- 1Department of Biosciences, Centre for Ecology and Conservation, College of Life and Environmental Sciences, University of Exeter, Exeter, United Kingdom
- 2Centre for Ecological Sciences, Indian Institute of Science, Bangalore, India
A well-established route to speciation in animals is via the evolution of divergent male mating signals and female preferences within a species. However, an open question is how common it is for near complete isolation to be achieved through a single signal-receiver system as opposed to multiple aspects of the mate-recognition system diverging simultaneously. The five highly divergent mate-attraction song types of the bush cricket Mecopoda elongata exemplify reproductive isolation in sympatry through long-distance mating signals. Female preference for their own song type has been established as a strong pre-mating reproductive barrier, but the potential existence of additional isolating mechanisms has not been investigated. We quantify divergence in cuticular lipid profiles and external genital structures between song types. These traits show significant variation among species of Orthoptera and are known to be used in mate recognition following contact. We show that divergence among sympatric Mecopoda song types in both cuticular lipid profiles and two external genital structures is sufficiently extensive that either of them can be used to identify individual song type with 90% accuracy. Our findings suggest that multiple isolating mechanisms are likely to evolve simultaneously facilitating a more robust reproductive isolation. Our study indicates a role for sexual selection in the divergence and potential future speciation of these populations and suggests that reproductive isolation may frequently evolve through simultaneous divergence across different aspects of mate recognition systems.
Introduction
Speciation occurs when populations acquire reproductive isolating mechanisms that are strong enough to reduce gene flow to a level that makes these populations independently evolving entities (Coyne and Orr, 2004). In animals, reproductive isolation is frequently the result of divergence in male mating signals and associated female preferences (Mullen and Shaw, 2013). If a genetic covariance between female preferences and male traits develops, sexual selection will act directly on these traits, which can theoretically drive very rapid divergence (Panhuis et al., 2001; Ritchie, 2007). However, whether isolation between populations typically requires divergence in only a single, or multiple signaling systems remains an open question (Butlin et al., 2011). In any study aiming to test this, it is important to establish (1) that signal traits are divergent among populations, (2) that receivers discriminate among signalers on the basis of those signals causing assortative mating, (3) that signaling traits have the potential to contribute independently to reproductive isolation (Via, 2001; Butlin et al., 2011). Theory suggests that sexual selection will often lead to divergence in secondary sexual characters among populations that do not differ much in their ecological adaptations (Ritchie, 2007). The striking example of five sympatric populations of the bush cricket Mecopoda elongata (denoted as Mecopoda hereafter) found in South India, which express completely different male calling songs appears to support this argument. In this study, we examine divergence of two additional putative sexual signals among these populations in order to test the prediction that multiple recognition traits diverge in concert.
Each of the Mecopoda song types, Chirper, Double Chirper, Two Part, Helicopter and Train, have calls with distinct temporal structures which they retain throughout their life. These are the only types of call known among Mecopoda from India so far (Nityananda and Balakrishnan, 2006) and are used by Mecopoda males (females do not call) to attract potential mates from long distance (Dutta et al., 2017). However, the five distinct song types overlap in mating seasons and distributions, occupy similar habitat and appear to be generalist in terms of diet (Nityananda and Balakrishnan, 2006; Dutta et al., 2017). Each song type is considered a cryptic population because the song types could not be reliably differentiated based on either qualitative or quantitative morphological characters (Nityananda and Balakrishnan, 2006). Our previous work using song playback experiments (Dutta et al., 2017) demonstrates that females exercise preferences for their own song type on the basis of calls alone, but also that in the absence of song, these preferences are retained, suggesting that independent signaling channels exist. Here, we examine the potential of two putative sexually selected traits: male genital characters and cuticular lipid profiles in contributing to reproductive isolation among Mecopoda song types. These traits have a well-established mechanistic basis and are known to act as sexual signals in other orthopteroid insects (Tregenza and Wedell, 1997; Tregenza, 2002; Schilthuizen, 2003; Holwell et al., 2009; Masly, 2012).
In contrast with the male mating calls that play a key role in long distance sexual communication, cuticular pheromones and genital morphology are traits that require contact between potential mates for their signaling role to be expressed. In Mecopoda, the primary isolating mechanism between song type populations appears to be female preference for their own mating call (Dutta et al., 2017). However, in sympatric Mecopoda song type populations, chance encounters between adults must occur occasionally in nature, and in the absence of additional short-range reproductive barriers, the integrity of song-type populations would be likely to break down. In this context, we need to identify the secondary sexual characters that may be used in population specific mate choice. Here we study the shape of two external genital structures and the cuticular lipid profiles of male Mecopoda individuals to investigate the extent of divergence in these putative mate-choice associated characters among Mecopoda song types.
In crickets and bush crickets, the male and female antennate (touch one another with their antennae) after the female has successfully located a male through phonotaxis to a long-range acoustic signal from the male. A successful mating commences only after a mating pair is satisfied with the sex and species/population specific chemical cues detected during this antennation event [as discussed in Nagamoto et al. (2005)]. Cuticular hydrocarbons (CHCs), which make up the majority of the cuticular lipids are known species specific chemical cue (Howard and Blomquist, 2005; Kather and Martin, 2012). Cuticular lipids also act as sex pheromones in crickets (Tregenza and Wedell, 1997), are crucial to the mate recognition process (Nagamoto et al., 2005) and are used by females to choose mates (Thomas and Simmons, 2009; Steiger et al., 2013). In addition, CHCs in two species of leaf beetles (Coleoptera) have been found to reinforce behavioral isolation between these species (Peterson et al., 2007). Studies showing the ability of cuticular lipid profiles to distinguish sympatric species have been conducted in ants (Akino et al., 2002; Lucas et al., 2002; Schlick-Steiner et al., 2006; Martin et al., 2008), termites (Haverty et al., 1990), beetles (Page et al., 1997; Peterson et al., 2007), and butterflies (Guillem et al., 2012). Chemical isolation through CHC mediated mate choice has been studied in sulfur butterflies (Grula et al., 1980) and Drosophila (Coyne et al., 1994; Coyne, 1996). Therefore, the potential existence of differentiated cuticular lipid profiles coinciding with acoustic signal types would be consistent with a role in reproductive isolation among song types.
