Abstract
Changing ecosystem conditions and diverse socio-economical events have contributed to an ingrained presence of non-native tree species (NNTs) in the natural and cultural European landscapes. Recent research endeavors have focused on different aspects of NNTs such as legislation, benefits, and risks for forestry, emphasizing that large knowledge gaps remain. As an attempt to fulfill part of these gaps, within the PEN-CAFoRR COST Action (CA19128) network, we established an open-access questionnaire that allows both academic experts and practitioners to provide information regarding NNTs from 20 European countries. Then, we integrated the data originating from the questionnaire, related to the country-based assessment of both peer-reviewed and grey literature, with information from available datasets (EUFORGEN and EU-Forest), which gave the main structure to the study and led to a mixed approach review. Finally, our study provided important insights into the current state of knowledge regarding NNTs. In particular, we highlighted NNTs that have shown to be less commonly addressed in research, raising caution about those characterized by an invasive behavior and used for specific purposes (e.g., wood production, soil recultivation, afforestation, and reforestation). NNTs were especially explored in the context of resilient and adaptive forest management. Moreover, we emphasized the assisted and natural northward migration of NNTs as another underscored pressing issue, which needs to be addressed by joint efforts, especially in the context of the hybridization potential. This study represents an additional effort toward the knowledge enhancement of the NNTs situation in Europe, aiming for a continuously active common source deriving from interprofessional collaboration.
Introduction
Non-native tree species in Europe: Context and aims of the work
Non-native tree species (NNTs) are not a novelty in European forests, where the diversity has been established through evolutionary mechanisms and human impact (Bradshaw, 2004). Although most NNTs have been purposefully introduced, in monoculture plantations, mixed managed stands, and native forests during the past centuries, defining NNTs is a demanding task (Brus et al., 2019). Alpert et al. (2000) defined non-native as species “having been transported into a region by humans across a barrier that has apparently prevented natural dispersal so far.” The early utilitarian introduction of species to serve as an additional food and wood source, like in the case of Corylus avellana, Castanea sativa, and Juglans regia, was later extended to introduction due to curiosity, for ornamental, and forest restoration purposes (Nyssen et al., 2016). However, the discussion regarding the use of NNTs has recently shifted from their forestry-benefiting services to their long-term effects on ecosystems, and notably toward their potential role in climate change mitigation (Frischbier et al., 2019; Pötzelsberger et al., 2020a). The integration of species that demonstrate higher tolerance and better adaptive potential to climate change is a key strategy in adaptive forest management (Bolte et al., 2009). Increasing the number and genetic diversity may help construct more resistant and adaptable forest ecosystems that are better suited to respond to climate changes (Royer-Tardif et al., 2021). However the potential risks of NNTs, including their invasive potential (Pötzelsberger et al., 2020a; Bindewald et al., 2021), are not negligible.
In particular, while forest ecosystems are fundamental for reducing the impacts of climate change, they are also severely impacted by extreme weather events, which restrict the ecosystem services they provide and the general tree species distribution across Europe (Nordén et al., 2014; Dyderski et al., 2018; Buras and Menzel, 2019; Montagnoli et al., 2022). These climate-change related modifications have contributed to increased abiotic stressors (e.g., drought, salinity, temperature extremes) and biotic risks in forests (e.g., pathogens and pests, invasive species) (Teshome et al., 2020). Along with the anthropogenic pressures, the structure and dynamics of the forest landscape are changing (Bolte et al., 2009), impacting both native and non-native trees which provide numerous ecosystem services, ranging from provisioning, to regulatory, cultural, and ecological (Castro-Díez et al., 2019). The current role of forestry as a climate change mitigation tool is widely recognized (Canadell and Raupach, 2008) and anticipating the forest’s response to these climatic challenges is crucial for ensuring the provisioning of ecosystem services (Royer-Tardif et al., 2021). Mixed forests with native and/or NNTs are regarded as more resilient to monocultures when it comes to the resistance against effects of climate change (Ammer, 2019). Therefore, NNTs have been and continue to be explored for the potential benefits (e.g., productivity, biodiversity) without disregarding the risks (e.g., potential invasiveness) (Bolte et al., 2009).
The status of a species, whether invasive or not, is determined not only by the economic and ecological characteristics, but also by the socio-cultural context, and it is not fixed but constantly evolving with time (Heger, 2016). On one hand, the anticipated potential of many NNTs (e.g., productivity potential, soil stabilization, and aesthetic function) has facilitated their unplanned and unmonitored distribution. In some contexts, this has already altered the perception of the native landscape along with the composition and functioning of existing ecosystems (Brundu and Richardson, 2016; Brus et al., 2019). On the other hand, only some NNTs have exhibited invasive potential, by severely repressing the native species and introducing new pathogens, while many have been shown to provide numerous ecosystem services, in forest and urban areas (Alpert et al., 2000; Blackburn et al., 2014; Bartz and Kowarik, 2019). Past works exploring the risks and benefits of NNTs agree that the task of comparing NNTs’ attributes and assessing their impact at varying spatial and temporal scales is a major challenge, which is highly context-dependent and limited to the tree-specific characteristics (Blackburn et al., 2014; Bartz and Kowarik, 2019; Castro-Díez et al., 2019; Bindewald et al., 2021). It is notoriously difficult to identify specific plant traits that can be consistently associated with higher invasive potential (Alpert et al., 2000) and generalizing NNTs behavior as a cautionary approach disregards the fact that it may greatly vary across environmental conditions (Bartz and Kowarik, 2019). Thus, the functional aspects and the services NNTs can provide under certain conditions–rather than the origin of the species–require more attention (Brus et al., 2019).
Despite their importance to forestry and agroforestry systems, detailed information focusing on NNTs’ occurrence, distribution, use, and risks for most European countries is currently incomplete and scattered across published and unpublished literature and databases, and often only available in the local languages (Krumm and Vitkova, 2016; Brus et al., 2019). As previously observed and what our experience has shown during the preparation of the present study, the role and acceptance of NNTs in Europe vary greatly among countries due to several factors, i.e., the degree of native biodiversity, the climatic conditions that encourage or limit the establishment of NNTs, forest management practices, types of forest ownership, legislation and standards, local communities motivation, and land-use history (Koskela et al., 2013; Nunes et al., 2019; Pötzelsberger et al., 2020a,b). Moreover, research efforts in the field of NNTs have been strongly focused on the most common ones (e.g., Robinia pseudoacacia, Prunus serotina, Acer negundo, Quercus rubra, Ailanthus altissima, Acacia spp., Eucalyptus spp., Pseudotsuga menziesii, Picea sitchensis) (Krumm and Vitkova, 2016), while disregarding numerous other NNTs. This is especially the case for the presumably less common NNTs, for which information is under reported in peer-reviewed journals (Castro-Díez et al., 2021).
Significant attempts for risk assessment frameworks that would allow for better overview and subsequent management improvement, as a risk mitigation strategy, in the case of NNTs have been made (Sandvik et al., 2013; Ennos et al., 2019). For instance, Bindewald et al. (2021) recently developed a new and more inclusive methodological framework for NNTs site-specific risk analysis; but as acknowledged by the authors, frameworks cannot replace research efforts and monitoring programs that provide the backbone information. Royer-Tardif et al. (2021) provided a framework for quantitative evaluation of five key components of tree adaptive capacity to climate change and used it to evaluate 26 north-eastern American tree species based on a literature review. Their results indicate that no species maintains a consistent score across the five components (adaptive capacity to climate change, phenotypic plasticity, phenotypic diversity, and genetic exchange between populations and species), indicating the need for an interdisciplinary collaboration (Royer-Tardif et al., 2021). These conclusions further emphasize the need for more inclusive and serious research efforts. In the European context, academia, and practice alike, face the lack of precise distribution data and a comprehensive inventory that reflects the actual on-field situation and includes all tree species. National Forest Inventories (NFI) have served as the main source of information for numerous works but obstacles such as grid variability in sampling design and purphoses and data focused on only certain aspects, i.e., wood production, have left considerable gaps (Alberdi et al., 2019). The different approaches to forest management and research scopes, necessitate a more uniform and inclusive methodology regarding distribution and experiences with NNTs. Furthermore, in Europe, the distribution shift northward and westward is a natural process as the southern peninsulas have probably served as a glacial refugium for numerous species (Fagus sylvatica, Populus alba, Populus tremula, Quercus spp. Abies alba, Pinus pinaster, etc.). Recent research results show that shifts in climate niches expected for 2060–2080 will be observed as early as 2040–2060 and significantly threaten the current biodiversity and forest management practices (Puchałka et al., 2021). Identifying regions with climatological properties analogous to projected conditions of a certain location (Buras and Menzel, 2019) could be one approach in terms of retrospective investigation of tree species suitability. Although not a novel approach, the climate analogues need to be taken alongside other indicators in terms of practical recommendation (Boiffin et al., 2017), especially considering the history of land use, social, political, and economic changes, along with regional climate conditions and forest management practices that have combined impact on tree species utilization and distribution across Europe (Nunes et al., 2019).
Nonetheless, species with a wider distribution range may be composed of populations with higher local adaptability and plasticity, and, thus, serve as promising candidates to face climate pressure (Benito Garzón et al., 2011; Alizoti et al., 2022; Prasad and Leites, 2022). Species migration has been greatly impacted by anthropogenic activities and therefore differs among species (Mattioni et al., 2013; Tíscar et al., 2018). These assisted migrations are foreseen as a tool to help compromised tree species since the velocity of climate change is faster than the ability of trees to migrate and potentially adapt (Williams and Dumroese, 2013; Leroy et al., 2020). Therefore, assisted migration is a crucial part of adaptive forestry management.
However, assisted migrations further open the hybridization question. Hybridization is a natural phenomenon, often observed in secondary contact zones during post-glacial colonization (Jaramillo-Correa et al., 2009; Fussi et al., 2010). Thus, hybridization can be a major source of adaptive potential by increasing genetic diversity, and allowing for preadapted alleles to introgress into a population (Broadhurst et al., 2008; Weeks et al., 2011; Aitken and Bemmels, 2016; Tigano and Friesen, 2016). Hybridization could also break up beneficial allele combinations, increase the risk of outbreeding depression, and lead to a reduction in offspring fitness compared to the parental generation (Edmands, 2006; Frankham et al., 2011). This may result in an accelerated extinction of lineages or even species (Rhymer and Simberloff, 1996; Todesco et al., 2016). Due to assisted migrations, the hybridization output is also very context-depended and can have both a negative (decline in reproductive fitness and introgression) and a positive impact (generating novel and more potent genetic combinations) (Pollegioni et al., 2013), the latter being especially relevant in mitigating climate change (Royer-Tardif et al., 2021). In Europe, the proximity and distribution of numerous congeneric tree species increase the possibilities for interspecific hybridization, a possible facilitator of adaptive introgression (Leroy et al., 2020). The NNTs hybridization potential is even more so enhanced by the loose approach of the early legislation and the ease of movement of plant material on a pan-European level (Pötzelsberger et al., 2020b). Thus, unpredictable outcomes are a reality in European forestry and their impact cannot be taken lightly.
