- School of Natural and Computational Sciences, Massey University, Auckland, New Zealand
Songbirds learn their songs culturally, through imitating tutors. The vocal culture of a songbird population changes as new song units (syllables) are introduced through immigration, copying errors, and innovation, while other syllables fall out of use. This leads to a diversification of the syllable pool across the species, much like the diversification and spatial patterns of human language. Vocal cultures have been well-studied in male songbirds but have been largely overlooked in females. Here we undertake one of the first comparisons of male and female song cultures across a songbird metapopulation—studying New Zealand bellbirds Anthornis melanura spanning a network of six islands. Having classified 20,700 syllables (702 types), we compare population syllable repertoire sizes and overlap between sites and sexes. We show that males and females—both with complex songs—have distinct song cultures, sharing only 6–26% of syllable types within each site. Furthermore, male and female syllable types can be statistically discriminated based on acoustic properties. Despite diverse syllable repertoires within sites, few syllable types were shared between sites (both sexes had highly distinct site-specific dialects). For the few types shared between sites, sharing decreased with distance only for males. Overall, there was no significant difference between sexes in degree of site–site repertoire overlap. These results suggest different cultural processes at play for the two sexes, underlining the inadequacy of male-centric song research and calling for comparisons of male and female song cultures in many more species.
Introduction
Culture is shared information or behaviour acquired through social learning from conspecifics (Dawkins, 1976), involving the transmission of memes (units of culture) by behavioural imitation. Vocal culture—the social learning of acoustic memes—has so far been observed in songbirds (oscines; Passeri), some suboscines (Procnias spp., Cotingidae), parrots, hummingbirds, cetaceans, elephants, seals, bats, and humans (Paton et al., 1981; Baptista and Schuchmann, 1990; Janik and Slater, 1997; Poole et al., 2005; Sanvito et al., 2007; Catchpole and Slater, 2008; Kroodsma et al., 2013). In these taxa, the vocal repertoire of a population changes as new memes are introduced through immigration, copying errors, and innovation, while other memes fall out of use (Catchpole and Slater, 2008). This leads to a diversification of the meme pool across the species, much like the diversification of human language—resulting in dialects (Podos and Warren, 2007).
Despite the high volume of studies on male birdsong culture and dialects (Jenkins, 1978; Whitehead and Rendell, 2014; Aplin, 2019), little is known about female song culture. This is partly due to a northern-hemisphere-biased view of sexual selection that emphasises male-male competition and female choice in driving elaborate traits in males (Darwin, 1871), but which does not provide a framework for understanding elaborate female traits (Riebel et al., 2019). Female song (and other elaborate female traits) have been overlooked as non-functional aberrations, resulting “accidentally” from shared genetic architecture with males (Darwin, 1871; Lande, 1980; see Tobias et al., 2012 for review). This view has now been roundly discounted. Female song is present in 64% of surveyed songbird species (Webb et al., 2016), has been recovered as the ancestral state (Odom et al., 2014), can evolve independently of the male song phenotype (Price, 2015) and has female-specific functions in territory and resource defence, mate attraction, mate defence, and pair bonding (reviewed in Austin et al., 2021).
Overlooking females has impeded development of a more general theory that explains song culture in both sexes. For instance, the possibility that females have song cultures distinct from conspecific males, with different geographic patterns of song sharing, has hardly been investigated at a population level (Graham et al., 2017a).
Studies have reported a wide degree of sexual song dimorphism in songbirds, from identical repertoires for males and females at one extreme [e.g., forest weaver Ploceus bicolor (Wickler and Seibt, 1980), magpie lark Grallina cyanoleuca (Hall, 2000)], to completely non-overlapping repertoires at the other [e.g., slate-colored boubou Laniarius funebris (Wickler and Seibt, 1988), many duetting wrens (Brown and Lemon, 1979; Levin, 1996; Mann et al., 2009)]. The degree of repertoire overlap between males and females is thought to be governed by genetic factors—such as innate auditory templates and capacity for production—modulated by epigenetic factors, such as social selection, which can cause a selective attrition of syllables that “belong to” the other sex (Wickler and Seibt, 1988; Lobato et al., 2015).
However, studies comparing the sexes have mostly focused on within-pair repertoires in duetting species. To our knowledge, only five studies (of just two species) have compared spatial variation of male and female songs at a population level.
First, Mennill and Rogers (2006) examined the songs of duetting male and female eastern whipbird Psophodes olivaceus across their geographic range. While male whipbird song was highly consistent over space, female song showed pronounced variation, with multiple distinct song types. The authors suggest eastern whipbirds have undergone a decoupling of male and female song learning strategies in response to different sex-specific selection pressures.
Four later studies on rufous-and-white wrens (Graham et al., 2017a, 2018a,b, 2021) showed that song cultures of males and females can be similar in complexity, and appear to evolve in similar directions via acoustic adaptation and cultural drift. However, males and females differ in the relationship between dispersal distance and song-sharing with parents (Graham et al., 2017a), the speed of cultural change, and the relationship between immigration rate and cultural diversity (Graham et al., 2021).
We know of no other studies to examine spatial variation in song culture at a population level, comparing both sexes.
The korimako, or New Zealand bellbird Anthornis melanura—hereafter “bellbird”—provides an ideal system for comparing male and female song cultures over space. The bellbird is an endemic honeyeater (Family Meliphagidae) with complex, geographically diverse song in both sexes (Roper, 2018). Bellbirds are socially monogamous, and sexually dimorphic in size, plumage, and song. Juveniles are easily distinguished from adults (Heather and Robertson, 2000; Roper, 2018). Males and females do not duet, but both sing aggressively throughout the year in the defence of territories and food (Craig and Douglas, 1986; Brunton and Li, 2006).
Brunton and Li (2006) provided the first formal characterisation of bellbird song, at one site (Tiritiri Matangi Island, also known as Tiri). Syllables were not classified at a fine scale, but the authors identified 17 exclusively male syllable groups, 12 exclusively female, and 7 shared. Males sang with continuous variety in prolonged bouts up to 5 min without gaps, whereas females sang discrete songs of about 2 s, with gaps in between (Brunton and Li, 2006).
Roper et al. (2018) studied song ontogeny in wild bellbirds on Tiri, showing that males and females develop song at similar rates, sounding similar during the motor learning phases but diverging into dimorphic crystallised adult song by 24 weeks. Though nothing is yet known about tutor choice in bellbirds, population densities are often high, especially around food sources, and young birds are likely to be exposed to songs from many individuals. Because bellbirds flexibly combine syllables into variable songs, the functional units of culture in this species appear to be syllable types, rather than song types.
Bellbird populations occur across a network of islands and peninsulas in the Hauraki Gulf, northeastern New Zealand. Population connectivity is substantial, and regulated by geographic isolation (Baillie, 2011; Baillie et al., 2014). Bellbirds have been observed dispersing between islands singly or in flocks, as juveniles and as adults (Brunton et al., 2008; Brunton, pers. obs.). Females disperse more frequently than males, resulting in higher female connectivity between sites (Baillie, 2011), as is typical for songbirds (Greenwood, 1980; Clarke et al., 1997; Paris et al., 2016).
Compared to continental-scale song studies (e.g., male yellowhammers Emberiza citronella across Europe; Diblíková et al., 2019) and studies localised within one site (e.g., male North Island saddleback Philesturnus rufusater; Jenkins, 1978), an archipelago of this intermediate scale provides a tractable system for comparing male and female song cultures while including the spatial complexity of meme flow between sites.
