- 1Division of Gastrointestinal Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China
- 3Gastric Cancer Center, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China
- 4Department of Medical Discipline Construction, West China Hospital, Sichuan University, Chengdu, China
Introduction: Transversus abdominis plane block (TAPB) is now commonly administered for postoperative pain control and reduced opioid consumption in patients undergoing major colorectal surgeries, such as colorectal cancer, diverticular disease, and inflammatory bowel disease resection. However, there remain several controversies about the effectiveness and safety of laparoscopic TAPB compared to ultrasound-guided TAPB. Therefore, the aim of this study is to integrate both direct and indirect comparisons to identify a more effective and safer TAPB approach.
Materials and methods: Systematic electronic literature surveillance will be performed in the PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov databases for eligible studies through July 31, 2023. The Cochrane Risk of Bias version 2 (RoB 2) and Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tools will be applied to scrutinize the methodological quality of the selected studies. The primary outcomes will include (1) opioid consumption at 24 hours postoperatively and (2) pain scores at 24 hours postoperatively both at rest and at coughing and movement according to the numerical rating scale (NRS). Additionally, the probability of TAPB-related adverse events, overall postoperative 30-day complications, postoperative 30-day ileus, postoperative 30-day surgical site infection, postoperative 7-day nausea and vomiting, and length of stay will be analyzed as secondary outcome measures. The findings will be assessed for robustness through subgroup analyses and sensitivity analyses. Data analyses will be performed using RevMan 5.4.1 and Stata 17.0. P value of less than 0.05 will be defined as statistically significant. The certainty of evidence will be examined via the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) working group approach.
Ethics and dissemination: Owing to the nature of the secondary analysis of existing data, no ethical approval will be required. Our meta-analysis will summarize all the available evidence for the effectiveness and safety of TAPB approaches for minimally invasive colorectal surgery. High-quality peer-reviewed publications and presentations at international conferences will facilitate disseminating the results of this study, which are expected to inform future clinical trials and help anesthesiologists and surgeons determine the optimal tailored clinical practice for perioperative pain management.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=281720, identifier (CRD42021281720).
1 Introduction
Recently, minimally invasive surgery (MIS) has been recommended to treat colorectal diseases, such as colorectal cancer, inflammatory bowel disease, and diverticular disease, because of its equivalent efficacy and improved functional recovery (1–3). Compared to traditional protocols, enhanced recovery after surgery (ERAS) pathways could significantly shorten the length of hospital stay (LOS) and reduce the healthcare costs without compromising surgical outcomes (4–6). Acute postoperative pain, however, remains the most common concern of ERAS. Meanwhile, regular administration of opioids is associated with postoperative ileus (POI), postoperative nausea and vomiting (PONV), delayed mobilization, acute urinary retention, and early-term somnolence and delirium (7). Despite the increasing popularity of the conception of opioid-sparing multimodal analgesia, consensus on optimal pain management after MIS is lacking.
Transversus abdominis plane block (TAPB) as a type of local anesthesia involves the injection of a local anesthetic between the transversus abdominis and internal oblique muscles to infiltrate the segmental nerves at the level of T8-L1 (8, 9). TAPB is now commonly employed during laparoscopic colorectal surgery and has proven to be effective in reducing postoperative opioid consumption (10). In the ERAS Society Guidelines for Perioperative Care in Elective Colorectal Surgery 2018, the use of TAPB is strongly recommended instead of epidural analgesia in colorectal MIS (11). Both laparoscopic (Lap-) and ultrasound-guided (US-) TAPB have allowed to reduce the risk of peritoneal penetration and facilitate the accurate identification of the tissue plane (12).
Currently, training in the use of ultrasonography among anesthesiologists is commonplace in tertiary referral centers (13). Characterized by ability to perform dynamic maneuvers and assess long segments of nerves, lack of radiation and contraindications, and portability, ultrasonography is recognized as one of the optimal imaging modalities for peripheral nerves (14), which contributes to its widespread application in perioperative nerve blocks. Conversely, due to additional human, time, and economic costs, techniques of ultrasound-guided nerve blocks might not be available in the primary hospitals. Furthermore, despite the guidance of ultrasound, procedure-related inadvertent visceral injury still should not be ignored (15, 16). Lap-TAPB can seemly be a potential alternative to reduce the waste of healthcare resource. Though visualization of laparoscopy minimizes intraperitoneal injection and visceral injury originated from peritoneal penetration, the precise positioning of the nerves and planes can be compromised by Lap-TAPB compared to US-TAPB (17).
