- 1Uppsala Archaeobotanical Group (UAG), Department of Archaeology, Ancient History and Conservation, Uppsala University, Uppsala, Sweden
- 2Department of Human Geography, Stockholm University, Stockholm, Sweden
- 3Department of Agriculture, Food and Forestry Sciences, University of Palermo, Palermo, Italy
- 4Institute of Biosciences and BioResources, National Research Council, Palermo, Italy
Within the multidisciplinary framework of historical ecology, in this study plant morphology, oral history, and soil analyses are combined with phytoliths to reconstruct shifts in management and environment of historical and living olive agroecosystems on the island of Sicily (Italy). The use of phytoliths in the study of historical agroecosystems is still a developing field. We present the collaborative work done on three historical olive agroecosystems (Bosco Pisano, a wild olive wood; Cozzo del Lampo, a Mediterranean olive orchard; Malìa, remnant of past agroforestry), where we have collected and analyzed phytolith assemblages to trace correlations between environmental dynamics and (agri)culture, as unfolding over the latest six millennia. We demonstrate that the cumulative ecological legacies in historical agroecosystems are traceable through phytolith analyses. Bosco Pisano allows for a calibration of the tree cover density, based on phytolith evidence. Meanwhile, Cozzo del Lampo and Malìa have evolved from a shrubland-type of environment to fruit gardens and open grasslands, with establishment of olive trees in between as key vegetation elements. Both these examples show the longevity of combined land uses, especially the grazing adapted olive cultivation in Malìa. In gaining clues on clear variations in land use, as abandonment and intensification, our results demonstrate that phytoliths can shed light also in local past intercultural exchange of knowledge. The integrated methodology presented here allows to appreciate how the biological and cultural diversity in historical agroecosystems has shaped their current state and inspires present-future management.
1 Introduction
Studies on historical agroecosystems are important for their management in face of current environmental issues (e.g., climate change, loss of biodiversity, soil impoverishment). Their cumulative ecological legacies also contain clues to the range of practices possible in the past and present. Currently, we still lack a deep understanding of how and to what extent the present biological and cultural diversity in historical agroecosystems has been shaped by land use processes over time (Cevasco et al., 2015; Agnoletti and Emanueli, 2016). Perspectives examining the correlations between the long-term presence of historical agroecosystems and the current states of certain rural landscapes are still missing. Similarly, there is a void of research on multiple baselines and drivers at different scales (Pauly, 1995; Ligtermoet et al., 2023). Historical agroecosystems tend to be studied by different disciplines separately, while they would be better understood through integrated and transdisciplinary approaches, which can appreciate their wider array of spatial and temporal processes. Studies have shown the potential in combining diverse methods and sources (oral history, paleoecology, spatial analysis), as epistemic perspectives, for the exploration of historical agroecosystems (Crumley, 2012; Barthel et al., 2013a, 2013b) and their key role for the maintenance of local biocultural diversity (Baiamonte et al., 2015; Cohen et al., 2023; Gkisakis et al., 2018; Ferrara, 2024).
Phytoliths have strong potential for the reconstruction and assessment of past and present agroecosystems (e.g., Pokrovsky et al., 2024; Witteveen et al., 2023), especially when combined with other sources (traditional ecological knowledge, geospatial data, historical maps, just to name a few). Research on the application of phytolith analysis for the investigation of agricultural practices, species domestication, food production, and their environmental impacts through the Holocene is not new (e.g., Kealhofer, 2003; Iriarte et al., 2010; Pearsall and Hastorf, 2011 for a general introduction on the method; Dickau et al., 2016; Hill et al., 2023). While this strand of research deals with the investigation of human-nature ecological interactions at certain points in the past, the use of phytoliths in the study of living historical agroecosystems is still a developing field. In such respect, our work - for the first time - applies phytolith research in the investigation of agroecosystems that have maintained certain vegetation elements (i.e., century-old olive trees) over millennia, which thus characterize these agroecosystems as historical elements in an always-changing landscape. The scope of our work is to trace the historical ecology of these agroecosystems by catching, through phytolith analysis, variation patterns (as new and abrupt shifts or gradual changes, both in land use and broader environmental dynamics) within long-term stability trends represented by the very persistence of these historical agroecosystems until today. From this derived knowledge, we could gain insights on how these agroecosystems have evolved and interacted with anthropogenic disturbances (land use) and environmental (microclimate, vegetation cover, local biodiversity) elements over time, while maintaining essential features of stability over centuries, if not millennia.
In our work, we combine plant morphology, oral history, and soil analyses with phytoliths (Ferrara et al., 2025). This paper in particular focuses on phytolith assemblage analysis in historical olive (Olea europaea L.) agroecosystems still present on the island of Sicily (Italy). These remnants of very old agroecosystems in today’s landscape allow studying land use dynamics and broader environmental changes over centennial and even millennial time scales. A single gram of olive leaf can produce around 40,000 phytoliths, and they have been extracted from leaves, bark, wood and fruits of Olea europaea L (Tsartsidou et al., 2007), as well as from olive oil sediments (Loeta Tyree, 1994). Nonetheless, no taxonomic demarcation by phytolith signature has been published so far to understand which phytolith morphotypes could be diagnostic of Olea. The main obstacle to research development on Olea taxonomic demarcation via phytolith signature is the difficulty of discriminating taxonomically phytoliths from woody dicotyledons (Bremond et al., 2005a; Testé et al., 2020), and only a few studies are available on the topic (An, 2016; Lisztes-Szabó et al., 2019; Liu et al., 2021; An and Xie, 2022; Boyd et al., 2024). Due to such limitations, in this paper we conceptualize plant microfossil biogenic silica (phytoliths) stored in the soil of historical olive agroecosystems as biocultural traces of past and present human-nature interactions and environmental dynamics. Phytolith assemblages are here considered as indicators of entangled cultural land use practices and ecological dynamics over the long term, in environmental contexts where other proxies (e.g., pollen, macrofossils, charcoal) are not available.
Furthermore, as will be argued, as biocultural traces of past local vegetation and land uses, phytoliths can inform about how the historical olive agroecosystems under investigation have responded to analogous shifts we face today or in the future. As such, they can provide relevant evidence we may want to consider in our management, when it comes to handle current and prospected climatic variations and other forms of stress.
Section 2 in the paper describes the three areas on the island of Sicily selected as case studies. These are remnants of historical olive agroecosystems, each of them is unique in the local history of land use and management of the olive tree. Section 3 is dedicated to our methodological approach, based on the assumption that phytolith assemblages stored in soil layers of historical olive agroecosystems are biocultural records from previous local vegetation dynamics and (agri)cultural practices. In Section 3, the local phytoliths ecology is introduced as well, together with details on the sampling method and analytical approaches adopted to investigate the extracted assemblages.
The potential of phytolith analysis to estimate the openness of historical olive agroecosystems, to understand how land uses in different historical periods have contributed to shape the current structure of ancient olive groves, and how biological diversity can be correlated to human presence on a site are presented in Section 4. These results are further interpreted in Section 5, within a perspective that considers soil-extracted phytolith assemblages as biocultural traces that can allow gaining new knowledge on historical and intertwined ecological-cultural phenomena happening on a site. The importance of past analogues to inform agroecosystems management and conservation policies is emphasized. The paper concludes by proposing further research on phytolith assemblages as biocultural proxies in agricultural soils with a deep history.
2 Study areas
In this paper, we propose phytoliths as biocultural traces in three historical olive agroecosystems on the island of Sicily chosen as study areas: Bosco Pisano, represented by a wild olive patch included in a mosaic forested area (in the municipality of Buccheri, Siracusa Province), Cozzo del Lampo, a hill covered by century-old olive trees in the municipality of Villarosa, Enna province, and Contrada Malìa, within a rural district in the municipality of San Mauro, Palermo province (Figure 1).

Figure 1. Location of the three study areas on the island of Sicily: Bosco Pisano, Cozzo del Lampo, Malìa (Image source: Ferrara, 2024).
The case study areas are remnants of three specifically different olive agroecosystems. Each represents individual spatial and temporal tree arrangements, resulting from distinct local historical land uses and intentionalities. Thus, each case study area per se exemplifies a unique case of management and maintenance history. Below, the morphological characteristics of these historical olive agroecosystems and their ecology are briefly introduced, based on surveys conducted in 2022, 2023 and 2024, in combination with collected oral history and traditional ecological knowledge.
2.1 Bosco Pisano
Bosco Pisano is a patch of woodland located on the slopes of the Iblei Mountains, nowadays a Nature 2000 site (ZSC ITA090022 – Bosco Pisano). The landscape unit is a mosaic where open woodlands and pasturelands alternate. The vegetation is dominated by the presence of Olea europaea var. sylvestris-dominated formations (EUNIS habitat type G2.41), Quercus suber (EU Habitat Directive 9330) and Quercus pubescens (EUNIS G1.732) woods, pseudo-steppe with grasses and annuals of the Thero-Brachypodietea (EU Habitat Directive 6220), Sarcopoterium spinosum phryganas (EU Habitat Directive 5420), and Western Mediterranean and thermophilus scree (EU Habitat Directive 8130). Bosco Pisano lies on volcanic rock outcrops (Carbone et al., 1986) with prevailing very shallow andic brown soils and lithosols, where rock outcrop is abundant (Fierotti et al., 1988). The area chosen as case study, 780 m2 large (Figure 2), includes an unusual almost monospecific wild olive cluster (Figure 3). It is a unique example of wild olive woodlands that might have been much more widespread in the past and currently represented by few fragmented patches occurring occasionally in the entire region.

Figure 2. Bosco Pisano case study location and its broader area (Image source: Ferrara, 2024).

Figure 3. (a) Bosco Pisano, with its cluster of wild olive trees, (b) Peculiar shapes of the olive trees branches in Bosco Pisano, due to the reiterated cuttings done in the past to provide in situ fodder for grazing cattle.
As part of the study, an assessment was made of the tree morphology and grafting events since the olive trees in Bosco Pisano, even though wild olive, have the typical multistemmed shape of coppiced cultivated individuals (as for instance in Cozzo del Lampo, cf. 2.2). The olive wood was likely used for fuelwood, fodder-branches, and timber. Coppicing practices (the cut of tree branches and trunks at or near the ground level) are already mentioned in written sources from the 17th century and there may also be charcoal kilns in the vicinity (Garfì and Di Pasquale, 1988). Historical reconstructions of the local land use (cf. Di Pasquale et al., 2004) dates from the 17th century onwards. Until the last century, the local landscape spatial patterns probably resembled present-day grazing areas, such as the Dehesas or Montados (in Spain and Portugal respectively). During the 1940s and 1950s, this territory became virtually abandoned, except for cork harvesting and grazing. The area is today used for overwintering of animals, as part of a transhumance practice (cf. Garfì and Di Pasquale, 1988).
Di Pasquale et al. (2004) suggest that the wild olive dominated stands are probably the most ancient and stable units, when compared to other more recent forest communities, as the macchia formations (estimated as c. 60-year-old), resulting from the decline of coppicing, and the cork oak stands, most likely originated in its current settings at the end of the 19th century, now largely senescent and unable to rejuvenate due to the lack of natural regeneration owed to overgrazing.
Garfì and Di Pasquale (1988) further suggest that this woodland is a relict of wider oak forests, existing until the classic age in Sicily (i.e., Diodorus Siculus, Bibliotheca, 4–84). This wood made of wild olive trees represents a now forgotten form of past land use of the olive, and for this reason is an important case study.
2.2 Cozzo del Lampo
Cozzo del Lampo is a hill of approximately 1.28 km2, located in a rural area of inner Sicily, and characterized by the abundance of large size (c. 12–15 m in circumference) domesticated century-old olive trees (Figure 4). These agroecosystems are Mediterranean formations of Olea europaea var. europaea (EUNIS G2.91), with presence of Mediterranean subnitrophilous grass communities (graminoid formations that may cover post-cultural or pasture lands, EUNIS E1.61), sub-Mediterranean deciduous thickets (EUNIS F3.2) and diss formations (EUNIS E1.433). Along the slopes of the hill, these agroecosystems are intermixed with Mediterranean tall-grasses (EUNIS E1.4), evergreen sclerophyllous scrubs (EUNIS E1.2A) and riparian thickets close to the water streams (EU Habitats Directive 92D0). The hill, as a mosaic of Olea europaea var. europaea groves in terms of land use, is then surrounded by cereal and fallow fields. Since the 1950s post-war land reorganization in Sicily, these olive groves have been managed as small family holdings maintained for self-consumption and/or recreation.

Figure 4. Cozzo del Lampo hill with its mosaic of different olive spatial arrangements (Image source: Ferrara, 2024).
Cozzo del Lampo is an elevated area with slopes and/or protruding rocks of the Terravecchia Formation (i.e., interbedded quartzite pebbles, sandstone and shale sequences, cf. Grasso and Pedley, 1988). The size and configuration of the olive trees suggest that they are century-old, though no exact dating is available. The olive trees are found in places where it was extremely hard or impossible to plough the land manually.
Field surveys were carried out in collaboration with farmers and pruners (cf. Ferrara and Ingemark, 2023; Ferrara et al., 2024b). Trunk and spout morphology was assessed, together with crown shape and size, past grafting and pruning events if visible on the tree. Some trees have likely been grafted from the roots of previous wild olive individuals (sensu La Mantia, 2005), since no signs of grafting is evident on the trunks. Several consecutive coppicing (tree cuts at or near the ground level) and pollarding (tree cuts higher at the trunk level) events are visible, resulting in the current multi-stemmed and multi-branches habit of these century-old olives. Both coppicing and pollarding as pruning techniques have the aim to foster self-renewal in the tree (Rackham, 2018). According to the size and shape criteria specified by Schicchi and Raimondo (2011), the olive trees in Cozzo del Lampo could potentially be as old as 800 or 1,000 years (Figure 5). Finally, their spatial arrangement is distinctive compared to more recent olive orchards, resembling a mosaic of diverse configurations unique to this area (Ferrara et al., 2024a).
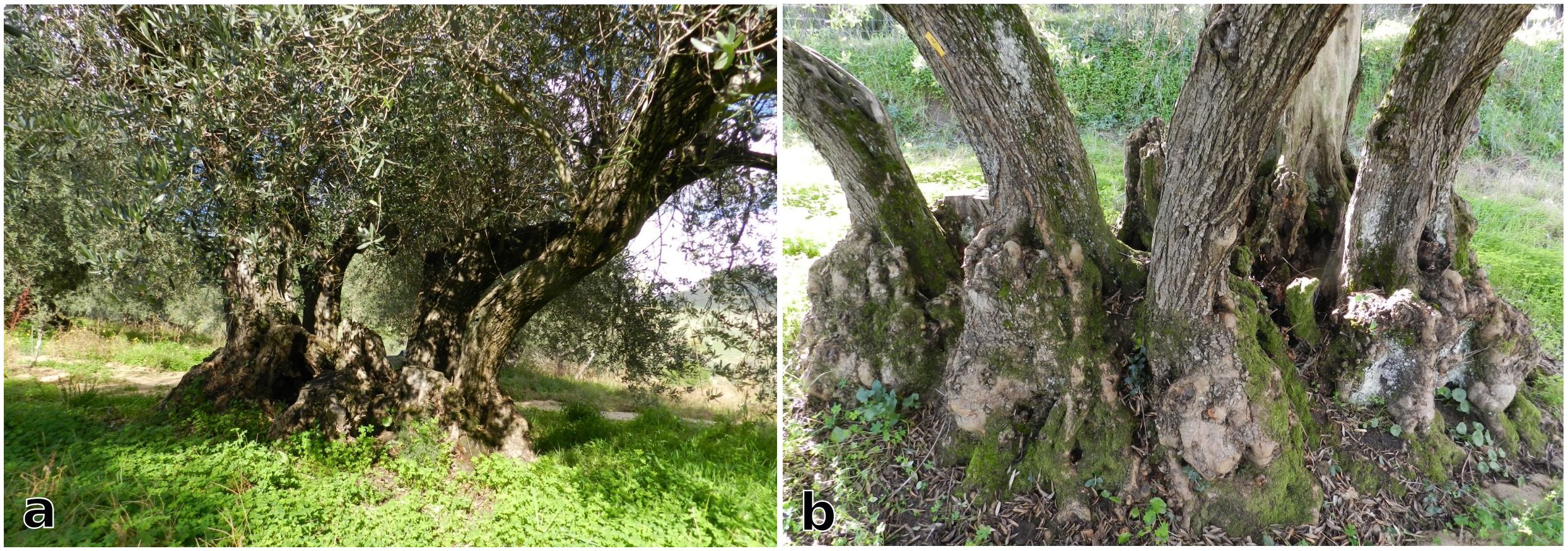
Figure 5. A century-old olive tree (a) with its multiple stems (b) surrounding the main decaying stump (Image source: Ferrara, 2024).
