- 1Idaho Department of Fish and Game, Salmon, ID, United States
- 2Washington Department of Fish and Wildlife, Olympia, WA, United States
- 3Idaho Department of Fish and Game, Moscow, ID, United States
Studies of monarch butterflies (Danaus plexippus) and their milkweed (Asclepias spp.) host plants in North America have focused primarily on monarch populations ranging east of the Rocky Mountains. We report the first systematic assessment of monarch butterfly and milkweed populations in the western states of Idaho and Washington, states at the northern tier of western monarch breeding range. Results of our 2-year study (2016–2017) offer new insights into monarch breeding habitat distribution, characteristics, and threat factors in our 2 states. We documented milkweeds and breeding monarchs in all 16 climate divisions in our study area. Milkweed and breeding monarch phenologies were examined with evidence supporting 2, and possibly 3 monarch generations produced in Idaho and Washington. Key monarch breeding habitats were moist-soil sites within matrices of grasslands, wetlands, deciduous forest, and shrub-steppe supporting large, contiguous, and high-density milkweed stands. Co-occurrence of showy milkweed (A. speciosa) and swamp milkweed (A. incarnata) was an important indicator of productive monarch breeding habitat in Idaho. Nectar plants were generally limited in quantity and richness across the study area, particularly in late summer, and included frequently-used non-native, invasive species. Primary threats at milkweed sites were invasive plant species, herbicide application, and mowing, followed by secondary threats of recreational disturbance, livestock grazing, insecticide application, loss of floodplain function, and wildfire. We provide management recommendations and research needs to address ongoing stressors and knowledge gaps in Idaho and Washington with the goal of conserving monarchs and their habitats in the West.
Introduction
Essential to the conservation of migratory species is understanding the full life-cycle ecology of populations across geographically disparate seasonal ranges (Webster et al., 2012; Small-Lorenz et al., 2013; Flockhart et al., 2015). The North American monarch butterfly (Danaus plexippus plexippus) is an iconic migratory insect that exemplifies the challenges of conserving highly mobile species. Two migratory populations of monarchs occur in North America (Urquhart and Urquhart, 1977). The larger eastern population breeds east of the Rocky Mountains and overwinters in high-elevation forests in Central Mexico (Flockhart et al., 2013), while the western population breeds west of the Rockies and overwinters at low-elevation wooded groves along the California coast (Dingle et al., 2005; Yang et al., 2015). Recent research indicates the boundary between populations is permeable with significant admixing occurring at breeding and overwintering sites (Vandenbosch, 2007; Pyle, 2015). The past decade has seen major advances in knowledge of monarchs, including studies focused on broad-scale population trends and factors driving recent and rapid population declines (Flockhart et al., 2015; Oberhauser et al., 2015; Schultz et al., 2017). Research focus has primarily centered on the eastern monarch population. While investigation of the western population has recently increased, knowledge of basic aspects of western monarch breeding biology, migratory connectivity, and threat factors remains rudimentary. As recently as 2015, significant gaps in knowledge of distribution of monarchs and their milkweed (Asclepias spp.) host plants existed for vast areas of the western U.S. (Jepsen et al., 2015). Addressing these key knowledge gaps is a crucial first step for conserving western monarch natal habitats and migratory connectivity (Martin et al., 2007; Webster et al., 2012).
Knowledge of milkweed and monarch breeding occurrence in the Pacific Northwest in general, and Idaho and Washington specifically, is derived from a limited body of research. Pyle (1999, 2015) described severely restricted monarch breeding incidence in the region due to patchy and low-density milkweed distributions, but noted historical occurrence of dense stands of showy milkweed (A. speciosa) in the Snake River Plain in southern Idaho and Columbia Basin in eastern Washington. Stevens and Frey (2010) identified 2 climate divisions corresponding to the lower Snake River Plain and Columbia Basin as sole regions in Idaho and Washington with potential to support western migrant monarchs. They posited in such northern-latitude states, monarch development was likely constrained by cold temperatures, and to a lesser degree, by low milkweed species diversity and bottom-up effects from drought. In 2011, the Xerces Society for Invertebrate Conservation initiated a project to compile milkweed and monarch breeding records in the western U.S. As of 2015, this database amassed >12,000 milkweed records from multiple sources. Of the 700 milkweed records collated for Idaho and Washington, 88% were collected pre-2000 or had low spatial accuracy or ambiguous species identification. A mere 7 high-quality monarch records existed across both states, with most records lacking life-stage information or spatial accuracy.
In 2016, prompted by concerns about western monarch declines and major knowledge gaps in the distribution and status of monarch breeding habitats in their states, the Idaho Department of Fish and Game (IDFG) and Washington Department of Fish and Wildlife (WDFW) initiated a 2-year study with objectives to (1) determine statewide distributions of monarchs and milkweeds, (2) describe characteristics of monarch breeding habitats, (3) identify primary threats to monarchs and their natal habitats, and (4) utilize these data to guide beneficial management and future research of the western monarch. Our study also presented an opportunity to gather information on aspects of monarch breeding ecology poorly understood for the inland Pacific Northwest, such as breeding phenology, important nectar plants, and whether roosting structure is an essential component of summer natal habitats. Here, we report the findings of our bi-state study offering new insights into the regional distribution and ecology of breeding monarchs and milkweed host plants in Idaho and Washington. In this regional context, we suggest management actions and research needs to mitigate further decline of the western monarch butterfly population.
Methods
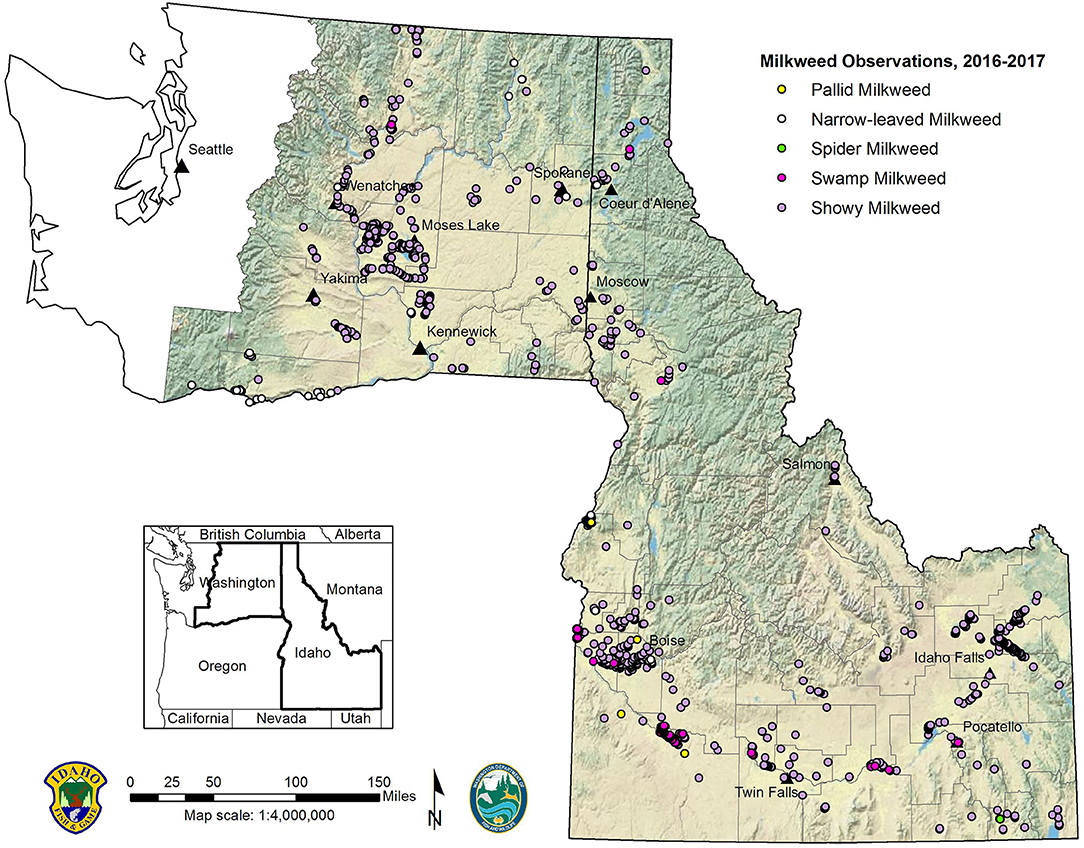
Our study encompassed the state of Idaho and that portion of Washington east of the Cascade Mountain Range coincident with native milkweed distribution (Xerces Society [Xerces], 2012; Hitchcock and Cronquist, 2018) (Figure 1). The 2 states share complex physiography dominated by several major mountain ranges, basaltic tablelands, basin and range deserts, and large river systems including the Snake, Salmon, and Columbia rivers. The study area spans 7 ecoregions (Bailey, 1976), with the Middle Rockies-Blue Mountains, Canadian Rocky Mountains, and Columbia-Snake Plateau ecoregions being contiguous between the states, and the latter ecoregion comprising the majority of the study area. Climates are highly variable, possessing both continental and marine characteristics, and temperature regimes are strongly mediated by latitude and altitude. The study area is positioned entirely west of the Continental Divide and in the northern latitudes (42–49°N) of the western monarch's breeding range. Idaho and Washington are often grouped with other adjacent states and provinces into western monarch subregions variably named “northern inland range” (Yang et al., 2015), “Cascadia” (Pyle, 2015), and “Pacific Northwest” (James, 2016). These terms not only confer geographic location, they infer certain ecological constraints for breeding monarchs comprising the “outermost immigrants and breeders of the entire western phenomenon” (Pyle, 2015). Milkweed species richness in the study area is comparatively low (≤6 species) among western states and milkweed distributions are characterized as patchy and sparse (Pyle, 1999, 2015), though locally dense stands of showy milkweed have been documented in both states (Pyle, 2015; James, 2016).
Implicit in our study was our aim to contribute current high-resolution milkweed and monarch occurrence records to a new modeling effort to map and characterize habitat for the western monarch butterfly (USFWS and Xerces, 2016; Dilts et al., 2018). Accordingly, IDFG and WDFW developed milkweed and monarch survey protocols and field forms using standardized definitions and categories portable to this modeling effort and its future iterations (see Supplementary Data Sheets S1–S3). We defined a milkweed patch as a discrete grouping of milkweed plants separated from other milkweed patches by ≥50 m, or by dense, tall shrubs or trees, lakes or rivers, buildings, roads, or other anthropogenic land demarcations. Data collected for each milkweed patch included milkweed species, patch structure, plant count, patch area, predominant phenophase, habitat type(s), habitat association(s), management activities, threat(s), and a GPS (Global Positioning System)-derived location. Management activities were known land management or other action(s) occurring on the site that may positively or negatively affect milkweed plants. Threats were defined as proximate stressors causing destruction, degradation, and/or impairment of milkweeds. Data collected for monarch observations included weather, time, life stage(s) observed and count, sex and behavior of adult(s), nectar species used, habitat type(s), habitat association(s), management activities, threat(s), and GPS location.