With this information and experiences in mind, regarding the pan-European region, the threefold aim of this study is:
- (i)
To provide an overview of the current occurrence of NNTs on a country-specific basis in terms of diversity and assisted migration potential, and the possible factors that have led to it;
- (ii)
To identify the knowledge gaps focusing on those NNTs that are less-common in terms of, both, occurrence and research efforts (less/minor case studies), which may hold potential for ecosystem and socio-economic services, and climate change mitigation;
- (iii)
To analyze all NNTs noted with the questionnaire from the point of view of hybridization with native species, in terms of risks and/or opportunities.
Analyzing non-native tree species in Europe through a mixed approach: The rationale behind
To achieve these aims, we took advantage of the network built within the COST Action “Pan-European Network for Climate Adaptive Forest Restoration and Reforestation” (PEN-CAFoRR, CA19128, 2020–2024) which contributes to research on climate-adapted forest restoration and reforestation. Considering the complexity of the issue and the potential to involve numerous experts from academia and practice, we adopted a mixed approach in terms of data collection, analysis, and interpretation, integrating the use of a country-based questionnaire and already existing datasets. In particular, the 26 questionnaire contributors, who represented 20 European countries, consensually agreed that peer-reviewed publications regarding NNTs would provide limited information, which can, to a certain extent, be surpassed by the inclusion of grey literature. While numerous definitions of grey literature exist (Mahood et al., 2014), we defined it as works that have not been peer-reviewed (e.g., various types of scientific reports, non-ISI papers, national reports, theses, conference proceedings, conference abstracts, posters, conference presentations, newspaper articles, unpublished work, books, literature reviews, and personal communications of an expert in academia and/or practice), and/or are written in a language other than English. The inclusion of grey literature is often questioned due to the validity and the quality of the reported data, but when used alongside peer-reviewed literature, it can contribute to more balanced and thorough information (Mahood et al., 2014) as long as experts have been involved in writing (Sandvik et al., 2013). Additionally, the size and scope of the work in a scientific publication format are often limited, and only a part of the results that are deemed significant in the context of the study are published (Andivia et al., 2019). In the case of NNTs, a bias is possible both in a positive context, e.g., reporting NNTs that grow significantly better than native species, and in a negative context, e.g., NNTs that have harmed local ecosystems. In extensive international literature review studies, this bias is expected to be larger, as the information comes from numerous countries whose language as well as peer-reviewed publication culture greatly varies. Considering that modern desktop-based studies can uncover only so much information that has been digitalized, further joint efforts to systematically reveal and provide access to previous research efforts are much needed. In addition to this, we also noted that mapping tree species distribution in Europe has been the primary focus of several previous pan-European projects and we included two in the scope of our study: (i) the EUFORGEN database, established and coordinated by experts, allows for a distinction of the native ranges of distribution of tree species in Europe,1 and (ii) the recent EU-Forest dataset that provides harmonized data regarding the tree occurrence of both native and NNTs in EU countries based on NFI, the Forest Focus and Biosoil databases (Mauri et al., 2017).
This manuscript summarized the outcomes of this mixed approach in service of the earlier defined aims as a way to illustrate its potential and call the readers to supplement and use our questionnaire, allowing us to build a comprehensive future pan-European database of NNTs (Questionnaire_PEN-CAFoRR_WG2D6).
Methodology and data collection
The questionnaire: Gathering country-based knowledge on non-native tree species
We constructed a questionnaire where country entries report the presence of NNTs and, when information is available, their period of introduction, distribution, use and interest in forestry, ecological risks, level of knowledge regarding the ecological risks, the genetic benefits or risks, the legal status, and the level of scientific knowledge (Figure 1). Additionally, details regarding the information origin of the entries (i.e., the type of gray literature used and its original language) were asked from each contributor (Figure 1 and Supplementary material 4). The questionnaire was distributed across 20 out of the 44 European countries of the involved co-authors participating in the WG2-D6 activity, covering 34% of the total European area and representing the four pre-dominant climatic regions (Northern, Central Western, Central Eastern, and Southern Europe), as differentiated by Härkönen et al. (2019). The terminology and the structure of the questionnaire were determined and defined over a series of virtual meetings between December 2020 and January 2021, before opening the questionnaire for the contributors who were able to make additions and changes in the period between January 2021 and October 2021. The contributors were reviewing literature regarding NNTs and pre-selected keywords (all defined in the section “Glossary” provided at the end of this paper). However, we acknowledge that our coverage in terms of countries and species is far from being complete, limited both by the time for voluntary contributions and the accessibility of grey literature. Therefore, the questionnaire remains open to access, and further entries and distribution will allow the construction of a reference database of European NNTs (Questionnaire_PEN-CAFoRR_WG2D6).
FIGURE 1
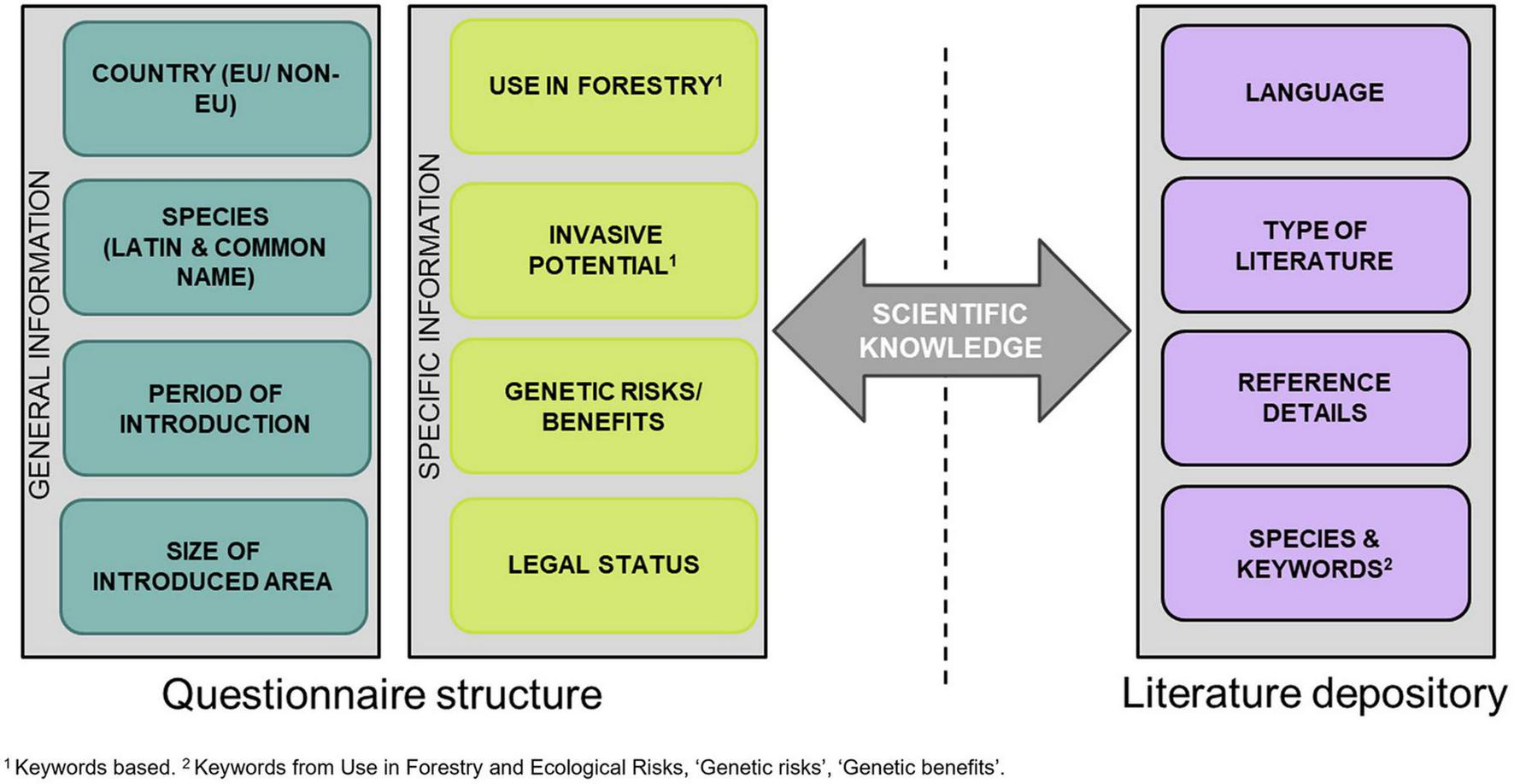
Summary of the WG2-D6 questionnaire structure and literature depository.
EUFORGEN and EU-forest datasets: Analysis of non-native tree species distribution
We extracted the list of species from both EUFORGEN and EU-Forest datasets with their distribution data. We consider that EUFORGEN provides data regarding the distribution of native tree species in Europe, while the EU-Forest dataset provides data regarding the overall (native and NNTs) distribution in the EU. We, therefore, used overlaps and differences between the two datasets for the construction of distribution maps regarding the number of native species, the number of all tree species, and the percentage gain in species diversity due to NNTs introduction. It must be noted that these data were limited to the EU countries. A detailed list of the species noted in both databases is provided as Supplementary material 6.