The Morello valley, where the hill is located, has been inhabited since prehistory (Giannitrapani et al., 2014). Archaeological surveys in one olive orchard were carried out by Tegerdal Hune (2022), resulting in the location of an undated site. Giannitrapani et al. (2014) suggest an abandonment phase in the entire valley from late Antiquity to the 14th century. This was followed by slow repopulation and, from the 18th century, the consolidation of an extensive feudal estate, characterized by intensive cereal cultivation (Verga, 1993; Carocci, 2010). A redistribution of land to peasants took place after the end of World War II. Based on recent collection of oral histories and geonarratives (cf. Ferrara et al., 2024b), until c. 1950 fruit trees (such as Pyrus pyraster L. (Burgsd.), Punica granatum L., Prunus spinosa L. subsp. spinosa, to name a few) were cultivated in between these olive trees, resembling the intercropping system of the “Mediterranean garden”. Outmigration from the area remains a problem today (Fondazione Migrantes, 2022), though small-scale farming activities persist, mainly involving cereal and livestock production (Ferrara and Lindberg, 2023).
2.3 Malìa
The third study area presented in this paper is located in a rural district called Malìa, in the Madonie Mountains, known as a biodiversity hotspot due to remnants of semi-natural vegetation (Baiamonte et al., 2015). The Olea europaea var. europaea formations (EUNIS habitat type G2.91) in the Malìa study area are found in permanent mesotrophic pastures and aftermath-grazed meadows (EUNIS E2.1) dominated by Mediterranean subnitrophilous grass communities (EUNIS E1.61), Calicotome infesta (EUNIS F5.515), Ampelodesmos mauritanica dominant formations (EUNIS F5.53), Medio-European rich-soil thickets (EUNIS F3.11) and Spartium junceum scrubs (EUNIS S53). The study area surveyed has an extension of approximately 550 m2 (Figure 6), and it is embedded in a tangled web of Quercus pubescens (EUNIS G1.732) Quercus suber (EU Habitat Directive 9330) woods and Italic poplar galleries (EUNIS G1.314). Here too, during fieldwork, we assessed the morphology and grafting events visible on the century-old olive trees. Evidence of past grafting, done on semi-mature wild and feral trunks at more than 1.5 meters from the ground (Figure 7), suggests an adaptation of protect cultivated olives to grazing animals. Based on their trunk size and overall physiognomy, these olive trees are estimated to be century-old.
Figure 6. Location of the study area and its surrounding landscape in contrada Malìa (Image source: Ferrara, 2024).

Figure 7. The difference between wild and domesticated olive trees can be appreciated from the different shades of green of the trees´ crown. Darker green (as in (a)) indicates wild and/or feral olive. Lighter green are leaves from domesticated olives. The olive tree in (b) shows the coexistence of both, wild and domesticated; it is also a good example of a grafting event happened on a semi-mature trunk at more than 1.5 meter of height. Note the slope (Image source: Ferrara, 2024).
Numidian Flysch, a rare Oligocene to mid-Miocene deep-marine sandstone and mudstone formation (Hubert Thomas, 2011; Pinter et al., 2016), emerges until the surface in certain places, making the topography very steep and soil layers shallow. In this agroecosystems, the spatial distribution of century-old olive trees appears random, growing directly from and among the bedrock, even in the steepest parts of the hill slope. In addition, numerous olive trees are located in the most remote and inaccessible places (Figure 8).
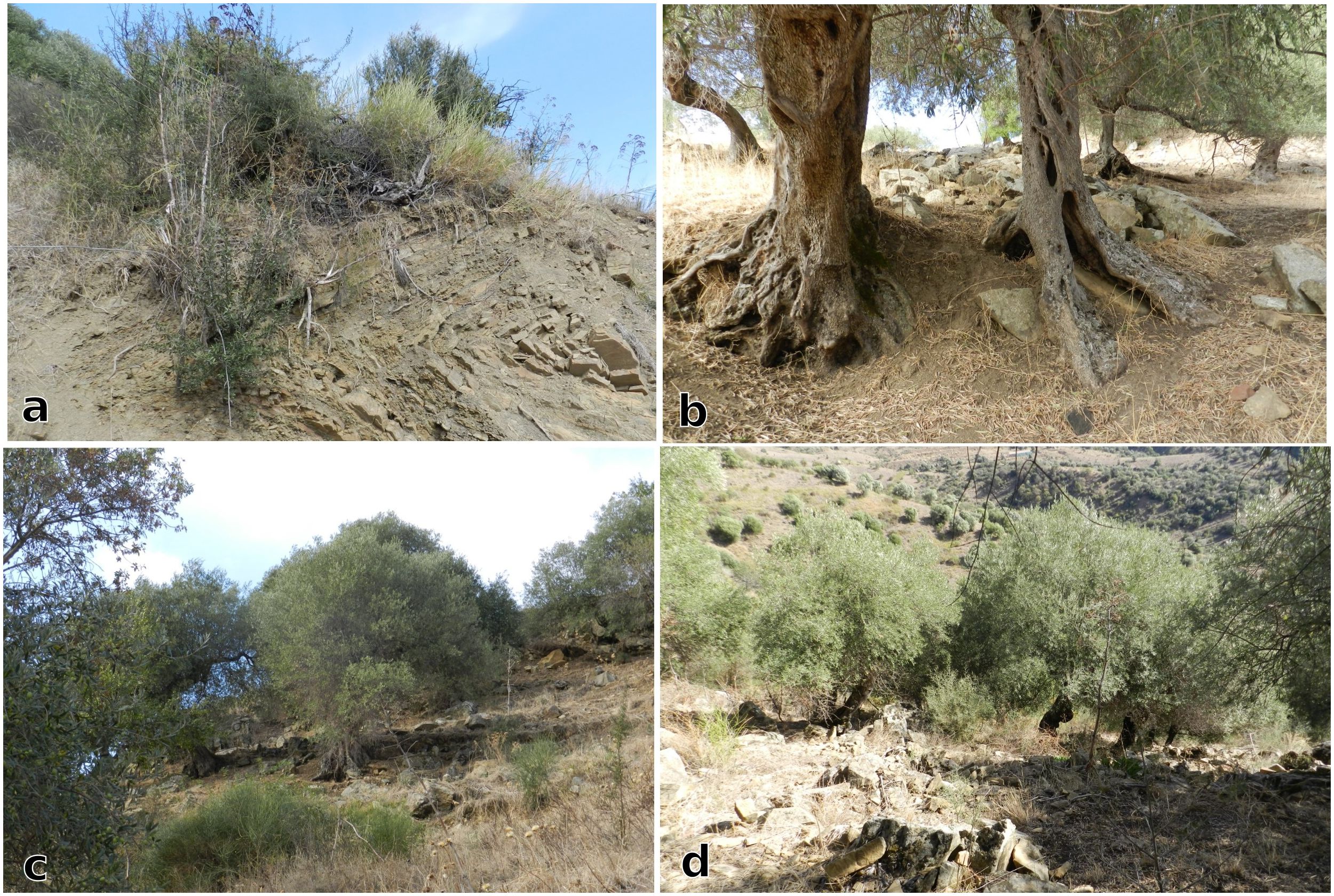
Figure 8. (a) Local vegetation, including wild olive, growing on Numidian Flysch bedrock; (b–d) Domesticated olive trees growing among the bedrock. Note the slope and the natural bedrock terraces in Figures 7c and d (Image source: Ferrara, 2024).
Local reconstructions of Holocene vegetation and fire dynamics show the expansion of open grasslands from the 5th millennium BCE onwards, on top of previously closed woodlands (Tinner et al., 2016). This grassland expansion, alongside other evidence, has been linked with human activities (Belvedere and Forgia, 2010; Forgia et al., 2013, 2021). Unfortunately, documentation for the more recent period remains scarce.
From interviews with local residents, the area had a similar socio-economic history as Cozzo del Lampo. It was part of a large estate, which after the end of World War II was parceled into small plots, bought by the farmers already working the land. Malìa represents a past multifunctional space, for cultivation and grazing, while also providing fuelwood and timber (cf. Ferrara et al., 2023), which suggests this case study to be representative of an old agroforestry model (similar to those in Antiquity), characterized by the high presence of grazing activity and sparsely planted olive trees.
3 Material and methods
A few scholars have tested the potential of multiproxy vegetation reconstructions in millennial old olive agroecosystems. Cohen et al. (2023) analyzed land use factors (spatial context, management type and intensity) and environmental conditions. Jouffroy-Bapicot et al. (2021) traced the transition from Mediterranean woodlands to olive agroecosystems. Meanwhile, Moriondo et al. (2013) used historical ranges of olive cultivation as a paleoclimate proxy to model future environmental and agricultural scenarios. These pioneering studies demonstrate how multiproxy methods and interdisciplinary approaches are crucial to reconstruct the historical ecology not just of olive agroecosystems, but of other types of agroecosystems with a deep history as well. As reviewed above, the case studies presented here have been investigated spatially (Ferrara and Wästfelt, 2021; Ferrara et al., 2024a; Ferrara and Wästfelt, 2025) and through oral narratives, local ecological knowledge and written sources (Ferrara and Ingemark, 2023; Ferrara and Lindberg, 2023; Ferrara et al., 2023; Ferrara et al., 2024b). In this paper, for the first time phytolith assemblage analysis is integrated into a historical ecology study of ancient olive agroecosystems still present in today’s landscape.
3.1 Phytoliths as biocultural traces
Phytoliths are used in this paper to reconstruct past vegetation, land use and broader environmental dynamics (Pearsall and Trimble, 1984; Strömberg et al., 2007; Aleman et al., 2014; Feng et al., 2017). Phytoliths have also been used to study erosion patterns and paleoclimate (see review in Qader et al., 2023). In old soils, phytoliths are preserved due to silica resistance to decay (Pearsall, 2015). Although phytoliths have an enormous potential in studying and assessing agricultural soils, such field of research is underdeveloped (though see Meunier et al., 1999; Fernández Honaine et al., 2006; Liang et al., 2015; Hussain et al., 2023; Pokrovsky et al., 2024; Benvenuto et al., 2025 for positive examples). Plants and soils act as a Si ‘filter’ (cf. Vandevenne et al., 2012, 243), transforming the dissolved silica (DSi) originated from mineral weathering into biogenic silica (BSi). Such biogenic silica is then returned to the soil when plants decay. Along this cycle, vegetation and soils changes caused by specific land use activities, such as the removal of biomass during harvest, affect the Si quantity and distribution (Barão et al., 2020). Through phytoliths analysis, we can thus trace how vegetation composition and assemblages have been shaped by land use practices. By approaching the historical olive agroecosystems present in the study areas as containing legacies of their past-present agricultural and silvicultural use, we developed a methodological approach that would allow investigating this biocultural heritage. By ideally following the roots of century-old olive trees underground, we explored the memory of the soil through the analysis of phytolith assemblages (Piperno, 2006). By comparing the relative frequencies through time of all diagnostic phytoliths (Strömberg, 2004), first of all we intended to observe if distinctive assemblages along the stratigraphic profiles could lead to distinguish patterns of change in vegetation and habitats structure (Strömberg et al., 2013). Secondly, we considered if the correlation between different vegetation communities represented by these assemblages could offer new interpretative perspectives about past land use practices (included successional trajectories along gradients of human disturbance, cf. Witteveen et al., 2023), climatic and broader environmental trends over the long term in these agroecosystems.
Therefore, testpits were opened in each of the case study areas, for the extraction of phytoliths from soil layers following a protocol developed by Mazuy et al. (2024) specifically for these types of agro-ecological contexts, as detailed in Section 3.2. Where available, 14C dates were modeled to provide a relative age-depth.
The identification of phytolith morphotypes (Figure 9 for examples) to categorize into assemblages has followed local phytoliths ecology (Section 3.3) and phytolith assemblages ratios have been interpreted according to four different indices (Section 3.4), in order to provide indication of specific plant communities structures, composition and variation patterns along each profile and when comparing the three different sites.

Figure 9. Examples of phytoliths morphotypes extracted from the case study areas: elongate sinuate (a), crenate (b), and dendritic (c) from Malìa (Image source: Ferrara, 2024); blocky (d) from Bosco Pisano (Image source: Ferrara et al., 2025).
3.2 Sampling and laboratory procedure
Soil sampling for phytoliths extraction and analysis was adapted to the specific geological features in each study area and the spatial composition of their century-old olive trees.
In Bosco Pisano, two profiles of 20 cm depth were opened (Figure 10a, Table 1), resulting in two samples. Sampling here aimed to estimate tree cover density through phytoliths signature in Olea wood formations (cf. 2.1 for habitats characterization), so to employ it as a local tree cover threshold when interpreting the results from the other two case studies.
