- 1Department of Evolution, Ecology, and Behavior, School of Integrative Biology, University of Illinois Urbana-Champaign, Urbana, IL, United States
- 2Department of Biological Sciences, Western Michigan University, Kalamazoo, MI, United States
Referential alarm calls that denote specific types of dangers are common across diverse vertebrate lineages. Different alarm calls can indicate a variety of threats, which often require specific actions to evade. Thus, to benefit from the call, listeners of referential alarm calls must be able to decode the signaled threat and respond to it in an appropriate manner. Yellow warblers (Setophaga petechia) produce referential “seet” calls that signal to conspecifics the presence of nearby obligate brood parasitic brown-headed cowbirds (Molothrus ater), which lay their eggs in the nests of other species, including yellow warblers. Our previous playback experiments have found that red-winged blackbirds (Agelaius phoeniceus), a species also parasitized by brown-headed cowbirds, eavesdrop upon and respond strongly to yellow warbler seet calls during the incubation stage of breeding with aggression similar to responses to both cowbird chatters and predator calls. To assess whether red-winged blackbird responses to seet calls vary with their own risk of brood parasitism, we presented the same playbacks during the nestling stage of breeding (when the risk of brood parasitism is lower than during incubation). As predicted, we found that blackbirds mediated their aggression toward both cowbird chatter calls and the warblers’ anti-parasitic referential alarm calls in parallel with the low current risk of brood parasitism during the nestling stage. These results further support that red-winged blackbirds flexibly respond to yellow warbler antiparasitic referential calls as a frontline defense against brood parasitism at their own nests.
Introduction
Acoustic signals are used across diverse lineages to signal a variety of information, such as food sources or predatory threats (Bradbury and Vehrencamp, 2011). Some acoustic signals, known as functionally referential calls, denote to specific objects in the environment, and are often used to warn conspecifics of particular predator types (e.g., flying vs. ground), each requiring different behavioral responses to evade (Evans et al., 1993; Oda and Masataka, 1996; Evans, 1997; Rainey et al., 2004a, b; Zuberbühler, 2009; Suzuki, 2012). Listeners to referential calls must therefore be able to understand what is being referenced to determine the appropriate response based on the risk posed to them by the specific predatory threat denoted. Heterospecific eavesdropping upon referential calls is common across birds and mammals (Sherman, 1977; Magrath et al., 2015). Heterospecific eavesdroppers often demonstrate the same abilities as intended conspecific receivers do in decoding and responding appropriately to the information contained within the referential signals (e.g., Oda and Masataka, 1996; Rainey et al., 2004b; Suzuki, 2012; see Magrath et al., 2015, 2020 for reviews). For example, Verreaux’s sifakas (Propithecus verreauxi) produce different referential alarm calls for aerial vs. terrestrial predators, which are heard by both intended conspecific receivers and by eavesdropping heterospecific black-casqued hornbills (Ceratogymna atrata) (Rainey et al., 2004a,b). Both types of listeners react to aerial alarm calls by hiding under cover, but, critically, the hornbills do not respond to the alarm calls signaling ground predators because they do not pose a threat to these birds.
Conspecific and heterospecific eavesdropping upon referential alarm calls occurs in songbirds within the context of improving nest defense or minimizing nest detection by predators (Gill and Sealy, 2003, 2004; Platzen and Magrath, 2005; Davies et al., 2006; Haff and Magrath, 2012; Suzuki, 2015; Yu et al., 2017). Avian nests can be threatened by at least two types of dangers: (1) nest predators that depredate eggs and nestlings, and (2) obligate brood parasites that solely lay their eggs in other species’ nests (i.e., hosts), leaving the hosts to care for the costly brood parasitic young (Davies, 2010). Many host species exhibit strong frontline defenses against both threat types, responding aggressively toward both predatory and parasitic intruders on the territories to prevent their direct access to the nest (Welbergen and Davies, 2009; Kilner and Langmore, 2011; Feeney et al., 2012; Feeney and Langmore, 2015). There is some overlap between these nest threats, in that brood parasites may depredate eggs (e.g., mafia hypothesis, farming; Hauber, 2014; reviewed in Soler et al., 2017), and nest predators may also threaten adult survival (e.g., genus Accipiter hawks; Winkler et al., 2020). The main distinction between threats is that nest predators are of risk to hosts throughout the nesting cycle (laying, incubation, and nestling stages), whereas brood parasites pose the gravest risk when nests have eggs. Hosts are generally aggressive toward brood parasites during laying and incubation, when the nest is at highest risk of successful brood parasitism, and less aggressive (compared to other threats such as nest predators) during either the pre-nesting or the nestling stages when the risk of parasitism is low (Neudorf and Sealy, 1992; Gill and Sealy, 1996; Fasanella and Fernández, 2009; Lawson et al., 2021b; see Lawson et al., 2021a for a meta-analysis). Conversely, nest predation costs remain high (even increasing) as the brood ages due to its unchanging outcome (i.e., partial or total reproductive failure; Gill and Sealy, 1996; Fasanella and Fernández, 2009; Ruiz et al., 2018).
There is an adaptive benefit for hosts facing both nest threats to be able to discriminate brood parasites from nest predators and respond based on current risk. For potential hosts, anti-parasitic defense hinges on the early detection of brood parasites prior to the parasites’ discovery of the host nests (Sealy et al., 1998). Thus, hosts of brood parasites should evolve to eavesdrop upon referential alarm calls that signal brood parasitism risk as an early warning system to maximize their frontline nest defenses. Yellow warblers (Setophaga petechia; hereafter “warblers”) emit a referential alarm call to signal the presence of a generalist obligate brood-parasite, the brown-headed cowbird (Molothrus ater; hereafter: cowbird) (Gill and Sealy, 2004). Specifically, warblers produce “seet calls” to warn conspecifics of nearby cowbirds. After hearing seet calls or producing them, female warblers return to and sit upon their nest, which may prevent the cowbird from inspecting or laying an egg into the nest (Gill et al., 2008; Lawson et al., 2021c). Seet calls are primarily produced in response to the sight and/or sound of cowbirds themselves or seet calls emitted by conspecific warblers, and almost exclusively during laying and incubation stages, when the nest is at the highest risk of parasitism, and not during the pre-nesting or nestling stages (Sealy et al., 1998; Gill and Sealy, 2004; Gill et al., 2008; Lawson et al., 2021b).
Our previous research found evidence that red-winged blackbirds (Agelaius phoeniceus, hereafter “blackbirds”), another North American host of brown-headed cowbirds (Searcy and Yasukawa, 1995; Strausberger, 2001; Shaffer and Goldade, 2003), eavesdrop upon and respond to nearby yellow warbler seet calls during their own laying and incubation stages (Lawson et al., 2020). Blackbirds are phylogenetically and vocally distinct from yellow warblers, but often nest within the same wetlands as the warblers, with greater proximity to blackbirds linked to lower parasitism upon nearby yellow warbler nests (Clark and Robertson, 1979). Blackbirds are larger than cowbirds and yellow warblers, and frontload their anti-parasitic nest defenses, using both vocal and physical aggression toward cowbirds to prevent them from accessing and parasitizing the nest (Robertson and Norman, 1976, 1977; Ortega and Cruz, 1988; Neudorf and Sealy, 1992; Gill et al., 1997, 2008; Strausberger and Horning, 1997; Cruz, 1999; Yasukawa et al., 2016). Blackbirds are not known to have a referential alarm call system of their own, but they do eavesdrop upon the seet calls of yellow warbler neighbors: in Lawson et al. (2020) we found that during the incubation stage, blackbirds of both sexes responded more often to the warblers’ seet calls relative to their generic “chip” alarm calls, and with similar urgency and vocal aggression toward playbacks of seet calls as to both cowbird chatters and nest predator calls. However, because there was equal response to both types of threats (brood parasite and nest predator), these findings implied that blackbirds do not perceive seet calls as a cowbird-specific referential signal per se, but rather as an alarm call for a nest threat.
Understanding how referential alarm calls are perceived by heterospecifics can be informed by testing under different conditions, such as varying levels of risk posed by the referent. For example, yellow warblers themselves respond less aggressively to referential seet calls during the nestling stage likely because there is little to no brood parasitism risk during this stage (Neudorf and Sealy, 1992; Gill and Sealy, 1996), and the same pattern can be seen across other hosts toward models of their respective brood parasites (Fasanella and Fernández, 2009; reviewed in Lawson et al., 2021a). Furthermore, blackbirds presented with taxidermy cowbird and nest predator models across nesting stages respond equally to both models during incubation, but more strongly to the nest predator during nestling stage (Neudorf and Sealy, 1992; also see Henger and Hauber, 2014). To determine whether blackbirds recognize seet calls as referential alarm calls denoting brood parasitism risk, we expanded on our previous playback study conducted during the incubation stage (Lawson et al., 2020), and presented playbacks of cowbird chatters, seet calls, nest predator calls, and a non-threatening control species to blackbird nests during the nestling stage, when the risk of brood parasitism is low. We predicted that if blackbirds respond to seet calls as a referent for brood parasites, aggressive responses toward cowbird chatter and seet calls should be lower than aggressive responses to nest predator calls, but comparable to each other, during the later stage of nesting.