Review outcomes on non-native tree species diversity, assisted migration, less common species, and hybridization
The impact non-native tree species have on tree species diversity in European countries
The EUFORGEN and EU-Forest datasets analysis resulted in distribution maps of native tree species (Figure 2A) and all–native and non-native–tree species (Figure 2B) along with the diversity percentage gain due to the introduction of NNTs (Figure 2C). These maps illustrated the current state of knowledge regarding species diversity and directed us to investigate the possible factors that have contributed to it. The summarized output of our questionnaire has provided additional insight in terms of the differences between the four European regions (Figure 3) regarding the use in forestry (Figure 3B) and the invasiveness potential (Figure 3C) of NNTs. These results indicate to five countries (Ireland, UK, Denmark, Netherlands, and Hungary) that appeared as most “inclusive” for NNTs, i.e., countries that have had the highest gain in terms of species diversity due to the introduction of NNTs (Figure 2C). These countries that exhibited a larger NNTs distribution and variety, mostly coincide with the countries characterized by low levels of restriction regarding the introduction of NNTs (Pötzelsberger et al., 2020b). For the case of the UK, our questionnaire output note only NNTs conifers (Supplementary material 1). In UK, intensive forest restoration programs that began in the early 20th century initially focused on timber production with the use of NNTs conifers (e.g., Picea sitchensis, Picea abies, Pseudotsuga menziesii, Larix kaempferi, Larix decidua, Pinus contorta, Pinus nigra ssp. laricio). These programs later shifted toward a multipurpose forestry approach and prioritized broadleaved species in attempts to conserve the native species and/or select new, non-native ones (Willoughby et al., 2007; Harmer et al., 2015). However, the lack of information revealed by the questionnaire raises the question if they have been monitored and researched enough. In the case of Denmark, the 19th-century afforestation efforts set the tone for a country open to NNTs species, but the selection largely depended on the country’s current goals and the particularities of the governance i.e., public and subsidized private afforestation (private landowners were also subsidized to participate in these afforestation efforts) (Madsen et al., 2005; Stanturf et al., 2018). As the quasi-entirely cleared landscapes and the heavily degraded soils (heathlands) were impossible to afforest with native species, more tolerant NNTs conifer species, such as Abies alba, Abies grandis, Abies nordmanniana, Abies procera, Larix decidua, Picea abies, Picea sitchensis, Pinus contorta, Pseudotsuga menziesii, were introduced. The use of these species, provision of timber and firewood, is considered a general priority in the northern countries included in our sampling efforts (Figure 3B). Yet, the species were marked as restricted in the aforementioned countries, in the sense that their use and occurrence are monitored and regulated, rather than free and sporadic (Supplementary material 1). The reason for these restrictions could be due to the fact that they have been intensively used as seed sources and for nursery production, but also due to a general tendency to be used as monocultures. Hungary is an interesting case with a relatively large contribution of NNTs to the total number of species diversity (Figure 2C) despite the strict legislation that limits the use of NNTs in the country (Pötzelsberger et al., 2020b). Our questionnaire also noted the occurrence of species as Acer negundo, Ailanthus altissima, Amorpha fruticosa, Elaeagnus angustifolia, Fraxinus pennsylvanica, Prunus serotina, and Robinia pseudoacacia but also restrictions in terms of their use (Supplementary material 1). This disharmony between the identified distribution and the legal restrictions supported our assumptions regarding the lack of updated and reliable on-field information which in the case of the invasive NNTs can be an issue for their sustainable uses. Figure 2 indicates that countries in southern Europe exhibit lower tendencies toward NNTs introduction. Additionally, the more restrictive legislation for the region noted in our questionnaire output (Supplementary material 1) and previously observed by Pötzelsberger et al. (2020b) might be due to two factors. First is the consideration of the Mediterranean basin as a hot spot for plant diversity (Myers et al., 2000), with forest genetic resources of high value and drought tolerance (Ducci, 2015; Médail et al., 2019) and a long history of the anthropogenic influence that has likely promoted tree species diversity. Thus, the native species have fulfilled various needs such as seed source, wood production, urban forestry, etc. However, the socio-economic changes of the last century have led to intensive forestland abandonment, non-sustainable and lack of management, and a lack of interest in the development of climate-smart forest ecosystems (Palahi et al., 2008; Fernandes et al., 2019; Forzieri et al., 2021). The reported NNTs in southern Europe were selected mainly for afforestation/reforestation purposes, soil recultivation, and the non-timber products (Figure 3). For example, in Italy, Portugal, and Spain, Eucalyptus spp. and Pinus spp. were selected due to their potential economic contribution in rural areas (Catry et al., 2015; Pra et al., 2019) and even more so for soil stabilization, fire mitigation, and non-wood products (Palahi et al., 2008). These rapid and intensive introductions, combined with the favorable climatic conditions, have led to some negative outcomes with NNTs, as in the case of Robinia pseudoacacia (Vítková et al., 2020) and Ailanthus altissima (Montecchiari et al., 2020).
FIGURE 2
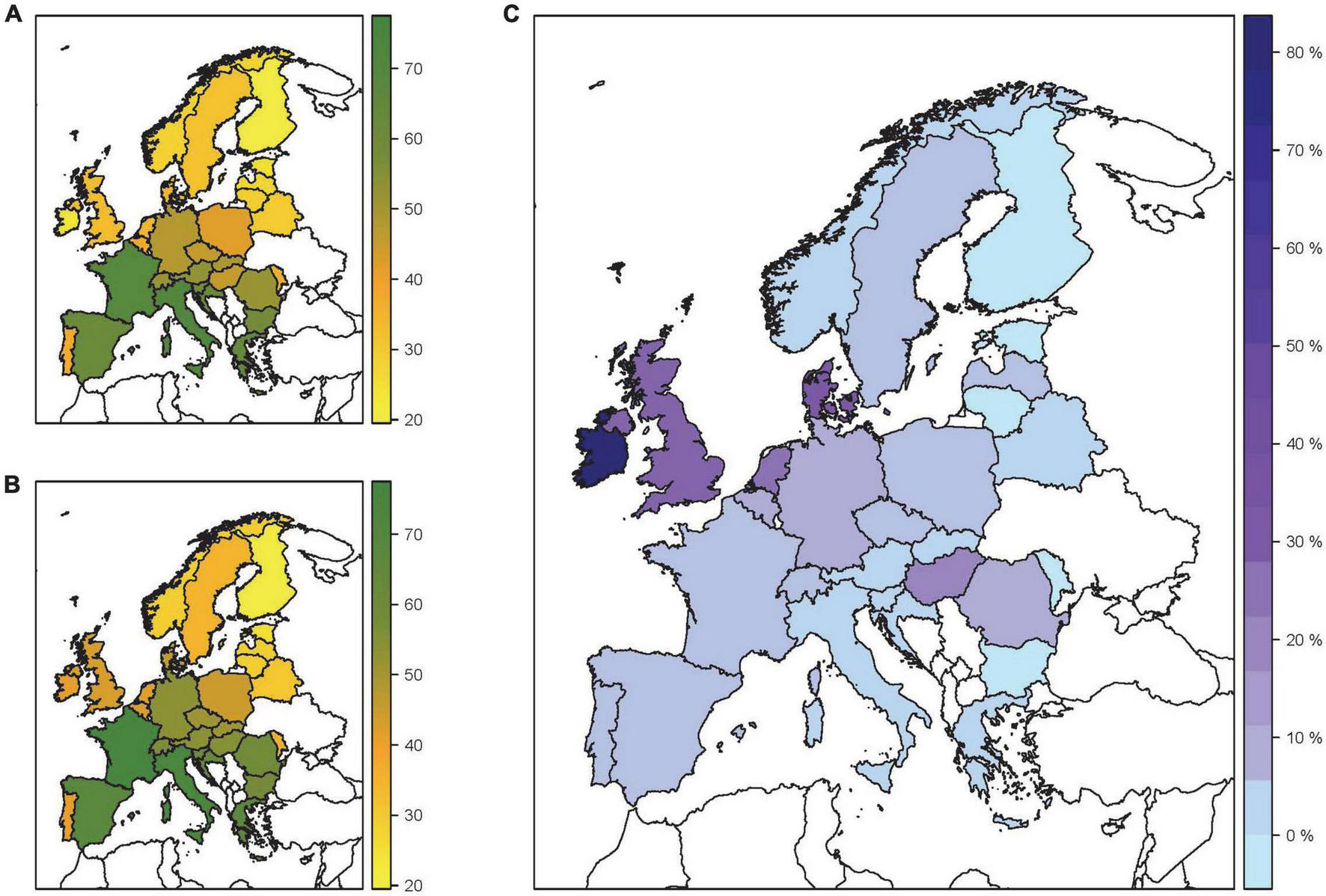
Species diversity distribution maps. (A) Map showing the number of native tree species across Europe. (B) Map showing the total number of native and NNTs across Europe. (C) Contribution of non-native species to the total number of tree species diversity in Europe (Percentage gain in species diversity). Tree species distribution data are taken from EUFORGEN and EU Forest databases.
FIGURE 3

Scope of study and summary of results from the questionnaire. (A) The participating countries in the WG2-D6, divided into climatic zones according to Härkönen et al. (2019). (B) Summarized relative frequency (%) of reported keywords regarding use in forestry of NNTs. (C) Summarized relative frequency (%) of reported keywords regarding invasive potential of NNTs. The reference definitions for all keywords are provided in the section “Glossary.”
Native to southern Europe, introduced to northern Europe: Potential for assisted migration
The analysis of the current NNTs occurrence highlighted a particular group of species: “native to some parts of Europe - generally to southern Europe, that have been introduced to other parts of Europe - generally to northern Europe.” The area occupancy of these species in the EU countries for which data was available is presented in Figure 4 and includes nine broadleaved (Acer platanoides, Acer pseudoplatanus, Aesculus hippocastanum, Alnus incana, Carpinus orientalis, Castanea sativa, Celtis australis, Fagus sylvatica, and Populus alba) and ten coniferous species (Abies nordmanniana, Cedrus atlantica, Cupressus sempervirens, Larix decidua, Picea abies, Picea omorika, Pinus mugo, Pinus nigra, Pinus pinaster, and Pinus sylvestris). As previously mentioned, these species are potential candidates for assisted migration which is defined as a deliberate species and population movement that serves to facilitate the natural range expansion. To highlight the current literature availability across the European countries, we also marked the overlaps with country-specific species entries from the questionnaire (Figure 4andSupplementary material 1). Our findings indicate that (i) numerous species that are suitable candidates for assisted migration are already part of the European landscape, and (ii) these species have been consistently underrepresented in both grey and published literature. However, we raise caution about the indicative nature of this figure, since, as previously mentioned, the information regarding the species occurrence is limited and probably not completely representative of the current on-field situation, in addition to the lack of information regarding non-EU countries.
