- 1Department of Biological Sciences, Auburn University, Auburn, AL, United States
- 2School of Biological Sciences, Monash University, Clayton, VIC, Australia
Understanding the evolution of mating display traits and preferences for them is a major aim of behavioral and evolutionary ecology. However, isolating the specific traits used as mate choice criteria and the possible genetic underpinnings of both trait and preference has proven difficult, particularly in natural systems offering little experimental control over key variables. In this study, we used discrete color morphs of otherwise phenotypically identical domestic canaries (Serinus canaria) in a mate choice apparatus to test whether breeding-condition female canaries show preference for males of varying color phenotypes (yellow, white, red, or wild-type green), using spatial association as a proxy for choice. We also used synthesized vocal recordings to examine whether females in our population exhibited mate choice for song characteristics, as has been demonstrated in this species. Contrary to previous study, we found that neither white nor yellow females in our colony showed any preference for males associated with songs of differing quality, and yellow females also did not prefer supernormal red or wild-type green males over yellow males. However, yellow—but not white—females demonstrated a preference to associate with yellow males over white males. We hypothesize that preference for brightly colored mates is ancestral in domestic canaries, but that strong artificial selection for white females to reproduce successfully with white males has eliminated the preference for color (along with color itself) in the white canaries.
Introduction
The coupling of mating preferences with ornamental displays is a key element of leading hypotheses for the evolution of ornamental traits (Lande, 1981; Kirkpatrick, 1982; Andersson and Simmons, 2006; Prum, 2010). Some versions of these models assume a genetic basis for both variation in ornament expression and for variation in mating preferences such that associations between preferences and display traits can arise from linkage of the genes that underlie the traits (Butlin and Ritchie, 1989; Boake, 1991; Kuijper et al., 2012; Wiley et al., 2012). However, much remains to be learned about the genetic basis of either preferences or ornaments.
Domestic canaries (Serinus canaria) provide an important system for exploring mate choice behavior (reviewed in Leboucher et al., 2012). Female canaries have been found consistently to prefer a particular aspect of song (the “A phrase”; Vallet et al., 1998; Leitner et al., 2001), and it is notable that this preference exists in both wild and domestic populations. Beyond song, recent research into the genes that underlie carotenoid-based colored signals in canaries (Lopes et al., 2016; Toomey et al., 2017) presents new opportunities to explore the interplay of male display traits and female preferences. Having lineages with fixed differences in expression of plumage coloration provides unprecedented opportunities for testing for correlated changes in coloration and color preferences.
Like song, carotenoid-based coloration appears to be frequently used as a criterion in mate choice in songbirds, with females tending to prefer males with more chromatic coloration and with hues shifted to longer wavelengths (Hill, 2006). However, the rich yellow coloration of the domestic canary has never before been studied with respect to mate choice; a single study tested female preference for males against backgrounds that matched or mismatched their plumage coloration, but did not examine preference for plumage coloration itself (Heindl and Winkler, 2003). Moreover, the value of domesticated populations of birds in studies of behavior has been well established in studies of mate choice for color and song quality in zebra finches (Taeniopygia guttata; Burley and Coopersmith, 1987; Collins et al., 1994; Riebel, 2009; Simons and Verhulst, 2011). The canary has the potential to be an invaluable study system for understanding the physiological and behavioral mechanisms underlying carotenoid-based plumage ornamentation and preferences for ornaments.
In this study, we tested the mating preference of female canaries for song quality and coloration of males. We used the discrete color variants present in the domestic canary to test female preference for carotenoid-based coloration, and we used broadcast of artificially constructed songs to test preferences relative to male song quality. Specifically, our comparisons allowed us to (1) establish whether these lines of canaries demonstrate the same strong preferences for song that have been described in other lines; (2) test if change in plumage color is tightly linked to change in preference such that females prefer males of their own type; and, (3) test if females show preference for a supernormal male trait (red coloration), as they appear to in song (Garcia-Fernandez et al., 2013), or if they may instead have retained preference for an ancestral plumage phenotype. These first tests of female canary mate choice for male plumage coloration are a step toward better understanding how strong selection on a male trait may shape the associated preference.
Methods
We performed mate choice trials on a long-term research colony of after-hatch-year canaries held at the Auburn University Avian Research Laboratory 1 in Auburn, AL. The protocols used in this study were approved by the Auburn University Institutional Animal Care and Use Committee (PRN 2014-2465, PRN 2015-2627).
