- Evolutionary and Organismal Biology Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bengaluru, India
We present a detailed study of male associations in the Asian elephant, using 6 years of data on identified, non-musth males. Adult males spent greater proportions of their time solitarily than in mixed-sex or in all-male groups. Old (over 30 years) males were sighted more frequently with their age-peers and less frequently with young (15–30 years) males than expected at random in all-male groups. Young males were not sighted more frequently with old males than with young males, and did not disproportionately initiate associations with old males. These results suggest that male associations, in the absence of females, may allow for old non-musth males to test strengths against age-peers. Social learning from older individuals did not seem to be important in male associations, unlike that observed in the African savannah elephant. We also found a constraint on the sizes of all-male groups, similar to that seen in female groups in our study population, and all-male groups were rarer and smaller than those in African savannah elephant. Although male associations were weak, most males had a significant top associate, with whom their association was the strongest, in female absence. In mixed-sex groups, male associations occurred at random, suggesting that males were tracking female groups independently. Differences in male social organization from that of the related African savannah elephant that occupies a similar niche possibly arise from differences in ecology.
Introduction
Adult males and females of many large mammals exhibit sexual dimorphism and strikingly different lifestyles, with female philopatry and male dispersal (see Greenwood, 1980; Ruckstuhl and Neuhaus, 2000). Following dispersal from their natal groups, males may follow different strategies. They may form relatively stable bonds with female groups [for example, Hrdy (1977) in Hanuman langur, Packer and Pusey (1987) in lions, Alberts and Altmann (1995) in baboons] or form mixed-sex groups during the breeding season and all-male groups outside of the breeding season [for example, Clutton-Brock et al. (1987) in red deer, Villaret and Bon (1995) in Alpine ibex, Mooring et al. (2003) in desert bighorn sheep]. Alternatively, males may rove between different female groups throughout the year, associating only temporarily with them [for example, Best (1979) in sperm whales, Poole (1982) in African savannah elephant, Desai and Johnsingh (1995) in Asian elephant, Baird and Whitehead (2000) in killer whales]. The interactions between males in polygynous species with these different kinds of lifestyles are expected to be competitive rather than affiliative due to intrasexual competition for mates (van Hooff and van Schaik, 1994). Therefore, strong male associations are not expected in these species and may occur primarily in the context of coalitions to defend or contest access to females [for example, Saayman (1971) in baboons, Connor et al. (1992) and Gerber et al. (2020) in bottlenose dolphins, van Hooff and van Schaik (1994) in non-human primates, Wagner et al. (2008) in hyaenas]. However, male-male interactions in all-male groups may be less competitive or aggressive than those within mixed-sex groups [Pusey and Packer (1987) in primates, Robbins (1996) in mountain gorillas]. All-male groups may provide individuals with opportunities to test their strength against competitors in a more relaxed setting [Bon et al. (1993) in mouflon sheep, Chiyo et al. (2011) in African savannah elephant]. Associations in all-male groups may also be motivated by the opportunities available for social learning from older, more experienced males [Evans and Harris (2008) and Chiyo et al. (2011, 2012) in African savannah elephant, Bercovitch and Berry (2015) in giraffes]. Increased efficiency in obtaining food resources (river otters, Blundell et al., 2002) and improved defense against predators (sperm whales, Curé et al., 2013) are also possible benefits from associating with other males. There has been little study on male association patterns in mammals overall, especially on those species that rove between female groups and form temporary associations.
The Asian elephant (Elephas maximus) is polygynous, with males and females exhibiting different morphologies and adult lifestyles. Females are organized into clans that show fission-fusion dynamics (de Silva et al., 2011; Nandini et al., 2017, 2018), while pubertal males disperse from their natal groups and thereafter associate only temporarily with other males or with female groups (McKay, 1973; Desai and Johnsingh, 1995). There are often no clearly defined bull areas (areas frequented by males and not by many female groups). Males are not known to form coalitions to defend females. Males can breed throughout the year, but females are sexually receptive only for a few days (Eisenberg et al., 1971) every 4–5 years due to long periods of gestation and nursing, making receptive females a rare resource, for which males are expected to compete intensely. Male–male dominance interactions have been observed, indicating contest competition (McKay, 1973; Daniel et al., 1987; Chelliah and Sukumar, 2013; Keerthipriya, 2018).
Male Asian and African elephants annually enter musth, a rut-like state during which they exhibit temporal gland secretion, sometimes urine dribbling, and increased serum testosterone levels (Jainudeen et al., 1972a, b; Poole, 1987). Musth seems to be a strategy for males, and particularly old males, to rove over long distances and search for receptive females (Taylor et al., 2020; Keerthipriya et al., 2020a). Although musth males have been found to have greater mating and reproductive success, non-musth males can also mate and reproduce (Hollister-Smith et al., 2007; Rasmussen et al., 2007; Chelliah and Sukumar, 2015; Kabini Elephant Project, unpublished data). Therefore, competition for females may also exist among non-musth males. African savannah elephant males nevertheless exhibit complex relationships, with males preferring to associate with age-peers (Chiyo et al., 2011; Goldenberg et al., 2014 – in the case of sexually inactive males) and related males (Chiyo et al., 2011). Male associations also facilitate social learning from older males (Chiyo et al., 2012), which may be preferred associates or more central to male society than young males and lead traveling male groups (Evans and Harris, 2008; Chiyo et al., 2011; Allen et al., 2020; Murphy et al., 2020). Thus, temporary all-male groups in African savannah elephants seem to provide an opportunity to spar and test strengths, and also possibly for younger males to learn from knowledgeable, older males.
Although Asian and African elephants were previously assumed to have similar societies, we now know that there are some differences between the female Asian elephant and African savannah elephant societies (de Silva and Wittemyer, 2012; Nandini et al., 2018), probably because of an ecological constraint on female group size in the Asian elephant (Nandini et al., 2017, 2018). If resources limit female group size due to within-group feeding competition, since Asian elephant males are larger than females (Sukumar et al., 1988) and hence, will have greater nutritional requirements, such a constraint might also exist in all-male groups, and lead to differences in male societies across species. Moreover, African savannah elephant males were known to return to the same bull areas when sexually inactive (Poole, 1982), providing an opportunity for repeated associations with specific individuals (although the selection of a bull area may also possibly be a decision to associate with other males in the area; see Lee et al., 2011). A restriction on group size and the absence of separate bull areas might weaken male associations in Asian elephant populations. We, therefore, wanted to examine associations among adult male Asian elephants to find out whether possible ecological differences were correlated with a different male social structure than in the African savannah elephant, despite the phylogenetic similarity and similarity in male reproductive strategies between species.
We analyzed associations of non-musth adult males (who are also capable of reproducing) in this paper because musth males spend only a small proportion of their time in all-male groups (Keerthipriya et al., 2020a) and because no clear indicators of active and inactive sexual states outside of musth have been recognized in the Asian elephant (unlike that in the African savannah elephant; Ganswindt et al., 2005; Rasmussen, 2005; Goldenberg et al., 2014). We aimed to examine the prevalence, strength, and stability of associations among non-musth Asian elephant males. We wanted to examine some potential reasons for male associations. Increased foraging efficiency was not likely to be a factor because individuals require large amounts of food and grouping is expected to create food competition. Defense against predators was also not likely to be important because adult male elephants do not have any natural predators and our work was carried out in protected areas. We did not examine genetic relatedness as a cause for associations. In this study, we considered two non-mutually exclusive reasons for male (adult, non-musth, male Asian elephants in this paper, unless specified otherwise) associations in all-male groups. We hypothesized that such associations might be based on opportunities available for (a) social learning from older individuals and/or (b) testing strengths, i.e., assessing relative dominance status through sparring or other agonistic interactions with their associates. In mixed-sex groups, male associations may be motivated by social learning from older males. They may also be affected by competition for females. Alternatively, males might be tracking and associating with female groups independent of the presence of other males. We also wanted to find out whether there is a constraint on all-male group sizes that could affect male associations, similar to that found in Asian elephant females (Nandini et al., 2017, 2018).
We set out to address the following specific questions:
(1) What are the proportions of time that non-musth males spend in all-male groups and mixed-sex groups and do these proportions vary with male age? African savannah elephant males spent greater proportions of their time in all-male groups than in mixed-sex groups (Chiyo et al., 2011) and older males had been seen more often than younger males in all-male groups (see Chiyo et al., 2011; Goldenberg et al., 2014). However, a restriction in group size could limit the time spent in all-male groups, changing these patterns among non-musth males in our study.
(2) How does male age and the presence or absence of females in the vicinity affect patterns of associations between non-musth males? We expected male age to affect associations differently depending on whether males associated primarily for social learning from older males or for testing strengths. We expected males to use their time in all-male groups, rather than in mixed-sex groups, to test strengths. However, social learning from older males could occur in female absence (learning related to resources) and/or female presence (learning related to resources and/or reproduction). If male associations were primarily based on social learning from older individuals, younger males would seek out older males more often than expected by chance alone, and be sighted more often with older males than with other young males. We constructed male social (association) networks in the presence and absence of females. Older males would be better connected in networks if they were preferred as associates by young and old males, or if old males had stronger age-peer associations than young males. If the primary purpose of male associations was to test strengths, males would be expected to associate with age-peers, rather than with much younger or older individuals. As Asian elephant males continue to grow in height several years after they reach adulthood and gain weight over most of their life (Sukumar et al., 1988; Mumby et al., 2015), old males are larger than young males, and their relative strengths are easily assessed by size differences, not necessitating prolonged association to evaluate their relative strengths. We did not have a priori expectations about whether old or young males would be more likely to associate amongst themselves to test strengths.
Since competition for females could play a major role in how males associated (as non-musth males also acquire matings), we compared the networks of male associations in the presence and absence of females. We expected males to meet fewer other males and their associations to be weaker in the presence of females than in their absence. Due to the expected competition among males, we also predicted that males would experience smaller group sizes (of adult males) in the presence, rather than the absence, of females. It was also possible that males approached female groups by tracking them independently of other males. In such a case, we expected males to associate with each other at random and had no a priori expectation about whether the group sizes of males would be higher in the presence or absence of females.
(3) Is there a restriction on male group size? If there was a constraint on the group sizes of all-male groups, we expected a trade-off between the number of associates of a male and how often the focal male was sighted with those associates. We also expected older males to spend less time in all-male groups or to form smaller all-male groups than younger males because of greater food competition (due to larger body size).
(4) Are there preferential associations between non-musth males and, if so, are they stable over time? As Asian elephant males are not known to form coalitions, we did not have any a priori expectation about whether preferred, stable associations would be present.
Materials and Methods
Field Data Collection
The field study was carried out in Nagarahole and Bandipur National Parks and Tiger Reserves (Nagarahole: 11.85304°–12.26089° N, 76.00075°–76.27996° E, 644 km2; Bandipur: 11.59234°–11.94884° N, 76.20850°–76.86904° E, 872 km2), southern India, from March 2009 to July 2014. Nagarahole and Bandipur National Parks are separated by the Kabini reservoir, and we refer to the elephants in the two parks as the Kabini population. Because of the high density of elephants around the reservoir and better visibility for behavioral observations, our sampling was centered around the reservoir, and extended to the forests in either direction with lower frequency of sampling (see Nandini et al., 2017). We sampled pre-selected routes (see Nandini et al., 2017 for details) in the study area from early morning to late evening (∼6:30 AM to 6:00-6:45 PM depending on field permits). There were no distinct bull areas in our study area. The adult sex ratio in the study area was 1 male: 4–5 females (Gupta et al., 2016).
