- 1US Department of Agriculture, Agricultural Research Service, Northern Plains Agricultural Research Laboratory, Sidney, MT, United States
- 2Missouri Department of Conservation, Columbia, MO, United States
- 3Department of Entomology, University of Wisconsin-Madison, Madison, WI, United States
- 4Saint Louis Zoo, St. Louis, MO, United States
Bison were historically a dominant large grazer in the Great Plains but were extirpated from much of their historic range. From reintroduction efforts, we understand bison and their associated activities have keystone effects on plants and wildlife in bison-grazed areas. Their specific activities modify the soil and plant community, but these effects on invertebrate communities are less explored, despite the diverse functional roles of grassland invertebrates. Wallowing, a unique behavior of bison in which they repeatedly roll on the ground and create bare depressions, may influence nesting resources of important ground-nesting pollinators (bees and wasps). This behavior provides one of the sources of bison-associated landscape heterogeneity, but how wallowing affects ground-nesting pollinators and other insects is not well-understood. Our broad objectives were to identify ground-nesting insects using wallows as nesting sites in north-central Montana and collate a list of other bison wallow-associated arthropods documented in the literature to understand the ecological interactions associated with bison-specific disturbances to the landscape. For our field study, we used emergence traps and sweep netting surveys to compare wallow and non-wallow prairie sites to determine differences of ground-nesting bee and wasp richness and abundance. Additionally, we surveyed surrounding vegetation communities and soil compaction at wallow and non-wallow sites. Our collections of 52 taxa were dominated by various wasp families (Mutillidae, Chrysididae, Crabronidae, Pompilidae), with few bees. Overall, we found higher abundance and taxonomic richness of ground-nesting pollinators emerging from within our adjacent prairie sites compared to within wallows. Vegetation surveys revealed distinct plant communities around bison wallows compared to adjacent prairie sites, with the most common forbs being non-native species. We found a small number of studies that collectively sampled 40 arthropod families associated with wallows, but our field study is the first published data on ground-nesting pollinator use of wallows. These data increase our knowledge of bison-engineered ecological interactions and how bison reintroductions might influence ground-nesting insects such as bees and wasps within the shortgrass prairie/sagebrush steppe ecosystem of the Northern Great Plains.
Introduction
Temperate grasslands are considered one of the most endangered biomes globally (Hoekstra et al., 2005) with much of the Great Plains region of the United States being converted to cropland (Olimb and Robinson, 2019). However, there has been a renewed effort in recent decades to preserve and restore some of these important grassland habitats. Restoration events often involve reintroducing native plant and animal species that have declined or were extirpated due to human activities. Bison (Bison bison), once one of the most common large land animals of the Great Plains, was nearly brought to extinction in the late 1800s due to commercial and subsistence hunting, exotic bovine diseases, and forage competition with domestic stock (Flores, 1991). Today, the reintroduction of bison to portions of North American prairies has been argued to be one of the most important conservation management practices for grassland ecosystems (Ratajczak et al., 2022). Bison are a keystone species (Knapp et al., 1999) and it is well established that their presence, in conjunction with other large fauna, played a significant role in shaping North America’s grassland ecosystems (Axelrod, 1985; Anderson, 2006). Beyond the ecological values of bison, they also have a historic and cultural value to Native Americans (Kolipinski et al., 2014). Historically, bison were critical for survival of Native Americans providing food, clothing, shelter, and tools. Indigenous cultures in North America also had (and continue to have) spiritual connections to bison and modern tribal nations that have bison herds are kept well beyond simple economic reasons (Sanderson et al., 2008).
Although domestic cattle (Bos taurus) have largely replaced bison as the primary bovine grazers throughout North America, there is clear evidence that the disturbance regimes of these domestic grazers do not sufficiently replicate those of their historic counterparts (Kohl et al., 2013). Cattle tend to stay relatively near water sources whereas bison move much greater distances away from water (Kohl et al., 2013). Additionally, wallowing by bison, a behavior cattle do not exhibit, is one of the most evident and observable differences between the two grazers (McMillan et al., 2011). Wallowing behavior involves rolling on dry ground which, if repeated in the same area, creates bare depressions (e.g., wallows) within the landscape. Numerous reasons have been proposed for wallowing and include relief from insects/skin irritation, shedding of winter coats, and various social behaviors (McMillan et al., 2000). Bison wallows, compared directly to adjacent grassland habitats, typically have greater soil compaction and water retention (Polley and Wallace, 1986). This can result in greater aboveground annual net primary production of plant communities occurring at the edge of wallows (McMillan et al., 2011) and overall increased plant diversity and community heterogeneity within bison-grazed grasslands (Knapp et al., 1999; Ratajczak et al., 2022).
Bison reintroductions into grassland habitats have also been shown to affect various species of birds (Boyce et al., 2022; Fagre et al., 2022), mammals (Matlack et al., 2001; Burke et al., 2020), and amphibians (Gerlanc and Kaufman, 2003). However, less information exists regarding how other key groups (e.g., various arthropods such as pollinators) respond to the presence of bison in prairie habitats, especially relating to the effects of wallowing behaviors. Previously, Nickell et al. (2018) found greater arthropod diversity on abandoned bison wallows than surrounding prairie but the opposite with active wallows, suggesting potential long-term benefits of bison disturbance to insects. Additionally, wallows in tallgrass prairie can contain standing water that support aquatic invertebrates (Gerlanc, 2004; Frazier et al., 2024).
As management to preserve and restore historic ecological interactions becomes increasingly necessary, so does the need for targeted research assessing the responses of native flora and fauna to conservation management practices. One important arthropod group, pollinators, are unfortunately in decline for numerous reasons, including increased pesticide use, deleterious agricultural practices (e.g., native habitat conversion), habitat fragmentation, invasive species colonization, spread of pathogens, urbanization, and climate change (Potts et al., 2010; Cameron et al., 2011; Abbate et al., 2019). Approximately one fifth of pollinator species is currently at risk of extinction (Cornelisse et al., 2025) and throughout the Northern Great Plains (NGP), habitat destruction and degradation for anthropogenic purposes is one of the primary drivers of native pollinator losses (Winfree et al., 2009; Koh et al., 2016). North American grasslands, including shortgrass prairie, have been fundamentally altered by the removal of historic grazers, including bison, and subsequent intensive management for agriculture and domestic grazers (Axelrod, 1985; Samson et al., 2004; Augustine et al., 2021). The few studies that have examined bison impacts on pollinating insects focused on bees and found conflicting responses. For example, Griffin et al. (2021) found decreases in bee abundance in bison pastures compared to pastures containing no bison, whereas Rosenberger and Conforti (2020) found increases in bumble bee abundance within bison pastures, thus exemplifying the need for future studies examining bison impact on the resources needed by bees and other pollinators.
