- 1Yale School of the Environment, Yale University, New Haven, CT, United States
- 2Department of Forestry and Environmental Science, University of Sri Jayewardenapura, Nugegoda, Sri Lanka
The Sinharaja rainforest in southwestern Sri Lanka is a protected forest in a largely agriculture-dominated landscape. In keeping with global UNESCO global biosphere reserves planning, the Sinharaja is surrounded by a buffer zone of regenerating forest and villages with small tea plots and multi-strata tree gardens (homegardens). Globally, however, conservation planning lacks standards on buffer zone management. We ask what relationships exist between village land use and bird assemblages, which are effective ecosystem indicators. Birds have been little studied across land use and vegetation structure in actively managed, large, protected forest buffer zones. To that end, we ran spatially- and temporally-replicated bird point counts across tree gardens, forest fragments, and tea plots within a Sinharaja village. Tree gardens held a greater abundance of birds across habitat association, conservation concern, diet, and endemic species than forest fragments or tea plots. Forest fragments and tree gardens hosted statistically similar numbers of birds in some subsets, but their species assemblages differed. In tea plots, greater shade tree species richness correlated with greater bird abundance and species richness. Our results support the argument for programs to support complex small-scale tree-based agroforestry embedded in buffer zone regenerating forest.
Introduction
Geological isolation and varied climate and topography produced the unique species assemblages of Sri Lanka, as in other parts of south Asia (Ripley and Beehler, 1990; Bossuyt, 2004; Kaluthota and Kotagama, 2005; Kotagama et al., 2006; Sodhi et al., 2009; Wikramanayake and Buthpitiya, 2017). In combination with the western Ghats of India, the island comprises a biodiversity hotspot with high endemism across taxa (Gunawardene et al., 2007). Twenty-seven of Sri Lanka’s observed birds are confirmed endemic and an additional six are proposed endemic (Warakagoda et al., 2013; Iucn., 2016). However, formerly largely forested, Sri Lanka has lost half its forest cover since 1930 (Naresa., 1991; Reddy et al., 2018). The loss in keeping with that of much of South Asia, which lost almost 30% of forest cover in the same period (Reddy et al., 2018). Reflecting Sri Lanka’s large-scale forest cover and habitat loss, seventeen of the island’s endemic birds are vulnerable, near threatened, or endangered (Warakagoda et al., 2013; Iucn., 2016; Abeyarama and Seneviratne, 2017). The Sinharaja rainforest, a National Park, UNESCO Man and the Biosphere Reserve, UNESCO World Heritage Site, and a Bird Life International Important Bird Area (IBA) in the southwest wet zone of Sri Lanka, is one of the only remaining stretches of relatively undisturbed, connected, lowland rainforest on the island (Gunatilleke et al., 2005). It contains significant avian phylogenetic diversity (Abeyarama and Seneviratne, 2017). In a nation of great climate and topographic heterogeneity, the Sinharaja lowland rainforest is particularly important to preserving Sri Lanka’s biodiversity, including avifauna (Gunatilleke and Gunatilleke, 1983; Gunatilleke et al., 2017).
Consistent with UNESCO’s global biosphere reserves organization, a buffer zone surrounds the Sinharaja rainforest (Unesco., 1996). Forest reserve buffer zones can help maintain local ecology (Robinson et al., 2013). However, there is a lack of global consensus on buffer zone management (Margles et al., 2010; Allan et al., 2017).
The Sinharaja’s buffer consists of regenerating forest and forest villages, which comprise homes, small tea plots, and multi-strata tree gardens (homegardens) (Wijesooriya and Gunatilleke, 2003; Dewi et al., 2013). Tree gardens reflect a system of indigenous tree culture common throughout South and Southeast Asia that is ancient but still evolving (Kumar and Nair, 2006; Martin et al., 2018). They provide medicine, food, construction material, fuel, fodder, and income to residents across the tropics from rural to urban settings. Tree gardens contribute to food security and local economies (Jaman et al., 2016; Sangakkara and Frossard, 2016; Paembonan et al., 2018; Rousta et al., 2018; Park et al., 2019). They are managed for resilience to climate and economic changes (Weerahewa et al., 2012). Tree gardens help maintain local and regional biodiversity through the tropics (Watson and Eyzaguirre, 2002; Kumar and Nair, 2006; Galluzzi et al., 2010; Kumar and Nair, 2011; Schroth et al., 2013), with specific research from Sri Lanka (Mohri et al., 2013), Indonesia (Michon et al., 1986; Mohri et al., 2013), Vietnam (Trinh et al., 2003; Mohri et al., 2013), Thailand (Timsuksai and Rambo, 2016), Malaysia (Moore et al., 2016), India (Das and Das, 2015), and Brazil (Peroni et al., 2016). In Sri Lanka, tree gardens make up almost half of total forest area and fourteen percent of the country’s total land area (Mattsson et al., 2013; Reddy et al., 2018). Research from 2010 estimated that they comprised one fifth of the country’s aboveground carbon stocks (Mattsson et al., 2013). They are actively supported by government policies (Fao., 2009; Pushpakumara et al., 2012; Chokkalingam and Vanniarachchy, 2013; Galhena et al., 2013; Mattsson et al., 2017). Understanding how and to what ends they contribute to conservation goals is necessary.
We studied bird use of a Sinharaja buffer zone village and its tree gardens. Birds provide ecological, economic, and scientific value, all of which make them important research subjects. The ecosystem services they provide include pollination, seed dispersal, and insect control (Sekercioglu, 2006). Bird-tourism is a significant and growing part of global eco-tourism, and draws foreign visitors specifically to the Sinharaja (Sekercioglu, 2002; Goodale et al., 2014; Arachchi, 2020). Birds are also effective ecosystem indicators across landscapes (Macarthur, 1964; O’Connell et al., 2000; Sekercioglu et al., 2004). Previous studies of bird populations in intensively managed tropical landscapes compared other agroforestry systems to intact forest (Thiollay, 1995; Perfecto et al., 1996; Calvo and Blake, 1998; Mas and Dietsch, 2004; Waltert et al., 2004; Beukema et al., 2007; Harvey and Villalobos, 2007; Van Bael et al., 2007; Tscharntke et al., 2008; Clough et al., 2009; Sekercioglu, 2012). Others examined tree gardens, agroforestry systems, and regenerating forest, but few did so in small-scale land use areas within the buffer zone of a large, undisturbed, conservation area (Sidhu et al., 2010; Kottawa-Arachchi and Gamage, 2015; Engelen et al., 2016; Prabowo et al., 2016; Perera et al., 2017). Yet tree gardens in buffer zones contain plants that provide food and shelter for birds (Martin et al., 2018). There is a need to quantify how the structure and size of buffer zone land use translates to bird presence around the Sinharaja (Waltert et al., 2011; Goodale et al., 2014).
To that end, we quantified the bird assemblages observed in small-scale tree gardens, forest fragments, and tea plots within the buffer zone village. We asked: (1) How does bird assemblage vary with land use? (2) Are there relationships between vegetation characteristics and bird assemblages? (3) What land use type hosts the greatest number of multi-species flocks?
Research suggests that the greater a habitat’s complexity, the greater the complexity of its species assemblage (Macarthur, 1964; Roth, 1976). We expected to see that tree gardens would host larger and more complex species assemblages than tea plots. We expected more complex vegetation would host more complex bird assemblages. Since multi-species flocks are a documented forest phenomenon, we expected to see more flocks in forest fragments (Goodale et al., 2014).
Materials and Methods
Study Site
Pitakele village is a community of thirty-plus households bordering the core of the Sinharaja Forest Reserve (6°26′N 80°21′E, 300–700 m in altitude), at the end of a jungle road in the northwest of the reserve’s buffer zone (Figure 1). It receives an average annual rainfall of 3 m with a dry season from January to March (Munidasa et al., 2002). The region’s average daily temperatures vary between 22 and 28°C (Ashton et al., 2001; Gunatilleke et al., 2005).
