- 1Centre for Ecology and Conservation, University of Exeter, Penryn Campus, Penryn, United Kingdom
- 2Department of Evolutionary Studies of Biosystems, The Graduate University for Advanced Studies (Sokendai), Hayama, Japan
- 3Department of Zoology, University of Cambridge, Cambridge, United Kingdom
- 4Department of Animal & Plant Sciences, University of Sheffield, Sheffield, United Kingdom
- 5Evolution & Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW, Australia
- 6Department of Biological Sciences, Macquarie University, Sydney, NSW, Australia
Recent theoretical and empirical work suggests that coordinating offspring provisioning plays a significant role in stabilizing cooperative care systems, with benefits to developing young. However, a warming and increasingly extreme climate might be expected to make contributions to, and so coordination of, care more challenging, particularly in cooperative breeding systems comprising multiple carers of varying age and pairwise relatedness. Here we investigated the interplay between breeding phenology, meteorological conditions and carer number on the individual rates and group-level coordination of nestling care in the cooperatively breeding chestnut-crowned babbler (Pomatostomus ruficeps) in outback south-eastern Australia. From 3 months since the last meaningful rain event, dominant male breeders and—to a lesser extent—related helpers showed reductions in their provisioning rates and increases in their day-to-day variation. Further, on days with high mean wind speed, dominant males contributed less and helpers were less likely to visit the nest on such days. Helpers also showed reduced visitation rates on days with high mean temperature. Provisioning rates were independent of the number of carers, and increasing numbers of carers failed to mitigate the detrimental effects of challenging environment on patterns of provisioning. Those helpers that were unrelated to broods often failed to help on a given day and tended to help at a low rate when they did contribute, with socio-environmental predictors having limited explanatory power. Given the marked variation in individual contributions to offspring care and the variable explanatory power of the socio-environmental predictors tested, babblers unsurprisingly had low levels of nest visitation synchrony. Large groups visited the nest more asynchronously on days of high mean temperature, suggesting that meteorological impacts on individual provisioning have consequences for group-level coordination. Our study has implications for the consequences of climate change on patterns of provisioning, the minimal role of group size in buffering against these challenges and the stabilization of cooperative care.
Introduction
Global trends for warming and increased climatic instability are suggested to be responsible for recent population declines in many bird species, but the underlying causes remain unresolved (Both et al., 2005, 2006; Visser and Both, 2005; McKechnie and Wolf, 2009; Saino et al., 2010). A popular explanation is provided by the “phenological mismatch hypothesis,” which proposes that organisms are increasingly mis-timing key life events as a result of general warming (Cushing, 1969; Thackeray et al., 2016; Cohen et al., 2018). For example, advancing springs are suggested to cause a mismatch between the breeding phenology of temperate passerine birds and the timing of peak food availability during nestling development (Both et al., 2006, 2009; Møller et al., 2008; Saino et al., 2010). While this hypothesis has significant explanatory power, an additional possibility is that changing weather patterns have a direct impact on the reproductive capacity of birds (Visser et al., 2015; Englert Duursma et al., 2019). For example, high temperatures are known to pose physiological challenges for many organisms, reducing foraging ability/success (Austin, 1976; Briga and Verhulst, 2015; Funghi et al., 2019) and presumably the ability to invest optimally in offspring (Speakman and Król, 2010; Wiley and Ridley, 2016; Andrew et al., 2017, 2018). Nevertheless, attempts to quantify the relative impacts of breeding phenology vs. meteorological conditions on drivers of breeding success in birds are lacking.
One viable means of elucidating the impacts of phenology vs. meteorological conditions on breeding success is to measure their impacts on patterns of nestling provisioning. Notably, carer provisioning of offspring is known to be costly, and so both its extent and timing are likely to be sensitive to phenologically-mediated variation in food availability (Both et al., 2005) and meteorological conditions (Bolton, 1995; Stienen et al., 2000; Luck, 2001; Hoset et al., 2004; Król et al., 2007; Rose, 2009; Visser et al., 2015). Further, the extent and timing of care have significant effects on offspring survival and recruitment, because in combination these can influence the growth and development of offspring (Hatchwell et al., 2004; Mariette and Griffith, 2015), levels of competition in the nest (Shen et al., 2010) and/or the risks of being detected by predators (Raihani et al., 2010; Leniowski and Wegrzyn, 2018). By extension, we would expect phenological mismatches and/or challenging meteorological conditions to be associated with reduced and/or more variable individual provisioning rates. And, in turn, reduced and more variable individual provisioning rates will make pair or group-level coordination of care more challenging by restricting care response rules or synchronization (Johnstone et al., 2014; Johnstone and Savage, 2019; Lejeune et al., 2019). Currently, however, phenological vs. meteorological impacts on individual patterns of provisioning and its group-level coordination consequences remain unclear.
Cooperative breeders provide a particularly interesting model for testing the impacts of breeding phenology and meteorological conditions on patterns of provisioning at the level of individuals and groups. On the one hand, cooperative groups typically comprise carers varying in their optimal investment patterns (Cockburn, 1998; Clutton-Brock et al., 2002; Russell et al., 2003; McAuliffe et al., 2015), and the challenge of coordinating investment at the level of the group is expected to increase with contrasting investment levels (Savage et al., 2013) and group size (McNamara et al., 2003). On the other hand, however, cooperative breeders are also over-represented in stochastic environments (Jetz and Rubenstein, 2011; Cornwallis et al., 2017; Griesser et al., 2017; Lukas and Clutton-Brock, 2017). While the reasons for this are not fully understood, two related possibilities of relevance here are that: (a) groups might be better able to find food or be more efficient at foraging than pairs, mitigating detrimental impacts of challenging environmental conditions; and so (b) groups might also be better able to coordinate nest-visits to the potential benefit of developing young.
Despite the long-appreciated association between the environment and cooperative breeding, surprisingly few studies have investigated phenological or meteorological impacts on individual contributions to offspring care in such systems (Wiley and Ridley, 2016). The growth of meerkat pups (Suricata suricatta), in the semi-arid zone of South Africa, is positively associated with recent rainfall and negatively impacted by high daytime temperature (Russell et al., 2002), but effects on contributions by carer classes were not assessed. In long-tailed tits (Aegithalos caudatus), all carers reduce their nestling provisioning rate on warm days (MacColl and Hatchwell, 2003), presumably because in the UK climate the energetic requirements of offspring are reduced on warm days. Finally, Wiley and Ridley (2016) showed that high temperatures in the semi-arid region of South Africa led to reductions of nestling provisioning by dominants but not helpers in pied babblers (Turdoides bicolor), and nestlings had reduced mass, although whether this was due to reduced provisioning and/or coordination was not clarified. Thus, the questions largely remain: How do breeding phenology and meteorological conditions impact the contributions of different classes of individuals? And what are the potential consequences on the coordination of care at the level of the group? Answering these questions will not only provide new insights into the socio-ecological dynamics of offspring care, but add to our general appreciation of the environmental factors underpinning the success of animal populations.
Here we investigate these two broad questions in the cooperatively breeding chestnut-crowned babbler (Pomatostomus ruficeps) in the arid zone of south-eastern Australia. This 50 g insectivore breeds in groups of 2–17 individuals (mean = 7.5), which we refer to as units, that typically comprise a breeding pair (although polyandry occurs in about 30% of groups) and male helpers of varying relatedness to the brood (Russell, 2016). Breeder and helper carers provide broods with a variety of invertebrates (mainly caterpillars, grubs, crickets, and spiders) and small vertebrates (lizards), delivered singly (Browning et al., 2012b). Further, helping is strongly kin directed, with unit members more likely to help and to do so at a higher rate when related to the brood by at least half-sib equivalents (Browning et al., 2012a). Breeding females reduce their provisioning rate by up to 80% across the range of unit sizes (Browning et al., 2012b), although breeding males and helpers do not do so on average (Browning et al., 2012b; Liebl et al., 2015). Unsurprisingly, prey availability is tied to rainfall in their arid environment: on-site light trap data suggests that “global” food availability peaks around 3 months post-rain (then wanes), while focal observations of foraging units suggest food resource depletion with progression of the breeding season, particularly for large units (evidence based on distances traveled for food, patch revisitation rates, and path tortuosity; Sorato et al., 2016). However, what are not known is: (a) how the patterns of provisioning of different classes of carer are affected by breeding phenology vs. meteorological conditions; (b) whether the impacts of breeding phenology or meteorological conditions are modified by the number of carers in the unit; and (c) how socio-environmental influences on patterns of provisioning translates into group-level coordination.
In this study, we address these unknowns by analyzing 1,742 h of nest visitation data at 29 nesting attempts of 26 breeding units. Analyses were conducted using mixed-effects models, incorporating zero inflation and heterogeneous variance in the contributions of different carer classes where necessary. First, we investigated the interplay among phenological, meteorological, and social (henceforth referred collectively as socio-environmental) variables on the patterns of nestling provisioning contributions by dominant breeding males (our monitoring methods unfortunately preclude the ability to do the same for breeding females, see section Methods). Second, we do the same for helpers, with this class further categorized by age (yearlings vs. adults) and relatedness to breeders (related vs. unrelated) (see section Methods). Dominant breeding males and helpers could not be analyzed in the same model due to differences in the distribution of data, but the models were set to allow direct comparison of the results. Third, we investigated the socio-environmental impacts on the coefficient of variation in the within-attempt provisioning rates of carers. Finally, we investigated the impacts of the socio-environmental predictors outlined on the level of nest visit synchronization by unit members. We predicted both phenology and meteorological conditions to impact patterns of provisioning by different classes of carer, and for larger units to buffer against detrimental environmental conditions.
Methods
Fieldwork was conducted at the University of New South Wales Arid Zone Research Station, Fowlers Gap (31°05′S, 141°43′E), New South Wales, Australia. The habitat consists of low and open chenopodiaceae shrubland, with tall shrubs and trees (mainly Acacia and Casuarina spp.) largely confined to short linear stands along usually dry creeks and drainage lines (Portelli et al., 2009). Rainfall, temperature and wind speed were measured hourly on-site by an Australian Bureau of Meteorology weather station. Although babblers have been recorded breeding in all months of the year, they usually begin laying their 3–5 egg-clutches in mid to late winter (July/August), with all young usually having fledged before the onset of summer at the end of November (incubation and nestling periods ca. 20 and 23 d, respectively), presumably because summers tend to be prohibitively hot (Andrew et al., 2017). In this study, we used provisioning data obtained from 9 August to 26 November in 2007 and 8 August to 7 November in 2008, for which molecular analyses (using 13 microsatellite loci) have been performed to determine breeding status, sex and relatedness of all individuals (for molecular methods see Holleley et al., 2009; Rollins et al., 2012).