We noted the age, sex, and identity of the elephants that we sighted. Asian elephants are sexually dimorphic, with males being taller and bulkier than females, apart from differences in genitalia. Females do not possess tusks, although some males are also tuskless. We estimated age (of male and female elephants) based on shoulder height, body length, skull size, and skin folds (see Vidya et al., 2014), with semi-captive elephants in the same area serving as a reference for aging older animals. We placed males into the following age categories (based on ages calculated at the mid-point of the study period; November 2012), based on their estimated ages: calves (<1 year), juveniles (1- < 5 years), sub-adults (5– < 15 years), young adults (15- < 30 years), and old adults (≥30 years). Old adult males are expected to be reproductively competitive and regularly enter musth. Their interactions with females were distinct from those of younger males (see Rasmussen et al., 2005; Chelliah and Sukumar, 2013), and old, but not young, males in the Kabini population used their musth period to increase association with females (Keerthipriya et al., 2020a). Thus, we wanted to examine non-musth associations of these two age-classes separately. We identified individuals based on a combination of ear, back, tail, tusk, and body characteristics (see Vidya et al., 2014). We recorded group size, GPS location, time of sighting, and whether adult males were in the presence or absence of females. Adult males were said to associate with a female group [one or more adult females (≥10 years of age) and their young that were in close proximity and showed coordinated movement; see Nandini et al., 2018] if they fed within 10 m (easy physical reach; males seem to check the reproductive status of females by touching or sniffing their genitals) of a group member or interacted with any group member. When two males associated with the same female group at the same time, they were said to be associating with each other in female presence. Males were said to associate with each other in female absence if they fed within about 50 m of each other and there were no females in the vicinity. At this distance, the males would be able to display or react to visual signals, apart from sensing one another through sound or smell. However, communication through acoustics or smell might also occur over longer distances. Males could indulge in sparring during their associations, but if males, upon encountering each other, displayed only aggressive interactions and moved away, they were not considered to be associating.
Ethical Note
Field data collection was observational in nature. Therefore, no animal handling or manipulation was involved. Fieldwork was carried out under permit numbers D/WL/CR-121/2008–2009 dated 23/01/09, 18/05/10, and 08/05/12, and PCCF(WL)/E2/CR-121/2014-15 dated 09/05/14, issued by the office of the Principal Chief Conservator of Forests (Wildlife) and Chief Wildlife Warden, Karnataka.
Data Analysis
Data analysis was carried out using only those sightings in which all the adult males were aged and identified and female group compositions (if applicable) were known. The GLMs (General Linear Models) and non-parametric tests described below were performed using Statistica 7 (StatSoft Inc, 2004), and GLMMs (Generalized Linear Mixed-Effects Models) and randomizations were carried out using MATLAB 7.12 (The MathWorks Inc, 2011) with codes written by the authors unless specified otherwise.
Proportions of Time Spent in All-Male and Mixed-Sex Groups and Their Relationship With Male Age
We calculated the number of minutes males (seen on at least five different days when not in musth; 16 old males and 22 young males) were observed in the following group types and calculated the proportions of each individual’s time spent in such groups (as the number of minutes the male was sighted in that group type divided by the total number of minutes the male was seen): (1) solitary, (2) all-male groups with only one adult male, but including subadult or juvenile males and, therefore, not solitary, (3) all-male groups with more than one adult male, and (4) mixed-sex groups. Since the four proportions add up to one and are, therefore, not independent, and the number of males seen in group type 2 was small, we compared only two of the four categories– group types 3 and 4. We carried out Wilcoxon’s matched-pairs tests, separately on old and young males, to test whether the proportions of time spent in the different group types differed significantly. Since the proportions were calculated for each male, each male was represented only once in this analysis. In order to find out whether old males spent a smaller proportion of their time in mixed-sex groups than young males did, we used a Mann–Whitney U test.
Changes in Male Associations and Time to Sighting Independence
Using data from the last 2 years of observation (2013–2014), we examined continuous observations of individual males that lasted at least 5 min and recorded the number of changes in that male’s associations in 5-min bins. If there were changes in the identities of the adult males in the focal male’s group or if the focal male’s association changed from female presence to absence (or vice versa), they were counted as changes in the focal male’s associations. We found that, at about 80 min, there was roughly equal probability of a male’s association changing or not changing (see Supplementary Material 1). We used this time interval to record independent sightings, in order to avoid pseudoreplication of data. For all subsequent analyses of sighting data, we included only independent sightings based on this time interval. In addition, in many of the analyses mentioned below, only males who were sighted in at least 10 independent sightings in that particular category (such as group composition type or female presence) when not in musth were used, as associations of males seen rarely are unlikely to represent their actual association patterns and may bias the results. Similarly, if there was a comparison between different categories (such as associations in female presence and absence), males common to both categories and sighted at least 10 times in each of the categories were used, unless otherwise mentioned.
Effect of Male Age and the Presence or Absence of Females on Male Association Patterns
Older males as preferred associates
We examined all the instances in which two males (all dyadic combinations in the small number of cases in which there were more than two males) of different ages were in close proximity and one approached the other and associated with it. It was possible under such circumstances for either of the males to approach the other. We randomly chose one of the males as the focal male and used a generalized linear mixed-effects model (GLMM) with a binomial dependent variable (0 if the focal male approached the associate and 1 if the focal male was approached by the associate), age difference (age of the focal male minus the age of the associate) as a continuous predictor variable, and dyad identity as a random factor. If younger males approached older males more often than expected, a significant positive effect of the age difference on the dependent variable would be found. We used the fitglme function in MATLAB R2011a, with a logit link function and Laplace estimation method, and the model was fit using maximum likelihood. For this analysis, we used data only from the years 2011–2014, during which detailed behavioral observations were available. We only used observations during which we were present before the beginning of the association and one of the males clearly moved toward the other.
We calculated, for each focal young male, the number of sightings in which that young male was seen along with old males, and divided that by the total number of sightings of the young male. Similarly, we also calculated, for each focal young male, the number of sightings in which that young male was seen with other young males, and divided that by the total number of sightings of the focal young male. We compared the proportions of young males’ sightings spent with old males with the proportions of the same young males’ sightings spent with other young males using Wilcoxon’s matched-pairs tests. These proportions were calculated, and the Wilcoxon’s matched-pairs tests were done, separately in the presence and absence of females. We expected that young males would spend a greater proportion of their sightings with old, rather than other young males if they preferred old males as associates (in the presence and/or absence of females), and a greater proportion of sightings with other young males, rather than old males, if their associations were based on testing strengths (in female absence).
We examined whether old males were better connected in male social networks. We first calculated the association index (AI; simple ratio index of Ginsberg and Young, 1992) between pairs of identified males as the number of times (independent sightings) two males were seen together (NAB) divided by the total number of (independent) sightings of the two males. AI between each pair of males was calculated separately in the presence and in the absence of females [for instance, AIAB(F_abs) = NAB(F_abs)/(NA(F_abs) + NB(F_abs) – NAB(F_abs)), where NA(F_abs) is the total number of sightings of A in female absence and NB(F_abs) is the total number of sightings of B in female absence]. We used these AIs to calculate association strength (or weighted degree) for each individual male, as the sum of the AI values between a male and all his associates. We calculated the difference in association strengths between males of the two age-classes (mean association strength of old males – mean association strength of young males) in the observed dataset. We then calculated this for each of 5000 permuted datasets (see the next section for details of permutations) and compared the value from the observed dataset with those from the permuted datasets (see section below; P < 0.025 for statistical significance as we had no prior expectation about whether the observed values would be lower or higher than the permuted values).
Age-peer associations among males
In order to find out whether males preferentially associated with age-peers more often than expected by chance, we used a permutation procedure following Whitehead (2008, p. 124), on the datasets of male sightings in the absence of females. We generated 5000 males permuted datasets by switching adult males across independent sightings, while keeping the group sizes and the number of sightings of each male constant in each permuted dataset. The number of flips performed in each permutation was five times the number of sightings in that dataset. We calculated the number of times old (≥30 years) and young (15–30 years) males were sighted with other males of the same or different age class in the observed dataset and compared these observed values with the values from the permuted datasets. The observed value was higher than expected by chance if it was >97.5% of the permuted values, and lower if it was <2.5% of the permuted values. We tested whether the observed value was higher than the permuted values by calculating P = proportion of permuted values that were higher than the observed value (P < 0.025 for statistical significance). Otherwise, we tested whether the observed value was lower than the permuted values (P = proportion of permuted values that were lower than the observed value; P < 0.025 for statistical significance).
We also compared the proportion of their sightings, in the absence of females, that old males spent associating with other old males (number of sightings in which a focal old male was seen with other old males in the absence of females, divided by the total number of sightings of the focal old male in the absence of females) with the proportion of their sightings that they spent associating with young males (number of sightings in which the focal old male was seen with young males in the absence of females, divided by the total number of sightings of the focal old male in the absence of females), using a Wilcoxon’s matched-pairs test. A higher proportion of sightings with other old males than young males would be seen if the testing-strengths hypothesis was supported.
Male Associations in the Presence and Absence of Females
We performed a similar permutation procedure as described in the section above on the female presence dataset. We also compared the following network statistics between male association networks in female presence and absence: mean degree (number of associates of a focal male), mean clustering coefficient (the proportion of the total possible connections between a male’s associates that exist), mean path length (the smallest number of connections between two individuals in the network), and network density (the proportion of all possible connections that exist in the network) (Latapy, 2008; see Wasserman and Faust, 1994). We compared these network statistics and mean AIs between female presence and absence using a sampled randomization test (Sokal and Rohlf, 1981, pp. 791–794). We created 5000 permuted datasets (permuted by randomly assigning sightings to female presence or absence, while conserving the sample sizes of both the categories and ensuring that at least ten sightings of each male were assigned to each category in each permutation). We then compared the observed differences in network statistics and AI between the original female presence and female absence datasets with the differences in statistics between the permuted ‘female presence’ and ‘female absence’ datasets. The probability of a significant difference between the observed values was calculated as the proportion of permutations in which the difference in statistic between the permuted datasets was greater than or equal to the difference in statistic between the observed datasets.
We compared the degree distributions of association networks in female presence and absence with their Poisson expectations (expected for an Erdös–Rényi random network; Erdös and Rényi, 1960) to test whether males were associating with each other at random.
We examined the effect of female presence on male group size by performing a GLM on group sizes experienced by individual adult males (counted as the number of adult males in each sighting of the focal male, including the focal male). We used female presence/absence as a fixed factor and male identity as a random factor.
Restriction on All-Male Group Size
We calculated the average of the proportions of sightings that focal males spent with their different associates: we divided the number of sightings in which a focal male was seen with a specific associate by the total number of sightings (including solitary sightings) of the focal male in female absence, and averaged this proportion across all associates of the focal male. We used a Spearman’s rank-order correlation to find out whether this averaged proportion of sightings spent by a male with other males was correlated with the number of associates of that male in female absence. Similarly, we also correlated the average of the proportion of all-male group sightings (solitary sightings of the focal male excluded) spent with an associate with the number of associates of those males. Similar to the comparison of time spent in mixed-sex groups in the section above (Proportions of time spent in all-male and mixed-sex groups and their relationship with male age), we examined whether old males spent a smaller proportion of their time (in minutes) than young males in all-male groups by performing a Mann–Whitney U test. We examined the relationship between the mean experienced group size (based on independent sightings) of a male and his age using Spearman’s rank-order correlation.
Preferred Male Associations and Stability of Associations
We determined a top associate (associate with the highest AI value) for males, separately in female presence and in female absence. Directed networks of males and their top associates were constructed using Gephi 0.8.2 (Bastian et al., 2009). In order to examine whether the identity and strength (AI) of a male’s association with his top associate was different from random, we again used the procedure for permuting associations (Whitehead, 2008, p. 124) explained in a section above. We permuted associations between males separately in the female presence and female absence datasets (5000 permutations on each dataset, with the number of flips in each permutation being five times the number of sightings in that dataset). We compared the AI between a male and his observed top associate with the AI values for the same dyad from the permuted dataset, and considered the association to be significant if the observed AI was greater than 95% of the permuted values. In each permutation, for each male, we also recorded the identity of his permuted top associate. We examined how often the male had the same top associate in the permuted datasets, and considered the identity of his top associate to be significantly different from random if the observed top associate was the top associate in fewer than 5% of the permuted datasets.
We compared AI matrices between consecutive years, using those males that were common to and sighted at least five times in both years, by performing Mantel tests of matrix correlation (Mantel, 1967), with 5000 permutations, in MATLAB (MATLAB R2011a; The MathWorks Inc, 2011). Due to limitations of the number of sightings, only data from 2011–2014 and only in female absence were used for this analysis.
Results
We used data from 878 days of field work between 2009 and 2014. Elephants were sighted on 853 days, and identified adult males were sighted on 718 days. Identified non-musth adult males were sighted on 681 days, and observed for a total of 1002.1 h.
