- 1Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW, Australia
- 2Kings Park Science, Biodiversity, and Conservation Science, Department of Biodiversity, Conservation, and Attractions, Perth, WA, Australia
Introduction: Many plant species have evolved to persist in fire-prone regions under specific fire regimes. Seeds have developed mechanisms, including the breaking of physical seed dormancy by fire-related heat shock, that synchronize germination and seedling emergence with post-fire conditions conducive to successful recruitment. Seeds with physical dormancy can have their dormancy released by high soil temperatures during fire, with documented thermal thresholds varying widely from 60°C to 150°C. Generally, these thresholds are believed to be highly phylogenetically conserved, but how ecosystems shape seed thermal thresholds within widespread, geographically diverse genera is unknown. In this study, we sought to understand how soil heating under different fire regimes, seed traits, and climate variables all shape pyro-thermal niche metrics, dormancy-break, and mortality of Acacia seeds.
Methods: Using 35 Acacia species from across 12 vegetation types in Australia, we explored the relationship between seed pyro-thermal niche characteristics and fire return interval (FRI), fuel type (as a proxy for soil heating), mean annual temperature, and total annual precipitation.
Results: Pyro-thermal niche metrics showed a hump-shaped relationship with both the minimum recommended FRI and fuel type, highlighting the role fire plays in shaping seed thermal thresholds. Climate variables showed no discernible relationship with pyro-thermal niche metrics.
Discussion: These results suggest that the mechanisms that shape the distribution of different seed dormancy classes are different from those that shape variation in pyro-thermal niche metrics. Understanding the processes driving plant population dynamics in fire-prone regions is essential for ecological understanding under a changing climate.
1 Introduction
Many plant species have evolved to persist in fire-prone regions under specific fire regimes (Whelan, 1995; Keeley et al., 2011). Fire-related heat shock can break seed dormancy for some species, allowing germination to occur in the post-fire environment to replace killed individuals and enable overall species persistence (Miller and Murphy, 2017). Accumulation of biomass within ecosystems is a function of its productivity, and in the context of fire, an increase in fuel load can directly influence soil heating and fire return intervals (FRIs) (Bradstock, 2010; Kreye et al., 2013). For physically dormant (PY) seeds in the soil seedbank, changes in soil temperatures directly determine whether seeds reach dormancy-breaking thresholds, and extreme soil temperatures can induce seed mortality. Generally, these thresholds are believed to be phylogenetically conserved at the genus level (Tangney et al., 2025), and seed germination responses are shaped by the fire regime (Monemizadeh et al., 2025). Despite this, understanding how fire shapes seed thermal thresholds is rarely quantified across broad ecological contexts.
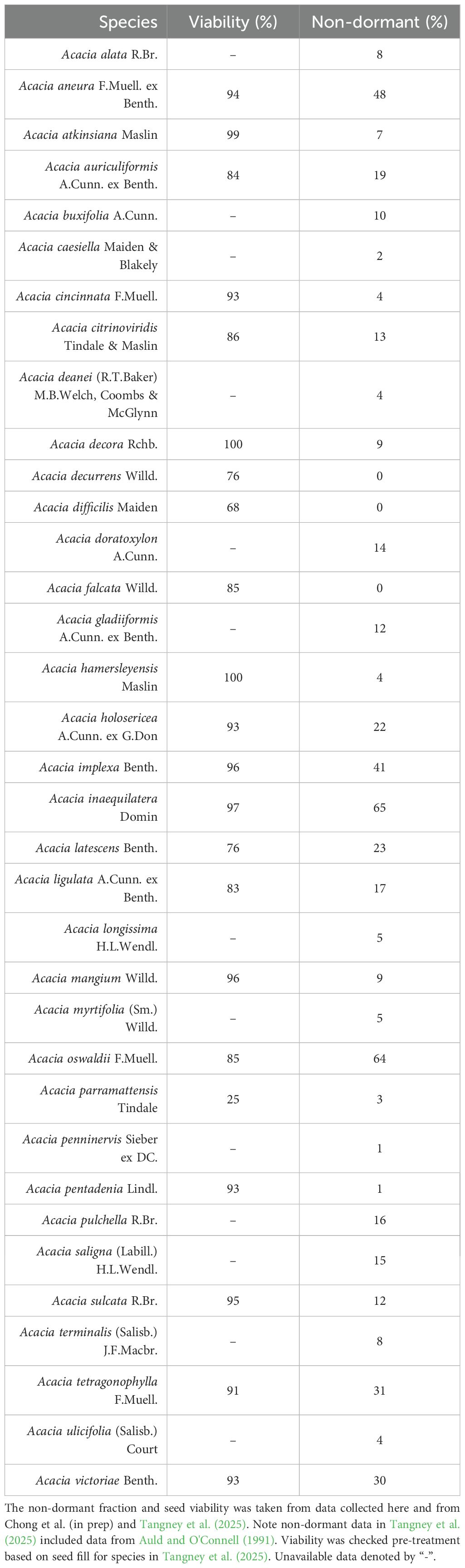
Seeds with physical dormancy in fire-prone regions can have dormancy broken by the passage of fire. Fire-related heat shock cracks the water-impermeable seed coat, allowing water entry and subsequent germination into the post-fire environment (Baskin and Baskin, 2014; Ooi et al., 2022). The range of fire-related temperatures across which seed dormancy is released and subsequently induces mortality for any particular species is known as the “pyro-thermal niche” and consists of three metrics: the dormancy release temperature (DRT50), optimal dormancy breaking temperature (Topt), and the lethal temperature (LT50; see Tangney et al., 2025). The DRT50 is the temperature at which 50% of viable, dormant seeds germinate, the Topt is the temperature that releases the most seeds from dormancy, and the LT50 is the temperature that induces 50% mortality (defined in Tangney et al., 2025). The pyro-thermal niche is variable and species-specific, often spanning over 50°C within a single metric (Tangney et al., 2025). For PY seeds stored in the soil seedbank, soil temperatures during fire determine whether each metric is reached. The LT50 must be high enough to prevent significant mortality, but the DRT50 and Topt must be low enough to break dormancy and facilitate post-fire seedling recruitment.
Fuel characteristics are a key determinant of the intensity of soil heating during fire. Different fuel types, total fuel load, the distribution of fuel (e.g., surface or elevated; fuel connectivity), and moisture content all impact soil temperatures during fire, generating a diversity in soil temperatures across different fire regimes (Busse et al., 2005; Kreye et al., 2013; Pausas and Paula, 2012; Murphy et al., 2013). Even within a single fire event in the same vegetation community, soil heating can vary due to heterogeneous fuels and dynamic fire behavior (Gimeno-García et al., 2004; Busse et al., 2013; Tangney et al., 2018).
Heating duration is a function of fuel size, thus, increasing surface fuel loads elevates soil temperatures (Bradstock and Auld, 1995; Hopkins et al., 2025). Densely packed fuels (e.g., peatland) burn for longer, contributing to soil heating to greater depths, while fine surface fuels burn faster and contribute to fire spread (Whelan, 1995; Catchpole, 2002). Grass-based fuels (e.g., savanna) are generally of a low intensity due to having low fuel density (Catchpole, 2002—although exceptions exist, see Archibald et al., 2013), whereas high-intensity fires predominantly occur in litter-based fuels in Australia (Murphy et al., 2013). Consequently, the structure and arrangement of surface fuels is an important driver of soil heating. The temperatures seeds stored in the soil seedbank are exposed to are tightly linked with fuel dynamics, with fuel characteristics (based on vegetation community) previously shown to influence seed heat shock tolerance across genera during fire (Ramos et al., 2016; Tangney et al., 2025).
Fire return interval (FRI) and soil heating are integrally linked, as FRI is positively correlated with biomass accumulation within an ecosystem. FRI is defined as the years between subsequent fires (Gill, 1975) and follows a hump-shaped relationship with the presence of available fuel (i.e., fuel that is burnable; see Bradstock, 2010). The amount of available fuel during a fire is determined by rainfall; high-moisture, high-biomass environments (e.g., rainforest) are too wet to burn, whereas in low-moisture, low-biomass environments (e.g., desert), fuel connectivity and production are too low to support frequent fire (Pausas and Bradstock, 2007; Pausas and Paula, 2012; Archibald et al., 2013). FRIs are shortest when there is enough moisture to support continuous fuel loads, but not enough moisture that fuel is too wet to burn (assuming an ignition source and fire weather are present; see Bradstock, 2010). As such, ecosystems that burn frequently or have poor fuel connectivity are expected to produce lower soil temperatures due to fuel loads being unable to accumulate. Where FRIs are long and fuel loads can accumulate, hotter soil temperatures are expected.
Variations in FRI (and fire regimes more broadly) select for different plant persistence strategies and germination responses (Keeley et al., 2011; Monemizadeh et al., 2025). The probability of obligate seeding (i.e., plants that are killed during the passage of fire and recruit post-fire from seed) for woody species follows a hump-shaped relationship with FRI (Yang et al., 2025). When FRIs are shorter than species juvenile periods and species are not in protected fire refugia, obligate seeding is an uncommon recruitment strategy, as plants are vulnerable to immaturity risk (i.e., the plant is killed by fire before new seeds are produced; Zedler, 1995). Where fires are a rare occurrence, PY break is disconnected from fire cues as it is not sustainable for overall species persistence (Auld, 1995; Baskin and Baskin, 2014). This is reflected in the distribution of PY within fire-prone regions, where PY is more common in woody ecosystems (e.g., temperate forests) than frequently burnt grassy vegetation communities, such as grasslands and savannas (Pausas and Lamont, 2022; Monemizadeh et al., 2025).
Seed mass is often reported as a driver of dormancy-breaking thresholds in fire-prone regions, based on the relationship between seed emergence depth and vertical heat flux through the soil during a fire (Bond et al., 1999). As soil depth increases, soil temperatures during fire decrease as heat fails to radiate deeper into the soil profile (Raison et al., 1986; Bradstock and Auld, 1995; Tangney et al., 2020; Pausas and Lamont, 2022). Large seeds that can emerge from deeper within the soil profile have lower heat shock tolerance than smaller seeds, which sit close to the surface and experience hotter soil temperatures during fire (Hanley et al., 2003; Liyanage and Ooi, 2018; Sano et al., 2025).
