- 1Integrative Insect Ecology Research Unit, Department of Biology, Chulalongkorn University, Bangkok, Thailand
- 2Centre for Biodiversity Genomics, Guelph, ON, Canada
- 3Department of Integrative Biology, University of Guelph, Guelph, ON, Canada
- 4Guanacaste Dry Forest Conservation Fund, Huntington, VT, United States
- 5Herbario Nacional de Costa Rica, Departamento de Historia Natural, Museo Nacional de Costa Rica, San José, Costa Rica
- 6Department of Entomology, National Museum Natural History, MRC 168, Smithsonian Institution, Washington, DC, United States
- 7Systematic Entomology Laboratory, Beltsville Agriculture Research Center, Agricultural Research Service, U.S. Department of Agriculture, c/o National Museum Natural History, MRC 168, Smithsonian Institution, Washington, DC, United States
- 8BioAlfa, Santo Domingo de Heredia, Costa Rica
- 9Department of Biology, University of Pennsylvania, Philadelphia, PA, United States
Caterpillar–food plant records collected over approximately 38 years in the Area de Conservación Guanacaste (ACG) in northwestern Costa Rica are described and summarized. The data comprise 431,212 individual rearing records, 197,366 of which represent unique plant–herbivore associations, i.e., same species pair found on separate dates and at different plants of the same species. These represent 29,187 different caterpillar–food plant associations between 2,489 plant and 7,160 Lepidoptera species. We evaluate changes in the taxonomic composition of the food plant flora and Lepidoptera fauna between 1990 and 2020 and across habitat/community types. Food plant and caterpillar community species richness in the rain forest changed considerably over the first 10 years but remained more stable since. Dry forest communities were more consistent than in rain forest. The cloud forest biota was the most consistent between 1995 and 2010, but as in dry forest, the caterpillar fauna changed considerably during 2015–2020. Plant species composition was more constant than caterpillar composition. The taxonomic distributions of diet specialists and generalists are explored. Most of the species-rich Lepidoptera families contain many specialists, variously concentrated throughout each family, though highly polyphagous collectively. The exceptions include Sphingidae, which show preference for Rubiaceae, Hesperiinae for monocotyledons, and non-Hesperiinae skippers for Fabaceae. Among plant families for which there are over 1,000 independent rearings, Acanthaceae, Apocynaceae, Arecaceae, Costaceae, Melastomataceae, Moraceae, Piperaceae, Poaceae, Rubiaceae, Rutaceae, and Solanaceae hosted the greatest proportion of specialists. However, the level at which dietary specialization corresponds to taxonomic rank varies with both caterpillar and plant taxon. Most fern-feeders are polyphagous with respect to fern families but still specialists on Polypodiopsida. A selection of plant families with conspicuous allelochemical and/or structural defenses and a selection of caterpillars and caterpillar families with equally conspicuous counter-defenses were examined. We determined that (1) unpalatable, aposematic herbivores tend to be specialists and (2) families of plants predominantly consumed by highly defended caterpillars host fewer polyphagous herbivores than families with less conspicuously defended plants. Highly toxic plant families with the fewest rearings, such as Aristolochiaceae and Zamiaceae, hosted many monophagous caterpillars. Biochemical and structural plant defenses appear to mediate herbivore diet breadth for many plant families.
1 Introduction
As primary consumers of vascular plants, prey to countless vertebrate and invertebrate predators and parasitoids, and important links in pollination networks, lepidoptera play crucial and unparalleled roles in nearly all terrestrial ecosystems worldwide. The complexity of their contributions to food webs and ecosystem diversity and stability is vast, and various aspects of these contributions have been described in numerous reviews (e.g., Hammond and Miller, 1998; Singer, 2016; Goldstein, 2017; Narango et al., 2020). Most lepidopteran caterpillars are phytophagous and assert strong selective pressure on their food plants which, in turn, drives the evolution of plant defenses.
Virtually all land plants have evolved some form of chemical and/or physical defense against herbivory (e.g., Mithöfer and Boland, 2012; Erb et al., 2012). Some of these defenses probably evolved specifically to protect them from large, vertebrate herbivores such as mammals, whereas others serve as protection against a variety of invertebrates (War et al., 2012). For exophytic caterpillars (those that feed on the outer surface of plants), the most common physical defenses include high cellulose and lignin content (which impede herbivory) and mechanical properties of leaves, such as pubescence, trichomes, toughness, gum, resin and/or sticky latex, and silica content. These physical features and low nutritional content function as deterrents, obstructing or inhibiting herbivory.
Many plants also produce secondary metabolites that help to protect them from insect herbivory by being directly entomotoxic or by acting as feeding deterrents. These secondary metabolites or allelochemicals are diverse and highly dynamic (War et al., 2012), providing plants with varying degrees of unpalatability (Janzen, 1979). The best-studied plant allelochemicals are terpenes (unsaturated hydrocarbons), phenolics (e.g., tannins), alkaloids, sesquiterpene lactones, cardenolides (e.g., cardiac glycosides), and sulphur-containing compounds (e.g., mustard oil glucosinolates) (e.g., Hopkins et al., 2009; Yoneyama and Natsume, 2010; Kong et al., 2019; Bachheti et al., 2019). However, there are many other such chemicals/chemical complexes, the detailed functions of which have hardly been investigated. Some trichomes bridge the chemical–physical spectrum, releasing glandular (Mustafa et al., 2018a) or mineralized toxins (Mustafa et al., 2018b; Weigend et al., 2018).
The interface between caterpillars and their food plants represents a boundless landscape for investigations into the complex interactions between caterpillars and plants. However, identification of overarching trends and/or patterns requires large amounts of data acquired over significant periods of time and space. Here we analyzed such a data set assembled over more than 35 years in a large, conserved area in northwestern Costa Rica, the Area de Conservación Guanacaste (ACG) (Janzen, 2004; Janzen et al., 2016; Janzen and Hallwachs, 2017, 2020).
The questions we sought to address in this study are many and include the following: (1) What is the degree of overlap among the caterpillar faunas of different ecosystems? (2) Which Lepidoptera families dominate the landscape in terms of number of individuals and species? (3) Has the caterpillar fauna of ACG changed over the last 30 years or so? (4) What is the degree of host specialization among ACG caterpillars, and which families exhibit the greatest host fidelity? (5) Is feeding on plants armed with conspicuously toxic host plant chemistry confined to specific caterpillar families? (6) Do caterpillar species feeding on a priori well-defended plant families have overall different dietary breadths than those feeding on palatable plants?
1.1 Nature of the data
The data explored here represents, to our knowledge, the largest wild-caught caterpillar rearing program ever conducted. To date, the project has accumulated more than 883,000 caterpillar–food plant records (Janzen, 1988; Janzen et al., 2009; Quicke et al., 2024). Records of larval food plants for the butterfly species (i.e., Papilionoidea) collected before 2007 were included in the catalogue by Beccaloni et al. (2008), which also categorized some of these recorded trophic associations as plausible versus dubious.
The scientific objective of the ACG caterpillar-rearing program has always been purely documentary in that it was to obtain a detailed snapshot of which plants are consumed by caterpillars in a diverse and successional tropical wildland. Thus, since there has been no standardization of sampling effort, the number of caterpillar rearings corresponds to the frequency with which their food plant was encountered. In addition, the intensity of survey effort varied throughout the course of the project, and sampling efficiency increased commensurate with the increased experience of the parataxonomists involved.
These features mean that it is not possible to compare directly all features of the present study with those of a number of other large field studies that have been conducted in recent years (e.g., Novotny et al., 2002a, 2004, 2010; Dyer et al., 2007), which usually restricted sampling to a particular group of plant species and/or standardized searching effort in relation to leaf area. Furthermore, most other studies have been restricted to relatively short time windows. In contrast, the data we make available may allow the extraction of subsets from which valid comparisons with other studies can be made.
1.2 Topics explored
Using a data set of 29,215 different caterpillar–food plant associations, we have compiled species accumulation curves, compared species richness among three different ecosystems in ACG, explored trophic interactions, quantified faunal turnover over time (largely due to successional changes in food plants), examined diet breadth and plant chemical defenses, and briefly reviewed a variety of other parameters among exophytic caterpillars revealed by the immense ACG database. Our overarching purpose is to investigate the factors mediating the diet breadth of the reared Lepidoptera species and families, and in this respect, we take two approaches. First, we examine the utilization of food plant families with well-known protection either physical or chemical against folivores. These families were selected based on scientific literature and investigated separately. Second, we investigate the food plant diversity of individual caterpillar species on each plant family as well as their diets across all other plant families. The latter approach revealed a number of rather unexpected trends which we discuss further.
2 Methods
2.1 Study system
Study site: The Área de Conservación (ACG) in northwestern Costa Rica is a 169,000-ha tract of land within the national system of protected areas. The site has been described in detail by Janzen (2004); Janzen et al. (2016), and Janzen and Hallwachs (2017). In general, ACG supports three primary ecosystems: dry forest (ca. 70% of the land area), rain forest (ca. 25%), and cloud forest (5%–10%). The last of these has diminished during the course of the study, most likely as a result of warming due to climate change (Smith, 2023). In ACG, dry forests occur predominantly at lower elevations and along the Pacific coast and are dominated by deciduous trees that often lose their leaves during the dry season. It is represented by a range of successional ages from very recent to centuries old rain forest is moister and characterized by dense canopies of evergreen trees, high humidity, and abundant rainfall, with many fewer and less abundant deciduous trees. Cloud forest occurs at the highest elevation in ACG (above 1,000 m elevation) and is characterized by a persistent cloud cover but exhibits considerable botanical overlap with the rain forest.
Field collecting and most caterpillar and plant identification were carried out by resident parataxonomists (“gusaneros” in the local language) (Janzen and Hallwachs, 2011). For those unfamiliar with this occupation, here is a brief description of their roles. A parataxonomist is broadly analogous to a paramedic or paralegal person but in the arena of field taxonomy/biology. They are resident men or women selected from the rural workforce, without a university degree. They are trained on the job to conduct an inventory by finding insects, knowing their names, knowing the plant species they are feeding on, rearing the caterpillars and their parasites, collecting and preserving the individual insects that emerge, and entering all the relevant data into a database. Most such parataxonomists began their career in the 1980–1990s by taking an intensive 6-month introductory course and then being continually coached and updated over the next 20–35 years while on the job. New parataxonomists are trained through an apprenticeship with the more experienced ones. Each is based at a particular field station where they have spent decades learning the taxonomy of their focal insects and plants.
During each collecting foray, parataxonomists would collectxmostly in only one of the aforementioned three ecosystems. However, sometimes they ventured into two (e.g., parataxonomists working at Estación Cacao might, for example, sample plants and caterpillars from dry forest/rain forest intergrade, rain forest, and cloud forest). Hence, the three ecosystems do intergrade, and some of the trails in each ecosystem have similar plants that are generally associated with recently disturbed habitats (ruderal species).
Caterpillar collecting: Methods of collecting, rearing, and vouchering external foliage-feeding caterpillars in the ACG have been described previously in detail elsewhere (e.g., Janzen et al., 2016; Janzen and Hallwachs, 2017; Quicke et al., 2024). Members of the experienced team of 10–30 resident parataxonomists physically searched for external feeding caterpillars, including those in rolled leaves or silk-leaf nests up to approximately 2 m above the ground level, primarily along trails (Janzen, 2004; Janzen and Hallwachs, 2011). Surveys were conducted both by day and night and all year around. Although this method is fairly effective for nearly all Macroheterocera (macro-Lepidoptera) as well as for those hidden in leaf rolls or nests, which reveal their presence, it proved less effective for discovering caterpillars that are concealed feeders. Despite the considerable experience accrued by the parataxonomists, sampling was inevitably biased toward more apparent or distinctive species and against least apparent, more cryptic ones or those that hide elsewhere during daylight hours. The parataxonomists did not collect leaf-miners, stem or gall borers, or any species feeding on rhizomes.
When a parataxonomists encountered multiple individuals of a caterpillar species on a given plant on the same occasion, they sometimes collected and reared up to around 20 of them to increase the chance of a successful rearing as well as the probability of rearing parasitoids. For the analyses presented here, we consider this as a single rearing event and refer to this as a unique or independent rearing.
Caterpillars discovered in the field were taken to “rearing barns” and singly placed in plastic bags with cuttings of the food plant and hung from clothes lines in the shade (photographs showing these are provided in Quicke et al. (2024)). New foliage was provided at 2–4-day intervals. Each caterpillar was labeled with a unique voucher number in the form of YY-SRNP-X…… (e.g., 09-SRNP-15328), where the prefix is the last two digits of the year (e.g., 2009), “SRNP” refers to the project “call letters” assigned in 1977 (when the initial project site was referred to as Santa Rosa National Park), and the suffix is a unique number assigned within the year. As adults emerged, they were frozen, pinned, and labeled. These voucher codes are provided in Supplementary Table S1 (also available at https://doi.org/10.5683/SP3/NX043G), which includes several additional fields to the ones used in these analyses, i.e., barcode BIN, parasitoids when reared, including latitude, longitude voucher fate, and taxonomic identifier information. Most specimens have been transferred to the United States National Entomological Collection (USNM), Smithsonian Institution, Washington DC, where they now reside, but due to capacity restrictions, species-level duplicates and some paratypes are also deposited in the Museo Nacional de Costa Rica in Santo Domingo de Heredia in the suburbs of San Jose, Costa Rica.
Species identification: The identification of ACG Lepidoptera has been dynamic, involving the recruitment of taxonomic experts worldwide. Many of the early “species concepts” based mainly or solely on morphology have been refined through more integrative methods relying on a combination of morphological and molecular data. Although some of the undescribed and cryptic species have been formally named (e.g., Burns and Janzen, 2005; Burns et al., 2008; Metz et al., 2017, 2020; Solis and Styer, 2003; Solis et al., 2005, 2020; Metz, 2024), most are now recognized based on morphology and/or food plant associations following revisiting them in the light of barcoding results (e.g., Hebert et al., 2004). Even so, members of many putative species complexes are still discriminated only by their barcode index number (BIN) in the Barcode of Life Data System (BOLD) (https://boldsystems.org) (Ratnasingham and Hebert, 2013). In the present dataset, the undescribed putative species are indicated by interim alphanumeric place-holder names usually with a researcher’s or project’s initials and a number. Sometimes, further evidence reveals that even certain of these comprise one or more distinct species, or that despite barcode differences, some putative species should be recombined with members of another BIN. In general, the result of barcoding and associated integrative taxonomy has revealed far higher levels of dietary specialization than earlier work suggested for Lepidoptera (Hebert et al., 2004; Janzen et al., 2011), their dipterous and hymenopterous parasitoids (Smith et al., 2006, 2007, 2008), and other insects (e.g. Dolson et al., 2020, 2021; Underwood et al., 2024). Hence, for many Neotropical Lepidoptera families, it should be noted that the current state of their taxonomy and generic classification is far from established, and in the future, many of the generic assignments of species will probably change.
For the purposes of this study, we describe diet breadth as a count of taxa (i.e., species as delimited by morphology and/or additional evidence as noted above classified in families) and do not infer phylogenetics other than assumed monophyly at the family level. Summarization at the family level is a matter of convenience and not an implication that families are comparable among the included taxa.
For DNA barcoding, DNA was extracted from tissue from the leg of an oven-dried individual adult lepidopteran (Hajibabaei et al., 2006; Janzen et al., 2009; Janzen and Hallwachs, 2011, 2016). Sequencing of the barcoding region of the mitochondrial cytochrome oxidase c subunit 1 (COI) gene was performed following standard methods at the Centre for Biodiversity Genomics, University of Guelph (Craft et al., 2010; Wilson, 2012).
With regard to the dataset (available in Supplementary Table S1), this comprised not only caterpillar–host associations for adults successfully reared from larvae discovered in the field but also for associations in which caterpillars succumbed to parasitoids. In the latter case, the identity of caterpillars was reliably determined either through morphological or behavioral distinctiveness or because other individuals of the same species collected from the same plant and on the same occasion yielded an adult butterfly or moth and/or was identified through comparative DNA barcoding (154,833 Lepidoptera DNA barcodes were generated in the course of the study). The data are more thoroughly vetted than those of our previous paper (Quicke et al., 2024) with only caterpillars that could be identified to species or assigned a confident interim name (or BIN) included.
2.2 Data analysis
Data analyses were performed using the R environment for statistical computing (version 4.4.1) (R Development Core Team, 2024). In addition to functions provided in base R, we also employed functions from the following packages: circlize (Gu et al., 2014), e1071 (Meyer et al., 2019), bipartite (Dormann et al., 2008), RColorBrewer (Neuwirth, 2014), pals (Wright, 2025), and VennDiagram (Chen and Boutros (2011).
Species accumulation curves were calculated by the standard method, plotting cumulative number of species (y-axis) against sequential number of independent rearings (x-axis). Four curves were compiled, one for the entire data set and one for each of the three ecosystems.
Shannon’s diversity index H (Shannon, 1948) was used to quantify species diversity within a sample (Stireman et al., 2017) as it is less strongly influenced by a small number of dominant species than is Simpson’s index. To estimate the overall degree of specialization in a network, we used the standardized two-dimensional Shannon entropy, H2’ (Blüthgen et al., 2006), which has values near zero in cases of extreme specialization and 1 when there is extreme generalism. Similarity among foodplant, caterpillars, and trophic interactions for the three ecosystems was calculated using Jaccard’s coefficient with the following formula: J(A, B) = |A∩B|/|A∪B|, which can be summarized as “the intersection of two sets/the union of two sets.”
Comparisons of species richness among lepidopteran families were illustrated using simple histograms with families on the x-axis and number of species on the y-axis. Histograms were compiled for the total data set and for each of the three individual ecosystems. The sequence of families along the x-axis was determined by species richness based on the total data set and retained for each of the ecosystems. In an attempt to quantify changes in similarity among caterpillars and foodplants over time, the data set was divided into six 5-year periods of comparable sampling effort (i.e., 1990–2020). Similarity among caterpillars and foodplants was calculated for each of the three ecosystems using Jaccard’s coefficient. Similarities were then divided into seven increments of 0.1, ranging from 0–0.1 to 0.6–0.7. For several analyses concerning unpalatable food plants, we treated the Arctiinae (tiger moths and allies) separately from other members of the Erebidae because their ability to feed on a wide range of toxic plants is well documented (Weller et al., 1999; Mason et al., 2014; Zaspel et al., 2014).
To examine the spectrum of plant defense against caterpillar herbivory, we created two lists of plant families, taxa that are conspicuously equipped with chemical and/or structural defenses, and one for families whose species generally lack such obvious protection. The well-protected list comprised those plant families that have latex, abundant silica, or notable toxins (e.g., aristolochic acid, alkaloids, cycasin, glucosinolates, and glycosides). The families considered well defended (with T = toxic, L = latex, S = silica) are Alismataceae (T), Amaryllidaceae (T, L), Annonaceae (S), Apocynaceae (T, L), Araceae (T, L), Arecaceae (S), Aristolochiaceae (S, T), Boraginaceae (S), Bromeliaceae (S), Burseraceae (S), Cactaceae (L, S), Cannabaceae (L, S), Cannaceae (S), Caricaceae (T, L), Chloranthaceae (S), Chrysobalanaceae (S), Costaceae (T, S), Celastraceae (T, L), Convolvulaceae (T, L), Cyperaceae (S), Dilleniaceae (S), Euphorbiaceae (T, L), Gesneriaceae (T), Heliconiaceae (S), Hernandiaceae (S), Icacinaceae (T, L), Loranthaceae (S), Magnoliaceae (S), Marantaceae (S), Menispermaceae (S), Moraceae (T, L), Nymphaeaceae (T), Musaceae (T, L, S), Olacaceae (T, L), Papaveraceae (T, L), Passifloraceae (T), Piperaceae (T, S), Poaceae (S), Sapotaceae (T, L, S), Solanaceae (T), Thymelaeaceae (S), Ulmaceae (S), Urticaceae (S), Zamiaceae (T), and Zingiberaceae (S). All remaining angiosperm families were classified as having largely palatable foliage.
The classification of angiosperm food plants follows APG IV (The Angiosperm Phylogeny Group et al., 2016). The classification of grasses follows GPWF II and GPWG III (GPWG II, 2012; GPWG III, 2025). The classification of ferns follows PPG I (Pteridphyte Phylogeny Group; Schuettpelz et al., 2016). Definitions of Lepidoptera families follow those of van Nieukerken et al. (2011).
3 Results and discussion
3.1 Species accumulation curves
We compiled 435,897 individual rearing records of which 197,366 represent unique, in other words independent, observations (i.e., caterpillar–plant associations that were collected on separate dates and from different plants). Within that data set, 2,489 plant species served as hosts for one or more caterpillars, and 7,160 species of Lepidoptera were reared. Caterpillar and food plant accumulation curves are shown for the whole data set (Figure 1) and for each of the three ecosystems (Figures 2A–C). For the total data set, trophic interactions showed the steepest curve, with accumulation curves for caterpillar species and plant species significantly more gradual. The accumulation curves for the three different ecosystems showed similar patterns, with caterpillar species far outnumbering plant species beyond the first few sampling bouts. In all cases, there was an initial steep accumulation of species with rearing effort, with a continuing nearly linear curve that never showed signs of reaching an asymptote, although rain forest Lepidoptera species accumulation did show a potential transition towards an asymptote. The rather relatively sharp increase in food plant species in the cloud forest data (Figure 2C) reflects, at least in part, changes that occurred during the period of COVID-19 restrictions (March 2020 to December 2021) as well as the transfer of a parataxonomist to and from another station.
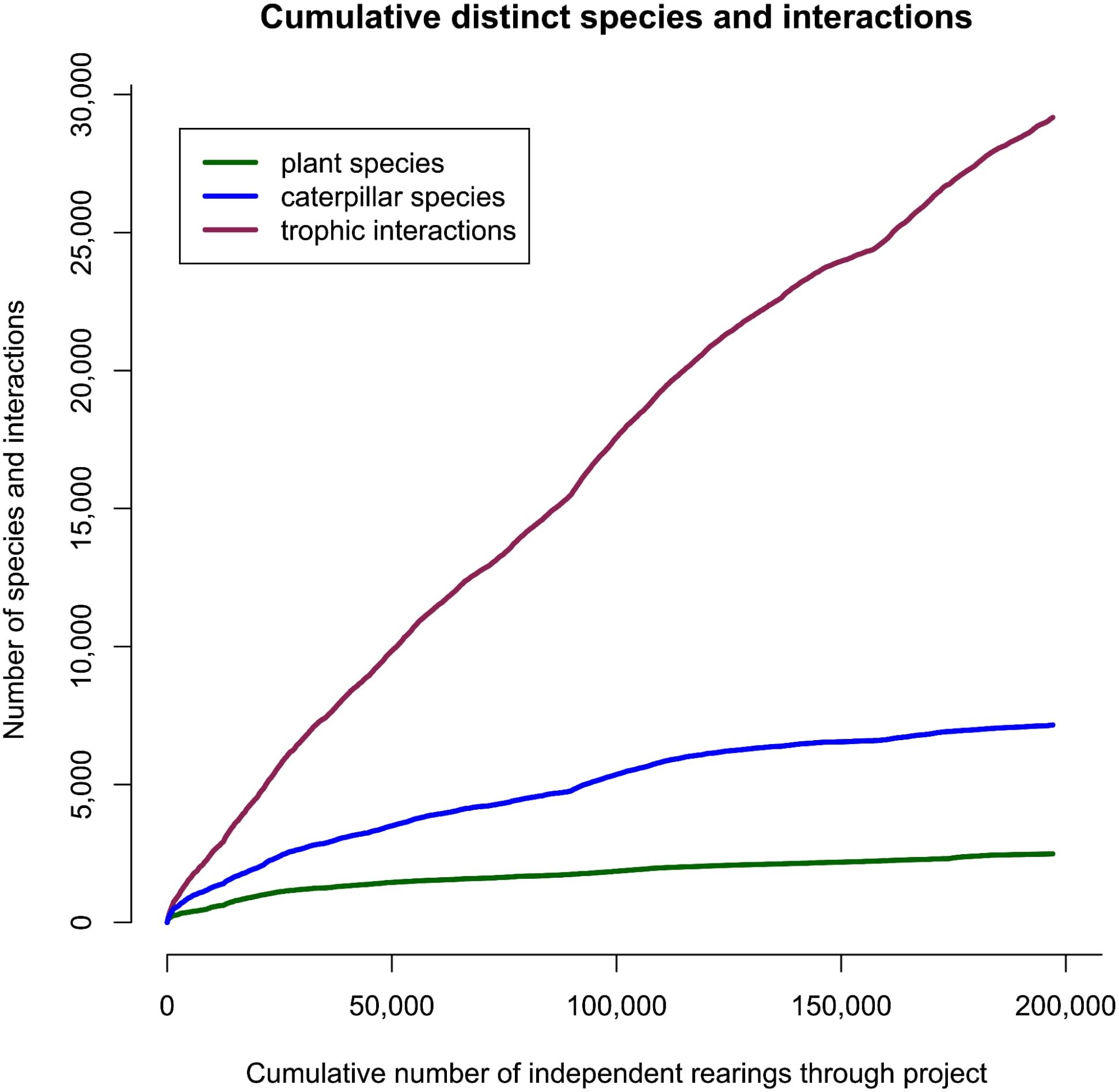
Figure 1. Accumulation of species with rearing effort for all ACG data pooled. Independent rearings mean that only a single record of a caterpillar species from an individual plant collected on a given date is included.
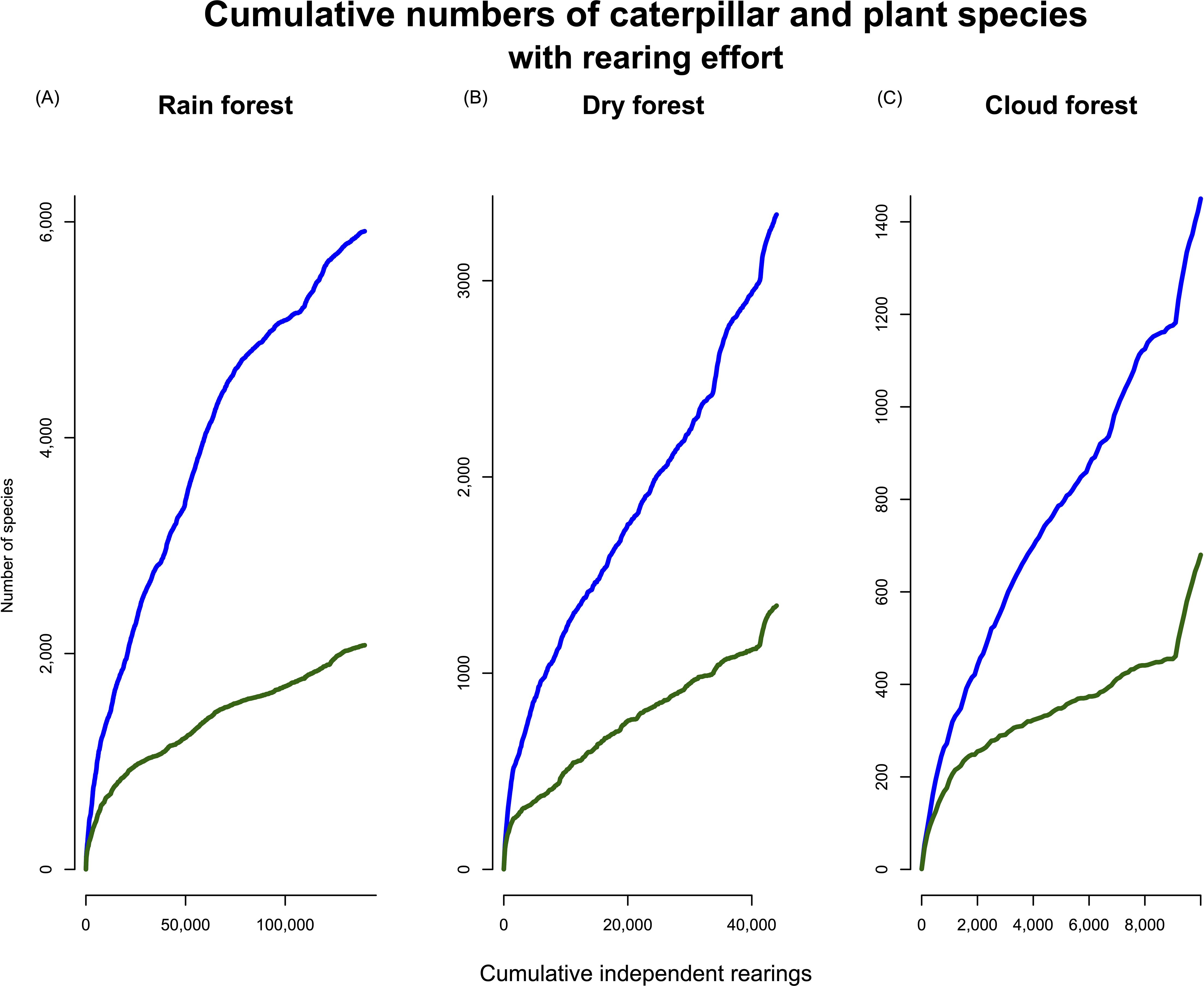
Figure 2. Separate species accumulation curves for each ecosystem: (A) rain forest, (B) dry forest, and (C) cloud forest. Independent rearings mean that only a single record of a caterpillar species from an individual plant collected on a given date is included. Colours of lines as in Figure 1.