Materials and Methods
This playback experiment was conducted during April–July 2020 and used the same sites, playback files, and playback methodology as in Lawson et al. (2020), which tested blackbirds’ responses to the same playbacks during the egg/incubation stages during the prior 2 years. The methodology is described briefly below; for more detailed methodology, see Lawson et al. (2020).
Sites and Study Species
Playbacks occurred at sites in Champaign (n = 3) and Vermilion counties (n = 3) in east central Illinois, United States, where blackbirds and yellow warblers both breed (Lawson et al., 2020). Both species are parasitized by cowbirds in Illinois (Rodewald, 2015; Merrill et al., 2017; pers. obs.). Blackbirds arrive as early as February but do not breed until late-April through late-July, with peak breeding season mid-May to mid-June (Lawson et al., 2020; Yasukawa and Searcy, 2020). Yellow warblers arrive on the breeding grounds in late-April with peak breeding mid-to-late May (overlapping with blackbirds; Kelly et al., 2019; Lawson et al., 2021b,c).
Playback Stimuli Construction
For our experiments, we used four of the playback treatments from Lawson et al. (2020): (1) female cowbird chatter (brood parasite), (2) yellow warbler seet calls [cowbird-specific anti-parasitic alarm call (Gill et al., 1997; Sealy et al., 1998; Gill and Sealy, 2003, 2004; Gill and Bierema, 2013)], (3) blue jay (Cyanocitta cristata, a nest predator commonly seen at our sites; Smith et al., 2020), calls and (4) wood thrush (Hylocichla mustelina, a non-threatening sympatric heterospecific control, Kelly et al., 2019) songs. Including a nest predator call along with a brood parasite was critical to determine whether the blackbirds’ responses to the seet call are antiparasitic or general (Rothstein and Robinson, 1998). Audio file construction is described in detail in Lawson et al. (2020). Briefly, audio files were edited and filtered in Adobe Audition CC 2019 and included five exemplar files for each treatment sourced from different individuals, with one exemplar chosen randomly for each playback trial to avoid pseudoreplication (Kroodsma et al., 2001). Each exemplar contained vocalizations from at least three individuals.
Playback Experiment
We conducted playback trials at active blackbird nests that were ≥50 m apart, which is the mean territory size for blackbirds (Searcy and Yasukawa, 1995). Blackbirds are polygynous harem breeders, and are highly defensive of territory boundaries with little to no overlap with other males, with females being site-faithful to male territories (Searcy and Yasukawa, 1995). Therefore, only testing nests ≥50 m apart (i) reduced the likelihood that we tested the same parents twice at different nests, as the subjects were not banded, and (ii) allowed us to accurately record the stages of any additional nests on the male’s territory. We searched sites 1–2 times weekly for active nests. Nest contents were checked every 3 days to ensure playback trials occurred during the nestling stage. We conducted playbacks at nests that only contained nestlings <9 days old to prevent forced fledging when inspecting the nest (blackbirds naturally fledge at 11–14 days old; Yasukawa and Searcy, 2020). Playbacks were conducted between 05:00 and 12:00 h local time with a FOXPRO NX4 game caller, placed ∼5 m from active nests. We placed the caller ∼1 m high in vegetation when possible and recorded data from >10 m away. Playback trials occurred for 10 min and were adjusted to broadcast at ∼90 dB at 1 m from the source (Lawson et al., 2020).
Blackbird nests received two of the four playback treatments, each on a separate day: cowbird chatter (n = 23), yellow warbler seet calls (n = 22), blue jay calls (n = 20), and wood thrush songs (n = 17), for a total of 82 playbacks. The time lapse in between the first and second playback at each territory ranged from 24 to 72 h later (mean = 30.4 h) to avoid habituation. Nests were randomly assigned treatments to minimize the potential for an effect of treatment order. Six nests were not retested as they were depredated between trials. Furthermore, the focal female did not appear within the playback range for two of the trials, and thus, these trials were dropped from the data analyses.
During the playback trial we recorded the following behavioral responses from both parents within 30 m of the speaker: (1) response latency (sec after the start of trial when a switch to behaviors signaling playback detection occurred: posturing, hopping, alarm calling, or attacking the speaker) (2) closest approach to the speaker (m); and (3) the number of alarm calls produced (“checks,” “chits,” “chonks” used interchangeably as nest defense alarm calls by both sexes, and “cheers” which are only produced by males, Beletsky et al., 1986; Knight and Temple, 1988; Yasukawa, 1989). We only recorded responses of the focal male and focal nesting female (determined by observing which female fed the nestlings), and not other females within the harem. The focal birds were visually tracked by an observer throughout the entire trial while another recorded the behaviors. The presence of additional nest(s) with eggs, as well as age of nestlings in the focal nest, were included as variables in our models (see section “Statistical Analyses”).
These studies were approved by the Animal Ethics Committee (IACUC) of the University of Illinois (#17259), and by United States federal (MB08861A-3) and Illinois state agencies (W20.6394).
Statistical Analyses
We evaluated whether playback treatment affected the same three response variables of interest (latency, total alarm calls, and closest approach) using a separate generalized linear model for each. Models were also separated by sex, due to the polygynous nature of blackbirds possibly leading to sex differences in nest defensive behaviors (Yasukawa and Searcy, 2020). For all latency and alarm call models we used a negative binomial general linear model to account for the large number of non-responses (0 s latency, no alarm calls produced) that varied by treatment. For the closest approach variable, we log-transformed the data after adding a small constant to obtain a normal distribution, and ran a linear model. All models included the following fixed effects: playback treatment, date (ordinal days after start of season – April 1st), trial order (to account for repeated playbacks at the same site), and age of nestlings (to account for the variation in ages of nests at time of playback). For models on male data, we also included the presence of another nest with eggs as another fixed effect, because males may have multiple females at different stages of nesting on their territory, and if there were differences in response over nest stages, presence of eggs may have affected the males’ responses. If the presence of eggs was significant, we ran the same generalized linear model with an interaction term (treatment × presence of eggs) to determine if responses to specific treatments were affected by presence of a nest with eggs on the male’s territory. For all models with significance, we ran post hoc Tukey tests to multiple compare treatment pairs of least-square means. All statistical tests were conducted in the statistical program R 4.0.5 (packages lme4, nlme, multcomp, emmeans, and car), with α = 0.05. Effect sizes were calculated in R for all significant and non-significant outcomes.
Results
Latency
Average latencies to respond varied significantly by treatment for both males (F3,81 = 8.95, p < 0.001; Figure 1) and females (F3,79 = 7.02, p < 0.001; Figure 2). Based on post hoc pairwise comparisons of least-square means, males responded more quickly to playbacks of blue jay calls compared to cowbird chatters (z = 4.44, p < 0.001), seet calls (z = 6.30, p < 0.001), and control wood thrush songs (z = 6.25, p < 0.001). Female latencies showed the same pattern, where females responded more quickly to playbacks of blue jay calls compared to cowbird chatters (z = 3.14, p < 0.01), seet calls (z = 3.06, p = 0.01), and control wood thrush songs (z = 2.92, p = 0.01). There was no significant difference in latency to respond to cowbird chatters compared to seet calls for either sex (males: z = −1.80, p = 0.27; females: z = 1.13, p = 0.99), and both sexes responded to cowbird and seet calls with similar latency to the control wood thrush (males: cowbird-wood thrush z = −2.02, p = 0.18, seet-wood thrush z = −3.99, p = 0.97; females: cowbird-wood thrush z = 0.005, p = 0.99, seet-wood thrush z = −0.11, p = 0.99; see Supplementary Table 1 for all post hoc comparisons). For both sexes, neither date of playback (males: F3,81 = 0.37, p = 0.54, estimate = −0.009; females: F3,79 = 1.10, p = 0.29, estimate <−0.001) nor trial order (males: F3,81 = 0.33, p = 0.56, estimate = 0.26; females: F3,79 < 0.01, p = 0.93, estimate = −0.01) affected latency responses. While age of nestlings did not significantly influence male latency (F3,81 = 3.07, p = 0.08, estimate = 0.22), females responded more quickly to playbacks with increasing age of nestlings (F3,79 = 3.93, p = 0.05, estimate = 0.12). For males, presence of a nest with eggs on the territory did not significantly affect latency responses (F3,81 = 3.15, p = 0.07, estimate = −1.4).