Climate is a driver of seed dormancy class occurrence, with PY strongly associated with dry, seasonal ecosystems (de Casas et al., 2017; Wyse and Dickie, 2018; Rosbakh et al., 2023). However, PY is not a binary trait, as considerable variation exists in mechanisms that break PY (Baskin and Baskin, 2014). While all methods of PY break are linked to canopy gap detection, PY seeds in regions that rarely experience fire use alternating seasonal temperatures to break dormancy (Baskin and Baskin, 2014). Conversely, PY seeds in fire-prone regions often require fire-related heat shock to render the seed coat water-permeable (Moreira and Pausas, 2012; Pausas and Lamont, 2022). Moreover, the heat shock temperature required to optimally break PY (Topt) in any batch of seeds during fire varies substantially, ranging from 60°C to 150°C depending on heating duration (Ooi et al., 2014; Pausas and Lamont, 2022; Tangney et al., 2025). While climate is related to seed dormancy distribution (Rosbakh et al., 2023), how it contributes to trait variation within a single seed dormancy class is unclear.
We hypothesize that pyro-thermal niche temperatures of PY seeds will follow a non-linear relationship with soil heating. For species from grass-type fuels and/or with short FRIs, pyro-thermal niche metrics are expected to be low. Thresholds will likely increase as FRIs lengthen and litter-based fuel loads accumulate in woodlands and forests before dropping again as vegetation shifts into fire-sensitive plant communities with long FRIs. Low thermal thresholds are also hypothesized to occur at very short and long FRIs because alternative persistence strategies are dominant over post-fire seedling recruitment from PY seeds (Yang et al., 2025; Monemizadeh et al., 2025). The non-linear relationship between pyro-thermal niche metrics and soil heating is hypothesized to be strongest in the LT50, as species cannot be expected to exist in these ecosystems otherwise.
In this study, we sought to explore whether fire shapes pyro-thermal niche variation using 35 Acacia Mill. species from ecosystems covering three broad fuel classifications across Australia (Figures 1, 2; Table 1). Acacia is a widespread genus in Australia with around 1,000 species (Australian National Botanic Gardens, 2024), occurring in a range of vegetation communities (Maslin and Pedley, 1988) and thus fuel regimes. Additionally, the 35 study species cover a wide distribution across Australia (Figure 2) and thus are broadly representative of the distribution of Australian Acacia species. The pyro-thermal niche was characterized for each species, and the DRT50, Topt, and LT50 were compared against the minimum FRI and fuel classification, alongside seed mass, total annual precipitation, and mean annual temperature, to determine how different drivers shape the pyro-thermal niche within a widespread, geographically diverse genus (Figure 1).
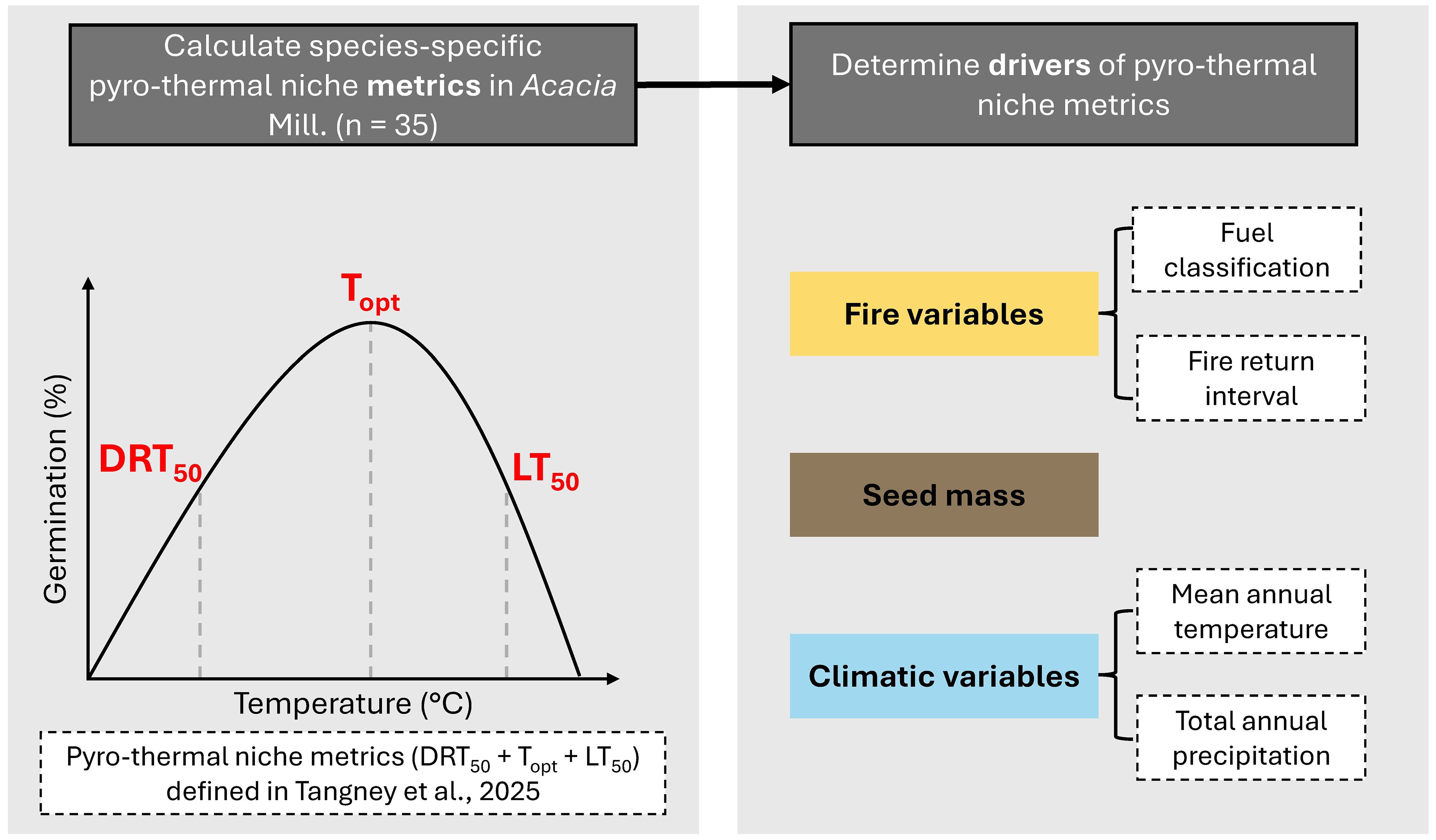
Figure 1. Study design. Pyro-thermal niche metrics were calculated for 35 Acacia species (Table 1) and modeled against hypothesized drivers of pyro-thermal niche metric variation. See Figure 2 and Supplementary Tables S1, S2.
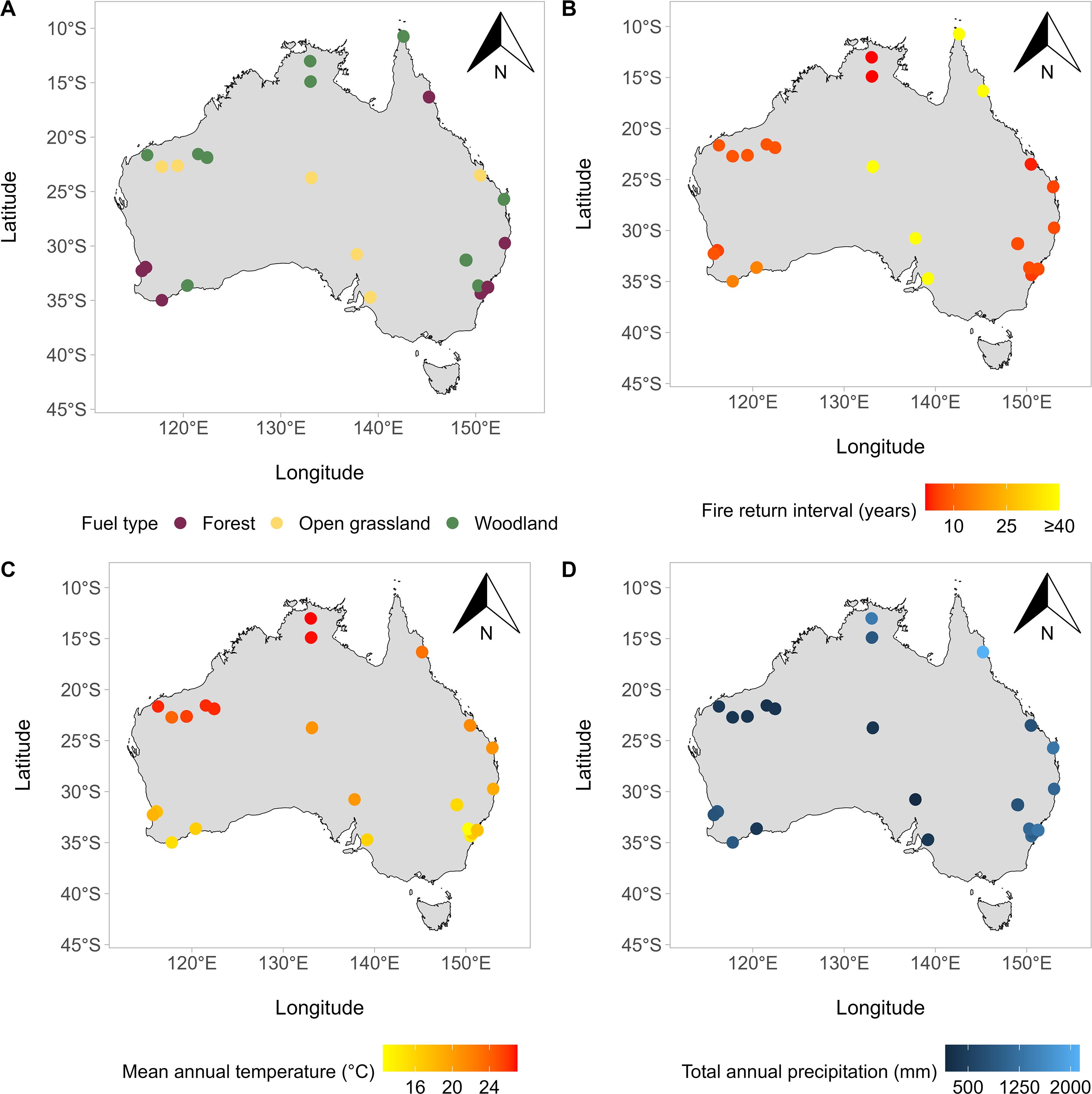
Figure 2. Spatial distribution of fuel type (A), fire return interval (B), mean annual temperature (C) and total annual precipitation (D) across the provenance of the 35 Acacia species studied. Note that A and B use the northern-most mainland Australia record for Acacia auriculiformis and C and D uses the original provenance of Papua New Guinea, which is not shown in this figure. However, climate variable values for this species are available in Supplementary Table S2, alongside all variable values for each species.
