- 1Graduate School of Bioresource and Bioenvironmental Sciences, Kyushu University, Fukuoka, Japan
- 2Kyushu University Forest, Kyushu University, Fukuoka, Japan
- 3Forest Tree Breeding Center, Forestry and Forest Products Research Institute, Ibaraki, Japan
Compared to trees, little is known about the respiratory characteristics of bamboo, especially culm respiration. In this study, we measured the respiration rates of current year, 2, 3, and above 4-year-old Moso bamboo (Phyllostachys pubescens) culms and examined its relation to culm morphology and anatomical structure. Current year culms had substantially higher respiration rates (1.9 ± 0.46 μmol m–2 s–1) compared to older culms (2, 3, and above 4-year-old average: 0.17 ± 0.09 μmol m–2 s–1). Culm wood density increased with age, with the concurrent thickening of parenchyma cell walls in the culm tissue. Nitrogen content in the culm tissue decreased with culm age. Both culm wood density and nitrogen content had significant relationships with culm respiration rate. On the other hand, culm height, wall thickness, and circumference did not affect culm respiration rate. Although bamboo culms did not change in size through the year, anatomical changes in the culm tissue that accompanied the aging of a culm affected the respiration. The culm age would have a significant effect on the evaluation of the respiratory characteristics of the bamboo forest. Our results suggested that young culms required a large amount of respiration to grow “inward” as cell wall thickening and also to maintain the relatively large amount of active tissue.
Introduction
Respiration is a fundamental and vital element of plant survivorship that partly determines the productivity and carbon balance of ecosystems. Understanding the respiratory characteristics of each component of the plant, such as the leaves, stem, and roots, are essential in order to accurately estimate the respiratory fluxes of a whole plant, and furthermore, an entire ecosystem. Studies on tree respiration have shown that tree stem respiration is an important component of the annual carbon balance of forest ecosystems (Ryan et al., 1994; Damesin et al., 2002; Zha et al., 2004). Variation in tree stem respiration has been shown to have strong relationships with morphological features such as diameter at breast height (DBH) (Cavaleri et al., 2006; Katayama et al., 2016), stem diameter increment (Ryan, 1990; Maier, 2001), and sapwood volume (Ryan, 1990; Lavigne et al., 1996) and anatomical traits such as number of living cells (Gruber et al., 2009) and xylem production (Lavigne et al., 2004).
Compared to trees, very little is known about the respiratory physiology of bamboo culm. Asian bamboo species constitute a non-timber forest product of major cultural and economic importance (Bystriakova et al., 2003). Moso bamboo is the most important and main bamboo species in China, and it is the main species for bamboo timber and bamboo shoot production. Moso bamboo also plays a very important role for the ecological environment (Fu, 2001). Moso bamboo stand usually consists of different age groups of culms, and culm age has been found to significantly influence plant functional traits especially leaf functional traits (Guo et al., 2021; Huang et al., 2021). However, only one study has referred to culm respiration (Isagi et al., 1997), although some studies have been reported on soil respiration in bamboo forest (Liu et al., 2018; Li et al., 2019; Zhang et al., 2021). In addition, the detailed age-related variation in culm respiration is still unknown. When considering the respiration of bamboo, it should be noted that there are large differences between trees and bamboo. Bamboos are a perennial woody grass, and morphological features such as height and culm diameter do not change after the culm has reached its full height in the first year (Shanmughavel and Francis, 1996). In the bamboo forest, the aboveground net primary production decreased upslope and the belowground net primary production allocation increased with decreasing upslope soil water availability. In contrast, leaf N content of the bamboo forest increased upslope, and N use efficiency decreased upslope with an increasing upslope soil N mineralization rate (Shimono et al., 2021). Considering the large differences, bamboo culm respiration may not be controlled by the same factors as tree stems, and therefore, features other than morphology need to be considered to explain any variation in respiration.
Although little information is available on the physiology of bamboo culms, some studies on culm quality and anatomical features exist due to versatile uses of culms as material. Studies on culms of different ages have reported increases in culm wood density (Wahab et al., 2010) and thickening of cell walls in the culm tissue (Alvin and Murphy, 1988) occurring over a period of several years after the culm has fully grown in height. Wahab et al. (2010) also suggested that the thickening of cell walls leads to the increase in culm wood density. However, yearly anatomical changes in bamboo culm tissue, has not been evaluated precisely due to the difficulty of age discrimination in perennial bamboo stands. Detailed anatomical analysis in relation to culm age could be a key to understand any age-related changes in culm respiration. Information on the respiration and anatomy of bamboo will not only advance our knowledge of the bamboo plant itself but will also be useful in estimating the respiratory fluxes of bamboo stands, which also helps to evaluate the productivity and capacity of capturing carbon of bamboo stands. The objective of this study was to examine the effect of bamboo culm anatomy and morphology on culm respiration, with reference to culm age. For this study we used Moso bamboo (Phyllostachys pubescens), which is the most common type of bamboo in Japan (Isagi et al., 1997) and is the most important bamboo species in China (Fu, 2001; Shimono et al., 2021). We set a bamboo stand and labeled newly sprouted culms every year to elucidate the age precisely.
Materials and Methods
Study Area and Sampling
This study was performed in a Moso bamboo stand at the Kasuya Research Forest of Kyushu University, Japan (33°37 ‘N, 130°31 ’E). The stand density was 10,600 culms ha–1. According to meteorological measurements 15 km from the site, mean annual air temperature between 1995 and 2005 was approximately 16°C, and the mean annual precipitation was 1790 mm (Kume et al., 2010). In 2013, a study area of 10 m by 10 m was selected within this bamboo stand. All culms within the plot into five age classes: current, 1, 2, 3-year-old, and above 4-year-old by yearly labeling of new culms between 2013 and 2015. Current year culms correspond to the culms sprouting in April 2015 and are referred to as “current year culms” in the present study. Incidentally, 1-year-old culms did not sprout in this plot in 2015 (Figure 1). Culm circumference in the plot ranged between 15 to 50 cm, with the highest frequency between 35 and 40 cm. Mean DBH in this plot was 11.6 cm (Katayama et al., 2018). In June 2015, three culms each from four age groups (current, 2, 3, and above 4-year-old) were randomly selected from the plot for respiration measurements. However, due to equipment failure, data from one culm each from the 3-year-old and above 4-year-old group could not be acquired, and therefore these two age groups have data from two culms. Respiration measurements on the ten culms were conducted once on each culm before harvesting. The culm circumference of the ten measured culms ranged between 26–40 cm, with an average circumference of 36.0 ± 4.3 cm.
