- 1Keller Science Action Center, The Field Museum of Natural History, Chicago, IL, United States
- 2Department of Biological Sciences, Auburn University, Auburn, AL, United States
- 3La Grange Park, IL, United States
The Guiana Shield in northeastern South America contains some of the largest tracts of intact forests on the globe. Guyana alone has more than 80% forest cover. In south-central Guyana a unique biogeographic feature allows for a hydrological connection between the Guiana Shield with the Amazon basin via the Rupununi savannas and wetlands (Rupununi Portal). This corridor allows for connectivity between two of the most biodiverse, carbon rich, and intact forests in the world. The significance of this (and other) hydrological corridors for terrestrial and aquatic species is underappreciated in the scientific literature. We attempt to determine the importance of the surrounding mosaic of habitats that influence fish assemblages in the Rupununi Portal. We extensively sampled fishes in this corridor over six expeditions. Multivariate analyses revealed significant trends in fish assemblage structure and environmental conditions. We found high species richness and diversity within the Takutu (Amazon River drainage) and Rupununi rivers (Essequibo River drainage). Fish assemblages were found to be most similar within the specific river drainages with some similarity within forest and savanna sites. A second Rupununi portal was revealed in the South Rupununi, at the upper Rupununi and Takutu Rivers. Ordination analyses found water body type, vegetation and water chemistry to be significantly structuring the fish assemblages of the Rupununi. Our study reveals the interdependent nature of the various habitats in the Rupununi that facilitate high biodiversity and maintain the ecosystem. With the increase in human activity in this region, and the increased connectivity of the region with the rest of the world through better roads, this area is in danger of being modified and fragmented to a point where ecosystem services begin to fail. We recommend demarcating a protected area in the region that considers the diversity of associated habitats and the importance of the ecological portal joining two diverse river basins.
Introduction
The Guiana Shield is an ancient geological formation of Proterozoic and Archean uplift located in northeastern South America. It spans across French Guiana, Suriname, Guyana, Venezuela to its western most terminus in Colombia and contains some of the largest tracts of intact forests on the globe (Potapov et al., 2017). Its dynamic and complex paleogeographic history has contributed to the tremendous biodiversity, spectacular table-top mountains called tepuis and remarkable waterfalls often cascading 500 m or more off the face of escarpments throughout the Shield (i.e., Angel Falls, Kaieteur Falls, Yutajé Falls). High levels of endemism are characteristic for the region particularly among several taxonomic groups (including cichlids, gymnotiform knifefishes, and siluriform catfishes) in the Guiana Shield with percentages as high as 80–95% (GAHEF, 1992; Duellman, 1999; Lopez-Fernandez et al., 2012; Maldonado Ocampo et al., 2013; Potapov et al., 2017; de Souza et al., 2019; Paz et al., 2019). Intriguing explorers for centuries, these highland regions of the Guiana Shield continue to remain poorly known due to remoteness and isolation. Of global importance, recent studies have shown the role the Guiana Shield can play in mitigating impacts from climate change due to the large tracts of intact forests and hydrological connection to the Amazonian forests (Bovolo et al., 2018).
The significance of hydrological corridors between large expanses of intact forests is scarce in the literature. River networks weave their way through forested landscapes bringing important nutrients to the soils, stabilizing hydrological regimes and supporting biodiversity (Vannote et al., 1980; Wang et al., 2015). Additionally, these forested landscapes have an important role on the ichthyofauna of the Guiana Shield and Amazon River basin, which is the focus of this study (Lujan and Armbruster, 2011). With ~6,000 described species, the Neotropical region harbors the greatest diversity of freshwater fishes on the planet (Reis, 2013). Contributing to this richness are diversification processes associated with the extensive geological history of South America, including paleoclimates, paleoenvironments, and the relatively recent (Miocene) time frame of the modern-day assembly of the Amazon basin as shown by geology, fossils and modern distributions of organisms (Hoorn et al., 1995; Räsänen and Linna, 1996; Lundberg et al., 1998; Lundberg, 2005; Lujan and Armbruster, 2011). The present-day configuration of the major river drainages in South America is based on major historical events associated with the tectonic evolution of the Amazon basin (Albert and Reis, 2011).
Located between the western and eastern portions of the Guiana Shield is an area drained by two of South America's largest rivers across a network of savanna, forests and wetlands. The Amazon and Essequibo Rivers are two major fluvial systems, covering ~7,000,000 and 182,000 km2, respectively (Albert et al., 2011). A hydrological connection forms between the Essequibo and Amazon rivers during the rainy season via the Rupununi wetlands of south-central Guyana, an area of 3,480 km2, termed the Rupununi Portal (de Souza et al., 2012). The Rupununi wetlands are an expansive network of ephemeral ponds, oxbow lakes, depression lakes, and creeks across vast savannas. Bordered by two main river drainages, the Takutu and Rupununi Rivers, the wetlands are intimately associated with the nearby forests, savannas, and indigenous people in the North Rupununi (Figure 1). These wetlands are an important site for migration and spawning of many aquatic taxa (including commercially and locally important fish species), and they harbor a tremendous amount of biodiversity including amphibians, reptiles, birds, plants, and fishes (Lowe-Mcconnell, 1964; Braun et al., 2007; de Souza et al., 2012; Cole et al., 2013). Globally, the Rupununi Portal is one of two known seasonal connections uniting two highly diverse species faunas (Winemiller et al., 2008; Albert et al., 2011; Lujan and Armbruster, 2011).
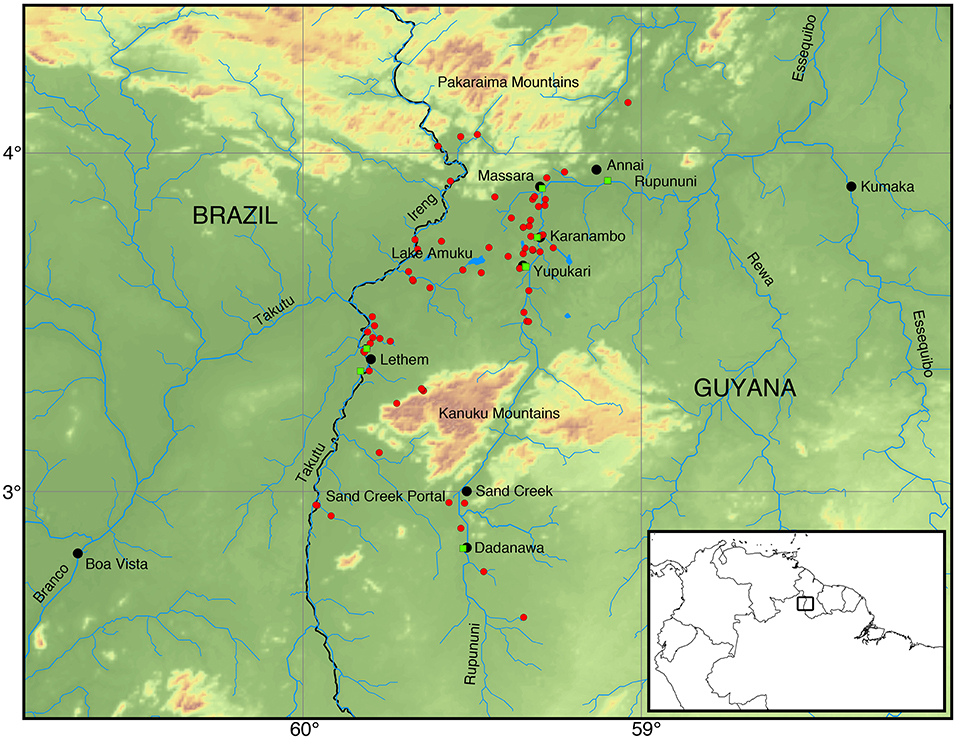
Figure 1. Map of the Rupununi region with red circles and green squares that represent collecting sites in the Rupununi. The green squares are sites with highest fish diversity. Lake Amuku represents the area that floods to provide the corridor between the Takutu and Rupununi rivers to form the Rupununi Portal. Location of the Sand Creek Portal is indicated and detailed further in Figure 5.
Research on the fish communities and adjacent landscapes in the Rupununi Portal is timely. Freshwater ecosystems are among the most imperiled on the globe, with declines in freshwater biodiversity greater than most affected terrestrial habitats (Sala et al., 2000; Strayer and Dudgeon, 2010; WWF, 2012). In addition, freshwater ecosystems cover <1% of the earth's surface, one-third of all vertebrates belong to freshwater ecosystems and freshwater fishes comprise 25% of all vertebrates (Lévêque et al., 2007). However, riverine habitats are rarely considered when setting aside protected areas, and aquatic biodiversity is largely ignored (Abell et al., 2011). Studies have suggested the influential role that the Rupununi Portal has played in structuring the flora and fauna of the region (Eigenmann, 1912; Lowe-Mcconnell, 1975; Hoogmoed, 1979; Turner et al., 2004; Mistry et al., 2008; de Souza et al., 2012), but no study has assessed how the mosaic of environmental characteristics that make up the Rupununi Portal influences fish assemblages.