The steep and continuous nature of the curves suggests that considering all measured parameters (caterpillars, host plants, and interactions), significant numbers remain undersampled. However, we included associations that were detected only once which some authors have argued against, suggesting that such rearings may be abnormal or atypical (Marohasy, 1998; Novotny et al., 2002b). While it is true that a female herbivorous insect might make occasional “mistakes”, that does not mean these events are unimportant. A shortage of the normal plant species, various types of stress or old age, might cause females to perform some egg dumping on possibly unsuitable plants on which their caterpillars have lower but nevertheless non-zero chance of developing successfully. If the resulting caterpillar does complete its development successfully on that plant, this is presumably how host ranges evolve. Furthermore, because of the non-targeted and opportunist nature of the caterpillar collection, the dataset includes a substantial number of Lepidoptera species that were encountered and reared only on a few occasions—for example, 1,751 caterpillar species were only reared on one occasion, 772 twice, and 494 three times.
In this light, we suspect that the steep increase in trophic interactions results from the fact that that species were only encountered rarely, and since very few species collected on numerous occasions were monophagous, increased rearing of uncommon taxa with more trophic links kept being revealed. Thus, both host specificity of caterpillars and the herbivore ranges of food plants based on these data are likely to be marked underestimates.
3.2 Distribution across “ecosystems”
The overlap in caterpillar, plant species, and trophic interactions among the three ecosystems is illustrated in Figures 3A–C. Jaccard coefficients of similarity are presented in Table 1. The overlap of food plant species among ecosystems was 13% (i.e., 324 shared species), and the overlap of lepidopteran species was 8.6% (i.e., 612 shared species). Only 1.5% of species–species interactions (i.e., 441) occurred in all three ecosystems.
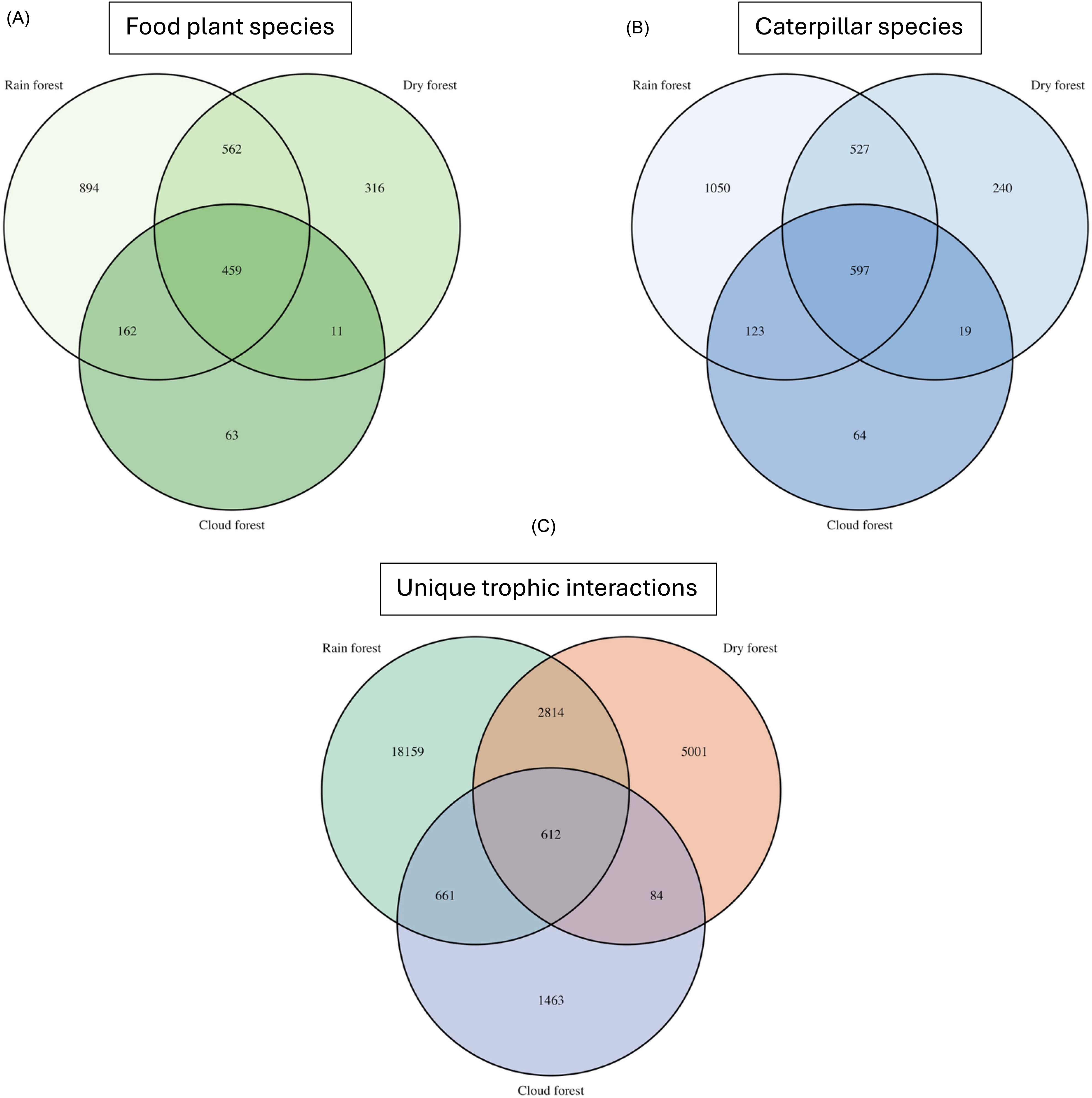
Figure 3. Venn diagrams showing the food plant, caterpillar species, and unique interactions recorded from the three ecosystems and the overlap between them: (A) food plant species, (B) caterpillar species, and (C) trophic interactions.
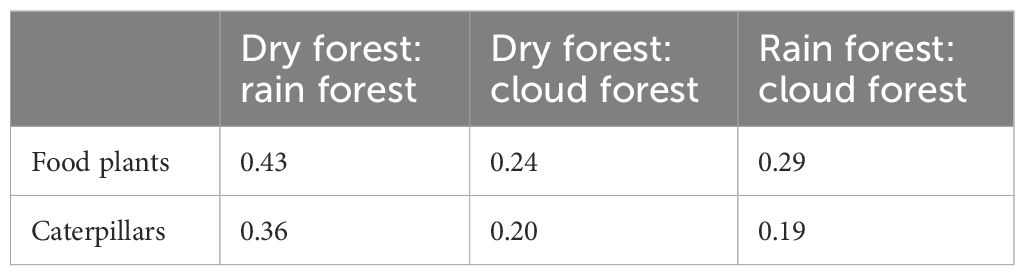
Table 1. Proportional overlap of plant and caterpillar species between the data from the regions designated as each of the three ecosystems.
Of the 439 most widely recovered trophic interactions, those linking only four combinations of caterpillar–food plant families were represented 10 or more times, and 39% of the family-level trophic links were found only once. The widespread caterpillar species were dominated by Hesperiidae, 29 of which fed on Fabaceae and 11 on Poaceae. Next were Sphingidae–Rubiaceae associations (n = 24) and the third were Notodontidae–Fabaceae links (n = 10), although the last is almost entirely due to the subfamily Hemiceratinae, specifically Hemiceras and its association with Inga species. Although Inga species are not ruderals, they are typically competitive and stress tolerant. While a total of 62 nymphalid species were reared from all three ecosystems, these were collectively reared from 22 different plant families.
Based on the total data set for ACG (Figure 4A), Erebidae (n = 1,212 species) was the most species-rich family, followed by Geometridae (n = 812), Hesperiidae (n = 666), Depressariidae (n = 659), and Crambidae (n = 497). The pattern of species richness in rain forest (Figure 4B) was similar to that of the total data set, with the same top 10 families (i.e., Erebidae, Geometridae, Depressariidae, Crambidae, Hesperiidae, Notodontidae, Nymphalidae, Noctuidae, Gelechiidae, and Tortricidae); however, there were a few slight differences in their relative positions—for example, Hesperiidae dropped from third to fourth most species-rich, Notodontidae dropped from sixth to seventh, and Noctuidae dropped from eighth to ninth.
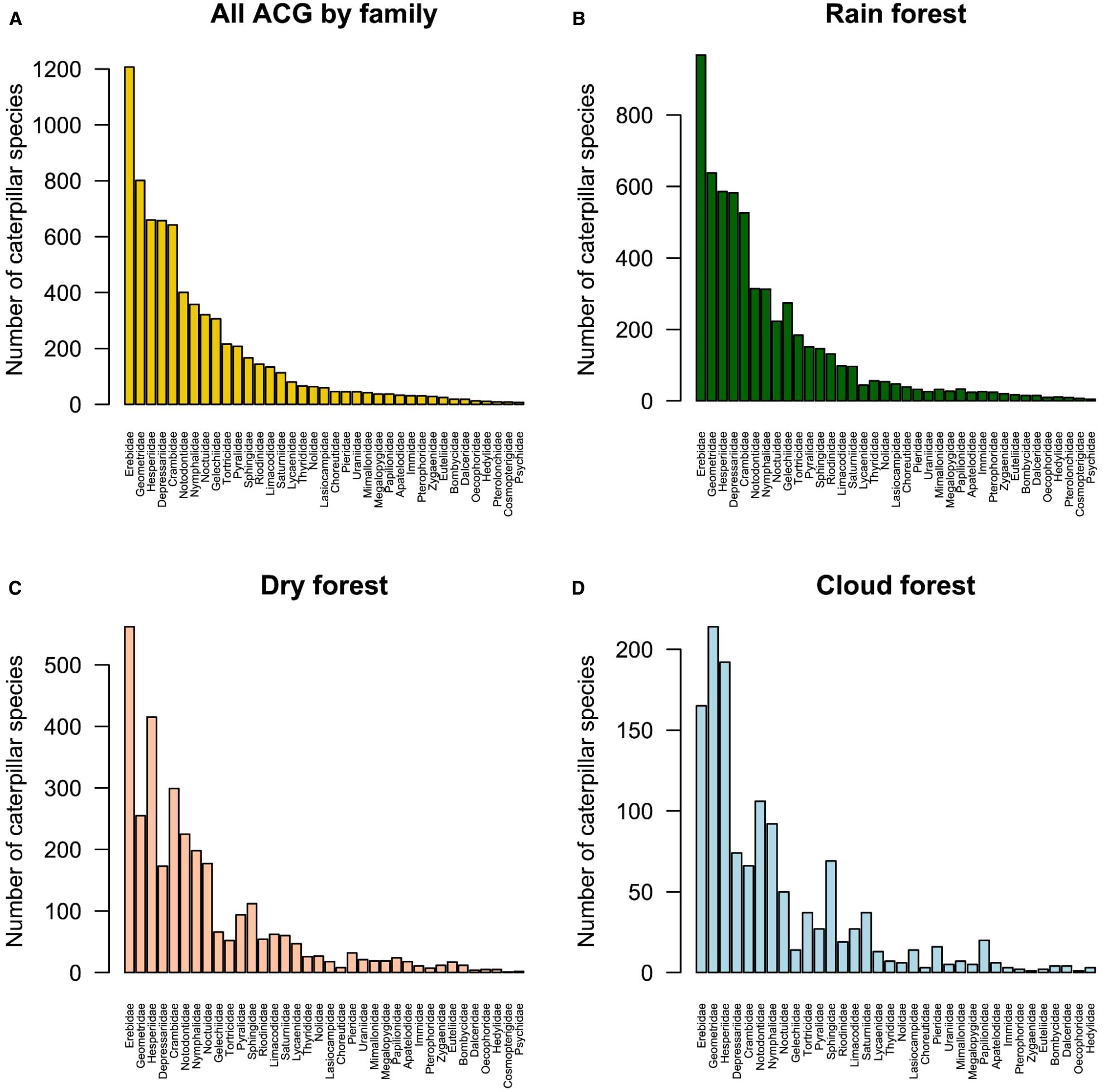
Figure 4. Ranked relative species richness of most abundant reared caterpillar families: (A) across all of the ACG, (B) rain forest, (C) dry forest, and (D) cloud forest. Lepidoptera families are presented in the same order as in the whole ACG data set to emphasize differences among ecosystems.
In dry forest (Figure 4C), Erebidae again were the most species-rich family (n = 497 species), but in this ecosystem, Hesperiidae was the second (n = 354) and Crambidae the third (n = 254). Cloud forest deviated the most from the overall ACG pattern (Figure 4D), with Geometridae (n = 194 species) representing the most species-rich family, followed by Erebidae (n = 126) and Hesperiidae (n = 120). Overall, Depressariidae were less species-rich in both dry and cloud forest compared to the overall ACG and rain forest ecosystem. Crambidae and Hesperiidae were comparatively more species-rich in dry forests, whereas Hesperiidae, Notodontidae, Nymphalidae, and Sphingidae were more species-rich in cloud forest in comparison to other ecosystems. As would be expected, many microlepidoptera families that typically feed on detritus, fungus, roots, or other cryptic microhabitats (e.g., Tineidae, Hepialidae, Blastobasidaae, Oecophoridae) are poorly represented in the reared samples. In this light, it was surprising that Tortricidae and Gelechiidae, microlepidopteran families that include many concealed feeders, are among the top 10 families sampled. It is noteworthy that nearly all of the gelechiids reared belonged to Dichomeridinae and Anacampsinae, with only a few belonging to Gelechiinae.
3.3 Persistence over time
Substantial parts of ACG are undergoing natural (i.e., benign neglect) succession from former agricultural land to forest (see Janzen, 1990), but climate change also contributes to changes in the vegetation through differences in rainfall patterns and increasing average temperatures (Herrera, 2016). Changes in floral and faunal composition over time are shown in Figure 5. It has now become apparent that for many years overall insect abundance, including Lepidoptera and the species that parasitize them, has shown a considerable decline since the 1980s (Janzen and Hallwachs, 2021). This follows an apparently global trend—for example, Hallmann et al. (2017) report greater than 75% decline in total flying insect biomass in just over 27 years in 63 protected areas in Germany. Furthermore, a recent metanalysis (Rumohr et al., 2023) indicates that the major contributing factors to this are anthropogenic in origin.
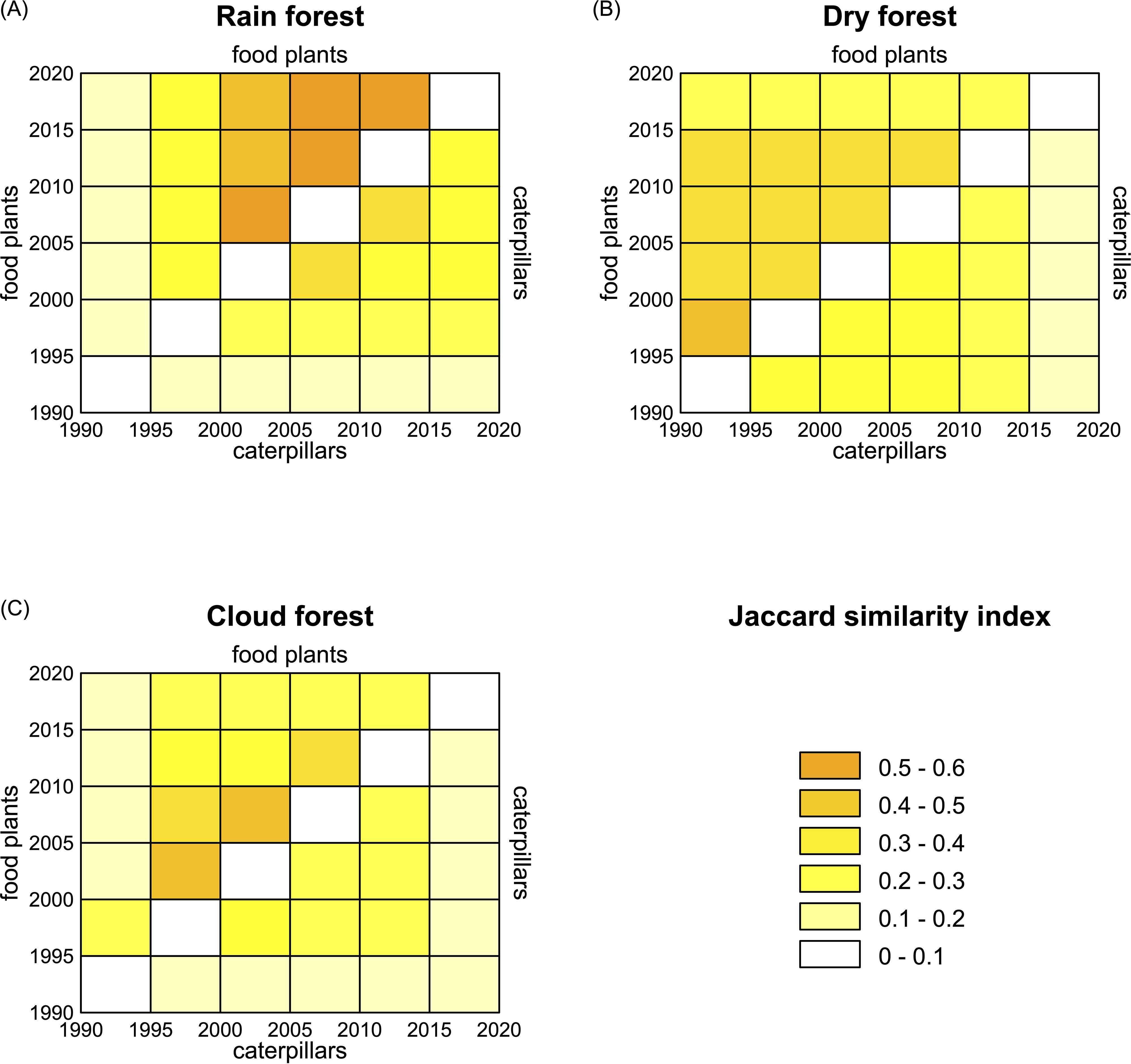
Figure 5. Separate plots for the three ACG ecosystems showing the sampled community similarity of sampled food plants (upper left triangle) and caterpillars (lower right) between 5-year time intervals: (A) rain forest, (B) dry forest, and (C) cloud forest.
Overall, food plant and caterpillar communities in rain forest (Figure 5A) showed the most change over the first 10 years but have remained more stable and consistently higher since the year 2000. However, it is possible that some of the consistency is a result of collecting bias, as parataxonomists grew increasingly familiar with the fauna and where to search for caterpillars and the intensity of collecting effort could not be standardized (in effect, a biased sampling effect). The floral and faunal composition of dry forest was more consistent than in rain forest throughout the sampling period. The cloud forest biota was the most consistent between 1995 and 2010 but, as in dry forest, the caterpillar fauna changed considerably during 2015–2020. In all cases, plant species composition was more constant than caterpillar composition.
In general, the number of caterpillars and food plant species, respectively, were comparatively low in the first 5-year period but subsequently increased, allowing us to more meaningfully calculate several standard food web statistics (connectivity, rescaled connectivity, link density, and generality) (see Bersier et al., 2002; Lewis et al., 2002; Blüthgen et al., 2006; Dormann et al., 2009; Gilbert, 2009; Kerkig et al., 2025), and these are presented in Supplementary Table S2 separately for each ecosystem and 5-year sampling windows. Overall, both generality and linkage density were highest in the rain forest and lowest in cloud forest, However, it should be noted that the reliability of the values might be reduced because of the incompleteness of the samples with many species represented by only a few individuals (singletons and doubletons) (Stireman et al., 2017).
3.4 Herbivory by caterpillar and angiosperm families
When examining family–family interactions, we included only those lepidopteran families with at least 40 distinct trophic interactions (Figure 6) and for food plant families with more than 100 distinct interactions. While the most species-rich lepidopteran families generally attack a similar range of food plant families, some distinct preferences are clear—for example, Pieridae were reared almost exclusively from Fabaceae due to the number of Dismorphinae rearings, with far smaller numbers from Capparaceae, Santalaceae (former Viscaceae clade thereof), and Bignoniaceae. Uraniids (all belonging to the Epipleminae) were predominantly reared from Bignoniaceae and as were many Sphingidae. Both of these plant families have species that produce iridoid glycosides (Nayar and Fraenkel, 1963; Quicke et al., 2024), suggesting that epiplemine uraniids and sphingids have evolved mechanisms to cope with these plants which usually involve suppressing enzymes in the gut that cleave them to release the unstable and toxic aglycone residue (Dobler et al., 2011). In this context, sphingids have been rather well-studied but not the uraniids.
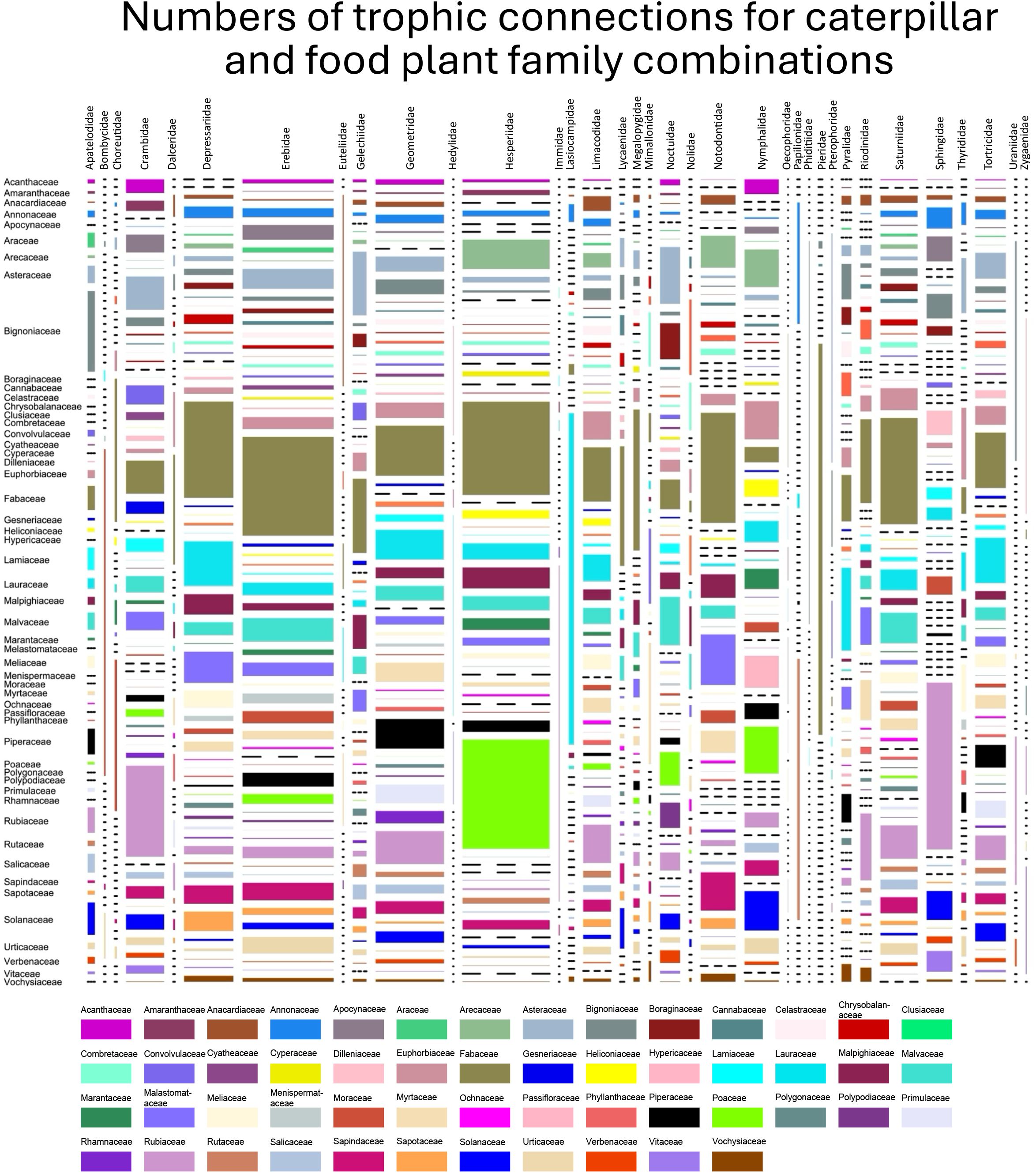
Figure 6. Mosaic plot of food plant preferences (unique caterpillar–food plant interactions) of the Lepidoptera families with >40 different trophic interactions and plant families with >100 interactions. Rectangles are colored according to the plant family key below. The width of each column represents the proportion of unique interactions involving the named Lepidoptera family, and the area of each rectangle is proportional to the proportion of unique interactions involving the combination of the named plant and Lepidoptera families.
Moths of the family Lasiocampidae (lappet moths) were predominantly reared from Lauraceae (870 unique rearings), Phiditiidae almost entirely from Bignoniaceae (n = 184) with a few from Lamiaceae, while more than 99.9% of Attevidae rearings were from Simaroubaceae (n = 265). The database confirmed that out of 13,627 unique Hesperiinae (grass skipper) rearings, not one was from a dicotyledonous plant family and predominantly from Poaceae and Arecaceae (Figure 6). Another conspicuous preference is the large number of trophic links between Sphingidae and Rubiaceae.
We explored the degree of specialization of those caterpillar species that were associated with the 40 plant families for which the data includes more than 1,000 independent rearings. For these taxa, we recorded the diet breadth of their herbivore species across all plant families to identify which plant families had the most specialist herbivores (Table 2). Here and throughout, we use the simple heuristic of the number of plant families (plant family “penetrance”), recognizing that the taxonomic range of any given group of herbivores does not track taxonomic rank.