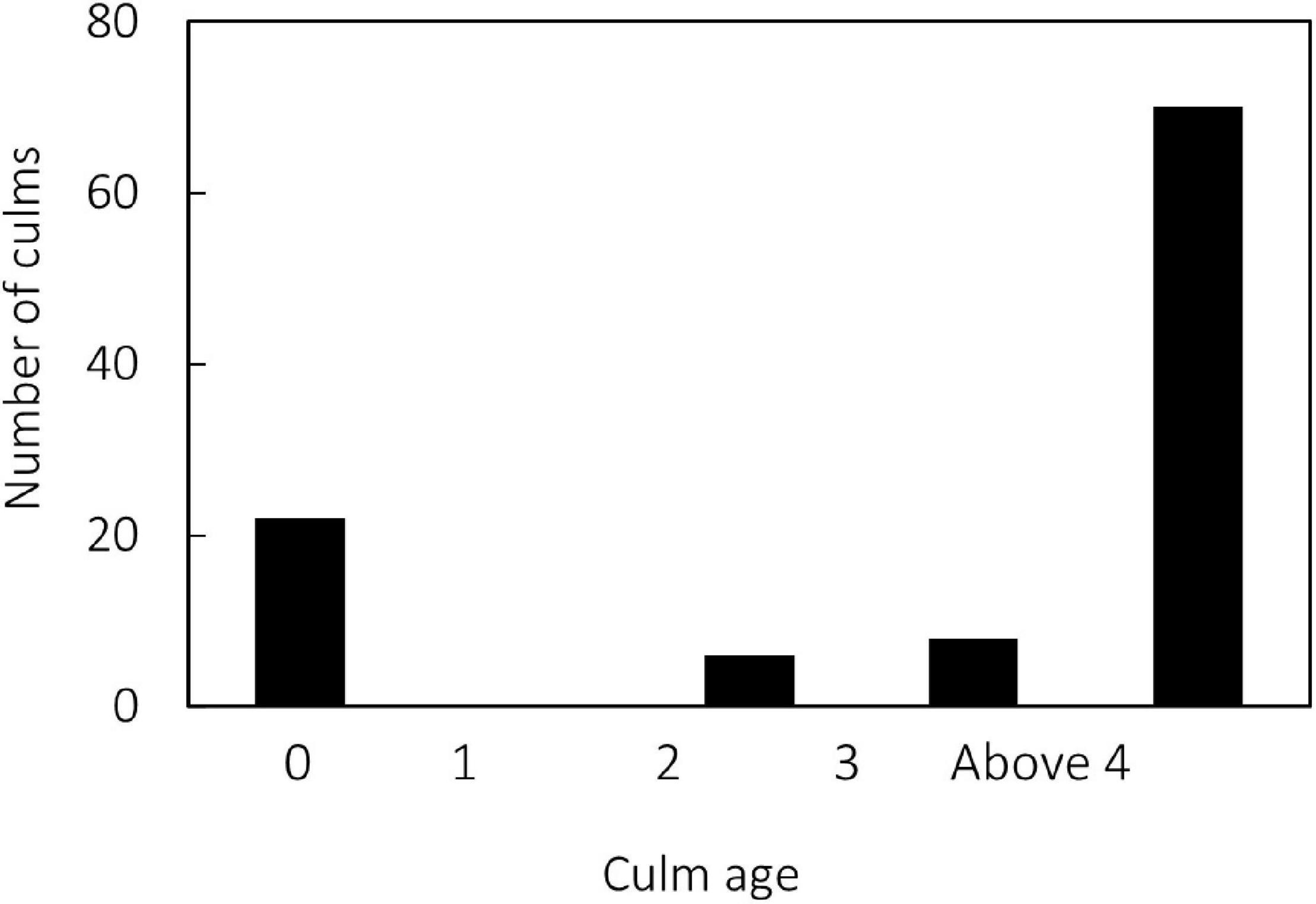
Figure 1. Number of culms in each age group of bamboo at the study area. “0-year-old” culms correspond to culms that sprouted in April 2015, “current year culm”.
Respiration Measurements
Respiration measurements were conducted based on the methods outlined by Katayama et al. (2014), using the closed system with an IRGA detector (GMP343, Vaisala, Helsinki, Finland). Prior to measurements, collars made of polyvinyl chloride were sealed to the culm surface with silicon sealant and non-drying clay, approximately 1.2 m high on the culm. During measurements, the IRGA detector was attached to the cylinder on the culm surface on the culm surface to measure the CO2 efflux of each culm. Air inside the chamber was mixed with a small fan. CO2 concentration inside the chamber was measured every 5 s and recorded by a monitor (M170, Vaisala, Finland). Air temperature and stem surface temperature were measured with a thermometer (TR- 52i; T&D Co. Ltd., Matsumoto, Japan). All respiration measurements were conducted before dawn to reduce the effects of sap flow and bark photosynthesis (Teskey et al., 2008).
Culm respiration rate (Rculm, μmol m–2 s–1) was calculated using Equation (1) (Katayama et al., 2014).
where V is the chamber volume (0.27 L), q is the air temperature inside the chamber (°C), A is the projected area of the chamber (0.3849 × 10–3 m2) and dc is the increment of CO2 concentration during the incubation time (dt). Equation (1) assumes that efflux rate is measured under standard barometric pressure. All measurements at each point were repeated two or three times and the means were used.
