- 1Monarch Joint Venture, Department of Fisheries, Wildlife, and Conservation Biology, University of Minnesota, St. Paul, MN, United States
- 2Environmental Incentives, Denver, CO, United States
- 3Department of Integrative Biology, Oklahoma State University, Stillwater, OK, United States
- 4Xerces Society for Invertebrate Conservation, Portland, OR, United States
- 5Institute on the Environment, University of Minnesota, St. Paul, MN, United States
- 6University of Wisconsin-Madison Arboretum, University of Wisconsin, Madison, WI, United States
- 7Department of Ecology, Evolution and Behavior, University of Minnesota, St. Paul, MN, United States
Sustaining native pollinator populations and reversing declines in species such as the monarch butterfly (Danaus plexippus) will require enhancing and maintaining habitats across many regions and land use sectors. Rights-of-way, such as the areas surrounding roads, have long been regarded as important habitat for pollinators due to their ubiquitous nature and management for herbaceous species including nectar plants and larval host plants. With better information regarding the quality of pollinator habitat in roadside rights-of-way, managers can identify the location of potential habitat and evaluate the effects of management activities. We conducted a survey of roadside managers to determine needs and limitations related to assessing and managing rights-of-way as monarch habitat. Survey results indicated that managers are often limited by time, funding, and expertise in plant identification. Based on survey results and consultations with roadside managers, we developed a protocol for rapid assessment of roadside rights-of-way (hereafter, Rapid Assessment) that can be easily implemented by managers and is flexible based on the expertise of the observer and the data needs of the roadside management authority. Using readily available software, the field data are automatically processed through a Roadside Monarch Habitat Evaluator to generate habitat quality scores that may be used by managers to describe the habitat resources and to inform management strategies. We field-tested the protocol at roadsides in Minnesota and compared results with a more intensive protocol for monarch habitat monitoring (the Integrated Monarch Monitoring Program). We found that the Rapid Assessment provided similar data as the more intensive protocol regarding milkweed densities, nectar plant species richness, and monarch use of sites (eggs and larvae, when detection levels were sufficient). Observed high habitat values in roadside rights-of-way confirm the potential of such habitat for pollinator and monarch conservation.
Introduction
Monarch butterflies (Danaus plexippus) are an important flagship species for insect conservation. Monarchs, insect pollinators, and indeed most insect species, have experienced steep population declines in recent decades (National Research Council, 2007; Cameron et al., 2011; Brower et al., 2012; Vidal and Rendón-Salinas, 2014; Goulson et al., 2015; Semmens et al., 2016; Hallmann et al., 2017; Schultz et al., 2017; Sánchez-Bayo and Wyckhuys, 2019). Multiple factors are driving monarch declines (Malcolm, 2018), but habitat loss is primary (Flockhart et al., 2015; Thogmartin et al., 2017a,b) and the United States, Mexico, and Canada have pledged to reverse declines by improving and expanding habitat (CEC, 2008; Pollinator Health Task Force, 2015). Two important components in monarch habitat are nectar sources for adult monarchs, provided by a wide variety of blooming plants that benefit pollinators in general, and plants for larval development, provided by plants in the milkweed subfamily (Apocynaceae: Asclepiadoideae), which are also important nectar plants for many insect pollinators. Demographic models of the North American eastern monarch population indicate that the breeding season is likely the phase of the monarch life cycle that contributes most to population dynamics (Flockhart et al., 2015; Oberhauser et al., 2017) and loss of milkweed in the core of its breeding range is implicated in population declines (Pleasants and Oberhauser, 2013; Pleasants, 2017; Thogmartin et al., 2017a,b; Zaya et al., 2017; Stenoien et al., 2018). This has led to the goal of adding 1.3–1.6 billion stems of milkweed in the United States to increase the monarch population to sustainable levels (Pleasants, 2017; Thogmartin et al., 2017a). To reach this goal, habitat conservation is needed across all land use sectors (e.g., agriculture, developed areas, rights-of-ways), not just in lands set aside for conservation (Thogmartin et al., 2017b).
Rights-of-way may provide suitable pollinator habitat if managed in ways that promote and maintain host and nectar plants (Munguira and Thomas, 1992; Ries et al., 2001; Saarinen et al., 2005; Hopwood, 2008; Skorka et al., 2013; Halbritter et al., 2015), although concerns exist about dangers from roads (Munoz et al., 2015) including collisions (McKenna et al., 2001; Skorka et al., 2013; Keilson et al., 2018) and chemical runoff (Kaspari et al., 2010; Snell-Rood et al., 2014). A growing number of transportation agencies have implemented pollinator habitat programs (e.g., Iowa Living Roadway Trust Fund, Illinois DOT Monarch Program, Monarch Highway, Ohio Pollinator Habitat Initiative), and best management practices have been developed for pollinator habitat in roadside rights-of-way (Hopwood et al., 2015, 2016a,b). However, critical information about the availability of milkweeds and nectar plants within rights-of-way habitats is largely missing (but see Hartzler and Buhler, 2000; Kasten et al., 2016; Pitman et al., 2018), both generally and specifically within roadside management authorities.
Roadside managers need information to decide where to invest limited resources for maintaining and developing additional monarch habitat, and data on how various management actions affect the extent and quality of monarch habitat within their jurisdictions. For example, mowing is needed to maintain safety strips along road margins and is used to control woody and invasive species. However, frequently mowed areas often have fewer species of blooming nectar plants (Halbritter et al., 2015), and mowing can detrimentally impact insects using mowed areas (Johst et al., 2006; Cizek et al., 2012). However, mowing can also stimulate growth of new milkweed leaves preferred by egg-laying monarchs (Baum and Mueller, 2015; Fischer et al., 2015; Alcock et al., 2016; Haan and Landis, 2019; Knight et al., 2019). Many roadside management authorities are implementing reduced mowing practices particularly when monarchs are breeding in their regions to protect habitat for monarchs and other pollinators. These managers are interested in assessing the habitat characteristics created by such programs. In addition, data are needed for landscape-level planning and broad conservation efforts such as the Mid-America Monarch Conservation Strategy (MAFWA, 2018) and the USFWS Monarch Conservation Database1.
We developed several tools to help rights-of-way managers develop, assess, and manage monarch habitat. Here we present a rapid field assessment methodology, the Rapid Assessment of Roadside Habitat for Monarchs (“Rapid Assessment”), designed for quick and easy implementation by rights-of-way vegetation managers and maintenance operators. The data from the Rapid Assessment automatically feeds into a habitat calculator that generates a habitat quality score for each site; the package together is the Roadside Monarch Habitat Evaluator.
To guide the design of the Rapid Assessment, we surveyed transportation managers to learn about their interest in pollinator habitat programs, their information needs, and the personnel resources that may be dedicated to habitat assessment. To calibrate the new Rapid Assessment, we collected data from the same roadside sites using both our rapid assessment protocol and a more intensive protocol from the national Integrated Monarch Monitoring Program2 (CEC, 2017; Cariveau et al., 2019; IMMP). Specifically, we compared results from the Rapid Assessment to those from the IMMP for milkweed densities, nectar plant species richness, indices of nectar plant abundance, and monarch observations (eggs and larvae). We were interested in whether both protocols would yield similar estimates for these key metrics, and in the correlation of measures from the two protocols.
In this paper, we explain features of the Rapid Assessment that facilitate its use by roadside managers in transportation departments. We relate these findings to other studies and discuss the results in the context of managing rights-of-way as pollinator habitat. We additionally provide, as Supplemental Material, the Rapid Assessment protocol and datasheet. The User Guide for the Roadside Monarch Habitat Evaluator that enables roadside practitioners to run it with standard Esri products is provided online3.
Materials and Methods
Survey of Managers
We created a 30-question survey about desired management tools in Qualtrics4 that we distributed to a network of roadside management authority representatives via email. The survey was reviewed by the Institutional Review Board at the University of Minnesota and determined not to constitute human subjects research, therefore not requiring IRB approval. It included questions about existing pollinator habitat programs; what types of information would be helpful for planning or implementing these programs; the availability of data about factors that could influence pollinator habitat quality, including noxious weeds, salt applications, mowing regimes, and herbicide applications; and manager interest in tracking management practices. The survey captured information about personnel resources available for conducting habitat assessments, including the number of people and number of days they could spend assessing habitat, and the expected skill levels of the personnel relative to assessing habitat. Answers were mostly categorical with some free response.
Semi-structured interviews with a subset of survey respondents who indicated that their organizations have established or were considering establishing pollinator habitat programs were held to elicit further input, better understand the context in which roadside managers make decisions, identify barriers to establishing habitat programs, and evaluate the usefulness of tools such as a Rapid Assessment protocol in managing roadside rights-of-way as habitat.
Design of Roadside Monarch Habitat Evaluator
We designed a Rapid Assessment protocol to assess rights-of-way as pollinator habitat, with an emphasis on monarchs. The protocol includes information on road type, adjacent land use, management practices, forb species richness and percent cover, noxious weed presence and percent cover, and milkweed species richness and abundance (Table 1; field data sheet and protocol instructions provided in Supplemental Material 1). We developed both a paper data sheet and an electronic data form that could be filled using a tablet or smartphone in the field.
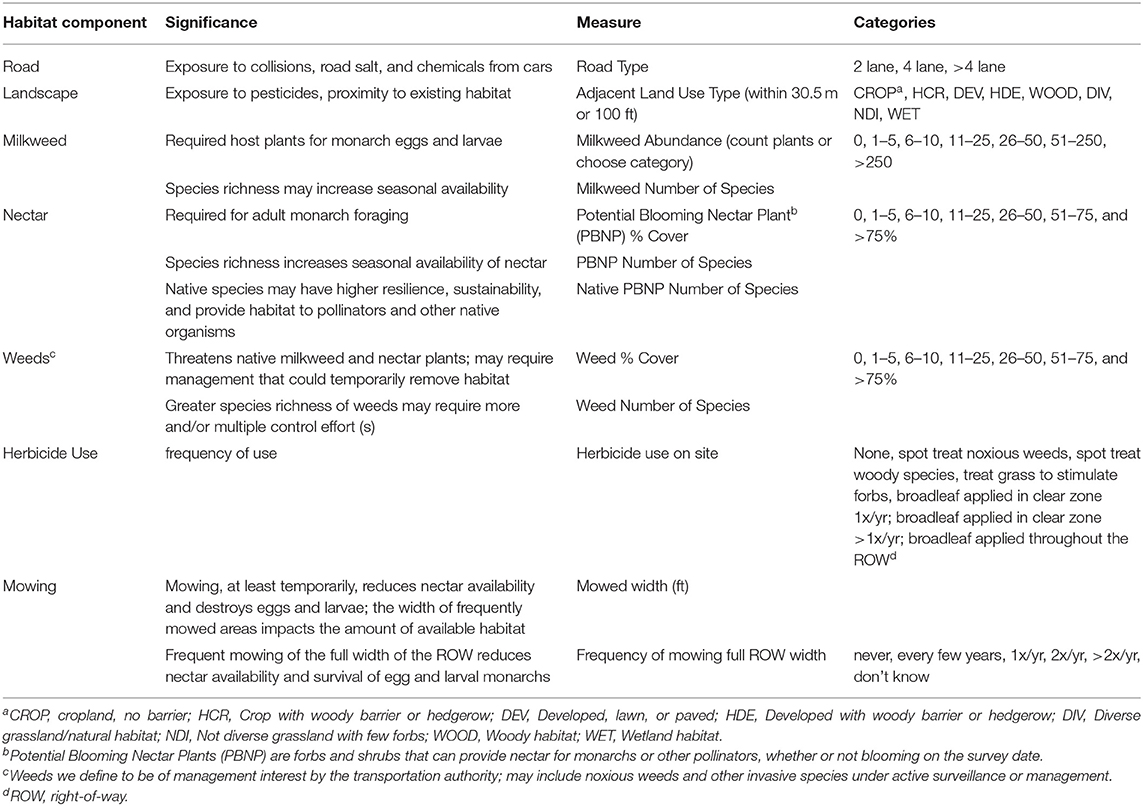
Table 1. Habitat components (and data collected) for roadside right-of-way habitats using the Rapid Assessment.