FIGURE 4
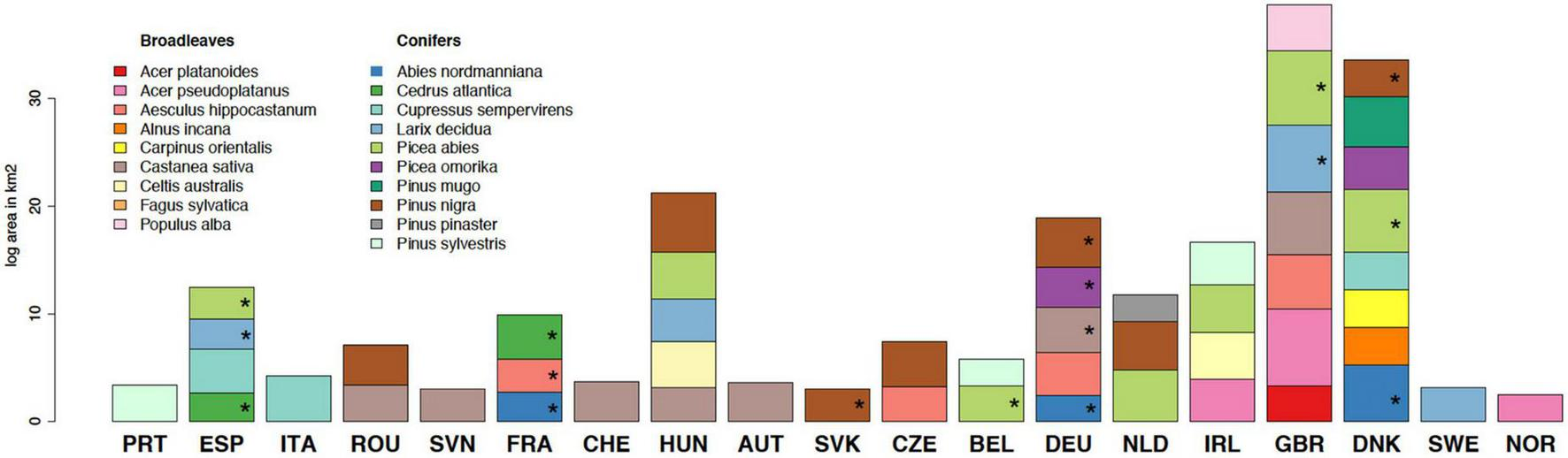
Difference in species status, native, and non-native in Europe. The log area of species native to some regions in Europe but introduced outside their natural range in other European countries. Tree species occurrence data are taken from EUFORGEN and EU-Forest databases. The area is calculated as the number of 1 km2 grid cells, where the species occurrences have been reported. Stars indicate species in given countries that were also reported in the questionnaire.
We deem this approach and information useful for understanding what has contributed to the factors affecting the occurrence of these species across Europe. For example, in Slovakia, Pinus nigra has been introduced from southern Europe and studied as a potential afforestation species in a magnesite mining air polluted area characterized by a mixture of dust particles (MgO and MgCO3) and gaseous compounds (SO2 and NOx) due to which soil is strongly alkaline (pH 8.8) with Cr, Mn, and Mg contents that exceed the toxicity limit, and reactive caustic magnesite which is aggressive in direct contact with crop moisture (Tucekova, 2000; Fazekašová et al., 2017; Fazekaš et al., 2018). In Latvia, this type of introduction has been done for several species. Populus alba along with other species of the Populus genus are widely distributed on abandoned former industrial areas for fast greening and along roadsides and forest edges (Jansons et al., 2017). Larches were also introduced for wood production and have shown high productivity, however inadequate management and cultivation have been limiting factors in terms of the successful establishment (Jansons et al., 2015; Jansone et al., 2018). Generally, the limited knowledge about the distribution of the majority of the south-European native species and their invasiveness potential put in question the impact they would have on the existing forests (Vítková et al., 2020). This uncovered a knowledge gap in the context of NNTs which has implications for their selection in future research and practical efforts.
Filling in the gaps: Shedding light on less common species by integrating peer-review and gray literature
The results from our questionnaire provided a total of 120 different tree species, characterized as non-native in at least one of the contributing countries (Table 1). The country-level approach and analysis of species occurrence allowed for identifying three NNTs categories based on their recurrence in the questionnaire output: “most common”—present in 11–19 countries (total of 5 species), “common”—present in 2–10 countries (total of 62 species), and “less common”—present in one country (total of 53 species) (Table 1). Indeed, most studies have focused on the NNTs identified as more common also by Krumm and Vitkova (2016). In our results, we identified as “most common”: Pseudotsuga menziesii, Quercus rubra, Robinia pseudoacacia, Ailanthus altissima, and Acer negundo. The rationale for the strong focus on these species is either due to their value in wood production and forestry (P. menziesii) or their invasiveness (A. altissima). Once introduced from North America, the favorable climatic conditions across Europe enabled P. menziesii to grow fast and form high wood quality in comparison to the native species (Bastien et al., 2013; Castaldi et al., 2020). P. menziesii was reported by 19 of the 20 participating countries in the questionnaire, noting its wide occurrence across climatic zones in Europe, adaptation for wood production and afforestation/reforestation purposes, and of interest for various research efforts (Supplementary material 3). Interestingly, from our questionnaire outputs, the legal status regarding the other four most commonly reported NNTs varies between European countries from “banned” to “no restrictions” (Supplementary material 1). In literature, these species have been denoted as invasive, or at least undesirable, due to their ecological characteristics (high regeneration potential and productivity, resistance to abiotic and biotic stressors) and negative impacts on native ecosystems (Langmaier and Lapin, 2020). In our questionnaire outputs, the reported uses and interests of these species vary across countries (Supplementary material 1). Both Q. rubra and A. negundo were initially introduced and are strongly used in urban forestry (Supplementary material 1). For Q. rubra, a species already naturalized in European forests, suitable management may provide the desired services while minimizing the potential risks (Jagodziński et al., 2018). For A. negundo, the strong colonization potential in riparian areas (Sikorska et al., 2019) complicates its control, hence making it less suitable to be considered a useful NNT. R. pseudoacacia and A. altissima are NNTs with high clonality, genotype plasticity, and adaptive potential, characteristics that were initially desired and of use for biomass production but today disliked due to the negative impact on the native species (Alpert et al., 2000; Bouteiller et al., 2019). This rationale explains the high frequency of the species in our questionnaire outputs as well as the wide range of uses in forestry (Supplementary material 1). However, the status of R. pseudoacacia, beneficial or invasive, is widely debated as the species is still purposefully cultivated across Europe and is part of the cultural landscape, especially in Central Europe (Nicolescu et al., 2020; Vítková et al., 2020). The impact of R. pseudoacacia, both in terms of benefits and risks, varies a lot depending on the local context (Cierjacks et al., 2013; Vítková et al., 2017; Bartz and Kowarik, 2019; Tölgyesi et al., 2020; Klisz et al., 2021).
TABLE 1
| Species category (number of recurrences)2 | Species (alphabetical order with the number of recurrences) |
| “most common” (11–19) | Acer negundo (11), Ailanthus altissima (12), Quercus rubra (14), Robinia pseudoacacia (14), Pseudotsuga menziesii (19); |
| “common” (2–10) | Abies alba (3), Abies cephalonica (2), Abies concolor (2), Abies grandis (8), Abies nordmanniana (4), Abies procera (3), Acacia dealbata (3), Acacia melanoxylon (2), Acer pseudoplatanus (2), Acer saccharinum (3), Aesculus hippocastanum (4), Amorpha fruticosa (2), Carya ovata (2), Castanea sativa (5), Cedrus atlantica (6), Cedrus libani (3), Chamaecyparis lawsoniana (4), Corylus colurna (3), Cryptomeria japonica (2), Cupressus arizonica (2), Eucalyptus camaldulensis (2), Eucalyptus globulus (3), Fagus sylvatica subsp. orientalis (3), Fraxinus americana (2), Fraxinus pennsylvanica (5), Gleditsia triacanthos (4), Juglans cinerea (2), Juglans nigra (9), Juglans regia (4), Larix decidua (9), Larix kaempferi (8), Larix sibirica (2), Larix X Eurolepis (3), Liriodendron tulipifera (3), Paulownia elongate (2), Paulownia tomentosa (4), Picea abies (4), Picea omorika (2), Picea pungens (3), Picea sitchensis (8), Pinus banksiana (2), Pinus contorta (9), Pinus nigra (6), Pinus peuce (3), Pinus ponderosa (2), Pinus radiata (3), Pinus strobus (8), Platanus X hispanica (2), Populus balsamifera (2), Populus deltoides (4), Populus trichocarpa (3), Populus X canadensis (5), Populus X euramericana (3), Populus X wettsteinii (2), Prunus serotina (6), Quercus cerris (2), Rhus typhina (2), Sequoia sempervirens (2), Sequoiadendron giganteum (3), Thuja plicata (6), Tsuga heterophylla (2), Ulmus pumila (4); |
| “less common” (1) | Abies balsamea, Abies bornmuelleriana, Abies borsii-regis, Abies homolepis, Abies pinsapo, Acacia longifolia, Acer rubrum, Alnus rubra, Araucaria araucana, Betula grossa, Betula maximowicziana, Broussonetia papyrifera, Calocedrus decurrens, Carya cordiformis, Cedrus deodara, Celtis occidentalis, Ceratonia siliqua, Chamaecyparis obtuse, Chamaecyparis pisifera, Elaeagnus angustifolia, Eucalyptus spp., Fagus sylvatica, Juglans ailanthifolia Juglans mandshurica, Juniperus virginiana, Koelreuteria paniculata, Larix gmelinii var. japonica, Larix rossica, Larix X marschlinsii, Leucaena leucocephala, Ligustrum lucidum, Maclura aurantiaca, Metasequoia glyptostroboides, Pinus brutia, Pinus halepensis, Pinus jeffreyi, Pinus nigra subsp. Laricio3, Pinus pinaster, Pinus pinea, Pinus rigida, Pinus taeda, Populus alba, Populus nigra, Prunus avium, Pseudotsuga macrocarpa, Salix viminalis, Sorbus domestica, Taxodium distichum, Thuja occidentalis, Platycladus orientalis, Tilia tomentosa, Tsuga canadensis. |
Species categorization based on recurrences in the questionnaire output.1
1Supplementary material 3 provides the complete overview while Supplementary material 1 provides a shortened version of the questionnaire results.
2The categories are determined by the range of recurrence of the species in Supplementary materials 1–3.
3Originally reported by the contributor as Pinus nigra var. Corsicana and present as such in Supplementary materials 1–3.