2 Materials and methods
2.1 Species selection and dormancy class
As seed pyro-thermal niche metrics are phylogenetically conserved across genera (Tangney et al., 2025), species selection was limited to Acacia to help control for phylogenetic signals and target soil heating as the factor under assessment. Moreover, most Acacia species produce seeds with PY. Given we were interested in the effect different dormancy drivers have on seed tolerance to heat shock, species that produce seeds with PY were suitable, as dormancy is broken by fire-related temperatures in fire-prone ecosystems.
Seeds were obtained from the Australian PlantBank, Australian Seed (www.australianseed.com), the Commonwealth Scientific and Industrial Research Organisation (CSIRO) Australian Tree Seed Centre (www.csiro.au/en/about/facilities-collections/collections/atsc), and Nindethana (www.nindethana.net.au). Before treatment, unfilled, non-viable seeds were discarded based on weight using a zig-zag aspirator (Frischie et al., 2020), and viability (based on seed fill) was confirmed via x-ray (Parameter Cabinet X-Ray System, Kubtec, Connecticut, USA) at the Australian PlantBank (Mount Annan, Sydney).
2.2 Heat shock germination trials and calculating pyro-thermal niche metrics
Pyro-thermal niche metrics were taken from Tangney et al. (2025) for 13 species and raw germination data from Chong et al. (in prep) for four species. Germination data post-heat shock was also collected to calculate the pyro-thermal niche metrics for a further 18 species. To characterize the DRT50, Topt, and LT50 of these 18 species, we used similar methods to those outlined in Tangney et al. (2025). Three replicates of 25 seeds were placed in metal mesh tea strainers and embedded in preheated sand for 10 min in a laboratory oven (D170fs, Steridium, Queensland, Australia). Heat treatments ranged from 40°C to 120°C and were increased in 20°C increments, along with an unheated control. Seeds that did not show significant mortality after heating at 120°C were additionally heated at 10°C increments until significant mortality occurred (up to 150°C). After heat shock, all samples were plated on 0.7% w/v agar and incubated (IPP110plus, Memmert, Schwabach, Germany) under a 12h/12h dark/light regime. Incubation temperatures were either 11/25°C, 20/30°C, or 15°C based on the germination temperatures associated with individual species provenance (Williams et al., 2003; Erickson et al., 2016; Mackenzie et al., 2016; Tangney et al., 2025). Germination was recorded twice a week for a minimum of 6 weeks, after which germination was recorded until no new germination was observed for two consecutive weeks. After germination had finished, all remaining seeds were scarified using either sandpaper or a scalpel, and germination followed to determine viability. To differentiate post-fire seed viability from experimental effects, the highest final viability (grouped by treatment) was taken for each species (Table 1). Species-specific percentage viability was multiplied with seed sample size across all treatments, thus correcting for final germination. This was repeated for species from Chong et al. (in prep), as raw germination data and post-treatment viability were available, and viability was corrected pre-treatment for species from Tangney et al. (2025).
Species-specific thermal response curves were created following heat shock to predict the DRT50, Topt, and LT50 from the curve using methods outlined in previous studies (Overton et al., 2024; Tangney et al., 2025). Thermal performance curves (TPCs) were created based on the viability-corrected final germination proportion after each heat shock treatment using the package rTPC v.1.0.4 (Padfield et al., 2024; Supplementary Figure S1; Supplementary Table S1). Non-linear models were fit to TPCs for each species, and the best model was selected based on the lowest Akaike information criterion ranking and visual assessment to remove spurious fits.
2.3 Species provenance
Specific seed provenance details were obtained from each seed supplier; however, broad regions (e.g., “South Australia”) were supplied as the provenance for A. aneura, A. inaequilatera, A. mangium, and A. victoriae. In these cases, a centroid of the species distribution within the broad region was taken to infer the most likely provenance. This was achieved through downloading and cleaning Australian Acacia species occurrence records from the Global Biodiversity Information Facility (GBIF; https://doi.org/10.15468/dl.v758n3) using the packages rgbif v3.8.1 (Chamberlain et al., 2025) and CoordinateCleaner v3.0.1 (Zizka et al., 2019). Occurrence data was cleaned following the procedure outlined in Yang et al. (2025), where records with no geospatial issues were downloaded from the GBIF website and only recent (post-1900) and precise (coordinate precision < 0.05 and coordinate uncertainty < 10,000 meters) were records kept. Records that had flagged coordinate mismatches, unlikely record dates, or were labelled as invasive, naturalized or introduced in GBIF were also removed. Additionally, records within 2 km of a country, capital city, or herbaria centroid were also excluded. Species names were confirmed using the Australian Plant Census (https://biodiversity.org.au/) and the GBIF species key extracted from the GBIF taxonomic backbone for each species to account for species synonyms during subsequent filtering. Cleaned occurrence data was then filtered using the GBIF species key to the 35 species of interest.
Further cleaning was undertaken through mapping each record to the National Vegetation Information System (NVIS) map of major extant vegetation types v7.0 (Department of Climate Change, Energy, the Environment and Water (DCCEEW), 2024) and extracting the associated vegetation type for each record using the terra v1.8-50 (Hijmans, 2025) and sf v1.0-21 (Pebesma and Bivand, 2023) packages. Records that occurred in urban or cleared areas, or had no vegetation type data available, were removed. Records were then filtered to the supplied broad provenance (e.g., “Northern Territory”) using ozmaps v0.4.5 (Sumner, 2021), and a centroid of the species distribution was taken for subsequent analysis. As these locations are not a ‘true’ provenance, they should be interpreted with the understanding that they were inferred based on species distribution within the supplied broad provenance.
2.4 Fuel type classification and fire return intervals
Fuel classifications were based on the CSIRO Bushfire Fuel Classification map (Figure 2; Joshi et al., 2025). The specific fuel type that aligned with species-specific single-point locations (i.e., the specific collection site provided by seed suppliers or the centroid calculated above) was extracted from the Bushfire Fuel Classification map using the terra v1.8–50 (Hijmans, 2025) package. The associated broad fuel type was then assigned (“open grassland,” “woodland,” “forest”) based on the groupings provided in Joshi et al. (2025). For species that did not correspond to a mapped fuel classification (e.g., coarse resolution placed some species within water bodies), the dominant fuel classification from the closest 50 records (using the cleaned species occurrence data described above) was taken. Acacia auriculiformis was sourced from Papua New Guinea (PNG); thus, the fuel classification and FRI assigned to this species were based on the northernmost occurrence record in mainland Australia. Provenance remained PNG for all other analyses.
FRIs were collated from the literature and Australian government fire management agencies (Figure 2; Supplementary Table S2). The minimum recommended FRI, which was based off the vegetation type each species occurred in, was used in analysis, as this metric was consistent between Australian states and territories.
2.5 Climatic variables
The WorldClim (www.worldclim.org) (Fick and Hijmans, 2017) bioclimatic variables mean annual temperature (MAT; BIO1), annual temperature range (TEMPR; BIO7), annual precipitation (TAP; BIO12) and precipitation seasonality (PRECIPS; BIO15), were selected to model against pyro-thermal niche metrics, as these variables have previously been shown to influence the distribution of PY (de Casas et al., 2017; Wyse and Dickie, 2018; Rosbakh et al., 2023). Climate data for each variable were downloaded from WorldClim (spatial resolution = 10 min) using the geodata v0.6–2 package (Hijmans et al., 2024). From this, the climate data for the specific provenance associated with each species location was extracted using terra v1.8-50 (Hijmans, 2025), and variable correlation was checked using the corrplot v0.95 (Wei and Simko, 2024) and Hmisc v5.2-3 (Harrell, 2025) packages. Moderate correlation existed between both MAT and PRECIPS alongside TEMPR and TAP (Supplementary Figure S2). The variance inflation factor (VIF) was checked for these two correlations using car v3.1-3 (Fox and Weisberg, 2019), resulting in VIFs of ~3 for both. TEMPR and PRECIPS were removed from the dataset, and VIFs were re-checked, resulting in VIFs ~1 and indicating no correlation. Thus, only MAT and TAP were included in subsequent analyses (Figure 2).
2.6 Statistical analysis
The relationships between pyro-thermal niche metrics and fire were modelled using a series of linear models and generalized linear models (GLMs) within the in-built stats v4.5.0 package. All model fits were checked for over-dispersion, normal residual distribution, and outliers using the model diagnostic functions within the packages DHARMa v0.4.7 (Hartig, 2024) and performance v0.13.0 (Lüdecke et al., 2021), alongside being visually assessed, to ensure appropriate fits. Any over-dispersed models were run using a negative binomial distribution, to account for the over-dispersion, using the MASS v7.3–65 package (Venables and Ripley, 2002). Pyro-thermal niche metrics were modeled against the fire variables (i) broad species fuel classifications using a linear model and (ii) the recommended minimum FRI using GLMs, with a negative binomial and quasi-Poisson distribution for the Topt and LT50, respectively. All models had an additional polynomial (second degree) argument to account for the non-linear relationship between fuel type and FRI against each pyro-thermal niche metric. The DRT50 was unable to be robustly modelled against the FRI. A linear model was used to model log-transformed seed mass against pyro-thermal niche metrics, and the interaction between MAT and TAP against the pyro-thermal niche was modelled using GLMs, with the DRT50 including a “log” link. Statistical significance in each model was determined using the ANOVA function within the car v3.1–3 package. A pairwise contrast between fuel type (open grassland, woodland, forest) and each pyro-thermal niche metric was also conducted using the emmeans v1.11.1 package (Lenth, 2025) to determine the impact of specific fuel types on thermal thresholds. All analyses and data cleaning in this study were conducted with R v4.5.0 (R Core Team, 2024).
