- 1Department of Hematology, Fujita Health University School of Medicine, Toyoake, Japan
- 2Department of Hematology, Osaka City General Hospital, Osaka, Japan
- 3Department of Hematology, Gifu Municipal Hospital, Gifu, Japan
- 4Department of Hematology, Hokkaido University Hospital, Sapporo, Japan
- 5Department of Hematology and Oncology, JA Aichi Konan Kosei Hospital, Konan, Japan
- 6Department of Hematology, Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital, Nagoya, Japan
- 7Department of Blood and Marrow Transplantation and Cellular Therapy, Fujita Health University School of Medicine, Toyoake, Japan
- 8Division of Hematology/Oncology, Department of Internal Medicine, Kameda Medical Center, Kamogawa, Japan
- 9Blood Disorders Center, Aiiku Hospital, Sapporo, Japan
- 10Department of Hematology and Oncology, Tosei General Hospital, Seto, Japan
- 11Department of Hematology, National Hospital Organization Nagoya Medical Center, Nagoya, Japan
- 12Department of Hematology and Oncology, Toyohashi Municipal Hospital, Toyohashi, Japan
- 13Division of Hematology, Department of Internal Medicine, Aichi Medical University School of Medicine, Nagakute, Japan
- 14Department of Hematology and Oncology, Nagoya City University, Nagoya, Japan
- 15Division of Hematology and Oncology, Department of Internal Medicine, Iwate Medical University School of Medicine, Morioka, Japan
- 16Department of Hematology and Oncology, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Nagoya, Japan
- 17Department of Hematology and Oncology, Anjo Kosei Hospital, Anjo, Japan
- 18Division of Hematology, Jichi Medical University Saitama Medical Center, Saitama, Japan
- 19Division of Hematology, Ichinomiya Municipal Hospital, Ichinomiya, Japan
- 20Department of Hematology, Kawasaki Medical School, Kurashiki, Japan
- 21Department of Hematology, Toyota Kosei Hospital, Toyota, Japan
- 22Department of Hematology/Oncology, Wakayama Medical University, Wakayama, Japan
- 23Department of Hematology and Immunology, Kanazawa Medical University, Kanazawa, Japan
- 24Japanese Data Center for Hematopoietic Cell Transplantation, Nagakute, Japan
- 25International Center for Cell and Gene Therapy, Fujita Health University, Toyoake, Japan
Background: Although the Omicron variant has been reported to reduce COVID-19 severity in the general population, its impact on patients with hematologic malignancies remains uncertain, and epidemiological investigation is warranted.
Methods: We conducted a multicenter retrospective cohort study of 1, 023 patients with hematologic diseases diagnosed with COVID-19 at 22 centers in Japan between January 2020 and January 2023. Outcomes within 60 days after diagnosis including severe and/or prolonged disease, COVID-19–related mortality, and overall survival (OS) were compared between the pre-Omicron and Omicron periods. Multivariable analysis was performed to identify independent adverse prognostic factors.
Results: Severe and/or prolonged disease occurred in 27.5% of patients, COVID-19–related mortality was 6.3%, and OS was 91.4%. Compared with the pre-Omicron period, the Omicron period was associated with significantly lower rates of severe/prolonged disease (26.0% vs. 48.0%, P<0.01) and COVID-19–related mortality (5.0% vs. 15.0%, P<0.01), but no significant difference in OS (92.0% vs. 84.0%, P = 0.62). Age ≥60 years was the strongest predictor of severe/prolonged disease (sHR 3.08, P<0.01) and mortality (HR 8.94, P<0.01). Male sex (sHR 1.38; HR 1.82, both P<0.01) and prior bendamustine exposure (sHR 1.83; HR 1.87, both P<0.01) were also associated with both outcomes, whereas anti-CD38 antibody therapy was linked only to mortality (HR 3.65, P<0.01).
Conclusion: In patients with hematologic diseases, the Omicron period was associated with reduced severity and COVID-19–related mortality but no improvement in OS. Older age and prior bendamustine exposure were strongly associated with adverse outcomes, highlighting the need for strict infection prevention and prompt, aggressive COVID-19 management in these high-risk populations.
Introduction
The epidemiology of coronavirus disease 2019 (COVID-19) has evolved over time. In Japan, the Omicron variant became predominant in January 2022, leading to three subsequent waves during the summer and winter of that year (1, 2). The Omicron variant has shown increased transmissibility in the general population (3, 4), although its associated mortality rate has declined (5–7). However, several reports have suggested that the Omicron variant might have a more profound impact on patients with hematologic diseases than in the general population (8–13). Recent large real-world registry studies, including the EPICOVIDEHA analysis in multiple myeloma and the Spanish GETH-TC registry in hematologic malignancies, have further confirmed that immunocompromised patients remain at considerable risk for severe and fatal COVID-19 even during the Omicron era (14, 15). Significant numbers of patients in the Omicron era still have severe disease requiring oxygen supplementation or admission to an intensive care unit, and COVID-19 has been fatal in some patients previously treated with bendamustine-containing regimens (13, 16). Others have had prolonged disease, with persistently positive SARS-CoV-2 viral loads requiring continued isolation in the hospital (13, 16, 17).
Many more therapeutic agents against SARS-CoV-2 became clinically available during the Omicron era than in earlier periods (18). Although the prognosis for patients is expected to improve, severe and/or prolonged and fatal cases are still being experienced in clinical practice. Several reports have indicated that prolonged disease may facilitate additional genetic mutations, leading to resistance against antiviral agents such as the anti-SARS-CoV-2 antibodies casirivimab/imdevimab, sotrovimab, and tixagevimab/cilgavimab (19–21); and to small molecules such as remdesivir (13, 22) and nirmatrelvir (23), potentially exacerbating disease severity and duration (13, 22, 24, 25). These phenomena suggest that in the clinical setting, it is critically important to identify the patient groups most vulnerable to severe and/or prolonged disease.
In addition, with the advent of the Omicron variant, multiple vaccines have become available and the situation has markedly changed compared to the early phase of the pandemic. However, patients with hematologic malignancies undergoing specific treatments, such as anti-CD20 antibodies and Bruton’s tyrosine kinase inhibitors, have exhibited markedly impaired humoral responses to COVID-19 vaccination (26–30). The extent to which immunodeficiency contributes to the risk of developing severe and/or prolonged disease, or mortality, during the Omicron era remains uncertain. Furthermore, as with antiviral drugs, especially in the Omicron era, reductions in the efficacy of both monovalent and bivalent vaccines due to additional mutations in the viral genome have also been reported (31–34). These factors are also considered to have a significant impact on patients with hematologic diseases.
Given the special circumstances of immunosuppression and the use of antitumor drugs in patients with hematologic diseases, we conducted a survey of actual clinical practice to assess whether the effects of COVID-19 in the Omicron era may be different in these patients than in the general population. The aim of this multicenter, large-scale retrospective cohort study was to evaluate the clinical outcomes of COVID-19 in patients with hematologic diseases, with a particular focus on the clinically significant composite event of severe disease requiring oxygen therapy and/or prolonged disease requiring isolation in the hospital for more than 3 weeks. These findings may help identify patients at high risk for severe COVID-19 who may require more intensive infection control measures and antiviral interventions during the Omicron era.
Methods
Patients
This multicenter retrospective study included patients who received treatment for hematologic diseases at 22 hospitals in Japan between January 1, 2020 and January 31, 2023, and who were newly diagnosed with SARS-CoV-2 infection by genetic testing (polymerase chain reaction) or antigen testing using nasal swab or saliva. Based on data from the Ministry of Health, Labour and Welfare and the National Institute of Infectious Diseases in Japan, the pre-Omicron era was defined as that up to December 31, 2021, and the Omicron era as beginning on January 1, 2022 (Figure 1A). Patients who had been diagnosed at institutions other than the participating centers and whose diagnoses were not confirmed at the study institutions were excluded. Clinical data were collected from electronic medical records using a structured research questionnaire. Due to the observational nature of the study, patient consent to participate was not required. However, information about the study was disclosed on each institution’s website, and patients could choose to opt out. The study was approved by the Ethics Review Committee of Fujita Health University School of Medicine (approval numbers: HM-23-010, HM-23-082) and affiliated institutions and was conducted according to the principles of the Declaration of Helsinki.