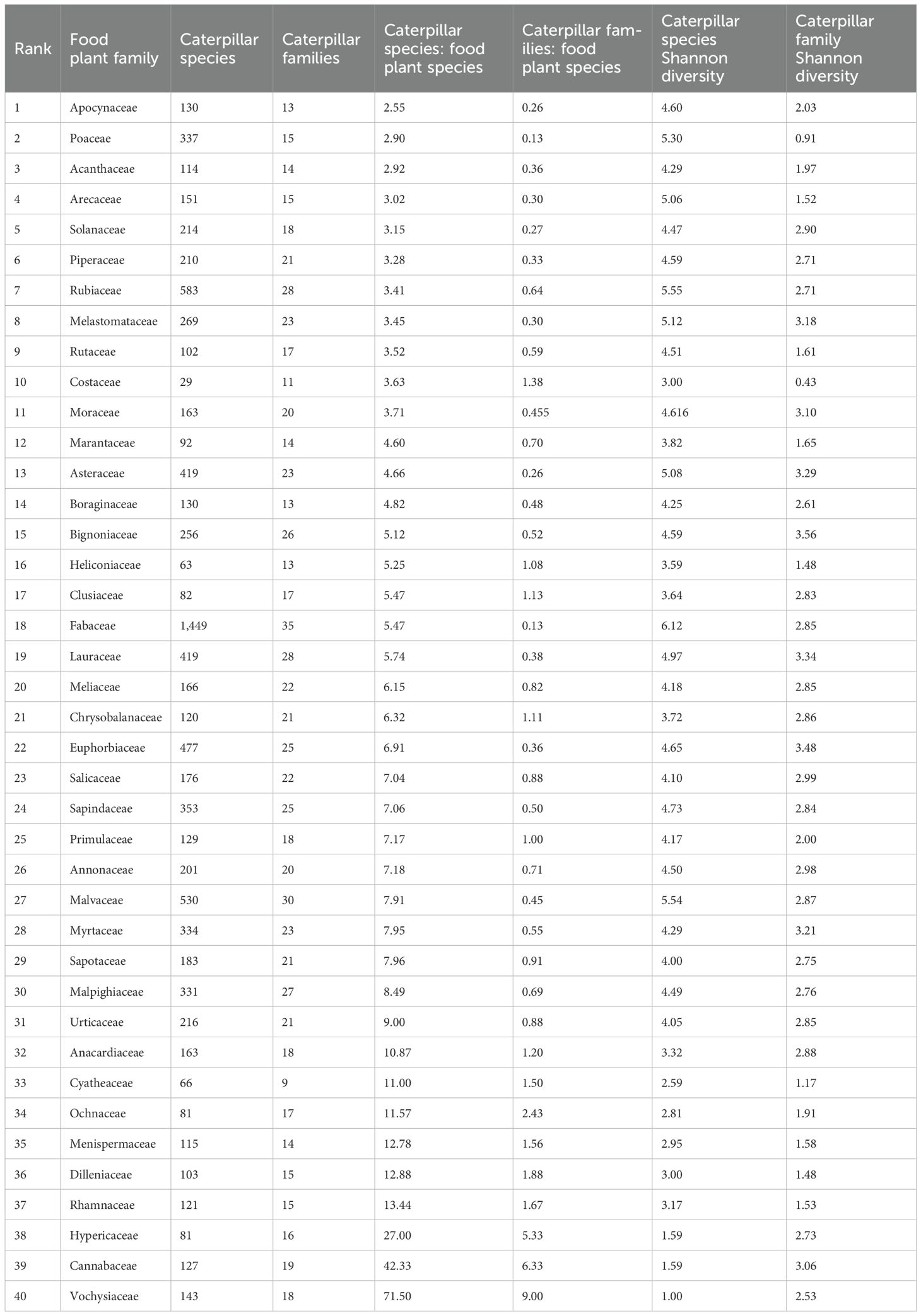
Table 2. Summary of the number of caterpillar taxa for the 40 plant families with more than 1,000 trophic associations in increasing order of the number of caterpillar species per food plant species.
Next we examined the overall recorded diet breadths, again as measured by overall taxonomic penetrance, exhibited by caterpillar species feeding on these families displayed across all of their range of food plant families (Figure 7). Plant families having the most specialist caterpillar species (lower left) are indicated in red, and ones with the most generalists are in green (towards right and top). The twelve families hosting the most specialist caterpillars were, in descending order, Apocynaceae, Poaceae, Acanthaceae, Arecaceae, Solanaceae, Piperaceae, Rubiaceae, Melastomataceae, Rutaceae, Costaceae, Moraceae and Marantaceae, in order of decreasing herbivore specialization.
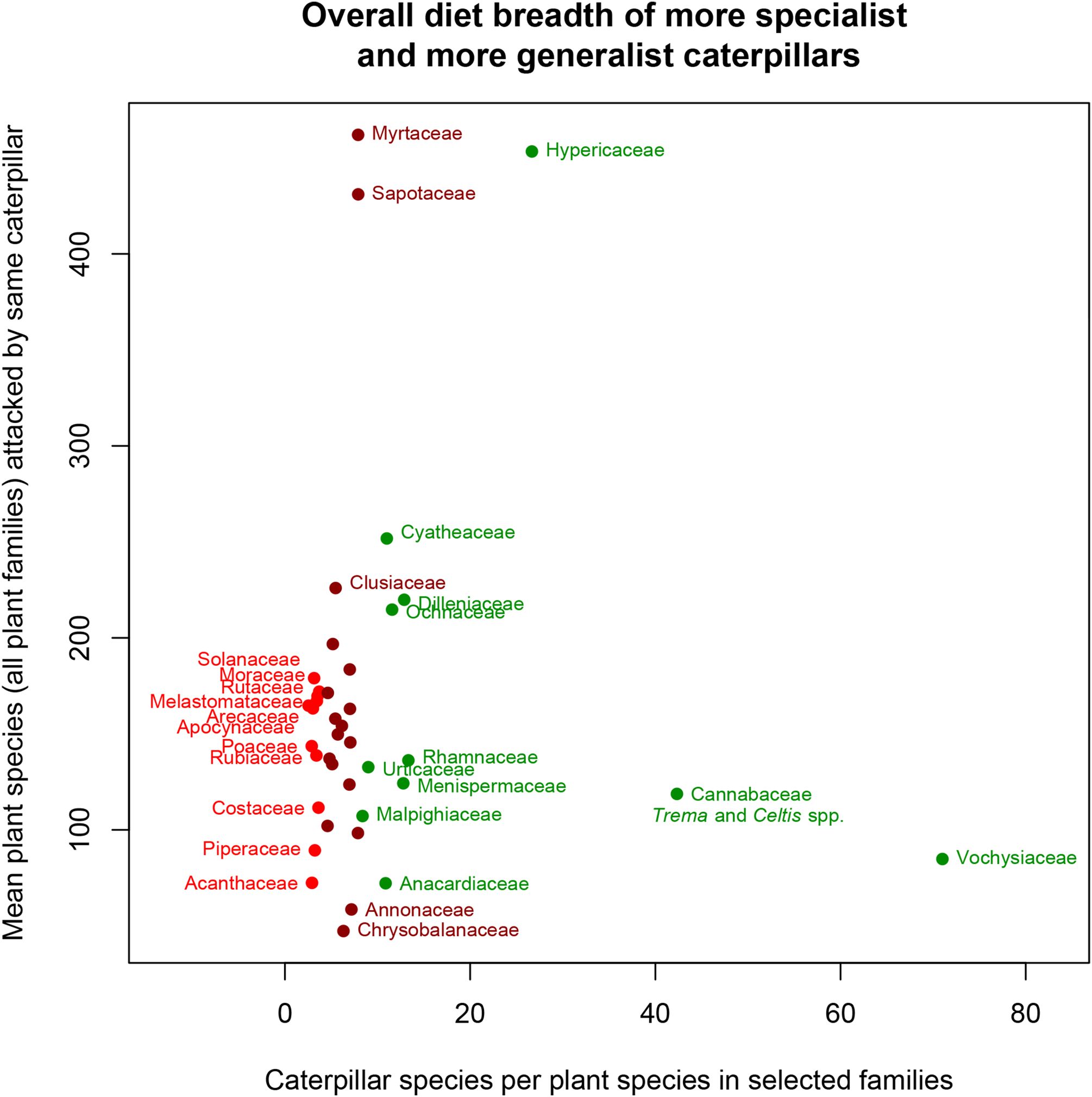
Figure 7. Relationship between mean herbivore load (the total number of caterpillar species recorded for each plant family divided by the number of recorded food plant species in that family; x-axis) and the measure of specialization (the mean number of all plant families recorded as hosts for those same species; y-axis). Only families with more than 2,000 independent rearings are shown. Red indicates families with most specialized caterpillar species, green the least specialized, and maroon intermediately specialized ones. The unlabeled maroon points represent the plant families Marantaceae, Asteraceae, Boraginaceae, Bignoniaceae, Heliconiaceae, Fabaceae, Lauraceae, Meliaceae, Euphorbiaceae, Salicaceae, Sapindaceae, Primulaceae, and Malvaceae.
From the standpoint of botanical understanding, Figure 7 suggests that several plant traits are associated with having trophic associations involving highly specialist caterpillars. Arecaceae, Costaceae, Piperaceae, and Poaceae have particularly tough leaves (sclerophyly) with particularly high silica content, Apocynaceae and Moraceae possess latex which contains various entomotoxic compounds, and Solanaceae and Piperaceae contain many toxic secondary metabolites, notably alkaloids. The picture is less clear as to why Melastomataceae and Rubiaceae are associated with particularly specialist caterpillar species, although both families have diverse secondary metabolites (some used in ethnopharmacology) (Ocampo Serna and Isaza Martínez, 2015; Martins and Nunez, 2015). Some Melastomataceae are aluminum accumulators (Timpone et al., 2025), including Miconia species which are represented in our data set by 42 species (Sittenfeld et al., 2002). Whether the presence of high concentrations of aluminum in their leaves affords them protection against caterpillar herbivory does not seem to have been investigated. However, in the cases of some other plant species, it appears to be a deterrent (Behmer et al., 2005; Ribeiro et al., 2017) although there is no overall consensus about the evolutionary selective value of metal hyperaccumulation in plants (Raskin et al., 1994; Jansen et al., 2002).
Several Lepidoptera are notorious for the broad range of plant families that serve as larval hosts. For example, Gelechiidae (twirler moths), a family with more than 4,000 described species, are documented from more than 80 plant families, with a proclivity for Asteraceae and Fabaceae (Powell, 1980). The 424 species of gelechiids reared in the ACG are recorded from 78 plant families but are represented in the data set only by species with exophytic caterpillars, whereas most gelechiids are leaf miners and stem borers. Although this family is under-sampled in our data set because the majority are leaf miners and stem borers, our data nevertheless reveals a remarkably broad range of host plant families.
3.5 Herbivory on non-angiosperm plant lineages
We analyzed rearing records from three taxonomic cohorts outside Angiospermae: Lycopodiopsida (clubmosses, spikemosses, and quillworts), Polypodiopsida (ferns), and Gymnospermae, the immediate sister group of the angiosperms. Ferns originated at least 423 million years ago (Ma) (Nitta et al., 2022), and gymnosperms diverged from their common ancestor with angiosperms between 337 and 208 Mya (Morris et al., 2018). We treat the cycad family Zamiaceae separately because of its well-known extreme toxicity. Utilization of these plant groups in terms of unique rearings and distinct trophic links are summarized by family in Figure 8. Caterpillars of only two families, Erebidae and Nymphalidae, utilized lycopods at ACG. Ferns, by contrast, are more widely used, with moth larvae of the Erebidae, Noctuidae, Crambidae, and Geometridae as the top four Lepidoptera families feeding on these primitive plants. There were too few rearings from non-cycad gymnosperms to discuss (Cupressaceae (n = 6), Pinaceae (n = 2), Podocarpaceae (n = 2)).
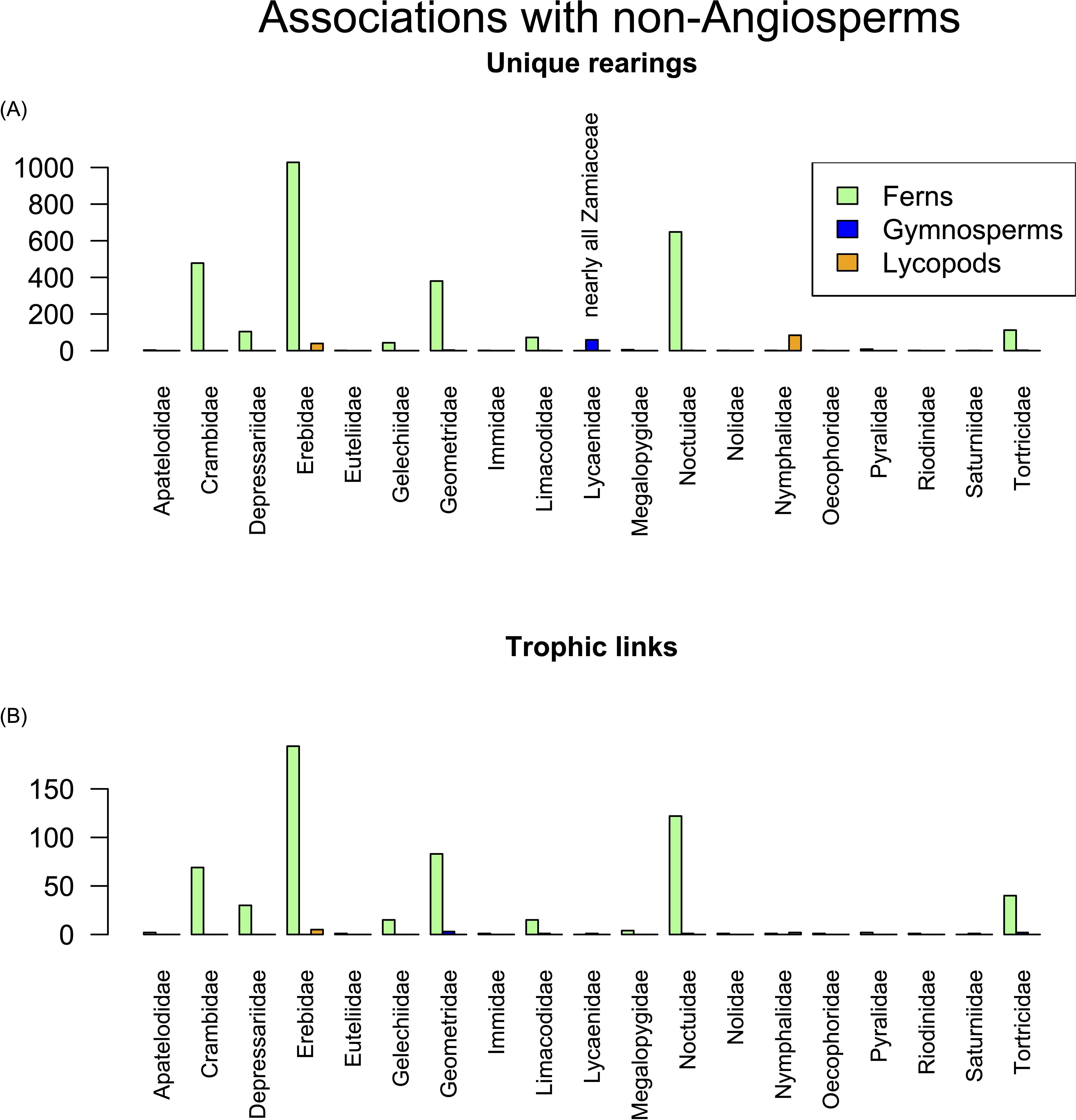
Figure 8. Caterpillar herbivory on ferns, gymnosperms, and lycopods by lepidopteran family: (A) number of unique rearings and (B) unique trophic links.
3.5.1 Lycopods (lycophytes)
The ACG data set includes rearings from includes 123 independent rearings from Selaginella arthritica (Selaginellaceae), collectively representing five species of Erebidae (Hypena, one species; Nicetas, three species; Salia, one species) and two species of the nymphalid genus Euptychia. Both Nicetas and Salia belong to the litter moth subfamily Herminiinae, most members of which feed on dead leaves, bryophytes, and fungi and also includes several genera with fern-feeding species (Goldstein et al., 2021, 2022; Sisson et al., 2025) and a few that feed on palms (Arecaceae).
3.5.2 Ferns
It has often been reported that ferns support a smaller insect herbivore fauna, and the composition of their insect herbivores differs from that of flowering plants (Cooper-Driver, 1978; Hendrix, 1980; Marquis, 2024). The ACG dataset includes rearings from 99 species, collectively representing 16 families of ferns of which 12 fall within the order Polypodiales (i.e., the families Aspleniaceae, Blechnaceae, Dennstaedtiaceae, Dryopteridaceae, Lomariopsidaceae, Nephrolepidaceae, Parkeriaceae, Polypodiaceae, Pteridaceae, Tectariaceae, Thelypteridaceae, and Woodsiaceae); the others each represent separate orders of ferns: Cyatheaceae (Cyatheales), Gleicheniaceae (Gleicheniales), Hymenophylaceae (Hymenophyllales), and Schizeaceae (Schizeales).
The total of 600 trophic links between ferns and caterpillars collectively represent 17 families of Lepidoptera. However, there is only one rearing record for six of these families, and only between three and eight for specific groups of Pyralidae, Megalopygidae, and Apatelodidae. Those associations detected more than 10 times involved only eight caterpillar families, and the number of trophic links and unique rearing events are given in Table 3, the family represented by most species and interactions being Erebidae. This differs somewhat from the global summary presented by Fuentes-Jacques et al. (2022) who found Lepidoptera families with the largest number of reported interactions including Noctuidae, Pyralidae, Geometridae, Tortricidae, Crambidae, and Stathmopodidae in descending order.
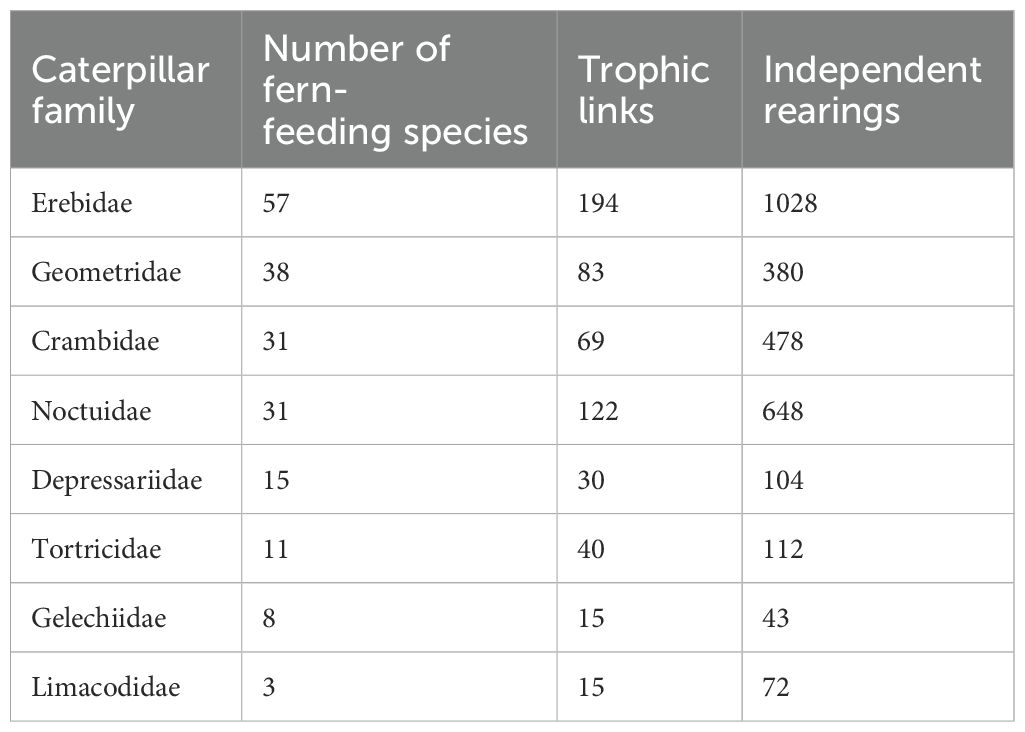
Table 3. Details on the number of associations and unique rearings from ferns for those caterpillar families having more than 10 trophic links with ferns.
Regarding Limacodidae, which include many species polyphagous on angiosperms, only three were reared from ferns: Epiperola vaferella (57 rearings from two species of Cyatheaceae, two from one species of Lomariopsidaceae, and one from Thelypteridaceae), Euclea bidiscalis (11 rearings across six fern families), and Natada miniscula (a single rearing). Thus, Epiperola vaferella appears largely to be a fern specialist. However, E. vaferella has also been reared from 24 angiosperm families, including nine families of monocots as well as Fabaceae, Malvaceae, Primulaceae, Rubiaceae, and Solanaceae. Interestingly, leaves of ferns, as well as of many monocots (see Section 3.8.2), are defended by having a high silica content (Trembath-Reichert et al., 2015), perhaps indicating that caterpillars of this moth have evolved specific mechanisms for overcoming this.
Although pteridivory has been documented in all herbivorous insect orders, it appears to be conserved within specific groups (Hendrix, 1980; Fuentes-Jacques et al., 2022). Moreover, most fern-feeders tend to specialize on leptosporangiate ferns, which include most of the fern families, and rarely specific fern families. As noted by Goldstein et al. (2021), ferns arose long before herbivorous insects; hence, their defensive chemistry was more likely driven by vertebrate herbivores, and thus ferns have been colonized by insects able to overcome those defenses through sequestration or detoxification. Powell (1980) concluded that while a few primitive Lepidoptera are associated with primitive plants (bryophytes, gymnosperms), the more species-rich, advanced superfamilies of both non-ditrysian and ditrysian Lepidoptera feed primarily on angiosperms, i.e., the largest radiations of herbivorous insects tracked that of angiosperms. No superfamily or higher taxon of Glossata is primarily or even primitively associated with non-flowering plants, though there are many secondary transfers to such hosts by unrelated species and genera (e.g., Goldstein et al., 2018, 2021; Brown, 2018).
3.5.3 Zamiaceae—Cycads
Zamiaceae was represented by a single species in our data set, Zamia neurophyllidia, which was only attacked by two species of Lepidoptera, the specialist cycad-feeding aposematic lycaenid butterfly Eumaeus godartii (white-tipped cycadian) (60 independent rearings) (Robbins et al., 2021) and a single rearing of the polyphagous limacodid moth, Parasa sandrae, which was also reared from 87 other food plant species in 37 other families. Cycads produce the carcinogenic and highly neurotoxic glucoside cycasin (methylazoxymethanol-β-D-glucoside) as well as a range of other toxins such as flavonoids, biflavonoids, phenolic acids, methylazoxymethanol (MAM) glycosides, and β-methylamino-L-alanine (BMAA) (Dossaji et al., 1973, 1975; El-Seadawy et al., 2023; Whitaker et al., 2023). Eumaeus species sequester cycasin and other secondary plant compounds from their cycad food plants, and the evolution of their toxin tolerance has been investigated using genomics (Robbins et al., 2021).
3.5.4 Non-cycad gymnosperms
While there are 225 New World species of non-cycad gymnosperms (Brown, 2018), they are extremely poorly represented in the ACG flora. Two of the four species present, Hesperocyparis lusitanica and Pinus caribaea, although New World in origin, are introduced to ACG. In total, only seven species of Lepidoptera were reared from non-cycad gymnosperms, each on only a single occasion. Pinaceae, Cupressaceae, and Podocarpaceae all serve as larval hosts to many specialist and generalist lepidopterans. In the New World alone, nearly 800 species of Lepidoptera are reported to feed on conifers, with about 500 specialists (i.e., restricted or nearly so to a single conifer family) and about 300 generalists (i.e., feeding on both conifers and angiosperms). Moths of the family Tortricidae include the greatest number of conifer feeders, followed by Geometridae and Noctuoidea. Whereas the majority of species of macrolepidoptera (e.g., Lasiocampoidea, Bombycoidea, Geometroidea, Noctuoidea) feeding on conifers are generalist herbivores that also utilize a variety of plant families (angiosperms and conifers), most microlepidopterans (e.g., species in Gracillarioidea, Yponomeutoidea, Gelechioidea, Pyraloidea) that feed on conifers are restricted to these plants (Brown, 2018). Although gymnosperms are food plants of some basal “microlepidoptera” (New, 2023), all the non-ditrysian and ditrysian Lepidoptera are principally herbivores of angiosperms, and none of them is primarily associated with gymnosperms (Powell, 1980; Powell et al., 1998).
3.6 Diets of selected groups of aposematic Lepidoptera
The data included a substantial number of species belonging to well-known, chemically defended Lepidoptera, such as the nymphalid tribe Heliconiini (including the Heliconius butterflies), Ithomiini (the glass wings), and Danaiini (including the milkweed butterflies) and the subfamilies Melitaeinae (Nymphalidae), Pierinae (Pieridae), Arctiinae (Erebidae), and the family Zygaenidae. We summarize these below.
3.6.1 Heliconiini (Nymphalidae)
The Heliconiini are represented by 29 species in seven genera (Agraulis, Dione, Dryadula, Dryas, Eueides, Heliconius, and Philaethria). All rearings are from Passiflora species, and the majority were oligophagous, with the maximum of 10 for Dryas moderata. Conversely, more than 97% (n = 503) independent caterpillar rearings from Passifloraceae were heliconiines. The only non-Heliconiini nymphalid genus reared from Passifloraceae was Euptoieta (i.e., E. meridiana). In addition, the data set includes only 16 rearings of caterpillars in other families (i.e., Crambidae, Depressariidae, Geometridae, Limacodidae, Noctuidae, Notodontidae, Pyralidae, Riodinidae, and Tortricidae), and some of these may represent incorrect larval identifications. We estimate the error rate at less than 1%.
3.6.2 Ithomiini (Nymphalidae)
A total of 15 genera of Ithomiini was reared: Callithomia, Ceratinia, Dircenna, Godyris, Greta, Hypoleria, Hyposcada, Hypothyris, Ithomia, Melinaea, Napeogenes, Oleria, Pseudoscada, Pteronymia, and Tithorea. Almost all rearings of this tribe were from Solanaceae, but Hyposcada was reared only from Gesneriaceae, and Tithorea from Apocynaceae.
3.6.3 Danaiini (Nymphalidae)
Danaini was represented by two genera: Danaus and Lycorea. All rearings of Danaus were from Apocynaceae (Asclepiadoideae), whereas Lycorea was additionally reared frequently from other Apocynaceae (i.e., Gonolobus, Macroscepis, Mandevilla, Prestonia), as well as Moraceae, Caricaceae (both the introduced papaya, Carica papaya, and endemic Jacartia spp.) and once also from Euphorbiaceae (Sebastiania pavoniana). All of these food plants possess potentially toxic latex and drip it copiously when ruptured. Some danaiines famously sequester protective cardiac glycosides (cardenolides) from their larval food plants, but females often creatures often select the individual food plants on the basis of them having low or no cardenolide content (Smith, 2014). Interestingly, males of some Danaus species actively forage for sources of pyrrolizidine alkaloids (PAs) which are sex pheromone precursors and, during copulation, pass the PAs to the female individual who thereby becomes toxic and subsequently passes the PAs to their eggs (and emerged larvae) (Smith, 2014).
3.6.4 Melitaeinae (Nymphalidae)
Melitaeines (checkerspots) include several well-studied toxic species. This subfamily was represented by 20 species, mostly of Chlosyne with a few records from Anthanassa (two spp.), Castilia (one sp.), Eresia (two spp.), Microtia (one sp.), and Tegosa (one sp.). Chlosyne species were mostly reared from Acanthaceae, but Chlosyne lacinia (discovered to comprise two cryptic species) is a well-known specialist on Asteraceae (Martucci and Gobbo-Neto, 2016; Gallon et al., 2019). In Europe and North America, several species have been studied extensively because of their ability to sequester iridoid glycosides from their larval food plants, notably Plantaginaceae (Wahlberg, 2001; Quicke et al., 2023). However, not all members of the subfamily are toxic, and in Costa Rica, Young (1973) suggested that our two species of Eresia are Batesian mimics of an ithomiine.
