- 1College of Health Sciences, Makerere University, Kampala, Uganda
- 2Burnet Institute, Melbourne, VIC, Australia
- 3Department of Infectious Diseases, University of Melbourne, Melbourne, VIC, Australia
- 4Department of Microbiology and School of Translational Medicine, Monash University, Melbourne, VIC, Australia
- 5Department of Laboratory Medicine, Lund University, Lund, Sweden
- 6Department of Infectious Diseases, Skåne University Hospital, Lund, Sweden
- 7Department of Biochemistry and Structural Biology, Lund University, Lund, Sweden
- 8Clinical Chemistry and Pharmacology, Laboratory Medicine, Skåne University Hospital, Lund, Sweden
Antibodies are important for protection against malaria. For optimal protective activity, it is thought that antibodies need to have high affinity. A longitudinal study conducted in Uganda followed newborn infants and their mothers for nine months. The study found that antibody affinity (here measured as dissociation rate constant, kd) against the merozoite antigens AMA1 and MSP2 decreased from birth to six months in the infants, then gradually increased to 9 months, but not reaching the level observed in the mothers. In contrast, affinity against the sporozoite antigen CSP, did not change throughout the study period. Among mothers, no significant changes in antibody affinity were observed for any antigen, which is consistent with expectations for adults in an endemic area. Comparing specific antibody affinities to total antibody levels revealed almost no correlations, indicating that antibody magnitude and affinity evolve differently during immune development. Significant correlations were observed between antibody affinities and some atypical memory B cells. In conclusion, our study shows that development of naturally acquired slowly dissociating (high affinity) antibodies against malaria can evolve separately across different antigens. This is important information for future vaccine development studies.
Introduction
Malaria remains a major global health challenge, causing significant morbidity and mortality. In 2023, an estimated 263 million malaria cases and 597,000 deaths were reported globally (1). Despite progress in reducing malaria incidence and mortality, challenges such as drug resistance, limited vaccine efficacy, and healthcare access persist, underscoring the need for continued innovations and investments (1).
Naturally-acquired immunity against malaria develops slowly and typically requires repeated exposure (2). While the precise mechanisms underlying the immunological responses are not fully understood, antibodies play a crucial role in mitigating clinical P. falciparum malaria. Studies as early as the 1960s demonstrated reduced parasitemia and clinical symptoms following the passive transfer of immunoglobulins from immune donors to infected individuals (3, 4). Antibodies targeting merozoite proteins, such as Merozoite Surface Protein 2 (MSP2) and Apical Membrane Antigen 1 (AMA1), or surface antigens on infected red blood cells, are integral to acquired immunity against malaria and are potential antigen candidates for vaccine development (5–10).
MSP2 is a 25–30 kDa protein (11) abundantly expressed on the surface of merozoites, playing a key role in red blood cell invasion (12). Both vaccine-induced and naturally acquired antibodies targeting MSP2 are strain-specific and protective (13, 14). These antibodies can mediate complement fixation to inhibit invasion (15) and promote opsonic phagocytosis (16). Similarly, AMA1 (82 kDa) is expressed on both sporozoites and on merozoites (17), with antibodies against it impacting the invasion of hepatocytes and erythrocytes (18, 19). The circumsporozoite protein (CSP) is the most abundant protein on the surface of the Plasmodium sporozoites and serves as the antigenic target of the RTS,S and R21 vaccines, which are recommended by WHO for implementation (1). However, the long-term efficacy of these vaccines still requires improvement (20–31). Antibodies to CSP can promote complement fixation, enhance opsonic phagocytosis and inhibit invasion by sporozoites (32–34).
Developing more efficacious vaccines requires a deeper understanding of the factors limiting their current efficacy. One significant challenge is the lack of an in vitro immunologic correlate of protection against malaria infection and disease (35). Understanding the development of both humoral and cellular immunity is crucial for designing effective malaria vaccines. Mere presence of antibodies does not suffice for immunity against malaria; the quality of the antibodies is equally essential (36). High-affinity antibodies, produced through effective priming by antigens or vaccines, mark the maturation of specific B cell clones (37). The generation of these high-affinity antibodies depends on repeated malaria exposure and clinical manifestations of the disease (38, 39).
Various methods have been used to evaluate antibody affinity. For example, an urea-based Enzyme-Linked Immunosorbent Assay (ELISA) has shown that individuals with complicated malaria has lower-affinity antibodies compared to those with asymptomatic or uncomplicated malaria (39). High-affinity antibodies targeting the parasite antigen VAR2CSA have been associated with reduced placental malaria (40). On the contrary, other studies have found no correlation between the affinity of antibodies against specific blood-stage malaria antigens and clinical protection (41–43). Of recent, high-affinity antibodies against the CSP N-terminal domain were found to fail in inhibiting P. falciparum activity (44).
These inconsistencies may be attributed to inadequacies in the methods used, or structural variations in the antigens studied (38). In previous work, we demonstrated that ELISA-based avidity assays strongly correlated with standard ELISA results but provided limited additional information for our antigens. This prompted us to explore alternative methods (45). Surface Plasmon Resonance (SPR) has been recommended for assessing vaccine efficacy in various diseases (46) and has shown promise in malaria research, demonstrating correlations between high-affinity antibodies and both malaria protection (45) and with invasion-inhibitory functions (38).
While numerous studies have explored antibody affinity against potential malaria antigen candidates for vaccine development, few have investigated how antibody affinity develops over extended periods in early infancy, particularly in comparison to maternal antibodies, This study aimed to evaluate the dissociation rate constant, kd (which correlates with affinity) of naturally acquired antibodies targeting key immune and vaccine antigens - AMA1, MSP2 and CSP. We conducted a longitudinal study in Uganda, analyzing samples collected from infants at birth and at 2.5, 6, and 9 months of age using SPR. Additionally, we compared antibody dissociation rate constants between mothers and infants, correlating the results with total antibody levels and the presence of different B cell subsets.
Materials and methods
Samples
Samples from 68 mother-infant pairs were randomly selected from a previously described cohort (47). Briefly, plasma samples were collected from mothers and babies in a malaria endemic area 20 km northeast of Kampala in Uganda, at the antenatal center at Kasangati Health Centre. In brief, blood was collected in lithium heparin vacutainer tubes and plasma was separated after centrifugation at room temperature, then the plasma samples were stored at -80°C. In this study area, malaria is meso-endemic with peak transmission after the two rainy seasons (February–March and September–October) every year, with an estimated malaria prevalence of 13% in children (48). Eligibility criteria were normal deliveries with healthy newborns and agreement to attend follow-up visits at 2.5, 6 and 9 months of the child’s age. Each pregnant woman received at least one dose of intermittent preventive treatment and was provided with a long-lasting insecticide-treated bed net. Detailed clinical examinations were conducted at recruitment and during follow-up visits, with data entered into the study questionnaire. Malaria rapid diagnostic tests (RDTs) were performed on all samples, and blood smear examinations were done upon a positive RDT. None of the individuals exhibited signs of severe infection during recruitment or sampling.
Surface plasmon resonance
The SPR dissociation rate constant measurements were performed using a Biacore 3000 instrument, (GE Healthcare Life sciences, Uppsala) as described before (45). Briefly, CM5 sensor chips (Pharmacia biosensor AB, Uppsala Sweden) were activated by using an amine coupling kit (GE Healthcare Bio-Sciences AB, Uppsala Sweden) using an injection pulse (10 min, 5 µL/min). The AMA1, MSP2 and CSP recombinant proteins were immobilized using a manual injection of 100 µg/mL in coating buffer (0.01 M sodium acetate buffer, pH 4.0) until the desired response units were achieved. The unoccupied activated sites were blocked by ethanolamine. All steps were carried out in a continuous flow of HBS-EP (10 mM Hepes, 150 mM NaCl, 3 mM EDTA, 0.005% polysorbate 20) running buffer at 5 µL/min. The residual antibodies that could remain attached to the immobilized antigens after measurement of kd were removed by washing with 10 mM glycine-HCl (pH 1.5) for 5 seconds at 5 µL/min to regenerate the surface before injection of the next plasma sample. An internal control, which was a pool of Ugandan adult (presumably immune) samples, was injected after a complete set of mother-baby pair samples (equal to 6 plasma samples) to test the stability of the immobilized protein and the reproducibility of the assay. The background level was determined by Swedish non-immune plasma and all the samples considered in the subsequent analysis were above the background level. The response was monitored as a function of time (sensogram) at 25 °C. The intra-assay coefficient of variation (CV), based on repeat measurements of the same sample within a single run, varied between 4-6% for the different antigens. The inter-assay CV, calculated from measurements across different days and chips, was below 5% for all three antigens. The BIAevaluation 4.1 software was used to fit a single exponential decay to the data to estimate the kd values. AMA1 and CSP were expressed in HEK293 cells with a his-tag and purified using nickel columns (49–51). MSP2 was expressed in E. coli and purified as described (52).