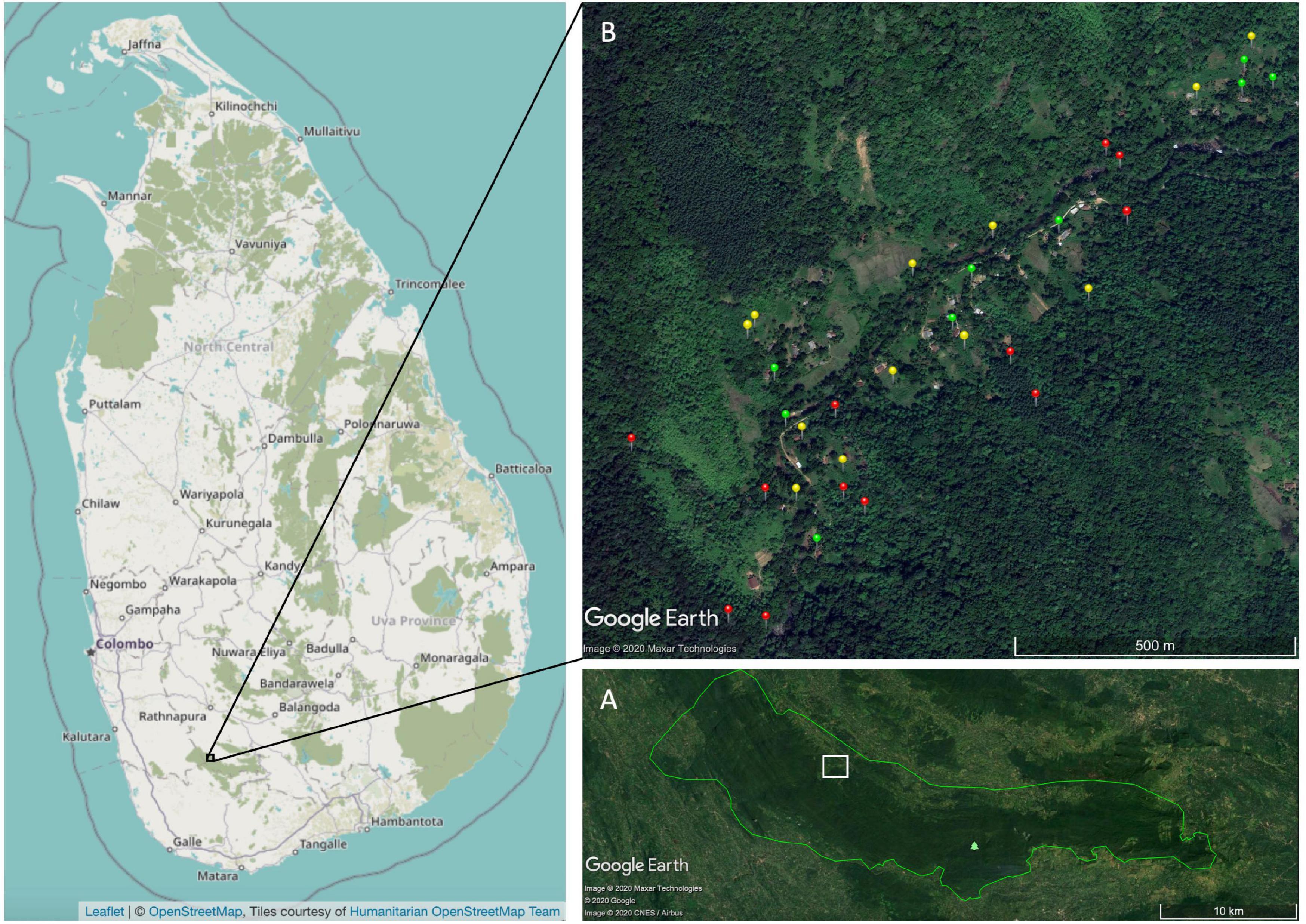
Figure 1. (A) Pitakele is located in southwest Sri Lanka. Satellite imagery via Google Earth clearly shows the dark band of the protected Sinharaja rainforest. (B) Bird point counts follow the Pitakele river valley. Green pins are tree gardens, yellow pins are tea, and red pins are forest fragment. Pitakele is embedded in the Sinharaja. Adapted from Google Earth and Open Street Map.
Like other rainforest-embedded villages in Sri Lanka, the homes of Pitakele lie along and upslope of a river, surrounded by tree gardens and often downslope of cultivated tea. Tea plots comprise 39% village land area, tree gardens comprise 27%, and forest fragments comprise 17% (Martin et al., 2018; Figure 1). Tea plots in Pitakele include shade trees, typically Gliricidia sepium, but also kitul (Caryota urens), other palms, and rainforest or cultivated tree garden species. Tea (Camellia sinensis) land borders second-growth rainforest, which was logged in the 1970s, or rubber (Hevea brasiliensis) or Caribbean pine (Pinus carribea) plantings (Kotagama and Goodale, 2004). The primary source of income for many Pitakele villagers is from picking tea. The village tree gardens average 0.12 ha in size with a mean species richness of 64 trees, shrubs, herbs, and climbers (Martin et al., 2018). At least 219 different species are grown across Pitakele’s homegardens (Martin et al., 2018). The overstories of these tree gardens are dominated by betel palm (Areca catechu), coconut (Cocos nucifera), and kitul palm (Caryota urens); lower strata by mango (Mangifera indica) and jack (Artocarpus heterophyllus) (Martin et al., 2018). Secondary forest fragments lie on rocky ground close to the river or steep slopes less favorable for growing crops. They average 0.12 ha in size (Martin et al., 2018). Some forest fragments connect to the Sinharaja’s core reserve. Our study area is approximately 100 ha. Pitakele village is representative of settlements that about the whole Sinharaja (Figure 1).
Sampling Design
We conducted thirty-three 20 m-radius bird point counts, with nine point counts in tree gardens, 12 in tea plots, and 12 in forest fragments. We recorded all birds seen and heard within a plot, including birds in flight, for 30 min (since it is a relatively small point count plot and flock observations can last for 10–60 min after first observation) (Farley et al., 2008). Other point counts within Sri Lanka have been conducted using the variable circular plot method (Wijesundara and Wijesundara, 2014). Previous studies within the Sinharaja buffer zone have been run along transects (Kotagama and Goodale, 2004). We used point counts to account for small-scale differences in land use. Bird point counts are non-invasive.
We ran each point count five times between January and March 2018. No plot was closer to the edge of the land use area than 20 m, the radius of the plot. We visited each site between 7:00 and 10:30 a.m. For every bird, we noted if they were part of a multi-species flock, i.e., moving with birds of other species in a close association. We conducted the point counts over the dry season, when winter migrants are present and many resident species breed (Jayarathna et al., 2013). We did not sample on mornings when it rained and randomized dates and times of the site visits.
Vegetation Surveys
We counted all trees within 10 m of the point count plot center and measured their diameter at breast height. We measured the total tree species richness of the plots, counting trees per species. We measured tree basal area from the center of the plot by using an angle gauge (metric basal area factor 2.5) in a variable radius plot, a standard forestry method (Packard and Radtke, 2007).
Analysis
For all variables, we calculated Moran’s I tests. Where Moran’s I test ruled out spatial autocorrelation, we ran further analyses. We tested all variables for normal distribution using the Shapiro-Wilks test (p > 0.05).