3.6.5 Arctiinae (Erebidae)
The data set includes 81 genera of Arctiinae which includes the former Arctiidae (tiger moths) and Ctenuchidae, representing 272 species (Table 4). Many species are highly polyphagous and most studies have focused on these. Here we limit our discussion to those genera that were reared on more than 40 independent occasions and those species that feed on more than 10 food plant species. The six most polyphagous genera represent three different subtribes, implying that generalists are phylogenetically widely distributed within the subfamily. Even so, species of most of the genera have a restricted diet, with more than one-half of the genera feeding on fewer than five plant families and more than two-thirds feeding on fewer than two food plant families per species. Of the 28 genera whose species were represented by more than 50 independent rearings, 12 were only ever associated with a single food plant order (Figure 9). However, 10 genera were reared from members of more than 10 orders, viz., Melese (26), Bertholdia (16), Pelochyta (19), Carales (18), Halysidota (11), Lophocampa (13), Hypercompe (24), Dysschema (14), Cosmosoma (11), and Gymnelia (13) (the number of food plant orders is given inside the parentheses).
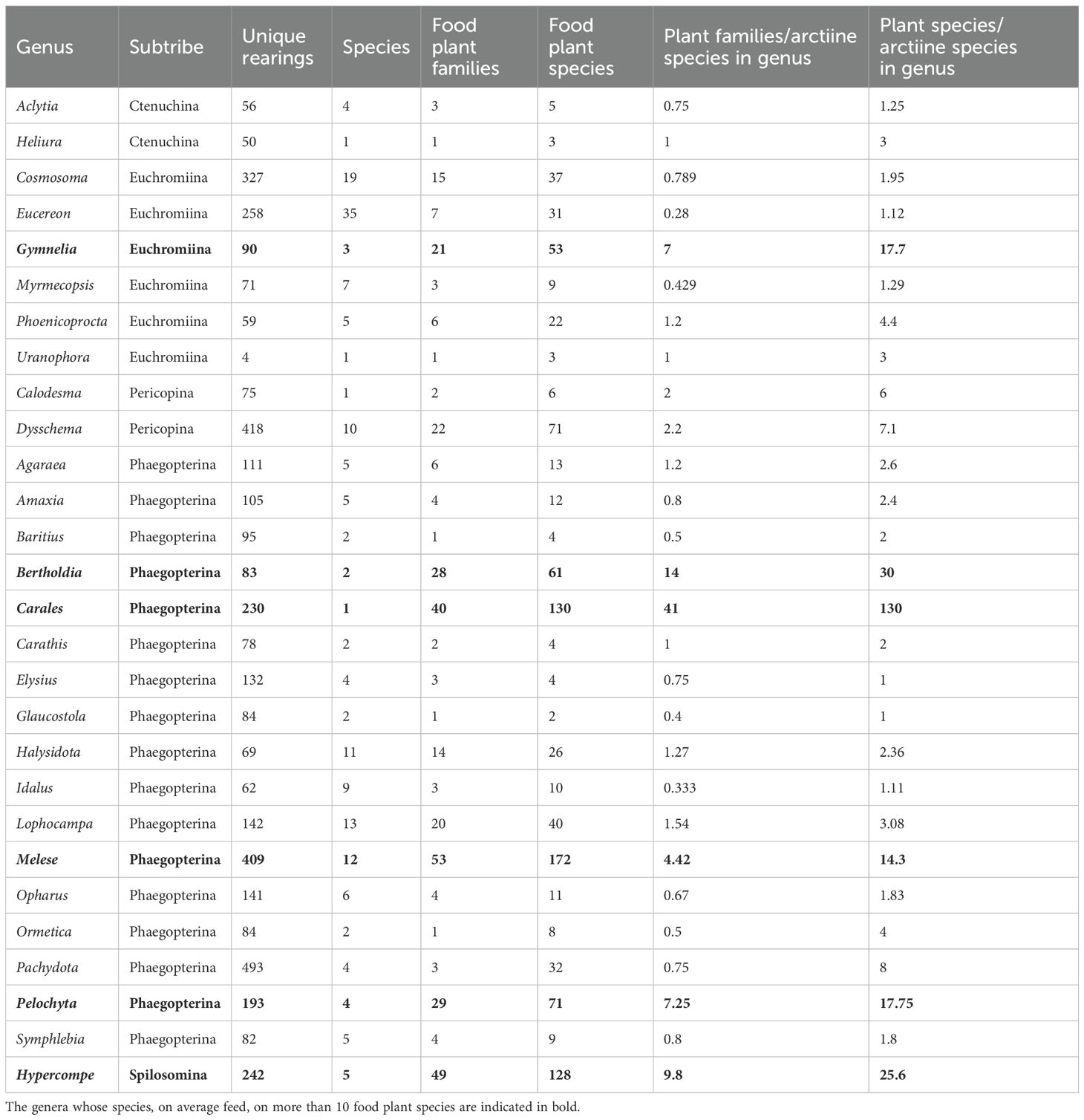
Table 4. Trophic interaction summary for Arctiinae (Erebidae) genera represented by 40 or more independent rearings.
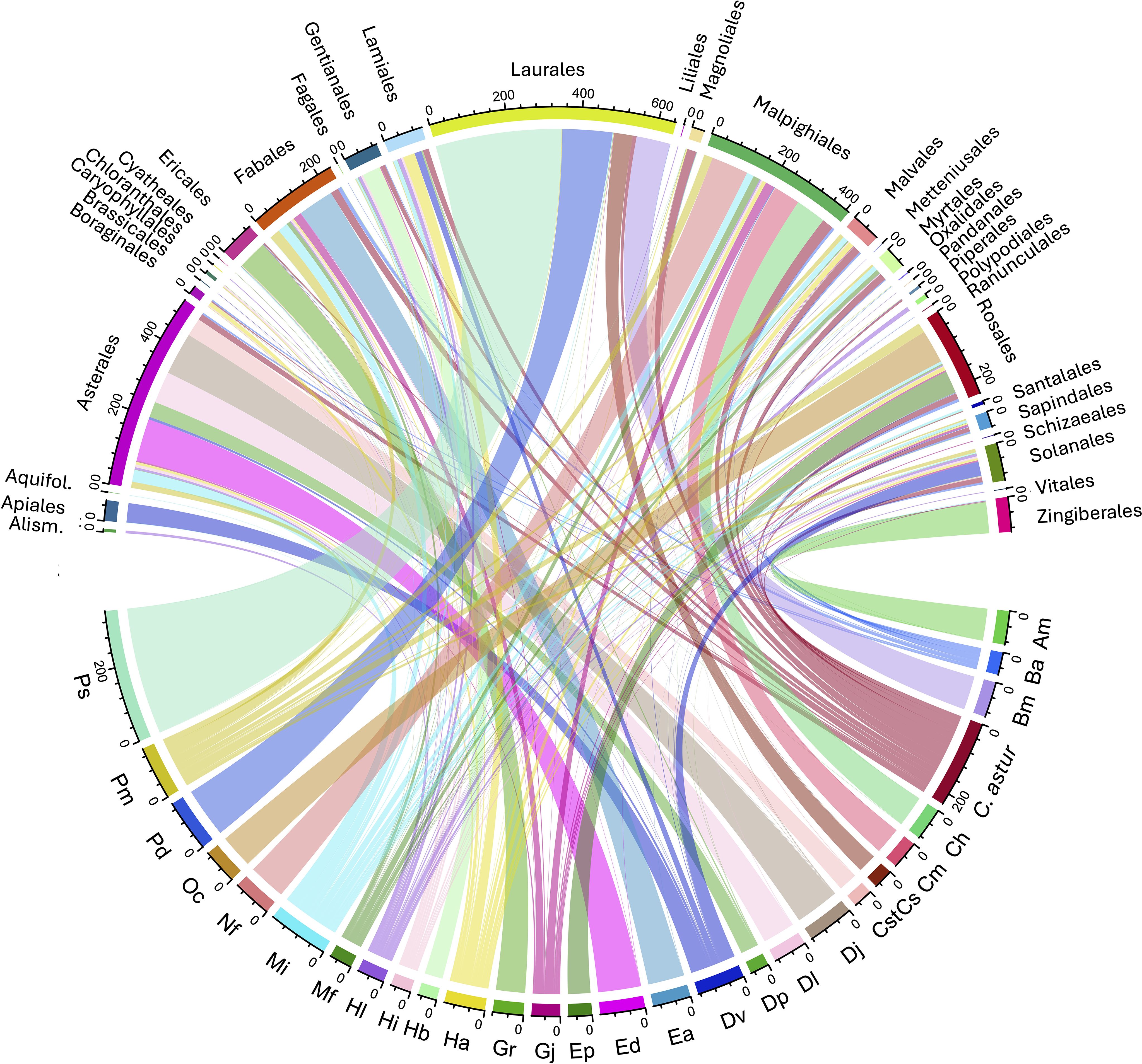
Figure 9. Bipartite network diagram for the species of Arctiinae represented by 50 or more independent rearings and the APG IV order of the food plants they consume where the thickness of the links represents the abundance of that taxon in the reduced matrix and the line width represents the frequency of each interaction. Am, Agaraea minuta; Bi, Bertholdi albipuncta, Bm, Baritius maribellealvarezae; Ch, Cosmosoma hercyna; Cm, Calodesma maculifrons; Cs, Carathis septentrionalisDHJ0; Cst, Cosmosoma stibosticta; Dj, Dysschema jansonis; Dl, Dysschema leucophaea; Dp, Dysschema panamensis; Dv, Dysschema viuda; Ea, Eucereon, aeolum; Ed, Elysius discoplagaDHJ01; Ep, Eucereon pseudarchias; Gj, Gymnelia jansonis; Gr, Glaucostola romula”; Ha, Hypercompe, albescens; Hb, Heliura banoca; Hi, Hypercompe icasiaDHJ0; Hl, Hypercompe laeta; Mf, Melese flavimaculataDHJ02; Mi, Melese incertus; Nf, Napata flaviceps; Oc, Opharus consimilis; Pd, Pachydota drucei; Pm, Pelochyta misera; Ps, Pachydota saduca.
The arctiine moth genus Carales, represented by the single species, C. astur, is particularly noteworthy as it was reared from 130 different plant species distributed across 41 families and 18 APG plant orders (Figure 9). These plants are broadly distributed across the major angiosperm branches including Magnoliidae, superasterids, and superrosids and with one record from the monocot family Marantaceae, although since this particular caterpillar was in its final instar when found, we cannot be absolutely certain that it fed on this plant.
The bipartite network of Erebidae species and host plants had a high network-wide estimate of specialisation, (H2’ = 0.894) where values close to zero indicate extreme generalization and those closer to 1 indicate specialisation (Blüthgen et al., 2006). However, when Arctiinae alone were considered, H2’ = 0.656, indicating that overall their diets are markedly more generalised.
Furthermore, despite some arctiines having long lists of food plant species, some appear to be highly specialized, for example, Pachydota drucei and P. saduca were reared only from Lauraceae (n = 137 and 347 respectively), and Opharus consimilis only from Urticaceae (n = 97). In some cases, ecological constraints are also apparent. For example, Hypercompe albescens and Hypercompe “icasiaDHJ01”, both have very long lists of food plant species (48 and 41, respectively), but those of the former feed on herbaceous plants or seedlings, whereas those of the latter feed on woody species.
3.6.6 Pieridae
Pieridae in our dataset are dominated by Dismorphinae, which were all reared from Fabaceae, nearly all from Inga spp. with and a few from Cojoba (Table 5). Braby and Trueman (2006) concluded that Fabaceae is likely the ancestral food plant group of the family, with members of the Pierinae mostly feeding on Brassicales, here represented by Brassicaceae, Capparaceae, Caricaceae, Dichapetalaceae and Resediaceae (including Stixaceae), but with the pierine genus Glutophrissa also reared from Putranjivaceae, and Melete from the ‘mistletoes’ (Loranthaceae and former Viscaceae clade of Santalaceae; see Haston et al., 2009; The Angiosperm Phylogeny Group et al., 2016). Members of the Coliadinae are also associated primarily with Fabaceae, but with some species feeding on a few other plant families. Notably, Zerene cesonia, which ranges from southern Canada to Costa Rica, feeds on the same Fabaceae genus (i.e., Dalea) throughout its range.
3.6.7 Zygaenidae
The data set includes 161 independent rearings of this family, and only six of the 29 species were reared on more than five separate occasions, limiting inferences concerning their host range. Only one species fed on more than one plant family: “Pampa Janzen04” was recorded from (Dilleniaceae) on 63 occasions, and from Sarcopera sessiliflora (Marcgraviaceae) on three occasions. However, this was by far the most commonly reared species of zyganeid with a caterpillar so distinctive that there is no ambiguity regarding its correct identification.
3.7 Herbivory on chemically defended angiosperms
Plant chemical defenses against insect herbivory include a broad range of toxic secondary metabolites such as alkaloids (Willaman and Schubert, 1961), glycosides (including cyanogenic glycosides which are then converted to cyanide) (Vetter, 2000; Park and Coats, 2002), terpenoids (Mumm et al., 2008; Kortbeek et al., 2019), glucosinolates and many others (Bennett and Wallsgrove, 1994). In addition to these smaller molecules, there are several entomotoxic proteins, e.g., sugar-binding lectins in both seeds and foliage production of that can be induced by herbivory (Van Damme et al., 2008). These molecules are attracting greater attention by virtue of our growing capacity to incorporate them into crops via genetic engineering (Carlini and Grossi-de-Sá, 2002; Grossi-de-Sá et al., 2015). Other secondary metabolites can still act as feeding deterrents even though they are less toxic (Simmonds, 2006) and some may have both insecticidal and antifeedant properties (e.g. Prota et al., 2014; War et al., 2020). The Apocynaceae, Rutaceae, Piperaceae, Solanaceae and many Rubiaceae are widely noted for their possession of toxic secondary plant compounds including various alkaloids, and in the case of the first of these, toxin-containing latex.
3.7.1 Cyanogenic food plants
The toxicity of cyanide is well known, and plants, in many families are capable of synthesizing precursors (e.g., the cyanogenic glycosides linemarin and lotustralin) that release hydrocyanic acid when the plant tissue is damaged or consumed. That this ability is an effective defense against herbivory is well established (Boter and Diaz, 2023).
Cyanogenesis is extremely widespread among plants having been documented in members of more than 100 plant families (Francisco and Pinotti, 2000; Vetter, 2000). Thomsen and Brimer (1997) surveyed 488 species in 79 families of woody plants in lowland rain forest in Costa Rica for cyanogenesis and found it present in 25 species collectively belonging to 16 families. Cyanogenesis positive species were identified in the families Achariaceae (as Flacourtiaceae), Annonaceae, Bignoniaceae, Elaeocarpaceae, Euphorbiaceae, Fabaceae, Lamiaceae, Malpighiaceae, Olacaceae, Passifloraceae, Proteaceae, Rubiaceae, Sapindaceae, Sapotaceae, and Violaceae.
The Passifloraceae were represented by 21 species of Passiflora, two species of Turnera, one of Erblichia plus two unidentified species. Their association with Heliconius butterflies has been studied extensively as a model system for the study of mimicry (Gilbert, 1971; Turner, 1971; Brown and Benson, 1974) and was one of the first examples put forth in support of the theory of coevolution (Ehrlich and Raven, 1964; Futuyma, 1983; de Castro et al., 2018). Passifloraceae are protected by cyanogenic glycosides, pyrrolizidine alkaloids, flavonoids, and saponins (de Castro et al., 2018) as well as by dense trichomes which appear to be particularly effective against caterpillars, though Heliconius have succeeded in overcoming these (Gilbert, 1971).
Zygaenidae (burnet and forester moths) are probably the best known for their own cyanogenic repertoire and are frequently associated with cyanogenic food plants (Nahrstedt, 1998; Zagrobelny et al., 2008). All zygaenids reared in the ACG belong to the Procridinae, which comprises a group of moths with cyanogenic caterpillars that are restricted to eight food plant families. The independent rearing data were as follows: Dilleniaceae (101 species), Marcgraviaceae (20), Vitaceae (24), Rubiaceae (17), Polygonaceae (8), Rosaceae (2), Fabaceae (1), and Urticaceae (1). Insofar as we know, cyanogenesis has never been detected in any member of the Dilleniaceae, Marcgraviaceae or Urticaceae. The single unique rearing from Fabaceae was based on many caterpillars of Neoilliberis thyesta (Zygaenidae) feeding on Mimosa tricephala. We do not know whether this food plant displays cyanogenesis, although its congener M. pudica contains cyanogenic glycosides (Ahuchaogu et al., 2017), but they do not appear to have been reported from numerous other members of the genus (Rizwan et al., 2022).
In addition to Zygaenidae, cyanogenesis and/or cyanogenic glycosides and/or the cyanide detoxification product (β-cyano-L-alanine, BCA) have also been detected in a wide range of other families that were reared in this study, viz. Limacodidae, Megalopygidae, Notodontidae, Geometridae, Hesperidae, Papilionidae, Erebidae (Arctiinae and Lymantriinae), Nymphalidae (at least some species of all tested tribes but abundant in most Heliconiina, many Nymphalina and Satyrinae) (Witthohn and Naumann, 1987; Zagrobelny et al., 2008), strongly suggesting that at least some of these are able to feed on cyanogenic plants.
3.7.2 Entomotoxic plant proteins
These proteins include lectins and hemilectins (carbohydrate-binding proteins) (Janzen, 1981; Carlini and Grossi-de-Sá, 2002; Van Damme et al., 2008; Grossi-de-Sá et al., 2015; Vandenborre et al., 2011), various enzymes which include ribosome-inactivating proteins, ureases and urease-derived encrypted peptides (Stanisçuaski and Carlini, 2012), chitinases (Bishop et al., 2000; Ramos et al., 2010), inhibitors of insect digestive enzymes (e.g., Wu and Haard, 2000), and other proteases/peptidases/proteinases whose entomotoxicity seems to result from their disruption of the insect herbivore’s peritrophic membrane (Konno et al., 2004; Domsalla and Melzig, 2008; Harrison and Bonning, 2010).
Ureases are large molecular weight, nickel-containing proteins which occur in some Fabaceae. Their entomotoxicity is particularly interesting as it does not depend on their enzymic activity (urea ⟶ CO2 + NH3) but instead the intact protein is neurotoxic, as are the peptide(s) produced as a result of insect protease action on it (Stanisçuaski and Carlini, 2012; Carlini and Ligabue-Braun, 2016).
The best-studied lectins occur in members of the plant families Amaranthaceae, Amarylidaceae, Araceae, Asteraceae, Euphorbiaceae, Fabaceae, Poaceae, and Solanaceae (Grossi-de-Sá et al., 2015; Macedo et al., 2015). Although they are usually produced and stored in seeds and storage organs such as rhizomes, they are also frequently expressed in leaves and their synthesis can be induced by jasmonic acid, a crucial plant hormone that is released in response to caterpillar herbivory (Chen et al., 2002; Vandenborre et al., 2011). Unfortunately, too little is known about the taxonomic distribution and prevalence of inducible lectins in plants for us to make firm conclusions as to their relationship with and to various lepidopteran caterpillars in terms of defense.
3.7.3 Tannins
Tannins are a chemically diverse group of polyphenolic compounds which bind to and precipitate proteins. They are ubiquitous secondary plant compounds often occurring at high concentrations, for example 5-10% dry weight, in the leaves of many woody dicotyledons, i.e., most trees, especially in the families Anacardiaceae, Combretaceae, Fabaceae, Fagaceae and Rhizophoraceae. Several studies have found evidence that high condensed tannin concentration may provide protection against caterpillar herbivory, and may differentially affect specialists more than generalists (Forkner et al., 2004), and some generalist caterpillar species have a high tannin tolerance (Barbehenn et al., 2009).
It has often been suggested that their entomotoxicity is due to complex negative effects on protein metabolism in insects feeding upon them, resulting in slower growth, lowering survival and probably also reducing the overall amount of food they consume (Yang et al., 2016; Adamczyk et al., 2017). However, this appears not to be the case (Fox and Macauley, 1977; Bernays, 1978) with damage reflecting the production of high levels of reactive oxygen species, such as semiquinone radicals (Barbehenn and Constabel, 2011). In addition to direct toxicity, tannins are also feeding deterrents to many herbivorous insects. However, because high tannin concentrations are generally less prevalent in herbaceous plant foliage and young tree leaves, we do not consider them to be a major driver in the caterpillar herbivory of the foliage sampled in this study.
Of particular interest with respect to tannins are the giant silk moths, Saturniidae. These moths are relatively omnivorous and many species feed on mature, tannin-rich leaves of woody plants. Janzen (2003) described the diet breadth of saturniid caterpillars in the ACG dry forest. At that time, 31 species of Saturniidae had been reared from the foliage of 77% of the 66 woody dicot families, 51% of the 240 woody dicot genera, and 47% of the 370 woody dicot species present. In the present data set, there are 64 species of saturniids reared from the dry forest, and these collectively have been reared from 269 food plant species in 58 families. This is an average of 13.9 food plants per moth species. However, there is a single species of dry forest saturniid caterpillar, Schausiella santarosensis, that massively (in irregular years) defoliates only a single species, Hymenaea courbaril (Fabaceae).
The life history of one hemileucine saturniid, Hylesia lineata, was described by Janzen (1984a). At that time, its caterpillars had been reared from 46 plant species in 17 families. The present data set expands this list to 86 plant species in 20 families, nearly all not typically regarded as chemically protected, the notable exception being 143 rearings from Euphorbiaceae. However, H. lineata caterpillars are gregarious, and these only represented 15 independent collection events, which involved one feeding on Acalypha diversifolia, and the rest feeding on various species of Croton (Fabaceae). Croton species contain di-, tri-, and tetraterpenoids, various alkaloids such as aporphine, morphinandienones, proaporphine, tetrahydroprotoberberines, flavonoids, and glycosides (Magwilu et al., 2022), though it is likely that their diverse diterpenoids are most important (Xu et al., 2018). Their essential oils may also have insecticidal properties (Lawal et al., 2017), and limonoids, musidunin, and musiduol act as insect antifeedants (Nihei et al., 2006). As noted by Janzen (1984a), despite its potential for generalism, H. lineata females oviposit only on a few plant species in non-outbreak years. Furthermore, while a female may oviposit hundreds of eggs in a cluster on a single plant (of many plant species in outbreak years), in the fourth and fifth instars the caterpillars wander widely as individuals, being able to complete development on many species of food plant.
Bernays and Janzen (1988) compared the way that two saturniid species (Orhorene purpurascens and Rothschildia lebeau) and two sphingid species (Pachylia ficus and Manduca dilucida) processed their food plants. In general, as mentioned before, Saturniidae snip their leaf bites, leaving the tannin vacuoles undamaged, thus avoiding tannin’s negative effects but thereby reducing the amount of nutrients that they obtain from the leaf. In contrast, Sphingidae grind the leaf much more finely with their mandibles and therefore have to cope with the secondary toxins in the food but grow more rapidly because of their increased nutrient extraction.
3.7.4 Plant families with other defensive compounds
Unlike some plant families that are famously chemically defended (Quicke et al., 2023), there are many that are seldom noted as being particularly entomotoxic. Nevertheless, plants in such families produce a diverse array of secondary compounds and many of them have been exploited in folk medicine (Kripasana and Xavier, 2020; Rattan, 2023; Braz et al., 2024). Their active compounds include phytosteroids 1,3,5(10)-estratrien-3,17β-diol, phytol, and a few contain alkaloids (Agriculture Research Service, 1961). The diterpene long-chain unsaturated alcohol phytol, in its free form, is produced by many plants and may have some insecticidal and feeding deterrent effects (Vencl and Morton, 1998; Benelli et al., 2020) as well as a wide range of other physiological activities. Here we discuss three plant families noted for their defensive chemistry; Zamiaceae were already discussed in Section 3.5.3.
3.7.4.1 Aristolochiaceae (birthworts)
Birthworts, so named because of their historic use in inducing abortions, produce aristolochic acid, a carcinogenic and nephrotoxic tetracyclic compound. It is also very entomotoxic and in ACG only 12 caterpillar species were reared from this family, 11 of which are troidine Papilionidae (represented by the genera Battus and Parides). The tribe Troidini includes the Old Word birdwing butterflies, all of which are Aristolochiaceae specialists (Quicke et al., 2023). In addition, one species of Pyralidae, Mapeta xanthomelas, was also reared, and this too is an Aristolochiaceae specialist. Caterpillars of M. xanthomelas sequester aristolochic acid (Durán et al., 2012) and its adults are highly aposematic.
3.7.4.2 Piperaceae
The black pepper family, Piperaceae, is represented by three genera in ACG: Manekia (one species), Peperomia (five species), and Piper (57 species). They are well known for their large range of secondary plant compounds, particularly alkaloids (Kato and Furlan, 2007; Scott et al., 2008; Gutierrez et al., 2013). These alkaloids include the particularly neurotoxic piperamide family of chemicals which display marked entomotoxicity (Ileke et al., 2020). More than 30 different piperamides have been extracted from one species (Piper nigrum) alone.
Bodner et al. (2012) found a wide variation in the number of species of caterpillars feeding on shrubs (Asteraceae and Piperaceae (Piper)) in the Ecuadorian Andes, ranging from 2 to 96. Piper species supported fewer caterpillars than Asteraceae, with one or two species of the genus Eois (Geometridae) being dominant. They also found that the one species of Asteraceae that possesses latex had a smaller caterpillar assemblage with higher dominance of a few species than the other two Asteraceae species.
3.7.4.3 Solanaceae
The nightshade family contains many species with a history of causing human and livestock deaths and psychoses. Many species are protected by pyrrolizidine alkaloids (PAs) which are also produced by various members of Apocynaceae, Fabaceae, Heliotropiaceae, and Boraginaceae, among others.
Most Ithomiini (Nymphalidae) are specialists on Solanaceae, although a few basal genera feed on other plant families including Apocynaceae. Most ithomiines are protected by PAs, but their caterpillars do not sequester them from their food plants. Instead, the adult males obtain them by foraging on various PA containing flowers, notably in the Asteraceae, Boraginaceae and Heliotropiaceae (Brown, 1984), and this ability is associated with a particular structure of the antennal lobe of the brain (Morris et al., 2021). However, the caterpillars of a few basal ithomiines, such as Tithorea harmonia, do sequester (Trigo and Brown, 1990), suggesting that after a shift to non-PA-containing food plants the need to have them was retained.
Several species of Sphingidae (Manduca species especially) specialize on Solanaceae and likely achieve this by enzymatically degrading the PAs and other small toxic molecules (Bernays and Janzen, 1988; Beck et al., 2006) as opposed to Saturniidae which bypass the tannins in their tannin-rich food plants by not breaking up the tannin-containing vacuoles with their mandibles, thereby growing more slowly by not extracting much nutrient from what they eat. In the field, saturniid fecal pellets are distinctive for breaking up into leaf chips, whereas sphingid fecal pellets are constituted of much more finely milled leaf matter, which takes place in the mandibular processing.
