Abstract
Understanding the cognitive and social foundations of healthcare behaviors in humans requires examining their evolutionary precursors in our closest living relatives. Investigating self-directed and other-directed healthcare in chimpanzees provides crucial insights into the origins of medicinal knowledge, identification of specific medicinal resources used for health maintenance, and the emergence of prosocial healthcare capacities. Here we document and analyze both previously reported and newly observed instances of self-directed and other-directed wound care, snare removal, and putatively medicinal hygiene behaviors in the Sonso and Waibira chimpanzee communities of the Budongo Forest in Uganda. Reports of these behaviors come from archival records collected from over thirty years of observation at the Budongo Conservation Field Station (BCFS), videos recorded by researchers at the site, and all-occurrence behavioral data collected over two 4-month periods of direct observation. We describe self-directed wound care behaviors such as wound licking, leaf-dabbing, pressing fingers to wounds, and the application of chewed plant material to wounds, as well as a successful self-directed snare removal. We also document self-directed hygiene behaviors including postcoital genital leaf wiping and post-defecation leaf wiping. For the first time in Budongo, we report the presence of prosocial wound care, adding to similar observations documented at other chimpanzee field sites. We present cases of individuals licking, finger pressing, and applying chewed plant material to the wounds of others. We also establish the presence of prosocial postcoital hygiene behaviors, specifically postcoital leaf wiping. Lastly, we report an additional unpublished case of prosocial snare removal. The presence of prosocial-care behaviors between both kin and non-kin individuals at Budongo adds another site to the growing list of locations where such behaviors have been documented, suggesting prosocial healthcare is more widespread across chimpanzee populations than previously recognized.
Introduction
Many species throughout the animal kingdom are capable of self-directed healthcare behaviors (Kessler and Aunger, 2022), otherwise known as self-medication. Non-human self-medication refers to self-directed actions performed by an individual from another species to treat an illness or injury and/or prevent future infection (Huffman, 2016). The study of non-human self-medication is also known as ‘Zoopharmacognosy’ (Huffman, 1997; Rodriguez and Wrangham, 1993). Many species across taxa have been reported to engage in self-medication, from Indo-Pacific bottlenose dolphins (Tursiops aduncus; Morlock et al., 2022) and Chinese lesser civets (Viverricula indica; Su et al., 2013) to African crested porcupines (Hystrix africaeaustralis; Huffman, 2022) and brown bears (Ursus arctos; (Blaise et al., 2023).
More surprising, perhaps, is that several species have also been found to engage in other-directed care behaviors (see Kessler and Aunger, 2022 for review), exhibiting prosocial tendencies. We use ‘prosocial’ here to refer to behaviors performed by an individual with the intent of benefitting another (van Leeuwen et al., 2021). Other-directed healthcare behaviors have been observed in several non-human animals including, amongst others: elephants (Loxodonta sp.; Douglas-Hamilton et al., 2006), dwarf mongooses (Helogale parvula;Rasa, 1983), lions (Panthera leo;Schaller, 2009), toque macaques (Macaca sinica; Dittus and Ratnayeke, 1989), and Florida carpenter ants (Camponotus floridanus; Frank et al., 2024). These prosocial healthcare behaviors, often related to wound care, occur most commonly between close kin (Kessler and Aunger, 2022). However, in a few notable species, including our two closest living relatives, the chimpanzee (Pan troglodytes) and bonobo (Pan paniscus), prosocial healthcare behaviors directed toward genetically distant or apparently unrelated individuals have also been documented (e.g., Mascaro et al., 2022; Amati et al., 2008; Tokuyama et al., 2012).
The ability to improve one’s own health or the health of a close family member has obvious evolutionary advantages, allowing related individuals to live longer and increase reproductive success, which assists in the survival of shared genes (Hart, 2011; Huffman, 2001; Kessler and Aunger, 2022). Healthcare behaviors directed at genetically unrelated or distantly related individuals may, however, require additional evolutionary mechanisms beyond inclusive fitness alone (Kessler and Aunger, 2022). This has led some to question whether factors such as empathy and altruism (which themselves may have evolved through natural selection) directed towards unrelated members of the same social unit or group could be driving these behaviors (e.g., Pruetz, 2011; Brooker et al., 2024). To understand the evolutionary origins of our own healthcare behaviors as well as to assess their potential uniqueness, it is crucial to investigate the self-care and prosocial healthcare behaviors of closely related species. Here we focus on the healthcare behaviors of the chimpanzee, one of our closest, extant, non-human relatives.
Framework for non-human healthcare systems
Most living beings co-evolve with pathogens in their environments (Kessler and Aunger, 2022). This dynamic has led to the emergence of complex behavioral defenses throughout the animal kingdom (Hart, 1988). When these defenses are directly employed to combat illness or infection they are referred to as ‘healthcare behaviors’ by Kessler and Aunger (2022). Amongst humans, healthcare behaviors are uniquely complex, with institutionalized systems like hospitals and government programs designed to elevate general health both indirectly and directly across a population. But what are the origins of these complex healthcare behaviors? In the evolutionary framework Kessler and Aunger (2022) created to better understand the origins of human and non-human healthcare systems, they outline two categories: ‘care behaviors’ and ‘community-health behaviors.’ Care behaviors in this context refer to actions that benefit the health of a targeted sick, injured, or vulnerable individual (e.g., providing medicine directly to a patient). Community-health behaviors refer to indirect actions which generate general benefits for the group without targeting specific individuals (e.g., latrine use to maintain a pathogen-free environment or self-isolation to reduce transmission of illness). However, in this study we focus only on care behaviors, as community-health behaviors have not yet been identified in non-human primates.
We elaborate on Kessler and Aunger’s framework by further defining within care behaviors: i) self-directed care (self-care) referring to behaviors undertaken by individuals to enhance their own health states, and ii) other-directed care (prosocial-care) referring to other-directed behaviors that enhance the health states of others without immediate physical benefits to the caregiver. Within the category of prosocial-care, we identify three additional sub-categories based on the relationship between the caregiver and recipient (Kessler and Aunger, 2022). Kessler and Aunger name two such relationships: kin-care (prosocial-care directed at genetically related kin) and stranger-care (care directed at unrelated and unfamiliar out-group individuals). We add a new relationship, non-kin-care, used to describe prosocial-care behaviors which occur between genetically distant, but familiar group members. Finally, within non-kin-care, we recognize the need to create two additional sub-categories: affiliate care, used here to describe non-kin group-members who are established social partners based on measures of affiliation such as proximity, associations, and grooming (e.g., Mitani et al., 2000), and non-affiliate care, describing non-kin group members who have weaker or less consistently positive social bonds. For scope, we do not directly address affiliate and non-affiliate care in this study; however, we encourage their consideration in future frameworks. A flowchart of our theoretical framework is shown in Figure 1.
Figure 1
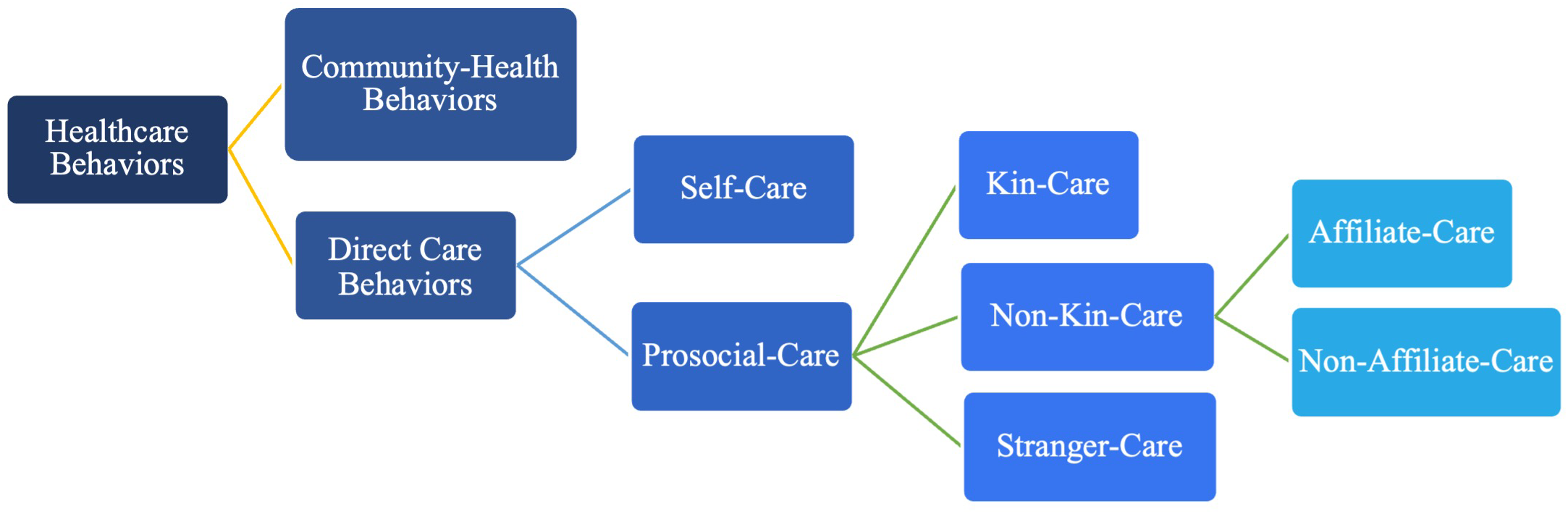
Adapted and modified from Kessler and Aunger (2022), showing hierarchical relationships between the terms used in this study.
Our integrated analysis of both self-care and prosocial-care behaviors in this study is theoretically motivated. Self-care provides a critical baseline for understanding prosocial-care because: 1) similar techniques and resources are often employed in both contexts, and 2) comparing the relative prevalence of these behaviors offers insights into the cognitive and social factors that might facilitate or inhibit prosocial healthcare. This integrated approach enhances our understanding of healthcare evolution in our closest living relatives.
Chimpanzee sociality
Chimpanzees are a highly social species, living in communities or unit groups with defined territorial boundaries and social composition (Mitani and Watts, 2005; Nishida, 1968; Wilson and Wrangham, 2003). Chimpanzees live in communities that show high fission‐fusion dynamics, in which subgroups of changing size and composition regularly form and reform throughout the day (Koops et al., 2024; Kummer, 1971). As offspring remain dependent on their mothers until they are ~10 years old (Boesch and Boesch-Achermann, 2000; Samuni et al., 2020), mother–offspring and maternal-sibling kinship relationships have been relatively well-documented in habituated chimpanzee communities (Langergraber et al., 2007; Péter et al., 2022; Reddy and Mitani, 2019). Paternity, however, is impossible to confirm without genetic testing, as chimpanzee females practice a ‘promiscuous’ mating strategy (Newton-Fisher, 2014; Watts, 2015). Female chimpanzees typically migrate upon reaching sexual maturity, while males are exclusively philopatric (Boesch and Boesch-Achermann, 2000; Luncz and Boesch, 2014). Given this structure, fathers of maternal kin are likely from the same community, while mothers of paternal kin are likely from different communities.
