- 1National Engineering Research Center of Eco - Environment Protection for Yangtze River Economic Belt, China Three Gorges Corporation, Wuhan, China
- 2Hubei Key Laboratory of Three Gorges Project for Conservation of Fishes, Chinese Sturgeon Research Institute, China Three Gorges Corporation, Yichang, China
Introduction: As a key protected species in the lower reaches of the Jinsha River, Schizothorax wangchiachii plays a vital role in maintaining aquatic ecosystem stability. Understanding habitat suitability conditions for its spawning grounds is critical for habitat restoration.
Methods: To systematically investigate habitat selection mechanisms and key drivers during its spawning period, this study induced its natural reproduction by enriching the natural habitat with diverse substrates, used acoustic telemetry to track movement trajectories, thereby clarifying environmental requirements.
Results: The results showed that the S. wangchiachii exhibited significant aggregation during spawning (Z > 2.58, P < 0.01), mainly gathering in the slow-flow beach area which belonged to the shallow flow type of slow-flow habitats. Notably, environmental preferences diverged between spawning phases, S. wangchiachii exhibited divergent environmental preferences, which were categorized into two distinct habitat types: pre- and post-spawning stages favored habitats with slower surface velocities (0.10–0.25 m/s), shallower depths (0.43–0.66 m), and small-pebble substrates. Active spawning, however, occurred exclusively in nest-like depressions characterized by higher surface velocities (0.32–0.42 m/s), reduced bottom velocities (0.04–0.24 m/s), greater depths (0.52–0.71 m), and finer gravel substrates. Random Forest-based importance analysis indicated that fluvial substrate composition and surface flow velocity were the key predictive variables for habitat selection model with MeanDecreaseGini being 23.3% and 22.6%, respectively.
Significance: These findings provide quantitative criteria for restoring natural spawning grounds and optimizing ecological operation strategies to support S. wangchiachii conservation in the lower Jinsha River.
1 Introduction
Freshwater ecosystems are among the most threatened globally, with fish populations in sharp decline due to increasing anthropogenic pressures. Habitat fragmentation, dam construction, water pollution, and flow regulation have collectively led to the degradation of lotic environments and the collapse of native fish diversity (Dudgeon et al., 2006; Liermann et al., 2012; Grill et al., 2019). Rheophilic fishes—species adapted to fast-flowing water—are particularly vulnerable. Altered flow regimes, sediment retention, and habitat homogenization diminish the hydraulic variability and oxygen-rich microhabitats that these species rely on for feeding, migration, and reproduction (Winemiller et al., 2016; Ziv et al., 2012). Consequently, many riverine fish species face population declines, local extirpation, or extinction, especially in heavily regulated watersheds.
To counteract these losses, spawning ground restoration has become a global conservation priority. However, successful restoration requires precise knowledge of species-specific habitat needs throughout the reproductive cycle (Cui et al., 2019; Zhou et al., 2022). A mismatch between behavioral requirements and restoration design can result in low efficiency or failure of restoration projects (Zhou, 2021).
The upper Yangtze River is a biodiversity hotspot in China and globally, home to a rich assemblage of cold-water rheophilic fish. Among them, the subfamily Schizothoracinae (Cyprinidae) represents an ecologically dominant lineage in western China’s plateau rivers and plays a vital role in sustaining ecological balance. In recent decades, overfishing, water pollution, and large-scale hydropower development have led to drastic declines in wild schizothoracine populations (Qing et al., 2019). Consequently, this group has been prioritized in conservation efforts including stock enhancement and habitat rehabilitation.
Although research has begun to characterize the spawning habitat of the subfamily Schizothoracinae (Cyprinidae), major knowledge gaps remain. Most studies use habitat suitability curves based on static variables like water depth or velocity (Shao et al., 2015; Fu et al., 2016), often based on short-term surveys or expert judgment, which may introduce biases or overlook behavioral nuances (Liu, 2020). Moreover, few studies have tracked habitat use across the entire spawning cycle, despite evidence that fish may shift their habitat preferences at different reproductive stages (Cui et al., 2019; Zhou et al., 2022).
Schizothorax wangchiachii, a representative species of the genus Schizothorax, has become a key conservation target in projects along the Jinsha River. Adults are herbivorous, primarily feeding on algae, and exhibit short-distance migratory behavior (Wu, 1982). Females typically mature at four years of age, while males mature at three. The spawning season occurs in winter (December to January) when water temperatures are low, and both sexes display distinct secondary sexual characteristics during this period. Spawning takes place in shallow (25–55 cm), fast-flowing gravel-sand shoals, with batch spawning and multiple egg releases per day (Yan, 2016). Mature eggs are pale yellow, measuring (3.18 ± 0.17) mm in diameter, swelling to (3.96 ± 0.25) mm after water absorption. At water temperatures between 12.7 and 14.0 °C, fertilized eggs hatch after approximately 192.5 hours (Liu et al., 2015). These reproductive traits highlight the species’ reliance on specific microhabitat conditions during spawning, yet systematic observations of environmental triggers and fine-scale habitat preferences remain lacking.
To systematically investigate habitat selection mechanisms and key drivers during its spawning period, this study induced natural reproduction by enriching riverbed substrate heterogeneity and employed acoustic telemetry to track the spawning behavior of sexually mature S. wangchiachii. Acoustic telemetry, which involves attaching transmitters to wild individuals and analyzing receiver data, enables the monitoring of fish location, physiological indicators, and surrounding habitat features. This method has been widely applied in studies on the life-history behavior of rare aquatic animals (Shi et al., 2022; Cooke and Wagner, 2004; Wang et al., 2010). The results of this study will contribute to the scientific basis for habitat restoration and ecological regulation in the lower Jinsha River.
2 Materials and methods
2.1 Experimental site
The experiments were carried out in a small river, a tributary of the Heishui River in Ningnan County, Sichuan province, China. This tributary flows from north to south and is a typical mountain river, which serves as the natural habitat of S. wangchiachii. The experimental river section selected in this study is 28.1 meters in length, with a width ranging from 3.2 to 8.6 meters and an average width of 5.8 ± 0.4 meters, exhibiting high habitat heterogeneity with alternating deep pools and riffles. The geographic coordinates of the study section extend from (102.64626105°E, 27.15775144°N) to (102.64632658°E, 27.15798904°N). Hydraulic measurements revealed the following: water depth ranged from 0.07 to 1.1 meters (mean ± SD: 0.56 ± 0.18 m), surface flow velocity ranged from 0.03 to 0.79 m/s (mean ± SD: 0.28 ± 0.12 m/s), and bottom flow velocity ranged from 0.03 to 0.44 m/s (mean ± SD: 0.12 ± 0.08 m/s). The substrate composition included gravel (2–64 mm), pebbles (64–256 mm), and boulders (>256 mm). Before the experiment, 2 cm mesh blocking nets were installed upstream and downstream of the study section to prevent the experimental fish from escaping. At the same time, substrate diversity was enhanced by enriching a suitable natural habitat with fine sand and small gravel to create heterogeneous benthic habitats. Throughout the experimental period, real-time flow discharge regulation was implemented to maintain hydraulic stability, ensuring that water depth and flow velocity within the test section remained within controlled thresholds (± 5% depth variation; velocity coefficient of variation < 0.15).