The above-reported knowledge is limited or not available in the case of the other NNTs listed in the questionnaire, which we grouped as “common” and “less common” depending on their occurrence (Table 1). These indicative results allowed us to underscore some species of potential interest due to their promising aspects in terms of adaptation to climate change and provision of various ecosystem services or potential risks. Moreover, we aim to emphasize the knowledge gaps, both regarding the species characteristics and occurrence in Europe. From the listed common species, for Central Europe, of interest could be familiar non-invasive NNTs such as the Turkish hazelnut—Corylus colurna, which is well adapted to the Mediterranean and the continental climate (Šeho et al., 2019) and Sequoia sempervirens due to its high adaptive potential (Breidenbach et al., 2020). Less promising in terms of productivity, but able to cope with harsh soil conditions and a warmer climate is Platanus × hispanica, a species often used in urban forestry (Willoughby et al., 2007) that might be worth exploring in various contexts. Caution needs to be raised toward NNTs that have exhibited some invasive behavior, reported in the questionnaire entries (Supplementary material 3) and further supported by literature. For example, Fraxinus pennsylvanica has been mentioned as a threat in the Central European floodplain forests (Schmiedel et al., 2013; Drescher and Prots, 2016). The Fabaceae family contains numerous invasive woody species (Fernandez et al., 2017). For instance, Gleditsia triacanthos, native to North America, has shown invasive behavior on several continents, including some Central and Eastern European countries (Fernandez et al., 2017), yet little is known about the distribution scale and possible management. Populus spp. and Paulownia spp. were introduced and extensively used for short-rotation forestry, biomass, and soil recultivation, and although reported as NNTs they are considered naturalized in some countries. However, non-native poplars are a threat to the native Populus nigra (Michalak et al., 2015). Concerns have also been raised regarding the invasive tendencies of Paulownia tomentosa, especially under climate changes (Essl, 2007) in addition to the species being recently pinpointed as a vector of pathogens as Phytophthora species (Aloi et al., 2021).
The NNTs reported in only one country (Table 1) were regarded as less common. Nonetheless, we cannot assume they are limited to these countries considering the logistic effort required to identify and provide details for these species, i.e., the participating countries, the time limitations, and information available in terms of the possibility to review literature. Thus, we used this data to emphasize the knowledge gaps and limited data available regarding numerous unreported NNTs in neither the EUFORGEN nor the EU-Forest database (Figure 5 and Supplementary material 6). These outputs are meant to serve as a supplementary addition to the current body of literature and even more so as an incentive for future research and management efforts regarding NNTs in European forests.
FIGURE 5

(A) Venn diagram of species overlaps and differences between EUFORGEN, EU-Forest and WG2-D6 questionnaire. (B) The number of “less common” species reported by only one country (countries with symbols) in the WG2-D6 questionnaire. Countries colored in blue are the ones that participated in the questionnaire.
Think twice about non-native tree species with a native relative—the question of hybridization
The analysis of the limited data regarding hybridization obtained from our questionnaire entries, together with the review of the literature regarding the species hybridization potential in forestry, and the information from the EUFORGEN dataset resulted in a table summary of tree genera from which both native and NNTs are present in Europe (Supplementary material 2). The information presented in this table indicates towards several species of significant importance in European forestry.
From the broadleaved species, we explored the hybridization potential for nine genera: Acer, Alnus, Corylus, Juglans, Populus, Prunus, Quercus, Tillia, and Ulmus (Supplementary material 2). In the Alnus genus, interspecific hybrids have been observed and in the context of species of interest to Europe, Alnus incana × Alnus glutinosa hybrids have been found more abundant at the northern limit of their range, possibly due to climate change-induced northward colonization (Banaev and Bažant, 2007). A. cordata, a species native to Corsica and southern Italy, has been observed to naturally hybridize with A. glutinosa, but the low occurrence frequency of A. cordata indicates low risk for its genetic pollution (Villani et al., 2021). Our questionnaire output indicate that A. rubra, a species used for various purposes, but reported only in Sweden (Supplementary material 2) raises a question regarding the presence and interaction between different Alnus spp. across Europe that has not been detected. In the Corylus genus, many interspecific hybrid combinations have been noted (Erdogan and Mehlenbacher, 2000), and considering the previously mentioned interest for the species in Europe, further consideration and monitoring during on-field introductions are needed. For species of high importance for forestry such as Juglans (Supplementary material 2), undertaken studies have revealed only a low frequency of hybrids occurrence even under favorable conditions, as J. nigra and J. regia are species that remain reproductively isolated (Pollegioni et al., 2013). In the poplar genus, both anthropogenic and natural hybridization is far from a novelty and has contributed to a detectable introgression of the wild population due to the possibility of spontaneous mating (Broeck et al., 2005; Tinschert et al., 2020). The native Populus nigra is considered one of the rarest native species in Central Europe, naturally present in the riparian forests. Despite exhibiting low levels of introgression, it seems to be dominated by planted poplars in its natural occurrence range (Supplementary material 2). Here, management approaches that consider the hydro-geomorphological characteristics of the site might be a tool for sustaining P. nigra regeneration (Tinschert et al., 2020).
Regarding the Prunus genus, hybridizations studies have been focused on optimizing the fruits used as food (e.g., Fresnedo-Ramírez et al., 2011; Ganopoulos et al., 2018), rather than in the forestry context, despite the reported invasive potential of P. serotina. In oaks, the hybridization with Q. robur has significantly contributed to the divergence of locally adapted Q. petraea (Leroy et al., 2020). The degree of hybridization between the two species is variable, as noted by Gerber et al. (2014) in a pan-European study, with a higher degree of hybridization in the two northernmost populations sampled, in this case, UK and Denmark, possibly because these regions were more recently colonized. However, data regarding the hybridization potential of the widely used and semi-invasive species in Europe, such as Q. rubra, is lacking. In its native range in North America, Q. rubra can potentially hybridize with other native Quercus species (Jensen et al., 1993), but species from its section are absent from Europe, so hybridization is less likely. Interesting is the case of Tilia tomentosa, which is native to south eastern Europe and exhibits a promising perspective for distribution in central Europe as a thermophilous and moderately drought-tolerant species (Heinrichs et al., 2021). Natural inter-specific hybrids do exist within the genus Tilia (e.g., T. cordata and T. platyphyllos) and as they are characterized by a higher drought tolerance (Radoglou et al., 2009), they can be of further interest in the context of climate change. While in North America, negative outcome from hybridization with the native species is not expected from Ulmus pumila (Zalapa et al., 2010), in Italy this type of hybridization has already taken place with the native U. minor (Brunet et al., 2013). This indicates caution when it comes to the use of the species and underlines the need for close monitoring.
Among the coniferous species, the presence of both native and non-native trees was explored for five genera: Abies, Cupressus, Larix, Picea, and Pinus (Supplementary material 2). For Abies, the high genetic compatibility and the success of field tests allowed for recommendations for hybrids selection (Krajmerová et al., 2016) and this is especially the case for the Mediterranean firs, which could be interesting to explore with pan-European efforts focused on the adaptive potential of fir hybrids (Kormut’ák et al., 2013). In the Cupressus genus, while other species have exhibited some scale of hybridization (Little, 2004; Gallis et al., 2007), the literature gave no information regarding the possibility of C. sempervirens/C. arizonica crosses. Larix might be considered the coniferous equivalent of poplar, whose hybrids have been preferred over the native L. decidua and for which coordinated efforts from IUFRO have allowed for provenance trials and larger research efforts (Pâques et al., 2013). For various Picea species in different parts of the world, the question of hybridization has been raised, e.g., P. abies and P. obovata in northern Eurasia (Krutovskii and Bergmann, 1995; Tsuda et al., 2016), P. omorika and P. abies in south western Europe (Siljak-Yakovlev et al., 2002), P. pungens and P. engelmannii, and P. sitchensis and P. glauca in North America (Daubenmire, 1972; Silim et al., 2001). Hybridization between native European Pinus species has also been observed, e.g., between P. sylvestris and P. mugo (Kormut’ák et al., 2005).
The questionnaire output along with the EUFORGEN data illustrated the varying degrees of hybridization success under controlled conditions and/or in nature (Supplementary material 2). The above-noted gaps in knowledge regarding the NNTs distribution have a direct impact on the knowledge regarding the hybridization outcomes, both positive and negative.
Conclusion: Climate change provides both opportunities and limitations for non-native tree species in Europe
The notable presence and different roles that NNTs have in European forestry make it of high importance to establish a sound backbone of knowledge regarding their characteristics and interplay with the native flora. This information needs to consider not only their distribution but also the pan-European experience of as many NNTs as possible and take into consideration both the potential benefits and risks. The outputs from our questionnaire, accompanied and integrated with the datasets analysis and review of literature, highlighted how numerous under-studied NNTs, not necessarily less distributed, hold different potential for the provision of ecosystem and socio-economic services, and climate change mitigation. The currently available, limited, and likely biased, knowledge needs to be addressed as appropriate species selection is crucial for the establishment of adaptable and resilient forests. Thus, large joint efforts that combine homogenized pan-European methodology for gathering information and well-established field experiment would enable a more informed decision-making process, valuable for researchers, practitioners, and policymakers.
Glossary
NNTs (Non-native tree species)—species transported into a region by humans across a barrier that has apparently prevented natural dispersal so far (Alpert et al., 2000). We consider NNTs as a synonym to exotic, non-indigenous, and introduced tree species.
Europe—Data mining context: European territory covered by the participating countries; Data interpretation context (especially the genetic risks): territory between the Atlantic and the Ural Mountains, including the European part of Turkey.
Seed source—The reported tree species is used as a seed source (mother tree) for further production of new individuals.
Nursery production—The reported tree species are used in nurseries for the production of new individuals.
Afforestation/reforestation—The reported tree species are used for afforestation and reforestation purposes. Afforestation refers to converting long-time non-forested land into forest. Reforestation refers to replanting trees on more recently deforested land (Definitions from European Climate Adaptation Platform Climate-ADAPT).
Wood production—The reported tree species are used for wood production and timber.
Bioenergy—The reported tree species are used as biomass for bioenergy production.
Non-timber products—The reported tree species are used for non-timber products further used for food or medicinal purposes.
Soil recultivation—The reported tree species are used for the restoration of the productivity of the soil and improvement of its structure and quality as desired properties.
Urban forestry—The reported tree species are used as part of the urban greenery. The presence of single individuals in botanical gardens/arboretums was not considered.
Reproductive potential—The relative capacity of a species to reproduce itself under optimum conditions.
Colonization potential—The capacity for the spread and development of an organism in a new area or habitat.