Idaho Surveys
We incorporated 3 approaches in our survey methods to maximize data collection opportunities. In 2016, we elected to use a spatially-balanced, stratified survey design to allow extrapolation of data across the landscape (Stohlgren et al., 1997). We selected “predicted milkweed habitat suitability” as our sampling strata to further target survey effort and efficiency. Our strata were derived from the Western Milkweed and Monarch Breeding Habitat Suitability Model (Phase I) for showy milkweed developed by the U.S. Fish and Wildlife Service (USFWS) and Xerces Society (USFWS and Xerces, 2016). Within modeled habitat, we created 4 strata to identify high (0.71–0.99), medium (0.21–0.70), low (0.06–0.20), and null (<0.06) probability of showy milkweed occurrence. We then created a grid sample frame of 270 × 270 m cells consistent with model resolution and considered a feasible size for survey effort. We applied a Generalized Random Tessellation Stratified (GRTS) sampling design to draw an ordered master sample and oversample of survey cells within the 3 suitable strata. We drew a sample of 250 primary sites (100 high, 100 medium, 50 low), the number of cells we estimated we could survey in one field season, with an overdraw of 120 sites to account for inaccessible cells (Figure 2A).
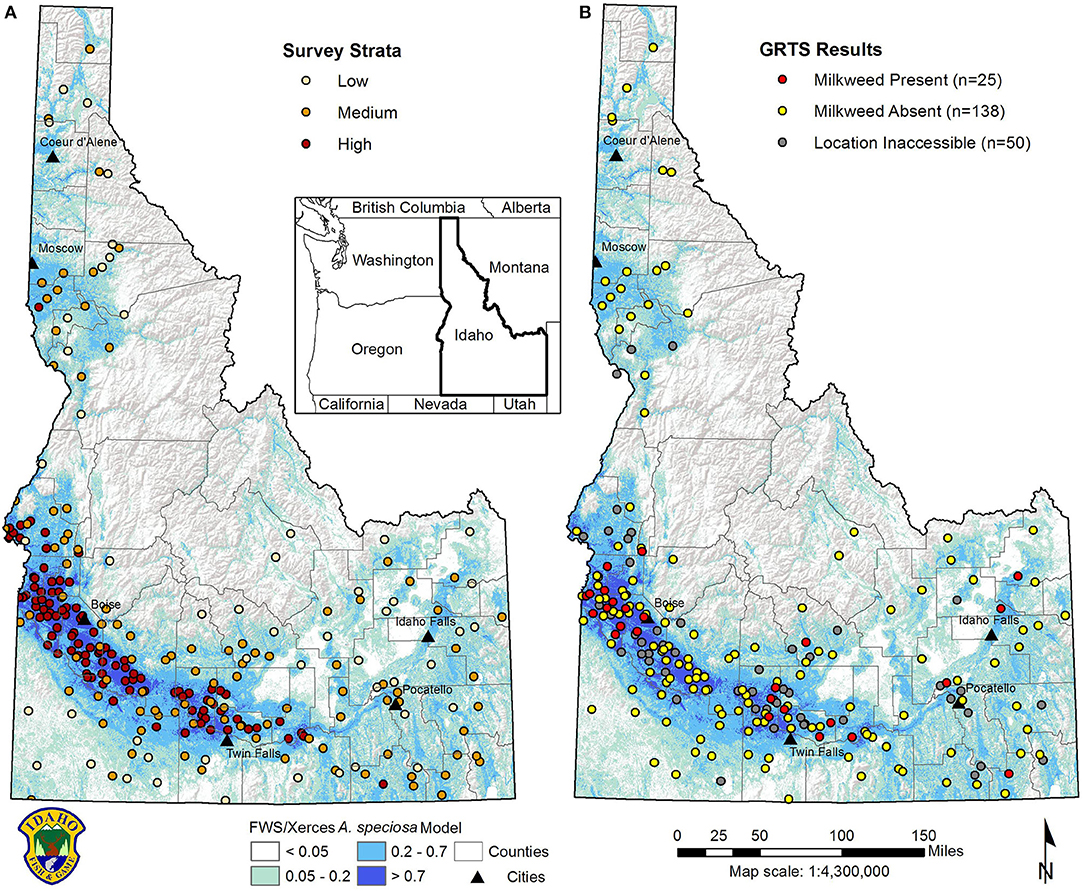
Figure 2. (A) GRTS sampling framework for milkweed and monarch surveys in Idaho stratified by high, medium, and low relative habitat suitability for showy milkweed (Asclepias speciosa) based on preliminary model developed by USFWS and Xerces (2016); (B) results of GRTS cell surveys in Idaho, 2016.
Standard field survey methods were developed for GRTS cells. Cell centroids and polygons were uploaded to GPS units to georeference and navigate in the field. At each GRTS cell, a team of 2 field technicians walking in tandem ≤15 m apart systematically searched for milkweeds and monarch butterflies (eggs, larvae, pupae, adults) along parallel and adjacent linear transects covering the entire cell. Milkweed plants encountered at 10-pace intervals were thoroughly searched for presence of eggs, larvae, and pupae. At each cell corner, technicians scanned adjoining cells with binoculars for 30 s to detect presence of milkweeds and monarchs.
In 2017, we conducted milkweed and monarch breeding habitat inventories at several IDFG Wildlife Management Areas (WMA) spanning the Snake River Plain region of southern Idaho. We used photo imagery and milkweed habitat suitability (USFWS and Xerces, 2016) digital layers to identify and delineate survey units at each WMA. Field technicians systematically searched for milkweeds and monarchs along parallel and adjacent transects covering the targeted unit using the same 10-pace protocol described above to search for monarch eggs, larvae, and pupae. At C.J. Strike WMA, 2 observers surveyed the reservoir perimeter by motor boat. Milkweed and monarch data were directly recorded in Collector for ArcGIS (Android) 17.0.2 (ESRI, 2017) supported by smartphone and tablet mobile devices. This application allowed collection of high-accuracy point or polygon data, other site information, and photo attachments. Our data was synchronized to the College of Western Idaho (CWI) ArcGIS geodatabase server.
Incidental observations can capture important data on where and when plants and animals occur, often at high spatial and temporal resolutions [e.g., eBird (Wood et al., 2012; Kelling et al., 2015), iNaturalist (iNaturalist.com., 2019)]. We recorded milkweed and monarch observations when in transit to target survey sites or in follow-up to reported sightings. Site data were recorded on a field form specifically developed for incidental observations as well as on Collector. Citizen scientists were invited to contribute observational data on field forms, via the Western Monarch Milkweed Mapper website, or Collector.
As opportunities presented, we netted wild adult monarchs to deploy tags from Washington State University's (WSU) Pacific Northwest monarch tagging program, and sampled adults for Ophryocystis elektroscirrha (OE), an obligate protozoan parasite of monarchs, in support of a CWI project. These field activities were the extent to which we handled monarchs in our study. Though butterfly taxa are not regulated under animal ethics and welfare guidelines, we practiced voluntary protocols parsimonious with ethical treatment of monarchs in the field.
Washington Surveys
We utilized a network of roving field technicians and geographically distinct WDFW District-based wildlife biologists to conduct surveys for milkweed and monarchs in open habitats of eastern Washington; 9 WDFW Districts cover the 21 counties in this region. Citizen scientists supplemented this information in under-surveyed areas, and WDFW Wildlife Area staff assisted with collection of milkweed and monarch data at their respective stations. In addition, we surveyed for milkweeds and monarchs along road-based transects. Potential survey sites included all ownerships except USFWS Refuges, where milkweed and monarch surveys were underway. Efforts emphasized coverage of WDFW-managed Wildlife Areas. Our approach focused on locating and characterizing milkweed and monarch habitat, and capitalized on staff and volunteers' local knowledge of vegetation and environmental conditions.
Biologists selected and visited sites and roadways known or suspected to support milkweed, and systematically searched for milkweed patches. Road-edge transects were conducted using a vehicle, driver, and passenger-observer. Traveling at ≤50 mph, roadsides were surveyed for milkweed patches, and where safe to pull over, observers collected milkweed patch data and surveyed for monarchs. Standardized time-limited surveys for monarch adults and immature stages were conducted in each patch. In areas with abundant milkweed patches (>20 patches), we surveyed a 50% subset of randomly-selected patches. Adult surveys entailed visually searching milkweed patches and immediate-adjacent areas while walking through patches at ≤10 m spacing, 5-min focal observations at flowering milkweed or other flowering plant patches, and observing any adults detected for ≥10 min or until they left the area. Literature review and local expert consultation (D. James, pers. comm.) indicated monarch eggs and larvae occur most often on undersides of leaves, within the upper third of a milkweed plant, and on plants at patch edges or in lower density stands (Zalucki and Suzuki, 1987). Thus, our surveys for immature stages focused on these expected patterns, with ≥3 min allotted to search a sample within 100 milkweed plants. Milkweed and monarch data were recorded on paper field forms and recreation-grade GPS units later entered into Access (2007) and ArcGIS. A subset of milkweed patches were photo documented using georeferencing software.
Results
Milkweed and Monarch Distributions
From 26 May to 3 August 2016, we surveyed 163 GRTS survey cells in Idaho in predicted suitable habitats for showy milkweed and monarchs (65 high, 68 medium, and 30 low probability A. speciosa strata) (Figure 2B). Surveys were attempted in an additional 50 cells, but access was impeded by private land ownership. Survey cells were generally located in lower elevation (≤2,000 m), unforested landscapes outside of the central Idaho mountains. Milkweed was detected in 25 (15%) cells. By strata, milkweed was detected in 17 of 65 (26%) high probability cells and 8 of 68 (12%) medium probability cells, with no detections of milkweed in low probability cells. Showy milkweed was the only Asclepias spp. found in this survey effort. Monarchs (adults, eggs, larvae) were detected in 6 (4%) cells, with 3 detections each in high and medium strata cells.
Our combined bi-state survey effort from 26 May to 1 September in 2016, and 3 June to 20 September in 2017, resulted in 3,616 milkweed patch observations across Idaho (n = 2,875) and eastern Washington (n = 741). In Washington, 149 milkweed patches were detected during road-edge surveys. We documented 5 milkweed species in the study area (Figure 1), with showy milkweed by far the most commonly reported species (92%). Swamp milkweed (A. incarnata) (6%), narrow-leaved milkweed (A. fascicularis) (1%), pallid milkweed (A. cryptoceras) (0.6%), and spider milkweed (A. asperula) (0.1%) were less commonly reported. All 5 milkweed species were found in Idaho and 3 milkweed species were found in Washington (A. speciosa, A. incarnata, and A. fascicularis). Milkweeds occurred in all 16 climate divisions (Figure 3) and 52 of 65 (80%) counties within the study area (Figure 1), with largest patches and greatest abundance of milkweeds found in the Columbia Plateau Ecoregion spanning both states. We documented first showy milkweed records for 12 Idaho counties (Blaine, Boise, Bonner, Bonneville, Boundary, Caribou, Clark, Jerome, Lewis, Lincoln, Minidoka, Oneida), and Franklin County, Washington. First swamp milkweed records were documented for Bannock, Bonner, Cassia, and Idaho counties, Idaho; and a first state record for Washington in Okanogan County. We also documented first narrow-leaved milkweed records for Payette County, Idaho; and Chelan, Ferry, Franklin, Skamania, and Stevens counties, Washington. Several localities with sympatric milkweed species were documented, including extensive areas supporting intermixed populations of showy and swamp milkweeds in Idaho's Snake River Plain (Supplementary Table S1). Milkweed elevations ranged from 0 to 1,686 m (0–855 m in Washington; 670–1,686 m in Idaho) with showy milkweed exhibiting the broadest elevational and ecological amplitude of milkweed species found in the study area.