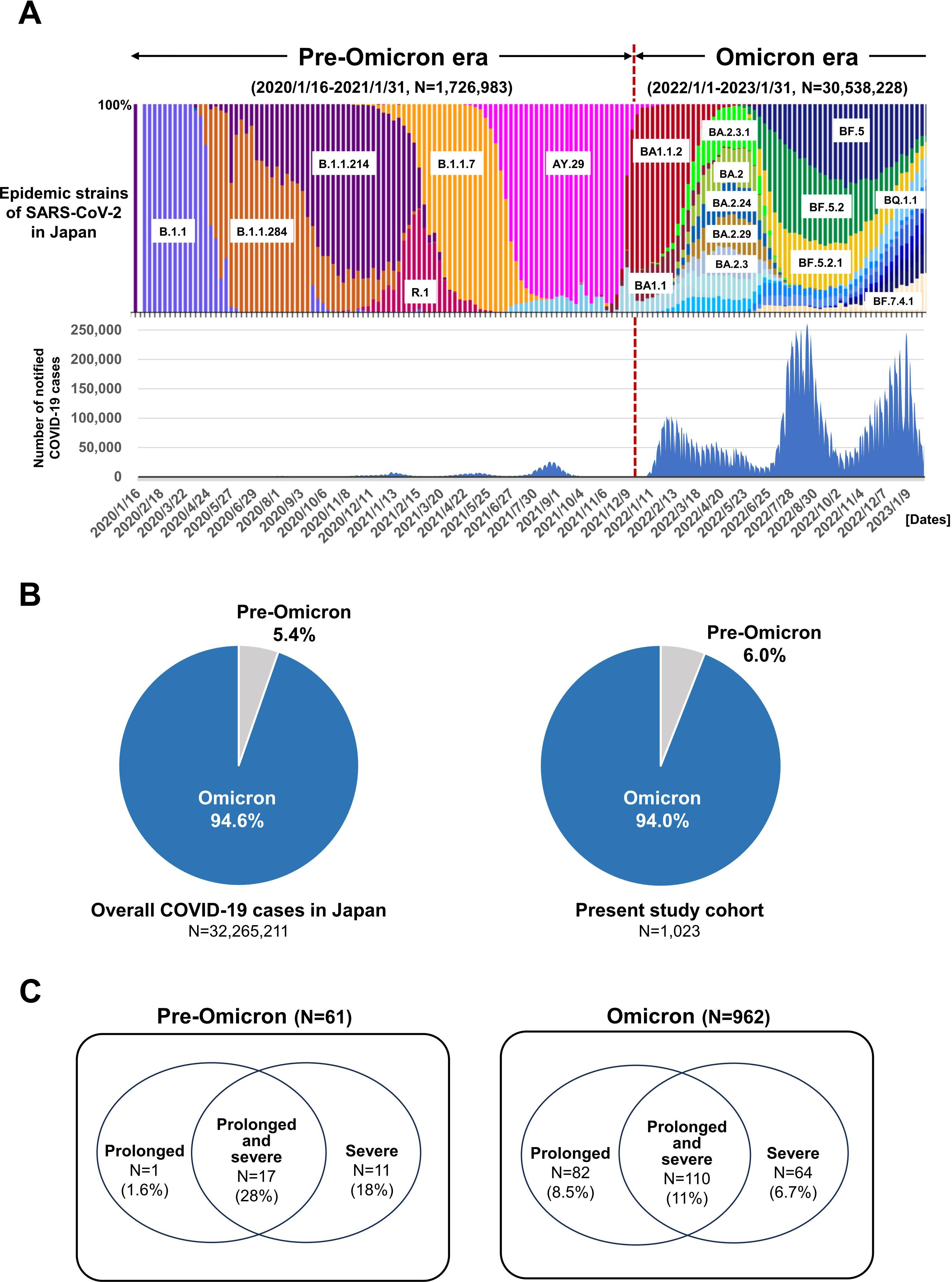
Figure 1. Trends in cases of COVID-19 during the pre-Omicron and Omicron eras in Japan. (A) Colored stacked bar graph shows nationwide trends in SARS-CoV-2 epidemic strains (upper panel). The corresponding numbers of COVID-19 patients are indicated by blue bars (lower panel). (B) Proportion of overall COVID-19 patients during the pre-Omicron and Omicron eras in Japan (left panel) and in the present study cohort (right panel). (C) Proportions of the present COVID-19 patients with prolonged, prolonged and severe, and severe disease during the pre-Omicron (left panel) and Omicron eras (right panel).
Definitions
The primary endpoint was the cumulative incidence of patients who experienced severe and/or prolonged disease within 60 days of COVID-19 diagnosis. The secondary endpoints were the overall survival rate at day 60 and COVID-19 related mortality. COVID-19 related mortality was defined as mortality during COVID-19 positivity or due to COVID-19-related complications. Risk factors associated with each endpoint were also analyzed. Severe disease was defined as COVID-19 requiring oxygen supplementation or admission to an intensive care unit. Prolonged disease was defined as continued isolation in hospital beyond 21 days after symptom onset, based on the attending physician’s judgment, due to persistent positivity on genetic or antigen testing or ongoing clinical symptoms. The hematologic diseases included in this study were classified into lymphoid malignancies, myeloid malignancies, and other benign hematologic disorders. To account for the potential impact of treatment regimens, lymphoid malignancies were further subdivided into B-cell lymphomas, plasma cell neoplasms, and other lymphoid malignancies for separate analyses (Table 1).
Statistical analysis
Fisher’s exact test was used to compare categorical variables between groups. The cumulative incidence of severe and/or prolonged disease was calculated from the date of COVID-19 diagnosis, treating death without severe or prolonged disease as a competing risk event. Gray’s test was used to compare the cumulative incidence of COVID-19-related mortality between groups. Cumulative incidence was calculated from the date of COVID-19 diagnosis, treating deaths due to causes other than COVID-19 as competing risks. Overall survival was calculated using the Kaplan–Meier method and was compared using the log-rank test.
Fine–Gray proportional hazards regression models were used for analyses of risk factors associated with severe and/or prolonged disease and COVID-19 related mortality. Cox proportional hazards regression models were used for analyses of factors associated with overall mortality. Factors with a p value<0.05 in univariate analysis were entered into multivariate analysis, and a backward elimination procedure was used in developing final models, using a p value threshold of 0.05. A two-sided p value of less than 0.05 was considered statistically significant. During the preparatory meeting prior to the initiation of the study, it was concluded based on clinical experience that bendamustine, anti-CD20 antibody therapy, allogeneic hematopoietic cell transplantation (allo-HCT), and autologous hematopoietic cell transplantation may result in prolonged immunosuppression lasting more than one year. Accordingly, treatment history within the past two years was considered for these therapies. In contrast, therapies such as anti-CD38 antibody therapy, Bruton’s tyrosine kinase inhibitors, Janus kinase inhibitors, and chimeric antigen receptor (CAR) T-cell therapy were evaluated based on treatment history within the past one year.
All statistical analyses were performed using EZR (Jichi Medical University Saitama Medical Center, Saitama, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (35).
Results
Patients
Based on the data from the Japanese Ministry of Health, Labour and Welfare and the National Institute of Infectious Diseases, there was a significant accumulation of COVID-19 cases associated with the Omicron variant after January 2023, with a wide variety of SARS-CoV-2 sub-lineages detected (Figure 1A) (1, 2). A total of 1, 023 COVID-19 patients with hematologic diseases were enrolled in this multicenter study (Table 1). The median age was 69 years (range, 13–97 years). Among them, 61 patients developed COVID-19 during the pre-Omicron era, and 962 during the Omicron era. The distribution of case numbers across the two eras was consistent with previously reported national data (Figure 1B). There were no significant differences in sex or age distribution, or in the distribution of underlying hematologic diseases, between the pre-Omicron and Omicron eras. A significant difference was observed in terms of treatment history. The frequency of use of drugs such as bendamustine (P = 0.010) and anti-CD20 antibodies (P = 0.039) was significantly higher during the Omicron era. Other treatment strategies, including Bruton’s tyrosine kinase inhibitors, anti-CD38 antibodies, Janus kinase 2 inhibitors, allogeneic and autologous SCT, and CAR-T therapy, were used at similar frequencies in both eras. These findings indicate that although baseline characteristics and disease distributions were generally similar, the use of bendamustine and anti-CD20 antibodies may have changed in the clinical setting, likely reflecting changes in the SARS-CoV-2 epidemic status and treatment modalities.
