- Institute of Biology, University of Neuchâtel, Neuchâtel, Switzerland
Introduction: Oxidative homeostasis plays a crucial role in physiology, as reactive oxygen species (ROS) regulate immunity and longevity, but also lead to damaging oxidative stress. ROS are therefore expected to influence host-parasite interactions. Previous studies have shown that supplementing mosquito diets with prooxidants (hydrogen peroxide) or antioxidants (ascorbic acid) disturbs their oxidative balance, particularly in uninfected individuals, which actively avoid these compounds when given a choice.
Methods: Here, we evaluated how such diet-induced shifts in oxidative status influence infection outcomes with the microsporidian parasite Vavraia culicis in the mosquito Anopheles gambiae. Mosquitoes were fed a standard sugar solution or one supplemented with a prooxidant or an antioxidant either early or late in life. We then measured longevity, fecundity, and parasite load 13 days after emergence or at death.
Results: Early prooxidant consumption increased longevity; this benefit was lower in infected mosquitoes. Antioxidant consumption increased fecundity irrespective of infection. Early intake of either supplement increased parasite load in 13-day old mosquitoes, while late intake promoted parasite growth later in life.
Discussion: These findings reveal context-dependent effects of oxidative status on host traits and parasite dynamics, emphasizing the crucial role of timing in shaping oxidative interventions.
Introduction
Nutrition is a crucial factor shaping host-parasite interactions because it delivers energy, micronutrients and other compounds that affect both the host’s and the parasite’s fitness (Calder and Jackson, 2000; Haydon et al., 2003; Cunningham-Rundles et al., 2005; Ponton et al., 2011, 2013). One mechanism is that nutrition modulates constitutive and inducible immune responses (Cunningham-Rundles et al., 2005; Povey et al., 2009; Cotter et al., 2011, 2019; Brunner et al., 2014; Kay et al., 2014; Galenza et al., 2016; Ponton et al., 2020). However, since nutrition influences immunity through various other direct and indirect pathways, including the interactions with the host’s endogenous microbiota (Ponton et al., 2011; Chambers and Schneider, 2012; Simpson and Raubenheimer, 2012), many details of the mechanism are poorly understood (Lazzaro and Little, 2008; Schmid-Hempel, 2021). Yet, a deeper understanding of the relationship between nutrition and immunity is important, for it has implications for morbidity and mortality (Kelley and Bendich, 1996; Samartín and Chandra, 2001; Cunningham-Rundles et al., 2005; Ritz and Gardner, 2006; Sorci and Faivre, 2008).
For many animals a large part of the nutrition is provided by flowering plants. Since these have coevolved with their pollinators and herbivores for millions of years (van der Kooi and Ollerton, 2020), they contain numerous volatile and non-volatile compounds (Leonhardt et al., 2024) in their nectar, pollen, resin and oils (Palmer-Young et al., 2019; Parachnowitsch et al., 2019). These include macronutrients (proteins, fats and carbohydrates), which can shape host–parasite interactions both directly and through their interactions with one another (Cheon et al., 2006; Adamo et al., 2008; Cotter et al., 2011; Brunner et al., 2014; Galenza et al., 2016; Ponton et al., 2020). However, their effects on immune function and host survival are further complicated by many other plant-derived compounds, which may also modulate these interactions (Povey et al., 2009; Kay et al., 2014; Cotter et al., 2019). Micronutrients – chemical substances like vitamins and minerals required in small quantities for normal growth, development, and immune function (Leonhardt et al., 2024) – are one such class of compounds. In addition, several phytochemicals in nectar or pollen have antimicrobial properties, so they protect not only the plants from pathogens (Huang et al., 2012; Junker and Tholl, 2013; Mcart et al., 2014), but also the animals that feed on them (Karban and English-Loeb, 1997; Singer et al., 2009; de Roode et al., 2013). Such properties may be particularly significant for pollinators and nectar-feeders, which are frequently exposed to the secondary metabolites.
As regular nectar-feeders (Ode, 2006) and vectors for parasites with major implications for human health, mosquitoes are particularly interesting for studying effects of secondary metabolites in nectar on host-parasite interactions. Indeed, nectar influences the outcome of mosquito infections by malaria parasites, likely due to a combination of toxic secondary metabolites and the nutritional profile of the nectar (Hien et al., 2016). Many of the compounds in nectar possess oxidative or antioxidative properties, which impact the production of reactive oxygen species (ROS) and the development of oxidative stress (OS) in mosquitoes (Forrester et al., 2018). Both ROS and OS play important roles in shaping mosquito life-history traits, such as aging and reproduction (Harman, 1992; Dowling and Simmons, 2009; Metcalfe and Alonso-Alvarez, 2010) while also regulating their immune responses (Pham-Huy et al., 2008). Redox cycles induced by prooxidants like hydrogen peroxide and by antioxidants like ascorbic acid are a likely defence mechanism against many bacteria and fungi in nectar (Carter and Thornburg, 2004; Schmitt et al., 2021). OS further acts as a physiological mediator linking environmental factors, including diet, to life-history trade-offs by modulating processes such as immune defence, reproduction, and aging (Costantini, 2019). Elevated ROS levels can both signal immune activation and cause cellular damage, requiring mosquitoes to maintain a finely balanced oxidative homeostasis that influences host survival and parasite resistance. Understanding how this balance is achieved may therefore provide valuable insights into the effects of mosquito nutrition on disease transmission. Furthermore, elucidating the roles of macro- and micronutrients in oxidative homeostasis and host-parasite interactions can clarify the behavioural ecology of self-medication in these insects (Clayton and Wolfe, 1993; de Roode et al., 2013; de Roode and Hunter, 2019).
In a recent study, we found that Anopheles gambiae mosquitoes dynamically adjust their dietary preferences in response to an infection by the microsporidian parasite Vavraia culicis (Zeferino et al., 2024). Infected individuals initially prefer diets containing prooxidants but later shift towards antioxidants as the infection progressed. This dynamic strategy suggests a trade-off between resistance and tolerance over time. In contrast, uninfected mosquitoes consistently avoid both prooxidant and antioxidant diets. These dietary choices significantly influence oxidative homeostasis, particularly in uninfected individuals, supporting the hypothesis of self-medication and indicating possible effects on other life-history traits. Building on these findings, we sought with this study to investigate the broader impacts of dietary supplementation with a prooxidant or an antioxidant on mosquitoes’ health and their possible implications for disease transmission. We thus examined the effects of consuming a standard sugar source or one supplemented with a prooxidant or an antioxidant, administered early or late in life (Figure 1), on the longevity, fecundity, and resistance of An. gambiae to infection by V. culicis. Additionally, we assessed whether these supplements impose a cost on uninfected mosquitoes. Based on our previous results, we hypothesised that infected individuals would benefit from oxidative supplements, thereby justifying their selective intake, while uninfected individuals would experience fitness costs when consuming these diets, which may explain their active avoidance. These assumptions provide a framework to evaluate the adaptive value of diet-mediated modulation of oxidative balance in the context of infection.