In this paper, we seek to advance understanding of female birdsong culture by comparing the patterns of male and female meme sharing across a network of six island and peninsula populations (Figure 1). Our questions are:
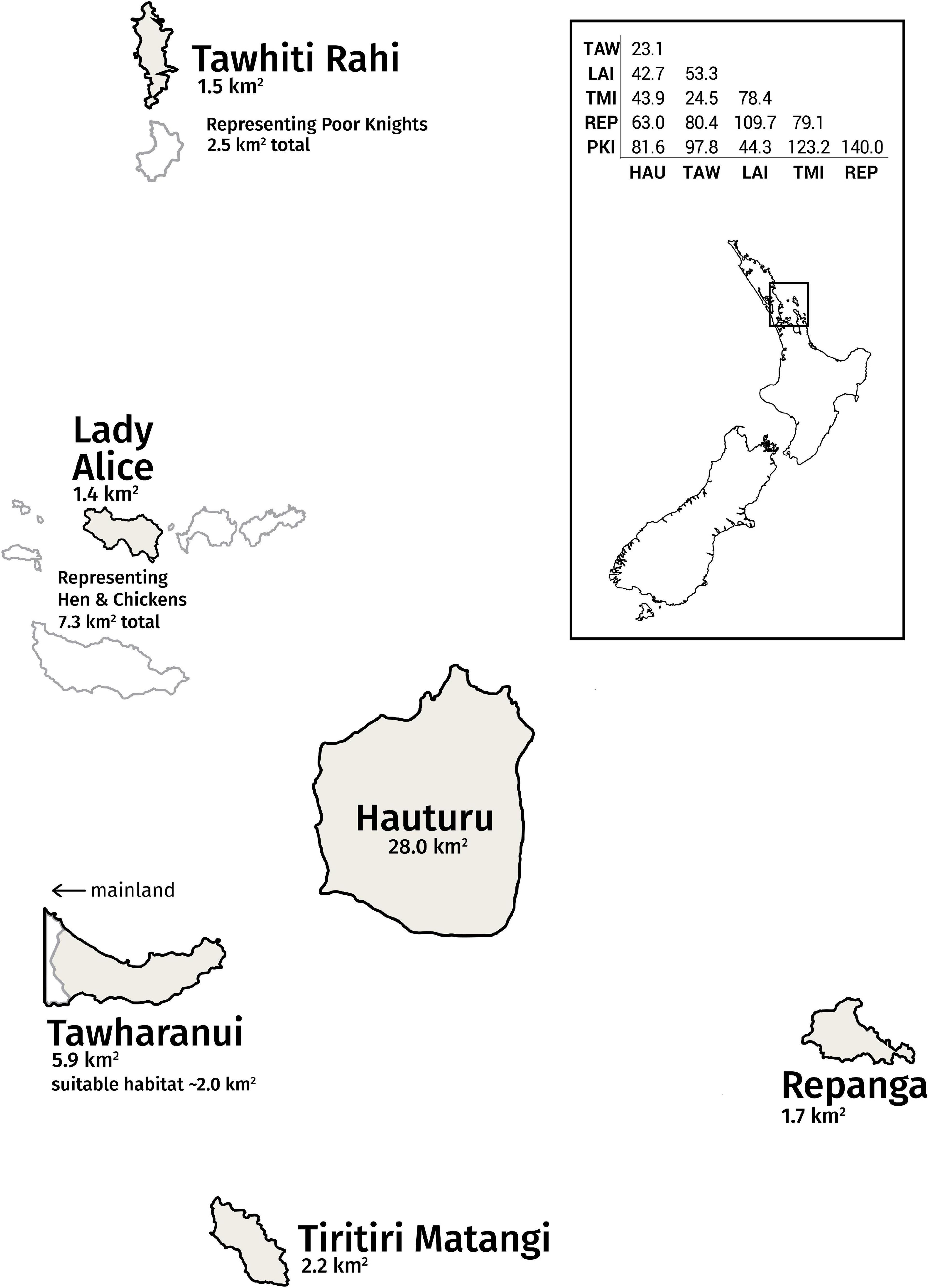
Figure 1. Simplified map of the Hauraki Gulf archipelago, home to a metapopulation of bellbirds. Tawhiti Rahi represents the Poor Knights Islands group; likewise, Lady Alice Island represents the Hen and Chickens Islands group. The other islands of these wider groups are shown in white. The distance matrix between sites (measured in km) is provided (HAU, Hauturu; TAW, Tawharanui; LAI, Lady Alice; TMI, Tiritiri Matangi; REP, Repanga, PKI, Tawhiti Rahi). Distances are not to scale on simplified map.
(1) Are there sexual differences in population syllable repertoire size?
(2) How much do male and female population repertoires overlap?
(3) What is the pattern of syllable type sharing between sites?
If geographic proximity drives cultural connectivity between islands, we expect sites that are closer together will share more memes (i.e., an isolation-by-distance pattern in vocal culture). We also expect higher female dispersal frequency will result in higher meme flow between sites, and thus higher sharing of syllable types between sites than for males.
Methods
Creating the Song Database
Recording Bellbird Song
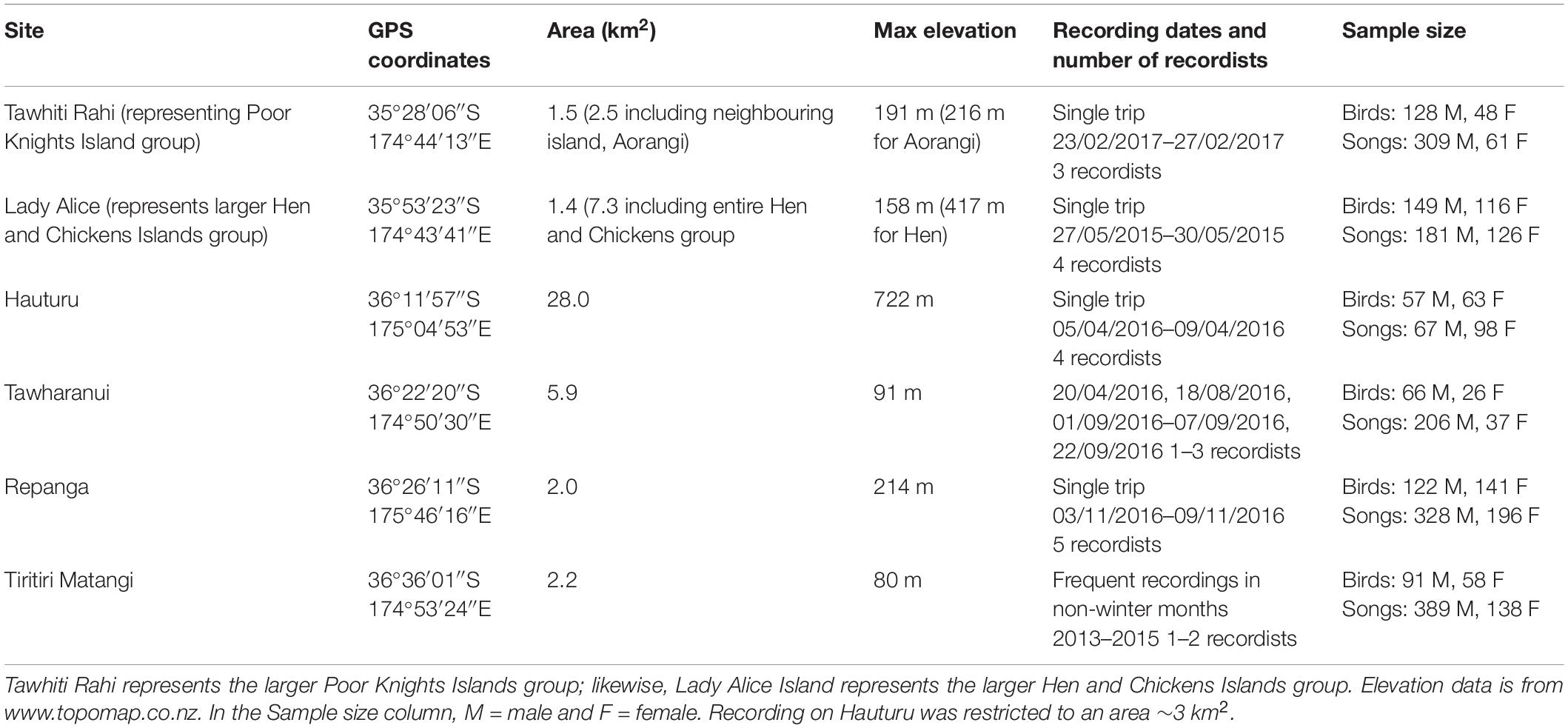
Recording trips were conducted to six island and mainland peninsula sites in the Hauraki Gulf 2013–2017 (Table 1), with iwi consent (Ngāti Wai, Ngāti Manuhiri) and permits from the New Zealand Department of Conservation (47948–FAU, 34833–FAU, 41756–FAU, 48000–FAU) and Massey University Animal Ethics Committee (permit number 15/21). The sites were chosen based on their large bellbird populations (good return for sampling effort) and their variety in connectivity. A team of 1–5 recordists per site collectively spent a total of over 1,000 recordist-hours actively tracking and recording wild bellbirds (per site: Tawhiti Rahi, 108; Lady Alice, 110; Hauturu, 140; Tawharanui, 132; Repanga, 260; Tiri, many hundreds of hours), with Marantz PMD661 portable solid-state recorders paired with handheld Sennheiser ME-66 shotgun microphones. Recordists coordinated movement to sample sites systematically and with maximal coverage, gathering 2,137 high-quality recorded songs (discrete vocalisation bouts) by adult individuals of known sex, during daylight hours. During recording, metadata including identity, age and sex of the focal bird was spoken into the microphone for later transcription.
Segmenting Songs Into Syllables
Songs were imported into a database in Koe bioacoustics software (Fukuzawa et al., 2020) and manually segmented into syllables by visually inspecting the spectrogram and setting syllable start/end points. Bellbirds mostly sing in discrete units that are easy to demarcate as syllables based on a gap either side. Occasionally there are fast-paced bursts where it is not clear where one unit ends and another begins; in these cases sounds were grouped together as a syllable if they were separated by <15 ms of silence. This value was chosen through trial and error for the best consistency of syllable groupings.