Self-care in chimpanzees
The best studied ingestion-based self-medicative behaviors in free-ranging chimpanzees are leaf swallowing (e.g., Huffman et al., 1996; Wrangham and Nishida, 1983) and bitter-pith chewing (Huffman and Seifu, 1989; Huffman et al., 1993). Both behaviors have been found to have anthelminthic effects (Huffman, 2016), therapeutically ameliorating the medicator’s internal parasite infections. Several other plants have been proposed as medicinal resources for chimpanzees (De la Fuente et al., 2022; Freymann et al., 2024a, 2024b; Pebsworth et al., 2006), as have certain soils and clays (Pebsworth et al., 2019).
Self-directed wound care has also been documented across many chimpanzee sites (Clark et al., 2021; Watts, 2008; Goodall, 1983; Sanz and Morgan, 2007; Mascaro et al., 2022), with reported examples including: 1) wound licking, 2) finger licking and pressing to wound, 3) leaf-dabbing, and 4) application of chewed organic matter to wounds (Table 1).
Table 1
| Behavior | Definition | Hypothesized function(s) | Reference |
|---|---|---|---|
| Lick wound | Licking wound directly with tongue. | Cleans or sterilizes wound by removing dirt or from antimicrobial properties of saliva. | Clark et al., 2021; Hart and Powell, 1990 |
| Lick finger and press to wound | Licking finger and pressing or applying it to wound. May then lick finger and repeat. | Cleans or sterilizes wound by removing dirt or from antimicrobial properties of saliva. | Clark et al., 2021 |
| Leaf-dab | Wiping or dabbing wound with single or multiple leaves, which are then usually sniffed and dropped, but may be licked and reused. | Cleans wound by removing dirt or could be purely exploratory. | Clark et al., 2021; Goodall, 1983 |
| Apply chewed organic material to wound | Masticating organic material (i.e., leaves or insects), and placing lips on wound with material still in mouth. | Promotes wound healing through possible pharmacological properties. | Mascaro et al., 2022 |
Definitions of self-directed wound care behaviors in wild chimpanzees.
The most commonly reported wound care behavior in chimpanzees is wound licking. The exact function of wound licking in this species has not yet been established, however, as reported for other animals, licking likely removes dirt and fly eggs and sterilizes wounds (Hart and Powell, 1990). Antimicrobial properties have been found in the saliva of many non-human animals, which may also facilitate more rapid wound-healing (Hart and Powell, 1990). Self-directed leaf-dabbing has thus far been reported in fewer sites, including Kibale, Uganda (Clark et al., 2021; Watts, 2008), Gombe, Tanzania (Goodall, 1983), and Goualougo, DRC (Sanz and Morgan, 2007), and, when present, appears to be observed infrequently (Clark et al., 2021). This behavior was also reported by Whiten et al. (1999) on their list of possible cultural variants. However, in their study, ‘leaf-dab’ was originally reported as absent amongst the chimpanzees of the Budongo Forest in Uganda. More recently, a new wound care behavior was reported in Loango National Park in Gabon, where chimpanzees were observed applying chewed insects to their wounds (Mascaro et al., 2022). While the species of insects remain unspecified, the authors suggest that these insects may have pharmacological properties that could facilitate wound healing or combat infection. This discovery was followed by a report of a Sumatran orangutan in the Suaq Balimbing research area in Indonesia applying chewed leaves of a medicinal plant (Fibraurea tinctoria) to a wound, which subsequently healed (Laumer et al., 2024). These cases suggest that the application of chewed organic material with medicinal properties is a medicinal wound care strategy that is likely present across great ape species. Across several sites, chimpanzees have also been observed wiping their genitals with leaves after copulation. This behavior has been hypothesized to be a hygienic behavior with a putative medicinal function, specifically for assessing or preventing the spread of sexually transmitted diseases or removing harmful external contaminants (O’Hara and Lee, 2006). This behavior has been reported at several chimpanzee long-term field sites including Budongo (O’Hara and Lee, 2006), Kibale (McGrew, 1992), and Gombe (Goodall, 1986).
Prosocial healthcare in chimpanzees
In captive chimpanzees, prosocial healthcare has long been established, with early observations of individuals helping others to remove splinters (Köhler, 1948; Yerkes, 1943). McGrew and Tutin (1972) similarly reported teeth cleaning and assisted tooth removal between chimpanzees in captivity. However, in the wild, reports of prosocial wound care amongst chimpanzees remain relatively rare, with only a few cases reported from sites including Gombe (Goodall, 1986), Taï, Côte d’Ivoire (Boesch, 1991), Kibale (Clark et al., 2021), Fongoli, Senegal (Pruetz, 2011), Mahale, Tanzania (Nishida, 2011) and, most recently, Loango (Mascaro et al., 2022). In most of these cases, reports involve an uninjured individual grooming or licking the wounds of related or unrelated individuals, although other-directed leaf-dabbing and application of insects to wounds have also been reported (Goodall, 1986; Clark et al., 2021; Mascaro et al., 2022). Prosocial healthcare in the form of assisted snare removal (Amati et al., 2008) has also been documented in chimpanzees. This behavior is not unique to chimpanzees, as bonobos have also been observed attempting to free a group member from a metal snare in Wamba, DRC (Tokuyama et al., 2012).
Prosocial healthcare behaviors in chimpanzees were originally suggested to occur only between directly-related maternal kin (i.e. mother–offspring, or maternal siblings; Goodall, 1986). However, recent findings have established the presence of other-directed wound care through leaf-dabbing (Clark et al., 2021) and the application of insects (Mascaro et al., 2022) between group members who are not known to be closely related. In Budongo, an adult male individual was observed helping an unrelated adult female remove a nylon snare (Amati et al., 2008; see section ‘Prosocial-care’ below). There are also reports of indirect forms of prosocial healthcare behaviors in chimpanzees. These include cases in which individuals provide indirect physical assistance to unrelated group members. In Fongoli, for example, Pruetz (2011) reported an adolescent male carrying the infant of an unrelated, injured female for two days until she recovered. There are also many documented cases of individuals adopting and caring for non-kin orphans (e.g., Boesch et al., 2010).
While chimpanzees are capable of at least two sub-categories of prosocial-care (kin-care and non-kin-care), the presence and frequency of these behaviors appear to vary between sites. Although the reason for this cross-site variation is still unknown—and may stem from observation opportunities and reporting biases—other factors such as predation risks, anthropogenic disturbances, and/or degree of social cohesion within the community may contribute to group differences in these behaviors (Boesch, 1991).
Healthcare behaviors in the Budongo Forest
While evidence of ingestion-based therapeutic healthcare behaviors has been identified amongst chimpanzees in the Budongo Forest (e.g., Freymann et al., 2024a, 2024b, 2024c), in this study we limit our evaluation to behaviors related to topical or external conditions. Specifically, we report and analyze behaviors related to wound care, snare removal, and hygiene behaviors with putative medicinal value in two communities of free-ranging Eastern chimpanzees (Sonso and Waibira) living in the Budongo Forest, Uganda. For scope, we do not include cases of self-grooming or social-grooming in this study, as these hygiene behaviors occur far more frequently than the other behaviors included here, are already well-documented, and have established non-hygiene related social functions including promoting intra-group alliances and strengthening group cohesion (e.g., Massen et al., 2010; Schino, 2007; Schino et al., 1988).
Wounds and injuries present a major health threat to chimpanzee communities in the Budongo Forest Reserve (Reynolds, 2005). Amongst the Sonso and Waibira communities, severe injuries are caused by intergroup and intragroup aggressions, encounters with other animals (i.e., bushpigs and colobus monkeys), and—most commonly—human-set snares (Fedurek et al., 2022). Snares, which are primarily intended for duiker and bushbuck, are typically made from wire or nylon, although large steel-jawed ‘mantraps’ can be found in nearby forest-edge areas (Reynolds, 2005). Snare-injured individuals in Budongo often develop problems with blood circulation, infections, necrosis, paralysis, mutilation, and/or heightened intestinal parasite loads (Yersin et al., 2017).
Amongst the Sonso and Waibira chimpanzee communities, self-directed leaf-dabbing behaviors have been reported (Lamon et al., 2018; Reynolds, 2005) but have not yet been systematically described. Self-directed hygiene behaviors, in the form of postcoital genital wiping, have been reported in the Sonso community, with 11 reported cases of postcoital genital wiping with leaves, and 29 reported cases of postcoital genital wiping with fingers (O’Hara and Lee, 2006). These observations are not re-reported in this study. A single case of prosocial snare removal (Amati et al., 2008) has thus far been the only published report of prosocial healthcare at this site, with no published cases of prosocial wound care or hygiene behaviors.
This study contributes to the literature on chimpanzee healthcare behaviors in three significant ways. First, we provide the first comprehensive documentation of both self-directed and prosocial healthcare behaviors in Budongo chimpanzees, compiling data systematically from multiple sources spanning over three decades (1993–2024). We also contextualize these findings with data on the types and frequencies of wounds and injuries observed during direct observational periods to better examine healthcare behaviors in relation to the actual health challenges these communities face. Our approach is systematic in that it combines historical records, researcher anecdotes, video archives, and direct observational data collected using standardized protocols, allowing for a more complete picture of these behaviors. Second, we identify and catalog the specific plant species used in healthcare contexts and connect these to their known ethnomedicinal uses and known pharmacological properties, providing insights into potential medicinal functions. Third, by documenting both kin-directed and non-kin-directed prosocial healthcare in Budongo, our findings add to the growing evidence that prosocial care, particularly toward non-kin, is more widespread across chimpanzee populations than previously recognized. These findings collectively advance our understanding of the evolutionary foundations of healthcare behaviors in our closest living relatives.
Materials and methods
Study site and subjects
Established by Vernon Reynolds in 1990, the Budongo Conservation Field Station (BCFS) is located within the Budongo Forest, Uganda. Within the forest, two East African chimpanzee communities, Sonso and Waibira have been habituated for research. These communities consisted of ~68 and ~105 identified individuals, respectively at the time of data collection. Sonso’s habituation began in 1990 (Reynolds, 2005), while Waibira’s began in 2011 (Samuni et al., 2014). During a recent assessment (2015–2019) Sonso’s home range was ~5.33 km2 and Waibira’s was 10.28 km2 (Badihi et al., 2022). The Budongo Conservation Field Station area is populated by continuous, semi-deciduous forest cover (Eggeling, 1947), with temperatures remaining relatively consistent throughout the year, ranging from 19°C to 32°C (Freymann et al., 2024a). Rainfall ranges between 1,200 and 2,200 mm and exhibits a bimodal pattern, with two wet seasons between March–May and September–November, a primary dry season between December and February, and an intermediate period with moderate rainfall between June and August (Freymann et al., 2024a; Reynolds, 2005; Soldati et al., 2022).