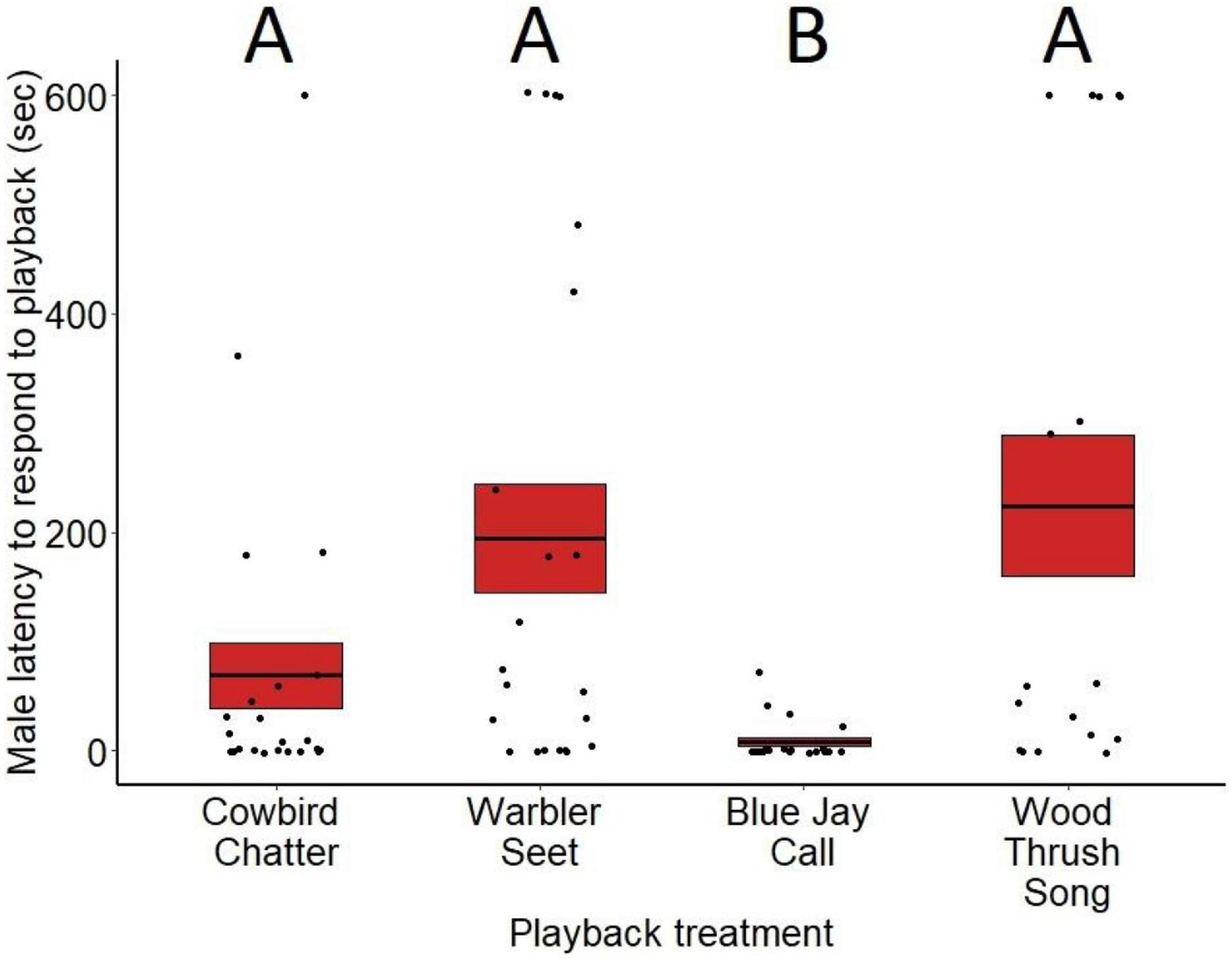
Figure 1. Latency (in seconds) for male red-winged blackbirds to respond to the playback treatments at nests during nestling stage. Means are shown with the bold line, and shaded boxes represent standard errors. Boxes with different letters denote post hoc statistical differences between treatments. For the p-values of post hoc comparisons, please refer to Supplementary Table 1.
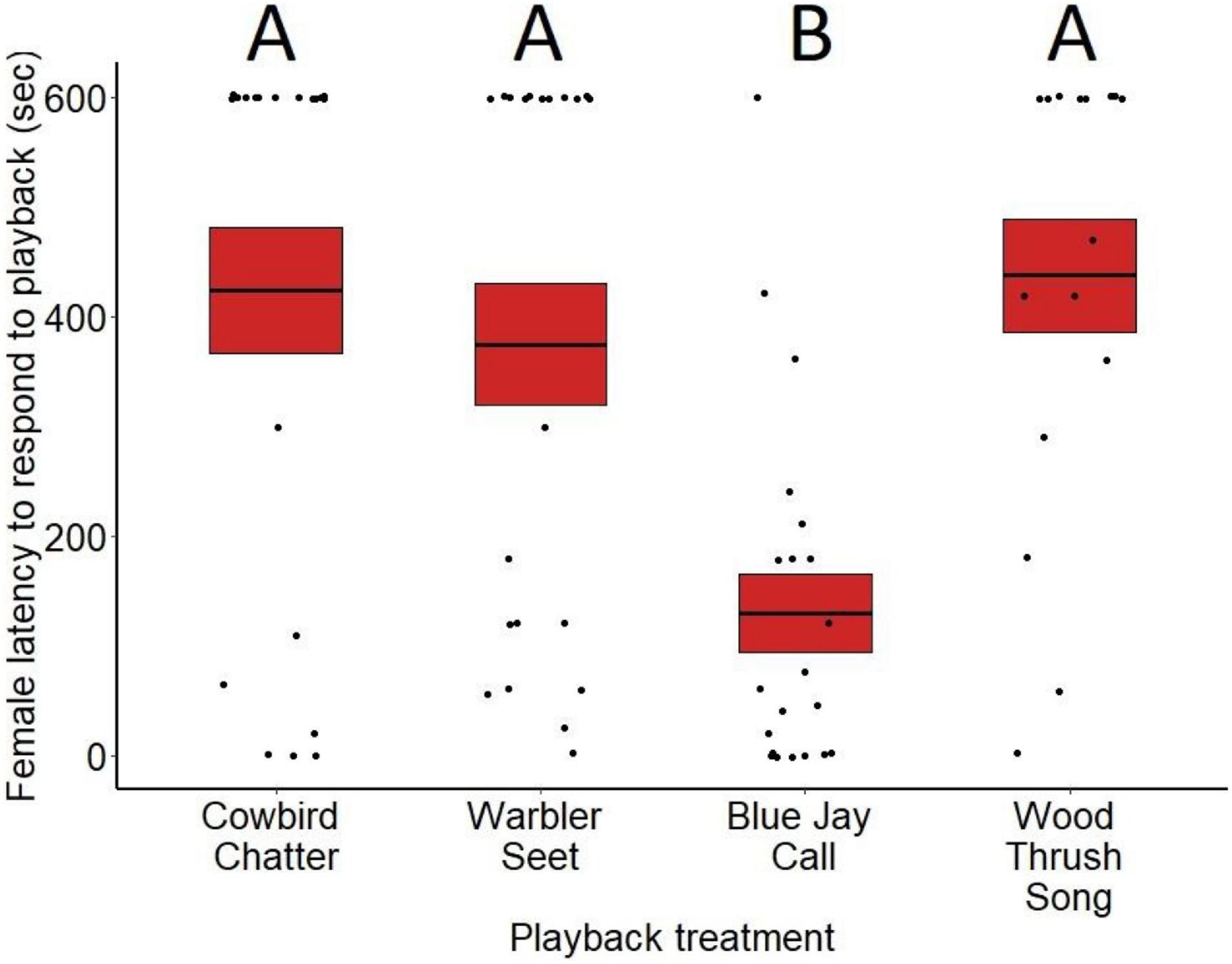
Figure 2. Latency (in seconds) for female red-winged blackbirds to respond to the playback treatments at nests during nestling stage. Means are shown with the bold line, and shaded boxes represent standard errors. Boxes with different letters denote post hoc statistical differences between treatments. For the p-values of post hoc comparisons, please refer to Supplementary Table 1.
Closest Approach
Closest approach varied significantly by treatment for males (F3,81 = 5.55, p < 0.01; Figure 3) but not females (F3,79 = 1.18, p = 0.32; Figure 4). Based on post hoc comparisons, males approached playbacks of cowbird chatters more closely than playbacks of seet calls (z = 2.73, p = 0.03) and control wood thrush songs (z = 3.81, p < 0.001). Males also approached blue jay calls more closely than wood thrush songs (z = 3.20, p = 0.05). Closest approach did not differ between any of the other playback comparisons (cowbird-blue jay z = −1.33, p = 0.54, blue jay-seet z = −1.34, p = 0.53, seet-wood thrush z = −1.35, p = 0.52; see Supplementary Table 2 for all post hoc comparisons). For both sexes, neither date of playback (males: F3,81 = 0.30, p = 0.58, estimate = 0.004; females: F3,79 < 0.001, p = 0.98, estimate <−0.001), trial order (males: F3,81 = 0.40, p = 0.52, estimate = −0.15; females: F3,79 < 0.68, p = 0.40, estimate = −0.16), nor age of nestlings (males: F3,81 = 0.17, p = 0.68, estimate = −0.02; females: F3,79 = 0.87, p = 0.98, estimate = 0.04) affected closest approach. For males, presence of a nest with eggs on the territory also did not significantly affect closest approach (F1,81 = 3.20, p = 0.07, estimate = −0.60).