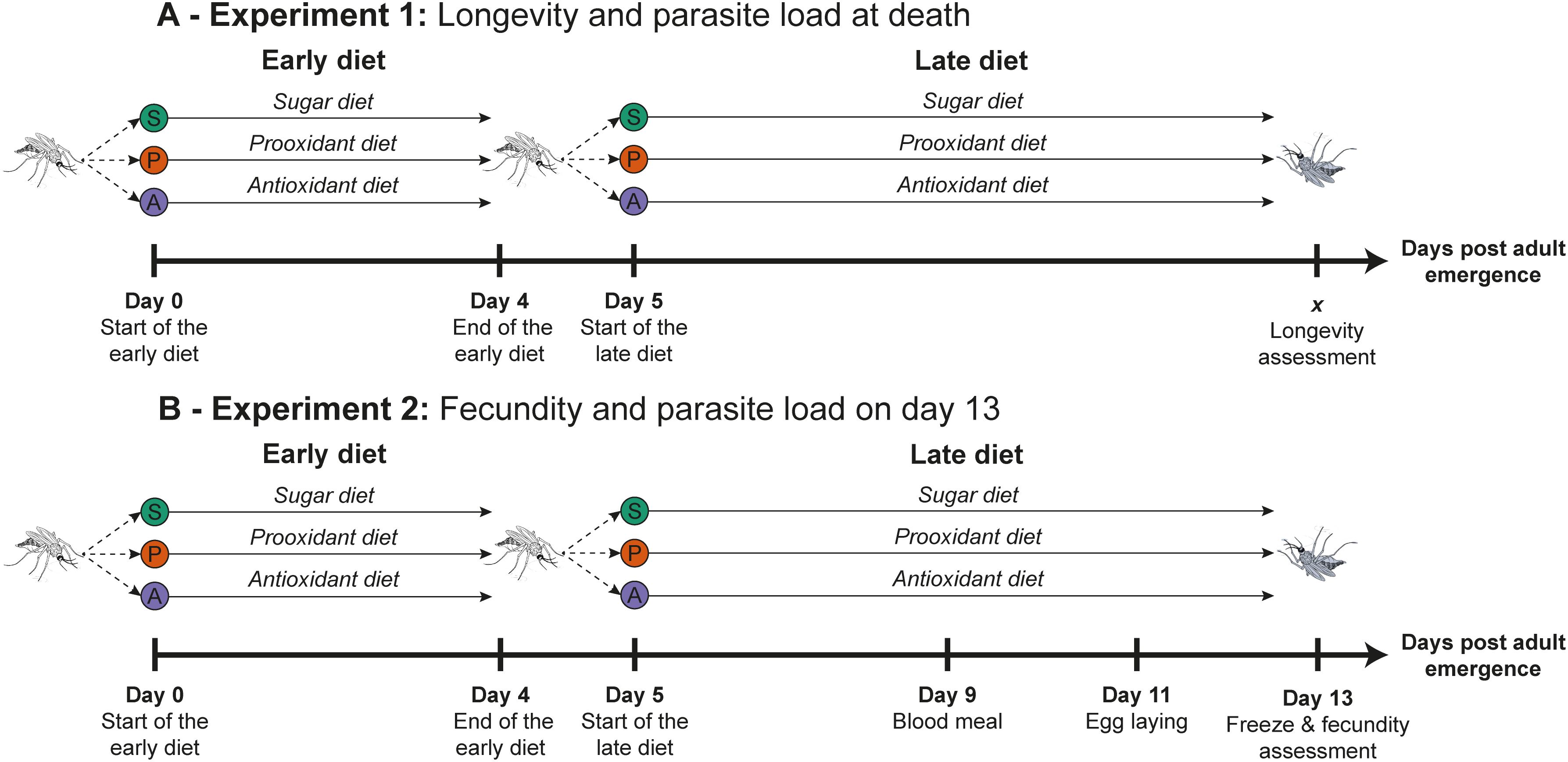
Figure 1. Experimental design. Schematic representation of the dietary treatments and overall setup for the (A) longevity and (B) fecundity experiments. In both experiments, each mosquito was assigned one of three dietary treatments from adult emergence until day 4 (early diet). From day 5 onwards, the same mosquito was switched to one of the three dietary options again (late diet), resulting in a full factorial 3×3 design. (A) In the longevity experiment, we recorded individual lifespan and spore load at death. (B) In the fecundity experiment, females received a blood meal on day 9, were transferred to individual oviposition cups on day 11, and were frozen on day 13 to assess fecundity and spore load.
Materials and methods
Experimental system
We used the Kisumu strain of An. gambiae s.s (Vulule et al., 1994), which had been maintained at our standard laboratory conditions (about 600 individuals per cage, constant access to 6% sucrose solution, 26 ± 1°C, 70 ± 5% relative humidity and 12 h light/dark) for many years before the experiments.
To infect mosquitoes, we used the microsporidian V. culicis floridensis, which had been provided by J.J Becnel (USDA, Gainesville, FL, USA). V. culicis is an obligate intracellular parasite of several mosquito genera, including Anopheles (Kelly et al., 1981; Fukuda et al., 1997; Andreadis, 2007). To maintain its status as a generalist parasite, we alternately infected Aedes aegypti and An. gambiae (Zeferino and Koella, 2024).
Mosquitoes become infected as larvae when they ingest spores. After several rounds of replication, the parasite produces infectious spores that spread to the gut and fat body cells. These spores are released into larval habitats when larvae die, when adults die on the water surface, or when eggs coated with spores are laid onto the water.
Mosquito rearing and maintenance
We conducted two experiments, one for longevity and one for fecundity, so that the two traits do not interfere with each other due to the life-history trade-off in An. gambiae (Dao et al., 2010). The design of the two experiments was identical. Freshly hatched (0 to 3-hour-old) larvae were individually placed into 12-well culture plates, each containing 3 ml of deionised water. The larvae received Tetramin Baby® fish food every day according to their age (0.04, 0.06, 0.08, 0.16, 0.32 and 0.6 mg/larva, respectively, at ages 0, 1, 2, 3, 4 and 5 or older (Kulma et al., 2013)). Two-day-old larvae were exposed to either 0 or 10,000 spores of V. culicis. Pupae were transferred to individual 50 ml falcon tubes with approximately 10 ml of deionised water (Dao et al., 2010). Upon emergence, females were maintained according to the experiment: for the longevity-experiment we kept females alone in 150 ml cups to prevent mating, whereas in the fecundity-experiment we kept mosquitoes in groups of 20 females and 20 males in 1.5 l jars to allow mating (see details below).
Longevity experiment
Adult females were individually moved to 150 ml cups (5.5 cm Ø x 10 cm) covered with a net immediately upon emergence (Figure 1A). The cups contained 50 ml deionised water covered by a petri dish (50 mm Ø) to prevent the mosquitoes from drowning. A 10 x 7 cm filter paper partly submerged in the water maintained humidity in the cup. The mosquitoes were provided with tightly compressed cotton balls (approx. 4 cm diameter) that were soaked with about 5–8 ml of the diet they were allocated to and placed onto the net of the cup. The cotton balls were replaced daily. The cups were surveyed daily, and dead individuals were collected and frozen at -20°C for later analysis.
Fecundity experiment
Females were moved to 1.5 l jars (15 cm Ø x 20 cm) in groups of 20 according to their treatment status immediately upon emergence. To enable mating, we also added 20 males. These were from our colony and were thus not infected, so that fecundity was not influenced by the infection of their mates. The mosquitoes were fed daily with cotton balls soaked in their respective diet. Nine days after emergence females were given the opportunity to blood-feed on TGZ’s arm for five minutes (Figure 1B). One day later females that were not fully engorged were excluded from the experiment. The next day the remaining females were transferred to individual cups containing 50 ml deionised water covered by a petri dish (50 mm Ø) to prevent the mosquitoes from drowning. The cup also contained a 94 mm Ø filter paper folded into the shape of a cone for egg laying. Four days after blood-feeding (so 13 days after emergence), the females were frozen at -20°C for later analysis and a picture was taken of each filter paper so that we could count the eggs.
Dietary treatments
We gave the mosquitoes one diet (referred to as early diet) from day 0 (the day of emergence) to day 4, and another diet (referred to as late diet) from day 5 up to the mosquitoes’ death (Figure 1). This categorization was based on our previous work (Zeferino et al., 2024) where we showed that infected mosquitoes exhibited a preference for different diets in early and late stages of life. At each of these stages, mosquitoes received either 10% sucrose (referred to as sugar diet), 10% sucrose supplemented with 8mM of hydrogen peroxide (prooxidant diet) or 10% sucrose supplemented with 1mg/mL of ascorbic acid (vitamin c; antioxidant diet). The diets were freshly prepared on the day they were given to the mosquitoes. The concentrations of hydrogen peroxide and ascorbic acid correspond to concentrations found in nectar (Herbert and Shimanuki, 1978; Herbert et al., 1985; Santos et al., 2017; Bartlett et al., 2022), and they had been tested before the experiment to ensure they did not kill the mosquitoes.
Measurements
The number of eggs was measured from the photos with the software ImageJ v1.54 (Abràmoff et al., 2004). The right wing of every mosquito was removed and its length was measured from the axillary incision to the wing’s tip (Koella and Lyimo, 1996; Petersen et al., 2016) with ImageJ. The remainder of the mosquito was put into a 2 ml Eppendorf tube containing 0.1 ml of deionised water. A stainless-steel bead (Ø 5 mm) was added to each tube and the samples were homogenised with a Qiagen TissueLyser LT at a frequency of 30 Hz for two minutes. The number of spores in each sample was counted from 0.1 µl of the sample with a haemocytometer under a phase-contrast microscope (400x magnification).
Statistical analysis
All analyses were conducted with R (Team, 2017) version 4.4.2, using the packages glmmTMB (Brooks et al., 2017) for model fitting, DHARMa (Hartig, 2022) for model diagnostics, car (Fox and Weisberg, 2020) for ANOVA, and emmeans (Lenth, 2023) and multcomp (Hothorn et al., 2008) for post hoc comparisons. We used generalized linear mixed-effects models (GLMMs) implemented in the glmmTMB package, selecting the distribution that best suited the biological nature of the data and provided the best model fit. Model assumptions, including overdispersion and residual structure, were checked using simulated residual diagnostics from the DHARMa package. Significance was assessed using the Anova function from the car package (Fox and Weisberg, 2020), with Type III ANOVA used in the presence of significant interactions, and Type II otherwise. Post-hoc multiple comparisons were conducted using emmeans with Tukey adjustment for multiple testing.
Age at death was analysed with a linear model with a Gaussian distribution of errors, where the response variable was age at death and the explanatory variables were infection status, early diet, late diet, and their interactions. Wing length was included as a covariate.
The proportion of individuals laying eggs was analysed with a generalised linear model with a binomial distribution of errors, where the response variable was the proportion of individuals that laid eggs and the explanatory variables were the infection status, early diet, late diet, and their interactions. Wing length was included as a covariate.