Secondly, we developed a habitat calculator that automatically computes habitat quality scores from the data collected in the Rapid Assessment. Together the Rapid Assessment and Habitat Calculator form the Roadside Monarch Habitat Evaluator (hereafter, “Habitat Evaluator”).
When developing the Habitat Evaluator, and in collaboration with the Rights of Way as Habitat Working Group facilitated by the Energy Resources Center of the University of Illinois – Chicago, we reviewed more than a dozen existing assessment tools including the Monarch Habitat Quantification Tool (Environmental Defense Fund, 2017), the Solar Site Pollinator Habitat Planning and Assessment Form (Minnesota Board of Soil Water Resources, 2016), and Bee Better Certified Farm Management Assessment Guide (Xerces Society for Invertebrate Conservation 2015). While none of these tools were created for use by transportation managers, they provided examples of ways in which pollinator habitat attributes were compiled into scores.
We designed the Habitat Evaluator tool in Survey123 for ArcGIS (Esri), a free product that affords several benefits for roadside management authorities. States or other entities can collect, manage, and view their own datasets using their own Esri Enterprise account. The Habitat Evaluator is installed within each agency's ArcGIS Online platform, when it is populated with a plant list for their state. Then managers may customize their assessment by selecting the noxious weeds they wish to track and set default answers regarding herbicide use and mowing practices, if desired. Within their own Survey123 website, transportation managers can view site locations, field data, and monarch habitat quality scores. A User Guide to the Roadside Monarch Habitat Evaluator is online3.
The electronic form of the Rapid Assessment provides the field user advantages such as the ability to automatically record the location, date, and time of the assessment. The survey also provides features such as a searchable drop-down list of plant species that enables one to type in letters from either the common name or the Latin name to select the species. It also includes choices based on genera, such as “Solidago/goldenrod species” for many groups. The assessment is flexible in that observers may also tally plant types they cannot identify and choose to estimate milkweed plant abundance in categories rather than count individual plants (e.g., depending on the abundance of the milkweed and time constraints). Observers also specify whether they are assessing the full right-of-way or just the unmowed areas, and whether or not they wish to collect optional data regarding the presence of monarch eggs and larvae. We incorporated several factors identified as important to roadside managers, including the need to assess sites quickly and once per growing or monarch breeding season, the ability to specify weeds of local or state importance, and the ability to specify the width of the area to be surveyed with regard to mowed areas, each of which we describe subsequently.
Given the strong preference of roadside managers for a protocol that could adequately characterize the habitat quality of a site in a single visit per year, we required a proxy for the availability of nectar throughout the growing season. We defined a term “Potentially Blooming Nectar Plants” (hereafter “nectar plants”) to describe forbs and shrubs that could provide nectar to pollinators (e.g., excluding grasses), whether or not blooming on the date of assessment. This broad categorization encompasses plants that may provide nectar, regardless of their nativity or the amount or quality of nectar they may provide. The numbers of nectar plant species may be important because a greater number of species may represent a greater variety of bloom times and thereby provision nectar for a greater proportion of a season of monarch use or use by other pollinators. We identified plants to species when possible and also estimated the aerial percent cover of nectar plants as a group. To make the protocol usable for people with varying skills in identifying plants to species, we included an option for tallying unidentified types of plants.
To accommodate variation in the list of invasive species, weeds, or non-native species of management concern from state to state, we created a customizable weed list. When transportation managers initially set up the protocol for their organization, they can list the weed species they want to include in the assessment. Observers will then report whenever those species are present on the assessment areas and estimate aerial cover for those species as a group to describe their prevalence.
Our survey of roadside managers indicated that the frequency and widths of mowing in the rights-of-way were highly variable; some routinely mow the full right-of-way width multiple times per growing season, while others mow the full right-of-way only once every several years. Some mow a safety strip (e.g., first 10–12 feet) monthly during the growing season, while others mow the strip only once per year (and some do not mow from May-July for wildlife and pollinators). Furthermore, some roadside managers expressed interest in using the Rapid Assessment to gain information about the effects of their mowing practices on pollinator habitat. In the first year of the project, we collected data across the entire right-of-way. In the second year, we focused our estimates of cover on the unmowed area for qualitative measures (such as percent cover) and collected milkweed and nectar plant richness in both the mowed and unmowed areas, which we sum in these analyses. The final Rapid Assessment protocol allows surveyors to choose whether to conduct their assessments in full rights-of-way, unmowed areas, or in mowed and unmowed areas separately.
Finally, because some departments of transportation were interested in monarch breeding activity in their roadside areas, we included optional fields for recording monarch eggs, larvae, and adults. This section also includes a place to record the species and number of milkweed plants searched.
We field-tested the Rapid Assessment protocols with representative users from the Illinois, Minnesota, and Wisconsin Departments of Transportation at sites that depicted high quality conditions, such as prairie remnants, as well as sites where restoration activities had been completed, to gain further feedback and refine the protocols and data forms.
Design of the Habitat Calculator
The Habitat Calculator is derived from the Monarch Habitat Quantification Tool (Monarch HQT, Anderson et al., 2017). The Monarch HQT is based on a modified Habitat Evaluation Procedure (HEP, see US Fish Wildlife Service, 1981) in which habitat characteristics (e.g., milkweed density) are translated to quality scores using suitability indices. Suitability indices approximate the relationship between a given habitat characteristic at a location and the location's suitability for monarchs. Suitability indices are weighted and summed to develop a Habitat Suitability Index (HSI), or habitat quality score. Habitat characteristics identified for important functional components of monarch habitat include breeding habitat (milkweed), foraging habitat (nectar plants) and factors that influence monarch habitat, including threats such as pesticide drift from agricultural fields.
For the Roadside Monarch Habitat Evaluator, the habitat characteristics evaluated were modified to match the data collected through the Rapid Assessment and expanded to include factors relevant to roadside rights-of-way. For example, the Rapid Assessment uses ocular estimates of cover of potentially blooming nectar plants whereas the Monarch HQT captures frequency of blooming nectar plants. The suitability indices were adapted as necessary based on expert opinion. In addition, the Habitat Evaluator includes additional indices of threats specific to roadside rights-of-way, including risk of collision with vehicles and chemical runoff and invasive weeds that may displace vegetation contributing to habitat quality. Finally, the Habitat Evaluator also incorporates vegetation management, including mowing and herbicide use. Measures of each variable are weighted and summed to produce a habitat quality score (see the online User Guide to the Monarch Roadside Habitat Evaluator3).
Rapid Assessment Field Technique
Rapid Assessments are completed for a 45.7 m (150 ft) length of roadway, implemented at random locations or systematically (e.g., every km or ten km) in a road system (see protocol in Supplemental Material 1). Upon arrival at a location of interest, the observer walks parallel to the road, toward traffic, pacing the 45.7 m distance (Figure 1). Next, the width of the vegetated right-of-way (perpendicular to the road) is measured or estimated (e.g., paced). These two distances bound the rectangular assessment area that extends from the road to the back of the right-of-way. The observer walks back through the right-of-way to the starting point, systematically zigzagging back and forth throughout the roadside habitat, while recording data. The observer records the number of milkweed plants by species, where stems separated by soil are counted as plants regardless of whether they are clonal or genetic individuals (following Kasten et al., 2016; CEC, 2017), the species or number of nectar plants (and notes for each species if it is blooming or not), and the presence of weeds (as defined by their roadside organization). Percent aerial cover is also estimated by classes for potential nectar plant species collectively (regardless of whether currently blooming) and for weeds of concern. In 2018 observers also estimated the percent cover by flowers for comparison to the IMMP blooming plant frequency. The observer records the dominant adjacent land use and mowing and herbicide application information. As an option, observers may examine milkweed plants by species for monarch eggs and larvae, recording the number of plants searched, and number of eggs and larvae detected. To maintain efficiency when milkweed is abundant, observers may choose to monitor every 2nd, 3rd, or 5th milkweed plant encountered to gain a sample size of 50–100 milkweed plants searched per site.
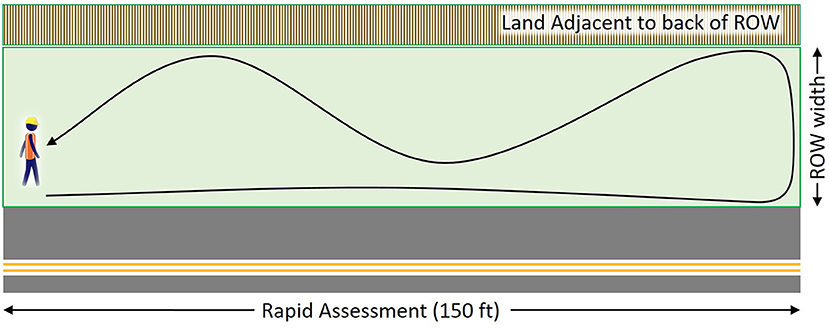
Figure 1. The Rapid Assessment of Roadside Habitat for Monarchs, showing an example of how an observer might move from a starting point 45.7 m along a roadside, then systematically zig-zagging throughout the right-of-way to characterize habitat conditions. The survey area for a Rapid Assessment is the 45.7 m by the width of the right-of-way (ROW) from the road to the adjacent land at the back of the ROW.
Integrated Monarch Monitoring Program Methods
IMMP sampling employs a total of 100 quadrats placed along ten transects arrayed diagonally from the road edge to the back of the right-of-way along a 400–500 m length of roadway (see Figure 2). Transects are 50 m in length and quadrats are placed every 5 m (however, in 2017, we placed quadrats every 2 m along 25 m transects, with 25 m between each transect). Quadrats consist of a 1.0 m by 0.5 m sampling frame placed to either side of the transect line for a 2.0 m by 0.5 m or 1 m2 quadrat area. Within each quadrat, observers count milkweed plants (same definition as above) to estimate milkweed density (milkweed plants/ha). All blooming plants are identified to species and assigned to the first subplot (area within the quadrat) in which they occur (first 0.5 x 0.5 m, 1.0 x 0.5 m, or 2.0 x 0.5 m) to generate a frequency score (proportion of subplots occupied). Plants that are not blooming on the date of the assessment are not recorded. The IMMP protocol is available on its website2.
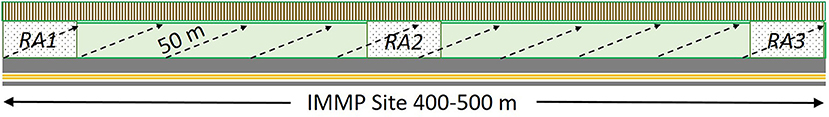
Figure 2. Overlay of Rapid Assessment (RA) and Integrated Monarch Monitoring Program (IMMP) for the comparison of protocols. The IMMP uses ten 50 m long transects arrayed diagonally 400–500 m along the roadway. In our comparison trials, 2–4 Rapid Assessments were completed for each IMMP site, typically established at the ends and middle of each IMMP site.
Field Trials to Compare Habitat Assessment Techniques
For 2017 field trials, we chose 14 sites from a set of randomly selected roadside sites in Minnesota that had been surveyed for milkweed and monarchs in 2015 (Figure 3; Kasten et al., 2016). We selected sites that contained milkweed in 2015. In 2018, we selected 15 new sites through the IMMP, which uses generalized random tessellated stratified sampling (GRTS) to identify random 10 x 10 km blocks and random point locations within them stratified by land use sector and prioritized to accommodate for variable inclusion probability (Cariveau et al., 2019). Sites in 2018 were randomly selected using the GRTS list of point locations; 13 sites were within the 15 highest ranked blocks in Minnesota (with vegetated roadsides at least 4 m wide) plus two additional sites within the 25 highest ranked blocks, for a total of 15 sites. Sites in 2018 also needed to have a minimum of 4 m width of vegetation in GIS preview for inclusion. Sites in both years represented variation in roadway types (except freeways which were excluded due to safety concerns).