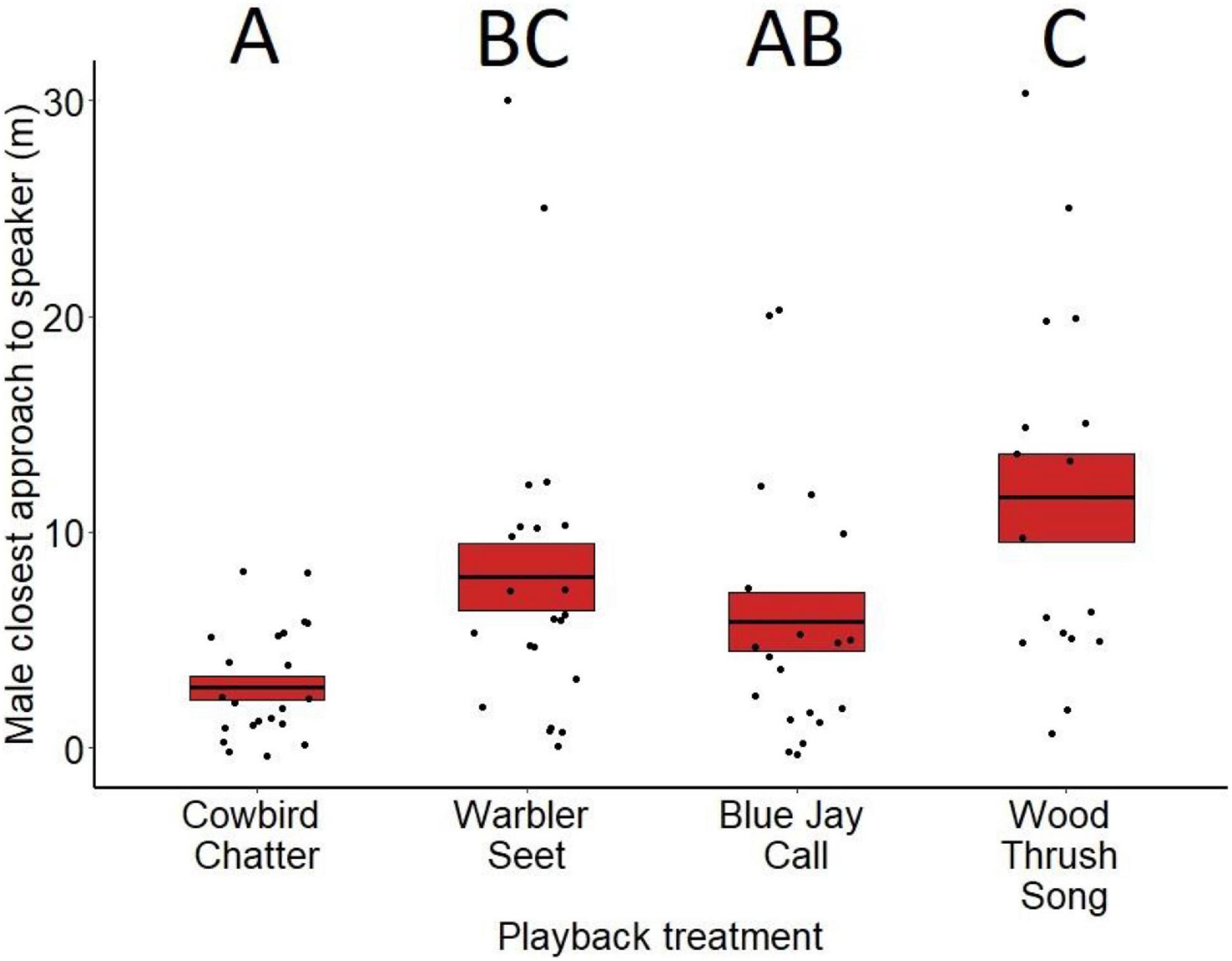
Figure 3. Closest approach to the playback speaker (in meters) by male red-winged blackbirds for the different treatments at nests during nestling stage. Means are shown with the bold line, and shaded boxes represent standard errors. Boxes with different letters denote post hoc statistical differences between treatments. For the p-values of post hoc comparisons, please refer to Supplementary Table 2.
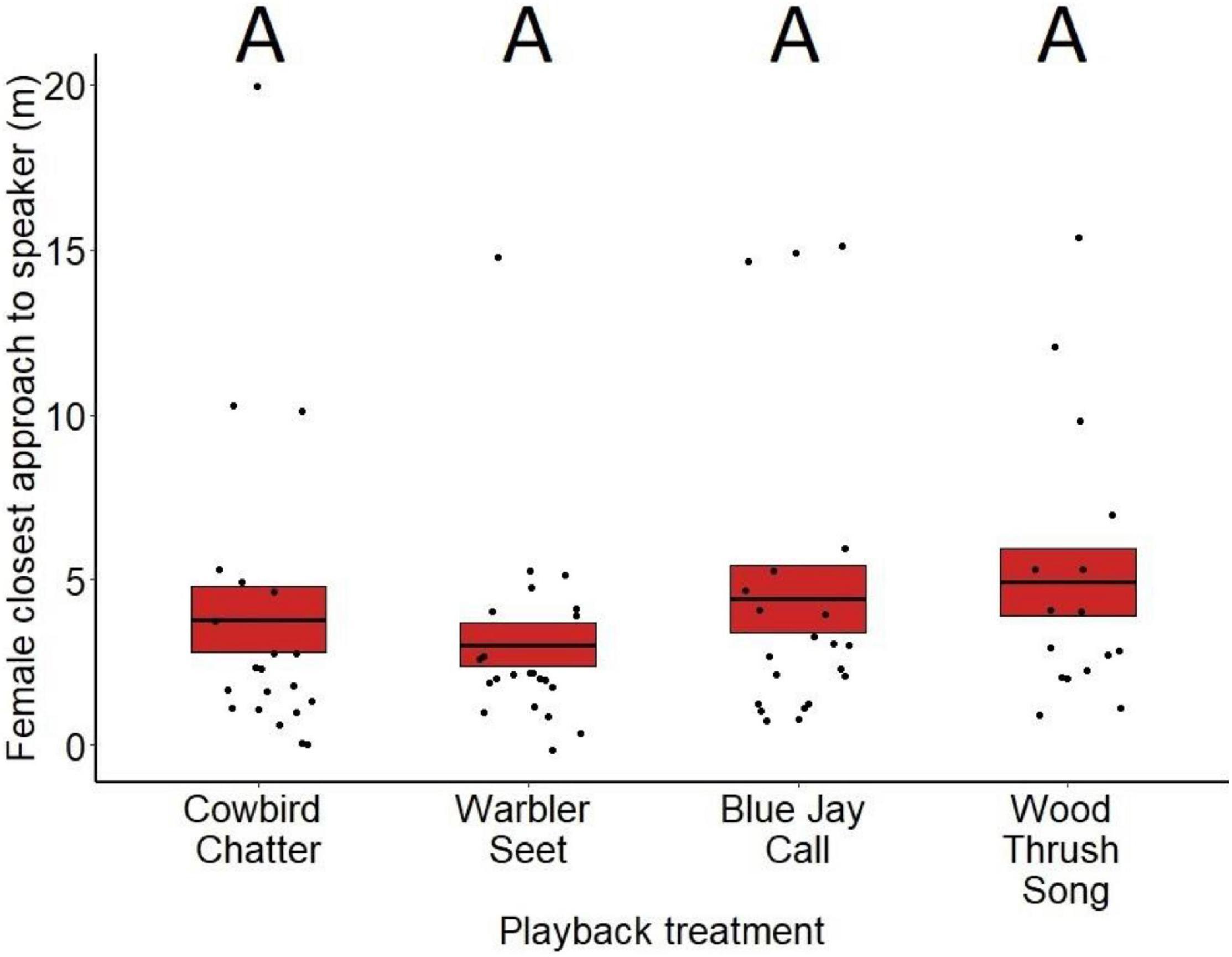
Figure 4. Closest approach to the playback speaker (in meters) by female red-winged blackbirds for the different treatments at nests during nestling stage. Means are shown with the bold line, and shaded boxes represent standard errors. There were no significant pairwise differences; for the p-values of post hoc comparisons, please refer to Supplementary Table 2.
Alarm Calling
Alarm calling varied significantly between treatments for both males (F3,81 = 6.55, p < 0.001; Figure 5) and females (F3,79 = 8.92, p < 0.001; Figure 6). Based on post hoc pairwise comparisons of least-squares means, males alarm called more toward playbacks of blue jay calls compared to cowbird chatters (z = 2.53, p = 0.05), seet calls (z = 3.31, p < 0.01), and control wood thrush songs (z = 3.75, p < 0.001). Female also alarm called more toward blue jay calls compared to cowbird chatters (z = 5.84, p < 0.001), seet calls (z = 5.99, p < 0.001), and control wood thrush songs (z = 3.33, p < 0.01). There was no significant difference in alarm calling responses toward cowbird chatters compared to seet calls for either sex (males: z = 0.73, p = 0.88; females: z = 0.05, p = 0.99), and both sexes alarm called similarly toward cowbird and seet calls compared to the control wood thrush (males: cowbird-wood thrush z = 1.34, p = 0.53, seet-wood thrush z = 0.69, p = 0.90; females: cowbird-wood thrush z = −2.11, p = 0.14, seet-wood thrush z = 2.16, p = 0.13; see Supplementary Table 3 for all post hoc comparisons). For both sexes, date of playback had a significant effect on alarm calling (males: F3,81 = 4.59, p = 0.03, estimate = 0.01; females: F3,79 = 8.28, p < 0.01, estimate = 0.03), with blackbirds producing more alarm calls later in the season. Females also alarm called significantly more with increasing age of the nestlings in her nest (F3,79 = 4.06, p = 0.04, estimate = −0.22), and while males showed this same pattern, it was non-significant (F3,81 = 3.65, p = 0.06, estimate = 0.12). Trial order did not significantly affect alarm call responses (males: F3,81 = 1.27, p = 0.26, estimate = −0.27; females: F3,79 = 2.56, p = 0.11, estimate = −0.75).