The number of eggs was analysed with a linear model with a Gaussian distribution of errors, where the response variable was egg count and the explanatory variables were infection status, early diet, late diet, and their interactions. Wing length was included as a covariate. Individuals that did not lay eggs were excluded from this analysis.
The infection dynamics of V. culicis in An. gambiae have been previously characterized (e.g., Zeferino and Koella, 2024). At the time of adult emergence (day 0), spores are not yet detectable. About 4 days after emergence spores become detectable in all exposed individuals, and parasite load increases until around day 8. Parasite growth then slows and typically plateaus after day 12. Because we assessed spore load on day 13 – during this plateau phase – we expected, and indeed found, that all exposed mosquitoes had detectable levels of infection. Spore load was measured using a haemocytometer with 0.1 µl of sample (i.e., 1/1000 of the total homogenate volume), resulting in a detection threshold of approximately 1000 spores. Counts were analysed using a generalized linear model with a negative binomial distribution to account for overdispersion (Venables and Ripley, 2002), where the response variable was spore load and the explanatory variables were early diet, late diet, their interaction, and wing length as a covariate. Since the number of spores increases throughout the mosquitoes’ life, we analysed the spore load at death (from the longevity experiment) using a generalised linear model with a negative binomial distribution of errors, where the response variable was spore load at death and the explanatory variables were age at death, early diet, late diet, their interaction, and wing length as a covariate.
Results
Longevity
In the longevity experiment, mosquitoes were housed individually and received their assigned dietary treatment daily until death (see Figure 1A). Infection by V. culicis shortened the lifespan from an average of 29.4 days to 24.8 days (χ2 = 83.11, df = 1, p < 0.001) and feeding on sugar supplemented with the prooxidant increased the lifespan, whether the consumption was early (prooxidant: 29.9 days; antioxidant: 26.2 days; sugar: 25.1 days; χ2 = 65.75, df = 2, p < 0.001) or late (prooxidant: 28.1 days; antioxidant: 26.9 days; sugar: 26.2 days; χ2 = 8.94, df = 2, p = 0.011, Figure 2). However, the impact of late diet was small, and its impact was not seen within groups defined by early diet and infection status (multiple comparisons of Figure 2). The effects of the early and the late diet were independent of each other (early diet * late diet: χ2 = 2.69, df = 4, p = 0.611). However, the early diet had a stronger impact on uninfected individuals than infected ones, with both supplemented diets increasing longevity (prooxidants: 32.8 vs 26.1 days; antioxidants: 29.2 vs 24.4 days; sugar 26.3 vs. 23.6 days; interaction infection status * early diet: χ2 = 10.11, df = 2, p = 0.006, Figures 2A–C), while the impacts of the late diet (infection status * late diet: χ2 = 0.96, df = 2, p = 0.617) and of the combination of the two diets (infection status * early diet * late diet: χ2 = 1.62, df = 4, p = 0.805) on infected and uninfected individuals were similar.
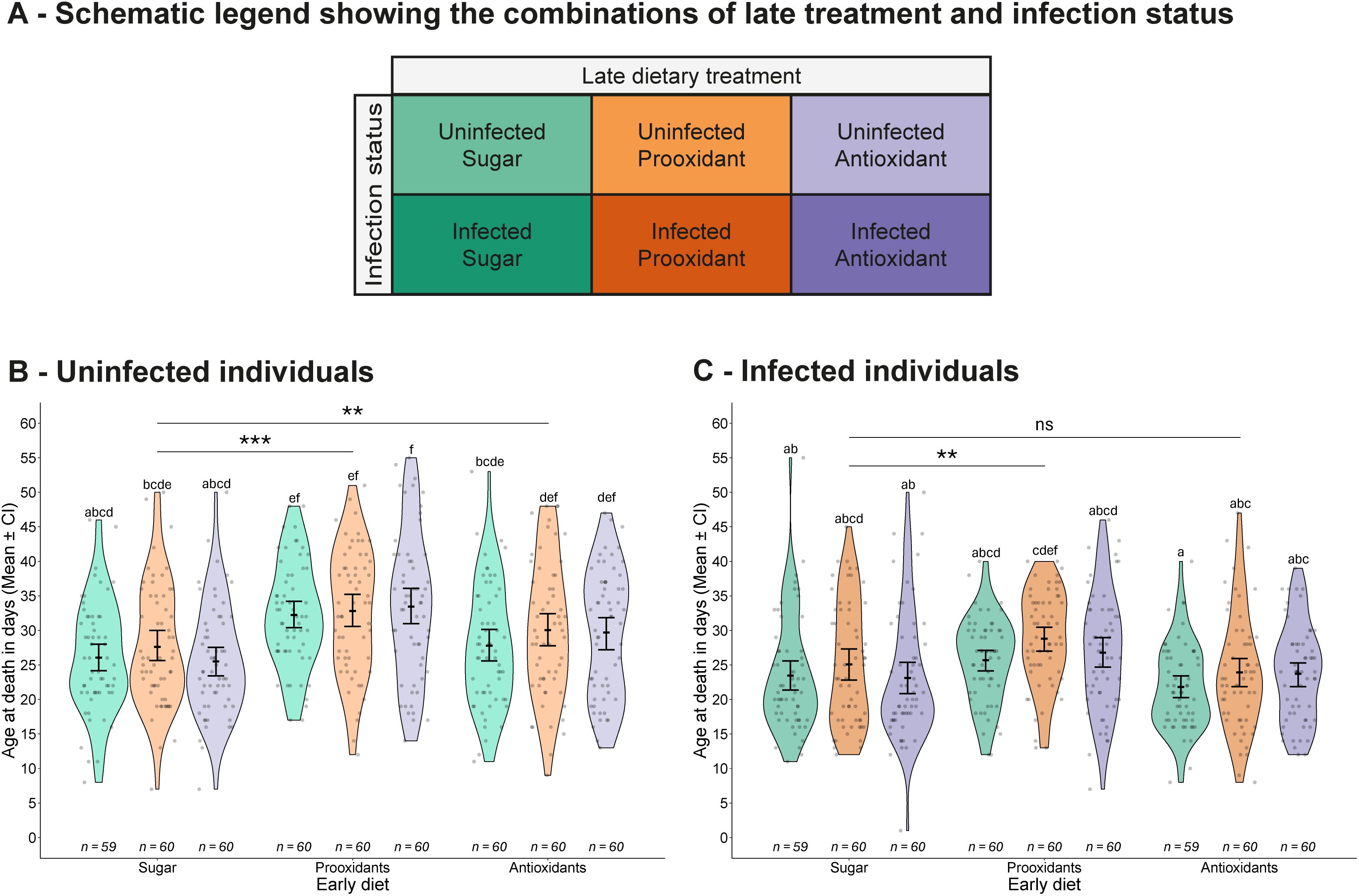
Figure 2. Longevity. (A) Schematic legend showing the combinations of late treatment and infection status. (B, C) Age at death of (B) uninfected and (C) infected mosquitoes, shown according to the combination of early and late dietary treatments. Each panel displays individual data points, means values with 95% confidence intervals, and violin plots representing the distribution within each treatment group. Sample sizes are shown below each treatment. Letters indicate statistically significant differences between groups based on Tukey-adjusted multiple comparisons. Asterisks highlight significant main effects of early diet compared to the early sugar diet: ns = not significant (p > 0.1), · = marginally non-significant (0.1 ≥ p > 0.05), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. The x-axis shows early dietary treatments, while late treatments are colour-coded and explained in the legend.
Egg-laying likelihood
In the fecundity experiment, mosquitoes were transferred to individual egg-laying cups and allowed to oviposit over a two-day period. They continued to receive their assigned dietary treatment daily until they were frozen on day 13 (see Figure 1B). 59.5% of the mosquitoes laid at least one egg, independently of the their infection status (57.8% vs 61.0%, χ2 = 1.12, df = 1, p = 0.290), early diet (62.3% vs 56.9% vs 59.0%, χ2 = 2.13, df = 2, p = 0.345), late diet (61.2% vs 60.1% vs 57.0%, χ2 = 1.25, df = 2, p = 0.536) or any of their interactions (infection status * early diet: χ2 = 3.95, df = 2, p = 0.139; infection status * late diet: χ2 = 1.76, df = 2, p = 0.414; early diet * late diet: χ2 = 5.91, df = 4, p = 0.206; infection status * early diet * late diet: χ2 = 2.64, df = 4, p = 0.619; Figures 3A–C).