Proportions of Time Spent in All-Male and Mixed-Sex Groups and Their Relationship With Male Age
We sighted 83 non-musth males (28 old males and 55 young males; see Supplementary Material 2) in all, of which 44 were seen in the presence of females and 81 in the absence of females. Based on the set of non-musth males seen on at least five different days (N = 38), we found that males spent an average (±SD) of 12.3% (±11.79%) of their time in all-male groups and 29.6% (±22.73%) of their time in mixed-sex groups (Figure 1A). Old males spent similar proportions of their time in all-male and mixed-sex groups (Wilcoxon’s matched-pairs test: T = 63.00, Z = 0.259, N = 16, P = 0.796), whereas young males spent a higher proportion of their time in mixed-sex groups than in all-male groups (Wilcoxon’s matched-pairs test: T = 9.00, Z = 3.815, N = 22, P < 0.001, see Figure 1B). Old males spent a smaller proportion of their time in mixed-sex groups than did young males (Mann–Whitney U test: U = 79.50, ZAdj = –2.853, P = 0.004, see Figure 1B).
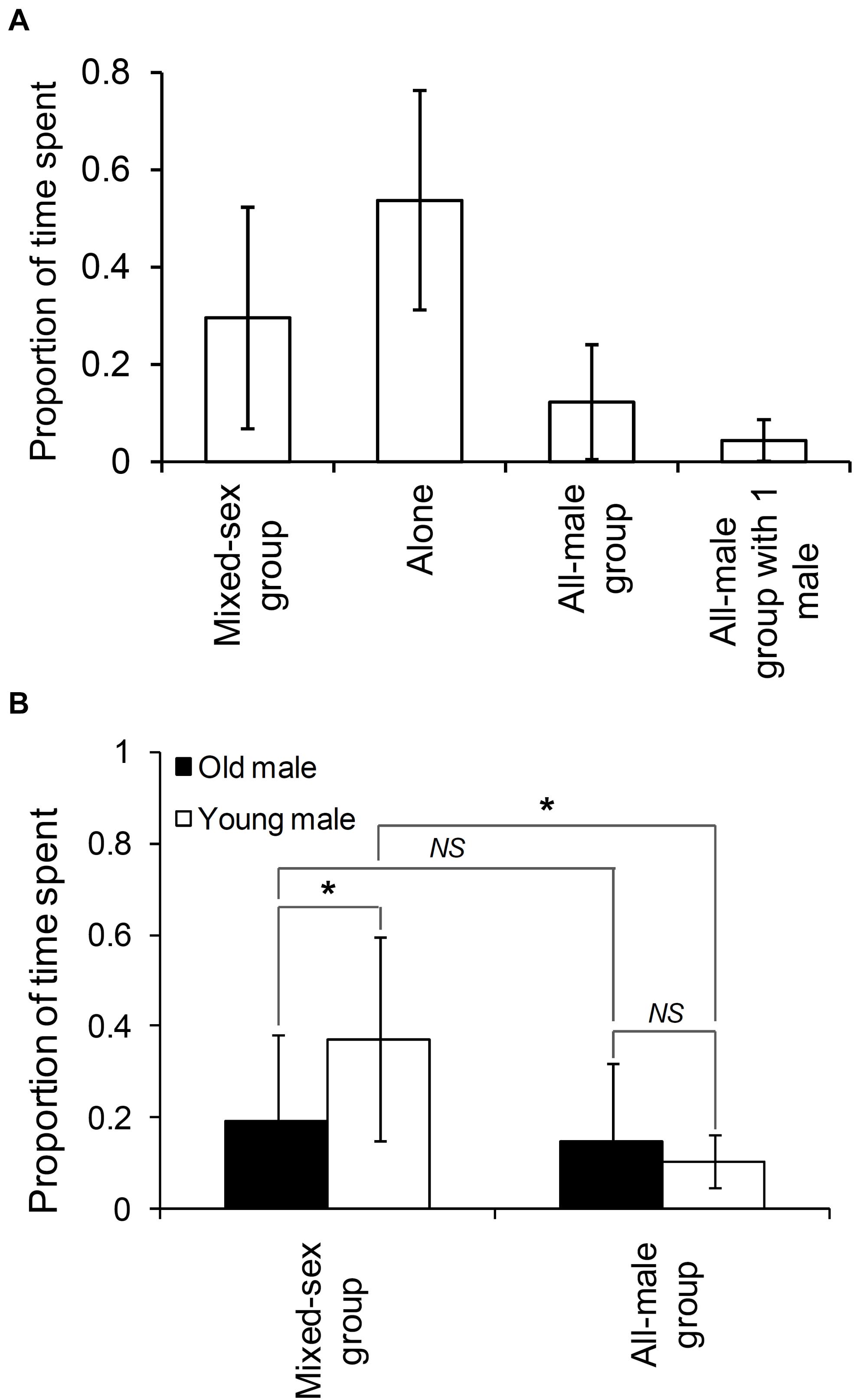
Figure 1. The proportion of time that (A) males (sighted on 5 days or more) spent in different group types and (B) old and young males (sighted on 5 days or more) spent in mixed-sex and adult all-male groups. Pairwise comparisons with significant results are marked with an asterisk, and those with non-significant results are marked as NS (Not Significant). Error bars are standard deviations.
Effect of Male Age and the Presence or Absence of Females on Male Association Patterns
Based on the 80-min cutoff, there were 2466 independent sightings of adult non-musth males (374 sightings in female presence and 2092 sightings in female absence) in the study period. Rarely (only three different sightings), males were seen to associate with subadult females (5–10 years old) in the absence of an adult female and this was also considered to be association in female presence. Fourteen males (4 old and 10 young males) were sighted at least 10 times in female presence, and 32 males (13 old males and 19 young males) were sighted at least 10 times in female absence. Only 12 males (called common males) were sighted at least 10 times each in the presence and absence of females.
Older Males as Preferred Associates
The age difference between the focal male and the other male did not significantly affect who approached whom either in the presence (GLMM: Estimated coefficientAge–difference = –0.035, tstat = –0.821, N = 19 approaches, df = 17, P = 0.423) or in the absence of females (GLMM: Estimated coefficientAge–difference = –0.026, tstat = –0.772, N = 48 approaches, df = 46, P = 0.444). Thus, old and young males were equally likely to approach each other to initiate association. In the presence of females, the proportions of their sightings that young males spent with old males (mean ± SD: 0.058 ± 0.035) and those that they spent with other young males (mean ± SD: 0.111 ± 0.090) were not significantly different from each other (Wilcoxon’s matched-pairs test: T = 6.50, Z = 1.610, N = 10 focal young males, P = 0.107; Figure 2). A lack of difference between the proportions of their sightings that young males spent with old males (mean ± SD: 0.073 ± 0.086) and those that they spent with other young males (mean ± SD: 0.065 ± 0.045) was also observed in the absence of females (Wilcoxon’s matched-pairs test: T = 58.00, Z = 0.114, N = 19 focal young males, P = 0.910; Figure 2).
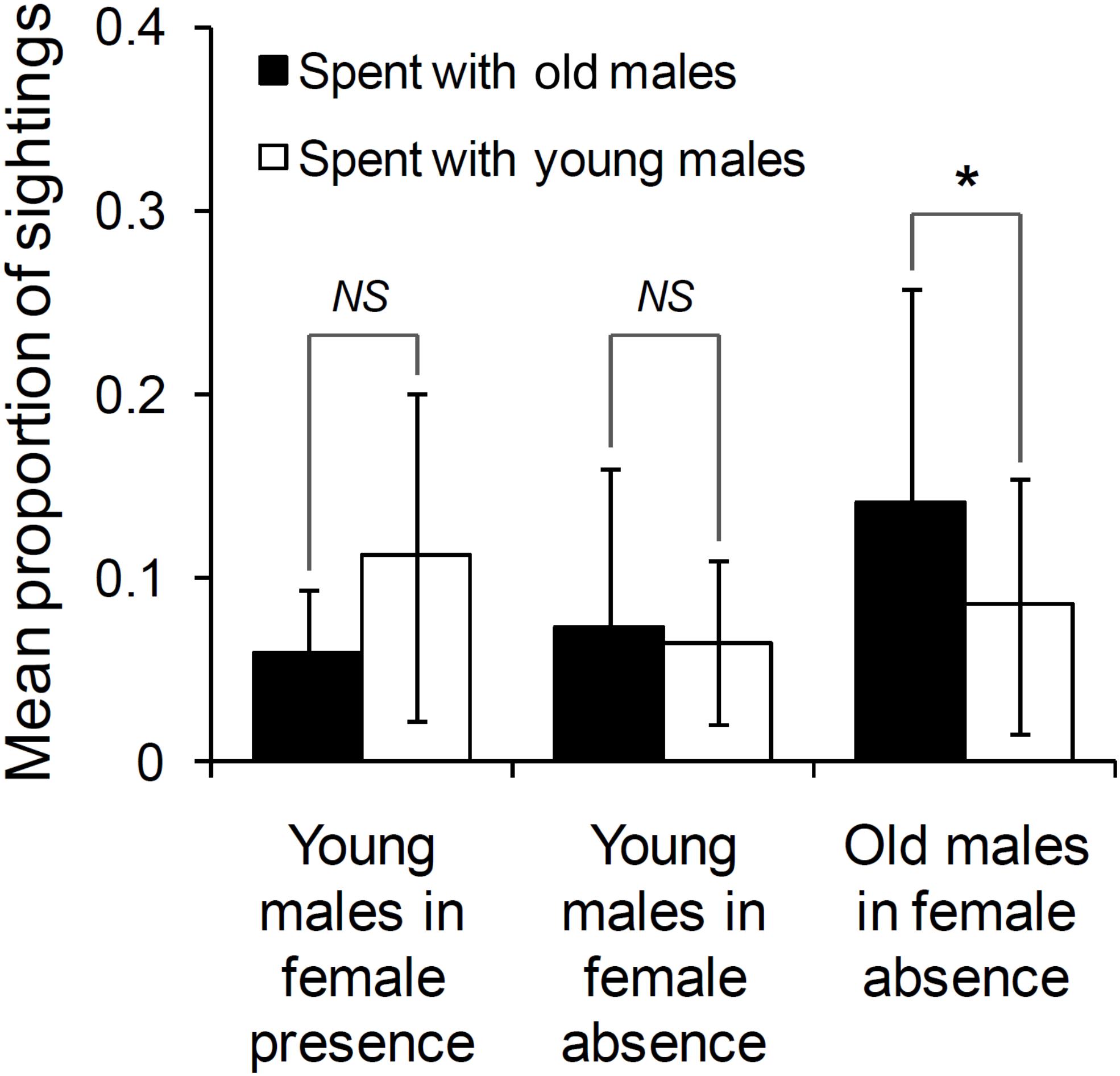
Figure 2. The proportion of sightings spent with old and young males, by young males in female presence and absence, and old males in female absence (sighted 10 times or more in that category). Pairwise comparisons with significant results are marked with an asterisk, and those with non-significant results are marked as NS (Not Significant). Error bars are standard deviations.
Examining the effect of male age-class on association strength, we found old males to have only a slightly higher association strength (mean = 0.087) than young males (mean = 0.052) in the absence of females [difference in mean association strength (old-young): observed dataset: 0.035; permuted datasets: mean (2.5, 97.5 percentiles): 0.003 (–0.015, 0.020), P < 0.001, Table 1]. The observed difference in mean association strengths between the age-classes was not different from the permuted values in the presence of females (see Table 1).

Table 1. Difference in mean association strength between males of the two age-classes in observed and permuted datasets.
Age-Peer Associations Among Males
In female absence, old males were sighted together more frequently than expected by chance [observed: 42 sightings, permuted: mean (2.5, 97.5 percentiles): 25.5 (18, 34) sightings, P < 0.001; Figure 3A]. Old and young males were sighted together less frequently than expected by chance [observed: 51 sightings, permuted: 69.4 (58, 80) sightings, P = 0.001], and young males were sighted together as expected by chance [observed: 38 sightings, permuted: 42.9 (33, 53) sightings, P = 0.178; Figure 3A]. Although these tests were based on small numbers of males, the results remained unchanged when we repeated the analysis using all the identified males (Supplementary Material 3).
