Abstract
Arctic breeding songbirds migrate early in the spring and can face winter environments requiring cold endurance throughout their journey. One such species, the snow bunting (Plectrophenax nivalis), is known for its significant thermogenic capacity. Empirical studies suggest that buntings can indeed maintain winter cold acclimatization into the migratory and breeding phenotypes when kept captive on their wintering grounds. This capacity could be advantageous not only for migrating in a cold environment, but also for facing unpredictable Arctic weather on arrival and during preparation for breeding. However, migration also typically leads to declines in the sizes of several body components linked to metabolic performance. As such, buntings could also experience some loss of cold endurance as they migrate. Here, we aimed to determine whether free-living snow buntings maintain a cold acclimatized phenotype during spring migration. Using a multi-year dataset, we compared body composition (body mass, fat stores, and pectoralis muscle thickness), oxygen carrying capacity (hematocrit) and metabolic performance (thermogenic capacity – Msum and maintenance energy expenditure – BMR) of birds captured on their wintering grounds (January–February, Rimouski, QC, 48°N) and during pre-breeding (April–May) in the Arctic (Alert, NU, 82°). Our results show that body mass, fat stores and Msum were similar between the two stages, while hematocrit and pectoralis muscle thickness were lower in pre-breeding birds than in wintering individuals. These results suggest that although tissue degradation during migration may affect flight muscle size, buntings are able to maintain cold endurance (i.e., Msum) up to their Arctic breeding grounds. However, BMR was higher during pre-breeding than during winter, suggesting higher maintenance costs in the Arctic.
Introduction
Arctic breeding birds typically migrate early in the spring to maximize the chances of successful reproduction during a relatively short summer (Love et al., 2010; Reneerkens et al., 2016; van Gils et al., 2016; Ramenofsky and Wingfield, 2017; Rakhimberdiev et al., 2018). For these species, early arrival allows for securing the best breeding territories and partners, and for breeding as soon as conditions become suitable (Drent et al., 2003; Ramenofsky and Wingfield, 2006), which can improve reproductive success (Lepage et al., 2000; Guindre-Parker et al., 2013). However, such early arrival implies migrating through cold winter conditions and, upon arrival on Arctic breeding grounds, birds are often faced with substantial snow accumulation, sub-zero temperatures and unpredictable weather that can persist for several weeks (e.g., Meltofte, 1983; Walsh et al., 2005; see also Morrison et al., 2007; Wingfield et al., 2011).
For species that overwinter in cold environments (e.g., temperate zones), these arrival conditions may be comparable or even more constraining than those encountered during winter (Meltofte, 1983; Wingfield et al., 2011; Snell et al., 2018). However, the physiological mechanisms involved in acclimatization to cold and to long-distance migration share similar physiology (Dawson et al., 1983; Zhang et al., 2015). For example, heart mass, blood oxygen-carrying capacity (hematocrit), and flight muscle size increase during cold acclimatization (Swanson, 1990b; O’Connor, 1995, 1996; Cooper, 2002; Zheng et al., 2008; Liknes and Swanson, 2011; Petit et al., 2013, 2014; Swanson and Vézina, 2015), and are thought to improve shivering heat production (Swanson, 1990a; Petit and Vézina, 2014). Similar changes also occur in preparation for migration (Battley and Piersma, 1997; Piersma et al., 1999; Krause et al., 2016) to support active flight (Battley and Piersma, 1997; Piersma et al., 1999). Therefore, birds migrating early in the spring could profit from a carryover of their winter phenotype to benefit migration in the cold.
Recently, Le Pogam et al. (2021) observed that snow buntings (Plectrophenax nivalis), Arctic breeding songbirds known for their cold endurance (Scholander et al., 1950; Le Pogam et al., 2020), were able to maintain a winter phenotype through most of summer when kept in outdoor aviaries at their wintering latitude (48°N), at a time when air temperatures can exceed 25°C. More precisely, the birds were found to maintain thermogenic capacity and cold endurance comparable to those from the peak of winter not only throughout migratory fattening, but also during the period corresponding to migration and Arctic breeding. Le Pogam et al. (2021) concluded that the winter phenotype in snow buntings transitioning into spring migration provided enough thermogenic capacity to migrate in cold, winter-like environments and to support thermoregulatory needs upon arrival on their breeding grounds. However, that study was based on captive individuals that could not migrate, and migration also has considerable physiological effects, such as the loss of body mass as well as declines in oxygen carrying capacity and muscle mass, which together could both potentially lead to a reduction in thermogenic capacity (O’Connor, 1995; Cooper, 2002; Jenni et al., 2006; Dubois et al., 2016). Thus, whether this cold specialist maintains winter-level cold endurance through spring migration remains to be tested.
The objective of this study was to compare peak of winter and pre-breeding phenotypes in free-living snow buntings to determine whether these birds show comparable metabolic performance and supporting traits at those stages, which would lend strength to the hypothesis that buntings maintain cold endurance throughout their spring migration (as proposed by Le Pogam et al., 2021). To test this hypothesis we compared phenotypic traits related to thermogenic capacity (Table 1) in buntings captured around Rimouski, Québec, Canada (48°N) during the peak of winter (i.e., January and February) to those of individuals captured in the weeks following arrival (i.e., May and early June) on breeding grounds at Alert, NU, Canada (82°N). Based on our previous observations (Le Pogam et al., 2021), we expected buntings would maintain a winter-type phenotype (Table 1) throughout both migration and pre-breeding. Birds would thus be expected to have comparable metabolic performance during winter in Québec and early spring in the Arctic. In contrast, if migration leads to body transformations that negatively impact metabolic performance at subsequent stages, we would instead expect to find lighter birds in the spring showing lower performance levels compared to individuals measured during winter.
TABLE 1
| Metabolic performance | |||
| Phenotypic traits | Interpreted as | Response to cold/winter | Pertinent references |
| Summit metabolic rate (Msum) | Maximum shivering thermogenic capacity, index of cold endurance | Typically higher | McKechnie and Swanson, 2010; Swanson, 2010; Petit et al., 2013; McKechnie et al., 2015; Le Pogam et al., 2020 |
| Basal metabolic rate (BMR) | Maintenance energy expenditure, index of physiological maintenance costs | Often higher but not in snow buntings | McKechnie and Swanson, 2010; Swanson, 2010; Petit et al., 2013; McKechnie et al., 2015 but see Le Pogam et al., 2020 |
| Phenotypic traits underlying metabolic performance | |||
| Phenotypic traits | Interpreted as | Response to cold/winter | Pertinent references |
| Body mass | Total body composition | Typically higher | Carey et al., 1978; Liknes and Swanson, 1996; Zheng et al., 2008; Petit et al., 2014; Le Pogam et al., 2020 |
| Fat store | Energy reserves | Typically higher | Blem, 1976; Lehikoinen, 1987; Gosler, 1996; Cooper, 2007; Le Pogam et al., 2020 |
| Hematocrit | Blood oxygen carrying capacity | Typically higher | Swanson, 1990b; O’Connor, 1996; Le Pogam et al., 2020 |
| Pectoralis muscle thickness | Shivering capacity | Typically increases with high metabolic rate | O’Connor, 1995; Cooper, 2002; Swanson and Merkord, 2012; Petit et al., 2013; Swanson and Vézina, 2015; Le Pogam et al., 2020 |
List of phenotypic traits measured in this study and their responses to cold and winter in passerine birds.