Morphological Analysis
After the culms were harvested, culm height, culm circumference and culm thickness were measured. One sample from breast height of each culm was used to measure wet volume for culm wood density (g cm–3) calculation. These samples were then dried for a week in an oven (20-802 P-1, IKEMOTO, Tokyo, Japan) set at 50°C until sample weights stabilized. Weight after 1 week was determined as the dry weight, which was used to calculate culm wood density. Small portions of the dried samples of each culm were ground with a mill. Nitrogen content (%) in each sample was determined using a CN analyzer (MT-600, Yanaco, Japan) to determine the nitrogen concentration. Nitrogen content per projected chamber area (g cm–2) was calculated by multiplying nitrogen content (%), culm wood density (g cm–3) and culm wall width (cm).
Anatomical Analysis
Samples 1 cm × 10 cm × culm thickness was obtained from one internode of each harvested culm at approximately DBH position, where the respiration measurement chamber was attached, avoiding areas with nodes. Embedding and sectioning of sample culm sections (approximately 2 mm × 10 mm × culm thickness) were performed following the protocols of Barbosa et al. (2010) and Ferreira et al. (2014). Following the methods outlined by Stockfors and Linder (1998), the sections were washed in distilled water, and stained with 0.05% Coomassie Brilliant Blue solution, which reacts with proteins. The sections were then consecutively stained with Iodine solution. The stained transverse sections were observed with a light microscope (ECLIPSE E600, Nikon, Tokyo, Japan). Cell wall thickness and starch content of ground parenchyma cells, which account for about 50% of bamboo internodes and have larger lumen diameter than fibers (Alvin and Murphy, 1988; Liese, 1998), were measured using ImageJ ver. 1.50b (National Institutes of Health, United States) on cross sectional images taken by a digital camera (NEX5N, Sony, Tokyo, Japan). For detailed examination of cell wall thickness, each section was divided into three from the epidermis side to lacuna side when as inner, middle, and outer region. Thirty parenchyma cells from each region were randomly selected for cell wall thickness and starch content measurement.
Results
Current year culms had substantially higher respiration rates compared to the culms of older ages (Figure 2). Average respiration rate of current year culms was 1.9 ± 0.46 μmol m2 s–1, as opposed to 0.17 ± 0.09 μmol m2 s–1 for all other age culms.
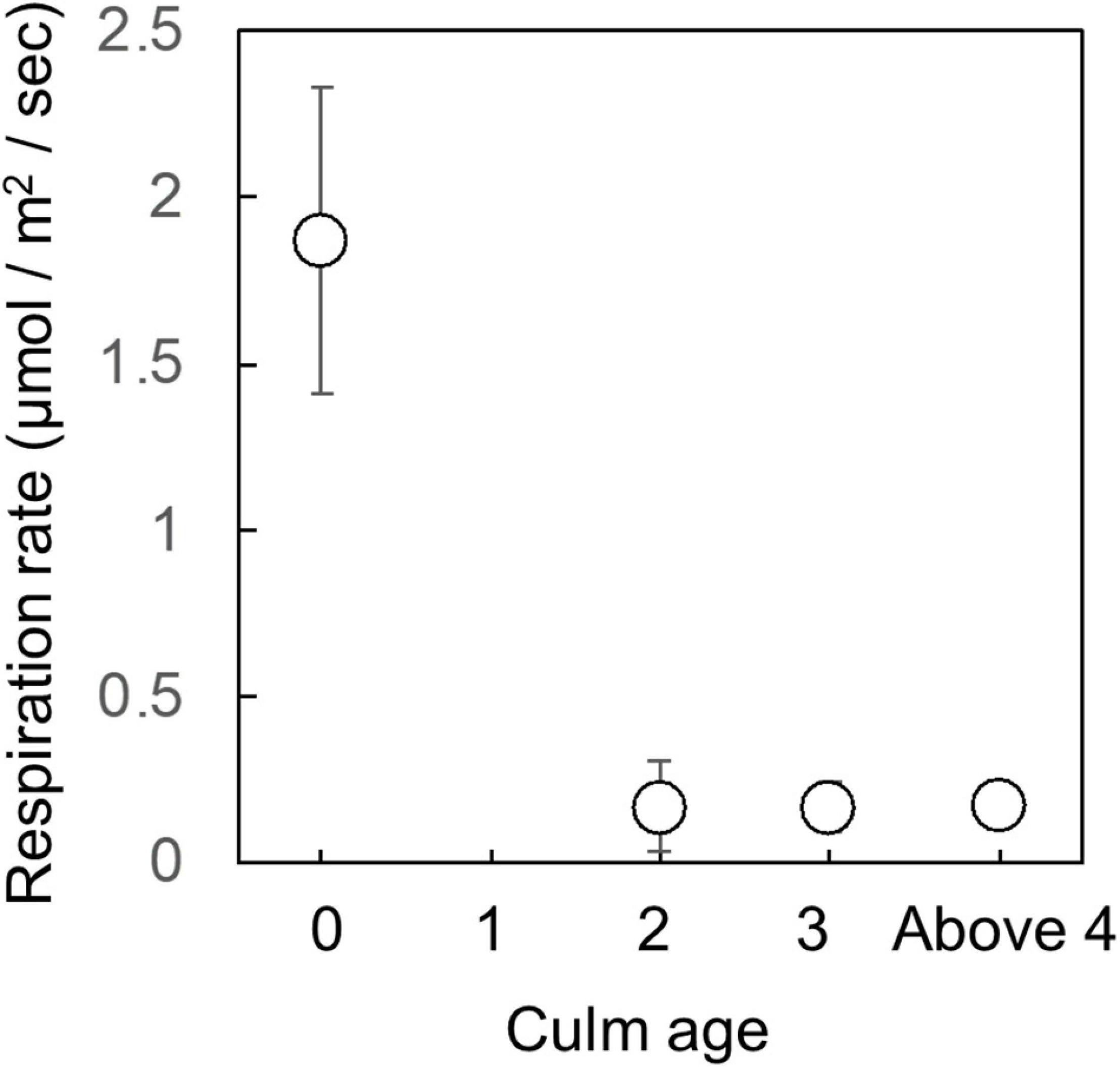
Figure 2. Age variation of culm respiration rate. Respiration measurements were conducted on 1 day in June, before dawn. Two current year culms had very similar values and therefore their data on the figure appears as one dot. Data from two culms (3 and above 4-year-old) is missing due to equipment failure. The respiration rate of current year culm was significantly larger than those of other years culm (p < 0.05, Kruskal-Wallis test).