In temperate zone species, breeding phenology is typically measured relative to a standard date, but in arid zone settings, phenology needs to be relative to the timing of meaningful rain events. Evidence over the past 16 years suggests that babblers generally initiated breeding following rain events of at least 18 mm over a 24 h period (AF Russell unpublished). In 2007, the first such meaningful rain events occurred on 15 May (22.2 mm) and 17 May (25.2 mm), and so for this year phenology was measured as the number of days from 17 May. However, in this year, another rain event occurred on 23 October (19.6 mm), and consequently, 5 days of provisioning collected 25–30 October retained the original date scale (i.e., calculated from 17 May), but 13 days of data collected from the 12 November to 26 November were assigned the number of days from 23 October; since light trap data suggested insect prey availability increased again ~3 weeks post rain (Fowlers Gap unpublished data). In 2008, the first meaningful rain events occurred on 6 June (18 mm) and 9 June (20 mm), with no other rain events until 19 November. As all days of provisioning were collected between the latter two rain events (i.e., 9 June and 19 November), days relative to rain were calculated from 9 June in this year.
Translating observation date into days since last meaningful rain showed that all data were collected 60–165 days since last meaningful rain, with first nesting attempts representing almost all of the data collected over the 2-month period before day 120, and subsequent attempts the 6-week period after day 120. Over the periods of data collection in the 2 years (August-November), both mean daytime temperature and wind speed were highly variable. Mean daytime temperatures ranged from 8 to 33°C (overall mean = 22°C; SD = 8), and daytime temperatures as low as −2°C and as high as 41°C were recorded. Additionally, daily means in wind speed ranged from 5 to 42 km/h (overall daily mean = 17 km/h, SD = 6.5) and winds of up to 59 km/h were recorded during the study. The correlation between days since last meaningful rain and temperature was strongly positive (Spearman's correlation coefficient rs = 0.7), with mean daytime temperature increasing by an average 0.2°C/d. Although wind speed also increased over the course of the study periods, the correlation with days since last meaningful rain was less strong (rs = 0.3), and the mean increase in wind speed was just 0.07 km/h per day.
Following data restrictions (see below), we analyzed 1,742 h of nest visitation data [mean ± standard deviation (SD) = 9 ± 3 h/day] at 29 nests of 26 breeding units. Nest visitations were recorded using our validated remote system (Browning et al., 2012b; Nomano et al., 2014). Briefly, during capture in mistnets (or before fledging) all individuals in the units used in this study were administered with a 2 × 12 mm (Trovan Ltd, UK, http://www.trovan.com) passive integrated transponder (PIT) tag, containing a unique hexadecimal code, subcutaneously in their flank. This PIT tag, which is equivalent to that used for pet identification purposes, remained functional in the birds for the duration of this study. Tags were registered, along with date and time, each time a bird passed through a copper coil antenna fitted to the entrance of their dome-shape nests and linked to an LID650 Trovan decoder at the bottom of the tree. Coils were ~0.5 cm thick, covered in non-shiny black tape, painted green and brown to blend with the nest and further disguised with vegetation; babblers routinely used the same nest in consecutive nesting attempts and across years with coil already in-position. This technology allowed us to record every nest visit for days at a time, although batteries (7.2 Ah NiCd gel batteries) varied in their running duration and we had far fewer decoders than nests, meaning that we had to rotate decoders around active nests to balance the number of days of observation with the number of nests observed.
The obvious benefits of this technology notwithstanding, there are three important caveats. First, nest visits were detected, irrespectively of whether or not food was delivered to offspring. By combining the PIT-tag system with nest cameras we have shown that, with the exception of the breeding female who regularly visited the nest without food (~40% of visits), food is brought to the nest in >90% of nest visits and babblers rarely false-feed (i.e., fail to successfully deliver food brought, <5% of nest visits; Young et al., 2013). Second, streams of “hits” occurred when birds entered and exited the nest. In this case, nest camera data showed that 99% of “hits” by the same individual within 1 min of each other represented a single visit, again with the exception of the breeding female, who spent variable amounts of time in the nest without being detected (Nomano et al., 2014); thus, we used gaps of >1 min to separate independent visits. Finally, although PIT tags do not capture variation in load size, we have shown previously using the nest cameras that babblers delivered a single prey item at a time, and that provisioning rate explained three times more variance in biomass delivered than did load size (Browning et al., 2012b). Thus, PIT tags can be used to capture provisioning behavior of all the members of breeding units with the exception of the breeding female, who was consequently removed from all analyses.
We made two further restrictions to our data. First, nests included in this study contained >3 days of monitoring from broods aged 9–21 d because we were primarily interested in climatic influences on among-day variability in provisioning, and provisioning rates were relatively constant between these brood ages (Browning et al., 2012a,b). Second, based on video data, any non-breeders visiting the nest <0.01 times/ h were not counted as “carers” and excluded from the nest visitation data. Of the 29 nesting attempts monitored, all except four contained a single dominant breeding male. In the four exceptions, the dominant male was assigned as the one with the greater share of paternity, while the five subordinate breeders were assigned to one of the two related helper categories depending on their age. Most the 89 non-breeding helpers of known sex (5 helpers were not sexed) were male (89%), of these 29% were assigned as relatives helping in the year subsequent to their birth (yearlings); 57% were relatives helping in their second or later season (adults); 3% were unrelated yearlings and 11% were unrelated adults (the two age classes of unrelated male were combined into a single “unrelated” category). Although strongly philopatric, males can quickly become distantly related to the breeders in their breeding unit owing to a combination of high breeder turnover and plural breeding (Rollins et al., 2012). Relatives were defined as those related to at least one dominant breeder by 0.25 in the pedigree or a pairwise relatedness coefficient of >0.2 (Queller and Goodnight, 1989) where pedigree information was not available, while non-relatives were defined as those less related. This cut-off was chosen based on known associations between kinship in pedigree and pairwise relatedness in this system: (a) in a sample of known non-relatives (n = 140), 95% had genetic relatedness values of <0.2; (b) all parent-offspring associations have relatedness values >0.2 (Rollins et al., 2012); and (c) in a sample of 87 known full sibs, 92% had relatedness values >0.2. We have shown previously that the key determinant of contributions to nestling provisioning is being a first-order relative of at least one parent (father, mother, or full sib), and that contributions are substantially reduced when carers are less or not related (Browning et al., 2012a). Finally, provisioning behavior was recorded for just 12 female helpers (present in 8 out of 25 unit-years), which included 4 yearling relatives, one yearling non-relative, five adult non-relatives, and two of unknown relatedness. Based on the similarities of these females' provisioning rates with those in each male category, we combined yearling related females with yearling related males (N = 4), yearling unrelated females with yearling unrelated males (N = 1), and the unrelated adult females with the unrelated adult males (N = 5). We excluded the single adult related female and the ungenotyped bird. We verified that including females in this way did not confound our analyses (Tables S1, S2, Figures S1–S4). Overall, the number of non-breeding helpers in the units ranged from 1 to 8 (mean = 4.3, SD = 1.9) and broods contained a mean of 4 offspring (SD = 0.9, range = 2–5) of 14 d old (SD = 3.4, range = 9–21 d old).
Socio-Environmental Effects on Nest Visitation by Dominant Breeding Males
We first modeled the effects of breeding phenology relative to meaningful rain events, mean daily meteorological conditions (temperature and wind speed) and carer number on the mean provisioning rates of dominant breeding males (nest visits per h) on each observation day. Two-way interactions between carer number and the three environmental parameters (days since last meaningful rain, temperature, wind) were also included. Dominant breeding males were modeled separately from subordinate members, because the distribution of provisioning rates was zero-inflated for subordinates but not for dominant males, although the results are comparable (see below). We analyzed hourly provisioning rate of dominant males by fitting a generalized linear mixed model (GLMM), with a Poisson distribution and log link function. The logarithm of daily observation time was included as an offset (also known as exposure) term with no coefficient, which allows for the evaluation of visits/h.
ID-nest was included as a random intercept to account for repeated observations of the same individuals in the same nest. Including OLRE, the observation-level random effect, allowed the model to account for overdispersion, and to elucidate the within-individual within-nest temporal variance. This analysis included 191 visit rates by 22 individuals observed in 29 nesting attempts by 26 unit-years.
Socio-Environmental Effects on Nest Visitation by Helpers
For testing the phenological, meteorological, and social effects on the provisioning rate of subordinate carers, we used a zero-inflated Poisson (ZIP) model, because helpers more often failed to visit the nest on a given day than would be expected under a Poisson distribution. Standard ZIP models are composed of two regression components corresponding to the two processes: an excess zero-generating process and a count-generating process. However, another feature of our data was that the coefficient of variation in nest visitation rates was different across carer classes (see section Results), which suggests the assumption of equal variance was violated (Cleasby and Nakagawa, 2011). To account for unequal variances, we added a third regression component to our ZIP models, which allowed us to account for heterogeneity in the observation-level variance of counts (Pinheiro and Bates, 2000; Zuur et al., 2009; Cleasby and Nakagawa, 2011).
The ZIP model contained three regression components for which coefficients were estimated simultaneously, and generated two sets of results pertaining to: (1) the probability of an individual showing an excess (zero-inflation) of non-visitation days; and (2) the provisioning rate of individuals expected under a Poisson distribution accounting for heterogeneity of variance (see below for fixed effects). To model the excess of zeros relative to expectation under a Poisson distribution, the probability of not observing excess zero values (ω) was estimated using a binomial distribution and a complementary log-log (cloglog) link function. The probability of not observing excess zeros with a cloglog link (rather than the more customary estimation of the probability of observing excess zeros with a logit link) was estimated because it improved model convergence. Random effects were not included in this part of the model, due to convergence problems. For the Poisson provisioning rate (which includes zero visits expected under Poisson), we used a Poisson distribution with log link function, and log observation time as an offset. In this Poisson part of the model, we were also able to include ID-nest as a random intercept to account for repeated measures of individuals provisioning over multiple days at a given nest. Further, by including OLRE, we again accounted for over-dispersion and could elucidate within-individual within-nest temporal variance. Finally, we added an extra regression component to the OLRE standard deviation to account for the heterogeneity of variance in patterns of carer provisioning rates, with OLRE following a normal distribution, with a mean of zero and an SD of σOLRE. The three regression components were formulated, respectively as:
For the third regression component: σ0 was the baseline standard deviation; the variance σOLRE2 represented variance unexplained by the predictors included in the second regression component; and coefficients of fixed effects represented an increase or decrease of the standard deviation σOLRE relative to the baseline σ0.
