- 1Institute of Vertebrate Biology of the Czech Academy of Sciences, Brno, Czechia
- 2Department of Evolution, Ecology, and Behavior, School of Integrative Biology, University of Illinois Urbana-Champaign, Urbana, IL, United States
The capability of hosts to reject the odd egg from their nest is one of the key defenses against avian brood parasitism. Considerable research effort has been devoted to exploring which phenotypic traits of eggshells facilitate to cue the recognition of the parasitic egg. Here we have reviewed studies addressing salient egg traits involved in the rejection of foreign eggs and used a formal meta-analysis to quantify their relative importance. Hosts appear to rely to a large extent on eggshell color traits, followed by maculation patterns. Hosts respond with similar rates of egg rejection to natural vs. model eggs and when breeding in both closed and open nests. Analyses of experiments on hosts of Cuculus and Molothrus parasites, the two best studied brood parasitic lineages with different co-evolutionary histories, yield similar conclusions. We also identify several poorly studied potential egg recognition cues, such as odor or weight, and recommend exploring even the visual traits in more detail, including chromatic and achromatic contrasts or experimentally manipulated egg maculation characteristics. Recent technological and sensory ecological advances open many new research avenues to experimentally examine the role of diverse egg characteristics in antiparasite defenses.
Introduction
The capability to perceive, recognize, and reject the parasitic egg(s) is a critical defense in hosts of avian brood parasites, which lay their eggs into the nests of other birds (Davies, 2000). To combat host defenses, some lineages of brood parasites have evolved sophisticated eggshell mimicry to fool the hosts, which in turn, have evolved fine-tuned abilities to discriminate and reject the foreign egg. This suite of antiparasite defense behaviors has attracted considerable observational, comparative, and experimental research attention in the last decades (e.g., Grim, 2007; Medina and Langmore, 2015), albeit the first such experiments had been performed by naturalists already more than a century ago (reviewed in Sealy and Underwood, 2012). Experiments usually involve adding to or exchanging one or more foreign eggs in the host nest and observing the host’s reaction. Stephen Rothstein was a pioneer of egg rejection experimentation (e.g., Rothstein, 1970), and his methods are still used by many researchers with only minor modifications (e.g., Canniff et al., 2018; Luro et al., 2018).
Since the time of some of the first egg rejection experiments ca. 100 years ago (e.g., Friedmann, 1929), multiple cues have been suggested to influence foreign-egg recognition. Accordingly, the host may rely on low intraclutch variation to facilitate the recognition of the distinct, outlier parasitic egg in the clutch (e.g., Davies and Brooke, 1989; Øien et al., 1995). In turn, according to the egg arrangement hypothesis, the host may examine disruptions to the arrangement of their eggs and use it to be alerted that their nest could be parasitized (Polačiková et al., 2013; but see Hanley et al., 2015b). Furthermore, placing a stuffed dummy of the adult parasite beside the host nest along with experimental parasitism may lead to the increased rejection of the parasitic egg suggesting that witnessing a parasitism event by the host may also narrow eggshell recognition thresholds and enable egg rejection (e.g., Bártol et al., 2002; Hanley et al., 2015c). Nest sanitation behavior, wherein the host removes debris from its nest, is also hypothesized to be responsible for recognition of differently shaped parasite eggs (e.g., Moskát et al., 2003; Guigueno and Sealy, 2012). Finally, the clutch size hypothesis predicts that psychophysically (e.g., according to Weber’s law), it is easier to recognize the odd-egg-out in smaller vs. larger clutches (Akre and Johnsen, 2014).
However, egg recognition and rejection, according to perceivable differences in the phenotypes between the parasite and the host eggs (Manna et al., 2017), are by far the best studied aspects of anti-parasitic defense behaviors (reviewed in Honza and Cherry, 2017). Thousands of completed egg rejection experiments suggest that hosts can use distinct egg traits to recognize parasitic egg, such as color, maculation, shape, size, odor, or weight (Honza and Cherry, 2017). The majority of studies examined visual traits, particularly eggshell color and maculation, with a general conclusion that magnitude of difference between self and foreign eggs increases the probability of rejection (e.g., Avilés et al., 2010; Honza and Cherry, 2017; but see Hauber et al., 2020). Taken together, the experiments also imply that specific eggshell traits differ in their importance for the recognition and rejection processes (Honza and Cherry, 2017). However, there are still missing quantitative estimates on overall eggshell characteristics and their effects on host behaviors that prevent us from further exploring and discussing their significance for egg rejection as an antiparasitic strategy (Turner and Hauber, 2021). Knowledge of the latter will help us to better understand the cognitive processes involved in the brood parasite – host coevolution and also to design informative future experiments to fill in the missing gaps.
Brood parasitism research has suggested a variety of factors affecting the egg recognition process in hosts (Soler, 2017). Ongoing debates concluded that experimental egg type (natural or model egg stimuli) used in an experiment considerably affects not only the host’s response but also the interpretation of results and a use of any stimulus type should be carefully justified (Hauber et al., 2015; Lahti, 2015; Stoddard et al., 2018). On the one hand, the use of natural stimuli allows researchers to observe biologically relevant reactions and these results can be generalized (e.g., Stevens et al., 2013). On the other hand, model (artificial) stimuli can be especially advantageous when planning a carefully designed experiment allowing exact alterations of the focal traits (Igic et al., 2015; Yang et al., 2019). Several review studies already examined the effect of egg type stimuli on their rejection probabilities (Honza and Cherry, 2017; Turner and Hauber, 2021) but we are still missing a comprehensive survey estimating such stimuli’s effects in a standardized comparison.
The study of Langmore et al. (2005) suggested that open nesters reject eggs more often than species breeding in closed nests. However, they also showed that the effect of nest type (open vs. closed nest) disappears after controlling for nest light availability suggesting a crucial role of illumination within the nest (see also Honza et al., 2014). Regarding visually-relevant traits, such as shell color and maculation, they may have less important function in birds utilizing closed nests, which might rely more on tactile traits (Mason and Rothstein, 1986; Langmore et al., 2003; Tosi-Germán et al., 2020). Quantitatively, it still remains to be explored if open or closed nesters allot different importance on visual and non-visual egg traits during the decision process.
Finally, the two most studied avian host-brood parasite systems, the Old World cuckoos (Cuculus spp.) and New World cowbirds (Molothrus spp.), have been shown to considerably differ in their coevolutionary and ecological relationships with their hosts (Winfree, 1999). Unlike in cuckoos, there is little evidence that the cowbird lays mimetic eggs (Rutledge et al., 2021), suggesting that the evolutionary arms-race in this brood parasite system has not escalated relative to their Eurasian counterparts. It is, thus, a critical question if the differences between the parasitic systems and their co-evolutionary histories are also reflected in the hosts’ emphasis on different egg traits when recognizing the foreign egg (Luro and Hauber, 2020).
In this study, we have built upon the previous review by Honza and Cherry (2017) with the aim to provide a formal meta-analysis through a quantitative measure of the magnitude of the experimental effect (effect sizes) for the egg characteristics involved in the recognition of parasitic egg in the host nest. Such a quantitative assessment across multiple host species and lineages of diverse parasitic species and lineages has not yet been conducted, although qualitative reviews of experiments on individual species’ egg rejection cues have begun to appear in the published literature (e.g., Turner and Hauber, 2021).
Here, we used a multi-host and -parasite approach to examine effects of three extrinsic factors (variation in egg type stimuli, differences in nest architecture types, and different co-evolutionary histories with a parasite) that had been previously proposed to play an important role in hosts’ egg-rejection responses and particularly relevant to parasitic egg traits. For this purpose, we employed recent meta-analytic statistical tools to provide unbiased quantitative estimates (Harrer et al., 2019a). The aim of this study is primarily exploratory, and thus we formulated predictions of major interests based on our overview above. We predicted that hosts use mainly visual traits (eggshell color and maculation characteristics) to recognize the foreign egg in their nest and this would be more pronounced in open nesting hosts. In line with the debate regarding artificial stimuli (e.g., Stoddard et al., 2018), we also assess the role of natural vs. model eggs’ use in egg rejection experiments. Specifically, we predict that model eggs will be rejected at lower rates compared to natural ones because artificial eggs are difficult or impossible to pierce and remove via puncture ejection (Antonov et al., 2009). Finally, we predicted that hosts of Old World cuckoos better discriminate by color and maculation relative to other traits than cowbird hosts due to several million years longer coevolutionary experience with more mimetic parasite eggs in the former group of hosts (Caves et al., 2017; Krüger and Pauli, 2017). We make this prediction because we know from prior research that egg rejection belongs to a different class of recognition systems compared to other recognition tasks faced by nesting birds (e.g., nest hygiene: Hauber et al., 2021).