Despite heavy pressure from gold, diamond, and bauxite mining, timber companies, exploratory oil drilling, and new road construction, only 8.74% of Guyana is protected, which is very small in contrast to the overall 22.75% of all of Latin America plus the Caribbean that is protected (International Union for the Conservation of Nature (IUCN; https://www.protectedplanet.net). A National Protected Areas System (NPAS) for Guyana has been in discussion since the Convention on Biological Diversity in 1994 (Persaud, 1997; World Bank, 1997). In these initial discussions the Rupununi savannas and wetlands have been proposed for protection due to high diversity across several taxa (Dalfelt, 1978; GAHEF, 1991, 1992). Dalfelt (1978) made particular note of the importance in the Rupununi that a protected area should attempt to incorporate the ecological gradient between the savannas and wetlands; however, the savanna is currently not protected.
There is a sense of urgency for this study in the Rupununi Portal, due to impending threats by oil drilling, gold/diamond mining, logging, and agriculture in the area. Fishes account for 60% of the protein in the diet of the Amerindians living in the area (Watkins et al., 2011). The health of the ecosystem is vital for the persistence of biological and cultural communities; however, we know very little about the ecosystem processes that maintain fisheries, nor how species are spread across the landscape. In order to better understand the region, the objectives of this study are to: (1) assess diversity of fish communities in the Rupununi Portal, including the Rupununi and Takutu River drainages; (2) examine geographical patterns of species distributions and assemblage structure within the region; (3) investigate correlation of fish assemblage structure to physical variables; and (4) identify important areas within the Rupununi that should be protected in order to retain the greatest amount of biodiversity.
Materials and Methods
Study Area
The Rupununi Savanna and its associated wetlands are located between the Eastern and Western Guiana Shield in central Guyana (Figure 1). Lake Amuku is a seasonal lake formed as the Rupununi Savannas fill. Lake Amuku facilitates a hydrological connection between the Amazon and Essequibo Rivers, referred to as the Rupununi Portal (de Souza et al., 2012). The peak of the rainy season is from June to August, while the dry-season peak is from November through March. The Rupununi region is inhabited by ~7,000 Makushi and Wapishana people who depend on the natural resources provided by the wetlands, forests, and savannas.
The Rupununi wetlands represent a feature related to the dissolution of the proto-Berbice paleodrainage. Through most of the Cenozoic, the proto-Berbice flowed along the southern flank of the Pakaraima mountains emptying at the mouth of the modern Berbice River. It included parts of the modern Orinoco, Branco (including the Takutu), Essequibo (including the Rupununi), Berbice, and Courantyne river systems. During the Pliocene and Pleistocene, rivers such as the Cotinga, Uraricoera, Ireng, and Takutu were captured by the Branco River (a tributary of the Negro-Amazon rivers). Perhaps due to continuing head cutting of the Amazon River and/or the fact that the Rupununi savannas occupy a slowly filling depression that in the early Cretaceous formed the paleo-Lake Maracanta, the Rupununi portal was formed (see Lujan and Armbruster, 2011 for review). The nature of this historical process of the Rupununi and Takutu rivers once sharing a connection, then having been split from one another, and now being connected seasonally has resulted in a complex aquatic fauna that contains proto-Berbice endemic species, species cognates where sister species are in the two drainages, species that contain both Amazonian- and Rupununi-derived DNA signatures, and Amazonia-endemic species with the Takutu being more enriched with Amazonian fauna than the Rupununi (de Souza et al., 2012).
Sampling
Extensive sampling in the Rupununi Portal, which includes the Rupununi and Takutu Rivers, and the Rupununi wetlands, was accomplished over six expeditions during October and November (2002, 2003, 2005, 2007, 2011, 2012) during the dry seasons (Figure 1). Fishes were collected by seine, gill net, cast net, hook and line, and by hand by teams of five to eight. Collections were made in the dry season, during the day and night. Sampling was intended to fully document the biodiversity of the area, and sampling was continued at a site at least until we were no longer were collecting additional species. No attempt was made to quantify effort at each location as this was not the original intent of the work. Localities included sites from the main river channel, tributaries, ponds, lakes, and borrow pits. The wide variety of habitats necessitated differing collecting methodologies at each site. Site locations were recorded using a handheld GPS. Individuals were sorted and identified using current taxonomic keys for the different groups and by having experts examine some taxa. Specimens were euthanized with an overdose of MS-222, fixed in 10% formalin solution, and later preserved in 70% ethanol. Over 400 species and 51,000 specimens from 111 localities were deposited at the Auburn University Museum (AUM) fish collection. An additional 95 species and 1,345 specimens from 21 localities were deposited in the Field Museum of Natural History (FMNH). All collection records are available on the individual museum web pages (aumnh.org, fmnh.org) as well as with data aggregators such as FishNet2 (fishnet2.net), iDigBio (idigbio.org), and GBIF (gbif.org).
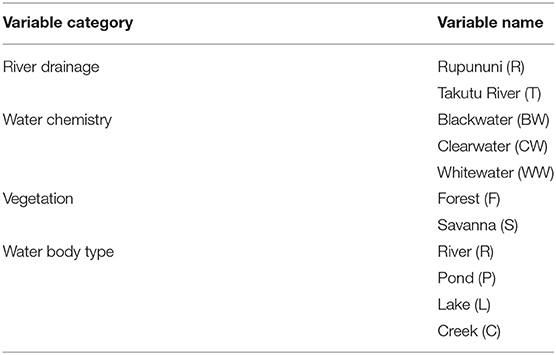
The composition of fish assemblages from collection sites was analyzed based on species presence or absence. Because collections were not made in a quantifiable way between expeditions, we could not perform abundance-based analyses. The inclusion of rare species and small samples can skew analyses, therefore, to avoid distortion, analyses were conducted after eliminating species that were found at <5 sites and sites that had fewer than five species (ter Braak and Simlauer, 1998). In this study the species assemblage matrix consisted of 104 survey sites and 233 species recorded as present (1) or absent (0). Physical variables for each site include river drainage, water chemistry, vegetation, and water body type (Table 1). River drainage at sites not in the main channel was determined by nearest river confluence with the main stem. Water chemistry categories follow descriptions in Sioli (1984) and include whitewater, blackwater, and clearwater. Vegetation describes the habitat surrounding the site as either forest or savanna via visual assessment. Four classifications of water body type were included: river (main river channels >25 m across), lake (>25 m across and >5 m deep), pond (<25 m across and <5 m deep), and creek (streams <25 m across) with sizes estimated visually.
Recently, communities and government agencies began developing a polygon for a proposed protected area in the North Rupununi which includes the sampled area of this study and is bounded by subnational boundaries. The polygon includes the Rupununi portal, Amerindian titled lands and state lands. We assess the suitability of the proposed conservation area and because all of our sampling sites fell within this polygon provide recommendations on fish diversity and connectivity.
Data Analysis
Species Richness and Diversity
Sample-based species accumulation curves were generated for both drainages using EstimateS ver. 8.2 (Colwell, 2013) including 95% confidence intervals in order to estimate the number of species in the sampled areas of the Rupununi region. These curves can be used to assess the adequacy of the collections with an asymptote suggesting sufficient sampling has been conducted to determine fish community structure. Species richness and Shannon's diversity were calculated for all localities to determine regions of high diversity using DIVERSITY in software program PRIMER-E version 6 (Clarke and Gorley, 2006).
Fish Assemblage Similarities Among Sites
Similarities among sites by means of fish assemblage structure were investigated with a cluster analysis (CLUSTER), using Jaccard's index of similarity and Euclidean distance to assess relationships among groups. Variation in fish assemblage structure among habitats was examined using non-metric multidimensional scaling (NMDS) ordination with two dimensions (k = 2) based on Jaccard's similarity matrices. Significant differences in assemblage structure were identified using one-way analysis of similarity (ANOSIM). The software program PRIMER-E version 6 (Clarke, 1993) was used to perform CLUSTER, NMDS, and ANOSIM.
Relationship Between Fish Assemblages and Habitat Characteristics
Canonical correspondence analysis (CCA) was used to investigate the relationships between fish assemblages and habitat characteristics. This multivariate method extracts environmental gradients in ecological datasets and uses ordination techniques to visualize these relationships (ter Braak and Verdonschot, 1995). To test whether the proportion of species variance explained by all the environmental constraints is significantly greater than expected by chance, 200 Monte Carlo permutations were performed on the canonical axes. Significance tests were performed on all variables simultaneously, by each canonical axis and by each variable. CCA analyses were performed in the VEGAN package in R (Oksanen et al., 2010).
Results
Species Richness and Diversity
At 104 sites throughout the Rupununi region, we documented 47 fish families, 120 genera, and 233 species including several undescribed taxa, that met the criteria of having been found in five localities or more. The species accumulation curve in the Rupununi region reached an asymptote indicating that each survey is a good representation of the fish assemblage within the watersheds of the Rupununi region (see also de Souza et al., 2012). Therefore, adding more sample sites would not likely uncover species not recorded in collections.
The Rupununi River at Karanambu (Figure 1), contained the highest diversity with 84 species (98 prior to removal of rare species) and Shannon's diversity (H') of 4.431. Of the 20 sites demonstrating the highest fish diversity (51–84 species), 15 were located in the Rupununi River drainage and five in the Takutu River drainage. All sites were associated with gallery-forested stretches of the main channel of the two drainages, and many were remote and difficult to access. The 20 sites with the lowest diversity (5–13 species) were associated with savanna and transitional forest with ephemeral ponds that shift in composition during the dry and rainy seasons.