The fixed effects of the first two regression components included: the three-level class of helpers (SAR: subordinate adult relative; SYR: subordinate yearling relative; and SU: subordinate unrelated (including also distant relatives); carer number; days since last meaningful rain as the phenological measure; and the two meteorological variables (mean daytime temperature and wind speed). We also included the two-way interactions between carer class and the three environmental parameters (days, temperature, and wind), as well as between carer number and the three environmental parameters. By contrast, the third (variance-level regression) component had the three-level individual class as a single fixed effect. Additionally, the proportion of variance explained by OLRE was calculated for each individual class following Nakagawa and Schielzeth (2013). This analysis included 813 visit rates by 89 individuals observed in 28 nesting attempts by 25 unit-years.
Socio-Environmental Effects on Among-Day Variation in Carer Contributions
Phenological, meteorological, and social variables might also be expected to impact among-day variation in individual nest visitation rates, either because food availability is declining (days since last meaningful rain) or because it is harder to obtain or more costly to deliver on days with high temperature or wind speeds. To test these possibilities, a coefficient of variation (CV) was calculated using among-day variation in nest visitation rates by each individual to capture their within-attempt temporal variability. We fitted to the data a GLMM with a gamma distribution and log link function. The explanatory terms were: the mean number of days since last meaningful rain; three meteorological variables (mean and SD of mean daily temperature over observation days of each attempt, and the SD of mean daily wind speed); carer number; and individual class (three subordinate classes: SAR, SYR SU, and dominant male: DM). We also included the two-way interactions between carer number and the four environmental parameters (4 not 3 because of interest in both mean and SD of temperature). Nest identity and the unit of observation (OLRE) were included as random intercepts. This analysis included 149 CV values by 105 carers at the 29 nesting attempts by 26 unit-years (7 helpers in 2007 became dominant in 2008, hence 105 not 111 carers).
Socio-Environmental Effects on Group-Level Coordination
Finally, we tested whether any variation in the patterns of contributions by different classes of carer and their socio-environmental predictors influenced the coordination of nest visitation among carers in a unit. During nestling provisioning, the whole unit invariably arrives in the nest vicinity, even though not all individuals provision every time they do so (Nomano et al., 2014). Further, those that do provision tend to do so in relatively quick succession (i.e., synchronously; Nomano et al., 2013, 2015), and this likely plays some role in observed turn-taking in this species (Savage et al., 2017). Visits by different individuals separated by <1 min were regarded as synchronous based on video observation in previous studies (Nomano et al., 2013, 2014). When more than two birds arrived with successive intervals of <1 min, all birds were judged as part of a single synchronous visiting cluster (even though the interval between the first and last visitor could be longer than 1 min). To quantify the overall level of synchrony by the unit and its variation among nests, we counted the number of visitation events (synchronous clusters plus asynchronous visits) separated by gaps of ≥1 min, and took the ratio of the number of visitation clusters to the total number of individual visits. The total number of visits sets the upper limit to the possible number of synchronous clusters, and therefore, this ratio becomes smaller when the level of synchrony is greater, and a value of 1 would indicate perfect asynchrony. We fitted a binomial GLMM with logit link function to test effects of phenology (days since last meaningful rain) and meteorological variables (mean daytime temperature, mean daytime wind speed) and carer number on this ratio, as well as the two-way interactions between carer number and the three environmental variables. Nest identity and the unit of observation (OLRE) were included as random intercepts. This analysis included n = 191 nest-days for 29 nesting attempts.
General Methods for Statistical Modeling
Each of the random intercepts in all models had a hierarchical normal prior N(0, σ2), and σ had a uniform distribution from 0 to 100 as a hyper-prior, and non-centering (Papaspiliopoulos et al., 2007) was applied to facilitate convergence of estimates. Because of the relatively large number of fixed effects in the model, all the fixed effect coefficients had independent Cauchy prior, Cauchy (0, 2.5). This prior distribution has greater density around zero and longer tails and shrinks non-influential coefficients toward zero compared to commonly used non-informative normal priors (Gelman et al., 2008). In effect, this alleviates problems of potential over-parameterization without resorting to stepwise model reduction. This method also has been suggested to be robust to collinearity (Gelman et al., 2008). A non-informative normal prior was given to the intercept. All of the continuous explanatory variables were standardized by subtracting the mean and dividing by 2 SD, so that intercepts and main effects of interaction terms were evaluated at the mean value of the other predictors, and coefficients in the same models were comparable on a common scale (Gelman, 2008). All models were fitted with Markov-chain Monte-Carlo (MCMC) in RStan (Stan Development Team, 2018). We took 800 MCMC samples that form a posterior distribution of the parameters. All the parameters showed convergence with split < 1.1 (Gelman et al., 2013).
Results
Socio-Environmental Effects on Nest Visitation by Dominant Breeding Males
When broods were aged 9–21 d, dominant breeding males visited nests at a mean rate of 4.4 times/h (SD = 2.3, range = 0.2–18.0) over the hours of daylight. The predictors of dominant male provisioning rates were phenological and meteorological (Table 1). Most notably, breeding males reduced their visitation rate by ~50% from a high of ~5 feeds/h around 80 d since last rain to a low of about ~2.5 feeds/h by 160 d since last rain (Figure 1A). Further, they also visited less frequently on days with high mean wind speed, although the magnitude of this effect was less than that of days since last meaningful rainfall (Figure 1B). By contrast, mean daytime temperature had no significant impact on visitation rates of dominant breeding males. Finally, we found no evidence to suggest that carer number modified the rate of visitation by dominant breeding males in challenging conditions, for none of the interactions between carer number and the three environmental variables was significant (Table 1). Thus, the provisioning rate of dominant males was negatively impacted by delayed breeding phenology and to a lesser extent high wind speeds, with the number of carers neither mitigating nor exacerbating the negative effects of challenging conditions.
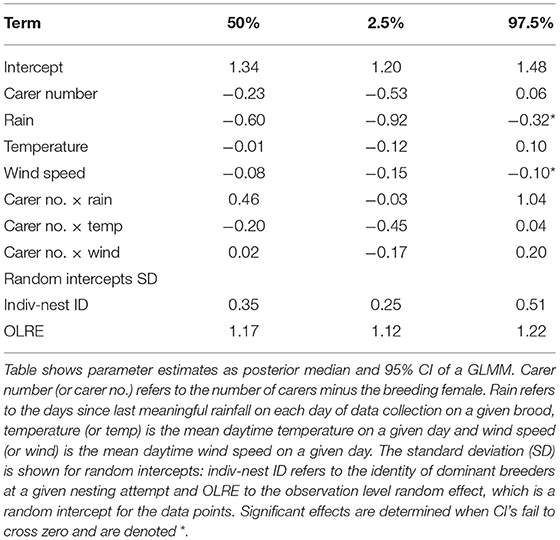
Table 1. Phenological, meteorological, and social effects on the provisioning rate of dominant breeding males.
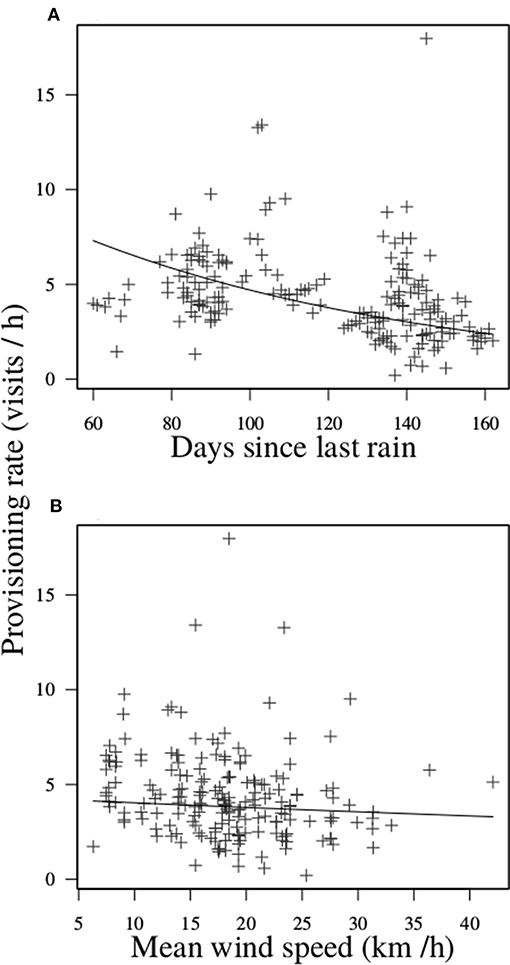
Figure 1. Daily provisioning rate of dominant males as a function of: (A) days since last meaningful rain; and (B) mean daily wind speed (km/h). Lines show predicted means.
Socio-Environmental Effects on Nest Visitation by Helpers
Helpers visited the nest on average 2.3 times/h (SD = 2.1, range = 0–13.1) over the course of days when broods were aged 9–21 d (Table 2). However, there was substantial variation in nest visitation patterns among helper classes. First, while those that were related to broods at the half-sib level or more, irrespective of their age, rarely showed zero inflation in their probability of visiting the nest (~8% of days more than expected by a Poisson distribution), those that were more distantly related commonly did so (~35% of days more than expected; Figure 2A). Second, while related helpers of both age categories visited the nest ~50% less often than dominant breeders, more distantly related helpers did so ~70% less often than related helpers and almost 70% less often than dominant breeders (Figure 2B). Exclusion of females from the analysis yielded similar effect sizes, and made only minor changes to the credible intervals (Table S1, Figure S1).