3 Results
3.1 General variability in the pyro-thermal niche
Pyro-thermal niche metrics varied considerably across Acacia (Supplementary Table S1). Twenty-three species had a DRT50 greater than 70°C, with the mean DRT50 across all Acacia species of 67 ± 4°C ( ± SE). Seven species associated with arid, semi-arid, and savanna ecosystems had a high non-dormant fraction (22%–65%; Table 1) and DRT50 between 25°C and 27°C (A. aneura, A. implexa, A. inaequilatera, A. latescens, A. oswaldii, A. tetragonophylla, and A. victoriae). The mean Topt was much higher at 97 ± 3°C (± SE), ranging from 53°C in the grassy woodland species A. implexa to 120°C in A. sulcata. The LT50 was the least variable of all metrics, with a mean value of 114 ± 2°C (± SE). The lowest LT50 was observed in the arid species A. oswaldii (79°C), and the highest was again A. sulcata, with an LT50 of 141°C. Acacia falcata and A. parramattensis both had low germination rates (Supplementary Figure S1), with the former not showing a strong response to heat shock and the latter having low initial viability of 25%.
3.2 FRI and fuel type against the pyro-thermal niche
The Topt and LT50 both had a significant, hump-shaped relationship with the minimum FRI (Figure 3A: χ² = 14.2, df = 2, p = 0.001, R2Efron = 0.31; Figure 3B: χ² = 9.54, df = 2, p = 0.009, R2Efron = 0.24. R2Efron = Efron’s pseudo R2; see Efron, 1978). The DRT50 was unable to be robustly modeled; however, the best-fitting model for the DRT50 showed a non-significant relationship with FRI.
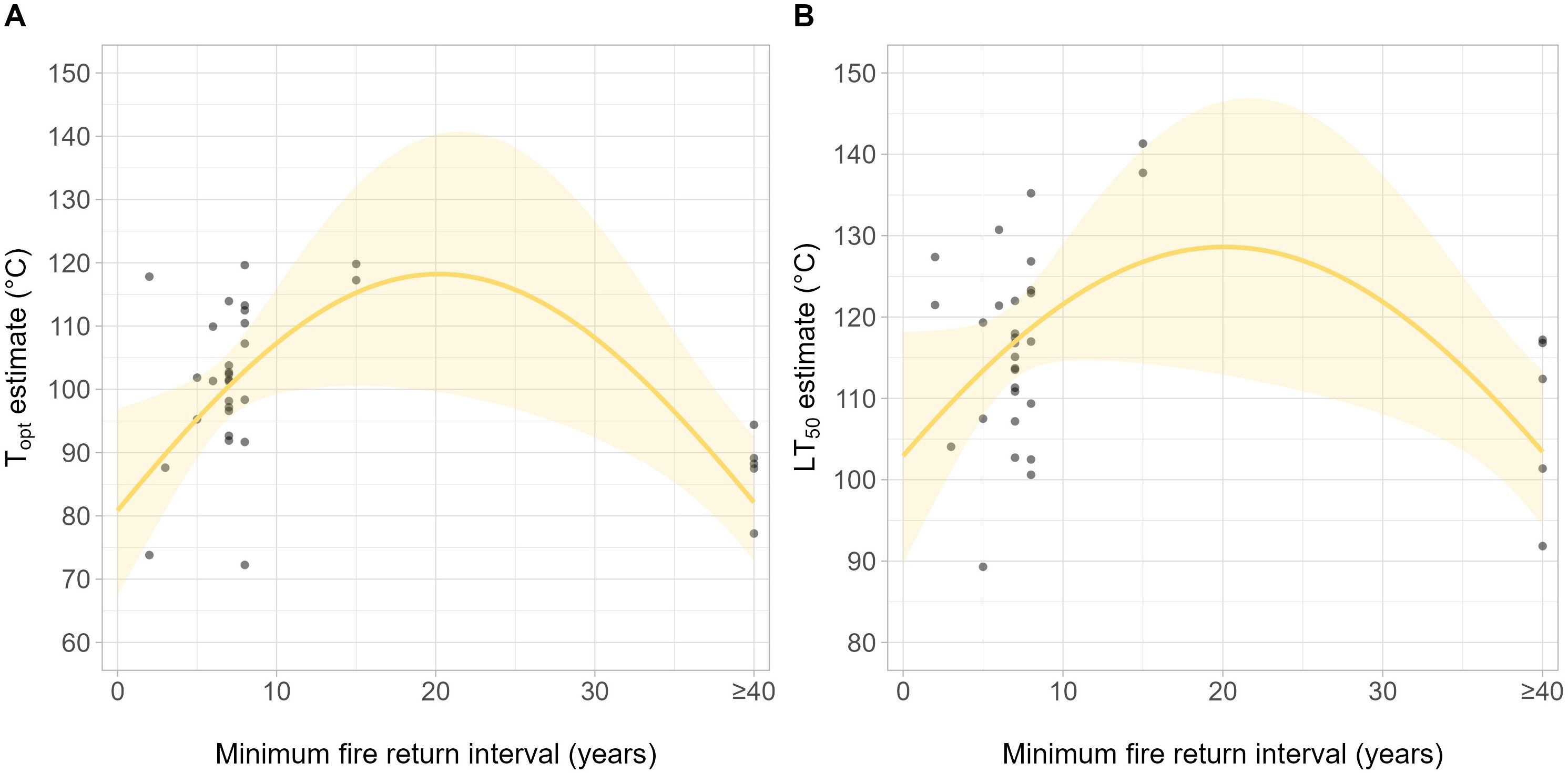
Figure 3. Topt (A) and LT50 (B) against the recommended minimum fire return interval (FRI) for the vegetation type associated with each species (Supplementary Table S2).
The DRT50 and LT50 both had a significant, hump-shaped relationship with broad fuel type (Figure 4; DRT50: F = 4.59, df = 2, p = 0.018, R2adj = 0.17; LT50: F = 4.52, df = 2, p = 0.019, R2adj = 0.17). No significant relationship existed between the Topt and fuel type (Figure 4; F = 1.83, df = 2, p = 0.177, R2adj = 0.05). These relationships were driven by the open grassland species (Figure 4, Table 2), which consistently had the lowest thermal thresholds. The mean DRT50 for open grassland species was 47°C, which was 25°C and 28°C lower than species from woodland (t-ratio = −2.61, p = 0.04) and forest (t-ratio = −2.83, p = 0.02) fuel types. The mean LT50 of open grassland species was also 15°C lower than woodland species (t-ratio = −2.96, p = 0.02). No significant difference existed between forest and woodland species across any pyro-thermal niche metrics, nor between any fuel types for the Topt (Table 2).
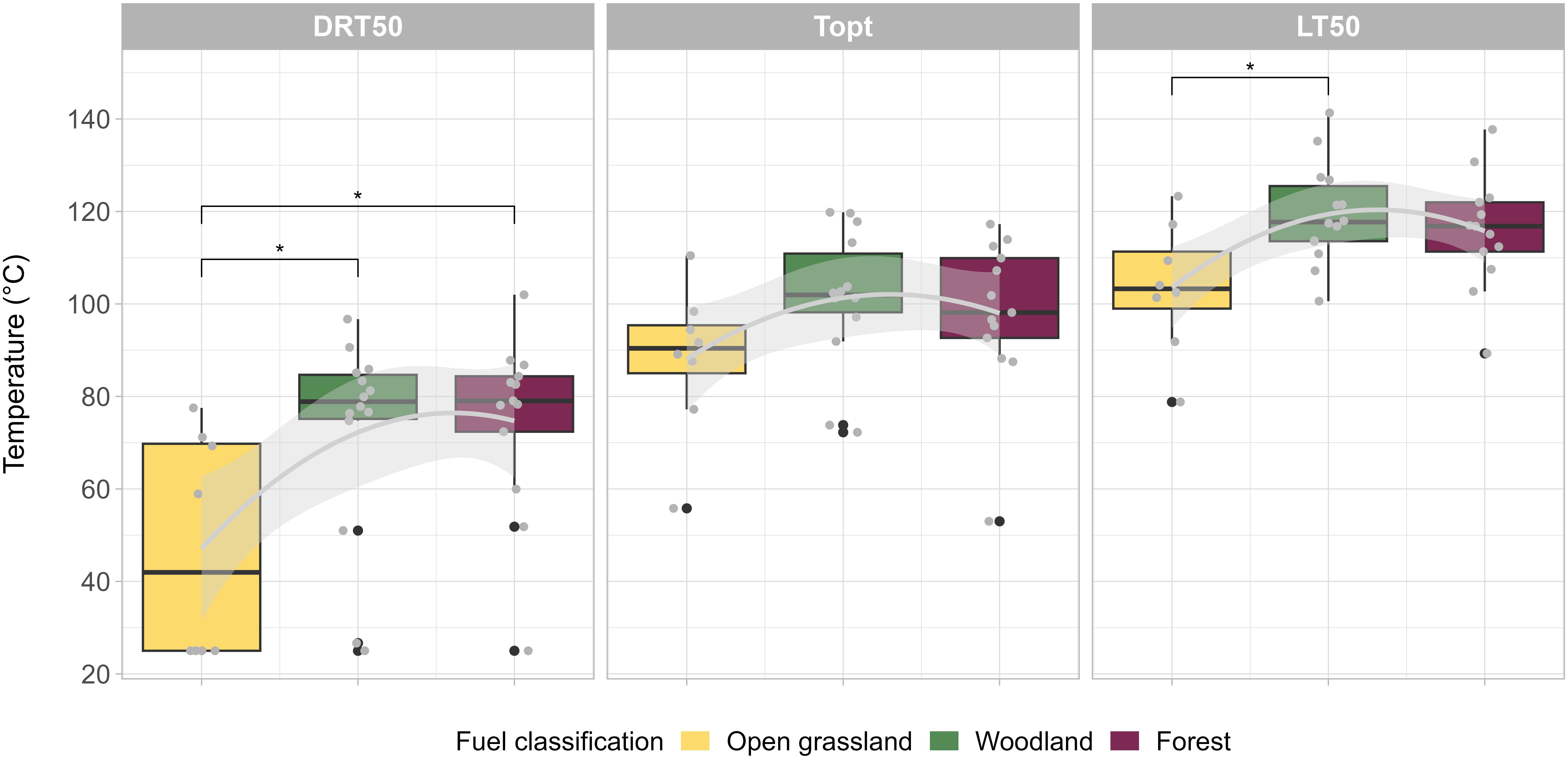
Figure 4. Pyro-thermal niche relationship with broad fuel classification. Significant relationships (p ≤ 0.05) are denoted by *.