The existing systematic reviews generally aimed to assess the differences between TAPB and no-TAPB locoregional analgesia or placebo control in colorectal surgery (18–21). Focusing on not all colorectal MIS but only laparoscopic colorectal surgery, a recently published meta-analysis cannot provide a convincing conclusion owing to the small sample size (3 studies, 219 patients) (22). Above all, there remain several controversies about the effectiveness and safety of Lap-TAPB compared with US-TAPB, and high-quality evidence is needed to guide individualized clinical practice (23–25). We hypothesize that surgeon-performed Lap-TAPB would be non-inferior to anesthesiologist-delivered US-TAPB. To verify this, we conduct the present meta-analysis to compare the effectiveness and safety of the two specific TAPB approaches for postoperative analgesia in colorectal MIS.
2 Materials and methods
On October 26, 2021, the present meta-analysis protocol was prospectively registered at the International Prospective Register of Systematic Reviews (PROSPERO) (registration ID: CRD42021281720). Besides, the protocol follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) 2015 checklist (Supplementary material) (26). The main text of our future meta-analysis will adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Collaboration’s standardized methodology (27, 28).
2.1 Eligibility criteria
2.1.1 Inclusion criteria
Detailed inclusion criteria will be developed using the PICOS description model (participants, intervention, controls, outcome measures, and study design) (29).
2.1.1.1 Type of participants (P)
Patients with cancer, inflammatory bowel disease, diverticular disease, or other diseases scheduled to undergo colorectal resection MIS (including laparoscopic, hand-assisted laparoscopic, robot-assisted, and trans-anal) will be included in this study. Other restrictions consist of age (≥ 18 years old) and American Society of Anesthesiologists physical status score (I - III).
2.1.1.2 Type of interventions (I)
Lap-TAPB performed by surgeons at the beginning or end of surgeries is administered by a traditional transcutaneous or intraperitoneal approach as the intervention. Under laparoscopic guidance, a bilateral TAPB with the “double pops” technique is performed using a total of 40 mL of local anesthetic. Particularly, a needle of 18 gauge is inserted under direct vision at the midpoint of the midaxillary line between the iliac crest and the lower costal margin, and then 2 mL of normal saline is injected to identify its position. The preplanned amount of local anesthetic will be injected at the same point after a bulge formation as a result of the internally pushed transversus abdominis muscle and peritoneum. The contralateral abdominal wall is treated with the same technique.
2.1.1.3 Type of controls (C)
US-TAPB delivered by anesthesiologists prior to surgery was set as the control.
2.1.1.4 Type of outcomes (O)
2.1.1.4.1 Primary outcomes
• opioid consumption at 24 hours postoperatively;
• pain scores at 24 hours postoperatively both at rest and at coughing and movement according to the numerical rating scale (NRS).
2.1.1.4.2 Secondary outcomes
• TAPB-related adverse events;
• overall postoperative 30-day complications (Clavien-Dindo classification grade II or higher) (30);
• postoperative 30-day POI;
• postoperative 30-day surgical site infection (SSI);
• postoperative 7-day PONV;
• LOS.
2.1.1.5 Type of study design (S)
Prospective randomized controlled trials (RCTs), quasi-RCTs (e.g., participants were assigned to groups according to alternate days of the week), case-control studies and cohort studies in which at least one outcome of interest was evaluated will be included.
2.1.2 Exclusion criteria
• patients undergoing only laparoscopic exploration, bypass or diverting ostomy, conversion to laparotomy, or without assignment to Lap-TAPB and US-TAPB groups;
• animal subjects;
• conference abstracts, case reports, letters, editorials, reviews, or non-controlled trials without available data;
• previously published literature or with overlapping data of the same clinical trial;
• studies with missing or insufficient data after contacting corresponding authors;
• literature in non-English languages.
