- The Koret School of Veterinary Medicine, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot, Israel
Introduction: Environmental crimes, such as illegal hunting, trade, smuggling, poisoning, and harvesting of protected wildlife, rank among the world’s top five illicit activities, contributing significantly to biodiversity loss. Wildlife forensic cases often involve multiple domestic and wild species and require a multidisciplinary approach for effective resolution. The enforcement of wildlife protection laws increasingly depends on molecular genetic methods.
Goals: In this study, I present three complex wildlife forensic cases involving the poisoning of the Eurasian griffon vulture (Gyps fulvus), critically endangered (CR) in Israel, and the poaching of wildlife, including the Nubian ibex (Capra nubiana) and gazelles (Gazella spp.), particularly the endangered mountain gazelle (Gazella gazella).
Results and Discussion: These cases underscore the importance of integrating methodologies, beginning with species identification, population assignment, and individual sample matching using public and local genetic databases to ensure comprehensive analysis. The local genetic databases play a crucial role in providing essential species and population validation. The involvement of both wild and domestic species in each case necessitates an efficient, accurate, rapid, and cost-effective protocol to differentiate wild from domestic species among exhibits seized at crime scenes and to confirm the identity of wild species beyond any doubt.
Introduction
Global climate change and rapid anthropogenic alterations to biotic and abiotic conditions are accelerating biodiversity loss, signaling the onset of the sixth mass extinction event (Gross, 2019). Human activities, particularly hunting pressures and the establishment of settlements, have had profound impacts on local ecosystems. These actions have led to the decline of large herbivore populations and their predators, caused pervasive habitat fragmentation, and reduced genetic diversity through bottlenecks and genetic drift (Ermini et al., 2015; Munshi-South et al., 2016; Puckett et al., 2016). Without intervention, many species could face extinction within this century. Currently, more than 46,300 animal species are threatened, accounting for about 30% of all known species worldwide (IUCN, 2024). The current rate of species loss today is 1,000 times higher than historical averages, primarily driven by human activities that disrupt ecosystem functions (Schickhoff et al., 2024).
The illegal wildlife industry, encompassing hunting, trade, smuggling, poisoning, and harvesting of protected species, significantly contributes to biodiversity loss, therefore, it is essential to address and eliminate these destructive practices. Enforcing wildlife protection laws often requires forensic investigations to address key questions: “What is it?” and “Where did it come from?” (Ogden, 2011). When these questions cannot be answered through morphological analysis alone, they are interpreted through molecular analysis of species identification, individual identification, and population assignment. Scientific DNA databases and molecular genetic and genomic methods are commonly used to answer these questions.
Molecular genetic methodologies have revolutionized forensic science by offering new tools for criminal investigations, although these methods come with advantages and limitations. A major advantage of these techniques is their ability to achieve accurate species identification from low-quality, low-quantity DNA collected from various specimens or exhibits. By establishing a unique genetic profile for a specimen, whether human, animal, or plant, molecular methods provide crucial evidence that can link a specimen or suspect to a crime scene or exclude them from involvement. However, these advanced molecular methods also have certain drawbacks, such as limited availability to a laboratory, high costs, lengthy processing times, the requirement for species-specific molecular primers, and the necessity of a comprehensive genetic/genomic database that accurately reflects local biodiversity.
Unlike human forensics, which focuses on a single species, wildlife forensics encompasses a diverse range of species. While global databases, such as GenBank, curate information on all organisms and are publicly accessible, they should be used meticulously, as not all data are quality-controlled, and sample origin information is often incomplete or missing. Wildlife analysts must consider these limitations when submitting reports or testifying in court based on public data (Mori and Matsumura, 2021). One way to address these limitations of molecular genetic technology is by focusing on the fundamental question, “What is it?” For species identification, wildlife forensics analysts commonly employ mitochondrial DNA (mtDNA) markers. The relatively high mutation rate of mtDNA in mammals (0.017*10−6; substitutions/site/year) allows for the distinction of recently diverged species and to define evolutionary events (Pakendorf and Stoneking, 2005). The chosen gene region to be used as a marker should have lower intra-species variability than inter-species variability, meaning the genetic diversity within the species should be lower than the diversity between species.
Israel, located at the crossroads of three continents, boasts a unique assemblage of species within its small yet highly heterogeneous landscape. Its diverse ecosystems, ranging from mountains and plains to coastal areas, create a variety of habitats, forming a significant biodiversity hotspot. This rich ecological region is home to approximately 2,800 plant species, over 500 bird species, and 100 mammal species. The country’s exceptional biodiversity is further enriched by the presence of local ecotypes, which enhance genetic variability within species, and by the fact that many populations occur at the periphery of their distribution range. In Israel, the rising threats of poaching and poisoning have contributed to significant declines in local wildlife populations, pushing them toward extinction. Notable examples include the Eurasian griffon vulture (Gyps fulvus), once abundant in large colonies in the Galilee and Carmel regions, which has seen its population shrink from hundreds of individuals in the late 19th and early 20th centuries to fewer than 230 today. This species is now classified as critically endangered by the International Union for Conservation of Nature (IUCN). Another example is the mountain gazelle (Gazella gazella), whose population has decreased from around 10,000 individuals in 1985 to approximately 3,000 today. Due to habitat loss, predation, and poaching pressures, it was recognized as an endangered species by the IUCN in 2017. Recognizing the importance of preserving this biodiversity, a local wildlife DNA database was established.
In the southern Levant, hunting of gazelle (G. gazella and Gazella dorcas) and Nubian ibex (Capra nubiana) dates back to the Epipaleolithic period (20,000 – 10,000 BC) and is documented in the archaeozoological assemblages through the Neolithic and Chalcolithic periods, even after the domestication of sheep (Ovis aries) and goats (Capra hircus) (Horwitz et al., 1999). Regardless of the availability of domestic animals as a food source, hunting of wild animals has continued throughout history evolving from a means of subsistence to a cultural role as observed today in puberty rites, pagan rituals, status rank, and as a recreational sport (Conover, 2001). In Israel, the Wildlife Protection Law was enacted in 1955. The law prohibits hunting, trade, possession, or transfer of protected wildlife species and their parts (Reserves, 1999). According to the Wildlife Protection Law, all wildlife (animals and plants) are protected unless declared to be an exception. Hence, all wildlife species described in the following are not exceptions to the law. The Wildlife Protection Law is enforced by the Israel Nature and Park Authority (INPA), yet poaching and wildlife trade still exist and are rising. When hunters are prosecuted, common defense arguments include their right to practice established tradition or claims that the seized evidence (such as bloodstains or meat) originates from livestock they own (e.g., sheep, goat, or cow).
Gazelles, Nubian ibex, domestic sheep, goats, and cows (Bos taurus) all belong to the Bovidae family. Domesticated livestock (sheep, goats, and cattle) share high genomic similarity with their wild ancestors, which allows for genetic introgression (Daly et al., 2018; Kahila Bar-Gal, 2022; Nayak et al., 2024). In the case of the domestic goat and the Nubian ibex, both species belong to the Capra genus and are capable of hybridization, as evidenced by the establishment of the “Yaez” (Rattner et al., 1994; Münger et al., 2024; Nayak et al., 2024). Consequently, to convict a suspect of poaching wild Bovidae species, such as gazelles or Nubian ibex, it is crucial to establish the identity of the evidence beyond a reasonable doubt.
In recent years, the Wildlife Forensic Laboratory (WFL) in Israel has handled complex cases involving both wild and domestic species, requiring a range of comprehensive molecular genetic approaches to support investigations and legal proceedings. The local genetic database established by the laboratory curates a wealth of information, including genetic profiles (mtDNA, nDNA, and STR), physical characteristics, and geographical distributions of various wild and domestic species. The comprehensive database has been pivotal in guiding restoration initiatives, evolutionary studies, public health management strategies and revolutionized wildlife forensic investigations in Israel (Magory Cohen et al., 2012; Hadas et al., 2015; 2016; Lapid et al., 2023; Martins et al., 2024). This enables evidence to be quickly and accurately matched to known species or populations. Notably, the inclusion of genetic data on domestic species has proven invaluable in addressing agricultural crimes, such as cattle theft (Blas, 2021), and in investigating incidents involving canid attacks by dogs or wolves (personal communication). Investigations of complex cases often constitute various disciplines, sample sources, and platforms. The use of multiple tools to solve these cases not only enhances the efficiency and accuracy of investigations but also revolutionizes interdisciplinary collaboration (see Case 3 below). The WFL collaborates closely with the law enforcement unit of the INPA, the Ministry of Agriculture’s Veterinary Services and Animal Health division (including pathology and toxicology departments), the Central Unit for Enforcement and Investigations, and the Israeli police. This coordinated effort has improved case resolution times and led to more successful prosecutions.
The developed protocol for cases involving multi-source samples starts by identifying the species of each specimen, evidence, or exhibit, followed by individual identification to determine whether samples originate from the same individual within a species. Finally, each species undergoes population assignment to provide further insights. This paper aims to demonstrate how our comprehensive approach leveraging our local DNA database has enabled the resolution of three complex wildlife forensic cases in Israel.
Materials and methods
Exhibits
The cases discussed in the current paper were resolved by the WFL at the Hebrew University of Jerusalem, Israel. Case and exhibit numbers were re-coded to maintain confidentiality of the original information. All exhibits were collected by rangers from the INPA, the governmental body responsible for enforcing wildlife protection laws in Israel. These cases met all the requirements for wildlife forensic investigations, and the files are securely maintained. The information presented was extracted from the designated files and summarized to ensure confidentiality.
DNA extraction
DNA extraction was performed using two different methods: (i) Guanidinium thiocyanate (GuSCN) and DNA capture in silica (Boom et al., 1990; Höss and Pääbo, 1993); (ii) Automated extraction using Promega Maxwell FSC DNA IQ (TM) Casework Kit, that was specifically designed for poor-quality and low-quantity DNA (Loten et al., 2018; Hakim et al., 2019). These two protocols were used to fit the nature of the sample and were approved by the court in Israel.
DNA amplification and genetic profile
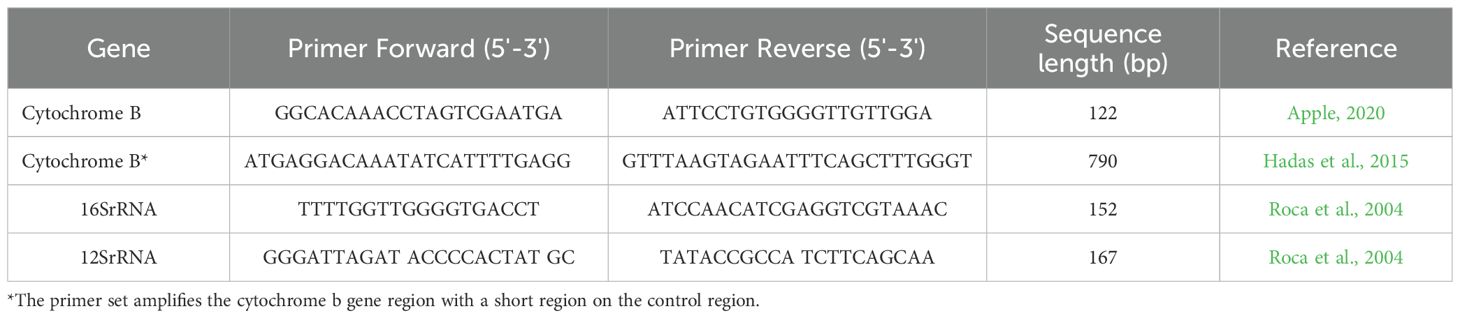
Prior to DNA amplification, we carefully planned the analysis to ensure that the correct markers were selected to answer whether the questions posed by law enforcement considered the quantity of available DNA (Figure 1). The four most widely used mtDNA markers for species identification in wildlife forensics are the Cytochrome oxidase subunit I gene (COI), the Cytochrome B gene (CytB), and the 12S and 16S rRNA genes (Catresana, 2001; Yang et al., 2014). The latter two are relatively conserved, evolving more slowly than other mitochondrial genes, and exhibit lower inter-species variation, with minimal differences between closely related species.
