- 1Programa de Pós-Graduação em Ecologia, Universidade Federal do Pará, Guamá, Brazil
- 2Programa de Pós-graduação em Zoologia, Universidade Federal do Pará, Av. Perimetral, 2-224 - Guamá, Belém, PA, Brazil
- 3Programa de Pós- Graduação em Ecologia, Laboratório de Biologia e Conservação de Morcegos, Departamento de Zoologia, Instituto de Ciências Biológicas, Universidade de Brasília, Brasília, DF, Brazil
- 4Programa de Pós-Graduação em Biologia Animal, Laboratório de Sistemática, Ecologia e Evolução (LSEE), Instituto de Biociências (INBIO), Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande, MS, Brazil
- 5Fundação Oswaldo Cruz de Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil
Introduction: The relationship between ectoparasitic flies and bats is influenced by anthropogenic changes in natural environments. In the Amazon, various forms of disturbance contribute to ecosystem imbalance, potentially leading to the local extinction and disruption of ecological functions. Cacao cultivation has been expanding in the Amazon, but studies on its impacts on bat diversity are still limited, hindering the development of effective conservation strategies. This study aims to assess bat and batfly species to understand how land-use changes affect antagonistic interaction networks between ectoparasitic flies and Phyllostomidae bats.
Methods: We sampled urban areas, natural vegetation, and cacao plantations using 10 mist nets over 69 nights at 36 sites across 10 municipalities in Pará.
Results: The overall interaction network showed 42 host species and 52 ectoparasite species, showing high specialization, low connectivity, and insignificant nesting and parasite vulnerability. The highest ectoparasites richness was observed in natural vegetation (47), followed by cacao (30) and urban areas (29). The cacao-dominated network exhibited high modularity, natural areas had the highest occurrence of infracommunities, followed by urban areas and cocoa. Five bat species were present in all three environments, each infested with infracommunities. Notably, infracommunal associations were common among ectoparasite genera Speiseria, Strebla, and Trichobius.
Discussion: The species richness observed highlights Pará as a region of exceptional diversity for dipteran ectoparasites of bats (with 60 species). Our study suggests that cacao plantations can serve as suitable habitats for both bats and flies. Notably, we conducted this research on a small, family-run farm. While these types of farms are not substitutes for natural vegetation, they may help mitigate the impacts of rapid land-use and land-cover change. In fact, these small, family-operated farms demonstrated habitat suitability close to that of natural vegetation areas, supporting greater biodiversity within agricultural landscapes.
1 Introduction
Parasites represent a large proportion of species diversity in ecosystems (Dobson et al., 2008), with associations so specific that they often drive co-speciation and mirror the phylogenetic relationships of their hosts (Hafner et al., 1994). In particular, bats and their ectoparasitic flies frequently form co-evolutionary or co-adaptive relationships (Poulin et al., 2011), shaped by ecological interactions (Janzen, 1980). Mammals are commonly parasitized by arthropods (Whitaker, 1988; Graciolli and Bianconi, 2007; Torres et al., 2019), especially ectoparasitic Diptera from the families Streblidae and Nycteribiidae, which are exclusive to Chiroptera (Graciolli, 2004; Rui and Graciolli, 2005; Santos et al., 2012). Bats, the only mammals capable of true flight, 1,487 species globally (Simmons and Cirranello, 2025), with 288 species in the Neotropics and 181 in Brazil (Garbino et al., 2024). Their morphological and dietary diversity allows them to occupy varied habitats and provide essential ecological services, such as pollination, seed dispersal, and insect control (Kunz et al., 2011; Aguiar et al., 2021), making them key species in tropical forests, like the (Silva et al., 2020).
Streblidae includes 299 species exclusive to the New World (Dick et al., 2016; Graciolli and Linardi, 2002). In Brazil, 24 genera, 101 species - 9 of which are endemic - and 2 subspecies are currently known (Graciolli, 2024). The Nycteribiidae family is distributed worldwide, with 28 species recorded in Brazil, 12 of which are endemic and belong to 2 genera (Graciolli and Hrycyna, 2024). These ectoparasitic flies reproduce through adrenotropic viviparity (Dittmar et al., 2015), where each female deposits a pre-pupa that develops in approximately three weeks (Dick and Patterson, 2007). The pre-pupa is deposited on the surface of the host’s shelter, and once it hatches, the young fly must quickly find a host to survive (Dittmar et al., 2009).
The development of these flies in limited by the shelter microclimatic (Dittmar et al., 2009; Morse et al., 2012) and behavior (Reckardt and Kerth, 2007), and host traits such as sex, age, size, and reproductive stage, which can influence the abundance of ectoparasitic flies (Patterson et al., 2008; Esbérard et al., 2012). These interactions are also influenced by human activity (Salkeld et al., 2013), which leads to habitat degradation and loss, reduced habitat host and parasite diversity (Bojsen and Jacobsen, 2003; Kleine and Trivinho-Strixino, 2005) and potentially causing local species extinctions (Ramírez-Mejía et al., 2020).
Alterations in parasitic relationships are also evident due to changes in land use and land cover, habitat loss and fragmentation, urbanization, and climate change (Davidson et al., 2012; Urbieta et al., 2014; Bolívar-Cimé et al., 2018; Palheta et al., 2020; Mendes, 2021). In fact, the subfamily Phyllostominae, which is primarily composed of insectivorous, carnivorous, and omnivorous gleaning bats, is described as a potential indicator of disturbed areas (Silva, 2012) and are highly adaptable, occupying both natural and anthropogenic environments (Morales et al., 2012; Heer et al., 2015).
In Pará, the natural landscape has been directly affected by climate change and rapid agricultural development (Galford et al., 2010). Changes in land use and cover are, in fact, the main drivers of biodiversity loss in the Neotropics (Meyer et al., 2016). In this context, numerous sources of anthropogenic disturbance are observed, such as deforestation, the opening of pastures, fires (Fearnside, 1995; Fearnside et al., 2013; Defries and Rosenzweig, 2010), short-cycle crops like soybeans (Nepstad et al., 2008; Barona et al., 2010), and long-cycle crops like cocoa, both in the cabruca system and in traditional monoculture (Brainer, 2021; França et al., 2023). The advance of cocoa farming in the Amazon is evident, despite the state of Pará having a smaller planted area than Bahia (152,881 ha vs. 440,050 ha, respectively) (IBGE/SIDRA/LSPA, 2023). Nevertheless, with a production of over 129,000 tons of cocoa in 2019, Pará became the largest cocoa producer in Brazil, surpassing Bahia, which produced 105,000 tons (Agencia Para, 2021).
Cocoa plantations are typically located near forest fragments, with cocoa trees ranging from three to twenty meters in height (SENAR, 2018). These areas, which exhibit low anthropogenic modification, represent productive environments with high bat activity (Faria, 2006). In Pará, cocoa is cultivated through conventional monoculture, extractivist systems, and cabruca-type Agroforestry Systems (AFS), where cocoa is grown alongside tall canopy trees. Cabruca AFS involve mixed and polyculture systems, often pairing cocoa with species like rubber, açaí, African mahogany, or Australian cedar, which can include native or exotic species of economic interest (SENAR, 2018; Ministério da Agricultura, Pecuária e Abastecimento, 2020).
Bat foraging in cocoa areas is common in both South America and Africa (Faria, 2006; Atagana et al., 2021; Ferreira et al., 2023a, Ferreira et al., 2023b). In the Amazon, however, studies in cocoa environments are limited, and comparisons between cocoa areas and other environments (urban and rural) regarding bat ecology and interaction networks remain understudied (Palheta et al., 2020). Bat sampling in the Amazon is among the lowest in Brazil, covering only 23% of the area (Bernard et al., 2012), which hampers the development of effective conservation strategies for Phyllostomidae bats (Cardoso et al., 2019).
This study aims to: (i) identify ectoparasitic fly species on bats from Cocoa, Natural Vegetation, and Urban areas in the Brazilian Amazon; and (ii) assess whether land-use changes impact the structure of interaction networks between ectoparasitic flies (Diptera: Streblidae, Nycteribiidae) and Phyllostomidae bats (Mammalia: Chiroptera). We analyze bat infracommunities, assemblage of parasite species co-occurring within individual host organisms (Bush et al., 1997), and predict that (i) cocoa area networks will show higher specialization and modularity, (ii) urban networks will be more vulnerable to extinction, and (iii) cocoa areas will have the lowest levels of network nesting. We also report four new occurrences of flies from Brazil and ten new occurrences for the state of Pará.
2 Material and methods
2.1 Study area
The study area includes ten municipalities in the state of Pará: Altamira, Anapú, Bragança, Brasil Novo, Medicilândia, Nova Timboteua, Placas, Rurópolis, Uruará, and Vitória do Xingu (Figure 1). Eight of these municipalities are in the region known as the ‘arc of deforestation,’ a phenomenon that began in 1974 with the opening of the Trans-Amazonian Highway and was intensified by the creation of the Belo Monte Hydroelectric Plant – UHBM, in the 2000s (Palheta et al., 2020). The predominant biome in Pará is the Amazon, with a tropical humid climate according to the Köppen-Geiger classification system (Peel et al., 2007). The average annual temperature ranges from 25.5°C to 27.1°C, and rainfall indices vary between 1900 mm and 3000 mm per year (SEMAS, 2021). Bats were sampled over 69 nights at 36 sites: 21 sites and 24 nights in vegetation areas (forest fragments), 5 sites and 25 nights in urban areas, and 10 sites and 20 nights in cocoa cultivation areas. For all analyses we use the nights as replicate.
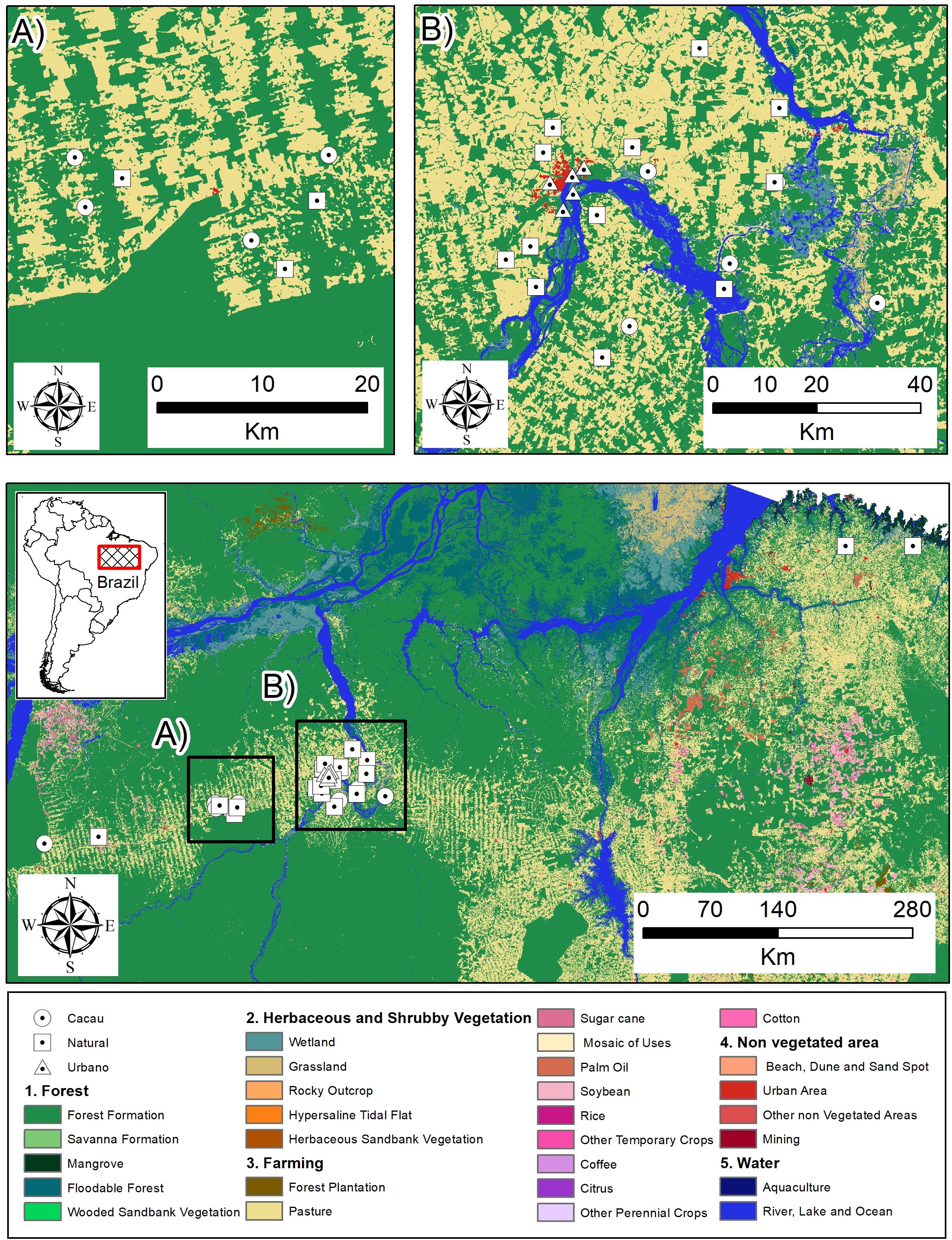
Figure 1. Spatial distribution of sampling sites across different environment types in the study area, 2017–2022. (A) shows the collection points in the municipalities of Uruará and Medicilândia, while (B) displays the collection points in the municipalities of Altamira, Anapu, and Vitória do Xingu.