2.2 Sources of information and strategies for searching
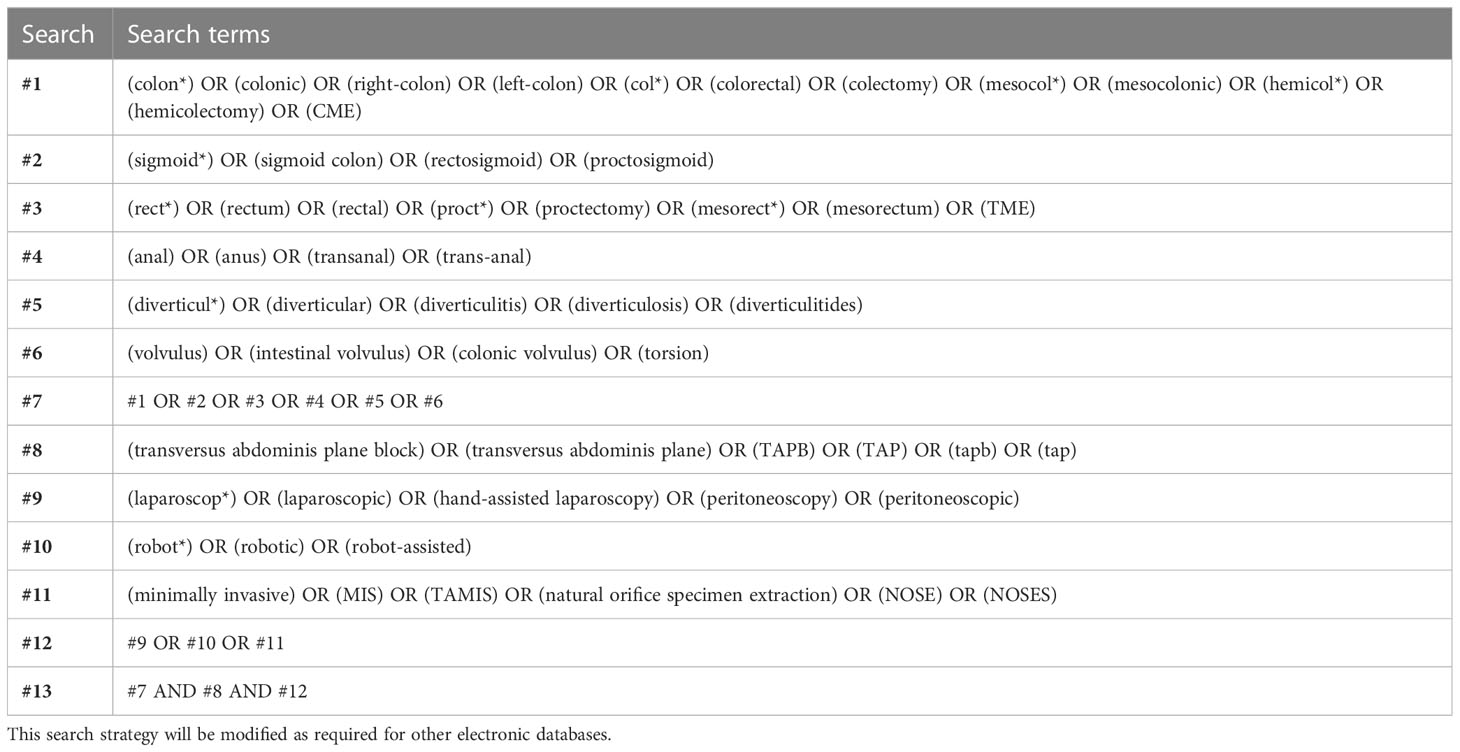
Systematic electronic literature surveillance will be conducted in the PubMed, Embase (OVID interface), and Cochrane Central Register of Controlled Trials (CENTRAL) databases utilizing TAPB-related text words and medical subject headings (MeSH) to obtain relevant studies published through July 31, 2023. All references of the included literature will be further retrieved to identify potential eligibility. Identifying some relevant studies through hand searching is planned to supplement searching whenever necessary. To review trials in progress, the ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP) databases will also be searched. The search will be confined to human subjects and the English language. The detailed and specific search strategy and syntax for the PubMed database are formulated (Table 1).
2.3 Study identification and data management
Records obtained following the search strategy will be collected and imported in Mendeley software (RELX Group, Amsterdam, Netherlands). A team of three reviewers (WY, TY, and ZC) will independently screen the searched titles and abstracts against the eligibility criteria. For potentially eligible studies, full texts will be reviewed thoroughly. If there are a series of reports on one clinical trial, the latest publication containing the most sufficient data is suitable for inclusion. For further information on eligibility, corresponding authors of the studies will be contacted via e-mail whenever necessary. Thereafter, the above-mentioned three reviewers will reevaluate the entire texts post initial identification and document the reasons for some records to be excluded. All disputes among the three reviewers in this process will be settled via a consultation with a senior author (XL, LY). A structured PRISMA 2020 flowchart will be drawn to display the overview of the study identification procedure (Figure 1).