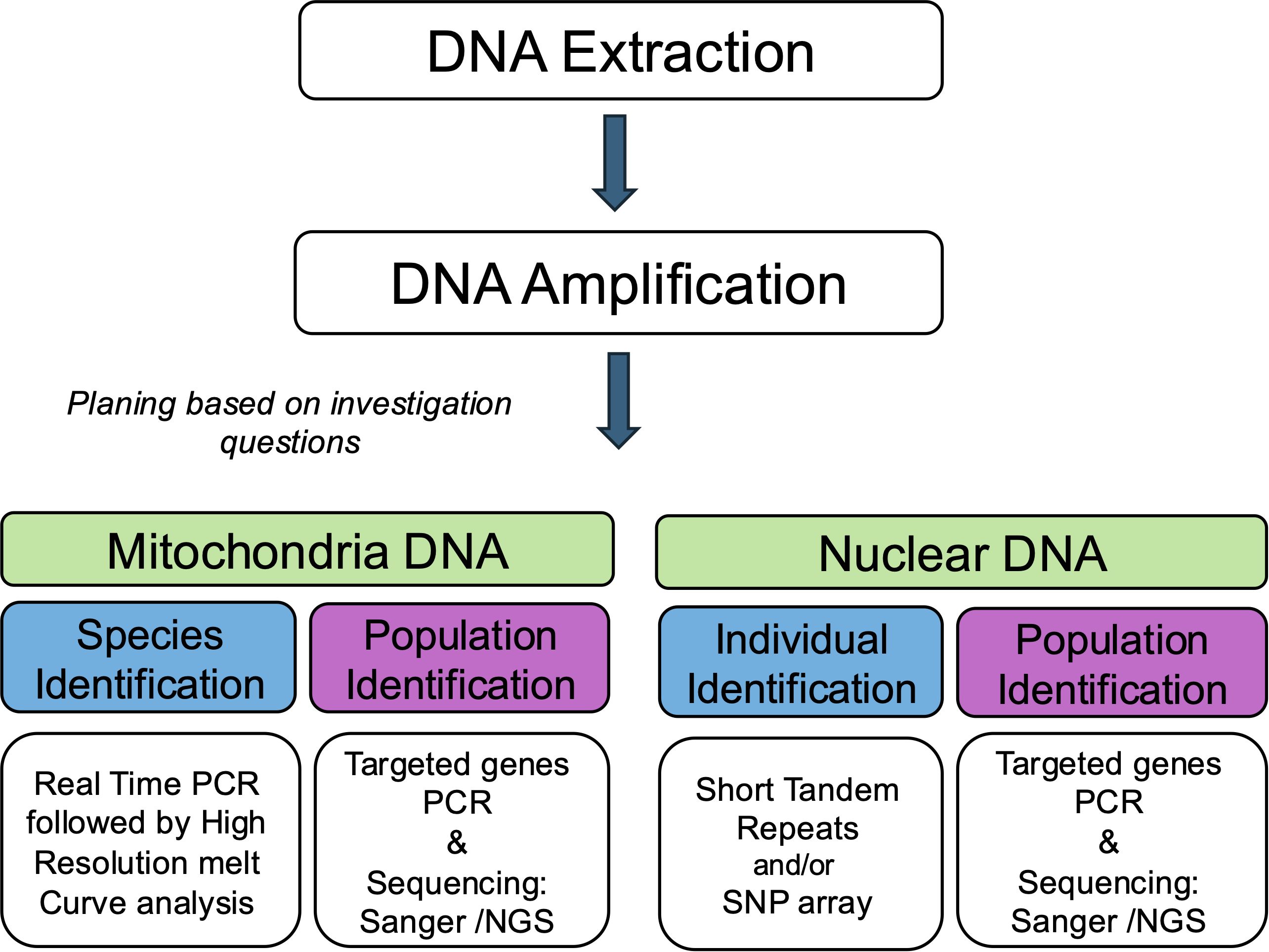
Figure 1. Research plan. The quantity and quality of DNA extracted from forensic exhibits are often limited due to poor preservation. Therefore, based on the investigation’s objectives, a well-thought-out plan is essential before laboratory work. The choice of markers and methodologies should align with the expected results required to solve the case.
A critical factor in choosing a mtDNA marker is that it must confirm the species’ identity with high certainty, beyond any doubt. The mtDNA marker should also distinguish between species of the same genus that share high genetic similarity and can hybridize, such as the wild boar (Sus scrofa) and the domestic pig (Sus scrofa domesticus) (Aravena and Skewes, 2007; Iacolina et al., 2018; Schleimer et al., 2022).
Species identification: At least two mtDNA gene regions, and in some cases three (12S, 16S, and CytB), were used to identify each sample (Roca et al., 2004; Hadas et al., 2015; Appel, 2022; Appendix 1). For these gene regions, we developed a Real-Time PCR (RT-PCR) followed by High-Resolution Melting (HRM) Curve analysis (RT-PCR-HRM). The assays were specifically designed to differentiate between the most commonly poached mammalian species in Israel, similar to an assay designed for African species (Ouso et al., 2020). This method is sensitive and efficient, functioning effectively even at very low DNA concentrations (0.05 ng, Figures 2A, B). The CytB assay, in particular, distinguishes between species and is especially effective in differentiating wild and domestic Bovidae species (Figure 2C).
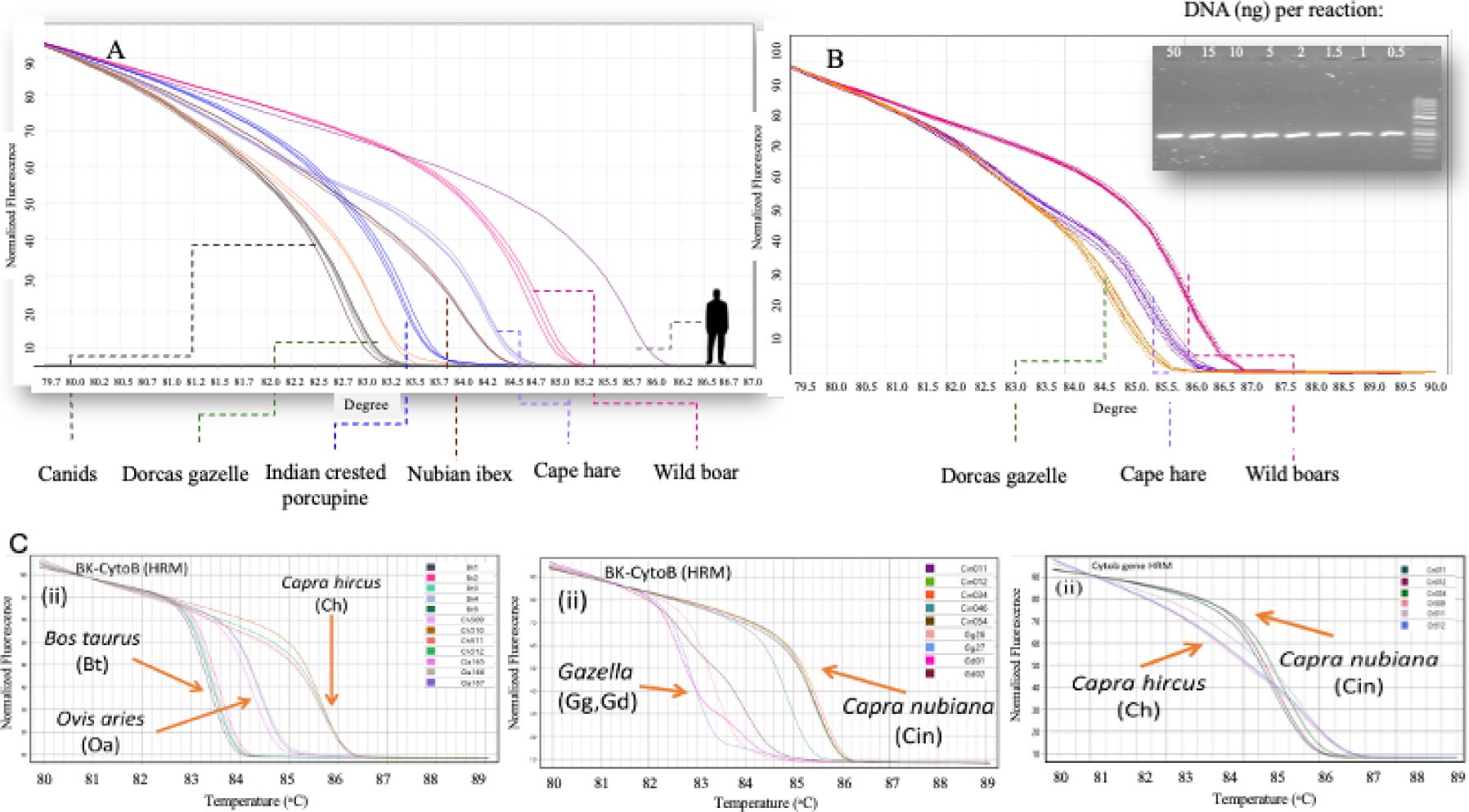
Figure 2. Species Identification using Real-Time PCR followed by High-Resolution Melting Curve Analysis. X = Temperature (in Celsius). Y= Normalise florescence. (A) HRM graph indicating the variation between main poached species in Israel based on the 12S gene region [Canids (Black), Dorcus gazelle (Orange), Indian crested porcupine (Blue), Nubian ibex (Brown), Cape hare (Purple), Wild boar (Red), Human (Grey)]; (B) HRM graph indicating the sensitivity of the reaction for the 12S gene region across three species [Dorcus gazelle (Orange), Cape hare (Purple) and Wild boar (Red)]. For each species, several lines represent the different concentrations of the DNA. (C) HRM graph indicating the variation between wild and domestic Bovidea species in Israel based on the CytoB gene region.
Amplification was conducted in a final volume of 25µl with 3.5µl MgCl2 (3.5mM Applied Biosystems, Massachusetts, USA), 2.5 µl dNTPs (0.3mM LAROVA, Germany), 2.5μl 10x buffer (Applied Biosystems, Massachusetts, USA), 1μl each primer (1μM, custom ordered from IDT, Syntezza Bioscience Ltd., USA), 0.75µl SYTO-9 (Thermo Fisher Scientific, MA, USA), 0.25µl Taq-Gold (1.25 units) (Applied Biosystems, Massachusetts, USA). PCR cycling conditions were as follows: initial denaturation at 95°C for 10 min followed by a total of 40 cycles of 15 sec at 95°C, 30-sec annealing at 58°C and elongation at 72°C for 30 sec. The 40 cycles were followed by a final extension step of 10 min at 72°C. HRM analysis was carried out on the Mic qPCR Cycler or Rotor-Gene 6000. The HRM analysis was as follows: holds at 90°C for 30 seconds, then 55°C for 60 seconds, finalizing the analysis by increasing the temperature from 55°C to 99°Cat 0.1°C c/s. PCR products were analyzed using Rotor-Gene 6000 software.