Eradication potential—The potential of the species in question to be eradicated from an area (in this context, determined by the country’s territory).
Resistance/adaptability—The potential of the species to resist unfavorable eco-climatic conditions and successfully adapt to ensure its establishment and survival.
The terms have been defined/adopted for the study and the establishment of the questionnaire by the authors.
Statements
Author contributions
AMonta: conceptualization and supervision and revision process and finalization. AD, KC, MK, ML, and AMonta: methodology. AD and KC: data analysis and figures. AD, KC, and AMonta: data interpretation. AD: writing – original draft. KC, MK, ML, SH, EA, MC, PM, and AMonta: writing – important insight. All contributors participated in the construction of the questionnaire, data collection, and in the final revision and editing.
Funding
This funding was provided by the COST Action CA19128 (PEN-CAFoRR) “Pan-European Network for Climate Adaptive Forest Restoration and Reforestation,” supported by COST (European Cooperation in Science and Technology) (www.cost.eu). AD acknowledges the University of Molise for providing the Ph.D. fellowship for AD.
Acknowledgments
This article was based upon work from the COST Action CA19128 (PEN-CAFoRR) “Pan-European Network for Climate Adaptive Forest Restoration and Reforestation,” supported by COST (European Cooperation in Science and Technology) (www.cost.eu). AM acknowledges the Department of Biotechnology and Life Science at the University of Insubria for providing the necessary support to the joint research project. The authors are grateful to the reviewers and guest editor for their careful reading and valuable suggestions and comments that helped to improve the manuscript.
Conflict of interest
PM was employed by company InNovaSilva ApS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.908464/full#supplementary-material
Footnotes
References
1
AitkenS. N.BemmelsJ. B. (2016). Time to get moving: Assisted gene flow of forest trees.Evol. Appl.9271–290. 10.1111/eva.12293
2
AlberdiI.NunesL.KovacM.BonhemeI.CañellasI.RegoF. C.et al (2019). The conservation status assessment of Natura 2000 forest habitats in Europe: Capabilities, potentials and challenges of national forest inventories data.Ann. For. Sci.76:34. 10.1007/S13595-019-0820-4/TABLES/4
3
AlizotiP.BastienJ.-C.ChakrabortyD.Miroslav KliszM.KroonJ.NeophytouC.et al (2022). Non-Native Forest Tree Species in Europe: The question of seed origin in afforestation.Forests13:273. 10.3390/f13020273
4
AloiF.RioloM.La SpadaF.BentivengaG.MoriccaS.SantilliE.et al (2021). Phytophthora root and collar rot of Paulownia, a new disease for Europe.Forests12:1664. 10.3390/f12121664
5
AlpertP.BoneE.HolzapfelC. (2000). Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants.Perspect. Plant Ecol. Evol.352–66.
6
AmmerC. (2019). Diversity and forest productivity in a changing climate.New Phytol.22150–66. 10.1111/NPH.15263
7
AndiviaE.Villar-SalvadorP.OlietJ. A.PuértolasJ.DumroeseR. K. (2019). How can my research paper be useful for future meta-analyses on forest restoration plantations?New For.50255–266. 10.1007/s11056-018-9631-y
8
BanaevE. V.BažantV. (2007). Study of natural hybridization between Alnus incana (L.) Moench. and Alnus glutinosa (L.) Gaertn.J. For. Sci.5366–73. 10.17221/2137-jfs
9
BartzR.KowarikI. (2019). Assessing the environmental impacts of invasive alien plants: A review of assessment approaches.NeoBiota4369–99. 10.3897/NEOBIOTA.43.30122
10
BastienJ.SanchezL.MichaudD. (2013). “Douglas-Fir (Pseudotsuga menziesii (Mirb .) Franco),” in Forest Tree Breeding In Europe, ed.PâquesL. E. (Bordrecht: Springer), 123–176. 10.1007/978-94-007-6146-9
11
Benito GarzónM.AlíaR.RobsonT. M.ZavalaM. A. (2011). Intra-specific variability and plasticity influence potential tree species distributions under climate change.Glob. Ecol. Biogeogr.20766–778. 10.1111/J.1466-8238.2010.00646.X
12
BindewaldA.BrunduG.SchuelerS.StarfingerU.BauhusJ.LapinK. (2021). Site-specific risk assessment enables trade-off analysis of non-native tree species in European forests.Ecol. Evol.1118089–18110. 10.1002/ece3.8407
13
BlackburnT. M.EsslF.EvansT.HulmeP. E.JeschkeJ. M.KuhnI.et al (2014). A unified classification of alien species based on the magnitude of their environmental impacts.PLoS Biol.12:e1001850. 10.1371/journal.pbio.1001850
14
BoiffinJ.BadeauV.BrédaN. (2017). Species distribution models may misdirect assisted migration: Insights from the introduction of Douglas-fir to Europe.Ecol. Appl.27446–457. 10.1002/EAP.1448
15
BolteA.SchallP.SpathelfP.RockJ. (2009). Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept.Scand. J. For. Res.24473–482. 10.1080/02827580903418224
16
BouteillerX. P.FrédériqueC.EmmiV.PaulA.DainouK.DelcampA.et al (2019). A few north Appalachian populations are the source of European black locust.Ecol. Evol.92398–2414. 10.1002/ece3.4776
17
BradshawR. H. W. (2004). Past anthropogenic influence on European forests and some possible genetic consequences.For. Ecol. Manag.197203–212. 10.1016/j.foreco.2004.05.025
18
BreidenbachN.GailingO.KrutovskyK. V. (2020). Genetic structure of coast redwood (Sequoia sempervirens [D. Don] Endl.) populations in and outside of the natural distribution range based on nuclear and chloroplast microsatellite markers.PLoS One15:e0243556. 10.1371/journal.pone.0243556
19
BroadhurstL. M.LoweA.CoatesD. J.CunninghamS. A.McDonaldM.VeskP. A.et al (2008). Seed supply for broadscale restoration: Maximizing evolutionary potential.Evol. Appl.1587–597. 10.1111/j.1752-4571.2008.00045.x
20
BroeckA. V.VillarM.Van BockstaeleE.Van SlyckenJ. (2005). Natural hybridization between cultivated poplars and their wild relatives: Evidence and consequences for native poplar populations.Ann. For. Sci.62601–613. 10.1051/forest
21
BrunduG.RichardsonD. M. (2016). Planted forests and invasive alien trees in Europe: A code for managing existing and future plantings to mitigate the risk of negative impacts from invasions.NeoBiota305–47. 10.3897/neobiota.30.7015
22
BrunetJ.ZalapaJ. E.PecoriF.SantiniA. (2013). Hybridization and introgression between the exotic Siberian elm, Ulmus pumila, and the native Field elm, U. minor, in Italy.Biol. Invasions152717–2730.
23
BrusR.PötzelsbergerE.LapinK.BrunduG.OrazioC.StraigyteL.et al (2019). Extent, distribution and origin of non-native forest tree species in Europe.Scand. J. For. Res.34533–544. 10.1080/02827581.2019.1676464
24
BurasA.MenzelA. (2019). Projecting tree species composition changes of european forests for 2061–2090 under RCP 4.5 and RCP 8.5 scenarios.Front. Plant Sci.9:1986. 10.3389/fpls.2018.01986
25
CanadellJ. G.RaupachM. R. (2008). Managing forests for climate change mitigation.Science3201456–1457.
26
CastaldiC.MarchiM.VacchianoG.CoronaP. (2020). Douglas-fir climate sensitivity at two contrasting sites along the southern limit of the European planting range.J. For. Res.312193–2204. 10.1007/s11676-019-01041-5
27
Castro-DíezP.AlonsoÁSaldaña-LópezA.GrandaE. (2021). Effects of widespread non-native trees on regulating ecosystem services.Sci. Total Environ.778:146141. 10.1016/j.scitotenv.2021.146141
28
Castro-DíezP.VazA. S.SilvaJ. S.Van LooM.BellinghamP. J.AponteC.et al (2019). Global effects of non-native tree species on multiple ecosystem services.Biol. Rev.941477–1501. 10.1111/brv.12511
29
CatryF. X.MoreiraF.DeusE.SilvaJ. S.ÁguasA. (2015). Assessing the extent and the environmental drivers of Eucalyptus globulus wildling establishment in Portugal: Results from a countrywide survey.Biol. Invasions173163–3181.
30
CierjacksA.KowarikI.JoshiJ.HempelS.RistowM.Von Der LippeM.et al (2013). Biological flora of the British Isles: Robinia pseudoacacia.J. Ecol.1011623–1640.
31
DaubenmireR. (1972). On the relation between Picea pungens and Picea engelmannii in the Rocky Mountains.Can. J. Bot.50733–742.
32
DrescherA.ProtsB. (2016). Fraxinus pennsylvanica – an invasive tree species in middle Europe: Case studies from the danube basin.Contrib. Bot.5155–69.
33
DucciF. (2015). Genetic resources and forestry in the Mediterranean region in relation to global change.Ann. Silvic. Res.3970–93. 10.12899/ASR-779
34
DyderskiM. K.PaźS.FrelichL. E.JagodzińskiA. M. (2018). How much does climate change threaten European forest tree species distributions?Glob. Chang. Biol.241150–1163. 10.1111/gcb.13925
35
EdmandsS. (2006). Between a rock and a hard place: Evaluating the relative risks of inbreeding and outbreeding for conservation and management: Relative risks of inbreeding and outbreeding.Mol. Ecol.16463–475. 10.1111/j.1365-294X.2006.03148.x
36
EnnosR.CottrellJ.HallJ.O’BrienD. (2019). Is the introduction of novel exotic forest tree species a rational response to rapid environmental change? – A British perspective.For. Ecol. Manag.432718–728. 10.1016/j.foreco.2018.10.018
37
ErdoganV.MehlenbacherS. (2000). Incompatibility in wild Corylus species.V Int. Congr. Hazelnut556163–170.
38
EsslF. (2007). From ornamental to detrimental? The incipient invasion of central Europe by Paulownia tomentosa.Preslia79377–389.
39
FazekašJ.FazekašováD.HronecO.BenkovaE.BoltižiarM. (2018). Contamination of soil and vegetation at a magnesite mining area in Jelšava-Lubeník (Slovakia).Ekológia37101–111.
40
FazekašováD.FazekašJ.HronecO.HorňakM. (2017). Magnesium contamination in soil at a magnesite mining area of Jelšava-Lubeník (Slovakia).IOP Conf. Ser. Earth Environ. Sci.921–5.