Materials and Methods
We sought out published studies exploring eggshell traits affecting antiparasitic egg rejection behavior. We searched the Web of Science Core Collection for studies published up to 31 December 2020. We used search terms using Basic Search and All Fields option: (brood parasitism or egg rejection or egg characteristics or defense∗ or defence∗) AND (cuckoo∗ or cowbird∗ or vidua∗ or honeyguide∗). This resulted in 1,608 studies we exported into Microsoft Excel Worksheet. We also noticed nine relevant studies published between 1972 and 1999 but not included in the search’s output and, thus, we manually entered these studies into analyses.
We screened all the studies identified and selected 62 studies fulfilling the following criteria for the analyses (see Supplementary Figure 1 for selection procedure): (a) only single trait at a time was manipulated, (b) there was a control treatment (i.e., referential baseline rejection rate) conducted or available from the study population (for three studies we sourced control data from the same population but published in a different study), (c) the host species is known to have <100% rejection rate of foreign eggs, (d) the study reported at least the total sample size and the count or proportion of rejected eggs. We a priori decided to apply these four search criteria to ensure that we obtain credible effect size estimates. In the studies performing a valid egg experiment, using a control treatment was particularly limiting selection criterion and led to a notable reduction of the selected studies for final analyses. However, modifying or even excluding any of the four criteria would directly prevent obtaining a valid result. If a study manipulated more egg traits in more experimental treatments in one species (each experimental treatment still manipulated only single egg trait) or an egg trait was tested in more species than we included all these experimental treatments as a separate unique report for calculating the effect size for each. Thus, some studies may have been used to generate several effect sizes. We also attempted to identify studies experimentally manipulating two traits at a time, while also meeting the rest of criteria above, but only eggshell color with maculation traits yielded a reasonable sample size (N = 12 studies). Therefore, we reported the estimates only for the color-maculation summation trait effect.
We found that identifying the trait as being experimentally manipulated was challenging in some studies. For the eggshell’s ground color trait, we excluded reports for which the authors did not state clearly the hue being used or the altered hue that was deemed as mimetic of host eggs. When using artificial eggs, the control eggs were painted to appear mimetic of the hosts’ own eggs. For natural eggs, only highly mimetic conspecific eggs were used as a control group. The experimental treatments for the maculation trait included creating new spots on both immaculate or already maculated eggs or in three reports also removing spots in hosts with maculated eggs. Egg material (type) stimuli varied from real eggs to those created from clay, wood, plaster, plastic, or plasticine. Experimental treatment for the shape trait was performed by creating eggs slimmer or more spherical than the natural egg shape. Experimental treatment for the ultraviolet reflectance (hereafter: UV) trait involved only decreasing UV for all but one study. Further, some studies reported only egg ejections but not desertions (or egg burial) and vice versa, likely because egg desertion is not always an outcome of natural or experimental parasitism (Grim et al., 2011; Croston and Hauber, 2014; Soler et al., 2015). For the effect size calculations, we, thus, always used the rejection rates if both ejection and desertion (or egg burial) events were reported and the ejection rates if only ejection events were reported.
Statistical Analysis
All the analyses were performed in R 3.4.4 (R Core Team, 2020). We identified 10 different egg traits in 62 studies with 128 effect size reports (Table 1) but for statistical analyses we chose only egg traits with representative number of reports (n ≥ 5), resulting in six egg traits from 56 studies.
We computed Cohen’s h effect size for each report from difference in rejection rates between control and manipulated treatment and using sample sizes data provided in studies (Cohen, 1988). We then examined and corrected for the high between-study heterogeneity (Higgin’s & Thompson’s I2 > 90% for all but one trait type) by detecting outlier reports, i.e., those in which the 95% confidence interval does not overlap with confidence interval of the pooled effect. We performed this test for each trait type separately using the function find. outliers implemented in R package dmetar (version 0.0.9000; Harrer et al., 2019b). After excluding studies identified as outliers, the between-study heterogeneity improved from substantial (I2 > 75%) to low or moderate (I2 < 75%; Higgins and Thompson, 2002) in four egg traits but remained substantial for egg color (I2 = 78%) and size (I2 = 82%). Thus, the effect size estimates for the two egg traits with the substantial between-study variability are under higher risk of producing biased overall estimates and should be interpreted with greater caution. To estimate the pooled confidence interval and each report’s confidence interval, we employed the random effect model using the function metagen implemented in the R package meta (version 4.15-1; Balduzzi et al., 2019). After correcting for the heterogeneity, the final dataset included 46 studies with 81 effect size reports from 30 species.
To account for phylogenetic non-independence between the species, we used a phylogenetic tree of the host species generated from BirdTree.org1 (Jetz et al., 2012). We applied a Bayesian random-effect model using the package brms (version 2.14.4; Bürkner, 2017) to calculate the pooled effect size for each egg trait. The identity of each effect size report was modeled as a random intercept effect. The covariance matrix of species relatedness was created using the package ape (version 5.4-1; Paradis and Schliep, 2019) and included as another random intercept effect. We set a weakly informative priors of Normal(μ = 0, σ = 1) for fixed predictors and Half-Cauchy(x0 = 0.3, γ = 0.3) for between-report heterogeneity (Williams et al., 2018; Harrer et al., 2019a). We ran 1 × 104 iterations with a burn-in phase of 1,000 to obtain >3,000 effective samples per parameter for posterior inference. The Potential Scale Reduction Factor (R̂) was always 1.00 suggesting a good convergence of chains.
We performed four main analyses, (i) examining overall effect of egg trait, (ii) comparing egg trait effects between hosts parasitized by natural eggs and artificial model eggs (egg type stimuli), (iii) comparing egg trait effects between open-nesters and those breeding in enclosed nests (domed, holes, cavities) and (iv) comparing egg trait effects only in hosts of Cuculus cuckoo or Molothrus cowbird parasitic species. In the first analysis, we included only egg trait type (categorical with six levels; color, maculation, material, shape, size, UV) as a fixed effect and in other three analyses it was the interaction of egg trait type with experimental egg stimuli (categorical with two levels; natural, artificial), egg trait type with nest type (categorical with two levels; open, closed) and egg trait type with parasite (categorical with two levels; cuckoo, cowbird), respectively. We then calculated median with 89% credible interval for each effect using the package emmeans (version 1.4.8; Lenth, 2020) and prefer this interval because it has been shown to be more stable as 95% credible intervals if effective sample size for a parameter <10,000 (Makowski et al., 2019). However, re-calculation with 95% credible intervals led to the same conclusions (results not shown). Additionally, we performed a Bayesian equivalence test to formally examine difference of each trait type from the null value and differences between trait types themselves. We computed these tests using function equivalence_test from the package bayestestR (version 0.8.0; Makowski et al., 2019). Due to lack of theoretical knowledge, the null value was set as the region of practical equivalence at δ = ± 0.1, which corresponds to the effect size at half of Cohen’s conventional definition for a small effect (Kruschke, 2018). Finally, we computed a Bayes factor using the package bayestest R and assumed that values of 3 and higher suggest an evidence for significant difference from the null value (e.g., Kruschke, 2018).
Results
After correcting for the between-study heterogeneity (see section “Materials and Methods”), a total of 46 studies with 81 effect size reports were entered into our final analyses. We found significant overall effect on egg rejection for egg color (Cohen’s h = 1.24, 89% credible intervals = 0.98–1.49), followed by maculation (h = 0.69 [0.45–0.94]) and UV (h = 0.43 [0.16–0.69]) (Figure 1 and Supplementary Table 1). Effect sizes of egg material (natural vs. artificial model), shape, and size were small and each of their credible intervals overlapped with 0 (Figure 1 and Supplementary Table 1). The same pattern of results was generated for eggshell trait types also when adding a fixed effect of stimulus type (natural vs. model), nest type (open vs. closed) or host-parasite system (Cuculus vs. Molothrus hosts; Figures 2–4 and Supplementary Tables 2–4).