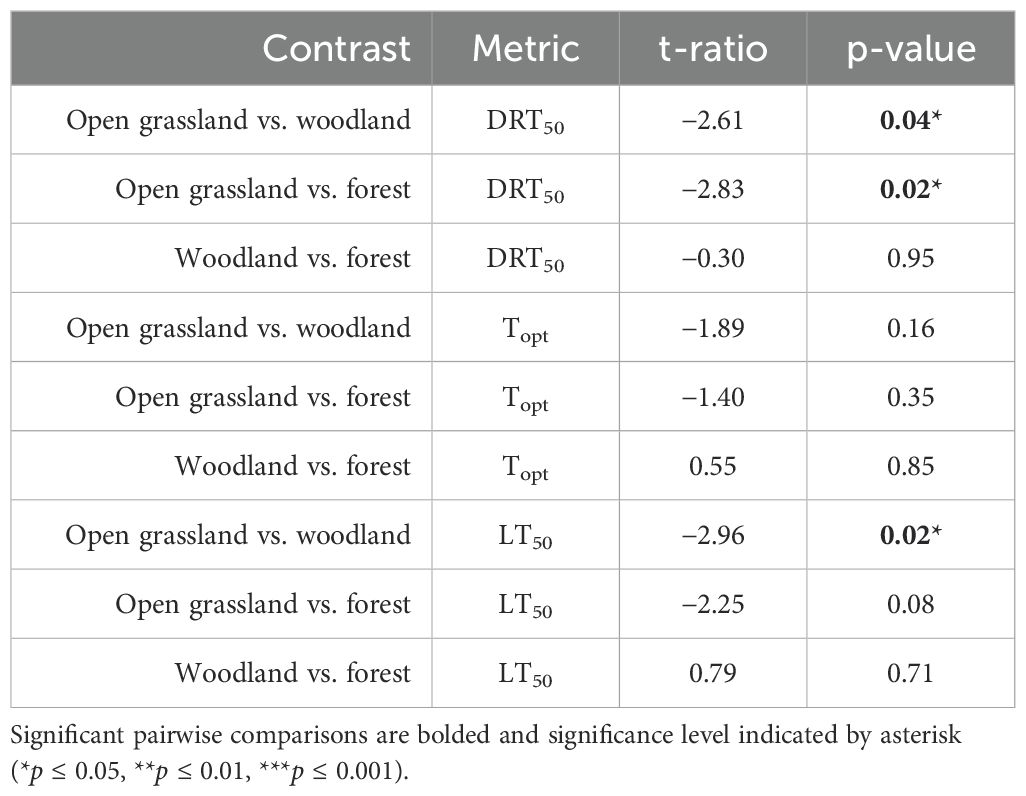
Table 2. Pairwise contrasts of fuel types (open grassland, woodland, forest) that explain each pyro-thermal niche metric (DRT50, Topt, LT50).
The DRT50 was the most varied metric across each fuel type, with a standard deviation of 24°C for species from open grasslands, 22°C for woodland, and 19°C for forests. The LT50 was the least variable, with standard deviations of 14°C, 12°C, and 11°C for open grasslands, forests, and woodlands, respectively.
3.3 Seed mass and climate against the pyro-thermal niche
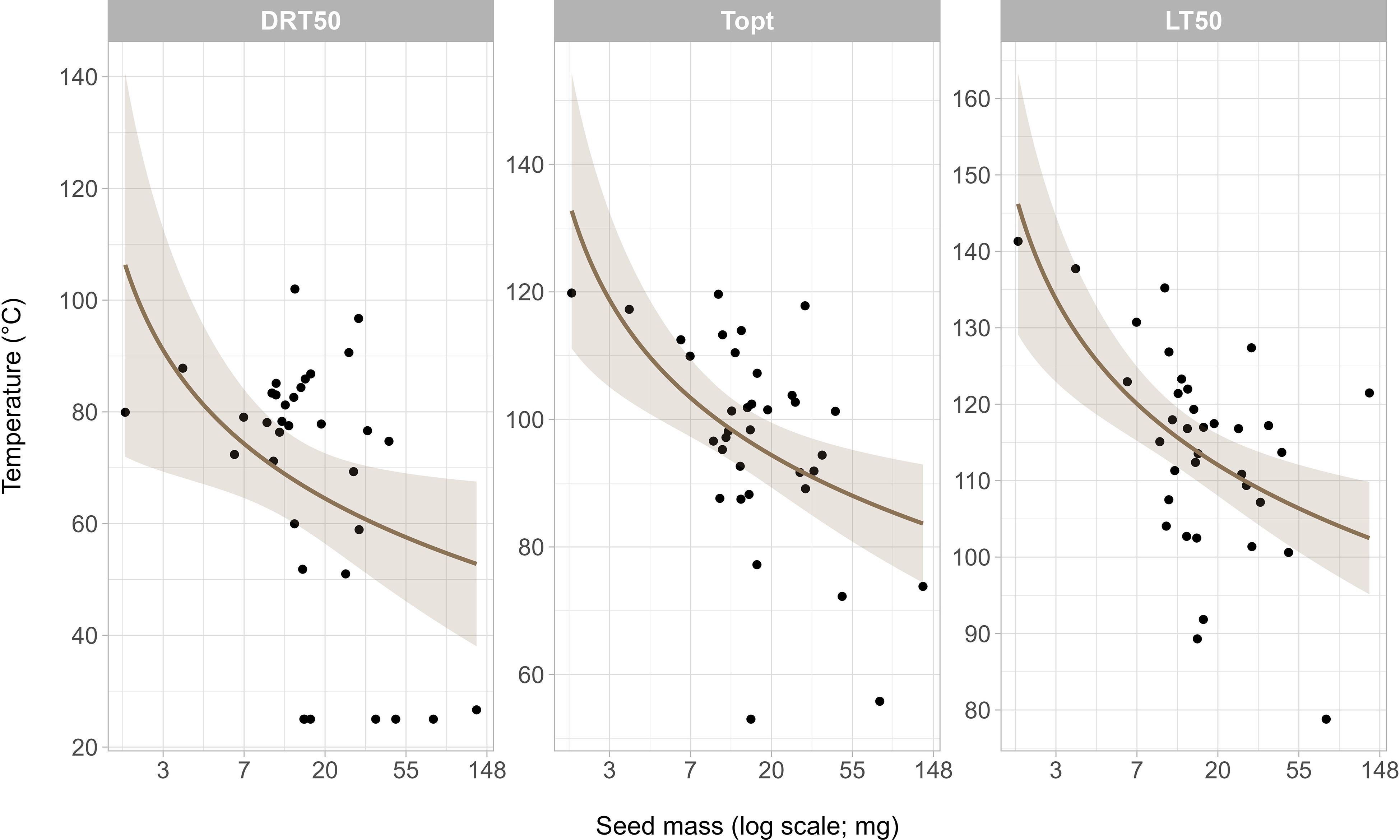
Seed mass had a negative relationship with all pyro-thermal niche metrics (Figure 5; DRT50: F = 10.1, df = 1, p = 0.003, R2adj = 0.21; Topt: F = 15.6, df = 1, p < 0.001, R2adj = 0.30; LT50: F = 12.4, df = 1, p = 0.001, R2adj = 0.25). Smaller seeds had higher temperatures across all pyro-thermal niche metrics than larger seeds, and the negative relationship between seed mass and pyro-thermal niche metrics was most significant for the Topt (p < 0.001, R2adj = 0.30).
No discernible patterns existed between the interaction of the climate variables, MAT and TAP, and the pyro-thermal niche (Figure 6). No significant relationships were found with any metric, nor when the variables were treated separately (Supplementary Figure S3).
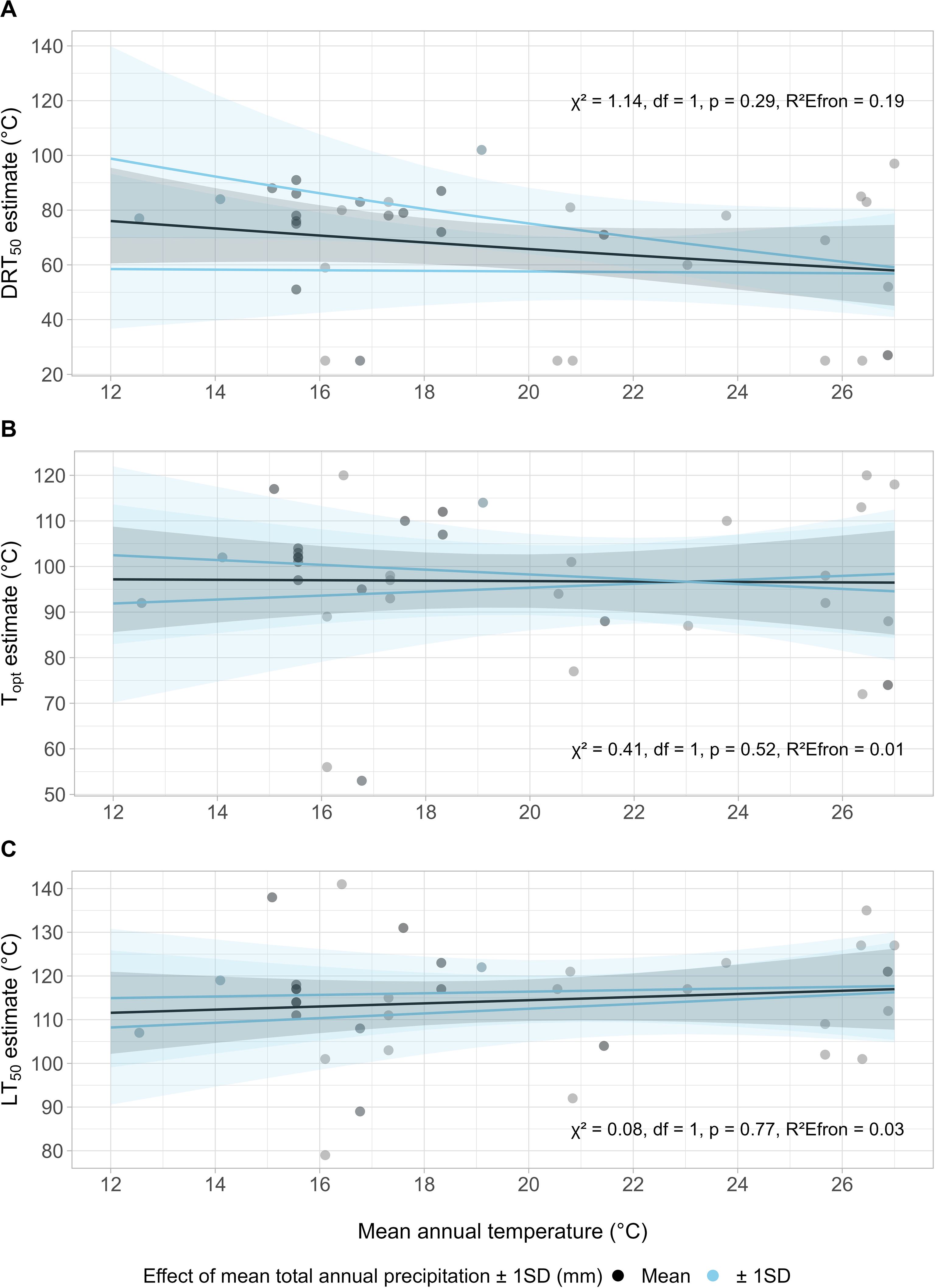
Figure 6. Non-significant relationship between the pyro-thermal niche metrics DRT50 (A), Topt (B) and LT50 (C) and the interacting climate variables mean annual temperature (MAT) and total annual precipitation (TAP). The effect of the moderator variable, TAP, on MAT and each pyro-thermal niche metric is presented across the mean ± 1 SD of TAP, alongside 95% confidence intervals. Relationships between pyro-thermal niche metrics and each variable separately (i.e., no interactions) are available in Supplementary Figure S3.
