oyama, K., and Denno, R. F. (1998). The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biol. Control 11, 130–140. doi: 10.1006/bcon.1997.0590
Birch, N., Southgate, B. J., and Fellows, L. E. (1985). “Wild and semi-cultivated legumes as potential sources of resistance to bruchid beetles for crop breeder: a study of Vigna/Phaseolus,” in Plants for Arid Lands, eds G. E. Wickens, J. R. Goodin, and D. V. Field (Dordrecht: Springer), 303–320.
Boeke, S. J., Van Loon, J. J., Van Huis, A., and Dicke, M. (2004). Host preference of Callosobruchus maculatus: a comparison of life history characteristics for three strains of beetles on two varieties of cowpea. J. Appl. Entomol. 128, 390–396. doi: 10.1111/j.1439-0418.2004.00827.x
Callejas, A. (1996). Variaciòn en la Conducta y Parámetros de Historia de Vida de Zabrotes Subfasciatus (Col.: Bruchidae) Sobre Tres Subespecies de Phaseolus coccineus. Master's thesis, Universidad Nacional Autónoma de México, Mexico.
Campan, E., and Benrey, B. (2004). Behavior and performance of a specialist and a generalist parasitoid of bruchids on wild and cultivated beans. Biol. Control 30, 220–228. doi: 10.1016/j.biocontrol.2004.01.002
Campan, E. D. M., and Benrey, B. (2006). Effects of seed type and bruchid genotype on the performance and oviposition behavior of Zabrotes subfasciatus (Coleoptera: Bruchidae). Insect Sci. 13, 309–318. doi: 10.1111/j.1744-7917.2006.00099.x
Chacón-Sánchez, M. I., and Martínez-Castillo, J. (2017). Testing domestication scenarios of Lima bean (Phaseolus lunatus L.) in Mesoamerica: insights from genome-wide genetic markers. Front. Plant Sci. 8:1551. doi: 10.3389/fpls.2017.01551
Chavan, P. D., Singh, Y., and Singh, S. P. (1997). Ovipositional preference of Callosobruchus chinensis for cowpea lines. Indian J. Entomol. 59, 295–303.
Chen, Y. H., Gols, R., and Benrey, B. (2015a). Crop domestication and naturally selected species interactions. Annu. Rev. Entomol. 60, 35–58. doi: 10.1146/annurev-ento-010814-020601
Chen, Y. H., Gols, R., Stratton, C. A., Brevik, K. A., and Benrey, B. (2015b). Complex tritrophic interactions in response to crop domestication: predictions from the wild. Entomol. Exp. App. 157, 40–59. doi: 10.1111/eea.12344
Colegrave, N. (1993). Does larval competition affect fecundity independently of its effect on adult weight? Ecol. Entomol. 18, 275–277. doi: 10.1111/j.1365-2311.1993.tb01101.x
Colegrave, N. (1995). The cost of exploitation competition in Callosobruchus Beetles. Funct. Ecol. 9, 191–196. doi: 10.2307/2390564
Cope, J. M., and Fox, C. W. (2003). Oviposition decisions in the seed beetle, Callosobruchus maculatus (Coleoptera: Bruchidae): effects of seed size on superparasitism. J. Stored Prod. Res. 39, 355–365. doi: 10.1016/S0022-474X(02)00028-0
Credland, P. F., and Dendy, J. (1992). Intraspecific variation in bionomic characters of the Mexican bean weevil, Zabrotes subfasciatus. Entomol. Exp. App. 65, 39–47. doi: 10.1111/j.1570-7458.1992.tb01625.x
Credland, P. F., Dick, K. M., and Wright, A. W. (1986). Relationships between larval density, adult size and egg production in the cowpea seed beetle, Callosobruchus maculatus. Ecol. Entomol. 11, 41–50. doi: 10.1111/j.1365-2311.1986.tb00278.x
Davies, J. C. (1972). A note on the occurrence of Zabrotes subfasciatus Boh., Coleoptera (Bruchidae) on legumes in Uganda. East Afr. Agric. J. 37, 294–299.
Delgado-Salinas, A. (1988). “Variation, taxonomy, domestication and germplasm potentialities in Phaseolus coccineus,” in Genetic Resources of Phaseolus Beans, ed P. Gepts (Boston: Kluwer Academic Publishers), 441–463.