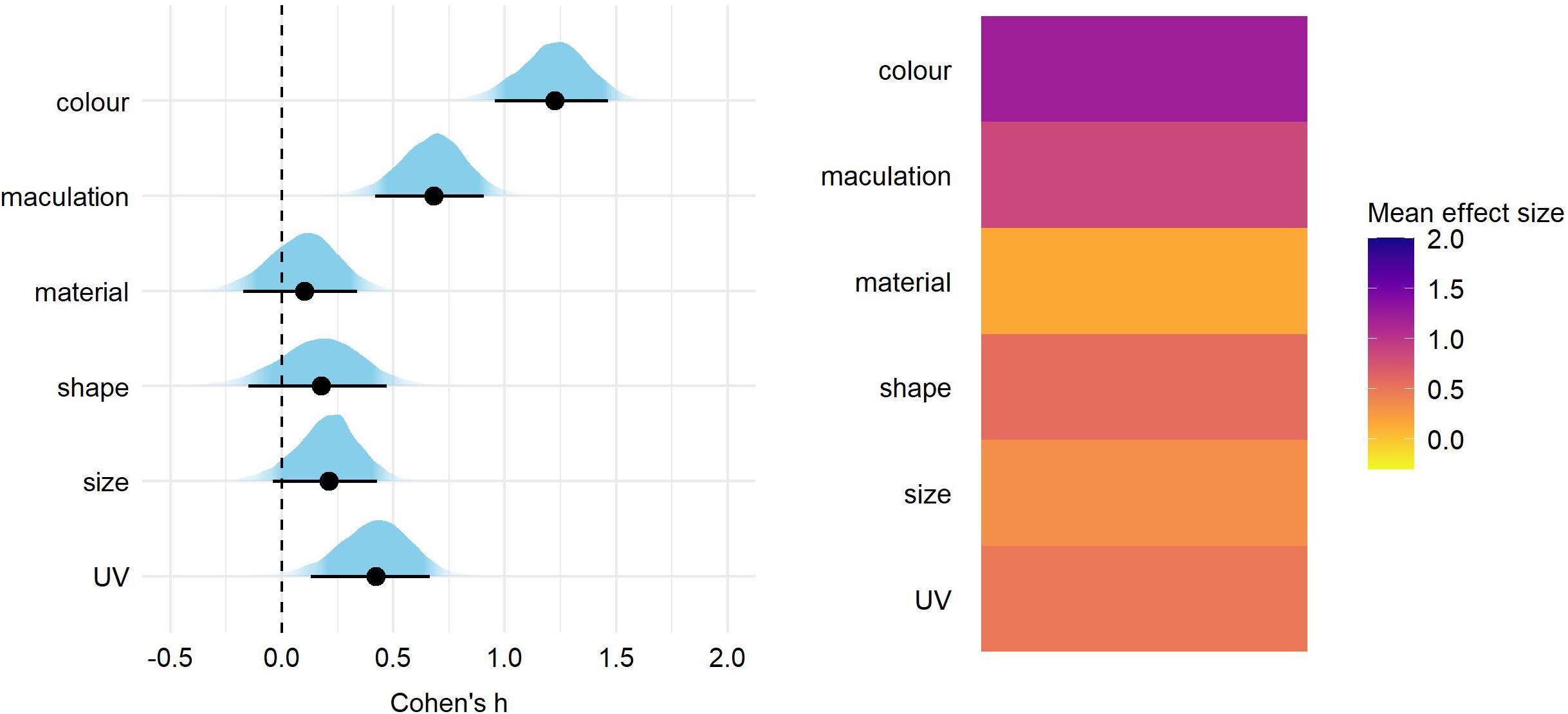
Figure 1. Predicted overall effect size for egg trait types. On the left, for each trait type we show the parameter distribution with its median (black dot) and 89% credible interval (black line). On the right, the median effect sizes are plotted as a heatmap for each trait type.
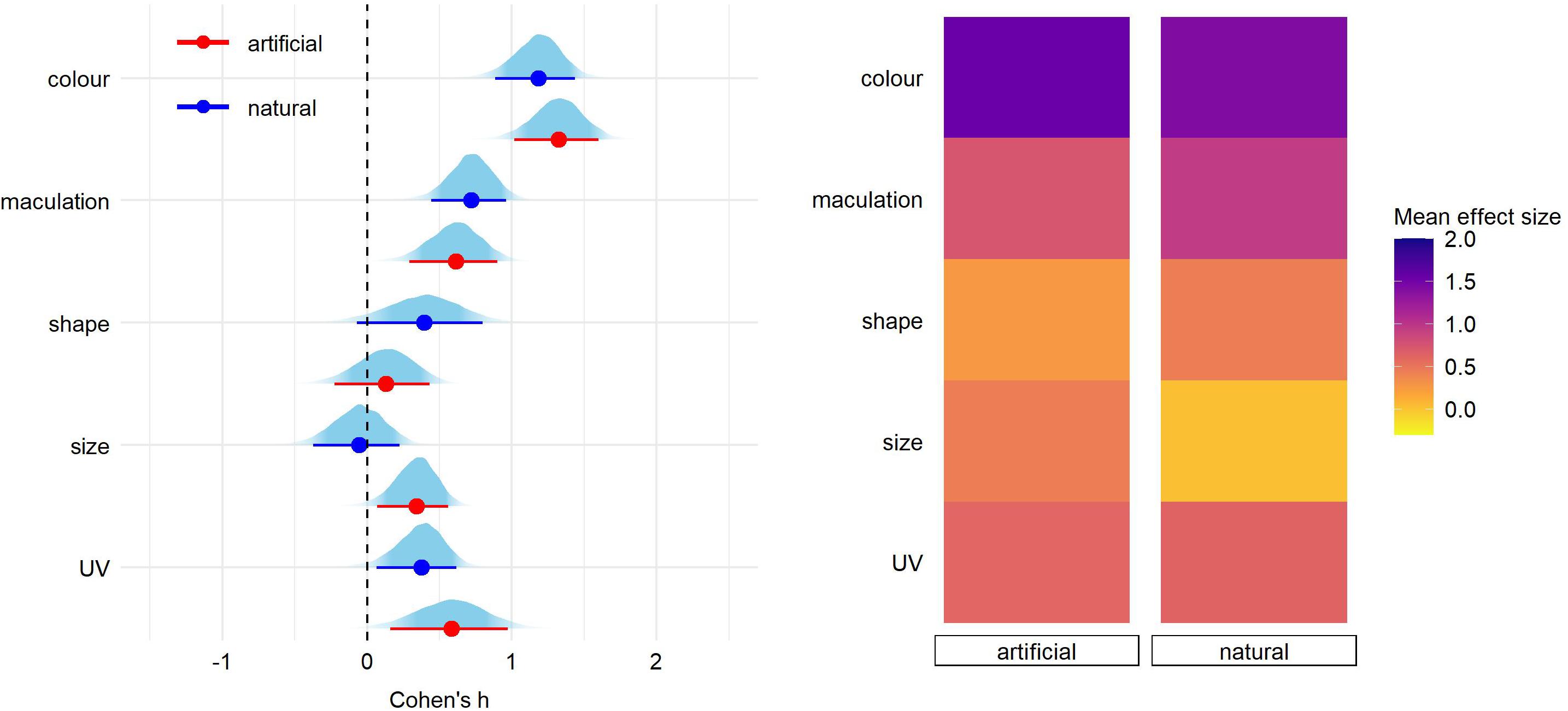
Figure 2. Predicted effect size for egg trait types according to the experimental egg stimuli (natural or model egg). On the left, for each trait type we show the parameter distribution with its median (dot) and 89% credible interval (lines). On the right, the median effect sizes are plotted as a heatmap for each trait type separated by egg type stimuli.