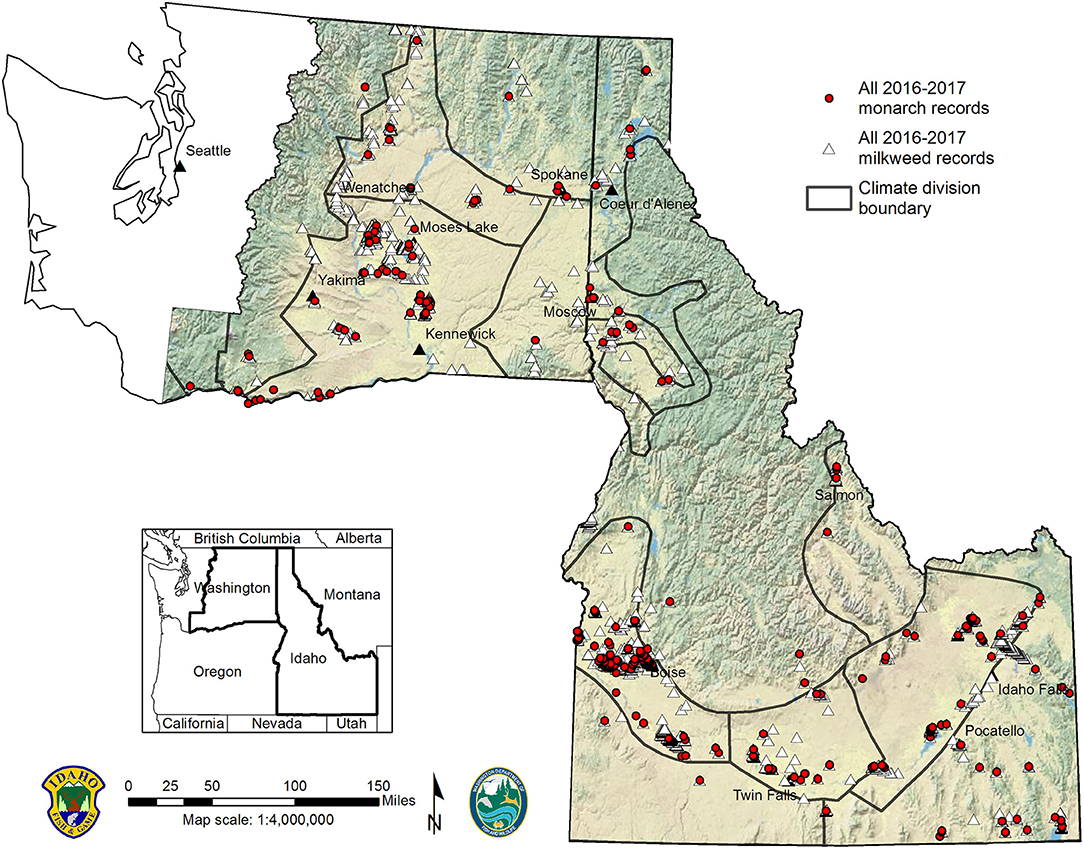
Figure 3. Distribution of monarch and milkweed observations by climate division based on IDFG and WDFW survey data, 2016–2017.
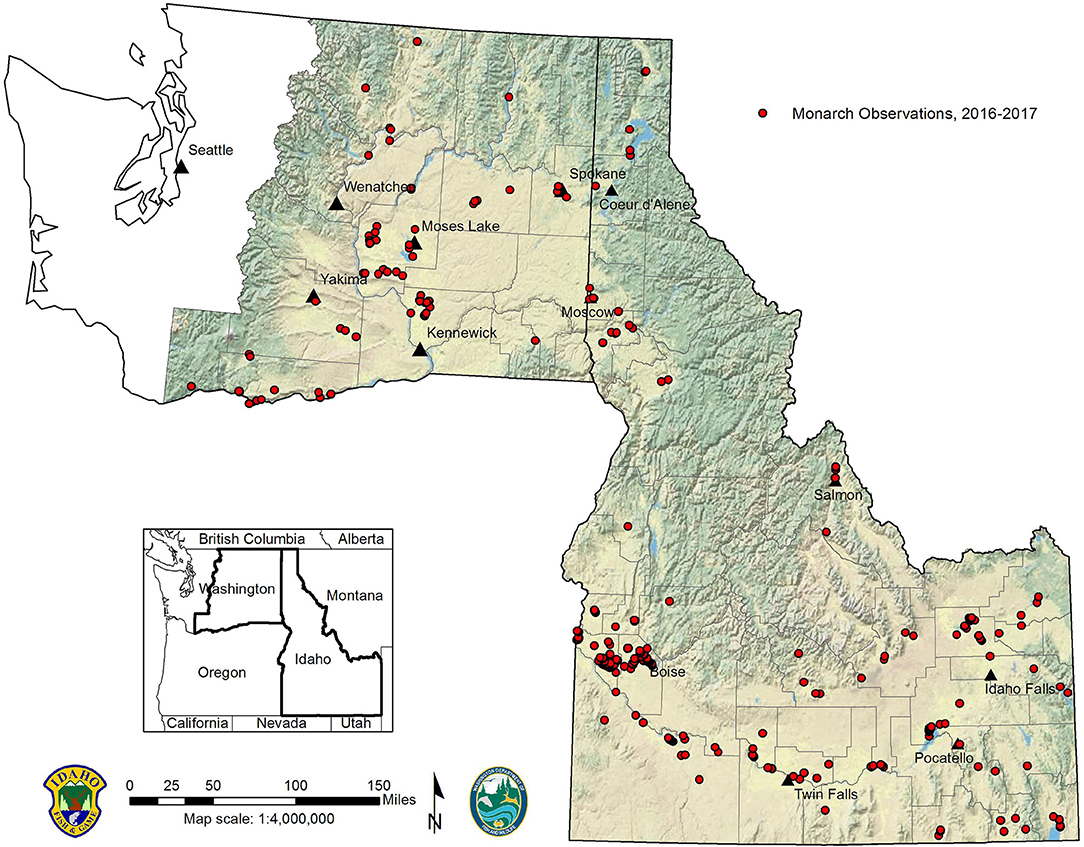
Our surveys generated 842 new breeding-season monarch records (n = 615 from Idaho; n = 227 from Washington) for the study area, including observations of monarch eggs (n = 178), larvae (n = 201), pupae (n = 4), and adults (n = 474). Monarchs were distributed across all 16 climate divisions (Figure 3) and 44 of 65 (68%) counties within the study area (Figure 4). Compared to available monarch records pre-project, our results demonstrated a 167% increase in climate divisions and 529% increase in counties occupied by breeding monarchs in the study area. Monarchs were observed at elevations from 0 to 1,686 m (0–573 m in Washington; 670–1,686 m in Idaho), with monarchs exhibiting lower elevational amplitude in Washington. Across both states, milkweed observations were more abundant and broadly distributed than monarch observations.
Monarch Life History
Monarch records collected during this study helped to fill life history data gaps on monarch breeding phenology (Figure 5) and use of nectar resources and roosting sites in Idaho and Washington. Arrival of first adults was typically the first week of June, and intriguingly, largely comprised fresh condition (immaculate, bright colors) migrants in Idaho and worn (torn or missing wing sections, faded colors) individuals in Washington. The former condition indicates recently emerged adults, and the latter, older butterflies that likely traveled a greater distance. A pattern of different wing wear between the two states suggests the geographic origin of newly arrived adults may differ. First eggs were observed in close succession with arrival of first adults, usually by mid-June. Early-instar larvae from this first generation were observed in mid- to late June, with an apparent lull in activity in early to mid-July during the pupal stage. The first locally-produced adults emerged in mid-July. Second local generation adults, considered to be the fall migrant generation, were observed in mid- to late August and commenced migration from mid-August through mid-September.
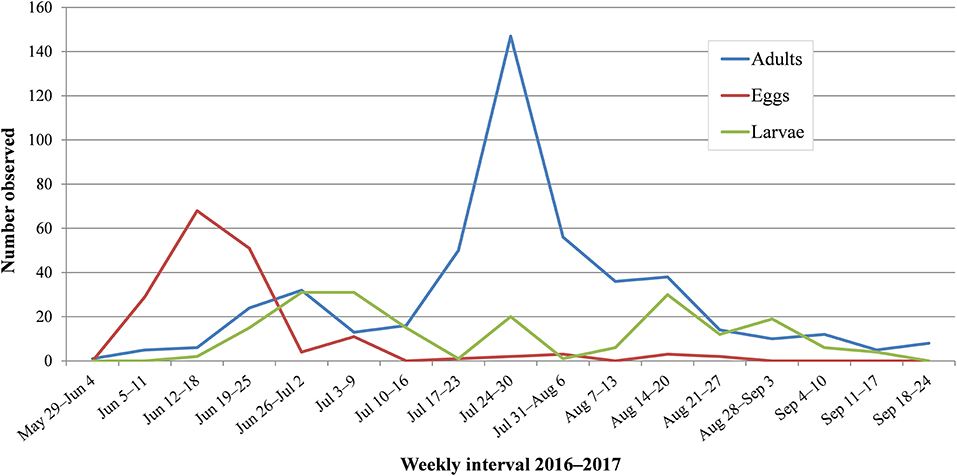
Figure 5. Life stage phenology of breeding monarchs in Idaho and Washington based on survey observations (n = 844) binned by weekly intervals in 2016–2017. Survey windows were 26 May−1 September in 2016, and 3 June−20 September in 2017.
We observed monarch oviposition, eggs, larvae, and pupae on showy and narrow-leaved milkweeds in both states. Oviposition, eggs, and larvae were reported on swamp milkweed in Idaho. We did not observe immature stages or ovipositing on pallid milkweed, however, S. McKnight (pers. comm.) of the Xerces Society reported late-instar larvae on pallid milkweed at one location in southwest Idaho in 2017. At the spider milkweed locality in Franklin County, Idaho, we did not detect evidence of the species' use as a monarch host plant, but did observe extensive, possibly damaging herbivory of seed pods by a variety of insects, primarily small milkweed bugs (Lygaeus kalmia) (see Supplementary Presentation S1 for photographs of milkweeds and monarch neonate life stages observed in the study area).