3.8 Herbivory on plants with structural defenses
3.8.1 Food plants with latex
Latex is present in many plant families and approximately 10% of plant species (Metcalf, 1967; Agrawal and Konno, 2009; Konno, 2011; Agrawal and Hastings, 2019) but is particularly prevalent in Alismataceae, Amaryllidaceae, Apocynaceae (including Asclepiadoideae), Araceae, Cactaceae, Campanulaceae, Cannabaceae, Caricaceae, Celastraceae, Convolvulaceae, Euphorbiaceae, Icacinaceae, Moraceae, Musaceae, Olacaceae, Papaveraceae, Sapotaceae, and the tribe Cichorieae (=Lactuceae) of Asteraceae. Latex is also significantly more prevalent in tropical taxa than in either temperate or widespread ones (Lewinsohn, 1991). The primary role of latex appears to be defense against herbivory, both by insects and mammals, and it often contains significant quantities of entomotoxins (Warowicka et al., 2020; Gracz-Bernaciak et al., 2021).
Latex may protect plants in two separate but not mutually exclusive ways; one is physical and the other is through its chemical constituents. Physically, the latex of many families is very sticky and can clog mandibles, impeding mastication and immobilizing mouthparts, and it can even kill first instar caterpillars to the leaf surface (Zalucki and Brower, 1992).
Caterpillars have evolved a variety of techniques to avoid or reduce feeding on latex and toxic plant compounds (Dillon et al., 1983; Dussourd, 1990, 1993, 1997, 1999; Dussourd and Denno, 1991, 1994) and other plant entomotoxins. These behaviors include trenching the leaf surface to cut tiny latex vessels, petiole constriction, and vein cutting (Betz et al., 2024). Trenching has been highlighted as being particularly important for generalists (Dussourd and Denno, 1994), although it is also important for specialists that feed on latex-containing plants (Bernays et al., 2004).
The caterpillar associations of these latex-bearing families are summarized in Table 6 and illustrated in Figure 10. Assuming this list represents the majority of latex-bearing plants in the data set, the number of species of latex bearing plants in each ecosystem does not differ significantly from those of non-latex bearing plants across ecosystems (χ2 = 0.86, df = 2, p = 0.65).
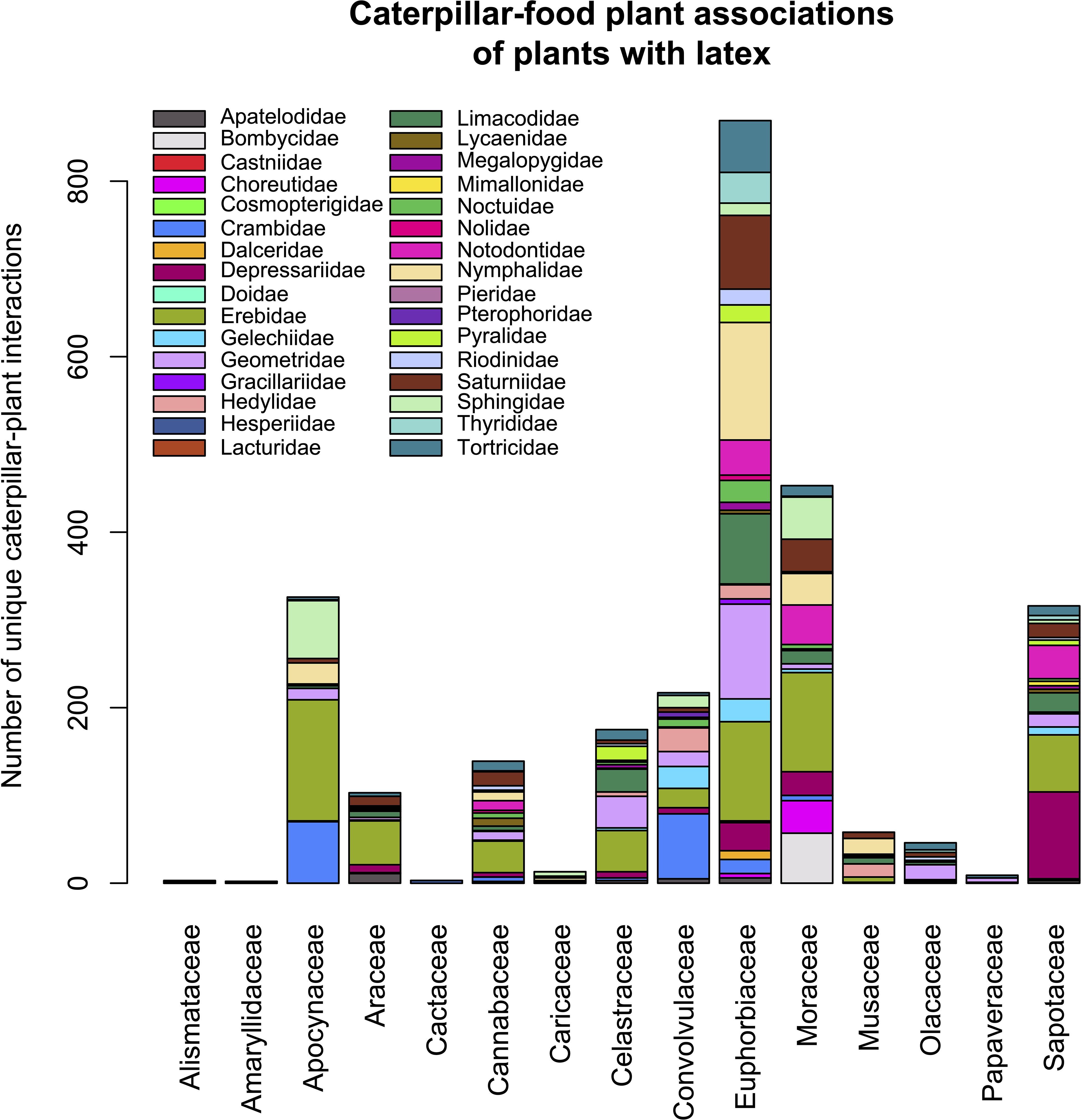
Figure 10. Bar chart showing the number of trophic associations for caterpillar families feeding on latex-bearing plant families. For clarity, the legend shows only the top 20 most represented caterpillar families.
The only representative Cichoriae (Asteraceae) in the ACG data was the introduced milk thistle, Sonchus oleraceus, from which two extremely generalist, crop pest species of Spodoptera (Noctuidae) were reared. Papaveraceae was represented only by Bocconia frutescens with the majority of rearings on that plant being of Anacrusis nephrodes (Tortricidae). All five unique rearings of Alismataceae were of Echinodorus subalatus, fed on by two cryptic species of Argyrogramma verruca (Noctuidae) and one by Platynota subargentea (Tortricidae). Unfortunately, most records of A. verruca were not separated as “verrucaDHJ01” or “verrucaDHJ02” because they were pre-DNA barcoding. All five unique rearings from Hymenocallis littoralis (Amarylidaceae) were of Xanthopastis timais (Noctuidae), and the other rearing from this plant family was one extreme generalist, Spodoptera latifascia (Noctuidae), feeding on Crinum erubescens. The introduced banana species (Musaceae) were collectively fed on by 47 Lepidoptera species in nine families, but most rearings involved four genera: the nymphalids Caligo (six species, 140 rearings) and Opsiphanes (three species, 367 rearings) and the hesperiids, Talides (six species, 190 rearings) and Thracides (one species, 74 rearings).
The latex of many Apocynaceae, Euphorbiaceae, and Moraceae contains lectins, including ricin in the case of Euphorbiaceae (Konozy et al., 2023), many of which have entomotoxic activity when ingested. Milkweed latex (Apocynaceae: Asclepiadoideae) famously contains cardiac glycosides (cardenolides), but the latex of some plants also includes abundant cysteine proteases that are highly insecticidal at very low doses (c. 0.1% w/w) (Ramos et al., 2010). Papaveraceae latex (Abarca et al., 2019) contains alkaloids (Gracz-Bernaciak et al., 2021). However, only one species of this plant family is represented in the present data set, with wild Papaveraceae (Bucconia) being essentially absent in ACG and its foliage characterized by experiencing almost no damage.
The chemistry of latex varies greatly. In most cases, it contains polysaccharides (Marinho and Teixeira, 2019). That of latex-bearing Cannabaceae is famously resinous in the case of Cannabis sativa. However, in ACG, the family is represented by two genera, Celtis and Trema, whose latex only includes proteins, lipids, polysaccharides, and terpenes (Leme et al., 2020), and of these, the latter can display relatively mild entomotoxicity in low doses.
3.8.1.1 Apocynaceae
The latex-bearing Apocynaceae includes many highly poisonous species such as oleander (Nerine spp.) in the tribe Nerieae (Janzen, 1978) and, in the same tribe, Strophanthus which is the source of the cardiac glycoside ouabain, an inhibitor of the Na+/K+-ATPase peritrophic matrix (membrane) pump and used on poison arrow tips.
3.8.1.2 Celastraceae
Celastraceae includes several species well known for their insecticidal and/or antifeedant secondary compounds (Spivey et al., 2002), perhaps most notably Azadirachta indica, the source of the commercial insecticide neem (azadirachtin), one of the many entomotoxic triterpenoids (Nisbet, 2000). The alkaloid wilfordine isolated from Tripterygium wilfordii also contains insecticidal/antifeedant sequiterpenoids (Acree and Haller, 1950; Shin-Foon and Yu-Tong, 1993; Luo et al., 2004; Ma et al., 2014).
3.8.1.3 Euphorbiaceae
This is a very large family of latex-producing plants. The chemistry of Euphorbiaceae latex has been well studied (Benjamaa et al., 2022). When it comes to specialized caterpillar species, this family was not as extreme as the ones indicated in red (Figure 7).
3.8.1.4 Fabaceae (Crotalaria)
Crotalaria species are generally protected by two kinds of pyrrolizidine alkaloids (PAs), monocrotaline and spectabiline (Flores et al., 2009), as well as by various flavonoids. While these compounds are highly concentrated in the seeds, all parts of the plant contain them. The aposematic ornate bella moth (Utetheisa ornatrix) (Erebidae: Arctiinae: Callimorphina) was the only species reared from Crotalaria, which appears to be its predominant (perhaps only) food plant (Wagner, 2005; Zhang et al., 2024), although numerous others have been cited in the literature. This is a PA specialist, and its larvae only feed on plants with PAs and oviposit on individual plants with higher PA concentrations (Hoina et al., 2013).
3.8.1.5 Moraceae
Just over one-third (667 out of 1,844) of the ACG Moraceae independent rearings were from figs, Ficus species, including three introduced species. Moraceae are well known producers of sticky latex that is used as a glue by many local communities (Shrestha et al., 1992). Ficus latex coagulation is relatively rapid and probably involves biochemical processes as well as evaporation (Bauer et al., 2014). Several entomotoxic proteins have been reported from Moraceae latex, e.g., the cysteine protease, ficin, is present in wild fig, Ficus virgata (Grossi-de-Sá et al., 2015), and two chitinases in mulberry (Morus spp.) (Kitajima et al., 2010). Moreover, in some species, the latex contains cardiac glycosides (cardenolides), and those of Maquira and Naucleopsis species are used as the active ingredient for poison darts (Shrestha et al., 1992). The ACG data set includes 1,413 rearings from Naucleopsis capirensis and 500 from Maquira guianensis.
3.8.2 Plants with high silica content
Silicon is present in all plants but varies widely in concentration from 0.1% to 10% dry mass (Kaufman et al., 1981; Epstein, 1999). It has a wide range of physiological functions in the soluble mono-silicic acid form, but when deposited as non-crystalline (amorphous) silica, e.g., as silicaceous phytoliths as well as being deposited on leaf surfaces, cell walls, and in specific cells, such as papillae, hairs, and silica cells where it may be abundant (Massey et al., 2006; Hartley and DeGabriel, 2016; Zexer et al., 2023), it provides protection from herbivory due to its very hard nature. It abrades caterpillar mandibles and also damages intestinal protection while slowing the release of nutrients from the plant tissue. Silica accumulation evolved early in land plants, and it is abundant in the leaves of ferns and lycopods but appears to have been lost and then regained on a number of independent occasions among the angiosperms (Trembath-Reichert et al., 2015).
In decreasing order of number of trophic interactions, the angiosperm plant families in the ACG data set that have particularly abundant silicaceous phytoliths (Supplementary Material in Katz, 2015) are Cactaceae, Poaceae, Piperaceae, Arecaceae, Annonaceae, Urticaceae, Sapotaceae, Marantaceae, Boraginaceae, Heliconiaceae, Dilleniaceae, Chrysobalanaceae, Menispermaceae, Cannabaceae, Cyperaceae, Bromeliaceae, Burseraceae, Zingiberaceae, Costaceae, Musaceae, Aristolochiaceae, Chloranthaceae, Cannaceae, Loranthaceae, Hernandiaceae, Thymelaeaceae, Magnoliaceae, and Ulmaceae. Ten of these 28 families fall within the monocotyledon orders Arecales, Poales, and Zingiberales. In addition, many ferns also have silica-rich leaves (Sharma et al., 2019).
3.8.2.1 Arecaceae (palms)
This family was among those with the most specialized caterpillars (Figure 7). Palm leaves have abundant silica, various flavonoids and their glycoside derivatives, phenolic acids and their derivatives, and sterols (Mohammed and Fouad, 2022). In addition to these, we also suspect that sclerophylly (tough foliage) plays a role.
Palms serve as the larval host of some of the most specialized (i.e., host specific) Lepidoptera species (Figure 11), but in some respects, these species do not appear to be morphologically or behaviorally specialized. The caterpillars reared from palms represented 17 families (Apatelodidae, Crambidae, Depressariidae, Erebidae, Eupterotidae, Gelechiidae, Hesperiidae, Lasiocampidae, Limacodidae, Megalopygidae, Noctuidae, Notodontidae, Nymphalidae, Pterolonchidae, Pterophoridae, Saturniidae, and Tortricidae). However, more than 93% of these rearings involved three families: Hesperiidae (the vast majority), Nymphalidae (two genera), and Notodontidae (two species), with the other 7% distributed among the remaining 14 Lepidoptera families.
3.8.2.2 Costaceae
Costaceae, the spiral ginger family, is represented by seven species of Costus and the introduced Hellenia speciosa. Members of this plant family serve as hosts for 33 species of Lepidoptera. The vast majority of rearings, however, were of the erebid, Agaraea minuta (n = 1,042), and the hesperiids, Calpodes esperi (n = 1,585) and Calpodes placens (n = 335). Thus, its apparently high use by herbivores is largely a reflection of the food plant preferences of these three caterpillars: Agaraea minuta was reared from five species of Costus and one species each of Fabaceae, Myristicaceae, and Sapindaceae. Calpodes placens was only reared from Costus (six species), Calathea lutea from six species of Costus, and one species each of Marantaceae and an introduced species of Canaceae, indicating that it likely is a specialist on monocots.
The spiral gingers are well known for their antioxidant, anticancer, antifungal, antidiabetic, and antimicrobial activities (Aruna et al., 2014; Bawakid et al., 2021; Sohrab et al., 2021). Little appears to have been published on their entomotoxicity, although Sohrab et al. (2021) do list insecticidal activity along with many health-related properties. Plant secondary compounds include flavonoids, saponins, tannins, phlobatannins, anthraquinone, phenol, alkaloids, resin, steroids, and terpenoids, including sesquiterpene lactones (Kim and Choi, 2019; Adesegun, 2021; Onanuga and Oloyede, 2021; Sohrab et al., 2021). Given the high degree of food plant specificity of their caterpillars, insecticidal activity in the family deserves further investigation.
3.8.2.3 Poaceae—grasses and bamboos (“woody grasses”)
The grass family is not generally noted for its insect anti-feedant or entomotoxic properties, although a few are known to contain saponins (Simmonds, 2006) and lectins (Han et al., 2018). Probably the best known grass secondary compounds are phytoalexins (Arruda et al., 2016), notably flavonoid phytoalexins and diterpene phytoalexins, which have principally been investigated from their antimicrobial activity (Ishihara, 2021). At least some phytoalexins have insect antifeedant activity (Sutherland et al., 1980). Some grass species (Poodieae in the BOP clade and Panicoideae in the PACMAD clade) (GPWG II, 2012; GPWG III, 2025) also produce benzoxazinoids, and these compounds are toxic to a wide range of plant pests such as insects, fungi, bacteria, and nematodes (Mikić and Ahmad, 2018). However, these compounds have not been found in all grass clades. Two benzoxazinoids, DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one) and DIBOA (2,4-dihydroxy-1,4-benzoxazin-3-one), are present in many members of the tribes Triticeae and Poeae (Gianoli and Niemeyer, 1998), including maize, wheat, and rye, and they have been demonstrated to provide defense against a range of insect herbivores including generalists such as the beet armyworm, Spodoptera exigua (Klun et al., 1970; Tzin et al., 2017).
However, grasses are well known for the concentration of silica in their foliage, which we treat here as a physical—as opposed to chemical—anti-herbivore deterrent. In fact, it has been fairly well established that grass specialization among herbivorous lepidopteran caterpillars has resulted in the evolution of highly developed mandibular muscles and concurrently large heads, much as the spread of grasslands have been implicated in vertebrate evolution (Simpson, 1951). Thus, it is not surprising that grasses and bamboos (Poaceae) are associated with highly specialist lepidopteran caterpillars, even though most work on them emphasizes the importance of their non-crystalline silicaceous phytoliths (Massey et al., 2006; Hartley and DeGabriel, 2016). This probably explains why many grass-feeding caterpillars other than Hesperiinae and Satyrinae are stem and rhizome borers rather than leaf feeders (Goldstein and Fibiger, 2005). Figure 11 suggests that relatively few groups of Lepidoptera have been able to specialize on grasses.
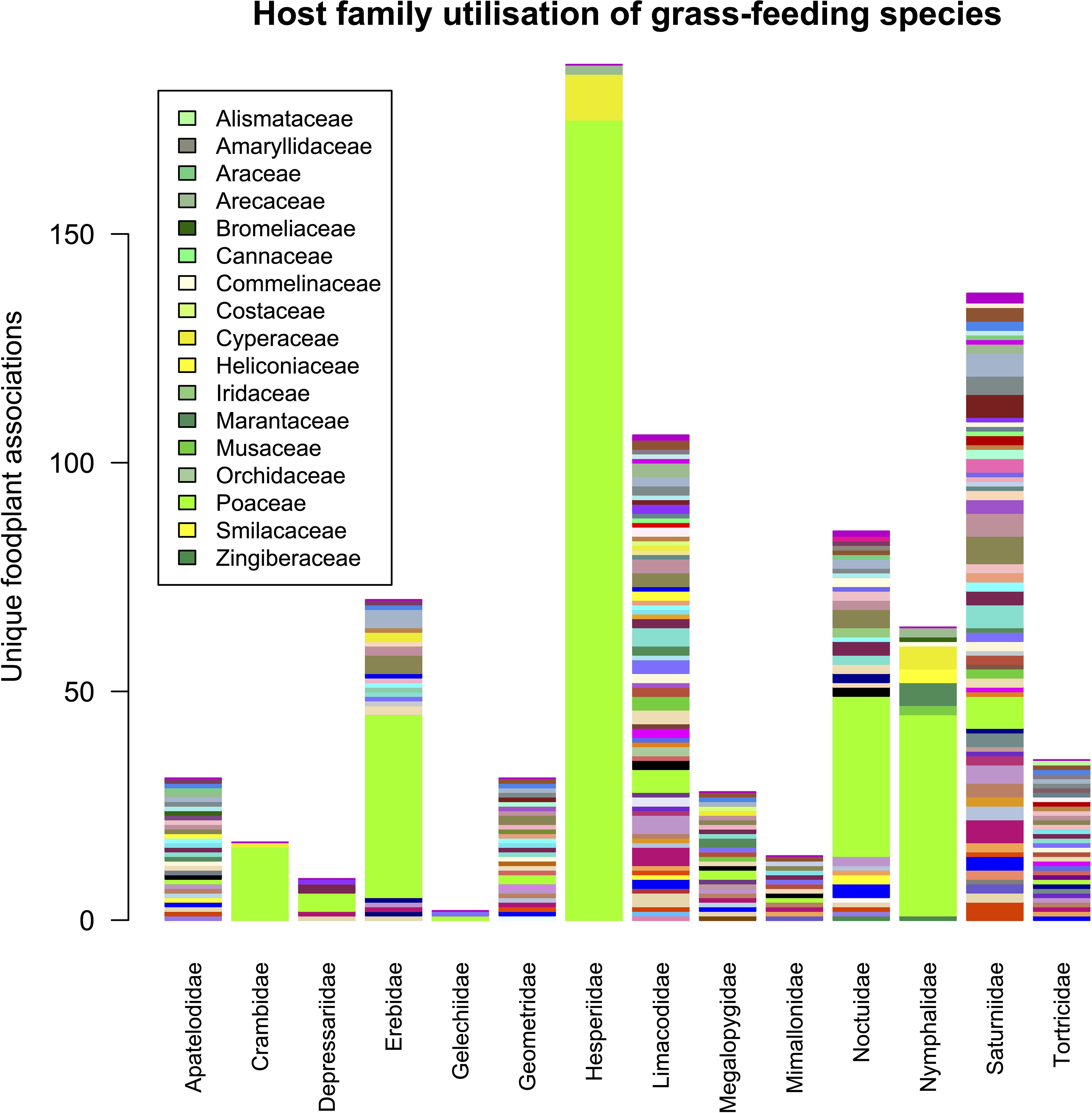
Figure 11. Bar chart showing the diet breadth at plant family level for species that were also reared from Poaceae (grasses, bamboos, etc.) with all monocot plant families indicated in the legend. In total, the dataset includes 17 monocot food plant families and 94 others. Monocot families have been assigned shades of green and yellow, while non-monocots have been indicated by oranges, red, purples, blues, and grays. The numbers are provided in Supplementary Table S3.
Nearly half of the species reared from grasses (n = 396) (Figure 11) were grass skipper butterflies (Hesperiidae: Hesperiinae) (n = 190), followed by Nymphalidae (n = 57) (predominantly Satyrini, but also including three genera of Brassolini and one each of Haeterini and Melanitini), Erebidae (n = 49), Noctuidae (n = 47), and Crambidae (Crambini) (n = 27). All other families were represented by fewer than 10 species of caterpillars on Poaceae. A marked contrast is apparent between those lepidopteran families strongly associated with Poaceae and those that are broad generalists—for example, the eight species of Saturniidae that were reared from one or more members of the Poaceae (15 out of 16 rearings being species of Automeris (Hemileucinae), some of which are notoriously polyphagous) collectively have been reared from 63 other plant families. The most common associations with families other than Poaceae consumed by grass feeders were with other monocyledons, most notably Cyperaceae (sedges), Arecaceae (palms), and the introduced banana species (Musaceae), each of which has a high silica content.
3.8.3 Trichomes
We have not been able to examine the importance of trichomes in detail because of the great interspecific—in some cases, intraspecific—variation in their development, including induction of their expression by herbivory itself. Stiff, biomineralized stinging trichomes, present in Urticaceae, some Euphorbiaceae, and a few other plant families, are primarily involved in defense against mammalian herbivores and have little, if any, deterrent effect against insects (Levin, 1973; Tuberville et al., 1996; Mustafa et al., 2018a). However, several experimental studies have shown that trichomes are effective at reducing caterpillar herbivory (e.g., Kariyat et al., 2017, 2018). In the case of non-glandular trichomes, protection against herbivory may result from the physical impedance of herbivore movement and access to edible foliage and/or by damaging the peritrophic membrane of the caterpillar after ingestion (Kariyat et al., 2017). The trichomes of Passiflora appear to be extremely effective against most caterpillars (Gilbert, 1971; Levin, 1973), although Heliconius and many other species have succeeded in overcoming these (Rathke and Poole, 1975; Cardoso, 2008).
Using ants as predators, Dyer and Floyd (1993) showed that, in general, specialists were significantly better protected from predation than generalists. This was corroborated by Dyer (1995) who prepared chemical extracts from numerous lyophilized caterpillars and offered these mixed with sugar solution to the ant colonies. It is now widely recognized that, in addition to primary defenses against herbivory (toxins, trichomes, protection by a resident ant colony; Janzen, 1966), many, if not most, plants have indirect defense mechanisms as well. These involve the release of volatile chemicals in response to herbivore damage that attract predators or parasitoids of the herbivore, sometimes even differentially in response to different herbivores (Aljbory and Chen, 2018). Farkas and Singer (2013), working on several generalist lepidopteran caterpillar species and their hymenopteran and dipteran parasitoids in the USA, found that the parasitism rate was probably largely determined by the production of plant-derived kairomones induced by herbivory that attract natural enemies rather than by plant chemistry directly.
3.9 Trophic links between caterpillar families and edible versus defended plants
To examine the spectrum of plant defense against caterpillar herbivory, we created two lists of families, one comprising 43 well-defended species as suggested in the previously published literature (see the footnote to Table 6) and one with little obvious protection. The well-protected list includes plant families that have latex, those with abundant silica, and those with notable levels of toxins (e.g., aristolochic acid, alkaloids, cycasin, glucosinolates, and glycosides) as detailed in Section 2.2.
There are many ways of ranking herbivores on a generalist–specialist scale, although any linear scale is at best a simplification. Since we are most interested in the utilization of very diverse food plants rather than the simple number of plant species consumed (or the behavioral or morphological adaptations to the morphology or behavior of food plants), we chose to use the Shannon diversity index of the number of trophic links with different plant families. This rank order is depicted in the central column (Figure 12). We then depict quantitatively the trophic links these have with the most abundantly represented largely edible food plants (Figure 12; left-hand column) and with the most abundantly represented defended plant (Figure 12; right-hand column). The least generalized lepidopteran caterpillar families show many more trophic links with generally edible plants, particularly with Fabaceae, Rubiaceae, and Lauraceae, than they do with any of the well-defended food plant families. The most polyphagous caterpillars have relatively few trophic links with defended plants, although geometrids and non-arctiine erebids show quite a strong association with Piperaceae and Nymphalidae with Passifloraceae (the Heliconiina), Solanaceae (the Ithomiini), and Euphorbiaceae.