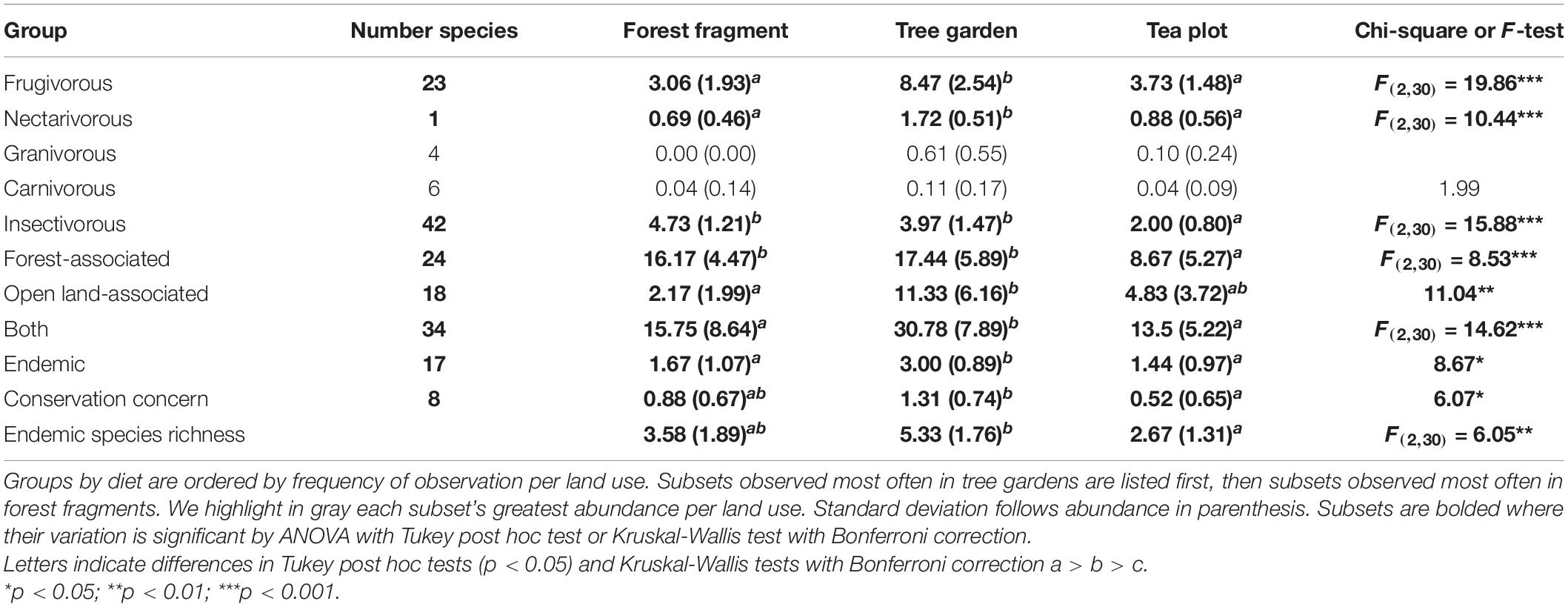
To answer how bird assemblage varied with land use, we analyzed point count data by total bird abundance, richness, and diversity; habitat association, conservation significance, and diet; and endemic species. Assemblage subsets by habitat association and diet reflect functional relationships between birds and habitat. Conservation significance shows if and how the landscape contributes to conservation goals. We looked at individual endemic species because Sri Lanka and the Sinharaja are important for endemic species. These subsets are commonly tested and can be used for cross-study comparison (Tscharntke et al., 2008; Maas et al., 2009; De Lima et al., 2012; Sekercioglu, 2012; Goodale et al., 2014). We tested species assemblage via multivariate analyses of variance (MANOVA).
We adopted habitat classifications (forest, open landscape, or both) from Goodale et al. (2014), which based its identifications on Ali and Ripley (1987) and Grimmett et al. (1999). Where bird-habitat associations were not identified by Goodale et al. (2014), we used Warakagoda et al. (2013) to classify them. We classified birds as of conservation concern if they were listed as “Near Threatened,” “Vulnerable,” or “Endangered” by the 2016 IUCN Red List. All of the birds identified as of conservation concern are declining in number (Iucn., 2016). We grouped birds by diet (frugivorous, nectarivorous, insectivorous, granivorous, or carnivorous) following Goodale et al. (2014), who used Rasmussen and Anderton (2012). We tested each subset’s mean abundance per plot. We tested mean endemic species richness per plot. We tested the mean abundance of individual endemic species observed at least three times. On normally distributed variables we ran analysis of variance (ANOVA) by land use type. If significant, we then ran a Tukey post hoc test. On non-normally distributed variables, we ran a Kruskal-Wallis test with a Bonferroni correction. We tested species assemblage variation per land use type by running three pair-wise MANOVAs of total bird species abundance and endemic bird species abundance. We square-root transformed the data for both MANOVAs to minimize the influence of the most common species. We used Bray-Curtis distance, 999 permutations, and a Bonferroni correction with alpha = 0.05. We ran a non-metric multidimensional scaling (NMDS) analysis (Figure 3) to visualize differences.
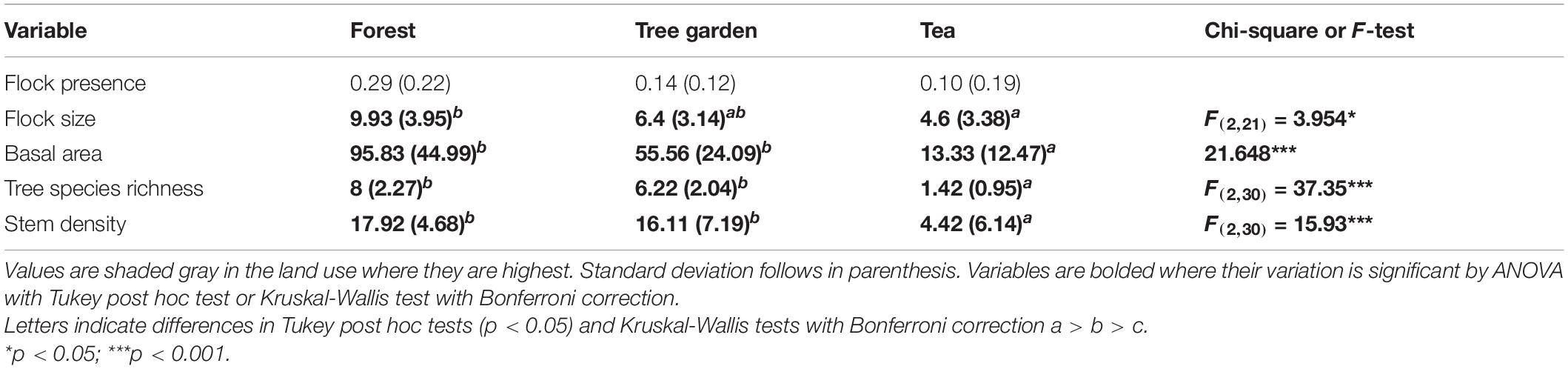
To answer question 2, whether bird assemblages varied with vegetation characteristics, we ran generalized linear regressions on total bird abundance, bird species richness, and abundance of conservation concern species by basal area measured by variable radius plot, and basal area, stem density, and tree species richness within 10 m of plot center. We used Gaussian distributions. To test for land use-specific correlations, we subdivided the data by land use. We ran GLMs by land use separately because other studies have observed that relationships between birds and vegetation characteristics can vary with type of habitat (Duguid et al., 2016). We ran ANOVA with Tukey post hoc analysis or Kruskal-Wallis with Bonferroni correction to check how basal area, tree species richness, and stem density varied with land use.
To answer question 3, what land use type hosted the greatest number of multi-species bird flocks, we analyzed flock presence, or the mean number of flocks observed in a plot, and flock size, the mean number of birds in a plot that were part of a flock. We ran the Kruskal-Wallis test with a Bonferroni correction on both variables, which were non-normally distributed.
Results
We observed 1,269 individual birds of 76 species across the 33 plots. We observed 59 species in tree gardens, 48 species in forest fragments, and 44 species in tea plots. We observed 17 endemic species.
Assemblage Variation
Bird abundance and Shannon-Weiner diversity were higher in tree gardens than in forest fragments or tea plots (Figure 2) [F(2, 30) = 20.79, p < 0.001] (Kruskal-Wallis chi-square = 16.39, df = 2, p < 0.001); and bird species richness was higher in forest fragments than in tea plots [F(2, 30) = 22.16, p < 0.001].