Dendy, J., and Credland, P. F. (1991). Development, fecundity and egg dispersion of Zabrotes subfasciatus. Entomol. Exp. App. 59, 9–17. doi: 10.1111/j.1570-7458.1991.tb01481.x
Ernest, E., and Kee, E. (2008). Lima bean breeding and genetics research at the University of Delaware. Annu. Rep. Bean Improv. Coop. 51, 54–55.
FAO (2013). FAO Production, Food and Agricultural Organization Statistics Division. Available online at: http://www.fao.org/docrep/018/i3107e/i3107e00.htm.
Fox, C. W., and Czesak, M. E. (2000). Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 45, 341–369. doi: 10.1146/annurev.ento.45.1.341
Fuller, D. Q. (2007). Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the Old World. Ann. Bot. 100, 903–924. doi: 10.1093/aob/mcm048
Gepts, P. (1988). “A Middle American and an Andean common bean gene pool,” in Genetic Resources of Phaseolus Beans, ed P. Gepts (Dordrecht; Holland: Kluwer Academic), 375–390.
Giga, D. P., and Smith, R. H. (1991). Intraspecific competition in the bean weevils Callosobruchus maculatus and C. rhodesianus (Col Bruchidae). J. Appl. Ecol. 28, 918–929. doi: 10.2307/2404217
Goossens, A., Quintero, C., Dillen, W., De Rycke, R., Flower Valor, J., De Clercq, J., et al. (2000). Analysis of bruchid resistance in the wild common bean accession G02771: no evidence for insecticidal activity of arcelin 5. J. Exp. Bot. 51, 1229–1236. doi: 10.1093/jexbot/51.348.1229
Guedes, N. M. P., Guedes, R. N. C., Campbell, J. F., and Throne, J. E. (2010). Contest behaviour of maize weevil larvae when competing within seeds. Anim. Behav. 79, 281–289. doi: 10.1016/j.anbehav.2009.10.022
Hernandez-Cumplido, J., Glauser, G., and Benrey, B. (2016). Cascading effects of early-season herbivory on late-season herbivores and their parasitoids. Ecology 97, 1283–1297. doi: 10.1890/15-1293.1
Janzen, D. H. (1977). How southern cowpea weevil larvae (Bruchidae: Callosobruchus maculatus) die on nonhost seeds. Ecology 58, 921–927. doi: 10.2307/1936229
Kenyon, S. G., Buerki, S., Hansson, C., Alvarez, N., and Benrey, B. (2015). Uncovering cryptic parasitoid diversity in Horismenus (Chalcidoidea, Eulophidae). PLoS ONE 10:e0136063. doi: 10.1371/journal.pone.0136063
Kluyver, T. A., Jones, G., Pujol, B., Bennett, C., Mockford, E. J., Charles, M., et al. (2017). Unconscious selection drove seed enlargement in vegetable crops. Evol. Lett. 1, 64–72. doi: 10.1002/evl3.6
Laurin-Lemay, S., Angers, B., Benrey, B., and Brodeur, J. (2013). Inconsistent genetic structure among members of a multitrophic system: did bruchid parasitoids (Horismenus spp.) escape the effects of bean domestication? Bull. Entomol. Res. 103, 182–192. doi: 10.1017/S000748531200051X
Leroi, B., Bonet, A., Pichard, B., and Biemont, J. C. (1990). Relaciones entre Bruchidae (Coleoptera) y poblaciones silvestres de Phaseolus (Leguminosae: Phaseolinae) en el norte de Morelos, Mexico. Acta Zool. Mex. 42, 1–28.