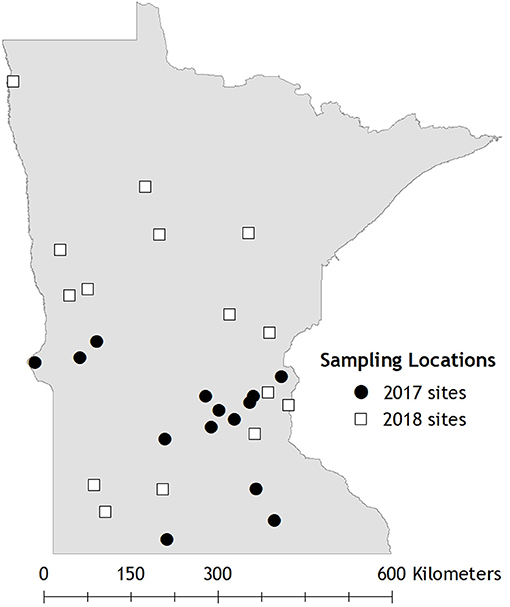
Figure 3. 29 field sampling locations in Minnesota where Rapid Assessments and Integrated Monarch Monitoring Program protocols were compared.
To account for the different sizes of the survey areas for each protocol, at each of these sites we completed one IMMP survey and typically three Rapid Assessments spaced 200–250 m apart within the footprint of the IMMP site (Figure 2). One site in 2017 had four Rapid Assessments and one site had only two; in 2018 three sites had only two Rapid Assessments.
Statistical Analyses
We calculated milkweed plants/ha based on the number of milkweed plants counted (all species combined) and the area searched at each site and converted to hectares. For the IMMP, the area searched was 100 m2 based on the 100 1-m2 quadrats. For the Rapid Assessment, the area searched was estimated as 45.7 m (the length of the plot) multiplied by the right-of-way width.
We present monarchs/plant as the sum of all monarch eggs and larvae observed, divided by the number of milkweed plants searched. For the IMMP protocol, the number of milkweed plants searched differed from the number of milkweed plants in the density estimate, because observers could search additional milkweed plants between the quadrats to look for monarch eggs and larvae. We focused analyses on sites with at least 10 milkweed plants examined by each method to ensure robustness of our monarchs/plant estimates (10 sites in 2017; 11 sites in 2018). We also estimated monarchs/ha by multiplying the average number of monarchs/plant times the average number of milkweed plants/ha using the IMMP method.
To represent nectar resource availability, we compared two indices: species richness and abundance. For species richness, we compared the number of blooming species. For the IMMP protocol, this is a list of all blooming species encountered in the quadrats. For Rapid Assessments, in 2017, we listed all of the blooming plant species encountered; in 2018, we identified all of the potentially blooming nectar plants and noted whether or not plants were blooming. Here we present the blooming subset to compare to the IMMP data. The nectar plant species lists across the several Rapid Assessments (RA) for each IMMP site were combined in two ways. First, the number of blooming species was determined for each RA, and then the number averaged across the several RA for each IMMP survey location; we call this RA averaged. Second, because of known relationships between species richness and area, we also depict the number of blooming species determined when summing the species across the RAs for each IMMP site (removing duplicates), which we call RA summed. For abundance, we compared the frequency of blooming nectar plants from the IMMP (number of quadrats out of 100 in which at least one blooming nectar plant was present) to percent cover by flowers from the Rapid Assessment, for 2018, the only year in which we estimated cover (averaged across the multiple Rapid Assessments per site).
We computed statistics using R version 3.5.1 (R Core Team, 2018). For milkweed plants/ha, and monarchs/plant, we compared the mean of the two to four Rapid Assessments to the IMMP measure for each site. To determine if protocol type had a significant effect on response variables, we ran generalized linear mixed models with year and protocol type as fixed effects and site as a random effect for each of the response variables of milkweed density, monarchs/plant, and number of blooming species (“nlme” package; Pinheiro et al., 2018). We report an interaction term for year and protocol type when significant. The sample size was 113 visits to 29 sites for the plant data; because we found no milkweed plants during 17 visits, the model for monarchs per plant contained 96 visits to 29 sites. For number of blooming species, we compared the estimates by the IMMP protocol to the RA averaged and RA summed in a generalized linear mixed model with year and protocol type as fixed effects, site as a random effect, and a year by protocol type interaction effect. For clarity, we also compare the numbers of blooming species by the IMMP protocol to the RA averaged and RA summed for each year separately. For milkweed density, monarchs/plant, nectar plant richness, and nectar plant abundance, we also compared the mean of the Rapid Assessments per IMMP site to the IMMP measure with a correlation coefficient. If variables met the Shapiro-Wilk normality test for normality, we computed a Pearson correlation; if they did not, then we used a Kendall rank correlation. We plotted data in Excel and ggplot2 (Wickham, 2016).
Results
Manager Survey Results
We received 79 responses to the survey; with respondents representing states (58%), counties (25%), regional or national entities (8%), local entities (9%), and other entities (5%) in19 states: Arizona, Arkansas, California, Illinois, Indiana, Iowa, Kansas, Maryland, Michigan, Minnesota, Nebraska, New Hampshire, Ohio, Oklahoma, South Dakota, Texas, Virginia, Washington, and Wisconsin. Respondents from 14 (74%) of the states from which we received responses indicted that they had a pollinator program. We asked if managers would like guidance about where to install or manage monarch habitat, tools for monitoring that habitat, or both. Of 33 respondents to this question, 39% wanted monitoring methods, 12% indicated that the planning information would be most valuable, and 39% wanted both (9% had other answers). We report their answers to questions about capacity for field work and management practices in Table 2.
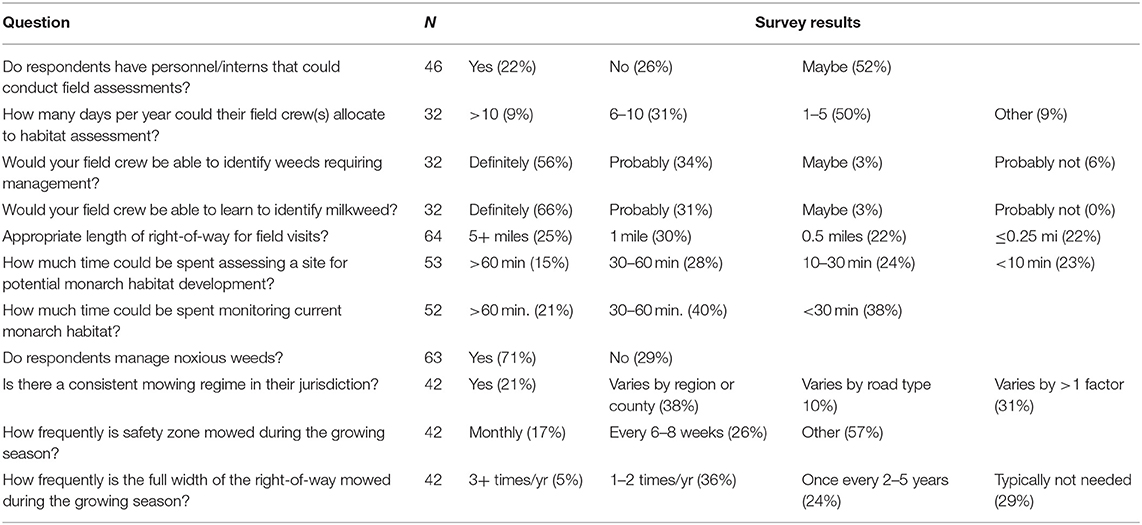
Table 2. Responses by roadside managers to questions regarding roadside vegetation assessment and management (N, number of respondents).
Field Surveys
In 2017, we assessed 14 sites between June 29 and August 22. All sites were located along paved roads, eleven along 2-lane roads, and three along 4-lane roads. Eight sites were adjacent to cropland, with two sites each by woodland, grassland, and developed land. Right-of-way widths from the Rapid Assessments varied from 3 to 21.5 m (mean = 12.35 m, standard deviation (SD) = 3.71); widths were not recorded by the IMMP protocol in 2017.
In 2018, we surveyed 15 sites between July 23 and August 29; all sampled sites were along two-lane roads; 12 were paved; and three were dirt/gravel. In 2018, adjacent land uses included: cropland (7), woodland (3), grassland (2), and wetland (3). The widths of the rights-of-ways by Rapid Assessments varied from 5 to 52 m (mean = 14.07, SD = 12.79). The average width of IMMP rights-of-way in 2018 was 9.43 m (SD = 3.70, range 3.5–19.5 m).
Single Rapid Assessments took an average of 22 min in 2017 (SD = 15 min; range 4–88 min) and 20 min in 2018 (SD = 12 min; range 5–59 min). IMMP visits took 134 min on average (SD = 67 min; range 68–345 min) in 2017 and 167 min in 2018 (SD = 56 min; range 92–274 min). Variation in the duration of visits was affected by the number of nectar plant species present and the number of milkweed plants counted and examined for monarch eggs and larvae.
Milkweed Density
We detected milkweed at all sites in 2017 and 14 of the 15 sites (93%) in 2018 using the IMMP protocol. The vast majority of milkweed was Asclepias syriaca (common milkweed; 96%); other species were A. incarnata (swamp milkweed, 3%), A. verticillata (whorled milkweed, 0.69%), A. sullivantii (Sullivant's milkweed, 0.2%), and A. tuberosa (butterfly weed, 0.01%). The mean milkweed density for all species of milkweed combined using the IMMP protocol was 1,242 plants/ha (SD = 1,303) in 2017, 2,807 plants/ha (SD = 4,864) in 2018, and for both years combined: 2,052 plants/ha (SD = 3,639; median = 800; range 0–18,000) (Figure 4A). Averaging the RAs per site, the mean milkweed density for all species of milkweed across sites in 2017 was 1,508 plants/ha (SD = 2,082), 1,545 plants/ha (SD = 2,377) in 2018, and 1,527 plants/ha for years combined (SD = 2,199; median = 625; range 0–8,966). Milkweed density did not vary with year (t27 = 0.415, p = 0.681) or survey type (t83 = −0.639; p = 0.524, df = 83). Milkweed density as estimated by the two protocols was correlated (Kendall's rank correlation tau = 0.568, z = 4.257, df = 27, p < 0.001; see Figure 5A).
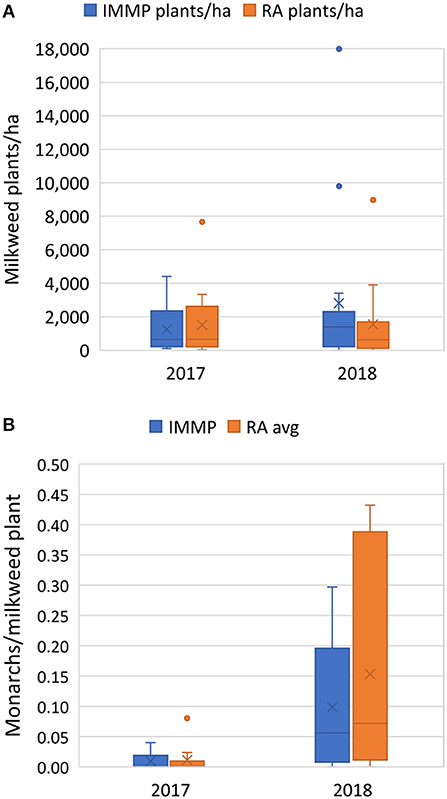
Figure 4. (A) Mean milkweed density (plants/ha) and (B) mean monarch eggs and larvae per milkweed plant examined for 29 roadside rights-of-way sites in Minnesota sampled in 2017 and 2018, comparing years and two sampling methodologies, the Integrated Monarch Monitoring Program (IMMP) and averaged values for 2–4 Roadside Habitat for Monarchs Rapid Assessment (RA) taken from the same sampling location. Milkweed density did not vary by protocol type (t83 = −0.639; p = 0.524, df = 83) or by year (t27 = 0.415, p = 0.681). Monarchs/plant did not differ by protocol type (t66 = 0.118; p = 0.906) but year was a significant factor (t27 = 2.373, p = 0.025). Mean values are indicated by the “x”; median values by a horizontal line, boxes indicate 25 and 75% quartiles, bars indicate the upper and lower quartiles, and outliers more than 1.5 the 75% quartile are depicted by dots.