Individual identification and population assessment: Short Tandem Repeats (STRs) were developed for the wild species, Nubian ibex and Gazelle spp., and were used to establish a genetic database. This database includes specimens from various geographic locations across Israel to better capture the genetic diversity within each species/population. The Nubian ibex population was characterized using 9–11 STRs (Appel, 2022; Tichon and Bar-David, 2020; Shemesh, 2015), while the Gazelle population was analyzed using 9 different STRs (Hadas et al., 2015, 2016). Domestic goat populations were characterized using the same primer sets as those used for Nubian ibex. The domestic dog population was characterized by 13 STRs based on the StockMarks™ for Dogs Genotyping Kit, system (Thermo Fisher Scientific, Massachusetts, USA).
The extracted DNA was amplified multiplexing the STRs (Holleley and Geerts, 2009). Reactions had a final volume of 15µl with 0.15µl Taq-Gold (1.25 units), 3.5µl MgCl2 (3.5mM Applied Biosystems, Massachusetts, USA), 2.5 µl dNTPs (0.3mM LAROVA, Germany), 0.6 µl of each primer (1μM, Rhenium, Modi’in Israel) and 2.5μl 10x buffer (Applied Biosystems, Massachusetts, USA); the volume was brought to 15μl with ddH2O. PCR cycling conditions were as follows: initial denaturation at 95°C for 10 min followed by a total of 45 cycles of 15 sec at 95°C, 30-sec annealing for 3 cycles each at 60°C, 58°C, 56°C, 54°C, 52°C, and 50°C, followed by 17 cycles at 48°C, and elongation at 72°C for 45 sec, with a final extension step of 10 min at 72°C. PCR products were genotyped on a Spectrum CE System (Promega Corporation, USA) at Prof. Kahila Bar-Gal’s laboratory.
mtDNA Sanger sequencing: Positive amplification products were purified using the EPPIC Fast kit (A&A Biotechnology, Gdańsk, Poland). Sanger sequencing was performed using the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Massachusetts, USA) as described by Crossley et al. (2020). The BigDye PCR conditions were as follows: an initial denaturation step at 96°C for 1 minute, followed by 30 cycles of 96°C for 10 seconds, 50°C for 5 seconds, and 60°C for 4 minutes.
The obtained mtDNA sequences were visually inspected for ambiguities and errors using Sequencher software (version 5.4.6, Gene Codes Corporation, Michigan, USA). For each sample, the sequences from both the sense and antisense strands of each gene fragment were aligned, and primer sequences were trimmed. A consensus sequence was then generated for each sample. Consensus sequences from all samples of the same gene region were aligned for further analysis. These sequences were compared to known sequences in the GenBank database using the Basic Local Alignment Search Tool (BLAST) and to the established database curated by the WFL to validate the species identification and confirm the gene region.
STR genotyping: STR genotyping was performed using GeneMapper software (Applied Biosystems, California, USA). Genotypes were manually scored. A unique profile for each sample (individual) was determined based on the successful amplification of loci. Determination of the number of individual(s) involved in the crime and the population assessment were conducted based on several statistical analyses using GenAlEx 6.5 software (Peakall and Smouse, 2006). These included the calculation of haplotype diversity (Hd), an analysis of variance (ANOVA), Principal Coordinate Analysis (PCoA), and an assignment test to determine population differentiation and genetic relationships.
Results
Three case studies are presented to achieve the stated goals. Each case approaches the topic from a different angle, providing a background overview, a detailed description of the materials and methods used, and a discussion of the results and conclusions.
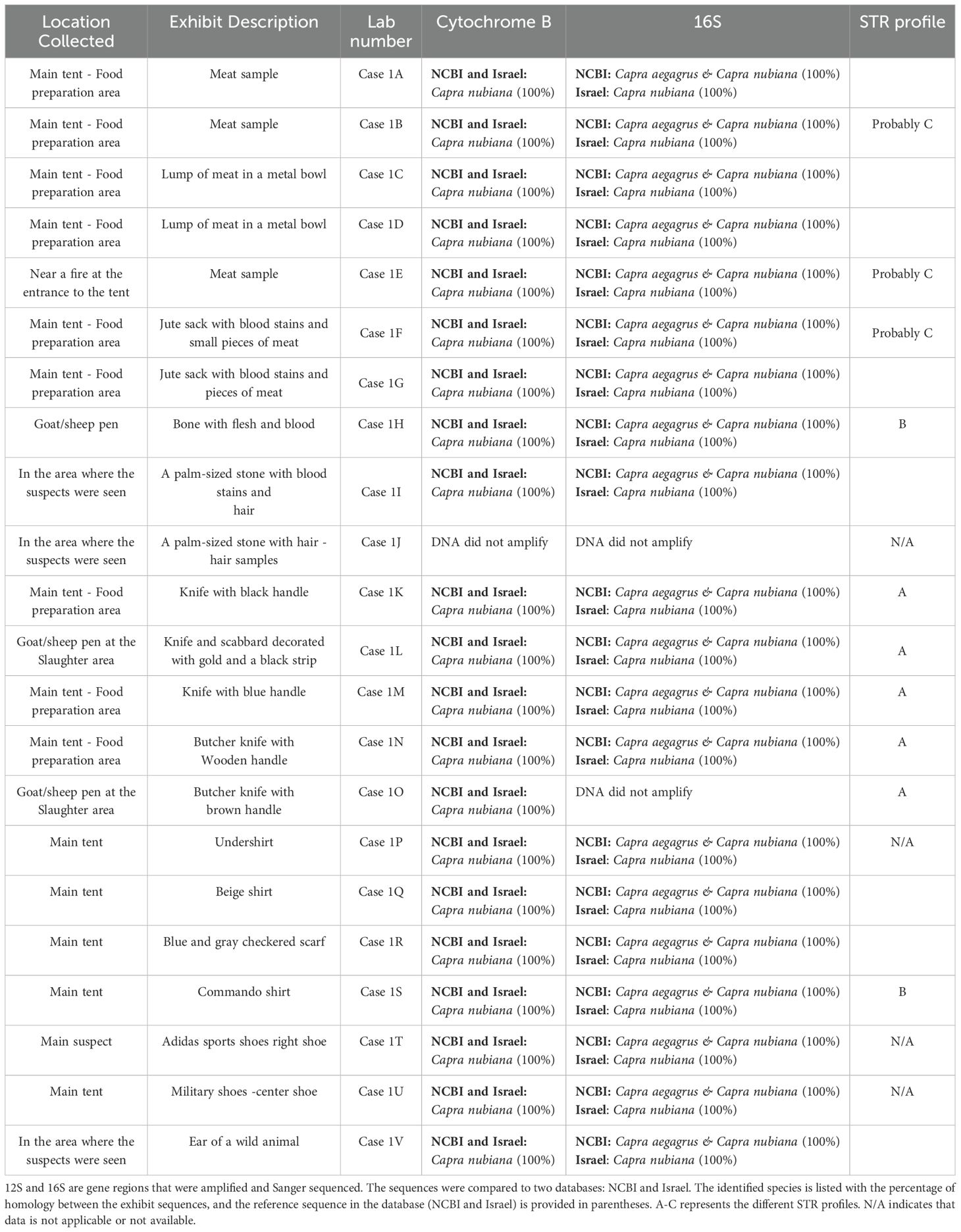
Case 1: The Nubian ibex (C. nubiana): the importance of distinguishing wild and domestic species
Background: Intelligence information regarding illegal hunting led to an investigation in a Bedouin camp in the Judean Desert, where INPA rangers conducted a thorough search. The search covered all areas of the camp, including the living quarters, kitchen, herd area (containing sheep and goats), and vehicles. A total of 22 samples were collected by the rangers from various locations within the camp, including areas where a suspect had been previously observed by a ranger. The exhibits collected were diverse and included knives, meat, stones, clothing, and shoes, all of which exhibited blood stains. These items were subsequently sent to the WFL accompanied by a letter from the Head of Investigation at the INPA Law Enforcement Unit with a request to address the following objectives using molecular genetic markers:
1. Species Identification of the 22 exhibits.
2. In the event that wildlife species are identified among the exhibits:
● Determine the genetic relationships between all samples belonging to the same species.
● Indicate the number of individual animals involved and if possible trace their origin.
Results:
Species Identification: RT-PCR-HRM assay, using two mitochondrial gene fragments, 16S (152 bp) and CytB (137 bp), was conducted for species identification. The assay successfully amplified 21 samples, all of which were determined to belong to the Capra genus (Table 1).
All positive amplifications were Sanger sequenced, and consensus sequences were determined for each sample and gene region. The consensus sequences for the 16S gene region were identical to either Capra aegagrus (wild goat) or C. nubiana in GenBank. Furthermore, a comparison of the same consensus sequences to a local database, which includes a wide range of regional mammalian species, revealed 100% homology with the C. nubiana from Israel. The difference in identification results stems from the length of the gene region aligned in each database. When using GenBank, a shorter gene region (114–120 bp) was aligned, whereas, in the local database, a longer region (152 bp) was aligned although the same consensus sequence was used. Increasing the number of base pairs compared enhances the accuracy of the results.
Similarly, the consensus sequences for the CytB gene region were found to be identical to C. nubiana in both GenBank and the local database, further confirming the results obtained from the RT-PCR-HRM assay (Table 1). Thus, the use of two mitochondrial DNA regions, along with the comparison to a comprehensive local wildlife database, conclusively supported the identification of all samples as Nubian ibex.
Individual and population assessment: The samples identified as C. nubiana (n=21) were genotyped using a set of nine STR markers. Despite amplifying all samples twice, only seven of the nine STR markers were successfully amplified in 18 of the samples. Two loci, which exhibited partial amplification, were excluded from the analysis. For the seven successfully amplified loci, there were a few cases where only one allele was amplified at a locus, raising the question of whether this represents a true homozygous genotype or an allele drop-out. In these cases, the analysis treated these situations as homozygous. The resulting genetic profiles revealed the following groupings (Table 1, Figure 3):
● Five samples (Case 1K, Case 1L, Case 1M, Case 1N, Case 1O) shared the same profile, Profile A.
● Two samples (Case 1H and Case 1S) shared the same profile, Profile B.
● Three samples (Case 1B, Case 1E, Case 1F) were similar, possibly forming Profile C.
● Seven samples (Case 1A, Case 1D, Case 1G, Case 1I, Case 1Q, Case 1R, Case 1V) each exhibit had a unique profile, they did not share a profile.