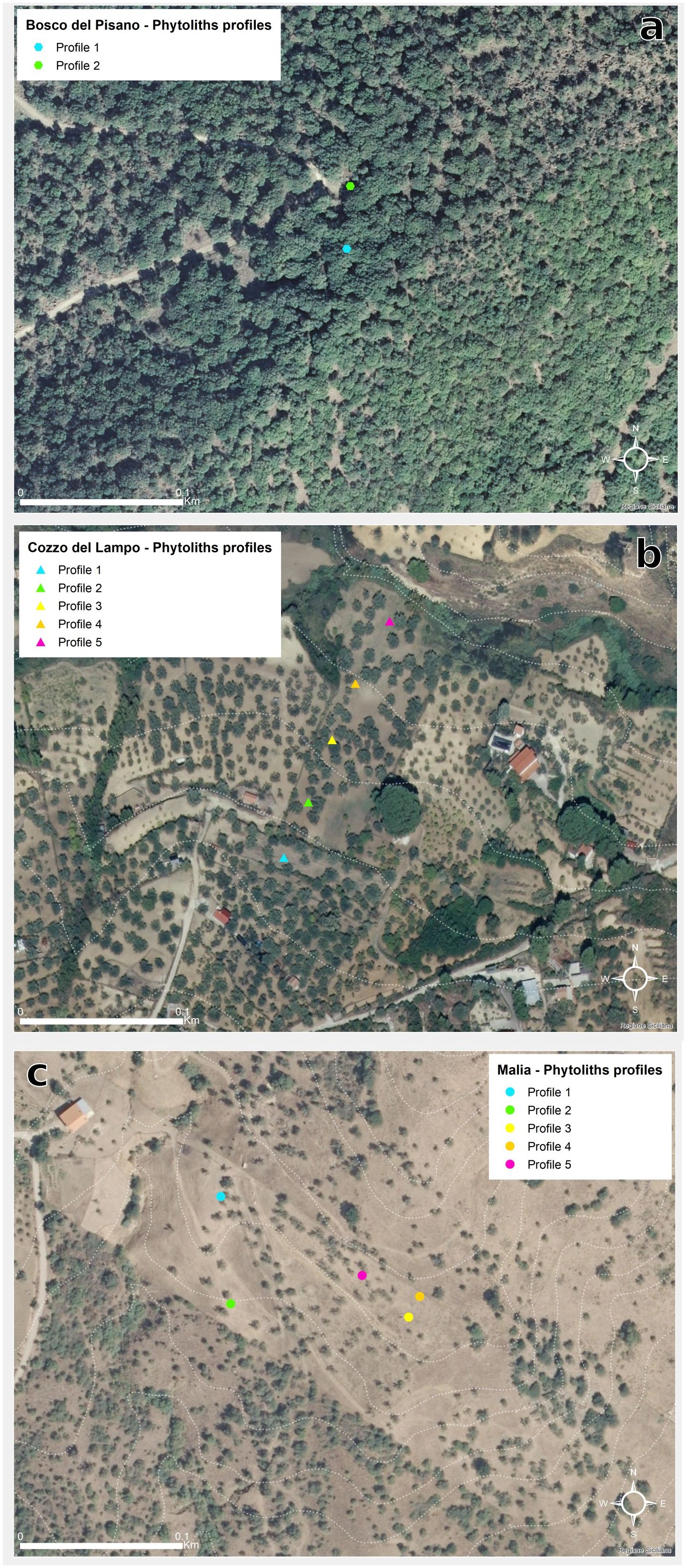
Figure 10. Location of the soil profiles excavated in each study area for sampling. (a) Bosco Pisano; (b) Cozzo del Lampo; (c) Malìa (Image source: Ferrara, 2024).
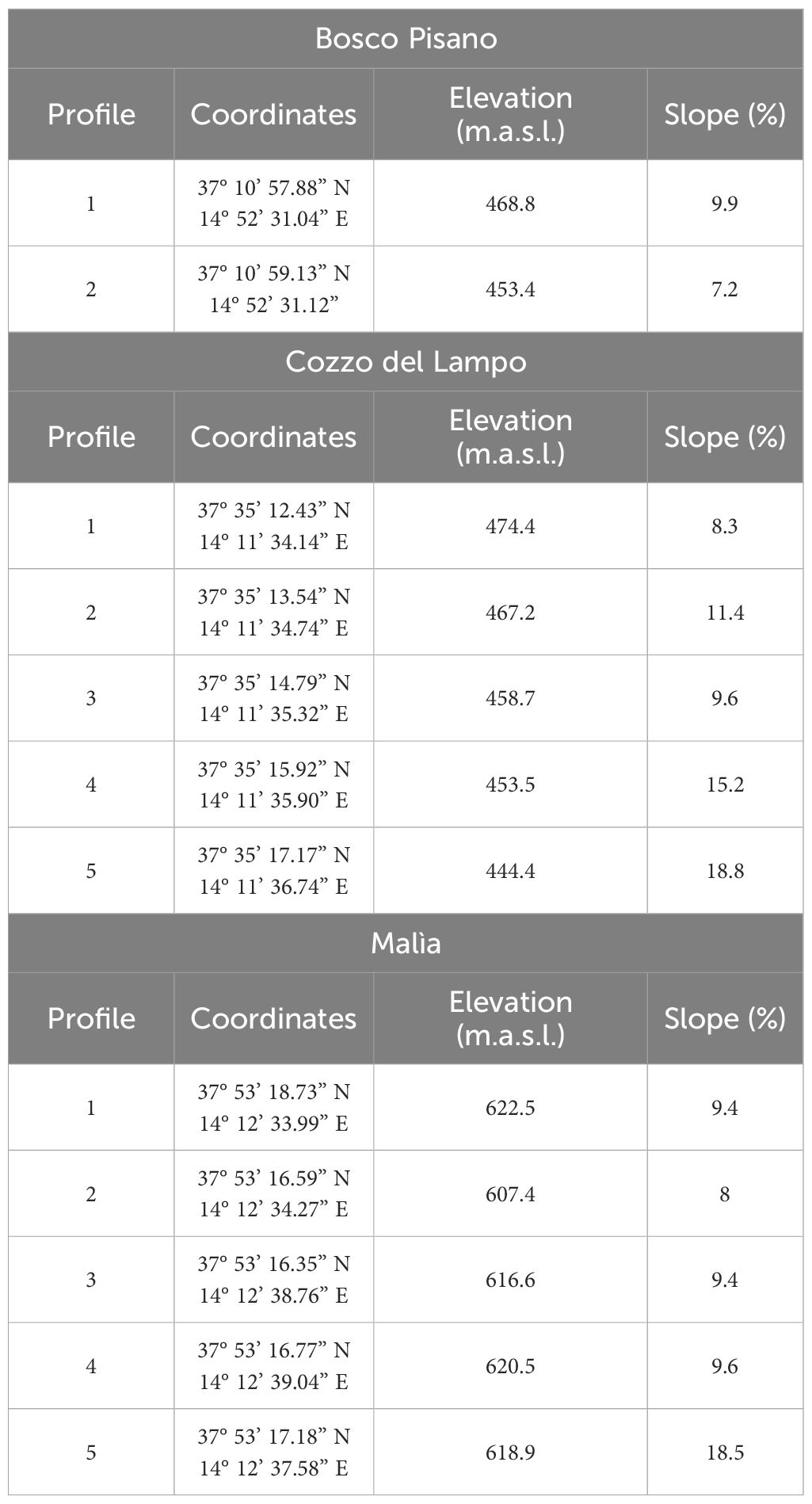
Table 1. Coordinates, elevation and slope values of soil profiles in each of the three study areas (Bosco Pisano, Cozzo del Lampo, Malìa) (Ferrara, 2024).
In the two study areas Cozzo del Lampo and Malìa, five testpits 1.5 m deep were opened in each area, and soil sampled every 10 cm, resulting in a total of 75 samples per study area (Table 1). In Cozzo del Lampo, the testpits followed the slope transect with an interval of 40 meters (Figure 10b). In Malìa, a transect was not possible due to the shallow soil depth in recurrent places. The testpits were placed near olive trees in an area with relative soil thickness and were local residents said there had been cereal and/or legumes cultivation and pasture (Figure 10c, Table 1).
Stratigraphic analyses were carried out for the profiles excavated in Cozzo del Lampo and Malìa (Supplementary Material S1). Three charcoal samples from Cozzo del Lampo and two samples from Malìa were submitted for radiocarbon dating at the Ångström Laboratory, Uppsala University. Dates were calibrated by the laboratory using IOSACal v0.4.1 and the IntCal20 calibration curve (Reimer et al., 2020). A Bayesian age-depth model was produced in R (R Core Team, 2021) using the package Bacon (Blaauw et al., 2022) and the uncalibrated dates for Cozzo del Lampo samples are published already in Ferrara and Wästfelt (2025).
Phytolith extraction was done at the Paleobiology Laboratory (Department of Earth Sciences, Uppsala University), following the protocol developed in Mazuy et al. (2024). This protocol allows extracting quantities of biogenic silica suitable for phytolith morphotypes analysis, from soils and contexts with low phytoliths concentrations. The samples have been first deflocculated through magnetic stirring. Thereafter, the fine fraction (200 μm) is treated with hydrochloric acid 33% (to remove carbonates) and then boiled in a hot bath (80°C) with potassium hydroxide 10% for 15 minutes (to remove organic matter). Phytoliths are then extracted by heavy liquid flotation (using sodium polytungstate, SPT at 2.35 density) and in a hot bath (80°C) with hydrogen peroxide 30% for 1 hour to remove further organic matter.
Being collected on the island of Sicily, where the volcano Etna has been active for thousands of years, our soil samples contain a high amount of cryptotephra, which is silica glass (GSi). The density of cryptotephra is similar to that of phytoliths and biogenic silica more in general (BSi) (≤ 2.3 g cm-3), consequently silica glass is also separated together with phytoliths during the heavy liquid extraction process. In our specific case, the final BSi residue represents thus the sum of phytoliths and cryptotephra. Consequently, whatever quantitative assessment of the silica fraction extracted from our samples would be misleading in terms of specifying the exact amount of phytoliths content. A further separation between tephra particles and phytoliths would have been necessary to obtain such specific measures, which however could have caused partial loss of the already phytolith-low content of the studied samples (cf. Mazuy et al., 2024). Since cryptotephra is clearly distinguishable from phytoliths under microscope and being mainly interested in analyzing relative abundance of phytolith morphotypes and their assemblages variability to gain qualitative information about changes in vegetation composition, land use and plant behavior, the quantitative determination of the biogenic silica content in our samples was out of the scope of the work presented in this paper.
3.3 Local phytoliths ecology
Though different parts of a plant produce diverse phytolith morphotypes, several phytoliths can be commonly identified to subfamily level (Table 2). Phytoliths attributed to dicotyledonous (plants having two embryonic leaves in their seeds, such as trees and shrubs) from temperate areas, as our case studies, are globular (or spheroid) (Bozarth, 1992; Alexandre et al., 1997; Albert et al., 1999; Runge, 1999; Delhon et al., 2003) and tracheary (Bozarth, 1992; Alexandre et al., 1997; Madella et al., 1998; Albert et al., 1999; Runge, 1999; Delhon et al., 2003; Jarl and Bruch, 2023). They can also be jigsaw (Carnelli et al., 2004; Kawano et al., 2006; Gu et al., 2008; An and Lu, 2015; Potì et al., 2019; Hayashi et al., 2021) and blocky (Tsartsidou et al., 2015; An, 2016; Boixadera et al., 2016; Ntinou and Tsartsidou, 2017; Burguet-Coca et al., 2020; Kraushaar et al., 2021; Tencariu et al., 2022). Even relatively modest percentages of these morphotypes can be interpreted as substantial, as dicots have a low phytolith production (Carnelli et al., 2004; Tsartsidou et al., 2007).
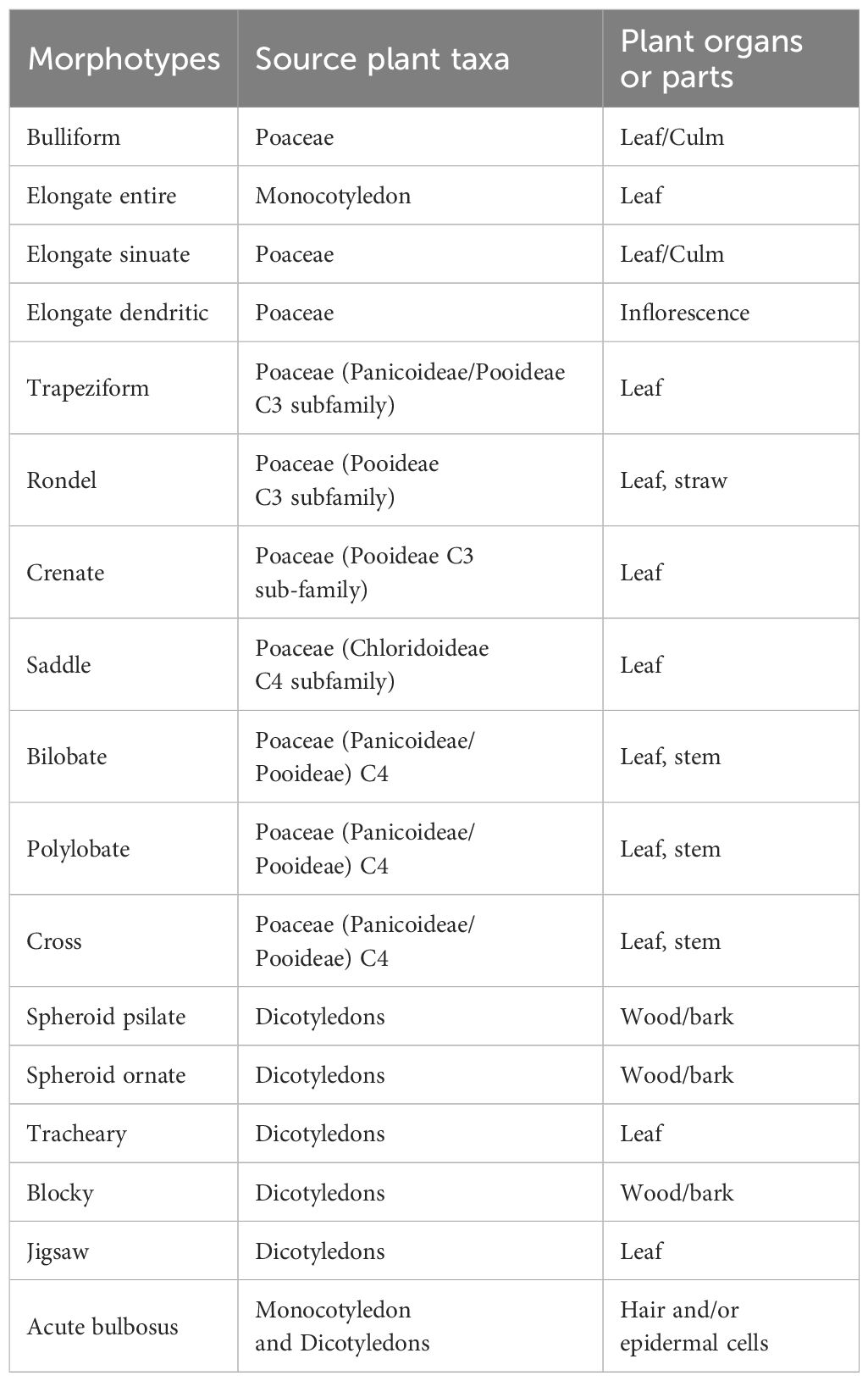
Table 2. Attribution of phytolith morphotypes to plant taxa and plant parts (Ferrara, 2024).
Grasses (monocotyledon plants, i.e. having only one embryonic leaf in the seeds) of the Poaceae family produce elongate, papillate, acute bulbosus and bulliform flabellate forms (Twiss et al., 1969; Fredlund and Tieszen, 1994; Piperno, 1988; Ball et al., 2001; Neumann et al., 2019). Elongate phytoliths present morphological variations in vegetative parts (the elongate sinuate type is formed in leaves, and simple elongate in stems). Elongate dendritic and papillate forms are produced by the flowering parts of Poaceae (glumes, lemma and palea, also named inflorescence). Dendritic long cells are commonly associated with the chaff of domesticated Pooideae, such as wheat, barley and oat (Rosen, 1992; Ball et al., 1999; Portillo et al., 2006; Albert et al., 2008). However, dendritic forms are also found in wild grasses (Novello and Barboni, 2015), thus their attribution must be carefully assessed.
Acute bulbosus is a morphotype produced in the interior part of the hair cells, above all in grasses (Alexandre et al., 1997; Barboni et al., 2007). Being produced in sedges and dicots as well, this morphotype is not considered here diagnostic. Nonetheless, it has been included in the counted morphotypes due to the limited amount of phytoliths in certain layers correlated with the stratigraphy (cf. Supplementary Material S1).
Bulliform flabellate and blocky morphotypes are produced in the bulliform cells of leaves (Mader et al., 2020), which usually silicify in a later stage of plant life (Moulia, 1994). Bulliform flabellate phytoliths allow leaves to bend to avoid excessive water loss, and their formation is directly influenced by environmental conditions such as high evapotranspiration (Bremond et al., 2005b; Novello et al., 2012).