The evolution of genital structures is known to be primarily driven by sexual selection (Schilthuizen, 2003; Hosken and Stockley, 2004; Eberhard, 2009, 2013; Masly, 2012; Arnqvist and Rowe, 2013). Nityananda and Balakrishnan (2006) studied the morphology of five Mecopoda song types based on 75 quantitative characters and 61 qualitative characters. In their traditional morphometric analysis, Chirper was the only song type to cluster separately from the other song types. The difference between Chirper and the other song types turned out to be the combined result of many characters, as even after the removal of certain characters (such as body size, file length, peg number) thought to play important roles in song production, the Chirper individuals did not cluster with other song types. Genital morphology often acts as a good diagnostic character that helps in differentiating species when external morphology is similar (Song, 2009). Nityananda and Balakrishnan (2006) did not examine the genital characters of Mecopoda song types in detail; only the length and width of the secondary sexual characters such as cerci and subgenital plate were considered for quantitative analysis. However, these characters failed to show up as diagnostic characters among a large number of characters examined. This study further included the shape of the subgenital plate and cerci of the Mecopoda in the qualitative analysis in the form of nominal variables. The study did not compare the actual shapes (in the form of coordinates) of these two structures due to limitations in the ability of traditional morphometric techniques to quantify and retain original shapes (Rohlf and Marcus, 1993). We use landmark-based geometric morphometric tools to study the difference in shapes of the subgenital plate and the cerci of the five different song types of Mecopoda. These traits are of particular interest both because genital morphology is commonly used to distinguish among closely related insect species (Shapiro and Porter, 1989; Mutanen et al., 2006; Mikkola, 2008). This indicates the potential for both of these characters to play a role as participatory organs in mechanical isolation among Mecopoda song types.
Mecopoda song types are known to be differentiated in male mating calls that are used to attract mates far away from the signaler. However, this evidence fails to explain how gene flow is prevented in events when mating calls are not involved, for instance, in chance encounters. Our hypothesis is that there must be other simultaneous barriers to matings that prevent hybridization among the song types. Since orthopteran reproductive behavior involves exchange of chemical cues during antennation and requires proper articulation of male and female genital structures for successful mating, cuticular lipid profiles and external genital characters are candidate secondary isolating traits among these song types. Therefore, we predict that cuticular lipid profiles and external genital morphology will be divergent among the song types of Mecopoda. The objectives of our study are:
1. To examine whether cuticular lipid profiles are differentiated among the Mecopoda song types.
2. To determine whether shape differences exist in the subgenital plate and cerci among the song types.
Materials and Methods
Specimen Collection
Most of the Mecopoda males were collected opportunistically from locations around Kadari field station (N13° 13′, E75 05′), Ullodu (N13° 38′, E77° 42′) and from within the Indian Institute of Science Campus (N13° 01′, E77° 34′), Bangalore. A few of the specimens were collected from Shimoga (N13° 55', E75° 35') while one each were collected from Gerusoppa (N14° 14′ E74° 44′) and Sagar (N14° 10′, E75° 02′) (see Supplementary Tables 1, 2). All these locations lie within the state of Karnataka, India where they have been previously recorded (Nityananda and Balakrishnan, 2006; Dutta et al., 2017). Males were tracked by listening to their calling songs or by following their movement when ground vegetation was disturbed. The songs of all these males were recorded to ascertain the song type of the individuals before the study was conducted. A total of 166 live Mecopoda specimens (41 Chirper, 45 each of Double Chirper and Two Part, 14 Helicopter and 21 Train) were used for cuticular lipid analysis. The total number of specimens analyzed for subgenital plate and cercal morphology was 130 (30 each of Mecopoda Chirper, Two-Part and Double Chirper; 21 Helicopter and 19 Train). Among these, only 60 individuals (belonging to Two Part and Chirper) were common between the two analyses i.e., cuticular lipid analysis and morphometric analysis. Hence, geometric morphometric analysis and cuticular lipid analyses were done separately.
Geometric Morphometric Analysis
Shapiro and Porter (1989) and Schilthuizen (2003) describe male genitalia as one of the most important characters for species level identification in insects (Mutanen et al., 2006). Until recently, utilization of genital structures was predominantly limited to external genital structures, which usually play a role in the early stages of mating (Mikkola, 2008). Rentz et al. (2006) used external genital structures such as cerci and the subgenital plate in delimitation of the Austromecopoda species complex. Cerci, hook-like bilaterally symmetrical structures at the end of the 10th tergite (Faucheux, 2012), are mostly a sensory organ acting as proprioceptors, sensitive to air movement (Snell and Killian, 2000; Faucheux, 2012; Chapman et al., 2013); apart from being vibroreceptors, they also host a small number of chemoreceptors and olfactory receptors (Faucheux, 2012; Chapman et al., 2013). Cerci have also been found to work as claspers during mating in crickets (Faucheux, 2012) and successful mating depends on their movement during mating (Sakai and Ootsubo, 1988; Snell and Killian, 2000). In bush crickets the subgenital plate, which is an elongated structure attached to the 9th sternite and bifurcated at the posterior end, acts as a protective organ for the male genitalia (Chapman et al., 2013) as well as a sensory organ (Faucheux, 2012). It also helps in the articulation of male genitalia onto female genitalia in crickets and bush crickets (Faucheux, 2012). Both cerci and the subgenital plate are involved in copulations and have tactile functions during mating in other Orthoptera (Faucheux, 2012) and therefore we used these characters in our geometric morphometric analysis. Previous attempts to compare the morphology of Mecopoda song types have employed traditional morphometrics (Nityananda and Balakrishnan, 2006). This approach has a number of disadvantages compared to recently developed geometric approaches. In particular, geometric morphometric analysis has the advantages that it retains more information about the shapes of structures by fitting smoothed functions between shape coordinates (Rohlf and Marcus, 1993). It also avoids the requirement that particular features are chosen for comparison prior to the analysis, or that the use of large numbers of independent characters dilutes the effects of singular variables increasing the risk of type 1 error.