Culm wood density for current culms was significantly higher than those for older culms (Figure 3A, P < 0.05, Tukey’s post ANOVA test). The average culm wood density for current year culms, 2-year-old culms, 3-year-old culms, and above 4-year-old culms was 0.26 ± 0.02, 0.53 ± 0.10, 0.64 ± 0.04, and 0.63 ± 0.04 g cm–3, respectively. The largest increase in density occurred during the first two years, with no large changes beyond the age of three. Nitrogen content in the culm tissue significantly differed between current and 3-year-old culms (P < 0.05, Tukey’s post ANOVA test) and decreased with culm age (Figure 3B). The average nitrogen content for current year culms, 2-year-old culms, and 3-year-old culms was 0.395 ± 0.169, 0.202 ± 0.147, and 0.053 ± 0.025 g cm–2, respectively. Nitrogen content in culms of ages above four were too low to be detected by the combustion analyzer.
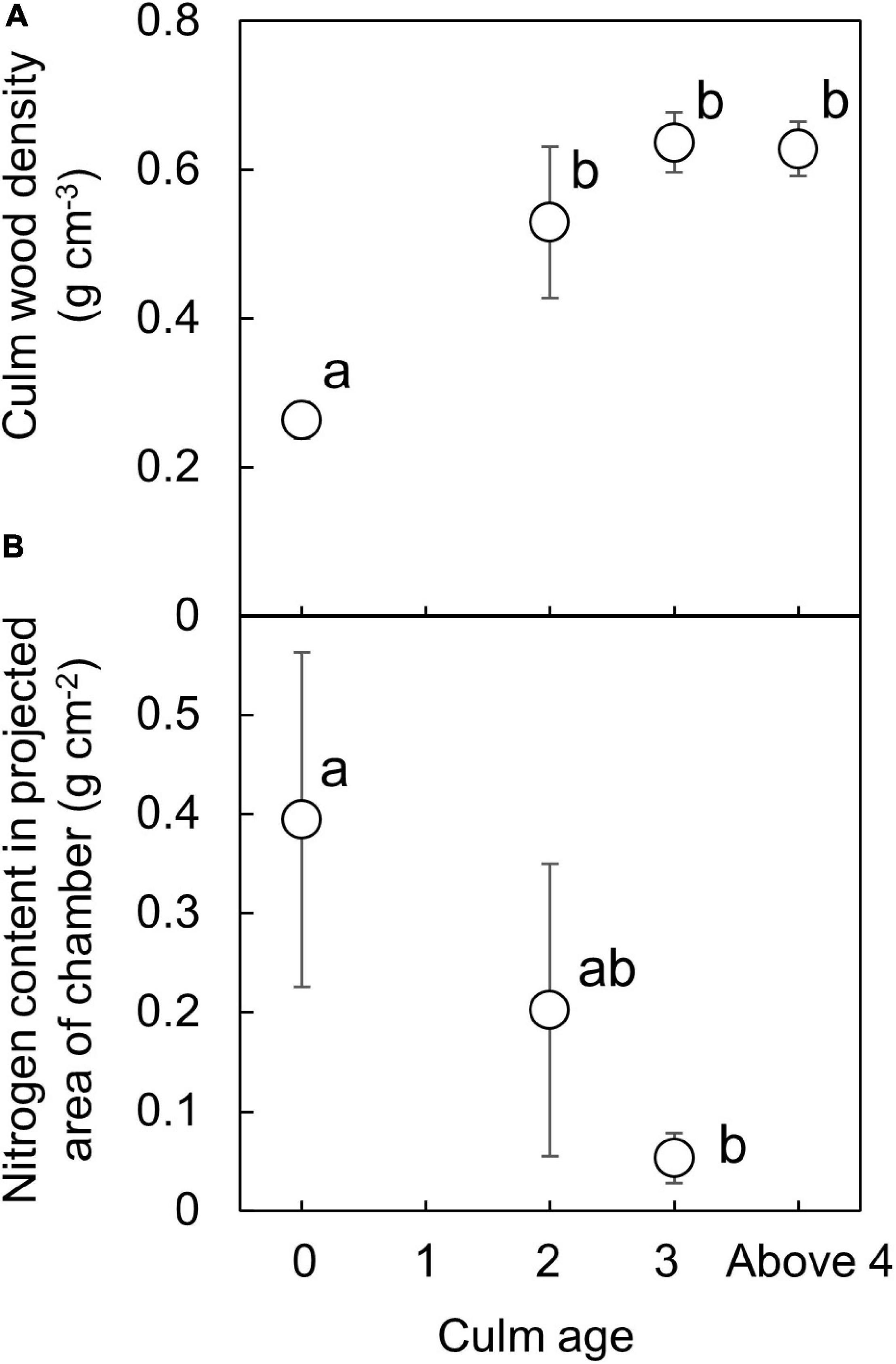
Figure 3. Age variation of culm wood density (A) and nitrogen content in projected chamber area (B). Data was obtained using culm samples taken on the harvesting day in June. Culm wood density increased with culm age, and more than doubled in two years (A). Nitrogen content (g/projected chamber area) in the culm tissue decreased with age (B). The same letter indicates no significant difference with ages (p > 0.05, Tukey’s post ANOVA test).