How the creation of bison wallows specifically affects bees and wasps, arguably the most important insect pollinators, is largely unknown. Considering most native bees (Harmon-Threatt, 2020; Antoine and Forrest, 2021) and many wasps (O'Neill, 2001) construct ground nests, bison wallows could be an overlooked yet important nesting resource for bees and wasps in bison-grazed grasslands due to the creation of bare ground and compacted soil. Addtionally, floral resource richness and abundance has been found to increase within bison grazed areas (Collins et al., 1998). Ground-nesting pollinator use of spatially dynamic landscapes created by bison cannot be easily predicted, as preferred nesting sites and soil requirements are unknown for the majority of bee and wasp species (Harmon-Threatt, 2020) despite their importance of pollination to wild and agricultural plants. Although numerous abiotic factors may play roles in bee preference for nest sites, many bees have been shown to prefer nesting sites within compacted soil (Antoine and Forrest, 2021).
We monitored bison wallows and adjacent prairie for ground-nesting wasps and bees in a shortgrass prairie/sagebrush steppe ecosystem to provide empirical evidence of associations between ground-nesting bees and wasps and bison wallows. Our objectives were (1) identify ground-nesting bees and wasps utilizing bison wallows for nesting sites, (2) determine if ground-nesting bees and wasps are more diverse and abundant near bison wallows compared to nearby non-wallowed areas and if any insect community differences existed between wallows and non-wallowed prairie, (3) assess differences in plant community and soil compaction between wallow and non-wallow sites. Additionally, we (4) conducted a review to identify all wallow-associated arthropods currently in the literature to further understand the resources bison wallowing creates for grassland arthropods.
Methods
Our study area was located within the 11,000 ha Sun Prairie unit of American Prairie (47.766236, - 107.770859) in central and northeastern Montana, USA which is comprised of a mix of shortgrass prairie and sagebrush steppe. Topography consists of gently rolling hills and soil type is clay-loam dominated by Harlake Clay. American Prairie is a private, non-profit initiative working to connect and restore public and private lands to create one of the largest nature reserves in the United States (https://americanprairie.org/). First stocked with 16 bison in 2005, Sun Prairie’s bison herd now numbers over 400. Bison were present within the Sun Prairie unit during the duration of our study. Our study site was chosen due to the numerous active and non-active wallows that can be found and comprised an approximately 400 ha area (see Supplementary Figure 1). To ensure all traps could be deployed and retrieved within a 72-hour period, only wallows located within 500 m of the main road traversing the Sun Prairie Unit were considered. Only active wallows were eligible for inclusion. Active wallows within the survey area were initially measured, with most having maximum diameters between approximately 2 and 5 m. To minimize potential effects of wallow size variability, only wallows within this size range were retained as potential sampling sites. Wallows lacking a feasible control area 20 m north or south (e.g., due to the presence of another wallow or the road) were excluded. The remaining wallows (approximately 35 total) were assigned unique identifiers, and the final study sites were selected using a random number generator. All 25 wallows chosen were slightly irregular, though roughly circular, and we measured the largest diameter of each wallow as well as the diameter of the wallow 90° from the first measurement. Overall, wallows chosen for this study had an average diameter of 3.31 meters (range 2.2 - 4.2 m). Individual wallows were located a minimum of 50 m from other wallows used in this study (Supplementary Figure 1).
To assess the use of bison wallows as nesting habitat by ground-nesting bees and wasps, soil emergence traps were deployed once per month for 72 hours. Emergence traps (MegaView Science Company, Talchung, Taiwan; Supplementary Figure 2) were comprised of an enclosed, four-sided mesh structure (0.62 m2) that tapered into a collection jar at its apex. In 2021, emergence traps were placed near the center on 12 bison wallows and paired control locations (adjacent prairie) May-August. In 2022, the same 12 wallows and an additional 13 wallows and paired controls were utilized for a total of 25 paired wallows/controls. In 2022, emergence traps were deployed June-August. This chosen time frame coincides with the main growing season and bee activity within our region. However, we do acknowledge that some early spring bee species (e.g., some andrenid species) would have been missed. Control plots were located 20 m from each study wallow in a standardized direction from the edge of the paired wallow (Figure 1). Wallows chosen were all active in 2021 and had similar characteristics (e.g., size, soil type, etc.). During trap deployment, we avoided placing traps on top of any potential pre-existing insect holes/nests identified through visual surveys in the wallows and control sites in order to only collect newly emerged insects. Based on area covered by emergence traps and average diameter of wallows, our emergence traps would have covered roughly 7% of the wallow or control area.
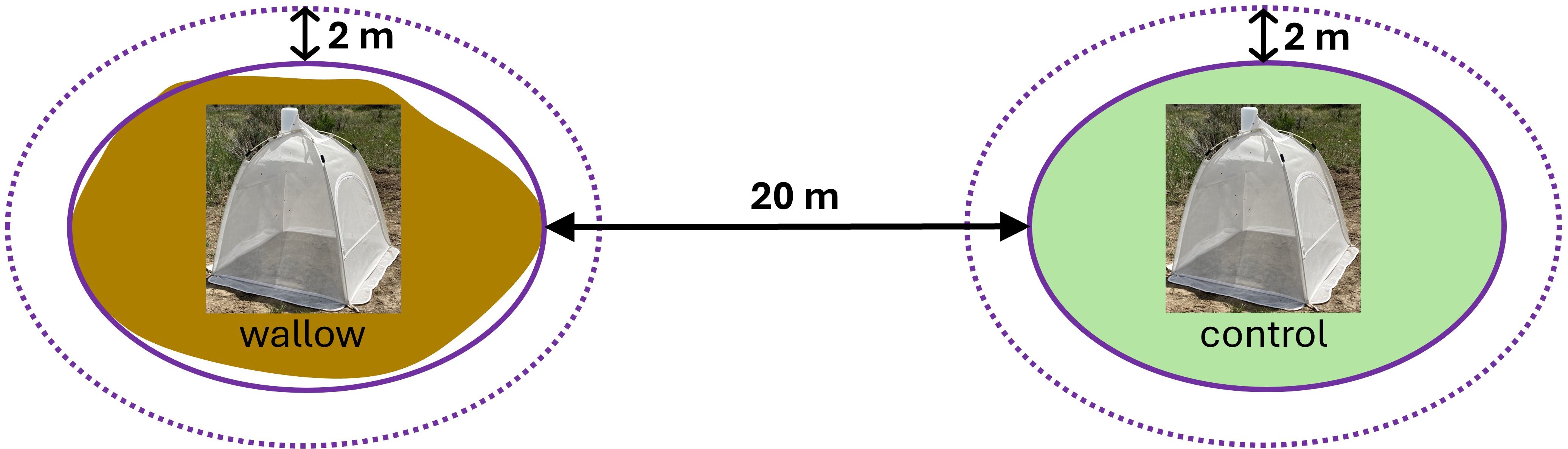
Figure 1. Schematic figure showing site set-up for emergence traps within wallows and undisturbed prairie sites (controls). Solid red lines indicate the general area of wallows and control sites. Dashed red lines indicate a 2 m buffer surrounding wallows/control, in which plant surveys and insect netting were conducted.
Bees and wasps were also collected via targeted sweep-netting flowering plants (Prendergast et al., 2020; Campbell et al., 2023) around the 25 wallow and control sites during the same time periods that emergence traps were active. During the insect survey, a researcher slowly walked around a 2-meter buffer zone (Figure 1) of each wallow and control site for 2 minutes and collected any potential pollinating insects that landed on vegetation or the ground. Insects captured in both collection methods were pinned, labeled, and identified to the most specific taxonomic designation (hereafter referred to as “taxa”).