Specific grass silica short cell phytoliths (hereafter GSSCPs, Neumann et al., 2019) can be diagnostic of the following Poaceae subfamilies (Table 2). C3 grasses in cool moist/temperate climate (Pooideae) are associated with rondels (Twiss et al., 1969; Fredlund and Tieszen, 1994; Piperno and Pearsall, 1998; Barboni and Bremond, 2009). Rondels are produced also in the Arundinoideae (cf. Tencariu et al., 2022). The trapezoid (Barboni and Bremond, 2009) and Crenate morphotypes (Twiss et al., 1969; Fredlund and Tieszen, 1994; Barboni et al., 2007) are mainly produced in Festucoid grasses (Tsartsidou et al., 2007).
Chloridoideae are C4 grasses found in dry and hot environments. These are dominated by saddle forms (Piperno, 2006; Madella et al., 2016) and rondels found together with saddles (cf. Bamford et al., 2006; Barboni and Bremond, 2009). Warm and wet environments tend to support C4 Panicoideae where species have many morphotypes as bilobate (Twiss et al., 1969; Fredlund and Tieszen, 1994; Barboni and Bremond, 2009), polylobate (Twiss et al., 1969; Fredlund and Tieszen, 1994; Neumann et al., 2019) and cross morphotypes. Bilobate phytoliths can also occur in a few Festucoid grasses and some Chloridoid grasses (as pointed out by Metcalfe, 1960; Danu et al., 2020).
3.4 Phytolith assemblage analysis
Phytoliths were identified and counted using light microscopy at ×400 magnification. Their morphotypes were categorized into plant taxonomic groups according to the International Code for Phytolith Nomenclature (ICPN) (Neumann et al., 2019) and the PhytCore online database (Albert et al., 2016). In addition, the UMR 7264 CEPAM CNRS-Université Côte d’Azur (Nice, France) was consulted for identifications. Only those recognized morphotypes listed in the International Code for Phytolith Nomenclature (ICPN) (Neumann et al., 2019) were counted, with a minimum of 200 phytoliths per sample. Such an amount is attested to give statistically interpretable assemblages (cf. Strömberg, 2009; Zurro Hernández, 2018), above all in soil samples coming from specific contexts (e.g., farming systems, arboriculture) where the concentration of phytoliths can be lower if compared with their higher concentration in soils of very specific environments, such as grasslands and archaeological sites (Cabanes et al., 2011).
The relative abundance of each phytolith morphotypes per soil layer was calculated based on the total sum. Along each excavated profile, phytolith assemblages were analyzed using four indices, to assess if the diachronic variability in their ratios could be informative of changes in vegetation structure and compositions, as well as land use practices and plant behavior to climatic disturbances.
The long/short cell index measures the ratio of long cells vs short cells. Short cells phytoliths are impregnated with silica as soon as they form (Sangster, 1970), while silicification of long cells become more intense with age (Rencheng et al., 2017). Thus, the ratio of long cells to short cells in a phytolith assemblage may provide information about the composition of grasses in terms of mature versus young grasses (Delhon et al., 2024). The formula used to calculate the long/short cell index here is Long/short cell index = long cells (elongate entire + sinuate + dentate + dendritic)/short cells (GSSCPs).
The Fs-index (Bremond et al., 2005b, 2008) can provide information on local microhabitats and/or climatic conditions, since an abundance of bulliform flabellate phytoliths is correlated with an increase in evapotranspiration and/or prolonged water stress (cf. Bremond et al., 2005b; Mader et al., 2020).
The formula used to calculate the Fs-index here is Fs-index = bulliform/GSSCPs (Bremond et al., 2005b, 2008).
The Dicot/Poaceae index (D/P index) measures the ratio of dicotyledons morphotypes (D) versus Poaceae morphotypes (P). This index allows to estimate the tree cover density (Alexandre et al., 1997), since based on the assumption that open habitats have a higher proportion of grass phytoliths (Strömberg et al., 2018). The lower the D/P value, the more open the habitat. In Mediterranean environments, 0.1 is the lower limit in the D/P index to distinguish between dicotyledon- and grass-dominated assemblages (Delhon et al., 2003).
Even though the D/P index has a standard formula (D/P) (cf. Alexandre et al., 1997), the variables indicating Poaceae and Dicots have been interpreted by scholars in slightly different ways (for a detailed review cf. Ferrara, 2024). Building on the standard D/P index (called here Da/P index, considering as diagnostic of dicots only spheroid and tracheary morphotypes), Ferrara (2024) has developed also a local D/P index (Db/P index), which includes also the blocky (blocky polyhedral and cubic) and jigsaw morphotypes as diagnostic of dicots from local samples.
The formulas used to calculate the D/P index are the following:
The inflorescence/culm-leaves index measures the ratio of Elongate dendritic phytoliths (from inflorescence bracts) versus Elongate entire and Elongate sinuate (from culms and leaves) (Piperno, 1988; Tsartsidou et al., 2007; Delhon et al., 2020). The ratio of the index varies according to the presence of the whole plant or residues from straws and spikelets, and this information can be indicative of local agricultural practices (e.g., harvest) and/or cereal processing (Delhon et al., 2020).
The formula used is Inflorescence/culm-leaves index = elongate dendritic + dentate/elongate entire + sinuate.
Results are presented in diagrams drawn using the software TILIA 3.03 (Grimm, 1993) (Section 4).
4 Results
Dating semi-natural soil profiles in open agricultural environments is difficult due to frequent and constant anthropogenic land use. Consequently, our age-depth model must be considered as tentative. The age depth model resulting from the radiocarbon dates suggests that the historical period under investigation through phytolith assemblage analysis may cover however several millennia.
4.1 Woodland (Bosco Pisano)
Two testpits (20 cm deep) were opened in two locations with a different density of tree cover (Figure 10a): Profile 1 is characterized by a dense tree cover; Profile 2 is located in a more open space but still inside the olive wood. Phytoliths are abundant in both the samples from Bosco Pisano, which is somewhat surprising considering its vegetation (woodland), but less surprising if we take into consideration the low historical anthropogenic impact on the soil of this area.
Pooideae morphotypes dominated with the high percentage of short cells phytoliths (on average 34% vs long cells 46%). The most common morphotype are rondel and trapezoid. These phytoliths were formed mainly in the leaves and the stems of grasses. Phytoliths from grass inflorescence (dendritic) are not present in the phytolith records. Phytoliths from woody and herbaceous dicotyledons are present in the two samples representing, on average, 9% (including also the blocky morphotype, cf. Section 3.3 for Db/P index specifications), nonetheless, phytoliths from grasses are still dominant. Morphotypes related to other monocotyledons are low, with C4 grass phytoliths at only 1.25%.
An analysis of phytolith morphotypes assemblages was done using the Da/P and Db/P indices and the results are indicative. Overall, both indices result above the standard threshold of 0.1 for woodland estimation, except for Da/P of Profile 2 (less dense tree cover), which is 0.08. Furthermore, Profile 1 (with dense tree cover) has higher values of both indices than Profile 2 (Da/P = 0.13 vs 0.08; Db/P = 0.34 vs 0.17). Results from Bosco Pisano, despite lack of soil depth, allowed for a calibration of the D/P index in relation to the other case study areas.
4.2 Shrubland to olive orchard (Cozzo del Lampo)
Phytolith morphotypes are abundant in most of the samples throughout the five profiles from Cozzo del Lampo. The only layers where it was not possible to extract phytoliths were samples taken in eroded bedrock (Profile 2, 56–150 cm; Profile 4; Profile 5, 92–150 cm) (cf. Strömberg et al., 2018). We begin here giving an overview of the results from all profiles analyzed and then focus on specific profiles.
4.2.1 General results
The phytolith morphotypes of Cozzo del Lampo are predominantly from the Pooideae subfamily (C3), with a high percentage of short cells phytoliths (in average 32.55% vs long cells 35.56%), above all rondel and trapezoid, formed mainly in the leaves and stems of these plants. Phytoliths from grass inflorescence (the dendritic morphotype, cf. Figure 9c) are present in very low percentages (0.6%). In the literature, dendritics are usually indicators of domestic crops and high water availability (Rosen and Weiner, 1994; Ball et al., 1999; Albert et al., 2008; Jenkins et al., 2011a, b). Their limited presence here may suggest that only wild grasses were predominant in the site.
Morphotypes related to other monocotyledons, such as C4 grasses phytoliths, are scarce (0.42%) or absent.
Meanwhile, phytoliths from woody and herbaceous dicotyledons are also present in all the samples, although in small amounts (7.15%). This value is, however, significant since dicotyledonous plants are minor phytolith producers (Albert and Weiner, 2001; Carnelli et al., 2001; Tsartsidou et al., 2007). Within this group, phytoliths from the wood and bark (spheroids) are more abundant (6.76%) than those from leaves (tracheary and jigsaw, 0.38%), quite probably due to preservation issues that dicots phytoliths have (Albert and Weiner, 2001; Tsartsidou et al., 2007).
4.2.2 Profile 1
Only Profile 1 had identifiable charcoal sufficient for dating. This profile was dated at 13 cm, 110 cm and 142 cm below the ground surface. Since the dates obtained at 110 cm (6475 ± 41 BP) and 142 cm (5619 ± 35 BP) show a reverse chronology, not unusual for these heavily impacted soils, an age-depth model was generated based on the younger date (Ferrara and Wästfelt, 2025). Based on the age-depth model the oldest age is c. 6700 years ago, with an average sediment accumulation rate (i.e., sedimentation time) of ca. 35 years/cm.
From the phytolith diagram of Profile 1, we can distinguish at least five different temporal phases (Figure 11). The oldest phase, Phase 1 (150–130 cm), is characterized by a slight increase of the three indices long/short cell index, Fs-index and D/P index. This tells that a higher presence of mature grasses and dicots (observed in the long/short cell index and D/P index curves) is accompanied by an increase in their evapotranspiration rates (suggested by the Fs-index curve). Such an increase in the curve of the Fs-index may attest the beginning of a substantial variation in local climatic conditions towards more warm and dry conditions. According to the age-depth model, this phase could have covered the period 4849–3854 BCE. What shown by the phytoliths evidence in this phase is coherent with published palynology and paleoclimate reconstructions, which have attested in the region droughts at around ca. 5550–4550 BCE (Zanchetta et al., 2007; Carroll et al., 2012; Peyron et al., 2013).

Figure 11. Phytolith diagram showing percentages of morphotypes from Cozzo del Lampo, Profile 1. Yellow and orange bars: grass phytoliths; green bars: dicots phytoliths. Dots indicate morphotypes < 1%. Inflorescence/culm-leaves index shown with exaggeration factor (60x). Note the difference in scale between the indices (Image source Ferrara and Wästfelt, 2025).
Phase 2 (130–110 cm, 3854 BCE – 2871 BCE, end of Neolithic - beginning of Bronze Age) is characterized by a marked correlation between land cover and climate, as we see peaks in both the D/P indices and the Fs-index. These peaks may signify the natural expansion of xerophilous taxa (e.g., Olea, Pistacia), attested by palynological and paleoclimatic reconstructions as well telling that, around ca. 4050 BCE, there were reduced rainfall, progressive increasing dryness and aridification (Zanchetta et al., 2007; Sadori et al., 2008; Carroll et al., 2012; Peyron et al., 2013).
In Phase 3 (110–90 cm, 2871–1882 BCE, Bronze Age), the presence of trees is slightly reduced, while there is an increase in both mature grasses (but no evidence of flowering) and grasses cut in their young stage. The water stress signal of plants remains however strong. Paleoclimatic records (Sadori et al., 2015) attest these dry conditions, predominant until a cooler phase starting approximately at 2600 BCE.
Phase 4 (90–40 cm) corresponds to a period between 1882 BCE to CE 576, covering a wide temporal range of Sicilian history (from Early Bronze Age to late Antiquity). This phase is characterised by the substantial reduction of trees (cf. Db/P index) and an increase in grasses reaching their maturing stage, but flowering evidence is absent (cf. long cell/short cell index). There is a notable increase in grasses cut instead in their young phase (indicated above all by the high presence of the rondel morphotype).
The last temporal phase (Phase 5, 40–0 cm) may cover the period from CE 576, thus from Late Antiquity, until today, according to the age-depth model. In this phase, we see a further reduction of dicot plants (attested by the reduction of blocky morphotypes) from the previous phase. There is, moreover, a reduction of grasses kept mature accompanied by an increase in the presence of young grasses.
In Profile 1 of Cozzo del Lampo, the stratigraphic layers from Phase 1 to Phase 4 are dominated by grass phytoliths produced in the stems and leaves of these grasses, while the representation of grass inflorescences is very low (only present on the most recent layer, 10–0 cm). This is not surprising in natural grassland contexts, where the proportion of elongate dendritic phytoliths (indicators of grass spikelets) usually remains low (Novello and Barboni, 2015). In Cozzo del Lampo, the very low input of grass inflorescences, either domesticated or wild, might suggest on one side the harvesting of grasses before the complete formation and silicification of the inflorescence bracts. On the other side, such a low amount of inflorescence bract phytoliths could also be evidence of intense grazing before grasses reach their mature inflorescence (Delhon et al., 2020). Furthermore, the long/short cell index confirms a higher percentage of mature grasses in the older phases of the profile, and their progressive reduction over time. This might be due to preservation issues of short cell phytoliths along the profile or be related to historical changes in land use towards practices that have favored a more increased presence of young grasses (this could have been the case of heavy tilling practices). However, even though elongate entire phytoliths are very common in grass leaves, especially stem and culm epidermis, they are also formed in non-grass vegetation (Neumann et al., 2019, 9). Unfortunately, this is an understudied issue, thus our interpretation of the results from the long/short cell index must be considered with caution.
Along the profile, the Fs-index curve indicate periods of water stress experienced by local vegetation, which may be linked with warmer and drier climatic conditions, particularly in Phase 2. In coincidence with this period of water stress, both the D/P indices peak and this may indicate the natural expansion of xerophilous taxa in the area. Furthermore, even though phytolith production is far more important in grass than in dicots (Delhon, 2010), the presence of high percentages of dicot phytoliths in Phase 2 and 3 can suggest the presence of a semi-open habitat resembling a shrubland-type of environment. As both the D/P indices curves significantly decrease with time from 90 cm (beginning of Phase 4) until today, we can then infer the disappearance of certain species of arboreal vegetation (shrubs)? and the further opening of this landscape. Similar patterns were observed by Blinnikov (2005) in the historical boundaries between open grasslands and conifer forests in USA, as well as by Silantyeva et al. (2018) and Solomonova et al. (2019) who, relying on the floristic composition of forest vs. meadow vs. steppe in the Russian Altay, demonstrated the utility of using rondels as indicators of open habitats.
4.2.3 Profile 3
Profile 3 (Figure 12) show the historical transition from agroforestry model of land use into the present olive orchard. This is attested by the prevalent presence of grasses, accompanied with a constant low presence of dicots phytoliths along the entire profile, if not in the uppermost layer.