Fish Assemblage Similarities Among Sites
Distinct species assemblages were identified in the Rupununi and Takutu Rivers using cluster analysis. The relationships coincided with proximity within drainage and connection at the Rupununi Portal. Two clusters consisted of upper Takutu River and upper Rupununi River localities suggesting the presence of a second seasonal connection near Dadanawa Ranch (Figure 2). Distinct clusters were identified between forest and savanna vegetation cover using cluster analysis, although many clusters were highly mixed. Relationships of clusters to vegetation were based on geographic proximity as well as through hydrological connections. We found no significant clustering among sites using the variables water chemistry or water body type.
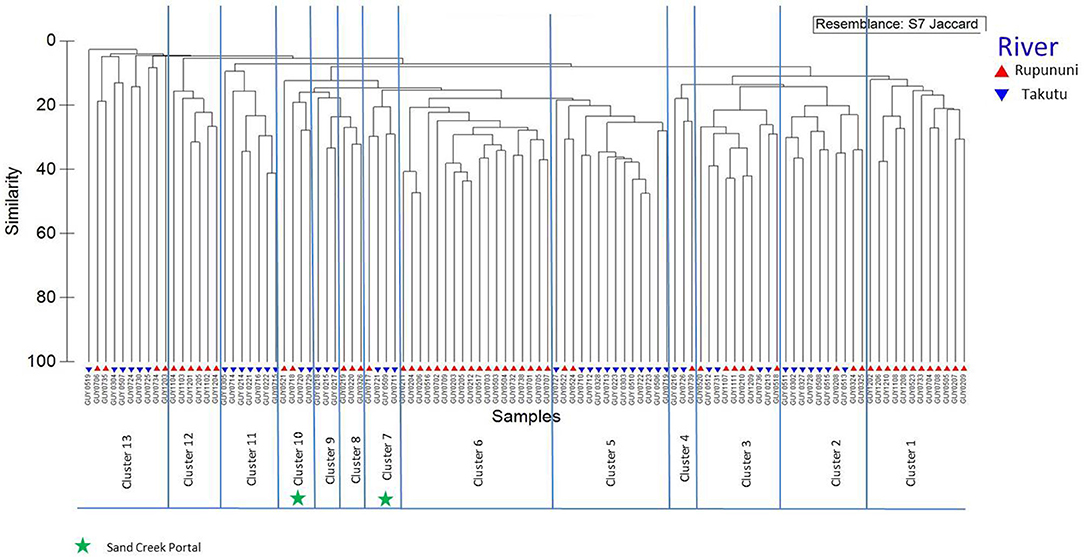
Figure 2. Dendrogram of Rupununi (58 sites) and Takutu River (46 sites) species assemblage relationships derived from cluster analysis (based on Jaccard's similarity matrix). Mixed clusters between the Takutu and Rupununi represent areas with similar species compositions and potential interconnectedness of the two rivers. These mixed clusters include known areas of mixing through the Rupununi Portal (i.e., Cluster 13). Clusters represented by green stars are evidence for connectedness through the proposed Sand Creek Portal in the South Rupununi.
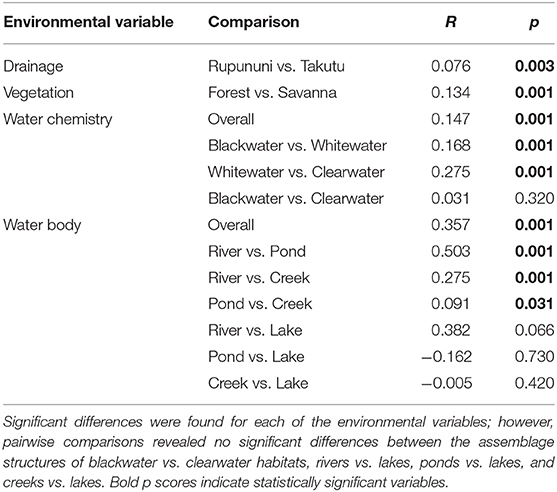
NMDS (k = 2) ordination analysis illustrates the variation of fish assemblage structure among the four variables: river drainage (Rupununi vs. Takutu; Figure 3A), vegetation (forest vs. savanna; Figure 3B), water body type (river, creek, lake or pond; Figure 3C), and water chemistry (blackwater, whitewater, or clearwater; Figure 3D). ANOSIM (Table 2) indicated that the Rupununi River was significantly different from the Takutu River and forested habitats were significantly different from those in savanna-dominated habitats. Fish assemblages were significantly different in blackwater and whitewater and whitewater and clearwater; however, fish assemblages were not significantly different between blackwater and clearwater. Pairwise comparisons of water body type between river and pond, river and creek and pond and creek were significantly different, while river and lake, pond and lake and creek and lake were not significantly different. Overall, water chemistry and water body were significant in their influence on fish assemblage structure.
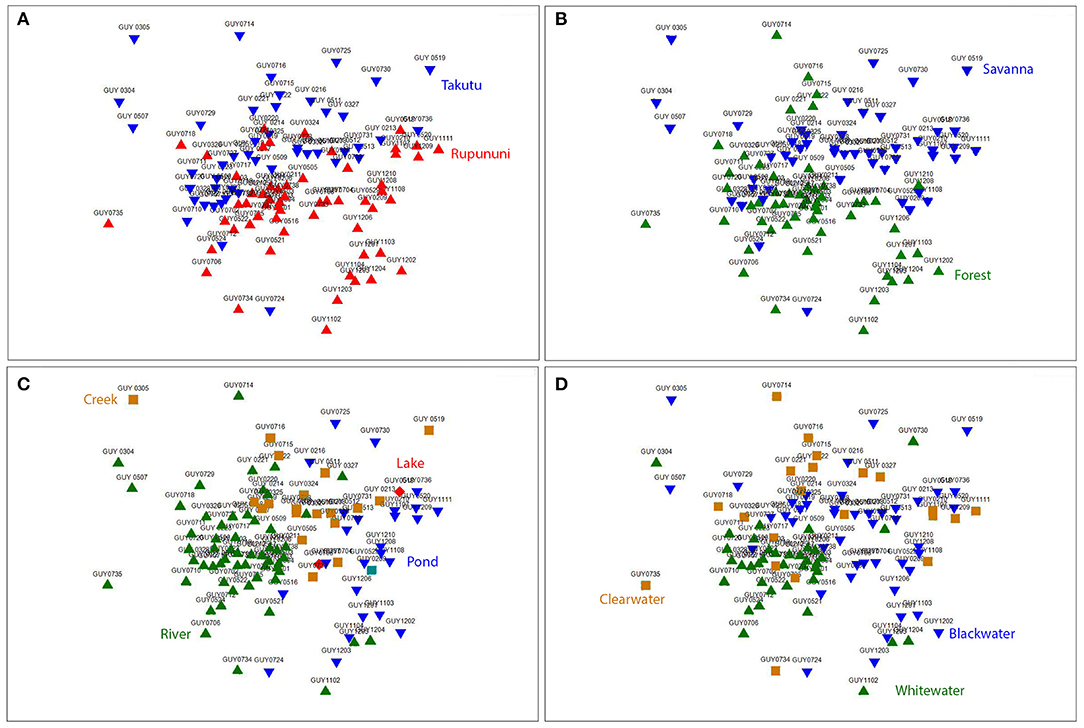
Figure 3. Nonmetric multidimensional scaling (NMDS) ordination (k = 2) based on Jaccard's similarity matrices for Takutu and Rupununi Rivers of fish communities, stress = 0.21. (A) river drainage (Rupununi vs. Takutu), (B) vegetation (forest vs. savanna), (C) water body type (river, creek, lake or pond), and (D) water chemistry (blackwater, whitewater, or clearwater).
Relationship Between Fish Assemblages and Habitat Characteristics
Using the variables of drainage, vegetation, water chemistry, and water body type in a canonical correspondence analysis (CCA) resulted in 6.55% of the variance explained by the environmental matrix. The triplot indicates that the variable “clearwater” had the strongest positive correlation to canonical axis 1 while the “Rupununi” is negatively correlated to canonical axis 1. Canonical axis 1 is also positively correlated to the variable “river” (water body type) and negatively correlated to variables “whitewater” and “forest” (Figure 4). Therefore, sites and species on the right side of the ordination are associated with “clearwater” and “river,” while species and sites on the left are associated with “forest,” “whitewater,” and within “Rupununi.” Direction and angle of environmental vectors indicate a close association between the three variables Rupununi drainage, whitewater and forest. Fish species with the large positive loadings on the first species assemblage axis, such as Melanocharacidium sp. and Parapristella aubynei tend to be associated with clearwater while fish species with large negative loadings, such as Loricariichhthys sp. and Hassar sp. are widely found in whitewater drainages surrounded by gallery forest (Table 3). To determine if the proportion of species variance is explained by the environmental constraints, Monte Carlo permutation tests were done for all variables simultaneously. Permutation tests by axes found CCA1 and CCA2 to be statistically significant and tests by variables resulted in three variables statistically significant: drainage, forest, and river (Table 4).
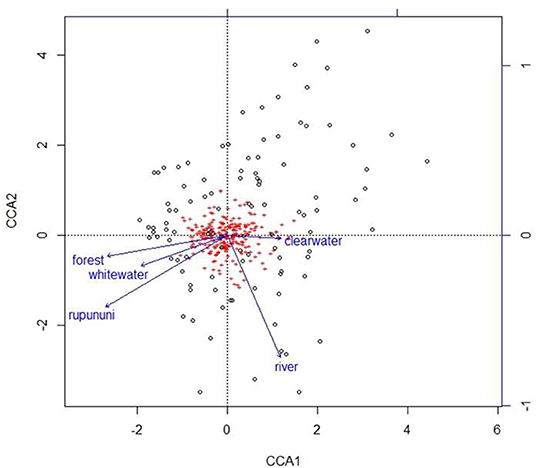
Figure 4. Plot from canonical correspondence analysis (CCA) scores on axis 1 and axis 2 based on the matrix of species assemblages and matrix of habitat variables. Red plus signs represent species and location on the plot indicates distributional similarity. Open circles represent sites and location on plot indicates compositional similarity.