Ground parenchyma cell walls in the culm tissue thickened with age, with the largest increase in the first two years. Ground parenchyma cells in the current year culm had thin cell walls (Figure 4A), whereas the ground parenchyma cells in 2-year-old culm had significantly thicker cell walls (Figure 2, P < 0.05, Kruskal-Wallis test, Figure 4B). In this period, the cell wall thickness more than doubled (Table 1). The cell wall thickness was gradually increased in 3-year-old (Figure 4C) and above 4-year-old (Figure 4D and Table 1). On the other hand, the diameter of cell lumina was decreased with age (Figure 4). Starch was found in the cell lumina of 3-year-old and above 4-year-old (Figure 4). Ground parenchyma cell wall thickness positively and significantly correlated with culm wood density (R = 0.89, P < 0.05). For current year culms, the outer region tended to have thicker cell walls, but inner region but in older culms, the inner region tended to have thicker wall (Table 1). Starch was present in culms above the age of two (Figures 4C,D).
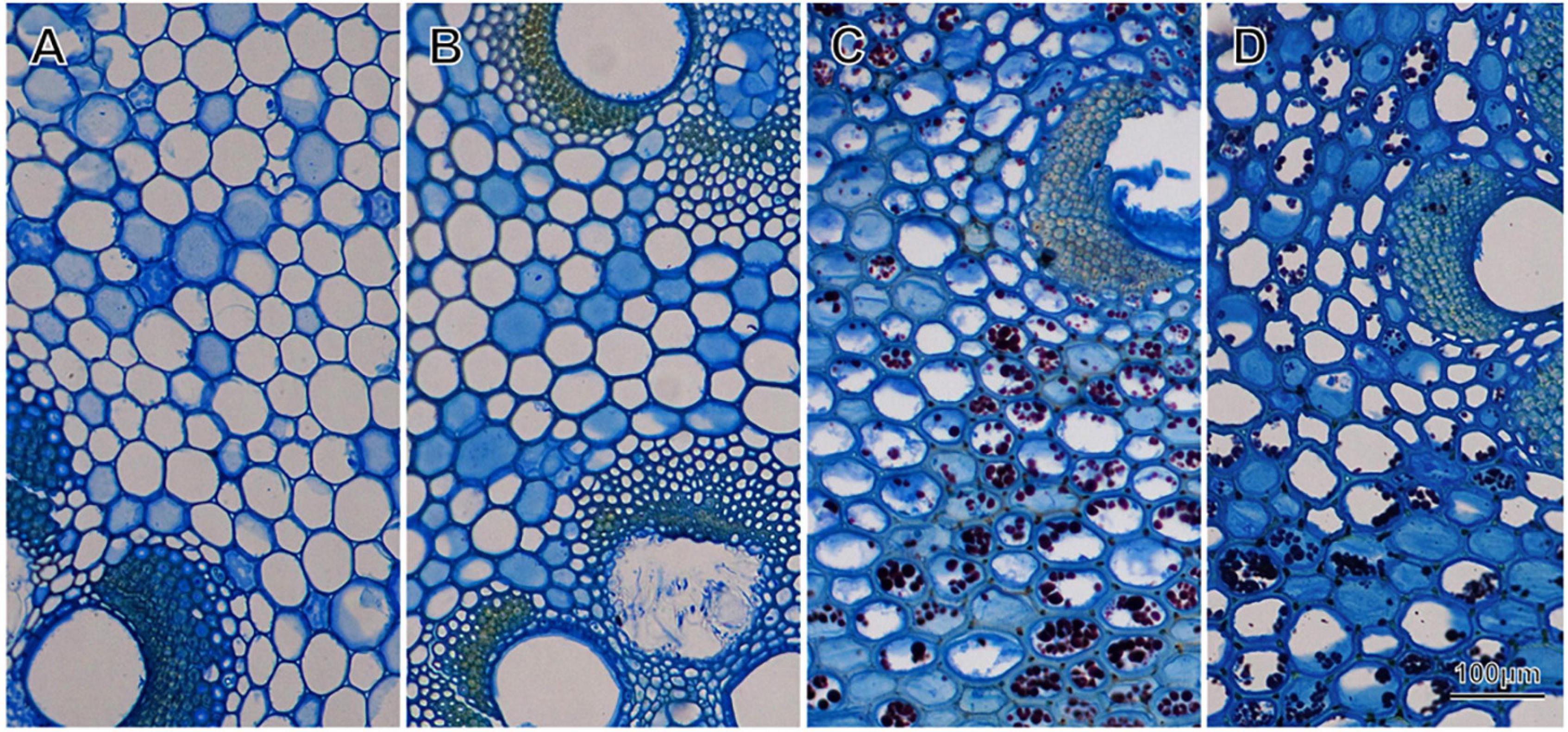
Figure 4. Horizontal sections of culm tissue from four different age groups stained with Coomassie blue and Iodine [(A): 0-year-old (current year), (B): 2-year-old, (C): 3-year-old, (D): above 4-year-old]. All images are from the middle portion of the culm section. The cell wall thickness of the parenchyma cells changes significantly in the first 2 years. Starch granules are stained dark purple inside the cell are only present in older culms.