In both years of study, we encountered aggregations of large numbers (100s) of fresh adults at sites in southern Idaho. Adult massings were reported from 28 July to 24 August on 7 state- and federally-managed natural areas located within modeled high-suitability milkweed habitat. We interpreted the early adult massings as synchronous enclosure events, and those in late-August likely migrating adults.
During Idaho fieldwork, 293 adult monarchs were tagged through the WSU monarch tagging program. Overall, sex ratios were male-biased, with males accounting for 63% of tagged monarchs in 2016 (n = 63), and 60% in 2017 (n = 113). No recoveries of our Idaho-tagged monarchs were reported (James et al., 2018). We sampled 170 adult monarchs from 6 WMAs for OE, of which 5 (3%) tested positive. All OE positive adults were collected at the Roswell Habitat Area of Fort Boise WMA (Canyon County) on 28 July 2017 (n = 4) and 9 September 2017 (n = 1). Prevalence of OE in 89 adult monarchs sampled at Roswell was 5.6% (84 negative; 5 positive), at the low range of OE infection rates (5–30%) estimated in the western monarch population (Altizer and de Roode, 2015).
We compiled 448 observations of breeding season nectaring use by adult monarchs on 32 plant species (Supplementary Table S2). Asclepias spp. (showy, swamp, and narrow-leaved milkweeds) were the primary nectar plants used by monarchs in the study area (53%; n = 237). Of 29 non-Asclepias nectar plants identified, 16 (55%) are native to the study area and accounted for 27% of nectaring observations (n = 120). Of native plants, common sunflower (Helianthus annuus) and goldenrods (Solidago spp., Euthania spp.) were frequently visited by nectaring monarchs and had particular value as late-season forage for migratory generation monarchs and other native pollinators, notably bumble bees (Bombus spp.). We documented monarchs nectaring from 13 non-native plants, with 20% of total observations (n = 91) on 3 species: bull thistle (Cirsium vulgare) in Idaho, purple loosestrife (Lythrum salicaria) in Washington, and Canada thistle (Cirsium arvense) in both states. Diversity of nectar plants used by monarchs was highest in August, although, this may simply reflect peak abundance of monarchs on the landscape or monarch opportunism as primary nectar plants (i.e., showy milkweed) senesce.
Occasional observations of roosting monarchs were reported during our study. In Washington, adults were observed day-roosting in Russian olive (Elaeagnus angustifolia) and other small trees and shrubs on multiple days and sites when ambient temperatures exceeded 32°C. At several survey sites in Idaho, adult monarchs were observed day-roosting in herbaceous vegetation, including hardstem bulrush, broadleaf cattail, basin wild rye (Leymus cinereus), and Nuttall's sunflower (Helianthus nuttallii), though trees (native and non-native) were available in close proximity. An observation of an adult monarch day-roosting in Wyoming big sagebrush (Artemesia tridentata wyomingensi) at an eastern Idaho locale was notable in that timing was late in the season (14 September 2017) and use of sagebrush as a day-roost has not been previously reported for the study area. We observed night-roosting monarchs on a few occasions in late August-early September, consisting of 1–3 monarchs roosting at a height of 2–3 m in Russian olive.
Characteristics of Monarch Breeding Habitat
Habitat types were determined for 3,429 milkweed occurrence records (2,875 in Idaho; 554 in Washington) collected within the study area in 2016–2017. We identified 6 primary habitat types (Figure 6) after excluding habitat types with ≤2% of all milkweed records. Types occurring with highest relative frequency were grassland-herbaceous and emergent herbaceous wetland habitats, followed by deciduous forest, shrub-scrub, irrigation canal, and woody wetland habitats. Of these types, native or naturalized grassland-wetland habitats managed as IDFG and WDFW WMAs, USFWS National Wildlife Refuges, and U.S. Forest Service (USFS) National Grasslands supported the largest, most contiguous, and highest density milkweed stands. Cottonwood (Populus spp.) riparian forests within grassland-wetland habitats also supported abundant stands of showy milkweed, as did agricultural lands in the Columbia Basin of Washington and Snake River Plain of Idaho. These irrigation landscapes contain extensive networks of canals used to deliver water for crop irrigation, including sites with regular accumulations of runoff water. Combined season-long availability of water and intermittent disturbance from canal maintenance, mowing, or tilling facilitates rapid colonization by showy milkweed (Figure 7). Notable differences between states were higher occurrence of grassland-herbaceous and irrigation canal habitats in Washington, and higher occurrence of emergent herbaceous wetland and deciduous forest habitats in Idaho.
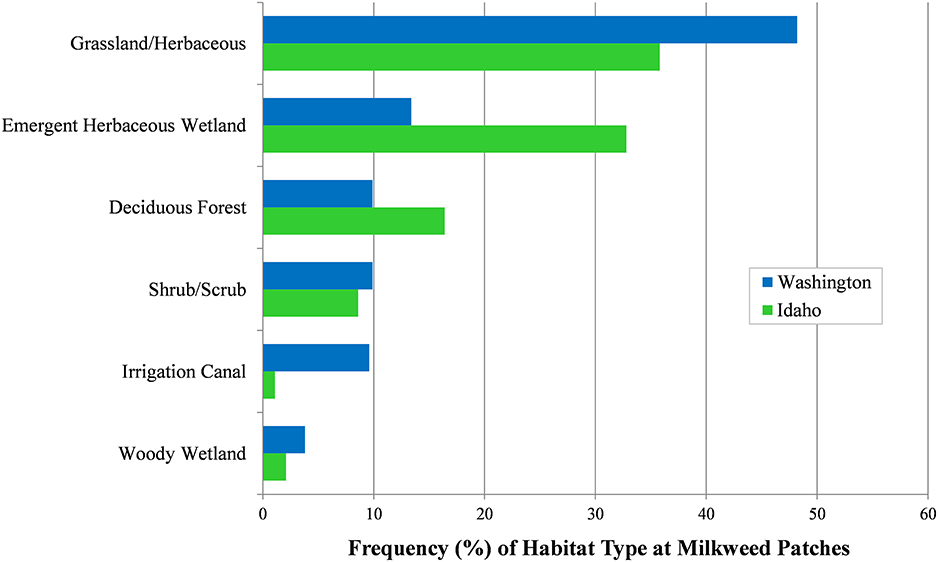
Figure 6. Habitat type frequency (%) among milkweed patches (n = 3,429), Idaho and Washington, 2016–2017. Observers could multi-select from 15 habitat type categories at each patch.
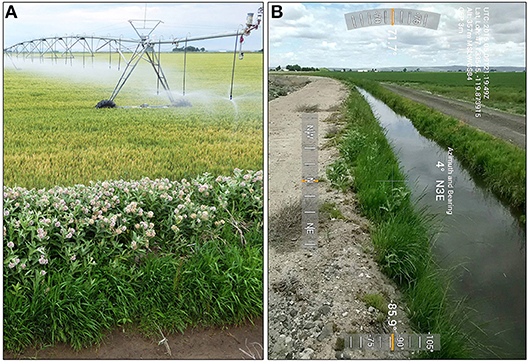
Figure 7. Examples of “indirect watering” management actions: (A) showy milkweed at edge of pivot-irrigated grain field, Jefferson County, Idaho, 2016; (B) showy milkweed established in an irrigation canal, Grant County, Washington, 2016.
Although shrub-scrub habitat was identified at about 18% of milkweed patches in our study area, it was infrequently the sole or dominant habitat type, and frequently co-occurred with grassland, riparian, and wetland habitat types. Notably, 32 of 163 (20%) randomly selected GRTS cells surveyed in Idaho with sagebrush-dominant shrub-scrub as the primary habitat did not contain A. speciosa or other milkweeds. Milkweed was rarely found in cultivated cropland, bare rock-gravel, developed, pasture-hay, garden, mixed forest, or evergreen forest habitat types.
Milkweed patch area (m2) was reported for 1,232 milkweed occurrence records (n = 653 in Idaho; n = 579 in Washington) and aggregated into 4 size classes (≤400, >400–4,000, >4,000–8,000, >8,000 m2; Figure 8). Nearly half (47%) of total milkweed patches were in the smallest size class (≤400 m2) and 39% fell within the next largest size class (>400–4,000 m2). The balance of milkweed patches (14%) was about equally aggregated between the 2 largest size classes. Milkweed patch areas were fairly consistent between states, with the exception of milkweed patches >8,000 m2, which although rare in both states, were more frequently reported in Idaho (n = 69) than in Washington (n = 16).
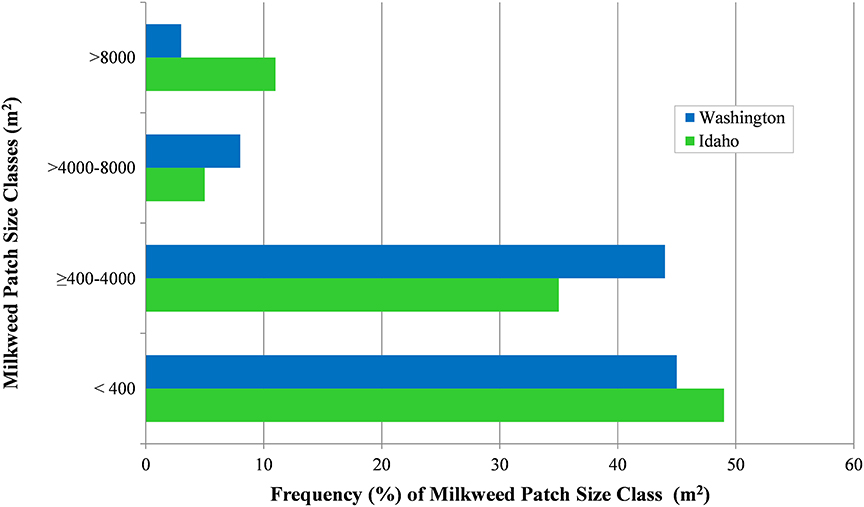
Figure 8. Patch size (m2) frequency (%) among milkweed patches (n = 1,232), Idaho and Washington, 2016–2017.