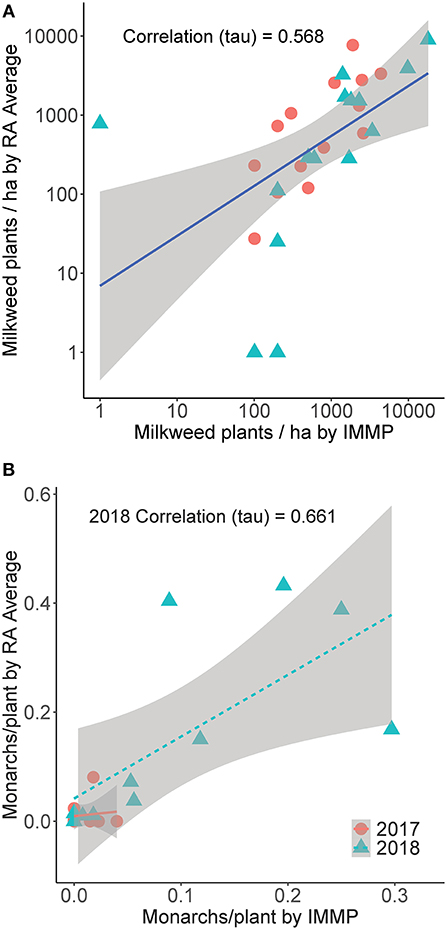
Figure 5. (A) Comparison of milkweed density (milkweed plants/ha), log10 transformed, for sites monitored in 2017 (circle) and 2018 (triangle), using the Integrated Monarch Monitoring Program (IMMP) and Roadside Habitat for Monarchs Rapid Assessment (RA) averaged for each site. 95% confidence interval indicated in gray. (B) Monarch eggs and larvae per milkweed plant searched (monarchs/plant), log10 transformed, for sites monitored in 2017 (circle) and 2018 (triangle), for the same two methodologies. 95% confidence interval indicated in gray. The correlation between techniques for 2017 was non-significant.
Monarch Eggs and Larvae
The mean number of milkweed plants searched for monarch eggs and larvae in 2017 was 40.93 (SD = 47.66) with the IMMP and 76.11 (SD = 91.15) with the RA. In 2018 the mean number of milkweed plants searched for monarch eggs and larvae was 113 (SD = 134.48) with the IMMP and 36.27 (SD = 44.38) with the RA. In 2017, using the IMMP method, monarch eggs or larvae were found at 6 of 14 sites (43%); with the RA monarch eggs or larvae were found at 7 of 14 sites (50%). In 2018, using the IMMP method or the RA, monarch eggs or larvae were found in 11 of 15 sites (73%), or in 11 of 14 sites containing milkweed (79%). If considering RAs independently from one another, then in 2017, monarch eggs or larvae were found in 11 of 42 (26%) RAs or 11 of 37 (30%) sites with milkweed, and in 2018, monarch eggs or larvae were found in 19 of 42 (45%) RAs or 19 of 30 (63%) sites with milkweed.
For monarchs/plant, year was a significant factor (t27 = 2.373, p = 0.025) with more eggs and larvae found in 2018 than 2017, but protocol type did not have a significant effect on estimates of monarch density (t66 = 0.118; p = 0.906; Figure 4B).
When restricting analysis to sites with at least ten milkweed plants examined by each protocol, in 2017, monarch egg or larvae were found at 40% of the sites with the IMMP protocol and 50% with the RA protocol (summed per site; 10 sites). At five sites monarchs were found with the RA protocol but not by the IMMP; at three sites monarchs were detected by the IMMP but not by the RA. In 2017, the mean number of monarchs/plant with the IMMP protocol was 0.010 (SD = 0.014) and 0.011 (SD = 0.025) with the RA (Figure 4B). In 2017, the monarchs/plant estimated by the two protocols were not correlated (Kendall's rank correlation tau = −0.216; z = −0.762, p = 0.446; Figure 5B).
In 2018, monarch eggs or larvae were found at 82% of the sites with the IMMP protocol and 91% with the RA (summed per site; 11 sites); on one site monarchs were found with the RA method but not by the IMMP. In 2018, the mean number of monarchs/plant was 0.099 (SD = 0.105) with the IMMP and 0.153 (SD = 0.173) with the RA (Figure 4B). In 2018, monarchs/plant measured with the two protocols were correlated (Kendall's rank correlation tau = 0.661, z = 2.81, p = 0.005; Figure 5B).
An estimate of the average number of monarch eggs and larvae per ha, using the overall IMMP mean was 115 monarchs/ha (2,052 plants/ha*0.056 monarchs/plant) across both years. Separating the 2 years, for 2017, the estimate was 12 monarchs/ha (1242*.010) and for 2018, 253 monarchs/ha (2807*.099). Using RA averages, the overall estimate was 131 monarchs/ha (1527*0.086); for 2017 it was 17 monarchs/ha (1508*0.011) and 2018 it was 236 (1545*0.153).
Blooming Nectar Plants
The average number of blooming species per site in 2017 was 6.71 (SD = 4.50, range 1–18) with the IMMP protocol, 6.72 (SD = 2.56, range 1–12.33) with RA averaged, and 12.14 (SD = 4.45, range = 5–19) with RA summed (Figure 6). In 2018, the average number of blooming species per site was 10.40 (SD = 6.40, range = 1–23) with the IMMP protocol, 6.57 (SD = 2.85, range 2–11.33) with RA averaged, and 12.00 (SD = 5.35, range = 1–20) with RA summed (Figure 6).
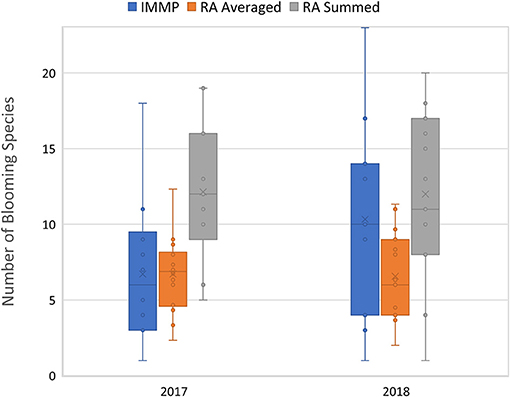
Figure 6. Mean number of blooming species as estimated by the Integrated Monarch Monitoring Program (IMMP), averaging across Rapid Assessments (RA averaged) per IMMP site, and summing across Rapid Assessment (RA summed) per IMMP site. Number of species did not differ by protocol type (t82 = −0.047; p = 0.963) but there was an effect of year (t27 = 2.33, p = 0.027) and protocol type by year interaction (t82 = −2.86; p = 0.005). Mean values are indicated by the “x”; median values by a horizontal line, boxes indicate 25 and 75% quartiles, and bars indicate the upper and lower quartiles.
Comparing the number of blooming species by IMMP to the RAs (taking each RA independently as in milkweed and monarch analyses), the significance of the factors in the model was as follows: year (t27 = 2.33, p = 0.027), protocol type (t82 = −0.047; p = 0.963), and protocol type by year interaction (t82 = −2.86; p = 0.005). In 2017, the number of blooming species estimated by IMMP did not differ from the RA averaged (t26 = 0.007, p = 0.995), but was lower than the RA summed (t26 = 6.247, p < 0.001). In 2018, for the same comparison, IMMP results did not differ from RA summed (t28 = 1.532, p = 0.136), but were higher than RA averaged (t28 = −3.463, p = 0.002).
In 2017, the number of blooming species by IMMP protocol was correlated with RA averaged (Pearson's r = 0.706, t12 = 3.453, p = 0.005) and RA summed (Pearson's r = 0.690, t12 = 3.302, p = 0.006; Figure 7A). In 2018, the number of blooming species by IMMP protocol was correlated with RA averaged (Pearson's r = 0.801, t13 = 4.829, p < 0.001) and RA summed (Pearson's r = 0.698, t13 = 3.515, p = 0.004; Figure 7B).
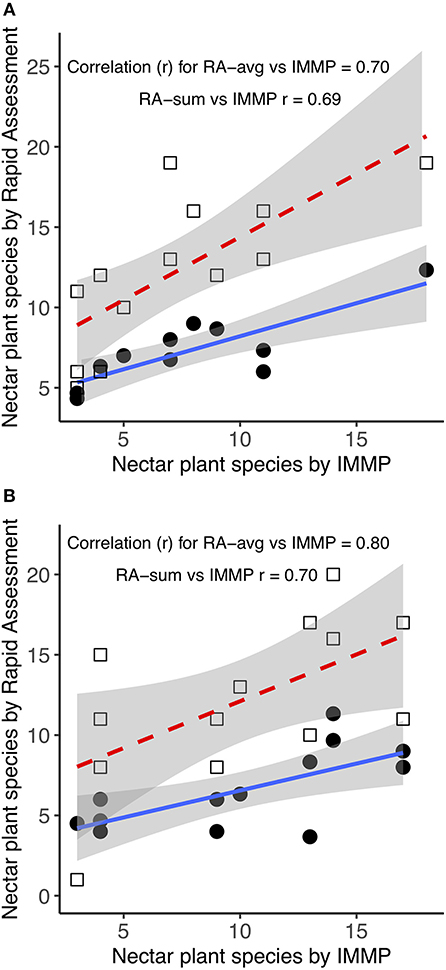
Figure 7. Number of blooming plant species in 2017 (A) and 2018 (B) comparing data by averaging from several Rapid Assessments (RA averaged, in orange) or when summed across the Rapid Assessments (RA summed, in gray), compared to the number derived from the Integrated Monarch Monitoring Protocol (IMMP) for each site.
The correlation of the estimate of percent cover by blooms in the Rapid Assessment plots (percent cover classes converted to midpoints, then averaged per site) to the frequency of nectar plants based on the IMMP method was 0.53 (Kendall's tau; n = 14, z = 2.477, p = 0.013; Figure 8).
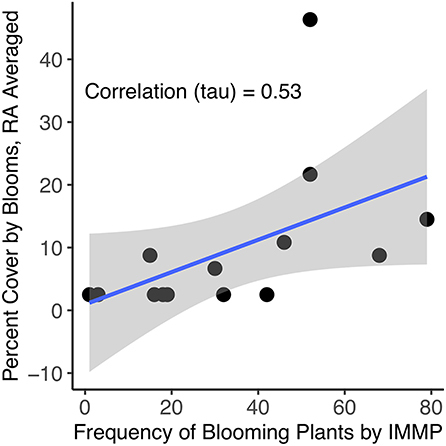
Figure 8. Comparing frequency of nectar plants to estimate of percent cover by blooms in the RA plots, averaged per site, for 2018 sites (n = 14); percent cover classes were converted to midpoints prior to averaging. Correlation (tau) = 0.53 (z = 2.477, p-value = 0.013).
Discussion
We designed and tested a Rapid Assessment protocol for monarch habitat within roadside rights-of-way. Observers focus on a small length along the roadway to count milkweed plants and types of nectar plants and estimate cover of nectar plants and noxious weeds. Rapid Assessment data are automatically calculated into habitat quality scores that provide information to managers about their habitat resources, enable them to compare conditions across sites, and inform their management decisions. This is similar to other applications of simple vegetation assessment methods to support natural resource management. One example is identifying groups of plants of particular interest, such as cool-season grasses, in a 25 m x 0.01 m belt transect (Grant et al., 2004) to provide data inputs for a decision support tool for adaptive management of native prairie (Hunt et al., 2016). Pywell et al. (2011) combined vegetation metrics and management information including seed mix and mowing practices to accurately predict use by bees and butterflies in the United Kingdom.
This project furthers conservation efforts for monarch butterflies by creating a tool that is tailored to the needs and preferences of state departments of transportation that manage an estimated 17 million acres of potential habitat for monarchs (Ament et al., 2014). Great attention has come to rights-of-ways for their ability to provide habitat for monarchs, such as the effort to provide habitat in roadside and energy rights-of-way through a National Candidate Conservation Agreement with Assurances (CCAA; Cardno, 2019). As an indication of the interest in this project, personnel at the Delaware Department of Transportation implemented the Rapid Assessment at nearly 100 locations in the summer of 2018 to learn about monarch habitat along their roadways.