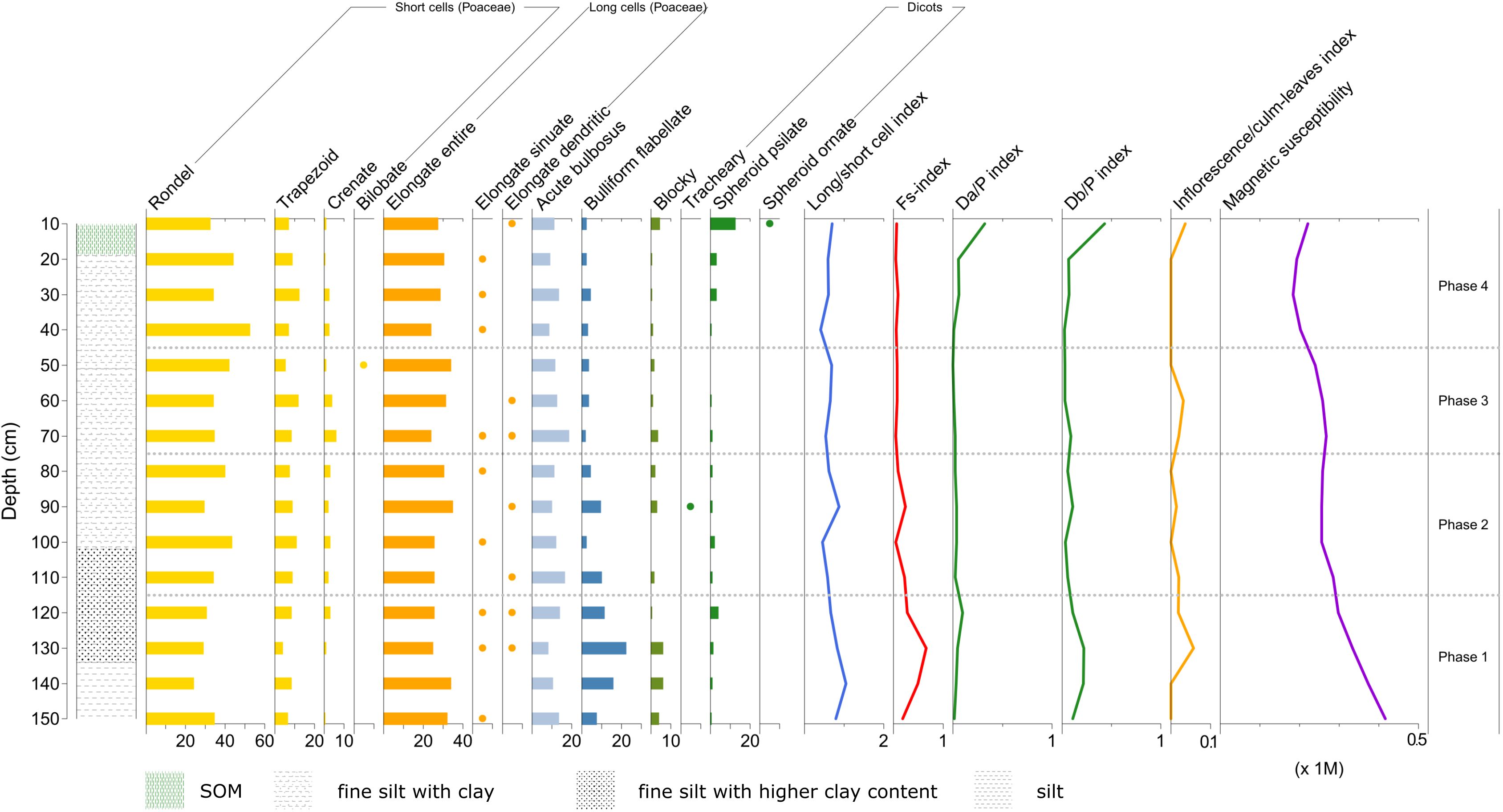
Figure 12. Phytolith diagram showing percentages of morphotypes from Cozzo del Lampo – Profile 3. See diagram legend in Figure 11 caption (Image source: Ferrara and Wästfelt, 2025).
At the bottom of the profile (from 150 to 111 cm), we see simultaneous peaks of all four indices, telling that in this phase land cover may have been characterized by the presence of both mature grasses (attested by the peak in the inflorescence/culm-leaves and long/short cells index) and arboreal vegetation (peak in the D/P indices), within an overall increase in dryness conditions (cf. peak of the Fs-index). At 90 cm (Phase 2), there are slight peaks in both the Fs-index and the Da/P index (above 0.1), again associated with a peak of the inflorescence/culm-leaves index and the long/short cell index. The potential land use pattern could resemble an agroforestry model, with intercropping practices.
Another interesting phase along the profile could be at 75–45 cm (Phase 3), characterized by a higher peak of the inflorescence/culm-leaves index, but now associated with a slight decrease of dicots and long cells grasses, transitioning into a further slight decrease of dicots (< 1%). This trend may suggest the progressive opening of the local environment, now featured by the presence of less trees. The soil in between 70 and 60 cm is also characterized by the inclusion of highly fragmented and minute pottery shards, which suggests anthropogenic presence at the site at those levels.
The topsoil layer in Profile 3 (Phase 4) is characterized by a higher presence of dicots phytoliths, which may attest the presence of fruit trees in the area until recently (Ferrara et al., 2024b; Ferrara and Wästfelt, 2025).
4.3 Agroforestry (Malìa)
In Malìa, the only 14C age obtained from collected charcoal is very recent, consequently it was not possible to date any of the profiles. Even with the absence of a tentative chronology, the phytolith record in Malìa still provides diachronic information.
Phytoliths are abundant in most samples, apart from those layers with eroded bedrock (Profile 1, 60–150 cm; Profile 2; Profile 3, 80 cm and 100–150 cm; Profile 4, 50 cm and 80–150 cm; Profile 5, 150 cm). Detailed stratigraphic descriptions of each single profile are provided in Supplementary Material S1.
Similar to Cozzo del Lampo, all the phytolith records in Malìa are characterized by a morphological predominance of grasses from the Pooideae subfamily (C3), given by the high percentage of short cells phytoliths (31.4%) vs long cells (46.76%). The most common morphotypes recognized are rondel and trapezoid, formed mainly in the leaves and the stems. Phytoliths from grass inflorescence (dendritic) are present in low percentages, values which are however higher than in Cozzo del Lampo (3% vs 0.6%). Morphotypes related to other monocotyledons are scarce (0.45%). Phytoliths from woody and herbaceous dicotyledons are omnipresent in small amounts (5.83%). Wood and bark types (spheroids) are more abundant (5.71%) than from leaves (tracheary, 0.11%).
The assemblage analysis of the phytolith record from Malìa indicates specific environmental and land use history dynamics, which can be correlated with alternate periods of land abandonment. Profiles 4 and 5, described in details below, represent the most indicative cases of these dynamics.
4.3.1 Profile 5
As in Cozzo del Lampo, in the lowest and earliest layers, the profiles in Malìa show simultaneous peaks in the three indices long/short cells, Fs-index and D/P index, which may indicate a phase of shrubland growth due to warmer and drier conditions (Figure 13). At that time, vegetation composition was dominated by trees/shrubs and mature grasses.
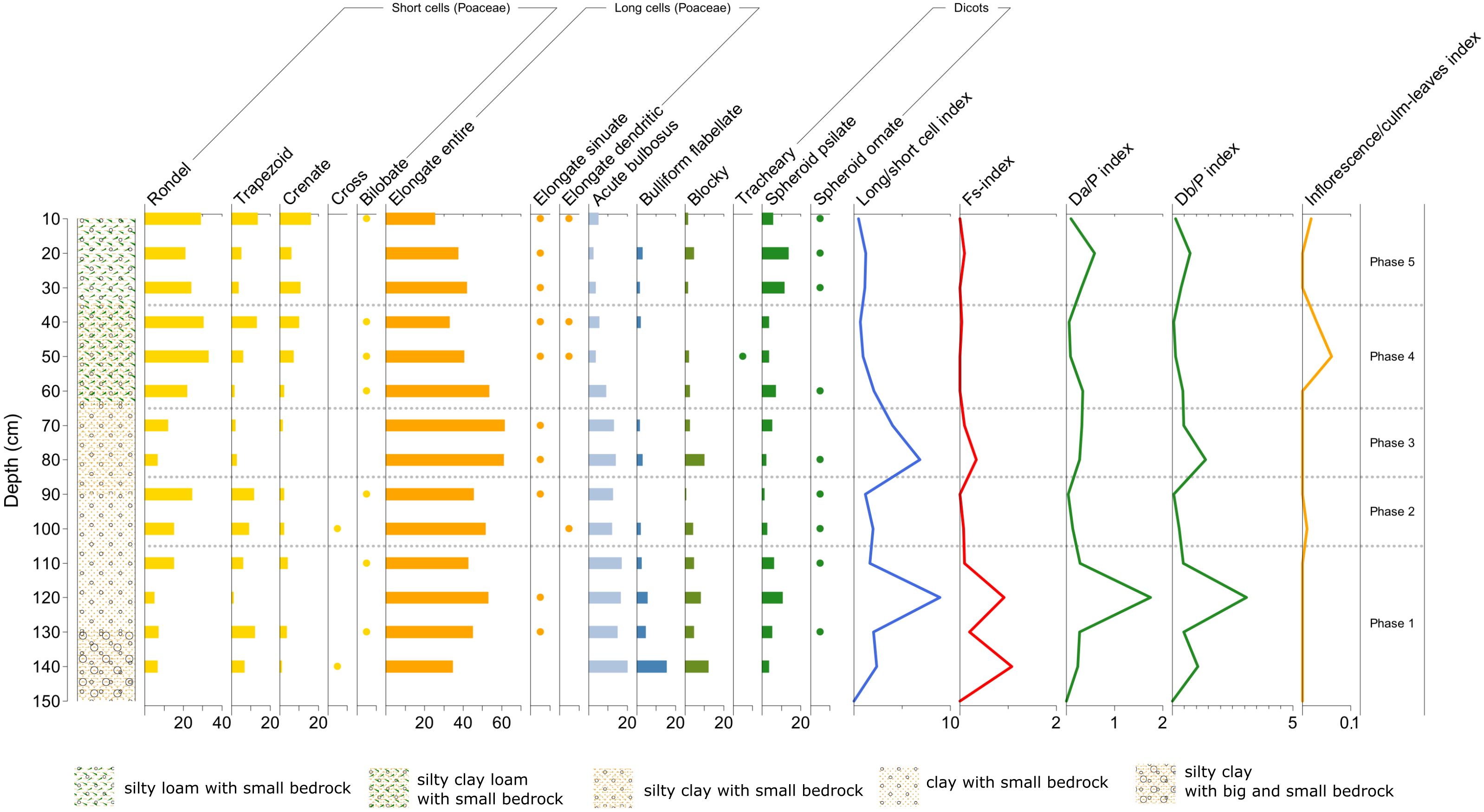
Figure 13. Phytolith diagram showing percentages of morphotypes from Malìa – Profile 5. See diagram legend in Figure 11 (Image source: Ferrara, 2024).
Phases 2 and 4 are both characterized by a reduction of the two indices featuring overgrowing of tall grasses, shrubs and trees (long/short cell index, D/P index), while there is an increase in the presence of inflorescence phytoliths, which may attest the use of the site for cultivation purposes.
The presence of inflorescence, associated with phytoliths indicating the clearing out of other grasses (cf. the long/short cell index decrease) and bushes (cf. the D/P index decrease), may be highly indicative of specific cultivation practices that kept certain grasses (crops)? reaching their maturation stage, while removing/clearing out some other types of grasses and bushes.
In between Phase 2 and 4, Phase 3 is on the contrary characterized by simultaneous peaks in long/short cells index, Fs-index and Db/P index, accompanied by a decrease in the inflorescence index. This may be linked to mature grasses being harvested or grazed before reaching their inflorescence stage.
In the last and more recent phase of Profile 5 (Phase 5), there is an increase in dicots (0.5) and long grass cells, followed by their reduction associated with an increase again in inflorescence, which may attest first an abandonment phase and then a re-use of the site (possibly for the combination of olive cultivation and grazing, based on the owners´ information on the land use since last century).
4.3.2 Profile 4
The location of this profile is a large open space among olive trees. Phases of use and abandonment, similar to Profile 5, can be deduced also here from the phytolith analysis (Figure 14). The oldest Phase 1 displays peaks in both the long/short cell index, Fs-index and D/P index, followed by their decrease in Phase 2. A second peak in all these three indices occurs in Phase 3, characterized also by the presence of inflorescence phytoliths that continues in Phase 4. Phase 3 shows a potential abandonment of the site, suggested by an increase in late-season grasses (i.e., in their year of growth; cf. long/short cell index) and in the presence of dicots (indicated by peaks in the long/short cell index, inflorescence index and D/P indices).
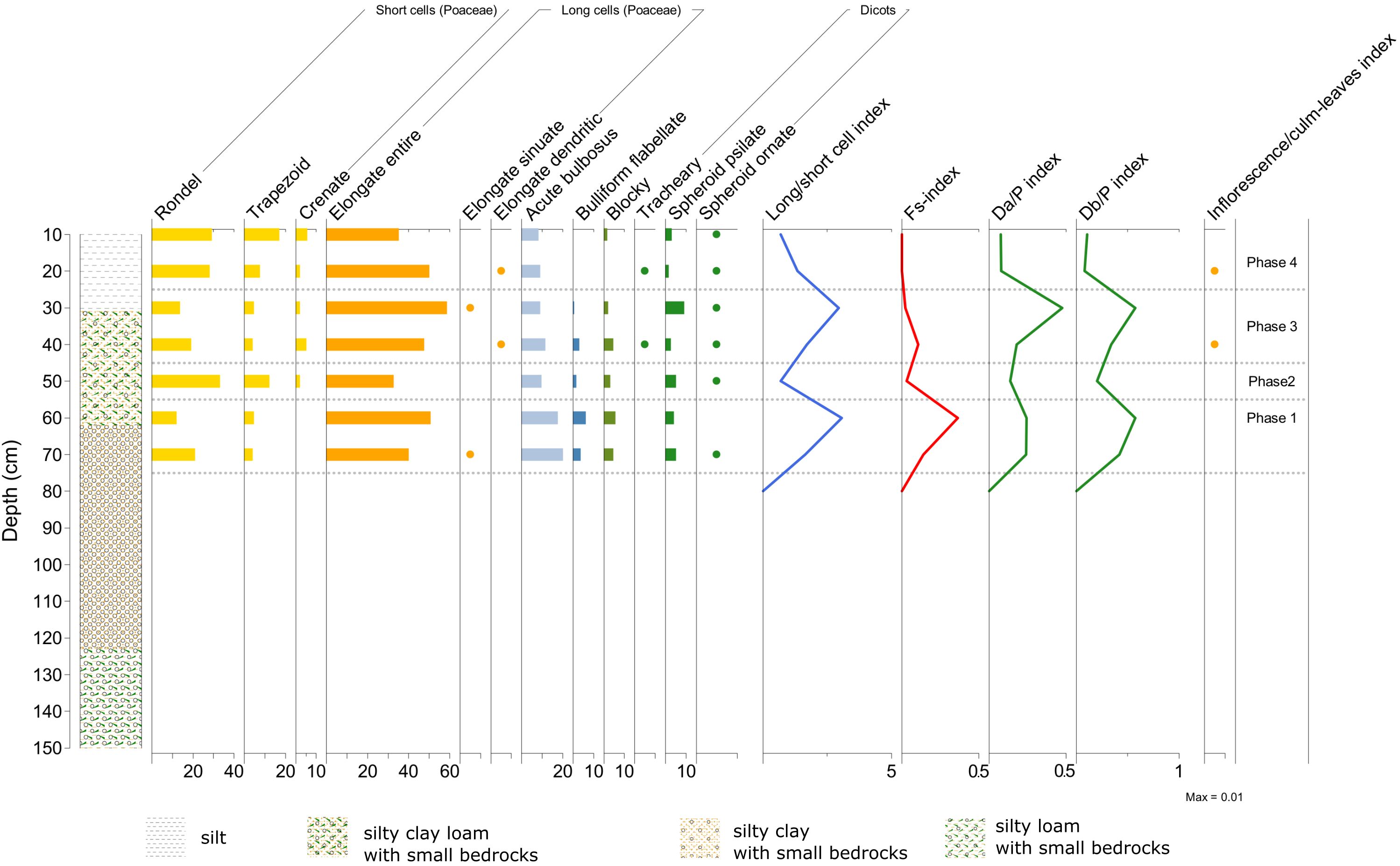
Figure 14. Phytolith diagram showing percentages of morphotypes from Malìa – Profile 4. See diagram legend in Figure 11 (Image source: Ferrara, 2024).