Milkweed species native to the study area are short-lived herbaceous perennials that senesce in late summer-early fall and are winter dormant, with new stems emerging in spring from established root systems. We sought to better describe milkweed phenology in the study area, not only to understand the changing availability of host plant resources for breeding monarchs, but to inform habitat management windows with least risk and greatest benefit potential to monarchs, and guide selection of milkweed species for restoration project success (Buisson et al., 2016). We found pallid and spider milkweeds to be the earliest phenology milkweed species, emerging in April, flowering in mid-May, fruiting in mid-June, and senescing by mid- to late July. Showy and narrow-leaved milkweed foliage began developing in early to mid-May, flowered over a prolonged period from late May to July, fruited in July to August, and senesced in September. However, milkweed phenology is also plastic and capable of response to environmental conditions; in 2017 we observed a narrow-leaved milkweed population (Kootenai County, Idaho) delayed ~5 weeks due to submergence by high flows in the Spokane River (Supplementary Presentation S1). Swamp milkweed exhibited the latest phenology of milkweeds in our study area, emerging in early to mid-June, with prolonged flowering in July-August, fruiting in August-September, and senescing in September-October. We documented multiple areas with sympatric milkweed species, but none more extensive and productive as monarch natal habitat than mixed showy and swamp milkweed stands in Idaho's Snake River Plain. Over the 2 years of our study, we observed phenological synchrony between adult monarch arrival (i.e., egg-laying) and bud burst/young expanding foliage of showy milkweed. Research on eastern monarchs has shown that phenological asynchrony with milkweed host plants can lead to high mortality of early instars and increased predation or poor nutrition in later instars (Zalucki et al., 2011), though how this mortality contributes to overall population dynamics remains unclear(Despland, 2017).
Management Activities and Threats in Monarch Habitats
We collected data on management activities and threats at milkweed sites where these factors were discernable. Management activities were identified for 644 milkweed occurrences in our study area (n = 270 in Idaho; n = 374 in Washington). Threat factors were recorded for 808 milkweed occurrences (n = 321 in Idaho; n = 487 in Washington) (Figure 9). Most management activity categories had corresponding threat categories (e.g., herbicide application was both a management activity and threat category). A commonly encountered management activity, indirect watering, described milkweed patches receiving supplemental watering from agricultural runoff, sprinkler systems, irrigation canals, roadside ditches, or agricultural ponds. Indirect watering was observed at 13% of Idaho milkweed occurrences (n = 34) and 49% of Washington occurrences (n = 183). All other reported management activity categories were also represented by threat categories.
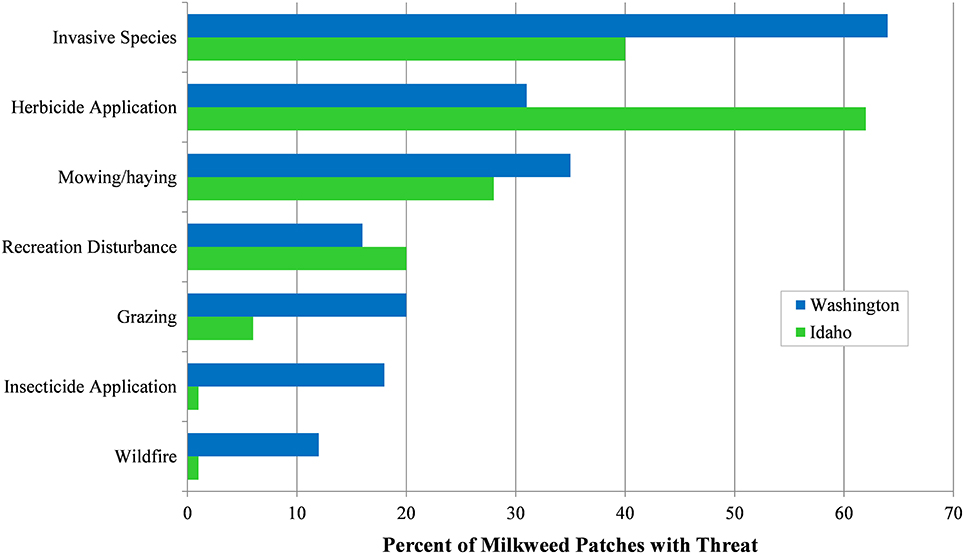
Figure 9. Threat factors identified at milkweed patches (n = 808) in Idaho and Washington, 2016–2017. Observers could multi-select from 11 threat categories at each patch.
We documented 1,625 threats at 808 milkweed patches. Primary threats were invasive plant species (n = 443), herbicide application (n = 348), and mowing (n = 259). Of primary threats, invasive plant species was more prevalent in Washington (64%) than in Idaho (40%), whereas herbicide application was considerably more frequent in Idaho (62%) than Washington (31%). Mowing occurred at 32% of milkweed occurrences in Washington and Idaho, usually for control of road-edge vegetation, or for harvest of hay or other crops. Secondary threats were recreational disturbance (n = 142), livestock grazing (n = 116), insecticide application (n = 88), flooding regimes (i.e., loss of floodplain function) (n = 68), and wildfire (n = 64). Disturbance of milkweed from recreation, typically from trampling or off-road vehicle use, was reported at 18% of milkweed patches across the study area. Livestock grazing occurred at 14% of sites, but was more common in Washington (20%) than Idaho (6%). Though livestock rarely consumed milkweed, they often trampled milkweed plants and grazed available nectar plants, thereby reducing or eliminating those resources. Washington reported notably higher frequencies of insecticide application, flooding regimes, and wildfire threats than Idaho. Other threat categories, such as irrigation canal maintenance, vegetation encroachment, and development, were less common in the study area (range 18–42 records).
Discussion
Milkweed and Monarch Distributions
Our study presents the first statewide inventories of milkweeds and breeding monarch distributions within the western monarch range. These data signify a major advancement over prior understanding of monarch breeding habitat extent and characteristics in Idaho and Washington, states at the northern tier of western monarch range. Our study documented much broader distribution of milkweed host plants and breeding monarchs than previously hypothesized based on suitable thermal regimes for monarch reproduction (Stevens and Frey, 2010). We increased our pre-project dataset of ~700 milkweed records by >400% and documented a first Washington state record for swamp milkweed, and first county records for showy milkweed (13 counties), swamp milkweed (5 counties), and narrow-leaved milkweed (5 counties). Of the 5 milkweed species documented in the study area, showy milkweed was most ubiquitous and wide-ranging owing to its adaptation to a wide range of soil types, moisture regimes, and disturbance agents (Stevens, 2000). Our surveys documented first records for breeding monarchs in 37 counties within the study area. Although we did not demonstrate range expansion for the western monarch population, we did produce a high-resolution baseline distribution for breeding monarchs scaled to the Idaho-Washington region.
Geographic and elevation ranges for the 40+ species of western milkweeds are highly variable, owing to the integrated effects of latitude and altitude, and their influence on temperature, precipitation, humidity, heat, and illumination (Xu et al., 2017). The 5 milkweed species native to our study area have geographic ranges extending a few 100 to 2,000+ km south of Idaho and Washington and occur at elevations up to ~2,700 m in southwestern states (e.g., Arizona, Nevada, Utah). The elevation range of the 5 milkweed species in our study area (0–1,686 m) is at the low to mid-range for these species across the western states, indicating that relatively high latitudes (42–49°N) and mountain habitats may be important determinants of milkweed distributions in Idaho and Washington. This is born out in the Western Monarch and Milkweed Habitat Suitability Modeling Project, Version 2 (Dilts et al., 2018), which ascribes low habitat suitability for milkweeds and breeding monarchs in mountainous regions of our study area. By refining elevational distributions of milkweeds (and by extension, breeding monarchs) in our study area, monarch conservation work can be appropriately targeted in areas with highest potential for success.
Monarch Life History
Although our research was not designed to examine detailed breeding phenology of monarchs, our surveys refined a temporal window for monarch breeding and life stages in Idaho and Washington (Figure 5; Supplementary Table S1) where few spatial or temporal data previously existed. An important caveat to these results is sampling efforts to identify monarch life stages were not equally distributed in time and space across the study area and thus have limitations regarding spatio-temporal precision. In addition, a larger sample of monarch life stage observations from Idaho may impart a geographic bias to our phenology dataset. With these caveats in mind, we sampled continuously during monarch breeding seasons across a heterogeneous geography within and between states and found strong correspondence of dates for adult arrival (early June), peak egg observation (mid-June), and peak adult observation (late July). James (2016) reported adult arrival time and systematically collected adult numbers over 3 breeding seasons (2013–2015) at a single central Washington study site. Our findings of regional adult arrival time in early June were harmonious with James's observations, and our observations of peak adult numbers in late-July were consistent with 2 of his 3 study years. Further work is needed to determine finer spatio-temporal resolution of monarch breeding phenology and identify effective windows to minimize risk to monarchs in Idaho and Washington.
Our observations of robust monarch production at numerous milkweed-abundant sites in Idaho and eastern Washington counter previous studies suggesting minor recruitment of Pacific Northwest migrant-generation monarchs to the western population (Stevens and Frey, 2010; Pyle, 2015). Rather, our study builds upon evidence of substantial natal contributions of monarchs from interior western states (i.e., “northern inland range”) to the California overwintering population (Yang et al., 2015). Furthermore, major pulses of monarch production documented in our study were consistent with similarly large populations of monarchs observed at a milkweed-rich study site in Central Washington, which James (2016) stated were “remarkable and challenge our concepts of summer breeding of D. plexippus in the Pacific Northwest.”
Whereas monarch larvae are specialists with respect to host plant use, adult monarchs are nectar generalists, feeding on a wide assortment of flowering plants (Brower et al., 2006). We found nectar species to be limited in quantity and richness in the study area, particularly in late summer. Inadequate nectar resources can reduce fecundity and lipid accumulations needed by monarchs to fuel the fall migration, overwintering period, and subsequent northward flight in spring (Brower, 1985; Alonso-Mejia et al., 1997; Brower et al., 2006). Although we disproportionately sampled predicted milkweed sites, 53% of nectar uses by monarchs were on milkweeds; slightly <60% reported by Xerces (2018) for the 11 western states. Our results underscore the importance of milkweeds not only as monarch host plants but as extended-season nectar resources for adults. Monarchs also nectared on non-native, invasive species, such as Canada and bull thistles and purple loosestrife, and in some locations these non-natives were the only nectar resources available after milkweeds flowered. James (2016) found purple loosestrife to be a principal late-season nectar resource at a monarch breeding site in eastern Washington and a key factor in the site's suitability as monarch natal habitat. In some cases, invasive species may provide significant nectar sources for migrating monarchs (Brower et al., 2006).
Although targeted surveys for roosting monarchs were not part of our study, we recorded roosting behavior when incidentally observed in the field. Previous studies and observations from eastern Washington (Pyle, 1999; James, 2016), Oregon (Cheryl Schultz, pers. comm.), Utah (Utah Lepidopterists' Society, pers. comm.), and Idaho (Rose Lehman, pers. comm.) suggest that tree and shrub roosting structure may be important to western monarch breeding and migration ecology. The dominant tree species noted in these reports is the introduced Russian olive, variably used by adult monarchs for daytime shade (James, 2016) and nighttime roosts (Pyle, 1999). Russian olive is a Class C noxious weed in Washington and considered an invasive plant species in Idaho and several other western states for its propensity to displace and hinder recruitment of native climax species in many waterways of the interior western U.S. (Lesica and Miles, 1999, 2001; Pearce and Smith, 2001). Given the plethora of negative ecological impacts linked to Russian olive, it is often targeted for control efforts by land managers. Research is needed to address whether roosting habitat is an essential component in western monarch breeding range, particularly if Russian olive control could result in unintentional but potentially harmful consequences for breeding and migrating monarchs.