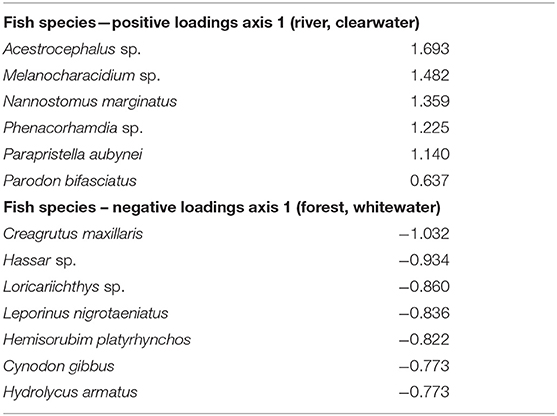
Table 3. Canonical correspondence analysis (CCA) for species with highest loadings on axis 1 listed illustrating the relationship with fish species and habitat variables.
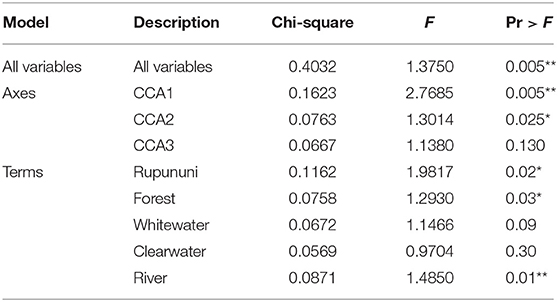
Table 4. Monte Carlo permutation tests on all variables, by axes, and by variable (significant codes: 0 “***,” 0.001 “**,” 0.01 “*”).
Discussion
The forest-savanna mosaic characteristic of the Rupununi is a transitory ecotone high in a diverse suite of habitats. As basement layer tilting was altering river drainages of the Guiana Shield, the climatic oscillations of the Quaternary caused contraction and expansion of the savannas during the interglacial periods (Rull, 2007). What ensued in the Rupununi are creeks lined with buriti palms (Mauritia flexuosa) flowing into main river channels, lakes, gallery forests, forest islands, and montane forests (Barbosa et al., 2007). This mosaic of habitats generates an ecosystem where these environments are interdependent allowing for dispersal and genetic exchange for several taxa. Unfortunately, the Rupununi is at risk from several threats (i.e., mining, logging, development), but agriculture in the Rupununi wetlands is of immediate concern. Throughout Amazonas, large development projects (e.g., soybean, irrigated rice, hydroelectric power plants) are altering the natural landscape and fragmenting the ecological interactions of these ecosystems. Therefore, it is with urgency that we attempt to explain the structure of fish communities in the Rupununi as a model for other taxa dependent on this ecosystem.
We found significant trends in fish assemblage structure and environmental conditions, particularly as relates to savanna vs. forested habitats. Areas with the highest fish diversity were found predominately in the Rupununi River drainage with some sites in the Takutu River drainage. Both areas of high diversity were concentrated in areas with a mix of forest and savanna, combining the unique faunas of those two areas. The 15 sites of high diversity in the Rupununi River drainage were along the main channel or tributaries that drain the Kanuku Mountains. The Kanuku Mountains have been demarcated as a protected area in the Rupununi for their rich biodiversity and ecological diversity (Montambault and Missa, 2002). The Rupununi River runs between the Western and Eastern Kanukus and this association could explain the concentration of sites with high fish diversity. Sites in the Takutu with high diversity were also along the forest-savanna ecotone providing an array of various habitats with possibilities for varied niches. This was evident in the main channel, where in addition to sandy stretches with woody debris and a healthy riparian edge, there are lateritic rocks that support a myriad of fishes.
The clustering of Rupununi and Takutu sites in clusters 7 and 10 (Figure 2) in the analysis suggest that these sites are very similar to one another. These sites are located to the south of the Kanuku Mountains in the South Rupununi savannas near Dadanawa Ranch and Sand Creek village (Figure 5). This is highly suggestive of another potential conduit between the Takutu and Rupununi rivers. Locals have suggested that this region is indeed a connection point between the two drainages and have personally observed fish moving across the savanna in the rainy season (Duane de Freitas, pers. comm.). The low-lying topography of this southern corridor is ~25 km across with wetlands and meandering creeks making this a plausible hydrological connection.
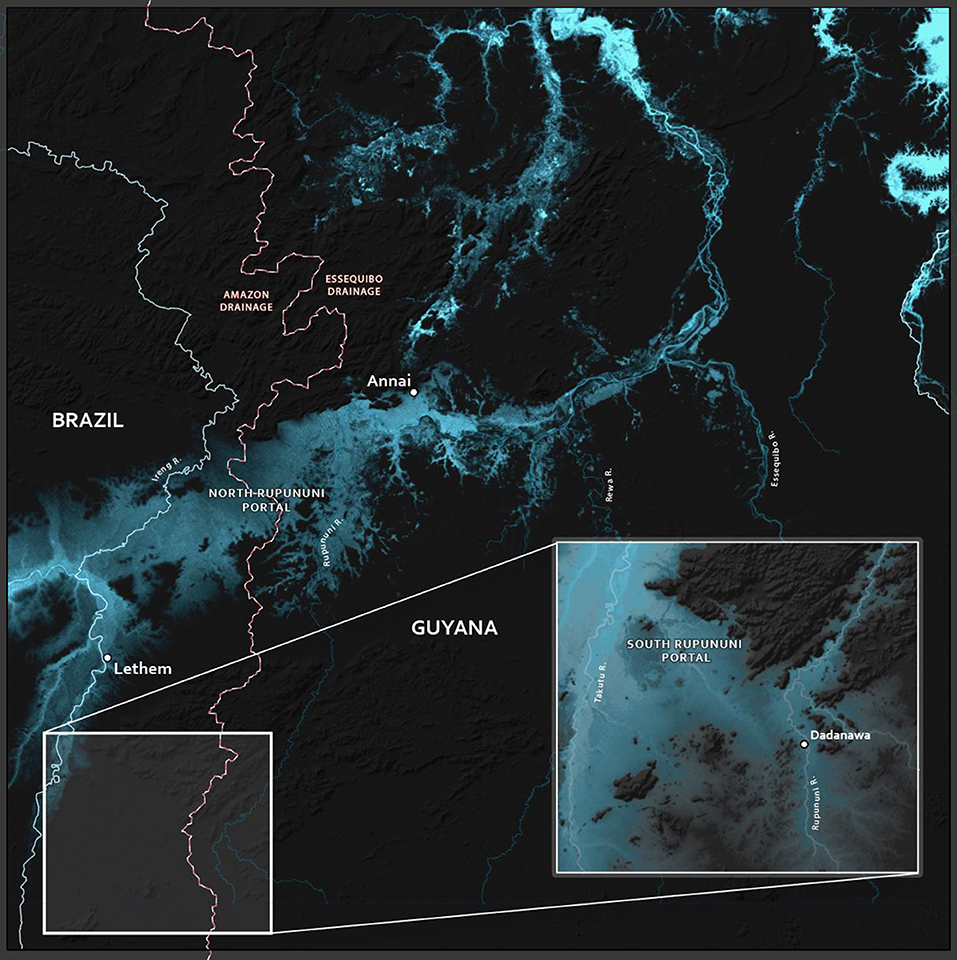
Figure 5. Map of the hydrological portals in the North and South Rupununi. The red/back dashed lines indicate the divide between the drainages of the Amazon and Essequibo Rivers.
This southern Rupununi portal, which we term the Sand Creek Portal (Figure 5), may be important for understanding the hydrological history of the proto-Berbice system, perhaps indicating that the upper Takutu once was a tributary of the upper Rupununi while the middle and lower Takutu and the lower Rupununi served as the main channel of the proto-Berbice. This potential split origin of the Takutu could be examined with genetics of highland fishes and other aquatic organisms from the Kamoa and Acarai mountains along the southern flank of Guyana, the Kanuku Mountains, and the Pakaraima Mountains that are north of the Rupununi savanna. We would predict that relatedness would be based on distance and not by drainage, and the divergence times between neighboring drainages would be as old or older than the split between the Takutu and Rupununi Rivers. More recent divergence times would indicate recent gene flow due to more recent hydrological connections and relationships related to drainage would indicate that this hydrological scenario is likely incorrect. A split origin of the Takutu would, in turn, have conservation implications. If the fauna of the Rupununi Portal and the Sand Creek Portal are distinct, then both sets would have to be preserved. Unfortunately, few samples for genetic analyses are available for most of the highlands flanking or within the Rupununi Savannas. The Kanuku Mountains as well as other, smaller highlands, represented a fragmented ecosystem between the Pakaraima and Kamoa/Acarai mountains. Although the Kanukus are protected, their aquatic fauna is not well-studied.