Incidence of severe and/or prolonged disease
Among the 1, 023 COVID-19 patients included in this study, a total of 281 (27.5%) met the criteria for severe and/or prolonged COVID-19 within 60 days (95% confidence interval (CI): 25.0%–30.1%). Of these, 29 cases (48.0%) occurred during the pre-Omicron period, and 252 cases (26.0%) during the Omicron period. The distribution of these cases by period is shown in Figure 1C.
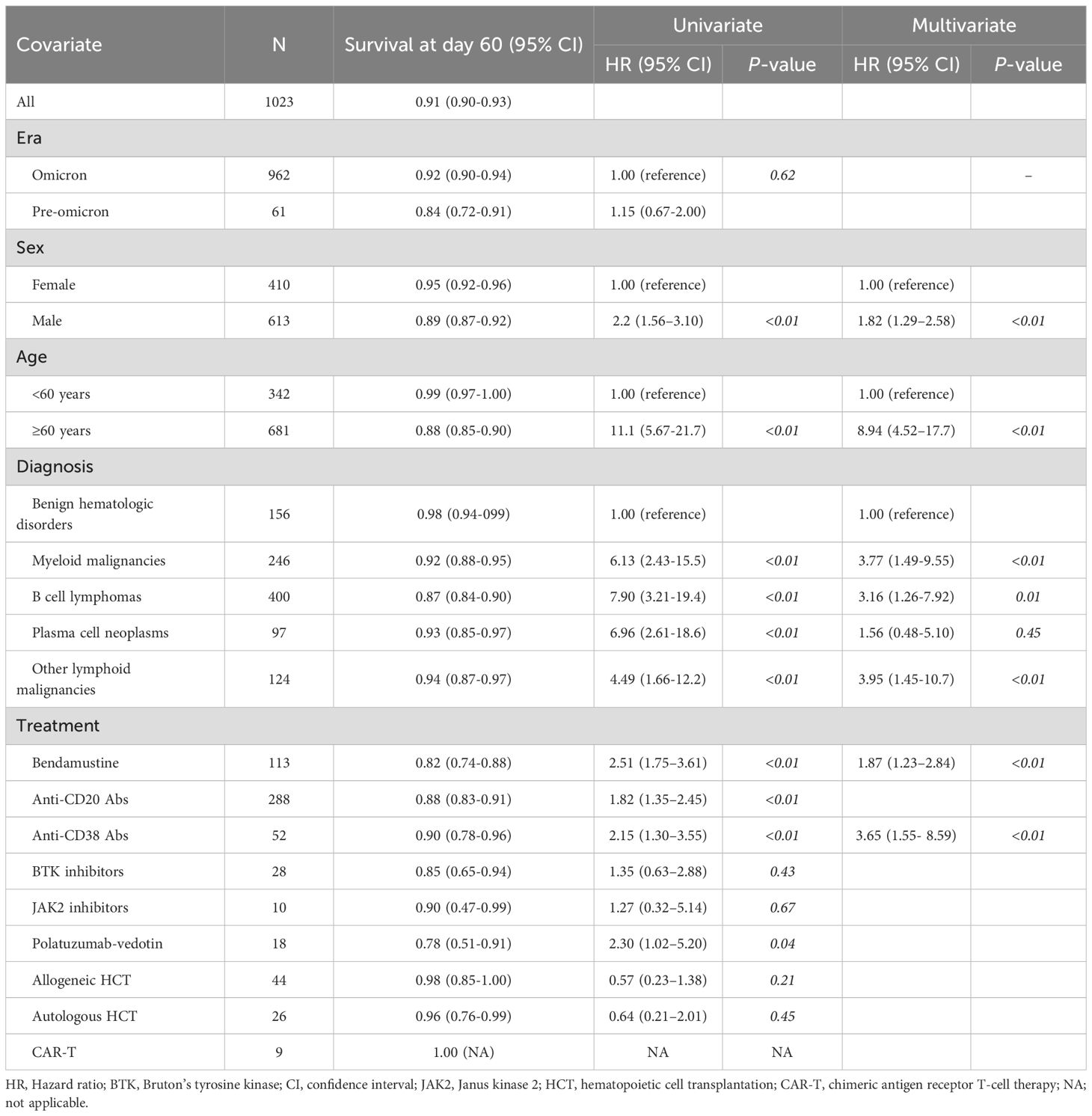
The cumulative incidence of severe and/or prolonged disease within 60 days after COVID-19 diagnosis (the primary endpoint) was 27.5% (95% CI, 25.0%–30.1%) (Table 2). As expected, the incidence increased as patient age increased, with 60 years considered an appropriate cut-off age for subsequent analysis (Figure 2A). Table 2 shows the results of regression analyses conducted to identify factors associated with severe and/or prolonged disease within 60 days. Univariate analysis identified pre-Omicron era, male sex, age ≥ 60 years, B-cell lymphoma, plasma cell neoplasms, bendamustine use, anti-CD20 antibody use, and polatuzumab vedotin use as statistically significant factors associated with severe and/or prolonged disease (P<0.01 for all). Multivariate analysis identified pre-Omicron era (sHR 2.32, 95% CI 1.56–3.45, P<0.01), male sex (sHR 1.38, 95% CI 1.08–1.75, P<0.01), age ≥ 60 years (sHR 3.08, 95% CI 2.16–4.39, P<0.01), bendamustine therapy (sHR 1.83, 95% CI 1.37–2.44, P<0.01), and anti-CD20 antibody therapy (sHR 1.48, 95% CI 1.12–1.95, P<0.01) as statistically significant factors associated with severe and/or prolonged disease. Figures 2B–F show cumulative incidence curves according to the presence or absence of these individual factors. These findings suggest that specific types of hematologic diseases and therapeutic strategies, in addition to older age and male sex, significantly correlate with the incidence of severe and/or prolonged disease.
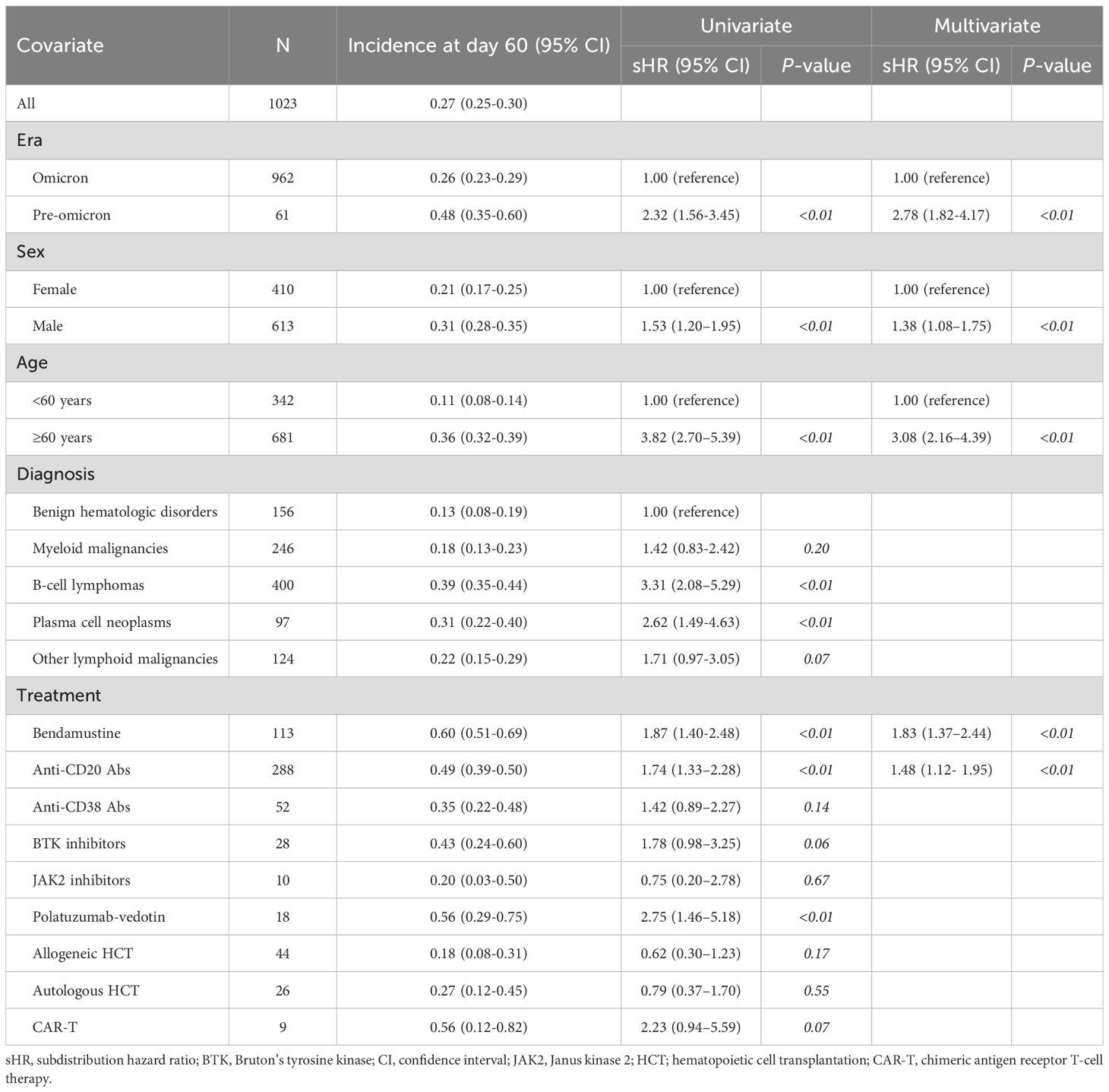
Table 2. Univariate and multivariate analysis of factors associated with severe and/or prolonged diseases.