Characteristics of Monarch Breeding Habitat
Although monarch breeding habitat is delimited by distributions of its obligate milkweed host plants, not all milkweed sites support breeding monarchs (Grant et al., 2018; Pitman et al., 2018). The relatively coarse scale of our study did not allow inferences about microsite attributes or preferred spatial configurations of habitat at monarch natal habitats. However, we did identify some common key characteristics of monarch breeding habitat in the study area. Highly productive monarch breeding habitats were moist-soil sites within a matrix of grasslands, wetlands, deciduous forest, and shrub-steppe habitats supporting large, contiguous, and high-density milkweed stands. These habitats were most often located on public lands managed for wildlife conservation or multiple uses. Common to these sites were presence of naturally-occurring or anthropogenic-sourced surface or ground water that resulted in increased soil moisture relative to surrounding landscapes, and maximum daytime temperatures agreeable with the thermal optimum for monarch life stage development (~28°C) (Zalucki and Kitching, 1982; York and Oberhauser, 2002). Most of the WMAs and refuges are located within irrigation landscapes and directly or indirectly rely on water delivery systems allocated by legal water right to maintain habitat conditions. WMAs and refuges in Idaho occur in the Snake River Plain where ~85% of total state water withdrawals support irrigated agriculture (Murray, 2018). Similarly, important monarch breeding habitats in the Columbia Basin of Washington spatially overlap the state's most concentrated region of irrigated cropland, which uses ~80% of state water withdrawals (McLain et al., 2017). The waterscapes of southern Idaho and eastern Washington are presently at risk of increased water deficits due to population growth, land-use change, and changes in cropping systems and commodities grown (Ryu et al., 2012; Hall et al., 2016; Kliskey et al., 2019). Projected hydroclimatic changes across the region in the next 50 years include a substantial warming (Rupp et al., 2017), decreased snowpack, shorter snow accumulation season, earlier snowmelt, and increased evapotranspiration leading to likely water and soil moisture deficits during summer months (Vano et al., 2015; Gergel et al., 2017). Such scenarios point to the inherent vulnerability of monarch breeding habitats in both states. The persistence and viability of these habitats will rely on adaptive, long-term water plans that recognize and value monarch and other wildlife habitat to proactively address the region's complex water challenges (Kliskey et al., 2019).
The Idaho GRTS survey helped to identify milkweed habitat suitability across a range of habitat types and indicated that sagebrush-steppe habitats in Idaho are generally unsuitable for showy milkweed. This result was unsurprising given seasonal aridity of sagebrush-steppe habitats and showy milkweed affinity for moist-soil sites. A key assumption of this finding is the dataset underlying the showy milkweed habitat suitability model (USFWS and Xerces, 2016) used in our GRTS survey framework was adequately robust in its Phase I iteration. In Washington, shrub-scrub habitats consist of shrub-steppe plant communities dominated by big sagebrush (A. tridentata), bitterbrush (Purshia tridentata), and rabbitbrush (Ericameria nauseosa, Chrysothamnus viscidiflorus) with perennial bunchgrass understory. Portions of Columbia Plateau shrub-steppe are underlain by deep alluvial and eolian sand deposits formed during Pleistocene deglaciation (Hallock et al., 2007). Washington's most productive milkweed stands occur in these mosaics of deep, sandy soils where supplemental irrigation water and resulting raised water tables, as well as naturally occurring lakes, ponds, and rivers, have facilitated establishment of herbaceous and woody vegetation.
While we located several large, high density milkweed patches during our surveys, over half of patches contained relatively few individuals (i.e., 1–50 plants). Our findings were consistent with Pyle (2015) and James (2016) who described milkweed distributions in Pacific Northwest states as patchy and low density. Whether this type of distribution pattern is characteristic across the West and how such patterns affect carrying capacity of monarch breeding habitat in terms of available milkweed resource or reproductive success remains unclear. Monarch females in eastern North America sought out smaller milkweed patches in agricultural, roadside, and non-agricultural areas and oviposited more heavily there (Zalucki, 1981; Zalucki and Kitching, 1982). These results were variably attributed to use of fertilizer, ability of females to detect milkweed in monocultures, and higher quality plants due to reduced competition for resources (Pleasants and Oberhauser, 2012; Pitman et al., 2018). We are uncertain whether a similar pattern would hold true for monarchs in western ecosystems with a different complement of milkweed species, cropping systems, and precipitation patterns.
In southwest Idaho, a key attribute of productive monarch habitat was sympatric occurrence of showy and swamp milkweeds, typically at the patch level. The mix of milkweed species with asynchronous phenologies extended the vegetative stage required for egg, larvae, and pupae development, and the bloom period for nectaring monarchs and other pollinator taxa. Mixed milkweed sites typically had dense, complex vegetative structure with abundant cover for immature monarch stages. Whether this structure or combination of milkweed species influences monarch vital rates (i.e., survival, individual growth, recruitment) are research questions meriting investigation in western monarchs.
Management Activities and Threats in Monarch Habitats
Though the western U.S. abounds with large natural areas and wilderness, we found milkweeds and monarchs in Idaho and Washington persist primarily in landscapes impacted by high human activity. Threats (e.g., factors that jeopardize persistence of milkweed and monarchs) were commonly observed during our study and most often directly or indirectly human-induced. In addition to sharing threats faced by eastern milkweeds and breeding monarch populations, the butterfly-host system in the West experiences unique threats, likely because occurrence is often restricted to moist-soil conditions within an otherwise arid landscape. Our research is the first assessment and body of data on milkweed and breeding monarch threats collected in western states.
Non-native and invasive grasses, shrubs, and trees assessed as likely milkweed competitors were documented at 55% of sites. Though not all invasive plants were identified to species, Russian olive and perennial and annual grasses (including cheatgrass [Bromus tectorum]) were commonly encountered. The abundance of invasive species at surveyed sites was likely a byproduct of milkweed occurrence in frequently disturbed and moist-soil habitats prone to invasion by non-native plants. In some situations, invasive plants benefit monarchs by providing essential habitat features (e.g., non-native thistles and purple loosestrife providing nectar sources). In some areas Russian olive provided day and night roosting sites and may benefit milkweed and monarchs by creating limited, partially-shaded microhabitats (Pyle, 1999; James, 2016). Because control of invasive plants is desirable from an ecosystem standpoint, herbicide application, mowing, or other practices targeting invasives have the potential to collaterally destroy host and nectar plants and immature monarch life stages (Xerces, 2018).
A primary threat to milkweeds and monarchs documented in both states was herbicide use (43% of all milkweed sites; Figures 9, 10). Herbicide use was likely more widespread, as detection was reliant on survey timing relative to applications. We regularly encountered evidence of herbicide use in milkweed habitat and direct impacts to milkweed plants. Discussions with several land managers and landowners confirmed milkweed is a situational target for control and eradication on some lands. However, much observed herbicide use was conducted for general vegetation control, along roadsides, railroad rights-of-way (ROW), parking areas, etc., and not specifically to affect milkweed. Monarchs rely on milkweed and floral nectar sources, and herbicide applications affecting these resources essentially destroys breeding habitat, even when it may not be effective in controlling or eradicating targeted plant species. Widespread use of herbicide to target or collaterally damage milkweeds illustrates a prevailing regional perspective that these native plants are considered “weeds.”

Figure 10. Herbicide use targeting milkweed observed in (A) Lemhi County, Idaho, and (B) Franklin County, Washington. Applications occurred in July during peak monarch breeding activity in the study area.
We documented insecticide application at 18% of Washington milkweed patches, but less frequently in Idaho. Primary insecticide treatment observed in both states was for adult mosquito control. In Washington, extensive stands of milkweed fall within regions regularly treated with mosquito adulticides by local mosquito control districts, including on lands managed by WDFW and the U.S. Bureau of Reclamation near Moses Lake (Grant County Mosquito Control District #1., 2015). In Idaho, insecticide application to control adult mosquitos occurs in a milkweed- and monarch-rich area within the Boise River Greenbelt. Mosquito adulticides, including pyrethrin- or permethrin-based pesticides used in Washington, were found toxic to butterfly larvae and adults (Hoang et al., 2011), and specifically monarchs (Oberhauser et al., 2006).
Showy milkweed was commonly found colonizing areas with indirect supplemental watering, including transportation ROW and irrigation waterway edges. Impervious surfaces of roadways serve to harvest and channel rainwater to roadside verges where plant growth is often profuse (Wojcik and Buchmann, 2012). Likewise, season-long availability of irrigation water can produce a hedgerow effect of milkweed along irrigation canals (Figure 7). In Idaho, 13% of milkweed patches received supplemental water from paved road and agricultural sources (i.e., paved roads, roadside ditches, sprinkler irrigation, irrigation canals, irrigation runoff, and agricultural ponds). Nearly one-half of milkweed patches in Washington received supplemental watering from these sources. These locations are within zones of high human activity maintained for user safety, visibility, accessibility, and, in the case of irrigation systems, efficient water delivery, thereby making milkweeds and monarchs occurring in these areas inherently vulnerable to loss and degradation.
Wildfire and human-caused fire frequency, size, and intensity has increased throughout western states, including Washington and Idaho (Abatzoglou and Williams, 2016). Monarchs and milkweeds can be directly threatened by fires during breeding season, and wildfire smoke may be an additive stressor to fall-migrating western monarchs (Pelton et al., 2016). In 2016, wild and human-caused fires burned milkweed habitat occupied by monarchs on 2 WDFW Wildlife Areas (Lower Crab Creek and Sinlahekin). Anthropogenic climate change is expected to continue driving increased wildfire activity in the West while fuels remain abundant on the landscape (Abatzoglou and Williams, 2016).
Comparison of threats to monarchs and their habitat in the arid western states of Idaho and eastern Washington to those in the Midwest shows commonalities and differences. Herbicide and insecticide use have been implicated in the loss of milkweed and monarchs in the Midwest (Pleasants and Oberhauser, 2012; Krischik et al., 2015). Threats related to herbicide use are being addressed in part by growing participation of state and county Departments of Transportation in Integrated Roadside Vegetation Management programs. Such programs recognize ROW landscapes offer important and overlooked conservation opportunities for monarchs and other pollinators (Hopwood et al., 2015; Xerces, 2018).
Management Recommendations and Research Needs
Key results of our study lead us to recommend management actions to abate threats to monarchs in Idaho and Washington. Paramount to monarch persistence in our states is continued protection and beneficial management of known monarch breeding habitats. Our study identified many of these high quality habitats, but other similarly important natal areas likely exist in Idaho and Washington and should be inventoried and assessed for long-term protection.
Managing quality monarch habitat often requires addressing invasive non-native plants and noxious weeds as part of public policy and ecosystem health directives. This can place land managers in a difficult position of navigating between conflicting resource management objectives (e.g., invasive plant nectar availability vs. ecosystem integrity). Where those conflicts exist, we suggest approaches that promote control vs. eradication of invasive plants providing nectar and roosting benefits to breeding monarchs and other pollinators.