Additionally, we conducted plant surveys to assess forb species richness, floral cover, and overall plant community cover on and off wallows. Survey areas included the two-meter buffer around wallows. To compare plant communities at the edges of wallows vs. adjacent prairie habitat, a comparably-sized area was surveyed as the control plot 20 meters from that wallow (Figure 1). Within each two-meter buffer area, we surveyed plant communities using four ½ x 1-m2 PVC quadrats placed 0.5 m from the edges of wallows/control areas and oriented along randomly selected cardinal and intermediate directions. During plant surveys, we recorded the percent cover of graminoid, woody plants, bare ground, litter, scat/patty, and forbs (both flowering and non-flowering at the time of surveys). To ensure randomization of quadrat placements throughout a given buffer area, we used a random number generator to select 4 of 16 possible locations placed along the axes of, and with the left or right edge of the quadrat oriented to, the cardinal and intermediate directions (i.e., N, S, E, W, NE, NW, SE, SW; Supplementary Figure 3). Depending on location, either the left or right quadrat side were placed 0.5 m from the edge of a wallow or control area to minimize potential edge effects. In 2021, we collected soil samples from wallows and controls (N = 12), and sent these to Ward Laboratories, Ltd. (Kearney, NE) where bulk density was measured.
We also conducted a search for literature that documented or collected arthropods within bison wallows using Google Scholar to identify currently known associations between arthropods and wallows. The following search phrases were used: “bison wallow insect”, “bison wallow arthropod”, “bison wallow invertebrate”, “bison wallow insect emergence”, “bison insect nest”, and “bison wallow insect nest”.
Analyses
For our second objective, we determined the differences of insect taxa richness and abundance collected on wallows and adjacent non-wallow sites in both methods using Wilcoxon rank sum tests due to the uneven sampling effort and similarly shaped distributions between treatments. Using emergence traps on wallows to collect ground-nesting bees and wasps is novel, so we accompanied these comparisons with the accumulation curves of collected taxa in our emergence trap surveys to understand our sampling completeness. We then performed permutational multivariate analysis of variance on the insects collected in emergence traps at the taxonomic family level to determine if the community composition of ground-nesting insects differed between wallow and non-wallowed areas.
Similarly, we used permutational multivariate analysis of variance on the vegetation cover groups per wallow and non-wallow pairing in both years to determine differences in ground cover between wallow and non-wallow areas. We then visualized the Bray-Curtis dissimilarity of wallow and non-wallow pairings in both years on non-metric multidimensional scaling ordination space. All analyses were conducted in R using packages BiodiversityR and vegan (Kindt and Coe, 2005; Oksanen et al., 2025).
Results
During the two year study, a total of 243 insects were collected from emergence traps with 85 collected from wallows and 158 from controls. Mutillidae was the most common family collected (N = 57, 23.5% of emergence trap collections), followed by Chrysididae (N = 50, 20.6% of all insects collected), Crabronidae (N = 48, 19.8% of all insects collected), and Pompilidae (N = 38, 15.6% of all insects collected). Only 12 individual bees (8 species) were collected from emergence traps (Table 1). Sweep net surveys around wallows and controls yielded an additional 67 insects (45 from wallows and 22 from controls) which included 13 bees. A total of 60 insect taxa were collected with emergence traps and sweep netting (Table 1). Emergence traps placed within controls had a greater taxa richness (W = 984, p-value=0.00098) and abundance (W = 991, p-value=0.00081) compared to wallows (Figures 2A, B) but no differences were observed with sweep netting (Figures 2C, D). Though taxa richness and abundance differed, insect community assemblages from emergence traps did not significantly differ between wallows and surrounding areas. The taxa accumulation curve indicated that more sampling is needed in our system to fully sample insects around wallow and non-wallow areas with emergence traps (Figure 2E).
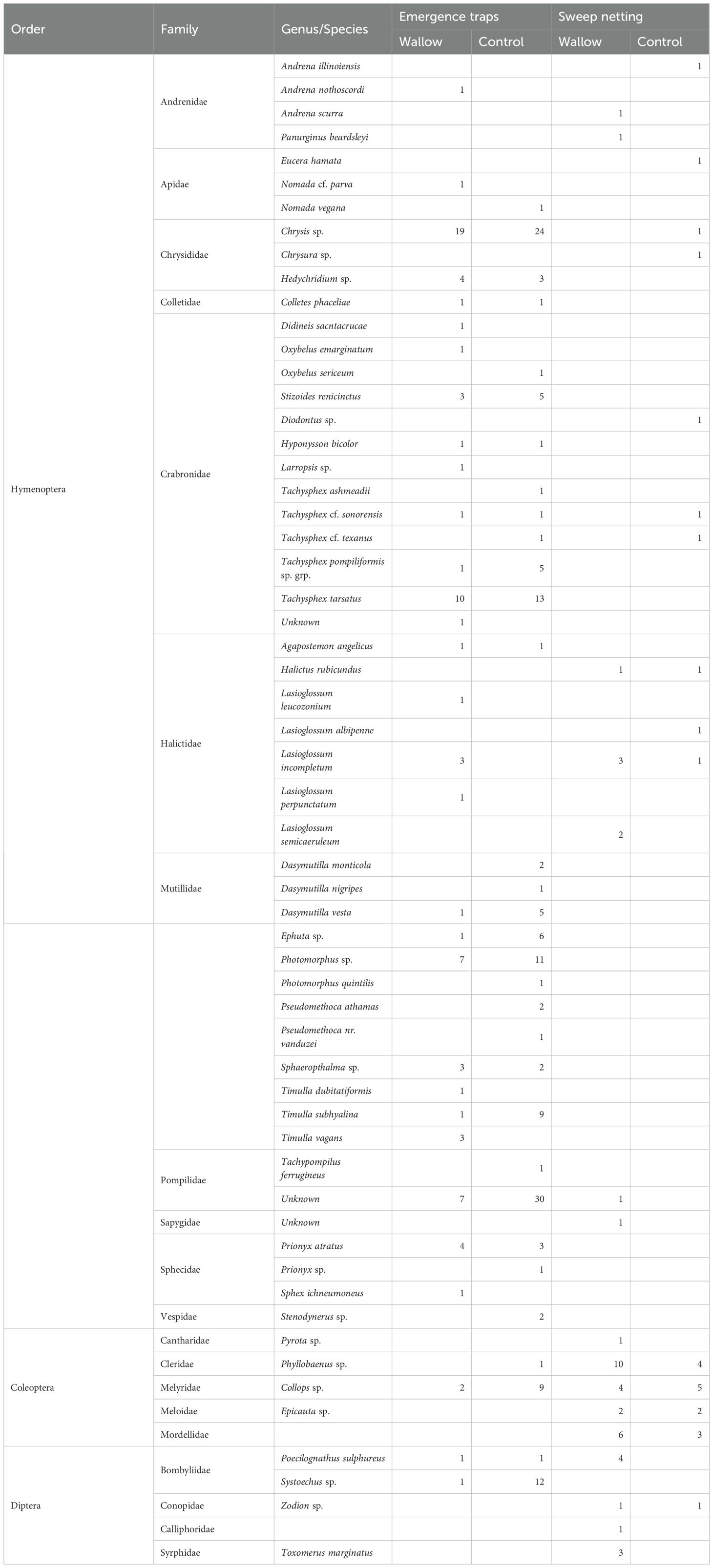
Table 1. List of all potential pollinating insects collected with emergence traps and sweep nets from bison wallows and paired controls.