● In four samples (Case 1J, Case 1P, Case 1T, and Case 1U) STR’s failed to amplify despite three attempts. In Case 1J, a hair sample, it is likely that the follicle was absent, as no amplification was observed for either mtDNA or nDNA, suggesting a lack of viable DNA. A low amount of DNA is conserved in hair shafts, hence the low success rate in DNA extraction from hair shafts is well-documented in forensic literature (Almeida et al., 2011). For the other three samples (Case 1P, Case 1T, and Case 1U), DNA was extracted from an undershirt and shoes (Table 1). STRs were partially amplified, with four loci detected for Case 1U and two loci for both Case 1P and Case 1T. The most plausible explanation for these partial results is the low quality and/or quantity of the extracted DNA.
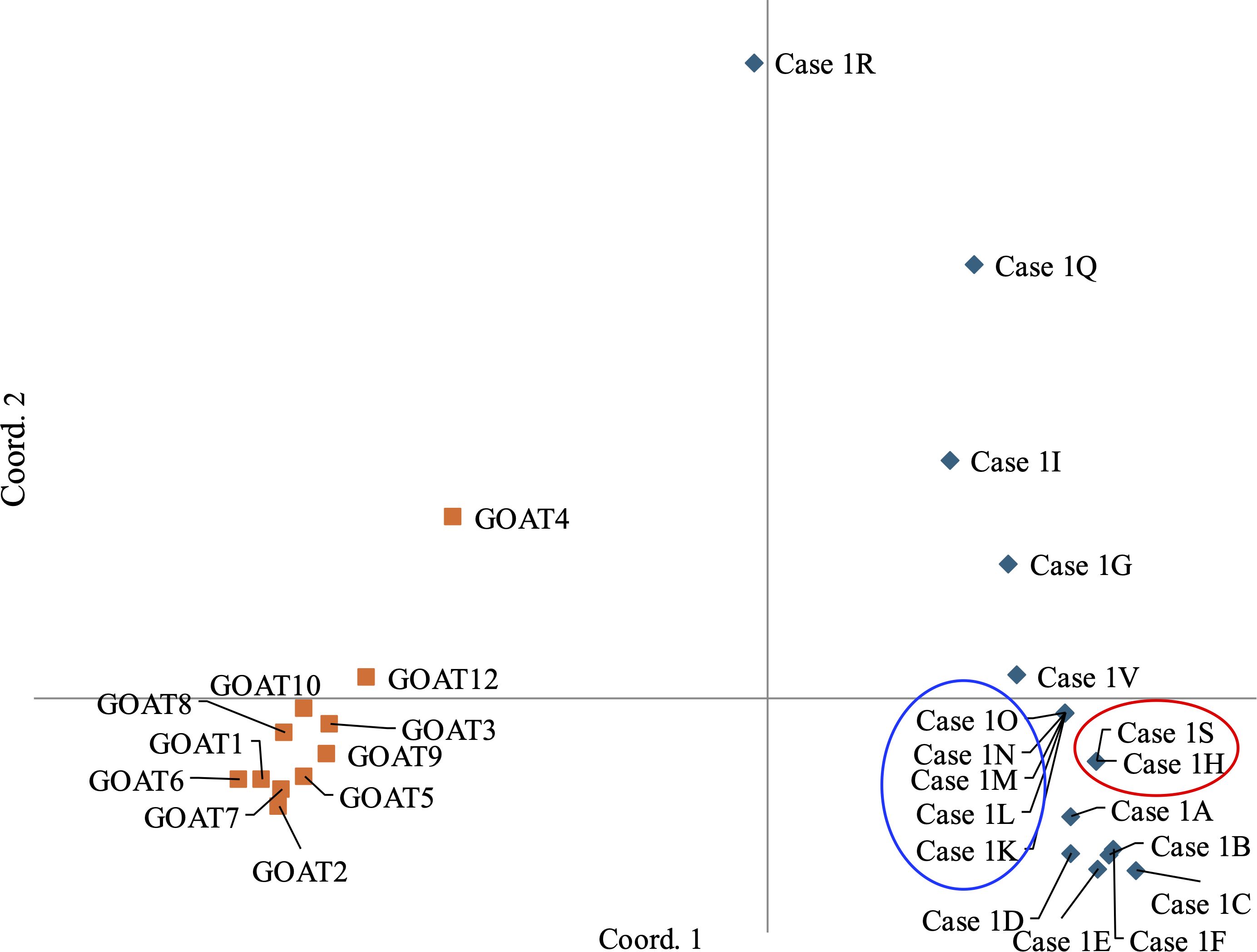
Figure 3. Genetic relationships between exhibits in Case 1 based on STR haplotype analysed. PCoA analysis was conducted using GenAlex software. Blue circle samples with identical Profile A, Red circle samples with identical Profile B, Orange – goat samples.
Since the genetic profiles were based on only seven STR markers, these results do not provide conclusive evidence regarding the number of individuals that were involved in the forensic case, whether there were more than one individual or more than two individuals. The probability that two individuals will have the same profile for the seven markers is 1 in 72 individuals. In Israel, there are roughly 1200 Nubian ibex.
A comparison of the obtained genetic data with a local database of domestic goats and Nubian ibex populations in Israel (n=75) confirmed without doubt that the exhibits represent wild Nubian ibex from the Judean Desert region. The limited number of loci used in the comparison, however, prevented a more precise determination of the origin of the individuals involved.
Conclusions:
The molecular genetic results supported the INPA rangers’ suspicion that the suspects were involved in the illegal hunting of a wild animal, specifically the Nubian ibex, protected under the Israel Wildlife Protection Law. This contradicted the suspects’ claim that all the blood stains and meat belonged to a sheep and/or goat that they had slaughtered from their herd. Notably, blood stains on two knives (Case 1L and Case 1O) found in the goat/sheep pen near the slaughter area were identified as having DNA of Nubian ibex (based on the mtDNA markers) and shared the same genetic profile (based on the STR markers) as blood stains on three knives (Case 1K, Case 1M, and Case 1N) found in the food preparation area in the main tent (Table 1). These findings strongly suggest that the suspects were involved in slaughtering and preparing Nubian ibex meat for cooking and/or sale in a market. Further supporting this conclusion, the blood stain found on a “commando” shirt (Case 1S) in the main tent and an exhibit (Case 1H) from the goat/sheep pen demonstrated matching STR profiles, reinforcing the claim of illegal hunting of a Nubian ibex by the individuals in the camp. Additionally, meat samples from the food preparation area (Case 1B and Case 1F) and from the campfire (Case 1E) were consistent with the possibility of originating from the same individual (STR profile), suggesting that the animal was used as a food resource.
It is important to note that STR genetic profiles obtained from exhibits collected at the area where the suspects were seen (Case 1I and Case 1V) (Figure 3) could not be linked to the exhibits collected in the camp, implying a possible separate criminal event. Despite these interpretations, it should be emphasized that the conclusions are based on a minimal number of STR markers, and increasing the number of markers could influence the results. Therefore, the WFL recommended that the INPA Law Enforcement Unit invest in a study to validate a new set of C. nubiana STR markers.
Summary: The key points highlighted in presenting this case are:
1. The significance of using multiple markers to establish species identification, especially when both domestic and wild species are involved and are from the same genus.
2. The importance of using markers that differentiate domestic animals from wild species.
3. The crucial role of local databases that curate genetic information on local wild species.
The results demonstrate that genetic identification of a variety of samples collected from different locations associated with the forensic case can either support or refute the investigator’s theory about the suspects and provide vital evidence in court.
Case 2: Mountain gazelle (G. gazella): illegal hunting using trained dogs
Background: In recent years, there has been evidence that poachers are becoming more sophisticated, using various technologies to increase their success rates (INPA report, 2020). To evade law enforcement rangers, poachers have become more cunning in illegal hunting practices, especially when the availability of target species is low, which often drives innovation. In Israel, the use of trained domestic dogs in illegal hunting, particularly for gazelles, has become common (INPA report, 2020). Working with trained hunting dogs minimizes direct contact with the hunter during the hunt, reducing the risk of detection.
This illustrated case involved the illegal hunting of gazelles using trained dogs. In Israel, there are three gazelle species: G. gazella, the most common endemic gazelle, found from the North of Israel to the Negev, listed by the IUCN as an endangered (EN) species (IUCN SSC Antelope Specialist Group, 2017); the Dorcas gazelle (G. dorcus), considered a vulnerable species (VU) by IUCN, is common in the south of Israel and the Acacia gazelle (G. arabica acaciae) confined to a nature reserve enclosure for protection (Hadas et al., 2015). The mountain and Dorcas gazelles overlap in the northern Negev. Gazelle hunting in the southern Levant has been practiced since prehistoric times (Horwitz et al., 1999), but the drastic decline in mountain gazelle populations over the last decade has led to the species reclassification as endangered by the IUCN in 2017 (IUCN SSC Antelope Specialist Group, 2017). This situation has prompted the INPA Enforcement Unit to implement stronger measures to combat gazelle poaching.
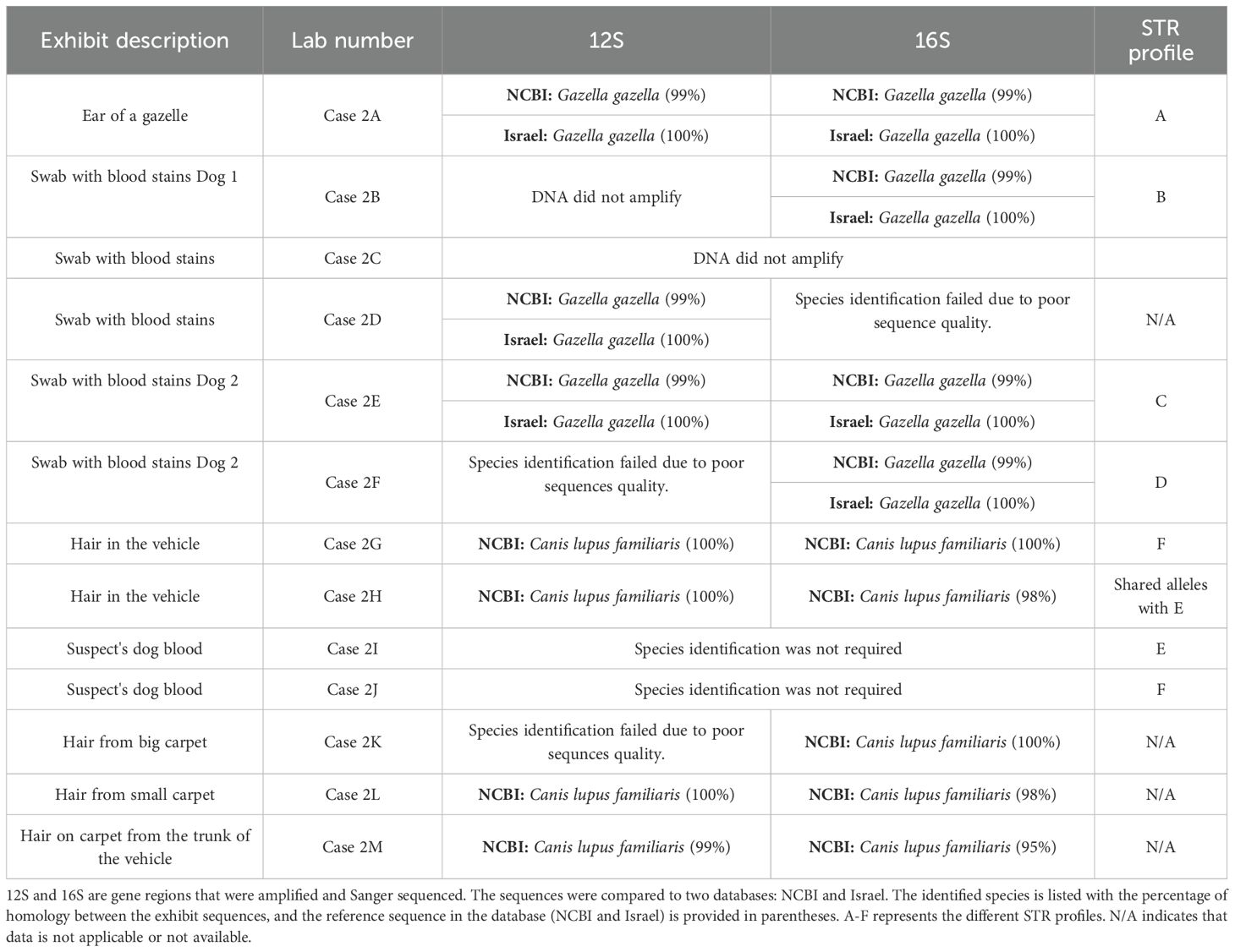
This case exemplifies the complexities surrounding gazelle poaching and the difficulties involved in bringing the suspect to court. Case 2 consisted of 17 exhibits collected by the INPA rangers, which were sent to the WFL. The exhibits were collected from the crime scene, in the field at a nature reserve, including the suspect’s car at the crime scene, and the dogs handled by the suspects were confiscated. A letter of request from the Head of Investigation at the INPA Law Enforcement Unit requested molecular genetic analysis for only 13 designated exhibits (Table 2, Figure 4):
● Species identification of the exhibits, excluding known dog blood samples.