The sampled areas included natural vegetation, consisting of forest fragments that have undergone anthropogenic modifications, primarily located in municipalities within the Trans-Amazonian region. The cocoa cultivation areas comprised conventional, non-intercropped plantations. The urban sites were sampled within the urban zone of the municipality of Altamira-PA.
2.2 Bats and bat flies sampling
The ChiroXingu research group (Center for Studies in Ecology and Conservation of Chiroptera) conducted bat collections between 2017 and 2022 under SISBIO license no. 57294-2. Collections were carried out during the dry season (between July and November) at sites selected for accessibility and spaced at least 5 km apart. At each sampling location, researchers deployed ten mist nets (9 m x 2.5 m) at sunset. The nets remained in place for six hours, with inspections conducted every 30 minutes (Bernard, 2001; Silva, 2012).
Captured bats were temporarily placed in 100% cotton fabric bags and for field sorting. For each individual, we recorded weight, sex, age, and forearm measurement. Bats not retained as specimens were marked with numbered bands and released. Voucher specimens were transported to the Laboratory of Chiroptera Studies (LABEQ) at the Laboratory of Ecology (LABECO) of the Federal University of Pará (UFPA), Altamira campus, there, we euthanized two individuals of each species from each locality, one male and one female. We recorded morphometric data, including total length, foot length, ear length, tragus length, forearm length, and weight. Following measurements, specimens were fixed in 10% formalin and subsequently preserved in 70% alcohol in the ChiroXingu Bat Collection.
In the field, we conducted thorough examinations of captured bats to collect ectoparasites. The process involved: 1. Active searches across the entire body of each bat including fur, wings, membranes, and ears. 2. Removal of ectoparasitic flies using tweezers and brushes moistened with 70% alcohol. Preservation of collected flies: placed in individual containers filled with 70% alcohol and labeled according to the host bat. Fly identification was carried out in the laboratory to the lowest taxonomic level, using identification keys proposed by Guerrero (1996), Wenzel (1976), and Graciolli and Carvalho (2001).
2.3 Data analysis
The lists of bat species and ectoparasitic flies were organized as follows: the flies were listed in alphabetical order and divided into the two sampled families, Nycteribiidae and Streblidae. The bats were categorized according to the flies captured in our study, those already recorded in the literature, and the total richness and abundance by genus (male and female) of the flies, as well as new occurrences for Brazil and the state of Pará. The interactions between fly and bat communities were described by constructing interaction networks, considering the abundance of flies on the bats as the frequency of interactions. This analysis was carried out for the entire dataset and for each environment investigated (Cocoa, Natural Vegetation, and Urban Area).
To characterize and analyze the structure of the networks, we calculated the degree of ectoparasites and hosts, as well as the metrics of Connectivity, Nesting, Specialization, and Modularity, along with parasites vulnerability, extinction slope, and species richness. The specification of each metric is described in the Supplementary Material. To assess the significance of the indices of Nesting (WNODF), Connectivity (C), Modularity (M), and Specialization (H2) obtained from the interaction networks, we used the Monte Carlo procedure based on random matrices according to the null model (Dormann et al., 2008), with 10,000 randomizations. The analysis of network metrics (Interaction Degree, Nesting, Connectivity, Specialization, Modularity, Parasite Vulnerability, Extinction Slope, and Species Richness) was conducted using the bipartite package (Dormann et al., 2008) in R (R Core Team, 2022).
Finally, for the description of the infracommunities, we considered only hosts that occurred in all environments and had an abundance of more than two individuals in each environment. For all infracommunities, we calculated the absolute number of occurrences and the relative frequency (the ratio between the absolute frequency of parasites and the total population of each host) of ectoparasites. We defined an infracommunity as the association of a host with two or more species of ectoparasitic flies (Bush et al., 1997).
3 Results
With 69 nights of sampling at 36 points (Figure 1), we achieved a sampling effort of 93,150 h.m², 32,400 h.m² in fragments, 33,750 h.m² in urban areas and 27,000 h.m² in cocoa plantation (Straube and Bianconi, 2002). A total of 1,091 bats were captured, with 774 individuals hosting ectoparasitic flies. We sampled 2,694 flies (see Supplementary Table S1). The bats belonged to six families and 42 species, with Phyllostomidae and Mormoopidae being the most abundant families (see Supplementary Table S1). The most abundant host species in the three environments were Carollia perspicillata (450 individuals), Pteronotus rubiginosus (50), Artibeus lituratus (44) and Artibeus obscurus (31) (Supplementary Table S1).
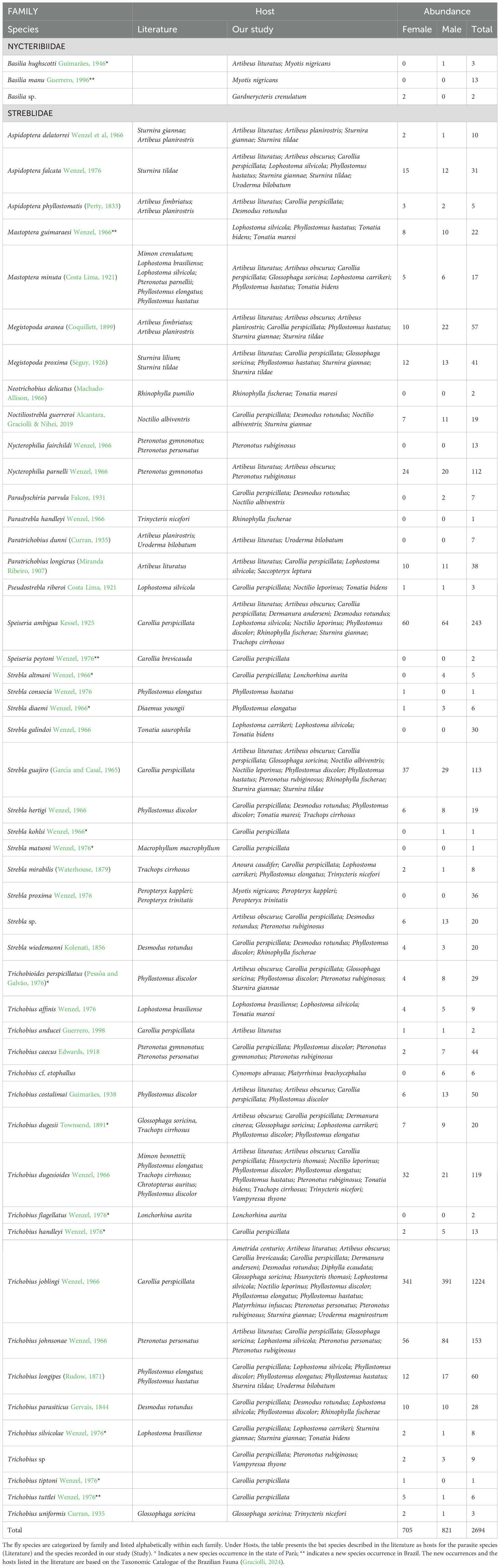
The captured ectoparasitic flies belonged to the family Nycteribiidae, with 18 individuals from three species of the genus Basilia, and to the family Streblidae, with 2,676 individuals from 49 species and 14 genera (Table 1). The most abundant species were Trichobius joblingi (1,224), Speiseria ambígua (243), Trichobius johnsonae (153), Trichobius dugensioides (119), Strebla guajiro (113) e Nycterophilia parnelli (112) (Table 1). Four new occurrences of flies were observed in Brazil, and ten new occurrences were recorded for the state of Pará (Table 1). All sampled ectoparasite species presented new associations with hosts (Table 1), with particular emphasis on Speiseria ambigua, Strebla guajiro, and Trichobius joblingi, which were associated with Carollia perspicillata according to the literature and observed in numerous other host species, including non-Phyllostomidae, in our study (Table 1). Among the species with the highest number of associations, we observed Trichobius joblingi associated with 20 host species, Strebla guajiro with 12 hosts, Trichobius dugesioides and Speiseria ambigua with 11 hosts, and Aspidoptera falcata and Megistopoda aranea with eight hosts (Supplementary Table S2; Figure 2).
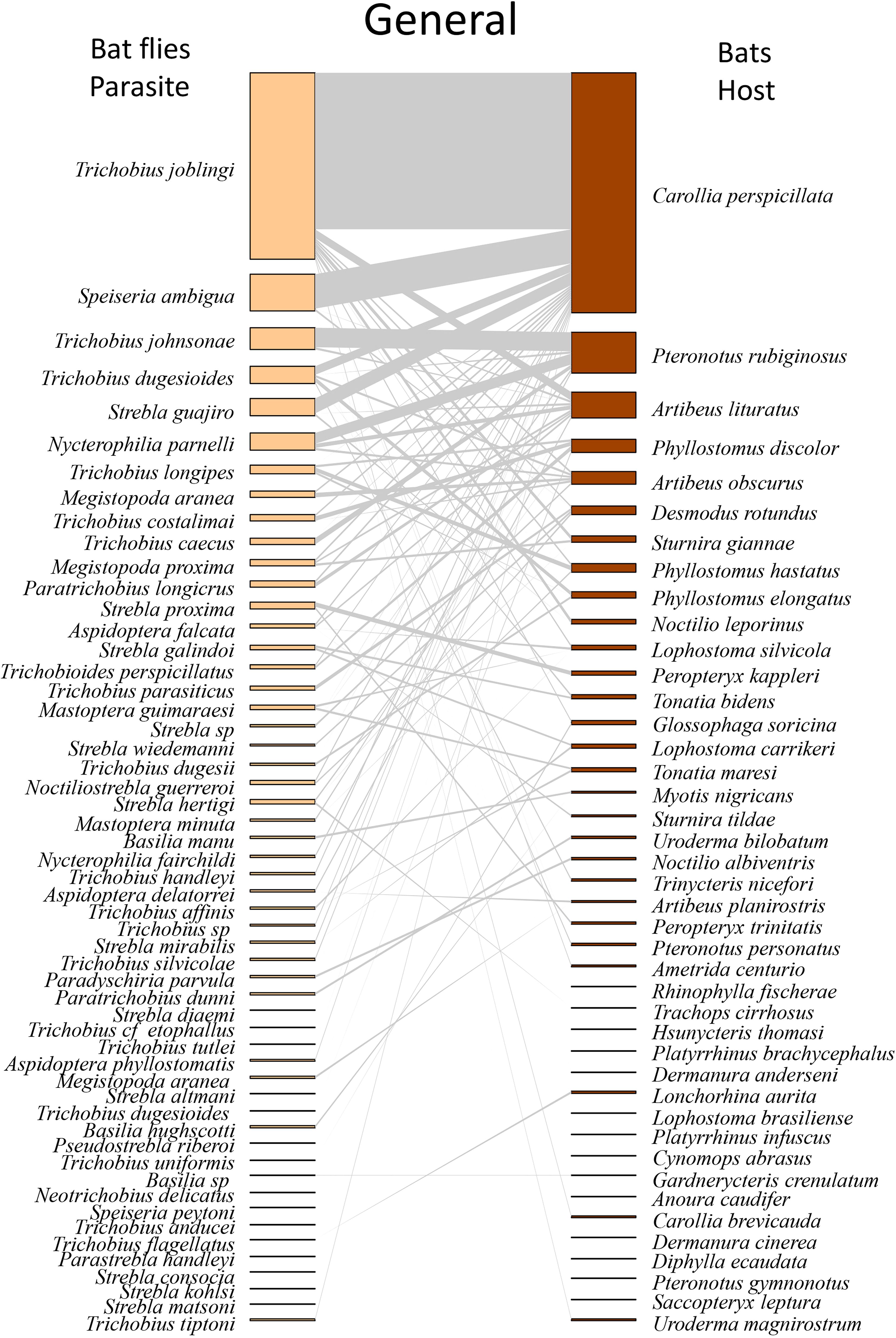
Figure 2. Network of ectoparasites (Diptera: Nycteribiidae and Streblidae) and host bats (Chiroptera: Phyllostomidae) observed as the sum of all sampled areas across the 10 municipalities in the study from 2017 to 2022. Species are arranged from most to least abundant. The darker sections on the host and parasite sides represent the abundances of each species, while the middle (gray part) illustrates the interaction network. The thickness of the lines is adjusted according to the number of interactions between the species.
When analyzed by type of environment, we found that Trichobius joblingi, Speiseria ambigua, Megistopoda aranea, and Aspidoptera falcata had the lowest degree of interaction with bats in the cacao area (Supplementary Table S2; Figure 3) and the highest degree in the natural vegetation area (Supplementary Table S2; Figure 4), followed by the urban area (Supplementary Table S2; Figure 5). The hosts with the highest degree of interaction (Supplementary Table S3) were Carollia perspicillata with 33 parasite species, Artibeus lituratus with 17 species, Phyllostomus discolor with 12 species, Sturnira giannae with 11 species, and Pteronotus rubiginosus with 10 parasite species (Supplementary Table S3; Figure 2). Carollia perspicillata showed high degrees of interaction across all three environments (Supplementary Table S3; Figures 3–5). Artibeus lituratus had the highest number of interactions in the Urban Area (Supplementary Table S3; Figure 5), Phyllostomus discolor in the Cacao areas (Supplementary Table S3; Figure 3), and Pteronotus rubiginosus and Sturnira giannae in Natural Vegetation (Supplementary Table S3; Figure 4).