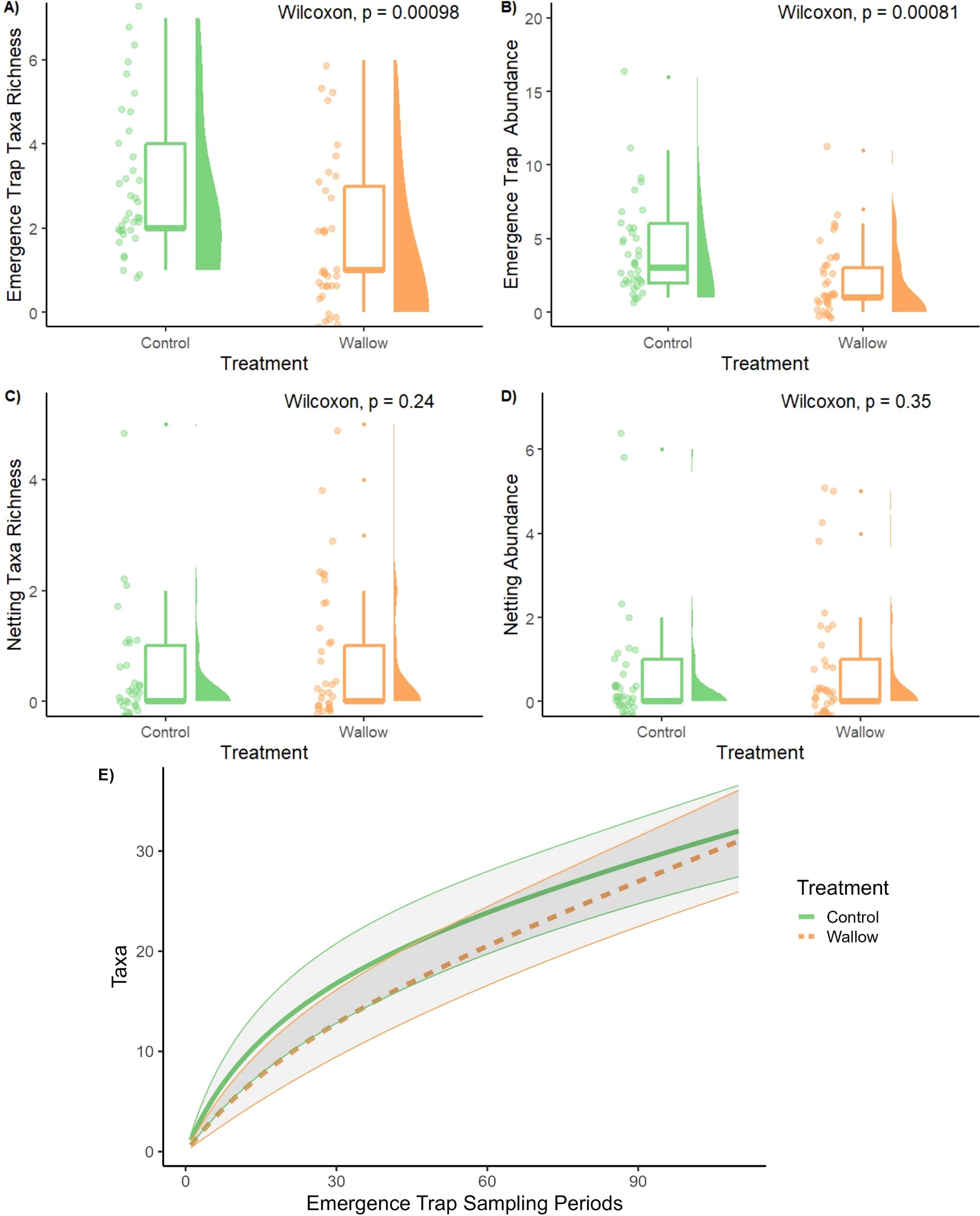
Figure 2. Distributions of taxa richness (A, C) and abundance (B, D) sampled in wallows and surrounding grassland controls in emergence traps (A, B) and netting surveys (C, D) compared using a Wilcoxon Rank Sum test. Distributions are shown by sampling points (dots), shaded distribution curves, and boxplots that show the median (middle line in box), interquartile range (box edges), and the smallest/largest values within 1.5x the interquartile ranges above/below the 25th and 75th percentiles (whiskers). (E) Sampling accumulation of insect taxa captured by emergence traps in 2021 and 2022 with 95% confidence intervals.
Vegetation surveys around wallows and paired controls did reveal a significant difference with wallows and controls having distinct ground cover composition (r2 = 0.202; F = 11.631; p=0.001) (Figure 3A). We documented nine actively flowering plant species within our field sites and generally the area around wallows contained a larger percent-cover of these plants (Figure 3B). Alyssum desertorum (non-native, desert madwort) was the most common flowering plant in controls and wallows. Achillea millefolium (western yarrow) was only located within controls whereas Polygonum aviculare (non-native, common knotgrass) and Opuntia polyacantha (Plains prickly pear) were only found adjacent to wallows. Soil bulk density was greater within wallows compared to controls but this was not significant (df=11, p= 0.12), with wallows having an average of 1.34 g/cm³ (SE ±0.03) and controls 1.28 g/cm³ (SE ±0.04).