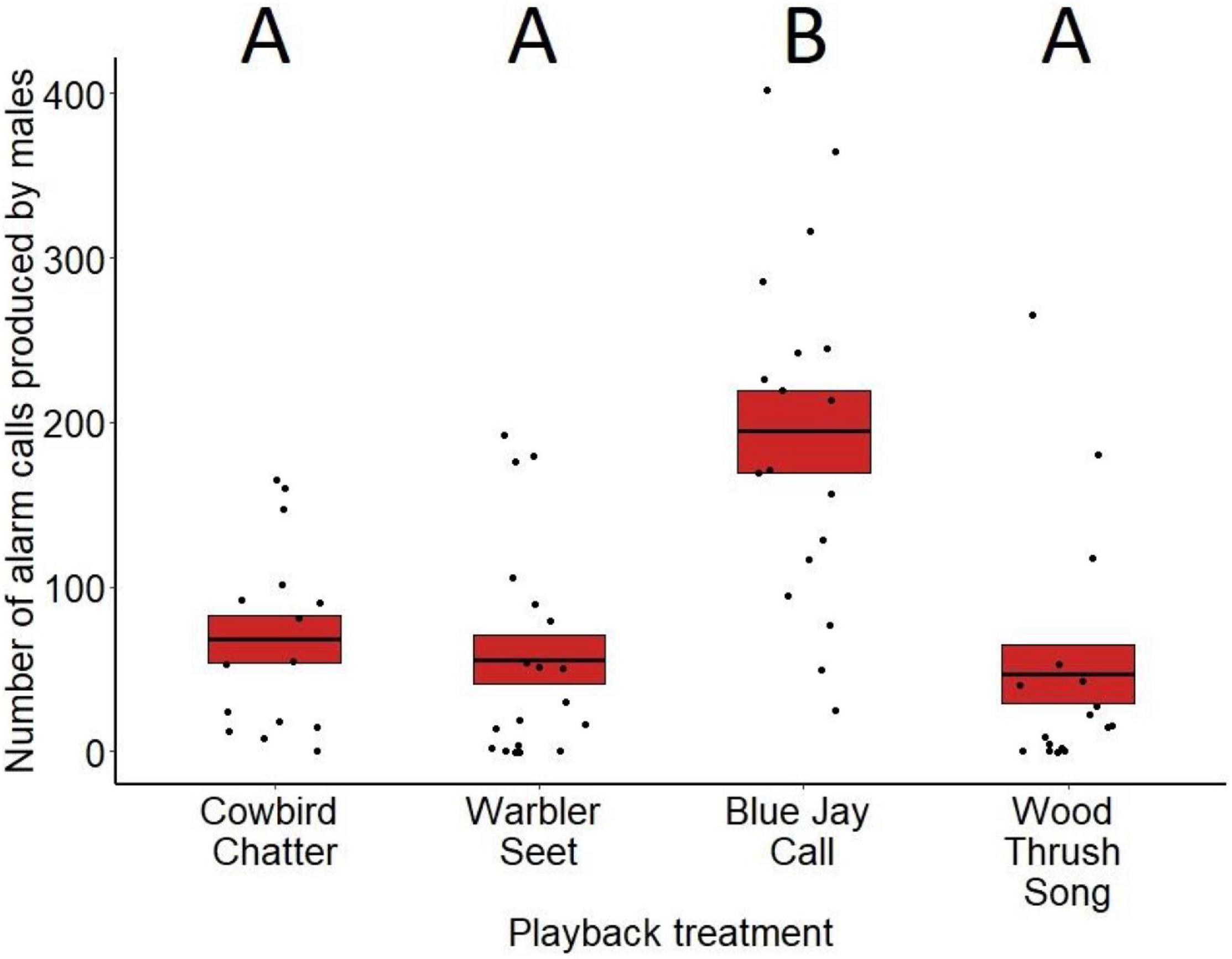
Figure 5. Number of alarm calls produced by male red-winged blackbirds in response to playbacks at nests during nestling stage. Trials where males had an additional nest on territory with eggs are excluded. Means are shown with the bold line, and shaded boxes represent standard errors. Boxes with different letters denote post hoc statistical differences between treatments. For the p-values of post hoc comparisons, please refer to Supplementary Table 3.
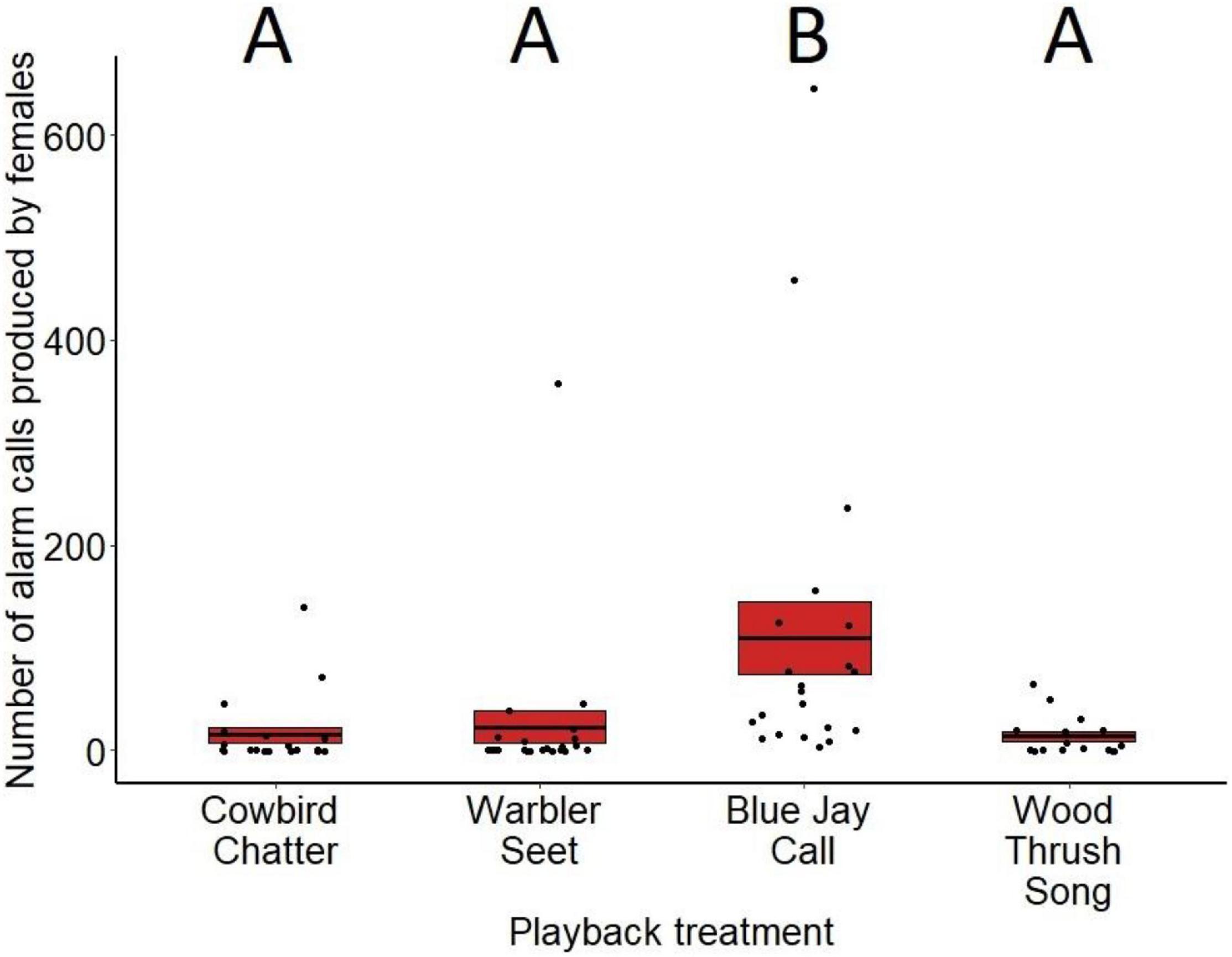
Figure 6. Number of alarm calls produced by female red-winged blackbirds in response to playbacks at nests during nestling stage. Means are shown with the bold line, and shaded boxes represent standard errors. Boxes with different letters denote post hoc statistical differences between treatments. For the p-values of post hoc comparisons, please refer to Supplementary Table 3.
For males, the presence of an additional nest with eggs on their territory significantly affected alarm calling responses (F3,81 = 8.25, p < 0.01, estimate = 0.95). When we ran the same generalized linear model with an interaction term we found a significant interaction between treatment and presence of a nest with eggs on alarm calling rates (treatment × eggs term: F3,64 = 5.01, p < 0.01). Specifically, males alarm called more toward playbacks of cowbird chatters (z ratio = 2.60, p < 0.01) and seet calls (z ratio = 2.63, p < 0.01) when there was an additional nest with eggs on their territory, while alarm calling toward blue jay playbacks was not significantly affected by presence of eggs vs. nestlings only (z ratio = −1.72, p = 0.08) (wood thrush playbacks were not included since no territories with the wood thrush playback had a blackbird nest with eggs). To determine how alarm calling between treatments varied without the influence of nests with eggs, we removed any trials with territories with eggs and reran the original general linear model. Male alarm calling still varied significantly by treatment (F3,81 = 6.57, p < 0.001), with post hoc comparisons showing the same patterns of higher alarm calling toward playbacks of blue jay calls compared to cowbird chatters (z = 2.64, p = 0.04), seet calls (z = 3.40, p < 0.01), and control wood thrush songs (z = 3.71, p = 0.001) as the model with no eggs. In addition, males showed no significant differences in responses between cowbird chatters and seet calls (z = 0.52, p = 0.95), between cowbird chatters and the control (z = 0.93, p = 0.78), and between seet calls and the control (z = 0.43, p = 0.97), similar to males with nestlings only.