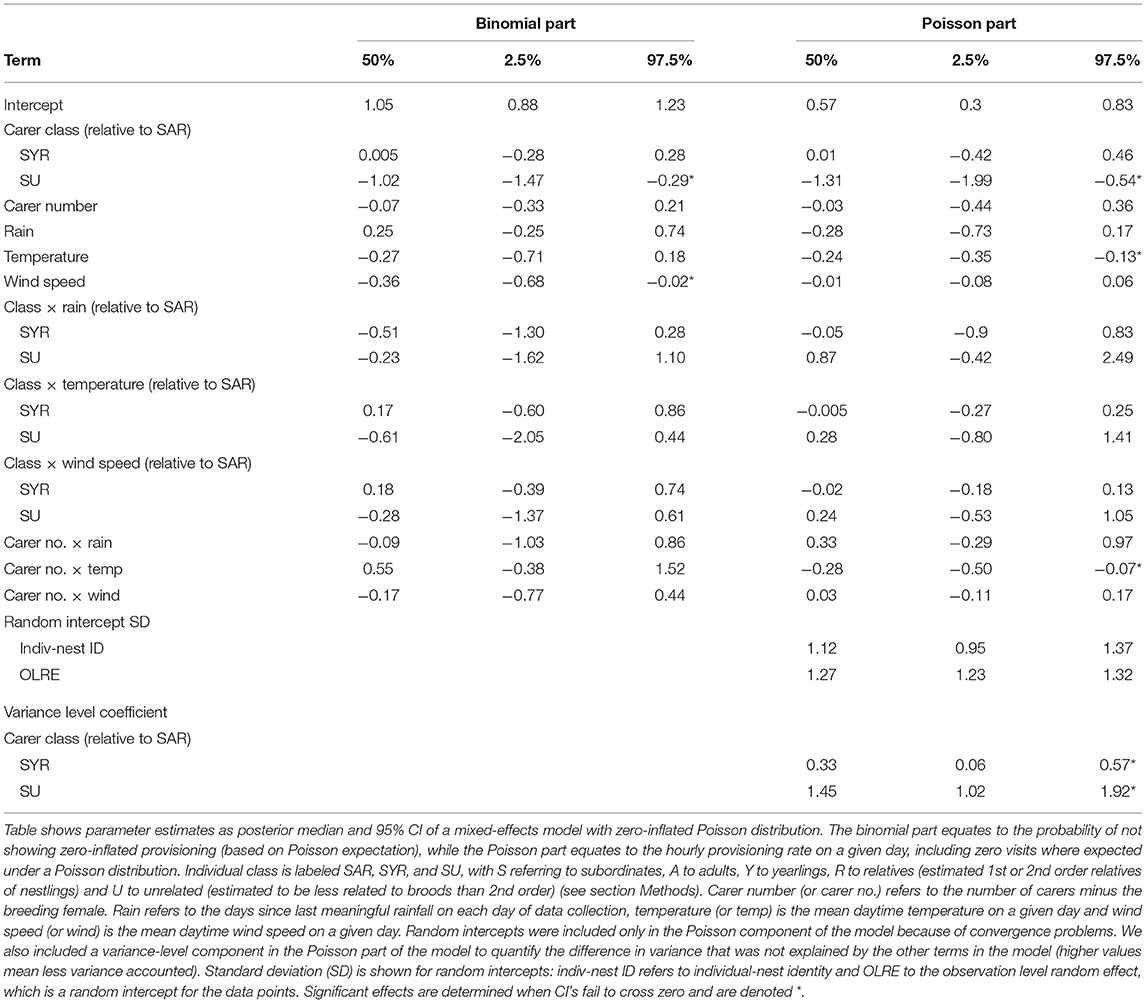
Table 2. Phenological, meteorological, and social effects on the provisioning rate of helpers as a function of their age-relatedness class.
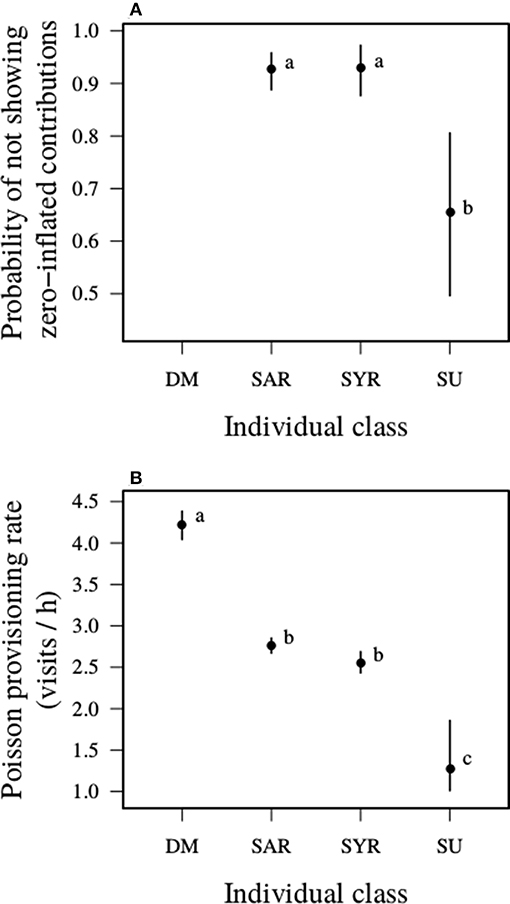
Figure 2. Daily provisioning rates of different classes of carers. Carer classes varied in: (A) their propensity for zero-inflation (i.e., visiting the nest less often on a given day than expected by a Poisson distribution); and (B) their provisioning rate based on a Poisson expectation. DM, dominant breeding males; SAR, helping adult relatives of at least one member of the breeding pair; SYR, helping yearling relatives; SU, unrelated helpers that were more distantly related or unrelated to either member of the breeding pair. The different letters inset indicate where the 95% CI of the difference between the categories did not include zero. Plots show marginalized prediction of means and 95% CI.
Patterns of helper nest visitation were impacted by some, but not all, of the socio-environmental predictors analyzed. Overall, the probability of helpers showing zero-inflated patterns of nest visitation was uninfluenced by days since last meaningful rain (Figure 3A) or mean daytime temperature (Figure 3B), but increased with increasing daytime wind speed (Figure 3C). In addition, the probability of zero inflation was uninfluenced by carer number (Table 2). In contrast to dominants, helpers showed reduced provisioning rates at high temperatures (Figure 3E), only showed a non-significant tendency to reduce provisioning rates with increasing phenology (Figure 3D) and showed no evidence of being impacted by wind speed (Figure 3F). While a lack of a wind speed effect on visitation rates might be due to the increased probability of zero-inflation in high winds, combining the regression estimates from the binomial and Poisson components of the model showed that the temperature effect on visitation rates was more salient for overall rates of nest visitation than was the wind effect on zero inflation (Figure 3G). Further, there was little firm evidence to suggest that different classes of helper contrasted in their responses to the three environmental parameters (Table 2), although there was a non-significant tendency for related (but not unrelated) helpers to reduce their provisioning rate with increasing days since last meaningful rain (Figure 3D). Finally, as was the case with dominants, there was no overall carer number effect on helper provisioning rates (Table 2), although helpers in larger units provisioned relatively more frequently than those in smaller units when temperatures were low but not high (Figure 3H). These results were affected little by the exclusion of females (Table S1, Figure S2).
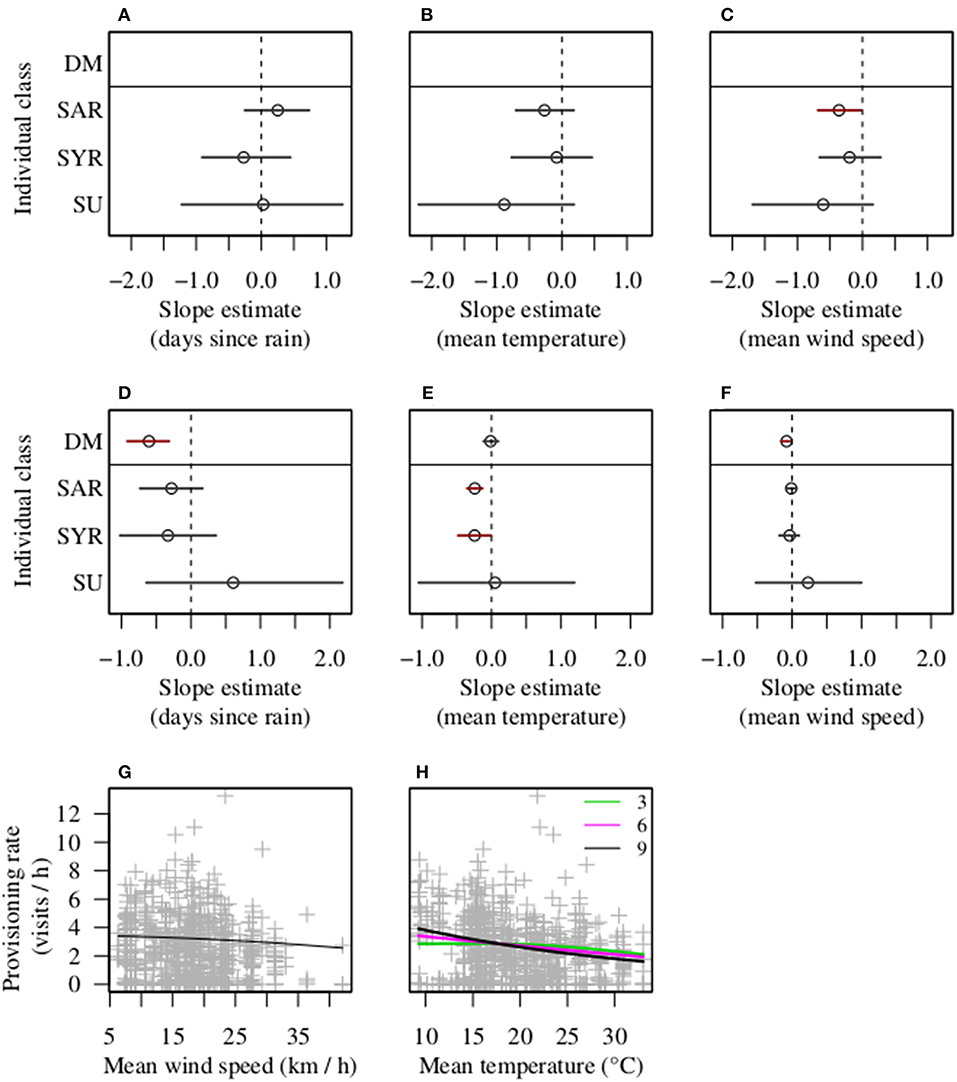
Figure 3. Environmental predictors of carer provisioning rates. Figures show the slope effect sizes for each carer class as a function of the three environmental variables tested, and provisioning rate as a function of meteorological variables and carer number. (A–C) Effects on the probability of not showing excess zero values for days since last meaningful rain, mean daily temperature and mean daily wind speed, respectively. (D–F) Effects on the provisioning rate explained by a Poisson distribution. The slope estimates for non-reference categories were calculated by combining interaction and main effect parameters. Acronyms and meteorological measures are as for Figure 2. Bars show 95% CI, with black bars overlapping zero and red bars not doing so. (G,H) Effects of wind speed on individual provisioning rate, and an interaction between the number of cares and temperature, respectively. Numbers inset in (H) are the number of carers excluding the breeding female. The curves are predicted expectations of zero-inflated Poisson distribution (i.e., based on both binomial part coefficients and Poisson part coefficients).