The birds used in our trials comprised three different types of the color-bred breed of canary and one song-bred breed. The former types included yellow lipochrome (“yellow”), white recessive lipochrome (“white”), and red factor lipochrome (“red”). Lipochrome canaries lack melanin pigmentation in their plumage, so these birds expressed entirely yellow, white, or red plumage over their entire body (Figure 1). Yellow canaries feature extensive bright yellow carotenoid pigment deposition in their feathers, while white recessive canaries have a knock-down mutation that severely depletes carotenoid pigment absorption from the diet, leaving feathers white (Toomey et al., 2017). Red canaries are uniformly deep orange to red in coloration due to the selective introgression of “redness genes” from hybridization in the early 1900s (Lopes et al., 2016). The last type of canary we used is the Spanish Timbrado, a song-bred breed with wild-type coloration (“green”; Figure 1). Records of all four canary types extend back at least 100 years (up to 100 generations)—in fact, a solid yellow canary is depicted as much as 500 years ago (Koch and Hill, 2015), though it is likely that breeders have created multiple lineages of yellow lipochromes in the centuries since; regardless, we anticipate that the color-bred lines have existed as unique strains under selection for coloration for many generations.
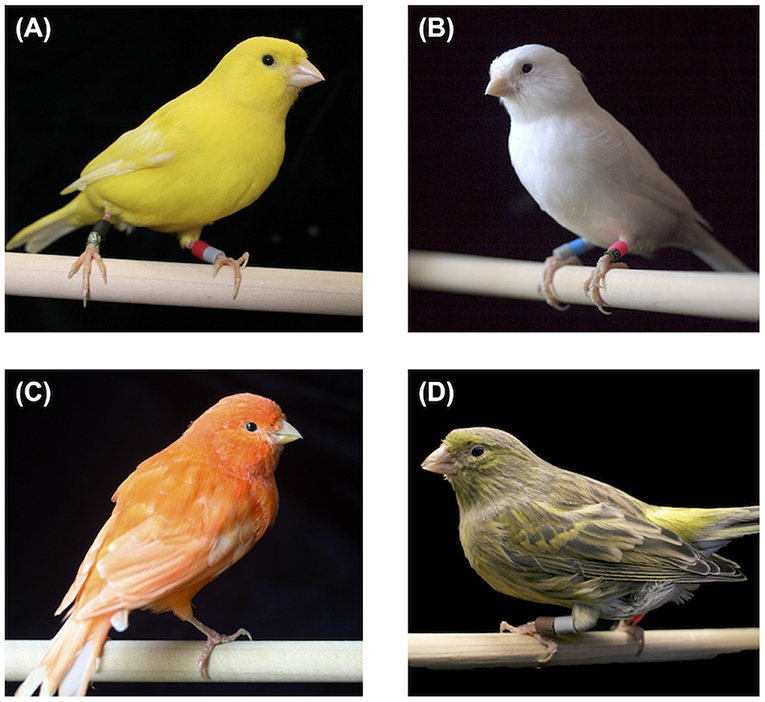
Figure 1. The four color types of canaries used in these experiments: yellow (A), white recessive (B), and red factor (C) color-bred lipochromes, and green song-bred Spanish Timbrado (D).
We performed mate choice experiments over two consecutive breeding seasons (2015 and 2016). Our mate choice apparatus was identical in both years: a female was released into a large central cage surrounded by four smaller cages, two of which contained experimental males (Figure S1). To control the song stimuli received by females, we placed speakers (Logitech Z130) behind each male's cage and played clips of recorded canary song (described below) in the direction of the female such that song emanated from each visible male.
Each trial was recorded from overhead by a high definition camera (Logitech C615). At the start of each trial, a female was released into the center of the central cage and allowed to move freely within the cage for 25 min. We tracked female movement from the video recordings, using JWatcher (v. 1.0; Blumstein et al., 2000) to create a quantified etholog of behavior. A female's choice for each experimental male in a trial was measured by the proportion of the trial that she spent in the corner of her cage nearest that male.
In 2015, we tested only yellow and white canaries. We began with a basic test of whether female canaries preferred to associate with males producing long, complex songs with rapid, broadband trills, as has been previously demonstrated in canaries (Leboucher et al., 2012). We tested yellow females with yellow males and white females with white males, each male with either a high or low quality song derived from recordings from our colony (see Supplementary Material). We then tested female preference for males of similar song quality but different plumage coloration by presenting white or yellow females each with one white and one yellow male, both with associated “high quality” song.