Through a survey and field visits with transportation personnel, we learned that a flexible survey design was needed to meet the departments' wide range of needs. We designed the assessment in Esri software typically used by transportation departments so that states could customize their assessments. For instance, some field staff are knowledgeable about vegetation and would like to quantify not only the number of nectar plant species present but also how many are native. Others are only able to quantify numbers of plants that look distinct from one another; we created a convenient lookup table from which a surveyor can pick plants from their state by either common or Latin names or simply tally unknown types. Departments differ also in the tracking of noxious weeds, from no tracking to extensive lists of species that differ state to state and sometimes by counties or bioregions within states, so we enabled the ability for road managers to specify a list of species they wish to track. Because managers indicated that only a limited number of days and people would be available for assessments, we designed a survey to be conducted once per growing season. To accommodate the single yearly sample, we created the term “potentially blooming nectar plants” to represent all of the plants that could provide nectar for monarchs and other pollinators, regardless of whether they were blooming on the date of the survey. This is consistent with a pollinator scorecard being designed by the Rights-of-way as Habitat Working Group of the Energy Resource Center at the University of Illinois-Chicago (A. Cariveau personal communication).
This Rapid Assessment fits into a suite of habitat assessment tools for monarch butterflies but is the only one designed for ready application in the roadside context with specific consideration of the needs and constraints of transportation managers. The Natural Resources Conservation Service (NRCS) Monarch Butterfly Wildlife Habitat Evaluation Guides (WHEG) and Decision Support Tools5 are designed to provide a qualitative rating of current monarch habitat and assess habitat management alternatives for working agricultural lands (USDA NRCS, 2018). The WHEG similarly focuses on milkweed presence and species richness of nectar producing plants, while focusing on specific plants known to be used by monarchs. The Habitat Quantification Tool is used to evaluate the quality of monarch habitat for protection and enhancement in a variety of land-use contexts including roadside rights-of-way (Environmental Defense Fund, 2017), but it is more time consuming to implement than the Rapid Assessment. Programs such as the Western Monarch and Milkweed Mapper6 use metrics similar to those in our protocol (e.g., milkweed counts and monarch presence) but rather than characterizing particular locations, the goal is enhanced understanding of the distribution of monarchs and their habitats to inform conservation, like the Integrated Monarch Monitoring Program (Cariveau et al., 2019).
Our testing results suggest that the Rapid Assessment provides a standardized and rapid way to describe habitat conditions for monarch butterflies in roadside rights-of-way and produces similar results to those of a more intensive protocol. Outcomes from other rapid assessments are mixed. A rapid technique for characterizing habitat in agricultural fields predicted the overall abundance and richness of butterfly species in Britain, though performed less well for predicting occurrence of some particular species (Pywell et al., 2004). For the Fender's blue, rapid assessment of vegetation did not align with more detailed assessments of host and nectar plant availability in determining habitat suitability for this rare butterfly (Schultz and Dlugosch, 1999). Our study did not focus on relating use by monarchs to the habitat, but rather on habitat availability and the ability for the rapid assessment to concur with a more intensive quantification method, the IMMP. The IMMP is designed to track changes in habitats throughout seasons and across years and to compare monarch habitat quality and use across land use sectors. The IMMP collects additional data, including a quantitative survey for adult monarchs and nectar plant diversity, and could be used to address questions such as whether monarchs prefer particular nectar plant species. However, our results suggest that for assessing and comparing rights-of-way habitat to inform roadside habitat restoration and management for monarchs and other pollinators, the Rapid Assessment produces sufficiently similar results.
It should be noted that we only compared the Rapid Assessment and the IMMP within the range of the eastern monarch population. However, we developed the Rapid Assessment with input from road managers in both western and eastern states. While little is known about how the relationship between monarchs and their habitats may differ between the two main North American populations, we expect that the Rapid Assessment should effectively depict habitat conditions across roadside sites in western states. Individual state managers may adjust and customize the Roadside Monarch Habitat Evaluator tool for appropriate plants and scoring for their bioregions. Given the recent population levels of the western monarch (Pelton et al., 2019), we encourage use and adjustment of these tools to learn more about habitat availability and use by monarchs in western roadsides.
The Rapid Assessment is efficient; our two-person field crew completed assessments in an average of 21 min, including time spent looking for monarch eggs and larvae, which many departments of transportation will elect to skip. It was much faster than the IMMP even when repeating the protocol three times over the footprint of the IMMP (sum of 62 min as compared to an average duration of 2 ½ h). The Rapid Assessment also appears easier to learn and may be spread out to sample from a larger landscape in the same amount of time as the IMMP. In 1 day, a crew can complete 10–15 assessments. Experienced crews, after learning how to identify the plants in the rights-of-way, are likely to be faster than employees who are conducting assessments for the first time, but observers typically become faster through practice.
There may be concerns about whether road crews could effectively collect the data required for the Rapid Assessment. However, volunteers with no formal research training have effectively contributed biological data to a multitude of programs, such as the Breeding Bird Survey, which has produced excellent information about the status and trends of North American birds (Hudson et al., 2017). Citizen scientist contributions were instrumental in years of research on butterflies in Britain (Roy et al., 2007) and in building a butterfly database in Florida (Jue and Daniels, 2015). For monarchs, citizen scientists have had a long history of contributing to research (Howard and Davis, 2009; Ries and Oberhauser, 2015), including a recent analysis of the population status of western North American monarchs (Schultz et al., 2017). In some studies, volunteer data were compared to data collected by researchers or by a more rigorous method. A study of stream monitors found high concurrence of data collected by volunteers and paid researchers (Fore et al., 2001), and collection of terrestrial invertebrate diversity data by volunteers and researchers were similarly satisfactory (Lovell et al., 2009). In contrast, volunteers were not very successful in identifying stream macroinvertebrates (Nerbonne and Vondracek, 2003). A comparison of pollinator data from citizen scientists and researchers found similar trends in detection for higher level bee taxa but not for detections of all species (Kremen et al., 2011). Thus, fine-scaled species identification is typically more difficult for non-researcher observers, but this should not pose a problem for roadside assessments that only rely on distinguishing types of plants and optionally identifying one distinctive butterfly. In particular to our protocol, volunteers successfully collect similar data on milkweed, monarchs, and nectar plants in the Monarch Larva Monitoring Project (MLMP)7 and the IMMP. Furthermore, our field-testing of the protocol with three departments of transportation indicated that their personnel could effectively collect these data.
The Rapid Assessment was effective for measuring milkweed density, nectar plant species richness, and for monarch eggs and larvae per plant in 2018 (the measures were not significantly correlated in 2017 when monarch detections were low). In general, averaging parameter estimates for multiple Rapid Assessments yielded more consistent results than any single Rapid Assessment from sites, suggesting that combining multiple Rapid Assessments to characterize areas is preferred over single samples. This is similar to a comparison of rapid qualitative score to quantitative scores of vegetative condition, where there was broad association in the scores across many sites, but rapid assessments were not reliable at the level of a specific site (Cook et al., 2010). Furthermore, this underscores our recommendations that managers sample multiple sites. In particular, we note that it is important for managers to pre-select random or systematic (e.g., every km or 1/2 km) sampling locations to effectively characterize larger areas without bias from sampling in locations where habitat quality is known to be or appears to be high.
When averaging Rapid Assessments across several sites, milkweed density estimates were not statistically different than those derived by the IMMP protocol, and the estimates by the two methods were correlated across sites (tau = 0.568; Figure 5A). Numbers of species of blooming nectar plants also were highly correlated between survey protocol types (r = 0.69–0.80, depending on the comparison; Figure 7). Differences between protocols likely reflect the patchiness of common milkweed, which often grows in clonal patches, as well as many nectar plants, rather than undesirable biases in either method. Due to this patchy distribution of milkweed across the landscape, the spatial distribution of quadrats sampled with the IMMP protocol (spread over 500 m) was more reliable for detection of milkweed than a single Rapid Assessment (50 m), although milkweed detection was similar when combining the several Rapid Assessments per site (two or three 50 m widths spread across 500 m). Also, we had predicted that the IMMP likely provided more accurate estimates of milkweed density by focusing observer attention into small areas, but the estimates obtained by the Rapid Assessment were similar. While the two assessments are not perfectly correlated, they result in a similar categorical quality ranking of habitat sites that would be relevant for management decisions. For example, managers could differentiate high-quality sites that would benefit from preservation, moderate sites that could benefit from enhancement, and low-quality sites that would be cost prohibitive to improve or might be good sites for full re-seeding.
The high milkweed density documented in this study in Minnesota (2,052 plants/ha by IMMP (834 plants/ac); 1,527 plants/ha (620 plants/ac) by Rapid Assessment) confirm that roadside rights-of-way can provide significant amounts of breeding habitat for monarchs (Kasten et al., 2016). And, adult monarch numbers are associated with percent cover of milkweed (Kinkead et al., 2019) and milkweed abundance has been associated with adult monarch abundance (e.g., Zalucki and Lammers, 2010; Pleasants and Oberhauser, 2013). Converted into linear miles of interest to road managers, for the average right-of-way width we surveyed (9.43 m), this is 2,316–2,641 milkweed plants/mile (using the range of IMMP and RA estimates). The 2017 milkweed density estimate could have been inflated due to the fact that we selected sites from a set that contained milkweed in a prior study, but the 2018 average milkweed density was higher and these sites were selected through a random process. These milkweed densities are higher than other studies in the upper Midwest (508 plants/ha, Kasten et al., 2016; 141 plants/ha, as converted from Hartzler and Buhler (2000) in Thogmartin et al. (2017b) and used to estimate levels in current roadside rights-of-way). However, our sample size was small and we did not sample all types of roads, such as those in developed areas that do not typically provide habitat or those that appeared to be <4 m wide when previewed online. Overall estimates of habitat availability must take into account different rights-of-way types and potential variation by region; data collected from more locations are needed in ongoing assessments of monarch habitat availability.
The levels of monarch use for reproduction suggest these roadside rights-of-way can serve a significant function for breeding habitat. The per plant density of monarch eggs and larvae ranged from 0.01 monarchs/plant in 2017 to 0.099 in 2018 (IMMP protocol), bracketing the 0.059 reported for roadsides by Kasten et al. (2016) and 0.043 eggs/plant reported by Nail et al. (2015); from Monarch Larva Monitoring Project data from non-roadside areas, primarily gardens.
We detected a strong difference among years in monarch egg and larval abundance, which is not surprising given high inter-annual variation in monarch numbers (Thogmartin et al., 2017a). In 2017, when monarch numbers were low, the two survey methods did not correlate well. In fact, monarchs were not detected with one or the other of the techniques in eight of ten sites. However, in 2018 when monarchs were detected at a higher rate, the two methods were correlated (tau = 0.661). Our findings suggest that single visits to describe monarch use are unreliable in years with lower monarch numbers. This coincides with recommendations from MLMP and IMMP to conduct monarch use surveys weekly. For roadside managers or others constrained to single visits, monarch use data may be regarded as descriptive rather than quantitative (i.e., eggs or larvae indicate breeding but their absence is not meaningful). Managers must be aware that monarch use data from 1 year may not be representative of other years. Furthermore, as monarch abundance also fluctuates within seasons, surveys should be conducted during similar dates within the season to compare monarch use among sites. If monarch use is a primary focus for a roadside manager, collecting data from repeat surveys within a year and across multiple years would greatly improve information about monarch use.
Similarly, we found inter-annual variation in nectar plant metrics, but only using the IMMP method, which may have been due to several factors. First, different sites were visited each year by different field crews. Secondly, the IMMP method recorded only plants blooming at the time of survey, which varies throughout the season, another factor that was not controlled for. The Rapid Assessment technique will be more resilient to seasonal effects because it includes all potentially blooming plants, including those that have already bloomed or will bloom after the survey. Because it is generally easier to identify plants when they are blooming, we recommend that surveys be conducted in peak blooming season within the period(s) of time when monarchs are present (usually mid- to late-summer), to facilitate identification, or at least differentiation, of plant species. Best practices will be to minimize variation by comparing habitat quality scores from visits to sites within the same year and season. For vegetation, it is likely that surveys may be done periodically, every several years, while for monarch use, extrapolation across years would be less representative.
