Abstract
Understanding patterns of species diversity along an altitudinal gradient is the major topic of much biogeographical and ecological research. The aim of this study was to explore how richness and density of orchid species and subspecies in terms of different categories of underground organ systems and pollination systems vary along an altitudinal gradient in the central Balkans. The altitudinal gradient of the study area was divided into 21 100-m vertical intervals. Data were analyzed using both non-linear and linear regressions with three data sets (total orchids, orchids of forest habitats, orchids of non-forest habitats) in the case of species richness and three data sets (total orchids—total area, forest orchids—forest area, and orchids of non-forest habitats—non-forest area) in the case of species density. The results showed a hump-shaped pattern of orchid richness and density, peaking at 900–1,000 m. The richness and density of orchids of forest habitats are generally slightly greater than the richness and density of orchids of non-forest habitats in lowland areas, whereas the orchids of herbaceous vegetation types dominating at high altitudes. Tuberous orchids dominate in low and mid-altitude areas, orchids with palmately lobed and fusiform tubers (“intermediate orchids”) dominate at high altitudes, while rhizomatous orchids are predominate in mid-altitude forest stands. Both deceptive and self-pollinated orchids show a unimodal trend with a peak at mid-altitude areas. This study underlines the importance of low and mid-altitude areas for the survival of deceptive orchids and the importance of mid- and high-altitude areas for the survival of rewarding orchids. In addition, forest habitats at mid-altitudes have been shown to be crucial for the survival of self-pollinated orchids. The results suggest that the altitudinal patterns of orchid richness and density in the central Balkans are determined by mechanisms related to land area size and habitat cover, partially confirming the species-area relationship (SAR) hypothesis. This study contributes significantly to a better understanding of the potential impacts of habitat changes on orchid diversity, thereby facilitating more effective conservation planning.
Introduction
The family Orchidaceae is one of the most species-rich families in the plant kingdom, with an estimated 26,000–28,000 species from 749 genera (Christenhusz and Byng, 2016; Chase et al., 2017). Although orchids grow in almost all terrestrial ecosystems, they are most diverse in the tropics and subtropics, where species of different life forms can be found. In Europe, orchids are exclusively terrestrial, inhabiting both forest habitats and herbaceous plant communities (Djordjević and Tsiftsis, 2022). Because of their germination limitation, mycorrhizal specificity, and pollinator specialization, many orchid species are particularly vulnerable to environmental change (Waterman and Bidartondo, 2008; Swarts and Dixon, 2009). Intensive anthropogenic impacts resulting in habitat alteration and loss have led to the extinction or decline in abundance and distribution of many orchids (Kull and Hutchings, 2006). Understanding patterns of orchid species richness and abundance along the geographical and environmental gradients is a central goal of much ecological and biogeographical research (Tsiftsis et al., 2008; Acharya et al., 2011; Zhang et al., 2015a; Djordjević et al., 2016, 2020). In addition, knowledge of diversity patterns along the altitudinal gradient and factors influencing these patterns can contribute not only to a better understanding of orchid ecology and distribution, but also to planning strategies necessary for a successful species conservation plan.
In general, there are two main patterns of species richness—an altitude relationship: a monotonic decrease in number of species with altitude; and a hump-shaped pattern with the highest species number at mid-altitudes (Rahbek, 1995; McCain and Grytnes, 2010; Timsina et al., 2021). Nearly half of the studies showed that the hump-shaped patterns are the most common ones, whereas other studies suggested either a monotonic decrease or an increase in the number of species with altitude (McCain and Grytnes, 2010; Timsina et al., 2021). Although several hypotheses have been proposed to explain patterns of orchid diversity along the altitudinal gradient, most studies address the influence of climatic factors, then the mid-domain effect (MDE), while less attention has been paid to the species-area relationship (SAR). The climatic gradient hypothesis predicts that species richness peaks at a particular altitude where a combination of growing conditions is optimal for the species. According to Colwell and Lees (2000), most species live in mid-altitude areas due to the geometric limit of the species’ range. This pattern, known as the “mid-domain effect” (MDE), results from random overlap of the altitudinal range of species (Colwell and Hurtt, 1994; Colwell et al., 2004; Dunn et al., 2007). The concept of a species-area relationship suggests that species richness varies depending on size of the area of a certain altitude range, i.e., that maximum species richness occurs in the altitudinal zones that cover the largest area (Acharya et al., 2011; Karger et al., 2011; Trigas et al., 2013).
There are some studies and books that provide detailed information on the altitudinal range of individual orchid species in Europe or specific countries, suggesting that the altitudinal range of the same species can vary considerably within the range of its distribution (Baumann et al., 2006; Delforge, 2006; Jersáková et al., 2015). To date, many studies provide important information on how altitudinal gradients affect orchid species richness, but most of them have been conducted for Asian (Acharya et al., 2011; Zhang et al., 2015a,b; Timsina et al., 2021), American (Cardelús et al., 2006; Ackerman et al., 2007; Štípková et al., 2016), and African countries (Jacquemyn et al., 2005b,2007), while there are just few studies on orchid richness along the altitudinal gradient in Europe (Tsiftsis et al., 2019; Štípková et al., 2020, 2021). The species-area relationship (SAR) has been investigated for some countries in Asia (Acharya et al., 2011; Zhang et al., 2015a), whereas in Europe there are only two studies (Štípková et al., 2020, 2021) that consider this relationship by analysis of density. However, it has not been studied in detail how the area of specific habitats affects the patterns of orchid diversity along the altitudinal gradient.
In recent years, the diversity patterns of species classified in different functional groups have been used to understand the relationships between these traits and environmental variation (Laughlin et al., 2012; Taylor et al., 2021). There are several studies on the distribution of certain orchid life forms, including commonly terrestrial, epiphytic, and saprophytic orchids (Cardelús et al., 2006; Acharya et al., 2011; Zhang et al., 2015a), while knowledge on the distribution patterns of orchid life forms in Europe is limited (Tsiftsis et al., 2019; Štípková et al., 2021). Species diversity patterns related to specific life forms along gradients (e.g., altitude, latitude) not only may be useful from a basic ecology perspective, but they can also contribute to a better understanding of orchid evolutionary history, prediction of their distribution, and effective orchid species conservation. Some studies have focused particularly on the distribution and species richness of orchids possessing certain floral traits and breeding systems, as well as pollination systems (Arroyo et al., 1982; Jacquemyn et al., 2005b; Pellissier et al., 2010; Štípková et al., 2020). Although it was found that the relative occurrence of food-deceptive orchids decreases with increasing altitude in the territory of Switzerland (Pellissier et al., 2010), there is a lack of knowledge on how orchid diversity patterns vary when it comes to other orchid pollination systems, including rewarding, self-pollinated and other deceptive orchids. Furthermore, there is a lack of knowledge about the relationship between altitude and richness/density of orchids, which are characterized by different life forms and pollination systems in different habitats (e.g., forests and herbaceous plant communities) and regions.
Although the Balkan Peninsula is one of the parts of Europe with the highest number of orchid taxa (Djordjević et al., 2020), the patterns of species richness and density along the altitudinal gradient in the central Balkans have not yet been explored. Therefore, our study aims to explore patterns of diversity of orchids along the altitudinal gradient in the central Balkans, with the goal of analyzing both the total orchid flora and the orchid flora of individual life forms and pollination systems. Special attention had to be paid to the analysis of the influence of the size of the area of individual altitudinal intervals on the patterns of diversity, focusing on the area of specific habitat types (forest and non-forest). Consequently, the importance of this study lies in the contribution to the knowledge of orchid life histories, ecology and distribution, but also in the creation of a good basis for more effective orchid conservation. We hypothesized that spatial patterns of forest and non-forest habitats along the altitudinal gradient affect orchid species diversity patterns. Moreover, we expected that orchids of different traits have different diversity patterns as well. Based on the evolutionary development of the underground organs of orchids (Averyanov, 1990; Tsiftsis et al., 2019), we assume that orchids with spheroid or ovoid tubers dominate at lower altitudes because they generally tolerate drought and warmer conditions best. On the other hand, orchids with palmately lobed and fusiform tubers are assumed to dominate at higher altitudes because their origin is related to the emergence of colder climates and they have the best adaptations that allow them to grow in habitats with low temperatures and high humidity characteristic of highland areas. Given the different pollination systems of orchids and the studies already published (Pellissier et al., 2010; Štípková et al., 2020), we expect that the richness of rewarding orchids is greater than that of deceptive ones in high-altitude areas.
The main objectives of this study were: (i) to determine altitudinal range size of individual orchids and compare altitudinal range size and mean altitude of occurrence of orchid species and subspecies with different life traits (underground organ systems, pollination systems); (ii) to analyze orchid species richness and density along the altitudinal gradient; (iii) to determine how the richness and density of orchid species with different life traits (underground organ systems, pollination systems) vary with altitude. Patterns of species richness and density along the altitudinal gradient were explored for the total orchid flora, as well as for the orchid flora recorded in forest and non-forest habitats.
Materials and methods
Study area
The study area covers the entire territory of western Serbia (19°09′-20°39′ E, 42°50′-44°58′ N) and encompasses approximately 18,000 km2 (Figure 1A). It is located in the central Balkans and belongs to the eastern Dinaric Alps. Two basic units are distinguished in the study area: (a) the flatlands of the southern part of the Pannonian Plain, which occupy the northern parts of western Serbia, and (b) the mountainous region, which belongs to the Dinaric mountain system. The altitude ranges from 65 m (Šabac) to 2,154 m (Mokra Gora—Pogled). The climate in Serbia can be described as temperate-continental. The average annual temperature varies from 6.7°C in the coldest parts to 11.6°C in the warmest parts, while the average annual temperature in the areas above 1,500 m above sea level is about 3.0°C. Annual precipitation varies from 726.4 mm in the lower-lying regions to about 1,500 mm in the mountainous areas of south-western Serbia (climatic data from the Hydrometeorological Service of the Republic of Serbia).