Pairwise comparisons within each egg trait type according to stimulus type (natural or model egg) were similar except of egg size trait (estimate = 0.39 [0.14–0.66]; Figure 2 and Supplementary Table 2). This sole difference was driven by a high effect size found for smaller artificial eggs (Cohen’s h = 0.59 [0.28–1.16], N = 10 reports) but not for other treatments (artificial larger = −0.14 [−0.55−0.27], N = 4; natural smaller = 0.03 [−0.29−0.43], N = 3; natural larger = -0.04 [−0.21−0.14], N = 4).
Finally, pairwise comparisons did not detect significant effect of nest type nor host-parasite system on any of egg trait (Figures 3, 4 and Supplementary Tables 3,4).
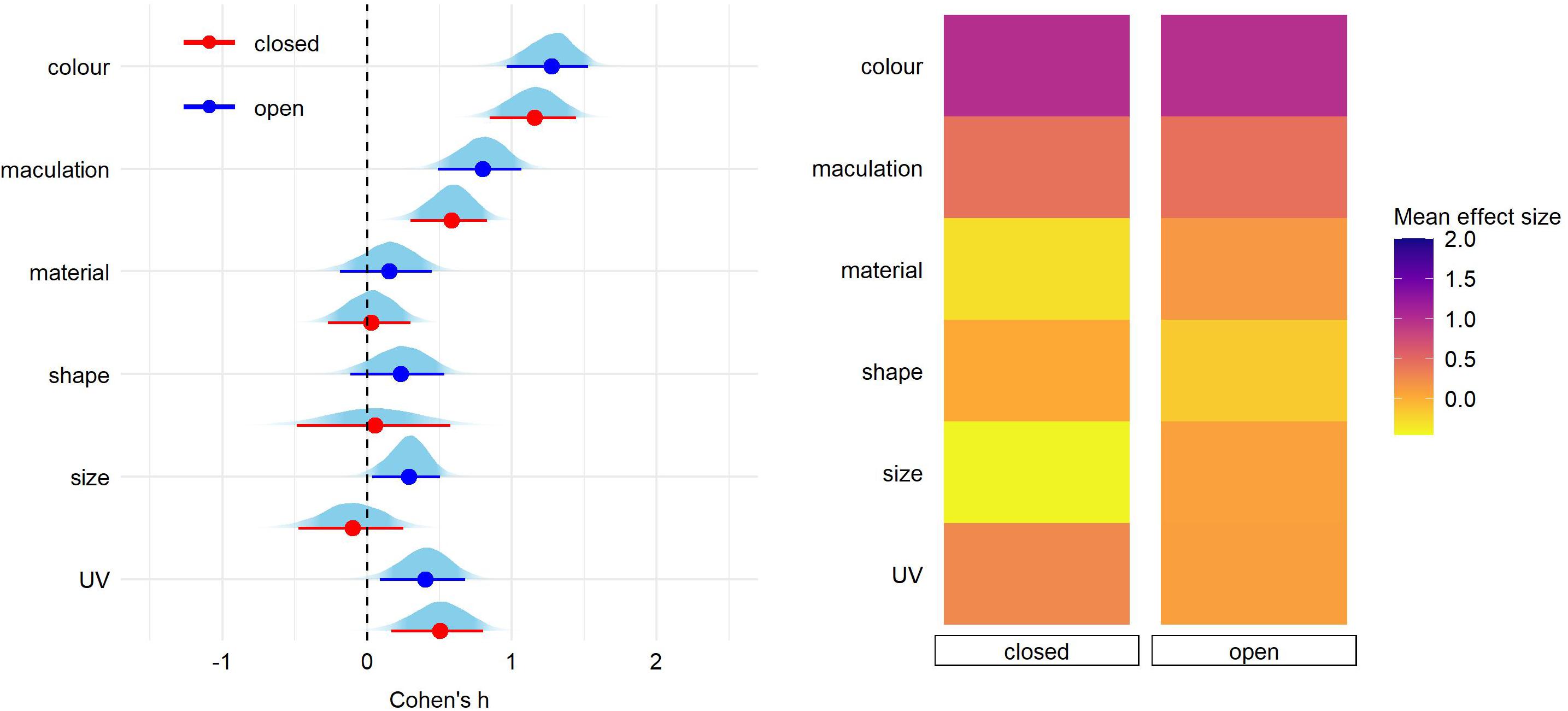
Figure 3. Predicted effect size for egg trait types according to the breeding strategy (nest type: open or closed). On the left, for each trait type we show the parameter distribution with its median (dot) and 89% credible interval (lines). On the right, the median effect sizes are plotted as a heatmap for each trait type separated by host nest architecture.
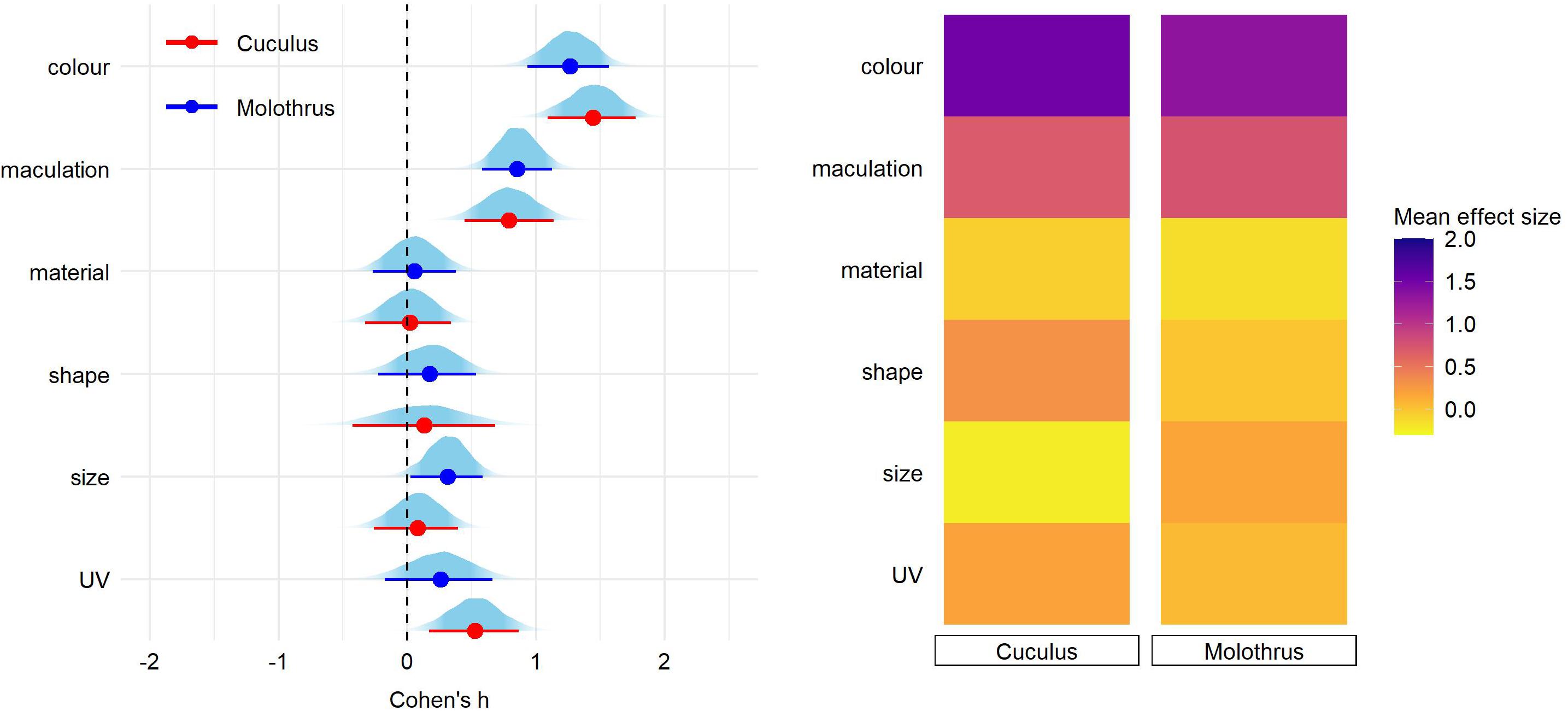
Figure 4. Predicted effect size for egg trait types in hosts of Cuculus and Molothrus parasite species. On the left, for each trait type we show the parameter distribution with its median (dot) and 89% credible interval (lines). On the right, the median effect sizes are plotted as a heatmap for each trait type separated by parasitic lineage type.