Classifying Syllables
To prepare for classification, the 20,700 segmented syllables were ranked by acoustic similarity in Koe. Similarity was calculated by extracting all available acoustic features for each unit and applying the UPGMA method (Sokal, 1958); see https://github.com/fzyukio/koe/wiki for details of extracted features and similarity index calculation.
Wesley H. Webb then compared syllables visually and aurally to classify them into types based on just-noticeable differences in pitch, timbre, and duration of the playback and visual appearance of the spectrogram. Manual perception-based classification is considered excellent for acoustic classification (Sayigh et al., 2007; Duda et al., 2012) and allowed finer scale than automated classification methods, such as multi-dimensional scaling (MDS) or principal component analysis (PCA). The high acuity of bird hearing relative to humans (Dooling, 2004) justifies a fine-scale classification approach. We validated our classification by having 74 inexperienced judges independently label a subset of syllables. Average match with our own labels was 89.6% (median 95.6%); see Fukuzawa et al. (2020) and Supplementary Article 1A for more details.
Sex Differences in Population-Level Repertoires
Male and Female Syllable Diversity
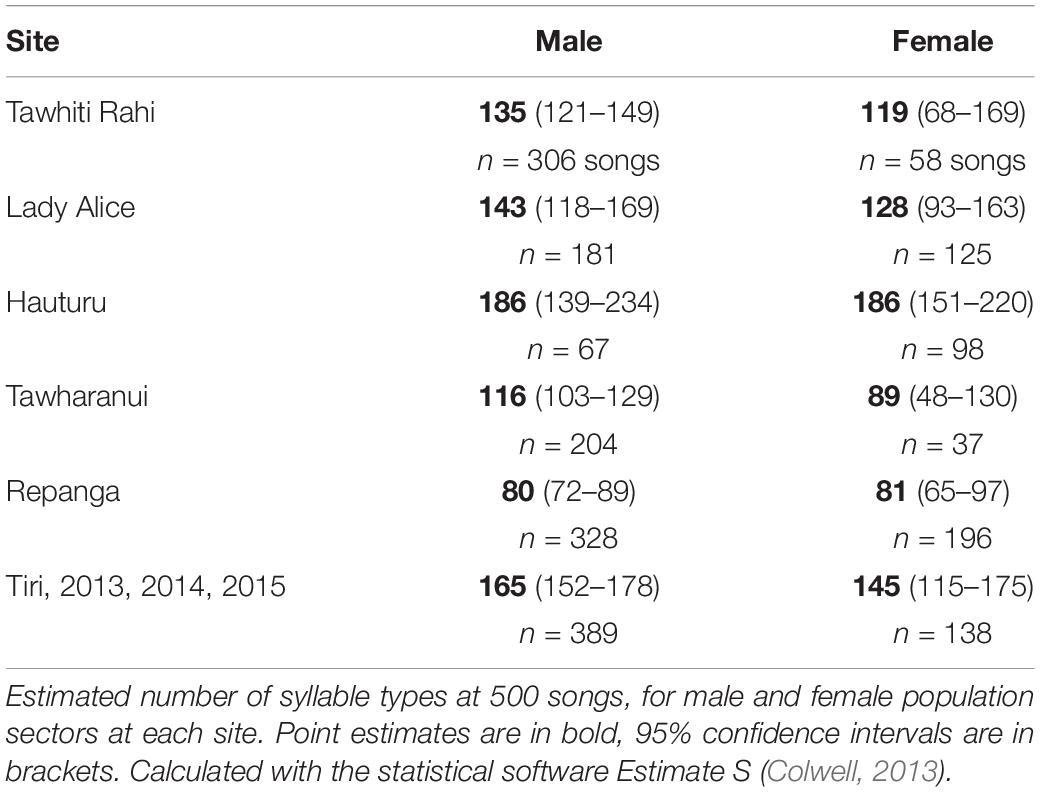
The recorded number of syllable types at each site are not directly comparable, due to the inevitable confounding of different sampling effort. To account for this, we used the statistical software EstimateS (Colwell, 2013) to produce syllable type accumulation curves and extrapolate an estimate of the total number of syllable types at each site (Supplementary Article 1B).
Repertoire Overlap of Male and Female Population Sectors
For each site we calculated the percentage overlap between male and female population repertoires, using the Jaccard similarity index (Hamers et al., 1989): the number of shared types divided by the total number of types.
Next, the acoustic feature measurements of all syllables (previously extracted to aid classification in Koe) were normalised by mean-centering and dividing by standard deviation. To test whether male-only, female-only, and shared syllable types form three distinguishable clusters in acoustic space, we classified syllables using a linear discriminant analysis (LDA) on the data, with leave-one-out cross-validation. If male-only, female-only, and shared syllables are randomly interspersed in acoustic space, then the LDA classification results will be similar to random assignment of groups. If the three groups occupy more distinct ranges, then the LDA classification will perform much better than random assignment.
The Pattern of Syllable Type Sharing Between Sites
We calculated repertoire overlaps between sites, for all pairwise combinations of site lists—comparing males against males, females against females. For each sex we tested for an isolation-by-distance pattern in repertoire overlaps between sites, in two ways.
First, we conducted a Hauturu-centric analysis. The large size, geographic centrality, and the long-term persistence of the Hauturu bellbird population make it likely to be a substantial source of dispersing bellbirds to other sites in the Hauraki Gulf archipelago. We calculated Spearman’s rank correlation coefficients between population repertoire overlap with Hauturu against geographic distance from Hauturu.
Next, to test for an overall isolation-by-distance pattern, we calculated Spearman’s rank correlation coefficients between percentage of site–site repertoire overlap against site–site geographic distance, for all pairwise combinations of sites in the archipelago. In this case, to account for non-independence of points (being pairwise combinations), we used the RELATE routine in PRIMER (version 7, Clarke and Gorley, 2015) with 9,999 permutations.
To test whether males or females share more syllables between sites overall, we used a two-tailed Sign Test (Dixon and Mood, 1946), with null hypothesis: the degree of site–site sharing is equal between sexes (Supplementary Article 1C).
Results
Sex Differences in Population Repertoires
Male and Female Syllable Diversity
Across the metapopulation we recorded a total of 702 syllable types: 337 male-only, 203 female-only, and 162 shared. See Supplementary Data 1 and Supplementary Table 1 for a complete catalogue. The entire Koe database of 2,137 songs (and segmented syllables) can be browsed online with spectrograms and playback; see section “Data Availability Statement.”
For each site and sex, the syllable type accumulation curve levelled off nearly or completely by 500 songs (i.e., approximating the true number of syllable types present), allowing meaningful comparison of syllable diversity (Supplementary Figure 1).
We found no evidence of a difference in repertoire size for males vs. females within sites; 95% confidence intervals overlap for males and females in all cases (Table 2). There was high variability between sites for both sexes, with male point estimates ranging from 80 (Repanga) to 186 (Hauturu) and females from 81 (Repanga) to 186 (Hauturu).
Repertoire Overlap of Male and Female Population Sectors
Male and female population sectors had largely separate repertoires, ranging from 6% total units shared at Tawhiti Rahi to 26% shared at Hauturu (Figure 2 and Supplementary Table 2). In other words, 74–94% of syllable types at each site were sung by one sex only.