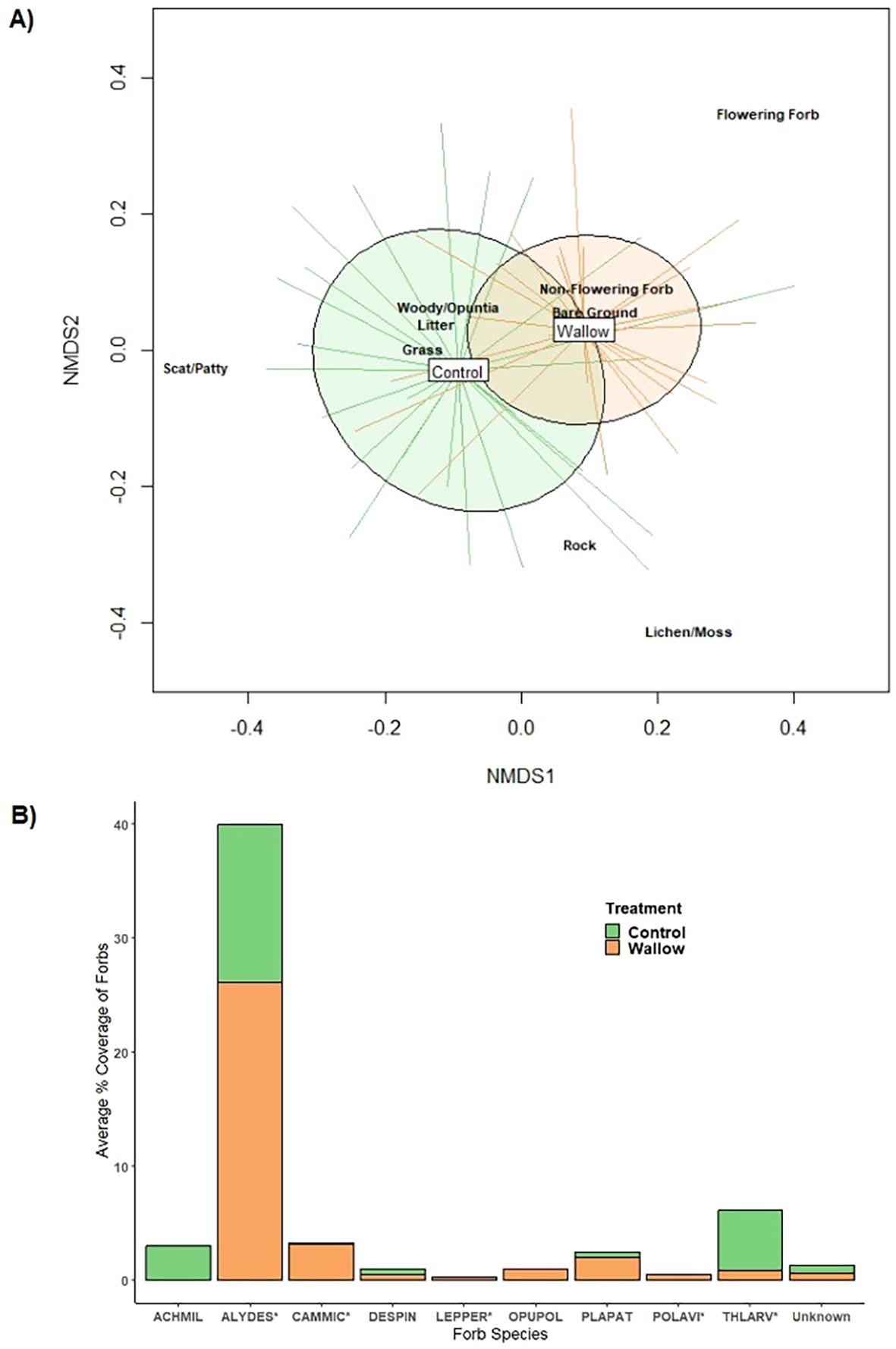
Figure 3. Non-metric multidimensional scaling ordination of wallow and surrounding grassland control survey plots indicated by the spider plot lines (A). Cover types estimated in vegetation cover surveys are overlaid. K = 2; Stress=0.1795. Average percent coverage of flowering forbs between bison wallows (orange) and surrounding grassland controls (green) in Central Montana (B). Species with asterisks are non-native species to the survey region. ACHMIL: Achillea millefolium, ALYDES*: Alyssum desertorum, CAMMIC*: Camelina macrocarpa, DESPIN: Descurainia pinnata, LEPPER*: Lepidium perfoliatum, OPUPOL: Opuntia polyacantha, PLAPAT: Plantago patagonica, POLAVI*: Polygonum aviculare, THLARV*: Thlaspi arvense, Unknown: flowering forbs that were unable to be identified to species.
While several studies have investigated arthropods within bison-grazed areas, our literature search only yielded six studies that collected insects either within or directly adjacent to wallows (Supplementary File 1). Three studies collected aquatic invertebrates from inundated wallows (Gerlanc, 2004; Frazier et al., 2024) or mud along the edges of wallows (Pfannenstiel and Ruder, 2015). The other studies observed butterflies (Hess et al., 2014), collected carabid beetles (Miller et al., 2014), and unidentified insects categorized as herbivores, carnivores, or detritivores (Nickell et al., 2018). See Supplementary data file for details of each paper.
Discussion
Our study is the first to examine insects from bison wallows within shortgrass prairie/sagebrush steppe, as all previous research was accomplished within tallgrass prairie sites. Our overall collection effort yielded numerous insect taxa within wallows and adjacent prairie. Our emergence traps generally found higher ground-nesting insect abundance and more taxa nesting within the adjacent prairie, but increased sampling effort is needed to fully understand these differences (Figure 2). Many of the ground-nesting taxa require access to bare ground for constructing nests (e.g., bees) (Harmon-Threatt, 2020; Antoine and Forrest, 2021; Gardein et al., 2022) and although wallows provided bare ground, bare ground may not be a limiting factor within the shortgrass prairie ecosystem (Figure 3A). Alternatively, bison wallowing behavior may destroy or prevent some insects from utilizing active wallows as nesting habitat. Regardless, we found that wallows provide important nesting resources in our system, primarily for wasps. Our results should be compared with future studies using emergence traps in other grassland systems, such as those with less bare ground available outside of wallows (e.g., tallgrass prairie).
The sweep netting survey yielded very few insects during the duration of this study. However, our sweep netting was aimed at collecting potential pollinators and although forbs had higher average cover around wallow edges, these plants were rarely flowering during our collection events. We posit that more forbs were found at wallow edges compared to adjacent prairie potentially due to increased plant nutrients from bison urine and feces and possibly increased water retention within wallows. However, Trager et al. (2004) found that plant richness was lower at the edges of bison wallows compared to adjacent prairie whereas McMillan et al. (2011) found varying differences with vegetation richness and composition but did find above ground net primary production to be greater around wallows compared to adjacent prairie. These differences in plants between wallow edges and adjacent prairie may be related to decreased plant competition, increased light availability at wallow edges, nutrient availability, and soil moisture (McMillan, 1999). However, these studies were accomplished in tallgrass prairie and may not be applicable to the shortgrass prairie/sagebrush steppe ecosystem. Additionally, wallows are known to have higher soil compaction and bulk density (McMillan et al., 2011) that may affect plant diversity/abundance but we did not find a statistical difference between wallows and adjacent prairie for bulk density. Thus, differences in plant communities around wallows compared to adjacent prairie in shortgrass prairie/sagebrush steppe may be driven by other parameters such as soil type or how frequent bison use specific areas for wallowing behaviors. Our plant surveys did reveal that non-native plants were the dominant forbs in the area around wallows and adjacent prairies with over 50% of the forbs being non-native. The wallows and immediate habitat surrounding wallows could be considered disturbed habitat due to the wallowing behavior and many of these non-native plants are commonly associated with disturbed habitats. Thus, wallows may be providing ideal habitat for many non-native forbs. Indeed, exotic plant species and plants with weedy lifestyles have been found to be more common adjacent to wallows than non-wallowed areas (Miller et al., 2014; Trager et al., 2004). Bison have also been documented to act as seed dispersers of both native and non-native grasses and forms through their feces or seeds sticking to their hair which can be dislodged via wallowing behavior (Constible et al., 2005; Rosas et al., 2008; Sigaud et al., 2020). Thus, wallowing behavior may be creating suitable habitat for plants that inhabit disturbed areas but also creating areas in which seeds are dispersed by bison.