Data collection
In this study we exploited multiple datasets to collate observed but unpublished cases of wound care, snare removal, and relevant hygiene behaviors. To add to these reports, we also employed several data collection methods for two 4-month periods of direct observation to systematically identify novel cases.
Site logbook
Researchers and field staff monitor chimpanzees in Sonso daily from 07:00 to 16:30 and in Waibira from 06:30 to 17:00. Field staff at BCFS collect focal sampling data and party composition scans conducted every 15-minutes. Noteworthy events are documented in community-specific logbooks. In this study, we included entries from the Sonso community’s logbook recorded between 1993 and 2021 which involved cases of external healthcare behaviors. We also included cases involving hygiene behaviors which have putative medicinal functions, or which involve the use of organic resources. In Waibira, daily summaries and noteworthy events are also recorded in a logbook; however, these data were not considered for this study as they are not yet digitized.
Collating site-specific self-medication anecdotes
The Chimpanzee Self-Medicative Anecdote Survey (CSMAS), hosted on Jisc Online Surveys, was activated and accepting entries from December 25, 2021, until September 25, 2022. The survey was advertised on X (formerly Twitter). More information on CSMAS, including criteria for submission, can be found on the host site: https://primobevolab.web.ox.ac.uk/article/chimpanzee-self-medicative-anecdote-database-survey. The survey’s purpose was primarily to collect anecdotes from different field sites to obtain additional information on cases of chimpanzee self-medication and to promote collaboration among researchers. CSMAS specifically called for cases of ingestion-based self-medication events, though space was provided for participants to submit cases involving topical or external healthcare behaviors. Any researchers who were currently working with or had worked with wild chimpanzees were eligible to participate. This study includes all anecdotes submitted to CSMAS involving topical and external healthcare behaviors from Budongo. In addition to CSMAS, researchers working at BCFS between 2021 and 2023 were invited to submit anecdotes on a voluntary basis.
Great Ape Dictionary video archive
The study also used data from the Great Ape Dictionary (GAD) video database (Hobaiter et al., 2021), which contains ~35,000 catalogued video clips, updated regularly. The videos have basic meta-data, including location, date, duration, individuals present, and notable behaviors. The Budongo chimpanzee population contributed 13,806 videos to the current version (1.0.0) of the database. For this study, we only included videos from Budongo marked as including cases of visible wound care.
Direct observational period
The study employed identical methods throughout two 4-month field periods of direct observation, with the first period in Sonso (June–October 2021) and the second in Waibira (June–October 2022), using identical methods, to ensure a comparable dataset. We used pseudo–random focal selection, with focal individuals selected each morning based on prioritization criteria (Hobaiter et al., 2017), including parasite load or health state, presence of sickness behaviors, recent unusual feeding behaviors, or inclusion in a dependent dyad (mother–infant/adopter–adoptee). If no individuals met the criteria in the party encountered in the morning, a focal was selected randomly. Behavioral data were recorded for focal individuals, and events of interest were noted ad libitum (Altmann, 1974). Individual health states were recorded opportunistically each day for all observed community members, including female estrous status, presence of sickness behaviors (including diarrhea, lethargy, day-nesting, and audible respiratory symptoms), and any visible wounds. All ad libitum observations of wound care and hygiene behaviors were recorded.
Age classes in this study were defined as follows: infant (0–4 years), juvenile (5–9 years), subadults (♀: 10–14 years; ♂: 10–15 years), and adults (♀: 15+; ♂: 16+; Reynolds, 2005). We considered individuals to be ‘kin’ if they were part of a direct maternal/paternal lineage or siblings related through parents, following Clark et al. (2021). Individuals were considered ‘non-kin’ if they were members of the same community, but not directly ‘kin’. Kinship relationships at the site were determined through genetic testing, although paternal kinships are more thoroughly established in Sonso than in Waibira. It remains unknown whether chimpanzees can recognize paternal kin, though there are reasonable data to suggest recognition is possible to a limited extent (Péter et al., 2022).
Plant collection and identification
Plants used by chimpanzees for suspected self-medication during direct observations were collected and identified by members of the BCFS phenology team. The current scientific names of each species were confirmed on https://powo.science.kew.org/ as of August 2024. Plant family assignments were done in accordance with the Angiosperm Phylogeny Group IV guidance (Angiosperm Phylogeny Group et al., 2016).
Permits and permissions
Data used in this study were collected with the approval of the Uganda Wildlife Authority (UWA). Data recorded during the direct observational period (June–October 2021/2022) were collected under UWA permit no. COD/96/05 and Uganda National Council for Science and Technology (UNCST) permit no. NS257ES. During the direct observational period, we adhered to the guidelines for best practice in field primatology (MacKinnon et al., 2014). All applicable international and national guidelines were followed. The authors report no conflict of interest.
Data availability
Videos of some events are available in the Supplementary Materials.
Results
Wounds and injuries
In Sonso, 12 injuries (♀ = 2; ♂ = 10) were documented over the 4-month direct observational period (mid-June to mid-October 2021), all of which were most likely caused by intragroup aggressions. In two cases, fresh wounds were observed on individuals the day of, or immediately following, infanticides. In one of these cases, wounds were found on the mother (UP) of the deceased infant. In the other, wounds were found on a high-ranking male (PS), whose role in the infanticide remains unknown. In another case, the aftermath of an intragroup aggression event was observed. During this event, an adult female, (KG), was badly beaten by several males, resulting in severe wounds on her vulva. No individuals with newly acquired open wounds or injuries caused by snares were documented during the study period.
In Waibira we observed five injured individuals (♀ = 1; ♂ = 4) over a 4-month period (mid-June to mid-October 2022). The most severe of these injuries was caused by a wire snare, attached to a dependent juvenile female (PAV). When this injury was first observed, the snare was still attached to PAV’s right foot, which appeared swollen and infected. After the initial sighting, PAV and her mother (PEN) were not seen again for a prolonged period. PEN re-joined the group without PAV 84 days later, at which point PAV was assumed to have died from her wounds. The four other reported injuries in Waibira involved adult males (LKU, MAC, ALF, ILA) and at least three of these likely resulted from intragroup aggressions. All wounds observed throughout both study periods are listed and characterized in Supplementary Table S1.
Healthcare behaviors in Budongo
From our combined databases, we report 23 cases of self-directed wound care, one case of self-directed snare removal, and ten cases of putatively medicinal hygiene behaviors across both chimpanzee communities. We included a case of self-directed post-defecation leaf wiping as a putatively medicinal hygiene behavior due to its similarity in form to postcoital genital wiping and its use of organic material, with potential functions of maintaining cleanliness and preventing infection. We also report four cases of prosocial wound care, three cases of prosocial snare removal, and one putatively medicinal prosocial hygiene behavior across both communities.
Self-care
From historic data and our observations, we report 34 total cases of self-care at Budongo (Table 2), 23 of which are wound-related (Sonso: 22; Waibira: 1). Many cases reported here involved multiple self-directed wound care behaviors within a single event. Specific self-directed wound care behaviors documented in Sonso included licking fingers and pressing them to wounds, direct wound licking, leaf-dabbing, and application of chewed leaves to wounds. An example of an observation involving both leaf-dabbing and application of chewed plant material to a wound is shown in Figure 2 (see Supplementary Video S5). In the single observation of self-directed wound care in the Waibira community, we observed finger licking, pressing fingers to a wound, and leaf-dabbing (Table 2: Observation 18). Self-directed snare removal attempts have thus far only been observed in Sonso (Sonso: 1; Waibira: 0), as have self-directed post-defecation leaf wiping (Sonso: 1; Waibira: 0). Of the nine cases of self-directed postcoital penis and vaginal leaf wiping, only one case was observed in Waibira (Sonso: 8; Waibira: 1). Overall, 21/34 (62%) of these events involved the use of plant material.