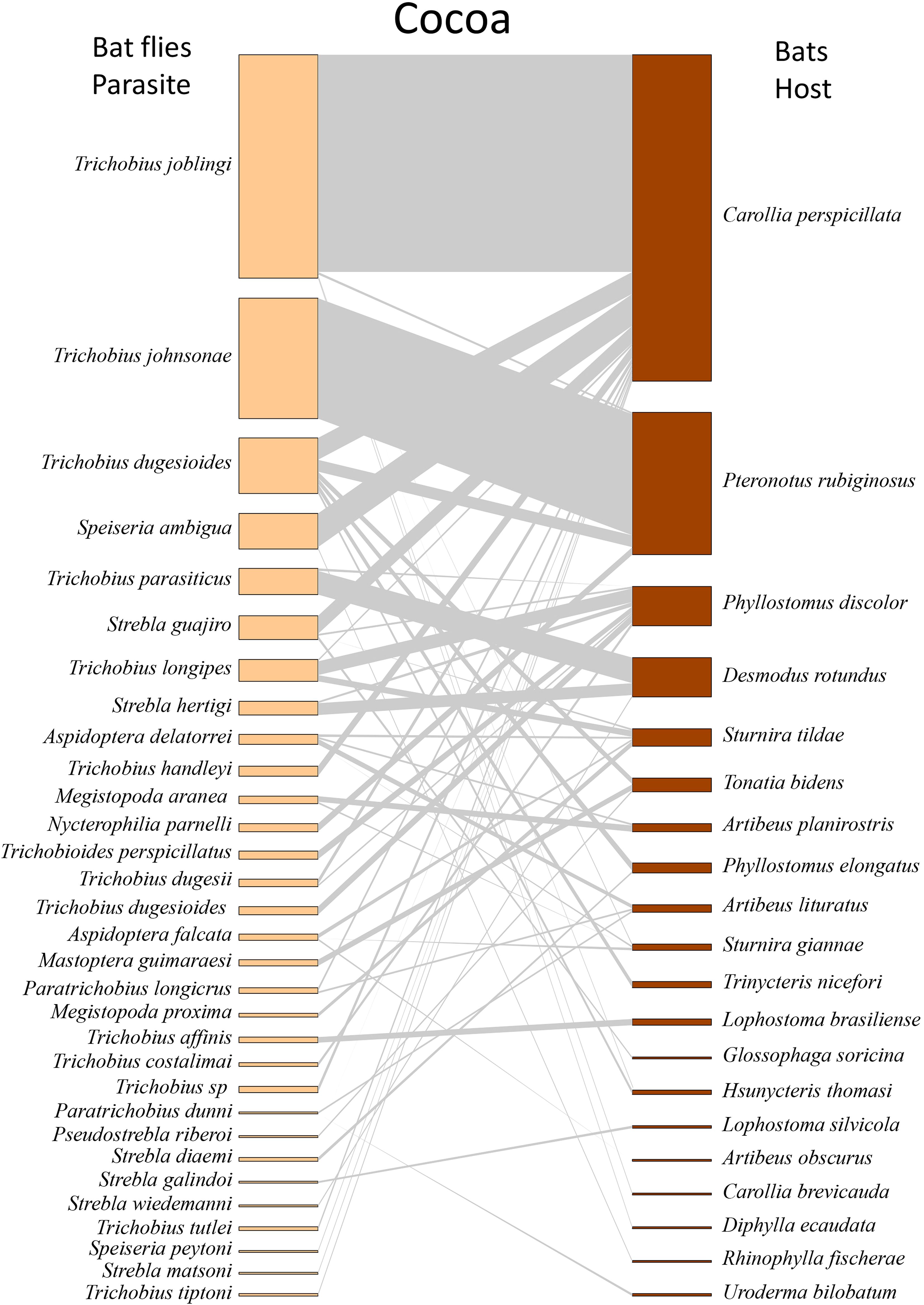
Figure 3. Network of ectoparasites (Diptera: Nycteribiidae and Streblidae) and host bats (Chiroptera: Phyllostomidae) observed in cocoa plantation areas of the municipalities of Altamira, Anapú, Medicilândia, Rurópolis, Uruará, and Vitória do Xingu, sampled from 2021 to 2022. Species are arranged from most to least abundant. The darker sections on the host and parasite sides represent the abundances of each species, while the middle (gray part) illustrates the interaction network. The thickness of the lines is adjusted according to the number of interactions between the species.
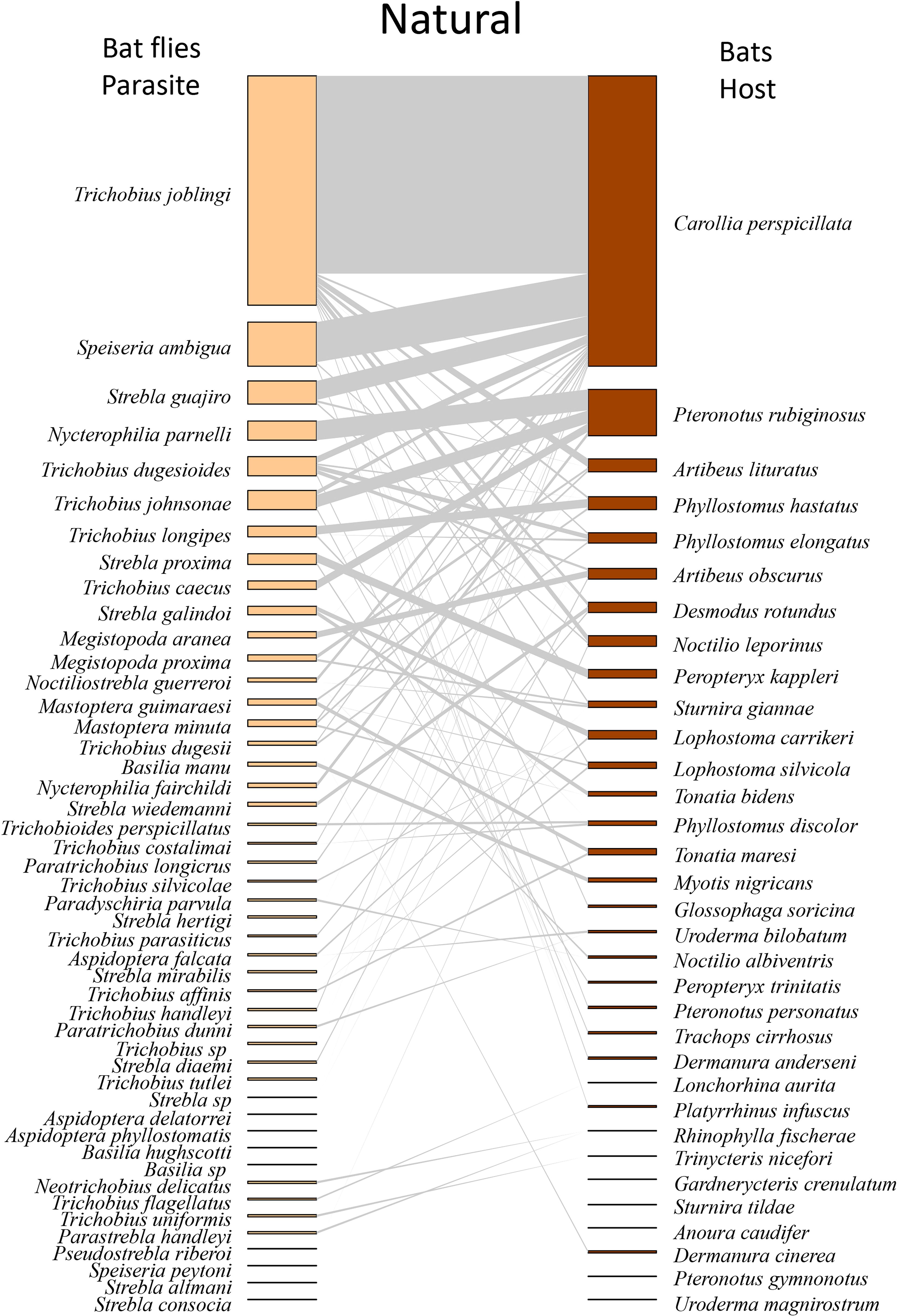
Figure 4. Network of ectoparasites (Diptera: Nycteribiidae and Streblidae) and host bats (Chiroptera: Phyllostomidae) observed in natural areas of the municipalities of Altamira, Bragança, Brasil Novo, Medicilândia, Nova Timboteua, Placas, and Vitória do Xingu, sampled from 2017 to 2022. Species are arranged from most to least abundant. The darker sections on the host and parasite sides represent the abundances of each species, while the middle (gray part) illustrates the interaction network. The thickness of the lines is adjusted according to the number of interactions between the species.
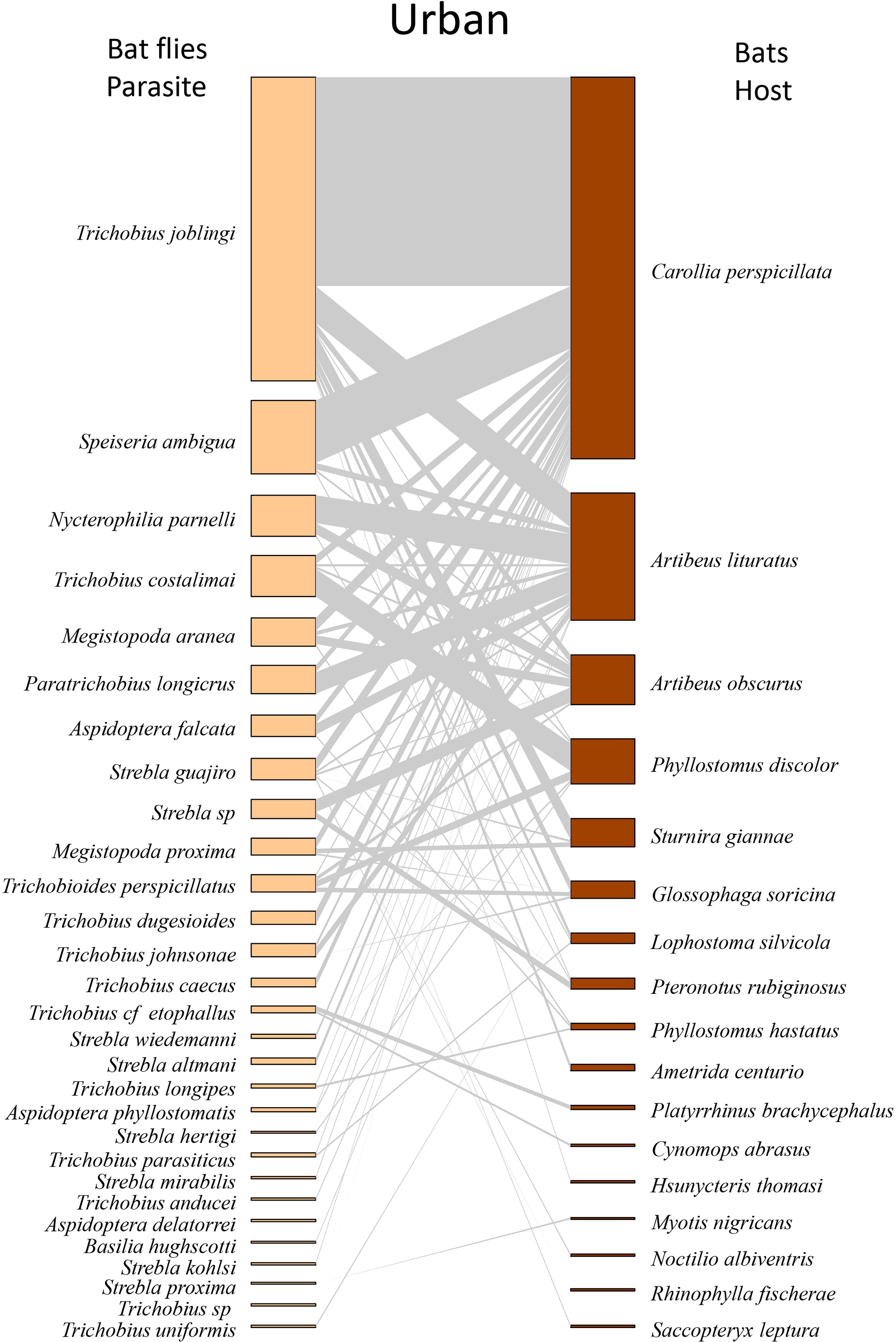
Figure 5. Network of ectoparasites (Diptera: Nycteribiidae and Streblidae) and host bats (Chiroptera: Phyllostomidae) observed in urban areas of Altamira, sampled from 2017 to 2022. Species are arranged from most to least abundant. The darker sections on the host and parasite sides represent the abundances of each species, while the middle (gray part) illustrates the interaction network. The thickness of the lines is adjusted according to the number of interactions between the species.
The overall interaction networks, including all types of land use and cover sampled (Cacao, Natural Vegetation, and Urban Area), showed a richness of 42 host species and 52 ectoparasite species (Figure 2; Table 2). When constructing the networks by type of land use and cover (Figures 3–5; Table 2), we observed that the highest richness of ectoparasites was in the natural vegetation areas, with 47 species, followed by cacao and urban areas with 30 and 29 species, respectively. This same pattern of richness was observed in the hosts, with the highest richness in natural vegetation, followed by cacao and urban areas.