The only summation effect allowing us to estimate effect size was the simultaneous change in egg color and maculation (N = 12 reports). Even after excluding five outlier reports (see section “Statistical Analysis” for details), the between-study heterogeneity remained high (I2 = 90%; 95% CI = 81.8–94.4) suggesting a caution for further interpretation of this overall effect size estimate. Bayesian random-effect model estimated high Cohen’s h of 1.52 (89% credible intervals 0.86–2.05; N = 7 reports).
Discussion
The results of this meta-analysis support earlier qualitative findings that visual traits play a dominant role in the recognition of parasitic egg in the host nest (Honza and Cherry, 2017; Turner and Hauber, 2021). All the three visually-related eggshell traits, including color, maculation, and UV, showed no overlap with null effect sizes. The effect of the shell’s ground coloration was particularly substantial and more important than either maculation or UV, and any other egg characteristic. However, we note that the visual egg traits are at the same time the most studied characteristic (55% of included reports; Table 1). Only partly vision-related traits such as egg shape and size showed small effects and the effect of other, also potentially partly tactile traits represented by natural vs. model materials, was negligible. Other hypothesized recognition cues, such as egg odor (e.g., Soler et al., 2014; Hauber, 2020) or weight (Ruiz-Raya et al., 2015) could not be statistically analyzed due to insufficient number of published reports (Figure 5). Therefore, our first recommendation is that more such studies address the potential roles of tactile-only or olfactory cued egg rejection behaviors in varied hosts of diverse avian brood parasites (also see Turner and Hauber, 2021). Finally, even in the studies performing a valid egg experiment, using a control treatment was particularly limiting selection criterion and led to a notable severe reduction of the selected studies for final analyses (Supplementary Table 1). However, modifying or even excluding any of the four selection criteria (see section “Materials and Methods”) would have directly prevented obtaining a valid meta-analytical result.
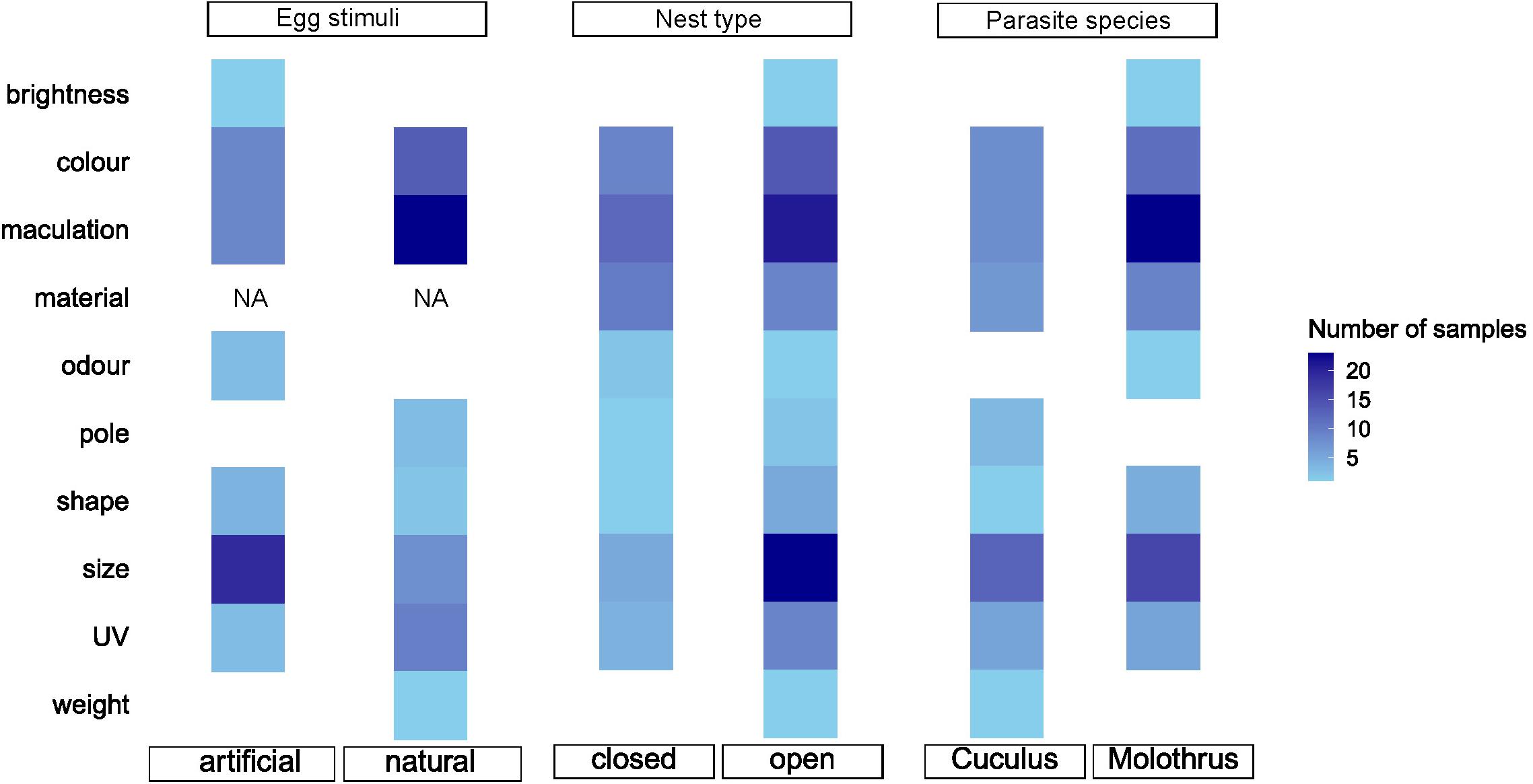
Figure 5. A heat map showing number of samples (number of effect size reports) in our meta-analysis dataset. Blank squares represent no experiments available to be included in our data set for a particular treatment. NA, treatment does not exist in our dataset.
Further, examining the three impactful extrinsic factors potentially affecting the relative importance within each egg trait showed that birds responded similarly regardless the bird is presented with artificial or natural experimental eggs, breeding in open or closed nest and parasitized by Old World cuckoos or New World cowbirds. The latter lack of difference between cuckoo vs. cowbird hosts may be due to the prior, naturally selected adaptations of the mostly insectivorous hosts of both parasite types, whereby visual discrimination of salient recognition cues, such as colors and patterns, may have been similarly preadapted to non-egg relevant traits, such as shared foraging contexts (e.g., Luro and Hauber, 2020).
In turn, the only within-egg trait difference was found for egg size, where model eggs with experimentally altered size were rejected more often than natural ones. More detailed exploration showed that this difference was caused by high rejection rates of artificial eggs. This was true particularly for experimental eggs smaller than the host egg. This treatment with smaller artificial eggs was also studied more often (N = 10 reports compared to smaller, N ≤ 4 in other three treatments, i.e., larger artificial, smaller and larger natural egg) and 7 out of 10 reports were performed on Turdus spp. These thrush species are also known to reject smaller egg models at generally high rates (e.g., Grim et al., 2011; Samas et al., 2014; Hanley et al., 2017; Luro et al., 2018). This bias for testing strong rejecters in the treatment with artificial egg sizes might thus explain the higher effect size compared to published experiments using natural eggs in moderately rejecter host species.