Table 2
| Self-care type | Event # | Community(S/W) | Date(M/D/Y) | Individual (age category, sex) | Wound location/cause | Description | Plant species (plant part) | Observer(s) |
|---|---|---|---|---|---|---|---|---|
| Wound care: Finger lick & wound press | 1 | S | 9/10/2015 | KT (adult, male) | Unknown/suspected intragroup aggression | Licked fingers and pressed large wound on left side of back. | none | Events Book |
| 2 | S | 9/8/2021 | KC (subadult, male) | Ear/unknown | Licked finger and pressed wound. | none | Current Study Supplementary Figure S3; Supplementary Video S1 | |
| 3 | S | 4/23/1998 | ZF (adult, male) | Puncture on left calf/unknown | Licked wound while being groomed by KW (adult, female). | none | Events Book | |
| Wound care: Wound lick | 4 | S | 11/2002 | JM (adult, male) | Neck/unknown | Picked and licked bloody scabs from raw wound. | none | Events Book |
| 5 | S | 2/2/2003 | KN (infant, female) | Right foot/snare | Licked and touched snare wound. | none | Events Book | |
| 6 | S | 12/13/2008 | JN (adult, female) JT (subadult, female) | JN: Back and finger JT: Right hand/unknown | Both individuals licked bleeding wounds. | none | Events Book | |
| 7 | S | 2/27/2011 | ZM (adult, female) | Hand, forearm/intragroup aggression | Licked bleeding wound after being aggressed by NK (adult, male). | none | GAD | |
| 8 | S | 8/25/2011 | NT (juvenile, female) | Right hand/wire snare | Licked and touched snare wound. | none | GAD | |
| 9 | S | 7/9/2012 | SQ (adult, male) | Face/unknown | Picked scab from near eye and ate it. | none | GAD | |
| 10 | S | 1/23/2015 | ZG (adult, male) | Unknown/intragroup aggression | Licked wound after being injured by KT (adult, male). | none | Events Book | |
| 11 | S | 6/26/2021 | HW (adult, male) | Arm/unknown | Licked wound on lower arm. | none | Current Study Supplementary Figure S1; Supplementary Video S2 | |
| 12 | S | 8/30/2021 | UP (adult, female) | Arm/suspected intragroup aggression related to infanticide | Licked wound while holding mummified carcass of dead infant. | none | Current Study Supplementary Figure S2; Supplementary Video S3 | |
| Wound care: Wound lick & Leaf-dab | 13 | S | 2/5/2014 | MS (adult, male) | Right foot/intragroup aggression | Licked wound and then dabbed with leaves. | Pseudospondias microcarpa (leaves) | Events Book |
| 14 | S | 7/22/2008 | FK (juvenile, male) | Swollen Eye/unknown | Detached leaf and dabbed eye, then discarded. Nearby juvenile (PS) picked up leaf and smelled/licked it. | Unidentified sp. (leaves) | GAD | |
| Wound care: Leaf-dab | 15 | S | 8/22/2011 | FK (adult, male) | Face/unknown | Detached branch of leaves, folded in mouth, dabbed on forehead to stop bleeding. | Unidentified sp. (leaves) | GAD |
| 16 | S | 12/19/2019 | KC (subadult, male) | Wrist/wire snare | Detached branch of leaves from tree and dabbed the end slowly to open wound on snared wrist. | Unidentified sp. (leaves) | CSMAS | |
| 17 | S | 4/26/2022 | MB (juvenile, male) | Left side of body/unknown | Detached leaf, licked and chewed leaf, dabbed on wound. | Unidentified sp. (leaves) | Researcher Observation | |
| Wound care: Leaf-dab + Finger lick & wound press | 18 | W | 05/27/2023 | ALF (adult, male) | Left foot | Detached leaf from shrub, licked leaf and leaf-dabbed with first partial, then whole leaves. Licked fresh leaves, chewed, and then finger licked and wound pressed. Repeated with old leaves from forest floor. | Argomuellera macrophylla (leaves) | Researcher Observation Supplementary Video S4 |
| 19 | S | 11/20/2008 | NB (adult, female) | Vagina/intragroup aggression | Detached leaf and applied to wounded area. Folded leaf and carefully chewed it, then dabbed repeatedly on wound. Also seen licking fingers and pressing them to wound. | Alchornea floribunda(?) (leaves) | GAD | |
| 20 | S | 2/20/2022 | KX (adult, female) | Head/unknown | Detached leaf and dabbed on wound, repeatedly licking and chewing it between dabs. Minutes later, licked fingers and pressed them to wound. | Lasiodiscus pervillei (leaves) | Researcher Observation | |
| Wound care: Apply chewed material to wound & Wound lick + Leaf-dab | 21 | S | 10/13/2021 | KO (juvenile, male) | Left knee/unknown | Chewed stem bark and then applied to wound with mouth. Licked wound, and dabbed leaves of same species on wounded left knee. | Argomuellera macrophylla (stem bark + leaves) | Current Study Supplementary Video S5 |
| 22 | S | 2/22/2002 | MA (adult, male) | Left leg + left hand/suspected intragroup aggression | Licked wound, picked up leaves, chewed them and then applied material to the wound. Licked wound again, then leaf-dabbed three times before dropping leaves and traveling away. | Acalypha sp. (leaves) | Events Book (Also reported by Reynolds, 2005). | |
| Wound care: Apply chewed material to wound | 23 | S | 10/6/2021 | PS (adult, male) | Right arm/unknown | Detached leaf from shrub, chewed leaf, and applied material to wound. | Unidentified sp. (leaves) | Current Study Supplementary Figure S4; Supplementary Video S6 |
| Snare removal | 24 | S | 11/25/2015 | KT (adult, male) | Unknown/wire snare | Removed his own snare. | none | Events Book |
| Hygiene: Postcoital genital leaf wipe | 25 | S | 7/14/2009 | ZD (juvenile, male) | Penis/copulation | Wiped penis with leaves after copulation. | Unidentified sp. (leaves) | GAD |
| 26 | S | 1/30/2011 | ZM (adult, female) | Vagina/copulation | Wiped vagina with leaves after copulation. | Unidentified sp. (leaves) | GAD | |
| 27 | S | 2/14/2011 | FD (adult, male) | Penis/copulation | Wiped penis with leaves in tree after copulation and then dropped them. | Unidentified sp. (leaves) | GAD Supplementary Video S7 | |
| 28 | S | 8/22/2011 | KM (subadult, female) | Vagina/unknown | Wiped vagina with leaves and sniffed/licked it. Unknown whether this occurred after copulation. | Unidentified sp. (leaves) | GAD | |
| 29 | S | 7/14/2012 | FK (adult, male) | Penis/copulation | Wiped erect penis with leaves after copulation. | Unidentified sp. (leaves) | GAD | |
| 30 | S | 10/6/2021 | ZL (adult, male) | Penis/copulation | Broke branch off, wiped penis with leaves after copulation, and then licked leaves. | Lasiodiscus pervillei (leaves) | Current Study Supplementary Video S8 | |
| 31 | W | 9/30/2022 | ALF (adult, male) | Penis/copulation | Used leaves from unknown tree to wipe penis after copulation while in branches. | Unidentified sp. (leaves) | Current Study | |
| 32 | S | 7/28/2022 | MS (adult, male) | Penis/copulation | Wiped penis with attached leaves after copulation, and then smelled leaves. | Argomuellera macrophylla (leaves) | Researcher Observation | |
| 33 | S | 1/14/2023 | KJ (juvenile, male) | Penis/copulation | Wiped penis with leaves after copulation. | Argomuellera macrophylla (leaves) | Researcher Observation | |
| Hygiene: Post-defecation leaf wipe | 34 | S | 3/8/2009 | KU (adult, female) | Anus/defecation | Used leaves from unknown species to wipe anus after defecating. | Unidentified sp. (leaves) | Events Book |
Observations of self-directed external care behaviors in Budongo.
(?), Species suspected but unconfirmed.
S, Sonso; W, Waibira.
NB: See Supplementary Material for list of contributing observers; See O’Hara and Lee (2006) for formerly published cases of postcoital genital wiping at Budongo.
Figure 2

(Table 2: Observation 21) (Supplementary Video S5) [Top left] KO chews stem bark of A. macrophylla. [Top right] KO detaches leaves from stem and dabs on wounded knee. [Bottom left] KO applies chewed stem bark to wound. [Bottom right] KO leaf-dabs with attached leaves.
Prosocial-care
In Sonso, we report the presence of both kin-care and non-kin-care in external healthcare contexts. Prosocial wound care was not directly observed during the study period but has previously been observed in Sonso by field staff and researchers. Table 3 summarizes the seven recorded events of prosocial healthcare in Sonso: two cases of assisted snare removal, four cases of prosocial wound care, and one case of prosocial postcoital penis wiping. The sexes of care recipients and providers vary across cases, with three involving a male caring for a female, one involving a female caring for a male, two involving a female caring for a female, and one involving a male caring for a male (Figure 3). Of these seven cases, there were three instances of kin-care, two of which occurred between the same mother and daughter pair (Table 3: Observations 2, 4). In the first case, it is suspected that NB provided physical assistance to her juvenile daughter (NT) who was stuck in a wire snare. In the second case, NT observed and copied her mother’s self-directed wound behaviors, folding and chewing leaves of a plant suspected to be Alchornea floribunda, and dabbing them onto NB’s wound. NT also licked her fingers and applied them to NB’s wound (Supplementary Video S9). In the third case, an adult male (ZG) licked the wound of his maternal sibling, an adult female (KY). Of the remaining cases, four are classified as non-kin-care. We report no cases of prosocial healthcare behaviors in Waibira. Across all prosocial healthcare events reported here, 2/7 (29%) involved the use of plant material.
Table 3
| Social-care type | Event # | Community (S/W) | Date (M/D/Y) | Caregiver→Receiver (age category, sex) | Relationship | Wound location/cause | Description | Species used | Observer |
|---|---|---|---|---|---|---|---|---|---|
| Snare removal | 1 | S | 7/18/2008 | NK (adult male)→ KW (adult, female) | Non-kin (M–F) | Unknown/ nylon snare | NK helped remove a nylon snare from KW. | none | Events Book Also reported in (Amati et al., 2008) |
| 2 | S | 3/11/2009 | NB (adult, female)→ NT (juvenile, female) | Kin (F–F) (Mother–Offspring) | Unknown/ nylon snare | NB (mother) seen chewing nylon snare after NT (daughter) got hand stuck. Suspected that she bit nylon from NT’s hand. | none | Events Book | |
| Wound care: Wound lick | 3 | S | 8/13/2003 | ZG (adult, male)→ KY (adult, female) | Kin (M–F) (Maternal Siblings) | Leg/colobus attack | After an attack, KY licked her wound and leaf-dabbed. ZG approached and licked blood from KY’s cut. | none | Events Book |
| Wound care: Apply chewed material to wound + Finger lick & wound press | 4 | S | 11/20/2008 | NT (juvenile, female)→NB (adult, female) | Kin (F–F) (Mother–Offspring) | Vagina (swelling)/intragroup aggression | After an attack, NB began applying a folded and chewed leaf to the wound. NT watched and then copied her mother, chewing a leaf and applying it and then licking her fingers and pressing them to NB’s wound. | Alchornea floribunda(?) (leaves) | GAD Supplementary Video S9 |
| Wound care: Wound lick | 5 | S | 3/8/2012 | PS (subadult, male)→ ZG (subadult, male) | Non-kin (M–M) | Leg/unknown | PS sucked a deep cut on ZG’s leg. | none | Events Book (Figure 3) |
| Wound care: Wound lick | 6 | S | 9/5/2018 | HW (adult, male)→ RS (adult, female) | Non-kin (M–F) | Unknown/ intragroup aggression | HW licked a wound on RS after she was aggressed with her new baby. | none | Events Book |
| Hygiene: Postcoital genital leaf wipe | 7 | S | 11/26/2007 | NR (adult, female)→ ZK (adult, male) | Non-kin (F–M) | Penis/copulation | NR wiped ZK’s penis with leaves after copulation. | Senna spectabilis (leaves) | Events Book |
Observations of prosocial healthcare in Budongo.
(?), Species suspected but unconfirmed.
S, Sonso; W, Waibira.
NB: See Supplementary Material for list of contributing observers.
Figure 3

(Table 3: Observation 5) PS Licking wound of ZG (Photographed by L. Samuni and A. Schel).
Discussion
Data collected during our direct observational periods reveals the diversity and frequency of external health challenges that Budongo chimpanzees currently face. These wounds and injuries range in severity from superficial to fatal, affecting multiple body regions including limbs, digits, head, genitalia, and trunk, and were more frequent in Sonso than Waibira. Primarily resulting from intragroup aggression and human-set snares, these injuries present diverse healing challenges.