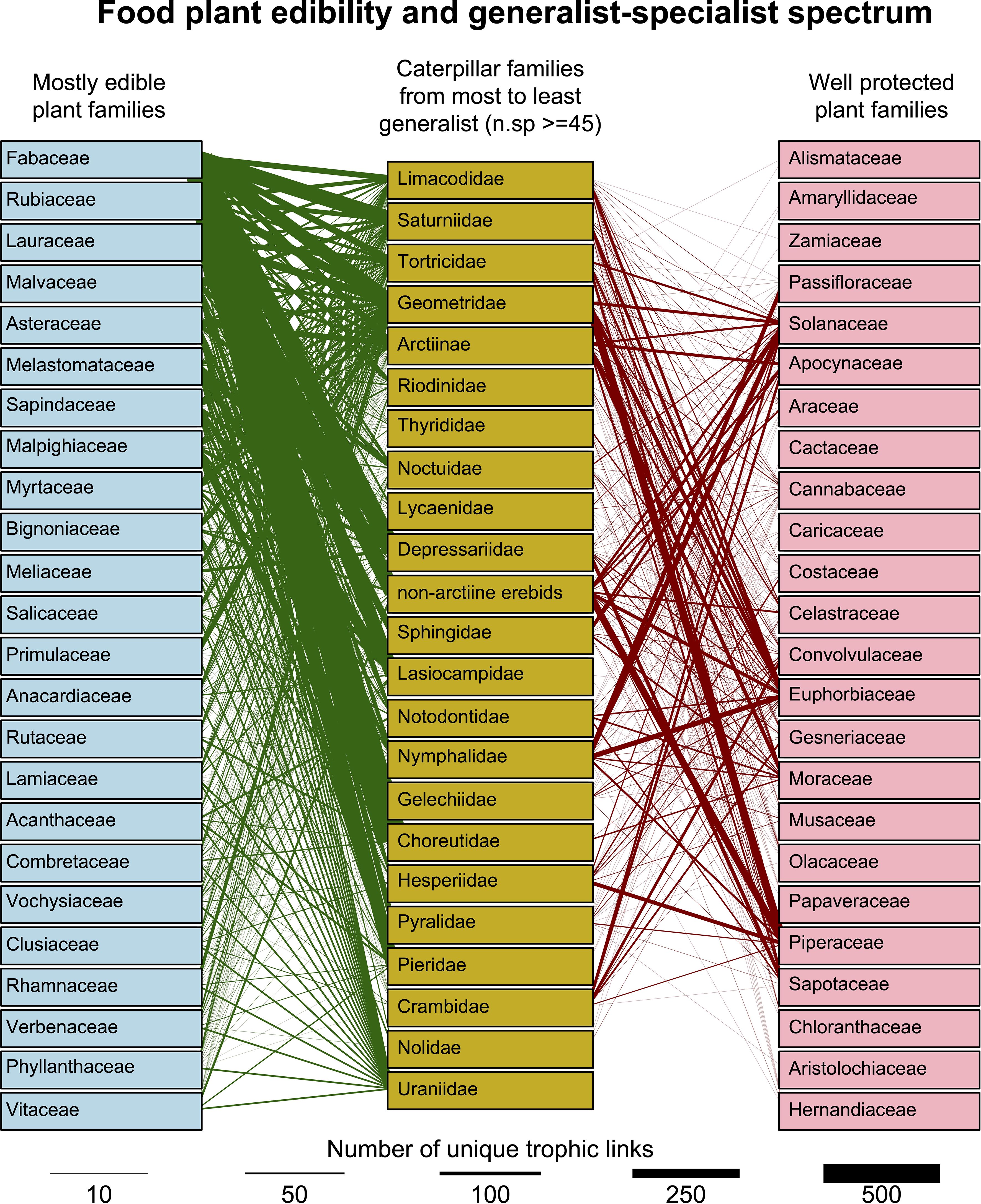
Figure 12. Relative number of distinct trophic links between caterpillar families (Arctiinae is treated separately from the other erebids) along a spectrum from most polyphagous (top) to least polyphagous (bottom), with broadly palatable plants (left) and well defended plants (right).
To test whether the difference in herbivore utilization pattern of the two plant palatability categories apparent in Figure 12 was statistically significant, we first compiled the lists of the caterpillar species that were recorded from each plant family on more than 40 separate occasions. Then, for each species in the list, we determined the number of food plant species and food plant families they were reared from as well as the Shannon diversity of the raw numbers (Figure 13). The mean values for the two palatability categories are given in Table 7. For both food plant species and food plant families, the mean values differed significantly between food plant groups (food plant species: t = 2.91, d.f. = 1,000, p < 0.005; food plant families: t = 6.21, d.f. = 1,041, p ≪ 0.001). As regards Shannon diversity, the food plant species ranges did not differ significantly (t = 1.3229, d.f. = 829, p = 0.19), but the diversity of food plant families was significantly greater for caterpillars feeding on the palatable category (t = 4.70, d.f. = 1029, p ≪ 0.001).
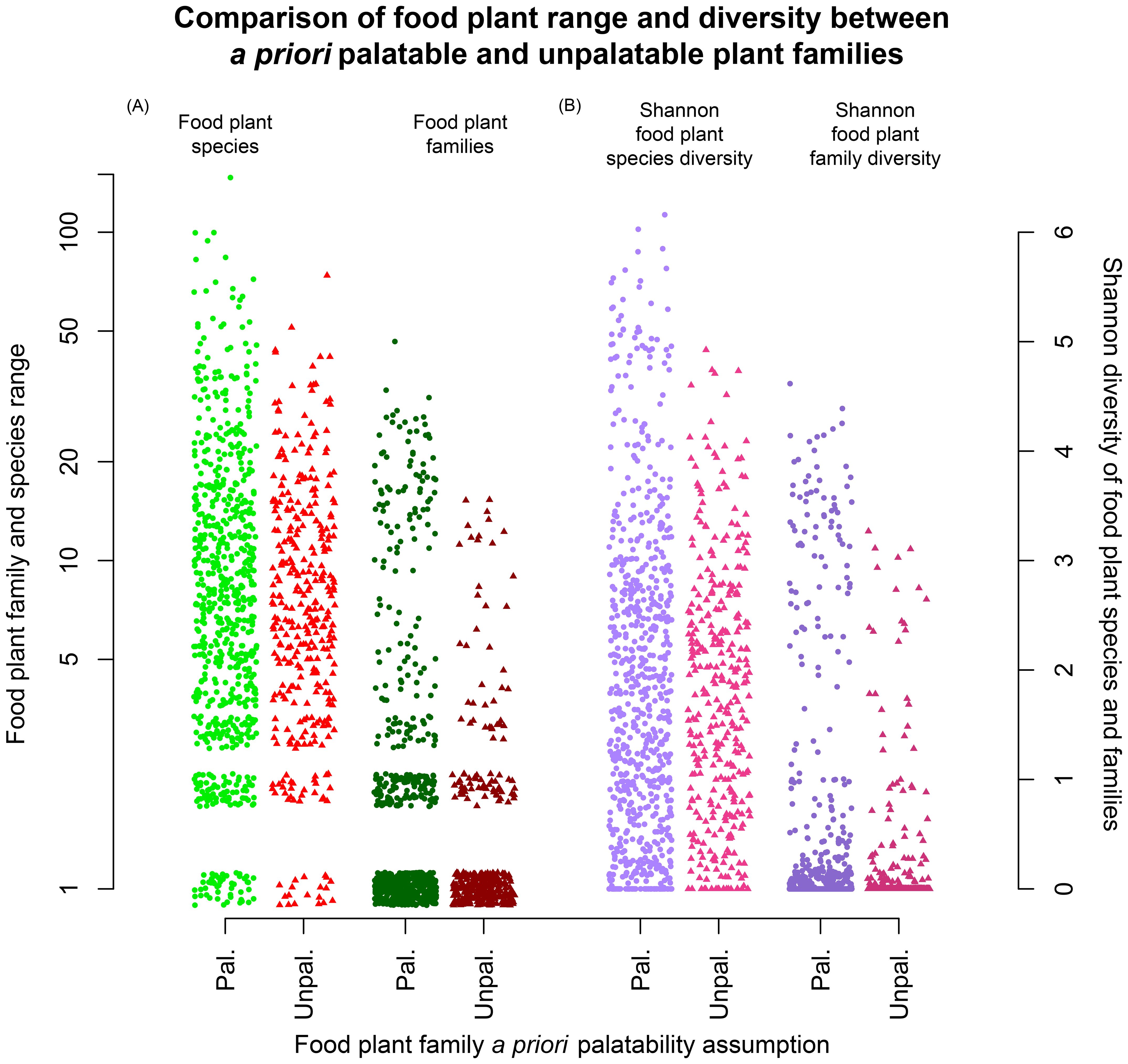
Figure 13. Food plant range and diversity of caterpillar species that were reared on more than 40 separate occasions from food plant families deemed a priori to be generally palatable (circles) versus those deemed to be well defended (triangles). (A) Number of food plant species (left) and families (right), on a logarithmic scale, for both plant palatability groups with x- and y-axis values jittered by a small amount to aid visualization. (B) Corresponding food plant Shannon diversities of each caterpillar species with only the x-axis values jittered. Pal., palatable; Unpal., unpalatable.
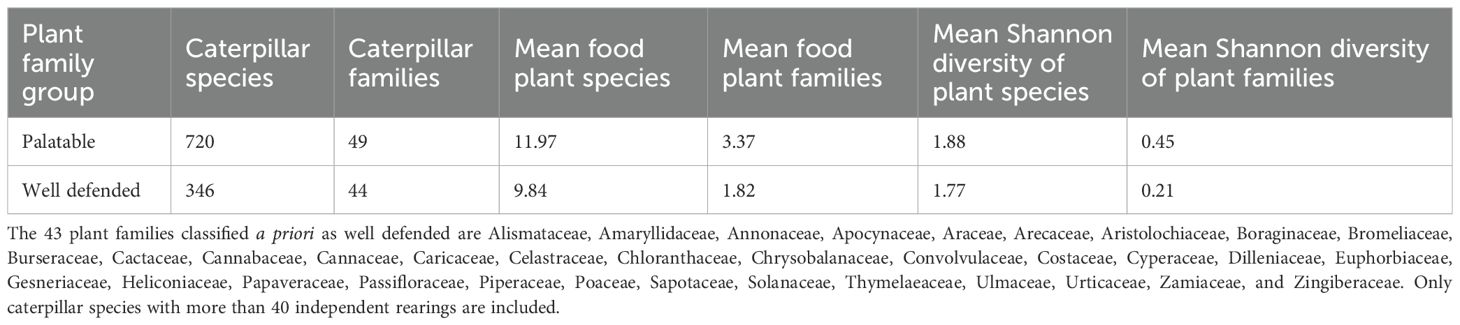
Table 7. Summary of food plant ranges and Shannon diversity index for Lepidoptera species reared from plant families categorized a priori as either well defended or broadly palatable (i.e., not well defended).
We interpret these results as showing that Lepidoptera species feeding on broadly palatable plant families are not particularly constrained in their food plant range by chemistry or sclerophyly and so, on average, utilize a wider range of food plant species and families than caterpillar species adapted to particular well-defended plant groups, which is not surprising. This is also indicated by the highly significant difference in Shannon food plant family diversity between the groups. However, the lack of statistical difference between groups in terms of Shannon food plant diversity may indicate that species adapted to cope with a particular class of chemical or physical defense mechanism can still utilize a similar range of food plant species within families with that defense mechanism.
4 Conclusions
Because of the 0–2 m (approx.) height range of the plant foliage that that parataxonomists sampled, the current data set represents only a portion of the species of Lepidoptera to be found in the ACG. Indeed light and Malaise trapping have revealed many more species than have been reared. Some caterpillars were collected from low-hanging tree branches and also from the foliage of tree saplings, but this is likely quite different from what may feed on mature leaves of mature trees in the canopy. The latter type of foliage is generally tougher, with more fiber and tannins and different water and nitrogen concentrations than found in the foliage of young trees and in the leaves of herbaceous plants (Scriber and Feeny, 1979). However, all those “old tough” leaves were at one time “young and tender” and conspicuously fed on by herbivores (especially many species of Erebidae (e.g., Eulepidotis) and Crambidae (e.g., Desmia)) especially at the beginning of the rainy season when trees flush their new foliage.
From this large field-based empirical study, we conclude that the caterpillar–plant food web structure largely reflects plant defenses against caterpillar herbivory, with generalists largely confined to consuming plant families with general and limited defenses. Specialization by a herbivore on a few species of plants in one family may come with a cost, i.e., their specialization often limiting how many food plant species in less defended families they are able to incorporate into their host range. Only a few groups include a large proportion of species that can feed on a wide range of well-defended plants (e.g., some arctiine Erebidae, Limacodidae, and Saturniidae). The protective effect of tannins is more general, and a specialist but low-reward chewing strategy by Saturniidae that allows them to consume leaves of a wide range of plants that are not otherwise well defended has evolved (Janzen, 1984b).
Most of the species-rich Lepidoptera families contain both specialists and generalists. Though moth families are highly polyphagous when evaluated collectively, the within-group variance is often as great—or greater—than that among groups. Every large family includes highly polyphagous taxa, highly specialized taxa, and everything in between. Exceptions include the Sphingidae, many of which show a preference for Rubiaceae; Hesperiinae, which feed primarily on monocotyledons; and non-hesperiine skippers (Hesperiidae), which exhibit a preference for Fabaceae. This study further highlights the importance of integrative taxonomy involving DNA barcoding in research involving poorly known but hyper-diverse faunas (Miller, 2007; Kaartinen et al., 2010). The conclusions reached now about levels of food plant specificity would probably differ greatly from what they would have been if the identifications had been based solely on pre-barcoding morphological concepts.
What about the future? Will there ever be a similar caterpillar food web study carried out on this scale and with basically the same methodology? It seems exceedingly unlikely. If there are any such projects, they will almost certainly be done in a very different and less labor-intensive way. DNA barcoding has been essential to our understanding of caterpillar–food plant associations in this study, but DNA technology has advanced enormously over recent years and will allow vastly more genetic signal to be collected than just the 658 base pairs of the standard insect barcode (Loxdale and Balog, 2025). Rearing is no longer necessary and perhaps not even killing the individuals, as it seems likely that a small-field-collected hemolymph sample would suffice not only to determine the identity of the caterpillar but also a small sample of the plant for its determination. The hemolymph would almost certainly also provide evidence of any parasitoids or pathogens.
However, the question remains as to whether it will ever be possible to determine a near complete tropical food web at all. The species and interaction accumulation curves presented here (Figures 2, 3) show that even after more than 800,000 caterpillar rearings, the data are still a long way from being complete. It has been estimated that in the ACG alone, there are probably between 1,200 and 1,500 species of trees. This is in stark contrast, for example, with the UK where there are only between 33 and 35 native tree species (depending on the definition).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material and are publicly available from https://doi.org/10.5683/SP3/NX043G.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
DQ: Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AB: Data curation, Resources, Writing – review & editing. MH: Validation, Visualization, Writing – review & editing. RM: Data curation, Software, Visualization, Writing – review & editing. SN: Data curation, Software, Visualization, Writing – review & editing. SR: Data curation, Resources, Software, Visualization, Writing – review & editing. JS: Data curation, Resources, Visualization, Writing – review & editing, Software. BJ: Validation, Visualization, Writing – review & editing, Data curation. MSm: Data curation, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. NZ: Resources, Validation, Visualization, Writing – review & editing. JWB: Data curation, Resources, Validation, Visualization, Writing – review & editing. TM: Data curation, Resources, Validation, Visualization, Writing – review & editing. SM: Data curation, Investigation, Resources, Validation, Visualization, Writing – review & editing. JMB: Data curation, Resources, Validation, Visualization, Writing – review & editing. PG: Data curation, Resources, Validation, Visualization, Writing – review & editing. MM: Data curation, Resources, Validation, Visualization, Writing – review & editing. RR: Data curation, Resources, Validation, Visualization, Writing – review & editing. MSo: Data curation, Resources, Validation, Visualization, Writing – review & editing. IC: Data curation, Validation, Visualization, Writing – review & editing. BE: Data curation, Resources, Validation, Visualization, Writing – review & editing. AP: Data curation, Investigation, Resources, Visualization, Writing – review & editing. EP-R: Data curation, Investigation, Resources, Writing – review & editing. PH: Data curation, Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. DJ: Data curation, Investigation, Resources, Validation, Visualization, Writing – review & editing. WH: Data curation, Investigation, Resources, Validation, Visualization, Writing – review & editing. BB: Data curation, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study has been supported by the U.S. National Science Foundation grants BSR 9024770 and DEB 9306296, 9400829, 9705072, 0072730, and 0515699, and grants from the Wege Foundation, International Conservation Fund of Canada, Jessie B. Cox Charitable Trust, Blue Moon Fund, Guanacaste Dry Forest Conservation Fund, Permian Global, individual donors, and the University of Pennsylvania (D.H.J. and W.H.). Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement. All of the specimens were collected, exported, and DNA barcoded under Costa Rican government permits issued to BioAlfa (Janzen and Hallwachs 2019) (R-054-2022-OT-CONAGEBIO; R-019-2019- CONAGEBIO; National Published Decree #41767), JICA-SAPI #0328497 (2014), and D.H.J. and W.H. (ACG-PI-036-2013; R-SINAC-ACG-PI-061-2021; Resolución No001–2004 SINAC; PI-028-2021). This research was funded by the National Research Council of Thailand (NRCT) (N42A650262) and Chulalongkorn University, RSPG-Chula, to B.A.B. D.L.J.Q. was supported by the Rachadaphisek Somphot Fund for postdoctoral fellowship, Graduate School, Chulalongkorn University. This study was also supported by the Government of Canada through its ongoing support to the Canadian National Collection, and by grants from Genome Canada and Ontario Genomics to P.D.N.H. in support of the Centre for Biodiversity Genomics at the University of Guelph, and to the Natural Sciences and Engineering Research Council of Canada. Sequence analysis was supported by the Walder Foundation and by a Transformation 2020 award to P.D.N.H. from the New Frontiers in Research Fund, while critical infrastructure at the CBG was acquired with grants from the Gordon and Betty Moore Foundation and the Canada Foundation for Innovation (CFI). A Major Science Infrastructure award MSI 42450 from CFI sustains the CBG’s capacity to provide informatics and sequencing support.
Acknowledgments
We are exceedingly indebted to the parataxonomists at ACG and to the many taxonomic experts who helped with identifications, without all of whom this study would not have been possible. This study was made possible because of awards from the New Frontiers in Research Fund (NFRFT-2020-00073) and CFI’s Major Science Infrastructure program (MSI 42450). They sustained the analytical capacity and informatics platforms at the Centre for Biodiversity Genomics at Guelph, Canada, needed to advance the overall BIOSCAN research program and its key initiatives such as BioAlfa and BOLD. We thank the ACG parataxonomists for sharing biological data and providing specimens and photographs that enabled the descriptions and identifications. All yy-SRNP-nnnnn vouchered specimens were collected, exported, and DNA barcoded under Costa Rican government permits issued to BioAlfa (Janzen and Hallwachs, 2019) (R-054-2022-OT-CONAGEBIO; R-019-2019-CONAGEBIO; National Published Decree #41767), JICASAPI #0328497 (2014), and DHJ and WH (ACGPI-036-2013; R-SINAC-ACG-PI-061-2021; Resolución N°001–2004 SINAC; PI-028-2021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1647436/full#supplementary-material
References
Abarca L. F. S., Klinkhamer P. G., and Choi Y. H. (2019). Plant latex, from ecological interests to bioactive chemical resources. Planta Med. 85, 856–868. doi: 10.1055/a-0923-8215, PMID: 31137048
Acree F. Jr and Haller H. L. (1950). Wilfordine, an insecticidal alkaloid from Tripterygium wilfordii Hook. J. Amer. Chem. Soc 72, 1608–1611. doi: 10.1021/ja01160a050
Adamczyk B., Simon J., Kitunen V., Adamczyk S., and Smolander A. (2017). Tannins and their complex interaction with different organic nitrogen compounds and enzymes: old paradigms versus recent advances. ChemistryOpen 16, 610–614. doi: 10.1002/open.201700113, PMID: 29046854
Adesegun O. O. (2021). Isolation and characterisation of secondary metabolites and essential oils from Costus lucanusianus J. Braun & K. Schum and their antimicrobial activity (Ibadan, Nigeria: UI Postgraduate College).
Agrawal A. A. and Hastings A. P. (2019). Trade‐offs constrain the evolution of an inducible defense within but not between plant species. Ecology 100, e02857.
Agrawal A. A. and Konno K. (2009). Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 40, 311–331. doi: 10.1146/annurev.ecolsys.110308.120307
Agriculture Research Service (1961). Alkaloid bearing plants and their contained alkaloids. Technical Bulletin No. 124 (Washington D.C.: USA Department of Agriculture), 287.
Ahuchaogu A., Chukwu J., Obike A., Ugonna Oha T., Bull J., and Echeme O. (2017). Quantitative determination of secondary metabolites and antibacterial activity of Mimosa pudica. Int. J. Med. Plants Nat. Prod. 3, 2454–7999. doi: 10.20431/2454-7999.0302001
Aljbory Z. and Chen M. S. (2018). Indirect plant defense against insect herbivores: a review. Insect Sci. 25, 2–23. doi: 10.1111/1744-7917.12436, PMID: 28035791
Arruda R. L., Paz A. T. S., Bara M. T. F., Côrtes M. V. D. C. B., Filippi M. C. C. D., and Conceição E. C. D. (2016). An approach on phytoalexins: function, characterization and biosynthesis in plants of the family Poaceae. cienc. Rural 46, 1206–1216. doi: 10.1590/0103-8478cr20151164
Aruna A., Nandhini R., Karthikeyan V., Bose P., and Vijayalakshmi K. (2014). Comparative anti-diabetic effect of methanolic extract of insulin plant (Costus pictus) leaves and its silver nanoparticle. Indo Amer. J. Pharmaceut. Res. 4, 3217–3230.
Bachheti A., Sharma A., Bachheti R. K., Husen A., and Pandey D. P. (2019). “Plant allelochemicals and their various applications,” in Co-Evolution of Secondary Metabolites. Eds. Mérillon J.-M. and Ramawat K. G. (Springer International, Cham, Switzerland), 441–465. doi: 10.1007/978-3-319-76887-8_14-1
Barbehenn R. V. and Constabel P. C. (2011). Tannins in plant-herbivore interactions. Phytochemistry 72, 1551–1565. doi: 10.1016/j.phytochem.2011.01.040, PMID: 21354580
Barbehenn R. V., Jaros A., Lee G., Mozola C., Weir Q., and Salminen J. P. (2009). Hydrolyzable tannins as “quantitative defenses”: Limited impact against Lymantria dispar caterpillars on hybrid poplar. J. Ins. Physiol. 55, 297–304. doi: 10.1016/j.jinsphys.2008.12.001, PMID: 19111746
Bauer G., Friedrich C., Gillig C., Vollrath F., Speck T., and Holland C. (2014). Investigating the rheological properties of native plant latex. J. R. Soc Interface 11, 20130847. doi: 10.1098/rsif.2013.0847, PMID: 24173604
Bawakid N. O., Abdel-Lateff A., El-Senduny F. F., and Alarif W. M. (2021). Costus speciosus J Koenig (Costaceae) exerts anti-proliferative effect on breast cancer cells via induction of cell cycle arrest and inhibition of activity of metalloproteinase-2. Trop. J. Pharmaceut. Res. 20, 1365–1372. doi: 10.4314/tjpr.v20i7.7
Beccaloni G. W., Viloria Á.L., Hall S. K., and Robinson G. S. (2008). Catalogue of the Hostplants of the Neotropical Butterflies (Zaragoza, Spain: Sociedad Entomológica Aragonesa), 536.
Beck J., Kitching I. J., and Linsenmair K. E. (2006). Diet breadth and host plant relationships of Southeast-Asian sphingid caterpillars. Ecotropica 12, 1–13.
Behmer S. T., Lloyd C. M., Raubenheimer D., Stewart-Clark J., Knight J., Leighton R. S., et al. (2005). Metal hyperaccumulation in plants: mechanisms of defence against insect herbivores. Funct. Ecol. 19, 55–66. doi: 10.1111/j.0269-8463.2005.00943.x
Benelli G., Pavela R., Drenaggi E., Desneux N., and Maggi F. (2020). Phytol, (E)-nerolidol and spathulenol from Stevia rebaudiana leaf essential oil as effective and eco-friendly botanical insecticides against Metopolophium dirhodum. Indust. Crop Product. 155, 112844. doi: 10.1016/j.indcrop.2020.112844
Benjamaa R., Moujanni A., Kaushik N., Choi E. H., Essamadi A. K., and Kaushik N. K. (2022). Euphorbia species latex: a comprehensive review on phytochemistry and biological activities. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1008881, PMID: 36275519
Bennett R. N. and Wallsgrove R. M. (1994). Secondary metabolites in plant defence mechanisms. New Phytol. 127, 617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x, PMID: 33874382
Bernays E. A. (1978). Tannins: an alternative viewpoint. Entomol. Exp. Appl. 24, 244–253. doi: 10.1111/j.1570-7458.1978.tb02779.x
Bernays E. A. and Janzen D. H. (1988). Saturniid and sphingid caterpillars: two ways to eat leaves. Ecology 69, 1153–1160. doi: 10.2307/1941269
Bernays E. A., Singer M. S., and Rodrigues D. (2004). Trenching behavior by caterpillars of the Euphorbia specialist, Pygarctia roseicapitis: a field study. J. Ins. Behav. 17, 41–52. doi: 10.1023/B:JOIR.0000025131.16275.0f
Bersier L. F., Banasěk-Richter C., and Cattin M. F. (2002). Quantitative descriptors of food-web matrices. Ecology 83, 2394–2407. doi: 10.1890/0012-9658(2002)083[2394:QDOFWM]2.0.CO;2
Betz A., Bischoff R., and Petschenka G. (2024). Late-instar monarch caterpillars sabotage milkweed to acquire toxins, not to disarm plant defence. Proc. R. Soc B Biol. Sci. 291, 20232721. doi: 10.1098/rspb.2023.2721, PMID: 38378155
Bishop J. G., Dean A. M., and Mitchell-Olds T. (2000). Rapid evolution in plant chitinases: molecular targets of selection in plant-pathogen coevolution. Proc. Natl. Acad. Sci. U.S.A. 97, 5322–5327. doi: 10.1073/pnas.97.10.532, PMID: 10805791
Blüthgen N., Menzel F., and Blüthgen N. (2006). Measuring specialization in species interaction networks. BMC Ecol. 6, 9. doi: 10.1186/1472-6785-6-9, PMID: 16907983
Bodner F., Strutzenberger P., Brehm G., and Fiedler K. (2012). Species richness and host specificity among caterpillar ensembles on shrubs in the Andes of southern Ecuador. Neotrop. Entomol. 41, 375–385. doi: 10.1007/s13744-012-0066-4, PMID: 23950087
Boter M. and Diaz I. (2023). Cyanogenesis, a plant defence strategy against herbivores. Int. J. Mol. Sci. 24, 6982. doi: 10.3390/ijms24086982, PMID: 37108149
Braby M. F. and Trueman J. W. H. (2006). Evolution of larval host plant associations and adaptive radiation in pierid butterflies. J. Evol. Biol. 19, 1677–1690. doi: 10.1111/j.1420-9101.2006.01109.x, PMID: 16910997
Braz D. M., dos Santos Tozin L. R., Gevú K. V., Lima H. R. P., Dos Santos V., de Oliveira R. A. M., et al. (2024). Folk medicine, biological activity, and chemical profiles of Brazilian Acanthaceae (Lamiales) – A review. J. Ethnopharmacol. 327, 117980. doi: 10.1016/j.jep.2024.117980, PMID: 38453098
Brown J. W. (2018). Patterns of Lepidoptera herbivory on conifers in the New World. J. Asia-Pacific Biodiv. 11, 1–10. doi: 10.1016/j.japb.2018.01.008
Brown K. S. (1984). Chemical ecology of dehydropyrolizidine alkaloids in adult Ithomiinae (Lepidoptera: Nymphalidae). Rev. Bras. Biol. 44, 435–460. doi: 10.1007/BF01239482
Brown K. S. and Benson W. W. (1974). Adaptive polymorphism associated with multiple Müllerian mimicry in Heliconius numata (Lepid.: Nymph.). Biotropica 6, 205–228. doi: 10.2307/2989666
Burns J. M. and Janzen D. H. (2005). Pan-neotropical genus Venada (Hesperiidae: Pyrginae) is not monotypic: four new species occur on one volcano in the Area de Conservación Guanacaste, Costa Rica. J. Lepid. Soc 59, 19–34.