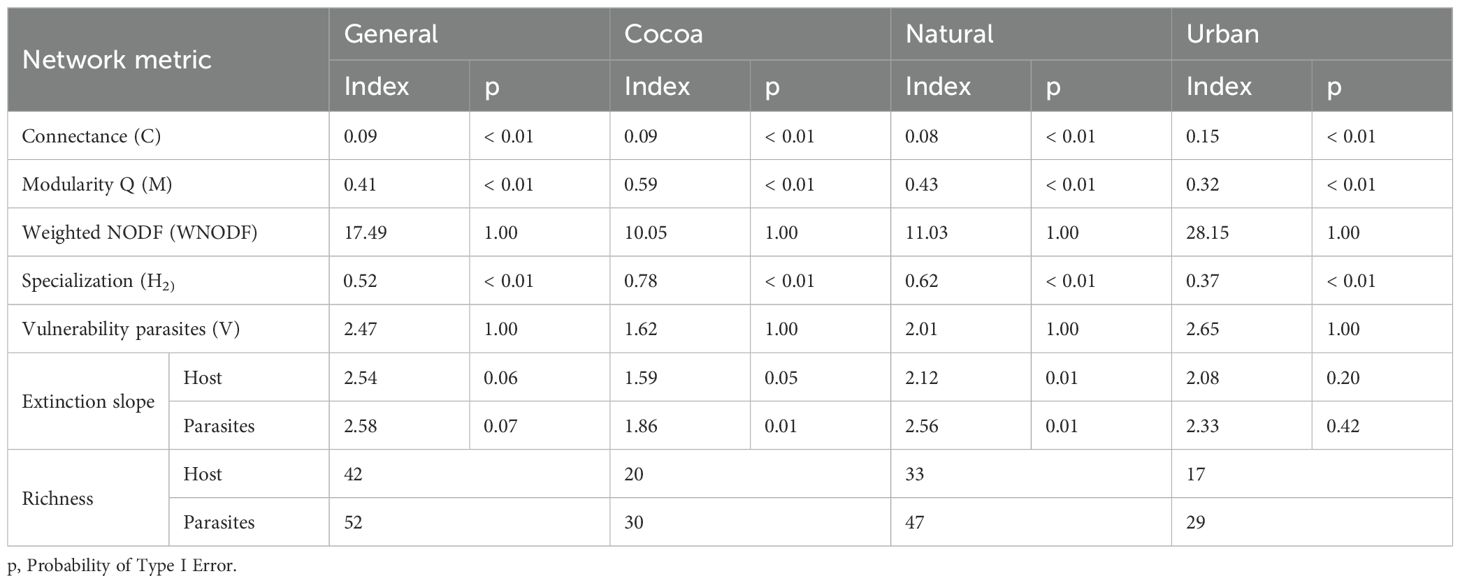
Table 2. Description of interaction network indices between parasites, bat flies (Diptera: Nycteribiidae and Streblidae), and hosts, bats (Chiroptera: Phyllostomidae).
The general network exhibited high specialization, low Connectance, and moderate Modularity (Table 2; Figure 2). When evaluating the networks separately, the cocoa interaction network exhibited the predicted structural properties consistent with our initial hypotheses, showing high specialization, low connectance, and high modularity (Table 2; Figure 3). In the natural vegetation interaction network, we observed high specialization and low Connectance and Modularity (Table 2; Figure 4). Finally, in the urban areas interaction network, we observed low specialization, Connectance, and Modularity (Table 2; Figure 5). The extinction slope values for both hosts and parasites were proportional or mirrored (Table 2). Natural and urban areas had the highest extinction slopes, with cacao areas showing the lowest value and thus the highest risk (Table 2). For all networks, we observed non-significant values of nestedness (WNODF) and parasite vulnerability (Table 2). With these results we were able to answer our second and third hypotheses (ii) the network in urban areas will face greater risk of extinction with a shallower extinction slope; and (iii) cocoa areas will display the lowest levels of network nesting.
In all the environments investigated, bats infested with more than one species of ectoparasite were observed, characterizing infestations by parasitic infracommunities (assemblage of parasite species co-occurring within individual host organisms; Bush et al., 1997) (Supplementary Tables S4–S6). We observed five host species occurring in all land use and cover types studied, which were infested with infracommunities of ectoparasites: Artibeus lituratus, Carollia perspicillata, Phyllostomus discolor, Pteronotus rubiginosus, and Sturnira giannae (Supplementary Tables S4–S6). Natural Vegetation Areas exhibited the highest composition of infracommunities, with 38 infracommunities, followed by Urban Areas with 26 infracommunities, and Cacao Areas with 22 infracommunities (Table 3). Notably, a large number of associations were observed among the ectoparasite genera Speiseria, Strebla, and Trichobius (Table 3). With these results we were able to answer our first hypothesis (i) the network in cocoa areas will exhibit a higher degree of specialization and modularity.
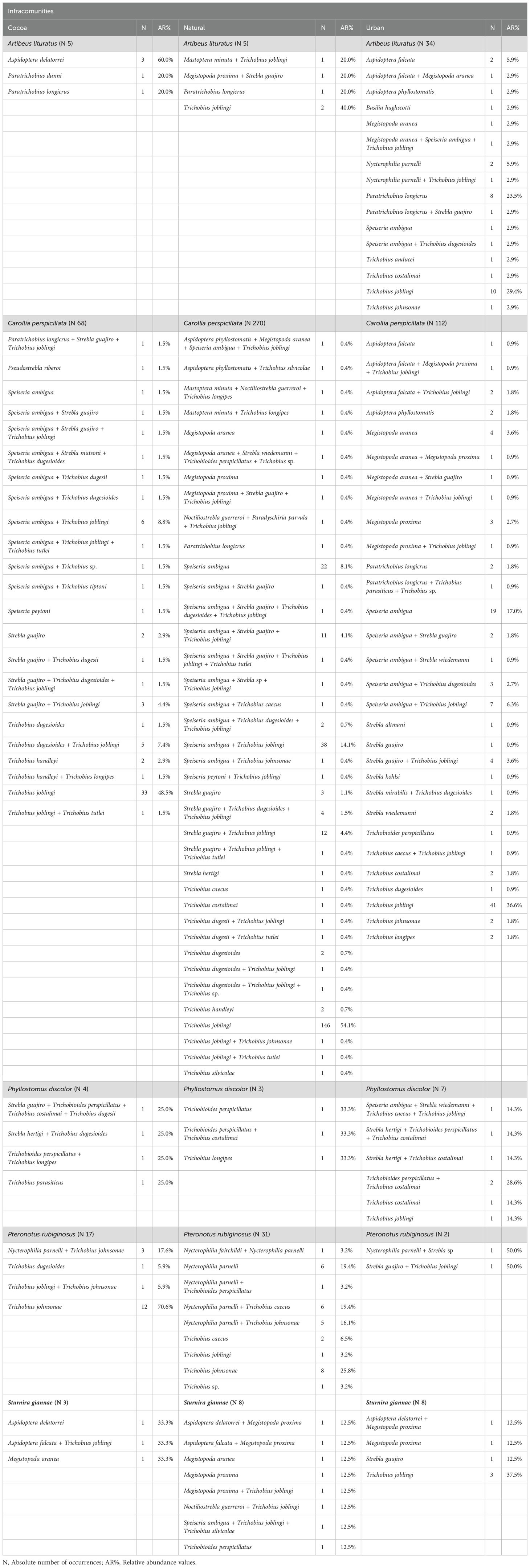
Table 3. Bat species with their respective infracommunities observed in cocoa, natural vegetation and urban areas of Altamira, sampled from 2017 to 2022.
Carollia perspicillata was the species with the highest number of interactions, with its association with Trichobius joblingi present in all three environments and showing the highest relative abundance (AR% = 54.1% in Natural Vegetation, 48.5% in Cacao areas, and 36.6% in Urban Areas). The second most significant association was with Speiseria ambigua (1.5%, 8.1%, and 17%, respectively). Regarding infracommunity associations, the highest relative abundance was observed for the association between Speiseria ambigua and Trichobius joblingi (8.8%, 14.1%, and 6.3%) and between Strebla guajiro and Trichobius joblingi (4.4%, 4.4%, and 3.6%) (Table 3). For Artibeus lituratus, no infracommunities were observed in Cacao areas (Table 3).
4 Discussion
As expected, the fly family with the highest richness was Streblidae, which can be attributed to the widespread abundance of Phyllostomidae bats, as these two families are strongly correlated (Dick and Graciolli, 2006; Barbier et al., 2019; Hrycyna et al., 2019). The genera Trichobius and Speiseria were the most common in this study, with Trichobius joblingi being the species with the highest abundance and degree of interaction within the overall network, followed by Speiseria ambigua. This pattern has also been observed in other regions of Brazil (Bertola et al., 2005; Eriksson et al., 2011; Aguiar and Antonini 2016; Urbieta et al., 2018; Palheta et al., 2020; Júnior et al., 2020; Urbieta et al., 2021; Falcão et al., 2022; Alcantara et al., 2023). This pattern observed in these flies can be attributed to their broad generality in parasitizing various hosts, consistent with the findings of Wenzel et al. (1966), Dick et al. (2009), and Trujillo-Pahua and Ibáñez-Bernal (2020). Despite T. joblingi and S. ambigua being found on various other bat hosts, their overall abundances were related to Carollia perspicillata, with this interaction being a primary association that has been recorded in other studies (Aguiar and Antonini 2016; Soares et al., 2016; Torres et al., 2019; Vasconcelos et al., 2016; Bezerra and Bocchiglieri, 2023).
The species Carollia perspicillata is frequently recorded throughout the Amazon region (Costa et al., 2018; Verde et al., 2018) and across the country (Bianconi et al., 2006; Aguiar and Marinho-Filho 2007; Barreto, 2020) due to its significant ecological plasticity and widespread distribution in the Neotropical region (Gardner, 2008). Additionally, C. perspicillata exhibits high adaptability to human-altered environments, such as secondary vegetation or areas with a high abundance of pioneer species. It is found across the country in habitats where Piper species, its primary food source, thrive. The widespread presence of Piper in disturbed and secondary habitats plays a crucial role in sustaining the high abundance of C. perspicillata in these environments (Aguiar and Marinho-Filho 2007; Silva et al., 2024).
Cacao plantations support a high abundance of Carollia perspicillata, a species that feeds on pioneer plants like Solanum spp. and various Cecropia species commonly found in these areas (Silva, 2024). The proximity of cacao to natural or regenerating vegetation also enhances food availability for Carollia and other frugivores, such as Sturnira guianensis, Sturnira tildae, and Artibeus lituratus, which rely on pioneer plants. Additionally, cacao environments provide access to nectar, insects, and pollen, further supporting these bat species (Silva, 2024).
The cacao environment exhibited the second highest richness of flies and bats, and the difference compared to the natural area was not as pronounced. This is because cacao cultivation combines agriculture with the planting of fruit trees, and the shade provided by the cover and height of these trees contributes to increased diversity, abundance, and richness of frugivorous, insectivorous, and nectarivorous bats. Consequently, agroforestry processes are an important and viable technique for maintaining biodiversity (França et al., 2023; Ferreira et al., 2023a; Palheta et al., 2020; Russo et al., 2023).
However, the highest richness of both fly and bat species was recorded in areas of natural vegetation. This result can be explained by the greater availability of food resources, foraging opportunities, and shelter (Galindo-González, 1998; Kerches-Rogeri et al., 2020; Vieira et al., 2024) typically found in denser vegetation. These findings underscore the importance of natural vegetation areas and forest fragments for the conservation of species and their antagonistic interaction networks. In urban environments, Carollia perspicillata and Artibeus lituratus were abundant, likely due to the availability of pioneer plants and insects attracted by artificial lighting (Palheta et al., 2020; Alencastre-Santos et al., 2024; Vieira et al., 2021). Both species show high tolerance to urban conditions (Silveira et al., 2024).
We recorded infracommunity associations involving up to four dipteran species in different bats from the Phyllostomidae family. Notably, Carollia perspicillata is parasitized by various species of flies and appears to play a central role in the parasite–host interaction network (Aguiar and Antonini 2016). The high abundance of C. perspicillata observed in this study can be explained by its ability to roost in a variety of shelters, such as caves, culverts, storm drains, and abandoned buildings (Trajano and Gimenez, 1998; Bredt et al., 1999), which increases the opportunities for interaction between different bat species and their parasites (Fagundes et al., 2017).
The species Artibeus lituratus and Pteronotus rubiginosus had primary associations with flies of the genus Trichobius. No infracommunity was found for A. lituratus in the cacao area, and in the natural vegetation area, we observed a low presence of infracommunities. This is due to the heterogeneity of these environments and the greater abundance of bats due to higher vegetation cover (Purificação et al., 2020). In the urban area, we observed a higher presence of infracommunities with both primary and secondary associations. Pteronotus rubiginosus showed infestations by infracommunities in all environments, with particular emphasis on the natural vegetation area, where associations such as (Nycterophilia parnelli + T. johnsonae and Nycterophilia parnelli + Trichobius caecus) were noted. These primary associations have been recorded in other studies (Hrycyna et al., 2019).
The species Sturnira guiannae and Phyllostomus discolor presented infracommunities with up to four species of dipterans. S. guiannae showed a higher incidence of infracommunities in natural vegetation areas. From this study, we can infer that the infracommunity formed by Aspidoptera delatorrei + Megistopoda próxima may be considered a primary association for S. guiannae. The main association found in P. discolor was the infracommunity formed by Trichobioides perspicillatus + Trichobius costalimai. It is worth noting that T. perspicillatus represents a new occurrence in the state of Pará; the species had been previously described in the North region, specifically in the state of Amapá (Hrycyna et al., 2019).