ELISA
ELISA was performed as described before, using schizont extract from P. falciparum cultures (47). In brief, microtiter plates were prepared by coating with schizont extract, blocking with 5% skimmed milk (Sigma) for the IgG assay and super block dry blend (Thermo Scientific) for the IgM assay. Plates were then incubated with peroxidase-conjugated goat anti-human IgG or IgM antibodies (Sigma). Antibody binding was detected using TMB (3,3′,5,5′-Tetramethylbenzidine) substrate (Promega), and the optical density (OD) was measured at 450 nm.
Flow cytometry of CD19+ B-cells
Studies of different populations of total and P. falciparum-positive B-cells was performed as described before (47). Subpopulations of cells were determined: total/Pf+ IgG memory B-cells (MBC) (IgG+ MBC) [CD19+CD20+CD27+IgG+), non-IgG+ MBC (CD19+CD20+CD27+IgG−), atypical MBC (CD19+CD20+CD27−IgG+), naïve B-cells (CD19+CD20+CD27−IgG−) and plasma cells/blasts (CD19+CD20−CD27+IgG−).
Statistical analysis
Continuous variables were presented as medians with interquartile ranges or estimated means with corresponding confidence intervals. To account for the repeated measurements, linear mixed models were employed to model how each protein changed over time with a first-order autoregressive covariance structure, considering individuals as a random effect. Correlations between protein levels at each time point were analyzed using Spearman’s correlation for each time point, separately for mothers and infants. False discovery rate adjustments were applied to correct for multiple comparisons. Antibody dissociation rate constants between groups were compared using non-parametric Mann-Whitney U Test. For multiple group and antigen comparisons, Kruskal-Wallis was employed. Two-sided p-values were calculated for all tests, with p < 0.05 considered statistically significant. All analyses were performed using R (v. 4.1.2) or GraphPad Prism, version 10 (GraphPad Software Inc., San Diego, CA, USA).
Results
Estimating antibody affinity through dissociation rate constant measurements
We used SPR to quantify the dissociation rate constant (kd) of antibody binding to immobilized antigens. We have previously evaluated this method for estimating affinity differences of polyclonal antibodies in human serum and confirmed the reproducibility of the method (45, 53). Three targets of acquired immunity and potential malaria antigen candidates for vaccine development, including two merozoite antigens (AMA1, MSP2) and one sporozoite protein (CSP), were immobilized through amine coupling to SPR senor chips. The dissociation rate constant (kd) for the complexes between antibody and these antigens in the individual mother-baby pair plasma samples was used to estimate the affinity. Plasma dilutions of 1:15 and 1:30 were used and it was found that the kd values were independent of the plasma concentration, as expected. A pool of plasma samples from a malaria endemic area was used regularly as an internal control to test the stability of the antigens on the sensor chip, and it was found that the kd values were not dependent on protein loss over time.
Total anti P. falciparum IgG and IgM
Antibody levels against P. falciparum were analyzed in all participants, as previously reported (47, 54). In this study, we re-evaluated the data using linear mixed models to assess the development of total anti-P. falciparum IgG and IgM in infants. As expected, infants exhibited high levels of anti-P. falciparum IgG levels at birth, which declined by 2.5 months and then stabilized over the next 9 months after birth. In contrast, anti-P. falciparum IgM levels increased gradually from 6 months onwards, reflecting the infants’ subsequent exposure to malaria. Among mothers, no significant differences in anti-P. falciparum IgG or IgM levels were observed between the two time points.
Development of high affinity antibodies during infancy
We conducted a linear mixed model to assess the changes in antibody affinities against specific malaria antigens in infants at various time points from birth until 9 months of age. The estimated means of dissociation rate constants for antibodies targeting AMA1 and MSP2 significantly increased from birth to six months, then decreased by nine months (Figure 1A). However, the estimated means of antibody dissociation rate constants for CSP did not show significant changes over the 9 months (Figure 1A).
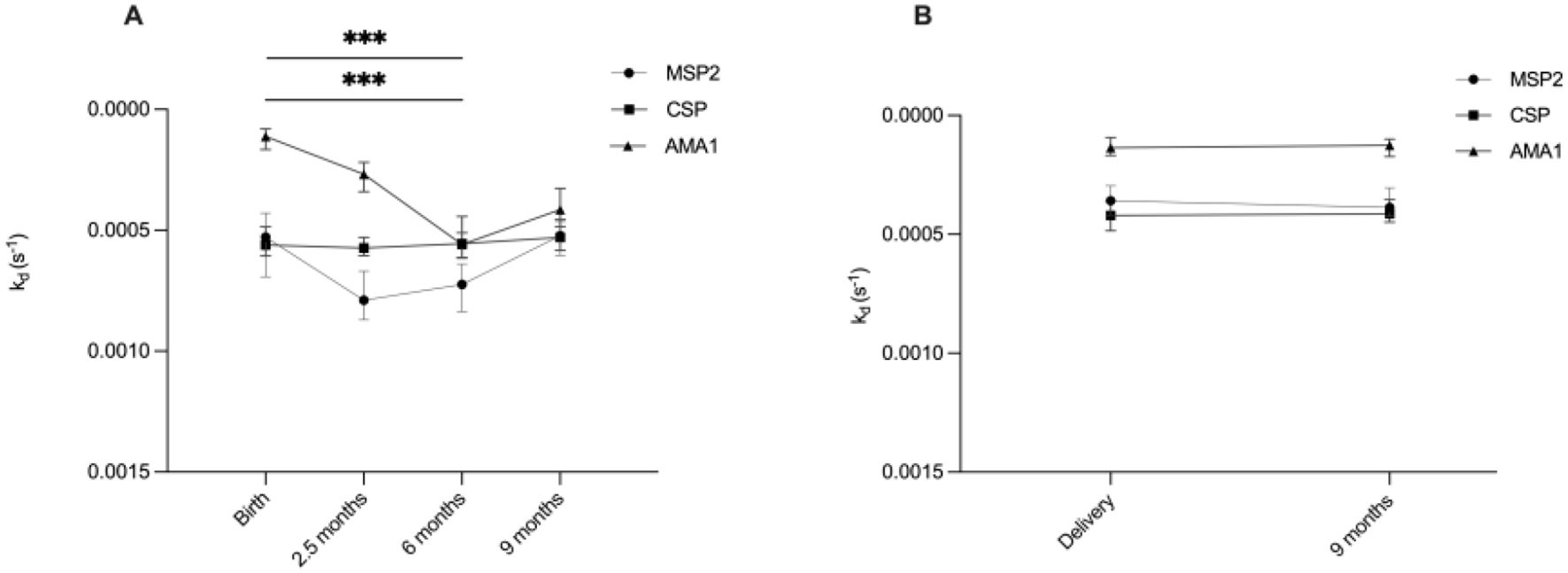
Figure 1. Overview of development of high affinity antibodies during infancy and in mothers. Graphs illustrate estimated means of antibody dissociation rate constants (kd) for MSP2, CSP and AMA1 during (A) infancy (at birth, 2.5, 6 and 9 months) and (B) in mothers (at delivery and after 9 months). Lower kd values indicate higher antibody affinity. *** indicates significant at P < 0.001, analyzed by linear mixed models.
Stable affinity in the mothers
The estimated means of antibody dissociation rate constants in the mothers for the antigens AMA1, MSP2 and CSP were the same at delivery and nine months postpartum (Figure 1B).
Higher antibody affinity in mothers compared to infants
We compared the antibody dissociation rate constants of mothers at delivery and nine months postpartum with those of their infants at the same time points. At both time points, mothers exhibited significantly lower median of kd (indicating higher affinity) for all three antigens AMA1, MSP2, and CSP compared to their infants, except for AMA1 at delivery, where no significant difference was observed (Figures 2A–C).