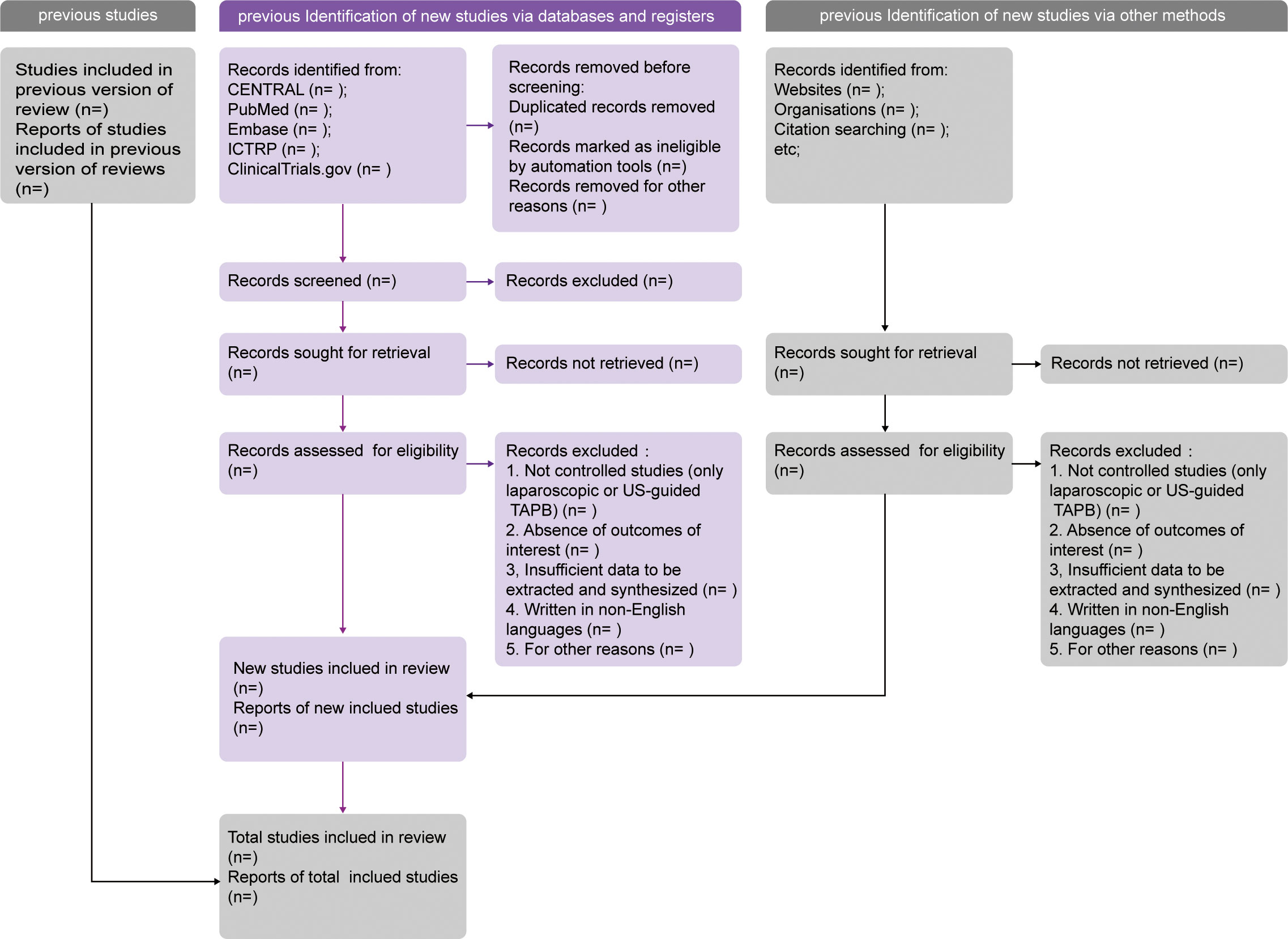
Figure 1 PRISMA 2020 flow diagram template of literature surveillance. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. CENTRAL, the Cochrane Central Register of Controlled Trials. ICTRP, the International Clinical Trials Registry Platform.