Materials and Methods
Study Species
Snow buntings are an Arctic-breeding, cold-associated migratory passerine. In the spring, these birds migrate through cold winter landscapes (Macdonald et al., 2012; McKinnon et al., 2016; Snell et al., 2018), with males arriving on the breeding grounds to secure territories (up to 83.6°N) up to a month before females (March–April, Cramp and Perrins, 1994; McKinnon et al., 2016; Snell et al., 2018; Montgomerie and Lyon, 2020). Arrival conditions can be comparable or even harsher than those experienced at the peak of winter, with extensive snow cover and air temperatures (Ta) reaching −30°C (Meltofte, 1983). After arrival, buntings can maintain winter-like behavior for several weeks before dispersing to defend breeding territories (Tinbergen, 1939; Meltofte, 1983). Although the wintering range of birds breeding at Alert has yet to be formally established as no banded individuals have been recovered so far, banding data analyzed by Meltofte (1983; Figure 1) and Macdonald et al. (2012) suggest that Alert birds could be wintering in either North America or in the Siberian steppes. Regardless, in both cases wintering conditions are similar to that experienced by birds in Eastern Québec. For example, Snell et al. (2018) tracked snow buntings breeding at Svalbard and reported a mean temperature on their wintering range (i.e., Siberian steppes) of −10.9°C to −3.6°C, which encompasses the mean temperature on the wintering ground for this study (−8.9°C in February, Table 2).
TABLE 2
| Ambient air temperature (°C) | |||
| Min | Mean | Max | |
| Winter (Rimouski, QC) | |||
| January | −12.5 ± 5.7 | −8.6 ± 5.7 | −4.0 ± 6.6 |
| February | −13.2 ± 6.6 | −8.9 ± 6.4 | −4.3 ± 6.8 |
| Spring (Alert, NU) | |||
| April | −26.3 ± 4.7 | −23.6 ± 4.6 | −20.9 ± 5.0 |
| May | −12.6 ± 4.8 | −10.7 ± 4.6 | −8.8 ± 4.7 |
Air temperatures recorded at the peak of winter at Rimouski (QC) averaged from 2015 to 2018 and in the spring at Alert (NU) averaged from 2016 to 2019.
Ethics Statement
All bird handling at Rimouski (QC) and at Alert (NU) was approved by the Animal Care Committee of the Université du Québec à Rimouski (Rimouski: CPA-54-13-130 and CPA-71-17-195 and Alert: CPA-61-15-163 and CPA-71-17-194) and was conducted under banding (10889E) and scientific (SC-48, NUN-SCI-15-05) permits from Environment and Climate Change Canada, and under scientific permits from the Department of Environment of Nunavut (WL 2016-006, 2017-021, 2018-010, 2019-002).
Study Sites, Capture, and Measurements Protocol
On the Breeding Grounds
Snow buntings were studied in the Arctic during the springs of 2016–2019 at Alert, NU, Canada (82°29′58″N, 62°28′5″W). Specifically, we studied birds from their arrival to their dispersal onto breeding territories (hereafter pre-breeding, n = 213 males and 53 females, see Supplementary Table 1 for detailed sample sizes per variable). Although single individuals or small groups (i.e., 2–4 birds) can be observed earlier, the bulk of arrivals at Alert occur in the last week of May (AL, FV, pers. obs.).
Birds were caught with homemade walk-in traps or potter traps (Third Wheel, Devon, England) baited with commercial seed-mix (crushed corn, wheat, sorghum, white millet, red millet, and black sunflower, Armstrong, Hagersville, ON, Canada).
Immediately after capture, a blood sample (<1% of body mass) was taken from the brachial vein. Blood samples were temporarily kept in cold storage and later centrifuged for 10 min at 8,000 RPM to obtain data on hematocrit (i.e., packed red blood cell volume). The birds were then weighed (±0.01 g) and sexed according to Smith (1992). We banded birds with a USGS numbered metal band as well as a unique combination of three darvic color bands to allow for individual identification from a distance. Right wing length was measured as an index of structural body size. The size of fat stores was also estimated visually using a standard fat score (from 0 = no visible fat in furculum area to 6 = fat overlapping pectoralis muscles according to Canadian Snow Bunting Network guidelines, Love et al., 2012). The birds were then brought into our field laboratory (less than 6 km distance from capture site, transport time <20 min) where we estimated pectoralis muscle thickness non-invasively by ultrasonography (Dietz et al., 1999; Royer-Boutin et al., 2015; Le Pogam et al., 2020, 2021) using a LOGIQ e ultrasound scanner fitted with a linear probe (12 MHz, GE Healthcare, Wauwatosa, WI, United States). Since the supracoracoideus muscle is very thin at the measured location, muscle thickness values essentially reflect thickness of the pectoralis muscle. Birds were then held in cages (76 cm W × 46 cm D × 45 cm H) with ad libitum water and seed (same mix as for captures) until metabolic performance measurements were complete (see below).
On the Wintering Grounds
Each winter between January and February from 2015 to 2018, wintering buntings were captured around Rimouski, QC, Canada (48°27′N, 68°30′W) as part of a snow bunting banding program. Snow buntings have a differential migration (Macdonald et al., 2016; McKinnon et al., 2019) and the wintering population around Rimouski is composed in very large proportion of males (total captures males = 508, females = 31, see Supplementary Table 1 for detailed sample sizes per variable). Birds were captured using walk-in traps baited with crushed corn. Upon capture, birds were subjected to the same measurement sequence as described above except that they were banded with only a USGS numbered metal band. Blood samples were taken on 51 individuals and later centrifuged for 10 min at 8,000 RPM (UNICO PowerSpin BX Centrifuge C886, Dayton, NJ, United States) to obtain hematocrit data. In total, 57 birds were transported to the avian facilities at the Université du Québec à Rimouski (less than 25 km distance from the capture site, transport time <25 min) for pectoralis muscle thickness and metabolic performance measurements. Birds were held in indoor cages (117 cm W × 310 cm D × 39 cm H) with access to ad libitum food and water while waiting for measurements.
Metabolic Performance
For both Alert and Rimouski, we used the set-up and protocol described by Le Pogam et al. (2020, 2021), except that the oxygen analyzers used at Alert were two Sable Systems Foxboxes (Sable Systems, Las Vegas, NV, United States) instead of the Servomex oxygen analyzer (gas purity analyzer, model 4100, Boston, MA, United States) used at Rimouski. The following key points are specific to this study.