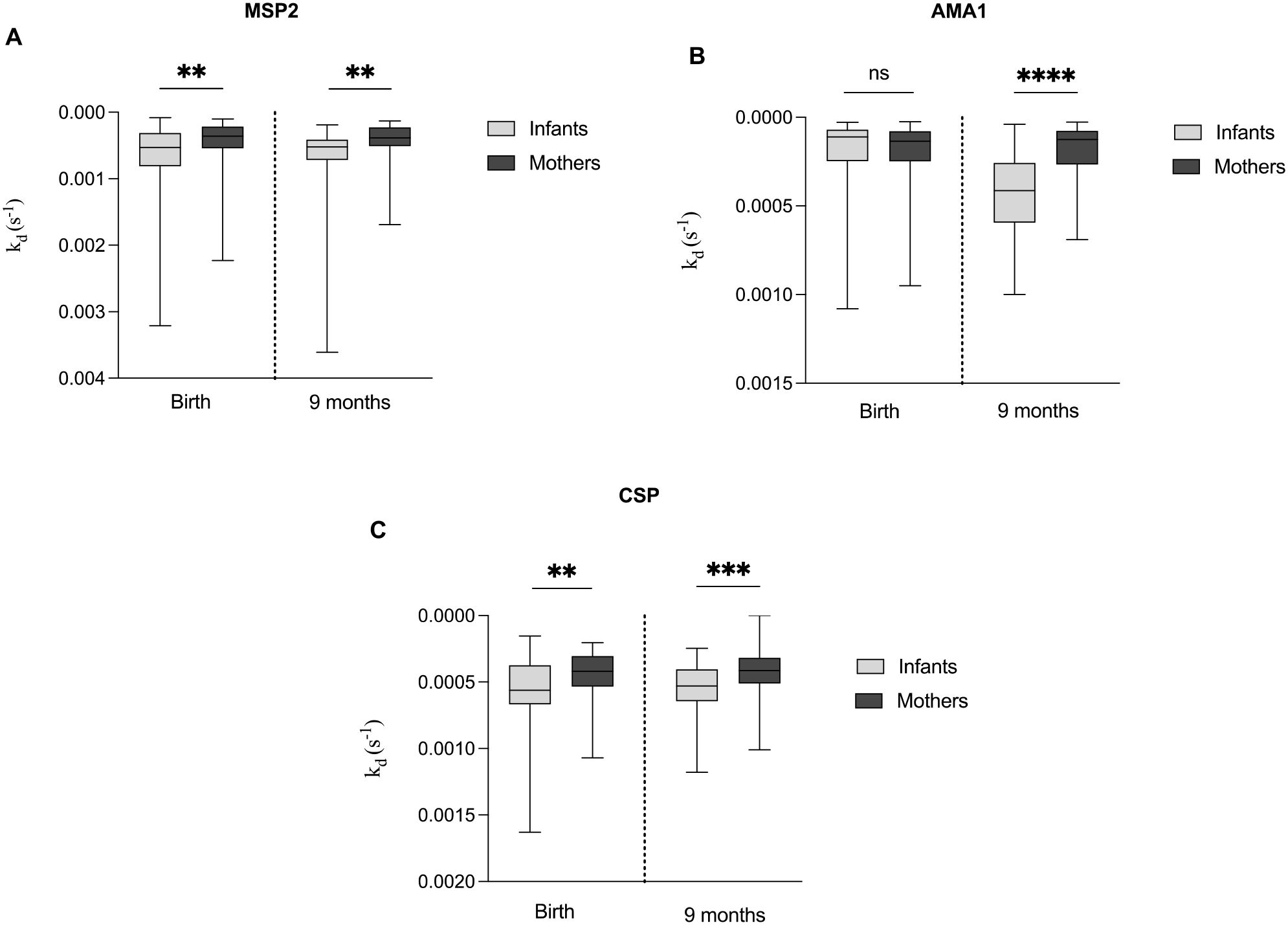
Figure 2. Comparative analysis of antibody dissociation rate constant (kd) for MSP2, AMA1 and CSP in infants and mothers. Box plots comparing the median kd of antibodies targeting the antigens (A) MSP2, (B) AMA1 and (C) CSP between mothers at delivery and nine months postpartum, and their infants at birth and nine months. Box plots represent interquartile range (IQR), whiskers the range and horizontal lines the median; **, ***, **** indicate significant differences (p < 0.01, 0.001, < 0.0001, respectively), and ns (p ≥ 0.05) indicates not significant tested by the Mann-Whitney U test.
Difference in antibody affinity between antigens
To investigate differences in antibody dissociation rate constants among the three tested antigens, we compared median dissociation rate constants to AMA1, MSP2, and CSP in both infants and mothers throughout the study period (Figures 3A–F). At birth and up to 2.5 months, infants exhibited significantly slower dissociation for AMA1 compared to MSP2 and CSP. By 6 and 9 months of age, no significant difference was observed between the dissociation rate constants for AMA1 and CSP, while MSP2 showed the highest dissociation rate constant (lowest affinity). For mothers, both at delivery and nine months postpartum, we observed similar patterns as for the infants, with significantly lower median kd values for AMA1 compared to MSP2 and CSP (Figures 3E, F).
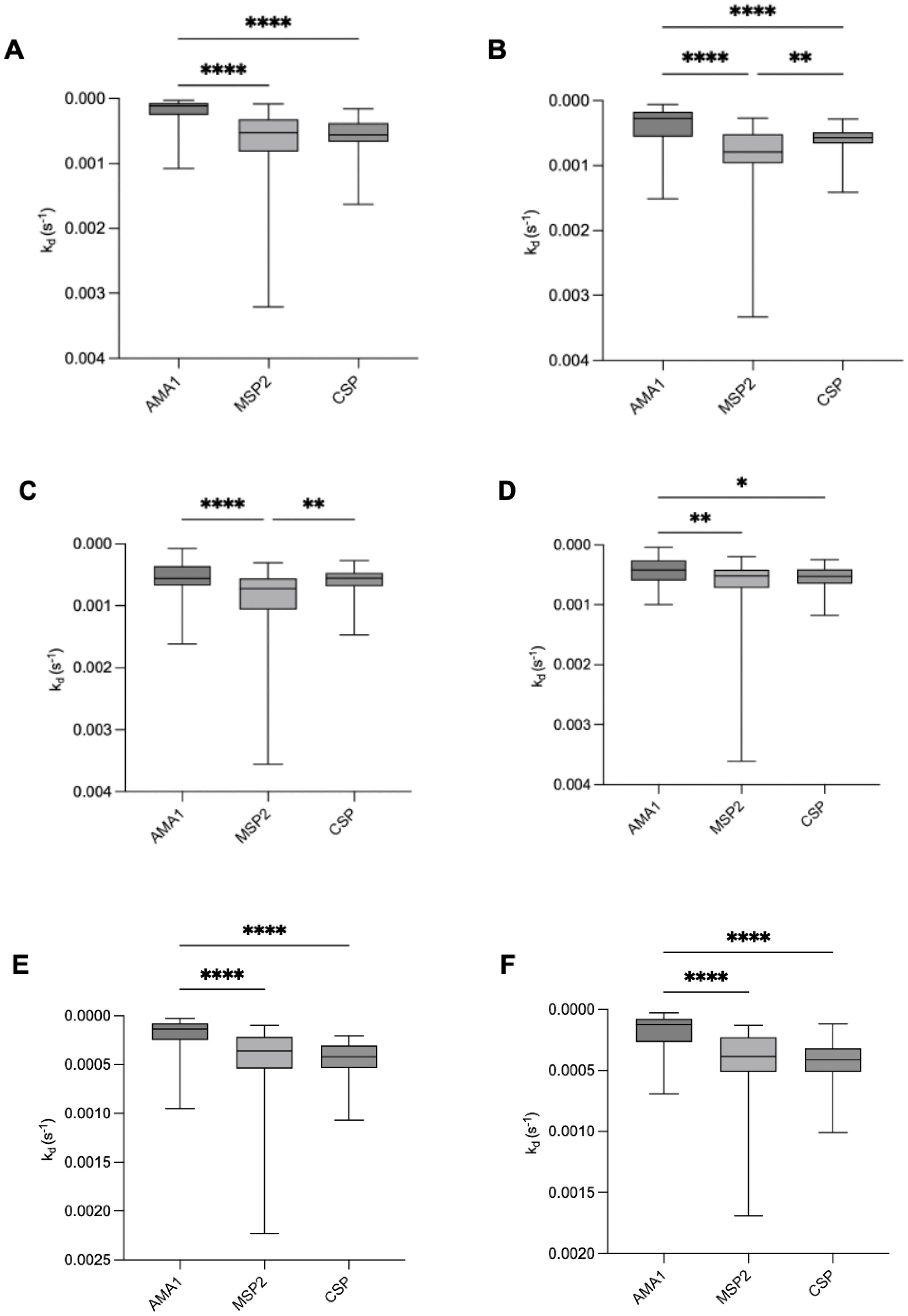
Figure 3. Comparison of antibody dissociation rate constants for AMA1, MSP2, and CSP in infants and mothers. Box plots illustrate the median antibody affinities (kd) against AMA1, MSP2, and CSP in infants at (A) birth, (B) 2.5 months, (C) 6 months, (D) 9 months, and in mothers at (E) delivery and (F) 9 months postpartum. Box plots represent interquartile range (IQR), whiskers the range, and horizontal lines represent the median; *,**, ***, and **** indicate significant differences (p < 0.05, 0.01, 0.001, and 0.0001, respectively) tested by Kruskal-Wallis test.