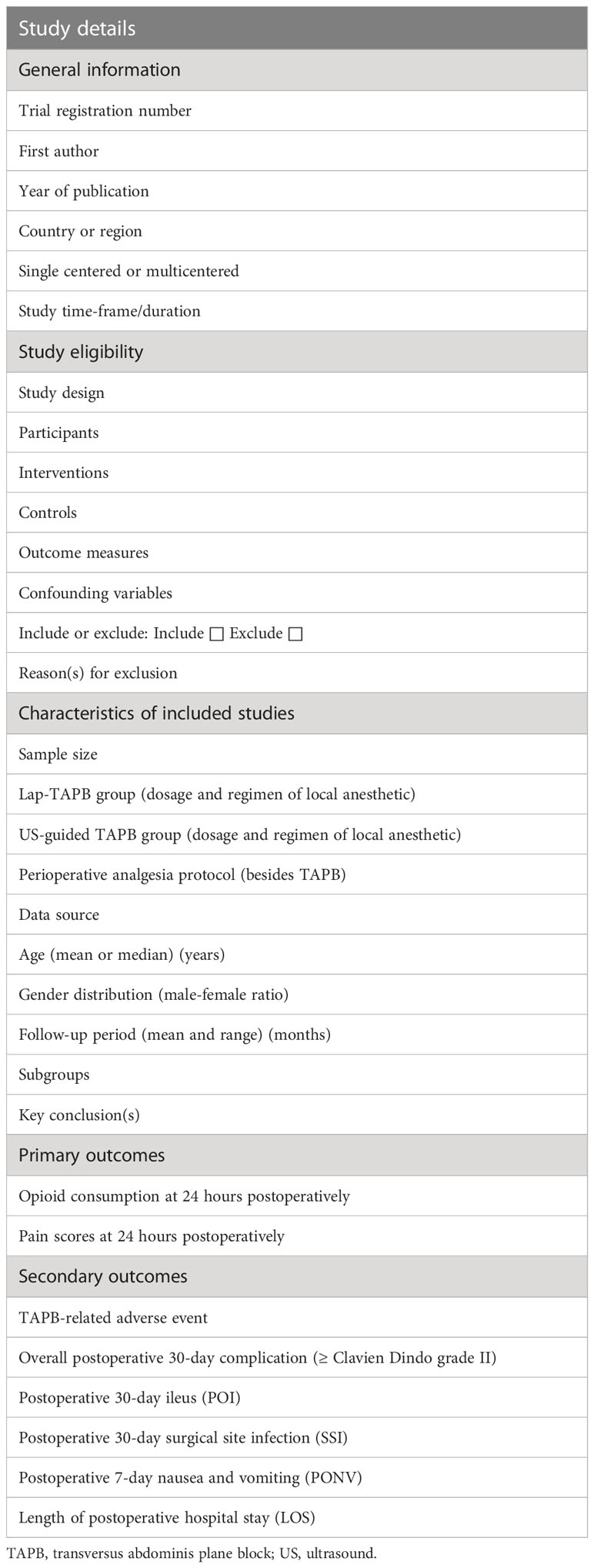
The review author pair (QM, HZ) will then extract general characteristic data of selected articles using a standardized electronic form designed by all authors (Table 2) for pooled analyses. A data set of first author’s name, publication year, country (or region), study design, study period, sample size, general characteristics of study population, TAPB technique and perioperative analgesia protocol, and all outcomes of interest will be collected from all eligible studies. Moreover, e-mails will be sent to the corresponding author to request adequate raw data in order to ensure the accuracy of the meta-analysis. If no effective response is received in 2 weeks, individual trials with missing data will be omitted from pooled analyses of the outcomes of interest. For high-quality management and synthesis, the cross-checked data will then be entered into Stata 17.0 software (StataCorp LLC, College Station, Texas, USA).
2.4 Methodological quality assessment
The included studies will be meticulously evaluated by a team of three reviewers (WY, TY, and ZC) for methodological quality. The Cochrane Risk of Bias version 2 (RoB 2) tool is supposed to validate the risk of bias for included RCTs based on bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result (31). The RoB 2 Excel Tool (available at: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 ) will be applied to implete RoB 2 for primary outcomes (31, 32). To quantify the risk of bias in each study, the Cochrane Handbook for Systematic Reviews of Interventions will be adopted (28). For non-randomized studies (NRSs), on the other hand, the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool will be utilized (33). Due to potential risk of bias in the selected studies, the findings generated from this meta-analysis will be interpreted with caution.