Several previous studies suggest the broad distribution of this species in the Amazon region (Barbier and Bernard, 2017) and many other yet-uncatalogued species in this biome, demonstrating that a significant portion of ectoparasitic fly diversity remains unknown to science (Graciolli and Linardi, 2002; Graciolli and Bernard, 2002; Vasconcelos, 2016). Specific studies aimed at filling knowledge gaps for the Amazon region, including the identification, biology, and ecology of bat ectoparasites and their interaction networks, are extremely necessary and important. They contribute to identifying the best strategies for the conservation of fruit-eating Phyllostomidae bats, a key group for ecosystem recovery and conservation.
Although we recorded new bat–parasite associations, determining host specificity is complex, as it involves multiple anatomical, physiological, evolutionary, and behavioral factors (Esbérard et al., 2005; Aguiar and Antonini, 2011). For instance, some Neotropical Strebla species, typically associated with Phyllostomidae bats (Guerrero, 1996; Wenzel, 1966; Guerrero 1994; Graciolli and Carvalho, 2001b), were found on non-phyllostomid hosts in our study. Such associations are likely accidental or transient, possibly resulting from shelter-sharing among different bat species (Aguiar and Antonini, 2011; Bejec et al., 2023; Ospina-Pérez et al., 2023; Silva Almeida et al., 2024; Barbier et al., 2024; López-Rivera et al., 2024). Further studies are needed to explore host preference and better understand the drivers of host specificity (Amarga and Phelps, 2021; da Silva Reis et al., 2022; Orlova et al., 2022).
The study region faces rapid land-use changes due to deforestation and wildfires, with about 16.05% of the Amazon biome losing conditions necessary for bat-provided ecosystem services (Silva et al., 2016; Velasco Gomez et al., 2015; Brasileiro et al., 2022). Currently, 17% of the biome has been converted to pastures and short-cycle crops (MapBiomas, 2020), potentially impacting ectoparasite-host dynamics, such as reproduction and mortality rates of ectoparasitic insects (Hrycyna et al., 2019; Trujillo-Pahua and Ibáñez-Bernal, 2020; Amarga and Phelps, 2021; da Silva Reis et al., 2022; Orlova et al., 2022).
In all networks, we observed non-significant values for Nestedness (WNODF), which does not support our third hypothesis that cacao areas would exhibit the lowest nestedness. All networks showed a poorly nested structure, similar to findings in other studies on interactions between flies and bats (Fagundes et al., 2017; Vieira et al., 2024; Patterson et al., 2008), as well as in interactions between bats and plants (Cordero-Schmidt et al., 2021; Silva, 2024) and plants with frugivorous animals (Paixão et al., 2023). According to Patterson et al (2008), nested networks are more common in host-parasite relationships involving long-term infestations, that is, species with a long evolutionary association. Thus, the presence of accidental or opportunistic associations in our study may have influenced the low nestedness values.
Nestedness describes how species with fewer interactions to associate with subset of the partners of more connected species (Almeida-Neto and Ulrich, 2011), enhancing network robustness by increasing resilience to disturbances or species loss (Menezes and Fernandez, 2013). Although our networks, did not show significant nestedness, its typically inverse relationship with specialization and modularity supports our results. These metrics, associated with species co-evolution (Krasnov et al., 2012), followed and anthropization gradient – lower specialization and modularity, alongside higher nestedness, were observed in the most disturbed (urban) environments.
According to our data, the network with a somewhat more nested structure compared to the others was the urban network. This nestedness may arise from various anthropogenic processes in the urban environment and could be due to the generalist interactions of the species, which allows connections between different bat species and the various flies present in this network. Overall, this location exhibited the lowest diversity (both parasites and hosts) and the lowest specialization (H2). Nested patterns generated by processes such as extinction in fragmented or anthropized landscapes are of particular interest for conservation because they imply a predictable order of species loss (Ganzhorn and Eisenbeiss, 2001; Martinez-Morales, 2005).
Connectivity Connectivity indices did not differ significantly among Cocoa, Natural, and Urban areas, with consistently low values across all networks. This reflects a dominance of generalist species, which reduces interspecific competition (Blüthgen, 2010). The uniformity in connectivity likely stems from the similar richness and composition of bats and flies across environments, as well as the ecological plasticity of bats in adapting to disturbed habitats. Similar patterns have been observed in mutualistic bat–plant networks (Almeida and Mikich, 2018). Despite low connectivity, modularity enhances network robustness by containing disturbances, such as disease spread or species loss, within isolated modules (Robinson and Strauss, 2020). Preserving key species within modules is thus vital for sustaining both the network structure and its associated ecosystem services (Messeder et al., 2020).
The overall network showed a high specialization index, with cacao areas exhibiting the highest values, followed by natural vegetation. Specialization reflects the exclusivity of interactions (Sebastián-González et al., 2015), and elevated values may result from reduced resource diversity, even in areas with greater vegetation cover (Zhang et al., 2023). In cacao landscapes, ongoing anthropogenic disturbances likely contribute to this pattern by simplifying network structures. Additionally, specialization may be shaped by the phylogenetic relationships between parasites and their hosts (Poulin et al., 2011; Palheta et al., 2020). Although specialization can signal co-evolution, high values indicate stronger interdependence, making specialist species more susceptible to extinction (Kaiser-Bunbury and Blüthgen, 2015).
The values of specialization and modularity in the Cocoa and Natural vegetation networks were high, not supporting our initial hypothesis that these metrics would be higher only in the Cocoa area. Regarding modularity, we observed that in the networks of all three environments, small modules were formed groups with more interactions among themselves due to preferential interactions between specific bat and fly species. The Cocoa area recorded the highest modularity index, followed by the Natural vegetation environment, indicating low nesting and low connectivity in these networks. The urban area exhibited a low specialization index, which aligns with findings from other studies (Ramalho et al., 2021), indicating that this network primarily consists of generalist species.
Although this trend is often attributed to the limited availability of shelters and resources in urban settings, leading to reduced selectivity among parasites (Urbieta et al., 2018), Ramalho et al. (2021) observed that, even in urban environments with relatively abundant roosting sites – due to various tree species and building features like expansion joints – the level of specialization in parasite–host interactions remains low. Their study suggests that anthropogenic changes may shape the structure of bat-bat fly interaction networks, resulting in a more hierarchical arrangement where parasites become less specialized and interact with a broader range of host species. This reduced specialization could impact parasite fitness and potentially increase the transmission of pathogens across different bat populations.
Contrary to our second hypothesis, extinction risk was highest in the cacao area, while natural and urban areas showed lower values. This suggests that, although urbanization acts as an environmental filter for bats and ectoparasitic flies (Palheta et al., 2020), it does not necessarily result in the highest extinction risk. Some bat species may persist or even thrive in urban environments, while others decline due to habitat loss - responses that are highly species-specific (Palheta et al., 2020; Bernard et al., 2023). Notably, the urban network exhibited the highest parasite vulnerability index, highlighting the complex and context-dependent effects of urbanization.
The loss of fly diversity in urban areas may be related to the reduction in the number and diversity of bats (Dick and Gettingert, 2005), supporting the idea that the network is fragile and at risk of local extinction of ectoparasitic dipterans due to the loss of bat species from anthropogenic factors. The cacao and natural vegetation areas had low vulnerability indices, with no significant differences between them. Indeed, bat and fly communities are more diverse in rural environments than in urban ones. Additionally, since these species are correlated, they seem to respond differently to the two environments, resulting in heterogeneity in rural areas (which contributes to the low parasite vulnerability index) and homogeneity in urban areas (which contributes to increased parasite vulnerability).
Changes in land use and cover likely influenced the structure of bat–fly networks. Host and parasite diversity are closely linked to environmental shifts, which affect ectoparasite reproduction and survival (Tlapaya-Romero et al., 2021; Biz et al., 2023). Vegetated areas, with their environmental heterogeneity and greater resource availability, supported higher diversity of both bats and flies. In contrast, urban areas, characterized by homogeneity and limited resources, showed reduced bat diversity and, consequently, lower fly diversity (Dick and Gettingert, 2005; Palheta et al., 2020). Our results reflect this gradient, with urban networks exhibiting lower specialization and diversity, but higher nestedness and vulnerability. This suggests that fewer bat species are hosting a broader array of ectoparasites, forming asymmetric networks where generalist hosts support specialist parasites (Mello et al., 2016; Ramalho et al., 2021).
We also highlight the discovery of four new occurrences of bat flies for Brazil and ten new species for the state of Pará. Additionally, all 51 parasite species identified in this study presented new host associations, emphasizing the need for increased investment in research focused on primary data collection and the documentation of parasite–host interactions. This knowledge gap regarding the geographical distribution of ectoparasitic flies is not unique and is also observed in bats, especially in the Amazon (Vieira et al., 2024). This indicates that the Brazilian Amazon remains understudied in terms of its chiropterofauna, with great potential for the discovery of new species (Aguiar et al., 2021). Furthermore, the conservation of bats is directly linked to human health, as their population decline could lead to an increase in infant mortality rates, due to the intensified use of pesticides for pest insect control in agricultural fields (Frank, 2024). The decline in bat populations also destabilizes production chains and compromises food security, as crop losses caused by pest insect attacks are exacerbated (Frank, 2024).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Sistema de Autorização e Informação em Biodiversidade (SISBIO) license no. 57294-2. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SD: Formal analysis, Methodology, Writing – original draft. RA: Writing – review & editing. LC: Data curation, Writing – review & editing. LA: Writing – review & editing. GG: Writing – review & editing. DA: Writing – review & editing. TV: Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Centro Nacional de Pesquisa e Conservação de Cavernas (Cecav/ICMBio)Instituto Brasileiro de Desenvolvimento e Sustentabilidade – IABS - IABS.VALE S.A.Sociedade Brasileira de Quirópteros – SBEQ.Foundation Coordination for the Improvement of Higher Education Personnel – CAPES.Amazon Foundation to Support Studies and Research – FAPESPA.
Acknowledgments
This research benefited from resources from Vale SA’s environmental compensation administered by the Centro Nacional de Pesquisa e Conservação de Cavernas (Cecav/ICMBio) and services to the Brazilian Society for the Study of Chiropterans – SBEQ, as part of the DD Program – The Species More Unknown in Brazil and with resources from the Termo de Compromisso de Compensação Espeleológica – TCCE VALE 1/2018 – Edital Ferruginosas 01/2021 under the administration of the Instituto Brasileiro de Desenvolvimento e Sustentabilidade – IABS – IABS. Amazon Foundation to Support Studies and Research – FAPESPA. Foundation Coordination for the Improvement of Higher Education Personnel – CAPES.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1499475/full#supplementary-material
References
Agencia Para (2021). Pará lidera a produção nacional de cacau pelo segundo ano consecutivo (Agência Pará). Available at: https://agenciapara.com.br/noticia/24646/para-lidera-a-producao-nacional-de-cacau-pelo-segundo-ano-consecutivo.
Aguiar L. and Marinho-Filho J. (2007). Bat frugivory in a remnant of Southeastern Brazilian Atlantic forest. Acta Chiropterologica, 9(1), 251–260.
Aguiar L. M. S. and Antonini Y. (2011). Descriptive ecology of bat flies (Diptera : Hippoboscoidea) associated with vampire bats (Chiroptera : Phyllostomidae) in the cerrado of central Brazil. Mem Inst Oswaldo Cruz 106, 170–176. doi: 10.1590/S0074-02762011000200009
Aguiar L. M. and Antonini Y. (2016). Prevalence and intensity of Streblidae in bats from a Neotropical savanna region in Brazil. Folia Parasitologica. 63, 1.
Aguiar L. M. S., Bueno-Rocha I. D., Oliveira G., Pires E. S., Vasconcelos S., Nunes G. L., et al. (2021). Going out for dinner-The consumption of agriculture pests by bats in urban areas. PloS One 16, e0258066. doi: 10.1371/journal.pone.0258066
Alcantara D. M. C., Graciolli G., and Nihei S. S. (2019). Revision of Noctiliostrebla (Diptera: Streblidae), parasites of bulldog bats (Chiroptera: Noctilionidae: Noctilio). Zootaxa 4560 (3), 483–521. [2019.02.26]
Alcantara D. M. C., Ikeda P., Souza C. S., De Mello V. V. C., Torres J. M., Lourenço E. C., et al. (2023). Multilayer networks assisting to untangle direct and indirect pathogen transmission in bats. Microbial Ecol. 86, 1292–1306. doi: 10.1007/s00248-022-02108-3
Alencastre-Santos A. B., Gonçalves R., Correia L. L., Brito D., Oprea M., and Vieira T. B. (2024). The effect of urbanization on species composition and trophic guilds of bats (Mammalia, Chiroptera) in the Brazilian Savanna. Braz. J. Biol. v. 84, e275828. doi: 10.1590/1519-6984.275828
Almeida A. and Mikich S. B. (2018). Combining plant–frugivore networks for describing the structure of neotropical communities. Oikos 127, 184–197. doi: 10.1111/oik.04774
Almeida J., Silva D. B., Pires T. D. A., Resende D., Mihsfeldt L. H., and Zanoni M. A. (2024). Occurrence of ectoparasitic flies (Diptera : Streblidae) of bats (Chiroptera : Mammalia) in a semideciduous forest remnant in Northern Paraná, Brazil Ocorrência de moscas ectoparasitas (Diptera : Streblidae) de morcegos (Chiroptera : Mammalia) em. 91–100. doi: 10.5433/1679-0367.2024v45n1p91
Almeida-Neto M. and Ulrich W. (2011). A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Model. Software 26, 173–178. doi: 10.1016/j.envsoft.2010.08.003
Amarga A. K. S. and Phelps K. L. (2021). New host and distribution records of bat flies (Diptera: Streblidae, Nycteribiidae) on cave-dwelling bats from Bohol Island, Philippines. Int. J. Trop. Insect Sci. 41, 3213–3222. doi: 10.1007/s42690-021-00584-7
Atagana P. J., Fils E. M. B., and Kekeunou S. (2021). Responses of bat communities (Mammalia: Chiroptera) to forest loss and habitat conversion in Southern Cameroon. Trop. Conserv. Sci. 14, 19400829211010360. doi: 10.1177/19400829211010360
Barbier E., André M. R., and Bernard E. (2024). A wingless fly on a winged mammal: host-parasite dynamics between Basilia travassosi (Diptera: Nycteribiidae) and Myotis lavali (Chiroptera: Vespertilionidae). Parasitol. Res. 123. doi: 10.1007/s00436-024-08294-y
Barbier E. and Bernard E. (2017). From the Atlantic Forest to the borders of Amazonia: species richness, distribution, and host association of ectoparasitic flies (Diptera: Nycteribiidae and Streblidae) in northeastern Brazil. Parasitol. Res. 116, 3043–3055. doi: 10.1007/s00436-017-5615-7
Barbier E., Graciolli G., and Bernard E. (2019). Structure and composition of Nycteribiidae and Streblidae flies on bats along an environmental gradient in northeastern Brazil. Can. J. Zoology 97, 409–418. doi: 10.1139/cjz-2018-0098
Barona E., Ramankutty N., Hyman G., and Coomes O. T. (2010). The role of pasture and soybean in deforestation of the Brazilian Amazon. Environ. Res. Lett. 5, 024002. doi: 10.1088/1748-9326/5/2/024002
Barreto J. O. M. (2020). Bionomia de Carollia perspicillata (linnaeu) em um remanescente florestal urbano (RIO DE JANEIRO, RJ). Rev. Multidisciplinar Educação e Meio Ambiente 1, 54. Available at: https://editoraime.com.br/revistas/index.php/rema/article/view/75 (Accessed December 20, 2024).