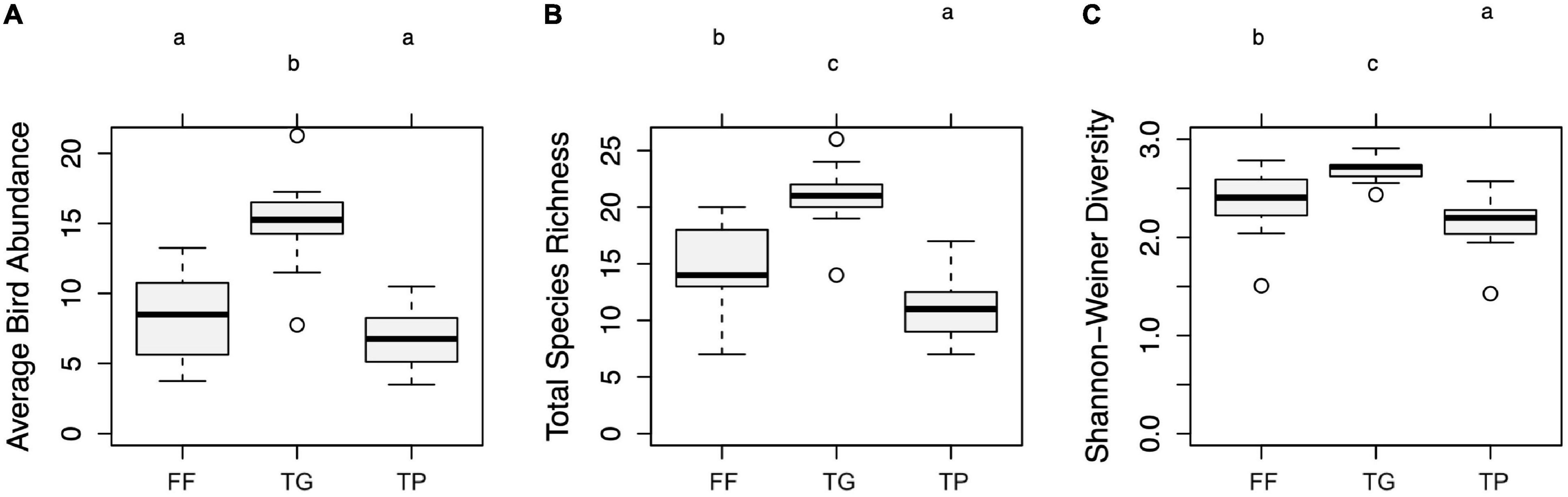
Figure 2. (A) Bird abundance, species richness per point count, and diversity by land use: FF = forest fragment, TG = tree garden, TP = tea. Letters indicate differences in Tukey post hoc tests (p < 0.05) and Kruskal-Wallis tests with Bonferroni correction a > b > c. (B) Bird abundance by habitat association and land use. (C) Endemic bird abundance and species richness and abundance of birds of conservation concern by land use.
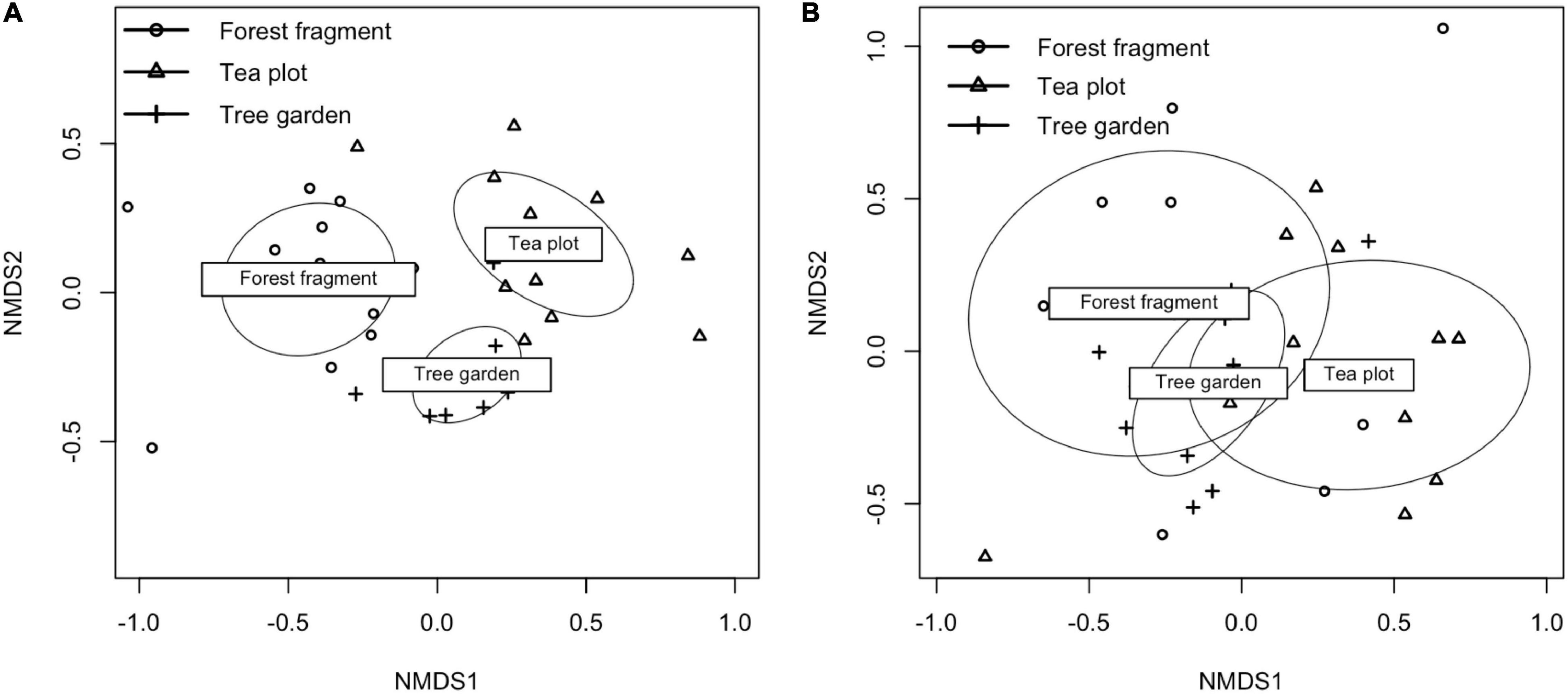
Figure 3. (A) Non-metric-multidimensional scaling of all bird species abundance by land use in two dimensions using Bray-Curtis distance, convergent solution reached after 20 runs. Ellipses capture standard deviation of point for each land use type. (B) Non-metric-multidimensional scaling of endemic bird species abundance by land use in two dimensions using Bray-Curtis distance.
Birds associated with both open lands and forest, endemic birds, frugivorous birds, and nectarivorous birds (only the purple-rumped sunbird, Leptocoma zeylonica) were observed in higher abundances in tree gardens than in either forest fragments or tea plots (Table 1). Forest-associated birds and insectivorous birds were more abundant in both tree gardens and forest fragments than in tea plots (Table 1). Species of conservation concern were more abundant in tree gardens than in tea plots, and their numbers in forest fragments statistically overlapped both (Table 1). Similarly, endemic bird species richness was greater in tree gardens than in tea plots and their numbers in forest fragments statistically overlapped both (Table 1). Open land-associated birds were more abundant in tree gardens than in forest fragments, and their abundance in tea plots statistically overlapped both (Table 1). Carnivorous birds did not vary across land use type.
MANOVA analysis showed that bird composition differed between land use [F(2, 30) = 5.22, p < 0.001, r2 = 0.26]. Multivariate means for bird assemblages differed between forest and tree garden, [F(1, 19) = 3.08, p = 0.001, r2 = 0.17], between tree garden and tea [F(1, 19) = 3.17, p < 0.001, r2 = 0.14], and between forest fragment and tea plots [F(1, 22) = 6.97, p < 0.001, r2 = 0.24] (see Figure 3). MANOVA analysis showed that endemic bird composition differed between land use [F(2, 30) = 5.22, p < 0.001, r2 = 0.26]. Multivariate means for endemic bird assemblages differed between forest and tree garden [F(1, 19) = 3.78, p = 0.003, r2 = 0.17], between tree garden and tea [F(1, 19) = 2.51, p = 0.02, r2 = 0.12], and between remnant forest fragment and tea [F(1, 22) = 2.97, p = 0.005, r2 = 0.12].
Of the endemic bird species that varied significantly across land use type according to ANOVA and the Tukey post hoc test, four species were observed most often in tree gardens and one in forest fragments (Supplementary Table 1).
Variation With Vegetation
Tree basal area, tree species richness, and stem density was statistically greater in forest fragments and tree gardens than in tea plots (Table 2).
GLMs showed significant but relatively weak correlations between bird abundance and tree species richness [F(1, 31) = 5.07, b = 7.17, r2 = 0.14, p = 0.03]; and bird species richness and tree species richness [F(1, 31) = 7.33, b = 11.72, r2 = 0.19, p = 0.01]. When regressions were run in subsets of land use type, bird abundance and species richness were strongly related to tree species richness in tea [F(1, 10) = 16.7, b = 4.35, r2 = 0.63, p = 0.002; F(1, 10) = 5.90, b = 8.66, r2 = 0.37, p = 0.04].