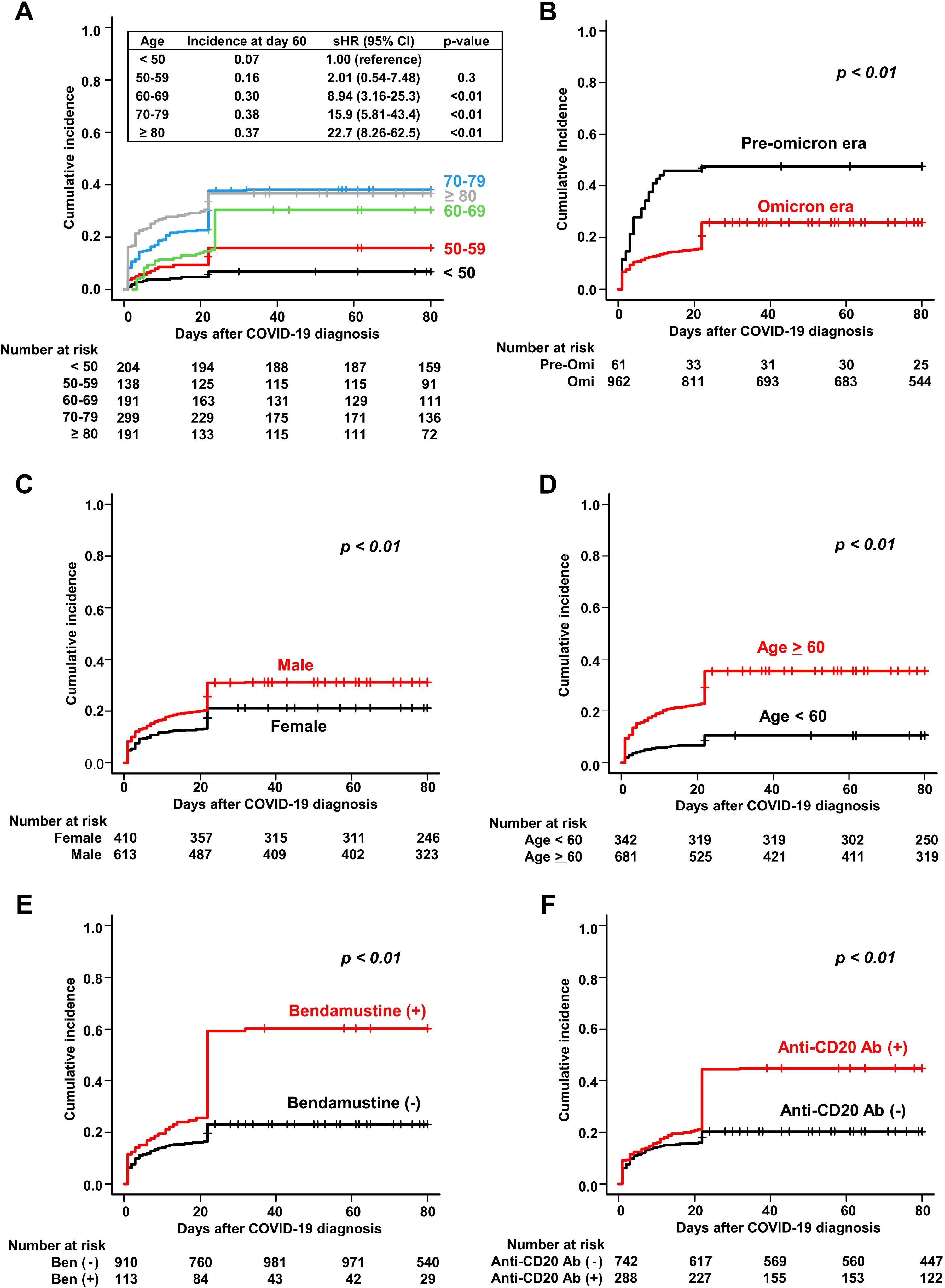
Figure 2. Cumulative incidence of severe and/or prolonged COVID-19 in patients with hematologic diseases. Cumulative incidence of severe and/or prolonged disease is stratified by (A) age, (B) time period of COVID-19 onset (pre-Omicron and Omicron), (C) sex, (D) age (≥60 and <60 years), (E) bendamustine use, and (F) anti-CD20 antibody use.
Overall survival
The probability of overall survival at 60 days after COVID-19 diagnosis was 91.4% (95% CI, 89.6%–92.9%) (Table 3). The probabilities of overall survival were similar during the pre-Omicron and Omicron periods (84.0% and 92.0%, respectively, P<0.62, Figure 3A, Table 3). Univariate analysis identified male sex, age ≥60 years, myeloid malignancies, B-cell lymphoma, other lymphoid malignancies, bendamustine use, anti-CD20 antibody use, and anti-CD38 antibody use as statistically significant risk factors (P<0.01 for all). Multivariate analysis identified male sex (HR, 1.82; 95% CI, 1.29–2.58; P<0.01), age ≥60 years (HR, 8.94; 95% CI, 4.52–17.70; P<0.01), myeloid malignancies (HR, 3.77; 95% CI, 1.49–9.55; P<0.01), B-cell lymphoma (HR, 3.16; 95% CI, 1.26–7.92; P = 0.01), other lymphoid malignancies (HR, 3.95; 95% CI, 1.45–10.7; P<0.01), prior bendamustine use (HR, 1.87; 95% CI, 1.23–2.84; P<0.01) and prior anti-CD38 antibody use (HR, 3.65; 95% CI, 1.55–8.59; P<0.01) as independently associated with overall survival. Figures 3B–F show survival curves according to the presence or absence of these individual factors.