The lack of socio-environmental influence on the unrelated helpers was reflected in the estimates of variance-level components of the model (Table 2). These estimates reflect the residual, unexplained variance remaining after consideration of the fixed effects and individual-nest random effect. While the proportion of such unexplained within-individual variance was similarly lower for dominant breeders and adult related helpers, and only slightly higher for yearling related helpers, it was markedly higher for non-relatives (Figure 4). Put another way, the socio-environmental predictors included in the models account for comparably large variation in dominant breeders and related adult helpers, slightly less variation in yearling helpers, but substantially less by non-relatives. The result was similar when female helpers were removed from the analysis (Table S2, Figure S3). These results suggest that young relatives and especially non-relatives, have more opportunistic patterns of nest visitation (e.g., sensitive to recent foraging success).
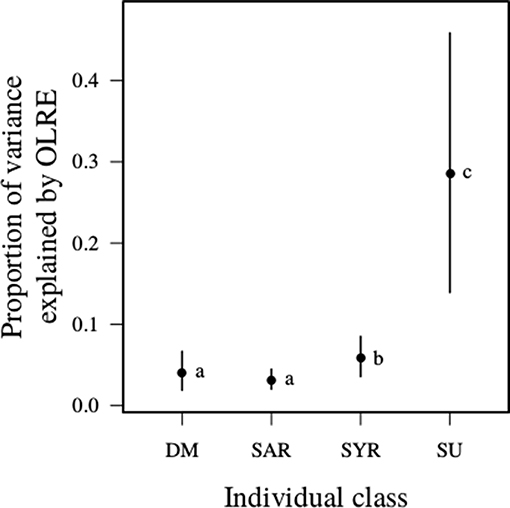
Figure 4. Proportion of variance explained by observation level random effect (OLRE) to total variance (i.e., sum of fixed effect variance, random effect variance, and distribution specific variance) in daily provisioning rate explained by a Poisson distribution (Table 2). Acronyms and meteorological measures are as for Figure 2. The different letters inset indicate where the 95% CI of the difference between the categories did not include zero. Plot shows predicted means and 95% CI.
Socio-Environmental Effects on Among-Day Variation in Carer Contributions
The coefficient of variation in individual nest visitation rates averaged 0.7 (SD = 0.7, range = 0.05–2.8). This variation was explained primarily by carer class and phenology, while social and meteorological conditions appeared to have had little or no influence (Table 3). While the mean daily visitation rate of dominant breeding males was ~4 times their standard deviation, the value for related helpers was half this, and the mean visitation rate of unrelated helpers was less than their standard deviation (Figure 5A). Further, the visitation rate of individuals varied more among consecutive days of the same nesting attempt as the mean number of days since last meaningful rain increased (Figure 5B). By contrast, we found no evidence to suggest that day-to-day variation in individual visitation rates within breeding attempts were influenced by the mean daytime temperature or the among-day variation (SD) in the mean daytime temperature within an attempt, or by the day-to-day variation in mean daytime wind speed. Nor was there any evidence of main effect of carer number, or evidence to suggest that the number of carers in the unit modified the environmental parameters considered. These results were unchanged following exclusion of female helpers (Table S2, Figure S4).
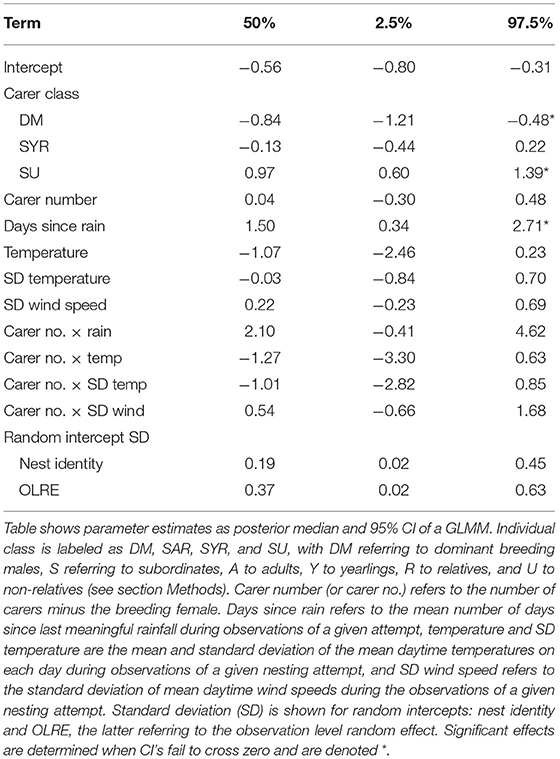
Table 3. Phenological, meteorological, and social effects on the coefficient of variation in daily provisioning rates across days within nesting attempts as a function of carer class.
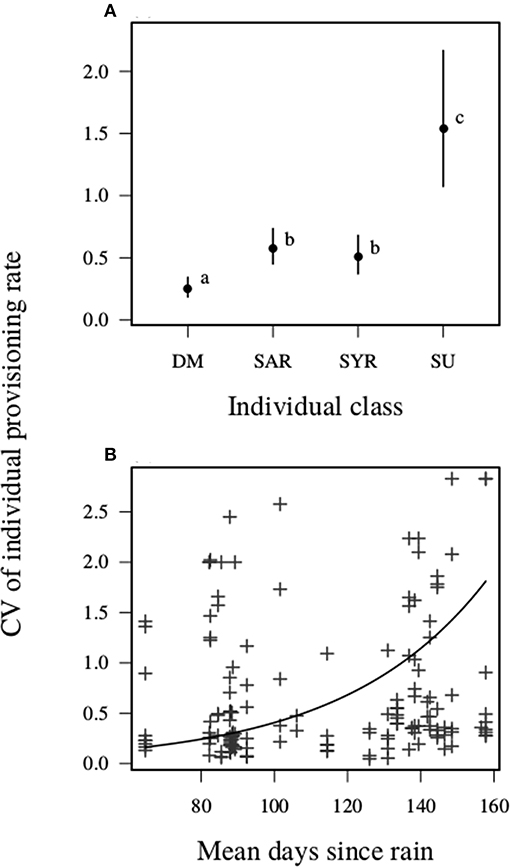
Figure 5. Coefficient of variation (CV) in carer nest visitation among days. (A) The CV of individual provisioning rate differed among different classes of individuals, and (B) increased as a function of mean days since last meaningful rain. Acronyms as for Figure 2, and letters inset indicate that 95% CI of the difference between the categories did not include zero. Plots show predicted means, and error bars show 95% CI.
Socio-Environmental Effects on Group-Level Coordination
Nest visit asynchrony, measured as the ratio of the number of runs of visits within 1 min of each other (clusters) to the number of individual visits, was relatively high (mean = 0.7), but variable (SD = 0.14, range = 0.38–1). The level of nest visit synchrony increased with carer number, which might be expected by chance since with more carers the probability that runs of nest visits within 1 min of each other will increase. While there were no main effects of the phenological or meteorological predictors, there was a significant interaction between carer number and mean daytime temperature (Table 4). Specifically, individual visits were more asynchronous in large units on days with high mean daytime temperatures (Figure 6). These results suggest that nest visit synchrony is generally low, and not compromised further by challenging conditions, except in large units on hot days.
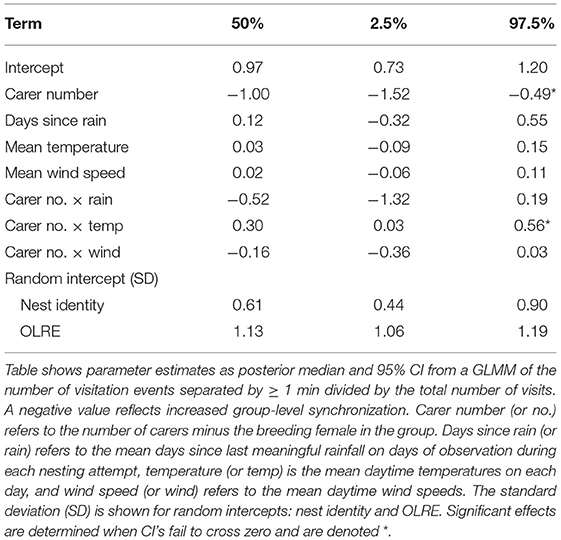
Table 4. Phenological, meteorological, and social effects on group-level synchronization of nest visits.
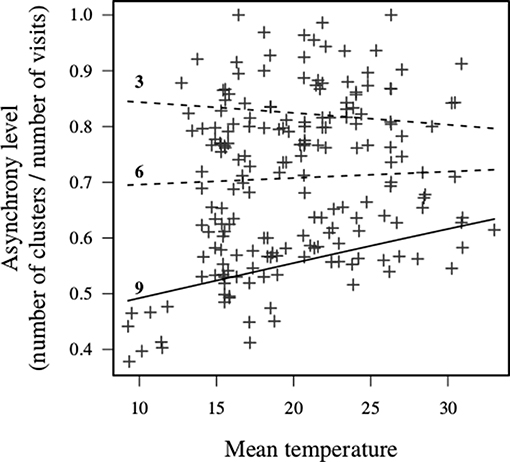
Figure 6. Interaction between carer number and mean daytime temperature on group-level synchrony. The numbers in the figures indicate the number of carers excluding the breeding female. Lines show predicted means for each value of the carer number. Solid line indicates that 95% CI of the slope estimate did not include zero, and dashed line indicates that it included zero. Only large units (e.g., 9+ carers) increased asynchrony with increased daily temperature, whilst smaller units showed no clear change.