In Phase 4, the phytoliths from dicots decrease, thus these dicot plants were not likely to be olive trees. The increasing (in Phase 3) and then decreasing trend of the D/P indices (in Phase 4) could signify that the dicot vegetation present in Phase 3 was rather the result of abandonment (e.g. shrubs), successively cleared out for cultivation purposes around the existing olive trees (visible in Phase 4 and potentially a similar shift also took place in the previous Phase 2).
5 Discussion
As shown from our results, phytolith assemblage analysis provides the possibility to distinguish between close and open environment in historical olive agroecosystems and, furthermore, to differentiate between periods of anthropogenic occupation and abandonment of a site. Phytoliths as biocultural traces can also reveal previously unknown dimensions of local land use, linked to traditional ecological practices of the past. Phytolith assemblage analysis can then allow us to understand how land uses in diverse historical times have contributed to shape the current configurations of ancient olive trees in present space.
The results from Bosco Pisano are reference for comparisons with the other study areas based on the agricultural use of Olea, since they can be representative of the tree cover density typical of an olive woodland. In the case of Bosco Pisano, phytolith assemblage analysis aimed to establish a reference for the local calibration of the threshold in tree cover density calculated by the D/P index, which was applied to interpret evidence from the other sites.
In Cozzo del Lampo, the results from phytolith analysis have shown that the site transitioned – through at least six millennia – from a shrubland into the olive orchard we see today. A similar transition is also shown in the Malìa site, where we gain further knowledge into how biological diversity in plant composition was positively correlated with human practices and negatively affected by anthropogenic abandonment. Phytoliths as biocultural proxies in the soil can therefore allow us to gain new knowledge on the land use and management of a site happening as a historical process, helping see more clearly how different historical phases are interlinked and dependent to each other.
The results presented here also suggest both shifts and maintenance in land use. In Malìa, the phytolith results show that a predominant grass vegetation cover has been the norm, as in the phytolith diagrams we see no drastic change from natural or semi-natural grassland to today´s olive orchards. This result suggests that a grass layer was maintained, either via grazing (thus we could advance also the hypothesis that phytoliths may have been introduced through the dung of grazing livestock) or by cutting grass (meadowing). This could have been probably also a practice in other olive agroecosystems in the Madonie Mountains. In this area of Sicily, olive orchards were organized to provide grazing and fodder, alongside cultigens; a multifunctional land use which may have been necessitated by the local extremely harsh topography. Moreover, the Malìa phytolith records, if compared with other proxies, may shed light on the correlation between certain land use practices and specific cultural phases in local history. In Profile 5, the peaks of the inflorescence/culm-leaves index are simultaneous with the presence of C4 phytoliths (bilobate and cross), indicative of certain plant varieties that may have been associated with specific cultural land use practices. This is the case, for instance, of the introduction of C4 plants for both humans and animals’ diet during early Medieval times (as attested in the archaeological record elsewhere in Sicily, cf. Egli et al., 2013), which in the Madonie Mountains may be linked to the Islamic settlers´ agricultural practices reshaping the local landscape during the 9th–11th centuries BCE (cf. Barbera, 2013). The Malìa case is therefore inspiring for further integrated investigations into correlation dynamics between plant diversity traced from soil layers of the past and the diverse historical phases of cultural contaminations on the island. Furthermore, since peaks in inflorescence with C4 grasses (thriving in more warm and wet environments) occur together with a decreasing trend of water stress (cf. Fs-index) in Profile 5, we may infer that these grasses reaching an inflorescence stage could have been favored somehow by local access to water.
In conclusion, our work demonstrates that phytoliths assemblages conceived as biocultural traces stored in soil layers can be analyzed for the investigation of past land uses in historical agroecosystems. Phytoliths in the soil reflect local plant communities and, studied as biocultural heritage and within a historical perspective, they can be interpreted as the accumulated outcomes of local plants´ responses to both external and internal inputs/stresses. Even though the deposition and preservation of phytoliths in agricultural soils are affected by several environmental and anthropogenic disturbances over time, the analysis presented here shows that phytoliths assemblages can be informative on how land use practices in a place at different periods are correlated to the alternate states and historical conditions of these agroecosystems over time. In such respect, results show that these historical agroecosystems have experienced significant shifts in both conditions and compositions of local vegetation over the past millennia (as indicated in particular in Cozzo del Lampo, where chronology is available). Such historical dimension can be highly informative not only to better understand past land use practices, but also to advance knowledge about how local plants (and humans managing them) have acted and re-acted in front of changing environmental circumstances (e.g., climate). In Cozzo del Lampo and Malìa profiles, the oldest changes in land cover, driven probably by environmental shifts (i.e., climate), have preceded and potentially partly driven the coming land use and its further changes. The phytolith records presented in this paper inform us of an initial shrubland, which could have been favored by certain climatic and environmental conditions. Among the local xerophilous taxa occurring naturally in this environment, the selection of Olea was surely shaped by pragmatism alongside cultural influences, since it is one of the most resistant trees/shrubs for agricultural and silvo-pastoral use. In simple words, the phytolith record in Cozzo del Lampo, when combined with local palynological and paleoclimatic data, suggests the hypothesis that, without the environmental conditions favoring a certain type of vegetation to spread at ca. 5550–4050 BCE (cf. Cozzo del Lampo, Profile 1), we would not have had the beginning of olive-linked land use. Furthermore, an herbaceous undergrowth has been the local land cover in these olive agroecosystems for centuries, until most recent phases in which grasses are present in their young stage.
The analysis of phytolith assemblages stored in the soil as biocultural heritage can, therefore, be used to infer land use dynamics at the whole agroecosystem level, particularly in remnants of old systems. This method opens up the possibility of expanding phytoliths research as proxies to understand past land use practices in historical agricultural soils. This allows for a better understanding of not only past land uses but, what is even more important, their ecological legacies on current soils and ecosystem conditions. Information on the silica cycle in agroecosystems over the long term is important to reconstruct long-term anthropogenic drivers and their impacts of land use practices. The analysis of phytoliths as biocultural evidence can thus operationalize the investigation of past land uses in historical agroecosystems to address current management issues, since along the (deep) history of a site we may find past analogues to future scenarios of local environmental changes. These we may want to bear in mind when reflecting on how future adaptations of land use and agriculture could be shaped. Knowing how these agroecosystems have locally responded to similar shifts and challenges we face today or we may face in the future, can provide us with crucial evidence that we can immediately operationalize in our current management. This could be the case, for instance, of ecosystems´ reaction to an increase in local temperatures and progressive dryness, or to the anthropogenic abandonment of a site. More importantly, we may start noticing the evidence of an incredible interdependence between gradients of variation along millennia (in microclimatic conditions, land cover, land use, biodiversity, occupation) and longer stability trends (represented by the maintenance of certain vegetation elements, i.e. century-old olive trees); and advance the interpretative hypothesis that exactly such persistence could have been the prolonged response to disturbances. The novel contribution of our work relies on showing that agriculture in itself is an ecological practice, which over the long term have contributed to maintain certain agroecosystems structures and functions, including their biocultural diversity. Such a historically informed perspective can inspire us practitioners for present day management and future adaptation, as well as inform public policies on the urgent need to promote the conservation of these historical agroecosystems.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: Zenodo, https://doi.org/10.5281/zenodo.15367550.
Author contributions
VF: Data curation, Writing – original draft, Investigation, Formal analysis, Resources, Visualization, Conceptualization, Writing – review & editing, Methodology. GS: Investigation, Writing – review & editing, Resources, Methodology. GG: Writing – review & editing, Investigation, Resources. TM: Methodology, Writing – review & editing, Investigation, Formal analysis, Resources. AE: Methodology, Investigation, Resources, Conceptualization, Project administration, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The research presented in this paper has received funding by Vetenskapsrådet (The Swedish Research Council), under the research project “Oliven och Siciliens bio-kulturella arv”, grant number 2020-02625. The orthophotos published in this paper have been granted by the Regione Siciliana, Assessorato Regionale Territorio e Ambiente, Dipartimento di Urbanistica, Area 2 Interdipartimentale, on 31/01/2023 with authorization number n. 2023-S-3466.
Acknowledgments
Special thanks go to all the local people who joined us as colleagues during the fieldwork done in the three case study areas. We are moreover thankful to all the colleagues from other departments and institutions who collaborated in various ways to this project: Anders Wästfelt, Maria Fernanda Salame González, Pascoal Gota, Frances Deegan, Sebastian Willman, Ian Brown, Claire Delhon, Rafael da Silveira Bueno, Johan Lindberg. Last but not least, we would like to express our deepest gratitude to the reviewers for their insightful comments and feedback on the earlier version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1625887/full#supplementary-material
Supplementary Material S1 | Stratigraphy of the study areas.
References
Agnoletti M. and Emanueli F. (2016). “Biocultural Diversity and Landscape in Europe: Framing the Issue,” in Biocultural Diversity in Europe. Eds. Agnoletti M. and Emanueli F. (Cham: Springer), 1–20.
Albert R. M., Lavi O., Estroff L., Weiner S., Tsatskin A., Ronen A., et al. (1999). Mode of occupation of Tabun Cave, Mt Carmel, Israel during the Mousterian period: A study of the sediments and phytoliths. J. Archaeological Sci. 26, 1249–1260. doi: 10.1006/jasc.1999.0355
Albert R. M., Ruiz J. A., and Sans A. (2016). PhytCore ODB: A new tool to improve efficiency in the management and exchange of information on phytoliths. J. Archaeological Sci. 68, 98–105. doi: 10.1016/j.jas.2015.10.014
Albert R. M., Shahack-Gross R., Cabanes D., Gilboa A., Lev-Yadun S., Portillo M., et al. (2008). Phytolith-rich layers from the Late Bronze and Iron Ages at Tel Dor (Israel): mode of formation and archaeological significance. J. Archaeological Sci. 35, 57–75. doi: 10.1016/j.jas.2007.02.015
Albert R. M. and Weiner S. (2001). “Study of phytoliths in prehistoric ash layers using a quantitative approach,” in Phytoliths: Applications in Earth Sciences and Human History. Eds. Meunier J. D. and Colin F. (Lisse: A.A. Balkema Publishers), 251–266.
Aleman J. C., Canal-Subitani S., Favier C., and Bremond L. (2014). Influence of the local environment on lacustrine sedimentary phytolith records. Palaeogeography Palaeoclimatology Palaeoecol. 414, 273–283. doi: 10.1016/j.palaeo.2014.08.030
Alexandre A., Meunier J. D., Lézine A. M., Vincens A., and Schartz D. (1997). Phytoliths: Indicators of grassland dynamics during the late Holocene in intertropical Africa. Palaeogeography Palaeoclimatology Palaeoecol. 136, 213–229. doi: 10.1016/S0031-0182(97)00089-8
An X. (2016). Morphological characteristics of phytoliths from representative conifers in China. Palaeoworld 25, 116–127. doi: 10.1016/j.palwor.2016.01.002
An X. and Lu H. Y. (2015). Surface soil phytoliths as vegetation and altitude indicators: a study from the southern Himalaya. Sci. Rep. 5, 15523. doi: 10.1038/srep15523
An X. and Xie B. (2022). Phytoliths from woody plants: A review. Diversity 14, 339. doi: 10.3390/d14050339
Baiamonte G., Domina G., Raimondo F. M., and Bazan G. (2015). Agricultural landscapes and biodiversity conservation: a case study in Sicily (Italy). Biodiversity Conserv. 24, 3201–3216. doi: 10.1007/s10531-015-0950-4
Ball T. B., Gardner J. S., and Anderson N. (1999). Identifying inflorescence phytoliths from selected species of wheat (Triticum monococcum, T. dicoccon, T. dicoccoides and T.aestivum) and barley (Hordeum vulgare and H. spontaneum) (Gramineae). Am. J. Bot. 86, 615–623. doi: 10.2307/2656798
Ball T. B., Gardner J. S., and Anderson N. (2001). “An approach to identifying inflorescence phytoliths from selected species of wheat and barley,” in Phytoliths: Applications in Earth Sciences and Human History. Eds. Meunier J. D. and Colin F. (Lisse: A.A. Balkema Publishers), 289–302.
Bamford M. K., Albert R. M., and Cabanes D. (2006). Plio–Pleistocene macroplant fossil remains and phytoliths from Lowermost Bed II in the eastern palaeolake margin of Olduvai Gorge, Tanzania. Quaternary Int. 148, 95–112. doi: 10.1016/j.quaint.2005.11.027
Barão L., Teixeira R., Vandevenne F., Ronchi B., Unzué-Belmont D., and Struy E. (2020). Silicon mobilization in soils: the broader impact of land use. Silicon 12, 1529–1538. doi: 10.1007/s12633-019-00245-y
Barbera G. (2013). “Chapter 24 Sicily,” in Italian Historical Rural Landscapes. Ed. Agnoletti M. (Dordrecht: Springer), 509–529.
Barboni D. and Bremond L. (2009). Phytoliths of East African grasses: An assessment of their environmental and taxonomic significance based on floristic data. Rev. Palaeobotany Palynology 158, 29–41. doi: 10.1016/j.revpalbo.2009.07.002
Barboni D., Bremond L., and Bonnefille R. (2007). Comparative study of modern phytolith assemblages from inter-tropical Africa. Palaeogeography Palaeoclimatology Palaeoecol. 246, 454–470. doi: 10.1016/j.palaeo.2006.10.012
Barthel S., Crumley C. L., and Svedin U. (2013a). Biocultural refugia: combating the erosion of diversity in landscapes of food production. Ecol. Soc. 18, 71. doi: 10.5751/ES-06207-180471
Barthel S., Crumley C. L., and Svedin U. (2013b). Bio-cultural refugia—Safeguarding diversity of practices for food security and biodiversity. Global Environ. Change 23, 1142–1152. doi: 10.1016/j.gloenvcha.2013.05.001
Belvedere O. and Forgia V. (2010). “Prehistoric settlement and population in the Madonie Mountains,” in Archéologie de la Montagne Européenne. Eds. Tzortzis S. and Delestre X. (Aix-en-Provence: Éditions Errance), 145–152.
Benvenuto M. L., De Rito M., Osterrieth M. L., and Honaine M. F. (2025). Analysis of phytolith inputs from natural plant communities and crops and soil silicon availability (Southeastern Pampean region,Argentina). Flora 322, 152640. doi: 10.1016/j.flora.2024.152640
Blaauw M., Christen J., and Aquino Lopez M. (2022). rbacon: age-depth modelling using Bayesian statistics. R package version 2.5.8.
Blinnikov M. S. (2005). Phytoliths in plants and soils of the interior Pacific Northwest, USA. Rev. Palaeobotany Palynology 135, 71398. doi: 10.1016/j.revpalbo.2005.02.006
Boixadera J., Riera S., Vila S., Esteban I., Albert R. M., Llop J. M., et al. (2016). Buried A horizons in old bench terraces in Les Garrigues (Catalonia). Catena 137, 635–650. doi: 10.1016/j.catena.2014.08.017
Boyd K. C., Cordova C. E., Cadd H. R., Rowe C., and Cohen T. J. (2024). Woody plant phytolith morphology and representation in surface sediments across the Northern Territory, Australia. Rev. Palaeobotany Palynology 329, 105158. doi: 10.1016/j.revpalbo.2024.105158
Bozarth S. (1992). “Classification of opal phytoliths formed in selected Dicotyledons native to the Great Plains,” in Phytolith Systematics. Advances in Archaeological and Museum Science, Vol 1. Eds. Rapp G. and Mulholland S. C. (Boston, MA: Springer), 193–214.