The substantial level of herbicide use at milkweed sites in our study area highlights a pressing need for expanded communication with key sectors within our states (e.g., transportation departments, utility companies, farmers, ranchers, irrigation districts, private landowners) to reframe pervasive negative perceptions about native milkweeds and encourage management practices that conserve monarch habitat. Potential actions could entail development of effective messaging for different audiences, promoting financial incentive and technical assistance programs, and developing Integrated Vegetation Management programs that achieve more cost-effective and environmentally-sustainable management of undesirable plants while considering monarch needs.
Our study to delineate milkweed and breeding monarch distributions in Idaho and Washington fortuitously interfaced with development of the Western Monarch and Milkweed Habitat Suitability Modeling Project, Version 2 (Dilts et al., 2018). We recommend regular updating of these west-wide models as well as development of new models at finer spatial scales. Efforts to enhance and restore monarch habitat should consult both milkweed and monarch breeding models to assess suitability of any site. Such analyses are particularly relevant for USFS and Bureau of Land Management (BLM) lands in sagebrush-steppe and forested land cover types of our study area. Sagebrush-steppe habitats in Idaho (with the exception of Curlew National Grassland, Oneida and Power counties) and forested habitats in Idaho and Washington exhibit low suitability as milkweed or breeding monarch habitats. Instead, we suggest these agencies focus conservation work on protection and restoration of migratory habitat and connectivity. Monarch migration in the West is tied to riparian corridors, which provide crucial nectaring habitats, particularly in years of drought (Dingle et al., 2005; Brower et al., 2006, 2015). Management directed to reducing threats (Figure 9) to these spatially limited, but highly productive riparian communities could improve quantity and richness of floral nectar sources and roosting structure for spring and fall migrating monarchs.
Finally, we provide suggestions for future research to improve our knowledge base of the western monarch life cycle, breeding habitat requirements, and threat factors:
• Continue to address data gaps in monarch and milkweed distributions in Idaho and Washington.
• Develop a demographic model for the full annual life-cycle of western monarchs and conduct a sensitivity analysis to validate model.
• Determine characteristics of monarch breeding and migratory habitat that promote reproductive performance and survivorship.
• Investigate type, prevalence, and demographic impact of predation, parasites, and disease on all monarch life stages.
• Determine if agricultural landscapes and roadsides function as source, sink, or ecological trap habitats for monarchs.
Based on the new body of knowledge acquired from our study and evidence of continuing declines of the western monarch overwintering population, we recommend the monarch butterfly retain its status as “Species of Greatest Conservation Need” (SGCN) in Idaho and Washington. Moreover, we encourage our western state partners to evaluate monarchs for SGCN designation and consider similar survey approaches to address regional data gaps with the goal of conserving monarch habitats and migratory connectivity in the West.
Ethics Statement
Though butterfly taxa are not regulated under animal ethics and welfare guidelines, we observed voluntary protocols parsimonious with ethical treatment of monarchs in the field.
Author Contributions
BW and AP conceptualized the study, coordinated and conducted field data collection, conducted data management and analyses, and wrote the initial drafts of the manuscript. LS designed the GRTS survey framework, developed ArcGIS products, and conducted data management and analyses. All authors contributed to manuscript revisions and read and approved the final manuscript.
Funding
Funding was provided by the USFWS State Wildlife Grant Program (grant number F16AP00020), Idaho Department of Fish and Game Nongame Trust Fund, and Washington Personalized License Plate Fund.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for collegiality and partnership with S. Jepsen, E. Pelton, C. Fallon, S. McKnight, and S. Hoffman Black of the Xerces Society for Invertebrate Conservation. We thank C. Schultz, D. Perkins, D. James, T. Dilts, and M. Forister for their research collaborations. We thank our IDFG and WDFW colleagues and field crews, numerous citizen scientists, and Spokane Chapter of the Washington Butterfly Association for their contributions in data collection. We are grateful to J. Jenkerson for WDFW database management, D. Smith of IDFG for grant facilitation, and T. Keegan and two reviewers for insightful discussion and comments on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00172/full#supplementary-material
References
Abatzoglou, J. T., and Williams, A. P. (2016). Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. U.S.A. 113, 11770–11775. doi: 10.1073/pnas.1607171113
Alonso-Mejia, A., Rendon-Salinas, E., Montesinos-Patino, E., and Brower, L. P. (1997). Use of lipid reserves by monarch butterflies overwintering in Mexico: implications for conservation. Ecol. Appl. 7, 934–947. doi: 10.1890/1051-0761(1997)007[0934:UOLRBM]2.0.CO;2
Altizer, S., and de Roode, J. C. (2015). “Monarchs and their debilitating parasites,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds. K. Oberhauser, S. Altizer, and K. Nail (Ithaca, NY: Cornell University Press), 83–93.
Bailey, R. G. (1976). Ecoregions of the United States (map). Ogden, UT: USDA Forest Service, Intermountain Region. 1:7,500,000.
Brower, L. P. (1985). “New perspectives on the migration biology of the monarch butterfly, Danaus plexippus L.,” in Migration: Mechanisms and Adaptive Significance, ed. M. A. Rankin (Austin, TX: University of Texas Press), 748–785.
Brower, L. P., Fink, L. S., Kiphart, R. J., Pocius, V., Zubieta, R. R., and Ramirez, M. I. (2015). “Effect of drought on lipids in migrating monarchs” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds. K. Oberhauser, S. Altizer, and K. Nail (Ithaca, NY: Cornell University Press), 117–129.
Brower, L. P., Fink, L. S., and Walford, P. (2006). Fueling the fall migration of the monarch butterfly. Integr. Comp. Biol. 46, 1123–1142. doi: 10.1093/icb/icl029
Buisson, E., Alvarado, S. T., Stradic, S. L., and Morellato, L. P. C. (2016). Plant restoration research enhances ecological restoration. Restoration Ecol. 25, 164–171. doi: 10.1111/rec.12471
Despland, E. (2017). Effects of phenological synchronization on caterpillar early-instar survival under a changing climate. Can. J. For. Res. 48, 247–254. doi: 10.1139/cjfr-2016-0537
Dilts, T. D., Steele, M., Black, S., Craver, D., Cruz, E., Engler, J., et al. (2018). Data from: Western Monarch and Milkweed Habitat Suitability Modeling Project Version 2 – Maxent Model Outputs. Xerces Society, U.S. Fish and Wildlife Service, University of Nevada Reno. Available online at: https://www.monarchmilkweedmapper.org/habitatsuitabilitymodels
Dingle, H., Zalucki, M. P., Rochester, W. A., and Armijo-Prewitt, T. (2005). Distribution of the monarch butterfly, Danaus plexippus (L.) (Lepidoptera:Nymphalidae) in western North America. Biol. J. Linnean Soc. 85, 491–500. doi: 10.1111/j.1095-8312.2005.00512.x
Flockhart, D. T. T., Pichancourt, J.-B., Norris, D. R., and Martin, T. G. (2015). Unravelling the annual cycle in a migratory animal: breeding-season habitat loss drives population declines of monarch butterflies. J. Anim. Ecol. 84, 155–165. doi: 10.1111/1365-2656.12253
Flockhart, D. T. T., Wassenaar, L. I., Martin, T. G., Hobson, K. A., Wunder, M. B., and Norris, D. R. (2013). Tracking multi-generational colonization of the breeding grounds by monarch butterflies in eastern North America. Proc. R. Soc. Lond. Biol. 280:20131087. doi: 10.1098/rspb.2013.1087
Gergel, D. E., Nijssen, B., Abatzoglou, J. T., Lettenmaier, D. P., and Stumbaugh, M. R. (2017). Effects of climate change on snowpack and fire potential in the western USA. Clim. Change 141, 287–299. doi: 10.1007/s10584-017-1899-y
Grant County Mosquito Control District #1. (2015). Integrated Pest Management Plan for Northern Leopard Frog Habitat. Moses Lake, WA.
Grant, T. J., Parry, H. R., Zalucki, M. P., and Bradbury, S. P. (2018). Predicting monarch butterfly (Danaus plexippus) movement and egg-laying with a spatially-explicit agent-based model: the role of monarch perceptual range and spatial memory. Ecol. Model. 374, 37–50. doi: 10.1016/j.ecolmodel.2018.02.011
Hall, S. A., Adam, J. C., Barik, M., Yoder, J., Brady, M. P., Haller, D., et al. (2016). 2016 Washington State Legislative Report. Columbia River Basin long-term water supply and demand forecast. Publication No. 16-12-001. Washington Department of Ecology, Olympia, WA. Available online at: https://fortress.wa.gov/ecy/publications/documents/1612001.pdf
Hallock, L. A., Haugo, R. D., and Crawford, R. (2007). Conservation Strategy for Washington State Inland Sand Dunes. Natural Heritage Report 2007-05, Washington State Department of Natural Resources, Olympia, WA.
Hitchcock, C. L., and Cronquist, A. (2018). “Milkweed (Asclepias) treatment,” in Flora of the Pacific Northwest: an Illustrated Manual, 2nd Edn, eds D. E. Giblin, B. S. Legler, P. F. Zika, and R. G. Olmstead (Seattle, WA: University of Washington Press), 419.
Hoang, T. C., Pryor, R. L., Rand, G. M., and Frakes, R. A. (2011). Use of butterflies as nontarget insect test species and the acute toxicity and hazard of mosquito control insecticides. Environ. Toxicol. Chem. 30, 997–1005. doi: 10.1002/etc.462
Hopwood, J., Black, S., and Fleury, S. (2015). Roadside Best Management Practices That Benefit Pollinators: Handbook for Supporting Pollinators Through Roadside Maintenance and Landscape Design. Federal Highway Administration. Report No. FHWA-HEP-16-020. Available online at: https://www.environment.fhwa.dot.gov/env_topics/ecosystems/Pollinators_Roadsides/BMPs_pollinators_roadsides.pdf
iNaturalist.com. (2019). Data From: iNaturalist Research-Grade Observations. Occurrence Dataset. Available online at: https://doi.org/10.15468/ab3s5x accessed via GBIF.org
James, D. (2016). Population biology of monarch butterflies, Danaus plexippus (L.) (Lepidoptera: Nymphalidae), at a milkweed-rich summer breeding site in central Washington. J. Lepid. Soc. 70, 182–193. doi: 10.18473/107.070.0303
James, D. G., James, T. S., Seymour, L., Kappen, L., Russell, T., Harryman, B., et al. (2018). Citizen scientist tagging reveals destinations of migrating monarch butterflies, Danaus plexippus (L.) from the Pacific Northwest. J. Lepid. Soc. 72, 127–144. doi: 10.18473/lepi.v72i2.a5
Jepsen, S., Schweitzer, D. F., Young, B., Sears, N., Ormes, M., and Black, S. H. (2015). Conservation Status and Ecology of Monarchs in the United States. Portland, OR: Xerces Society for Invertebrate Conservation; NatureServe.