In 2016, we chose to further pursue questions of color preference by testing female choice for additional male color phenotypes and using more carefully controlled song stimuli. We acquired standardized clips of canary song recordings (from Tudor Drăganŏiu, Université Paris Nanterre) that each contained slightly different (but functionally identical) variants of the “A phrase” that has induced copulation solicitation behavior in previous experimentation on female canaries (Drăgănoiu et al., 2002). We first again tested white or yellow females presented with white and yellow males, and then we additionally tested yellow females presented with red (a supernormal signal) or yellow males and yellow females presented with green (a wild-type signal) or yellow males.
Because the proportional preference data within a single trial type often failed Shapiro-Wilk normality tests, we used Wilcoxon signed rank tests to test for significant differences between the proportion of time females spent near a male of one type compared to the other (i.e., to test for significant preferences within one trial type). To compare strength of preferences among trial types, we then performed mixed model analyses, using the package “lmerTest” to approximate P-values (Kuznetsova et al., 2017). We first compared the preferences of yellow and white females for yellow or white males on a pooled data set comprising results from both 2015 and 2016, which did meet the assumption of normality (Shapiro-Wilk test: W = 0.92, p < 0.001). In this analysis, we tested for the effects of female color, male color, and their interaction (as fixed effects) on proportion of time spent near a male, with year and female identity as random effects. If the association preference of a female for male color depends on the female's own color (e.g., if only yellow females prefer yellow males), then we would expect see a significant interaction term. Secondly, we performed a similar analysis of a pooled dataset for all three color comparisons with yellow females in 2016 (yellow male vs. green, vs. red, or vs. white; Shapiro-Wilk test: W = 0.87, p < 0.001); here, we tested for the effects of male color, trial type (i.e., yellow male vs. green), and their interaction (as fixed effects) on proportion of time spent near a male, including female identity as a random effect. This analysis tests more directly for whether yellow females significantly prefer particular male color phenotypes, and whether the strength of that preference depends on the color of the other male presented.
All analyses were performed in R [version 3.4.1; (R Core Team, 2018)].
Results
We found that female canaries did not show a significant association preference for songs of either quality (average percent of total time spent near male ± SE; high quality song: 20.1% ± 3.5%; low quality song: 32.8% ± 4.9%; n = 13, V = 22, p = 0.11; Figure S2).
In 2015, we found no significant difference in association preference for white or yellow males by either white females or yellow females (p > 0.2; Figure 2, Table S1). However, we noted that the behavior of a single yellow female, who spent 75% of her trial near the white male, was highly influential to the results; when that female was excluded from the analysis, we found that yellow females did show a significant association preference for yellow compared to white males (p < 0.05; Table S1). In 2016, we observed that yellow females showed a statistically significant association preference for yellow males compared to white males (p = 0.04), but white females showed no such preference (p = 0.77; Table S1, Figure 2).
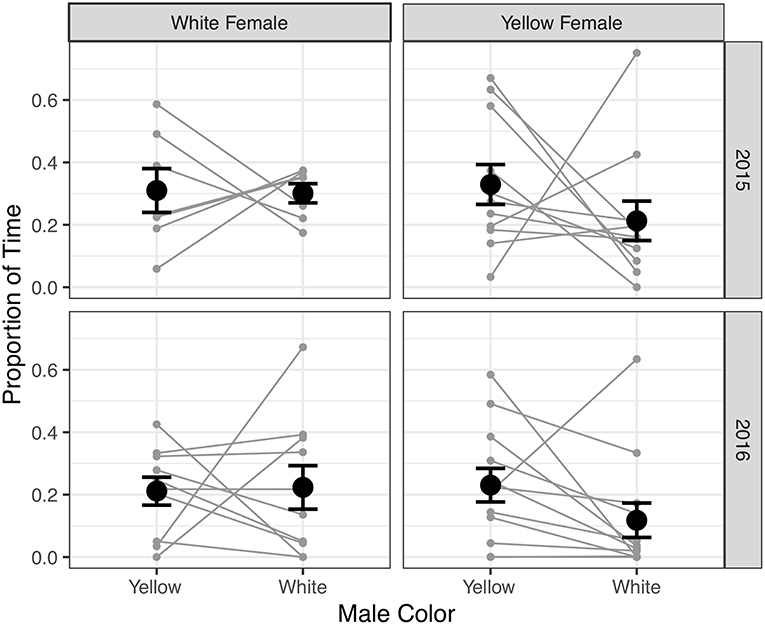
Figure 2. The mean ± SE proportion of time white (left column) and yellow (right column) females spent near yellow or white males in the trials during 2015 (top row) and 2016 (bottom row). Each pair of gray points connected by a line indicates the values for a particular female such that lines with a negative slope indicate females who spent a greater proportion of trial time near yellow males.