FIGURE 1
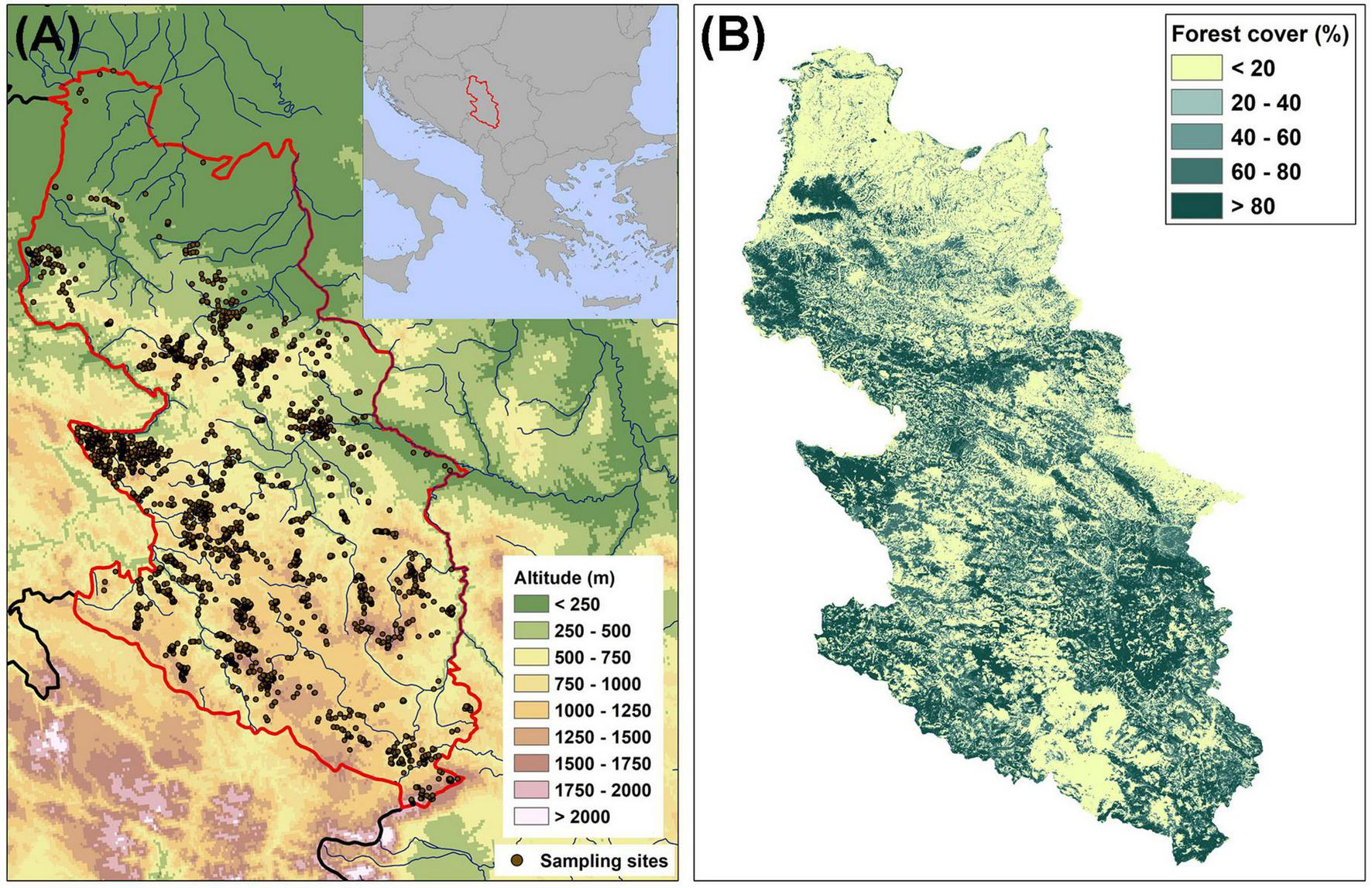
(A) Map of the study area (central Balkans: western Serbia) with sampling sites where orchids were found (the boundaries of the study area are marked by the red line); (B) the density of forest cover in the study area.
In general, vegetation in the study area is structured according to climatic differentiation. In the northernmost parts of western Serbia, near the Sava and Kolubara rivers, there are floodplain (Fraxino-Quercion roboris) forests, while in the rest of the study area (especially at low to medium altitudes) oak (Quercion confertae and Quercion petraeo-cerridis) forests predominate. Mesophilous deciduous beech and hornbeam (Fagion sylvaticae and Carpinion betuli) forests are predominant in the zone of middle altitudes, while coniferous (Vaccinio-Piceetea) forests are found in the high-mountain regions. The density of forest cover in the study area is shown in Figure 1B. Western Serbia is geologically diverse, with a large occurrence of carbonate and ultramafic rocks and various types of silicate rocks (Djordjević and Tsiftsis, 2019).
Data collection
The total database consists of data on 55 orchid species and subspecies recorded at 3,580 sites (Supplementary Table 1). Data on 53 orchid species and subspecies from 2,610 sites were collected during field observations between 1995 and 2021. In addition, the dataset included published data on 48 species and subspecies from 683 sites and herbarium data on 44 taxa from 287 sites collected in the Herbarium of the University of Belgrade (BEOU) and the Herbarium of the Natural History Museum in Belgrade (BEO). The number of sampling sites for each altitudinal interval is shown in Figure 2. This number does not include the sites we visited and did not find any orchids there. Orchid taxa were identified according to Delforge (2006), while Djordjević et al. (2021) was used for nomenclature. During field surveys, geographic coordinates (longitude, latitude) and altitude were determined by a Garmin eTrex 30 hand-held GPS device in the WGS 84, while reliable data from published sources and herbarium collections were georeferenced using Ozi Explorer 3.95.4s software.
FIGURE 2
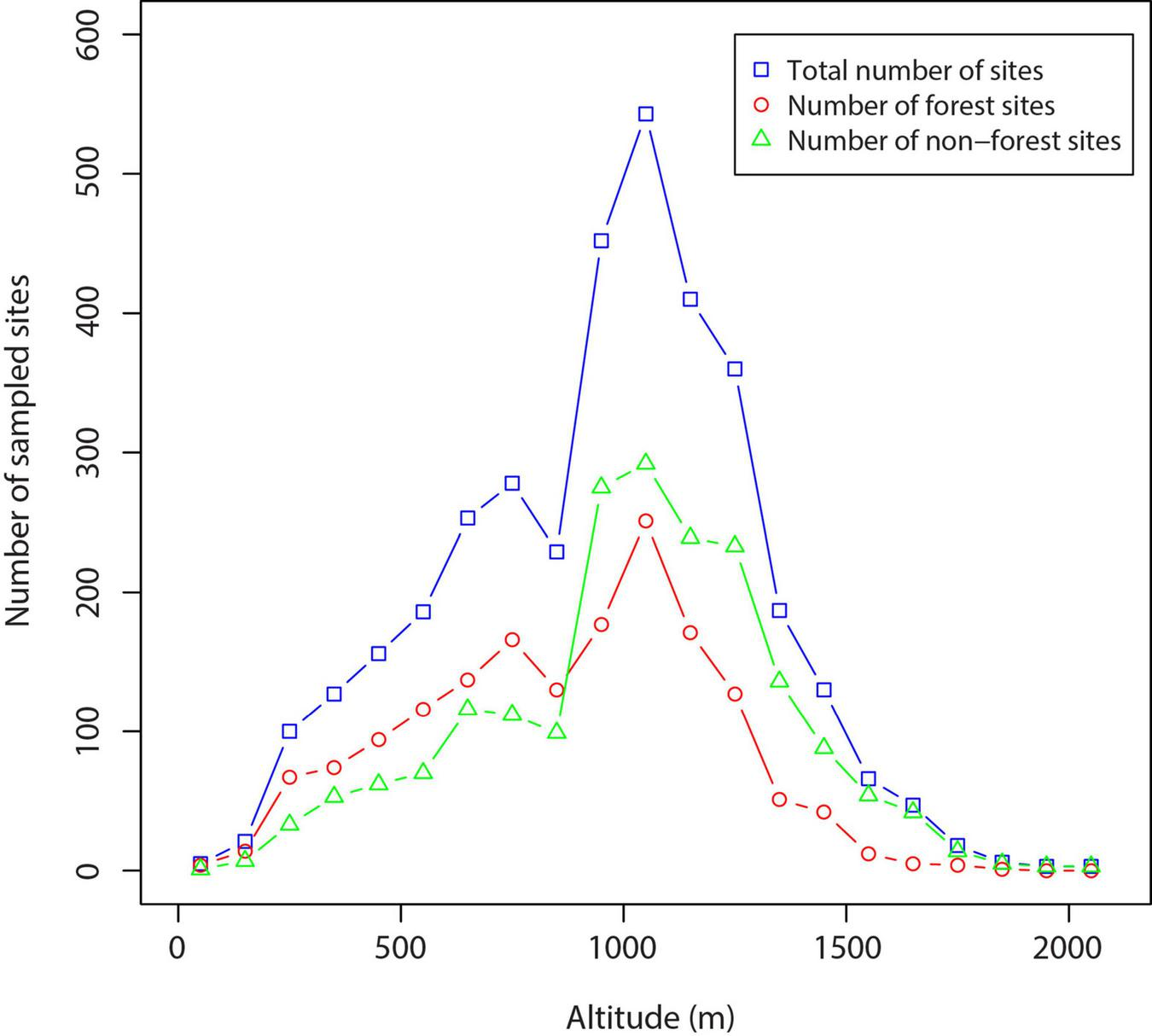
The number of sampling sites for each altitudinal interval where orchids were found.
We studied each altitudinal interval with the same effort, number of days spent in the field, and mileage. The minimum distance between sites was 250 m (i.e., two populations found closer than 250 m from each other were considered as one site), except in the case when large differences in altitude and habitats of the studied places were observed. Due to the relatively small size of the area searched and the long duration of the study, we are sure that the number of sites we missed during the search is negligible and therefore cannot have affected the outcome. For the same reason, we can expect that the sampling effort was uniform throughout the region. For subsequent calculations, we only considered sites where orchids were found.
Subdivision of orchid species by habitat type, life forms (underground organs), and pollination systems
Orchid species were divided into two categories based on habitat type: (1) orchids that were recorded in forest habitats and (2) orchids that inhabit non-forest habitats (grasslands and herbaceous wetlands) (Supplementary Table 1). Orchids that occurred in both forest and non-forest habitats were included in both habitat categories (counted twice). In addition, orchids were relegated to various categories based on their underground organs and pollination systems (Supplementary Table 1). We classified orchids as belonging to one of three underground organ systems, following the concept presented by Tsiftsis et al. (2019) and Štípková et al. (2021): (1) rhizomatous orchids (the most primitive ones); (2) “intermediate orchids,” i.e., orchids with palmate, fusiform, or stoloniferous tubers (intermediate in evolutionary history between rhizomatous orchids and orchids with spheroid tubers); and (3) tuberous orchids, i.e., orchids with spheroid tubers (considered the most specialized and advanced orchids). Species of the genera Cephalanthera, Corallorhiza, Epipactis, Epipogium, Goodyera, Limodorum, and Neottia were classified in the rhizomatous orchid group, while species of the genera Coeloglossum, Dactylorhiza, Gymnadenia, Nigritella, Platanthera, and Pseudorchis were placed in the intermediate group. In addition, species of the genera Anacamptis, Herminium, Himantoglossum, Neotinea, Ophrys, Orchis, Spiranthes, and Traunsteinera were classified as tuberous orchids.