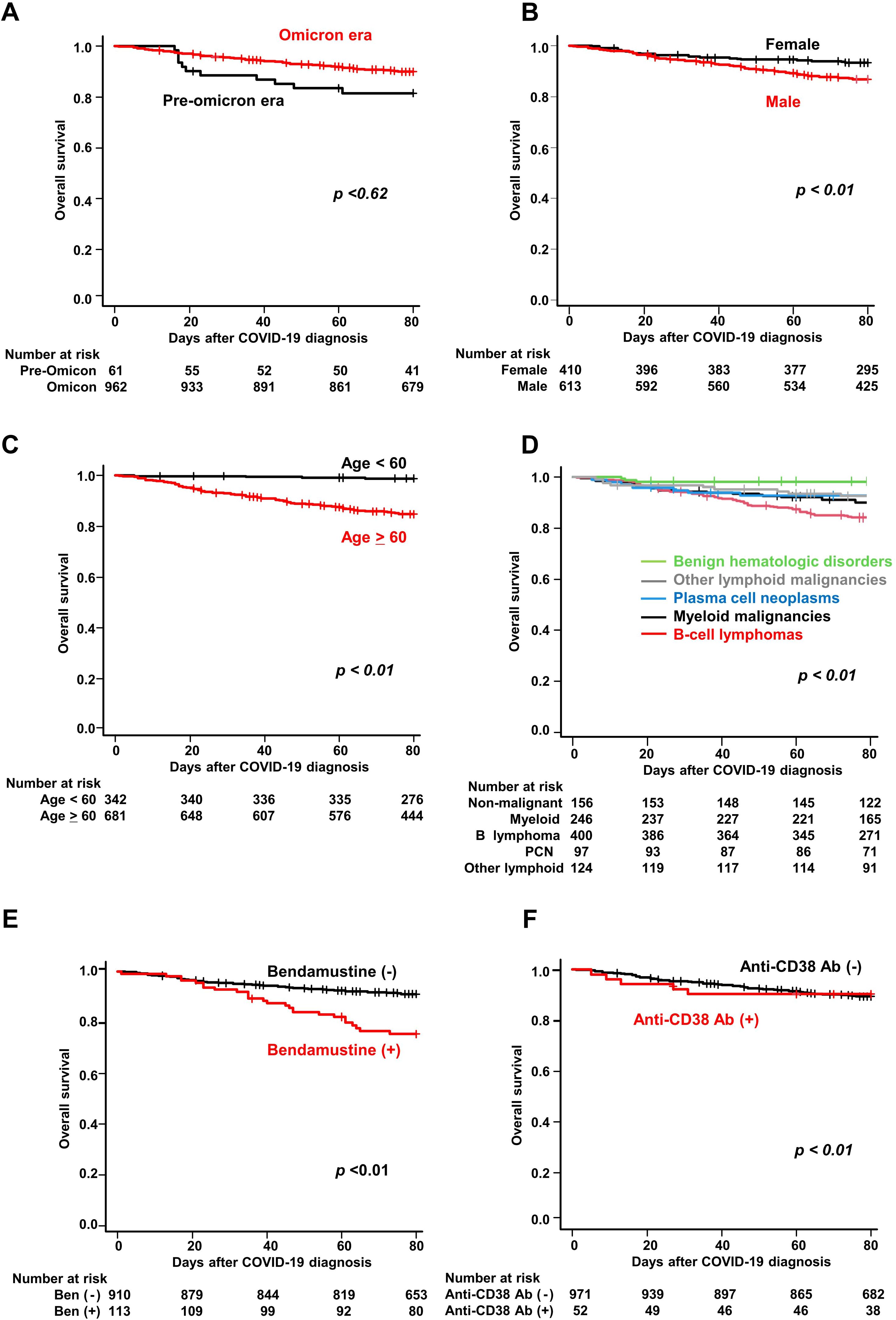
Figure 3. Overall survival of COVID-19 patients with hematologic diseases. Kaplan–Meier curves of overall survival stratified by (A) time period of COVID-19 onset (pre-Omicron and Omicron), (B) sex, (C) age (≥60 vs <60 years), (D) hematologic disease group, (E) bendamustine use, and (F) anti-CD38 antibody use.
Incidence of COVID-19 related mortality
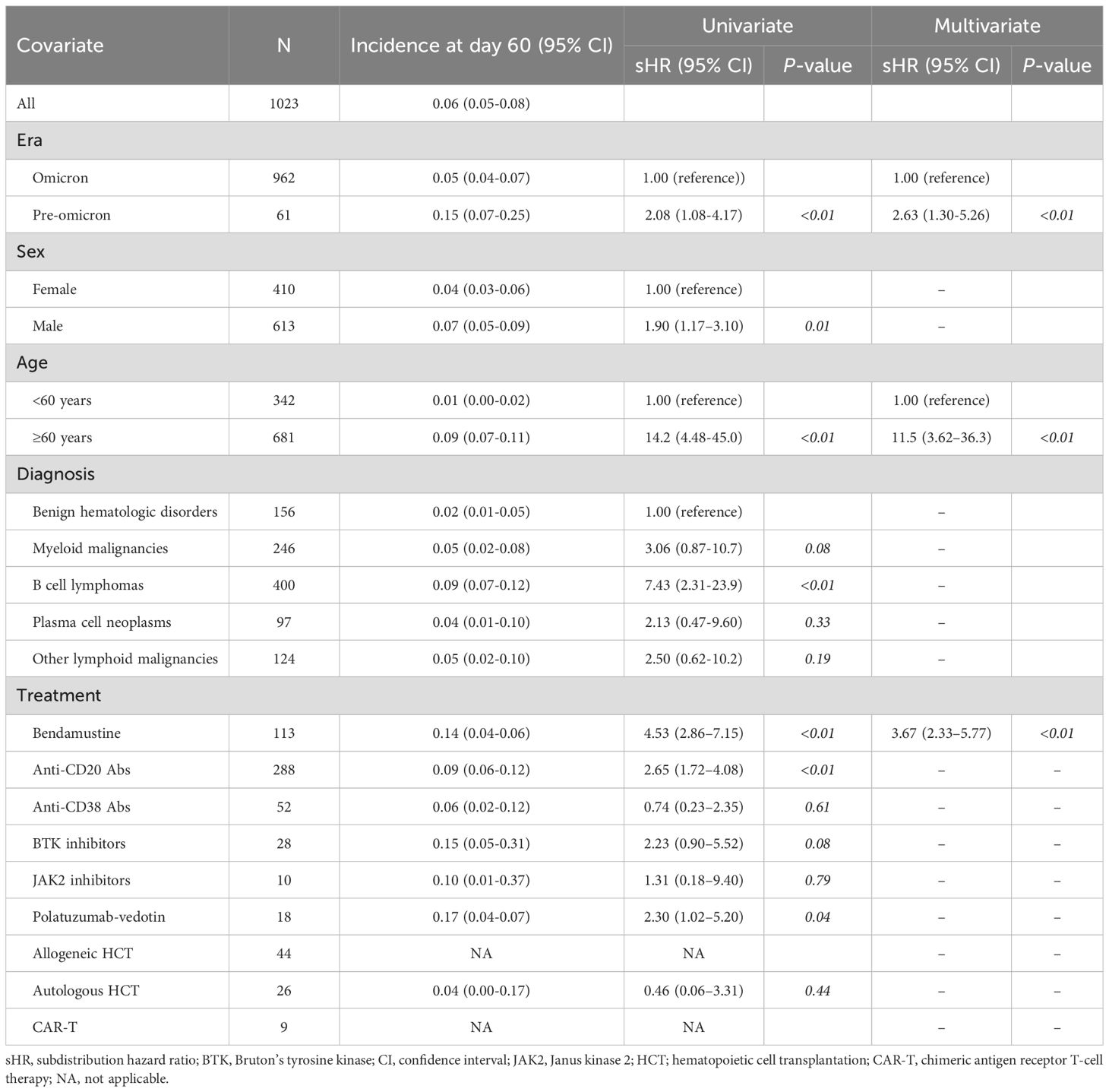
The cumulative incidence of COVID-19-related mortality was 6.3% (95% CI, 5.0%–7.7%) at 60 days after COVID-19 diagnosis (Table 4). The incidence was significantly lower in the Omicron era than that in the pre-Omicron era (5.0% vs. 15.0%, P<0.01). Table 4 lists the factors associated with COVID-19 related mortality. Univariate analysis identified pre-Omicron era, male sex, age ≥60 years, B-cell lymphoma, bendamustine use, anti-CD20 antibody use, and polatuzumab vedotin use as factors associated with increased COVID-19 mortality. In multivariate analysis, age ≥60 years was the strongest independent predictor of COVID-19 related mortality (9.0% vs. 1.0%; sHR, 11.47; 95% CI, 3.62–36.33; P<0.01). Prior bendamustine use was also independently associated with significantly higher mortality (14.0%; sHR, 3.67; 95% CI, 2.33–5.77; P<0.01) (Table 4). Male sex and B-cell lymphoma were not retained in the multivariate model. No deaths were observed among patients who underwent allogeneic hematopoietic cell transplantation (n = 44) or CAR-T cell therapy (n = 9) during the observation period. Among patients in the present cohort who died, the proportion of deaths classified as COVID-19–related was approximately 90.0% (9/10) during the pre-Omicron era, which decreased to approximately 65.4% (51/78) during the Omicron era. These data suggest that although specific types of hematologic diseases and therapeutic strategies are also significantly correlated with COVID-19-related mortality, the risk of mortality due to COVID-19 showed a decreasing tendency in the Omicron era compared with that in the pre-Omicron era.
Post-COVID-19 complications
Post-COVID-19 complications were confirmed in 79 patients (8.5%), of whom 57 had respiratory complications, the most frequent of which was interstitial pneumonia (n = 42) (Supplementary Table S1). Among patients with interstitial pneumonia as a post-COVID-19 complication, bendamustine was used in 13 (31%) (P<0.01, 95% CI 1.82–8.13, odds ratio 3.94). Severe fatigue, a common symptom of long COVID (36), occurred in five patients (0.5%).