2.5 Data synthesis and statistical analysis
Stata 17.0 and Review Manager 5.4.1 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) will be employed to conduct the present meta-analysis. For continuous outcomes (opioid consumption at 24 hours postoperatively, pain scores at 24 hours postoperatively, and LOS), pooled weighted (WMDs) or standardized mean differences (SMDs) with their respective 95% confidence intervals (CIs) will be calculated due to the uniformity of scales used in studies. In addition, pooled odds ratios (ORs) will be worked out with corresponding 95% CIs for dichotomous variables (TAPB-related adverse events, overall postoperative 30-day complications, POI, SSI, and PONV). As a way to summarize the findings across the studies, statistical significance level will be set at a p value of less than 0.05. Given that NRSs with large sample sizes could dominate and reverse the pooled effect estimates, data synthesis for the RCT group and NRS group will be performed separately. We will identify the statistical heterogeneity among studies using the χ² test and quantify it with Cochrane’s Inconsistency (I²)-statistic. We set 50% as a cutoff value, such that substantial heterogeneity is defined as I² exceeding 50% and/or p value less than 0.10. It is preferable to adopt a random-effect model (REM) if heterogeneity is considerable, or else a regular fixed-effect model (FEM) will be the alternative. Meanwhile, to investigate the potential sources of substantial heterogeneity, sensitivity analysis and significative subgroup analyses will be conducted. Whenever clinical heterogeneity is considerable, we will undertake a narrative review rather than a meta-analysis.
2.6 Sensitivity analysis and subgroup analyses
A leave-one-out sensitivity analysis will be carried out, which aims to verify the robustness of the primary outcomes’ measure effects regarding study design, sample size, heterogeneity qualities, and non-informative prior distribution for heterogeneity parameters.
The subgroup analyses listed below will be arranged if homogeneous outcomes are reported in multiple studies within the matched subgroups:
1) region/country: Asia versus other places;
2) type of MIS: laparoscopic versus robot-assisted;
3) natural orifice specimen extraction surgery: yes versus not;
4) neoadjuvant therapy: yes versus not;
5) NRSs with propensity-score matched analysis: yes versus not.
2.7 Publication bias
To ascertain the possibility of publication bias, we will firstly check whether the RCT protocol was published prior to the enrollment of patients for the study. Studies published after July 1, 2005 will be checked at the World Health Organization-affiliated ICTRP. The presence of outcome reporting bias (selective reporting of outcomes) will also be evaluated. The visual symmetry of a funnel plot will be considered as the primary predictor of publication bias when more than ten studies are included (34).
2.8 Confidence in cumulative evidence
In order to grade the certainty of evidence for each outcome, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) working group approach will be chosen, which mainly contains the dimensions of study limitations, publication bias, imprecision, inconsistency, and indirectness (35). The evidence’s strength will be ranked as four levels: high (very confident that the effect estimate lies close to the true effect), moderate (moderately confident in the effect estimate), low (limitedly confident in the effect estimate), and very low (very little confident in the effect estimate) (36). In order to make the table and the process easier to be understood, all decisions to downgrade or upgrade the certainty of evidence will be accompanied by clear arguments in footnotes whenever necessary.
2.9 Ethical approval and dissemination
Owing to the nature of secondary analysis of existing data, there will be no patients involved in this study, and ethical approval will be not needed. High-quality peer-reviewed publications and presentations at international conferences will facilitate disseminating the results of this study.
3 Discussion
This protocol for a meta-analysis complies with the PRISMA-P guidelines. The subsequent meta-analysis will explore the effectiveness and safety of Lap-TAPB compared with US-TAPB on postoperative analgesia in colorectal MIS by summarizing the published studies. Furthermore, the meta-analysis is supposed to determine which subgroups benefit more from Lap-TAPB. The statistical analyses and other methodological processes will follow the PRISMA guidelines. The risk of bias will be examined via the Cochrane RoB 2 or ROBINS-I tool at the study level as well as the GRADE approach at the outcome level. Therefore, its findings are expected to build the foundation for future research and provide evidence-based tailored guidance on postoperative pain management for patients undergoing colorectal MIS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LY, WY, TY, and ZC conceptualized and designed this study. QM, HZ, and SQ made contributions to the inclusion and exclusion criteria, search strategy and syntax, and data extraction and summary method. ZC and XL provided methodological advice and statistical expertise. WY and TY drafted the manuscript. LY supervised the work and polished the manuscript for important intellectual content to be published. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project (Grant No. 2022HXFH028) from West China Hospital, Sichuan University and Sichuan Provincial Administration of Traditional Chinese Medicine (Grant No. 2023MS173).