Based on their pollination system, orchids were divided into three categories: (1) rewarding orchids, i.e., those that produce nectar and offer it as a reward to their pollinators, (2) deceptive, and (3) self-pollinated species (Supplementary Table 1). Information on the pollination mechanism of orchids was obtained from Jacquemyn et al. (2005a); Jersáková et al. (2006), Vereecken et al. (2010), and Inda et al. (2012), while for the genus Epipactis the AHO-Bayern webpage (Aho-Bayern, 2021) was used. Orchids that have nectar and thus could be rewarding but can also be self-pollinated (e.g., Epipactis spp.) were classified in both categories (rewarding and self-pollinated plants).
Data analysis
The altitudinal gradient of the study area was divided into twenty-one 100 m vertical intervals (i.e., 0–100 m, 101–200 m, etc.). Species richness was calculated for each 100-m altitudinal interval as the total number of orchid species and subspecies in that interval. The area (in km2) of each 100-m interval was estimated by counting the number of 100-m grid cells of a Digital Altitude Model (DEM) having altitude values at a specific vertical interval. To achieve the 100-m map, the 25-m European Digital Altitude Model (Copernicus, EU-DEM version 1.1) was used by carrying out an aggregation process. The Tree Cover Density layer (2015 was used as the reference year) at a 100-m resolution (available through the Copernicus Land Monitoring Service) was used to calculate the forest area at each 100-m vertical interval, and then the non-forest area was calculated by removing the forested area from the total area in each vertical interval.
An orchid was considered as present in a 100-m interval only if it was recorded at least once in this vertical interval. After constructing the total matrix for all the orchid taxa recorded in the study area, two series of orchid matrices were generated according to the traits studied (underground organ system category, pollination system). Specifically, for each orchid category the number of orchid taxa occurring in each vertical interval was calculated.
To explore whether (a) the altitudinal range and (b) the mean altitude of occurrence of the orchids with different underground organ systems and pollination systems are statistically different, we used the Kruskal-Wallis test, followed by Dunn’s post-hoc test with Bonferroni correction carried out on each pair of groups. The altitudinal range for each orchid was defined as the difference between the highest and the lowest site where each orchid has been recorded, whereas the mean altitude was calculated on the basis of all altitude values of the sites where each orchid has been recorded.
Orchid species density (D) at each altitude interval was calculated using the formula:
where S is the number of orchid species recorded in each vertical interval and A is the area of each vertical interval in km2.
Orchid density was calculated using (a) the total orchid flora and total area of the vertical intervals, (b) orchids of forest habitats and the forest area, and (c) orchids of non-forest habitats and the non-forest area of the vertical intervals. The three data sets were used to identify possible specific patterns in the orchids of these broad habitat categories.
The associations between richness and density of orchid species and subspecies and altitude were explored by analyzing the data sets using regressions. As we did not have any a priori hypothesis about the functions describing the shape of the dependences studied, polynomial regressions were used. We first used third-degree polynomials and always tested significance of the cubic terms in order to determine whether a second-degree or a linear regression would not be sufficient for fitting the data. Linear regression was used in cases where both cubic and quadratic terms were insignificant (Tsiftsis et al., 2019; Štípková et al., 2020, 2021).
All analyses were performed in R version 4.0.5 (R Core Team, 2021), whereas variable extraction was done using ArcGIS 10.6 (ESRI, 2017). Kruskal-Wallis and Dunn’s post-hoc tests were performed using the packages “stats” and “FSA” (Ogle et al., 2022), respectively.
Results
Altitudinal range size
Altitudinal range profiles of orchid species and subspecies of the central Balkans (western Serbia) showed that most species occurred over wide altitudinal ranges (Figure 3 and Supplementary Table 1). Thus, 12 species and subspecies (21.82%) had altitudinal ranges less than 500 m, 13 species and subspecies (23.64%) had altitudinal ranges from 500 to 1,000 m, 20 taxa (36.36%) had altitudinal ranges from 1,000 to 1,500 m, while 10 taxa (18.18%) had altitudinal ranges of more than 1,500 m (Figure 3 and Supplementary Table 1).
FIGURE 3
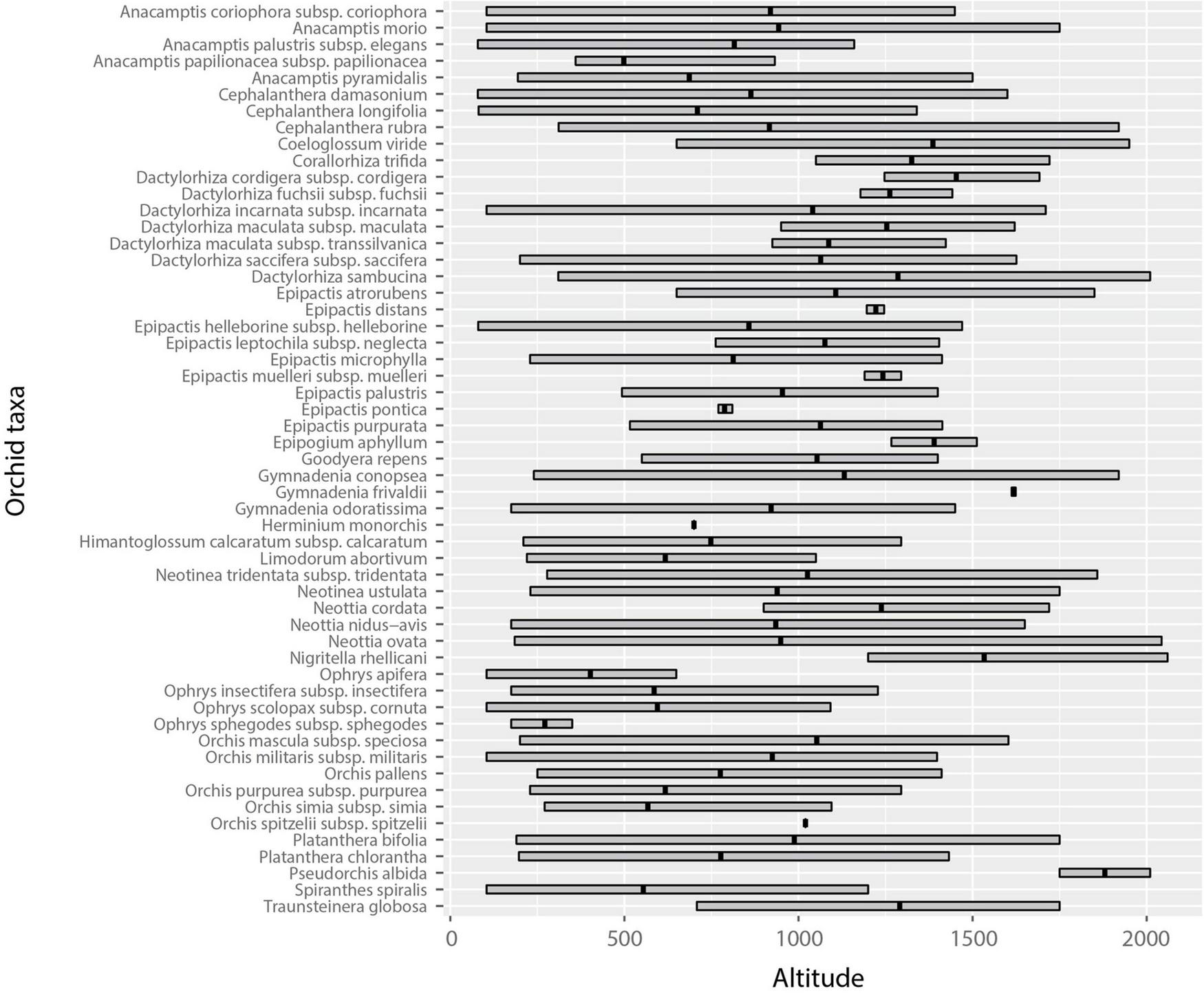
The altitudinal range of orchids in western Serbia (the central Balkans).
There was no significant difference in altitudinal range between orchids with different types of underground organs (Kruskal-Wallis χ2 = 0.314, p = 0.854) or pollination systems (χ2 = 1.635, p = 0.441). In contrast, the mean altitude of occurrence differed significantly between orchid species with different underground organs (χ2 = 21.617, p < 0.001). In particular, intermediate orchids had a significantly higher mean altitude of occurrence than rhizomatous orchids (Z = 2.01, p < 0.05) and tuberous orchids (Z = 4.589, p < 0.001). Moreover, rhizomatous orchids had a significantly higher mean altitude than tuberous orchids (Z = 2.707568, p < 0.01). Similarly, the mean altitude of occurrence differed between orchid species with different pollination systems (χ2 = 6.6227, p < 0.05), with the mean altitude of occurrence of deceptive orchids being significantly lower that of rewarding orchids (Z = –2.573, p < 0.05). No significant difference was found between the mean altitude of occurrence of deceptive and self-pollinated orchids (Z = –0.861, p = 0.973), and rewarding and self-pollinated orchids (Z = 1.086, p = 0.832).
Altitudinal patterns of orchid species richness
Regression analysis showed a strong influence of altitude on orchid species richness in western Serbia (Table 1 and Figure 4). The total orchid species richness showed a hump-shaped relationship along the altitudinal gradient. Species richness reached its maximum value in the mid-altitude zone between 901 and 1,000 m (41 orchid species and subspecies) and then decreased to reach its minimum at high-altitude sites (Table 1 and Figure 4).
TABLE 1
| Orchid group | R2 | P-value |
| Total orchids | 0.9267 (c) | <0.001 |
| Orchids of forest habitats | 0.9295 (c) | <0.001 |
| Orchids of non-forest habitats | 0.9061 (c) | <0.001 |
Overall statistics of the polynomial regressions used to determine the relationship between orchid species richness and altitude.
(c): 3rd order polynomial regression.