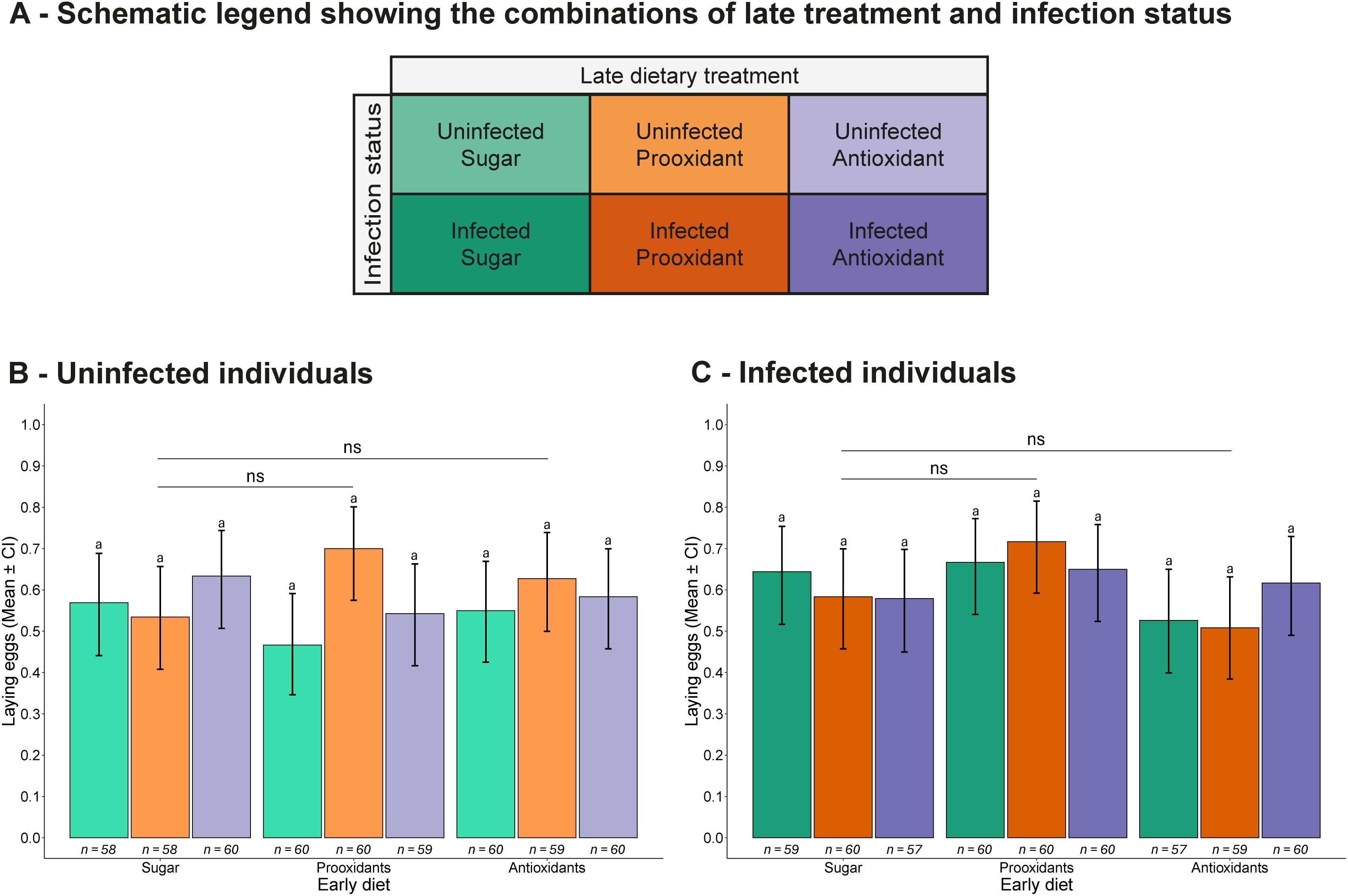
Figure 3. Egg-laying likelihood. (A) Schematic legend showing the combinations of late treatment and infection status. (B, C) Proportion of (B) uninfected and (C) infected individuals that laid eggs, shown according to the combination of early and late dietary treatments. Bars represent the mean proportion of egg-laying mosquitoes, with error bars showing the 95% confidence intervals. Sample sizes are displayed below each bar. Letters denote statistically significant differences between groups based on Tukey-adjusted multiple comparisons. Asterisks highlight significant main effects of early diet compared to the early sugar diet: ns = not significant (p > 0.1), · = marginally non-significant (0.1 ≥ p > 0.05), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. The x-axis shows early dietary treatments, while late treatments are colour-coded and explained in the legend.
Fecundity output
In contrast, if the mosquitoes did lay eggs, infection by V. culicis reduced the number of eggs from an average of 87.5 eggs to 81.7 eggs (χ2 = 4.06, df = 1, p = 0.044) and consuming sugar supplemented with either a prooxidant or, in particular, an antioxidant increased the number of eggs, whether the supplement was given early (prooxidant: 85.9 eggs; antioxidant: 95.3 eggs; sugar: 73.6 eggs; χ2 = 23.91, df = 2, p < 0.001) or late (prooxidant: 89.4 eggs; antioxidant: 87.8 eggs; sugar: 76.9 eggs; χ2 = 13.06, df = 2, p = 0.001). These effects were independent of any combination of the treatments (infection status * early diet: χ2 = 3.66, df = 2, p = 0.160; infection status * late diet: χ2 = 2.55, df = 2, p = 0.280; early diet * late diet: χ2 = 4.42, df = 4, p = 0.352; infection status * early diet * late diet: χ2 = 5.62, df = 4, p = 0.229; Figures 4A–C).
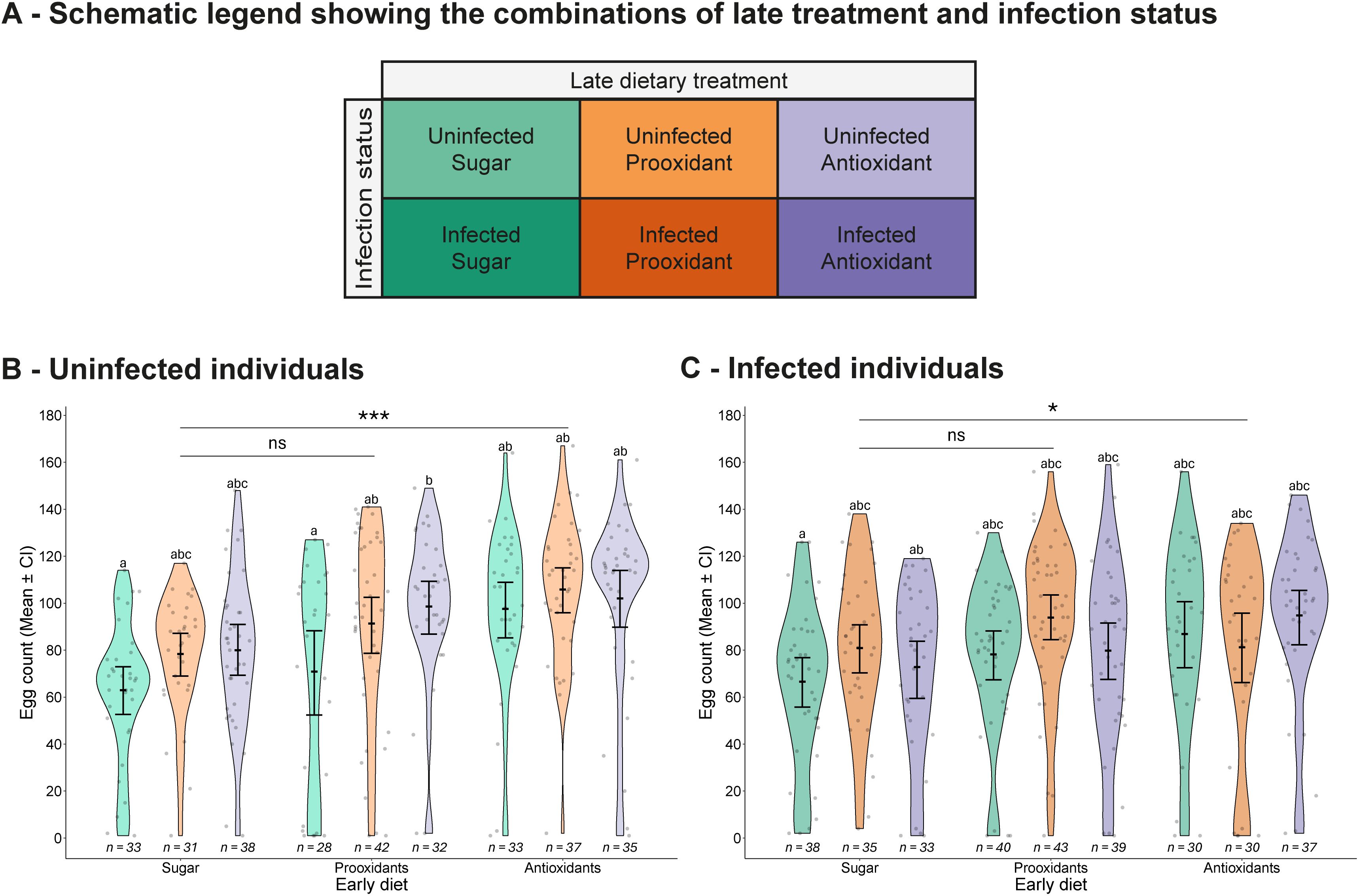
Figure 4. Fecundity output. (A) Schematic legend showing the combinations of late treatment and infection status. (B, C) Number of eggs laid by (B) uninfected and (C) infected individuals, shown according to the combination of early and late dietary treatments. Each panel displays individual data points, means values with 95% confidence intervals, and violin plots representing the distribution within each treatment group. Sample sizes are shown below each treatment. Letters indicate statistically significant differences between groups based on Tukey-adjusted multiple comparisons. Asterisks highlight significant main effects of early diet compared to the early sugar diet: ns, not significant (p > 0.1), · = marginally non-significant (0.1 ≥ p > 0.05), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. The x-axis shows early dietary treatments, while late treatments are colour-coded and explained in the legend.