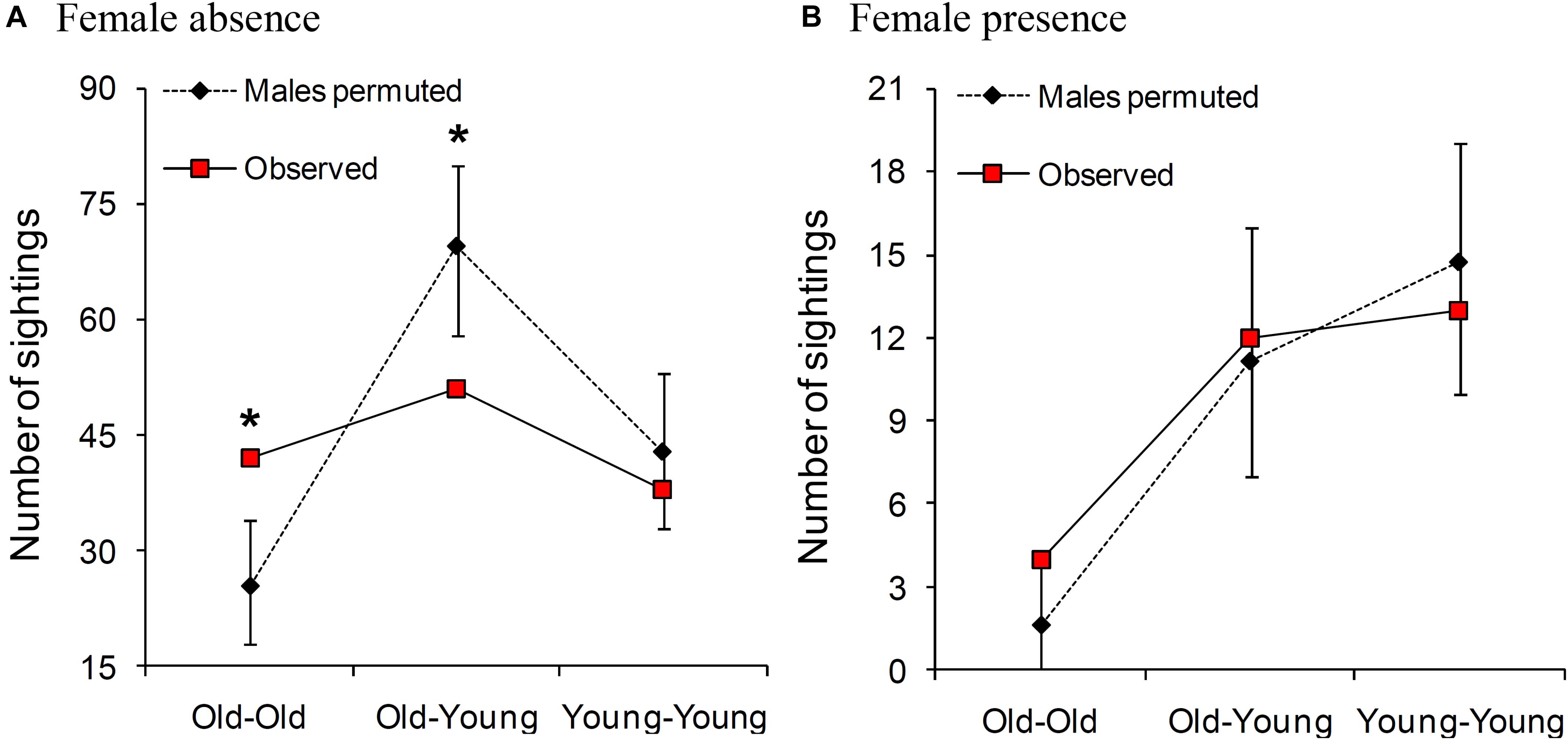
Figure 3. Permuted and observed numbers of times adult males (sighted 10 times or more in that category) of the same and different age-classes were sighted together in (A) female absence and (B) female presence. Comparisons where the observed value was significantly different from those of the permuted datasets are marked with an asterisk. Old males are ≥30 years and young males are 15- < 30 years old. Please note that the Y axis is on different scales in the two panels.
The proportions of their sightings that old males spent with other old males (mean ± SD: 0.141 ± 0.116) was higher than those that they spent with young males (mean ± SD: 0.085 ± 0.069; Wilcoxon’s matched-pairs test: T = 5.00, Z = 2.489, N = 13, P = 0.013; Figure 2), in female absence.
Male Associations in the Presence and Absence of Females
We found that old males were sighted together as expected by chance in the presence of females [observed: four sightings, permuted: mean (2.5 and 97.5 percentiles): 1.6 (0, 4) sightings, P = 0.069]. The numbers of sightings were not different from those expected by chance in the case of old and young males seen together [observed: 12 sightings, permuted: 11.1 (7, 16) sightings, P = 0.439], or young males seen together either [observed: 13 sightings, permuted: 14.7 (10, 19) sightings, P = 0.289] (Figure 3B). These results remained unchanged when we repeated the analyses using all the identified males without a sighting cutoff (Supplementary Material 3). All three age-class combinations of males were sighted together more often in female absence than in female presence (Figure 3). However, while the observed male association network appeared more connected and denser (higher mean degree, mean clustering coefficient, and density, and lower mean path length) in female absence than in female presence, none of the statistics we examined was significantly different based on sampled randomization tests [all P > 0.05, 12 males seen at least 10 times each in female presence (249 sightings) and in female absence (1237 sightings), Table 2]. There was also no significant difference in mean AI between males in the presence and absence of females (Table 2 and see Supplementary Material 4 for distributions). Thus, the seemingly more connected network in female absence was probably a result of the greater time spent by males in the absence of females.
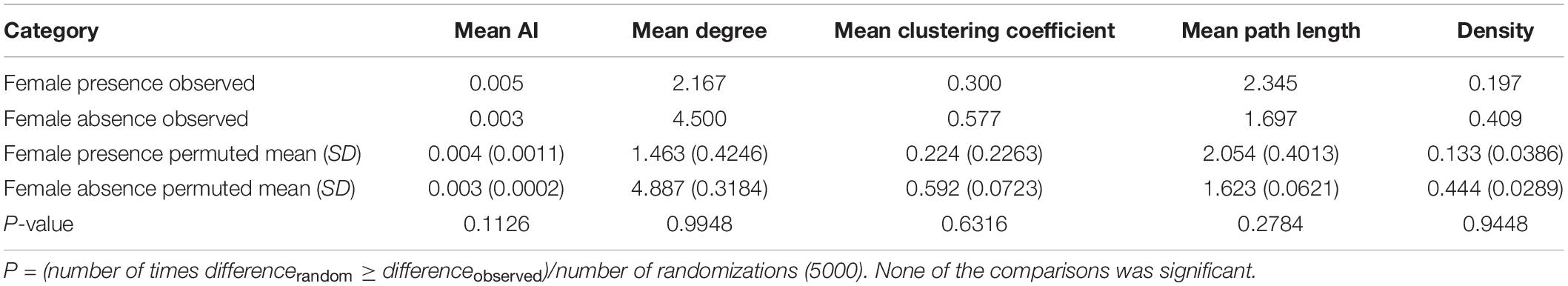
Table 2. Network statistics based on observed and permuted male associations in female presence and female absence.
The association network of adult males in female presence was not significantly different from a random network (χ2 = 6.122, df = 8, P = 0.633), but the network in female absence was different from random (χ2 = 184.647, df = 15, P < 0.001) (see Supplementary Material 5).
Group sizes (number of non-musth adult males in the sighting) were small in general, with the modal group size of all groups that had an adult male being 1 and the modal group size of all multi-male groups being 2 (Figure 4A). The experienced group sizes of common males (N = 12 males, seen 273 times in total in the presence of females and 1304 times in total in the absence of females, see Figure 4A) were larger in female presence (mean ± SD: 1.6 ± 0.80) than in female absence (mean ± SD: 1.2 ± 0.52; F1,1553 = 48.520, P < 0.001). There was a significant main effect of male identity (F11,1553 = 2.918, P = 0.045) and a significant interaction effect between male identity and female presence (F11,1553 = 2.157, P = 0.014; Figure 4B).
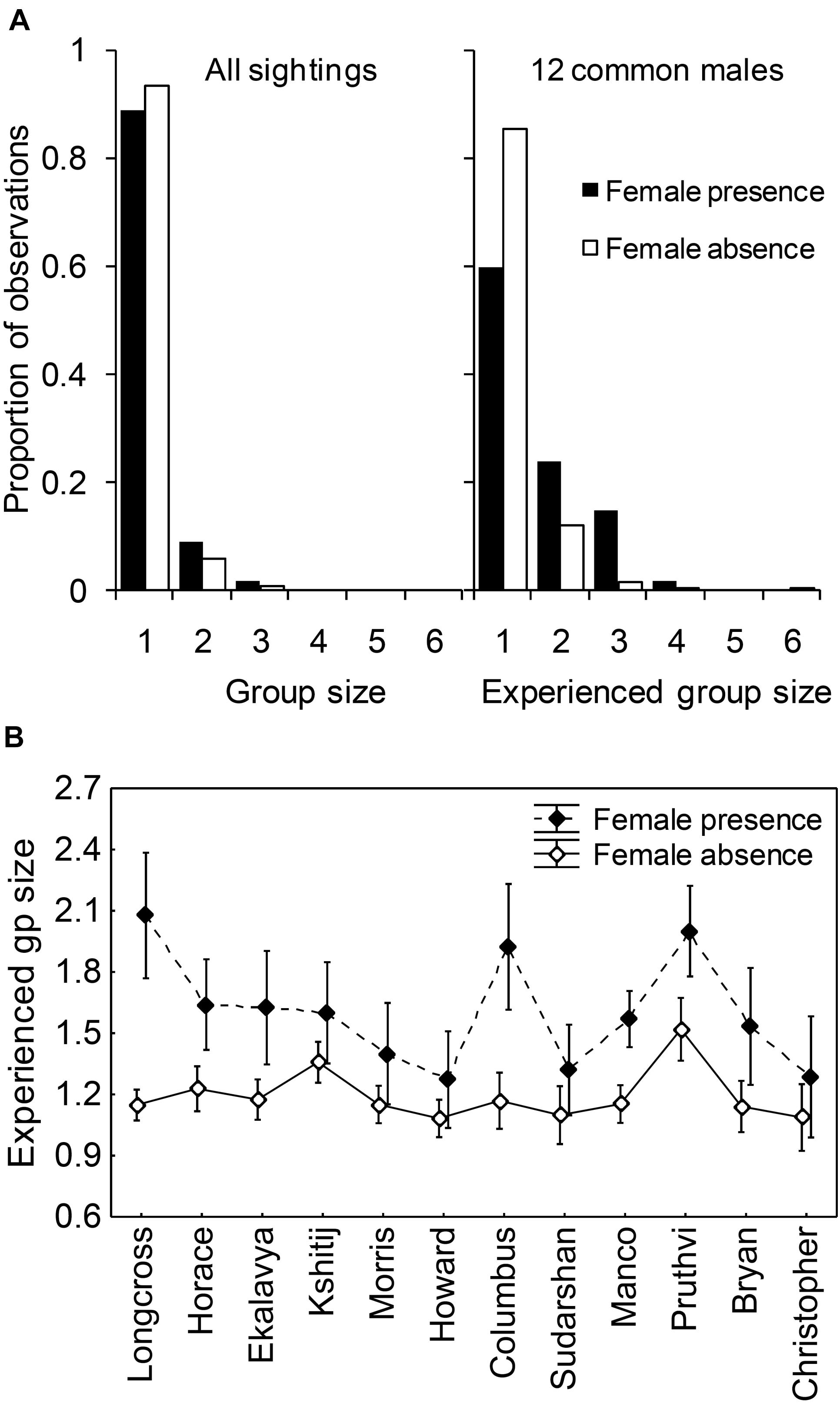
Figure 4. (A) Proportion of all sightings (containing at least one male), and proportion of experienced group sizes of common males, of different group sizes, and (B) the experienced group sizes of individual common males in female presence and absence. Group size was measured as the number of adult males in the sighting. Error bars are 95% CI.
Restriction on All-Male Group Size
The mean proportions of sightings that a male spent with an associate was negatively correlated with the number of associates of that male (Spearman’s rank-order correlation: N = 31 males, each with at least one associate and seen at least ten times in female absence, rs = –0.582, P < 0.001; Figure 5A). The relationship was even more negative when the mean proportion of all-male group sightings (rather than all sightings) spent with an associate was correlated with the number of associates of the focal male (rs = –0.984, P < 0.001; Figure 5B). Thus, in the absence of females, males that associated with a greater number of males spent a smaller proportion of their sightings on average with each of their associates. However, contrary to expectation, old and young males did not spend significantly different proportions of their time in all-male groups (Mann–Whitney U test: U = 157.50, ZAdj = 0.547, NOld = 16, NYoung = 22, P = 0.584), and there was no significant relationship between male age and mean experienced group size (Spearman’s rank-order correlation: N = 31 males, each with at least one associate and seen at least ten times in female absence, rs = 0.213, P > 0.05).