Acknowledgments
We would like to acknowledge the professional and valuable suggestions for this manuscript from Dr. Pan Nie (Department of Gastrointestinal Surgery, The Third People’s Hospital of Chengdu).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1080327/full#supplementary-material
References
1. Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, et al. The American society of colon and rectal surgeons clinical practice guidelines for the management of colon cancer. Dis Colon Rectum (2022) 65(2):148–77. doi: 10.1097/DCR.0000000000002323
2. You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, et al. The American society of colon and rectal surgeons clinical practice guidelines for the management of rectal cancer. Dis Colon Rectum (2020) 63(9):1191–222. doi: 10.1097/DCR.0000000000001762
3. Hall J, Hardiman K, Lee S, Lightner A, Stocchi L, Paquette IM, et al. The American society of colon and rectal surgeons clinical practice guidelines for the treatment of left-sided colonic diverticulitis. Dis Colon Rectum (2020) 63(6):728–47. doi: 10.1097/DCR.0000000000001679
4. Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American society of colon and rectal surgeons and society of American gastrointestinal and endoscopic surgeons. Dis Colon Rectum (2017) 60(8):761–84. doi: 10.1097/DCR.0000000000000883
5. Grant MC, Yang D, Wu CL, Makary MA, Wick EC. Impact of enhanced recovery after surgery and fast track surgery pathways on healthcare-associated infections: results from a systematic review and meta-analysis. Ann Surg (2017) 265(1):68–79. doi: 10.1097/SLA.0000000000001703
6. Molenaar CJ, van Rooijen SJ, Fokkenrood HJ, Roumen RM, Janssen L, Slooter GD. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database Syst Rev (2022) 5(5):CD013259. doi: 10.1002/14651858.CD013259.pub2
7. McEvoy MD, Scott MJ, Gordon DB, Grant SA, Thacker JKM, Wu CL, et al. American Society for enhanced recovery (ASER) and perioperative quality initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: part 1-from the preoperative period to PACU. Perioper Med (Lond) (2017) 6:8. doi: 10.1186/s13741-017-0064-5
8. Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia (2001) 56(10):1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x
9. McDonnell JG, O'Donnell BD, Farrell T, Gough N, Tuite D, Power C, et al. Transversus abdominis plane block: a cadaveric and radiological evaluation. Reg Anesth Pain Med (2007) 32(5):399–404. doi: 10.1016/j.rapm.2007.03.011
10. Mukhtar K, Singh S. Transversus abdominis plane block for laparoscopic surgery. Br J Anaesth (2009) 102(1):143–4. doi: 10.1093/bja/aen338
11. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS®) society recommendations: 2018. World J Surg (2019) 43(3):659–95. doi: 10.1007/s00268-018-4844-y
12. Hamid HK, Emile SH, Saber AA, Ruiz-Tovar J, Minas V, Cataldo TE. Laparoscopic-guided transversus abdominis plane block for postoperative pain management in minimally invasive surgery: systematic review and meta-analysis. J Am Coll Surg (2020) 231(3):376–386.e15. doi: 10.1016/j.jamcollsurg.2020.05.020
13. Yeung J, Small C. Impact of regional analgesia in surgery. Br J Surg (2021) 108(9):1009–10. doi: 10.1093/bjs/znab214
14. Ali ZS, Pisapia JM, Ma TS, Zager EL, Heuer GG, Khoury V. Ultrasonographic evaluation of peripheral nerves. World Neurosurg (2016) 85:333–9. doi: 10.1016/j.wneu.2015.10.005
15. Lancaster P, Chadwick M. Liver trauma secondary to ultrasound-guided transversus abdominis plane block. Br J Anaesth (2010) 104(4):509–10. doi: 10.1093/bja/aeq046
16. ECRI top 10 health technology hazards for 2020. J Radiol Nurs (2020) 39(1):6. doi: 10.1016/S1546-0843(20)30009-2
17. Neal JM, Brull R, Horn JL, Liu SS, McCartney CJ, Perlas A, et al. The second American society of regional anesthesia and pain medicine evidence-based medicine assessment of ultrasound-guided regional anesthesia: executive summary. Reg Anesth Pain Med (2016) 41(2):181–94. doi: 10.1097/AAP.0000000000000331