Infection outcome
In both experiments, mosquitoes were collected on day 13 or at death for spore load assessment (see Figures 1A, B). The mosquitoes that were assayed 13 days after emergence harboured, on average, about 1.5x105 spores (Figures 5A, B), and early consumption of sugar supplemented with either a prooxidant or an antioxidant increased the spore load (prooxidant: 1.7x105 spores; antioxidant: 1.6x105 spores; sugar: 1.1x105 spores; χ2 = 8.35, df = 2, p = 0.015). However, although spore load was not influenced by the late diet (prooxidant: 1.4x105 spores; antioxidant: 1.6x105 spores; sugar: 1.3x105 spores; χ2 = 2.31, df = 2, p = 0.315), early consumption of prooxidants followed by late consumption of antioxidants led to the higher parasite load (early diet * late diet: χ2 = 12.15, df = 4, p = 0.016).
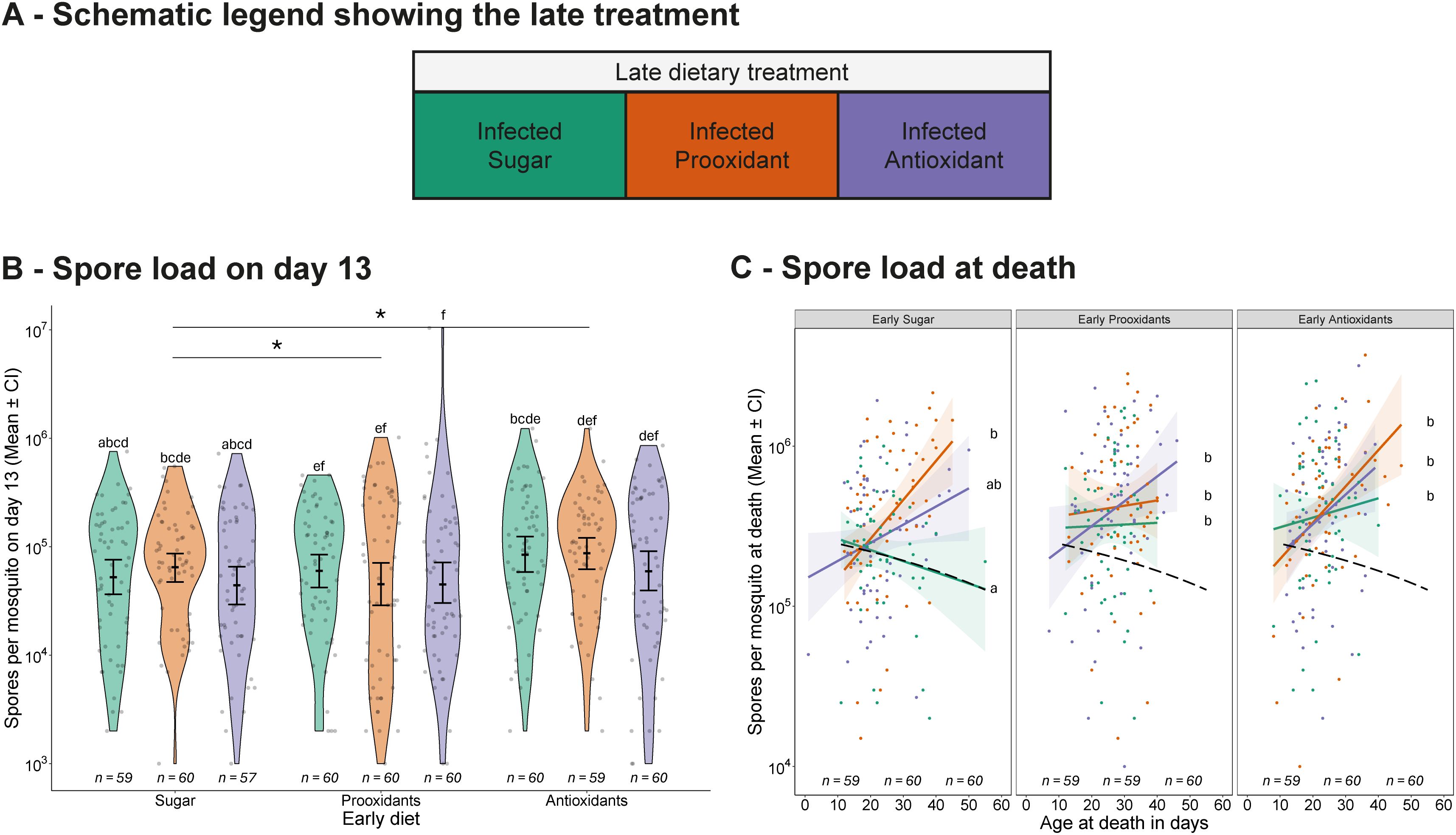
Figure 5. Infection outcome. (A) Schematic legend showing the late treatment. (B, C) Spore load measured (B) on day 13 and (C) at death, shown according to the combination of early and late dietary treatments. (B) displays individual data points, means values with 95% confidence intervals, and violin plots representing the distribution within each treatment group. (C) displays individual spore load values at death (log10 scale) plotted against age at death, with smoothed regression lines fitted for each dietary treatment using the “lm” method in ggplot2. Shaded regions represent 95% confidence intervals around the regression lines. A dashed black line highlights the sugar-only treatment group (early and late sugar diet). Sample sizes are shown below each treatment. Letters indicate statistically significant differences between groups based on Tukey-adjusted multiple comparisons. Asterisks highlight significant main effects of early diet compared to the early sugar diet: ns = not significant (p > 0.1), · = marginally non-significant (0.1 ≥ p > 0.05), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. The x-axis shows early dietary treatments, while late treatments are colour-coded and explained in the legend.
The spore load at death increased with age at death (χ2 = 25.40, df = 1, p < 0.001) and was higher in mosquitoes consuming either supplemented diet provided early (χ2 = 25.30, df = 2, p < 0.001, Figure 5C), with an average spore load of 5.9x105 per day in mosquitoes feeding on the prooxidant and antioxidant diet, and 4.1x105 spores on the sugar diet. Notably, while the late diet had a modest effect on spore load (χ2 = 9.10, df = 2, p = 0.010), it also changed the relationship between spore load and age, with supplemented diets increasing spore load with age at death (age * late diet: χ2 = 9.36, df = 2, p = 0.009, Figure 5C).
Discussion
Supplementing the sugar meals of mosquitoes with prooxidants or antioxidants had significant effects on their longevity, fecundity and parasite growth, likely increasing their reproductive success. These effects depended on the timing of the supplementation, with early-life diets having a particularly strong influence. However, some of the observed outcomes – especially those related to longevity and fecundity – deviated from our initial expectations, particularly in uninfected individuals. Rather than experiencing strong costs from consuming these supplemented diets, uninfected mosquitoes sometimes showed neutral or even unexpectedly beneficial responses. In the following sections, we explore possible explanations for these patterns, discuss how they relate to oxidative homeostasis and previously observed dietary preferences (Zeferino et al., 2024), and reflect on their broader ecological and epidemiological implications.
Consuming a prooxidant or an antioxidant together with sugar, particularly early in life, significantly extended both the longevity (Figure 2) and the fecundity (Figure 4) of uninfected mosquitoes. These results were unexpected, as uninfected individuals actively avoided these diets when given a choice (Zeferino and Koella, 2024). However, the beneficial effect of antioxidants aligns with findings from other studies. For instance, antioxidants enhance longevity in mealworm beetles by suppressing immune activity and reducing immunopathology (Dhinaut et al., 2017). Similarly, feeding crickets with vitamin C reduces several aspects of oxidative damage, including damaged DNA in haemocytes (Flasz et al., 2021). In contrast, the positive effect of prooxidants observed in our study differs from previous findings in An. arabiensis, where hydrogen peroxide reduced lifespan. This discrepancy may be explained by the low, naturally occurring concentration of hydrogen peroxide used in our experiment. At such levels, dietary ROS may have hormetic effects – where mild oxidative stress induces beneficial cellular responses – enhancing physiological function and extending lifespan (Ristow, 2014; Berry and López-Martínez, 2020). These effects may involve the upregulation of cellular defence mechanisms, enhanced viral resistance (Pan et al., 2012) or modulation of insulin-signalling pathway to extend longevity (Zhang et al., 2017).