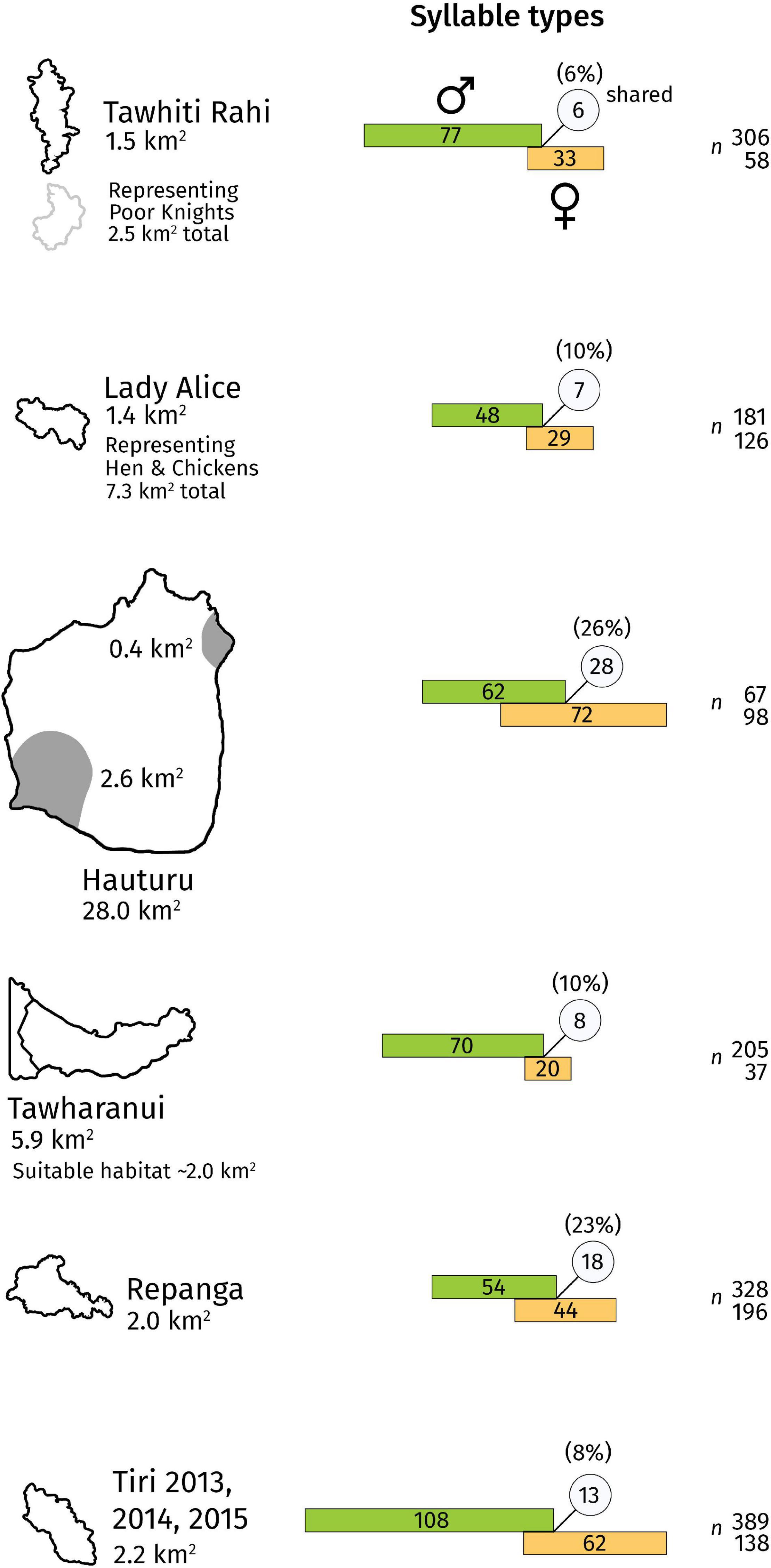
Figure 2. Overlap of male and female population syllable repertoires. Each diagram indicates the recorded number of male (green bar) and female (orange bar) syllable types, and the number of types common to both sexes (circle). The percentage overlap is given above the circle, calculated as the Jaccard similarity index: number of shared types divided by total number of types. For robustness, repertoires exclude types with fewer than three occurrences within that site-and-sex group. Bar length is proportional to the number of syllable types, and overlap length is proportional to the number of shared syllable types. Sample sizes (number of songs) for the male and female bars are given to the right of the bars. Note that repertoire sizes are recorded values, not extrapolated, and therefore are not directly comparable due to differing sample sizes. However, relative overlap percentage is more robust to differing sample sizes and thus meaningful to compare. Site outlines on the left of the figure are to scale. The shaded grey area in the Hauturu outline indicates the sampled region of the island.
In the LDA ordination Figure 3, male-only and female-only syllables form two clear, partially overlapping clusters, with shared syllables in between, when data from all sites are pooled. For individual sites the configuration of the three clusters varies but shows a tendency for female-only syllables to occur at lower LD1 values, male-only to occur at higher LD1 values, with shared syllables in between.
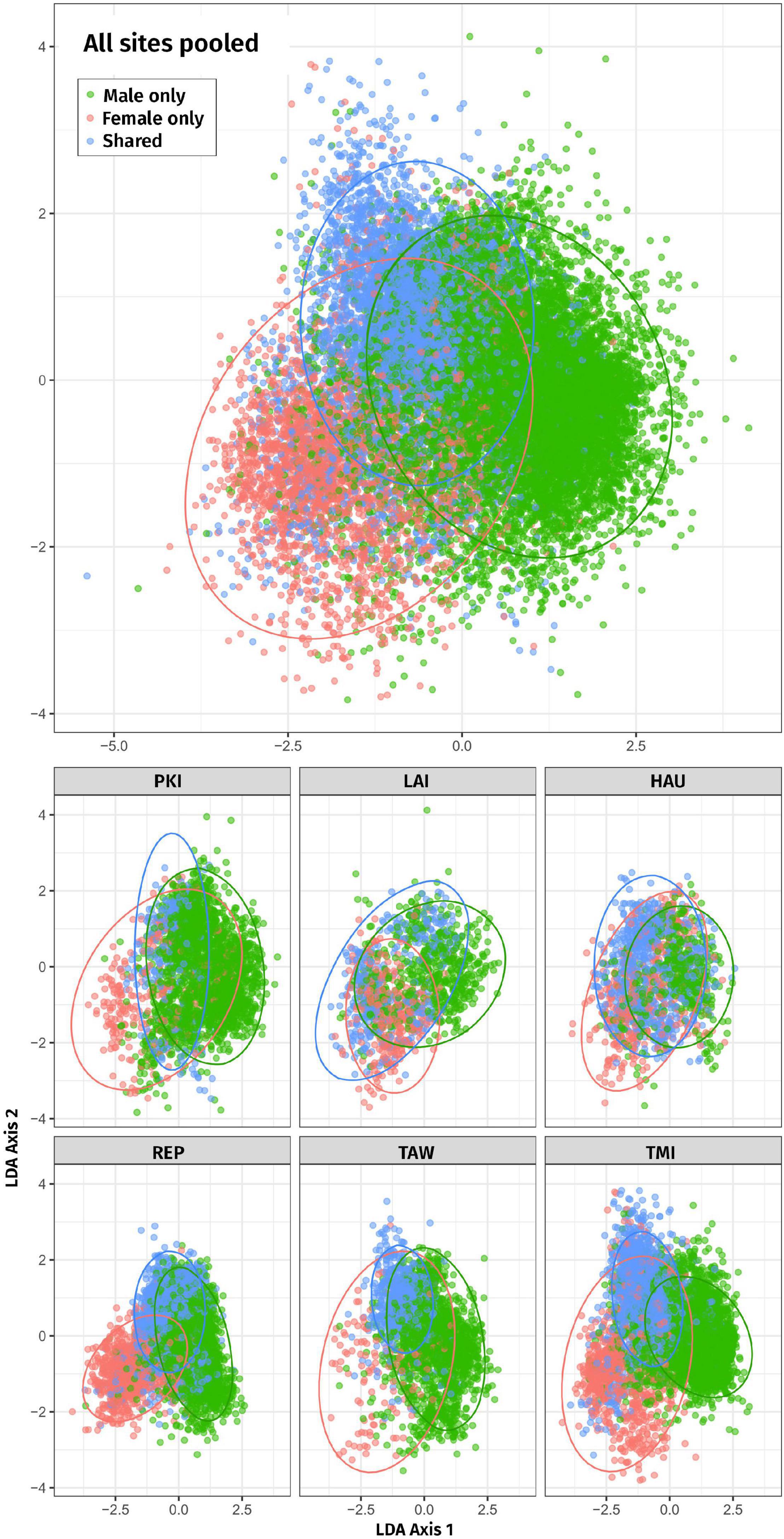
Figure 3. Two-dimensional linear discriminant analysis (LDA) of male-only, female-only, and shared syllables for all sites combined (top) and each site separately (bottom). Ellipses represent the regions covering 95% of the data (according to the fitted normal). Each site is plotted using the same axes to be comparable. Site abbreviations are as follows: PKI, Tawhiti Rahi, representing Poor Knights Islands; LAI, Lady Alice Island, representing Hen & Chickens; HAU, Hauturu; REP, Repanga; TAW, Tawharanui; TMI, Tiri.
Success of the LDA leave-one-out classifier had an overall lift of 1.78; that is, the LDA assigned labels (male-only, female-only, and shared) to syllables overall 1.78 times better than random allocation of labels. Lift was 1.51 for male-only syllables, 3.99 for female-only syllables, and 2.15 for shared syllables. P-values for all lift tests were <0.001; i.e., in all cases none of the 4,999 null distributions had a lift as large as the observed lift. Therefore, male-only, female-only, and shared types can be separated on the basis of acoustic features.
The Pattern of Meme Sharing Between Sites
Site–Site Repertoire Overlap
Matrices of site–site repertoire overlap are presented in Figure 4. The degree of site-site repertoire overlap between male populations was low, ranging 3–18% (median = 6%); for females: 1–12% (median = 5%). Remarkably, for both sexes, the syllable types shared between sites were almost exclusively pure-tone whistle syllables or simple stutter-like syllables. The one exception was Hauturu–Tawharanui, which additionally shared some more complex types following the recent Hauturu → Tawharanui founder event (Brunton et al., 2008; See Supplementary Data 1, 2).