Ordination analysis revealed significant relationships in species assemblage structure among physical variables: drainage, vegetation, water body type, and water chemistry meaning that different habitat types have different fish assemblages and that conservation areas must take into account a variety of these habitats in order to fully protect species in the area. Concordant with the cluster analysis and canonical correspondence analysis, river drainage is a factor in structuring the fish fauna of the Rupununi. While a majority of the fishes are shared between the two drainages (de Souza et al., 2012), these results could be indicative of the isolation that occurred during the breakup of the ancient proto-Berbice. The current connection between the drainages via the Rupununi Portal is a stream capture event in progress of the Amazon River capturing the Essequibo River (Lujan and Armbruster, 2011). Despite the powerful pull from the largest river in the world, there are factors that are simultaneously limiting the dispersal of fish across the divide and structuring the fish fauna by drainage. Some species may have entered the Rupununi from the Amazon (such as the candirus, Vandellia, family Trichomycteridae) and some proto-Berbice endemics may have been preserved in the Rupununi that have been extirpated from the Takutu due to competition with Amazonian endemics (such as some headstanders, family Anostomidae; de Souza et al., 2012). Some of these factors could be related to variables our study revealed to be significantly influencing fish assemblages: water body type, vegetation, and water chemistry.
Water Body Type, Vegetation, and Water Chemistry
Ecosystem size and water flow were the most important delimiting factors for fish communities. We found significant differences between larger rivers and ponds, rivers and creeks, and pond and creeks; however, rivers and lakes, creeks and lakes, and ponds and lakes were not significantly different. The lack of distinction between ponds and lakes is understandable as they are different only in size, and there are not likely to be significant differences in habitat. Where we failed to find significance between flowing and still waters can be a result of the fact that permanent still water habitats are not likely to be the norm for the region. Although the Paleolake Marakanta filled the region until the end of the Cretaceous, the area has been dominated by flowing waters since then (Lujan and Armbruster, 2011). This means that the ponds and lakes are inhabited by secondarily lacustrine species, which are those species that are also found in pools and backwaters of rivers and creeks.
Water body type directly influences these characteristics and consequently the structure of fish communities. Factors in lakes/ponds show variation in a vertical orientation, while rivers/creeks are longitudinally arranged. For example, in lakes/ponds fish communities distribute vertically with some species (most catfishes) found at the bottom, some species (the arowana, Osteoglossum bicirrhosum) occupying the top of the water column, and many species in the middle and along the edges. In rivers, communities are more strongly segregated along the fluvial gradient between riffles and pools. For instance, some armored catfish (for example, Peckoltia cavatica and Ancistrus leucostictus) are found in swift flow associated with rocks while the large pimelodid catfish (like Pseudoplatystoma fasciatum) are found in pools within the river channel. Driven by competition, habitat segregation is the most prevalent type of resource partitioning shaping fish communities (Grossman and Sabo, 2010).
Lake and river morphology affect local temperature, flow regime, depth, and substrate irregularities. Lakes/ponds in the Rupununi savannas exhibit low dissolved oxygen and high temperatures, especially during the dry season when there are large fluctuations in size and depth of lakes/ponds (Lowe-Mcconnell, 1975). Fluctuations increase the physiological demands on fish in the savanna lakes/ponds, thus experiencing a physiological winter due to intense crowding and heavy predation (Lowe-Mcconnell, 1975). These conditions alter water chemistry in the savanna ponds/lakes, increasing acidity. The combination of increased acidity and low dissolved oxygen results in a greater degree of air-breathing fishes in tropical systems relative to temperate, reflecting selective pressure due to the hypoxic conditions (Lowe-Mcconnell, 1975; Kramer, 1983), and sites with these conditions were dominated by predatory species and species tolerant of these conditions, including Pygocentrus natteri, Acestrorhynchus falcirostris, Hoplias malabaricus, and Megalechis thoracata (Figure 6). These hypoxia-tolerant fishes are those that we would predict would move more easily across the Rupununi Portal, and such has been shown for needlefish (Potamorrhaphis; Lovejoy and De Araújo, 2000; de Souza et al., 2012), the trahira (Hoplias malabaricus; de Souza et al., 2012), the peacock bass (Cichla; Willis et al., 2010), the eartheater cichlid (Geophagus surinamensis; de Souza et al., 2012), the flannel-mouth characin (Prochilodus; Turner et al., 2004); two species of suckermouth armored catfish (Ancistrus nudiceps and Hypostomus emarginatus; de Souza et al., 2012, 2019). Meanwhile, the slow waters of the flooded savanna likely limit some fishes from crossing. Species cognates (sister species pairs found either in the Rupununi or the Takutu) have been identified in species that appear to be intolerant of still waters including some suckermouth armored catfishes (Peckoltia cavatica vs. P. braueri and Ancistrus leucostictus vs. A. saudades), some species of pencil catfishes (two probably undescribed species of Acanthopoma and two of Branchioica), some wood catfishes (Auchenipterus demerarae vs. A. ambyiacus), and some toothless characins (Curimata sp. vs. C. roseni) (de Souza et al., 2012, 2019).
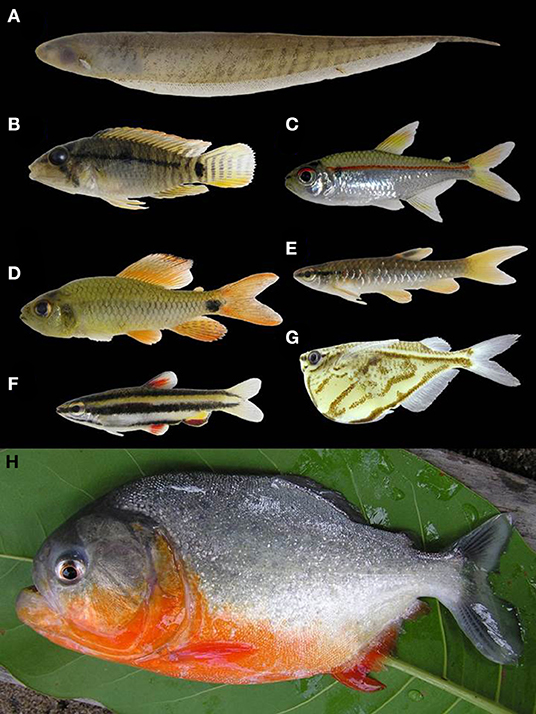
Figure 6. Examples of fishes found in the Rupununi, Guyana. (A) Brachyhypopomus pinnicaudatus, (B) Apistogramma steindachneri, (C) Hemigrammus erythrozonus, (D) Crenuchus spilurus, (E) Pyrrhulina stoli, (F) Nannostomus marginatus, (G) Carnegiella strigata, (H) Pygocentrus nattereri. Photos by: P. Willink.
Habitats surrounding and within these water bodies shape the corresponding fish communities. Flora of the savannas or forests directly influences soils, and water chemistry, while aquatic vegetation increases habitat complexity within rivers or lakes (Carter, 1934; Sarmiento, 1984). Aquatic vegetation provides important habitats for a majority of fish species in the lowlands, for reproduction, food resources and protection from predation (Willis et al., 2005). Several species move between heavily vegetated areas to open water habitats, but others like the knifefish Gymnotus and the cichlid Apistogramma are associated with vegetation for their entire life cycle (Kullander, 1986; Crampton et al., 2005). Other species were collected in open water but were often found feeding along the edge of aquatic vegetation, like macrophyte-feeder Schizodon cf. vittatus and algivore/detrivore Steindachnerina planiventris (Vari, 1991; Vari and Raredon, 1991). Armored catfishes Hypostomus macushi and Panaqolus claustellifer are closely associated with woody debris and most often found in the rivers associated with the forest (Armbruster and de Souza, 2005; Tan et al., 2016).
We found significant differences between blackwater and whitewater and clearwater and whitewater, but not between blackwater and clearwater. Future conservation efforts will need to be able to maintain a mix of black/clearwater habitats and whitewater habitats in order to conserve the greatest number of species. Water chemistry types are a result of the hydrology, soils, and vegetation of the area (Winemiller and Jepsen, 1998) indicating that preserving a wide variety of river types would also preserve a wide array of terrestrial surfaces as well.
Several factors contribute to the various types of water chemistries found in tropical systems, including previously discussed vegetation and water body type. Our study revealed the role that water chemistry plays in structuring the fish assemblages of the Rupununi and likely acts as an ecological barrier to dispersal between aquatic habitats for some species. Extremely acidic conditions of blackwater habitats likely pose physiological constraints on fish species. Low pH influences ionic balance as well as other physiological processes in fishes such as osmotic balance, oxygen affinity for hemoglobin, and digestion (Wilson et al., 1999; Matsuo and Val, 2002). Therefore, blackwater fishes are adapted to the specific environmental physiochemical characteristics of their environment (Val and Almeida-Val, 1995). The various water chemistry types in the Rupununi could be functioning similar to the Casiquiare connection at the Amazon and Orinoco Rivers. The Casiquiare at the Orinoco River connection is a whitewater river slowly transitioning to a blackwater river as it flows into the Rio Negro, a blackwater river. Examination of this connection revealed distribution of fishes was based on an environmental gradient found along the Casiquiare with shifts in water chemistry, habitat, and food resources (Winemiller et al., 2008). Our data suggest that water chemistry and habitat are also structuring the fish fauna in the Rupununi.
Understanding the role environmental conditions have in shaping fish assemblages of the Rupununi is valuable for insights into the diversification processes of Neotropical fishes, habitat assessment, and managing or conserving fish populations. The ecological interactions of microhabitats across the Rupununi are working in concert to maintain ecosystem services that aquatic and terrestrial species depend on. Therefore, our management recommendation to protect fish populations and maintain fish diversity in the Rupununi is to demarcate an area that connects the Rupununi and Takutu Rivers including the Rupununi wetlands as well as most of the most speciose sites.