Discussion
This study demonstrates that even during the Omicron era, patients with hematologic malignancies remained at substantial risk for severe and/or prolonged COVID-19 and death. Multivariate analysis identified age >65 years and bendamustine use as major risk factors for adverse outcomes, whereas male sex and anti-CD38 antibody therapy were associated with increased overall mortality. These findings are consistent with earlier studies that have reported higher risks among older adults and male patients in the pre-Omicron era (37).
The proportion of deaths due to COVID-19 among all deaths decreased from 89.7% in the pre-Omicron era to 65.4% during the Omicron era. Decreasing virulence of Omicron strains and improvements in COVID-19 treatment strategies may be considered as possible reasons for this trend. However, the lack of a direct correlation between lower proportion of deaths due to COVID-19 and an improvement in overall survival suggests that indirect effects, such as clinical deterioration or delays in treatment for hematologic diseases, may have also contributed to mortality. These findings highlight the importance of considering both direct and indirect consequences of COVID-19 in managing immunocompromised patients.
It is important to note that prolonged disease beyond 60 days after COVID-19 diagnosis can occur in immunocompromised patients with hematologic diseases. In particular, patients with a history of anti-CD20 antibody and bendamustine combination therapy have been reported to exhibit persistent infections lasting 3–6 months or longer (13, 16, 38, 39). In such cases, persistent SARS-CoV-2 infection may facilitate the accumulation of genetic mutations in the viral genome, leading to resistance to antiviral drugs. Furthermore, secondary bacterial and/or fungal infections in the setting of severe immunodeficiency also represent serious complications in these patients (13, 22, 40). These findings strongly suggest the necessity of intensive management not only for prolonged SARS-CoV-2 infections but also for prevention and treatment of other infectious diseases.
This study demonstrated that five factors (older age, male sex, hematologic disease type, prior bendamustine use, and anti-CD38 antibody use) were significantly associated with poorer overall survival. Among these, age emerged as the most prominent predictor, indicating that patients aged 60 years or older with additional adverse prognostic factors may be critical candidates for timely antiviral therapy and intensive monitoring. Given the strong association between bendamustine use and adverse outcomes such as severe disease and mortality, its use warrants careful consideration, particularly in older or immunocompromised patients, when clinically appropriate. In COVID-19 patients with a history of bendamustine use, early intervention and ongoing close follow-up may support recovery from SARS-CoV-2 infection and reduce the risk of other infectious diseases. A risk-adapted, individualized management approach may prove beneficial not only during the Omicron era but also in the future, even if the virulence of SARS-CoV-2 decreases.
The impact of bendamustine on COVID-19 outcomes remains under-investigated, although several reports have suggested associations with severe and/or prolonged disease (41–43). Bendamustine can cause prolonged lymphocytopenia, particularly of CD4+ T cells (44–47), and has been linked to other viral infections, such as cytomegalovirus (48). Reduced vaccine responsiveness in patients receiving bendamustine has been also demonstrated (49). A recent multicenter retrospective study from Spain showed that patients with follicular lymphoma and mantle cell lymphoma who received bendamustine induction followed by rituximab maintenance had a higher risk of COVID-19 related death compared to those treated with cyclophosphamide-based regimens (50). Furthermore, Iriyama et al. and others have reported prolonged and/or severe COVID-19 in patients with marked reductions in CD19+ B-cells and CD4+ T-cells, and an increased proportion of exhausted CD4+ T-cells following treatment with bendamustine combined with anti-CD20 antibodies (13, 16). These findings may suggest that transient but prolonged impairment of T-cell function induced by bendamustine may in part contribute to deficiency of SARS-CoV-2 clearance. Recent reports, together with our findings, suggest that the use of bendamustine should be carefully evaluated, particularly in elderly or immunocompromised patients. In such patients, prompt initiation of antiviral therapy may be advisable immediately upon COVID-19 diagnosis.
In the present study, anti-CD20 antibody use was significantly associated with an increased risk of severe and/or prolonged COVID-19, but not with overall mortality. One possible explanation for this finding is the advancement of various therapeutic strategies for COVID-19. Another may be the limited impact of anti-CD20 antibody therapy on cellular immunity, which plays a critical role in viral clearance. We have previously demonstrated that treatment with anti-CD20 antibodies temporarily impairs the humoral immune response to SARS-CoV-2 vaccination, with anti-SARS-CoV-2 spike protein antibody titers remaining largely undetectable for approximately 9–12 months after the last administration. This phenomenon is closely associated with delayed B-cell recovery (26). During the acute phase of SARS-CoV-2 infection, cytotoxic responses mediated by CD8+ T cells are induced within 1 to 2 weeks of symptom onset and are known to contribute substantially to viral control and milder clinical outcomes (51–54). In addition to the importance of viral clearance, chronic inflammation driven by cellular immunity may play a critical role in the development of severe and/or prolonged disease and death. In this context, T-cell function may be particularly important. As anti-CD20 antibody treatment has minimal impact on cellular immune function, its use may have had little impact on patient survival.
Although allo-HCT is generally considered a high-risk factor for infection (55), there was no significant association of allo-HCT with severe infection or a higher mortality rate in our cohort. Immune reconstitution and improved vaccine responses in transplant recipients may contribute to reduced COVID-19 severity (56), suggesting that allo-HCT recipients may no longer be a high-risk population in the Omicron era, as previously reported (57, 58).
Limitations of this study include the absence of detailed information on COVID-19 treatment and its effect on clinical outcomes. In addition, selection bias may be present, as the analysis included only patients treated at participating hospitals that submitted data, potentially excluding milder cases that were managed at non-participating or community-based facilities. Information bias due to the retrospective data collection and unmeasured confounding factors, such as vaccination status, antiviral treatment, and degree of immunosuppression, also warrant consideration. Future research should incorporate standardized treatment data and evaluate the effectiveness of antiviral interventions in patients with hematologic diseases.
In conclusion, despite the milder nature of the Omicron variant, patients with certain types of hematologic diseases and specific conditions remain at risk for severe and/or prolonged COVID-19. Particular attention should be given to older patients, male patients, and those with B-cell malignancies treated with bendamustine, not only in preventing SARS-CoV-2 infection but also in ensuring prompt and active treatment when COVID-19 is contracted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of the Fujita Health University and was conducted according to the principles of the Declaration of Helsinki (HM23-082). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AO: Investigation, Supervision, Writing – review & editing, Methodology, Validation, Conceptualization, Funding acquisition, Software, Formal analysis, Writing – original draft, Project administration, Data curation, Visualization, Resources. MY: Investigation, Writing – review & editing. SeK: Writing – review & editing, Investigation. TA: Investigation, Writing – review & editing. KO: Writing – review & editing, Investigation. TM: Investigation, Writing – review & editing. DI: Writing – review & editing, Investigation. MK: Writing – review & editing, Investigation. TK: Writing – review & editing, Investigation. YS: Investigation, Writing – review & editing. ShK: Investigation, Writing – review & editing. TH: Writing – review & editing, Investigation. YM: Writing – review & editing, Investigation. TO: Investigation, Writing – review & editing. SS: Investigation, Writing – review & editing. HS: Investigation, Writing – review & editing. SKi: Investigation, Writing – review & editing. TN: Writing – review & editing, Investigation. EK: Writing – review & editing, Investigation. JH: Investigation, Writing – review & editing. HH: Writing – review & editing, Investigation. YM: Investigation, Writing – review & editing. YA: Methodology, Investigation, Writing – review & editing, Formal analysis. HY: Investigation, Conceptualization, Writing – review & editing. TM: Investigation, Writing – review & editing. NG: Writing – review & editing, Investigation, Conceptualization. CI: Investigation, Writing – review & editing, Conceptualization. KM: Writing – review & editing, Investigation. YI: Methodology, Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AT: Methodology, Investigation, Conceptualization, Writing – original draft, Funding acquisition, Writing – review & editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors would like to thank all the participating patients and staff members involved in this study, Ms. Keiko Hattori, Ms. Ryoko Kajiya, Ms. Sayoko Ogata, and Ms. Saori Takagi for their laboratory work. We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of interest
SK received honoraria from Asahi Kasei Pharma and Pfizer. EK received honoraria from Kyowa Kirin, Takeda, Otsuka and Sumitomo Pharma. YI received honoraria from Meiji Seika Pharma, Novartis Pharma and Janssen Pharmaceutical, and received research support from Meiji Seika and Amgen. AT received honoraria from Chugai, Nippon-Shinyaku, Ono, AstraZeneca and AbbVie, and received research support from Chugai.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1687204/full#supplementary-material
Abbreviations
allo-HCT, allogeneic hematopoietic cell transplantation; BTK, Bruton’s tyrosine kinase; CI, confidence interval; COVID 19, coronavirus disease 2019; CAR-T, chimeric antigen receptor T-cell therapy; HR, hazard ratio; JAK, Janus kinase; SCT, stem cell transplantation; sHR, subhazard ratio.