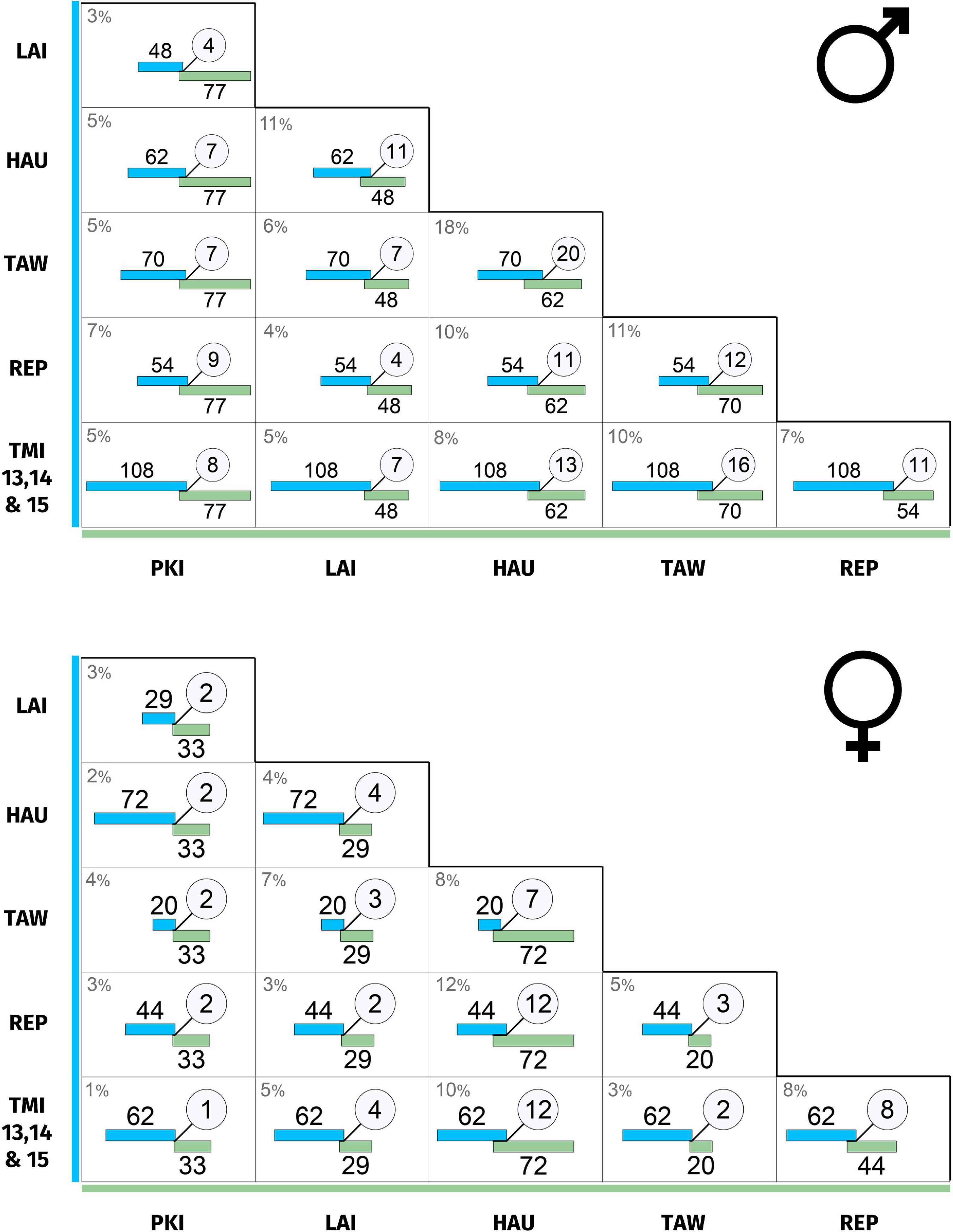
Figure 4. (Top) Male population repertoire overlap between sites. (Bottom) Female population repertoire overlap between sites. Each cell indicates the recorded number of syllable types for the two sites (blue and green bars), and the number of types common to both (circle). The percentage overlap is given at the top left of each cell and is calculated as Jaccard similarity index: number of shared types divided by total number of types. For robustness, repertoires exclude types with fewer than three occurrences within that site and sex. Bar length is proportional to the number of syllable types, and overlap length is proportional to the shared number of syllable types. Site abbreviations are as follows: PKI, Tawhiti Rahi (representing Poor Knights Islands); LAI, Lady Alice Island (representing Hen and Chickens); HAU, Hauturu; TAW, Tawharanui; REP, Repanga; TMI, Tiri.
Repertoire Overlap vs. Distance
The relationship between repertoire overlap with Hauturu vs. geographic distance from Hauturu is shown in Figure 5A. There was a negative correlation between sharing and distance for males (rs = -0.90, df = 3, 0.03 < P < 0.05) but no relationship for females (rs = -0.10, df = 3, P > 0.10). Contrary to predictions, sites at increasing distances from Hauturu did not share a progressively diminishing subset of syllable types with Hauturu, but different (apparently unrelated) subsets; this was true for both males and females (Supplementary Data 2).
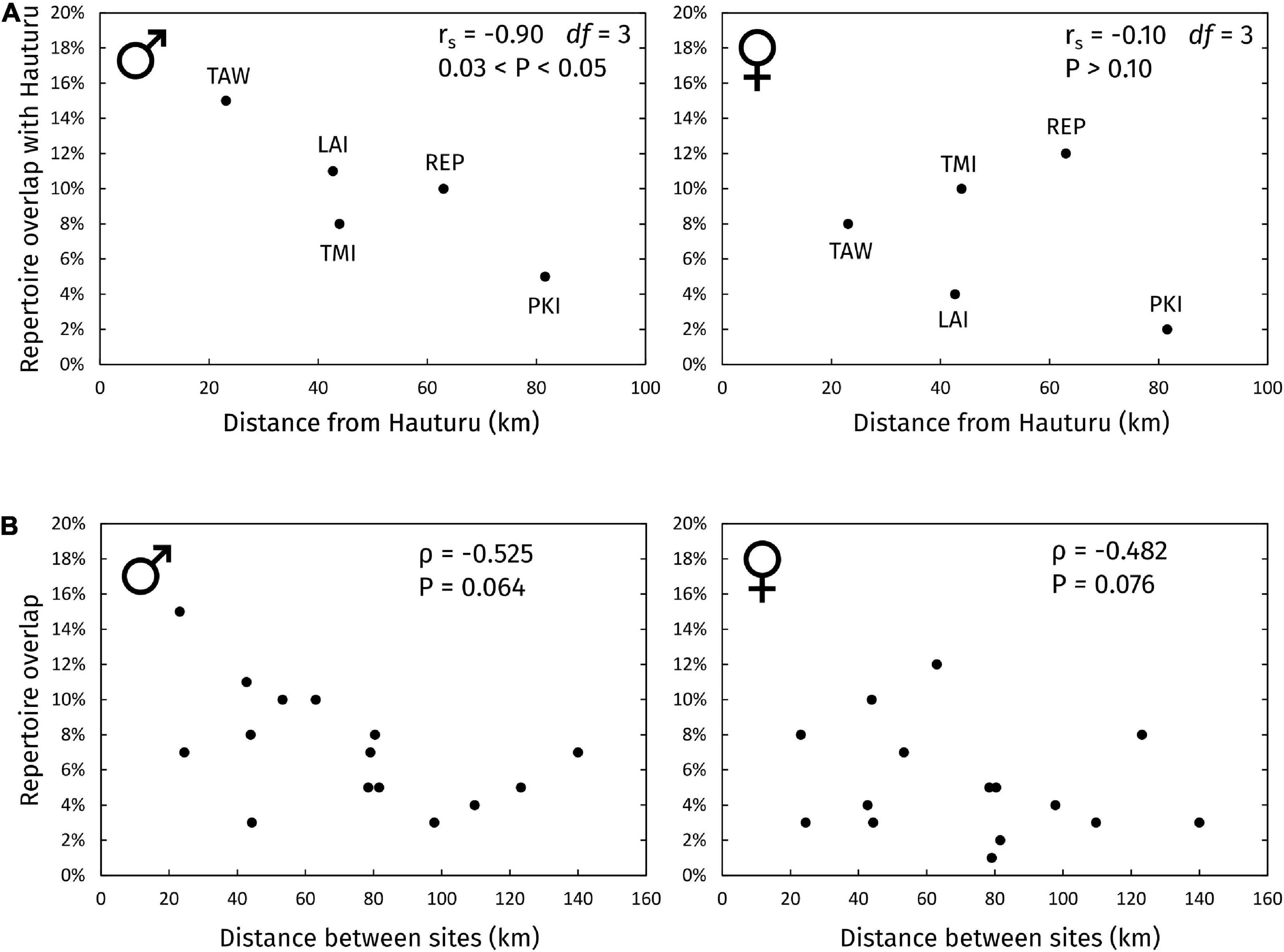
Figure 5. Repertoire overlap vs. distance between sites. (A) Repertoire overlap of all subpopulations with Hauturu vs. their distance from Hauturu. Overlaps were calculated on repertoire lists of types with 3+ occurrences within each site-and-sex group. Site abbreviations: TAW, Tawharanui; LAI, Lady Alice Island (representing Hen and Chickens); TMI, Tiri; REP, Repanga; PKI, Tawhiti Rahi (representing Poor Knights Islands). (B) Site-site repertoire overlap vs. site-site geographic distance, for all pairs of sites. Spearman’s rank correlation coefficients (rs) were calculated using the RELATE routine in PRIMER (version 7) with 9,999 permutations, accounting for the non-independence of points.