Flocks
Flock presence did not vary significantly with land use (Table 2). Flock size was higher in forest fragments than in tea plots (Table 2).
Discussion
Across habitat association, conservation concern, and diet group, more birds and a greater variety of them used tree gardens than forest fragments or tea plots in the buffer zone village. Tree gardens hosted the most endemic species and species of conservation concern. Species assemblages differed between forest fragments, tree gardens, and tea plots. More frugivores, species associated with both forests and open lands, and endemic species were observed in tree gardens than in forest fragments or tea plots. Within tea plots, greater bird abundance and species richness correlated with greater tree species richness.
The literature shows that tree gardens can host similar amounts of birds to protected forests but lose species from their assemblages, and that bird use of agroforestry more generally is driven by proximity to protected forest. Tree gardens can host the same amount of or more birds than protected forest (Kottawa-Arachchi and Gamage, 2015; Prabowo et al., 2016). Some species observed in forest landscapes are lost in agriculture or tea (Engelen et al., 2016; Perera et al., 2017). Other studies have compared differences in bird assemblages between other agroforestry systems and forests, reporting results that vary with place and system (Thiollay, 1995; Beukema et al., 2007). For example, while one study from Sumatra, Indonesia, reported less species richness in complex agroforests than in primary forest (Thiollay, 1995), another Sumatra study in different agroforests showed little difference in bird diversity (Beukema et al., 2007). While Scales and Marsden (2008) of biodiversity change across habitats reported that small-scale agroforestry usually holds less biodiversity than retained forest, it depended on the system. In reviews, Waltert et al. (2004) and Clough et al. (2009) observed that abundance and species richness declines with monodominance and distance from intact rainforest. In single studies Anand et al. (2008) and Goodale et al. (2014) found that distance to intact rainforest had a larger effect on bird abundance and species richness than specific land use. Engelen et al. (2016) did not observe a difference in homegarden bird assemblages at different distances from protected forest. Based on this literature, Pitakele needs to be seen specifically as a buffer zone village with all plots proximate to intact forest.
Bird Abundance by Habitat Association and Diet
While tree gardens host different bird communities than forest fragments, birds of varied habitat association, including forest-associated species, use tree gardens in the buffer zone. Research from the Sinharaja and India’s western Ghats found that the bird community in buffer zones outside of conserved forests retained more than 70% of forest interior species (Goodale et al., 2014). The high number of forest-associated birds in tree gardens could be due to the variety of food and shelter provided by a multi-strata tree garden (Tews et al., 2004; Perera et al., 2017). Open land-associated birds may also use these resources. Most of the tree gardens border open areas but not forest fragments. However, second-growth rainforest around the Sinharaja’s core is becoming dense, entering the late stem exclusion phase of forest development which, locals observed, propels forest birds to its edges (Oliver and Larson, 1996). This may increase the use of tree gardens by forest species. Future research can further identify specific vegetation to support birds.
Flock size in Pitakele was observed to be highest in forest fragments. Goodale et al. (2014), in comparing bird flocks and communities between primary forest, pine plantations, and open agriculture, observed that flock densities in buffer areas in general were similar to those of intact primary forests, but flocks were smaller. Our results show that flocks are present in buffer zone tree gardens with fewer birds than in forest fragments. While buffer zone tree gardens host multi-species flocks, tree gardens do not replace forest fragments in bird flock use.
Tscharntke et al. (2008) and Sekercioglu (2012) report that species richness of large frugivorous and insectivorous birds decline in agroforests as compared to original primary rain forest; whereas nectarivores, small-to-medium frugivores, omnivores, and sometimes granivores and small frugivorous birds do better in agroforestry landscapes. Our results differ in that we observed statistically similar numbers of insectivores in tree gardens as forest fragments. This may be due to the closeness of Pitakele’s tree gardens to intact rainforest.
Tea Shade Tree Diversity
Our results show that diversity in shade tree systems benefits bird abundance and species richness in this protected area buffer zone. More complex agroforestry systems result in more complex bird communities (Perfecto et al., 1996; Calvo and Blake, 1998; Mas and Dietsch, 2004; Waltert et al., 2004; Harvey and Villalobos, 2007; Van Bael et al., 2007; Anand et al., 2008; Clough et al., 2009). The direct relationships between tree species richness and bird diversity and abundance within tea plots demonstrates that this principle holds true on small land use areas in the conservation buffer zone.
Endemic Birds
Endemism is globally high on islands, where biodiversity is at high risk (Scharlemann et al., 2004; Ricketts et al., 2005; Kier et al., 2009). Agriculture has disproportionately expanded in Endemic Bird Areas (Scharlemann et al., 2004). Studies show that endemic birds decrease with the intensity of land use in human-modified tropical landscapes (Maas et al., 2009; Waltert et al., 2011; De Lima et al., 2012; Davies et al., 2015). Tropical forest conversion to farmland can result in the replacement of endemics by widespread species (Maas et al., 2009; Waltert et al., 2011; Dallimer et al., 2012; De Lima et al., 2012; Sekercioglu, 2012).
We observed endemics in greater abundance within tree gardens than in the other land uses and in greater species richness in tree gardens than in tea plots. The Sri Lanka gray hornbill (Ocyceros gingalensis), and the Sri Lanka junglefowl (Gallus lafayetii) were observed significantly more in tree gardens than in tea plots or forest fragments. The white-throated flowerpecker (near threatened, Dicaeum vincens) was observed significantly more in tree gardens than in tea plots, with statistically intermediate values in forest fragments. The Sri Lanka hanging parrot (Loriculus beryllinus) was observed significantly more in tree gardens and tea plots than forest fragments. We observed five other endemics most often in tree gardens without statistical significance (Supplementary Table 1). The endemic, vulnerable red-faced malkoha (Phaenicophaeus pyrrhocephalus) was observed only in forest fragments. We note that while Perera et al. (2017), who also examined birds in a SW Sri Lankan forest buffer zone, did not observe forest endemics in buffer zone tree gardens, their study included tea plots as tree garden, had only five of these plots, and ran their point counts outside a smaller protected area. A Sri Lanka-wide survey of birds observed half the number of endemic species in tree gardens across the country as this study (Bambaradeniya, 2003). Since most of this study’s observed endemics are forest-associated birds, this data is buffer-zone specific. Waltert et al. (2011) observed correlations between endemic species richness in agroforestry with tree abundance and tree species richness. We suggest that habitat diversity may contribute to Pitakele tree gardens hosting endemic bird species. Our results and the literature suggest that endemic birds are present in the buffer zone tree gardens due to proximity to the large protected area and tree garden habitat complexity. We do not suggest that individual land uses support entire species or subsets. The complete buffer zone matrix and extensive edge habitat available in the village buffer define these habitats.
Management Implications
Tropical reserve systems are often encroached. Successful conservation depends on buffer land cover that is also profitable for residents—but conservation planning lacks global standards on buffer zone management (Budhathoki, 2004; Margles et al., 2010; Waltert et al., 2011; Dewi et al., 2013; Robinson et al., 2013; Goodale et al., 2014; Lui and Coomes, 2016; Allan et al., 2017). Programs like the EU’s Small Grants Program for Operations to Promote Tropical Forests Program have focused on developing resilient and self-supporting communities within tropical conservation buffer zones (RECOFTC., 2008; Rands et al., 2010). Agroforestry can integrate forests in a multifunctional landscape for conservation, including specifically in the Sinharaja (Michon et al., 1986; Bhagwat et al., 2008; Jose, 2009; Dewi et al., 2013; Kothalawala et al., 2013). Complex cover benefits birds. This study shows that programs for tree garden agroforestry in buffer zones like that of the Sinharaja can support habitat for vulnerable and endemic birds, including forest-associated ones. However, tree gardens do not replace forest fragments or protected core forest. We suggest pursuing further research on the ecological significance of tree gardens broadly in tropical protected area buffer zones.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MA conceived the study. MA, BS, and JH planned methodology. MA and JH planned analyses. JH collected the data, and analyzed and wrote the manuscript with input from MA and BS. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Tropical Forest Conservation and Management Fund at the Yale School of the Environment, Yale University. This study received logistical support from the Sri Lanka Program for Forest Conservation, University of Sri Jayewardenepura, and the Forest Department, Sri Lanka.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the villagers of Pitakele for participation and for allowing us access to their tree gardens and working lands. We thank the reviewers of all drafts of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.608434/full#supplementary-material
References
Abeyarama, D. K., and Seneviratne, S. S. (2017). Evolutionary distinctness of important bird areas (IBAs) of Sri Lanka: do the species-rich wet zone forests safeguard Sri Lanka’s genetic heritage? Ceylon J. Sci. 46:89. doi: 10.4038/cjs.v46i5.7456
Ali, S., and Ripley, S. D. (1987). Compact handbook of the birds of India and Pakistan: Together with those of Bangladesh, Nepal, Bhutan and Sri Lanka. Delhi: Oxford University Press.