There is substantial literature that has supported the importance of human-designed habitat corridors for wildlife (Rosenberg et al., 1997; Noss and Beier, 1998; Bennett, 2003). The unique nature of the Rupununi functioning as both a natural terrestrial and aquatic corridor (Figure 7) increases the importance of its preservation as an intact system. The relationship between environment and fish diversity is complex, especially because waterways are nested within a larger fluvial hierarchy, where environmental variables at multiple spatial scales ultimately shape local fish assemblages (Schlosser, 1987). Our recognition of the Sand Creek Portal further complicates the region. As the southern Rupununi portal was supported by a unique, shared fauna between the Takutu and Rupununi, the southern and northern portals cannot be considered duplicates, and the connectivity of both must be maintained.

Figure 7. North Rupununi savanna and wetlands during the rainy season with the Kanuku Mountains in the distance. Photo by: E. Paemelaere.
Not only are the corridors used for movement, they are also where many species dwell continuously and where others reproduce especially during the dry season (Lowe-Mcconnell, 1964). During this time the fish fauna of the wetlands is composed of fishes that can withstand hypoxic conditions, including Hoplias malabaricus, Platydoras hancockii, and Potamorrhaphis guianensis, as well as the world's largest scaled-freshwater fish and obligate air-breather Arapaima arapaima, which is endemic to the Rupununi and currently endangered. Several bird and plant species are endemic to the Rupununi savannas and persist due its fluctuating nature (Boggan et al., 1993; Braun et al., 2007). Fragmentation (via agriculture, road building, or logging) of an interdependent ecosystem like the Rupununi can adversely affect genetics, physiology, behavior, and abundance of many species, including such economically important fish species like Prochilodus rubrotaeniatus that move into such habitats as juveniles (Silva, 2000; Hermann et al., 2016).
A Protected Area in the North Rupununi—North Rupununi Conservation Area
The Rupununi Portal is a unique biogeographic region as one of the only seasonal connections between highly diverse rivers. We have shown that complex habitats that mix forest and savanna are the most biodiverse areas and that the Rupununi Savanna serves as a corridor for some species and a barrier to others. This complexity of ecosystems leads to high diversity that is deserving of protection. Meanwhile, the same geographical feature that has led to the diversity of species is also an effective corridor for humans. Brazil desires a Caribbean port, and has worked to improve the road to and through the Rupununi.
In order to protect ecologically important regions in Guyana in a more scientific manner than is typical, Bicknell et al. (2017) used a combination of species occupancy modeling for terrestrial vertebrates and the program Marxan (Ball et al., 2009) in order to identify priority areas for conservation. They found that the grasslands of the Rupununi Savanah was an area of conservation concern. Combining our data on fish distributions, the priority areas identified by Bicknell et al. (2017), and what we know of the geography, geology, and connectiveness of the river systems of the Rupununi, we have outlined an area within the North Rupununi region as a suggested area for protection (Figure 8). This includes areas with the highest freshwater fish diversity and incorporates an ecological gradient between habitats of the savanna, wetlands, and forests interface. This area should be contiguous with the Kanuku Mountains Protected Area as well as areas protected in Brazil as indigenous peoples' territory. With the increase in human activity in this region, and the increased connectivity of the region with the rest of the world through better roads, this area is in danger of being modified and fragmented to a point where ecosystem services begin to fail. This could lead to profound changes in the biota and the loss of critical food fishes that use the flooded savanna for spawning. The proposed protected area would preserve the greatest percentage of fish species and maximize the ability for the ecosystem services for all taxa to continue undisturbed in spite of future development in the region. Based on (IUCN) protected area categories, we recommend that the Rupununi savanna and wetlands be placed under Category VI (protected area with sustainable use of natural resources) because of the importance of the area to locals and the Lake Amuku region (connection between the Amazon & Essequibo Rivers) under Category IV (habitat/species management area) because this area is critical to the movement of fishes between the two basins and as a support area for juvenile game fishes. Currently, we are completing a study similar to that of Bicknell et al. (2017) with the addition of fish data to the vertebrate data to further refine the borders of this proposed protected area.
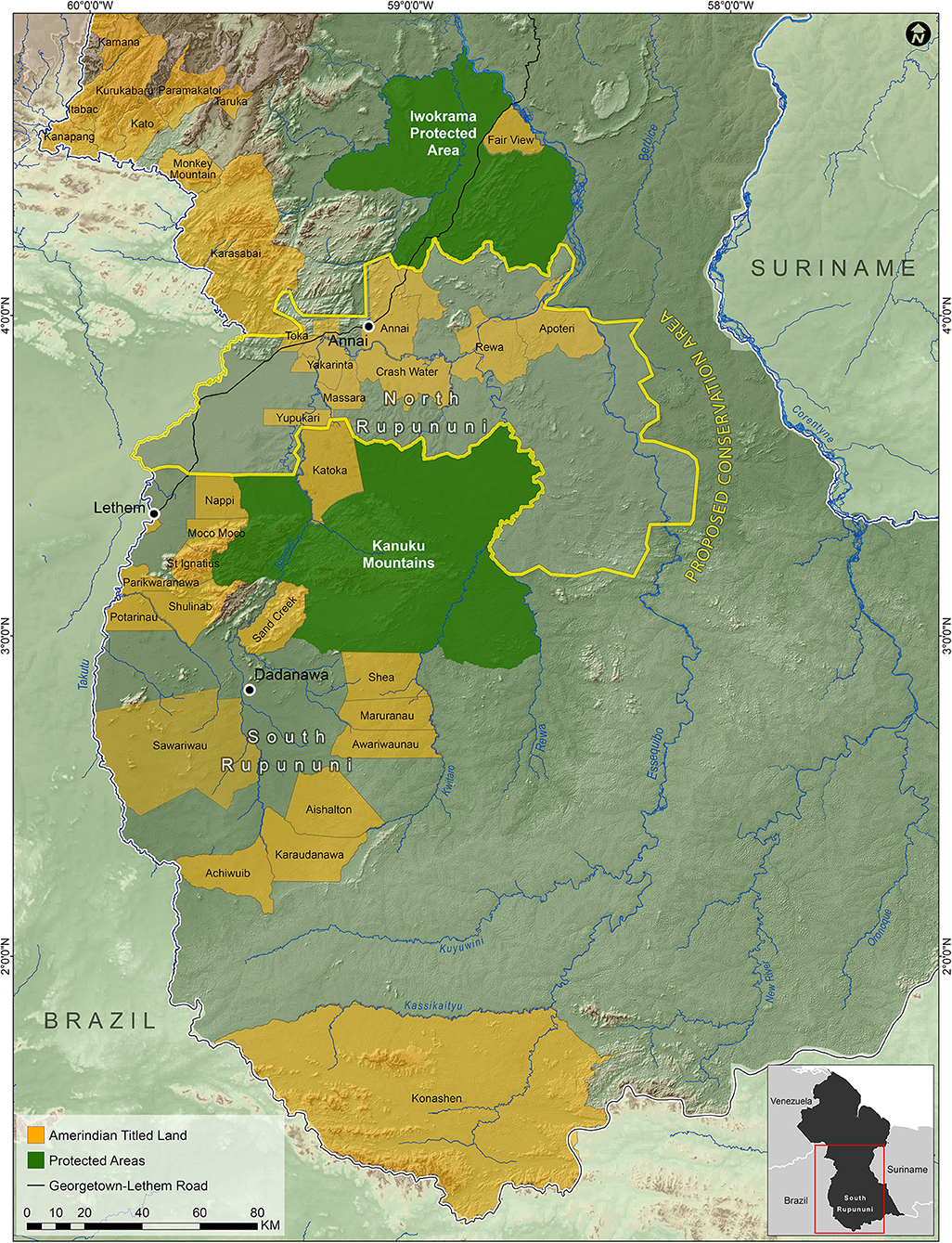
Figure 8. Map of the suggested North Rupununi Conservation Area outlined in yellow which includes the Rupununi Portal and the ecological gradient of forests, savannas and wetlands.
The discovery of the Sand Creek Portal (Figure 5) indicates another potentially critical area for the maintenance and preservation of biodiversity. We found the region to contain fishes not found in the North Rupununi. Similar to the North Rupununi, this region is inhabited by indigenous people dependent on the natural resources for their livelihood with impending threats primarily from gold mining. Few collections of aquatic organisms have been made south of the Kanuku mountains. A more focused study in the South Rupununi, including the Sand Creek Portal, would give us more insight into areas in need of protection in the South Rupununi. With the threat of gold mining to this region, it is imperative that this region be further studied as the impact of gold mining to streams is extreme.
Conclusion
This study demonstrates the importance of the Rupununi Savanna for the maintenance of aquatic biodiversity in the region, and provides support to the development of a protected area in the North Rupununi so that this tremendous biodiversity can be maintained. We are currently working to refine those borders by combining our data with terrestrial vertebrate data presented in Bicknell et al. (2017). The newly discovered Sand Creek Portal represents a second important hydrological connection between the Takutu and Rupununi rivers, and suggests a further refinement of the biogeographic scenario of the Proto-Berbice. Further collections within the Kanuku Mountains and the areas to the south are necessary to better understand the region and the functioning of the southern portal. This would provide insights to historical river drainage patterns, fish diversity and inform conservation management that include aquatic habitats.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Auburn University IACUC Committee.