Burns J. M., Janzen D. H., Hajibabaei M., Hallwachs W., and Hebert P. D. (2008). DNA barcodes and cryptic species of skipper butterflies in the genus Perichares in Area de Conservacion Guanacaste, Costa Rica. Proc. Natl. Acad. Sci. U.S.A. 105, 6350–6355. doi: 10.1073/pnas.07121811, PMID: 18436645
Cardoso M. Z. (2008). Herbivore handling of a plant’s trichome: the case of Heliconius charithonia (L.) (Lepidoptera: Nymphalidae) and Passiflora lobata (Killip) Hutch. (Passifloraceae). Neotrop. Entomol. 37, 247–252. doi: 10.1590/S1519-566X2008000300002, PMID: 18641894
Carlini C. R. and Grossi-de-Sá M. F. (2002). Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon 40, 1515–1539. doi: 10.1016/s0041-0101(02)00240-4, PMID: 12419503
Carlini C. R. and Ligabue-Braun R. (2016). Ureases as multifunctional toxic proteins: a review. Toxicon 110, 90–109. doi: 10.1016/j.toxicon.2015.11.020, PMID: 26690979
Chen H. and Boutros P. C. (2011). VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinf. 12, 1–7. doi: 10.1186/1471-2105-12-35, PMID: 21269502
Chen Y., Peumans W. J., Hause B., Bras J., Kumar M., Proost P., et al. (2002). Jasmonate methyl ester induces the synthesis of a cytoplasmic/nuclear chitooligosaccharide-binding lectin in tobacco leaves. FASEB J. 16, 905–907. doi: 10.1096/fj.01-0598fje, PMID: 12039875
Cooper-Driver G. A. (1978). Insect-fern associations. Entomol. Exp. Applicata 24, 310–316. doi: 10.1111/j.1570-7458.1978.tb02787.x
Craft K. J., Pauls S. U., Darrow K., Miller S. E., Hebert P. D. N., Helgen L. E., et al. (2010). Population genetics of ecological communities with DNA barcodes: An example from New Guinea Lepidoptera. Proc. Natl. Acad. Sci. U.S.A. 107, 5041–5046. doi: 10.1073/pnas.09130841, PMID: 20202924
de Castro É.C., Zagrobelny M., Cardoso M. Z., and Bak S. (2018). The arms race between heliconiine butterflies and Passiflora plants–new insights on an ancient subject. Biol. Rev. 93, 555–573. doi: 10.1111/brv.12357, PMID: 28901723
Dillon P. M., Lowrie S., and McKey D. (1983). Disarming the "Evil Woman": petiole constriction by a sphingid larva circumvents mechanical defenses of its host plant, Cnidoscolus urens (Euphorbiaceae). Biotropica 15, 112–116. doi: 10.2307/2387953
Dobler S., Petschenka G., and Pankoke H. (2011). Coping with toxic plant compounds–the insect’s perspective on iridoid glycosides and cardenolides. Phytochem. 72, 1593–1604. doi: 10.1016/j.phytochem.2011.04.015, PMID: 21620425
Dolson S. J., Loewen E., Jones K., Jacobs S. R., Solis A., Hallwachs W., et al. (2021). Diversity and phylogenetic community structure across elevation during climate change in a family of hyperdiverse neotropical beetles (Staphylinidae). Ecography 44, 740–752. doi: 10.1111/ecog.05427
Dolson S. J., McPhee M., Vizquez C., Hallwachs W., Janzen D. H., and Smith M. A. (2020). Diversity, abundance, and phylogenetic structure of spiders (Araneae) across a Costa Rican elevation gradient. Biotropica 52, 1092–1102. doi: 10.1111/btp.12874
Domsalla A. and Melzig M. F. (2008). Occurrence and properties of proteases in plant latices. Planta Med. 74, 699–711. doi: 10.1055/s-2008-1074530, PMID: 18496785
Dormann C. F., Fründ J., Blüthgen N., and Gruber B. (2009). Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24. doi: 10.2174/1874213000902010007
Dormann C. F., Gruber B., and Fründ J. (2008). Introducing the bipartite package: analysing ecological networks. R News 8, 8–11.
Dossaji S. F., Bell E. A., and Wallace J. W. (1973). Biflavones of dioon. Phytochem. 12, 371–373. doi: 10.1016/0031-9422(73)80020-2
Dossaji S. F., Mabry T. J., and Bell E. A. (1975). Biflavanoids of the cycadales. Biochem. Syst. Ecol. 2, 171–175. doi: 10.1016/0305-1978(75)90057-5
Durán J., Fagua G., Robles J., and Gil E. (2012). Sequestration of aristolochic acid I from Aristolochia pilosa by Mapeta xanthomelas Walke. J. Chem. Ecol. 38, 1285–1288. doi: 10.1007/s10886-012-0187-6, PMID: 22968784
Dussourd D. E. (1993). “Foraging with finesse: caterpillar adaptations for circumventing plant defenses,” in Caterpillars: Ecological and Evolutionary Constraints on Foraging. Eds. Stamp N. E. and Casey T. (Chapman and Hall, New York), 92–131.
Dussourd D. E. (1997). Plant exudates trigger leaf-trenching by cabbage loopers, Trichoplusia ni. Oecologia 112, 362–369. doi: 10.1007/s004420050321, PMID: 28307485
Dussourd D. E. (1999). Behavioral sabotage of plant defense: do vein cuts and trenches reduce insect exposure to exudate? J. Ins. Behav. 12, 501–515. doi: 10.1146/annurev-ento-031616-035030, PMID: 27649317
Dussourd D. E. and Denno R. F. (1991). Deactivation of plant defense: correspondence between insect behavior and secretory canal architecture. Ecology 72, 1383–1396. doi: 10.2307/1941110
Dussourd D. E. and Denno R. F. (1994). Host range of generalist caterpillars: trenching permits feeding on plants with secretory canals. Ecology 75, 69–78. doi: 10.2307/1939383
Dyer L. A. (1995). Tasty generalists and nasty specialists? Antipredator mechanisms in tropical lepidopteran larvae. Ecology 76, 1483–1496. doi: 10.2307/1938150
Dyer L. A. and Floyd T. (1993). Determinants of predation on phytophagous insects: the importance of diet breadth. Oecologia 96, 575–582. doi: 10.1007/BF00320516, PMID: 28312465
Dyer L. A., Singer M. S., Lill J. T., Stireman J. O., Gentry G. L., Marquis R. J., et al. (2007). Host specificity of Lepidoptera in tropical and temperate forests. Nature 448, 696–699. doi: 10.1038/nature05884, PMID: 17687325
Ehrlich P. R. and Raven P. H. (1964). Butterflies and plants: a study in coevolution. Evolution 18, 586–608. doi: 10.2307/2406212
El-Seadawy H. M., Abo El-Seoud K. A., El-Aasr M., and Ragab A. E. (2023). Phytochemical profile, ethnobotanical and biological impacts of various Zamia species: a mini-review. J. Adv. Med. Pharmaceut. Res. 4, 63–73. doi: 10.21608/jampr.2023.202326.1053
Epstein E. (1999). Silicon. Annu. Revi. Plant Physiol. Plant Mol. Biol. 50, 641–664. doi: 10.1146/annurev.arplant.50.1.641, PMID: 15012222
Erb M., Meldau S., and Howe G. A. (2012). Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 17, 250–259. doi: 10.1016/j.tplants.2012.01.003, PMID: 22305233
Farkas T. E. and Singer M. S. (2013). Can caterpillar density or host-plant quality explain host-plant-related parasitism of a generalist forest caterpillar assemblage? Oecologia 173, 971–983. doi: 10.1007/s00442-013-2658-z, PMID: 23620347
Flores A. S., de Azevedo Tozzi A. M. G., and Trigo J. R. (2009). Pyrrolizidine alkaloid profiles in Crotalaria species from Brazil: chemotaxonomic significance. Biochem. Syst. Ecol. 37, 459–469. doi: 10.1016/j.bse.2009.06.001
Forkner R. E., Marquis R. J., and Lill J. T. (2004). Feeny revisited: condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecol. Ent. 29, 174–187. doi: 10.1111/j.1365-2311.2004.0590.x
Fox L. R. and Macauley B. J. (1977). Insect grazing on Eucalyptus in response to variation in leaf tannins and nitrogen. Oecologia 29, 145–162. doi: 10.1007/BF00345794, PMID: 28308647
Francisco I. A. and Pinotti M. H. P. (2000). Cyanogenic glycosides in plants. Braz. Archiv. Biol. Technol. 43, 487–492. doi: 10.1590/S1516-89132000000500007
Fuentes-Jacques L. J., Hanson-Snortum P., Hernández-Ortiz V., Díaz-Castelazo C., and Mehltreter K. (2022). A global review and network analysis of phytophagous insect interactions with ferns and lycophytes. Plant Ecol. 223, 27–40. doi: 10.1007/s11258-021-01187-5
Futuyma D. J. (1983). “Evolutionary interactions among herbivorous insects and plants,” in Coevolution. Eds. Futuyma D. J. and Slatkin M. (Sinauer Associates, Sunderland, MA), 207–231.
Gallon M. E., Silva-Junior E. A., Amaral J. G., Lopes N. P., and Gobbo-Neto L. (2019). Natural products diversity in plant-insect interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae). Molecules 24, 3118. doi: 10.3390/molecules24173118, PMID: 31466223
Gianoli E. and Niemeyer H. M. (1998). DIBOA in wild Poaceae: sources of resistance to the Russian wheat aphid (Diuraphis noxia) and the greenbug (Schizaphis graminum). Euphytica 102, 317–321. doi: 10.1023/A:1018323121213
Gilbert L. E. (1971). Butterfly-plant coevolution: has Passiflora adeniopoda won the selectional race with heliconiine butterflies? Science 172, 585–586. doi: 10.1126/science.172.3983.585, PMID: 17802223
Gilbert A. J. (2009). Connectance indicates the robustness of food webs when subjected to species loss. Ecol. Indic. 9, 72–80. doi: 10.1016/j.ecolind.2008.01.010
Goldstein P. Z. (2017). “Diversity and significance of Lepidoptera: a phylogenetic perspective,” in Insect Biodiversity: Science and Society. Eds. Foottit R. G. and Adler P. H. (Wiley, Chichester, U.K), 463–495.
Goldstein P. Z. and Fibiger M. F. (2005). Biosystematics and evolution of the Apameini: a global synopsis. Noctuidae Europ. 8, 15–23.
Goldstein P. Z., Janzen D. H., and Hallwachs W. (2021). A novel origin of pteridivory among the New World Noctuoidea: fern-feeding “litter moths” (Erebidae: Herminiinae). Proc. Entomol. Soc Wash. 123, 314–333. doi: 10.4289/0013-8797.123.2.314
Goldstein P. Z., Janzen D. H., Hallwachs W., and Proshek B. (2022). New species in Rejectaria Guene (Lepidoptera: Erebidae: Herminiinae) with a focus on the Cyclanthaceae-feeders. Zootaxa 5087, 451–483. doi: 10.11646/zootaxa.5087.3.4, PMID: 35391278
Goldstein P. Z., Janzen D. H., Proshek B., Dapkey T., and Hallwachs W. (2018). “A review of Leucosigma Druce 1908: a newly discovered case of fern-feeding and a description of three new species (Lepidoptera, Noctuidae),” in Contributions to the Systematics of New World Macro-moths VII, vol. 788 . Eds. Schmidt B. C. and Lafontaine J. D. ZooKeys, 87–133. (Pensoft Publishers, Sofia, Bulgaria). doi: 10.3897/zookeys.788.21222, PMID: 30337826
GPWG II (2012). New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol. 193, 304–312., PMID: 22115274
GPWG III (2025). A nuclear phylogenomic tree of grasses (Poaceae) recovers current classification despite gene incongruence. New Phytol. 245, 818–834., PMID: 39568153
Gracz-Bernaciak J., Mazur O., and Nawrot R. (2021). Functional studies of plant latex as a rich source of bioactive compounds: focus on proteins and alkaloids. Int. J. Mol. Sci. 22, 12427. doi: 10.3390/ijms222212427, PMID: 34830309
Grossi-de-Sá M. F., Pelegrini P. B., Vasconcelos I. M., Carlini C. R., and Silva M. S. (2015). “Entomotoxic plant proteins: potential molecules to develop genetically modified plants resistant to insect-pests,” in Plant Toxins. Toxinology, vol. 10. (Springer Science, Dordrecht), 415–448. doi: 10.1007/978-94-007-6728-7_13-1
Gu Z., Gu L., Eils R., Schlesner M., and Brors B. (2014). Circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812. doi: 10.1093/bioinformatics/btu393, PMID: 24930139
Gutierrez M. P., Maria Neira Gonzalez A., and Hoyo-Vadillo C. (2013). Alkaloids from Piper: a review of its phytochemistry and pharmacology. Mini Rev. Med. Chem. 13, 163–193. doi: 10.2174/138955713804805148, PMID: 23279257
Hajibabaei M., Janzen D. H., Burns J. M., Hallwachs W., and Hebert P. D. (2006). DNA barcodes distinguish species of tropical Lepidoptera. Proc. Natl. Acad. Sci. U.S.A. 103, 968–971. doi: 10.1073/pnas.05104661
Hallmann C. A., Sorg M., Jongejans E., Siepel H., Hofland N., Schwan H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PloS One 12, e0185809. doi: 10.1371/journal.pone.0185809, PMID: 29045418
Hammond P. C. and Miller J. C. (1998). Comparison of the biodiversity of Lepidoptera within three forested ecosystems. An. Entomol. Soc Amer. 91, 323–328. doi: 10.1093/aesa/91.3.323
Han Y. J., Zhong Z. H., Song L. L., Stefan O., Wang Z. H., and Lu G. D. (2018). Evolutionary analysis of plant jacalin-related lectins (JRLs) family and expression of rice JRLs in response to Magnaporthe oryzae. J. Integ. Agric. 17, 1252–1266. doi: 10.1016/S2095-3119(17)61809-4
Harrison R. L. and Bonning B. C. (2010). Proteases as insecticidal agents. Toxins 2, 935–953. doi: 10.3390/toxins2050935, PMID: 22069618
Hartley S. E. and DeGabriel J. L. (2016). The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 30, 1311–1322. doi: 10.1111/1365-2435.12706
Haston E., Richardson J. E., Stevens P. F., Chase M. W., and Harris D. J. (2009). The linear Angiosperm Phylogeny Group (LAPG) III: a linear sequence of the families in APG III. Bot. J. Linn. Soc 161, 128–131. doi: 10.1111/j.1095-8339.2009.01000.x
Hebert P. D. N., Penton E. H., Burns J. M., Janzen D. H., and Hallwachs W. (2004). Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. U.S.A. 101, 14812–14817. doi: 10.1073/pnas.040616610, PMID: 15465915
Hendrix S. D. (1980). An evolutionary and ecological perspective of the insect fauna of ferns. Am. Nat. 115, 171–196. doi: 10.1086/283554
Herrera W. (2016). “Climate of Costa Rica,” in Costa Rican Ecosystems. Ed. Kappelle M. (The University of Chicago Press, Chicago, IL, USA), 19–29.
Hoina A., Martins C. H. Z., Trigo J. R., and Cogni R. (2013). Preference for high concentrations of plant pyrrolizidine alkaloids in the specialist arctiid moth Utetheisa ornatrix depends on previous experience. Arthrop.-Plant Interact. 7, 169–175. doi: 10.1007/s11829-012-9232-1
Hopkins R. J., van Dam N. M., and van Loon J. J. (2009). Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Ent. 54, 57–83. doi: 10.1146/annurev.ento.54.110807.090623, PMID: 18811249
Ileke K. D., Adesina J. M., Nwosu L. C., and Olagunju A. (2020). Perforation index assessment of cowpea seeds against cowpea bruchid, Callosobruchus maculatus (Fabricius) [Coleoptera: Chrysomelidae], infestation using Piper guineense. J. Basic Appl. Zool. 81, 1–10. doi: 10.1186/s41936-020-00195-7
Ishihara A. (2021). Defense mechanisms involving secondary metabolism in the grass family. J. Pesticide Sci. 46, 382–392. doi: 10.1584/jpestics.J21-05, PMID: 34908899
Jansen S., Watanabe T., and Smets E. (2002). Aluminium accumulation in leaves of 127 species in Melastomataceae, with comments on the order Myrtales. Ann. Bot. 90, 53–64. doi: 10.1093/aob/mcf142, PMID: 12125773
Janzen D. H. (1966). Coevolution of mutualism between ants and acacias in Central America. Evolution 20, 249–275. doi: 10.2307/2406628, PMID: 28562970
Janzen D. H. (1978). Cicada (Diceroprocta apache Davis) mortality by feeding on Nerium oleander. Pan-Pacif. Entomol. 54, 69–70.
Janzen D. H. (1979). “New horizons in the biology of plant defenses,” in Herbivores. Their Interaction with Secondary Plant Metabolites. Eds. Rosenthal G. A. and Janzen D. H. (Academic Press, New York), 331–350.
Janzen D. H. (1981). Lectins and plant-herbivore interactions. Recent Adv. Phytochem. 15, 241–258. doi: 10.1007/978-1-4684-3986-1_10
Janzen D. H. (1984a). Natural history of Hylesia lineata (Saturniidae: Hemileucinae) in Santa Rosa National Park, Costa Rica. J. Kans. Entomol. Soc 57, 490–514.
Janzen D. H. (1984b). Two ways to be a tropical big moth: Santa Rosa saturniids and sphingids. Oxf. Surv. Evol. Biol. 1, 85–140.
Janzen D. H. (1988). Ecological characterization of a Costa Rican dry forest caterpillar fauna. Biotropica 20, 120–135. doi: 10.2307/2388184
Janzen D. H. (2003). “How polyphagous are Costa Rican dry forest saturniid caterpillars?,” in Arthropods of Tropical Forests. Spatio-temporal Dynamics and Resource use in the Canopy. Eds. Basset Y., Novotny V., Miller S. E., and Kitching R. L. (Cambridge University Press, Cambridge, UK), 369–379.
Janzen D. H. (2004). Setting up tropical biodiversity for conservation through non-damaging use: participation by parataxonomists. J. Appl. Ecol. 41, 181–187. doi: 10.1111/j.1365-2664.2004.00879.x
Janzen D. H. and Hallwachs W. (2011). Joining inventory by parataxonomists with DNA barcoding of a large complex tropical conserved wildland in northwestern Costa Rica. PloS One 6, e18123. doi: 10.1371/journal.pone.0018123, PMID: 21857894
Janzen D. H. and Hallwachs W. (2016). DNA barcoding the Lepidoptera inventory of a large complex tropical conserved wildland, Area de Conservacion Guanacaste, northwestern Costa Rica. Genome 59, 641–660. doi: 10.1139/gen-2016-0005, PMID: 27584861
Janzen D. H. and Hallwachs W. (2017). Dynamic database for an inventory of the macrocaterpillar fauna, and its food plants and parasitoids, of Area de Conservación Guanacaste (ACG), northwestern Costa Rica. Available online at: http://janzen.sas.upenn.edu (Accessed August 21, 2015).
Janzen D. H. and Hallwachs W. (2020). Área de Conservación Guanacaste, northwestern Costa Rica: converting a tropical national park to conservation via biodevelopment. Biotropica 52, 1017–1029. doi: 10.1111/btp.12755
Janzen D. H. and Hallwachs W. (2021). To us insectometers, it is clear that insect decline in our Costa Rican tropics is real, so let’s be kind to the survivors. Proc. Natl. Acad. Sci. U.S.A. 118, e2002546117. doi: 10.1073/pnas.2002546117, PMID: 33431562
Janzen D. H., Hallwachs W., Blandin P., Burns J. M., Cadiou J. M., Chacon I., et al. (2009). Integration of DNA barcoding into an ongoing inventory of complex tropical biodiversity. Mol. Ecol. Res. 9, 1–26. doi: 10.1111/j.1755-0998.2009.02628.x, PMID: 21564960
Janzen D. H., Hallwachs W., Burns J. M., Hajibabaei M., Bertrand C., and Hebert P. D. N. (2011). Reading the complex skipper butterfly fauna of one tropical place. PloS One 6, e19874. doi: 10.1371/journal.pone.0019874, PMID: 21857895
Janzen D. H., Hallwachs W., and Kappelle M. (2016). “Biodiversity conservation history and future in Costa Rica: the case of Área de Conservación Guanacaste (ACG),” in Costa Rican Ecosystems. Ed. Kappelle M. (The University of Chicago Press, Chicago, Illinois and London), 290–341.
Kaartinen R., Stone G. N., Hearn J., Lohse K., and Roslin T. (2010). Revealing secret liaisons: DNA barcoding changes our understanding of food webs. Ecol. Ent. 35, 623–638. doi: 10.1111/j.1365-2311.2010.01224.x
Kariyat R. R., Hardison S. B., Ryan A. B., Stephenson A. G., De Moraes C. M., and Mescher M. C. (2018). Leaf trichomes affect caterpillar feeding in an instar-specific manner. Commun. Integ. Biol. 11, 1–6. doi: 10.1080/19420889.2018.1486653, PMID: 30214672
Kariyat R. R., Smith J. D., Stephenson A. G., De Moraes C. M., and Mescher M. C. (2017). Non-glandular trichomes of Solanum carolinense deter feeding by Manduca sexta caterpillars and cause damage to the gut peritrophic matrix. Proc. R. Soc B Biol. Sci. 284, 20162323. doi: 10.1098/rspb.2016.2323, PMID: 28228510
Kato M. J. and Furlan M. (2007). Chemistry and evolution of the piperaceae. Pure Appl. Chem. 79, 529–538. doi: 10.1351/pac200779040529
Katz O. (2015). Silica phytoliths in angiosperms: phylogeny and early evolutionary history. New Phytol. 208, 642–646. doi: 10.1111/nph.2015.208.issue-3, PMID: 26134931
Kaufman P. B., Dayanandan P., Takeoka Y., Bigelow W. C., Jones J. D., and Iler R. (1981). “Silica in shoots of higher plants,” in Silicon and Siliceous Structures in Biological Systems. Eds. Simpson T. L. and Volcani B. E. (Springer New York, New York, NY), 409–449.
Kerkig P., Quicke D. L. J., and Butcher B. A. (2025). Molecular food web of lepidopteran hosts and their parasitoids in a tropical secondary forest in Thailand. Trop. Nat. Hist. 25, 37–56.
Kim D. Y. and Choi B. Y. (2019). Costunolide—a bioactive sesquiterpene lactone with diverse therapeutic potential. Int. J. Mol. Sci. 20, 2926. doi: 10.3390/ijms20122926, PMID: 31208018
Kitajima S., Kamei K., Taketani S., Yamaguchi M., Kawai F., Komatsu A., et al. (2010). Two chitinase-like proteins abundantly accumulated in latex of mulberry show insecticidal activity. BMC Biochem. 11, 1–7. doi: 10.1186/1471-2091-11-6, PMID: 20109180
Klun J. A., Guthrie W. D., Hallauer A. R., and Russell W. A. (1970). Genetic nature of the concentration of 2,4-dihydroxy-7-methoxy 2H-l,4-benzoxazin- 3(4H)-one and resistance to the European corn borer in a diallel set of eleven maize inbreds. Crop Sci. 10, 87–90. doi: 10.2135/cropsci1970.0011183X001000010032x
Kong C. H., Xuan T. D., Khanh T. D., Tran H. D., and Trung N. T. (2019). Allelochemicals and signaling chemicals in plants. Molecules. 24, 2737. doi: 10.3390/molecules24152737, PMID: 31357670
Konno K., Hirayama C., Nakamura M., Tateishi K., Tamura Y., Hattori M., et al. (2004). Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J. 37, 370–378. doi: 10.1046/j.1365-313X.2003.01968.x, PMID: 14731257
Konno K. (2011). Plant latex and other exudates as plant defense systems: roles of various defense chemicals and proteins contained therein. Phytochemistry 72 (13), 1510–1530. doi: 10.1016/j.phytochem.2011.02.016, PMID: 21450319
Konozy E. H. E., Osman M. E. M., and Dirar A. I. (2023). A comprehensive review on Euphorbiaceae lectins: structural and biological perspectives. Biochem. (Mosc.) 88, 1956–1969. doi: 10.1134/S0006297923110238, PMID: 38105212
Kortbeek R. W., van der Gragt M., and Bleeker P. M. (2019). Endogenous plant metabolites against insects. Eur. J. Plant Pathol. 154, 67–90. doi: 10.1007/s10658-018-1540-6
Kripasana K. and Xavier J. (2020). Phytochemical analysis and antioxidant activity of leaf extracts of some selected plants of the family Acanthaceae. Plant Sci. Today 7, 264–274. doi: 10.14719/pst.2020.7.2.717
Lawal O. A., Ogunwande I. A., Osunsanmi F. O., Opoku A. R., and Oyedeji A. O. (2017). Croton gratissimus leaf essential oil composition, antibacterial, antiplatelet aggregation, and cytotoxic activities. J. Herbs Spices Med. Plants. 23, 77–87. doi: 10.1080/10496475.2016.1270245,2-s2.0-85009740577
Leme F. M., Borella P. H., Marinho C. R., and Teixeira S. P. (2020). Expanding the laticifer knowledge in Cannabaceae: distribution, morphology, origin, and latex composition. Protoplasma 257, 1183–1199. doi: 10.1007/s00709-020-01500-5, PMID: 32212022
Levin D. A. (1973). The role of trichomes in plant defense. Quart. Rev. Biol. 48, 3–15. doi: 10.1086/407484
Lewinsohn T. M. (1991). The geographical distribution of plant latex. Chemoecol. 2, 64–68. doi: 10.1007/BF01240668
Lewis O. T., Memmott J., Lasalle J., Lyal C. H., Whitefoord C., and Godfray H. C. J. (2002). Structure of a diverse tropical forest insect–parasitoid community. J. Anim. Ecol. 71, 855–873. doi: 10.1046/j.1365-2656.2002.00651.x
Loxdale H. D. and Balog A. (2025). Preface: the paradox of generalism, a philosophical perspective. Front. Ecol. Evol. 13. doi: 10.3389/fevo.2025
Luo D. Q., Zhang X., Tian X., and Liu J. K. (2004). Insecticidal compounds from Tripterygium wilfordii active against Mythimna separata. Z. Naturforsch. C 59, 421–426. doi: 10.1515/znc-2004-5-624, PMID: 18998413
Ma Z., Li Y., Wu L., and Zhang X. (2014). Isolation and insecticidal activity of sesquiterpenes alkaloids from Tripterygium wilfordii Hook f. Indust. Crops Prod 52, 642–648. doi: 10.1016/j.indcrop.2013.11.029
Macedo M. L. R., Oliveira C. F., and Oliveira C. T. (2015). Insecticidal activity of plant lectins and potential application in crop protection. Molecules 20, 2014–2033. doi: 10.3390/molecules20022014, PMID: 25633332
Magwilu K. D., Nguta J. M., Mapenay I., and Matara D. (2022). Phylogeny, phytomedicines, phytochemistry, pharmacological properties, and toxicity of Croton gratissimus Burch (Euphorbiaceae). Adv. Pharmacol. Pharmaceut. Sci. 2022, 1238270. doi: 10.1155/2022/1238270, PMID: 35619875
Marinho C. R. and Teixeira S. P. (2019). Novel reports of laticifers in Moraceae and Urticaceae: revisiting synapomorphies. Plant Syst. Evol. 305, 13–31. doi: 10.1007/s00606-018-1548-6
Marohasy J. (1998). The design and interpretation of host-specificity tests for weed biological control with particular reference to insect behaviour. Biocont. News Inform. 19, 13N–20N.