Summit metabolic rate (Msum – the maximum metabolic rate in response to the cold, considered a measure of cold endurance; Dutenhoffer and Swanson, 1996) was measured on up to two birds simultaneously, allowing for two trials per day. Depending on the time of capture, measurements began between 10:04 and 22:45 (average: 15:09 ± 3.27 h; duration: 1.68 ± 0.89 h) at Alert, and between 10:14 and 17:10 (average: 12:26 ± 2.05 h; duration: 1.88 ± 0.67 h) at Rimouski. Measurements took place at least 1 h after ultrasound measurements. Birds were placed inside stainless steel metabolic chambers (effective volume 1.5 L) and exposed to dry, CO2-free air for 10 min at −18°C (flow rate of 1,200 mL.min–1), before switching to a helox gas mixture (21% oxygen, 79% helium, Rosenmann and Morrison, 1974). Chamber temperature was then lowered by 3°C every 20 min until birds became hypothermic [decline of birds’ oxygen consumption () for several minutes] or reached the end of the trial programmed time (125 min). At Alert, 5 measurements out of 30 (16.6%) involved birds that were not hypothermic at the end of their trial (cloacal temperature ≥37°C, Swanson and Liknes, 2006). There were no such cases at Rimouski. However, since hypothermia is not a prerequisite to confirm Msum (Dutenhoffer and Swanson, 1996), we included these five individuals in our analyses (removing them did not affect results). Basal metabolic rates (BMR – metabolic rate at rest considered a measure of physiological maintenance; McNab, 1997; Swanson et al., 2017) were measured overnight on a maximum of four birds simultaneously. At Alert, BMR trials began between 18:56 and 0:22 (average start time: 21:12 ± 1.49 h) and at Rimouski between 17:03 and 21:19 (average start time: 18:58 ± 1.27 h). Since Msum and BMR were assessed consecutively, we ensured a minimum 1 h of rest after the Msum measurements. Using the same metabolic chambers as for Msum, birds were exposed to 25°C, a temperature within snow bunting’s thermoneutral zone (Scholander et al., 1950) and received dry CO2-free air (650 ml min–1) for the duration of trials (10.88 ± 1.81 h on average at Alert, 13.49 ± 1.84 h at Rimouski). Birds were weighed (±0.01 g) before and after measurements, and average body mass (Mb) was used in BMR analyses. We used a sampling frequency of 5 s for Msum and 20 s for BMR. Both Msum and BMR were calculated from the highest and lowest averaged 10 min trace of , respectively, using equation 10.1 from Lighton (2019) and using the instantaneous measurement technique (Bartholomew et al., 1981) for Msum. The duration of BMR trials ensured that birds were post-absorptive at the time of BMR measurements. We estimated energy expenditure for all metabolic measurements using a constant equivalent of 19.8 kJ L–1O2 and converted units to Watts (Gessaman and Nagy, 1988).
Weather Data
Weather data for both field sites were obtained from nearby weather stations. For Rimouski, we used data from Pointe-au-Père station (48°30′50″N; 68°28′06″W, Government of Canada)1. For Alert, we used data collected by Environment and Climate Change Canada at their Alert weather station (i.e., our study site).
Statistical Analyses
Our objective was to determine whether snow buntings retain a winter phenotype up to their pre-breeding stage. Given the known differences between sexes in phenotypic adjustments for breeding and wintering (see below), we also considered potential differences in patterns between sexes for body mass and fat score (not enough data in females for the other variables in winter). We therefore used linear mixed-effect models with Mb, fat score, hematocrit, pectoralis muscle thickness, Msum and BMR as separate response variables. All models included “period” (i.e., winter or pre-breeding) as categorial predictor variables. The variable “year” was treated as a random parameter because we did not have measurements for all years in the two periods.
For Mb and fat score, we had sufficient data to consider the potential (fixed) effect of sex and the interaction “sex × period” in models. This is pertinent as mass and fat load could differ between sexes (Laplante et al., 2019). We also considered a potential effect of daily fattening on Mb and fat (e.g., Laplante et al., 2019) by including a covariate “time at capture.” Analyses on Mb further considered the potential influence of structural body size by including wing length in models.
For pectoralis muscle thickness, “keel height” measured by ultrasound was included as a covariate to control for variation in muscle thickness due to probe positioning (Le Pogam et al., 2020, 2021).
We first analyzed metabolic performance parameters considering whole-animal Msum and BMR. We then included Mb as a covariate in models to examine “mass-independent” variation. Under scenarios when whole and mass-independent results were similar, mass-independent values are presented. Since the birds could be caught at any time of the day, Msum and BMR measurements could not always be conducted on the day of capture. Therefore, models also included “length of captivity.”
Visual inspection of residuals confirmed assumptions of normality and homogeneity of residuals for all models. All analyses were conducted using JMP pro (14.0.0) and data are presented as mean ± standard error of the mean (SEM) in the text and 95% confidence intervals (CI) in graphs. Effects were considered significant and retained in models when P < 0.05. However, for one model, the interaction was marginally non-significant at P = 0.06. We therefore opted to keep this interaction term in the final model.
Results
Weather Conditions
Air temperatures were lower at Alert during pre-breeding (April and May, Table 2) than at Rimouski during the coldest period of the winter (January and February, Table 2).
Phenotype Comparisons Between Winter and Pre-breeding
After considering the significant effects of body size and time at capture, Mb and fat score were comparable between winter and pre-breeding (Mb: winter = 38.8 ± 0.5 g, pre-breeding = 38.2 ± 0.5 g; fat score: winter = 3.3 ± 0.15 units, pre-breeding = 3.4 ± 0.15 units) despite a non-significant trend for birds being slightly heavier in winter (1.5%, Table 3). A significant sex effect was observed for Mb (Table 3), with males (39.6 ± 0.4 g) being heavier than females (37.4 ± 0.6 g) for their size. There was also a tendency for females to have higher fat reserves on the breeding ground while males remained unchanged (Table 3), but since there was a large overlap in data, this trend (not shown) was very weak. Hematocrit was lower during pre-breeding (52.5 ± 0.5%) than in winter (54.6 ± 0.6%; Table 3 and Figure 1A). Pectoralis muscle thickness differed between periods (Table 3) with values being 3.12% higher during winter (5.44 ± 0.06 mm) than pre-breeding (5.27 ± 0.04 mm, Figure 1B).