18. Hain E, Maggiori L, Denise JPA, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: a meta-analysis. Colorectal Dis (2018) 20(4):279–87. doi: 10.1111/codi.14037
19. Peltrini R, Cantoni V, Green R, Greco PA, Calabria M, Bucci L, et al. Efficacy of transversus abdominis plane (TAP) block in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol (2020) 24(8):787–802. doi: 10.1007/s10151-020-02206-9
20. Viderman D, Aubakirova M, Abdildin YG. Transversus abdominis plane block in colorectal surgery: a meta-analysis. Front Med (Lausanne) (2022) 8:802039. doi: 10.3389/fmed.2021.802039
21. Liu KY, Lu YJ, Lin YC, Wei PL, Kang YN. Transversus abdominis plane block for laparoscopic colorectal surgery: a meta-analysis of randomised controlled trials. Int J Surg (2022) 104:106825. doi: 10.1016/j.ijsu.2022.106825
22. Wong DJ, Curran T, Poylin VY, Cataldo TE. Surgeon-delivered laparoscopic transversus abdominis plane blocks are non-inferior to anesthesia-delivered ultrasound-guided transversus abdominis plane blocks: a blinded, randomized non-inferiority trial. Surg Endosc (2020) 34(7):3011–9. doi: 10.1007/s00464-019-07097-y
23. Zaghiyan KN, Mendelson BJ, Eng MR, Ovsepyan G, Mirocha JM, Fleshner P. Randomized clinical trial comparing laparoscopic versus ultrasound-guided transversus abdominis plane block in minimally invasive colorectal surgery. Dis Colon Rectum (2019) 62(2):203–10. doi: 10.1097/DCR.0000000000001292
24. Park SY, Park JS, Choi GS, Kim HJ, Moon S, Yeo J. Comparison of analgesic efficacy of laparoscope-assisted and ultrasound-guided transversus abdominis plane block after laparoscopic colorectal operation: a randomized, single-blind, non-inferiority trial. J Am Coll Surg (2017) 225(3):403–10. doi: 10.1016/j.jamcollsurg.2017.05.017
25. Copperthwaite A, Sahebally SM, Raza ZM, Devane L, McCawley N, Kearney D, et al. A meta-analysis of laparoscopic versus ultrasound-guided transversus abdominis plane block in laparoscopic colorectal surgery. Ir J Med Sci (2022) 192(2):795–803. doi: 10.1007/s11845-022-03017-7
26. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-p) 2015: elaboration and explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
28. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li TJ, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: John Wiley & Sons (2019). doi: 10.1002/9781119536604
29. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. framing the question and deciding on important outcomes. J Clin Epidemiol (2011) 64(4):395–400. doi: 10.1016/j.jclinepi.2010.09.012
30. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
31. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
32. Cai Z, Liu C, Ji G, Chen J, Mu M, Jiang Z, et al. Uncut roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Cochrane Database Syst Rev (2022) 6:CD015014. doi: 10.1002/14651858.CD015014
33. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (2016) 355:i4919. doi: 10.1136/bmj.i4919
34. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
35. Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (2014) 349:g5630. doi: 10.1136/bmj.g5630
Keywords: transversus abdominis plane block, postoperative pain management, minimally invasive, colorectal surgery, data synthesis
Citation: Yang W, Yuan T, Cai Z, Ma Q, Liu X, Zhou H, Qiu S and Yang L (2023) Laparoscopic versus ultrasound-guided transversus abdominis plane block for postoperative pain management in minimally invasive colorectal surgery: a meta-analysis protocol. Front. Oncol. 13:1080327. doi: 10.3389/fonc.2023.1080327
Received: 26 October 2022; Accepted: 09 May 2023;
Published: 22 May 2023.
Edited by:
Ye Zhou, Fudan University, ChinaReviewed by:
Narimantas Samalavicius, Vilnius University, LithuaniaZhen He, The Sixth Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2023 Yang, Yuan, Cai, Ma, Liu, Zhou, Qiu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lie Yang, bGllXzIyMkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Wenming Yang
Wenming Yang Tao Yuan2†
Tao Yuan2† Zhaolun Cai
Zhaolun Cai Qin Ma
Qin Ma Lie Yang
Lie Yang