FIGURE 4
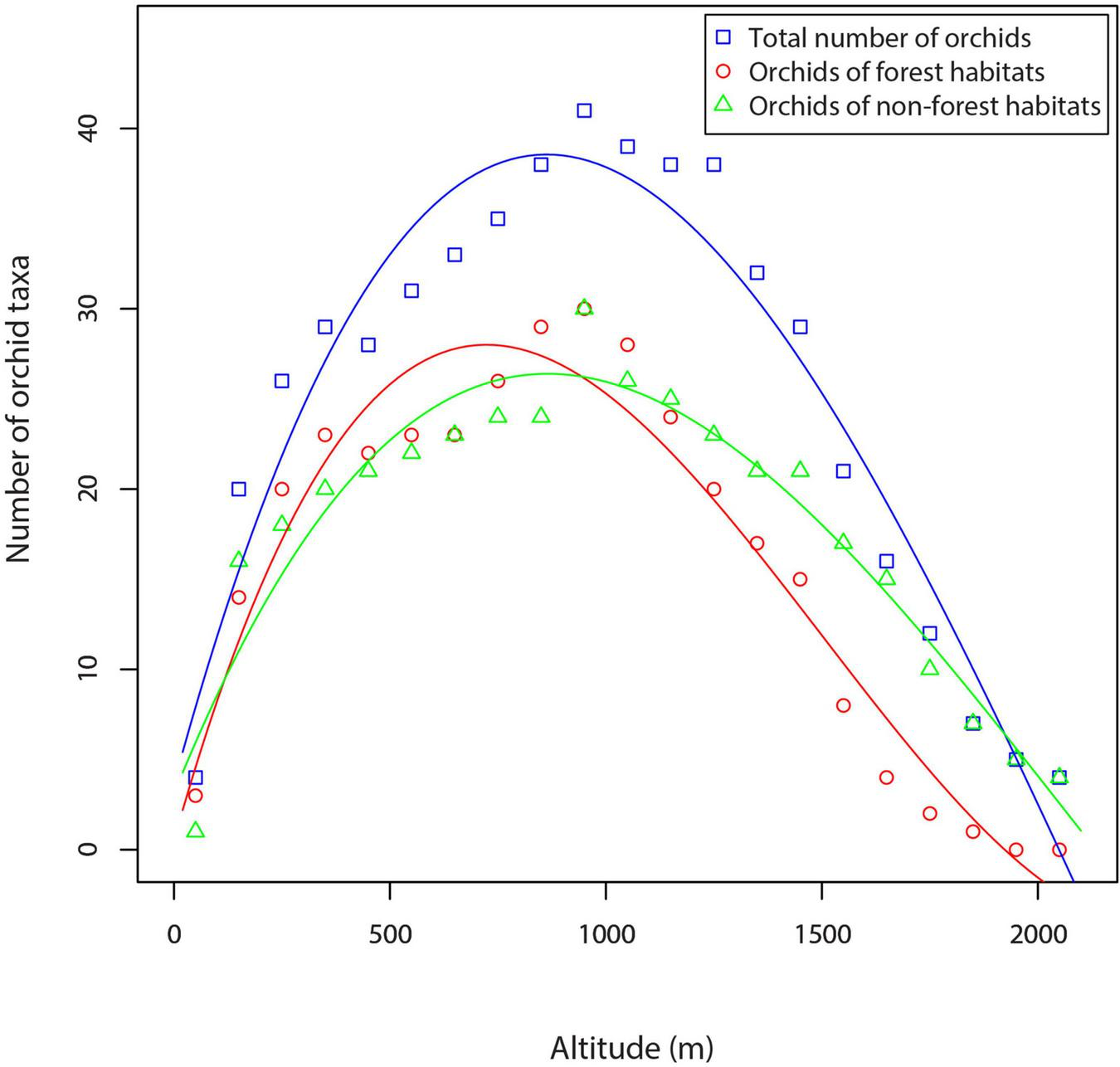
Orchid species richness along an altitudinal gradient in the central Balkans (western Serbia).
Regression analysis showed a significant effect of altitude on orchid species richness in both forest and non-forest habitats (Table 1 and Figure 4). Both orchids of forest habitats and orchids of non-forest habitats showed a hump-shaped relationship with the altitudinal gradient, peaking between 901 and 1,000 m (Figure 4). In the altitudinal zone from 0 to 1,100 m, the richness of orchids of forest habitats is generally higher than the richness of orchids of non-forest habitats (Figure 4). However, with increasing altitude (from 1,101 to 2,100 m), the richness of orchids of non-forest habitats is higher than the richness of orchids of forest habitats (Figure 4).
Orchid richness in terms of the number of rhizomatous, intermediate, and tuberous orchid taxa for the three data sets (total orchids, orchids of forest habitats, orchids of non-forest habitats) are shown in Figure 5. The regression lines of orchids of each orchid life form have rather the same shape (a hump-shaped pattern). Tuberous species dominate at low and mid-altitude zone, the rhizomatous orchids present their highest richness at c. 1,100–1,300 m, whereas intermediate orchids dominate at high-altitude areas (Figure 5A). The results concerning orchids of the forest habitats were of the similar shape for all three orchid groups (Figure 5B). However, tuberous orchids dominate at low altitude areas, whereas the rhizomatous orchids dominate from mid-altitude area to high-altitude zone (Figure 5B). In the case of orchids of non-forest habitats, tuberous orchids dominate in low and mid-altitudinal zone (0–1,200 m), whereas the intermediate orchids dominate between 1,200 and 2,100 m (Figure 5C).
FIGURE 5
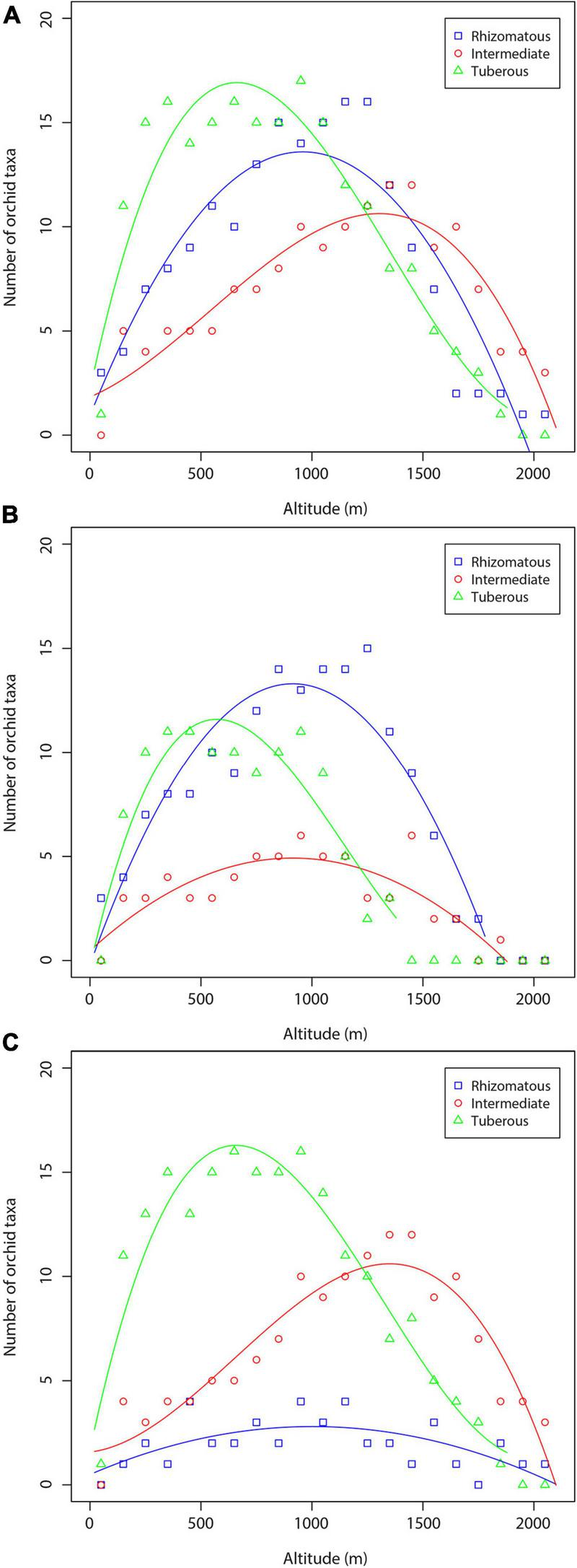
Richness of orchid species and subspecies with certain underground organ systems along the altitudinal gradient: (A) total number of orchid taxa; (B) orchids of forest habitats; (C) orchids of non-forest habitats.
The trends in orchid species richness along the altitudinal gradient based on the three pollination mechanisms are shown in Figure 6. The orchids of each orchid pollination system showed a hump-shaped relationship with the altitudinal gradient, peaking at mid-altitude zone (Figure 6A). In general, the richness of deceptive orchids is greater than the richness of rewarding and self-pollinated orchids at altitudes between 0 and 1,700 m, whereas the richness of rewarding orchids is higher than the richness of deceptive and self-pollinated orchids at altitudes between 1,700 and 2,100 m (Figure 6A). The altitudinal patterns of orchid species richness of specific pollination systems were hump-shaped also in cases when orchids of forest and non-forest habitats were considered separately (Figures 6B,C). All the correlations between orchid species richness and altitude using the three datasets were statistically significant (P < 0.001 or P < 0.01) (Tables 2, 3), whereas the predictive power was very high in almost all regressions.
FIGURE 6
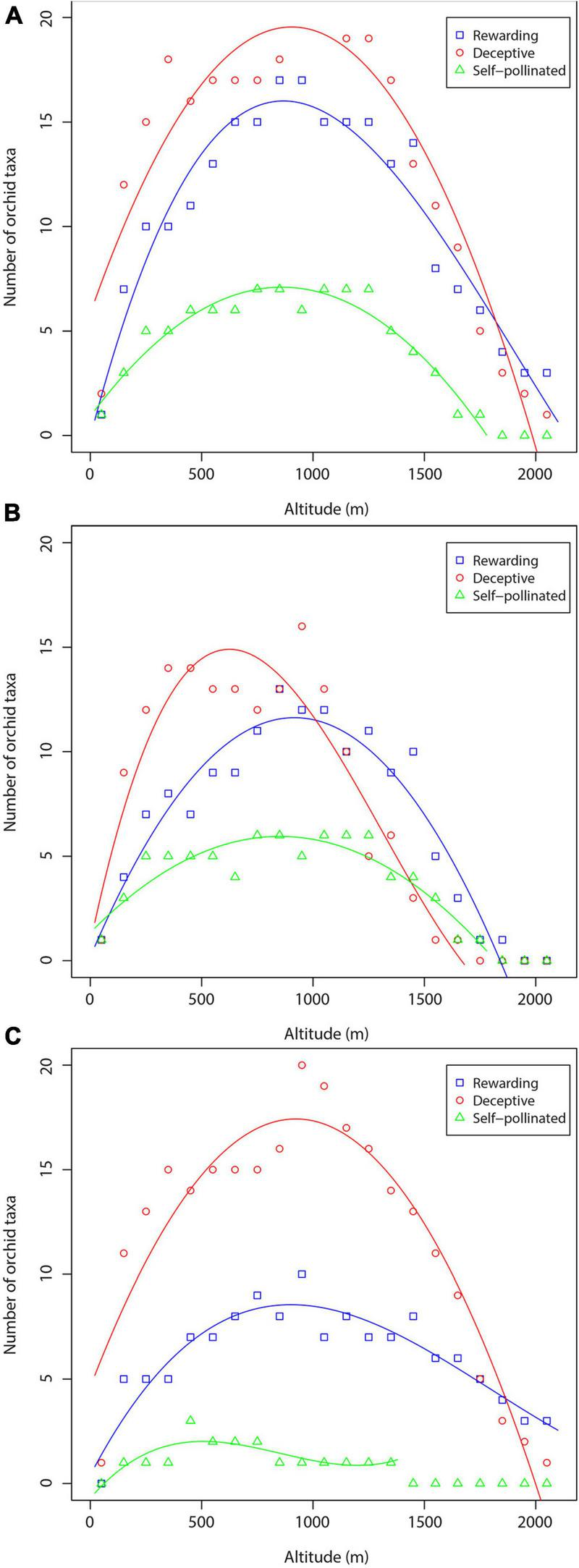
Richness of orchid species and subspecies of specific pollination systems along the altitudinal gradient: (A) total number of orchid taxa; (B) orchids of forest habitats; (C) orchids of non-forest habitats.