TABLE 3
| Variable | Body mass | Fat score | ||||
| df | F | P | df | F | P | |
| Period | 1, 626 | 3.53 | 0.06 | 1, 735 | 1.08 | 0.30 |
| Sex | 1, 793 | 27.19 | <0.0001 | 1, 802 | 0.0054 | 0.94 |
| Sex × Period | 1, 802 | 3.45 | 0.06 | |||
| Structural size | 1, 792 | 60.57 | <0.0001 | |||
| Time at capture | 1, 790 | 8.79 | 0.003 | 1, 800 | 4.83 | 0.03 |
| Variable | Hematocrit | Pectoralis muscle thickness | ||||
| df | F | P | df | F | P | |
| Period | 1, 26 | 11.96 | 0.002 | 1, 9 | 5.24 | 0.05 |
| Keel | 1, 98 | 380.54 | <0.0001 | |||
| Variable | Msum | BMR | ||||
| df | F | P | df | F | P | |
| Period | 1, 20 | 1.32 | 0.26 | 1, 92 | 87.23 | <0.0001 |
| Mass | 1, 63 | 13.71 | 0.0005 | 1, 108 | 55.50 | <0.0001 |
| Length of captivity | 1, 129 | 6.90 | 0.01 | |||
Linear mixed-effects models comparing phenotypic traits in snow buntings measured during wintering at Rimouski and during pre-breeding at Alert.
Models also considered the effect of sex on body mass and fat scores and included covariates meaningful to specific dependent variables. See text for details.
FIGURE 1
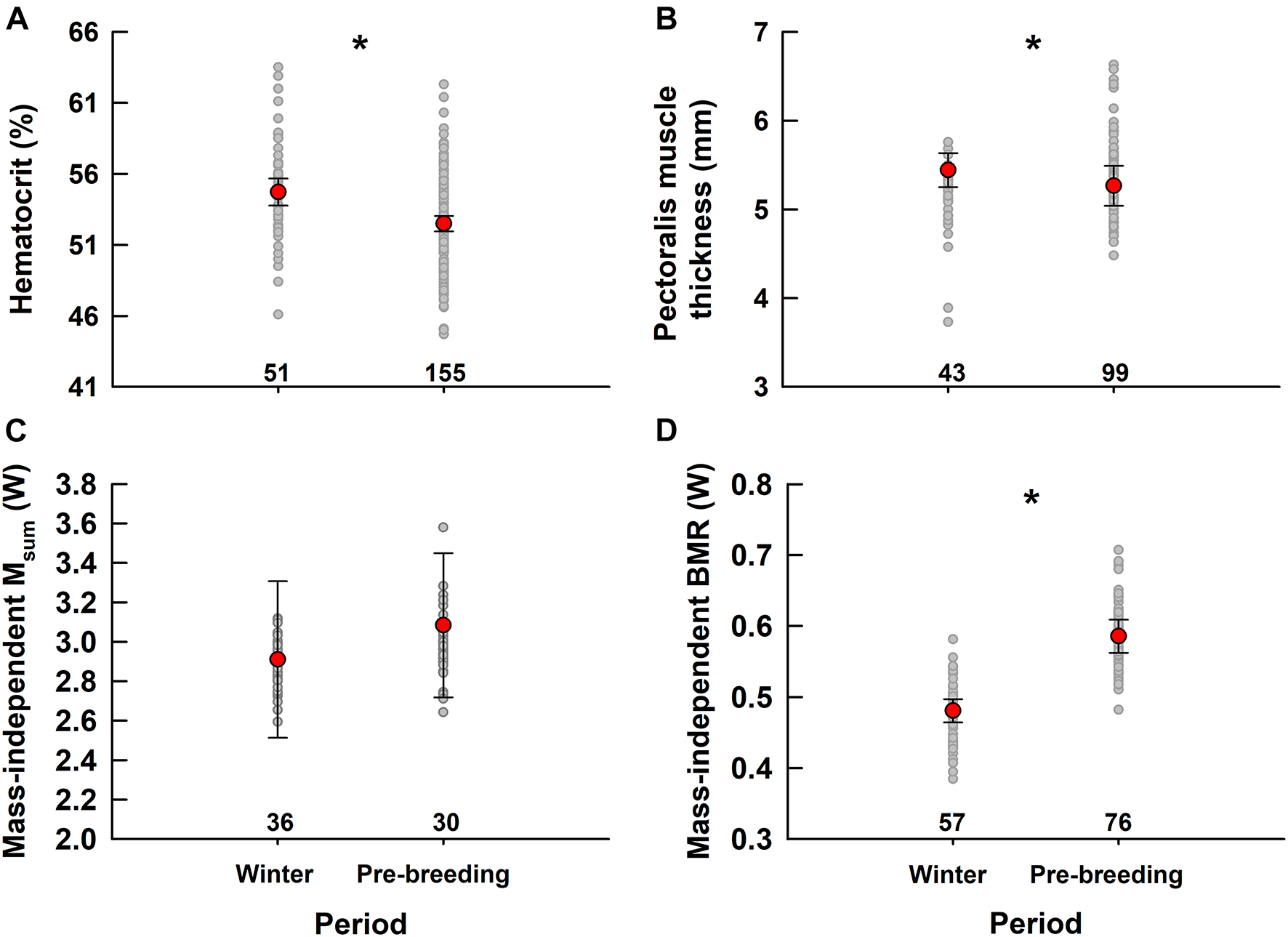
Comparing hematocrit (A), pectoralis muscle thickness (B), mass-independent Msum(C) and mass-independent BMR (D) in snow buntings measured in winter in Rimouski, Québec, Canada and during pre-breeding at Alert, NU, Canada. A star indicates significant differences. Numbers indicate sample size for each group. Values in red are least square means ± CI controlling for the random effect of year and for covariates in models including body mass for Msum and BMR, thus making these variables “mass-independent.” Gray dots show data predicted by models including fixed and random effects.
Summit metabolic rate (Msum), whether considered whole (not shown) or corrected for Mb, remained stable between winter and pre-breeding (Table 3 and Figure 1C, winter = 2.9 ± 0.1W, pre-breeding = 3.1 ± 0.1W). After considering the significant effect of captivity duration (BMR decreases with captivity duration, data not shown), BMR, whole (not shown) or corrected for Mb, was 22.9% higher during pre-breeding (0.59 ± 0.01W) than in winter (0.48 ± 0.01W, Table 3 and Figure 1D).
Discussion
This study reports the northernmost measurements of thermogenic capacity ever measured in birds (i.e., Alert, NU, Canada – only 817 km from North Pole). We aimed to determine whether pre-breeding snow buntings expressed a phenotype comparable to that observed while wintering at lower latitudes, which would support the hypothesis that these birds maintain winter-level metabolic performance and cold endurance throughout migration (Le Pogam et al., 2021). Overall, although all traits did not show the same pattern, our results suggest that snow buntings indeed maintain winter-like cold endurance during migration and during the pre-breeding period in the Arctic.