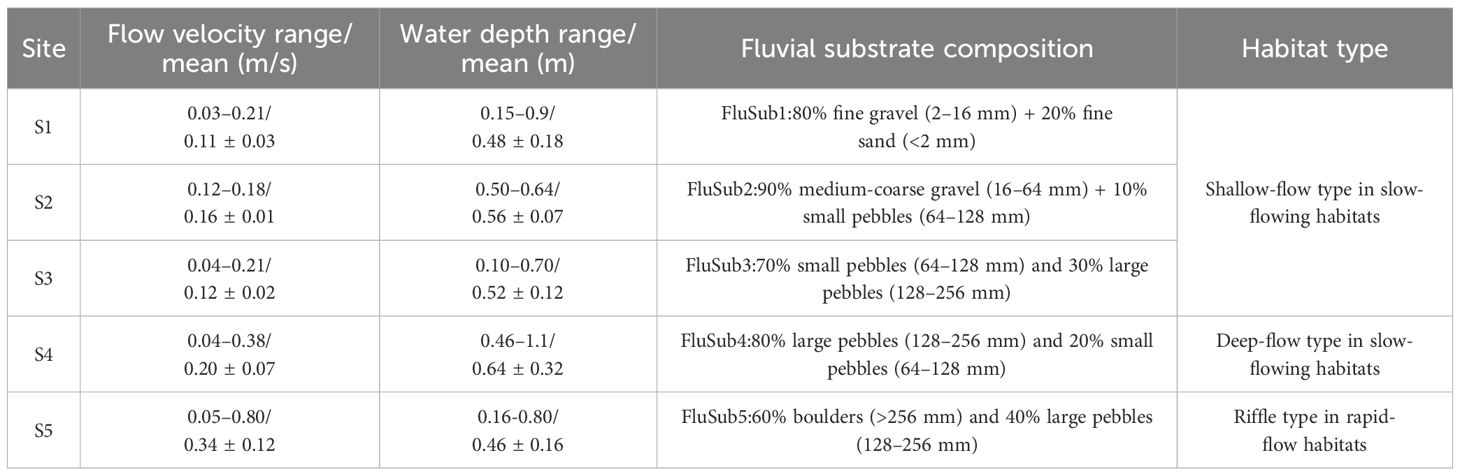
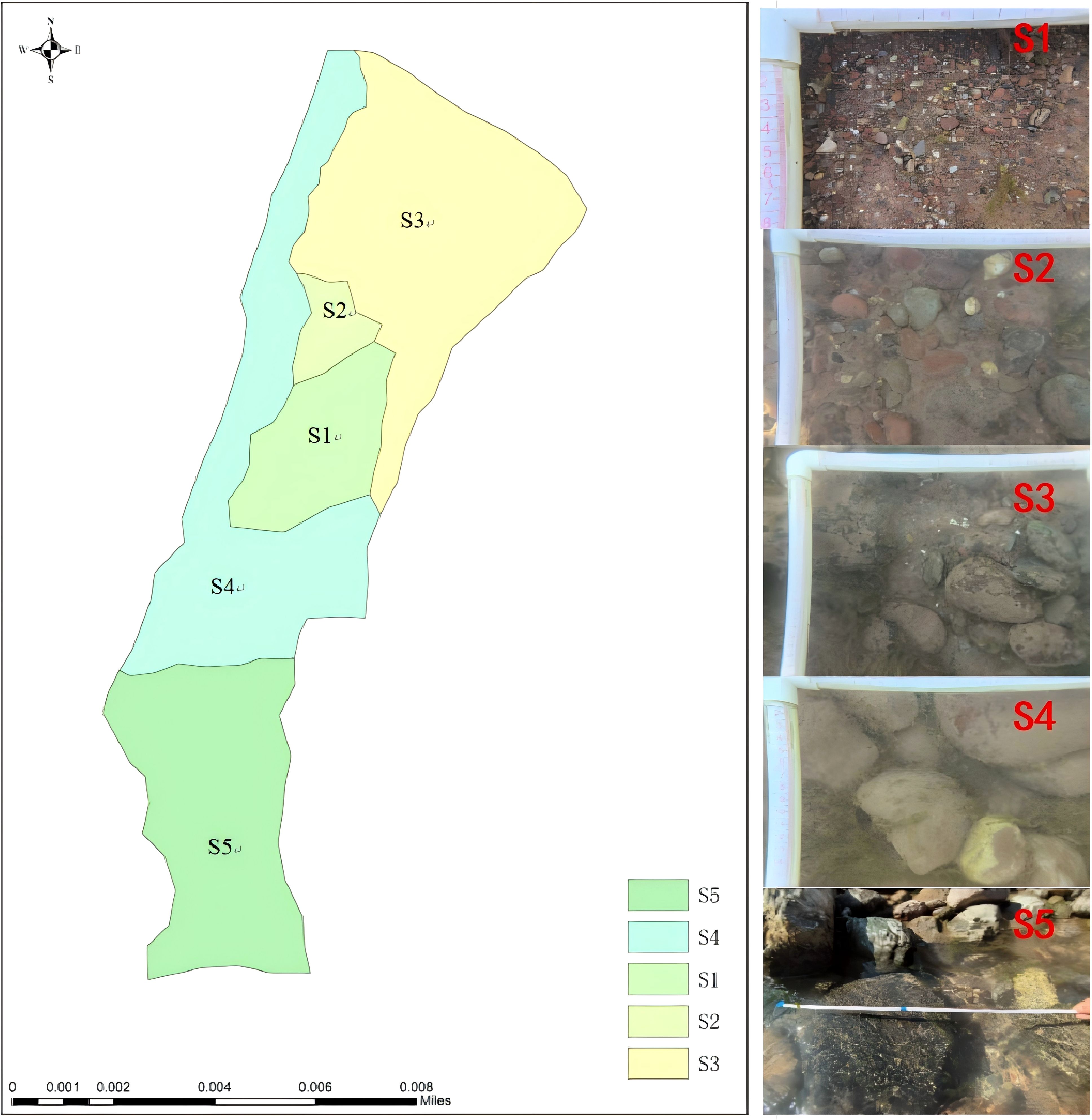
Referring to existing literature (Yang et al., 2017), the experimental river beach was divided into five zones (S1–S5) based on substrate particle size classification. The substrate of S1 was composed of 80% fine gravel (2–6 mm) and 20% fine sand (< 2 mm); S2, 90% medium-coarse gravel (16–64 mm) and 10% small pebbles (64–128 mm); S3, 70% small pebbles (64–128 mm) and 30% large pebbles (128–256 mm); S4, 80% large pebbles (128–256 mm) and 20% small pebbles (64–128 mm); and S5, 60% boulders (> 256 mm) and 40% large pebbles (128–256 mm).
2.2 Experimental methods
2.2.1 Acoustic tags and receivers
This study used 795-series acoustic tags (HTI, Seattle, USA) with a transmission frequency of 307 kHz. The tag shell was a transparent pressure-resistant acrylic tube with an external size of 6.8 mm in diameter and 17.5 mm in length. The tag weighed 0.65 g in air and only 0.34 g in water, with a service life of 4 months. Before use, the tag was placed in the magnetic field coil of the programmer for its activation (Shi et al., 2022).
The receiver used in this study was model HR3, which can continuously receive and store the uniquely coded ultrasonic signals emitted in the surroundings. A single receiver can receive and store 10 million tag signals. The receiver is powered by a built-in lithium battery and can be used for about 6 months at a time. A total of 6 receivers were used in this study, arranged in a W-shaped in the test river section (Figure 1). The interval between two neighbouring receivers was 5–7 m, and these receivers responded to each other to achieve microsecond time synchronization so as to ensure accurate positioning of the transmitter. Before the experiment, the receiving performance of the receiver was tested. The results showed that the signal receiving rate of the 5 receivers reached 100%. The receiver was fixed 0.5 m below the water surface to the anchor with a nylon rope, and the anchor was inserted into the riverbed with an insertion depth of > 0.5 m to ensure stability of the whole system without shaking.
2.2.2 Experimental fish and tagging methods
The fish used in this experiment were caught from the main stream of the Heishui River using a trawl with a mesh of 3 cm, a length of 10 m, and a height of 2 m. After being caught, the fish were temporarily kept in the nearby Xingfu Farm for one week. The aquaculture water was from the Xingfu River to ensure the same water source as in the experimental area. A total of 16 healthy broodstock (4 females and 12 males) with strong physique and obvious secondary sexual characteristics were selected for the experiment, and the selected male fish had a large number of obvious pearl organs on their snouts and a rough body, while female fish had a smooth body, an expanded abdomen, a red genital pore, and eggs would flow out when the female fish were pressed (Yan, 2016). Ten were selected for acoustic tagging (Table 1). The tagged fish had a body length range of 335–420 mm, an average body length of 390 mm, a weight range of 750–1250 g, and an average weight of 985 g, meeting the requirement that the weight of the transmitter must be less than 2% of the fish body weight (Guillemette et al., 2002; Bridger and Booth, 2003; Luo et al., 2013).
In order to minimize the impact of the tagging operation on the fish to be tested, the dorsal fin tag attachment method rather than the abdominal implantation method was used. This adopted method was similar to the tag hanging method, in which MS-222 was used as an anesthetic. The optimal anesthesia period was when the fish lay showing their stomach; stopped tail swinging; and made no response to slight touch (Shi et al., 2022). At this time, tagging was performed with the following procedures: The base of the dorsal fin was disinfected with iodine, and the tag was tied to the back with a needle and thread through the gap between the first and second fin bones of the dorsal fin of the fish. Afterwards, the wound was disinfected with antibiotics, and then the fish were placed into a pond next to the experimental site for temporary rearing.
2.2.3 Experimental process record
After 24-hour temporary rearing, fish were observed. The tagged fish with no abnormal responses were released into the experimental site at 17:00 on January 22, 2024. The tagged fish swam actively after their release, and no stress response was observed. The natural spawning of S. wangchiachii was observed from 13:40 to 14:20 on January 26, 2024, during which, five spawning and mating events were observed, with each mating lasting 15–20 seconds. The riverbank and underwater monitoring equipment simultaneously captured and recorded the spawning behaviors. The experiment ended on January 30, the tagged fish were recovered, and the equipment sites were cleaned up.