41
FernandesP. M.GuiomarN.RossaC. G. (2019). Analysing eucalypt expansion in portugal as a fire-regime modifier.Sci. Total Environ.66679–88. 10.1016/j.scitotenv.2019.02.237
42
FernandezR. D.CeballosS. J.MaliziaA.AragónR. (2017). Gleditsia triacanthos (Fabaceae) in argentina: A review of its invasion.Aust. J. Bot.65203–213. 10.1071/BT16147
43
Fresnedo-RamírezJ.SeguraS.Muratalla-LúaA. (2011). Morphovariability of capulín (Prunus serotina Ehrh.) in the central-western region of Mexico from a plant genetic resources perspective. Genet. Resour. Crop Evolut. 58, 481–495.
44
ForzieriG.GirardelloM.CeccheriniG.SpinoniJ.FeyenL.HartmannH.et al (2021). Emergent vulnerability to climate-driven disturbances in European forests.Nat. Commun.12:1081. 10.1038/s41467-021-21399-7
45
FrankhamR.BallouJ. D.EldridgeM. D. B.LacyR. C.RallsK.DudashM. R.et al (2011). Predicting the probability of outbreeding depression.Conserv. Biol.25465–475. 10.1111/j.1523-1739.2011.01662.x
46
FrischbierN.NikolovaP. S.BrangP.KlumppR.AasG.BinderF. (2019). Climate change adaptation with non-native tree species in central European forests: Early tree survival in a multi-site field trial.Eur. J. For. Res.1381015–1032. 10.1007/s10342-019-01222-1
47
FussiB.LexerC.HeinzeB. (2010). Phylogeography of Populus alba (L.) and Populus tremula(L.) in Central Europe: Secondary contact and hybridisation during recolonisation from disconnected refugia.Tree Genet. Genomes6439–450. 10.1007/s11295-009-0262-5
48
GallisA. T.DoulisA. G.PapageorgiouA. C. (2007). Variability of cortex terpene composition in Cupressus sempervirens L. provenances grown in Crete, Greece.Silvae Genet.56:294.
49
GanopoulosI.TourvasN.XanthopoulouA.AravanopoulosF. A.AvramidouE.ZambounisA.et al (2018). Phenotypic and molecular characterization of apple (Malusx domestica Borkh) genetic resources in Greece. Sci. Agricola75, 509–518.
50
GerberS.ChadoeufJ.GugerliF.LascouxM.BuiteveldJ.CottrellJ.et al (2014). High rates of gene flow by pollen and seed in oak populations across Europe.PLoS One9:e85130. 10.1371/journal.pone.0085130
51
HarmerR.WattsK.RayD. (2015). “A hundred years of woodland restoration in Great Britain: changes in the drivers that influenced the increase in woodland cover,” in Restoration Of Boreal And Temperate Forests, 2nd Edn, ed.StanturfJ. A. (Boca Raton, FL: CRC Press), 299–320.
52
HärkönenS.NeumannM.MuesV.BerningerF.BroniszK.CardelliniG.et al (2019). A climate-sensitive forest model for assessing impacts of forest management in Europe. Environ. Modelling Softw. 115, 128–143.
53
HegerT. (2016). “Can we predict whether a species will become invasive?,” in Introduced Tree speCies in European Forests: Opportunities and Challenges, edsKrummF.VítkováL. (Joensuu: European Forest Institute), 78–86.
54
HeinrichsS.ÖderV.IndreicaA.BergmeierE.LeuschnerC.WalentowskiH. (2021). The influence of Tilia tomentosa Moench on plant species diversity and composition in mesophilic forests of Western Romania–a potential tree species for warming forests in Central Europe?Sustain13:7996. 10.3390/su13147996
55
JagodzińskiA. M.DyderskiM. K.HorodeckiP.RawlikK. (2018). Limited dispersal prevents Quercus rubra invasion in a 14-species common garden experiment.Divers. Distrib.24403–414. 10.1111/ddi.12691
56
JansoneB.SkrastinsK.KapostinsR.RācenisE.JansoneL. (2018). Potential of hybrid larch (Larix x eurolepis) in forest regeneration in Latvia.Int. Multidiscip. Sci. Geoconf.18741–747.
57
JansonsA.Rieksts-Riekstin̨šJ.SenhofaS.KatrevicsJ.LazdinaD.SisenisL. (2017). Above-ground biomass equations of Populus hybrids in Latvia.Balt. For.23507–514.
58
JansonsĀMatisonsR.Purin̨aL.NeimaneU.JansonsJ. (2015). Relationships between climatic variables and tree-ring width of European beech and European larch growing outside of their natural distribution area.Silva Fenn.49:1255.
59
Jaramillo-CorreaJ. P.BeaulieuJ.KhasaD. P.BousquetJ. (2009). Inferring the past from the present phylogeographic structure of North American forest trees: Seeing the forest for the genes.Can. J. For. Res.39286–307. 10.1139/X08-181
60
JensenR. J.HokansonS. C.IsebrandsJ. G.HancockJ. F. (1993). Morphometric variation in oaks of the Apostle Islands in Wisconsin: Evidence of hybridization between Quercus rubra and Q. ellipsoidalis (Fagaceae).Am. J. Bot.801358–1366. 10.1002/J.1537-2197.1993.TB15375.X
61
KliszM.PuchałkaR.NetsvetovM.ProkopukY.VítkováM.SádloJ.et al (2021). Variability in climate-growth reaction of Robinia pseudoacacia in Eastern Europe indicates potential for acclimatisation to future climate.For. Ecol. Manag.492:119194.
62
Kormut’ákA.OstroluckáM.VookováB.Pret’ováA.FečkováM. (2005). Artificial hybridization of Pinus sylvestris L. and Pinus mugo turra.Acta Biol. Cracov. Ser. Bot.47129–134.
63
Kormut’ákA.VookováB.ČamekV.SalajT.GalgóciM.MaňkaP.et al (2013). Artificial hybridization of some Abies species.Plant Syst. Evol.2991175–1184. 10.1007/s00606-013-0787-9
64
KoskelaJ.LefèvreF.SchuelerS.KraigherH.OlrikD. C.HubertJ.et al (2013). Translating conservation genetics into management: Pan-European minimum requirements for dynamic conservation units of forest tree genetic diversity.Biol. Conserv.15739–49. 10.1016/j.biocon.2012.07.023
65
KrajmerováD.PauleL.ZhelevP.VolekováM.EvtimovI.GagovV.et al (2016). Natural hybridization in eastern-Mediterranean firs: The case of Abies borisii-regis.Plant Biosyst.1501189–1199. 10.1080/11263504.2015.1011723
66
KrummF.VitkovaL. (2016). “Introduced tree species in European forests: opportunities and challenges,” in Focus - Managing Forests in Europe, edsKrummF.VitkovaL. (Freiburg: European forest institute (EFI)), 423.
67
KrutovskiiK. V.BergmannF. (1995). Introgressive hybridization and phylogenetic relationships between Norway, Picea abies (L.) Karst., and Siberian, P. obovata Ledeb., spruce species studied by isozyme loci.Heredity74464–480.
68
LangmaierM.LapinK. (2020). A systematic review of the impact of invasive alien plants on forest regeneration in European temperate forests.Front. Plant Sci.11:524969. 10.3389/fpls.2020.524969
69
LeroyT.LouvetJ. M.LalanneC.Le ProvostG.LabadieK.AuryJ. M.et al (2020). Adaptive introgression as a driver of local adaptation to climate in European white oaks.New Phytol.2261171–1182. 10.1111/nph.16095
70
LittleD. P. (2004). Documentation of hybridization between californian cypresses: Cupressus macnabiana× sargentii.Syst. Bot.29825–833.
71
MadsenP.JensenF. A.FodgaardS. (2005). “Afforestation in Denmark,” in Restoration Of Boreal And Temperate Forests, edsStanturfJ.MadsenP. (Boca Raton, FL: CRC PRESS), 211–224.
72
MahoodQ.Van EerdD.IrvinE. (2014). Searching for grey literature for systematic reviews: Challenges and benefits.Res. Synth. Methods5221–234. 10.1002/jrsm.1106
73
MattioniC.Angela MartinM.PollegioniP.CherubiniM.VillaniF. (2013). Microsatellite markers reveal a strong geographical structure in European populations of Castanea sativa (Fagaceae): Evidence for multiple glacial refugia.Am. J. Bot.100951–961. 10.3732/ajb.1200194
74
MauriA.StronaG.San-miguel-ayanzJ. (2017). Data Descriptor: EU-Forest, a high-resolution tree occurrence dataset for Europe.Sci. Data4:160123. 10.1038/sdata.2016.123
75
MédailF.MonnetA. C.PavonD.NikolicT.DimopoulosP.BacchettaG.et al (2019). What is a tree in the mediterranean basin hotspot? A critical analysis.For. Ecosyst.6:17. 10.1186/S40663-019-0170-6/TABLES/4
76
MichalakM.PlittaB. P.TylkowskiT.ChmielarzP.SuszkaJ. (2015). Desiccation tolerance and cryopreservation of seeds of black poplar (Populus nigra L.), a disappearing tree species in Europe.Eur. J. For. Res.13453–60. 10.1007/s10342-014-0832-4
77
MontagnoliA.ChiatanteD.GodboldD. L.KoikeT.RewaldB.DumroeseR. K. (2022). Editorial: Modulation of growth and development of tree roots in forest ecosystems.Front. Plant Sci.13:850163. 10.3389/fpls.2022.850163
78
MontecchiariS.TeseiG.AllegrezzaM. (2020). Ailanthus altissima forests determine a shift in herbaceous layer richness: A paired comparison with hardwood native forests in sub-Mediterranean Europe.Plants9:1404. 10.3390/plants9101404
79
MyersN.MittermelerR. A.MittermelerC. G.Da FonsecaG. A. B.KentJ. (2000). Biodiversity hotspots for conservation priorities.Nature403853–858. 10.1038/35002501
80
NicolescuV. N.RédeiK.MasonW. L.VorT.PöetzelsbergerE.BastienJ. C.et al (2020). Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests.J. For. Res.311081–1101. 10.1007/s11676-020-01116-8
81
NordénB.DahlbergA.BrandrudT. E.FritzÖEjrnaesR.OvaskainenO. (2014). Effects of ecological continuity on species richness and composition in forests and woodlands: A review.Ecoscience2134–45. 10.2980/21-1-3667
82
NunesL. J. R.MeirelesC. I. R.Pinto GomesC. J.Almeida RibeiroN. M. (2019). Historical development of the portuguese forest: The introduction of invasive species.Forests10:974. 10.3390/F10110974
83
NyssenB.Den OudenJ.VerheyenK.VanhellemontM. (2016). “Integrating black cherry in forest management in the Netherlands and Belgium,” in Introduced Tree Species In European Forests: Opportunities And Challenges, edsKrummF.VitkovaL. (Joensuu: European Forest Institute), 362–372.