When considering sharing between all pairwise combinations of sites in the archipelago (Figure 5B), we did not find evidence of an isolation-by-distance pattern, for males (ρ = -0.525, P = 0.064) or females (ρ = -0.525, P = 0.076).
The recent founder event, where bellbirds from Hauturu colonised Tawharanui (Brunton et al., 2008) could possibly inflate the degree of sharing between the two sites, compared to other sites where both populations are well-established. Therefore, we tested robustness of the correlations to removal of the Hauturu–Tawharanui datapoint, but found little change in the slope or strength of the correlations.
There was no evidence of an overall difference between males and females in the degree of site-site repertoire overlap; out of 15 site-site comparisons, males shared a higher percentage of syllables than females in 9 cases, lower in 4 cases, and equal in 2 cases (Sign Test P = 0.17).
Discussion
This is one of the first comparisons of male and female song cultures across a metapopulation (Mennill and Rogers, 2006; Graham et al., 2017a, 2018a,b, 2021). We found that male and female bellbirds have comparable repertoire sizes (at a population level; Table 2), and sexually distinct vocal cultures, sharing only a small percentage of syllable types between sexes (6–26% within each site; Figure 2). Whether a type is male-specific, female-specific, or shared between sexes can be predicted based on its acoustic properties (Figure 3). Furthermore, song cultures of both sexes differ dramatically between sites—demonstrating male and female song dialects (Figure 4 and Supplementary Data 1). Despite a large and varied repertoire within sites, generally the only types shared between sites were flat-contour, pure-tone whistle syllables (Supplementary Article 1A) or simple stutter-like syllables. Between-site sharing of these syllable types decreased with distance for males but not females. These contrasting patterns of sharing across the archipelago may result from sex differences in dispersal, meme mutation rates and song-learning modes.
Comparisons of male and female repertoire sizes typically focus on individual-level repertoires. Such studies have found smaller female repertoires in some cases (e.g., rufous-and-white wrens Thryophilus rufalbus, Mennill and Vehrencamp, 2005; banded wrens Thryophilus pleurostictus, Hall et al., 2015), equal-sized repertoires in other cases (e.g., bay wren Thryothorus nigricapillus, Levin, 1996), and at least one case of larger female repertoire size (stripe-headed sparrow Peucaea r. ruficauda, Illes, 2014). The drivers of individual repertoire size are thought to differ between sexes, with males under selection from male–male competition and female choice (Catchpole, 1987; Hill et al., 2018), and females perhaps primarily from female–female competition for non-sexual breeding resources (Tobias et al., 2012). In the present study, similar male and female repertoire sizes may be explained by bellbird social ecology. Bellbirds are socially monogamous, and both sexes are highly social and aggressive in singing interactions (Roper, 2018). It is plausible, therefore, that similar intensity of competition and social interaction drive the evolution of similar syllable diversity in the two sexes. However, our analysis is on population-level repertoires, which are a product of both individual repertoire sizes and variation between individuals. For example, equal male and female population repertoire sizes could also result if male individual repertoires are larger and more consistent between individuals (cultural conformity; Aplin et al., 2015), and females smaller and more variable (cultural non-conformity; Riebel et al., 2015). Additional focused recording of banded individuals is required to quantify these two sources of diversity.
It is interesting that population repertoire sizes varied greatly between sites. Adaptation to differing acoustic environments (Potvin and Clegg, 2015; Graham et al., 2017b) seems an unlikely explanation, as all sites were coastal, with similar vegetation (though soundscape was not measured). Another potential explanation is that genetic diversity has driven population repertoire size. However, sites with low genetic diversity (Baillie, 2011) did not have correspondingly low syllable diversity, suggesting song culture is not tightly constrained by genetic diversity in bellbirds. We suspect that between-site differences in repertoire size are more likely driven by competition level. For example, sites with higher population density may have elevated competition for food, or sites with high population connectivity may experience increased competitive encounters with migrants—selecting for bigger individual repertoires.
The discovery of sexually distinct, yet partly overlapping syllable repertoires raises questions about transmission and function. Logically, all sex-specific syllable types must be learnt male-to-male or female-to-female (as in rufous-and-white wrens, for example; Mennill and Vehrencamp, 2005). But what of types common to both sexes? These must reflect inter-sexual learning in some form, whether accidental or intentional (Geberzahn and Gahr, 2013; Evans and Kleindorfer, 2016). Once learnt inter-sexually, memes may be transmitted intra-sexually to become an established part of the repertoire for that sex.
In blue-capped cordon-bleus Uraeginthus cyanocephalus, young birds of both sexes can learn from both male and female tutors. Not only so, but tutees housed together (two birds of the same age) strongly converge in their songs, learning from each other regardless of the sex of their peer (Geberzahn and Gahr, 2013). Inter-sexual learning can happen even in species with sexually contrasting song learning strategies; for example, tutoring experiments found that female northern cardinals Cardinalis cardinalis learn songs much faster than males, require auditory experience to develop normal song (whereas males do not), and, unlike males, do not change songs after dispersal. Despite such differences, both sexes learn equally well from male or female tutor recordings, without preference (Yamaguchi, 2001).
In bellbirds, juvenile male and female song is spectrally similar, then diverges prior to crystallisation (Roper et al., 2018). Perhaps both sexes of bellbird are physiologically capable of overlapping in acoustic space, but other factors (e.g., sexual/social selection) prevent it in the wild. This appears true in slate-colored boubous; male and female boubous share no syllable types in the wild, but birds hand-raised under experimental conditions are capable of developing syllables of both sexes (Wickler and Seibt, 1988). Wild birds may choose to express sex-specific memes to help avoid being mistaken for the other sex, which risks attracting same-sex rivals or repelling potential mates (Logue et al., 2007). However, the benefit of shared syllables is unclear. Might sex-specific syllables function in intra-sexual communication, and shared syllables in inter-sexual communication? Sophisticated field experiments with banded individuals are needed to resolve meme functions and transmission modes in bellbirds.
We found that male-specific and female-specific syllables do occupy two largely distinct regions of acoustic space, with shared syllables occupying a cluster between in a “sex-neutral” range (Figure 3). In the ordination, the separation of male-specific and female-specific memes could be due to universal morphological constraints of body size and syrinx structure, as males are 20% larger than females (Heather and Robertson, 2000) and have different syrinx morphologies (Roper, 2018). At the same time, the spread and shape of the clusters vary widely between sites, which may reflect site-specific cultures.
The pattern of meme sharing between sites defied our expectations. Repertoire similarity was not strongly related to geographic proximity between islands; only males showed some evidence of isolation with distance from Hauturu (Figure 5). However, for both sexes the percentage of syllable types shared between sites was small, and almost totally limited to pure-tone whistle syllables and simple stutter-like syllables (except for Hauturu–Tawharanui, which shared more types, likely due to the recent founder event described in Brunton et al., 2008). The observed pattern suggests that immigrants abandon most source memes after arrival, retaining only pure-tone and stutter-like types. We speculate that perhaps, all but these simple types incur high aggression at new sites (“colony password” hypothesis; Feekes, 1977) and so are dropped in favour of the local dialect.
The large size and geographic centrality of the Hauturu population make it a likely source of dispersing bellbirds to other sites in the archipelago. Thus, one might expect the islands around Hauturu to form a chain of “stepping stones” for bellbird dispersal, leading to progressively more dissimilar repertoires away from the central source population. There are many examples of such chains (Irwin et al., 2005; Parker et al., 2012; Lachlan et al., 2013). In contrast, we found that for both male and female bellbirds, sites at increasing distances from Hauturu did not share a progressively diminishing subset of syllable types with Hauturu, but different (apparently unrelated) subsets (Supplementary Data 2). Therefore, our results suggest direct dispersal to each site from Hauturu, rather than serial dispersal along the island chain.
We predicted that female-biased dispersal in bellbirds should result in higher female meme flow and thus higher inter-site sharing for females than for males. Instead, we found no evidence for a sexual difference in amount of sharing between sites. That females do not show higher inter-site sharing suggests that other processes—such as a higher turnover rate or weaker retention of source memes—counteract the greater connectivity of females between sites.