4 Discussion
Pyro-thermal niche metrics of Acacia species are shaped by fire regime and seed traits, highlighting the role fire plays in shaping trait expression in fire-prone ecosystems (Overton et al., 2024). Metrics had a non-linear relationship with FRI and fuel classification, with lower thermal thresholds observed in species with extreme FRIs and from grass-fuel types. However, contrary to our hypothesis, the non-linear relationship between fire metrics and the pyro-thermal niche was not strongest in the LT50, as the DRT50 and Topt showed stronger relationships with fuel type and FRI, respectively. Pyro-thermal niche metrics were highest at intermediate FRIs and for species from woodland and forest fuel types, mirroring the distribution of PY in fire-prone ecosystems (Monemizadeh et al., 2025). Climate variables showed no discernible relationship with pyro-thermal niche metrics, suggesting that climate is a broader driver of fuel regimes and dormancy class occurrence rather than within-class variation.
4.1 Acacia seed heat shock tolerance is highest in woody ecosystems with intermediate fire
Pyro-thermal niche metrics showed a hump-shaped relationship with both the minimum recommended FRI and fuel type, highlighting the role fire plays in shaping seed thermal thresholds. Pyro-thermal niche temperatures were highest at intermediate FRIs of approximately 20 years and in woodland and forest-type fuels, although this appears to largely be driven by two data points with a 15-year FRI; thus, additional data is needed to fill the gap of FRIs between 15 and 40 years to assess this relationship more robustly. Nevertheless, this is within the typical FRI of temperate fire-prone sclerophyll forests and woodlands in southeast Australia (National Parks and Wildlife Services (NPWS) and NSW Biodiversity Strategy, 2024), suggesting that pressure for PY seed heat shock tolerance is highest in fire-prone woody ecosystems that experience fire every few decades. This is supported by the distribution of PY within fire-prone ecosystems, as it is more prominent in woody ecosystem types than grassy vegetation communities (Pausas and Lamont, 2022).
4.2 Fire intensity and the pyro-thermal niche
Implicit to the relationship between the pyro-thermal niche metrics, FRI, and fuel type is the shared bond they all have with fire intensity. Fire intensity is the output of energy during a fire (Keeley, 2009) and is correlated with FRI (Bradstock, 2010). Ecosystems that support high-frequency fires generate lower intensity fires, such as in northern Australian savannas that have grass-based fuel loads that re-establish rapidly post-fire (supported by strong seasonal rainfall) and have an FRI < 5 years (Murphy et al., 2013). Conversely, when fuels accumulate due to longer FRIs and sufficient rainfall that supports high levels of biomass production, high-intensity fires are generated, such as Eucalpytus regnans-dominated (mountain ash) forests that burn at high to extreme intensity every 75–100 years (McCarthy et al., 2002). Higher fuel loads associated with high-intensity fires might be expected to generate more soil heating and thus select for higher threshold temperatures. However, the highest metrics observed here were from vegetation types with intermediate FRIs and fire intensity; thus, pyro-thermal niche metrics do not appear to have a linear relationship with fire intensity. This is likely due to PY-break being decoupled from fire cues where fire is a rare occurrence, as PY is broken through seasonal temperature fluctuations rather than heat shock (Baskin and Baskin, 2014).
4.3 Low thermal thresholds and alternate persistence strategies in regions with extreme FRIs or grass fuels
The comparatively low temperature thresholds for species with long or short FRIs and/or from grass-based fuels emphasizes that different fire regimes select for different plant persistence strategies and life forms (Keeley et al., 2011; Simpson et al., 2021).
Species from grass-fuel types and with the shortest FRIs had some of the lowest pyro-thermal niche temperatures, indicating weak selection for high thermal thresholds when fire is frequent and soil heating low. Grass-based fuels generally produce frequent fires of a lower intensity than litter fuels, which are present in woodlands and forests (Archibald et al., 2013; Murphy et al., 2013; Simpson et al., 2021). Resprouting is also the dominant persistence strategy for woody plant species in frequently burnt ecosystems, as woody seeders are vulnerable to immaturity risk (Zedler, 1995), with woody resprouters showing a positive linear relationship with increasingly frequent fire (Yang et al., 2025). For example, A. oncinocarpa seedlings in the savannas of northern Australia, where FRIs are <5 years (Murphy et al., 2013), devote resources to root development upon germination to allow for the ability to resprout after fire (Setterfield, 2002). While lower soil heating from grass fuels may drive lower pyro-thermal niche metrics, the mean LT50 of species from open grasslands was 104°C, which, although lower than species from woodland (119°C) and forest fuels (116°C), is still considerably high. This high LT50, alongside the high variability in pyro-thermal niche metrics in grass-fuel types, may be because grass fuels such as Triodia, can produce fires of a high intensity (Archibald et al., 2013; Murphy et al., 2013).
The remaining species with low pyro-thermal niche temperatures were associated with long unburnt ecosystems (e.g., rainforests and deserts) where minimum FRIs were ≥ 40 years. Post-fire seedling recruitment is not the dominant plant persistence strategy where wildfires are not a regular occurrence (Yang et al., 2025) and fire-sensitive species are dominant, which could explain the lower Topt and LT50 observed here, as species are less tolerant to heat shock. Within the arid zone, Acacia seedbanks are more strongly regulated by rainfall rather than fire to best take advantage of sporadic rainfall events (Auld, 1990; Nano et al., 2012), and persistence strategies include moderate resprouting (A. aneura, A. loderi, A. melleodora; Auld, 1995; Nano et al., 2012) and seedling recruitment through bird digestion scarifying seeds and breaking dormancy (A. ligulata; Letnic et al., 2000). Additionally, the high non-dormant fraction in the arid species A. oswaldii has previously been reported to allow the species to better take advantage of sporadic rainfall events (Auld, 1995). In this study, A. oswaldii, alongside the arid and semi-arid species A. aneura, A. inaequilatera, A. tetragonaphylla and A. victoriae had non-dormant fractions ranging from 30% to 65%, indicating germination is cued to stochastic rainfall events in these species to allow for recruitment. However, arid Acacia species from northern Australia (A. atkinsiana, A. citrinoviridis, A. hamersleyenesis, A. holosericea) generally had higher pyro-thermal niche metrics compared to southern arid species (A. ligulata, A. oswaldii, A. victoriae, A. aneura). The higher observed thresholds in the northern arid Acacia species are likely due to increased regularity and intensity of fire in hummock grasslands compared to southern Acacia and chenopod shrublands (Supplementary Table S2; Archibald et al., 2013; Murphy et al., 2013), as the exclusion of fire can lower thermal thresholds of PY seeds (Overton et al., 2024). This is supported by research showing Acacia species in arid regions that are solely post-fire recruiters tend to be restricted to hummock grasslands in northern Australia (Nano et al., 2012), and that fire regime characteristics follow a latitudinal gradient of rainfall (Murphy et al., 2013).
Although pyro-thermal niche metrics were generally highest in woodland-fuel types, these were not meaningfully different from forest-fuel types. This is likely due to the coarse fuel classifications used in this study not accounting for fuel moisture, which is critical for distinguishing between overall biomass and burnable biomass in the context of fire regimes (Bradstock, 2010). Further separation of fuel types (particularly woodland and forest fuels) along a fuel moisture and connectivity gradient would provide further clarification to the relationship that fuel type shares with pyro-thermal niche metrics. This is particularly important for the forest-fuel type classification used here, as temperate forests were grouped with tropical closed forests where rainfall patterns are substantially different. This limitation is not a reflection of the fuel types outlined in Joshi et al. (2025), as the authors do provide more detailed breakdowns for each broad category. Rather, the use of coarse fuel types is due to the limited sample size in this study (n = 35), as adequate sample sizes could not be obtained when using more detailed fuel-type categorizations. Additionally, while seed age data were not available as seeds were acquired from external seed collectors, seed age can impact PY break as thermal thresholds lower over time (Liyanage and Ooi, 2017) and should be considered when interpreting results.
4.4 Seed mass shapes pyro-thermal niche metrics
Seed mass showed a negative correlation with pyro-thermal niche metrics. This supports the concept that larger seeds are associated with lower thresholds, as fire-related soil temperatures decrease with soil depth, and only large seeds are able to successfully emerge from deep within the soil profile (Hanley et al., 2003; Liyanage and Ooi, 2018; Sano et al., 2025). Between genera, the negative correlation between seed mass and heat shock tolerance has only applied to the LT50 previously (Tangney et al., 2025) and not been present in the DRT50 of species from the Faboideae subfamily of Fabaceae (McInnes et al., 2025). However, this study found a clear negative relationship between seed mass and pyro-thermal niche metrics within Acacia, suggesting that the factors that shape the DRT50 and Topt across genera may be different than those that shape variation within a genus.