All these general limitations had a bearing on the previous morphometric study on Mecopoda and therefore, we use landmark based geometric morphometrics in this study. For procuring images of the two external genital characters, all specimens were chosen such that the genital structures to be studied were intact and undamaged. The pictures (in TIFF format) of the subgenital plate (SGP) and the cerci of Mecopoda specimens were taken using a digital camera (Leica DFC290) connected to a microscope (Leica M165C). The subgenital plates were photographed by placing each individual Mecopoda ventral side up. The cerci were photographed similarly with the dorsal side of the Mecopoda facing the camera. The software, tpsUtil (Rohlf, 2015) was then used to list the photographs into a.tps file. The first step for the landmark based geometric morphometric analysis involved identifying specific landmarks that are homologous, lie in the same plane, adequately cover the structure and which can be found reliably and repeatedly at similar positions with respect to adjacent landmarks in all samples (Zelditch et al., 2012). Landmarks that satisfy these criteria, can be classified into three types: type 1 corresponds to distinct meeting points of two structures (separate tissues/organs), type 2 corresponds to maxima or minima of curves in a localized structure, and type 3 corresponds to artificial points such as center/outermost points of a structure in relation to a different landmark (Bookstein, 1997). The landmarks in this study were chosen according to these criteria (Zelditch et al., 2012) and their classification into types (Bookstein, 1997) were tabulated (see Supplementary Table 3). Nineteen anatomical landmarks were used to define the coordinates for the shape of subgenital plate while nine landmarks were used in the case of right cercus (see Figures 1A,B). The landmarks were digitized on the photographs with tpsdig2 software (Rohlf, 2015) and coordinate values were stored in the same.tps file. The TPS software package fits a thin plate spline function between two adjacent landmark coordinates (Rohlf, 2015). Relative warp analysis which was performed using tpsrelw software for this study is akin to principal component analysis where the relative warps are the principal components of a set of thin-plate spline transformations (Rohlf, 1994; Zelditch et al., 2012). This program produces a consensus shape also called the reference shape through the generalized Procrustes method (GPA), which produces the most unbiased mean configuration, closest to the true shape (Rohlf, 2003). The difference between the original shapes of the specimen and the reference shape generated are then used to tests intra-population variation with respect to inter-population variation through relative warp analysis.
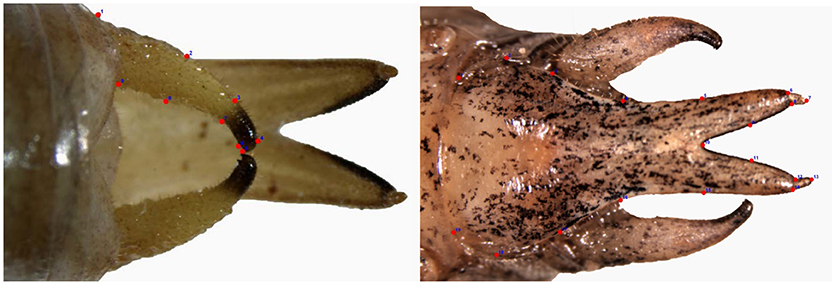
Figure 1. (A) Cerci. (B) Sub-genital plate. Approximate locations of landmarks denoting the boundary of the right cercus (A) and the sub-genital plate (B) used in landmark-based geometric morphometrics.
For 25 randomly selected individuals spread equally among five song types, 2 photographs were taken at different times and analyzed twice separately to quantify within individual variability and to confirm the repeatability of the measurements. We performed linear regression analysis between corresponding relative warps derived separately from two different photographs as well as between corresponding relative warps from two different analyses of the same photographs following the method used in House et al. (2013). Based on relative warp analysis, the number of relative warp components and the landmarks that explain most of the variation were identified. Random forest analyses (see below) were performed with the individual relative warp scores of each individual to see how Mecopoda individuals form groups.
Cuticular Lipid Extraction and GCMS
Any study involving cuticular lipid profiling follows a common analytical procedure (Lockey, 1991):
1. Organic solvents are used in extraction of cuticular lipids.
2. The extracted samples are analyzed using gas chromatography to separate out different components and create a cuticular lipid profile.
3. The cuticular lipids are identified, in our case, by mass spectrometry (GCMS).
Before extraction, both containers and forceps were washed in Hexane to remove any contamination. A Mecopoda male was put in a 10 ml glass vial, which was then filled completely with Hexane such that the Mecopoda specimen was totally submerged. After 5 min, the male was pulled out with the forceps and the remaining hexane was left to evaporate to about 1 ml. Using a pipette, this 1 ml of Hexane and dissolved cuticular lipids was transferred to 1.5 ml GC vial. Hexane was again allowed to evaporate completely so that the content of the vial was dry: the cuticular lipid extracts would have been deposited on the wall of the GC vial. After this, the GC vial was capped and stored in refrigerator until GCMS was conducted.
A mass spectrometer (Agilent 5975B MSD) enabled gas chromatography instrument (Agilent 7890A) with Agilent J&W DB-1MS capillary column (30 m × 250 μm × 0.25 μm) was used in the cuticular lipid profiling. Before the GCMS run, the air-dried sample was returned to solution by adding 500 μl of hexane. A known volume (1 ml of 10 ng/μl pentadecane) of internal standard was also added to this solution and this sample solution was vortexed for 10 s. The amount of sample injected into the capillary column was 2 ul via an autosampler (G6500-CTC). Highly pure helium was used in the analysis and its flow in the column was maintained at 3 ml/min. A 27 min programme was used with the temperature rising from 50°C to 280°C at 30°C/min continuing from 280°C to 350°C at 5°C/min and then maintained at 350°C for 5 min (the DB-1 capillary column used here can reach up to 360°C). The peaks detected by the mass spectrometer were analyzed using Agilent ChemStation software.
A total of 27 peaks and that of the internal standard (peak 1: pentadecane) occurred in the majority of our 166 sample extracts belonging to five Mecopoda song types. Of these 27, 6 were frequently found at such low concentrations that their relative abundance could not be reliably quantified (see Supplementary Table 4). Peaks 15 and 16 were dropped from further analysis since peak 16 merged with peak 15 in many cases making them impossible to distinguish. The relative abundance of the remaining 19 peaks were calculated by standardizing the area under each peak relative to the area under the internal standard peak. Fourteen peaks were positively identified as methyl alkanes (alternatively hydrocarbons) (see Supplementary Table 5) by their mass spectra and retention index [following the references in Steiger et al. (2007)]. The rest of the peaks are also very likely to be hydrocarbons following the trends in results from searching probable peak identity in the NIST 08 library (blast results predicted hydrocarbons ranging from C27 to C44). For random forest analysis (see below) we used log transformed standardized area under the peak data for 19 peaks of all 166 samples.
Random Forest Analysis
Random forest is increasingly used in exploratory studies with complex data where the number of variables is larger than the sample size (Strobl et al., 2007; Ranganathan and Borges, 2011). These complex data tend to vary in scale, contain missing data and zeroes, data points in them can be highly correlated and the structure for these data is usually unknown (Cutler et al., 2007; Ranganathan and Borges, 2011). Random forest is suitable for the present study mainly because:
1. The number of samples does not exceed the number of variables in morphometric analysis. In case of cuticular lipid profiling, the sample size for Chirper, Double Chirper, Two Part and Train are little more than the number of variables while that of Helicopter song type is less than the number of variables.
2. Both morphometric and cuticular lipid data points cannot be considered independent with surety especially since they were part of the same structure or underlying biochemical process.