Author Contributions
LS wrote the manuscript with input from co-authors. LS, JA, and PW did fieldwork to collect data. LS led ecological analysis with input from JA and PW. JA created map of fish diversity. PW contributed photos of fish species. LS created remaining figures and maps. The work in Guyana was supported and facilitated by LS, JA, and PW.
Funding
We would like to thank the National Science Foundation award DEB-0107751 and DEB-0315963 to JA, Auburn University graduate fellowship to LS, and Grainger Bioinformatics Center at the Field Museum of Natural History to LS for funding that supported this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank D. Arjoon, C. Bernard, C. Chin, E. Liverpool, S. Lord, and the staff of the University of Guyana, Center for Biodiversity for assisting in fieldwork in Guyana. Special thanks to M. Tammasar and the people at the Guyana EPA, in addition to the Ministry of Amerindian Affairs for obtaining permits. We deeply thank the Macushi and Wapishana people, the North Rupununi District Development Board, Karanambu Trust, and Iwokrama for their enthusiastic help and support during our collections in Guyana. Collecting was further aided by M. Sabaj, M. Hardman, N. Lujan, J. Hartsell, J. Hwan, T. Geerinckx, J. Baskin, D. Taphorn, C. Allison, M. Thomas, and D. Werneke. Thank you to John G. Shedd Aquarium for support during post-doctoral work in Guyana. We thank Grainger Bioinformatics Center for support to finalize this study. Thank you to colleagues in the Keller Science Action Center who gave feedback on versions of this manuscript, especially to N. Kotlinski for support on portal figure and map. Lead author thanks N. Pitman for fruitful discussions that improved this manuscript as well as your sustained support.
References
Abell, R., Thieme, M., Ricketts, T.H., Olwero, N., Ng, R., Petry, P., et al. (2011). Concordance of freshwater and terrestrial biodiversity. Conserv. Lett. 4, 127–136. doi: 10.1111/j.1755-263X.2010.00153.x
Albert, J. S., Petry, P., and Reis, R. E. (2011). “Major biogeographic and phylogenetic patterns,” in Historical Biogeography of Neotropical Freshwater Fishes, eds J. S. Albert and R. E. Reis (Berkeley, CA: University of California Press), 21–56.
Albert, J. S., and Reis, R. E. (2011). Historical Biogeography of Neotropical Freshwater Fishes. Berkeley, CA: University of California Press.
Armbruster, J. W., and de Souza, L. S. (2005). Hypostomus macushi, a new species of the Hypostomus cochliodon group from Guyana. Zootaxa 920, 1–12. doi: 10.11646/zootaxa.920.1.1
Ball, I. R., Possingham, H. P., and Watts, M. (2009). “Marxan and relatives: software for spatial conservation prioritization,” in Spatial Conservation Prioritisation: Quantitative Methods and Computational Tools, eds A. Moilanen, K. A. Wilson and H. Possingham (Oxford: Oxford University Press), 185–195.
Barbosa, R. I., Campos, C., and Pinto, F. Fearnside, P. M. (2007). The “Lavrados” of Roraima: biodiversity and conservation of Brazil's Amazonian savannas. Funct. Ecosyst. Commun. 1, 29–41.
Bennett, A. F. (2003). Linkages in the Landscapes: The Role of Corridors and Connectivity in Wildlife Conservation. Gland: World Conservation Union.
Bicknell, J. E., Collins, M. B., Pickles, R. S., McCann, N. P., Bernard, C. R., Fernandes, D. J., et al. (2017). Designing protected area networks that translate international conservation commitments into national action. Biol. Conserv. 214, 168–175. doi: 10.1016/j.biocon.2017.08.024
Boggan, J., Funk, V., Kelloff, C., Hoff, M., Cremers, G., and Feuillet, C. (1993). A Checklist of the Plants of the Guianas (Guyana, Surinam, French Guiana). Washington, DC: Smithsonian Institution.
Bovolo, C.I., Wagner, T., Parkin, G., Hein-Griggs, D., Pereira, R., and Jones, R. (2018). The Guiana Shield rainforests–overlooked guardians of South American climate. Environ. Res. Lett. 13:074029. doi: 10.1088/1748-9326/aacf60
Braun, M. J., Finch, D. W., Robbins, M. B., and Schmidt, B. K. (2007). A Field Checklist of the Birds of Guyana. 2nd ed. Washington, DC: Smithsonian Institution.
Carter, G. S. (1934). Results of the Cambridge expedition to British Guiana, 1933. The fresh waters of the rainforest areas of British Guiana. Zoo. J. Linn. Soc. 39, 147–193. doi: 10.1111/j.1096-3642.1934.tb00068.x
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Cole, C. J., Townsend, C. R., Reynolds, R. P., MacCulloch, R. D., and Lathrop, A. (2013). Amphibians and reptiles of Guyana, South America: illustrated keys, annotated species accounts, and a biogeographic synopsis. Proc. Biol. Soc. Wash. 125, 317–578. doi: 10.2988/0006-324X-125.4.317
Colwell, R. K. (2013). EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. Available online at: http://purl.oclc.org/estimates
Crampton, W.G., Thorsen, D.H., and Albert, J. S. (2005). Three new species from a diverse, sympatric assemblage of the electric fish Gymnotus (Gymnotiformes: Gymnotidae) in the lowland Amazon Basin, with notes on ecology. Copeia 2005, 82–99. doi: 10.1643/CI-03-242R2
Dalfelt, A. (1978). Nature Conservancy Survey of the Republic of Guyana. Report presented to The Nature Conservancy.
de Souza, L. S., Armbruster, J. W., and Werneke, D. C. (2012). The influence of the Rupununi portal on distribution of freshwater fish in the Rupununi district, Guyana. Cybium 36, 31–43. doi: 10.26028/cybium/2012-361-004
de Souza, L. S., Taphorn, D. C., and Armbruster, J. W. (2019). Review of Ancistrus (Siluriformes: Loricariidae) from the northwestern Guiana Shield, Orinoco Andes, and adjacent basins with description of six new species. Zootaxa 4552, 1–67. doi: 10.11646/zootaxa.4552.1.1
Duellman, W. E. (1999). “Distribution patterns of amphibians in South America,” in Patterns of Distribution of Amphibians: A Global Perspective, ed W. E. Duellman (Baltimore, MD: Hopkins University Press), 255–328.
Eigenmann, C. H. (1912). The Freshwater Fishes of British Guiana, Including a Study of the Ecological Grouping of Species and the Relation of the Fauna of the Plateau to that of the Lowlands. Pittsburgh, PA: Carnegie Institute.
GAHEF. (1991). National Forestry Action Plan, Project No.22: Development of a Protected Area System. Report by the Guyana Agency for Health, Environmental Education and Food Policy.
GAHEF. (1992). Guyana/UNEP Country Study of Biological Diversity. Report presented to the UNEP, Report by the Guyana Agency for Health, Environmental Education and Food Policy.
Grossman, G.D., and Sabo, J. L. (2010). “Structure and dynamics of stream fish assemblages,” in Community Ecology of Stream Fishes: Concepts, Approaches and Techniques, eds D.A. Jackson and K. B. Gido, (Bethesda, MD: American Fisheries Society), 401–406.
Hermann, T. W., Stewart, D. J., and Limburg, K. E. and Castello, L. (2016). Unravelling the life history of Amazonian fishes through otolith microchemistry. R. Soc. Open Sci. 3:160206. doi: 10.1098/rsos.160206
Hoogmoed, M.S. (1979). “The herpetofauna of the Guianan region,” in The South American Herpetofauna: Its Origin, Evolution, and Dispersal, ed. W. E. Duellman (Lawrence, KS: University of Kansas Museum of Natural History Monograph), 241–279.
Hoorn, C., Guerrero, J., Sarmiento, G. A., and Lorente, M. A. (1995). Andean tectonics as a cause for changing drainage patterns in Miocene northern South America. Geology 23, 237–240. doi: 10.1130/0091-7613(1995)023<0237:ATAACF>2.3.CO;2
Kramer, D.L. (1983). Aquatic surface respiration in the fishes of Panama: distribution in relation to risk of hypoxia. Env. Biol. Fish. 8, 49–54. doi: 10.1007/BF00004945
Kullander, S.O. (1986). Cichlid Fishes of the Amazon River Drainage of Peru. Stockholm: Department of Vertebrate Zoology, Research Division, Swedish Museum of Natural History.
Lévêque, C., Oberforff, T., Paugy, D., Stiassny, M. L. J., and Tedesco, P.A. (2007). Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595, 545–567. doi: 10.1007/s10750-007-9034-0
Lopez-Fernandez, H., Taphorn, D. C, and Liverpool, E. (2012). Phylogenetic diagnosis and expanded description of the genus Mazarunia Kullander 1990 (Teleostei: Cichlidae) from the upper Mazaruni River, Guyana, with description of two new species. Neotrop. Ichthyol. 10, 465–486. doi: 10.1590/S1679-62252012000300001
Lovejoy, N. R., and De Araújo, L. G. (2000) Molecular systematics, biogeography and population structure of Neotropical freshwater needlefishes of the genus Potamorrhaphis. Mol. Ecol. 9, 259–268.
Lowe-Mcconnell, R.H. (1964). The fishes of the Rupununi savanna district of British Guiana, South America. J. Linn. Soc. 45, 103–144. doi: 10.1111/j.1096-3642.1964.tb00490.x
Lowe-Mcconnell, R. H. (1975). Fish Communities in Tropical Freshwaters, Their Distribution, Ecology and Evolution. London: Longman.