4.5 Climate does not drive variation for physical dormancy in fire-prone ecosystems
MAT and TAP did not show any discernible relationship with pyro-thermal niche metrics; however, they are likely to shape fuel dynamics (and thus fire regimes) more broadly. While bioclimatic variables such as MAT and TAP are strong contributors to the presence of different dormancy classes in particular ecosystems (de Casas et al., 2017; Wyse and Dickie, 2018; Rosbakh et al., 2023), they do not appear to explain the variability in dormancy release found within Acacia species. These results suggest that the mechanisms that shape the distribution of different seed dormancy classes are different from those that shape variation in pyro-thermal niche metrics. Additionally, the finding of fire regime shaping the expression of PY contrasts the work of Rosbakh et al. (2023), who were unable to associate PY with fire season length at a global scale. This may not only be due to the variety of mechanisms that break PY (e.g., alternating temperatures, scarification; Baskin and Baskin, 2014) but also because of the inherent variability of thermal thresholds that break PY in fire-prone ecosystems (Tangney et al., 2025). Further study of associations between PY and fire-related cues requires more detailed assessments of seed thermal tolerance that also account for fire regime variability, such as the approach used here. While alternative climate variables other than those used here could potentially shape pyro-thermal niche variation in fire-prone ecosystems, this does not seem likely. Vegetation and fire, strong determinants of fuel characteristics and subsequent soil heating, can decouple each other from climate, as very different fire regimes and plant communities can occur within the same climatic region (e.g., rainforest and savanna; Bond et al., 2005; Murphy et al., 2013; Murphy and Bowman, 2012).
These results show considerable variation in pyro-thermal niche temperatures within Acacia, indicating that pyro-thermal niche metrics may be closely aligned with species’ habitat and reflecting other studies that report low phylogenetic signals in germination traits (e.g., base water potential) when microhabitat is considered (Arène et al., 2017). However, it should be emphasized that some species and families have higher pyro-thermal niche metrics than others, even when habitat is accounted for (Tangney et al., 2025).
Understanding the drivers of dormancy-break in Acacia is important in predicting how Acacia species will respond to changing fire regimes within their natural range, alongside how they persist in their invasive range—particularly in Mediterranean ecosystems. Overall, these results suggest that fire regime—specifically elements that drive soil heating—and seed traits shape variation within PY release in fire-prone ecosystems. This highlights the need to consider individual seed dormancy classes not as a binary trait but as a spectrum shaped by individual species’ ecology to allow for persistence in unpredictable landscapes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SM: Investigation, Conceptualization, Writing – review & editing, Visualization, Formal Analysis, Writing – original draft. RT: Writing – review & editing, Supervision, Conceptualization. KC: Investigation, Writing – review & editing. NA: Investigation, Writing – review & editing. MO: Supervision, Funding acquisition, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by an Australian Research Council Linkage Project (LP180100741) to MO, who is also supported by funding from the New South Wales Bushfire and Natural Hazards Research Centre.
Acknowledgments
We would like to thank the PlantBank at Mount Annan for providing seed material and allowing us to use their laboratory to determine initial seed viability. We would also like to thank Bethlea Bell for providing seed material and Thomas Williams, Azlïn Auckburally, and Harrison Windsor for their assistance with scoring germination.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1690756/full#supplementary-material
References
Archibald S., Lehmann C. E. R., Gómez-Dans J. L., and Bradstock R. A. (2013). Defining pyromes and global syndromes of fire regimes. Proc. Natl. Acad. Sci. United States America 110, 6442–6447. doi: 10.1073/pnas.1211466110
Arène F., Affre L., Doxa A., and Saatkamp A. (2017). Temperature but not moisture response of germination shows phylogenetic constraints while both interact with seed mass and lifespan. Seed Sci. Res. 27, 110–120. doi: 10.1017/S0960258517000083
Auld T. D. (1990). Regeneration in populations of the arid zone plants Acacia carnei and A. oswaldii. Proc. Ecol. Soc. Aust. 16, 267–272.
Auld T. D. (1995). Soil seedbank patterns of four trees and shrubs from arid Australia. J. Arid Environments 29, 33–45. doi: 10.1016/S0140-1963(95)80062-X
Auld T. D. and O'Connell M. A. (1991). Predicting patterns of postfire germination in 35 eastern Australian Fabaceae. Aust. J. Ecol. 16, 53–70. doi: 10.1111/j.1442-9993.1991.tb01481.x
Australian National Botanic Gardens (2024). Wattles – genus Acacia. Available online at: https://www.anbg.gov.au/acacia/ (Accessed October 24, 2025).
Baskin C. C. and Baskin J. M. (2014). Seeds: ecology, biogeography, and evolution of dormancy and germination. 2nd edn (San Diego: Academic Press).
Bond W. J., Honig M., and Maze K. E. (1999). Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia 120, 132–136. doi: 10.1007/s004420050841
Bond W. J., Woodward F. I., and Midgley G. F. (2005). The global distribution of ecosystems in a world without fire. New Phytol. 165, 525–537. doi: 10.1111/j.1469-8137.2004.01252.x
Bradstock R. A. (2010). A biogeographic model of fire regimes in Australia: current and future implications. Global Ecol. Biogeography 19, 145–158. doi: 10.1111/j.1466-8238.2009.00512.x
Bradstock R. A. and Auld T. D. (1995). Soil temperatures during experimental bushfires in relation to fire intensity - consequences for legume germination and fire management in south-eastern Australia. J. Appl. Ecol. 32, 76–84. doi: 10.2307/2404417
Busse M. D., Bussea M. D., Fiddler G. O., Shestak C. J., and Powers R. F. (2005). Lethal soil temperatures during burning of masticated forest residues. Int. J. Wildland Fire 14, 267–276. doi: 10.1071/WF04062
Busse M. D., Shestak C. J., and Hubbert K. R. (2013). Soil heating during burning of forest slash piles and wood piles. Int. J. Wildland Fire 22, 786–796. doi: 10.1071/WF12179
Catchpole W. (2002). “Fire properties and burn patterns in heterogeneous landscapes,” in Flammable Australia: the fire regimes and biodiversity of a continent. Eds. Bradstock R. A., Williams J. E., and Gill M. A. (Cambridge University Press, Cambridge), 49–75.
Chamberlain S., Barve V., Mcglinn D., Oldoni D., Desmet P., Geffert L., et al. (2025). rgbif: Interface to the Global Biodiversity Information Facility API, version 3.8.2. Available online at: https://CRAN.R-project.org/package=rgbif (Accessed August 14, 2025).
de Casas R. R., Willis C. G., Pearse W. D., Baskin C. C., Baskin J. M., and Cavender-Bares J. (2017). Global biogeography of seed dormancy is determined by seasonality and seed size: a case study in the legumes. New Phytol. 214, 1527–1536. doi: 10.1111/nph.14498
Department of Climate Change, Energy, the Environment and Water (DCCEEW) (2024). Australia - Present Major Vegetation Groups and Subgroups – NVIS, version 7.0. Available online at: https://fed.dcceew.gov.au/datasets/5e70b5afc36a4c458a2cceb313eb3889/about (Accessed August 14, 2025).
Efron B. (1978). Regression and ANOVA with zero-one data: measures of residual variation. J. Am. Stat. Assoc. 73, 113–121. doi: 10.1080/01621459.1978.10480013
Erickson T. E., Merritt D. J., and Turner S. R. (2016). Overcoming physical seed dormancy in priority native species for use in arid-zone restoration programs. Aust. J. Bot. 64, 401–416. doi: 10.1071/BT16059
Fick S. E. and Hijmans R. J. (2017). WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int. J. Climatology 37, 4302–4315. doi: 10.1002/joc.5086
Frischie S., Miller A. L., Pedrini S., and Kildisheva O. A. (2020). Ensuring seed quality in ecological restoration: native seed cleaning and testing. Restor. Ecol. 28, S239–S248. doi: 10.1111/rec.13217
Gill A. M. (1975). Fire and the Australian flora: a review. Aust. Forestry 38, 4–25. doi: 10.1080/00049158.1975.10675618
Gimeno-García E., Andreu V., and Rubio J. L. (2004). Spatial patterns of soil temperatures during experimental fires. Geoderma 118, 17–38. doi: 10.1016/S0016-7061(03)00167-8
Hanley M. E., Unna J. E., and Darvill B. (2003). Seed size and germination response: a relationship for fire-following plant species exposed to thermal shock. Oecologia 134, 18–22. doi: 10.1007/s00442-002-1094-2
Hartig F. (2024). DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models, version 0.4.7. https://doi.org/10.32614/CRAN.package.DHARMa (Accessed August 14, 2024).
Hijmans R. (2025). terra: spatial data analysis, version 1.8-50. https://doi.org/10.32614/CRAN.package.terra (Accessed August 14, 2024).
Hijmans R. J., Barbosa M., Ghosh A., and Mandel A. (2024). geodata: download geographic data, version 0.6-2. https://doi.org/10.32614/CRAN.package.geodata (accessed August 14, 2024).
Hopkins J. R., Semenova-Nelsen T. A., Huffman J. M., Jones N. J., Robertson K. M., Platt W. J., et al. (2025). Fuel accumulation shapes post-fire fuel decomposition through soil heating effects on plants, fungi, and soil chemistry. Sci. Total Environ. 961, 178386. doi: 10.1016/j.scitotenv.2025.178386
Joshi R. C., Cruz M. G., and Donohue R. J. (2025). Nationally consistent mapping of wildland fuel types across Australia using satellite-derived vegetation structural data. Int. J. Wildland Fire 34, WF24224. doi: 10.1071/WF24224
Keeley J. E. (2009). Fire intensity, fire severity and burn severity: a brief review and suggested usage. Int. J. Wildland Fire 18, 116–126. doi: 10.1071/WF07049
Keeley J. E., Pausas J. G., Rundel P. W., Bond W. J., and Bradstock R. A. (2011). Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 16, 406–411. doi: 10.1016/j.tplants.2011.04.002
Kreye J. K., Kobziar L. N., and Zipperer W. C. (2013). Effects of fuel load and moisture content on fire behaviour and heating in masticated litter-dominated fuels. Int. J. Wildland Fire 22, 440–445. doi: 10.1071/WF12147
Lenth R. (2025). emmeans: estimated marginal means, aka least-squares means, version 1.11.1. https://doi.org/10.32614/CRAN.package.emmeans (accessed August 14, 2025).