Lindig-Cisneros, R., Benrey, B., and Espinosa-García, F. J. (1997). Phytoalexins, resistance traits, and domestication status in Phaseolus coccineus and Phaseolus lunatus. J. Chem. Ecol. 23, 1997–2011. doi: 10.1023/B:JOEC.0000006485.38713.8c
Martínez-Castillo, J., Camacho-Pérez, L., Villanueva-Viramontes, S., Andueza-Noh, R. H., and Chacón-Sánchez, M. I. (2014). Genetic structure within the Mesoamerican gene pool of wild Phaseolus lunatus (Fabaceae) from Mexico as revealed by microsatellite markers: implications for conservation and the domestication of the species. Am. J. Bot. 101, 851–864. doi: 10.3732/ajb.1300412
Martínez-Castillo, J., Colunga-García Marín, P., and Zizumbo-Villarreal, D. (2008). Genetic erosion and in situ conservation of Lima bean (Phaseolus lunatus L.) landraces in its Mesoamerican diversity center. Genet. Resour. Crop Evol. 55, 1065–1077. doi: 10.1007/s10722-008-9314-1
Messina, F. J., and Renwick, J. A. A. (1985). Ability of ovipositing seed beetles to discriminate between seeds with differing egg loads. Ecol. Entomol. 10, 225–230. doi: 10.1111/j.1365-2311.1985.tb00552.x
Meyer, R. S., DuVal, A. E., and Jensen, H. R. (2012). Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytol. 196, 29–48. doi: 10.1111/j.1469-8137.2012.04253.x
Mitchell, C., Brennan, R. M., Graham, J., and Karley, A. J. (2016). Plant defense against herbivorous pests: exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 7:1132. doi: 10.3389/fpls.2016.01132
Moraes, R. A., Sales, M. P., Pinto, M. S. P., Silva, L. B., Oliveira, A. E. A., Machado, O. L. T., et al. (2000). Lima bean (Phaseolus lunatus) seed coat phaseolin is detrimental to the cowpea weevil (Callosobruchus maculatus). Braz. J. Med. Biol. Res. 33, 191–198. doi: 10.1590/S0100-879X2000000200005
Moreira, X., Abdala-Roberts, L., Hernandez-Cumplido, J., Rasmann, S., Kenyon, S. G., and Benrey, B. (2015). Plant species variation in bottom-up effects across three trophic levels: a test of traits and mechanisms. Ecol. Entomol. 40, 676–686. doi: 10.1111/een.12238
Motta-Aldana, J. R., Serrano-Serrano, M. L., Hernández-Torres, J., Castillo-Villamizar, G., and Debouck, D. G. (2010). Multiple origins of lima bean landraces in the Americas: evidence from chloroplast and nuclear DNA polymorphisms. Crop Sci. 50, 1773–1787. doi: 10.2135/cropsci2009.12.0706
Nienhuis, J., Tivang, J., Skroch, P. W., and dos Santos, J. B. (1995). Genetic relationships among cultivars and landraces of lima bean (Phaseolus lunatus L.) as measured by RAPD markers. J. Am. Soc. Hortic. Sci. 120, 300–306.
Oliveira, S. O. D., Rodrigues, A. S., Vieira, J. L., Rosi-Denadai, C. A., Guedes, N. M. P., and Guedes, R. N. C. (2015). Bean type modifies larval competition in Zabrotes subfasciatus (Chrysomelidae: Bruchinae). J. Econ. Entomol. 108, 2098–2106. doi: 10.1093/jee/tov107
Paukku, S., and Kotiaho, J. S. (2008). Female oviposition decisions and their impact on progeny life-history traits. J. Insect Behav. 21, 505–520. doi: 10.1007/s10905-008-9146-z
Piñero, D., and Eguiarte, L. (1988). The origin and biosystematic status of Phaseolus coccineus ssp. polyanthus: electrophoretic evidence. Euphytica 37, 199–203. doi: 10.1007/BF00015116
Plaza, S. (2001). Patrons D'oviposition de Zabrotes Subfasciatus (Coleoptera: Bruchidae): Facteurs Biotiques et Abiotiques. master's thesis, University of Neuchâtel, Neuchatel.