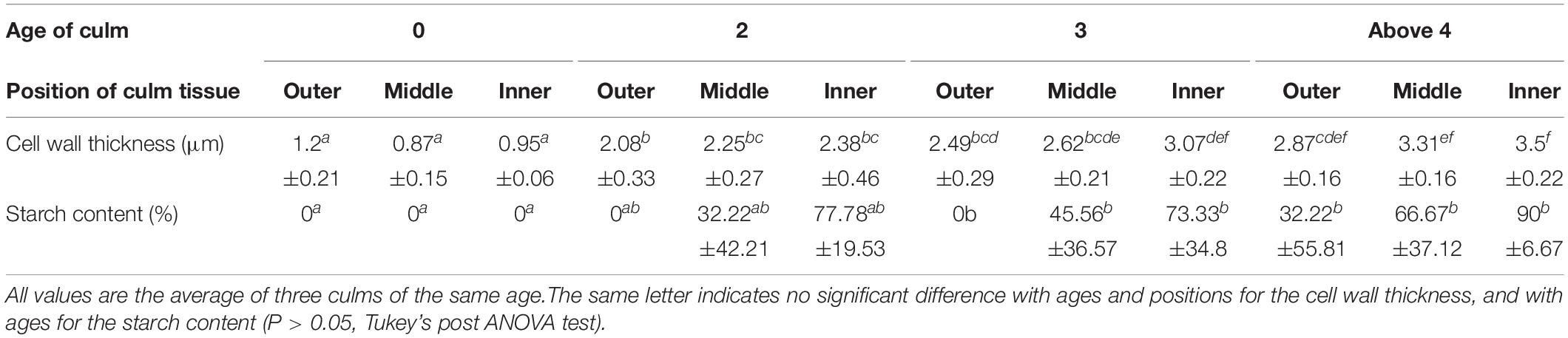
Table 1. Age variation of cell wall thickness (μm) and starch content (%) of parenchyma cells in culm tissue.
Culm wood density, cell wall thickness, and nitrogen content had positive and significant relationships with culm respiration rates (R = –0.84, P < 0.01, R2 = 0.79, P < 0.01, and R = 0.67, P < 0.05, respectively, Table 2). Morphological characteristics such as culm circumference, wall thickness, and height showed no significant relationships with culm respiration rate (Table 2). Culm circumference and thickness had a positive relationship (R = 0.86, P < 0.01).
Discussion
Morphological and Anatomical Effect on Culm Respiration
We did not find any relationships of culm respiration with morphological traits for Moso bamboo. For trees, morphological factors such as DBH and sapwood volume determine stem respiration rate (Ryan, 1990; Lavigne et al., 1996; Cavaleri et al., 2006; Katayama et al., 2016). Therefore, it is presumable that larger bamboo culms may have higher culm respiration. However, our results did not agree with this assumption, and morphological properties such as culm circumference, culm thickness, and culm height did not show any relation to the respiration rate of all culms (Table 2). Tree increases the amount of living cell in their sapwood for several years although it has variations between species (Bamber, 1987). On the other hand, moso bamboo has only the xylem formed in the current year. The no relationships between culm respiration and morphological traits can be attributed to the growth property of moso bamboo as an annual herbaceous plant.
Age-Based Variation of Culm Respiration
We found high variation among age in culm respiration for Moso bamboo: respiration rate for current year culms were much higher than that of older culms (Figure 2). This result was different from the previous study of Isagi et al. (1997), who reported that culm respiration decreased rather linearly with culm age. However, in our study, a sudden drop-in respiration rate was observed between current year culms and older culms. The respiration rates of older culms were between four to ten times smaller than that of younger culms (Figure 2). The differences in results may be due to different sample designs and respiration measurement methods. Isagi et al. (1997) used five culms, which have different age between 0.5 and 12.5, and measured their respiration after harvesting.
The respiration rates for current year culms (1.9 ± 0.46 μmol m2 s–1) was surprisingly high for their diameter, leveling the respiration rates of large trees in tropical rain forests [approximately 2.0 μmol m2 s–1, 25°C, DBH > 60 cm, Katayama et al. (2014)]. On the other hand, respiration rates of older culms (0.17 ± 0.09 μmol m2 s–1) were lower than that of 50-year-old Chamaecyparis obtusa trees growing in Japan, whose respiration rate was measured in July [approximately 0.40 μmol m2 s–1, DBH = 14.9 cm, Araki et al. (2010)]. Thus, the considerable change among culm age in respiration can be one of characteristics of bamboo species.
Factors Which Affected Culm Respiration
Culm wood density increased with age, and the largest increase occurred in the first two or three years (Figure 3). Strong relationships between cell wall thickness of ground parenchyma cells and culm wood density (R = 0.89, P < 0.01) suggested that density increase was mainly caused by the increase in the cell wall thickening of culm cells (Table 1 and Figure 4). Although this study only examined the parenchyma cells, previous studies suggested the increase of fiber wall thickness with age (Liese and Weiner, 1996; Huang et al., 2015), which is also a factor likely to have contributed to the density increase. The increase of culm wood density and the cell wall thickness by aging were consistent with earlier studies conducted on other bamboos (Alvin and Murphy, 1988; Wahab et al., 2010), although these studies did not conduct the annual culm sampling from current year. The presence of starch in the tissues of the bamboo has been reported before (Liese, 1998; Lian et al., 2021). In this study, starch was observed in the older culms but not in the one or two-year-old culms. This suggests the younger culms were metabolically active and consume carbohydrate immediately.
A substantial amount of respiration must have been required for the large increase in biomass of culms. In previous studies of tree stem respiration, growth respiration has often been shown in relation with stem diameter increment (Ryan, 1990; Maier, 2001). Whereas trees require growth respiration to grow outward (as stem diameter growth), this study suggests that bamboos require respiration to grow inward (as cell wall thickening) with no change in diameter, which may be defined as a type of growth respiration.
Young culms would also have comparatively higher maintenance respiration than old culms. The thickening of parenchyma cell walls with age (Table 1) causes limitation in cytoplasm area in ground parenchyma cells (Figure 4). A decrease in cytoplasm area with culm age would have resulted in a decrease in respiration, because respiration is conducted in the cytoplasm. Current year culms with a larger area of cytoplasm potentially needed larger maintenance respiration than older culms. In addition, many previous studies on tree stem respiration have shown strong positive relationships between stem respiration and nitrogen content (Lavigne et al., 1996; Maier et al., 1998; Ceschia et al., 2002). The present study revealed a significant relationship between culm respiration rate and nitrogen content (Table 2). Ryan et al. (1996) has pointed out that nitrogen is tightly linked with cellular activity and result in higher maintenance respiration. Higher nitrogen content in current year culms would be related to the high maintenance respiration rates in the current year culms. Larger live tissue volume and higher nitrogen concentration in current year culms potentially contribute to higher growth respiration and maintenance respiration for the rapid increase in the culm biomass.