Random forest is a machine learning algorithm that creates a set of classification schemes (also called base learners) using a random bootstrap set of samples and a random subset of variables for predicting group affinity of individuals within a data set (Strobl et al., 2009; Touw et al., 2013). We used the “cforest” algorithm from the “party” package (R Core Team, 2014) which is an advanced implementation of random forest in R. It produces conditional inference trees as base learners which can overcome the bias among correlated variables in the analysis (Hothorn et al., 2006; Strobl et al., 2007, 2008). We checked the stability of the random forest analysis by increasing the number of iterations for classification trees (ntree) from a smaller number to a larger number. The number of predictor variables should be equal to the square root of the total number of variables as suggested by Strobl et al. (2009). We fixed the ntree and the mtry (also called hyper parameters) before the analysis. Random forest analysis produces an error estimate for the classification of data under study. This is done by comparing the value of the known independent variables with the predicted value of the same independent variables from the random forest analysis. Proximity is the measure of similarity between any two individual observations from a data set and the proportion of times these two individuals occurred at the same node of the classification tree during random forest analysis. We performed proximity analysis before doing a multi-dimensional scaling (MDS) of the calculated proximity matrix to produce a scatterplot which can help visualize how the individuals group together. This is equivalent to plotting multivariate data to detect clustering and is also similar to principal component analysis.
Results
Landmark Based Geometric Morphometrics
In relative warp analysis of 130 Mecopoda specimens, 34 relative warp components were obtained for the subgenital plate and 14 relative warp components were obtained for the right cercus. The first 3 components for subgenital plate explained 70% of variation while the first 2 components for right cercus explained 88% of variation. The test also confirmed that landmarks 6, 8, 12, and 14 (see Figure 1B) out of 19 landmarks on the subgenital plate contribute most to the variation in shape whereas landmarks 5 and 6 (see Figure 1A) out of 9 landmarks contribute most to the shape variation of right cercus. In other terms, the maximum contribution to the variation of the shape of the sub-genital plate came from the posterior apical projection on the sub-genital plates. Similarly, the maximum contribution to the variation of the shape of cerci involved the two subapical hook-like structures on posterior projection of cerci. The relative warp analysis failed to differentiate between the song types adequately when sub-genital plate and right cercus were analyzed and visualized in graphs separately (see Supplementary Figures 1, 2). However, there was a possibility that the relative warp scores from both the shapes analyzed together could separate the Mecopoda song types where either shape failed singly. Due to limitations of the tpsrelw software, relative warp scores of sub-genital plate and right cercus could not be used together for a single multivariate analysis. This necessitated the use of random forest analysis that could use relative warp scores from both sub-genital plate and right cercus originally derived in previous relative warp analysis to perform multivariate analysis. Therefore, we used relative warp scores (a continuous variable) of all 130 Mecopoda samples as a substitute for the original discrete Cartesian coordinates (Webster and Sheets, 2010; Zelditch et al., 2012) for the random forest analysis of the morphometric data.
The hyper parameters for the random forest analysis were fixed at ntree = 5000 and mtry = 7 (approximate square root of 48, the number of relative warps) in the cforest algorithm. The seed was set at 698 (a random number that helps check repeatability of the result and helps others reproduce the analysis). When “cforest” analysis predicted song types of the same Mecopoda individuals used for the analysis, it was found that the prediction from the “cforest” analysis differed in only 15 individuals out of the total 130 individuals. The error estimate in predicting the song type of each Mecopoda individual from this random forest analysis was 11.5% (15 out of 130). This means almost 9 out of 10 times the algorithm was able to correctly predict the song type of an individual Mecopoda. It is also interesting to note that greatest number of errors (10 out of the 15 wrong predictions) occurred while predicting whether an individual belonged to the Helicopter song type. The first two components from multi-dimensional scaling of the proximity matrix were able to substantially segregate the song types (see Figure 2). A 3-dimesional scatter-plot (not shown here) from three components of the multi-dimensional scaling of the proximity matrix showed even clearer Mecopoda song types clusters in 3D space.
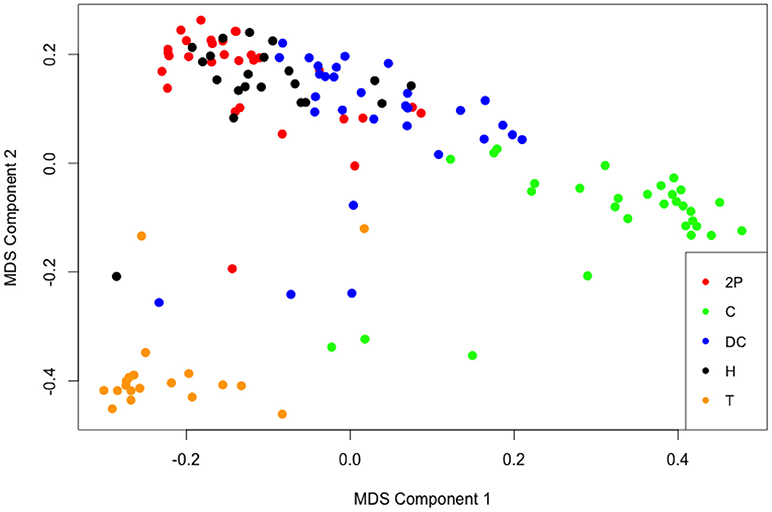
Figure 2. Multi-dimensional scaling (MDS) of the proximity matrix showing Mecopoda song type clusters based on geometric morphometrics of subgenital plate and right cercus of 130 Mecopoda individuals. The different colored circles represent Mecopoda song types: 2P, Two Part; C, Chirper; DC, Double Chirper; H, Helicopter; T, Train.
Cuticular Lipid Analysis
The hyper-parameters during the random forest analysis were ntree = 10000 and mtry = 4 (rounded square root of 19, the number of log transformed cuticular lipid peaks). The seed was set at 874. The “cforest” analysis of the log transformed cuticular lipid peak data predicted song type of the each Mecopoda individual with an error estimate of 7.2 % (i.e., 12 errors out of total 166). We should again note that the greatest number of errors (8 out of the 12 wrong predictions) occurred while predicting whether an individual belonged to the Helicopter song type. After proximity analysis, multi-dimensional scaling of the proximity matrix was able to differentiate all the five song types (see Figure 3). While Chirper and Double Chirper formed distinct clusters, Train, Two Part and Helicopter formed clusters with very few overlaps. This graphical overlap concords with the prediction from the random forest analysis where 5 Helicopter individuals were predicted as Two Part individuals and 3 Helicopter individuals were predicted as Train individuals. A 3-dimesional scatter-plot (not shown here) from three components of the multi-dimensional scaling of the proximity matrix based on abundance of cuticular lipids in Mecopoda samples showed even starker Mecopoda song types clusters in 3D space. It appears that Chirper, Double Chirper, Two Part and Train are diverged in 4 different directions while Helicopter song types occupying somewhat central position to these 4 axes.