The previous overview (Honza and Cherry, 2017) and our current meta-analysis both show a notable preponderance of studies to examine egg color and maculation characteristics (about a half of all studies in our data set, Figure 5). Yet, our second recommendation is that the potential for future studies on both of these visual traits still remains vast due to recent development of new analytical tools for data collection and approaches to statistical analyses (Stevens, 2011; Weinstein, 2018). For example, the eggshell color signal was recently assessed from two vision aspects, chromatic (hue) and achromatic contrasts (saturation; e.g., Avilés et al., 2010; Croston and Hauber, 2014; Hanley et al., 2017; Abolins-Abols et al., 2019; Manna et al., 2020). Similarly, egg patterning has been explored in a greater detail using advanced analytical tools (e.g., Schmitz Ornés et al., 2014; Stoddard et al., 2014). These studies provide important new insights into the sensory and cognitive processes of the hosts and show that the potential for future studies remains vast. Also, other recently emerged technologies, such as 3D printing (Igic et al., 2015), thermochromic coats (Hauber et al., 2019), or multispectral cameras (Attisano et al., 2018) open additional and novel research avenues to examine in greater detail various potential eggshell trait effects. Future meta-analyses can benefit from the more detailed studies by exploring each trait in greater depth. The direct quantification of the change in the manipulated egg trait will allow to include into analyses also the effect of magnitude of the manipulation. Such more detailed analysis was beyond the scope of this study here but we also note that the current level of methodological details and diversity in the methods would hinder these attempts. We recommend that the future studies should provide specific information, which allows to estimate the magnitude of egg trait manipulation whenever it is possible. For example, the magnitude of color contrast between control and manipulated eggs could be expressed in just noticeable difference units (JND; Vorobyev and Osorio, 1998) or as a simple proportion of change in quantities expressed with the International System of Units for some other traits.
Rothstein (1982) formally suggested that only one egg trait may play less important role on rejection than the summation of several egg characteristics. This “stimulus summation” hypothesis was supported by several other studies (Bártol et al., 2002; López-de-Hierro and Moreno-Rueda, 2010; de la Colina et al., 2012), including a biological replication of Rothstein’s own study on American robins (Turdus migratorius) (Luro et al., 2018), while Underwood and Sealy (2006) concluded that in warbling vireos (Vireo gilvus) it was egg maculation itself that was a sufficient cue to recognize the cowbird egg. We found that Cohen’s h of 1.52 (89% credible intervals 0.86–2.05) for the simultaneous change in egg color and maculation was somewhat higher, but still highly overlapping in its intervals with the effect size for the egg color trait only (Cohen’s h = 1.23; 0.96–1.47). This single result does not provide a quantitative support for the “stimulus summation” hypothesis and more focally designed studies are clearly necessary before drawing any conclusions.
The greater importance of the color trait effect than any other eggshell traits (Figure 1) suggests the highest reliance of avian cognition processes on this particular visual parameter, irrespective of the nest’s lighting milieu (Figure 3). At least from a human perspective, eggshell colors are diverse (Hauber, 2014; but see Hanley et al., 2015a), whereas other traits, including egg size and shape, are more limited in their variability (but see Stoddard et al., 2017). Also, all bird eggs have a ground coloration but not all of them are maculated, which might contribute to generally lower importance of maculation traits compared to the color as a reliable recognition cue. In turn, the effect of UV has been studied relatively often but it is rather assumed as a part of the color characteristic than a distinct trait (Cassey et al., 2008; Stoddard and Hauber, 2017). We classified the UV as a separate trait because this meta-analysis reflected the viewpoint and efforts in the field of brood parasitism research, whereby UV-sensitivity and -spectral reflectance are often treated as a critically avian-relevant perceptual cue (e.g., Honza et al., 2007; Croston and Hauber, 2014; Abernathy and Peer, 2015). Here we also examined the effect of egg material, which did not appear to generate reliably distinct effect sizes between model and natural egg stimuli (Figure 2). This conclusion is still important from a methodological point of view, because various materials are used to manufacture the artificial egg models. However, what is still missing from the experimental repertoire is a model egg stimulus that can be pierced by hosts whose beaks are too small for grasp rejection (e.g., Roncalli et al., 2017). Finally, we compared the eggshell traits’ impact on egg rejection by hosts parasitized by Old World cuckoos vs. cowbirds and, contrary to expectations, found no statistical differences between these diverse set of hosts (Figure 4). This may be due to the use of artificial colors, rather than naturally mimetic cuckoo egg coloration, in studying the responses of hosts of both types of parasites, whereby even control treatments can be rejected by some hosts at unnaturally high rates (e.g., Abolins-Abols et al., 2019).
Vision is assumed to be the most important sense in birds (Martin, 2017). Accordingly, it is increasingly accepted that hosts recognize the foreign egg in their nest according to color and maculation (Honza and Cherry, 2017). Our results confirm quantitatively that visual components are essential during interactions with brood parasites during the egg stage. However, we must be reminded that egg characteristics that are not sensed visually have also attracted much lower research attention. Noticeably egg odor, weight, or surface texture remain unstudied (Turner and Hauber, 2021; Figure 5), and their relative impact on egg rejections remains mostly unknown and unquantifiable by us, too. Recent technological advances also open new ways to study in more depth any of the egg’s visual characteristics and promise novel insights in the near future. We encourage continuing research efforts in this fascinating field of coevolutionary and ecological interactions.
Data Availability Statement
The dataset generated for this meta-analysis is deposited in the Figshare digital repository (Dataset – Meta-analysis of avian egg traits; https://figshare.com/s/7320f0fb44c4f188c9eb).
Author Contributions
MH conceived the study. MH and PS designed the study. PS analyzed the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Many thanks to Shelby Lawson for statistical advice. MH thanks the support of the Hanse-Wissenschaftskolleg, Germany. Computational resources were supplied by the project “e-Infrastruktura CZ” (e-INFRA LM2018140) provided within the program Projects of Large Research, Development and Innovations Infrastructures.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.703208/full#supplementary-material
Footnotes
References
Abernathy, V. E., and Peer, B. D. (2015). Reduced ultraviolet reflectance does not affect egg rejection by Northern Cardinals (Cardinalis cardinalis). Wilson J. Ornithol. 128, 334–342. doi: 10.1676/wils-128-02-334-342.1
Abolins-Abols, M., Hanley, D., Moskát, C., Grim, T., and Hauber, M. E. (2019). Anti-parasitic egg rejection by great reed warblers (Acrocephalus arundinaceus) tracks differences along an eggshell color gradient. Behav. Proc. 166:103902. doi: 10.1016/j.beproc.2019.103902
Akre, K. L., and Johnsen, S. (2014). Psychophysics and the evolution of behavior. Trends Ecol. Evol. 29, 291–300. doi: 10.1016/j.tree.2014.03.007
Antonov, A., Stokke, B. G., Moksnes, A., and Røskaft, E. (2009). Evidence for egg discrimination preceding failed rejection attempts in a small cuckoo host. Biol. Lett. 5, 169–171. doi: 10.1098/rsbl.2008.0645
Attisano, A., Sato, N. J., Tanaka, K. D., Okahisa, Y., Kuehn, R., Gula, R., et al. (2018). Visual discrimination of polymorphic nestlings in a cuckoo-host system. Sci. Rep. 8:10359. doi: 10.1038/s41598-018-28710-5
Avilés, J. M., Vikan, J. R., Fossøy, F., Antonov, A., Moksnes, A., Røskaft, E., et al. (2010). Avian colour perception predicts behavioural responses to experimental brood parasitism in chaffinches. J. Evol. Biol. 23, 293–301. doi: 10.1111/j.1420-9101.2009.01898.x
Balduzzi, S., Rücker, G., and Schwarzer, G. (2019). How to perform a meta-analysis with R: a practical tutorial. Evid. Based Ment. Health 22, 153–160. doi: 10.1136/ebmental-2019-300117
Bártol, I., Karcza, Z., Moskát, C., Røskaft, E., and Kisbenedek, T. (2002). Responses of great reed warblers Acrocephalus arundinaceus to experimental brood parasitism: the effects of a cuckoo Cuculus canorus dummy and egg mimicry. J. Avian Biol. 33:420–425. doi: 10.1034/j.1600-048X.2002.02945.x
Bürkner, P. C. (2017). brms: an R Package for bayesian multilevel models using stan. J. Stat. Soft. 80, 1–28. doi: 10.18637/jss.v080.i01
Canniff, L., Dainson, M., López, A. V., Hauber, M. E., Grim, T., Samaš, P., et al. (2018). Probing the limits of egg recognition using egg rejection experiments along phenotypic gradients. J. Vis. Exp. 138:e57512. doi: 10.3791/57512
Cassey, P., Honza, M., Grim, T., and Hauber, M. E. (2008). The modelling of avian visual perception predicts behavioural rejection responses to variable egg colours. Biol. Lett. 4, 515–517. doi: 10.1098/rsbl.2008.0279
Caves, E. M., Stevens, M., and Spottiswoode, C. N. (2017). Does coevolution with a shared parasite drive hosts to partition their defences among species? Proc. R. Soc. Lond. B 284:20170272. doi: 10.1098/rspb.2017.0272
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Hillsdale, NJ: Law-rence Erlbaum.