Kelling, S., Fink, D., La Sorte, F. A., Johnston, A., Bruns, N. E., and Hochachka, W. M. (2015). Taking a “Big Data” approach to data quality in a citizen science project. AMBIO 44, 601–611. doi: 10.1007/s13280-015-0710-4
Kliskey, A., Abatzoglou, J., Alessa, L., Kolden, C., Hoekema, D., Moore, B., et al. (2019). Planning for Idaho's waterscapes: a review of historical drivers and outlook for the next 50 years. Environ. Sci. Policy 94, 191–201. doi: 10.1016/j.envsci.2019.01.009
Krischik, V., Rogers, M., Gupta, G., and Varshney, A. (2015). Soil-applied Imidacloprid translocates to ornamental flowers and reduces survival of adult Coleomegilla maculata, Harmonia axyridis, and Hippodamia convergens lady beetles, and larval Danaus plexippus and Vanessa cardui butterflies. PLoS ONE 10:3. doi: 10.1371/journal.pone.0119133
Lesica, P., and Miles, S. (1999). Russian olive invasion into cottonwood forests along a regulated river in north-central Montana. Can. J. Bot. 77, 1077–1083. doi: 10.1139/b99-088
Lesica, P., and Miles, S. (2001). Natural history and invasion of Russian olive along eastern Montana rivers. West. N. Am. Naturalist 61, 1–10. Available online at: https://scholarsarchive.byu.edu/wnan/vol61/iss1/1
Martin, T. G., Chadès, I., Arcese, P., Marra, P. P., Possingham, H. P., and Norris, D. R. (2007). Optimal conservation of migratory species. PLoS ONE 2:e751. doi: 10.1371/journal.pone.0000751
McLain, K., Hancock, J., and Drennan, M. (2017). 2015 Drought and Agriculture: A Study by the Washington State Department of Agriculture. AGR PUB 104-495 (N/2/17). Available online at: https://agr.wa.gov/FP/Pubs/docs/104-495InterimDroughtReport2015.pdf
Murray, E. M. (2018). Water Use by Source and Category in Idaho Counties, 2015. U.S. Geological Survey data release.
Oberhauser, K. S., Brinda, S. J., Weaver, S., Moon, R. D., Manweiler, S. A., and Read, A. (2006). Growth and survival of monarch butterflies (Lepidoptera: Danaidae) after exposure to permethrin barrier treatments. Environ. Entomol. 35, 1626–1634. doi: 10.1093/ee/35.6.1626
Oberhauser, K. S., Nail, K. R., and Altizer, S. (2015). Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly. Ithaca, NY: Cornell University Press.
Pearce, C. M., and Smith, D. G. (2001). Plains cottonwood's last stand: can it survive invasion of Russian olive onto the Milk River, Montana floodplain? Environ. Manage. 28, 623–637. doi: 10.1007/s002670010248
Pelton, E., Jepsen, S., Schultz, C., Fallon, C., and Black, S. H. (2016). State of the Monarch Butterfly Overwintering Sites in California. Xerces Society for Invertebrate Conservation, Portland, OR. Available online at: http://www.xerces.org/wp-content/uploads/2016/07/StateOfMonarchOverwinteringSitesInCA_XercesSoc_web.pdf
Pitman, G. M., Flockhart, D. T. T., and Norris, D. R. (2018). Patterns and causes of oviposition in monarch butterflies: implications for milkweed restoration. Biol. Conserv. 217, 54–65. doi: 10.1016/j.biocon.2017.10.019
Pleasants, J. M., and Oberhauser, K. S. (2012). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect. Conserv. Divers. 6, 135–144. doi: 10.1111/j.1752-4598.2012.00196.x
Pyle, R. M. (1999). Chasing Monarchs: Migrating with the Butterflies of Passage. New York, NY: Houghton Mifflin Company.
Pyle, R. M. (2015). “Monarchs in the mist: new perspectives on monarch distribution in the Pacific Northwest” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds. K. S. Oberhauser, K. R. Nail, and S. Altizer (Ithaca, NY: Cornell University Press), 236–247.
Rupp, D. E., Abatzoglou, J. T., and Mote, P. W. (2017). Projections of 21st century climate of the Columbia River Basin. Clim. Dyn. 49, 1–17. doi: 10.1007/s00382-016-3418-7
Ryu, J., Contor, B., Johnson, G., Allen, R., and Tracy, J. (2012). System dynamics to sustainable water resources management in the eastern Snake Plain Aquifer under water supply uncertainty. J. Am. Water. Resour. Assoc. 48, 1204–1220. doi: 10.1111/j.1752-1688.2012.00681.x
Schultz, C. B., Brown, L. M., Pelton, E., and Crone, E. E. (2017). Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol. Conserv. 214, 343–346. doi: 10.1016/j.biocon.2017.08.019
Small-Lorenz, S. L., Culp, L. A., Brandt Ryder, T., Will, T. C., and Marra, P. P. (2013). A blind spot in climate change vulnerability assessments. Nat. Clim. Chang. 3, 91–93. doi: 10.1038/nclimate1810
Stevens, M. (2000). Plant Guide for Showy Milkweed (Asclepias speciosa). USDA-Natural Resources Conservation Service, National Plant Data Center. Available online at: https://plants.usda.gov/plantguide/pdf/pg_assp.pdf
Stevens, S. R., and Frey, D. F. (2010). Host plant pattern and variation in climate change predict the location of natal grounds for migratory monarch butterflies in western North America. J. Insect Conserv. 14, 731–744. doi: 10.1007/s10841-010-9303-5
Stohlgren, T. J., Chong, G. W., Kalkhan, M. A., and Schell, L. D. (1997). Rapid assessment of plant diversity patterns: a methodology for landscapes. Environ. Monit. Assess. 48, 25–43. doi: 10.1023/A:1005796618823
U.S. Fish Wildlife Service (USFWS) Xerces Society for Invertebrate Conservation (Xerces). (2016). Data From: Western Monarch Milkweed Habitat Suitability Modeling Project-Maxent Model Outputs. Available online at: https://catalog.data.gov/dataset/western-monarch-and-milkweed-habitat-suitability-modeling-project-maxent-model-outputs
Urquhart, F. A., and Urquhart, N. R. (1977). Overwintering areas and migratory routes of monarch butterfly (Danaus plexippus, Lepidoptera Danaidae) in North America, with special reference to the western population. Can. Entomol. 109, 1583–1589. doi: 10.4039/Ent1091583-12
Vandenbosch, R. (2007). What do monarch population time series data tell us about eastern and western population mixing? J. Lepid. Soc. 61, 28–31. Available online at: http://images.peabody.yale.edu/lepsoc/jls/2000s/2007/2007(1)28-Vandenbosch.pdf
Vano, J. A., Nijssen, B., and Lettenmaier, D. P. (2015). Seasonal hydrologic responses to climate change in the Pacific Northwest. Water Resour. Res. 51, 1959–1976. doi: 10.1002/2014WR015909
Webster, M. S., Marra, P. P., Haig, S. M., Bensch, S., and Holmes, R. T. (2012). Links between worlds: unraveling migratory connectivity. Trends Ecol. Evol. 17, 76–83. doi: 10.1016/S0169-5347(01)02380-1
Wojcik, V. A., and Buchmann, S. (2012). Pollinator conservation and management on electrical transmission and roadside rights-of-way: a review. J. Pollinat. Ecol. 7, 16–26. doi: 10.26786/1920-7603(2012)5
Wood, C., Sullivan, B., Iliff, M., Fink, D., and Kelling, S. (2012). eBird: Engaging birders in science and conservation. PLoS Biol. 9:12. doi: 10.1371/journal.pbio.1001220
Xerces Society (Xerces). (2018). Managing for Monarchs in the West: Best Management Practices for Conserving the Monarch Butterfly and its Habitat. The Xerces Society for Invertebrate Conservation, Portland, OR. Available online at: https://xerces.org/wp-content/uploads/2018/04/18-009_01-Monarch_BMPs_Final_Web.pdf
Xerces Society [Xerces]. (2012). A Guide to the Native Milkweeds of Washington. The Xerces Society for Invertebrate Conservation, Portland, OR. Available online at: https://www.xerces.org/wp-content/uploads/2011/10/WA-milkweed-guide_XercesSoc1.pdf
Xu, M., Ma, L., Jia, Y., and Liu, M. (2017). Integrating the effects of latitude and altitude on the spatial differentiation of plant community diversity in a mountainous ecosystem in China. PLoS ONE 12:3. doi: 10.1371/journal.pone.0174231
Yang, L. H., Ostrovsky, D. M., Rogers, M. C., and Welker, J. M. (2015). Intra-population variation in the natal origins and wing morphology of overwintering western monarch butterflies (Danaus plexippus). Ecography 39, 998–1007. doi: 10.1111/ecog.01994
York, H. A., and Oberhauser, K. S. (2002). Effects of duration and timing of heat stress on monarch butterfly (Danaus plexippus) (Lepidoptera: Nymphalidae) development. J. Kans. Entomol. Soc. 75, 290–298. Available online at: http://www.jstor.org/stable/25481789
Zalucki, M. P. (1981). Temporal and spatial variation of parasitism in Danaus plexippus (L.) (Lepidoptera: Nymphalidae, Danaidae). Aust. Entomol. Mag. 8, 3–9.
Zalucki, M. P., and Kitching, R. L. (1982). Temporal and spatial variation of mortality in field populations of Danaus plexippus L. and D. chrysippus L. larvae (Lepidoptea: Nymphalidae). Oecologia 53, 201–207. doi: 10.1007/BF00545664
Zalucki, M. P., Malcolm, S. B., Hanlon, C. C., and Paine, T. D. (2011). First-instar monarch larval growth and survival on milkweeds in Southern California: Effects of latex, leaf hairs and cardenolides. Chemoecology 22, 75–88. doi: 10.1007/s00049-011-0099-x
Keywords: monarch butterfly, Danaus plexippus, milkweed, Asclepias, Idaho, Washington, monarch breeding habitat, milkweed and monarch threats
Citation: Waterbury B, Potter A and Svancara LK (2019) Monarch Butterfly Distribution and Breeding Ecology in Idaho and Washington. Front. Ecol. Evol. 7:172. doi: 10.3389/fevo.2019.00172
Received: 30 December 2018; Accepted: 30 April 2019;
Published: 22 May 2019.
Edited by:
Jay E. Diffendorfer, United States Geological Survey, United StatesReviewed by:
Emily L. Weiser, United States Geological Survey, United StatesJohn Pleasants, Iowa State University, United States
Copyright © 2019 Waterbury, Potter and Svancara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beth Waterbury, a2VlZ2FuQGNlbnR1cnl0ZWwubmV0
 Beth Waterbury
Beth Waterbury Ann Potter
Ann Potter Leona K. Svancara
Leona K. Svancara