TABLE 2
| Orchid group | R2 | P-value |
| Total orchids | ||
| Tuberous | 0.906 (c) | <0.001 |
| Rhizomatous | 0.842 (b) | <0.001 |
| Intermediate | 0.829 (b) | <0.001 |
| Orchids of forest habitats | ||
| Tuberous | 0.822 (c) | <0.001 |
| Rhizomatous | 0.861 (b) | <0.001 |
| Intermediate | 0.676 (b) | <0.001 |
| Orchids of non-forest habitats | ||
| Tuberous | 0.923 (c) | <0.001 |
| Rhizomatous | 0.420 (b) | <0.01 |
| Intermediate | 0.885 (c) | <0.001 |
Overall statistics of the polynomial regressions used to determine the relationship between the richness of orchid species with specific underground organ systems and altitude.
(b): 2nd order polynomial regression; (c): 3rd order polynomial regression.
TABLE 3
| Orchid group | R2 | P-value |
| Total orchids | ||
| Rewarding | 0.938 (c) | <0.001 |
| Deceptive | 0.889 (b) | <0.001 |
| Self-pollinated | 0.924 (b) | <0.001 |
| Orchids of forest habitats | ||
| Rewarding | 0.914 (b) | <0.001 |
| Deceptive | 0.882 (c) | <0.001 |
| Self-pollinated | 0.821 (b) | <0.001 |
| Orchids of non-forest habitats | ||
| Rewarding | 0.869 (c) | <0.001 |
| Deceptive | 0.891 (b) | <0.001 |
| Self-pollinated | 0.664 (c) | <0.01 |
Overall statistics of the polynomial regressions used to determine the relationship between the richness of orchid species with specific pollination systems and altitude.
(b): 2nd order polynomial regression; (c): 3rd order polynomial regression.
Altitudinal patterns of orchid species density
Regression analysis showed a strong influence of altitude on orchid species density in the central Balkans (Table 4 and Figure 7). The regression lines of total orchids and orchids of forest habitats have rather the same shape (a hump-shaped pattern). Total species density reached its maximum value in the mid-altitude zone (at c. 1,000 m) and then decreased to reach its minimum in high-altitude areas (Figure 7). Species density of orchids of non-forest habitats increased with increase in altitude, peaking at about 800 m, then slightly decreased or stabilized and slightly increased up to the highest altitudes. In the in lowland areas, the density of orchids of forest habitats is generally higher than the density of orchids of non-forest habitats, whereas the density of orchids of non-forest habitats is higher than the density of orchids of forest habitats at mid- to high-altitude zone (Figure 7).
TABLE 4
| Orchid group | R2 | P-value |
| Total orchids | 0.8967 (c) | <0.01 |
| Orchids of forest habitats | 0.938 (c) | <0.001 |
| Orchids of non-forest habitats | 0.7711 (c) | <0.001 |
Overall statistics of the polynomial regressions used to determine the relationship between orchid species density and altitude.
(c): 3rd order polynomial regression.
FIGURE 7
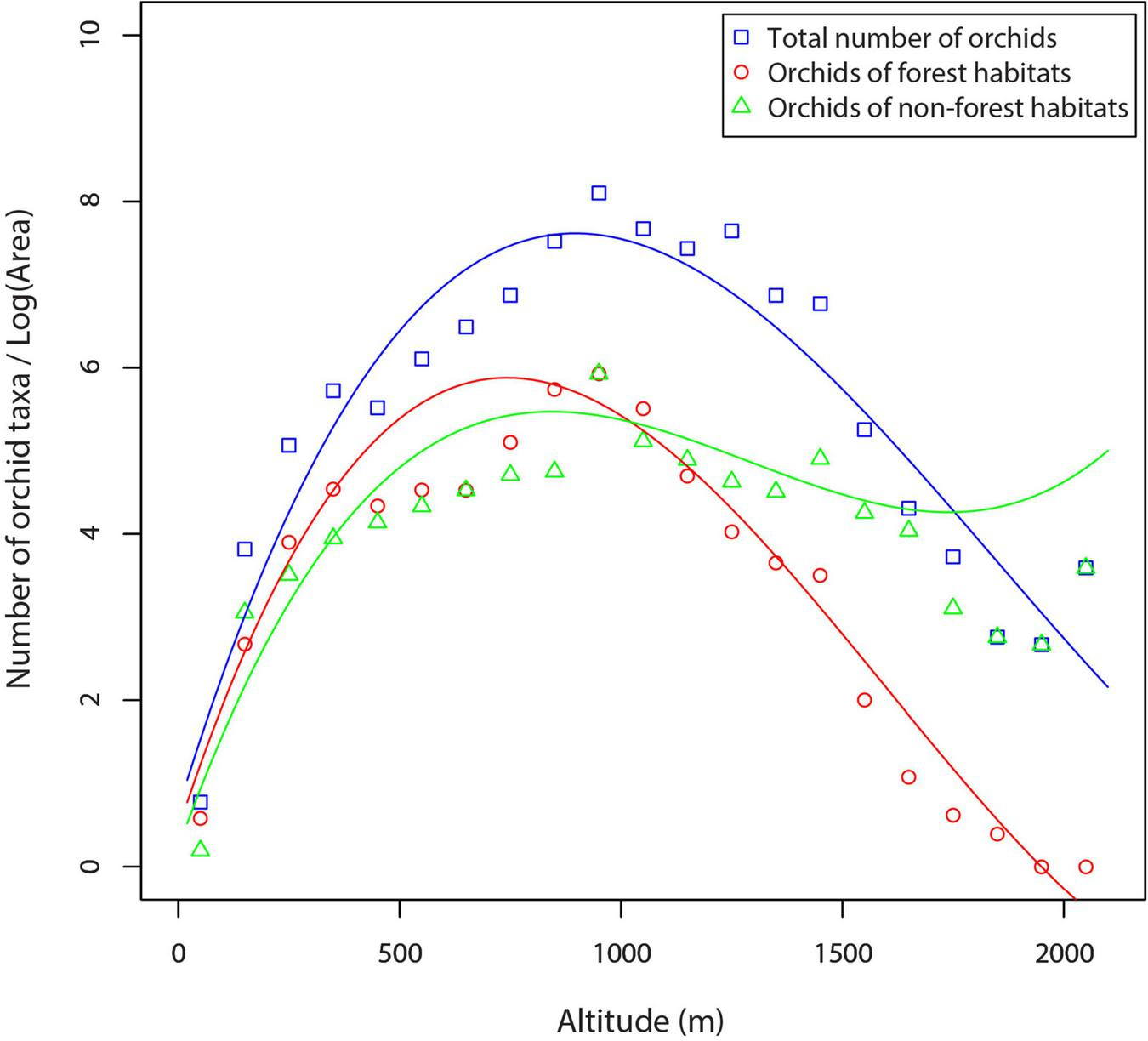
Orchid species density along an altitudinal gradient in the central Balkans (western Serbia).
Orchid densities in terms of the number of rhizomatous, intermediate, and tuberous orchid taxa for the three data sets (total orchids—total area, forest orchids—forested area, non-forest orchids—non-forested area) are shown in Figure 8. When the orchid density was calculated based on the total orchid flora and the total area at each vertical interval, the curves of rhizomatous and tuberous orchids have rather the same shape (a hump-shaped pattern), but the number of tuberous species is slightly higher (Figure 8A). Contrary to these two species groups, the intermediate orchids show an increasing trend, and their density is gradually stabilized above 1,500 m. The results concerning orchids of the forest area were of the same shape for all three orchid groups (Figure 8B). In the case of orchid density calculated using non-forest orchids and the non-forest area, the intermediate orchids showed a sharp increase along the altitudinal gradient, whereas the tuberous orchids showed a hump-shaped pattern (Figure 8C). Rhizomatous orchids have the lowest species density compared to the other two orchid groups.
FIGURE 8
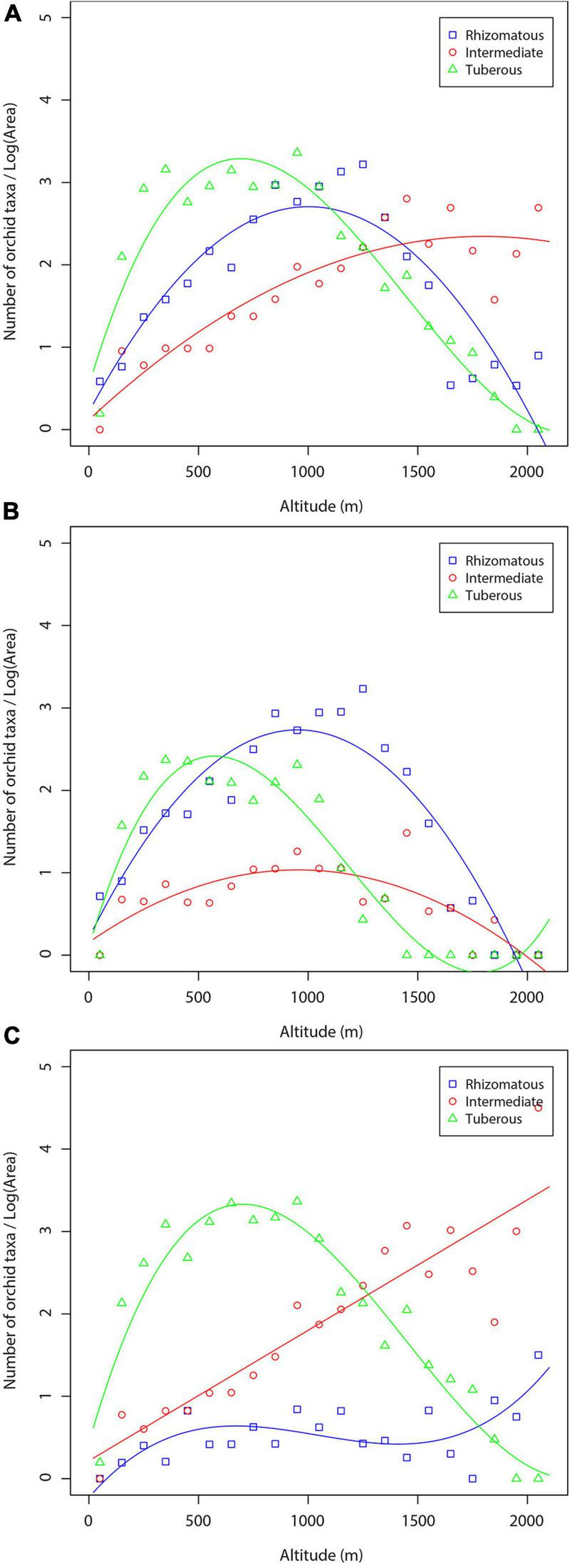
Density of orchid species and subspecies with certain underground organ systems along the altitudinal gradient: (A) total number of orchid taxa—total area; (B) forest orchids—forest area; (C) non-forest orchids—non-forest area.