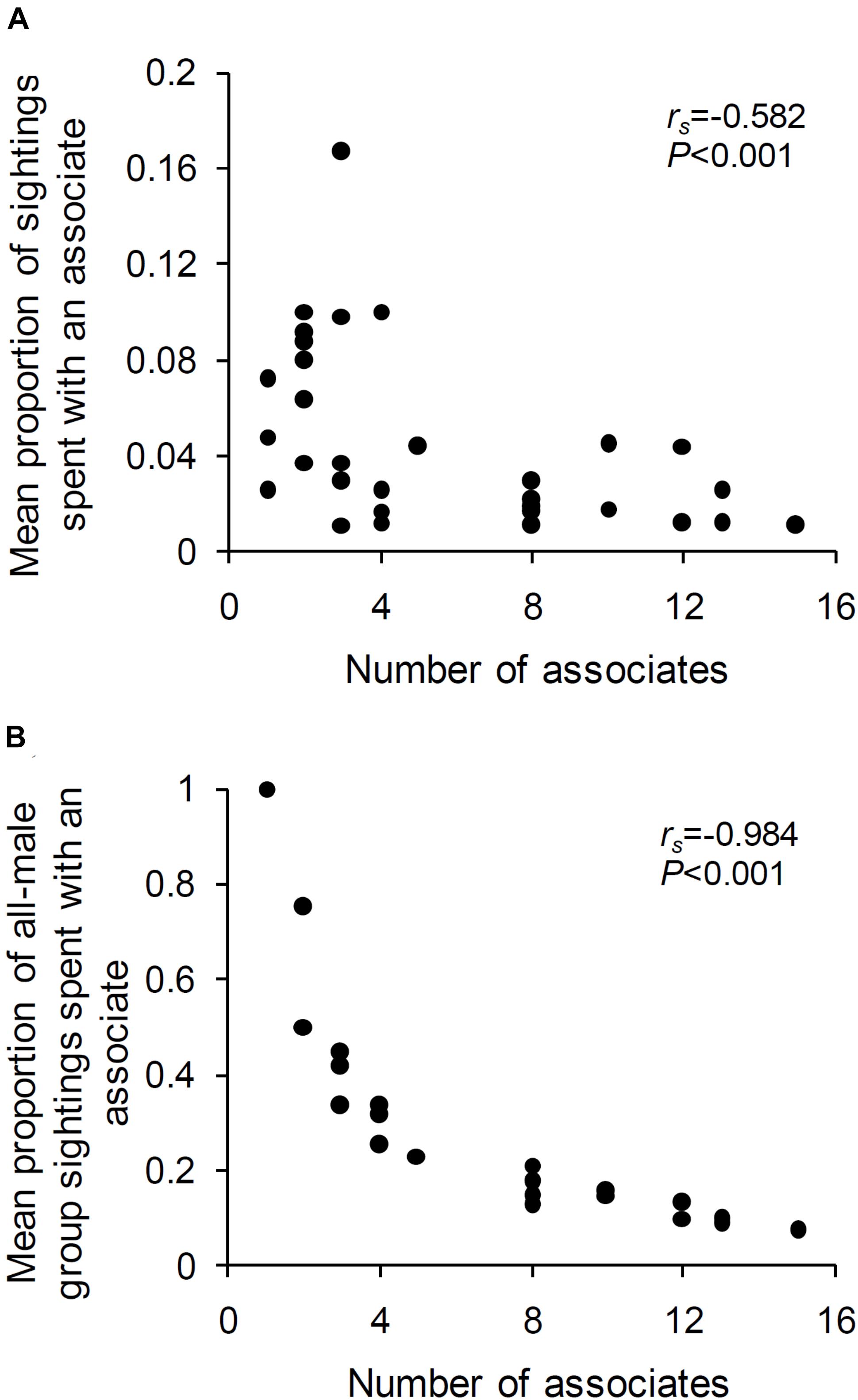
Figure 5. The mean proportion of (A) sightings (out of the total sightings of the male) and (B) all-male group sightings (out of the total all-male group sightings of the male), that a male spent with an associate plotted against the number of associates of that male. Results from Spearman’s rank-order correlations are written in the plot.
Preferred Male Associations and Stability of Associations
Only 14.3% (2 out of 14 males; 2 out of 15 dyads as one male had two top associates) of the males had a significant association with their top associates in female presence, whereas 61.3% (19 of 31 males; one male did not associate with any of the other males) of the males had a significant association with their top associate in female absence (mean significant AI with top associate in female absence = 0.032, see Supplementary Material 4 for AI distribution; networks shown in Figure 6). We found that the identity of the focal male-top associate dyad was different from random in 13.3% (2 out of 15) of the dyads in female presence and in 83.9% (26 out of 31) of the dyads in female absence.
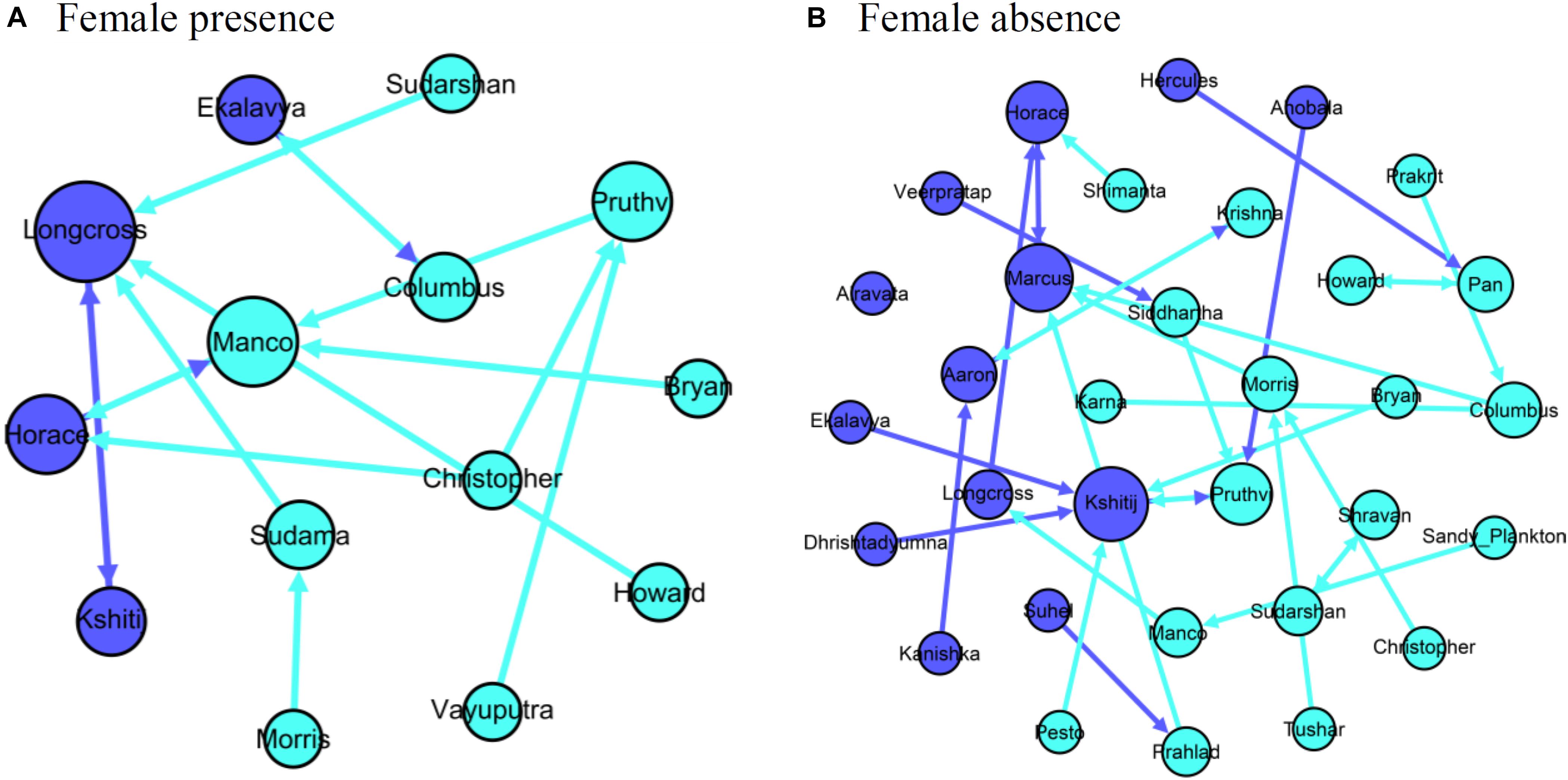
Figure 6. Networks of males and their top associates in (A) female presence and (B) female absence. Nodes representing old males are colored dark blue and those representing young males are colored light blue.
Mantel tests showed no significant correlation between association matrices across years, in two out of the three comparisons (Supplementary Material 6).
Discussion
This is the first detailed study of non-musth adult male associations in the Asian elephant in a relatively undisturbed natural habitat. As expected (because non-musth males also acquire matings, and hence, may experience competition), male-male affiliative associations were weak.
Proportions of Time Spent in All-Male and Mixed-Sex Groups and Their Relationship With Male Age
Non-musth adult males spent only ∼12% of their time in all-male groups in Kabini. In contrast, in the Amboseli African savannah elephant population, this value was ∼63% after adjusting for age-effects (Chiyo et al., 2011) and 30.5% of all male sightings (Lee et al., 2011). As Chiyo et al. (2011) used observations of males in an area used by both males and females, the observed difference cannot be attributed to the lack of bull areas in Kabini. We found evidence for a restriction of all-male group sizes in Kabini (see below); this restriction may contribute to the smaller time spent in all-male groups. In the Kabini population, when we examined associations of males of all musth statuses, male age did not affect the time spent in mixed-sex groups (Keerthipriya, 2018). However, old males increased their association with females, while young males decreased their association with females, when they were in musth, compared to when they were not in musth (Keerthipriya et al., 2020a). Therefore, our present result that old non-musth males spent a smaller proportion of their time with female groups than did young non-musth males was expected. Whereas old males spent a similar proportion of their time in all-male and mixed-sex groups, young males spent more of their time in mixed-sex than in all-male groups. As young males invest less in a competitive breeding strategy (expected in roving males, see Whitehead, 1994) like musth (see Keerthipriya et al., 2020a), their greater associations with females might facilitate learning about female groups in areas they have dispersed to and also allow for opportunistic matings (see Chelliah and Sukumar, 2015; Kabini Elephant Project, unpublished data). In another Asian elephant population, 15–20-year-old non-musth males engaged in sneak matings and 20–30-year-old non-musth males engaged in consortships more than expected by chance (Chelliah and Sukumar, 2013). As there is evidence for females preferring old musth males over young non-musth males in both African savannah (Poole, 1989) and Asian elephants (Chelliah and Sukumar, 2013), it would be interesting to find out how much mating and reproductive success is acquired by young non-musth males in this population. In the Samburu population of African savannah elephants, young males spent most of their sexually active state out of musth. The sexually active state shortened with age, and older (35 years of age and above) males were mostly in musth during their sexually active state (Rasmussen, 2005). Young males, thus, seemed to have a longer low-investment (mostly non-musth) sexually active state compared to old males. It is possible that young non-musth males in Kabini are also sexually active more often than old non-musth males, leading to the greater association with females. Although matings are very rarely seen, future studies involving hormonal analysis may help us understand this better. Young males might also associate with female groups to build social bonds with them, which could help in later reproduction. Alternatively, the greater time that young males spent in mixed-sex groups may reflect a reduced cost of feeding alongside females compared to that faced by old males. Further data are required to examine these possibilities.