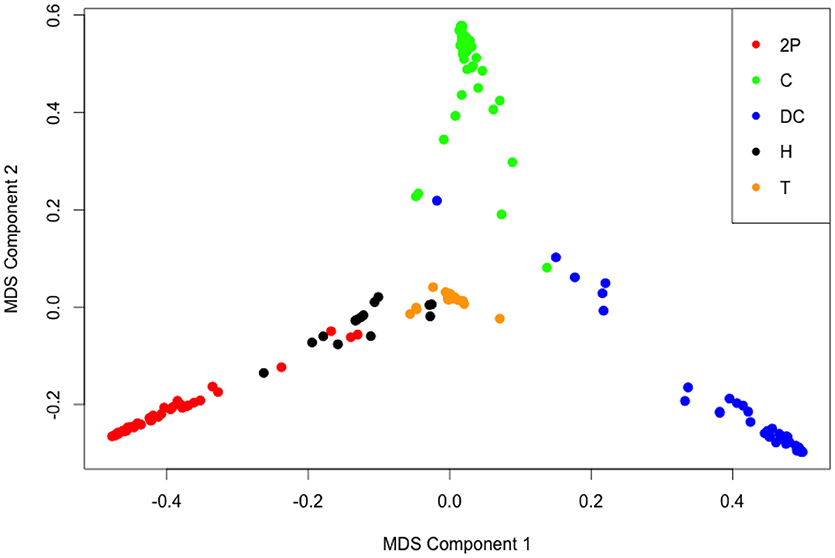
Figure 3. Multi-dimensional scaling (MDS) of the proximity matrix showing Mecopoda song type clusters based on log transformed standardized cuticular lipid peak areas of 166 Mecopoda individuals. The different colored circles represent Mecopoda song types: 2P, Two Part; C, Chirper; DC, Double Chirper; H, Helicopter; T, Train.
Discussion
Divergence in Cuticular Lipid Profiles and External Genital Characters Among Mecopoda Song Types
Reproductive isolation among sympatric Mecopoda song types is facilitated by disruptive sexual selection due to assortative mating based on their mating calls (Dutta et al., 2017). Lack of differentiation in ecological adaptations among the song types suggests that sexual selection among each song type is particularly strong in its ability to conserve their identity. In mating trials involving Chirper and Double Chirper males (to which Chirper females show differential phonotaxis while not responding to any of the other song types), Chirper females showed significant preference for males belonging to their own song type (Dutta et al., 2017). Such preferential mating without the involvement of long distance mating calls indicates the existence of other population identifying traits that are also under sexual selection. We, therefore, predicted that Mecopoda population differentiation based on these traits will correspond to the differentiation in Mecopoda mating calls. To test this prediction, we studied potential contact sexual signals in the form of cuticular lipid profiles and morphological secondary sexual characters (the subgenital plate and cerci).
Cuticular hydrocarbons are known to play an important role in conspecific identification in other orthopterans (Howard and Blomquist, 2005; Blomquist and Bagnères, 2010; Kather and Martin, 2012) and are a major discriminating factor among divergent populations (Linn et al., 1997; Peterson et al., 2007; Maroja et al., 2014). Identification of chromatographic peaks reveals that the cuticular lipids of Mecopoda range between C27 and C44, meaning they are unlikely to be volatile. Fourteen out of Twenty-Five peaks turned out to be methyl alkanes that are more likely to be involved in signaling because of their 3D branched structures, than are simple alkanes, which are more likely to act primarily as waterproofing agents (Blomquist and Bagnères, 2010; Kather and Martin, 2012). The composition of cuticular lipids also shows a greater divergence and complexity among species than within species both in terms of presence-absence and relative abundance of different lipid components (Howard and Blomquist, 2005; Kather and Martin, 2012). We found Mecopoda song types differ in the relative abundance of 19 different cuticular lipid peaks. This indicates that the song types, although closely related, have garnered substantial differences in cuticular lipid profiles.
Relative warp analysis of our morphological measurements failed to distinguish between the song types although patterns of divergence similar to those identified using traditional morphometrics [using a much larger panel of traits (Nityananda and Balakrishnan, 2006)] were noticeable as Chirpers tended to group together distinct from other song types (see Supplementary Figures 1, 2). The sensitivity of the relative warp analysis depends on the independence of landmark coordinates. Because the landmarks were not typically independent of each other, correcting for this non-independence reduced the power of the relative warp analysis to capture differences of the characters among the song types. One way to ameliorate these problems is to use bootstrap-based data mining statistical analysis such as “cforest” implementation of random forest analysis suitable for correlated predictor variables and “small n large p” datasets (Strobl et al., 2009). Our random forest analysis, revealed that the morphological data, do provide sufficient information to differentiate among song types if analyzed appropriately.
Random forest analysis on morphological relative warp scores and log transformed standardized peak area identified the correct Mecopoda song type in 9 out of 10 individuals separately. This confirms our prediction that divergence has occurred among song types in additional intersexual communication channels. Theory suggests that the success of sympatric speciation often depends on synergistic role of disruptive sexual selection and disruptive natural selection in sustaining incremental population differentiation by blocking genetic flow among subpopulations (van Rijssel et al., 2018). However, it appears that considerable divergence among Mecopoda song types has been achieved even when they are ecologically identical. Our study supports the potential for strong disruptive sexual selection to maintain and potentially to increase population differentiation to near complete isolation.
Reproductive Isolation in Mecopoda Song Types
We do not expect divergence in genital structures within species due to stabilizing selection (Schilthuizen, 2003; Mutanen et al., 2006; Richmond et al., 2012; Eberhard, 2013). However, sexual selection has the potential to increase intra-population variance (Richmond et al., 2012) such that there may be formation of cryptic species complexes if female choice of genital structure coevolves with genital structure. Genitalia have been shown to evolve faster than other morphological features (Mutanen et al., 2006). This may select for the neurological changes associated with the response of females to the detection of male genitalia (Coyne and Orr, 2004). Tactile isolation is based on the ability to detect conspecific or non-conspecific mating structures and behavior (Coyne and Orr, 2004; Masly, 2012). This kind of isolation may be expected in situations where one of the mating partners is able to sense aberrant copulatory organs or behavior of a heterospecific individual and subsequently avoid mating. There are very few studies attributing speciation solely to tactile isolation (Masly, 2012). In these few cases (LORKOVIC, 1958; Eberhard, 1992; Coyne, 1993), tactile isolation seems to act where males differ in their copulatory structures across the two groups while females do not and where females are able to control mating events and prevent a successful heterospecific mating. The sub-genital plate and cerci play an important role in articulation of male-female genitalia as well as have sensory functions in stimulating mating in crickets (Snell and Killian, 2000; Faucheux, 2012; Chapman et al., 2013). In the case of Mecopoda, we speculate that the difference in the shapes of these two characters in males of five song types, and the sensory capacities of these organs could potentially help females discriminate among males according to their genital morphology.