Thermogenic Capacity, Body Mass, and Fat Stores Remain High Up to the Breeding Grounds
We found that body mass, fat scores, and summit metabolic rate were globally comparable between wintering and pre-breeding stages in snow buntings. These results are consistent with the hypothesis that buntings likely maintain winter-level cold endurance and energy stores throughout migration and certainly into early spring on the breeding grounds as suggested previously by Le Pogam et al. (2021). In fact, pre-breeding birds at Alert faced environmental conditions colder than those encountered during winter at Rimouski (Table 2). Therefore, the maintenance of these traits likely allows buntings to cope with cold and unpredictable conditions during migration and on arrival on the breeding grounds (Ramenofsky and Wingfield, 2006). Winter-level thermogenic capacity could be particularly beneficial for early arriving males that are known to secure the best breeding territories well before the onset of breeding (Meltofte, 1983; Macdonald et al., 2012; Snell et al., 2018).
We also observed that for a given structural size, males tended to be heavier in winter, but did not differ in fat stores between winter and pre-breeding. In contrast, females did not differ in Mb, but did tend to have larger fat reserves during pre-breeding. This observation is similar to that reported by Laplante et al. (2019) in wintering buntings, and suggests that males and females have a different relative body composition in terms of lean and fat mass, possibly due to differing wintering strategies (Macdonald et al., 2016). As male buntings typically arrive before females on the breeding grounds (Cramp and Perrins, 1994; McKinnon et al., 2016; Snell et al., 2018; Montgomerie and Lyon, 2020), they likely face colder temperatures (Table 2), which may require adjustments in body composition to sustain thermoregulatory demands that differ from females. For example, life in the cold leads to increased daily food consumption and consequently larger digestive organs (Barceló et al., 2017), which could increase lean body mass with no effect on relative fat storage.
Lower Oxygen Carrying Capacity and Smaller Pectoralis Muscles on the Breeding Grounds
Not all traits representative of a snow bunting winter phenotype (Table 1, Le Pogam et al., 2020) were fully similar when contrasting winter and pre-breeding birds. In fact, both pectoralis muscle thickness and oxygen carrying capacity (hematocrit) were lower in pre-breeding individuals than in birds measured during winter. The difference in muscle thickness could potentially be a direct consequence of tissue degradation during migration as observed in several species (Battley et al., 2000; Bauchinger and Biebach, 2001; Bauchinger et al., 2005; Bauchinger and McWilliams, 2010). However, pectoralis muscles in shorebirds have also been reported to decline in size and mass in the first week after arrival at Alert (Morrison, 2006; Vézina et al., 2012) which has been suggested to act as protein stores to facilitate migratory recovery and transition into the breeding phenotype (Morrison, 2006; Vézina et al., 2012). Although the bulk of snow buntings seem to arrive at Alert around the end of May (AL, FV, pers. obs.), which matches our measurements of pre-breeding birds, local sightings have also confirmed that some individuals arrive much earlier (end of April), albeit in very small numbers (AL, FV, pers. obs.). We therefore cannot completely rule out the possibility that some proportion of the pectoralis muscles might be lost post arrival. Interestingly, the smaller pectoralis size of pre-breeding birds was not reflected in lower shivering heat production measured as Msum. However, this is not necessarily surprising since recent studies have suggested that Msum can be upregulated without changes in muscle size (Stager et al., 2015; Barceló et al., 2017; Milbergue et al., 2018), including in snow buntings (Le Pogam et al., 2020).
The reduced oxygen carrying capacity (lower hematocrit level) measured in pre-breeding buntings relative to wintering individuals is surprising. Birds faced with high energy demands, such as prolonged exercise for migration (Viscor et al., 1985; Bairlein and Totzke, 1992; Morton, 1994; Piersma et al., 1996) or high thermoregulatory requirements, typically show high oxygen transport capacity (Swanson, 1990b; Morton, 1994; O’Connor, 1996; Le Pogam et al., 2020), and birds measured at Alert were living in a considerably colder environment than those measured in winter. The reason for this discrepancy is not immediately clear. However, other studies have also observed a decline in hematocrit during migration (e.g., Piersma et al., 1996; Landys-Ciannelli et al., 2002; Jenni et al., 2006). One possible explanation suggested by Jenni et al. (2006) is that birds could increase their plasma volume by hemodilution to reduce blood viscosity, which could reduce the heart’s energy expenditure during migration.
Physiological Maintenance Costs Are Higher on the Breeding Grounds
We observed that physiological maintenance costs measured as BMR were higher during pre-breeding than during winter. This result contrasts with our predictions (i.e., same or lower BMR at Alert), but matches observations by Le Pogam et al. (2021) who reported a spring related increase in BMR in outdoor captive buntings kept on their wintering range. This elevated BMR was in fact maintained in captive birds throughout the periods corresponding to spring migration and most of breeding (Le Pogam et al., 2021). Several studies on migratory shorebirds have also reported an elevated BMR during the arrival period or breeding season in the Arctic (Kvist and Lindström, 2001; Lindström and Klaassen, 2003; Vézina et al., 2012) and this increase in BMR has been interpreted as a result of high thermoregulatory demands on the breeding grounds (Kersten and Piersma, 1987; Kvist and Lindström, 2001; Jetz et al., 2008). While the species in these studies typically winter in relatively warm environments (Kvist and Lindström, 2001; Lindström and Klaassen, 2003; Vézina et al., 2012), the snow buntings compared here were also exposed to colder temperatures in the spring at Alert than in eastern Québec in winter. As far as we know, this is the first report of high maintenance costs at Arctic latitudes in a pre-breeding passerine species. Furthermore, as BMR increased independently from body mass, our results suggest that the higher maintenance costs in the Arctic results from tissue level metabolic activity (i.e., metabolic intensity, Swanson et al., 2017). Interestingly, as this result was also observed in snow buntings unable to migrate and exposed to much warmer temperatures, it could be that some of the underlying variation is driven by endogenous circannual cycles (Vézina et al., 2011; Karagicheva et al., 2016).
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All bird handling at Rimouski (QC) and at Alert (NU) was approved by the Animal Care Committee of the Université du Québec à Rimouski (Rimouski: CPA-54-13-130 and CPA-71-17-195 and Alert: CPA-61-15-163 and CPA-71-17-194) and was conducted under banding (10889E) and scientific (SC-48, NUN-SCI-15-05) permits from Environment and Climate Change Canada, and under scientific permits from the Department of Environment of Nunavut (WL 2016-006, 2017-021, 2018-010, 2019-002).
Author contributions
AL and FV conceived the ideas and designed the methodology. AL, M-PL, JD, LR, GR, and FV collected the data. AL analyzed the data. AL, RO’C, FV, and OL led the writing of the manuscript. All authors contributed to the drafts and gave final approval for publication.
Funding
This research benefited from a generous donation from the Kenneth M. Molson Foundation. It was also supported by NSERC Discovery grants to FV and OL, Canada Foundation for Innovation (CFI) awards to FV and OL, Canada Research Chair funding to OL, as well as logistical support and funding from the Department of National Defence to FV and DB.