Letnic M., Dickman C. R., and McNaught G. (2000). Bet-hedging and germination in the Australian arid zone shrub Acacia ligulata. Austral Ecol. 25, 368–374. doi: 10.1046/j.1442-9993.2000.01047.x
Liyanage G. S. and Ooi M. K. J. (2017). Do dormancy-breaking temperature thresholds change as seeds age in the soil seed bank? Seed Sci. Res. 27, 1–11. doi: 10.1017/S0960258516000271
Liyanage G. S. and Ooi M. K. J. (2018). Seed size-mediated dormancy thresholds: a case for the selective pressure of fire on physically dormant species. Biol. J. Linn. Soc. 123, 135–143. doi: 10.1093/biolinnean/blx117
Lüdecke D., Ben-Shachar M., Patil I., Waggoner P., and Makowski D. (2021). performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Software 6, 3139. doi: 10.21105/joss.03139
Mackenzie B. D. E., Auld T. D., Keith D. A., Hui F. K. C., and Ooi M. K. J. (2016). The effect of seasonal ambient temperatures on fire-stimulated germination of species with physiological dormancy: a case study using Boronia (Rutaceae). PloS One 11, e0156142. doi: 10.1371/journal.pone.0156142
Maslin B. R. and Pedley L. (1988). Patterns of distribution of acacia in Australia. Aust. J. Bot. 36, 385–393. doi: 10.1071/BT9880385
McCarthy M., Gill M., Lang S., and Moore P. H. R. (2002). “Local extinction or persistence of Mountain Ash due to fire regimes,” in Wildlife, fire and future climate: a forest ecosystem analysis. Eds. Mackey B., Lindenmayer D., Gill M., McCarthy M., and Lindesay J. (CSIRO Publishing, Collingwood), 23–43.
McInnes S. J., Tangney R., and Ooi M. K. J. (2025). Seed fatty acid composition and physical dormancy in fire-prone ecosystems. Ann. Bot. 00, 1–14. doi: 10.1093/aob/mcaf225
Miller B. P. and Murphy B. P. (2017). “Fire and Australian vegetation,” in Australian vegetation, 3rd edn. Ed. Keith D. A. (Cambridge University Press, Cambridge), 113–134.
Monemizadeh Z., Siahmarguee A., Soltani E., Torabi B., Baskin C. C., Azimmohseni M., et al. (2025). Fire and seed dormancy: a global meta-analysis. Ann. Bot. 135, 1059–1074. doi: 10.1093/aob/mcae229
Moreira B. and Pausas J. G. (2012). Tanned or burned: the role of fire in shaping physical seed dormancy. PloS One 7, e51523. doi: 10.1371/journal.pone.0051523
Murphy B. P. and Bowman D. M. J. S. (2012). What controls the distribution of tropical forest and savanna? Ecol. Lett. 15, 748–758. doi: 10.1111/j.1461-0248.2012.01771.x
Murphy B. P., Bradstock R. A., Boer M. M., Carter J., Cary G. J., Cochrane M. A., et al. (2013). Fire regimes of Australia: a pyrogeographic model system. J. Biogeography 40, 1048–1058. doi: 10.1111/jbi.12065
Nano C. E., Clarke P. J., and Pavey C. R. (2012). “Fire regimes in arid hummock grasslands and Acacia shrublands,” in Flammable Australia: fire regimes, biodiversity and ecosystems in a changing world. Eds. Bradstock R. A., Gill A. M., and Williams R. J. (CSIRO Publishing, Collingwood), 195–214.
National Parks and Wildlife Services (NPWS), NSW Biodiversity Strategy (2004). Guidelines for ecologically sustainable fire management. Available online at: https://www.environment.nsw.gov.au/resources/biodiversity/FireGuidelinesReport.pdf (Accessed August 14, 2025).
Ooi M. K. J., Denham A. J., Santana V. M., and Auld T. D. (2014). Temperature thresholds of physically dormant seeds and plant functional response to fire: variation among species and relative impact of climate change. Ecol. Evol. 4, 656–671. doi: 10.1002/ece3.973
Ooi M. K. J., Tangney R., and Auld T. D. (2022). “Fire and regeneration from seeds in a warming world, with emphasis on Australia,” in Plant regeneration from seeds: a global warming perspective. Eds. Baskin C. C. and Baskin J. M. (Academic Press, London), 229–242.
Overton J., Ooi M. K. J., and Tangney R. (2024). Some like it hot: seed thermal threshold variation in obligate seeding Acacia pulchella along a climate gradient. Sci. Total Environ. 948, 174929. doi: 10.1016/j.scitotenv.2024.174929
Padfield D., O'Sullivan H., and Windram F. 2024. rTPC: fitting and analysing thermal performance curves. R package version 1.0-2. URL https://CRAN.Rproject.org/package=rTPC (Accessed January 29, 2025).
Pausas J. G. and Bradstock R. A. (2007). Fire persistence traits of plants along a productivity and disturbance gradient in mediterranean shrublands of south-east Australia. Global Ecol. Biogeography 16, 330–340. doi: 10.1111/j.1466-8238.2006.00283.x
Pausas J. G. and Lamont B. B. (2022). Fire-released seed dormancy - a global synthesis. Biol. Rev. 97, 1612–1639. doi: 10.1111/brv.12855
Pausas J. G. and Paula S. (2012). Fuel shapes the fire-climate relationship: evidence from Mediterranean ecosystems. Global Ecol. Biogeography 21, 1074–1082. doi: 10.1111/j.1466-8238.2012.00769.x
Pebesma E. and Bivand R. (2023). Spatial data science: with applications in R (Abingdon: CRC Press).
R Core Team. (2024). R: A Language and Environment for Statistical Computing, version 4.5.0. Vienna, Austria: R foundation for Statistical Computing. URL https://www.R-project.org. (Accessed January 29, 2025).
Raison R. J., Woods P. V., Jakobsen B. F., and Bary G. A. V. (1986). Soil temperatures during and following low-intensity prescribed burning in a Eucalyptus pauciflora forest. Aust. J. Soil Res. 24, 33–47. doi: 10.1071/SR9860033
Ramos D. M., Liaffa A. B. S., Diniz P., Munhoz C. B., Ooi M. K. J., Borghetti F., et al. (2016). Seed tolerance to heating is better predicted by seed dormancy than by habitat type in Neotropical savanna grasses. Int. J. Wildland Fire 25, 1273–1280. doi: 10.1071/WF16085
Rosbakh S., Carta A., Fernández-Pascual E., Phartyal S. S., Dayrell R. L. C., Mattana E., et al. (2023). Global seed dormancy patterns are driven by macroclimate but not fire regime. New Phytol. 240, 555–564. doi: 10.1111/nph.19173
Sano M., Tangney R., Thomsen A., and Ooi M. K. J. (2025). Extreme fire severity interacts with seed traits to moderate post-fire species assemblages. Am. J. Bot. 112, e70012. doi: 10.1002/ajb2.70012
Setterfield S. A. (2002). Seedling establishment in an Australian tropical savanna: effects of seed supply, soil disturbance and fire. J. Appl. Ecol. 39, 949–959. doi: 10.1046/j.1365-2664.2002.00772.x
Simpson K. J., Jardine E. C., Archibald S., Forrestel E. J., Lehmann C. E., Thomas G. H., et al. (2021). Resprouting grasses are associated with less frequent fire than seeders. New Phytol. 230, 832–844. doi: 10.1111/nph.17069
Sumner M. (2021). ozmaps: Australia maps, version 0.4.5. https://doi.org/10.32614/CRAN.package.ozmaps (Accessed August 14, 2025).
Tangney R., Issa N. A., Merritt D. J., Callow J. N., and Miller B. P. (2018). A method for extensive spatiotemporal assessment of soil temperatures during an experimental fire using distributed temperature sensing in optical fibre. Int. J. Wildland Fire 27, 135–140. doi: 10.1071/WF17107
Tangney R., McInnes S. J., Dalziell E. L., Cornwell W. K., Miller B., Auld T. D., et al. (2025). Defining the pyro-thermal niche: do seed traits, ecosystem type and phylogeny influence thermal thresholds in seeds with physical dormancy? New Phytol. 246, 1567–1582. doi: 10.1111/nph.70061
Tangney R., Merritt D. J., Callow J. N., Fontaine J. B., and Miller B. (2020). Seed traits determine species' responses to fire under varying soil heating scenarios. Funct. Ecol. 34, 1967–1978. doi: 10.1111/1365-2435.13623
Venables W. N. and Ripley B. D. (2002). Modern Applied Statistics with S. 4th edn (New York: Springer).
Wei T. and Simko V. (2024). R package 'corrplot': visualization of a correlation matrix, version 0.95. Available online at: https://github.com/taiyun/corrplot (Accessed August 14, 2025).
Williams P. R., Congdon R. A., Grice A. C., and Clarke P. J. (2003). Fire-related cues break seed dormancy of six legumes of tropical eucalypt savannas in north-eastern Australia. Austral Ecol. 28, 507–514. doi: 10.1046/j.1442-9993.2003.01307.x
Wyse S. V. and Dickie J. B. (2018). Ecological correlates of seed dormancy differ among dormancy types: a case study in the legumes. New Phytol. 217, 477–479. doi: 10.1111/nph.14777
Yang S., Ooi M. K. J., Falster D. S., and Cornwell W. K. (2025). Continental-scale empirical evidence for relationships between fire response strategies and fire frequency. New Phytol. 246, 528–542. doi: 10.1111/nph.20464
Zedler P. H. (1995). “Fire frequency in southern California shrublands: biological effects and management options,” in Brushfires in California wildlands: ecology and resource management. Eds. Keeley J. E. and Scott T. (International Association of Wildland Fire, Washington), 101–112.
Keywords: heat shock, seed persistence, pyro-thermal niche, Acacia, fuel characteristics, fire return interval, fire intensity, germination
Citation: McInnes SJ, Tangney R, Chong KGJ, Allen NE and Ooi MKJ (2025) Fire as a driver of pyro-thermal niche variation in Acacia. Front. Ecol. Evol. 13:1690756. doi: 10.3389/fevo.2025.1690756
Received: 22 August 2025; Accepted: 31 October 2025;
Published: 24 November 2025.
Edited by:
Arian Correa-Diaz, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, MexicoReviewed by:
Francisco Alejandro López-Núñez, University of Coimbra, PortugalCarlos A. Ordóñez-Parra, Universidade Federal de Minas Gerais, Brazil
Copyright © 2025 McInnes, Tangney, Chong, Allen and Ooi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah J. McInnes, cy5tY2lubmVzQHVuc3cuZWR1LmF1
 Sarah J. McInnes
Sarah J. McInnes Ryan Tangney1,2
Ryan Tangney1,2 Mark K. J. Ooi
Mark K. J. Ooi