Marquis R. J. (2024). On the uniqueness of fern–insect interactions. New Phytol. 2024, 1–3. doi: 10.1111/nph.20361, PMID: 39722210
Martins D. and Nunez C. V. (2015). Secondary metabolites from Rubiaceae species. Molecules 20, 13422–13495. doi: 10.3390/molecules200713422, PMID: 26205062
Martucci M. E. P. and Gobbo-Neto L. (2016). Differential secondary metabolite accumulation and performance of Chlosyne lacinia f with Tithonia diversifolia or Vernonia polyanthes. Biochem. Syst. Ecol. 68, 156–162. doi: 10.1016/j.bse.2016.07.009
Mason P. A., Bernardo M. A., and Singer M. S. (2014). A mixed diet of toxic plants enables increased feeding and anti-predator defense by an insect herbivore. Oecologia 176, 477–486. doi: 10.1007/s00442-014-3029-0, PMID: 25106116
Massey F. P., Ennos A. R., and Hartley S. E. (2006). Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 75, 595–603. doi: 10.1111/j.1365-2656.2006.01082.x, PMID: 16638012
Metcalf C. R. (1967). Distribution of latex in the plant kingdom. Econ. Bot. 21, 115–127. doi: 10.1007/BF02897859
Metz M. A. (2024). Accurate classification of the species formerly combined in Teuchophanes Meyrick with the description of new species of Helcystogramma Zeller and Dichomeris Hübner (Lepidoptera: Gelechiidae: Dichomeridinae). Proc. Entomol. Soc Wash. 126, 317–336. doi: 10.4289/0013-8797.126.3.317
Metz M. A., Janzen D. H., and Hallwachs W. (2017). Descriptions of four new species of Struthoscelis Meyrick (Lepidoptera: Oecophoridae: Oecophorinae), one from Area De Conservación Guanacaste, Northwestern Costa Rica, providing the first known biology for the genus, and discovery of a novel wing morphology in males. Proc. Entomol. Soc Wash. 119, 442–458. doi: 10.4289/0013-8797.119.3.442
Metz M. A., Janzen D. H., and Hallwachs W. (2020). Four new gelechioid species to honor Costa Rica's conservation of wild biodiversity (Lepidoptera). Zootaxa 4810, 45–64. doi: 10.11646/zootaxa.4810.1.2, PMID: 33055909
Meyer D., Dimitriadou E., Hornik K., Weingessel A., Leisch F., Chang C.-C., et al. (2019). Package ‘e1071’, The R Journal, 2019. 1–66.
Mikić S. and Ahmad S. (2018). Benzoxazinoids-protective secondary metabolites in cereals: Biochemistry and genetic control. Field Veg. Crops Res. 55, 39–48. doi: 10.5937/ratpov55-12210
Miller S. E. (2007). DNA barcoding and the renaissance of taxonomy. Proc. Natl. Acad. Sci. U.S.A. 104, 4775–4776. doi: 10.1073/pnas.0700466104, PMID: 17363473
Mithöfer A. and Boland W. (2012). Plant defense against herbivores: chemical aspects. Ann. Rev. Plant Biol. 63, 431–450. doi: 10.1146/annurev-arplant-042110-103854, PMID: 22404468
Mohammed M. H. H. and Fouad M. A. (2022). Chemical and biological review on various classes of secondary metabolites and biological activities of Arecaceae, (2021-2006). J. Adv. Biomed. Pharmaceut. Sci. 5, 113–150. doi: 10.21608/jabps.2022.126338.1149
Morris B. J., Couto A., Aydin A., and Montgomery S. H. (2021). Re-emergence and diversification of a specialized antennal lobe morphology in ithomiine butterflies. Evolution 75, 3191–3202. doi: 10.1111/evo.14324, PMID: 34383301
Morris J. L., Puttick M. N., Clark J. W., Edwards D., Kenrick P., Pressel S., et al. (2018). The timescale of early land plant evolution. Proc. Natl. Acad. Sci. U.S.A. 115, E2274–E2283. doi: 10.1073/pnas.1719588115, PMID: 29463716
Mumm R., Posthumus M. A., and Dicke M. (2008). Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 31, 575–585. doi: 10.1111/j.1365-3040.2008.01783.x, PMID: 18208515
Mustafa A., Ensikat H.-J., and Weigend M. (2018a). Stinging hair morphology and wall biomineralization across five plant families: conserved morphology versus divergent cell wall composition. Amer. J. Bot. 105, 1109–1122. doi: 10.1002/ajb2.1136, PMID: 30080249
Mustafa A., Ensikat H.-J., and Weigend M. (2018b). Mineralized trichomes in Boraginales: complex microscale heterogeneity and simple phylogenetic patterns. Ann. Bot. 121, 741–751. doi: 10.1093/aob/mcx191, PMID: 29325008
Nahrstedt A. (1998). Cyanogenesis in the Zygaenidae (Lepidoptera): a review of the state of the art. Theses Zoologicae 30, 17–30.
Narango D. L., Tallamy D. W., and Shropshire K. J. (2020). Few keystone plant genera support the majority of Lepidoptera species. Nat. Comms 11, 5751. doi: 10.1038/s41467-020-19565-4, PMID: 33188194
Nayar J. K. and Fraenkel G. (1963). The chemical basis of the host selection in the catalpa sphinx, Ceratomia catalpae (Lepidoptera, Sphingidae). Ann. Entomol. Soc Amer. 56, 119–122. doi: 10.1093/aesa/56.1.119
Neuwirth E. (2014). RColorBrewer: ColorBrewer Palettes. R Package Version 1.1-3. Available online at: https://CRAN.R-project.org/package=RColorBrewer (Accessed August 21, 2015).
New T. R. (2023). “Introducing moth variety and diversity,” in The Other Lepidoptera: Moth Conservation in Australia (Springer International Publishing, Cham, Switzerland), 1–20. doi: 10.1007/978-3-031-32103-0_1
Nihei K., Asaka Y., Mine Y., Yamada Y., Iigo M., and Yanagisawa T. (2006). Musidunin and musiduol, insect antifeedants from Croton jatrophoides. J. Nat. Prod. 69, 975–977. doi: 10.1021/np060068d, PMID: 16792423
Nisbet A. J. (2000). Azadirachtin from the neem tree Azadirachta indica: its action against insects. Anais Sociedad. Entomol. Brasil 29, 615–632. doi: 10.1590/S0301-80592000000400001
Nitta J. H., Schuettpelz E., Ramırez-Barahona S., and Iwasaki W. (2022). An open and continuously updated fern tree of life. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.909768, PMID: 36092417
Novotny V., Basset Y., Miller S. E., Drozd P., and Cizek L. (2002b). Host specialization of leaf-chewing insects in a New Guinea rainforest. J. Anim. Ecol. 71, 400–412. doi: 10.1046/j.1365-2656.2002.00608.x
Novotny V., Basset Y., Miller S. E., Weiblem G. D., Bremer B., Cizek L., et al. (2002a). Low host specificity of herbivorous insects in a tropical forest. Nature 416, 841–844. doi: 10.1038/416841a, PMID: 11976681
Novotny V., Miller S. E., Baje L., Balagawi S., Basset Y., Cizek L., et al. (2010). Guild-specific patterns of species richness and host specialization in plant-herbivore food webs from a tropical forest. J. Anim. Ecol. 79, 1193–1203. doi: 10.1111/j.1365-2656.2010.01728.x, PMID: 20673235
Novotny V., Miller S. E., Leps J., Basset Y., Bito D., Janda M., et al. (2004). No tree an island: the plant–caterpillar food web of a secondary rain forest in New Guinea. Ecol. Lett. 7, 1090–1100. doi: 10.1111/j.1461-0248.2004.00666.x
Ocampo Serna D. M. and Isaza Martínez J. H. (2015). Phenolics and polyphenolics from Melastomataceae species. Molecules 20, 17818–17847. doi: 10.3390/molecules201017818, PMID: 26404220
Onanuga A. O. and Oloyede G. K. (2021). Two new biologically active steroids from Costus lucanusianus (Costaceae). Steroids 175, 108913. doi: 10.1016/j.steroids.2021.108913, PMID: 34481815
Park D. S. and Coats J. R. (2002). Cyanogenic glycosides: alternative insecticides? Kor. J. Pesticide Sci. 6, 51–57.
Powell J. A. (1980). Evolution of larval food preferences in microlepidoptera. Ann. Rev. Entomol. 25, 133–159. doi: 10.1146/annurev.en.25.010180.001025
Powell J. A., Mitter C., and Farrell B. (1998). “Evolution of larval food preferences,” in Handbook of Zoology part 35, Lepidoptera. In Lepidoptera, Moths and Butterflies, part 4. Ed. Kristensen N. P. (de Gruyter, Berlin), 403–422.
Prota N., Bouwmeester H. J., and Jongsma M. A. (2014). Comparative antifeedant activities of polygodial and pyrethrins against whiteflies (Bemisia tabaci) and aphids (Myzus persicae). Pest Manage. Sci. 70, 682–688. doi: 10.1002/ps.3610, PMID: 23868321
Quicke D. L. J., Ghafouri Moghaddam M., and Butcher B. A. (2023). Dietary challenges for parasitoid wasps (Hymenoptera: Ichneumonoidea); coping with toxic hosts, or not? Toxins 15, 424. doi: 10.3390/toxins15070424, PMID: 37505693
Quicke D. L. J., Janzen D. H., Hallwachs W., Sharkey M. J., Hebert P. D. N., and Butcher B. A. (2024). Forty-five years of caterpillar rearing in Area de Conservación Guanacaste (ACG) northwestern Costa Rica: DNA barcodes, BINs, and a first description of plant–caterpillar– ichneumonoid interactions detected. Diversity 16, 683. doi: 10.3390/d16110683
Ramos M. V., Grangeiro T. B., Freire E. A., Sales M. P., Souza D. P., Araújo E. S., et al. (2010). The defensive role of latex in plants: detrimental effects on insects. Arthrop.-Plant Interact. 4, 57–67. doi: 10.1007/s11829-010-9084-5
Raskin I., Kumar P. B. A. N., Dushenkov S., and Salt D. E. (1994). Bioconcentration of heavy metals by plants. Curr. Op. Biotech. 5, 285–290. doi: 10.1016/0958-1669(94)90030-2
Rathke B. J. and Poole R. W. (1975). Coevolutionary race continues: butterfly larval adaptation to plant trichomes. Science 187, 175–176. doi: 10.1126/science.187.4172.175, PMID: 17736541
Ratnasingham S. and Hebert P. D. N. (2013). A DNA-based registry for all animal species: The Barcode Index Number (BIN) System. PloS One 8, e66213. doi: 10.1371/journal.pone.0066213, PMID: 23861743
Rattan R. (2023). Bioactive phytochemicals from Acanthaceae – mini review. J. Emerg. Technol. Innov. Res. 10, 6.
R Development Core Team (2024). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://cran.r-project.org.
Ribeiro S. P., Londe V., Bueno A. P., Barbosa J. S., Corrêa T. L., Soeltl T., et al. (2017). Plant defense against leaf herbivory based on metal accumulation: examples from a tropical high altitude ecosystem. Plant Sp. Biol. 32, 147–155. doi: 10.1111/1442-1984.12136
Rizwan K., Majeed I., Bilal M., Rasheed T., Shakeel A., and Iqbal S. (2022). Phytochemistry and diverse pharmacology of genus Mimosa: a review. Biomolecules 12, 83. doi: 10.3390/biom12010083, PMID: 35053231
Robbins R. K., Cong Q., Zhang J., Shen J., Quer Riera J., Murray D., et al. (2021). A switch to feeding on cycads generates parallel accelerated evolution of toxin tolerance in two clades of Eumaeus caterpillars (Lepidoptera: Lycaenidae). Proc. Natl. Acad. Sci. U.S.A. 118, e2018965118. doi: 10.1073/pnas.2018965118, PMID: 33568532
Rumohr Q., Baden C. U., Bergtold M., Marx M. T., Oellers J., SChade M., et al. (2023). Drivers and pressures behind insect decline in Central and Western Europe based on long-term monitoring data. PloS One 18, e0289565. doi: 10.1371/journal.pone.0289565, PMID: 37611013
Schuettpelz E., Schneider H., Smith A. R., Hovencamp P., Prado J., Pouhan G., et al. (2016). A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 54, 563–603. doi: 10.1111/jse.12229
Scott I. M., Jensen H. R., Philogène B. J., and Arnason J. T. (2008). A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem. Rev. 7, 65–75. doi: 10.1007/s11101-006-9058-5
Scriber J. M. and Feeny P. (1979). Growth of herbivorous caterpillars in relation to feeding specialization and to the growth form of their food plants. Ecology 60, 829–850. doi: 10.2307/1936618
Shannon C. E. (1948). A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Sharma R., Kumar V., and Kumar R. (2019). Distribution of phytoliths in plants: a review. Geol. Ecolo. Landscape. 3, 123–148. doi: 10.1080/24749508.2018.1522838
Shin-Foon C. and Yu-Tong Q. (1993). Experiments on the application of botanical insecticides for the control of diamondback moth in South China. J. Appl. Entomol. 116, 479–486. doi: 10.1111/j.1439-0418.1993.tb01224.x
Shrestha T., Kopp B., and Bisset N. G. (1992). The Moraceae-based dart poisons of South America. Cardiac glycosides of Maquira and Naucleopsis species. J. Ethnopharmacol. 37, 129–143. doi: 10.1016/0378-8741(92)90071-X, PMID: 1434687
Simmonds M. S. (2006). The search for plant-derived compounds with antifeedant activity. Adv. Phytomed. 3, 291–324. doi: 10.1016/S1572-557X(06)03013-3
Simpson G. G. (1951). Horses: the Story of the Horse Family in the Modern World and through Sixty Million Years of History (Oxford, UK: Oxford University Press).
Singer M. S. (2016). Behaviorally plastic host-plant use by larval Lepidoptera in tri-trophic food webs. Curr. Opin. Ins. Sci. 14, 56–60., PMID: 27436647
Sisson M. S., Dowdy N. J., Fisher M. L., Gall L. F., Goldstein P. Z., Homziak N. T., et al. (2025). Erebidae systematics: past, present, and future—progress in understanding a diverse lepidopteran lineage. Ins. Syst. Diver. 9, 5. doi: 10.1093/isd/ixaf018
Sittenfeld A., Uribe-Lorio L., Mora M., Nielsen V., Arrieta G., and Janzen D. H. (2002). Does a polyphagous caterpillar have the same gut microbiota when feeding on different species of food plants? Rev. Biol. Trop. 50, 547–560., PMID: 12298285
Smith D. A. S. (2014). African Queens and their Kin: A Darwinian Odyssey (Brambleby Books, Taunton, UK).
Smith M. A. (2023). Temperature, precipitation and soil characteristics of Volcan Cacao - Area de Conservacion Guanacaste (ACG), Costa Rica. doi: 10.5683/SP3/LQYQJZ (Accessed August 25, 2025).
Smith M. A., Rodriguez J. J., Whitfield J. B., Deans A. R., Janzen D. H., Hallwachs W., et al. (2008). Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc. Natl. Acad. Sci. U.S.A. 105, 12359–12364. doi: 10.1073/pnas.0805319105, PMID: 18716001
Smith M. A., Wood D. M., Janzen D. H., Hallwachs W., and Hebert P. D. N. (2007). DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (Diptera, Tachinidae) are not all generalists. Proc. Natl. Acad. Sci. U.S.A. 104, 4967–4972. doi: 10.1073/pnas.0700050104, PMID: 17360352
Smith M. A., Woodley N., Hallwachs W., Janzen D. H., and Hebert P. D. N. (2006). DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proc. Natl. Acad. Sci. U.S.A. 103, 3657–3662. doi: 10.1073/pnas.0511318103, PMID: 16505365
Sohrab S., Mishra P., and Mishra S. K. (2021). Phytochemical competence and pharmacological perspectives of an endangered boon—Costus speciosus (Koen.) Sm.: a comprehensive review. Bull. Natl. Res. Centre 45, 1–27. doi: 10.1186/s42269-021-00663-2
Solis M. A., Davis D. R., and Nishida K. (2005). Biology and systematics of Albusambia elaphaglossumae, a new genus and species of Crambidae (Lepidoptera: Pyraloidea: Crambidae) mining the fronds of Elaphoglossum conspersum (Pteridophyta: Lomariopsidaceae) in Costa Rica. Int. J. Trop. Biol. 53, 487–501. doi: 10.15517/rbt.v53i3-4.14617, PMID: 17354458
Solis M. A., Phillips-Rodríguez E., Hallwachs W., Dapkey T., and Janzen D. H. (2020). Asturodes Amsel (Lepidoptera: Crambidae: Spilomelinae): three new species from the Western Hemisphere and food plant records from Area de Conservación Guanacaste, Costa Rica. Proc. Entomol. Soc Wash. 122, 147–171. doi: 10.4289/0013-8797.122.1.147
Solis M. A. and Styer L. (2003). Revision and phylogenetic analysis of Accinctapubes Solis (Lepidoptera: Pyralidae: Epipaschiinae), including a new species from Costa Rica and a larval description of A. albifasciata (Druce) that feeds on avocado. J. Lepid. Soc 57, 121–136.
Spivey A. C., Weston M., and Woodhead S. (2002). Celastraceae sesquiterpenoids: biological activity and synthesis. Chem. Soc Rev. 31, 43–59. doi: 10.1039/b000678p, PMID: 12108982
Stanisçuaski F. and Carlini C. R. (2012). Plant ureases and related peptides: understanding their entomotoxic properties. Toxins 4, 55–67. doi: 10.3390/toxins4020055, PMID: 22474566
Stireman J. O. III, Dyer L. A., and Greeney H. F. (2017). Specialised generalists? Food web structure of a tropical tachinid-caterpillar community. Ins. Conserv. Divers. 10, 367–384. doi: 10.1111/icad.12238
Sutherland O. R., Russell G. B., Biggs D. R., and Lane G. A. (1980). Insect feeding deterrent activity of phytoalexin isoflavonoids. Biochem. Syst. Ecol. 8, 73–75. doi: 10.1016/0305-1978(80)90029-0
The Angiosperm Phylogeny Group, Chase M. W., Christenhusz M. J., Fay M. F., Byng J. W., Judd W. S., et al. (2016). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc 181, 1–20. doi: 10.1111/boj.12385
Thomsen K. and Brimer L. (1997). Cyanogenic constituents in woody plants in natural lowland rain forest in Costa Rica. Bot. J. Linn. Soc 124, 273–294. doi: 10.1111/j.1095-8339.1997.tb01793.x
Timpone L. T., Bacci L. F., Goldenberg R., and Habermann G. (2025). Aluminum accumulation in Miconia species of the Atlantic rainforest: phylogenetic insights and soil interactions. Trees 39, 47. doi: 10.1007/s00468-025-02623-z
Trembath-Reichert E., Wilson J. P., McGlynn S. E., and Fischer W. W. (2015). Four hundred million years of silica biomineralization in land plants. Proc. Natl. Acad. Sci. U.S.A. 112, 5449–5454. doi: 10.1073/pnas.1500289112, PMID: 25825729
Trigo J. R. and Brown K. S. Jr (1990). Variation of pyrrolizidine alkaloids in Ithomiinae: A comparative study between species feeding on Apocynaceae and Solanaceae. Chemoecol. 1, 22–29. doi: 10.1007/BF01240582
Tuberville T. D., Dudley P. G., and Pollard A. J. (1996). Responses of invertebrate herbivores to stinging trichomes of Urtica dioica and Laportea canadensis. Oikos 75, 83–88. doi: 10.2307/3546324
Turner J. R. G. (1971). “Studies of Müllerian mimicry and its evolution in burnet moths and heliconid butterflies,” in Ecological Genetics and Evolution. Ed. Creed E. R. (Blackwell, Oxford), 224–260.
Tzin V., Hojo Y., Strickler S. R., Bartsch L. J., Archer C. M., Ahern K. R., et al. (2017). Rapid defense responses in maize leaves induced by Spodoptera exigua caterpillar feeding. J. Exp. Bot. 68, 4709–4723. doi: 10.1093/jxb/erx274, PMID: 28981781
Underwood K. D., Puschendorf R., Bilton D. T., Hallwachs W., Janzen D. H., and Smith M. A. (2024). Decoding Anotylus (Thomson 1859) beetle diversity: DNA and external morphology match in Área de Conservaciόn Guanacaste, Costa Rica. Diversity 16, 441. doi: 10.3390/d16080441
Van Damme E. J. M., Lannoo N., and Peumans W. J. (2008). Plant lectins. Adv. Bot. Res. 48, 107–209. doi: 10.1016/S0065-2296(08)00403-5
Vandenborre G., Smagghe G., and Van Damme E. J. (2011). Plant lectins as defense proteins against phytophagous insects. Phytochem. 72, 1538–1550. doi: 10.1016/j.phytochem.2011.02.024, PMID: 21429537
van Nieukerken E. J., Kaila L., Kitching I. J., Kristensen N. P., Lees D. C., Minet J, et al. (2011). Order Lepidoptera Linnaeus, 1758. Zootaxa 3148, 212–221.
Vencl F. V. and Morton T. C. (1998). The shield defense of the sumac flea beetle, Blepharida rhois (Chrysomelidae: Alticinae). Chemoecol. 8, 25–32. doi: 10.1007/PL00001800
Vetter J. (2000). Plant cyanogenic glycosides. Toxicon 38, 11–36. doi: 10.1016/S0041-0101(99)00128-2, PMID: 10669009
Wagner D. L. (2005). Caterpillars of Eastern North America: A Guide to Identification and Natural History (Princeton: Princeton University Press).
Wahlberg N. (2001). The phylogenetics and biochemistry of host-plant specialization in melitaeine butterflies (Lepidoptera: Nymphalidae). Evolution 55, 522–537. doi: 10.1111/j.0014-3820.2001.tb00786.x, PMID: 11327160
War A. R., Buhroo A. A., Hussain B., Ahmad T., Nair R. M., and Sharma H. C. (2020). “Plant defense and insect adaptation with reference to secondary metabolites,” in Co-Evolution of Secondary Metabolites, Reference Series in Phytochemistry. Eds. Mérillon J. M. and Ramawat K. (Springer International Publishing, Cham, Switzerland), 795–822.
War A. R., Paulraj M. G., Ahmad T., Buhroo A. A., Hussain B., Ignacimuthu S., et al. (2012). Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 7, 1306–1320. doi: 10.4161/psb.21663, PMID: 22895106
Warowicka A., Nawrot R., and Goździcka-Józefiak A. (2020). “Pharmacologically active compounds from latex-bearing plants,” in Advances in Botanical Research, vol. 93 . Ed. Nawrot R. (Elsevier, Netherlands), 119–151.
Weigend M., Mustafa A., and Ensikat H. J. (2018). Calcium phosphate in plant trichomes: the overlooked biomineral. Planta 247, 277–285. doi: 10.1007/s00425-017-2826-1, PMID: 29234879
Weller S. J., Jacobsen N. L., and Conner W. E. (1999). The evolution of chemical defenses and mating systems in tiger moths (Lepidoptera: Arctiidae). Biol. J. Linn. Soc 68, 557–578. doi: 10.1111/j.1095-8312.1999.tb01188.x
Whitaker M. R., Banack S. A., Mescher M. C., Cox P. A., and De Moraes C. M. (2023). BMAA in cycad-feeding Lepidoptera: defensive sequestration or bioaccumulation? Front. Ecol. Evol. 11. doi: 10.3389/fevo.2023.1114636
Willaman J. J. and Schubert B. (1961). Alkaloid-bearing plants and their contained alkaloids (No. 1234) (Washington D.C.: US Department of Agriculture).
Wilson J. J. (2012). “DNA barcodes for insects,” in DNA Barcodes: Methods and Protocols. Eds. Kress W. J. and Erickson D. L. (Springer, New York), 17–46. doi: 10.1007/978-1-61779-591-6_3, PMID: 22684951
Witthohn K. and Naumann C. M. (1987). Cyanogenesis—A general phenomenon in the Lepidoptera. J. Chem. Ecol. 13, 1789–1809., PMID: 24302389
Wright K. (2025). Overview of the 'pals' package. Available online at: https://cran.r-project.org/web/packages/pals/vignettes/pals_examples.html (Accessed June 14, 2025).
Wu J. W. and Haard N. F. (2000). Purification and characterization of a cystatin from the leaves of methyl jasmonate treated tomato plants. Comp. Biochem. Physiol. C 127, 209–220. doi: 10.1016/S0742-8413(00)00145-6, PMID: 11083031
Xu W. H., Liu W. Y., and Liang Q. (2018). Chemical constituents from croton species and their biological activities. Molecules 23, 9. doi: 10.3390/molecules23092333,2-s2.0-85053633929, PMID: 30213129
Yang Y. H., Michaud J. P., Guan X. M., Cao J. J., Li Z., Yang Q. P., et al. (2016). Direct and indirect consumption of tannic acid impedes the development and survival of parasitoid when parasitizing cotton bollworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc Am. 109, 839–844. doi: 10.1093/aesa/saw062
Yoneyama K. and Natsume M. (2010). “Chapter 4.13 - Allelochemicals for plant–plant and plant–microbe interactions,” in Comprehensive Natural Products II. Eds. Liu H.-W. and Mander L. (Elsevier, Netherlands), 539–561.
Young A. M. (1973). Notes on the biology of Phyciodes (Eresia) eutropia (Lepidoptera: Nymphalidae) in a Costa Rican mountain forest. J. N. Y. Entomol. Soc 81, 87–100.
Zagrobelny M., Bak S., and Møller B. L. (2008). Cyanogenesis in plants and arthropods. Phytochem. 69, 1457–1468. doi: 10.1016/j.phytochem.2008.02.019, PMID: 18353406
Zalucki M. P. and Brower L. P. (1992). Survival of first instar larvae of Danaus plexippus (Lepidoptera: Danainae) in relation to cardiac glycoside and latex content of Asclepias humistrata (Asclepiadaceae). Chemoecol. 3, 81–93. doi: 10.1007/BF01245886
Zaspel J. M., Weller S. J., Wardwell C. T., Zahiri R., and Wahlberg N. (2014). Phylogeny and evolution of pharmacophagy in tiger moths (Lepidoptera: Erebidae: Arctiinae). PloS One 9, e101975. doi: 10.1371/journal.pone.0101975, PMID: 25036028
Zexer N., Kumar S., and Elbaum R. (2023). Silica deposition in plants: scaffolding the mineralization. Ann. Bot. 131, 897–908. doi: 10.1093/aob/mcad056, PMID: 37094329
Keywords: trophic interactions, latex, toxins, diet breadth, Lepidoptera, food plants, specialism
Citation: Quicke DLJ, Brown A, Hajibabaei M, Manjunath R, Naik S, Ratnasingham S, Sones J, Jacques BS, Smith MA, Zamora N, Brown JW, Matson TA, Miller SE, Burns JM, Goldstein PZ, Metz MA, Robbins R, Solis MA, Chacon I, Espinoza B, Picado A, Phillips-Rodriguez E, Hebert PDN, Janzen DH, Hallwachs W and Butcher BA (2025) Caterpillar diet breadth in Área de Conservación Guanacaste, a large and diverse Neotropical wildland in northwestern Costa Rica: toxins, silica, aluminum, and sclerophylly. Front. Ecol. Evol. 13:1647436. doi: 10.3389/fevo.2025.1647436
Received: 15 June 2025; Accepted: 05 August 2025;
Published: 18 September 2025.
Edited by:
Hugh David Loxdale, Cardiff University, United KingdomReviewed by:
Vojtech Novotny, Academy of Sciences of the Czech Republic (ASCR), CzechiaDavid Heckel, Max Planck Institute for Chemical Ecology, Germany
Copyright © 2025 Quicke, Brown, Hajibabaei, Manjunath, Naik, Ratnasingham, Sones, Jacques, Smith, Zamora, Brown, Matson, Miller, Burns, Goldstein, Metz, Robbins, Solis, Chacon, Espinoza, Picado, Phillips-Rodriguez, Hebert, Janzen, Hallwachs and Butcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Buntika A. Butcher, QnVudGlrYS5hQGNodWxhLmFjLnRo
 Donald L. J. Quicke1
Donald L. J. Quicke1 Mehrdad Hajibabaei
Mehrdad Hajibabaei Suresh Naik
Suresh Naik Sujeevan Ratnasingham
Sujeevan Ratnasingham Paul Z. Goldstein
Paul Z. Goldstein Robert Robbins
Robert Robbins Daniel H. Janzen
Daniel H. Janzen Buntika A. Butcher
Buntika A. Butcher