We then gave yellow female canaries a choice between yellow males and males with red plumage (red factor canaries) or greenish wild-type plumage coloration (Spanish Timbrado canaries). Yellow females showed no significant association preference for either red vs. yellow males or for green males vs. yellow males (both p > 0.6; Table S1, Figures S3, S4).
However, when we tested more directly for a differences in preference between white and yellow females, we found no significant effect of female color, male color, or their interaction on the proportion of time spent near a male (all p > 0.1; Table S2). Similarly, we found no significant effects of male color, trial type, or their interaction on the proportion of time yellow females spent near red, green, or white males in 2016 (all p > 0.08; Table S3). These results indicate that the significant versus non-significant preferences we detected within certain trial types (i.e., yellow females preferring yellow males) did not translate into significant differences in preferences among trial types (i.e., yellow females preferring yellow males more than did white females), though the effect estimates conformed to predictions derived from the nonparametric tests.
Discussion
It has been well documented that wild cardueline finches (family Fringillidae) show a mating preference for carotenoid coloration that is more chromatic and, in many species, more red-shifted (Hill, 1990, 1991, 1994; Johnson et al., 1993; Toomey and McGraw, 2012). In canaries, researchers have convincingly documented female choice for specific renditions of male song (Vallet et al., 1998; Drăgănoiu et al., 2002; Leboucher et al., 2012; Amy et al., 2015). To our knowledge, however, this study is the first to test whether female canaries use color as a criterion in mate choice.
In contrast to previous studies, we found that females showed no preference for males associated with higher quality song in 2015 trials, and displayed no copulatory behavior in response to the “stimulus” songs broadcast in 2016 trials. We conclude that the strong sexual preference for elaborate song that was observed in other canary studies was not present here.
One explanation for this discrepancy is that our tests were performed exclusively with females from color-bred lines of canaries. The color and song canaries lines have been bred as independent populations for hundreds of years; color canaries have been selected strongly for specific aspects of feather coloration with no overt selection on song. It is possible that preference for song exists in song-bred canaries and mate choice for color (but not song) exists in color-bred canaries. To our knowledge, our study is the first to isolate the preferences of color-bred canaries.
It is also important to note that our observations indicate heterogeneity in preference such that some females in every trial type demonstrated strong preference for either male phenotype, and several females showed little interest in associating with males at all. Repeated measures of the same females' preferences is necessary to reveal whether this heterogeneity reflects differences among individuals, or flexibility in individual response due to variation in factors we did not explicitly control for, such as precise stage of reproductive receptivity or subtle variation in environment. This variation may have obscured the existence of preferences within our tested birds, and as such, the detection of any significant preference at all becomes more notable.
In contrast to the rich literature describing canary song, no study has tested whether female canaries assess the feather coloration of prospective mates. We found that, at least in our more carefully controlled trials in 2016, yellow females preferred to associate with yellow rather than white males, but white females showed no preference for either white or yellow males. Because the preference for yellow plumage over white plumage by yellow females did not extend to a preference for yellow feathers over other carotenoid-based plumage coloration (red or green), our results suggest that yellow female canaries demonstrate a preference for plumage coloration itself rather than yellow per se.
We propose that this choice of colored plumage in yellow female canaries likely results from the retention of a choice for colored feathers from the wild ancestors of domesticated canaries. If the patterns were instead from imprinting, we would have expected to detect positive assortative preferences for coloration (e.g., white females should have preferred white males).
If female preference for colored males is the ancestral state for canaries, then it is plausible that the strong artificial selection that eliminated plumage coloration in white recessive canaries also eliminated the preference for that colored plumage. The question then becomes whether (1) the genetic basis of the trait and preference for it are genetically linked such that modifying the trait modifies the preference, or (2) artificial selection modified first the male trait and then female preference for it, such as through selecting females that bred quickly and rapidly with males of the new white phenotype. These possibilities demand further study, ideally capturing how preferences change in the generations immediately after the evolution of a trait like white plumage and before further selection. In either case, connecting a knock-down color mutation to the elimination of a mating preference provides tantalizing evidence for the genetic basis of female preference as well as male trait.