2.3 Data acquisition and analysis
2.3.1 Environmental data
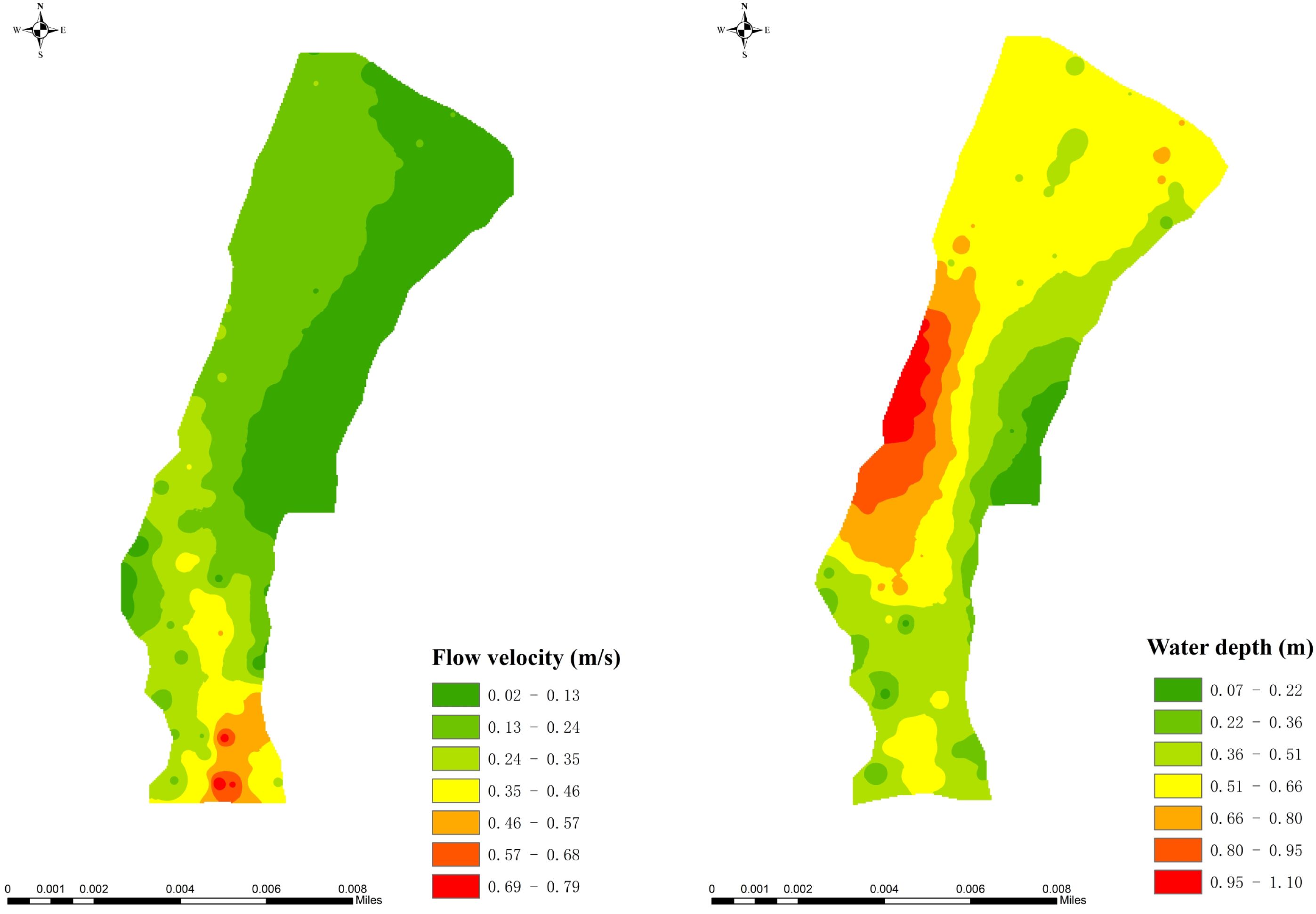
Before the experiments, water depth and flow velocity were measured at intervals of 0.5 m across the study area. Surface velocity was defined as the flow rate at 0.1 m below the water surface, while bottom velocity measurements, taken at 0.8 relative water depth, were exclusively performed in locations exceeding 0.5 m depth. Flow velocity was measured using a portable acoustic Doppler velocimeter (ADV, FlowTracker2, SonTek/YSI Inc., USA), which provides high-resolution measurements of three-dimensional flow components in shallow water environments. Spatial distribution maps of flow velocity and water depth were subsequently generated using the Inverse Distance Weighting interpolation method in ArcGIS 10.6 (Deng, 2008).
2.3.2 Fish trajectory data
The data were preprocessed and analyzed using Vemco’s VUE software to acquire receiver reception rates and performance metrics. The geospatial trajectory data of tagged fish, comprising sequential latitude-longitude coordinates within the experimental domain, were obtained through Fathom Position software (Vemco Inc.) (Shi et al., 2022). The Fishnet Tool in ArcGIS 10.6 was utilized to grid the experimental sites with a size of 0.6 × 0.6 m. Based on the longitudinal trajectory coordinate dataset, fish occurrence frequency per grid was quantified by counting trajectory intersections within each cell, followed by spatial frequency distribution calculations.
2.3.3 Spatial autocorrelation analysis
Spatial weight matrices based on polygon-contiguity relationships (Geng, 2018; Longley and Frank Goodchild, 2020) were employed to conduct global spatial autocorrelation and hotspot analysis (also known as high/low clustering analysis) for tagged fish distributions using ArcGIS 10.2. Specifically, the global spatial autocorrelation was evaluated through Moran’s I index, while the hotspot analysis was conducted using the Getis-Ord Gi* statistic (Piatt et al., 2006). The Moran’s I index ranges from [-1, 1], where values approaching 1 indicate significant spatial clustering of the target attribute, and values approaching -1 denote pronounced spatial dispersion. Both analytical results were subjected to significance test using the permutation test (permutation = 999, α = 0.05), with spatial distribution patterns categorized as: random distribution (Z < 1.65, P > 0.10), clustered distribution (1.65 < Z < 1.96, P < 0.10), and high clustered distribution (1.96 < Z < 2.58, P < 0.05 and Z > 2.58, P < 0.01) (Mitchell, 2005).
The predictive model for fish habitat utilization was developed using the random forest classification algorithm: First, the model framework incorporated three predictive variable categories: 1) biological features including individual variation (modeled as random intercepts via individual ID, IndVar) and Sexual dimorphism (Sx); 2) environmental variables at grid-cell resolution comprising Fluvial substrate composition (FluSub), water depth (WD), surface flow velocity (SurVel), and bottom flow velocity (BotVel); 3) habitat use intensity, a response variable derived from occurrence frequency within grid cells, factorized via K-means clustering into three distinct classes: Class I (Absence, 0% frequency, n = 872), Class II (Occasional occurrence, > 0% to ≤ 3.1%, n = 803), and Class III (Frequent occurrence, > 3.1%, n = 95). The dataset was then partitioned through stratified random sampling into training (70%) and independent testing (30%) subsets, followed by systematic optimization of critical hyperparameters—mtry (variables per node split) and ntree (total decision trees)—via grid search. Finally, model performance was rigorously evaluated using classification metrics (Cohen’s Kappa, accuracy, precision, recall) and feature importance rankings (MDG% reflecting impurity reduction), ensuring methodological transparency while maintaining ecological relevance through biologically interpretable predictors and validation protocols.
The model construction was implemented using the randomForest package in RStudio software (version 4.1.0), while mapping was performed with ArcGIS 10.6 (ESRI, USA) and OriginPro 2022 (OriginLab, USA).
3 Results
3.1 Habitat characteristics of experiment site
The spatial distribution of flow velocity and water depth in the experiment river section is shown in Figure 2. Referring to existing literature (Yang et al., 2017), the experimental river section was divided into five zones (S1–S5) based on substrate particle size classification(Figure 3). The habitat characteristics of each site are shown in Table 1. In terms of river physical habitat type (Li, 2009), S1, S2, and S3 belonged to the shallow-flow type in slow-flowing habitats, S4 belonged to the deep-flow type in slow-flowing habitats, and S5 belonged to the riffle type in rapid-flow habitats.
3.2 Spatial distribution of S.wangchiachii during spawning
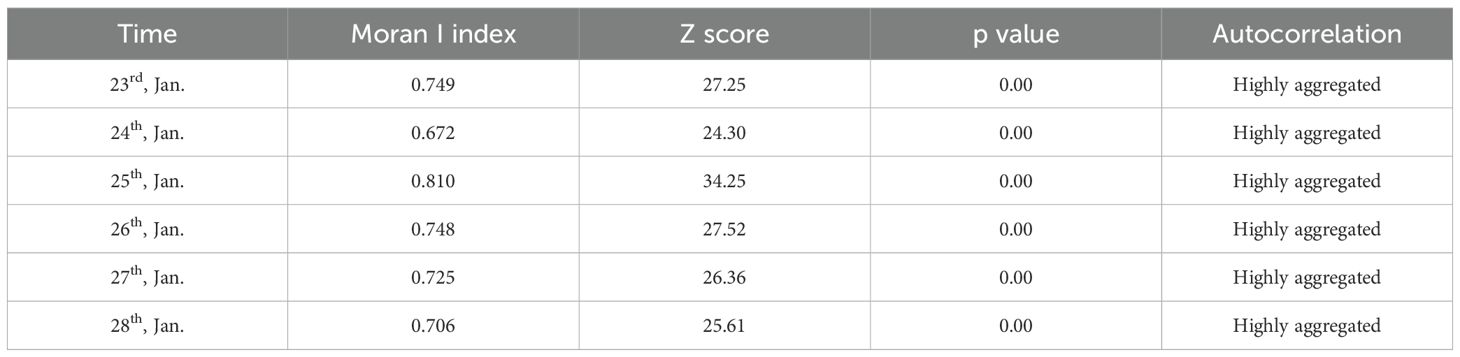
During the experiment, 61,949 signals were collected from 10 tagged fish, with corresponding longitude-latitude coordinates derived through interpretation. Fish occurrence signals were quantified per grid (0.6 m × 0.6 m) at the experimental site based on georeferenced coordinates. Results indicated that 203 of 375 total grids exhibited S. wangchiachii tracking signals, with a maximum single-grid signal count of 2,287. Spatial autocorrelation analysis revealed Moran’s I indices for S. wangchiachii distribution ranging from 0.672 to 0.810, alongside Z scores of 25.61–34.25 (Table 2), both peaking on 25 January 2024 (pre-spawning) before gradually declining post-spawning. Throughout the experiment, S. wangchiachii exhibited significant aggregation (Z > 2.58, P < 0.01). Hotspot analysis identified high-aggregation zones predominantly in S3 during the spawning phase, except for S1 and S3 clusters on 23 January (Figure 4), confirming that spawning-phase aggregation areas of S. wangchiachii were primarily classified as shallow-flow type in slow-flowing habitats.