84
PalahiM.MavsarR.GraciaC.BirotY. (2008). Mediterranean forests under focus.Int. For. Rev.10676–688. 10.1505/ifor.10.4.676
85
PâquesL. E.García-CasasM.delC.CharpentierJ. P. (2013). Distribution of heartwood extractives in hybrid larches and in their related European and Japanese larch parents: Relationship with wood colour parameters.Eur. J. For. Res.13261–69. 10.1007/S10342-012-0654-1/FIGURES/4
86
PollegioniP.OlimpieriI.WoesteK. E.De SimoniG.GrasM.MalvoltiM. E. (2013). Barriers to interspecific hybridization between Juglans nigra L. and J. regia L. species.Tree Genet. Genomes9291–305. 10.1007/s11295-012-0555-y
87
PötzelsbergerE.SpieckerH.NeophytouC.MohrenF.GazdaA.HasenauerH. (2020a). Growing non-native trees in European forests brings benefits and opportunities but also has its risks and limits.Curr. For. Rep.6339–353. 10.1007/s40725-020-00129-0
88
PötzelsbergerE.LapinK.BrunduG.AdriaensT.AndonovskiV.AndraševS.et al (2020b). Mapping the patchy legislative landscape of non-native tree species in Europe.Forestry93567–586. 10.1093/forestry/cpaa009
89
PraA.MasieroM.BarreiroS.ToméM.De AranoI. M.OrradreG.et al (2019). Forest plantations in Southwestern Europe: A comparative trend analysis on investment returns, markets and policies.For. Pol. Econ.109:102000.
90
PrasadA.LeitesL. (2022). Ecological analysis of intraspecific variability of eastern white pine (Pinus strobus) under climate change by combining provenance and demographic data.Landsc. Ecol.37109–128. 10.1007/s10980-021-01333-4
91
PuchałkaR.DyderskiM. K.VítkováM.SádloJ.KliszM.NetsvetovM.et al (2021). Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate.Glob. Change Biol.271587–1600. 10.1111/gcb.15486
92
RadoglouK.DobrowolskaD.SpyroglouG.NicolescuV. N. (2009). A review on the ecology and silviculture of limes: (Tilia cordata Mill., Tilia platyphyllos Scop, and Tilia tomentosa Moench.) in Europe.Bodenkultur609–20.
93
RhymerJ. M.SimberloffD. (1996). Extinction by hybridization and introgression.Annu. Rev. Ecol. Syst.2783–109. 10.1146/annurev.ecolsys.27.1.83
94
Royer-TardifS.Boisvert-MarshL.GodboutJ.IsabelN.AubinI. (2021). Finding common ground: Toward comparable indicators of adaptive capacity of tree species to a changing climate.Ecol. Evol.1113081–13100. 10.1002/ece3.8024
95
SandvikH.SætherB. E.HolmernT.TuftoJ.EngenS.RoyH. E. (2013). Generic ecological impact assessments of alien species in Norway: A semi-quantitative set of criteria.Biodivers. Conserv.2237–62. 10.1007/s10531-012-0394-z
96
SchmiedelD.HuthF.WagnerS. (2013). Using data from seed-dispersal modelling to manage invasive tree species: The example of Fraxinus pennsylvanica Marshall in Europe.Environ. Manag.52851–860. 10.1007/s00267-013-0135-4
97
ŠehoM.AyanS.HuberG.KahveciG. (2019). A review on Turkish hazel (Corylus colurna L.): A promising tree species for future assisted migration attempts.South East Eur. For.1053–63. 10.15177/seefor.19-04
98
SikorskaD.SikorskiP.ArchicińskiP.ChormańskiJ.HopkinsR. J. (2019). You can’t see the woods for the trees: Invasive Acer negundo L. in Urban riparian forests harms biodiversity and limits recreation activity.Sustain11:5838. 10.3390/su11205838
99
SilimS.GuyR.PattersonT.LivingstonN. (2001). Plasticity in water-use efficiency of Picea sitchensis, P. glauca and their natural hybrids.Oecologia128317–325.
100
Siljak-YakovlevS.CerbahM.CoulaudJ.StoianV.BrownS. C.ZoldosV.et al (2002). Nuclear DNA content, base composition, heterochromatin and rDNA in Picea omorika and Picea abies.Theor. Appl. Genet.104505–512. 10.1007/s001220100755
101
StanturfJ. A.MadsenP.Sagheb-TalebiK.HansenO. K. (2018). Transformational restoration: Novel ecosystems in Denmark.Plant Biosyst.152536–546.
102
TeshomeD. T.ZharareG. E.NaidooS. (2020). The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate.Front. Plant Sci.11:1874. 10.3389/FPLS.2020.601009/BIBTEX
103
TiganoA.FriesenV. L. (2016). Genomics of local adaptation with gene flow.Mol. Ecol.252144–2164. 10.1111/mec.13606
104
TinschertE.EggerG.WendelgaßJ.HeinzeB.RoodS. B. (2020). Alternate reproductive strategies of Populus nigra influence diversity, structure and successional processes within riparian woodlands along the Allier River, France.J. Hydro Environ. Res.30100–108. 10.1016/j.jher.2020.03.004
105
TíscarP. A.Lucas-BorjaM. E.Candel-PérezD. (2018). Lack of local adaptation to the establishment conditions limits assisted migration to adapt drought-prone Pinus nigra populations to climate change.For. Ecol. Manag.409719–728. 10.1016/j.foreco.2017.12.014
106
TodescoM.PascualM. A.OwensG. L.OstevikK. L.MoyersB. T.HübnerS.et al (2016). Hybridization and extinction.Evol. Appl.9892–908. 10.1111/eva.12367
107
TölgyesiC.TörökP.HábenczyusA. A.BátoriZ.ValkóO.DeákB.et al (2020). Underground deserts below fertility islands? Woody species desiccate lower soil layers in sandy drylands.Ecography43848–859.
108
TsudaY.ChenJ.StocksM.KällmanT.SønstebøJ. H.ParducciL.et al (2016). The extent and meaning of hybridization and introgression between Siberian spruce (Picea obovata) and Norway spruce (Picea abies): Cryptic refugia as stepping stones to the west?Mol. Ecol.252773–2789. 10.1111/mec.13654
109
TucekovaA. (2000). Growth of Austrian pine (Pinus nigra) plants in alkaline air-polluted region Jelsava-Lubenik [Slovak Republic] under the effect of reclamation.J. For. Sci.46356–368.
110
VillaniF.CastellanaS.BeritognoloI.CherubiniM.ChiocchiniF.BattistelliA.et al (2021). Genetic variability of Alnus cordata (Loisel.) duby populations and introgressive hybridization with A. glutinosa (l.) Gaertn. in Southern Italy: Implication for conservation and management of genetic resources.Forests12:655. 10.3390/f12060655
111
VítkováM.MüllerováJ.SádloJ.PerglJ.PyšekP. (2017). Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe.For. Ecol. Manag.384287–302. 10.1016/j.foreco.2016.10.057
112
VítkováM.SádloJ.RolečekJ.PetříkP.SitziaT.MüllerováJ.et al (2020). Robinia pseudoacacia-dominated vegetation types of Southern Europe: Species composition, history, distribution and management.Sci. Total Environ.707:134857. 10.1016/j.scitotenv.2019.134857
113
WeeksA. R.SgroC. M.YoungA. G.FrankhamR.MitchellN. J.MillerK. A.et al (2011). Assessing the benefits and risks of translocations in changing environments: A genetic perspective.Evol. Appl.4709–725. 10.1111/j.1752-4571.2011.00192.x
114
WilliamsM. I.DumroeseR. K. (2013). Preparing for climate change: Forestry and assisted migration.J. For.111287–297. 10.5849/jof.13-016
115
WilloughbyI.StokesV.PooleJ.WhiteJ. E. J.HodgeS. J. (2007). The potential of 44 native and non-native tree species for woodland creation on a range of contrasting sites in lowland Britain.Forestry80531–553. 10.1093/forestry/cpm034
116
ZalapaJ. E.BrunetJ.GuriesR. P. (2010). The extent of hybridization and its impact on the genetic diversity and population structure of an invasive tree, Ulmus pumila (Ulmaceae).Evol. Appl.3157–168. 10.1111/j.1752-4571.2009.00106.x
Summary
Keywords
climate change, forestry, invasive species, distribution, hybridization, database, grey literature, assisted migration
Citation
Dimitrova A, Csilléry K, Klisz M, Lévesque M, Heinrichs S, Cailleret M, Andivia E, Madsen P, Böhenius H, Cvjetkovic B, De Cuyper B, de Dato G, Ferus P, Heinze B, Ivetić V, Köbölkuti Z, Lazarević J, Lazdina D, Maaten T, Makovskis K, Milovanović J, Monteiro AT, Nonić M, Place S, Puchalka R and Montagnoli A (2022) Risks, benefits, and knowledge gaps of non-native tree species in Europe. Front. Ecol. Evol. 10:908464. doi: 10.3389/fevo.2022.908464
Received
30 March 2022
Accepted
10 October 2022
Published
28 October 2022
Volume
10 - 2022
Edited by
Orsolya Valkó, Hungarian Academy of Sciences, Hungary
Reviewed by
Giuseppe Brundu, University of Sassari, Italy; Csaba Tölgyesi, University of Szeged, Hungary
Updates
Copyright
© 2022 Dimitrova, Csilléry, Klisz, Lévesque, Heinrichs, Cailleret, Andivia, Madsen, Böhenius, Cvjetkovic, De Cuyper, de Dato, Ferus, Heinze, Ivetić, Köbölkuti, Lazarević, Lazdina, Maaten, Makovskis, Milovanović, Monteiro, Nonić, Place, Puchalka and Montagnoli.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Montagnoli, antonio.montagnoli@uninsubria.it
†ORCID: Marcin Klisz, orcid.org/0000-0001-9486-6988; Radoslaw Puchalka, orcid.org/0000-0002-4764-0705
This article was submitted to Conservation and Restoration Ecology, a section of the journal Frontiers in Ecology and Evolution
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.