The presence of starch in only older parenchyma suggests the older culms store excess photosynthate as starch. Previous studies have shown that even 12-year-old culms of Phyllostachys viridiglaucescen can store starch (Liese and Weiner, 1996). Magel et al. (2005) indicated a relocation of carbon toward another growing culms during the growing period in Sasa palmata culms. Younger culms would consume the starch for metabolic processes, and have no starch stored in their cells as suggested by Liese and Weiner (1996).
This study showed the large difference in the respiratory mechanism between bamboo and trees. Factors used to explain variations in tree stem respiration, such as sapwood volume and stem diameter increment, cannot be applied to bamboo culms. In contrast, changes in the culm anatomical structure and physiological properties, such as cell wall thickness, culm wood density, and nitrogen content, which accompany the aging of culms, contribute to the large variation in respiration rate among individual culms. In this study, respiration measurements were conducted only before dawn. However, the green bamboo culms would conduct photosynthesis during daytime, and cause lowering the overall CO2 efflux from the culm. Diurnal measurements of culm respiration and their correspondence with anatomical and morphological changes of culm will lead to a better understanding of the unique respiratory characteristics of bamboo culms.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
AK and YU designed the study. KO coordinated the study. EU conducted all experiments. YY and SK supported the anatomical analysis. TE supported Nitrogen and statistical analysis. All authors contributed to writing the manuscript and approved the submitted version.
Funding
This work was supported by grants from the JSPS KAKENHI (Grant No. JP20K20730).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Kasuya Research Forest, Kyushu University for managing the sample stand. We also thank Naoaki Tashiro and Sachiko Inoue for acquiring culm samples, and Atsushi Kume for his valuable comments.
References
Alvin, K. L., and Murphy, R. J. (1988). Variation in fibre and parenchyma wall thickness in culms of the bamboo Sinobambusa tootsik. IAWA J. 9, 353–361.
Araki, M. G., Utsugi, H., Kajimoto, T., Han, Q., Kawasaki, T., and Chiba, Y. (2010). Estimation of whole-stem respiration, incorporating vertical and seasonal variations in stem CO2 efflux rate, of Chamaecyparis obtusa trees. J. For Res. 15, 115–122.
Barbosa, A. C. F., Pace, M. R., and Witovisk, L. A. V. (2010). A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA J. 31, 373–383.
Bystriakova, N., Kapos, V., Lysenko, I., and Stapleton, C. (2003). Distribution and conservation status of forest bamboo biodiversity in the Asia-Pacific Region. Biodiv. Conserv. 12, 1833–1841.
Cavaleri, M. A., Oberbauer, S. F., and Ryan, M. G. (2006). Wood CO2 efflux in a primary tropical rain forest. Glob. Change Biol. 12, 2442–2458.
Ceschia, É, Damesin, C., Lebaube, S., Pontailler, J. Y., and Dufrêne, É (2002). Spatial and seasonal variations in stem respiration of beech trees (Fagus sylvatica). Ann. For. Sci. 59, 801–812.
Damesin, C., Ceschia, E., Le, Goff, N., Ottorini, J. M., and Dufrêne, E. (2002). Stem and branch respiration of beech: from tree measurements to estimations at the stand level. New Phytol. 153, 159–172.
Ferreira, B. G., Teixeira, C. T., and Isaias, R. M. S. (2014). Efficiency of the Polyethylene-Glycol (PEG) Embedding Medium for Plant Histochemistry. J. Hisochem. Cytohem. 62, 1–7. doi: 10.1369/0022155414538265
Gruber, A., Wieser, G., and Oberhuber, W. (2009). Intra-annual dynamics of stem CO2 efflux in relation to cambial activity and xylem development in Pinus cembra.”. Tree Physiol. 29, 641–649. doi: 10.1093/treephys/tpp001
Guo, X., Shi, P., Niinemets, Ü, Hölscher, D., Wang, R., Liu, M., et al. (2021). “Diminishing returns” for leaves of five age-groups of Phyllostachys edulis culms. Am. J. Bot. 108, 1662–1672. doi: 10.1002/ajb2.1738
Huang, L., Niinemets, Ü, Ma, J., Schrader, J., Wang, R., and Shi, P. (2021). Plant age has a minor effect on non-destructive leaf area calculations in moso bamboo (Phyllostachys edulis). Symmetry 13:369. doi: 10.3390/sym13030369
Huang, X. Y., Qi, J., Xie, J. L., Hao, J. F., Qin, B. D., and Chen, S. (2015). Variation in anatomical characteristics of bamboo. Bambusa rigida. Sains Malaysiana 44, 17–23. doi: 10.1093/treephys/tpab017
Isagi, Y., Kawahara, T., Kamo, K., and Ito, H. (1997). Net production and carbon cycling in a bamboo Phyllostachys pubescens stand. Plant Ecol. 130, 41–52.
Katayama, A., Kume, T., Komatsu, H., Ohashi, M., Matsumoto, K., Ichihashi, R., et al. (2014). Vertical variations in wood CO2 efflux for live emergent trees in a Bornean tropical rainforest. Tree Physiol. 34, 503–512. doi: 10.1093/treephys/tpu041
Katayama, A., Kume, T., Ohashi, M., Matsumoto, K., Nakagawa, M., Saito, T., et al. (2016). Characteristics of wood CO 2 efflux in a Bornean tropical rainforest. Agr. For Meteorol. 220, 190–199.
Katayama, A., Shimono, K., Inoue, S., Ogi, D., Osaki, S., and Ohigashi, K. (2018). Aboveground biomass in managed and unmanaged bamboo forests for Phyllostachys pubescens and Phyllostachys bambusoides. Bull. Kyushu Univers. Fores. 99, 13–17.