● Individual identification of the exhibits identified as wild species.
● Individual identification of the trained dogs at the crime scene.
● Determination of the genetic relationship within each species - wild species and trained dog.
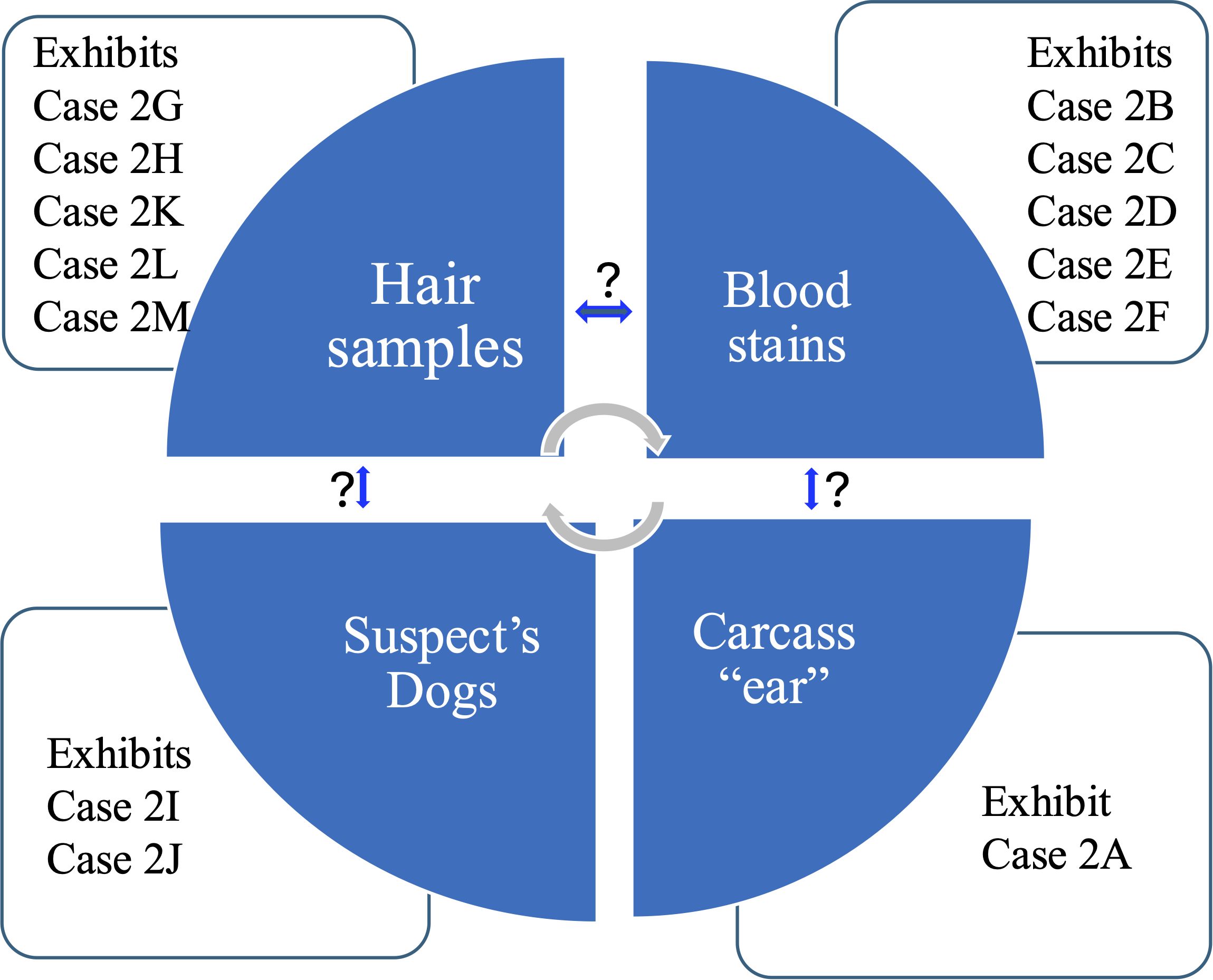
Figure 4. Specific INPA Law Enforcement Investigator Requests in Case 2. This diagram illustrates the samples studied and the relationships between different exhibits, as questioned by the investigator.
The specific requests included:
1. Individual identification of two dogs (Exhibit Case 2I and Case 2J) suspected of being involved in the hunting (Table 2, Figure 4).
2. Determination of the connection between the trained dogs, the owner, the crime scene, and the exhibits. Specifically, determining the genetic relationships between:
● Exhibit Case 2A (Carcass ear) and exhibits Cases 2B-F (Blood stains on dogs’ mouths) (Table 2, Figure 4).
● Exhibits Case 2G, Case 2H, Case 2K, Case 2L, and Case 2M (Hair samples) and exhibits Case 2I and Case 2J (suspected trained dog blood sample) (Table 2, Figure 4).
Results:
Species identification: RT-PCR-HRM assay, using two mitochondrial gene fragments (16S [152 bp] and 12S [161 bp]), revealed that the successfully amplified samples (n=9) were from a wild species, Gazelle (n=5), and a Canid species (n=5) (Table 2). Sanger sequencing of the successfully amplified DNA confirmed the RT-PCR-HRM findings, indicating that exhibits Case 2A, Case 2B, Case 2D, Case 2E, and Case 2F were identified as G. gazella based on one or two sequences (Table 2). The remaining exhibits (Case 2G, Case 2H, Case 2K, Case 2L, Case 2M) were identified as domestic dogs (Canis lupus familiaris) with 98-100% homology comparing via BLAST to the NCBI database (Table 2). The species identification results for the mountain gazelle highlight the significance of using a local database, as it showed 100% homology to the mountain gazelle sequences, whereas the GenBank homology was only 99% (Table 2). In both mtDNA gene regions, there is one SNP difference. For the 12S gene fragment, the GenBank sequence (JX647811.1) originates from a skin specimen with an uncertain origin and contains missing data (unknown bp, N) (Bärmann et al., 2013). In the 16S gene, there is a substitution from G (in the exhibits) to A (JX647811.1). The GenBank sequence originates from Saudi Arabia (AWWP 4587 - Gazella gazella erlangeri), which is outside the primary distribution range of the mountain gazelle (Hassanin et al., 2012). Furthermore, G. g. erlangeri from Saudi Arabia is likely a distinct species from G. gazella, as genetic phylogenetic and population analyses indicate a potential species boundary separating mountain gazelle from Arabian gazelles (Lerp et al., 2013).
Individual Identification:
Mountain gazelle: Nine STR markers were used to characterize genetically five gazelle samples, including the gazelle ear sample (Case 2A) found at the crime scene. Due to poor DNA quality in the samples, as indicated by the success rate of the mtDNA analysis (Table 2), only partial profiles of eight STR markers were obtained for three samples (Cases 2B, 2E, and 2F). A full profile across nine loci was obtained only for the gazelle ear sample. Comparison of ear profile (Case 2A) with the partial amplified (two, four, and three loci, respectively) profiles of the three gazelle revealed allele differences in the genotyped loci (62.5%), ruling out a match. Consequently, the blood stains found on the dog’s mouths (Cases 2B, 2E, 2F) are of G. gazella but not from the carcass found by INPA rangers. The low success rate of amplified loci for each sample prevents any statistical analysis, including the calculation of probabilities. Therefore, the population database used was based on the samples themselves.
Dogs: The two blood samples from the suspect’s trained dogs (Case 2I and Case 2J), along with hair samples identified as domestic dogs, were genetically characterized using 13 STR markers from the StockMarks™ for Dogs Genotyping Kit. The profiles of the suspect’s two dogs differed from each other (Table 2). Comparison of these profiles with those obtained from the four hair exhibits in the vehicle identified as dog hairs revealed a match between Case 2G (hair) and Case 2J (suspect’s dog). Exhibit Case 2H shared alleles with the other suspected dog (Case 2I), but a partial profile prevents a definitive conclusion regarding a match between the samples.
Conclusions: The molecular genetic findings supported the INPA Law Enforcement Unit’s suspicion that the suspect was involved in the illegal hunting of mountain gazelles using trained dogs:
1. Matching STR profiles generated from one of the suspect’s dog’s blood samples and a hair sample found in the vehicle (match between Case 2J and Case 2G) (Table 2) confirmed the connection of one dog to the suspect.
2. Identification of the blood stains on the swabs that were taken from blood samples found around the dogs’ mouths as mountain gazelle using mtDNA indicated that the dogs had direct contact with a gazelle species.
3. There was no evidence to associate the dogs with the gazelle ear found at the crime scene due to the different genetic profiles obtained from the gazelle samples.
The genetic findings established only partial links between the trained dogs, the owner, the crime scene, and the exhibits. The presence of dog hair in the suspect’s car, which demonstrated a matching STR genotype to the blood of a dog at the crime scene with gazelle blood around its mouth, suggested the possible involvement of the suspect in illegal gazelle hunting. Consequently, the dogs were confiscated from the owner. It is important to note that trained hunting dogs are highly valuable and difficult to replace
Summary: The key points highlighted in this case are:
1. When DNA is degraded, obtaining complete genetic profiles becomes challenging, which can impact the accuracy and ability to fulfill analysis requests.
2. Using multiple markers can be essential for species identification, particularly with degraded DNA of poor quality and quantity. In such cases, a single gene region often fails to amplify.
3. A local database, built using native voucher specimens, is important for accurate species identification.
4. There is a need to establish genetic databases for trained hunting dogs, as this information can be valuable for future cases.
The results demonstrate that genetic identification of a variety of samples collected from different locations associated with the forensic case can either support or refute the investigator’s theory and provide vital evidence in court.