Bejec G. A., Bucol L. A., Ancog A. B., Pagente A. C., Panerio J. J. M., Bejec A. L. N., et al. (2023). Diversity of bat ectoparasites from the caves of selected Key Biodiversity Areas in Central Visayas, Philippines. Biodiversitas J. Biol. Divers. 24, 1693–1703. doi: 10.13057/biodiv/d240343
Bernard E. (2001). Vertical stratification of bat communities in primary forests of Central Amazon, Brazil. J. Trop. Ecol. 17, 115–126. doi: 10.1017/S0266467401001079
Bernard E., Aguiar L. M. S., Brito D., Cruz-Neto A., Gre – Gorin R., Machado R., et al. (2012). Uma análise de horizontes sobre a conservação de morcegos no Brasil. Mamíferos do Brasil: genética sistemática ecologia e conservação 2, 19–35.
Bernard E., Barbier E., Leal E. S.B., dos Santos F. I., Pimentel N. T., Pereira J. D.S.B., and Souza-Motta C. M. (2023). Morcegos no Parque Nacional do Catimbau, Pernambuco, Brasil: síntese de uma década (2012-2022) de pesquisas. Biodiversidade Brasileira, 13(2).
Bertola P., Aires B., Favorito C. C. S., Graciolli E., Amaku G., and Pinto-Da-Rocha M. R. (2005). Bat flies (Diptera: Streblidae, Nycteribiidae) parasitic on bats (Mammalia: Chiroptera) at Parque Estadual da Cantareira, São Paulo, Brazil: parasitism rates and host-parasite associations. Mem Inst Oswaldo Cruz (Rio de Janeiro) 100, 25–32. doi: 10.1590/S0074-02762005000100005
Bezerra R. H. S. and Bocchiglieri A. (2023). Ectoparasitic flies of bats (Mammalia: Chiroptera) in urban green areas of northeastern Brazil. Parasitol. Res. 122, 117–126. doi: 10.1007/s00436-022-07703-4
Bianconi G. V., Mikich S. B., and Pedro W. A. (2006). Movements of bats (Mammalia, Chiroptera) in Atlantic Forest remnants in southern Brazil. Rev. Bras. zoologia 23, 1199–1206. doi: 10.1590/S0101-81752006000400030
Biz L. S., Bastazini V. A., Carvalho F., and Ramos Pereira M. J. (2023). Network and parasitological analyses reveal latitudinal gradient in bats-ectoparasitic fly interactions across the Neotropic. Ecol. Evol. 13, e10527. doi: 10.1002/ece3.10527
Blüthgen N. (2010). Why network analysis is often disconnected from community ecology: a critique and an ecologist’s guide. Basic Appl. Ecol. 11, 185–195. doi: 10.1016/j.baae.2010.01.001
Bojsen B. H. and Jacobsen D. (2003). Effects of deforestation on macroinvertebrate diversity and assemblage structure in Ecuadorian Amazon streams. Archiv fur Hydrobiologie 158, 317–342. doi: 10.1127/0003-9136/2003/0158-0317
Bolívar-Cimé B., Cuxim-Koyoc A., Reyes-Novelo E., Morales Malacara J. B., Laborde J., and Flores-Peredo R. (2018). Habitat fragmentation and the prevalence of parasites (Diptera, Streblidae) on three phyllostomid bat species. Biotropica. 50, 90–97. doi: 10.1111/btp.2018.50.issue-1
Brainer M. S., De C. P., and Caderno Setorial ETENE (2021). “Produção de cacau: crescer é preciso!,” in Fortaleza: BNB, 20. Available at: https://g20mais20.bnb.gov.br/s482dspace/bitstream/123456789/1042/1/2021_CDS_199.pdf.
Brainer de M. S.C. (2021) PRODUÇÃO DE CACAU: CRESCER É PRECISO! v. 6 n. 199. Caderno Setorial ETENE, Fortaleza, 6, 2024. Available at: https://www.bnb.gov.br/revista/cse/article/view/2871. (Accessed December 2024).
Brasileiro L. A.M., Machado R. B., and Aguiar L. M.S. (2022). Ecosystems services provided by bats are at risk in Brazil. Frontiers in Ecology and Evolution, 10, 852177.
Bredt A., Uieda W., and Magalhães E. D. (1999). Morcegos cavernícolas da região do Distrito Federal, centro-oeste do Brasil (Mammalia, Chiroptera). Revista Brasileira de Zoologia, 16, 731–770.
Bush A. O., Lafferty K. D., Lotz J. M., and Shostak A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol., 575–583. doi: 10.2307/3284227
Cardoso K., Gadelha K., Ferreira G., Silva K., Oliveira P. A., Moy K., et al. (2019). Efeito da abundância dos adultos sobre as ninfas de Gerromorpha (Heteroptera) em riachos de Cerrado no estado de Goiás. Biotema 32, p.71–p.77. doi: 10.5007/2175-7925.2019v32n2p71
Coquillett D. W. (1899). New genera and species of Nycteribidae and Hippoboscidae. The Canadian Entomologist 31 (11), 333–336.
Cordero-Schmidt E., Maruyama P. K., Vargas-Mena J. C., Pereira Oliveira P., de Assis R., Santos F., et al. (2021). Bat–flower interaction networks in Caatinga reveal generalized associations and temporal stability. Biotropica 53, 1546–1557. doi: 10.1111/btp.13007
Costa L. M., Novaes R. L. M., Amorim J. A., Kuzel M. A., and Moratelli R. (2018). Bat assemblages from rural localities in Guarapari, Espírito Santo state, southeastern Brazil. Boletim da Sociedade Bras. Mastozoologia 82, 102–107.
da Silva Reis A., de Almeida Zampaulo R., Dornelles G. D. P., Graciolli G., and Talamoni S. A. (2022). Variation of dipteran ectoparasites (Streblidae) on Anoura geoffroyi Gra(Phyllostomidae) in two caves in southeastern Brazil. Parasitol. Res. 121, 255–265. doi: 10.1007/s00436-021-07385-4
Davidson E. A., Artaxo A., Balch P., Brown J., Bustamante I., Coe M., et al. (2012). The Amazon basin in transition. Nature 481, 321–328. doi: 10.1038/nature10717
Defries R. and Rosenzweig C. (2010). Toward a whole-landscape approach for sustainable land use in the tropics. Proc. Natl. Acad. Sci. 107, 19627–19632. doi: 10.1073/pnas.1011163107
Dick C. W., Esbérard C. E. L., Graciolli G., Bergallo H. G., and Gettinger D. (2009). Avaliação da especificidade do hospedeiro de ectoparasitas obrigatórios na ausência de barreiras de dispersão. Parasitol. Res. 105, 1345–1349. doi: 10.1007/s00436-009-1563-1
Dick C. W. and Gettingert D. (2005). A faunal survey of streblid flies (Diptera: Streblidae) associated with bats in Paraguay. J. Parasitology. 91, 1015–1024. doi: 10.1645/GE-536R.1
Dick C. W. and Graciolli G. (2006). Família streblidae kolenat (SIBBr). Available at: https://ala-bie.sibbr.gov.br/ala-bie/species/220191?lang=pt_BR (Accessed January 12, 2024).
Dick C. W., Graciolli G., and Guerrero R. (2016). Família streblidae. Zootaxa 4122, 784–802. doi: 10.11646/zootaxa.4122.1.67
Dick C. W. and Patterson B. D. (2007). Against all odds: explaining high host specificity in dispersal-prone parasites. Int. J. Parasitol. 3, 871–876. doi: 10.1016/j.ijpara.2007.02.004
Dittmar K., Dick C. W., Whiting M. F., and Gruwell M. E. (2009). Pupal deposition and ecology of bat flies (Diptera: Streblidae): Trichobius sp.(caecus group) in a Mexican cave habitat. J. Parasitol. 95, 308–314. doi: 10.1645/GE-1664.1
Dittmar K., Morse S., Dick C., and Patterson B. (2015). Evolução da mosca do morcego do Eoceno ao Presente (Hippoboscoideia, Streblidae e Nycteribiidae) (Cambridge: Cambridge University Press), 246–264.
Dobson A., Lafferty K. D., Kuris, Hechinger A. M., and Jetz R. F. W. (2008). Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl. Acad. Sci. 105, 11482–11489. doi: 10.1073/pnas.0803232105
Dormann C., Gruber B., and Fründ J. (2008). Introducing the bipartite package: analysing ecological networks. Interaction 1, 2413793. Available at: http://www.biom.unifreiburg.de/Dateien/PDF/dormann2008rnews.pdf(Accessed August 13, 2024).
Edwards F. W. (1918). Two new Diptera from Trinidad. Annals and Magazine of Natural History (9) 1, 424–425.
Esbérard C. E., Motta J. A., and Perigo C. (2005). Morcegos cavernícolas da Área de Proteção Ambiental (APA) Nascentes do Rio vermelho, goiás. Revista brasileira de Zoociências, 7(2).
Eriksson A., Graciolli G., and Fischer E. (2011). Bat flies on phyllostomid hosts in the Cerrado region: component community, prevalence and intensity of parasitism. Memórias do Instituto Oswaldo Cruz 106, 274–278. doi: 10.1590/S0074-02762011000300004
Esbérard C. E. L., Astúa D., Geise L., Costa L. M., and Pereira L. G. (2012). Do young Carollia perspicillata (Chiroptera: Phyllostomidae) present higher infestation rates of Streblidae (Diptera)? Braz. J. Biol. 72, 617–621. doi: 10.1590/S1519-69842012000300027
Fagundes R., Antonini Y., and Aguiar L. M. S. (2017). Overlap in cave usage and period of activity as factors structuring the interactions between bats and ectoparasites. Zoological Stud., 56. doi: 10.6620/ZS.2017.56-22
Falcão L., Araújo W., Leite L., Fagundes M., Espírito-Santo M., Borges M., et al. (2022). Network structure of bat-ectoparasitic interactions in tropical dry forests at two different regions in Brazil. Acta Chiropterologica. 24, 239–248. doi: 10.3161/15081109ACC2022.24.1.019
Faria D. (2006). Phyllostomid bats of a fragmented landscape in the north-eastern Atlantic forest, Brazil. J. Trop. Ecol. 22, 531–542. doi: 10.1017/S0266467406003385
Fearnside P. M. (1995). Quem desmata a Amazônia: Os pobres ou os ricos? Ciec. Hoje 19, 26–33. Available at: http://philip.inpa.gov.br/publi_livres/1995/Quem desmataaAmazonia20os20pobresou20osricos.pdf (06/09/23).
Fearnside P. M., Barbosa R. I., and Pereira V. B. (2013). Greenhouse gas emissions from deforestation and forest fires in Roraima: sources and sinks. Agro@mbiente On-line 7, 95–111. doi: 10.18227/1982-8470ragro.v7i1.971
Ferreira D. F., Darling A., Jarrett C., Atagana P. J., Sandjo P. R., Taedoumg H., et al. (2023b). Not all farms are created equal: Shady African cocoa farms promote a richer bat fauna. Biol. Conserv. 284, 110191. doi: 10.1016/j.biocon.2023.110191
Ferreira D. F., Jarrett C., Wandji A. C., Atagana P. J., Rebelo H., Maas B., et al. (2023a). Birds and bats enhance yields in Afrotropical cacao agroforests only under high tree-level shade cover. Agriculture Ecosyst. Environ. 345, 108325. doi: 10.1016/j.agee.2022.108325
França J. D. O., Alexandre R. J. R., Correia L. L., Souza L. M., Graciolli G., Aguiar L. M. S., et al. (2023). Bat flies (Diptera: Streblidae) of Phyllostominae and Stenodermatinae (Chiroptera: Phyllostomidae) bats from cocoa and natural areas of Amazonia. Stud. Neotropical Fauna Environ., 1–10. doi: 10.1080/01650521.2023.2266190
Frank E. G. (2024). The economic impacts of ecosystem disruptions: Costs from substituting biological pest control. Science 385 (6713), eadg0344.