Discussion
Individual nest visitation rates were primarily predicted by carer class and environment. Dominant breeding males visited the nest most, and showed the least day-to-day variation in their visitation rates, while related helpers showed intermediate visitation rates and among-day variation, and unrelated helpers were much less likely to visit the nest than expected by a Poisson distribution, showed low visitation rates and high among-day variation. Environmental, but not social, variation generally played an important role in explaining variation in patterns of nest visitation. From 3 months since the last meaningful rainfall, dominant breeding males (and to a lesser extent related helpers) showed reduced visitation rates and all carers showed increased day-to-day variation. Dominant males also contributed less on days with high mean wind speed, while helpers showed zero inflation on such days and further showed reduced visitation rates on days with higher mean temperature. Carer number had no overall effect on patterns of nest visitation, although those in larger units synchronized their visits less than those in smaller units on days with high mean temperature. Together, these results suggest that high wind speeds, high temperatures, and protracted periods without sufficient rain are all likely to increase the costs of nestling care with detrimental impacts for developing offspring. Finally, given that different classes of individuals varied markedly in their patterns and predictability of nest visitation, as well as their sensitivity to differing environmental conditions, babbler units likely face a significant coordination problem in provisioning. Indeed, despite units visiting the general nesting area together (Nomano et al., 2014; Sorato et al., 2016), nest visitations were relatively asynchronous; especially for large units on hot days.
Before discussing the functional explanations and implications of these results, it is important to consider potential confounding sources of variation. Most notably, while we were able to obtain a comprehensive set of nest visitation data using our automated PIT-tag system, we were not able to measure actual feeding rate. We are not overly concerned about variation in load size, for our nest-camera evidence shows that provisioning rate is a substantially more important metric of biomass delivered than more slight variation in the single prey loads delivered (Browning et al., 2012b). Nor do we think that false-feeding is confounding, since it is very low (<5% of visits) and occurs when broods reject the food despite repeated attempts (Young et al., 2013). More of a potential concern is the rate of non-feeding, where carers visit the nest without food, and which occurs in ~10% of nest visits by the carers included in the analyses on average (Young et al., 2013). Nevertheless, we have shown previously that non-feeding rate is independent of carer number and immigrant status, is weakly but positively associated with individual provisioning rate, and is insufficiently variable among individuals to alter the rank order of individual provisioning rates (Young et al., 2013). Thus, our available evidence suggests that the method of data collection in this study captures individual provisioning rates.
This is the third study of ours to report the association between carer class and provisioning behavior using data collected in 2007 and 2008, although each has been to a different end and so with methodological and/or analytical distinctions. Using nest-video data, Browning et al. (2012a) showed that breeding males provisioned at the fastest rate, followed by adult helpers, yearling helpers, and breeding females. Further, using PIT-tag data, Browning et al. (2012b) showed that helpers related to broods at first and second order levels visited the nest three times more frequently that those more distantly related, and again that adults showed higher nest visitation rates than yearlings. Here using a PIT-tag based data set restricted to broods aged 9–21 d and nests with at least 4 days of data, as well as a contrasting statistical approach, we found that dominant males visited the nest most frequently, that adult and yearling helpers related to either dominant by at least second-order visited the nest with intermediate frequency and those helpers more distantly related to the brood did so with least frequently. Why we failed to detect a significant difference between adult and yearling related helpers is not known, but it suggests that the age difference is not general. A key advance of this study was to remove the assumption that different classes of carer are drawn from the same statistical population and to account for unequal variance structures through variance-level regression coefficients (see section Methods). Doing so was justified by our results, for not only did unrelated helpers show significantly increased zero inflation but the explanatory terms considered explained substantially less of the marked variation in nest visitation rates by such helpers. Not accounting for both of these issues will confound the predictive power of fixed effects, and we suggest that the approach we adopt might be used fruitfully in future studies of individual contributions in other cooperative breeders.
Despite this study necessarily being conducted during sufficiently favorable conditions, carer provisioning rates were nonetheless significantly influenced by breeding phenology relative to the last meaningful rain event and daily meteorological conditions. First, dominant breeding males, and to some extent related helpers, were negatively impacted by delayed breeding phenology. An obvious explanation is that these effects were caused by “global” reductions in food availability and food depletion by babblers, for which we have evidence (Sorato et al., 2016). By contrast, we do not think late breeding by inferior units on low quality habitat offers a viable explanation, since chestnut-crowned babblers are weakly territorial (Sorato et al., 2015) and almost all incidences of late breeding were second attempts. However, because they were second attempts, the phenology effects on provisioning could stem from costs of prior investment (Russell et al., 2003). Contrary to this hypothesis, however, increasing days since last meaningful rain was associated with an increase in among-day variation in carer provisioning rates, which would be expected if food availability were declining, whereas we would expect the reverse under a prior cost of investment hypothesis (Mathot et al., 2009). Second, patterns of provisioning were influenced to a varying degree by both wind speed and temperature. That dominant males reduced their provisioning rate on days with high winds and helpers showed increased zero inflation on such days were unsurprisingly in this weakly flying species inhabiting an open environment, since both the costs of flying and the risk of aerial predation likely increase in high winds. The temperature effect on helpers, but not dominants, was more ambiguous. On the one hand a negative relationship between temperature and provisioning might reflect reduced energetic demand of nestlings with increasing temperatures (see also MacColl and Hatchwell, 2003), but on the other hand it might reflect an increasing difficulty of provisioning in high temperatures (Wiley and Ridley, 2016). Further work is required to disentangle these effects, but given the mean 9–33°C daytime temperature range during provisioning observations (let alone the −2 to 41°C total range), it is likely that the decline in provisioning by helpers is initially driven by reduced brood demand under increasing temperatures and only latterly by the costs of provisioning as temperatures become prohibitively hot toward the summer months (du Plessis et al., 2012; Wiley and Ridley, 2016; Andrew et al., 2017; Funghi et al., 2019).
Despite the significant environmental impacts on individual provisioning rates documented here (and elsewhere, Wiley and Ridley, 2016), cooperative breeding systems are disproportionately represented in challenging climatic environments (Jetz and Rubenstein, 2011; Cornwallis et al., 2017; Griesser et al., 2017; Lukas and Clutton-Brock, 2017). One suggested advantage of group-breeding in such environments is that it helps to buffer against climatic variation (Rubenstein and Lovette, 2007; Kennedy et al., 2018), although how this might manifest is not clear. One possibility in the context of patterns of provisioning is that individuals in groups have improved foraging efficiency because they are better able to locate and/or obtain food (Clark and Mangel, 1986; Beauchamp, 1998; Clutton-Brock et al., 1999; Ridley et al., 2013). This foraging hypothesis leads to the predictions that increasing numbers of carers mitigate the impact of detrimental environmental conditions. On the contrary, we found little evidence to suggest that the number of carers in breeding units: (a) impacted the mean or among-day variation in provisioning rates of dominant breeding males or helpers; (b) mitigated the negative effects of days since last meaningful rain or wind speed on provisioning; or (c) reduced the positive effects of days since last meaningful rain on among-day variation in provisioning rates. Indeed, the only statistically significant interaction was between carer number and mean temperature on helper provisioning rates, but, as discussed above, this was likely to be driven more by the benefits of providing offspring with more food during cold conditions than mitigating the costs of provisioning at high temperatures. Together, these results suggest that any mechanism of environmental buffering in chestnut-crowned babblers is not mediated by unit size effects on foraging ability or success in challenging conditions.
Where carer classes vary in their patterns of nest visitation and are influenced by contrasting environments, coordinating nest visits can become challenging. Coordinating provisioning events not only provides a mechanism to reduce conflict over allocations to brood care within the group (Johnstone et al., 2014), but can also reduce sibling competition (Shen et al., 2010) and the risk of nest predation (Raihani et al., 2010; Leniowski and Wegrzyn, 2018). During the nestling phase, babbler units forage on average ~200 m from the nest and show a mean daily maximal distance from the nest of ~550 m, although larger units forage further away than smaller units (Sorato et al., 2016). Because this distance can be traveled in any direction from their relatively centrally-placed nest, unit members risk becoming detached if they leave the unit to provision alone, which, along with the predation risk during flight in the open habitat (Sorato et al., 2012), probably explains why all unit members invariably fly back to the nest area during provisioning bouts, even if they do not provision the nestlings (Nomano et al., 2014). How returns to the nest are orchestrated is not known, but presumably it requires a threshold proportion of individuals to “agree” to return to provision. However, with units comprising individuals likely varying in their cost and/or benefit functions of providing care (McAuliffe et al., 2015), chestnut-crowned babbler units presumably suffer a coordination problem during provisioning. This problem is supported by the substantial variation in group-level asynchrony observed, which varied from <0.5 to almost 1 (SD = 0.15), indicating that on some days almost half the provisioning events were synchronized provisioning events, but on other days, almost none was. Because increasing unit size can lead to increased estimates of synchrony by chance, the positive effect of carer number on synchrony is ambiguous. Nevertheless, that large units visited the nest more asynchronously on days with high daytime temperatures and the provisioning rate of helpers is also negatively impacted on such days, suggests that climatic impacts on individuals can have group-level consequences for coordination. Further work is required to clarify the role of coordination in stabilizing individual contributions to cooperation in this system (Savage et al., 2017) and the consequences for offspring development, which are known to be impacted in other systems (Shen et al., 2010).
The results of this study have at least four important implications. First, increasing temperatures and stochastic weather events, including continuing patterns of reduced rainfall and increasing wind speed in this desert environment, are likely to have significant effects on patterns of provisioning in chestnut-crowned babbler, with likely ramifications for the costs of helping and the quality of developing young (see also Wiley and Ridley, 2016). Second, while there is much interest in explaining the occurrence of unrelated helpers with adaptive explanations (e.g., Clutton-Brock et al., 2002; Bergmüller et al., 2007; Riehl, 2013), the evidence from this study using specific statistical approaches suggests that their contributions are not only low, but largely random. Third, that individuals vary in their contributions and are variably sensitive to different environmental variables suggest that changing climates will also have detrimental effects on group-level synchronization of nest visits, with further implications for both the stable contribution by individuals (Johnstone et al., 2014; Savage et al., 2017) and offspring development (Shen et al., 2010). Finally, while cooperative breeders are suggested to be adapted to dealing with climatic challenges (Cornwallis et al., 2017; Griesser et al., 2017; Kennedy et al., 2018), the mediating mechanism is unclear. Our results suggest that improved foraging efficiency at the individual level, as measured by individual contributions to provisioning, is not the key means of buffering against environmental challenges in this system.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Fieldwork was approved by the UNSW Animal Care and Ethics Committee (license number 06/40A) and NSW National Parks and Wildlife Service and the Australian Bird and Bat Banding Scheme. We did not detect any harmful effects of the tags on the birds, which is consistent with other studies on similarly sized passerines (Nicolaus et al., 2008; Schroeder et al., 2011).