Croston, R., and Hauber, M. E. (2014). Spectral tuning and perceptual differences do not explain the rejection of brood parasitic eggs by American robins (Turdus migratorius). Behav. Ecol. Sociobiol. 68, 351–362. doi: 10.1007/s00265-013-1649-8
Davies, N. B., and Brooke, M. D. L. (1989). An experimental study of coevolution between the Cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 58, 225–236. doi: 10.2307/4996
de la Colina, M. A., Pompilio, L., Hauber, M. E., Reboreda, J. C., and Mahler, B. (2012). Different recognition cues reveal the decision rules used for egg rejection by hosts of a variably mimetic avian brood parasite. Anim. Cogn. 15, 881–889. doi: 10.1007/s10071-012-0515-9
Friedmann, H. (1929). The Cowbirds. A Study in the Biology of Social Parasitism. Springfield: C. C. Thomas.
Grim, T., Samaš, P., Moskát, C., Kleven, O., Honza, M., Moksnes, A., et al. (2011). Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? J. Anim. Ecol. 80, 508–518. doi: 10.1111/j.1365-2656.2010.01798.x
Guigueno, M. F., and Sealy, S. G. (2012). Nest sanitation in passerine birds: implications for egg rejection in hosts of brood parasites. J. Ornithol. 153, 35–52. doi: 10.1007/s10336-011-0731-0
Hanley, D., Grim, T., Cassey, P., and Hauber, M. E. (2015a). Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Biol. Lett. 11:20150087. doi: 10.1098/rsbl.2015.0087
Hanley, D., Samaš, P., Hauber, M. E., and Grim, T. (2015b). Who moved my eggs? An experimental test of the egg arrangement hypothesis for the rejection of brood parasitic eggs. Anim. Cogn. 18, 299–305. doi: 10.1007/s10071-014-0800-x
Hanley, D., Samaš, P., Heryán, J., Hauber, M. E., and Grim, T. (2015c). Now you see it, now you don’t: flushing hosts prior to experimentation can predict their responses to brood parasitism. Sci. Rep. 5:9060. doi: 10.1038/srep09060
Hanley, D., Grim, T., Igic, B., Samas, P., López, A. V., Shawkey, M. D., et al. (2017). Egg discrimination along a gradient of natural variation in eggshell coloration. Proc. R. Soc. Lond. B 284:20162592. doi: 10.1098/rspb.2016.2592
Harrer, M., Cuijpers, P., Furukawa, T. A., and Ebert, D. D. (2019a). Doing Meta-Analysis in R: A Hands-on Guide. Avaliable online at: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/ (accessed August 4, 2021).
Harrer, M., Cuijpers, P., Furukawa, T., and Ebert, D. D. (2019b). dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’. R Package Version 0.0.9000. Avaliable online at: http://dmetar.protectlab.org/ (accessed August 4, 2021).
Hauber, M. E. (2020). Natural and artificial scents do not increase egg rejection rates of model brood parasitic eggs by American Robins (Turdus migratorius). Acta Zool. Academ. Sci. Hung. 66, 309–317. doi: 10.17109/AZH.66.4.309.2020
Hauber, M. E., Dainson, M., Luro, A., Louder, A. A., and Hanley, D. (2019). When are egg-rejection cues perceived? A test using thermochromic eggs in an avian brood parasite host. Anim. Cogn. 22, 1141–1148. doi: 10.1007/s10071-019-01306-w
Hauber, M. E., Kim, C. R., Goethe, C., and Hanley, D. (2020). Self-referent phenotype matching is a poor predictor of egg rejection by American Robins. J. Field Ornithol. 91, 254–262. doi: 10.1111/jofo.12339
Hauber, M. E., Tong, L., Bán, M., Croston, R., Grim, T., Waterhouse, G., et al. (2015). The value of artificial stimuli in behavioral research: making the case for egg rejection studies in avian brood parasitism. Ethology 121, 521–528. doi: 10.1111/eth.12359
Hauber, M. E., Winnicki, S. K., Hoover, J. P., Hanley, D., and Hays, I. R. (2021). The limits of egg recognition: testing acceptance thresholds of American robins in response to decreasingly egg-shaped objects in the nest. Roy. Soc. Open Sci. 8:201615. doi: 10.1098/rsos.201615
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Honza, M., Polačiková, L., and Procházka, P. (2007). Ultraviolet and green parts of the colour spectrum affect egg rejection in the song thrush (Turdus philomelos). Biol. J. Linn. Soc. Lond. 92, 269–276. doi: 10.1111/j.1095-8312.2007.00848.x
Honza, M., and Cherry, I. M. (2017). “Egg characteristics affecting egg rejection,” in Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution, ed. M. Soler (Cham: Springer International Publishing), 401–419. doi: 10.1007/978-3-319-73138-4_22
Honza, M., Šulc, M., and Cherry, M. I. (2014). Does nest luminosity play a role in recognition of parasitic eggs in domed nests? A case study of the red bishop. Naturwissenschaften 101, 1009–1015. doi: 10.1007/s00114-014-1240-9
Igic, B., Nunez, V., Voss, H. U., Croston, R., Aidala, Z., López, A. V., et al. (2015). Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. PeerJ 3:e965. doi: 10.7717/peerj.965
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., and Mooers, A. O. (2012). The global diversity of birds in space and time. Nature 491, 444–448. doi: 10.1038/nature11631
Krüger, O., and Pauli, M. (2017). “Evolution of avian brood parasitism and phylogenetic history of brood parasites,” in Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution, ed. M. Soler (Cham: Springer International Publishing), 43–59. doi: 10.1007/978-3-319-73138-4_3
Kruschke, J. K. (2018). Rejecting or accepting parameter values in Bayesian estimation. Adv. Methods Pract. Psychol. Sci. 1, 270–280. doi: 10.1177/2515245918771304
Lahti, D. (2015). The limits of artificial stimuli in behavioral research: the umwelt gamble. Ethology 121, 529–537. doi: 10.1111/eth.12361
Langmore, N. E., Hunt, S., and Kilner, R. M. (2003). Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160. doi: 10.1038/nature01460
Langmore, N. E., Kilner, R. M., Butchart, S. H. M., Maurer, G., Davies, N. B., Cockburn, A., et al. (2005). The evolution of egg rejection by cuckoo hosts in Australia and Europe. Behav. Ecol. 16, 686–692. doi: 10.1093/beheco/ari041
Lenth, R. V. (2020). emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.4.8. Avaliable online at: https://CRAN.R-project.org/package=emmeans (accessed August 4, 2021).
López-de-Hierro, M. D. G., and Moreno-Rueda, G. (2010). Egg-spot pattern rather than egg colour affects conspecific egg rejection in the house sparrow (Passer domesticus). Behav. Ecol. Sociobiol. 64, 317–324. doi: 10.1007/s00265-009-0811-9
Luro, A., and Hauber, M. E. (2020). Avian diet and foraging ecology constrain foreign egg recognition and rejection. Avian Biol. Res. 13, 24–31. doi: 10.1177/1758155920914575
Luro, A., Igic, B., Croston, R., Lopez, A. V., Shawkey, M. D., and Hauber, M. E. (2018). Which egg features predict egg rejection responses in American robins? Replicating Rothstein’s (1982) study. Ecol. Evol. 8, 1673–1679. doi: 10.1002/ece3.3759
Makowski, D., Ben-Shachar, M., and Lüdecke, D. (2019). bayestestR: describing effects and their uncertainty, existence and significance within the bayesian framework. J. Open Source Softw. 4:1541. doi: 10.21105/joss.01541
Manna, T., Moskát, C., and Hauber, M. E. (2017). “Cognitive decision rules for egg rejection,” in Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution, ed. M. Soler (Cham: Springer International Publishing), 438–448.