Large-scale studies of song culture in the wild are challenging. In contrast to a laboratory situation, it is difficult to amass data, the identity of individuals is not known, and there is little control over the social context of singing—which limits assessment of individual-level mechanisms. However, our population-level analyses reveal distinct and complex female culture with different spatial patterns to male culture. This underlines the inadequacy of the male-centric research paradigm and calls for comparisons of male and female repertoires in many more species (Riebel et al., 2019). Sophisticated field experiments are now needed to resolve the mechanisms of dispersal, selection and learning modes that give rise to the pattern we have uncovered.
Data Availability Statement
The interactive Koe database can be accessed online at koe.io.ac.nz with username korimako and password Bellbird_Culture. See Supplementary Data 3 for a table of all acoustic units and annotations and Supplementary Data 4 for a table of all songs and their metadata. Measurements of all syllables can be accessed at 10.5281/zenodo.5072580.
Ethics Statement
The animal study was reviewed and approved by the Massey University Animal Ethics Committee (permit number 15/21).
Author Contributions
DB and WW conceived of the study. WW, MR, DB, and AH conducted the fieldwork with help from volunteers. WW, YF, and MR created the song database. WW, YF, and MP analysed the data, with guidance from DB and AH. WW wrote the manuscript with guidance from the other authors. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by a Marsden Fund grant (13-MAU-004) from the Royal Society of New Zealand Te Apārangi.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Ngāti Wai and Ngāti Manuhiri iwi for their support, and Barbara Evans, Jessica Patiño-Pérez, and Mehrnaz Tavasoli for their help with recording song. The manuscript was improved by feedback from Rebecca Webb.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.755633/full#supplementary-material
References
Aplin, L. M. (2019). Culture and cultural evolution in birds: a review of the evidence. Anim. Behav. 147, 179–187. doi: 10.1016/j.anbehav.2018.05.001
Aplin, L. M., Farine, D. R., Morand-Ferron, J., Cockburn, A., Thornton, A., and Sheldon, B. C. (2015). Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538–541. doi: 10.1038/nature13998
Austin, V. I., Dalziell, A. H., Langmore, N. E., and Welbergen, J. A. (2021). Avian vocalisations: the female perspective. Biol. Rev. 96, 1484–1503. doi: 10.1111/brv.12713
Baillie, S. M. (2011). Population Genetics, Biogeography and Ecological Interactions of the New Zealand bellbird (Anthornis melanura) and Their Avian Malaria Parasites: A Thesis Presented in Partial Fulfilment of the Requirements. Ph.D. thesis. Albany: Zoology at Massey University.
Baillie, S. M., Ritchie, P. A., and Brunton, D. H. (2014). Population genetic connectivity of an endemic New Zealand passerine after large-scale local extirpations: a model of re-colonization potential. Ibis 156, 826–839. doi: 10.1111/ibi.12182
Baptista, L. F., and Schuchmann, K.-L. (1990). Song learning in the Anna hummingbird (Calypte anna). Ethology 84, 15–26.
Brown, R. N., and Lemon, R. E. (1979). Structure and evolution of song form in the wrens Thryothorus sinaloa and T. felix. Behav. Ecol. Sociobiol. 5, 111–131. doi: 10.1007/BF00293301
Brunton, D. H., Evans, B. A., and Ji, W. (2008). Assessing natural dispersal of New Zealand bellbirds using song type and song playbacks. N. Z. J. Ecol. 32, 147–154.
Brunton, D. H., and Li, X. (2006). The song structure and seasonal patterns of vocal behavior of male and female bellbirds (Anthornis melanura). J. Ethol. 24, 17–25. doi: 10.1007/s10164-005-0155-5
Catchpole, C. K. (1987). Bird song, sexual selection and female choice. Trends Ecol. Evol. 2, 94–97. doi: 10.1016/0169-5347(87)90165-0
Catchpole, C. K., and Slater, P. J. B. (2008). Bird Song: Biological Themes and Variations, 2nd Edn. New York, NY: Cambridge University Press. doi: 10.1017/CBO9780511754791
Clarke, A. L., Saether, B.-E., and Røskaft, E. (1997). Sex biases in avian dispersal: a reappraisal. Oikos 79, 429–438. doi: 10.2307/3546885
Colwell, R. (2013). EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Available online at: http://purl.oclc.org/estimates (accessed October 29, 2018).
Craig, J. L., and Douglas, M. E. (1986). Resource distribution, aggressive asymmetries and variable access to resources in the nectar feeding bellbird. Behav. Ecol. Sociobiol. 18, 231–240. doi: 10.1007/BF00290827
Diblíková, L., Pipek, P., Petrusek, A., Svoboda, J., Bílková, J., Vermouzek, Z., et al. (2019). Detailed large-scale mapping of geographical variation of yellowhammer Emberiza citrinella song dialects in a citizen science project. Ibis 161, 401–414. doi: 10.1111/ibi.12621
Dixon, W. J., and Mood, A. M. (1946). The statistical sign test. J. Am. Stat. Assoc. 41, 557–566. doi: 10.1080/01621459.1946.10501898
Dooling, R. (2004). “Audition: can birds hear everything they sing?,” in Nature’s Music, eds P. Marler, and H. Slabbekoorn (San Diego, CA: Elsevier), 206–225. doi: 10.1016/B978-012473070-0/50010-4
Duda, R. O., Hart, P. E., and Stork, D. G. (2012). Pattern Classification. Hoboken, NJ: John Wiley & Sons.
Evans, C., and Kleindorfer, S. (2016). Superb fairy-wren (Malurus cyaneus) sons and daughters acquire song elements of mothers and social fathers. Front. Ecol. Evol. 4:9. doi: 10.3389/fevo.2016.00009
Feekes, F. (1977). Colony-specific song in Cacicus cela (Icteridae, Aves) – password hypothesis. Ardea 65, 197–202.
Fukuzawa, Y., Webb, W. H., Pawley, M. D. M., Roper, M. M., Marsland, S., Brunton, D. H., et al. (2020). Koe: web-based software to classify acoustic units and analyse sequence structure in animal vocalizations. Methods Ecol. Evol. 11, 431–441. doi: 10.1111/2041-210X.13336
Geberzahn, N., and Gahr, M. (2013). Song learning in male and female Uraeginthus cyanocephalus, a tropical songbird species. J. Comp. Psychol. 127, 352–364. doi: 10.1037/a0033154
Graham, B. A., Heath, D. D., and Mennill, D. J. (2017a). Dispersal influences genetic and acoustic spatial structure for both males and females in a tropical songbird. Ecol. Evol. 7, 10089–10102. doi: 10.1002/ece3.3456
Graham, B. A., Sandoval, L., Dabelsteen, T., and Mennill, D. J. (2017b). A test of the acoustic adaptation hypothesis in three types of tropical forest: degradation of male and female Rufous-and-white Wren songs. Bioacoustics 26, 37–61. doi: 10.1080/09524622.2016.1181574
Graham, B. A., Heath, D. D., and Mennill, D. J. (2021). Female and male song exhibit both parallel and divergent patterns of cultural evolution: a long-term study of song structure and diversity in tropical wrens. Ornithology 138:ukaa067. doi: 10.1093/ornithology/ukaa067
Graham, B. A., Heath, D. D., Walter, R. P., Mark, M. M., and Mennill, D. J. (2018a). Parallel evolutionary forces influence the evolution of male and female songs in a tropical songbird. J. Evol. Biol. 31, 979–994. doi: 10.1111/jeb.13279
Graham, B. A., Heath, D. D., Walter, R. P., and Mennill, D. J. (2018b). Immigrant song: males and females learn songs after dispersal in a tropical bird. Behav. Ecol. 29, 711–723. doi: 10.1093/beheco/ary029
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
Hall, M. L. (2000). The function of duetting in magpie-larks: conflict, cooperation, or commitment? Anim. Behav. 60, 667–677. doi: 10.1006/anbe.2000.1517
Hall, M. L., Rittenbach, M. R. D., and Vehrencamp, S. L. (2015). Female song and vocal interactions with males in a neotropical wren. Behav. Evol. Ecol. 3:12. doi: 10.3389/fevo.2015.00012
Hamers, L., Hemeryck, Y., Herweyers, G., Janssen, M., Keters, H., Rousseau, R., et al. (1989). Similarity measures in scientometric research: the Jaccard index versus Salton’s cosine formula. Inf. Process. Manag. 25, 315–318. doi: 10.1016/0306-4573(89)90048-4
Heather, B. D., and Robertson, H. A. (2000). The Field Guide to the Birds of New Zealand. Auckland: Viking.
Hill, S. D., Brunton, D. H., Anderson, M. G., and Ji, W. (2018). Fighting talk: complex song elicits more aggressive responses in a vocally complex songbird. Ibis 160, 257–268.