While our study was focused on wallowing behavior and relatively small portions of prairie (e.g., strips around wallows), other studies have compared bison and cattle grazed areas. Most of these studies have been accomplished in tallgrass prairies and have found varying results. For example, Towne et al. (2005) found only minor plant community differences between bison and cattle grazed pastures whereas others have found that differences can be complex and may depend on the plant species of interest (Damhoureyeh and Hartnett, 1997). Other studies have compared bison grazed areas with ungrazed areas and have found distinct differences with bison grazed areas containing great plant diversity (Hartnett et al., 1996). Within shortgrass prairie habitat in the Northern Great Plains, behavioral differences have been documented between cattle and bison grazing (Kohl et al., 2013) and Yu et al. (2023) found that bison grazing increased native vegetation diversity within riparian areas compared to areas grazed with cattle. Thus, much more work is needed on how bison may restructure vegetation within prairie habitats.
The use of emergence traps is a relatively novel way of collecting ground-nesting pollinators and has been utilized in forested habitats, agricultural fields, chaparral, and prairie habitats (Sardiñas and Kremen, 2014; Pane and Harmon-Threatt, 2017; Cope et al., 2019; Ulyshen et al., 2021). However, how to use emergence traps is ambiguous (Pane and Harmon-Threatt, 2017) and possibly habitat dependent. Despite uncertainties regarding trap deployment techniques and our low number of bees collected, our emergence traps did collect numerous other ground-nesting insects and several insect groups that sweep netting did not. For example, numerous wasp families (e.g., Crabronidae, Sphecidae, Mutillidae, Pompilidae) were only collected with emergence traps and wasps comprised over 80% of our emergence trap collections. Mutillid wasps, the most collected insect family from our emergence traps, are important components of pollinator communities where adults visit flowers for nectar but also act as parasitoids on bees and other wasps (Brothers et al., 2000). Overall, ecological knowledge of Mutillidae is scarce but mutillids are known to visit a number of flowering plant families with female mutillids being less generalistic compared to their male counterparts (Parejo-Pulido et al., 2025). Scant mutillid ecology has also been attributed to a lack of standardized collection methods (Aranda and Graciolli, 2016) and, thus, our use of emergence traps could be a useful tool that could inform mutillid and other wasp nesting habits that other passive collection methods (e.g., pitfall traps) cannot.
Our insect collections from bison wallows and adjacent prairie have added to the overall knowledge of wallow use by insects. While several studies have examined insect communities within bison grazed pastures (e.g., Moran, 2014; Alaniz et al., 2024), very few studies have collected or observed insects from bison wallows. Additionally, a third of studies that did collect wallow-associated invertebrates were focused on aquatic or semi-aquatic invertebrates from wallows within tallgrass prairies. The efforts and proposals to reintroduce bison into the prairie landscape (especially within shortgrass prairie/sagebrush steppe) across their former range are increasing (Freese et al., 2007; Sanderson et al., 2008) and led by governmental agencies, NGOs and Native American Nations (Torbit and LaRose, 2001; Shamon et al., 2022). However, these efforts are covering only a small portion of the historic range of bison that once encompassed much of North America (Martin et al., 2022). These reintroductions offer unique opportunities to see how insects and plants interact with these large grazers and their distinctive wallowing behavior. However, bison behaviors after reintroductions are now interacting with altered grasslands with native and non-native plant assemblages. Future research should expand the exploration of how bison-specific behaviors are affected by the numerous non-native plants that are now commonplace and how bison-engineered modifications affect arthropod and other animal communities that reside in these altered shortgrass/sagebrush steppe prairie landscapes.
Data availability statement
The dataset presented in this study can be found online through the National Agricultural Library.
Ethics statement
The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft. CP: Data curation, Formal Analysis, Investigation, Writing – review & editing. AM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing – review & editing. CB: Data curation, Investigation, Writing – review & editing. ES: Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Financial support was received for the research through the Center of Excellence for Bison Studies-South Dakota State University and the USDA-Agricultural Research Service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1665879/full#supplementary-material
Supplementary Figure 1 | Location of the 25 wallows and paired controls (yellow dots) within the Sun Prairie unit of American Prairie.
Supplementary Figure 2 | A soil emergence trap, covering a ground area of 60 cm2.
Supplementary Figure 3 | Possible quadrat locations placed within 2-m buffer areas. The perimeter of a wallow or control area is given by the innermost brown polygon. The outermost red circle indicates the extent of the 2-m buffer. The red dashed lines separate the buffer area into four quadrants. For each survey round, locations (direction in degrees) of ½ x 1 m2 quadrats within each quadrant were selected using a random number generator. One edge of the quadrat was then oriented along each of these directions, ½ m from the wallow edge, for each wallow and paired control.
Supplementary Figure 4 | Photograph of wallow (right) and control (left) with emergence trap.
References
Abbate A., Campbell J. W., Kimmel C. B., and Kern W. H. Jr (2019). Urban development decreases bee abundance and diversity within coastal dune systems. Glob. Ecol. Conserv. 20. doi: 10.1016/j.gecco.2019.e00711
Alaniz M. N., Padilla S., Hosler S. C., Jones H. P., and Barber N. A. (2024). Ground- dwelling invertebrate community responses to bison and prescribed fire management in tallgrass prairies. J. Insect Conserv. 28, 1161–1170. doi: 10.1007/s10841-024-00614-y
Anderson R. C. (2006). Evolution and origin of the Central Grassland of North America: climate, fire, and mammalian grazers. J. Torrey. Bot. Soc 133, 626–647. doi: 10.3159/1095-5674(2006)133[626:EAOOTC]2.0.CO;2
Antoine C. M. and Forrest J. R. (2021). Nesting habitat of ground-nesting bees: a review. Ecol. Entomol. 46, 143–159. doi: 10.1111/een.12986
Aranda R. and Graciolli G. (2016). Protocol for collecting Mutillidae (Hymenoptera, Aculeata) in ecological studies: species-area effects on Mutillidae communities. Rev. Bras. Entomol. 60, 312–318. doi: 10.1016/j.rbe.2016.08.003