Acknowledgments
We thank François Fournier from Environment and Climate Change Canada for his help with logistical support in the initial Alert phase of this project, and Claire Bottini, Yann Bouchez, Emily Cornelius Ruhs, Polan-Devi Darboven, Alexandre Paiement, Kim Régimbald Bélanger, Charles Richard, Laurence Rondeau, and Josianne Ruest for their help in catching birds in winter as well as Gratien Bélanger and Sylvie Foucault for granting access to their land for snow bunting captures. We are grateful to Jonathan Coudé for technical support and Alain Caron for statistical advice. We also thank Chris McRae and Nathan Koutroulides for their help with logistics and the personnel from CFS Alert for their support during fieldwork. We thank the two reviewers for their constructive comments on the earlier versions of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.724876/full#supplementary-material
Footnotes
References
1
BairleinF.TotzkeU. (1992). New aspects on migratory physiology of trans-Saharan passerine migrants.Ornis Scand23244–250. 10.2307/3676645
2
BarcelóG.LoveO. P.VézinaF. (2017). Uncoupling basal and summit metabolic rates in white-throated sparrows: digestive demand drives maintenance costs, but changes in muscle mass are not needed to improve thermogenic capacity.Physiol. Biochem. Zool.90153–165. 10.1086/689290
3
BartholomewG. A.VleckD.VleckC. M. (1981). Instantaneous measurements of oxygen consumption during pre-flight warm-up and post-flight cooling in sphingid and saturniid moths.J. Exp. Biol.9017–32. 10.1242/jeb.90.1.17
4
BattleyP. F.PiersmaT. (1997). Body composition of lesser knots (Calidris canutus rogersi) preparing to take off on migration from northern New Zealand.Notornis44137–150.
5
BattleyP. F.PiersmaT.DietzM. W.TangS.DekingaA.HulsmanK. (2000). Empirical evidence for differential organ loss during trans-oceanic bird flight.Proc. R. Soc. Lond. B267191–195. 10.1098/rspb.2000.0986
6
BauchingerU.BiebachH. (2001). Differential catabolism of muscle protein in garden warblers (Sylvia borin): Flight and leg muscle act as a protein source during long-distance migration.J. Comp. Physiol. B Biochem. Syst. Environ. Physiol.171293–301. 10.1007/s003600100176
7
BauchingerU.McWilliamsS. R. (2010). Extent of phenotypic flexibility during long-distance flight is determined by tissue-specific turnover rates: A new hypothesis.J. Avian Biol.41603–608. 10.1111/j.1600-048X.2010.05137.x
8
BauchingerU.WohlmannA.BiebachH. (2005). Flexible remodeling of organ size during spring migration of the garden warbler (Sylvia borin).Zoology10897–106. 10.1016/j.zool.2005.03.003
9
BlemC. R. (1976). Patterns of lipid storage and utilization in birds.Am. Zool.16671–684. 10.1093/icb/16.4.671
10
CareyC.DawsonW. R.MaxwellL. C.FaulknerJ. A. (1978). Seasonal acclimatization to temperature in cardueline finches.J. Comp. Physiol.125101–113. 10.1007/BF00686746
11
CooperS. J. (2002). Seasonal metabolic acclimatization in mountain chickadees and juniper titmice.Physiol. Biochem. Zool.75386–395. 10.1086/342256
12
CooperS. J. (2007). Daily and seasonal variation in body mass and visible fat in mountain chickadees and juniper titmice.Wilson J. Ornithol.119720–724. 10.1676/06-183.1
13
CrampS.PerrinsC. M. (1994). The birds of the Western Palearctic: Vol. IX. Buntings and New World warblers.Oxford: Oxford University Press.
14
DawsonW. R.MarshR. L.YacoeM. E. (1983). Metabolic adjustments of small passerine birds for migration and cold.Am. J. Physiol. Integr. Comp. Physiol.245R755–R767. 10.1152/ajpregu.1983.245.6.R755
15
DietzM. W.DekingaA.PiersmaT.VerhulstS. (1999). Estimating organ size in small migrating shorebirds with ultrasonography: An intercalibration exercise.Physiol. Biochem. Zool.7228–37. 10.1086/316648
16
DrentR.BothC.GreenM.MadsenJ.PiersmaT. (2003). Pay-offs and penalties of competing migratory schedules.Oikos103274–292.
17
DuboisK.HallotF.VézinaF. (2016). Basal and maximal metabolic rates differ in their response to rapid temperature change among avian species.J. Comp. Physiol. B186919–935. 10.1007/s00360-016-1001-5
18
DutenhofferM. S.SwansonD. L. (1996). Relationship of basal to summit metabolic rate in passerine birds and the aerobic capacity model for the evolution of endothermy.Physiol. Zool.691232–1254. 10.1086/physzool.69.5.30164255
19
GessamanJ. A.NagyK. A. (1988). Energy metabolism: errors in gas-exchange conversion factors.Physiol. Zool.61507–513. 10.1086/physzool.61.6.30156159
20
GoslerA. G. (1996). Environmental and social determinants of winter fat storage in the great tit Parus major.J. Anim. Ecol.651–17. 10.2307/5695
21
Guindre-ParkerS.Grant GilchristH.BaldoS.DoucetS. M.LoveO. P. (2013). Multiple achromatic plumage ornaments signal to multiple receivers.Behav. Ecol.24672–682. 10.1093/beheco/ars215
22
JenniL.MüllerS.SpinaF.KvistA.LindströmÅ (2006). Effect of endurance flight on haematocrit in migrating birds.J. Ornithol.147531–542. 10.1007/s10336-006-0076-2
23
JetzW.FreckletonR. P.McKechnieA. E. (2008). Environment, migratory tendency, phylogeny and basal metabolic rate in birds.PLoS One3:e3261. 10.1371/journal.pone.0003261
24
KaragichevaJ.RakhimberdievE.DekingaA.BruggeM.KoolhaasA.Ten HornJ.et al (2016). Seasonal time keeping in a long-distance migrating shorebird.J. Biol. Rhythms31509–521. 10.1177/0748730416655929
25
KerstenM.PiersmaT. (1987). High levels of energy expenditure in shorebirds; metabolic adaptations to an energetically expensive way of life.Ardea75175–187. 10.5253/arde.v75.p175
26
KrauseJ. S.NémethZ.PérezJ. H.ChmuraH. E.RamenofskyM.WingfieldJ. C. (2016). Annual hematocrit profiles in two subspecies of white-crowned sparrow: a migrant and a resident comparison.Physiol. Biochem. Zool.8951–60. 10.1086/684612
27
KvistA.LindströmÅ (2001). Basal metabolic rate in migratory waders: Intra-individual, intraspecific, interspecific and seasonal variation.Funct. Ecol.15465–473. 10.1046/j.0269-8463.2001.00549.x
28
Landys-CiannelliM. M.JukemaJ.PiersmaT. (2002). Blood parameter changes during stopover in a long-distance migratory shorebird, the bar-tailed godwit Limosa lapponica taymyrensis.J. Avian Biol.33451–455. 10.1034/j.1600-048X.2002.03051.x
29
LaplanteM.-P.McKinnonE. A.LoveO. P.VézinaF. (2019). Flexible response to short-term weather in a cold-adapted songbird.J. Avian Biol.501–10. 10.1111/jav.01766
30
Le PogamA.LoveO. P.RégimbaldL.DuboisK.HallotF.MilbergueM.et al (2020). Wintering snow buntings elevate cold hardiness to extreme levels but show no changes in maintenance costs.Physiol. Biochem. Zool.93417–433. 10.1086/711370
31
Le PogamA.O’ConnorR. S.LoveO. P.PetitM.RégimbaldL.VézinaF. (2021). Coping with the worst of both worlds: phenotypic adjustments for cold acclimatization benefit northward migration and arrival in the cold in an Arctic-breeding songbird.Funct. Ecol.351240–1254. 10.1111/1365-2435.13793
32
LehikoinenE. (1987). Seasonality of the daily weight cycle in wintering passerines and its consequences.Ornis Scand18216–226. 10.2307/3676769
33
LepageD.GauthierG.MenuS. (2000). Reproductive consequences of egg-laying decisions in snow geese.J. avian Ecol.69414–427.