Case 3: The Eurasian griffon vulture (G. fulvus): poisoning events
Background: In Israel, the G. fulvus is listed as critically endangered by the IUCN. As of today, ~230 individuals remain in the wild (Mayrose et al., 2017; King personal communication). The species is under protection, and significant national conservation efforts are underway to save the population. Pesticide poisoning is the main cause of mortality in Griffon vulture populations in Israel, where 48 out of 107 (45%) known injury/mortality cases in 2010–2021 were caused by poisoning (Anglister et al., 2023). The use of organophosphate and carbamate pesticides is controlled by the Ministry of Agriculture to prevent the poisoning of wildlife and minimize risks to humans. Regulations limit the types of toxins that can be used, their application methods, and the locations where they can be deployed. Violating these regulations, especially when poisoning threatens humans and innocent wildlife, is considered a serious offense.
Wildlife poisoning is a major threat to conservation efforts, with heavy metal toxicity, particularly lead (Pb), among the most widespread dangers. It is estimated that poisoning kills millions of wildfowl, terrestrial birds, raptors, and scavengers worldwide each year and results in sub-lethal poisoning for millions more (Olea et al., 2022). Typically, pesticides or toxins are placed in bait to target specific species. However, most toxins impact multiple species, leading to both direct and indirect effects. Secondary poisoning occurs when scavengers, such as raptors and vultures, consume the carcasses of poisoned animals, extending the toxic impact across species (Olea et al., 2022).
In November 2021, seven carcasses of G. fulvus were discovered by INPA rangers in the south of Israel. Near the crime scene, the rangers found a domestic goat carcass, raising the suspicion that this might be a case of secondary poisoning—where the vultures were poisoned after feeding on the goat carcass. Toxicological tests on both the goat and the vultures confirmed that they were poisoned with the same toxin, carbamate. During a pathological post-mortem examination, a piece of meat was found in the goiter of one of the vultures.
For the INPA Law Enforcement Unit to press charges, they need to establish, beyond any doubt, the connection between the suspect, the toxin, the goat used as bait, and the cause of the vultures’ deaths, i.e. resulting from feeding on the poisoned goat. Exhibits were sent to the WFL following a pathological post-mortem that determined poisoning as the cause of death. A letter of request from the investigator of the INPA Law Enforcement Unit was attached to the exhibits requesting molecular genetic analysis of the following:
1. Determine the genetic relationships between the goat exhibits (skin [2021A] and hair [2021B]) and the meat sample found in the G. fulvus’s goiter (2021C).
2. If all exhibits are found to be from the same species, determine the genetic relationship between these samples and individual samples from a goat herd owned by the suspect.
Results:
Species Identification: Species identification was conducted using two mtDNA markers (16S and CytB) and RT-PCR-HRM analysis. Successful mtDNA amplification was achieved for all three samples, and Sanger sequencing revealed high homology (99%) with the published mitochondrial genome of the domestic goat (GenBank ID: MH165338). The identification of the meat found in the Eurasian griffon vulture’s goiter (2021C) as a domestic goat required determining its relationship to the poisoned goat carcass.
Individual identification: The Thermo Fisher Scientific Bovine Genotypes Panel 3.1 Kit together with an additional 12 STR’s was used to generate genetic profiles for the three samples (2021A-C). Genetic profiles, based on the panel of 22 STR markers, were generated for each sample. A comparison of the genetic profiles showed that the two samples of the domestic goat carcass (2021A and 2021B) were identical, as expected. These STR profiles also matched the meat found in the vulture’s goiter (2021C) (Figure 5). The results supported the conclusion that all three samples originated from the same individual, suggesting that the Eurasian griffon vulture died after feeding on the poisoned carcass of a domestic goat found at the crime scene.
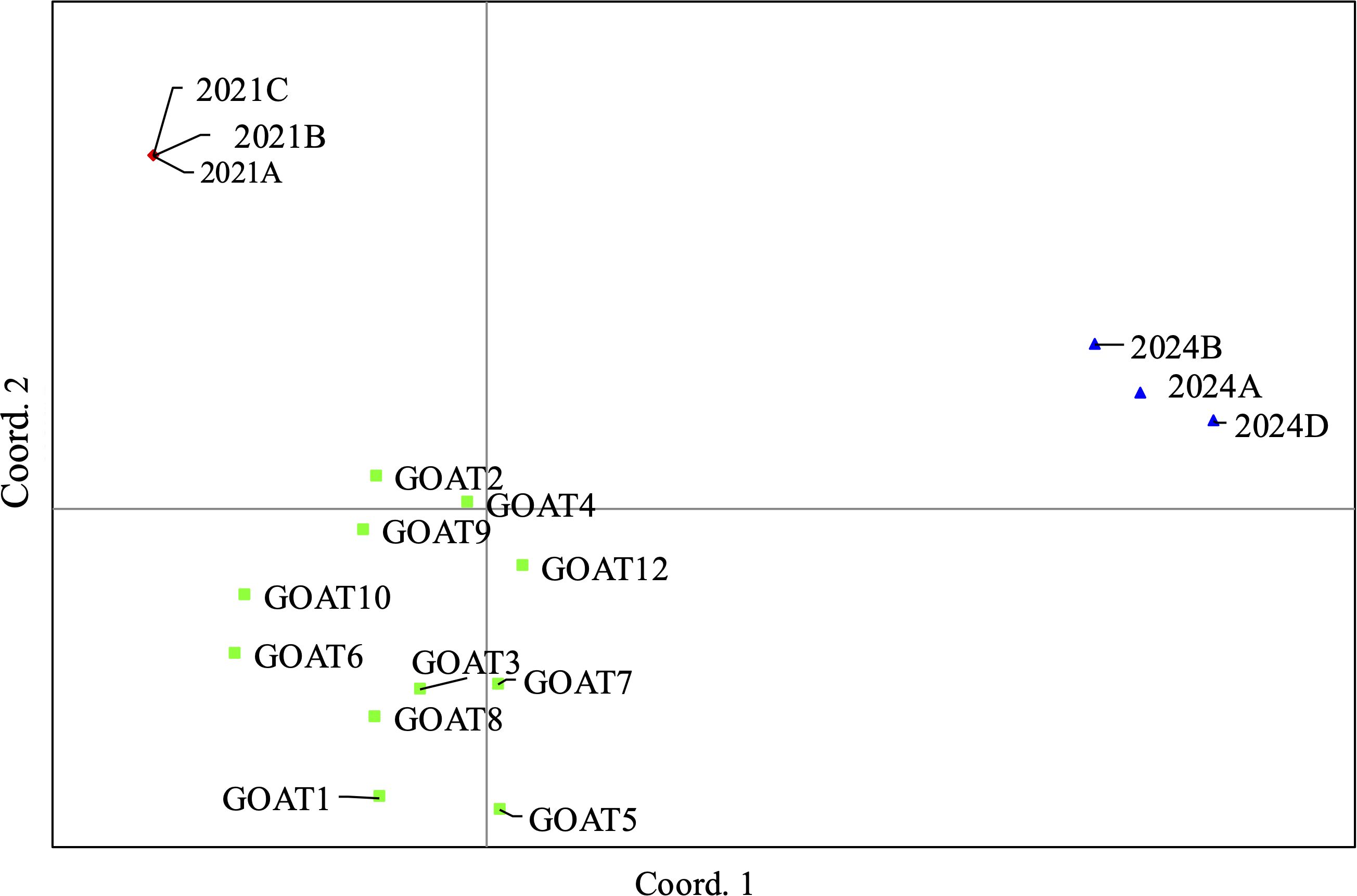
Figure 5. Genetic Relationships Between Exhibits in Case 3 based on PCoA analysis conducted using GenAlex software.
To link the poisoning event to the suspect, we analyzed the genetic relationship between the carcass and 12 goats from the herd owned by the suspect. DNA was extracted from blood samples of the 12 goats, amplified, and genotyped using the same 22 STR markers used previously. The genetic profiles of the 12 goats showed clear divergence from the three samples from the crime scene (Fst = 0.181, P < 0.001). PCoA and assignment tests (100%) further confirmed the genetic differences between the two populations (Figure 5). The probability that two individuals will have the same profile in the 22 markers is 1 in 4e9 individuals.
Conclusions: The deaths of the seven Eurasian griffon vultures were likely caused by secondary poisoning due to feeding on the poisoned goat carcass. The genetic data obtained from the exhibits did not establish a direct link between the poisoning event and the suspect’s herd. Therefore, based on the available genetic evidence, it was not possible to conclusively link the suspect to the crime scene. While no direct evidence was found, there is a high probability that other wildlife, especially raptors, were affected by this poisoning event.
In April 2024, as part of the efforts of the INPA to control and reduce poisoning events that pose a significant threat to wildlife populations in Israel, especially endangered species and raptors, a new case was sent to the WFL for genetic analysis. The case involved three vulture carcasses, two Eurasian griffon vultures (2024B and 2024C) and one Egyptian vulture (Neophron percnopterus) (2024D), that were found at the same location. Post-mortem analysis confirmed that all three birds had been poisoned. Meat samples from their goiters, along with a sample of the suspected bait (2024A) found at the crime scene were sent to the WFL. The INPA Law Enforcement Unit requested the following:
1. Determine the genetic relationships between the four samples (meat samples from the goiters and the suspected bait).
2. Investigate the connection between the samples in the current case and the goat herd involved in the previous poisoning event in 2021.
Results:
Species Identification: DNA was extracted, amplified and Sanger sequenced for two mitochondrial gene fragments, 12S and 16S using RT-PCR-HRM. The genetic analysis identified the bait (2024A), the meat from the goiters of one Eurasian griffon vulture (2024B), and the Egyptian vulture (2024D) as domestic goat. However, the meat sample from the goiter of the second Eurasian griffon vulture (2024C) was identified as domestic sheep (Ovis aries).
Individual identification: A genetic profile was determined for the three goat samples from the goiter (2024A, 2024B, and 2024D) based on 12 STR markers, the same markers used in the 2021 poisoning case. A comparison of these genotypes revealed an 85% genetic similarity between the suspected bait (2024A) and the meat in the goiter of the Eurasian griffon vulture (2024B). In contrast, the meat in the goiter of the Egyptian vulture (2024D) was more divergent, with only 69% homology to the suspected bait, indicating that the bait was not the same individual that the raptors were feeding on.
Further comparison of the 2024 samples with the 2021 exhibits and the goat herd linked to the previous poisoning event revealed that the samples belonged to three distinct populations. The results, supported by Fst values (Fst = 0.093, P < 0.009), the assignment test (100%), and PCoA, confirmed that the 2024 and 2021 populations were genetically different. The probability that two individuals will have the same profile for the 12 markers is 1 in 1613 individuals.
Conclusions:
The Eurasian griffon vultures and the Egyptian vulture died from secondary poisoning after feeding on poisoned carcasses. However, it is unlikely that the suspected bait found at the crime scene was the carcass the vultures were preying on. The genetic profiles of the goat herd owned by the suspect were distinct from the profiles of the poisoned bait and the meat found in the vultures’ goiters. Therefore, we cannot conclusively confirm or deny that the poisoned goat carcass belonged to the INPA investigator’s suspect.