The effects of dietary supplementation were also observed in infected mosquitoes, though to a lesser extent. Two main explanations may account for this difference. First, parasites may reduce the beneficial impacts of the supplements. For antioxidants, this could occur because parasitic infections stimulate the immune system, leading to oxidative damage. Although antioxidant intake typically reduces oxidative stress and limits cellular damage (Sternberg et al., 2012; de Roode et al., 2013), infection-induced immune activation might counteract these benefits. For prooxidants, the combined ROS increase from the diet and parasite-induced immune stimulation may push OS beyond manageable levels, disrupting oxidative homeostasis and leading to oxidative damage. Second, these dietary supplements may reduce the pathological effects of infection on longevity and fecundity, although increasing parasite load (Figure 5). This increase, observed both at day 13 and at death, suggests that the diets help hosts to better cope with the physiological costs of infection, enabling them to live despite a more efficiently parasite proliferation. This aligns with a form of tolerance where the host is not reducing the parasite’s growth (as in resistance) but is instead managing the infection’s impact on its own survival (longevity-tolerance (Strauss and Agrawal, 1999; Stowe et al., 2000; Jackson et al., 2014; Kutzer and Armitage, 2016)), fecundity (fecundity-tolerance (Corby-Harris et al., 2007; Råberg et al., 2007, 2008; Howick and Lazzaro, 2014; Kutzer and Armitage, 2016)) and potentially other life-history traits. These results also highlight a potential dual effect of these diets: they increased resistance early in life (i.e., reduced parasite load on day 8 (Zeferino et al., 2024)) while promoting tolerance later in life (i.e., increased parasite load by day 13 without apparent costs).
Although both diets disrupt the mosquito’s oxidative homeostasis (Zeferino et al., 2024), their effects diverged. Prooxidants may boost immune function, perhaps by raising ROS levels, which helps to combat infections (Costantini et al., 2009; Schneeberger et al., 2013), and increases longevity (Figure 2). In contrast, antioxidants appeared to suppress immune responses by decreasing ROS (Godfrey, 1957; Krungkrai and Yuthavong, 1987; Marva et al., 1989; Awodele et al., 2007; Isah and Ibrahim, 2014). While this boosted fecundity (Figure 4), it had no effect on longevity and appeared to compromise the ability to contain infections, highlighting the immuno-suppressant effects of antioxidants diets. While these dietary items did not clear the infection, as is often the case in the wild (Baracchi et al., 2015; Richardson et al., 2015), they significantly improved the overall fitness of the host, as reflected in increased longevity and fecundity (Clayton and Wolfe, 1993; Singer and Bernays, 2009; Singer et al., 2009; Abbott, 2014). Notably, the timing of supplementation played a critical role, with early diet interventions showing stronger effects than late supplementation. This temporal difference aligns with the infection dynamics of V. culicis, which undergoes exponential growth around day four before slowing and reaching a plateau after day twelve (Zeferino and Koella, 2024).
Our findings suggest that our dietary treatments are most effective when administered before day five, during the early, rapid replication phase of the parasite. Prooxidant intake during this period likely increases oxidative stress, which, while causing some cellular damage (Råberg et al., 2008; Graham et al., 2011; Schmid-Hempel, 2021), may also inhibit V. culicis replication, thus extending host longevity (Sternberg et al., 2012; Howick and Lazzaro, 2014). In contrast, antioxidant intake reduces oxidative stress, mitigating cellular damage but potentially facilitating V. culicis replication. This could explain the observed trade-off between increased fecundity and shorter lifespan in mosquitoes consuming antioxidants (Sternberg et al., 2012; de Roode et al., 2013). These results emphasize the importance of the interaction between dietary timing and the physiological dynamics of both the host and the parasite. Our findings also underscore the complex interplay between prooxidants and antioxidants in mosquito physiology, challenging the traditional notion of oxidative stress as solely damaging. Instead, oxidative stress appears to play a dual role, where its benefits and costs are carefully balanced by the mosquitoes, influencing their survival and reproduction. This nuanced perspective highlights the adaptive strategies mosquitoes employ to regulate oxidative stress in response to dietary factors, emphasizing the importance of oxidative balance in their biology.
Understanding this complexity provides valuable insights into mosquito ecology and physiology, offering potential avenues for innovative vector control strategies. However, our results also raise important considerations for the use of V. culicis as a biological control agent. Infected mosquitoes showed an ability to mitigate the fitness costs of parasitism, potentially reducing the effectiveness of microsporidians. These findings emphasise the need to account for host-parasite dynamics and dietary influences when designing biological control programs.
A key limitation of this study is that we did not directly measure oxidative stress or immune parameters alongside the life-history traits we examined. Such measurements would have strengthened our interpretation of how dietary treatments modulate mosquito physiology, particularly in relation to switching diets mid-life. Capturing oxidative stress at multiple time points—rather than a single snapshot—would have allowed us to trace the temporal dynamics of oxidative balance and better understand the progression of physiological responses. Including additional oxidative damage markers or broader immune indicators would also have provided a more complete mechanistic picture, but these were beyond the logistical scope of this study. Finally, examining the potential transgenerational costs of these diets, especially in uninfected individuals, could reveal important hidden trade-offs. We believe these are promising directions for future research to deepen our understanding of the interplay between diet, immunity, and infection in mosquitoes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
TZ: Project administration, Formal analysis, Writing – review & editing, Methodology, Conceptualization, Data curation, Writing – original draft, Investigation. AM: Conceptualization, Writing – review & editing, Methodology. JK: Funding acquisition, Writing – review & editing, Conceptualization, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. TGZ, ARM and the project were supported by SNF grant 310030_192786.
Acknowledgments
We thank Luís M. Silva for his advice and technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1628611/full#supplementary-material
References
Abbott J. (2014). Self-medication in insects: Current evidence and future perspectives. Ecol. Entomol 39, 273–280. doi: 10.1111/een.12110
Abràmoff M. D., Magalhães P. J., and Ram S. J. (2004). Image processing with imageJ. Biophotonics Int. 11, 36–42.
Adamo S. A., Roberts J. L., Easy R. H., and Ross N. W. (2008). Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J. Exp. Biol. 211, 531–538. doi: 10.1242/JEB.013136
Andreadis T. G. (2007). Microsporidian parasites of mosquitoes. J. Am. Mosq. Control Assoc. 23, 3–29. doi: 10.2987/8756-971X(2007)23[3:MPOM]2.0.CO;2
Awodele O., Emeka P. M., Akintonwa A., and Aina O. O. (2007). Antagonistic effect of vitamin E on the efficacy of artesunate against Plasmodium berghei infection in mice. Afr. J. Biomed. Res. 10, 51–57. doi: 10.4314/ajbr.v10i1.48971
Baracchi D., Brown M. J. F., and Chittka L. (2015). Behavioural evidence for self-medication in bumblebees? F1000Res 4, 1–20. doi: 10.12688/F1000RESEARCH.6262.3
Bartlett L. J., Martinez-Mejia C., and Delaplane K. S. (2022). Honey bees (Apis mellifera Hymenoptera: Apidae) preferentially avoid sugar solutions supplemented with field-relevant concentrations of hydrogen peroxide despite high tolerance limits. J. Insect Sci. 22, 1–6. doi: 10.1093/jisesa/ieab102
Berry R. and López-Martínez G. (2020). A dose of experimental hormesis: When mild stress protects and improves animal performance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 242, 110658. doi: 10.1016/J.CBPA.2020.110658
Brooks M. E., Kristensen K., van Benthem K. J., Magnusson A., Berg C. W., Nielsen A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9. doi: 10.32614/rj-2017-066