34
LightonJ. R. B. (2019). Measuring Metabolic Rates: a manual for scientists, 2nd Edn. Oxford: Oxford University Press.
35
LiknesE. T.SwansonD. L. (1996). Seasonal variation in cold tolerance, basal metabolic rate, and maximal capacity for thermogenesis in white-breasted nuthatches Sitta carolinensis and downy woodpeckers Picoides pubescens, two unrelated arboreal temperate residents.J. Avian Biol.27279–288. 10.2307/3677259
36
LiknesE. T.SwansonD. L. (2011). Phenotypic flexibility of body composition associated with seasonal acclimatization in passerine birds.J. Therm. Biol.36363–370. 10.1016/j.jtherbio.2011.06.010
37
LindströmÅKlaassenM. (2003). High basal metabolic rates of shorebirds while in the Arctic: a circumpolar view.Condor105420–427. 10.1650/7222
38
LoveO. P.GilchristH. G.DescampsS.SemeniukC. A.BêtyJ. (2010). Pre-laying climatic cues can time reproduction to optimally match offspring hatching and ice conditions in an Arctic marine bird.Oecologia164277–286. 10.1007/s00442-010-1678-1
39
LoveO. P.MacdonaldC. A.MckinnonE. A. (2012). Canadian Snow Bunting Banding Network Protocol.doi: 10.6084/m9.figshare1588581.
40
MacdonaldC. A.FraserK. C.GilchristH. G.KyserT. K.FoxJ. W.LoveO. P. (2012). Strong migratory connectivity in a declining arctic passerine.Anim. Migr.123–30. 10.2478/ami-2012-0003
41
MacdonaldC. A.MckinnonE. A.GilchristH. G.LoveO. P. (2016). Cold tolerance, and not earlier arrival on breeding grounds, explains why males winter further north in an Arctic-breeding songbird.J. Avian Biol.477–15. 10.1111/jav.00689
42
McKechnieA. E.NoakesM. J.SmitB. (2015). Global patterns of seasonal acclimatization in avian resting metabolic rates.J. Ornithol.156367–376. 10.1007/s10336-015-1186-5
43
McKechnieA. E.SwansonD. L. (2010). Sources and significance of variation in basal, summit and maximal metabolic rates in birds.Curr. Zool.56741–758. 10.1038/35131
44
McKinnonE. A.LaplanteM.-P.LoveO. P.FraserK. C.MackenzieS.VézinaF. (2019). Tracking landscape-scale movements of snow buntings and weather-driven changes in flock composition during the temperate winter.Front. Ecol. Evol.7:329. 10.3389/fevo.2019.00329
45
McKinnonE. A.MacdonaldC. A.GilchristH. G.LoveO. P. (2016). Spring and fall migration phenology of an Arctic-breeding passerine.J. Ornithol.157681–693. 10.1007/s10336-016-1333-7
46
McNabB. K. (1997). On the utility of uniformity in the definition of basal rate of metabolism.Physiol. Zool.70718–720. 10.1086/515881
47
MeltofteH. (1983). Arrival and pre-nesting period of the snow bunting Plectrophenax nivalis in East Greenland.Polar Res.1185–198. 10.1111/j.1751-8369.1983.tb00702.x
48
MilbergueM. S.BlierP. U.VézinaF. (2018). Large muscles are beneficial but not required for improving thermogenic capacity in small birds.Sci. Rep.8:14009. 10.1038/s41598-018-32041-w
49
MontgomerieR.LyonB. (2020). “Snow bunting (Plectrophenax nivalis), version 1.0,” in Birds of the World, edsBillermanS.KeeneyB.RodewaldP.SchulenbergT. (Ithaca, NY: Cornell Lab of Ornithology).
50
MorrisonR. I. G. (2006). Body transformations, condition, and survival in red knots Calidris canutus travelling to breed at Alert, Ellesmere Island, Canada.Ardea94607–618.