Allan, J., Venter, O., Maxwell, S., Bertzky, B., Jones, K., Shi, Y., et al. (2017). Recent increases in human pressure and forest loss threaten many natural world heritage sites. Biol. Conser. 206, 47–55. doi: 10.1016/j.biocon.2016.12.011
Anand, M. O., Krishnaswamy, J., and Das, A. (2008). Proximity to forests drives bird conservation value of coffee plantations: implications for certification. Ecol. Appl. 18, 1754–1763. doi: 10.1890/07-1545.1
Arachchi, T. (2020). Exploring the potential for avitourism in Sri Lanka as a lucrative ecotourism niche market -a working paper, Horizon Campus, Faculty of Management.
Ashton, M. S., Gunatilleke, C., Singhakumara, B., and Gunatilleke, I. (2001). Restoration pathways for rain forest in southwest sri lanka: a review of concepts and models. Forest Ecol. Manag. 154, 409–430. doi: 10.1016/s0378-1127(01)00512-6
Bambaradeniya, C. (2003). “Traditional home garden and rice agro-ecosystems in Sri Lanka: an integrated managed landscape that sustains a rich biodiversity,” in Proceedings of the International Symposium on perspectives of the biodiversity research in the western pacific and Asia in the 21st Century, Kyoto.
Beukema, H., Danielsen, F., Vincent, G., Hardiwinoto, S., and Andel, J. V. (2007). Plant and bird diversity in rubber agroforests in the lowlands of sumatra, indonesia. Agroforestry Syst. 70, 217–242. doi: 10.1007/s10457-007-9037-x
Bhagwat, S. A., Willis, K. J., Birks, H. J., and Whittaker, R. J. (2008). Agroforestry: a refuge for tropical biodiversity? Trends Ecol. Evol. 23, 261–267. doi: 10.1016/j.tree.2008.01.005
Bossuyt, F. (2004). Local endemism within the western ghats-sri lanka biodiversity hotspot. Science 306, 479–481. doi: 10.1126/science.1100167
Budhathoki, P. (2004). Linking communities with conservation in developing countries: buffer zone management initiatives in nepal. Oryx 38, 334–341. doi: 10.1017/s0030605304000584
Calvo, L., and Blake, J. (1998). Bird diversity and abundance on two different shade coffee plantations in guatemala. Bird Conser. Int. 8, 297–308. doi: 10.1017/s0959270900001945
Chokkalingam, U., and Vanniarachchy, S. A. (2013). Sri Lanka’s REDD potential: Myth or reality? Forest carbon Asia country Profile Report No. 1. Sri Lanka: Forest Carbon Asia.
Clough, Y., Putra, D. D., Pitopang, R., and Tscharntke, T. (2009). Local and landscape factors determine functional bird diversity in indonesian cacao agroforestry. Biol. Conser. 142, 1032–1041. doi: 10.1016/j.biocon.2008.12.027
Dallimer, M., Parnell, M., Bicknell, J. E., and Melo, M. (2012). The importance of novel and agricultural habitats for the avifauna of an oceanic island. J. Nat. Conser. 20, 191–199. doi: 10.1016/j.jnc.2012.04.001
Das, T., and Das, A. K. (2015). Conservation of plant diversity in rural homegardens with cultural and geographical variation in three districts of barak valley, northeast india1. Eco. Bot. 69, 57–71. doi: 10.1007/s12231-015-9299-6
Davies, T., Clarke, R., Ewen, J., Fazey, I., Pettorelli, N., Cresswell, W., et al. (2015). The effects of land-use change on the endemic avifauna of makira, solomon islands: endemics avoid monoculture. Emu - Austral Ornithol. 115, 199–213. doi: 10.1071/MU14108
De Lima, R. F., Dallimer, M., Atkinson, P. W., and Barlow, J. (2012). Biodiversity and land-use change: 559 understanding the complex responses of an endemic-rich bird assemblage. Diver. 560 Distri. 19, 411–422. doi: 10.1111/ddi.12015
Dewi, S., Noordwijk, M. V., Ekadinata, A., and Pfund, J. (2013). Protected areas within multifunctional landscapes: squeezing out intermediate land use intensities in the tropics? Land Use Policy 30, 38–56. doi: 10.1016/j.landusepol.2012.02.006
Duguid, M. C., Morrell, E. H., Goodale, E., and Ashton, M. S. (2016). Changes in breeding bird abundance and species composition over a 20year chronosequence following shelterwood harvests in oak-hardwood forests. Forest Ecol. Manag. 376, 221–230. doi: 10.1016/j.foreco.2016.06.010
Engelen, D., Lemessa, D., şekercioǧlu, ÇH., and Hylander, K. (2016). Similar bird communities in homegardens at different distances from afromontane forests. Bird Conserv. Int. 27, 83–95. doi: 10.1017/s0959270916000162
Fao. (2009). Sri Lanka Forestry Outlook Study. Asia Pacific Forestry Outlook Sector Outlook Study II, Working Paper No. APFSOS II/WP/2009/29, Bangkok, FAO Regional Office for Asia and the Pacific. Bangkok: FAO.
Farley, E. A., Sieving, K. E., and Contreras, T. A. (2008). Characterizing complex mixed-species bird flocks using an objective method for determining species participation. J. Ornithol. 149, 451–468. doi: 10.1007/s10336-008-0284-z
Galhena, D. H., Freed, R., and Maredia, K. M. (2013). Home gardens: a promising approach to enhance household food security and wellbeing. Agric. Food Security 2:8. doi: 10.1186/2048-7010-2-8
Galluzzi, G., Eyzaguirre, P., and Negri, V. (2010). Home gardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodiv. Conserv. 19, 3635–3654. doi: 10.1007/s10531-010-9919-5
Goodale, E., Kotagama, S. W., Raman, T. S., Sidhu, S., Goodale, U., Parker, S., et al. (2014). The response of birds and mixed-species bird flocks to human-modified landscapes in Sri Lanka and southern India. Forest Ecol. Manage. 329, 384–392. doi: 10.1016/j.foreco.2013.08.022
Grimmett, R., Inskipp, C., and Inskipp, T. (1999). A guide to the birds of India, Pakistan, Nepal, Bangladesh, Bhutan, Sri Lanka, and the Maldives. Princeton, NJ: Princeton University Press.
Gunatilleke, C., and Gunatilleke, I. (1983). “A forestry case study of the Sinharaja rainforest in Sri Lanka,” in Forest and watershed development and conservation in Asia and the Pacific, ed. L. S. Hamilton (Boulder, CO: Westview Press).
Gunatilleke, I., Gunatilleke, C., and Dilhan, M. (2005). Plant biogeography and conservation of the south-western hill forests of sri lanka. Raffles Bull. Zoo. 12, 9–22.
Gunatilleke, N., Gunatilleke, S., and Ashton, P. S. (2017). South-west sri lanka: a floristic refugium in south asia. Ceylon J. Sci. 46:65. doi: 10.4038/cjs.v46i5.7454
Gunawardene, N., Daniels, A., Gunatilleke, I., Gunatilleke, C., Karunakaran, P., Nayak, K., et al. (2007). A brief overview of the western ghats – sri lanka hotspot. Curr. Sci. 93, 1567–1572.
Harvey, C. A., and Villalobos, J. A. (2007). Agroforestry systems conserve species-rich but modified assemblages of tropical birds and bats. Biodiv. Conserv. 16, 2257–2292. doi: 10.1007/s10531-007-9194-2
Iucn. (2016). IUCN Red List of Threatened Species. Available online at www.iucnredlist.org (Accessed July 2020)
Jaman, M., Hossain, M., Islam, M., Helal, M., and Jamil, M. (2016). Quantification of carbon stock and tree diversity of homegardens in rangpur district, bangladesh.. Int. J. Agric. Forestry 6, 169–180.