The presence of late instar larvae indicates that monarchs are developing in these habitats. Providing more milkweed dispersed across the landscape may improve monarch larval survival in lower density patches of milkweed (Zalucki and Kitching, 1982), and having access to milkweed across the landscape should increase the number of eggs females lay (Zalucki and Lammers, 2010; Zalucki et al., 2016; Grant et al., 2018). However, monarch eggs and larvae experience high levels of mortality due to predation, weather, disease, and other factors (Nail et al., 2015). Additionally, milkweed in roadside areas may support lower densities of monarchs than milkweed found in adjoining agricultural habitats (Pitman et al., 2018), although it is not known if these patterns reflect differences in habitat quality or other factors, such as behavioral responses to linear landscape features or opportunistic use of the few milkweed plants remaining in an agricultural matrix dominated by genetically-modified crop fields treated with glyphosate. Therefore, more information about the survival of monarch eggs and larvae in roadside habitats compared to other habitat types will be important for assessing the relative benefits of roadside habitat for producing monarchs.
The species richness of blooming nectar plants in each small roadside site ranged up to 18 species, suggesting roadside areas could serve an important function in providing foraging resources for pollinators. Flowering plant diversity is associated with greater frequency of visits by pollinators and pollinator diversity (Potts et al., 2003; Ebeling et al., 2008) and increases the likelihood of nectar availability throughout the season of monarch use. Also, the frequency of blooming plants ranged up to 79% of plots occupied, with estimates of the area covered by flowers as high as 46%. Floral display is well known to relate to pollinator use (e.g., Hegland and Totland, 2005; Gunnarsson and Federsel, 2014). In restored mine sites, nectar plant diversity and nectar abundance related to butterfly numbers and diversity (Holl, 1995), and similarly in roadsides in England, abundance of flowering plants was related to butterfly richness (Munguira and Thomas, 1992). While the Fender's blue was associated with the availability of native plant nectar sources (Thomas and Schultz, 2015), in many studies butterflies appear to be generalists, for instance using many nectar sources regardless of sugar content (Pavlik et al., 2018). In an experimental study of pollinator gardens, butterfly use increased with number of flowering plants; monarchs nectared on non-native flowers more than native (Majewska et al., 2018). In particular, monarchs may be limited by access to nectar in the fall that is critical for gaining lipids sufficient for successful overwintering (Brower et al., 2006; Inamine et al., 2016). Indeed, greater numbers of fall migrant monarchs were found in association with greater abundances of flowers on fire-restored pine-grasslands than on control sites or those more than 3 years since burned in Arkansas (Rudolph et al., 2006).
Our approaches to describing nectar availability were limited; practices such as counting and measuring flowers, and measuring nectar quantity and quality in them, would be much more informative (e.g., Denisow et al., 2014; Hicks et al., 2016; Szigeti et al., 2016, 2018). However, these do not fit within the constraints of a rapid assessment. Additional research that relates more intensive measures of nectar availability to simpler indices would also be helpful to many future studies of pollinator habitat. Work has been done on the relative nutritional value (e.g., sugars, amino acids) of different nectar sources (e.g., Gottsberger et al., 1984; Baker and Baker, 1986; Abrahamczyk et al., 2017). However, for monarchs in particular, few quantitative studies investigate relative use or nutrition of different nectar sources (Malcolm, 2018), which could vary among years, locations, and with environmental conditions. The Nectar Plant Guides produced by USDA NRCS and The Xerces Society report species used by monarchs; species reported as of “outstanding value” or mentioned by multiple sources were rated “very high”; species “cited as attractive to monarchs but with less frequency” were rated “high” (USDA NRCS, 2016). Further work on preference and nutritional value (including pyrrolizidine alkaloids used in pheromone production; Boppré, 1990) of various types of nectar sources for monarchs would help to guide conservation efforts.
While our results and a handful of previous studies highlight the promise of roadsides as monarch habitats, these areas also bring a suite of threats to monarchs and other pollinators including collisions with vehicles and chemical inputs (Skorka et al., 2013; Snell-Rood et al., 2014; Keilson et al., 2018; Pitman et al., 2018). However, larger butterflies such as monarchs may sustain a lower rate of mortality from car collisions than smaller butterflies (Skorka et al., 2013). Furthermore, mortality from cars is lower in roadside habitats with certain characteristics, such as greater plant species richness (Ries et al., 2001; Skorka et al., 2013). The width of the right-of-way habitat as well as the composition of adjacent lands also may affect collision mortality rates, such that wider habitats with greater access to adjoining habitats may reduce collision mortality (Munguira and Thomas, 1992; Skorka et al., 2013, but see Saarinen et al., 2005). In addition, collision risk appears greater in areas where monarchs funnel together during migration, such as in southern Texas and northern Mexico (Kantola et al., 2019; Tracy et al., 2019). Chemicals, including sodium and heavy metal run-off from roadways, are incorporated into roadside vegetation (Snell-Rood et al., 2014; Munoz et al., 2015). These chemicals could affect the development of monarch eggs and larvae or even affect adults through contamination of nectar resources. Further study of roadside areas to profile monarch egg and larval survival in relation to chemical or traffic-induced mortality would allow better understanding of how roadside habitats perform as monarch breeding areas.
Roadside management authorities are becoming aware of the impact of management policies on roadside habitat, and exemplary programs with deferred mowing, re-establishment of native plants, control of noxious weeds, and integrated vegetation management occur around the country. Mowing, in particular, is a complex topic. This ubiquitous management action is required to provide safety in roadside rights-of-ways and can be instrumental in the control of invasive plants. However, mowing can harm animals inhabiting mowed areas (Dale et al., 1997; Johst et al., 2006; Cizek et al., 2012) and can reduce floral cover (Halbritter et al., 2015) or cover by native plants (Entsminger et al., 2017). Indeed, reduced mowing during the monarch breeding season is recommended to reduce direct mortality for monarch eggs and caterpillars and to preserve more plant blooms as nectar sources (Monarch Joint Venture, 2019). On the other hand, a single mowing of milkweed in early-mid growing season can increase oviposition by monarchs (Haan and Landis, 2019; Knight et al., 2019). More information about the effects of deferred mowing practices on nectar availability and the prevalence of invasive species is needed. Several of the managers advising this project expressed their interest in recording the effects of new mowing practices on the habitat in their roadside rights-of-ways. The Roadside Monarch Habitat Evaluator allows managers to track milkweed, nectar plants, and monarchs under different management, such as comparing mowed and unmowed portions of their rights-of-way. Data about monarch habitat quality will help managers to make management decisions to benefit monarchs and pollinators generally. Challenges remain in balancing the multiple management needs for rights-of-way and communicating the benefits of native, uncut vegetation to shift public preferences for well-manicured turf grass along roadways. Future research on optimal mowing regimes and effects on milkweed, nectar availability, and use by monarchs continue to be particularly pertinent.
Because of the importance of the breeding season to the monarch annual cycle (Oberhauser et al., 2017), the strong connection between habitat loss in the core of the eastern population's breeding range and low monarch numbers (Thogmartin et al., 2017a), and use of roadsides for monarch breeding (Kasten et al., 2016), roadside restoration and management of existing habitat is promising for monarch conservation. Furthermore, roadside areas managed for monarch habitat provide native plants that could benefit other wildlife, such as small mammals, birds, pollinators and other beneficial insects. Ongoing communication and research around the potential conservation benefits of well-managed roadside rights-of-way will be highly beneficial.
Author Contributions
AC directed the study, conducted data analysis, and led the writing of the manuscript. EA, KB, JH, EL, CN, KO, KT, and ES-R advised on the design of the project. KO was involved with the inception of the project. ES-R later became the primary investigator overseeing the project at the University of Minnesota. KB collected data using the Rapid Assessment for another dataset. KT designed and implemented the data collection system in Survey123.
Funding
This work was funded by the National Cooperative Highway Research Program of the Transportation Research Board, grant 20-119 Evaluating the Suitability of Roadway Corridors for Use by Monarch Butterflies. The Snell-Rood lab is additionally supported by funding through the Environment and Natural Resources Trust Fund as recommended by the Legislative- Citizen Commission on Minnesota Resources.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The project benefited greatly from contributions by the advisory panel, research advisors, and roadside managers at departments of transportation who assisted us throughout the project. For interviews, we thank Rob Roman (Linn County, Iowa), Kayti Ewing (Arkansas DOT), Dan MacSwain (Washington County, MN), and Stephanie Dobbs (IL DOT). For field visits, we thank Chris Smith and Tina Markeson at MNDOT, Stephanie Dobbs and Susan Hargrove at ILDOT, and Alyssa Barette and Christa Schaefer at WISCDOT. We appreciate Nicholas Haas, Alexandra Grace Haynes, and Patrick Perish for their dedicated work in collecting high quality field data, Daniel Cariveau for statistical assistance, and Holly Holt, Wendy Caldwell, Kyle Kasten, and Laura Lukens for their input into the design and execution of the project. We thank Iris Caldwell and Klaudia Kuklinski at the Energy Resources Center (University of Illinois – Chicago) for their roles in facilitating the review of other pollinator habitat rating systems.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00386/full#supplementary-material
Footnotes
1. ^https://www.fws.gov/savethemonarch/MCD.html
2. ^https://monarchjointventure.org/immp
3. ^https://monarchjointventure.org/roadsidehabitat
4. ^Qualtrics Version 12/17 © 2107; Available online at: https://umn.ca1.qualtrics.com/
5. ^https://www.nrcs.usda.gov/wps/portal/nrcs/detail/national/plantsanimals/pollinate/?cid=nrcseprd402207
References
Abrahamczyk, S., Kessler, M., Hanley, D., Karger, D. N., Meuller, M. P. J., Knauer, A. C., et al. (2017). Pollinator adaptation and the evolution of floral nectar sugar composition. J. Evol. Biol. 30, 112–127. doi: 10.1111/jeb.12991
Alcock, J., Brower, P., and Williams, E. H. (2016). Monarch butterflies use regenerating milkweeds for reproduction in mowed hayfields in northern virginia. J. Lepidopterists Soc. 70, 177–181. doi: 10.18473/107.070.0302
Ament, R., Begley, J., Powell, S., and Stoy, P. (2014). Roadside Vegetation and Soils on Federal Lands-Evaluation of the Potential for Increasing Carbon Capture and Storage and Decreasing Carbon Emissions. Report for the Federal Highway Administration, # DTFH68-07-E-00045.
Anderson, E. T., Oberhauser, K. S., Stenoien, C., Caldwell, W., Nail, K. R., Wolfe, D., et al. (2017). Monarch Habitat Quantification Tool Specifications Document. Environmental Incentives, LLC. Available online at: http://monarchhabitatexchange.org/about/resources (accessed June 27, 2019).
Baker, H. G., and Baker, I. (1986). The occurrence and significance of amino acids in floral nectar. Plant System. Evol. 151:175. doi: 10.1007/BF02430273
Baum, K. A., and Mueller, E. (2015). “Grassland and roadside management practices affect milkweed abundance and opportunities for monarch recruitment,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds K. S. Oberhauser, K. R. Nail, and S. M. Altizer (Ithaca, NY: Cornell University Press, 197–202.
Boppré, M. J. (1990). Lepidoptera and pyrrolizidine alkaloids: exemplification of complexity in chemical ecology. Chem. Ecol. 16:165. doi: 10.1007/BF01021277
Brower, L. P., Fink, L. S., and Walford, P. (2006). Fueling the fall migration of the monarch butterfly. Integr. Compar. Biol. 46, 1123–1142. doi: 10.1093/icb/icl029
Brower, L. P., Taylor, O. R., Williams, E. H., Slayback, D. A., Zubieta, R. R., and Ramirez, M. I. (2012). Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv. Divers. 5, 95–100. doi: 10.1111/j.1752-4598.2011.00142.x
Cameron, S. A., Lozier, J. D., Strange, J. P., Koch, J. B., Cordes, N., Solter, L. F., et al. (2011). Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U.S.A. 108, 662–667. doi: 10.1073/pnas.1014743108
Cardno (2019). Nationwide Candidate Conservation Agreement for Monarch Butterfly on Energy and Transportation Lands April 2019 Draft. Monarch CCAA/CCA Development Advisory Team and the Energy Resources Center at The University of Illinois at Chicago. Available online at: https://www.fws.gov/savethemonarch/pdfs/Monarch%20CCAA-CCA%20Public%20Comment%20Documents/Monarch-Nationwide_CCAA-CCA_Draft.pdf (accessed September 2, 2019).