51
MorrisonR. I. G.DavidsonN. C.WilsonJ. R. (2007). Survival of the fattest: body stores on migration and survival in red knots Calidris canutus islandica.J. Avian. Biol.38479–487. 10.1111/j.2007.0908-8857.03934.x
52
MortonM. L. (1994). Hematocrits in montane sparrows in relation to reproductive schedule.Condor96119–126. 10.2307/1369069
53
O’ConnorT. P. (1995). Metabolic characteristics and body composition in house finches: Effects of seasonal acclimitatization.J. Comp. Physiol. B165298–305. 10.1007/BF00367313
54
O’ConnorT. P. (1996). Geographic variation in metabolic seasonal acclimatization in house finches.Condor98371–381. 10.2307/1369155
55
PetitM.LewdenA.VézinaF. (2013). Intra-seasonal flexibility in avian metabolic performance highlights the uncoupling of basal metabolic rate and thermogenic capacity.PLoS One8:e68292. 10.1371/journal.pone.0068292
56
PetitM.LewdenA.VézinaF. (2014). How does flexibility in body composition relate to seasonal changes in metabolic performance in a small passerine wintering at Northern latitude?Physiol. Biochem. Zool.87539–549. 10.1086/676669
57
PetitM.VézinaF. (2014). Phenotype manipulations confirm the role of pectoral muscles and haematocrit in avian maximal thermogenic capacity.J. Exp. Biol.217824–830. 10.1242/jeb.095703
58
PiersmaT.EveraartsJ. M.JukemaJ. (1996). Build-up of red blood cells in refuelling bar-tailed godwits in relation to individual migratory quality.Condor98363–370. 10.2307/1369154
59
PiersmaT.GudmundssonG. A.LilliendahlK. (1999). Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird.Physiol. Biochem. Zool.72405–415. 10.1086/316680
60
RakhimberdievE.DuijnsS.KaragichevaJ.CamphuysenC. J.CastricumV. R. S.DekingaA.et al (2018). Fuelling conditions at staging sites can mitigate Arctic warming effects in a migratory bird.Nat. Commun.91–10. 10.1038/s41467-018-06673-5
61
RamenofskyM.WingfieldJ. C. (2006). Behavioral and physiological conflicts in migrants: The transition between migration and breeding.J. Ornithol.147135–145. 10.1007/s10336-005-0050-4
62
RamenofskyM.WingfieldJ. C. (2017). Regulation of complex behavioural transitions: migration to breeding.Anim. Behav.124299–306. 10.1016/j.anbehav.2016.09.015
63
ReneerkensJ.SchmidtN. M.GilgO.HansenJ.HansenL. H.MoreauJ.et al (2016). Effects of food abundance and early clutch predation on reproductive timing in a high Arctic shorebird exposed to advancements in arthropod abundance.Ecol. Evol.67375–7386. 10.1002/ece3.2361
64
RosenmannM.MorrisonP. (1974). Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2.Am. J. Physiol.226490–495. 10.1152/ajplegacy.1974.226.3.490
65
Royer-BoutinP.CortésP. A.MilbergueM. S.PetitM.VézinaF. (2015). Estimation of muscle mass by ultrasonography differs between observers and life states of models in small birds.Physiol. Biochem. Zool.88336–344. 10.1086/680016
66
ScholanderP. F.HockR.WaltersV.JohnsonF. (1950). Heat regulation in some Arctic and tropical mammals and birds.Biol. Bull.99237–258. 10.2307/1538741
67
SmithR. D. (1992). Age determination, wing-feather colour and wing-length change in snow buntings plectrophenax nivalis.Ringing Migr.1343–51. 10.1080/03078698.1992.9674014
68
SnellK. R. S.StokkeB. G.MoksnesA.ThorupK.FossoyF. (2018). From Svalbard to Siberia: Passerines breeding in the High Arctic also endure the extreme cold of the Western steppe.PLoS One13:e0202114. 10.1371/journal.pone.0202114
69
StagerM.SwansonD. L.ChevironZ. A. (2015). Regulatory mechanisms of metabolic flexibility in the dark-eyed junco (Junco hyemalis).J. Exp. Biol.218767–777. 10.1242/jeb.113472
70
SwansonD. L. (1990a). Seasonal variation in cold hardiness and peak rates of cold-induced thermogenesis in the dark-eyed junco (Junco hyemalis).Auk107561–566. 10.1093/auk/107.3.561
71
SwansonD. L. (1990b). Seasonal variation of vascular oxygen transport in the dark-eyed junco.Condor9262–66. 10.2307/1368185
72
SwansonD. L. (2010). “Seasonal metabolic variation in birds: functional and mechanistic correlates,” in Current Ornithology, ed.ThompsonC. F. (New York: Springer-Verlag), 75–129.
73
SwansonD. L.LiknesE. T. (2006). A comparative analysis of thermogenic capacity and cold tolerance in small birds.J. Exp. Biol.209466–474. 10.1242/jeb.02024
74
SwansonD. L.McKechnieA. E.VézinaF. (2017). How low can you go? An adaptive energetic framework for interpreting basal metabolic rate variation in endotherms.J Comp Physiol B1871039–1056. 10.1007/s00360-017-1096-3
75
SwansonD. L.MerkordC. (2012). Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements.J. Ornithol.154119–127. 10.1007/s10336-012-0877-4
76
SwansonD. L.VézinaF. (2015). Environmental, ecological and mechanistic drivers of avian seasonal metabolic flexibility in response to cold winters.J. Ornithol.156377–388. 10.1007/s10336-015-1192-7
77
TinbergenN. (1939). The behavior of the snow bunting in spring, Vol. V.New York: Transaction of the Linnaean society of New-York.
78
van GilsJ. A.LisovskiS.LokT.MaissnerW.OżarowskaA.de FouwJ.et al (2016). Body shrinkage due to Arctic warming reduces red knot fitness in tropical wintering range.Science352819–821. 10.1002/ece3.2361
79
VézinaF.DekingaA.PiersmaT. (2011). Shorebirds’ seasonal adjustments in thermogenic capacity are reflected by changes in body mass: how preprogrammed and instantaneous acclimation work together.Integr. Comp. Biol.51394–408. 10.1093/icb/icr044
80
VézinaF.WilliamsT. D.PiersmaT.MorrisonR. I. G. (2012). Phenotypic compromises in a long-distance migrant during the transition from migration to reproduction in the High Arctic.Funct. Ecol.26500–512. 10.1111/j.1365-2435.2011.01955.x
81
ViscorG.MarquésM. S.PalomequeJ. (1985). Cardiovascular and organ weight adaptations as related to flight activity in birds.Comp. Biochem. Physiol. A Physiol.82597–599. 10.1016/0300-9629(85)90439-6
82
WalshJ. E.ShapiroI.ShyT. L. (2005). On the variability and predictability of daily temperatures in the Arctic.Atmos Ocean43213–230. 10.3137/ao.430302
83
WingfieldJ. C.KelleyJ. P.AngelierF. (2011). What are extreme environmental conditions and how do organisms cope with them?Curr. Zool.57363–374. 10.1093/czoolo/57.3.363
84
ZhangY.EysterK.LiuJ.-S.SwansonD. L. (2015). Cross-training in birds: cold and exercise training produce similar changes in maximal metabolic output, muscle masses and myostatin expression in house sparrows (Passer domesticus).J. Exp. Biol.2182190–2200. 10.1242/jeb.121822
85
ZhengW.-H.LiM.LiuJ.-S.ShaoS.-L. (2008). Seasonal acclimatization of metabolism in Eurasian tree sparrows (Passer montanus).Comp. Biochem. Physiol. A151519–525. 10.1016/j.cbpa.2008.07.009
Summary
Keywords
Arctic bird, Arctic breeding, body composition, basal metabolic rate, cold acclimatization, migration, phenotypic flexibility, summit metabolic rate
Citation
Le Pogam A, O’Connor RS, Love OP, Drolet J, Régimbald L, Roy G, Laplante M-P, Berteaux D, Tam A and Vézina F (2021) Snow Buntings Maintain Winter-Level Cold Endurance While Migrating to the High Arctic. Front. Ecol. Evol. 9:724876. doi: 10.3389/fevo.2021.724876
Received
14 June 2021
Accepted
06 September 2021
Published
23 September 2021
Volume
9 - 2021
Edited by
Lucy Alice Hawkes, University of Exeter, United Kingdom
Reviewed by
Yufeng Zhang, University of Memphis, United States; Theunis Piersma, University of Groningen, Netherlands
Updates
Copyright
© 2021 Le Pogam, O’Connor, Love, Drolet, Régimbald, Roy, Laplante, Berteaux, Tam and Vézina.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audrey Le Pogam, audreylp@wanadoo.fr
This article was submitted to Behavioral and Evolutionary Ecology, a section of the journal Frontiers in Ecology and Evolution
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.