Jayarathna, A., Kotagama, S., and Goodale, E. (2013). The seasonality of mixed-species bird flocks in a Sri Lankan rainforest in relation to the breeding of the nuclear species, orange-billed babbler turdoides rufescens. Forktail 29, 138–139.
Jose, S. (2009). Agroforestry for ecosystem services and environmental benefits: an overview. Adv. Agroforestry 76, 1–10. doi: 10.1007/978-90-481-3323-9_1
Kaluthota, C., and Kotagama, S. (2005). Important bird areas of Sri Lanka present status. Proc. Int. Forestry Environ. Symp. 10:48.
Kier, G., Kreft, H., Lee, T. M., Jetz, W., Ibisch, P. L., Nowicki, C., et al. (2009). A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. U S A. 106, 9322–9327. doi: 10.1073/pnas.0810306106
Kotagama, S., De Silva, R., Wijayasinha, A., and Abeygunawardane, V. (2006). The fauna of Sri Lanka: Status of taxonomy, research, and conservation. Colombo: The World Conservation Union.
Kotagama, S., and Goodale, E. (2004). The composition and spatial organization of mixed-species flocks in a Sri Lankan rainforest. Forktail 20, 63–70.
Kothalawala, K. I., Pushpakumara, D. K., and Sivananthwerl, T. (2013). Agro- forestry system to protect both the Sinharaja forest and the peripheral villages (a case study of the southern part of Sinharaja). Proc.Int. Forestry Environ. Symp. 16:1666. doi: 10.31357/fesympo.v0i0.1666
Kottawa-Arachchi, J. D., and Gamage, R. N. (2015). Avifaunal diversity and bird community responses to man-made habitats in St. Coombs Tea Estate, Sri Lanka. J. Threatened Taxa 7, 6878–6890. doi: 10.11609/jott.o3483.6878-90
Kumar, B. M., and Nair, P. K. (2011). Carbon sequestration potential of agroforestry systems opportunities and challenges. Dordrecht: Springer.
Kumar, B. M., and Nair, P. K. R. (2006). Tropical home gardens, a time-tested example of sustainable agroforestry. Dordrecht: Springer.
Lui, G. V., and Coomes, D. A. (2016). Tropical nature reserves are losing their buffer zones, but leakage is not to blame. Environ. Res. 147, 580–589. doi: 10.1016/j.envres.2015.11.008
Maas, B., Putra, D. D., Waltert, M., Clough, Y., Tscharntke, T., and Schulze, C. H. (2009). Six years of habitat modification in a tropical rainforest margin of Indonesia do not affect bird diversity but endemic forest species. Biol. Conser. 142, 2665–2671. doi: 10.1016/j.biocon.2009.06.018
Macarthur, R. H. (1964). Environmental factors affecting bird species diversity. Am. Naturalist 98, 387–397. doi: 10.1086/282334
Margles, S. W., Masozera, M., Rugyerinyange, L., and Kaplin, B. A. (2010). Participatory planning: using swot-ahp analysis in buffer zone management planning. J. Sust. Forestry 29, 613–637. doi: 10.1080/10549811003769483
Martin, M., Geiger, K., Singhakumara, B. M., and Ashton, M. S. (2018). Quantitatively characterizing the floristics and structure of a traditional homegarden in a village landscape, Sri Lanka. Agroforestry Syst. 93, 1439–1454. doi: 10.1007/s10457-018-0254-2
Mas, A. H., and Dietsch, T. V. (2004). Linking shade coffee certification to biodiversity conservation: butterflies and birds in chiapas, mexico. Ecol. Appl. 14, 642–654. doi: 10.1890/02-5225
Mattsson, E., Ostwald, M., and Nissanka, S. P. (2017). What is good about Sri Lankan homegardens with regards to food security? a synthesis of the current scientific knowledge of a multifunctional land-use system. Agroforestry Syst. 92, 1469–1484. doi: 10.1007/s10457-017-0093-6
Mattsson, E., Ostwald, M., Nissanka, S. P., and Marambe, B. (2013). Homegardens as a multi-functional land-use strategy in sri lanka with focus on carbon sequestration. Ambio 42, 892–902. doi: 10.1007/s13280-013-0390-x
Michon, G., Mary, F., and Bompard, J. (1986). Multistoried agroforestry garden system in west sumatra. Indonesia. Agroforestry Syst. 4, 315–338. doi: 10.1007/bf00048106
Mohri, H., Lahoti, S., Saito, O., Mahalingam, A., Gunatilleke, N., Herath, S., et al. (2013). Assessment of ecosystem services in homegarden systems in indonesia, sri lanka, and vietnam. Ecosyst. Ser. 5, 124–136. doi: 10.1016/j.ecoser.2013.07.006
Moore, J. H., Sittimongkol, S., Campos-Arceiz, A., Sumpah, T., and Eichhorn, M. P. (2016). Fruit gardens enhance mammal diversity and biomass in a southeast asian rainforest. Biol. Conser. 194, 132–138. doi: 10.1016/j.biocon.2015.12.015
Munidasa, B., Gunatilleke, C., and Gunatilleke, I. (2002). Climate of sinharaja rain forest, Sri Lanka: an attempt to understand the El-Nino and La-Nina events. Cey. J. Sci. 30, 37–54.
Naresa. (1991). Natural resources of Sri Lanka: conditions and trends. Colombo: Natural Resources, Energy and Science Authority.
O’Connell, T. J., Jackson, L. E., and Brooks, R. P. (2000). Bird guilds as indicators of ecological condition in the central appalachians. Ecol. Appl. 10, 1706–1721. doi: 10.1890/1051-0761(2000)010[1706:bgaioe]2.0.co;2
Packard, K. C., and Radtke, P. J. (2007). Forest sampling combining fixed- and variable-radius sample plots. Can. J. Forest Res. 37, 1460–1471. doi: 10.1139/x06-321
Paembonan, S. A., Millang, S., Dassir, M., and Ridwan, M. (2018). Species variation in home garden agroforestry system in south sulawesi, indonesia and its contribution to farmers’ income. IOP Confer. Serie Earth Environ. Sci. 157:012004. doi: 10.1088/1755-1315/157/1/012004
Park, J. H., Woo, S. Y., Kwak, M. J., Lee, J. K., Leti, S., Soni, T., et al. (2019). Assessment of the diverse roles of home gardens and their sustainable management for livelihood improvement in west java, indonesia. Forests 10:970. doi: 10.3390/f10110970
Perera, P., Wijesinghe, S., Dayawansa, N., Marasinghe, S., and Wickramarachchi, C. (2017). Response of tropical birds to habitat modifications in fragmented forest patches: A case from a tropical lowland rainforest in south-west Sri Lanka. Comm. Ecol. 18, 175–183. doi: 10.1556/168.2017.18.2.7
Perfecto, I., Rice, R. A., Greenberg, R., and Voort, M. E. (1996). Shade coffee: a disappearing refuge for biodiversity. BioScience 46, 598–608. doi: 10.2307/1312989
Peroni, N., Hanazaki, N., Begossi, A., Zuchiwschi, E., Lacerda, V. D., and Miranda, T. M. (2016). Homegardens in a micro-regional scale: contributions to agrobiodiversity conservation in an urban-rural context. Ethnobiol. Conser. 5, 1–17. doi: 10.15451/ec2016-8-5.6-1-17
Prabowo, W. E., Darras, K., Clough, Y., Toledo-Hernandez, M., Arlettaz, R., Mulyani, Y. A., et al. (2016). Bird responses to lowland rainforest conversion in sumatran smallholder landscapes, indonesia. Plos One 11:0154876. doi: 10.1371/journal.pone.0154876
Pushpakumara, D. K. N. G., Marambe, B., Gllp, S., Weerahewa, J., and Bvr, P. (2012). A review research on homegardens in Sri Lanka: the status, importance and future perspective. Tropical Agric. 160, 55–125.
Rands, M. R., Adams, W. M., Bennun, L., Butchart, S. H., Clements, A., Coomes, D., et al. (2010). Biodiversity conservation: challenges beyond 2010. Science 329, 1298–1303. doi: 10.1126/science.1189138
Rasmussen, P. C., and Anderton, J. (2012). Birds of South Asia: The Ripley guide. Washington, D.C: National Museum of Natural History.