Author Contributions
FN and AR conceived this specific study and wrote the paper. Data were collected by LB, JS, and AR. SG conducted the molecular work. FN conducted the analyses. All authors approved the submission.
Funding
The study was supported by Natural Environment Research Council (studentship, LB; New Investigators, AR NE/D000394/1), Australian Research Council (AR, SG, DP1094295), the Royal Society University Fellowship Scheme, UK (AR), Irish Research Council (JS), and Japan Society for the Promotion of Science (FN, 18J00154).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to David Croft, Keith Leggett, and the Dowling family for logistical support at Fowlers Gap, Lee Ann Rollins for lab work. Elena Berg, Sam Patrick, Bec Rose, and Beth Woodward helped with fieldwork.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00423/full#supplementary-material
References
Andrew, S. C., Awasthy, M., Griffith, A. D., Nakagawa, S., and Griffith, S. C. (2018). Clinal variation in avian body size is better explained by summer maximum temperatures during development than by cold winter temperatures. Auk 135, 206–217. doi: 10.1642/AUK-17-129.1
Andrew, S. C., Hurley, L. L., Mariette, M. M., and Griffith, S. C. (2017). Higher temperatures during development reduce body size in the zebra finch in the laboratory and in the wild. J. Evol. Biol. 30, 2156–2164. doi: 10.1111/jeb.13181
Beauchamp, G. (1998). The effect of group size on mean food intake rate in birds. Biol. Rev. 73, 449–472. doi: 10.1017/S0006323198005246
Bergmüller, R., Johnstone, R. A., Russell, A. F., and Bshary, R. (2007). Integrating cooperative breeding into theoretical concepts of cooperation. Behav. Proc. 76, 61–72. doi: 10.1016/j.beproc.2007.07.001
Bolton, M. (1995). Food delivery to nestling storm petrels: limitation or regulation? Funct. Ecol. 9, 161–170. doi: 10.2307/2390560
Both, C., Bouwhuis, S., Lessells, C. M., and Visser, M. E. (2006). Climate change and population declines in a long distance migratory bird. Nature 441, 81–83. doi: 10.1038/nature04539
Both, C., van Asch, M., Bijlsma, R. G., Van Den Burg, A. B., and Visser, M. E. (2009). Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J. Anim. Ecol. 78, 73–83. doi: 10.1111/j.1365-2656.2008.01458.x
Both, C. G., Bijlsma, R. E., and Visser, M. (2005). Climatic effects on timing of spring migration and breeding in a long-distance migrant, the pied flycatcher Ficedula hypoleuca. J. Avian. Biol. 36, 368–373. doi: 10.1111/j.0908-8857.2005.03484.x
Briga, M., and Verhulst, S. (2015). Large diurnal temperature range increases bird sensitivity to climate change. Sci. Rep. 5:16600. doi: 10.1038/srep16600
Browning, L. E., Patrick, S. C., Rollins., L. A, Griffith, S. C., and Russell, A. F. (2012a). Kin selection, not group augmentation, predicts helping in an obligate cooperatively breeding bird. Proc. R. Soc. Lond. B 279, 3861–3869. doi: 10.1098/rspb.2012.1080
Browning, L. E., Young, C. M., Savage, J. L., Russell, D. J. F., Barclay, H., Griffith, S. C., et al. (2012b). Carer provisioning rules in an obligate cooperative breeder: prey type, size and delivery rate. Behav. Ecol. Sociobiol. 66, 1639–1649. doi: 10.1007/s00265-012-1419-z
Clark, C. W., and Mangel, M. (1986). The evolutionary advantages of group foraging. Theor. Pop. Biol. 30, 45–75. doi: 10.1016/0040-5809(86)90024-9
Cleasby, I. R., and Nakagawa, S. (2011). Neglected biological patterns in the residuals. Behav. Ecol. Sociobiol. 65, 2361–2372. doi: 10.1007/s00265-011-1254-7
Clutton-Brock, T. H., O'Riain, M. J., Brotherton, P. N., Gaynor, D., Kansky, R., Griffin, A. S., et al. (1999). Selfish sentinels in cooperative mammals. Science 284, 1640–1644. doi: 10.1126/science.284.5420.1640
Clutton-Brock, T. H., Russell, A. F., Sharp, L. L., Young, A. J., Balmforth, Z., and McIlrath, G.M. (2002). Evolution and development of sex differences in cooperative behaviour in meerkats. Science 297, 253–256. doi: 10.1126/science.1071412
Cockburn, A. (1998). Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177. doi: 10.1146/annurev.ecolsys.29.1.141
Cohen, J. M., Lajeunesse, M. J., and Rohr, J. R. (2018). A global synthesis of animal phenological responses to climate change. Nat. Clim. Change 8, 224–228. doi: 10.1038/s41558-018-0067-3
Cornwallis, C. K., Botero, C. A., Rubenstein, D. R., Downing, P. A., West, S. A., and Griffin, A. S. (2017). Cooperation facilitates the colonization of harsh environments. Nat. Ecol. Evol. 1:57. doi: 10.1038/s41559-016-0057
Cushing, D. H. (1969). The regularity of the spawning season of some fishes. ICES J. Mar. Sci. 33, 81–92. doi: 10.1093/icesjms/33.1.81
du Plessis, K. L., Martin, R. O., Hockey, P. A. R., Cunningham, S. J., and Ridley, A. R. (2012). The costs of keeping cool in a warming world: implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob. Change Biol. 18, 3063–3070. doi: 10.1111/j.1365-2486.2012.02778.x
Englert Duursma, D., Gallagher, R. V., and Griffith, S. C. (2019). Variation in the timing of avian egg-laying in relation to climate. Ecography 42, 535–548. doi: 10.1111/ecog.03602
Funghi, C., McCowan, L. S., Schuett, W., and Griffith, S. C. (2019). High air temperatures induce temporal, spatial and social changes in the foraging behaviour of wild zebra finches. Anim. Behav. 149, 33–43. doi: 10.1016/j.anbehav.2019.01.004
Gelman, A. (2008). Scaling regression inputs by dividing by two standard deviations. Stat. Med. 27, 2865–2873. doi: 10.1002/sim.3107
Gelman, A., Carlin, J. B., Stern, H. S., Dunson, D. B., Vehtari, A., and Rubin, D. B. (2013). Bayesian Data Analysis, 3rd Edn. London: Chapman & Hall; CRC Press.
Gelman, A., Jakulin, A., Pittau, M. G., and Su, Y. S. (2008). A weakly informative default prior distribution for logistic and other regression models. Ann. Appl. Stat. 2, 1360–1383. doi: 10.1214/08-AOAS191
Griesser, M., Drobniak, S. M., Nakagawa, S., and Botero, C. A. (2017). Family living sets the stage for cooperative breeding and ecological resilience in birds. PLoS Biol. 15:e2000483. doi: 10.1371/journal.pbio.2000483
Hatchwell, B. J., Russell, A. F., MacColl, A. D., Ross, D. J., Fowlie, M. K., and McGowan, A. (2004). Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav. Ecol. 15, 1–10. doi: 10.1093/beheco/arg091
Holleley, C. E., Russell, A. F., and Griffith, S. C. (2009). Isolation and characterization of polymorphic tetranucleotide microsatellite loci in the chestnut-crowned babbler (Pomatostomus ruficeps). Mol. Ecol. Res. 9, 993–995. doi: 10.1111/j.1755-0998.2009.02528.x
Hoset, K. S., Espmark, Y., Moksnes, A., Haugan, T., Ingebrigtsen, M., and Lier, M. (2004). Effect of ambient temperature on food provisioning and reproductive success in snow buntings Plectrophenax nivalis in the high arctic. Ardea 92, 239–246.