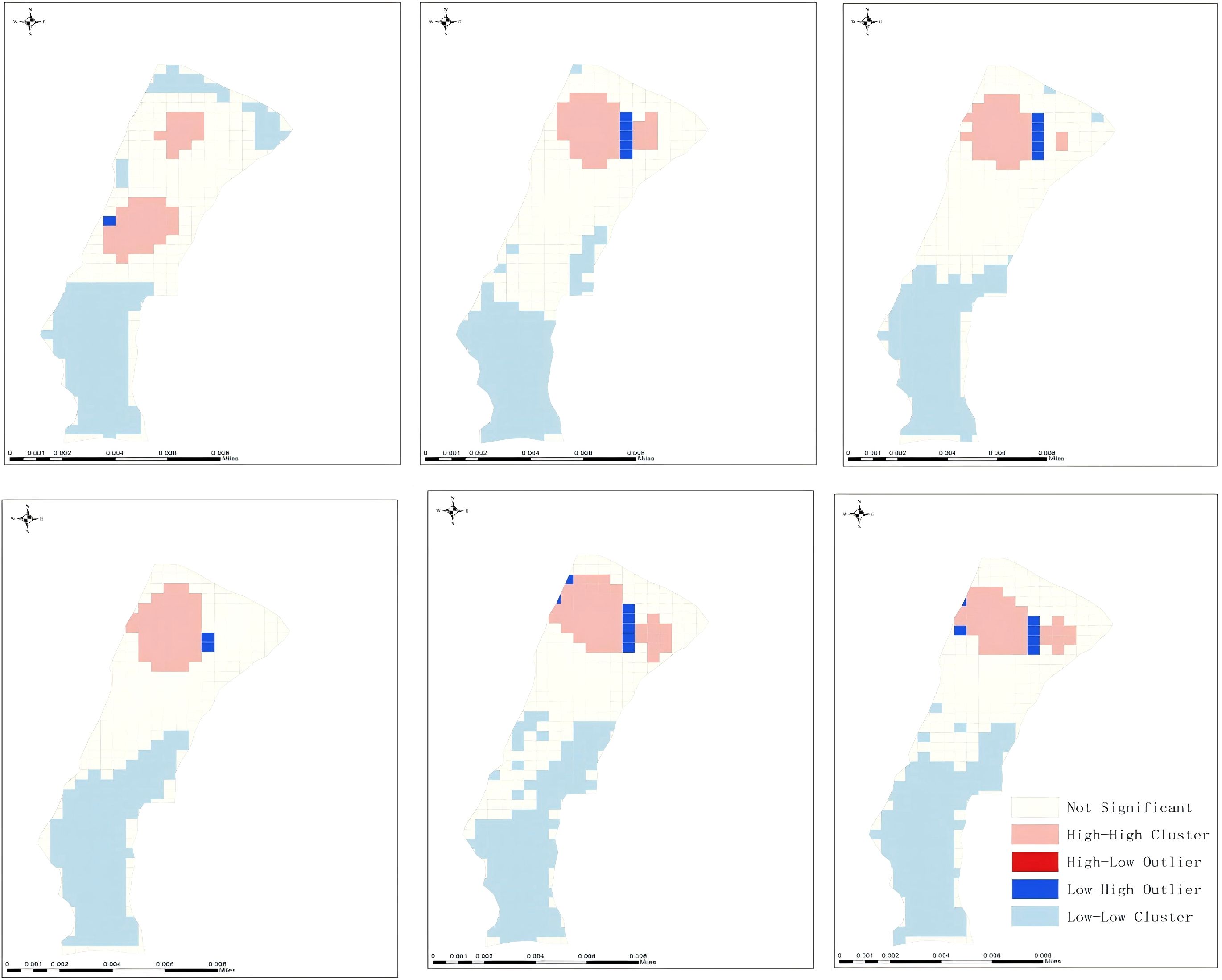
Figure 4. Hot spot diagram of spatial distribution of S.wangchiachii in Jan. 23rd-28th, High-High Cluster – High-value areas surrounded by other high values, denoting significant hotspot clusters; High-Low Outlier – High-value areas adjacent to low values, representing spatial; Low-High Outlier – Low-value areas adjacent to high values, representing spatial outliers. Low-Low Cluster – Low-value areas surrounded by other low values, denoting significant coldspot clusters; Not Significant – No significant spatial association with neighboring areas, indicating a random pattern.
3.3 Habitat characteristics of distribution area of S.wangchiachii
Analysis of habitat characteristics in the distribution areas of S. wangchiachii revealed distinct substrate preferences, with occurrence frequencies ranked as FluSub3 (44,855 records, averaging 760 per grid) > FluSub1 (10,366 records, averaging 518 per grid) > FluSub2 (1,278 records, averaging 213 per grid) > FluSub4 (5,449 records, averaging 102 per grid) > FluSub5 (51records, averaging 1.3 per grid), indicating a strong selection for substrate type FluSub3 characterized by 70% small pebbles + 30% large pebbles. Hydrologically, these fish inhabited waters with depths of 0.17–1.02 m (primarily 0.43–0.66 m) and surface flow velocities of 0.03–0.80 m/s, though higher abundances were observed in slower flows between 0.1–0.25 m/s (Figure 5).
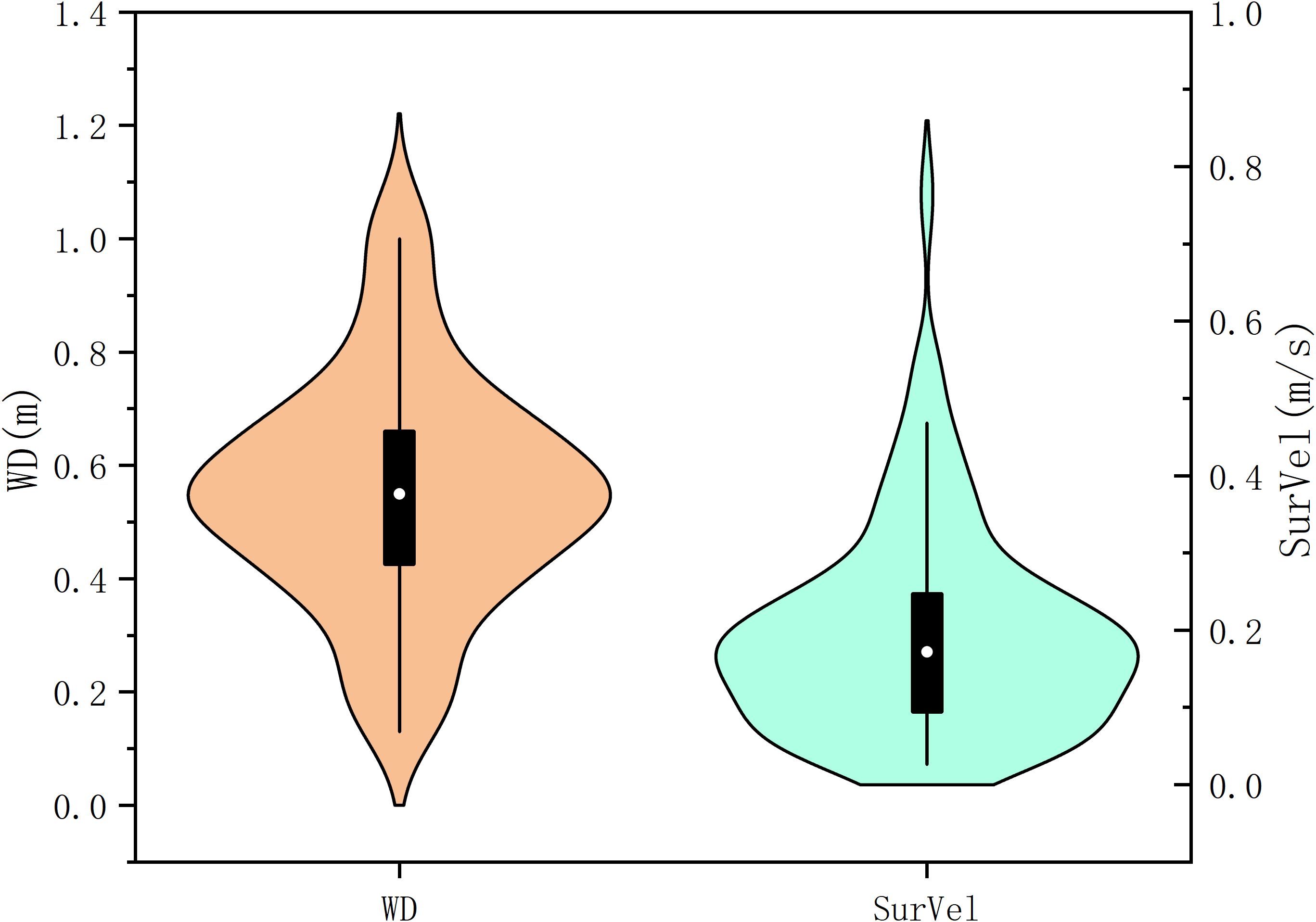
Figure 5. Statistical analysis of water depth and surface flow velocity in the distribution area of S.wangchiachii.