References
1. Ministry of Health LaW. Visualizing the data: information on COVID-19 infections (2023). Available online at: https://covid19.mhlw.go.jp/ (Accessed April 14, 2025).
2. Security JIfH. SARS-coV-2 variants: historical data (2023). Available online at: https://id-info.jihs.go.jp/diseases/sa/covid-19/170/cepr-topics-log.html (Accessed April 14, 2025).
3. Lyngse FP, Mortensen LH, Denwood MJ, Christiansen LE, Moller CH, Skov RL, et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun. (2022) 13:5573. doi: 10.1038/s41467-022-33328-3
4. Klompas M, Pandolfi MC, Nisar AB, Baker MA, and Rhee C. Association of omicron vs wild-type SARS-coV-2 variants with hospital-onset SARS-coV-2 infections in a US regional hospital system. JAMA. (2022) 328:296–8. doi: 10.1001/jama.2022.9609
5. Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. (2022) 399:437–46. doi: 10.1016/S0140-6736(22)00017-4
6. Ward IL, Bermingham C, Ayoubkhani D, Gethings OJ, Pouwels KB, Yates T, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. (2022) 378:e070695. doi: 10.1136/bmj-2022-070695
7. Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, and Tartof SY. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med. (2022) 28:1933–43. doi: 10.1038/s41591-022-01887-z
8. Lund LC, Rahbek MT, Brown P, Obel N, Hallas J, and Frederiksen H. Mortality and clinical outcomes following SARS-CoV-2 infection among individuals with haematological Malignancies: A Danish population-based cohort study. Eur J Haematol. (2023) 111(6):946–50. doi: 10.1111/ejh.14108
9. Kevlicius L, Sablauskas K, Maneikis K, Juozapaite D, Ringeleviciute U, Vaitekenaite V, et al. Immunogenicity and clinical effectiveness of mRNA vaccine booster against SARS-CoV-2 Omicron in patients with haematological Malignancies: A national prospective cohort study. Br J Haematol. (2023) 204(2):497–506. doi: 10.1111/bjh.19126
10. Blennow O, Salmanton-Garcia J, Nowak P, Itri F, Van Doesum J, Lopez-Garcia A, et al. Outcome of infection with omicron SARS-CoV-2 variant in patients with hematological Malignancies: An EPICOVIDEHA survey report. Am J Hematol. (2022) 97:E312–E7. doi: 10.1002/ajh.26626
11. Pagano L, Salmanton-Garcia J, Marchesi F, Blennow O, Gomes da Silva M, Glenthoj A, et al. Breakthrough COVID-19 in vaccinated patients with hematologic Malignancies: results from the EPICOVIDEHA survey. Blood. (2022) 140:2773–87. doi: 10.1182/blood.2022017257
12. Visentin A, Chatzikonstantinou T, Scarfo L, Kapetanakis A, Demosthenous C, Karakatsoulis G, et al. The evolving landscape of COVID-19 and post-COVID condition in patients with chronic lymphocytic leukemia: A study by ERIC, the European research initiative on CLL. Am J Hematol. (2023) 98:1856–68. doi: 10.1002/ajh.27093
13. Iriyama C, Ichikawa T, Tamura T, Takahata M, Ishio T, Ibata M, et al. Clinical and molecular landscape of prolonged SARS-CoV-2 infection with resistance to remdesivir in immunocompromised patients. PNAS Nexus. (2025) 4:pgaf085. doi: 10.1093/pnasnexus/pgaf085
14. Musto P, Salmanton-Garcia J, Sgherza N, Bergantim R, Farina F, Glenthoj A, et al. Survival in multiple myeloma and SARS-COV-2 infection through the COVID-19 pandemic: Results from the EPICOVIDEHA registry. Hematol Oncol. (2024) 42:e3240. doi: 10.1002/hon.3240
15. Pinana JL, Vazquez L, Heras I, Aiello TF, Lopez-Corral L, Arroyo I, et al. Omicron SARS-CoV-2 infection management and outcomes in patients with hematologic disease and recipients of cell therapy. Front Oncol. (2024) 14:1389345. doi: 10.3389/fonc.2024.1389345
16. Ikeda D, Fukumoto A, Uesugi Y, Tabata R, Miura D, Narita K, et al. Clinical and immunological characteristics of prolonged SARS-CoV-2 Omicron infection in hematologic disease. Blood Cancer J. (2023) 13:133. doi: 10.1038/s41408-023-00897-5
17. Kang SW, Kim JW, Kim JY, Lim SY, Jang CY, Chang E, et al. Characteristics and risk factors of prolonged viable virus shedding in immunocompromised patients with COVID-19: a prospective cohort study. J Infect. (2023) 86:412–4. doi: 10.1016/j.jinf.2023.01.024
18. Li G, Hilgenfeld R, Whitley R, and De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. (2023) 22:449–75. doi: 10.1038/s41573-023-00672-y
19. Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, Zhou D, Ginn HM, Selvaraj M, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. (2022) 185:2422–33 e13. doi: 10.1016/j.cell.2022.06.005
20. Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, et al. Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. (2022) 387:468–70. doi: 10.1056/NEJMc2207519
21. Planas D, Staropoli I, Planchais C, Yab E, Jeyarajah B, Rahou Y, et al. Escape of SARS-coV-2 variants KP.1.1, LB.1, and KP3.3 from approved monoclonal antibodies. Pathog Immun. (2024) 10:1–11. doi: 10.20411/pai.v10i1.752
22. Gandhi S, Klein J, Robertson AJ, Pena-Hernandez MA, Lin MJ, Roychoudhury P, et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat Commun. (2022) 13:1547. doi: 10.1038/s41467-022-29104-y
23. Duan Y, Zhou H, Liu X, Iketani S, Lin M, Zhang X, et al. Molecular mechanisms of SARS-CoV-2 resistance to nirmatrelvir. Nature. (2023) 622:376–82. doi: 10.1038/s41586-023-06609-0
24. Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case study: prolonged infectious SARS-coV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. (2020) 183:1901–12 e9. doi: 10.1016/j.cell.2020.10.049
25. Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of SARS-coV-2 in an immunocompromised host. N Engl J Med. (2020) 383:2291–3. doi: 10.1056/NEJMc2031364
26. Okamoto A, Fujigaki H, Iriyama C, Goto N, Yamamoto H, Mihara K, et al. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv. (2022) 6:3230–3. doi: 10.1182/bloodadvances.2021006302
27. Maneikis K, Sablauskas K, Ringeleviciute U, Vaitekenaite V, Cekauskiene R, Kryzauskaite L, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological Malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. (2021) 8:e583–e92. doi: 10.1016/S2352-3026(21)00169-1
28. Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. (2021) 5:3053–61. doi: 10.1182/bloodadvances.2021005094
29. Lim SH, Campbell N, Johnson M, Joseph-Pietras D, Collins GP, O’Callaghan A, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. (2021) 8:e542–e4. doi: 10.1016/S2352-3026(21)00199-X
30. Shree T, Shankar V, Lohmeyer JJK, Czerwinski DK, Schroers-Martin JG, Rodriguez GM, et al. CD20-targeted therapy ablates de novo antibody response to vaccination but spares preestablished immunity. Blood Cancer Discov. (2022) 3:95–102. doi: 10.1158/2643-3230.BCD-21-0222
31. Miller J, Hachmann NP, Collier AY, Lasrado N, Mazurek CR, Patio RC, et al. Substantial neutralization escape by SARS-coV-2 omicron variants BQ.1.1 and XBB.1. N Engl J Med. (2023) 388:662–4. doi: 10.1056/NEJMc2214314
32. Planas D, Staropoli I, Michel V, Lemoine F, Donati F, Prot M, et al. Distinct evolution of SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat Commun. (2024) 15:2254. doi: 10.1038/s41467-024-46490-7
33. Wang Q, Guo Y, Bowen A, Mellis IA, Valdez R, Gherasim C, et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe. (2024) 32:315–21 e3. doi: 10.1016/j.chom.2024.01.014