Bremond L., Alexandre A., Hely C., and Guiot J. (2005a). A phytolith index as a proxy of tree cover density in tropical areas: Calibration with Leaf Area Index along a forest–savanna transect in southeastern Cameroon. Global Planetary Change 45, 277–293. doi: 10.1016/j.gloplacha.2004.09.002
Bremond L., Alexandre A., Peyron O., and Guiot J. (2005b). Grass water stress estimated from phytoliths in West Africa. J. Biogeography 32, 311–327. doi: 10.1111/j.1365-2699.2004.01162.x
Bremond L., Alexandre A., Wooller M. J., Hély C., Williamson D., Schäfer P. A., et al. (2008). Phytolith indices as proxies of grass subfamilies on East African tropical mountains. Global Planetary Change 61, 209–224. doi: 10.1016/j.gloplacha.2007.08.016
Burguet-Coca A., Polo-Díaz A., Martínez-Moreno J., Benito-Calvo A., Allué E., Mora R., et al. (2020). Pen management and livestock activities based on phytoliths, dung spherulites, and minerals from Cova Gran de Santa Linya (Southeastern pre-Pyrenees). Archaeological Anthropological Sci. 12, 148. doi: 10.1007/s12520-020-01101-6
Cabanes D., Weiner S., and Shahack-Gross R. (2011). Stability of phytoliths in the archaeological record: a dissolution study of modern and fossil phytoliths. J. Archaeological Sci. 38, 2480–2490. doi: 10.1016/j.jas.2011.05.020
Carbone S., Grasso M., and Lentini F. (1986). Carta Geologica del Settore Nord-orientale Ibleo (Sicilia SE), Scala 1:50.000 (Firenze: S.EL.CA.).
Carnelli A. L., Madella M., and Theurillat J. P. (2001). Biogenic silica production in selected alpine plants species and plant communities. Ann. Bot. 87, 425–434. doi: 10.1006/anbo.2000.1355
Carnelli A. L., Theurillat J. P., and Madella M. (2004). Phytolith types and type-frequencies in subalpine-alpine plant species of the European Alps. Rev. Palaeobotany Palynology 129, 39–65. doi: 10.1016/j.revpalbo.2003.11.002
Carocci S. (2010). ““Metodo regressivo“ e possessi collettivi: i “demani“ del Mezzogiorno (sec. XII-XVIII),” in Écritures de l’Espace Social: Mélanges d’Histoire Médiévale Offerts à Monique Bourin. Eds. Boisseuil D., Chastang P., Feller L., and Morsel J. (Paris: Éditions de la Sorbonne), 541–556.
Carroll F. A., Hunt C. O., Schembri P. J., and Bonanno A. (2012). Holocene climate change, vegetation history and human impact in the Central Mediterranean: evidence from the Maltese Islands. Quaternary Sci. Rev. 52, 24–40. doi: 10.1016/j.quascirev.2012.07.010
Cevasco R., Moreno D., and Hearn R. (2015). Biodiversification as an historical process: An appeal for the application of historical ecology to bio-cultural diversity research. Biodiversity Conserv. 24, 3167–3183. doi: 10.1007/s10531-015-0943-3
Cohen M., Godron M., Cretin-Pablo R., and Pujos R. (2023). Plant biodiversity in Mediterranean orchards is related to historical land use: perspectives for biodiversity friendly olive production. Regional Environ. Change 23, 70. doi: 10.1007/s10113-023-02088-1
Crumley C. L. (2012). “A heterarchy of knowledge: Tools for the study of landscape histories and futures,” in Resilience and the Cultural Landscape: Understanding and Managing Change in Human-Shaped Environments. Eds. Plieninger T. and Bieling C. (Cambridge: Cambridge University Press), 303–313.
Danu M., Delhon C., and Weller O. (2020). Could the grasses have played a role in the earliest salt exploitation? Phytoliths analysis of prehistoric salt spring from Hălăbutoaia -Ţolici (Romania). Archaeological Anthropological Sci. 12, 270. doi: 10.1007/s12520-020-01228-6
Delhon C. (2010). Phytoliths and taphonomy, the contribution of experimentation to the quantification of phytoliths in wood ashes. Palethnologie 2:93–104. doi: 10.4000/palethnologie.8655
Delhon C., Alexandre A., Berger J. F., Thiébault S., Brochier J. L., and Meunier J. D. (2003). Phytolith assemblages as a promising tool for reconstructing Mediterranean Holocene vegetation. Quaternary Res. 59, 48–60. doi: 10.1016/S0033-5894(02)00013-3
Delhon C., Binder D., Verdin P., and Mazuy A. (2020). Phytoliths as a seasonality indicator? The example of the Neolithic site of Pendimoun, south-eastern France. Vegetation History Archaeobotany 29, 229–240. doi: 10.1007/s00334-019-00739-0
Delhon C., Martin L., and Thiébault S. (2024). Neolithic shepherds and sheepfold caves in Southern France and adjacent areas: An overview from 40 years of bioarchaeological analyses. Quaternary Int. 683-684, 61–75. doi: 10.1016/j.quaint.2023.03.004
Dickau R., Iriarte J., Quine T., Soto D., and Mayle F. (2016). Reconstructing pre-Colombian agricultural practices in the Bolivian savannah: Stratigraphic and phytolith evidence from raised fields at campo España, western llanos de moxos. Cadernos Do LEPAARQ (UFPEL) 13, 223–267. doi: 10.15210/lepaarq.v13i25.7391
Di Pasquale G., Garfì G., and Migliozzi A. (2004). “Landscape Dynamics in South-eastern Sicily in the last 150 years: the case of the Iblei Mountains,” in Recent Dynamics of the Mediterranean Vegetation and Landscape. Eds. Mazzoleni S., Pasquale G., Mulligan M., Martin P., and Rego F. (Chichester: Wiley & Sons), 73–80.
Egli M., Gristina L., Wiesenberg G. L., Civantos J. M., Rotolo A., Novara A., et al. (2013). From pedologic indications to archaeological reconstruction: deciphering land use in the Islamic period in the Baida district (north-western Sicily). J. Archaeological Sci. 40, 2670–2685. doi: 10.1016/j.jas.2013.02.001
Feng Y., Jie D., Guo M., Dong S., Chen X., Liu H., et al. (2017). Phytolith loss and enrichment in soil phytolith assemblages revealed by comparisons of phytoliths in vegetation and surface soils of altitudinal belts in the Changbai Mountains, Northeast China. Flora 236-237, 84–93. doi: 10.1016/j.flora.2017.08.005
Fernández Honaine M., Zucol A. F., and Osterrieth M. L. (2006). Phytolith assemblages and systematic associations in grassland species of the South-Eastern Pampean Plains, Argentina. Ann. Bot. 98, 1155–1165. doi: 10.1093/aob/mcl207
Ferrara V. (2024). Historical Olive Agroecosystems of Sicily. Operationalising Biocultural Heritage for Sustainable Futures. Studies in Global Archaeology 29 (Uppsala: Acta Universitatis Upsaliensis).
Ferrara V. and Ingemark D. (2023). The entangled phenology of the olive tree: A compiled ecological calendar of Olea europaea L. over the last three millennia with Sicily as a case study. GeoHealth 7, e2022GH000619. doi: 10.1029/2022GH000619
Ferrara V. and Lindberg J. (2023). “Chapter 6. Climate and environmental change perceptions. A case from rural Sicily (Italy),” in Handbook of Climate Change and Local Social-Ecological Systems. Eds. Reyes-Garcia V., Alvarez-Fernandez S., Benyei P., L. Calvet-Mir L., García-del-Amo D., Junqueira A. B., Li X., Porcher V., Porcuna-Ferrer A., Schlingmann A., and Soleymani R. (London: Routledge), 109–123.
Ferrara V., Lindberg J., and Wästfelt A. (2024a). CONTEXTS.PY (CS.py): A supervised contextual post-classification method to access multiple dimensions of complex geospatial objects. MethodsX 12, 102753. doi: 10.1016/j.mex.2024.102753
Ferrara V., Sala G., Ingemark D., and La Mantia T. (2023). The “green“ granary of the Empire? Insights into olive agroforestry in Sicily from the Roman past and the present. Ital. J. Agron. 18, 2184. doi: 10.4081/ija.2023.2184
Ferrara V., Sala G., and La Mantia T. (2024b). Change and persistence in an olive landscape of Sicily. Geospatial insights into biocultural heritage. Hum. Ecol. 52, 353–366. doi: 10.1007/s10745-024-00498-1
Ferrara V. and Wästfelt A. (2021). Unpacking layers of space-time complexity in land use dynamics. A case study from the olive agrosystems of Sicily (Italy). GI_FORUM. J. Geographic Inf. Sci. 9, 108–121. doi: 10.1553/giscience2021_02_s108
Ferrara V. and Wästfelt A. (2025). An ancient olive tree in the garden. Mapping the deep history of land use from a single image. Geocarto Int. 40(1):1–28. doi: 10.1080/10106049.2025.2471090
Ferrara V., Wästfelt A., and Ekblom A. (2025). “A modern agroecology of the long term: Historical olive agroecosystems in Sicily as case study,” in Encyclopedia of Agriculture and Food Systems, 3rd Edition. (Elsevier, Academic Press). doi: 10.1016/B978-0-443-15976-3.00105-7
Fondazione Migrantes (2022). Rapporto Italiani nel Mondo 2022. Parte Quarta. Allegati Socio-Statistici. (Todi: TAU Editrice).
Fierotti G., Dazzi C., and Raimondi S. (1988). Carta dei suoli della Sicilia (Palermo: Regione Sicilia, Assessorato Territorio e Ambiente).
Forgia V., Martín P., López-García J. M., Ollé A., Vergès J. M., Allué E., et al. (2013). New data on Sicilian prehistoric and historic evolution in a mountain context, Vallone Inferno (Scillato, Italy). Comptes Rendus Palevol 12, 115–126. doi: 10.1016/j.crpv.2012.11.002
Forgia V., Ollé A., and Vergès J. M. (2021). Early pastoral communities in the mountains of Sicily. Prehistoric evidence from Vallone Inferno (Scillato) in the palaeoenvironmental framework of the Madonie mountain range. J. Anthropological Archaeology 61, 101238. doi: 10.1016/j.jaa.2020.101238
Fredlund G. and Tieszen L. (1994). Modern phytoliths assemblages from the North American Great Plains. J. Biogeography 21, 321–335. doi: 10.2307/2845533
Garfì G. and Di Pasquale G. (1988). “First results of an ecological case-history in Sicily: the woods of Buccheri,” in Human Influence on Forest Ecosystems Development in Europe. Ed. Salbitano F. (Bologna: Pitagora Editrice), 353–356.
Giannitrapani E., Ianni F., Chilardi S., and Anguilano L. (2014). Case Bastione: A prehistoric settlement in the Erei uplands (central Sicily). ORIGINI. XXXVI, 181–211.
Gkisakis V. D., Bàrberi P., and Kabourakis E. M. (2018). Olive canopy arthropods under organic, integrated, and conventional management. The effect of farming practices, climate and landscape. Agroecology Sustain. Food Syst. 42, 843–858. doi: 10.1080/21683565.2018.1469066
Grasso M. and Pedley H. M. (1988). The sedimentology and development of Terravecchia Formation carbonates (Upper Miocene) of North Central Sicily: Possible eustatic influence on facies development. Sedimentary Geology 57, 131–149. doi: 10.1016/0037-0738(88)90022-X
Grimm E. C. (1993). Tilia Software (Springfield, IL: Illinois State Museum – Research and Collections Centre).
Gu Y. S., Pearsall D. M., Xie S. C., and Yu J. X. (2008). Vegetation and fire history of a Chinese site in southern tropical Xishuangbanna derived from phytolith and charcoal records from Holocene sediments. J. Biogeography 35, 325–341. doi: 10.1111/j.1365-2699.2007.01763.x
Hayashi N., Inoue Y., Kawano T., and Inoue J. (2021). Phytoliths as an indicator of change in vegetation related to the huge volcanic eruption at 7.3 ka in the southern-most part of Kyushu, southern Japan. Holocene 31, 709–719. doi: 10.1177/0959683620988057
Hill J., Black S., Araujo-Murakami A., Boot R., Brienen R., Feldpausch T., et al. (2023). An assessment of soil phytolith analysis as a palaeoecological tool for identifying pre-columbian land use in amazonian rainforests. Quaternary 6, 33. doi: 10.3390/quat6020033
Hubert Thomas M. F. (2011). Sedimentology and Basin Context of the Numidian FlyschFormation; Sicily and Tunisia. Ph.D. thesis, University of Manchester, England.
Hussain B., Riaz L., Li K., Hayat K., Akbar N., Hadeed M. Z., et al. (2023). Abiogenic silicon: interaction with potentially toxic elements and its ecological significance in soil and plant systems. Environ. pollut. 338, 122689. doi: 10.1016/j.envpol.2023.122689
Iriarte J., Glaser B., Watling J., Wainwright A., Birk J. J., Renard D., et al. (2010). Late Holocene Neotropical agricultural landscapes: phytolith and stable carbon isotope analysis of raised fieldsfrom French Guianan coastal savannahs. J. Archaeological Sci. 37, 2984–2994. doi: 10.1016/j.jas.2010.06.016
Jarl J. and Bruch A. A. (2023). Modern phytolith assemblages as indicators of vegetation in the southern Caucasus. Vegetation History Archaeobotany 32, 561–581. doi: 10.1007/s00334-023-00921-5
Jenkins E., Baker A., and Elliott S. (2011a). “Past plant use in Jordan as revealed by archaeological and ethnoarchaeological phytolith signatures,” in Water, Life and Civilisation: Climate, Environment and Society in the Jordan Valley. Eds. Mithen S. and Black E. (Cambridge: Cambridge University Press), 381–400.
Jenkins E., Jamjoum K., and Nuimat S. A. (2011b). “Irrigation and phytolith formation: an experimental study,” in Water, Life and Civilisation: Climate, Environment and Society in the Jordan Valley. Eds. Mithen S. and Black E. (Cambridge University Press, Cambridge), 347–372.
Jouffroy-Bapicot I., Pedrotta T., Debret M., Field S., Sulpizio R., Zanchetta G., et al. (2021). Olive groves around the lake. A ten-thousand-year history of a Cretan landscape (Greece) reveals the dominant role of humans in making this Mediterranean ecosystem. Quaternary Sci. Rev. 267, 107072. doi: 10.1016/j.quascirev.2021.107072
Kawano T., Kawano K., Udatsu T., and Fujiwara H. (2006). Relationship between modern tree phytoliths assemblages and tree composition in lucidophyllous forests in the southern part of Miyazaki prefecture. Japanese J. Historical Bot. 14, 3–14. doi: 10.1016/j.quaint.2025.109754
Kealhofer L. (2003). Looking into the gap: land use and the tropical forests of southern Thailand. Asian Perspectives 42(1), 72–95. doi: 10.1353/asi.2003.0022
Kraushaar S., Konzett M., Kiep J., Siebert C., and Meister J. (2021). Suitability of phytoliths as a quantitative process tracer for soil erosion studies. Earth Surface Processes Landforms 46, 1797–1808. doi: 10.1002/esp.5121
La Mantia T. (2005). “L´innesto delle radici nell´ulivo: una tecnica agronomica per la costituzione di sistemi agroforestali olivicoli,” in Atti del Convegno Europeo “Il Futuro dei Sistemi Olivicoli in Aree Marginali: Aspetti Socio-economici, Gestione delle Risorse Naturali e Produzione di Qualità” – Matera, 12 e 13 Ottobre 2004 (Potenza: Edizioni L´Aquilone), 483–493.