In this study, we provide an overview of wound care, snare removal, and hygiene behaviors with putative medicinal functions in Budongo chimpanzees. Self-directed wound care behaviors documented here include wound licking, leaf-dabbing, licking fingers and pressing to a wound, and application of chewed materials to a wound. We also report the presence of self-directed snare removal and two forms of hygiene behaviors with putative medicinal functions, including postcoital leaf wiping and post-defecation leaf wiping.
We also report, for the first time, the presence of prosocial wound care and putatively medicinal hygiene behaviors at this site. Consistent with findings from Ngogo (Clark et al., 2021) and Loango (Mascaro et al., 2022), we found that Budongo chimpanzees are capable not only of recognizing and tending to their own wounds, but also to the wounds of both kin and non-kin within their community. Prosocial wound care behaviors documented in this study include wound licking, licking fingers and pressing to a wound, and application of chewed materials to a wound. We also report the presence of other-directed snare removal and one form of hygiene behavior, postcoital genital leaf wiping. Our findings further suggest that prosocial healthcare behaviors and putatively medicinal hygiene behaviors are not limited to dyads of particular ages, sexes or relatedness. Our findings add to the recent literature on external healthcare behaviors, particularly wound care (e.g., Laumer et al., 2024; Mascaro et al., 2022), across the primate order.
Botanical species used for wound care
Identified plant species used for leaf-dabbing wounds included Acalypha sp., Pseudospondias microcarpa, and Lasiodiscus pervillei in Sonso, and Argomuellera macrophylla in Waibira. Alchornea floribunda leaves were suspected to have been used in an additional case in Sonso in which the individual chewed the leaf and applied the plant material to her wound, but identification remains unconfirmed. We also suspect A. floribunda leaves were chewed and applied to the wound of the same individual by her daughter in one of the prosocial wound care events. In two cases, we observed individuals chewing organic material and applying the masticated bolus to their wounds with their lips. One of the species involved in these events could not be identified, in the other, the stem bark of A. macrophylla was used, in combination with leaf-dabbing using leaves from the same plant. A. macrophylla leaves have therefore been observed being used in wound care contexts in both Sonso and Waibira. Identified species used for postcoital penis wiping after copulation include Senna spectabilis, A. macrophylla, and L. pervillei leaves.
Several of the species used in wound care (e.g., Acalypha sp., A. floribunda, P. microcarpa) have already been shown to exhibit antibacterial, antifungal, anti-inflammatory, and/or analgesic properties on pharmacological assays which could aid in wound healing or pain relief (Table 4). Acalypha sp., A. floribunda, and P. microcarpa have also been reported to be specifically used for healing wounds and/or ulcers in traditional medicinal practices (Table 4). S. spectabilis has demonstrated strong antifungal and antimicrobial activity, suggesting its efficacy as a cleaning agent after coitus or defecation. This species also has traditional uses in human communities as an antimicrobial and for treating skin lesions (Table 4).
Table 4
| Scientific name | Family | Pharmacological properties | Traditional uses in human medicine |
|---|---|---|---|
| Acalypha sp. | Euphorbiaceae | Several tested Acalypha species have been reported to possess antimicrobial, antidiabetic, antioxidant, anti-inflammatory, larvidal, pupicidal, hepatoprotective, anticancer, leishmanicidal, antihyperglycemic, antihypertensive, anti-venom, analgesic, anthelmintic, antiemetic, laxative, expectorant, diuretic, antifertility and/or wound healing effects (Seebaluck et al., 2015). Some Acalypha species (e.g., A. fruticose) have specifically been shown to contain compounds with wound healing properties (e.g., Gopalakrishnan et al., 2010). | Acalypha species are traditionally used in the treatment of ailments such as diabetes, jaundice, hypertension, fever, liver inflammation, schistosomiasis, dysentery, and respiratory problems (e.g., bronchitis, asthma and pneumonia). They are also used to treat several skin conditions including scabies, eczema and mycoses (Seebaluck et al., 2015). Some species such as A. fruticose have been shown to be used for wound healing (PROTA, 2023). |
| A. macrophylla | Euphorbiaceae | n.a. | In Côte d’Ivoire, A. macrophylla leaf sap is taken as a purgative and emetic to treat poisoning and ascites. Powdered dried leaves are used as an aphrodisiac (Burkill et al., 1995; PROTA, 2023). |
| A. floribunda | Euphorbiaceae | Secondary metabolites found in A. floribunda have been shown to exhibit anti-inflammatory, antioxidant, immunomodulatory, antimicrobial and antiprotozoan activities (reviewed in Agbo et al., 2020). A patent has allegedly been obtained for the use of the leaf alkaloid as a spasmolytic, and clinical experiments have reported positive results for the use of root and leafy stem extracts to treat hepatitis (PROTA, 2023). | In West and Central Africa (e.g., Côte d’Ivoire, Gabon and DR Congo), A. floribunda root is used as a stimulating intoxicant and aphrodisiac, thought to provide a state of excitement followed by a deep depression. Similar effects have been documented in gorillas and chimpanzees after eating the root of this species. In several of these countries’ leaf/root sap is also rubbed over affected areas to treat wounds, ringworm, and eczema (Burkill et al., 1995). In DR Congo a leaf maceration is taken against pains in the heart. A decoction of the young leaves is used to treat diarrhea. A leaf decoction is drunk, or the leaves are eaten to treat ovarian problems, stomach problems and intestinal disorders. In Congo the leaves are eaten as an antidote to poison. Roots and fruits are also eaten for urinary, respiratory, and intestinal problems. In Nigeria and Gabon, root sap is used as eye drops to treat ophthalmia and conjunctivitis. In Cameroon the ash of burnt roots mixed with palm oil is applied to scarifications to treat chest pain and headache. Root bark powder is eaten daily to cure impotence. In Equatorial Guinea the leaf pulp is applied to wounds (PROTA, 2023). |
| L. pervillei | Rhamnaceae | n.a. | In Uganda the bark is administered to children as a purgative (PROTA, 2023). |
| P. microcarpa | Anarcadiaceae | Previous pharmacological reports showed that P. microcarpa possesses antioxidant (Yondo et al., 2009) antimicrobial (Kisangau et al., 2007), anticonvulsant (Adongo et al., 2017), antidepressant (Adongo et al., 2015) anxiolytic (Adongo et al., 2016), sedative, analgesic (Adongo et al., 2014), and cytotoxic and antiplasmodial effects (Malebo et al., 2009). Specific phytochemical constituents possibly responsible for these properties were identified by Yondo et al. (2009) and Adongo et al. (2014). See also Guetchueng et al. (2020) for review. | P. microcarpa is used in Ghana and other parts of Africa as medication for different diseases. This plant is traditionally used as a sedative and for treating general central nervous system disorders, arthritis, rheumatism, eye problems, kidney disorders, nasopharyngeal infections, stomach problems, malaria, and jaundice (Burkill et al., 1995; PROTA, 2023). It is used in combination with other plants to externally treat ulcers on the soles of the feet. |
| S. spectabilis | Fabaceae | Leaf extracts exhibit significant antifungal activity against Candida albicans (Jothy et al., 2012), Saccharomyces cerevisiae and Aspergillus niger (Jothy et al., 2012). S. spectabilis is known to possess piperidine alkaloids as major constituents of leaves known to exhibit strong antitumor, leishmanicidal, analgesic, antimicrobial, purgative and anticonvulsant effects (Jothy et al., 2012). This species has also shown antibacterial effects against several bacteria (Jothy et al., 2012), as well as anti-biofilm activity (Sangetha et al., 2009). | S. spectabilis has been used to treat ringworm and skin diseases (Burkill et al., 1995; Jothy et al., 2012). In eastern medicine, this plant is traditionally used as laxative, antimicrobial, anti-inflammatory and antiulcerogenic. In northeast Brazilian folk medicine, S. spectabilis is used for the above conditions and additionally used as an analgesic and purgative (Jothy et al., 2012). In Asiatic countries, it is also used for treating rheumatism, pain and skin lesions (Selegato et al., 2017). |
Pharmacological properties and traditional uses of species used in wound care and hygiene behaviors.
Bold text indicates relevance to healthcare use by chimpanzees in this study.
Of the 41 total cases (including both self-care and prosocial-care) reported here, 23/41 (56%) involved plant materials. This raises important questions about the functional differences between plant-mediated and non-plant-mediated healthcare/hygiene behaviors. Self-directed behaviors that do not incorporate plants, such as direct wound licking and finger licking followed by wound pressing, likely serve primarily mechanical functions of wound cleaning and debris removal, while potentially delivering antimicrobial compounds present in saliva (Hart and Powell, 1990). In contrast, plant-mediated behaviors may serve additional pharmacological functions beyond mechanical cleaning, as suggested by the documented bioactive properties and ethnomedicinal uses of several selected plants (Table 4). The co-occurrence of both types of behaviors, sometimes within the same wound care event, suggests these approaches may be complementary rather than functionally redundant. Individuals may select different care strategies based on wound severity, location, environmental context (including plant availability), or prior experience with particular treatment methods. Further research should investigate whether chimpanzees show preferences for specific treatment methods based on wound characteristics or whether they employ a hierarchical approach to wound treatment, beginning with simple mechanical cleaning before progressing to plant-based applications for more persistent or severe injuries.
Socio-ecological variables and prosocial healthcare behaviors
Chimpanzees have a well-demonstrated capacity for other-regarding prosocial behaviors (e.g., adoption: Hobaiter et al., 2014; babysitting for incapacitated mothers: Huffman and Seifu, 1989; food sharing: Mitani and Watts, 2001; post-conflict consolation from uninvolved by-standers: de Waal and Suchak, 2010). Recent findings suggest that some prosocial behaviors are expressed in group-specific ways (van Leeuwen et al., 2021). Based on accounts across several free-ranging chimpanzee sites (e.g., Clark et al., 2021; Mascaro et al., 2022; Pruetz, 2011), this variation may also apply to healthcare behaviors. But what could cause the differences in these behaviors across communities and/or sites? While Budongo chimpanzees do not have any direct predators, they experience high risk of human-caused injuries. In Sonso, across all known chimpanzees to have been active members of the community, ~40% have been observed with confirmed snare injuries (D. Taylor, pers. comm.). Several other individuals have permanent disabilities which appear to have been caused by snares, but whose origins are unconfirmed. If increased predation is thought to enhance social-cohesion and cooperation (Boesch, 1991), then elevated anthropogenic risks may have a similar effect, resulting in the more frequent presence of non-kin prosocial-care in sites with high rates of exposure to human hunting practices. While the current absence of prosocial healthcare cases reported in Waibira is most likely a result of observation bias due to differences in observation opportunity between the communities, future studies should seek to systematically investigate potential correlations between rates of snare and human-caused injuries and social wound care behaviors at this site.