Jetz, W., and Rubenstein, D. R. (2011). Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr. Biol. 21, 72–78. doi: 10.1016/j.cub.2010.11.075
Johnstone, R. A., Manica, A., Fayet, A. L., Stoddard, M. C., Rodriguez-Gironés, M. A., and Hinde, C. A. (2014). Reciprocity and conditional cooperation between great tit parents. Behav. Ecol. 25, 216–222. doi: 10.1093/beheco/art109
Johnstone, R. A., and Savage, J. L. (2019). Conditional cooperation and turn-taking in parental care. Front. Ecol. Evol. 7:335. doi: 10.3389/fevo.2019.00335
Kennedy, P., Higginson, A. D., Radford, A. N., and Sumner, S. (2018). Altruism in a volatile world. Nature 15, 359–362. doi: 10.1038/nature25965
Król, E., Murphy, M., and Speakman, J. R. (2007). Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J. Exp. Biol. 210, 4233–4243. doi: 10.1242/jeb.009779
Lejeune, L. A., Savage, J. L., Bruendl, A. C., Thiney, A., Russell, A. F., and Chaine, A. S. (2019). Environmental effects on parental care visitation patterns in blue tits Cyanistes caeruleus. Front. Ecol. Evol. 7:356. doi: 10.3389/fevo.2019.00356
Leniowski, K., and Wegrzyn, E. (2018). Synchronisation of parental behaviours reduces the risk of nest predation in a socially monogamous passerine bird. Sci. Rep. 8:7385. doi: 10.1038/s41598-018-25746-5
Liebl, A. L., Nomano, F. Y., Browning, L. E., and Russell, A. F. (2015). Experimental evidence for fully additive care among male carers in the cooperatively breeding chestnut-crowned babbler. Anim. Behav. 115, 47–53. doi: 10.1016/j.anbehav.2016.02.024
Luck, G. W. (2001). Variability in provisioning rates to nestlings in the cooperatively breeding Rufous Treecreeper, Climacteris rufa. Emu 101, 221–224. doi: 10.1071/MU00028
Lukas, D., and Clutton-Brock, T. H. (2017). Climate and the distribution of cooperative breeding in mammals. R. Soc. Open Sci. 4:160897. doi: 10.1098/rsos.160897
MacColl, A. D. C., and Hatchwell, B. J. (2003). Sharing is caring: nestling provisioning behaviour of long-tailed tit, Aegithalos caudatus, parents and helpers. Anim. Behav. 66, 955–964. doi: 10.1006/anbe.2003.2268
Mariette, M. M., and Griffith, S. C. (2015). The adaptive significance of provisioning and foraging coordination between breeding partners. Am. Nat. 185, 270–280. doi: 10.1086/679441
Mathot, K. J., Godde, S., Careau, V., Thomas, D. W., and Giraldeau, L. A. (2009). Testing dynamic variance-sensitive foraging using individual differences in basal metabolic rates of zebra finches. Oikos 118, 545–552. doi: 10.1111/j.1600-0706.2009.17357.x
McAuliffe, K., Wrangham, R., Glowacki, L., and Russell, A. F. (2015). When cooperation begets cooperation: the role of key individuals in galvanizing support. Philos. Trans. Lond. B 370:20150012. doi: 10.1098/rstb.2015.0012
McKechnie, A. E., and Wolf, B. O. (2009). Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256. doi: 10.1098/rsbl.2009.0702
McNamara, J. M., Houston, A. I., Barta, Z., and Osorno, J. L. (2003). Should young ever be better off with one parent than with two? Behav. Ecol. 14, 301–310. doi: 10.1093/beheco/14.3.301
Møller, A. P., Rubolini, D., and Lehikoinen, E. (2008). Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl. Acad. Sci. U.S.A. 105, 16195–16200. doi: 10.1073/pnas.0803825105
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Nicolaus, M., Bouwman, K. M., and Dingemanse, N. J. (2008). Effect of PIT tags on the survival and recruitment of great tits Parus major. Ardea 96, 286–293. doi: 10.5253/078.096.0215
Nomano, F. Y., Browning, L. E., Nakagawa, S., Griffith, S. C., and Russell, A. F. (2014). Validation of an automated data collection method for quantifying social networks in collective behaviours. Behav. Ecol. Sociobiol. 68, 1379–1391. doi: 10.1007/s00265-014-1757-0
Nomano, F. Y., Browning, L. E., Rollins, L. A., Nakagawa, S., Griffith, S. C., and Russell, A. F. (2013). Feeding nestlings does not function as a signal of social prestige in cooperatively breeding chestnut-crowned babblers. Anim. Behav. 86, 277–289 doi: 10.1016/j.anbehav.2013.05.015
Nomano, F. Y., Browning, L. E., Savage, J. L., Rollins, L. A., Griffith, S. C., and Russell, A. F. (2015). Unrelated helpers neither signal contributions nor suffer retribution in chestnut-crowned babblers. Behav. Ecol. 26, 986–995. doi: 10.1093/beheco/arv023
Papaspiliopoulos, O., Roberts, G. O., and Sköld, M. (2007). A general framework for the parametrization of hierarchical models. Stat. Sci. 22, 59–73. doi: 10.1214/088342307000000014
Pinheiro, J., and Bates, D. (2000). Mixed Effects Models in S and S-Plus. New York, NY: Springer-Verlag. doi: 10.1007/978-1-4419-0318-1
Portelli, D. J., Barclay, H., Russell, D. J. F., Griffith, S. C., and Russell, A. F. (2009). Social organisation and foraging ecology of the cooperatively breeding chestnut-crowned babbler (Pomatostomus ruficeps). Emu 109, 153–162 doi: 10.1071/MU08065
Queller, D. C., and Goodnight, K. F. (1989). Estimating relatedness using genetic markers. Evolution 43, 258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x
Raihani, N. J., Nelson-Flower, M. J., Moyes, K., Browning, L. E., and Ridley, A. R. (2010). Synchronous provisioning increases brood survival in cooperatively breeding pied babblers. J. Anim. Ecol. 79, 44–52. doi: 10.1111/j.1365-2656.2009.01606.x
Ridley, A. R., Nelson-Flower, M. J., and Thompson, A. M. (2013). Is sentinel behaviour safe? An experimental investigation. Anim. Behav. 85, 137–142. doi: 10.1016/j.anbehav.2012.10.017
Riehl, C. (2013). Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280:20132245. doi: 10.1098/rspb.2013.2245
Rollins, L. A., Browning, L. E., Holleley, C. E., Savage, J. L., Russell, A. F., and Griffith, S. C. (2012). Building genetic networks using relatedness information: a novel approach for the estimation of dispersal and characterization of group structure in social animals. Mol. Ecol. 21, 1727–1740 doi: 10.1111/j.1365-294X.2012.05492.x
Rose, A. P. (2009). Temporal and individual variation in offspring provisioning by tree swallows: a new method of automated nest attendance monitoring. PLoS ONE 4:e4111. doi: 10.1371/journal.pone.0004111
Rubenstein, D. R., and Lovette, I. J. (2007). Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr. Biol. 17, 1414–1419. doi: 10.1016/j.cub.2007.07.032
Russell, A. F. (2016). “Chestnut-crowned babblers: dealing with climatic adversity and uncertainty in the Australian arid zone,” in Cooperative Breeding in Vertebrates, eds W. D. Koenig and J. L. Dickinson (Cambridge, MA: University Press), 150–164. doi: 10.1017/CBO9781107338357.010
Russell, A. F., Clutton-Brock, T. H., Brotherton, P. N. M., Sharpe, L. L., McIlrath, G. M., Dalerum, F. D., et al. (2002). Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J. Anim. Ecol. 71, 700–709. doi: 10.1046/j.1365-2656.2002.00636.x
Russell, A. F., Sharp, L. L., Brotherton, P. N. M., and Clutton-Brock, T. H. (2003). Cost minimization by helpers in cooperative vertebrates. Proc. Natl. Acad. Sci. U.S.A. 100, 3333–3338. doi: 10.1073/pnas.0636503100
Saino, N., Ambrosini, R., Rubolini, D., von Hardenberg, J., Provenzale, A., Hüppop, K., et al. (2010). Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B 278, 835–842. doi: 10.1098/rspb.2010.1778
Savage, J. L., Browning, L. E., Manica, A., Russell, A. F., and Johnstone, R. A. (2017). Turn- taking in cooperative offspring care: by-product of individual provisioning behavior or active response rule? Behav. Ecol. Sociobiol. 71:162. doi: 10.1007/s00265-017-2391-4
Savage, J. L., Russell, A. F., and Johnstone, R. A. (2013). Intra-group relatedness affects parental and helper investment rules in offspring care. Behav. Ecol. Sociobiol. 67, 1855–1865. doi: 10.1007/s00265-013-1595-5
Schroeder, J., Cleasby, I. R., Nakagawa, S., Ockendon, N., and Burke, T. (2011). No evidence for adverse effects on fitness of fitting passive integrated transponders (PITs) in wild house sparrows Passer domesticus. J. Avian. Biol. 42, 271–275. doi: 10.1111/j.1600-048X.2010.05271.x
Shen, S. F., Chen, H. C., Vehrencamp, S. L., and Yuan, H. W. (2010). Group provisioning limits sharing conflict among nestlings in joint-nesting Taiwan yuhinas. Biol. Lett. 6, 318–321. doi: 10.1098/rsbl.2009.0909
Sorato, E., Griffith, S. C., and Russell, A. F. (2016). The price of associating with breeders in the cooperatively breeding chestnut-crowned babbler: foraging constraints, survival and sociality. J. Anim. Ecol. 85, 1340–1351. doi: 10.1111/1365-2656.12539
Sorato, E., Gullet, P. R., Griffith, S. C., and Russell, A. F. (2012). Effects of predation risk on foraging behaviour and group size: adaptations in a social cooperative species. Anim. Behav. 84, 823–834. doi: 10.1016/j.anbehav.2012.07.003
Sorato, E., Gullett, P. R., Creasey, M. J., Griffith, S. C., and Russell, A. F. (2015). Plastic territoriality in group-living chestnut-crowned babblers: roles of resource value, holding potential and predation risk. Anim. Behav. 101, 155–168. doi: 10.1016/j.anbehav.2014.12.012
Speakman, J. R., and Król, E. (2010). Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J Anim. Ecol. 79, 726–746. doi: 10.1111/j.1365-2656.2010.01689.x
Stan Development Team (2018). RStan: the R Interface to Stan. R Package Version 2.17.3. Available online at: https://mc-stan.org (accessed May 31, 2019).
Stienen, E. W., Van Beers, P. W., Brenninkmeijer, A., Habraken, J. M. P. M., Raaijmakers, M. H. J. E., and Van Tienen, P. G. (2000). Reflections of a specialist: patterns in food provisioning and foraging conditions in Sandwich Terns Sterna sandvicensis. Ardea 88, 33–49.
Thackeray, S. J., Henrys, P. A., Hemming, D., Bell, J. R., Botham, M. S., Burthe, S., et al. (2016). Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. doi: 10.1038/nature18608
Visser, M. E., and Both, C. (2005). Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569. doi: 10.1098/rspb.2005.3356
Visser, M. E., Gienapp, P., Husby, A., Morrisey, M., de la Hera, I., Pulido, F., et al. (2015). Effects of spring temperatures on the strength of selection on timing of reproduction in a long-distance migratory bird. PLoS Biol. 13:e1002120. doi: 10.1371/journal.pbio.1002120
Wiley, E. M., and Ridley, A. R. (2016). The effects of temperature on offspring provisioning in a cooperative breeder. Anim. Behav. 117, 187–195. doi: 10.1016/j.anbehav.2016.05.009
Young, C. M., Browning, L. E., Savage, J. L., Griffith, S. C., and Russell, A. F. (2013). No evidence for deception over allocation to brood care in a cooperative bird. Behav. Ecol. 24, 70–81. doi: 10.1093/beheco/ars137
Keywords: environmental buffering, climate change, compensation, helpers, kin selection, phenological mismatch, synchronous provisioning
Citation: Nomano FY, Savage JL, Browning LE, Griffith SC and Russell AF (2019) Breeding Phenology and Meteorological Conditions Affect Carer Provisioning Rates and Group-Level Coordination in Cooperative Chestnut-Crowned Babblers. Front. Ecol. Evol. 7:423. doi: 10.3389/fevo.2019.00423
Received: 31 May 2019; Accepted: 21 October 2019;
Published: 08 November 2019.
Edited by:
Eric L. Walters, Old Dominion University, United StatesReviewed by:
Vittorio Baglione, Universidad de León, SpainAllison Johnson, University of Nebraska-Lincoln, United States
Copyright © 2019 Nomano, Savage, Browning, Griffith and Russell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fumiaki Y. Nomano, bm9tYW5vX2Z1bWlha2lAc29rZW4uYWMuanA=
 Fumiaki Y. Nomano
Fumiaki Y. Nomano James L. Savage
James L. Savage Lucy E. Browning3,5
Lucy E. Browning3,5 Simon C. Griffith
Simon C. Griffith Andrew F. Russell
Andrew F. Russell