In this study, we present evidence that yellow female canaries prefer colored males, but that white female canaries show no such preference. Our evidence for a genetic basis for preference for the plumage coloration of prospective males is indirect, but the prospect of a gene for a mating preference for colored plumage in canaries is intriguing.
Ethics Statement
This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals (NRC 2011) and the Guide for the Care and Use of Agricultural Animals in Research and Training (FASS 2010). The protocol was approved by the Auburn University Institutional Animal Care and Use Committee (PRN 2014-2465, PRN 2015-2627).
Author Contributions
RK planned and executed the experiments, analyzed data, and wrote and revised manuscript draft. GH assisted in experimental design and revised the manuscript.
Funding
This project was supported by a National Science Foundation Doctoral Dissertation Improvement Grant (number 1501560) to RK and GH. RK was supported during analysis and writing by the Australian Research Council (DP170100165 to D.K. Dowling and DE190100831 to RK).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank T. Drăgănoiu (Université Paris Nanterre) for providing the stimulus canary songs for our 2016 experiments, R.F. Koch for assistance in video behavior recording, and Auburn University undergraduates for assisting with bird husbandry and experimental trials.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00106/full#supplementary-material
References
Amy, M., Salvin, P., Naguib, M., and Leboucher, G. (2015). Female signalling to male song in the domestic canary, Serinus canaria. R. Soc. Open Sci. 2:140196. doi: 10.1098/rsos.140196
Andersson, M., and Simmons, L. W. (2006). Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. doi: 10.1016/j.tree.2006.03.015
Blumstein, D., Daniel, J., and Evans, C. (2000). JWatcher. Available online at: www.jwatcher.ucla.edu (accessed March 18, 2018).
Boake, C. R. (1991). Coevolution of senders and receivers of sexual signals: Genetic coupling and genetic correlations. Trends Ecol. Evol. 6, 225–227. doi: 10.1016/0169-5347(91)90027-U
Burley, N., and Coopersmith, C. B. (1987). Bill color preferences of zebra finches. Ethology 76, 133–151. doi: 10.1111/j.1439-0310.1987.tb00679.x
Butlin, R. K., and Ritchie, M. G. (1989). Genetic coupling in mate recognition systems: what is the evidence? Biol. J. Linn. Soc. 37, 237–246. doi: 10.1111/j.1095-8312.1989.tb01902.x
Collins, S. A., Hubbard, C., and Houtman, A. M. (1994). Female mate choice in the zebra finch—the effect of male beak colour and male song. Behav. Ecol. Sociobiol. 35, 21–25. doi: 10.1007/BF00167055
Drăgănoiu, T. I., Nagle, L., and Kreutzer, M. (2002). Directional female preference for an exaggerated male trait in canary (Serinus canaria) song. Proc. R. Soc. B Biol. Sci. 269, 2525–2531. doi: 10.1098/rspb.2002.2192
Garcia-Fernandez, V., Draganoiu, T. I., Ung, D., Lacroix, A., Malacarne, G., and Leboucher, G. (2013). Female canaries invest more in response to an exaggerated male trait. Anim. Behav. 85, 679–684. doi: 10.1016/j.anbehav.2013.01.007
Heindl, M., and Winkler, H. (2003). Female canaries (Serinus canaria) associate more with males that contrast strongly against the background. Ethology 109, 259–271. doi: 10.1046/j.1439-0310.2003.00869.x
Hill, G. E. (1990). Female house finches prefer colorful males - sexual selection for a condition-dependent trait. Anim. Behav. 40, 563–572. doi: 10.1016/S0003-3472(05)80537-8
Hill, G. E. (1991). Plumage coloration is a sexually selected indicator of male quality. Nature 350, 337–339.
Hill, G. E. (1994). Geographic variation in male ornamentation and female mate preference in the house finch: a comparative test of models of sexual selection. Behav. Ecol. 5, 64–73. doi: 10.1093/beheco/5.1.64
Hill, G. E. (2006). “Female mate choice for ornamental coloration,” in Bird Coloration: Function and Evolution, eds G. E. Hill and K. J. McGraw (Cambridge, MA: Harvard University Press), 137–200.