Augustine D., Davidson A., Dickinson K., and Van Pelt B. (2021). Thinking like a grassland: challenges and opportunities for biodiversity conservation in the Great Plains of North America. Rangel. Ecol. Manage. 78, 281–295. doi: 10.1016/j.rama.2019.09.001
Axelrod D. I. (1985). Rise of the grassland biome, central North America. Bot. Rev. 51, 163–201. doi: 10.1007/BF02861083
Boyce A. J., Shamon H., and McShea W. J. (2022). Bison reintroduction to mixed- grass prairie is associated with increases in bird diversity and cervid occupancy in riparian areas. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.821822
Brothers D. J., Tschuch G., and Burger F. (2000). Associations of mutillid wasps (Hymenoptera, Mutillidae) with eusocial insects. Insectes. Soc 47, 201–211. doi: 10.1007/PL00001704
Burke A. M., Barber N. A., and Jones H. P. (2020). Early small mammal responses to bison reintroduction and prescribed fire in restored tallgrass prairies. Nat. Areas. J. 40, 35–44. doi: 10.3375/043.040.0105
Cameron S. A., Lozier J. D., Strange J. P., Koch J. B., Cordes N., Solter L. F., et al. (2011). Pattern of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U.S.A. 108, 662–667. doi: 10.1073/pnas.1014743108
Campbell J. W., Abbate A., West N. M., Straub L., and Williams G. R. (2023). Comparing three collection methods for pollinating insects within electric transmission rights-of-ways. J. Insect Conserv. 27, 377–387. doi: 10.1007/s10841-023-00460-4
Collins S. L., Knapp A. K., Briggs J. M., Blair J. M., and Steinauer E. M. (1998). Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 280, 745–747. doi: 10.1126/science.280.5364.745
Constible J. M., Sweitzer R. A., Vuren D. V., Schuyler P. T., and Knapp D. A. (2005). Dispersal of non-native plants by introduced bison in an island ecosystem. Biol. Invasions. 7, 699–709. doi: 10.1007/s10530-004-5859-x
Cope G. C., Campbell J. W., Grodsky S. M., and Ellis J. D. (2019). Evaluation of nest- site selection of ground-nesting bees and wasps (Hymenoptera) using emergence traps. Can. Entomol. 151, 260–271. doi: 10.4039/tce.2019.3
Cornelisse T., Inouye D. W., Irwin R. E., Jepsen S., Mawdsley J. R., Ormes M., et al. (2025). Elevated extinction risk in over one-fifth of native North American pollinators. Proc. Natl. Acad. Sci. 122, e2418742122. doi: 10.1073/pnas.2418742122
Damhoureyeh S. A. and Hartnett D. C. (1997). Effects of bison and cattle on growth, reproduction, and abundances of five tallgrass prairie forbs. Am. J. Bot. 84, 1719–1728. doi: 10.2307/2446471
Fagre D. A., Janousek W. M., and Dreitz V. J. (2022). Avian species richness and abundance show stronger responses to bison grazing intensity than to ecosystem productivity. Ecosphere 13. doi: 10.1002/ecs2.4299
Flores D. (1991). Bison ecology and bison diplomacy: the southern plains from 1800–1850. J. Am. Hist. 78, 465–485. doi: 10.2307/2079530
Frazier C. F., Karlin A. T., and Thorp J. H. (2024). Bison act as habitat engineers for large branchiopod crustaceans in the Great Plains. Trans. Kans. Acad. Sci. 127, 43–48. doi: 10.1660/062.127.0105
Freese C. H., Aune K. E., Delaney P. B., Derr J. D., Forrest S. C., C. Gates C., et al. (2007). Second chance for the plains bison. Biol. Conserv. 136, 175–184. doi: 10.1016/j.biocon.2006.11.019
Gardein H., Fabian Y., Westphal C., Tscharntke T., and Hass A. (2022). Ground-nesting bees prefer bare ground areas on calcareous grasslands. Glob. Ecol. Conserv. 39, e02289. doi: 10.1016/j.gecco.2022.e02289
Gerlanc N. M. (2004). Bison wallows: community assembly and population dynamics in isolated ephemeral aquatic habitats of the tallgrass prairie (Manhattan, Kansas: Kansas State University).
Gerlanc N. M. and Kaufman G. A. (2003). Use of bison wallows by anurans on Konza Prairie. Am. Midl. Nat. 150, 158–168. doi: 10.1674/0003-0031(2003)150[0158:UOBWBA]2.0.CO;2
Griffin S. R., Bruninga-Socolar B., and Gibbs J. (2021). Bee communities in restored prairies are structured by landscape and management, not local floral resources. Basic Appl. Ecol. 50, 144–154. doi: 10.1016/j.baae.2020.12.004
Harmon-Threatt A. (2020). Influence of nesting characteristics on health of wild bee communities. Annu. Rev. Entomol. 65, 39–56. doi: 10.1146/annurev-ento-011019-024955
Hartnett D. C., Hickman K. R., and Walter L. E. (1996). Effects of bison grazing, fire, and topography on floristic diversity in tallgrass prairie. J. Range Manage. 49, 413–420. doi: 10.2307/4002922
Hess A. N., Hess R. J., Hess J. L. M., Paulan B., and Hess J. A. M. (2014). American bison influences on lepidopteran and wild blue lupine distribution in an oak savanna landscape. J. Insect Conserv. 18, 327–338. doi: 10.1007/s10841-014-9640-x
Hoekstra J. M., Boucher T. M., Ricketts T. H., and Roberts C. (2005). Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 8, 23–29. doi: 10.1111/j.1461-0248.2004.00686.x
Kindt R. and Coe R. (2005). Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies (World Agroforestry Centre (ICRAF). Available online at: http://www.worldagroforestry.org/output/tree-diversity-analysis (Accessed September 15, 2025).
Knapp A. K., Blair J. M., Briggs J. M., Collins S. L., Hartnett D. C., Johnson L. C., et al. (1999). North keystone role of bison in American tallgrass prairie bison increase habitat heterogeneity processes. BioScience 49, 39–50. doi: 10.1525/bisi.1999.49.1.39
Koh I., Lonsdorf E. V., Williams N. M., Brittain C., Isaacs R., Gibbs J., et al. (2016). Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. U.S.A. 113, 140–145. doi: 10.1073/pnas.1517685113
Kohl M. T., Krausman P. R., Kunkel K., and Williams D. M. (2013). Bison versus cattle: are they ecologically synonymous? Rangel. Ecol. Manage. 66, 721–731. doi: 10.2111/REM-D-12-00113.1
Kolipinski M., Borish S., Scott A., Kozlowski K., and Ghosh S. (2014). Bison: yesterday, today, and tomorrow. Nat. Areas. J. 34, 365–375. doi: 10.3375/043.034.0312
Martin J. M., Short R. A., Plumb G. E., Markewicz L., Van Vuren D. H., Wehus-Tow B., et al. (2022). Integrated evidence-based extent of occurrence for North American bison (Bison bison) since 1500 CE and before. Ecology 104, e3864. doi: 10.1002/ecy.3864
Matlack R. S., Kaufman D. W., and Kaufman G. A. (2001). Influence of grazing by bison and cattle on deer mice in burned tallgrass prairie. Am. Midl. Nat. 146, 361–368. doi: 10.1674/0003-0031(2001)146[0361:IOGBBA]2.0.CO;2
McMillan B. R. (1999). Bison wallowing and its influence on the soil environment and vegetation characteristics in tallgrass prairie (Manhattan, Kansas: Kansas State University).
McMillan B. R., Cottam M. R., and Kaufman D. R. (2000). Wallowing behavior of American bison (Bos bison) in tallgrass prairie: an examination of alternate explanations. Am. Midl. Nat. 144, 159–167. doi: 10.1674/0003-0031(2000)144[0159:WBOABB]2.0.CO;2
McMillan B. R., Pfeiffer K. A., and Kaufman D. W. (2011). Vegetation responses to an animal-generated disturbance (bison wallows) in tallgrass prairie. Am. Midl. Nat. 165, 60–73. doi: 10.1674/0003-0031-165.1.60
Miller K., Foster J., Nielsen K., and O'Loughlin M. (2014). Potential impacts of bison wallows on a restored tallgrass prairie community. Prairie. Nat. 46, 29–39.