Relationship between antibody affinity and total levels of anti-P. falciparum IgG and IgM
We compared the antibody dissociation rate constant for the tested antigens with total levels of P. falciparum IgG and IgM (Figure 4). No significant correlations were observed between antibody dissociation rate constants and total IgG or IgM at any time point, except for a negative correlation between CSP dissociation rate constant and P. falciparum IgM in infants at 9 months of age (rho= -0.47, p < 0.0001).
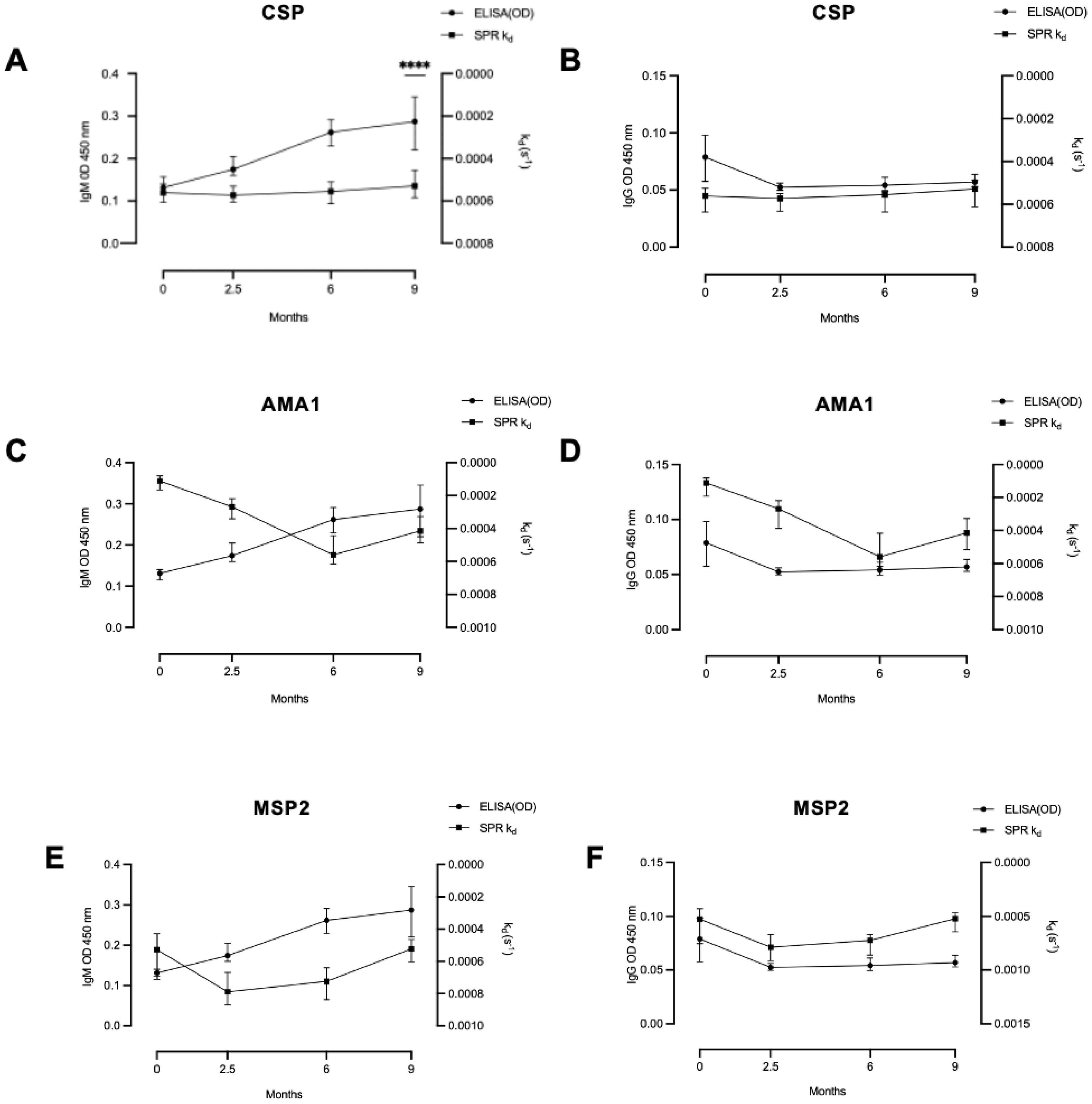
Figure 4. Relationship between antibody dissociation rate constant and ELISA-determined antibody levels. In infants there was (A) a significant correlation between antibody level and dissociation rate constant only for IgM and CSP at 9 months, but not for any other tested correlations: (B) IgG and CSP, (C) IgM and AMA1, (D) IgG and AMA1, (E) IgM and MSP2 and (F) IgG and MSP2. Coefficient of correlation, rho, and P values were calculated using Spearman’s correlation test. **** significant at P < 0.0001.
Correlation between antibody affinity and total CD19-positive B cell and various P. falciparum specific B cells
We previously quantified levels of total CD19-positive B cells and P. falciparum specific (Pf+) B cell subsets in this cohort (47). In this study we performed a statistical reanalysis to investigate correlations between antibody dissociation rate constant and various Pf+ B cell subsets, namely, Pf+ IgG MBC, Pf+ non-IgG+ MBC, Pf+ atypical MBC, Pf+ naïve B-cells and Pf+ plasma cells/blasts. Our analysis revealed correlations between antibody dissociation rate constant for MSP2 and atypical memory B cells (MBCs) in infants at 9 months (rho = -0.36, p = 0.042) (Figure 5A). Additionally, in mothers 9 months postpartum, there were correlations between AMA1 and non-IgG MBCs (rho = 0.39, p = 0.024), as well as between AMA1 and Pf+ atypical MBCs (rho = -0.4, p = 0.02 (Figure 5B).