Brunner F. S., Schmid-Hempel P., and Barribeau S. M. (2014). Protein-poor diet reduces host-specific immune gene expression in Bombus terrestris. Proc. R. Soc. B: Biol. Sci. 281, 1–11. doi: 10.1098/RSPB.2014.0128
Calder P. C. and Jackson A. A. (2000). Undernutrition, infection and immune function. Nutr. Res. Rev. 13, 3–29. doi: 10.1079/095442200108728981
Carter C. and Thornburg R. W. (2004). Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci. 9, 320–324. doi: 10.1016/j.tplants.2004.05.008
Chambers M. C. and Schneider D. S. (2012). Pioneering immunology: insect style. Curr. Opin. Immunol. 24, 10–14. doi: 10.1016/J.COI.2011.11.003
Cheon H. M., Sang W. S., Bian G., Park J. H., and Raikhel A. S. (2006). Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aEgypti. J. Biol. Chem. 281, 8426–8435. doi: 10.1074/JBC.M510957200/ASSET/21B18318-D046-4B08-BEF8-AC5CE6637E03/MAIN.ASSETS/GR7.JPG
Clayton D. H. and Wolfe N. D. (1993). The adaptive significance of self-medication. Trends Ecol. Evol. 8, 60–63. doi: 10.1016/0169-5347(93)90160-Q
Corby-Harris V., Habel K. E., Ali F. G., and Promislow D. E. L. (2007). Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J. Evol. Biol. 20, 526–533. doi: 10.1111/J.1420-9101.2006.01267.X
Costantini D. (2019). Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 222. doi: 10.1242/JEB.194688/2696
Costantini C., Ayala D., Guelbeogo W. M., Pombi M., Some C. Y., Bassole I. H. N., et al. (2009). Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles Gambiae. BMC Ecol. 9, 1–27. doi: 10.1186/1472-6785-9-16
Cotter S. C., Reavey C. E., Tummala Y., Randall J. L., Holdbrook R., Ponton F., et al. (2019). Diet modulates the relationship between immune gene expression and functional immune responses. Insect Biochem. Mol. Biol. 109, 128–141. doi: 10.1016/J.IBMB.2019.04.009
Cotter S. C., Simpson S. J., Raubenheimer D., and Wilson K. (2011). Macronutrient balance mediates trade-offs between immune function and life history traits. Funct. Ecol. 25, 186–198. doi: 10.1111/J.1365-2435.2010.01766.X
Cunningham-Rundles S., McNeeley D. F., and Moon A. (2005). Mechanisms of nutrient modulation of the immune response. J. Allergy Clin. Immunol. 115, 1119–1128. doi: 10.1016/J.JACI.2005.04.036
Dao A., Kassogue Y., Adamou A., Diallo M., Yaro A. S., Traore S. F., et al. (2010). Reproduction-longevity trade-off in Anopheles Gambiae (Diptera: Culicidae). J. Med. Entomol 47, 769–777. doi: 10.1093/jmedent/47.5.769
de Roode J. C. and Hunter M. D. (2019). Self-medication in insects: when altered behaviors of infected insects are a defense instead of a parasite manipulation. Curr. Opin. Insect Sci. 33, 1–6. doi: 10.1016/j.cois.2018.12.001
de Roode J. C., Lefèvre T., and Hunter M. D. (2013). Self-medication in animals. Sci. (1979) 340, 150–151. doi: 10.1126/science.1235824
Dhinaut J., Balourdet A., Teixeira M., Chogne M., and Moret Y. (2017). A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-12769-7
Dowling D. K. and Simmons L. W. (2009). Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B: Biol. Sci. 276, 1737–1745. doi: 10.1098/rspb.2008.1791
Flasz B., Dziewięcka M., Kędziorski A., Tarnawska M., Augustyniak J., and Augustyniak M. (2021). Multigenerational selection towards longevity changes the protective role of vitamin C against graphene oxide-induced oxidative stress in house crickets. Environ. pollut. 290, 117996. doi: 10.1016/J.ENVPOL.2021.117996
Forrester S. J., Kikuchi D. S., Hernandes M. S., Xu Q., and Griendling K. K. (2018). Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122, 877–902. doi: 10.1161/CIRCRESAHA.117.311401
Fukuda T., Willis O. R., and Barnard D. R. (1997). Parasites of the Asian tiger mosquito and other container-inhabiting mosquitoes (Diptera: Culicidae) in northcentral Florida. J. Med. Entomol 34, 226–233. doi: 10.1093/jmedent/34.2.226
Galenza A., Hutchinson J., Campbell S. D., Hazes B., and Foley E. (2016). Glucose modulates Drosophila longevity and immunity independent of the microbiota. Biol. Open 5, 165–173. doi: 10.1242/BIO.015016
Godfrey D. G. (1957). Antiparasitio Action of Dietary God Liver Oil upon Plasmodium berghei and its Reversal by Vitamin” E. Exp. Parasitol. 6, 555–565. doi: 10.1016/0014-4894(57)90038-3
Graham A. L., Shuker D. M., Pollitt L. C., Auld S. K. J. R., Wilson A. J., and Little T. J. (2011). Fitness consequences of immune responses: strengthening the empirical framework for ecoimmunology. Funct. Ecol. 25, 5–17. doi: 10.1111/j.1365-2435.2010.01777.x
Harman D. (1992). Role of free radicals in aging and disease. Ann. N Y Acad. Sci. 673, 126–141. doi: 10.1111/j.1749-6632.1992.tb27444.x
Hartig F. (2022).DHARMa: residual diagnostics for hierarchical (Multi-level/mixed) regression models. Available online at: https://CRAN.R-project.org/package=DHARMa (Accessed June 10, 2023).
Haydon D. T., Matthews L., Timms R., and Colegrave N. (2003). Topndash;down or bottom–up regulation of intra–host blood–stage malaria: do malaria parasites most resemble the dynamics of prey or predator? Proc. R Soc. Lond B Biol. Sci. 270, 289–298. doi: 10.1098/RSPB.2002.2203
Herbert E. W. and Shimanuki J. H. (1978). “Chemical composition and nutritive value of bee collected and bee-stored pollen,” in Apidologie (Italy: Springer Science+Business Media), 9 (1), 33–40.
Herbert E. W., Vanderslice J. T., and Higgs D. J. (1985). Vitamin C enhancement of brood rearing by caged honeybees fed a chemically defined diet. Arch. Insect Biochem. Physiol. 2 (1), 29–37. doi: 10.1002/arch.940020104
Hien D. F., d. S., Dabiré K. R., Roche B., Diabaté A., Yerbanga R. S., et al. (2016). Plant-mediated effects on mosquito capacity to transmit human malaria. PloS Pathog. 12. doi: 10.1371/journal.ppat.1005773
Hothorn T., Bretz F., and Westfall P. (2008). Simultaneous inference in general parametric models. Biom J. 50, 346–363. doi: 10.1002/bimj.200810425
Howick V. M. and Lazzaro B. P. (2014). Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol. Biol. 14, 1–13. doi: 10.1186/1471-2148-14-56/FIGURES/5
Huang M., Sanchez-Moreiras A. M., Abel C., Sohrabi R., Lee S., Gershenzon J., et al. (2012). The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 193, 997–1008. doi: 10.1111/J.1469-8137.2011.04001.X
Isah M. B. and Ibrahim M. A. (2014). The role of antioxidants treatment on the pathogenesis of malarial infections: a review. Parasitol. Res. 113, 801–809. doi: 10.1007/s00436-014-3804-1
Jackson J. A., Hall A. J., Friberg I. M., Ralli C., Lowe A., Zawadzka M., et al. (2014). An immunological marker of tolerance to infection in wild rodents. PloS Biol. 12, e1001901. doi: 10.1371/JOURNAL.PBIO.1001901
Junker R. R. and Tholl D. (2013). Volatile organic compound mediated interactions at the plant-microbe interface. J. Chem. Ecol. 39, 810–825. doi: 10.1007/S10886-013-0325-9
Karban R. and English-Loeb G. (1997). Tachinid parasitoids affect host plant choice by caterpillars to increase caterpillars to increase caterpillar survival. Ecology 78, 603–611. doi: 10.1890/0012-9658(1997)078
Kay A. D., Bruning A. J., van Alst A., Abrahamson T. T., Hughes W. O. H., and Kaspari M. (2014). A carbohydrate-rich diet increases social immunity in ants. Proc. R. Soc. B: Biol. Sci. 281. doi: 10.1098/RSPB.2013.2374
Kelley D. S. and Bendich A. (1996). Essential nutrients and immunologic functions. Am. J. Clin. Nutr. 63, 994S–996S. doi: 10.1093/AJCN/63.6.994
Kelly J. F., Anthony D. W., and Dillard C. R. (1981). A laboratory evaluation of the microsporidian Vavraia culicis as an agent for mosquito control. J. Invertebr Pathol. 37, 117–122. doi: 10.1016/0022-2011(81)90064-1
Koella J. C. and Lyimo E. O. (1996). Variability in the relationship between weight and wing length of Anopheles Gambiae (Diptera: Culicidae). J. Med. Entomol 33, 261–264. doi: 10.1093/jmedent/33.2.261
Krungkrai S. R. and Yuthavong Y. (1987). The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans. R Soc. Trop. Med. Hyg 81, 710–714. doi: 10.1016/0035-9203(87)90003-4
Kulma K., Saddler A., and Koella J. C. (2013). Effects of age and larval nutrition on phenotypic expression of insecticide-resistance in Anopheles mosquitoes. PloS One 8, e58322. doi: 10.1371/journal.pone.0058322
Kutzer M. A. M. and Armitage S. A. O. (2016). The effect of diet and time after bacterial infection on fecundity, resistance, and tolerance in Drosophila melanogaster. Ecol. Evol. 6, 4229–4242. doi: 10.1002/ECE3.2185
Lazzaro B. P. and Little T. J. (2008). Immunity in a variable world. Philos. Trans. R. Soc. B: Biol. Sci. 364, 15–26. doi: 10.1098/RSTB.2008.0141
Lenth R. V. (2023).emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online at: https://CRAN.R-project.org/package=emmeans (Accessed June 10, 2023).