Differences in stability of the social hierarchy between communities could also impact the frequency of injuries in a group, impacting opportunities for both self-care and prosocial-care, as well as any social transmission of these behaviors. Exploring the impact of social variables on relatively infrequently observed behaviors in long-lived species requires deep multi-decade datasets. Where these datasets could be assembled, it would be of particular interest to investigate both social factors (e.g., rate of female migration, size of core-group, level of habituation, hierarchy stability) and ecological variables (e.g., availability of medicinal resources and duration of seasonal periods with increased pathogenic risk) that could shape community-level differences.
Evolutionary implications of prosocial healthcare
Free-ranging chimpanzees provide prime models for studying the evolution of human healthcare systems. Our findings establish the presence of prosocial wound care, snare removal, and hygiene behaviors in Budongo chimpanzees. These observations contribute to the growing number of cases which suggest that chimpanzees have the cognitive ability to understand the needs of wounded group members, and selectively provide appropriate care (Fábrega, 1997). The presence of prosocial healthcare at this site, between individuals who are genetically distant or unrelated, provides further evidence that chimpanzees have a capacity for empathy (e.g., Brooker et al., 2024; Campbell and de Waal, 2014; Webb et al., 2017), a trait thought to underly directed altruism in humans (Batson, 2014). Wound care contexts provide a particularly interesting case study for empathic behavior, as a care giver may experience potential costs in cases when licking wounds/infections and handling snares may also expose them to possible diseases and physical danger.
While we did not measure or address the effect that established social bonds may have on these selective prosocial behaviors, we encourage future research into the effects of affiliation and social bonding. We predict that, if measured, non-kin affiliate-care would occur more frequently than non-kin non-affiliate-care, as social bonds likely affect a care giver’s tolerance to risk (here, exposure to infection or proximity to physical danger).
Lastly, we found that prosocial healthcare behaviors amongst the Budongo chimpanzees appear to be adaptive and context-specific. For example, the cases in which individuals helped others remove snares (Table 3: Observations 1, 2) suggest that at least some individuals within the Sonso community have developed adaptive care behaviors in response to anthropogenic risks. This further demonstrates chimpanzees’ cognitive flexibility in addressing novel environmental challenges. Despite the prevalence of snare injuries at Budongo, however, prosocial snare removal remains infrequent, the reasons for which remain unclear and warrant further investigation.
Conclusion
This paper gives an overview of self-directed and other-directed healthcare and hygiene behaviors, observed over thirty cumulative years, amongst two chimpanzee communities in the Budongo Forest. Our findings into non-human healthcare systems deepen our understanding of primate behavior and cognition while providing additional evidence for empathic capacities in our closest evolutionary relatives. We strongly encourage more long-term investigations into external healthcare behaviors at free-ranging chimpanzee sites and highlight the need for further exploration into which selective pressures drive the presence and frequency of these behaviors. While we currently do not know the extent to which wound care, snare removal, or hygiene behaviors are socially learned and/or transmitted, establishing this will be an important step for understanding whether any components of non-human healthcare systems are influenced by local medicinal cultures. We highlight the importance of this research for understanding the evolutionary origins of modern human healthcare systems and encourage continued research into this exciting and rapidly evolving field.
As chimpanzee habitats become increasingly disrupted, and primate populations inch closer to extinction, understanding the socio-ecological pressures on chimpanzee healthcare behaviors could play a critical role in informing conservation strategies. By uncovering and protecting the resources chimpanzees need to keep themselves healthy, as well as guarding against anthropogenic risks (e.g., snares) known to negatively impact chimpanzee wellbeing (Yersin et al., 2017), we can help buffer free-ranging chimpanzees from environmental and climatic disturbances that increasingly threaten their survival.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Uganda Wildlife Authority (UWA); Uganda National Council for Science and Technology (UNCST); University of Oxford; Budongo Conservation Field Station. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. CH: Data curation, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing. MH: Supervision, Writing – review & editing. HK: Investigation, Writing – review & editing. GM: Investigation, Methodology, Writing – review & editing. VR: Data curation, Resources, Writing – review & editing. NS: Investigation, Writing – review & editing. AS: Investigation, Writing – review & editing. EY: Writing – review & editing, Investigation, Methodology. KZ: Resources, Writing – review & editing. SC: Supervision, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. EF was funded by the Clarendon Fund, Keble College, Oxford, and the Explorers Club. CH received funding from the European Union’s 8th Framework Program, Horizon 2020, under grant agreement no. 802719. Open Access to this article is financed by FCT – Fundação para a Ciência e a Tecnologia, within the scope of the project UID/04211: Centro Interdisciplinar de Arqueologia e Evolução do Comportamento Humano (ICArEHB).
Acknowledgments
We are grateful to all the present and past field colleagues and staff working with the Sonso and Waibira communities who provided invaluable instruction and guidance in the field. Specifically, we would like to thank Chandia Bosco, Monday Mbotella Gideon, Adue Sam, Asua Jackson, Eguma Robert Yikii, Steven Mugisha, Atayo Gideon, and Kizza Vincent. We would also like to thank all Budongo researchers whose observations over the years contributed to the results reported in this study. We are also thankful to the BCFS management, including Walter Akankwasa and David Eryenyu as well as the other researchers working at the site for their support. We also extend our gratitude to Vernon Reynolds who founded the field station and to the Royal Zoological Society of Scotland which provides core funding that keeps the station operational. Thank also to the Uganda Wildlife Authority and the Uganda National Council for Science and Technology for granting permission to work in Uganda.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1540922/full#supplementary-material
References
1
AdongoD. W.KukuiaK. K. E.ManteP. K.AmeyawE. O.WoodeE. (2015). Antidepressant-like effect of the leaves of Pseudospondias microcarpa in mice: evidence for the involvement of the serotoninergic system, NMDA receptor complex, and nitric oxide pathway. BioMed. Res. Int2015, 397943. doi: 10.1155/2015/397943.
2
AdongoD. W.ManteP. K.Edem KukuiaK. K.AmeyawE. O.WoodeE.AziI. H. (2016). Anxiolytic-like effect of the leaves of Pseudospondias microcarpa (A. Rich.) Engl. in mice. J. Basic Clin. Physiol. Pharmacol.27, 533–546. doi: 10.1515/jbcpp-2015-0067
3
AdongoD. W.ManteP. K.KukuiaK. K. E.BineyR. P.Boakye-GyasiE.BennehC. K.et al. (2017). Anticonvulsant activity of Pseudospondias microcarpa (A. Rich) Engl. hydroethanolic leaf extract in mice: The role of excitatory/inhibitory neurotransmission and nitric oxide pathway. J. Ethnopharmacology206, 78–91. doi: 10.1016/j.jep.2017.05.017
4
AdongoD. W.ManteP. K.WoodeE.AmeyawE. O.KukuiaK. K. E. (2014). Effects of hyrdroethanolic leaf extract of Pseudospondias microcarpa (A. Rich.) Engl. (Anacardiaceae) on the central nervous system in mice. J. Phytopharmacol.3 (6), 410–417. Available online at: https://www.phytopharmajournal.com/Vol3_Issue6_07.pdf.
5
AgboM. O.OkoyeF. B.EbiG. C.OsadebeP. O. (2020). Alchornea floribunda (Müll. Arg.)—A review of its phytochemistry and biological activities. Trop. J. Pharm. Res.19, 1113–1120. doi: 10.4314/tjpr.v19i5.30
6
AltmannJ. (1974). Observational study of behavior: sampling methods. Behaviour49 (3), 227–267. doi: 10.1163/156853974x00534
7
AmatiS.BabweteeraF.WittigR. M. (2008). Snare removal by a chimpanzee of the Sonso community, Budongo Forest (Uganda). Pan Afr. News15, 6–8. doi: 10.5134/143488
8
Angiosperm Phylogeny GroupChaseM. W.ChristenhuszM. J. M.FayM. F.ByngJ. W.JuddW. S.et al. (2016). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical J. Linn. Soc.181, 1–20. doi: 10.1111/boj.12385
9
BadihiG.BoddenK.ZuberbühlerK.SamuniL.HobaiterC. (2022). Flexibility in the social structure of male chimpanzees (Pan troglodytes schweinfurthii) in the Budongo Forest, Uganda. R. Soc. Open Sci.9, 220904. doi: 10.1098/rsos.220904
10
BatsonC. D. (2014). The altruism question: toward a social-psychological answer (1st ed.). (Psychology Press). doi: 10.4324/9781315808048
11
BlaiseA.KiewraD.ChrząścikK.SelvaN.PopiołekM.SergielA. (2023). Anti-parasitic function of tree-rubbing behaviour in brown bears suggested by an in vitro test on a generalist ectoparasite. J. Zool.319, 296–307. doi: 10.1111/jzo.13045
12
BoeschC. (1991). The effects of leopard predation on grouping patterns in forest chimpanzees. Behaviour117, 220–241. doi: 10.1163/156853991X00544
13
BoeschC.Boesch-AchermannH. (2000). The chimpanzees of the Taï Forest: behavioural ecology and evolution (Oxford: Oxford University Press).
14
BoeschC.BoléC.EckhardtN.BoeschH. (2010). Altruism in forest chimpanzees: the case of adoption. PloS One5, e8901. doi: 10.1371/journal.pone.0008901
15
BrookerJ. S.WebbC. E.de WaalF. B. M.ClayZ. (2024). The expression of empathy in human’s closest relatives, bonobos and chimpanzees: current and future directions. Biol. Rev.99, 1556–1575. doi: 10.1111/brv.13080
16
BurkillH. M.DalzielJ. M.HutchinsonJ. (1995). “The useful plants of west tropical Africa,” in The useful plants of west tropical Africa, 2nd ed, vol. 1–3. (Royal Botanic Gardens, Kew).
17
CampbellM. W.de WaalF. B. M. (2014). Chimpanzees empathize with group mates and humans, but not with baboons or unfamiliar chimpanzees. Proc. Biol. Sci.281, 20140013. doi: 10.1098/rspb.2014.0013
18
ClarkI. R.SandelA. A.ReddyR. B.LangergraberK. E. (2021). A preliminary analysis of wound care and other-regarding behavior in wild chimpanzees at Ngogo, Kibale National Park, Uganda. Primates62, 697–702. doi: 10.1007/s10329-021-00925-7
19
De la FuenteM. F.SoutoA.AlbuquerqueU. P.SchielN. (2022). Self-medication in nonhuman primates: A systematic evaluation of the possible function of the use of medicinal plants. Am. J. Primatology84, e23438. doi: 10.1002/ajp.23438
20
de WaalF. B. M.SuchakM. (2010). Prosocial primates: selfish and unselfish motivations. Philos. Trans. R. Soc. London. Ser. B Biol. Sci.365, 2711–2722. doi: 10.1098/rstb.2010.0119
21
DittusW. P.RatnayekeS. M. (1989). Individual and social behavioral responses to injury in wild toque macaques (Macaca sinica). Int. J. Primatology10, 215–234. doi: 10.1007/BF02735201
22
Douglas-HamiltonI.BhallaS.WittemyerG.VollrathF. (2006). Behavioural reactions of elephants towards a dying and deceased matriarch. Appl. Anim. Behav. Sci.100, 87–102. doi: 10.1016/j.applanim.2006.04.014
23
EggelingW. J. (1947). Observations on the ecology of the Budongo rain forest, Uganda. J. Ecol.34, 20–87. doi: 10.2307/2256760
24
FábregaH.Jr. (1997). Evolution of Sickness and Healing. Berkeley: University of California Press. Available online at: http://ark.cdlib.org/ark:/13030/ft1j49n6b2/.