Manna, T. J., Hanley, D., Honza, M., Čapek, M., Rutila, J., Samaš, P., et al. (2020). Fitting different visual models to behavioral patterns of parasitic egg rejection along a natural egg color gradient in a cavity-nesting host species. Vis. Res. 167, 54–59. doi: 10.1016/j.visres.2019.12.007
Mason, P., and Rothstein, S. I. (1986). Coevolution and avian brood parasitism: cowbird eggs show evolutionary response to host discrimination. Evolution 40, 1207–1214. doi: 10.1111/j.1558-5646.1986.tb05745.x
Medina, I., and Langmore, N. E. (2015). The costs of avian brood parasitism explain variation in egg rejection behaviour in hosts. Biol. Lett. 11:20150296. doi: 10.1098/rsbl.2015.0296
Moskát, C., Székely, T., Kisbenedek, T., Karcza, Z., and Bártol, I. (2003). The importance of nest cleaning in egg rejection behaviour of great reed warblers Acrocephalus arundinaceus. J. Avian Biol. 34, 16–19. doi: 10.1034/j.1600-048X.2003.02919.x
Øien, I. J., Moksnes, A., and Røskaft, E. (1995). Evolution of variation in egg color and marking pattern in European passerines: adaptations in a coevolutionary arms race with cuckoo. Cuculus canorus. Behav. Ecol. 6, 166–171. doi: 10.1093/beheco/6.2.166
Paradis, E., and Schliep, K. (2019). ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. doi: 10.1093/bioinformatics/bty633
Polačiková, L., Takasu, F., Stokke, B. G., Moksnes, A., Røskaft, E., Cassey, P., et al. (2013). Egg arrangement in avian clutches covaries with the rejection of foreign eggs. Anim. Cogn. 16, 819–828. doi: 10.1007/s10071-013-0615-1
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Roncalli, G., Ibáñez-Álamo, J. D., and Soler, M. (2017). Size and material of model parasitic eggs affect the rejection response of western Bonelli’s warbler Phylloscopus bonelli. Ibis 159, 113–123. doi: 10.1111/ibi.12431
Rothstein, S. I. (1970). An Experimental Investigation of the Defenses of the Hosts of the Parasitic Brown-Headed Cowbird (Molothrus ater). PhD dissertation, New Haven: Yale University.
Rothstein, S. I. (1982). Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav. Ecol. Sociobiol. 11, 229–239. doi: 10.1007/BF00299299
Ruiz-Raya, F., Soler, M., Sánchez-Pérez, L. L., and Ibáñez-Álamo, J. D. (2015). Could a factor that does not affect egg recognition influence the decision of rejection? PLoS One 10:e0135624. doi: 10.1371/journal.pone.0135624
Rutledge, S., Carr, D. E., Hauber, M. E., and Hanley, D. (2021). Best of a bad job or masters of illusion: do nest light conditions make the eggs of brood parasitic brown-headed cowbirds (Molothrus ater) more similar to the eggs of its hosts? Ethology 127, 117–124. doi: 10.1111/eth.13109
Samas, P., Hauber, M. E., Cassey, P., and Grim, T. (2014). Host responses to interspecific brood parasitism: a by-product of adaptations to conspecific parasitism? Front. Zool. 11:34. doi: 10.1186/1742-9994-11-34
Schmitz Ornés, A., Herbst, A., Spillner, A., Mewes, W., and Rauch, M. (2014). A standardized method for quantifying eggshell spot patterns. J. Field Ornithol. 85, 397–407. doi: 10.1111/jofo.12079
Sealy, S. G., and Underwood, T. J. (2012). Egg discrimination by hosts and obligate brood parasites: a historical perspective and new synthesis. Chin. Birds 3, 274–294. doi: 10.5122/cbirds.2012.0042
Soler, J. J., Pérez-Contreras, T., De Neve, L., Macías-Sánchez, E., Møller, A. P., and Soler, M. (2014). Recognizing odd smells and ejection of brood parasitic eggs. An experimental test in magpies of a novel defensive trait against brood parasitism. J. Evol. Biol. 27, 1265–1270. doi: 10.1111/jeb.12377
Soler, M. (2017). Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Cham: Springer International Publishing.
Soler, M., Ruiz-Raya, F., Roncalli, G., and Ibáñez-Álamo, J. D. (2015). Nest desertion cannot be considered an egg-rejection mechanism in medium- or large-sized hosts: an experimental study with the common blackbird (Turdus merula). J. Avian Biol. 46, 1–9. doi: 10.1111/jav.00571
Stevens, M. (2011). Avian vision and egg colouration: concepts and measurements. Avian Biol. Res. 4, 168–184. doi: 10.3184/175815511X13207790177958
Stevens, M., Troscianko, J., and Spottiswoode, C. N. (2013). Repeated targeting of the same hosts by a brood parasite compromises host egg rejection. Nat. Comm. 4:2475. doi: 10.1038/ncomms3475
Stoddard, M., Kilner, R., and Town, C. (2014). Pattern recognition algorithm reveals how birds evolve individual egg pattern signatures. Nat. Comm. 5:4117. doi: 10.1038/ncomms5117
Stoddard, M. C., and Hauber, M. E. (2017). Colour, vision and coevolution in avian brood parasitism. Phil. Trans. R. Soc. B 372:20160339. doi: 10.1098/rstb.2016.0339
Stoddard, M. C., Miller, A., Eyster, H. N., and Akkaynak, D. (2018). I see your false colors: how artificial stimuli appear to different animal viewers. Roy. Soc. Int. Foc. 9:20180053. doi: 10.1098/rsfs.2018.0053
Stoddard, M. C., Yong, E. H., Akkaynak, D., Sheard, C., Tobias, J. A., and Mahadevan, L. (2017). Avian egg shape: form, function, and evolution. Science 356, 1249–1254. doi: 10.1126/science.aaj1945
Tosi-Germán, R. A., Tassinoa, B., and Reboreda, J. C. (2020). Female and male rufous horneros eject shiny cowbird eggs using a mental template of the size of their own eggs. Behav. Proc. 178:1041152. doi: 10.1016/j.beproc.2020.104152
Turner, A. M., and Hauber, M. E. (2021). The American robin (Turdus migratorius): a focal species for anti-parasitic egg rejection studies among hosts of the brown-headed cowbird (Molothrus ater). Ethology 127, 490–503. doi: 10.1111/eth.13158
Underwood, T. J., and Sealy, S. G. (2006). Parameters of brown-headed cowbird Molothrus ater egg discrimination in warbling vireos Vireo gilvus. J. Avian Biol. 37, 457–466. doi: 10.1111/j.2006.0908-8857.03583.x
Vorobyev, M., and Osorio, D. (1998). Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358. doi: 10.1098/rspb.1998.0302
Weinstein, B. G. (2018). A computer vision for animal ecology. J. Anim. Ecol. 87, 533–545. doi: 10.1111/1365-2656.12780
Williams, D. R., Rast, P., and Bürkner, P. C. (2018). Bayesian meta-analysis with weakly informative prior distributions. PsyArXiv [Preprint]. doi: 10.31234/osf.io/7tbrm
Winfree, R. (1999). Cuckoos, cowbirds and the persistence of brood parasitism. Trends Ecol. Evol. 14, 338–343. doi: 10.1016/S0169-5347(99)01643-2
Keywords: meta-analysis, brood parasitism, egg rejection, egg traits, egg color, egg maculation
Citation: Samaš P, Hauber ME and Honza M (2021) A Meta-Analysis of Avian Egg Traits Cueing Egg-Rejection Defenses Against Brood Parasitism. Front. Ecol. Evol. 9:703208. doi: 10.3389/fevo.2021.703208
Received: 30 April 2021; Accepted: 30 July 2021;
Published: 25 August 2021.
Edited by:
Cynthia Ursino, Princeton University, United StatesReviewed by:
Jesús Miguel Avilés, Consejo Superior de Investigaciones Científicas (CSIC), SpainMaria Cecilia De Mársico, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2021 Samaš, Hauber and Honza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Samaš, cHNhbWFzQHNlem5hbS5jeg==
 Peter Samaš
Peter Samaš Mark E. Hauber
Mark E. Hauber Marcel Honza
Marcel Honza