3.4 Spawning habitat characteristics of S.wangchiachii
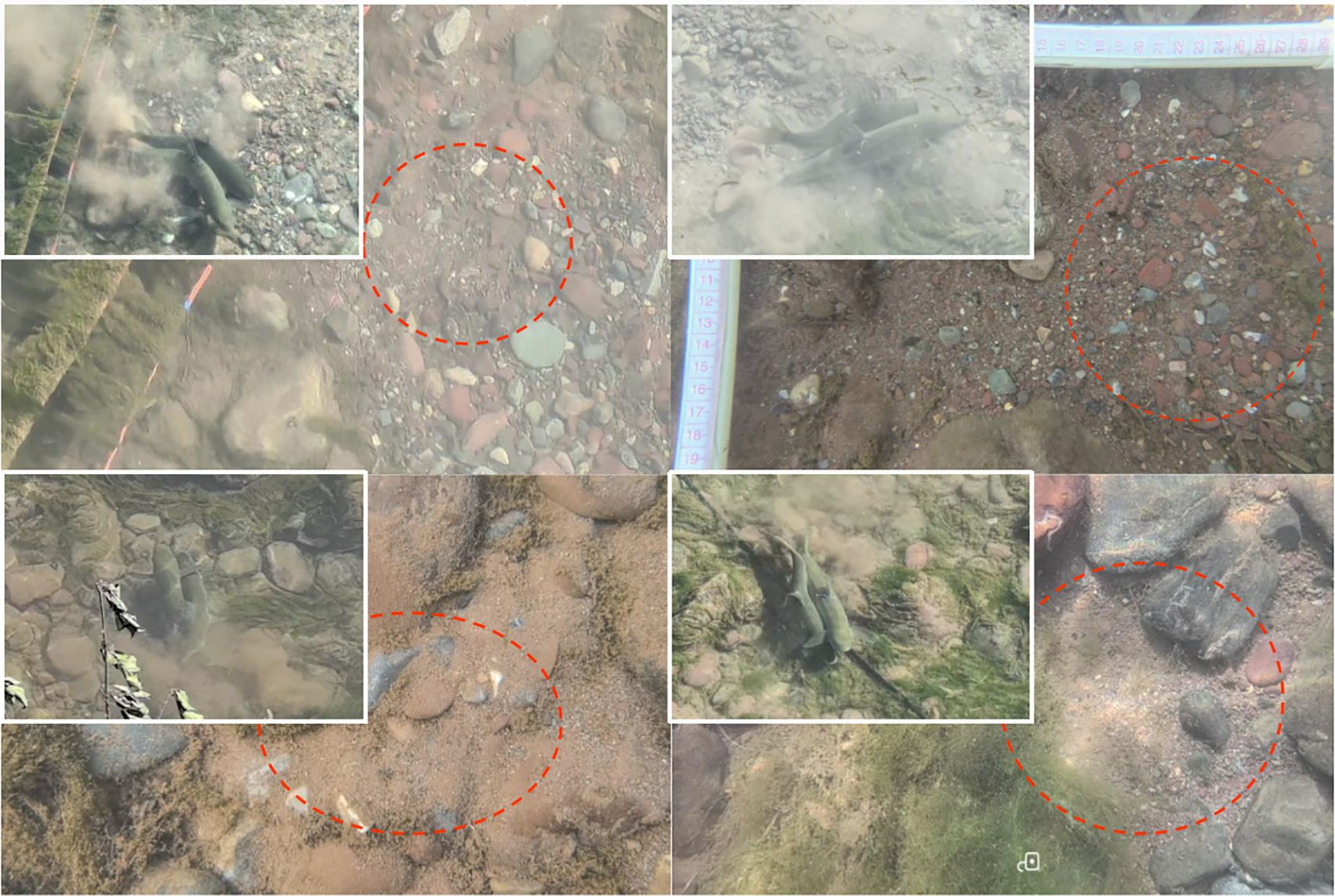
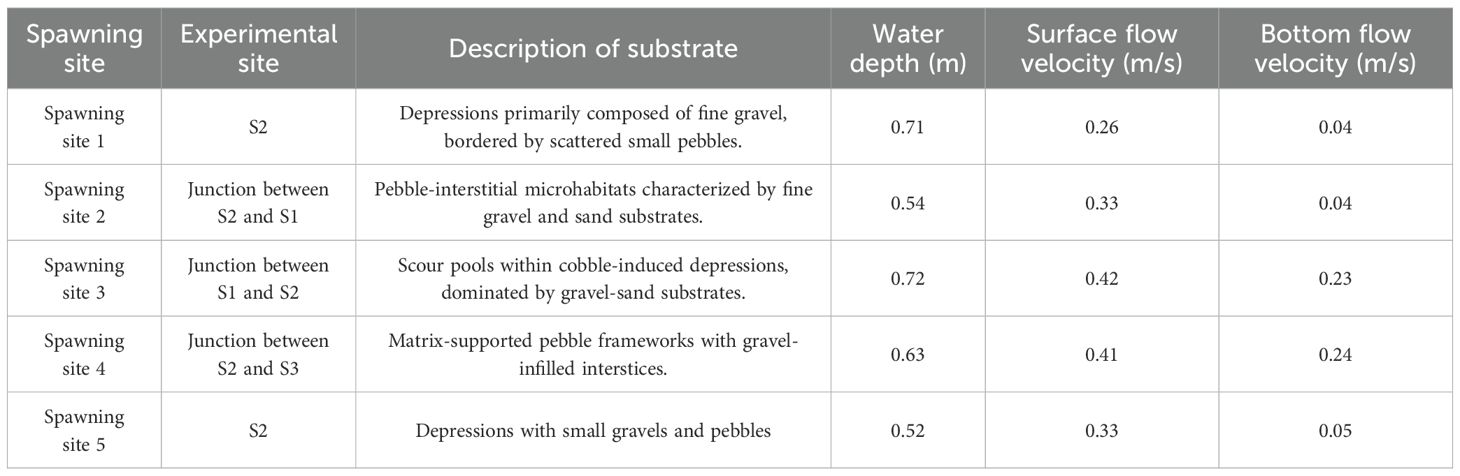
During the experiment, a total of five spawning and mating events of S.wangchiachii were observed, with each lasting 15–20 seconds. The spawning process raised a large amount of gravel, and the fertilized eggs were scattered in the gaps among the gravels or shallowly buried in fine sand. The reproductive spawning of S.wangchiachii primarily occurred in S2 and at the junctions between S2 and S1, and between S3 and S4, and spawned in the depression (nest-like places) of the riverbed, where substrate was mainly composed of fine gravel and sand (Figure 6); the water depth ranged from 0.52 m to 0.71 m; the surface flow velocity ranged from 0.26 m/s to 0.42 m/s; and the bottom flow velocity ranged from 0.04 m/s to 0.24 m/s; with a notable velocity gradient was observed between surface and bottom layers (Table 3).
3.5 Influencing factors of S. wangchiachii habitat selection during spawning
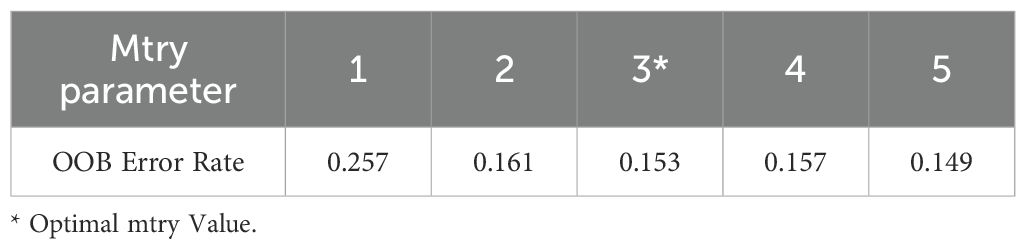
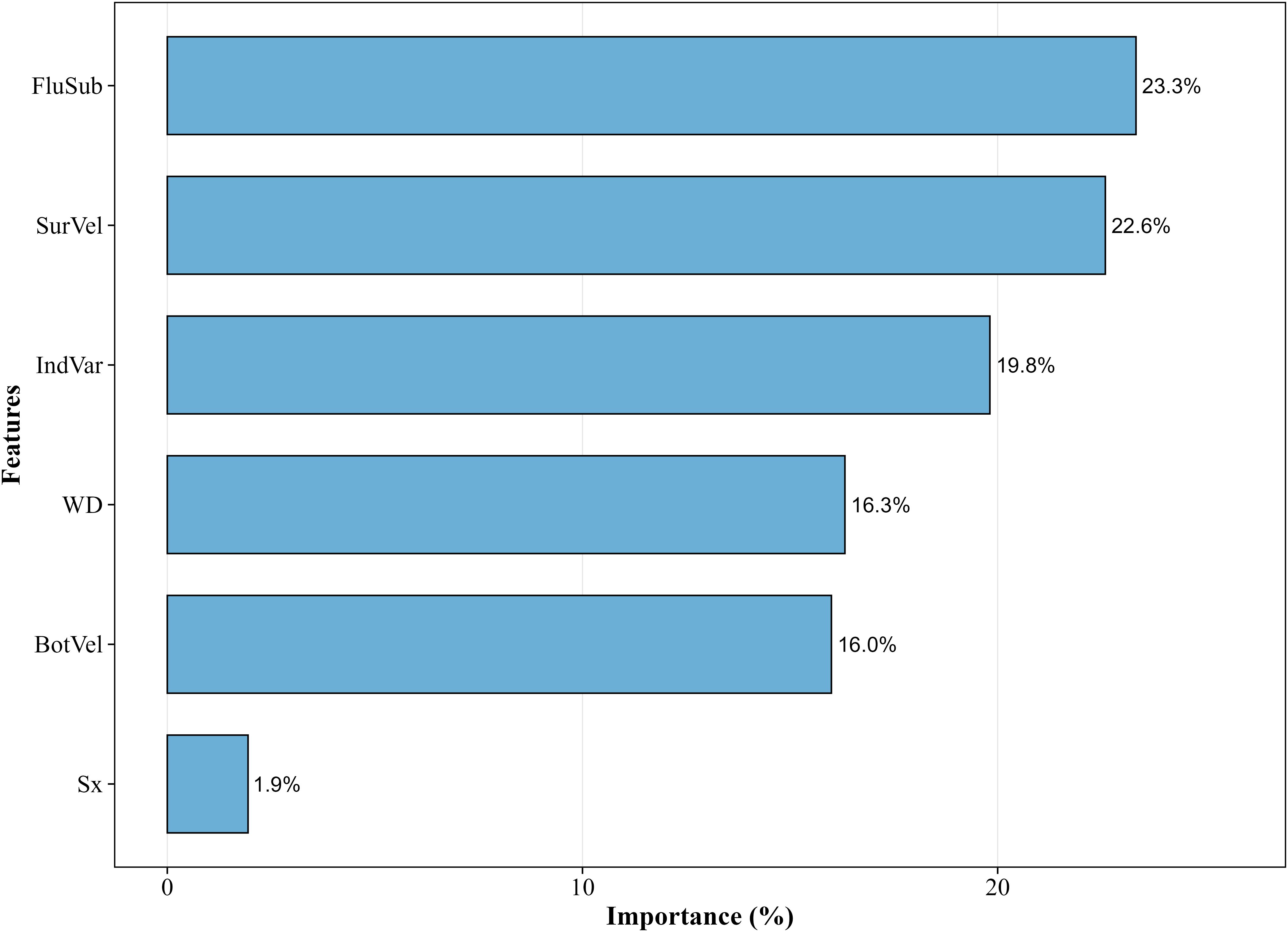
After conducting a comprehensive grid search of mtry parameters (1-5), the optimal mtry value was determined as 3 (Table 4). The ntree was fixed at 200 based on Out-of-Bag (OOB) error stabilization analysis. The Random Forest model demonstrated strong performance on an independent test set: overall accuracy 85.3% (95% CI: 81.9–88.2%), recall 84.7%, and Cohen’s kappa of 0.73, indicating substantial agreement between predictions and ground-truth labels (Landis and Koch, 1977). Feature importance analysis (Figure 7) highlighted fluvial substrate composition (mean Gini decrease: 23.3%) and surface flow velocity (mean Gini decrease: 22.6%) as key drivers of fish habitat selection.