Effect of Male Age on Association Patterns: Reasons for Adult Male Associations
Adult male associations in the Kabini elephant population showed preferential age-peer associations among old, but not young, males, and no evidence for young males preferentially associating with old males, thus favoring the testing-strengths hypothesis. Similar-aged males preferentially associate with each other and test strengths in all-male groups of other species (Villaret and Bon, 1995 – Alpine ibex; Cransac et al., 1998 – mouflon sheep) and all-male groups in Kabini may similarly provide a relaxed setting for old non-musth males to test strengths with age-peers. We had no a priori expectation about which age-class would have stronger preferential associations under the testing strength hypothesis, and found that only old males showed preferential association with age-peers. As old males regularly enter musth, it may be more important for them than for young males to clarify their relationships when they are not in musth. Similar to our results, old (30 + years) males associated more with age-peers, while the within age-class associations were as expected by chance in younger age-classes in all-male groups in the Amboseli African savannah elephant population (Chiyo et al., 2011). It would be interesting to find out if dominance relationships formed when not in musth have a bearing on the spatiotemporal distribution of musth males. Old males, having spent more time in their post-dispersal home ranges, might have formed significant associations with familiar age-peers. African savannah elephant males in Amboseli picked familiar age-peers as sparring partners (Chiyo et al., 2011), and less aggressive interactions with familiar than with unfamiliar opponents have been found in other species (Smith et al., 1999; López and Martín, 2001; Wich and Sterck, 2007). Examining the relationship between familiarity and the frequency of dominance interactions in the Kabini population would further help us understand the role of male associations in testing strengths. Murphy et al. (2020) suggested that, perhaps, younger (20–30-year-old African savannah male elephants in their study) males had not yet established a consistent non-musth home range, leading to lowered stability in association strength compared to older males. However, we found no difference between the turnover rates of young and old non-musth males (see Supplementary Material 2); therefore, this is unlikely to be the reason for the difference observed in our six-year dataset.
We found no evidence that male associations were primarily driven by opportunities for younger individuals to learn from experienced older individuals about the location of food resources in all-male groups or about food resources and/or interactions with females in mixed-sex groups. Young males did not prefer old males as associates or seek out old males to initiate association, either in female presence or absence. Moreover, none of the common young males met more old males than expected by chance in either female presence or absence (see Supplementary Material 7). Although old males had higher association strengths than young males in female absence, this was due to the stronger old male-old male associations, rather than old male-young male associations. It is worth noting that social learning about food resources or safety outside protected areas might occur between any pair of associating males, and we have not explicitly examined social learning between age-peers in the current study. The low associations between the age-classes may also be, in part, due to young males avoiding potentially risky conflict with the larger old males.
In African savannah elephants, older males were important in male networks. Older males spent more time with other males than younger males did (Poole, 1982; Chiyo et al., 2011; Lee et al., 2011) and had a greater number of associates in all-male groups (Chiyo et al., 2011) in the Amboseli population. In the Samburu population, sexually inactive older males showed significant affiliation with a higher proportion of available dyads compared with sexually inactive younger males (Goldenberg et al., 2014). Old males had higher centrality in association networks based on all-male groups in Amboseli (Chiyo et al., 2011), although when males were classified based on their sexual state in the Samburu population, age did not affect centrality in sexually inactive networks and was negatively correlated with centrality in sexually active networks (Goldenberg et al., 2014). In a recent study on non-musth male associations in South Africa, older males showed higher stability in association strength than younger males across sampling periods; however, age of the male did not affect the stability of the identity of top ranked associates across sampling periods, eigenvector centrality, or association strength of individual males in the network (Murphy et al., 2020). Further, younger males preferentially associated with older males, unlike what we found in Kabini. In Amboseli, males of the 20–29-year age-class associated with older (30+) males more than expected by chance (Chiyo et al., 2011), and male associations seemed to facilitate social learning; males who had an older crop raider as a top associate were more likely to raid themselves (Chiyo et al., 2012). Older African savannah elephant males (≥36 years of age in their study) were preferred as nearest physical neighbors during associations by males of all ages, based on observations in a bull area in Okavango Delta, Botswana (Evans and Harris, 2008), and have been considered analogous to the knowledgeable matriarchs of female groups in the species (Evans and Harris, 2008). In a bull area in Botswana, older males (≥26 years of age in their study) were more likely to lead all-male group movements than expected by chance, possibly serving as repositories for ecological knowledge (Allen et al., 2020). More generally, preferred association with, and social learning from older, more experienced individuals has been observed in the context of mating behavior (Pereira, 1988; Beecher et al., 1994), and foraging strategies (Rajpurohit et al., 1995; Biro et al., 2003) in other species.
There were some differences in age-classification and methodologies [males of a group were within about 100 m of one another in Chiyo et al. (2011) and Murphy et al. (2020), and about 500 m in Goldenberg et al. (2014)] between the studies on African savannah elephants, yet preferential associations with old males were observed across these different methods. We used the same age-classification for old males (30 + years) as Chiyo et al. (2011), Goldenberg et al. (2014), and Murphy et al. (2020). Although we used slightly smaller distances [50 m between males in the group, compared to 100 m in Chiyo et al. (2011) and Murphy et al. (2020), and 500 m in Goldenberg et al. (2014)], our results are unlikely to have been different if we had used a slightly larger distance as we did not find many instances of males within a 100-m radius but outside a 50-m radius.
The greater social role of older males in the African savannah elephant compared to the Asian elephant in female absence may stem from differences in the habitats they occupy. Relative to the African savannah elephant, Asian elephants occupy moister, more forested habitats, in which food is possibly more dispersed and unpredictable in space at a very local scale (for example, zones within the Kabini backwaters area showed significant differences in grass biomass; Gautam and Vidya, 2019), but possibly more predictable at a larger spatiotemporal scale due to clear seasonality. This local heterogeneity, along with possibly limited food in a feeding patch, and greater predictability at a larger scale might make older males no more knowledgeable about resource availability than young males. We also did not find any evidence for preferred associations with older males in female presence. As young male Asian elephants have been observed to obtain some mating success through opportunistic matings (see Chelliah and Sukumar, 2015; Kabini Elephant Project, unpublished data), it is possible that learning in the context of reproductive behaviors occurs at a younger age-class, such as subadult males. In our study population, matriarchs of female clans were not the most central individuals in association networks (Shetty, 2016), which has also been suggested in Uda Walawe in Sri Lanka (de Silva et al., 2011). Female clan members are split up across small groups due to group size constraints (Nandini et al., 2017), which might result in the matriarch not being central to the clan. However, although males also associated in small group sizes, young males did not prefer older age-class males as associates even within those small groups. Therefore, it appears that social learning from older males is not the primary reason for adult male associations, even accounting for limitations on group size.
Male Associations in the Presence and Absence of Females
The association network of males was non-random in female absence but random in female presence. Network statistics and AI values of males were not significantly different between female presence and absence, after accounting for the different numbers of sightings in the two categories. Thus, the seemingly better-connected network in female absence was due to the greater amount of time that males spent in the absence of females. Contrastingly, the association networks of sexually inactive African savannah elephant males in Samburu were significantly denser and more clustered than those of sexually active males (Goldenberg et al., 2014). However, sexually active males in Samburu included musth males and it is not clear to what extent this might have contributed to the significant difference between the networks. Our results suggest that males approach female groups solely due to the presence of females and independent of the presence of other males. This is in contrast to the previous finding that young musth males were never found with old non-musth males in the presence of females (Keerthipriya et al., 2020a). Thus, not investing in musth may allow young males to associate with females more freely and, perhaps, obtain the occasional mating. Moss and Poole (1983) observed that African savannah elephant males in Amboseli also associated at random when in mixed-sex groups. Males in multi-male mixed-sex groups of other species have also been found to invest in affiliations with females rather than with other males (for example, mountain gorillas, see Sicotte, 1994). The slightly larger male group size in female presence than in female absence could be a result of multiple males tracking the same resource (female groups).
Restriction on All-Male Group Size
We found a negative relationship between how many associates a male had and how much time he spent with them, indicating that all-male groups have a constraint on their group size. The group size of sightings of all-male groups was lower in Kabini than in the Amboseli population of African savannah elephant (mean ± SD group size of all-male groups with more than one male: Kabini: 2.159 ± 0.502, Amboseli: 3.325 ± 1.995; Supplementary Material 8, see Chiyo et al., 2011). This is similar to what was found in female elephants in Kabini (Nandini et al., 2017, 2018). We note here that since many sightings of male elephants came from around the Kabini backwaters area, if males encountered each other more often due to the high grass biomass compared to the forest habitat (Gautam and Vidya, 2019), male associations inside the forest would be even weaker, leading to even smaller average group sizes and a greater restriction on group size. Low male group sizes have also been reported in other Asian elephant populations. The average group size of adult males (including solitary males) was 1.1 in Mudumalai Wildlife Sanctuary, southern India (Daniel et al., 1987). The maximum all-male group size was 2 in Mudumalai (Daniel et al., 1987), 6 in Kabini (observed in only one sighting), and 5 in Gal Oya in Sri Lanka (McKay, 1973). In contrast, the maximum group size in Amboseli was 40 (Lee et al., 2011). Lee et al. (2011) classified gregarious males (who spend most of their non-musth time in all-male groups) as promiscuous (males who spent a lot of time with many associates) or discriminating (males who associated with fewer males for a lot of time). The presence of promiscuous males, along with much larger group sizes, suggest that there is no strong negative relationship between how many associates a male has and how strong his associations are with them in Amboseli.
There are methodological differences between the current study and the one in Amboseli. Apart from the difference in the radius for male association used in the two studies (see above), unlike this study, Chiyo et al. (2011) included males regardless of musth status/sexual state (which may artificially reduce strength of association; see Goldenberg et al., 2014). However, since musth males in Amboseli associated more with female groups than in all-male groups, and the reverse was true for non-musth males (Poole, 1987), removal of musth males from the data is unlikely to lead to a substantial reduction in the all-male group sizes reported in Chiyo et al. (2011).
We expected a group size constraint to affect old males to a greater extent than young males due to their greater food requirements. However, male age had no effect on the time spent in all-male groups. Preferential age-peer associations among old but not young males could have dampened this effect. We also found no relationship between male age and the mean experienced group size, but the mean experienced group sizes were close to 1.2, precluding much further reduction in group sizes. Our study was carried out in well-protected areas with little human disturbance. Thus, the adult male elephants here do not face any predation risk, even from humans. Larger Asian elephant male groups are reported elsewhere when they raid crops in risky, but resource-rich environments (see Srinivasaiah et al., 2012), which would relax any constraints on group size. Anthropogenic threats could also result in larger group sizes under those circumstances. Intra-group feeding competition has been discussed as a constraint on group sizes in primates (see Chapman et al., 1995), including in all-male groups (Rajpurohit, 1995; Steenbeek et al., 2000).
Preferred Male Associations and Stability of Associations
As male associations were weak (and we found no evidence for overall preferred associations among males across 2-week intervals in either female absence or presence; Supplementary Material 9), the lack of highly correlated associations between males across years is not surprising. However, preferred top associates were seen in female absence, with the identity of the top associate being different from random for most (84%) males and a majority of the pairs (61%) showing a higher AI (although low absolute value) than expected by chance. These numbers suggest that there were males who showed preference in their choice of top associate but did not or could not (possibly due to group size restriction) spend more time with those associates. In all-male groups in Amboseli, less than 10% of all the observed AI values were greater than that predicted (AI = 0.1) under a model of random associations (Chiyo et al., 2011). Similar to our findings in Kabini, older (>20 years old) adult males in Amboseli also had at least one significant top associate (Lee et al., 2011). Stable and significant affiliation among adult males have been observed in several other species (Packer and Pusey, 1982; Connor et al., 2001; Mitani, 2009; Berghänel et al., 2011) but as mentioned in the Introduction, such relationships are often thought to be a means to form coalitions to defend females. Adult male coalitions have not been observed in Asian elephants and are unlikely, given the low probability of finding a receptive female and the small sizes of female groups (Nandini et al., 2017). It will be interesting to explore other possible reasons for the significant top male associates we find in Kabini. It is possible that significant affiliations occur between males who are related (see Vidya and Sukumar, 2005).
It is worth noting that, as we did not observe any overt affiliative behavior between males, we could only use feeding within relevant distances as a criterion for male associations in the current study. We classified males over the age of 30 years as old males, similar to some of the previous studies as mentioned above. However, this meant that males who were aged slightly younger than 30 years and those aged slightly older than 30 years would be classified into different age-classes in our study, even though they were close in age. The analysis on approaches of males used age difference and would not be affected by this. In examining age-peer associations and other analyses, only a small proportion of dyads of the common males fell within the 26–34 year-old age class (21 out of 496 dyads in female absence and 3 out of 91 dyads in female presence). Therefore, these analyses would also not be affected significantly by this age-classification.