RECOFTC. (2008). Forest lives: Lessons on sustaining communities and forests from the Small Grants Programme for Operations to Promote Tropical Forests (SGPPTF). Bangkok: SEAMEO, SEARCA.
Reddy, C. S., Saranya, K., Pasha, S. V., Satish, K., Jha, C., Diwakar, P., et al. (2018). Assessment and monitoring of deforestation and forest fragmentation in south asia since the 1930s. Global Planetary Change 161, 132–148. doi: 10.1016/j.gloplacha.2017.10.007
Ricketts, T. H., Dinerstein, E., Boucher, T., Brooks, T. M., Butchart, S. H., Hoffmann, M., et al. (2005). Pinpointing and preventing imminent extinctions. Proc. Natl. Acad. Sci. U S A. 102, 18497–18501. doi: 10.1073/pnas.0509060102
Ripley, S. D., and Beehler, B. M. (1990). Patterns of speciation in indian birds. J. Biogeography 17, 639. doi: 10.2307/2845145
Robinson, E. J., Albers, H. J., and Busby, G. M. (2013). The impact of buffer zone size and management on illegal extraction, park protection, and enforcement. Ecol. Eco. 92, 96–103. doi: 10.1016/j.ecolecon.2012.06.019
Roth, R. R. (1976). Spatial heterogeneity and bird species diversity. Ecology 57, 773–782. doi: 10.2307/1936190
Rousta, I., Sarif, M., Gupta, R., Olafsson, H., Ranagalage, M., Murayama, Y., et al. (2018). Spatiotemporal analysis of land use/land cover and its effects on surface urban heat island using landsat data: a case study of metropolitan city tehran (1988–2018). Sustainability 10:4433. doi: 10.3390/su10124433
Sangakkara, U., and Frossard, E. (2016). Characteristics of south asian rural households and associated home gardens - a case study from Sri Lanka. Tropical Ecol. 57, 765–777.
Scales, B., and Marsden, S. (2008). Biodiversity in small-scale tropical agroforests: a review of species richness and abundance shifts and the factors influencing them. Environ. Conser. 35, 160–172. doi: 10.1017/s0376892908004840
Scharlemann, J. P. W., Green, R. E., and Balmford, A. (2004). Land-use trends in endemic bird areas: global expansion of agriculture in areas of high conservation value. Global Change Biol. 10, 2046–2051. doi: 10.1111/j.1365-2486.2004.00860.x
Schroth, G., Harvey, C. A., Gascon, C., Vasconcelos, H. L., and Izac, A. N. (2013). “Complex agroforests: Their structure, diversity, and potential role in landscape conservation,” in Agroforestry and Biodiversity Conservation in Tropical Landscapes, (Washington: Island Press).
Sekercioglu, C. (2006). Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471. doi: 10.1016/j.tree.2006.05.007
Sekercioglu, C. H. (2002). Impacts of birdwatching on human and avian communities. Environ. Conser. 29, 282–289. doi: 10.1017/s0376892902000206
Sekercioglu, C. H. (2012). Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J. Ornithol. 153, 153–161. doi: 10.1007/s10336-012-0869-4
Sekercioglu, C. H., Daily, G. C., and Ehrlich, P. R. (2004). Ecosystem consequences of bird declines. Proc. Natl. Acad. Sci. 101, 18042–18047. doi: 10.1073/pnas.0408049101
Sidhu, S., Raman, T. R. S., and Goodale, E. (2010). Effects of plantations and home-gardens on tropical forest bird communities and mixed-species bird flocks in the southern Western Ghats. J. Bombay Nat. Hist. Soc. 107, 91–108.
Sodhi, N. S., Posa, M. R., Lee, T. M., Bickford, D., Koh, L. P., and Brook, B. W. (2009). The state and conservation of southeast asian biodiversity. Trends Ecol. Evol. 19, 317–328. doi: 10.1007/978-94-007-0168-7_2
Tews, J., Brose, U., Grimm, V., Tielbörger, K., Wichmann, M. C., Schwager, M., et al. (2004). Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeography 31, 79–92. doi: 10.1046/j.0305-0270.2003.00994.x
Thiollay, J. (1995). The role of traditional agroforests in the conservation of rain forest bird diversity in sumatra. Conser. Biol. 9, 335–353. doi: 10.1046/j.1523-1739.1995.9020335.x
Timsuksai, P., and Rambo, A. T. (2016). The influence of culture on agroecosystem structure: a comparison of the spatial patterns of homegardens of different ethnic groups in thailand and vietnam. Plos One 11:0146118. doi: 10.1371/journal.pone.0146118
Trinh, L., Watson, J., Hue, N., De, N., Minh, N., Chu, P., et al. (2003). Agrobiodiversity conservation and development in vietnamese home gardens. Agric. Ecosyst. Environ. 97, 317–344. doi: 10.1016/s0167-8809(02)00228-1
Tscharntke, T., Sekercioglu, C. H., Dietsch, T. V., Sodhi, N. S., Hoehn, P., Tylianakis, J. M., et al. (2008). landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecology 89, 944–951. doi: 10.1890/07-0455.1
Unesco. (1996). Biosphere reserves: The Seville Strategy and the statutory framework of the world network. New York: Oceana Publications, Inc.
Van Bael, S. A., Bichier, P., Ochoa, I., and Greenberg, R. (2007). Bird diversity in cacao farms and forest fragments of western Panama. Biodiv.Conser. 16, 2245–2256. doi: 10.1007/s10531-007-9193-3
Waltert, M., Bobo, K. S., Kaupa, S., Montoya, M. L., Nsanyi, M. S., and Fermon, H. (2011). Assessing conservation values: biodiversity and endemicity in tropical land use systems. PloS one 6:e16238. doi: 10.1371/journal.pone.0016238
Waltert, M., Mardiastuti, A., and Mühlenberg, M. (2004). Effects of land use on bird species richness in sulawesi, indonesia. Conser. Biol. 18, 1339–1346. doi: 10.1111/j.1523-1739.2004.00127.x
Warakagoda, D., Inskipp, C., Inskipp, T., and Grimmett, R. (2013). Birds of Sri Lanka. London: Christopher Helm.
Watson, J. W., and Eyzaguirre, P. (2002). “Home gardens agrobiodiversity: an overview across regions,” in Home gardens and in situ conservation of plant genetic resources in farming systems: Proceedings of the Second International Home Gardens Workshop, 17-19 July 2001, Witzenhausen, Federal Republic of Germany, eds J. W. Watson and P. Eyzaguirre (Rome: International Plant Genetic Resources Institute).
Weerahewa, J., Pushpakumara, G., and Silva, P. (2012). Are homegarden ecosystems resilient to climate change? an analysis of the adaptation strategies of homegardeners in sri lanka. APN Sci. Bull. 2, 22–27.
Wijesooriya, W. A., and Gunatilleke, C. V. (2003). Buffer zone of the sinharaja biosphere reserve in sri lanka and its management strategies. J. Natl. Sci. Found. Sri Lanka 31:57. doi: 10.4038/jnsfsr.v31i1-2.3023
Wijesundara, C., and Wijesundara, M. (2014). bird diversity of dekinda forest reserve, balana, sri lanka: implications for conservation. Ceylon J. Sci. 43:137. doi: 10.4038/cjsbs.v43i1.7283
Keywords: agroforestry, tree garden, forest fragment, homegarden, rainforest, Sinharaja, tea, buffer zone
Citation: Hanle J, Singhakumara BMP and Ashton MS (2021) Complex Small-Holder Agriculture in Rainforest Buffer Zone, Sri Lanka, Supports Endemic Birds. Front. Ecol. Evol. 9:608434. doi: 10.3389/fevo.2021.608434
Received: 20 September 2020; Accepted: 25 January 2021;
Published: 19 February 2021.
Edited by:
Craig Barnett, Kyoto University, JapanReviewed by:
Swati Sidhu, Nature Conservation Foundation, IndiaJörn Theuerkauf, Museum and Institute of Zoology (PAN), Poland
Copyright © 2021 Hanle, Singhakumara and Ashton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juliana Hanle, SnVsaWFuYS5oYW5sZUBnbWFpbC5jb20=
 Juliana Hanle
Juliana Hanle Balangoda M. P. Singhakumara2
Balangoda M. P. Singhakumara2