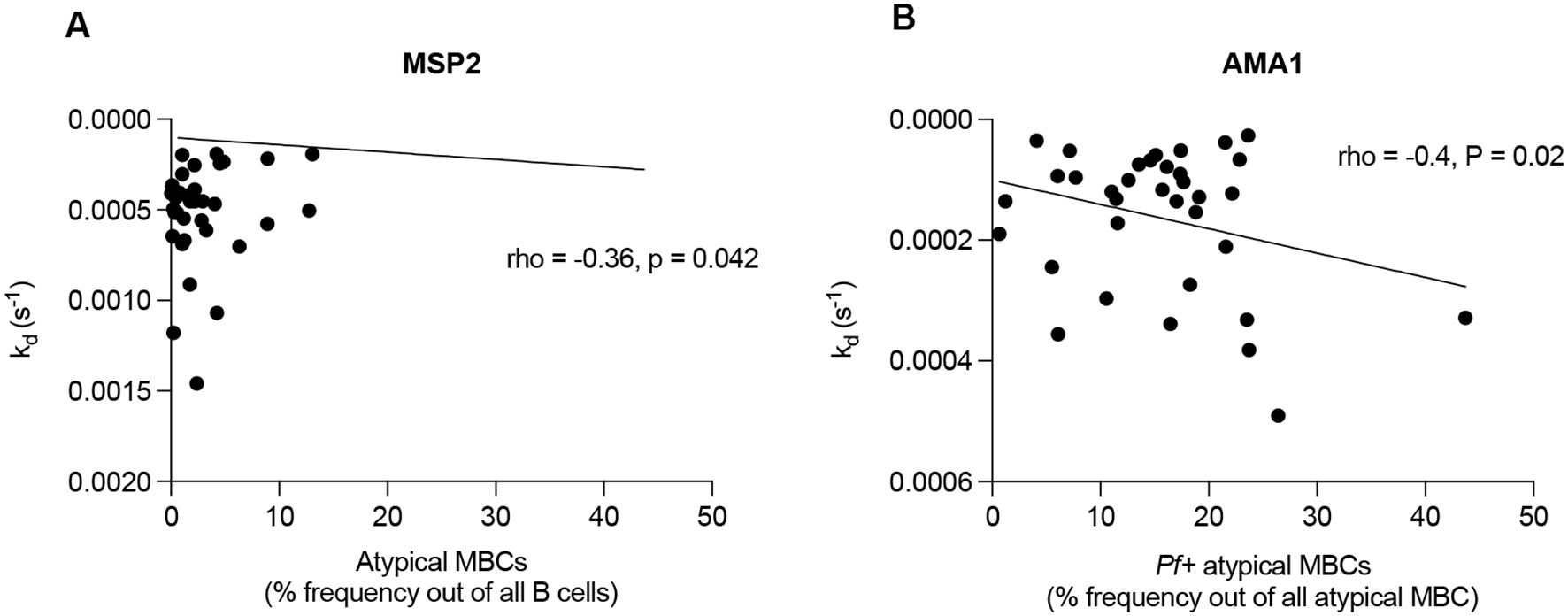
Figure 5. Correlations between antibody affinity and B cell subsets. Figures representing (A) antibody dissociation rate constant for MSP2 and atypical memory B cells (MBCs) in infants at 9 months and (B) antibody dissociation rate constant for AMA1 and Pf+ atypical MBCs in mothers postpartum. Pearson’s coefficient was used to detect correlations between plasma antibody affinity and B cell subsets.
Discussion
In this study, we investigated the development of antibody affinity against three antigen candidates for vaccine development: MSP2, AMA1 and CSP through measurements of dissociation rate constants. Our results show that antibodies targeting AMA1 exhibited the slowest dissociation, followed by CSP and then MSP2. This finding aligns with previous research indicating that antibodies from residents in malaria-endemic areas tend to develop higher affinity antibodies to AMA1 compared to MSP2 (45, 55). In vaccine trials, it has also been shown that maintaining high affinity antibodies against MSP2 over time can be challenging (53). Moreover, the kd values for AMA1 observed in our study were comparable to those of monoclonal antibodies generated from B cells of a donor living in an endemic area (56). The slower dissociation observed for AMA1 may be due to its structural stability, which is attributed to multiple intramolecular disulphide bonds linking its globular domains (57, 58).
In contrast, MSP2, is considered an unstructured protein, which likely contributes to its lower stability. MSP2 has been evaluated in a vaccine trial called Combination B, which included recombinant P. falciparum ring-infected erythrocyte surface antigen alongside MSP1 and MSP2. This trial demonstrated a reduction in parasite density and showed that the vaccine exerted selective pressure on infecting P. falciparum strains (59). A phase 1 trial for the MSP2-C1 vaccine, containing recombinant forms of both msp2 alleles families (3D7 and FC27), found that MSP2-specific antibodies did not directly inhibit parasite growth in vitro. Instead, these antibodies acted via antibody-dependent cellular inhibition (ADCI) to inhibit parasite growth (60), as well as activated complement on the merozoite surface and promoted opsonic phagocytosis (15, 16). Similarly, human anti-AMA1 antibodies have been shown to inhibit P. falciparum in vitro (56). A field trial using the FMP2.1/AS02 (A) vaccine, which contains AMA1 from the 3D7 strain of P. falciparum, did not provide significant protection against clinical malaria but exhibited evidence of strain-specific efficacy (61). These findings suggest that AMA1 could be a valuable component of a multi-component vaccine. We did not observe strong correlations between total levels of anti-P. falciparum antibodies and the specific dissociation rate constants measured. This lack of correlation may reflect insufficient levels of individual specific antibodies, or that the antibody levels evolved differently compared to antibody affinities.
Our study aimed to assess the development of antibody affinity during infancy and compare it with that of mothers. We measured the dissociation rate constant (kd) of antibody binding to immobilized antigens because kd is independent of concentration and reliable values are more easily accessible than affinity, which requires precise knowledge of plasma concentrations for accurate quantification. Moreover, the lifetime of the antigen-antibody complex, i.e. the inverse of the dissociation rate constant, may be the determinant of function. In infants, we observed a significant increase in antibody dissociation rate from AMA1 and MSP2 from birth to six months, likely due to the gradual waning of maternal IgG antibodies transferred transplacentally (62, 63). This aligns with previous findings showing that maternal antibodies against malaria typically wane within the first 3 to 6 months of life (64–66). After six months, antibody dissociation rate constants to AMA1 and MSP2 decreased, probably as a result of exposure to malaria parasites in this endemic area. Concurrently, overall antibody levels also increased. Previous studies have demonstrated that malaria exposure enhances antibody affinities to AMA1 and MSP2 (38, 39), in line with the exposure-dependent nature of malaria immunity (14, 67).
In mothers, antibody dissociation rates from AMA1 and MSP2 were lower than those observed in infants and were the same at delivery and nine months postpartum. This stability reflects the well-documented phenomenon in endemic regions where repeated malaria exposures lead to a plateau in antibody affinity among adults. Interestingly, significant differences in antibody dissociation rate constants between mothers and infants at birth were observed for MSP2 and CSP, with a non-significant trend for AMA1. Mothers exhibited lower dissociation rate constants (higher affinities), suggesting that transplacental transfer does not preferentially transfer the highest affinity antibodies. This difference may be explained by the fact that infants only receive IgG through transplacental transfer, whereas maternal samples contain both IgG and IgM. Anti-malarial IgM could exhibit higher affinity than IgG (68), this difference may reflect the absence of IgM in infants.
Previous studies have shown that high-avidity antibodies for antigens such as tetanus toxoid and type 3 pneumococcal antigen are preferentially transferred through the placenta. This selectivity may depend on the antigen rather than the degree of antigenic exposure (69). Similarly, a study on pertussis toxin antibodies confirmed preferential placental transfer of high-avidity antibodies but noted decreased transfer in HIV-positive mothers (70). In our cohort, none of the mothers were HIV-positive. Additionally, the transplacental transfer of IgG3 has been associated with a reduced risk of clinical malaria (71, 72). However, preferential transfer generally follows the order: IgG1 >IgG4 >IgG3 >IgG2 (73). This raises the possibility that high-affinity antibodies for MSP2 and CSP may belong to subclasses that are less efficiently transferred. Selective transfer mechanisms or other unknown factors may influence the quality and subclass distribution of antibodies passed from mother to infant. To help elucidate the affinity and functional relevance of antibodies, IgG subclass-specific affinity assays could be performed, which would include functional assays such as opsonic phagocytosis and parasite growth inhibition to link affinity maturation to protective efficacy. Previous studies have shown that high-affinity antibodies against AMA1 and MSP2 correlate with greater opsonic and inhibitory activity (15, 74). Even though we did not include such assays in this study, our findings provide a basis for future research to investigate whether the observed affinity profiles, particularly for AMA1 and MSP2, translate into functional protection.
In comparison to the observed changes in antibody dissociation rate constants for AMA1 and MSP2 in infants during the 9 months study, no significant changes in antibody dissociation rate constant for CSP were observed, either in infants or in adults. Previous research has shown that most natural antibody responses to CSP target the central NANP repeat region (28, 75–77), which is intrinsically disordered (78) and tends to elicit low affinity antibodies (79), as well as the C-terminal region (80). Moreover, earlier studies have highlighted challenges in maintaining a robust response for CSP over time and in achieving high efficacy (81–83). We speculate that the difficulty in inducing high-affinity antibodies may contribute to CSP’s modest success as a vaccine candidate. Even in mothers, antibodies against CSP showed higher dissociation rate constant (lower affinity) compared to AMA1, although they were similar to those against MSP2. This suggests that the structural characteristics of CSP may be of importance when selecting and combining antigens for optimal vaccine design.
Despite variations in antibody affinities for different antigens, the responses in mothers remained the same at the time of delivery and nine months later for all tested antigens. This implies that in adults, each of the three antigens elicits an immunological response from germinal center B cells that have undergone both somatic hypermutation and clonal selection (84, 85). These activated B cells appear to reach a threshold level of antigen-specific affinity maturation, beyond which no further increase in affinity is observed (38). This phenomenon has also been observed in other diseases, such as vesicular stomatitis virus (86), and in vaccination against meningococcus using a recombinant vaccine, particularly after the third and fourth doses (87).