Moran M. D. (2014). Bison grazing increases arthropod abundance and diversity in a tallgrass prairie. Environ. Entomol. 43, 1174–1184. doi: 10.1603/EN14013
Nickell Z., Varriano S., Plemmons E., and Moran M. D. (2018). Ecosystem engineering by bison (Bison bison) wallowing increases arthropod community heterogeneity in space and time. Ecosphere 9. doi: 10.1002/ecs2.2436
O'Neill K. M. (2001). Solitary wasps: behavior and natural history (New York: Cornell University Press).
Oksanen J., Simpson G., Blanchet F., Kindt R., Legendre P., Minchin P., et al. (2025). vegan: Community Ecology Package. R package version 2.7-0. Available online at: https://github.com/vegandevs/vegan (Accessed September 15, 2025).
Olimb S. K. and Robinson B. (2019). Grass to grain: Probabilistic modeling of agricultural conversion in the North American Great Plains. Ecol. Indic. 102, 237–245. doi: 10.1016/j.ecolind.2019.02.042
Pane A. M. and Harmon-Threatt A. N. (2017). An assessment of the efficacy and peak catch rates of emergence tents for measuring bee nesting. Appl. Plant Sci. 5. doi: 10.3732/apps.1700007
Parejo-Pulido D., Díaz-Calafat J., and Robla J. (2025). Shedding light on overlooked pollinators: Global insights into floral interactions of velvet ants (Hymenoptera: Mutillidae and Myrmosidae). J. Appl. Entomol. 149, 453–465. doi: 10.1111/jen.13306
Pfannenstiel R. S. and Ruder M. G. (2015). Colonization of bison (Bison bison) wallows in a tallgrass prairie by Culicoides spp (Diptera: Ceratopogonidae). J. Vector. Ecol. 40, 187–190. doi: 10.1111/jvec.12150
Polley W. and Wallace L. (1986). The relationship of plant species heterogeneity to soil variation in buffalo wallows. Southwest. Nat. 31, 493–501. doi: 10.2307/3671703
Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., and Kunin W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Prendergast K. S., Menz M. H., Dixon K. W., and Bateman P. W. (2020). The relative performance of sampling methods for native bees: an empirical test and review of the literature. Ecosphere 11, e03076. doi: 10.1002/ecs2.3076
Ratajczak Z., Collins S. L., Blair J. M., Koerner S. E., Louthan A., Smith M. D., et al. (2022). Reintroducing bison results in long-running and resilient increases in grassland diversity. Proc. Natl. Acad. Sci. U.S.A 119. doi: 10.1073/pnas.2210433119
Rosas C. A., Engle D. M., Shaw J. H., and Palmer M. W. (2008). Seed dispersal by Bison bison in a tallgrass prairie. J. Veg. Sci. 19, 769–778. doi: 10.3170/2008-8-18447
Rosenberger D. W. and Conforti M. L. (2020). Native and agricultural grassland use by stable and declining bumble bees in Midwestern North America. Insect Conserv. Divers. 13, 585–594. doi: 10.1111/icad.12448
Samson F. B., Knopf F. L., and Ostlie W. R. (2004). Great Plains ecosystems: past, present, and future. Wildl. Soc Bull. 32, 6–15. doi: 10.2193/0091-7648(2004)32[6:GPEPPA]2.0.CO;2
Sanderson E. W., Redford K. H., Weber B., Aune K., Baldes D., Berger J., et al. (2008). The ecological future of the North American bison: conceiving long-term, large-scale conservation of wildlife. Conserv. Biol. 22, 252–266. doi: 10.1111/j.1523-1739.2008.00899.x
Sardiñas H. S. and Kremen C. (2014). Evaluating nesting microhabitat for ground- nesting bees using emergence traps. Basic Appl. Ecol. 15, 161–168. doi: 10.1016/j.baae.2014.02.004
Shamon H., Cosby O. G., Andersen C. L., Augare H., BearCub Stiffarm J., Bresnan C. E., et al. (2022). The potential of bison restoration as an ecological approach to future tribal food sovereignty on the Northern Great Plains. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.826282
Sigaud M., Mason T. H. E., Barnier F., Cherry S. G., and Fortin D. (2020). Emerging conflict between conservation programmes: when a threatened vertebrate facilitates the dispersal of exotic species in a rare plant community. Anim. Conserv. 23, 660–669. doi: 10.1111/acv.12579
Torbit S. and LaRose L. (2001). A commentary on bison and cultural restoration: partnership between the National Wildlife Federation and the Intertribal Bison Cooperative. Great. Plains. Res. 11, 175–182. Available online at: https://www.jstor.org/stable/23775646 (Accessed September 15, 2025).
Towne E. G., Hartnett D. C., and Cochran R. C. (2005). Vegetation trends in tallgrass prairie from bison and cattle grazing. Ecol. Appl. 15, 1550–1559. doi: 10.1890/04-1958
Trager M. D., Wilson G. W. T., and Hartnett D. C. (2004). Concurrent effects of fire regime, grazing and bison wallowing on tallgrass prairie vegetation. Am. Mid. Nat. 152, 237–247. doi: 10.1674/0003-0031(2004)152[0237:CEOFRG]2.0.CO;2
Ulyshen M. D., Wilson A. C., Ohlson G. C., Pokswinksi S. M., and Hiers J. K. (2021). Frequent prescribed fires favour ground-nesting bees in southeastern US forests. Insect Conserv. Divers. 14, 527–534. doi: 10.1111/icad.12484
Winfree R., Aguilar R., Vázquez D. P., LeBuhn G., and Aizen M. A. (2009). A meta- analysis of bees' responses to anthropogenic disturbance. Ecology 90, 2068–2076. doi: 10.1890/08-1245.1
Keywords: pollinators, Mutillidae, bison wallows, Northern Great Plains, emergence traps, ground-nesting wasps
Citation: Campbell JW, Pei CK, Morphew AR, Brabant CM and Spevak EM (2025) Bison wallowing alters pollinator nesting and foraging resources in shortgrass sagebrush steppe in the Northern Great Plains. Front. Ecol. Evol. 13:1665879. doi: 10.3389/fevo.2025.1665879
Received: 14 July 2025; Accepted: 29 October 2025;
Published: 11 November 2025.
Edited by:
Jeff M. Martin, South Dakota State University, United StatesReviewed by:
Julia B. Leone, Friends of the Mississippi River, United StatesEva Horne, Kansas State University Olathe, United States
Copyright © 2025 Campbell, Pei, Morphew, Brabant and Spevak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joshua W. Campbell, am9zaHVhLmNhbXBiZWxsQHVzZGEuZ292
 Joshua W. Campbell
Joshua W. Campbell C. K. Pei1
C. K. Pei1 Craig M. Brabant
Craig M. Brabant