Galford G. L., Melillo J. M., Kicklighter D. W., Cronin T. W., Cerri C. E., Mustard J. F., et al. (2010). Greenhouse gas emissions from alternative futures of deforestation and agricultural management in the southern Amazon. Proc. Natl. Acad. Sci. 107, 19649–19654. doi: 10.1073/pnas.1000780107
Galindo-González J. (1998). Dispersión de semillas por murciélagos: su importância em la conservación y regeneración del bosque tropical. Acta Zoológica Mexicana 73, 57–74. doi: 10.21829/azm.1998.73731727
Ganzhorn J. and Eisenbeiss B. (2001). The concept of nested species assemblages and its utility for understanding effects of habitat fragmentation. Basic Appl. Ecol. 2 (1), 87–99. doi: 10.1078/1439-1791-00040
Garbino G. S. T., Gregorin R., Lima I. P., Loureiro L., Moras L., Moratelli R., et al. (2024). “Updated checklist of Brazilian bats: versão 2024,” in Comitê da Lista de Morcegos do Brasil—CLMB (Sociedade Brasileira para o Estudo de Quirópteros (Sbeq). (Accessed May 30, 2025).
Garcia M. and Casal O. H. (1965). Revision de las especies del genero Euctenodes Waterhouse, 1879 (Diptera, Acalipterae, Streblidae). Notas Biol. Fac. Cienc. exactas, fisicas Nat. (Zool.) 5, 23.
Gardner A. (2008). Mammals of south america, volume 1: marsupials, xenarthrans, shrews, and bats (Universityof Chicago Press), 1481.
Graciolli G. (2004). Nycteribiidae (Diptera, Hippoboscoidea) no Sul do Brasil. Rev. Bras. Zoologia 21, 971–985. doi: 10.1590/S0101-81752004000400035
Graciolli G. (2024). Streblidae in Catálogo Taxonômico da Fauna do Brasil (PNUD. Available in). Available at: http://fauna.jbrj.gov.br/fauna/faunadobrasil/2624.
Graciolli G. and Bernard E. (2002). Novos registros demoscas ectoparasitas (Diptera, Streblidae e Nycteribiidae) em morcegos (Mammalia, Chiroptera) do Amazonas e Pará, Brasil. Revista Brasileira de Zoologia. Curitiba, 77–86. doi: 10.1590/S0101-81752002000500003
Graciolli G. and Bianconi G. V. (2007). Moscas ectoparasitas (Diptera, Streblidae e Nycteribiidae) em morcegos (Mammalia, Chiroptera) em área de Floresta com Araucária no Estado do Paraná, sul do Brasil. Rev. Bras. Zoologia 24, 246–249. doi: 10.1590/S0101-81752007000100033
Graciolli G. and Carvalho C. J. B. (2001). Moscas ectoparasitas (Diptera, Hippoboscoidea, Nycterybiidae) de morcegos (Mammalia: Chiroptera) do Estado do Paraná, Brasil. I. Basilia, taxonomia e chave pictórica para as espécies. Revta Bras. Zool 18, 907–960. doi: 10.1590/S0101-81752001000300026
Graciolli G. and Hrycyna G. (2024). Nycteribiidae in Catálogo Taxonômico da Fauna do Brasil (PNUD). Available at: http://fauna.jbrj.gov.br/fauna/faunadobrasil/1145 (Accessed June 27, 2024).
Graciolli G. and Linardi P. M. (2002). Some streblidae and nycteribiidae (Diptera: hippoboscoidea) from maracá Island, roraima, Brazil. . Memórias do Instituto Oswaldo Cruz 97, 139–141. doi: 10.1590/S0074-02762002000100026
Guerrero R. (1994). Catalogo de los streblidae (Diptera: pupipara) parásitos de murcielagos (Mammalia: chiroptera) del nuevo mundo II: los grupos: pallidus, caecus, major, uniformis y longipes del género Trichobius gervais, 1844. Acta biol. venez, 1–18.
Guerrero R. (1996). Catálogo de los Streblidae (Diptera: Pupipara) parásitos de murcielagos (Mammalia: Chiroptera) del Nuevo Mundo. VI. Streblinae. Acta Biológica Venezolana 16, 1–26.
Guerrero R. (1998). Notes on Neotropical batflies (Diptera, Streblidae). 1. The genus Trichobius, with description of two new species and one new subspecies from Venezuela. Acta Parasit.
Guimarães L. R. (1946). Revisão das espécies sulamericanas do gênero Basilia (Diptera, Nycteribiidae). Arquivos de Zoologia do Estado de S. Paulo, Brasil 5, 1–88, 98 figs.
Guimarães L. R. (1938). Sobre as especies sul Americanas do genero Trichobius (Diptera-Streblidae). Revta Mus. Paulista.
Hafner M. S., Sudman P. D., Villablanca F. X., Spradling T. A., Demastes J. W., and Nadler S. A. (1994). Disparate rates of molecular evolution in cospeciating hosts and parasites. Science (New York, N.Y.) 265 (5175), 1087–1090. doi: 10.1126/science.8066445
Heer K., Helbig-Bonitz M., Fernandes R. G., Mello M. A., and Kalko E. K. (2015). Effects of land use on bat diversity in a complex plantation–forest landscape in northeastern Brazil. J. Mammalogy 96, 720–731. doi: 10.1093/jmammal/gyv068
Hrycyna G., Martins A. C. M., and Graciolli G. (2019). Infracommunities of bat flies (Diptera: Streblidae and Nycteribiidae) of bats (mammalia: Chiroptera) in three conservation units in the State of Amapá, Brazil. Biota Neotrop. 19. doi: 10.1590/1676-0611-bn-2018-0715
IBGE-Instituto brasileiro de geografia e estatística (2023). “Levantamento Sistemático da Produção Agrícola, maio 2023 -Tabela 1618 - Área plantada, área colhida e produção, por ano da safra e produto das lavouras/safra 2022-2023,” in Sistema IBGE de Recuperação Automática- SIDRA. Available at: https://sidra.ibge.gov.br/tabela/1618resultado (Accessed February 22, 2024).
Janzen D. H. (1980). When is it coevolution? Evolution 34, 611–612. doi: 10.1111/j.1558-5646.1980.tb04849.x
Júnior L. F., de Araújo W. S., and Falcão L. A. D. (2020). Structure of the interaction networks between bats (Mammalia: Chiroptera) and ectoparasite flies (Diptera: Streblidae, Nycteribiidae) on a latitudinal gradient. Acta Chiropterologica 22, 187–196. doi: 10.3161/15081109ACC2020.22.1.018
Kaiser-Bunbury C. N. and Blüthgen N. (2015). Integrating network ecology with applied conservation: a synthesis and guide to implementation. AoB Plants 7, plv076. doi: 10.1093/aobpla/plv076
Kerches-Rogeri P., Niebuhr B. B., Muylaert R. L., and Mello M. A. R. (2020). Individual specialization in the use of space by frugivorous bats. J. Anim. Ecol. 89, 2584–2595. doi: 10.1111/1365-2656.13339
Kessel Q. C. (1925). A synopsis of the Streblidae of the World. Journal of the New York Entomological Society 33, 11–33, 4.
Kleine P. A. N. D. and Trivinho-Strixino S. (2005). Chironomidae and other aquatic macroinvertebrates of a first order stream: community response after habitat fragmentation. Acta Limnologica Brasiliensia 17, 81–90.
Krasnov B. R., Fortuna M. A., Mouillot D., Khokholva I. S., Shenbrot G. I., and Poulin R. (2012). Phylogenetic signal in module composition and species connectivity in compartmentalized host-parasite networks. Am. Nat. 179, 501–511. doi: 10.1086/664612
Kunz T. H., Torrez E. B., Bauer D., Lobova T., and Fleming T. H. (2011). Ecosystem services provided by bats. Ann. New York Acad. Sci. 1223, 1–38. doi: 10.1111/j.1749-6632.2011.06004.x
López-Rivera C., Robayo-Sánchez L. N., Ramírez-Hernández A., Cortés-Vecino J. A., Cuéllar-Sáenz J. A., Villar J. D., et al. (2024). Hyperparasitism in bat flies (Diptera: Streblidae): new records and interaction networks in the Neotropics. Parasitol. Res. 123. doi: 10.1007/s00436-024-08221-1
Machado-Allison C. E. (1966). Notas sobre Streblidae (Diptera) de Venezuela I. Las especies del genero Pterellipsis Coquillett. Acta Biol. Venez.
Martinez-Morales M. A. (2005). Nested species assemblages as a tool to detect sensitivity to forest fragmentation: the case of cloud forest birds. Oikos 108 (3), 634–642.
MapBiomas. (2020). Projeto de Mapeamento Anual da Cobertura e Uso do Solo do Brasil. Available online at: https://brasil.mapbiomas.org/. Acess in: fevereiro 2024.
Mello M. A. R., Muylaert R. L., Pinheiro R. B. P., and Ferreira G. M. F. (2016). Guia para análise de Redes Ecológicas. Belo Horizonte, 112. Available at: https://marcomellolab.wordpress.com (Accessed April 28, 2024).
Mendes P. (2021). Effects of land-use changes on Brazilian bats: a review of current knowledge. Mammal Rev. 51, 127–142. doi: 10.1111/mam.12227
Menezes J. and Fernandez F. (2013). Nestedness in forest mammals is dependent on area but not on matrix type and sample size: an analysis on different fragmented landscapes. Braz. J. Biol. 73, 465–470. doi: 10.1590/S1519-69842013000300002
Messeder J. V. S., Guerra T. J., Dáttilo W., and Silveira F. A. (2020). Searching for keystone plant resources in fruit-frugivore interaction networks across the Neotropics. Biotropica 52, 857–870. doi: 10.1111/btp.12804
Meyer C. F. J., Struebig M. J., and Willig M. R. (2016). “Responses of tropical bats to habitat fragmentation, logging, and deforestation,” in Bats in the anthropocene: Conservation of bats in a changing world (Springer, Cham), 63–103.
Ministério da Agricultura and Pecuária e Abastecimento (2020). Cartilha de boas práticas na lavoura cacaueira no estado do Pará/Ministério da Agricultura, Pecuária e Abastecimento, Secretaria de Inovação, Desenvolvimento Rural e Irrigação, Comissão Executiva do Plano da Lavoura Cacaueira (Belém: Mapa/CEPLAC).
Miranda-Ribeiro de A. (1907). Alguns Dipteros interessantes. Arch. Mus. Nac. Rio de Janeiro 14, 229–239.
Morales R. G., Chapa-Vargas L., Galindo-González J., and Badano E. I. (2012). Seed dispersal among three different vegetation communities in the Huasteca region, Mexico, analyzed from bat feces. Acta Chiropterologica 14, 357–367. doi: 10.3161/150811012X661675
Morse S. F., Dick C. W., Patterson B. D., and Dittmar K. (2012). Some like it hot: evolution and ecology of novel endosymbionts in bat flies of cave-roosting bats (Hippoboscoidea, Nycterophiliinae). Appl. Environ. Microbiol. 78, 8639–8649. doi: 10.1128/AEM.02455-12
Nepstad D. C., Stickler C. M., Filho B. S., and Merry F. (2008). Interactions among Amazon land use, forests and climate: prospects for a near-term forest tipping point. Philos. Trans. R. Soc. B: Biol. Sci. 363, 1737–1746. doi: 10.1098/rstb.2007.0036
Orlova M. V., Larchanka A. I., Dolgova I. G., and Dziamianchyk V. V. (2022). Unusual findings of fleas (Siphonaptera: Ctenophthalmidae, Ceratophyllidae) on bats (Chiroptera: Vespertilionidae) in Belarus: case report. Ecol. Montenegrina 57, 37–43. doi: 10.37828/em.2022.57.5
Ospina-Pérez E. M., Rivera-Páez F. A., and Ramírez-Chaves H. E. (2023). Exploring the relationship between bats (Mammalia, Chiroptera) and ectoparasitic flies (Diptera, Hippoboscoidea) of the Orinoquia Region in South America. Zookeys 1179, 1–34. doi: 10.3897/zookeys.1179.103479
Paixão V. H. F., Gomes V. G. N., De Souza C. S., and Venticinque E. M. (2023). Cactus height increases the modularity of a plant–frugivore network in the Caatinga dry forest. Biotropica 55, 877–887. doi: 10.1111/btp.13239
Palheta L. R., Urbieta G. L., Brasil L. S., Dias-Silva K., Da Silva J. B., Graciolli G., et al. (2020). The effect of urbanization on bats and communities of bat flies (Diptera: Nycteribiidae and Streblidae) in the Amazon, northern Brazil. Acta Chiropterologica 22, 403–416. doi: 10.3161/15081109ACC2020.22.2.014
Patterson B. D., Dick C. W., and Dittmar K. (2008). Sex biases in parasitism of neotropical bats by bat flies (Diptera: Streblidae). J. Trop. Ecol. 24, 387–396. doi: 10.1017/S0266467408005117
Peel M. C., Finlayson B. L., and McMahon T. A. (2007) Updated world map of the Köppen-Geiger climate classification. Hydrology and earth system sciences. 11(5), 1633–1644.