Lujan, N.K., and Armbruster, J. W. (2011). “The Guiana shield,” in: Historical Biogeography of Neotropical Freshwater Fishes, eds J. S. Albert and R. E. Reis (Berkeley, CA: University of California Press), 211–224.
Lundberg, J. G. (2005). Brachyplatystoma promagdalena, new species, a fossil goliath catfish (Siluriformes: Pimelodidae) from the Miocene of Colombia, South America. Neotrop. Ichthyol. 3, 597–605. doi: 10.1590/S1679-62252005000400017
Lundberg, J. G., Marshall, L. C., Guerrero, J., Horton, B., Malabarba, M. C. S. L., and Wesselingh, F. (1998). “The stage for Neotropical fish diversification: a history of tropical South American rivers,” in Phylogeny and Classification of Neotropical Fishes, eds L. R. Malabarba, R. E. Reis, R. P. Vari, Z. M. Lucena, and C. A. S. Lucena, (Porto Alegre: Edipucrs), 14–48.
Maldonado Ocampo, J., López-Fernandez, H., Taphorn, D. C., Bernard, C., Crampton, W., and Lovejoy, N. (2013). Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool. Scr. 43, 24–33. doi: 10.1111/zsc.12035
Matsuo, A. Y., and Val, A. L. (2002). Low pH and calcium effects on net Na+ and K+ fluxes in two catfish species from the Amazon River (Corydoras: Callichthyidae). Braz. J. Med. Biol. Res. 35, 361–367. doi: 10.1590/S0100-879X2002000300012
Mistry, J., Berardi, A., and Simpson, M. (2008). Birds as indicators of wetland status and change in the North Rupununi, Guyana. Biodivers. Conserv. 17, 2383–2409. doi: 10.1007/s10531-008-9388-2
Montambault, J. R., and Missa, O. (2002). A biodiversity assessment of the eastern Kanuku mountains, lower Kwitaro River, Guyana. Rapid Assess. Prog. Bulletin Biol. Assess. 26, 1–88.
Noss, R. F., and Beier, P. (1998). Do habitat corridors provide connectivity? Conserv. Biol. 12, 1241–1252. doi: 10.1111/j.1523-1739.1998.98036.x
Oksanen, J., Blanchet, F., Kindt, R., Legendre, P., and O'Hara, R. B. (2010). Vegan: community ecology package. Available online at: http://vegan.r-forge.r-project.org (accessed January, 2019).
Paz, H. J., Hayes, M. M., Stout, C. C., Werneke, D. C., and Armbruster, J. W. (2019). A hotspot atop: rivers of the Guyana highlands hold diversity of endemic catfish. bioRxiv. doi: 10.1101/640821
Persaud, D. (1997). “Policies, legislation and organizational structure for protected areas in Guyana,” in Guyana National Report, ed M. Hoosein (Georgetown: EPA), 2–5.
Potapov, P., Hansen, M. C., Laestadius, L., Turubanova, S., Yaroshenko, A., Thies, C., et al. (2017). The last frontiers of wilderness: tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3:e1600821. doi: 10.1126/sciadv.1600821
Räsänen, M.E., and Linna, A. M. (1996). Miocene deposits in the Amazonian foreland basin—reply. Science 273, 124–125. doi: 10.1126/science.273.5271.124
Reis, R. E. (2013). Conserving the freshwater fishes of South America. Int. Zoo Yearb. 47, 65–70. doi: 10.1111/izy.12000
Rosenberg, D. K., Noon, B. R., and Meslow, E. C. (1997). Biological corridors: form, function, and efficacy. Bioscience 47, 677–687. doi: 10.2307/1313208
Rull, V. (2007). Holocene global warming and the origin of the Neotropical Gran Sabana in the Venezuelan Guayana. J. Biogeogr. 34, 279–288. doi: 10.1111/j.1365-2699.2006.01620.x
Sala, O. E., Chapin, F. S. III., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., et al. (2000). Global biodiversity scenarios for 2100. Science 287:1700 doi: 10.1126/science.287.5459.1770
Schlosser, I. J. (1987). “A conceptual framework for fish communities in small warmwater streams,” in Community and Evolutionary Ecology of North American Stream Fishes, eds W. J. Matthews and D. C. Heins (Norman, OK: University of Oklahoma Press), 17–24.
Silva, E. A. (2000). Life History and Migration Patterns of the Commercial Fish Prochilodus nigricans (bocachico) in Northeastern Ecuador (MS Thesis), State University of New York, College of Environmental Science and Forestry, Syracuse, NY, United States.
Sioli, H. (1984). “The Amazon and its main affluents: hydrography, morphology of the river courses and river types,” in The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and its Basin, eds H. Sioli and W. Junk (Dordrecht: Springer), 127–165.
Strayer, D., and Dudgeon, D. (2010). Freshwater biodiversity conservation: recent progress and future challenges. J. N. Am. Benthol. Soc. 29, 344–358. doi: 10.1899/08-171.1
Tan, M., de Souza, L.S., and Armbruster, J.W. (2016). A new species of Panaqolus (Siluriformes: Loricariidae) from the rio Branco. Neotrop. Ichthyol. 14:e150033. doi: 10.1590/1982-0224-20150033
ter Braak, C. J. F., and Simlauer, P. (1998). CANOCO Reference Manual. Wageningen: Centre for Biometry.
ter Braak, C. J. F., and Verdonschot, P. F. M. (1995). Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 57, 255–289. doi: 10.1007/BF00877430
Turner, T. F., Mcphee, M. V., Campbell, P., and Winemiller, K. O. (2004). Phylogeography and interspecific genetic variation of prochilodontid fishes endemic to rivers of northern South America. J. Fish Biol. 64, 186–201. doi: 10.1111/j.1095-8649.2004.00299.x
Val, A. L., and Almeida-Val, V. (1995). Fishes of the Amazon and Their Environment. Berlin: Springer-Verlag. doi: 10.1007/978-3-642-79229-8
Vannote, R. L., Minshall, G. W., Cummins, K. W., Sedell, J. R., and Cushing, C. E. (1980). The river continuum concept. Can. J. Fish. Aquat. Sci. 37, 130–137. doi: 10.1139/f80-017
Vari, R. P. (1991). Systematics of the Neotropical characiform genus Steindachnerina Fowler (Pisces, Ostariophysi). Smithson. Contr. Zool. 507, 1–118. doi: 10.5479/si.00810282.507
Vari, R. P., and Raredon, S. J. (1991). The genus Schizodon (Teleostei: Ostariophysi: Anostomidae) in Venezuela, a reappraisal. Proc. Biol. Soc. Wash. 104, 12–22.
Wang, Z. Y., Lee, J. H. W., and Melching, C. S. (2015). River Dynamics and Integrated River Management. Berlin: Springer.
Watkins, G., Oxford, P., and Bish, R. (2011). Rupununi: Rediscovering a Lost World. Petaluma, CA: Earth in Focus Editions.
Willis, S. C., Nunes, M., Montaña, C. G., Farias, I. P., Ortí, G., and Lovejoy, N. R. (2010). The Casiquiare river acts as a corridor between the Amazonas and Orinoco river basins: biogeographic analysis of the genus Cichla. Mol. Ecol. 19, 1014–1030.
Willis, S. C., Winemiller, K. O., and Lopez-Fernandez, H. (2005). Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142, 284–295. doi: 10.1007/s00442-004-1723-z
Wilson, R. W., Wood, C. M., Gonzalez, R. J., Patrick, M. L., Bergman, H. L., Narahara, A., et al. (1999). Ion and acid-base balance in three species of Amazonian fish during gradual acidification of extremely soft water. Physiol. Biochem. Zool. 72, 277–285. doi: 10.1086/316672
Winemiller, K.O., and Jepsen, D.B. (1998). Effects of seasonality and fish movement on tropical river food webs. J. Fish Biol. 53(Suppl. A), 267–296. doi: 10.1111/j.1095-8649.1998.tb01032.x
Winemiller, K.O., López-Fernández, H., Taphorn, D. C., Nico, L. G., and Duque, A. B. (2008). Fish assemblages of the Casiquiare River, a corridor and zoogeographical filter for dispersal between the Orinoco and Amazon basins. J. Biogeogr. 35, 1551–1563. doi: 10.1111/j.1365-2699.2008.01917.x
World Bank (1997). Guyana: National Protected Areas Systems (Drafts Proposal). Washington, DC: World Bank.
Keywords: intact forests, fish diversity, multivariate analysis, protected area, Rupununi portals, Amerindians
Citation: de Souza LS, Armbruster JW and Willink PW (2020) Connectivity of Neotropical River Basins in the Central Guiana Shield Based on Fish Distributions. Front. For. Glob. Change 3:8. doi: 10.3389/ffgc.2020.00008
Received: 17 August 2019; Accepted: 17 January 2020;
Published: 17 February 2020.
Edited by:
Matthew Struebig, University of Kent, United KingdomReviewed by:
Clare Wilkinson, National University of Singapore, SingaporeStefanie Marcella Rog, Wetlands International, Wageningen, Netherlands
Copyright © 2020 de Souza, Armbruster and Willink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lesley S. de Souza, bGRlc291emFAZmllbGRtdXNldW0ub3Jn
 Lesley S. de Souza
Lesley S. de Souza Jonathan W. Armbruster2
Jonathan W. Armbruster2