It has been shown that both classical and atypical memory B cells generated following natural P. falciparum infection produce neutralizing antibodies against blood stage P. falciparum parasites. However, only atypical memory B cells appear to show evidence for active antibody secretion (88). These atypical memory B cells are largely maintained by chronic malaria exposure (89–91), with subsets such as IgD+IgMlo and IgD-IgG+ displaying a high threshold for antigen avidity during activation. This mechanism likely helps prevent autoimmune responses during chronic malaria (92)., highlighting the important role of these cells in the development of malaria immunity (47, 93). Furthermore, we identified positive correlations between atypical memory B cells and antibody affinities against the tested parasite antigens, emphasizing their significance in malaria immunity. This finding is worth considering in future vaccine designs aimed at improving efficacy, especially given the modest efficacy observed with current vaccines like RTS, S and R21.
In conclusion, our study demonstrates a distinct and gradual development of antibody affinity for the merozoite antigens AMA1 and MSP2 during the first nine months of life, but not for the sporozoite antigen CSP. In adults, antibody affinity was relatively the same at delivery and nine months post-partum. Additionally, we identified correlations between antibody dissociation rate constant and atypical memory B cells. These findings contribute to our understanding of naturally acquired immunity to malaria and provide valuable insights for selecting optimal antigen candidates for vaccine development in future clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Research and Ethics Committee (SOMREC) of Makerere University School of Medicine, The Uganda National Council of Science and Technology (approval 2011–114), Regionala Etikprövningsnämnden in Stockholm, Sweden 2014/478-32. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. RI: Data curation, Investigation, Writing – review & editing. SK: Data curation, Investigation, Writing – review & editing. JB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Validation, Writing – review & editing. DD: Investigation, Methodology, Resources, Writing – review & editing. SM: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Writing – review & editing. KP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work received funding from Sida and a grant, number D43TW010132, supported by Office of the Director, National Institutes of Health (OD), National Institute of Dental & Craniofacial Research (NIDCR), National Institute of Neurological Disorders and Stroke (NINDS), National Heart, Lung, and Blood Institute (NHLBI), Fogarty International Center (FIC), National Institute on Minority Health and Health Disparities (NIMHD) and ALF (Region Skåne/Lund University). The SPR instrumentation was funded by the Swedish Research Council (VR). JB is supported by the National Health and Medical Research Council of Australia.
Acknowledgments
We are grateful to all the study participants. We thank all the members of the study team for their tireless effort and excellent work. We thank Robin Anders (LaTrobe University) for providing recombinant MSP2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1562671/full#supplementary-material
References
1. World Health Organization. World Malaria Report 2024. Geneva: World Health Organization (2024). Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (Accessed December, 2024).
2. Marsh K and Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. (2006) 28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x
3. Cohen S, Mc GI, and Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. (1961) 192:733–7. doi: 10.1038/192733a0
4. Mayxay M, Chotivanich K, Pukrittayakamee S, Newton P, Looareesuwan S, and White NJ. Contribution of humoral immunity to the therapeutic response in falciparum malaria. Am J Trop Med Hyg. (2001) 65:918–23. doi: 10.4269/ajtmh.2001.65.918
5. Felger I, Marshal VM, Reeder JC, Hunt JA, Mgone CS, and Beck HP. Sequence diversity and molecular evolution of the merozoite surface antigen 2 of Plasmodium falciparum. J Mol Evol. (1997) 45:154–60. doi: 10.1007/PL00006215
6. Hoffmann EH, Malafronte RS, Moraes-Avila SL, Osakabe AL, Wunderlich G, Durham AM, et al. Origins of sequence diversity in the malaria vaccine candidate merozoite surface protein-2 (MSP-2) in Amazonian isolates of Plasmodium falciparum. Gene. (2006) 376:224–30. doi: 10.1016/j.gene.2006.03.011
7. Ferreira MU and Hartl DL. Plasmodium falciparum: worldwide sequence diversity and evolution of the malaria vaccine candidate merozoite surface protein-2 (MSP-2). Exp Parasitol. (2007) 115:32–40. doi: 10.1016/j.exppara.2006.05.003
8. Richards JS and Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. (2009) 87:377–90. doi: 10.1038/icb.2009.27
9. Kusi KA, Remarque EJ, Riasat V, Walraven V, Thomas AW, Faber BW, et al. Safety and immunogenicity of multi-antigen AMA1-based vaccines formulated with CoVaccine HT and Montanide ISA 51 in rhesus macaques. Malar J. (2011) 10:182. doi: 10.1186/1475-2875-10-182
10. Drew DR, Hodder AN, Wilson DW, Foley M, Mueller I, Siba PM, et al. Defining the antigenic diversity of Plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PLoS One. (2012) 7:e51023. doi: 10.1371/journal.pone.0051023
11. Adda CG, MacRaild CA, Reiling L, Wycherley K, Boyle MJ, Kienzle V, et al. Antigenic characterization of an intrinsically unstructured protein, Plasmodium falciparum merozoite surface protein 2. Infect Immun. (2012) 80:4177–85. doi: 10.1128/IAI.00665-12
12. Boyle MJ, Langer C, Chan JA, Hodder AN, Coppel RL, Anders RF, et al. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun. (2014) 82:924–36. doi: 10.1128/IAI.00866-13
13. Langhorne J, Ndungu FM, Sponaas AM, and Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. (2008) 9:725–32. doi: 10.1038/ni.f.205
14. Doolan DL, Dobaño C, and Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. (2009) 22:13–36. doi: 10.1128/CMR.00025-08
15. Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. (2015) 42:580–90. doi: 10.1016/j.immuni.2015.02.012
16. Feng G, Boyle MJ, Cross N, Chan JA, Reiling L, Osier F, et al. Human immunization with a polymorphic malaria vaccine candidate induced antibodies to conserved epitopes that promote functional antibodies to multiple parasite strains. J Infect Dis. (2018) 218:35–43. doi: 10.1093/infdis/jiy170
17. Narum DL and Thomas AW. Differential localization of full-length and processed forms of PF83/AMA-1 an apical membrane antigen of Plasmodium falciparum merozoites. Mol Biochem Parasitol. (1994) 67:59–68. doi: 10.1016/0166-6851(94)90096-5
18. Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. (2004) 279:9490–6. doi: 10.1074/jbc.M311331200
19. Uddin N, Hoessli DC, Butt A, Kaleem A, Iqbal Z, Afzal I, et al. O-GlcNAc modification of the anti-malarial vaccine candidate PfAMA1: in silico-defined structural changes and potential to generate a better vaccine. Mol Biol Rep. (2012) 39:4663–72. doi: 10.1007/s11033-011-1258-4
20. Espinosa DA, Gutierrez GM, Rojas-Lopez M, Noe AR, Shi L, Tse SW, et al. Proteolytic cleavage of the plasmodium falciparum circumsporozoite protein is a target of protective antibodies. J Infect Dis. (2015) 212:1111–9. doi: 10.1093/infdis/jiv154
21. Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. (2009) 200:337–46. doi: 10.1086/600120
22. Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS One. (2009) 4:e6465. doi: 10.1371/journal.pone.0006465
23. Datoo MS, Natama HM, Somé A, Bellamy D, Traoré O, Rouamba T, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. (2022) 22:1728–36. doi: 10.1016/S1473-3099(22)00442-X