Johnson, K., Rosetta, D., and Burley, D. N. (1993). Preferences of female American goldfinches (Carduelis tristis) for natural and artificial male traits. Behav. Ecol. 4, 138–143. doi: 10.1093/beheco/4.2.138
Kirkpatrick, M. (1982). Sexual selection and the evolution of female choice. Evolution 36, 1–12. doi: 10.1111/j.1558-5646.1982.tb05003.x
Koch, R. E., and Hill, G. E. (2015). Rapid evolution of bright monochromatism in the domestic Atlantic Canary (Serinus canaria). Wilson J. Ornithol. 127, 615–621. doi: 10.1676/14-172.1
Kuijper, B., Pen, I., and Weissing, F. J. (2012). A guide to sexual selection theory. Annu. Rev. Ecol. Evol. Syst. 43, 287–311. doi: 10.1146/annurev-ecolsys-110411-160245
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). {lmerTest} Package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Lande, R. (1981). Models of speciation by sexual selection on polygenic traits. Proc. Natl. Acad. Sci. U.S.A. 78, 3721–3725. doi: 10.1073/pnas.78.6.3721
Leboucher, G., Vallet, E., Nagle, L., Béguin, N., Bovet, D., Hallé, F., et al. (2012). “Chapter 5 - studying female reproductive activities in relation to male song: the domestic canary as a model,” in Advances in the Study of Behavior, Vol. 44, eds H. Jane Brockmann, T. J. Roper, M. Naguib, J. C. Mitani, and L. W. Simmons (San Diego, CA: Academic Press), 183–223. doi: 10.1016/B978-0-12-394288-3.00005-8
Leitner, S., Voigt, C., and Gahr, M. (2001). Seasonal changes in the song pattern of the non-domesticated island canary (Serinus canaria), a field study. Behaviour 138, 885–904. doi: 10.1163/156853901753172700
Lopes, R. J., Johnson, J. D., Toomey, M. B., Ferreira, M. S., Araujo, P. M., Melo-Ferreira, J., et al. (2016). Genetic basis for red coloration in birds. Curr. Biol. 26, 1427–1434. doi: 10.1016/j.cub.2016.03.076
Prum, R. O. (2010). The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for meaning, honesty, and design in intersexual signals. Evolution 64, 3085–3100. doi: 10.1111/j.1558-5646.2010.01054.x,
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available online at: http://www.R-project.org.
Riebel, K. (2009). Chapter 6 song and female mate choice in zebra finches: a review. Adv. Study Behav, 40, 197–238. doi: 10.1016/S0065-3454(09)40006-8.
Simons, M. J. P., and Verhulst, S. (2011). Zebra finch females prefer males with redder bills independent of song rate—a meta-analysis. Behav. Ecol. 22, 755–762. doi: 10.1093/beheco/arr043
Toomey, M. B., Lopes, R. J., Araújo, P. M., Johnson, J. D., Gazda, M. A., Afonso, S., et al. (2017). High-density lipoprotein receptor SCARB1 is required for carotenoid coloration in birds. Proc. Natl. Acad. Sci. U.S.A. 114, 5219–5224. doi: 10.1073/pnas.1700751114
Toomey, M. B., and McGraw, K. J. (2012). Mate choice for a male carotenoid-based ornament is linked to female dietary carotenoid intake and accumulation. BMC Evol. Biol. 12:3. doi: 10.1186/1471-2148-12-3
Vallet, E., Beme, I., and Kreutzer, M. (1998). Two-note syllables in canary songs elicit high levels of sexual display. Anim. Behav. 55, 291–297. doi: 10.1006/anbe.1997.0631
Keywords: carotenoid-based coloration, mate choice, artificial selection, ornamentation, Serinus canaria, sexual selection
Citation: Koch RE and Hill GE (2019) Loss of Carotenoid Plumage Coloration Is Associated With Loss of Choice for Coloration in Domestic Canaries. Front. Ecol. Evol. 7:106. doi: 10.3389/fevo.2019.00106
Received: 23 January 2019; Accepted: 18 March 2019;
Published: 05 April 2019.
Edited by:
Ann Valerie Hedrick, University of California, Davis, United StatesReviewed by:
Conor Taff, Cornell University, United StatesUdo M. Savalli, Arizona State University, United States
Copyright © 2019 Koch and Hill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca E. Koch, cmViZWNjYS5hZHJpYW5AbW9uYXNoLmVkdQ==
 Rebecca E. Koch
Rebecca E. Koch Geoffrey E. Hill
Geoffrey E. Hill