Illes, A. E. (2014). Context of female bias in song repertoire size, singing effort, and singing independence in a cooperatively breeding songbird. Behav. Ecol. Sociobiol. 69, 139–150. doi: 10.1007/s00265-014-1827-3
Irwin, D. E., Bensch, S., Irwin, J. H., and Price, T. D. (2005). Speciation by distance in a ring species. Science 307, 414–416. doi: 10.1126/science.1105201
Jenkins, P. F. (1978). Cultural transmission of song patterns and dialect development in a free-living bird population. Anim. Behav. 26, 50–78. doi: 10.1016/0003-3472(78)90007-6
Kroodsma, D., Hamilton, D., Sánchez, J. E., Byers, B. E., Fandiño-Mariño, H., Stemple, D. W., et al. (2013). Behavioral evidence for song learning in the suboscine bellbirds (Procnias spp.; Cotingidae). Wilson J. Ornithol. 125, 1–14. doi: 10.1676/12-033.1
Lachlan, R. F., Verzijden, M. N., Bernard, C. S., Jonker, P.-P., Koese, B., Jaarsma, S., et al. (2013). The progressive loss of syntactical structure in bird song along an island colonization chain. Curr. Biol. 23, 1896–1901. doi: 10.1016/j.cub.2013.07.057
Lande, R. (1980). Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305. doi: 10.2307/2407393
Levin, R. N. (1996). Song behaviour and reproductive strategies in a duetting wren, Thryothorus nigricapillus: I. Removal experiments. Anim. Behav. 52, 1093–1106. doi: 10.1006/anbe.1996.0257
Lobato, M., Vellema, M., Gahr, C., Leitão, A., de Lima, S. M. A., Geberzahn, N., et al. (2015). Mismatch in sexual dimorphism of developing song and song control system in blue-capped cordon-bleus, a songbird species with singing females and males. Front. Ecol. Evol. 3:117. doi: 10.3389/fevo.2015.00117
Logue, D. M., Droessler, E. E., Roscoe, D. W., Vokey, J. R., Rendall, D., and Kunimoto, R. M. (2007). Sexually antithetical song structure in a duet singing wren. Behaviour 144, 331–350. doi: 10.1163/156853907780425749
Mann, N. I., Dingess, K. A., Barker, F. K., Graves, J. A., and Slater, P. J. B. (2009). A comparative study of song form and duetting in neotropical Thryothorus wrens. Behaviour 146, 1–43.
Mennill, D. J., and Rogers, A. C. (2006). Whip it good! Geographic consistency in male songs and variability in female songs of the duetting eastern whipbird Psophodes olivaceus. J. Avian Biol. 37, 93–100. doi: 10.1111/j.0908-8857.2006.03548.x
Mennill, D. J., and Vehrencamp, S. L. (2005). Sex differences in singing and duetting behavior of neotropical rufous-and-white wrens (Thryothorus rufalbus). Auk 122, 175–186.
Odom, K. J., Hall, M. L., Riebel, K., Omland, K. E., and Langmore, N. E. (2014). Female song is widespread and ancestral in songbirds. Nat. Commun. 5:3379. doi: 10.1038/ncomms4379
Paris, D., Nicholls, A. O., Hall, A., Harvey, A., and Massaro, M. (2016). Female-biased dispersal in a spatially restricted endemic island bird. Behav. Ecol. Sociobiol. 70, 2061–2069. doi: 10.1007/s00265-016-2210-3
Parker, K. A., Anderson, M. J., Jenkins, P. F., and Brunton, D. H. (2012). The effects of translocation-induced isolation and fragmentation on the cultural evolution of bird song. Ecol. Lett. 15, 778–785. doi: 10.1111/j.1461-0248.2012.01797.x
Paton, J. A., Manogue, K. R., and Nottebohm, F. (1981). Bilateral organization of the vocal control pathway in the budgerigar, Melopsittacus undulatus. J. Neurosci. 1, 1279–1288.
Podos, J., and Warren, P. S. (2007). “The evolution of geographic variation in birdsong,” in Advances in the Study of Behavior, eds H. J. Brockmann, T. J. Roper, M. Naguib, K. E. Wynne-Edwards, C. Barnard, and J. Mitani (Maryland Heights, MO: Academic Press), 403–458.
Poole, J. H., Tyack, P. L., Stoeger-Horwath, A. S., and Watwood, S. (2005). Animal behaviour: elephants are capable of vocal learning. Nature 434, 455–456.
Potvin, D. A., and Clegg, S. M. (2015). The relative roles of cultural drift and acoustic adaptation in shaping syllable repertoires of island bird populations change with time since colonization. Evolution 69, 368–380. doi: 10.1111/evo.12573
Price, J. J. (2015). Rethinking our assumptions about the evolution of bird song and other sexually dimorphic signals. Behav. Evol. Ecol. 3:40. doi: 10.3389/fevo.2015.00040
Riebel, K., Lachlan, R. F., and Slater, P. J. B. (2015). “Chapter six – Learning and cultural transmission in chaffinch song,” in Advances in the Study of Behavior, eds M. Naguib, H. J. Brockmann, J. C. Mitani, L. W. Simmons, L. Barrett, S. Healy, et al. (Cambridge, MA: Academic Press), 181–227. doi: 10.1016/bs.asb.2015.01.001
Riebel, K., Odom, K. J., Langmore, N. E., and Hall, M. L. (2019). New insights from female bird song: towards an integrated approach to studying male and female communication roles. Biol. Lett. 15:20190059.
Roper, M. M. (2018). Sexual Dimorphism of Song and Life History Trade-Offs in the New Zealand Bellbird: A Thesis Presented in Partial Fulfilment of the Requirements. Ph. D. thesis. Albany: Massey University.
Roper, M. M., Harmer, A. M. T., and Brunton, D. H. (2018). Developmental changes in song production in free-living male and female New Zealand bellbirds. Anim. Behav. 140, 57–71. doi: 10.1016/j.anbehav.2018.04.003
Sanvito, S., Galimberti, F., and Miller, E. H. (2007). Observational evidences of vocal learning in southern elephant seals: a longitudinal study. Ethology 113, 137–146. doi: 10.1111/j.1439-0310.2006.01306.x
Sayigh, L. S., Esch, H. C., Wells, R. S., and Janik, V. M. (2007). Facts about signature whistles of bottlenose dolphins, Tursiops truncatus. Anim. Behav. 74, 1631–1642. doi: 10.1016/j.anbehav.2007.02.018
Sokal, R. R. (1958). A statistical method for evaluating systematic relationship. Univ. Kans. Sci. Bull. 28, 1409–1438.
Tobias, J. A., Montgomerie, R., and Lyon, B. E. (2012). The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2274–2293. doi: 10.1098/rstb.2011.0280
Webb, W. H., Brunton, D. H., Aguirre, J. D., Thomas, D. B., Valcu, M., and Dale, J. (2016). Female song occurs in songbirds with more elaborate female coloration and reduced sexual dichromatism. Front. Ecol. Evol. 4:22. doi: 10.3389/fevo.2016.00022
Whitehead, H., and Rendell, L. (2014). The Cultural Lives of Whales and Dolphins. Chicago, IL: University of Chicago Press.
Wickler, W., and Seibt, U. (1980). Vocal duetting and the pair bond. Z. Tierpsychol. 52, 217–226. doi: 10.1111/j.1439-0310.1980.tb00713.x
Wickler, W., and Seibt, U. (1988). “Gender dialects” — a new phenomenon from birdsong epigenesis. Naturwissenschaften 75, 51–52. doi: 10.1007/BF00367445
Keywords: female song, dialects, cultural evolution, birdsong, avian, bioacoustics, oscine passerine, song culture
Citation: Webb WH, Roper MM, Pawley MDM, Fukuzawa Y, Harmer AMT and Brunton DH (2021) Sexually Distinct Song Cultures Across a Songbird Metapopulation. Front. Ecol. Evol. 9:755633. doi: 10.3389/fevo.2021.755633
Received: 09 August 2021; Accepted: 12 October 2021;
Published: 17 November 2021.
Edited by:
Ann Valerie Hedrick, University of California, Davis, United StatesReviewed by:
Tomasz S. Osiejuk, Adam Mickiewicz University, PolandManfred Gahr, Max-Planck-Gesellschaft (MPG), Germany
Copyright © 2021 Webb, Roper, Pawley, Fukuzawa, Harmer and Brunton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wesley H. Webb, d2VzbGV5dGhld2ViYkBnbWFpbC5jb20=
 Wesley H. Webb
Wesley H. Webb Michelle M. Roper
Michelle M. Roper Matthew D. M. Pawley
Matthew D. M. Pawley Yukio Fukuzawa
Yukio Fukuzawa Aaron M. T. Harmer
Aaron M. T. Harmer Dianne H. Brunton
Dianne H. Brunton