Cariveau, A. B., Holt, H. L., Ward, J. P., Lukens, L., Kasten, K., Thieme, J., et al. (2019). The integrated monarch monitoring program: from design to implementation. Front. Ecol. Evol. 7:167. doi: 10.3389/fevo.2019.00167
CEC (2008). North American Monarch Conservation Action Plan. Montreal: Commission for Environmental Cooperation.
CEC (2017). Monitoring Monarch Butterflies and Their Habitat across North America: Inventory and Monitoring Protocols and Data Standards for Monarch Conservation. Montreal, QC: Commission for Environmental Cooperation.
Cizek, O., Zamecnik, J., Tropek, R., Kocarek, P., and Knovicka, M. (2012). Diversification of mowing regime increases arthropods diversity in species-poor cultural hay meadows. J. Insect Conserv. 16, 215–226. doi: 10.1007/s10841-011-9407-6
Cook, C. N., Wardell-Johnson, G., Keatley, M., Gowans, S. A., Gibson, S. M., Westbrooke, E., et al. (2010). Is what you see what you get? Visual vs. measured assessments of vegetation condition. J. Appl. Ecol. 47, 650–661. doi: 10.1111/j.1365-2664.2010.01803.x
Dale, B. C., Martin, P. A., and Taylor, P. S. (1997). Effects of hay management on grassland songbirds in Saskatchewan. Wildlife Soc. Bull. 616–626.
Denisow, B., Monika Strzałkowska-Abramek, M., Bozek, M., and Jezak, A. (2014). Early spring nectar and pollen and insect visitor behavior in two Corydalis species (Papveraceae). J. Apicult. Sci. 58, 93–103. doi: 10.2478/jas-2014-0009
Ebeling, A., Klein, A. M., Schumacher, J., Weisser, W. W., and Tscharntke, T. (2008). How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 117, 1808–1815. doi: 10.1111/j.1600-0706.2008.16819.x
Entsminger, E. D., Jones, J. C., Guyton, J. W., Strickland, B. K., and Leopold, B. D. (2017). Evaluation of mowing frequency on right-of-way plant communities in Mississippi. J. Fish Wildlife Manag. 8, 125–139. doi: 10.3996/062016-JWFM-051
Environmental Defense Fund (2017). Monarch Habitat Quantification Tool—User's Guide v1.0—North Central Region. Environmental Defense Fund, Environmental Incentives, and the Monarch Lab at the University of Minnesota.
Fischer, S. J., Williams, E. H., Brower, L. P., and Palmiotto, P. A. (2015). Enhancing monarch butterfly reproduction by mowing fields of common milkweed. Am. Midland Nat. 173, 229–240. doi: 10.1674/amid-173-02-229-240.1
Flockhart, D. T., Pichancourt, J. B., Norris, D. R., and Martin, T. G. (2015). Unravelling the annual cycle in a migratory animal: breeding-season habitat loss drives population declines of monarch butterflies. J. Anim. Ecol. 84, 155–165. doi: 10.1111/1365-2656.12253
Fore, L. S., Paulsen, K., and O'Laughlin, K. (2001). Assessing the performance of volunteers in monitoring streams. Freshw. Biol. 46, 109–123. doi: 10.1111/j.1365-2427.2001.00640.x
Gottsberger, G., Schrauwen, J., and Linskens, F. (1984). Amino acids and sugars in nectar, and their putative evolutionary significance. Syst. Evol. 145, 55–77. doi: 10.1007/BF00984031
Goulson, D., Nicholls, E., Botías, C., and Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. doi: 10.1126/science.1255957
Grant, T. A., Madden, E. M., Murphy, R. K., Smith, K. A., and Nenneman, M. P. (2004). Monitoring native prairie vegetation: the belt transect method. Ecol. Restor. 22, 106–111. doi: 10.3368/er.22.2.106
Grant, T. J., Parry, H. R., Zalucki, M. P., and Bradbury, S. P. (2018). Predicting monarch butterfly (Danaus plexippus) movement and egg-laying with a spatially-explicit agent-based model: the role of monarch perceptual range and spatial memory. Ecol. Model. 374, 37–50. doi: 10.1016/j.ecolmodel.2018.02.011
Gunnarsson, B., and Federsel, L. M. (2014). Bumblebees in the city: abundance, species richness and diversity in two urban habitats. J. Insect. Conserv. 18, 1185–1191. doi: 10.1007/s10841-014-9729-2
Haan, N. L., and Landis, D. A. (2019). Grassland disturbance increases monarch butterfly oviposition and decreases arthropod predator abundance. Biol. Conserv. 233, 85–192. doi: 10.1016/j.biocon.2019.03.007
Halbritter, D. A., Daniels, J. C., Whitaker, D. C., and Huang, L. (2015). Reduced mowing frequency increases floral resource and butterfly (Lepidoptera: Hesperioidea and Papilionoidea) abundance in managed roadsides. Florida Entomol. 98, 1081–1092. doi: 10.1653/024.098.0412
Hallmann, C. A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. doi: 10.1371/journal.pone.0185809
Hartzler, R. G., and Buhler, D. D. (2000). Occurrence of common milkweed (Asclepias syriaca) in cropland and adjacent areas. Crop Prot. 19 363–366. doi: 10.1016/S0261-2194(00)00024-7
Hegland, S. J., and Totland, O. (2005). Relationships between species' floral traits and pollinator visitation in a temperate grassland. Oecologia 145, 586–594. doi: 10.1007/s00442-005-0165-6
Hicks, D. M., Ouvrard, P., Baldock, K. C. R., Baude, M., Goddard, M. A., Kunin, W. E., et al. (2016). Food for pollinators: quantifying the nectar and pollen resources of urban flower meadows. PLoS ONE 11:e0158117. doi: 10.1371/journal.pone.0158117
Holl, K. D. (1995). Nectar resources and their influence on butterfly communities on reclaimed coal surface mines. Restor. Ecol. 3, 76–85. doi: 10.1111/j.1526-100X.1995.tb00080.x
Hopwood, J., Black, S., and Fleury, S. (2016a). Pollinators and Roadsides: Best Management Practices for Managers and Decision Makers. Washington, DC: Federal Highway Administration. Available online at: https://www.environment.fhwa.dot.gov/env_topics/ecosystems/Pollinators_Roadsides/BMPs_pollinators_roadsides.aspx
Hopwood, J., Black, S., and Fleury, S. (2016b). Roadside Best Management Practices that Benefit Pollinators: Handbook for Supporting Pollinators through Roadside Maintenance and Landscape Design. Washington, DC: Federal Highway Administration. Available online at: https://www.environment.fhwa.dot.gov/env_topics/ecosystems/Pollinators_Roadsides/BMPs_pollinators_landscapes.pdf
Hopwood, J., Black, S. H., Lee-Mader, E., Charlap, A., Preston, R., Mozumder, K., et al. (2015). Literature Review: Pollinator Habitat Enhancement and Best Management Practices in Highway Rights-of-Way. Washington, DC: Federal Highway Administration.
Hopwood, J. L. (2008). The contribution of roadside grassland restorations to native bee conservation. Biol. Conserv. 141, 2632–2640. doi: 10.1016/j.biocon.2008.07.026
Howard, E., and Davis, A. K. (2009). Investigating long-term changes in the spring migration of monarch butterflies (Lepidoptera: Nymphalidae) using 18 years of data from Journey North, a citizen science program. Ann. Entomol. Soc. Am. 108, 664–669. doi: 10.1093/aesa/sav061
Hudson, M. A. R., Francis, C. M., Campbell, K. J., Downes, C. M., Smith, A. C., Pardieck, K. L., et al. (2017). The role of the North American Breeding Bird Survey in conservation. Condor 119, 526–545. doi: 10.1650/CONDOR-17-62.1
Hunt, V. M., Jacobi, S. K., Gannon, J. J., Zorn, J. E., Moore, C. T., and Lonsdorf, E. V. (2016). A decision support tool for adaptive management of native prairie ecosystems. J. Appl. Anal. 46, 281–363. doi: 10.1287/inte.2015.0822
Inamine, H., Ellner, S. P., Springer, J. P., and Agrawal, A. A. (2016). Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos 125, 1081–1091. doi: 10.1111/oik.03196
Johst, K., Drechsler, M., Thomas, J., and Settele, J. (2006). Influence of mowing on the persistence of two endangered large blue butterfly species. J. Appl. Ecol. 43, 333–342. doi: 10.1111/j.1365-2664.2006.01125.x
Jue, D. K., and Daniels, J. C. (2015). A successful model for citizen scientist involvement in building a statewide at-risk butterfly database. J. Insect. Conserv. 19, 421–431. doi: 10.1007/s10841-014-9733-6
Kantola, T., Tracy, J. L., Baum, K. A., Quinn, M. A., and Coulson, R. A. (2019). Spatial risk assessment of eastern monarch butterfly road mortality during autumn migration within the southern corridor. Biol. Conserv. 231, 150–160. doi: 10.1016/j.biocon.2019.01.008
Kaspari, M., Chang, C., and Weaver, J. (2010). Salted roads and sodium limitation in a northern forest ant community. Ecol. Entomol. 35, 543–548. doi: 10.1111/j.1365-2311.2010.01209.x
Kasten, K., Stenoien, C., Caldwell, W., and Oberhauser, K. S. (2016). Can roadside habitat lead monarchs on a route to recovery? J. Insect Conserv. 20, 1047–1057. doi: 10.1007/s10841-016-9938-y
Keilson, W., Narango, D. L., and Tallamy, D. W. (2018). Roadside habitat impacts insect tra?c mortality. J. Insect Conserv. 22, 183–188. doi: 10.1007/s10841-018-0051-2
Kinkead, K. E., Harms, T. M., Dinsmore, S. J., Frese, P. W., and Murphy, K. T. (2019). Design implications for surveys to monitor monarch butterfly population trends. Front. Ecol. Evol. 7:195. doi: 10.3389/fevo.2019.00195
Knight, S. M., Norris, D. R., Derbyshire, R., and Flockhart, D. T. T. (2019). Strategic mowing of roadside milkweeds increases monarch butterfly oviposition. Global Ecol. Conserv. 19:e00678. doi: 10.1016/j.gecco.2019.e00678
Kremen, C., Ullmann, K. S., and Thorp, R. W. (2011). Evaluating the quality of citizen-scientist data on pollinator communities. Conserv. Biol. 25, 607–617. doi: 10.1111/j.1523-1739.2011.01657.x
Lovell, S., Hamer, M., Slotow, R., and Herbert, D. (2009). An assessment of the use of volunteers for terrestrial invertebrate biodiversity surveys. Biodivers. Conserv. 18, 3295–3307. doi: 10.1007/s10531-009-9642-2
MAFWA (2018). Mid-America Monarch Conservation Strategy, 2018–2038, Version 1.0. Midwest Association of Fish and Wildlife Agencies.
Majewska, A. A., Sims, S., Wenger, S. J., Davis, A. K., and Altizer, S. (2018). Do characteristics of pollinator-friendly gardens predict the diversity, abundance, and reproduction of butterflies? Insect Conserv. Divers. 11, 370–382. doi: 10.1111/icad.12286
Malcolm, S. B. (2018). Anthropogenic impacts on mortality and population viability of the monarch butterfly. Ann. Rev. Entomol. 63, 277–302. doi: 10.1146/annurev-ento-020117-043241
McKenna, D., McKenna, K., Malcom, S. B., and Berenbaum, M. R. (2001). Mortality of lepidoptera along roadways in Central Illinois. J. Lepid. Soc. 55, 63–68.