25
FedurekP.AkankwasaJ. W.DanelD. P.FensomeS.ZuberbühlerK.MuhanguziG.et al. (2022). The effect of warning signs on the presence of snare traps in a Ugandan rainforest. Biotropica54, 721–728. doi: 10.1111/btp.13088
26
FrankE. T.BuffatD.LibertiJ.AibekovaL.EconomoE. P.KellerL. (2024). Wound-dependent leg amputations to combat infections in an ant society. Curr. Biol.34, 3273–3278.e3. doi: 10.1016/j.cub.2024.06.021
27
FreymannE.BadihiG.HobaiterC.HuffmanM. A.MuhumuzaG.OrbellS.et al. (2024a). The adaptive role of bark in the diet of Budongo chimpanzees (Pan troglodytes schweinfurthii). Int. J. Prim.45, 1229–1263. doi: 10.1007/s10764-024-00445-3
28
FreymannE.CarvalhoS.GarbeL. A.Dwi GhazheliaD.HobaiterC.HuffmanM. A.et al. (2024b). Pharmacological and behavioral investigation of putative self-medicative plants in Budongo chimpanzee diets. PloS One19, e0305219. doi: 10.1371/journal.pone.0305219
29
FreymannE.d’Oliveira CoelhoJ.HobaiterC.HuffmanM. A.MuhumuzaG.ZuberbühlerK.et al. (2024c). Applying collocation and APRIORI analyses to chimpanzee diets: Methods for investigating nonrandom food combinations in primate self-medication. Am. J. Primatol.86, e23603. doi: 10.1002/ajp.23603
30
GoodallJ. (1983). Population dynamics during a 15 year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Z. Fuer Tierpsychologie61, 1–60. doi: 10.1111/j.1439-0310.1983.tb01324.x
31
GoodallJ. (1986). The chimpanzees of Gombe: patterns of behavior (Cambridge, Mass: Belknap Press of Harvard University Press).
32
GopalakrishnanS.SarojaK.ElizabethJ. D. (2010). Chemical investigation of aerial parts of Acalypha fruticosa forssk. Der Pharma Chemica2 (5), 383–389. Available online at: https://www.derpharmachemica.com/pharma-chemica/chemical-investigation-of-aerial-parts-of-acalypha-fruticosa-forssk.pdf.
33
GuetchuengS. T.NaharL.RitchieK. J.Daud IsmailF. M.DempsterN. M.NnangaE. N.et al. (2020). Phenolic compounds from the leaves and stem bark of Pseudospondias microcarpa (A. Rich.) Engl. (Anacardiaceae). Biochem. Systematics Ecol.91, 104078. doi: 10.1016/j.bse.2020.104078
34
HartB. (1988). Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev.12, 123–137. doi: 10.1016/S0149-7634(88)80004-6
35
HartB. L. (2011). Behavioural defences in animals against pathogens and parasites: parallels with the pillars of medicine in humans. Philos. Trans. R. Soc. London. Ser. B Biol. Sci.366, 3406–3417. doi: 10.1098/rstb.2011.0092
36
HartB. L.PowellK. L. (1990). Antibacterial properties of saliva: role in maternal periparturient grooming and in licking wounds. Physiol. Behav.48, 383–386. doi: 10.1016/0031-9384(90)90332-X
37
HobaiterC.BadihiG.DalyG. B. D. M.EleuteriV.GrahamK. E.GrundC.et al. (2021). The Great Ape Dictionary video database (1.0.0). doi: 10.5281/zenodo.5600472
38
HobaiterC.SamuniL.MullinsC.AkankwasaW. J.ZuberbühlerK. (2017). Variation in hunting behaviour in neighbouring chimpanzee communities in the Budongo forest, Uganda. PloS One12, e0178065–e0178065. doi: 10.1371/journal.pone.0178065
39
HobaiterC.SchelA. M.LangergraberK.ZuberbühlerK. (2014). Adoption by maternal siblings in wild chimpanzees. PloS One9, e103777. doi: 10.1371/journal.pone.0103777
40
HuffmanM. A. (1997). Current evidence for self-medication in primates: A multidisciplinary perspective. Am. J. Phys. Anthropology104, 171–200. doi: 10.1002/(sici)1096-8644(1997)25+<171::aid-ajpa7>3.3.co;2-k
41
HuffmanM. A. (2001). Self-medicative behavior in the African great apes: an evolutionary perspective into the origins of human traditional medicine: in addition to giving us a deeper understanding of our closest living relatives, the study of great ape self-medication provides a window into the origins of herbal medicine use by humans and promises to provide new insights into ways of treating parasite infections and other serious diseases. BioScience51, 651–661. doi: 10.1641/0006-3568(2001)051[0651:SMBITA]2.0.CO;2
42
HuffmanM. A. (2016). Primate self-medication, passive prevention and active treatment—A brief review. Int. J. Multidiscip. Stud.3, 1–10. doi: 10.4038/ijms.v3i2.1
43
HuffmanM. A. (2022). Folklore, animal self-medication, and phytotherapy—something old, something new, something borrowed, some things true. Planta Med.88, 187–199. doi: 10.1055/a-1586-1665
44
HuffmanM. A.PageJ. E.SukhdeoM. V. K. K.GotohS.KalundeM. S.ChandrasiriT.et al. (1996). Leaf-swallowing by chimpanzees: A behavioral adaptation for the control of strongyle nematode infections. Int. J. Primatology17, 475–503. doi: 10.1007/BF02735188
45
HuffmanM. A.SeifuM. (1989). Observations on the illness and consumption of a possibly medicinal plant Vernonia amygdalina (DEL.), by a wild chimpanzee in the Mahale Mountains National Park, Tanzania. Primates. 30, 51–63. doi: 10.1007/BF02381210
46
HuffmanM. A.ShunjiG.DaisukeI.KoichiK.KalundeM. S. (1993). Further obervations on the use of the medicinal plant, Vernonia amygdalina (Del), by a wild chimpanzee, its possible effect on parasote load, and its phytochemistry. Afr. Study Monogr.14, 227–240. Available online at: https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/68112/1/ASM_14_227.pdf.
47
JothyS. L.ToreyA.DarahI.ChoongY. S.SaravananD.ChenY.et al. (2012). Cassia spectabilis (DC) Irwin et Barn: a promising traditional herb in health improvement. Molecules (Basel Switzerland)17 (9), 10292–10305. doi: 10.3390/molecules170910292
48
KesslerS. E.AungerR. (2022). The evolution of the human healthcare system and implications for understanding our responses to COVID-19. Evolution Medicine Public Health10, 87–107. doi: 10.1093/emph/eoac004
49
KisangauD. P.HoseaK. M.JosephC. C.LyaruuH. V. M. (2007). In vitro antimicrobial assay of plants used in traditional medicine in Bukoba rural district, Tanzania. Afr. J. Traditional Complementary Altern. Medicines: AJTCAM4 (4), 510–523. doi: 10.4314/ajtcam.v4i4.31245
50
KöhlerW. (1948). The mentality of apes 1917. In DennisW. (Ed.), Readings in the history of psychology (pp. 497–505). Appleton-Century-Crofts.