Liang Y., Nikolic M., Bélanger R., Gong H., and Song A. (2015). Silicon in Agriculture. From Theory to Practice (Dordrecht: Springer Science).
Ligtermoet E., Narndal Gumurdul J., Nayinggul C., and Baker R. (2023). The return of the kinga (saltwater crocodile): Population ‘bust then boom’ shapes shifting baselines in Indigenous biocultural knowledge in northern Australia. Biol. Conserv. 277, 109746. doi: 10.1016/j.biocon.2022.109746
Lisztes-Szabó Z., Braun M., Csík A., and Pető Á. (2019). Phytoliths of six woody species important in the Carpathians: characteristic phytoliths in Norway spruce needles. Vegetation History Archaeobotany 28, 649–662. doi: 10.1007/s00334-019-00720-x
Liu Y., Liu H., Jie D., Gao G., Meng M., and Zhang G. (2021). Phytolith morphotypes of woody plants and their preservation in soil in the warm temperate humid zones of China. Quaternary Int. 599–600, 158–169. doi: 10.1016/j.quaint.2021.03.017
Loeta Tyree E. (1994). Phytolith analysis of olive oil and wine sediments for possible identification in archaeology. Can. J. Bot. 72, 499–504. doi: 10.1139/b94-067
Madella M., Powers-Jones A. H., and Jones M. K. (1998). A simple method of extraction of opal phytoliths from sediments using a non-toxic heavy liquid. J. Archaeological Sci. 25 (8), 801–803.
Madella M., Lancelotti C., and García-Granero J. J. (2016). Millet microremains—an alternative approach to understand cultivation and use of critical crops in Prehistory. Archaeological Anthropological Sci. 8, 17–28. doi: 10.1007/s12520-013-0130-y
Mader A., Langer M., Knippers J., and Speck O. (2020). Learning from plant movements triggered by bulliform cells: the biomimetic cellular actuator. J. R. Soc. Interface 17, 20200358. doi: 10.1098/rsif.2020.0358
Mazuy A., Ferrara V., Ekblom A., and Delhon C. (2024). A rapid and simple method for the extraction of biogenic silica (BSi) in phytolith-poor sediments and soils. MethodsX 12, 102634. doi: 10.1016/j.mex.2024.102634
Meunier J. D., Colin F., and Alarcon C. (1999). Biogenic silica storage in soils. Geology 27, 835–838. doi: 10.1130/0091-7613(1999)027<0835:BSSIS>2.3.CO;2
Moriondo M., Trombi G., Ferrise R., Brandani G., Dibari C., Ammann C. M., et al. (2013). Olive trees as bio-indicators of climate evolution in the Mediterranean Basin. Global Ecol. Biogeography 22, 818–833. doi: 10.1111/geb.12061
Neumann K., Strömberg C. A. E., Ball T., Albert R. M., Vrydaghs L., and Scott Cummings L. (2019). International code for phytolith nomenclature (ICPN) 2.0. Ann. Bot. 124, 189–199. doi: 10.1093/aob/mcz064
Novello A. and Barboni D. (2015). Grass inflorescence phytoliths of useful species and wild cereals from sub-Saharan Africa. J. Archaeological Sci. 59, 10–22. doi: 10.1016/j.jas.2015.03.031
Novello A., Barboni D., Berti-Equille L., Mazur J., Poilecot P., and Vignaud P. (2012). Phytolith signal of aquatic plants and soils in Chad, Central Africa. Rev. Palaeobotany Palynology 178, 43–58. doi: 10.1016/j.revpalbo.2012.03.010
Ntinou M. and Tsartsidou G. (2017). Domestic and ritual use of plants and fuels in the neolithic cave of Alepotrypa, southern Peloponnese, Greece: The wood charcoal and phytolith evidence. Quaternary Int. 457, 211–227. doi: 10.1016/j.quaint.2016.11.028
Pauly D. (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430. doi: 10.1016/s01695347(00)89171-5
Pearsall D. M. and Hastorf C. A. (2011). "Reconstructing Past Life-Ways with Plants II: Human-Environment and Human-Human Interactions," in Ethnobiology. Eds. E.N. Anderson D. M. and Pearsall E. S. Hunn and N.J. Turner (Hoboken, NJ: Wiley-Blackwel), 173–187. doi: 10.1002/9781118015872.ch11
Pearsall D. M. and Trimble M. K. (1984). Identifying past agricultural activity through soil phytoliths analysis: a case study from the Hawaiian Islands. J. Archaeological Sci. 11, 119–133. doi: 10.1016/0305-4403(84)90047-5
Peyron O., Magny M., Goring S., Joannin S., de Beaulieu J. L., Brugiapaglia E., et al. (2013). Contrasting patterns of climatic changes during the Holocene across the Italian Peninsula reconstructed from pollen data. Climate Past 9, 1233–1252. doi: 10.5194/cp-9-1233-2013
Pinter P. R., Butler R. W. H., Hartley A. J., Maniscalco R., Baldassini N., and Di Stefano A. (2016). The Numidian of Sicily revisited: a thrust-influenced confined turbidite system. Mar. Petroleum Geology 78, 291–311. doi: 10.1016/j.marpetgeo.2016.09.014
Piperno D. R. (1988). Phytolith Analysis: An Archaeological and Geological Perspective (San Diego: Academic Press).
Piperno D. R. (2006). Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists (Lanham: AltaMira Press).
Piperno D. R. and Pearsall D. M. (1998). The silica bodies of tropical American grasses: morphology, taxonomy, and implications for grass systematics and fossil phytolith identification. Smithsonian Contributions to Bot. 85, 1–40. doi: 10.5479/si.0081024X.85
Pokrovsky O. S., Akerman A., Fraysse F., Olonova M. V., Kuznetzov A. A., Loiko S. V., et al. (2024). Elemental composition of grass phytoliths: Environmental control and effect on dissolution. Sci. Total Environ. 913, 169764. doi: 10.1016/j.scitotenv.2023.169764
Portillo M., Ball T., and Manwaring J. (2006). Morphometric analysis of inflorescence phytoliths produced by Avena sativa L. Avena strigos schreb. Economic Bot. 60, 121–129. doi: 10.1663/0013-0001(2006)60[121:MAOIPP]2.0.CO;2
Potì A., Kehl M., Broich M., Marco Y. C., Hutterer R., Jentke T., et al. (2019). Human occupation and environmental change in the western Maghreb during the Last Glacial Maximum (LGM) and the Late Glacial. New evidence from the Iberomaurusian site Ifri El Baroud (northeast Morocco). Quaternary Sci. Rev. 220, 87–110. doi: 10.1016/j.quascirev.2019.07.013
Qader W., Mir S. H., Meister J., Dar R. A., Madella M., and Rashid I. (2023). Sedimentological perspective on phytolith analysis in palaeoecological reconstruction. Earth- Sci. Rev. 244, 104549. doi: 10.1016/j.earscirev.2023.104549
Rackham O. (2018). “Archaeology of Trees, Woodland, and Wood-Pasture,” in Ancient woodlands and trees: a guide for landscape planners and forest managers. Eds. Çolak A. H., Kırca S., and Rotherham I. D. (Ankara: TÜBA), 39–60.
R Core Team. (2021). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Reimer P., Bard E., Bayliss A., Blackwell P. G., Ramsey C. B., Butzin M., et al. (2020). The IntCal20 Northern Hemisphere radiocarbon age calibration curve 0–55 cal kbp. Radiocarbon 62, 725–757. doi: 10.1017/RDC.2020.41
Rencheng L., Fan J., Carter J., Jiang N., and Gu Y. (2017). Monthly variation of phytoliths in the leaves of the bamboo Dendrocalamus ronganensis (Poaceae: Bambusoideae). Rev. Palaeobotany Palynology 246, 62–69. doi: 10.1016/j.revpalbo.2017.06.006
Rosen A. M. (1992). “Preliminary identification of silica skeletons from near eastern archaeological sites: an anatomical approach,” in Phytolith Systematics. Eds. Rapp G. and Mulholland S. C. (New York: Plenum), 129–147.
Rosen A. M. and Weiner S. (1994). Identifying ancient irrigation: a new method using opaline phytoliths from emmer wheat. J. Archaeological Sci. 21, 125–132. doi: 10.1006/jasc.1994.1013
Runge F. (1999). The opal phytolith inventory of soils in Central Africa. Quantities, shapes, classification and spectra. Rev. Palaeobotany Palynology 107, 23–53. doi: 10.1016/S0034-6667(99)00018-4
Sadori L., Masi A., and Ricotta C. (2015). Climate-driven past fires in central Sicily. Plant Biosyst. 149, 166–173. doi: 10.1080/11263504.2014.992996
Sadori L., Zanchetta G., and Giardini M. (2008). Last Glacial to Holocene palaeoenvironmental evolution at Lago di Pergusa (Sicily, Southern Italy) as inferred by pollen, microcharcoal, and stable isotopes. Quaternary Int. 181, 4–14. doi: 10.1016/j.quaint.2007.02.024
Sangster A. G. (1970). Intracellular silica deposition in immature leaves in three species of the Gramineae. Ann. Bot. 34 (1), 245–257.
Schicchi R. and Raimondo M. F. (2011). Schede per il censimento degli alberi monumentali di Sicilia 37-43. Quaderni di Botanica Ambientale Applicata 22, 135–150.
Silantyeva M. M., Solomonova M. Y., Speranskaja N. Y., Blinnikov M., and Blinnikov M. (2018). Phytoliths of temperate forest-steppe: A case study from the Altay, Russia. Rev. Palaeobotany Palynology 250, 1–15. doi: 10.1016/j.revpalbo.2017.12.002
Solomonova M. Y., Blinnikov M. S., Speranskaja N. Y., Elesova N. V., and Silantyeva M. M. (2019). Phytolith assemblages in modern top soils under plant communities of Northern and Western Altay, Russia. Ukrainian J. Ecol. 9, 429–435. doi: 10.15421/2019_122
Strömberg C. A. (2004). Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the great plains of North America during the late Eocene to early Miocene. Palaeogeography Palaeoclimatology Palaeoecol. 207, 239–275. doi: 10.1016/j.palaeo.2003.09.028
Strömberg C. A. E. (2009). Methodological concerns for analysis of phytolith assemblages: Does count size matter? Quaternary Int. 193, 124–140. doi: 10.1016/j.quaint.2007.11.008
Strömberg C. A. E., Dunn R. E., Crifò C., and Harris E. B. (2018). “Phytoliths in paleoecology: analytical considerations, current use, and future directions,” in Methods in Paleoecology. Eds. Croft D. A., Su D. F., and Simpson S. W. (Cham: Springer), 235–287.
Strömberg C. A., Dunn R. E., Madden R. H., Kohn M. J., and Carlini A. A. (2013). Decoupling the spread of grasslands from the evolution of grazer-type herbivores in South America. Nat. Commun. 4, 1–8. doi: 10.1038/ncomms2508
Strömberg C. A. E., Werdelin L., Friis E. M., and Saraç G. (2007). The spread of grass-dominated habitats in Turkey and surrounding areas during the Cenozoic: Phytolith evidence. Palaeogeography Palaeoclimatology Palaeoecol. 250, 18–49. doi: 10.1016/j.palaeo.2007.02.012
Tegerdal Hune J. (2022). Valley of Connections: Networks and Spatialities in the Archaeological Landscape of the Morello Valley in Central Sicily. Department of Archaeology and Ancient History, Uppsala University, Uppsala.
Tencariu F. A., Delhon C., Vornicu D. M., Asăndulesei A., Braşoveanu C., and Danu M. (2022). Science revealing ancient magic: Phytolith evidence from the early Chalcolithic site of Isaiia (Eastern Romania). Biology 11, 1102. doi: 10.3390/biology11081102
Testé M., Garnierc A., Limondin-Lozouet N., Oxlaj E., Castanet C., Purdue L., et al. (2020). The phytoliths of Naachtun (Petén, Guatemala): Development of a modern reference for the characterization of plant communities in the Maya Tropical Lowlands. Rev. Palaeobotany Palynology 272, 104–130. doi: 10.1016/j.revpalbo.2019.104130
Tinner W., Vescovi E., van Leeuwen J. F. N., Colombaroli D., Henne P. D., Kaltenrieder P., et al. (2016). Holocene vegetation and fire history of the mountains of Northern Sicily (Italy). Vegetation History Archaeobotany 25, 499–519. doi: 10.1007/s00334-016-0569-8
Tsartsidou G., Karkanas P., Marshall G., and Kyparissi-Apostolika N. (2015). Palaeoenvironmental reconstruction and flora exploitation at the Palaeolithic cave of Theopetra, central Greece: the evidence from phytolith analysis. Archaeological Anthropological Sci. 7, 169–185. doi: 10.1007/s12520-014-0183-6
Tsartsidou G., Lev-Yadun S., Albert R. M., Miller-Rosen A., Efstratiou N., and Weiner S. (2007). The phytolith archaeological record: strengths and weaknesses evaluated based on a quantitative modern reference collection from Greece. J. Archaeological Sci. 34, 1262–1275. doi: 10.1016/j.jas.2006.10.017
Twiss P. C., Suess E., and Smith R. (1969). Morphological classification of grass phytoliths. Soil Sci. Soc. America J. 33, 109–115. doi: 10.2136/sssaj1969.03615995003300010030x
Vandevenne F., Struyf E., Clymans W., and Meire P. (2012). Agricultural silica harvest: have humans created a new loop in the global silica cycle? Front. Ecol. Environ. 10, 243–248. doi: 10.1890/110046
Verga M. (1993). La Sicilia dei Grani. Gestione dei Feudi e Cultura Economica fra Sei e Settecento (Firenze: Olschki).
Witteveen N. H., White C. S., Sanchez Martinez B. A., Booij R., Philip A., Gosling W. D., et al. (2023). Phytolith assemblages reflect variability in human land use and the modern environment. Veg. Hist. Archaeobot. 33, 221–236.
Zanchetta G., Borghini A., Fallick A. E., Bonadonna F. P., and Leone G. (2007). Late Quaternary paleohydrology of Lake Pergusa (Sicily, southern Italy) as inferred by stable isotopes of lacustrine carbonates. J. Paleolimnology 38, 227–239. doi: 10.1007/s10933-006-9070-1
Keywords: plant morphology, Olea, past analogues, biocultural heritage, soil analysis, historical ecology, land use change
Citation: Ferrara V, Sala G, Garfì G, La Mantia T and Ekblom A (2025) Down with the roots. Phytoliths as biocultural traces in historical olive agroecosystems of Sicily. Front. Ecol. Evol. 13:1625887. doi: 10.3389/fevo.2025.1625887
Received: 30 May 2025; Accepted: 18 August 2025;
Published: 15 September 2025.
Edited by:
Ofir Katz, Dead Sea and Arava Science Center, IsraelReviewed by:
Debora Zurro, Spanish National Research Council (CSIC), SpainMikhail S. Blinnikov, St. Cloud State University, United States
Copyright © 2025 Ferrara, Sala, Garfì, La Mantia and Ekblom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenza Ferrara, dmluY2VuemEuZmVycmFyYUBhcmtlb2xvZ2kudXUuc2U=
 Vincenza Ferrara
Vincenza Ferrara Giovanna Sala
Giovanna Sala Giuseppe Garfì
Giuseppe Garfì Tommaso La Mantia3
Tommaso La Mantia3 Anneli Ekblom
Anneli Ekblom