Since cuticular lipids can act as mating signals in close range encounters (Simmons et al., 2013; Maroja et al., 2014), it is possible that differences in the chemical profile of Mecopoda could provide the basis for sexual selection in Mecopoda as shown in other insects (Mullen et al., 2007; Peterson et al., 2007; Martin et al., 2008). The females of the five song types may exercise mate discrimination against individuals of divergent cuticular composition, analogous to the pattern observed in two distinct populations of D. montana (Veltsos et al., 2011) or two sibling species of Drosophila (Coyne, 1996), in two species of leaf beetles in hybrid zones (Peterson et al., 2007) or in closely related sympatric population of closely related sympatric species of Colias butterflies (Grula et al., 1980).
Conclusion
We have demonstrated that Mecopoda song types previously only known to be divergent in long distance male mating calls, are also divergent in two other potential signaling traits. The role of these traits in mate choice in closely related species makes them leading candidates to provide additional and simultaneous barriers to mating in Mecopoda. This suggests that divergence among song types involves simultaneous evolution of reproductive barriers based on multiple independent signal and receiver systems. Further behavioral assays investigating female preference in relation to genital morphology and cuticular composition have the potential to provide unequivocal evidence for the role of these traits as isolating mechanisms among Mecopoda song types.
Ethics Statement
All protocols for data collection adhered to the national guidelines for the ethical treatment of animals laid out by the National Biodiversity Authority (Government of India). Mecopoda is not mentioned in both IUCN red list and Indian schedule species database. It is a widely distributed species in India and occur in both protected and non protected areas near human habitation. All fieldwork during this study was conducted with the full consent and permission of the local stakeholders.
Author Contributions
Study design was done by RD, RB, and TT. Investigation, data curation and analysis were done by RD. The study was supervised by RB and TT. Original draft manuscript was written by RD. Review and editing of the manuscript was done by RB and TT.
Funding
The research was funded by University of Exeter, UK and necessary logistical support was provided by Indian Institute of Science, Bangalore, India. We thank DST-FIST for funding the equipment used in the study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Sudhakara Gowda and Manjunatha Reddy for helping us with Mecopoda field collection, and Manjunatha Reddy for helping us with Mecopoda culture maintenance. Christopher Mitchell and Melia Burdon provided immense support for the cuticular lipid profiling work and we remain indebted to them for the generous help. Initial instructions on how to use of TPS software for morphometric analysis from Clarissa House was invaluable.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00158/full#supplementary-material
References
Akino, T., Terayama, M., Wakamura, S., and Yamaoka, R. (2002). Intraspecific variation of cuticular hydrocarbon composition in Formica japonica Motschoulsky (Hymenoptera: Formicidae). Zool Sci. 19, 1155–1165. doi: 10.2108/zsj.19.1155
Blomquist, G. J., and Bagnères, A. G. (2010). Insect Hydrocarbons. Cambridge, UK: Cambridge University Press.
Bookstein, F. L. (1997). Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge, UK: Cambridge University Press.
Butlin, R., Debelle, A., Kerth, C., Snook, R. R., Beukeboom, L. W., Cajas, R. F. C., et al. (2011). What do we need to know about speciation? TREE 27, 27–39. doi: 10.1016/j.tree.2011.09.002
Chapman, R. F., Simpson, S. J., and Douglas, A. E. (2013). The Insects: Structure and Function. Cambridge, UK: Cambridge University Press.
Coyne, J. A. (1993). The genetics of an isolating mechanism between two sibling species of Drosophila. Evolution 47, 778–788. doi: 10.1111/j.1558-5646.1993.tb01233.x
Coyne, J. A. (1996). Genetics of a difference in male cuticular hydrocarbons between two sibling species, Drosophila simulans and D. sechellia. Genetics 143, 1689–1698.
Coyne, J. A., Crittenden, A. P., and Mah, K. (1994). Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science 265, 1461–1464. doi: 10.1126/science.8073292
Cutler, D. R., Edwards, T. C. Jr, Beard, K. H., Cutler, A., Hess, K. T., Gibson, J., et al. (2007). Random forests for classification in ecology. Ecology 88, 2783–2792. doi: 10.1890/07-0539.1
Dutta, R., Tregenza, T., and Balakrishnan, R. (2017). Reproductive isolation in the acoustically divergent groups of tettigoniid, Mecopoda elongata. PLoS ONE 12:e0188843. doi: 10.1371/journal.pone.0188843
Eberhard, W. G. (1992). Species isolation, genital mechanics, and the evolution of species-specific genitalia in three species of Macrodactylus beetles (coleoptera, scarabeidae, melolonthinae). Evolution 46:1774.
Eberhard, W. G. (2009). Evolution of genitalia: theories, evidence, and new directions. Genetica 138, 5–18. doi: 10.1007/s10709-009-9358-y
Eberhard, W. G. (2013). Sexual Selection and Animal Genitalia. Cambridge, MA; London: Harvard University Press.