4 Discussion
4.1 Experimental site and fish tagging method
Habitat refers to the sum of environmental conditions in a specific space occupied by an organism, a population, a community, or an ecosystem. Specifically, the habitat includes five aspects, namely, geography, physics, chemistry, biology, and time, and their integration and separation in space form a variety of habitat structures (Mellas and Haynes, 1985). Different from the existing indoor artificial simulation of fish habitats, this study employed the river section of natural habitat of the S.wangchiachii as the experimental site. The naturalness of its environmental conditions, including the horizontal diversity and vertical diversity of the habitat structure, was superior to that of artificially simulated habitats, enabling the experimental fish to better adapt to the environment and making the research results more authentic and reliable.
It is essential for the physiological condition and behavioral characteristics of the fish are not affected by the ultrasonic transmitter tagging method so as to obtain representative data that reflect the actual life of fish in their natural environment (Bridger and Booth, 2003). Currently, there are mainly three methods for tagging fish with ultrasonic transmitters: surgical implantation, gastric insertion, and external attachment (Lv, 2024; Welch et al., 2007). Considering that the experimental fish in this study were sexually mature (gonadal development in stage IV or above), the dorsal fin attachment method was adopted to minimize the impact of acoustic tagging on the reproduction of the experimental fish for the following reasons: (1) Compared with tagging methods such as abdominal implantation involving muscle damage, the base of the fish’s dorsal fin contains less blood vessels and nerves, thereby minimize the duration of negative effects; (2) Dorsal fin tagging does not occupy the abdominal cavity space, nor cause additional pressure on the body wall or intestines, thus reducing the impact on the gonads of the parental fish (Luo et al., 2013; Bridger and Booth, 2003). In addition, to decrease the impact of tagging, this study also took multiple measures such as selecting large and strong test fish (body length > 350 mm; weight > 750 g), using small and lightweight transmitters (< 0.05% of fish body weight), adopting strict and effective sterilization measures during the tagging process, temporary rearing, and ensuring same source experimental water. During the experiment, all the individuals remained highly active and in good physiological condition.
4.2 Habitat selection for natural reproduction of S.wangchiachii
The spawning grounds of S.wangchiachii are generally located in shallow streams or shoals (Rong et al., 2022), Similar patterns are observed in other rheophilic Cyprinids, such as Gymnodiptychus dybowskii (Dybowski’s naked osman; Cai et al., 2013), Schizothorax kozlovi (Kozlov’s snowtrout; Chen and Luo, 1997), Schizothorax lissolabiatus (smooth-lipped schizothoracin; Sun, 2018), Schizopygopsis malacanthus baoxingensis (Baoxing soft-spine schizothoracin; Zhou, 2007) and Gymnocypris przewalskii (Przewalski’s naked carp; Zhou et al., 2022). In this study, the spawning sites of S. wangchiachii exhibited the following microhabitat characteristics: shallow shoals with slow water flow, gentle river slope, shallow depth, and substrate dominated by small pebbles and gravels.
Using acoustic telemetry and direct in situ observations, we found that S. wangchiachii exhibits a biphasic microhabitat selection strategy during the reproductive period. In the pre- and post-spawning stages, adults aggregated in low-velocity areas (surface velocity: 0.10–0.25 m/s; depth: 0.43–0.66 m) with substrates consisting of 70% small pebbles and 30% large pebbles. Individuals remained relatively stationary or foraged in these zones to conserve energy. This behavior is similar to that of Barbus barbus (European Barbel), which selects low-velocity habitats outside its main spawning migrations (Lucas et al., 2001).
During active spawning, adults moved to riverbed depressions (“nests”) characterized by greater depth (0.52–0.71 m), elevated surface flow (0.32–0.42 m/s), reduced bottom velocity (0.04–0.24 m/s), and substrates composed of fine gravel and sand. These microhabitats facilitated mating and egg deposition. After spawning, females typically returned to resting areas, while males remained to guard embryos. A similar nest-site selection has been reported for Catostomus commersonii (white sucker), which uses gravel depressions in moderate-flow environments for reproduction (Cooke and Bunt, 2001).
Collectively, these findings suggest that S. wangchiachii selects two functionally distinct reproductive habitats: (1) feeding/resting areas (shallow, slow-flowing, pebble-dominated), which are essential for energy conservation; and (2) spawning/incubation sites (deeper, higher surface flow, fine gravel–sand mixture), which provide stable conditions for embryo development. The latter habitat type enables micro-adhesive eggs to settle into gravel crevices, reducing predation, shielding from UV radiation, and preventing downstream displacement into unsuitable environments (Yan et al., 2017).
Compared to previous work, our study refines the microhabitat characteristics of S. wangchiachii by pinpointing specific depth, velocity, and substrate size ranges. This refinement is facilitated by high-resolution telemetry data that capture fine-scale movements, unlike earlier studies relying on expert surveys or coarse habitat suitability indices (Shao et al., 2015; Fu et al., 2016; Liu, 2020).
4.3 Key factors influencing S.wangchiachii habitat selection during the spawning period
The natural reproduction activities of many fish species need to be carried out under specific environmental conditions, among which environmental parameters such as water temperature, water flow, water depth, riverbed quality, and light are commonly considered the most important environmental factors (Seesholtz et al., 2014; Ban et al., 2011; Coutant, 2004). For demersal fish, the riverbed substrate is an important ecological factor affecting its spawning, and its reproductive activities have strict demands on the particle size, composition, and layout of substrate. For example, the reproduction of salmon and trout relies on gravel substrate, and the particle size and layout of gravel directly affect parent fish spawning and juvenile fish hatching (Soulsby et al., 2001). Gymnocypris przewalskii (Przewalski’s naked carp) only selects pebble-based substrate habitat for its spawning since it is almost impossible to induce reproduction in a pebble-free substrate habitat (Zhou et al., 2022).
In this study, random forest model analysis revealed that substrate is the primary factor influencing spawning site selection by sexually mature S. wangchiachii. Although spawning can occur in areas with various substrate types, the fish displayed significant differences in substrate preferences at different reproductive stages: before and after spawning, they preferred small cobble substrates; during spawning, they significantly favored substrates of finer gravel or sand. This variation reflects their stage-specific microhabitat requirements and is consistent with observations in other Schizothoracinae fishes (Zhou et al., 2022; Yan, 2016; Chen and Luo, 1997).
Nevertheless, distinct interspecific differences in substrate preferences exist, shaped largely by ecological habits and native habitat conditions. For instance, Gymnocypris przewalskii (Przewalski’s naked carp) prefers sandy substrates during pre- and post-spawning stages but switches to cobble-dominated substrates during spawning (Zhou et al., 2022). This likely reflects adaptations to their respective environments: S. wangchiachii inhabits rivers in China’s southwestern mountains (e.g., Yalong, Dadu, Qingyi, and Minjiang Rivers), characterized by high bed roughness (0.25–0.60) and cobble-gravel substrates (Wang et al., 2010). In contrast, G. przewalskii originates from Qinghai Lake, which features muddy and sandy lakebeds. The spawning preference of S. wangchiachii for finer gravel and sand may be associated with its reproductive strategy and egg traits—specifically, the adhesive and demersal nature of its eggs. Finer substrates facilitate nest formation and enhance egg adhesion and burial. Meanwhile, G. przewalskii may rely on the micro-hydrodynamic stimulation generated within cobble beds to promote gonadal development and the initiation of reproductive activity (Hu et al., 1975). These species-specific differences in substrate preference reflect long-term ecological adaptation strategies.