The trends in orchid species density along the altitudinal gradient based on the three pollination mechanisms are shown in Figure 9. In the case of orchid density calculated based on the total number of orchids and the total area, both deceptive and self-pollinated orchids show a unimodal trend with a peak at about 900–1,000 m, the deceptive orchids being the richest in terms of species (Figure 9A). On the contrary, density of rewarding orchids increases sharply up to 900 m and then slightly decreases. The graph of orchid density of the forest habitat types is presented in Figure 9B. Here, all orchid groups show a unimodal trend. When analyzing non-forest orchids using the non-forested area, we found that the deceptive orchids showed a unimodal trend, reaching a maximum density at c. 1,000 m, much higher than density of the other orchid groups (Figure 9C). The density of rewarding orchids increases with increase in altitude, peaking at about 500 m, then is stabilized or slightly decreases up to c. 1,400 m before increasing again up to the highest altitudes. Self-pollinated species are only poorly represented in non-forested areas and show a slight hump-shaped pattern.
FIGURE 9
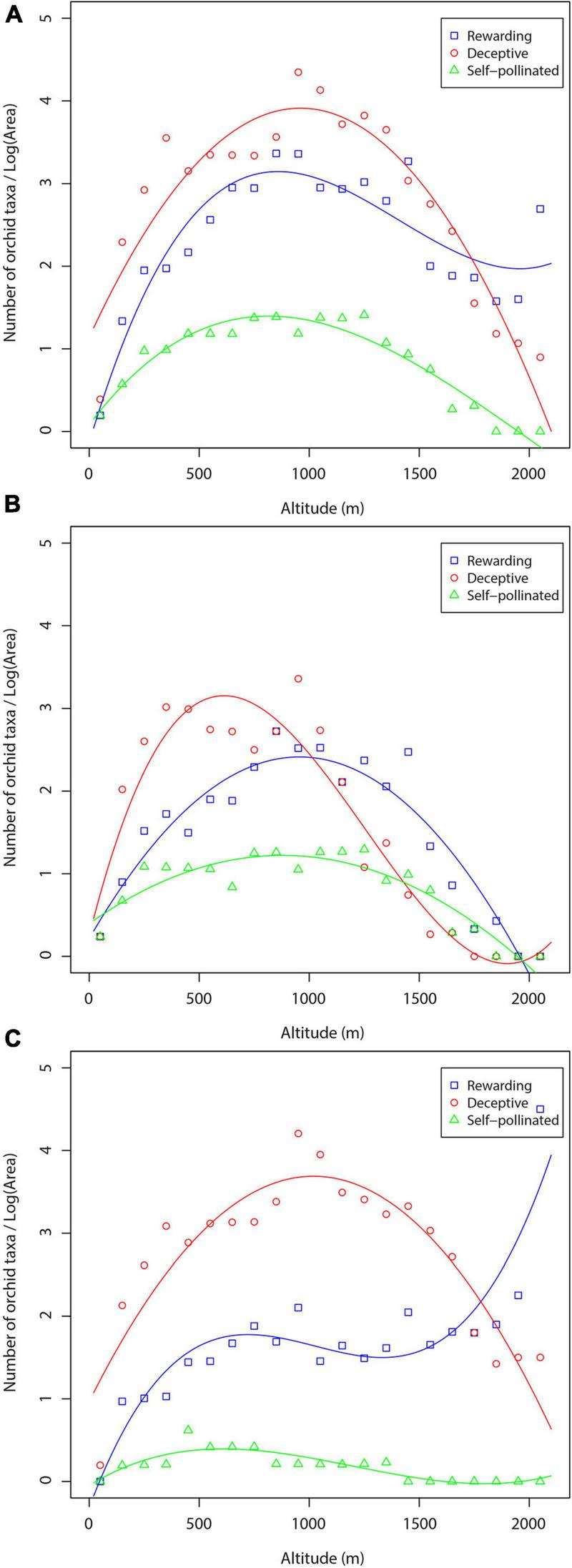
Density of orchid species and subspecies of specific pollination systems along the altitudinal gradient: (A) total number of orchid taxa—total area; (B) forest orchids—forest area; (C) non-forest orchids—non-forest area.
All the correlations between orchid species density and altitude using the three datasets were statistically significant (P < 0.001 or P < 0.01) (Tables 5, 6). Moreover, the predictive power was very high in almost all regressions. Specifically, the predictive power in the three datasets in the analyses performed using the underground organ system categories was 51.4–92.5%, whereas when analyzing orchids on the basis of their pollination mechanisms the predictive power of the regressions was 77.5–93.1%.
TABLE 5
| Orchid group | R2 | P-value |
| Total orchids | ||
| Tuberous | 0.919 (c) | <0.001 |
| Rhizomatous | 0.789 (b) | <0.001 |
| Intermediate | 0.829 (b) | <0.001 |
| Orchids of forest habitats | ||
| Tuberous | 0.912 (c) | <0.001 |
| Rhizomatous | 0.874 (b) | <0.001 |
| Intermediate | 0.667 (b) | <0.001 |
| Orchids of non-forest habitats | ||
| Tuberous | 0.925 (c) | <0.001 |
| Rhizomatous | 0.514 (c) | <0.01 |
| Intermediate | 0.831 (a) | <0.001 |
Overall statistics of the polynomial regressions used to determine the relationship between the density of orchid species with specific underground organ systems and altitude.
(a): 1st order polynomial regression; (b): 2nd order polynomial regression; (c): 3rd order polynomial regression.
TABLE 6
| Orchid group | R2 | P-value |
| Total orchids | ||
| Rewarding | 0.822 (c) | <0.001 |
| Deceptive | 0.867 (b) | <0.001 |
| Self-pollinated | 0.931 (c) | <0.001 |
| Orchids of forest habitats | ||
| Rewarding | 0.893 (b) | <0.001 |
| Deceptive | 0.920 (c) | <0.001 |
| Self-pollinated | 0.857 (b) | <0.001 |
| Orchids of non-forest habitats | ||
| Rewarding | 0.807 (c) | <0.001 |
| Deceptive | 0.826 (b) | <0.001 |
| Self-pollinated | 0.775 (c) | <0.001 |
Overall statistics of the polynomial regressions used to determine the relationship between the density of orchid species with specific pollination systems and altitude.
(b): 2nd order polynomial regression; (c): 3rd order polynomial regression.
Discussion
In this study we investigated how the richness and density of orchids vary along the altitudinal gradient in the central Balkans. Specifically, we explored whether the forest and non-forest areas along the altitudinal gradient affect patterns of orchids richness and density using different functional traits. Our results showed a hump-shaped pattern of orchid richness and density, peaking in the mid-altitude area. In addition, the richness and density of orchids of forest habitats are generally slightly higher than the richness and density of orchids of non-forest habitats in lowland areas, while orchids of non-forest habitats dominate in high-altitude areas. The results showed that the diversity patterns of orchid species with different underground organs and pollination systems differ significantly along the altitudinal gradient when the orchid flora of specific habitat types was analyzed.
Altitudinal range size
The results of this study show that 10 orchid taxa have the largest altitudinal ranges (above 1,500 m) in the central Balkans (Figure 3 and Supplementary Table 1), highlighting their ecological plasticity and adaptability, as well as a lower degree of specialization.
Our results show that orchids belonging to the Central European and boreal chorological groups (Coeloglossum viride, Dactylorhiza fuchsii, Goodyera repens, Epipactis leptochila subsp. neglecta, Epipactis muelleri, Epipactis purpurata, Epipogium aphyllum, and Neottia cordata) occur in the middle and high altitudes of the central Balkans. In contrast, in Central and Northern Europe, these species have higher altitude ranges, from lowlands to high-mountain areas (Baumann et al., 2006; Delforge, 2006). In addition, in the central Balkans, orchids characteristic primarily of Central and Northern Europe have a greater elevational range or occur at lower altitudes compared to northeastern Greece (Tsiftsis et al., 2008). Furthermore, some Mediterranean-submediterranean orchids (e.g., Anacamptis papilionacea, Neotinea tridentata, and Orchis simia) have a lower altitudinal range and occur mainly at lower altitudes in western Serbia than in northeastern Greece (Tsiftsis et al., 2008), which can be explained primarily by the climatic differences between these two study areas. Indeed, north-eastern Greece is under strong influence of the Mediterranean climate and has a significant presence of thermophilous habitats along the altitudinal gradient. In contrast, in the central Balkans (western Serbia), due to the humid and continental climate, thermophilous habitats are mainly present at lower and middle altitudes.
Altitudinal patterns of orchid species richness and density
The results of this study show hump-shaped patterns of orchid richness and density along the altitudinal gradient in western Serbia, both in the case of total orchids and in the cases of orchids of specific habitat types. In all cases, the highest species richness and density were observed between 500 and 1,200 m. This is consistent with previous studies indicating that orchid species richness is highest at mid-altitudes and decreases with increasing altitude (Acharya et al., 2011; Chen et al., 2014; Liu et al., 2015; Zhang et al., 2015a). It is assumed that patterns of orchid species richness and density along the altitudinal gradient in the central Balkans (western Serbia) are primarily determined by climatic factors and breadth of the climatic niche of species composing the orchid pool in western Serbia. The highest orchid species richness and density at mid-altitudes in the central Balkans can be explained by the fact that most species tolerate the moderate environmental conditions in the middle altitudes better than the extreme environmental conditions, in terms of temperature, precipitation, relative air humidity, ultraviolet radiation, atmospheric pressure, partial pressure of all atmospheric gases, and anthropogenic influences in the low and high-altitudes (Lomolino, 2001; van der Meulen et al., 2001; Körner, 2007). The hump-shaped patterns of orchid richness and density along the altitudinal gradient can also be explained by size of the area. In western Serbia, area of the high-altitude zones (from 1,500 to 2,100 m) is rather restricted compared to areas at middle altitudes. On the contrary, although low-altitude areas (e.g., <500 m) are extensive in the study area, species richness and density in such areas are quite low because a large part of these areas has been converted to cultivated land and the landscape is not very heterogeneous in terms of habitats and geological substrates. Similarly, previous research has indicated that habitat heterogeneity overrides the species-area relationship and is the most important predictor of species richness (Báldi, 2008; Tsiftsis, 2020). In addition, it is assumed that the lower richness and density of orchids of non-forest habitats at lower altitudes can be explained by the intense anthropogenic influences. In general, the species richness of a given altitudinal range is related to its extent. However, this is correct for orchids of forest habitats, but not for orchids of non-forest habitats. Thus, our study partially confirms the SAR hypothesis (Karger et al., 2011). We could assume that the lower richness of orchid species in the high-altitude areas of western Serbia is determined by the lower diversity of their pollinators (Arroyo et al., 1982; Jacquemyn et al., 2005b), as well as by a smaller pollen load of pollinators (Bingham and Orthner, 1998). Furthermore, the greater richness and density of orchid species of forest habitat types compared to the species richness and density of orchids of non-forest habitats in lowland areas can be explained primarily by the high diversity of forest communities in this altitude range. On the other hand, the greater richness and density of orchid species of herbaceous vegetation in relation to the richness and density of orchid species of forest vegetation in the high-altitude regions of western Serbia can be explained by the great diversity and high heterogeneity of grasslands and herbaceous wetlands, as well as by the lower diversity of forest vegetation.