24. Datoo MS, Dicko A, Tinto H, Ouédraogo JB, Hamaluba M, Olotu A, et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet. (2024) 403:533–44. doi: 10.1016/S0140-6736(23)02511-4
25. Cawlfield A, Genito CJ, Beck Z, Bergmann-Leitner ES, Bitzer AA, Soto K, et al. Safety, toxicity and immunogenicity of a malaria vaccine based on the circumsporozoite protein (FMP013) with the adjuvant army liposome formulation containing QS21 (ALFQ). Vaccine. (2019) 37:3793–803. doi: 10.1016/j.vaccine.2019.05.059
26. Almeida MEM, Vasconcelos MGS, Tarrago AM, and Mariuba LAM. Circumsporozoite Surface Protein-based malaria vaccines: a review. Rev Inst Med Trop Sao Paulo. (2021) 63:e11. doi: 10.1590/s1678-9946202163011
27. Julien JP and Wardemann H. Antibodies against Plasmodium falciparum malaria at the molecular level. Nat Rev Immunol. (2019) 19:761–75. doi: 10.1038/s41577-019-0209-5
28. Dobano C, Sanz H, Sorgho H, Dosoo D, Mpina M, Ubillos I, et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun. (2019) 10:2174. doi: 10.1038/s41467-019-10195-z
29. Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med. (2015) 373:2025–37. doi: 10.1056/NEJMoa1505819
30. Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, and Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. (2010) 6:90–6. doi: 10.4161/hv.6.1.9677
31. Fries LF, Gordon DM, Schneider I, Beier JC, Long GW, Gross M, et al. Safety, immunogenicity, and efficacy of a Plasmodium falciparum vaccine comprising a circumsporozoite protein repeat region peptide conjugated to Pseudomonas aeruginosa toxin A. Infect Immun. (1992) 60:1834–9. doi: 10.1128/iai.60.5.1834-1839.1992
32. Kurtovic L, Behet MC, Feng G, Reiling L, Chelimo K, Dent AE, et al. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med. (2018) 16:61. doi: 10.1186/s12916-018-1054-2
33. Feng G, Wines BD, Kurtovic L, Chan JA, Boeuf P, Mollard V, et al. Mechanisms and targets of Fcγ-receptor mediated immunity to malaria sporozoites. Nat Commun. (2021) 12:1742. doi: 10.1038/s41467-021-21998-4
34. Kisalu NK, Idris AH, Weidle C, Flores-Garcia Y, Flynn BJ, Sack BK, et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med. (2018) 24:408–16. doi: 10.1038/nm.4512
35. Laurens MB. The promise of a malaria vaccine-are we closer? Annu Rev Microbiol. (2018) 72:273–92. doi: 10.1146/annurev-micro-090817-062427
36. Courtin D, Oesterholt M, Huismans H, Kusi K, Milet J, Badaut C, et al. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One. (2009) 4:e7590. doi: 10.1371/journal.pone.0007590
37. Good-Jacobson KL and Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. (2010) 185:3117–25. doi: 10.4049/jimmunol.1001155
38. Tijani MK, Reddy SB, Langer C, Beeson JG, Wahlgren M, Nwuba RI, et al. Factors influencing the induction of high affinity antibodies to Plasmodium falciparum merozoite antigens and how affinity changes over time. Sci Rep. (2018) 8:9026. doi: 10.1038/s41598-018-27361-w
39. Leoratti FM, Durlacher RR, Lacerda MV, Alecrim MG, Ferreira AW, Sanchez MC, et al. Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar J. (2008) 7:186. doi: 10.1186/1475-2875-7-186
40. Tutterrow YL, Salanti A, Avril M, Smith JD, Pagano IS, Ako S, et al. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS One. (2012) 7:e40049. doi: 10.1371/journal.pone.0040049
41. Akpogheneta OJ, Dunyo S, Pinder M, and Conway DJ. Boosting antibody responses to Plasmodium falciparum merozoite antigens in children with highly seasonal exposure to infection. Parasite Immunol. (2010) 32:296–304. doi: 10.1111/j.1365-3024.2009.01193.x
42. Ibison F, Olotu A, Muema DM, Mwacharo J, Ohuma E, Kimani D, et al. Lack of avidity maturation of merozoite antigen-specific antibodies with increasing exposure to Plasmodium falciparum amongst children and adults exposed to endemic malaria in Kenya. PLoS One. (2012) 7:e52939. doi: 10.1371/journal.pone.0052939
43. Olotu A, Clement F, Jongert E, Vekemans J, Njuguna P, Ndungu FM, et al. Avidity of anti-circumsporozoite antibodies following vaccination with RTS,S/AS01E in young children. PLoS One. (2014) 9:e115126. doi: 10.1371/journal.pone.0115126
44. Thai E, Costa G, Weyrich A, Murugan R, Oyen D, Flores-Garcia Y, et al. A high-affinity antibody against the CSP N-terminal domain lacks Plasmodium falciparum inhibitory activity. J Exp Med. (2020) 217:e20200061. doi: 10.1084/jem.20200061
45. Reddy SB, Anders RF, Beeson JG, Farnert A, Kironde F, Berenzon SK, et al. High affinity antibodies to Plasmodium falciparum merozoite antigens are associated with protection from malaria. PLoS One. (2012) 7:e32242. doi: 10.1371/journal.pone.0032242
46. Hearty S, Conroy PJ, Ayyar BV, Byrne B, and O’Kennedy R. Surface plasmon resonance for vaccine design and efficacy studies: recent applications and future trends. Expert Rev Vaccines. (2010) 9:645–64. doi: 10.1586/erv.10.52
47. Lugaajju A, Reddy SB, Wahlgren M, Kironde F, and Persson KE. Development of Plasmodium falciparum specific naive, atypical, memory and plasma B cells during infancy and in adults in an endemic area. Malar J. (2017) 16:37. doi: 10.1186/s12936-017-1697-z
48. International UBoSaI. Uganda Malaria Indicator Survey 2014-15. Kampala, Uganda and Rockville, Maryland, USA: Uganda Bureau of Statistics (UBOS) and ICF International (2015).
49. Drew DR, Wilson DW, Weiss GE, Yeoh LM, G. Henshall I, Crabb BS, et al. Defining species-specific and conserved interactions of apical membrane protein 1 during erythrocyte invasion in malaria to inform multi-species vaccines. Cell Mol Life Sci. (2023) 80:74. doi: 10.1007/s00018-023-04712-z
50. Kurtovic L, Feng G, Hysa A, Haghiri A, O’Flaherty K, Wines BD, et al. Antibody mechanisms of protection against malaria in RTS,S-vaccinated children: a post-hoc serological analysis of phase 2 trial. Lancet Microbe. (2024) 5. doi: 10.1016/S2666-5247(24)00130-7
51. Kearney EA, Amratia P, Kang SY, Agius PA, Alene KA, O’Flaherty K, et al. Geospatial joint modeling of vector and parasite serology to microstratify malaria transmission. Proc Natl Acad Sci. (2024) 121:e2320898121. doi: 10.1073/pnas.2320898121
52. McCarthy JS, Sekuloski S, Griffin PM, Elliott S, Douglas N, Peatey C, et al. A pilot randomised trial of induced blood-stage plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One. (2011) 6:e21914. doi: 10.1371/journal.pone.0021914
53. Persson KEM, Horton JL, Kurtovic L, McCarthy JS, Anders RF, and Beeson JG. Declining antibody affinity over time after human vaccination with a plasmodium falciparum merozoite vaccine candidate. J Infect Dis. (2024) 230:e753–e7. doi: 10.1093/infdis/jiae259
54. Mortazavi SE, Lugaajju A, Kaddumukasa M, Tijani MK, Kironde F, and Persson KEM. Osteopontin and malaria: no direct effect on parasite growth, but correlation with P. falciparum-specific B cells and BAFF in a malaria endemic area. BMC Microbiol. (2021) 21:307. doi: 10.1186/s12866-021-02368-y
55. Reddy SB, Anders RF, Cross N, Mueller I, Senn N, Stanisic DI, et al. Differences in affinity of monoclonal and naturally acquired polyclonal antibodies against Plasmodium falciparum merozoite antigens. BMC Microbiol. (2015) 15:133. doi: 10.1186/s12866-015-0461-1
56. Maskus DJ, Krolik M, Bethke S, Spiegel H, Kapelski S, Seidel M, et al. Characterization of a novel inhibitory human monoclonal antibody directed against Plasmodium falciparum Apical Membrane Antigen 1. Sci Rep. (2016) 6:39462. doi: 10.1038/srep39462
57. Crewther PE, Matthew ML, Flegg RH, and Anders RF. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect Immun. (1996) 64:3310–7. doi: 10.1128/iai.64.8.3310-3317.1996
58. Cortes A, Mellombo M, Masciantonio R, Murphy VJ, Reeder JC, and Anders RF. Allele specificity of naturally acquired antibody responses against Plasmodium falciparum apical membrane antigen 1. Infect Immun. (2005) 73:422–30. doi: 10.1128/IAI.73.1.422-430.2005
59. Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. (2002) 185:820–7. doi: 10.1086/339342
60. McCarthy JS, Marjason J, Elliott S, Fahey P, Bang G, Malkin E, et al. A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide(R) ISA 720. PLoS One. (2011) 6:e24413. doi: 10.1371/journal.pone.0024413
61. Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. (2011) 365:1004–13. doi: 10.1056/NEJMoa1008115
62. Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol. (2014) 5:446. doi: 10.3389/fimmu.2014.00446
63. Simon AK, Hollander GA, and McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. (2015) 282:20143085. doi: 10.1098/rspb.2014.3085
64. Achidi EA, Perlmann H, Salimonu LS, Perlmann P, Walker O, and Asuzu MC. A longitudinal study of seroreactivities to Plasmodium falciparum antigens in Nigerian infants during their first year of life. Acta Trop. (1995) 59:173–83. doi: 10.1016/0001-706X(95)00076-Q
65. Campbell CC, Martinez JM, and Collins WE. Seroepidemiological studies of malaria in pregnant women and newborns from coastal El Salvador. Am J Trop Med Hyg. (1980) 29:151–7. doi: 10.4269/ajtmh.1980.29.151
66. Biggar RJ, Collins WE, and Campbell CC. The serological response to primary malaria infection in urban Ghanaian infants. Am J Trop Med Hyg. (1980) 29:720–4. doi: 10.4269/ajtmh.1980.29.720
67. Beadle C, McElroy PD, Oster CN, Beier JC, Oloo AJ, Onyango FK, et al. Impact of transmission intensity and age on Plasmodium falciparum density and associated fever: implications for malaria vaccine trial design. J Infect Dis. (1995) 172:1047–54. doi: 10.1093/infdis/172.4.1047
68. Boyle MJ, Chan JA, Handayuni I, Reiling L, Feng G, Hilton A, et al. IgM in human immunity to Plasmodium falciparum malaria. Sci Adv. (2019) 5:eaax4489. doi: 10.1126/sciadv.aax4489
69. Avanzini MA, Pignatti P, Chirico G, Gasparoni A, Jalil F, and Hanson LA. Placental transfer favours high avidity IgG antibodies. Acta Paediatr. (1998) 87:180–5. doi: 10.1111/j.1651-2227.1998.tb00972.x
70. Taton M, Willems F, Widomski C, Georges D, Martin C, Jiang Y, et al. HIV-related immune activation attenuates polyfunctional IgG and memory B-cell responses to Tdap immunization during pregnancy. EBioMedicine. (2024) 104:105179. doi: 10.1016/j.ebiom.2024.105179
71. Roussilhon C, Oeuvray C, Müller-Graf C, Tall A, Rogier C, Trape JF, et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. (2007) 4:e320. doi: 10.1371/journal.pmed.0040320
72. Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. (2009) 77:1165–74. doi: 10.1128/IAI.01129-08
73. Clements T, Rice TF, Vamvakas G, Barnett S, Barnes M, Donaldson B, et al. Update on transplacental transfer of igG subclasses: impact of maternal and fetal factors. Front Immunol. (2020) 11:1920. doi: 10.3389/fimmu.2020.01920
74. Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med. (2014) 12:108. doi: 10.1186/1741-7015-12-108
75. Kastenmuller K, Espinosa DA, Trager L, Stoyanov C, Salazar AM, Pokalwar S, et al. Full-length Plasmodium falciparum circumsporozoite protein administered with long-chain poly(I.C) or the Toll-like receptor 4 agonist glucopyranosyl lipid adjuvant-stable emulsion elicits potent antibody and CD4+ T cell immunity and protection in mice. Infect Immun. (2013) 81:789–800. doi: 10.1128/IAI.01108-12
76. Bongfen SE, Ntsama PM, Offner S, Smith T, Felger I, Tanner M, et al. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine. (2009) 27:328–35. doi: 10.1016/j.vaccine.2008.09.097
77. Murugan R, Scally SW, Costa G, Mustafa G, Thai E, Decker T, et al. Evolution of protective human antibodies against Plasmodium falciparum circumsporozoite protein repeat motifs. Nat Med. (2020) 26:1135–45. doi: 10.1038/s41591-020-0881-9
78. Patra AP, Sharma S, and Ainavarapu SR. Force spectroscopy of the plasmodium falciparum vaccine candidate circumsporozoite protein suggests a mechanically pliable repeat region. J Biol Chem. (2017) 292:2110–9. doi: 10.1074/jbc.M116.754796
79. Oyen D, Torres JL, Wille-Reece U, Ockenhouse CF, Emerling D, Glanville J, et al. Structural basis for antibody recognition of the NANP repeats in Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci U.S.A. (2017) 114:E10438–E45. doi: 10.1073/pnas.1715812114
80. Kurtovic L, Drew DR, Dent AE, Kazura JW, and Beeson JG. Antibody targets and properties for complement-fixation against the circumsporozoite protein in malaria immunity. Front Immunol. (2021) 12:775659. doi: 10.3389/fimmu.2021.775659
81. Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young african children. N Engl J Med. (2016) 374:2519–29. doi: 10.1056/NEJMoa1515257
82. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. (2015) 386:31–45. doi: 10.1016/S0140-6736(15)60721-8
83. Mugo RM, Mwai K, Mwacharo J, Shee FM, Musyoki JN, Wambua J, et al. Seven-year kinetics of RTS, S/AS01-induced anti-CSP antibodies in young Kenyan children. Malar J. (2021) 20:452. doi: 10.1186/s12936-021-03961-2
84. Victora GD and Nussenzweig MC. Germinal centers. Annu Rev Immunol. (2012) 30:429–57. doi: 10.1146/annurev-immunol-020711-075032
85. Murugan R, Buchauer L, Triller G, Kreschel C, Costa G, Pidelaserra Marti G, et al. Clonal selection drives protective memory B cell responses in controlled human malaria infection. Sci Immunol. (2018) 3:eaap8029. doi: 10.1126/sciimmunol.aap8029
86. Bachmann MF, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, et al. The role of antibody concentration and avidity in antiviral protection. Science. (1997) 276:2024–7. doi: 10.1126/science.276.5321.2024
87. Longworth E, Borrow R, Goldblatt D, Balmer P, Dawson M, Andrews N, et al. Avidity maturation following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine in UK infants. Vaccine. (2002) 20:2592–6. doi: 10.1016/S0264-410X(02)00151-2
88. Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, et al. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med. (2013) 210:389–99. doi: 10.1084/jem.20121970
89. Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. (2009) 183:2176–82. doi: 10.4049/jimmunol.0901297
90. Weiss GE, Clark EH, Li S, Traore B, Kayentao K, Ongoiba A, et al. A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PLoS One. (2011) 6:e15983. doi: 10.1371/journal.pone.0015983
91. Ayieko C, Maue AC, Jura WG, Noland GS, Ayodo G, Rochford R, et al. Changes in B Cell Populations and Merozoite Surface Protein-1-Specific Memory B Cell Responses after Prolonged Absence of Detectable P. falciparum Infection. PLoS One. (2013) 8:e67230. doi: 10.1371/journal.pone.0067230
92. Holla P, Dizon B, Ambegaonkar AA, Rogel N, Goldschmidt E, Boddapati AK, et al. Shared transcriptional profiles of atypical B cells suggest common drivers of expansion and function in malaria, HIV, and autoimmunity. Sci Adv. (2021) 7:eabg8384. doi: 10.1126/sciadv.abg8384
Keywords: malaria, falciparum, affinity, antibodies, CSP, AMA1, MSP2
Citation: Lugaajju A, Idro R, Kiwuwa S, Beeson JG, Drew DR, Mortazavi SE, Linse S and Persson KEM (2025) Development of high affinity antibodies to Plasmodium falciparum merozoite and sporozoite antigens during infancy and adulthood. Front. Immunol. 16:1562671. doi: 10.3389/fimmu.2025.1562671
Received: 17 January 2025; Accepted: 18 June 2025;
Published: 02 July 2025.
Edited by:
Wang Nguitragool, Mahidol University, ThailandReviewed by:
Maria Elizbeth Alvarez Sanchez, Universidad Autónoma de la Ciudad de México, MexicoThitinan Kitisin, Mahidol University, Thailand
Copyright © 2025 Lugaajju, Idro, Kiwuwa, Beeson, Drew, Mortazavi, Linse and Persson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristina E. M. Persson, a3Jpc3RpbmEucGVyc3NvbkBtZWQubHUuc2U=
 Allan Lugaajju
Allan Lugaajju Richard Idro
Richard Idro Stephen Kiwuwa1
Stephen Kiwuwa1 Susanne E. Mortazavi
Susanne E. Mortazavi Sara Linse
Sara Linse Kristina E. M. Persson
Kristina E. M. Persson