Perty M. (1833). De insectorum in America meridionali habitantium vitae genere, moribus ac distributione geographica observationes nonnullae.
Pessõa S.B., Ayrossa, and Galvão A.L. (1937). Descripcao de uma nova especie do genero Trichobius. Folia Clin. Biol., Sao Paulo.
Poulin R., Krasnov B. R., and Mouillot D. (2011). Host specificity in phylogenetic and geographic space. Trends Parasitol. 27, 355–361. doi: 10.1016/j.pt.2011.05.003
Purificação K. N., Pascotto M. C., Pedroni F., Mews H. A., and Lima-Junior D. P. (2020). Disentangling the architecture of the frugivorous bird-plant interaction networks in a savanna-forest mosaic in the Neotropical savanna. Acta Oecologica 107, 103601. doi: 10.1016/j.actao.2020.103601
Ramalho D. F., Diniz U. M., and Aguiar L. M. S. (2021). Anthropization affects the assembly of bat-bat fly interaction networks. Front. Environ. Sci. 9. doi: 10.3389/fenvs.2021.752412
Ramírez-Mejía A. F., Urbina-Cardona J. N., and Sánchez F. (2020). Functional diversity of phyllostomid bats in an urban–rural landscape: A scale-dependent analysis. Biotropica 52, 1168–1182. doi: 10.1111/btp.12816
R Core Team (2022). “R: A language and environment for statistical Computing.,” in R foundation for statistical computing(Vienna, Austria). Available at: https://www.R-project.org/ (Accessed 31, 2024).
Reckardt K. and Kerth G. (2007). Roost selection and roost switching of female Bechstein’s bats (Myotis bechsteinii) as a strategy of parasite avoidance. Oecologia 154, 581–588. doi: 10.1007/s00442-007-0843-7
Robinson M. L. and Strauss S. Y. (2020). Generalists are more specialized in low-resource habitats, increasing stability of ecological network structure. Proc. Natl. Acad. Sci. 117, 2043–2048. doi: 10.1073/pnas.1820143117
Rui A. M. and Graciolli G. (2005). Moscas ectoparasitas (Diptera, Streblidae) de morcegos (Chiroptera, Phyllostomidae) no sul do Brasil: associações hospedeiros-parasitos e taxas de infestação. Rev. Bras. Zoologia 22, 438–445. doi: 10.1590/S0101-81752005000200021
Russo D., Coleman J. L., Ancillotto L., and Korine C. (2023). “Ecosystem services by bats in urban areas,” in Urban bats: biology, ecology, and human dimensions (Springer International Publishing, Cham), 167–180. doi: 10.1007/978-3-031-13173-8_12
Salkeld D. J., Padgett K. A., and Jones J. H. (2013). A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett., 679–686. doi: 10.1111/ele.2013.16.issue-5
Santos F. G. A., Calouro A. M., Souza S. F., Lague B. M., Marciente R., Faustino R. C. L., et al. (2012). Ectoparasitismo em uma assembléia de morcegos em um fragmento florestal no estado do Acre, Brasil. Acta Veterinaria Brasilica, 211–217.
Sebastián-González E., Dalsgaard B., Sandel B., and Guimaraes P. R. (2015). Macroecological trends in nestedness and modularity of seed-dispersal networks: human impact matters. Global Ecol. Biogeography, 293–303. doi: 10.1111/geb.12270
SEMAS - Secretaria de Meio Ambiente e Sustentabilidade (2021) Boletim informativo de zoneamento agroclimático das principais culturas temporárias produzidas no estado do Pará (Belém-PA). Available at: https://www.semas.pa.gov.br/hidromet/files/BOLETIM-INFORMATIVO-ZONEAMENTO-OUT-2021.pdf.
SENAR- Serviço Nacional de Aprendizagem Rural (2018). “Cacau: produção, manejo e colheita,” in Coleção SENAR(Brasília), 145. Available at: https://www.cnabrasil.org.br/assets/arquivos/215-CACAU.pdf.
Silva I. M. S. (2012). Estratificação vertical e efeito da fragmentação numa comunidade de morcegos (Chiroptera, Mammalia) na Amazónia central. In: Dissertação de Mestrado. Programa de pós-graduação em Biologia da Conservação (Lisboa-Portugal: Universidade de Lisboa (Departamento de Biologia Animal).
Silva Z. D. (2024). Dispersão de semente e redes mutualísticas entre morcegos da família phyllostomidae (Mammalia: Chiroptera) e plantas de paisagens dominadas por cultivo de cacau na Amazônia Brasileira. 2024 (Bélem-PA: Dissertação (Mestrado em Zoologia) - Programa de Pós-Graduação em Zoologia), 30.
Silva Z. D., Gurgel E. S. C., Correia L. L., and Vieira T. B. (2024). Seed dispersal by bats (Chiroptera: Phyllostomidae) and mutualistic networks in a landscape dominated by cocoa in the Brazilian amazon. Glob. Ecol. Conserv. 55, e03252. doi: 10.1016/j.gecco.2024.e03252
Silva J., da Silva L. C., Dias-Silva K., Oliveira Júnior A., da Silva B. T., Veloso G. K., et al. (2020). Nota sobre morcegos (Mammalia, Chiroptera) e moscas ectoparasitas (Insecta, Diptera) do Parque Nacional da Serra do Pardo, estado do Pará, Brasil. Boletim do Museu Paraense Emílio Goeldi - Ciências Naturais 15, 829–841. doi: 10.46357/bcnaturais.v15i3.263
Silveira M. C. D., Silveira M., Medeiros L. S., and Aguiar L. M. S. (2024). The role of feeding roosts in seed dispersal service bats provide in urban areas. Biotropica. 56, e13291. doi: 10.1111/btp.13291
Silva M. E.S., Pereira G., and da Rocha R. P. (2016). Local and remote climatic impacts due to land use degradation in the Amazon “arc of deforestation”. Theoretical and Applied Climatology, 125(3–4), 609–623.
Soares F. A., Graciolli G., Ribeiro C. E., Bandeira R. S., Moreno J. A., and Ferrari S. F. (2016). Bat (Mammalia: Chiroptera) diversity in an area of mangrove forest in southern Pernambuco, Brazil, with a new species record and notes on ectoparasites (Diptera: Streblidae). Papéis Avulsos Zoologia 56, 63–68. doi: 10.1590/0031-1049.2016.56.06
Straube F. C. and Bianconi G. V. (2002). Sobre a grandeza e a unidade utilizada para estimar esforço de captura com utilização de redes-de-neblina. Chiroptera Neotropical 8, 150–152.
Tlapaya-Romero L., Santos-Moreno A., and Ibáñez-Bernal S. (2021). Effect of seasonality and microclimate on the variation in bat-fly load (Diptera: Streblidae) in a cave bat assemblage in a dry forest. . Mammalia 85, 345–354. doi: 10.1515/mammalia-2020-0115
Torres J. M., Urbieta G. L., Almeida L. B. M., Soares D. K. F., and Anjos E. A. C. (2019). Moscas ectoparasitas (Diptera, Streblidae) de morcegos (Mammalia, Chiroptera) em um remanescente periurbano de Cerrado: composição da comunidade, prevalência, intensidade de infestação e especificidade. Iheringia. Série Zoologia 109, e2019006. doi: 10.1590/1678-4766e2019006
Trajano E. and Gimenez E. E. (1998). Bat community in a cave from eastern Brazil, including a new record of Lionycteris (Phyllostomidae, Glossophaginae). Studies on Neotropical Fauna and Environment, 33(2), 69–75.
Trujillo-Pahua L. and Ibáñez-Bernal S. (2020). Bat flies (Diptera: Streblidae) of phyllostomid bats (Chiroptera: Phyllostomidae) from the mountainous central region of Veracruz, Mexico. Systematic Parasitol. 97, 743–777. doi: 10.1007/s11230-020-09951-3
Urbieta G. L., Graciolli G., and Vizentin-Bugoni J. (2021). Modularity and specialization in bat–fly interaction networks are remarkably consistent across patches within urbanized landscapes and spatial scales. Curr. Zoology 67, 403–410. doi: 10.1093/cz/zoaa072
Urbieta G. L., Torres J. M., Almeida L. B. M. D., Shinohara A., and Dos Anjos E. A. C. (2014). Infestação de morcegos (Mammalia, Chiroptera) por moscas do gênero Megistopoda (Diptera, Streblidae) em um fragmento urbano de Cerrado de Campo Grande, Mato Grosso do Sul. Boletim da Sociedade Bras. Mastozoologia 69, 10–13.
Urbieta G. L., Torres J. M., Anjos E. A. C., Carvalho C. M. E., and Graciolli G. (2018). Parasitism of bat flies (Nycteribiidae and streblidae) on bats in urban environments: lower prevalence, infracommunities, and specificity. Acta Chiropterologica 20, 511–518. doi: 10.3161/15081109ACC2018.20.2.021
Vasconcelos P. F., Falcão L. A. D., Graciolli G., and Borges M. A. Z. (2016). Parasite-host interactions of bat flies (Diptera: Hippoboscoidea) in Brazilian tropical dry forests. Parasitol. Res. 115, 367–377. doi: 10.1007/s00436-015-4757-8
Velasco Gomez Beuchle R., Shimabukuro Y., Grecchi R., Simonetti D., Eva H. D., and Achard F. (2015). A long-term perspective on deforestation rates in the Brazilian Amazon. The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, 40, 539–544.
Verde R. S., Silva R. C., and Calouro A. M. (2018). Activity patterns of frugivorous phyllostomid bats in an urban fragment in southwest Amazonia, Brazil. Iheringia. Série Zoologia 108, e2018016. doi: 10.1590/1678-4766e2018016
Vieira T. B., Alexandre R. J. R., Dias S. V., Pena S. A., Silva Z. D., Da, Correia L. L., et al. (2024). Association between bats (Mammalia: Chiroptera) and bat flies (Streblidae, Hippoboscoidea) from urban fragments of Amazon. Mastozoología Neotropical 31, e0990. doi: 10.31687/saremMN.24.31.01.18.e0990
Waterhouse C. O.. (1879). On the affinity of the genus Polyctenes Giglioli with the description of new species. Trans. Ent. Soc. London.
Wenzel R. L.. (1966). [new taxa.] In Wenzel R. L., Tipton V. J., and Kiewlicz A. The Streblid batflies of Panama (Diptera Calypterae: Streblidae). Pp. 405–675. In Wenzel R. L., Tipton V. J., and Kiewlicz A. (eds.), Ectoparasites of Panama. Field Museum, Chicago. xii + 861 pp.
Vieira T. B., Silva L. C. N. D., Aguiar L. M. D. S., Oprea M., Mendes P., and Ditchfield A. D. (2021). Bat species composition associated with restinga lagoons from the Paulo César vinha state park, Espírito Santo, Brazil. Papéis Avulsos Zoologia 61, e20216132. doi: 10.11606/1807-0205/2021.61.32
Wenzel R. L. (1976). The streblid batflies of Venezuela (Diptera: Streblidae). Brigham Young University Science Bulletin. Biol. Ser. 20, 1.
Wenzel R. L., Tipton V. J., and Kiewlicz A. (1966). “The streblid bat flies of Panama (Diptera: Calyptera: Streblidae,” in Ectoparasites of Panama. Eds. Wenzel R. L. and Tipton V. J. (Field Museum of Natural History, Chicago), 405–675.
Whitaker J. J.O. (1988). Collecting and preserving ectoparasites for ecological study. Ecol. Behav. Methods Study Bats (Washington), 459–474.
Zhang X., Dalsgaard B., Staab M., Zhu C., Zhao Y., Gonçalves F., et al. (2023). Habitat fragmentation increases specialization of multi-trophic interactions by high species turnover. Proc. R. Soc. B 290, 20231372. Available at: https://www.researchgate.net/publication/374948563 (Accessed July 15, 2024).
Keywords: Streblidae, Nycteribiidae, infracommunity, Phyllostomidae, Trichobius tuttlei, Speiseria peytoni, Mastoptera guimaraesi, Basilia manu
Citation: Dias SV, Alexandre RJR, Correia LL, Aguiar LMS, Graciolli G, Alcantara DMC and Vieira TB (2025) Are cocoa plantations suitable habitats? Network between parasites (Diptera: Hippoboscidea) and hosts (Mammalia: Chiroptera) in cocoa-dominated landscapes of the Brazilian Amazon. Front. Ecol. Evol. 13:1499475. doi: 10.3389/fevo.2025.1499475
Received: 20 September 2024; Accepted: 22 May 2025;
Published: 24 June 2025.
Edited by:
Pavel Kindlmann, Charles University, CzechiaReviewed by:
Bruno Garcia Luize, State University of Campinas, BrazilValeria Salinas-Ramos, University of Naples Federico II, Italy
Copyright © 2025 Dias, Alexandre, Correia, Aguiar, Graciolli, Alcantara and Vieira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Letícia Lima Correia, bGV0aWNpYWxpbWFjb3JyZWlhMTZAZ21haWwuY29t
 Samantha Valente Dias
Samantha Valente Dias Rafaela Jemely Rodrigues Alexandre
Rafaela Jemely Rodrigues Alexandre Letícia Lima Correia
Letícia Lima Correia Ludmilla M. S. Aguiar
Ludmilla M. S. Aguiar Gustavo Graciolli
Gustavo Graciolli Daniel Maximo Correa Alcantara
Daniel Maximo Correa Alcantara Thiago Bernardi Vieira
Thiago Bernardi Vieira