Conclusion
We examined male associations in the Asian elephant, an endangered species with high male competition, where males are not known to form coalitions, and rove between female groups. Associations among non-musth adult male Asian elephants, although weak and temporary, were affected by male age and the immediate presence of females. They reflected the different reproductive strategies that old and young males were expected to follow in species with roving males. Old males are competitive breeders and regularly enter musth. When not in musth, they preferentially associated with their age-peer competitors in all-male groups, possibly to settle dominance relationships. On the other hand, young males, who might be sexually active out of musth, spent more of their non-musth time associating with females than in all-male groups. Young males did not preferentially associate with older males in Kabini, unlike in African savannah elephants. We posit that this is due to the difference in the dispersion of food resources in the habitats they occupy. In comparison with African savannah elephants, males in Kabini spent a much smaller proportion of their time in all-male groups, of smaller sizes, possibly due to group size constraints. Thus, differences in ecology possibly shaped the differences in male societies, despite the two species being phylogenetically related and showing similar male reproductive strategies. Differences in the nature of male associations between phylogenetically related species have been observed in other taxa: chimpanzees and bonobos show different levels of agonism (Furuichi and Ihobe, 1994), macaque populations/species differ in the frequencies of male affiliations depending on group sizes and group sex ratios (Hill, 1994). However, while differences between related species in overall social organization (dolphin species: Parra et al., 2011, Grevy’s zebra and onager: Rubenstein et al., 2015) or female social organization (colobine species: Korstjens et al., 2002, African savannah and Asian elephants: Nandini et al., 2018) that are consistent with resource distributions are known, there is little information on food resource distributions differently affecting male social organization in related species of large mammals. Studies on the foraging ecology of male elephants are required in the future to further understand the differences in social organization between species.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This observational study was carried out under permits issued by the offices of the PCCF(WL), Karnataka Forest Department, and of the Conservators of Forests of Nagarahole and Bandipur National Parks and Tiger Reserves.
Author Contributions
PK and TV conceived this work. PK collected field data during February 2011–July 2014 and carried out the analyses. SN helped with field data collection during January 2011–May 2013. TV helped with initial field data collection in 2009–2010. PK primarily and TV wrote the manuscript. All the authors read and finalized the manuscript. This work is part of PK’s Ph.D. thesis.
Funding
This work was supported by the Department of Science and Technology’s (DST; Government of India) Ramanujan Fellowship (to TV) under Grant No. SR/S2/RJN-25/2007 (dated 09/06/2008), Council of Scientific and Industrial Research (CSIR), Government of India, under Grant No. 37(1375)/09/EMR-II and No. 37(1613)/13/EMR-II, National Geographic Society (NGS), United States, under Grant #8719-09 and #9378-13, the Department of Biotechnology (DBT, Government of India) under Grant No. BT/INF/22/SP27679/2018 (dated 12/09/2018 to JNCASR), and JNCASR. PK was supported as a Ph.D. student by CSIR [No. 09/733(0152)/2011-EMR-I]. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank DST, CSIR, NGS, DBT, and JNCASR for funds and JNCASR for logistic support. We thank the offices of the PCCF (WL), Karnataka Forest Department, and of the Conservators of Forests of Nagarahole and Bandipur National Parks and Tiger Reserves for field permits. We also thank various officials, from various PCCFs and APCCFs, to the Conservators of Forests and Range Forest Officers, to the staff of Nagarahole and Bandipur National Parks for their support across the years. We also thank Krishna, Althaf, Ranga, Shankar, Gunda, Rajesh, Binu, and others for field assistance, and Deepika Prasad and Arjun Ghosh for help with the initial field data collection. We also thank Ajay Desai, who sadly passed away last year, for useful discussions. This manuscript has been released as a pre-print at bioRxiv (Keerthipriya et al., 2020b).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.616666/full#supplementary-material
References
Alberts, S. C., and Altmann, J. (1995). Balancing costs and opportunities: dispersal in male baboons. Am. Nat. 145, 279–306. doi: 10.1086/285740
Allen, C. R., Brent, L. J., Motsentwa, T., Weiss, M. N., and Croft, D. P. (2020). Importance of old bulls: leaders and followers in collective movements of all-male groups in African savannah elephants (Loxodonta africana). Sci. Rep. 10:13996. doi: 10.1038/s41598-020-70682-y
Baird, R. W., and Whitehead, H. (2000). Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105. doi: 10.1139/z00-155
Bastian, M., Heymann, S., and Jacomy, M. (2009). “Gephi: an open source software for exploring and manipulating networks,” in Proceedings of the 3rd International AAAI Conference on Weblogs and Social Media, San Jose, CA, 361–362. doi: 10.1136/qshc.2004.010033
Beecher, M. D., Campbell, S. E., and Stoddard, P. K. (1994). Correlation of song learning and territory establishment strategies in the Song Sparrow. Proc. Natl. Acad. Sci. U.S.A. 91, 1450–1454. doi: 10.1073/pnas.91.4.1450
Bercovitch, F. B., and Berry, P. S. (2015). The composition and function of all−male herds of Thornicroft’s giraffe, Giraffa camelopardalis thornicrofti, in Zambia. Afr. J. Ecol. 53, 167–174. doi: 10.1111/aje.12169
Berghänel, A., Ostner, J., Schröder, U., and Schülke, O. (2011). Social bonds predict future cooperation in male Barbary macaques, Macaca sylvanus. Anim. Behav. 81, 1109–1116. doi: 10.1016/j.anbehav.2011.02.009
Best, P. B. (1979). “Social organization in sperm whales, Physeter macrocephalus,” in Behavior of Marine Animals, eds H. E. Winn and B. L. Olla (New York, NY: Springer US), 227–289. doi: 10.1007/978-1-4684-2985-5_7
Biro, D., Inoue-Nakamura, N., Tonooka, R., Yamakoshi, G., Sousa, C., and Matsuzawa, T. (2003). Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim. Cogn. 6, 213–223. doi: 10.1007/s10071-003-0183-x
Blundell, G. M., Ben-David, M., and Bowyer, R. T. (2002). Sociality in river otters: cooperative foraging or reproductive strategies? Behav. Ecol. 13, 134–141. doi: 10.1093/beheco/13.1.134
Bon, R., Dubois, M., and Maublanc, M. L. (1993). Does age influence between-rams companionship in mouflon (Ovis gmelini)? Rev. Ecol. Terre Vie 48, 57–64.
Chapman, C. A., Chapman, L. J., and Wrangham, R. (1995). Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behav. Ecol. Sociobiol. 36, 59–70. doi: 10.1007/BF00175729
Chelliah, K., and Sukumar, R. (2013). The role of tusks, musth and body size in male–male competition among Asian elephants, Elephas maximus. Anim. Behav. 86, 1207–1214. doi: 10.1016/j.anbehav.2013.09.022
Chelliah, K., and Sukumar, R. (2015). Interplay of male traits, male mating strategies and female mate choice in the Asian elephant, Elephas maximus. Behaviour 152, 1113–1144. doi: 10.1163/1568539X-00003271
Chiyo, P. I., Archie, E. A., Hollister-Smith, J. A., Lee, P. C., Poole, J. H., Moss, C. J., et al. (2011). Association patterns of African elephants in all-male groups: the role of age and genetic relatedness. Anim. Behav. 81, 1093–1099. doi: 10.1016/j.anbehav.2011.02.013
Chiyo, P. I., Moss, C. J., and Alberts, S. C. (2012). The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLoS One 7:e31382. doi: 10.1371/journal.pone.0031382
Clutton-Brock, T. H., Iason, G. R., and Guinness, F. E. (1987). Sexual segregation and density−related changes in habitat use in male and female Red deer (Cervus elaphus). J. Zool. 211, 275–289. doi: 10.1111/j.1469-7998.1987.tb01534.x
Connor, R. C., Heithaus, M. R., and Barre, L. M. (2001). Complex social structure, alliance stability and mating access in a bottlenose dolphin ‘super-alliance.’. Proc. Biol. Sci. 268, 263–267. doi: 10.1098/rspb.2000.1357
Connor, R. C., Smolker, R. A., and Richards, A. F. (1992). Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl. Acad. Sci. U.S.A. 89, 987–990. doi: 10.1073/pnas.89.3.987
Cransac, N., Gerard, J. F., Maublanc, M. L., and Pépin, D. (1998). An example of segregation between age and sex classes only weakly related to habitat use in mouflon sheep (Ovis gmelini). J. Zool. 244, 371–378. doi: 10.1111/j.1469-7998.1998.tb00042.x
Curé, C., Antunes, R., Alves, A. C., Visser, F., Kvadsheim, P. H., and Miller, P. J. (2013). Responses of male sperm whales (Physeter macrocephalus) to killer whale sounds: implications for anti-predator strategies. Sci. Rep. 3:1579. doi: 10.1038/srep01579
Daniel, J. C., Desai, A. A., Sivaganesan, N., Datye, H. S., Rameshkumar, S., Baskaran, N., et al. (1987). Ecology of the Asian Elephant (Final Report). Bombay: Bombay Natural History Society.
de Silva, S., Ranjeewa, A., and Kryazhimskiy, S. (2011). The dynamics of social networks among female Asian elephants. BMC Ecol. 11:17. doi: 10.1186/1472-6785-11-17
de Silva, S., and Wittemyer, G. (2012). A comparison of social organization in Asian elephants and African savannah elephants. Int. J. Primatol. 33, 1125–1141. doi: 10.1007/s10764-011-9564-1
Desai, A. A., and Johnsingh, A. J. T. (1995). “Social organization and reproductive strategy of the male Asian elephant (Elephas maximus),” in A Week With Elephants, eds J. C. Daniel and H. Datye (Bombay: Bombay Natural History Society), 532–532.
Eisenberg, J. F., McKay, G. M., and Jainudeen, M. R. (1971). Reproductive behavior of the Asiatic elephant. Behaviour 38, 193–224. doi: 10.1163/156853971X00087
Erdös, P., and Rényi, A. (1960). On the evolution of random graphs. Pub. Math. Inst. Hung. Acad. Sci. 5, 17–61.
Evans, K. E., and Harris, S. (2008). Adolescence in male African elephants, Loxodonta africana, and the importance of sociality. Anim. Behav. 76, 779–787. doi: 10.1016/j.anbehav.2008.03.019
Furuichi, T., and Ihobe, H. (1994). Variation in male relationships in bonobos and chimpanzees. Behaviour 130, 211–228. doi: 10.1163/156853994X00532
Ganswindt, A., Rasmussen, H. B., Heistermann, M., and Hodges, J. K. (2005). The sexually active states of free-ranging male African elephants (Loxodonta africana): defining musth and non-musth using endocrinology, physical signals, and behavior. Horm. Behav. 47, 83–91. doi: 10.1016/j.yhbeh.2004.09.002
Gautam, H., and Vidya, T. N. C. (2019). A test of the socioecological model in female Asian elephants: the effects of food abundance, food distribution, and competitor density on within-clan and between-clan contests. bioRxiv [Preprint]. doi: 10.1101/754515
Gerber, L., Connor, R. C., King, S. L., Allen, S. J., Wittwer, S., Bizzozzero, M. R., et al. (2020). Affiliation history and age similarity predict alliance formation in adult male bottlenose dolphins. Behav. Ecol. 31, 361–370. doi: 10.1093/beheco/arz195
Ginsberg, J. R., and Young, T. P. (1992). Measuring association between individuals or groups in behavioural studies. Anim. Behav. 44, 377–379. doi: 10.1016/0003-3472(92)90042-8
Goldenberg, S. Z., de Silva, S., Rasmussen, H. B., Douglas-Hamilton, I., and Wittemyer, G. (2014). Controlling for behavioural state reveals social dynamics among male African elephants, Loxodonta africana. Anim. Behav. 95, 111–119. doi: 10.1016/j.anbehav.2014.07.002
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. doi: 10.1016/s0003-3472(80)80103-5
Gupta, M., Ravindranath, S., Prasad, D., and Vidya, T. N. C. (2016). Short-term variation in sex ratio estimates of Asian elephants due to space use differences between the sexes. Gajah 44, 5–15.
Hill, D. A. (1994). Affiliative behaviour between adult males of the genus Macaca. Behaviour 130, 293–308. doi: 10.1163/156853994X00578
Hollister-Smith, J. A., Poole, J. H., Archie, E. A., Vance, E. A., Georgiadis, N. J., Moss, C. J., et al. (2007). Age, musth and paternity success in wild male African elephants, Loxodonta africana. Anim. Behav. 74, 287–296. doi: 10.1016/j.anbehav.2006.12.008
Hrdy, S. B. (1977). The Langurs of Abu- Female and Male Strategies of Reproduction. Cambridge, MA: Harvard University Press.