Discussion
Our current playback study found support for the hypothesis that both male and female red-winged blackbirds eavesdrop upon and respond to yellow warbler’s seet calls specifically as a referent for “brood parasite” and not as a general nest-threat heterospecific alarm call. On the one hand, the risk of brood parasitism for hosts is highest when nests have eggs, and lower after the eggs hatch; on the other hand, the risk of predation remains high and often increases across nest stages, as the fitness outcome generally is the same – partial or total reproductive failure (Gill and Sealy, 1996; Fasanella and Fernández, 2009; Ruiz et al., 2018). In our own work, blackbirds demonstrated markedly different patterns of response toward brood parasitic vs. predatory threat playbacks depending on the risk posed by them across nest stages. During the nestling stage in the current study, blackbirds of both sexes responded equally and with low aggression toward calls signaling brood parasites (cowbird and seet calls) as to the control wood thrush songs, responding instead most aggressively toward nest-predatory blue jay calls. Contrastingly, when the same playbacks were presented during the incubation stage, blackbirds responded with equally strong aggression toward playbacks of cowbird chatters, seet calls, and blue jay calls (Lawson et al., 2020). Thus, blackbirds mediated in parallel their urgency to respond and aggression toward calls signaling brood parasitic danger, including referential seet calls of yellow warblers, depending on the level of the current threat of parasitism to their nest(s). Blackbirds mediated aggression depending on date in season and the age of their nestlings as well, showing increased aggression as the breeding season progressed and with increased age of their nestlings. Costs of renesting increase as the season progresses and reproductive value of offspring due to increased chance of survival increases (Montgomerie and Weatherhead, 1988; Gill and Sealy, 1996; Fasanella and Fernández, 2009; Ruiz et al., 2018) parents are thus expected to increase aggression toward threats toward their offspring with the greater age of their young. Similar patterns have been observed in other presentation studies with alarm-calling species (Regelmann and Curio, 1983; Montgomerie and Weatherhead, 1988; Campobello and Sealy, 2010; Lawson et al., 2021a,b).
A relevant distinction between male and female blackbirds is that in this polygynous mating system (Searcy and Yasukawa, 1995), males may have multiple nests at once on their territory, some even at different stages of development, while females only actively care for one nest. Males do not incubate eggs and provide limited paternal provisions for nestlings (e.g., Li and Hauber, 2021), but rather perform a sentinel role of protecting the territory from threats (Yasukawa and Searcy, 2020). However, our findings suggest that males actively monitor the progress of all nests within their harem, as those with additional nests with eggs still vulnerable to brood parasitism responded more strongly to cowbird chatters and seet calls compared to males with nests solely at the nestling stage. This statistical effect was not seen in blackbirds with eggs responding to blue jay calls, indicating a specifically anti-parasitic nest defense. This corresponds with previous work that showed male blackbirds alter provisioning rates based on age of nestlings, even after nests were swapped, supporting that male blackbirds actively monitor all the nests within their harem (Yasukawa et al., 1993). Blackbird males are also known to pay attention to social and vocal cues of females on their territories (Yasukawa, 1989), and this may also include cues from females regarding brood parasitism risk, leading to adjustments in the male’s responses to cowbirds and cowbird-signaling calls.
Personal information vs. social information on risk likely affects host responses, as each has different reliability and cost. For example, we found that males approached cowbird and blue jay calls more closely than seet calls. The pattern of male closest approach was similar to that found by blackbirds during the incubation stage (Lawson et al., 2020), as well as to male yellow warblers during the incubation stage as well (Lawson et al., 2021b). Though both cowbird chatters and seet calls indicate brood parasitism risk, cowbird chatters directly indicate cowbird presence, whereas seet calls indirectly do so. Yellow warblers and blackbirds alike appear to more closely approach playbacks that directly signal threats (cowbird and blue jay calls) compared to social information of risk (seet calls) as acoustic presentations alone provide no visual target for responding subjects to direct physical aggression toward. Campobello and Sealy (2011a) found similar patterns in responses of yellow warblers presented with personal (cowbird model on nest, nest parasitized) or social information (conspecifics mobbing cowbird) on brood parasitism risk, where warblers responded more strongly to individually learned information. Conversely, reed warblers (Acrocephalus scirpaceus) in a similar experiment showed preference for social information on brood parasitism risk by common cuckoos compared to personal information (Campobello and Sealy, 2011b). Therefore, cost of acquiring personal information may also affect reliance on and responses to it, as common cuckoo nestlings eject all host eggs/nestlings from the nest unlike cowbirds (Campobello and Sealy, 2011b). Treatments did not influence female closest approach because females spent most of the time alarm calling near or on the nest during playbacks, resulting in an average of ∼5 m approaches across treatments, as this was the distance the speaker was placed from the nest.
Our combined set of blackbird playback studies brings to light new questions in the blackbird-warbler eavesdropping system that should be addressed in future studies. Yellow warblers nesting in close proximity to blackbirds experience lower rates of parasitism (Clark and Robertson, 1979), due to the blackbirds’ aggressive frontline defenses toward cowbirds near their territories. Do blackbirds that nest near yellow warblers themselves experience a decrease in brood parasitism rate as well? Blackbirds that nest closer to yellow warblers show increased alarm calling responses to chatters and seet calls (Lawson et al., 2020), suggesting a “neighborhood watch effect” where blackbirds that have access to the yellow warblers’ referential system are more primed to respond to their cowbird-signaling calls. Thus, the relationship between yellow warblers and blackbirds appears mutualistic, yet it is unknown whether blackbirds experience a similar decrease in parasitism of their nests when in proximity to yellow warblers. Our study also encourages future research into how blackbird males and many other host species mechanistically make the switch in behavior toward cowbirds as their nests transition from eggs to chicks. Yellow warblers of both sexes also demonstrate a shift in response toward cowbirds from incubation to nestling stage, but warblers of both sexes also interact with and care for the young, unlike most blackbird males at most nests (Li and Hauber, 2021). The mechanism underlying these shifts in behavior is unknown for either species, although endocrine factors, particularly testosterone and prolactin, play a strong role in parental (including paternal) care and different nest-attentive behaviors across the breeding stages in birds (Wingfield et al., 1990; Schoech et al., 1998; Van Roo et al., 2003; Ketterson et al., 2005; Møller et al., 2005; O’Neal et al., 2008).
Our set of playbacks conducted across nesting stages has led to firm support for heterospecific eavesdropping on a referential call signaling the presence of obligate brood parasites (also see Yu et al., 2019). Blackbirds appear to perceive the seet call as a warning specifically for brood parasitic danger, priming them for defensive responses to actual cowbirds. Moreover, blackbirds respond to warbler seets and cowbird chatters based on current risk of brood parasitism to their nests. Future research is needed to measure parasitism rates and fitness benefits of blackbirds nesting near yellow warblers; our study suggests that red-winged blackbirds may have a communicative and possibly mutualistic relationship with the warblers, whereby warblers provide the early warning system for cowbirds, and blackbirds keep cowbirds away from nearby nests.
Heterospecific eavesdropping on alarm calls signaling threats to fitness are seen across diverse taxa, including networks of co-existing species (e.g., tropical mixed-species bird flocks: Martínez et al., 2021). Eavesdropping in multi-species networks could improve threat detection in many biologically meaningful contexts (see Magrath et al., 2015 for review), including foraging (e.g., Batcheller, 2017), habitat selection (e.g., Mönkkönen and Forsman, 2002), and offspring defense (this study). It still remains to be seen, however, whether the symmetrical (whereby each interacting species recognizes the other’s referential alarm call; Walton and Kershenbaum, 2019) or asymmetrical (whereby only one actor recognizes the other’s call; this study) systems are more likely to evolve and be maintained by mutualistic selective forces. Both theoretical modeling and more empirical and meta-analytic work may be able to resolve these broader scale questions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Illinois Institutional Animal Care and Use Committee (IACUC) protocol #18040.
Author Contributions
SL and MH conceived the study and drafted the manuscript. SL, SG, and MH participated in the design of the study and obtained funding. SL and JE carried out the field work and playbacks. SL conducted the data analysis. All authors critically edited and revised the manuscript, gave final approval for publication, and agreed to be held accountable for the work performed therein.
Funding
This project was supported by the American Ornithological Society (to SL) the National Geographic Society (to MH, NGS-60453R-19), the United States National Science Foundation (IOS #1952726 to SG and IOS #1953226 to MH), and by the Harley Jones Van Cleave Professorship (to MH) and the School of Integrative Biology (to SL, Clark Research Support Grant, Lebus Fund Award, Dissertation Travel Grant) at the University of Illinois Urbana-Champaign. MH was also supported by the Wissenschaftskolleg zu Berlin, Germany.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KY declared a past collaboration with one of the authors MH to the handling editor.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Champaign, and Vermilion counties in Illinois for permitting us to use their parks for our research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.706170/full#supplementary-material
References
Batcheller, H. J. (2017). Interspecific information use by army-ant–following birds. Auk 134, 247–255. doi: 10.1642/AUK-16-93.1
Beletsky, L. D., Higgins, B. J., and Orians, G. H. (1986). Behavioral Ecology and Sociobiology Communication by changing signals: call switching in red-winged blackbirds. Behav. Ecol. Sociobiol. 18, 221–229. doi: 10.1007/BF00290826
Bradbury, J. W., and Vehrencamp, S. L. (2011). Principles Of Animal Communication, 2nd Edn. Sunderland, MA: Sinauer.