Orchid species richness and density in terms of different underground organ systems
The results of this study show that tuberous orchids dominate in areas of lower and middle altitudes (Figures 5, 8). This result was expected, bearing in mind that orchids of this life form are best adapted to dry, semi-dry, and warm habitat conditions, such as those found at the lower and middle altitudes of the study area (Dafni, 1987; Averyanov, 1990; Tsiftsis et al., 2019; Štípková et al., 2021). Rhizomatous orchids are predominant in mid-altitude areas, indicating that moderate environmental conditions are appropriate for them. However, the results showed that altitude strongly influences rhizomatous orchid species richness and density in forest habitats, whereas the influence of altitude is relatively weak when it comes to the richness and density of rhizomatous orchids in non-forest habitats. This can be explained by the fact that representatives of the orchids of this life form, as the most primitive representatives of European orchids, primarily grow in forest habitats (Averyanov, 1990; Delforge, 2006).
This study shows that intermediate orchids dominate in high-altitude areas, which is consistent with previous studies suggesting that these orchids prefer lower temperatures and higher humidity in their habitats and therefore occur in high-altitude areas (Averyanov, 1990; Delforge, 2006; Pillon et al., 2006; Tsiftsis et al., 2019). The results are understandable, bearing in mind the evolutionary development of orchids. Specifically, the evolution of the first intermediate orchids was associated with the Alpine orogenesis, and the formation of mountain habitats with lower temperatures (Averyanov, 1990).
Orchid species richness and density in terms of different pollination systems
The results of this study show that the richness and density of deceptive orchids are higher through almost all the altitudinal gradient studied, and that only in the highest regions of the investigated area do rewarding orchids prevail (Figures 6, 9). This result is consistent with those of Pellissier et al. (2010), who found that the relative occurrence of food-deceptive orchids decreases with increasing altitude in the territory of Switzerland and in the Vaud mountains, suggesting that deception may be less profitable at high compared to low altitudes. This may be explained by climatic factors expressed through altitude, such as temperature, precipitation, or seasonality (Körner, 2007), as well as by factors that influence the decrease of pollinator diversity and visitation rate at high altitudes (Arroyo et al., 1982; Jacquemyn et al., 2005b; Pellissier et al., 2010).
Štípková et al. (2020) used nectarless and nectariferous orchids of the Czech Republic and found that both groups showed a hump-shaped pattern of species density, with a maximum between 300 and 900 m, i.e., at lower altitudes compared to orchids in the central Balkans. Similarly to our results, species density of both nectariferous and nectarless orchids along the altitudinal gradient in the Czech Republic was found to depend on habitat cover, i.e., the spatial distribution of forest and non-forest habitats. Earlier studies of orchids have shown that most self-pollinated orchids occur in high-altitude areas (Catling, 1990; Jacquemyn et al., 2005b). Self-pollinated orchids in the central Balkans mostly inhabit forest vegetation types, so the density of these orchids is highest in mid-altitude areas, in which forest orchids dominate.
Implications for conservation
This study shows that forest and non-forest habitats at low and mid- altitudes have high conservation value for tuberous orchids, while forest habitats at mid-altitudes are important for the survival of rhizomatous orchids. In addition, non-forest habitats at mid- and high-altitudes are most important for the survival of intermediate orchids. Given the resulting diversity patterns and the fact that intermediate orchids inhabit colder and higher precipitation areas (Tsiftsis et al., 2019; Štípková et al., 2021), our study suggests that these orchids may be affected by the rise of temperature and lower precipitation at lower altitudes due to climate change.
Our study suggests that forest and non-forest habitats at low and mid-altitudes are most important for the survival of deceptive orchids. On the other hand, mid- and high-altitudinal areas are important for the survival of rewarding orchids. Since rewarding orchids are rarer at lower altitudes, they are at high risk of extinction in these areas. In view of the fact that the rewarding orchids in western Serbia occur in almost equal numbers in forest and non-forest habitats, it is necessary to carefully plan their conservation. Deceptive orchids in the central Balkans occur in slightly higher numbers in non-forest habitats (grasslands and meadows), a circumstance that requires careful conservation of these habitats. Finally, our study indicates that most orchid species grow in mid-altitude areas, which coincide with the strong presence of tourist sites and facilities in the study area. It is therefore necessary to work on a carefully designed plan for protection of these areas, including the application of ecologically sustainable tourism that does not threaten orchids to extinction.
Conclusion
This study demonstrates a hump-shaped pattern of orchid richness and density peaking at 900–1,000 m and the fact that orchid species diversity patterns differ significantly along the altitudinal gradient when comparing forest vs. non-forest habitats. In general, our results confirm the SAR hypothesis, i.e., that the richness and density of orchid species along the altitudinal gradient are significantly affected by size of the area of a given altitudinal interval. However, this does not hold true for the orchids of non-forest habitats. Furthermore, it does not hold true for the intermediate orchids in non-forest habitats or for the rewarding orchids in the same habitats because the species density of these groups increases with altitude.
Our study suggests that the diversity patterns of orchid species with different underground organs and pollination systems differ significantly along the altitudinal gradient when considering the total flora in the whole area, but also when analyzing the orchid flora of specific habitat types. In general, tuberous orchids dominate in low and mid-altitude areas, intermediate orchids dominate at high altitudes, while rhizomatous orchids are predominate in mid-altitude forest stands. This confirms the hypothesis of evolutionary development of orchids with different underground organs and their specific ecological requirements (Averyanov, 1990; Tsiftsis et al., 2019; Štípková et al., 2021). Our study highlights the importance of low and mid-altitude areas for the survival of deceptive orchids and the importance of mid- and high-altitude areas for the survival of rewarding orchids. In addition, forest habitats at mid-altitudes have been shown to be crucial for the survival of self-pollinated orchids.
In general, our study shows that the strategies required to protect orchids change along the altitudinal gradient and depend on both functional traits of species and habitat cover. In addition, our results suggest that changes in habitat cover may be reflected in patterns of orchid diversity along the altitudinal gradient. Future research should reveal which climatic and other environmental factors are crucial for the changes in orchid species richness and density along the altitudinal gradient in the central Balkans.
Statements
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
VD: fieldwork. VD and ST: methodology and formal analysis. All authors conceptualization, writing – review and editing and read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (number 451-03-68/2022-14/200178).
Acknowledgments
We are grateful to the Tara National Park, the Forest Estate Golija Ivanjica, and the Tourist Organization of Čačak for logistical field support. We thank reviewers for their useful suggestions and comments on a previous version of the manuscript. We would also like to thank Raymond Dooley for proofreading the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.929266/full#supplementary-material
References
1
Acharya K. P. Vetaas O. R. Birks H. J. B. (2011). Orchid species richness along Himalayan elevational gradients.J. Biogeogr.381821–1833. 10.1111/j.1365-2699.2011.02511.x
2
Ackerman J. D. Trejo-Torres J. C. Crespo-Chury Y. (2007). Orchids of the West Indies: predictability of diversity and endemism.J. Biogeogr.34779–786. 10.1111/j.1365-2699.2006.01676.x
3
Aho-Bayern (2021). ArbeitskreisHeimischeOrchideen Bayern e.V.Available online at:http://www.aho-bayern.de(accessed 26 June, 2021)
4
Arroyo M. T. K. Primack R. Armesto J. (1982). Community studies in pollination ecology in the high temperate Andes of Central Chile. I. pollination mechanisms and altitudinal variation.Am. J. Bot.6982–97. 10.1002/j.1537-2197.1982.tb13237.x
5
Averyanov L. V. (1990). “A review of the genus Dactylorhiza,” in Orchid Biology: Reviews and Perspectives V, ed.ArdittiJ. (Portland, OR: Timber Press), 159–206.
6
Báldi A. (2008). Habitat heterogeneity overrides the species–area relationship.J. Biogeogr.35675–681. 10.1111/j.1365-2699.2007.01825.x
7
Baumann H. Künkele S. Lorenz R. (2006). Die Orchideen Europas. Mit angrenzenden Gebieten.Stuttgart: Eugen Ulmer KG.
8
Bingham R. A. Orthner A. R. (1998). Efficient pollination of alpine plants.Nature391238–239. 10.1038/34564
9
Cardelús L. C. Colwell R. K. Watkins J. E. Jr. (2006). Vascular epiphyte distribution patterns: explaining the mid-elevation richness peak.J. Ecol.94144–156. 10.1111/j.1365-2745.2005.01052.x
10
Catling P. (1990). “Auto-pollination in the Orchidaceae,” in Orchid Biology: Reviews and Perspectives, ed.ArdittiJ. E. (Portland, OR: Timber Press), 121–158.
11
Chase M. Christenhusz M. Mirenda T. (2017). The Book of Orchids: A Life-Size Guide to Six Hundred Species from Around the World.London: Ivy Press.
12
Chen Y. K. Yang X. B. Yang Q. Li D. H. Long W. X. Luo W. Q. (2014). Factors affecting the distribution pattern of wild plants with extremely small populations in Hainan Island, China.PLoS One9:e97751. 10.1371/journal.pone.0097751
13
Christenhusz M. J. M. Byng J. W. (2016). The number of known plants species in the world and its annual increase.Phytotaxa261201–217. 10.11646/phytotaxa.261.3.1
14
Colwell R. K. Hurtt G. C. (1994). Nonbiological gradients in species diversity and a spurious Rapoport effect.Am. Nat.144570–595. 10.1086/285695
15
Colwell R. K. Lees D. C. (2000). The mid-domain effect: geometric constraints on the geography of species diversity.Trends Ecol. Evol.1570–76. 10.1016/S0169-5347(99)01767-X
16
Colwell R. K. Rahbek C. Gotelli N. J. (2004). The mid-domain effect and species diversity patterns: what have we learned so far?Am. Nat.163E1–E23. 10.1086/382056
17
Dafni A. (1987). “Pollination in Orchis and related genera: evolution from reward to deception,” in Orchid biology, Reviews and Perspectives IV, ed.AdrittiJ. (Ithaca, NY: Cornell University Press), 79–104.