Kume, T., Onozawa, Y., Komatsu, H., Tsuruta, K., Shinohara, Y., Umebayashi, T., et al. (2010). Stand-scale transpiration estimates in a Moso bamboo forest:(I) Applicability of sap flux measurements. For. Ecolog. Manage. 260, 128–1294.
Lavigne, M. B., Franklin, S. E., and Hunt, E. R. (1996). Estimating stem maintenance respiration rates of dissimilar balsam fir stands. Tree Physiol. 16, 687–695. doi: 10.1093/treephys/16.8.687
Lavigne, M. B., Little, C. H., and Riding, T. (2004). Changes in stem respiration rate during cambial reactivation can be used to refine estimates of growth and maintenance respiration. New Phytol. 162, 81–93.
Li, Q., Song, X., Chang, S. X., Peng, C., Xiao, W., Zhang, J., et al. (2019). Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agri. For. Met. 268, 48–54.
Lian, C., Chen, H., Zhang, S., Liu, R., Wu, Z., and Fei, B. (2021). Characterization of ground parenchyma cells in Moso bamboo (Phyllostachys edulis–Poaceae). IAWA J. doi: 10.1163/22941932-bja10076
Liu, Y., Zhou, G., Du, H., Berninger, F., Mao, F., Li, X., et al. (2018). Soil respiration of a Moso bamboo forest significantly affected by gross ecosystem productivity and leaf area index in an extreme drought event. PeerJ. 6:e5747. doi: 10.7717/peerj.5747
Magel, E., Kruse, S., Lütje, G., and Liese, W. (2005). Soluble carbohydrates and acid invertases involved in the rapid growth of developing culms in Sasa palmata (Bean) Camus. Bamboo Sci. Cult. 19, 23–29.
Maier, C. A. (2001). Stem growth and respiration in loblolly pine plantations differing in soil resource availability. Tree Physiol. 21, 1183–1193. doi: 10.1093/treephys/21.16.1183
Maier, C. A., Zarnoch, S. J., and Dougherty, P. M. (1998). Effects of temperature and tissue nitrogen on dormant season stem and branch maintenance respiration in a young loblolly pine (Pinus taeda) plantation. Tree Physiol. 18, 11–20. doi: 10.1093/treephys/18.1.11
Ryan, M. G. (1990). Growth and maintenance respiration in stems of Pinus contorta and Picea engelmannii. Can. J. For. Res. 20, 48–57.
Ryan, M. G., Hubbard, R. M., Clark, D. A., and Sanford, R. L. Jr. (1994). Woody-tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits. Oecologia 100, 213–220. doi: 10.1007/BF00316947
Ryan, M. G., Hubbard, R. M., Pongracic, S., Raison, R. J., and McMurtrie, R. E. (1996). Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol. 16, 333–343. doi: 10.1093/treephys/16.3.333
Shanmughavel, P., and Francis, K. (1996). Above ground biomass production and nutrient distribution in growing bamboo (Bambusa bambos (L.) Voss). Biomass Bioenergy 10, 383–391.
Shimono, K., Katayama, A., Kume, T., Enoki, T., Chiwa, M., and Hish, T. (2021). Differences in net primary production allocation and nitrogen use efficiency between Moso bamboo and Japanese cedar forests along a slope. J. Fores. Res. 27, 28–35. doi: 10.1080/13416979.2021.1965280
Stockfors, J. A. N., and Linder, S. (1998). Effect of nitrogen on the seasonal course of growth and maintenance respiration in stems of Norway spruce trees. Tree Physiol. 18, 155–166. doi: 10.1093/treephys/18.3.155
Teskey, R. O., Saveyn, A., Steppe, K., and McGuire, M. A. (2008). Origin, fate and significance of CO2 in tree stems. New Phytol. 177, 17–32. doi: 10.1111/j.1469-8137.2007.02286.x
Wahab, R., Mustapa, M., Sulaiman, O., Mohamed, A., Hassan, A., and Khalid, I. (2010). Anatomical and physical properties of cultivated two-and four-year-old Bambusa vulgaris. Sains Malays. 39, 571–579.
Zha, T., Kellomäki, S., Wang, K. Y., Ryyppö, A., and Niinistö, S. (2004). Seasonal and annual stem respiration of Scots pine trees under boreal conditions. Ann. Bot. 94, 889–896. doi: 10.1093/aob/mch218
Zhang, S., Li, Y., Singhd, B. P., Wange, H., Cai, X., Chen, J., et al. (2021). Contrasting short-term responses of soil heterotrophic and autotrophic respiration to biochar-based and chemical fertilizers in a subtropical Moso bamboo plantation. App. Soil Ecol. 157:103758. doi: 10.1016/j.apsoil.2020.103758
Keywords: bamboo, culm age, respiration, nitrogen content, wood density, cell wall thickness
Citation: Uchida EM, Katayama A, Yasuda Y, Enoki T, Otsuki K, Koga S and Utsumi Y (2022) Age-Related Changes in Culm Respiration of Phyllostachys pubescens Culms With Their Anatomical and Morphological Traits. Front. For. Glob. Change 5:868732. doi: 10.3389/ffgc.2022.868732
Received: 03 February 2022; Accepted: 09 March 2022;
Published: 15 April 2022.
Edited by:
Tingting Mei, Zhejiang A&F University, ChinaReviewed by:
Peijian Shi, Nanjing Forestry University, ChinaLiwei Zhu, South China Botanical Garden (CAS), China
Copyright © 2022 Uchida, Katayama, Yasuda, Enoki, Otsuki, Koga and Utsumi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasuhiro Utsumi, dXRzdW1pQGZvcmVzdC5reXVzaHUtdS5hYy5qcA==; Ayumi Katayama, a2F0YXlhbWEwOTIwQGdtYWlsLmNvbQ==
 Eiko M. Uchida
Eiko M. Uchida Ayumi Katayama
Ayumi Katayama Yuko Yasuda3
Yuko Yasuda3 Tsutomu Enoki
Tsutomu Enoki Shinya Koga
Shinya Koga Yasuhiro Utsumi
Yasuhiro Utsumi