Campobello, D., and Sealy, S. G. (2010). Enemy recognition of reed warblers (Acrocephalus scirpaceus): threats and reproductive value act independently in nest defence modulation. Ethology 116, 498–508. doi: 10.1111/j.1439-0310.2010.01764.x
Campobello, D., and Sealy, S. G. (2011a). Nest defence against avian brood parasites is promoted by egg-removal events in a cowbird–host system. Anim. Behav. 82, 885–891. doi: 10.1016/j.anbehav.2011.07.028
Campobello, D., and Sealy, S. G. (2011b). Use of social over personal information enhances nest defense against avian brood parasitism. Behav. Ecol. 22, 422–428. doi: 10.1093/beheco/arq225
Clark, K. L., and Robertson, R. J. (1979). Spatial and temporal multi-species nesting aggregations in birds as anti-parasite and anti-predator defenses. Behav. Ecol. Sociobiol. 5, 359–371. doi: 10.1007/BF00292524
Cruz, A. (1999). Aggressive responses of red-winged blackbirds (Agelaius phoeniceus) Toward brown-headed cowbirds (Molothrus ater) in areas of recent and long-term sympatry. Bird Behav. 13, 1–7. doi: 10.3727/096020199389662
Davies, N. B., Madden, J. R., Butchart, S. H. M., and Rutila, J. (2006). A host-race of the cuckoo Cuculus canorus with nestlings attuned to the parental alarm calls of the host species. Proc. R. Soc. B Biol. Sci. 273, 693–699.
Evans, C. S. (1997). Referential Signals: Communication. Boston, MA: Springer, 99–143. doi: 10.1007/978-1-4899-1745-4_5
Evans, C. S., Evans, L., and Marler, P. (1993). On the meaning of alarm calls: functional reference in an avian vocal system. Anim. Behav. 46, 23–38. doi: 10.1006/anbe.1993.1158
Fasanella, M., and Fernández, G. J. (2009). Alarm calls of the Southern House Wren Troglodytes musculus: variation with nesting stage and predator model. J. Ornithol. 150, 853–863. doi: 10.1007/s10336-009-0406-2
Feeney, W. E., and Langmore, N. E. (2015). Superb Fairy-wrens (Malurus cyaneus) increase vigilance near their nest with the perceived risk of brood parasitism. Auk 132, 359–364. doi: 10.1642/AUK-14-218.1
Feeney, W. E., Welbergen, J. A., and Langmore, N. E. (2012). The frontline of avian brood parasite-host coevolution. Anim. Behav. 84, 3–12. doi: 10.1016/j.anbehav.2012.04.011
Gill, S. A., and Bierema, A. M. K. (2013). On the meaning of alarm calls: a review of functional reference in avian alarm calling. Ethology 119, 449–461. doi: 10.1111/eth.12097
Gill, S. A., and Sealy, S. G. (1996). Nest defence by yellow warblers: recognition of a brood parasite and an avian nest predator. Behaviour 133, 263–282. doi: 10.1163/156853996X00143
Gill, S. A., and Sealy, S. G. (2003). Tests of two functions of alarm calls given by yellow warblers during nest defence. Can. J. Zool. 81, 1685–1690. doi: 10.1139/z03-162
Gill, S. A., and Sealy, S. G. (2004). Functional reference in an alarm signal given during nest defence: seet calls of yellow warblers denote brood-parasitic brown-headed cowbirds. Behav. Ecol. Sociobiol. 56, 71–80. doi: 10.1007/s00265-003-0736-7
Gill, S. A., Neudorf, D. L. H., and Sealy, S. G. (1997). Host responses to cowbirds near the nest: cues for recognition. Anim. Behav. 53, 1287–1293. doi: 10.1006/anbe.1996.0362
Gill, S. A., Neudorf, D. L. H., and Sealy, S. G. (2008). Do hosts discriminate between sexually dichromatic male and female brown-headed cowbirds? Ethology 114, 548–556. doi: 10.1111/j.1439-0310.2008.01501.x
Haff, T. M., and Magrath, R. D. (2012). Learning to listen? Nestling response to heterospecific alarm calls. Anim. Behav. 84, 1401–1410. doi: 10.1016/j.anbehav.2012.09.005
Hauber, M. E. (2014). Mafia or Farmer? Coevolutionary consequences of retaliation and farming as predatory strategies upon host nests by avian brood parasites. Coevolution 2, 18–25.
Henger, C. S., and Hauber, M. E. (2014). Variation in antiparasitic behaviors of Red-winged Blackbirds in response to simulated Brown-headed Cowbirds. Wilson J. Ornithol. 126, 488–499. doi: 10.1676/13-193.1
Kelly, J. K., Suckow, N. M., and Ward, M. P. (2019). Preferential settling at sites with higher conspecific density does not protect Yellow Warblers (Setophaga petechia) from brood parasitism. Oecologica 96, 24–28. doi: 10.1016/j.actao.2019.03.003
Ketterson, E. D., Nolan, V., and Sandell, M. (2005). Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 166, 85–98. doi: 10.1086/444602
Kilner, R. M., and Langmore, N. E. (2011). Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852. doi: 10.1111/j.1469-185X.2010.00173.x
Knight, R. L., and Temple, S. A. (1988). Nest-defense behavior in the red-winged blackbird. Condor 90, 193–200. doi: 10.2307/1368448
Kroodsma, D. E., Byers, B. E., Goodale, E., Johnson, S., and Liu, W. C. (2001). Pseudoreplication in playback experiments, revisited a decade later. Anim. Behav. 61, 1029–1033. doi: 10.1006/anbe.2000.1676
Lawson, S. L., Enos, J. K., Mendes, N. C., Gill, S. A., and Hauber, M. E. (2021b). Pairing status moderates both the production of and responses to anti-parasitic referential alarm calls in male yellow warblers. Ethology 127, 385–394. doi: 10.1111/eth.13139
Lawson, S. L., Enos, J. K., Mendes, N. C., Gill, S. A., and Hauber, M. E. (2020). Heterospecific eavesdropping on an anti-parasitic referential alarm call. Commun. Biol. 3, 1–8. doi: 10.1038/s42003-020-0875-7
Lawson, S. L., Enos, J. K., Antonson, N. D., Gill, S. A., and Hauber, M. E. (2021a). Do hosts of avian brood parasites discriminate parasitic vs. predatory threats? A meta-analysis. Adv. Study Behav. 53, 63–94. doi: 10.1016/bs.asb.2021.03.002
Lawson, S. L., Enos, J. K., Mendes, N. C., Gill, S. A., and Hauber, M. E. (2021c). Responses of female yellow warblers to playbacks signaling brood parasitism or predation risk: a quasi-replication study. Anim. Behav. Cogn. 8, 216–230. doi: 10.26451/abc.08.02.08.2021
Li, D., and Hauber, M. E. (2021). Parasitic begging calls of nestmate-evictor common cuckoos stimulate more parental provisions by red-winged blackbirds than calls of nest-sharing brown-headed cowbirds. Behav. Ecol. Sociobiol. 75, 1–9. doi: 10.1007/s00265-020-02955-5
Magrath, R. D., Haff, T. M., and Igic, B. (2020). “Interspecific communication: gaining information from heterospecific alarm calls,” in Coding Strategies in Vertebrate Acoustic Communication, eds T. Aubin and N. Mathevon (Cham: Springer International Publishing), 287–314. doi: 10.1007/978-3-030-39200-0_12
Magrath, R. D., Haff, T. M., Fallow, P. M., and Radford, A. N. (2015). Eavesdropping on heterospecific alarm calls: from mechanisms to consequences. Biol. Rev. 90, 560–586. doi: 10.1111/brv.12122
Martínez, A. E., Parra, E., Gomez, J. P., and Vredenburg, V. T. (2021). Shared predators between primate groups and mixed species bird flocks: the potential for forest-wide eavesdropping networks. Oikos 1–17. doi: 10.1111/oik.08274
Merrill, L., Chiavacci, S. J., Paitz, R. T., and Benson, T. J. (2017). Rates of parasitism, but not allocation of egg resources, vary among and within hosts of a generalist avian brood parasite. Oecologia 184, 399–410. doi: 10.1007/s00442-017-3870-z
Møller, A. P., Garamszegi, L. Z., Gil, D., Hurtrez-Boussès, S., and Eens, M. (2005). Correlated evolution of male and female testosterone profiles in birds and its consequences. Behav. Ecol. Sociobiol. 58, 534–544. doi: 10.1007/s00265-005-0962-2
Mönkkönen, M., and Forsman, J. T. (2002). Heterospecific attraction among forest birds: a review. Ornithol. Sci. 1, 41–51. doi: 10.2326/osj.1.41
Montgomerie, R. D., and Weatherhead, P. J. (1988). Risks and rewards of nest defence by parent birds. Q. Rev. Biol. 63, 167–187. doi: 10.1086/415838
Neudorf, D. L. H., and Sealy, S. G. (1992). Reactions of four passerine species to threats of predation and cowbird parasitism: enemy recognition or generalized responses? Behaviour 123, 84–105. doi: 10.1163/156853992X00138
O’Neal, D. M., Reichard, D. G., Pavilis, K., and Ketterson, E. D. (2008). Experimentally-elevated testosterone, female parental care, and reproductive success in a songbird, the Dark-eyed Junco (Junco hyemalis). Horm. Behav. 54, 571–578. doi: 10.1016/j.yhbeh.2008.05.017
Oda, R., and Masataka, N. (1996). Interspecific responses of ringtailed lemurs to playback of antipredator alarm calls given by verreaux’s sifakas. Ethology 102, 441–453. doi: 10.1111/j.1439-0310.1996.tb01138.x
Ortega, C. P., and Cruz, A. (1988). Mechanisms of egg acceptance by marsh-dwelling blackbirds. Condor 90, 349–358. doi: 10.2307/1368563
Platzen, D., and Magrath, R. D. (2005). Adaptive differences in response to two types of parental alarm call in altricial nestlings. Proc. R. Soc. B. 272, 1101–1106. doi: 10.1098/rspb.2005.3055
Rainey, H. J., Zuberbühler, K., and Slater, P. J. B. (2004a). Hornbills can distinguish between primate alarm calls. Proc. R. Soc. B. 271, 755–759. doi: 10.1098/rspb.2003.2619
Rainey, H. J., Zuberbühler, K., and Slater, P. J. B. (2004b). The responses of black-casqued hornbills to predator vocalisations and primate alarm calls. Behaviour 141, 1263–1277. doi: 10.1163/1568539042729658
Regelmann, K., and Curio, E. (1983). Determinants of brood defence in the great tit Parus major L. Behav. Ecol. Sociobiol. 13, 131–145. doi: 10.1007/BF00293803
Robertson, R. J., and Norman, R. F. (1976). Behavioral defenses to brood parasitism by potential hosts of the brown-headed cowbird. Condor 78, 166–173. doi: 10.2307/1366851
Robertson, R. J., and Norman, R. F. (1977). The function and evolution of aggressive host behavior towards the brown-headed cowbird (Molothrus ater). Can. J. Zool. 55, 508–518. doi: 10.1139/z77-066
Rodewald, P. (2015). The Birds of North America. Ithaca, NY: Laboratory of Ornithology. Available online at: https://birdsna.org.Cornell
Rothstein, S. I., and Robinson, S. K. (1998). Parasitic Birds And Their Hosts. Oxford: Oxford University Press.