Summary: In recent years, diurnal raptors, such as eagles, buzzards, and harriers, particularly Eurasian griffon vultures, have been poisoned after feeding on carcasses, often goats or sheep, that were laced with toxins. In response to these ongoing poisoning events, the WFL recommends that the INPA Law Enforcement Unit establish a database of genetic profiles from goat and sheep herds in the region. Such a database would be invaluable for future poisoning cases, providing more accurate and reliable evidence.
Discussion
In Israel, poaching is driven by factors distinct from those seen in other countries, where poverty and corruption are often significant motivators. In contrast, poaching in Israel is primarily fueled by cultural traditions, social status, greed, and sport hunting (Duffy et al., 2016; INPA report, 2020; Harper, 2023). Cultural practices, such as the consumption of hunted wildlife during celebratory events like weddings, can elevate the social status of hunters and create a unique demand for hunting protected wildlife species. This cultural context presents significant challenges for law enforcement, particularly for the INPA, which finds itself in a continuous “arms race” with poachers. Criminals are increasingly developing sophisticated methods to evade detection, prompting authorities to adapt and refine enforcement strategies. This situation reflects broader global trends in wildlife crime, where poachers rapidly adopt advanced technologies and complex schemes to circumvent law enforcement efforts (Nellemann et al., 2016; InterPol, 2018; Harper, 2023). As poachers adapt, investigations become more complicated, necessitating the integration of multidisciplinary approaches to effectively resolve these cases. One such approach is the use of molecular genetic tools, which are instrumental in addressing the complex nature of wildlife crimes. These crimes often involve multiple species, making it essential to identify the species involved in illegal activities (Webster et al., 2024).
Forensic DNA analysis plays a critical role in these investigations by enabling species identification, tracking illegal trade, and supporting legal actions against poachers. For effective species identification, two key aspects must be emphasized: (1) the use of robust DNA markers for species, individual, and population identification, and (2) the establishment of local genetic databases representing the fauna and flora of the region, particularly for species that are vulnerable to poaching. Unlike human forensics, wildlife forensic cases often involve unknown species, making universal primers for species identification essential (Nellemann et al., 2016; Smart et al., 2021). Subsequently, DNA markers must be designed to distinguish between closely related species to prevent ambiguities in identification (Linacre, 2021; Webster et al., 2024).
The mitochondrial gene markers used in our study were aligned with the most commonly used gene regions for species identification in wildlife forensics (Mori and Matsumura, 2021, 2022). Over the years these markers were used in various methodologies, including fluorescent multiplex PCR analysis, which simultaneously identifies mammalian and poultry species. Each method had its advantages and disadvantages, evaluated based on reliability, cost-effectiveness, and the availability of the necessary equipment and software (Mori and Matsumura, 2021, 2022). In our study, we developed a set of primers capable of distinguishing between the most frequently poached species in Israel at both the genus and species levels using additional methodologies, such as Next Generation Sequencing, to those previously published (Roca et al., 2004; Hadas et al., 2015; Appel, 2022). Our fluorescent RT-PCR-HRM followed by Sanger sequencing of the amplified PCR products targeting the CytB gene region, proved particularly effective in distinguishing closely related species within the Bovidae family, such as the domestic goat and the Nubian ibex. In comparison to the fluorescent multiplex PCR analysis (Mori and Matsumura, 2022) our method does not require special equipment or software. Furthermore, we found that using at least two mtDNA markers for species identification is crucial. This approach ensures that complementary results are obtained, especially when one marker fails due to DNA degradation or when dealing with closely related species.
The importance of local genetic databases cannot be overstated especially for species frequently involved in wildlife crime. Such databases are essential for accurate species identification and population monitoring, both of which are vital for identifying poaching hotspots and tracing illegal trade routes (InterPol, 2018; Mori and Matsumura, 2021; Harper, 2023). A local database provides not only genetic information but also contextual knowledge regarding the history, ecology, and conservation status of the species involved. For example, our population genetic study on the gazelle population in Israel (Hadas et al., 2015) has proven invaluable in forensic cases (Hadas et al., 2016) and conservation actions (IUCN, 2017). Species that have undergone significant population declines, resulting in reduced genetic diversity, may require additional markers or alternative marker systems to enhance the accuracy of forensic profiling and to ensure adequate statistical match probabilities (Harper, 2023). This can be achieved only by establishing and continually updating a local genetic database.
A key conclusion from our studies is that current databases remain insufficient for the demands of wildlife forensic science. There is often a trade-off between the number of individuals and the number of markers used to characterize species, particularly populations and/or individuals. High-throughput genomic sequencing approaches generally require smaller sample sizes compared to traditional genotyping studies (Nazareno et al., 2017). In situations where high-throughput genomic sequencing is either cost-effective and/or unavailable, we recommend increasing both the number of genetic markers, particularly STR markers, and the number of samples from different areas and habitats in the country within local databases. This is especially important for domesticated species involved in wildlife crimes. The diversity of breeds within domesticated species can play a crucial factor, reflected in significant genetic variation between breeds of the same species, such as the Italian goats (Cortellari et al., 2021). Moreover, the incorporation of advanced molecular technologies, such as long-read portable sequencing, is essential for facilitating rapid, in situ identification of species and individuals. This approach is suggested to be particularly beneficial when working with degraded DNA, as it enables the recovery of partial profiles, which may otherwise hinder the accuracy of forensic analysis (Smart et al., 2021).
Conclusion
The cases presented in this study highlight the methodological challenges in complex forensic investigations. The use of multiple genetic markers is critical for accurate species identification, particularly when dealing with degraded DNA or closely related species. The establishment and expansion of local genetic databases, which include both wild and domestic species frequently involved in wildlife crime, is essential for improving forensic capabilities. It is important to note that while advancements in DNA analysis hold great promise, they also introduce significant challenges. These include ensuring data security, maintaining the integrity of evidence, and addressing ethical and legal concerns surrounding genetic data usage (Alketbi, 2004). Ultimately, the integration of forensic science into wildlife conservation provides authorities with the tools needed to gather evidence, disrupt illegal activities, and develop strategies to protect endangered species. This multidisciplinary approach not only strengthens law enforcement efforts but also contributes to the broader goal of biodiversity conservation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author (Z2lsYS5rYWhpbGFAbWFpbC5odWppLmFjLmls).
Ethics statement
The study was approved by the HUVTH Committee of Animal Handling and Experimentation (permit KSVM-VTH/08_2015). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The wildlife forensic cases were investigated as part of a paid service provided to the Israel Nature and Parks Authority (INPA), not a grant.Some of the local database information is from research supported by grant of INPA no. 3011006018.
Acknowledgments
I would like to thank the Law Enforcement Unit of the Israel National Park Authority for our long collaboration in solving wildlife forensic crime in the last 20 years. I thank my two dedicated students, Mrs. Lia Hadas and Dr. Galit Eakteiman, who helped me carry out the laboratory work and stood next to me in court. I would like to thank Mr. Amizor Boldo and Dr. Roni King for their essential help in establishing the wildlife forensic database and providing samples. Finally, I would like to thank my friend, Dr. Marylin Reymond for her comments and help in editing the paper.
Conflict of interest
The authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1525957/full#supplementary-material
References
Alketbi S. K. (2024). Emerging technologies in forensic DNA analysis. PLFS 1, 10007. doi: 10.70322/plfs.2024.10007
Almeida M., Betancor E., Fregel R., Suárez N. M., and Pestano J. (2011). Efficient DNA extraction from hair shafts. Forensic. Sci. Int. Genet. 3, e319–e320. doi: 10.1016/j.fsigss.2011.09.022
Anglister N., Gonen-Shalom S., Shlanger P., Blotnick-Rubin E., Rosenzweig A., Horowitz I., et al. (2023). Plasma cholinesterase activity: A benchmark for rapid detection of pesticide poisoning in an avian scavenger. Sci. Total Environ. 877, 162903. doi: 10.1016/j.scitotenv
Appel N. (2022). Species identification in solving wildlife crime: establishment of an efficient, accurate, and cost-effective methodology for the Bovidae family (Israel: The Hebrew University of Jerusalem).
Apple N. (2020). Species identification in solving wildlife crime: establishment of an efficient, accurate, and cost-effective methodology for the Bovidae family (Ms.c thesis The Hebrew University of Jerusalem, Israel).
Aravena P. and Skewes O. (2007). European wild boar purebred and Sus scrofa intercrosses. Discrimination proposals. A review. Agro-Ciencia 23, 133–147.
Bärmann E. V., Wronski T., Lerp H., Azanza B., Borner S., Erpenbeck D., et al. (2013). A morphometric and genetic framework for the genus Gazella de Blainville 1816 (Ruminantia: Bovidae) with a special focus on Arabian and Levantine mountain gazelles. J. Zool. J. Linn. Soc 169, 673–696. doi: 10.1111/zoj.12066
Blas S. (2021). The use of genetic approach in the fight against agricultural crime in Israel: Dairy Cattle as a case study (Israel: The Hebrew University of Jerusalem).
Boom R., Sol C. J. A., Salimans M. M. M., Jansen C. L., Wertheim-van Dillen P. M., and Van Der Noordaa J. (1990). Rapid and simple method for purification of nucleic acids. JCM 28, 495–503. doi: 10.1128/jcm.28.3.495-503.1990
Catresana J. (2001). Cytochrome b phylogeny and the taxonomy of Great Apes and mammals. Mol. Biol. Evol. 18, 465–471. doi: 10.1093/oxfordjournals.molbev.a003825
Conover M. R. (2001). Effect of hunting and trapping on wildlife damage. Wildl. Soc Bull. 29, 521–532. doi: 10.2307/3784176
Cortellari M., Barbato M., Talenti A., Bionda A., Carta A., Ciampolini R., et al. (2021). The climatic and genetic heritage of Italian goat breeds with genomic SNP data. Sci. Rep. 11, 10986. doi: 10.1038/s41598-021-89900-2
Crossley B. M., Bai J., Glaser A., Maes R., Porter E., Killian M. L., et al. (2020). Guidelines for Sanger sequencing and molecular assay monitoring. J. Vet. Diagn. Invest. 32, 767–775. doi: 10.1177/1040638720905833
Daly K. G., Delser P. M., Mullin V. E., Scheu A., Mattiangeli V., Teasdale M. D., et al. (2018). Domestication in the fertile crescent. Science 361, 24–27. doi: 10.1126/science.aas9411
Duffy R., St John F. A. V., Büscher B., and Brockington D. (2016). Toward a new understanding of the links between poverty and illegal wildlife hunting. Conserv. Biol. 30, 14–22. doi: 10.1111/cobi.2016.30.issue-1
Ermini L., Der Sarkissian C., Willerslev E., and Orlando L. (2015). Major transitions in human evolution revisited: A tribute to ancient DNA. J. Hum. Evol. 79, 4–20. doi: 10.1016/j.jhevol.2014.06.015
Gross M. (2019). Hunting wildlife to extinction. Curr. Biol. 29, R551–R554. doi: 10.1016/j.cub.2019.05.063
Hadas L., Hermon D., Boldo A., Arieli G., Gafny R., King R., et al. (2015). Wild gazelles of the southern Levant: Genetic profiling defines new conservation priorities. PLoS One 10, 1–19. doi: 10.1371/journal.pone.0116401
Hadas L., Hermon D., and Kahila Bar-Gal G. (2016). Before they are gone - Improving gazelle protection using wildlife forensic genetics. Forensic. Sci. Int. Gene. 24, 51–54. doi: 10.1016/j.fsigen.2016.05.018
Hakim H. M., Khan H. O., Ismail S. A., Ayob S., Lalung J., Kofi E. A., et al. (2019). Assessment of autosomal and male DNA extracted from casework samples using Casework Direct Kit, Custom and Maxwell 16 System DNA IQ Casework Pro Kit for autosomal-STR and Y-STR profiling. Sci. Rep. 9, 14558. doi: 10.1038/s41598-019-51154-4
Harper C. K. (2023). Poaching forensics: animal victims in the courtroom. Annu. Rev. Anim Bios 11, 269–286. doi: 10.1146/annurev-animal-070722-084803
Hassanin A., Delsuc F., Ropiquet A., Hammer C., Van Vuuren B. J., Matthee C., et al. (2012). Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. C. R. Biol. 335, 32–50. doi: 10.1016/j.crvi.2011.11.002
Holleley C. E. and Geerts P. G. (2009). Multiplex Manager 1.0: A cross-platform computer program that plans and optimizes multiplex PCR. Biotechniques 46, 511–517. doi: 10.2144/000113156
Horwitz L. K., Tchernov E., Ducos P., Becker C., Von Den Driesch A., Martin L., et al. (1999). Animal domestication in the southern Levant. Paléorient 25 (2), 63–80. doi: 10.3406/paleo.1999.4687
Höss M. and Pääbo S. (1993). DNA extraction from Pleistocene bones by purification method. Nucleic Acids Res. 21, 3913–3914. doi: 10.1093/nar/21.16.3913
Iacolina L., Pertoldi C., Amills M., Kusza S., Megens H. J., Bâlteanu V. A., et al. (2018). Hotspots of recent hybridization between pigs and wild boars in Europe. Sci. Rep. 8, 17372. doi: 10.1038/s41598-018-35865-8
INPA Science Division Nature and Parks Authority (INPA) (2020). Israel wildlife report 2020 (Israel: INPA).