Romero, J., and Johnson, C. D. (2000). Revision of the genus Zabrotes Horn of Mexico (Coleoptera: Bruchidae: Amblycerinae). T. Am. Entomol. Soc. 126, 221–274. Available online at: http://www.jstor.org/stable/25078712
Rowen, E., and Kaplan, I. (2016). Eco-evolutionary factors drive induced plant volatiles: a meta-analysis. New Phytol. 210, 284–294. doi: 10.1111/nph.13804
Savalli, U. M., and Fox, C. W. (1998). Sexual selection and the fitness consequences of male body size in the seed beetle Stator limbatus. Anim. Behav. 55, 473–483. doi: 10.1006/anbe.1997.0622
Schoonhoven, A. V., Cardona, C. V., and Valor, J. (1983). Resistance to the bean weevil and the Mexican bean weevil (Coleoptera: Bruchidae) in noncultivated common bean accessions. J. Econ. Entomol. 76, 1255–1259. doi: 10.1093/jee/76.6.1255
Serrano-Serrano, M. L., Andueza-Noh, R. H., Martínez-Castillo, J., Debouck, D. G., and Chacón S, M. I. (2012). Evolution and domestication of Lima Bean in Mexico: evidence from Ribosomal DNA. Crop Sci. 52, 1698–1712. doi: 10.2135/cropsci2011.12.0642
Shlichta, J. G., Glauser, G., and Benrey, B. (2014). Variation in cyanogenic glycosides across populations of Wild Lima Beans (Phaseolus lunatus) has no apparent effect on bruchid beetle performance. J. Chem. Ecol. 40, 468–475. doi: 10.1007/s10886-014-0434-0
Silva, L. B., Sales, M. P., Oliveira, A. E., Machado, O. L. T., Fernandes, K. V., and Xavier-Filho, J. (2004). The seed coat of Phaseolus vulgaris interferes with the development of the cowpea weevil [Callosobruchus maculatus (F.) (Coleoptera: Bruchidae)]. Anais da Academia Brasileira de Ciências 76, 57–65. doi: 10.1590/S0001-37652004000100006
Silva, R. N. O., Burle, M. L., Pádua, J. G., de Almeida Lopes, A. C., Gomes, R. L. F., and Martínez-Castillo, J. (2017). Phenotypic diversity in lima bean landraces cultivated in Brazil, using the Ward-MLM strategy. Chilean J. A. R. 77:35. doi: 10.4067/S0718-58392017000100004
Smartt, J. (1988). “Morphological, physiological and biochemical changes in Phaseolus beans under domestication,” in Genetic Resources of Phaseolus Beans ed P. Gepts (Dordrecht: Kluwer Academic), 143–161.
Sotelo, A., Sousa, H., and Sánchez, M. (1995). Comparative study of the chemical composition of wild and cultivated beans (Phaseolus vulgaris). Plant Foods Hum. Nutr. 47, 93–100. doi: 10.1007/BF01089257
Teixeira, I. R. V., and Zucoloto, F. S. (2012). Intraspecific competition in Zabrotes subfasciatus: physiological and behavioral adaptations to different amounts of host. Insect Sci. 19, 102–111. doi: 10.1111/j.1744-7917.2011.01425.x
Van Valen, L. (1965). Morphological variation and width of ecological niche. Am. Nat. 99, 377–390. doi: 10.1086/282379
Westoby, M., Falster, D. S., Moles, A. T., Vesk, P. A., and Wright, I. J. (2002). Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 33, 125–159. doi: 10.1146/annurev.ecolsys.33.010802.150452
Whitehead, S. R., Turcotte, M. M., and Poveda, K. (2017). Domestication impacts on plant–herbivore interactions: a meta-analysis. Philos. Trans. R. Soc. B 372:20160034. doi: 10.1098/rstb.2016.0034
Keywords: plant-insect interactions, bean weevil, seed pest, intraspecific competition, Phaseolus lunatus, seed size, domestication syndrome
Citation: Cuny MAC, Shlichta GJ and Benrey B (2017) The Large Seed Size of Domesticated Lima Beans Mitigates Intraspecific Competition among Seed Beetle Larvae. Front. Ecol. Evol. 5:145. doi: 10.3389/fevo.2017.00145
Received: 18 July 2017; Accepted: 09 November 2017;
Published: 23 November 2017.
Edited by:
Alejandro Casas, Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México, MexicoReviewed by:
Maria Pappas, Democritus University of Thrace, GreecePaul Gepts, University of California, Davis, United States
Copyright © 2017 Cuny, Shlichta and Benrey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Betty Benrey, YmV0dHkuYmVucmV5QHVuaW5lLmNo
 Maximilien A. C. Cuny
Maximilien A. C. Cuny Gwen J. Shlichta2
Gwen J. Shlichta2 Betty Benrey
Betty Benrey