Ruiz, N. M. D., Fasanella, M., and Ferna´ndez, G. J. (2018). Breeding southern house wrens exhibit a threat-sensitive response when exposed to different predator models. J. Ethol. 36, 43–53. doi: 10.1007/s10164-017-0528-6
Schoech, S. J., Ketterson, E. D., Nolan, V., Sharp, P. J., and Buntin, J. D. (1998). The effect of exogenous testosterone on parental behavior, plasma prolactin, and prolactin binding sites in dark-eyed juncos. Horm. Behav. 34, 1–10. doi: 10.1006/hbeh.1998.1455
Sealy, S., Neudorf, D., Hobson, K., and Gill, S. (1998). Nest defense by potential hosts of the Brown-headed Cowbird: methodological approaches, benefits of defense, and coevolution. Oxford Ornithol. Ser. 9, 194–211.
Searcy, W. A., and Yasukawa, K. (1995). Polygyny and Sexual Selection in Red-Winged Blackbirds. Princeton, NJ: Princeton University Press. doi: 10.1515/9781400863938
Shaffer, J., and Goldade, C. (2003). Brown-headed Cowbirds in grasslands: their habitats, hosts, and response to management. USGS North. Prairie Wildl. Res. Cent. 150, 1–40.
Sherman, P. W. (1977). Nepotism and the evolution of alarm calls. Science 197, 1246–1253. doi: 10.1126/science.197.4310.1246
Smith, K. G., Tarvin, K. A., and Woolfenden, G. E. (2020). “Blue jay (Cyanocitta cristata), version 1.0,” in Birds of the World, ed. A. F. Poole (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bow.blujay.01
Soler, M., Pérez-Contreras, T., and Soler, J. J. (2017). “Brood parasites as predators: farming and mafia strategies,” in Avian Brood Parasitism. Fascinating Life Sciences, ed. M. Soler (Cham: Springer). doi: 10.1007/978-3-319-73138-4
Strausberger, B. M. (2001). The relationship of habitat and spatial distribution of nests with brown-headed cowbird parasitism of red-winged blackbirds. Wilson Bull. 113, 129–133. doi: 10.1676/0043-5643(2001)113[0129:TROHAS]2.0.CO;2
Strausberger, B. M., and Horning, M. E. (1997). Responses of nesting song sparrows (Melospiza melodia) and red-winged blackbirds (Agelaius phoeniceus) to models of parasitic cowbirds and nonthreatening towhees. Bird Behav. 12, 71–78. doi: 10.3727/015613897797141038
Suzuki, T. N. (2012). Referential mobbing calls elicit different predator-searching behaviours in Japanese great tits. Anim. Behav. 84, 53–57. doi: 10.1016/j.anbehav.2012.03.030
Suzuki, T. N. (2015). Assessment of predation risk through referential communication in incubating birds. Sci. Rep. 5, 1–6. doi: 10.1038/srep10239
Van Roo, B. L., Ketterson, E. D., and Sharp, P. J. (2003). Testosterone and prolactin in two songbirds that differ in paternal care: the blue-headed vireo and the red-eyed vireo. Horm. Behav. 44, 435–441. doi: 10.1016/j.yhbeh.2003.07.001
Walton, B., and Kershenbaum, A. (2019). Heterospecific recognition of referential alarm calls in two species of lemur. Bioacoustics 28, 592–603. doi: 10.1080/09524622.2018.1509375
Welbergen, J. A., and Davies, N. B. (2009). Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240. doi: 10.1016/j.cub.2008.12.041
Wingfield, J. C., Hegner, R. E., Dufty, A. M., and Ball, G. F. (1990). The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846. doi: 10.1086/285134
Winkler, D. W., Billerman, S. M., and Lovette, I. J. (2020). “Hawks, eagles, and kites (Accipitridae),” in Birds of the World, eds S. M. Billerman, B. K. Keeney, P. G. Rodewald, and T. S. Schulenberg (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bow.accipi1.01
Yasukawa, K. (1989). The costs and benefits of a vocal signal: the nest-associated ‘Chit’of the female red-winged blackbird, Agelaius phoeniceus. Anim. Behav. 38, 866–874. doi: 10.1016/S0003-3472(89)80118-6
Yasukawa, K., and Searcy, W. A. (2020). “Red-winged blackbird (Agelaius phoeniceus), version 1.0,” in Birds of the World, ed. P. G. Rodewald (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bow.rewbla.01
Yasukawa, K., Leanza, F., and King, C. D. (1993). An observational and brood-exchange sudy of paternal provisioning in the red-winged blackbird, Agelaius phoeniceus. Behav. Ecol. 4, 78–82. doi: 10.1093/beheco/4.1.78
Yasukawa, K., Lindsey-Robbins, J., Henger, C. S., and Hauber, M. E. (2016). Antiparasitic behaviors of red-winged blackbirds (Agelaius phoeniceus) in response to simulated brown-headed cowbirds (Molothrus ater): further tests of the frontloaded parasite-defense hypothesis. Wilson J. Ornithol. 128, 475–486. doi: 10.1676/1559-4491-128.3.475
Yu, J., Lu, H., Sun, W., Liang, W., Wang, H., and Møller, A. P. (2019). Heterospecific alarm-call recognition in two warbler hosts of common cuckoos. Anim. Cogn. 22, 1149–1157. doi: 10.1007/s10071-019-01307-9
Yu, J., Xing, X., Jiang, Y., Liang, W., Wang, H., and Møller, A. P. (2017). Alarm call-based discrimination between common cuckoo and Eurasian sparrowhawk in a Chinese population of great tits. Ethology 123, 542–550. doi: 10.1111/eth.12624
Keywords: brood parasitism, host-parasite interactions, heterospecific eavesdropping, playback presentations, referential alarm calling
Citation: Lawson SL, Enos JK, Gill SA and Hauber ME (2021) Eavesdropping on Referential Yellow Warbler Alarm Calls by Red-Winged Blackbirds Is Mediated by Brood Parasitism Risk. Front. Ecol. Evol. 9:706170. doi: 10.3389/fevo.2021.706170
Received: 06 May 2021; Accepted: 09 September 2021;
Published: 05 October 2021.
Edited by:
James Rivers, Oregon State University, United StatesReviewed by:
Ken Yasukawa, Beloit College, United StatesMichael L. Morrison, Texas A&M University, United States
Copyright © 2021 Lawson, Enos, Gill and Hauber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shelby L. Lawson, c2xhd3NvbjNAaWxsaW5vaXMuZWR1
 Shelby L. Lawson
Shelby L. Lawson Janice K. Enos
Janice K. Enos Sharon A. Gill
Sharon A. Gill Mark E. Hauber
Mark E. Hauber