InterPol (2018). Global wildlife enforcement: Strengthening Law Enforcement Cooperation against Wildlife Crime (Lyon, France: I- NTERPOL General Secretariat). 13.
IUCN (2024).The IUCN red list of threatened species. In: Version 2024-2, Background and History. Available online at: https://www.iucnredlist.org (Accessed January 20, 2025).
IUCN SSC Antelope Specialist Group (2017). Gazella arabica (IUCN Red List Threat). Species e.T117582065A88018124.
Kahila Bar-Gal G. (2022). “Animal domestication in the near east,” in The origins of plant domestication in the ancient near east. Eds. Abbo S., Gopher A., and Kahila Bar-Gal G. (Cambridge University Press, Cambridge CB2 8BS, United Kingdom).
Lapid R., Motro Y., Craddock H., Khalfin B., King R., Kahila Bar-Gal G., et al. (2023). Fical microbiota of the synanthropic Golden Jackal (Canis aureus). Anim. Microb. 5, 37. doi: 10.1186/s42523-023-00259-3
Lerp H., Wronski T., Plath M., Schröter A., and Pfenninger M. (2013). Phylogenetic and population genetic analyses suggest a potential species boundary between Mountain (Gazella gazella) and Arabian Gazelles (G. arabica) in the Levant. Mamm. Biol. 78, 383–386. doi: 10.1016/j.mambio.2012.11.005
Linacre A. (2021). Wildlife crime in Australia. Emerg. Top. Life Sci. 5, 487–494. doi: 10.1042/ETLS20200288
Loten M., Graham E. K., Thompson J., Wieczorek D., Kevin Brewer K., Tullis A., et al. (2018). Developmental validation of the maxwell® FSC DNA IQ™ Casework kit on the maxwell® FSC instrument. White paper.
Magory Cohen T., Narkiss T., Dolev A., Ben-Ari Y., Kronfeld-Schor N., Guter A., et al. (2012). Genetic diversity of the Eurasian otter (Lutra lutra) population in Israel and its implications for conservation. J. Hered. 104, 192–201. doi: 10.1093/jhered/ess094
Martins N. G., Sun X., Hennelly L., Lanigan L. T., Alemany C. F., Madrigal J. R., et al. (2024). Unraveling the golden jackal's genomic journey: insights into origin, expansion, and hybridization with other canids across Eurasia. iScience. doi: 10.2139/ssrn.4878887
Mayrose A., Vine G., Labinger Z., Steinitz O., Hazofe O., Haviv E., Perlman Y., Alon D., and Leader N. (2017). The Israeli Red List of Threatened Breeding Birds. The Society for the Protection of Nature in Israel and the Israel Nature and Parks Authority. https://redlist.parks.org.il/aves/;.
Mori C. and Matsumura S. (2021). Current issues for mammalian species identification in forensic science: a review. Int. J. Legal Med. 135, 3–12. doi: 10.1007/s00414-020-02341-w
Mori C. and Matsumura S. (2022). Development and validation of simultaneous identification of 26 mammalian and poultry species by a multiplex assay. Int. J. Legal Med. 136, 1–12. doi: 10.1007/s00414-021-02711-y
Münger X., Robin M., Dalén L., and Grossen C. (2024). Facilitated introgression from domestic goat into Alpine ibex at immune loci. Mol. Ecol. 33, e17429. doi: 10.1111/mec.v33.14
Munshi-South J., Zolnik C. P., and Harris S. E. (2016). Population genomics of the Anthropocene: Urbanization is negatively associated with genome-wide variation in white-footed mouse populations. Evol. Appl. 9, 546–564. doi: 10.1111/eva.12357
Nayak S. S., Rajawat D., Jain K., Sharma A., Gondro C., Tarafdar A., et al. (2024). A comprehensive review of livestock development: insights into domestication, phylogenetics, diversity, and genomic advances. Mamm. Genome. 35, 577–599. doi: 10.1007/s00335-024-10075-y
Nazareno A. G., Bemmels J. B., Dick C. W., and Lúcia G. L. (2017). Minimum sample sizes for population genomics: an empirical study from an Amazonian plant species. Mol. Ecol. Resour. 17, 1136–1147. doi: 10.1111/1755-0998.12654
Nellemann C., Henriksen R., Kreilhuber A., Stewart D., Kotsovou M., Raxter P., Mrema E., and Barrat S. (Eds.) (2016). “The rise of environmental crime – A growing threat to natural resources peace, development and security,” in A UNEPINTERPOL rapid response assessment. United nations environment programme and RHIPTO rapid response–norwegian center for global analyses (RHIPTO), 102. Available at: www.rhipto.org, ISBN: 978-82-690434-1-9.
Ogden R. (2011). Unlocking the potential of genomic technologies for wildlife forensics. Mol. Ecol. Resour. 11, 109–116. doi: 10.1111/j.1755-0998.2010.02954.x
Olea P. P., Fernández-García M., Vicente López-Bao J., Viñuela J., Valente e Santos J. P., Rodríguez-Pérez J., et al. (2022). Unraveling the real magnitude of illegal wildlife poisoning to halt cryptic biodiversity loss. Biol. Conserv. 273, 109702. doi: 10.1016/j.biocon.2022.109702
Ouso D. O., Otiende M. Y., Jeneby M. M., Oundo J. W., Bargul J. L., and Miller S. E. (2020). Three-gene PCR and high-resolution melting analysis for differentiating vertebrate species mitochondrial DNA for biodiversity research and complementing forensic surveillance. Sci. Rep. 10, 4741. doi: 10.1038/s41598-020-61600-3
Pakendorf B. and Stoneking M. (2005). Mitochondrial DNA and human evolution. Annu. Rev. Genomics Hum. Genet. 6, 165–183. doi: 10.1146/annurev.genom.6.080604.162249
Peakall R. and Smouse P. E. (2006). GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. notes 6, 288–295. doi: 10.1111/j.1471-8286.2005.01155.x
Puckett E. E., Park J., Combs M., Blum M. J., Bryant J. E., Caccone A., et al. (2016). Global population divergence and admixture of the brown rat (Rattus norvegicus). Proc. R. Soc B. 283, 20161762. doi: 10.1098/rspb.2016.176
Rattner D., Riviere J., and Bearman J. E. (1994). Factors affecting abortion, stillbirth, and kid mortality in the Goat and Yaez (Goat×ibex). Small Rumin. Res. 13, 33–40. doi: 10.1016/0921-4488(94)90028-0
Roca A. L., Bar-Gal G. K., Eizirik E., Helgen K. M., Maria R., Springer M. S., et al. (2004). Mesozoic origin for West Indian insectivores. Nature 429, 649–651. doi: 10.1038/nature02597
Schickhoff U., Bobrowski M., Offen I. A., and Suraj M. A. L. (2024). “The biodiversıty crisis in the anthropocene,” in Geography and the anthropocene. Eds. Gönençgil B. and Meadows M. E. (Istanbul University Press, Istanbul, Turkiye), 79–112.
Schleimer A., Richart L., Drygala F., Casabianca F., Maestrini O., Weigand H., et al. (2022). Introgressive hybridization between domestic pigs (Sus scrofa domesticus) and endemic Corsican wild boars (S. s. meridionalis): effects of human-mediated interventions. Hered 128, 279–290. doi: 10.1038/s41437-022-00517-1
Shemesh M. (2015). Development of genetic markers for differentiating domestic goat (Capra hircus) breeds in Israel: applications in parentage identification and forensics (Israel: The Hebrew University of Jerusalem).
Smart U., Cihlar J. C., and Budowle B. (2021). International Wildlife Trafficking: A perspective on the challenges and potential forensic genetics solutions. Forensic. Sci. Int. Genet. 54, 102551. doi: 10.1016/j.fsigen.2021.102551
Tichon Y. and Bar-David S. (2020). The Nubian ibex population in the Dead Sea region: demographic, genetic spatial structure and threat assessment.
Webster L. M., Prigge T. L., and Frankham G. J. (2024). A guide for the validation of DNA-based species identification in forensic casework. FSIAE 5, 100080. doi: 10.1016/j.fsiae.2023.100080
Yang L., Tan Z., Wang D., Xue L., Guan M. X., Huang T., et al. (2014). Species identification through mitochondrial rRNA genetic analysis. Sci. Rep. 4, 1–11. doi: 10.1038/srep04089
Appendix
Keywords: wildlife forensic, Nubian ibex (Capra nubiana), mountain gazelle (Gazella gazella), Eurasian griffon vulture (Gyps fulvus), illegal hunting, complex cases, poisoning
Citation: Kahila Bar-Gal G (2025) Beyond species identification: integrative methodologies for solving complex wildlife forensic cases. Front. Ecol. Evol. 13:1525957. doi: 10.3389/fevo.2025.1525957
Received: 10 November 2024; Accepted: 14 April 2025;
Published: 18 July 2025.
Edited by:
Chloe Hatten, The University of Hong Kong, ChinaReviewed by:
McDonald Shiri, Victoria Falls Wildlife Trust, ZimbabweTasha Bauman, Wyoming Game and Fish Department, United States
Copyright © 2025 Kahila Bar-Gal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gila Kahila Bar-Gal, Z2lsYS5rYWhpbGFAbWFpbC5odWppLmFjLmls
 Gila Kahila Bar-Gal
Gila Kahila Bar-Gal