Faucheux, M. J. (2012). The structure of the male postabdomen and associated sensilla of Phaneroptera nana Fieber 1853, and remarks on uniporous sensilla of genitalia (Orthoptera: Tettigoniidae: Phaneropterinae). Israbatacma 34, 107–114. Available online at: http://www.israbat.ac.ma/?page_id=257
Grula, J. W., McChesney, J. D., and Taylor, O. R. Jr (1980). Aphrodisiac pheromones of the sulfur butterflies Colias eurytheme and C. philodice (Lepidoptera, Pieridae). J. Chem. Ecol. 6, 241–256. doi: 10.1007/BF00987543
Guillem, R. M., Drijfhout, F. P., and Martin, S. J. (2012). Using chemo-taxonomy of host ants to help conserve the large blue butterfly. Biol. Conser. 148, 39–43. doi: 10.1016/j.biocon.2012.01.066
Haverty, M. I., Thorne, B. L., and Page, M. (1990). Surface hydrocarbon components of two species of Nasutitermes from Trinidad. J. Chem. Ecol. 16, 2441–2450. doi: 10.1007/BF01017467
Holwell, G. I., Winnick, C., Tregenza, T., and Herberstein, M. E. (2009). Genital shape correlates with sperm transfer success in the praying mantis Ciulfina klassi (Insecta: Mantodea). Behav. Ecol. Sociobiol. 64, 617–625. doi: 10.1007/s00265-009-0879-2
Hosken, D. J., and Stockley, P. (2004). Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93. doi: 10.1016/j.tree.2003.11.012
Hothorn, T., Bühlmann, P., Dudoit, S., Molinaro, A., and van der Laan, M. J. (2006). Survival ensembles. Biostatistics 7, 355–373. doi: 10.1093/biostatistics/kxj011
House, C. M., Lewis, Z., Hodgson, D. J., Wedell, N., Sharma, M. D., Hunt, J., et al. (2013). Sexual and natural selection both influence male genital evolution. PLoS ONE 8:e63807. doi: 10.1371/journal.pone.0063807
Howard, R. W., and Blomquist, G. J. (2005). Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393. doi: 10.1146/annurev.ento.50.071803.130359
Kather, R., and Martin, S. J. (2012). Cuticular hydrocarbon profiles as a taxonomic tool: advantages, limitations and technical aspects. Physiol. Entomol. 37, 25–32. doi: 10.1111/j.1365-3032.2011.00826.x
Linn, C. E., Young, M. S., Gendle, M., Glover, T. J., and Roelofs, W. L. (1997). Sex pheromone blend discrimination in two races and hybrids of the European corn borer moth, Ostrinia nubilalis. Physiol. Entomol. 22, 212–223. doi: 10.1111/j.1365-3032.1997.tb01161.x
Lockey, K. H. (1991). Insect hydrocarbon classes: implications for chemotaxonomy. Insect Biochem. 21, 91–97. doi: 10.1016/0020-1790(91)90068-P
LORKOVIC Z, Z. (1958). Some peculiarities of spatially and sexually restricted gene exchange in the Erebia tyndarus group. Cold Spring Harb. Symp. Quant. Biol. 23, 319–325.
Lucas, C., Fresneau, D., Kolmer, K., Heinze, J., Delabie, J. H. C., and Pho, D. B. (2002). A multidisciplinary approach to discriminating different taxa in the species complex Pachycondyla villosa (Formicidae). Biol. J. Linnean Soc. 75, 249–259. doi: 10.1111/j.1095-8312.2002.tb01425.x
Maroja, L. S., McKenzie, Z. M., Hart, E., Jing, J., Larson, E. L., and Richardson, D. P. (2014). Barriers to gene exchange in hybridizing field crickets: the role of male courtship effort and cuticular hydrocarbons. BMC Evol. Biol. 14:65. doi: 10.1186/1471-2148-14-65
Martin, S. J., Helantera, H., and Drijfhout, F. P. (2008). Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linnean Soc. 95, 131–140. doi: 10.1111/j.1095-8312.2008.01038.x
Masly, J. P. (2012). 170 years of “lock-and-key”: genital morphology and reproductive isolation. Int. J. Evol. Biol. 2012, 1–10. doi: 10.1155/2012/247352
Mikkola, K. (2008). The lock-and-key mechanisms of the internal genitalia of the Noctuidae (Lepidoptera): how are they selected for? Eur. J. Entomol. 105, 13–25. doi: 10.14411/eje.2008.002
Mullen, S. P., Mendelson, T. C., Schal, C., and Shaw, K. L. (2007). Rapid evolution of cuticular hydrocarbons in a species radiation of acoustically diverse Hawaiian crickets (Gryllidae: Trigonidiinae: Laupala). Evolution 61, 223–231. doi: 10.1111/j.1558-5646.2007.00019.x
Mullen, S. P., and Shaw, K. L. (2013). Insect speciation rules: unifying concepts in speciation research. Annu Rev Entomol. 59, 339–61. doi: 10.1146/annurev-ento-120710-100621
Mutanen, M., Kaitala, A., and Monkkonen, M. (2006). Genital variation within and between three closely related Euxoa moth species: testing the lock-and-key hypothesis. J. Zool. 268, 109–119. doi: 10.1111/j.1469-7998.2005.00029.x
Nagamoto, J., Aonuma, H., and Hisada, M. (2005). Discrimination of conspecific individuals via cuticular pheromones by males of the cricket Gryllus bimaculatus. Zool. Sci. 22, 1079–1088. doi: 10.2108/zsj.22.1079
Nityananda, V., and Balakrishnan, R. (2006). A diversity of songs among morphologically indistinguishable katydids of the genus Mecopoda (Orthoptera: Tettigoniidae) from Southern India. Bioacoustics 15, 223–250. doi: 10.1080/09524622.2006.9753552
Page, M., Nelson, L. J., Blomquist, G. J., and Seybold, S. J. (1997). Cuticular hydrocarbons as chemotaxonomic characters of pine engraver beetles (Ips spp.) in the grandicollis subgeneric group. J. Chem. Ecol. 23, 1053–1099. doi: 10.1023/B:JOEC.0000006388.92425.ec
Panhuis, T. M., Butlin, R., Zuk, M., and Tregenza, T. (2001). Sexual selection and speciation. TREE 16, 364–371. doi: 10.1016/S0169-5347(01)02160-7
Peterson, M. A., Dobler, S., Larson, E. L., Juárez, D., Schlarbaum, T., Monsen, K. J., et al. (2007). Profiles of cuticular hydrocarbons mediate male mate choice and sexual isolation between hybridising Chrysochus (Coleoptera: Chrysomelidae). Chemoecology 17, 87–96. doi: 10.1007/s00049-007-0366-z
Ranganathan, Y., and Borges, R. M. (2011). To transform or not to transform: that is the dilemma in the statistical analysis of plant volatiles. Plant Signal Behav. 6, 113–116. doi: 10.4161/psb.6.1.14191
Rentz, D. C. F., Su, Y. N., and Ueshima, N. (2006). Studies in Australian Tettigoniidae: the Mecopodine katydids (Orthoptera: Tettigoniidae; Mecopodinae; Mecopodini). Trans. Am. Entomol. Soc. 132, 1–24. doi: 10.3157/0002-8320(2006)132[229:SIATTM]2.0.CO;2
Richmond, M. P., Johnson, S., and Markow, T. A. (2012). Evolution of reproductive morphology among recently diverged taxa in the Drosophila mojavensis species cluster. Ecol. Evol. 2, 397–408. doi: 10.1002/ece3.93
Ritchie, M. G. (2007). Sexual selection and speciation. Annu. Rev. Ecol. Evol Syst. 38, 79–102. doi: 10.1146/annurev.ecolsys.38.091206.095733
Rohlf, F. J. (2003). Bias and error in estimates of mean shape in geometric morphometrics. J. Hum. Evol. 44:665. doi: 10.1016/S0047-2484(03)00047-2
Rohlf, F. J., and Marcus, L. F. (1993). A revolution morphometrics. Trends Ecol. Evol. 8, 129–132. doi: 10.1016/0169-5347(93)90024-J
Sakai, M., and Ootsubo, T. (1988). Mechanism of execution of sequential motor acts during copulation behavior in the male cricket Gryllus bimaculatus DeGeer. J. Comp. Physiol. A 162, 589–600. doi: 10.1007/BF01342634
Schilthuizen, M. (2003). Shape matters: the evolution of insect genitalia. Proc. Exper. Appl. Entomol. Nev. 14, 9–15.