34. Kaku Y, Yo MS, Tolentino JE, Uriu K, and Okumura K. Genotype to Phenotype Japan C, et al. Virological characteristics of the SARS-CoV-2 KP.3, LB.1, and KP.2.3 variants. Lancet Infect Dis. (2024) 24:e482–e3. doi: 10.1016/S1473-3099(24)00415-8
35. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
36. Davis HE, McCorkell L, Vogel JM, and Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
37. Visco C, Marcheselli L, Mina R, Sassone M, Guidetti A, Penna D, et al. A prognostic model for patients with lymphoma and COVID-19: a multicentre cohort study. Blood Adv. (2022) 6:327–38. doi: 10.1182/bloodadvances.2021005691
38. Ichikawa T, Tamura T, Takahata M, Ishio T, Ibata M, Kasahara I, et al. Prolonged shedding of viable SARS-CoV-2 in immunocompromised patients with haematological Malignancies: A prospective study. Br J Haematol. (2024) 204:815–20. doi: 10.1111/bjh.19143
39. Franceschini E, Pellegrino M, Todisco V, Dolci G, Bettelli F, Meschiari M, et al. Persistent SARS-CoV-2 infection with multiple clinical relapses in two patients with follicular lymphoma treated with bendamustine and obinutuzumab or rituximab. Infection. (2023) 51:1577–81. doi: 10.1007/s15010-023-02039-2
40. DeWolf S, Laracy JC, Perales MA, Kamboj M, van den Brink MRM, and Vardhana S. SARS-CoV-2 in immunocompromised individuals. Immunity. (2022) 55:1779–98. doi: 10.1016/j.immuni.2022.09.006
41. Lamure S, Dulery R, Di Blasi R, Chauchet A, Laureana C, Deau-Fischer B, et al. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: A retrospective multicentric cohort study. EClinicalMedicine. (2020) 27:100549. doi: 10.1016/j.eclinm.2020.100549
42. Nakajima Y, Ogai A, Furukawa K, Arai R, Anan R, Nakano Y, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. (2021) 27:387–9. doi: 10.1016/j.jiac.2020.12.001
43. Sepulcri C, Dentone C, Mikulska M, Bruzzone B, Lai A, Fenoglio D, et al. The longest persistence of viable SARS-coV-2 with recurrence of viremia and relapsing symptomatic COVID-19 in an immunocompromised patient-A case study. Open Forum Infect Dis. (2021) 8:ofab217. doi: 10.1093/ofid/ofab217
44. Martinez-Calle N, Hartley S, Ahearne M, Kasenda B, Beech A, Knight H, et al. Kinetics of T-cell subset reconstitution following treatment with bendamustine and rituximab for low-grade lymphoproliferative disease: a population-based analysis. Br J Haematol. (2019) 184:957–68. doi: 10.1111/bjh.15722
45. Gafter-Gvili A and Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological Malignancies. Leuk Lymphoma. (2016) 57:512–9. doi: 10.3109/10428194.2015.1110748
46. Yutaka T, Ito S, Ohigashi H, Naohiro M, Shimono J, Souichi S, et al. Sustained CD4 and CD8 lymphopenia after rituximab maintenance therapy following bendamustine and rituximab combination therapy for lymphoma. Leuk Lymphoma. (2015) 56:3216–8. doi: 10.3109/10428194.2015.1026818
47. Grigg A, Dyer MJ, Diaz MG, Dreyling M, Rule S, Lei G, et al. Safety and efficacy of obinutuzumab with CHOP or bendamustine in previously untreated follicular lymphoma. Haematologica. (2017) 102:765–72. doi: 10.3324/haematol.2016.152272
48. Pezzullo L, Giudice V, Serio B, Fontana R, Guariglia R, Martorelli MC, et al. Real-world evidence of cytomegalovirus reactivation in non-Hodgkin lymphomas treated with bendamustine-containing regimens. Open Med (Wars). (2021) 16:672–82. doi: 10.1515/med-2021-0274
49. Ishio T, Tsukamoto S, Yokoyama E, Izumiyama K, Saito M, Muraki H, et al. Anti-CD20 antibodies and bendamustine attenuate humoral immunity to COVID-19 vaccination in patients with B-cell non-Hodgkin lymphoma. Ann Hematol. (2023) 102:1421–31. doi: 10.1007/s00277-023-05204-7
50. Serna A, Navarro V, Iacoboni G, Lopez L, Sancho JM, Gonzalez-Barca E, et al. Rituximab maintenance after bendamustine-based treatment for follicular lymphoma and mantle cell lymphoma may exert a negative influence on SARS-CoV-2 infection outcomes. Haematologica. (2025) 110:173–8. doi: 10.3324/haematol.2024.285219
51. Lucas C, Klein J, Sundaram ME, Liu F, Wong P, Silva J, et al. Author Correction: Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat Med. (2021) 27:1309. doi: 10.1038/s41591-021-01416-4
52. Bergamaschi L, Mescia F, Turner L, Hanson AL, Kotagiri P, Dunmore BJ, et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. (2021) 54:1257–75 e8. doi: 10.1016/j.immuni.2021.05.010
53. Notarbartolo S, Ranzani V, Bandera A, Gruarin P, Bevilacqua V, Putignano AR, et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci Immunol. (2021) 6(62):eabg5021. doi: 10.1126/sciimmunol.abg5021
54. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-coV-2 in acute COVID-19 and associations with age and disease severity. Cell. (2020) 183:996–1012 e19. doi: 10.1016/j.cell.2020.09.038
55. Randi BA, Higashino HR, Silva VPD, Xavier EM, Rocha V, and Costa SF. COVID-19 in hematopoietic stem-cell transplant recipients: A systematic review and meta-analysis of clinical characteristics and outcomes. Rev Med Virol. (2023) 33:e2483. doi: 10.1002/rmv.2483
56. Takagi E, Terakura S, Fujigaki H, Okamoto A, Miyao K, Sawa M, et al. Antibody response after third dose of COVID-19 mRNA vaccination in allogeneic hematopoietic stem cell transplant recipients is comparable to that in healthy counterparts. Int J Hematol. (2023) 118:462–71. doi: 10.1007/s12185-023-03648-1
57. Pinana JL, Martino R, Vazquez L, Lopez-Corral L, Perez A, Chorao P, et al. SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination. Bone Marrow Transplant. (2023) 58:567–80. doi: 10.1038/s41409-023-01946-0
Keywords: COVID-19, hematologic diseases, Omicron variant, prognosis, immunocompromised patients, multicenter study
Citation: Okamoto A, Yoshida M, Kasahara S, Ara T, Ozeki K, Morishita T, Ikeda D, Kanaya M, Kajiguchi T, Suzuki Y, Kurahashi S, Horio T, Marumo Y, Oyake T, Saito S, Sawa H, Kimura S-i, Nishiyama T, Kondo E, Hiraga J, Hosoi H, Masaki Y, Atsuta Y, Yamamoto H, Miyama T, Goto N, Iriyama C, Mihara K, Inamoto Y and Tomita A (2025) Severe and/or prolonged COVID-19 in hematologic diseases: clinical implications before and during the omicron era. Front. Oncol. 15:1687204. doi: 10.3389/fonc.2025.1687204
Received: 17 August 2025; Accepted: 22 October 2025;
Published: 05 November 2025.
Edited by:
Nicola Sgherza, AOU Policlinico Consorziale di Bari, ItalyReviewed by:
Francesco Tarantini, University of Bari Aldo Moro, ItalyAntonella Russo Rossi, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Italy
Copyright © 2025 Okamoto, Yoshida, Kasahara, Ara, Ozeki, Morishita, Ikeda, Kanaya, Kajiguchi, Suzuki, Kurahashi, Horio, Marumo, Oyake, Saito, Sawa, Kimura, Nishiyama, Kondo, Hiraga, Hosoi, Masaki, Atsuta, Yamamoto, Miyama, Goto, Iriyama, Mihara, Inamoto and Tomita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akinao Okamoto, YW9rYW1vdG9AZnVqaXRhLWh1LmFjLmpw
 Akinao Okamoto
Akinao Okamoto Masahiro Yoshida
Masahiro Yoshida Senji Kasahara3
Senji Kasahara3 Takahide Ara
Takahide Ara Minoru Kanaya
Minoru Kanaya Tatsuo Oyake
Tatsuo Oyake Eisei Kondo
Eisei Kondo Yasufumi Masaki
Yasufumi Masaki Yoshiko Atsuta
Yoshiko Atsuta Akihiro Tomita
Akihiro Tomita