Jainudeen, M. R., Katongole, C. B., and Short, R. V. (1972a). Plasma testosterone levels in relation to musth and sexual activity in the male Asiatic elephant, Elephas maximus. J. Reprod. Fertil. 29, 99–103. doi: 10.1530/jrf.0.0290099
Jainudeen, M. R., McKay, G. M., and Eisenberg, J. F. (1972b). Observations on musth in the domesticated Asiatic elephant (Elephas maximus). Mammalia 36, 247–261. doi: 10.1515/mamm.1972.36.2.247
Keerthipriya, P. (2018). Associations, Dominance Interactions, and Musth in Male Asian Elephants in Nagarahole and Bandipur National Parks, Southern India. Ph.D. dissertation. Bengaluru: Jawaharlal Nehru Centre for Advanced Scientific Research.
Keerthipriya, P., Nandini, S., Gautam, H., Revathe, T., and Vidya, T. N. C. (2020a). Musth and its effects on male-male and male-female associations in Asian elephants in Nagarahole-Bandipur, southern India. J. Mammal. 101, 259–270. doi: 10.1093/jmammal/gyz190
Keerthipriya, P., Nandini, S., and Vidya, T. N. C. (2020b). Effects of male age and female presence on male associations in a large, polygynous mammal in southern India. bioRxiv [Preprint]. doi: 10.1101/485144v2 bioRxiv:485144
Korstjens, A. H., Sterck, E. H., and Noë, R. (2002). How adaptive or phylogenetically inert is primate social behaviour? A test with two sympatric colobines. Behaviour 139, 203–226. doi: 10.1163/156853902760102654
Latapy, M. (2008). Main-memory triangle computations for very large (sparse (power-law)) graphs. Theor. Comput. Sci. 407, 458–473. doi: 10.1016/j.tcs.2008.07.017
Lee, P. C., Poole, J. H., Njiraini, N., Sayialel, C. N., and Moss, C. J. (2011). “Male social dynamics: independence and beyond,” in The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal, eds C. J. Moss, H. Croze, and P. C. Lee (Chicago, IL: University of Chicago Press), 224–237.
López, P., and Martín, J. (2001). Fighting rules and rival recognition reduce costs of aggression in male lizards, Podarcis hispanica. Behav. Ecol. Sociobiol. 49, 111–116. doi: 10.1007/s002650000288
Mantel, N. (1967). The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220.
McKay, G. M. (1973). Behavior and ecology of the Asiatic elephant in southeastern Ceylon. Smithson. Contrib. Zool. 125, 1–113. doi: 10.5479/si.00810282.125
Mitani, J. C. (2009). Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 77, 633–640. doi: 10.1016/j.anbehav.2008.11.021
Mooring, M. S., Fitzpatrick, T. A., Benjamin, J. E., Fraser, I. C., Nishihira, T. T., Reisig, D. D., et al. (2003). Sexual segregation in desert bighorn sheep (Ovis canadensis mexicana). Behaviour 140, 183–207. doi: 10.1163/156853903321671497
Moss, C. J., and Poole, J. H. (1983). “Relationships and social structure of African elephants,” in Primate Social Relationships: An Integrated Approach, ed. R. A. Hinde (London: Blackwell Scientific Publications), 315–325.
Mumby, H., Chapman, S. N., Crawley, J. A. H., Mar, K. U., Htut, W., Soe, A. T., et al. (2015). Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Evol. Biol. 15:214. doi: 10.1186/s12862-015-0487-x
Murphy, D., Mumby, H. S., and Henley, M. D. (2020). Age differences in the temporal stability of a male African elephant (Loxodonta africana) social network. Behav. Ecol. 31, 21–31. doi: 10.1093/beheco/arz152
Nandini, S., Keerthipriya, P., and Vidya, T. N. C. (2017). Seasonal variation in female Asian elephant social structure in Nagarahole-Bandipur, southern India. Anim. Behav. 134, 135–145. doi: 10.1016/j.anbehav.2017.10.012
Nandini, S., Keerthipriya, P., and Vidya, T. N. C. (2018). Group size differences may mask underlying similarities in social structure: a comparison of female elephant societies. Behav. Ecol. 29, 145–159. doi: 10.1093/beheco/arx135
Packer, C., and Pusey, A. E. (1982). Cooperation and competition within coalitions of male lions: kin selection or game theory? Nature 296, 740–742. doi: 10.1038/296740a0
Packer, C., and Pusey, A. E. (1987). The evolution of sex-biased dispersal in lions. Behaviour 101, 275–310. doi: 10.1163/156853987X00026
Parra, G. J., Corkeron, P. J., and Arnold, P. (2011). Grouping and fission-fusion dynamics in Australian snubfin and Indo-Pacific humpback dolphins. Anim. Behav. 82, 1423–1433. doi: 10.1016/j.anbehav.2011.09.027
Pereira, M. E. (1988). Effects of age and sex on intra-group spacing behaviour in juvenile savannah baboons, Papio cynocephalus cynocephalus. Anim. Behav. 36, 184–204. doi: 10.1016/S0003-3472(88)80262-8
Poole, J. H. (1982). Musth and Male-Male Competition in the African Elephant. Ph.D. dissertation. Cambridge: University of Cambridge.
Poole, J. H. (1987). Rutting behavior in elephants: the phenomenon of musth in African elephants. Anim. Behav. 102, 283–316. doi: 10.1163/156853986X00171
Poole, J. H. (1989). Mate guarding, reproductive success and female choice in African elephants. Anim. Behav. 37, 842–849. doi: 10.1016/0003-3472(89)90068-7
Pusey, A. E., and Packer, C. (1987). “Dispersal and philopatry,” in Primate Societies, eds B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, and T. T. Struhsaker (Chicago, IL: University of Chicago Press), 250–266.
Rajpurohit, L. S. (1995). Temporary splitting or subgrouping in male bands of Hanuman langurs, Presbytis entellus, around Jodhpur, western India. Mammalia 59, 3–8. doi: 10.1515/mamm.1995.59.1.3
Rajpurohit, L. S., Mohnot, S. M., and Sommer, V. (1995). Wanderers between harems and bachelor bands: male Hanuman langurs (Presbytis entellus) at Jodhpur in Rajasthan. Behaviour 132, 255–299. doi: 10.1163/156853995X00739
Rasmussen, H. B. (2005). Reproductive Tactics of Male African Savannah Elephants (Loxodonta africana). Ph.D. dissertation. Oxford: University of Oxford.
Rasmussen, H. B., Okello, J. B. A., Wittemyer, G., Siegismund, H. R., Arctander, P., Vollrath, F., et al. (2007). Age-and tactic-related paternity success in male African elephants. Behav. Ecol. 19, 9–15. doi: 10.1093/beheco/arm093
Rasmussen, L. E. L., Sukumar, R., and Krishnamurthy, V. (2005). Behavioural and chemical confirmation of the preovulatory pheromone, (Z)-7-dodecenyl acetate, in wild Asian elephants: its relationship to musth. Behaviour 142, 351–396. doi: 10.1163/1568539053778300
Robbins, M. M. (1996). Male−male Interactions in heterosexual and all−male wild mountain gorilla groups. Ethology 102, 942–965. doi: 10.1111/j.1439-0310.1996.tb01172.x
Rubenstein, D. I., Sundaresan, S. R., Fischhoff, I. R., Tantipathananandh, C., and Berger-Wolf, T. Y. (2015). Similar but different: dynamic social network analysis highlights fundamental differences between the fission-fusion societies of two equid species, the onager and Grevy’s zebra. PLoS One 10:e0138645. doi: 10.1371/journal.pone.0138645
Ruckstuhl, K. E., and Neuhaus, P. (2000). Sexual segregation in ungulates: a new approach. Behaviour 137, 361–377. doi: 10.1163/156853900502123
Saayman, G. S. (1971). Behaviour of the adult males in a troop of free-ranging chacma baboons (Papio ursinus). Folia Primatol. 15, 36–57. doi: 10.1159/000155366
Shetty, N. (2016). Social Structure, Genetic Relatedness, and Dominance Relationships in Female Asian Elephants in Nagarahole and Bandipur National Parks, Southern India. Ph.D. dissertation. Bengaluru: Jawaharlal Nehru Centre for Advanced Scientific Research.
Sicotte, P. (1994). Effect of male competition on male−female relationships in bi−male groups of mountain gorillas. Ethology 97, 47–64. doi: 10.1111/j.1439-0310.1994.tb01028.x
Smith, L. K., Fantella, S. L., and Pellis, S. M. (1999). Playful defensive responses in adult male rats depend on the status of the unfamiliar opponent. Aggress. Behav. 25, 141–152. doi: 10.1002/(SICI)1098-2337199925:2<141::AID-AB6<3.0.CO;2-S
Srinivasaiah, N. M., Anand, V. D., Vaidyanathan, S., and Sinha, A. (2012). Usual populations, unusual individuals: insights into the behavior and management of Asian elephants in fragmented landscapes. PLoS One 7:e42571. doi: 10.1371/journal.pone.0042571
StatSoft Inc (2004). STATISTICA (Data Analysis Software System), Version 7. Available online at: www.statistica.com
Steenbeek, R., Sterck, E. H. M., deVries, H., and van Hooff, J. A. R. A. M. (2000). “Costs and benefits of the one-male, age-graded, and all-male phases in wild Thomas’s langur groups,” in Primate Males, ed. P. M. Kappeler (Cambridge: Cambridge University Press), 130–145.
Sukumar, R., Joshi, N. V., and Krishnamurthy, V. (1988). Growth in the Asian elephant. Proc. Anim. Sci. 97, 561–571. doi: 10.1007/BF03179558
Taylor, L. A., Vollrath, F., Lambert, B., Lunn, D., Douglas−Hamilton, I., and Wittemyer, G. (2020). Movement reveals reproductive tactics in male elephants. J. Anim. Ecol. 89, 57–67. doi: 10.1111/1365-2656.13035
van Hooff, J. A., and van Schaik, C. P. (1994). Male bonds: affiliative relationships among nonhuman primate males. Behaviour 130, 309–337. doi: 10.1163/156853994X00587
Vidya, T. N. C., Prasad, D., and Ghosh, A. (2014). Individual identification in Asian elephants. Gajah 40, 3–17.
Vidya, T. N. C., and Sukumar, R. (2005). Social organization of the Asian elephant (Elephas maximus) in southern India inferred from microsatellite DNA. J. Ethol. 23, 205–210. doi: 10.1007/s10164-005-0144-8
Villaret, J. C., and Bon, R. (1995). Social and spatial segregation in Alpine ibex (Capra ibex) in Bargy, French Alps. Ethology 101, 291–300. doi: 10.1111/j.1439-0310.1995.tb00366.x
Wagner, A. P., Frank, L. G., and Creel, S. (2008). Spatial grouping in behaviourally solitary striped hyaenas, Hyaena hyaena. Anim. Behav. 75, 1131–1142. doi: 10.1016/j.anbehav.2007.08.025
Wasserman, S., and Faust, K. (1994). Social Network Analysis: Methods and Applications. Cambridge: Cambridge University Press.
Whitehead, H. (1994). Delayed competitive breeding in roving males. J. Theor. Biol. 166, 127–133. doi: 10.1006/jtbi.1994.1011
Whitehead, H. (2008). Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. Chicago, IL: University of Chicago Press.
Keywords: Asian elephant, all-male groups, association networks, Kabini Elephant Project, testing-strengths hypothesis, social learning from older males hypothesis, female presence/absence, group size constraint
Citation: Keerthipriya P, Nandini S and Vidya TNC (2021) Effects of Male Age and Female Presence on Male Associations in a Large, Polygynous Mammal in Southern India: The Asian Elephant. Front. Ecol. Evol. 9:616666. doi: 10.3389/fevo.2021.616666
Received: 12 October 2020; Accepted: 10 May 2021;
Published: 07 June 2021.
Edited by:
Rita Covas, Centro de Investigacao em Biodiversidade e Recursos Geneticos (CIBIO-InBIO), PortugalReviewed by:
Imke Lüders, University of Pretoria, South AfricaShifra Z. Goldenberg, Smithsonian Institution, United States
Copyright © 2021 Keerthipriya, Nandini and Vidya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. N. C. Vidya, dG5jdmlkeWFAam5jYXNyLmFjLmlu; dG5jdmlkeWFAZ21haWwuY29t; orcid.org/0000-0002-7143-9008
†Present address: S. Nandini, Azim Premji Foundation, Bengaluru, India
 P. Keerthipriya
P. Keerthipriya S. Nandini
S. Nandini T. N. C. Vidya
T. N. C. Vidya