18
Delforge P. (2006). Orchids of Europe, North Africa and the Middle East.London: A. & C. Black.
19
Djordjević V. Niketić M. Stevanović V. (2021). “Orchids of Serbia: taxonomy, life forms, pollination systems, and phytogeographical analysis,” in Orchidaceae: Characteristics, Distribution and Taxonomy, ed.DjordjevićV. (New York, NY: Nova Science Publishers), 57–163.
20
Djordjević V. Tsiftsis S. (2019). Patterns of orchid species richness and composition in relation to geological substrates.Wulfenia261–21.
21
Djordjević V. Tsiftsis S. (2022). “The role of ecological factors in distribution and abundance of terrestrial orchids,” in Orchids Phytochemistry, Biology and Horticulture, Reference Series in Phytochemistry. Fundamentals and Applications, edsMérillonJ.-M.KodjaH. (Cham: Springer International Publishing), 3–72.
22
Djordjević V. Tsiftsis S. Lakušić D. Jovanović S. Jakovljević K. Stevanović V. (2020). Patterns of distribution, abundance and composition of forest terrestrial orchids.Biodivers. Conserv.294111–4134. 10.1007/s10531-020-02067-6
23
Djordjević V. Tsiftsis S. Lakušić D. Jovanović S. Stevanović V. (2016). Factors affecting the distribution and abundance of orchids in grasslands and herbaceous wetlands.Syst. Biodivers.14355–370. 10.1080/14772000.2016.1151468
24
Dunn R. R. McCain C. M. Sanders N. (2007). When does diversity fit null model predictions? Scale and range size mediate the mid-domain effect.Glob. Ecol. Biogeogr.16305–312. 10.1111/j.1466-8238.2006.00284.x
25
ESRI (2017). ArcGIS – ArcMap: ArcInfo (version 10.6).Redlands, CA: Environmental Science Research Institute.
26
Inda L. A. Pimentel M. Chase M. W. (2012). Phylogenetics of tribe Orchideae (Orchidaceae: Orchidoideae) based on combined DNA matrices: inferences regarding timing of diversification and evolution of pollination syndromes.Ann. Bot.11071–90. 10.1093/aob/mcs083
27
Jacquemyn H. Brys R. Hermy M. Willems J. H. (2005a). Does nectar reward affect rarity and extinction probabilities of orchid species? an assessment using historical records from Belgium and the Netherlands.Biol. Conserv.121257–263. 10.1016/j.biocon.2004.05.002
28
Jacquemyn H. Micheneau C. Roberts D. L. Pailler T. (2005b). Elevational gradients of species diversity, breeding system and floral traits of orchid species on Réunion Island.J. Biogeogr.321751–1761. 10.1111/j.1365-2699.2005.01307.x
29
Jacquemyn H. Honnay O. Pailler T. (2007). Range size variation, nestedness and species turnover of orchid species along an altitudinal gradient on Réunion Island: Implications for conservation.Biol. Conserv.136388–397. 10.1016/j.biocon.2006.12.008
30
Jersáková J. Johnson S. D. Kindlmann P. (2006). Mechanisms and evolution of deceptive pollination in orchids.Biol. Rev.81219–235. 10.1017/S1464793105006986
31
Jersáková J. Traxmandlová I. Ipser Z. Matthias K. Pellegrino G. Schatz B. et al (2015). Biological flora of Central Europe: Dactylorhiza sambucina (L.) Soó.Perspect. Plant Ecol. Evol. Syst.17318–329. 10.1016/j.ppees.2015.04.002
32
Karger D. N. Kluge J. Krömer T. Hemp A. Lehnert M. Kessler M. (2011). The effect of area on local and regional elevational patterns of species richness.J. Biogeogr.381177–1185. 10.1111/j.1365-2699.2010.02468.x
33
Körner C. (2007). The use of ‘altitude’ in ecological research.Trends Ecol. Evol.22569–574. 10.1016/j.tree.2007.09.006
34
Kull T. Hutchings M. J. (2006). A comparative analysis in decline in the distribution ranges of orchid species in Estonia and the United Kingdom.Biol. Conserv.12931–39. 10.1016/j.biocon.2005.09.046
35
Laughlin D. C. Joshi C. van Bodegom P. M. Bastow Z. A. Fulé P. Z. (2012). A predictive model of community assembly that incorporates intraspecific trait variation.Ecol. Lett.151291–1299. 10.1111/j.1461-0248.2012.01852.x
36
Liu Q. Chen J. Corlett R. T. Fan X. Yu D. Yang H. et al (2015). Orchid conservation in the biodiversity hotspot of southwestern China.Conserv. Biol.291563–1572. 10.1111/cobi.12584
37
Lomolino M. V. (2001). Elevation gradients of species-density: historical and prospective views.Glob. Ecol. Biogeogr.103–13. 10.1046/j.1466-822x.2001.00229.x
38
McCain C. M. Grytnes J. A. (2010). Elevational Gradients in species richness. Encyclopedia of Life Sciences (ELS).Chichester: John Wiley & Sons, Ltd.
39
Ogle D. H. Doll J. C. Wheeler P. Dinno A. (2022). FSA: Fisheries Stock Analysis. R Package Version0.9.3. Available online at:https://github.com/fishR-Core-Team/FSA(accessed June 9, 2022).
40
Pellissier L. Vittoz P. Internicola A. I. Gigord L. D. B. (2010). Generalized food-deceptive orchid species flower earlier and occur at lower altitudes than rewarding ones.J. Plant Ecol.3243–250. 10.1093/jpe/rtq012
41
Pillon Y. Fay M. F. Shipunov A. B. Chase M. W. (2006). Species diversity versus phylogenetic diversity: a practical study in the taxonomically difficult genus Dactylorhiza (Orchidaceae).Biol. Conserv.1294–13. 10.1016/j.biocon.2005.06.036
42
R Core Team (2021). R: A Language And Environment Statistical Computing.Vienna: R Foundation for Statistical Computing.Available online at:https://www.r-project.org/
43
Rahbek C. (1995). The elevational gradient of species richness: a uniform pattern?Ecography18200–205. 10.1111/j.1600-0587.1995.tb00341.x
44
Štípková Z. Traxmandlová I. Kindlmann P. (2016). Determinants of orchid species diversity in Latin America.Lankesteriana16293–297. 10.15517/lank.v16i2.26013
45
Štípková Z. Tsiftsis S. Kindlmann P. (2020). Pollination mechanisms are driving orchid distribution in space.Sci. Rep.10:850. 10.1038/s41598-020-57871-5
46
Štípková Z. Tsiftsis S. Kindlmann P. (2021). Distribution of orchids with different rooting systems in the Czech Republic.Plants10:632. 10.3390/plants10040632
47
Swarts N. D. Dixon K. W. (2009). Terrestrial orchid conservation in the age of extinction.Ann. Bot.104543–556. 10.1093/aob/mcp025
48
Taylor A. Keppel G. Weigelt P. Zotz G. Kreft H. (2021). Functional traits are key to understanding orchid diversity on islands.Ecography44703–714.
49
Timsina B. Kindlmann P. Subedi S. Khatri S. Rokaya M. B. (2021). Epiphytic orchid diversity along an altitudinal gradient in central Nepal.Plants10:1381. 10.3390/plants10071381
50
Trigas P. Panitsa M. Tsiftsis S. (2013). Elevational gradient of vascular plant species richness and endemism in Crete – the effect of post-isolation mountain uplift on a continental island system.PLoS One8:e59425. 10.1371/journal.pone.0059425
51
Tsiftsis S. (2020). The complex effect of heterogeneity and isolation in determining alpha and beta orchid diversity on islands in the Aegean archipelago.Syst. Biodivers.18281–294. 10.1080/14772000.2020.1738584
52
Tsiftsis S. Štípková Z. Kindlmann P. (2019). Role of way of life, latitude, elevation and climate on the richness and distribution of orchid species.Biodivers. Conserv.2875–96. 10.1007/s10531-018-1637-4
53
Tsiftsis S. Tsiripidis I. Karagiannakidou V. Alifragis D. (2008). Niche analysis and conservation of the orchids of east Macedonia (NE Greece).Acta Oecol.3327–35.
54
van der Meulen M. A. Hudson A. J. Scheiner S. M. (2001). Three evolutionary hypotheses for the hump-shaped productivity–diversity curve.Evol. Ecol. Res.3379–392.
55
Vereecken N. J. Dafni A. Cozzolino S. (2010). Pollination syndromes in mediterranean orchids – implications for speciation, taxonomy and conservation.Bot. Rev.76220–240. 10.1007/s12229-010-9049-5
56
Waterman R. J. Bidartondo M. I. (2008). Deception above, deception below: linking pollination and mycorrhizal biology of orchids.J. Exp. Bot.591085–1096. 10.1093/jxb/erm366
57
Zhang S.-B. Chen W.-Y. Huang J.-L. Bi Y.-F. Yang X.-F. (2015a). Orchid species richness along elevational and environmental gradients in yunnan, China.PLoS One10:e0142621. 10.1371/journal.pone.0142621
58
Zhang Z. Yan Y. Tianb Y. Lib J. Hea J.-S. Tanga Z. (2015b). Distribution and conservation of orchid species richness in China.Biol. Conserv.18164–72. 10.1016/j.biocon.2014.10.026
Summary
Keywords
Orchidaceae, ecology, altitudinal patterns, distribution, life history strategies, species richness, species diversity, Balkan Peninsula
Citation
Djordjević V, Tsiftsis S, Kindlmann P and Stevanović V (2022) Orchid diversity along an altitudinal gradient in the central Balkans. Front. Ecol. Evol. 10:929266. doi: 10.3389/fevo.2022.929266
Received
26 April 2022
Accepted
04 July 2022
Published
09 August 2022
Volume
10 - 2022
Edited by
Isabel Marques, University of Lisbon, Portugal
Reviewed by
Hans Jacquemyn, KU Leuven, Belgium; Zhifeng Ding, Institute of Zoology, Guangdong Academy of Science (CAS), China
Updates
Copyright
© 2022 Djordjević, Tsiftsis, Kindlmann and Stevanović.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vladan Djordjević, vdjordjevic@bio.bg.ac.rs
This article was submitted to Conservation and Restoration Ecology, a section of the journal Frontiers in Ecology and Evolution
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.