Minnesota Board of Soil Water Resources (2016). Solar Site Pollinator Habitat Assessment Form for Project Planning. Available online at: https://bwsr.state.mn.us/sites/default/files/2019-02/Project%20Planning%20Assessment%20Form.pdf (accessed June 19, 2019).
Monarch Joint Venture (2019). Mowing and Management: Best Practices for Monarchs. Available online at: https://monarchjointventure.org/images/uploads/documents/MowingForMonarchs.pdf (accessed August 1, 2019).
Munguira, M. L., and Thomas, J. A. (1992). Use of road verges by butterfly and burnet populations, and the effect of roads on adult dispersal and mortality. J. Appl. Ecol. 29, 316–329. doi: 10.2307/2404501
Munoz, P. T., Torres, F. P., and Megias, A. G. (2015). Effects of roads on insects: a review. Biodivers. Conserv. 24, 659–682. doi: 10.1007/s10531-014-0831-2
Nail, K. R., Stenoien, C., and Oberhauser, K. S. (2015). Immature monarch survival: effects of site characteristics, density, and time. Ann. Entomol. Soc. Am. 108, 680–690. doi: 10.1093/aesa/sav047
National Research Council (2007). Status of Pollinators in North America. Washington, DC: National Academies Press.
Nerbonne, J. F., and Vondracek, B. (2003). Volunteer macroinvertebrate monitoring: assessing training needs through examining error and bias in untrained volunteers. J. N. Am. Benthol. Soc. 22, 152–163. doi: 10.2307/1467984
Oberhauser, K., Wiedenholt, R., Diffendorfer, J., Semmens, D., Ries, L., Thogmartin, W., et al. (2017). A trans-national monarch butterfly population model and implications for regional conservation priorities. Ecol. Entomol. 42, 51–60. doi: 10.1111/een.12351
Pavlik, D. T., Fleishman, E., Wang, N., Boswell, P., and Blair, R. B. (2018). Sugars in nectar sources and their use by butterflies (Hesperioidea and Papilionoidea) in the Sierra Nevada, California. J. Lepidopterists Soc. 72, 165–174. doi: 10.18473/lepi.v72i2.a10
Pelton, E. M., Schultz, C. B., Jepsen, S. J., Black, S. H., and Crone, E. E. (2019). Western monarch population plummets: status, probable causes, and recommended conservation actions. Front. Ecol. Evol. 7:258. doi: 10.3389/fevo.2019.00258
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., and R Core Team. (2018). _nlme: Linear and Nonlinear Mixed Effects Models_. R package Version 3. Available online at: https://CRAN.R-project.org/package=nlme
Pitman, G. M., Flockhart, D. T. T., and Norris, D. R. (2018). Patterns and causes of oviposition in monarch butterflies: implications for milkweed restoration. Biol. Conserv. 217, 54–65. doi: 10.1016/j.biocon.2017.10.019
Pleasants, J. (2017). Milkweed restoration in the Midwest for monarch butterfly recovery: estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the monarch population. Insect Conserv. Divers. 10, 42–53. doi: 10.1111/icad.12198
Pleasants, J. M., and Oberhauser, K. S. (2013). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Divers. 6, 135–144. doi: 10.1111/j.1752-4598.2012.00196.x
Pollinator Health Task Force (2015). National Strategy to Promote the Health of Honey Bees and Other Pollinators. Available online at: https://obamawhitehouse.archives.gov/sites/default/files/microsites/ ostp/Pollinator%20Health%20Strategy%202015.pdf (accessed December 17, 2018).
Potts, S. G., Vulliamy, B., Dafni, A., Ne'eman, G., and Willmer, P. (2003). Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84, 2628–2642. doi: 10.1890/02-0136
Pywell, R. F., Meek, W. R., Hulmes, L., Hulmes, S., James, K. L., Nowakowski, M., et al. (2011). Management to enhance pollen and nectar resources for bumblebees and butterflies within intensively farmed landscapes. J. Insect Conserv. 15, 853–864. doi: 10.1007/s10841-011-9383-x
Pywell, R. R., Warman, E. A., Sparks, T. H., Greatorex-Davies, J. N., Walker, K. J., Meek, W. R., et al. (2004). Assessing habitat quality for butterflies on intensively managed arable farmland. Biol. Conserv. 118, 313–325. doi: 10.1016/j.biocon.2003.09.011
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Ries, L., Debinski, D. M., and Wieland, M. L. (2001). Conservation value of roadside prairie restoration to butterfly communities. Conserv. Biol. 15, 401–411. doi: 10.1046/j.1523-1739.2001.015002401.x
Ries, L., and Oberhauser, K. (2015). A citizen-army for science: quantifying the contributions of citizen scientists to our understanding of monarch butterfly biology. Bioscience 65, 419–430. doi: 10.1093/biosci/biv011
Roy, D. B., Rothery, P., and Brereton, T. (2007). Reduced effort schemes for monitoring for monitoring butterfly populations. J. Appl. Ecol. 44, 993–1000. doi: 10.1111/j.1365-2664.2007.01340.x
Rudolph, D. C., Ely, C. A., Schaefer, R. R., Williamson, J. H., and Thill, R. E. (2006). Monarch (Danaus plexippus L. Nymphalidae) migration, nectar resources and fire regimes in the Ouachita Mountains of Arkansas. J. Lepidopterists Soc. 60, 165–170. Available online at: http://images.peabody.yale.edu/lepsoc/jls/2000s/2006/2006(3)165-Rudolf.pdf
Saarinen, K., Valtonen, A., Jantunen, J., and Saarnio, S. (2005). Butterflies and diurnal moths along road verges: does road type affect diversity and abundance? Biol. Conserv. 12, 403–412. doi: 10.1016/j.biocon.2004.12.012
Sánchez-Bayo, F., and Wyckhuys, K. A. G. (2019). Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 232, 8–27. doi: 10.1016/j.biocon.2019.01.020
Schultz, C. B., Brown, L. M., Pelton, E., and Crone, E. E. (2017). Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol. Conserv. 214, 343–346. doi: 10.1016/j.biocon.2017.08.019
Schultz, C. B., and Dlugosch, K. M. (1999). Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia 119, 231–238. doi: 10.1007/s004420050781
Semmens, B. X., Semmens, D. J., Thogmartin, W. E., Wiederholt, R., López-Hoffman, L., Diffendorfer, J. E., et al. (2016). Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6:23265. doi: 10.1038/srep23265
Skorka, P., Lenda, M., Moron, D., Kalarus, K., and Tryjanowski, P. (2013). Factors affecting road mortality and the suitability of road verges for butterflies. Biol. Conserv. 159, 148–157. doi: 10.1016/j.biocon.2012.12.028
Snell-Rood, E. C., Espeset, A., Boser, C. J., White, W. A., and Smykalski, R. (2014). Anthropogenic changes in sodium affect neural and muscle development in butterflies. Proc. Natl Acad. Sci. U.S.A. 111, 10221–10226. doi: 10.1073/pnas.1323607111
Stenoien, C., Nail, K. R., Zalucki, J. M., Parry, H., Oberhauser, K. S., and Zalucki, M. P. (2018). Monarchs in decline: a collateral landscape-level effect of modern agriculture. Insect Sci. 25, 528–541. doi: 10.1111/1744-7917.12404
Szigeti, V., Korösi, Á., Harnos, A., and Kis, J. (2018). Temporal changes in floral resource availability and flower visitation in a butterfly. Arthropod-Plant Interact. 12, 177–198. doi: 10.1007/s11829-017-9585-6
Szigeti, V., Korösi, Á., Harnos, A., Nagy, J., and Kis, J. (2016). Measuring floral resource availability for insect pollinators in temperate grasslands—a review. Ecol. Entomol. 41, 231–240. doi: 10.1111/een.12298
Thogmartin, W. E., López-Hoffman, L., Rohweder, J., Diffendorfer, J., Drum, R., Semmens, D., et al. (2017b). Restoring monarch butterfly habitat in the Midwestern US: all hands on deck. Environ. Res. Lett. 12:74005. doi: 10.1088/1748-9326/aa7637
Thogmartin, W. E., Wiederholt, R., Oberhauser, K., Drum, R. G., Diffendorfer, J. E., Altizer, S., et al. (2017a). Monarch butterfly population decline in North America: identifying the threatening processes. R. Soc. Open Sci. 4:170760. doi: 10.1098/rsos.170760
Thomas, R. C., and Schultz, C. B. (2015). Resource selection in an endangered butterfly: females select native nectar species. J. Wildilfe Manag. 80, 171–180. doi: 10.1002/jwmg.987
Tracy, J. L., Kantola, T., Baum, K. A., and Coulson, R. N. (2019). Modeling fall migration pathways and spatially identifying potential migratory hazards for the eastern monarch butterfly. Landscape Ecol. 34, 443–458. doi: 10.1007/s10980-019-00776-0
US Fish Wildlife Service (1981). Standards for the Development of Habitat Suitability Index Models. United States Fish and Wildlife Service. Washington, DC: Department of the Interior. Available online at: https://www.fws.gov/policy/ESMindex.html
USDA NRCS (2016). Important Plants of the Monarch Butterfly. Central National Technology Support Center. US Department of Agriculture (USDA) Natural Resources Conservation Service (NRCS). Available online at: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/national/plantsanimals/pollinate/?cid=nrcseprd402207 (accessed July 31, 2019).
USDA NRCS (2018). USDA NRCS Monarch Butterfly Habitat Evaluation Guide (WHEG) and Decision Support Tool: Midwest Edition 2.0. US Department of Agriculture (USDA) Natural Resources Conservation Service (NRCS). Available online at: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/national/plantsanimals/pollinate/?cid=nrcseprd402207/ (accessed July, 31, 2019).
Vidal, O., and Rendón-Salinas, E. (2014). Dynamics and trends of overwintering colonies of the monarch butterfly in Mexico. Biol. Conserv. 180, 165–175. doi: 10.1016/j.biocon.2014.09.041
Zalucki, M. P., and Kitching, R. L. (1982). Temporal and spatial variation in mortality in field populations of Danaus plexippus L., and D. chrsippus L. larvae (Lepidoptera: Nymphalidae). Oecologia 53, 201–207. doi: 10.1007/BF00545664
Zalucki, M. P., and Lammers, J. H. (2010). Dispersal and egg shortfall in Monarch butterflies: what happens when the matrix is cleaned up? Ecol. Entomol. 35, 84–91. doi: 10.1111/j.1365-2311.2009.01160.x
Zalucki, M. P., Parry, H., and Zalucki, J. M. (2016). Movement and egg laying in monarchs: to move or not to move, that is the equation. Aust. Ecol. 41, 154–167. doi: 10.1111/aec.12285
Keywords: rights-of-way, roadside vegetation management, habitat assessment, butterflies, milkweed, nectar, host plants, Danaus plexippus
Citation: Cariveau AB, Anderson E, Baum KA, Hopwood J, Lonsdorf E, Nootenboom C, Tuerk K, Oberhauser K and Snell-Rood E (2019) Rapid Assessment of Roadsides as Potential Habitat for Monarchs and Other Pollinators. Front. Ecol. Evol. 7:386. doi: 10.3389/fevo.2019.00386
Received: 14 January 2019; Accepted: 24 September 2019;
Published: 16 October 2019.
Edited by:
Cheryl Schultz, Washington State University Vancouver, United StatesReviewed by:
Nathan L. Haan, Michigan State University, United StatesKatherine Kral-O'Brien, North Dakota State University, United States
Copyright © 2019 Cariveau, Anderson, Baum, Hopwood, Lonsdorf, Nootenboom, Tuerk, Oberhauser and Snell-Rood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison B. Cariveau, YWxpc29uLmNhcml2ZWF1QGdtYWlsLmNvbQ==
 Alison B. Cariveau
Alison B. Cariveau Erik Anderson2
Erik Anderson2 Kristen A. Baum
Kristen A. Baum Eric Lonsdorf
Eric Lonsdorf Chris Nootenboom
Chris Nootenboom Karen Tuerk
Karen Tuerk Emilie Snell-Rood
Emilie Snell-Rood