It is worth noting that this shift in substrate preference may be influenced not only by microenvironmental needs but also by changes in flow velocity and reproductive physiology. Studies in drift-egg-producing species such as Ctenopharyngodon idellus (grass carp) have shown that elevated flow velocity can stimulate the hypothalamic–pituitary–gonadal (HPG) axis, leading to increased expression of gonadotropin-related genes and secretion of steroid hormones, which in turn promote gonadal development and spawning readiness (Shu et al., 2024; Wang et al., 2023). However, since these species differ fundamentally from benthic fishes that produce adhesive demersal eggs, such as S. wangchiachii, there is currently no direct evidence supporting a similar mechanism in the latter. Thus, the hypothesis that hydrodynamic stimulation might regulate reproduction in adhesive-egg species remains plausible but unverified, and further physiological research is necessary to test this interaction between flow conditions, reproductive hormones, and substrate selection.
While this study primarily compares S. wangchiachii with other Chinese Schizothoracinae species, future research could be extended to cyprinid fishes from Europe and North America that exhibit similar reproductive strategies. Many European cyprinids display flow-induced reproductive migrations and distinct substrate preferences during spawning. For instance, Barbus barbus (European barbel) migrates from slow-flowing habitats to gravel-rich, fast-flowing reaches for spawning (Lucas et al., 2001). Similarly, Chondrostoma nasus (nase) migrates to tributaries where females deposit adhesive eggs in gravel nests, selecting spawning sites characterized by specific gravel substrate structures (Horváth and Specziár, 2013). Both Leuciscus leuciscus (common dace) and Leuciscus idus (ide) prefer to spawn in shallow, fast-flowing gravel or sand beds, where eggs adhere to the substrate; substrate composition and hydrodynamic conditions significantly influence their spawning success (Demers and Magnan, 1992; Kottelat and Freyhof, 2007). In North America, Notropis chrosomus (rainbow shiner) shows a comparable migratory spawning pattern, aggregating in fast-flowing, gravel-bottomed shoals (Jelks et al., 2008). These examples suggest that despite geographical and phylogenetic differences, many cyprinid species worldwide exhibit convergent reproductive adaptations to flow regimes and substrate characteristics.
This evolutionary convergence in reproductive behavior enhances fertilization and hatching success and reduces the predation risk to fertilized eggs, reflecting the high adaptability of benthic fishes to their reproductive microhabitats.
5 Conclusion
This study facilitated the natural reproduction of S. wangchiachii by enhancing their natural habitat with diverse substrates in controlled river sections, combined with acoustic telemetry tracking of their spawning movements. Our findings resolve critical habitat selection patterns and identify key environmental drivers during their reproductive cycle:
1. S. wangchiachii exhibited significant aggregation behavior during spawning seasons, predominantly in Zone S3 characterized by substrate composition (70% small pebbles + 30% large cobbles), water depth 0.43–0.66 m, and surface velocity 0.1–0.25 m/s.
2. Spawning pairs preferentially selected riverbed depressions with distinct hydraulic conditions: higher surface velocity (0.26–0.42 m/s) contrasting with reduced bottom velocity (0.04–0.24 m/s), greater depth (0.52–0.71 m), and finer gravel substrates. This microhabitat configuration provides optimal hydraulic stability for the incubation of semi-adhesive demersal eggs.
3. Surface velocity and substrate composition emerged as dominant factors influencing spawning habitat selection. Current debates persist regarding whether Schizothorax spawning substrate selection is driven by physical substrate characteristics (Zhang et al., 2007; Riley et al., 2019) or substrate-modulated hydrodynamics. Future investigations will employ numerical modeling of substrate-microflow field interactions to elucidate the coupling mechanisms among substrate morphology, microhydraulic patterns, and spawning preferences. These findings will inform both natural spawning habitat restoration in the lower Jinsha River and ecological regulation strategies targeting Schizothorax reproductive success.
Based on the findings of this study, we propose the following targeted recommendations for habitat management and restoration: (1) In habitat restoration projects for Schizothorax, mixed-size substrates dominated by small pebbles and coarse sand should be added, and riverbed depressions with hydraulic heterogeneity should be reconstructed to improve the suitability and stability of spawning microhabitats; (2) During the peak spawning season, ecological flow regulation should aim to maintain suitable surface flow velocities (0.2–0.4 m/s) across a greater number of river sections to expand the area of potential spawning habitats. In key spawning reaches, water level fluctuations should be controlled within 0.5 m to ensure the normal incubation of fertilized eggs; (3) It is recommended to incorporate acoustic telemetry and high-resolution microhabitat mapping technologies into future habitat monitoring and management practices, in order to enable continuous assessment and science-based regulation of spawning habitat suitability under dynamic environmental conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
BL: Conceptualization, Writing – original draft, Formal analysis, Investigation, Project administration, Software, Data curation, Writing – review & editing. FH: Writing – original draft, Investigation, Validation, Supervision, Methodology. WL: Investigation, Writing – review & editing. WS: Writing – review & editing, Validation, Software. JZ: Investigation, Supervision, Software, Visualization, Writing – review & editing. WJ: Writing – review & editing, Data curation, Project administration, Supervision, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the National Key Research and Development Plan (2022YFC3204200), and by the Scientific Research Project of China Three Gorges Corporation (Grant/Award Number: NBWL202200489).
Acknowledgments
Throughout the writing of this dissertation, a great deal of support and assistance was received. Professor Ping Liu is acknowledged for the discussions and English review.
Conflict of interest
Authors BL, FH, WL, WS, JZ, and WJ were employed by the company China Three Gorges Corporation.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1615081/full#supplementary-material
References
Ban X., Du Y., Liu H.-Z., and Ling F. (2011). Applying instream flow incremental method for the spawning habitat protection of Chinese sturgeon (Acipenser sinensis). River Res. Appl. 27, 87–98. doi: 10.1002/rra.1341
Bridger C. J. and Booth R. K. (2003). The effects of biotelemetry transmitter presence and attachment procedures on fish physiology and behavior. Rev. Fisheries Sci. 11, 13–34. doi: 10.1080/16226510390856510
Cai L.-G., Niu J.-G., Li H., and Liu J. (2013). Study on micro-environment at the spawning fields of Gymnodiptychus dybowskii and Diptychus maculates in the Kunes River. Arid Zone Res. 30, 144–148. doi: 10.13866/j.azr.2013.01.013
Chen Y.-X. and Luo Q.-S. (1997). Studies on the reproductive eco-biology of Schizothorax prenanti in Sichuan: V. Reproductive groups and breeding habits. J. Bijie Normal Coll. 1997, 1–5.
Cooke S. J. and Bunt C. M. (2001). Nest-site selection by white sucker (Catostomus commersonii): Use of shallow gravel depressions in moderate-flow runs for spawning. J. Freshw. Ecol. 16, 413–421. doi: 10.1111/j.1600_0633.2001.tb00194.x
Cooke S. J. and Wagner G. N. (2004). Training, experience, and opinions of researchers who use surgical techniques to implant telemetry devices into fish. Fisheries 29, 10–18. doi: 10.1577/1548-8446(2004)29[10:TEAOOR]2.0.CO;2
Coutant C. C. (2004). A riparian habitat hypothesis for successful reproduction of white sturgeon. Rev. Fisheries Sci. 12, 23–73. doi: 10.1080/10641260490273023
Cui K.-C., Liu W., Gao W.-Y., Li P.-L., Wang J.-L., and Tang F.-J. (2019). Suitability index construction and weight analysis for the spawning ground of Oncorhynchus keta. Chin. J. Ecol. 38, 3762–3770. doi: 10.13292/j.1000-4890.201912.031
Demers E. and Magnan P. (1992). Substrate preference and spawning success of common dace in relation to riverbed structure. Can. J. Fisheries Aquat. Sci. 49, 846–854. doi: 10.1139/f92-095
Deng X.-B. (2008). Comparison between two space interpolation methods based on ArcGIS. Geospatial Inf. 6, 85–87.
Dudgeon D., Arthington A. H., Gessner M. O., Kawabata Z. I., Knowler D. J., Lévêque C., et al. (2006). Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182. doi: 10.1017/S1464793105006950, PMID: 16336747
Fu J.-J., Huang B., Rui J.-L., Tan S.-K., and Zhao S. (2016). Application of habitat simulation to fish habitat protection in Heishui River. J. Aquat. Ecol. 37, 70–75. doi: 10.15928/j.1674-3075.2016.03.010
Geng Z. (2018). The environment adaptability and conservation strategy of Eriocheir sinensis in estuarine life stages. (Master's Thesis). East China Normal University, China (Shanghai).
Grill G., Lehner B., Thieme M., Geenen B., Tickner D., Antonelli F., et al. (2019). Mapping the world's free-flowing rivers. Nature 569, 215–221. doi: 10.1038/s41586-019-1111-9, PMID: 31068722
Guillemette M., Woakes A. J., Flagstad A., and Butler P. J. (2002). Effects of data-loggers implanted for a full year in female common eiders. Condor 104, 448–452. doi: 10.1093/condor/104.2.448
Horváth Z. and Specziár A. (2013). Spawning habitat use by nase (Chondrostoma nasus) in a regulated lowland river. J. Appl. Ichthyology 29, 47–53. doi: 10.1111/jai.12010
Hu A., Tang S. S., and Gong S. X. (1975). “Research on the reproductive biology of Gymnocypris przewalskii (Kessler).” in The fish fauna of Qinghai Lake region and biology of Gymnocypris przewalskii przewalskii (Kessler). eds. Institute of Biology, Qinghai Province. (Science Press), pp. 49–62.