51
KoopsK.AkankwasaW.CamaraH. D.FitzgeraldM.KeirA.MamyG.et al. (2024). Flexible grouping patterns in a western and eastern chimpanzee community. Am. J. Primatology86, e23593. doi: 10.1002/ajp.23593
52
KummerH. (1971). Primate societies: group techniques of ecological adaptation (1st ed.). (Routledge). doi: 10.4324/9781315127415
53
LamonN.NeumannC.ZuberbühlerK. (2018). Development of object manipulation in wild chimpanzees. Anim. Behav.135, 121–130. doi: 10.1016/j.anbehav.2017.11.003
54
LangergraberK. E.MitaniJ. C.VigilantL. (2007). The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl. Acad. Sci.104, 7786–7790. doi: 10.1073/pnas.0611449104
55
LaumerI. B.RahmanA.RahmaetiT.AzhariU.HermansyahAtmokoS. S. U.et al. (2024). Active self-treatment of a facial wound with a biologically active plant by a male Sumatran orangutan. Sci. Rep.14, 8932. doi: 10.1038/s41598-024-58988-7
56
LunczL. V.BoeschC. (2014). Tradition over trend: Neighboring chimpanzee communities maintain differences in cultural behavior despite frequent immigration of adult females. Am. J. Primatology76, 649–657. doi: 10.1002/ajp.22259
57
MacKinnonK.RileyE.GarberP.SetchellJ.Fernandez-DuqueE. (2014). Code of best practices for field primatology. International Primatological Society. doi: 10.13140/2.1.2889.1847
58
MaleboH. M.TanjaW.CalM.SwalehS. A. M.OmoloM. O.HassanaliA.et al. (2009). Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzan. J. Health Res.11 (4), 226–234. doi: 10.4314/thrb.v11i4.50194
59
MascaroA.SouthernL. M.DeschnerT.PikaS. (2022). Application of insects to wounds of self and others by chimpanzees in the wild. Curr. Biol.32, R112–R113. doi: 10.1016/j.cub.2021.12.045
60
MassenJ.SterckE.de VosH. (2010). Close social associations in animals and humans: functions and mechanisms of friendship. Behaviour147, 1379–1412. doi: 10.1163/000579510X528224
61
McGrewW. C. (1992). Chimpanzee material culture: implications for human evolution. Cambridge University Press. doi: 10.1017/CBO9780511565519
62
McGrewW. C.TutinC. E. G. (1972). Chimpanzee dentistry. J. Am. Dental Assoc.85, 1198–1204. doi: 10.14219/jada.archive.1972.0540
63
MitaniJ. C.MerriwetherD. A.ZhangC. (2000). Male affiliation, cooperation and kinship in wild chimpanzees. Anim. Behav.59, 885–893. doi: 10.1006/anbe.1999.1389
64
MitaniJ. C.WattsD. P. (2001). Why do chimpanzees hunt and share meat? Anim. Behav.61, 915–924. doi: 10.1006/anbe.2000.1681
65
MitaniJ. C.WattsD. P. (2005). Correlates of territorial boundary patrol behaviour in wild chimpanzees. Anim. Behav.70, 1079–1086. doi: 10.1016/j.anbehav.2005.02.012
66
MorlockG. E.ZiltenerA.GeyerS.TersteegenJ.MehlA.SchreinerT.et al. (2022). Evidence that Indo-Pacific bottlenose dolphins self-medicate with invertebrates in coral reefs. IScience25, 104271. doi: 10.1016/j.isci.2022.104271
67
Newton-FisherN. E. (2014). Roving females and patient males: a new perspective on the mating strategies of chimpanzees. Biol. Rev.89, 356–374. doi: 10.1111/brv.12058
68
NishidaT. (1968). The social group of wild chimpanzees in the Mahali Mountains. Primates9, 167–224. doi: 10.1007/BF01730971
69
NishidaT. (2011). Chimpanzees of the lakeshore: natural history and culture at Mahale (Cambridge University Press). doi: 10.1017/CBO9781139059497
70
O’HaraS. J.LeeP. C. (2006). High frequency of postcoital penis cleaning in Budongo chimpanzees. Folia Primatologica; Int. J. Primatology77, 353–358. doi: 10.1159/000093700
71
PebsworthP. A.HillierS.WendlerR.GlahnR.TaC. A. K.ArnasonJ. T.et al. (2019). Geophagy among East African Chimpanzees: consumed soils provide protection from plant secondary compounds and bioavailable iron. Environ. Geochemistry Health41, 2911–2927. doi: 10.1007/s10653-019-00366-8
72
PebsworthP.KriefS.HuffmanM. A. (2006). The role of diet in self-medication among chimpanzees in the Sonso and Kanyawara communities, Uganda. In: Newton-FisherN. E.NotmanH.PatersonJ. D.ReynoldsV. (eds) Primates of Western Uganda. Developments in primatology: progress and prospects (New York, NY: Springer), 105–133. doi: 10.1007/978-0-387-33505-6_7
73
PéterH.LaporteM.Newton-FisherN. E.ReynoldsV.SamuniL.SoldatiA.et al. (2022). Recognition of visual kinship signals in chimpanzees (Pan troglodytes) by humans (Homo sapiens). J. Comp. Psychol.136, 255–269. doi: 10.1037/com0000327
74
PROTA (2023). PROTA4U. Available online at: https://prota.prota4u.org/ (Accessed May 20, 2024).
75
PruetzJ. D. (2011). Targeted helping by a wild adolescent male chimpanzee (Pan troglodytes verus): evidence for empathy? J. Ethology29, 365–368. doi: 10.1007/s10164-010-0244-y
76
RasaO. A. E. (1983). A case of invalid care in wild dwarf mongooses. Z. Für Tierpsychologie62, 235–240. doi: 10.1111/j.1439-0310.1983.tb02153.x
77
ReddyR. B.MitaniJ. C. (2019). Social relationships and caregiving behavior between recently orphaned chimpanzee siblings. Primates; J. Primatology60, 389–400. doi: 10.1007/s10329-019-00732-1
78
ReynoldsV. (2005). The chimpanzees of the budongo forest: ecology, behaviour and conservation (Oxford, 2005; online edn, Oxford Academic, 1 Sept. 2007), doi: 10.1093/acprof:oso/9780198515463.001.0001
79
RodriguezE.WranghamR. (1993). Zoopharmacognosy: the use of medicinal plants by animals. In: DownumK. R.RomeoJ. T.StaffordH. A. (eds) Phytochemical potential of tropical plants. Recent advances in phytochemistry, vol 27. Springer, Boston, MA.
80
SamuniL.MundryR.TerkelJ.ZuberbühlerK.HobaiterC. (2014). Socially learned habituation to human observers in wild chimpanzees. Anim. Cogn.17, 997–1005. doi: 10.1007/s10071-014-0731-6
81
SamuniL.TkaczynskiP.DeschnerT.LöhrrichT.WittigR. M.CrockfordC. (2020). Maternal effects on offspring growth indicate post-weaning juvenile dependence in chimpanzees (Pan troglodytes verus). Front. Zoology17, 1. doi: 10.1186/s12983-019-0343-8
82
SangethaS.ZurainiZ.SuryaniS.SasidharanS. (2009). In situ TEM and SEM studies on the antimicrobial activity and prevention of Candida albicans biofilm by Cassia spectabilis extract. Micron40, 439–443. doi: 10.1016/j.micron.2009.01.003
83
SanzC. M.MorganD. B. (2007). Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J. Hum. Evol.52, 420–433. doi: 10.1016/j.jhevol.2006.11.001
84
SchallerG. B. (2009). The Serengeti lion: a study of predator-prey relations. Chicago: University of Chicago Press. Available online at: https://books.google.com/books?id=7ann2dYn9iYC.
85
SchinoG. (2007). Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behav. Ecol.18, 115–120. doi: 10.1093/beheco/arl045
86
SchinoG.ScucchiS.MaestripieriD.TurillazziP. G. (1988). Allogrooming as a tension-reduction mechanism: A behavioral approach. Am. J. Primatology16, 43–50. doi: 10.1002/ajp.1350160106
87
SeebaluckR.Gurib-FakimA.MahomoodallyF. (2015). Medicinal plants from the genus Acalypha (Euphorbiaceae)—A review of their ethnopharmacology and phytochemistry. J. Ethnopharmacology159, 137–157. doi: 10.1016/j.jep.2014.10.040
88
SelegatoD. M.MonteiroA. F.VieiraN. C.CardosoP.PavaniV. D.BolzaniV. S.et al. (2017). Update: biological and chemical aspects of Senna spectabilis. J. Braz. Chem. Soc.28 (3), 415–426. doi: 10.21577/0103-5053.20160322
89
SoldatiA.FedurekP.CrockfordC.AdueS.AkankwasaJ. W.AsiimweC.et al. (2022). Dead-infant carrying by chimpanzee mothers in the Budongo Forest. Primates63, 497–508. doi: 10.1007/s10329-022-00999-x
90
SuH.SuY.HuffmanM. A. (2013). Leaf swallowing and parasitic infection of the Chinese lesser civet Viverricula indica in northeastern Taiwan. Zoological Stud.52, 22. doi: 10.1186/1810-522X-52-22
91
TokuyamaN.EmikeyB.BafikeB.IsolumboB.IyokangoB.MulavwaM. N.et al. (2012). Bonobos apparently search for a lost member injured by a snare. Primates53, 215–219. doi: 10.1007/s10329-012-0298-2
92
van LeeuwenE. J. C.DeTroyS. E.KaufholdS. P.DuboisC.SchütteS.CallJ.et al. (2021). Chimpanzees behave prosocially in a group-specific manner. Sci. Adv.7 (9), eabc7982. doi: 10.1126/sciadv.abc7982
93
WattsD. P. (2008). Tool use by chimpanzees at ngogo, kibale national park, Uganda. Int. J. Primatology29, 83–94. doi: 10.1007/s10764-007-9227-4
94
WattsD. P. (2015). Mating behavior of adolescent male chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Primates56, 163–172. doi: 10.1007/s10329-014-0453-z
95
WebbC. E.RomeroT.FranksB.de WaalF. B. M. (2017). Long-term consistency in chimpanzee consolation behaviour reflects empathetic personalities. Nat. Commun.8, 292. doi: 10.1038/s41467-017-00360-7
96
WhitenA.GoodallJ.McGrewW. C.NishidaT.ReynoldsV.SugiyamaY.et al. (1999). Cultures in chimpanzees. Nature399, 682–685. doi: 10.1038/21415
97
WilsonM. L.WranghamR. W. (2003). Intergroup relations in chimpanzees. Annu. Rev. Anthropology32, 363–392. doi: 10.1146/annurev.anthro.32.061002.120046
98
WranghamR. W.NishidaT. (1983). Aspilia spp. leaves: a puzzle in the feeding behavior of wild chimpanzees. Primates24, 276–282. doi: 10.1007/BF02381090
99
YerkesR. M. (1943). “Chimpanzees: a laboratory colony,” in Chimpanzees: a laboratory colony (Yale University Press, New Haven, CT, US).
100
YersinH.AsiimweC.VoordouwM. J.ZuberbühlerK. (2017). Impact of snare injuries on parasite prevalence in wild chimpanzees (Pan troglodytes). Int. J. Primatology38, 21–30. doi: 10.1007/s10764-016-9941-x
101
YondoJ.FomekongG. I. D.M-C. KontanguiJ. P.WaboO. F.TankouaJ.-R. K. (2009). “In vitro antioxidant potential and phytochemical constituents of three Cameroonian medicinal plants used to manage parasitic diseases,” in Annals of nutrition and metabolism (Karger, Basel, Switzerland: Allschwilerstrasse).
Summary
Keywords
Pan troglodytes, self-medication, prosociality, wound care, animal healthcare
Citation
Freymann E, Hobaiter C, Huffman MA, Klein H, Muhumuza G, Reynolds V, Slania NE, Soldati A, Yikii ER, Zuberbühler K and Carvalho S (2025) Self-directed and prosocial wound care, snare removal, and hygiene behaviors amongst the Budongo chimpanzees. Front. Ecol. Evol. 13:1540922. doi: 10.3389/fevo.2025.1540922
Received
06 December 2024
Accepted
26 March 2025
Published
14 May 2025
Volume
13 - 2025
Edited by
Tom Langen, Clarkson University, United States
Reviewed by
Sharon Kessler, University of Stirling, United Kingdom
Jacob Negrey, University of Arizona, United States
Updates
Copyright
© 2025 Freymann, Hobaiter, Huffman, Klein, Muhumuza, Reynolds, Slania, Soldati, Yikii, Zuberbühler and Carvalho.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elodie Freymann, elodie.freymann@anthro.ox.ac.uk
†ORCID: Elodie Freymann, orcid.org/0000-0002-5484-8643; Catherine Hobaiter, orcid.org/0000-0002-3893-0524; Michael Alan Huffman, orcid.org/0000-0003-2115-7923; Harmonie Klein, orcid.org/0000-0003-1925-5049; Nora E. Slania, orcid.org/0009-0005-9717-4182; Adrian Soldati, orcid.org/0000-0002-3618-5022; Klaus Zuberbühler, orcid.org/0000-0001-8378-088X; Susana Carvalho, orcid.org/0000-0003-4542-3720
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.