Schlick-Steiner, B. C., Steiner, F. M., Moder, K., Seifert, B., Sanetra, M., Dyreson, E., et al. (2006). A multidisciplinary approach reveals cryptic diversity in Western Palearctic Tetramorium ants (Hymenoptera: Formicidae). Mol. Phylogene. Evol. 40, 259–273. doi: 10.1016/j.ympev.2006.03.005
Shapiro, A. M., and Porter, A. H. (1989). The lock-and-key hypothesis: evolutionary and biosystematic interpretation of insect genitalia. Annu. Rev. Entomol. 34, 231–245. doi: 10.1146/annurev.en.34.010189.001311
Simmons, L. W., Thomas, M. L., Simmons, F. W., and Zuk, M. (2013). Female preferences for acoustic and olfactory signals during courtship: male crickets send multiple messages. Behav. Ecol. 24, 1099–1107. doi: 10.1093/beheco/art036
Snell, L., and Killian, K. (2000). The role of cercal sensory feedback during spermatophore transfer in the cricket, Acheta domesticus. J. Insect Physiol. 46, 1017–1032. doi: 10.1016/S0022-1910(99)00213-9
Song, H. (2009). Species-specificity of male genitalia is characterized by shape, size, and complexity. Insect Syst. Evol. 40, 159–170. doi: 10.1163/187631209X424571
Steiger, S., Ower, G. D., Stökl, J., Mitchell, C., Hunt, J., and Sakaluk, S. K. (2013). Sexual selection on cuticular hydrocarbons of male Sagebrush crickets in the wild. Proc. R. Soc. B. 280:20132353. doi: 10.1098/rspb.2013.2353
Steiger, S., Peschke, K., Francke, W., and Müller, J. K. (2007). The smell of parents: breeding status influences cuticular hydrocarbon pattern in the burying beetle Nicrophorus vespilloides. Proc. R. Soc. Lond. 274, 2211–2220. doi: 10.1098/rspb.2007.0656
Strobl, C., Boulesteix, A.-L., Kneib, T., Augustin, T., and Zeileis, A. (2008). Conditional variable importance for random forests. BMC Bioinform. 9:307. doi: 10.1186/1471-2105-9-307
Strobl, C., Boulesteix, A. L., Zeileis, A., and Hothorn, T. (2007). Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinform. 8:25. doi: 10.1186/1471-2105-8-25
Strobl, C., Malley, J., and Tutz, G. (2009). An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 14, 323–348. doi: 10.1037/a0016973
Thomas, M. L., and Simmons, L. W. (2009). Sexual selection on cuticular hydrocarbons in the Australian field cricket, Teleogryllus oceanicus. BMC Evol. Biol. 9:162. doi: 10.1186/1471-2148-9-162
Touw, W. G., Bayjanov, J. R., Overmars, L., Backus, L., Boekhorst, J., Wels, M., et al. (2013). Data mining in the Life Sciences with Random Forest: a walk in the park or lost in the jungle? Briefings Bioinform. 14, 315–326. doi: 10.1093/bib/bbs034
Tregenza, T. (2002). Divergence and reproductive isolation in the early stages of speciation. Genetica 116, 291–300. doi: 10.1023/A:1021257114996
Tregenza, T., and Wedell, N. (1997). Definitive evidence for cuticular pheromones in a cricket. Anim. Behav. 54:979. doi: 10.1006/anbe.1997.0500
van Rijssel, J. C., Moser, F. N., Frei, D., and Seehausen, O. (2018). Prevalence of disruptive selection predicts extent of species differentiation in Lake Victoria cichlids. Proc. R. Soc. Lond. 285:20172630. doi: 10.1098/rspb.2017.2630
Veltsos, P., Wicker-Thomas, C., Butlin, R. K., Hoikkala, A., and Ritchie, M. G. (2011). Sexual selection on song and cuticular hydrocarbons in two distinct populations of Drosophila montana. Ecol. Evol. 2, 80–94. doi: 10.1002/ece3.75
Via, S. (2001). Sympatric speciation in animals: the ugly duckling grows up. Trends Ecol. Evol. 16, 381–390. doi: 10.1016/S0169-5347(01)02188-7
Webster, M., and Sheets, H. (2010). A Practical Introduction to Landmark-Based Geometric Morphometrics. Paleontol. Soc. Pap. 16, 163–188. doi: 10.1017/S1089332600001868
Keywords: sympatric speciation, divergence, sexual selection, reproductive isolation, geometric morphometrics (GM), cuticular lipids, random forest analysis, bush cricket
Citation: Dutta R, Balakrishnan R and Tregenza T (2018) Divergence in Potential Contact Pheromones and Genital Morphology Among Sympatric Song Types of the Bush Cricket Mecopoda elongata. Front. Ecol. Evol. 6:158. doi: 10.3389/fevo.2018.00158
Received: 26 July 2018; Accepted: 18 September 2018;
Published: 09 October 2018.
Edited by:
Ann Valerie Hedrick, University of California, Davis, United StatesReviewed by:
Raine Kortet, University of Eastern Finland, FinlandFrancisco Garcia-Gonzalez, Estación Biológica de Doñana (EBD), Spain
Copyright © 2018 Dutta, Balakrishnan and Tregenza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rochishnu Dutta, cm9jaGlzaG51ZHV0dGFAZ21haWwuY29t
†Present Address: Rochishnu Dutta, Department of Biological Sciences, Indian Institute of Science Education and Research, Mohali, India
 Rochishnu Dutta
Rochishnu Dutta Rohini Balakrishnan
Rohini Balakrishnan Tom Tregenza
Tom Tregenza