Leonhardt S. D., Chui S. X., and Kuba K. (2024). The role of non-volatile chemicals of floral rewards in plant-pollinator interactions. Basic Appl. Ecol. 75, 31–43. doi: 10.1016/J.BAAE.2024.01.002
Marva E., Cohen A., Saltman P., Chevion M., and Golenser J. (1989). Deleterious synergistic effects of ascorbate and copper on the development of Plasmodium falciparum: an in vitro study in normal and in G6PD-deficient erythrocytes. Int. J. Parasitol. 19, 779–785. doi: 10.1016/0020-7519(89)90066-0
Mcart S. H., Koch H., Irwin R. E., and Adler L. S. (2014). Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 17, 624–636. doi: 10.1111/ELE.12257
Metcalfe N. B. and Alonso-Alvarez C. (2010). Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996. doi: 10.1111/j.1365-2435.2010.01750.x
Ode P. J. (2006). Plant chemistry and natural enemy fitness: Effects on herbivore and natural enemy interactions. Annu. Rev. Entomol 51, 163–185. doi: 10.1146/annurev.ento.51.110104.151110
Palmer-Young E. C., Farrell I. W., Adler L. S., Milano N. J., Egan P. A., Junker R. R., et al. (2019). Chemistry of floral rewards: intra- and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecol. Monogr. 89, e01335. doi: 10.1002/ECM.1335
Pan X., Zhou G., Wu J., Bian G., Lu P., Raikhel A. S., et al. (2012). Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aEgypti. Proc. Natl. Acad. Sci. U.S.A. 109, E23–E31. doi: 10.1073/PNAS.1116932108/SUPPL_FILE/ST09.DOCX
Parachnowitsch A. L., Manson J. S., and Sletvold N. (2019). Evolutionary ecology of nectar. Ann. Bot. 123, 247–261. doi: 10.1093/AOB/MCY132
Petersen V., Marchi M. J., Natal D., Marrelli M. T., Barbosa A. C., and Suesdek L. (2016). Assessment of the correlation between wing size and body weight in captive Culex quinquefasciatus. Rev. Soc. Bras. Med. Trop. 49, 508–511. doi: 10.1590/0037-8682-0039-2016
Pham-Huy L. A., He H., and Pham-Huy C. (2008). Free radicals, antioxidants in disease and health. Int. J. BioMed. Sci. 4, 89–96. doi: 10.59566/IJBS.2008.4089
Ponton F., Morimoto J., Robinson K., Kumar S. S., Cotter S. C., Wilson K., et al. (2020). Macronutrients modulate survival to infection and immunity in Drosophila. J. Anim. Ecol. 89, 460–470. doi: 10.1111/1365-2656.13126
Ponton F., Wilson K., Cotter S. C., Raubenheimer D., and Simpson S. J. (2011). Nutritional immunology: A multi-dimensional approach. PloS Pathog. 7, e1002223. doi: 10.1371/JOURNAL.PPAT.1002223
Ponton F., Wilson K., Holmes A. J., Cotter S. C., Raubenheimer D., and Simpson S. J. (2013). Integrating nutrition and immunology: A new frontier. J. Insect Physiol. 59, 130–137. doi: 10.1016/J.JINSPHYS.2012.10.011
Povey S., Cotter S. C., Simpson S. J., Lee K. P., and Wilson K. (2009). Can the protein costs of bacterial resistance be offset by altered feeding behaviour? J. Anim. Ecol. 78, 437–446. doi: 10.1111/J.1365-2656.2008.01499.X
Råberg L., Graham A. L., and Read A. F. (2008). Decomposing health: tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. B: Biol. Sci. 364, 37–49. doi: 10.1098/RSTB.2008.0184
Råberg L., Sim D., and Read A. F. (2007). Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814. doi: 10.1126/SCIENCE.1148526
Richardson L. L., Adler L. S., Leonard A. S., Andicoechea J., Regan K. H., Anthony W. E., et al. (2015). Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B: Biol. Sci. 282. doi: 10.1098/RSPB.2014.2471
Ristow M. (2014). Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med. 20, 709–711. doi: 10.1038/NM.3624;SUBJMETA=2366,631,80,86;KWRD=STRESS+SIGNALLING
Ritz B. W. and Gardner E. M. (2006). Malnutrition and energy restriction differentially affect viral immunity. J. Nutr. 136, 1141–1144. doi: 10.1093/JN/136.5.1141
Samartín S. and Chandra R. K. (2001). Obesity, overnutrition and the immune system. Nutr. Res. 21, 243–262. doi: 10.1016/S0271-5317(00)00255-4
Santos V. H. M., Minatel I. O., Reco P. C., Garcia A., Lima G. P. P., and Silva R. M. G. (2017). Peptide composition, oxidative and insecticidal activities of nectar from flowers of Spathodea campanulata P. Beauv. Ind. Crops Prod 97, 211–217. doi: 10.1016/j.indcrop.2016.12.025
Schmid-Hempel P. (2021). Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics (Oxford, UK: Oxford University Press).
Schmitt A., Roy R., and Carter C. J. (2021). Nectar antimicrobial compounds and their potential effects on pollinators. Curr. Opin. Insect Sci. 44, 55–63. doi: 10.1016/j.cois.2021.03.004
Schneeberger K., Czirják G.Á., and Voigt C. C. (2013). Inflammatory challenge increases measures of oxidative stress in a free-ranging, long-lived mammal. J. Exp. Biol. 216, 4514–4519. doi: 10.1242/jeb.090837
Simpson S. J. and Raubenheimer D. (2012). The nature of nutrition. Princeton University Press. doi: 10.1515/9781400842803/HTML
Singer M. S. and Bernays E. A. (2009). “Specialized generalists: behavioral and evolutionary ecology of polyphagous woolly bear caterpillars,” in Tiger moths and woolly bears: behavior, ecology, and evolution of the Arctiidae (Oxford: Oxford University Press), 103–114.
Singer M. S., Mace K. C., and Bernays E. A. (2009). Self-medication as adaptive plasticity: increased ingestion of plant toxins by parasitized caterpillars. PloS One 4, e4796. doi: 10.1371/journal.pone.0004796
Sorci G. and Faivre B. (2008). Inflammation and oxidative stress in vertebrate host–parasite systems. Philos. Trans. R. Soc. B: Biol. Sci. 364, 71–83. doi: 10.1098/RSTB.2008.0151
Sternberg E. D., Lefèvre T., Li J., de Castillejo C. L. F., Li H., Hunter M. D., et al. (2012). Food plant derived disease tolerance and resistance in a natural butterfly-plant-parasite interaction. Evol. (N Y) 66, 3367–3376. doi: 10.1111/J.1558-5646.2012.01693.X
Stowe K. A., Marquis R. J., Hochwender C. G., and Simms E. L. (2000). The evolutionary ecology of tolerance to consumer damage. Annu. Rev. Ecol. Syst. 31, 565–595. doi: 10.1146/ANNUREV.ECOLSYS.31.1.565/CITE/REFWORKS
Strauss S. Y. and Agrawal A. A. (1999). The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 14, 179–185. doi: 10.1016/S0169-5347(98)01576-6
van der Kooi C. J. and Ollerton J. (2020). The origins of flowering plants and pollinators. Sci. (1979) 368, 1306–1308. doi: 10.1126/SCIENCE.AAY3662/ASSET/D58F6410-481C-4AC3-8529-9F758E59C4B7/ASSETS/GRAPHIC/368_1306_F2.JPEG
Venables W. N. and Ripley B. D. (2002). Modern Applied Statistics with S (New York, NY: Springer). doi: 10.1007/978-0-387-21706-2
Vulule J. M., Beach R. F., Atieli F. K., Roberts J. M., Mount D. L., and Mwangi R. W. (1994). Reduced susceptibility of Anopheles Gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med. Vet. Entomol 8, 71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x
Zeferino T. G. and Koella J. C. (2024). Host-specific effects of a generalist parasite of mosquitoes. Sci. Rep. 14, 18365. doi: 10.1038/s41598-024-69475-4
Zeferino T. G., Mora A. R., Vallat A., and Koella J. C. (2024). Mosquitoes self-medicate according to the dynamics of a microsporidian infection. bioRxiv. doi: 10.1101/2024.12.12.628192
Keywords: oxidative stress, life-history trade-offs, parasite tolerance, host-parasite interactions, Anopheles mosquitoes, self-medication, infection resistance, oxidative ecology
Citation: Zeferino TG, Mora AR and Koella JC (2025) Timing of prooxidant and antioxidant intake shapes life-history and parasite tolerance in Anopheles mosquitoes. Front. Ecol. Evol. 13:1628611. doi: 10.3389/fevo.2025.1628611
Received: 14 May 2025; Accepted: 18 July 2025;
Published: 04 August 2025.
Edited by:
Francisco Garcia-Gonzalez, Spanish National Research Council (CSIC), SpainReviewed by:
Marko D. Prokic, University of Belgrade, SerbiaPepijn Luijckx, Trinity College Dublin, Ireland
Copyright © 2025 Zeferino, Mora and Koella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiago G. Zeferino, dGlhZ28uZ29uY2FsdmVzQHVuaW5lLmNo
 Tiago G. Zeferino
Tiago G. Zeferino Alfonso Rojas Mora
Alfonso Rojas Mora Jacob C. Koella
Jacob C. Koella