Jelks H. L., Walsh S. J., Burkhead N. M., Contreras-Balderas S., Díaz-Pardo E., Hendrickson D. A., et al. (2008). Conservation status of imperiled North American freshwater and diadromous fishes. Fisheries 33, 372–407. doi: 10.1577/1548-8446-33.8.372
Kottelat M. and Freyhof J. (2007). Handbook of European freshwater fishes (Cornol, Switzerland, Publications Kottelat).
Landis J. R. and Koch G. G. (1977). The measurement of observer agreement for categorical data. Biometrics 33, 159–174. doi: 10.2307/2529310
Li X. (2009). Study on ecology of mountain river habitat – Pengxi River of Three Gorges Reservoir area as a case study (China (Chongqing): Chongqing University).
Liermann C. R., Nilsson C., Robertson J., and Ng R. Y. (2012). Implications of dam obstruction for global freshwater fish biodiversity. Bioscience 62 (6), 539–548. doi: 10.1525/bio.2012.62.6.5
Liu S. W. (2020). Study on the application of habitat assessment and restoration technology of Qingyi River based on priority conservation fish (China (Henan): Zhengzhou University).
Liu Y., Zhu T.-B., Wu X.-B., Yan W.-B., and Yang D.-G. (2015). Observations on embryonic and early larval development of Schizothorax wangchiachii. Fisheries Sci. (Shuichan Kexue) 34, 683–689. doi: 10.16378/j.cnki.1003-1111.2015.11.003
Longley P. A. and Goodchild M. F. (2020). “Geographic Information Science and Systems,” in International Encyclopedia of Human Geography (Oxford, UK, Elsevier), 29–36. doi: 10.1016/b978-0-08-102295-5.10557-8
Lucas M. C., Baras E., and Smith C. (2001). Migration and spawning of the European barbel (Barbus barbus): behavioral adaptations to flow conditions. Freshw. Biol. 46, 117–132.
Luo H. W., Duan X. B., Liu S. P., and Chen D. Q. (2013). Effects of transmitter’s surgical implantation on fish: Research progress. Chin. J. Appl. Ecol. 24, 1160–1168. doi: 10.13287/j.1001-9332.2013.0276, PMID: 23898679
Lv S. L. (2024). Improvements of fish tagging methods and its application on yellowfin seabream (Acanthopagrus latus) (China (Zhanjiang: Guangdong Ocean University).
Mellas E. J. and Haynes J. M. (1985). Swimming performance and behavior of rainbow trout (Salmo gairdneri) and white perch (Morone americana): Effects of attaching telemetry transmitters. Can. J. Fisheries Aquat. Sci. 42, 488–493. doi: 10.1139/f85-066
Piatt J. F., Wetzel J., Bell K., DeGange A. R., Balogh G. R., Drew G. S., et al. (2006). Predictable hotspots and foraging habitat of the endangered short-tailed albatross (Phoebastria albatrus) in the North Pacific: Implications for conservation. Deep Sea Res. Part II: Topical Stud. Oceanography 53, 387–398. doi: 10.1016/j.dsr2.2006.01.008
Qing J., Cheng B. X., Yan X., Yang S. R., and Zhu D. Z. (2019). “Discussion on key technical problems of fish habitat ecological restoration in Heishui River,” in Proceedings of the 2019 Annual Science and Technology Conference of the Chinese Society for Environmental Sciences – Sub-forum on Technological Innovation and Application in Environmental Engineering, (Shanghai, China, Shanghai Investigation, Design & Research Institute Co., Ltd.; China Three Gorges Construction Management Co., Ltd.), Vol. 3. 154–157. doi: 10.26914/c.cnkihy.2019.071164
Riley S. C., Marsden J. E., Ridgway M. S., Konrad C. P., Farha S. A., Binder T. R., et al. (2019). A conceptual framework for the identification and characterization of lacustrine spawning habitats for native lake charr Salvelinus namaycush. Environ. Biol. Fishes 102, 1533–1557. doi: 10.1007/s10641-019-00928-w
Rong Y. F., Ban X., Zhou Y. H., Liu W. C., Qi H. F., Yang J. X., et al. (2022). The spawning habitat characteristics of Gymnocypris przewalskii and its identification by the UAV in tributary of Qinghai Lake—Taking Quanji River as an example. Acta Ecologica Sin. 42, 9371–9382. doi: 10.5846/stxb202107191949
Seesholtz A. M., Manuel M. J., and Van Eenennaam J. P. (2014). First documented spawning and associated habitat conditions for green sturgeon in the Feather River, California. Environ. Biol. Fishes 98, 905–912. doi: 10.1007/s10641-014-0325-9
Shao T., Wang Y. R., and Xu S. (2015). Response relationships between flow changing and habitat indicators of Schizothorax prenanti. Resour. Environ. Yangtze Basin 24, 85–91. doi: 10.11870/cjlyzyyhj2015Z10012
Shi L., Ye S. W., Zhu H., Ji X., Wang J. C., Liu C. A., et al. (2022). The spatial-temporal distribution of fish in lake using acoustic tagging and tracking method. Acta Hydrobiologica Sin. 46, 611–620. doi: 10.7541/2022.2021.004
Shu T.-T., Yang J., Yu Z.-X., Xiao K., Huang H.-T., and Dai L.-Q. (2024). Influences of water velocity on ovarian maturation and antioxidant capacity in adult grass carp (Ctenopharyngodon idellus). Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1441426
Soulsby C., Youngson A. F., Moir H. J., and Malcolm I. A. (2001). Fine sediment influence on salmonid spawning habitat in a lowland agricultural stream: A preliminary assessment. Sci. Total Environ. 265, 295–307. doi: 10.1016/s0048-9697(00)00672-0, PMID: 11227273
Sun J. (2018). Hatch dates and growth of larval Schizothorax lissolabiatus in the Lancang river based on otolith microstructure. Yunnan University, China (Kunming).
Wang L., Li Y., Zhang L., and Shu Y. (2023). Effects of short-term water velocity stimulation on the biochemical and transcriptional responses of grass carp (Ctenopharyngodon idellus). Front. Physiol. 14. doi: 10.3389/fphys.2023.1248999, PMID: 37719458
Wang C. Y., Wei Q. W., Du H., Zhang H., and Liu Z. G. (2010). Applications of ultrasonic telemetry in aquatic animal ecology. Chin. J. Ecol. 29, 2286–2292. doi: 10.13292/j.1000-4890.2010.0338
Welch D. W., Batten S. D., and Ward B. R. (2007). Growth, survival, and tag retention of steelhead trout (Oncorhynchus mykiss) surgically implanted with dummy acoustic tags. Hydrobiologia 582, 289–299. doi: 10.1007/s10750-006-0553-x
Winemiller K. O., McIntyre P. B., Castello L., Fluet-Chouinard E., Giarrizzo T., Nam S., et al. (2016). Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 351, 128–129. doi: 10.1126/science.aac7082, PMID: 26744397
Wu X. W. (1982). Monograph on Cyprinidae fishes of China (Shanghai: Shanghai Scientific and Technical Press).
Yan W. B. (2016). Studies on reproductive behavioural ecology of Schizothorax wangchiachii (China (Shanghai): Shanghai Ocean University).
Yan W. B., Zhu T. B., Wu X. B., Yang D. G., and Chen L. (2017). Observation on the spawning behavior of Schizothorax wangchiachii. Freshw. Fisheries 47, 9–15. doi: 10.13721/j.cnki.dsyy.2017.03.002
Yang Z., Zhang P., Tang H. Y., Gong Y., Dong C., Chen X. J., et al. (2017). The formation of habitat suitability curves for Coreius guichenoti (Sauvage & Dabry de Thiersant 1874) of the lower Jinsha River. Ecol. Sci. 36, 129–137. doi: 10.14108/j.cnki.1008-8873.2017.05.017
Zhang H., Wei Q. W., Yang D. G., Du H., Zhang H. J., and Chen X. H. (2007). Topography research on spawning grounds of Acipenser sinensis below Gezhouba Dam. Acta Ecologica Sin. 10, 3945–3955.
Zhou C. P. (2007). Reproductive biology of Schizopygopsis malacanthus baoxingensis (China (Sichuan: Sichuan Agricultural University).
Zhou Y. H. (2021). The requirement of natural breeding environment of Gymnocypris przewalskii and the construction technology of artificial (China (Wuhan): Huazhong Agricultural University).
Zhou Y. H., Rong Y. F., Zhou W. G., Liu H. X., Wang P. Y., Yu L. X., et al. (2022). Environmental requirements of natural reproduction of Gymnocypris przewalskii under artificial simulated conditions. Acta Hydrobiologica Sin. 46, 779–787. doi: 10.7541/2022.2021.0370
Keywords: natural reproduction, spawning ground habitat, tags and track, fluvial substrate composition, Schizothorax wangchiachii
Citation: Li B, Hu F, Li W, Su W, Zhu J and Jiang W (2025) Spawning habitat selection in Schizothorax wangchiachii using acoustic tagging and tracking. Front. Ecol. Evol. 13:1615081. doi: 10.3389/fevo.2025.1615081
Received: 21 April 2025; Accepted: 01 July 2025;
Published: 01 September 2025.
Edited by:
Hanxi Wang, Harbin Normal University, ChinaReviewed by:
Chao Song, Chinese Academy of Fishery Sciences (CAFS), ChinaJinming Wu, Chinese Academy of Fishery Sciences (CAFS), China
Daniel Bartoň, Biology Centre CAS, Czechia
Copyright © 2025 Li, Hu, Li, Su, Zhu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jiang, amlhbmd3ZWlfNkBjdGcuY29tLmNu; Bo Li, bGlfYm8xMUBjdGcuY29tLmNu
 Bo Li
Bo Li Fanxu Hu1,2
Fanxu Hu1,2